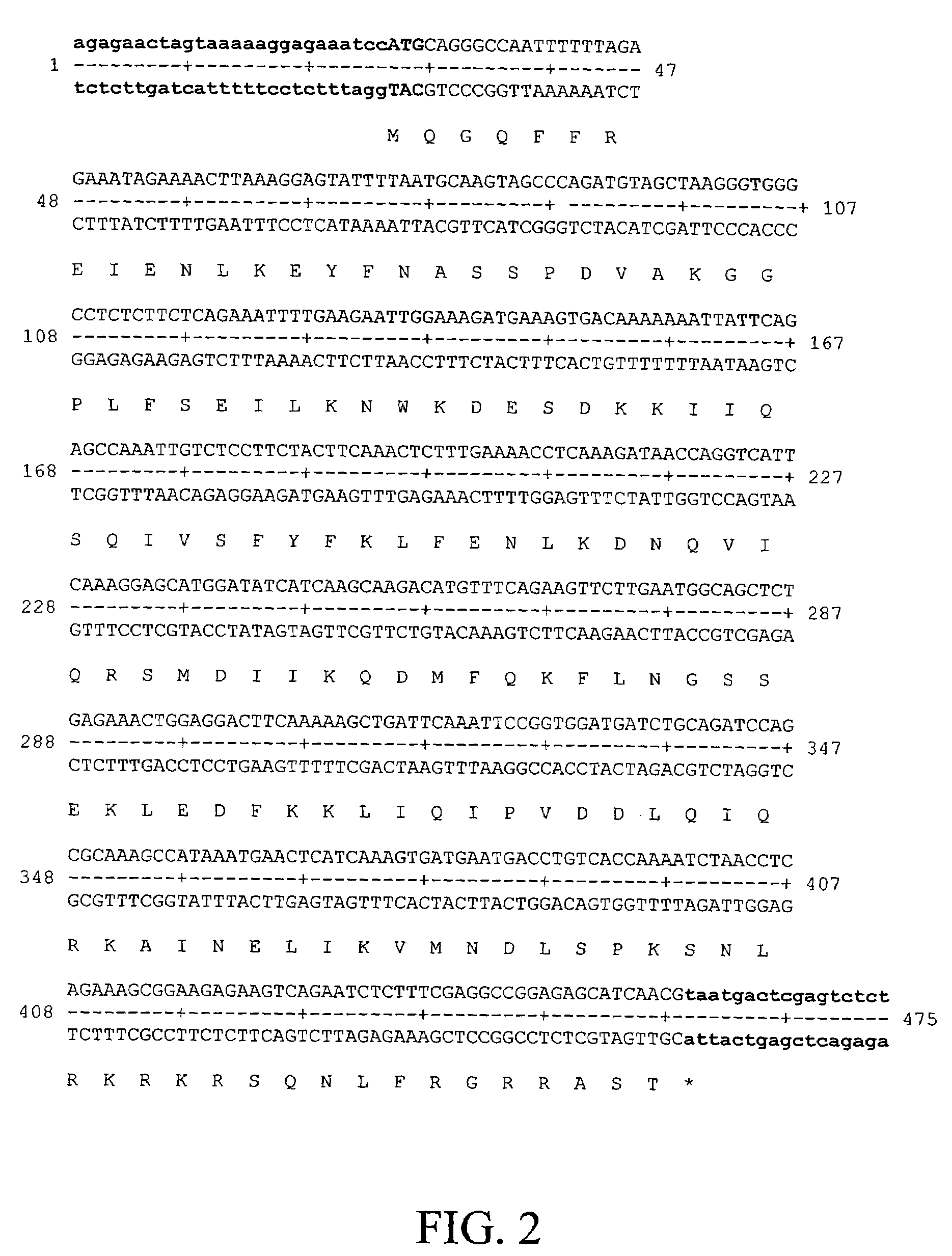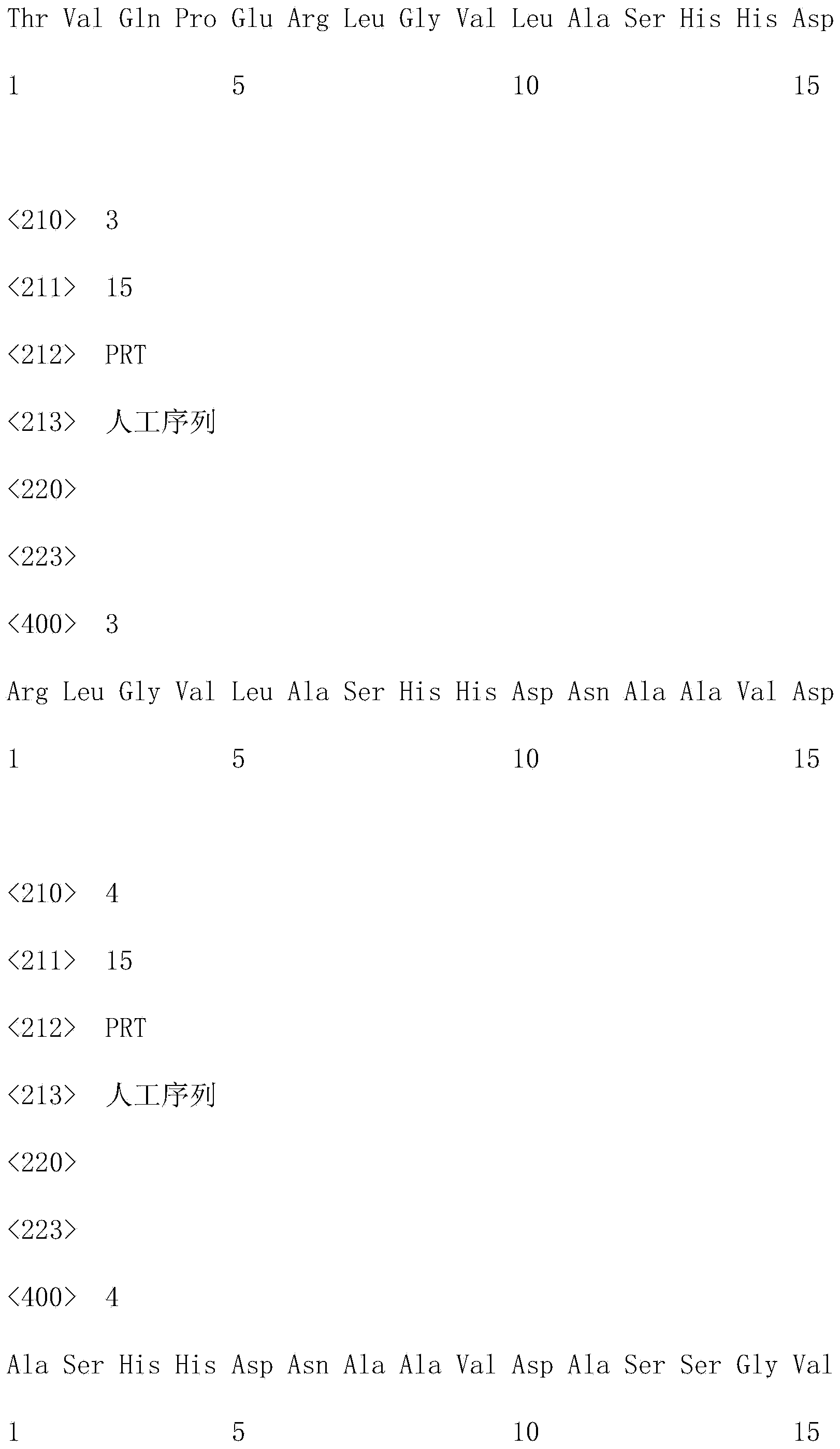Patents
Literature
191 results about "Gamma interferon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of gamma interferon. : an interferon that is produced by T cells, regulates the immune response, and in a form produced by recombinant DNA technology is used especially to control infections due to inability of white blood cells to destroy certain bacteria and fungi — compare alpha interferon, beta interferon.
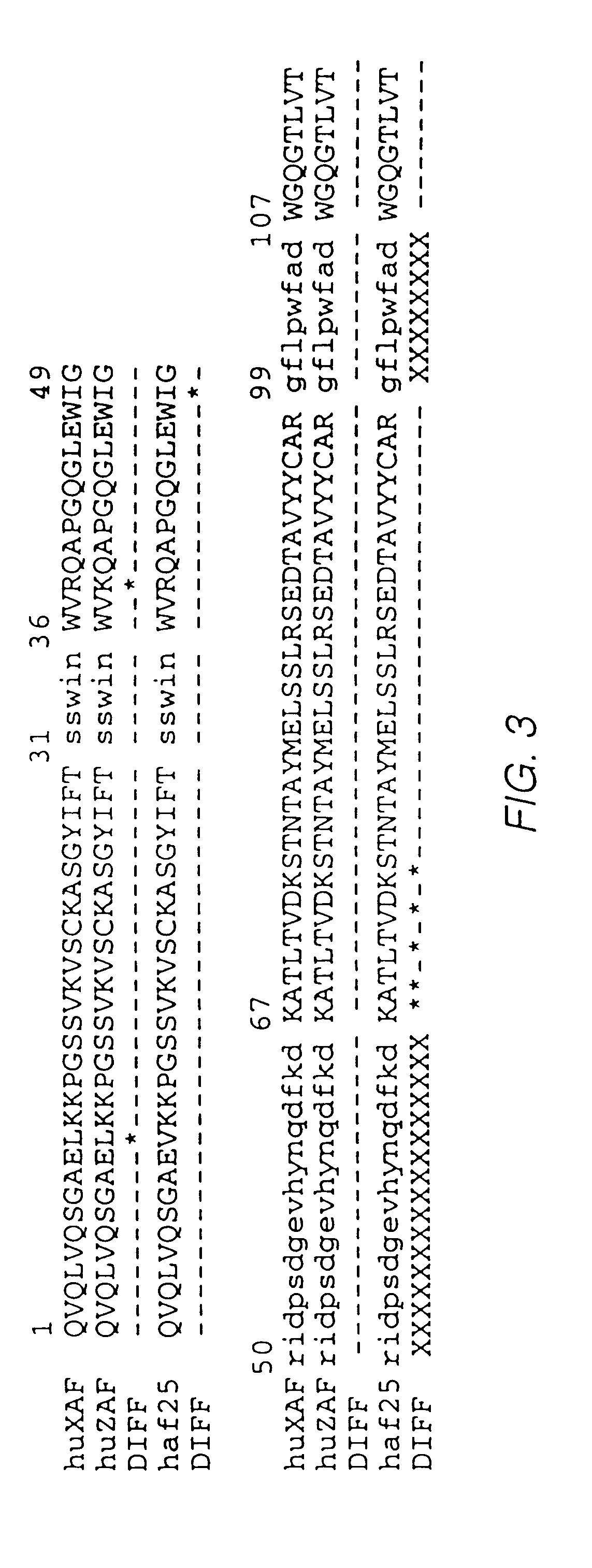
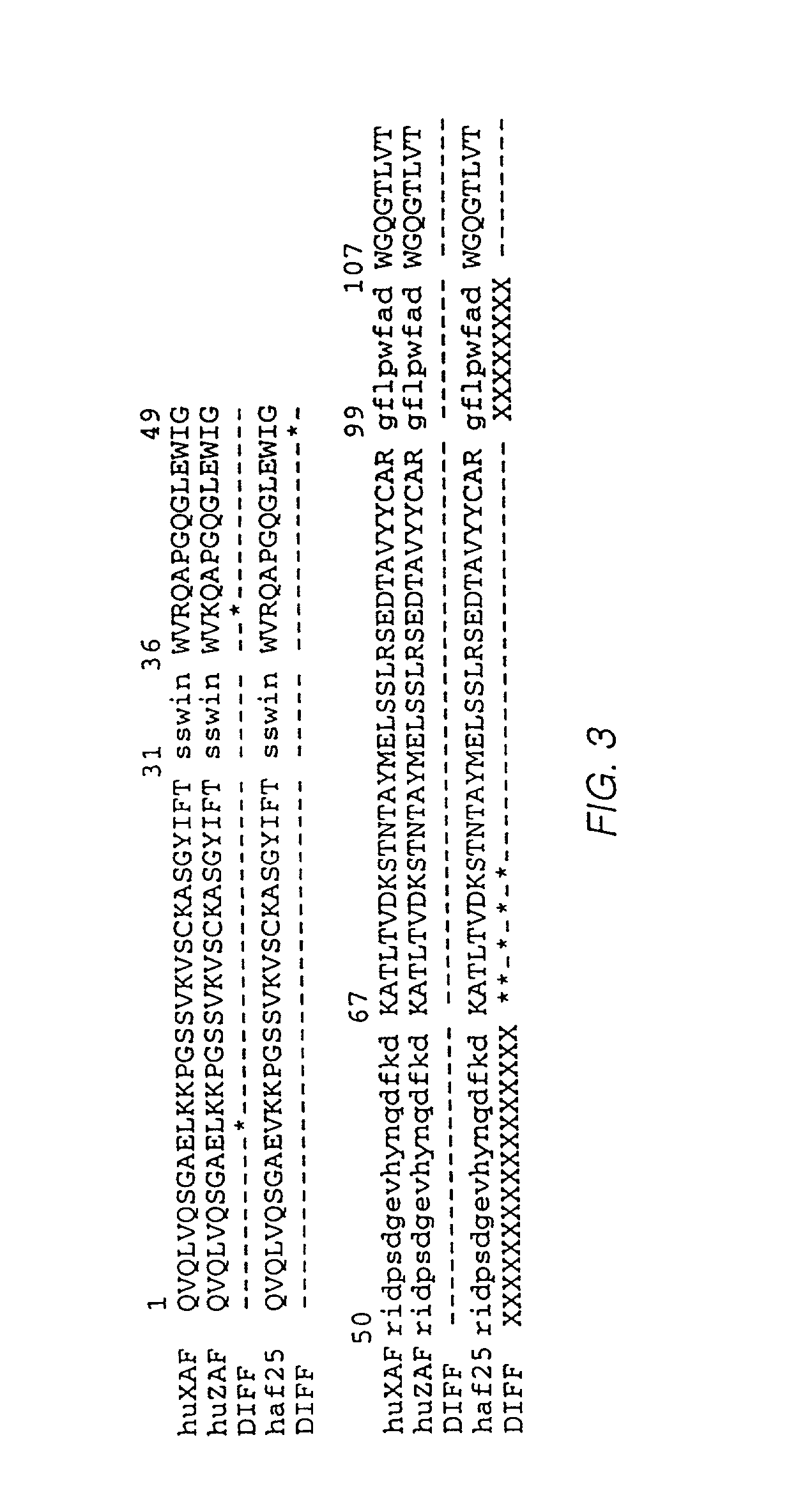
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Treatment of autism
InactiveUS20060188505A1Immunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsGreek letter betaGamma interferon
The invention includes methods of treating autistic spectrum disorders, including autism, in a patient where the method includes administration of an inhibitor of gamma interferon, an inhibitor of IL-1 beta, an inhibitor of IL-6, an inhibitor of IL-12, an inhibitor of IL-18, an inhibitor of TNF-alpha, and the administration of IL-10, alone or in combination, to the patient.
Owner:ADVANCED BIOTHERAPY
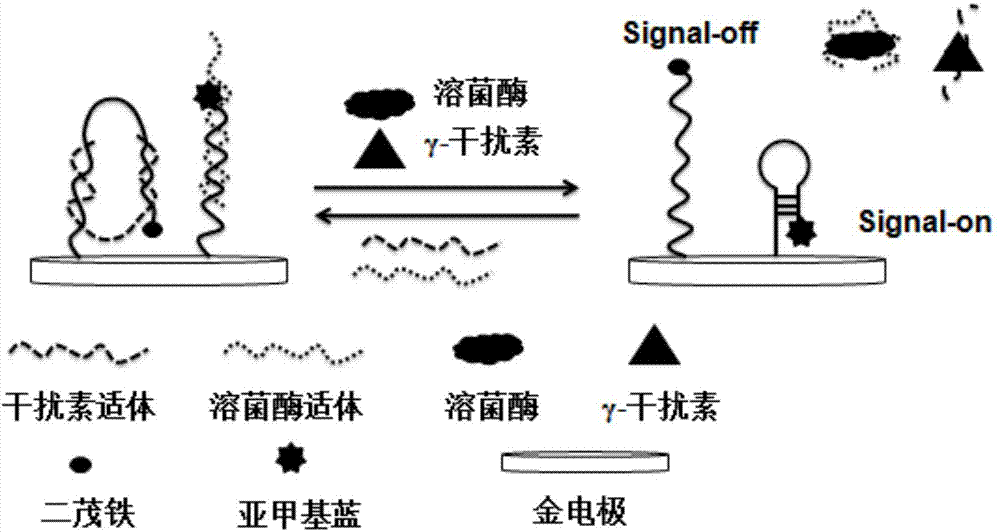
Preparation method and application of electrochemical sensor capable of simultaneously detecting two acute leukemia markers
ActiveCN103940872AAchieving High Sensitivity DetectionHigh selectivityMaterial electrochemical variablesTear lysozymeAcute leukemia
The invention discloses a preparation method of an electrochemical sensor capable of simultaneously detecting two acute leukemia markers. The method comprises the following steps: respectively standing a lysozyme report probe and a gamma-interferon report probe in a liquid containing trichloroethyl phosphate (TCEP), so as to open a disulfide bond and respectively form a double-chain structure together with a lysozyme aptamer and a gamma-interferon aptamer; preprocessing a gold electrode, and immersing the processed gold electrode into the pre-processed mixed liquid of lysozyme report probe-aptamer double chains and gamma-interferon report probe-aptamer double chains; standing at room temperature over the night, and then cleaning by using secondary distilled water and a cleanout fluid; immersing the electrode into the liquid containing MCH to seal the electrode, and then cleaning the secondary distilled water and the cleanout fluid; and taking the electrode processed by the above steps as a work electrode to be connected on a chemical work station together with a reference electrode and a counter electrode, so as to obtain the electrochemical sensor. The electrochemical sensor can be applied to simultaneous detection of two acute leukemia markers, namely lysozyme and gamma-interferon.
