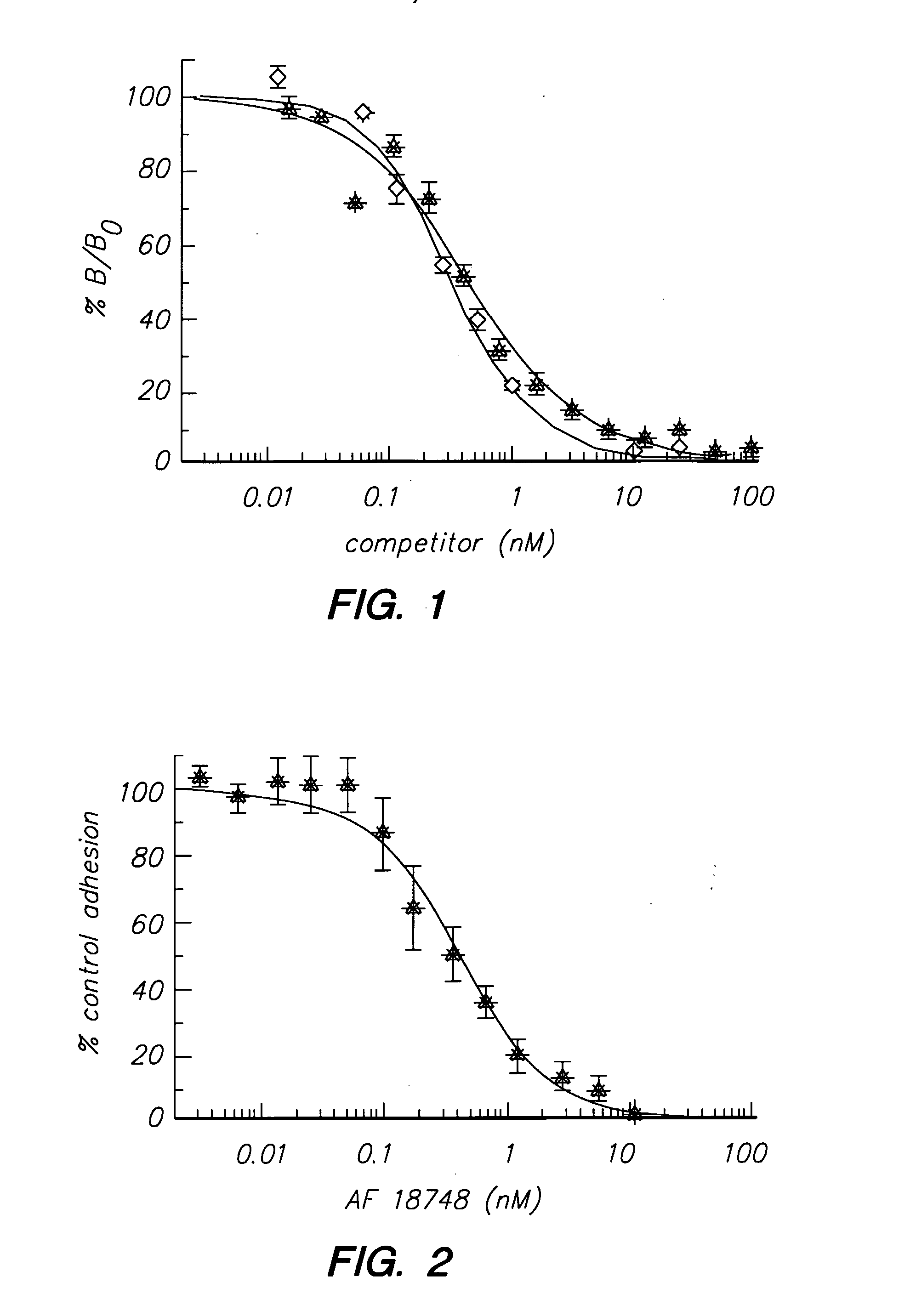
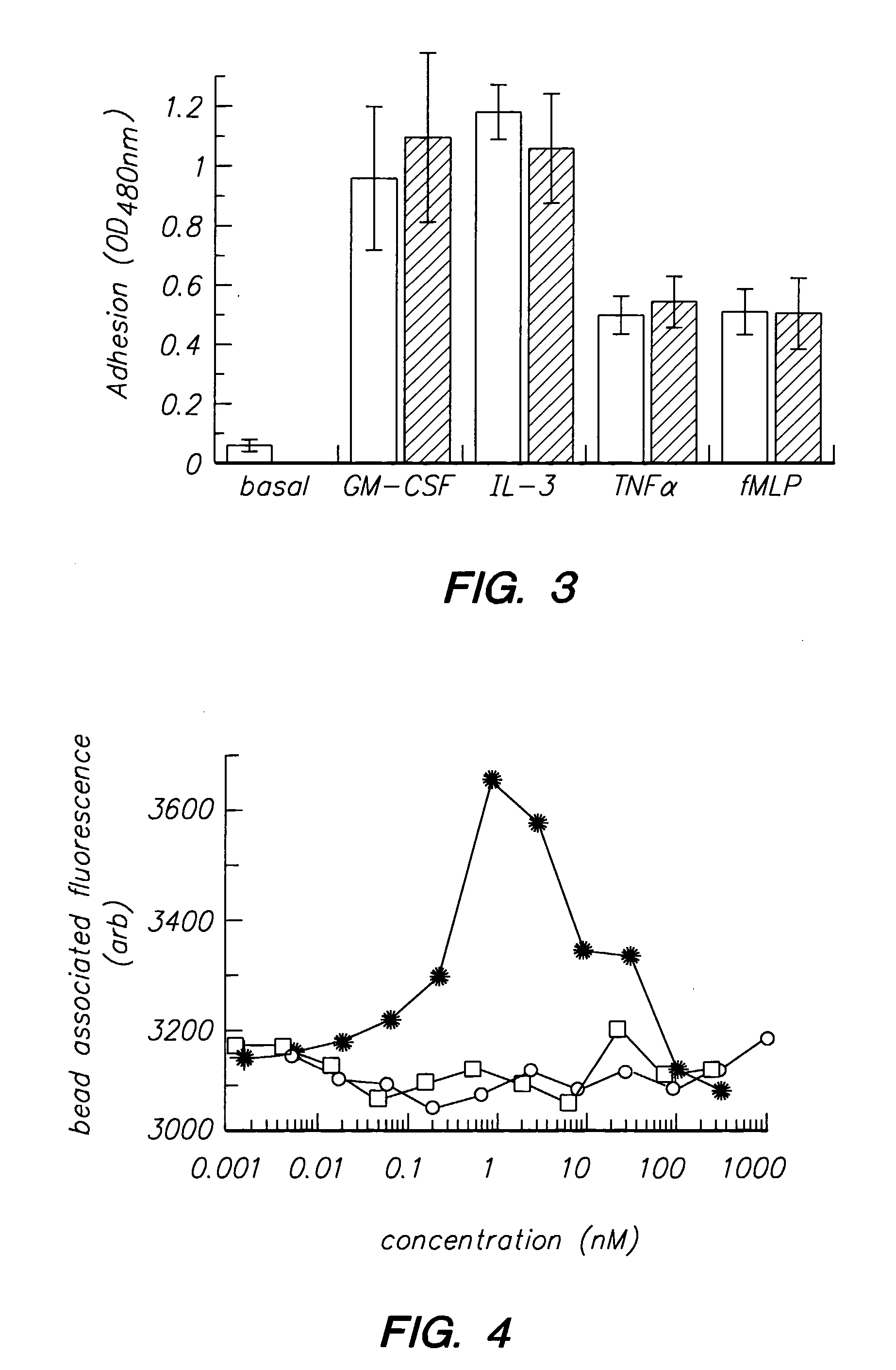
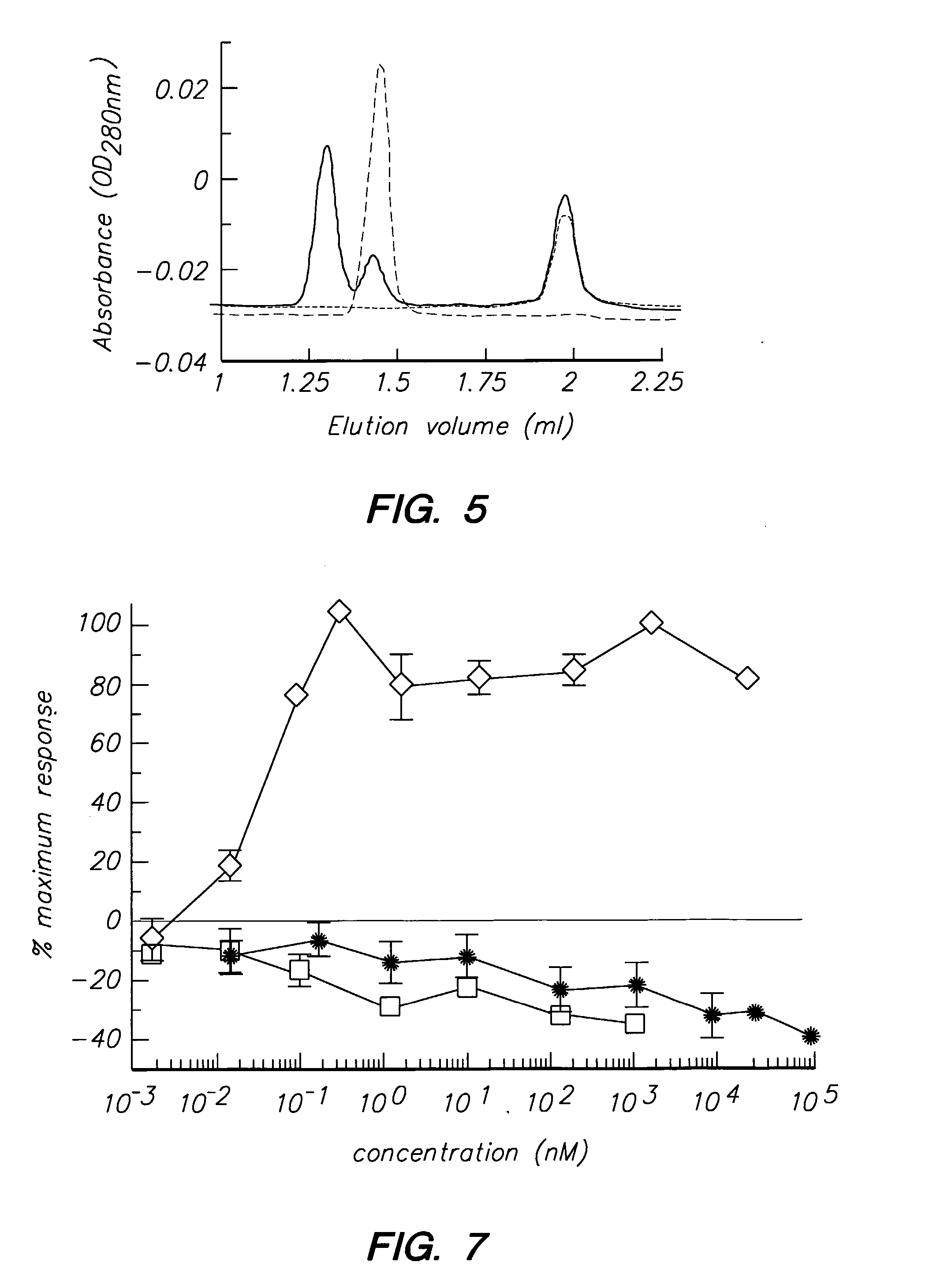
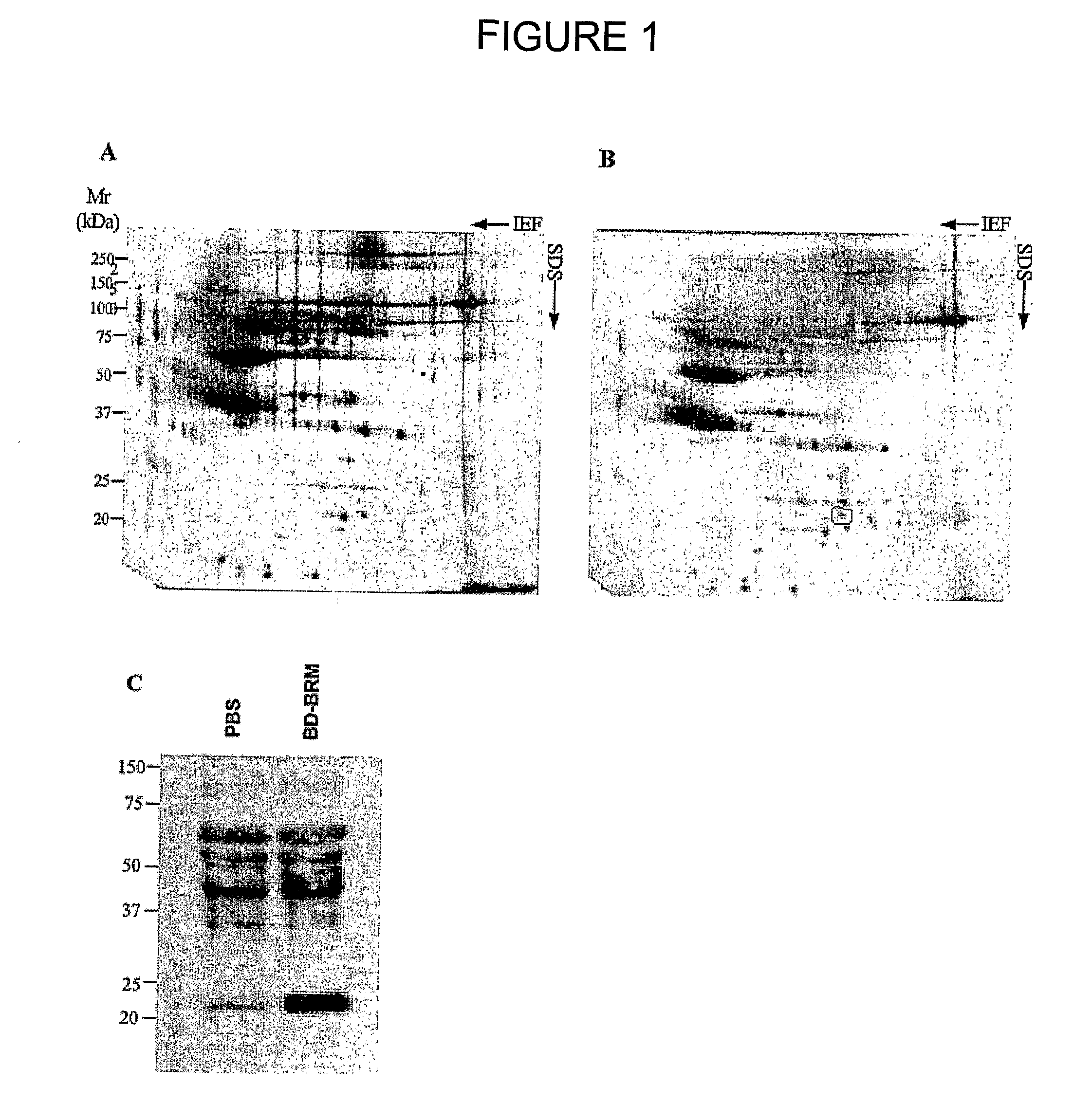
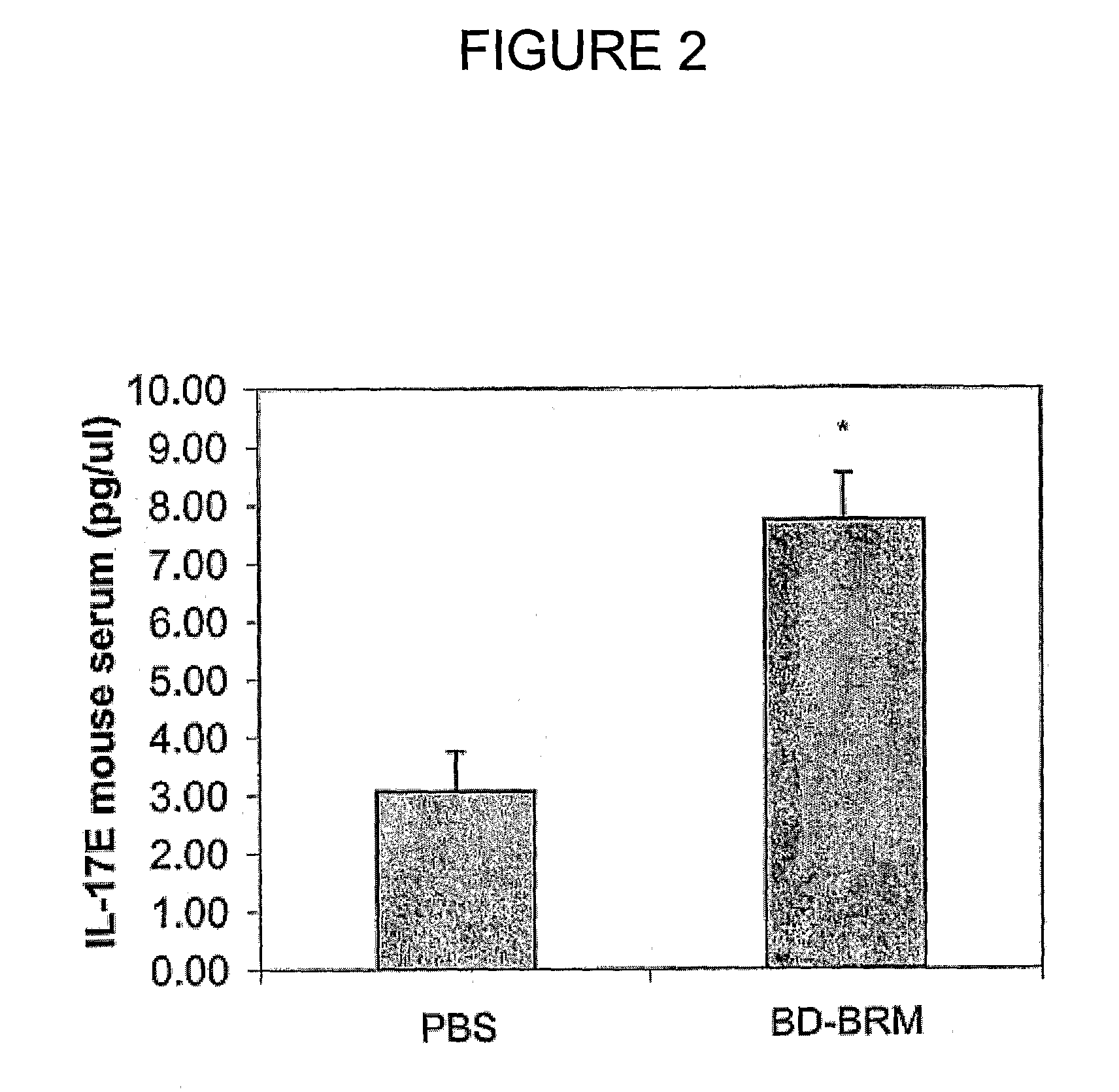
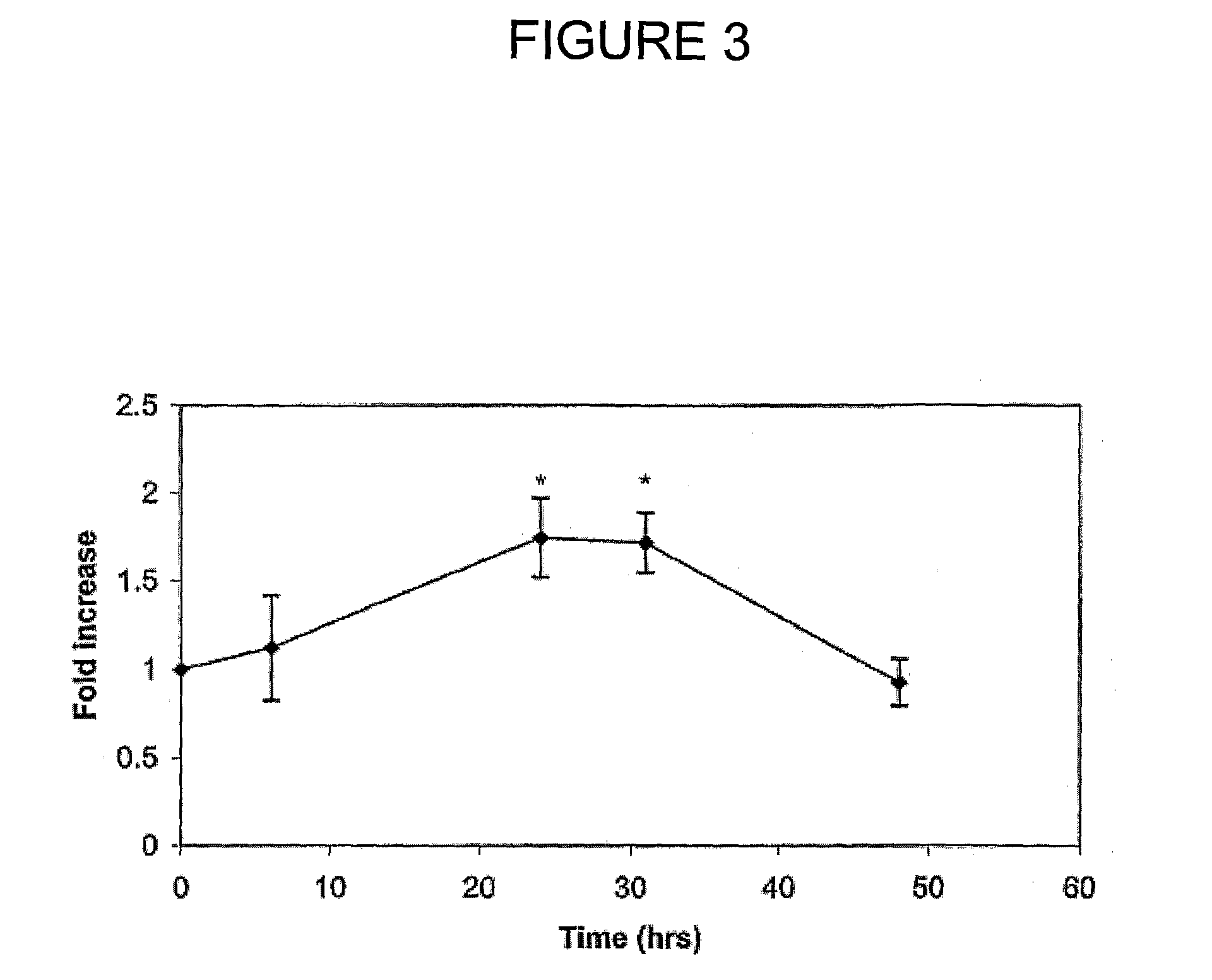
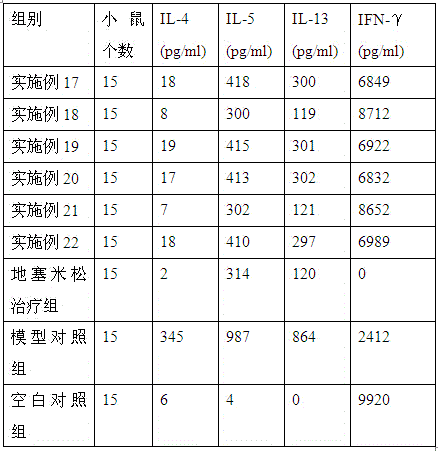
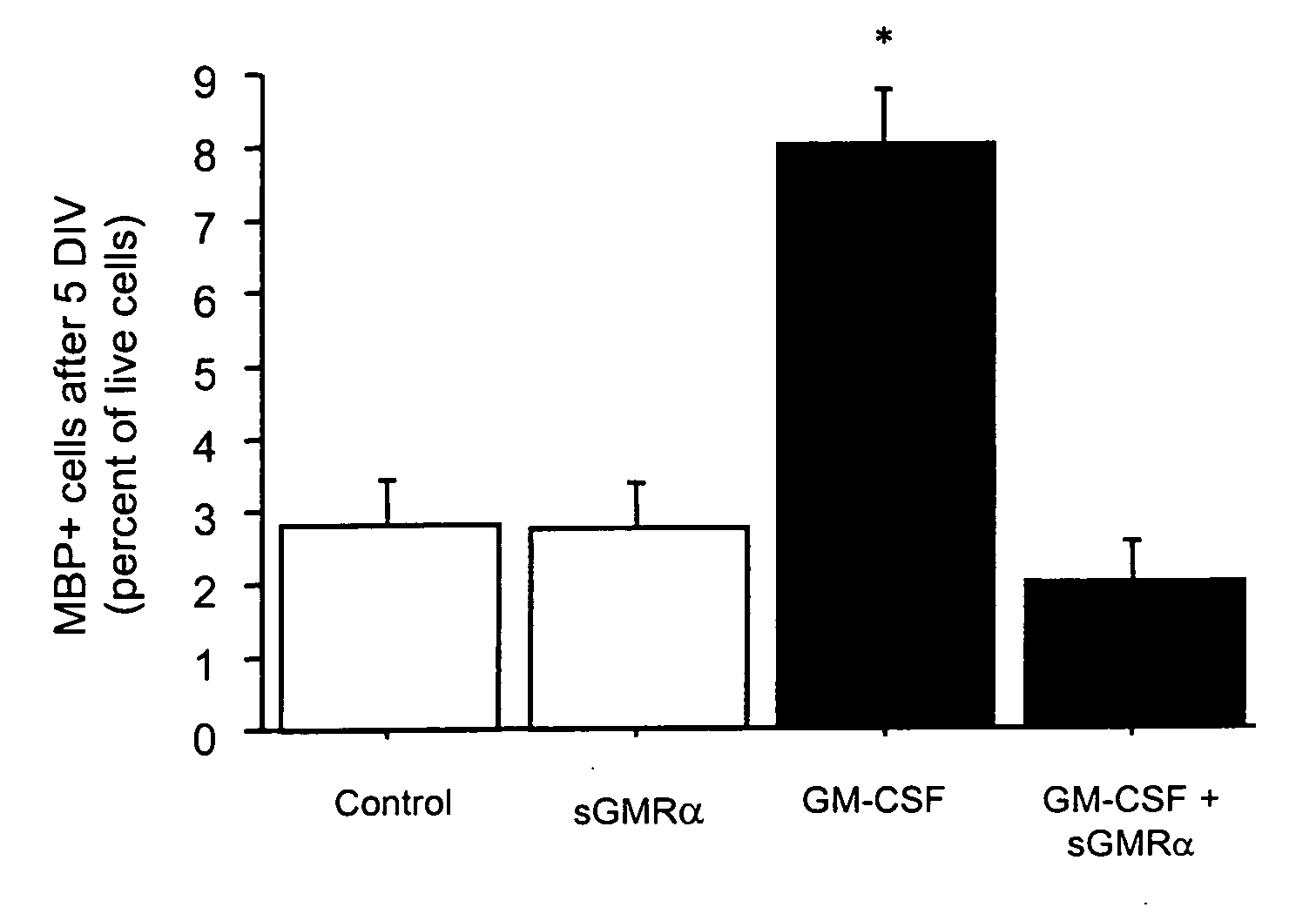
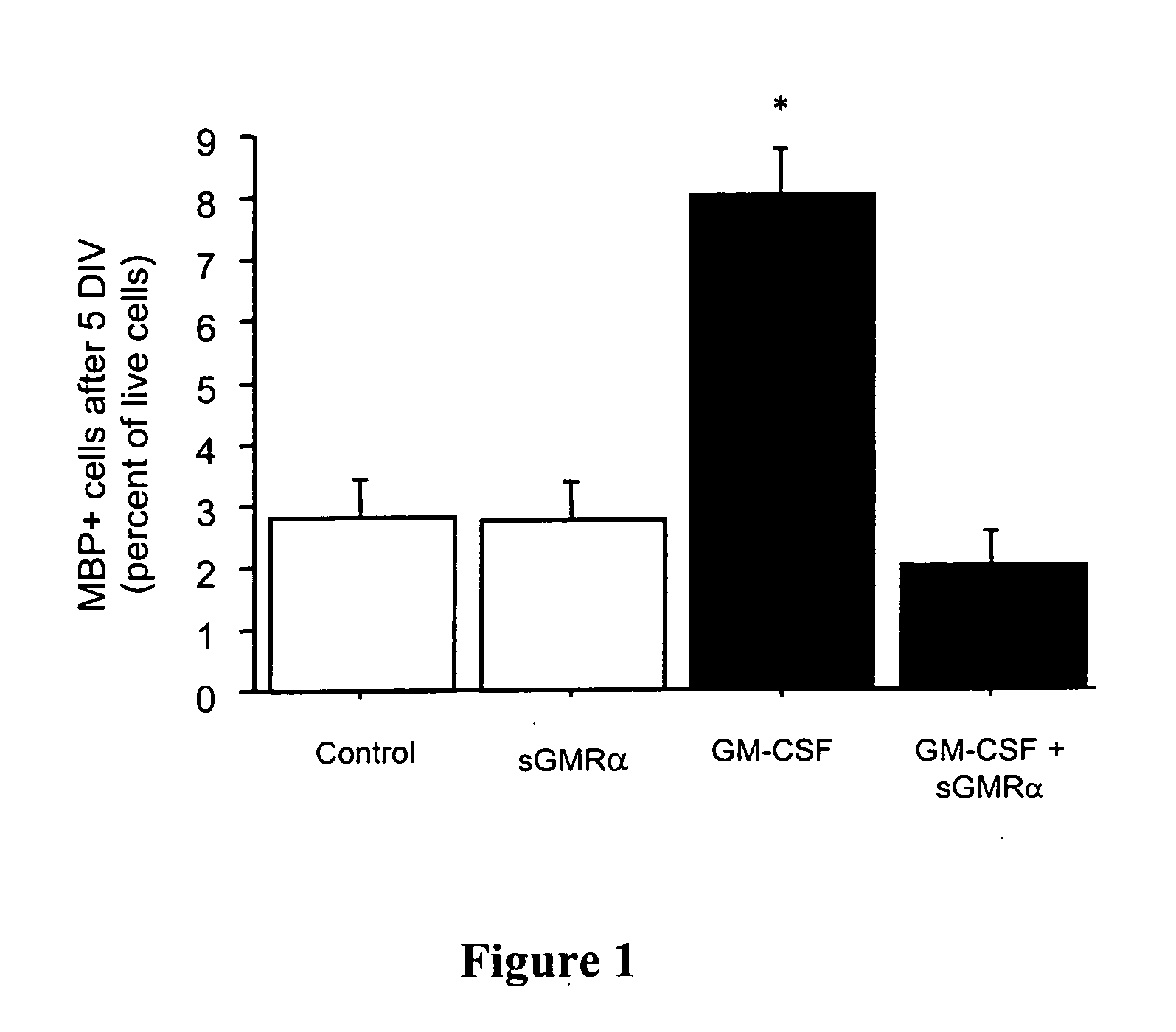
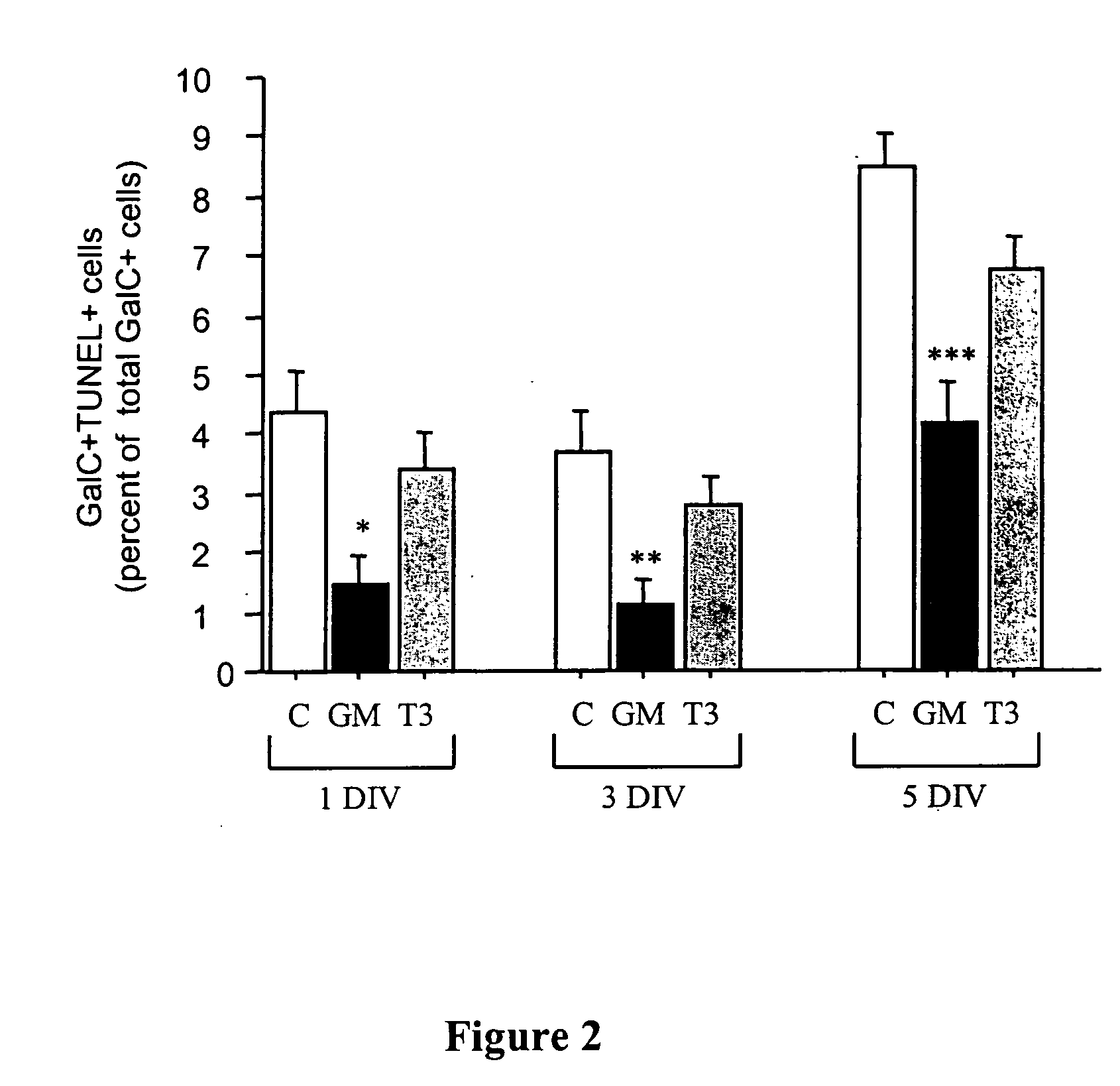
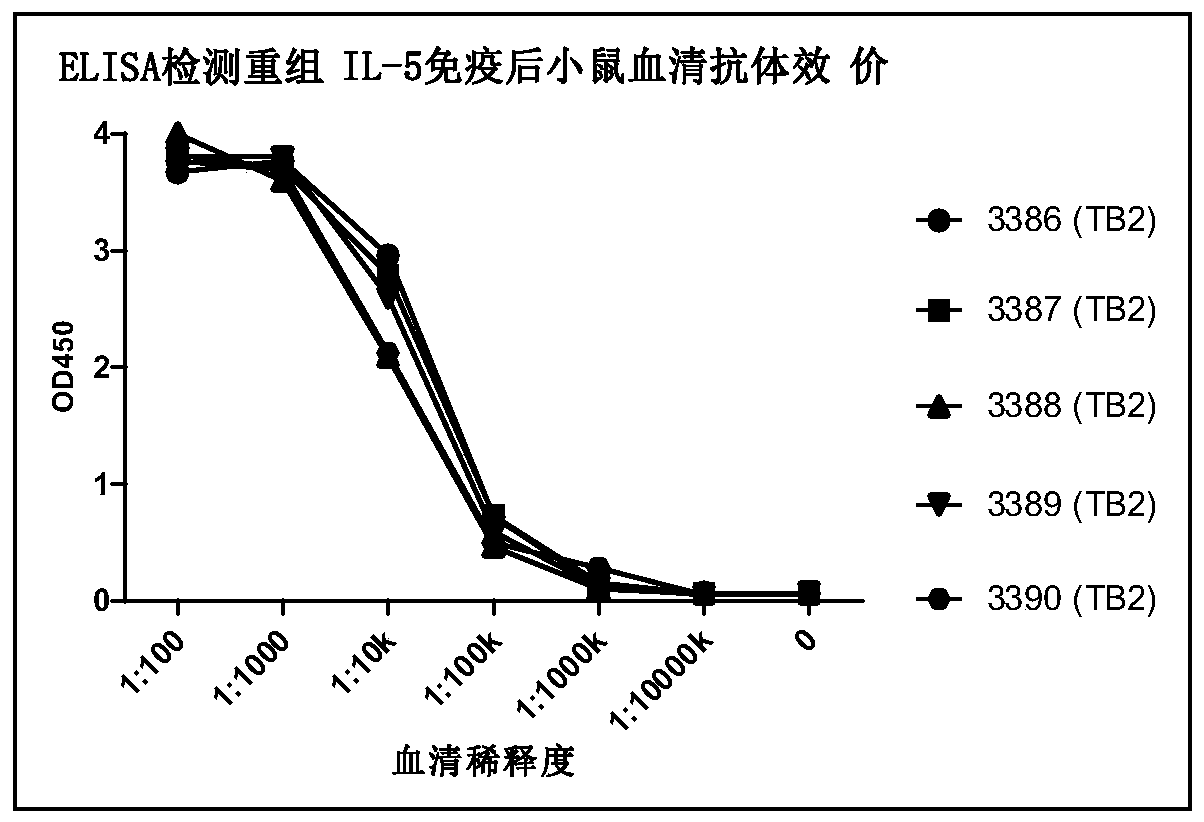
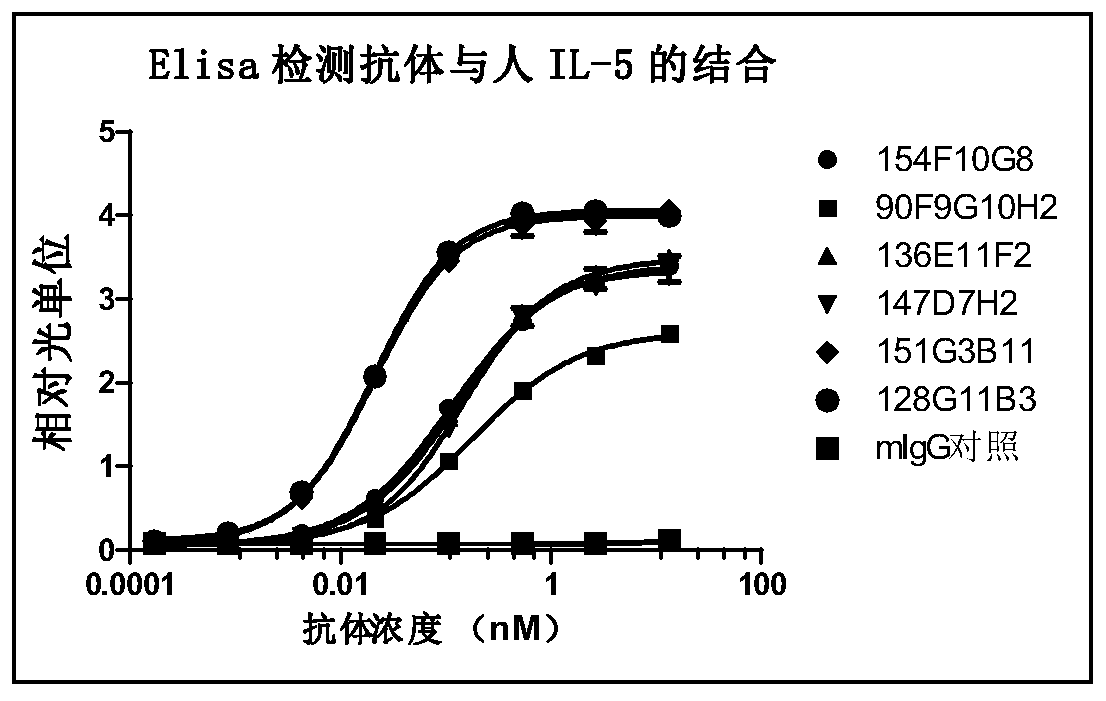
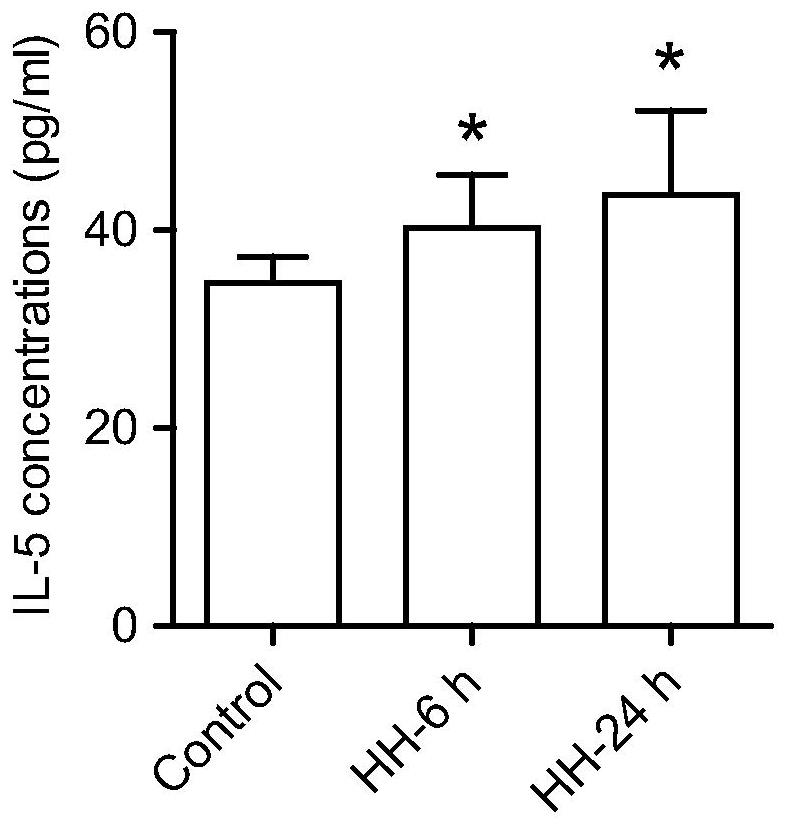
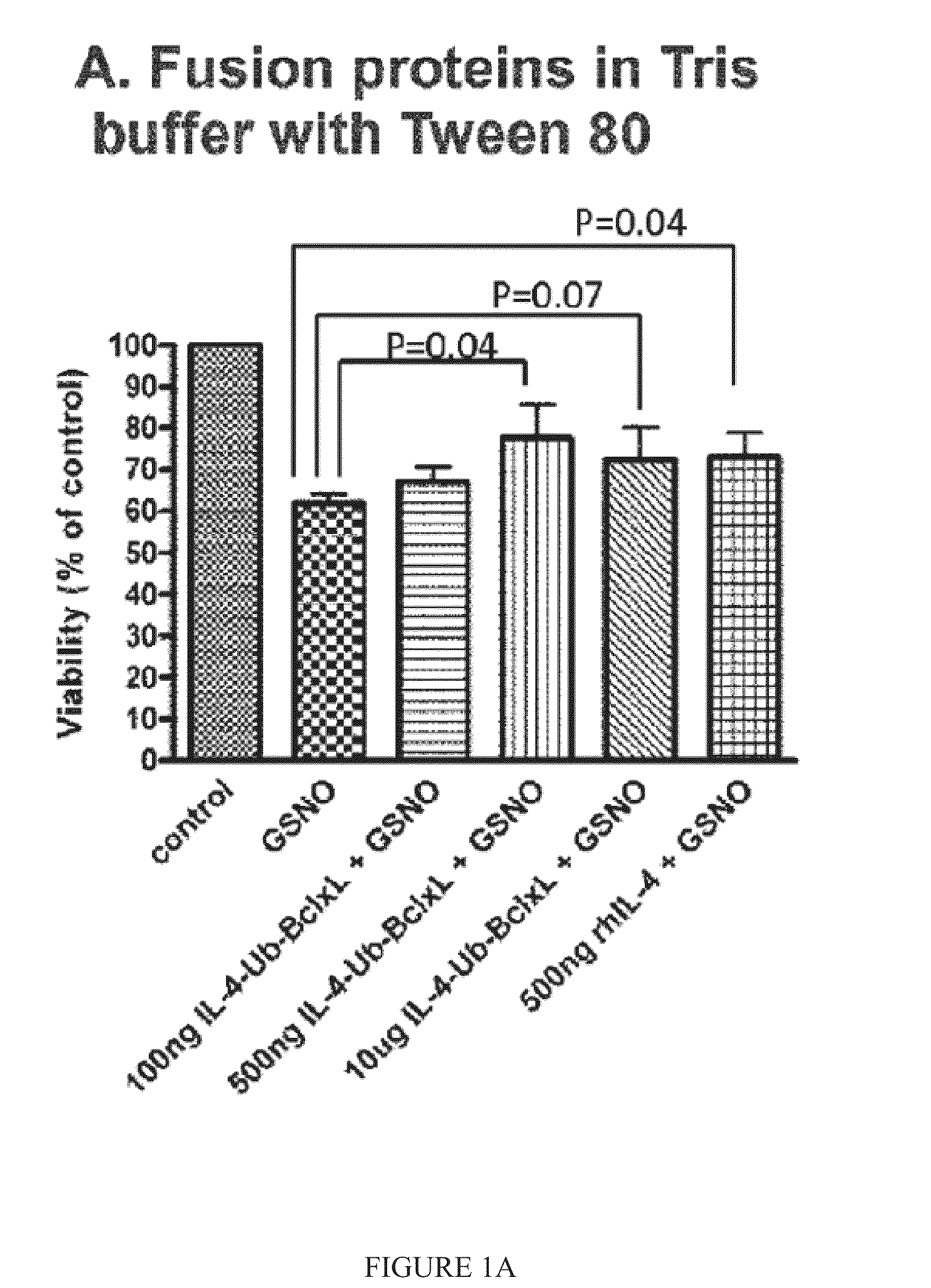
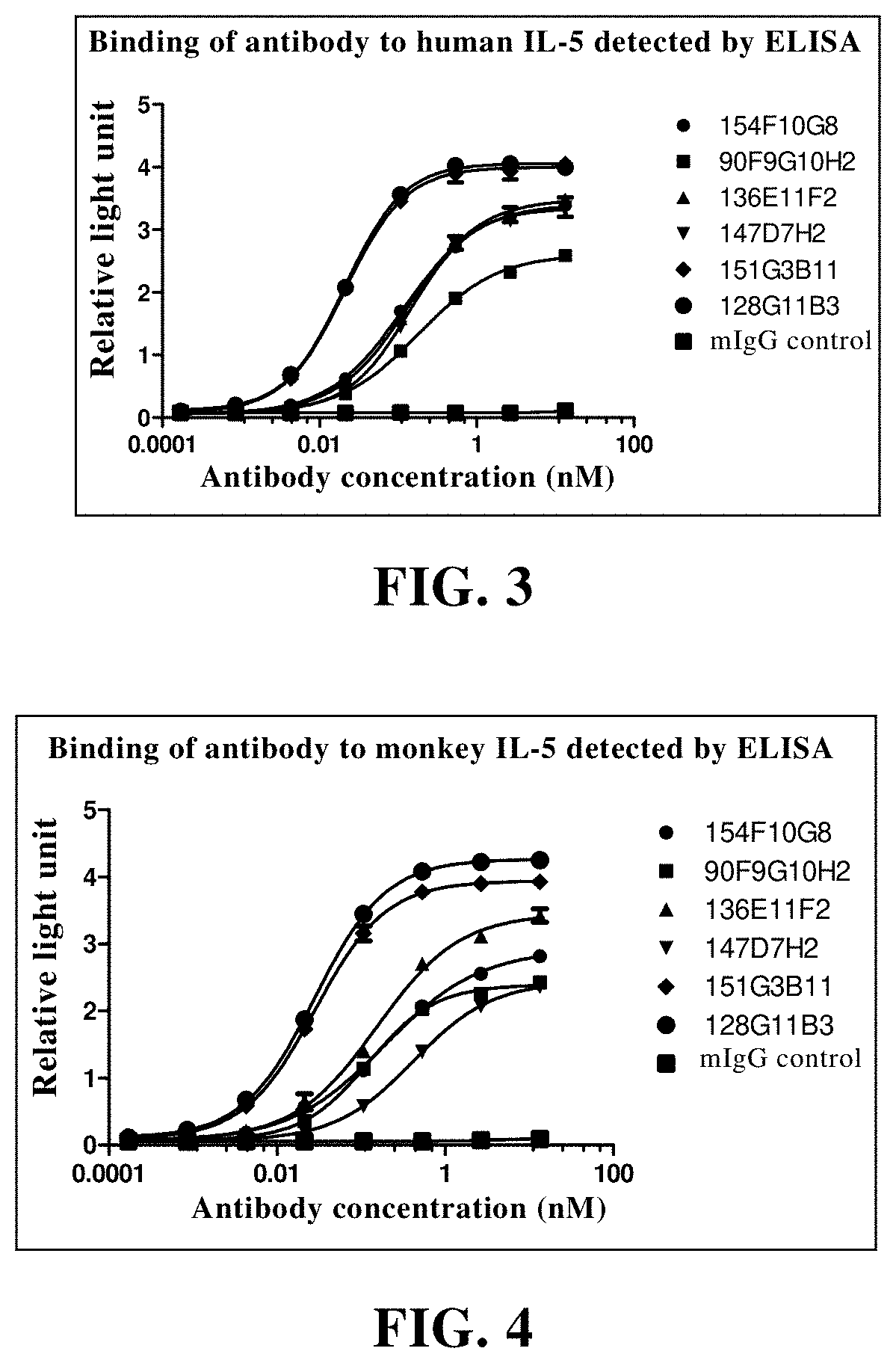
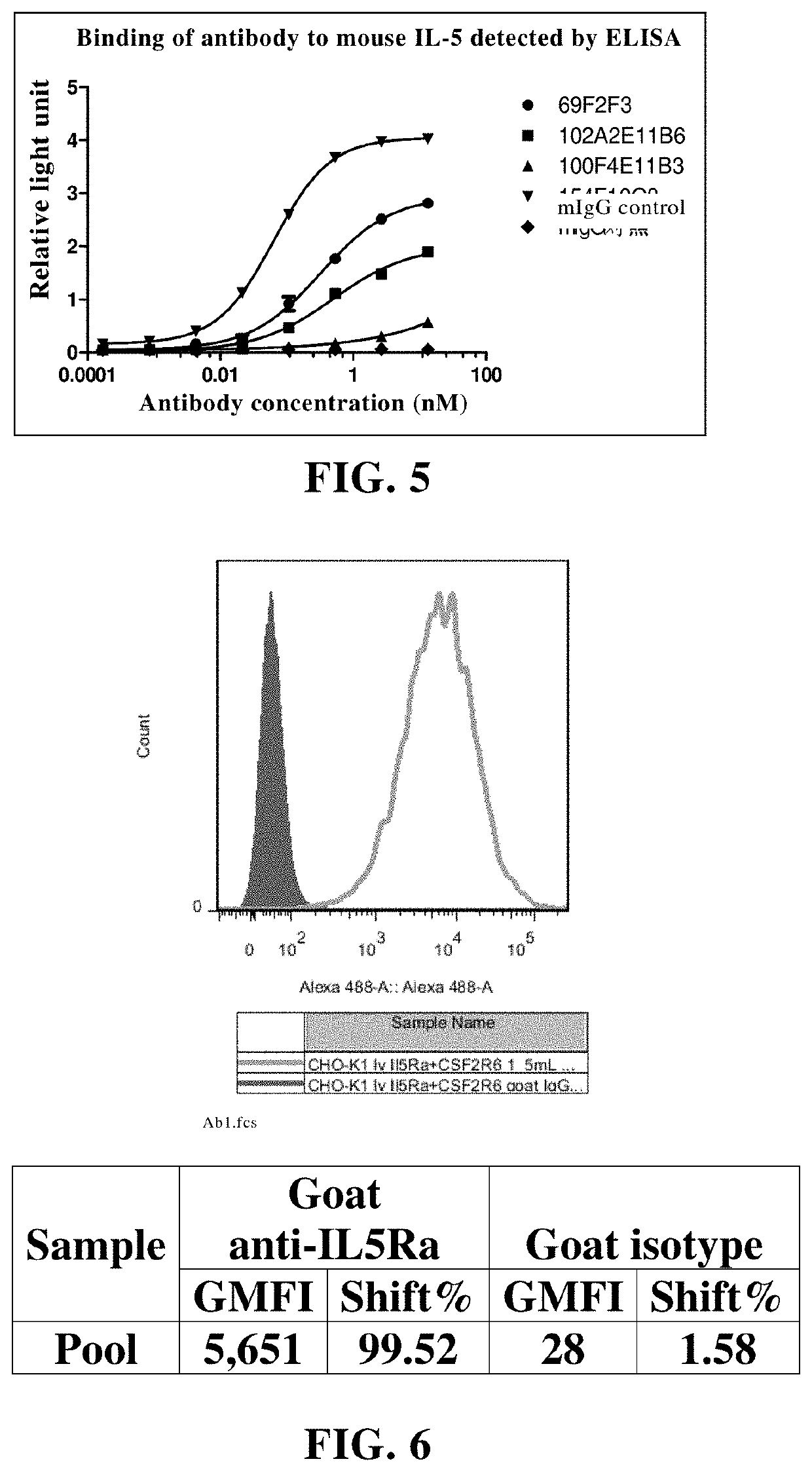
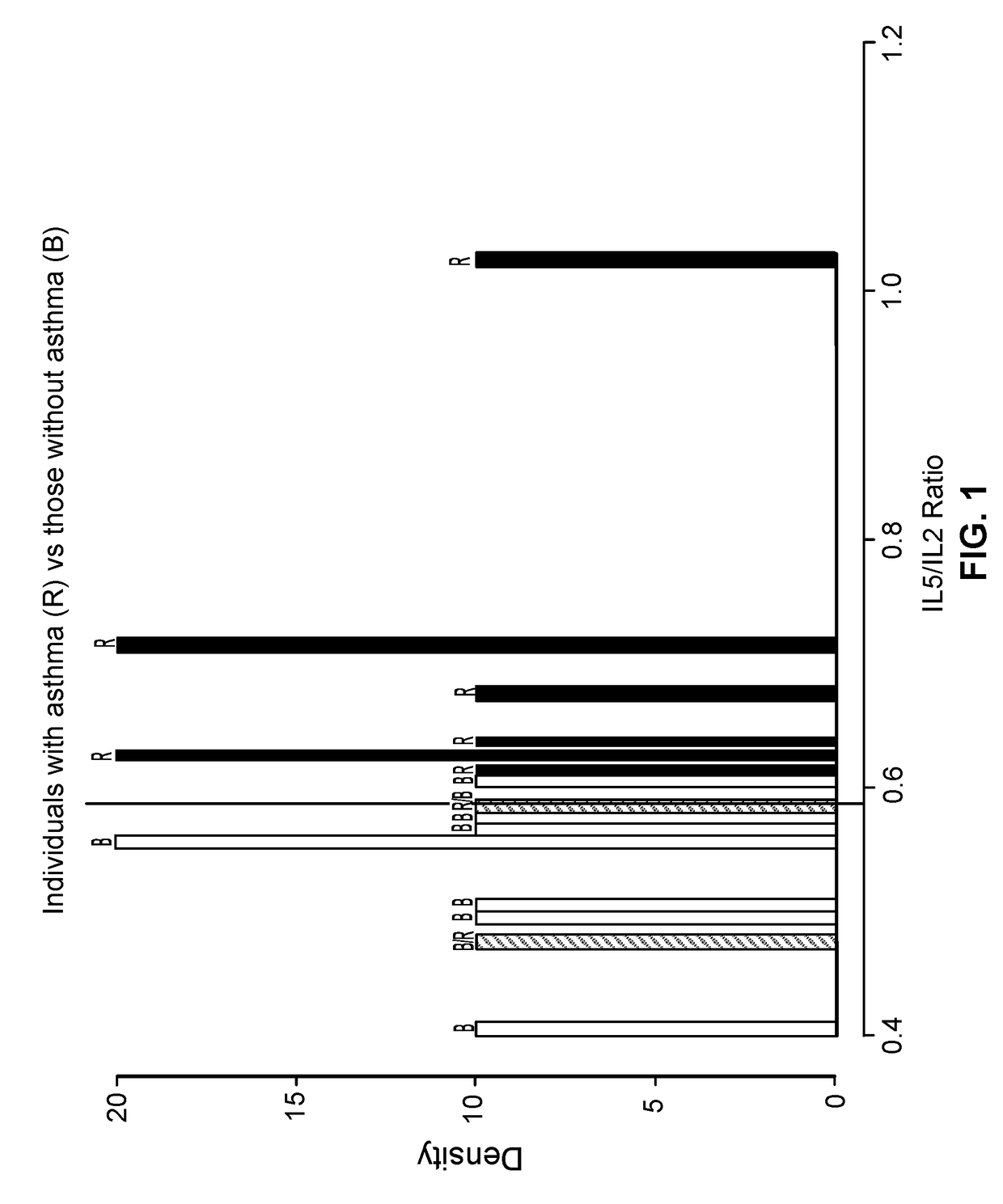
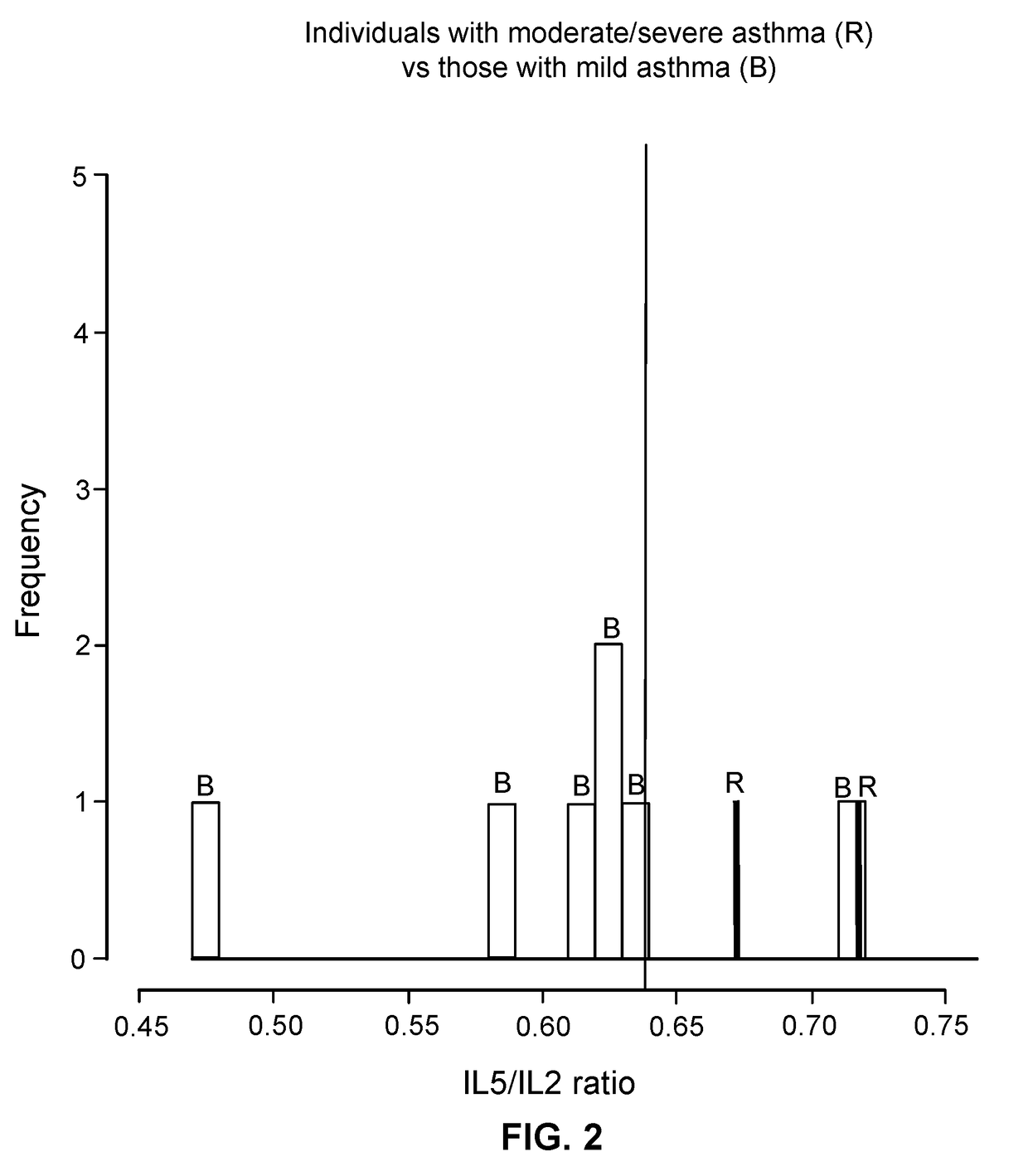
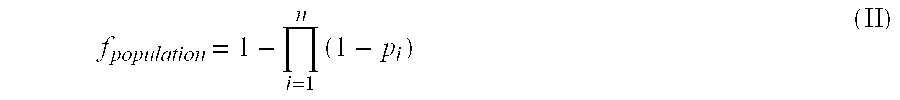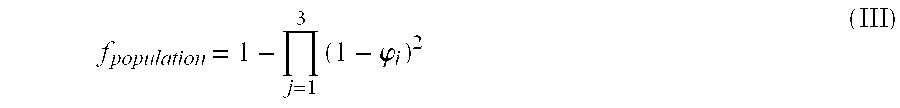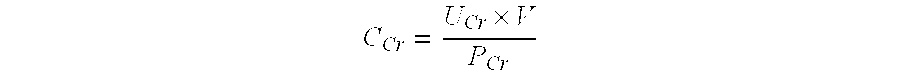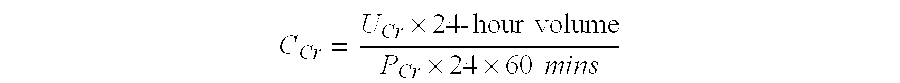Patents
Literature
41 results about "Interleukin 5" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin 5 (IL5) is an interleukin produced by type-2 T helper cells and mast cells.
Human monoclonal antibodies to interleukin-5
The present invention relates to antibodies and antigen-binding portions thereof that specifically bind to interleukin 5 (IL-5), which is preferably human IL-5. The invention also relates to human anti-IL-5 antibodies, including chimeric, bispecific, derivatized, single chain antibodies or portions of fusion proteins. The invention also relates to isolated heavy and light chain immunoglobulin molecules derived from anti-IL-5 antibodies and nucleic acid molecules encoding such molecules. The present invention also relates to methods of making anti-IL-5 antibodies, pharmaceutical compositions comprising these antibodies and methods of using the antibodies