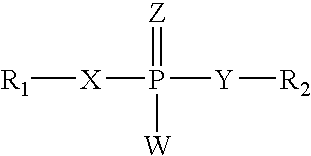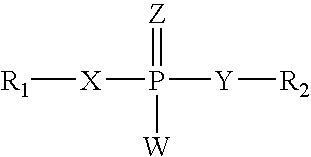Patents
Literature
767 results about "Greek letter beta" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Beta (UK: /ˈbiːtə/, US: /ˈbeɪtə/; uppercase Β, lowercase β, or cursive ϐ; Ancient Greek: βῆτα, translit. bē̂ta or Greek: βήτα vita) is the second letter of the Greek alphabet. In the system of Greek numerals it has a value of 2.
Diphenylimidazopyrimidine and -imidazole amines as inhibitors of beta-secretase
InactiveUS20050282826A1Elevated β-amyloid levelTreatment, prevention or amelioration of a disease orBiocideNervous disorderGreek letter betaMedicine
The present invention provides a compound of formula I and the use thereof for the therapeutic treatment, prevention or amelioration of a disease or disorder characterized by elevated β-amyloid deposits or β-amyloid levels in a patient.
Owner:WYETH
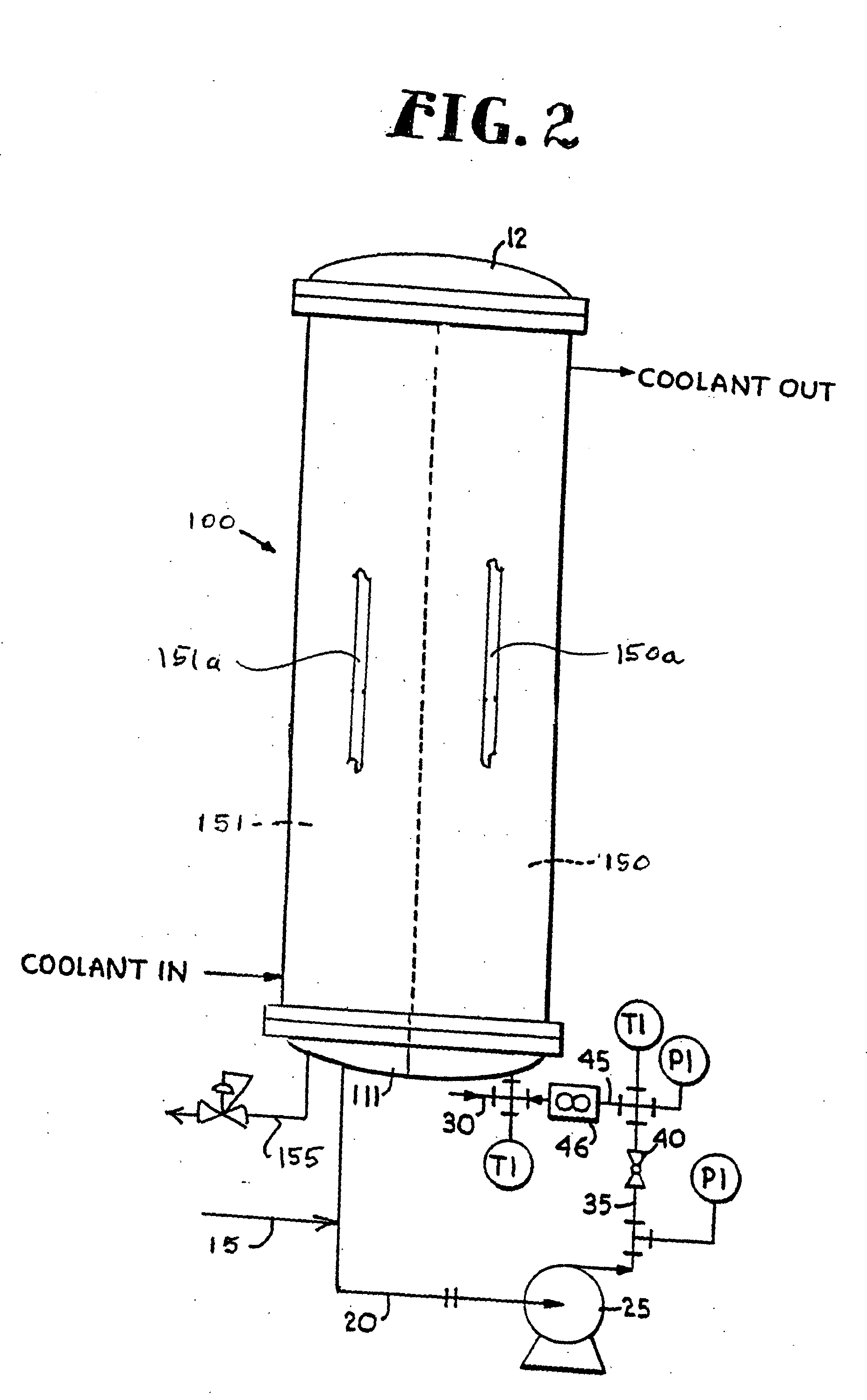
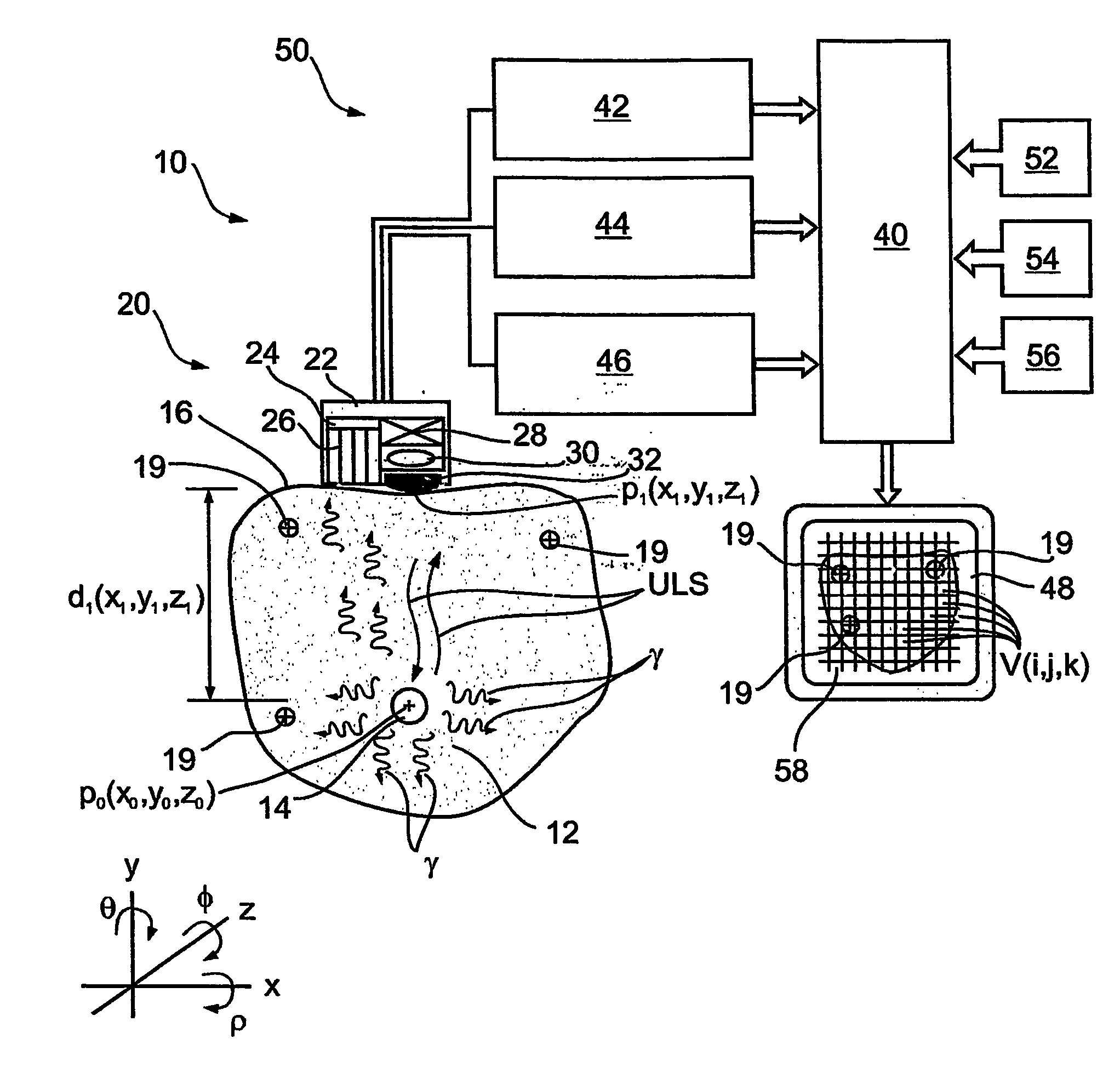
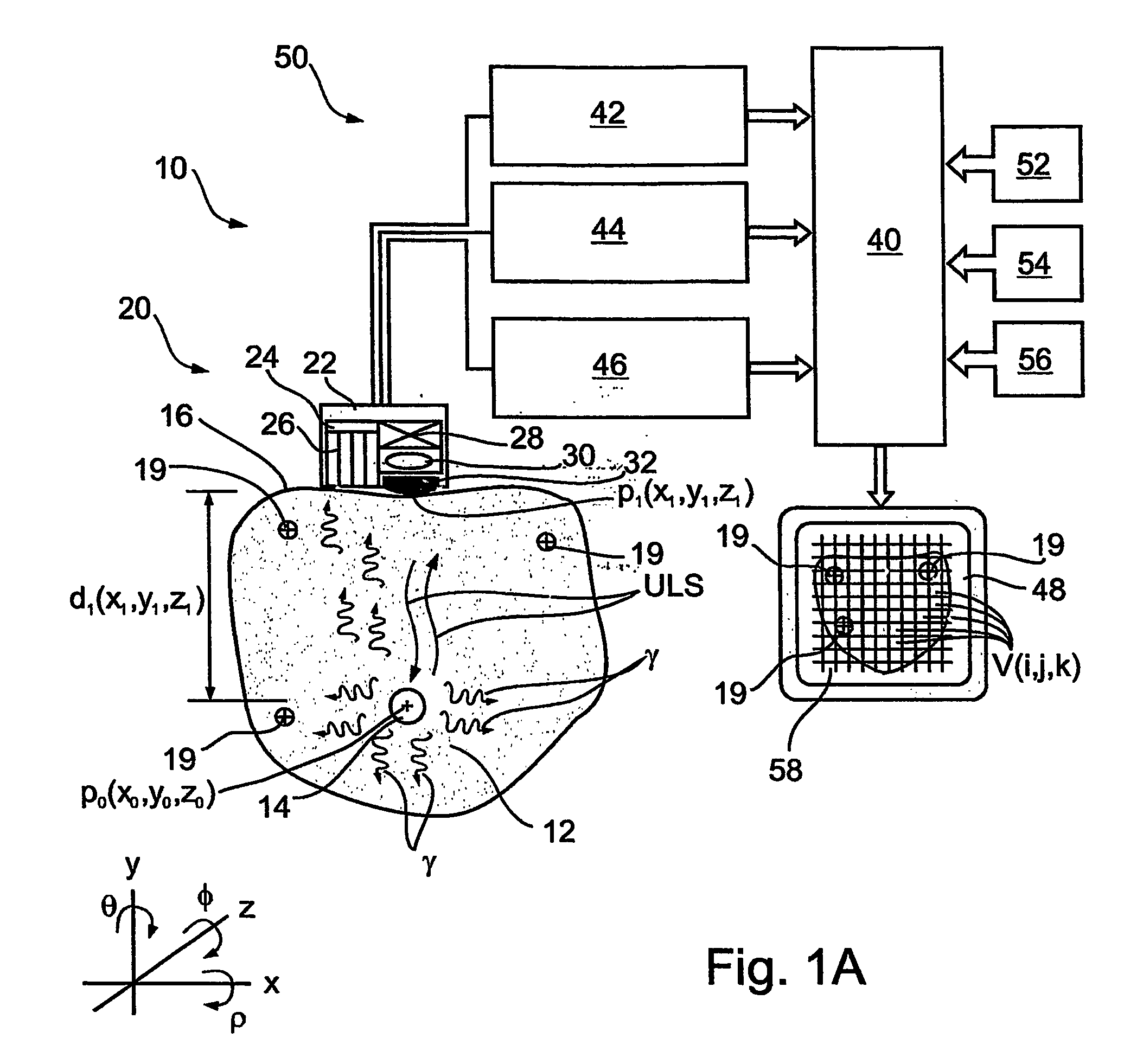
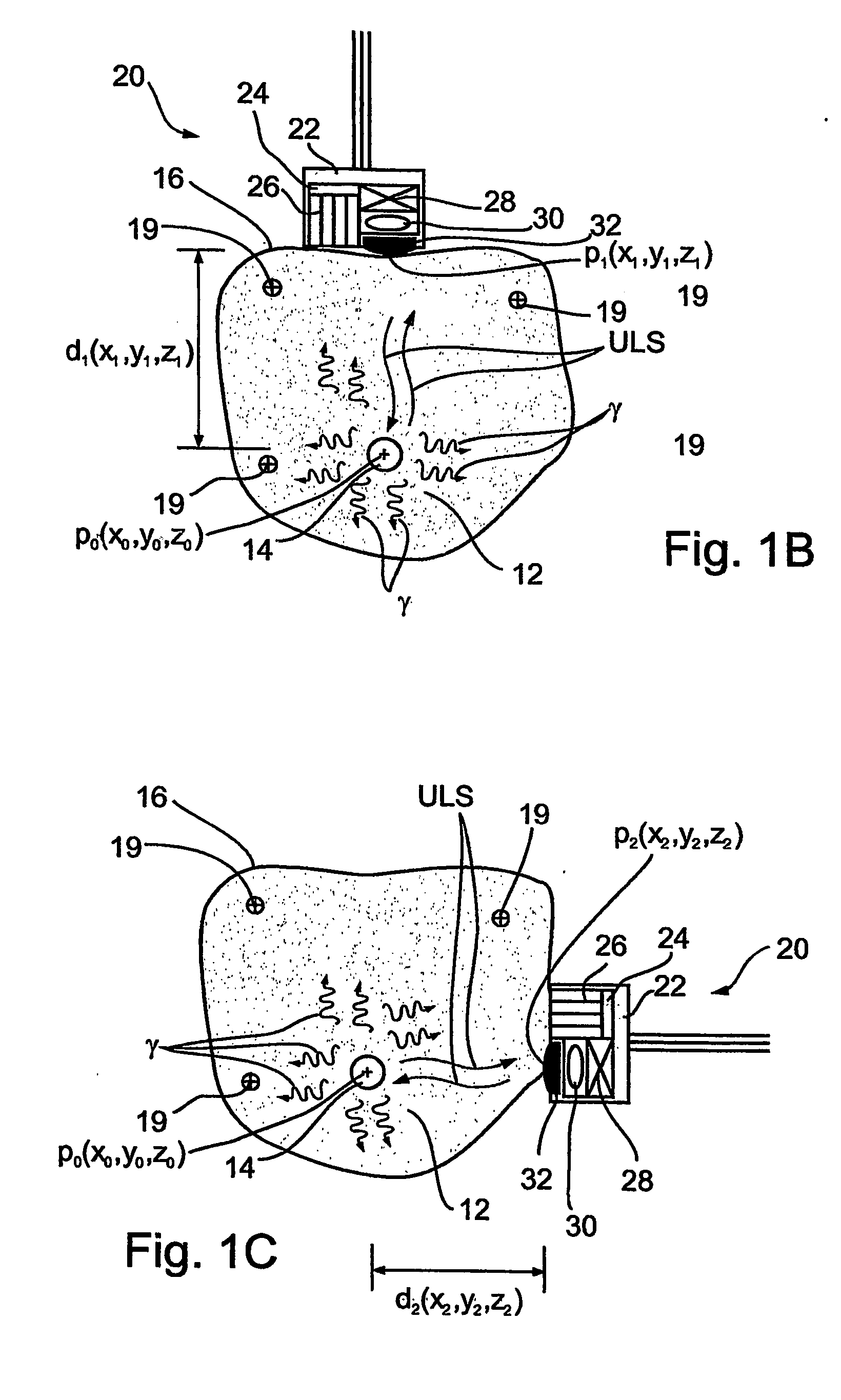
Apparatus and methods for imaging and attenuation correction
Imaging apparatus, is provided, comprising a first device, for obtaining a first image, by a first modality, selected from the group consisting of SPECT, PET, CT, an extracorporeal gamma scan, an extracorporeal beta scan, x-rays, an intracorporeal gamma scan, an intracorporeal beta scan, an intravascular gamma scan, an intravascular beta scan, and a combination thereof, and a second device, for obtaining a second, structural image, by a second modality, selected from the group consisting of a three-dimensional ultrasound, an MRI operative by an internal magnetic field, an extracorporeal ultrasound, an extracorporeal MRI operative by an external magnetic field, an intracorporeal ultrasound, an intracorporeal MRI operative by an external magnetic field, an intravascular ultrasound, and a combination thereof, and wherein the apparatus further includes a computerized system, configured to construct an attenuation map, for the first image, based on the second, structural image. Additionally, the computerized system is configured to display an attenuation-corrected first image as well as a superposition of the attenuation-corrected first image and the second, structural image. Furthermore, the apparatus is operative to guide an in-vivo instrument based on the superposition.
Owner:SPECTRUM DYNAMICS MEDICAL LTD
Amino-5,5-diphenylimidazolone derivatives for the inhibition of beta-secretase
InactiveUS20050282825A1Elevated β-amyloid levelTreatment, prevention or amelioration of a disease orBiocideNervous disorderGreek letter betaMedicine
The present invention provides a compound of formula I and the use thereof for the therapeutic treatment, prevention or amelioration of a disease or disorder characterized by elevated β-amyloid deposits or β-amyloid levels in a patient.
Owner:WYETH LLC
Modulators of 11- beta hydroxyl steroid dehydrogenase type 1, pharmaceutical compositions thereof, and methods of using the same
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1 and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1.
