Patents
Literature
648 results about "Ige reactivity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunoglobulin E (IgE) are antibodies produced by the immune system. If you have an allergy, your immune system overreacts to an allergen by producing antibodies called Immunoglobulin E (IgE). These antibodies travel to cells that release chemicals, causing an allergic reaction.
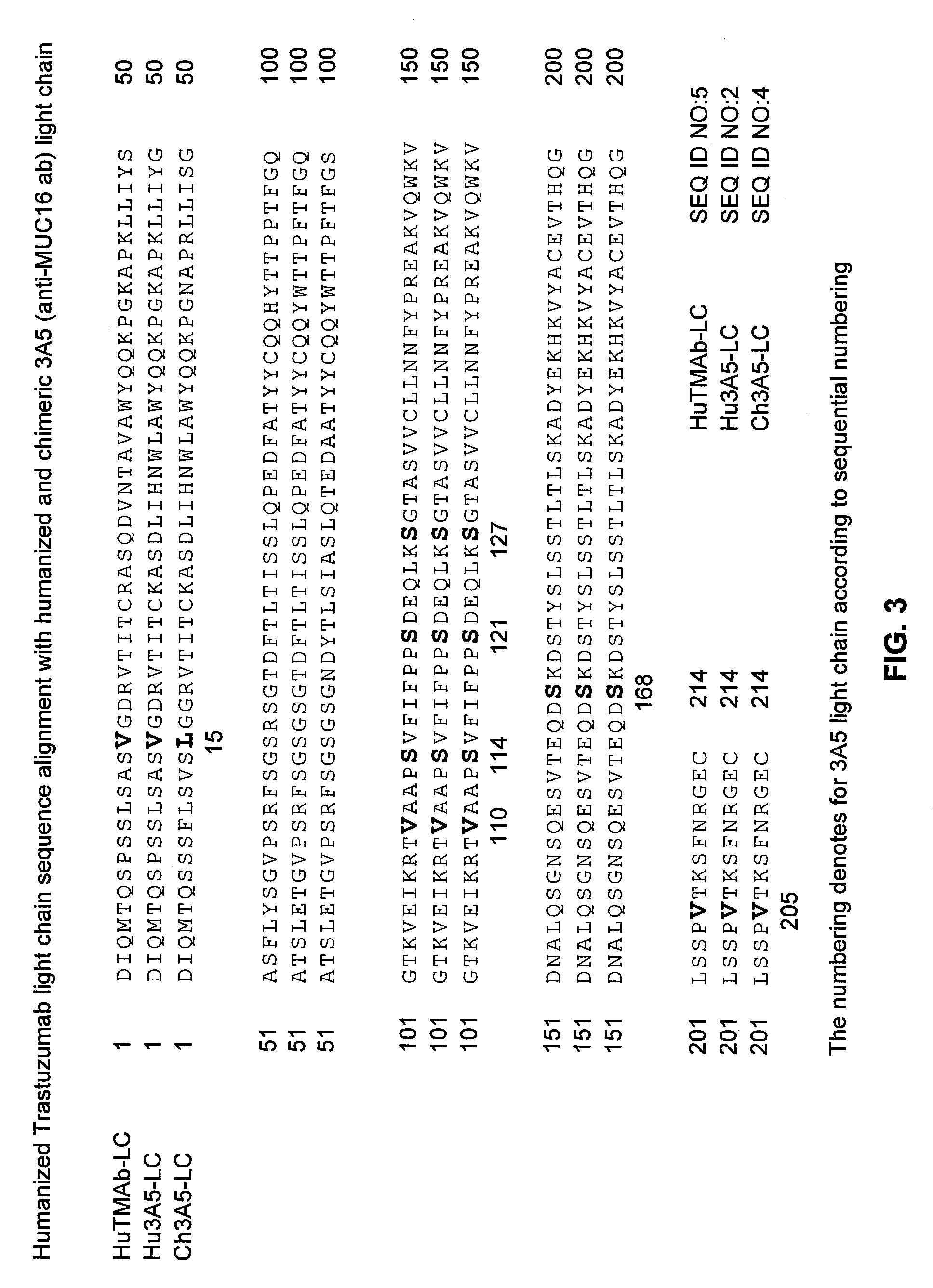
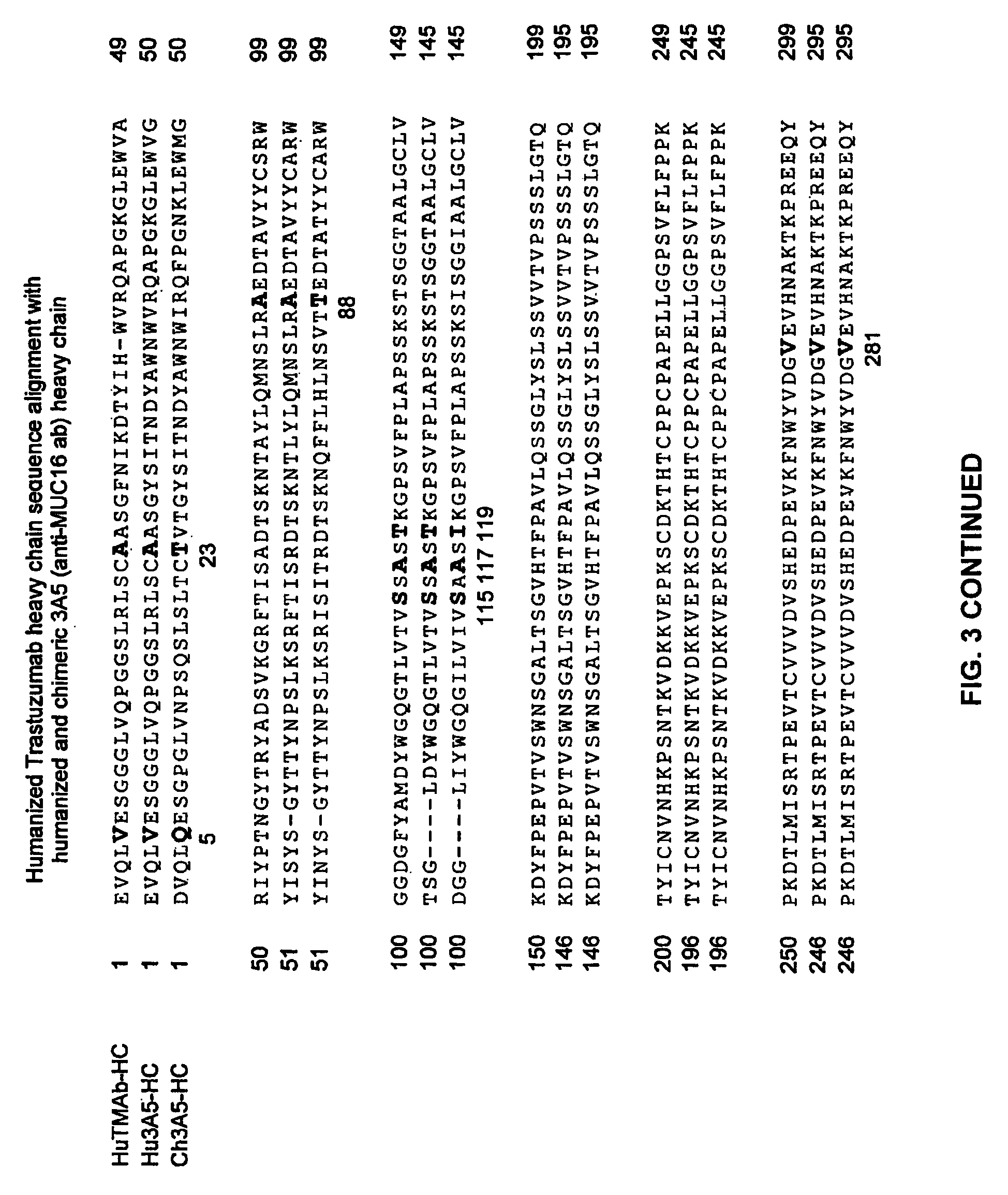
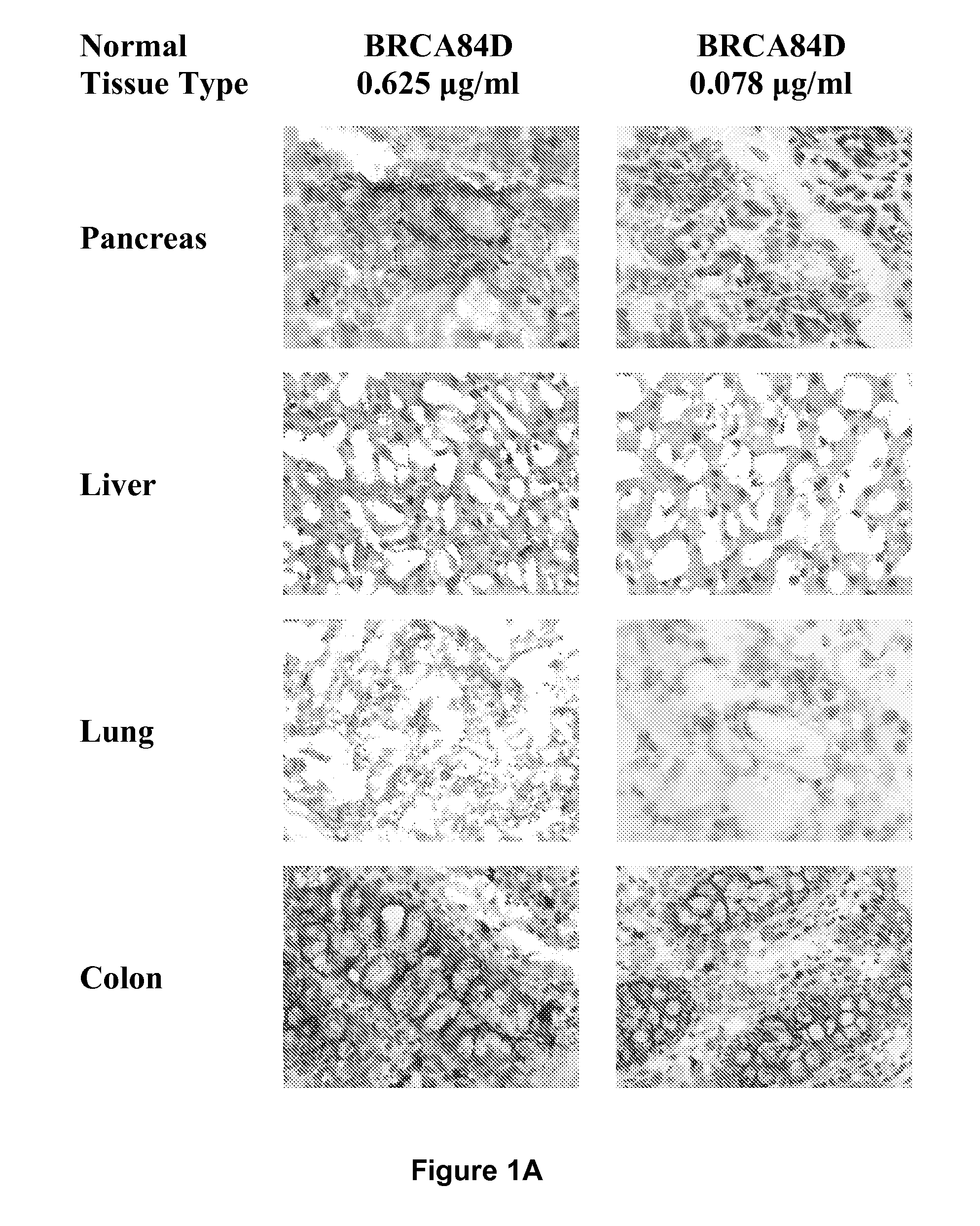
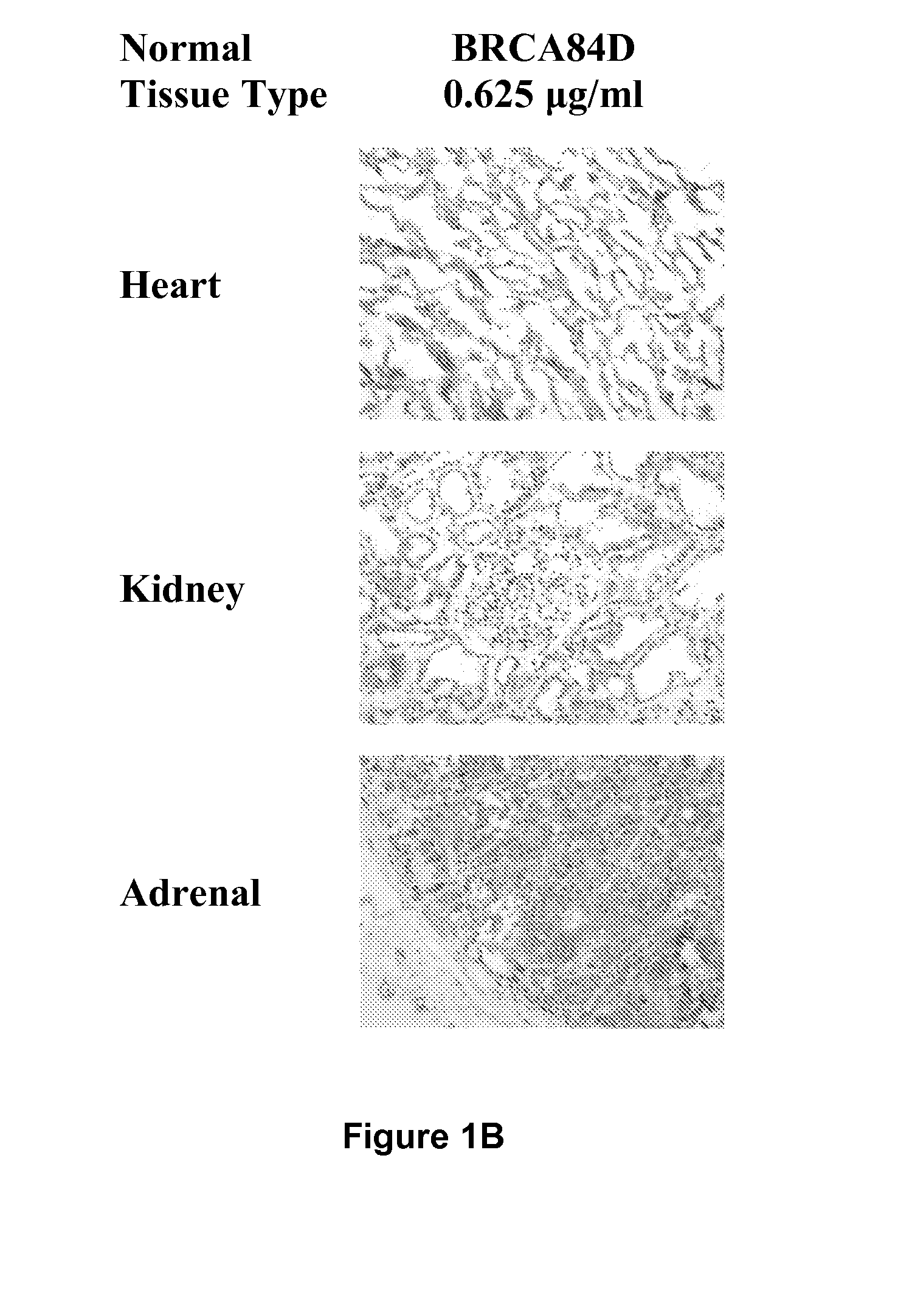
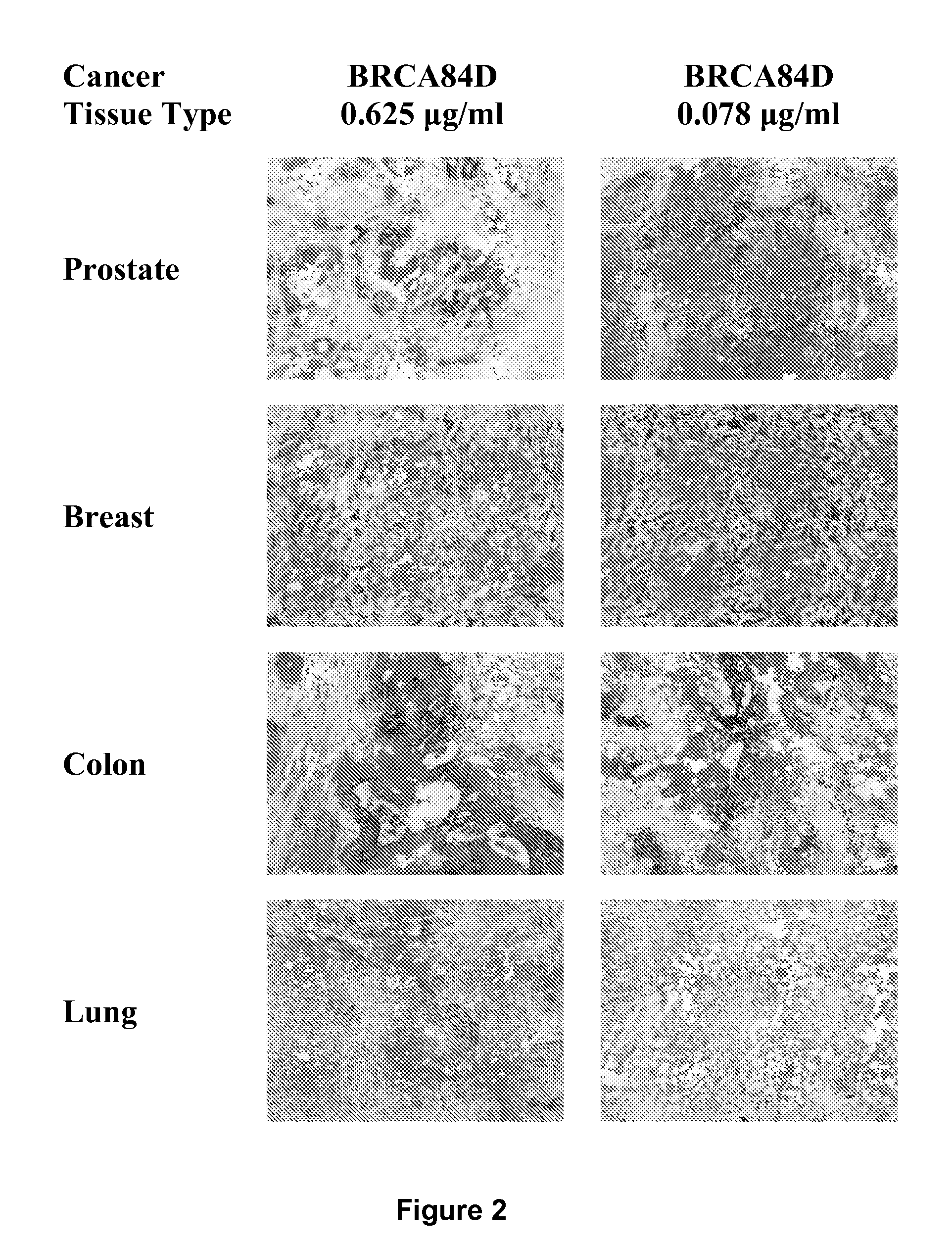
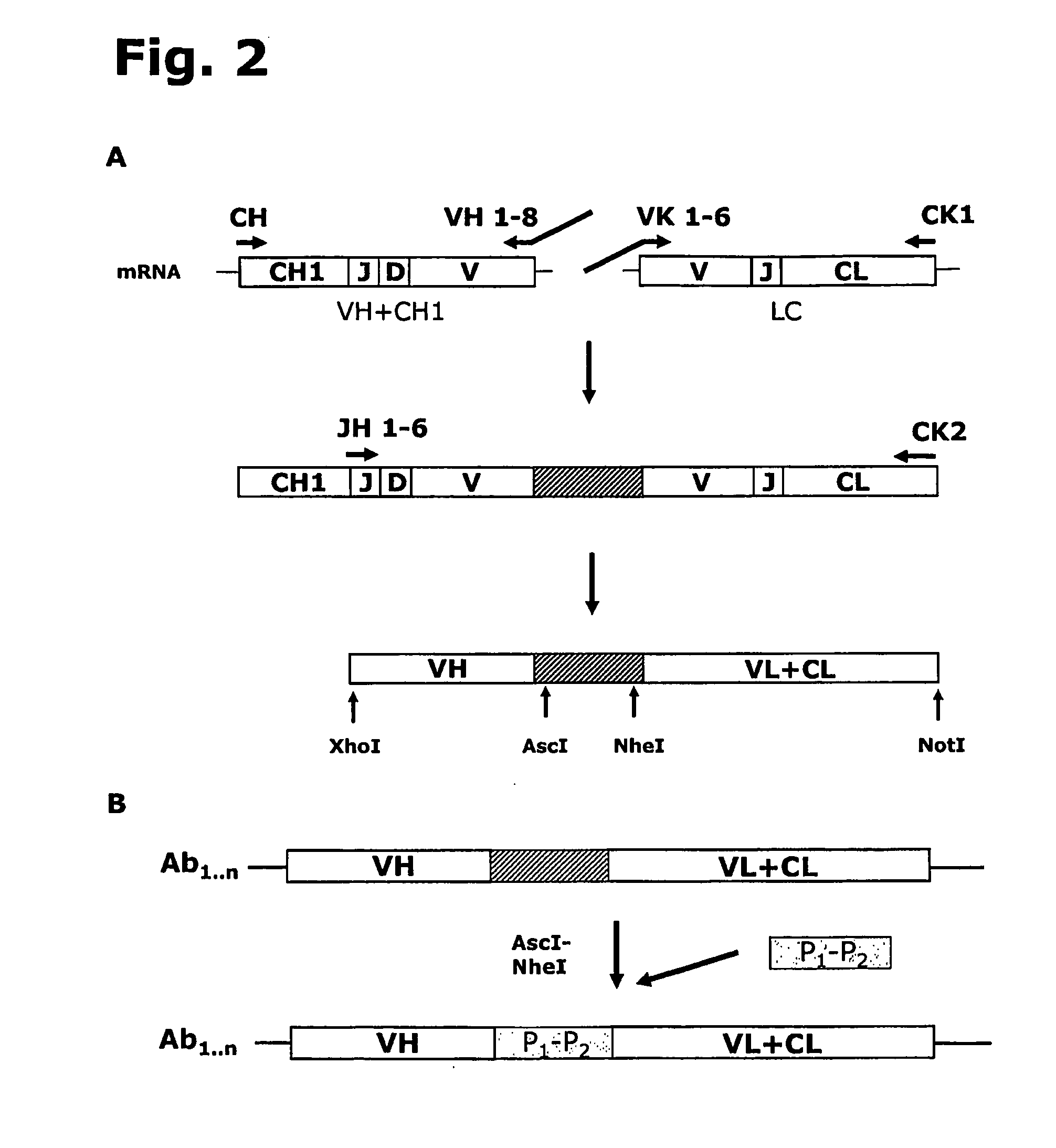
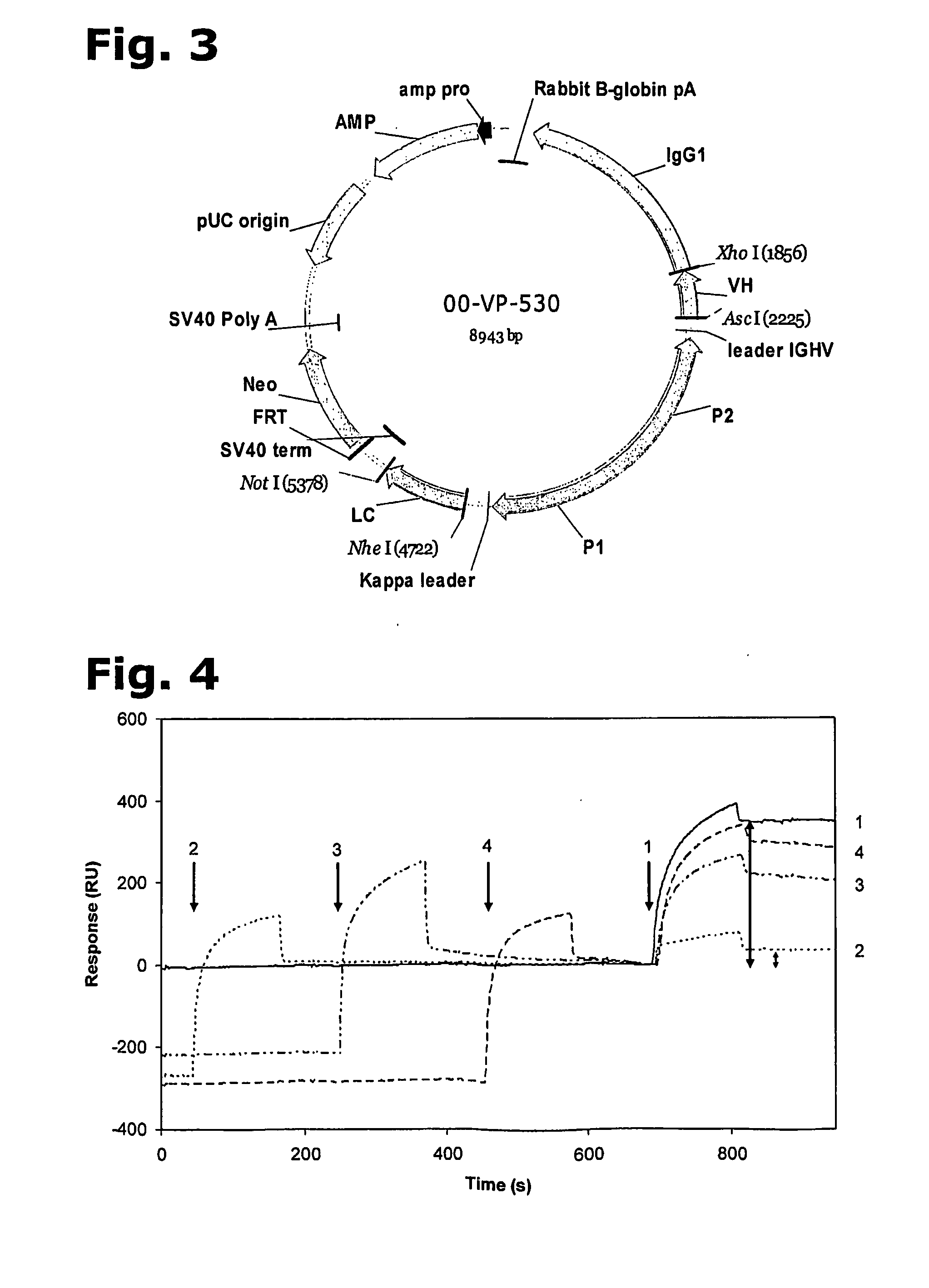
Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates
ActiveUS7723485B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkDrug compound
Cysteine engineered anti-MUC16 antibodies are engineered by replacing one or more amino acids of a parent anti-MUC16 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-MUC16 antibodies are provided. Cysteine engineered anti-MUC16 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-MUC16 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
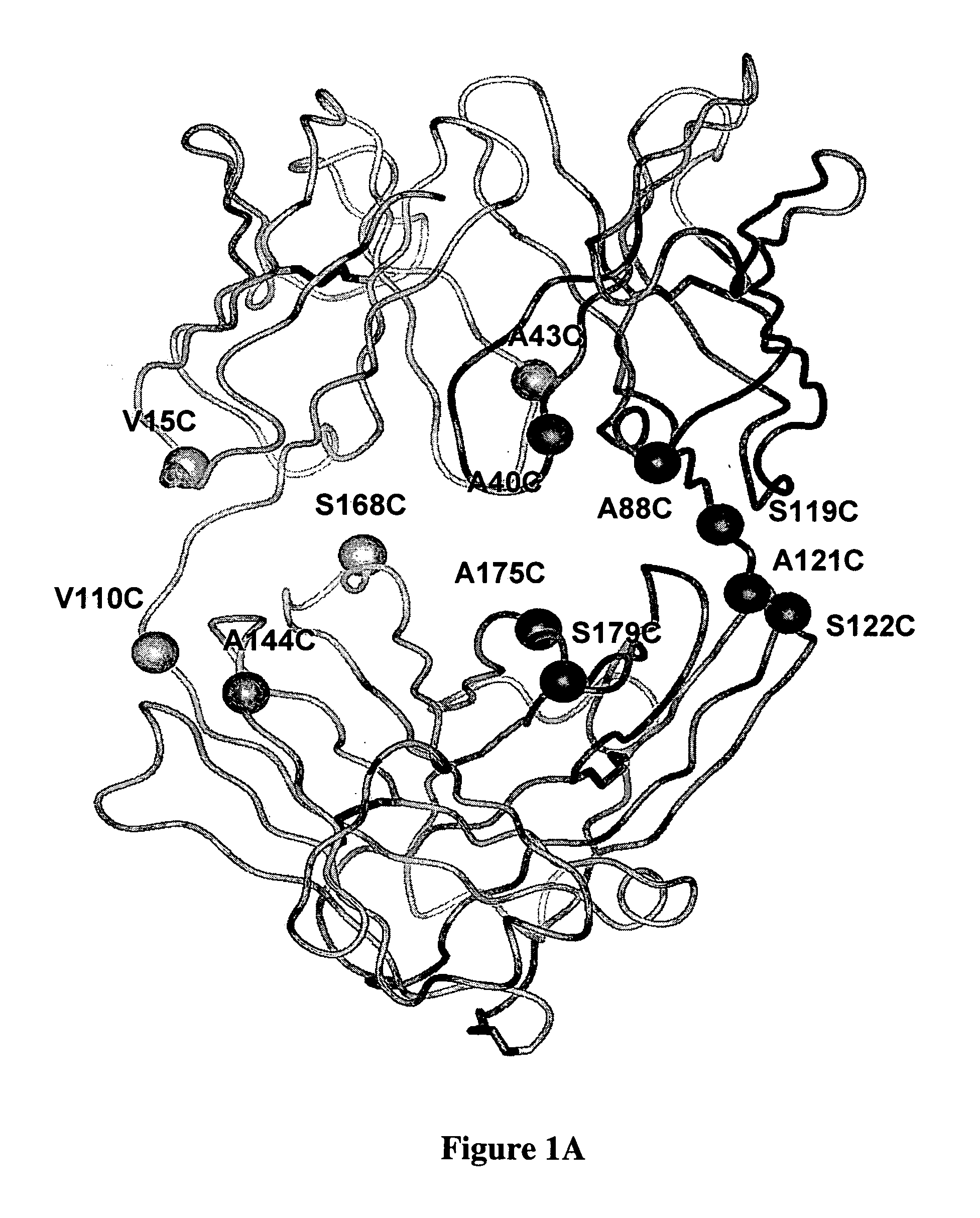
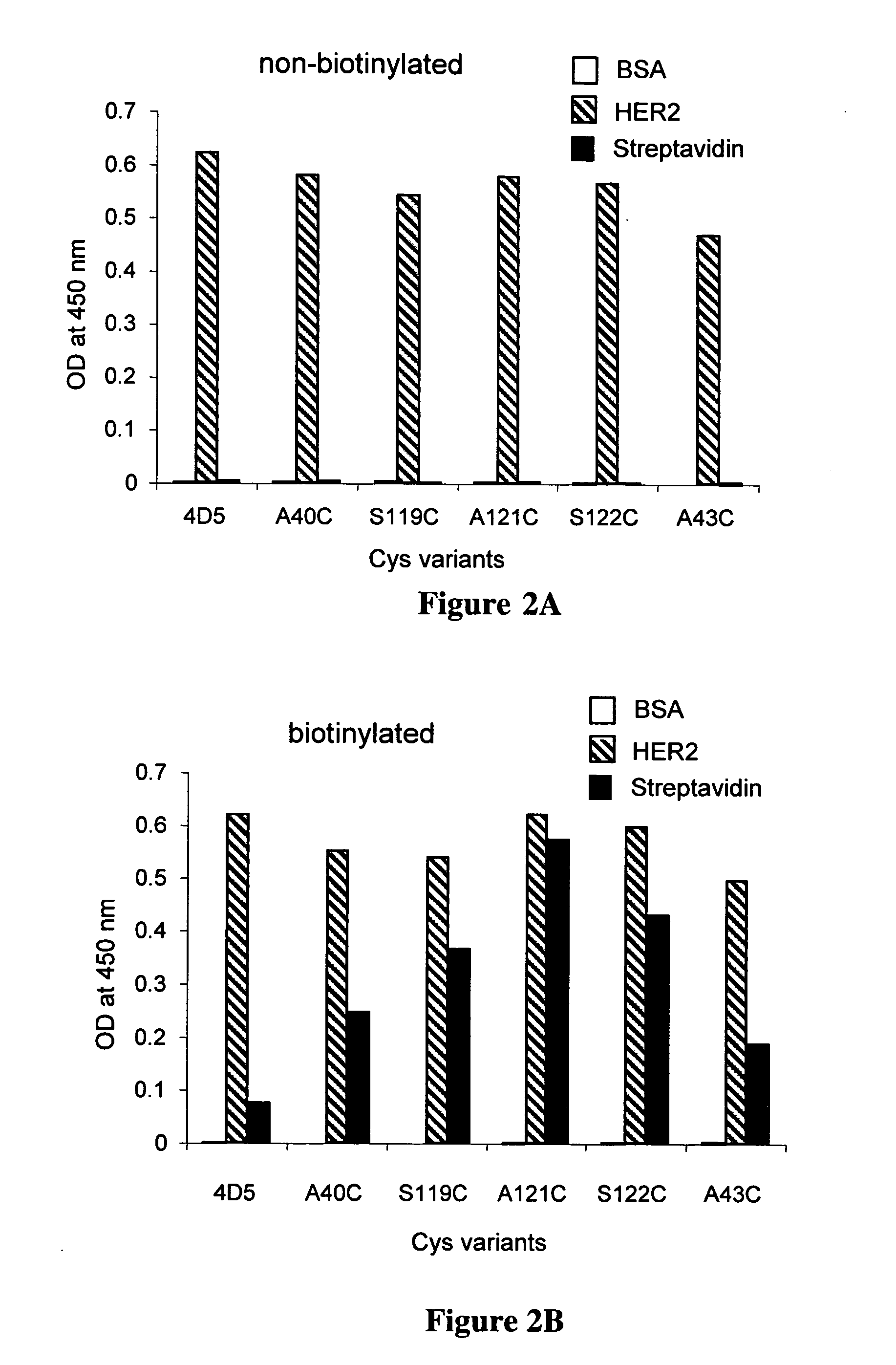
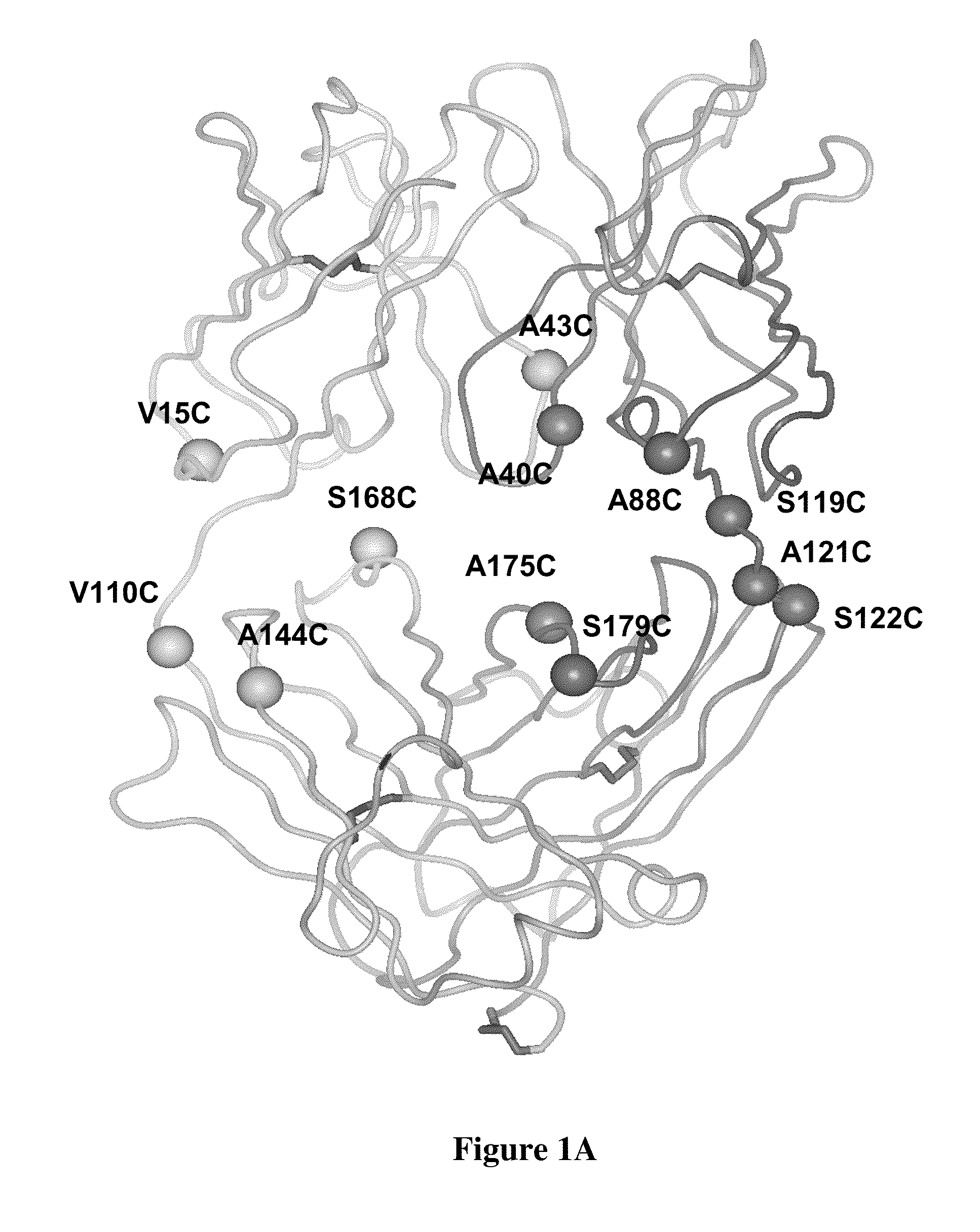
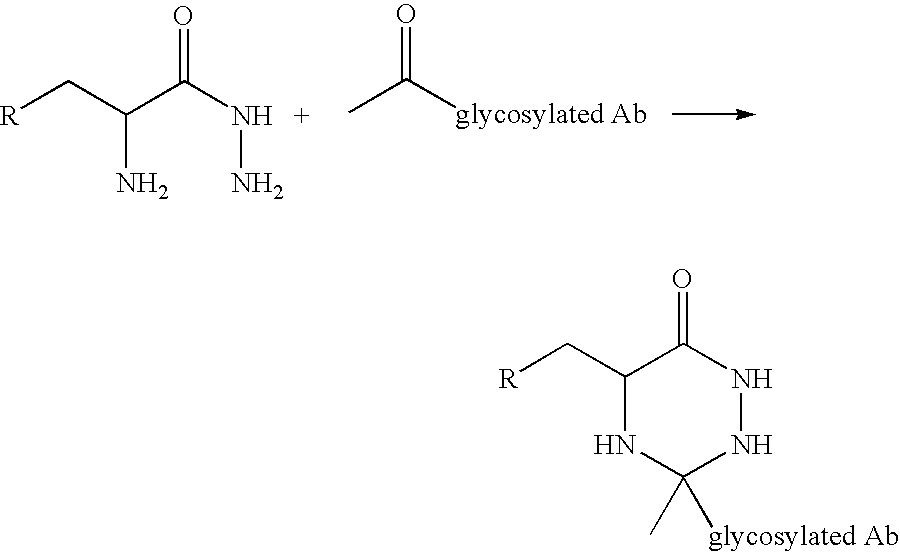
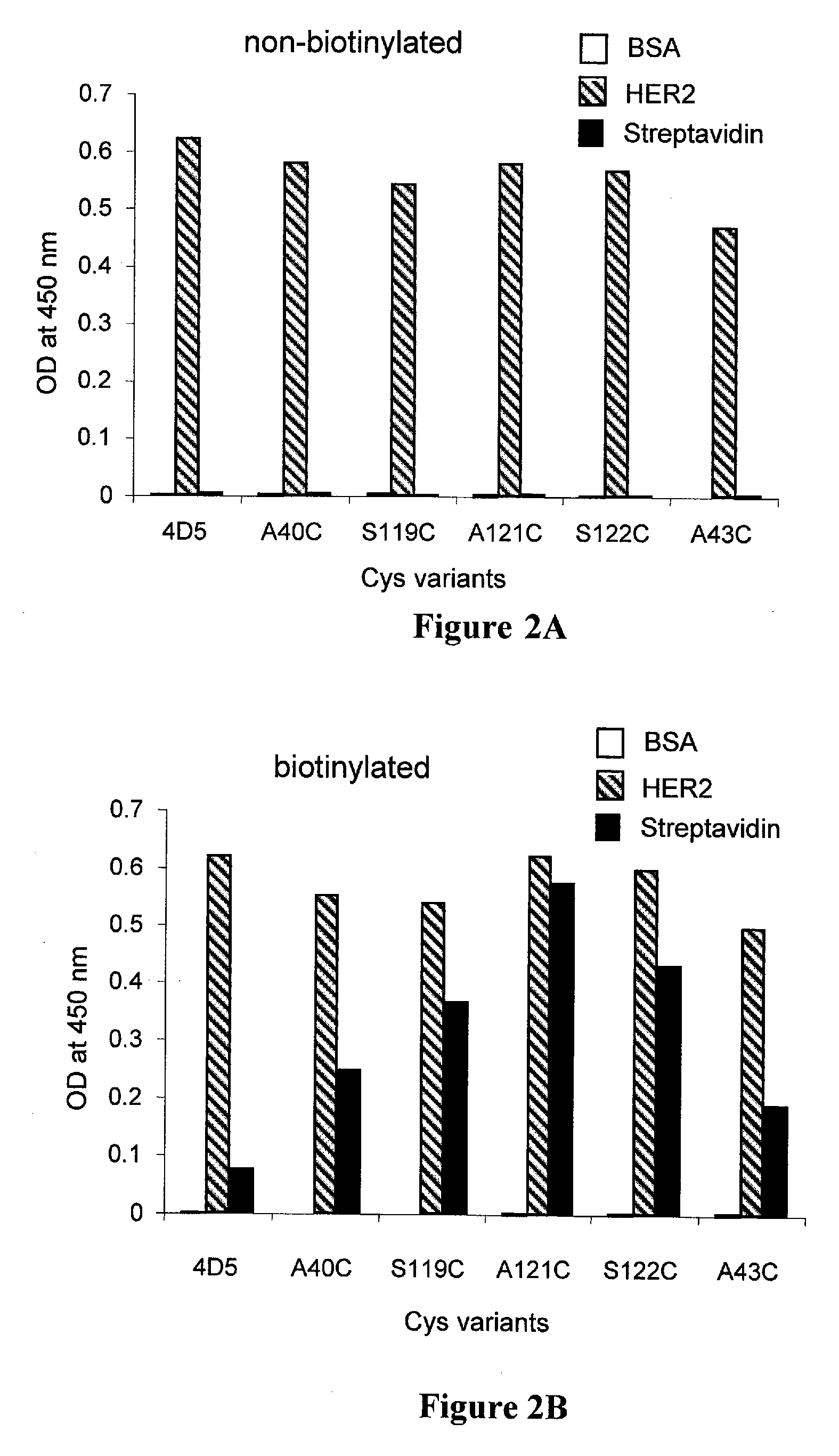
Cysteine engineered antibodies and conjugates
ActiveUS20070092940A1Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkCysteine thiolate