and compositions thereof for diagnosis and treatment. The invention also provides gene therapy methods using nucleic acid molecules encoding the heavy and / or light immunoglobulin molecules that comprise the human anti-IL-5 antibodies. The invention also relates to gene therapy methods and transgenic animals comprising nucleic acid molecules of the present invention.
Owner:MERCK SHARP & DOHME LLC +1
High affinity fully human monoclonal antibodies to interleukin-8
The present embodiments are related to high-affinity antibodies directed to IL-8, methods of making and characterizing such antibodies and uses of such antibodies. Isolated polynucleotide sequences encoding, and amino acid sequences comprising, heavy and light chain immunoglobulin molecules, particularly sequences corresponding to contiguous heavy and light chain sequences spanning the framework regions (FR's) and / or complementarity determining regions (CDR's), are provided.
Owner:AMGEN FREMONT INC
Novel immunogenic mimetics of multimer proteins
InactiveUS20030185845A1High yieldLow toxicityCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsInterleukin 5ADAMTS Proteins
The present invention relatas to novel immunogenic variants of multimeric proteins such as immunogenic variants of interleukin 5 (IL5) and tumour necrosis factor alpha (TNF, TNFalpha). The variants are, besides from being immunogenic in the autologous host, also highly similar to the native 3D structure of the proteins from which they are derived. Certain variants are monomeric mimics of the multimers, where peptide linkers (inert or T helper epitope containing) ensure a spatial organisation of the monomomer units that facilitate correct folding. A subset of variants are monomer TNFalpha variants that exhibit a superior capability of assembling into multimers with a high structural similarity to the native protein. Also disclosed are methods of treatment and production of the variants as well as DNA fragments, vectors, and host cells.
