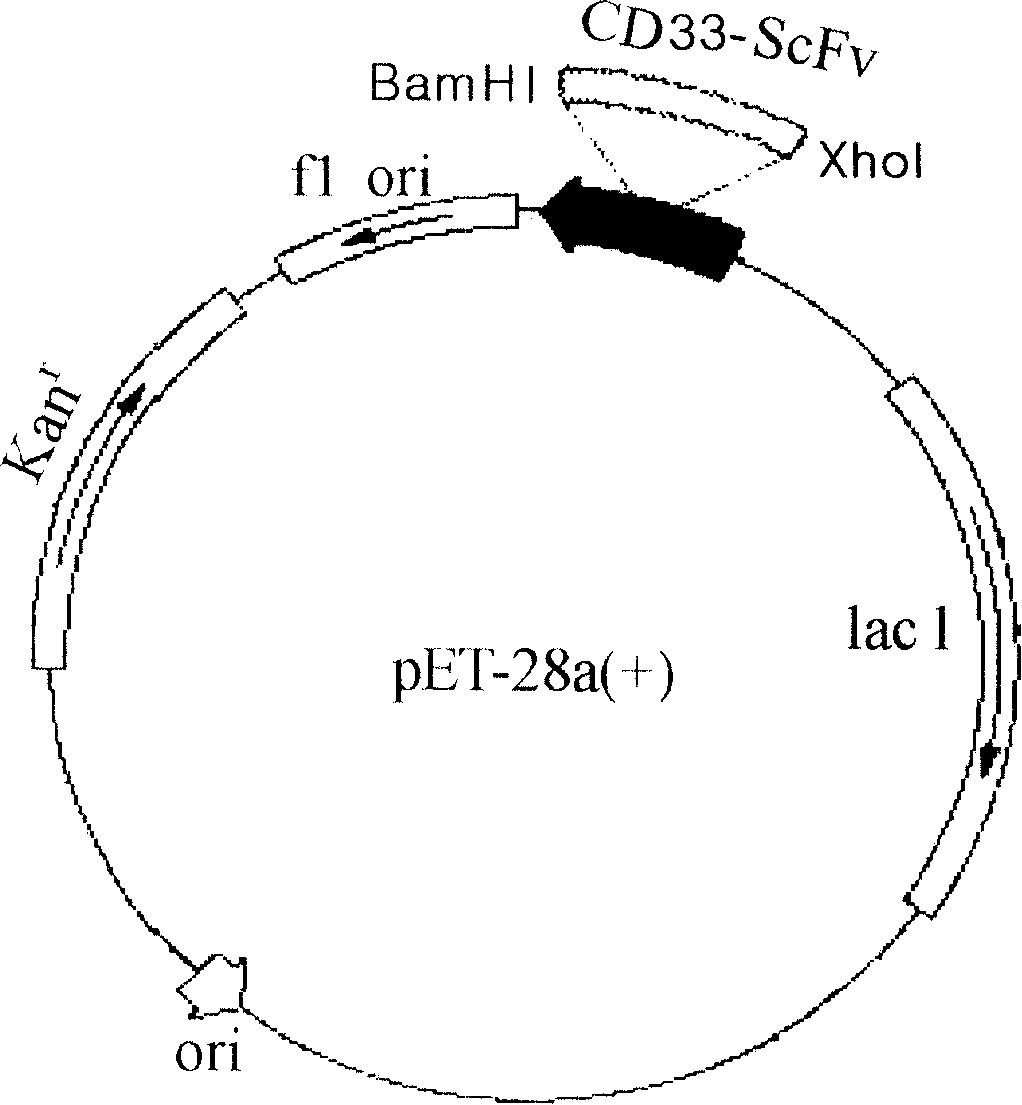
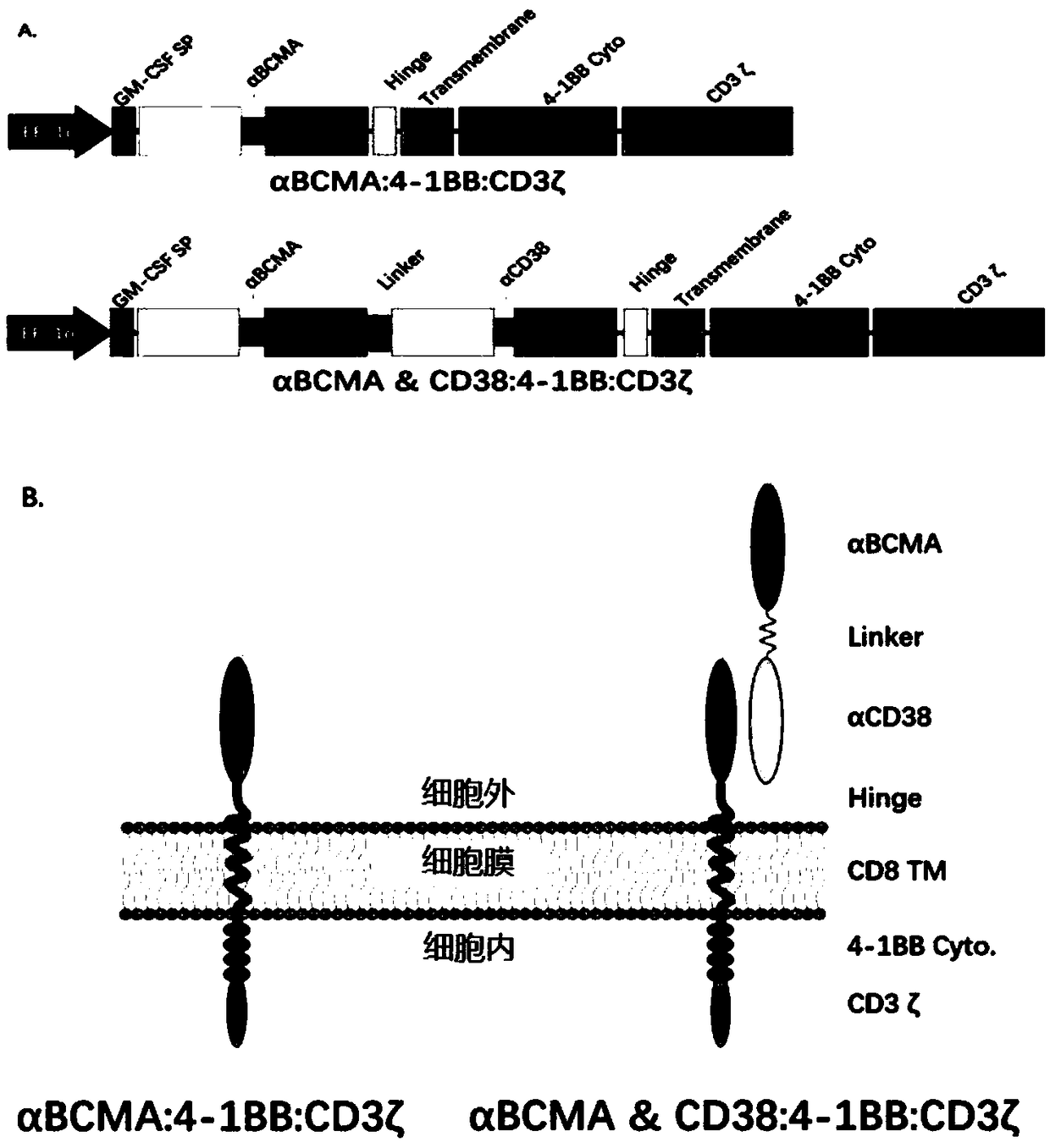
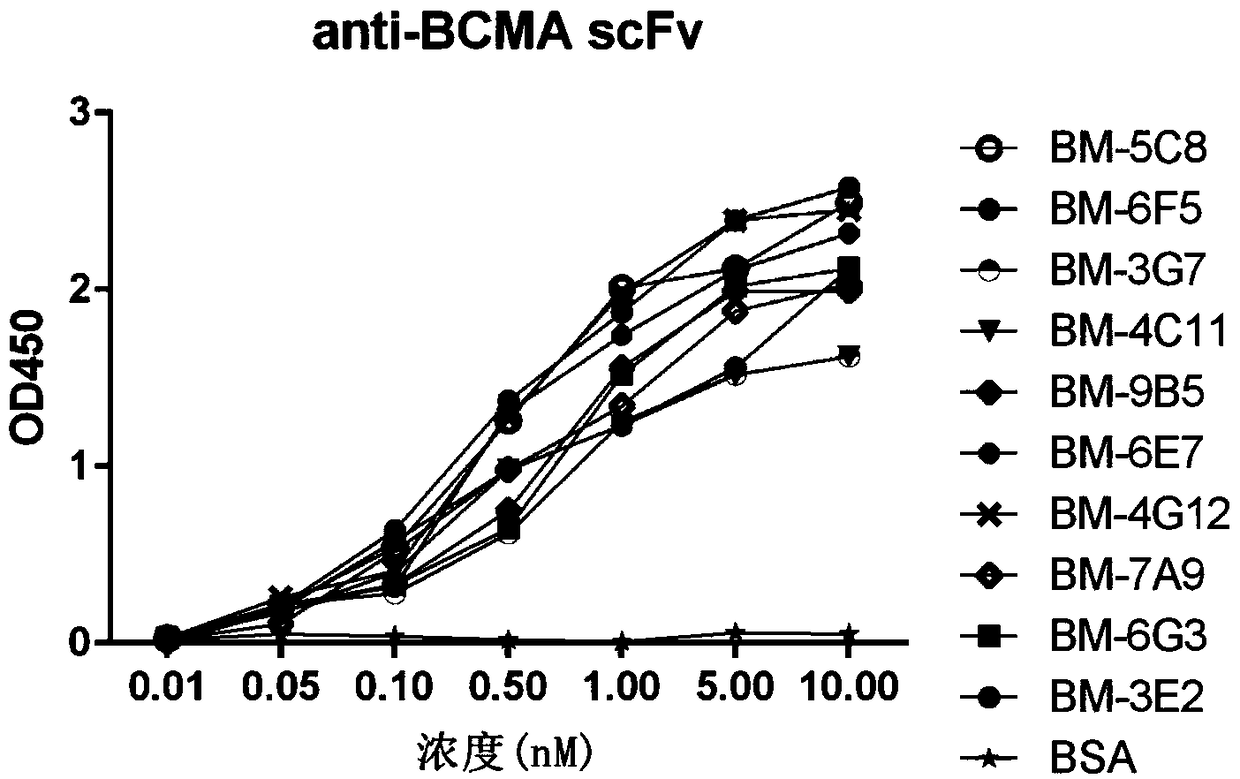
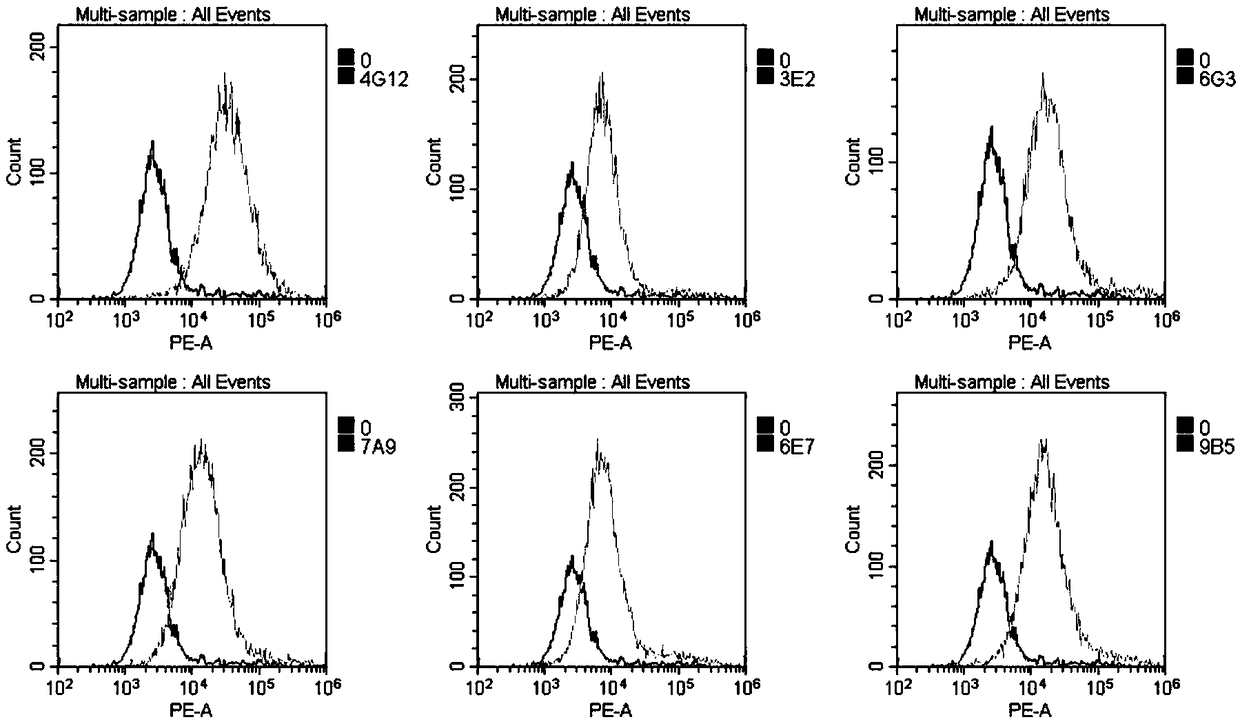
Patents
Literature
138 results about "CD33" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CD33 or Siglec-3 (sialic acid binding Ig-like lectin 3, SIGLEC3, SIGLEC-3, gp67, p67) is a transmembrane receptor expressed on cells of myeloid lineage. It is usually considered myeloid-specific, but it can also be found on some lymphoid cells.
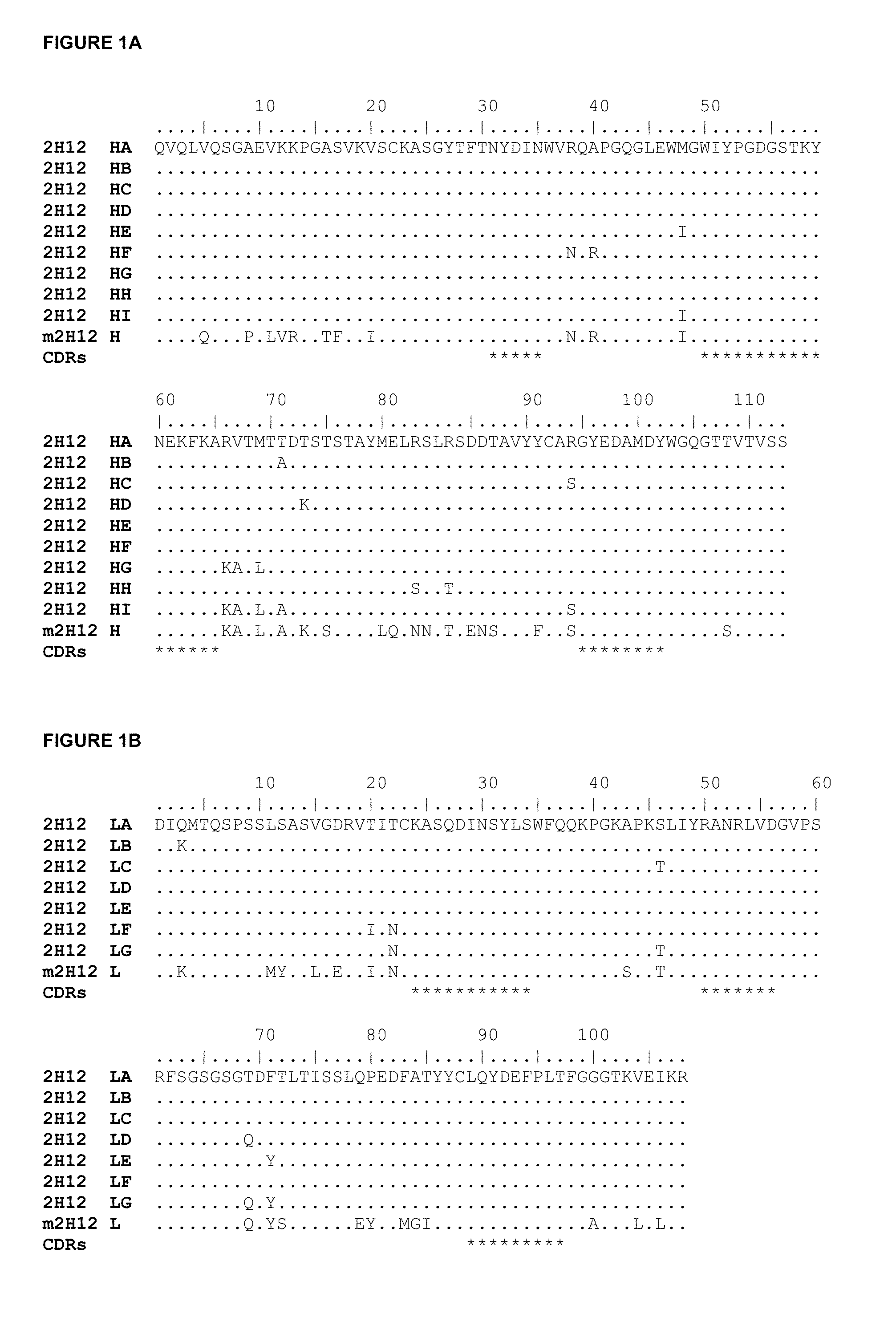
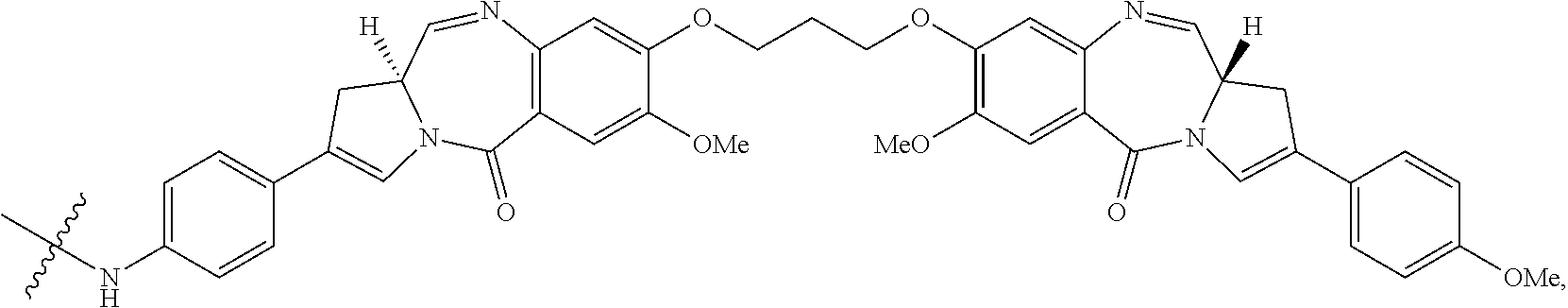
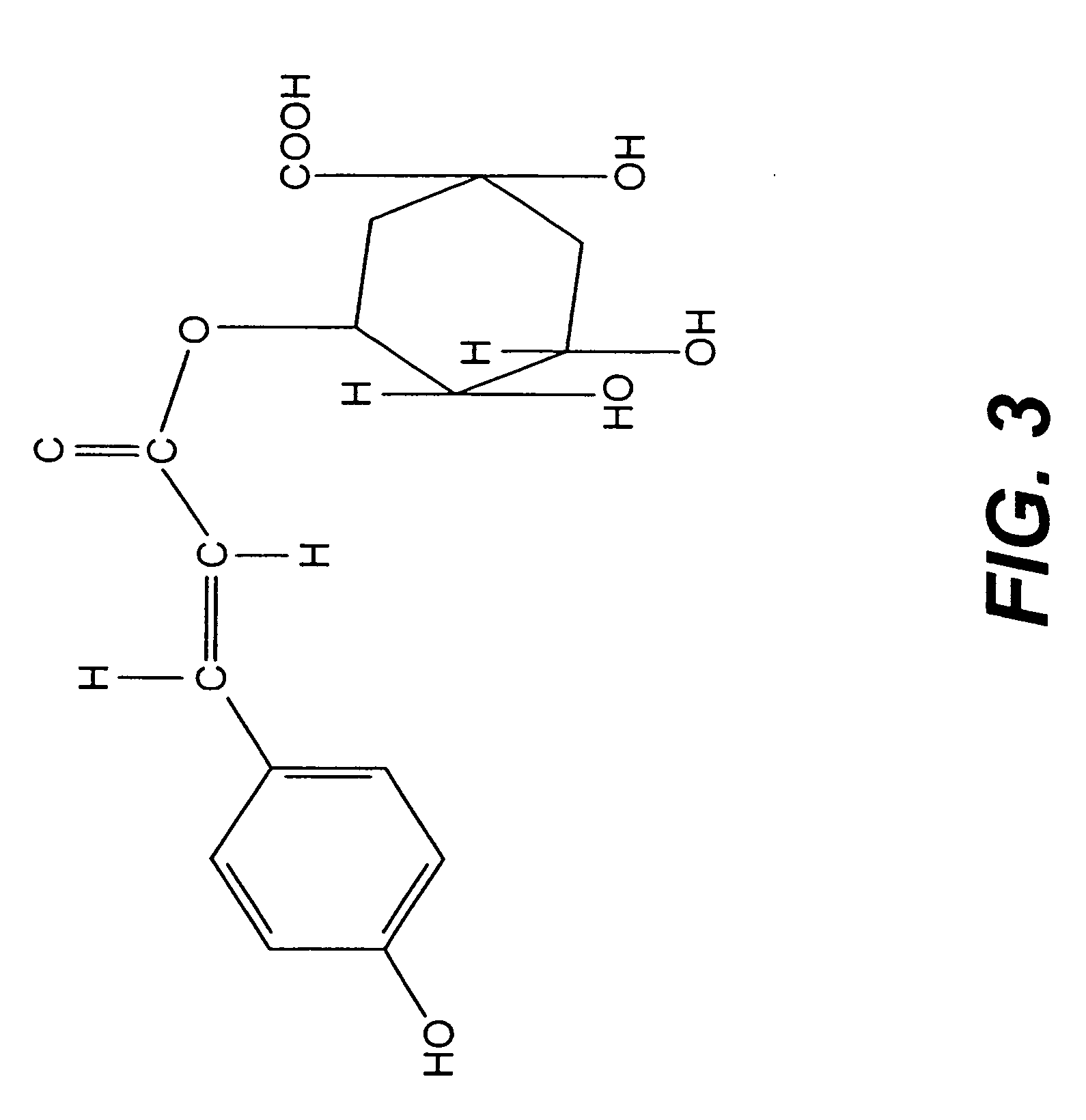
CD33 Antibodies And Use Of Same To Treat Cancer
Owner:SEATTLE GENETICS INC
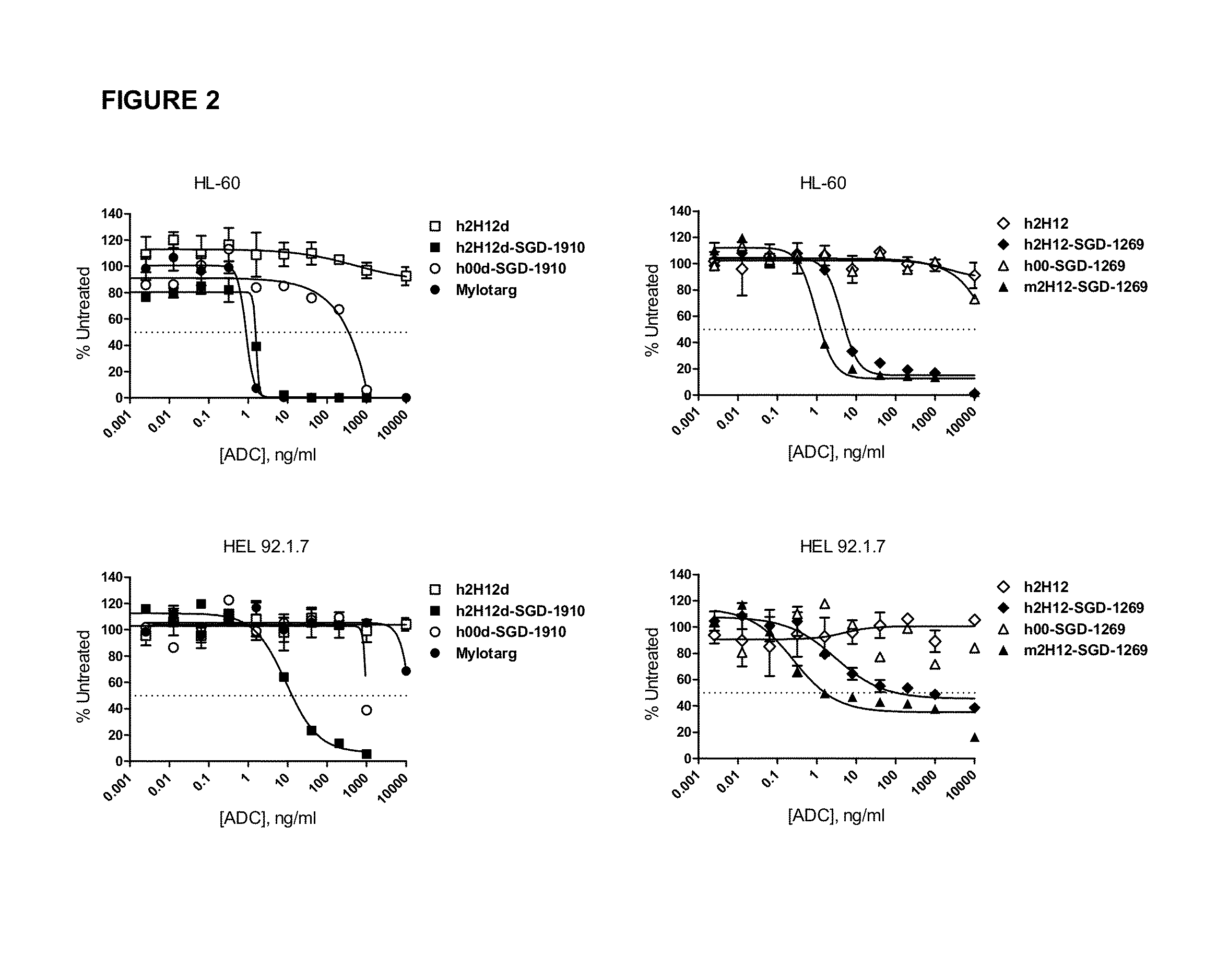
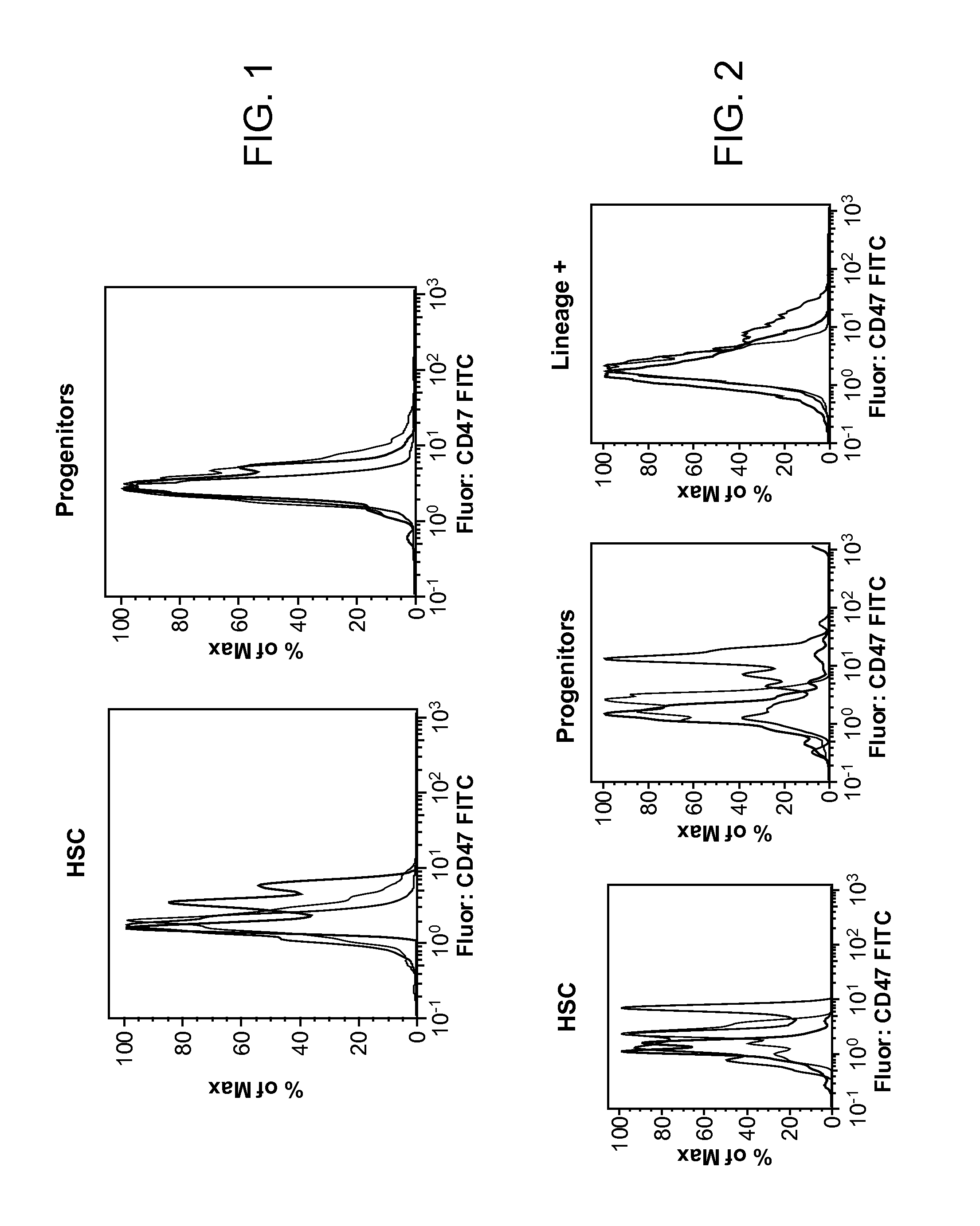
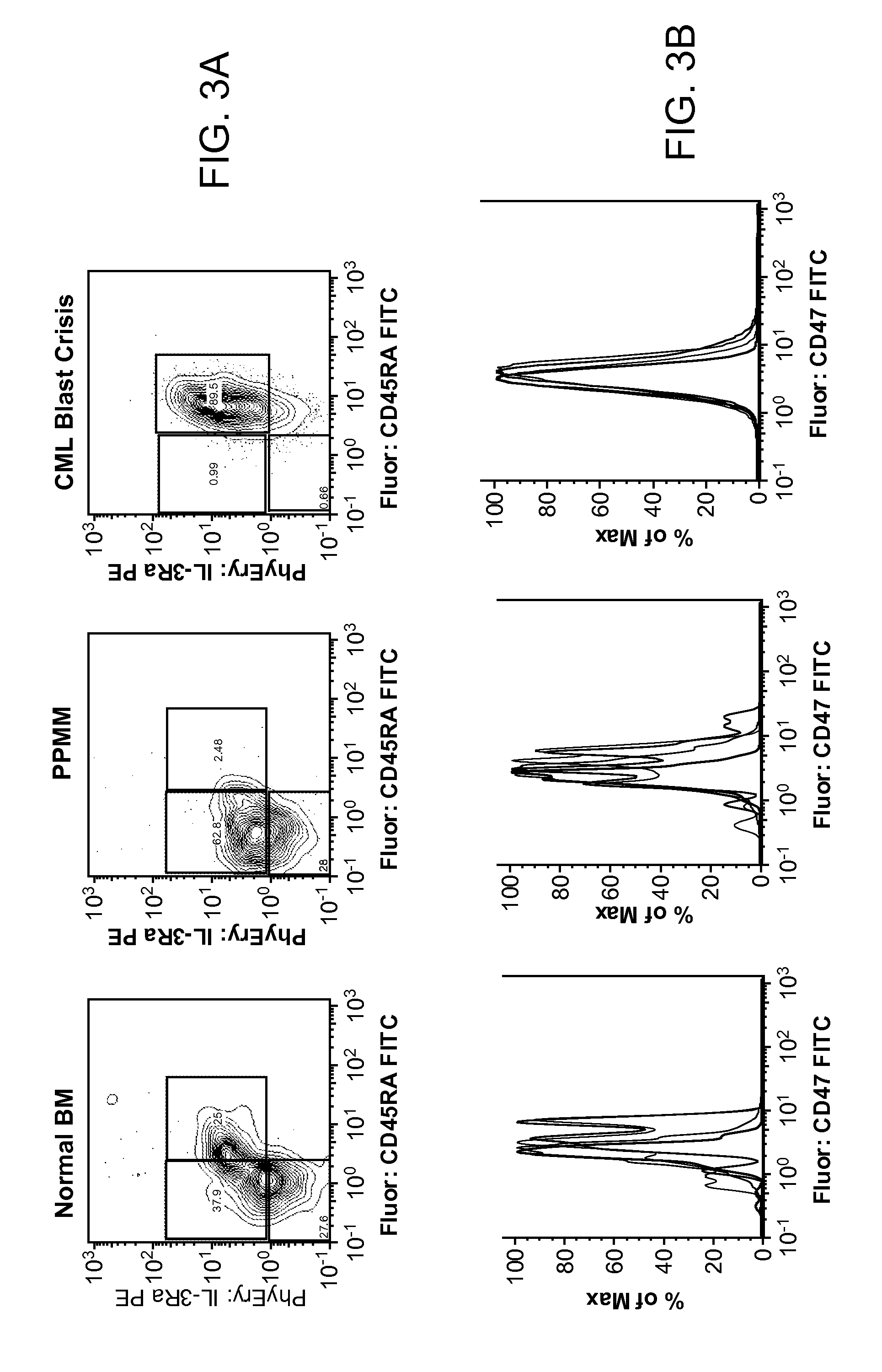
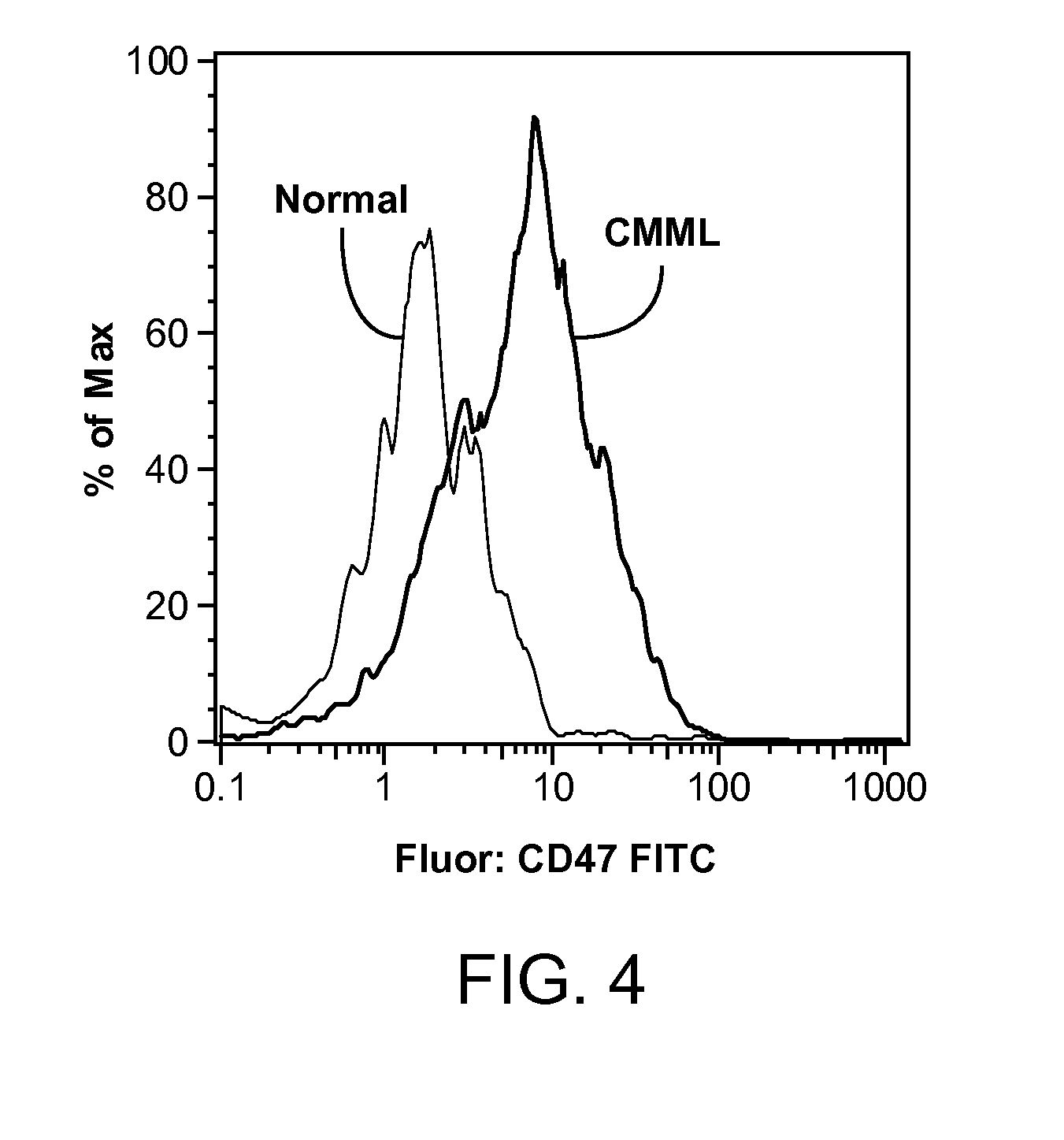
Synergistic Anti-CD47 Therapy for Hematologic Cancers
ActiveUS20120282174A1Immunoglobulins against cell receptors/antigens/surface-determinantsRadioactive preparation carriersSurface markerCD20
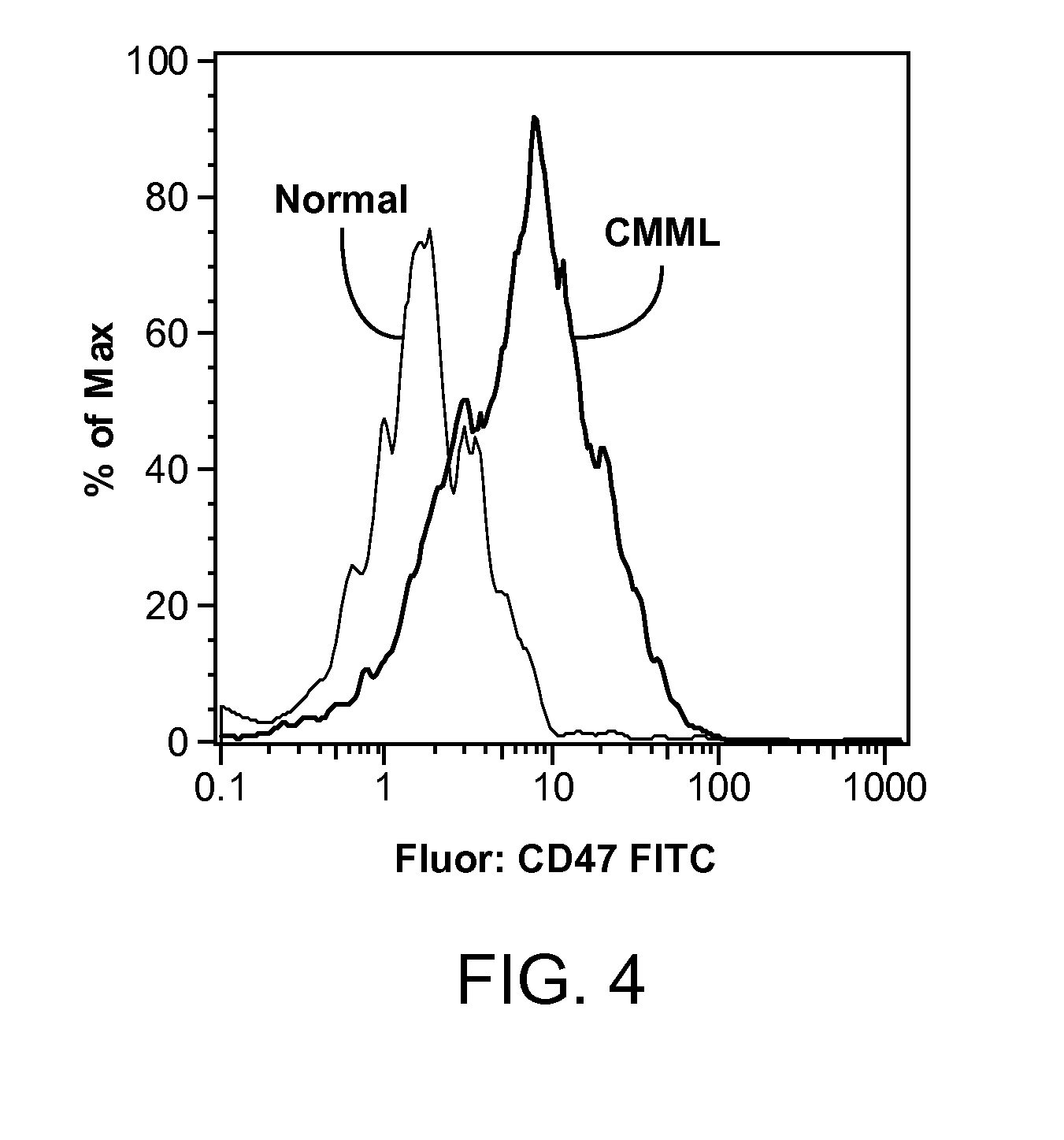
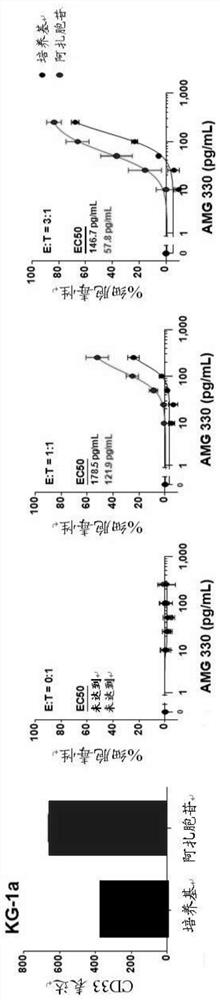
Methods are provided for treatment of hematologic cancers, particularly lymphomas and leukemias, including without limitation myelogenous and lymphocytic leukemias. A combination of antibodies specific for CD47; and specific for a cancer associated cell surface marker are administered to the patient, and provide for a synergistic decrease in cancer cell burden. The combination of antibodies may comprise a plurality of monospecific antibodies, or a bispecific or multispecific antibody. Markers of interest include without limitation, CD20, CD22, CD52, CD33; CD96; CD44; CD123; CD97; CD99; PTHR2; and HAVCR2.