Owner:INCYTE CORP
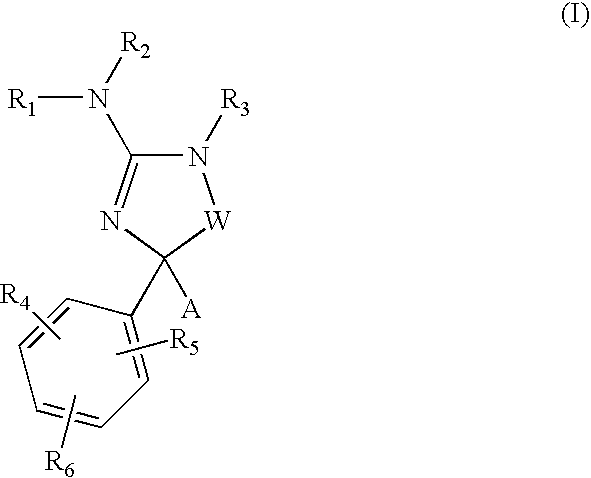
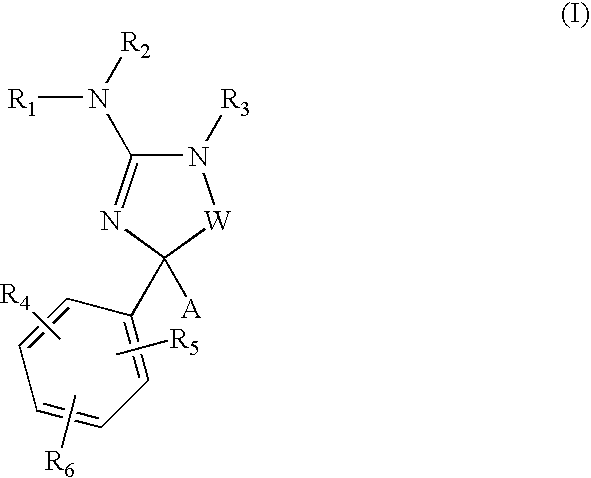
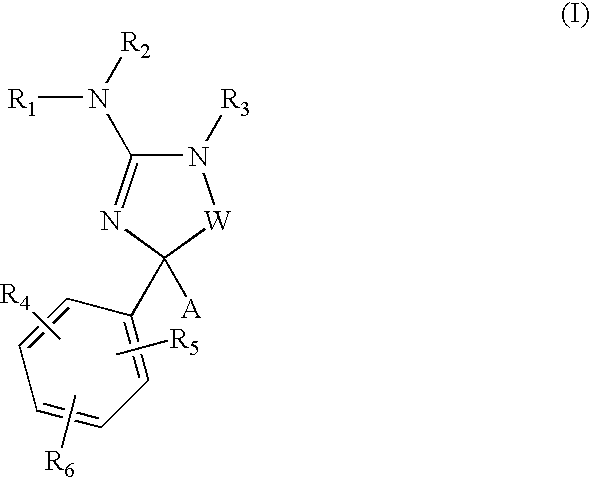
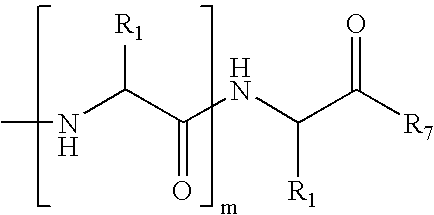
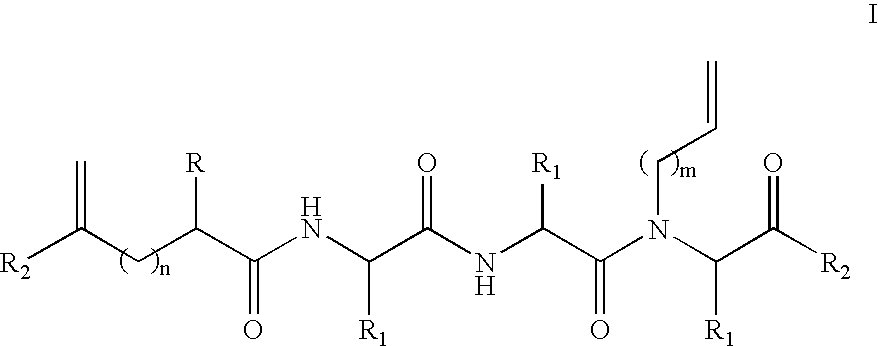
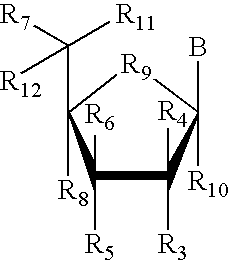
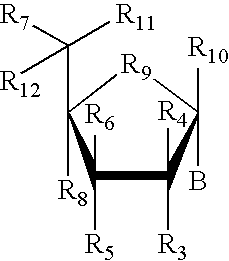
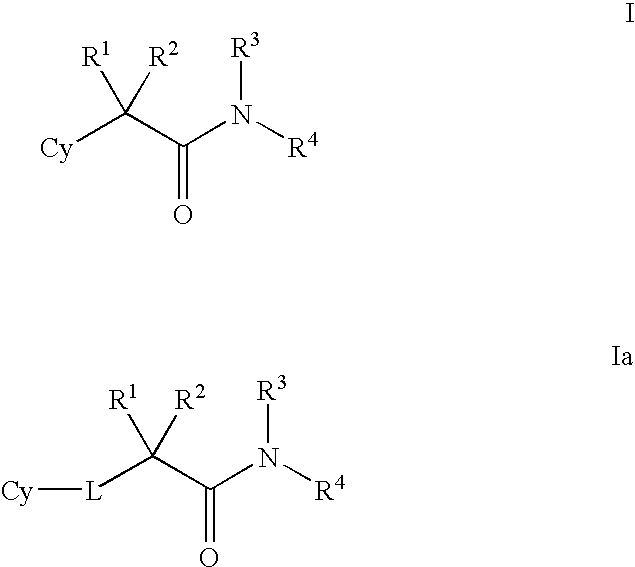
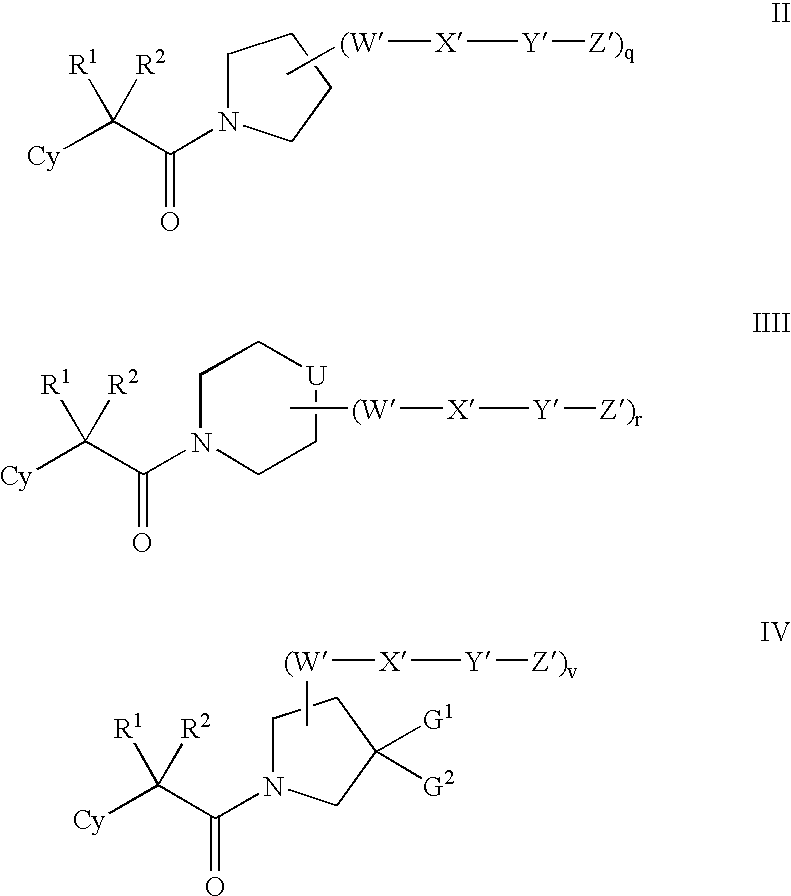
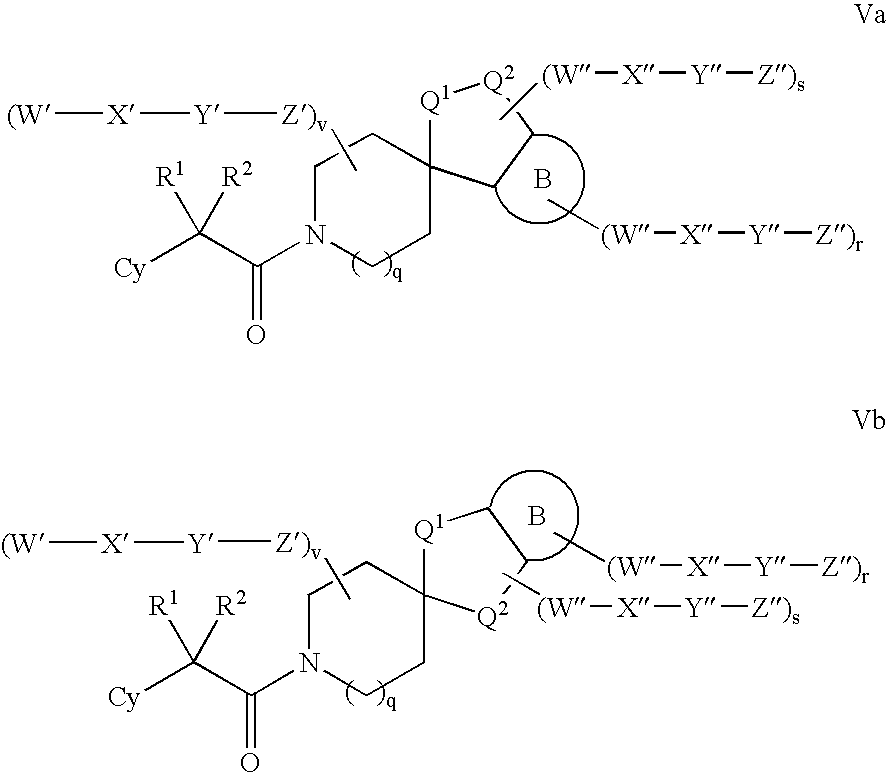
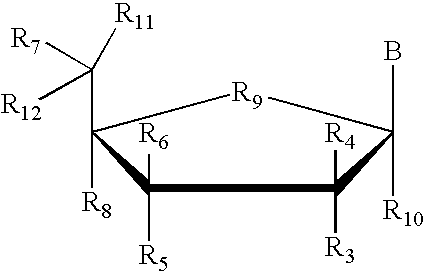
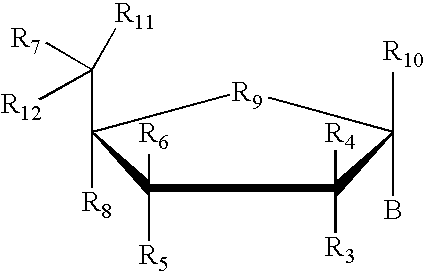
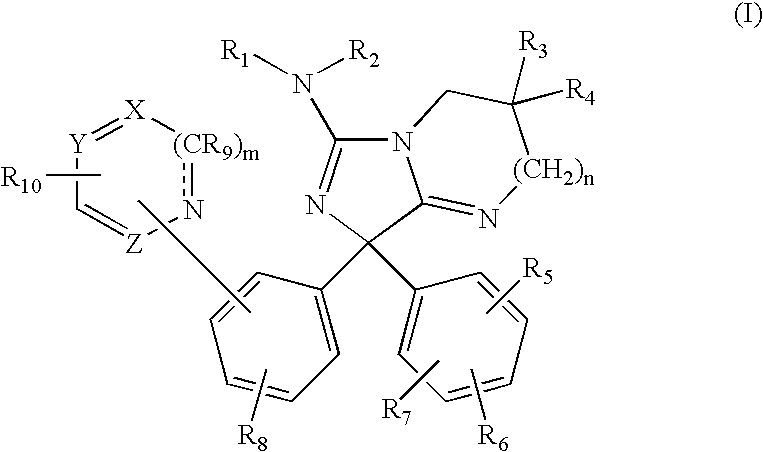
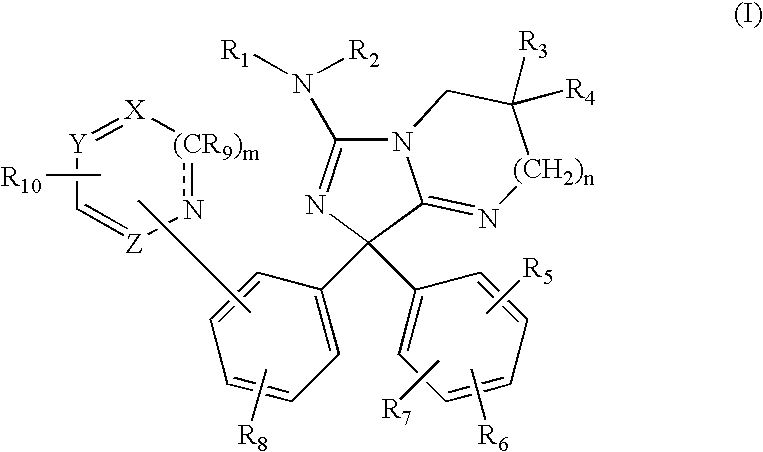
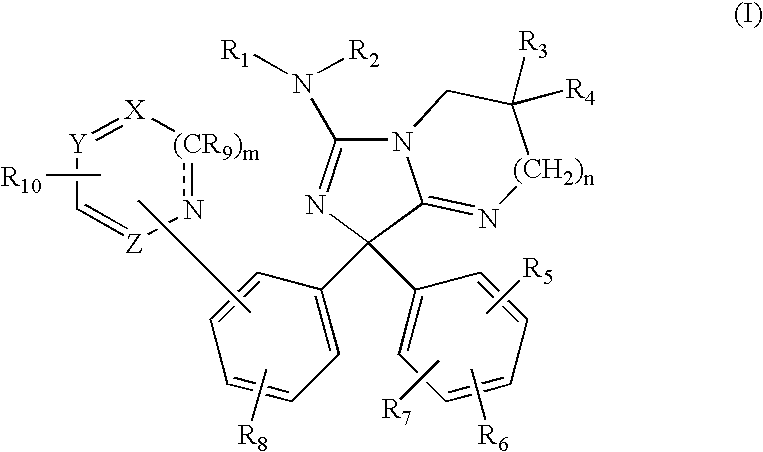
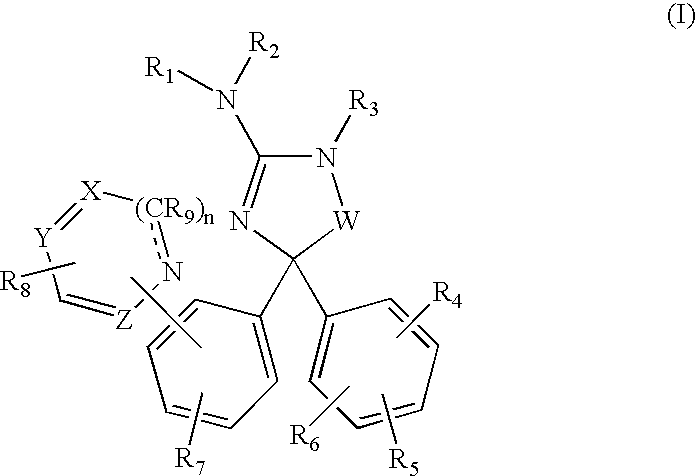
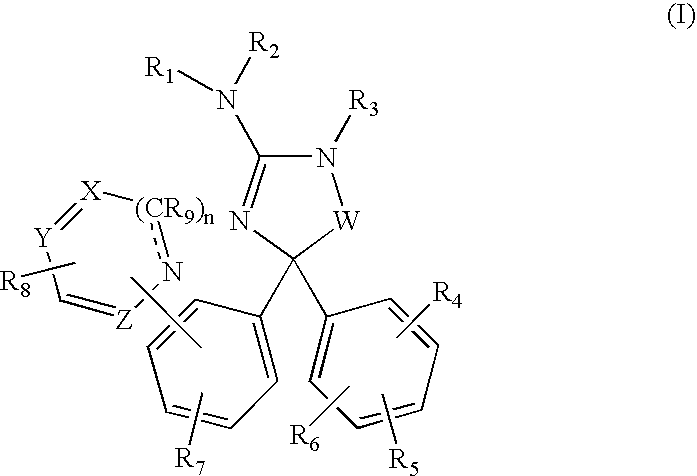
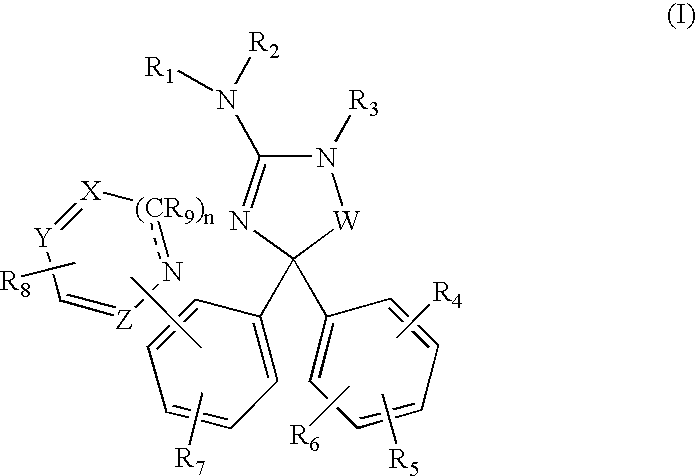
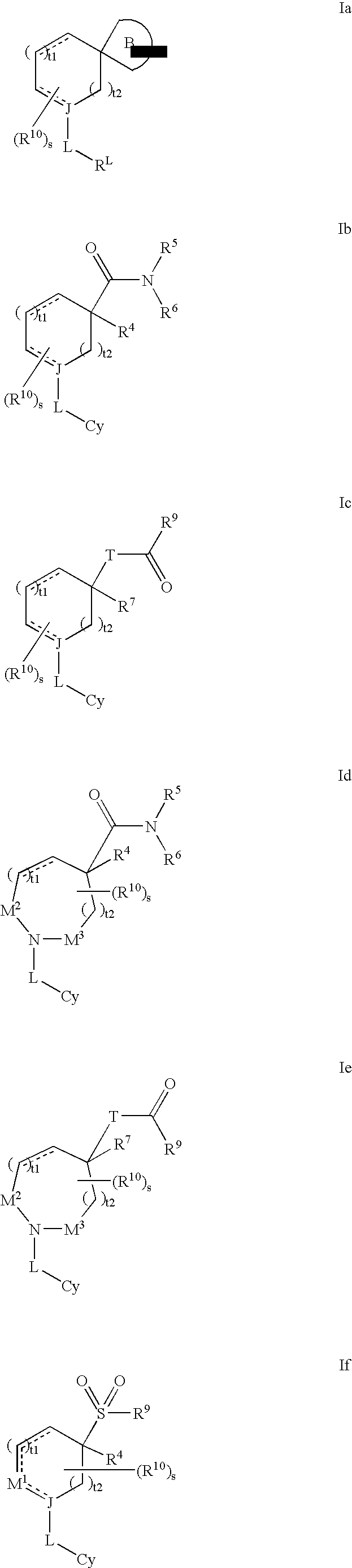
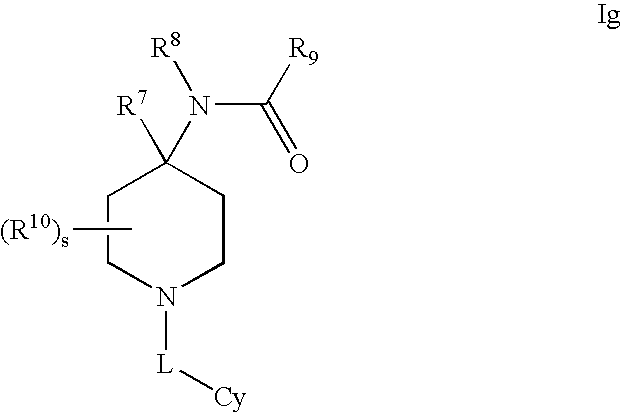
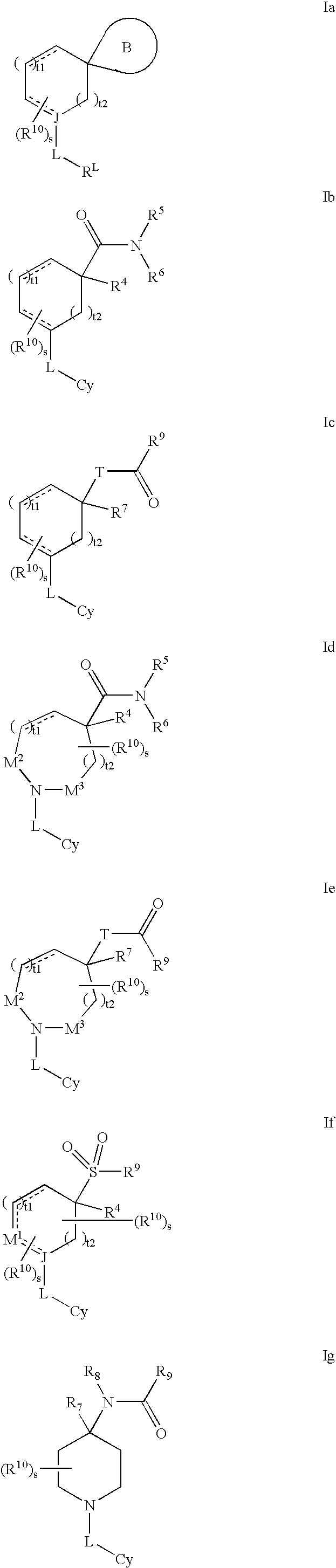
Amino-5-[4-(difluoromehtoxy)phenyl]-5-phenylimidazolone compounds for the inhibition of beta-secretase
Owner:WYETH LLC
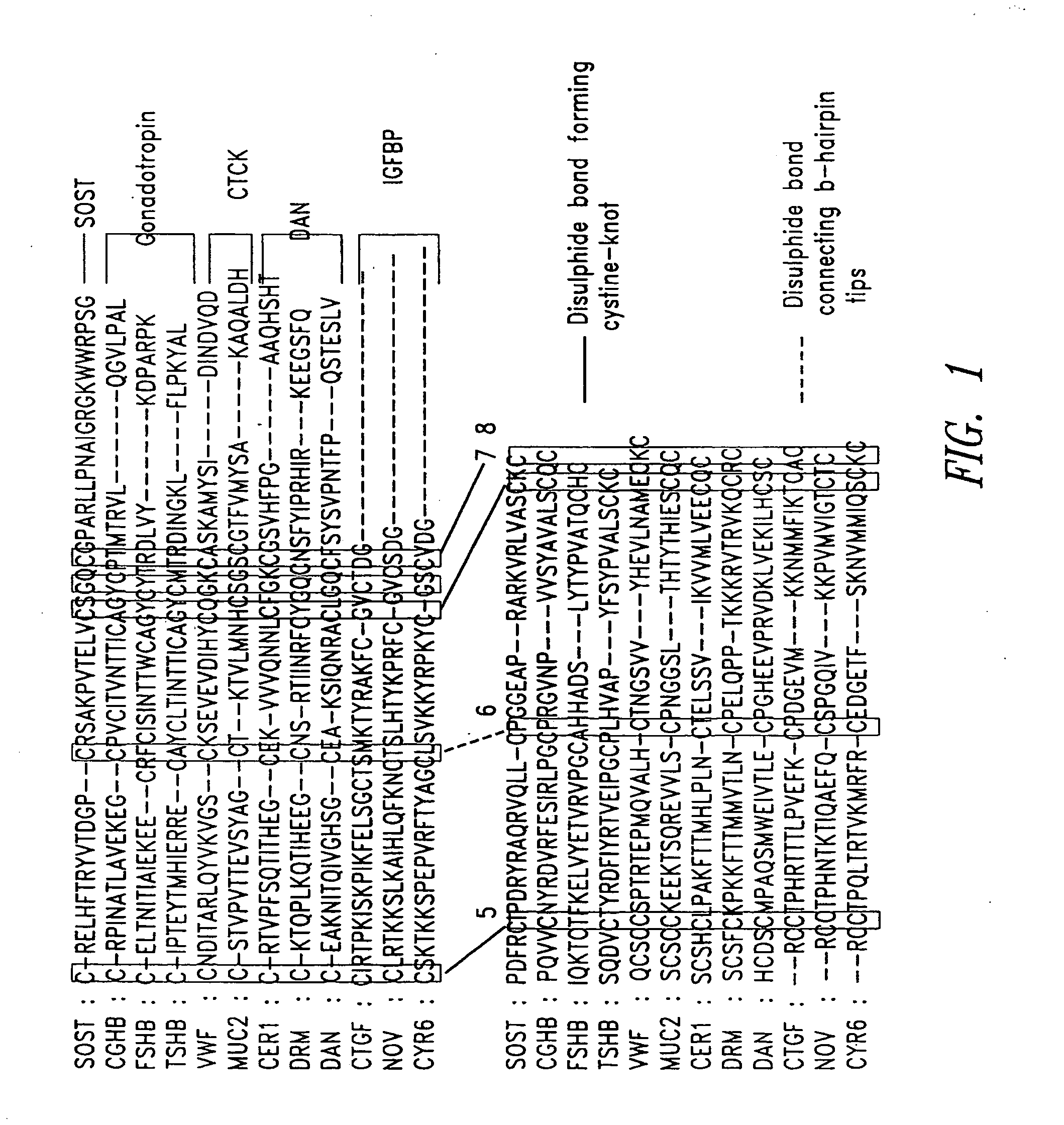
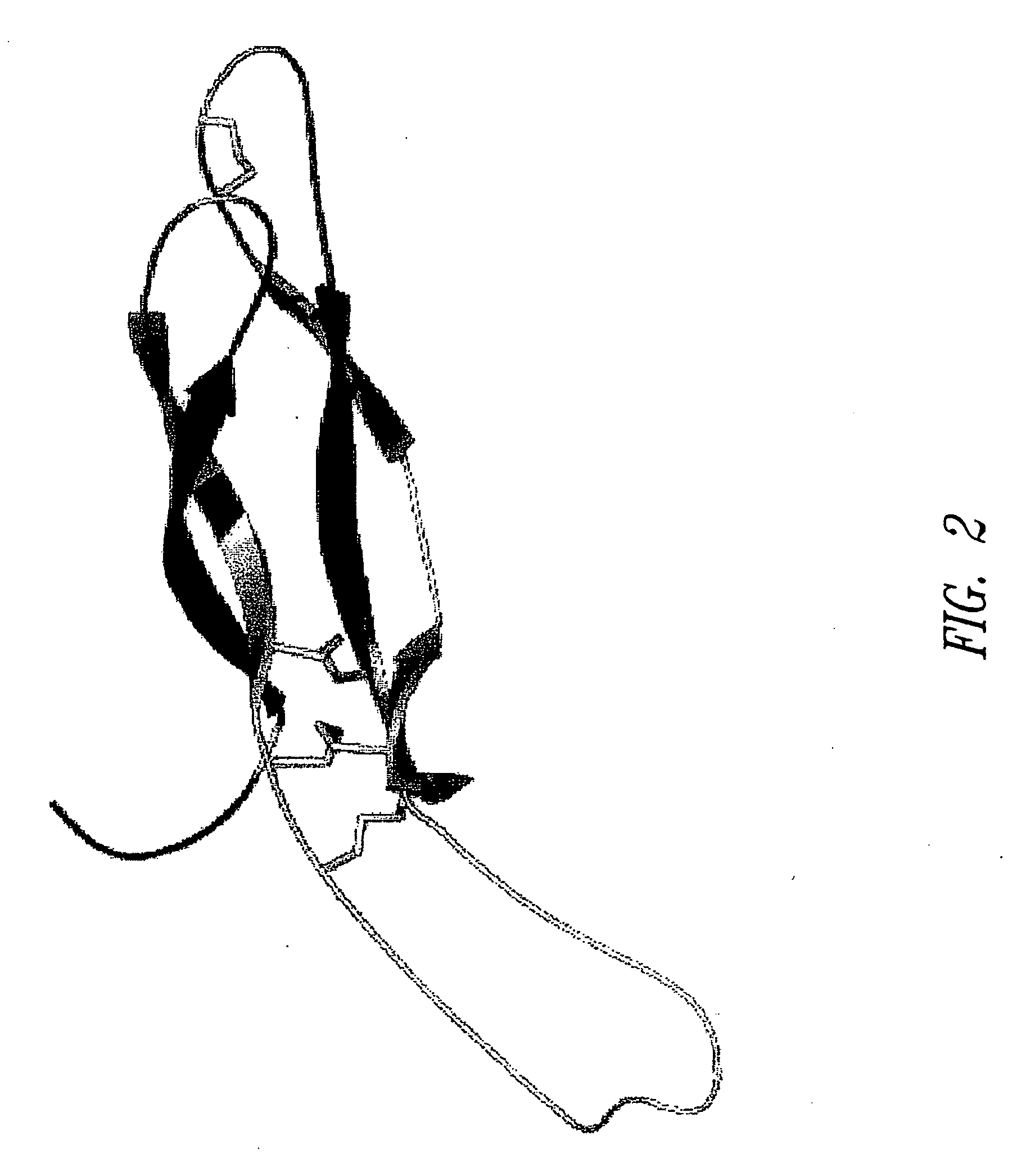
