Treatment of cancer using a cd33 chimeric antigen receptor
A technology of chimeric antigen receptors and binding domains, which can be used in the fields of antibody medical components, antibodies, gene therapy, etc., and can solve problems such as poor persistence and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0520]Additional examples of CAR molecules or antibody fragments thereof are provided in Example 3. Murine and humanized forms of anti-CD33 antibody 2213 are disclosed. For example, Example 3 provides the following: 2213 nucleotide sequence of murine anti-CD33 IgG4 (SEQ ID NO: 138); 2213 CAR nucleotide sequence (SEQ ID NO: 139); 2213 CAR amino acid sequence (SEQ ID NO: 140) ; 2213 scFv nucleotide sequence (SEQ ID NO: 141); and 2213 scFv amino acid sequence (SEQ ID NO: 142); 2218 humanized anti-CD33 IgG4H nucleotide sequence (SEQ ID NO: 143).
[0521] Other embodiments disclosed in Example 3 include CAR molecules and anti-CD33 antibody fragments of Gemtuzumab ozogamicin previously marketed as Mylotarg (e.g., referred to herein as "human Humanized form as described in "My96"). The amino acid sequence of the anti-CD33 scFv of gemtuzumab ozogamicin (immunoconjugate targeting CD33) with 41BB and CD3ζ signaling domains is described in Example 3 and SEQ ID NO:145. The correspondin...
Embodiment 1
[1167] Example 1: Humanized CAR constructs
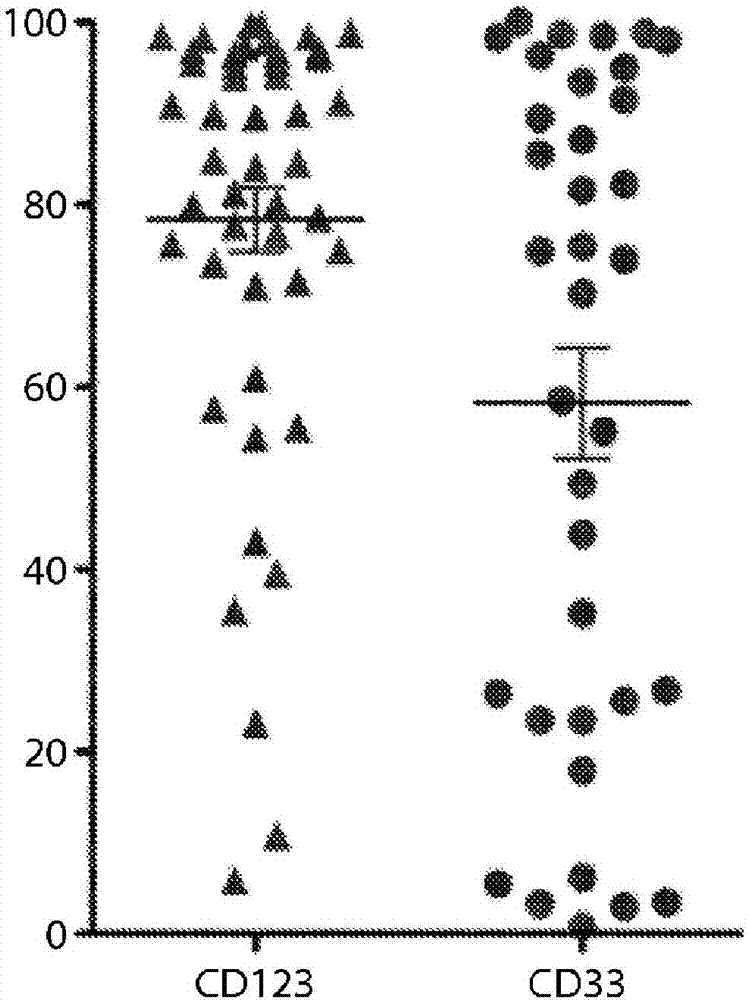
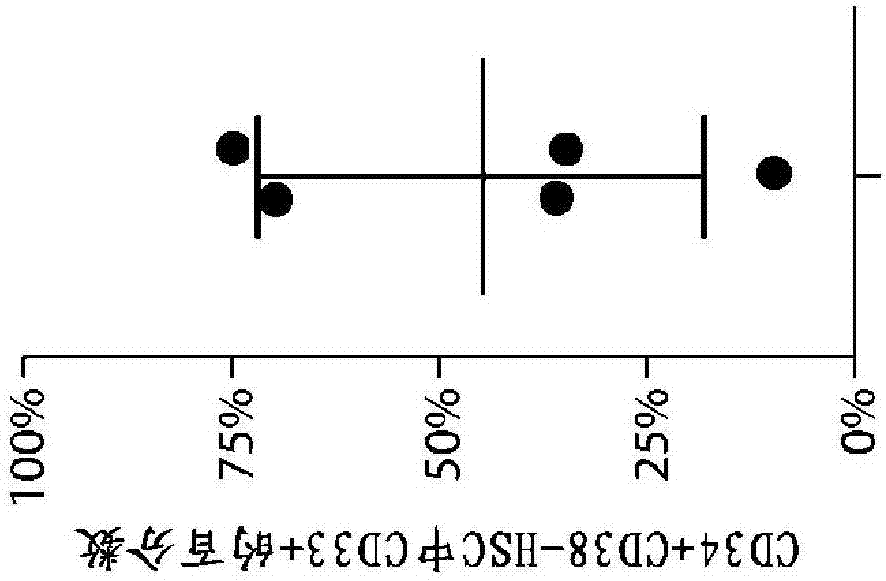
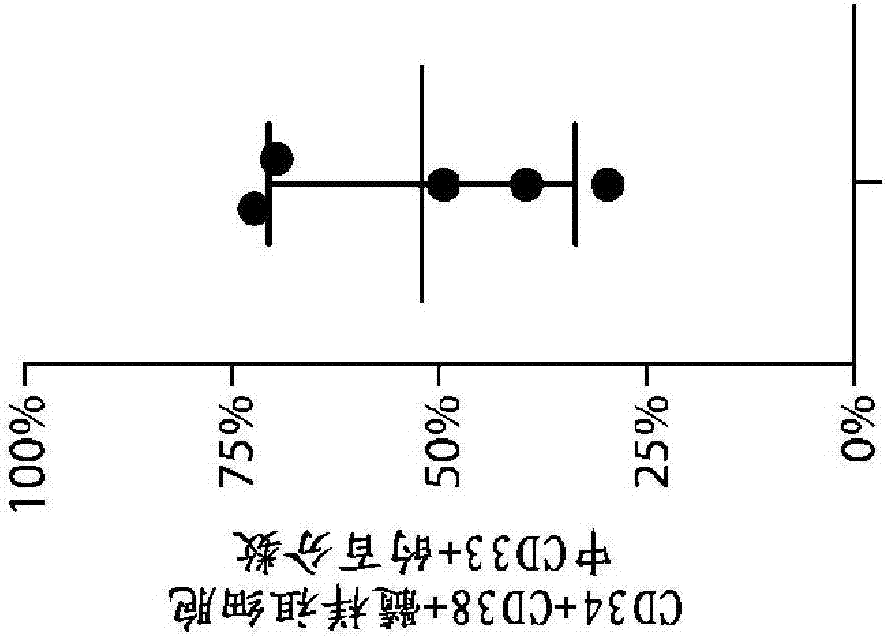
[1168] CD33 levels were measured by flow cytometry in primary proprietary samples from AML patients using commercially available antibodies (clone HIM3-4, eBioscience; or clone WM53, Biolegend). The results presented here demonstrate that CD33 is expressed in many primary AML patient samples (using standard side scatter 低 CD45 暗 Features (gated on AML blasts); n = 35-46 per group).