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
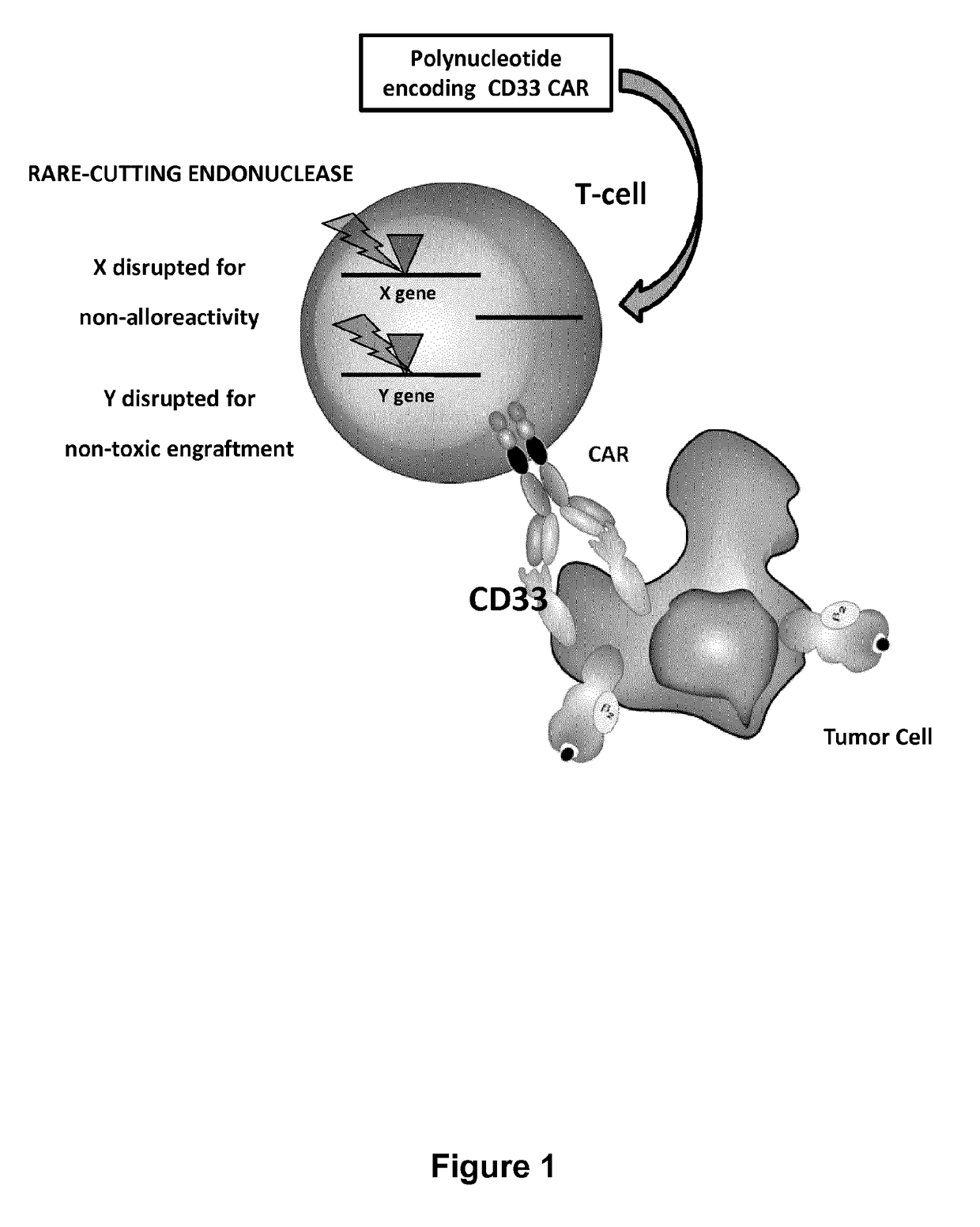
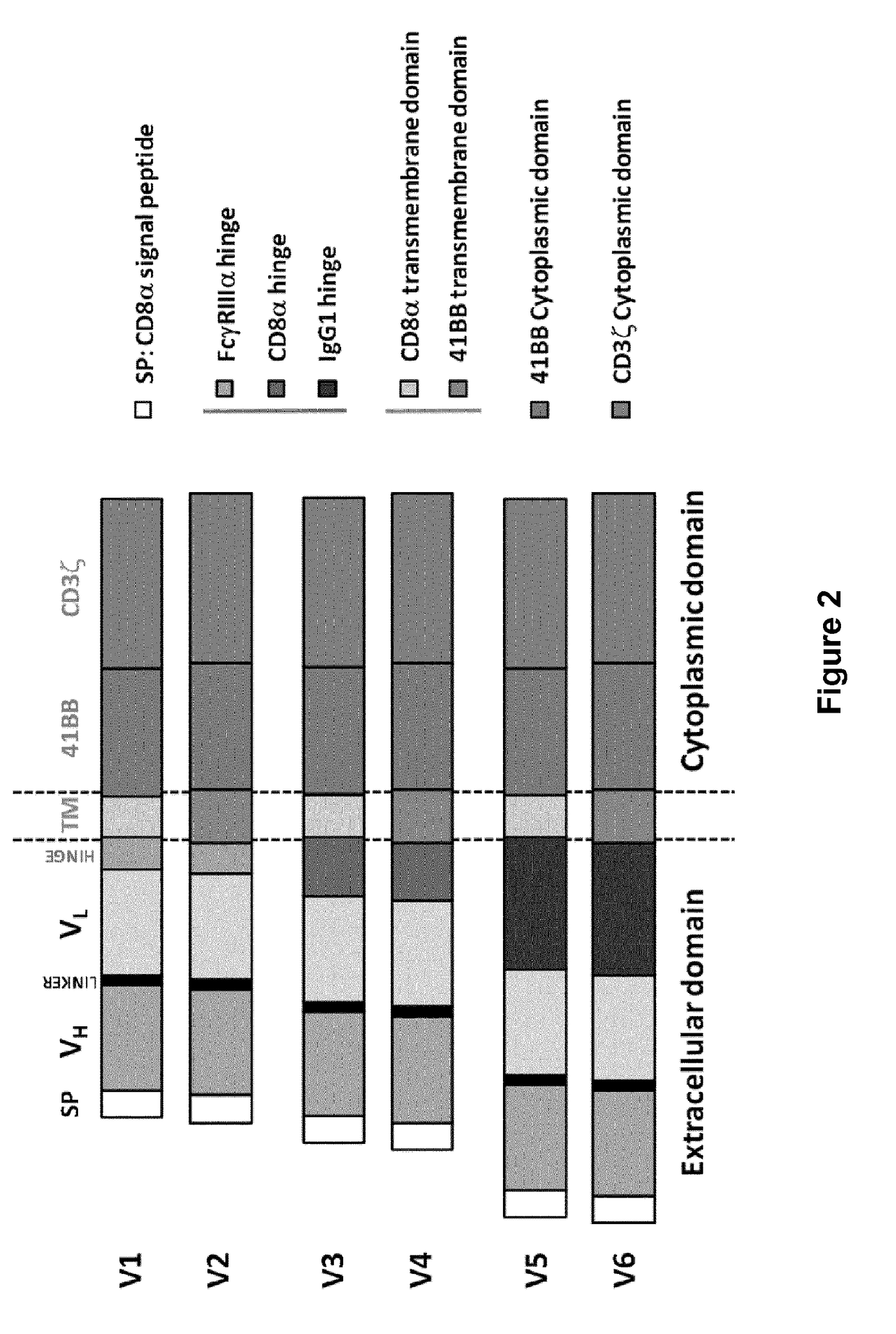
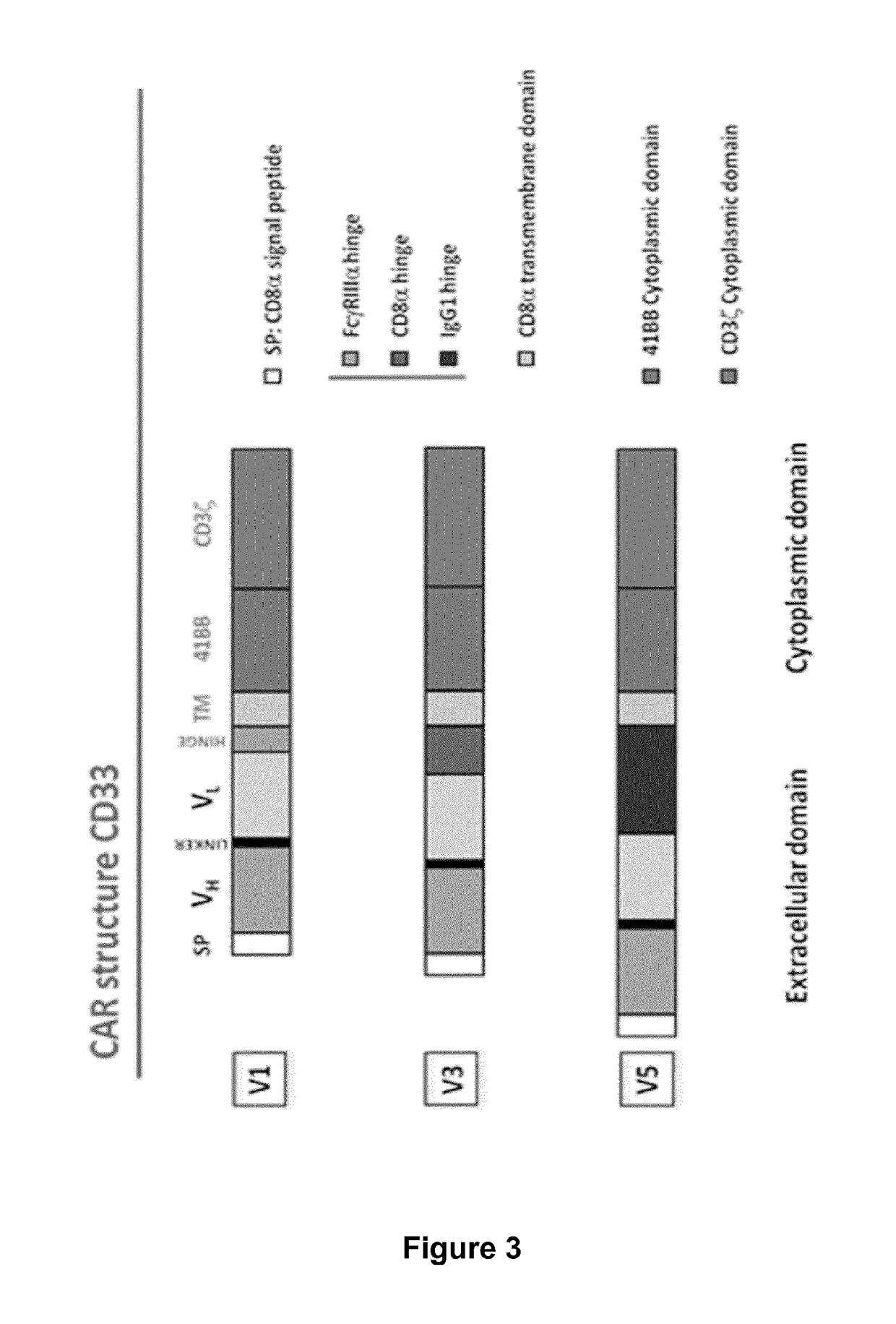
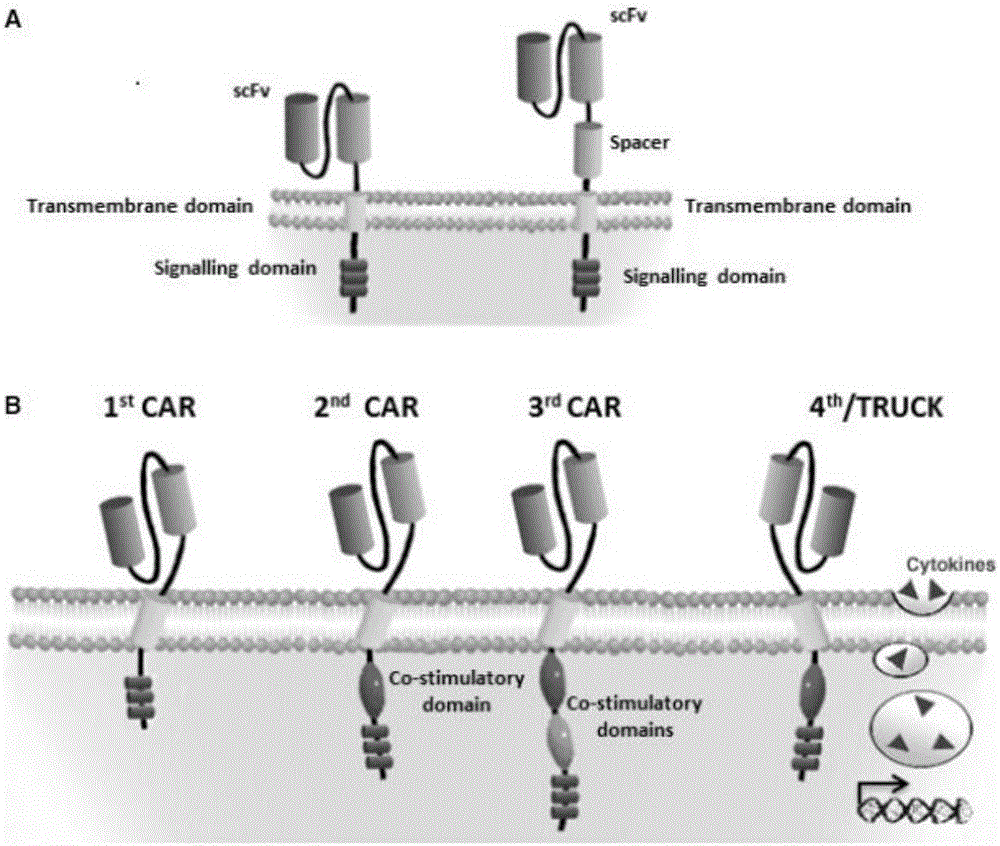
Cd33 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS20170145094A1Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD33
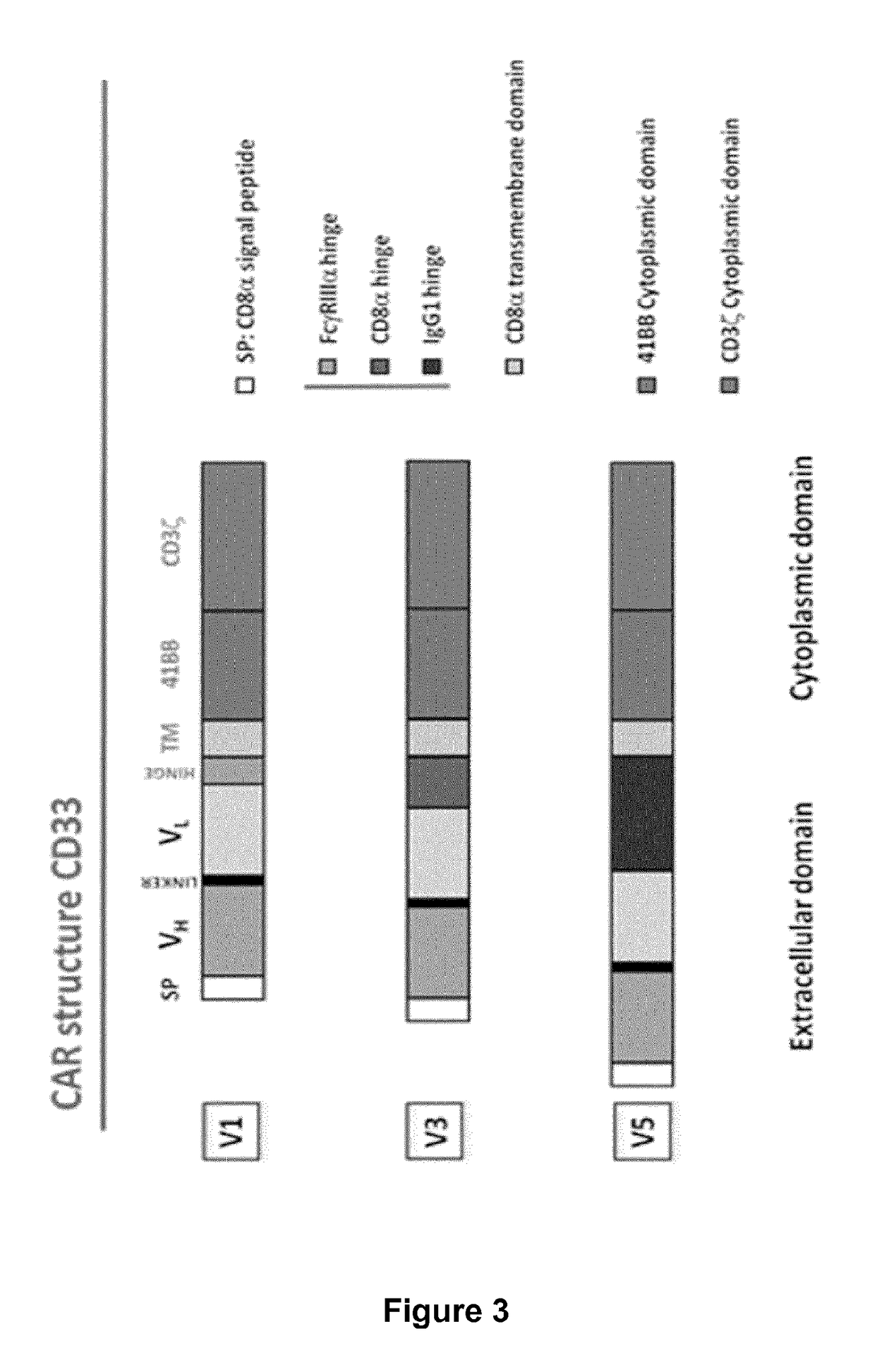
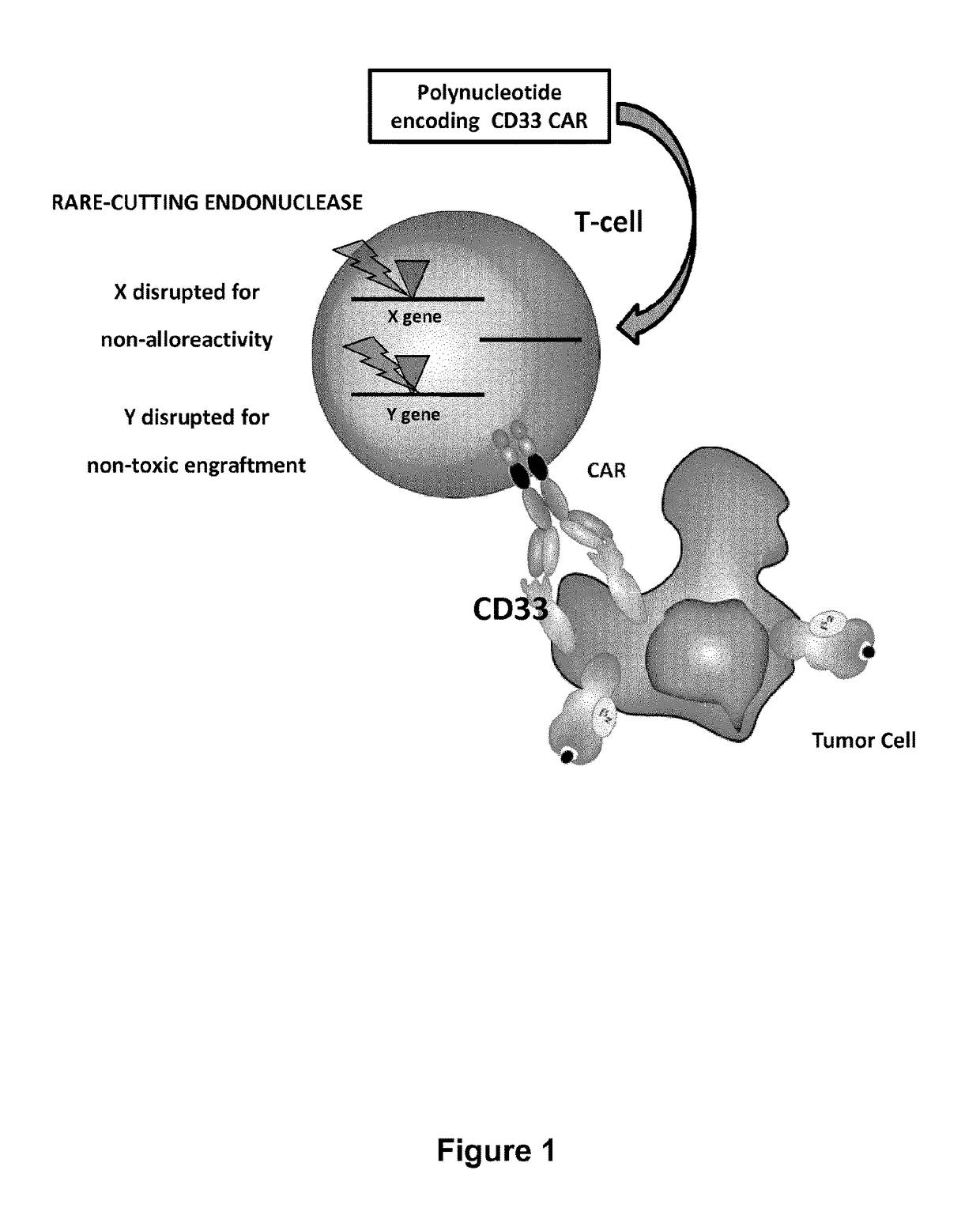
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD33 monoclonal antibody, conferring specific immunity against CD33 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
Synergistic anti-CD47 therapy for hematologic cancers
Methods are provided for treatment of hematologic cancers, particularly lymphomas and leukemias, including without limitation myelogenous and lymphocytic leukemias. A combination of antibodies specific for CD47; and specific for a cancer associated cell surface marker are administered to the patient, and provide for a synergistic decrease in cancer cell burden. The combination of antibodies may comprise a plurality of monospecific antibodies, or a bispecific or multispecific antibody. Markers of interest include without limitation, CD20, CD22, CD52, CD33; CD96; CD44; CD123; CD97; CD99; PTHR2; and HAVCR2.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Cd33 specific chimeric antigen receptors