Owner:QINGDAO UNIV
Kit and method for detecting mycobacterium tuberculosis infection and application
ActiveCN102004155ALow costQuality assuranceBiological testingMycobacterium InfectionsLatent tuberculosis
The invention belongs to the field of biomedicine examination, and particularly relates to a kit and a method for detecting mycobacterium tuberculosis infection and application. The invention discloses a novel mycobacterium tuberculosis detection reagent by screening specific T cell epitope of mycobacterium tuberculosis, wherein the reagent contains polypeptide or analog thereof represented by SEQ ID No.1-10. The method detects cell factors released from T cells by using single or more SEQ ID No.1-10 polypeptides to contact the T cells of mycobacterium tuberculosis infected individuals. The method can effectively detect active tuberculosis or latent tuberculosis infection, and is free from disturbance of Bacilli Calmette Guerin (BCG) inoculation vaccines. The invention also discloses a diagnostic kit and other application based on the polypeptide and the method. Compared with the gamma interferon release experiments in the prior art, the method can obviously improve the detection rate without reducing the specificity and has high clinical application value.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
C-class oligonucleotide analogs with enhanced immunostimulatory potency
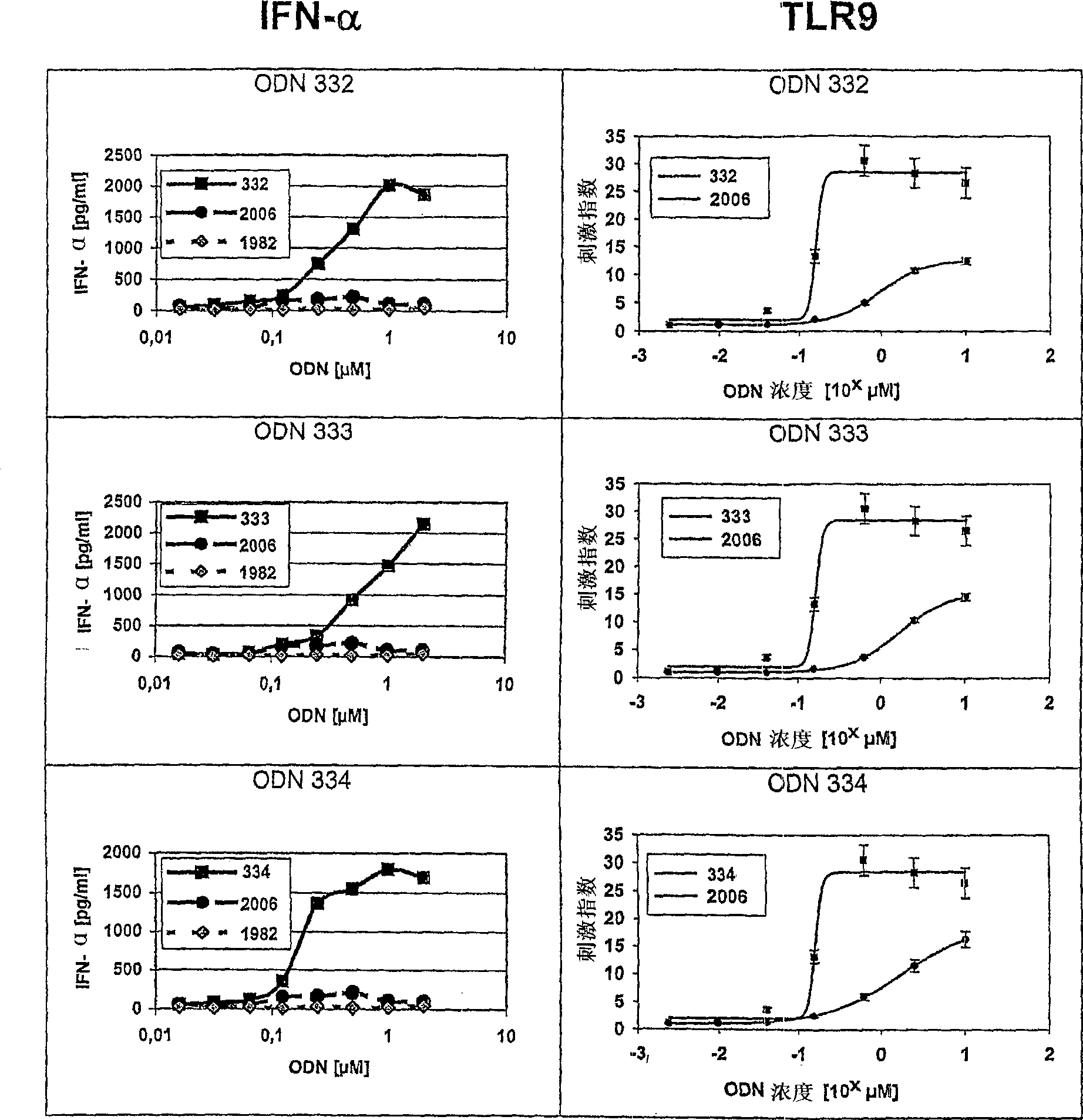
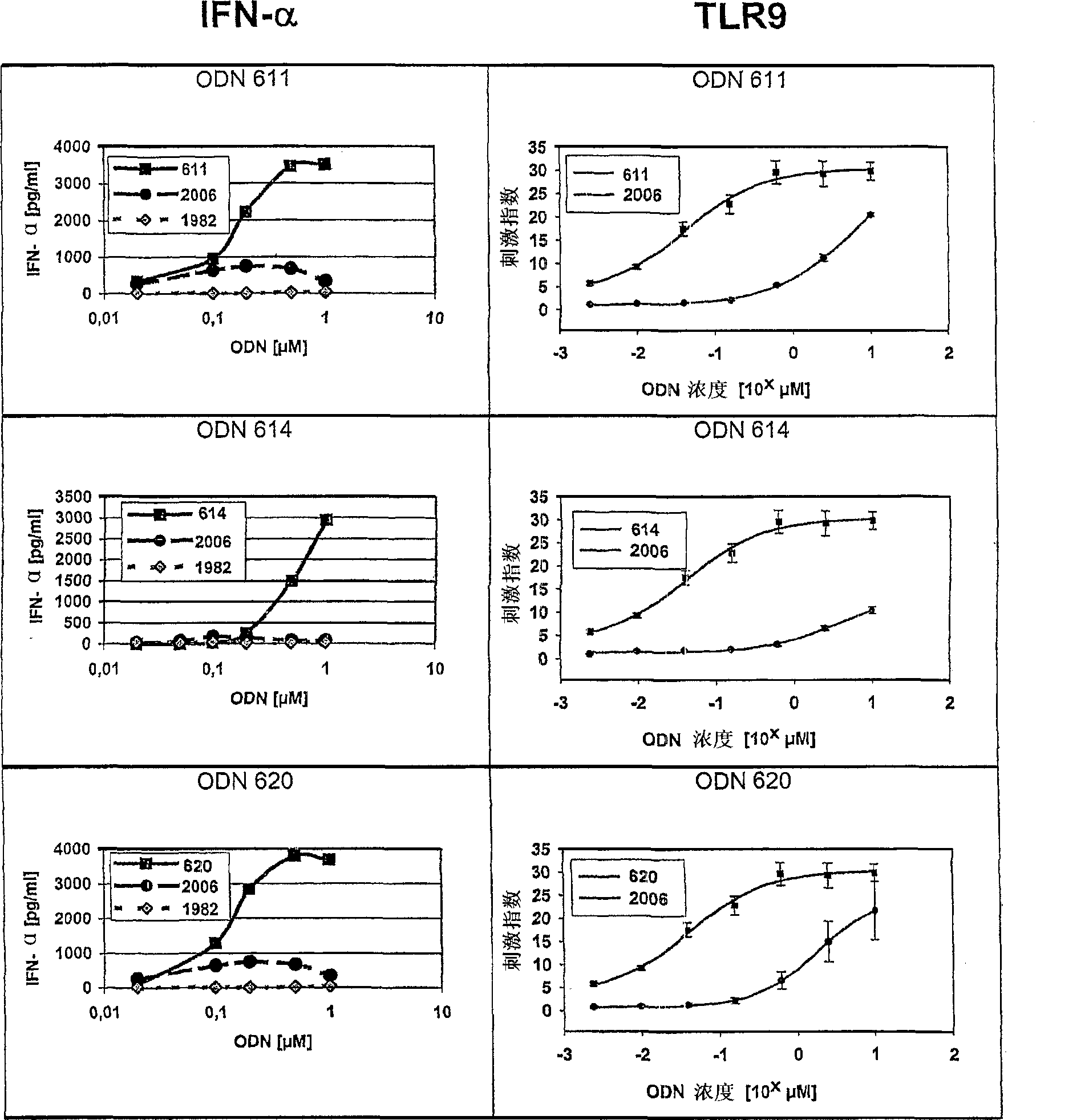
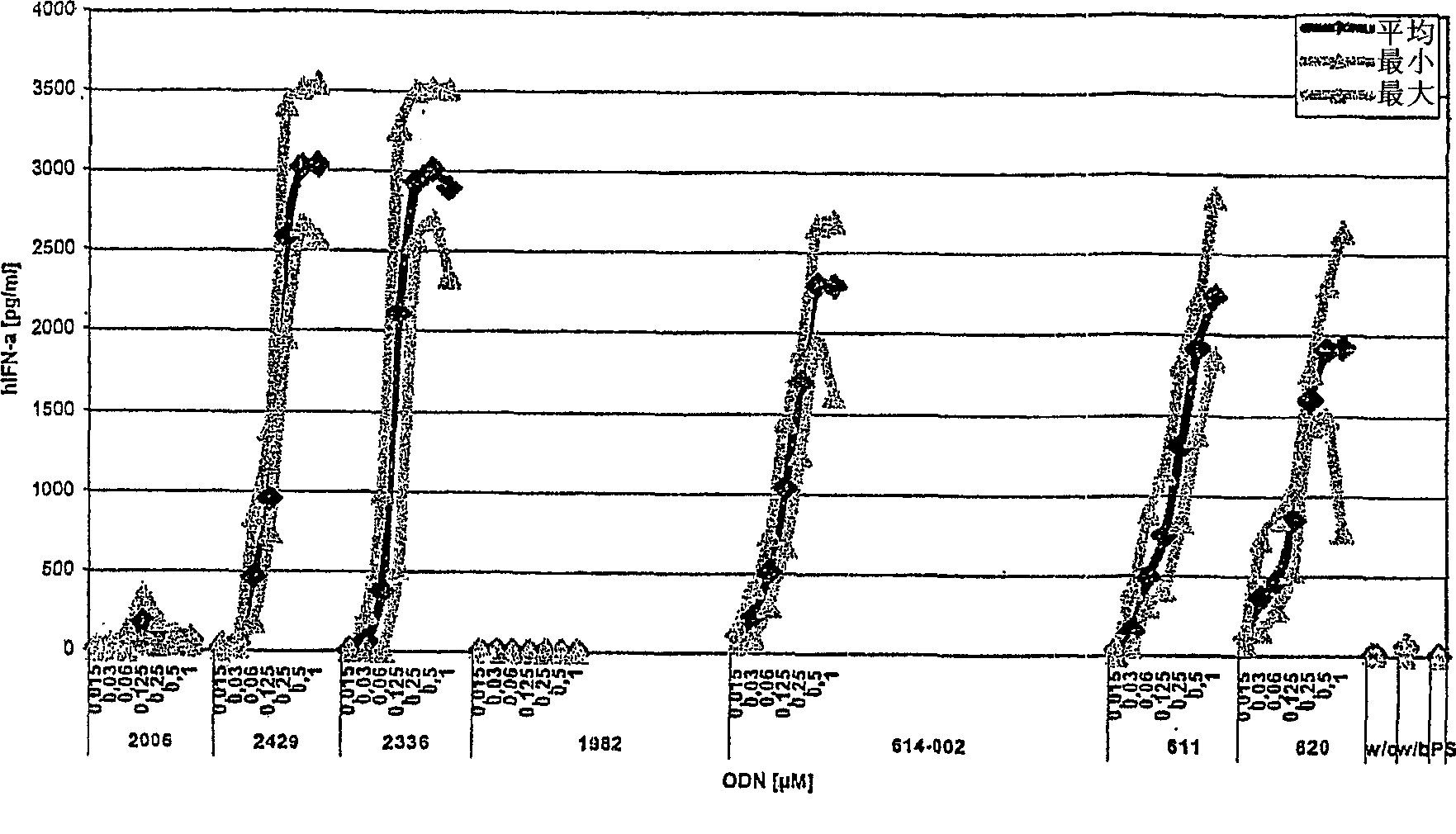
The invention relates to a class of CpG immunostimulatory oligonucleotides containing a CpG immunostimulatory motif and a second motif which is capable of forming secondary structure, including duplex and higher order structures, in vitro and in vivo. The oligonucleotides of the invention are useful as adjuvants in vaccination. The oligonucleotides are also useful for inducing an immune response, inducing expression of a type I interferon (IFN), inducing expression of gamma interferon (IFN-gamma), and for treating a variety of conditions, including allergy, asthma, infection, and cancer.
Owner:科勒制药有限公司 +1
Bovine tuberculosis antigen specific gamma-interferon enzyme-linked immuno sorbent assay (ELISA) kit
InactiveCN102183649ASupernatant with high titerStrong specificityMaterial analysisProtein solutionGamma interferon
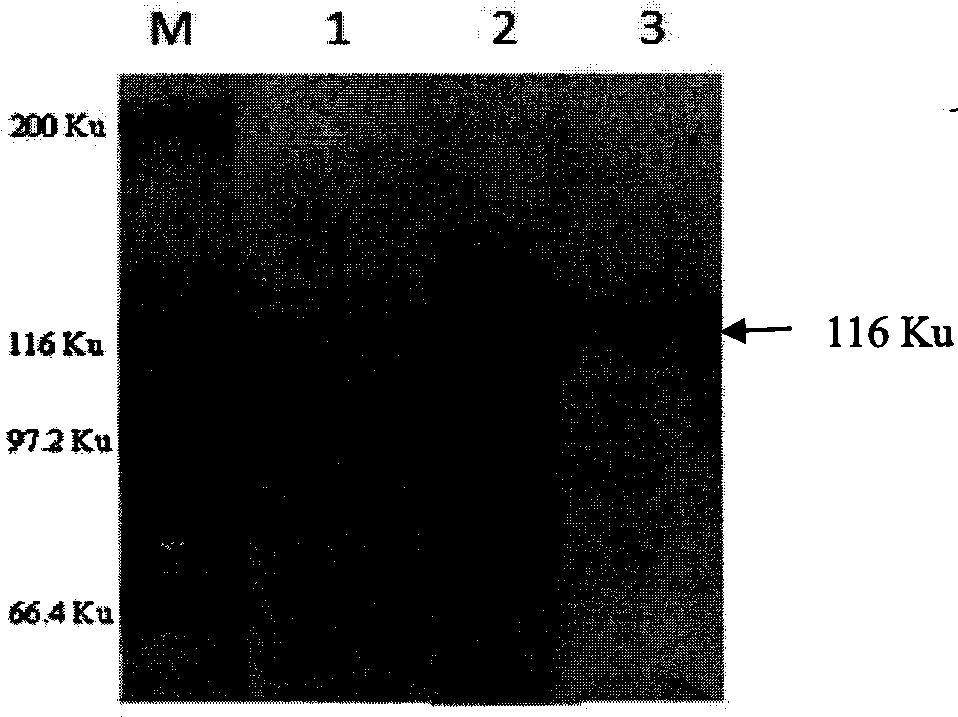
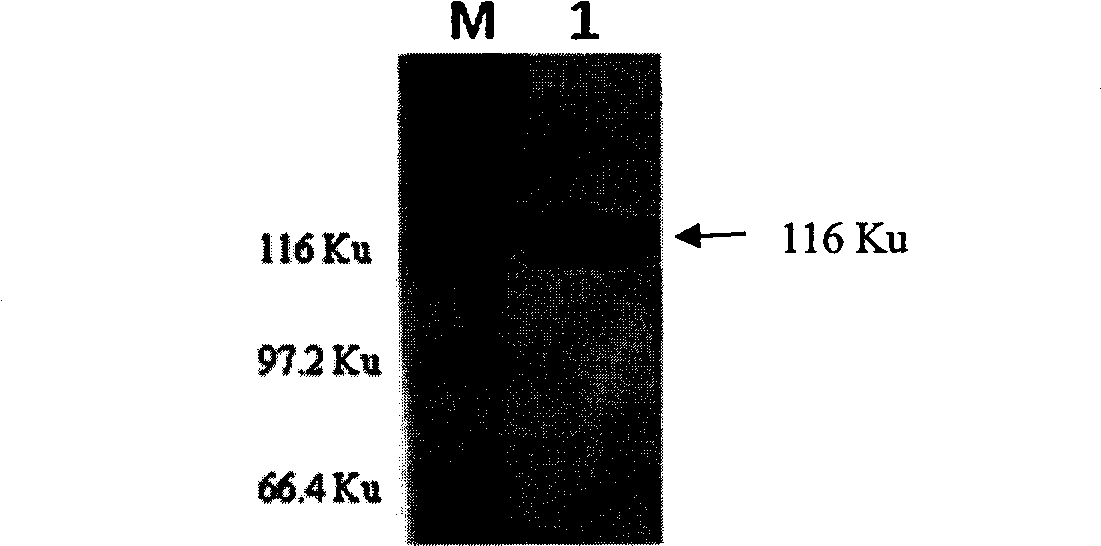
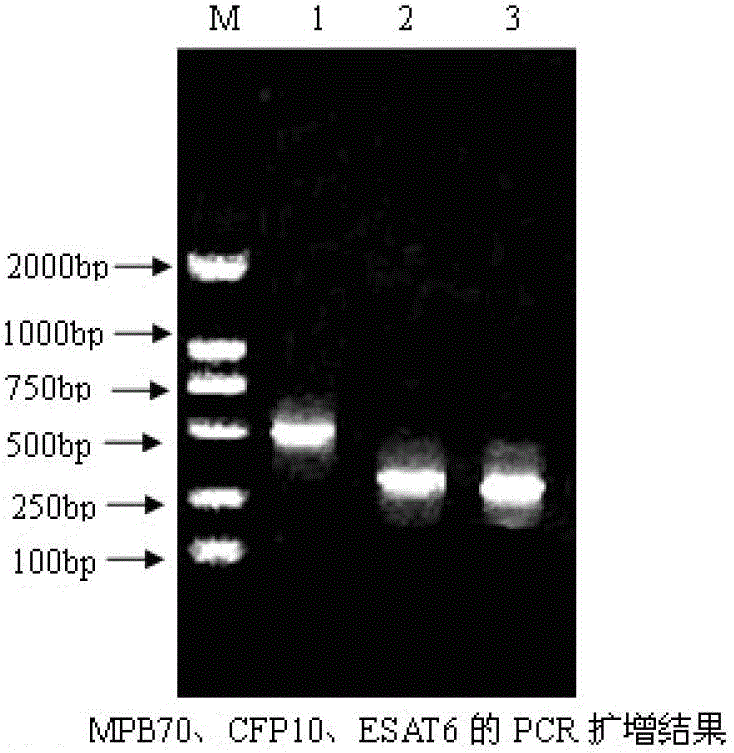
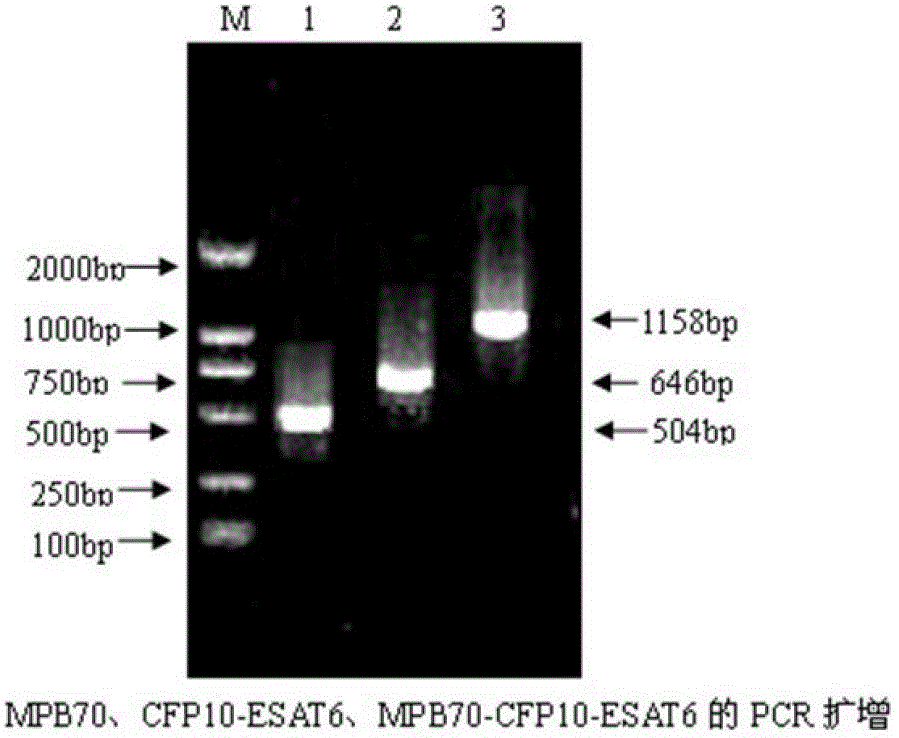
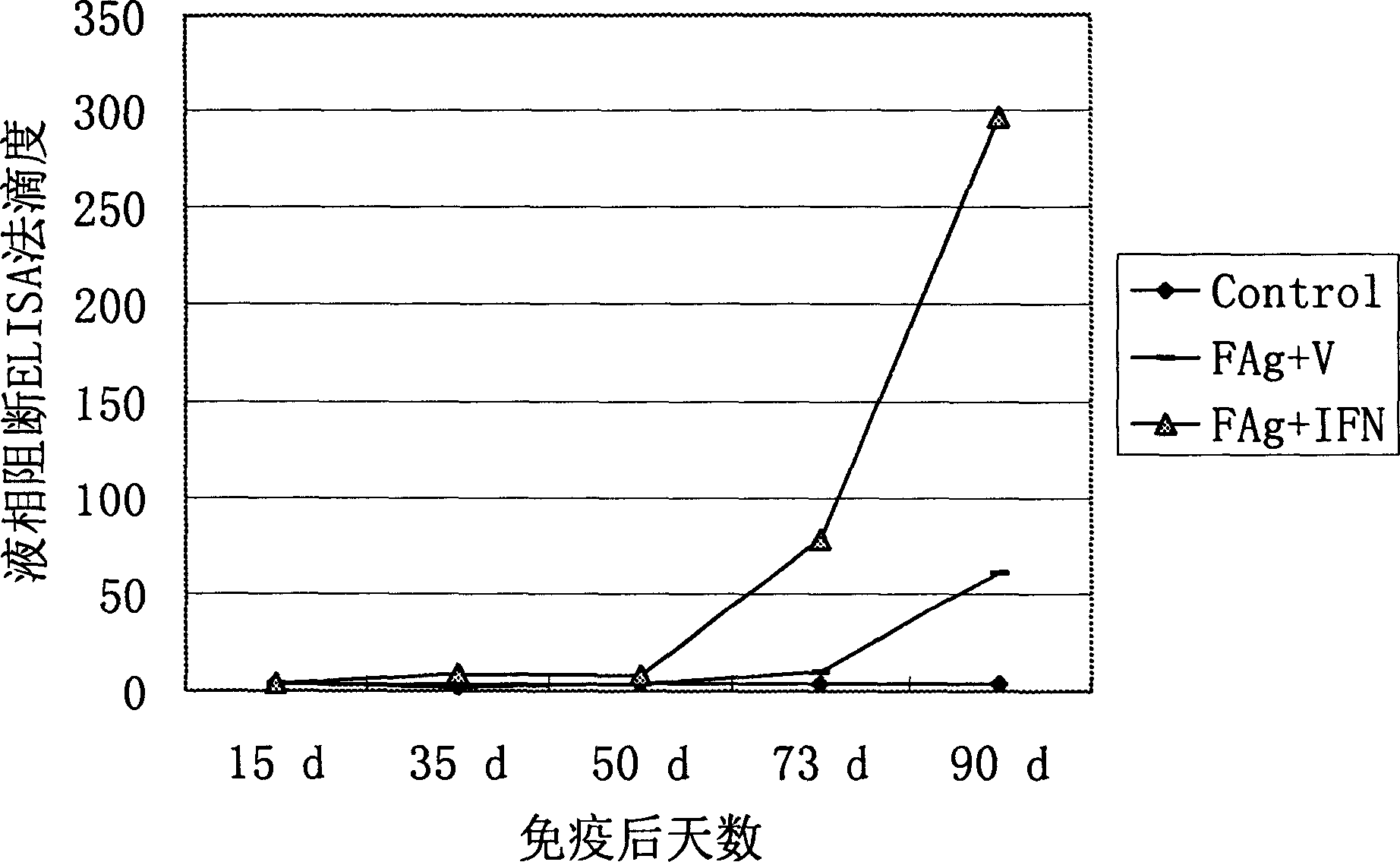
The invention provides a bovine tuberculosis antigen specific gamma-interferon enzyme-linked immuno sorbent assay (ELISA) kit. The kit comprises a bovine gamma-interferon monoclonal antibody coated ELISA plate, enzyme-labeled bovine gamma-interferon monoclonal antibodies, and protein solution containing mycobacterium bovis MPB83 and MPB70 and mycobacterium tuberculosis ESAT6 and CFP10. The antibodies are captured and detected for different antigen surfaces of gamma-interferon respectively. A bovine tuberculosis antigen specific gamma-interferon ELISA detection method established by using the mycobacterium bovis recombinant proteins MPB83 and MPB70 and mycobacterium tuberculosis recombinant proteins ESAT6 and CFP10 is relatively stable; the specificity of the method is 96 percent, and the sensitivity of the method is 88.6 percent; and the specificity and the sensitivity of the gamma-interferon ELISA detection method for the bovine tuberculosis are greatly improved.