[1169] image 3 A schematic diagram of the CAR construct used in this example is shown in . All are second-generation CARs using 41BB and CD3ζ signaling. The scFv of CART33 was derived from clone MY9-6.
[1170] In vitro activity of CART33
[1171] The experiments described here measure CART33-mediated degranulation of T cells. CART33-transduced T cells and UTDT cells were incubated with the CD33+ cell line MOLM14 and the control ALL cell line NALM6 and CD107a degranulation was measured by flow cytometry. Expression of both the murine CART33 con...
Embodiment 2
[1197] Example 2: CAR Constructs
[1198] A fully human anti-CD33 single chain variable fragment (scFv) was generated and cloned into a lentiviral expression vector along with the intracellular CD3ζ chain and the intracellular co-stimulatory domain 4-1BB and given the names described in Table 1 (this is described in the Invention Details). shown in the description).
[1199] The order in which the VL domain and the VH domain appear in the scFv changes (i.e., VL-VH, or VH-VL orientation), and 3 or 4 copies of the "G4S" (SEQ ID NO: 25) subunit ( Wherein each subunit comprises the sequence GGGGS (SEQ ID NO:25) (for example, (G4S) 3 (SEQ ID NO:28) or (G4S) 4 (SEQ ID NO:27)) to link the variable domains to create the integrity of the scFv domains, as shown in Table 3.
[1200] The sequences of the human scFv fragments (SEQ ID NO: 39-83, including optional leader sequences) are provided in Table 2 herein (in the Detailed Description of the Invention). The sequences of the leader...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com