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab), optionally with an albumin-binding peptide (ABP) sequence, are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered antibody-drug conjugates having Formula I: Ab-(L-D)p I where p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Antibodies Reactive with B7-H3 and Uses Thereof
ActiveUS20120294796A1Increased activationImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationHuman cancerCancer cell
The present invention relates to antibodies that are immunoreactive to the mammalian, and more particularly, the human B7-H3 receptor and to uses thereof, particularly in the treatment of cancer and inflammation. The invention thus particularly concerns humanized B7-H3-reactive antibodies that are capable of mediating, and more preferably enhancing the activation of the immune system against cancer cells that are associated with a variety of human cancers.
Owner:MACROGENICS INC
Cysteine engineered antibodies and conjugates
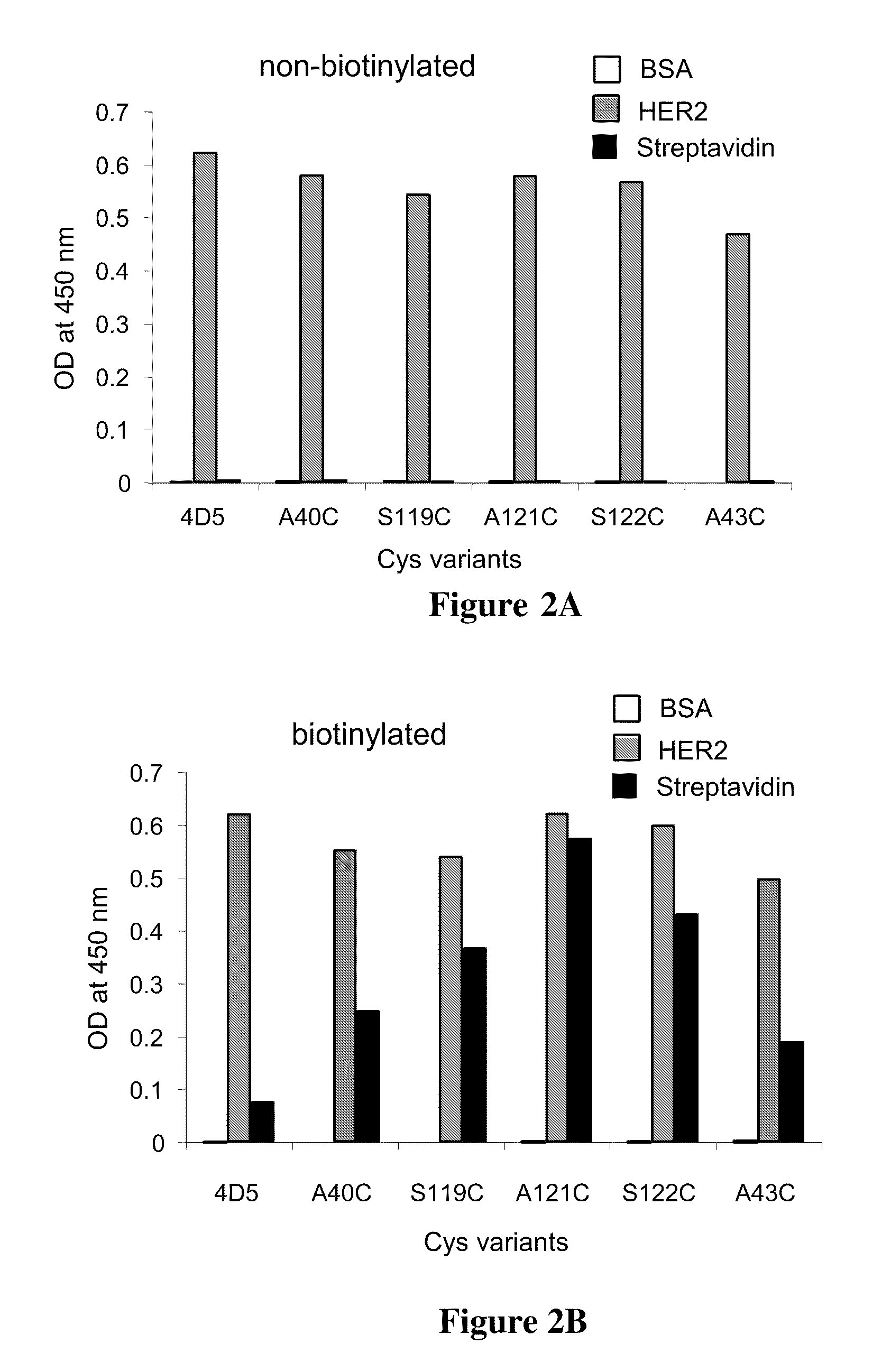
Cysteine engineered antibodies comprising a free cysteine amino acid in the heavy chain or light chain are prepared by mutagenizing a nucleic acid sequence of a parent antibody and replacing one or more amino acid residues by cysteine to encode the cysteine engineered antibody; expressing the cysteine engineered antibody; and isolating the cysteine engineered antibody. Certain highly reactive cysteine engineered antibodies were identified by the PHESELECTOR assay. Isolated cysteine engineered antibodies may be covalently attached to a capture label, a detection label, a drug moiety, or a solid support.