Owner:CHINA AGRI UNIV
Method and compositions for treatment of cancers
InactiveUS7854717B1Improve inflammationHigh activityHeavy metal active ingredientsPeptide/protein ingredientsSystemic chemotherapyWhole body
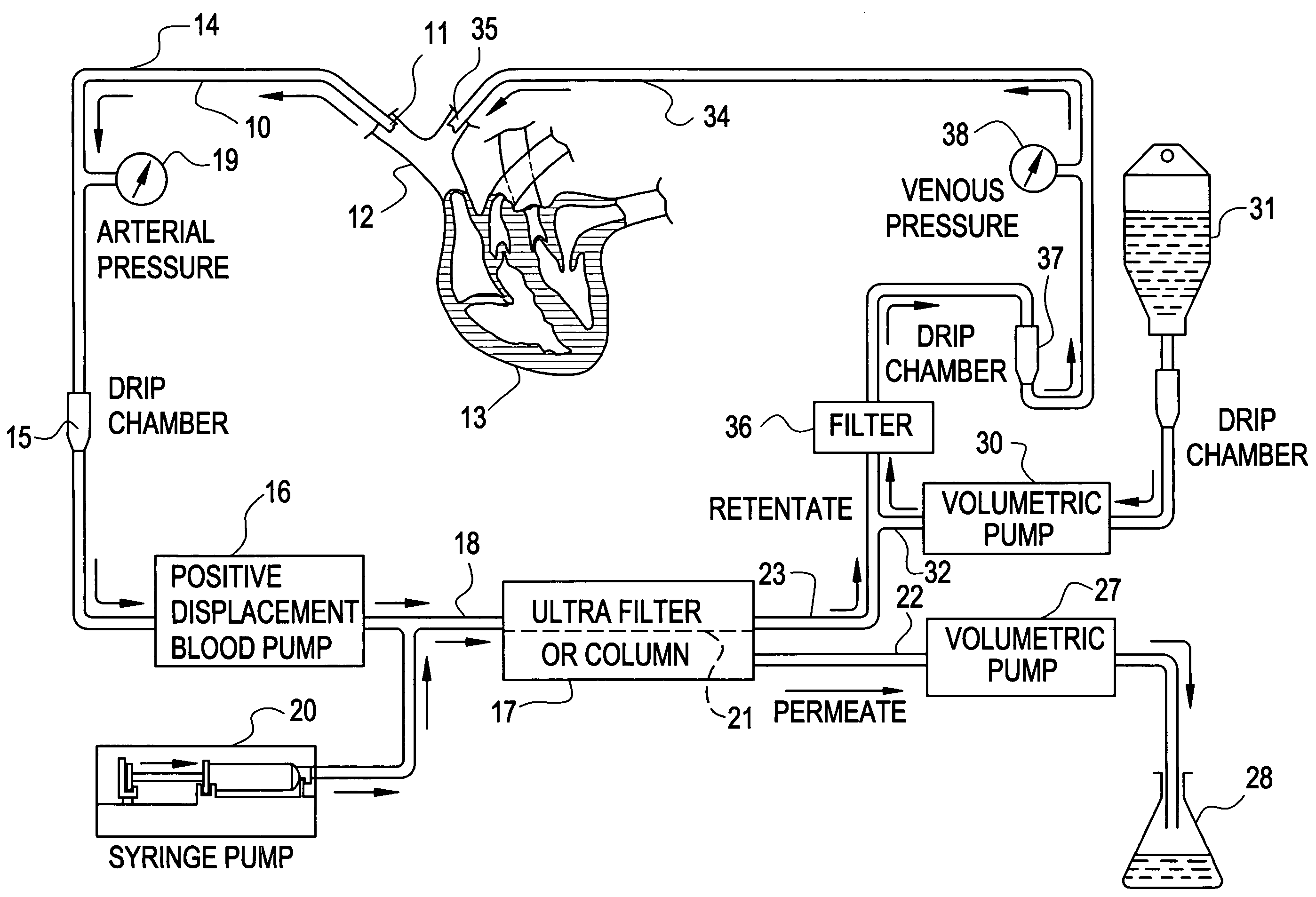
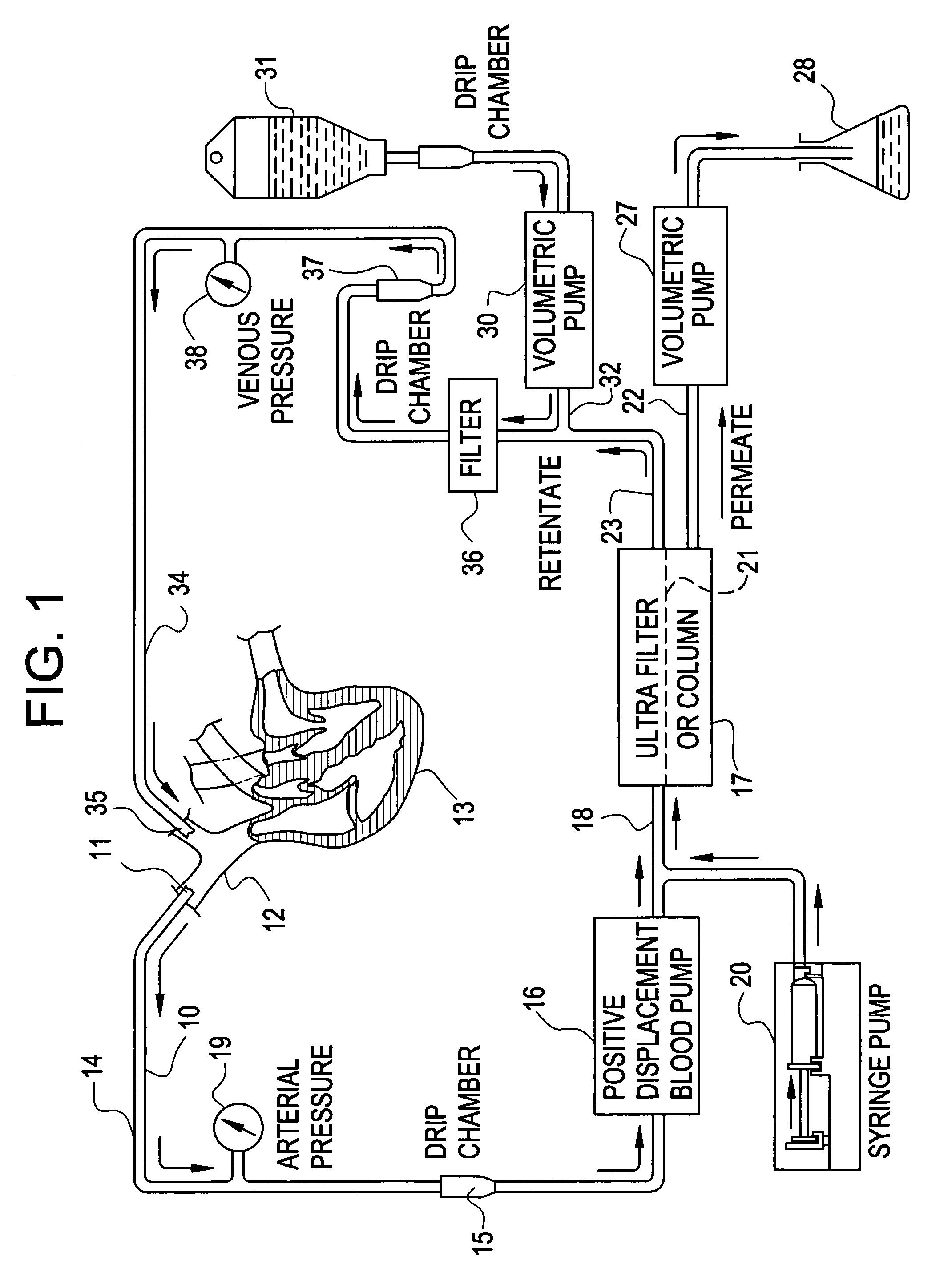
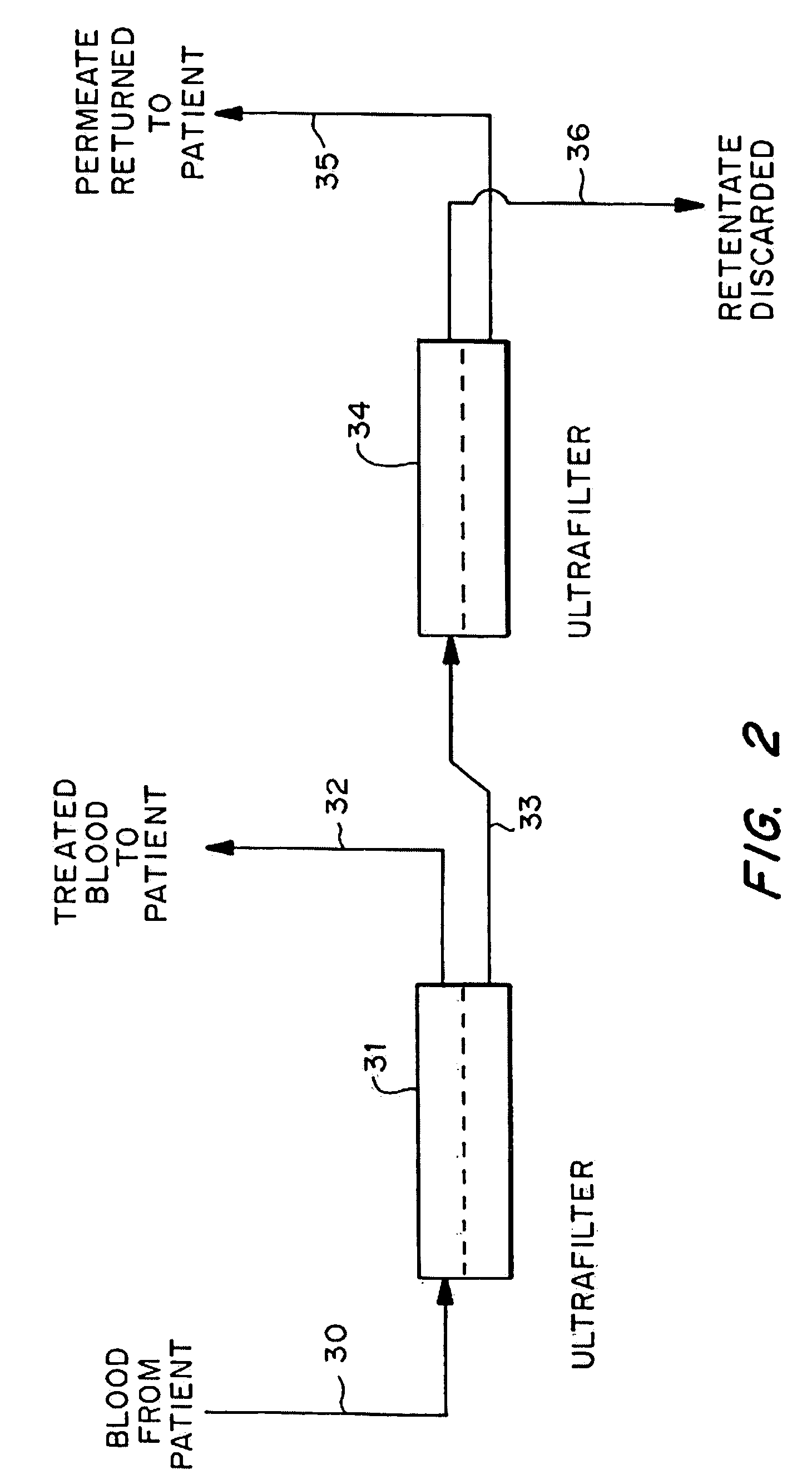
A method to treat cancer uses ultrapheresis, refined to remove compounds of less than 120,000 daltons molecular weight, followed by administration of replacement fluid, to stimulate the patient's immune system to attack solid tumors. In the preferred embodiment, the patient is ultrapheresed using a capillary tube ultrafilter having a pore size of 0.02 to 0.05 microns, with a molecular weight cutoff of 120,000 daltons, sufficient to filter one blood volume. The preferred replacement fluid is ultrapheresed normal plasma. The patient is preferably treated daily for three weeks, diagnostic tests conducted to verify that there has been shrinkage of the tumors, then the treatment regime is repeated. The treatment is preferably combined with an alternative therapy, for example, treatment with an anti-angiogenic compound, one or more cytokines such as TNF, gamma interferon, or IL-2, or a procoagulant compound. The treatment increases endogenous, local levels of cytokines, such as TNF. This provides a basis for an improved effect when combined with any treatment that enhances cytokine activity against the tumors, for example, treatments using alkylating agents, doxyrubicin, carboplatinum, cistplatimum, and taxol. Alternatively, the ultrapheresis treatment can be combined with local chemotherapy, systemic chemotherapy, and / or radiation.
Owner:INNATUS CORP
Method and system to remove cytokine inhibitor in patients
InactiveUS20050244371A1Induce inflammationOrganic active ingredientsPeptide/protein ingredientsAbnormal tissue growthSystemic chemotherapy
A method to treat cancer uses ultrapheresis, refined to remove compounds of less than 120,000 daltons molecular weight, followed by administration of replacement fluid, to stimulate the patient's immune system to attack solid tumors. In the preferred embodiment, the patient is ultrapheresed using a capillary tube ultrafilter having a pore size of 0.02 to 0.05 microns, with a molecular weight cutoff of 120,000 daltons, sufficient to filter one blood volume. The preferred replacement fluid is ultrapheresed normal plasma. The patient is preferably treated daily for three weeks, diagnostic tests conducted to verify that there has been shrinkage of the tumors, then the treatment regime is repeated. The treatment is preferably combined with an alternative therapy, for example, treatment with an anti-angiogenic compound, one or more cytokines such as TNF, gamma interferon, or IL-2, or a procoagulant compound. The treatment increases endogenous, local levels of cytokines, such as TNF. This provides a basis for an improved effect when combined with any treatment that enhances cytokine activity against the tumors, for example, treatments using alkylating agents, doxyrubicin, carboplatinum, cisplatinum, and taxol. Alternatively, the ultrapheresis treatment can be combined with local chemotherapy, systemic chemotherapy, and / or radiation.