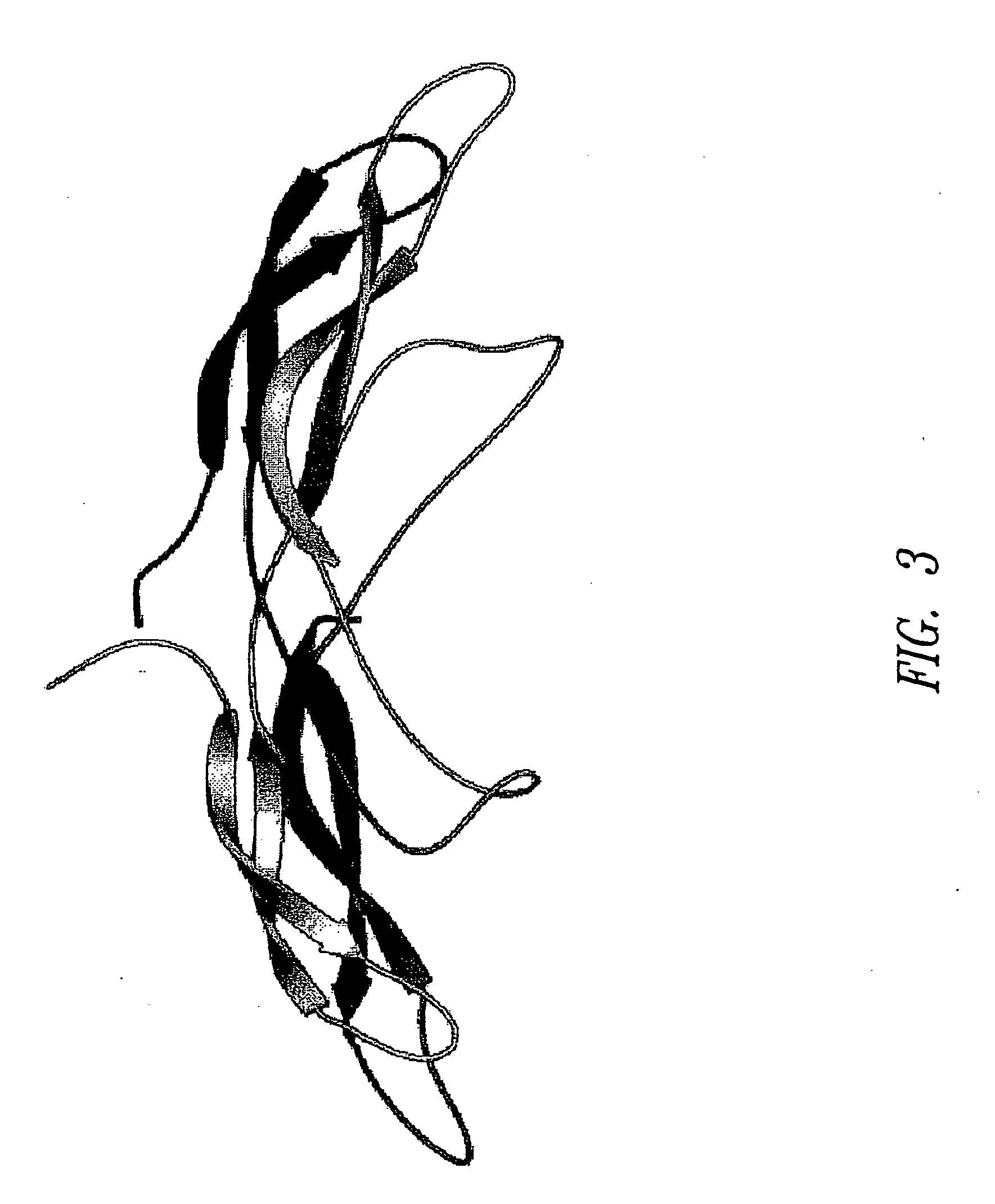
Antibodies specific for sclerostin and methods for increasing bone mineralization
ActiveUS20050106683A1Skeletal disorderImmunoglobulins against growth factorsGreek letter betaIncreased bone mineral density
Compositions and methods relating to antibodies that specifically bind to TGF-beta binding proteins are provided. These methods and compositions relate to altering bone mineral density by interfering with the interaction between a TGF-beta binding protein sclerostin and a TGF-beta superfamily member, particularly a bone morphogenic protein. Increasing bone mineral density has uses in diseases and conditions in which low bone mineral density typifies the condition, such as osteopenia, osteoporosis, and bone fractures.
Owner:UCB PHARMA SA
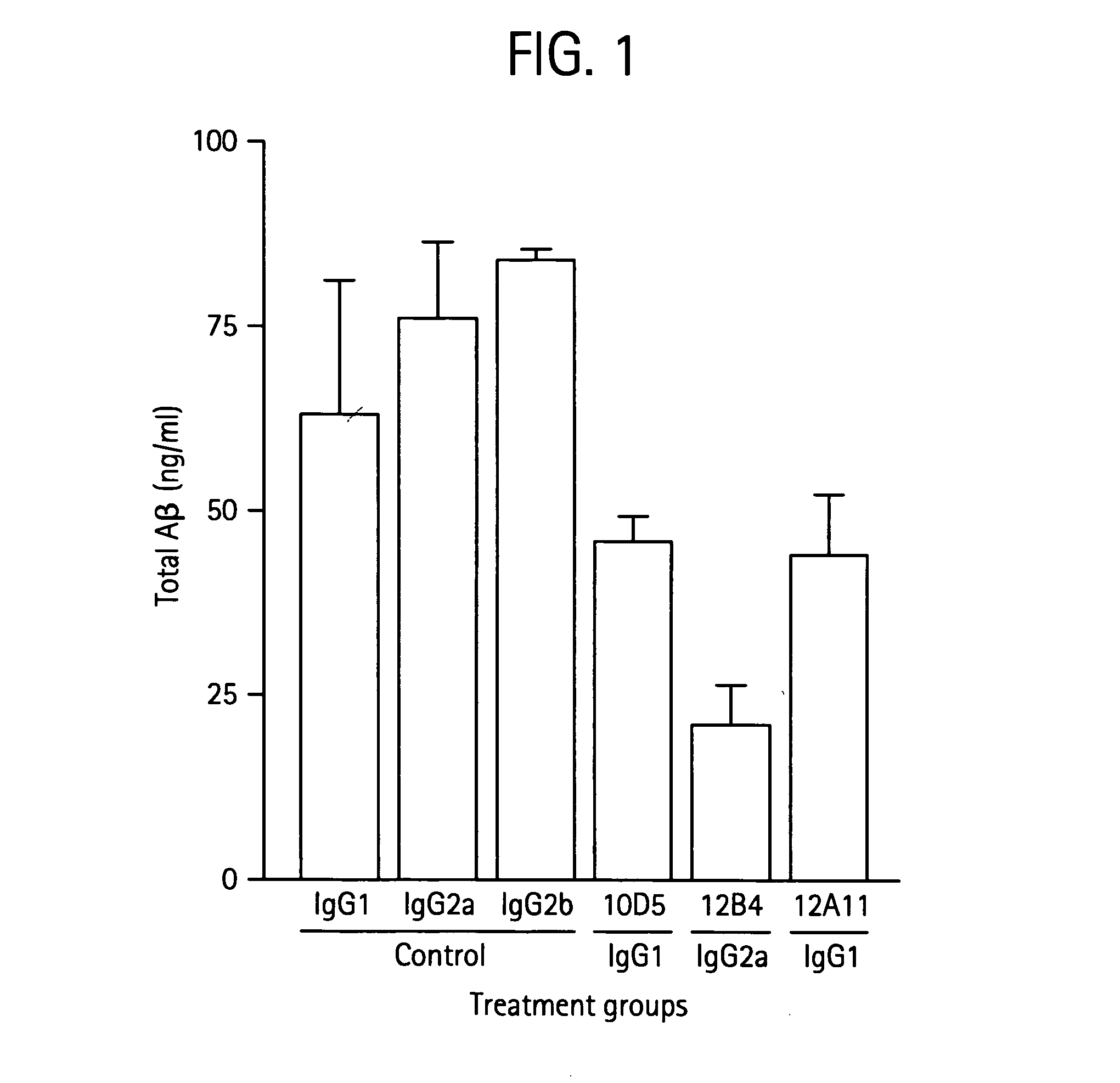
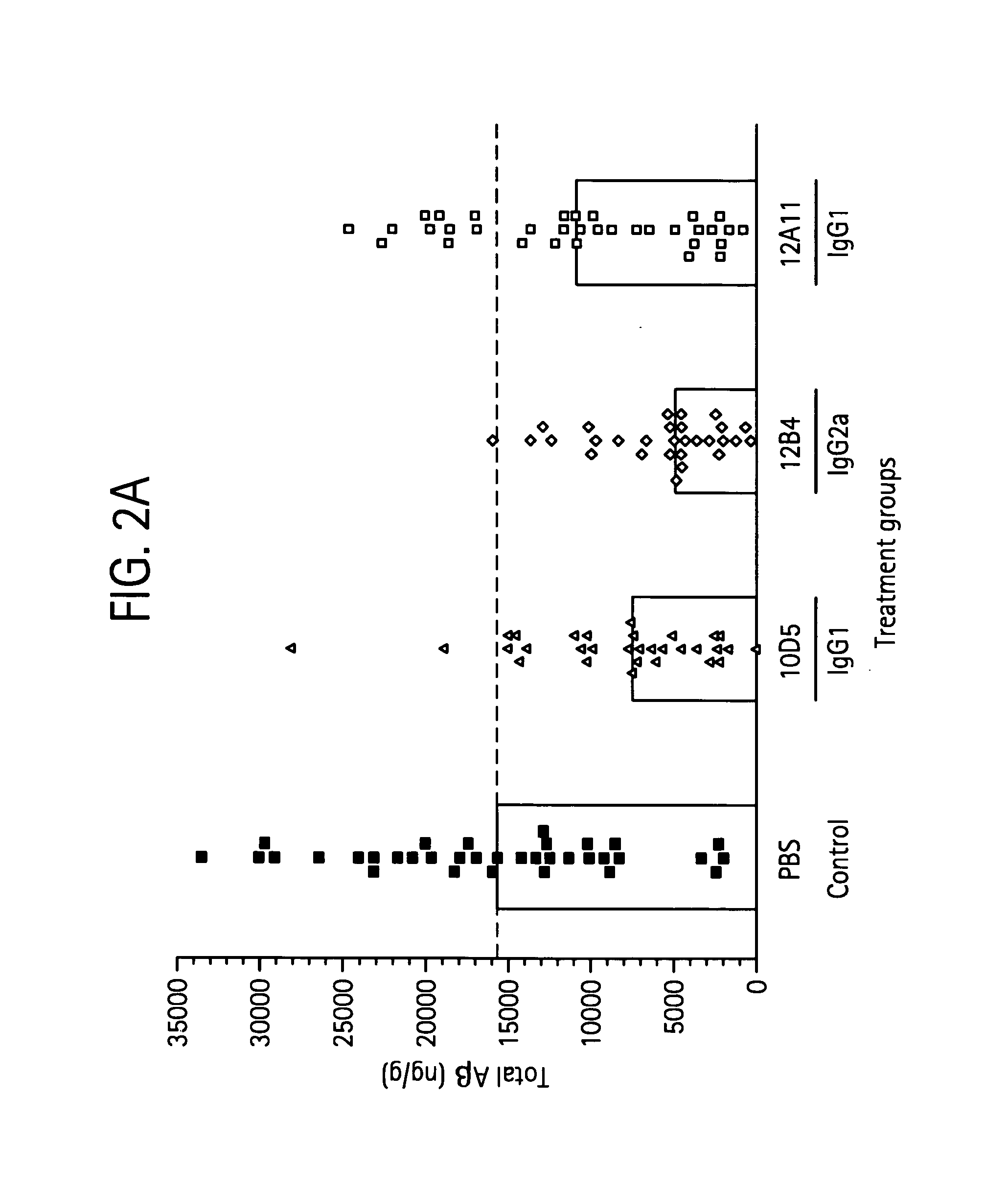
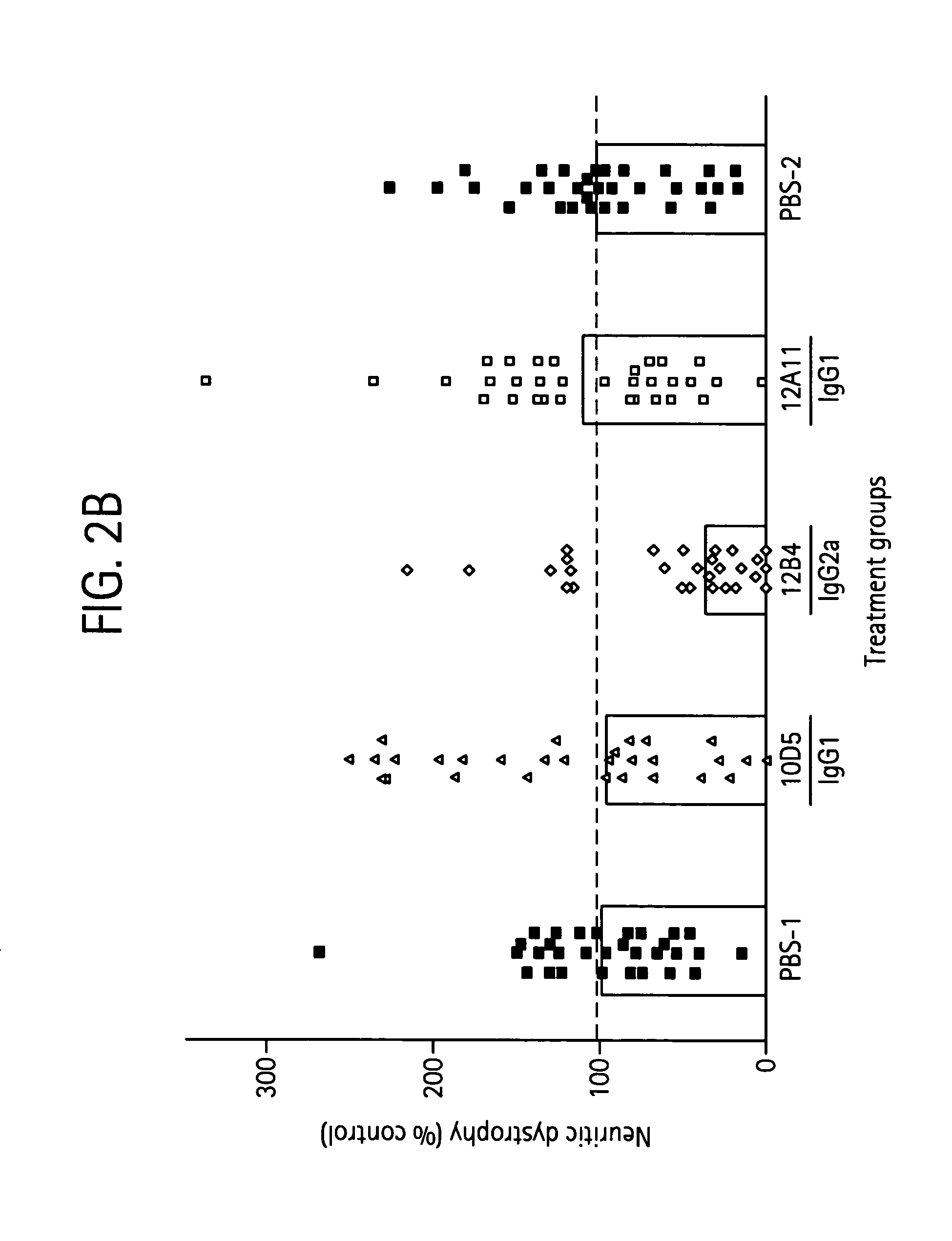
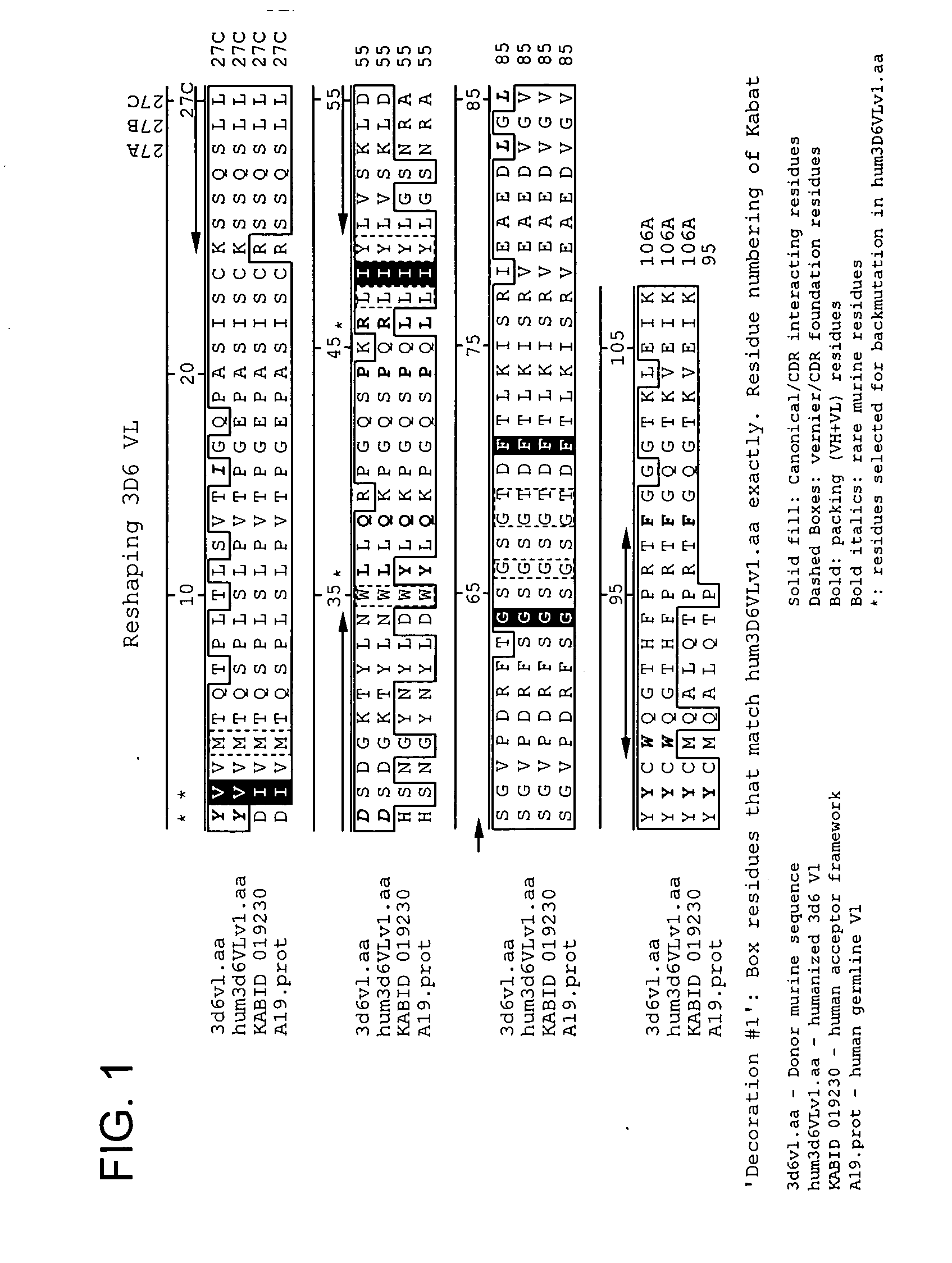
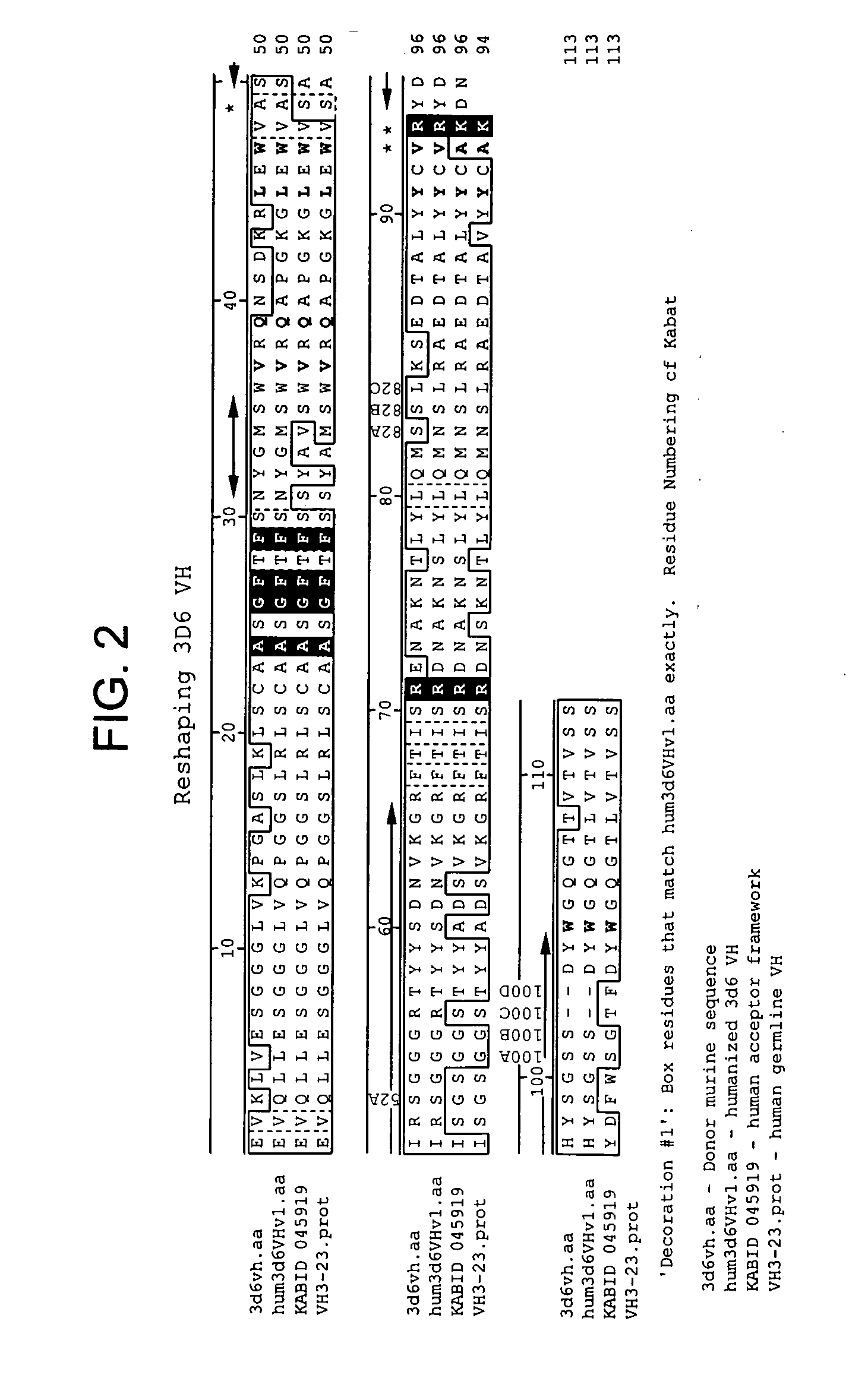
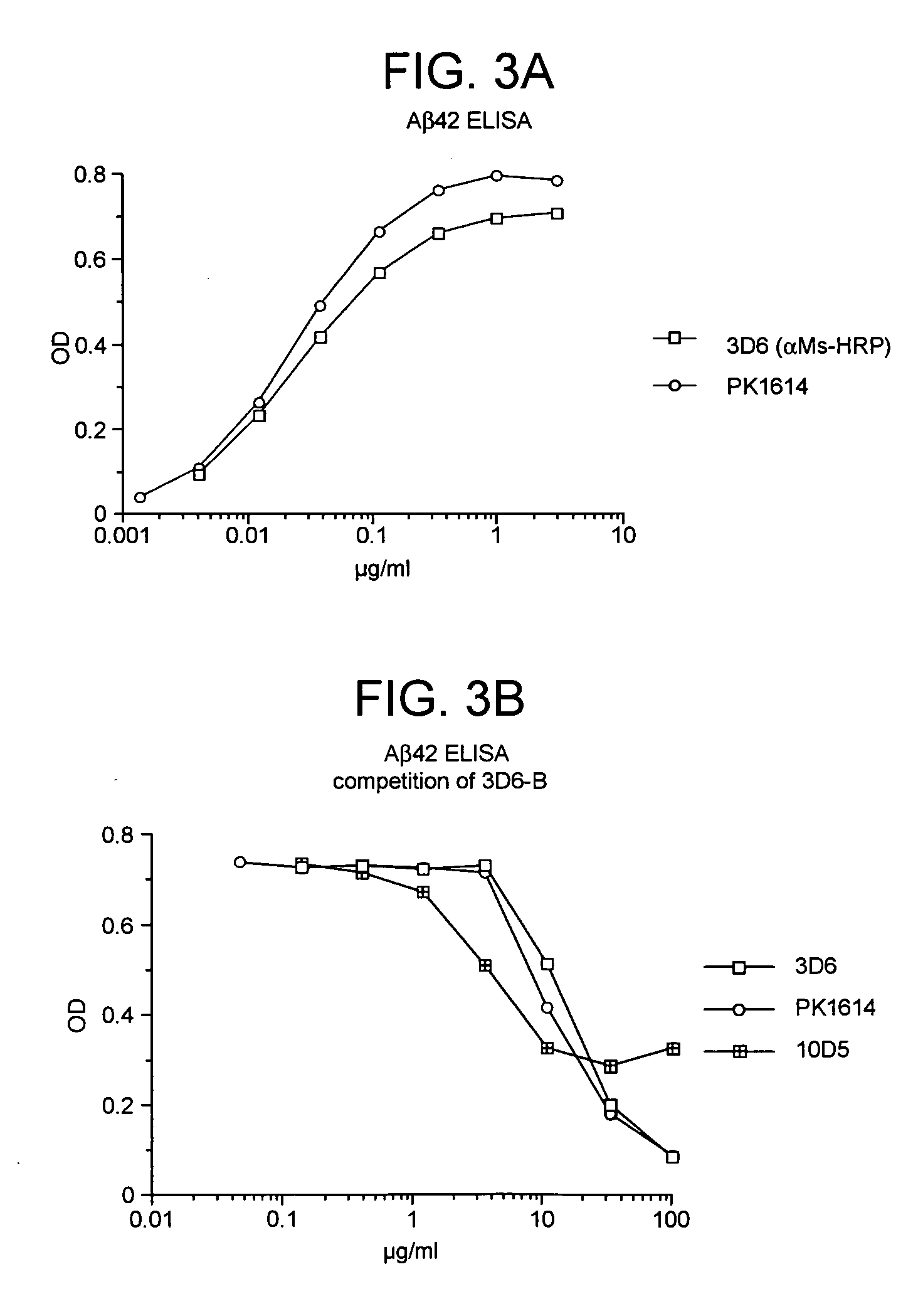
Humanized antibodies that recognize beta amyloid peptide