Owner:GENENTECH INC
Method for treating an IgE-mediated disease in a patient using anti-CD40 monoclonal antibodies
InactiveUS6899879B2Inhibition of differentiationInhibit growthOrganic active ingredientsVirusesDiseaseEpitope
Methods for preventing or treating an IgE-mediated allergic disease in a patient are presented, the methods comprising administration of a monoclonal antibody capable of binding to a human CD40 antigen located on the surface of a human B cell, wherein binding of the antibody to the CD40 antigen prevents the growth or differentiation of the B cell. Monoclonal antibodies useful in these methods, and epitopes immunoreactive with such monoclonal antibodies are also presented.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Methods and compositions for determining neoplastic disease responsiveness to antibody therapy
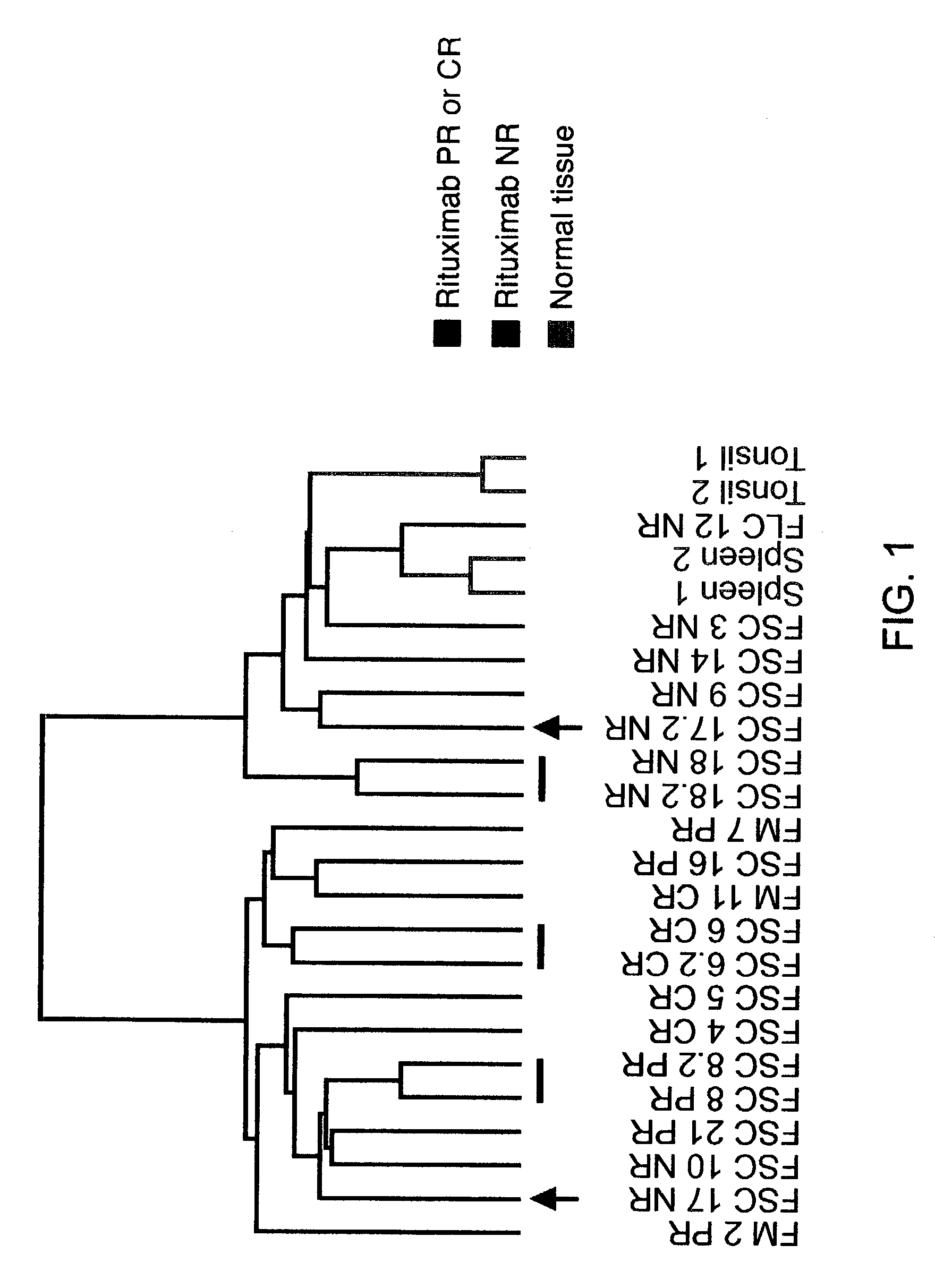
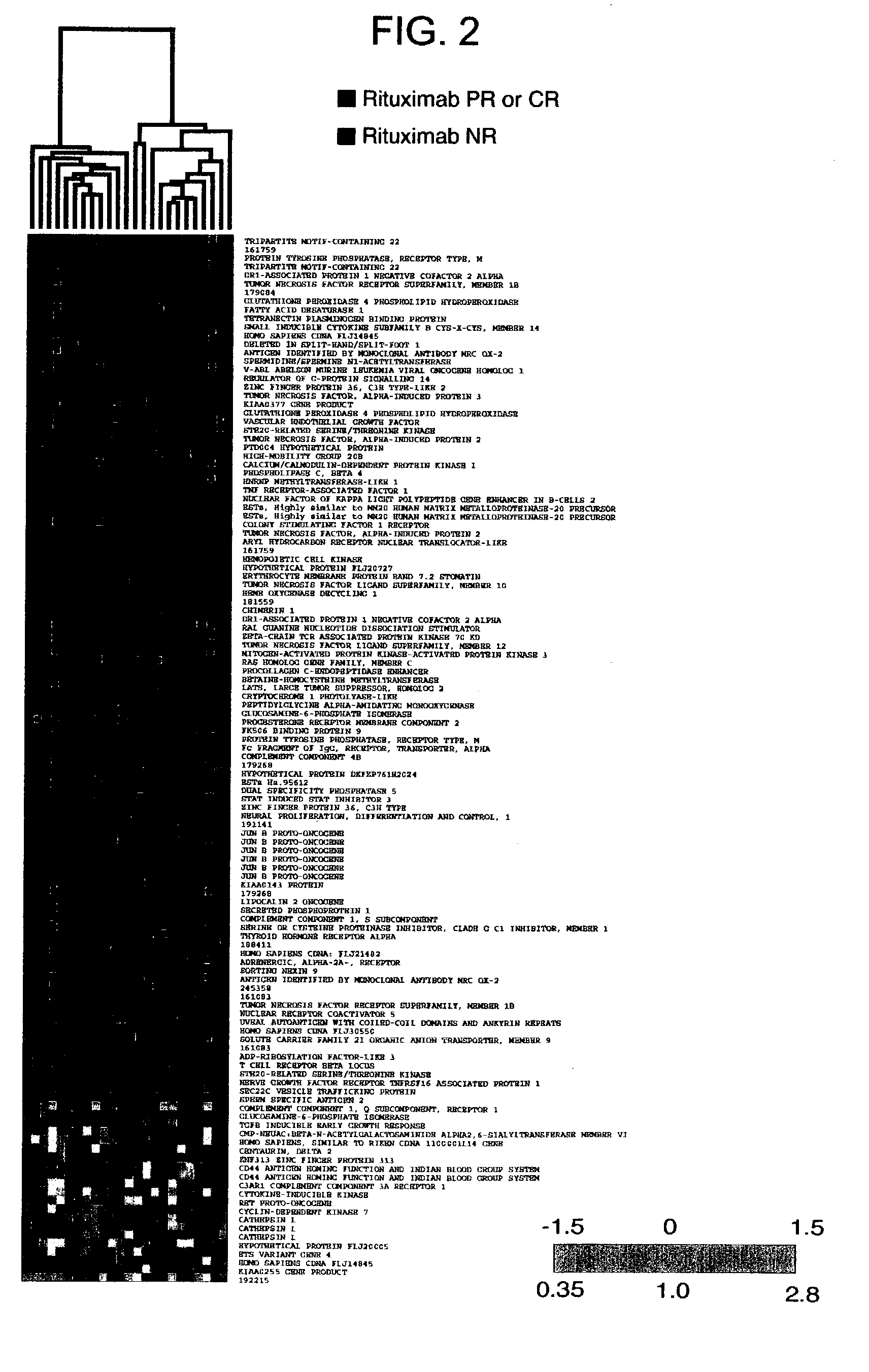
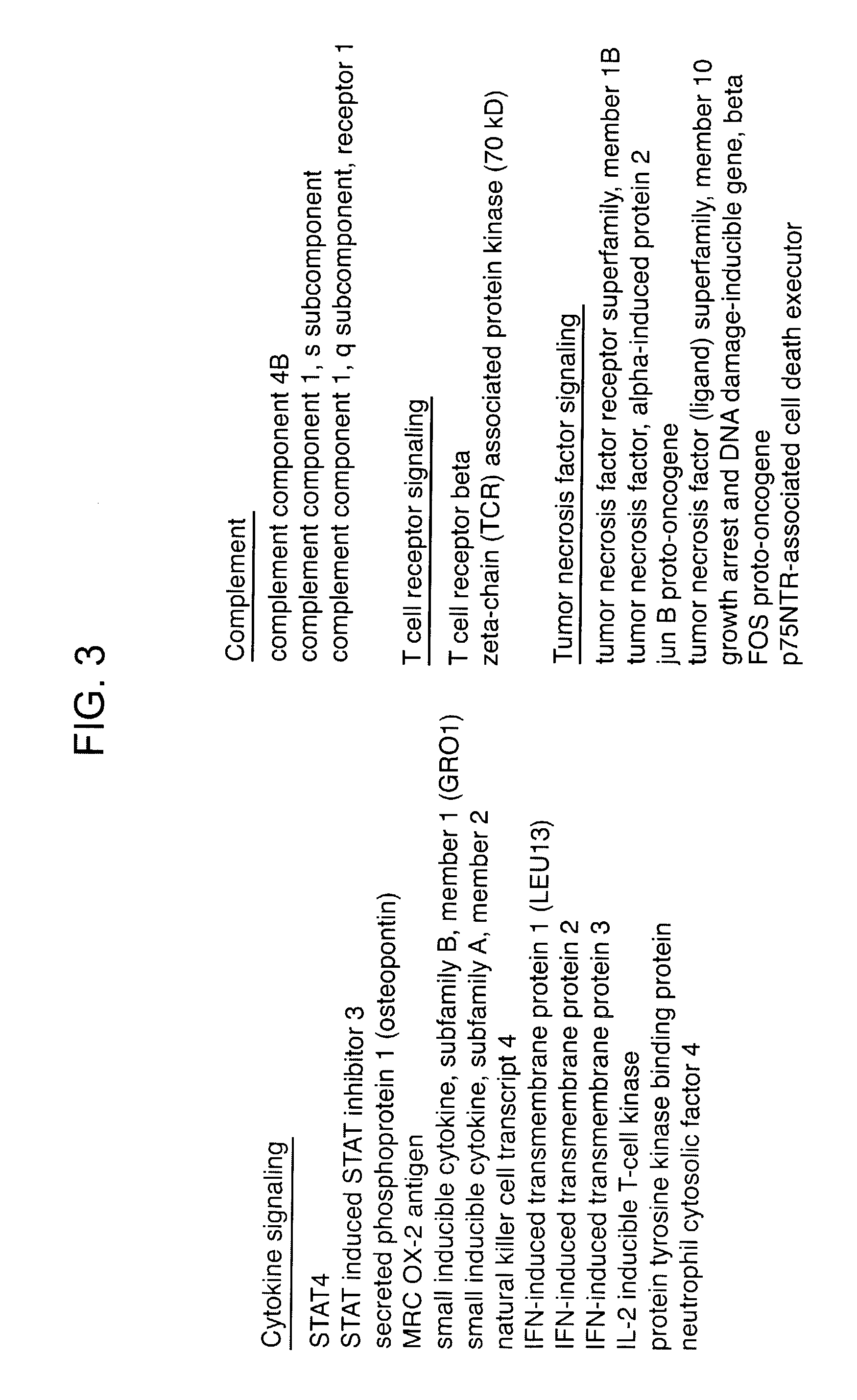
InactiveUS20030219818A1Microbiological testing/measurementDisease diagnosisFollicular lymphoma grade IIWilms' tumor
Methods are provided for determining whether a subject suffering from a neoplastic condition, e.g., non-Hodgkin's lymphoma (NHL), such as follicular lymphoma, is responsive to antineoplastic therapy, such as antibody therapy, e.g., Rituximab. In practicing the subject methods, an expression profile is obtained from the subject suffering from NHL and employed to determine whether the subject is responsive to antineoplastic therapy. In addition, reagents and kits thereof that find use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
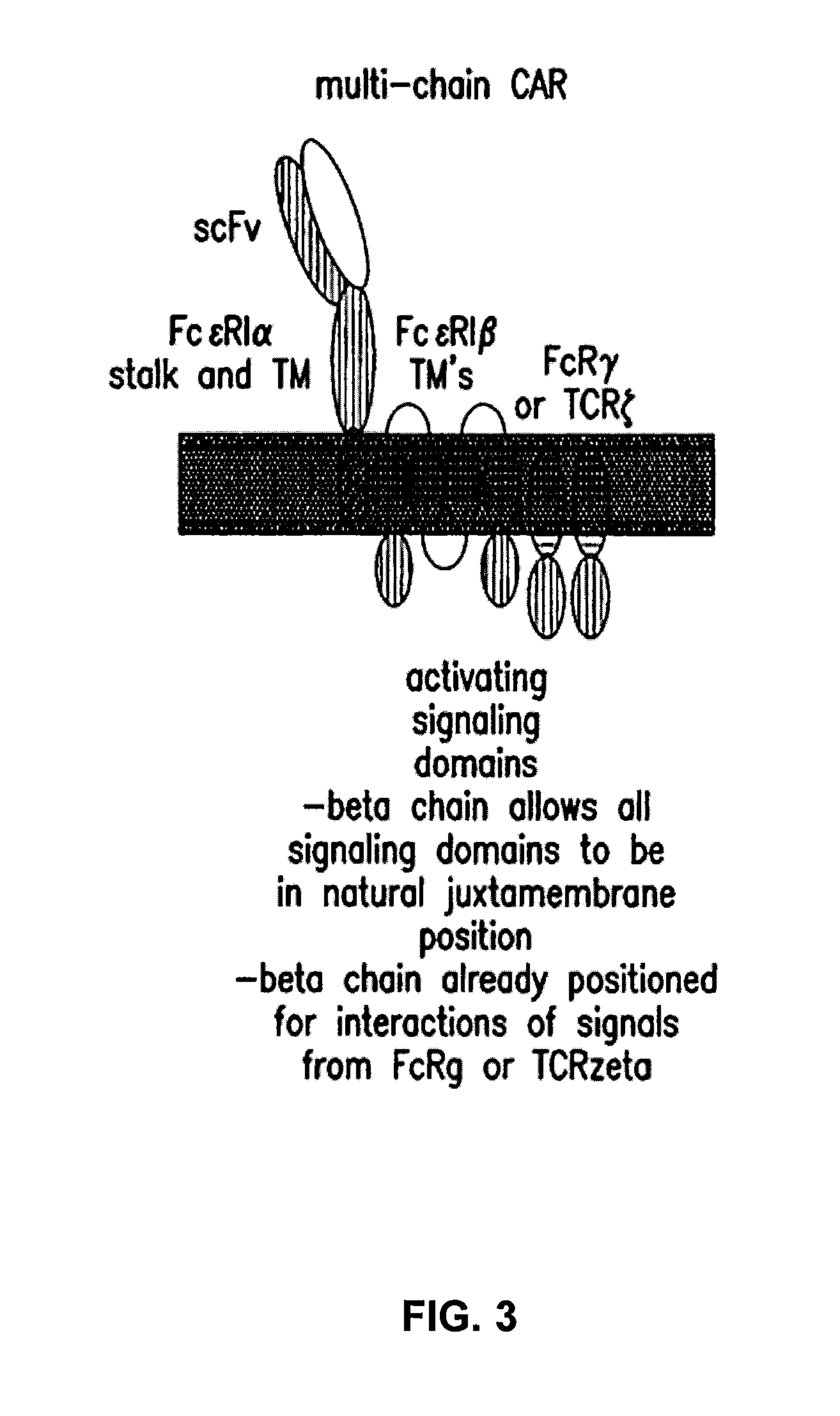
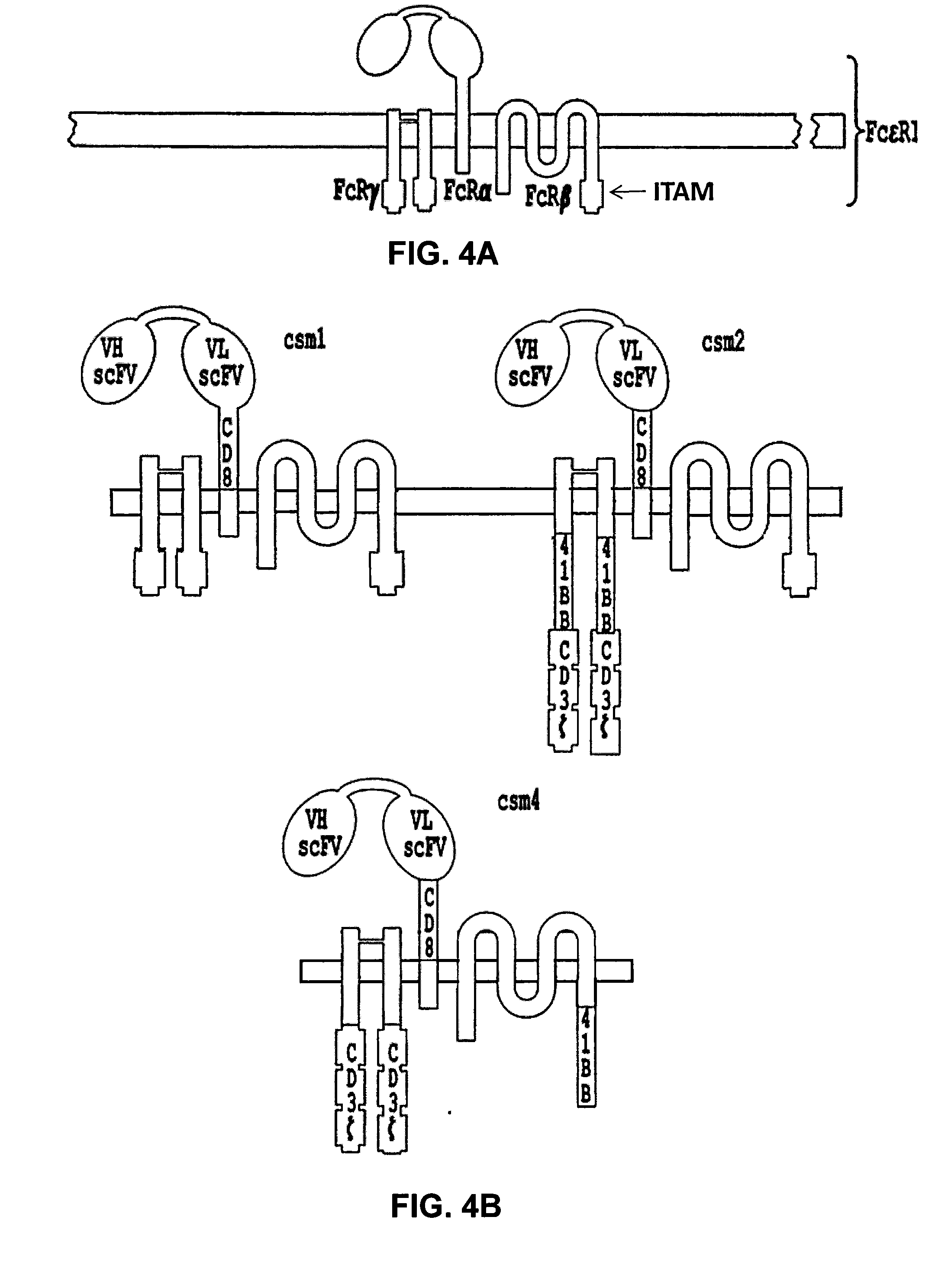
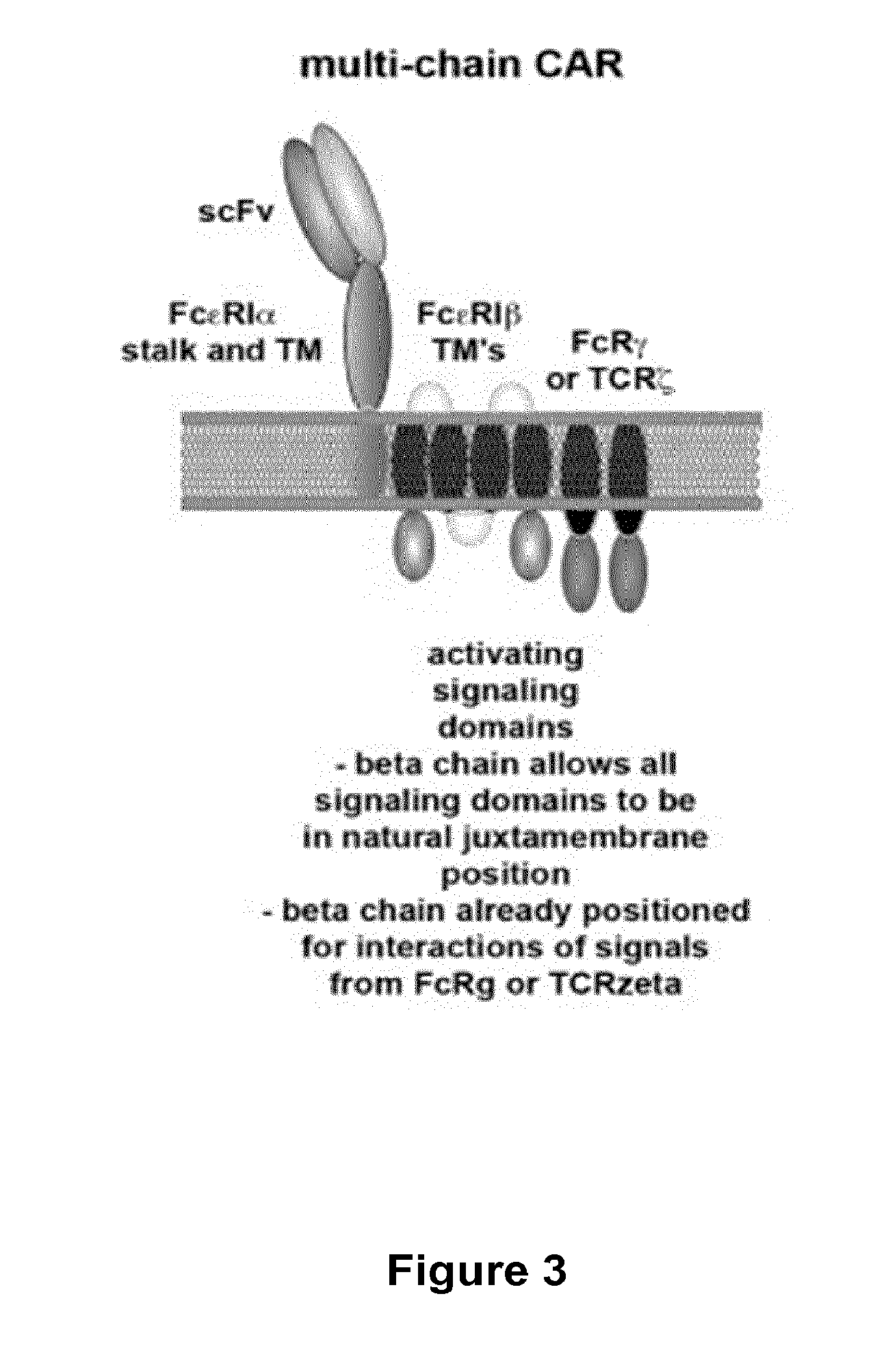
Multi-Chain Chimeric Antigen Receptor and Uses Thereof
ActiveUS20140134142A1Modulate activityStrong specificityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigen receptorReceptor
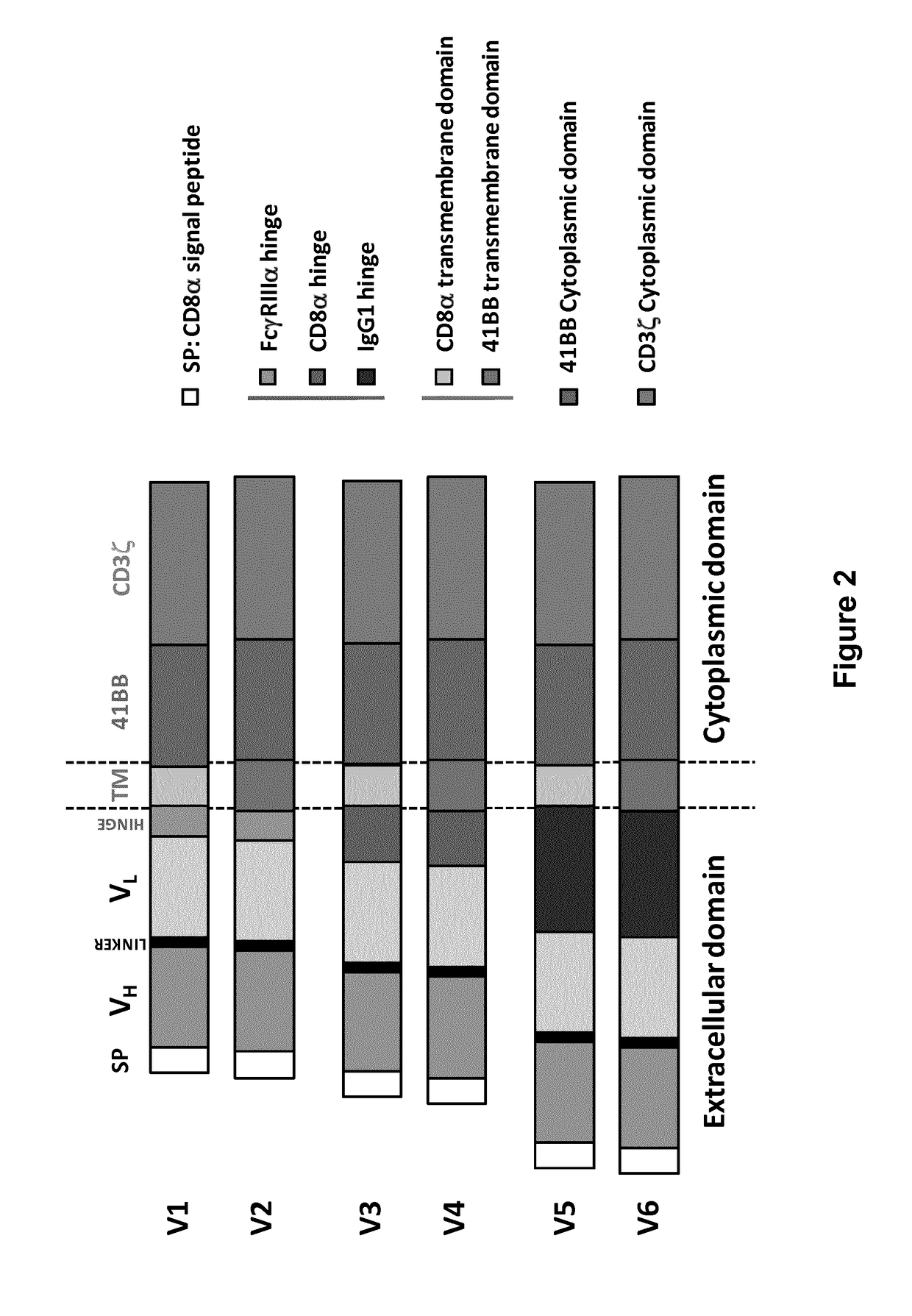
The present invention relates to a new generation of chimeric antigen receptors (CAR) referred to as multi-chain CARs. Such CARs, which aim to redirect immune cell specificity and reactivity toward a selected target exploiting the ligand-binding domain properties, comprise separate extracellular ligand binding and signaling domains in different transmembrane polypeptides. The signaling domains are designed to assemble in juxtamembrane position, which forms flexible architecture closer to natural receptors, that confers optimal signal transduction. The invention encompasses the polynucleotides, vectors encoding said multi-chain CAR and the isolated cells expressing them at their surface, in particularly for their use in immunotherapy. The invention opens the way to efficient adoptive immunotherapy strategies for treating cancer and viral infections.
Owner:CELLECTIS SA
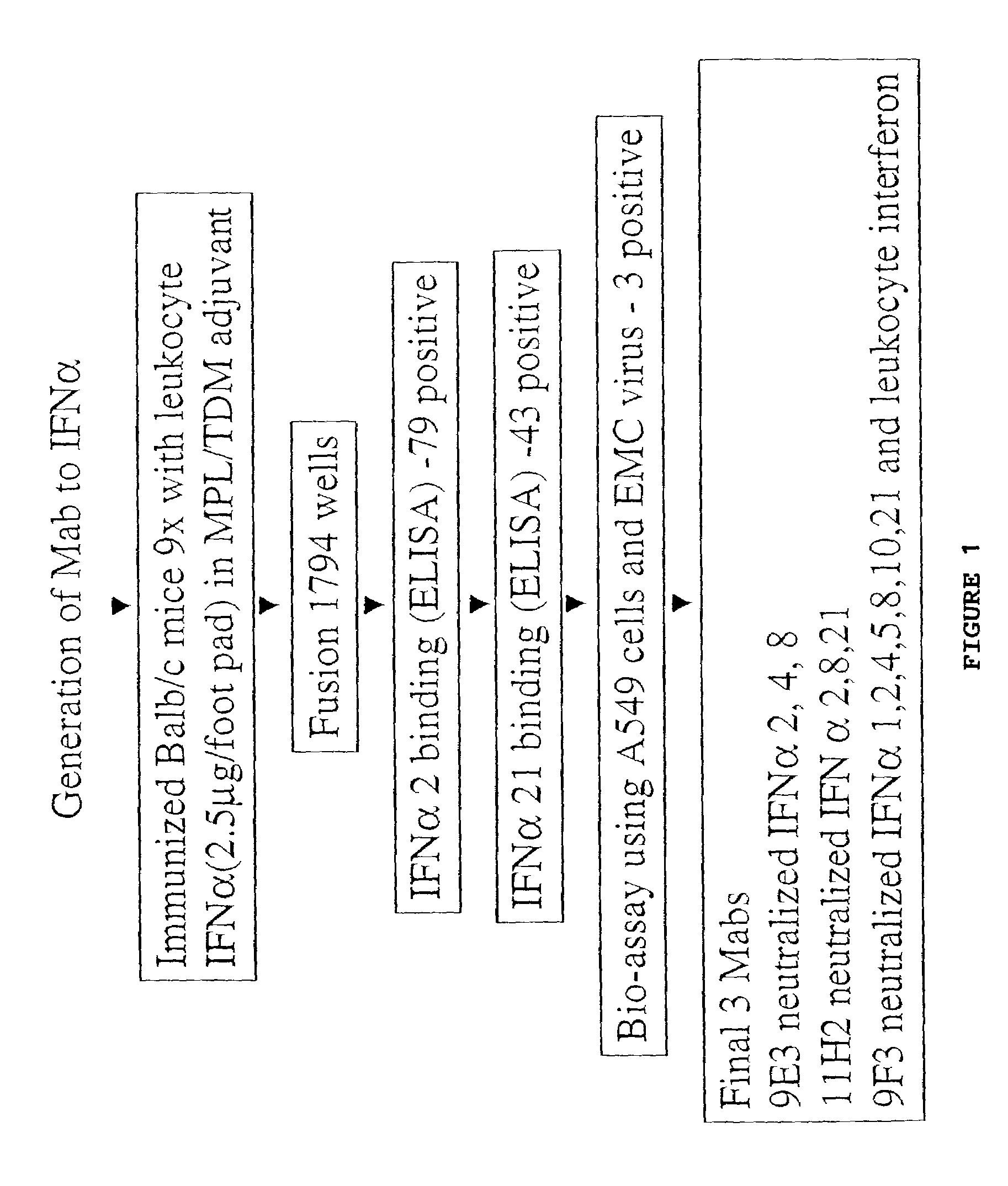
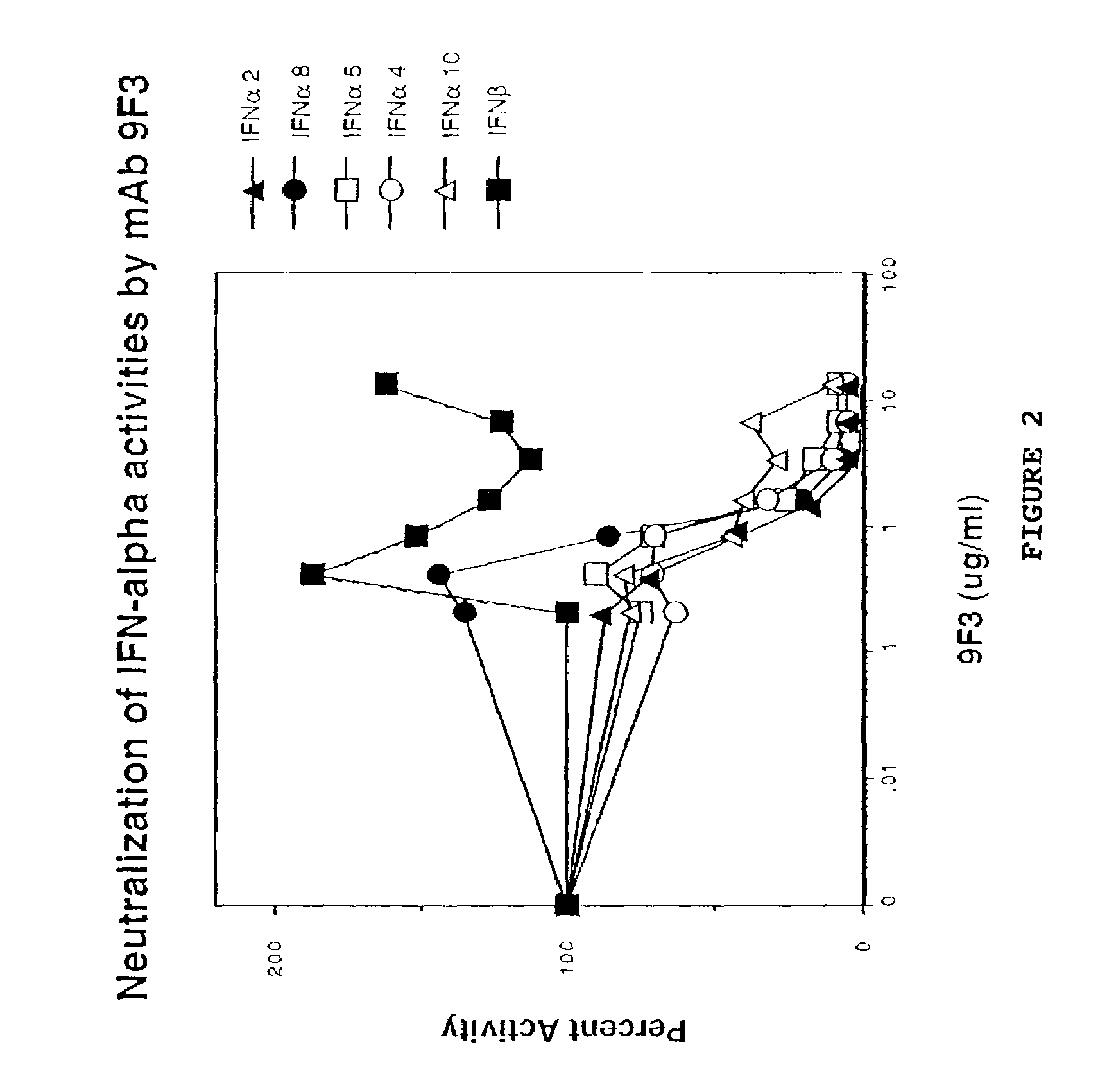
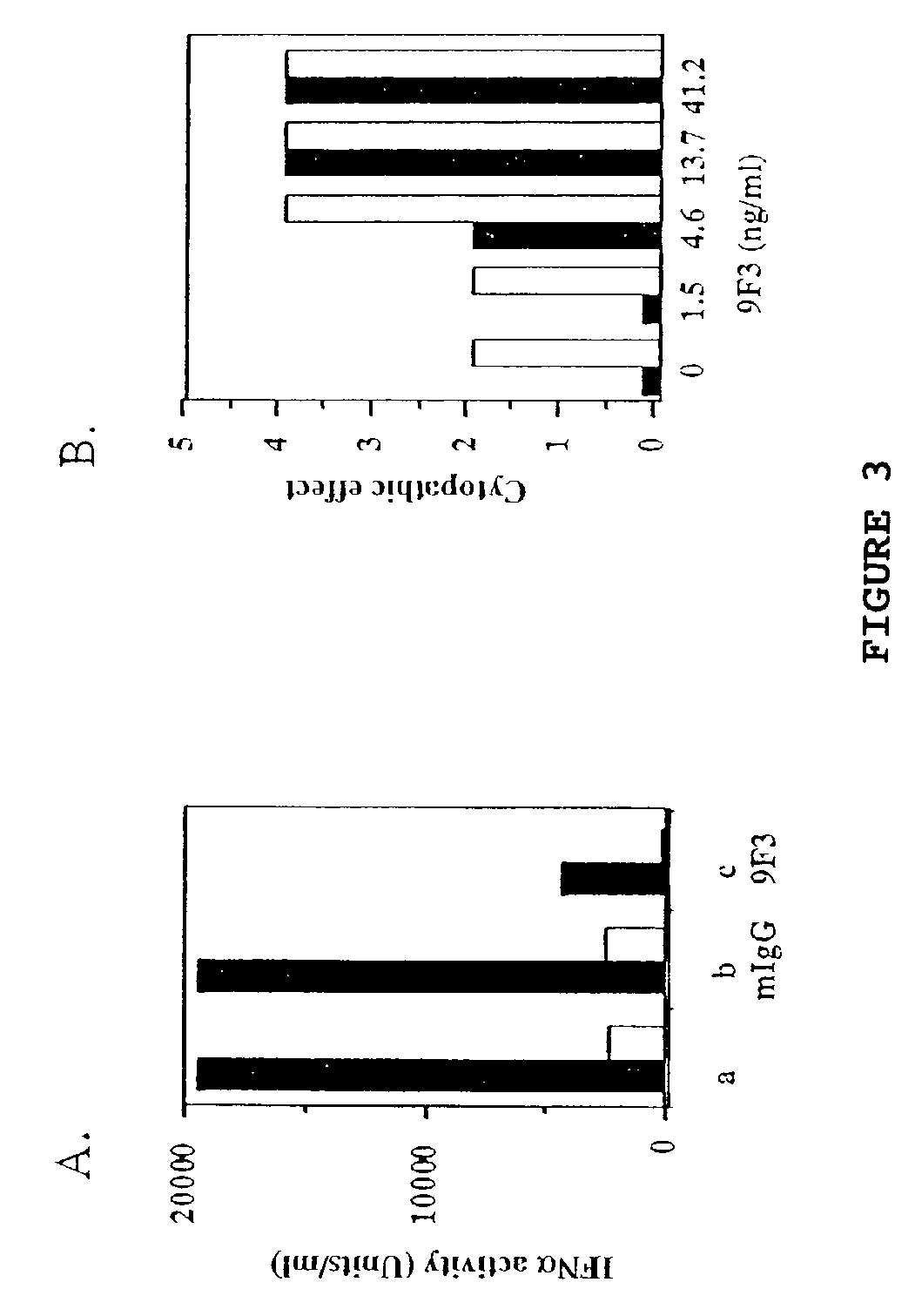
Anti-interferon-α antibodies
InactiveUS7087726B2Reduce and eliminate biological activityFungiBacteriaDiseaseAntiendomysial antibodies
The present invention relates generally to the generation and characterization of neutralizing anti-IFN-α monoclonal antibodies with broad reactivity against various IFN-α subtypes. The invention further relates to the use of such anti-IFN-α antibodies in the diagnosis and treatment of disorders associated with increased expression of IFN-α, in particular, autoimmune disorders such as insulin-dependent diabetes mellitus (IDDM) and systemic lupus erythematosus (SLE).