PendingUS20180002397A1Easy SurvivalEfficiently and specifically eliminatePolypeptide with localisation/targeting motifImmunoglobulin superfamilyMyeloid leukemiaCD33
Provided herein are chimeric antigen receptors (CARs) for cancer therapy, and more particularly, CARs containing a scFv from a CD33 monoclonal antibody. Provided are immune effector cells containing such CARs, and methods of treating proliferative disorders such as acute myeloid leukemia (AML), and relapsed or refractory AML.
Owner:PRECIGEN INC
Stem Cell Populations and Methods of Use
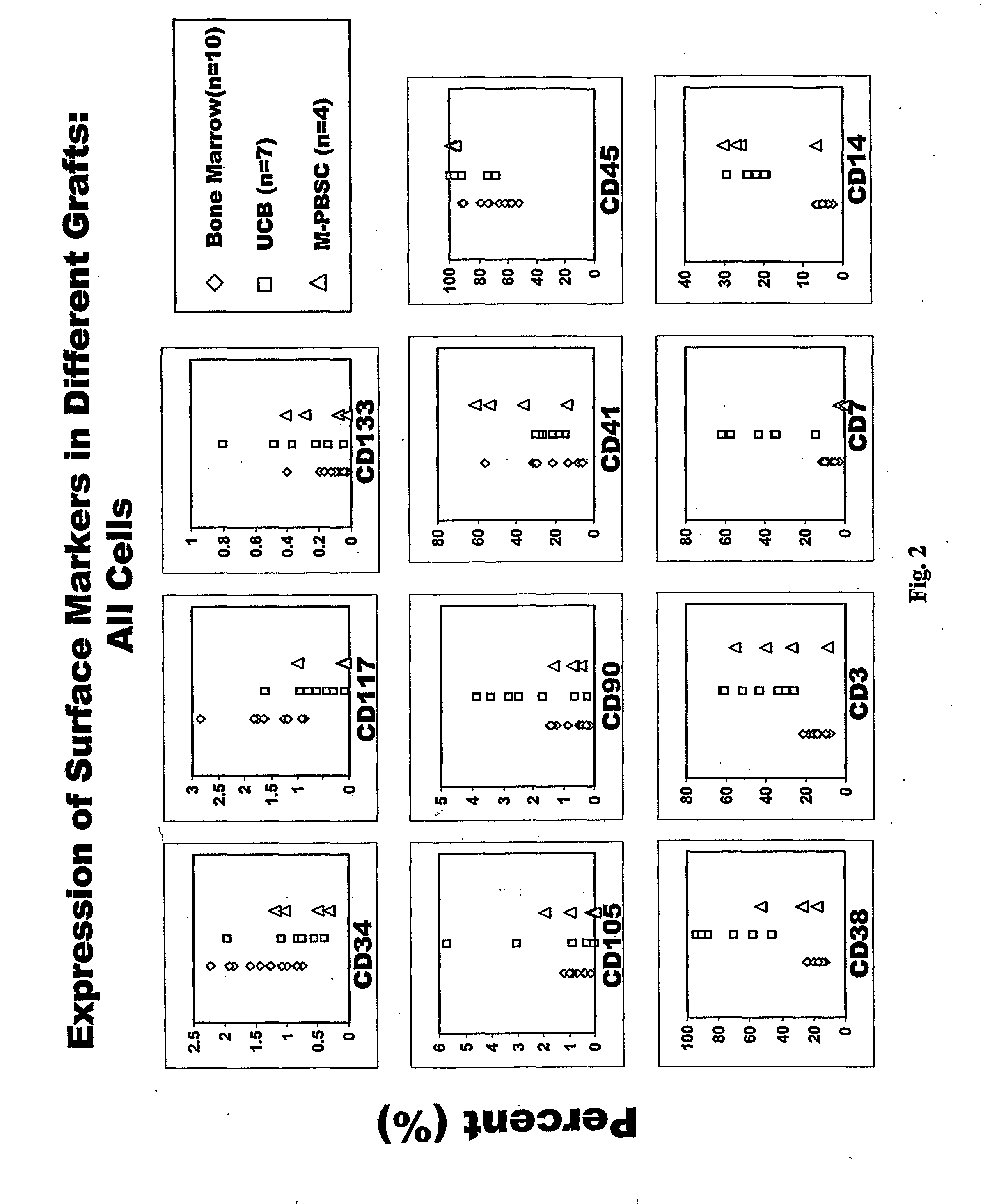
Populations of stem cells and methods for their isolation and use are provided. These stem cell populations comprise aldehyde dehydrogenase positive (ALDHbr) cells isolated from bone marrow, and ALDHbr CD105+ cells derived from any stem cell source. These populations may also comprise cells expressing such surface markers as CD34, CD38, CD41, CD45, CD105, CD133, CD135, CD117, and HLA-DR, and / or are substantially free from such cell surface markers as CD3, CD7, CD 10, CD 13, CD 14, C1319, CD33, CD35, CD56, CD 127, CD 138, and glycophorin A. The population may also comprise cells expressing CD90. The stem cell populations of the invention are isolated from a stem cell source such as bone marrow, peripheral blood, umbilical cord blood, and fetal liver. Methods of the invention comprise isolating and purifying stem cell populations from stem cell sources, and methods of using these cells to reconstitute, repair, and regenerate tissues.