Owner:INNATUS CORP
Compositions and methods for treating hyperimmune response in the eye
The present invention comprises and utilizes methods and compositions for treating hyperimmune reactions in the eye. Compositions comprising antibodies to gamma interferon alone and in combination with other drugs are described. Also disclosed in the invention are methods of applying a composition comprising interferon gamma antibodies topically to the eye to treat hyperimmune reactions, such as transplant rejection, uveitis, autoimmune diseases of the eye, and ocular disorders incidental to or connected with autoimmune diseases.
Owner:ADVANCED BIOTHERAPY
Amended recombinant cells for the production and delivery of gamma interferon as an antiviral agent, adjuvant and vaccine accelerant
The present invention provides active cytokine and / or chemokine compositions, as well as inexpensive means for the production, amended-cell encasement of active cytokine and / or chemokine compositions, processing, and delivery of active cytokine and / or chemokine compositions. The subject invention also provides methods of treatment and methods of accelerating an immune response comprising the administration of amended recombinant cell (ARC) containing cytokine and / or chemokine compositions to animals or humans.
Owner:CORTEVA AGRISCIENCE LLC
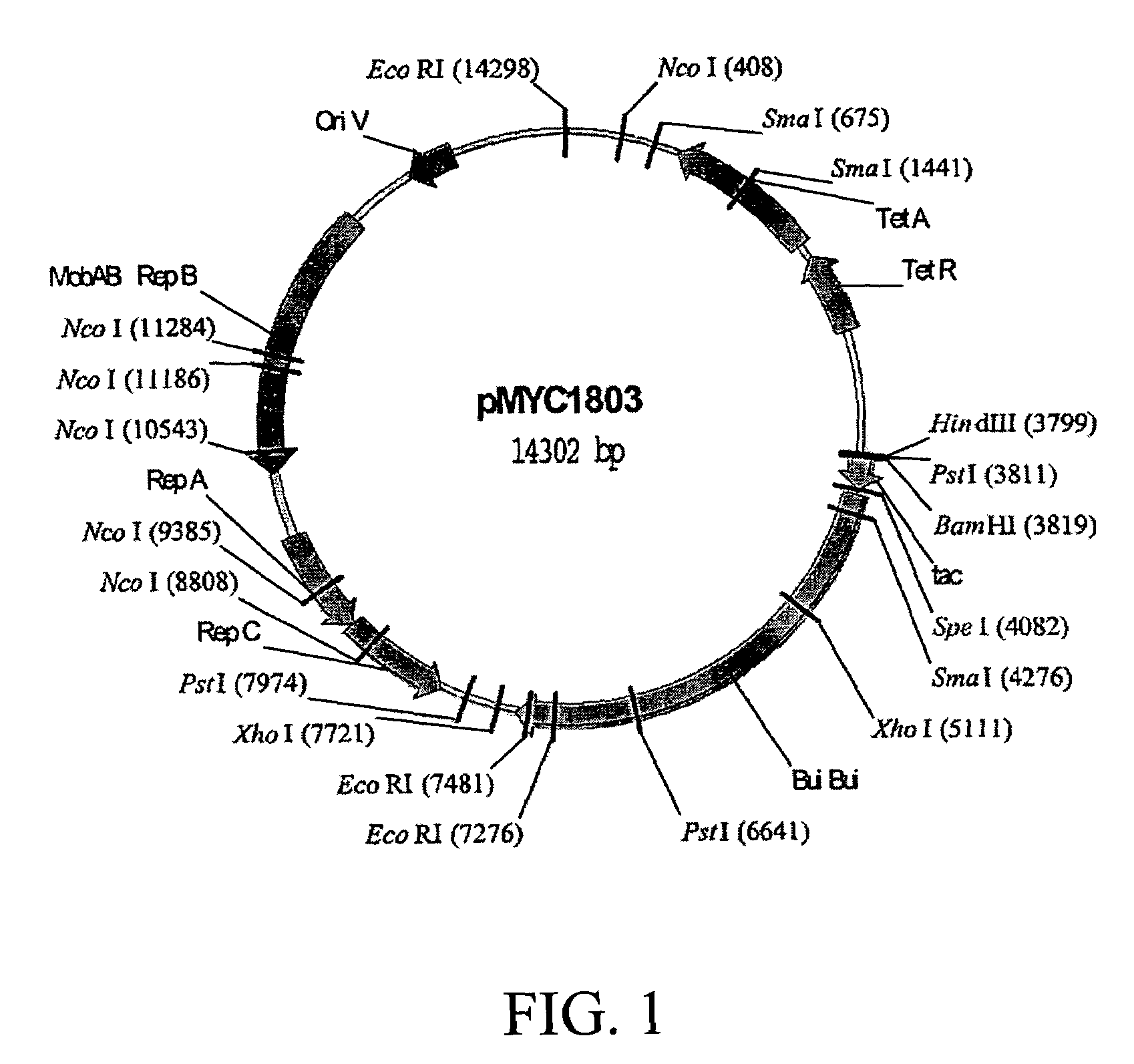
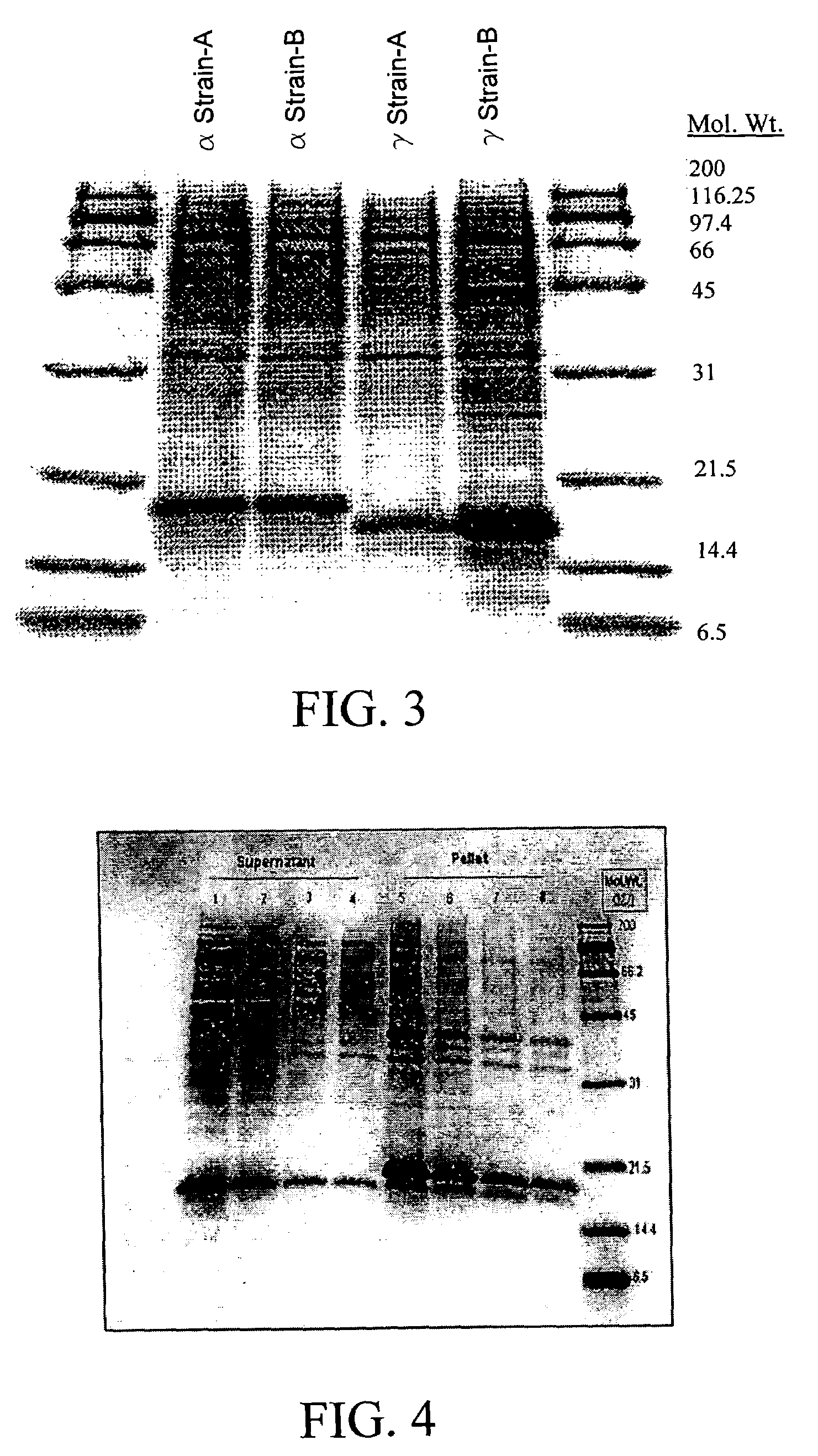
Spotted deer gamma-interferon double-antibody sandwich ELISA detection method, kit thereof and application of kit
ActiveCN101788563AMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationMicroorganismGamma interferon
The invention belongs to the field of agricultural micrological gene-engineering and animal borne diseases, and relates to a spotted deer gamma-interferon double-antibody sandwich ELISA detection method, a kit thereof and application of the kit. A cell strain (CerIFN-gamma4C) capable of stably secreting a spotted deer gamma-interferon monoclonal antibody is obtained, and the storage number of the cell strain is CCTCC NO:C200966. The invention also establishes a spotted deer gamma-interferon double-sandwich ELISA detection method, which is characterized by establishing the spotted deer gamma-interferon monoclonal antibody, preparing a spotted deer gamma-interferon polyclonal antibody and the like. The kit of the invention comprises the spotted deer gamma-interferon monoclonal antibody, the spotted deer gamma-interferon polyclonal antibody, bovine tuberculosis specific three-gene fusion antigenic proteins RCE and other reagents. The invention also discloses the detection method and the application of the kit. The kit has the advantages of high specificity, high sensitivity, simple and convenient operations and quick diagnosis.
