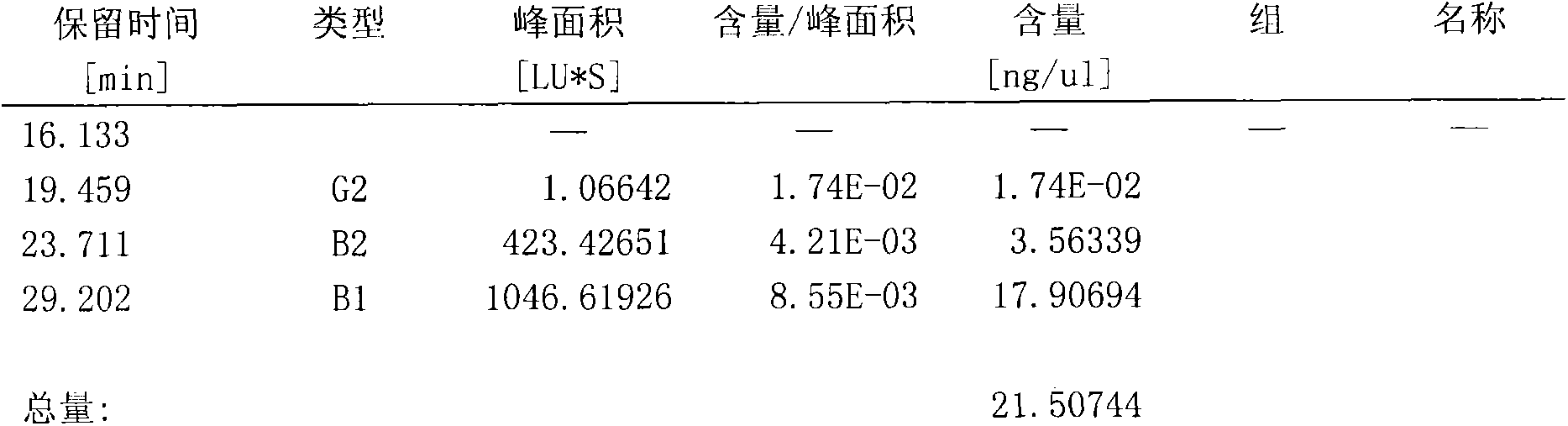
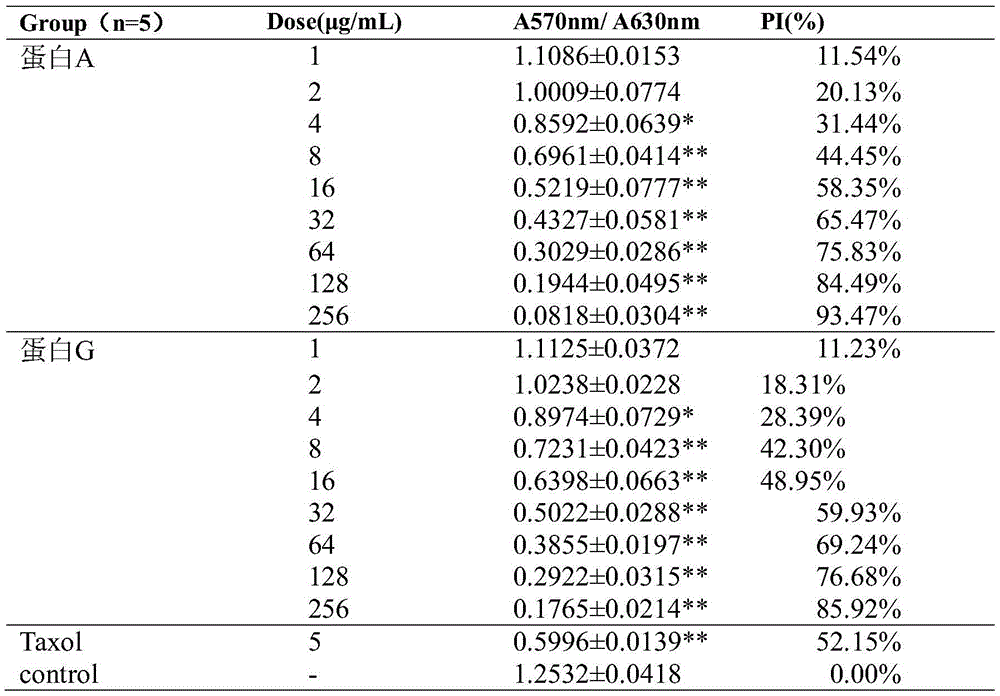
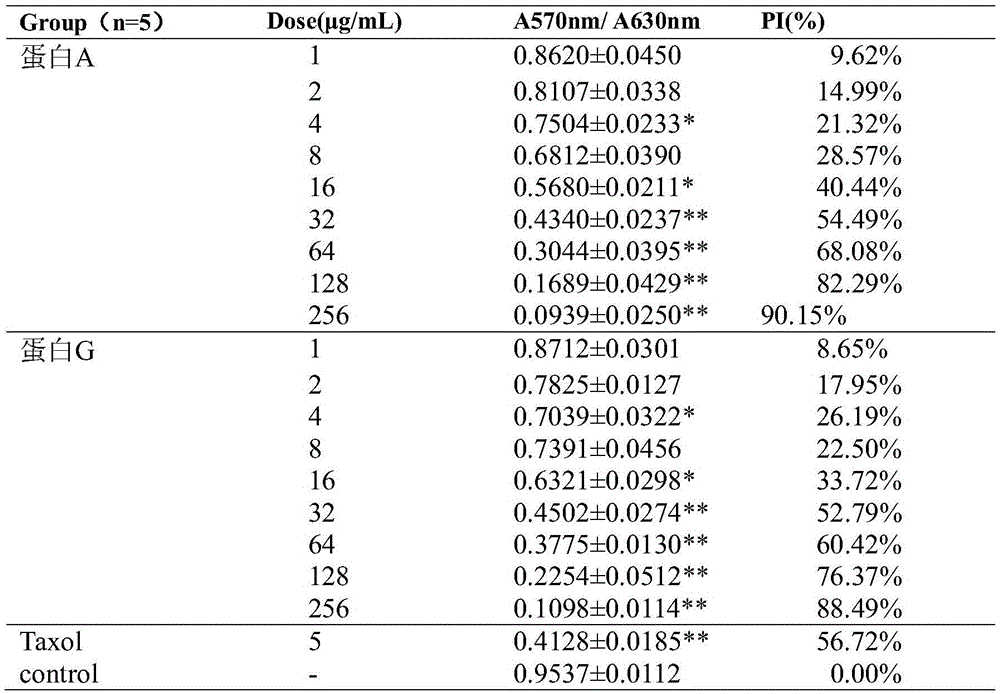
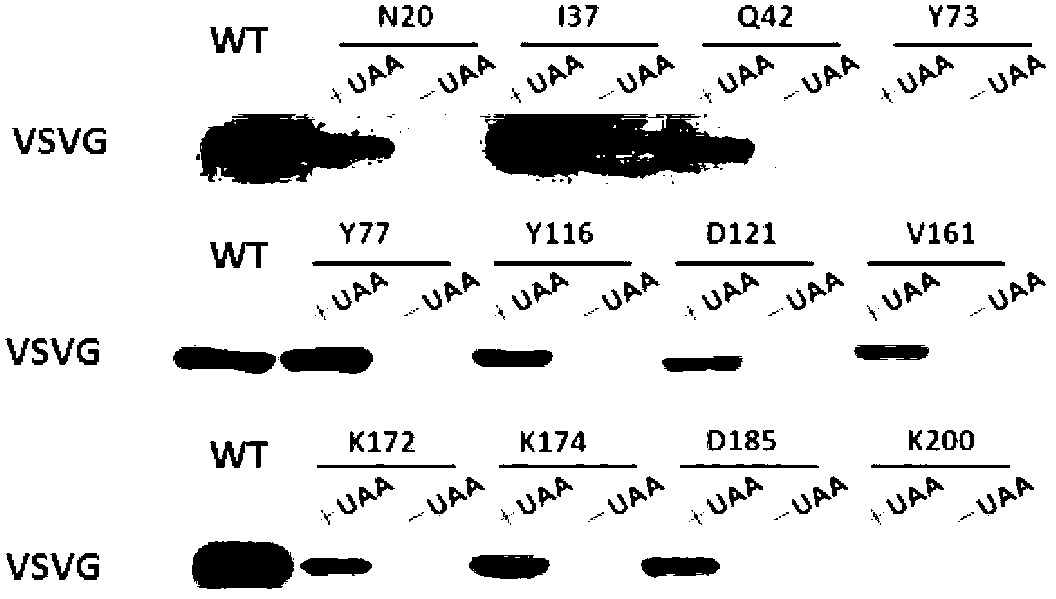Patents
Literature
157 results about "Protein G" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
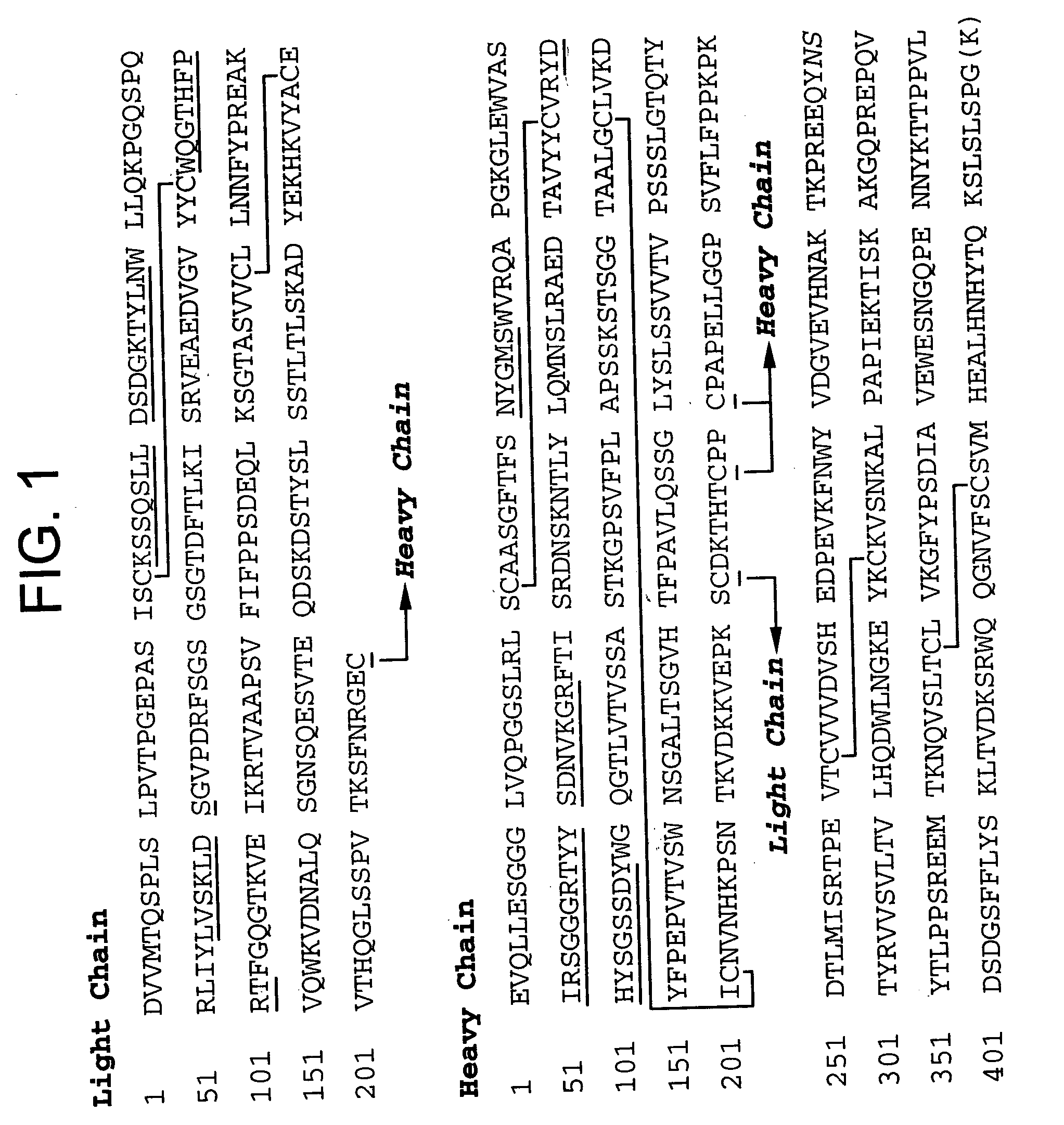
Protein G is an immunoglobulin-binding protein expressed in group C and G Streptococcal bacteria much like Protein A but with differing binding specificities. It is a 65-kDa (G148 protein G) and a 58 kDa (C40 protein G) cell surface protein that has found application in purifying antibodies through its binding to the Fab and Fc region. The native molecule also binds albumin, but because serum albumin is a major contaminant of antibody sources, the albumin binding site has been removed from recombinant forms of Protein G. This recombinant Protein G, either labeled with a fluorophore or a single-stranded DNA strand, was used as a replacement for secondary antibodies in immunofluorescence and super-resolution imaging.
Recombinant polyclonal antibody for treatment of respiratory syncytial virus infections
InactiveUS20100040606A1Reduce the possibilityMinimizing developmentSsRNA viruses negative-senseSugar derivativesEpitopeProtein G
Disclosed are novel polyclonal antibodies, which target respiratory syncyticilal virus (RSV), and novel high affinity antibody molecules reactive with RSV. The polyclonal antibodies may comprise antibody molecules which are reactive with both RSV protein F and RSV protein G, and preferably the polyclonal antibodies target a variety of epitopes on these proteins. The single antibody molecules of the invention are shown to exhibit affinities which provide for dissociation constants as low as in the picomolar range. Also disclosed are methods of producing the antibodies of the invention as well as methods of their use in treatment for RSV infection.
Owner:SYMPHOGEN AS
Methods of purifying Fc region containing proteins
ActiveUS20070072307A1Efficient removalDecrease and prevent nonspecific adherenceImmunoglobulins against animals/humansPeptide preparation methodsProtein GFc binding
The present application provides methods of purifying polypeptides having a Fc region, for example, antibodies or antibody fusions, by adsorbing the polypeptides to a Fc binding agent, such as, for example, Protein A or Protein G, followed by a wash with a divalent cation salt buffer to remove impurities and subsequent recovery of the adsorbed polypeptides. The present application also features methods of eluting the purified polypeptide as well as the incorporation of the methods within a purification train. Kits comprising components for carrying out the methods and instructions for use are also provided.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
Immunosensors: scFv-linker design for surface immobilization
InactiveUS20110201032A1Bioreactor/fermenter combinationsMaterial nanotechnologySingle-Chain AntibodiesSide chain
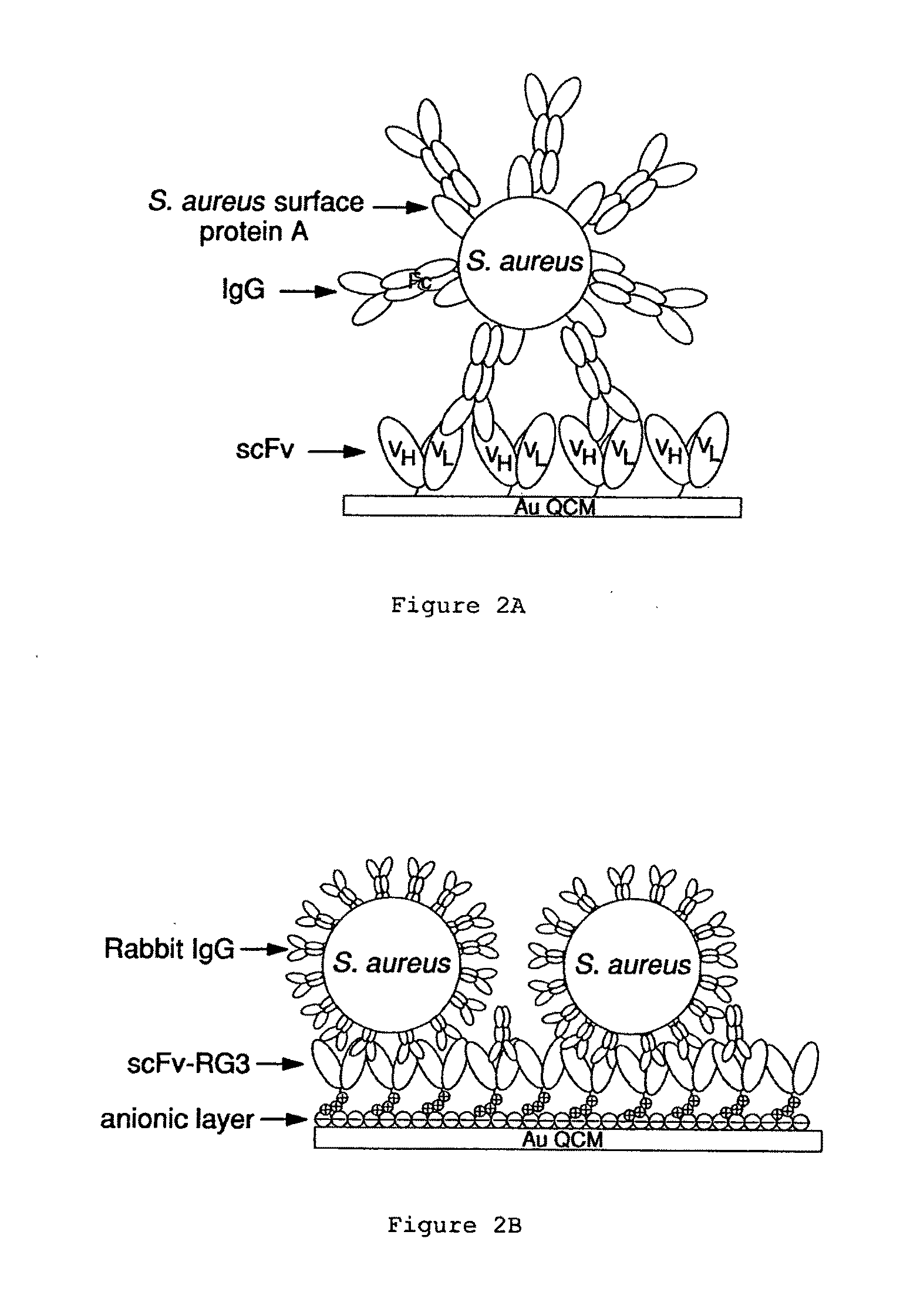
An apparatus and methods for binding an analyte of interest in a sample are provided. The apparatus comprises a substrate with an exposed surface with an compound, that is electrostatically charged or capable of forming hydrogen bonds, provided bound to the solid substrate. A recombinant single chain antibody (scFv) molecule specific for the analyte of interest, having one or more amino acids with charged or hydrogen-bond forming sidechains in a linker polypeptide portion, is bound to the layer on the solid substrate. When the analyte of interest is present in the sample the scFv binds the analyte to the solid substrate. The apparatus can be used with an immunoglobulin layer to detect Fc receptors, so as to detect microorganisms such as Staphylococcus aureus having protein A or protein G.