Owner:GENENTECH INC
Recombinant polyclonal antibody for treatment of respiratory syncytial virus infections
InactiveUS20100040606A1Reduce the possibilityMinimizing developmentSsRNA viruses negative-senseSugar derivativesEpitopeProtein G
Disclosed are novel polyclonal antibodies, which target respiratory syncyticilal virus (RSV), and novel high affinity antibody molecules reactive with RSV. The polyclonal antibodies may comprise antibody molecules which are reactive with both RSV protein F and RSV protein G, and preferably the polyclonal antibodies target a variety of epitopes on these proteins. The single antibody molecules of the invention are shown to exhibit affinities which provide for dissociation constants as low as in the picomolar range. Also disclosed are methods of producing the antibodies of the invention as well as methods of their use in treatment for RSV infection.
Owner:SYMPHOGEN AS
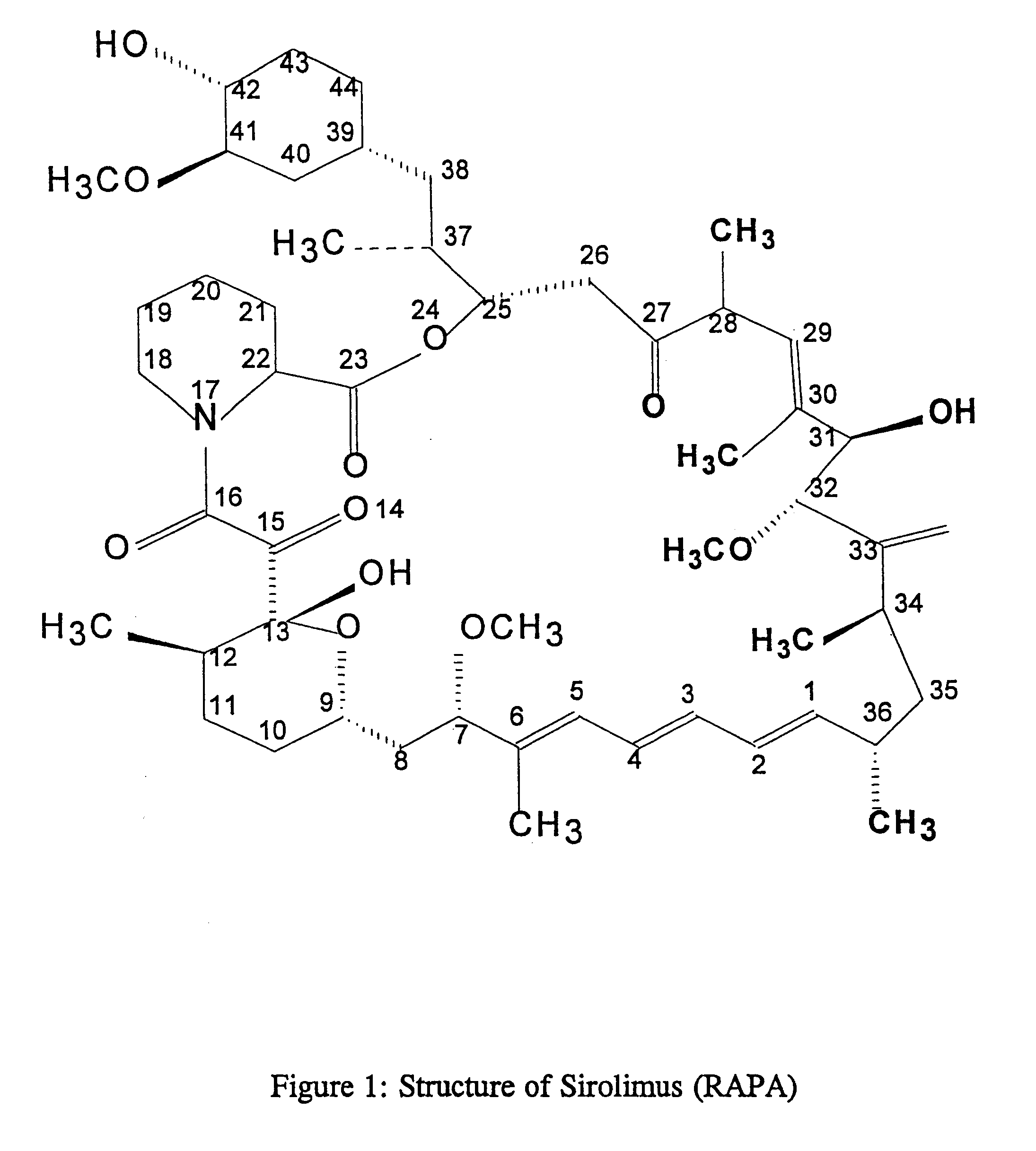
Method for production of antibodies to specific sites of rapamycin
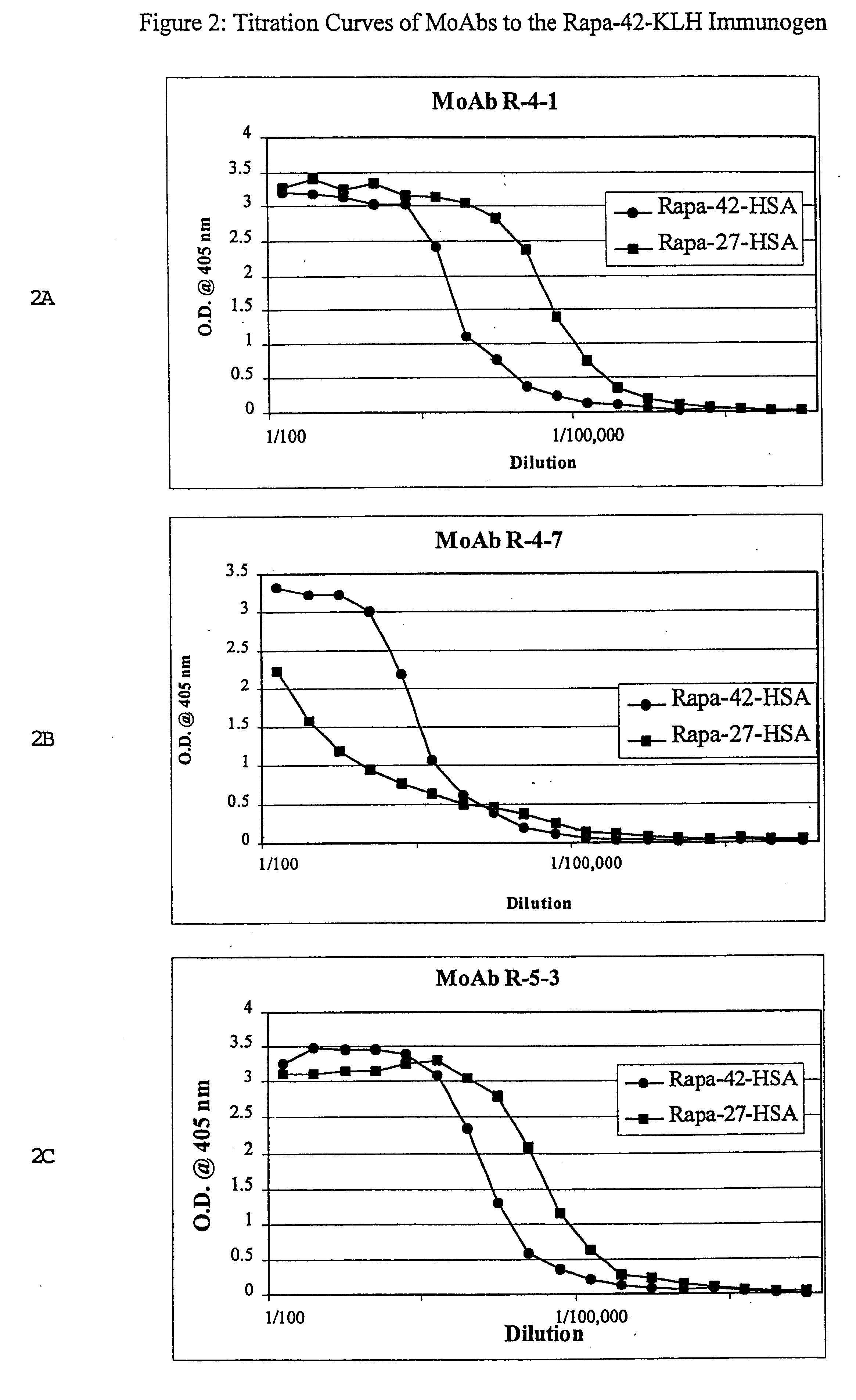
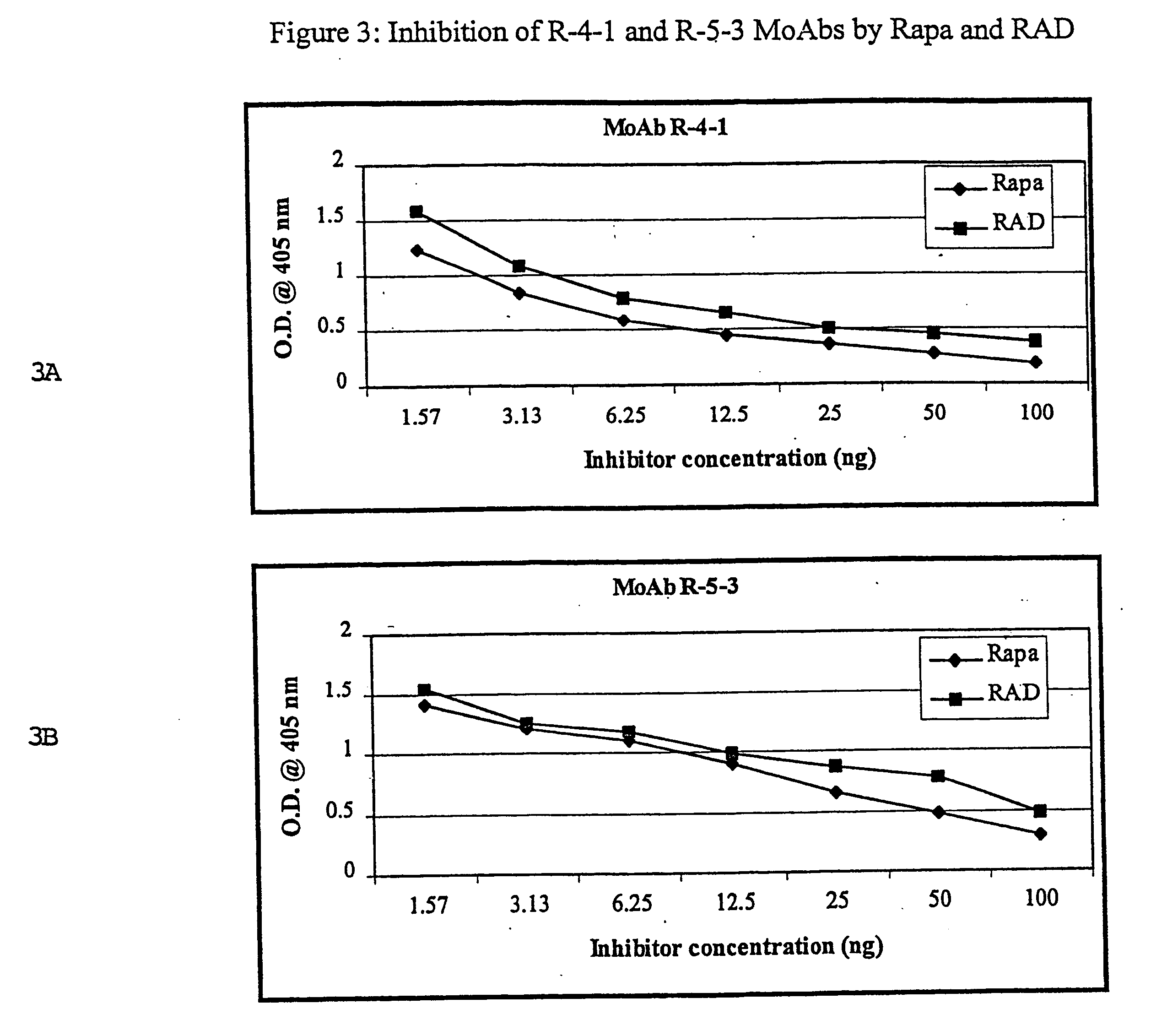
InactiveUS6709873B1Immunoglobulins against fungi/algae/lichensBiological testingPolyclonal antibodiesDivinyl sulfone
Owner:ISODIAGNOSTIKA
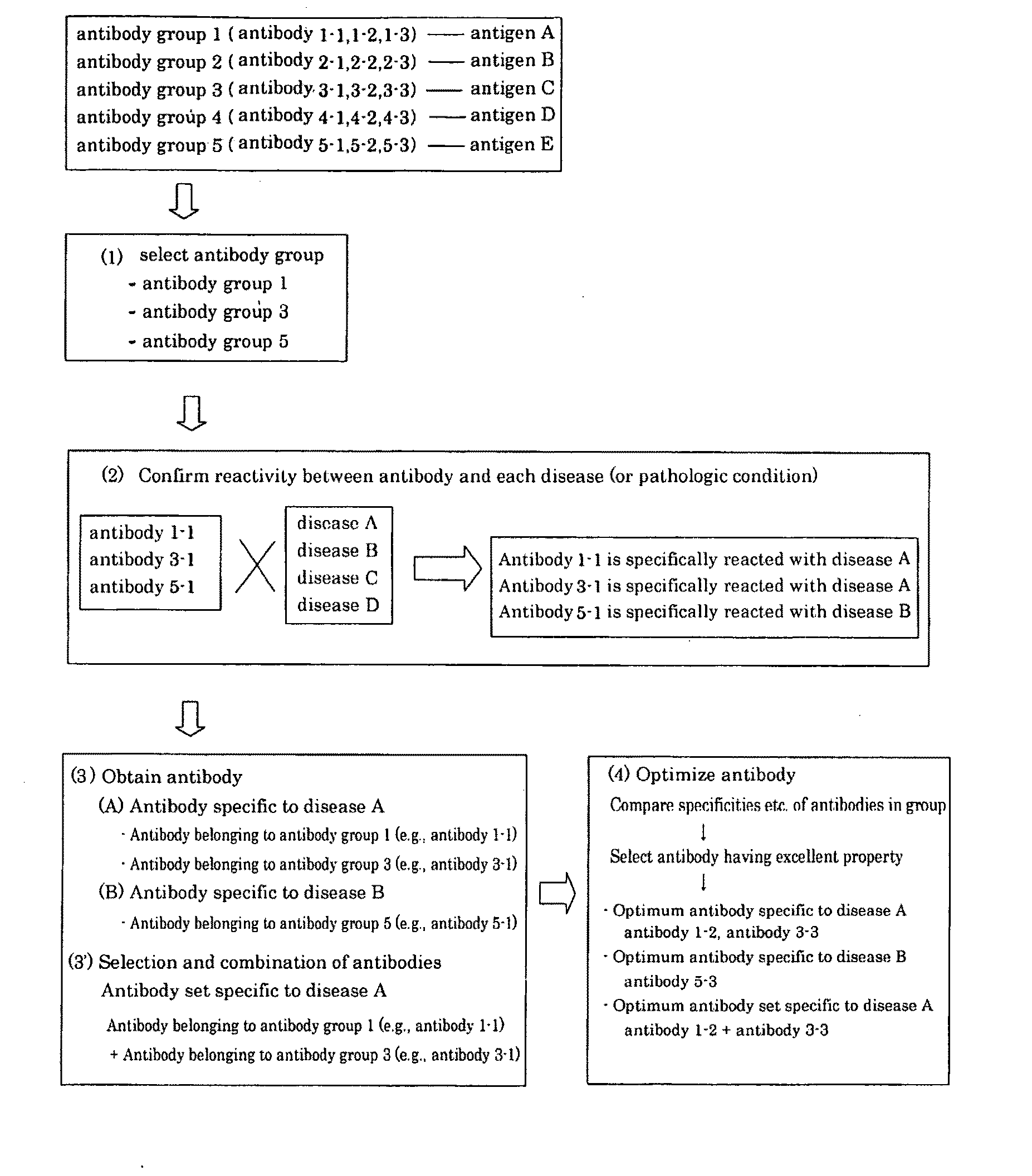
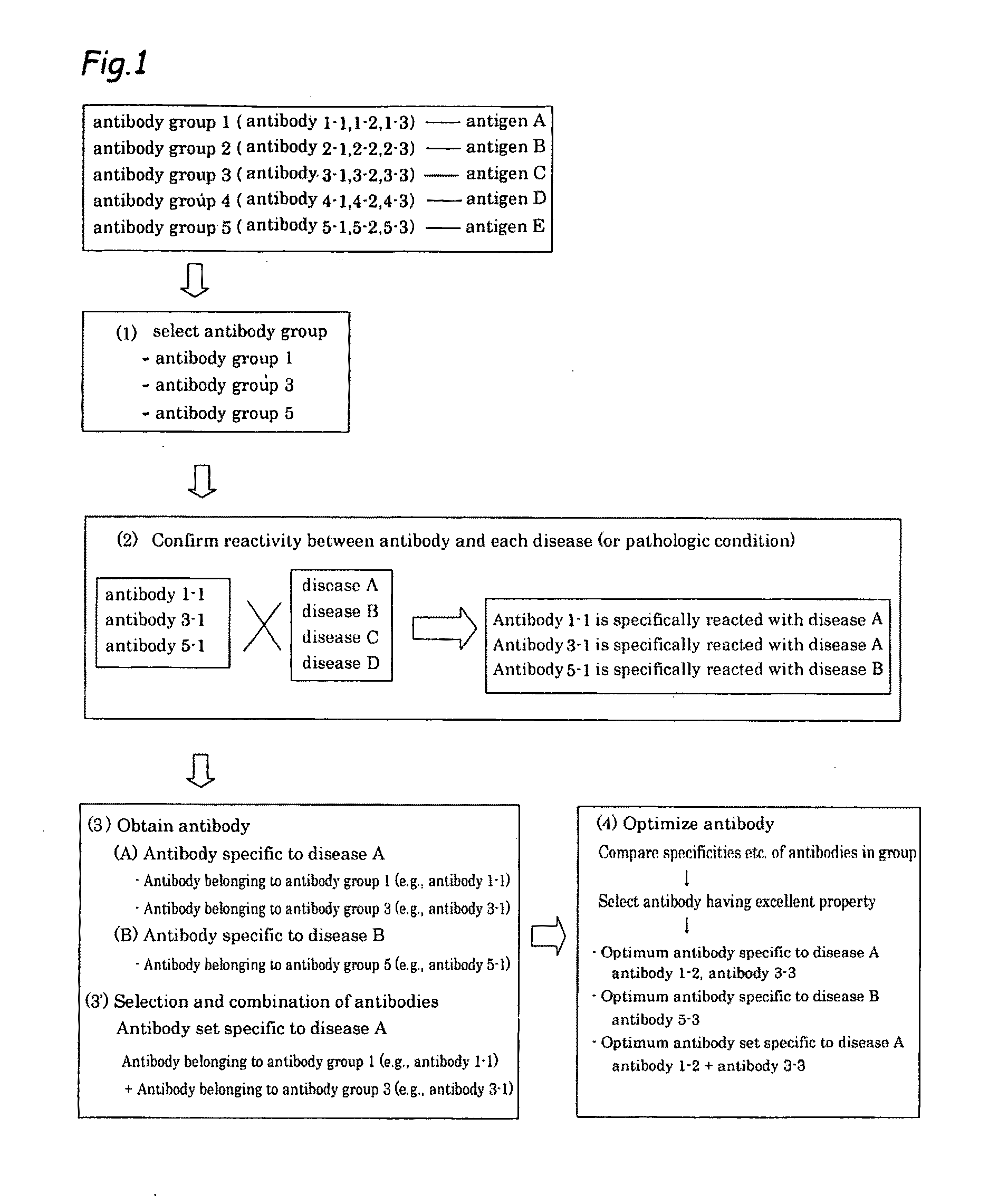
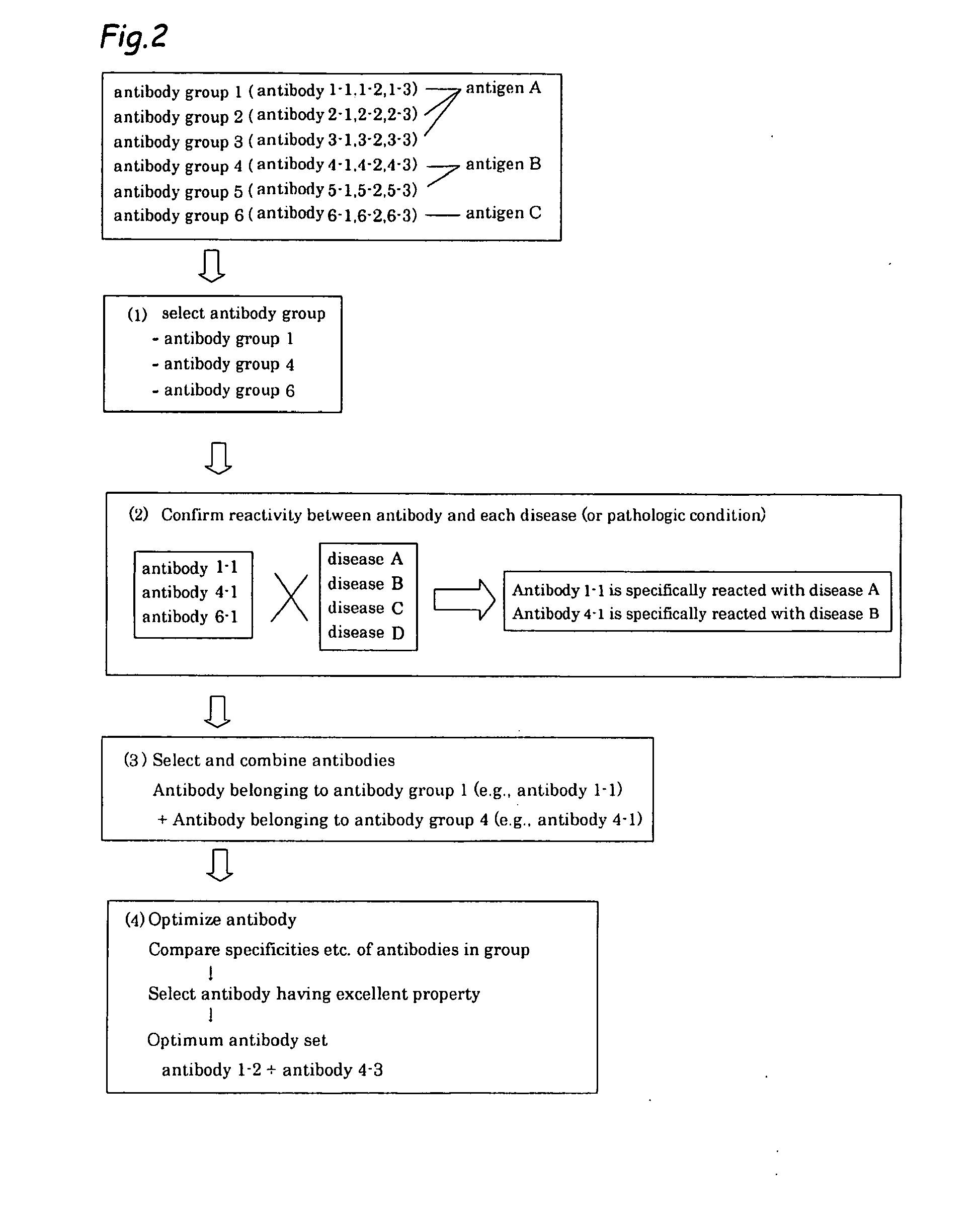
Method of classifying antibody, method of identifying antigen, method of obtaining antibody or antibody set, method of constructing antibody panel and antibody or antibody set and use of the same
InactiveUS20090203538A1Labor moreMany timesPeptide librariesLibrary screeningAntigenCell Surface Antigens
It is intended to provide a method whereby a plural number of antibodies against cell surface antigens are quickly classified and to provide a method whereby antigens of the thus classified antibodies are quickly identified. Further, it is intended to provide a method of promoting the utilization of the useful data obtained by the above methods. Furthermore, it is intended to provide an antibody which is effective in treating or diagnosing cancer. Namely, a method of classifying antibodies which comprises: (1) the step of preparing a plural number of antibodies respectively recognizing cell surface antigens; (2) the step of bringing each of these antibodies into contact with a cell of the same species; (3) the step of analyzing each of the cells having been treated in the step (2) by flow cytometry and thus obtaining data indicating the reactivity of each antibody with its cell surface antigen; and (4) the step of comparing the thus obtained data and classifying the individual antibodies depending on the similarity. A method of identifying antigens which further comprises: (5) the step of selecting one to several antibodies from each antibody group formed in the step (4) and identifying antigens thereof; and (6) on the assumption that antigens of the antibodies belonging to a single antibody group are the same or highly related to one another, making relations between the antigens having been identified in the step (5) and the antibody groups to thereby identify the antigens. An antibody against HER1, an antibody against HER2, an antibody against CD46, an antibody against ITGA3, an antibody against ICAM1, an antibody against ALCAM, an antibody against CD147, an antibody against C1qR, an antibody against CD44, an antibody against CD73, an antibody against EpCAM and an antibody against HGFR, each obtained by using the above methods.