Owner:HUAZHONG AGRI UNIV
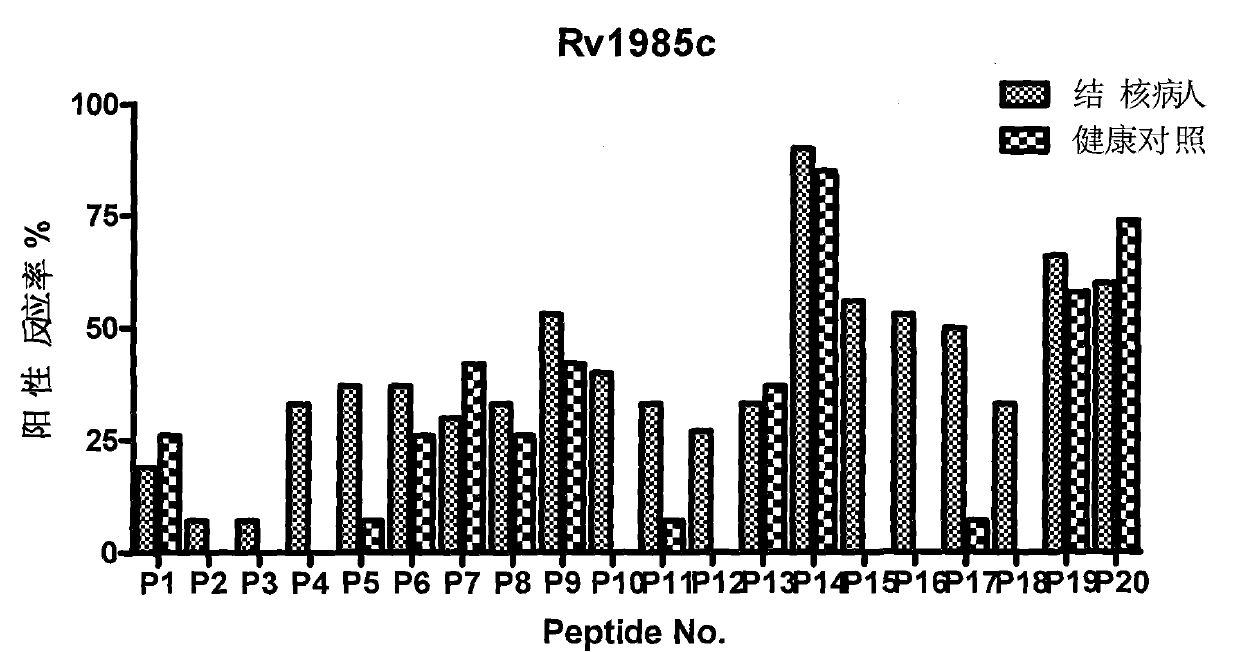
Epitope polypeptide applicable to mycobacterium tuberculosis infection detection and application thereof
ActiveCN102516356AHigh T cell reactivityDiagnosis of infectionAntibacterial agentsPeptide/protein ingredientsEpitopeMycobacterium Infections
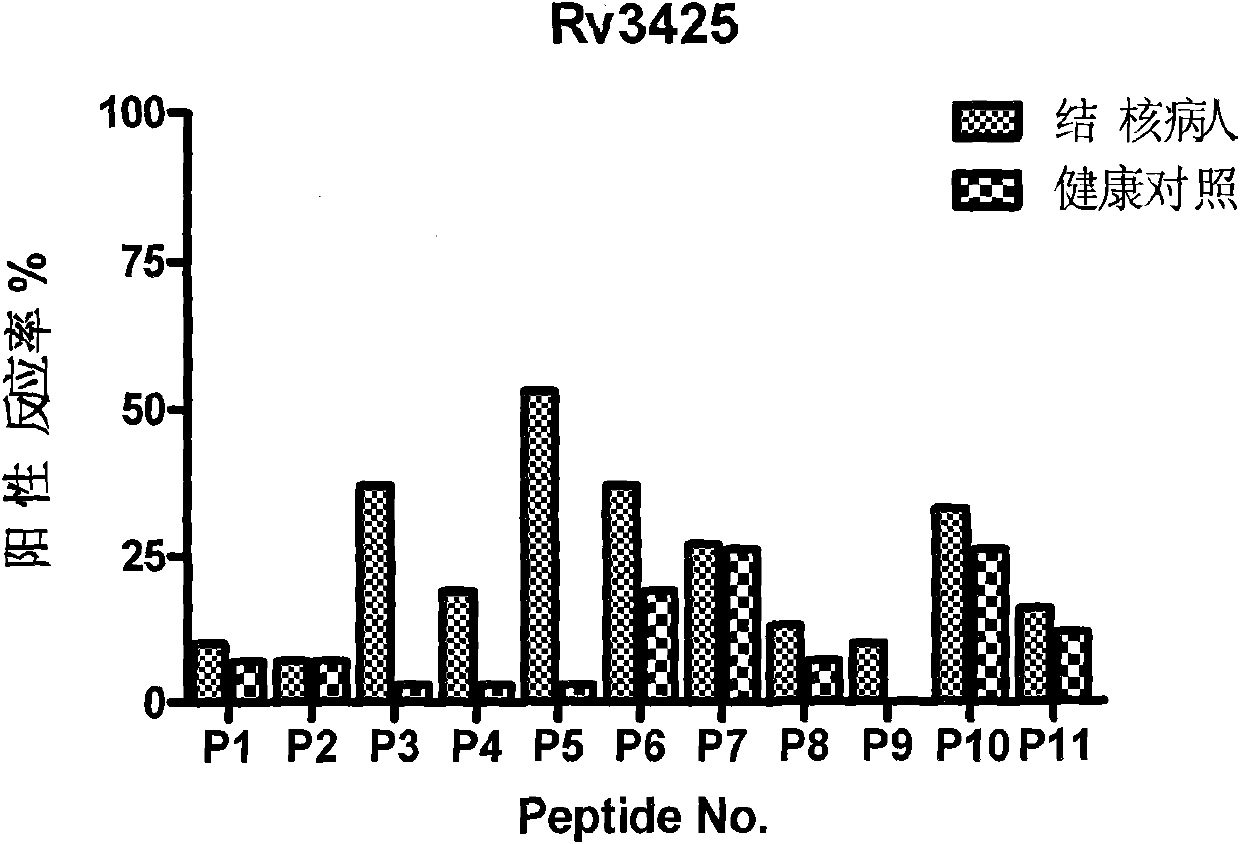
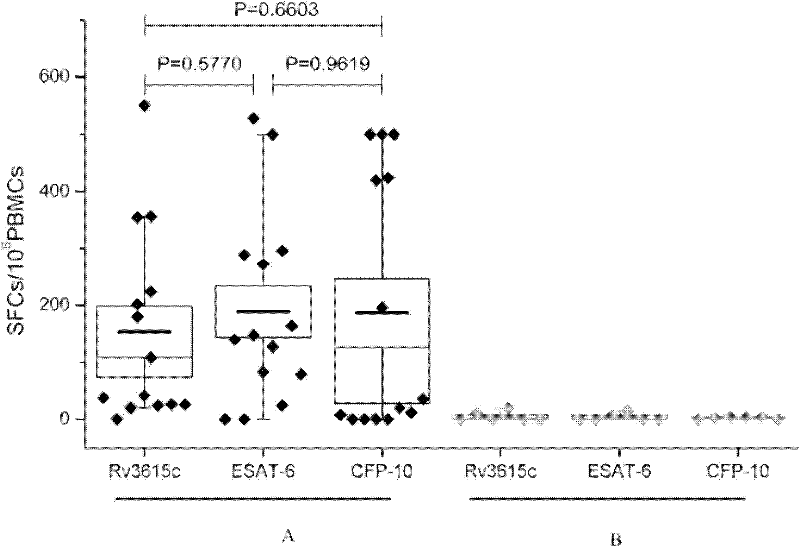
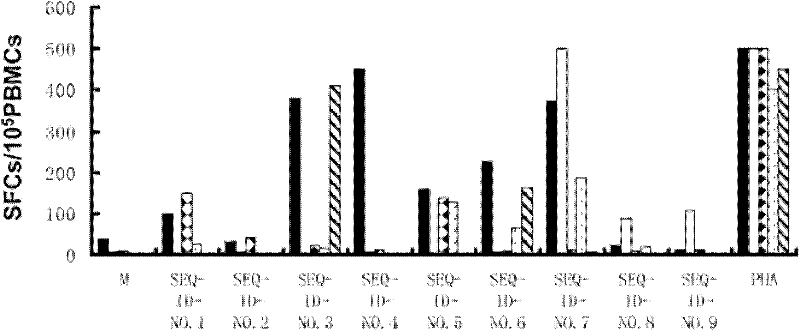
The invention discloses certain mycobacterium tuberculosis specific antigen epitope polypeptides, and particularly discloses an antigen Pv3615c antigen epitope polypeptide consisting of 8-11 continuous polypeptide fragments. The clinical detection practicability of the antigen polypeptide is evaluated according to the reaction sensitivity and specificity of the antigen polypeptide in a tuberculosis case. As proved by a result, an antigen polypeptide has high T cell reactivity in a tuberculosis patient, and the positive rate is up to 64 percent (9 / 14). Compared with the result of a healthy volunteer, the antigen epitope polypeptide has the specificity of up to 100 percent (6 / 6). Mycobacterium tuberculosis infection can be diagnosed effectively by applying an antigen peptide library specific T cell gamma interferon.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
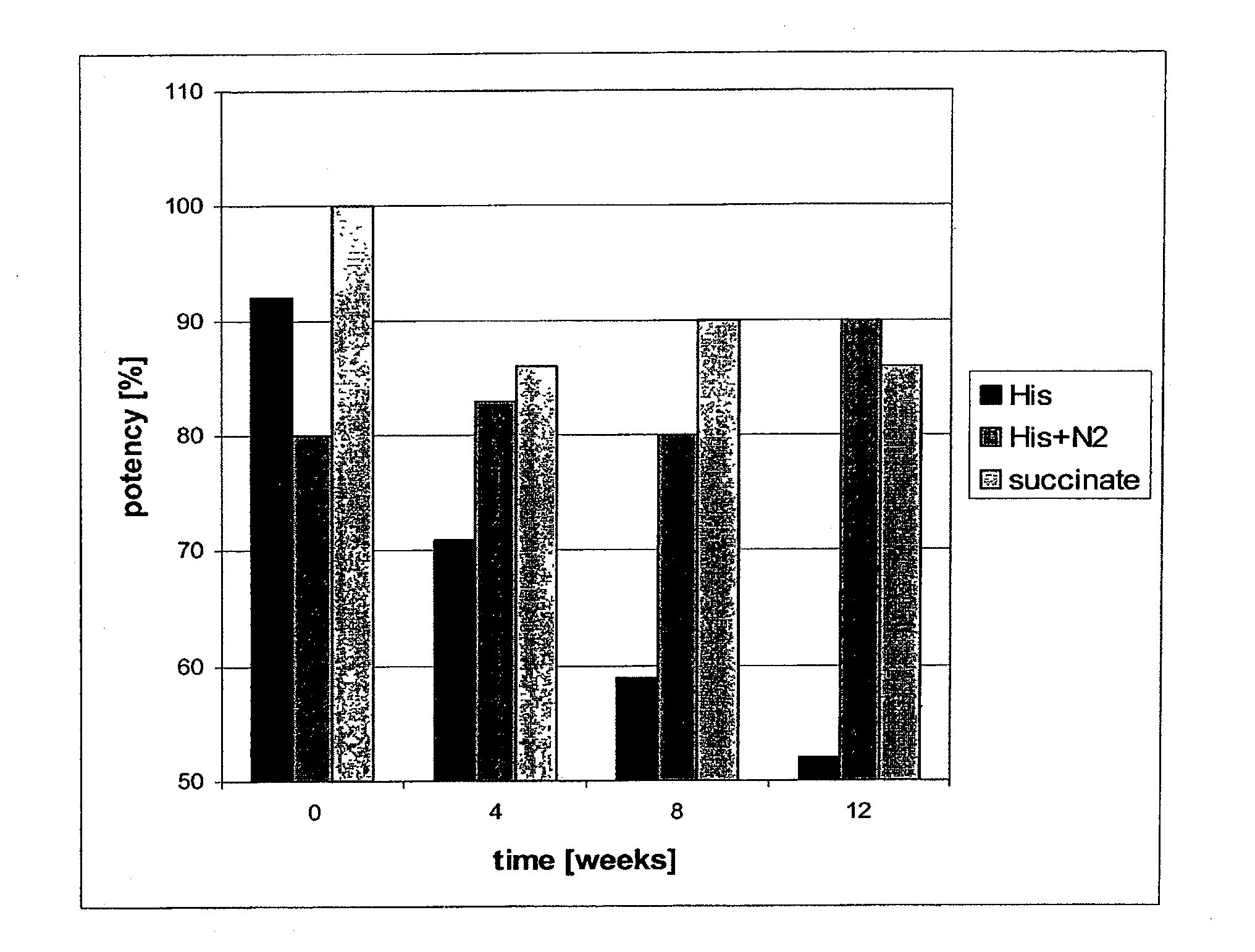
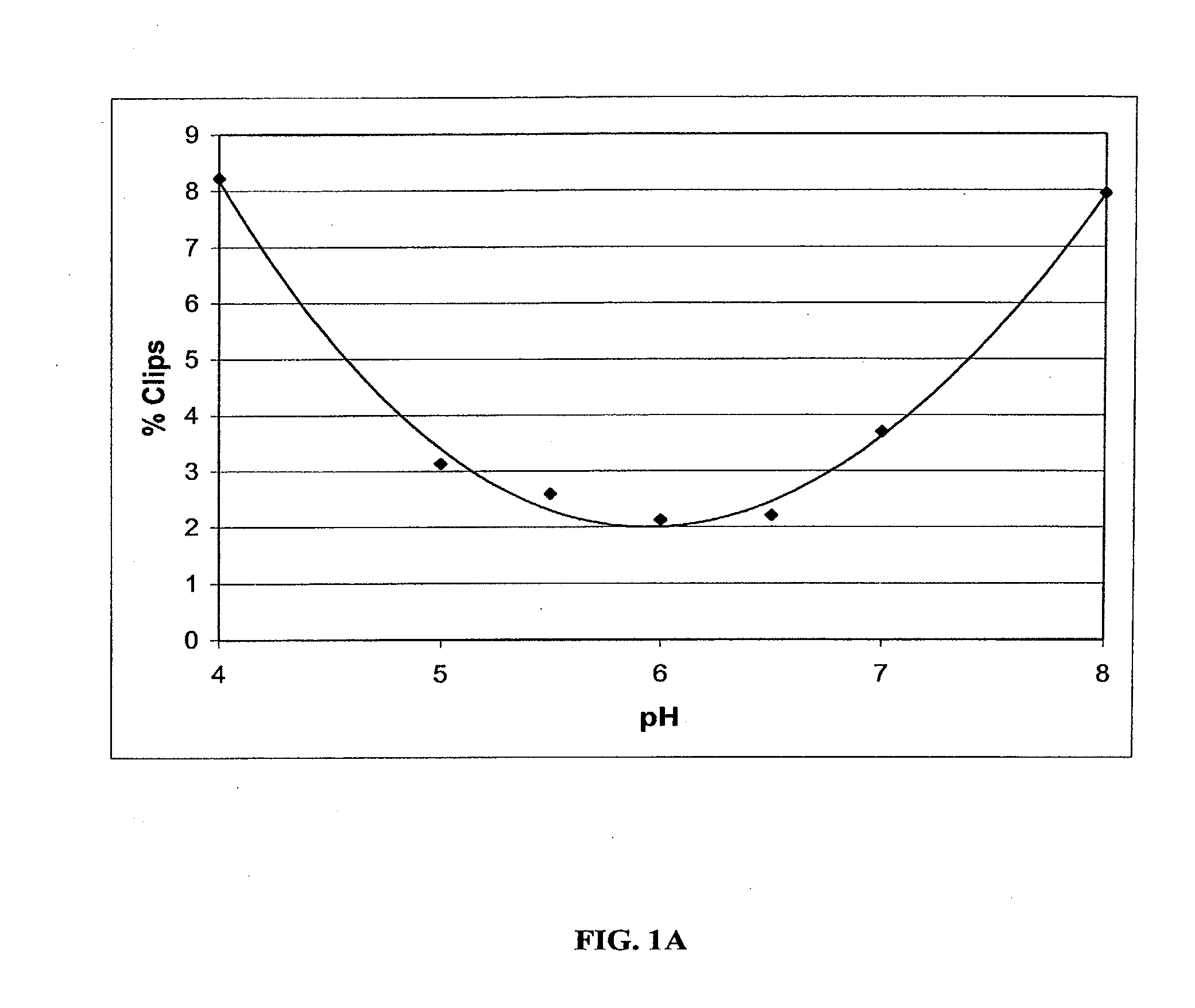
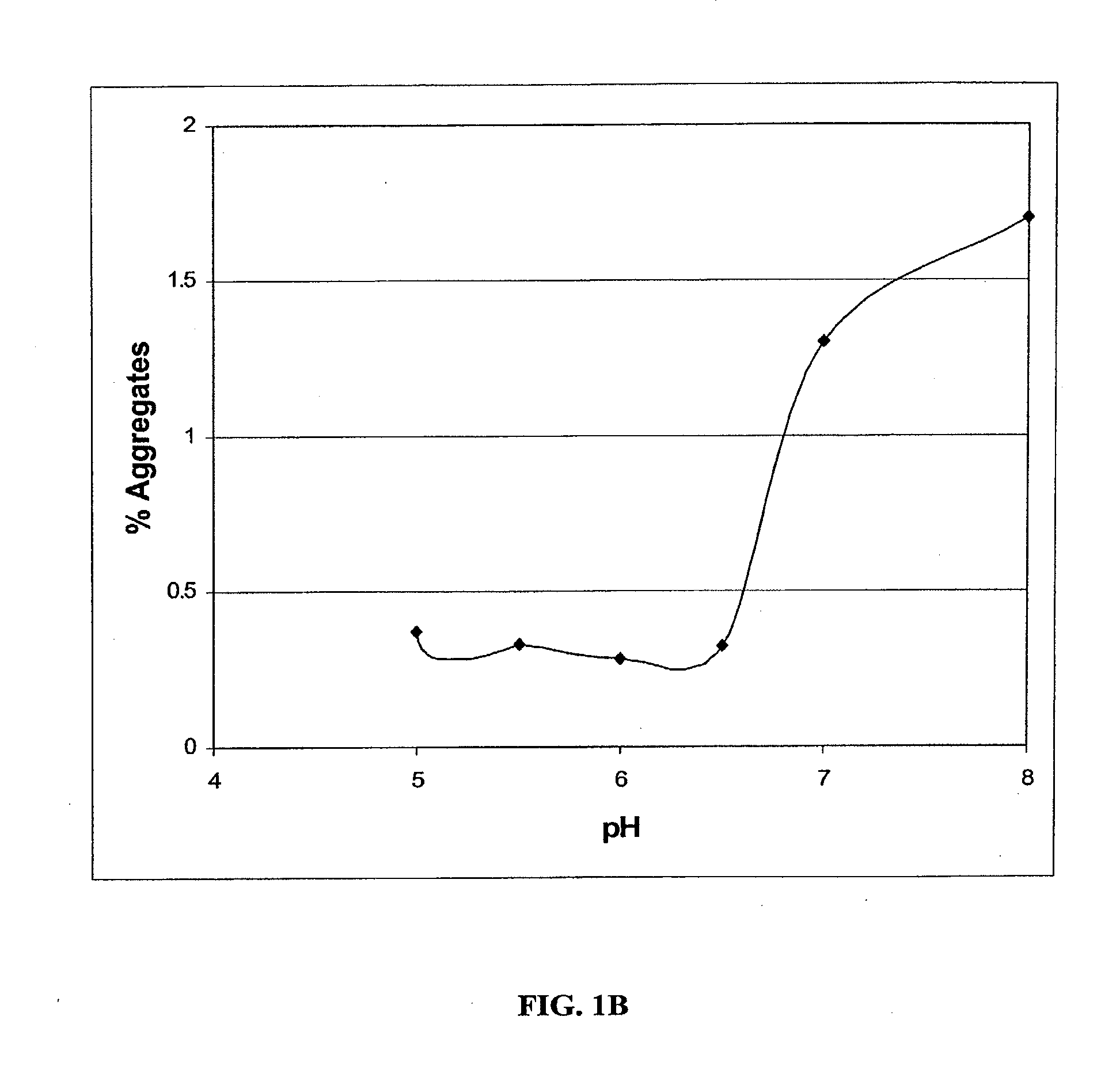
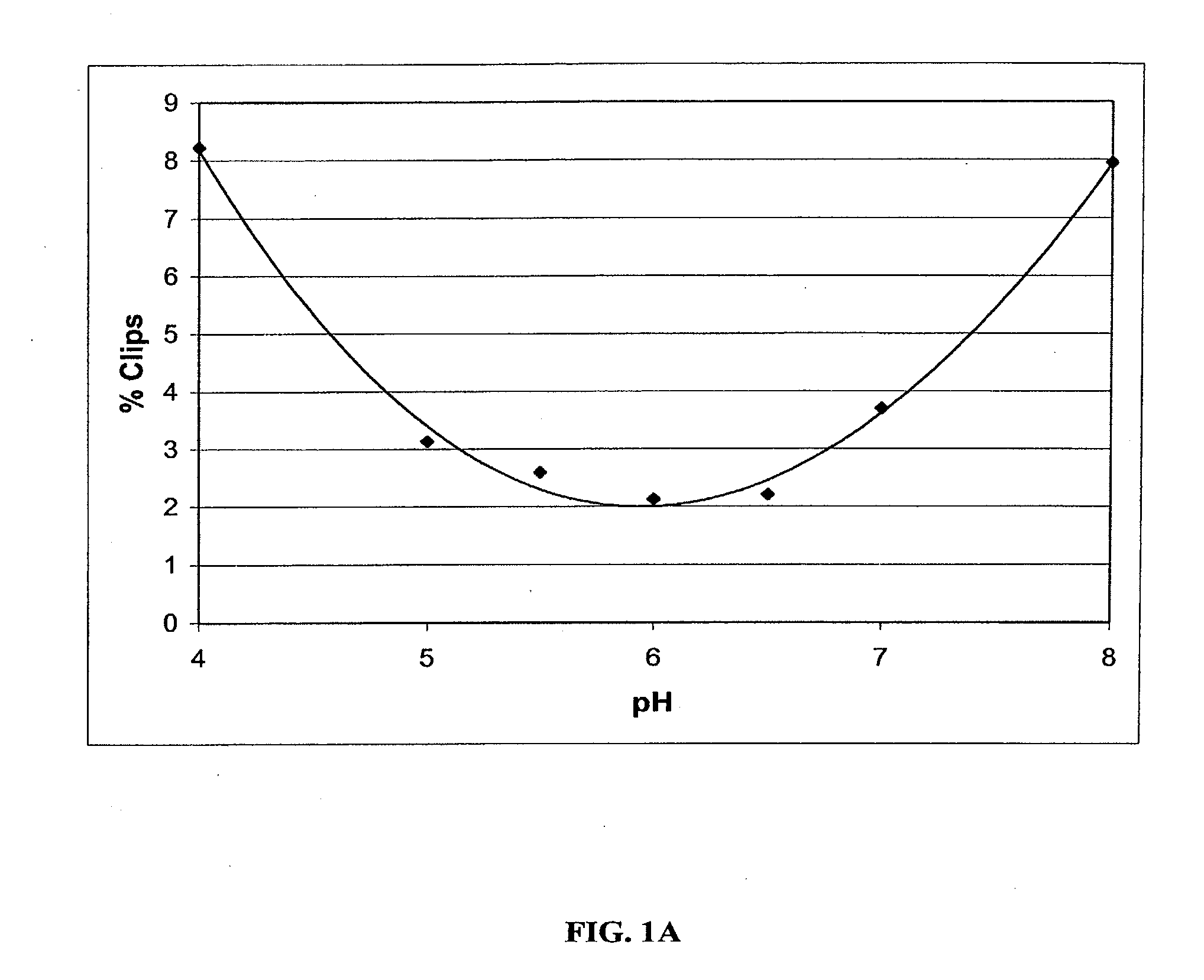
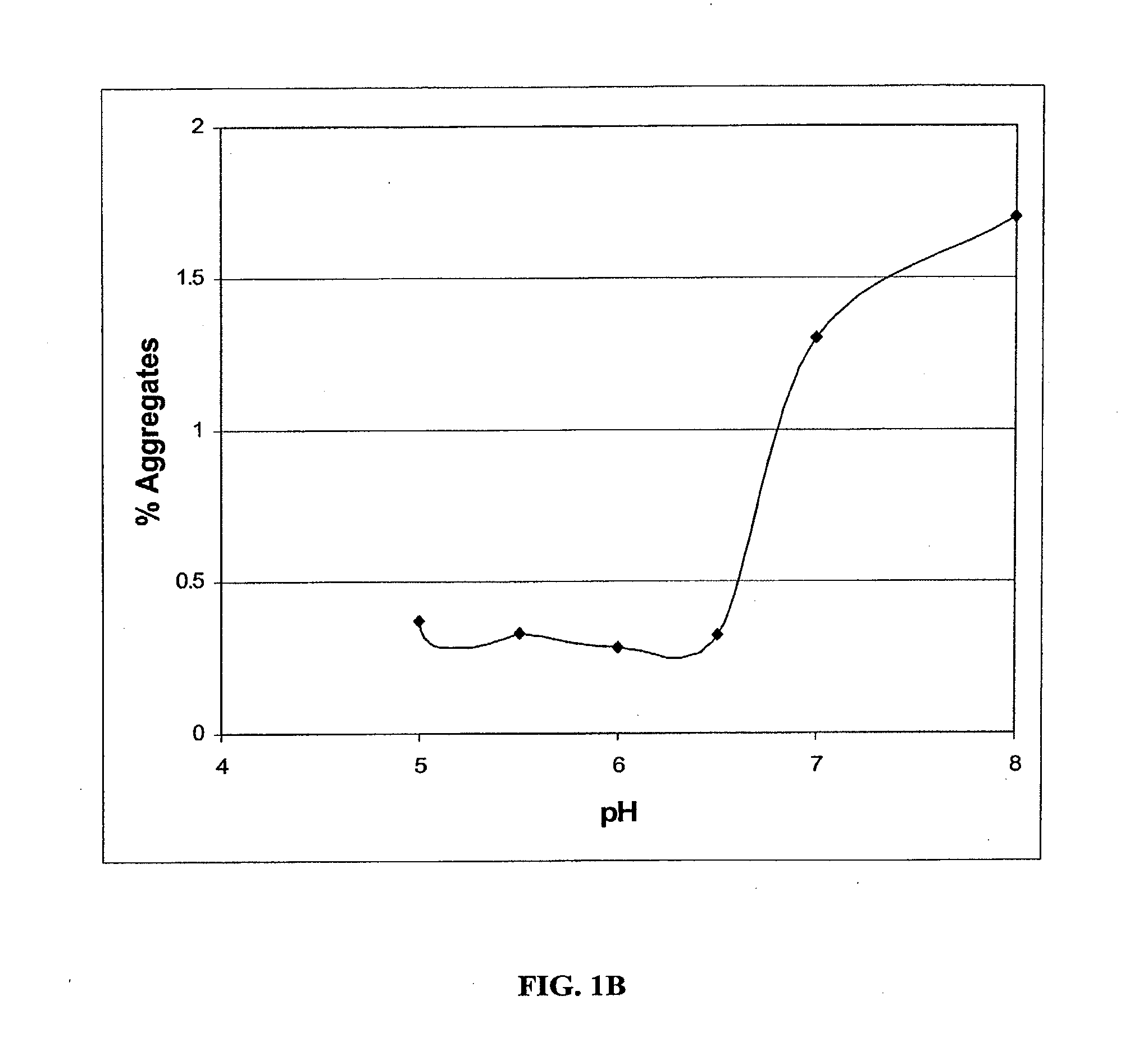
Stable Liquid Pharmaceutical Formulation Of IgG Antibodies
InactiveUS20110318343A1Avoid formingInorganic non-active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsHigh concentrationIL-2 receptor
This invention is directed to a stable liquid pharmaceutical formulation comprising a high concentration, e.g. 50 mg / ml or more, of antibody in about 20-60 mM succinate buffer or 30-70 mM histidine buffer, having pH from about pH 5.5 to about pH 6.5, about 0.01-0.1% polysorbate, and a tonicity modifier that contributes to the isotonicity of the formulation. This liquid formulation is stable at refrigerated temperature (2-8° C.) for at least 1 year, and preferably 2 years. This liquid formulation is suitable for subcutaneous injection. The preferred antibodies include Daclizumab, a humanized anti-IL-2 receptor monoclonal antibody; HAIL-12, a humanized anti-IL-12 monoclonal antibody; HuEP5C7, a humanized anti-L selectin monoclonal antibody; and Flintozumab, a humanized anti-gamma interferon monoclonal antibody.
