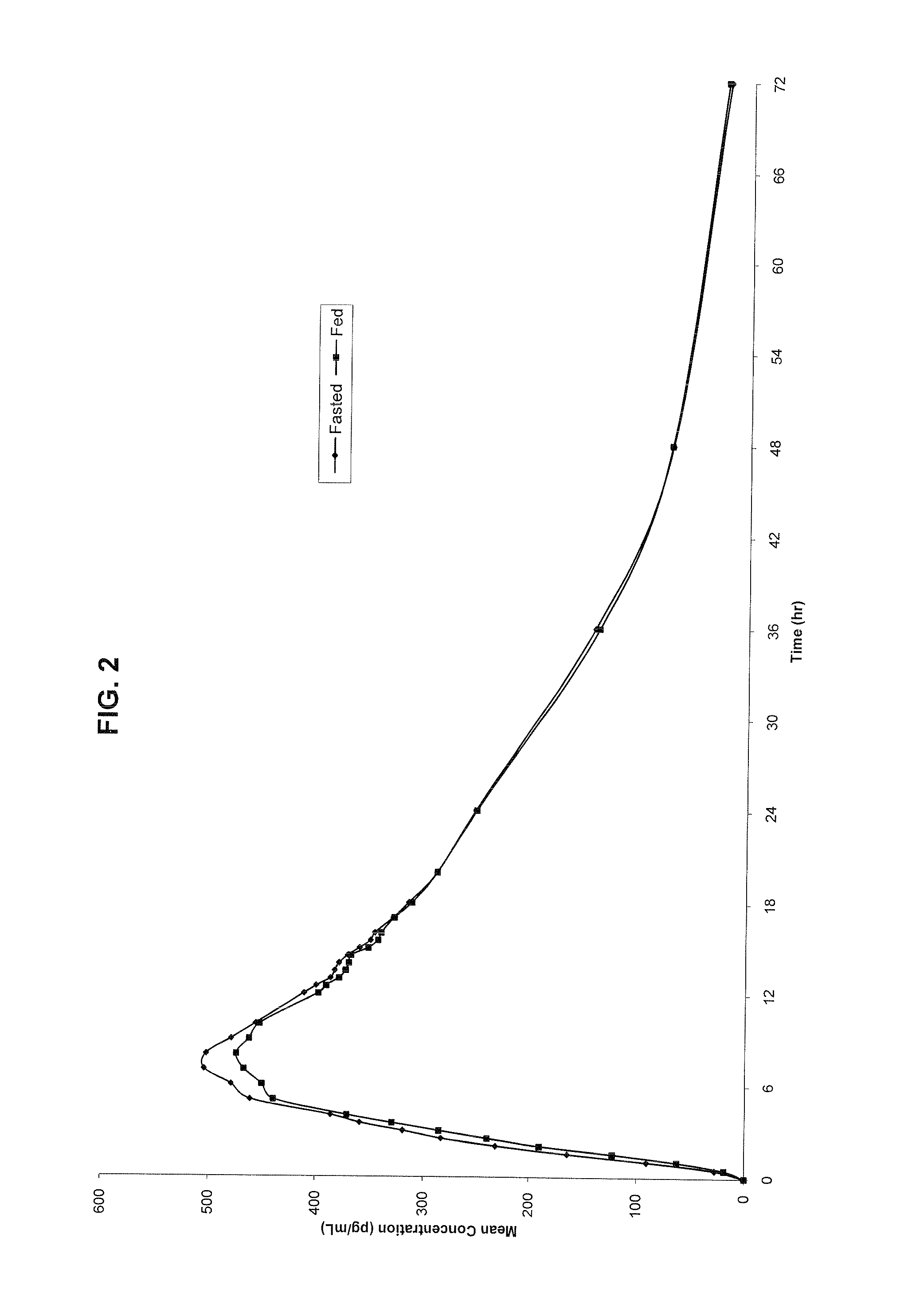
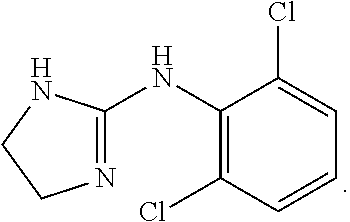
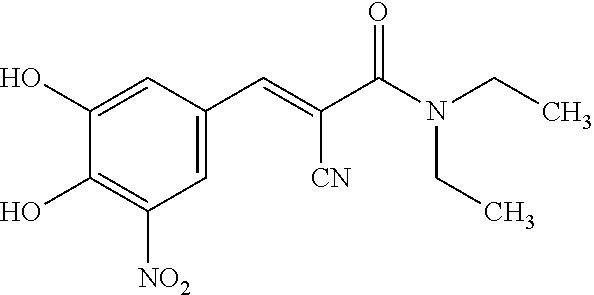
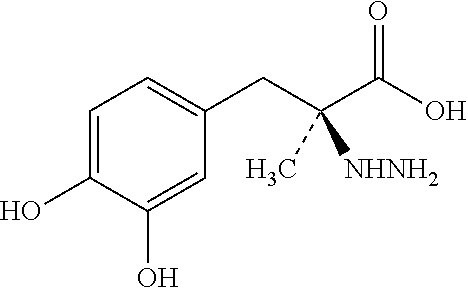
Patents
Literature
598 results about "Extended release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Official Answer. Extended-release means the pill is formulated so that the drug is released slowly over time. This has the advantage of taking pills less often. It also means that there may be fewer side-effects as the levels of the of drug in the body are more consistent in extended- release formulations.