Owner:OAKLAND UNIVESITY +1
Methods and means for targeted gene delivery
InactiveUS7033834B2Efficient introductionEfficiently introducedBiocideGenetic material ingredientsGene deliveryDelivery vehicle
A method for producing viral gene delivery vehicles which can be transferred to pre-selected cell types by using targeting conjugates. The gene delivery vehicles comprise: 1) the gene of interest; and 2) a viral capsid or envelope carrying a member of a specific binding pair, the counterpart of which is not directly associated with the surface of the target cell. These vehicles can be rendered unable to bind to their natural cell receptor. The targeting conjugates include the counterpart member of the specific binding pair, linked to a targeting moiety which is a cell-type specific ligand (or fragments thereof). The number of the specific binding pair present on the viral vehicles can be, for example, an immunoglobulin binding moiety (e.g., capable of binding to a Fc fragment, protein A, protein G, FcR or an anti-Ig antibody), or biotin, avidin or streptavidin. The virus' outer membrane or capsid may contain a substance which mediates entrance of the gene delivery vehicle into the target cell. Due to the specificity of the ligand, the binding pair's high affinity, and the gene delivery vehicle's inability to be targeted when used alone, the universality of the method for gene delivery, together with its high cell type selectively can be achieved by using various targeting conjugates.