ActiveUS20050118651A1Reduce the burden onAnimal cellsNervous disorderGreek letter betaHumanized antibody
The invention provides improved agents and methods for treatment of diseases associated with amyloid deposits of Aβ in the brain of a patient. Preferred agents include antibodies, e.g., humanized antibodies.
Owner:WYETH LLC +1
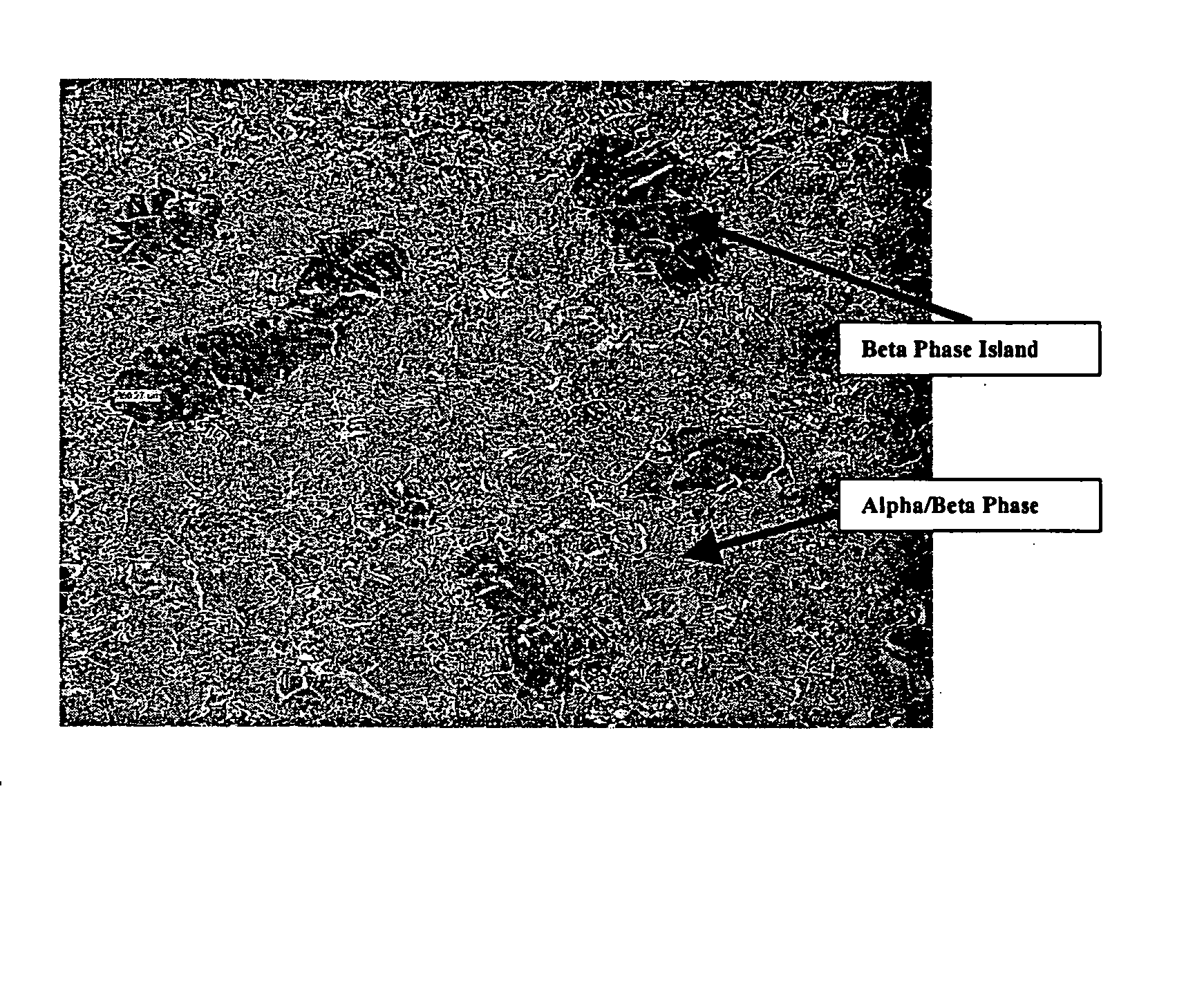
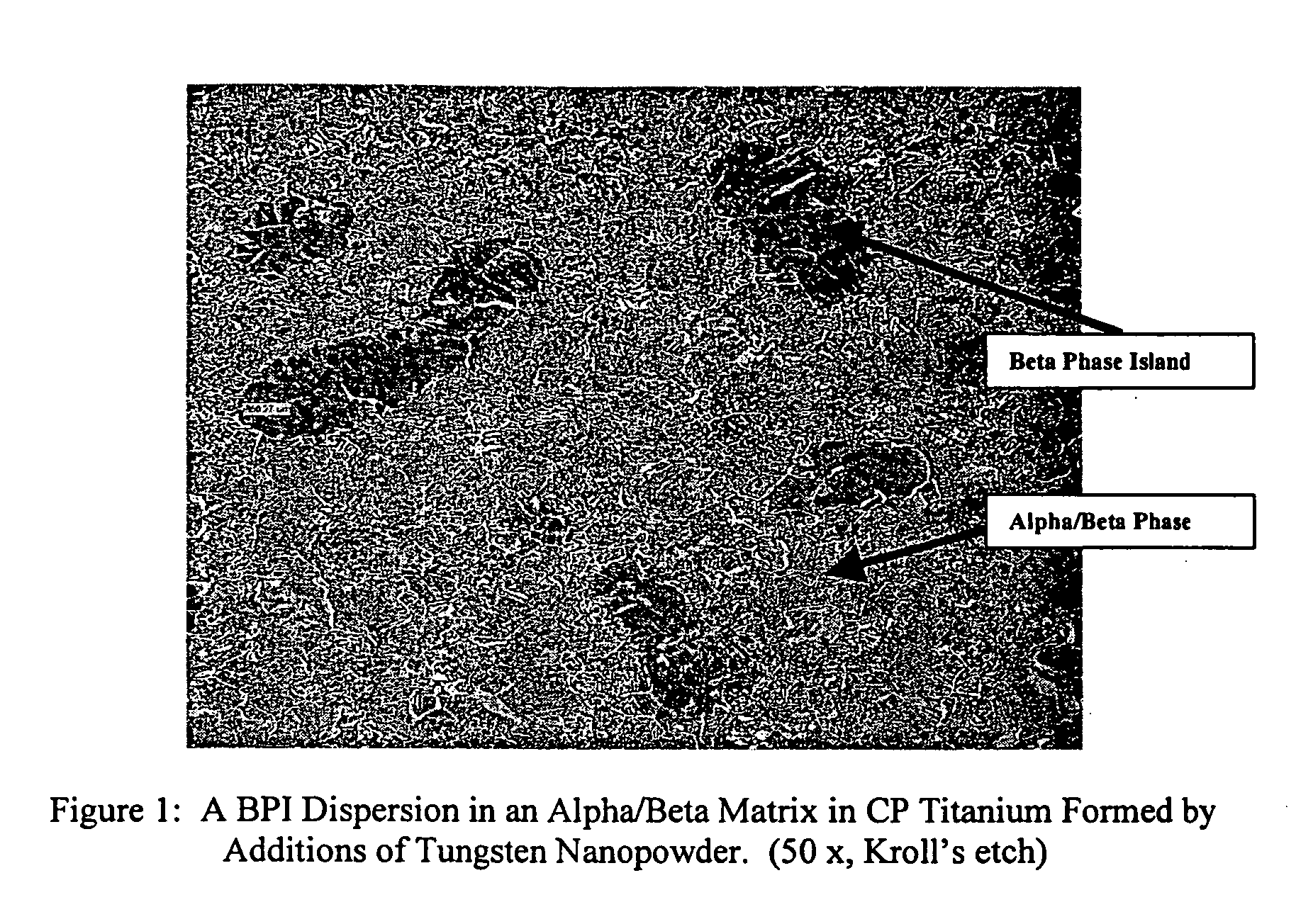
Titanium tungsten alloys produced by additions of tungsten nanopowder
Disclosed herein are titanium-tungsten alloys and composites wherein the tungsten comprises 0.5% to 40% by weight of the alloy. Also disclosed is a method of making such alloys and composites using powders of tungsten less then 3 μm in size, such as 1 μm or less. Also disclosed is a method of making the titanium alloy by powder metallurgy, and products made from such alloys or billets that may be cast, forged, or extruded. These methods of production can be used to make titanium alloys comprising other slow-diffusing beta stabilizers, such as but not limited to V, Nb, Mo, and Ta.
