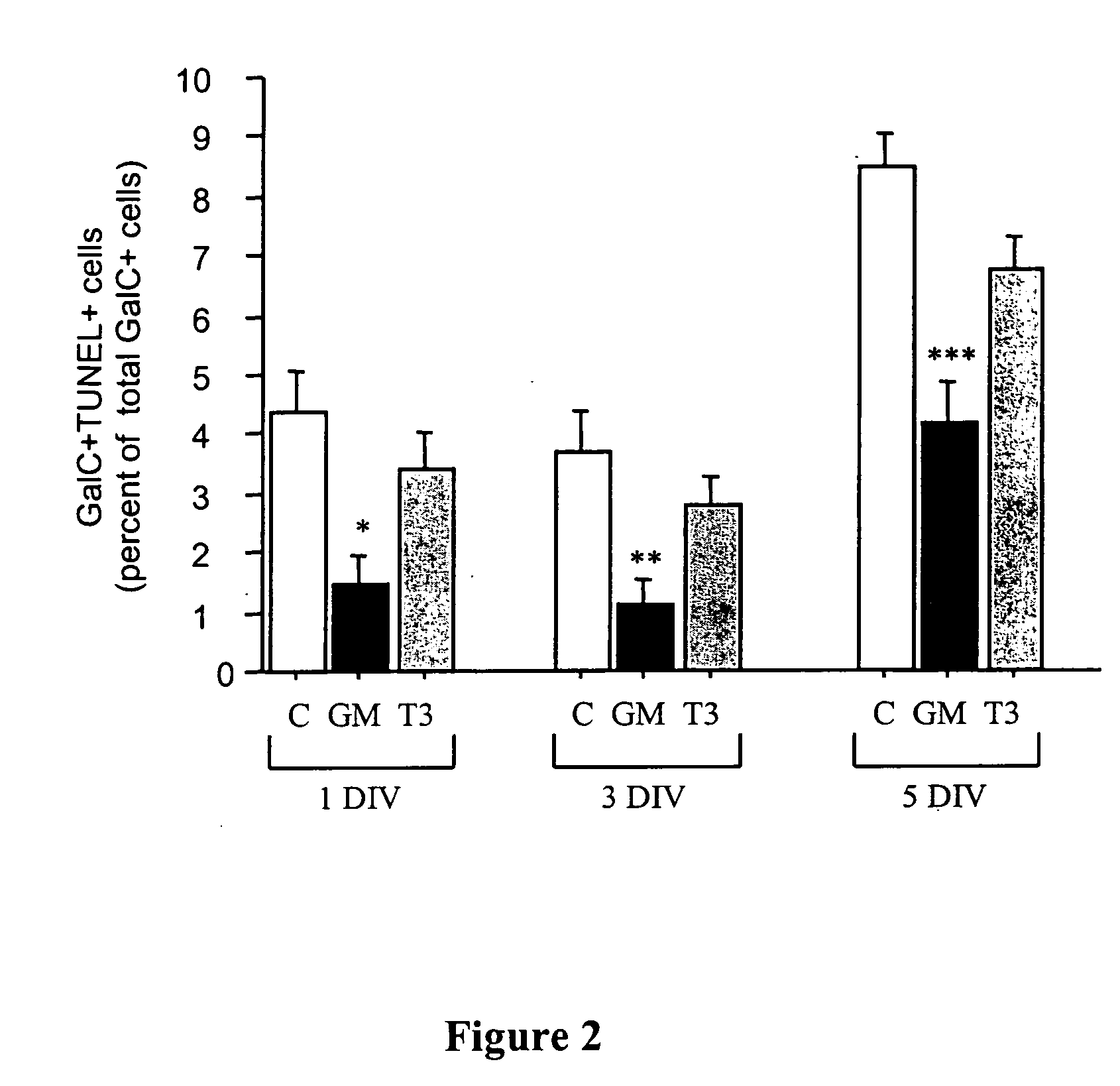
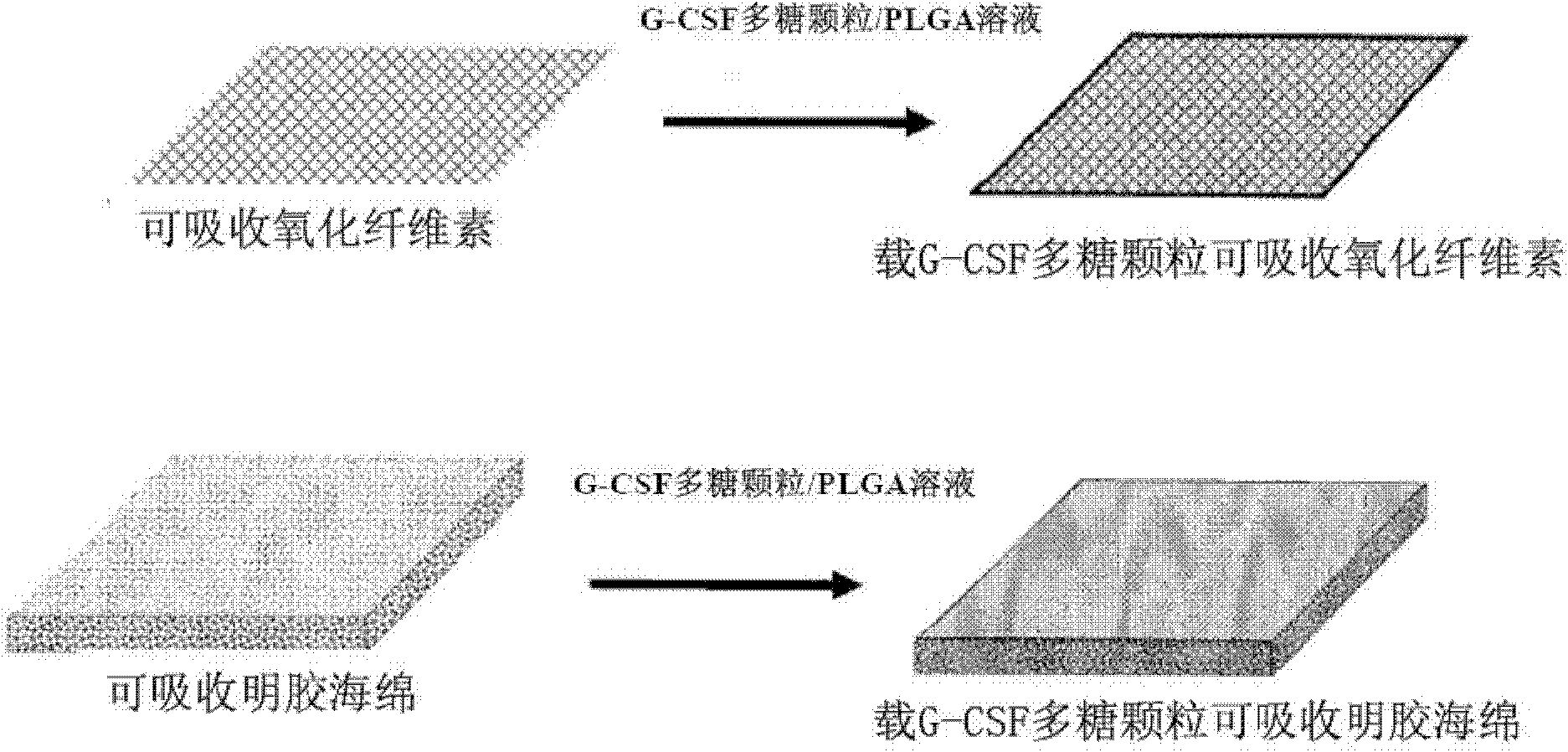
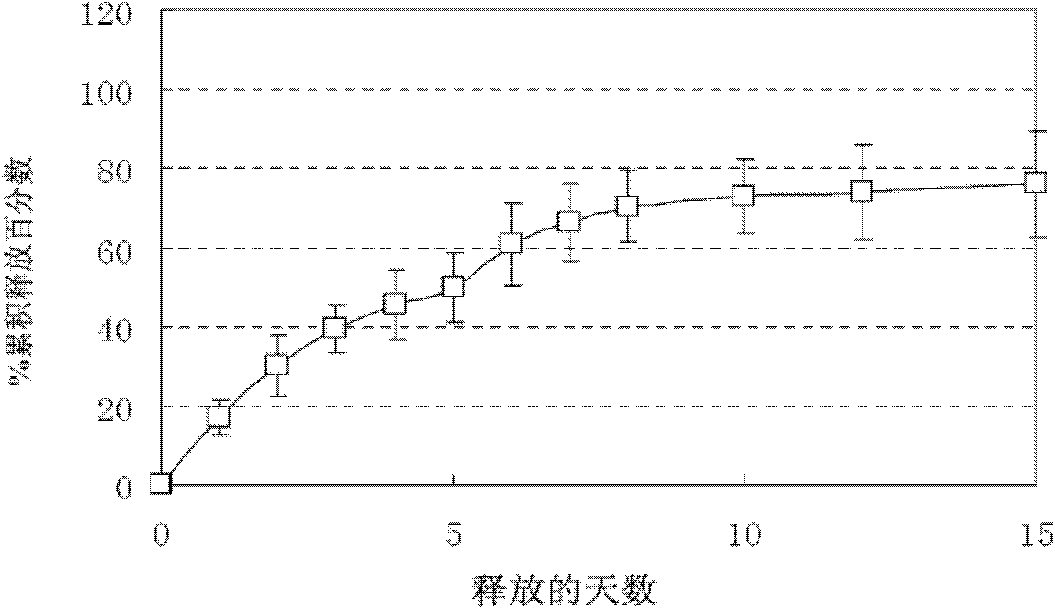
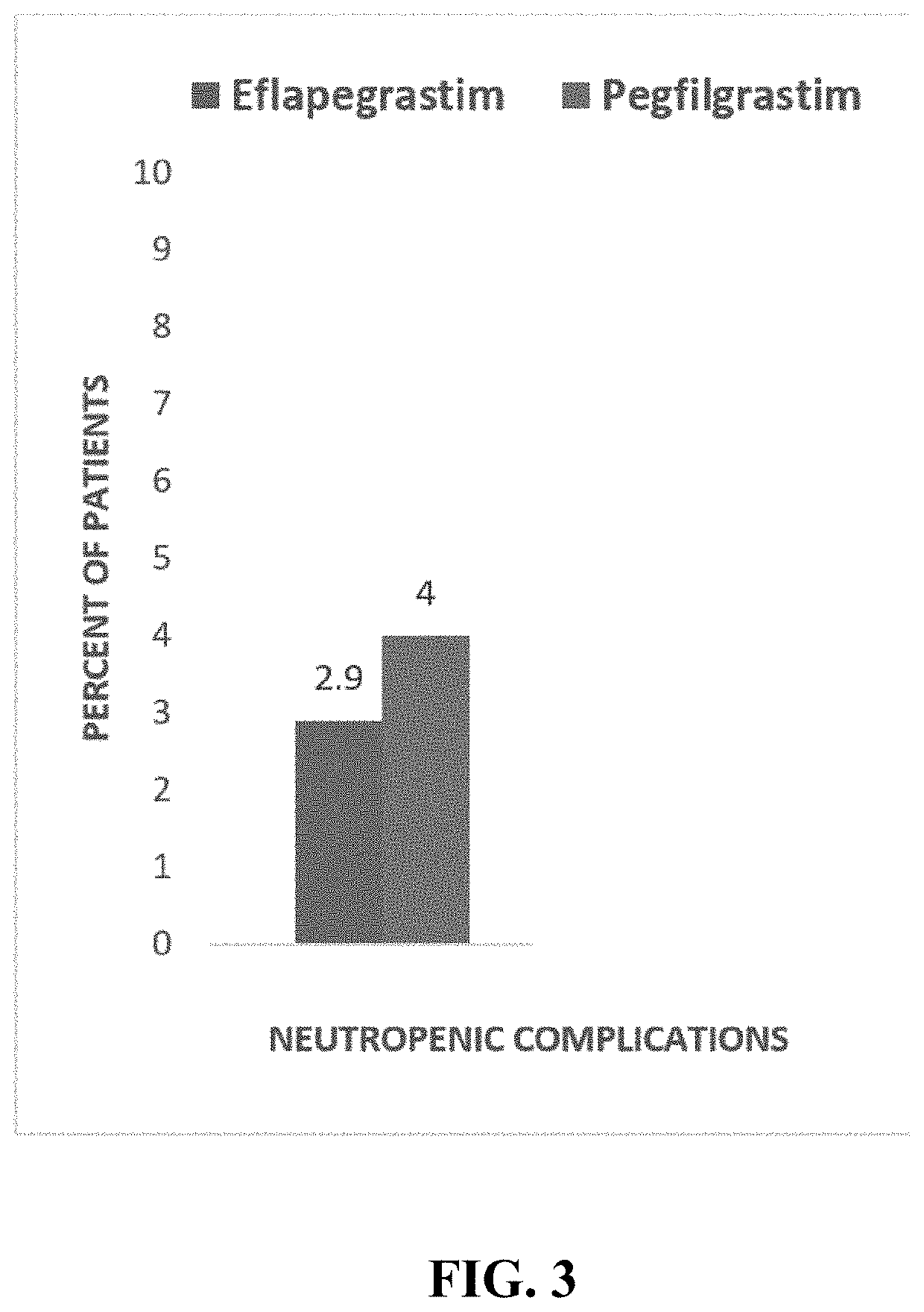
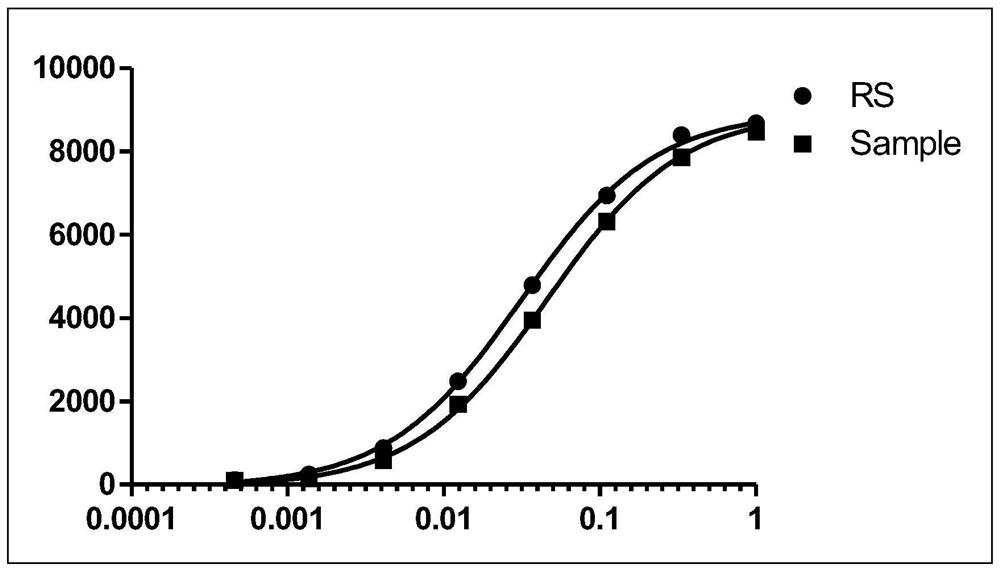
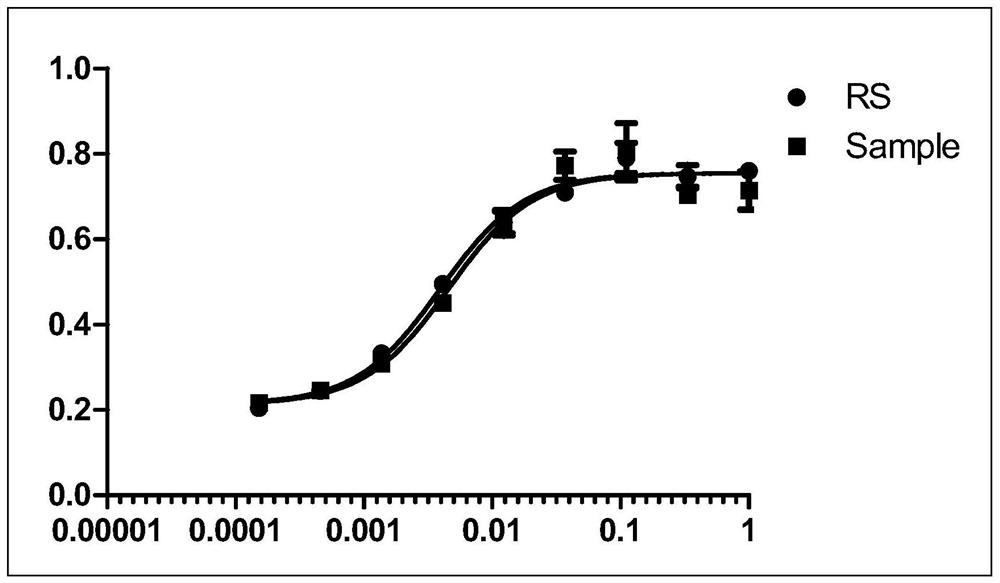
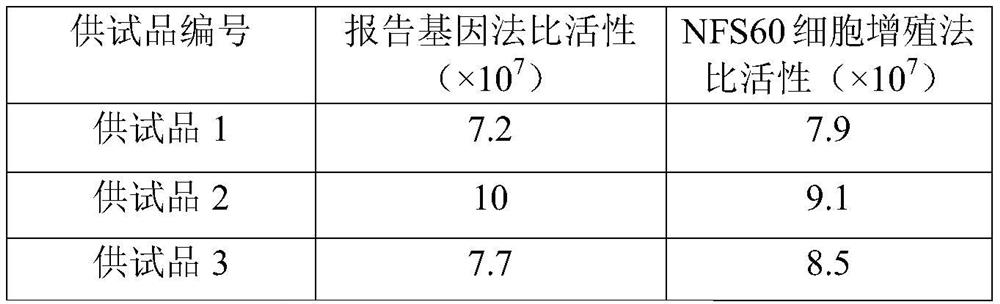
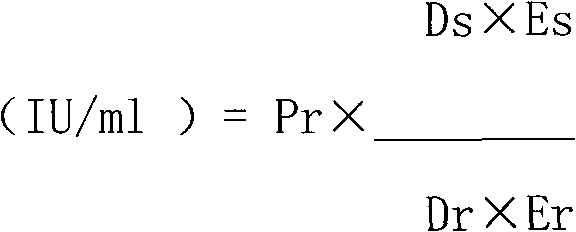Patents
Literature
50 results about "Granulocyte colony-stimulating factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Granulocyte-colony stimulating factor (G-CSF or GCSF), also known as colony-stimulating factor 3 (CSF 3), is a glycoprotein that stimulates the bone marrow to produce granulocytes and stem cells and release them into the bloodstream.