Owner:FUJITA HEALTH UNIVERSITY
Antibodies immunoreactive with mutant 5-enolpyruvlshikimate-3-phosphate synthase
Antibodies immunoreactive to double mutant EPSPS are provided, and in an embodiment the double mutant EPSPS is one in which the wild-type EPSPS is substituted at residue 102 with isoleucine and at residue 106 with serine. Also provided are hybridomas producing the antibodies, as well as methods of making and using the antibodies.
Owner:M S TECH
Antibodies Reactive with B7-H3, Immunologically Active Fragments Thereof and Uses Thereof
ActiveUS20130149236A1Increased activationAnimal cellsIn-vivo radioactive preparationsHuman cancerCancer cell
The present invention relates to antibodies and their fragments that are immunoreactive to the mammalian, and more particularly, the human B7-H3 receptor and to uses thereof, particularly in the treatment of cancer and inflammation. The invention thus particularly concerns humanized B7-H3-reactive antibodies and their immunoreactive fragments that are capable of mediating, and more preferably enhancing the activation of the immune system against cancer cells that are associated with a variety of human cancers.
Owner:MACROGENICS INC
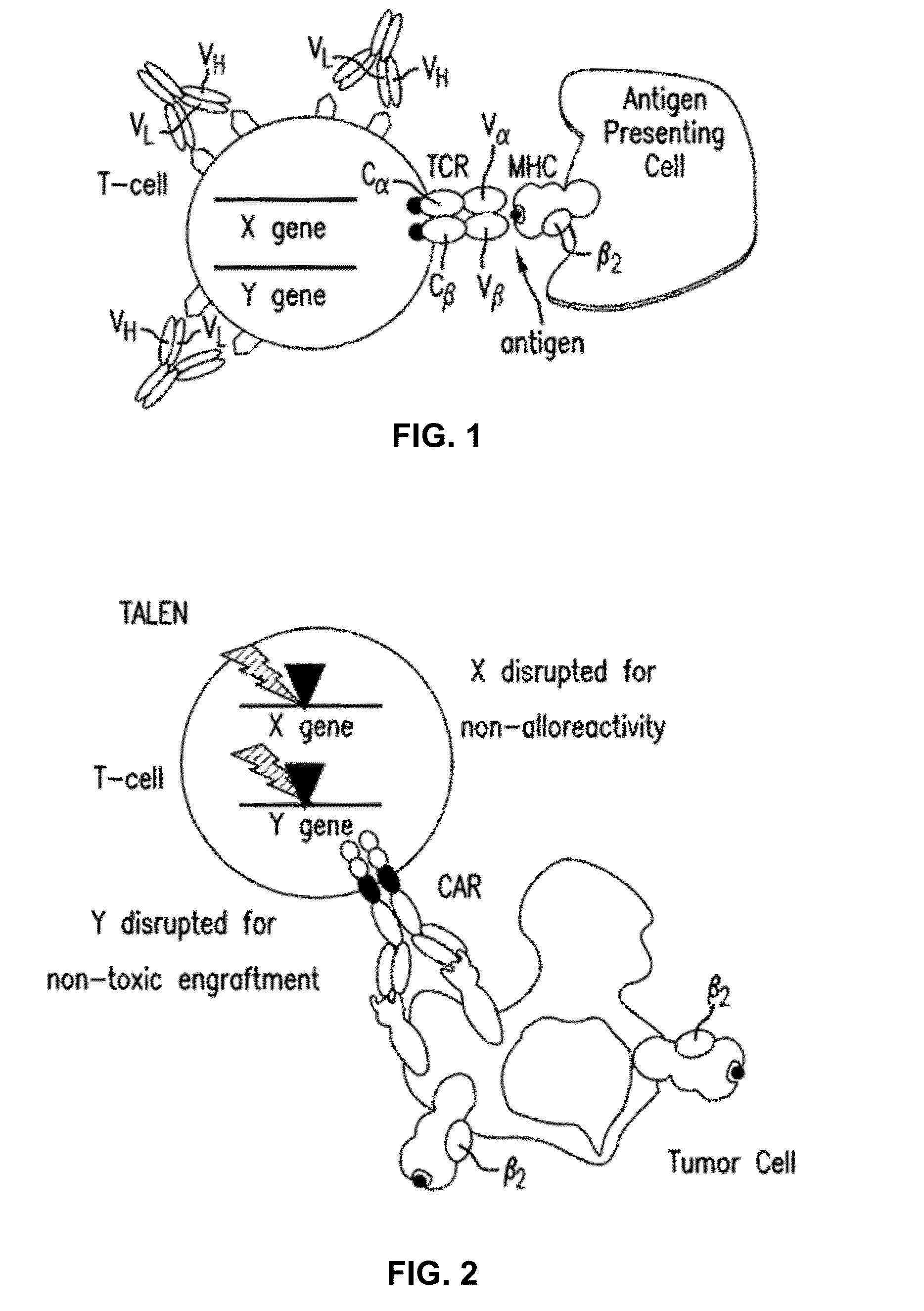
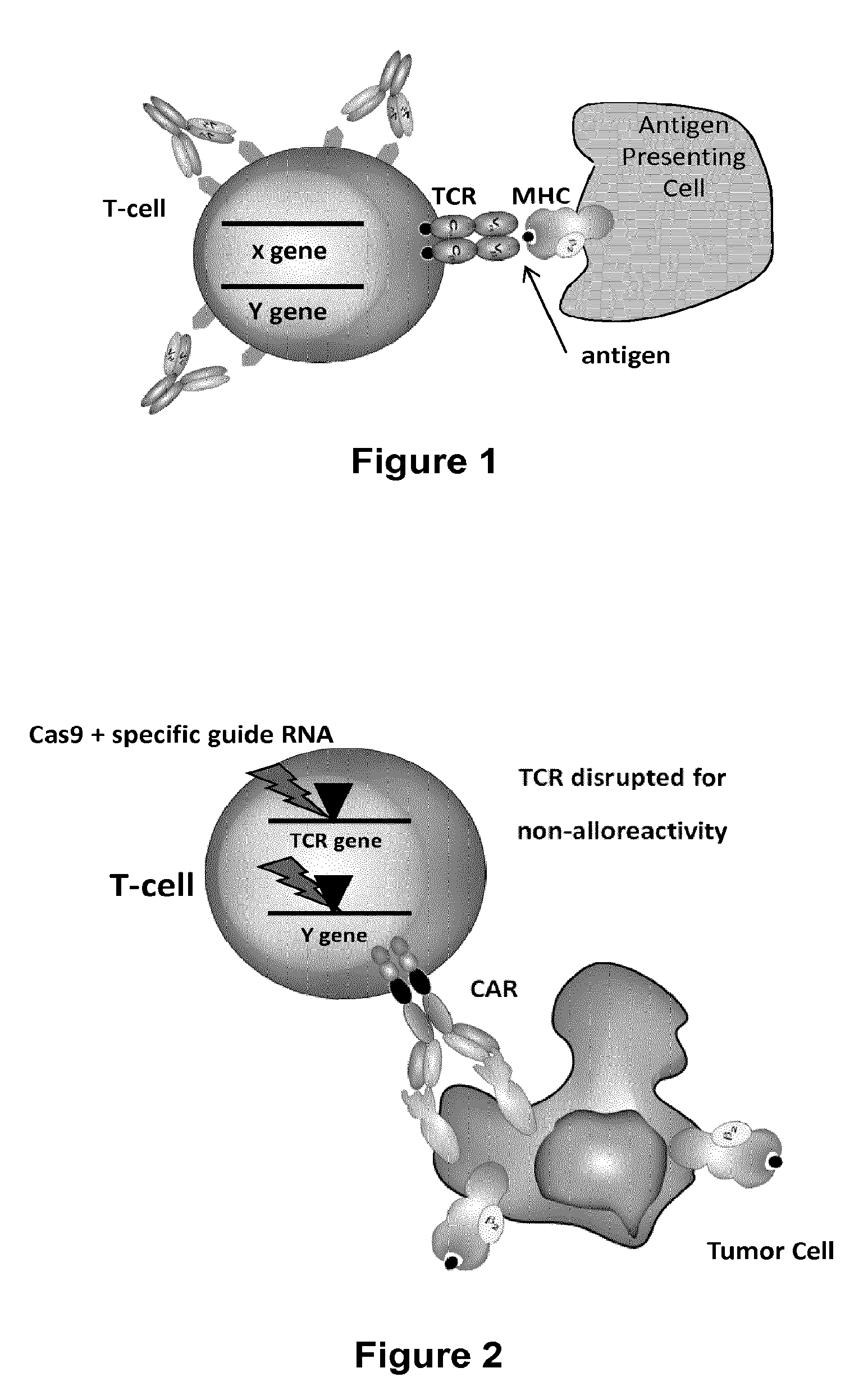
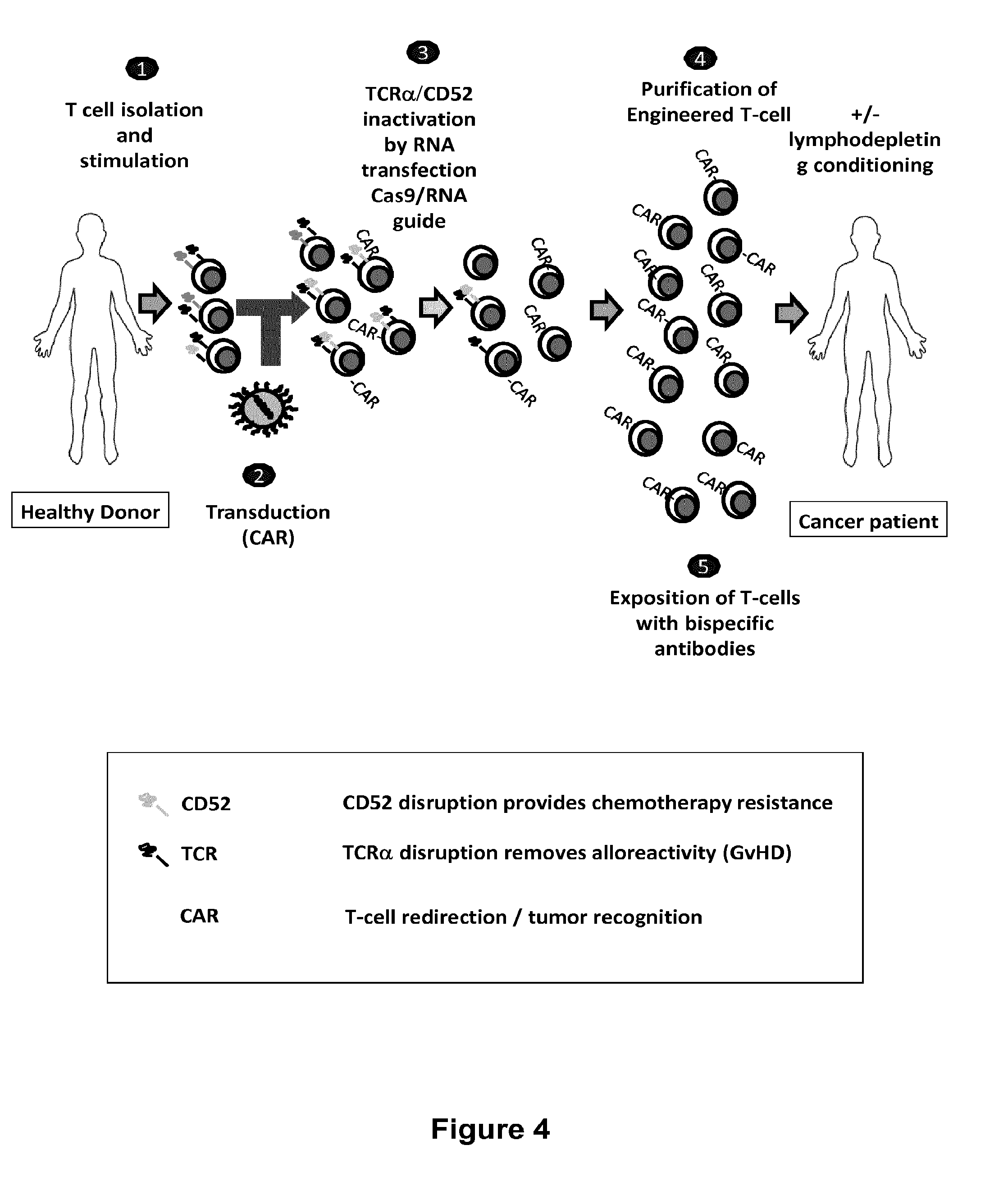
Methods for engineering t cells for immunotherapy by using rna-guided cas nuclease system
ActiveUS20160184362A1Accurate modificationHydrolasesGenetically modified cellsInfected cellAntigen receptors
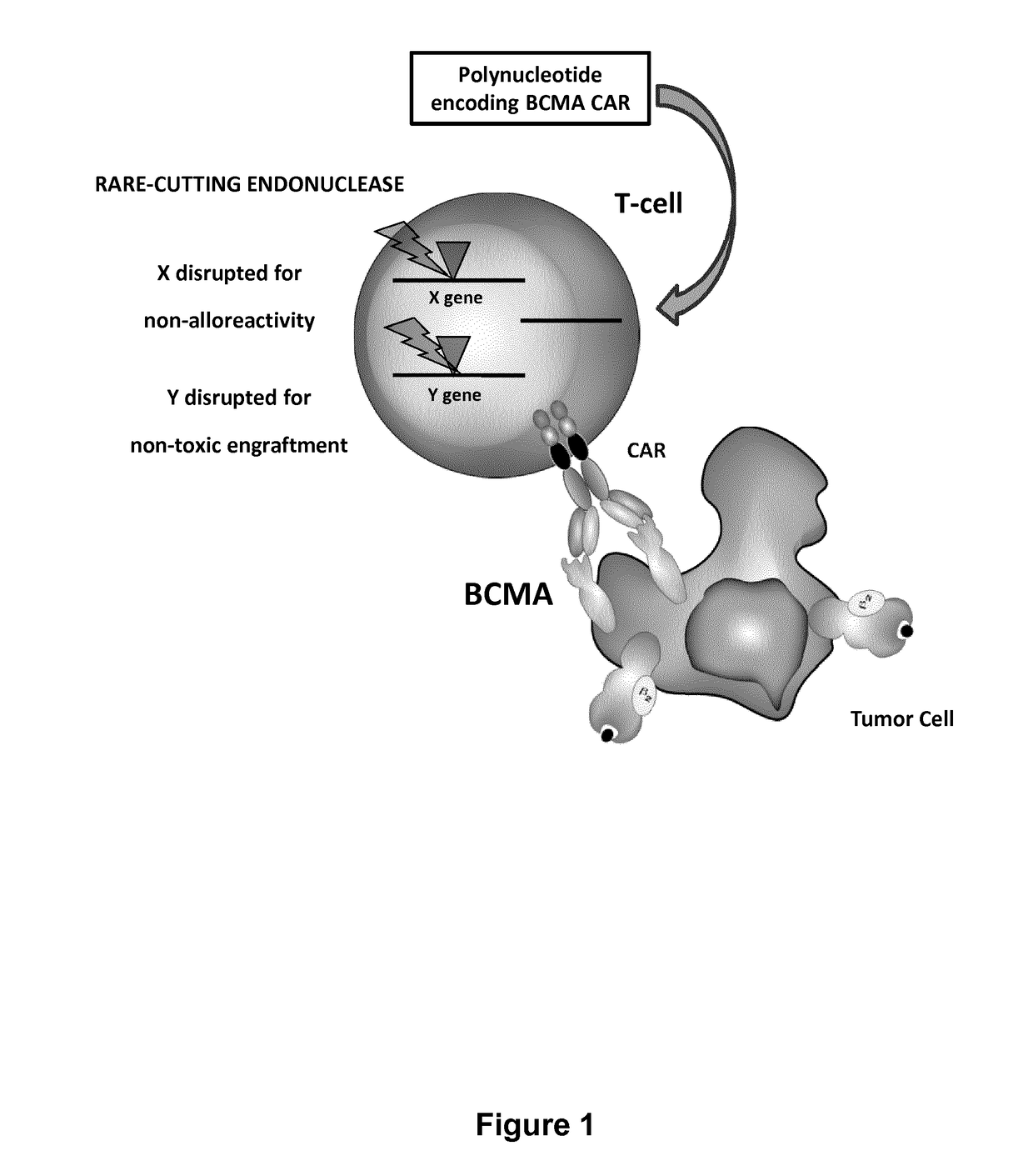
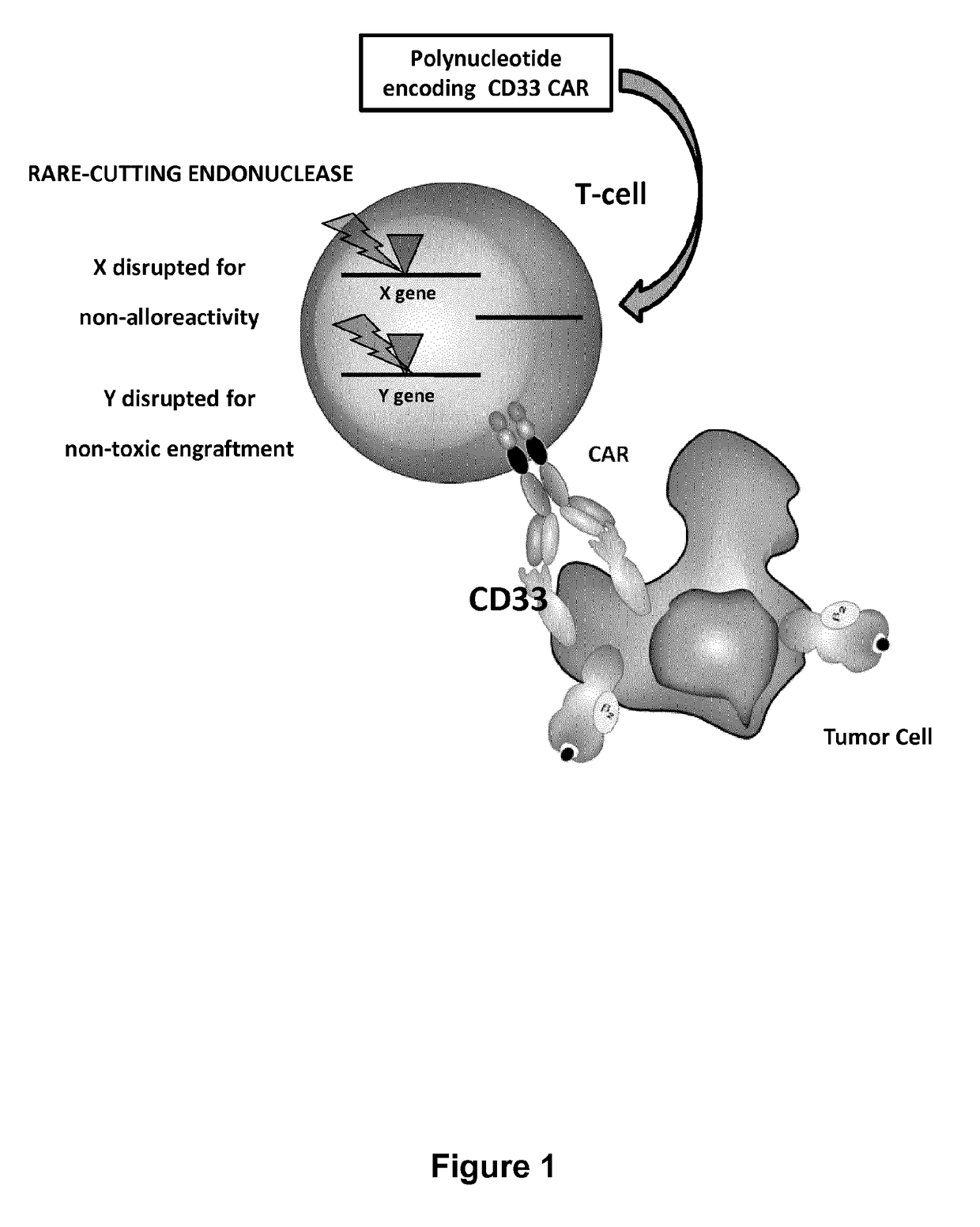
The present invention relates to methods of developing genetically engineered, preferably non-alloreactive T-cells for immunotherapy. This method involves the use of RNA-guided endonucleases, in particular Cas9 / CRISPR system, to specifically target a selection of key genes in T-cells. The engineered T-cells are also intended to express chimeric antigen receptors (CAR) to redirect their immune activity towards malignant or infected cells. The invention opens the way to standard and affordable adoptive immunotherapy strategies using T-Cells for treating cancer and viral infections.