Owner:DYNAMET TECH
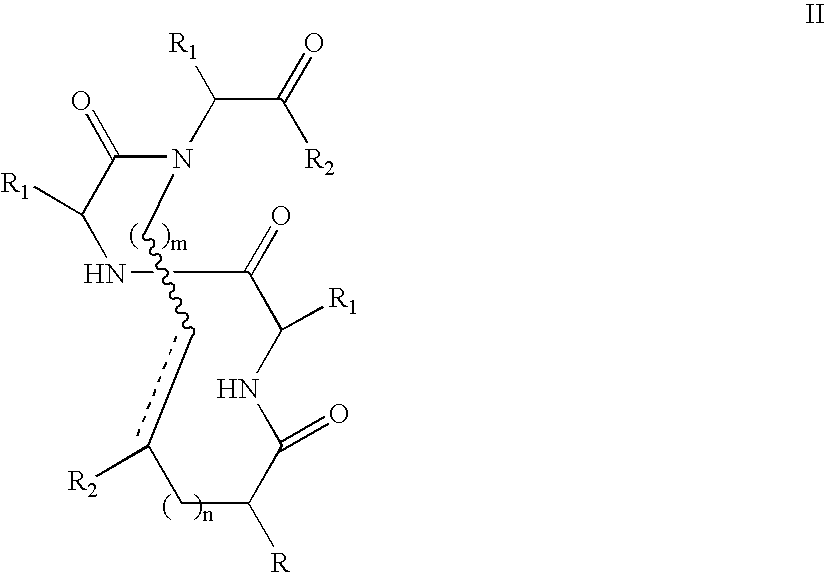
Methods for preparing internally constrained peptides and peptidomimetics
ActiveUS20060014675A1Promote cell deathAvoid interactionPeptide preparation methodsImmunoglobulinsGreek letter betaBeta sheet
The present invention relates to a method for preparing a peptide having a stable, internally constrained alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region and a method of stabilizing an alpha-helical, beta-sheet / beta-turn, 310-helical, or pi-helical region within a peptide structure. The resulting peptides and methods of using them are also disclosed.
Owner:NEW YORK UNIV
Molded porous ceramic article containing beta-SiC and process for the production thereof
ActiveUS20070032370A1Reduce energy consumptionReduce abrasionPhysical/chemical process catalystsLayered productsGreek letter betaPolymer science
The invention relates to a process for the production of a molded porous ceramic article containing β-SiC, which process comprises the following steps: the preparation of a molded article containing silicon and carbon and the subsequent pyrolysis and siliconization of the article containing silicon and carbon to form SiC. The invention further relates to a molded porous ceramic article containing SiC which has been produced from a molded article containing silicon and carbon.
Owner:HELSA AUTOMOTIVE GMBH & CO KG +1
Ionomer/rubber/polyolefin blend and uses thereof
A thermoplastic ionomer blend or alloy exhibiting advantageous properties upon molding or extrusion and / or thermoforming, consisting essentially of the following components:A. about 15 to 85 parts by weight of a thermoplastic copolymer containing about 91 to 80 weight percent of alpha-olefin units and about 9 to 20 weight percent of alpha, beta-ethylenically unsaturated carboxylic acid units said carboxylic acid units being about 20 to 90 percent neutralized with metal ions, preferably zinc,B. about 10 to 80 parts by weight of a rubber, preferably a thermoplastic elastomer selected from the group consisting of (a) crosslinked ethylene-propylene-diene copolymers and equivalent polyolefin copolymers such as ethylene-butene, hexene, or octene, (b) acrylonitrile-butadiene copolymers, (c) styrene-butadiene copolymers, and (d) styrene acrylonitrile graft-crosslinked butadiene rubbers, andC. about 5 to 40 parts by weight of a thermoplastic polymer selected from the group consisting of polyethylene and polypropylene copolymers and homopolymers, the total number of parts being 100,and molded or extruded and / or thermoformed products produced from the same.
Owner:LYONDELLBASELL ADVANCED POLYMERS INC
Interferon alpha antibodies and their uses
ActiveUS20070014724A1Inhibit biological activityInhibiting surface expressionPeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The present invention provides isolated anti-interferon alpha monoclonal antibodies, particularly human monoclonal antibodies, that inhibit the biological activity of multiple interferon (IFN) alpha subtypes but do not substantially inhibit the biological activity of IFN alpha 21 or the biological activity of either IFN beta or IFN omega. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for inhibiting the biological activity of IFN alpha using the antibodies of the invention, as well as methods of treating disease or disorders mediated by IFN alpha, such as autoimmune diseases, transplant rejection and graft versus host disease, by administering the antibodies of the invention.
Owner:MEDAREX LLC
Process for producing mid-range vinylidene content polyisobutylene polymer products
A process for making a relatively low molecular weight, mid-range vinylidene content PIB polymer product comprising a liquid phase polymerization process conducted in a loop reactor at a temperature of at least 60° F. using a BF3 / methanol catalyst complex and a contact time of no more than 4 minutes. At least about 90% of the PIB molecules present in the product comprise alpha or beta position isomers. The vinylidene (alpha) isomer content of the product may range from 20% to 70% thereof, and the content of tetra-substituted internal double bonds is very low, advantageously no more than about 10%, preferably less than about 5% and ideally less than about 1-2%.
Owner:TPC GROUP
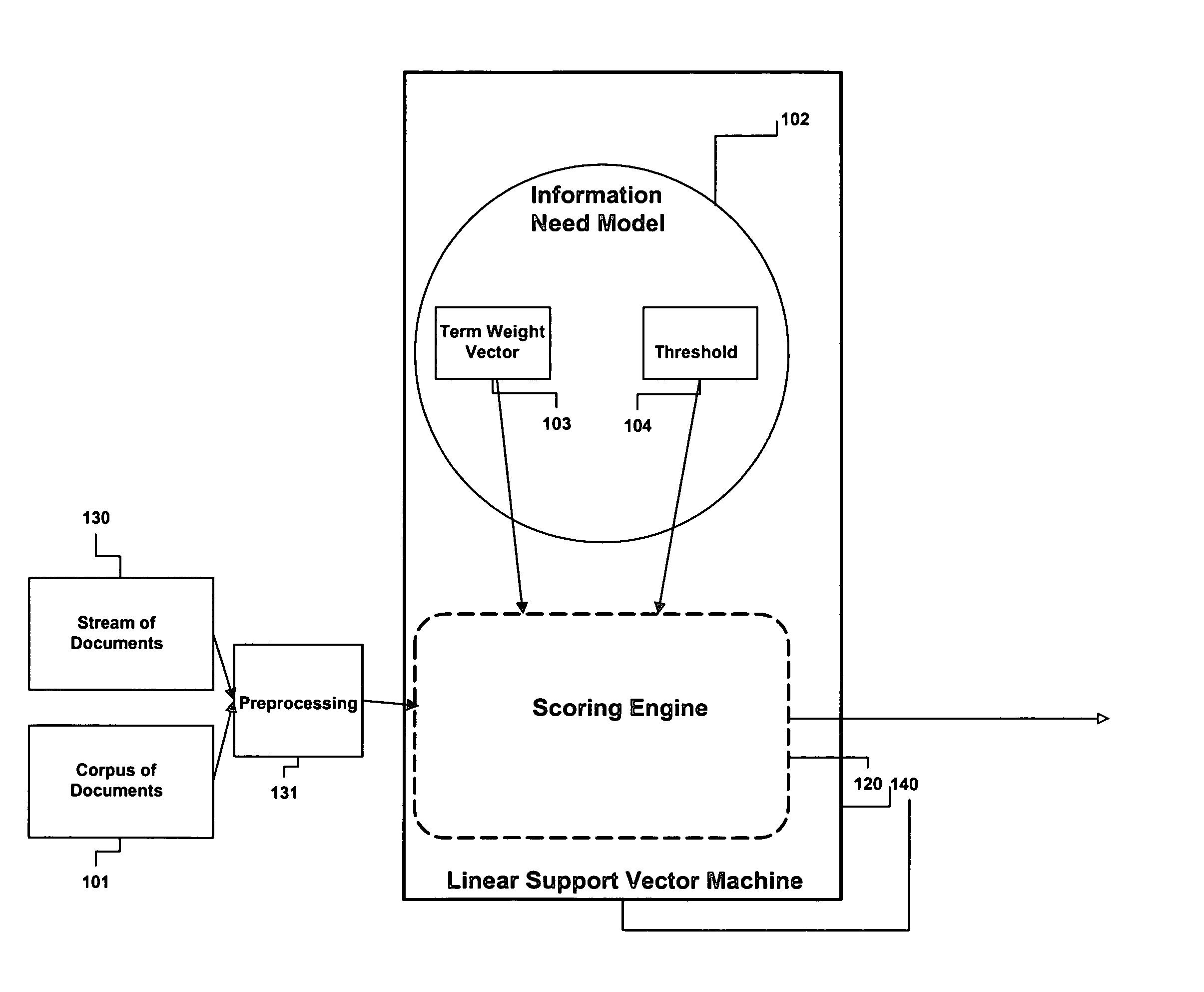
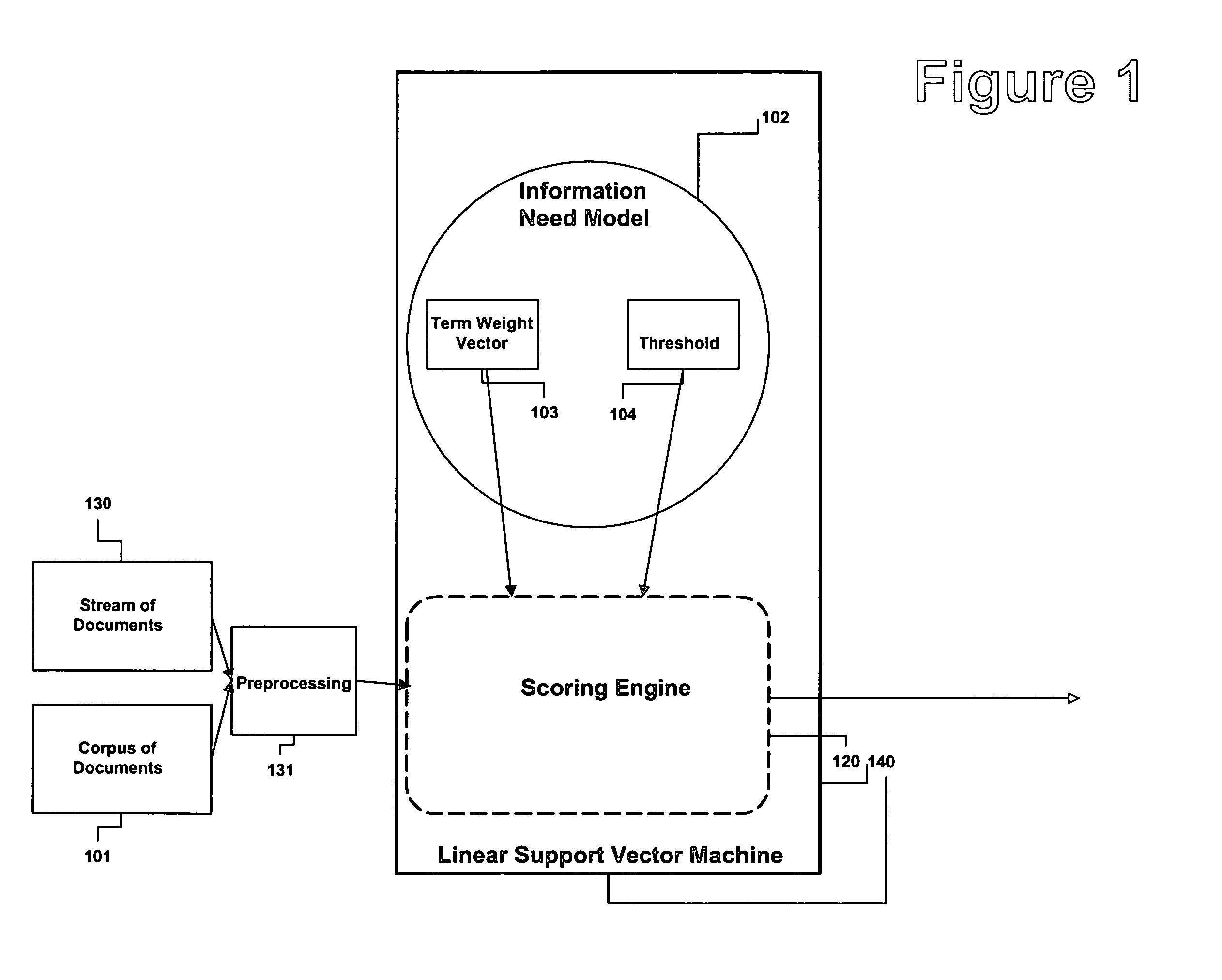
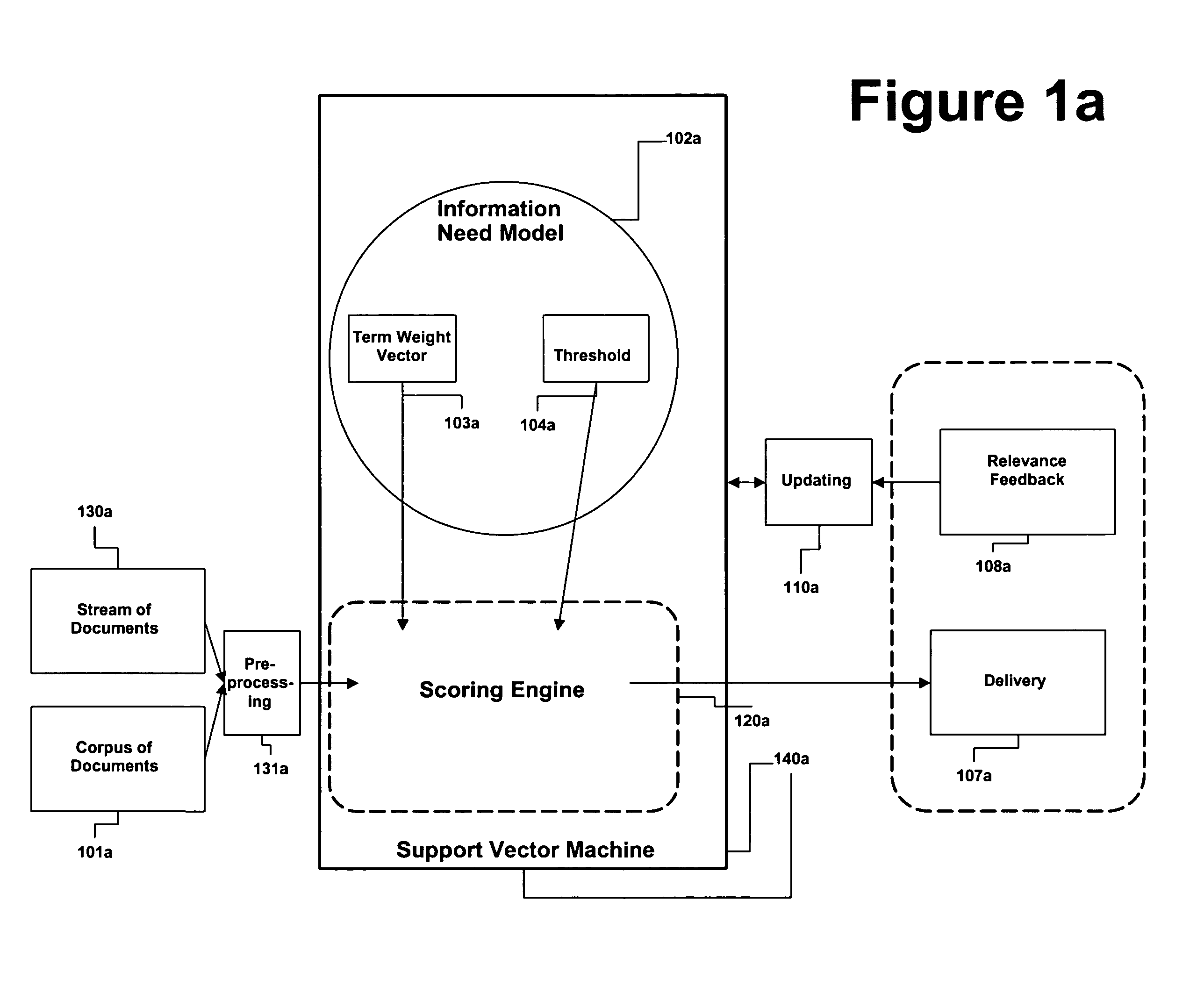
Method and apparatus for adjusting the model threshold of a support vector machine for text classification and filtering
InactiveUS20050228783A1Improve abilitiesReduce marginDigital data information retrievalDigital data processing detailsGreek letter betaAlgorithm
An information need can be modeled by a binary classifier such as support vector machine (SVM). SVMs can exhibit very conservative precision oriented behavior when modeling information needs. This conservative behavior can be overcome by adjusting the position of the hyperplane, the geometric representation of a SVM. The present invention describes a couple of automatic techniques for adjusting the position of an SVM model based upon a beta-gamma thresholding procedure, cross fold validation and retrofitting. This adjustment technique can also be applied to other types of learning strategies.