Owner:JANSSEN VACCINES & PREVENTION BV
Methods of purifying anti a beta antibodies
InactiveUS20070082367A1Efficient removalDecrease and prevent nonspecific and adherenceAnimal cellsSugar derivativesGreek letter betaProtein G
The present application provides methods of purifying Aβ binding proteins having a Fc region, for example, anti-Aβ antibodies or antibody fusions, by adsorbing the Aβ binding protein to a Fc binding agent, such as, for example, Protein A or Protein G, followed by a wash with a divalent cation salt buffer to remove impurities and subsequent recovery of the adsorbed Aβ binding protein. The present application also features methods of eluting the purified Aβ binding protein as well as the incorporation of the methods within a purification train. Kits comprising components for carrying out the methods and instructions for use are also provided.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
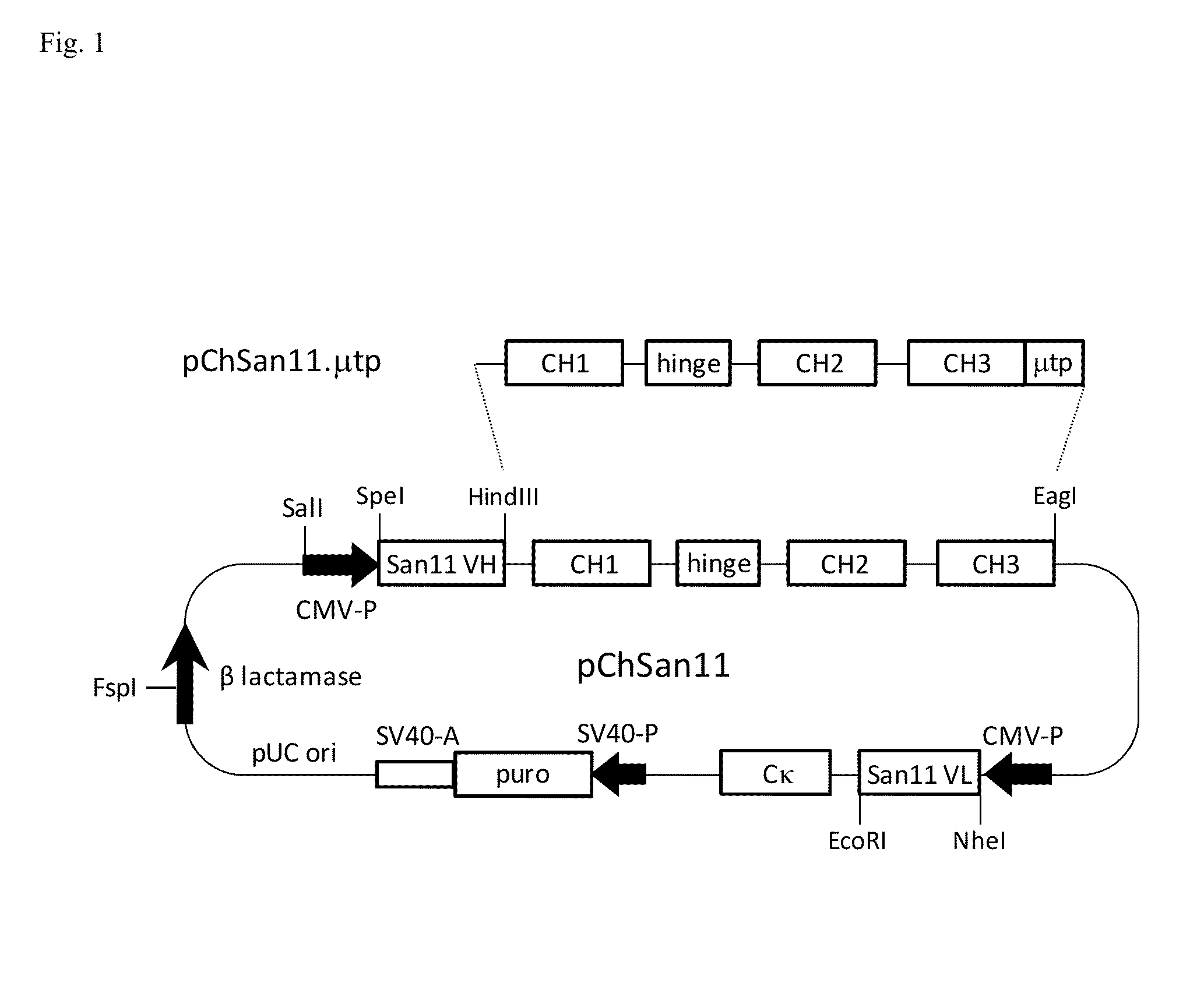
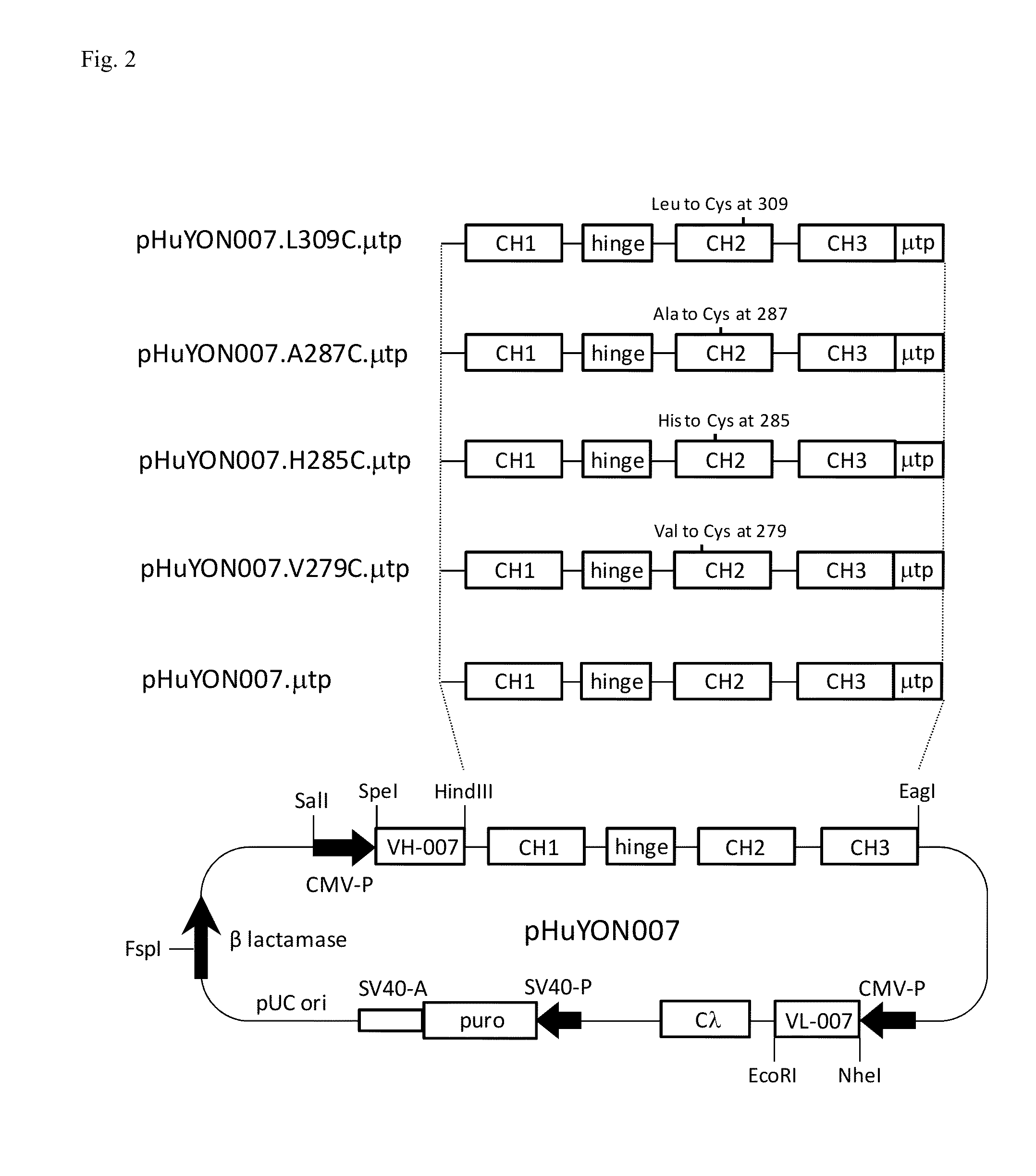
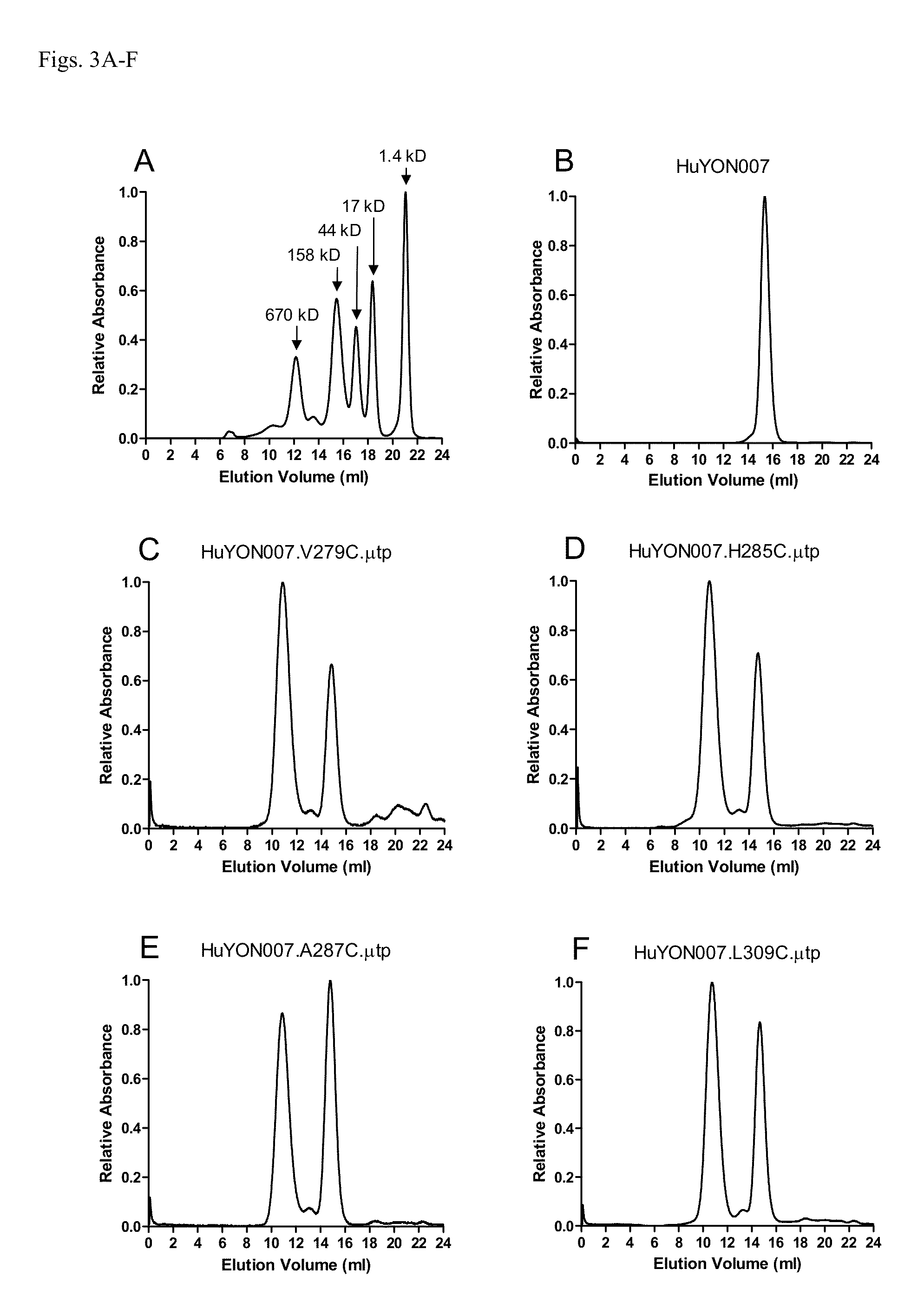
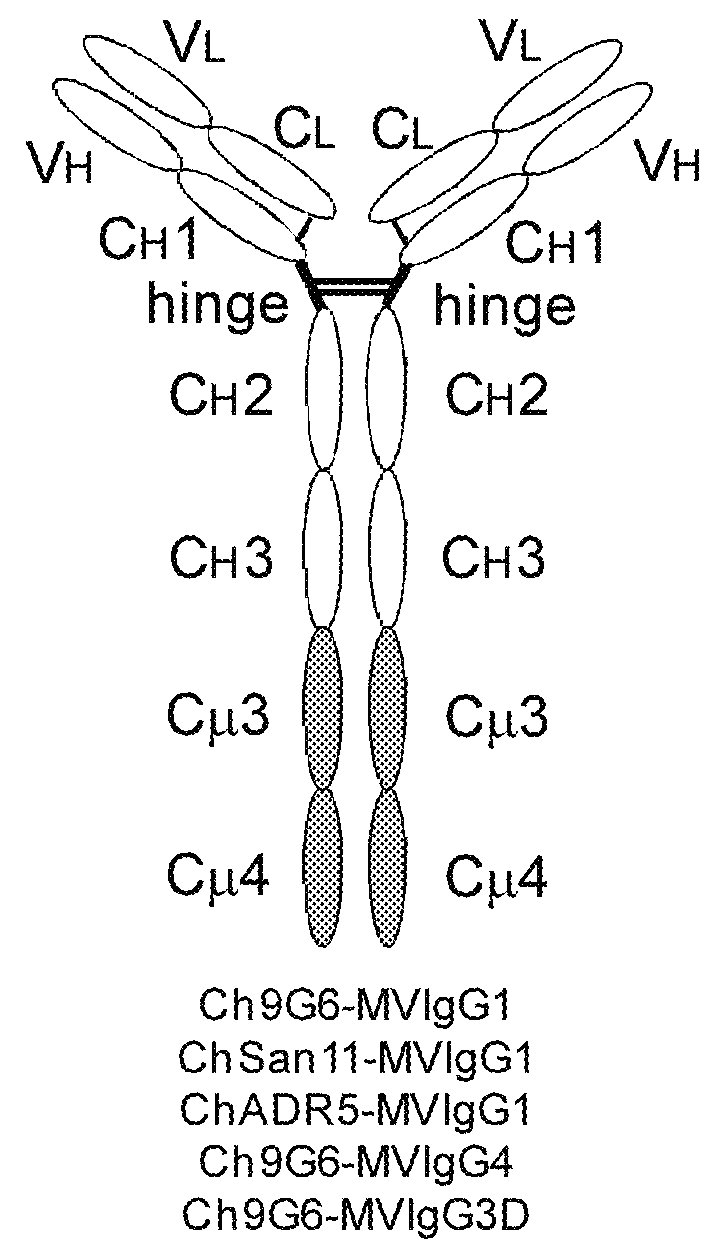
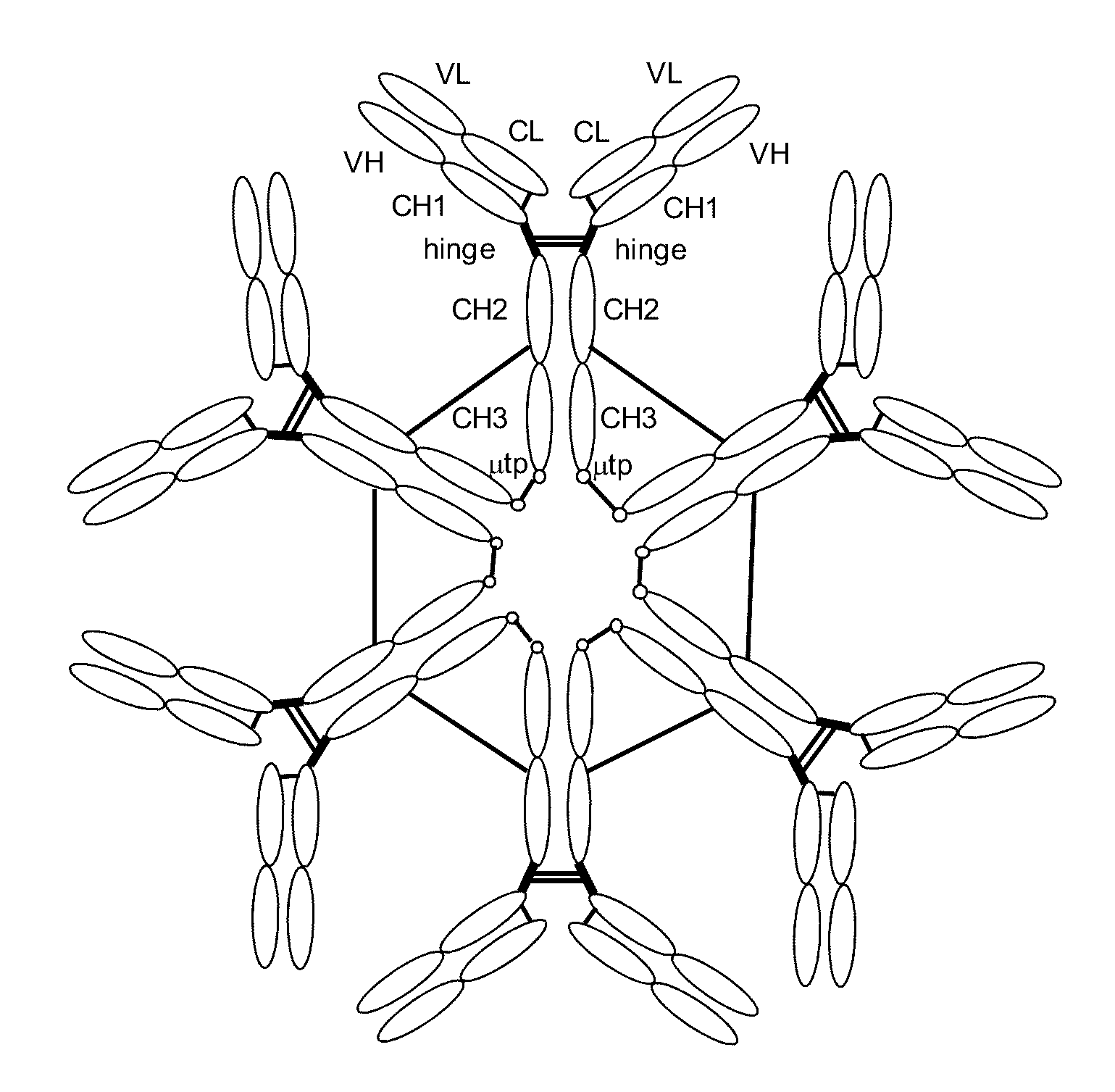
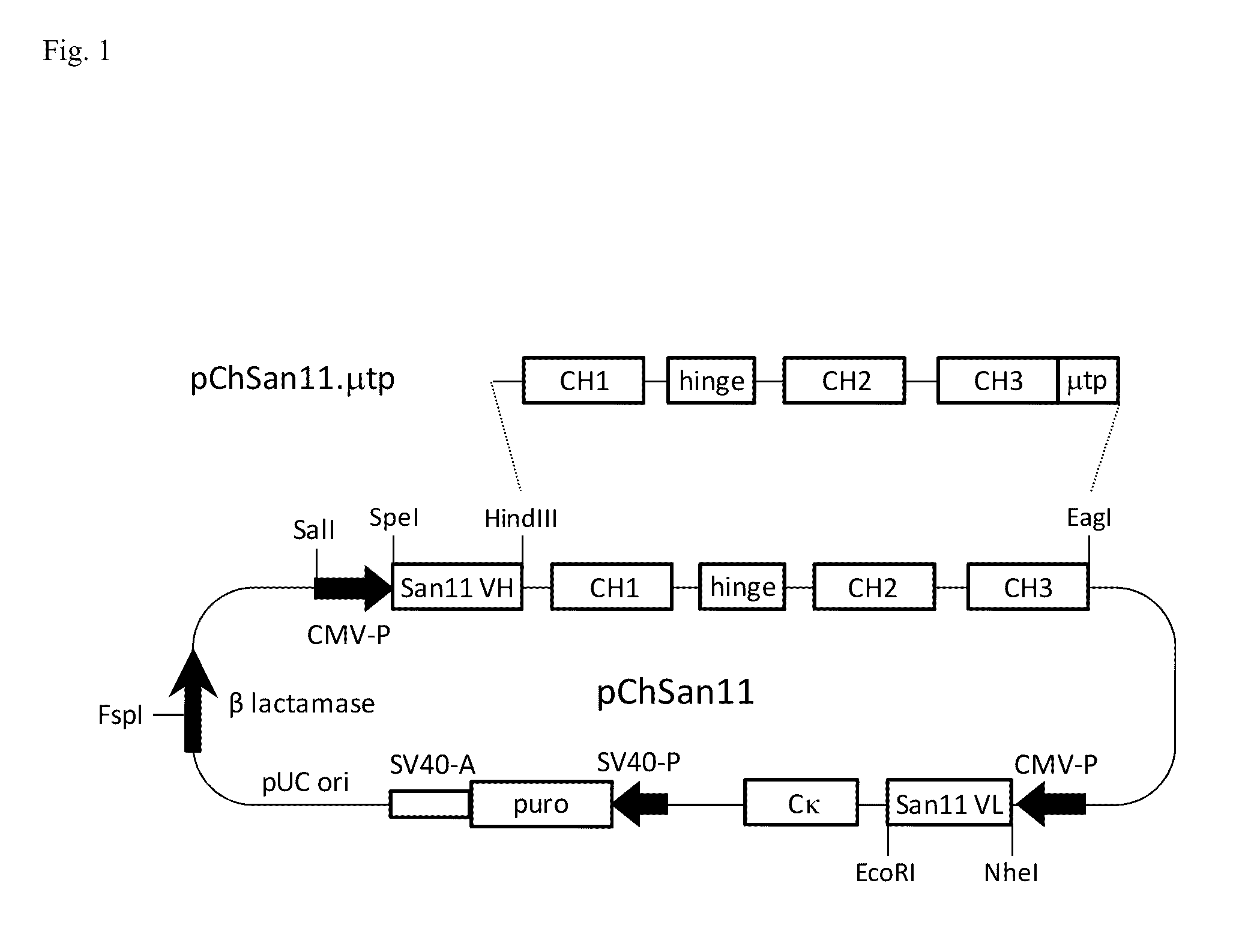
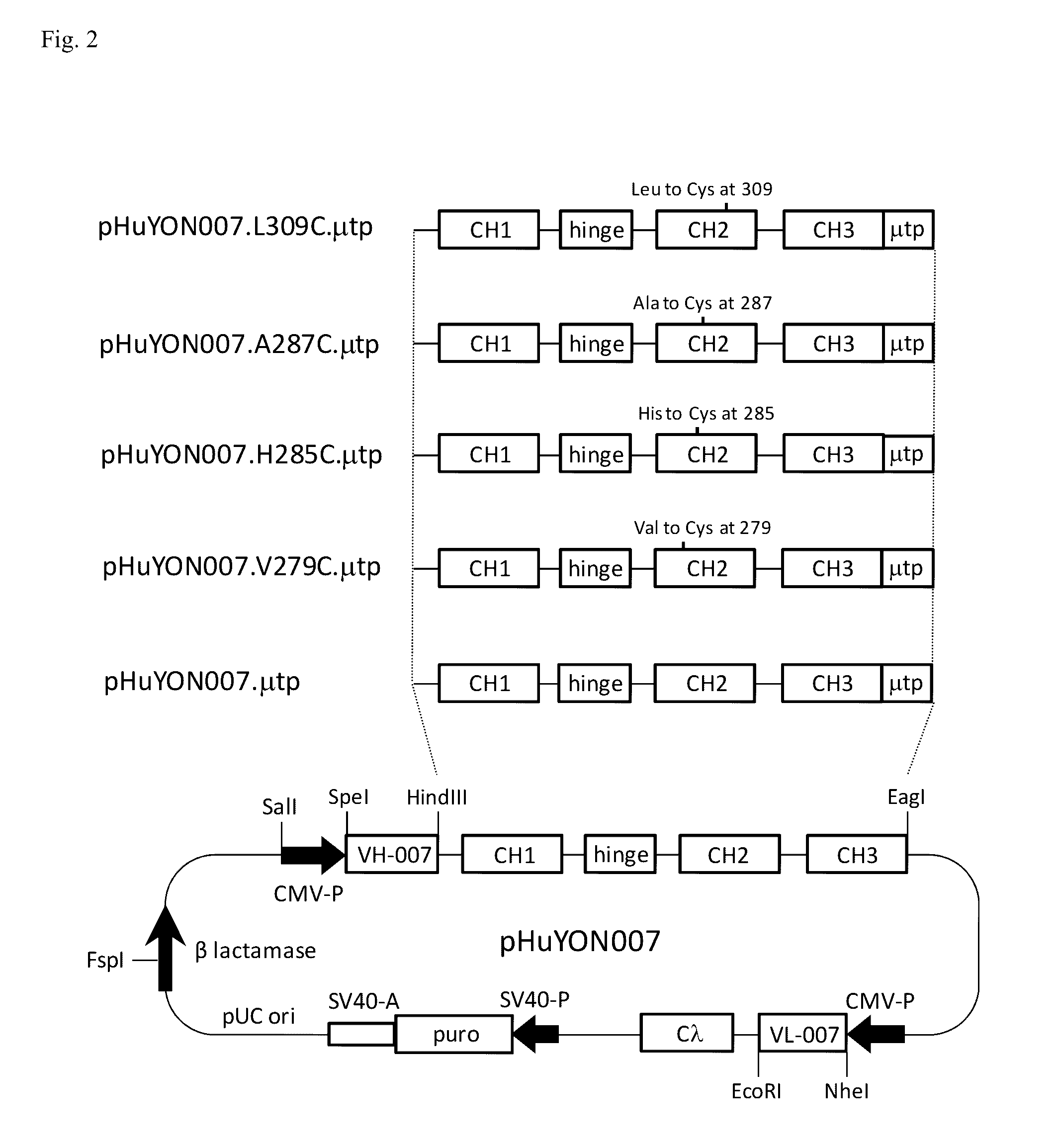
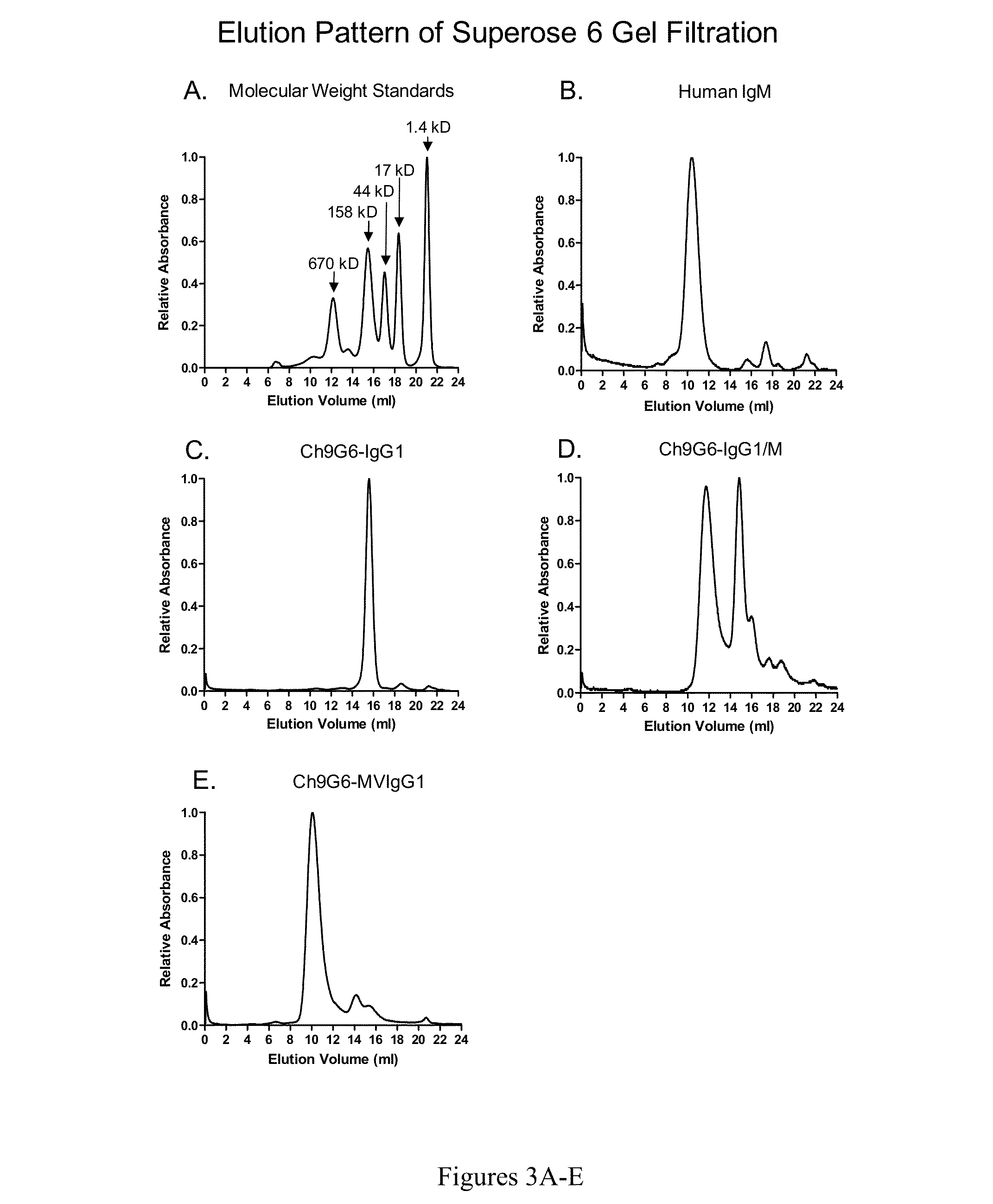
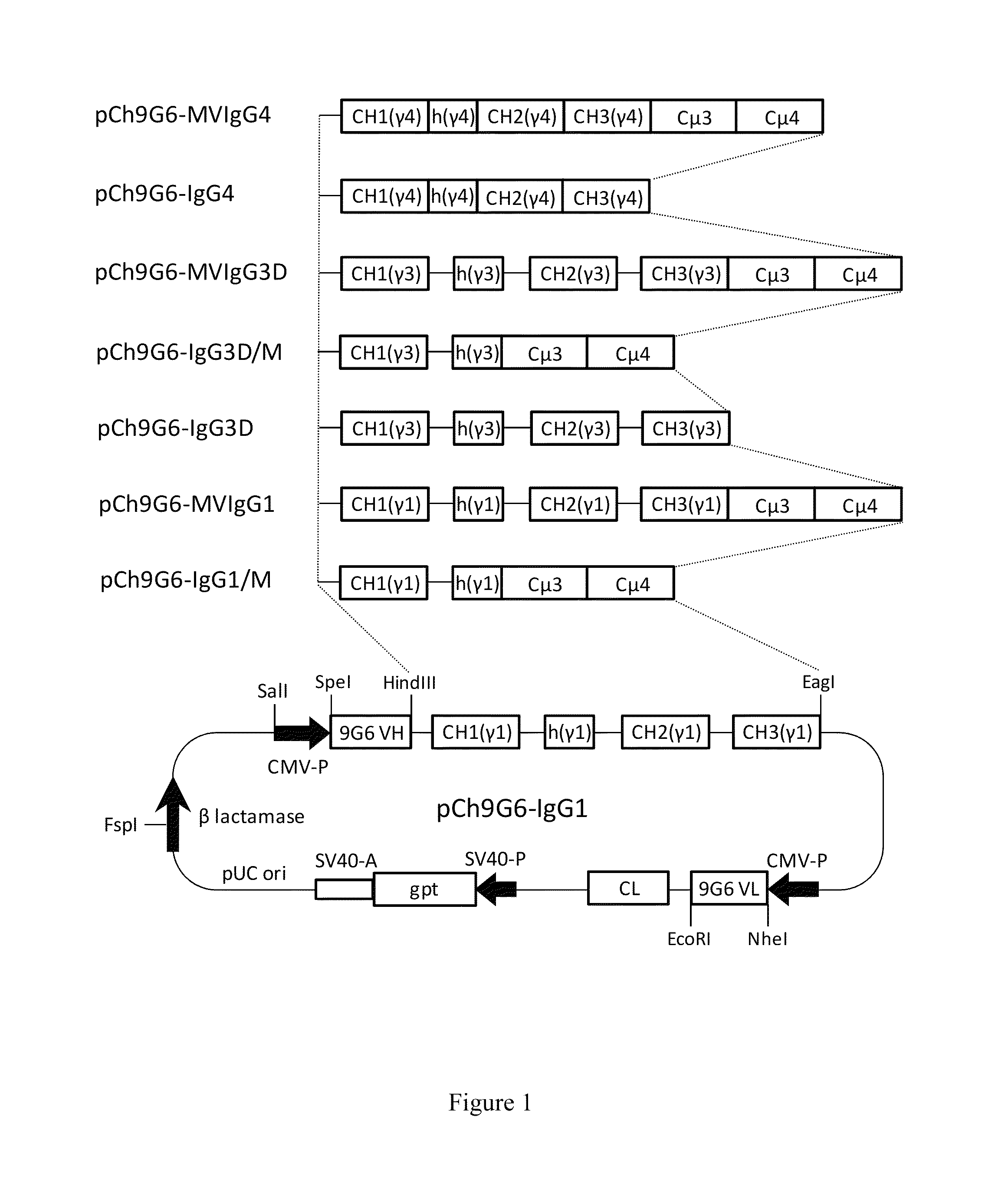
Antibodies or fusion proteins multimerized via cysteine mutation and a mu tailpiece
