C-class oligonucleotide analogs with enhanced immunostimulatory potency
An immunostimulatory, nucleotide technology, used in medical preparations containing active ingredients, microorganisms, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
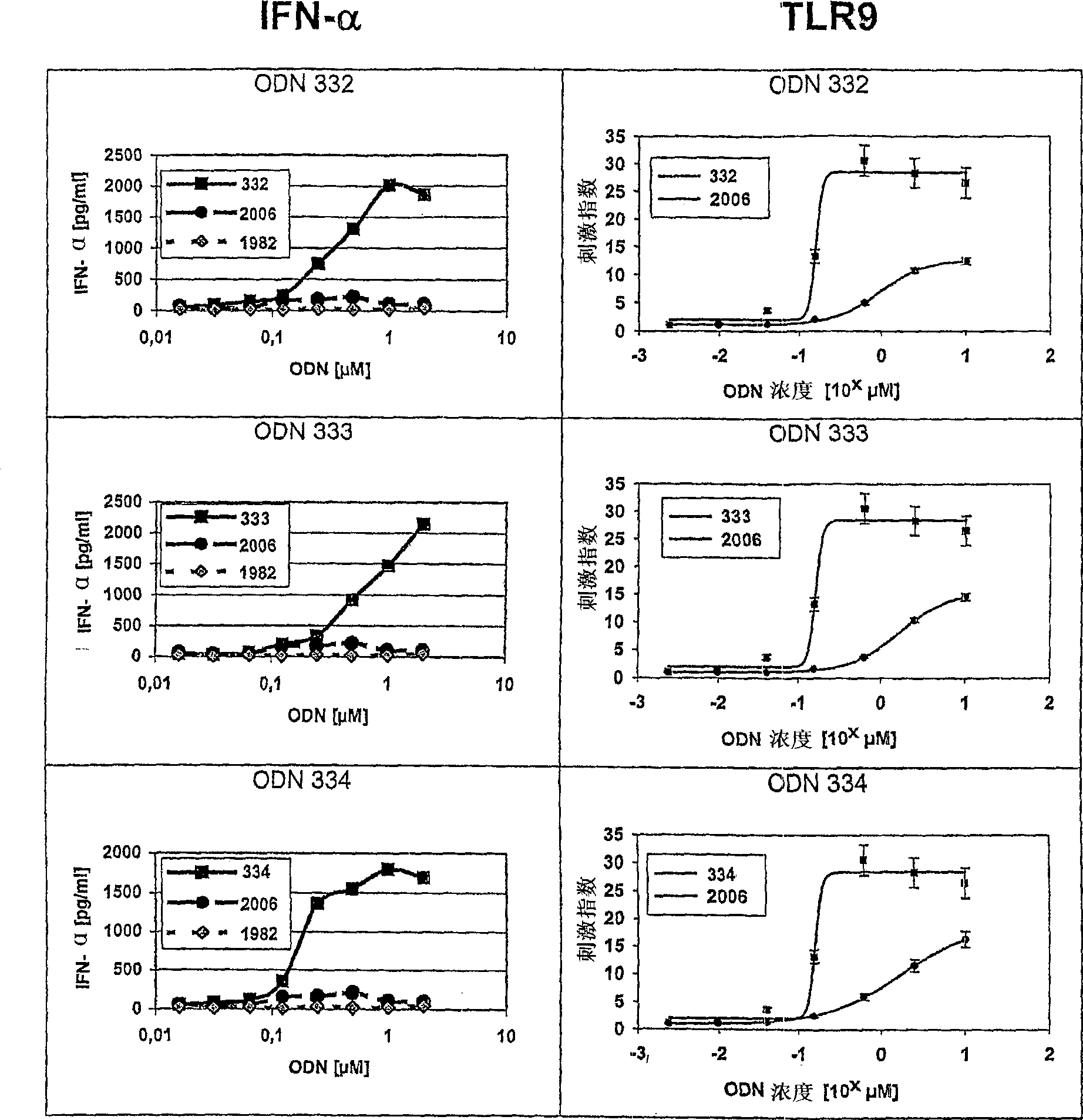
[0341] Class C ODN analogs induce IFN-α secretion and human TLR9 activity in vitro
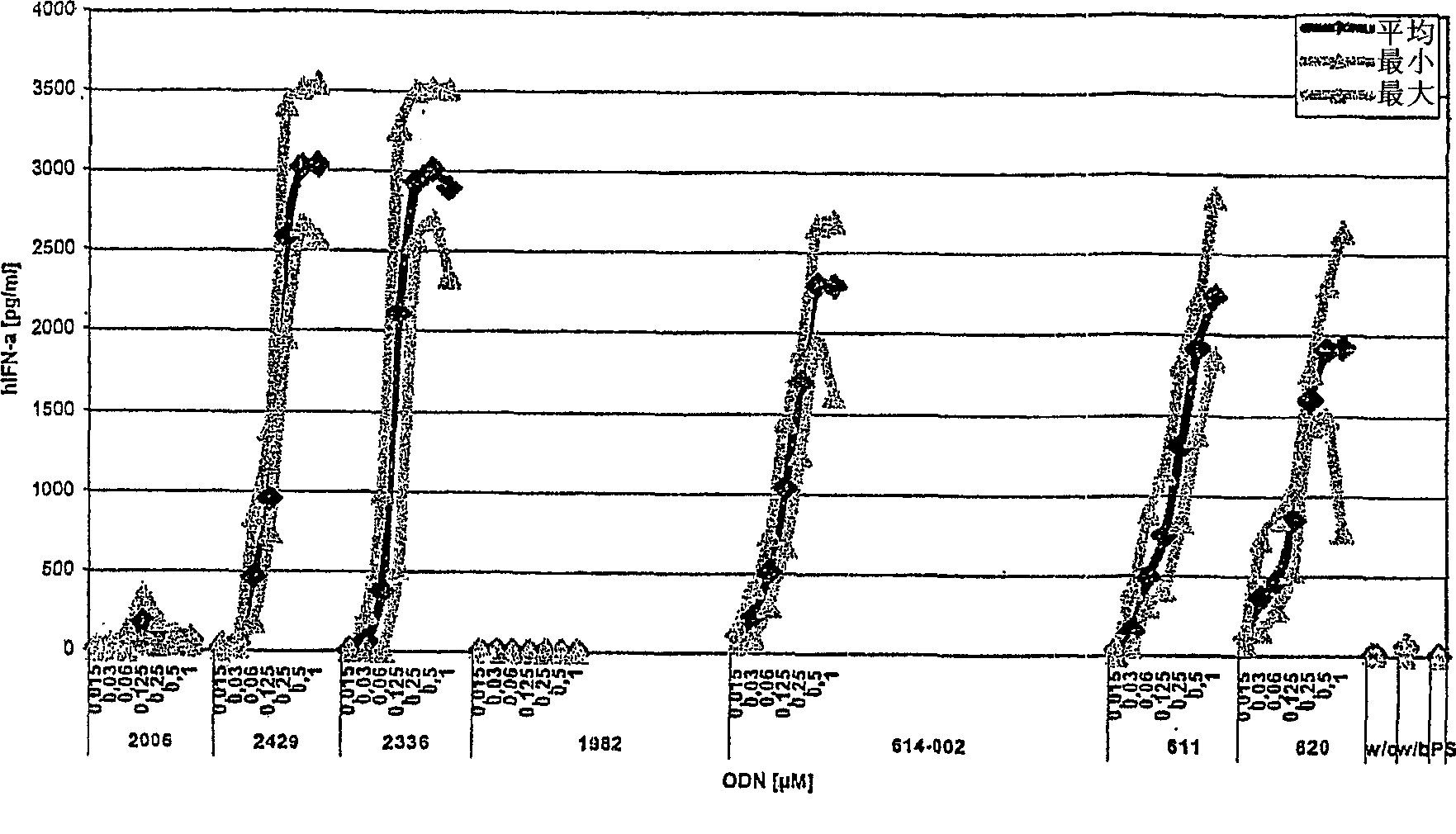
[0342] In this series of experiments, the class C ODN analogs of the present invention were tested in vitro for their ability to stimulate human peripheral blood mononuclear cells (PBMC) to secrete IFN-α and to stimulate HEK293 cells stably transfected with human TLR9 and NF-KB reporter gene constructs Shows the ability of TLR9 signaling.
[0343] ODN was purchased from Biospring (Frankfurt, Germany) and its identity and purity were controlled by Coley Pharmaceutical GmbH (Langenfeld, Germany). ODN was diluted in phosphate buffer (Sigma, Germany) and stored at -20°C. All dilutions were performed with pyrogen-free reagents. Test ODN includes the following:
[0344] 128T * C_G * T * C * G * T * T * T * T * A * C * G * G * C * G * T * C * G * T * G * C * C * G (SEQ ID NO: 48)
[0345] 611 T * C * G * T * C * G * T * T * T * T * A * C_G * G * C_G * C * C_G ...
Embodiment 2
[0372] Other Class C ODN analogs induce IFN-α secretion in vitro
[0373]In this series of experiments, other Class C ODN analogs of the invention were tested in vitro for their ability to induce IFN-[alpha] secretion. Class C ODN analogs in these assays were characterized in part by the presence of AT-rich interrupted inverted repeats, or by the presence of interrupted inverted repeats containing dSpacer residues without conventional nucleotide residues.
[0374] ODN was obtained as in Example 1. Test ODN includes the following:
[0375] 645T * C * G * T * C_G * T * T * T * T * T * A * A * T * A * T * T * T * A * T * T * A SEQ ID NO: 59
[0376] 646 T * C * G * T * C_G * T * T * T * T * C * A * A * T * A * T * T * T * A * T * T * G SEQ ID NO: 50
[0377] 647 T * C * G * T * C_G * T * T * T * T * T * A * A * T * A * T * C * C * A * T * T * A SEQ ID NO: 58
[0378] 649 T * C * G * T * C_G * T * T * T * T *...