Owner:PHARMEXA
Immunogenic mimetics of multimer proteins with promiscuous T cell epitope inserts
InactiveUS20040258660A1High expressionImprove purification effectPeptide/protein ingredientsAntibody mimetics/scaffoldsInterleukin 5ADAMTS Proteins
The present invention relatas to novel immunogenic variants of multimeric proteins such as immunogenic variants of interleukin 5 (IL5) and tumour necrosis factor alpha (TNF, TNFalpha). The variants are, besides from being immunogenic in the autologous host, also highly similar to the native 3D structure of the proteins from which they are derived. Certain variants are monomeric mimics of the multimers, where peptide linkers (inert or T helper epitope containing) ensure a spatial organisation of the monomomer units that facilitate correct folding. A subset of variants are monomer TNFalpha variants that exhibit a superior capability of assembling into multimers with a high structural similarity to the native protein. Also disclosed are methods of treatment and production of the variants as well as DNA fragments, vectors, and host cells.
Owner:PHARMEXA
Human monoclonal antibodies to interleukin-5 and methods and compositions comprising same
Owner:MERCK SHARP & DOHME LLC
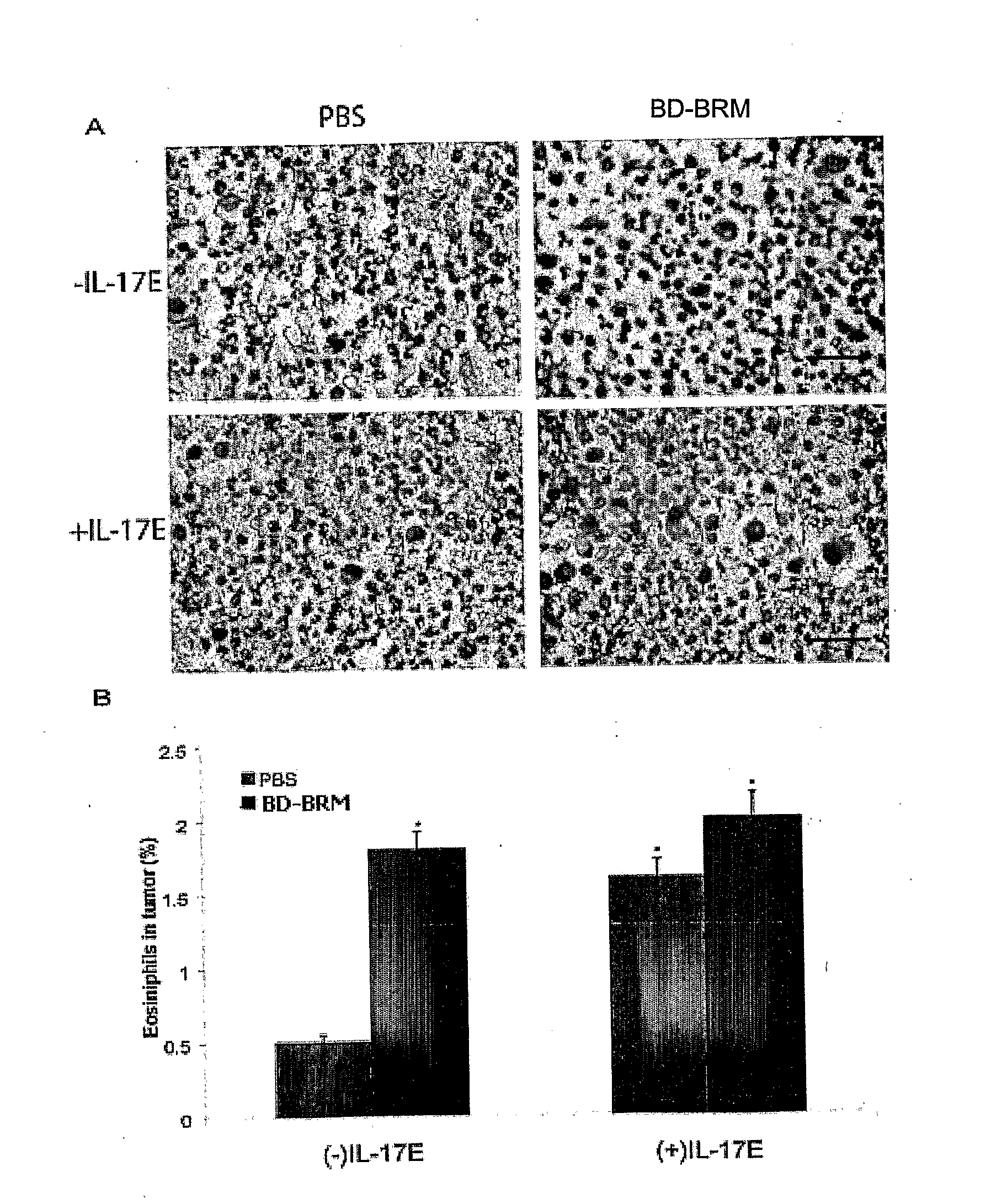
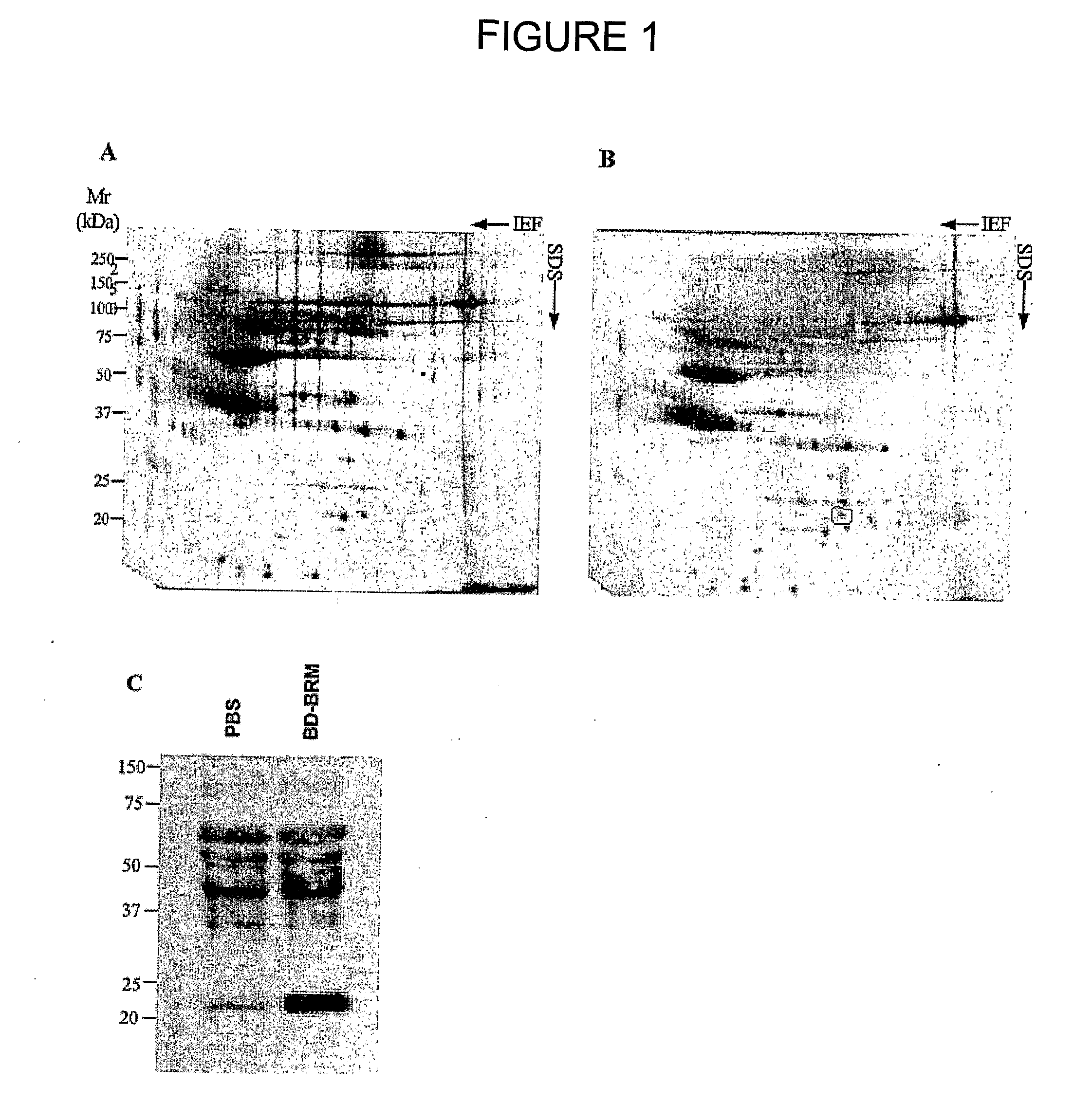
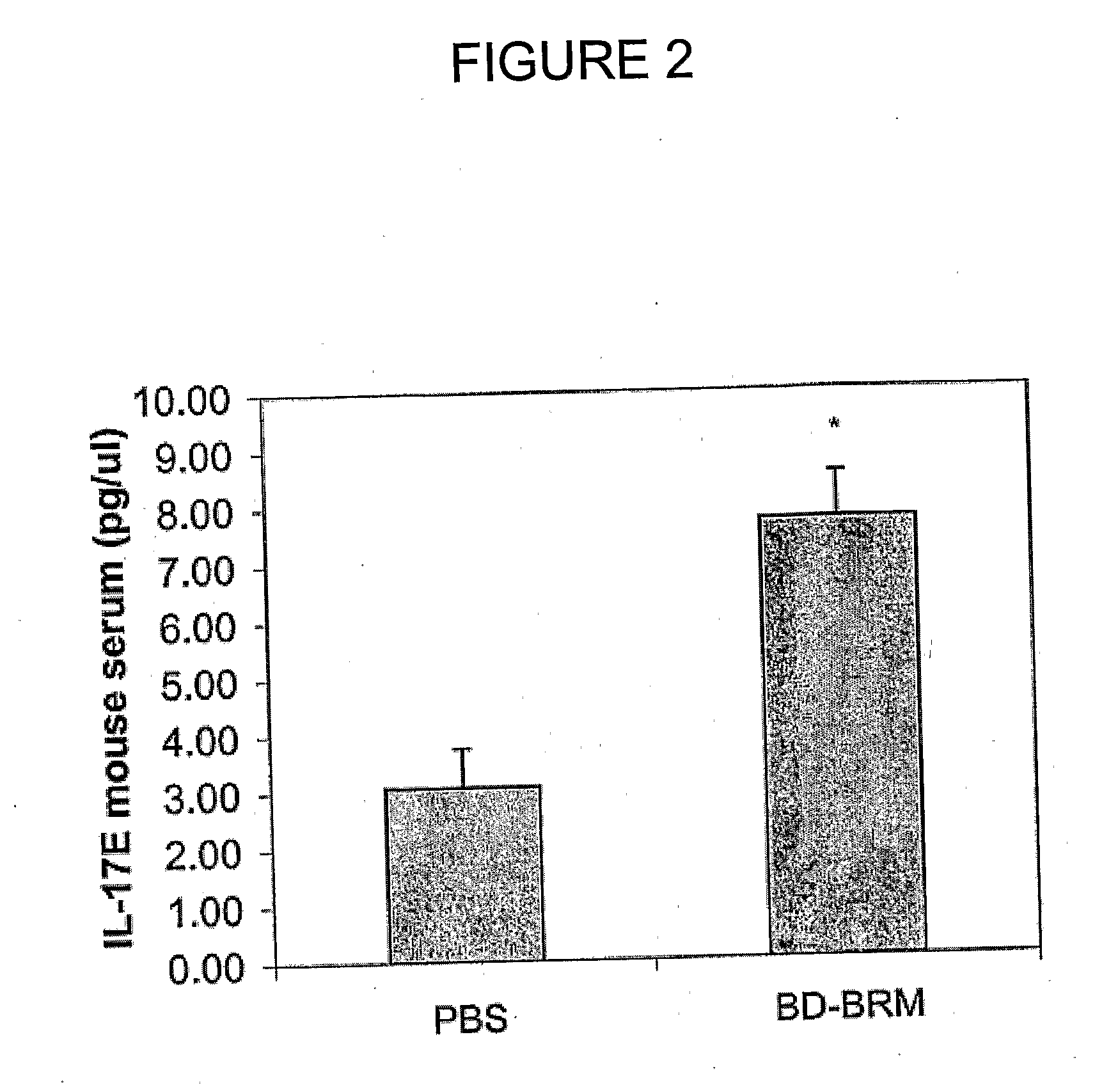
Use of interleukin 17E for the treatment of cancer
InactiveUS8287851B2Growth inhibitionImprove the level ofOrganic active ingredientsSugar derivativesInterleukin 10Nucleotide
Owner:APTOSE BIOSCIENCES INC