Recombinant human granulocyte colony stimulating factor and one-step purifying process for chemical modifications thereof
InactiveCN1663962AHigh purityCarrier-bound/immobilised peptidesCytokines/lymphokines/interferonsG-csf therapyHigh activity
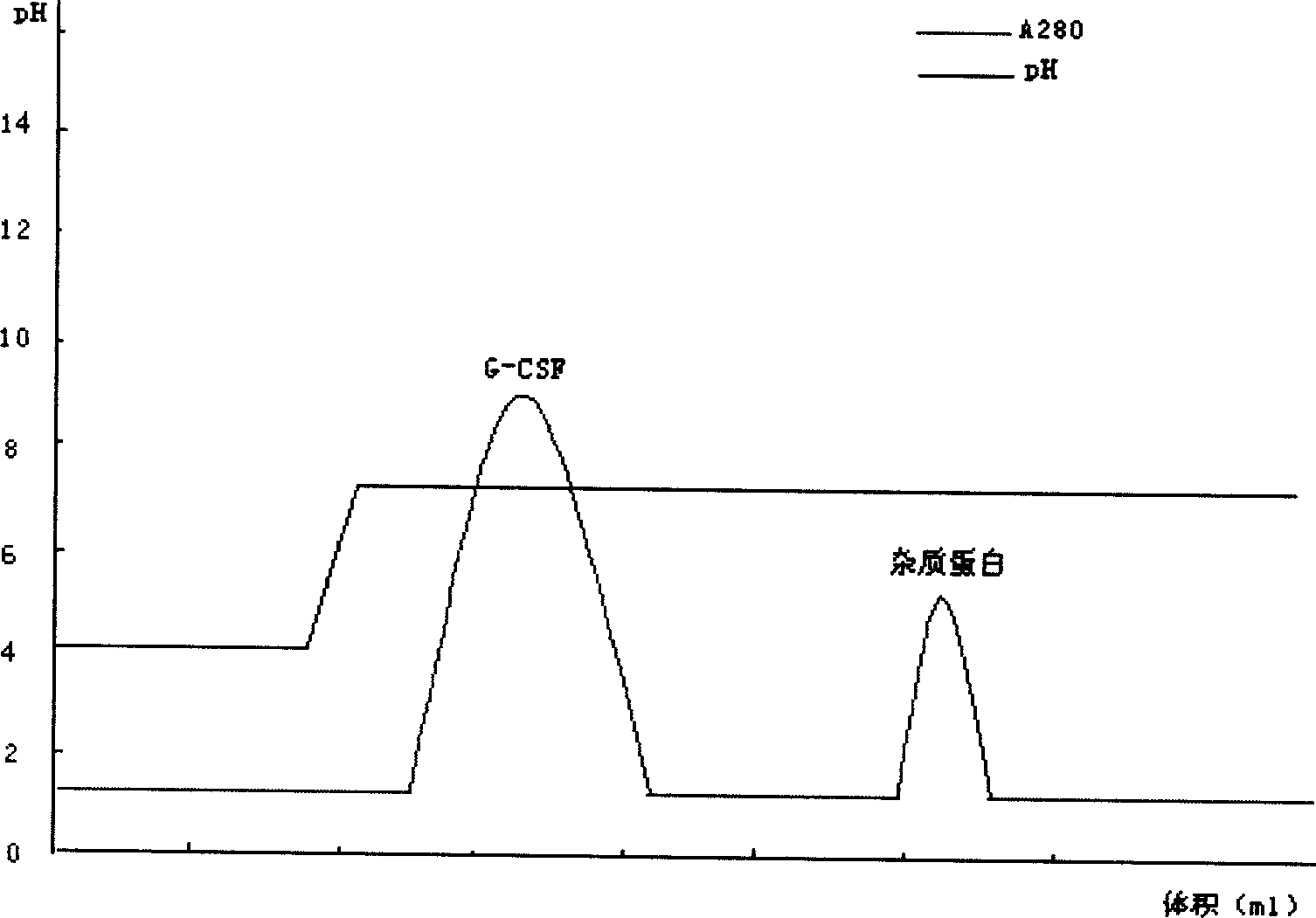
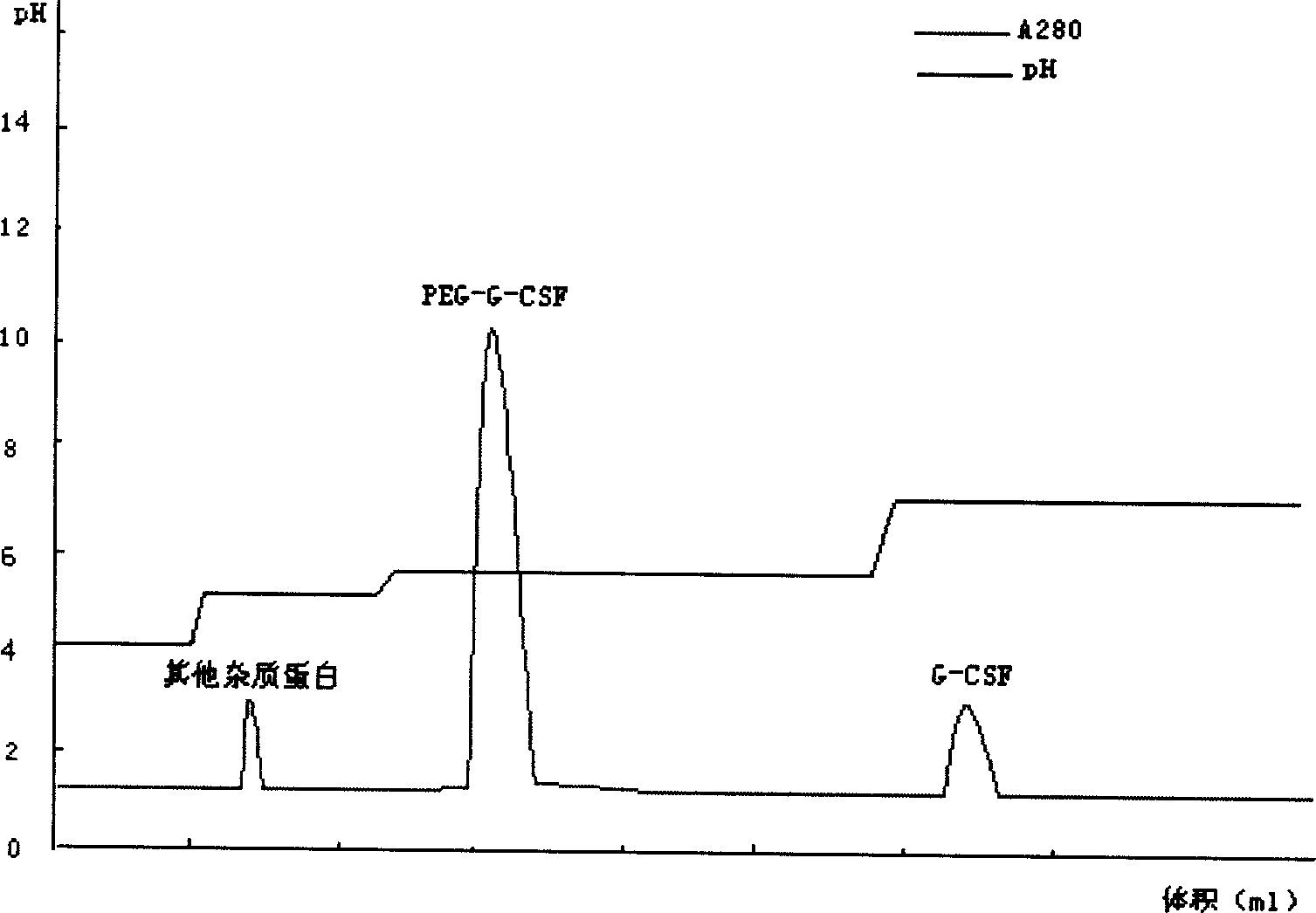
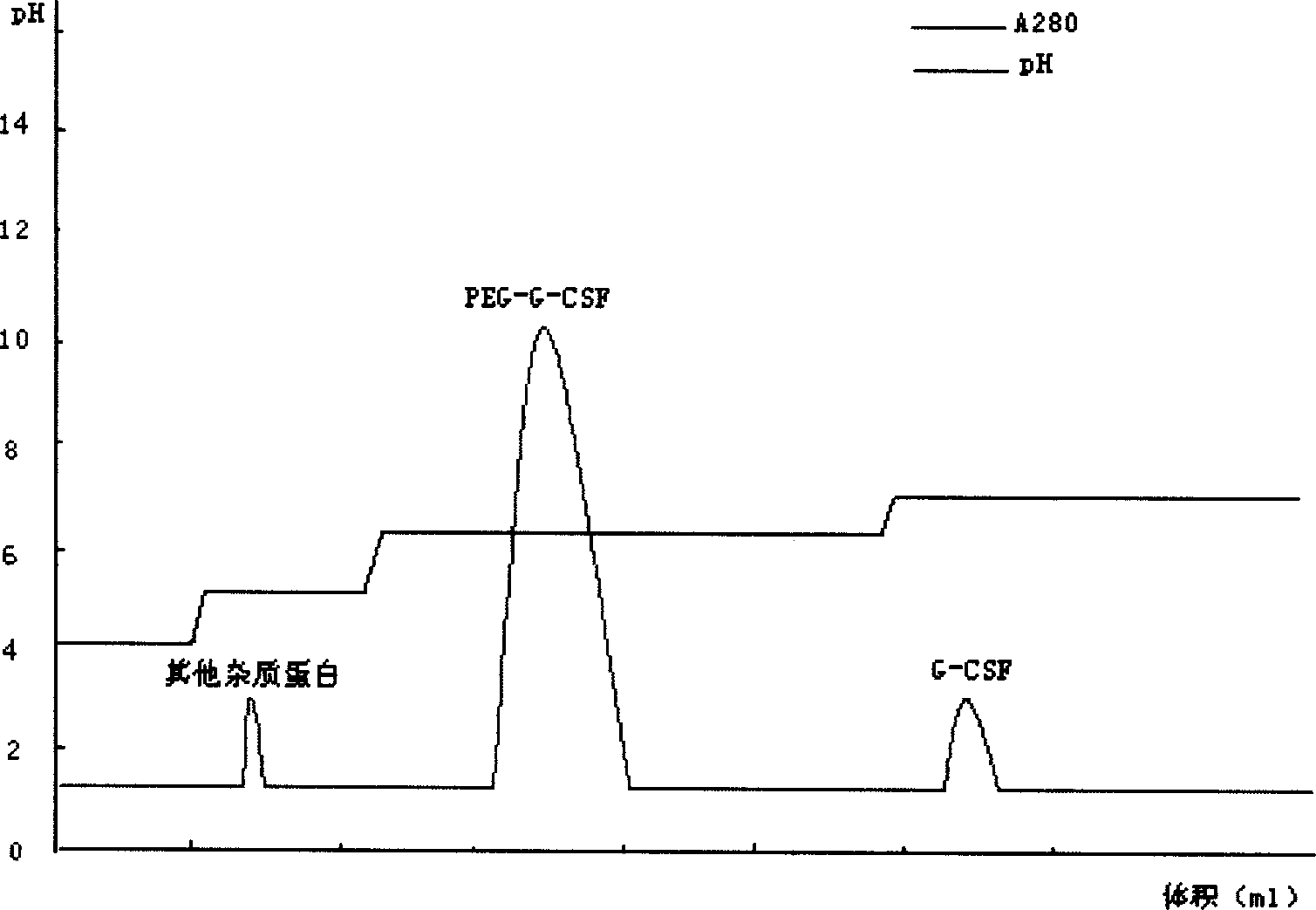
The invention relates to a recombinant human granulocyte colony stimulating factor and one-step purifying process for chemical modifications, which employs the method of cation exchange chromatography, wherein one-step chromatography is utilized for obtaining high activity, high purity and high recovery ratio rhG-CSF and polyethylene glycol chemically modified rhG-CSF. The process is especially suitable for industrial production.