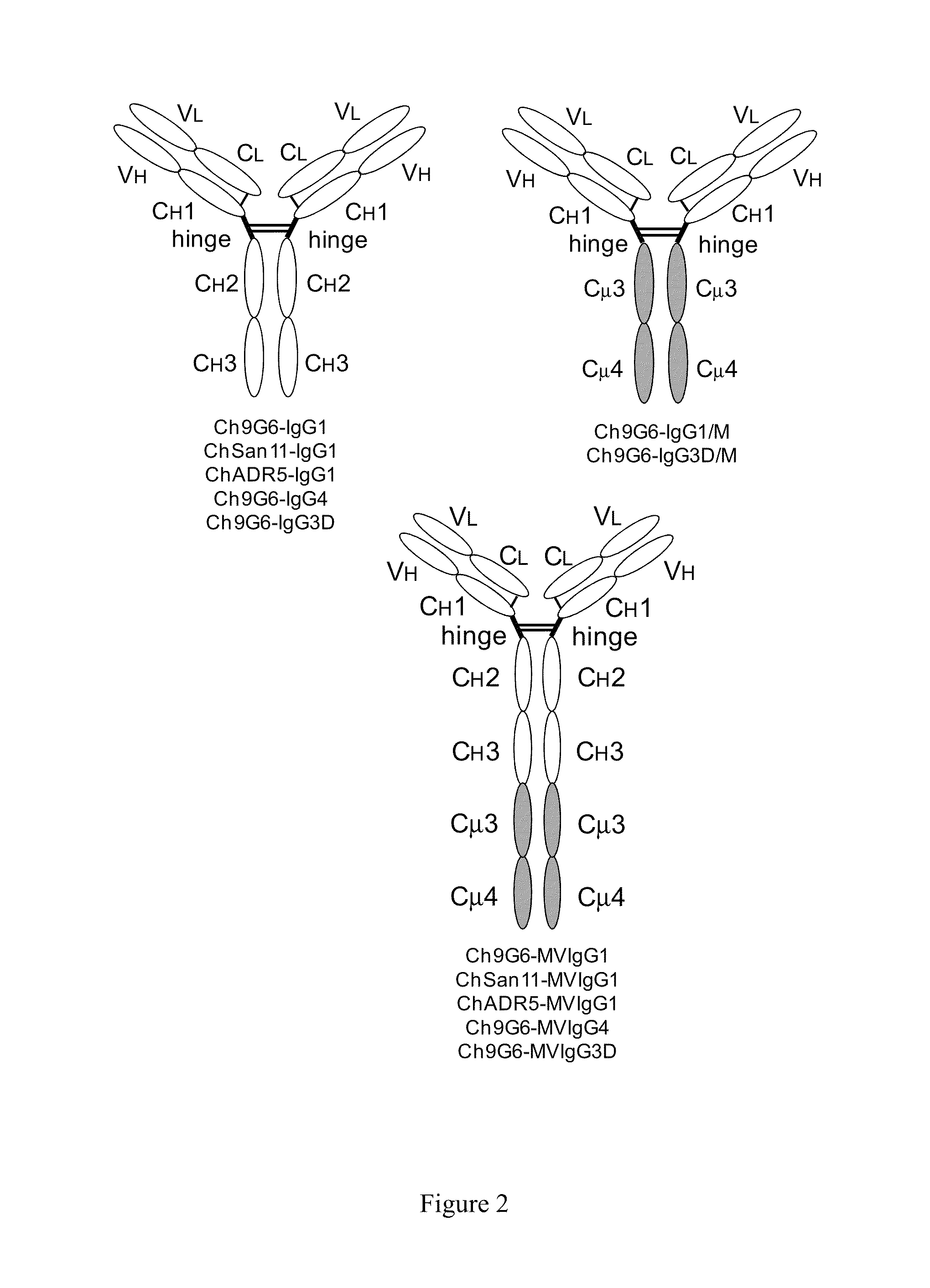
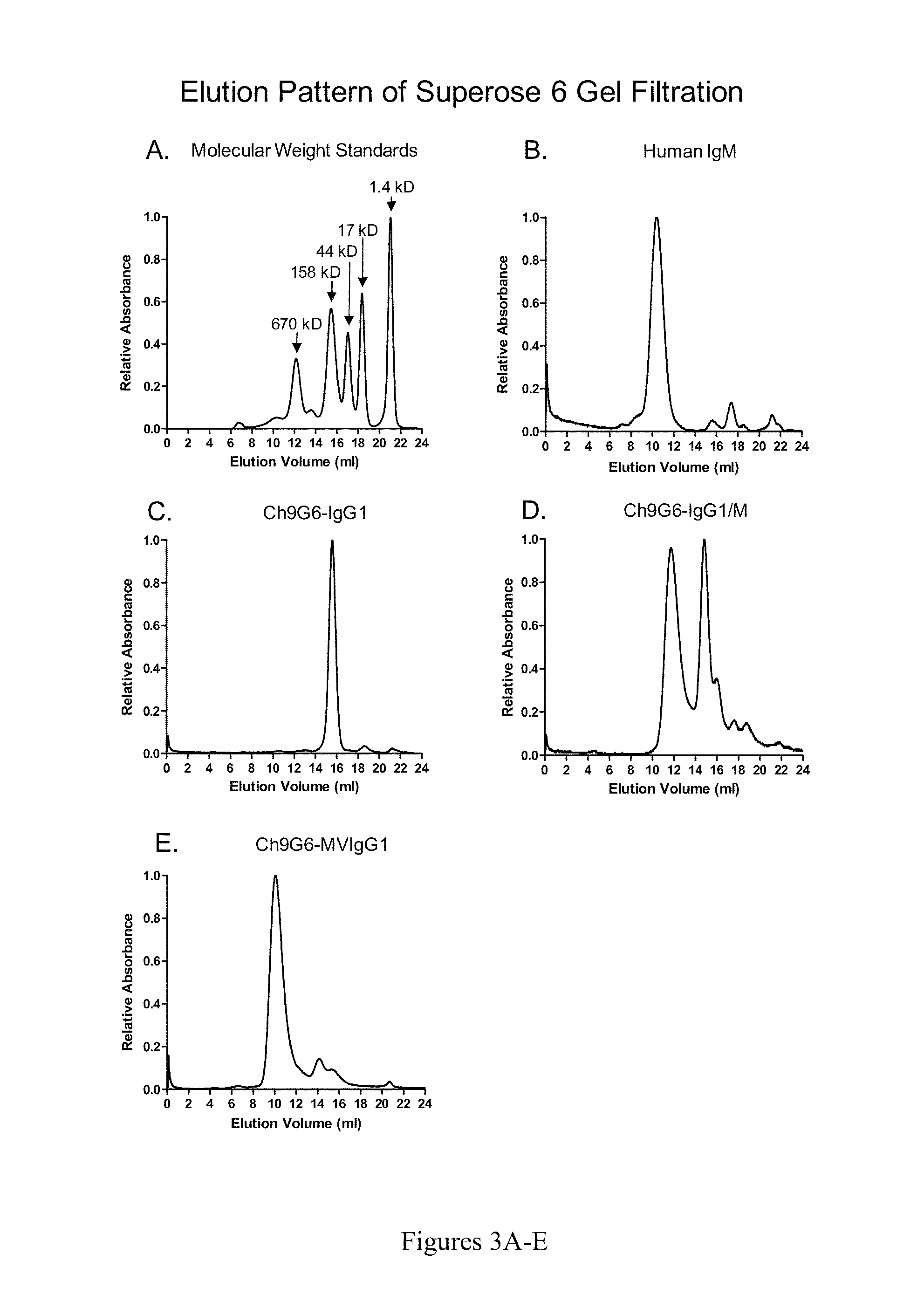
ActiveUS20140037621A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenIgG.heavy chain
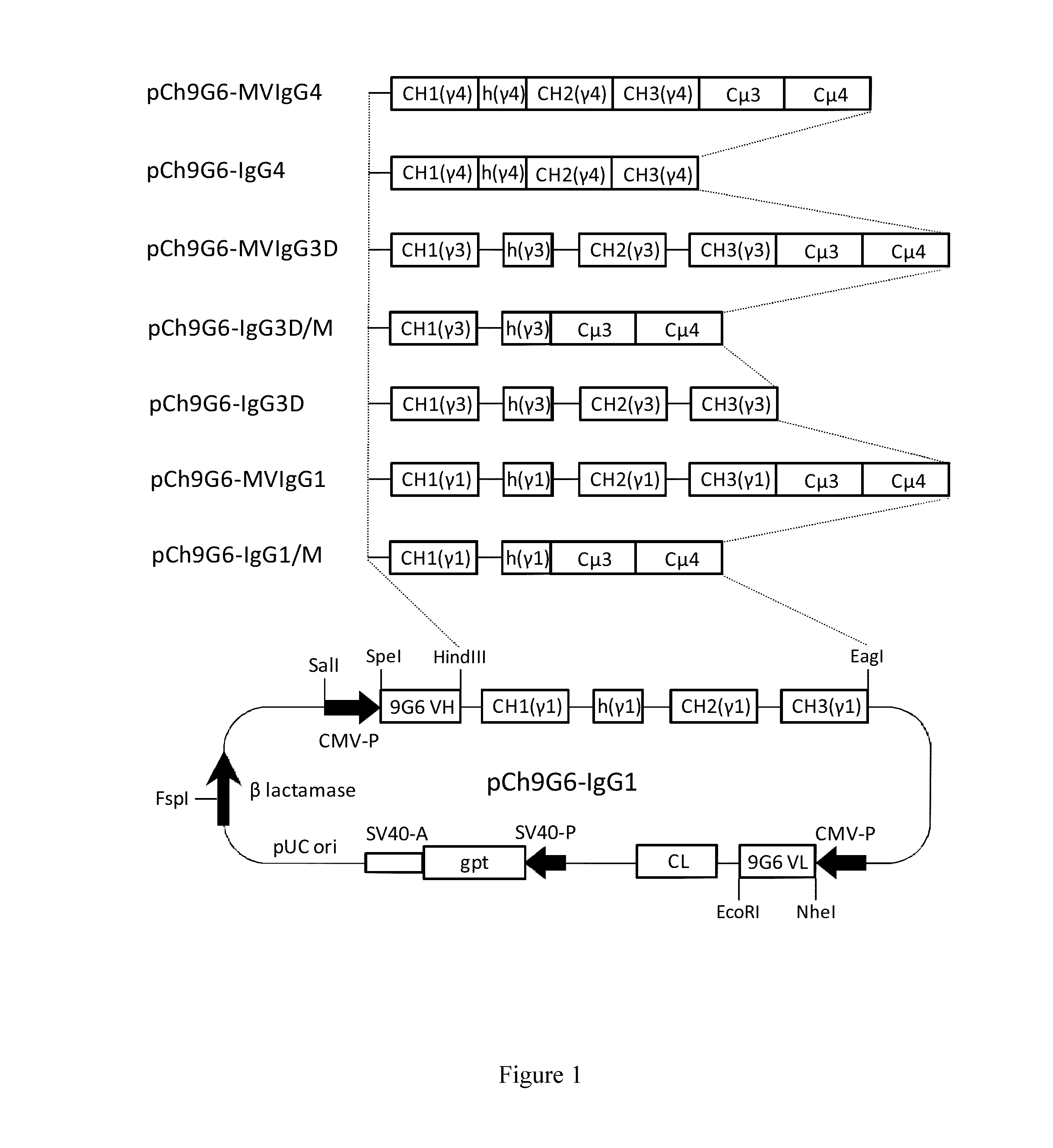
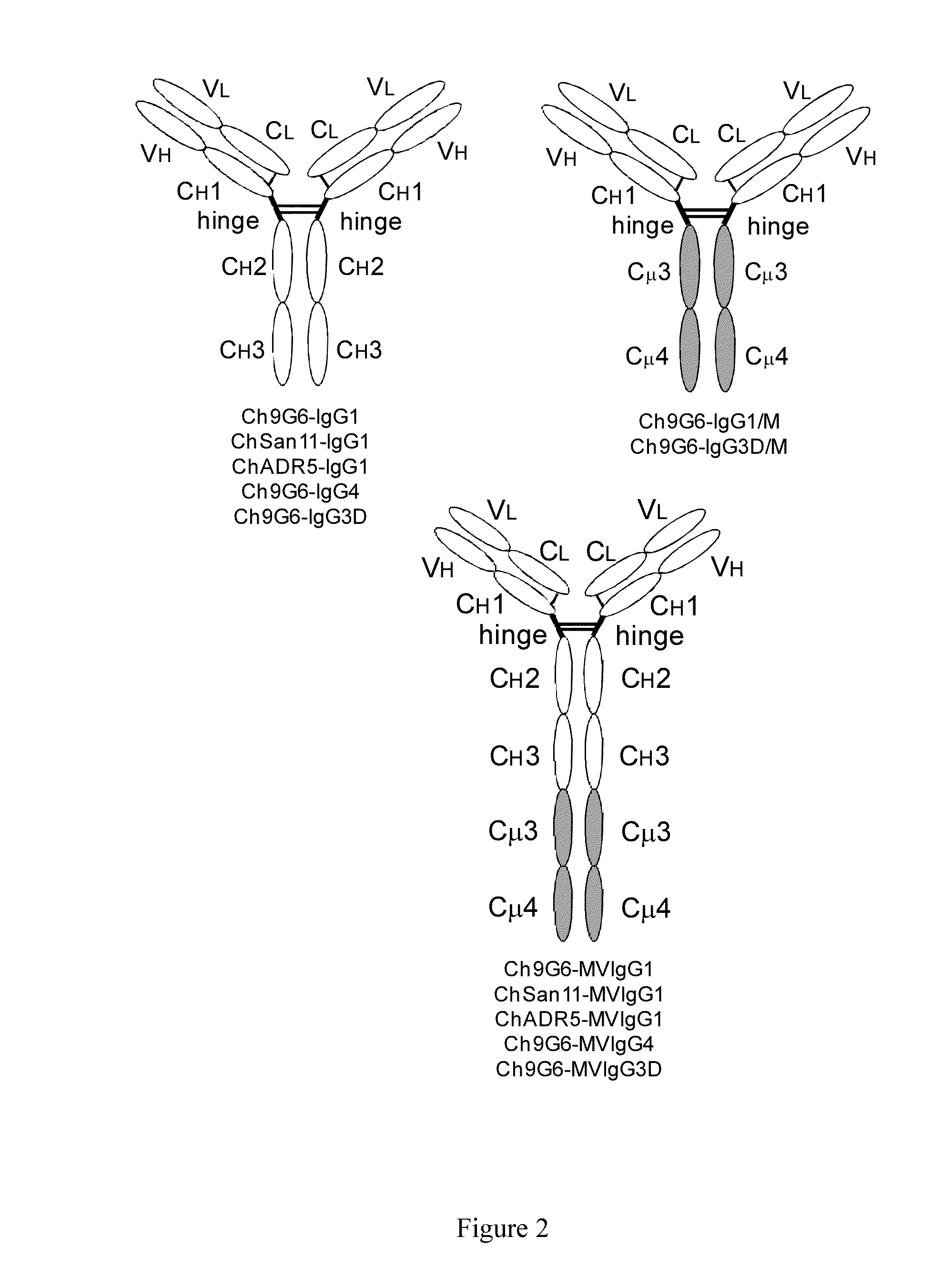
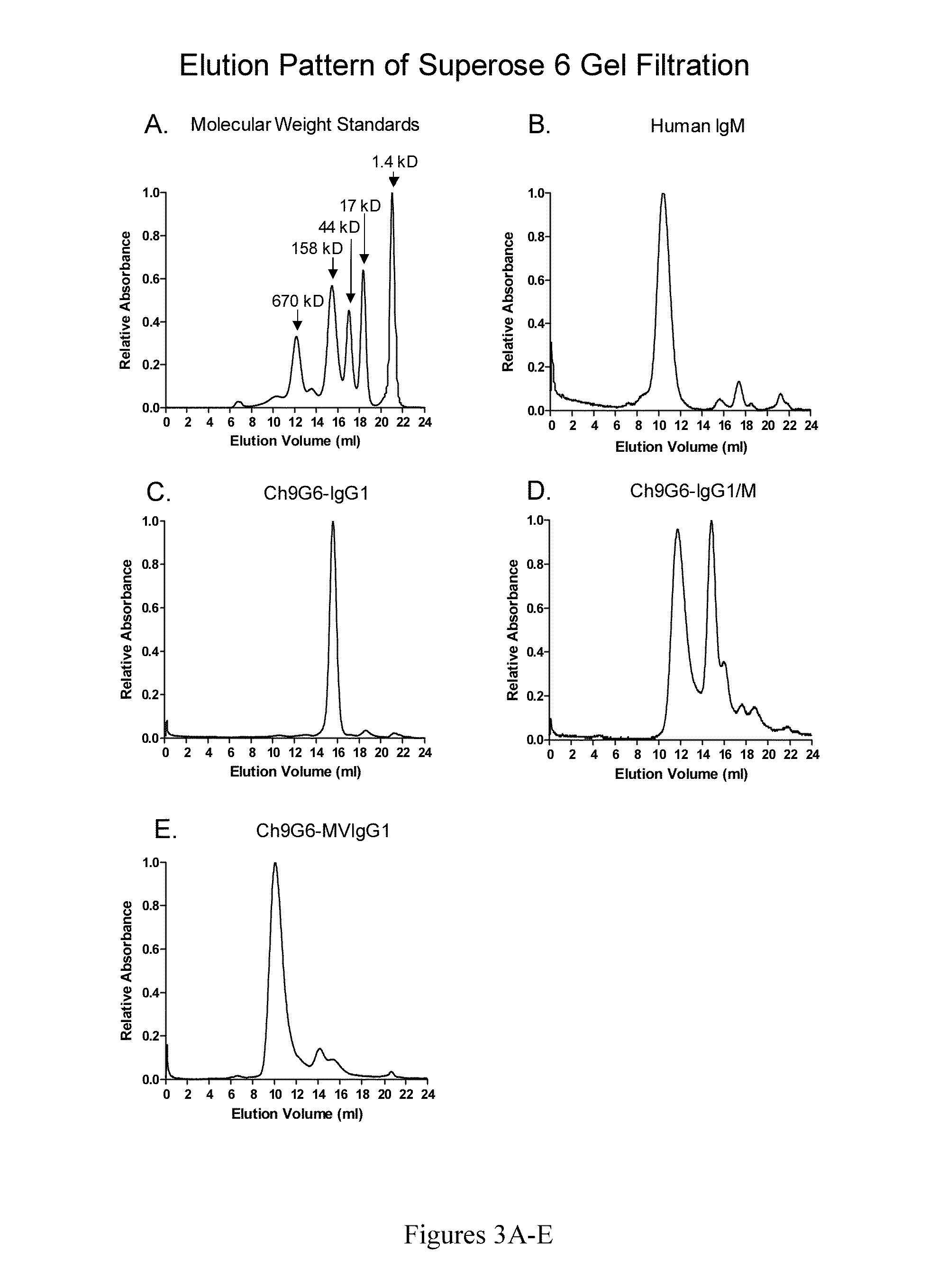
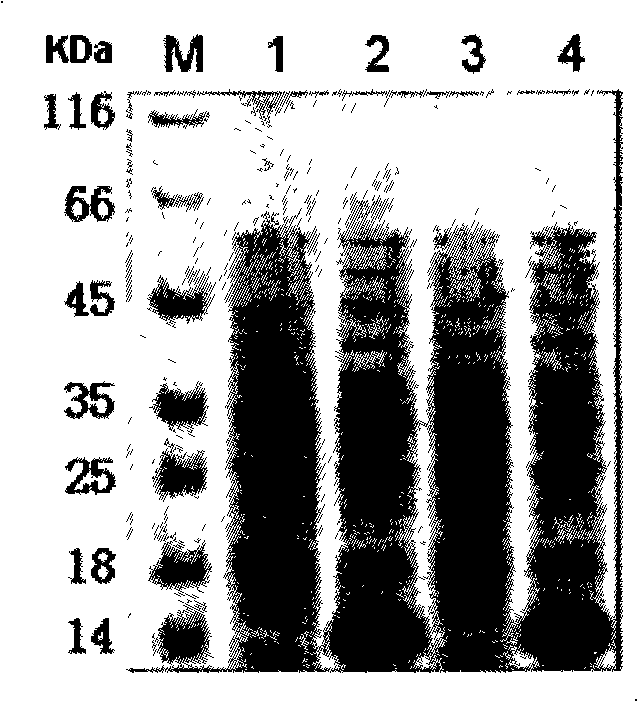
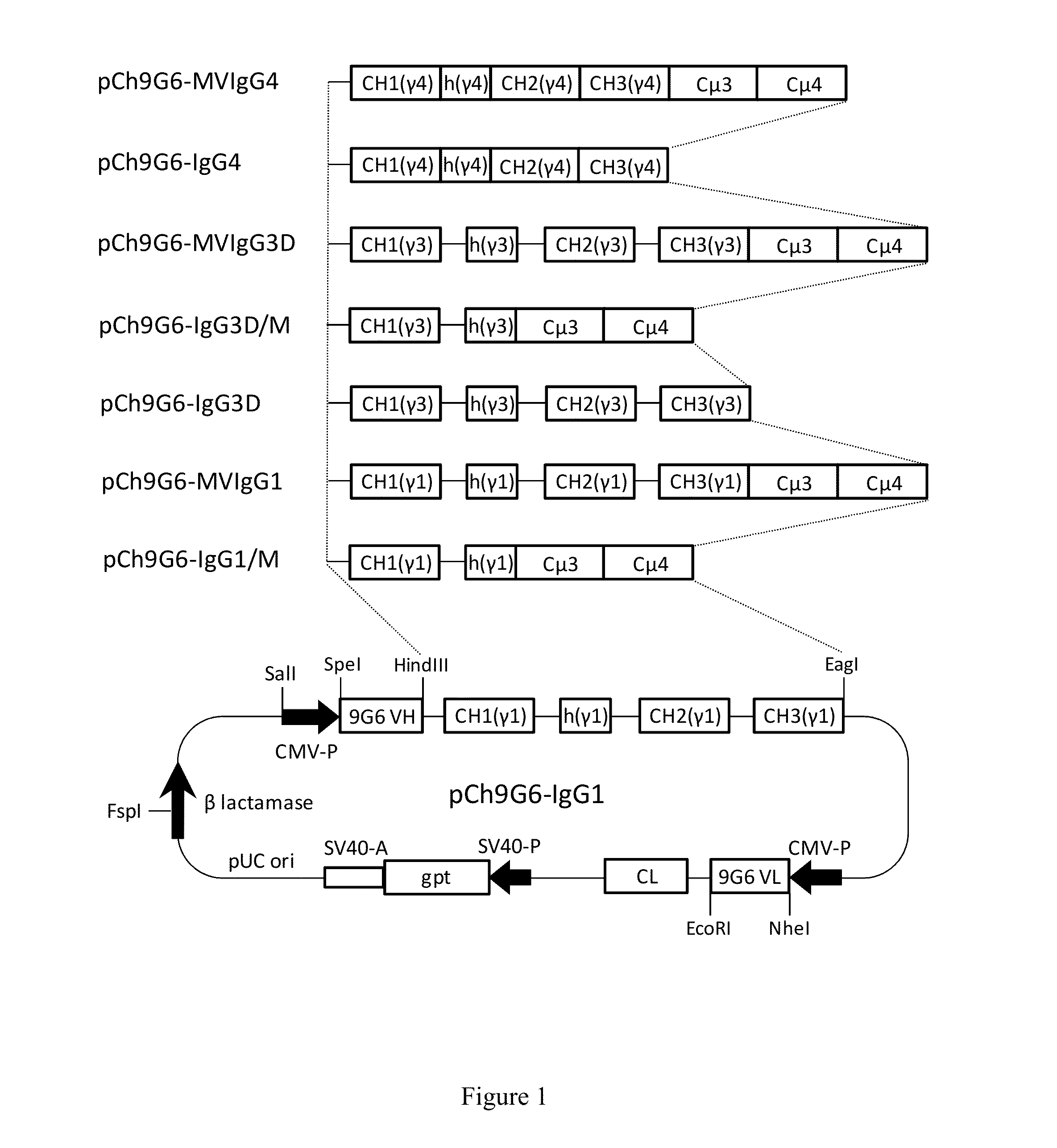
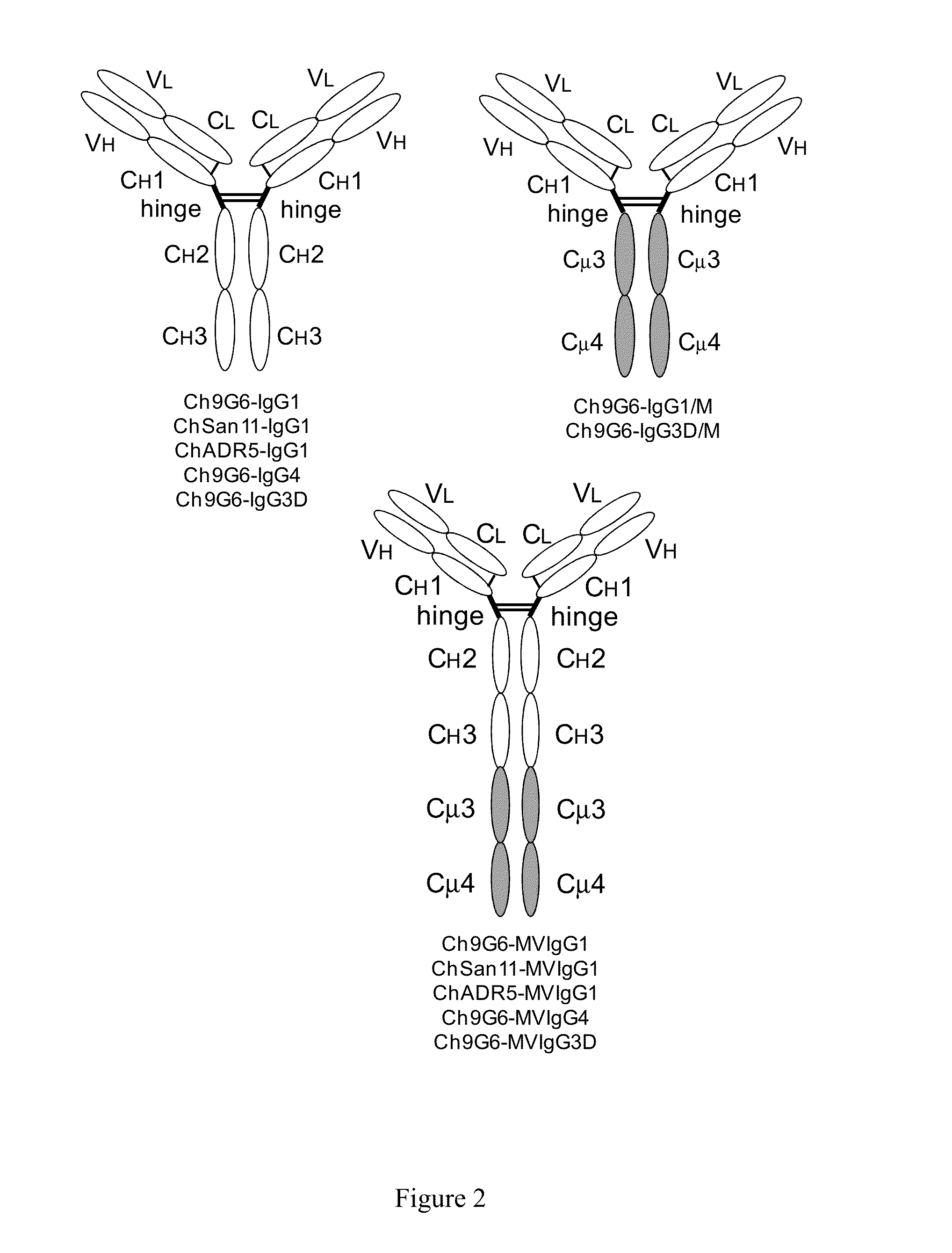
The invention provides constant regions incorporating a cysteine mutation and linked to a μ tailpiece and antibodies or fusion proteins incorporating the same. The constant regions include at least CH2 and CH3 regions of an IgG heavy chain constant region including a cysteine mutation and μ tailpiece. Antibodies or fusion proteins incorporating the constant regions gains the ability to form multivalent complexes, e.g., pentameric or hexameric structures. Antibodies or fusion proteins incorporating the constant regions also retain IgG properties including specific binding to protein G, which facilitates purification and may exhibit pH-dependent FcRn binding, which is associated with a relatively long in vivo half-life. Depending on the isotype and subtype, the nature of the antigen and presence of an additional IgG hinge domain, such antibodies or fusion proteins may also have properties of specific binding to protein A, and effector functions such as ADCC, CDC and opsonization.