Embodiment 3
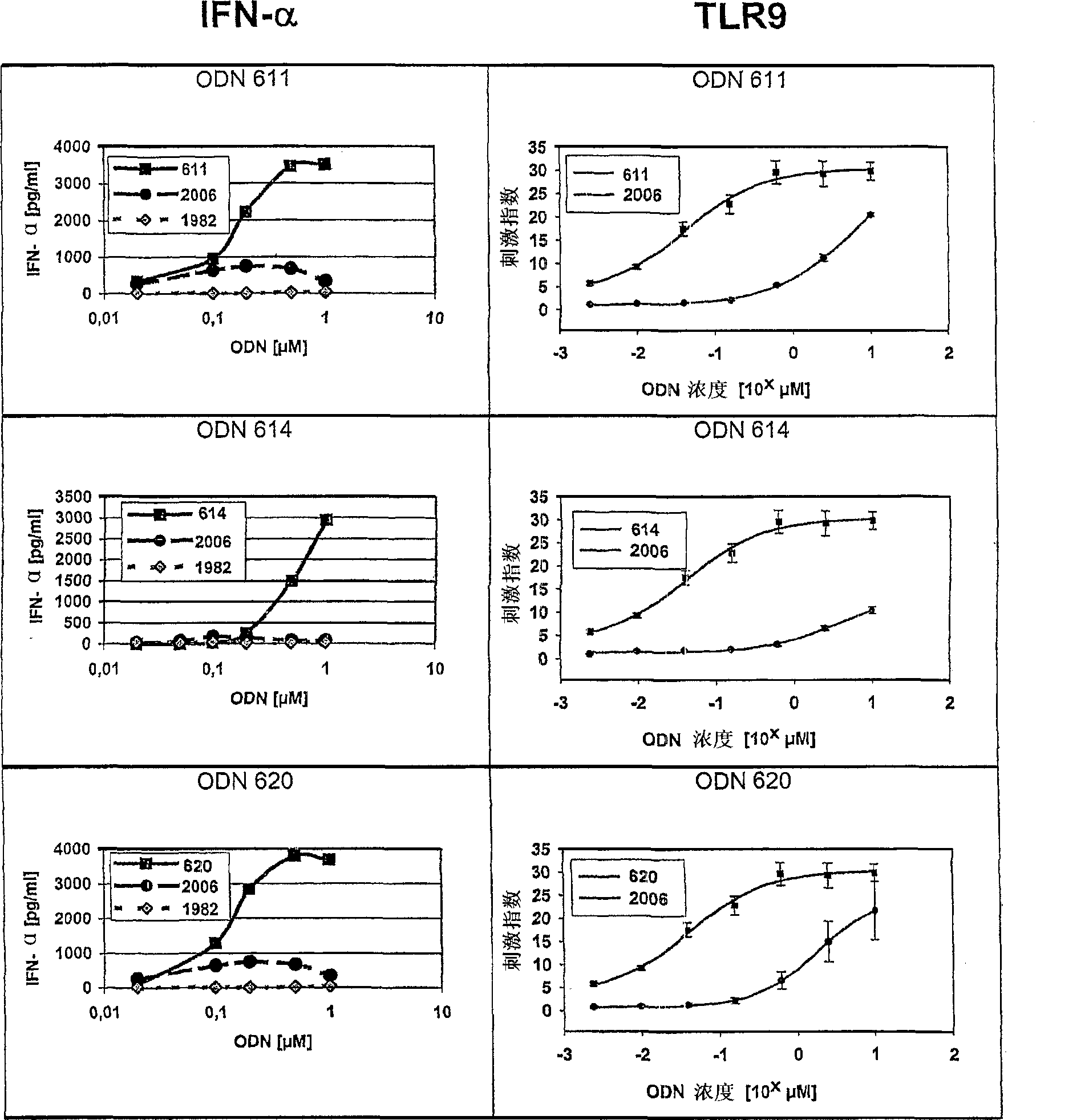
[0387] Additional class C ODN analogs induce IFN-α secretion and human TLR9 activity in vitro
[0388] In this series of experiments, the ability of the class C ODN analogs of the present invention to stimulate the secretion of IFN-α from human peripheral blood mononuclear cells (PBMC) and to stimulate HEK293 cells stably transfected with human TLR9 and NF-κB reporter gene constructs was tested in vitro Shows the ability of TLR9 signaling. The basic operation scheme is as described in Example 1, except that the test ODN includes the following:
[0389] 664 T * C * G * A * C * G * T * C * G * A * C * G * T * G * A * C * G * T * G (SEQ ID NO: 62)
[0390] 376T * C * G * A * C * G * T * C * G * A * C * G * T * G * A * C * G (SEQ ID NO: 61)
[0391] 801T * C_G * T * C_G * A * C_G * T * T * C_G * G * C * G * C * C_G * T * G * C * C * G (SEQ ID NO: 65)
[0392] 893 T * C * G * T * C_G * T * A * C_G * G * C * G * C * ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com