Owner:CHONGQING FAGEN BIOMEDICAL
Tumor vaccines
InactiveUS7247310B1Easy to handleImprove anti-tumor effectPeptide/protein ingredientsMammal material medical ingredientsAbnormal tissue growthGranulocyte colony-stimulating factor
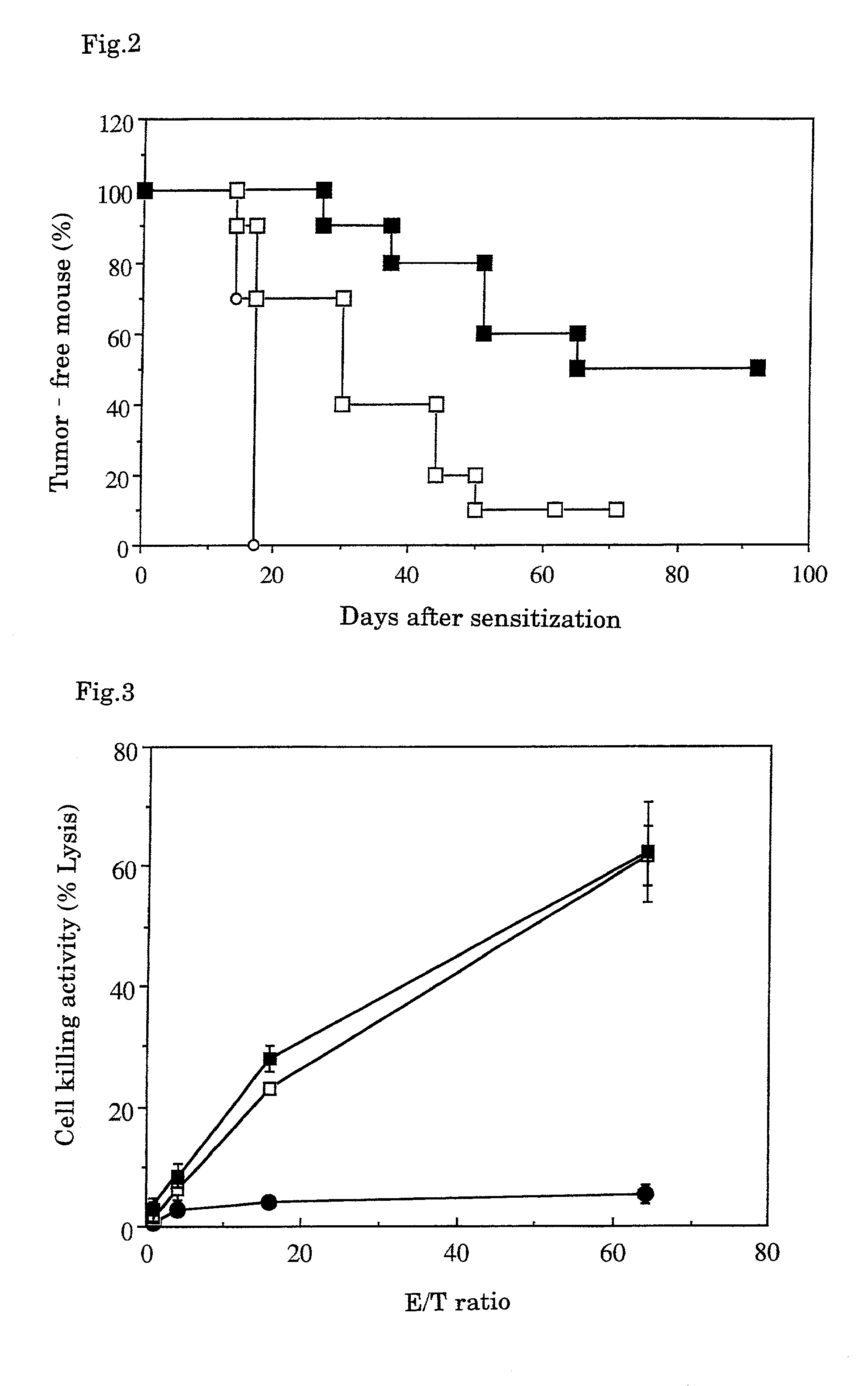
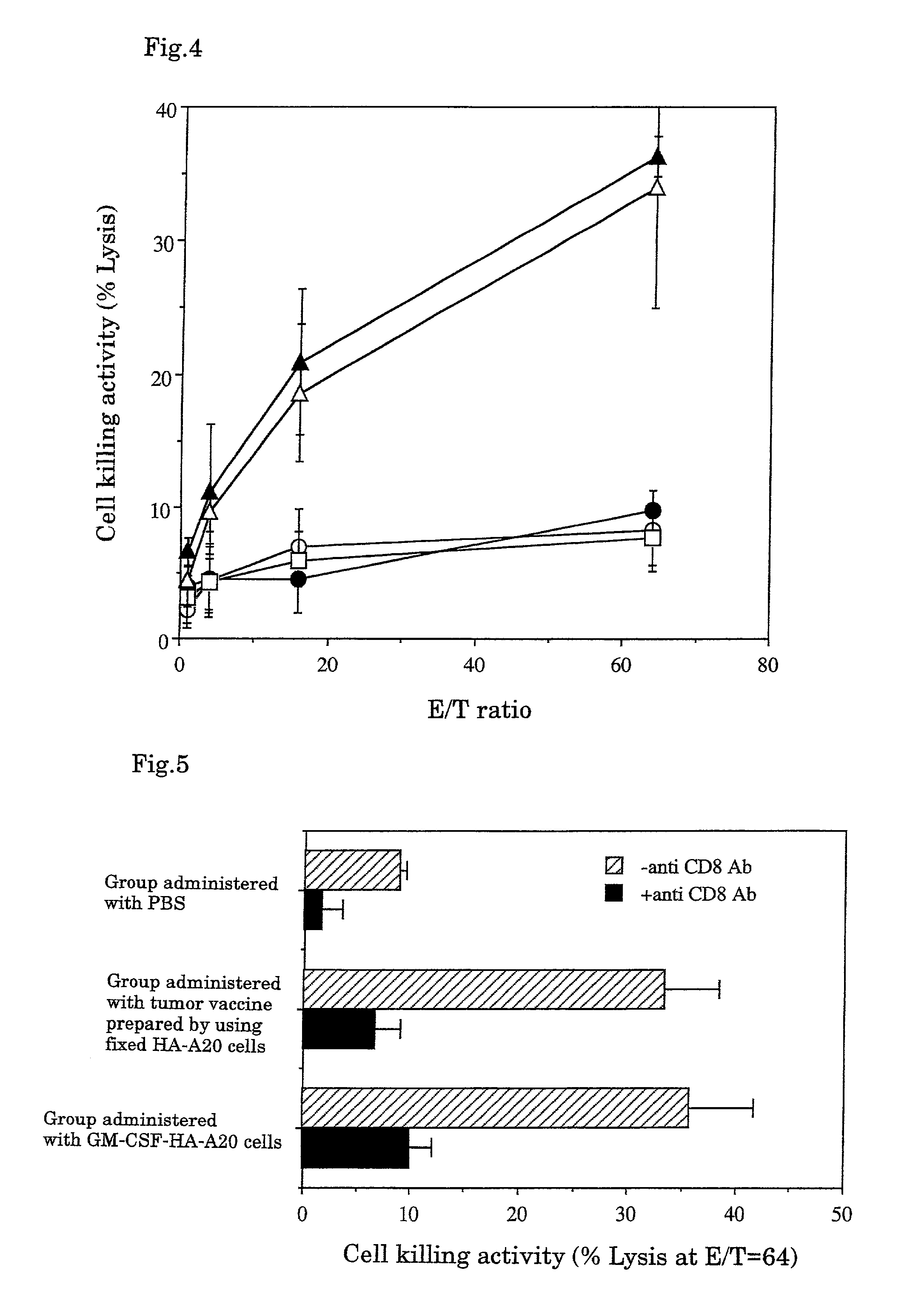
A tumor vaccine which comprises a microparticle or a lysate prepared from a solidified tumor material selected from the group consisting of a tumor tissue, a tumor cell, and a component thereof, and at least one cytokine and / or cytokine-inducing agent (e.g., a granulocyte-macrophage-colony stimulating factor and / or interleukin-2 and the like), and optionally an adjuvant. The vaccine can be easily prepared and widely applied for prevention of recurrence, inhibition of metastasis and therapeutic treatment regardless of a type of a tumor, and has excellent antitumor effect.
Owner:RIKEN +1
Combination of epigenetic factors and bispecific compounds targeting CD33 and CD3 in the treatment of myeloid leukemia
InactiveCN111888474AOrganic active ingredientsHybrid immunoglobulinsGranulocyte colony-stimulating factorMyeloid leukemia
Owner:AMGEN INC +1
Agent for preventing and/or treating tissue disruption-accompanied diseases
InactiveUS20070172447A1Promote absorptionHigh activityAntibacterial agentsNervous disorderG-csf therapyBULK ACTIVE INGREDIENT
The present invention relates to an agent for preventing and / or treating diseases accompanied by tissue disruption, which comprises a polypeptide having granulocyte colony-stimulating factor activity as an active ingredient, and a medicament for mobilizing a multipotent stem cell from a tissue into peripheral blood, which comprises a polypeptide having granulocyte colony-stimulating factor activity as an active ingredient.
Owner:KYOWA HAKKO KIRIN CO LTD
Inhibitors of colony stimulating factors
InactiveUS20070059280A1Inhibiting and minimizing capabilityInhibiting and minimizing accumulationPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsGranulocyte colony-stimulating factorHematopoietic factor
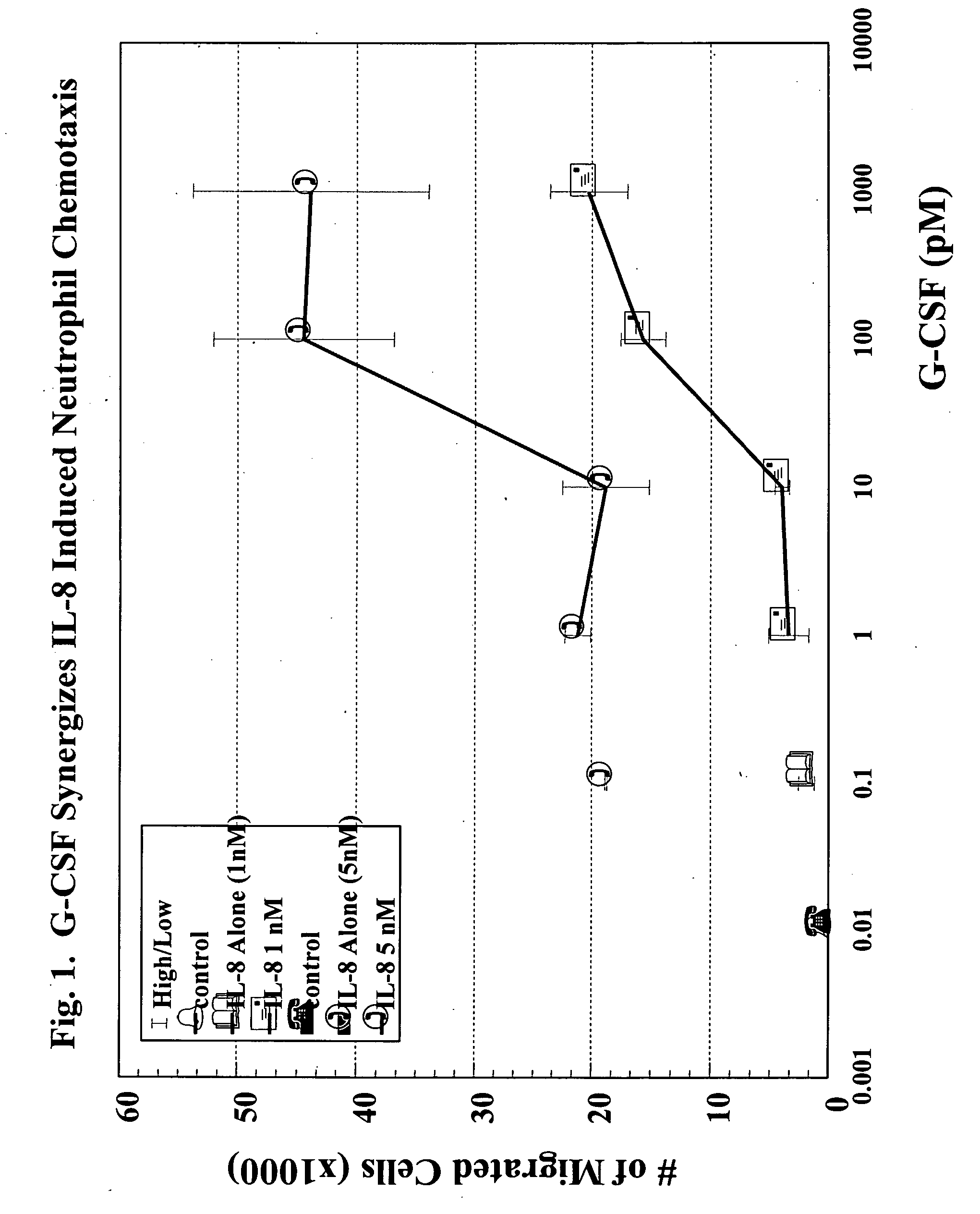
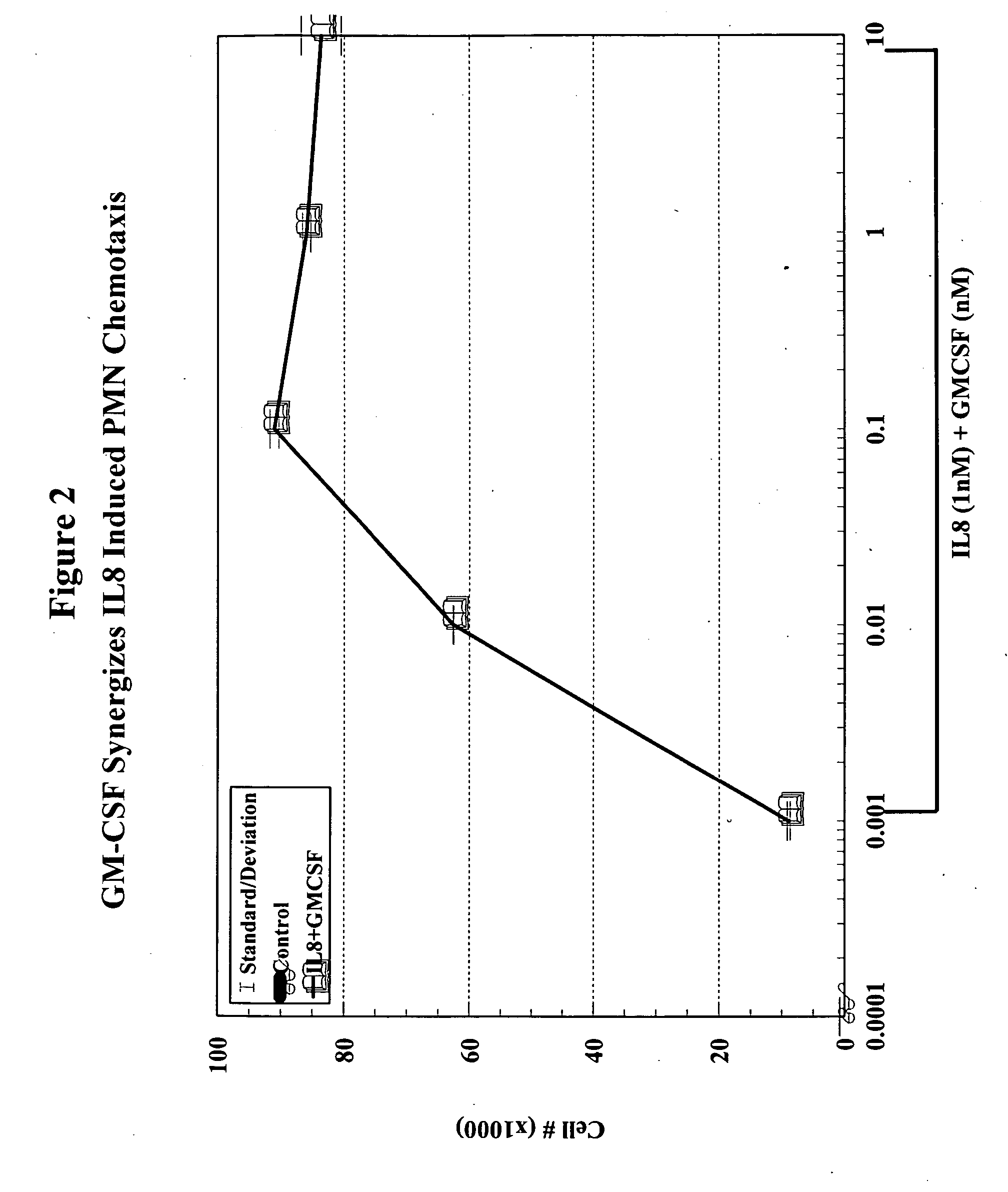
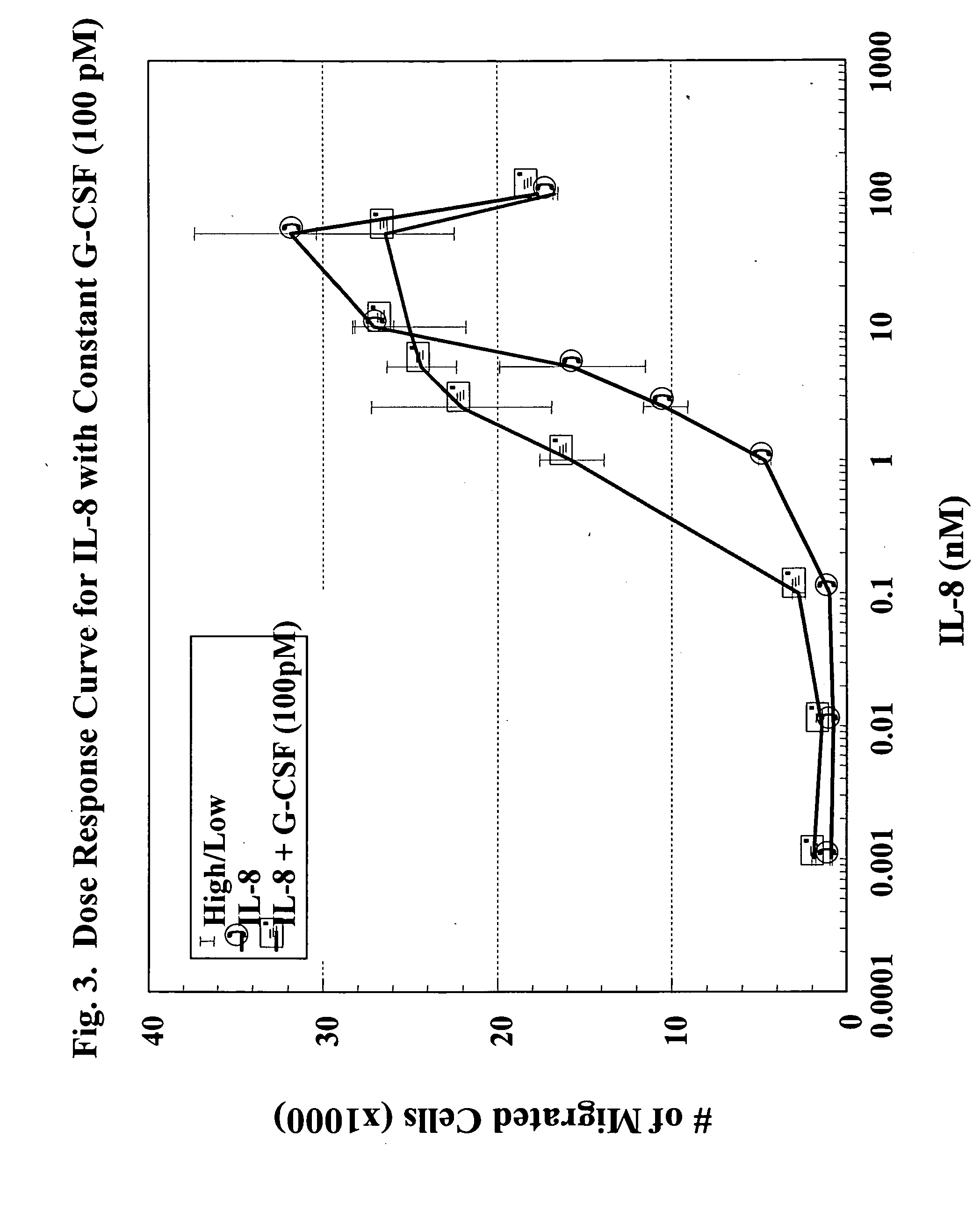
A hematopoetic factor called “colony stimulating factor” (CSF) is capable of synergizing the attracting capabilities of chemokines and of inducing the accumulation and / or activation in vitro and in vivo of key components of inflammatory responses. Various types of agents that inhibit or otherwise hinder the production, release or activity of CSF could be used therapeutically in the treatment of ischemia and other inflammatory diseases, such as autoimmune disease, and various chronic inflammatory diseases such as rheumatoid arthritis and psoriasis.