Owner:ABBVIE BIOTHERAPEUTICS
Expression method of animal alpha interferon and gamma interferon
ActiveCN102628062AConvenient timeIncrease resistanceFermentationGenetic engineeringGamma interferonInterferon alpha
The invention discloses an expression method of an animal alpha interferon and a gamma interferon. The method comprises the following step of: performing combined expression or co-expression on the alpha interferon and gamma interferon of a human being or an animal of the same type in a bioreactor. The alpha interferon gene and gamma interferon gene of a human being or an animal of the same type are subjected to combined expression or co-expression in the same insect rhabdovirus bioreactor, and an expressed recombinant alpha interferon product and recombinant gamma interferon have cooperative and synergistic actions, so that the valence and stability of a recombinant interferon product can be enhanced. The method has the advantages of high expression efficiency, high product valence, high stability and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
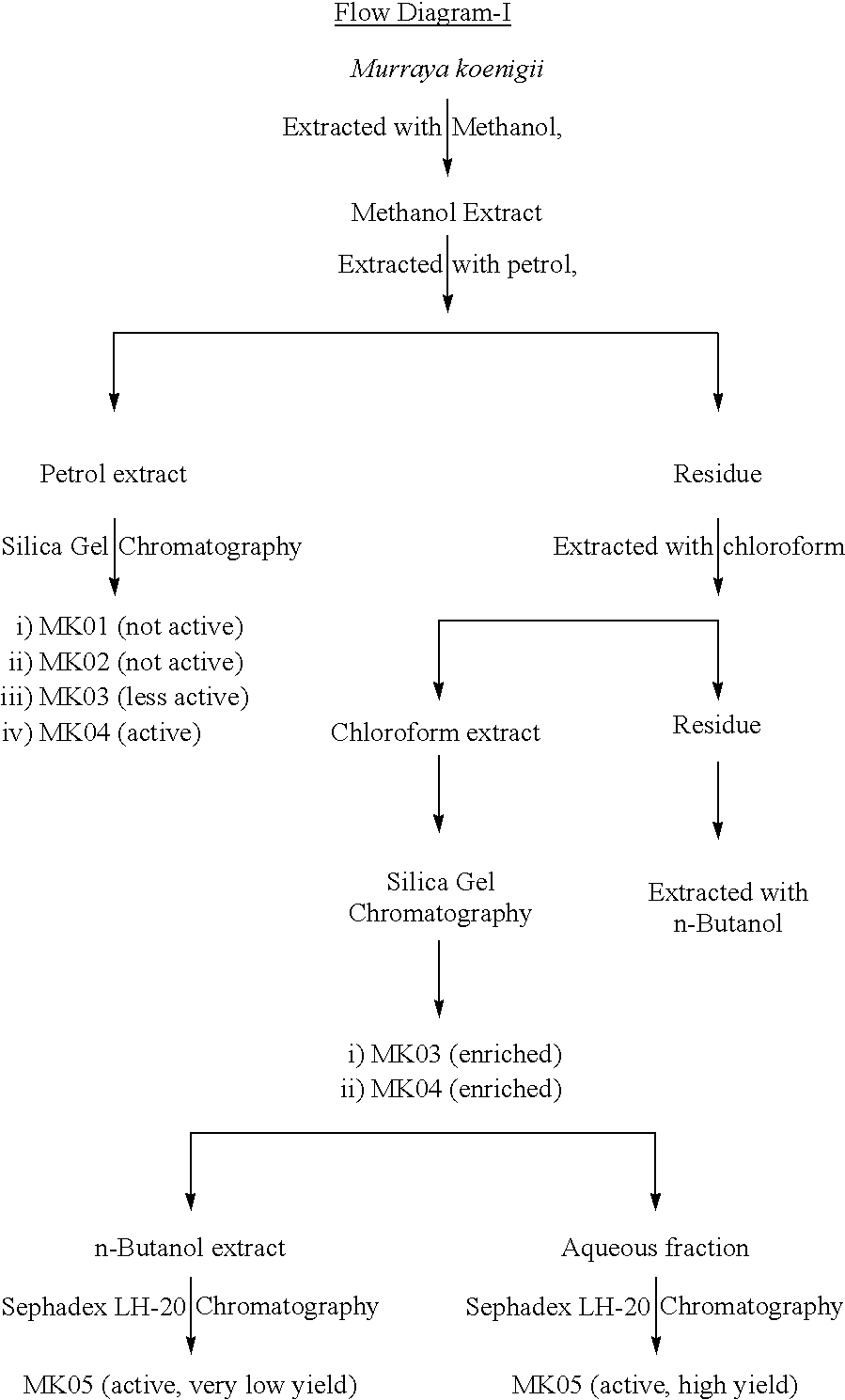
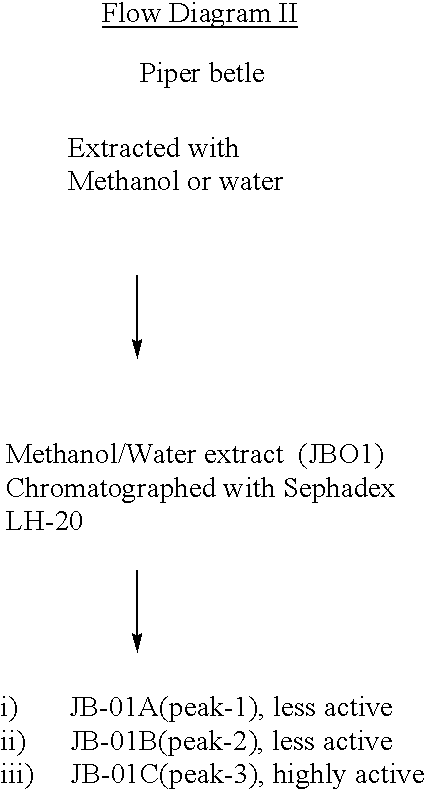
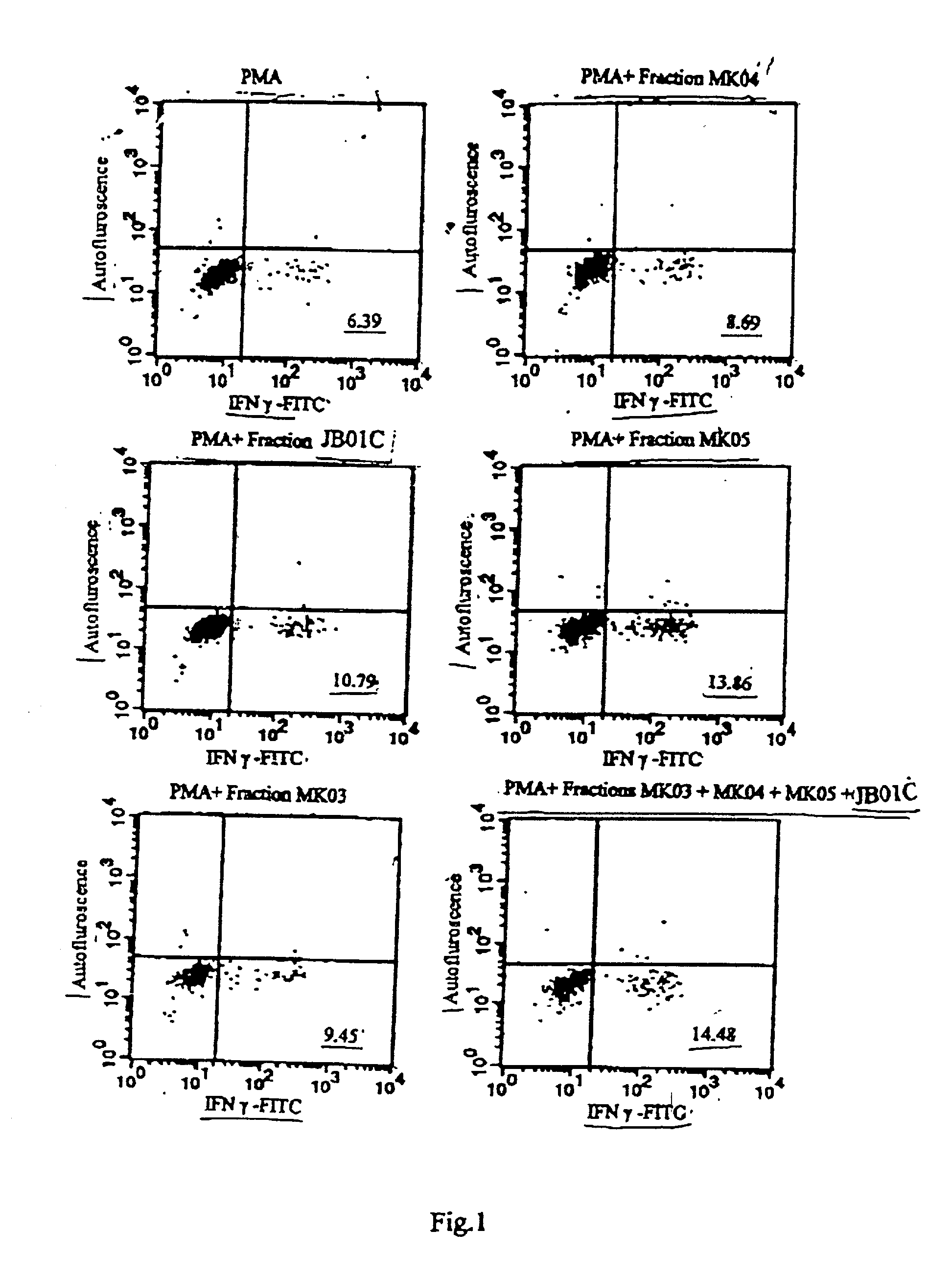
Herbal composition of blend of active components prepared from murrya koenigii and piper betle useful for blocking of 5 lipoxygenase activity leading to the inhibition of leukotriene synthesis, suppression of interleukin-4 production, and enhancement of gamma interferon release
InactiveUS20020086068A1Improve responseReduce IL- responseBiocideUnknown materialsDiseaseWhite blood cell
This invention relates to an herbal composition for the treatment and remedy of bronchial respiratory difficulties, more particularly this invention describes the process of separation, physicochemical characterization and biological response evaluation of active components obtained from extracts of any plant parts including leaves, barks, roots and seeds of plants M. koenigii and P. betle in order to establish their role on the treatment and remedy of bronchial respiratory troubles.
Owner:COUNCIL OF SCI & IND RES
Stable liquid pharmaceutical formulation of igg antibodies
InactiveUS20110070231A1Avoid formingInorganic non-active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsHigh concentrationSubcutaneous injection
This invention is directed to a stable liquid pharmaceutical formulation comprising a high concentration, e.g. 50 mg / ml or more, of antibody in about 20-60 mM succinate buffer or 30-70 mM histidine buffer, having pH from about pH 5.5 to about pH 6.5, about 0.01-0.1% polysorbate, and a tonicity modifier that contributes to the isotonicity of the formulation. This liquid formulation is stable at refrigerated temperature (2-8° C.) for at least 1 year, and preferably 2 years. This liquid formulation is suitable for subcutaneous injection. The preferred antibodies include Daclizumab, a humanized anti-IL-2 receptor monoclonal antibody; HAIL-12, a humanized anti-IL-12 monoclonal antibody; HuEP5C7, a humanized anti-L selectin monoclonal antibody; and Flintozumab, a humanized anti-gamma interferon monoclonal antibody.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Chlamydia trachomatis antigens for vaccine and diagnostic use
InactiveUS20090304722A1High expressionImprove abilitiesAntibacterial agentsOrganic active ingredientsGamma interferonWhole cell lysate
The present invention is related to antigens from Chlamydia trachomatis which are recognized by specific antibodies from individuals infected with Chlamydia or which can induce T cells from the same individuals to secrete gamma-interferon. The T cell reactive antigens are present in a whole-cell lysate and have apparent molecular weights of 5-12, 16-20, 25-35 and 58-74 kDa as determined by SDS-PAGE. The antigens of the invention are useful in vaccines but also as diagnostic compositions.
Owner:STATENS SERUM INST
Novel assay for detecting immune responses involving antigen specific cytokine and/or antigen specific cytokine secreting T-cells
InactiveUS20030143641A1Less laborAddressing slow performanceBiological material analysisBiological testingGamma interferonBiological activation
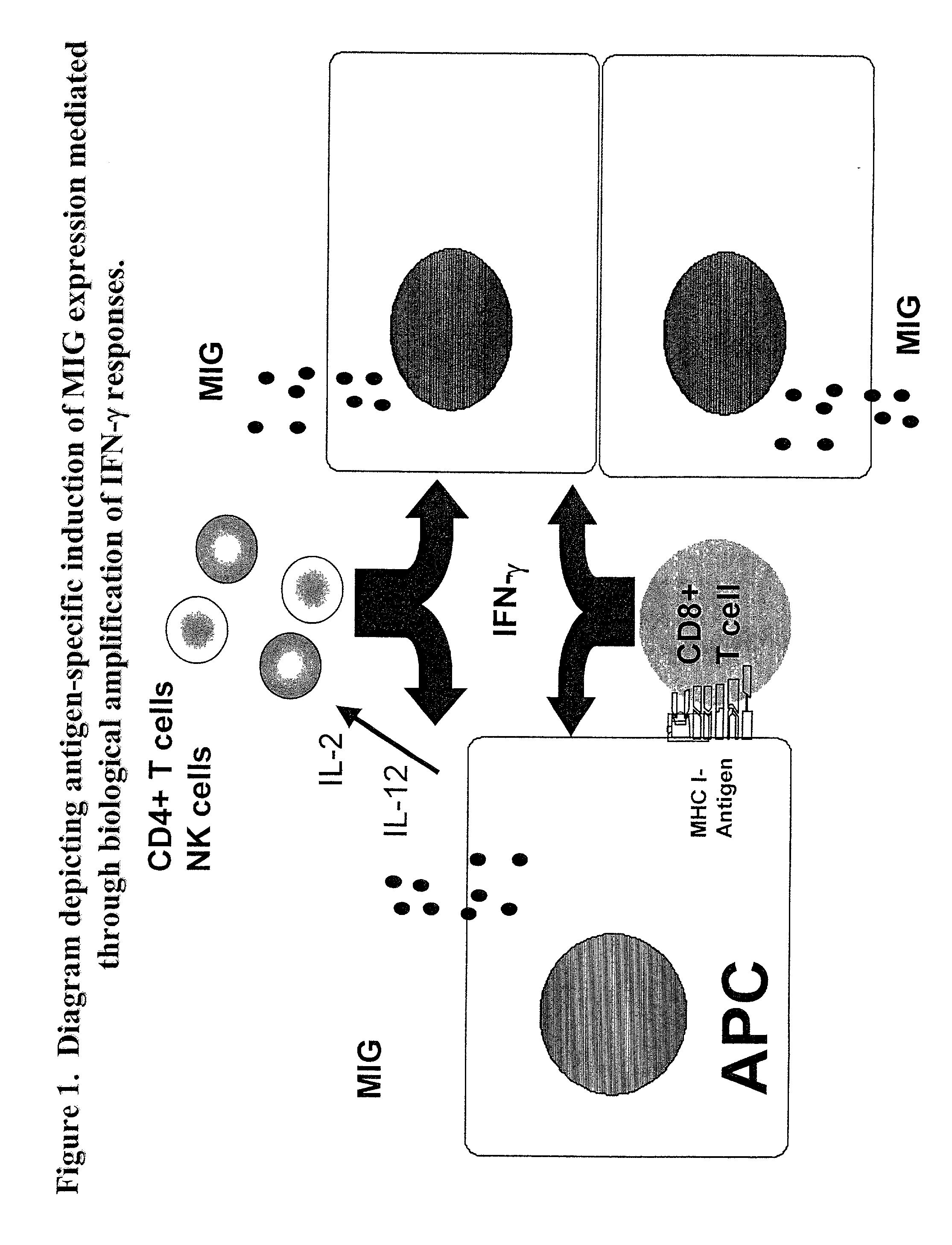
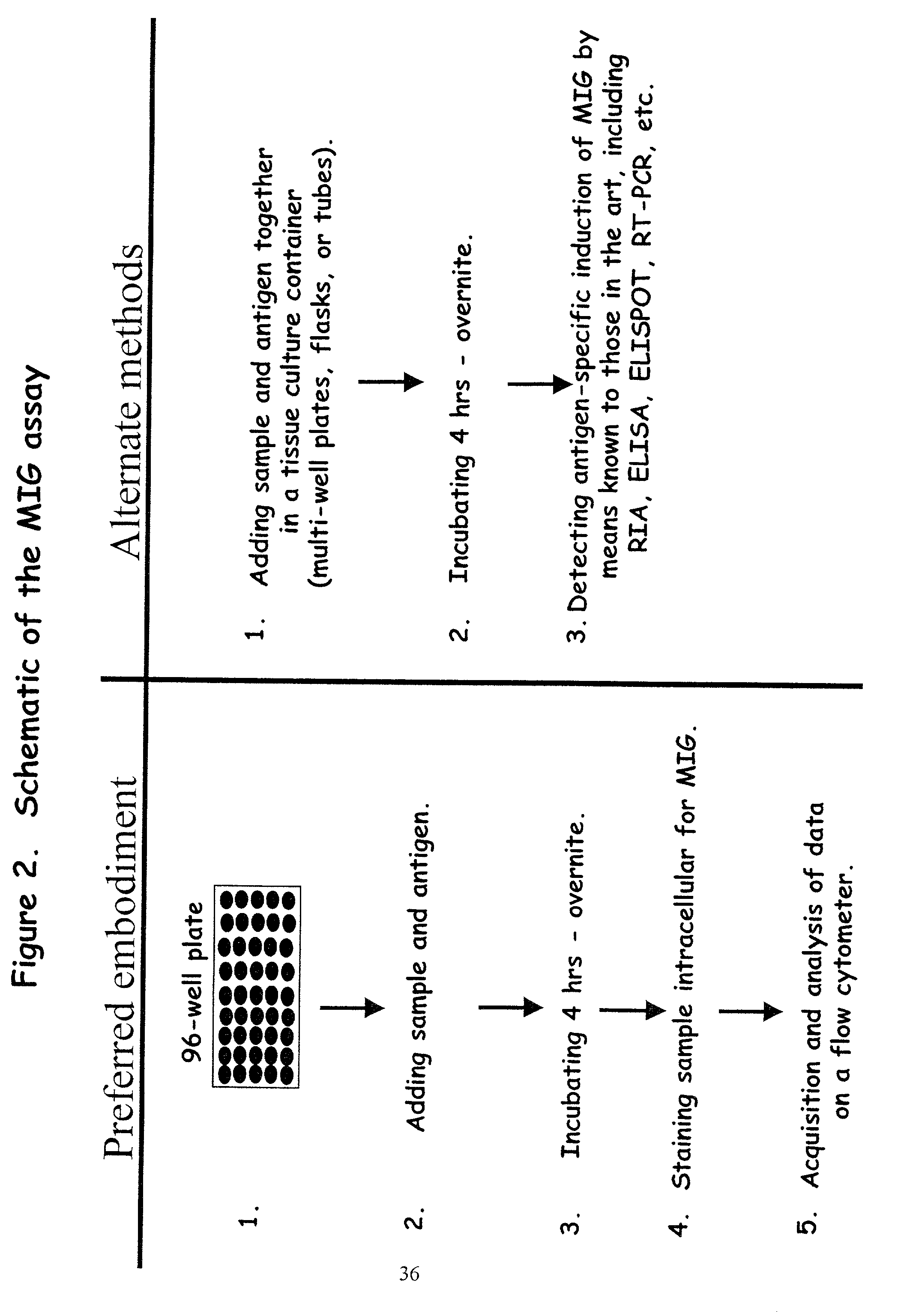
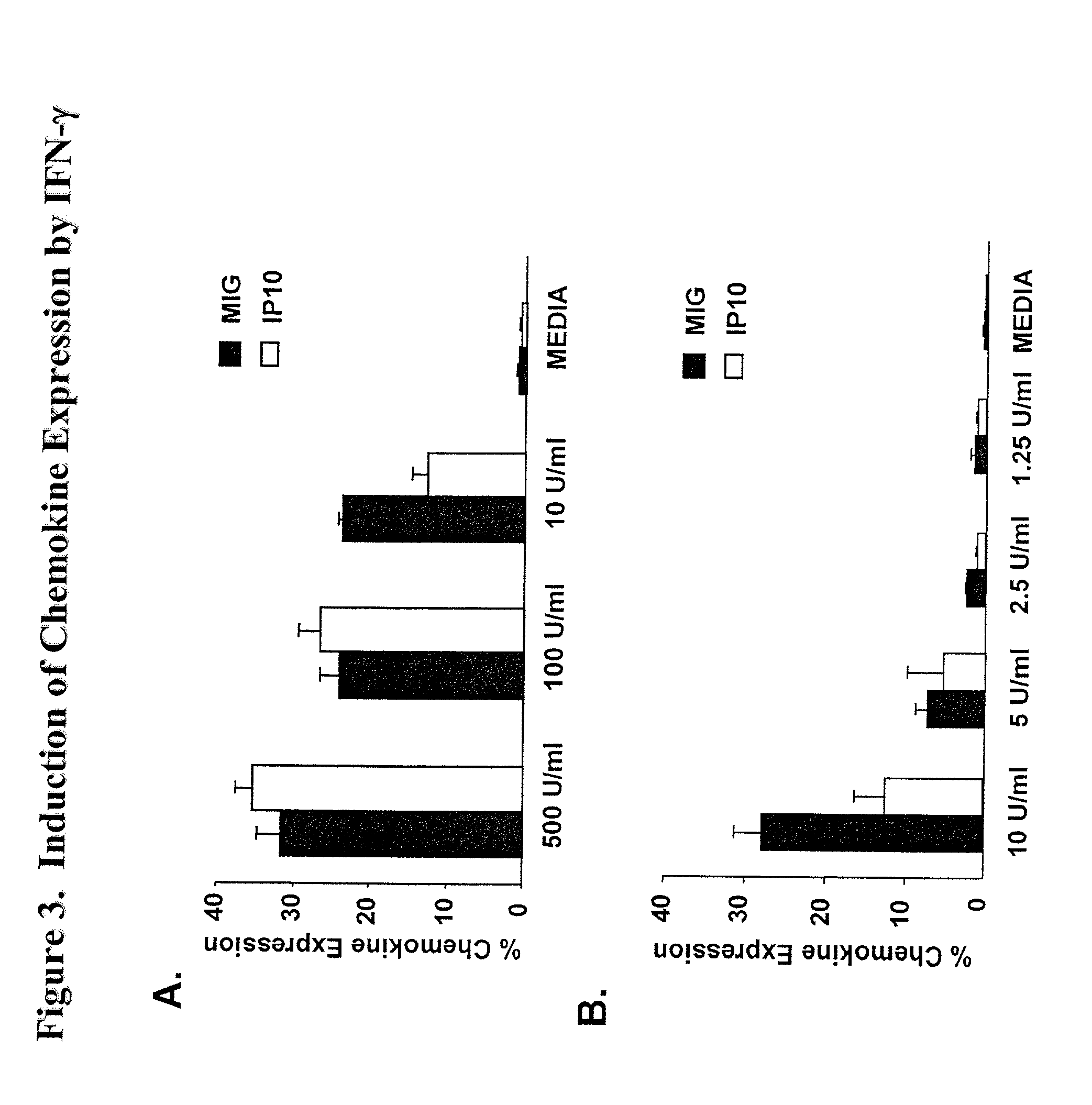
Here, we describe a sensitive and specific assay and kit for the detection of chemokines having activity that is upregulated by Th-1 cytokines (such IFN-gamma) and chemokines that upregulate the activity of Th-1 cytokines (such as IFN-gamma). In a typical embodiment, detection of the chemokine monokine induced by gamma interferon (MIG) provides a measure of the biological effect of IFN-gamma rather than direct quantitation of IFN-gamma or IFN-gamma secreting cells per se. Upregulation of MIG expression was observed following in vitro activation of PBMC with defined CD8+ T cell epitopes derived from influenza virus, CMV, or EBV, and in all cases this was antigen-specific, genetically restricted and dependent on both CD8+ T cells and IFN-gamma. Responses as assessed by the MIG assay paralleled those detected by conventional IFN-gamma ELISPOT, but the magnitude of response and sensitivity of the MIG assay were superior. Our data validate this novel method for the detection of high as well as low levels of antigen-specific and genetically restricted IFN-gamma activity or MIG.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY NAVAL RES LAB WASHINGTON
Method of treating psoriasis using anti-gamma interferon antibody
InactiveUS20030056233A1Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsAntigenInterferon alpha
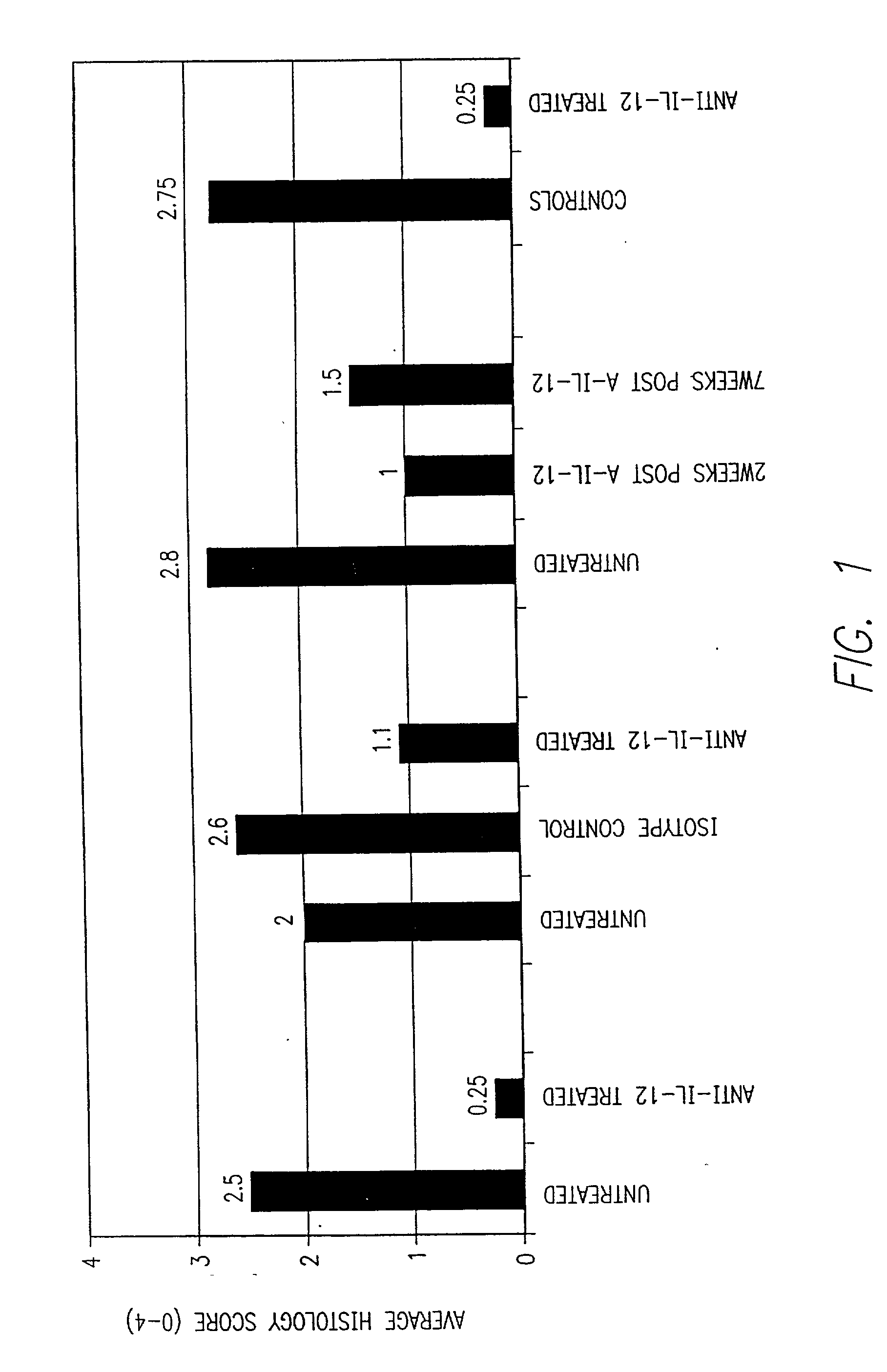
