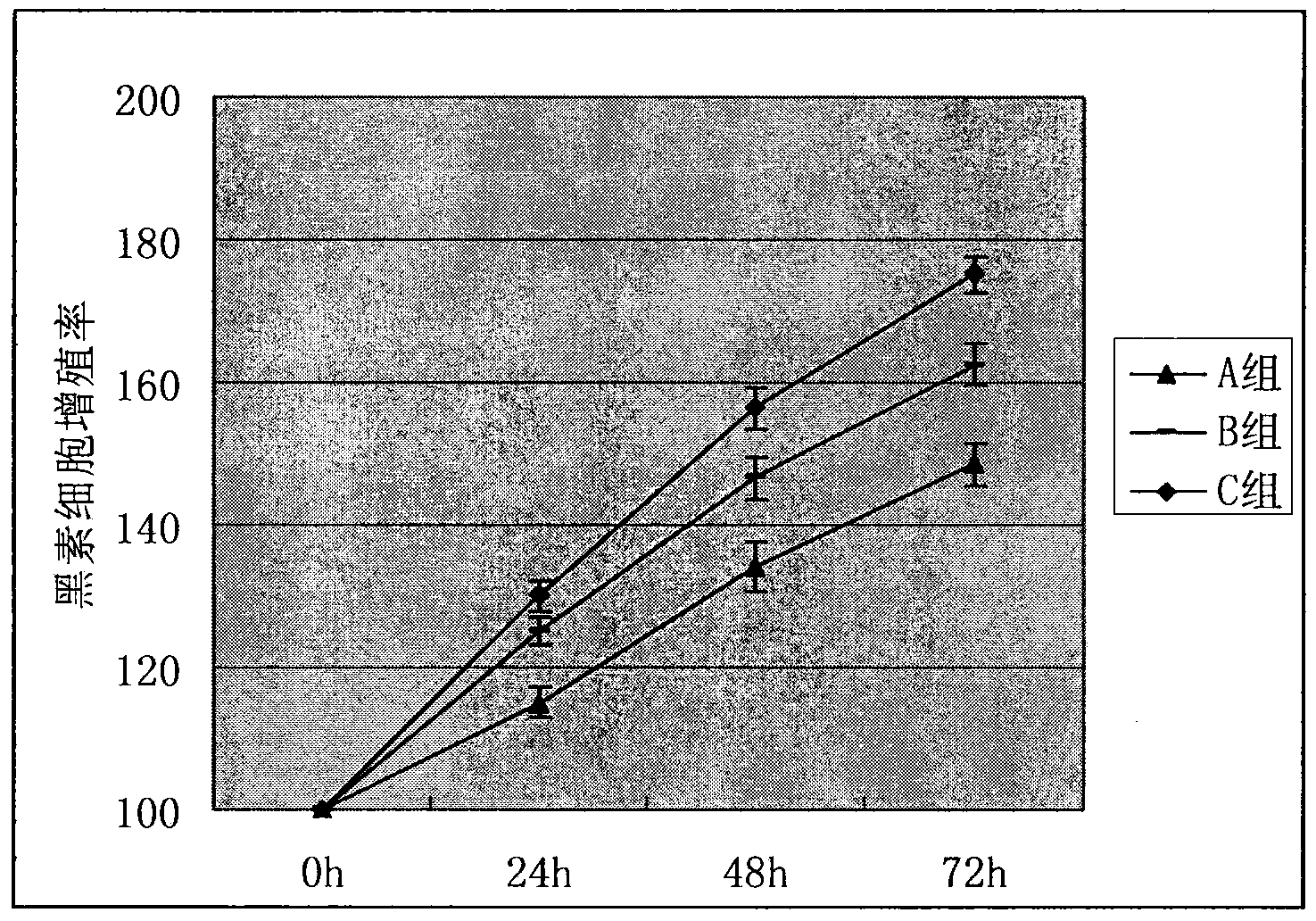
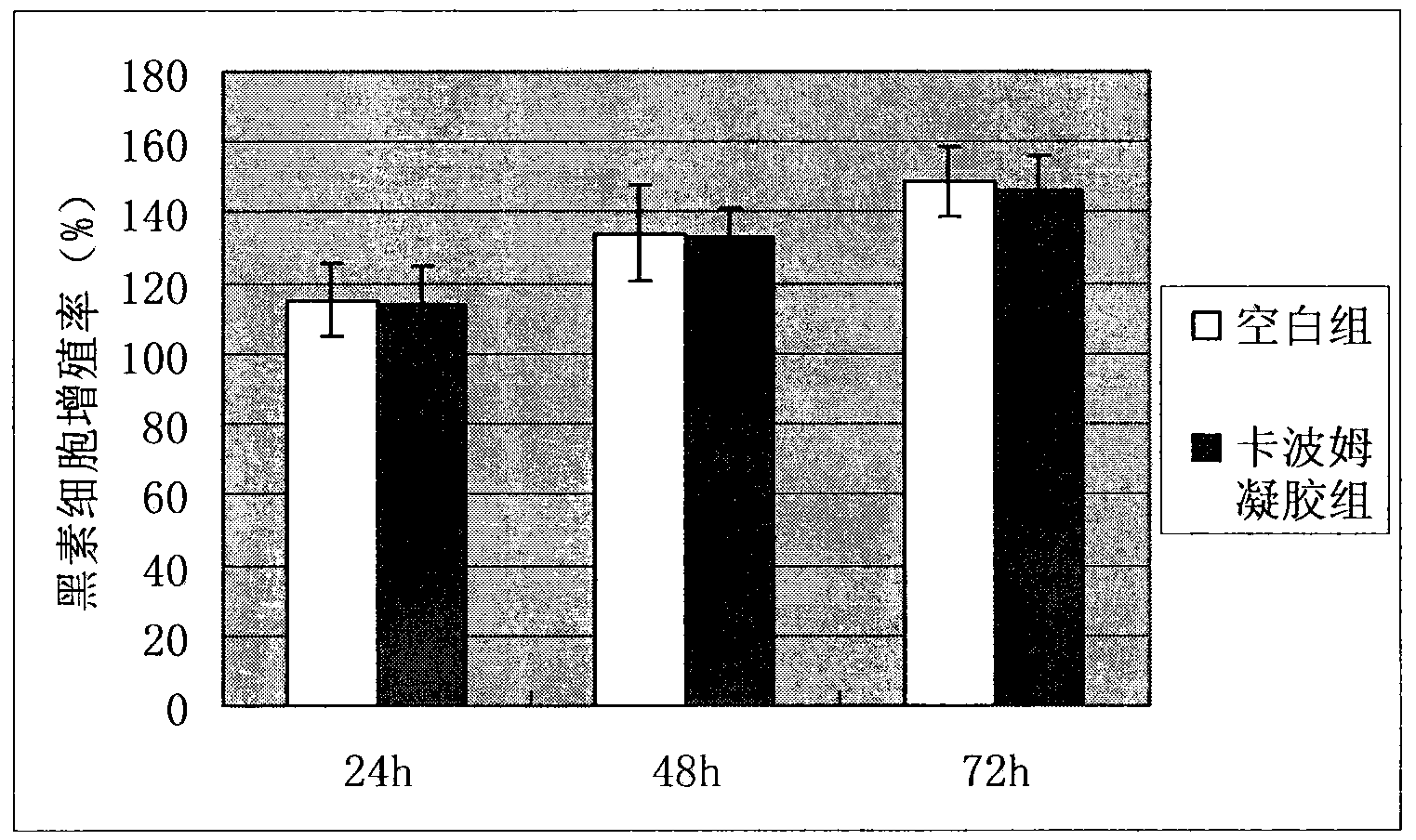
Preparation method of leucoderma melanocyte transplanting coating carrier system
A melanocyte and carrier system technology is applied in the field of preparation of vitiligo melanocyte transplantation coating carrier system to achieve the effects of solving the easy loss of cell suspension, reducing the loss and improving the implantation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of melanocyte culture medium:
[0026] Take 100 milliliters (mL) of melanocyte basal culture medium (Hams F12), add 10 mL of fetal bovine serum (FBS), 200 microliters (μL) of human granulocyte colony-stimulating factor G-CSF (trade name: Ruibai), the concentration 100 μL of melanopoietin α-MSH at 100 nanograms / milliliter (ng / mL), 100 μL of isomethylxanthine IBMX, 1.0 mL of a mixture of adrenaline and vitamin C at a concentration of 1.5 μg / mL (μg / mL) , 1.0 mL of penicillin-streptomycin solution, stored in a refrigerator at 4°C.
[0027] (2) In vitro culture of melanocytes:
[0028] The skin donor area was routinely disinfected, and autologous skin samples were obtained under sterile conditions; after washing with phosphate buffered saline (PBS), 9 mL of 0.25% trypsin-EDTA digestion solution was added, and digested in a 37°C incubator for 14 minutes.
[0029] Stop digestion with fetal bovine serum (1:1 volume ratio) or medium containing serum, pipette to...
Embodiment 2
[0035] (1) Preparation of melanocyte culture medium:
[0036] Take 120 milliliters (mL) of melanocyte basal culture medium (Hams F12), add 12 mL of fetal bovine serum (FBS), and 170 microliters (μL) of human granulocyte colony-stimulating factor G-CSF (trade name: Ruibai), the concentration Melanopoietin α-MSH 120 μL at 100 nanograms / ml (ng / mL), isomethylxanthine IBMX 110 μL, a mixture of epinephrine and vitamin C at a concentration of 1.5 μg / ml (μg / mL) 0.8 mL , 1.2 mL of penicillin-streptomycin solution, stored in a refrigerator at 4°C.
[0037] (2) In vitro culture of melanocytes:
[0038] The skin donor area was routinely disinfected, and autologous skin samples were obtained under sterile conditions; after washing with phosphate buffered saline (PBS), 8 mL of 0.25% trypsin-EDTA digestion solution was added, and digested in a 37°C incubator for 10 minutes.
[0039]Use fetal bovine serum (1:1 volume ratio) or serum-containing medium to stop digestion, pipette to obtain cel...
Embodiment 3
[0045] (1) Preparation of melanocyte culture medium:
[0046] Take 80 milliliters (mL) of melanocyte basal culture medium (Hams F12), add 8 mL of fetal bovine serum (FBS), 230 microliters (μL) of human granulocyte colony-stimulating factor G-CSF (trade name: Ruibai), the concentration Melanopoietin α-MSH 80 μL at 100 nanograms / milliliter (ng / mL), isomethylxanthine IBMX 90 μL, a mixture of epinephrine and vitamin C at a concentration of 1.5 μg / mL (μg / mL) 1.2 mL , 0.8 mL of penicillin-streptomycin solution, stored in a refrigerator at 4°C.
[0047] (2) In vitro culture of melanocytes:
[0048] The skin donor area was routinely disinfected, and autologous skin samples were obtained under sterile conditions; after washing with phosphate buffered saline (PBS), 10 mL of 0.25% trypsin-EDTA digestion solution was added, and digested in a 37°C incubator for 15 minutes.
[0049] Use fetal bovine serum (1:1 volume ratio) or serum-containing medium to stop digestion, pipette to obtain c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com