Owner:WARNER LAMBERT CO LLC
Mouse primary hepatocyte perfusion type separating and in-vitro culturing method
PendingCN109628377AGood perfusion effectNot easy to clotCell dissociation methodsCulture processGranulocyte colony-stimulating factorSingle cell suspension
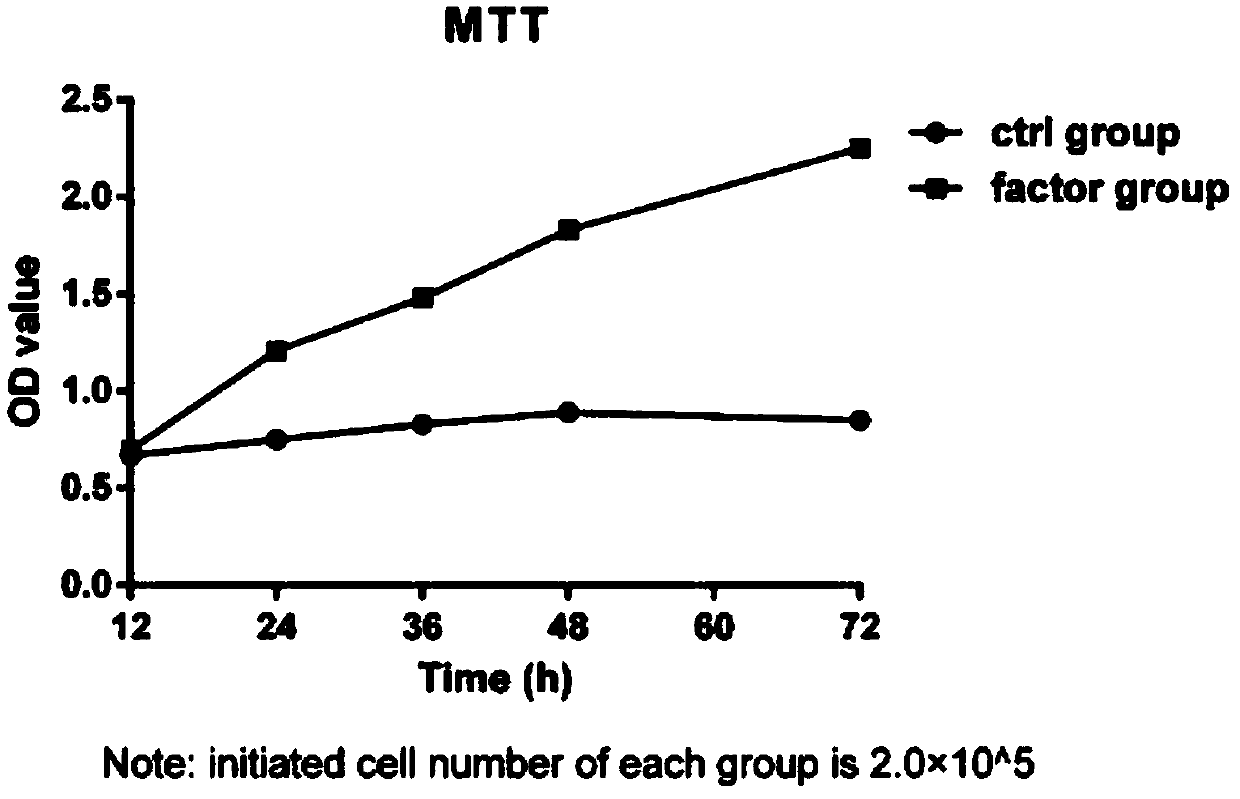
The invention discloses a mouse primary hepatocyte perfusion type separating and in-vitro culturing method. The perfusion type separating of mouse primary hepatocytes is conduced through an in-situ living body perfusion method, a high-yield single-cell suspension can be obtained, and therefore a basis is provided for in-vitro long-time culturing of the primary hepatocytes. An applied stimulating culture solution contains mouse colony stimulating factors (CSF), mouse IL-2, mouse IL-6 and mouse recombinant hepatocyte growth factors r-mHGF and other cell factors; the primary hepatocytes can be separated through stimulation according to the reasonable ratio for in-vitro proliferation, and the number can reach 2.25 times the number of initially-added primary hepatocytes 72 hours later. The cellproliferation activity can be kept for at least 1-2 weeks under the in-vitro conditions, the problem that the primary hepatocytes cannot be cultured for a long time in vitro is solved, and thereforethe method can be used for infection of retroviruses, provides a basis for over-expression target genes or knockout target genes in in-vitro primary cells, and greatly expands the application field ofthe primary hepatocytes.
Owner:GUIZHOU PROVINCIAL PEOPLES HOSPITAL
Pegylated recombinant human granulocyte colony stimulating factor freeze-dried powder/injection and preparation method thereof
ActiveCN102028661AAvoid sex changeSuitable for large-scale productionPowder deliveryPeptide/protein ingredientsSodium acetateGranulocyte colony-stimulating factor
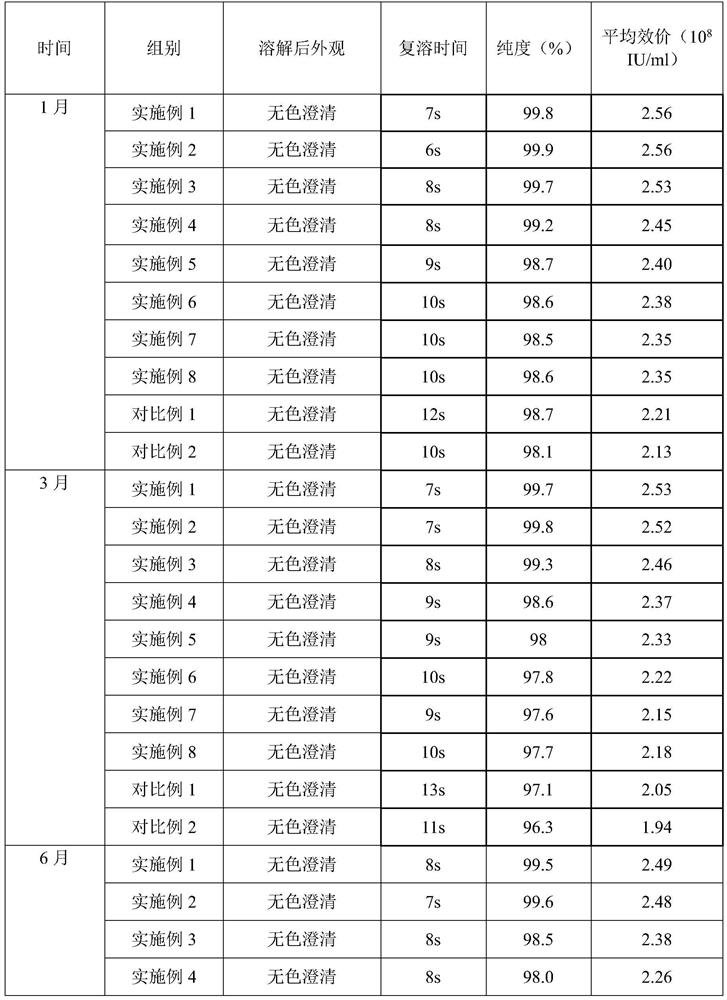
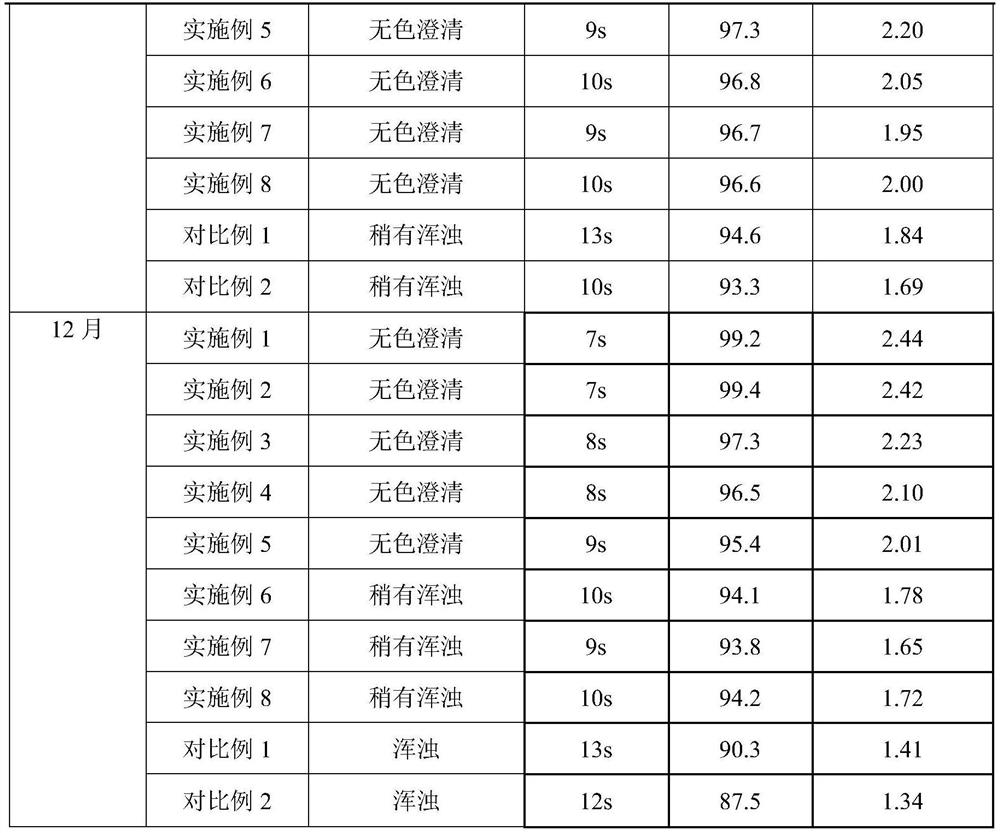
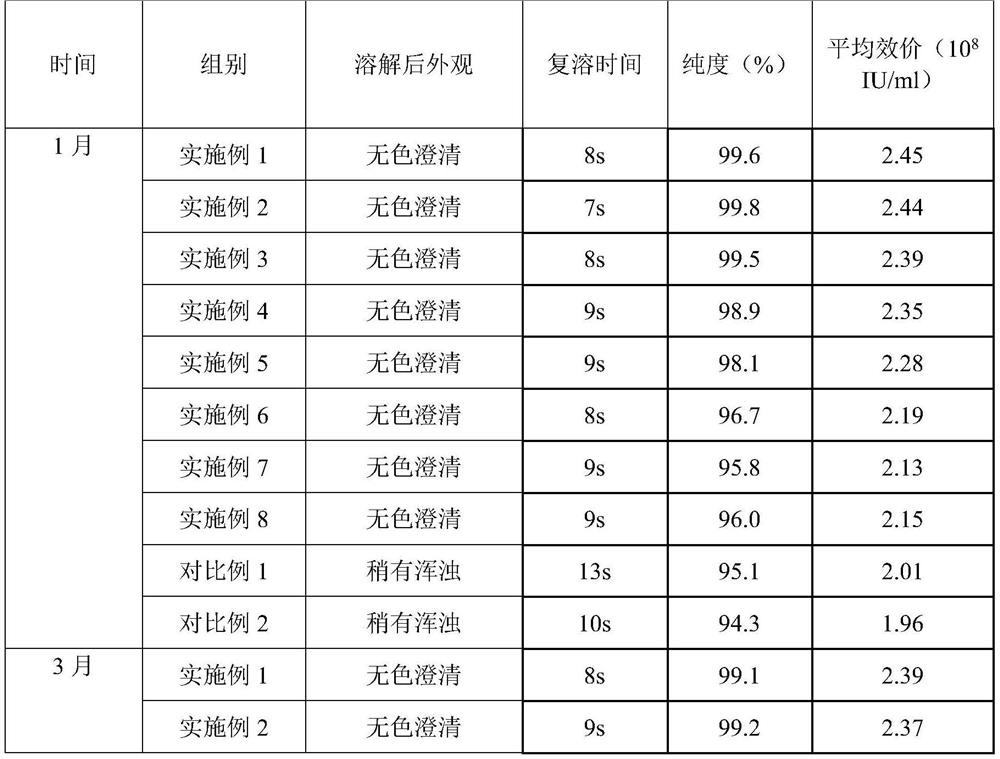
The invention belongs to the field of biomedicine, and particularly relates to pegylated recombinant human granulocyte colony stimulating factor freeze-dried powder / injection and a preparation method thereof. The freeze-dried powder / injection comprises pegylated recombinant human granulocyte colony stimulating factor serving as an active ingredient, freeze-drying protective agent, glacial acetic acid and sodium acetate. The preparation method comprises the following steps of: preparing diluent from auxiliary materials, then diluting the stock solution containing the active ingredient to the required concentration by using the diluent, filling the diluted solution into a clean amoxicillin bottle, sealing the bottle by using a rubber stopper, putting the bottle into a vacuum freeze dryer, and freeze-drying the solution in vacuum to obtain the freeze-dried powder / injection. The prepared pegylated recombinant human granulocyte colony stimulating factor freeze-dried powder / injection has long-term stability on appearance, purity and average potency.
Owner:LUNAN PHARMA GROUP CORPORATION
Chewing gum for preventing and treating oral ulcer resulted from chemotherapy
InactiveCN103083649AAvoid damageReduce dosageOrganic active ingredientsPeptide/protein ingredientsGranulocyte colony-stimulating factorOral ulcers
The invention relates to a chewing gum for preventing and treating oral ulcer resulted from chemotherapy and a preparation method of the chewing gum. The chewing gum comprises chewing gum ingredients and medicaments for preventing and treating oral ulcer; every 100 grams of chewing gum for preventing and treating oral ulcer comprise the following medicaments for preventing and treating oral ulcer by weight: 5mg-10mg of recombinant human granulocyte colony-stimulating factors, 5mg-10mg of folic acid, 5mg-10mg of metronidazole, 20mg-50mg of lidocaine, 5mg-20mg of cimetidine, 2.5mg-10mg of vitamin B2, and the balance being chewing gum ingredients. The chewing gum ingredients comprise the following components in percentage by weight: 25%-30% of edible gum base, 20%-25% of malt syrup, 35%-45% of xylitol and 10%-20% of sorbitol. The chewing gum disclosed by the invention can be used for effectively preventing and treating oral ulcer resulted from different disease causes after chemotherapy, further can be used for reducing stimulus of medicaments to gastrointestinal tract, reducing adverse reactions and increasing safety. Moreover, the chewing gum is good in mouth-feel and convenient to take.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Oligodendrocyte production from multipotent neural stem cells
InactiveUS20050244965A1Increase percentageMaximize productionOrganic active ingredientsNervous disorderOligodendrocyteInterleukin 5
This invention relates to methods of producing oligodendrocytes from multipotent neural stem cells by using at least one oligodendrocyte promoting factor, particularly granulocyte-macrophage colony stimulating factor, granulocyte colony stimulating factor, interleukin 3 or interleukin 5. The neural stem cells may optionally be expanded prior to being subjected to the oligodendrocyte promoting factor.
Owner:STEM CELL THERAPEUTICS
Preparation with double functions of stopping bleeding and sustainedly releasing granulocyte colony stimulating factor and preparation method thereof
InactiveCN102178977AFacilitated releaseEasy to manufacturePeptide/protein ingredientsPharmaceutical non-active ingredientsGranulocyte colony-stimulating factorControl release
The invention discloses a preparation with double functions of stopping bleeding and sustainedly releasing a granulocyte colony stimulating factor and a preparation method thereof in the technical field of medicine preparation. The preparation consists of glucan particles containing the granulocyte colony stimulating factor, a degradable sustained / controlled release material and a hemostatic material framework. By combining surgical hemostatic gauze with a medicine sustained-release system consisting of the glucan particles containing the granulocyte colony stimulating factor and a degradable sustained / controlled release material coating, a medicine loading procedure is added in a hemostatic gauze production process in a preparation process; and the method is simple and practicable and is suitable for various medicines.