Owner:JUSTSYST EVANS RES
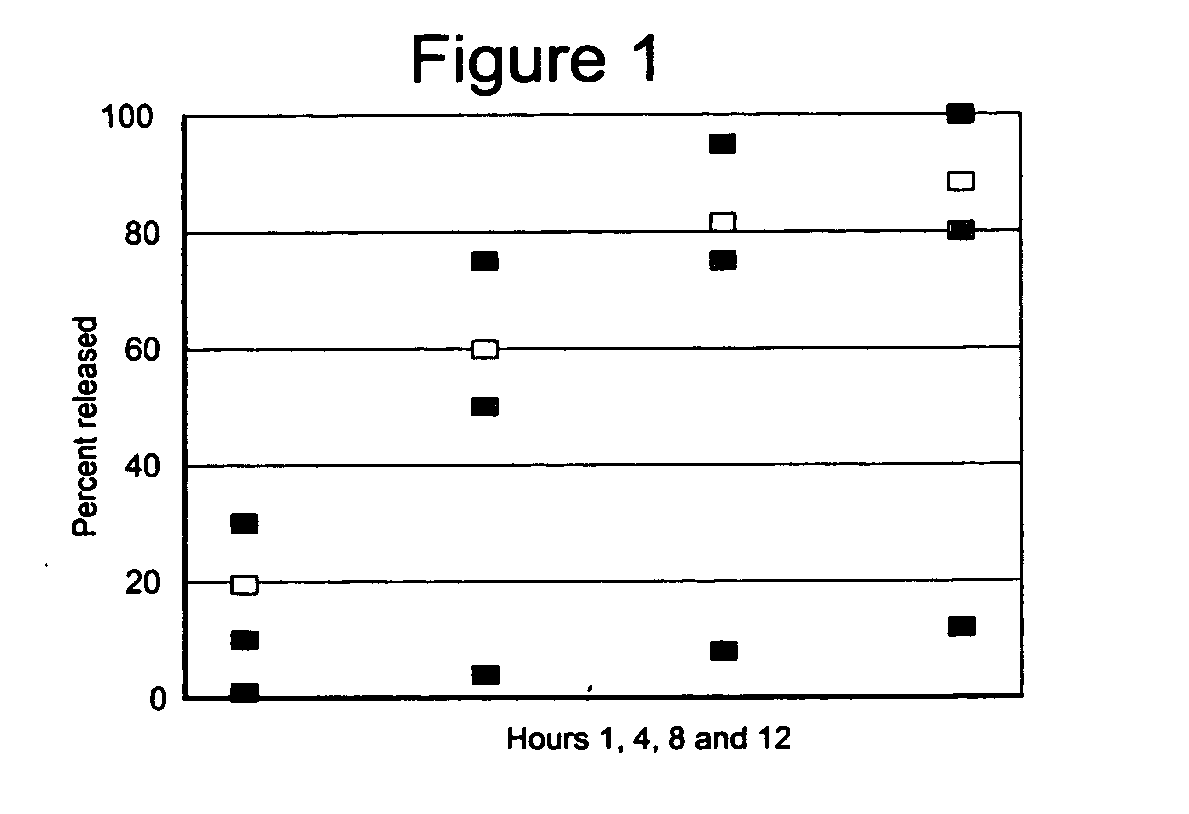
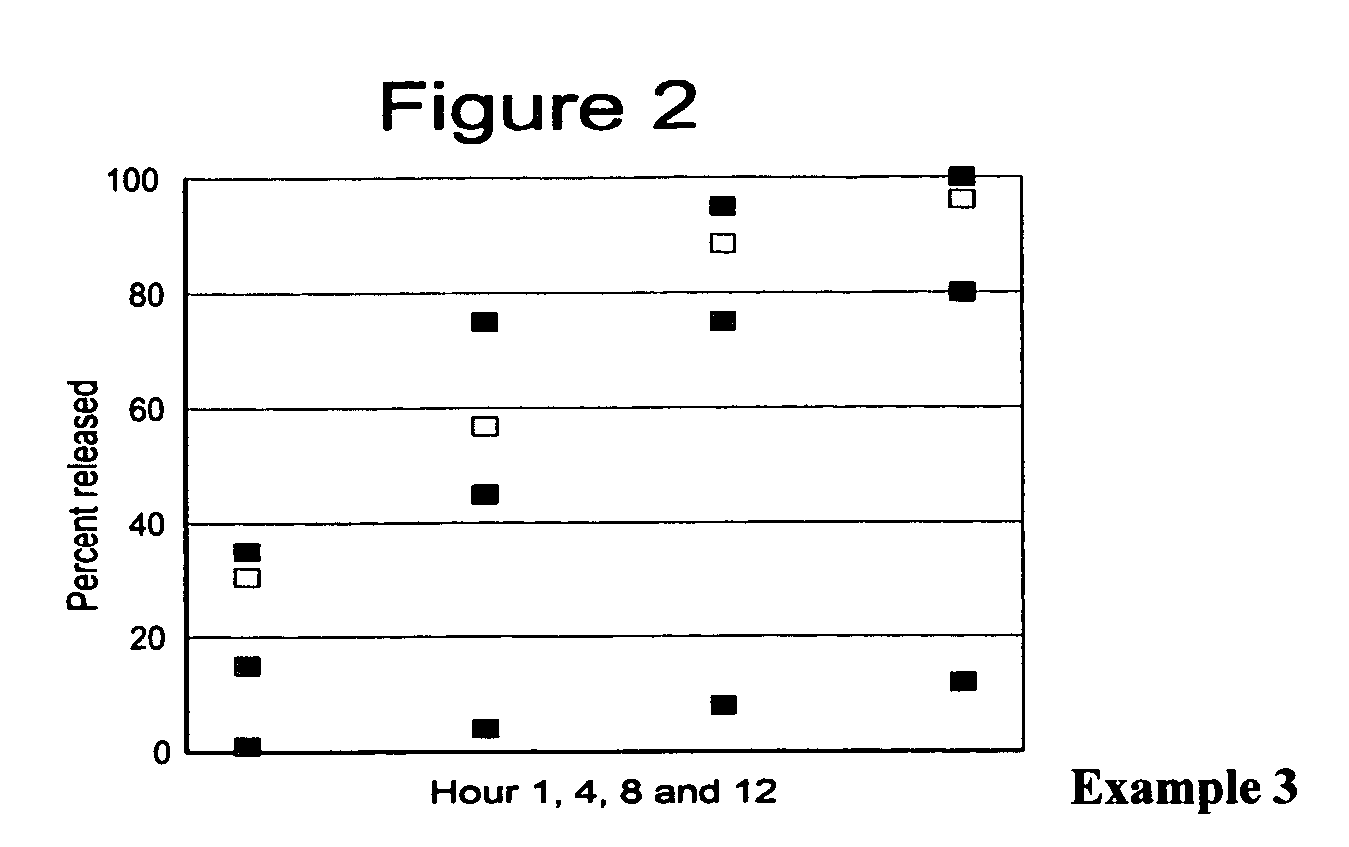
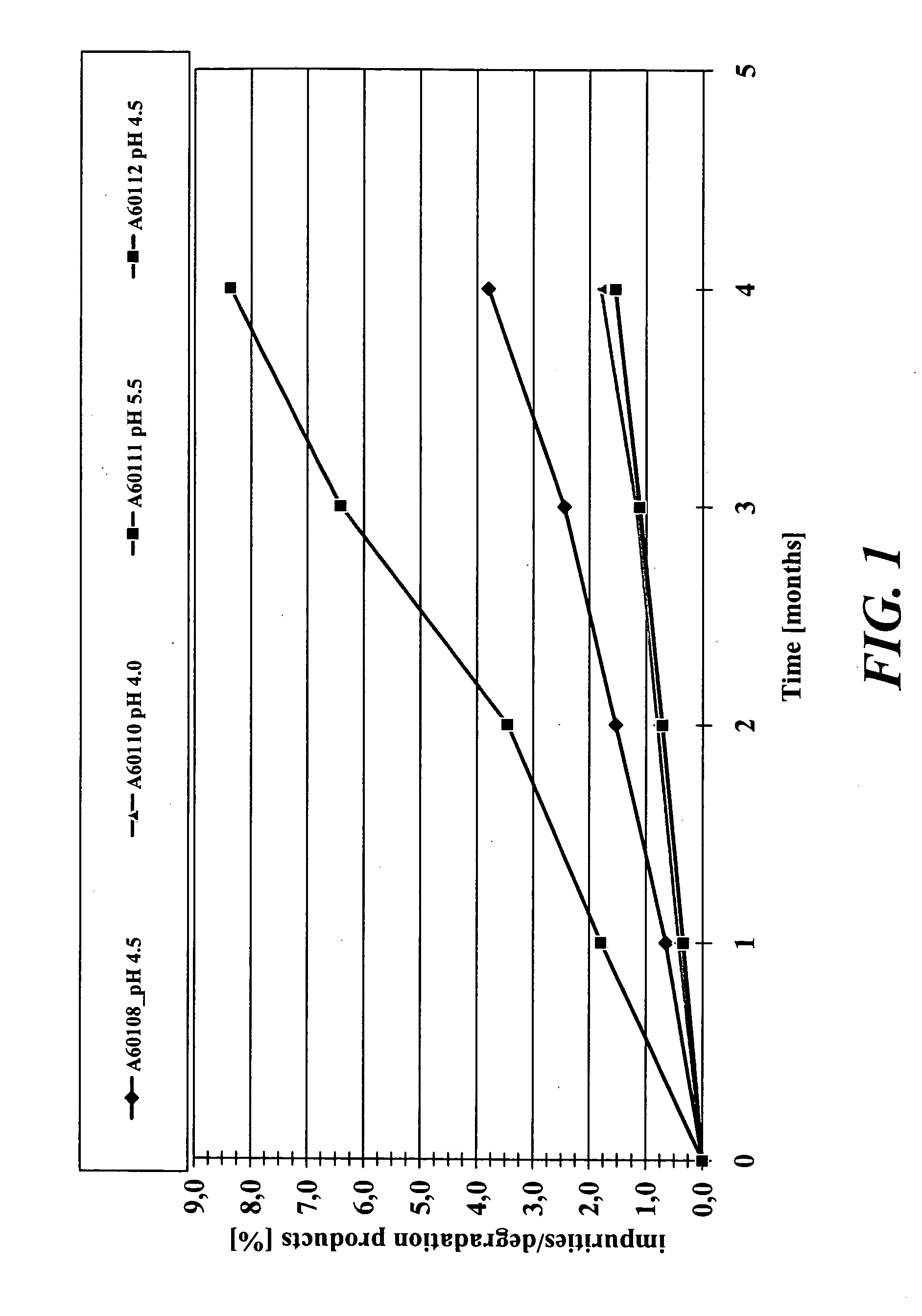
Stabilized extended release pharmaceutical compositions comprising a beta-adrenoreceptor antagonist
InactiveUS20070092573A1Well formedStrengthen matrixBiocideOrganic active ingredientsGreek letter betaAdrenergic receptor sites
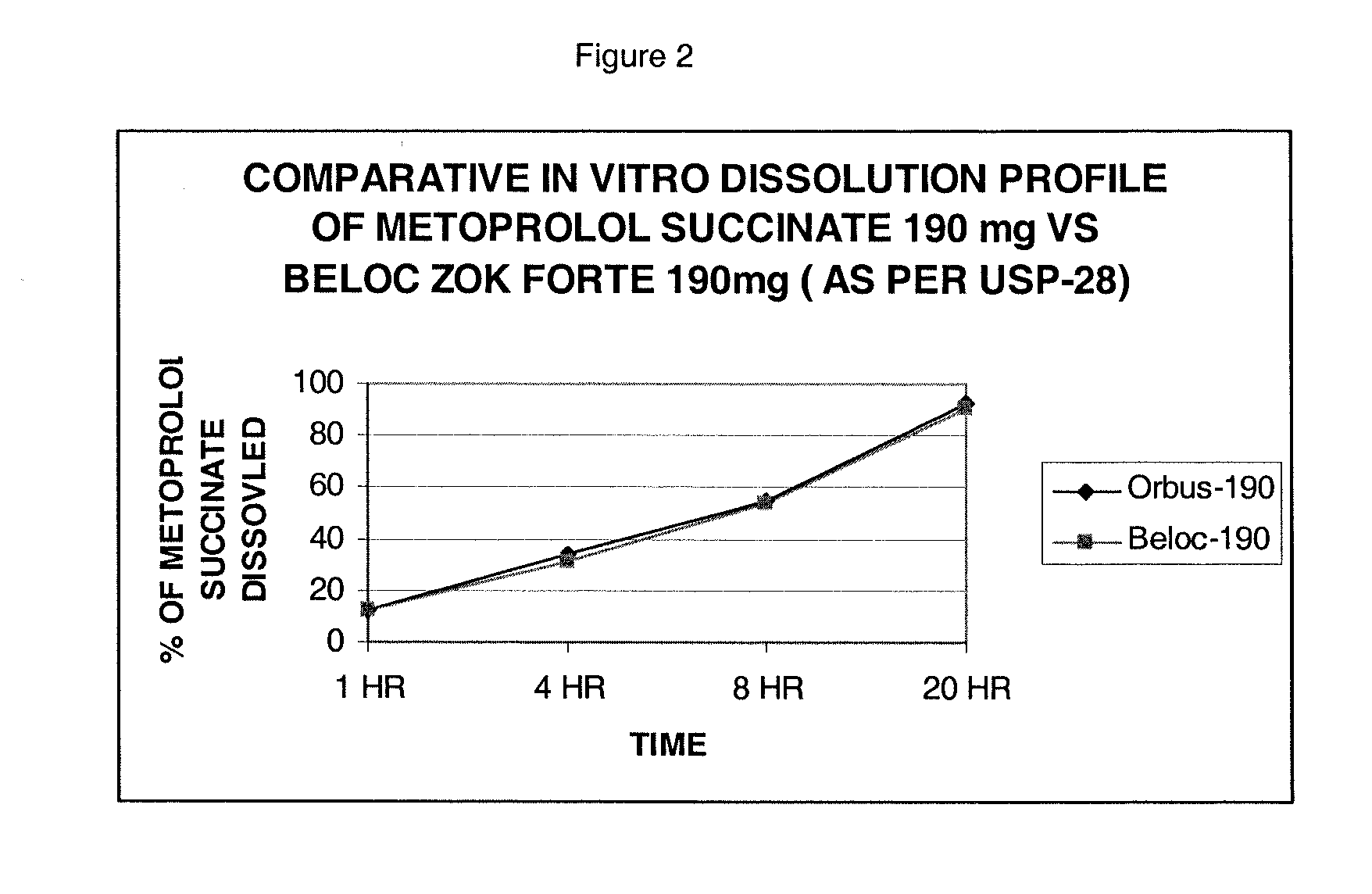
The present invention is a new stable extended release drug composition particularly suitable for use as a beta-adrenoreceptor antagonist agent. The present invention is specifically a drug composition comprising a pharmaceutical, a methacrylic acid copolymer and a matrix forming agent, and a method for manufacturing same. When applied to highly soluble drugs like metoprolol succinate, the resulting drug composition is characterized by an extended-release profile.
Owner:ORBUS PHARMA INC
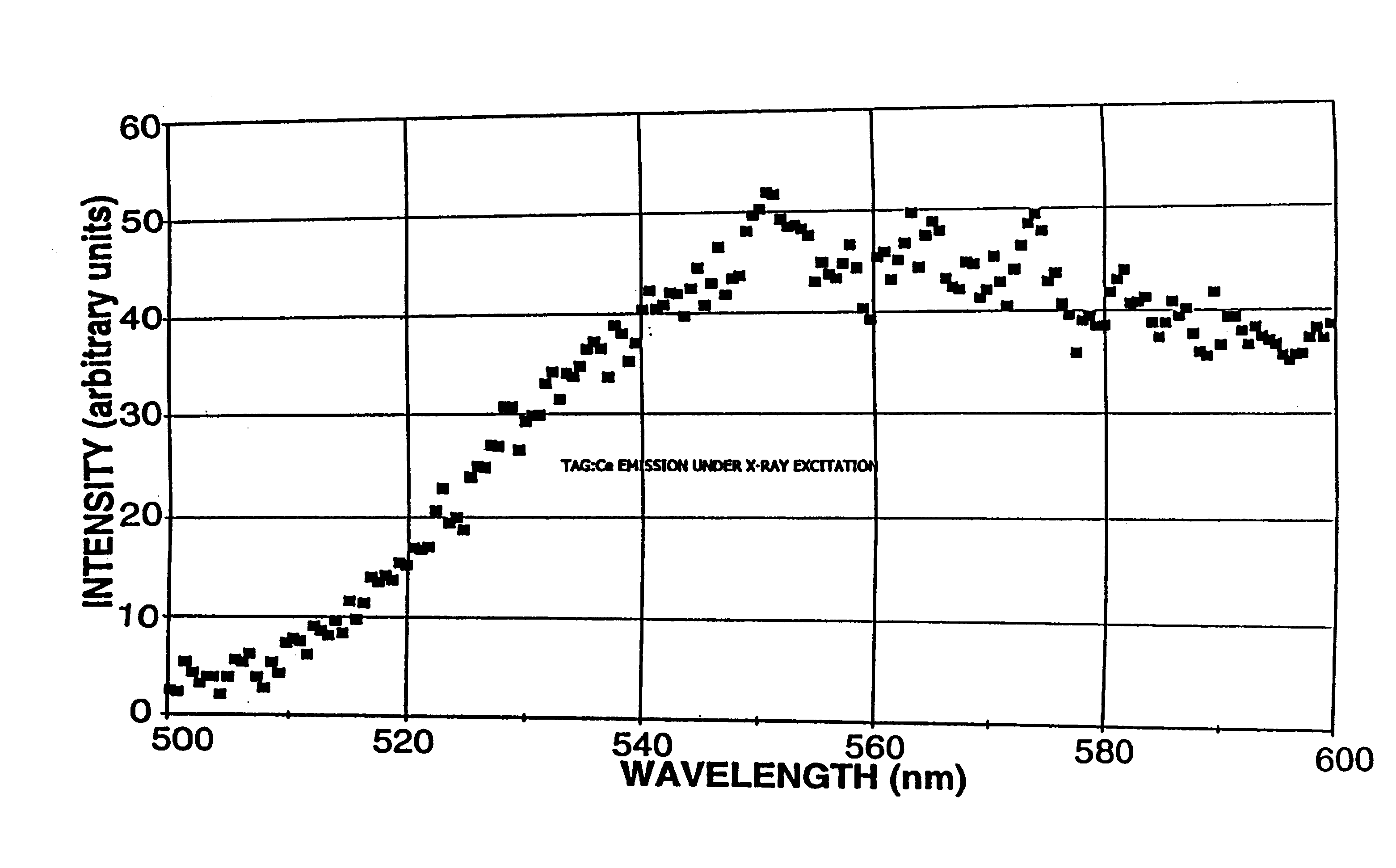
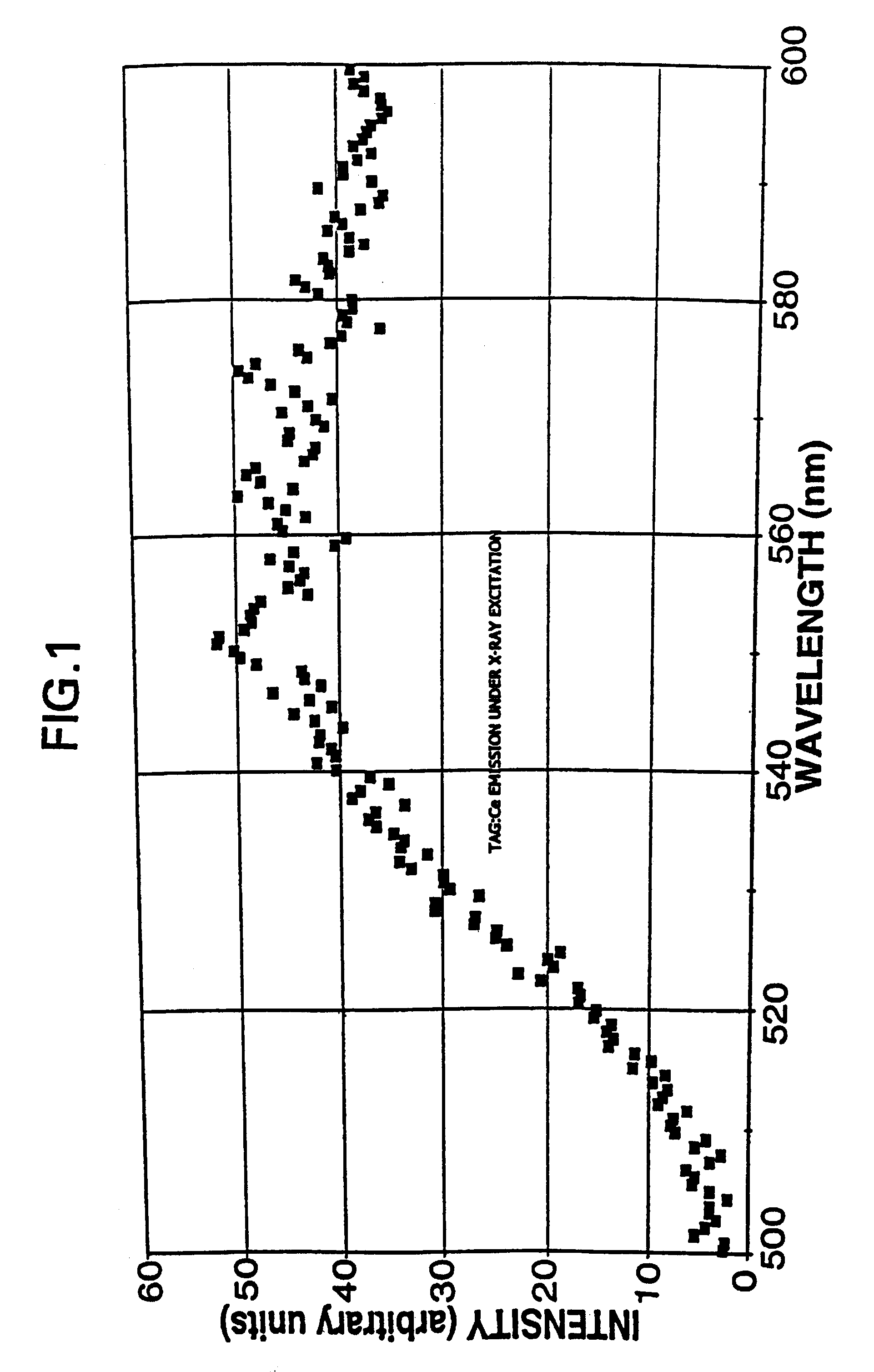
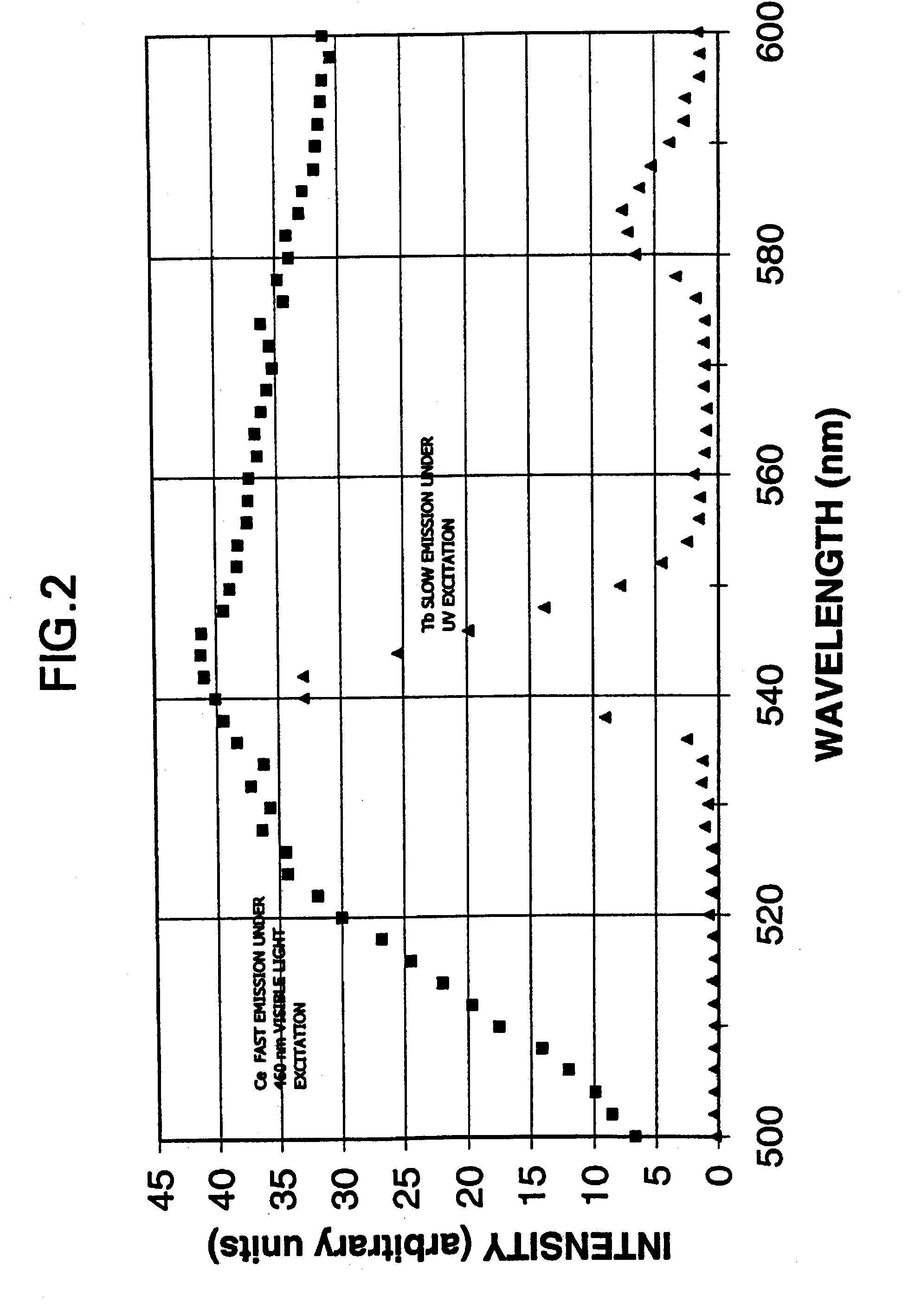
Terbium- or lutetium - containing garnet phosphors and scintillators for detection of high-energy radiation
InactiveUS6630077B2Improve light outputShort decay timePolycrystalline material growthMaterial analysis using wave/particle radiationLutetiumHigh energy
Owner:GENERAL ELECTRIC CO
RNA interference mediated inhibition of TGF-beta and TGF-beta receptor gene expression using short interfering nucleic acid (siNA)
InactiveUS20050227936A1Improves various propertyImprove the immunitySugar derivativesGenetic material ingredientsGreek letter betaDouble strand
This invention relates to compounds, compositions, and methods useful for modulating transforming growth factor beta (TGF-beta) and / or transforming growth factor beta receptor (TGF-betaR) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of TGF-beta and / or TGF-betaR gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of TGF-beta and / or TGF-betaR genes.