Owner:ALDAGEN
Pharmaceutical combinations comprising cd33 antibodies and de-methylating agents
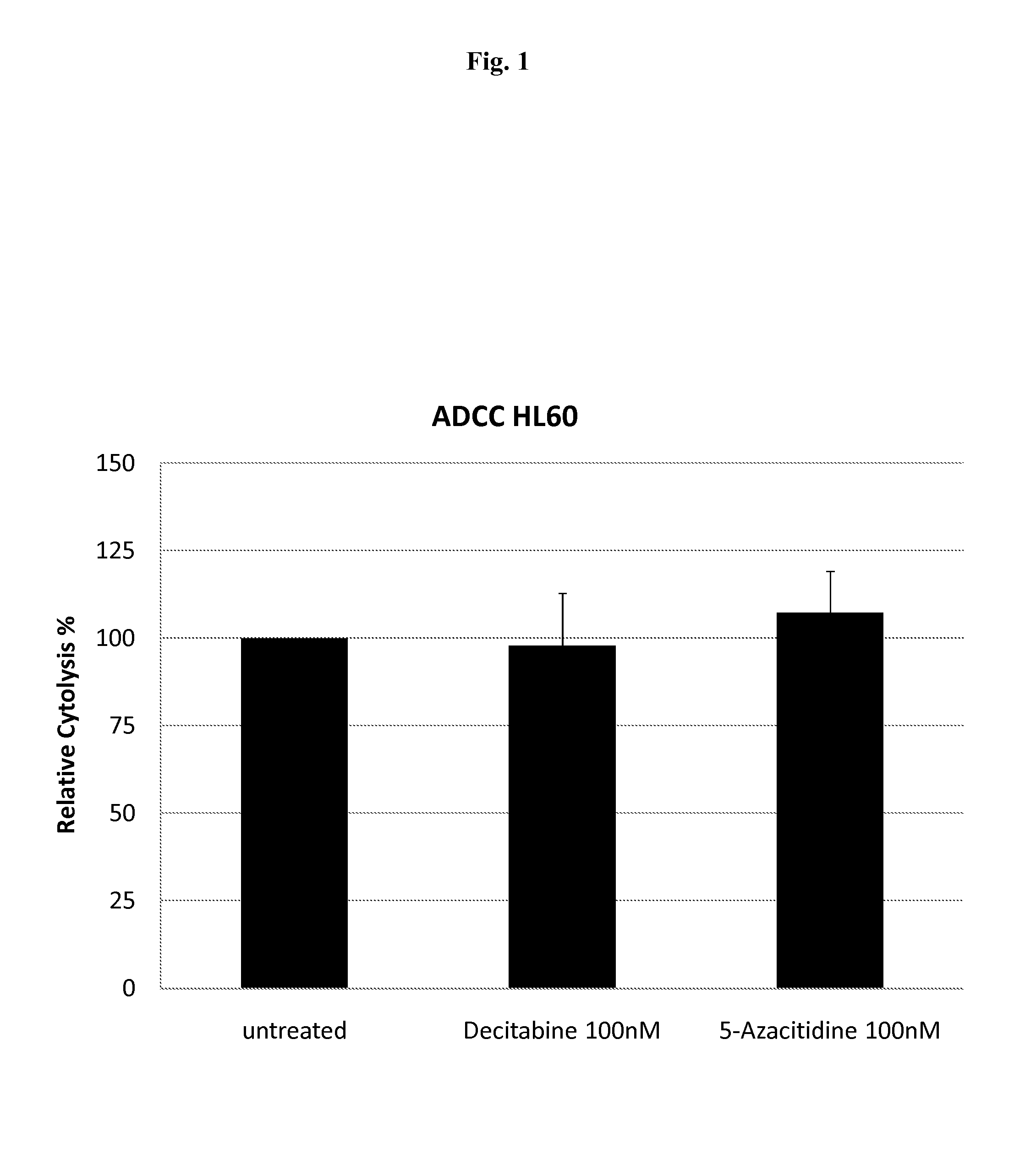
InactiveUS20150125447A1Enhanced ADCC activityADCC activityOrganic active ingredientsAntibody ingredientsDiseaseMethylating Agent
The present invention relates to pharmaceutical combinations CD33 antibodies and de-methylating agents for use in treating diseases like MDS and cancer, especially AML.
Owner:BOEHRINGER INGELHEIM INT GMBH
CD33 specific chimeric antigen receptors for cancer immunotherapy
ActiveUS9944702B2Useful for immunotherapyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenCD33
The present invention relates to Chimeric Antigen Receptors (CAR) that are recombinant chimeric proteins able to redirect immune cell specificity and reactivity toward selected membrane antigens, and more particularly in which extracellular ligand binding is a scFV derived from a CD33 monoclonal antibody, conferring specific immunity against CD33 positive cells. The engineered immune cells endowed with such CARs are particularly suited for treating lymphomas and leukemia.
Owner:CELLECTIS SA
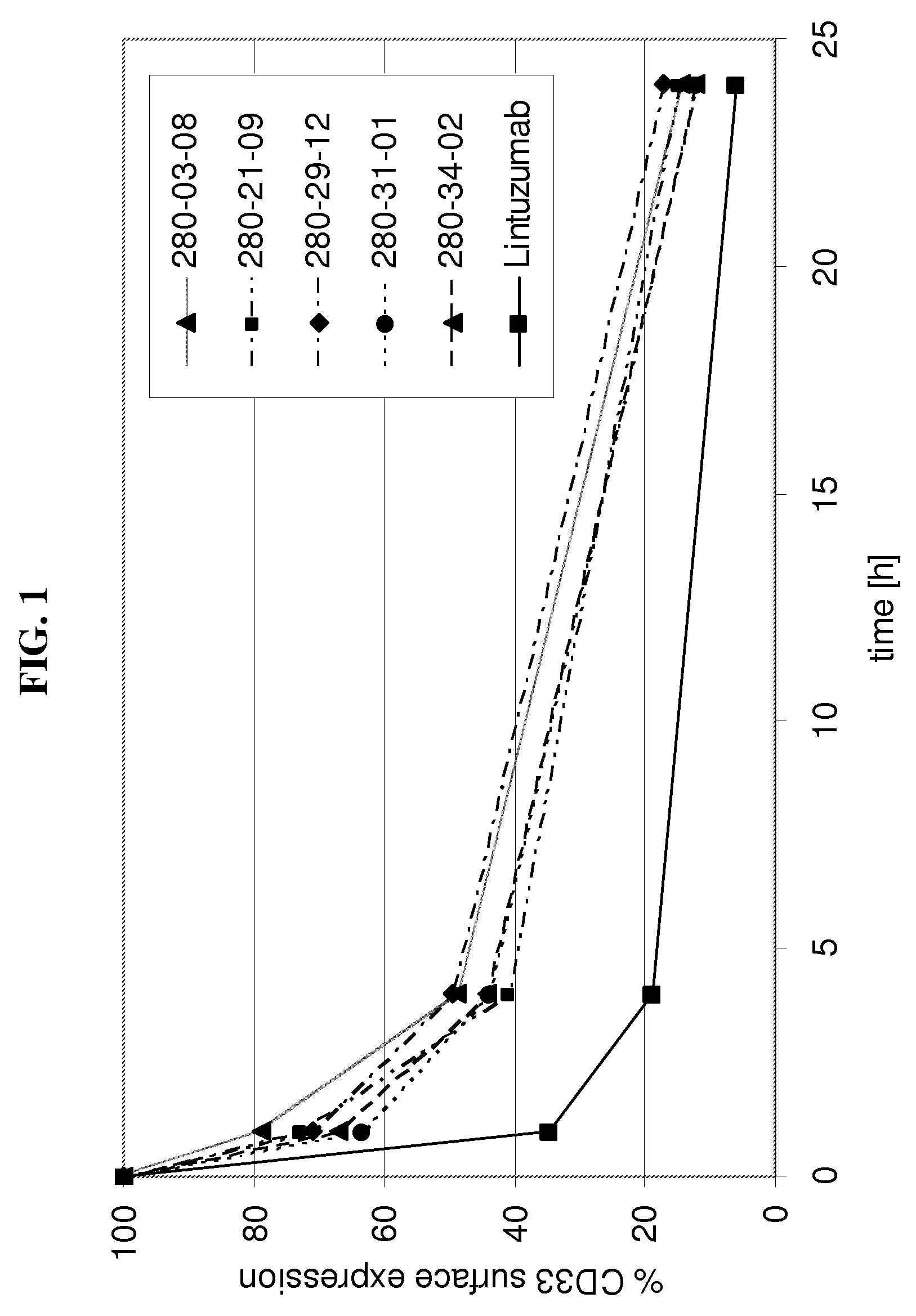
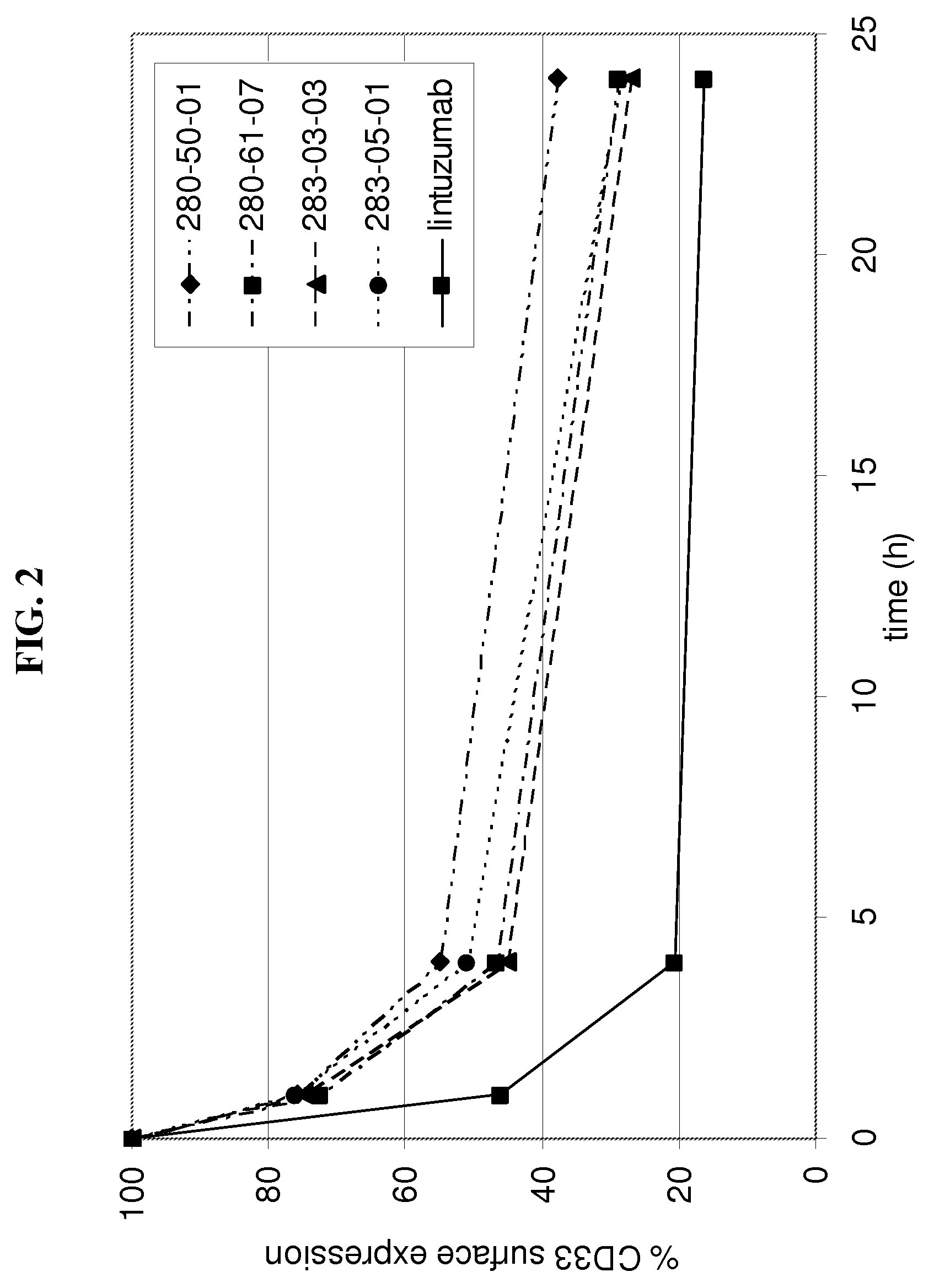
Anti-cd33 antibodies and method for treatment of acute myeloid leukemia using the same
The present invention relates to antibodies that bind CD33. More particularly, the invention relates to anti-CD33 antibodies, fragments and homologues of said antibodies, humanized and resurfaced versions of said antibodies, functional equivalents and improved versions of said antibodies, immunoconjugates and compositions comprising said antibodies, and the uses of same in diagnostic, research and therapeutic applications. The invention also relates to a polynucleotide encoding the antibodies, vectors comprising the polynucleotides, host cells transformed with polynucleotides and methods of producing the antibodies.