Owner:SHANGHAI JIAO TONG UNIV
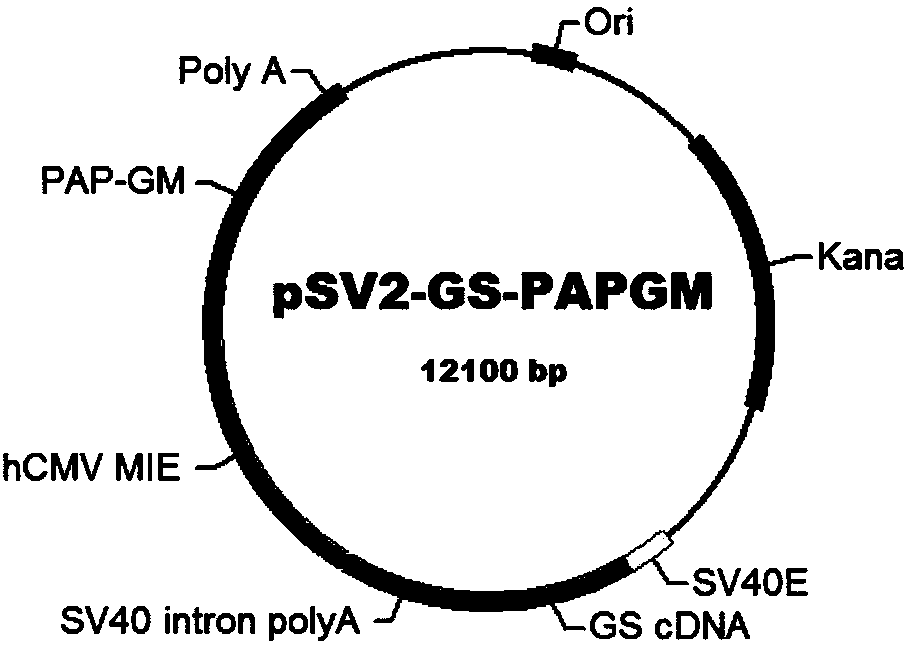
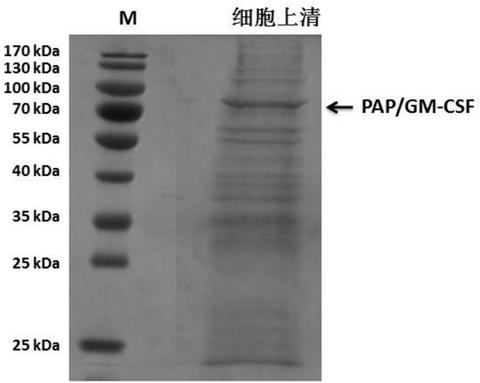
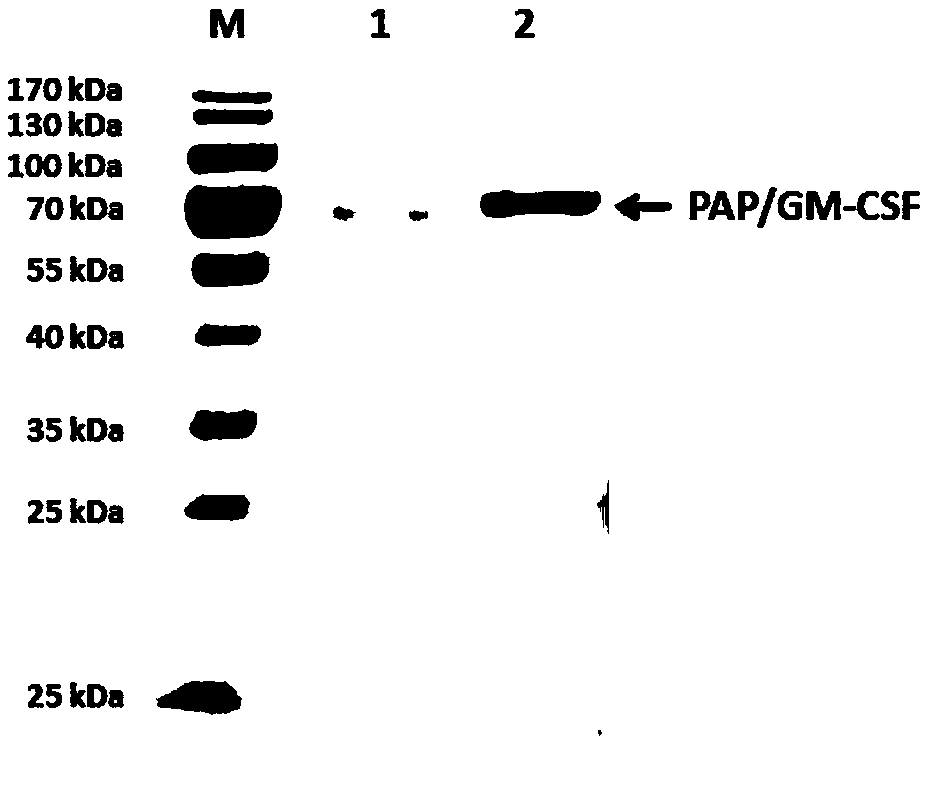
Preparation method of PAP/GM-CSF (prostatic acid phosphatase/grain macrophage-colony stimulating factor)
ActiveCN110079539AHydrolasesAntibody mimetics/scaffoldsGranulocyte colony-stimulating factorNucleotide
The invention relates to a preparation method of PAP / GM-CSF (prostatic acid phosphatase / grain macrophage-colony stimulating factor), in particular to a nucleotide sequence for encoding the PAP / GM-CSF.The amino acid sequence is encoded as the amino acid sequence shown in SEQ ID No.:1; preferably, the nucleotide sequence is shown in SEQ ID NO.:2. The invention also provides an expression carrier for high-efficiency expression of PAP / GM-CSF, engineering cell and a preparation method. The constructed PAP / GM-CSF has the advantages that when the PAP / GM-CSF is used for expressing the engineering cell, the expression amount is high, the expression is stable, and the like; the purifying technology is stable and reliable, the yield rate is high, and the large-scale production is convenient.
Owner:SHANGHAI HUIMMUTECH BIOTECHNOLOGY CO LTD
Medical preparation containing recombinant human granulocytecolony stimulating factor
InactiveCN101766810APeptide/protein ingredientsMicroorganism based processesHuman bodyMethionine biosynthesis
The invention discloses a medical preparation containing a recombinant human recombination granulocytecolony stimulating factor (G-CSF) with the same structure as a natural human granulocytecolony stimulating factor and a preparation method thereof. The active ingredient of the preparation is G-CST with proline at the N terminal, is 173-numbered amino acids and is one of the natural structures of G-CST in human body. The preparation overcomes the defect that the N terminal of the existing recombinant human G-CSF product contains methionine. The invention also provides the method for preparing the preparation.
Owner:BEIJING SL PHARMA
Purifying method of recombinant human granulocyte colony stimulating factors
InactiveCN101597319AHigh purityHigh activityPeptide preparation methodsGranulocyte colony-stimulating factorElectrophoreses
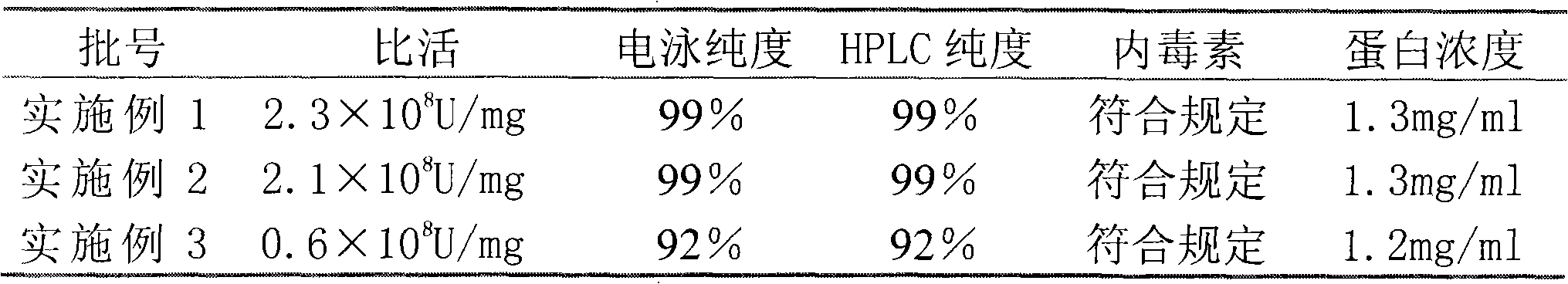
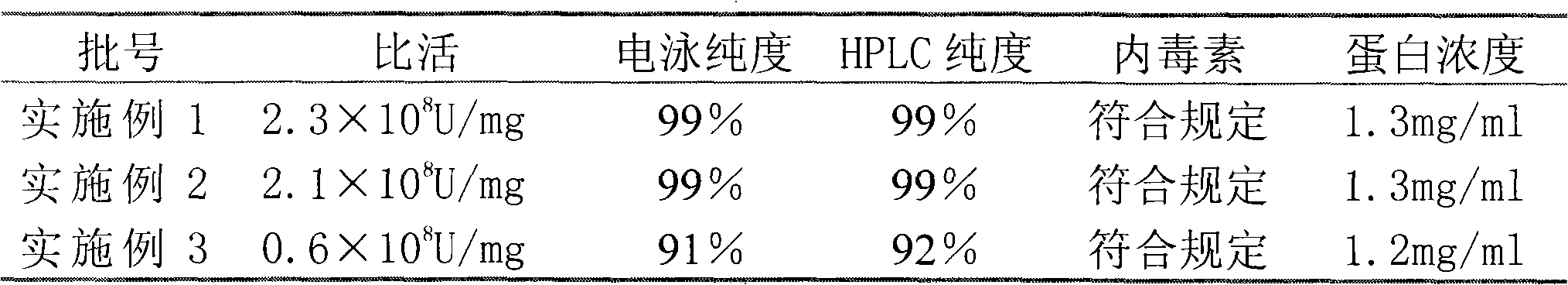
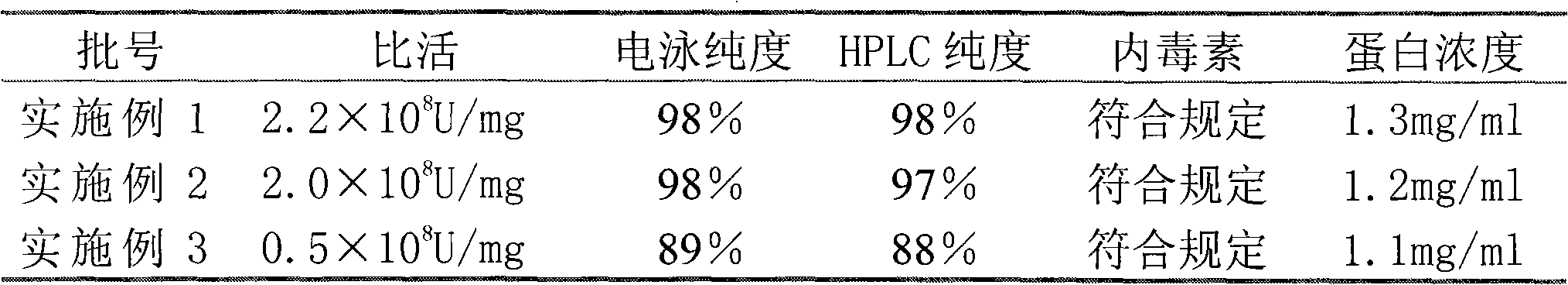
The invention relates to a purifying method of recombinant human granulocyte colony stimulating factors and applies a fine separating step of opposite-phrase filling to a purifying technology of the recombinant human granulocyte colony stimulating factors for the first time. Compared with the prior art, the method adopts the opposite-phrase filling for the first time to finely separate the recombinant human granulocyte colony stimulating factors, the electrophoresis purity is increased to 99 percent from 90 percent, the HPLC purity is increased to 99 percent from 90 percent, and the specific activity is increased to 2.3*10U / mg from 6.1*10U / mg. The purifying technology of the recombinant human granulocyte colony stimulating factors is adopted to greatly increase the purity, the specific activity and the stability of the recombinant human granulocyte colony stimulating factors and can effectively reduce the clinical side effect of the recombinant human granulocyte colony stimulating factors.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Method for purifying human granulocyte-colony stimulating factor from recombinant e. coli
ActiveUS20130211054A1Improve purification effectHigh yieldPeptide/protein ingredientsPeptide preparation methodsEscherichia coliHuman body
The present invention provides a method for purifying a large amount of human granulocyte-colony stimulating factors (hG-CSFs) from a recombinant E. coli with high yield and purity. According to the method of the present invention, human granulocyte-colony stimulating factor, identical to the native form expressed in the human body, can be easily purified with high yield and purity without an additional activation process. In particular, according to the purification method of the present invention, hG-CSF variants expressed in E. coli are efficiently removed to obtain physiologically active hG-CSFs with high purity.
Owner:HANMI SCI CO LTD
Method for preparing recombinant human granulocyte colony stimulating factors screened by microorganisms, and medicament composition and preparation thereof
ActiveCN103114115AOvercoming technical difficulties of low yieldSuitable for large-scale industrial productionPeptide/protein ingredientsColony-stimulating factorMicroorganismInclusion bodies
The invention relates to the field of bio-pharmaceuticals, and in particular relates to a method for preparing recombinant human granulocyte colony stimulating factors screened by microorganisms. The preparation method comprises the following steps of: selecting engineering bacteria of the recombinant human granulocyte colony stimulating factors to ferment and culture; ultrasonically pulverizing bacteria bodies obtained by fermentation medium; collecting inclusion bodies; washing the inclusion bodies with inclusion body washing solution and centrifuging at a low temperature; adding inclusion body dissolution liquid into the inclusion bodies, standing for 8-12 hours at 4 DEG C and centrifuging to obtain inclusion body extraction liquid; separating the extraction liquid by using a Sephacry1S-200 gel column; collecting a plurality of recombinant human granulocyte colony stimulating factor fractions; and separating by using a SephadexG25 column to obtain the recombinant human granulocyte colony stimulating factor. The invention also relates to a medicament composition and a preparation of the recombinant human granulocyte colony stimulating factors.
Owner:福建亿懿兴华生物技术开发有限公司
Cell culture medium and cell culture method
InactiveCN105838666APromote growthGuaranteed normal growthEpidermal cells/skin cellsCulture processGranulocyte colony-stimulating factorNormal growth
The invention discloses a cell culture medium, comprising a basic culture medium and a growth factor composition added to the basic culture medium, wherein the basic culture medium includes 0-1% of serum. The growth factor composition includes human keratinocyte growth factor, fibroblast growth factor, colony stimulating factor and fibronectin. The cell culture medium is low in cost, can promote normal growth of cells, and is suitable for large-scale culture of cells. In addition, the invention also discloses a method for culturing cells by using the cell culture medium.