Owner:CELLECTIS SA
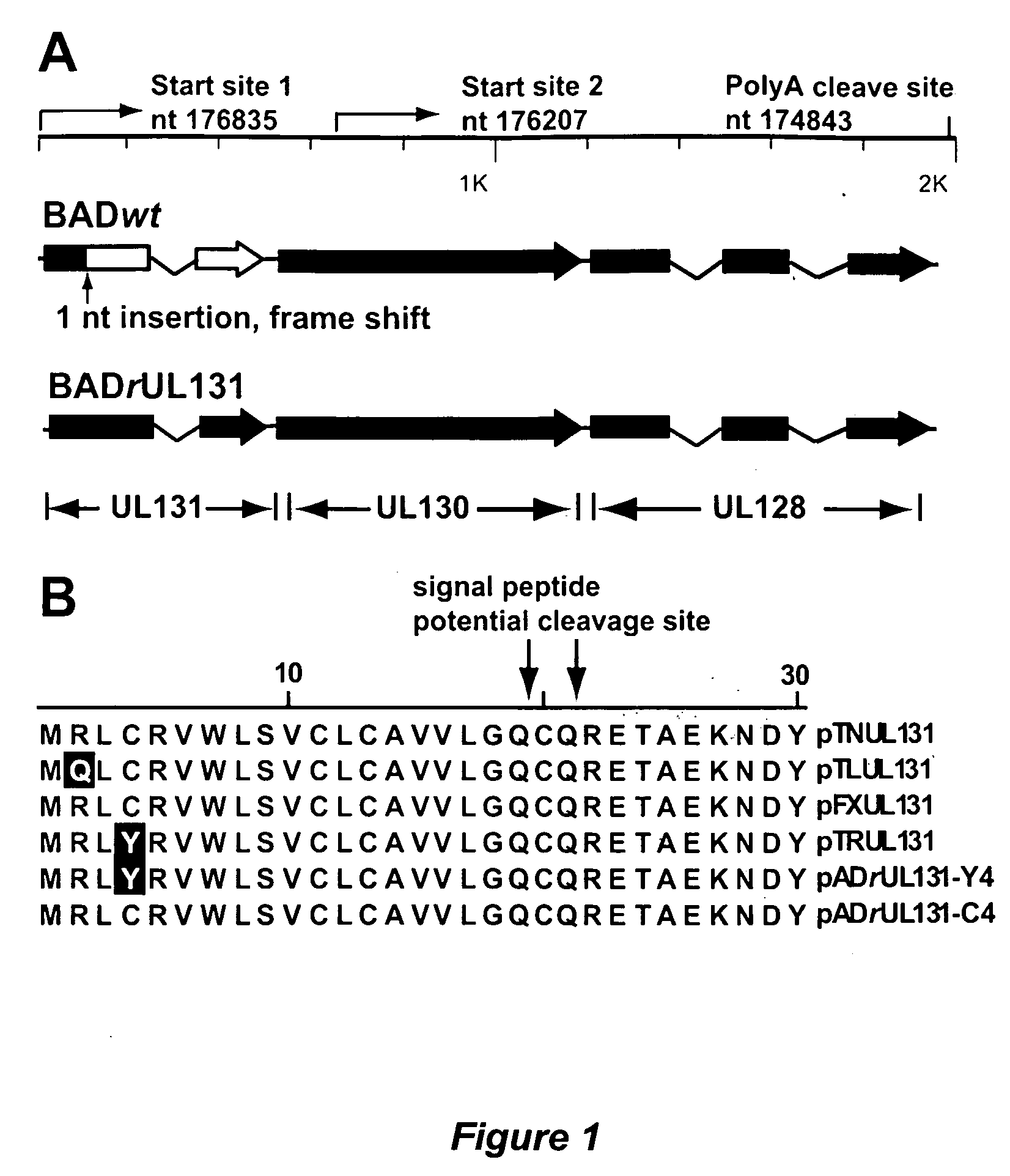
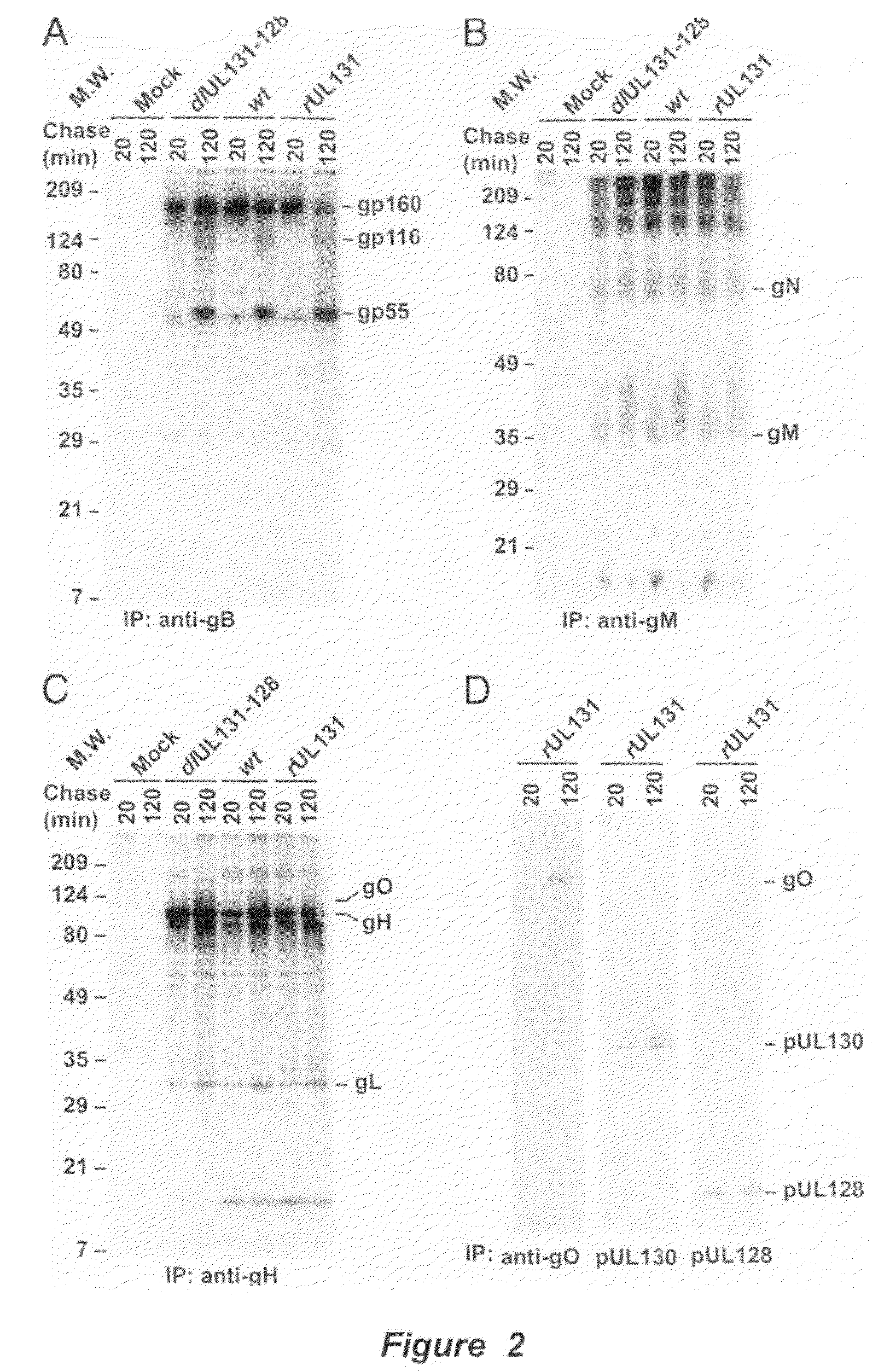
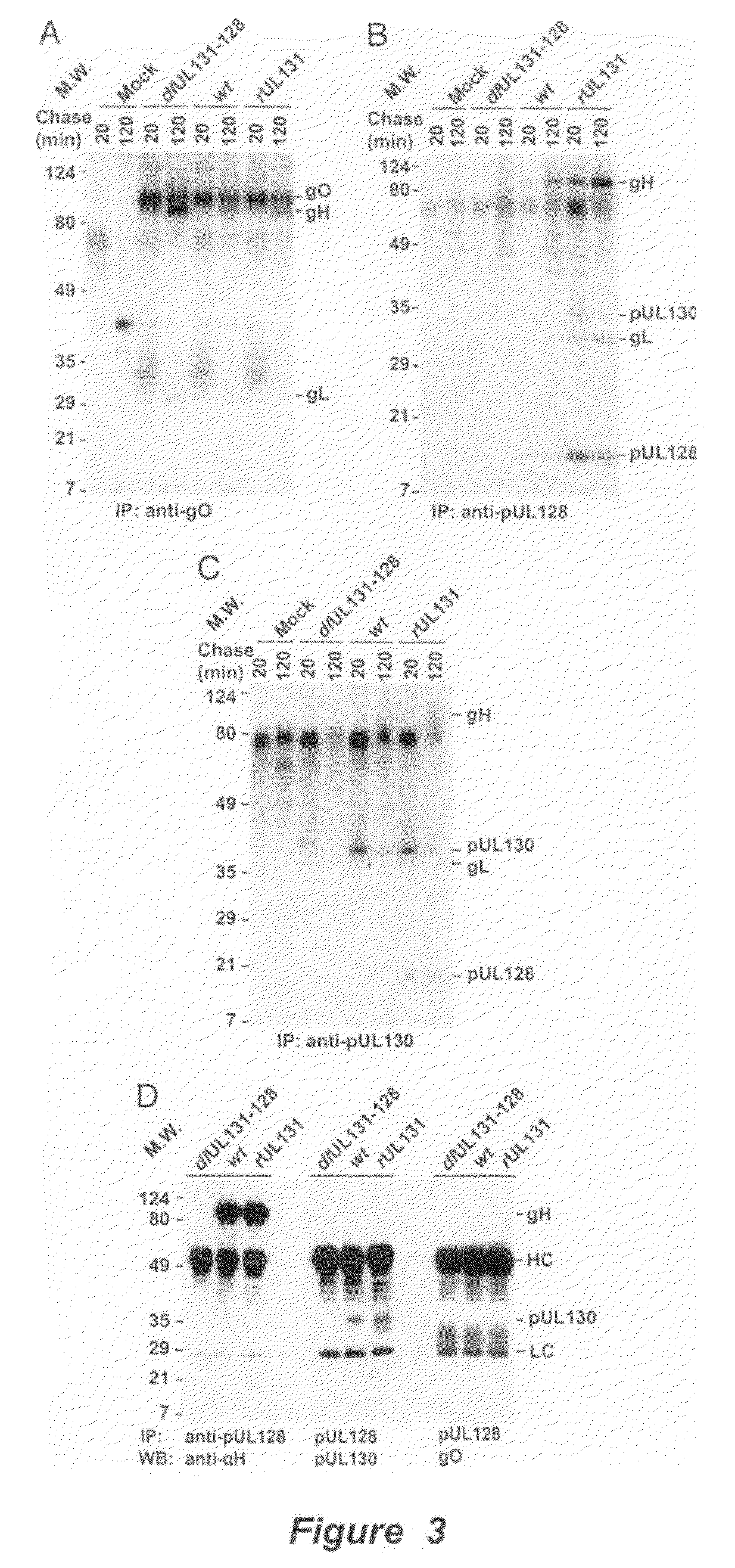
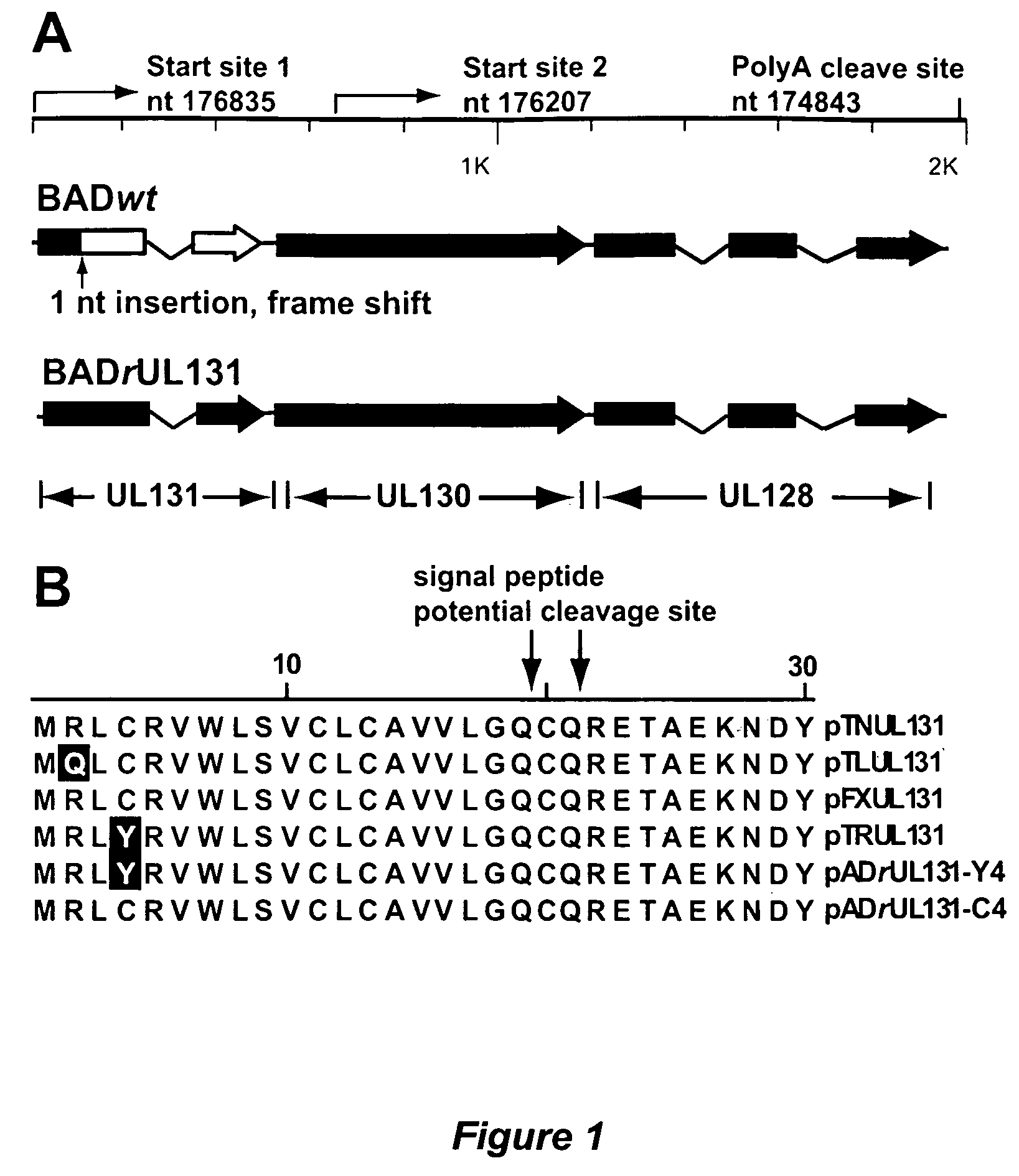
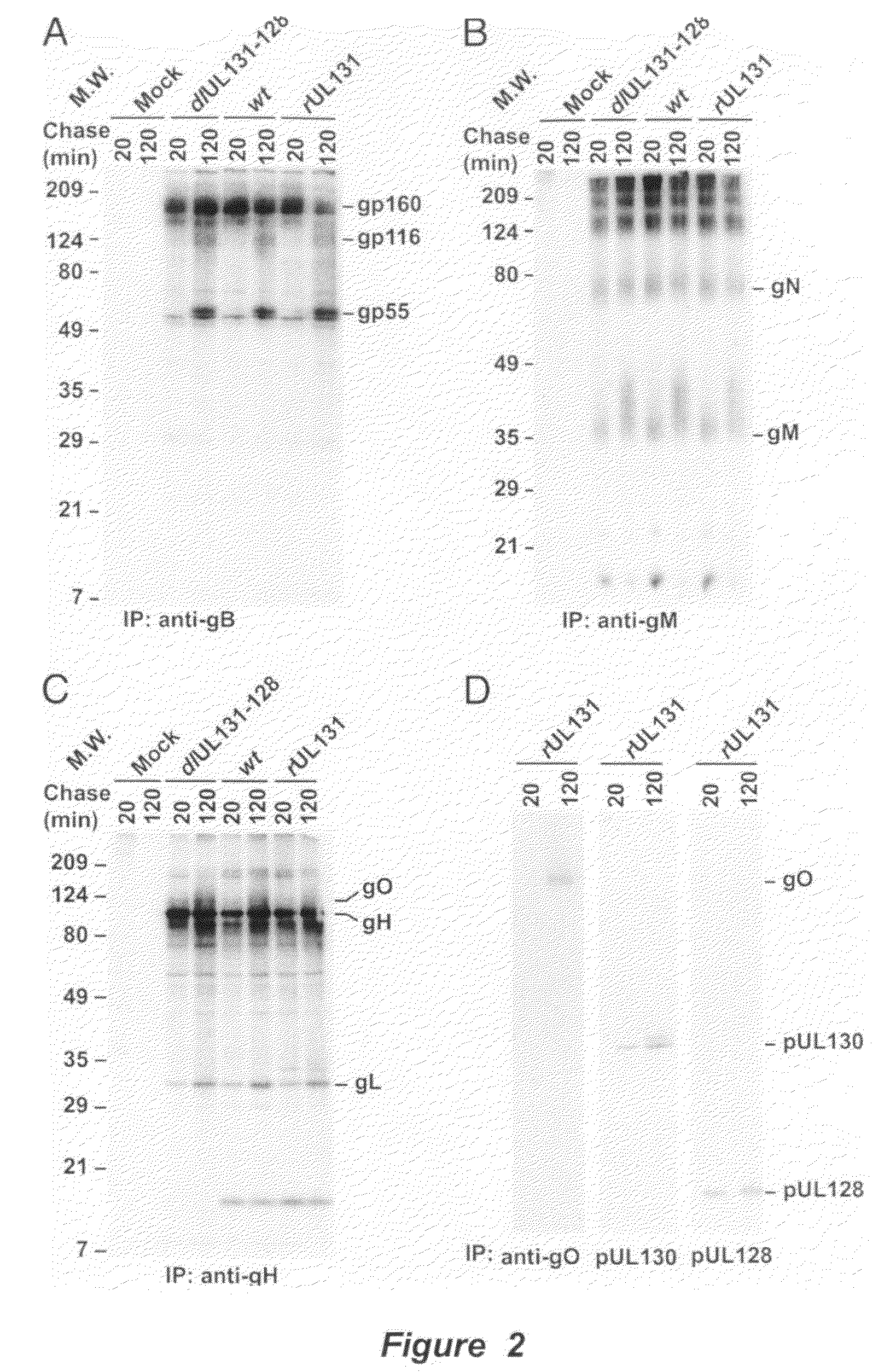
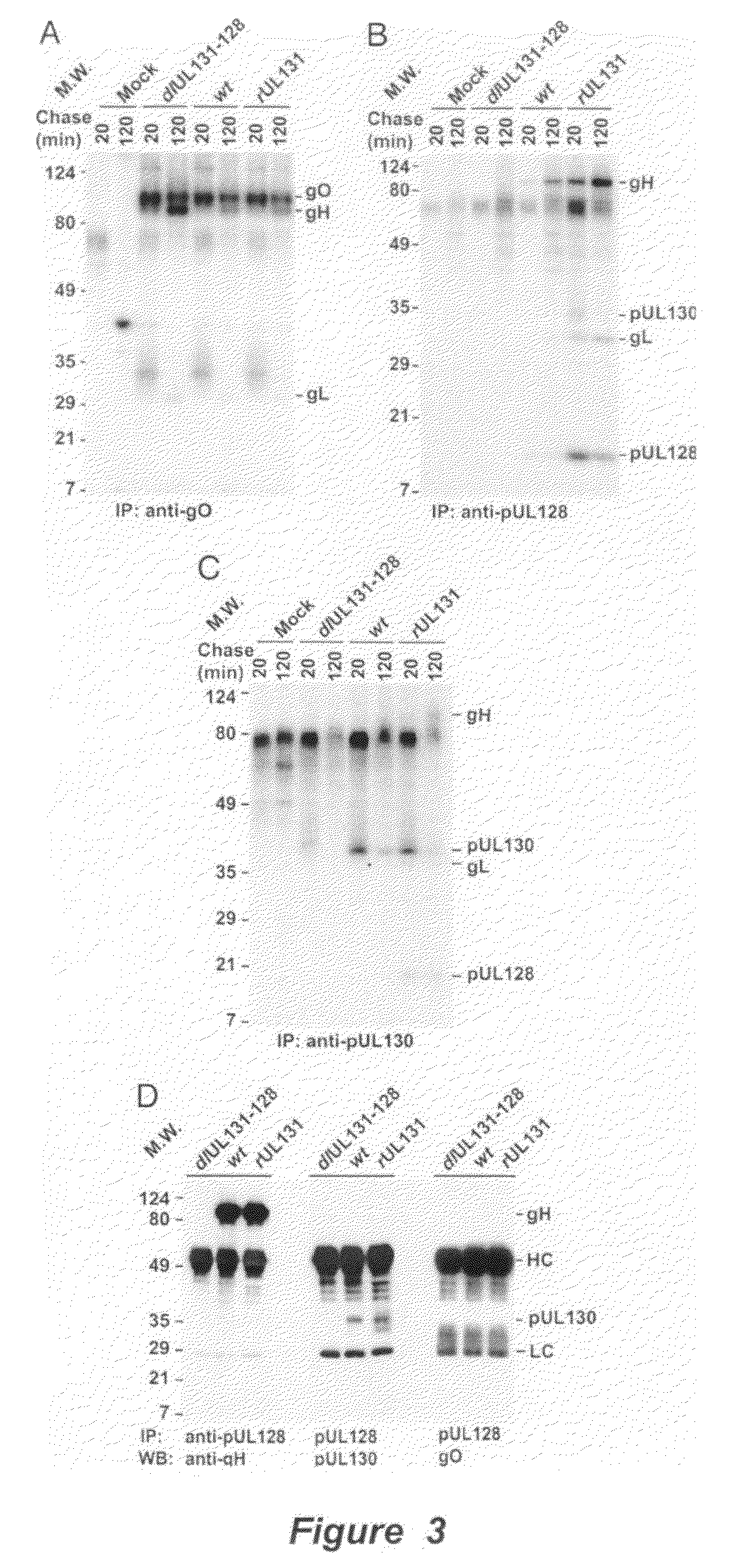
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Immunogenic compositions and prophylactic or therapeutic vaccines for use in protecting and treating against human cytomegalovirus (CMV) are disclosed. Subunit vaccines comprising a human CMV protein complex comprising pUL128 or pUL130, and nucleic acid vaccines comprising at least one nucleic acid encoding a CMV protein complex comprising pUL128 or pUL130 are described. Also disclosed are therapeutic antibodies reactive against a CMV protein complex comprising pUL128 or pUL130, as well as methods for screening compounds that inhibit CMV infection of epithelial and endothelial cells, methods for immunizing a subject against CMV infection, methods for determining the capability of neutralizing antibodies to inhibit human CMV infection of cell types other than fibroblasts, and methods of diminishing an CMV infection.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176258A1Improve efficiencyIncrease resourcesBiological material analysisDepsipeptidesImmunodominant EpitopesMonoclonal antibody
Disclosed herein among other MN / CA IX-related inventions are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Subsets of the new antibodies are to either the proteoglycan-like (PG) domain or to the carbonic anhydrase (CA) domain of MN / CA IX, and methods are provided by which antibodies can be prepared to the other MN / CA IX domains. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
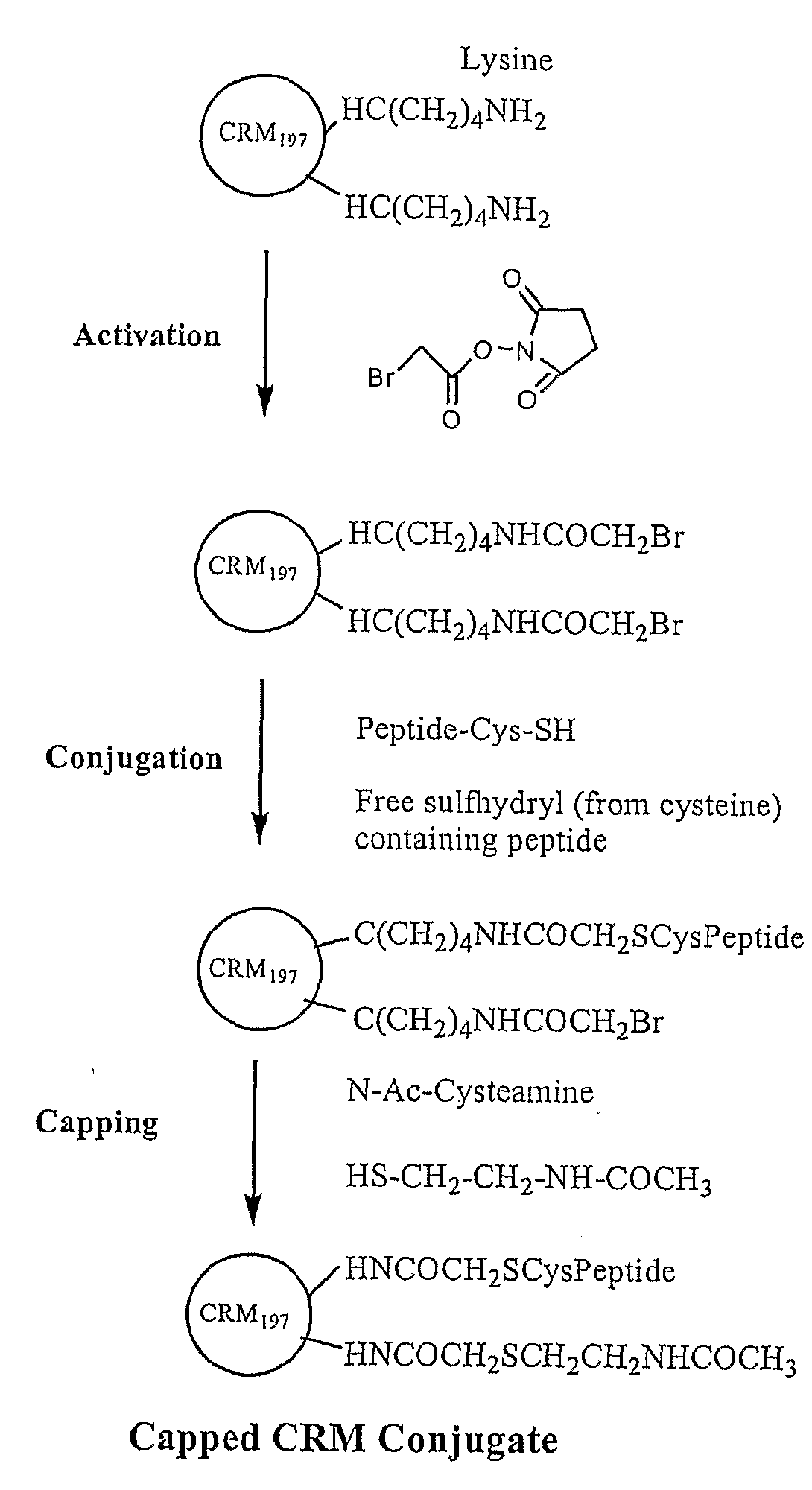
A-beta immunogenic peptide carrier conjugates and methods of producing same
InactiveUS20080145373A1Nervous disorderNervous system antigen ingredientsImmunogenicityImmunogenic peptide
Owner:JANSSEN SCI IRELAND UC +1
Modulation of NKT Cell Activity with Antigen-Loaded CD1d Molecules
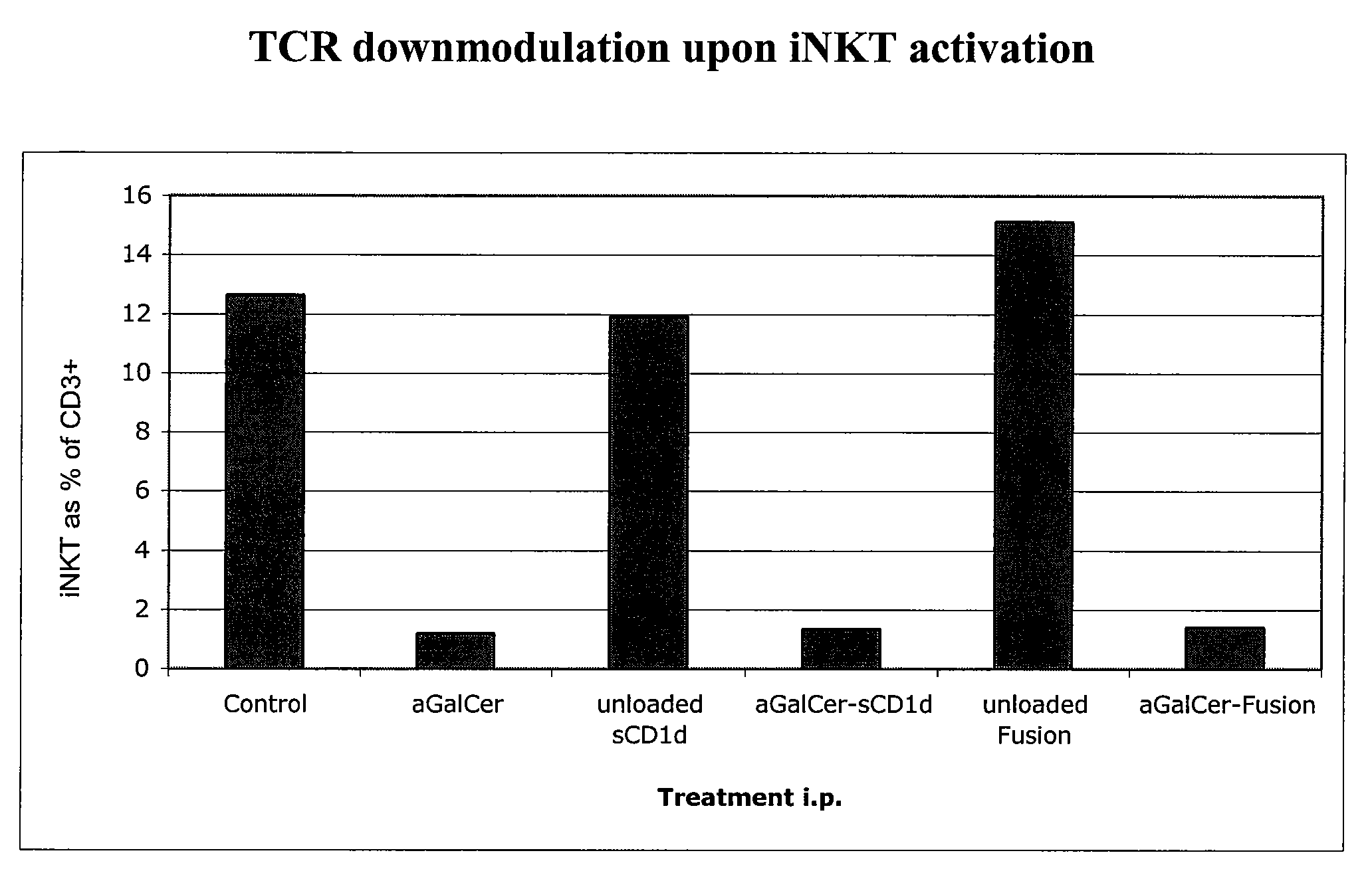
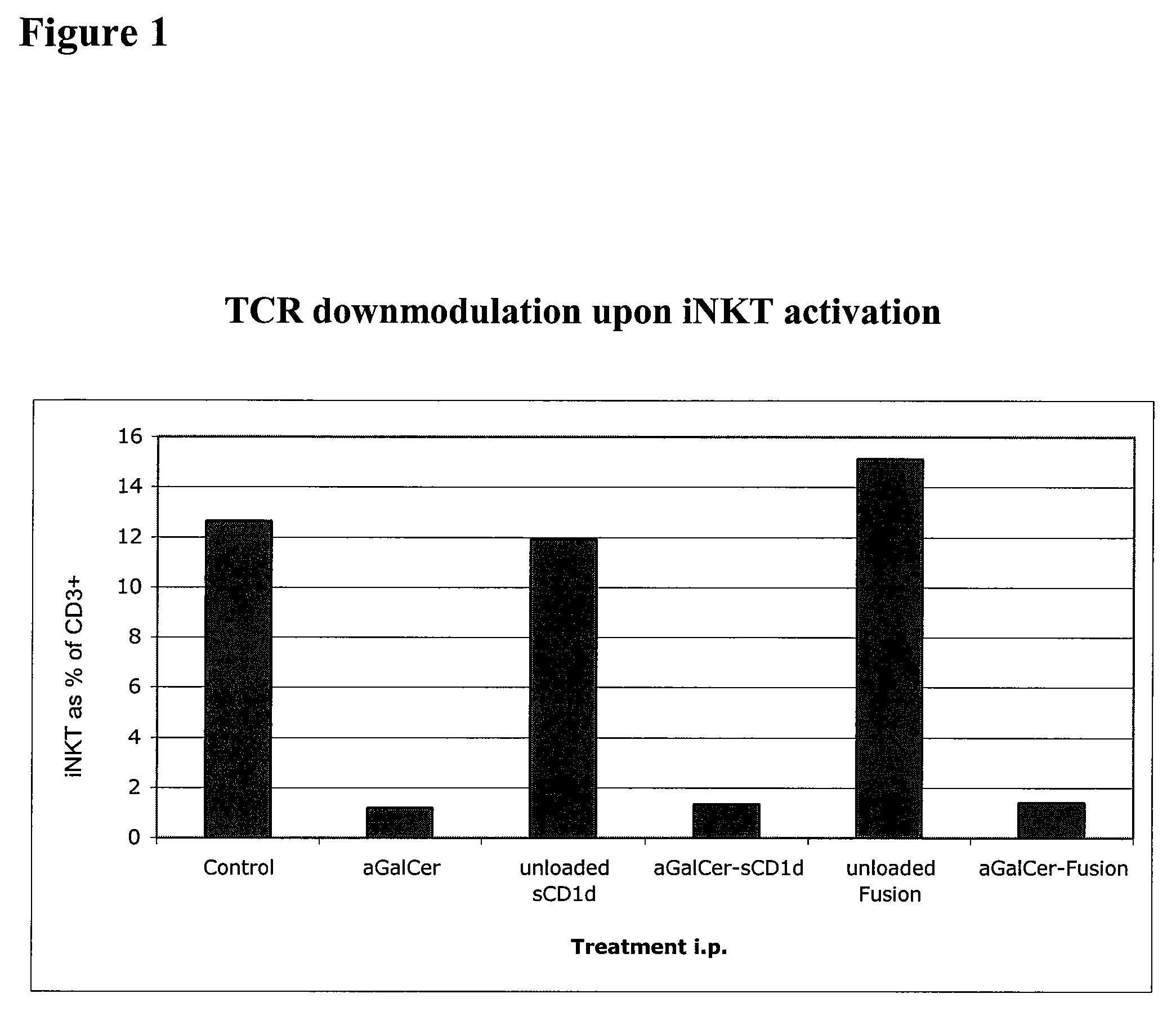
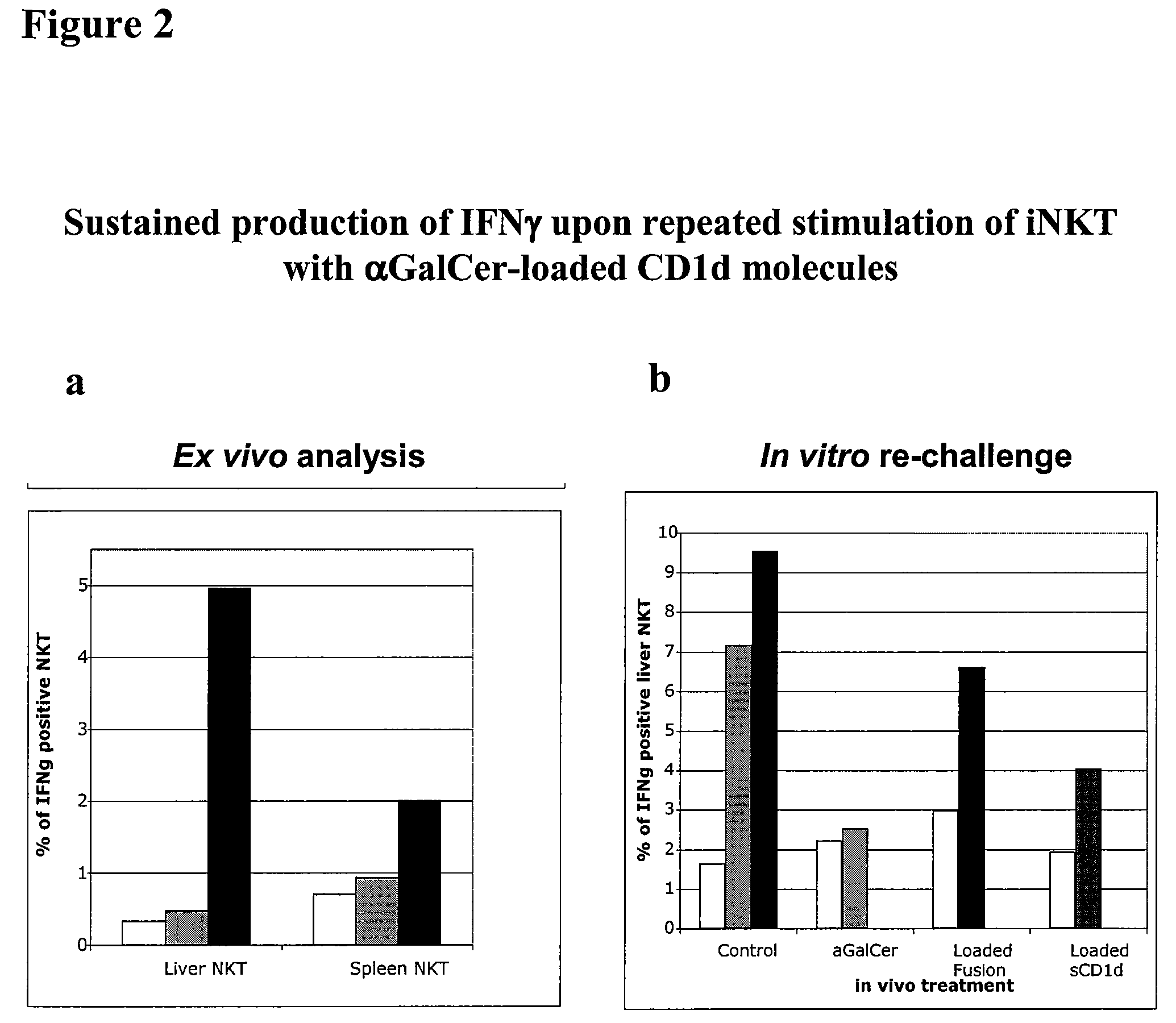
The invention is directed to methods of modulating an immune response in an animal, comprising administering a composition comprising one or more soluble CD1d complexes, in particular non-specific soluble CD1d complexes. Soluble CD1d complexes comprise a soluble CD1d polypeptide, a β2-microglobulin polypeptide, and a ceramide-like glycolipid antigen bound to the CD1d antigen binding groove, and in certain embodiments, an immunogen. The administration of compositions of the present invention affects the activity of CD1d-restricted NKT cells, and in particular, allows for multiple administrations without causing CD1d-restricted NKT cell anergy.