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20130230871A1Easy to adaptDisease diagnosisBiological testingAbnormal macrophageInterleukin 5
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Interleukin-5, Interleukin-6 receptor subunit beta, Tissue factor, Sex hormone-binding globulin, Alpha-2-macroglobulin, Apolipoprotein A-I, Calcitonin, Thrombopoietin, C-reactive protein, Intercellular adhesion molecule 3, Macrophage metalloelastase, Apolipoprotein B-100, and Fibrinogen as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Medicine for treating asthma
PendingCN105617356AReduce airway hyperresponsivenessRelieve spasmsOrganic active ingredientsInorganic active ingredientsInterleukin 5Interferon alpha
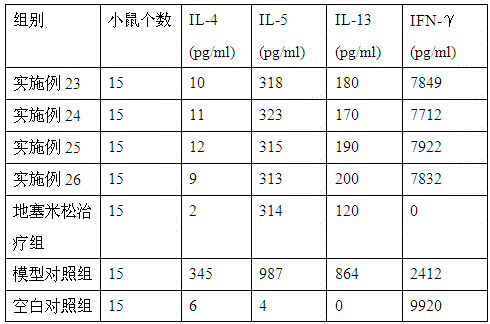
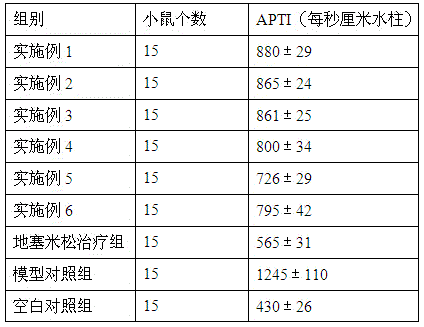
The invention provides medicine for treating asthma. The medicine is glycoprotein, mixture of polysaccharide and protein, polypeptide or protein. The medicine has the advantages that the bronchial hyperresponsiveness of a mouse asthma model can be effectively lowered, the tracheospasm can be relieved, and airway pressure-time index (APTI) is 726-880 per second centimeter water column; the inflammation in the lung can be treated effectively, inflammatory cell infiltration can be reduced, and the percentage of eosinophilic granulocyte is 14.6-16.6%; the gamma-interferon (IFN-gamma) and antibody which are capable of inhibiting asthmatic attack can be increased, and the contents of interleukin-4 (IL-4), interleukin-5 (IL-5) and interleukin-13 (IL-13) which can induce the asthma can be lowered; the medicine is safe, efficient, free of side effect, quick in action during asthma preventing and treatment, capable of relieving chest distress and short of breath and evident in curative effect on the asthma.