Methods and compositions are provided for the creation and screening of non-human animal models having many of the histologic characteristics of human psoriasis. Immunocompromised host animals are injected with a purified population of CD45Rb positive cells, which are tolerant of the host major histocompatibility antigens, but are mismatched at one or more minor antigens. The injected cells are stimulated with a pro-inflammatory cytokine, e.g. IL-12, and a polyclonal activating agent. The injected animals develop a chronic skin disorder that includes histological features observed in human psoriasis, e.g. rete pegs, severe acanthosis and infiltration of Th1 cells into the dermis.
Owner:FACET BIOTECH CORP
Oral ulcer ointment prepared by umbilical cord mesenchymal stem cell to secrete cytokine and preparation method thereof
InactiveCN109010523AEffective treatmentReduce releaseAerosol deliveryDigestive systemFreeze-dryingOral ulcers
The invention discloses an oral ulcer ointment prepared by umbilical cord mesenchymal stem cell to secrete cytokine and a preparation method thereof. The ointment can significantly improve the recovery rate of patients with oral ulcers and effectively prevent the recurrence rate. The method comprises the following specific steps: stimulating umbilical cord mesenchymal stem cells with calcium chloride and gamma-interferon to secrete more immunoregulatory cytokines, collecting a culture supernatant to prepare freeze-drying powder, mixing honeysuckle flower, isatis root, dandelion, folium isatidis, polyethylene glycol, maltodextrin and xanthan gum, and stirring a mixture under low temperature conditions to form an oral ulcer ointment. The oral ulcer ointment of the present invention is applied to the local part of patients with the oral ulcer, the treatment time and the recurrence rate are statistically determined, and the result indicates that the oral ulcer ointment can effectively promote the healing time of patients with the oral ulcer and effectively prevents recurrence.
Owner:北京壹典壹生生物技术有限公司
Modified elapid venoms as stimulators of the immune reaction
ActiveUS20080107752A1Avoid seizuresSuppress continued developmentBiocidePeptide/protein ingredientsCobra venomIMMUNE STIMULANTS
Detoxified cobra venom and its constituent neurotoxins have been reported to have potent antiviral activity. Others have reported that snake venoms were generally immune stimulants. Recent research has revealed more specific details on the effects of detoxified venoms and isolated alpha-neurotoxins on cells of the immune system. Exposure of the immune cells to these detoxified proteins yields a strong response in the innate immune reaction that represents the immune systems initial response to infectious agents. Of particular relevance is the marked increase in the expression of genes associated with the production of gamma interferon, a potent antiviral agent and regulator of the immune response. The ability to induce this strong innate response has significant application to those with weakened immune systems where their ability to fight infection has been compromised. It also has the potential application to act as a method to protect individuals from contagious infectious agents as a substitute for anti-viral vaccines.
Owner:RECEPTOPHARM
Detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and method thereof
ActiveCN101533018AStrong characteristicIncreased sensitivityHybrid peptidesMaterial analysisBCG immunizationMycobacterium Infections
The invention belongs to the field of immunodetection and relates to a detection kit for distinguishing cow pathogenic mycobacteria infection from non-pathogenic mycobacteria infection and a method thereof. The detection reagent comprises combined fusion protein rE6-M63-H70 used as a specific stimulation origin, the combined fusion protein can effectively stimulate sensitized peripheral blood lymphocyte cultured in vitro to release Gamma-interferon (IFN-Gamma) at a high level. The cow IFN-Gamma release test established by using the detection reagent rE6-M63-H70 combined fusion protein as the stimulation origin overcomes the insufficiencies of serology detection method and the IFN-Gamma release test with PPD as the stimulation origin, thus enjoying very high sensitivity and specificity and distinguishing cow pathogenic mycobacteria ( such as mycobacterium bovis) infection from non-pathogenic mycobacteria (such as mycobacterium avium or non-pathogenic mycobacteria) infection and even distinguishing the cow pathogenic mycobacteria infection from BGG immunity; therefore, the detection kit and the method of the invention can be effectively used to detect the clinical cow pathogenic mycobacteria infection.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Gamma-interferon sandwich ELISA detection method based on recombinant fusion antigen protein
ActiveCN103333251AImprove stabilityImprove accuracyMicroorganism based processesBiological testingMedical testingTuberculosis diagnostics
The present invention relates to a gamma-interferon sandwich ELISA detection method based on recombinant fusion antigen protein, further relates to a recombinant fusion antigen protein preparation method and a gamma-interferon monoclonal antibody preparation method, and belongs to the technical field of medical examination determination. According to the present invention, the recombinant fusion antigen protein is formed by linking secretory antigen protein MPB70, antigen protein CFP10 and antigen protein ESAT6 through peptide bonds, the gamma-interferon monoclonal antibody is prepared through immunization of animals, cell fusion, hybridoma cell screening, cloning and purification, and the recombinant fusion antigen protein and the gamma-interferon monoclonal antibody are adopted to establish a sandwich ELISA detection method; and the recombinant fusion antigen protein can rapidly stimulate a bovine blood sample to secret gamma-interferon, and the gamma-interferon monoclonal antibody can quickly and specifically identify bovine gamma-interferon, such that strong sensitivity and strong specificity are provided, and broad application prospects are provided in the field of buffalo tuberculosis diagnosis.