Owner:IMMUNOGEN INC
Use of siglec-7 or siglec-9 antibodies for the treatment of cancer
InactiveUS20190023786A1Reduces and inhibits ligand dependent calcium mobilizationInhibition of activationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD33Cancer research
The present application provides compositions and methods for treating a patient with cancer, and in particular epithelial tumors and carcinomas, with antibodies directed to CD33-like Siglecs.
Owner:PALLEON PHARMA INC
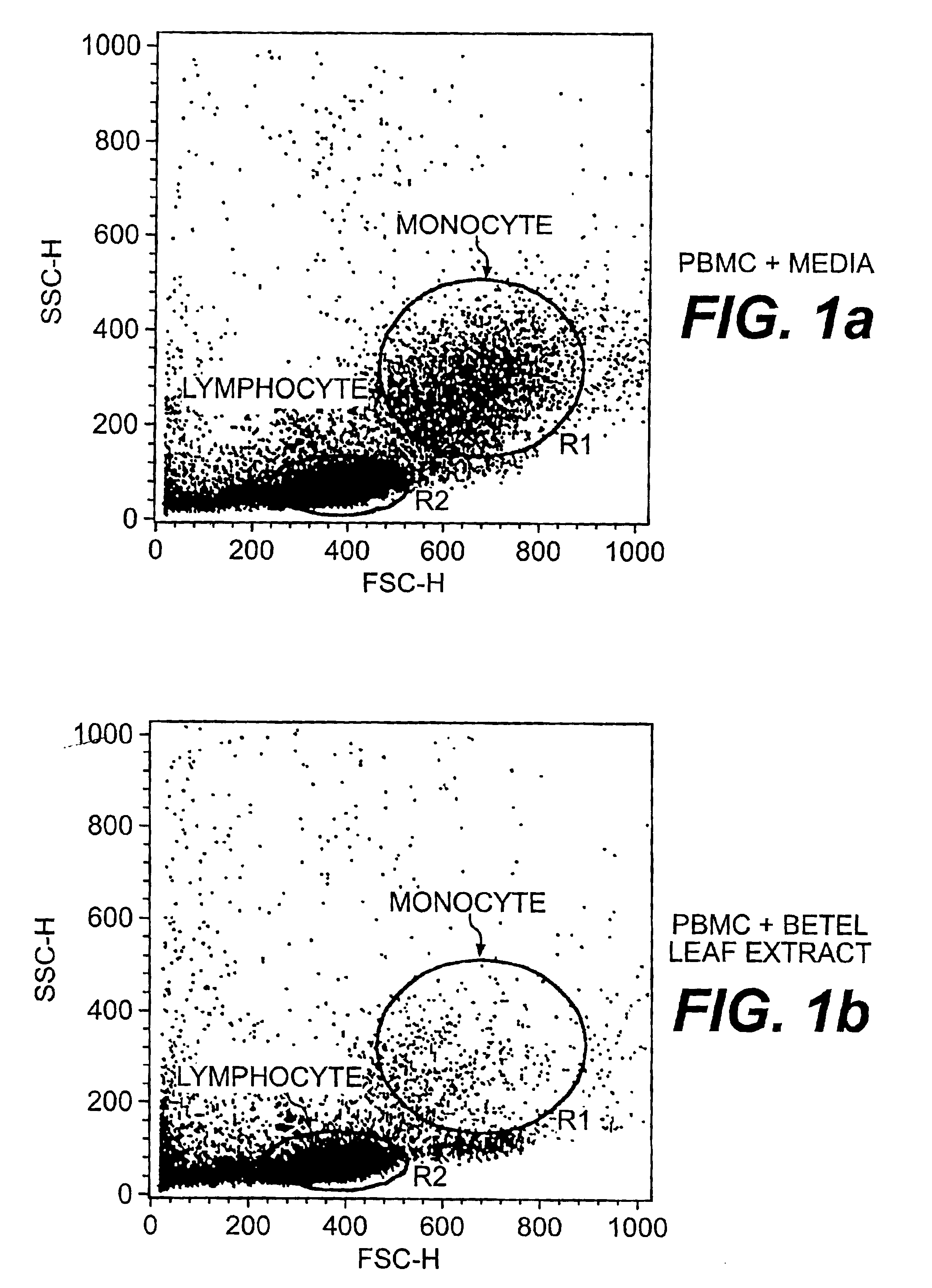
Herbal composition for treating CD33+ acute and chronic myeloid leukemia and a method thereof
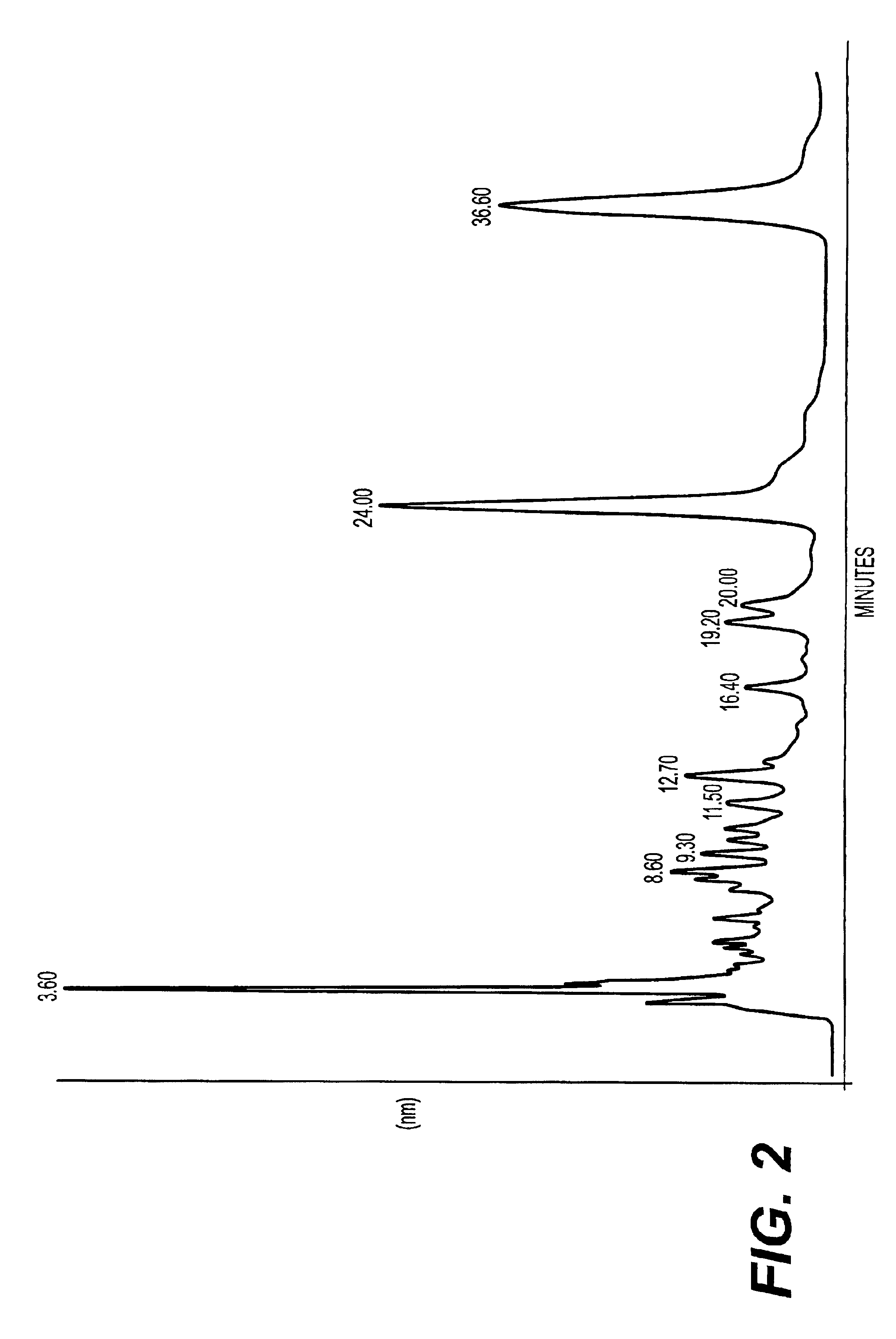
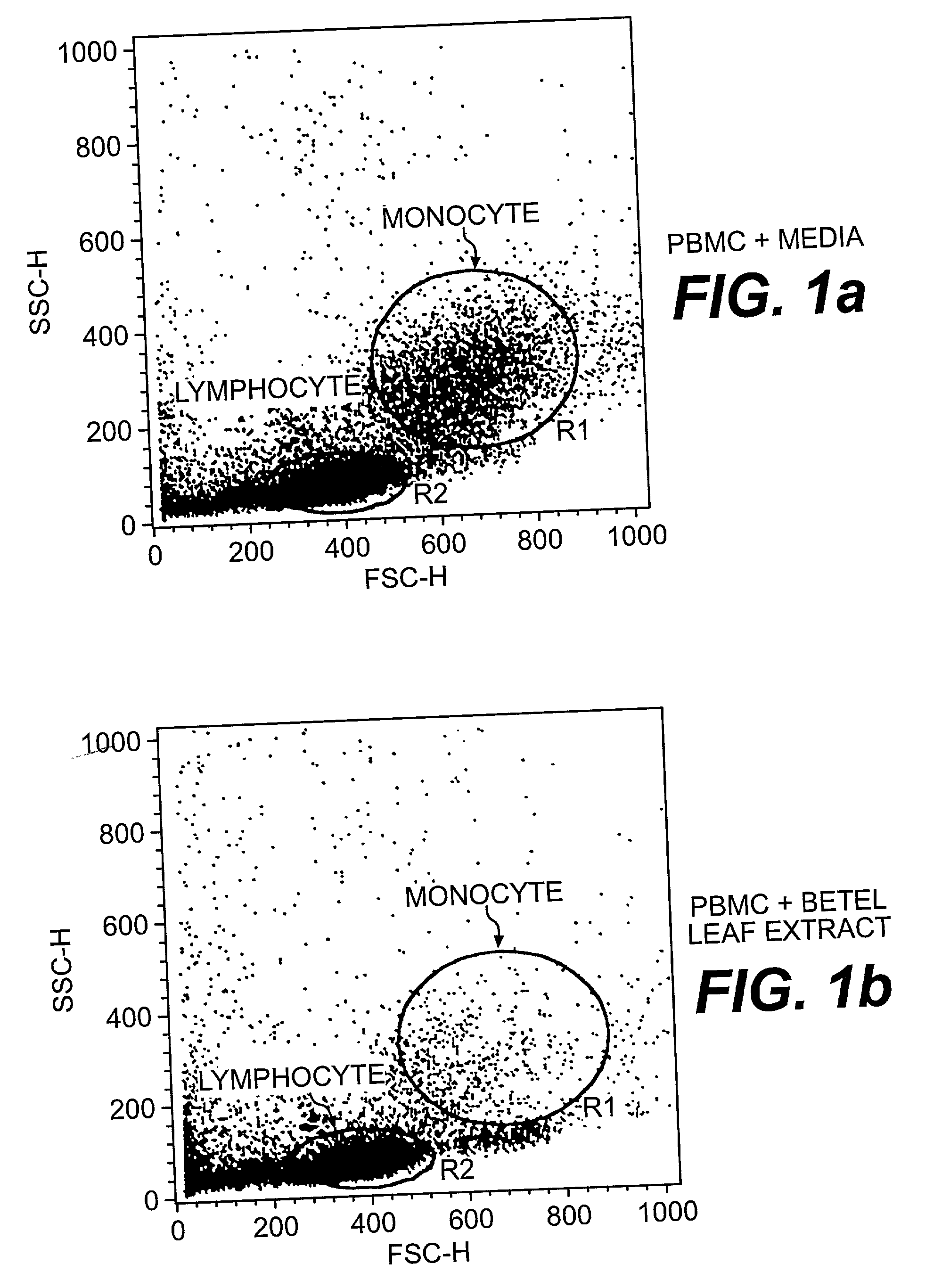
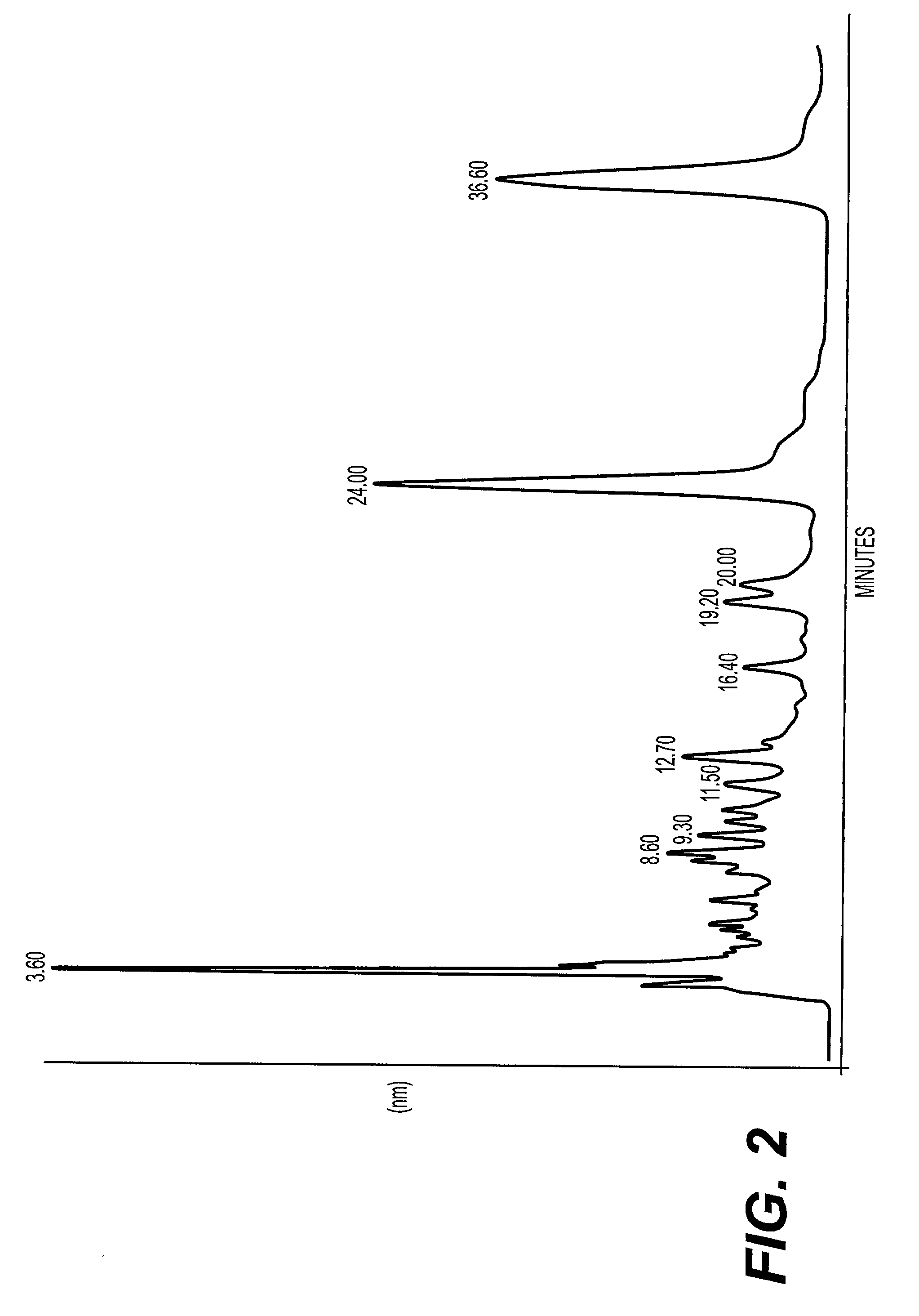
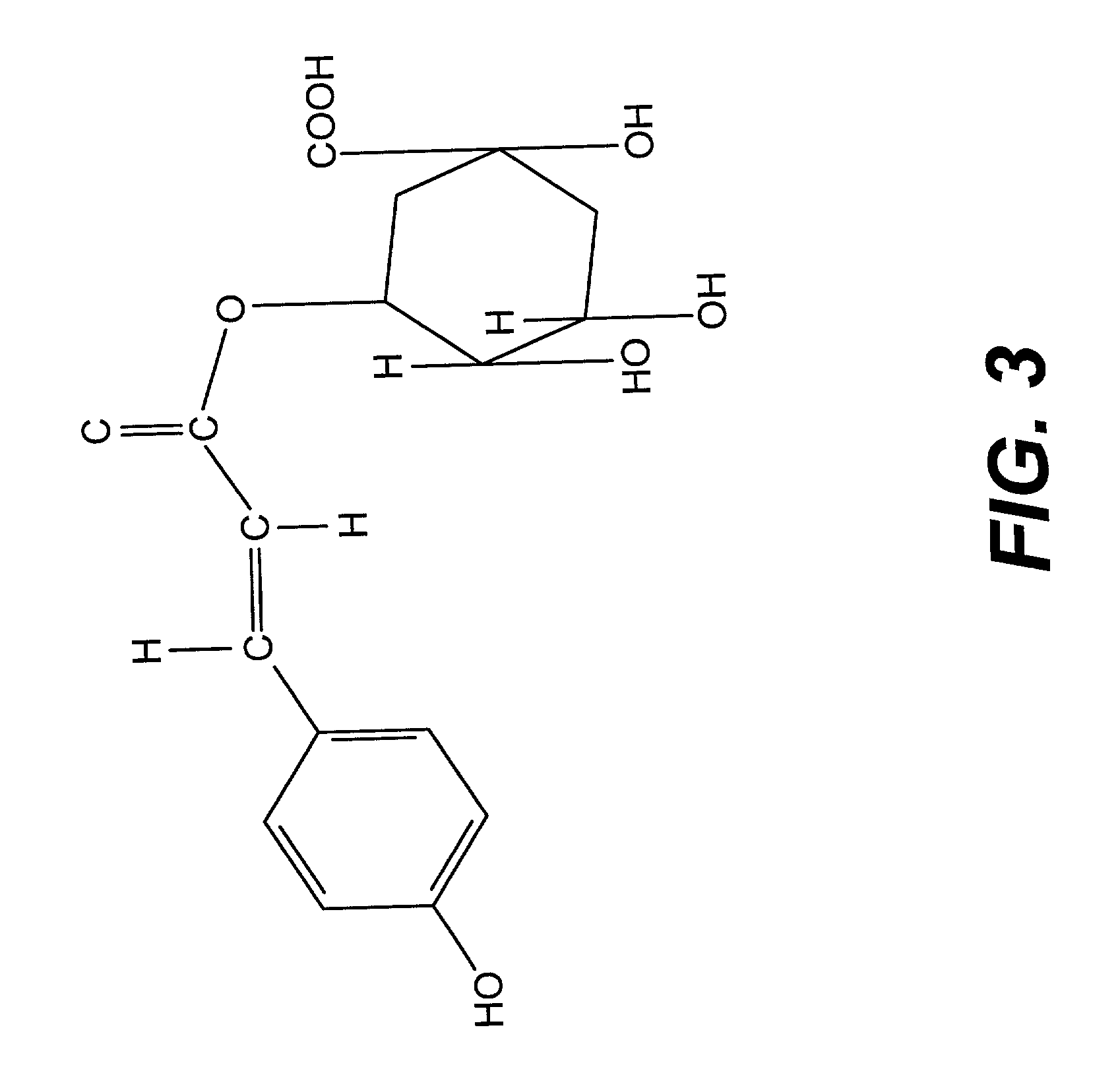
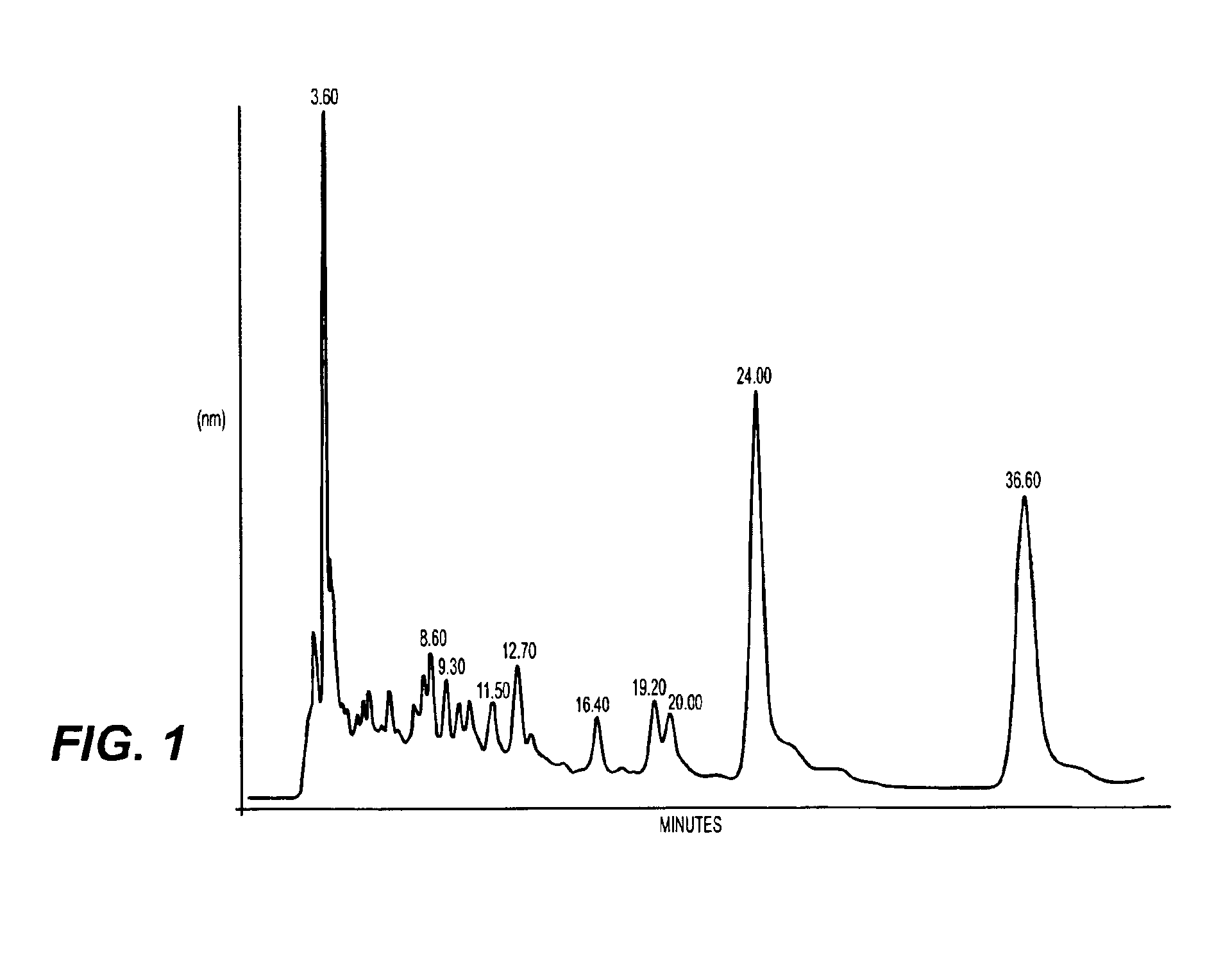
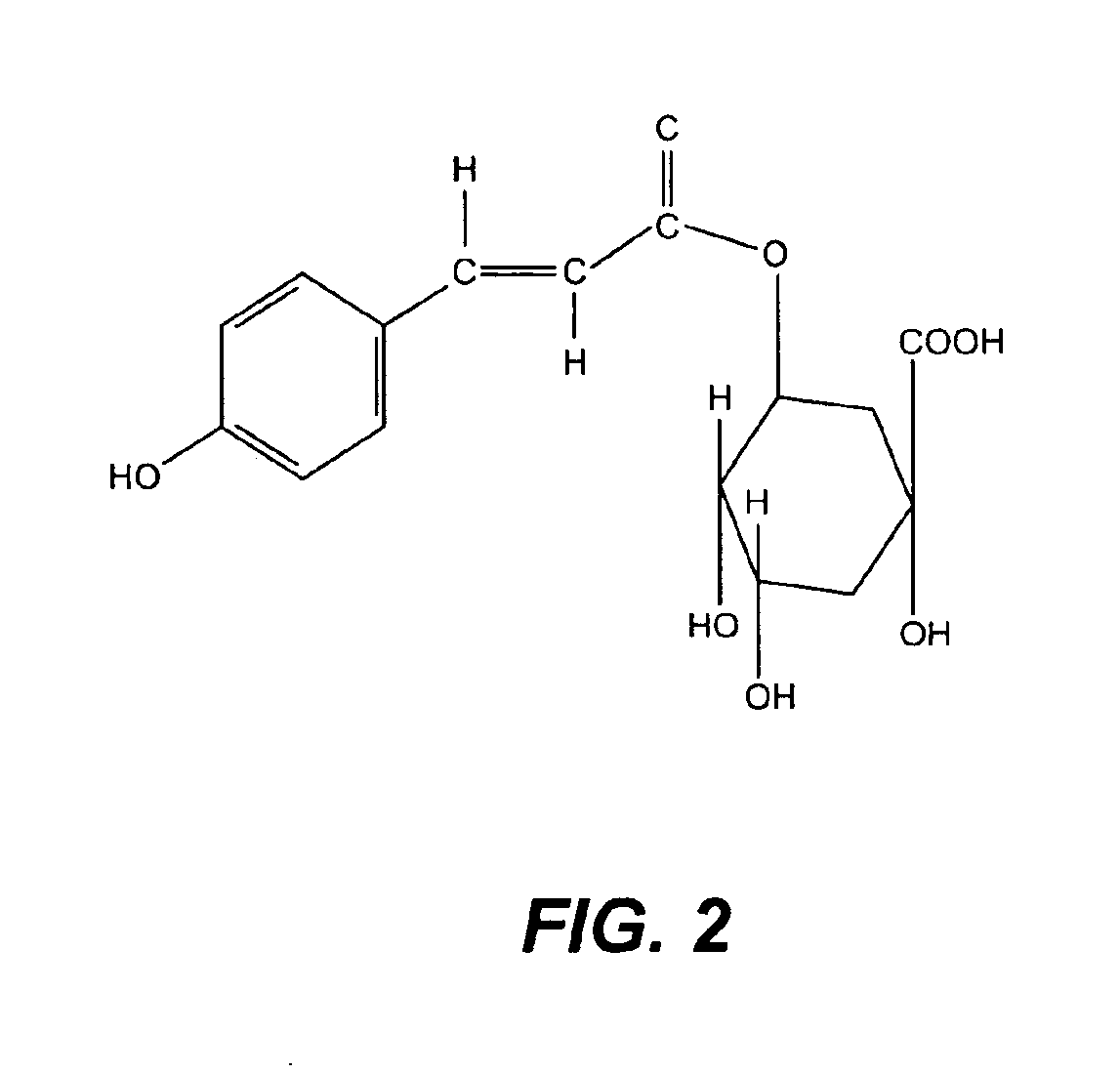
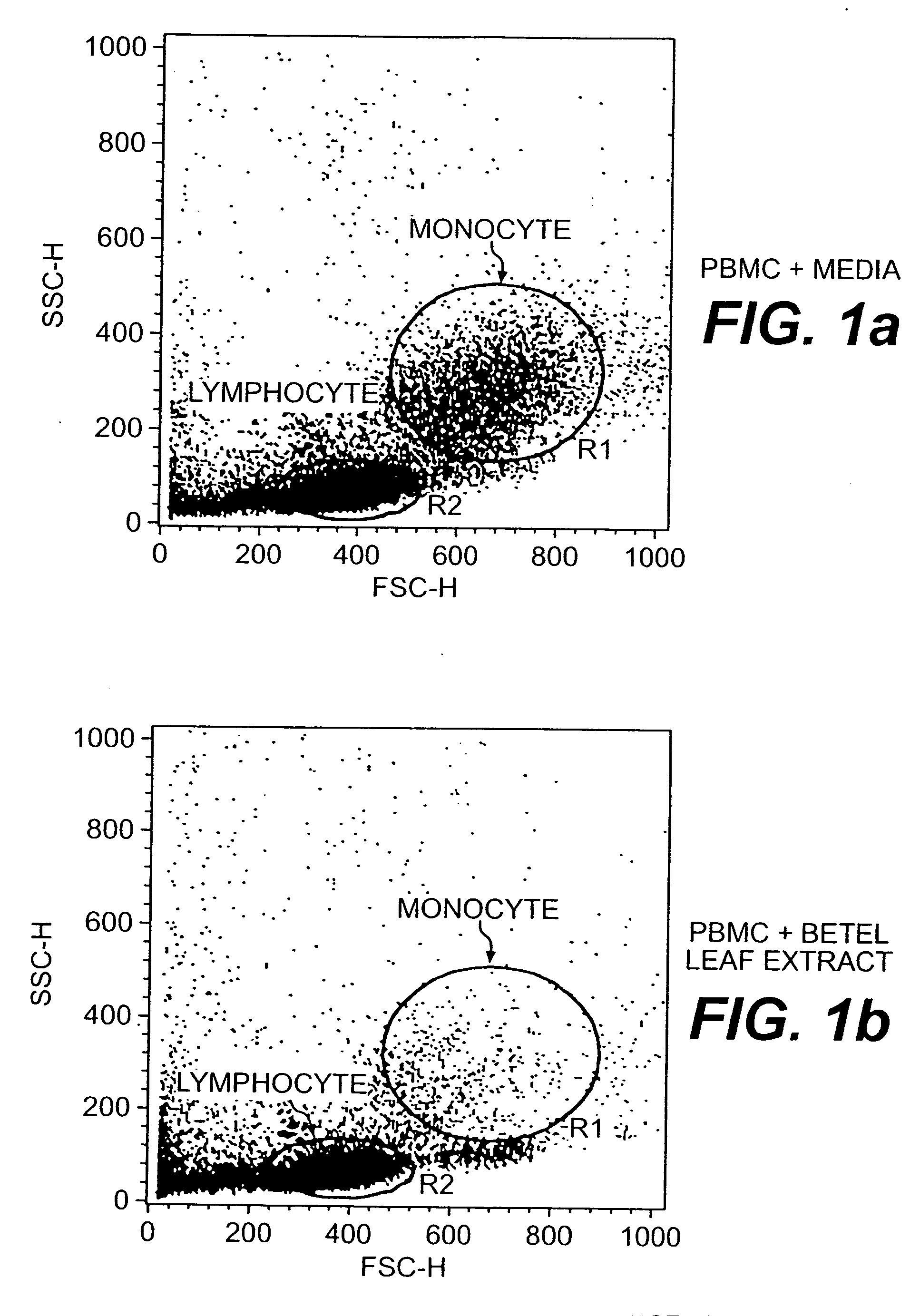
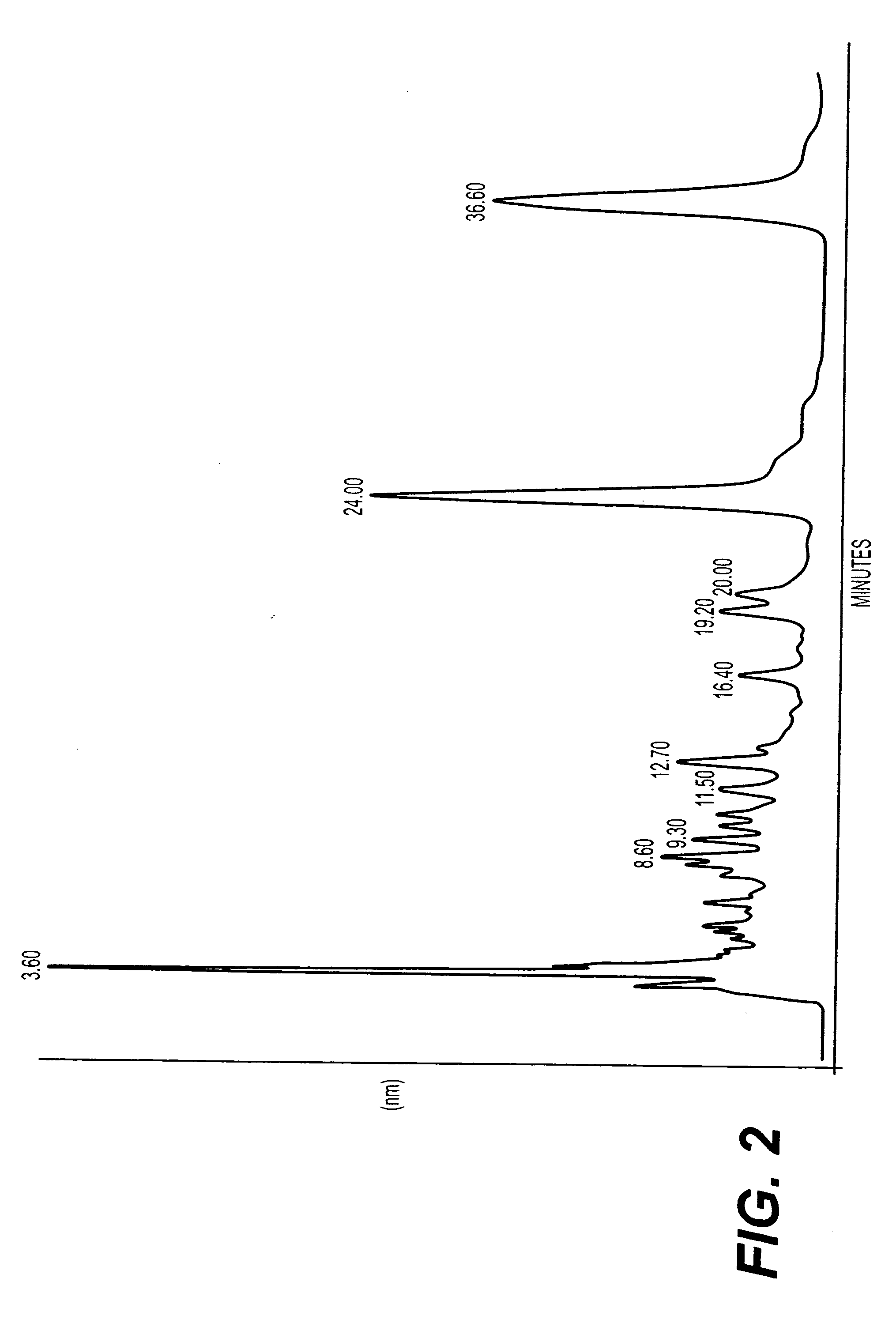
A method of treating CD33+ acute and chronic myeloid leukemia in animals including humans, using fraction nos. 1 and 9 obtained from water:methanol fraction by column chromatography, with ratio of water and methanol ranging between 1:5 to 5:1, wherein said water:methanol fraction is obtained from the polar extract of Piper betel by HPLC, with retention time of 3.6 and 24.0 minutes respectively, with the fractions used both individually, and in combination, and a composition including the fraction nos. 1 and 9.