Owner:JN BIOSCI
Purification of hetero-dimeric immunoglobulins
InactiveUS20150239991A1Reduces eliminates bindingReduces or eliminates binding of the hetero-dimeric immunoglobulinHybrid immunoglobulinsSolid sorbent liquid separationHeterologousDimer
The present invention describes novel hetero-dimeric immunoglobulinvariants or fragments thereof, which have reduced or eliminated binding to Protein A, Protein G or both Protein A and Protein G. Also encompassed in the present invention are methods for the selective purification of hetero-dimeric immunoglobulins or fragments thereof using Protein A and Protein G.
Owner:GLENMARK PHARMA SA
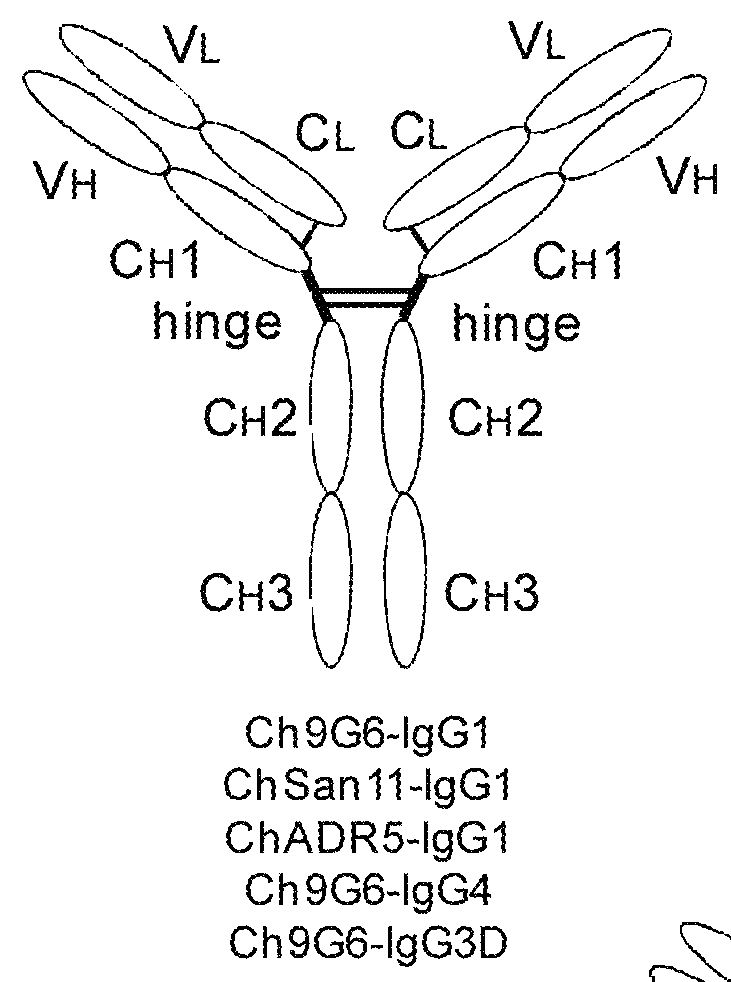
Hybrid constant regions
Owner:JN BIOSCI
Antibodies or fusion proteins multimerized via cysteine mutation and a mu tailpiece
ActiveUS9540442B2Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenPentamer
The invention provides constant regions incorporating a cysteine mutation and linked to a μ tailpiece and antibodies or fusion proteins incorporating the same. The constant regions include at least CH2 and CH3 regions of an IgG heavy chain constant region including a cysteine mutation and μ tailpiece. Antibodies or fusion proteins incorporating the constant regions gains the ability to form multivalent complexes, e.g., pentameric or hexameric structures. Antibodies or fusion proteins incorporating the constant regions also retain IgG properties including specific binding to protein G, which facilitates purification and may exhibit pH-dependent FcRn binding, which is associated with a relatively long in vivo half-life. Depending on the isotype and subtype, the nature of the antigen and presence of an additional IgG hinge domain, such antibodies or fusion proteins may also have properties of specific binding to protein A, and effector functions such as ADCC, CDC and opsonization.
Owner:JN BIOSCI
Viromembrane protein with site-specific mutagenesis and site-specific decoration, preparation method and applications of viromembrane protein
ActiveCN102838663AAvoid dissociationSsRNA viruses negative-senseBacteriaViral Membrane ProteinsUltraviolet lights
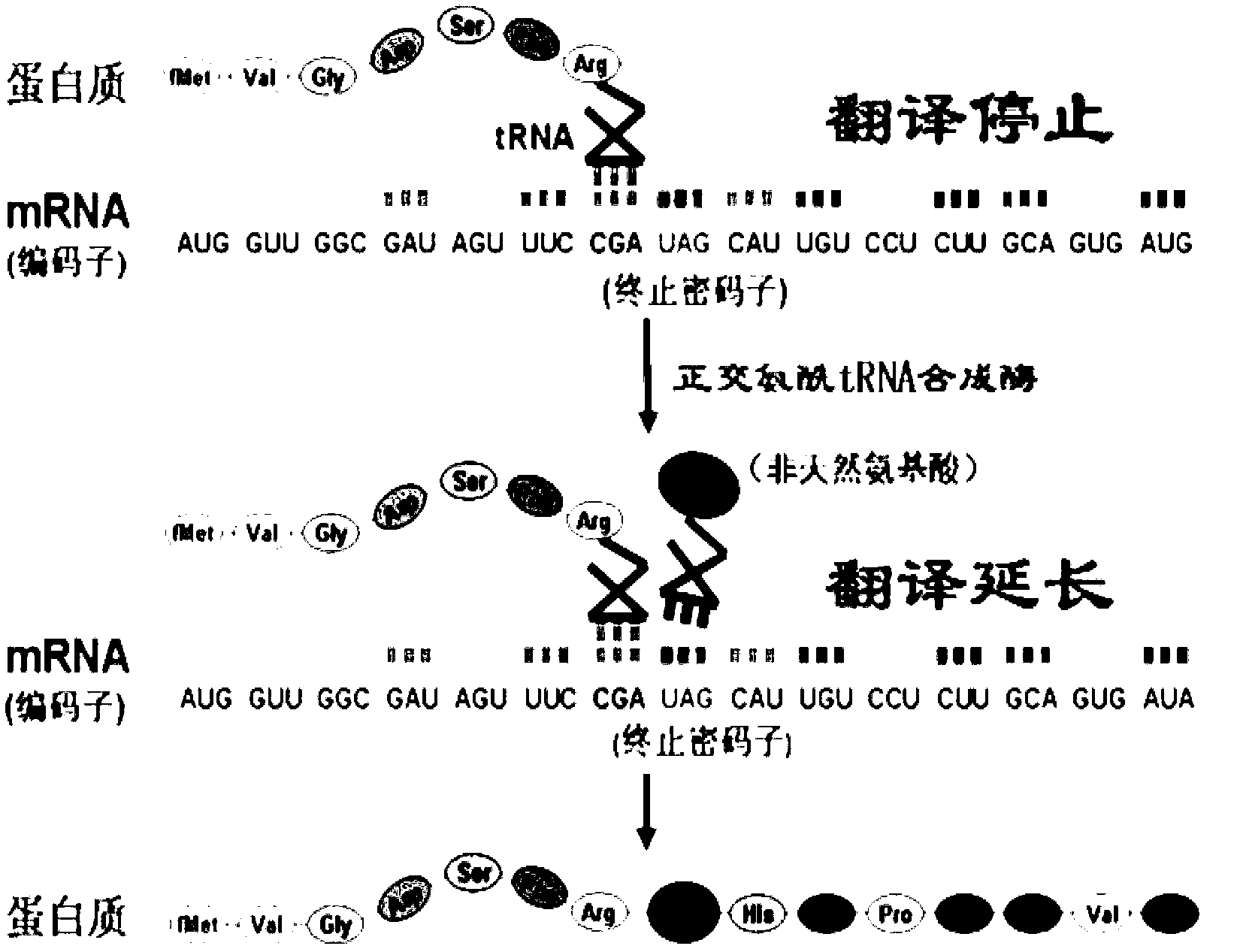
The invention discloses viromembrane protein with site-specific mutagenesis and site-specific decoration, a preparation method and applications of the viromembrane protein. Amber codon TAG is introduced to the specific site of envelope protein G (VSV G) gene of vesicular stomatitis virus, unnatural amino acid DiZPK with photo-crosslinking property is introduced to the specific site of VSV G by utilizing the orthorhombic aminoacyl-tRNA synthetase-tRNA through site-specific mutagenesis. In the interaction process of virus and host cells, the DiZPK photo-crosslinking reaction is triggered by 365nm ultraviolet light, the covalent bond is formed between the VSV G and interacting protein, the interaction dissociation can be avoided, and the powerful measure is provided for the research of the interaction of the virus and protein of the host cells.
Owner:PEKING UNIV
Hybrid constant regions
ActiveUS20140294825A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenHexamerins
The invention provides hybrid constant regions and antibodies or fusion proteins incorporating the same. The hybrid constant regions include at least CH2 and CH3 regions of an IgG or IgA constant region and Cμ3 and Cμ4 regions of a Cμ constant region. The hybrids retain properties of both component constant regions. The hybrids retain the ability of a Cμ constant region to form multivalent complexes, e.g., pentameric or hexameric structures. IgG hybrids also retain IgG properties including pH-dependent FcRn binding, which is associated with a relatively long in vivo half-life, and specific binding to protein G, which facilitates purification. Depending on the isotype and subtype, the nature of the antigen and presence of additional IgG CH1 and hinge domains, IgG hybrids may also retain properties of specific binding to protein A, and effector functions ADCC, CDC and opsonization. IgA hybrids retain the property of IgA of binding to an Fc-alpha receptor CD89.
Owner:JN BIOSCI
Polypeptide immunoassay kit and detection method thereof
InactiveCN102062780AStrong specificityStrong elution abilityImmunoglobulins against animals/humansMaterial analysis by electric/magnetic meansAntigenSolid phases
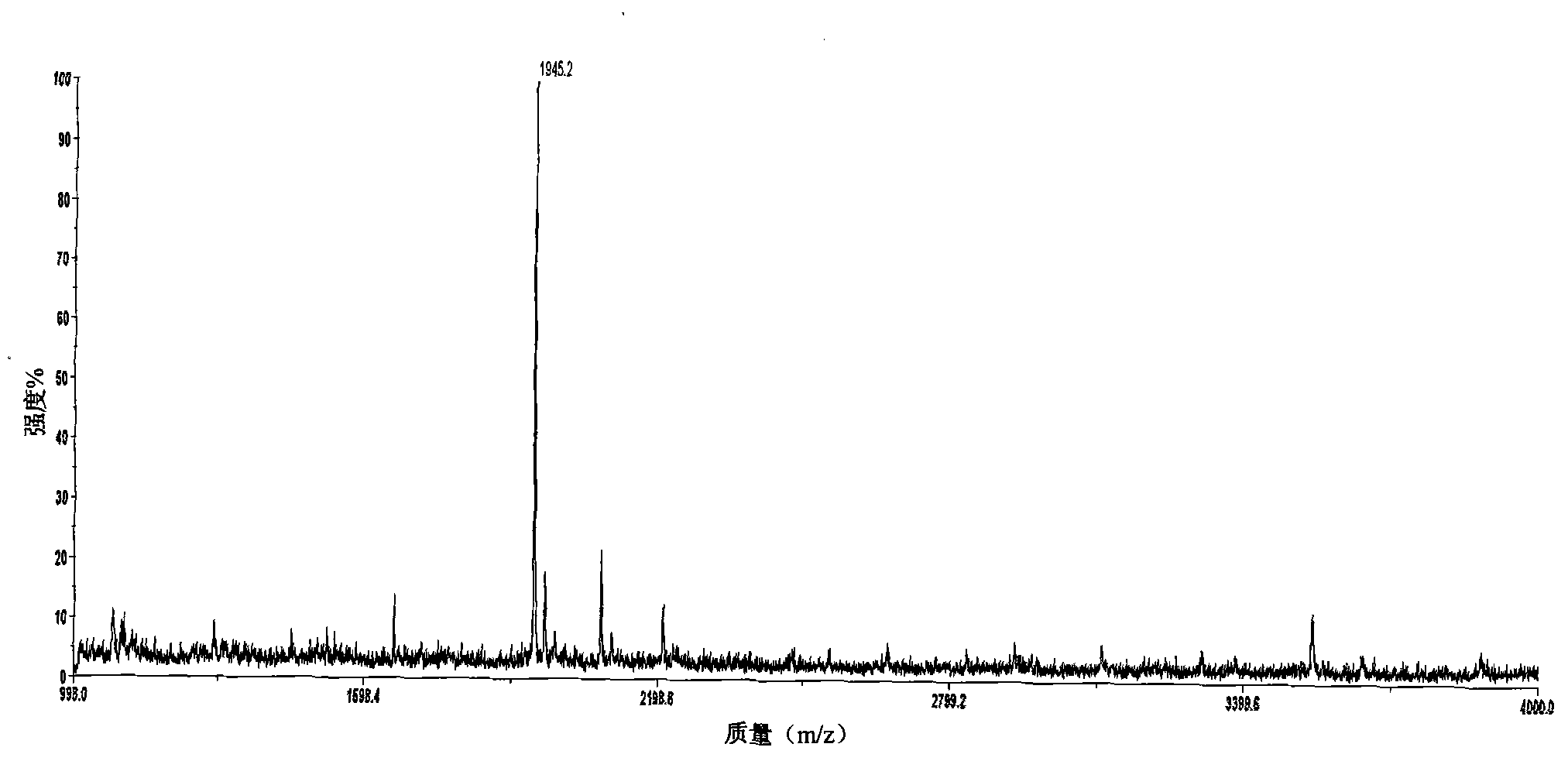
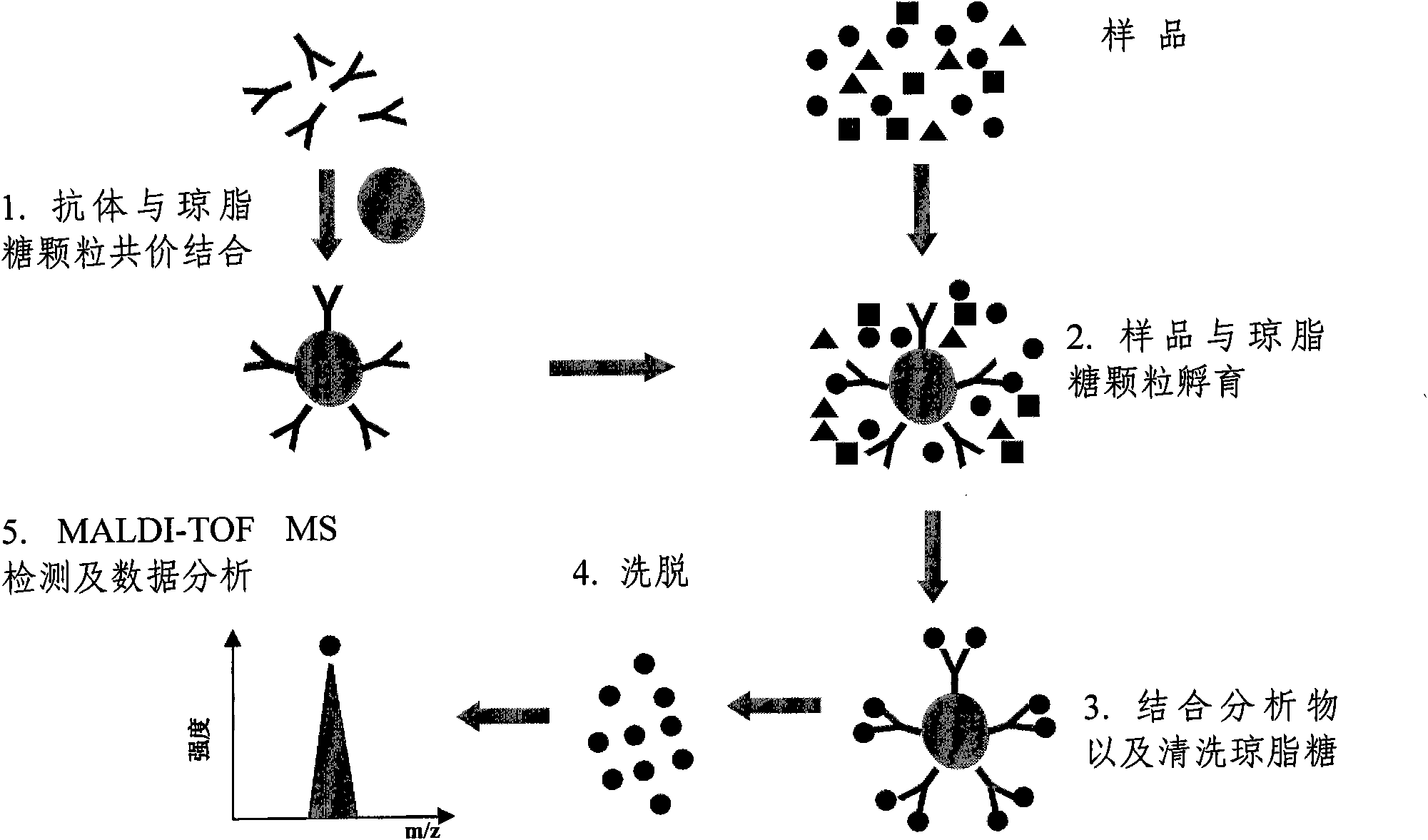
The invention relates to a polypeptide immunoassay kit, which comprises a protein G or protein A agarose serving as a solid phase carrier, a purified serum polypeptide antibody and PBS buffer solution. The invention also relates to a polypeptide immunomic spectrometry analysis method, which comprises the following steps: coupling a purified serum antibody with a proper solid carrier; allowing the coupled product to bond with a polypeptide marker antigen specifically, and separating the antigen from the carrier by using eluent; and finally, detecting polypeptide antigen in the eluent by high-pass matrix-assisted laser desorption / ionization time of flight mass spectrometry (MALDI-TOFMS). Thus, the complete immunomic spectrometry analysis method is established.