Owner:程潜
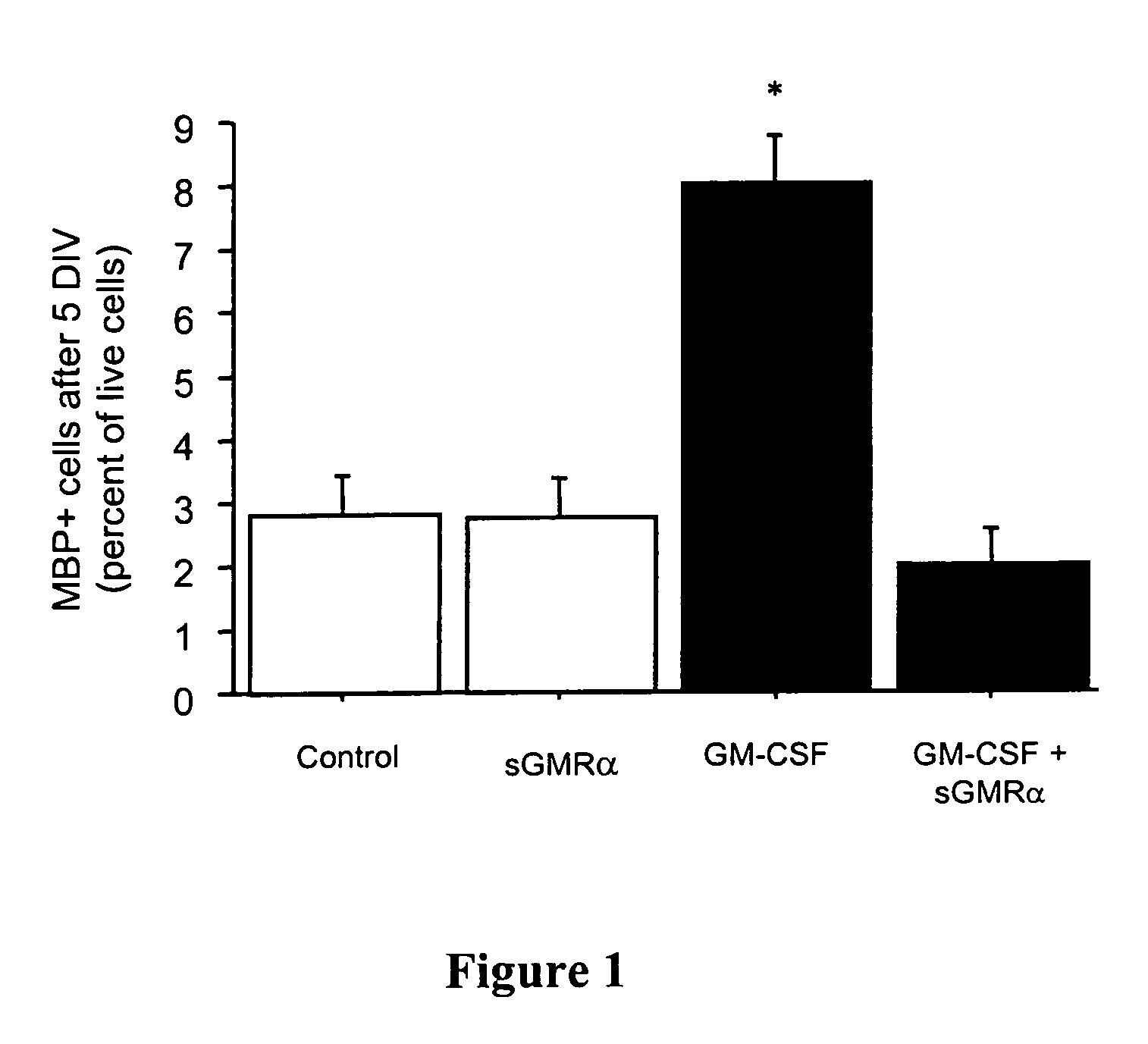
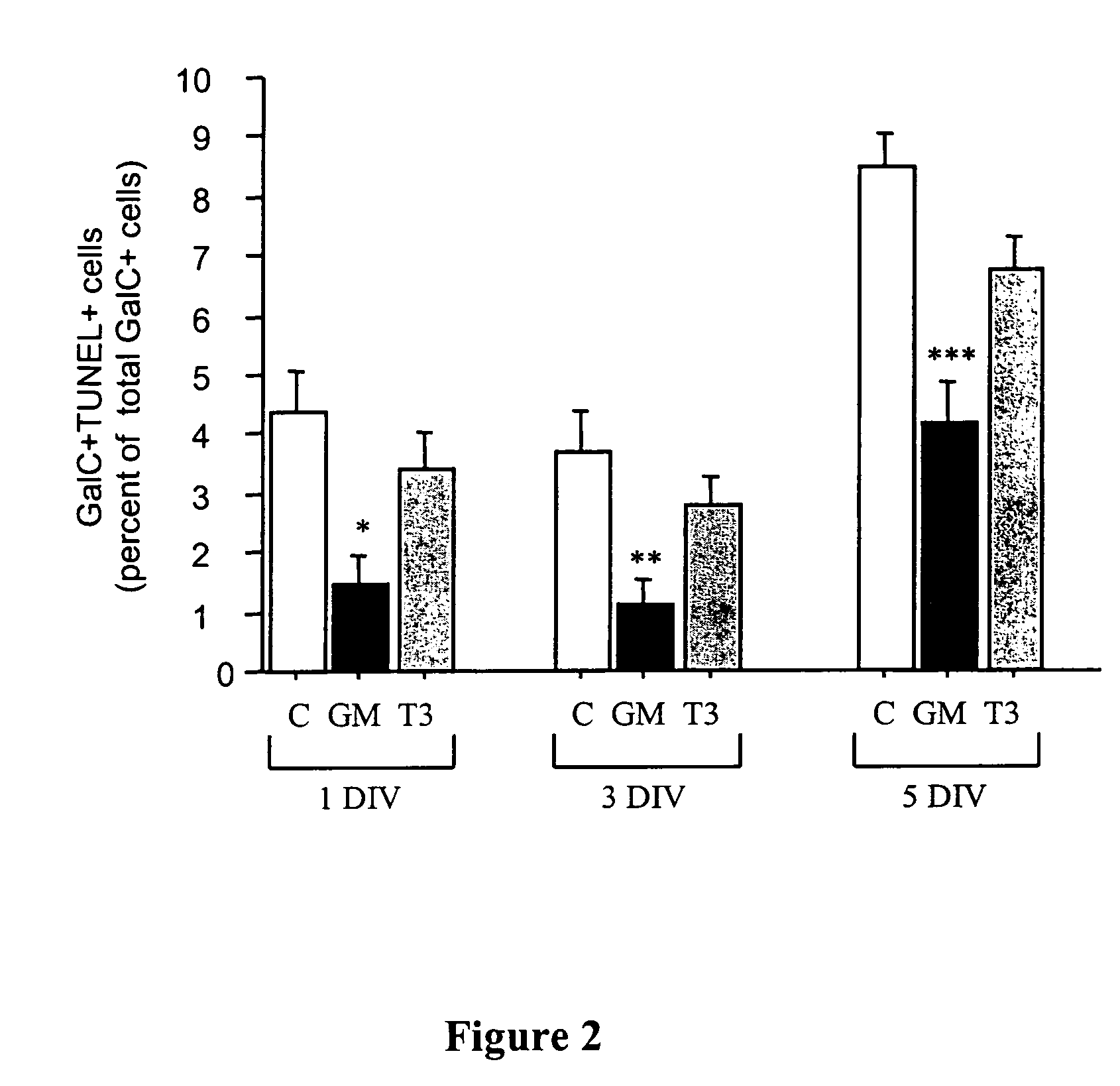
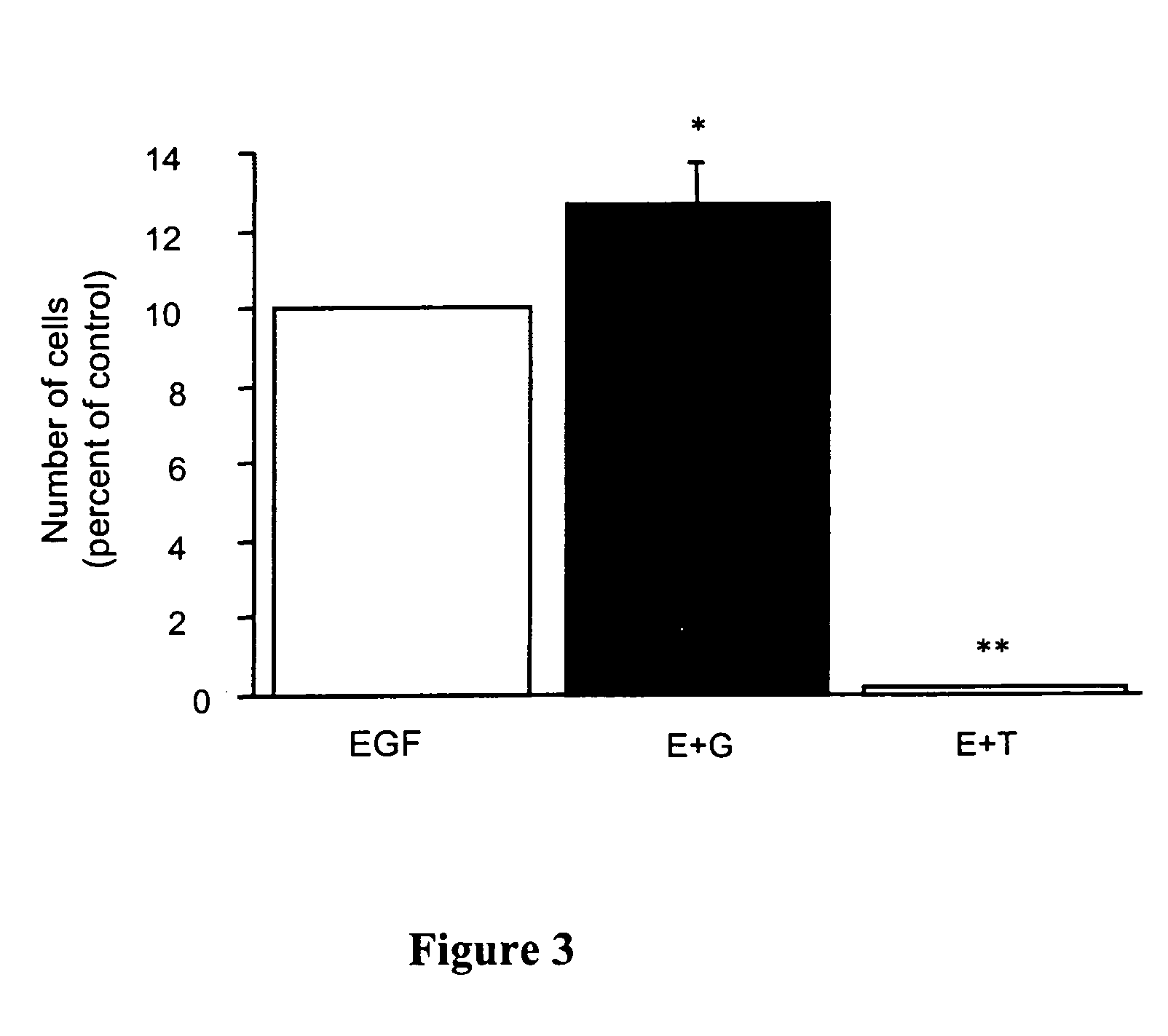
Oligodendrocyte production from multipotent neural stem cells
InactiveUS20050244965A1Increase percentageMaximize productionOrganic active ingredientsNervous disorderOligodendrocyteInterleukin 5
This invention relates to methods of producing oligodendrocytes from multipotent neural stem cells by using at least one oligodendrocyte promoting factor, particularly granulocyte-macrophage colony stimulating factor, granulocyte colony stimulating factor, interleukin 3 or interleukin 5. The neural stem cells may optionally be expanded prior to being subjected to the oligodendrocyte promoting factor.