Owner:COUNCIL OF SCI & IND RES INDIAN REGISTERED BODY INC UNDER THE REGISTRATION SOCIES ACT ACT XXI OF 1860
Combined reagent for detecting acute myelocytic leukemia cells and system thereof
ActiveCN109655616AWide coverageThere is no problem of reciprocal inhibition of expressionMaterial analysisCD33CD15
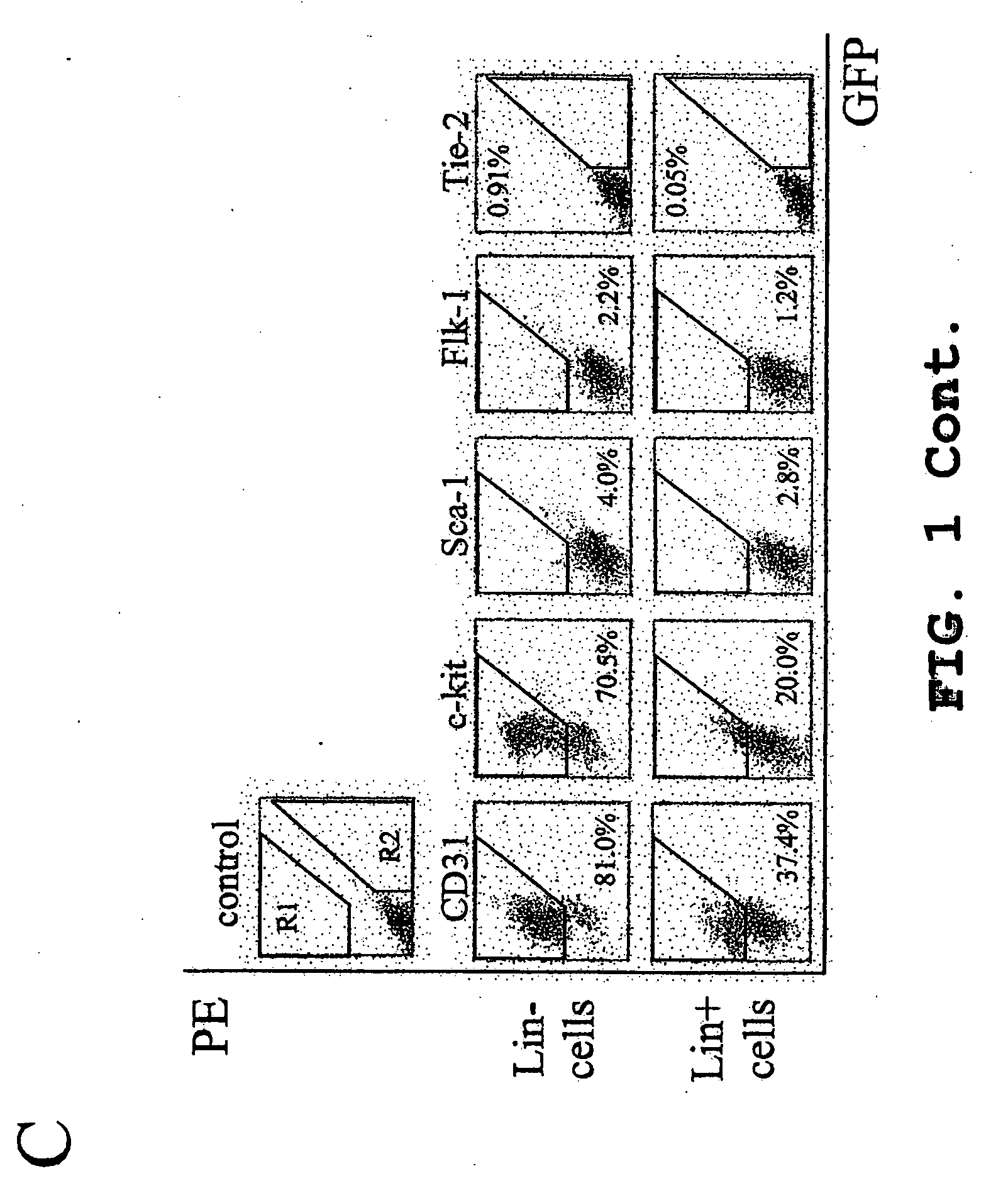
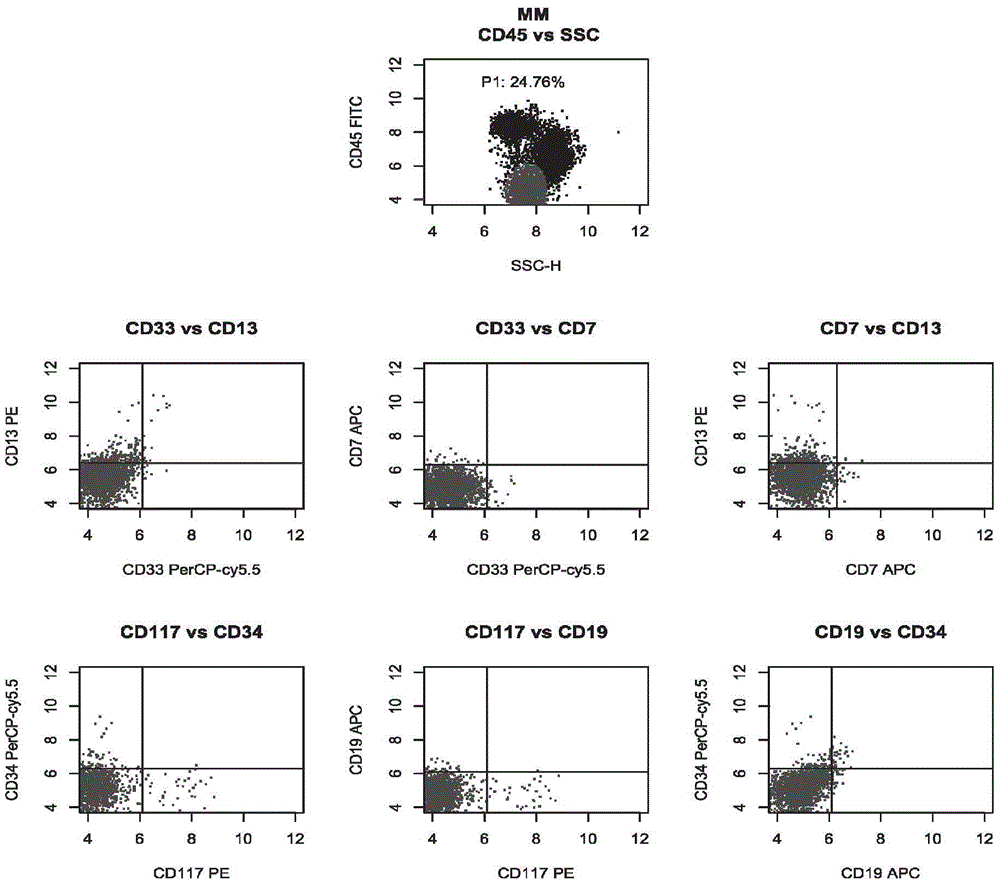
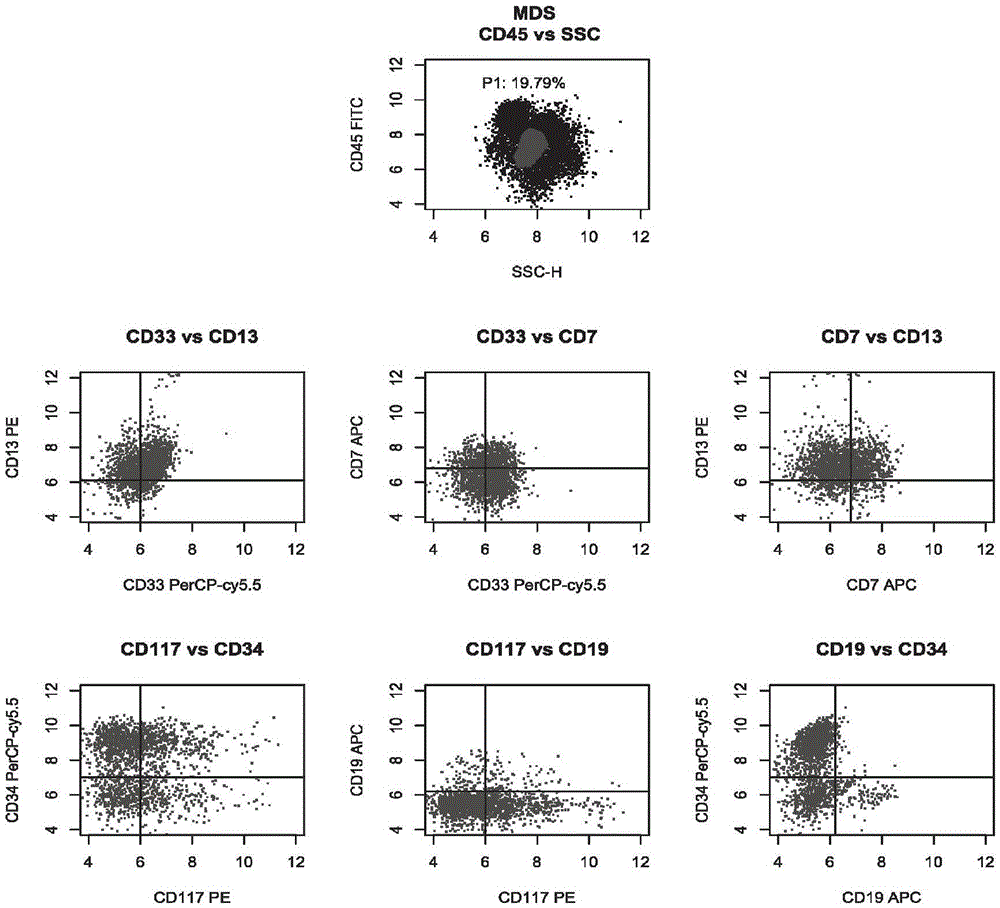
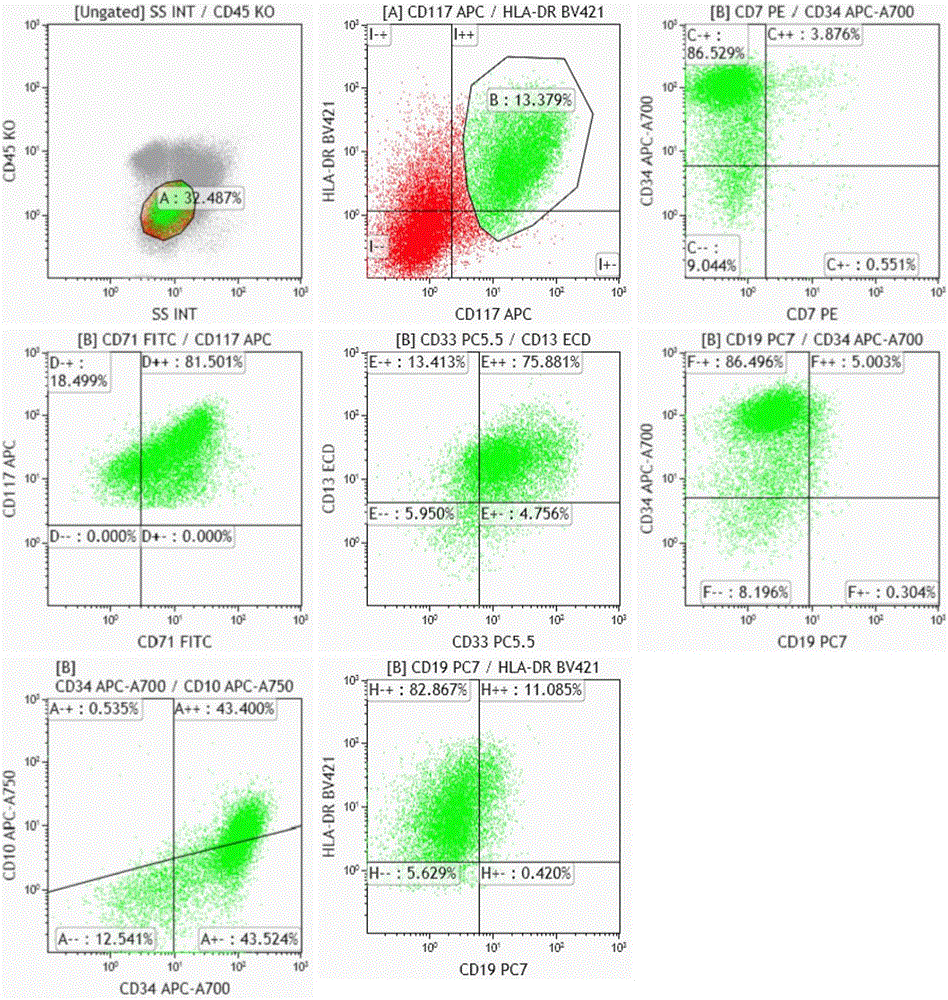
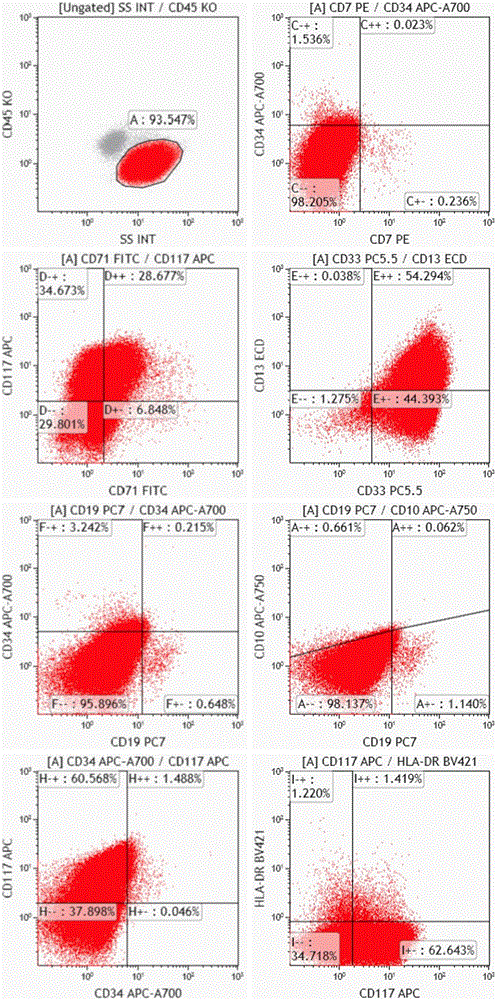
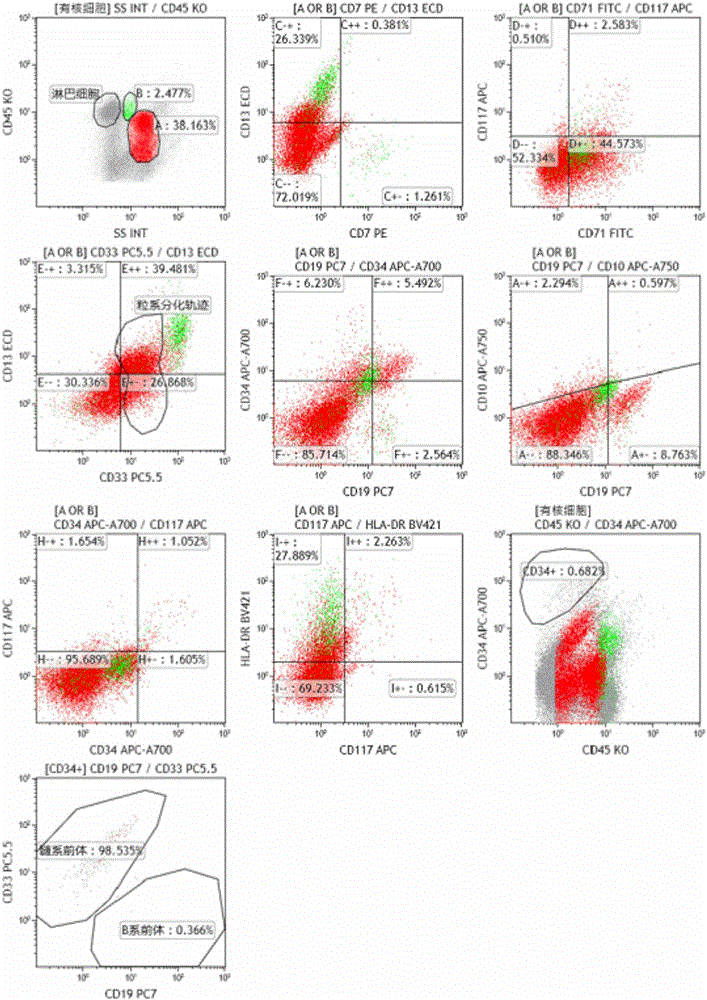
The invention relates to a combined reagent for detecting acute myelocytic leukemia cells and a system thereof, wherein the combined reagent and the system thereof belong to the field of medical technology. The combined reagent comprises at least one selected from the following antibody combinations: a first antibody combination which comprises CD38, CD13, CD34, CD117, CD33, CD19, HLA-DR and CD45antibodies; a second antibody combination which comprises CD38, CD64, CD34, CD123, CD56, CD14, HLA-DR and CD45 antibodies; and a third antibody combination which comprises CD38, CD7, CD34, CD5, CD11b,CD15 and CD45 antibodies. The antibody combinations of the invention cover the expression marks of three systems of granulocyte, single cell and lymphocyte. A normal antibody expression mode is established. Tumor cells can be identified maximally. Furthermore, through a large number of experiment data, the antibodies in each combination have no problem of mutual expression inhibition. FurthermoreAML-MRD can be comprehensively and quickly detected with high sensitivity through multi-parameter flow type cell analysis.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Compositions and methods related to therapeutic cell systems for tumor growth inhibition
InactiveUS20190160102A1Impairing synthesisReduce concentrationHydrolasesAntibody mimetics/scaffoldsCD33Degradative enzyme
The disclosure provides, e.g., enucleated erythroid cells comprising an amino acid degradative enzyme such as asparaginase and a targeting moiety such as an anti-CD33 antibody molecule. The cells may be used, e.g., to treat cancers such as AML.
Owner:RUBIUS THERAPEUTICS
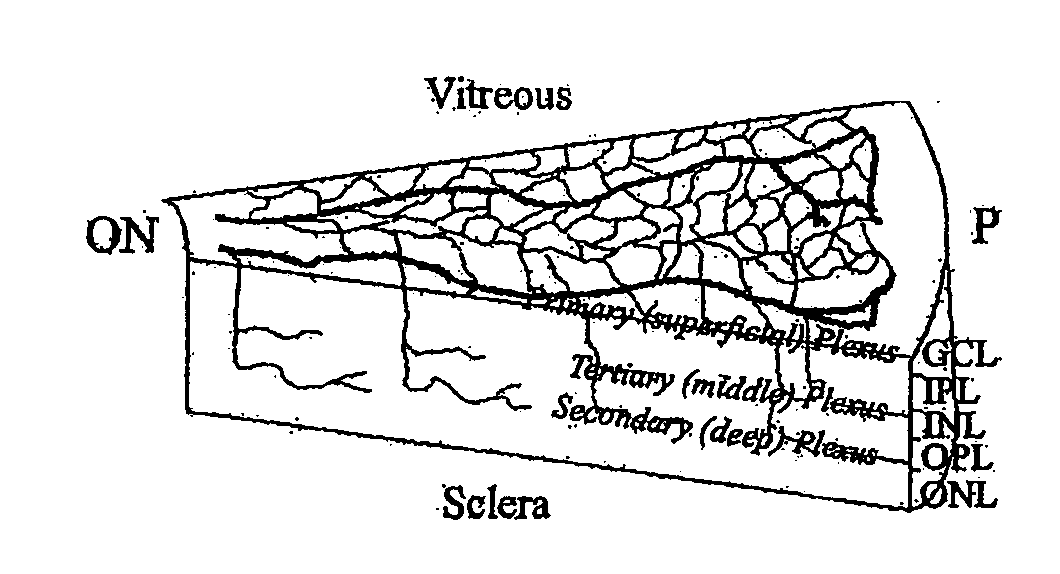
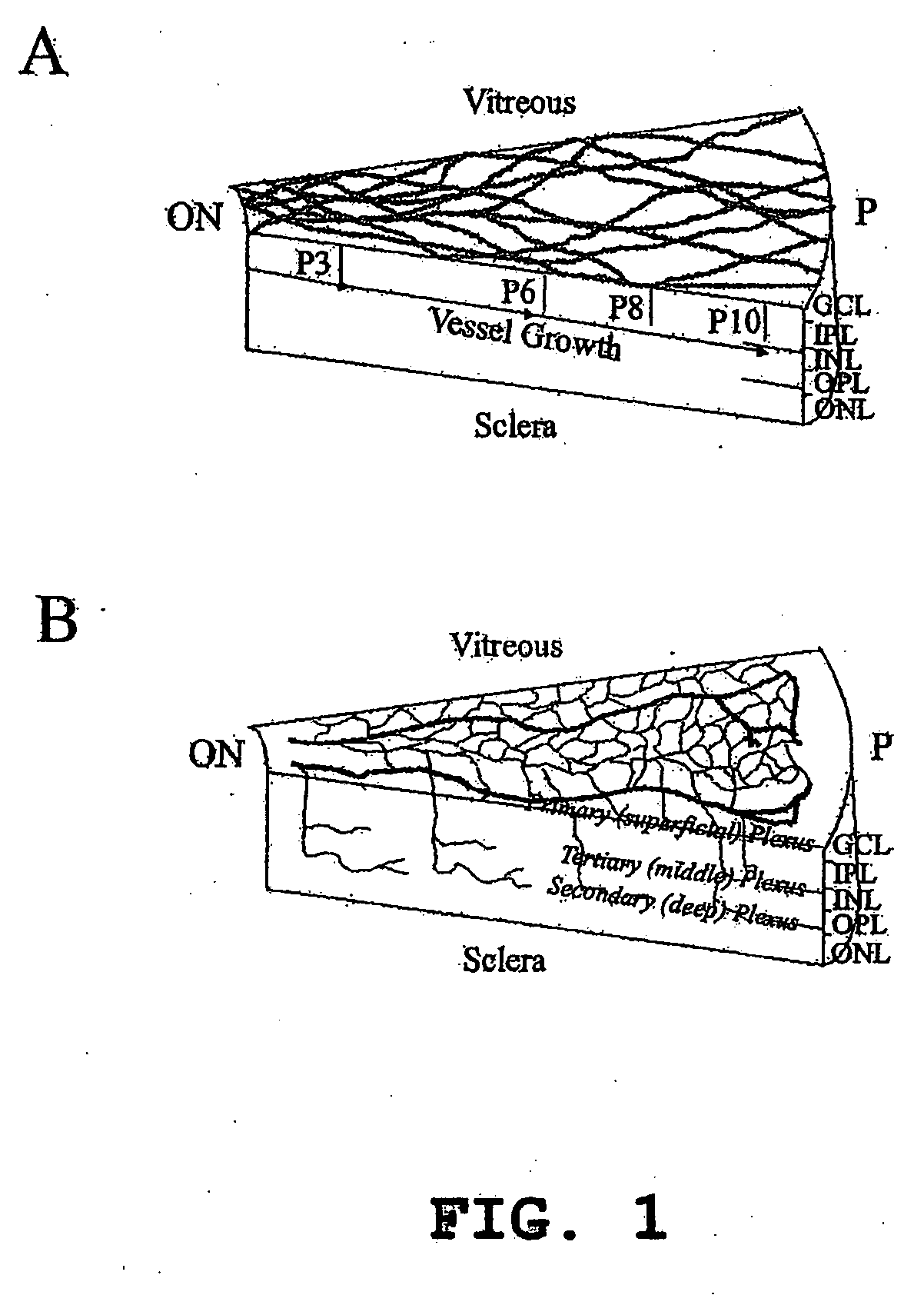
Isolated myeloid-like cell populations and methods of treatment therewith
The present invention provides an isolated myeloid-like cell population comprising a majority of cells that are lineage negative, and which express both CD44 antigen, CD11b antigen, and hypoxia inducible factor 1α (HIF-1α). These cells have beneficial vasculotrophic and neurotrophic activity when intraocularly administered to the eye of a mammal, particularly a mammal suffering from an ocular degenerative disease. The myeloid-like cells are isolated by treating bone marrow cells, peripheral blood cells or umbilical cord cells with an antibody against CD44 (hyaluronic acid receptor), against CD11b, CD14, CD33, or against a combination thereof and using flow cytometry to positively select CD44 and / or CD11b expressing cells therefrom. The isolated myeloid-like bone marrow cells of the invention can be transfected with a gene encoding a therapeutically useful protein, for delivering the gene to the retina.
