Medicine for treating asthma
A technology for drugs and asthma, applied in the field of medicine, can solve the problems of difficulty in guaranteeing the quality of drugs and inconvenience in industrialized production of drugs, etc., and achieve the effects of reducing airway hyperresponsiveness, relieving bronchial spasm, and treating pulmonary inflammation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
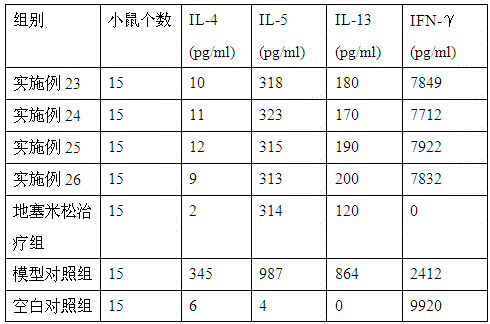
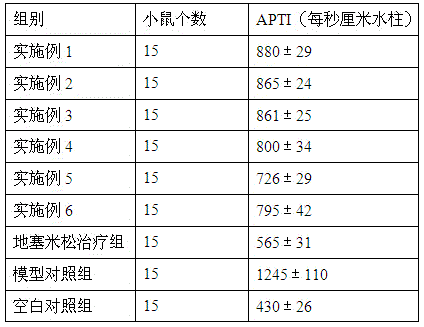
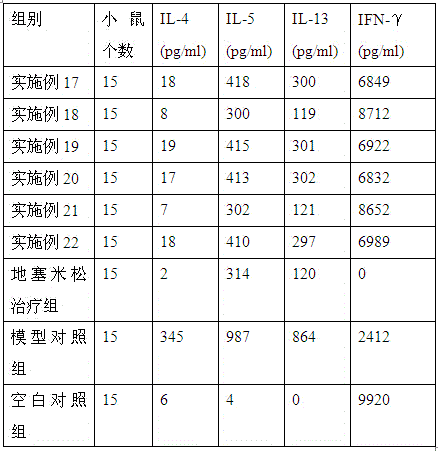
Image
Examples
Embodiment 1
[0030] Embodiment 1 A kind of medicine for the treatment of asthma
[0031] The drug is marine algae glycoprotein;
[0032] Said marine algae glycoprotein, in parts by weight, includes sugar 1%, protein 99%,
[0033] The molecular weight is 0.2kDa;
[0034] The marine algae are: cyanobacteria;
[0035] The sugar is a polysaccharide;
[0036] The polysaccharides include: xylose, fucose, arabinose, glucose, galactose, mannose, rhamnose;
[0037] The protein includes: asparagine, cysteine, lysine, arginine, serine, threonine;
Embodiment 2
[0038] Embodiment 2 A kind of medicine for the treatment of asthma
[0039] The drug is marine algae glycoprotein;
[0040] Said marine algae glycoprotein, in parts by weight, comprises sugar 25%, protein 75%,
[0041] The molecular weight is 55kDa;
[0042] The marine algae is: green algae;
[0043] The sugar is a polysaccharide;
[0044] The polysaccharides include: xylose, fucose, arabinose, glucose, galactose, mannose, rhamnose;
[0045] The protein includes: asparagine, cysteine, lysine, arginine, serine, threonine.
Embodiment 3
[0046] Embodiment 3 A kind of medicine for the treatment of asthma
[0047] The drug is marine algae glycoprotein;
[0048] Said marine algae glycoprotein, in parts by weight, comprises sugar 41%, protein 59%,
[0049] The molecular weight is 3kDa;
[0050] The marine algae are: cyanobacteria;
[0051] The sugar is a polysaccharide;
[0052] The polysaccharides include: xylose, fucose, arabinose, glucose, galactose, mannose, rhamnose;
[0053] The protein includes: asparagine, cysteine, lysine, arginine, serine, threonine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com