Owner:CHENGDU YUANRUI BIOTECH CO LTD
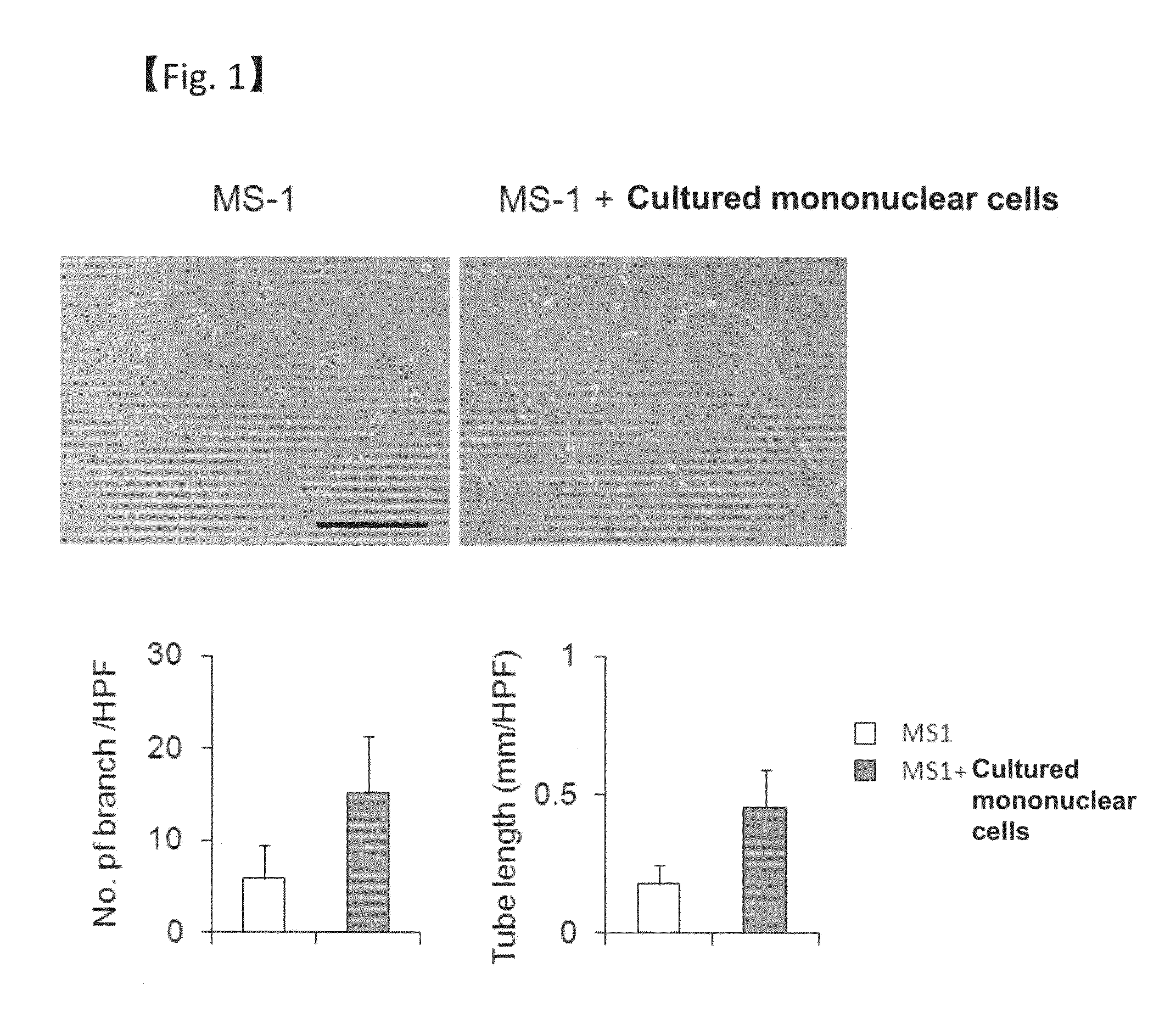
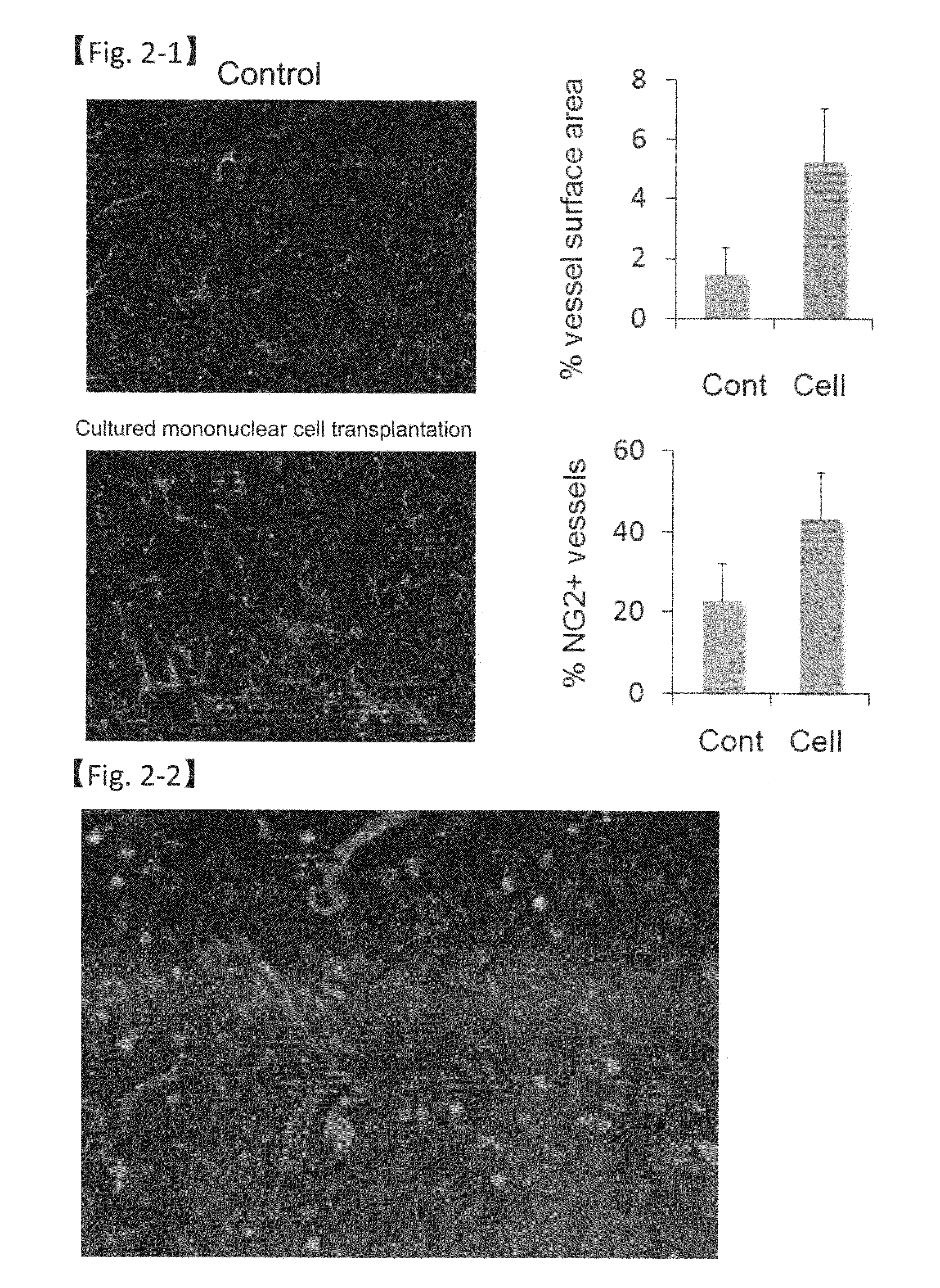
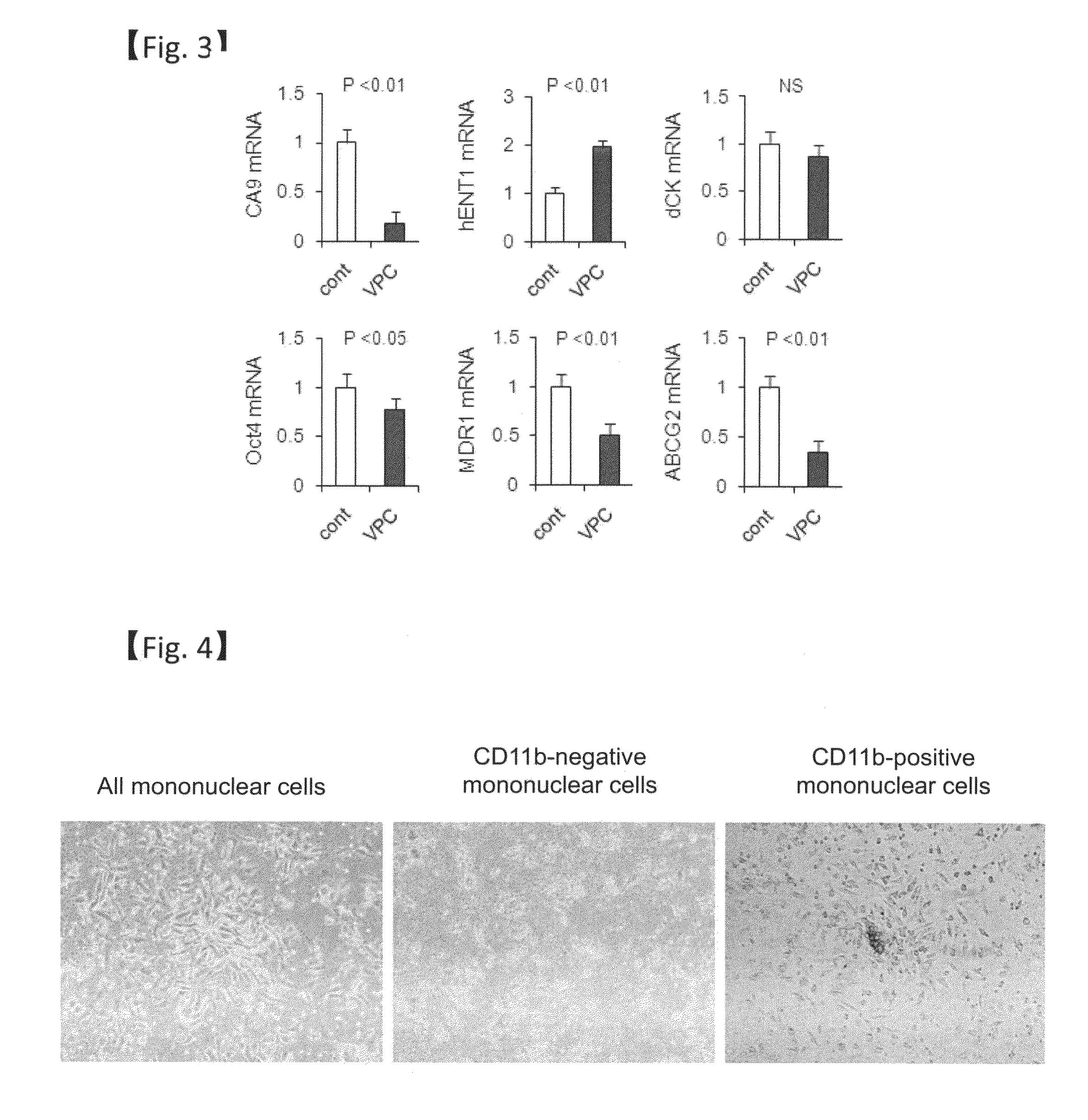
Revascularization cells derived from mononuclear cells, and method of inducing differentiation thereof
InactiveUS20120100610A1Promote lumen formationNo risk of causing infectionMammal material medical ingredientsArtificial cell constructsSerum free mediaTissue repair
The present invention relates to a method of safely and simply inducing differentiation of mononuclear cells into cells that promote neovascular stabilization and maturation, and lead to recovering from ischemia or tissue repair. The cells according to the present invention are obtained by inducing differentiation of a mononuclear cell by culturing the mononuclear cell in a medium (particularly a serum-free medium) containing one or more selected from vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), thrombopoietin (TPO), granulocyte-colony stimulating factor (G-CSF) and FMS-like tyrosine kinase 3 ligand (FLT3L), and collecting a cell population expressing CD11b.
Owner:NAT UNIV ASAHIKAWA MEDICAL UNIV
Preparation method of recombinant human granulocyte colony-stimulating factor
ActiveCN110066331AImprove plasmid retentionFix stability issuesColony-stimulating factorMicroorganism based processesRecombinant escherichia coliG-csf therapy
The invention relates to a preparation method of a recombinant human granulocyte colony-stimulating factor. Specifically, according to the preparation method, a fermentation culture medium containingan inorganic nitrogen source and at least one amino acid is used for culturing recombinant escherichia coli expression strains. By adopting the preparation method, the stability of plasmids is high, and the yield of rhG-CSF in a fermentation liquor is high. In addition, the provided culture medium which is clear in component and simple in control strategy simplifies the process, reduces the cost,increases the protein expression quantity, improves the product quality and is beneficial to realizing industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Traditional Chinese medicine preparation for relieving myelosuppression caused by chemotherapy and preparation method of traditional Chinese medicine preparation
InactiveCN111671864AAvoid side effectsOvercome all the shortcomings of the above drugs with side effectsDigestive systemAntineoplastic agentsGranulocyte colony-stimulating factorRegimen
The invention provides a traditional Chinese medicine preparation for relieving myelosuppression caused by chemotherapy and a preparation method of the traditional Chinese medicine preparation. The traditional Chinese medicine is prepared from the following drugs in parts by weight: 10-30 parts of caulis spatholobi, 20 to 30 parts of rhizoma drynariae, 10 to 30 parts of radix polygoni multiflori preparata, 20 to 30 parts of radix astragali, 15 to 30 parts of malt, 20 to 30 parts of herba epimedii, 20 to 30 parts of ginseng, 15 to 30 parts of Chinese yam, 25 to 30 parts of radix rehmanniae preparata, 20 to 30 parts of radix angelicae sinensis, 5-10 parts of rhizoma chuanxiong, and 10-20 parts of rhizoma atractylodis macrocephalae. The traditional Chinese medicine preparation for relieving myelosuppression caused by chemotherapy overcomes side effects of recombinant human granulocyte colony stimulating factors, improves the physique of a patient while nourishing blood, not only ensures the progress of radiotherapy and chemotherapy courses, but also can increase the curative effect of radiotherapy and chemotherapy, and is low in price, good in curative effect, and convenient to take,and has popularization value.
Owner:中科诺金科技有限公司
Fermentation method of engineering bacteria for preparing recombination human granular cell colony stimulation factor
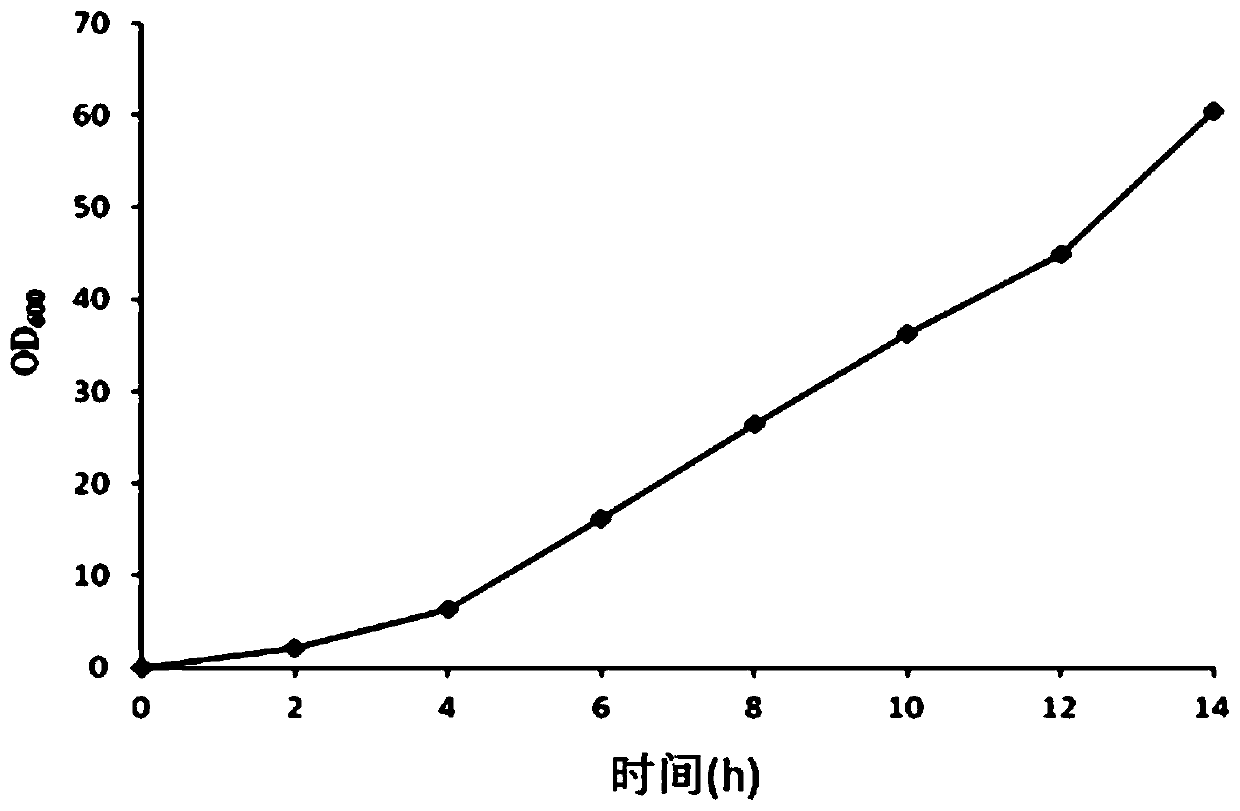
A fermenting method for preparing the engineering bacterium DH5 alpha-PBV220-hGCSF used to prepare the recombinant human granulocyte colony stimulating factor includes such steps as screening bacterial strain, culturing in the class-one seed liquid, culturing in class-two seed liquid, inoculating basic fermenting and induced fermenting while supplementing material every one hour when the OD600 is 2, and terminating the fermentation. Its advantages are high output and high activity.