Owner:THE SCRIPPS RES INST
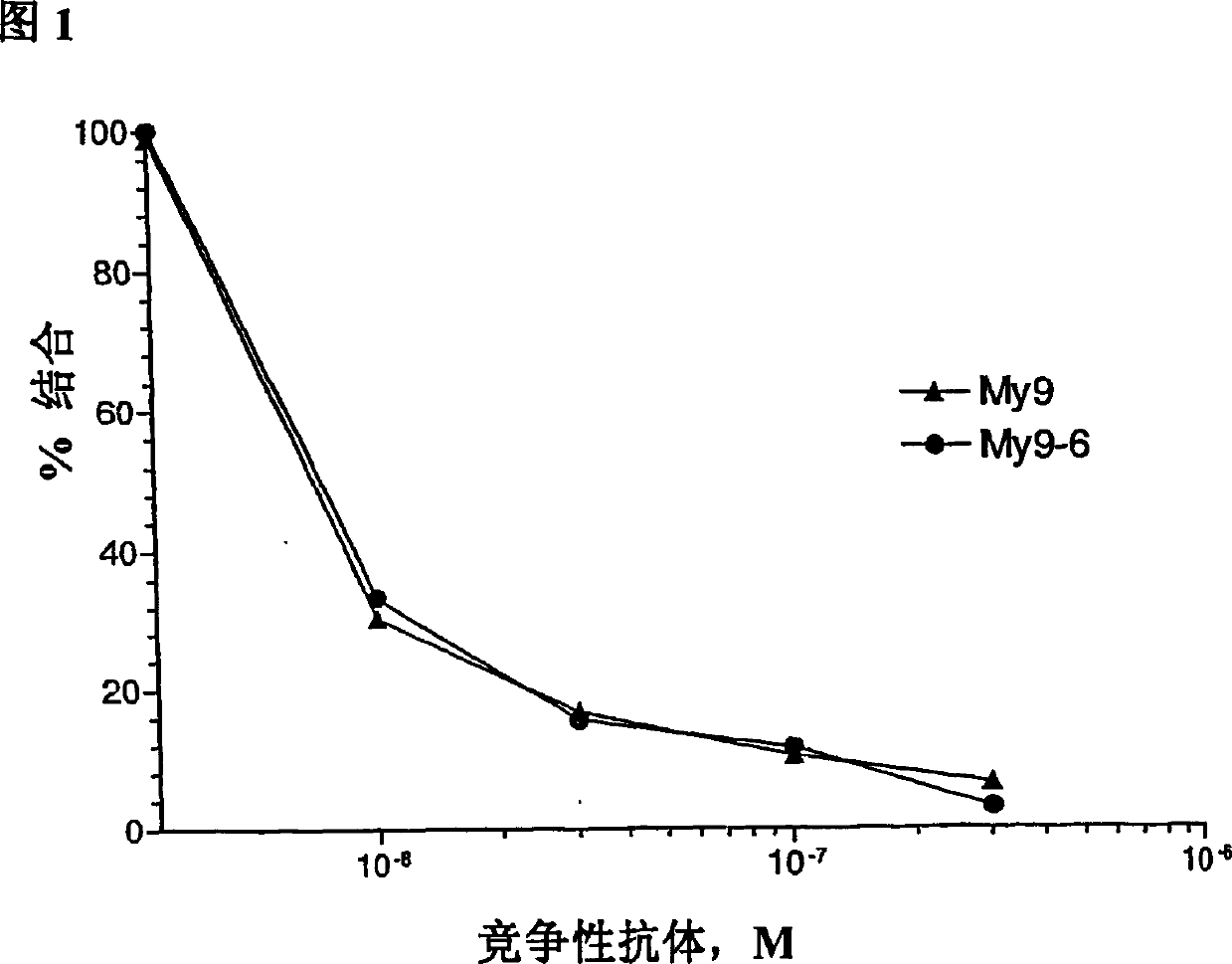
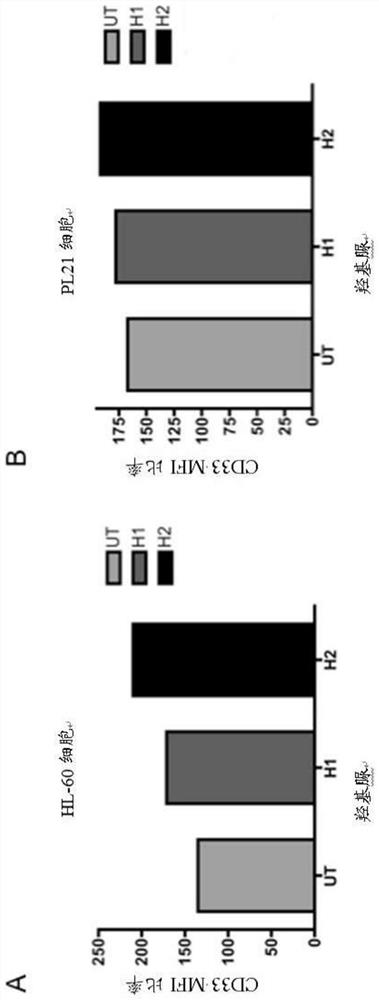
Anti-CD33 engineering antibody for target conjugated marrow series leukemia cell and its expression vector and use
InactiveCN101210048ALow immunogenicityIncrease distribution rateImmunoglobulins against animals/humansAntibody ingredientsEscherichia coliMyeloid leukemia
The invention discloses an engineered antibody that is used in targeting combination with combining myeloid leukemia cells to resist CD33 and the expression vector and application thereof, which comprises SEQ ID NO.3 heavy-chain variable region amino acid sequence and SEQ ID NO.4 light-chain variable region amino acid sequence. By applying RT-PCR technology, the light and heavy chain variable genes that resist CD33 resisting antibody is cloned successfully from hybridoma and light and heavy chain variable genes that resists CD33 antibody is cloned to prokaryotic expression vector to establish single-chain antibody that resists CD33 resisting antibody and the immunogenicity of mouse original CD33 resisting antibody is lowered, so as to be expressed efficiently in colibacillus, thus improving the output thereof and lowering the producing cost.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
Duplex-specific chimeric antigen receptor molecule and application thereof to tumor therapy
ActiveCN109503716AIncreased persistenceImprove targetingPeptide/protein ingredientsMammal material medical ingredientsSequence signalCD20
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
Herbal composition for treating CD33+ acute and chronic myeloid leukemia and a method thereof
InactiveUS20030049334A1Simple methodReduced viabilityBiocideOrganic active ingredientsMyeloid leukemiaMedicine
The present invention relates to a method of treating CD33+ acute and chronic myeloid leukemia in animals including humans, using fraction nos. 1 and 9 obtained from water:methanol fraction by column chromatography, with ratio of water and methanol ranging between 1:5 to 5:1, wherein said water:methanol fraction is obtained from the polar extract of piper betel by HPLC, with retention time of 3.6 and 24.0 minutes respectively, with said fractions used both individually, and in combination, and a composition comprising the said fraction nos. 1 and 9.
Owner:COUNCIL OF SCI & IND RES INDIAN REGISTERED BODY INC UNDER THE REGISTRATION SOCIES ACT ACT XXI OF 1860
Anti-CD33 chimeric antigen receptor, coding gene, recombinant expression vector and construction method and application of recombinant expression vector
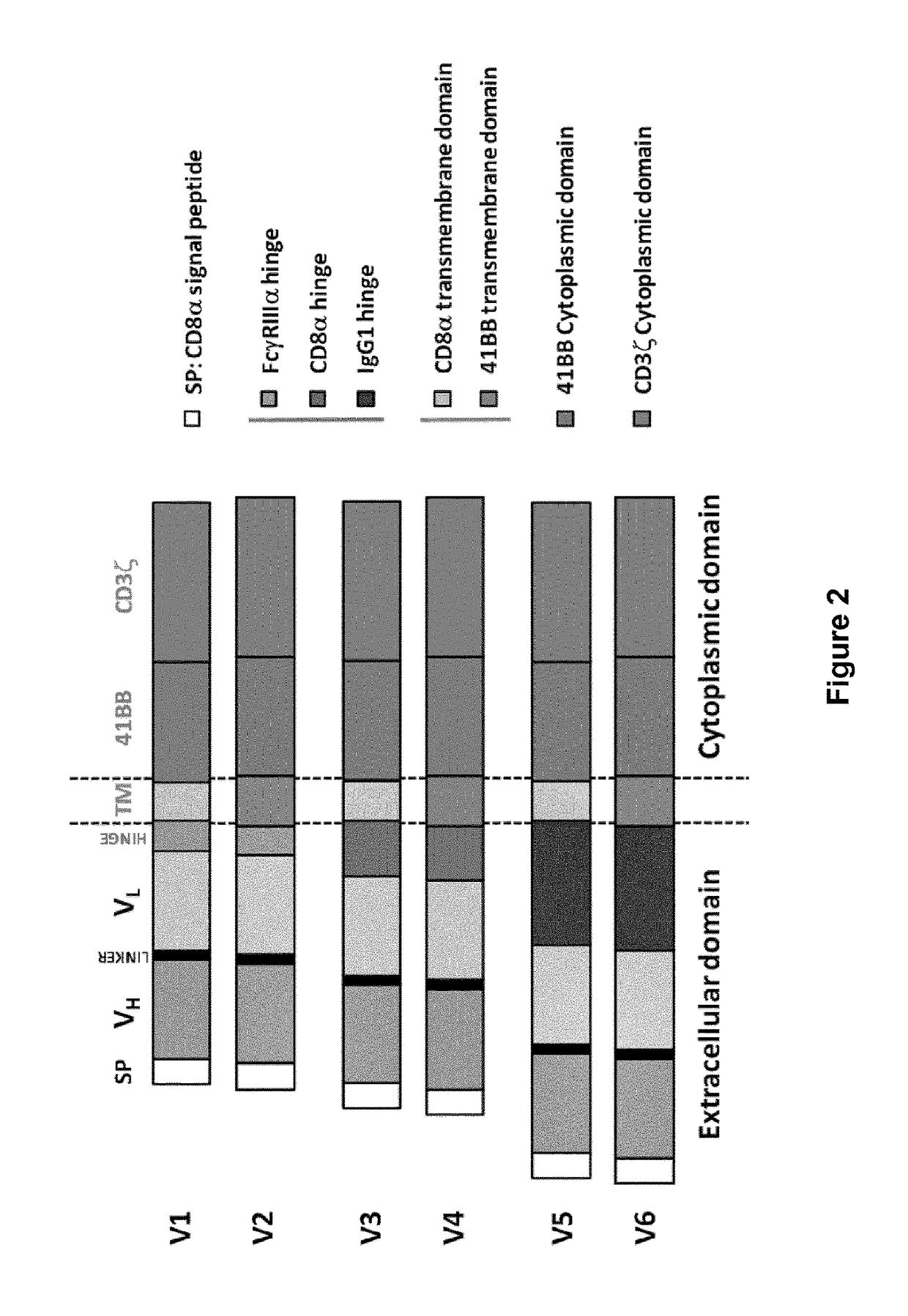
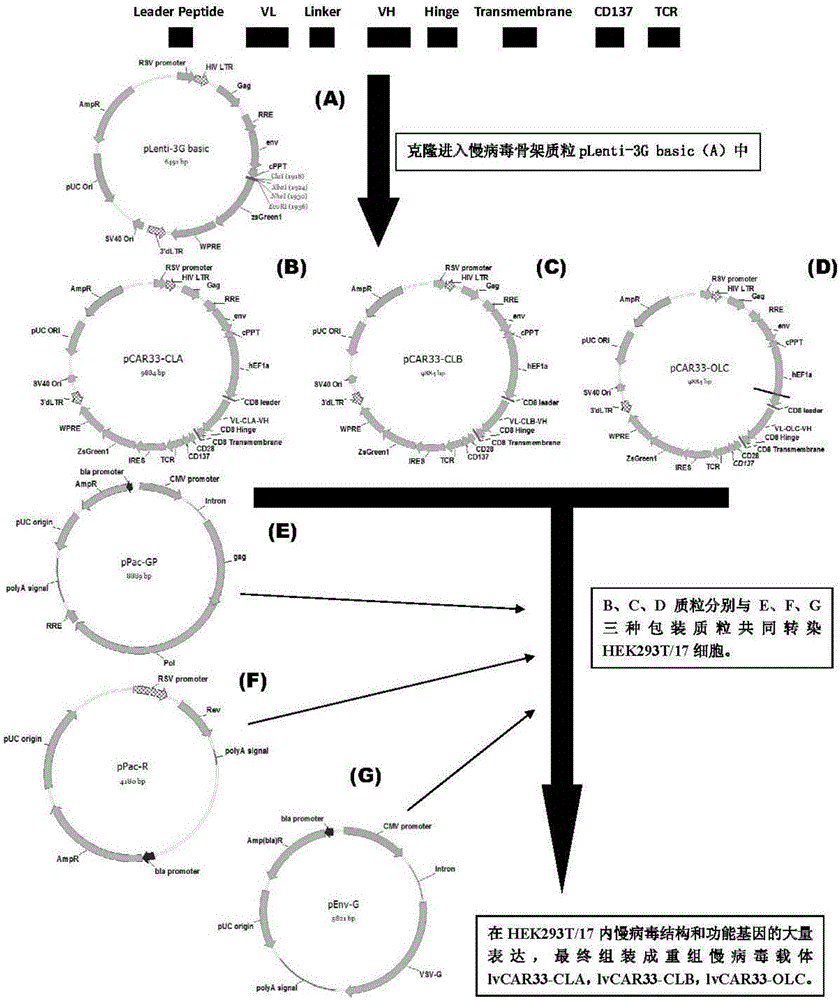
ActiveCN105820255AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsMammal material medical ingredientsSingle-Chain AntibodiesCD33
The invention discloses an anti-CD33 chimeric antigen receptor, a coding gene, a recombinant expression vector and a construction method and an application of the recombinant expression vector. The anti-CD33 chimeric antigen receptor comprises a CD8leader chimeric receptor signal peptide, a heavy chain VL of a CD33 single-chain antibody, an Optimal Linker C, a light chain VH of the CD33 single-chain antibody, a CD8Hinge chimeric receptor hinge, a CD8Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulation factor and a TCR chimeric receptor T-cell activation domain, which are serially connected in sequence. In addition, the invention discloses the coding gene of the anti-CD33 chimeric antigen receptor, the recombinant expression vector and the construction method and the application of the recombinant expression vector. According to the anti-CD33 chimeric antigen receptor, the secretion of cell factors and the in vitro killing effect of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Antibody composition and application of antibody composition in leukemia and lymphoma typing
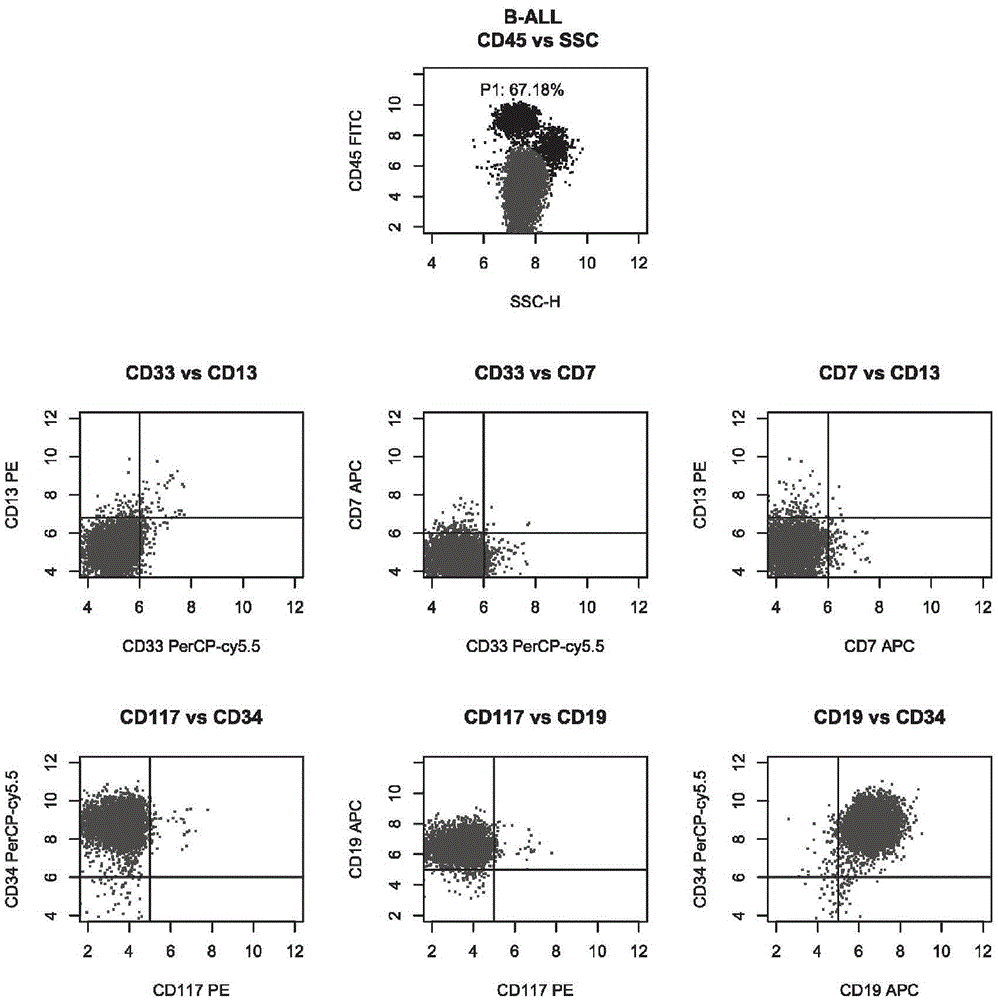
ActiveCN105606797AOptimizing Fluorescent Labeling CombinationsSimple methodMaterial analysisTypingCD33
The invention belongs to the technical field of antibody medicines and provides two types of antibody compositions including CD45 antibody, CD13 antibody, CD33 antibody and CD7 antibody or including CD45 antibody, CD117 antibody, CD34 antibody and CD19 antibody. The invention further provides a leukemia and lymphoma typing kit comprising the two types of antibody compositions and capable of being used with a flow cytometry to achieve typing of various hematological neoplasms.
Owner:ZHEJIANG BOZHEN BIOTECH CO LTD
Treatment of cancer using a cd33 chimeric antigen receptor
Owner:NOVARTIS AG +1
Herbal-based composition for treating acute and chronic myeloid leukemia
A new herbal-based composition and method for treatment of CD33+ acute and chronic myeloid leukemia by Piper betel leaf extracts, and to provide a process for the isolation of active fractions from leaves or any other plant parts of Piper betel to treat CD33+ AML and CML with a simplified method of isolation of active components from all plant parts of Piper betel possessing biological activities relevant to the treatment of CD33+ AML and CML.