Owner:常州同泰生物药业科技股份有限公司
The pIFN-gamma gene adjuvant for pig vaccine and its prepn process
ActiveCN1772297AActivity is not affectedEasy to makeProtozoa antigen ingredientsGenetic material ingredientsAdjuvantGamma interferon
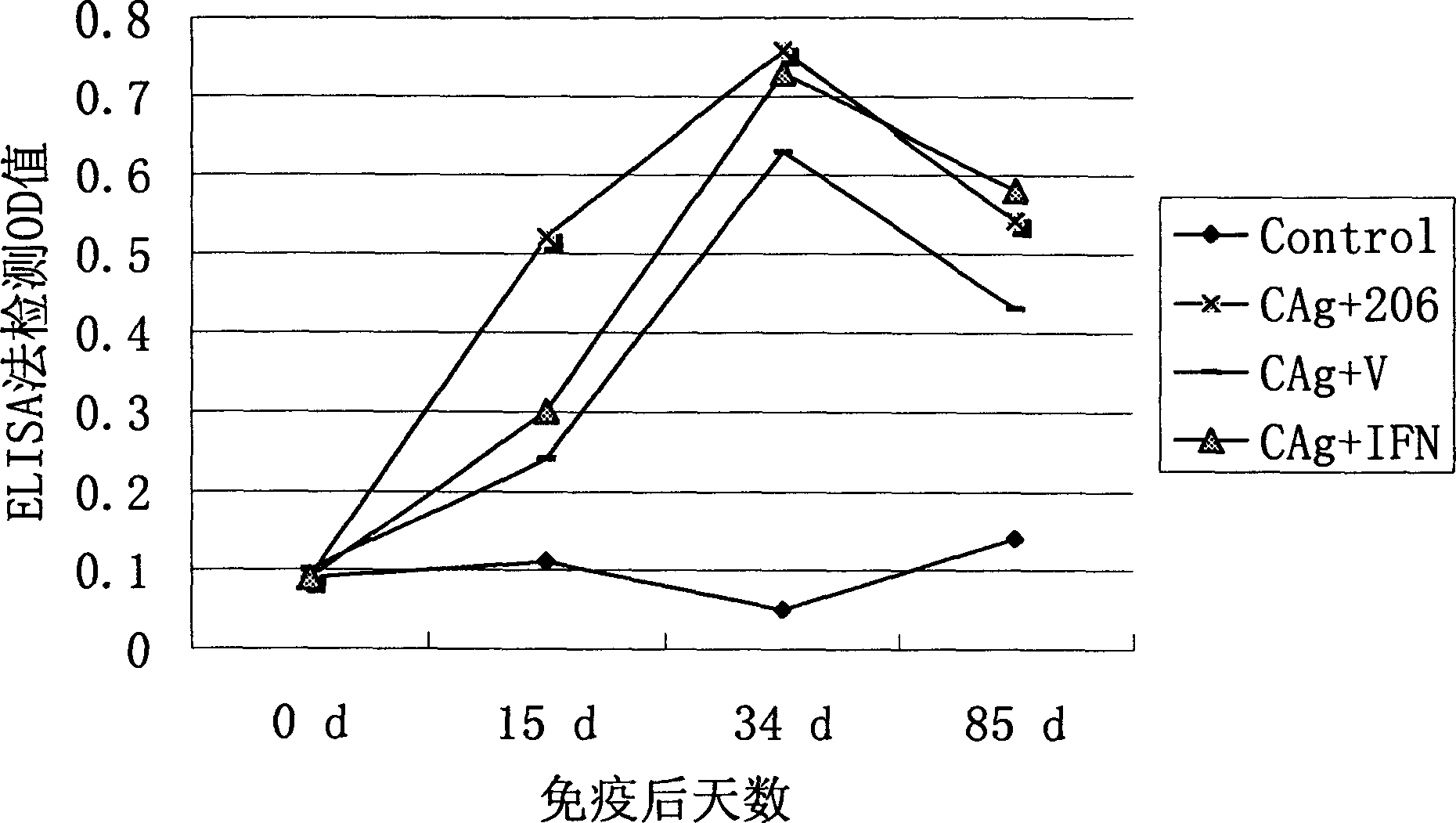
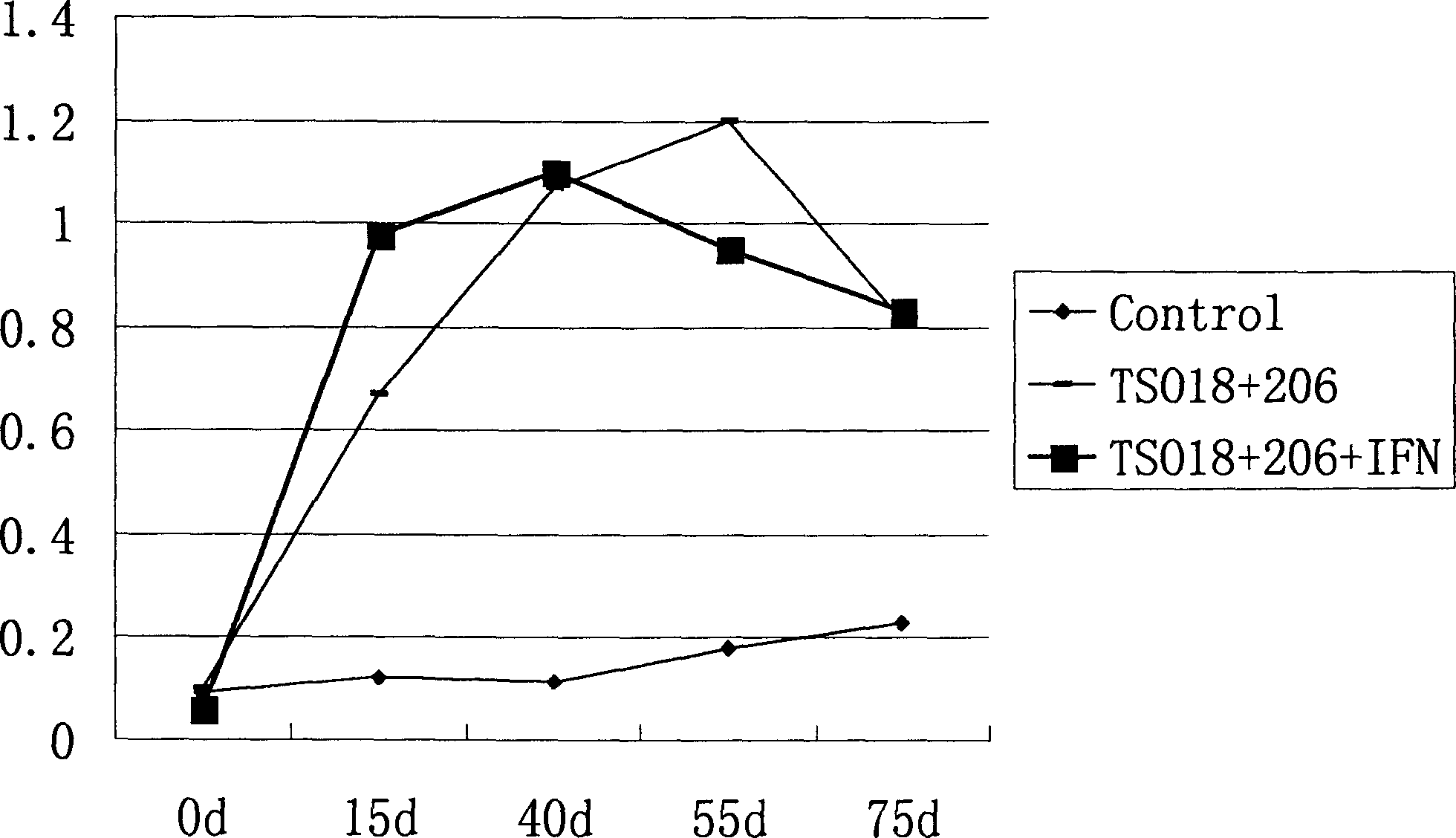
The present invention discloses one kind of adjuvant for pig vaccine and its preparation process and constituted pig vaccine. The gene adjuvant for pig vaccine is adjuvant including pig gamma-interferon gene (pIFN-gamma), or recombinant plasmid pcDNA-pIFN-gamma of animal cell expression plasmid pcDNA3.1 and pig gamma-interferon gene (pIFN-gamma), or the combination of pcDNA-pIFN-gamma and No. 206 adjuvant. The pig vaccine is composition of pigí»s cysticercus-resisting vaccine composition; pigí»s deactivated foot-and-mouth disease virus vaccine and the gene adjuvant. The gene adjuvant of the present invention is prepared through gene cloning, recombination, recombinant plasmid proliferation, extraction and purification and other processes.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
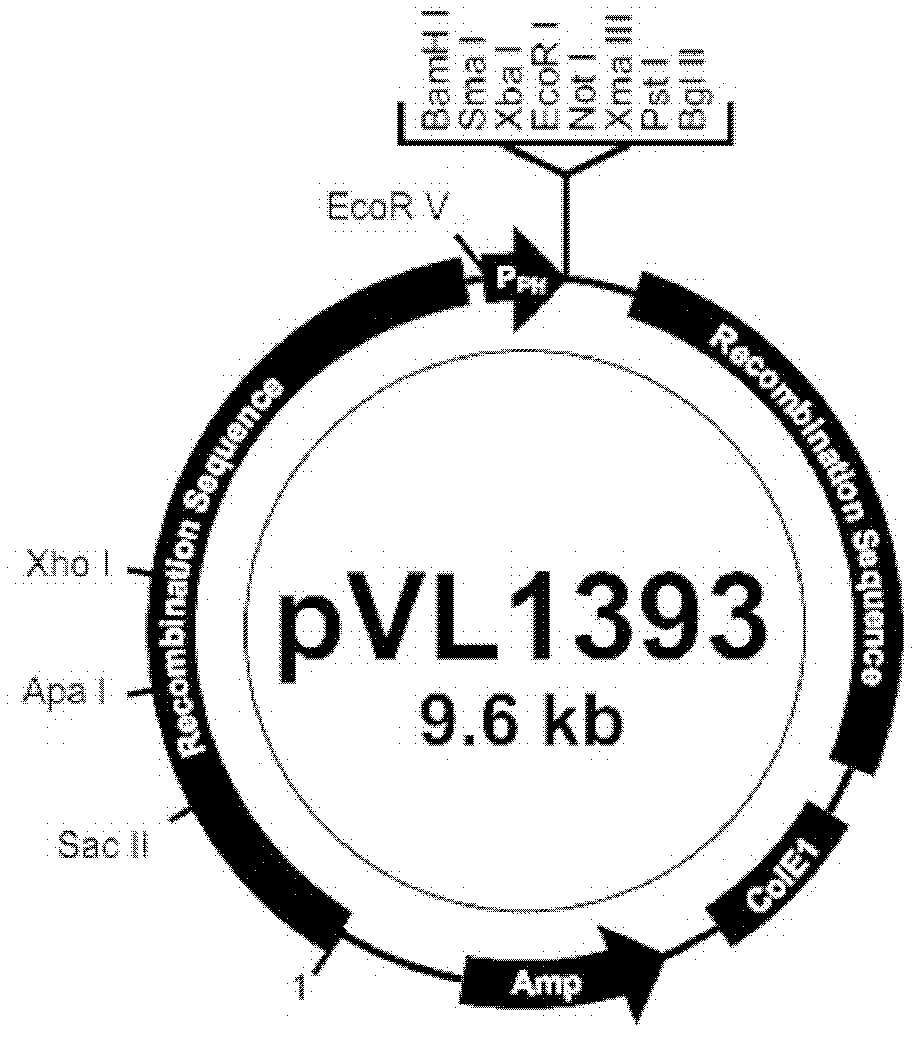
Efficiently expressed series porcine alpha and gamma interferon genes and application of expressed protein thereof
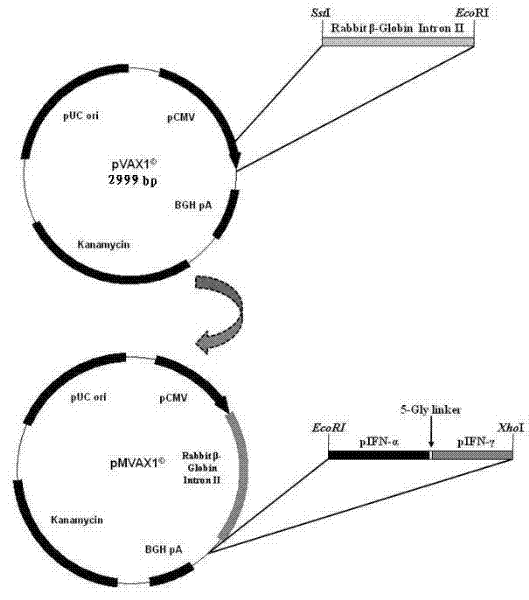
ActiveCN102212539AHigh expression activityPrevent proliferationPeptide/protein ingredientsAntiviralsPig farmsSynthesis methods
The invention relates to the field of gene expression, in particular to efficiently expressed series porcine alpha and gamma interferon genes. The nucleotide sequence of the interferon genes is shown as a sequence 2. The genes of the sequence 2 are cloned to eukaryotic expression vectors to obtain recombinant plasmids. A synthesis method of the series porcine alpha and gamma interferon genes comprises the following steps of: inserting Rabbit beta-GlobinIntronII, a pCMV immediate early promoter and a T7 promoter into pVAX1 to obtain pMVAX1, and inserting fusion protein genes pIFN-alpha / gamma into pMVAX1 to obtain pMVAX1-pIFN-alpha / gamma. The invention also relates to application of expression protein of the interferon genes in preparation of a medicament for treating porcine reproductive and respiratory syndrome. Through the interferon genes, the limitations of low expression quantity and poor activity when pIFN-alpha and pIFN-gamma are expressed through the pVAX1 are broken, the activity reaches 1*10<8.0>U / 0.1mL, the multiplication of high pathogenic porcine reproductive and respiratory syndrome viruses (PRRSV) can be obviously inhibited, and the economic loss of a pig farm is reduced.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Reagent for mycobacterium tuberculosis infection detection, clinical treatment effect tracking and antituberculous vaccine development and application thereof
InactiveCN104004069AGood tracerGood diagnostic value for tuberculosisAntibacterial agentsBacterial antigen ingredientsAntigenMycobacterium Infections
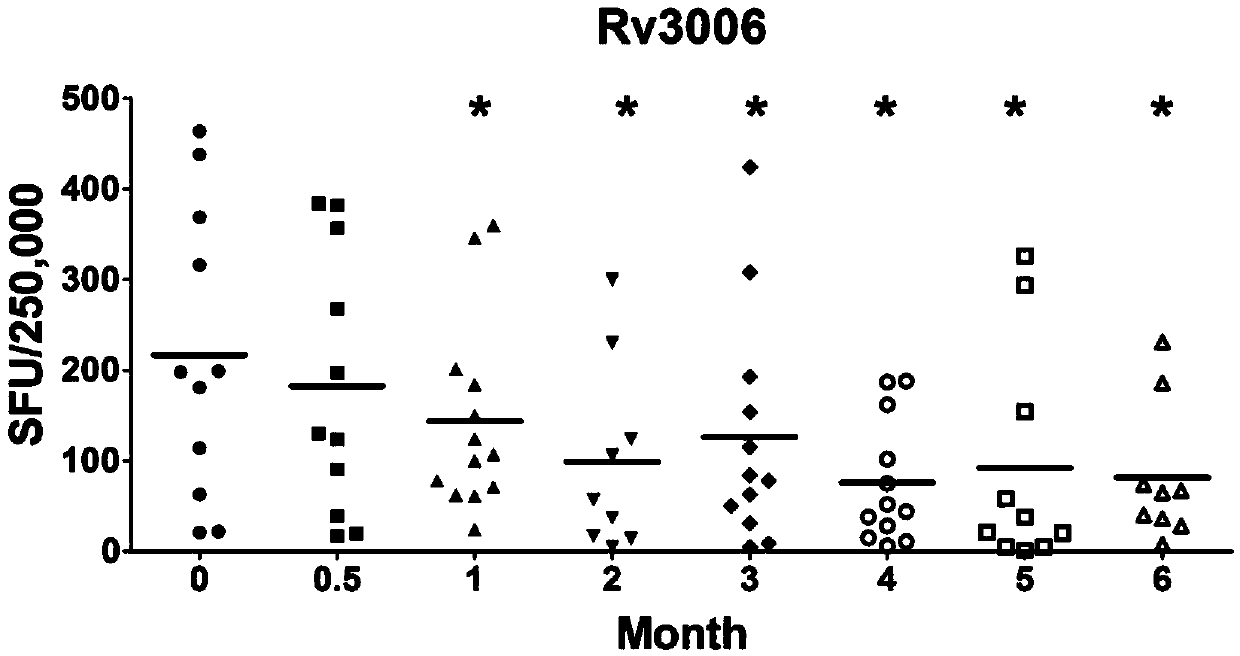
The invention discloses a reagent for mycobacterium tuberculosis infection detection, clinical treatment effect tracking and antituberculous vaccine development. The novel mycobacterium tuberculosis derived reagent Rv3006 (LPPZ) prepared by a gamma-interferon gamma release assay and enzyme linked immunosorbent assay comprises antigen or polypeptide and analogues thereof, wherein terminal C to terminal N in the amino acid sequence of the Rv3006 antigen is as shown in SEQ ID NO:1. By utilizing the reagent, tuberculous patients in the active phase and tuberculous latent infectors can be well detected; the specific immune response of Rv3006 is reduced along with treatment development and disease condition remission, which indicates that the reagent can be used for well tracking the clinical treatment situation of a tuberculous patient, thus having a good clinical application prospect. Furthermore, the reagent has a protective effect on a mouse infected by a mycobacterium tuberculosis standard toxic strain H37Rv, thus having a good development prospect of antituberculous vaccines.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Application of rainbow trout IFN-gamma2 (Interferon-gamma2) in preparing anti-IHN (Infectious Haematopoietic Necrosis) virus product
ActiveCN104043111AHas immunomodulatory activityInhibits plaque formationBacteriaPeptide/protein ingredientsAdjuvantGamma interferon
The invention discloses an application of rainbow trout IFN-gamma2 (Interferon-gamma2) in preparing an anti-IHNV (Infectious Haematopoietic Necrosis Virus) product. The invention provides an application of rtIFN-gamma2 protein, an encoding gene or a recombinant carrier with the encoding gene in preparing a product for inhibiting infectious hemopoietic organ necrosis viruses. The amino acid sequence of the rtIFN-gamma2 protein is a sequence 2 as shown in a sequence table. Experiment shows that IFN-gamma2 has the anti-IHNV activity, the situation that no effective anti-IHNV preparation is available in China at present is changed, meanwhile the interferon-gamma also has the immunity adjustment activity, and the rtIFN-gamma2 can be further developed into a natural immunity enhancing agent or an adjuvant for rainbow trout.
Owner:HEILONGJIANG RIVER FISHERY RES INST CHINESE ACADEMY OF FISHERIES SCI
Kit for detecting mycobacterium tuberculosis infection by using peripheral blood and application of kit
The invention discloses a kit for detecting mycobacterium tuberculosis infection by using peripheral blood. The kit provided by the invention comprises the following substances: (1) a mycobacterium tuberculosis Rv3615c mixed polypeptide library which consists of all or a part of 19 polypeptides consisting of amino acid sequences shown as sequences 1 to 19 in a sequence table, (2) an anti-CD3 antibody labeled with a fluorescent dye A, (3) an anti-gamma-interferon antibody labeled with a fluorescent dye B, and (4) a Golgi apparatus blocking agent, wherein the fluorescent dye A and the fluorescent dye B emit different fluorescent colors. A novel method which can be used for detecting mycobacterium tuberculosis infection is provided. As proved by experiments, antigenic stimulation can be performed by directly using the peripheral blood without separating single karyocytes of the peripheral blood. As indicated by experimental data, the kit has high sensitivity and high specificity when being used for detecting mycobacterium tuberculosis infection. Moreover, the kit is easy and convenient to operate and low in cost, and has high clinical application value.
Owner:北京同生时代生物技术有限公司
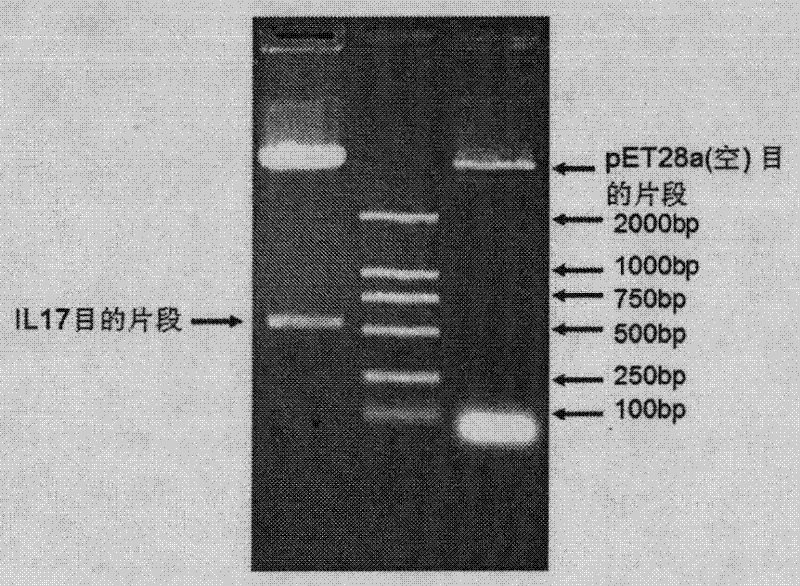
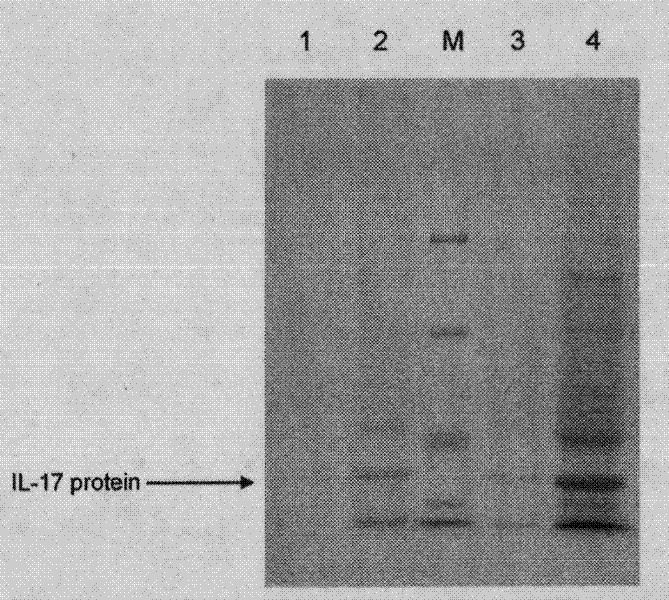
Medicament for treating and/or preventing viral infection
The invention provides a medicament for treating and / or preventing virus. The medicament for treating and / or preventing the virus comprises interleukin 17A and has the medicament effect generated by taking the interleukin 17A as an adjuvant together with a vaccine for enhancing the immunization effect of the vaccine, and the sequences of amino acid residues are as shown in SEQ (sequence) ID (identity) No. 2 and SEQ ID No. 3. The action mechanism of the medicament for treating and / or preventing the virus is to enable the medicament to excrete high-level gamma-interferon, Preforin and other effective immunization components by activating and improving cell immune reaction of an organism, especially by inducing high-level CD8 positive CTL (cytotoxic T lymphocyte) cell activity so as to achieve the purpose of treating and / or preventing the virus. Compared with the prior art, the medicament for treating and / or preventing the virus is not only convenient to use, low in cost and safe, but also easy to popularize.
Owner:BEIJING ADVACCINE BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com