Owner:VACCINEX
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176310A1Improve efficiencyIncrease resourcesOxidoreductasesFermentationKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with Her-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080177046A1Good curative effectImprove efficiencyImmunoglobulins against animals/humansBiological material analysisKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The Predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Immunogenic peptide carrier conjugates and methods of producing same
InactiveUS20070134762A1Peptide/protein ingredientsMicrobiological testing/measurementImmunogenicityImmunogenic peptide
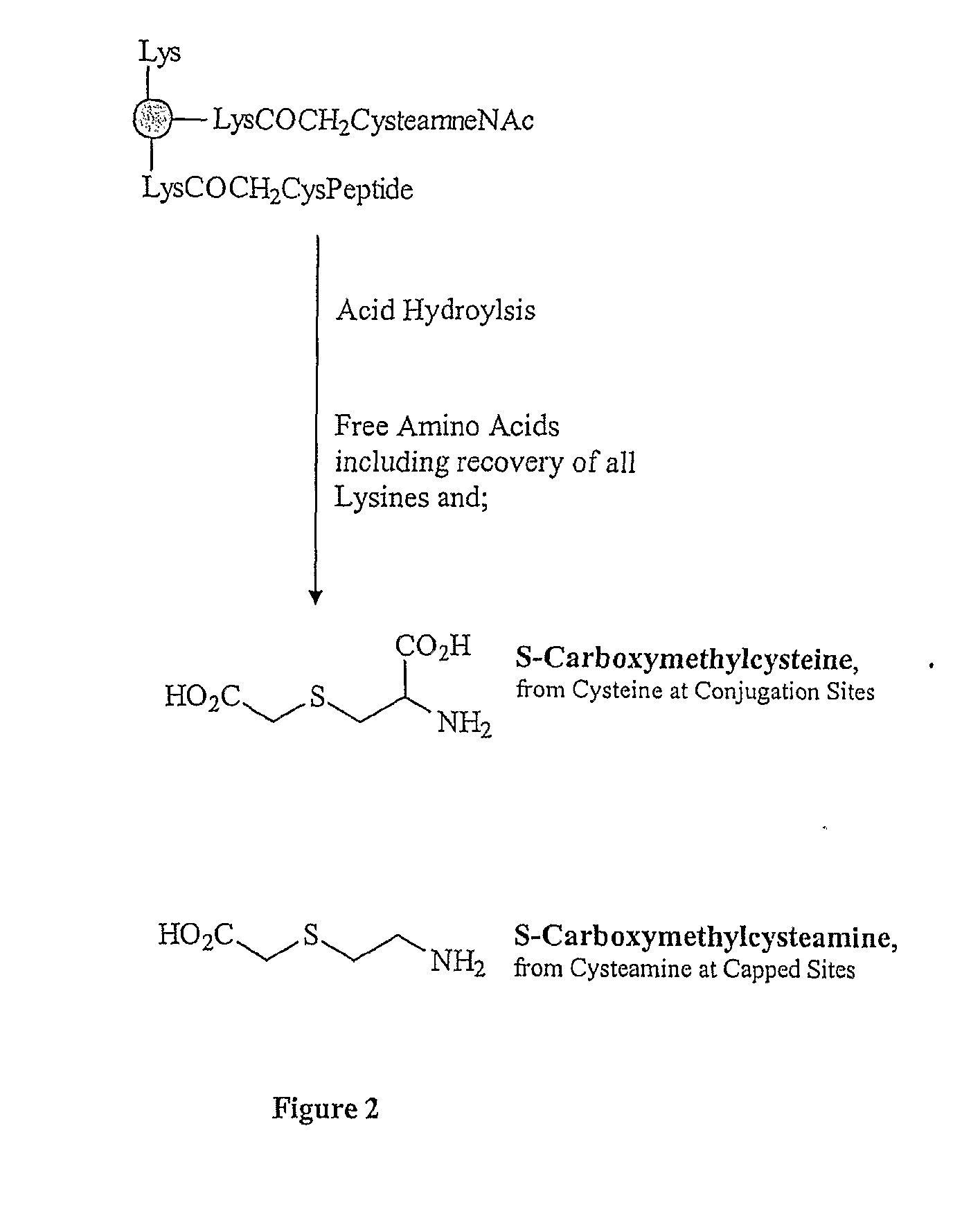
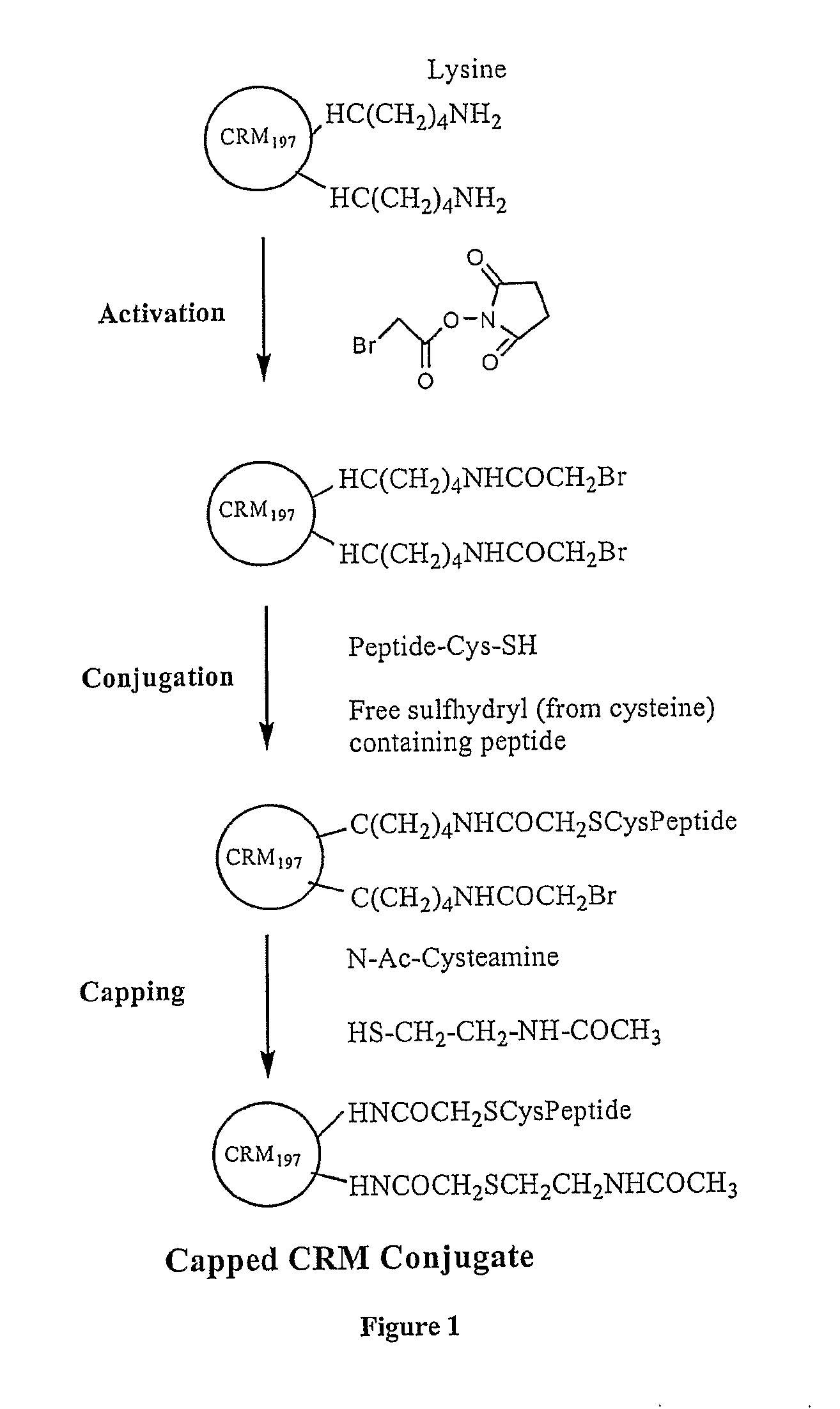
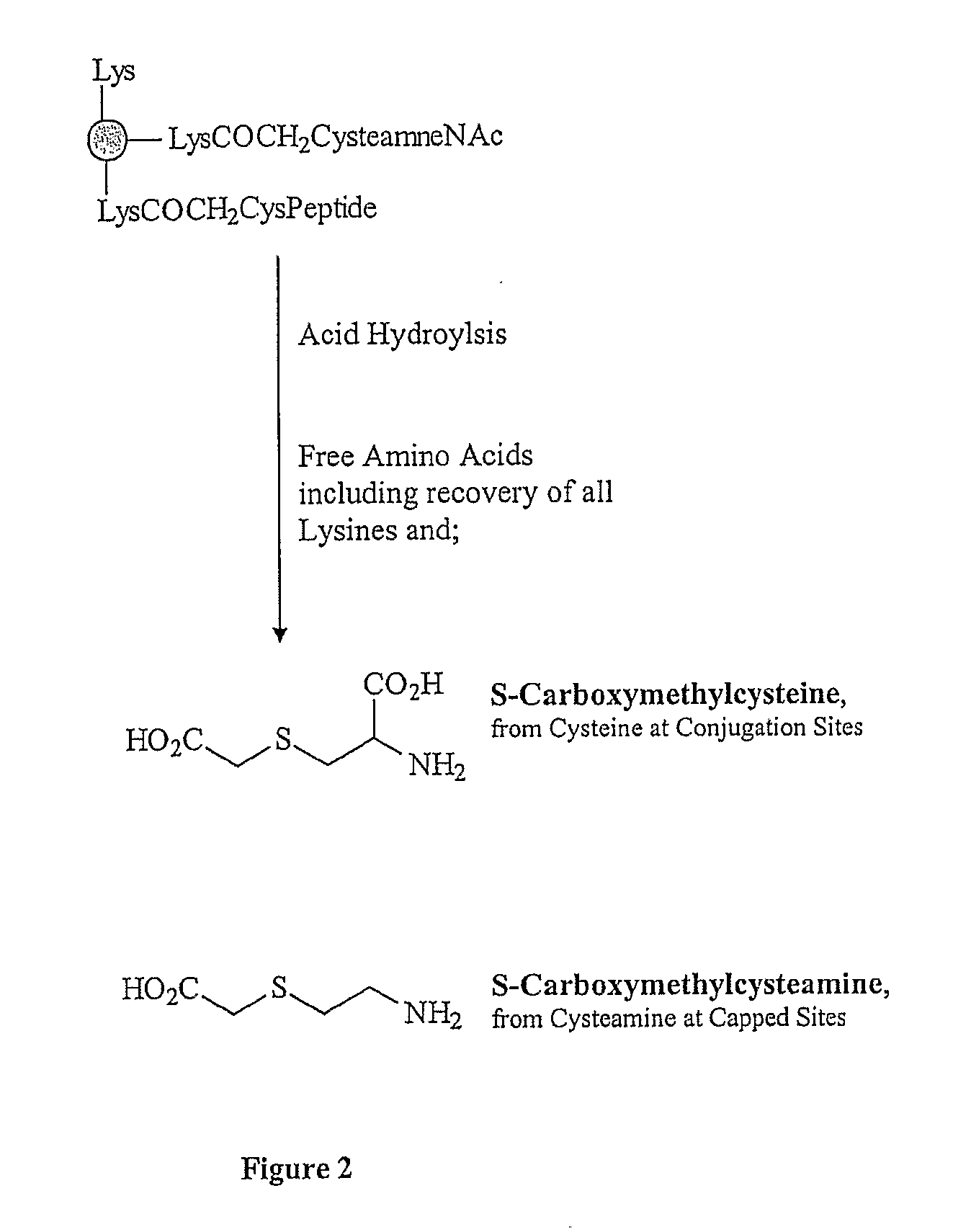
The present invention is directed to methods of producing conjugates of peptide immunogens with protein / polypeptide carrier molecules, which are useful as immunogens, wherein peptide immunogens are conjugated to protein carriers via activated functional groups on amino acid residues of the carrier or of the optionally attached linker molecule, and wherein any unconjugated reactive functional groups on amino acid residues are inactivated via capping, thus retaining the immunological functionality of the carrier molecule, but reducing the propensity for undesirable reactions that could render the conjugate less safe or effective. Furthermore, the invention also relates to such immunogenic products and immunogenic compositions containing such immunogenic products made by such methods.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
Bcma (CD269) specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170183418A1Useful for immunotherapyHigh selectivityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigenSpecific immunity
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a BCMA monoclonal antibody, conferring specific immunity against BCMA positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas, multiple myeloma and leukemia.
Owner:CELLECTIS SA
Polypeptides and polynucleotides for enhancing immune reactivity to HER-2 protein
Compositions for stimulating the immune system and for treating malignancies associated with overexpression of the HER-2 protein are provided. Such compositions include immunogenic epitopes of the HER-2 proteins and chimeric and multivalent peptides which comprise such epitopes. The present invention also relates to polynucleotides which encode the chimeric peptides. Also provided are pharmaceutical compositions comprising such immunogenic compositions. Methods for stimulating an immune response to HER-2 protein are provided. Methods for treating breast cancer, ovarian cancer, prostate cancer, colon cancer and lung cancer are provided.
Owner:OHIO STATE INNOVATION FOUND
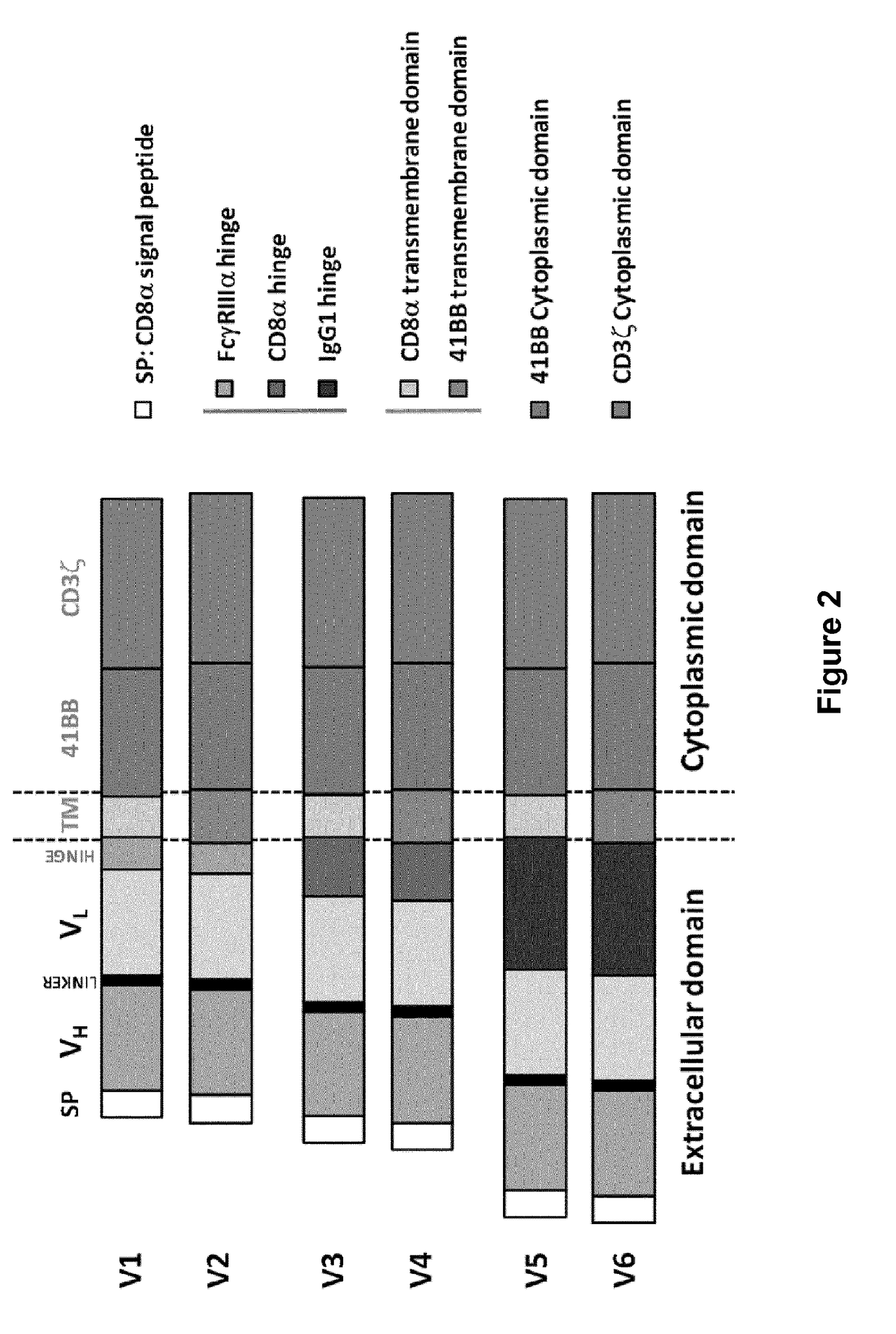
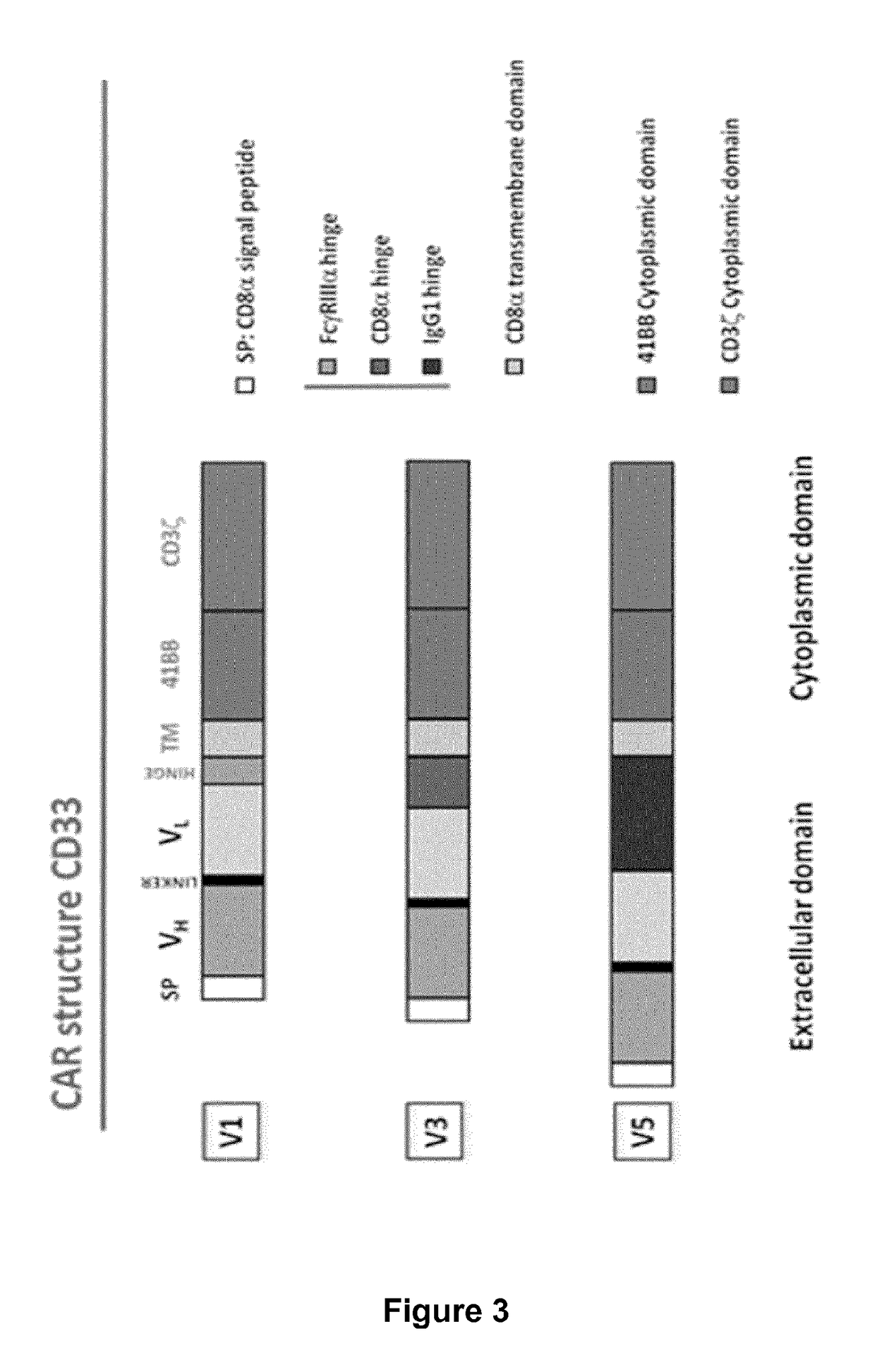
Cd33 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170145094A1Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD33
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD33 monoclonal antibody, conferring specific immunity against CD33 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
Landscaped antibodies and antibody fragments for clincal use
InactiveUS20020193572A1Simple and efficientIncrease productionPeptide/protein ingredientsAntibody mimetics/scaffoldsAntibody fragmentsAntigen binding
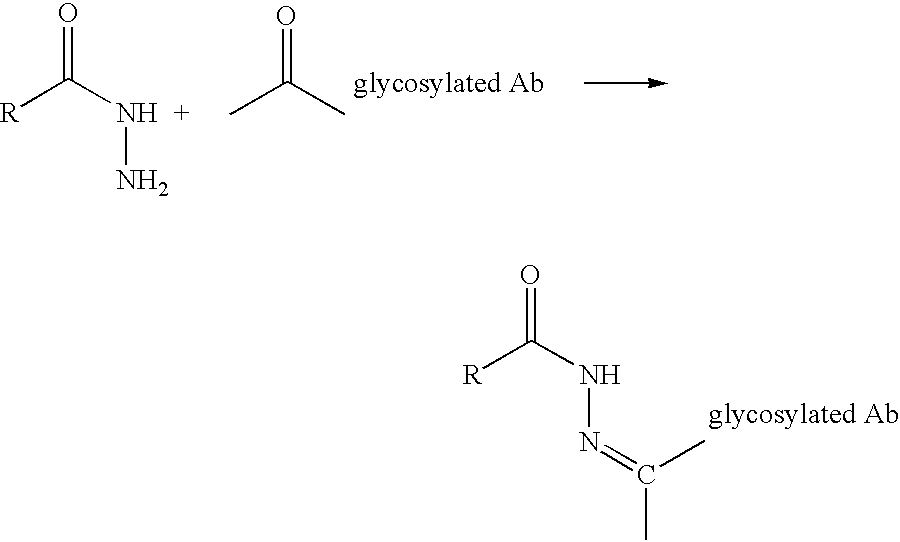
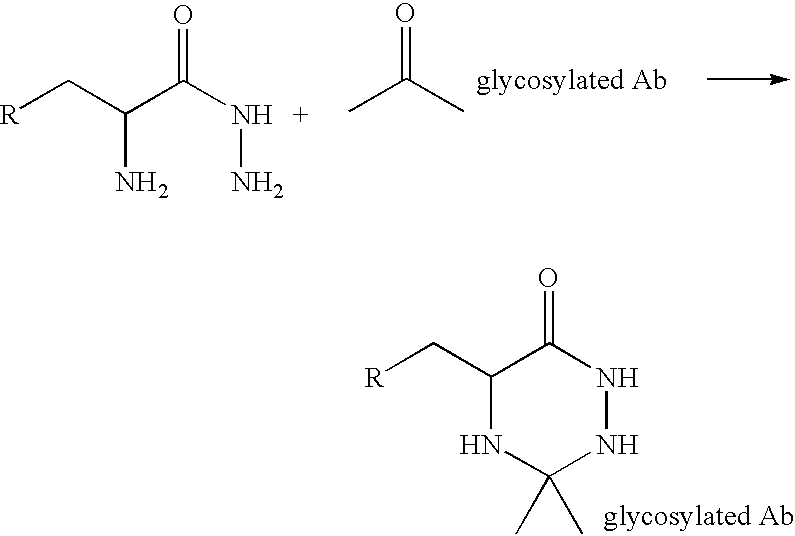
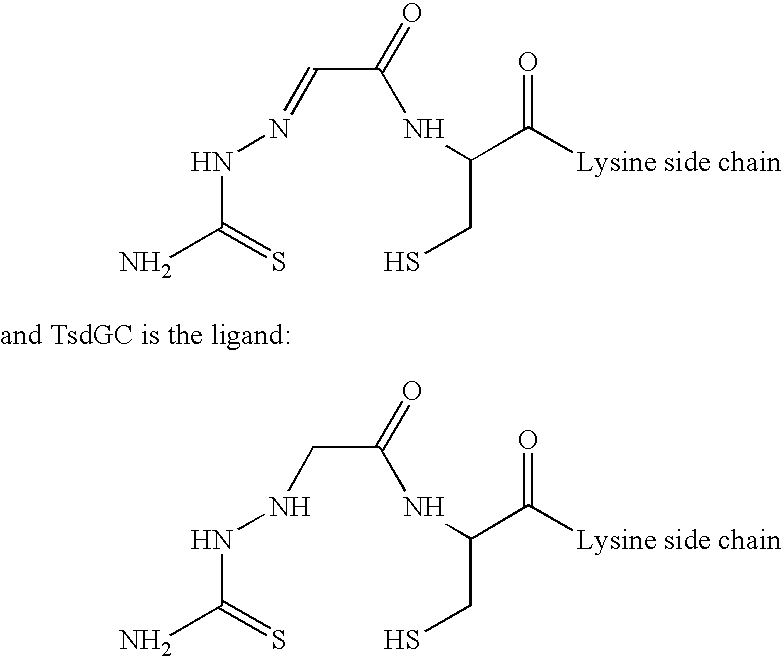
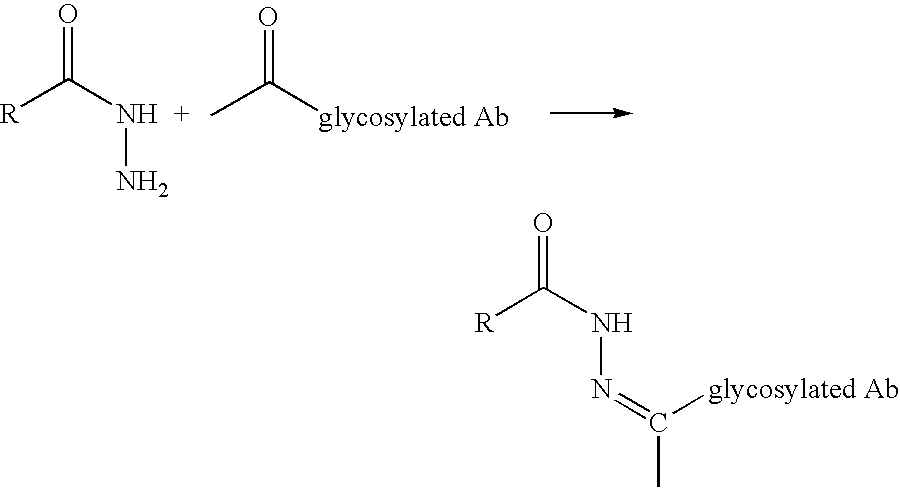
A method of making a glycosylated antibody or antibody fragment having a reactive ketone group on the glycosylated site is provided. The method comprises expressing a cell transfected with a vector encoding an antibody having one or more glycosylation sites in a culture medium comprising a ketone derivative of a saccharide or saccharide precursor and, in the case of an antibody fragment, fragmenting the resulting antibody into an antigen-binding antibody fragment. Methods of making immunoconjugates comprising the glycosylated antibodies or antibody fragments also are provided, wherein the antibody or antibody fragment is reacted with an agent comprising a ketone-reactive group. Glycosylated antibodies and antibody fragments having a reactive ketone group on the glycosylated site, immunoconjugates comprising such antibodies and antibody fragments and in vivo targeting methods using such antibodies, antibody fragments and immunoconjugates also are provided.