Owner:SIRNA THERAPEUTICS INC
Compositions and methods for timed release of water-soluble nutritional supplements
InactiveUS20050181047A1Great flexibility in designingIncrease in plasma levelSulfur/selenium/tellurium active ingredientsFood shapingGreek letter betaGlucosamine Sulfate
The present invention relates to compositions of and methods for producing timed or retarded release formulations that contain glucosamine sulfate, beta-(1,4)-2-amino-2-deoxy-D-glucose, and chondroitin, (C14H19NO14SNa2)n; N-acetylchondrosanine (2-acetamide-2-deoxi-D-galactopiranose) and D-gluoronic acid copolymer and / or their dietary and nutraceutically acceptable salts of the same and / or hydrates of the active substance that provide a timed release formulation of the active substance.
Owner:OSMOPHARM USA
Modulators of 11-beta hydroxyl steroid dehydrogenase type 1, pharmaceutical compositions thereof, and methods of using the same
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1 and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1.
Owner:INCYTE
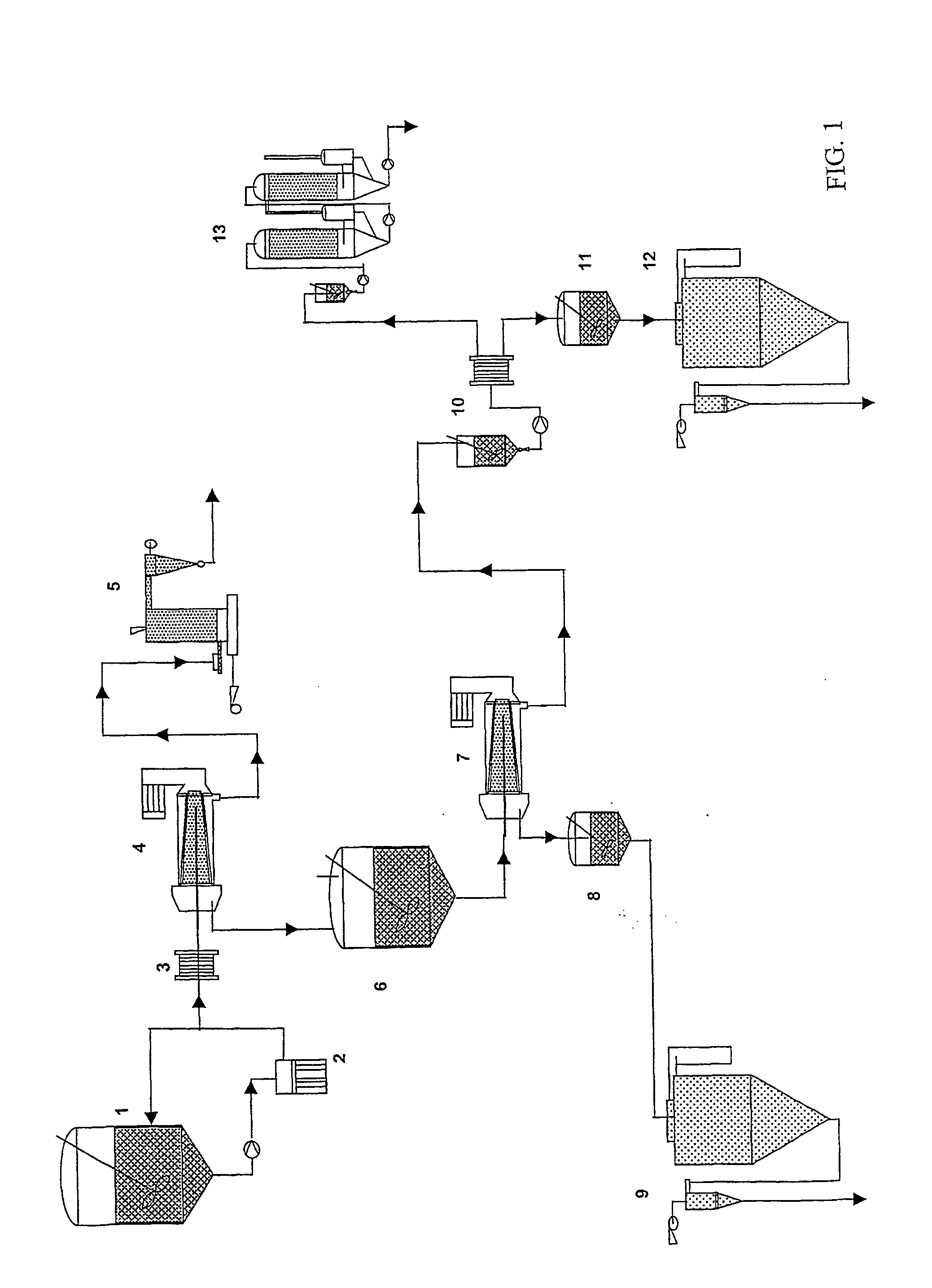
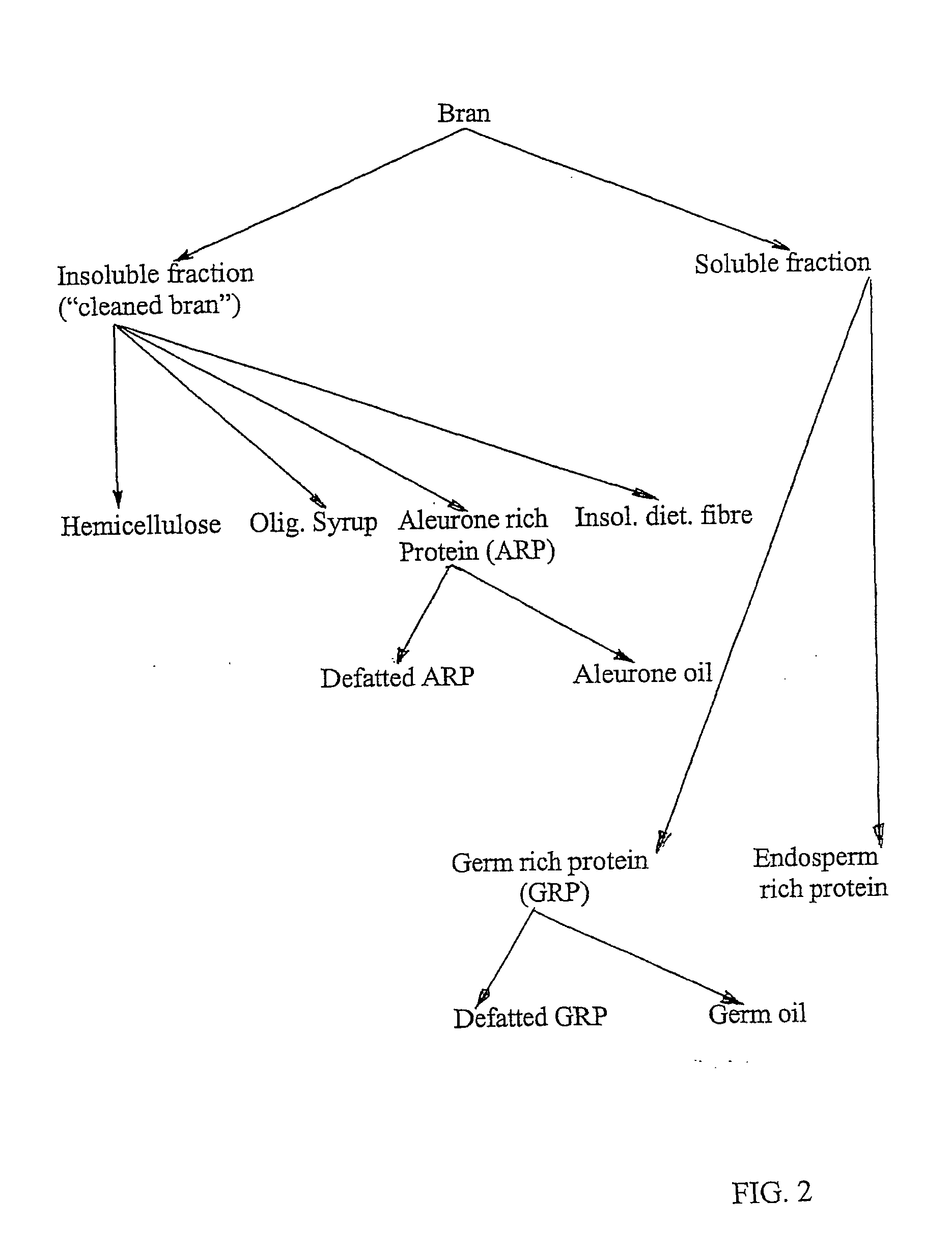
Process for the fractionation of cereal brans
InactiveUS20050089602A1Minimal contaminationImprove efficiencyTea extractionProtein composition from vegetable seedsUltrafiltrationHordeum vulgare
A process for the fractionation of valuable fractions from cereal brans (e.g. wheat, barley and oat brans, and rice polish) is described. In particular, this invention describes a two step process, in which the said bran is first subjected to a combination of enzymatic treatment and wet milling, followed by sequential centrifugation and ultrafiltration, which aims at physically separating the main bran factions, i.e. insoluble phase (pericarp and aleurone layer), germ-rich fraction, residual endosperm fraction and soluble sugars. A second step consists of fractionating cereal brans substantially free of soluble compounds, hence insoluble phase from the above-mentioned first step, by enzymatic treatment with xylanases and / or beta-glucanase and wet milling, followed by sequential centrifugation and ultrafiltration, which aims at physically separating the main fractions, i.e. insoluble phase (remaining cell wall components), protein-rich fraction, soluble hemicellulose and oligosaccharide, and therefore maximizes the extraction rate of valuable cell wall components and aleurone cells from previously cleaned bran.
Owner:LANTMANNEN OATS AB
Solution forms of cyclodextrins for nasal or throat delivery of essential oils
This invention further relates to a method for preventing or treating diseases or conditions of the oral cavity, throat or nose of warm-blooded animals including humans. More particularly, the invention pertains to a composition and method for spraying essential oils to the oral cavity, throat or nasal mucosa as cyclodextrin inclusion complexes. The spray composition includes a cyclodextrin in an amount of from about 0.1% w / v to about 20% w / v; at least one essential oil in an amount of from about 0.001% w / v to about 5.0% w / v; an effective amount of an antimicrobial preservative composition; and water. The composition may further comprise an alcohol co-solvent, a thickening agent, a sweetener, an antitussive, an anticholinergic, a decongestant, an antihistamine, an astringent, an anti-inflammatory steroid composition, a vitamin, a respiratory stimulant, a mucolytic agent, a bronchodilator, a beta-antagonist, an antidiarrheal agent, or combinations thereof.
Owner:QPHARMA
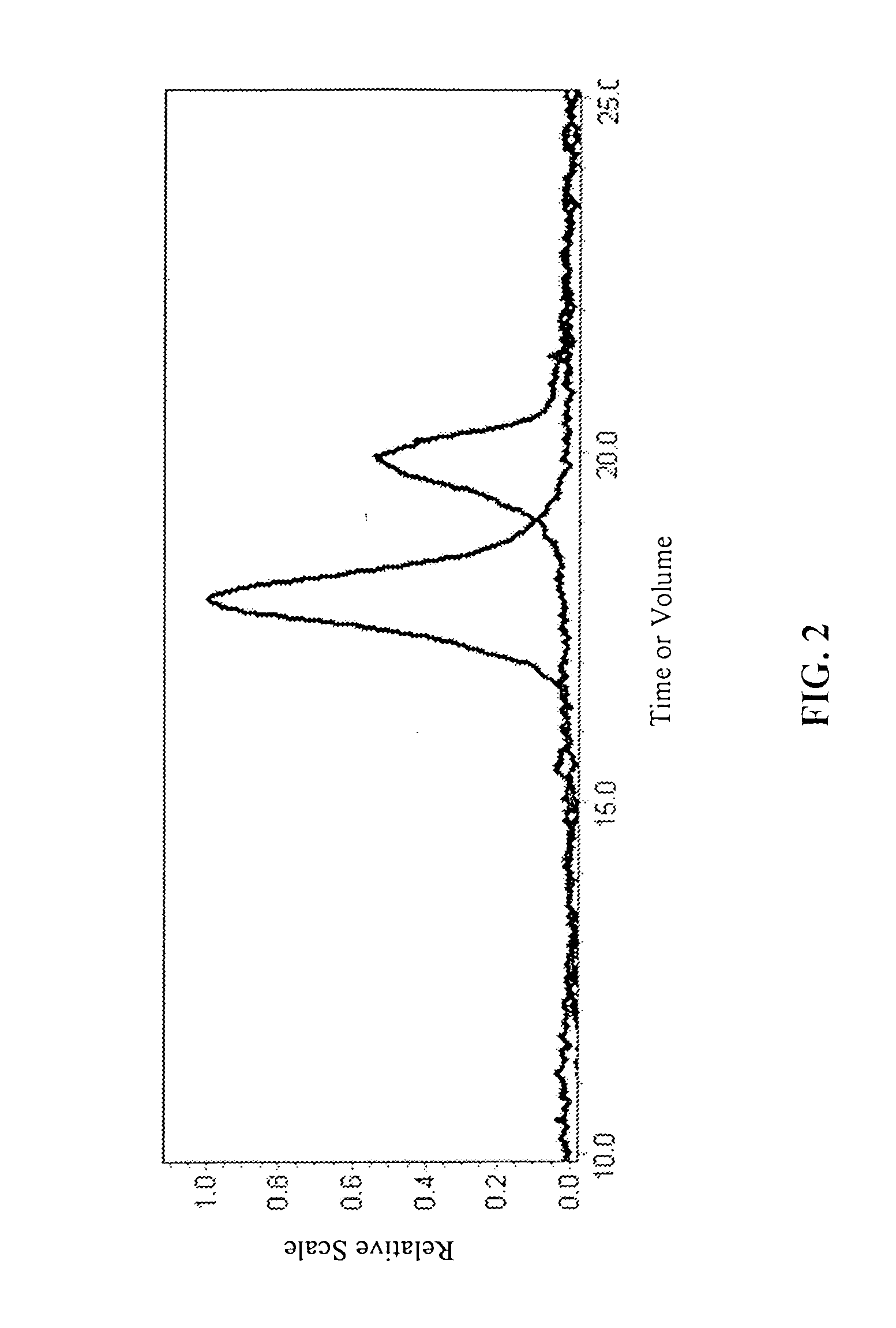
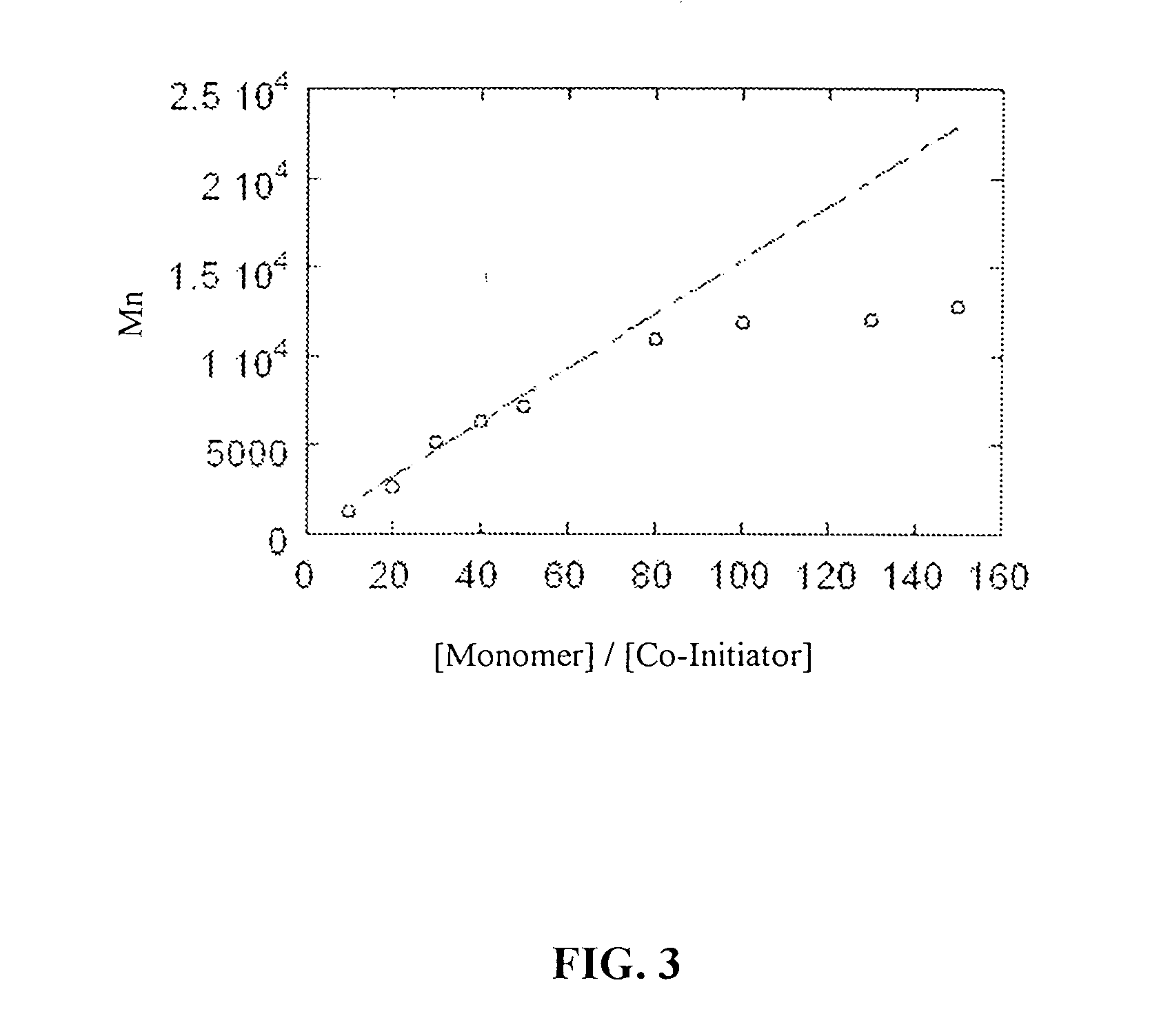
Poly-beta-peptides from functionalized beta-lactam monomers and antibacterial compositions containing same
InactiveUS20070087404A1Control over polymerization conditionLarge molecular weightAntibacterial agentsPeptide/protein ingredientsMonomerTetrahydrofuran
Disclosed is a method of making β-polypeptides. The method includes polymerizing β-lactam-containing monomers in the presence of a base initiator and a co-initiator which is not a metal-containing molecule to yield the product β-polypeptides. Specifically disclosed are methods wherein the base initiator is potassium t-butoxide, lithium bis(trimethylsilyl)amide (LiN(TMS)2), potassium bis(trimethyl-silyl)amide, and sodium ethoxide, and the reaction is carried out in a solvent such as chloroform, dichloromethane, dimethylsulfoxide, or tetrahydrofuran.