Owner:STEM CELL THERAPEUTICS
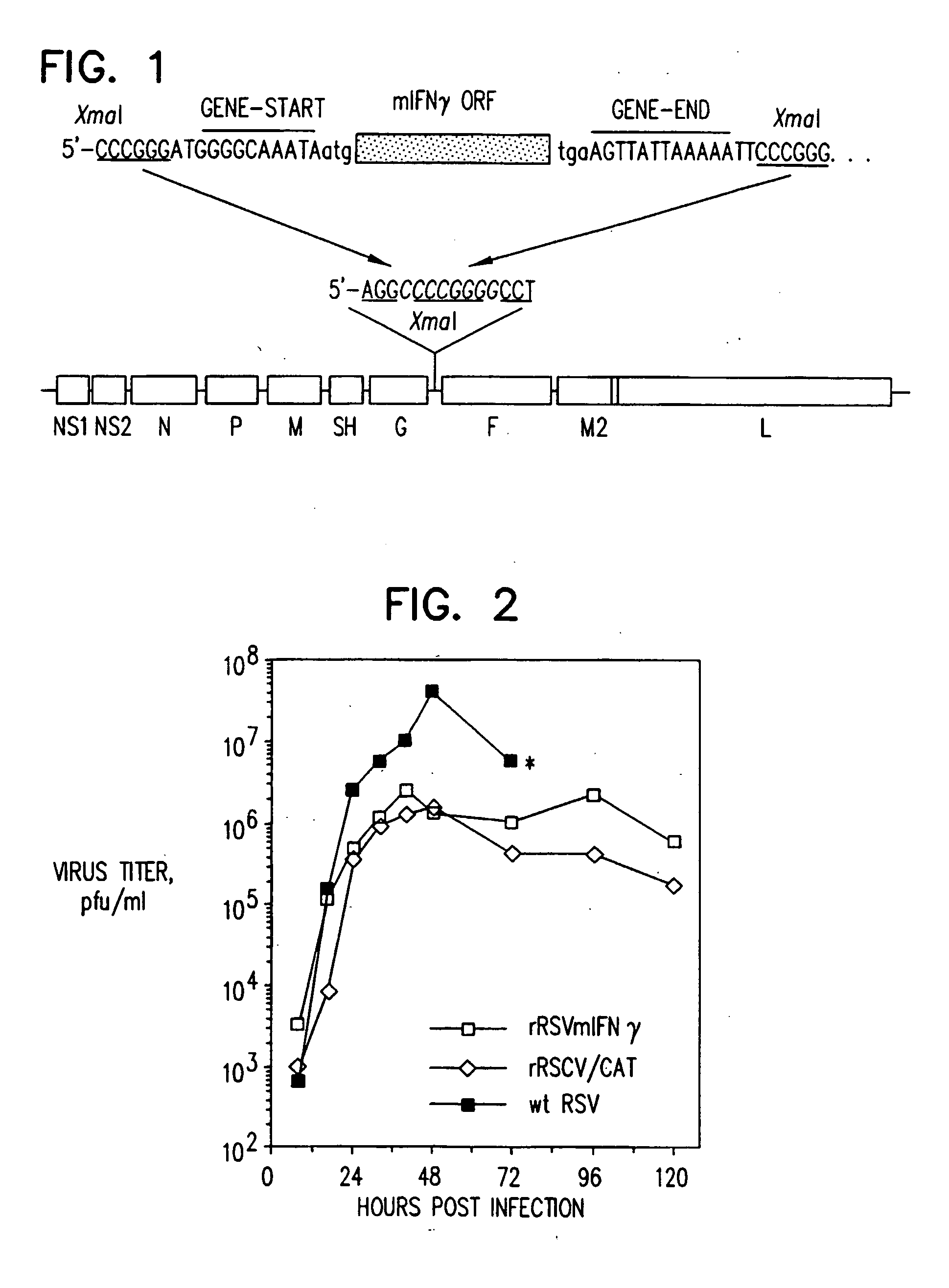
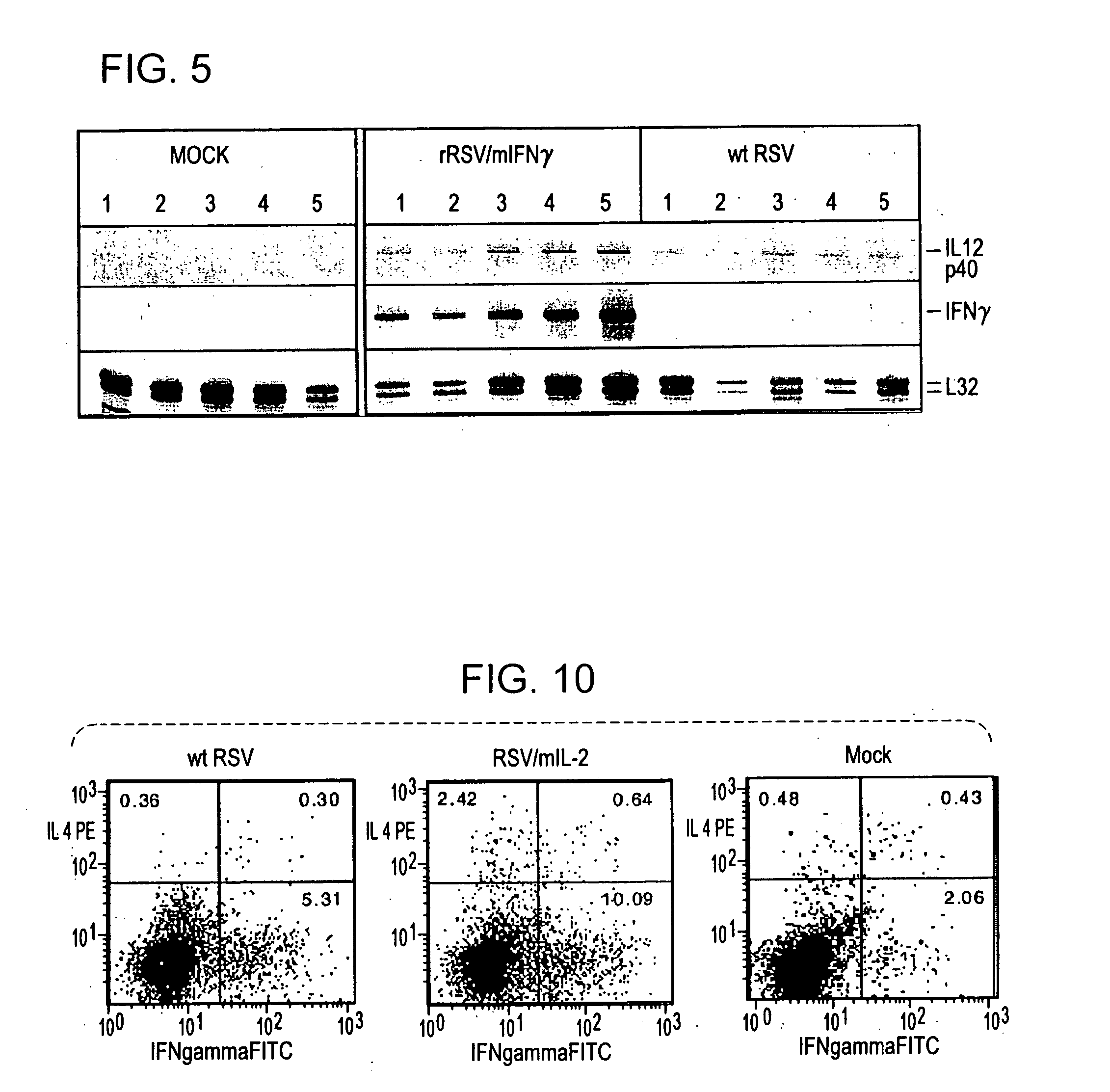
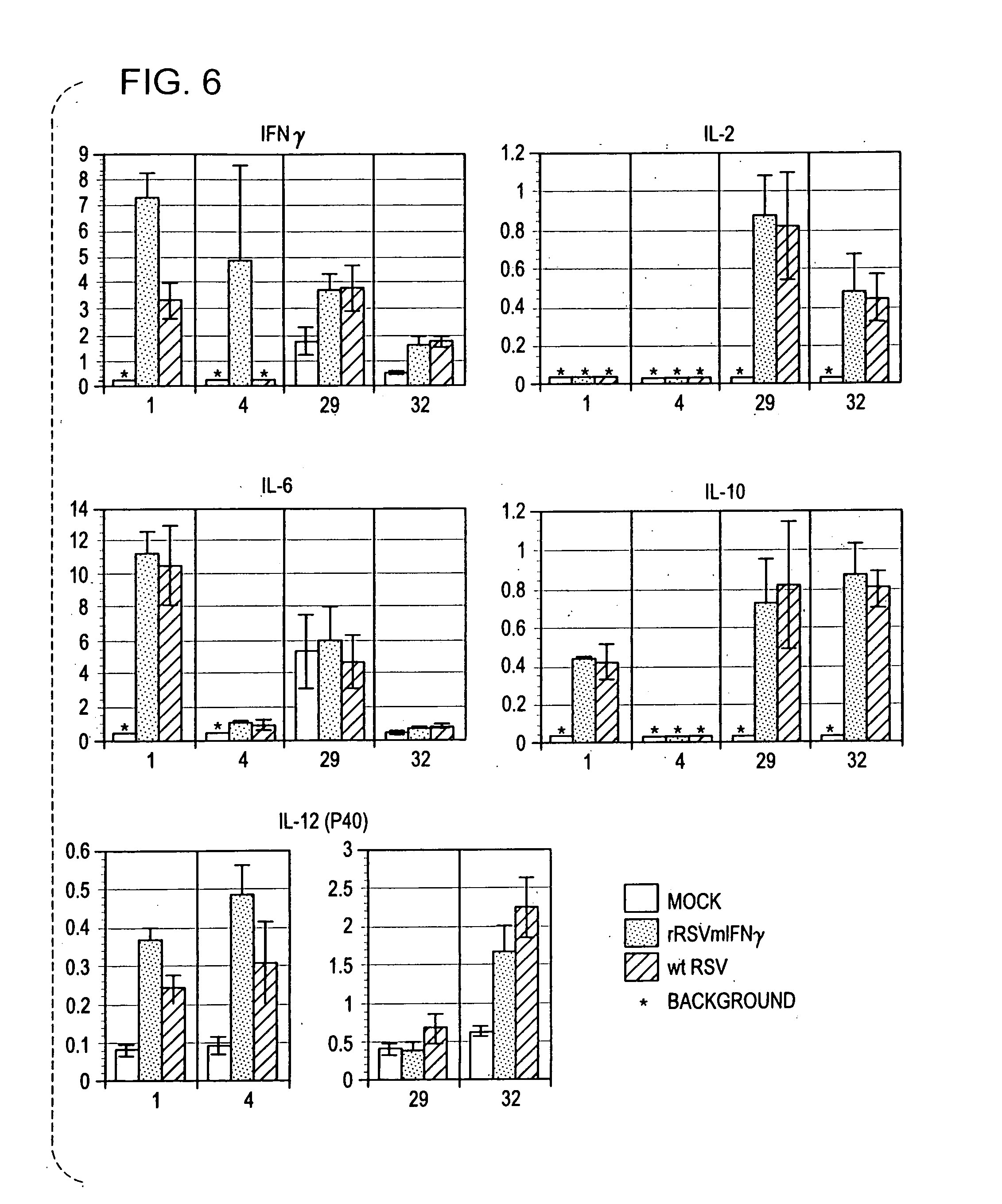
Production of recombinant respiratory syncytial viruses expressing immune modulatory molecules
InactiveUS20050220767A1Altered biological propertyImprove replication efficiencySsRNA viruses negative-senseBiocideInterleukin 6Interleukin 5
Recombinant respiratory syncytial virus (RSV) are provided which express one or more immune modulatory molecules. The recombinant virus is modified by addition or substitution of a polynucleotide sequence encoding the immune modulatory molecule, which is preferably a cytokine. Introduction of the cytokine increase, decrease, or otherwise enhances aspects of viral biology and / or host immune responses to RSV to facilitate vaccine use of the virus. Cytokines for use within the invention include but are not limited to interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), or interleukin 18 (IL-18), tumor necrosis factor (TNF) alpha, interferon gamma (IFN), and granulocyte-macrophage colony stimulating factor (GM-CSF). The polynucleotide or immune modulatory molecule is preferably added or substituted into the recombinant viral genome or antigenome, typically at an intergenic or other non-coding site, as a separate gene but may be otherwise expressed, for example as a fusion protein.
Owner:COLLINS PETER +3
Immune cell culture medium
InactiveCN105936891AHigh speedImprove cell functionBlood/immune system cellsCell culture active agentsInterleukin 5Cell culture media
The invention discloses an immune cell culture medium. The immune cell culture medium comprises a serum-free basal culture medium, plasma, cytokines and cistanchis glycosides. The content of cistanchis glycosides is 5-15 mg / mL, and the volume ratio of plasma to serum-free basal culture medium is (1:15)-(1:25). The cytokines include interleukin, monoclonal antibodies and interferon, the interleukin is one or more of interleukin-1, interleukin-4, interleukin-5, interleukin-12, interleukin-15 and interleukin-23, the monoclonal antibodies are anti-CD3 antibodies or anti-CD28 antibodies or both, and the interferon is gamma-interferon. By the adoption of the immune cell culture medium, different types of immune cells can be cultured, the amplification speed of immune cells is increased greatly, and a good cell function is maintained.
Owner:GUANGZHOU ZISHENG BIOLOGICAL TECH CO LTD
Anti-human interleukin 5 (1L-5) monoclonal antibody and application thereof
PendingCN111303284AHigh affinityStrong specificityBacteriaAntipyreticAntigenAntiendomysial antibodies
The invention discloses an IL-5-targeted antibody, and a preparation method and application thereof. Specifically, the invention discloses a novel IL-5-targeted murine or chimeric monoclonal antibody.The invention also discloses a method for preparing the monoclonal antibody. The monoclonal antibody can be combined with an IL-5 antigen with high specificity, has high affinity, and can well alleviate a series of asthma symptoms caused by IL-5, thereby playing a role in treating asthma.
Owner:SHANGHAI PHARMAEXPLORER
Biomarker for detecting plateau hypoxia and application thereof
PendingCN114878834AEasy to acceptFacilitates the progression of hypoxic injuryDisease diagnosisBiological testingSerum markersInterleukin 5
The invention discloses a biomarker for detecting plateau hypoxia injury and application of the biomarker. The biomarker is interleukin 5 or a specific antibody thereof, and is used for preparing a detection reagent or kit for plateau hypoxia. The biomarker provided by the invention can be used for serum marker detection to judge the plateau hypoxia degree of an organism, and is beneficial to detection or auxiliary detection of the hypoxia state, so that corresponding treatment measures can be taken as early as possible. The marker is convenient and rapid to detect, can be easily accepted by patients, and is more convenient for dynamically monitoring the progress of hypoxia injury of plateau people.
Owner:ACADEMY OF MILITARY MEDICAL SCI
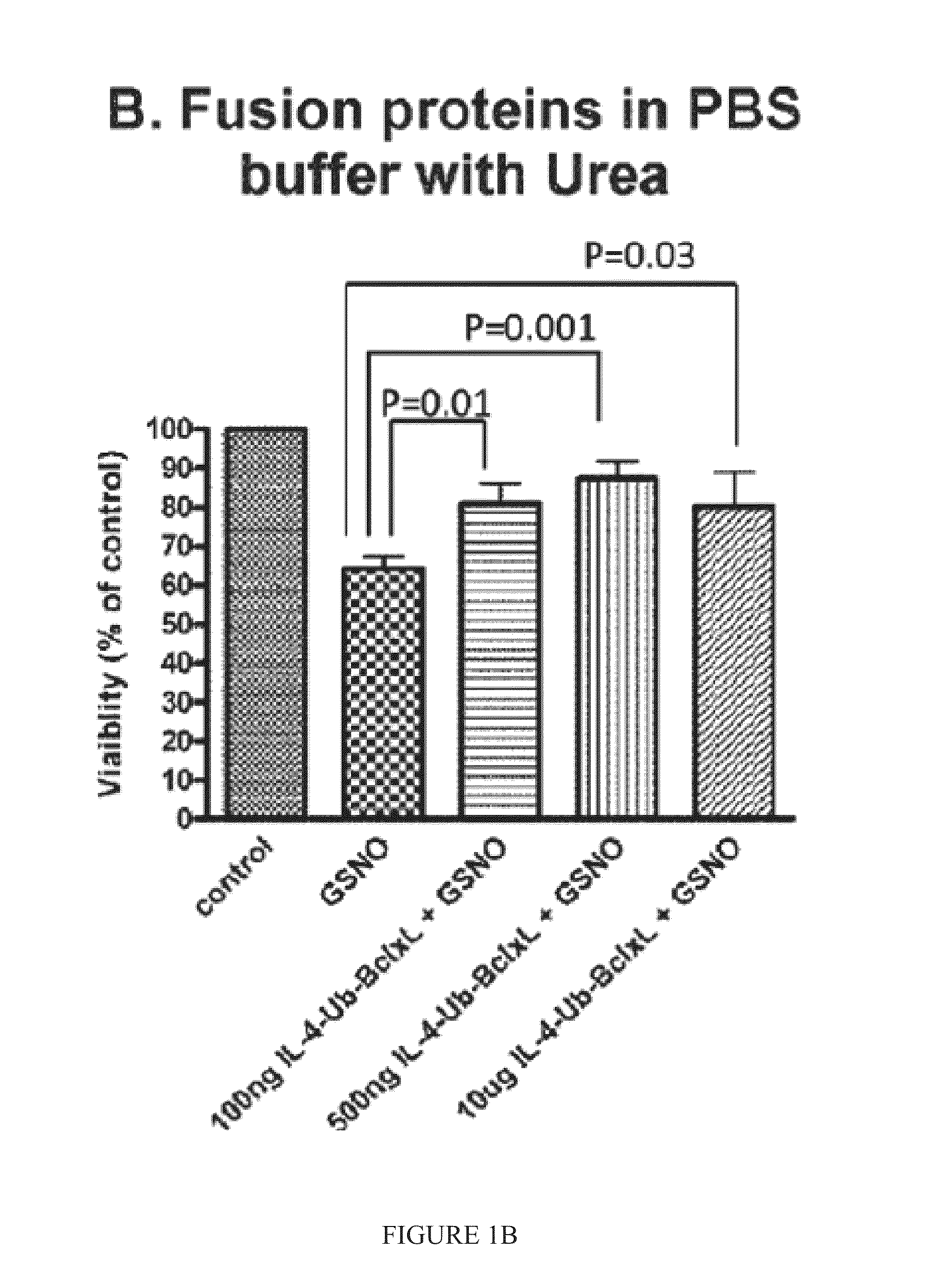
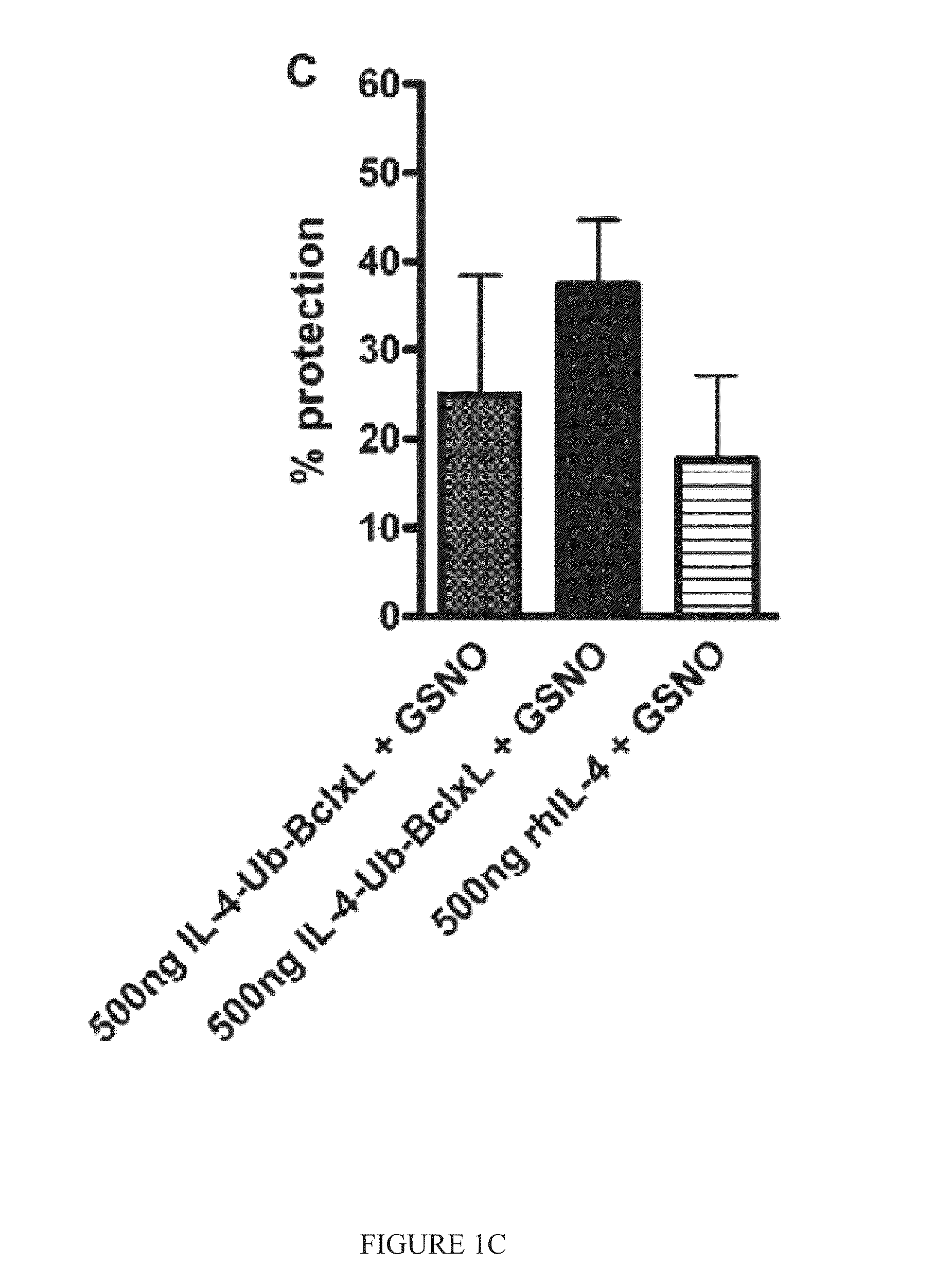
Interleukin-4 receptor-binding fusion proteins and uses thereof
ActiveUS20160237135A1Enhance cell viabilityIncreased activationSenses disorderNervous disorderWhite blood cellInterleukin 5
The present invention relates to interleukin-4 receptor-binding fusion proteins. More specifically, the invention provides, in part, fusion proteins that include an interleukin-4 or interleukin-13 protein moiety joined to an anti-apoptotic Bcl-2 family member protein moiety.