Owner:BEIJING C & N INT SCI TECH +1
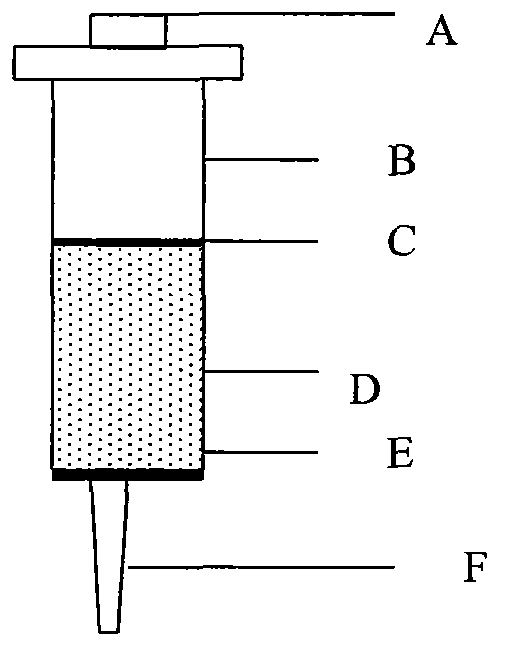
Aflatoxin immunoaffinity column as well as preparation method and application thereof
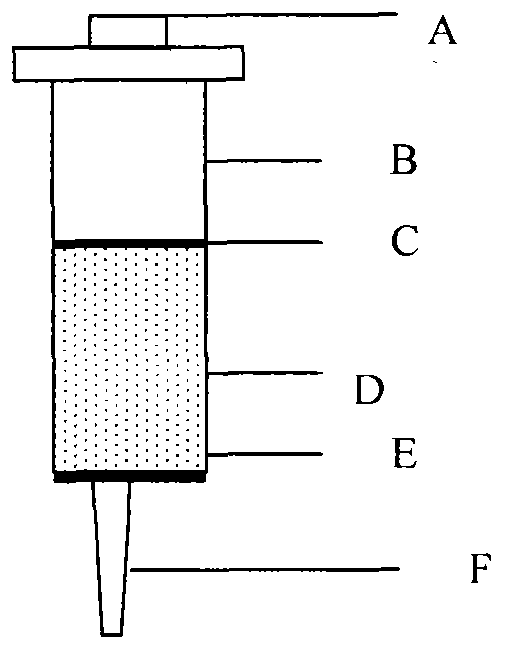
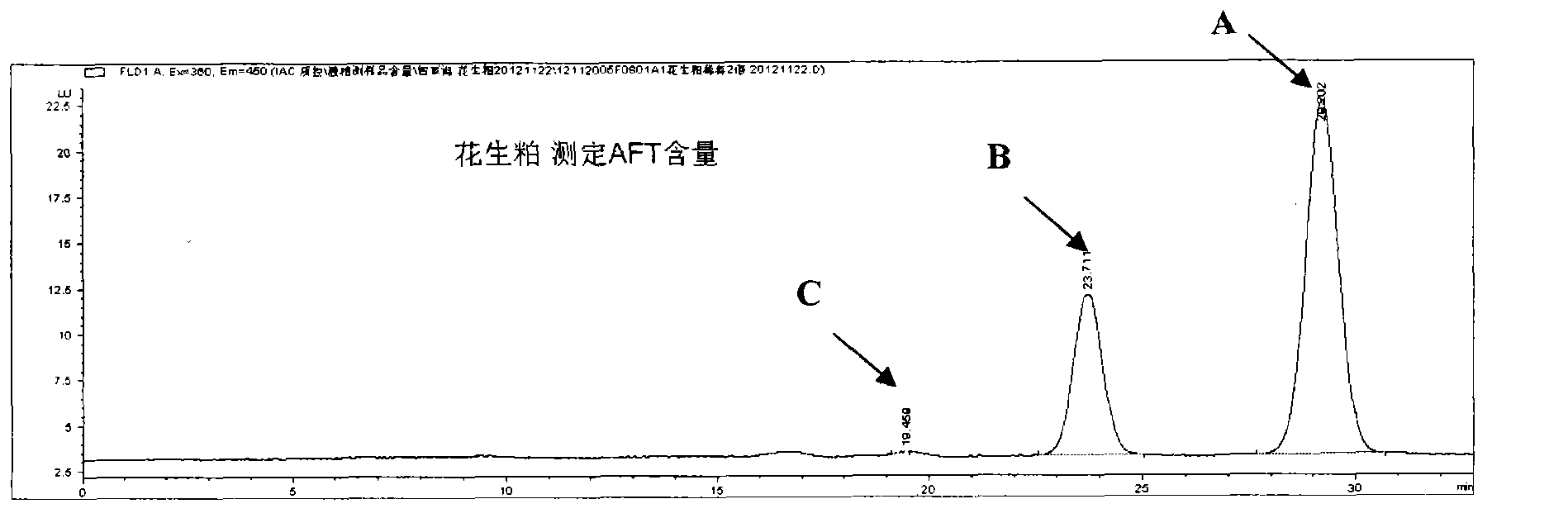
ActiveCN104117229AImprove capture abilityImprove purification efficiencyOther chemical processesPreparing sample for investigationCross-linkFluorescence
The invention relates to an aflatoxin immunoaffinity column as well as a preparation method and application of the aflatoxin immunoaffinity column. The preparation method of the immunoaffinity purification column comprises the following steps: coupling protein G onto an agarose gel carrier, and then coupling an antibody against aflatoxin with the protein G on agarose; crosslinking the aflatoxin antibody-protein G-agarose gel carrier by utilizing a cross-linking agent, and then filling the affinity column. The immunoaffinity column is mainly used for purifying aflatoxin in food, forage, milk, blood samples and other various samples so as to facilitate HPLC detection and fluorescence detection for aflatoxin in the samples in the later stage.
Owner:山东美正生物科技有限公司
Fusion protein with anti-tumor, anti-inflammation and oculopathy-treatment functions and preparation method and application thereof
ActiveCN105418769AInhibition of newbornsImprove ductilitySenses disorderPeptide/protein ingredientsTumor angiogenesisHalf-life
The invention discloses fusion protein with anti-tumor, anti-inflammation and oculopathy-treatment functions and a preparation method and application thereof, which belong to the technical field of biological pharmacy. Specifically, the invention relates to the fusion protein of an integrin blocking agent, which has the function of inhibiting tumor angiogenesis and has the integrin affinity and the combining capacity. A rigid (R) or flexible (F) linker is adopted to fuse two polypeptides, so as to respectively obtain protein A and protein G, the medicine effect can be increased, the half-life period can be extended, the stability can be enhanced, and the fusion protein has the characteristics of strong action effect, low toxicity and the like and can be used for preventing and treating solid tumors, all kinds of inflammation and angiogenesis oculopathy. The fusion protein structurally comprises two angiogenesis inhibiting polypeptides and the amino acid linker between the two angiogenesis inhibiting polypeptides, and the fusion protein is expressed in colibacillus or eukaryocyte by using a genetic engineering method and is obtained through separation and purification of a GST (Glutathione S Transferase) affinity column.
Owner:CHINA PHARM UNIV
Recombinant antibodies for treatment of respiratory syncytial virus infections
Disclosed are novel polyclonal antibodies, which target respiratory syncyticilal virus (RSV), as well as novel high affinity antibody molecules reactive with RSV. The polyclonal antibodies may comprise antibody molecules which are reactive with both RSV protein F and RSV protein G, and preferably the polyclonal antibodies target a variety of epitopes on these proteins. The antibody molecules of the invention have shown superior efficacy in vitro and / or in vivo. Also disclosed are methods of producing the antibodies of the invention as well as methods of their use in treatment or prevention ofRSV infection.
Owner:SYMPHOGEN AS
Respiratory syncytial virus sub-units vaccine, preparation and application
InactiveCN101264323ALimit developmentEnhance cellular immune responseDepsipeptidesAntiinfectivesEscherichia coliCtl epitope
The invention relates to a respiratory syncytial virus vaccine, and the preparation method and application, in particular to an application of escherichia coli to express and recombinant respiratory syncytial virus G protein and mutation G protein, and preparation vaccine with nontoxic typed escherichia coli heat labile enterotoxin, for human or animal preventive inoculation, and for respiratory syncytial virus resistance, belonging to the field of biotechnology. The respiratory syncytial virus vaccine is characterized in that: the respiratory syncytial virus subunits vaccine comprises lopped respiratory syncytial virus RSV protein G, and further comprises nontoxic typed escherichia coli heat labile enterotoxin LT adjuvant. The vaccines are lopped respiratory syncytial virus RSV protein G containing amino acid between aa130 and 230 of the original G protein, and substitutes the amino acid CAWIC (CX3C module ordered) between aa182 and 186 with the amino acid YLEKESIYY (CTL epitope) on the RSV M protein, forming the GCIL protein. The respiratory syncytial virus vaccine has the advantages of remarkable practical significance for preventing human respiratory syncytial virus infection.
Owner:KUNMING UNIV OF SCI & TECH
Hybrid Constant Regions
The invention provides hybrid constant regions and antibodies or fusion proteins incorporating the same. The hybrid constant regions include at least CH2 and CH3 regions of an IgG or IgA constant region and Cμ3 and Cμ4 regions of a Cμ constant region. The hybrids retain properties of both component constant regions. The hybrids retain the ability of a Cμ constant region to form multivalent complexes, e.g., pentameric or hexameric structures. IgG hybrids also retain IgG properties including pH-dependent FcRn binding, which is associated with a relatively long in vivo half-life, and specific binding to protein G, which facilitates purification. Depending on the isotype and subtype, the nature of the antigen and presence of additional IgG CH1 and hinge domains, IgG hybrids may also retain properties of specific binding to protein A, and effector functions ADCC, CDC and opsonization. IgA hybrids retain the property of IgA of binding to an Fc-alpha receptor CD89.