Owner:WISCONSIN ALUMNI RES FOUND
Antibacterial and anti-inflammatory oral care composition
ActiveUS20060134024A1Inhibiting plaque formationMaintain Oral HealthBiocideCosmetic preparationsMagnololToothpaste
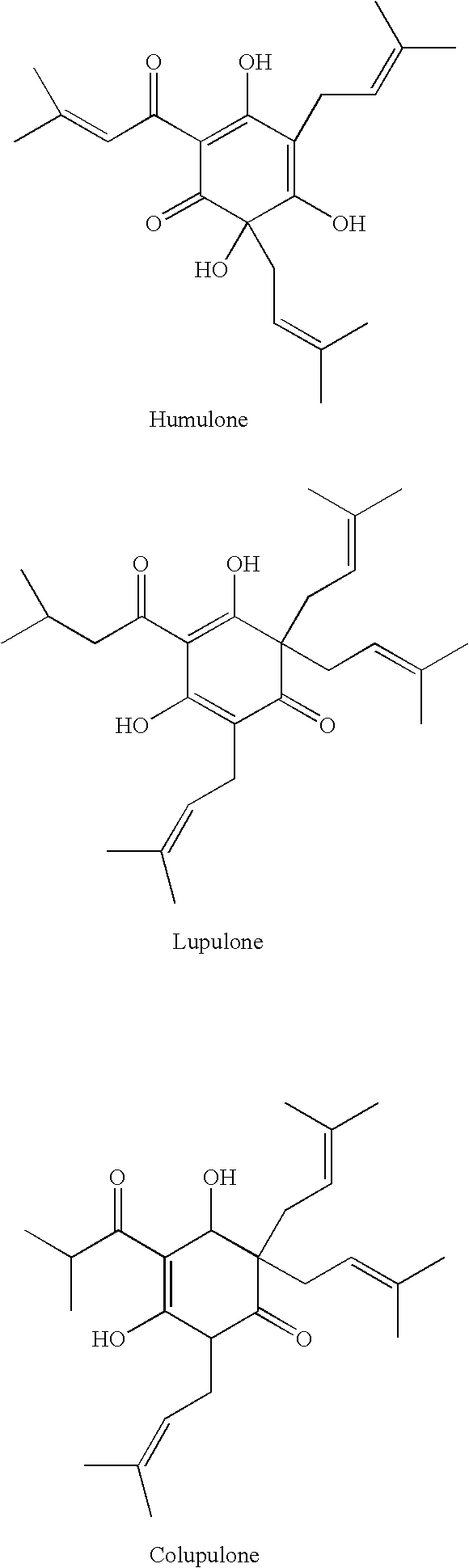
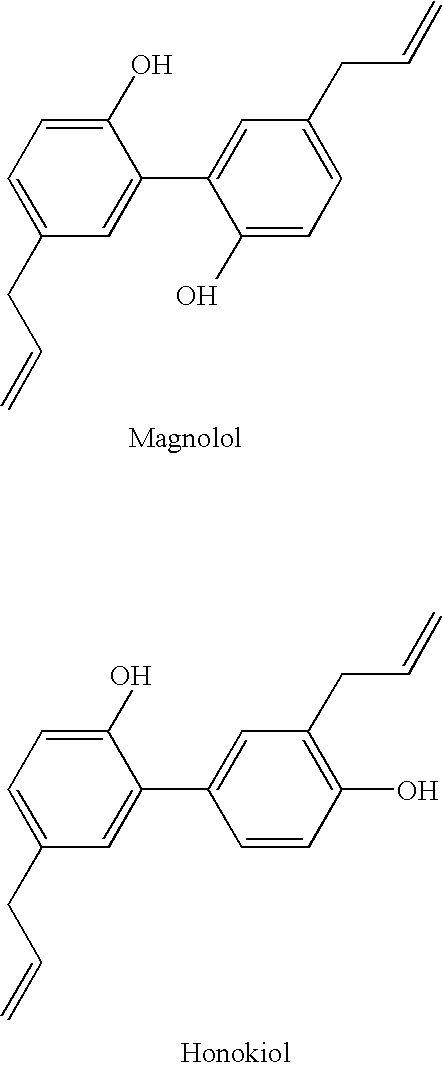
An efficacious antibacterial and anti-inflammatory oral composition is provided having an active ingredient combination comprising one or more active compounds from an extract of magnolia and an extract of hops. Preferably, the active compounds from magnolia extract comprise honokiol and magnolol, and the active compounds from hops extract comprise hexahydrogenated beta acids. The oral composition can be in the form of a mouth rinse or dentifrice, including toothpaste, gels, powders, confectionaries, lozenges, animal products, and the like. Methods of making and using the oral composition are also provided.
Owner:COLGATE PALMOLIVE CO
RNA interference mediated inhibition of TGF-beta and TGF-beta receptor gene expression using short interfering nucleic acid (siNA)
InactiveUS20050287128A1Improve bioavailabilityMinimize the possibilityBiocideGenetic material ingredientsDouble strandOrganism
This invention relates to compounds, compositions, and methods useful for modulating TGF-beta and / or TGF-betaR gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of TGF-beta and / or TGF-betaR gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of TGF-beta and / or TGF-betaR genes. Such small nucleic acid molecules are useful, for example, for treating, preventing, inhibiting, or reducing inflammatory, respiratory, autoimmune, and / or proliferative diseases, disorders, conditions, or traits in a cell, subject or organism and any other disease, condition, trait or indication that can respond to the level of TGF-beta and / or TGF-betaR in a cell or tissue; or alternately in providing long term hematopeitic reconstitution in a subject or organism.
Owner:SIRNA THERAPEUTICS INC
Perfume for capsule composition
InactiveUS20070149424A1Improving capsule hardnessCosmetic preparationsToilet preparationsGreek letter betaFlavor
The invention relates to a core shell capsule containing in the core an oil or waxy solid, wherein the oil or waxy solid comprises: (1) 50-100% by weight of a perfume composition, which is a mixture of at least two perfume ingredients-selected from: a) aldehydes, including alpha beta unsaturated aldehydes, which constitute 0-20% by weight of the perfume composition; b) primary or secondary amines constituting 0-10% by weight of the perfume composition; c) perfume ingredients having ClogP>4.0, which constitute 0-25% by weight of the perfume composition; d) perfume ingredients having ClogP>5.0, which constitute 0-20% by weight of the perfume composition; and e) perfume ingredients having ClogP<2.0, which constitute 0-20% by weight of the perfume composition, and (2) 0-50% by weight of benefit agents other than perfume ingredients.
Owner:TAKASAGO INTERNATIONAL CORPORATION
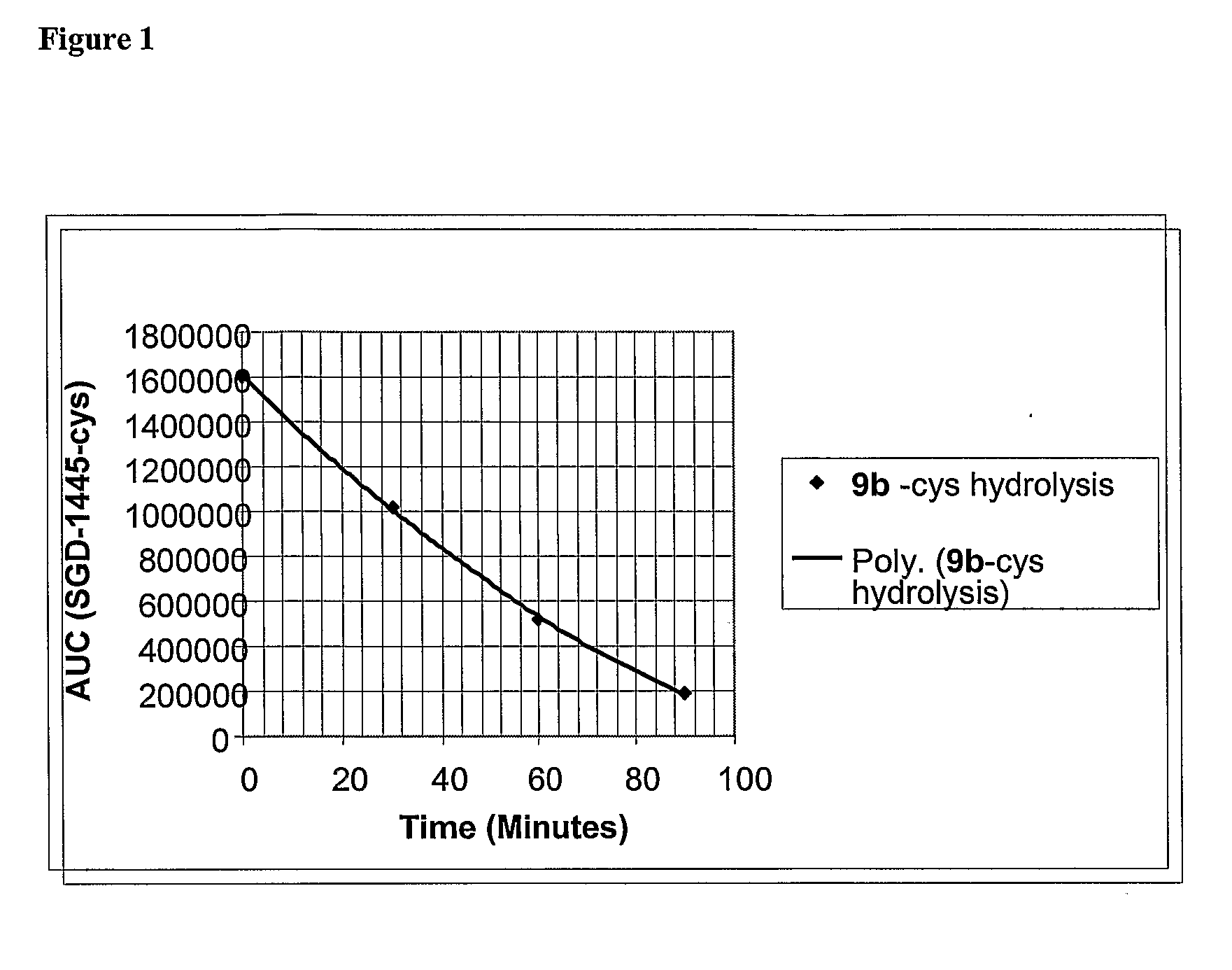
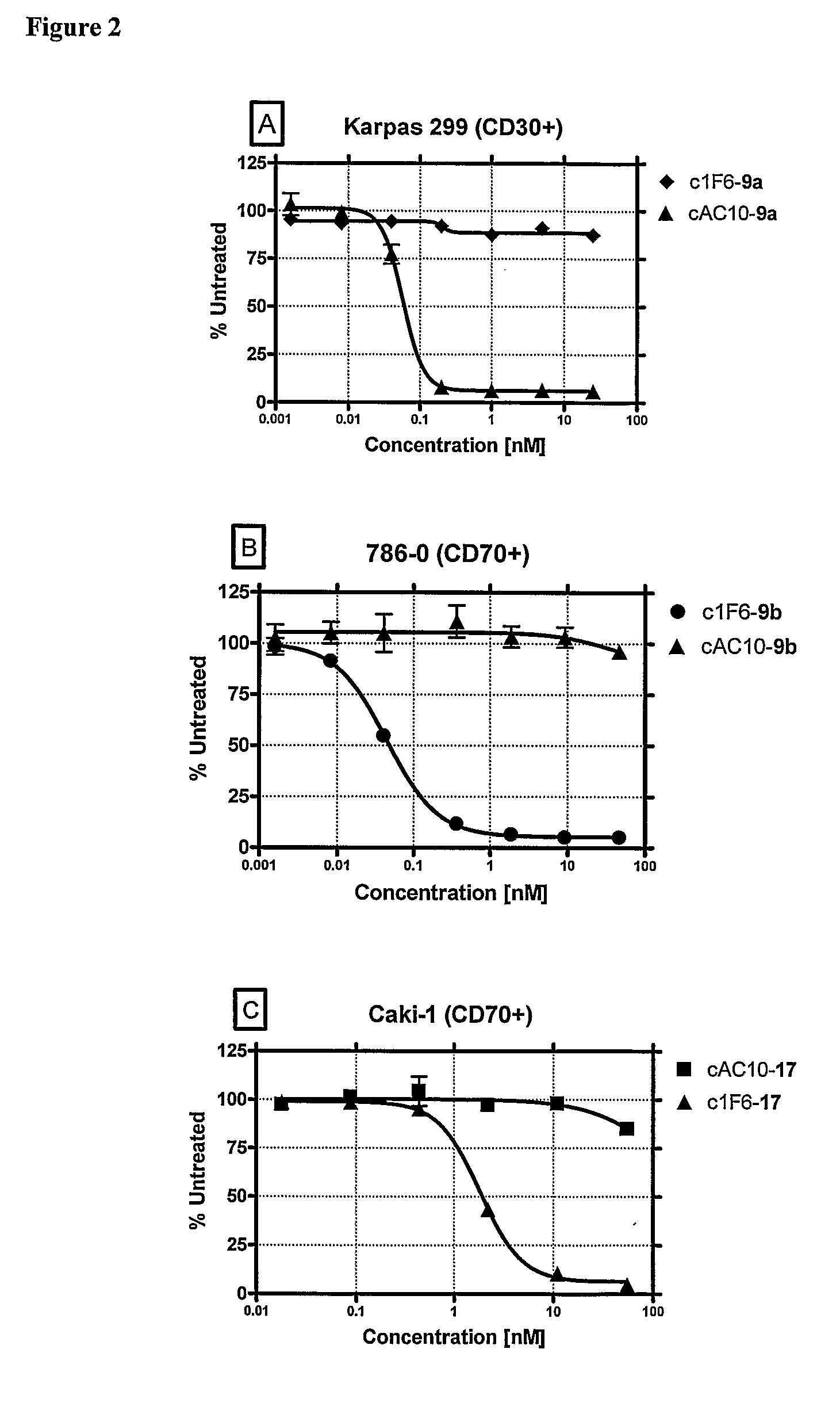
Beta-Glucuronide-Linker Drug Conjugates
ActiveUS20080241128A1Effective treatmentKill or inhibit the proliferation of the tumor cellsTetrapeptide ingredientsTissue cultureGreek letter betaDrug conjugation
Ligand Drug conjugate compounds comprising a β-glucuronide-based linker and methods of using such compounds are provided.
Owner:SEAGEN INC
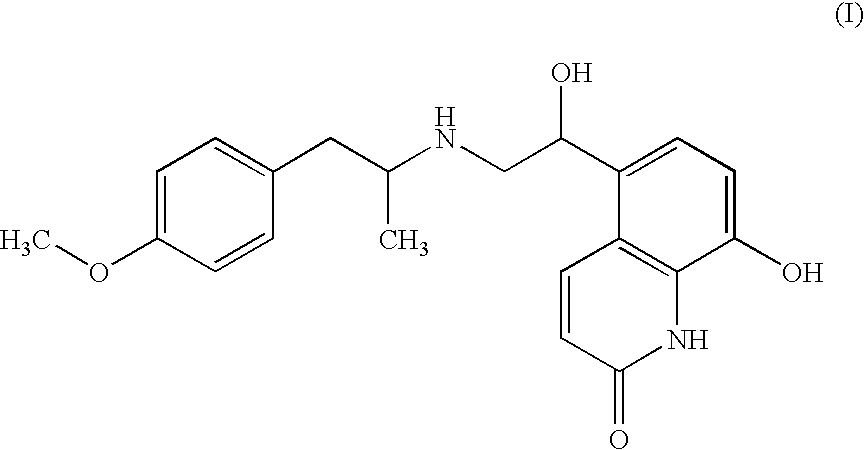
Pharmaceutical formulations comprising a long-acting beta2-agonist for administration by nebulisation
The invention relates to a liquid, propellant-free pharmaceutical formulation in the form of ready-to-use preparation for administration by nebulisation comprising a water soluble salt of the beta2-agonist 8-hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]-2(1H)-quinolinone as active ingredient. The active ingredient is chemically stable in the formulation, and said formulation is provided of an adequate shelf-life suitable for commercial distribution, storage and use.
Owner:CHIESI FARM SPA
Humanized antibodies that recognize beta amyloid peptide
InactiveUS20060280743A1Reducing plaque burdenReduce the burden onAnimal cellsNervous disorderGreek letter betaHumanized antibody
The invention provides improved agents and methods for treatment of diseases associated with amyloid deposits of Aβ in the brain of a patient. Preferred agents include humanized antibodies.
Owner:WYETH LLC +1
Beta-nucleation concentrates for film applications
InactiveUS20060177632A1Improve propertiesSynthetic resin layered productsThin material handlingGreek letter betaNucleation
In one aspect, the invention is directed to a concentrate comprising a polypropylene resin and a beta-nucleating agent, wherein the nucleating agent is present in a concentration in the concentrate in a range of from about 0.005% to about 2.0%. In another aspect, the invention is directed to an extruded polypropylene sheet comprising a beta-nucleating agent concentrate in a concentration of between about 0.5% to about 50% by weight of the total polypropylene content. In a further aspect, the invention is directed to a method of manufacture of a beta-nucleating agent / polypropylene concentrate comprising the step of blending a substantially pure beta-nucleating agent with a substantially non-nucleated polypropylene resin to provide a concentration in the concentrate of from about 0.005% to about 2.0%. In yet another aspect, the invention is directed to a film produced by stretching the sheet.
Owner:MAYZO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Amino-5-[4-(difluoromehtoxy)phenyl]-5-phenylimidazolone compounds for the inhibition of beta-secretase Amino-5-[4-(difluoromehtoxy)phenyl]-5-phenylimidazolone compounds for the inhibition of beta-secretase](https://images-eureka.patsnap.com/patent_img/9b458263-f0d6-4bd1-8869-72f2e272686a/US20070072925A1-20070329-C00001.png)
![Amino-5-[4-(difluoromehtoxy)phenyl]-5-phenylimidazolone compounds for the inhibition of beta-secretase Amino-5-[4-(difluoromehtoxy)phenyl]-5-phenylimidazolone compounds for the inhibition of beta-secretase](https://images-eureka.patsnap.com/patent_img/9b458263-f0d6-4bd1-8869-72f2e272686a/US20070072925A1-20070329-C00002.png)
![Amino-5-[4-(difluoromehtoxy)phenyl]-5-phenylimidazolone compounds for the inhibition of beta-secretase Amino-5-[4-(difluoromehtoxy)phenyl]-5-phenylimidazolone compounds for the inhibition of beta-secretase](https://images-eureka.patsnap.com/patent_img/9b458263-f0d6-4bd1-8869-72f2e272686a/US20070072925A1-20070329-C00003.png)