Owner:IMMUNOMEDICS INC
Landscaped antibodies and antibody fragments for clinical use
InactiveUS6953675B2Readily conjugatedAntibody mimetics/scaffoldsNanomedicineAntibody fragmentsKetone
A method of making a glycosylated antibody or antibody fragment having a reactive ketone group on the glycosylated site is provided. The method comprises expressing a cell transfected with a vector encoding an antibody having one or more glycosylation sites in a culture medium comprising a ketone derivative of a saccharide or saccharide precursor and, in the case of an antibody fragment, fragmenting the resulting antibody into an antigen-binding antibody fragment. Methods of making immunoconjugates comprising the glycosylated antibodies or antibody fragments also are provided, wherein the antibody or antibody fragment is reacted with an agent comprising a ketone-reactive group. Glycosylated antibodies and antibody fragments having a reactive ketone group on the glycosylated site, immunoconjugates comprising such antibodies and antibody fragments and in vivo targeting methods using such antibodies, antibody fragments and immunoconjugates also are provided.
Owner:IMMUNOMEDICS INC
Cysteine engineered antibodies and conjugates
Owner:GENENTECH INC
Cytomegalovirus surface protein complex for use in vaccines and as a drug target
Owner:THE TRUSTEES FOR PRINCETON UNIV
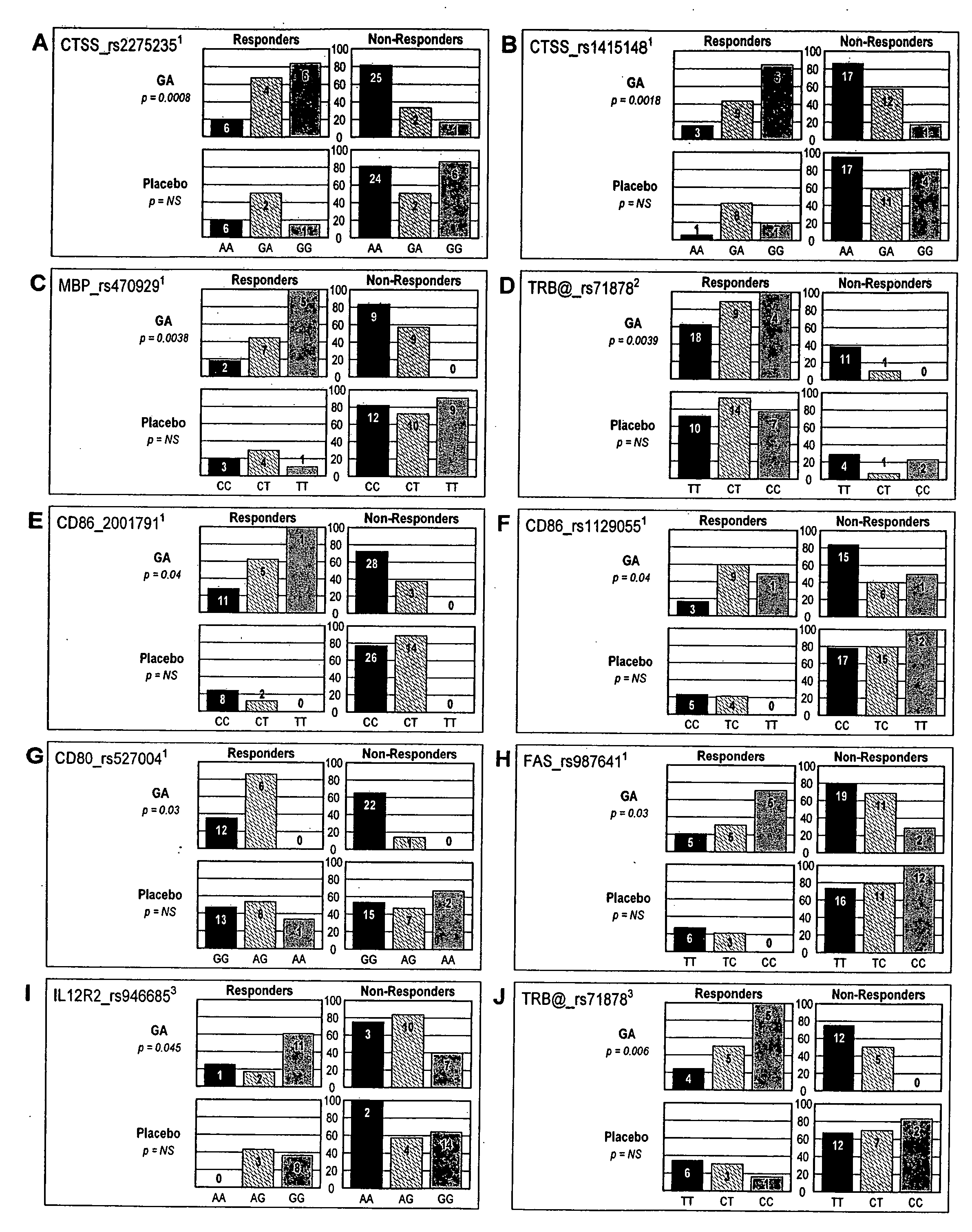
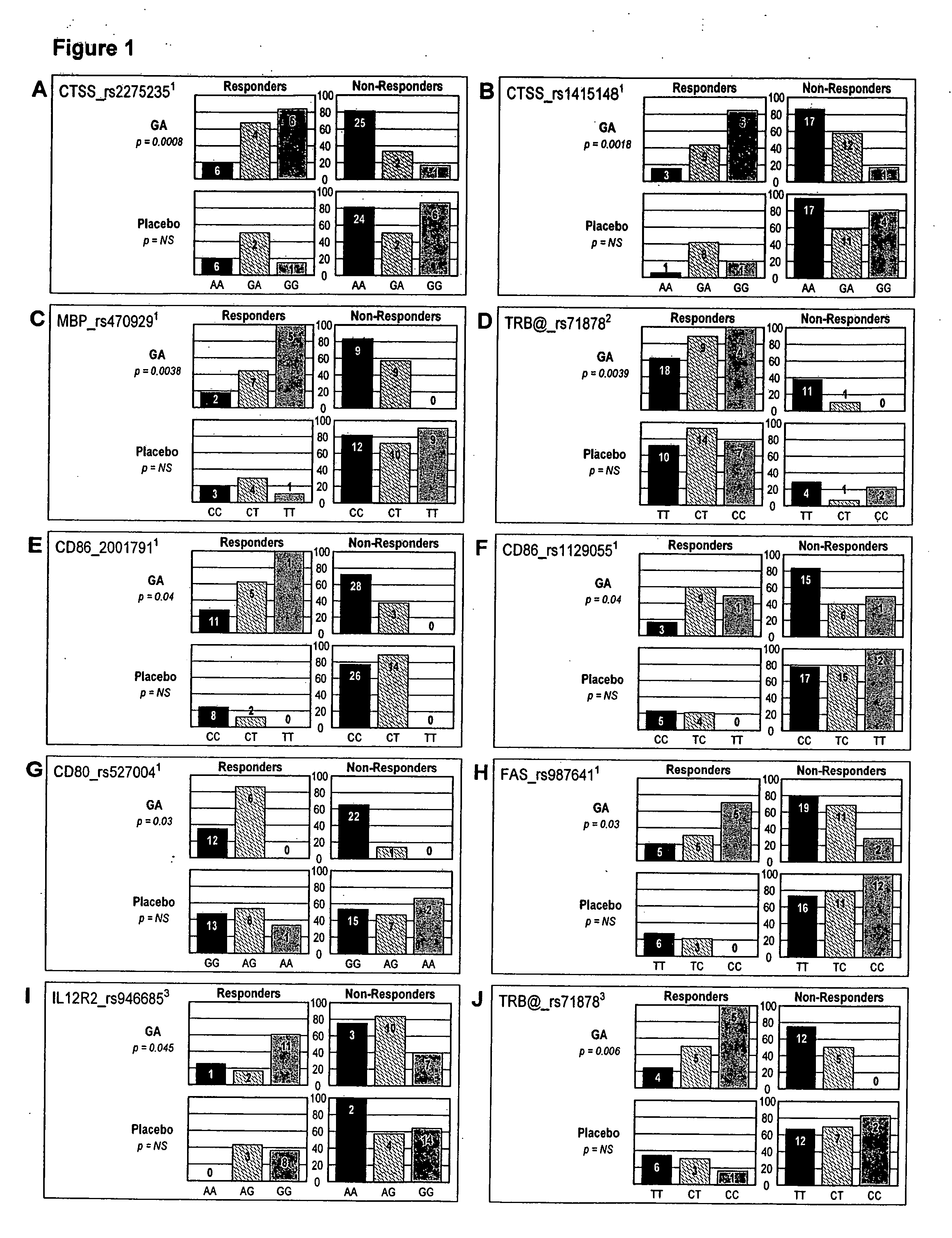
Markers associated with the therapeutic efficacy of glatiramer acetate
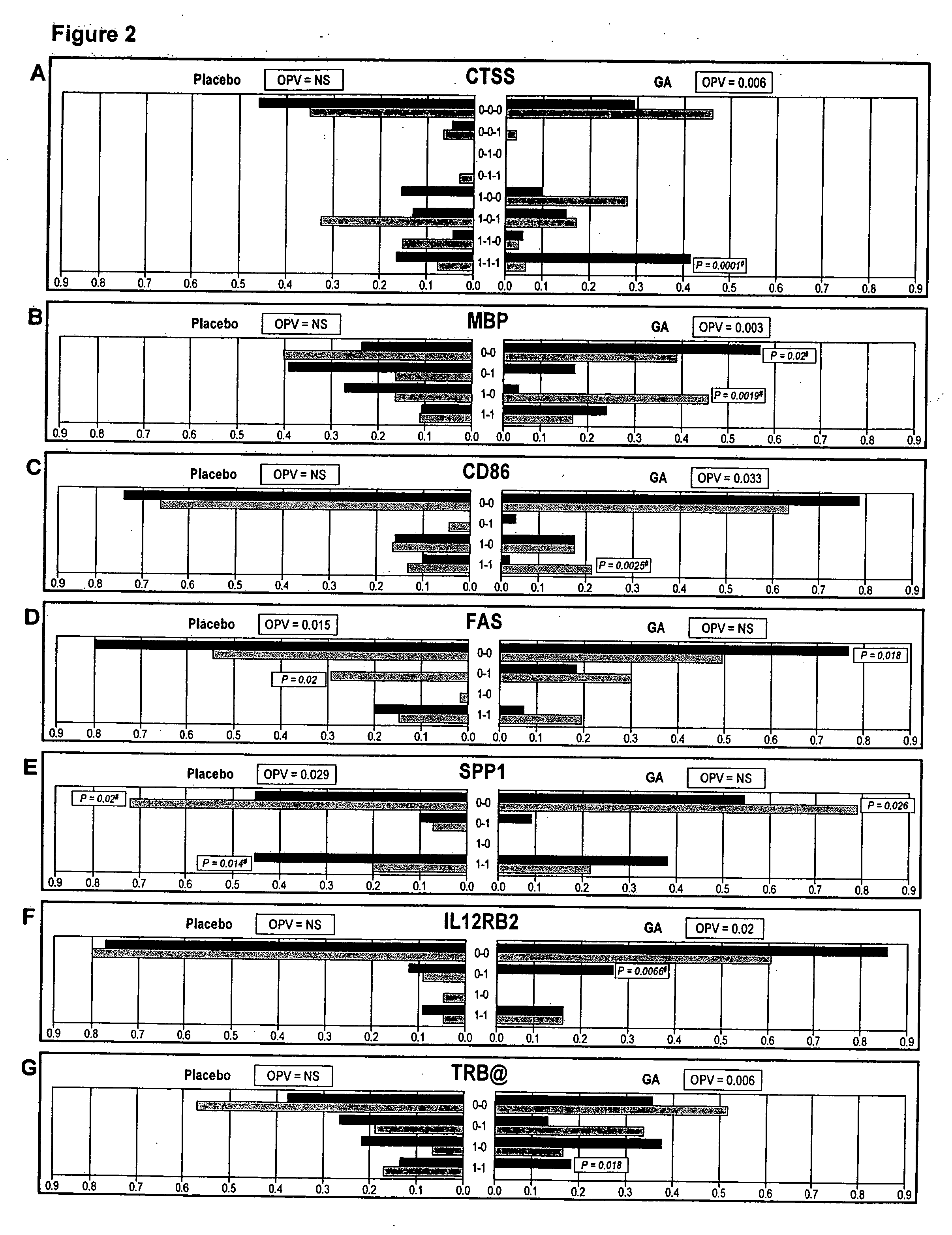
The present invention is directed to methods and kits based, at least in part, on the identification of allele-specific responsiveness or non-responsiveness to glatiramer acetate for the treatment of immune disorders, such as relapsing-remitting multiple sclerosis. The allele-specific responsiveness or non-responsiveness is based on polymorphisms in the following genes, CTSS, MBP, TCRB, CD95, CD86, IL-1R1, CD80, SCYA5, MMP9, MOG, SPP1 and IL-12RB2.
Owner:RAPPAPORT FAMILY INSTITUTE FOR RESEACH IN THE MEDICAL SCIENCES +2
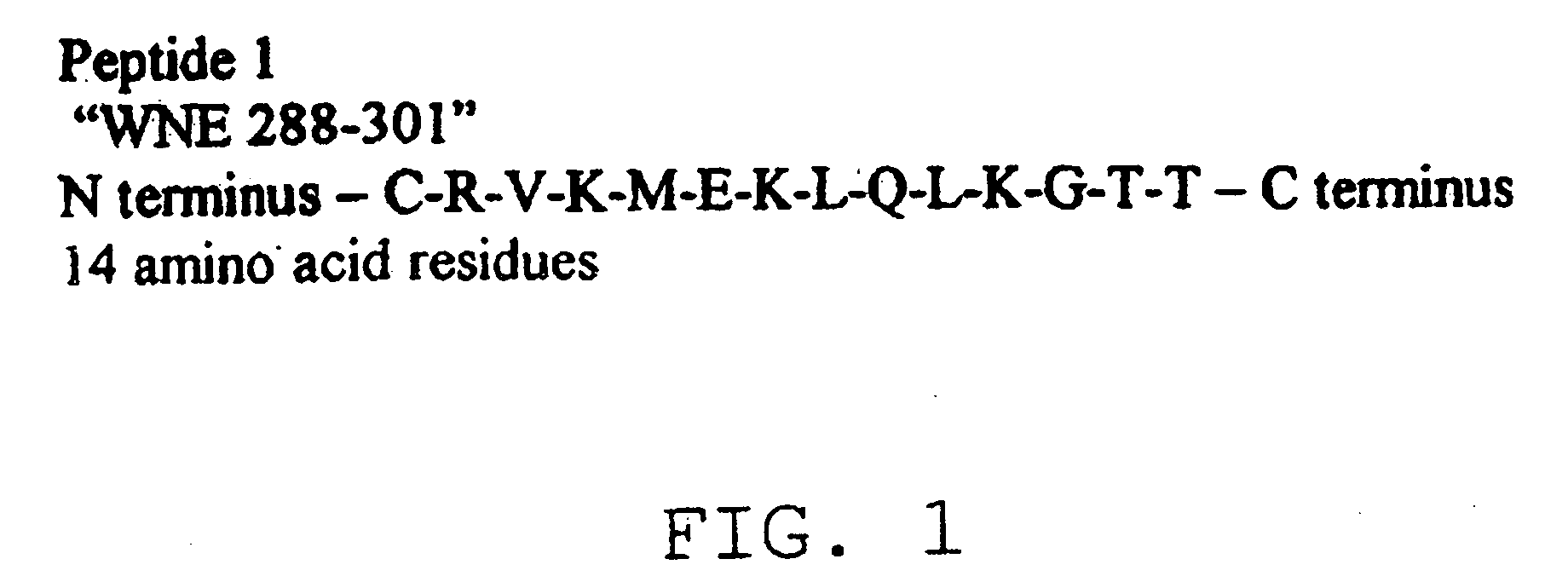
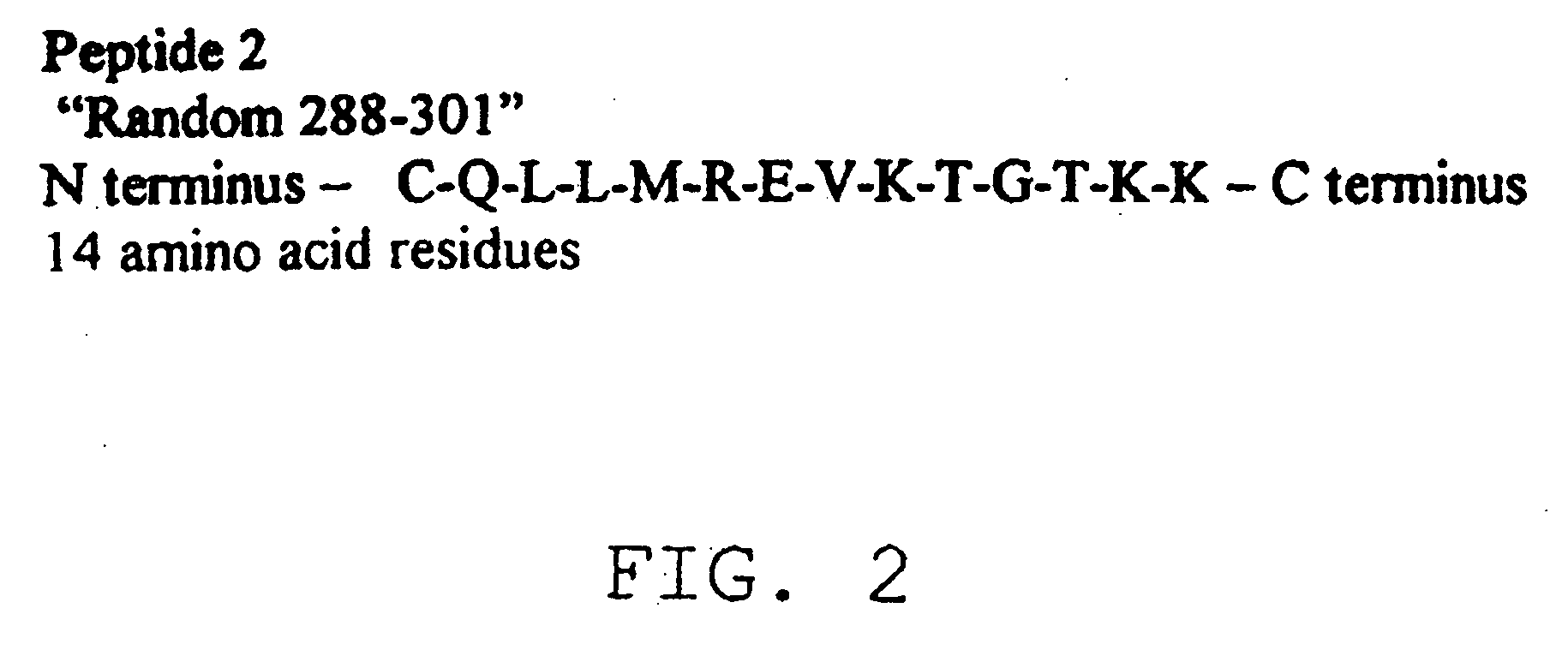
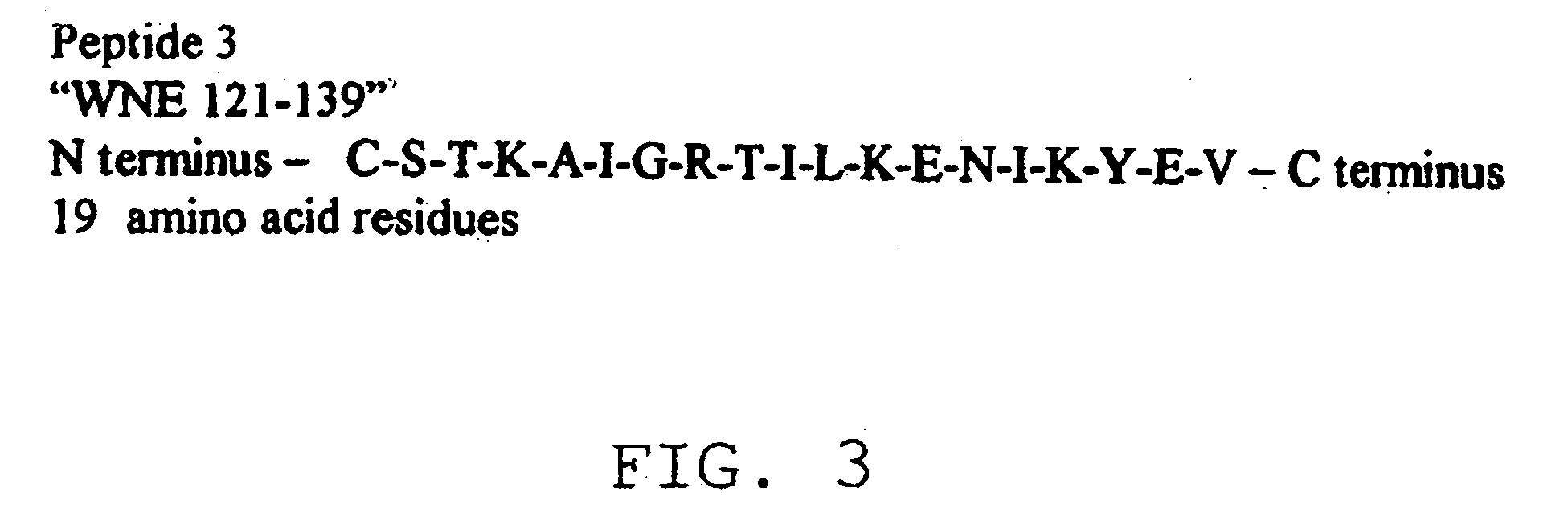
Diagnostic test for West Nile virus
InactiveUS20040197769A1More sensitiveEasy to useViral antigen ingredientsMicrobiological testing/measurementSt Louis encephalitis virusSerum ige
The present invention provides a rapid and sensitive method for the detection of a West Nile virus (WNV), Japanese encephalitis virus (JEV), St. Louis encephalitis virus (SLEV) and Dengue virus (DENV) and antibodies directed against thereof involving contacting a biological specimen suspected of being infected with WNV, JE, SLE or DEN with a substantially purified and isolated WNV E glycoprotein or subfragment thereof having a native conformation wherein the E glycoprotein or subfragment thereof has a reactivity with antibodies against WNV and a cross-reactivity with antibodies against JEV, SLEV and DENV. The instant invention further provides a rapid, sensitive, and consistent method for the specific detection of WNV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-WNV antibodies but not cross-reactive with antibodies against other flaviviruses such as JEV, SLEV, or DENV. The present invention also provides a rapid, sensitive, and consistent method for the specific detection of DENV by employing diagnostic assays having the antigen NS5 which is specifically reactive with anti-DENV antibodies but do not cross-react with antibodies against other flaviviruses such as JEV, SLEV, or WNV. Further, the DENV NS5 antigens are serospecific and do not cross react with antibodies to other DENV strains. Thus, the method of the present invention provides a manner by which to discriminate infections by each DENV strain. Further, diagnostic kits for carrying out the methods are provided. The methods and kits for carrying out the methods of the invention are rapid and require as little as 10 minutes to detect a result.
Owner:HEALTH RES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com