Owner:JN BIOSCI
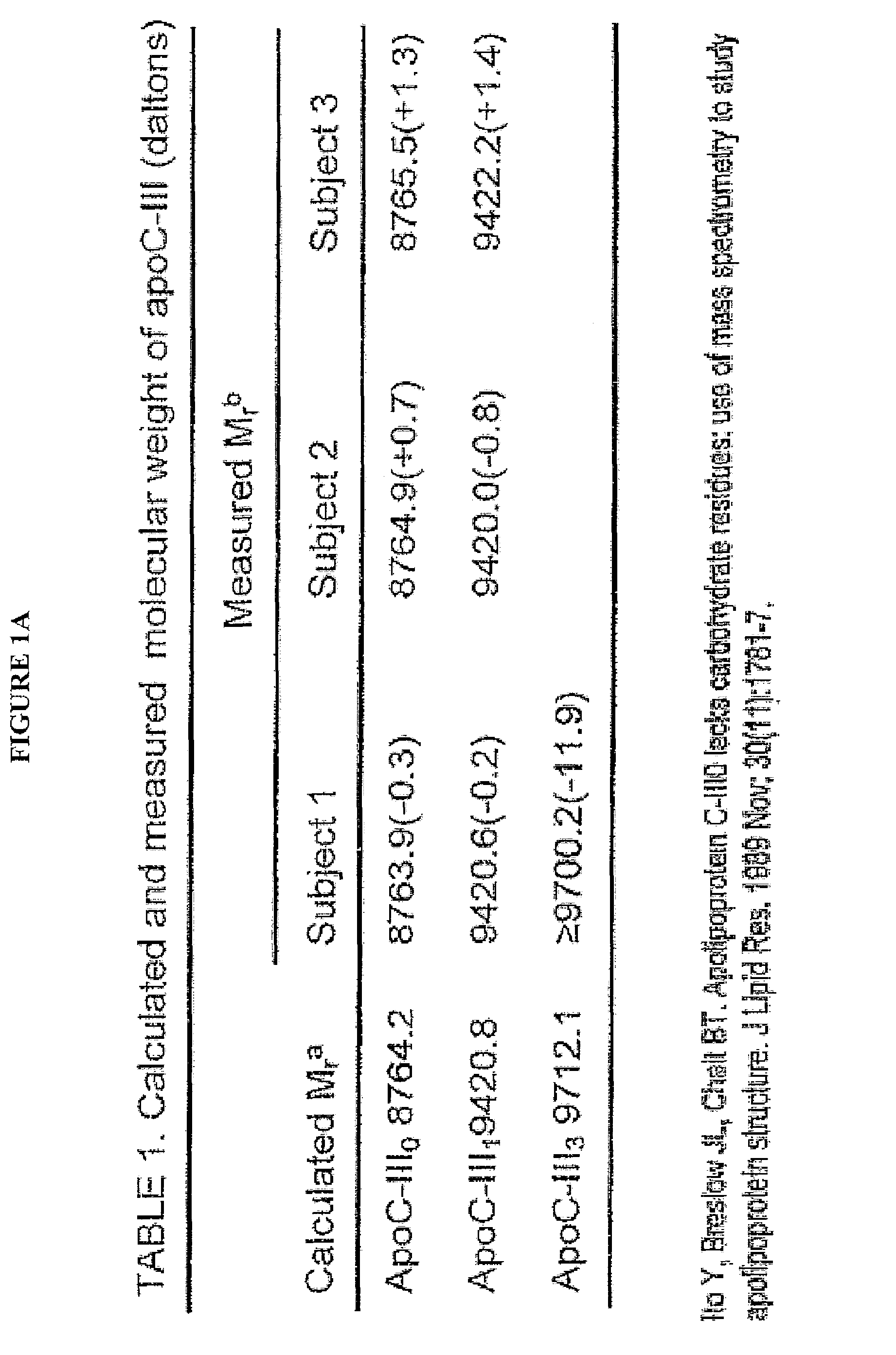
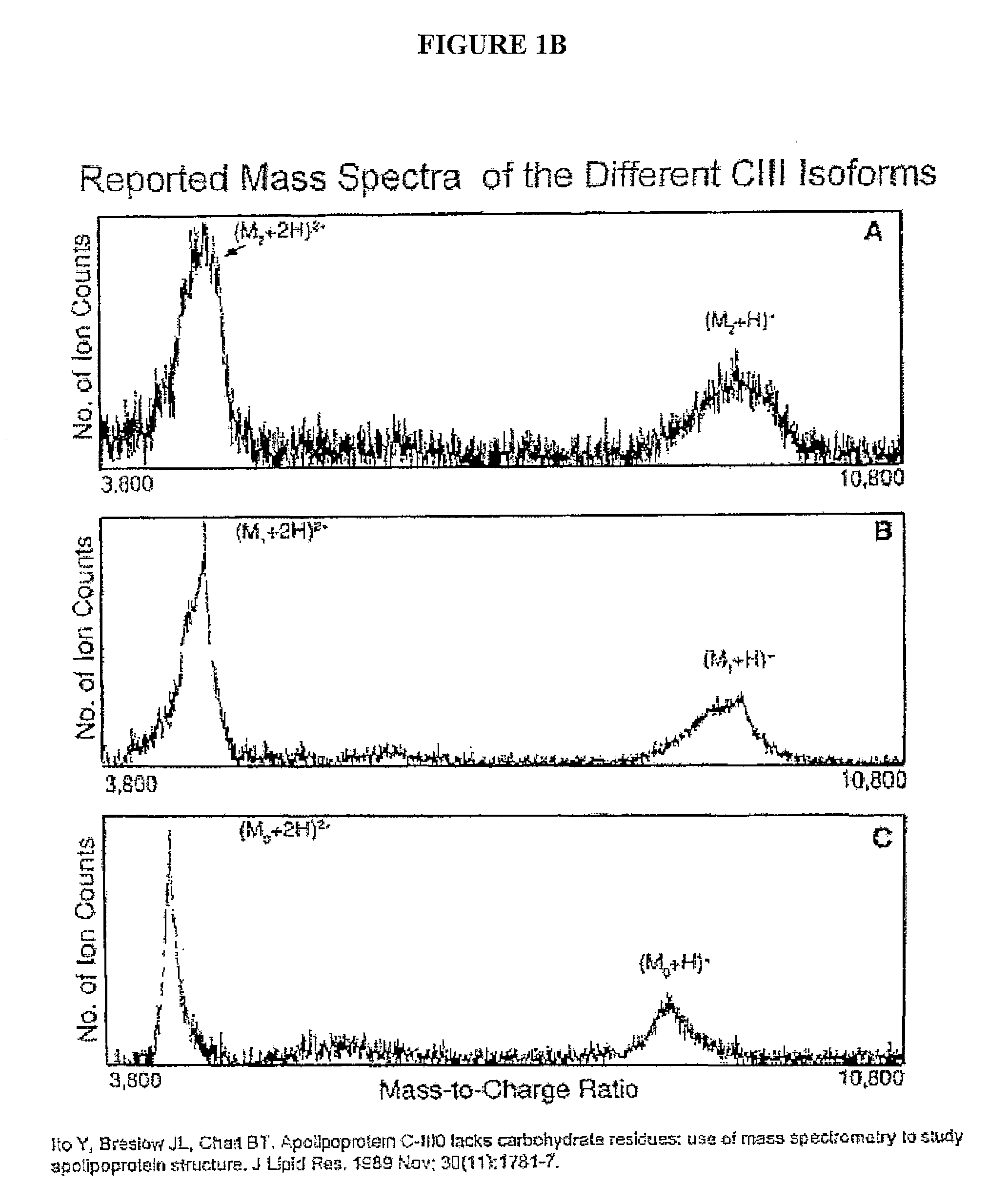
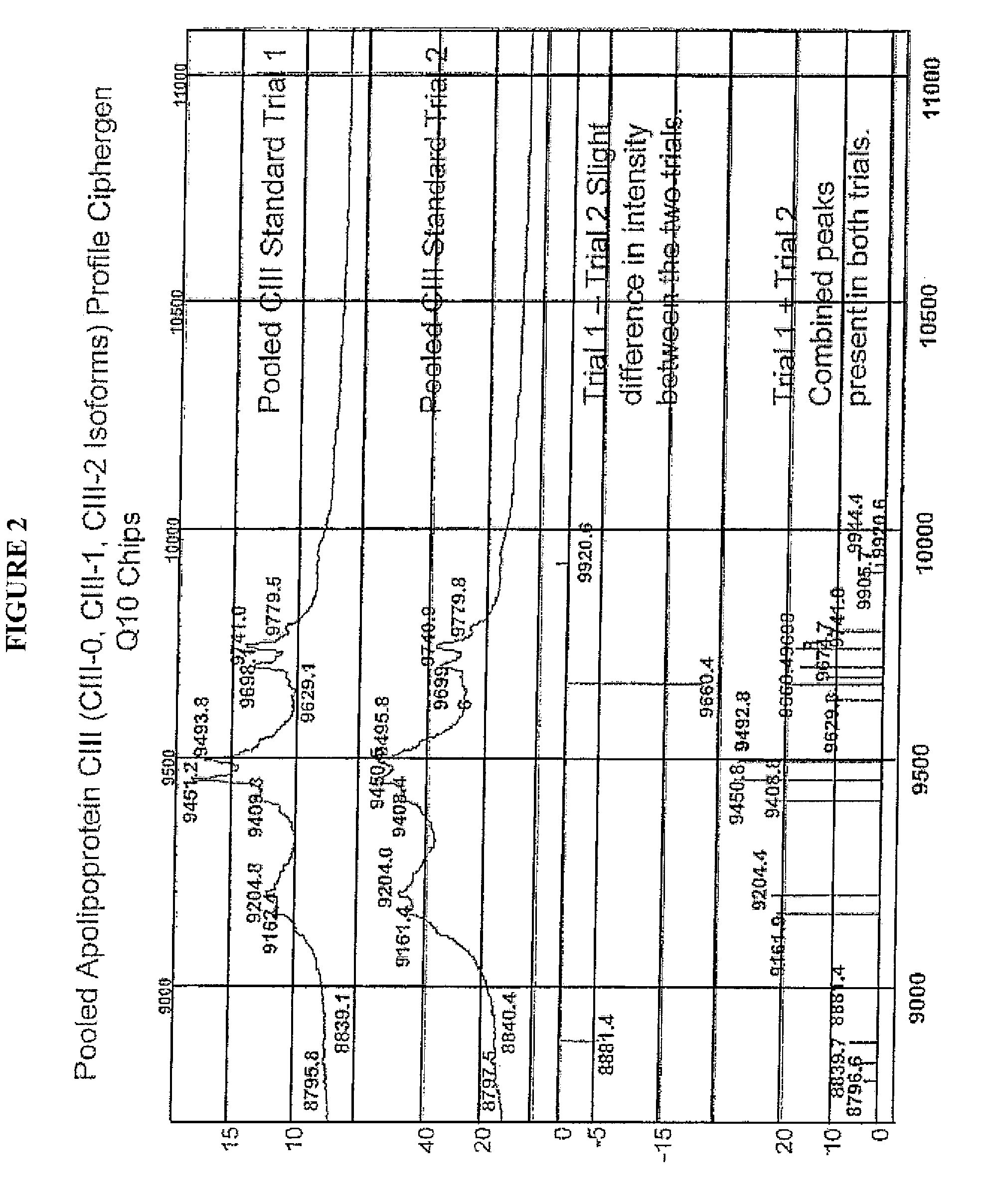
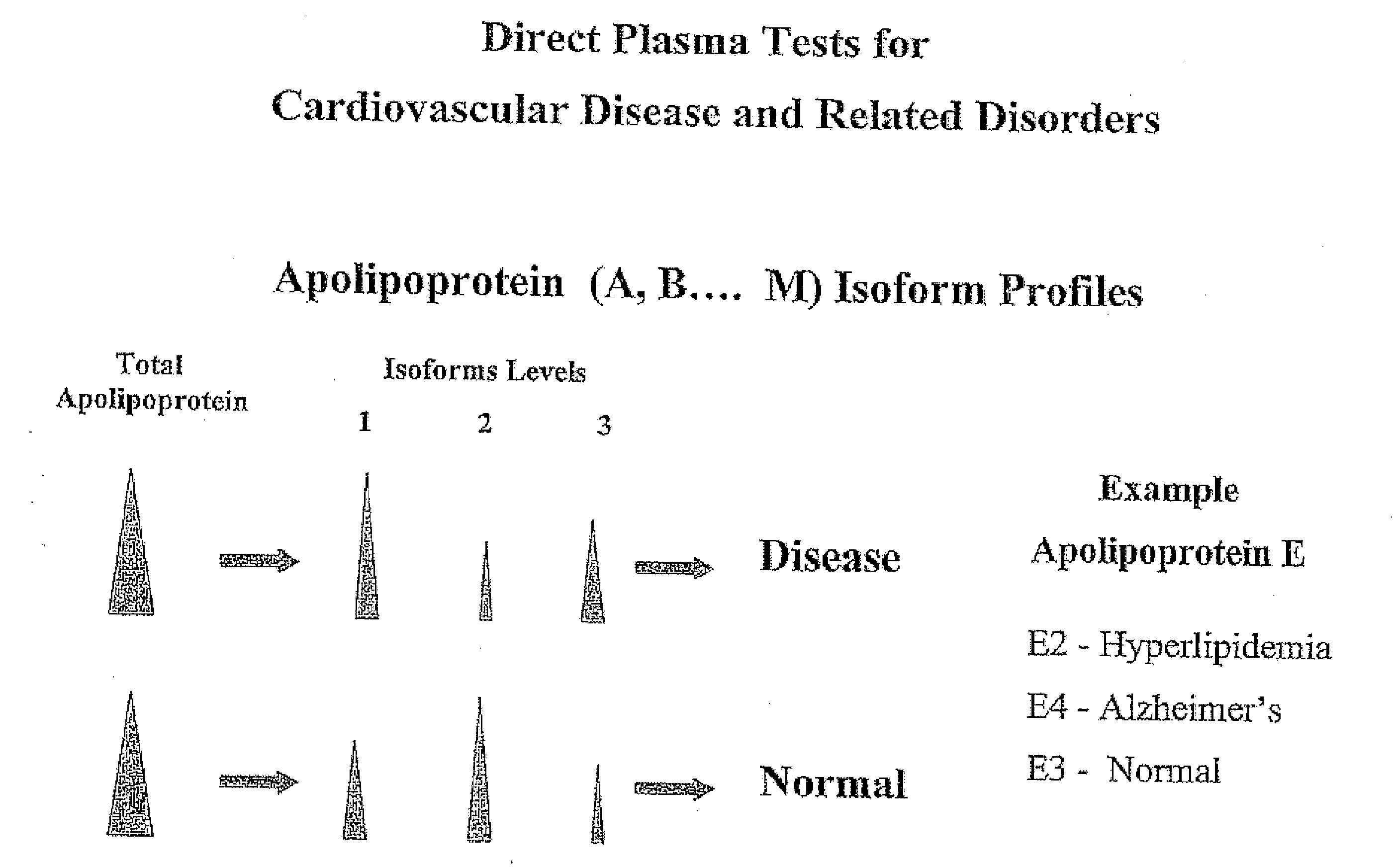
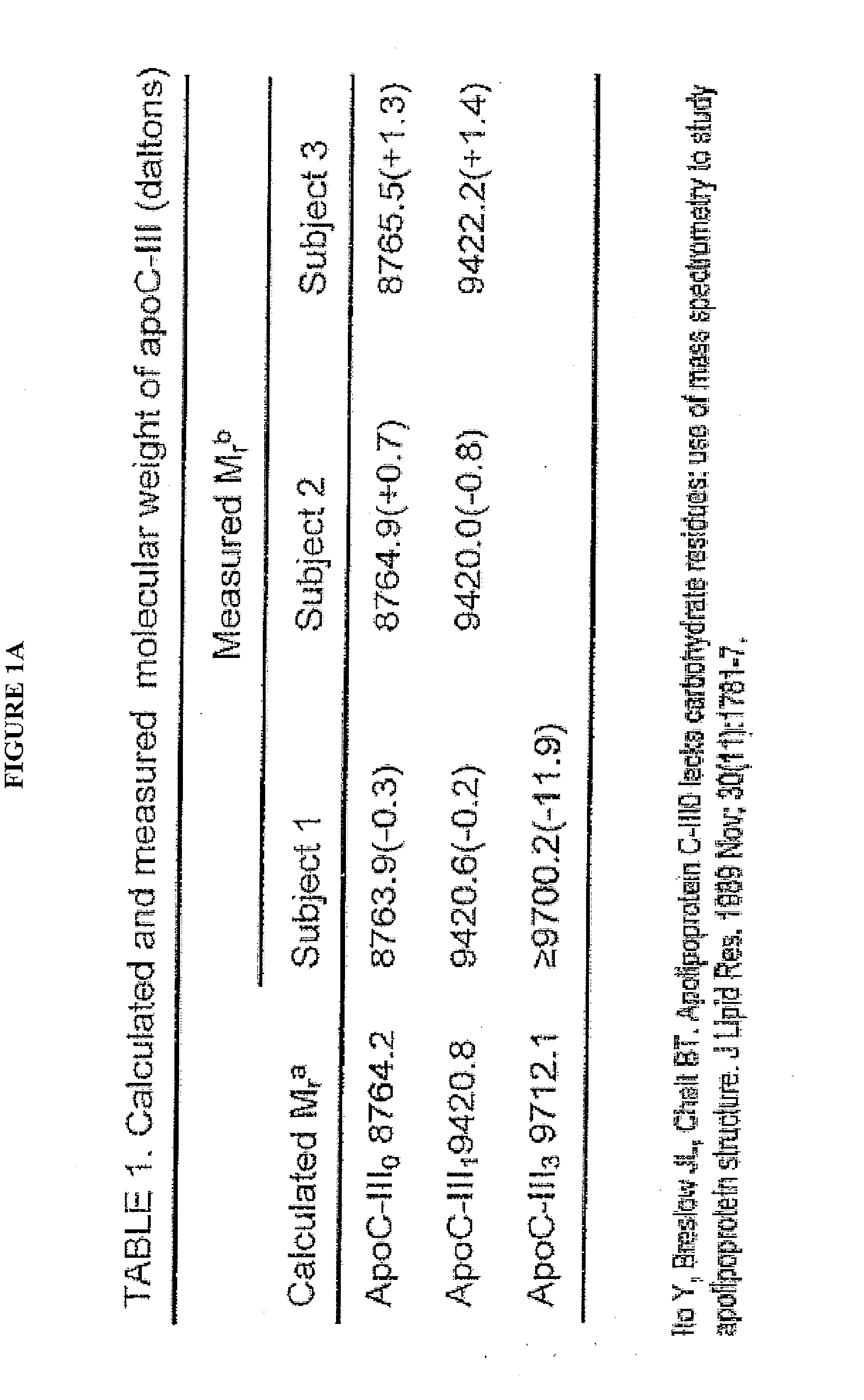
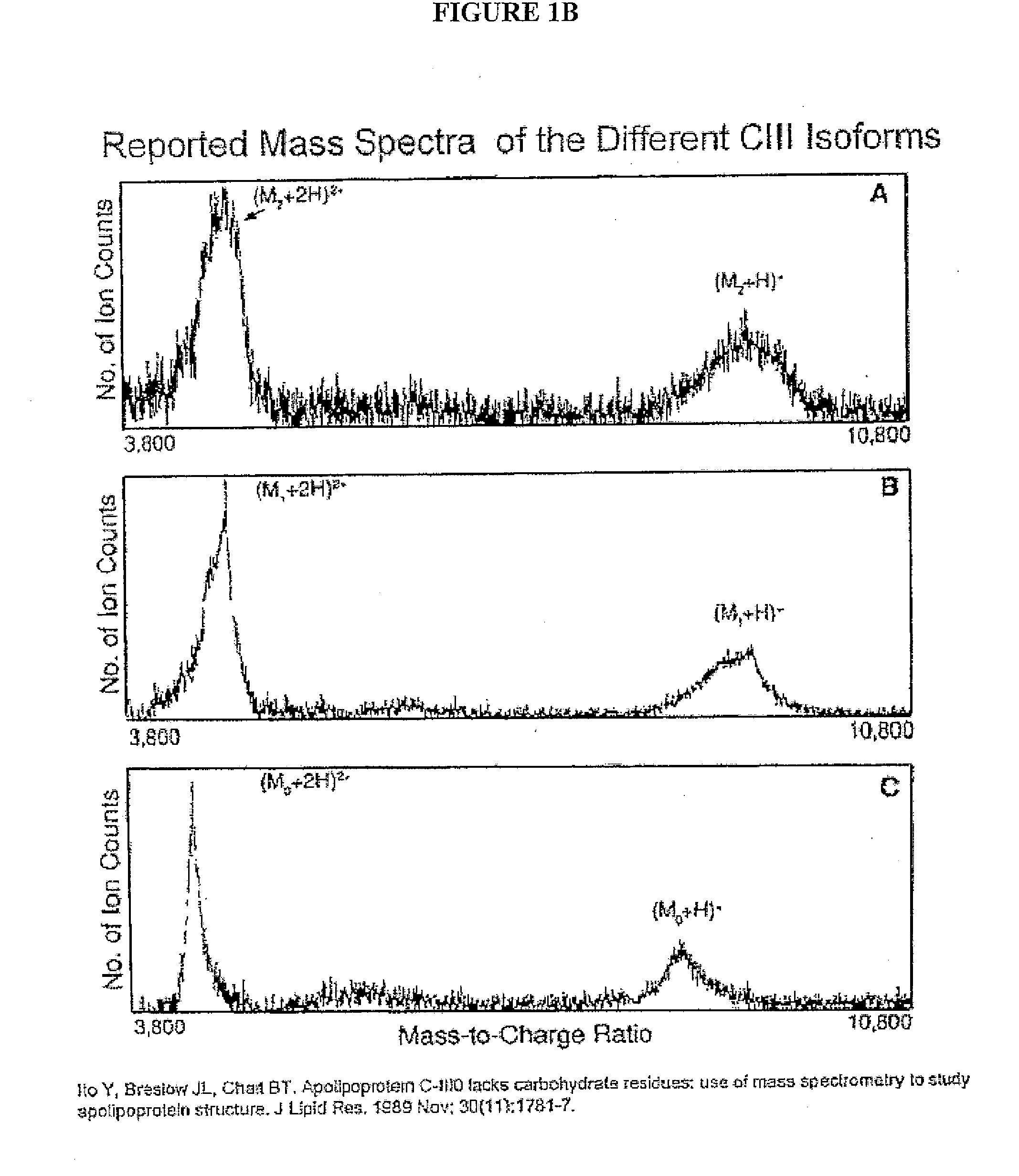
Apolipoprotein fingerprinting technique and methods related thereto
InactiveUS8389222B2Facilitate detection and measurementConfidenceBiological material analysisBiological testingAntiendomysial antibodiesA lipoprotein
A method for determining the concentration and modifications of apolipoprotein in biological samples including plasma, serum, and lipoprotein fractions, by obtaining a sample from a patient, adding a specific volume of an internal standard to the sample, applying the sample to a surface-enhanced, Protein G-coated, antibody-bound chip and removing unbound sample components, analyzing the sample by mass spectrometry, determining the concentration of the apolipoprotein using values of internal standards, and evaluating the concentration of the apolipoprotein, its isoforms, amino acid substitutions and modifications for use as a tool for diagnosing cancer, diabetes, stroke, stress, Alzheimer's disease, inflammation, neurological disease and cardiovascular diseases.
Owner:DE GUZMAN BREYER EMELITA +1
Functionalized bacterium magnetic particles for expressing specific recombinant proteins and preparation method thereof
PendingCN105624081ASpatial structure is stableOvercoming technical issues affecting each otherBacteriaHybrid cell preparationProtein GCell Membrane Proteins
The invention provides functionalized bacterium magnetic particles for expressing specific recombinant proteins. The bacterium magnetic particle membrane protein is fused with a flexible polypeptide chain to express the specific recombinant proteins. The specific recombinant proteins are Z structure field dimers improved on the basis of recombinant protein A or C structure field dimers improved on the basis of recombinant protein G, and the gene sequences are particularly modified, so that the prepared functionalized bacterium magnetic particles have excellent IgG combining capacity.