Owner:COUNCIL OF SCI & IND RES
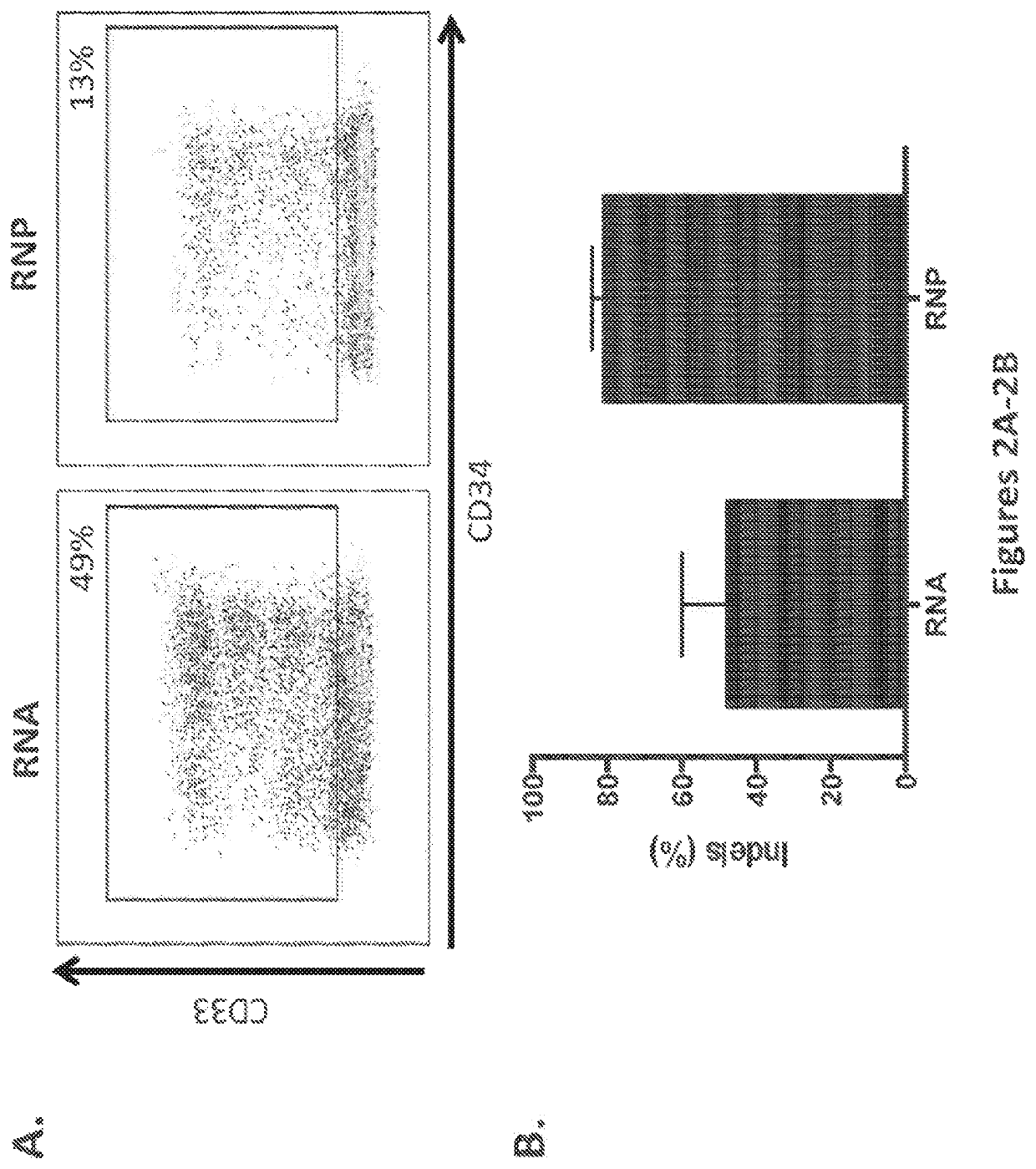
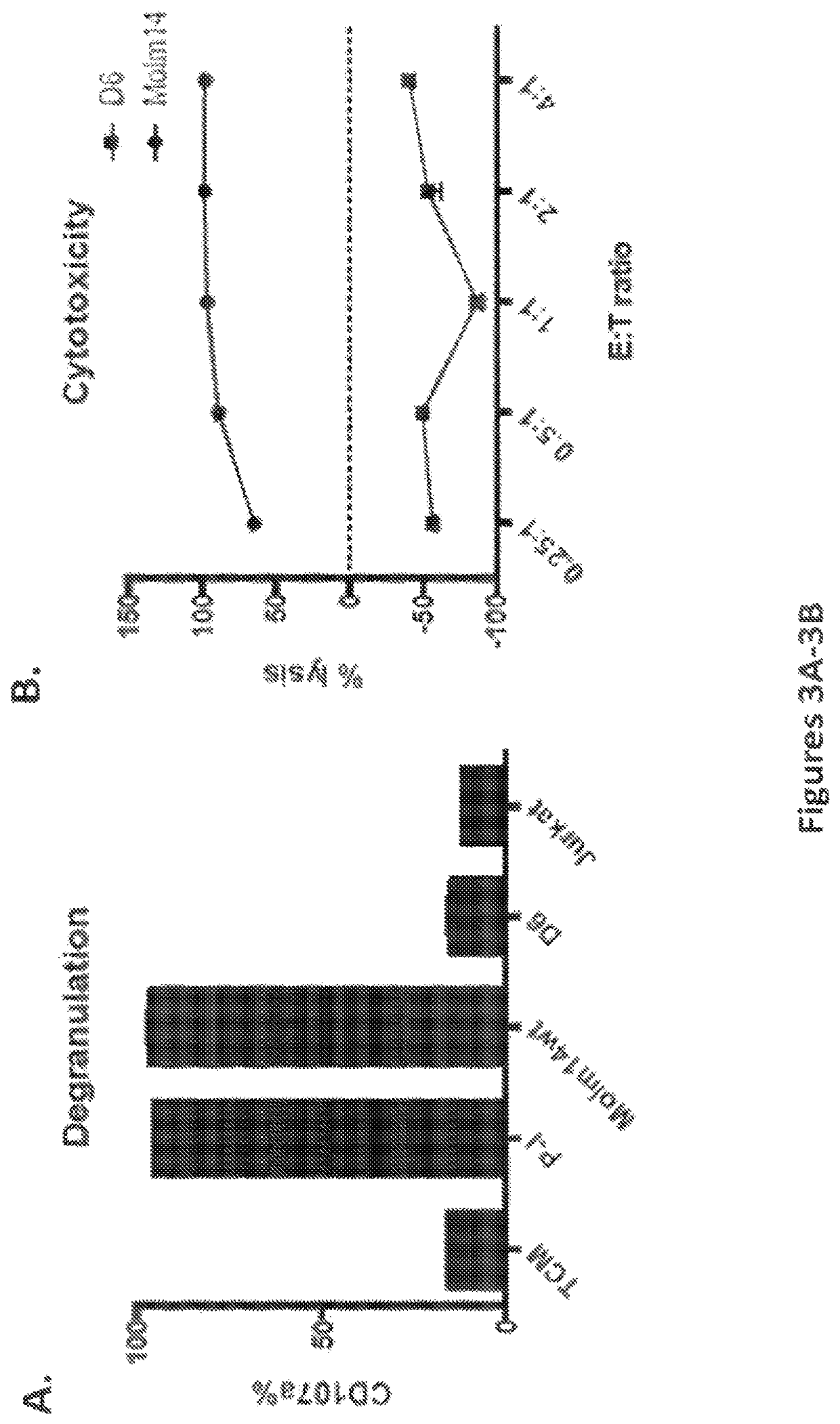
Methods and compositions for gene editing in hematopoietic stem cells
The present invention relates to compositions and methods of generating modified hematopoietic stem or progenitor cells. One aspect of the invention includes a modified hematopoietic stem or progenitor cell comprising a nucleic acid capable of decreasing expression of an endogenous gene or a portion thereof, wherein the endogenous gene encodes a polypeptide comprising an antigen domain targeted by a chimeric antigen receptor (CAR). Another aspect of the invention includes a method for generating a modified hematopoietic stem or progenitor cell. Also included are methods and pharmaceutical compositions comprising the modified cell for adoptive therapy and treating a condition, such as an autoimmune disease or cancer. In certain embodiments, the invention includes a modified hematopoietic stem or progenitor cell comprising a nucleic acid capable of decreasing expression of CD33. In certain embodiments, the invention includes compositions and methods for treating cancer comprising administering CD33 CAR-T cells and modified hematopoietic stem or progenitor cells that are resistant to CD33-targeted therapy.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
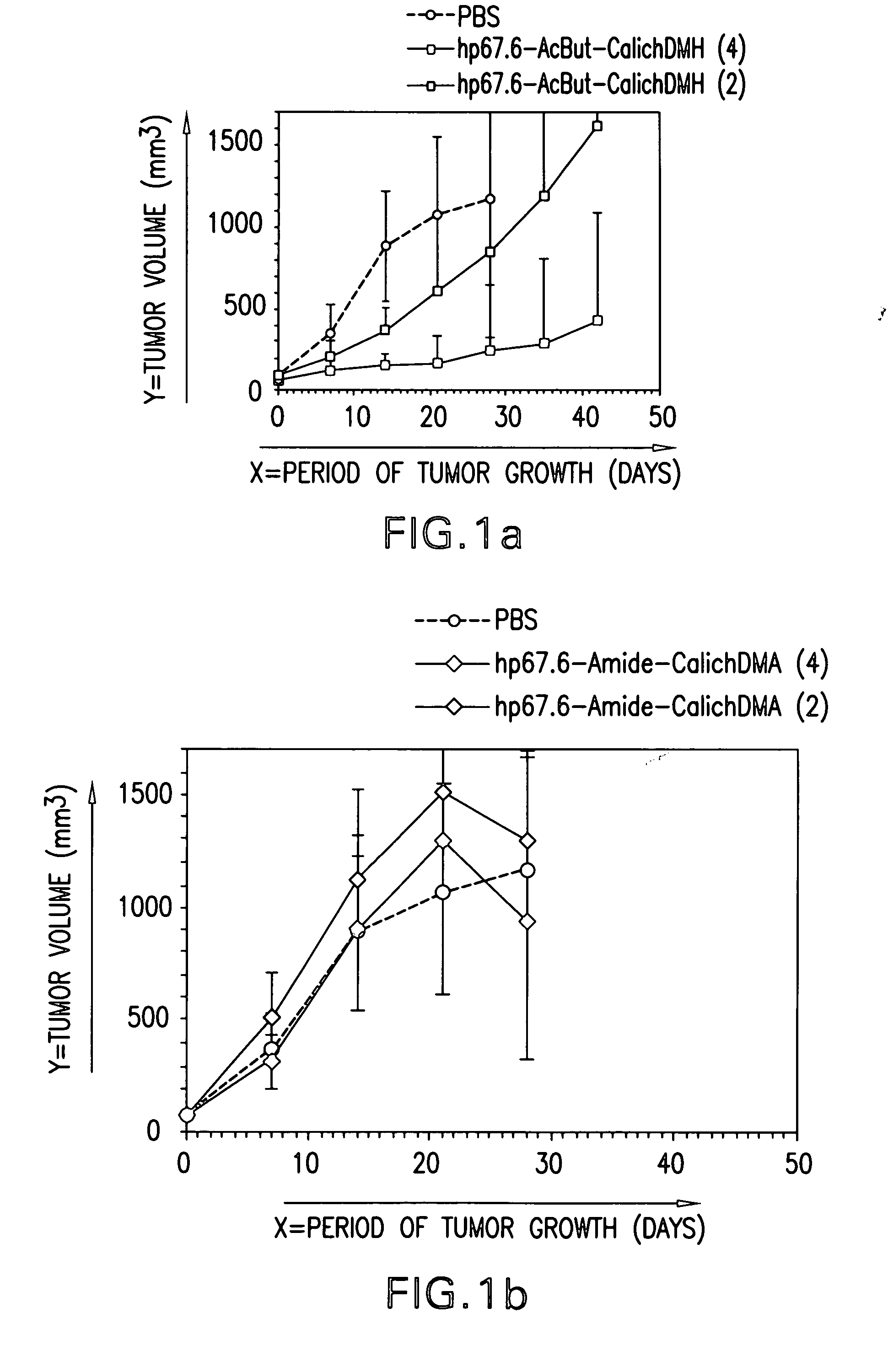
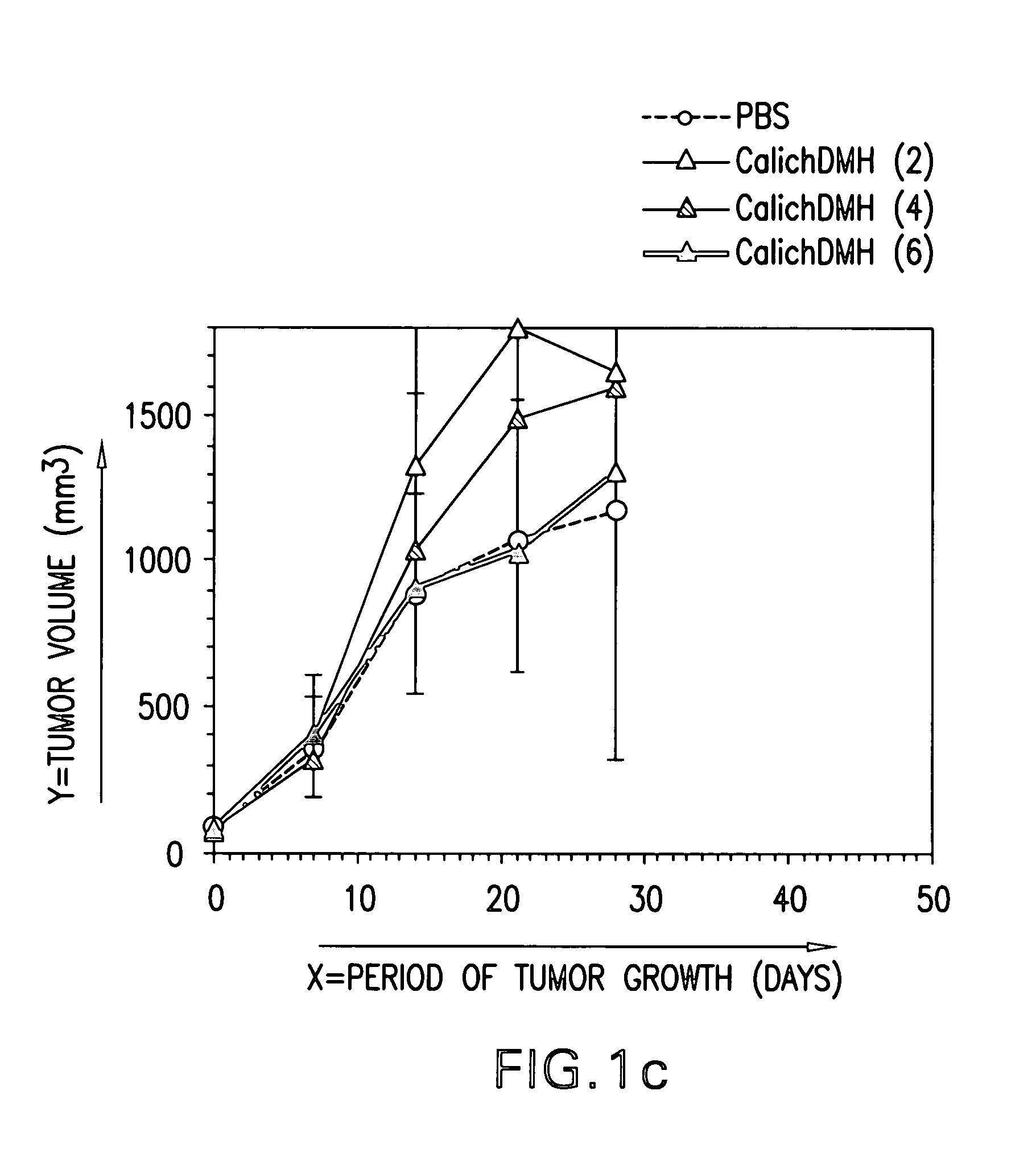
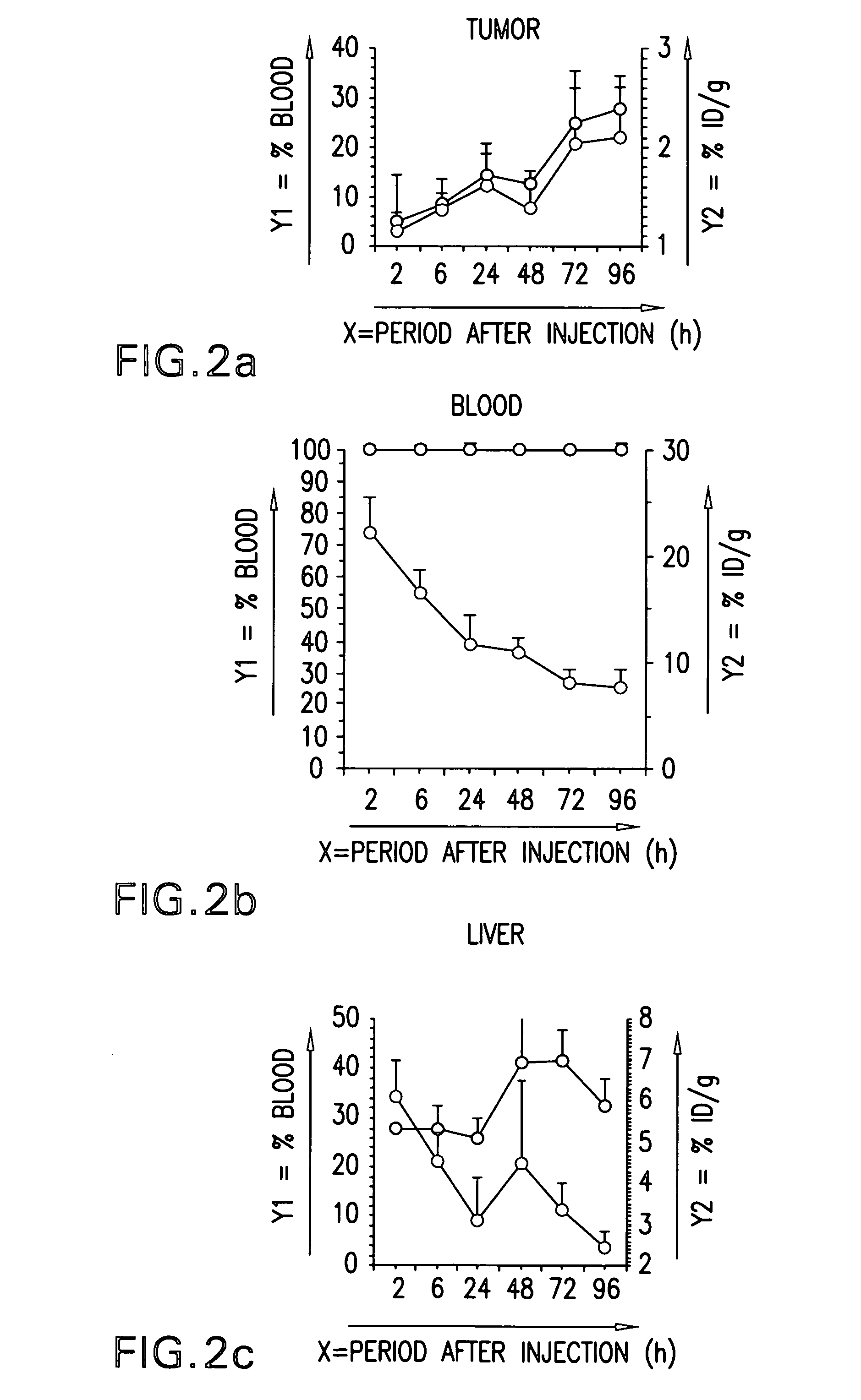
Passive targeting of cytotoxic agents
InactiveUS20070190060A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsProstate cancer cellAnticarcinogen
The present invention provides methods of treating cancer cells comprising administering to a patient in need thereof a therapeutically effective amount of a non-specific antibody conjugated to a cytotoxin, wherein the cancer cells do not express an antigen to which the non-specific antibody binds. In one embodiment, the non-specific antibody is an anti-CD33 antibody (e.g., hp67.6), an anti-CD22 antibody (e.g., g5 / 44), or an anti-CD20 antibody (e.g., rituximab). In another embodiment, the non-specific antibody does not bind a human antigen. The cancer cells treated can be, e.g., gastric, colon, non-small cell lung (NSCLC), breast, epidermoid, or prostate carcinoma cells. In one embodiment, the cytotoxin is calicheamicin. Calicheamicin can be conjugated to the non-specific antibody using a 4-(4′-acetylphenoxy)butanoic acid (AcBut) or (3-Acetylphenyl)acetic acid (AcPAc) linker. In another embodiment, the antibody to the non-specific antigen conjugated to a cytotoxin is administered in combination with a bioactive agent, e.g., an anti-cancer agent.
Owner:WYETH LLC
Ten-color antibody composition and application thereof in leukemia-lymphomas subtype
The invention belongs to the technical field of antibodies, and provides an antibody composition composed of ten antibodies, wherein the tan antibodies comprises CD71 resistant antibody, CD7 resistant antibody, CD13 resistant antibody, CD33 resistant antibody, CD19 resistant antibody, CD117 resistant antibody, CD34 resistant antibody, CD10 resistant antibody, HLA-DR resistant antibody, and CD45 resistant antibody. The invention provides a leukemia-lymphomas primary screening kit containing the antibodies, and the application thereof.
Owner:ZHEJIANG BOZHEN BIOTECH CO LTD
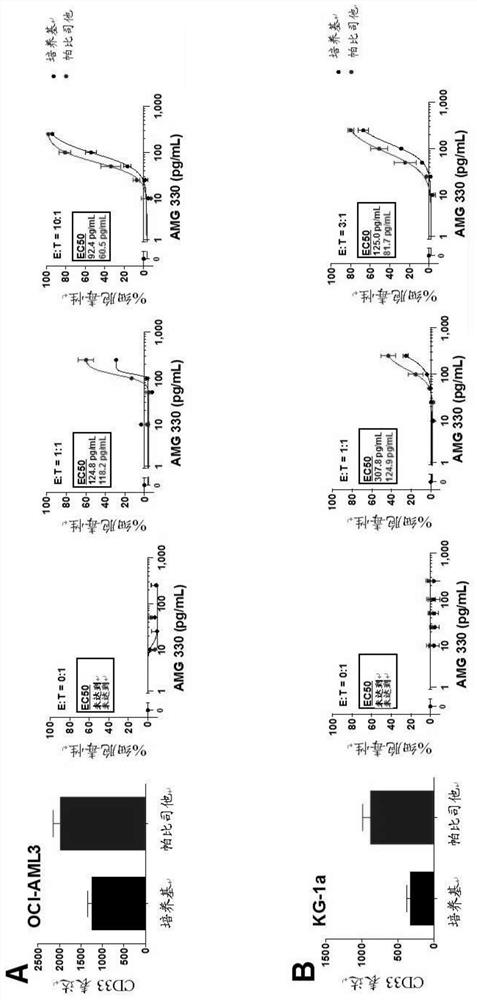
Combination of epigenetic factors and bispecific compounds targeting CD33 and CD3 in the treatment of myeloid leukemia
InactiveCN111888474AOrganic active ingredientsHybrid immunoglobulinsGranulocyte colony-stimulating factorMyeloid leukemia
Owner:AMGEN INC +1
Herbal composition for treating CD33and chronic myeloid leukemia and a method thereof
InactiveUS20050089585A1Simple methodReduced viabilityOrganic active ingredientsBiocideMyeloid leukemiaMedicine
The present invention relates to a method of treating CD33+ acute and chronic myeloid leukemia in animals including humans, using fraction nos. 1 and 9 obtained from water:methanol fraction by column chromatography, with ratio of water and methanol ranging between 1:5 to 5:1, wherein said water:methanol fraction is obtained from the polar extract of piper betel by HPLC, with retention time of 3.6 and 24.0 minutes respectively, with said fractions used both individually, and in combination, and a composition comprising the said fraction nos. 1 and 9.
Owner:COUNCIL OF SCI & IND RES
Anti-cd33 antibodies and methods of use thereof
ActiveUS20190002560A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsEpitopeCD33
The present disclosure is generally directed to compositions that include antibodies, e.g., monoclonal, chimeric, humanized antibodies, antibody fragments, etc., that specifically bind one or more epitopes within a CD33 protein, e.g., human CD33 or a mammalian CD33, and use of such compositions in preventing, reducing risk, or treating an individual in need thereof.
Owner:ALECTOR LLC
Cd33 antibodies and use of same to treat cancer
Owner:SEAGEN INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com