Owner:MEDICENNA THERAPEUTICS +1
Use of interleukin 17e for the treatment of cancer
InactiveUS20110158936A1Growth inhibitionImprove the level ofOrganic active ingredientsSugar derivativesInterleukin 10Interleukin 5
The use of interleukin 17E to inhibit tumour growth in a subject is provided. The interleukin 17E can be provided to the subject exogenously, as an interleukin 17E polypeptide or a polynucleotide encoding an interleukin 17E polypeptide, or it can be provided by stimulating production of endogenous interleukin 17E. Also provided is the use of interleukin 17E in combination with one or more anti-cancer therapeutics for inhibiting tumour growth in a subject. Anti-cancer therapeutics include, for example, standard chemotherapeutic drugs, immunotherapeutics, radiation, gene therapy, hormone manipulation and antisense therapy.
Owner:APTOSE BIOSCIENCES INC
Porcine circovirus bivalent genetic engineering vaccine
ActiveCN109053896AImproving immunogenicityAvoid infectionViral antigen ingredientsVirus peptidesInterleukin 5Protein subunit
The invention relates to preparation of a porcine circovirus bivalent genetic engineering vaccine. The vaccine is prepared from a PCV1 structural protein Cap protein subunit, a PCV3 structural proteinCap protein, a porcine interleukin-5, and a purification tag. The preparation process of the vaccine is stable and suitable for large-scale production. Animal experiments show that the porcine circovirus bivalent genetic engineering vaccine provided by the invention is good in safety, can induce a stronger immune response in an animal body, and effectively prevents the infection of a porcine circovirus type 1 and a porcine circovirus type 3.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Oligodendrocyte production from multipotent neural stem cells
InactiveUS7704737B2Increase percentageProliferating neural stem cells were decreasedOrganic active ingredientsNervous disorderOligodendrocyteInterleukin 5
This invention relates to methods of producing oligodendrocytes from multipotent neural stem cells by using at least one oligodendrocyte promoting factor, particularly granulocyte-macrophage colony stimulating factor, granulocyte colony stimulating factor, interleukin 3 or interleukin 5. The neural stem cells may optionally be expanded prior to being subjected to the oligodendrocyte promoting factor.
Owner:STEM CELL THERAPEUTICS
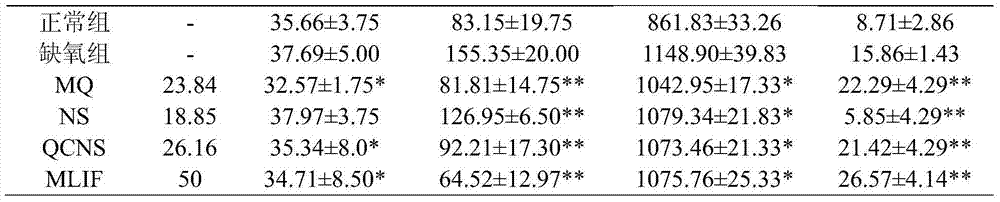
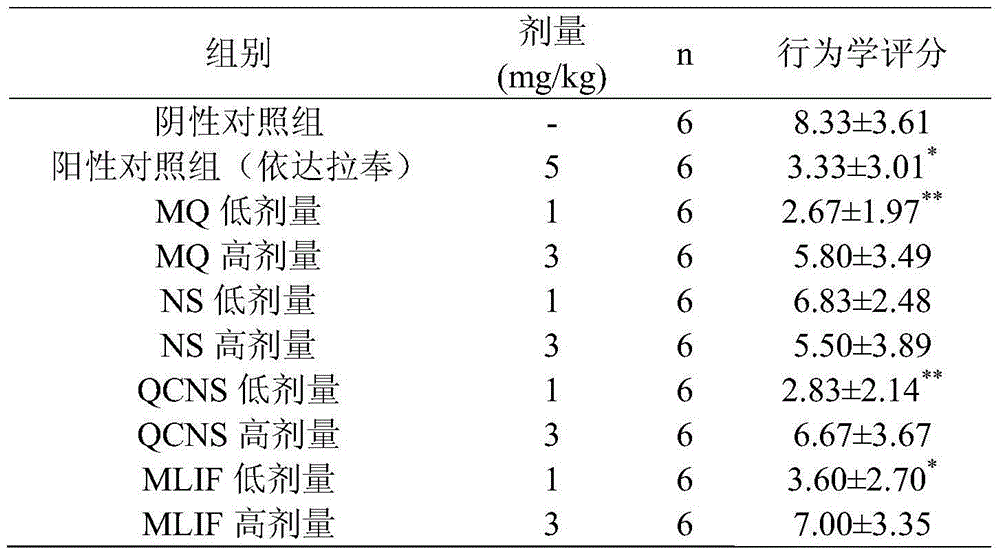
Pentapeptide metabolite and application of pentapeptide metabolite in preparation of medicament for preventing and treating ischemic cerebrovascular diseases
ActiveCN103611149AImprove protectionHigh transparencyDipeptide ingredientsAntipyreticInterleukin 6Disease
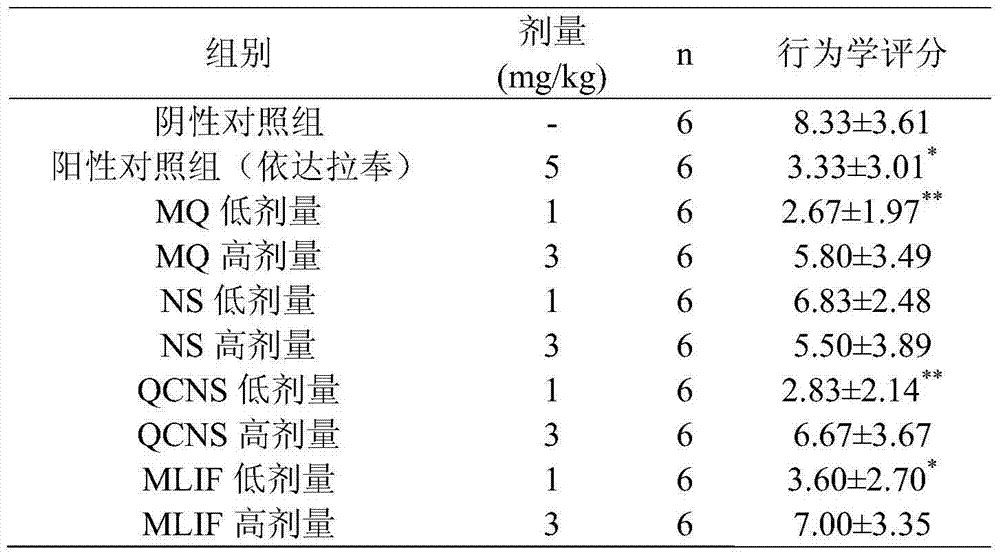
The invention relates to the technical field of medicines. A monocyte locomotion inhibiting factor, which is called pentapeptide or MLIF for short, has the effects of inhibiting locomotion of monocytes and inhibiting expression of IL-1beta (interleukin-1beta), IFN-gamma (interferon-gamma), IL-5 (interleukin-5), IL-6 (interleukin-6) and other inflammation factors, and has the effects of preventing and treating ischemic cerebrovascular diseases and myocardial ischemia. The invention further discovers that partial metabolites dipeptide Met-Gln and tetrapeptide Gln-Cys-Asn-Ser in the body of MLIF have the effect of preventing and treating ischemic cerebrovascular diseases. The invention provides application of the pentapeptide metabolite shown in SEQ ID No:2 or SEQ ID NO:4 in prevention of medicaments or health-care foods for prevention or treating ischemic cerebrovascular diseases.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Preparation and application of fusion protein genetically engineered bacterium of coccidium antigen peptide/IL5
ActiveCN114736308ASignificant preventionSignificant adjustmentBacteriaPeptide/protein ingredientsCoccidiosisAntigen
The invention discloses preparation and application of a fusion protein genetically engineered bacterium of coccidium antigen peptide / IL5. The amino acid sequence of the fusion protein is shown as SEQ ID No. 1. The sequence of the coding gene of the gene is shown as SEQ ID No.2. The protein provided by the invention has remarkable prevention, treatment and regulation effects on avian coccidiosis. The cationic polypeptide compound expressed by the food-grade bacillus subtilis causes acquired immune response of animal intestinal tracts through mucosal immunity, and an anti-coccidiosis antibody is generated. The interleukin 5 is an eosinophilic granulocyte activating factor and can activate eosinophilic granulocytes in intestinal tracts, secrete anti-parasitic active substances and repair intestinal mucosa damage caused by coccidium infection.
Owner:四川宜美康科技有限公司
Production of recombinant respiratory syncytial viruses expressing immune modulatory molecules
InactiveUS20050147622A1High expressionImprove propertiesSsRNA viruses negative-senseAntibody mimetics/scaffoldsInterleukin 6Interleukin 5
Recombinant respiratory syncytial virus (RSV) are provided which express one or more immune modulatory molecules. The recombinant virus is modified by addition or substitution of a polynucleotide sequence encoding the immune modulatory molecule, which is preferably a cytokine. Introduction of the cytokine increase, decrease, or otherwise enhances aspects of viral biology and / or host immune responses to RSV to facilitate vaccine use of the virus. Cytokines for use within the invention include but are not limited to interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL6), or interleukin 18 (IL-18), tumor necrosis factor (TNF) alpha, interferon gamma (IFN), and granulocyte-macrophage colony stimulating factor (GM-CSF). The polynucleotide or immune modulatory molecule is preferably added or substituted into the recombinant viral genome or antigenome, typically at an intergenic or other non-coding site, as a separate gene but may be otherwise expressed, for example as a fusion protein.
Owner:UNITED STATES OF AMERICA
Novel immunogenic mimetics of multimer proteins
InactiveCN1615316AAntibody mimetics/scaffoldsGenetic material ingredientsInterleukin 5White blood cell
The present invention relates to novel immunogenic variants of multi-subunit proteins such as interleukin 5 (IL5) and tumor necrosis factor alpha (TNF, TNF alpha). In addition to being immunogenic in the autologous host, the variants also highly resemble the native 3D structure of the protein from which they were derived. Certain variants are monomeric mimics of multimers in which a peptide linker (containing an inert or T helper epitope) ensures spatial organization of the monomeric unit that facilitates correct folding. One subtype of variant is the monomeric TNFα variant, which displays a superior ability to assemble into multimers with a high structural similarity to the native protein. Methods of processing and producing variants as well as DNA fragments, vectors and host cells are also disclosed.
Owner:PHARMEXA
Method for predicting liver cancer transfer relapse of primary liver cancer patient after operation
InactiveCN101430323ASuitable for detectionSimple and non-invasive assayBiological testingLymphatic SpreadPhosphate
The invention provides a method for forecasting metastasis and recurrence of liver cancer after the operation of a primary liver cancer patient which comprises the followig concrete steps: protein extraction reagent is prepared by dissolving 3-[(3-cholanidopropyl)dimethylammonio]-1-propanesulfonate, dithiothreitol, ethylene diamine tetraacetic acid, phenylmethylsulfonyl fluoride, gastric inhibitory polypeptide and leupeptin in phosphate buffered solution with a pH value ranging from 7.0 to 7.5; a frozen liver cancer surrounding tissue is put in liquid nitrogen and smashed into powder, and a protein extraction reagent is added into the mixture which is then blended in a mixer; ultrasound treatment and centrifugation are carried out on the mixture; supernate is sucked and stored at the temperature ranging from 20 DEG C to 80 DEG C; and the concentrations of interleukin-2, interleukin-15 and interleukin-5 of the supernate are detected, for purpose of forecasting the metastasis and recurrence as well as survival or death after the operation of a liver cancer patient. The concentrations of the interleukin-2, the interleukin-15 and the interleukin-5 are obviously superior to the existing clinical indicator that is used for forecasting the recurrence and the survival of the liver cancer patient.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Anti-human interleukin 5(il-5) monoclonal antibody and use thereof
PendingUS20220010008A1Relieving asthma symptomHigh affinityAntipyreticAnalgesicsAntigenAntiendomysial antibodies
Disclosed in the present invention are an antibody targeting IL-5, a preparation method therefor and use thereof. In particular, disclosed in the present invention is a novel murine-derived or chimeric monoclonal antibody targeting IL-5. Also disclosed in the present invention is a method for preparing said monoclonal antibody. The monoclonal antibody of the present invention is capable of binding IL-5 antigen with high specificity, has high affinity and can well alleviate a series of asthma symptoms caused by IL-5, thereby achieving the effect of treating asthma.
Owner:SHANGHAI PHARMAEXPLORER
Bionic affinity purifying process of interleukin I natural agonist
InactiveCN1772765AReduce steps for large-scale purificationLow costAntipyreticDigestive systemInterleukin 5Interleukin 8
The bionic affinity purifying process of natural interleukin I agonist belongs to the field of biotechnology. The present invention includes the following steps: 1. the separate reaction of basic chromatographic medium, after being activated with trichloro triazozine, to tyrosine and aminobenzo formamidine to synthesize bionic affinity separating material; and 2. preparing affinity chromatographic column with the synthesized bionic affinity separating material, making the protein sample containing human natural interleukin I agonist flow through the affinity chromatographic column for the protein containing human natural interleukin I agonist to be adsorbed onto the column, flushing the column to eliminate hetero protein, altering the buffering liquid for flushing the column so as to elute the protein containing human natural interleukin I agonist and to obtain purified protein containing human natural interleukin I agonist. The present invention has less purifying steps, long separating material life and low production cost.
Owner:上海荣君生物医药科技有限公司
Methods and materials for treating medical conditions
ActiveUS20170183402A1Rapidly and reliably identifiedReduce severityImmunoglobulins against cytokines/lymphokines/interferonsDisease diagnosisDiseaseInterleukin 5
This document provided methods and materials involved in treating medical conditions. For example, methods and materials for using anti-Interleukin 4, anti-Interleukin 5, and / or anti-Interleukin 13 antibodies to treat asthma in a mammal identified as having a Th2 immune response using a whole blood cell-based cytokine whole blood cell-based cytokine assay are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Liver cancer patient postoperative transferring recrudescence polymolecular forecasting reagent kit based on inflammation factor
ActiveCN101441213APredict metastatic recurrencePredicted survivalMaterial analysisInflammatory factorsWhite blood cell
The invention provides a multimolecular prediction kit for postoperative recurrence and transfer for the patients with liver cancer based on inflammatory factor, characterized by comprising a coating agent, a washing liquid, a horseradish peroxidase-labeled antibody, a substrate solution, a stop solution, and prediction reference values, wherein, the coating agent is composed of a carbonate coating buffer solution of PH9.6, and an interleukin 2 antibody or an interleukin 15 antibody or an interleukin 5 antibody; based on the 4mug / mul of protein concentration of extract from liver peri-cancer tissue, the prediction reference values of interleukin 2, interleukin 15, and interleukin 5 are respectively 20-30pg / ml, 32-43pg / ml, and 18-28pg / ml, and the prediction reference values vary proportionably along with the sample concentration. In the invention, by using the interleukin 2, interleukin 15, and interleukin 5 in liver peri-cancer tissue, the postoperative recurrence and transfer for the patients with liver cancer can be accurately predicted.