Owner:BEIJING GUO KE RONG ZHI BIOTECH CO LTD
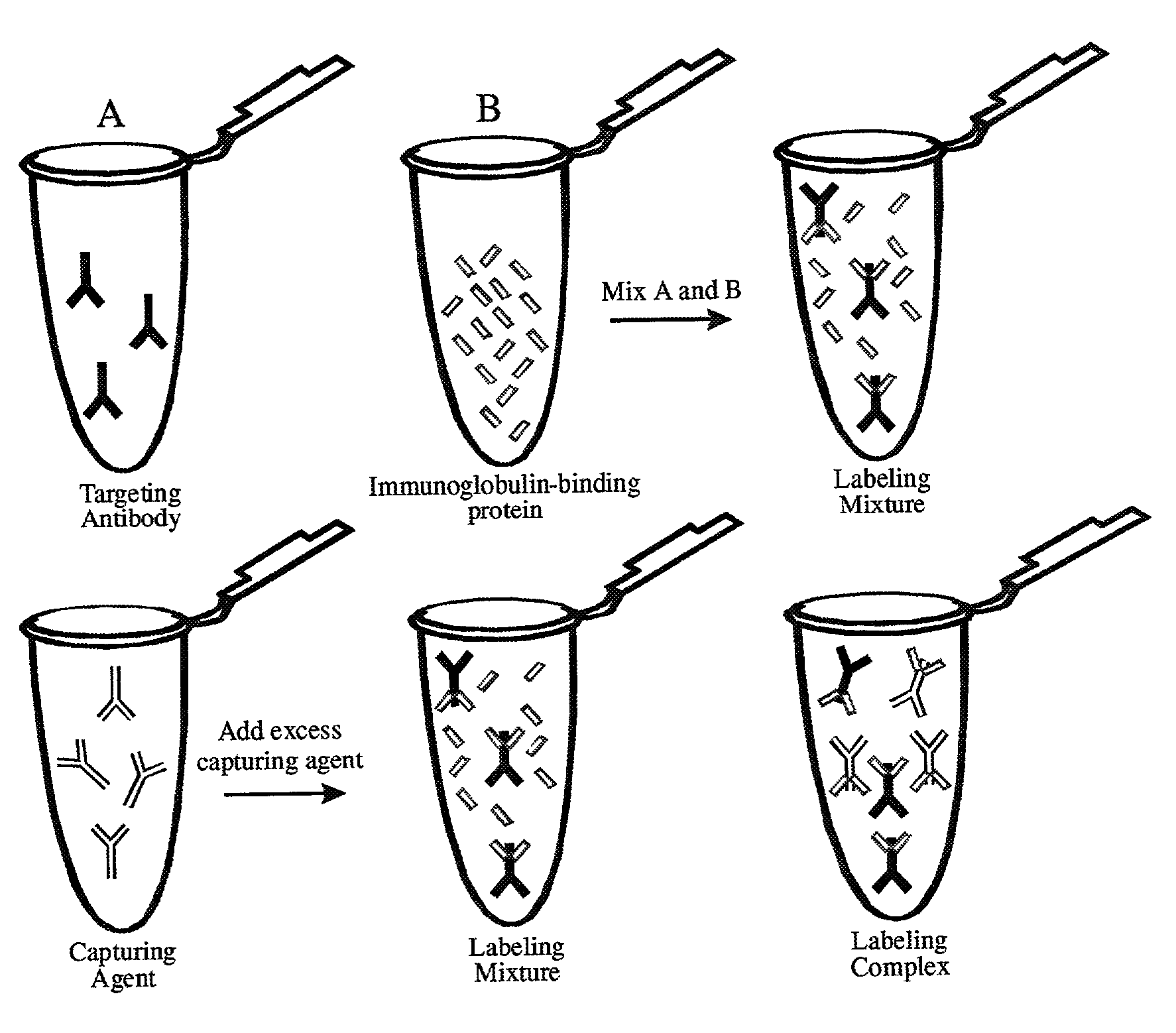
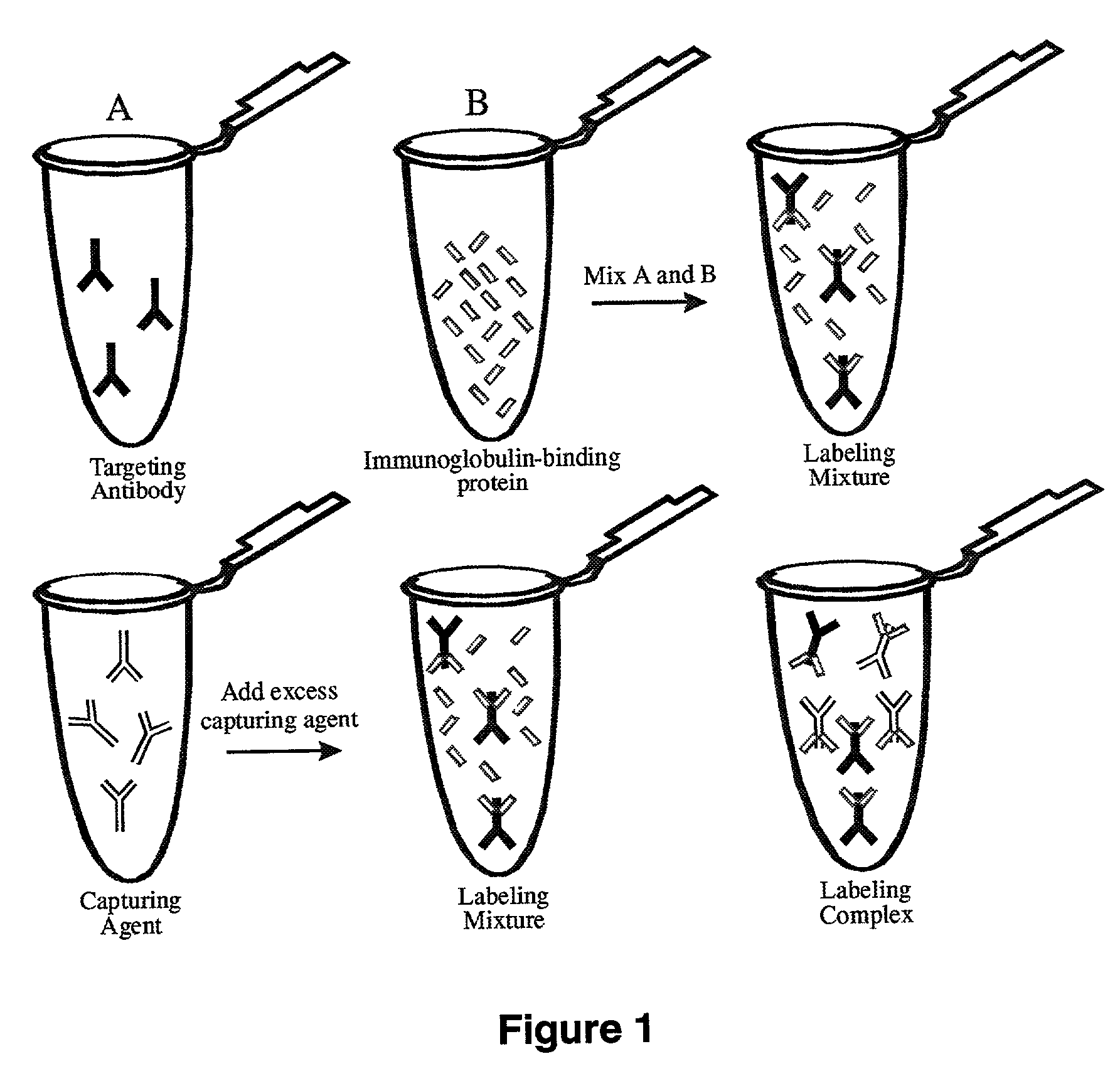
Antibody complexes and methods for immunolabeling
InactiveUS8323903B2The process is convenient and fastEasy to adjustMaterial nanotechnologyNanoinformaticsAntibody fragmentsProtein G
The present invention provides novel immunolabeling complexes and certain components of such complexes, as well as methods of preparing and using such complexes, and kits for use in preparing labeling proteins and for immunolabeling. The pre-formed immunolabeling complexes of the invention comprise both a target-binding antibody and a labeling protein that contains covalently attached labels, where the labeling protein binds selectively and with high affinity to a selected region of the target-binding antibody. Novel labeling proteins of the invention include non-antibody peptides and proteins, such as a complex of protein G and a labeled albumin, and monovalent antibody fragments, such as labeled Fab fragments of an anti-Fc antibody. In methods of the invention, the preformed immunolabeling complexes are added to the sample alone or in combination, for purposes of labeling and optionally detecting the target of interest.
Owner:LIFE TECH CORP
Methods for Quantifying Protein Leakage From Protein Based Affinity Chromatography Resins
InactiveUS20100112597A1Minimize PrA leakageLarge capacityPeptide preparation methodsDepsipeptidesProtein insertionADAMTS Proteins
The present invention provides methods of quantifying protein leakage from a protein based affinity chromatography media (e.g., protein A, protein G and protein L based affinity chromatography media), where such a protein is used for isolating and / or removing a molecule which binds the protein (e.g., an immunoglobulin).
Owner:MILLIPORE CORP
Deoxynivalenol immune affinity column and preparation method and use thereof
ActiveCN104117227AImprove capture abilityImprove purification efficiencyOther chemical processesPreparing sample for investigationProtein GSepharose
The invention relates to a deoxynivalenol immune affinity column and a preparation method and use thereof. The immune affinity purifying column is coupled to a sepharose supporter by protein G, and a deoxynivalenol antibody is coupled to the protein G on agarose; a crosslinking agent is used for crosslinking the deoxynivalenol antibody, the protein G and the sepharose supporter, and then the crosslinked deoxynivalenol antibody-protein G-sepharose supporter fills the affinity column. The immune affinity column is mainly used for purifying deoxynivalenol in food, feed, seasonings, wine, beverages and other various samples.
Owner:山东美正生物科技有限公司
Preparation method and application of fusion protein with broad spectrum adsorption capacity to antibodies
ActiveCN102676562AImprove bindingAvoid non-specific adsorptionIon-exchange process apparatusBacteriaIntravenous gammaglobulinAnion-exchange chromatography
The invention discloses a preparation method and application of fusion protein with broad spectrum adsorption capacity to antibodies. The method includes the steps: obtaining a protein A and a protein G immune globulin binding zone gene by means of T vector connection and double digestion by the genetic engineering means and via PCR (polymerase chain reaction) amplification; then connecting onto an expression vector pET-23a to form a recombinant plasmid, converting E.coli BL21(DE3), and obtaining a great number of thalli containing the fusion protein AG after inducible expression; and finally, performing ultrasonication, high-temperature heating, precipitation of DNA (deoxyribonucleic acid) and purification of weak anion exchange chromatography and affinity chromatography so that the fusion protein AG is obtained. The fusion protein has dual advantages of the protein A and the protein B and is wider in spectrum combination and low in nonspecific adsorption of non-immune globulin substances of albumin in serum and the like. The fusion protein serving as a ligand is connected to a solid-phase vector matrix to be prepared into adsorbent so that the shortcoming of poor IgG3 binding force of protein A adsorbent is overcome, and can be applied to the fields of antibody purification, blood purification and the like.
Owner:DALIAN UNIV OF TECH
Protein G staphylococcus aureus enterotoxin kit and preparation method thereof
InactiveCN105353129AIncrease profitReduce dosageMaterial analysisAbzymeStaphylococcus aureus enterotoxin B
The invention relates to a protein G staphylococcus aureus enterotoxin kit and a preparation method thereof. Protein G is adopted to preprocess an enzyme labeling plate, and quick and accurate detection on staphylococcus aureus enterotoxin A / B is realized by combining with an ELISA double-antibody sandwich method. The kit consists of the 96-hole enzyme labeling plate, a staphylococcus aureus enterotoxin A-resistant protein G recombinant protein monoclonal antibody, a staphylococcus aureus enterotoxin B-resistant monoclonal antibody, a staphylococcus aureus enterotoxin antibody enzyme labeling object containing a horseradish peroxidase label, a sample diluent, a cleaning solution and a stop buffer. The protein G staphylococcus aureus enterotoxin kit can quickly and effectively detect the residual level of tony red in a to-be-detected sample, and also has the characteristics of simple operation, good specificity, high sensitivity, and the like.
Owner:HUAAN MAGNECH BIO TECH
Hybrid constant regions
The invention provides hybrid constant regions and antibodies or fusion proteins incorporating the same. The hybrid constant regions include at least CH2 and CH3 regions of an IgG or IgA constant region and Cμ3 and Cμ4 regions of a Cμ constant region. The hybrids retain properties of both component constant regions. The hybrids retain the ability of a Cμ constant region to form multivalent complexes, e.g., pentameric or hexameric structures. IgG hybrids also retain IgG properties including pH-dependent FcRn binding, which is associated with a relatively long in vivo half-life, and specific binding to protein G, which facilitates purification. Depending on the isotype and subtype, the nature of the antigen and presence of additional IgG CH1 and hinge domains, IgG hybrids may also retain properties of specific binding to protein A, and effector functions ADCC, CDC and opsonization. IgA hybrids retain the property of IgA of binding to an Fc-alpha receptor CD89.
Owner:JN BIOSCI
Preparation method and application of NDM-1 monoclonal antibody
ActiveCN113150137AHigh purityStrong specificityAntibacterial agentsImmunoglobulins against bacteriaBALB/cIndirect elisa
The invention discloses a preparation method and application of an NDM-1 monoclonal antibody. The preparation method comprises the following steps: synthesizing a Klebsiella pneumoniae NDM-1 gene, expressing and purifying NDM-1 protein, taking recombinant NDM-1 protein as immunogen, immunizing Balb / c mice, taking spleen cells of the immunized mice to fuse with myeloma NS-1 cells of the mice, screening hybridoma cells secreting specific McAb through an indirect ELISA method, inducing the mice to generate ascites by using a hybridoma cell strain, purifying antibodies by using protein G affinity chromatography to finally obtain seven NDM-1 protein monoclonal antibodies, and sequencing to obtain amino acid sequences of four monoclonal antibodies. A foundation is laid for establishing an NDM-1 detection method.
Owner:深圳市龙华区疾病预防控制中心
G-eGFP protein, preparation method, and application
This invention discloses a method for preparing G-eGFP fusion protein and it application. G-eGFP fusion protein has a nucleotide sequence as shown in SEQ ID No.1. G-eGFP fusion protein is fused from protein G and protein eGFP, and the expression product can be used after purification without coupling problem. G-eGFP fusion protein has potential application in high-efficiency immobilization of subcellular protein. Besides, G-eGFP fusion protein also has potential application in biological detection by using antibody, such as protein blot hybridization and flow cytometry detection.
Owner:ZHEJIANG UNIV
Apolipoprotein fingerprinting technique and methods related thereto
InactiveUS20090155812A1Easy to detectEasy to measureBiological material analysisBiological testingApolipoproteins EBlood plasma
A method for determining the concentration and modifications of apolipoprotein in biological samples including plasma, serum, and lipoprotein fractions, by obtaining a sample from a patient, adding a specific volume of an internal standard to the sample, applying the sample to a surface-enhanced, Protein G-coated, antibody-bound chip and removing unbound sample components, analyzing the sample by mass spectrometry, determining the concentration of the apolipoprotein using values of internal standards, and evaluating the concentration of the apolipoprotein, its isoforms, amino acid substitutions and modifications for use as a tool for diagnosing cancer, diabetes, stroke, stress, Alzheimer's disease, inflammation, neurological disease and cardiovascular diseases.
Owner:DE GUZMAN BREYER EMELITA +1
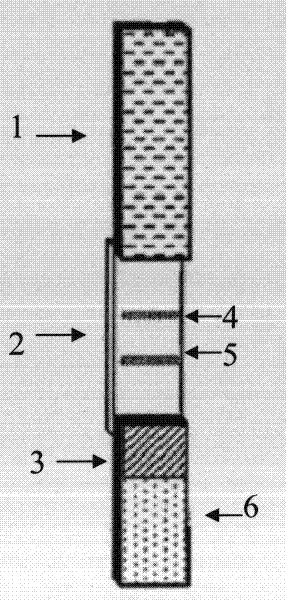
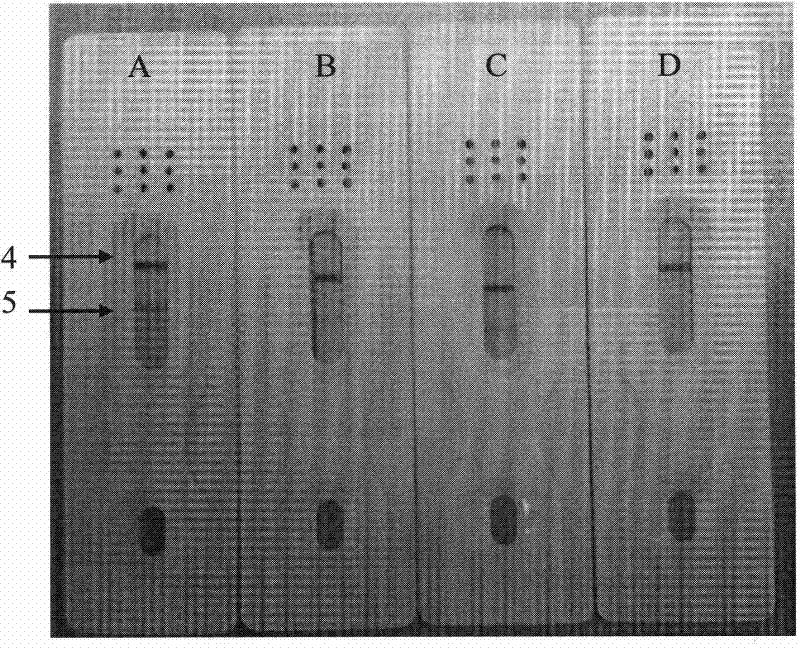
Colloidal gold testing card for testing sjogren's syndrome alpha-fodrin antibody
The invention relates to a colloidal gold testing card for testing sjogren's syndrome alpha-fodrin autoantibody. The testing card comprises orderly arranged components of, from left to right: a sample pad, glass fiber, a nitrocellulose membrane, and absorbent paper. Recombinant protein G labeled by nano-sized colloidal gold is applied on the glass fiber. Macromolecular-protein-coupled alpha-fodrin epitope polypeptide and recombinant protein G resistant antibody are fixed on the nitrocellulose membrane, wherein the sequence of alpha-fodrin epitope polypeptide is represented by SEQ ID NO: 1. Recombinant protein G resistant antibody fixed on the end close to the absorbent paper forms a control line, and macromolecule-protein-coupled alpha-fodrin epitope polypeptide fixed on the end close to the glass fiber forms a test line. The colloidal gold testing card provided by the present invention has advantages of easy preparation and quick testing. With the testing card, no professional technicist or expensive equipment is required. The testing card is suitable for large batch testing, and is suitable to be used in primary areas. The testing card has auxiliary diagnosis functions on early-stage sjogren's syndrome, and intermediate and advanced stage sjogren's syndromes.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Bacterial magnetic particles with recombinant protein G expression capability and application thereof
The invention provides bacterial magnetic particles with recombinant protein G expression capability. The bacterial magnetic particle with recombinant protein G expression capability are obtained by performing enlarged culture and separation and purification on magnetotactic bacteria MSR-1 recombinant strains. The invention further provides application of the bacterial magnetic particles with recombinant protein G expression capability in detection of blood type irregular antibody IgG. By use of the magnetic property of the bacterial magnetic particles and the effect of an applied magnetic field, the bacterial magnetic particles with recombinant protein G expression capability, provided by the invention, are capable of assisting agglutination of sensitized erythrocytes, so that on the one hand, the usage amount of the bacterial magnetic particles is reduced to the greatest extent, and on the other hand, the detection sensitivity of low-potency IgG antibodies is improved to the greatest extent.
Owner:BEIJING GUO KE RONG ZHI BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com