Owner:深圳未名新鹏生物医药有限公司
Pharmaceutical composition containing recombinant human granulocyte colony-stimulating factors
InactiveCN105311625APeptide/protein ingredientsPharmaceutical non-active ingredientsGranulocyte colony-stimulating factorChlorogenic acid
The invention relates to a pharmaceutical composition containing recombinant human granulocyte colony-stimulating factors. The pharmaceutical composition is prepared from a G-CSF stock solution, mannitol, chlorogenic acid, propylene glycol and water for injection.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
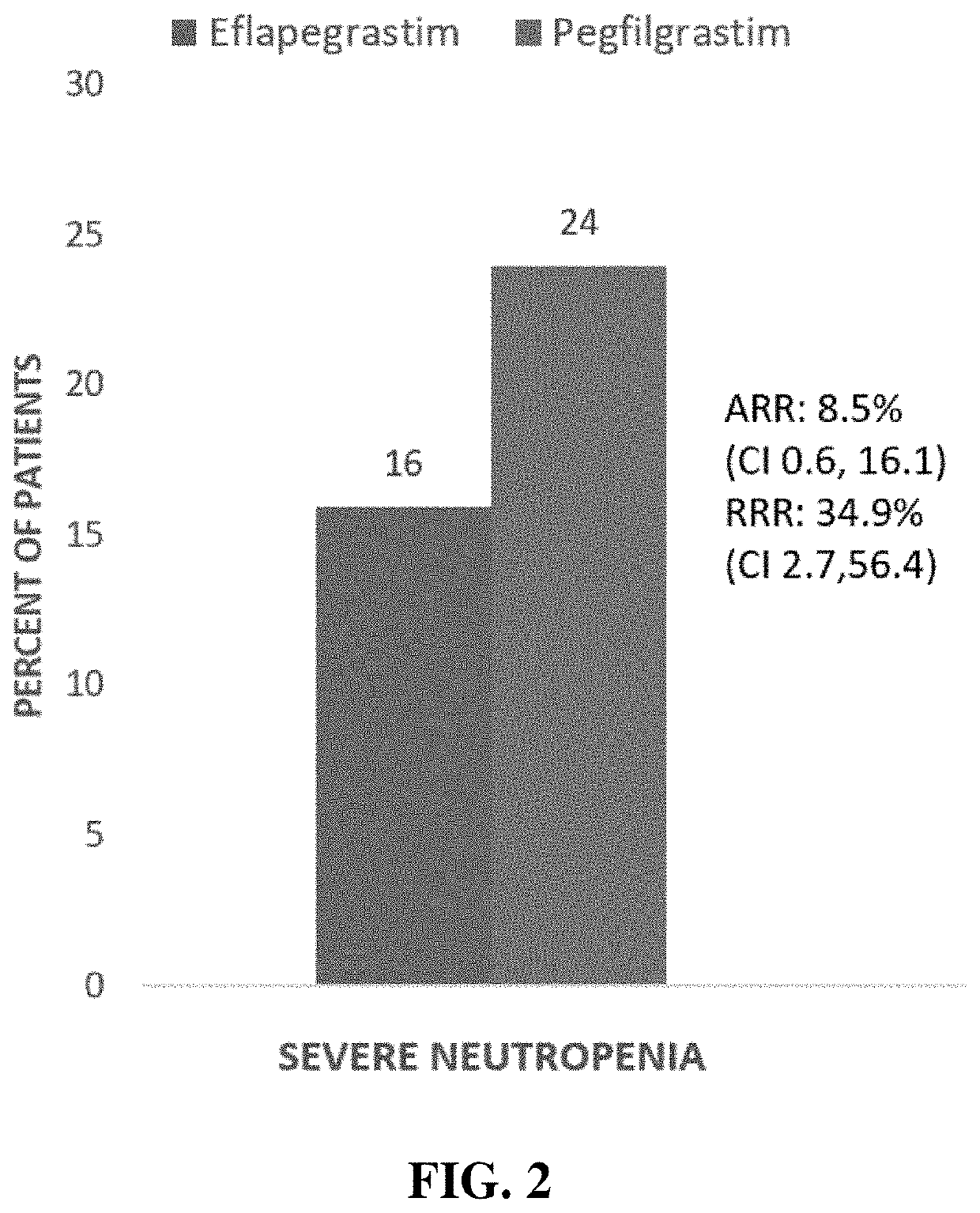
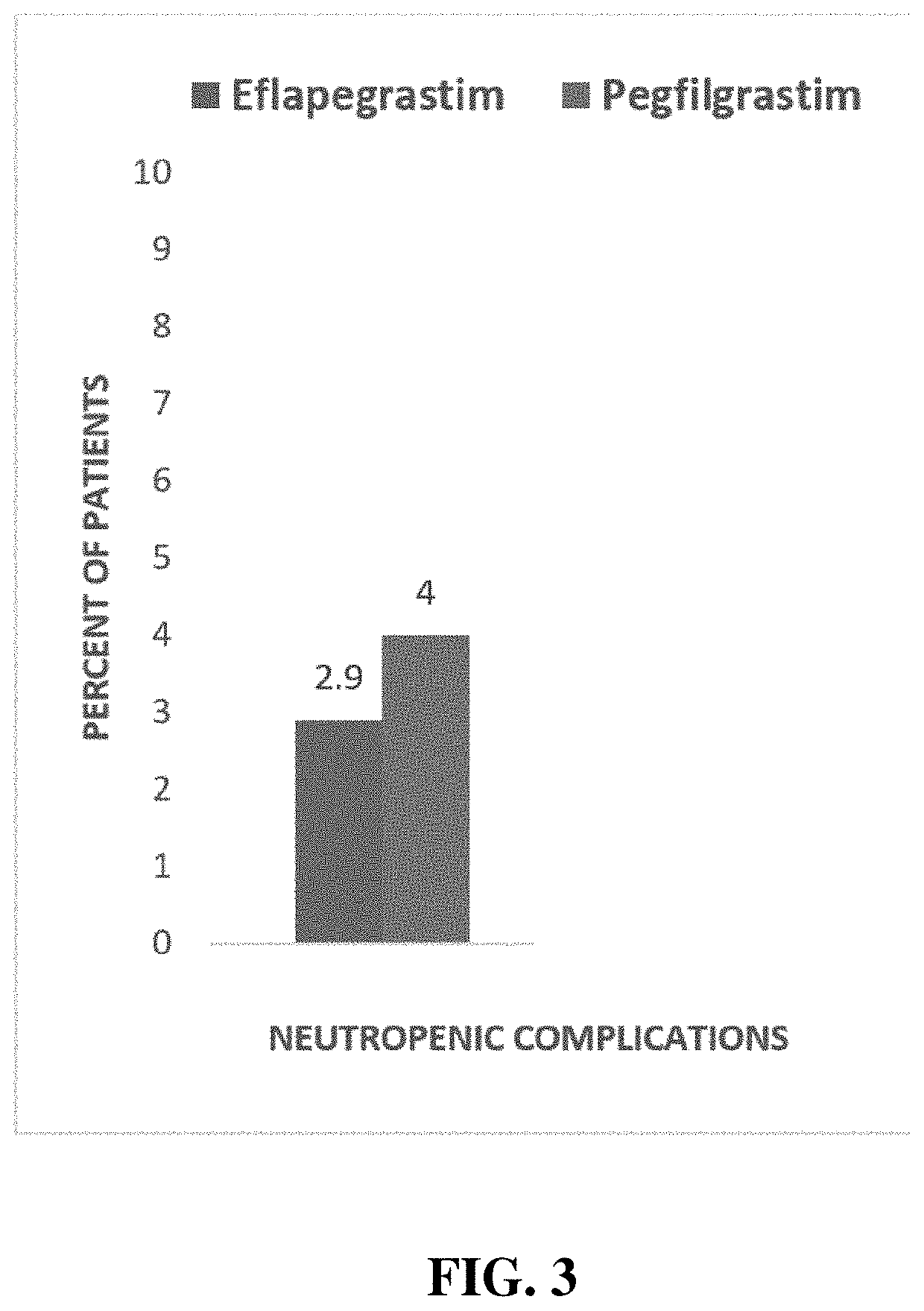
Methods of treatment using G-CSF protein complex
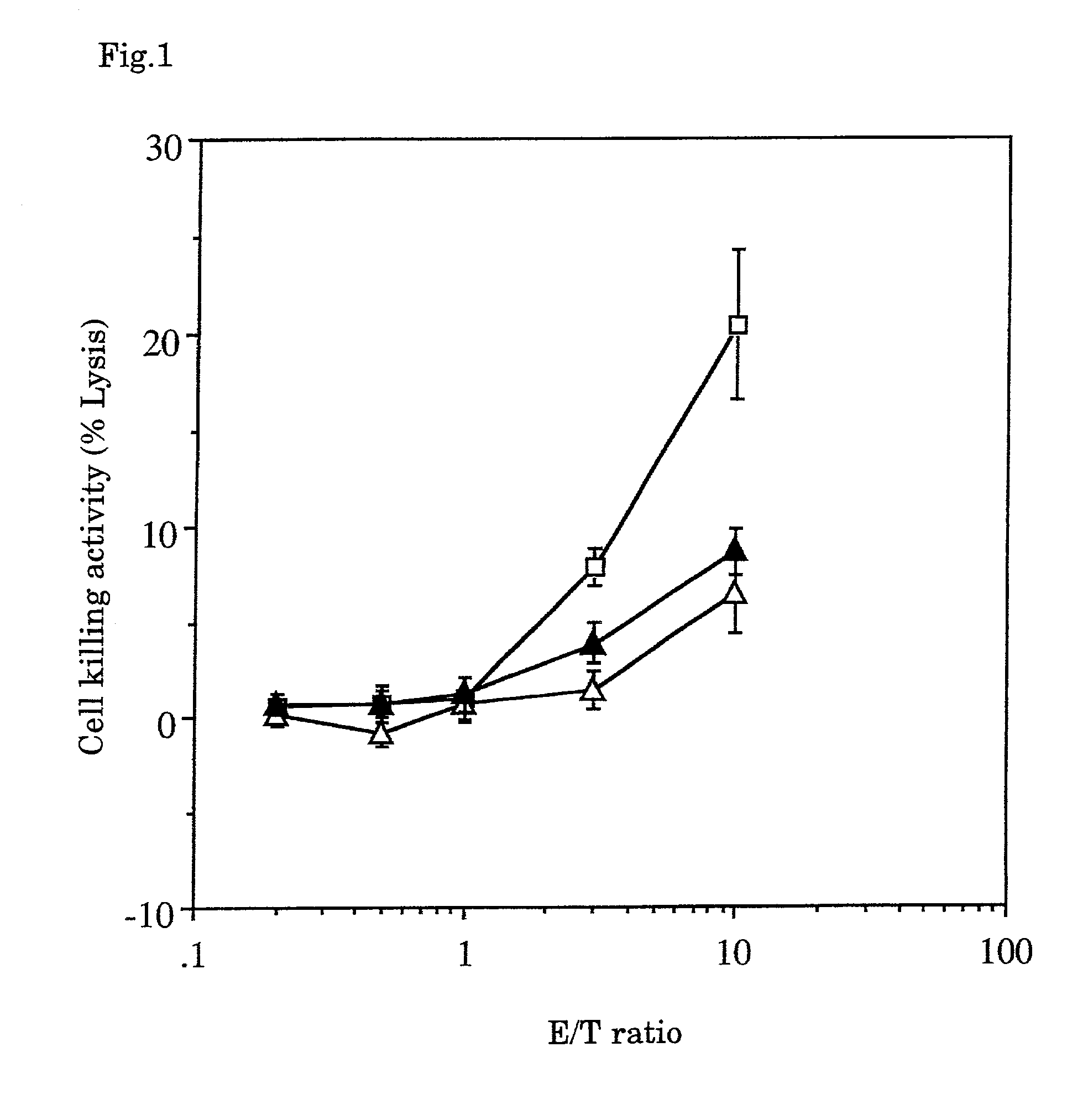
ActiveUS11267858B2Improving in vivo durationImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsGranulocyte colony-stimulating factorWhite blood cell
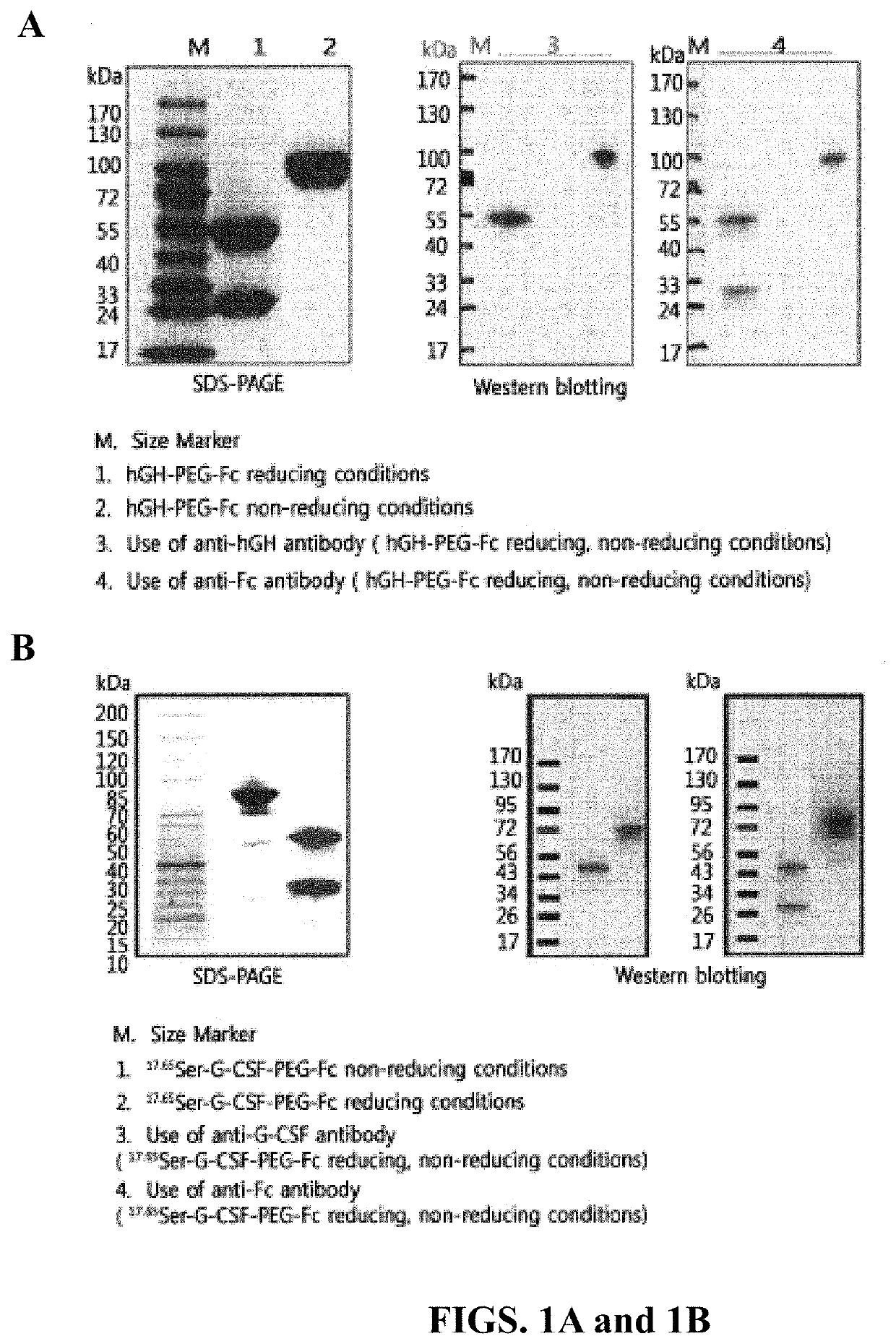
This disclosure provides a method of preventing, alleviating, or treating a condition (i.e., neutropenia) in a patient in need thereof, the condition characterized by compromised white blood cell production in the patient. The method includes administering to the patient a therapeutically effective amount of a protein complex comprising a modified human granulocyte-colony stimulating factor (hG-CSF) covalently linked to an immunoglobulin Fc region via a non-peptidyl polymer. The non-peptidyl polymer is site-specifically linked to an N-terminus of the immunoglobulin Fc region, and the modified hG-CSF comprises substitutions in at least one of Cys17 and Pro65.
Owner:SPECTRUM PHARMA INC
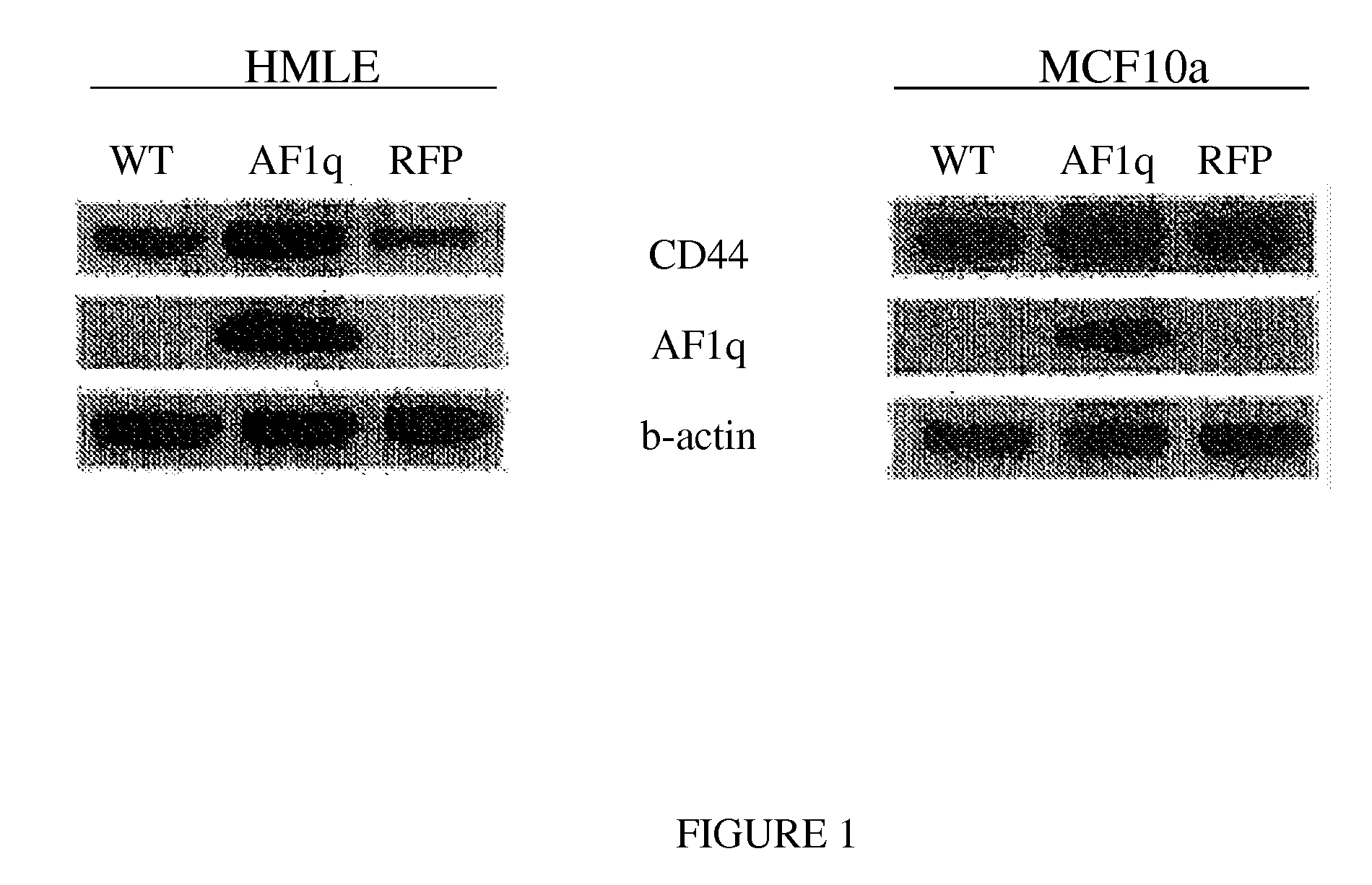
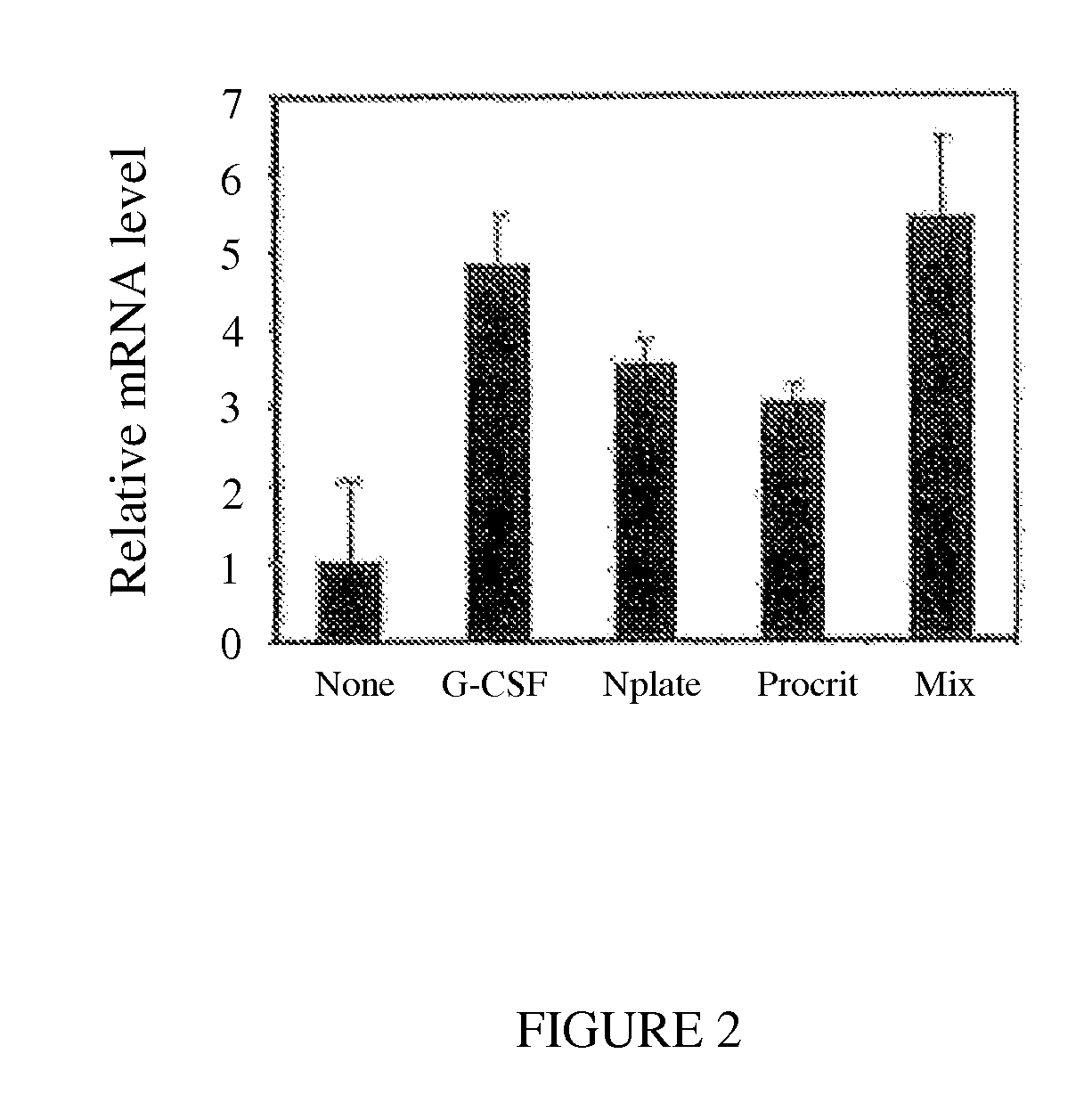
Method for Enhancing Umbilical Cord Blood Engraftment
InactiveUS20130259825A1Easy to implantBone marrow stroma cellsSkeletal/connective tissue cellsStem cell productRed blood cell
A method for enhancing bone marrow cells, peripheral blood, and umbilical cord blood engraftment is disclosed wherein at least one of a granulocyte colony stimulating factor and a granulocyte macrophage colony stimulating factor, and optionally romiplostim, and optionally an erythropoiesis-stimulating agent, are added thereto for enhancing the engraftment of CD44 cells within the bone marrow cells, peripheral blood, and umbilical cord blood. Also provided is a method for enhancing a stem cell infusion by activating an up-regulation of a AF1q / CD44 signaling pathway. A stem cell product is disclosed having treated stem cells conditioned for activating an up-regulation of a AF1q / CD44 signaling pathway.