Owner:SHANGHAI DIDA BIOTECH
Pentapeptide metabolite and application of pentapeptide metabolite in preparation of medicament for preventing and treating ischemic cerebrovascular diseases
ActiveCN103611149BImprove protectionReduce levels of inflammatory factorsDipeptide ingredientsAntipyreticInterleukin 6Disease
The invention relates to the technical field of medicines. A monocyte locomotion inhibiting factor, which is called pentapeptide or MLIF for short, has the effects of inhibiting locomotion of monocytes and inhibiting expression of IL-1beta (interleukin-1beta), IFN-gamma (interferon-gamma), IL-5 (interleukin-5), IL-6 (interleukin-6) and other inflammation factors, and has the effects of preventing and treating ischemic cerebrovascular diseases and myocardial ischemia. The invention further discovers that partial metabolites dipeptide Met-Gln and tetrapeptide Gln-Cys-Asn-Ser in the body of MLIF have the effect of preventing and treating ischemic cerebrovascular diseases. The invention provides application of the pentapeptide metabolite shown in SEQ ID No:2 or SEQ ID NO:4 in prevention of medicaments or health-care foods for prevention or treating ischemic cerebrovascular diseases.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY +1
Methods for treating medical conditions by anti-type 2 therapy
ActiveUS10654923B2Immunoglobulins against cytokines/lymphokines/interferonsDisease diagnosisDiseaseAntiendomysial antibodies
This document provided methods and materials involved in treating medical conditions. For example, methods and materials for using anti-Interleukin 4, anti-Interleukin 5, and / or anti-Interleukin 13 antibodies to treat asthma in a mammal identified as having a Th2 immune response using a whole blood cell-based cytokine whole blood cell-based cytokine assay are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Method
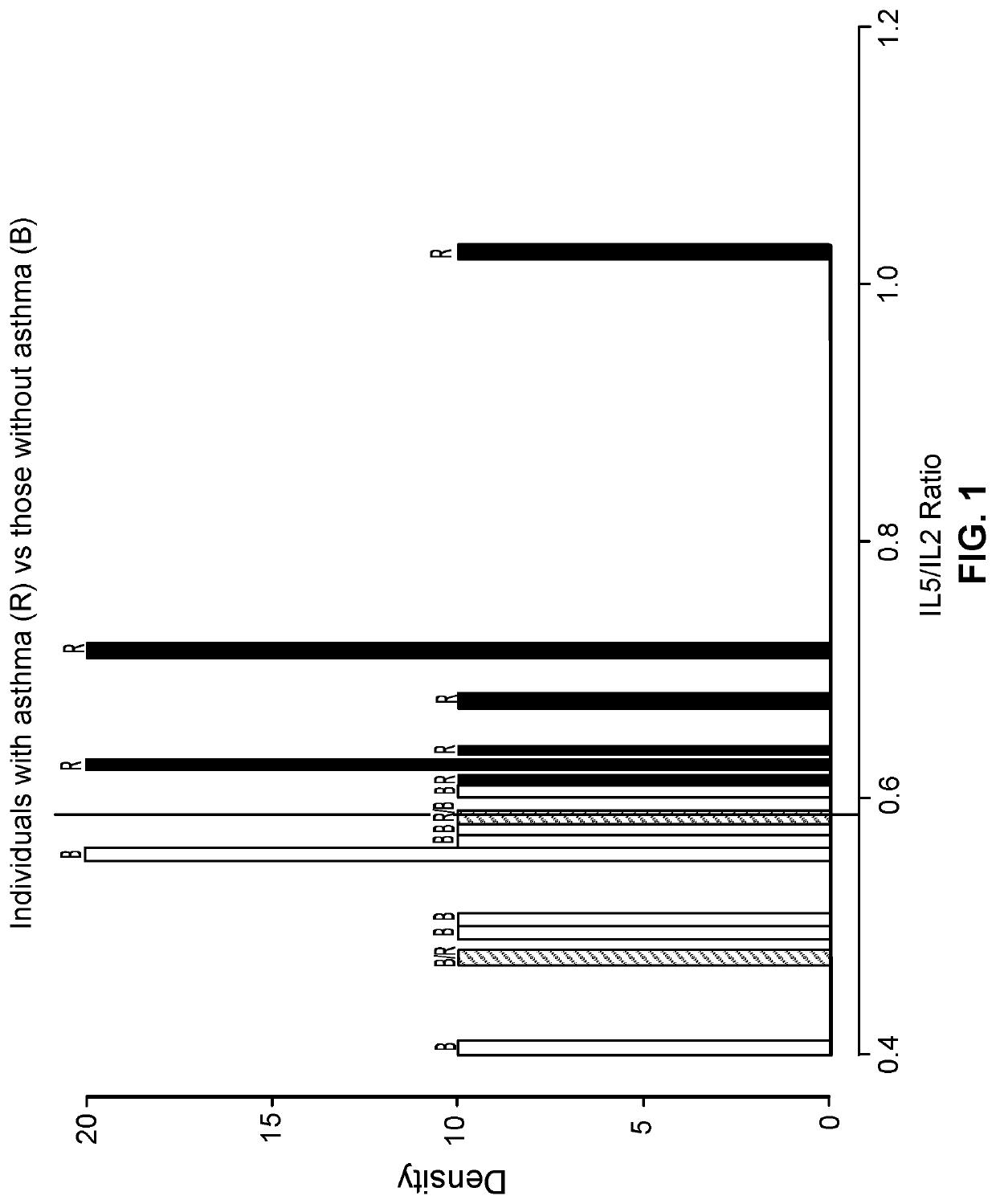
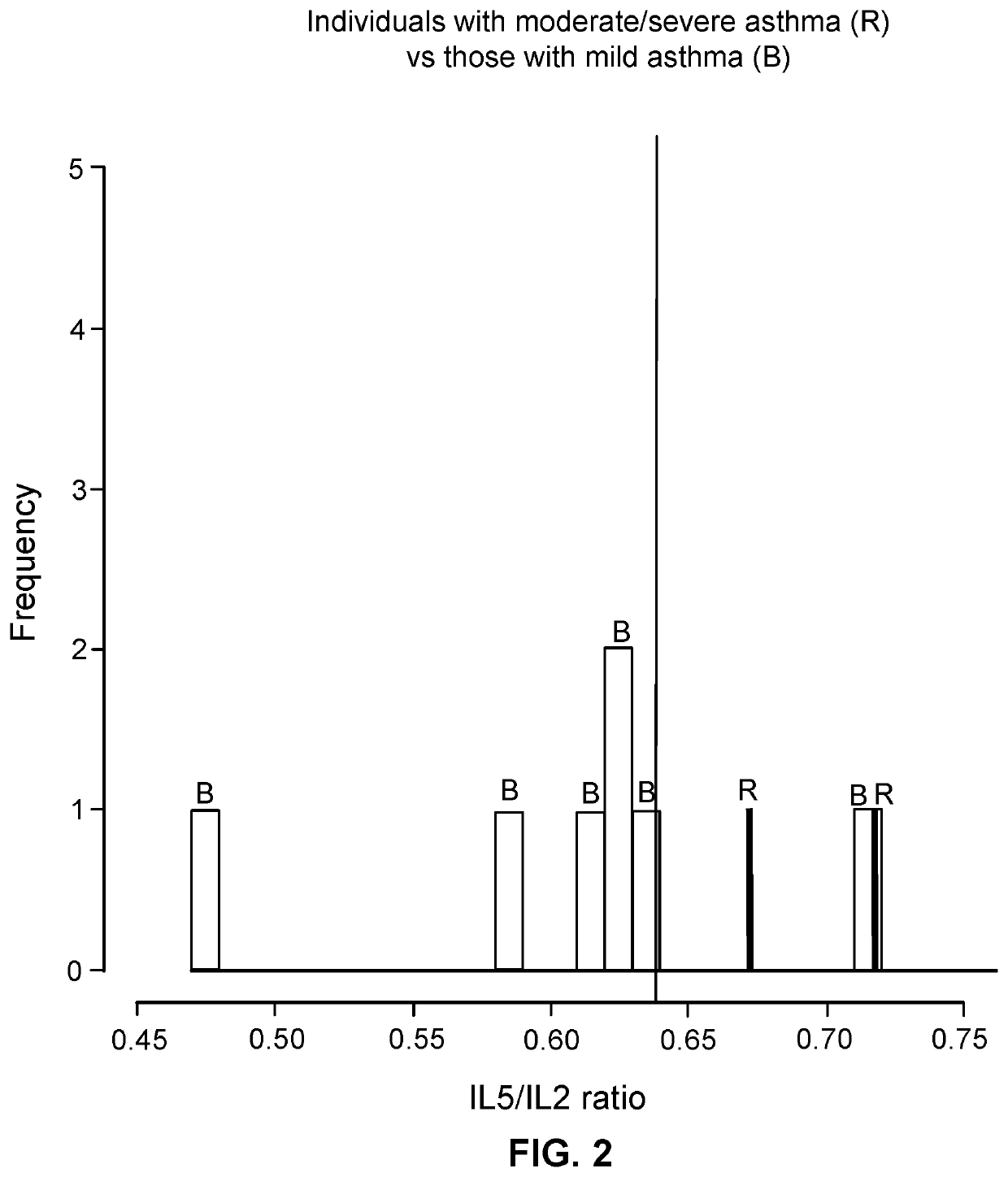
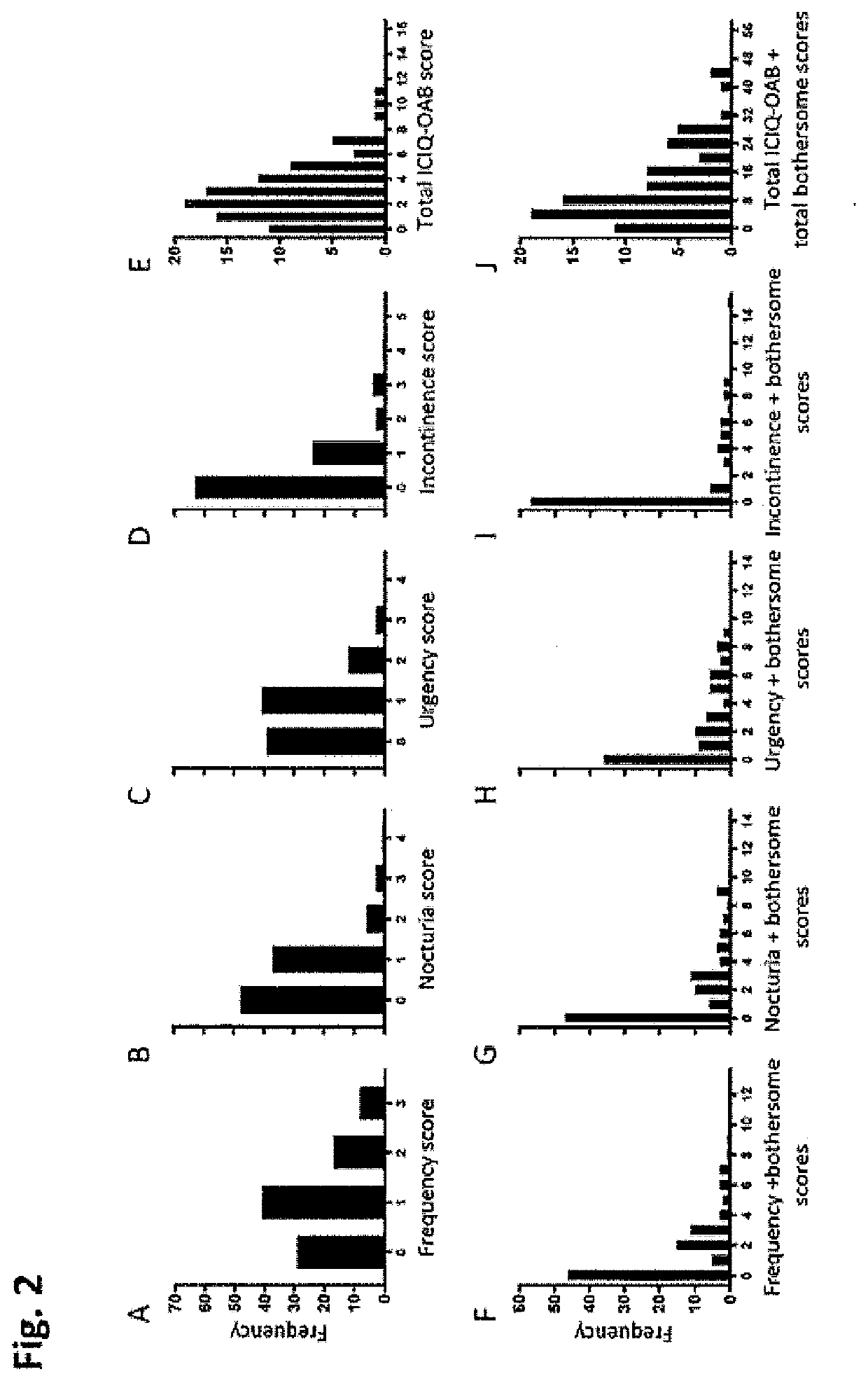
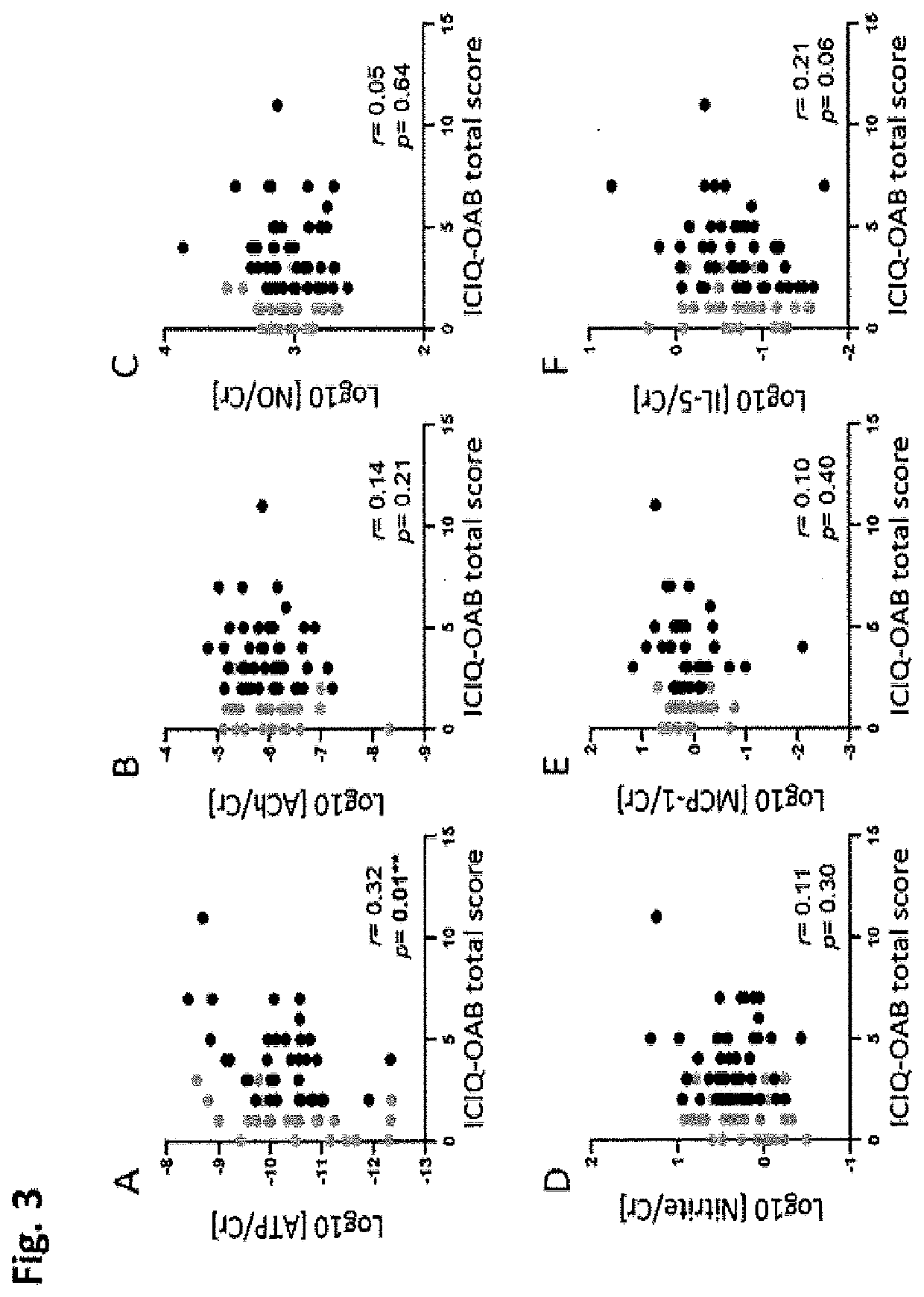
PendingUS20220099685A1Constant rateHigh negative predictive valueDisease diagnosisUrinary disorderAdenosineInterleukin 5
The invention provides a method of diagnosing overactive bladder disorder (OAB), the method comprising: measuring the concentrations of one or more of adenosine triphosphate (ATP), acetylcholine (ACh), nitric oxide (NO) and interleukin 5 (IL-5) in a sample obtained from a subject; normalising the concentrations to the concentration of creatinine (Cr) in the sample; range standardising the normalised concentrations and subject's age to the following values: Age to 120 years old; ATP / Cr to 0.000001; ACh / Cr to 0.1; NO to 20000; IL-5 / Cr to 100; wherein the likelihood of having OAB (pOAB)=1 / 1+e−x, where X=one or more of the following: (a) (−2.688±1.050)+5.472±2.098×subject's age+1.356±0.559×Gender (Female=1, Male=0)+(−7.998±40.273)×[IL-5 / Cr]; (b) (−2.141±0.966)+4.506±1.902×subject's age+1.034±0.519×Gender (Female=1, Male=0)+(−5294.063±9075.456)×[ACh / Cr]; (c) (−2.825±1.072)+5.964±2.167×subject's age+1.312±0.562×Gender (Female=1, Male=0)+17.790±58.762×[IL-5 / Cr]+(−9180.821±12700.057)×[ACh / Cr]; (d) (−2.993±1.197)+5.580±2.309×subject's age+1.724±0.719×Gender (Female=1, Male=0)+63.571±73.444×[IL-5 / Cr]+(−0908.523±13606.752)×[ACh / Cr]+(−566.991±636.589)×[ATP / Cr]; (e) (−3.090±1.200)+5.393±2.256×subject's age+1.797±0.717×Gender (Female=1, Male=0)+34.767±56.331×[IL-5 / Cr]+(−562.743±629.316)×[ATP / Cr]; or (f) (−2.650±1.067)+5.516±2.120×subject's age+1.389±0.583×Gender (Female=1, Male=0)+(−4.060±45.238)×[IL-5 / Cr]+(−1.456±6.833)×[NO / Cr]; and wherein a pOAB above a threshold indicates that the subject has a high likelihood of having or developing OAB and a pOAB below a threshold indicates that the subject does not have OAB.
Owner:UNIV OF PORTSMOUTH HIGHER EDUCATION CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com