Owner:WEST VIRGINIA UNIVERSITY
Preparation method of leucoderma melanocyte transplanting coating carrier system
InactiveCN102266586BReduce churnWon't hurtArtificial cell constructsVertebrate cellsGranulocyte colony-stimulating factorMelanocyte
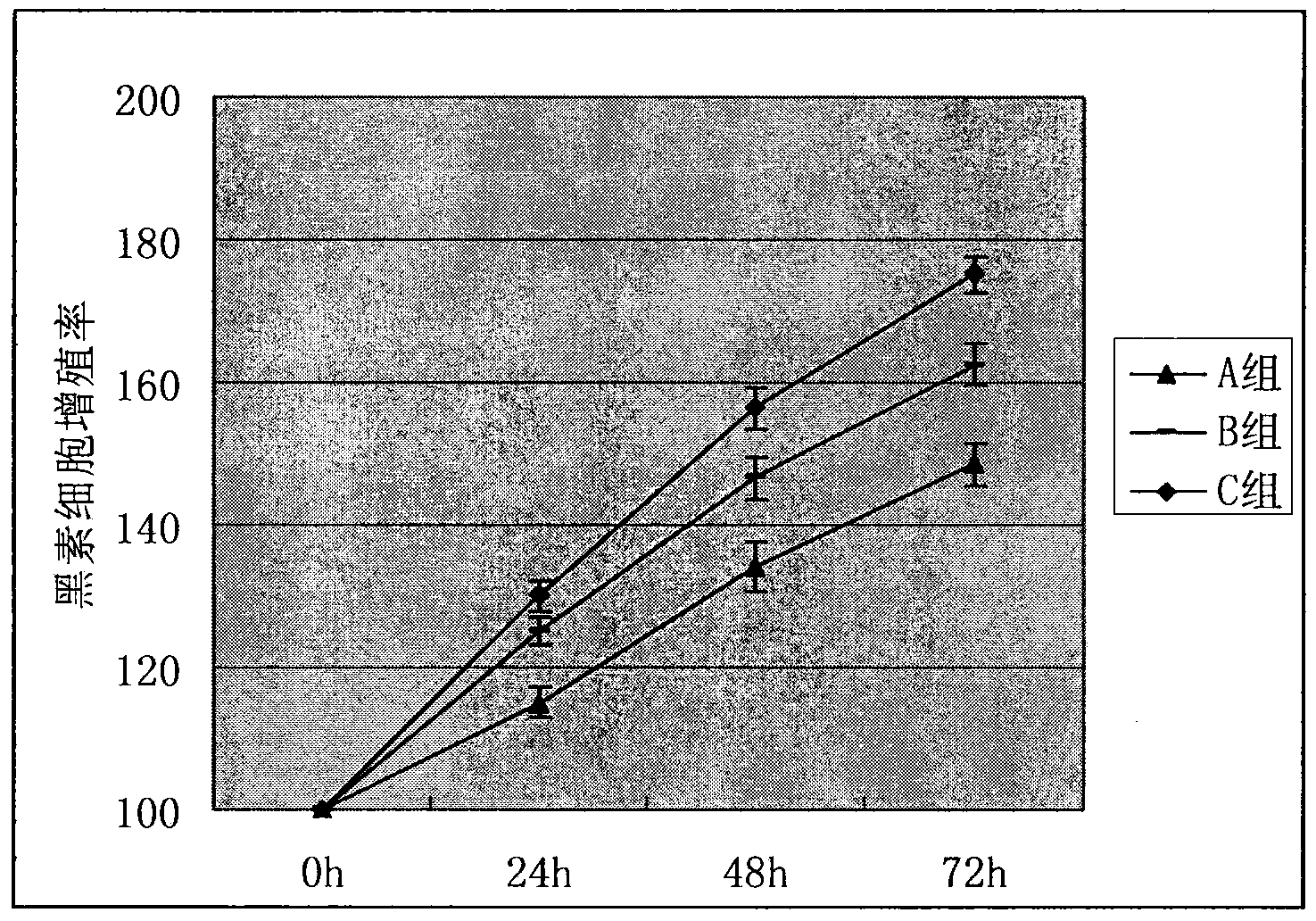
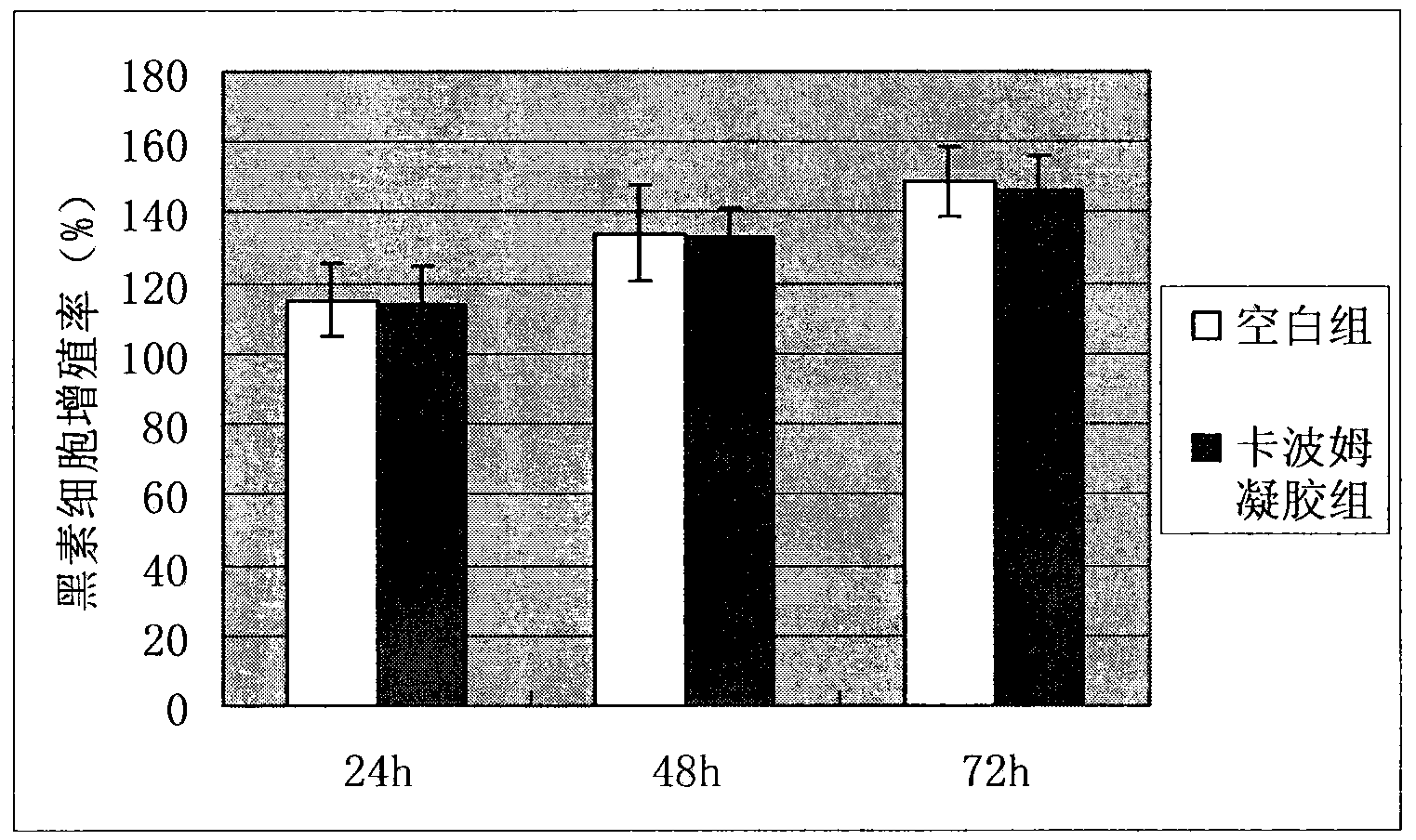
The invention relates to a method for preparing a coating carrier system for vitiligo melanocyte transplantation. , epinephrine and vitamin C mixed solution, pentamycin to configure melanocyte culture medium for the in vitro culture of autologous melanocytes, and use Carbomer gel and the above melanocyte culture medium to form a coating Cloth carrier system. The present invention uses the melanocyte culture medium containing human granulocyte colony-stimulating factor G-CSF to make the melanocyte proliferate in large quantities in vitro; The problem of easy loss of cell suspension during cell transplantation can reduce the loss of melanocytes, increase the implantation rate without causing damage to melanocytes, and reduce the cost of surgery. Treatment offers new avenues.
Owner:JIANGSU PROVINCE HOSPITAL
PEGylated recombinant human granulocyte colony stimulating factor freeze-dried preparation
ActiveCN113797171AImprove medication safetyImprove stabilityPowder deliveryPeptide/protein ingredientsGranulocyte colony-stimulating factorFreeze-drying
The invention provides a PEGylated recombinant human granulocyte colony stimulating factor (PEG-rhG-CSF) freeze-dried preparation. The PEG-rhG-CSF and an excipient are subjected to spray freeze-drying treatment, when the prepared PEG-rhG-CSF freeze-dried preparation is clinically used, a special solvent containing sodium chloride and acetate is prepared, and the freeze-dried preparation can be used after being mixed and dissolved with the special solvent. The PEG-rhG-CSF freeze-dried preparation prepared by the invention does not contain a surfactant namely Tween 20, is high in medication safety and good in stability, can be stored for a long time, and is simple in preparation process and short in production period.
Owner:SHANDONG NEWTIME PHARMA
Methods of Treatment Using G-CSF Protein Complex
PendingUS20220153799A1Easy to prepareStable protein complexPeptide/protein ingredientsAntibody mimetics/scaffoldsGranulocyte colony-stimulating factorWhite blood cell
This disclosure provides a method of preventing, alleviating, or treating a condition (i.e., neutropenia) in a patient in need thereof, the condition characterized by compromised white blood cell production in the patient. The method includes administering to the patient a therapeutically effective amount of a protein complex comprising a modified human granulocyte-colony stimulating factor (hG-CSF) covalently linked to an immunoglobulin Fc region via a non-peptidyl polymer. The non-peptidyl polymer is site-specifically linked to an N-terminus of the immunoglobulin Fc region, and the modified hG-CSF comprises substitutions in at least one of Cys17 and Pro65.
Owner:SPECTRUM PHARMA INC
Method for detecting biological activity of granulocyte colony stimulating factors
PendingCN113702340AEasy to operateNo misadditionFluorescence/phosphorescenceGranulocyte colony-stimulating factorTest sample
The invention provides a method for detecting biological activity of granulocyte colony stimulating factors. The method comprises the following three steps: constructing a cell line, preparing a standard substance and a test sample, and determining. The detection method is more convenient to operate, cells do not need to be specially treated, the operation time is shortened, and the experiment operation time is shortened by 1-2 days; meanwhile, according to the method, the detection flux is improved, the sample is diluted by 6-12 concentration points, the sample can be vertically arranged in a 96-well plate, and the sample detection number of each cell plate is increased.
Owner:江苏奥赛康生物医药有限公司
Protein with chemical modified groups and preparation method thereof
ActiveCN106589051AUniform compositionPeptide preparation methodsGenetic engineeringGranulocyte colony-stimulating factorHuman growth hormone
The invention relates to a technology for modifying chemical groups to the C-terminal of protein in a fixed-point mode through a protein trans-splicing technology. Concretely, by the utilization of the protein trans-splicing technology, polyethylene glycol (PEG) is modified to the C terminal of a granulocyte colony-stimulating factor (G-CSF), the C terminal f a human growth hormone (hGH), the C terminal of human interferon alpha 2b (IFN alpha 2b), the C terminal of interleukin-15 (ILK-15) and the C terminal of urate oxidase (UOX). The invention provides a method for preparing fixed-point C-end modified medicine. Meanwhile, the invention provides a protein intron sequence for fixed-point modified protein polypeptide drugs.
Owner:戴旭东 +1
Derivatives of recombinant proteins, homo-multimers of granulocyte colony-stimulating factor and method of preparation thereof
ActiveUS9243049B2Prolong circulation time in the bodyExtended circulation timeAntibody mimetics/scaffoldsDepsipeptidesActive proteinG-csf therapy
Owner:UAB PROFARMA
Method for improving immune effect of newcastle disease inactivated vaccine by utilizing adenovirus for expressing GM-CSF (granulocyte macrophagecolony stimulating factor) and kit
InactiveCN106177941AImprove immunityImprove efficiencyViral antigen ingredientsPharmaceutical delivery mechanismGranulocyte colony-stimulating factorFowl
The invention relates to the technical field of microorganisms and biology, in particular to a method for improving an immune effect of newcastle disease inactivated vaccine by utilizing an adenovirus for expressing a GM-CSF (granulocyte macrophagecolony stimulating factor), and a kit. The method comprises the following steps: uniformly mixing a recombinant adenovirus for expressing the GM-CSF and the newcastle disease inactivated vaccine to obtain a vaccine immunity adjuvant mixture; then, performing immunization on poultry by utilizing the vaccine immunity adjuvant mixture. The invention also relates to an immunity adjuvant which is formed by the recombinant adenovirus for expressing the GM-CSF, and the kit which consists the recombinant adenovirus for expressing the GM-CSF and the newcastle disease inactivated vaccine. The method and the kit can be used for the poultry culturing industry.
Owner:王兴龙
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com