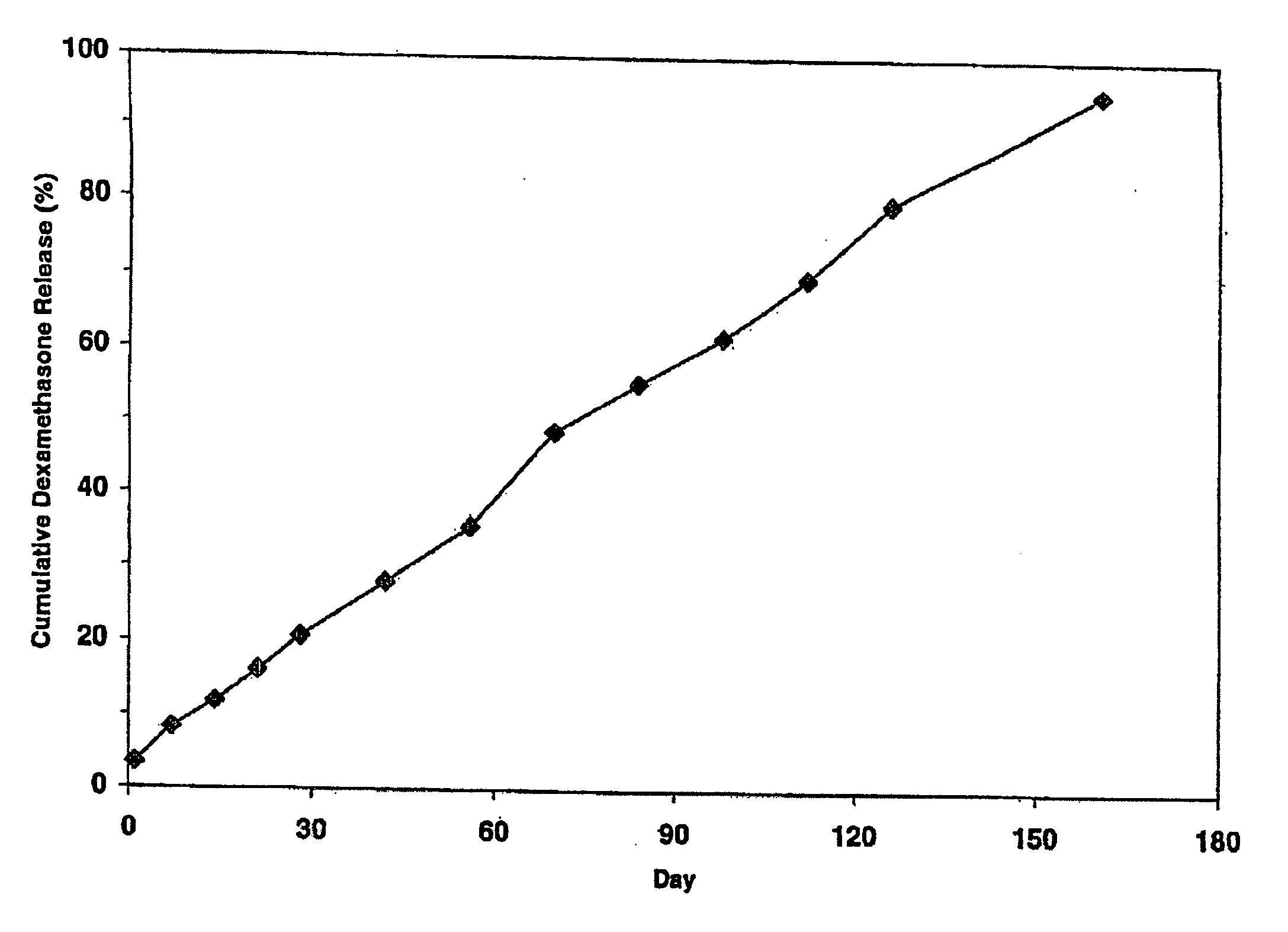
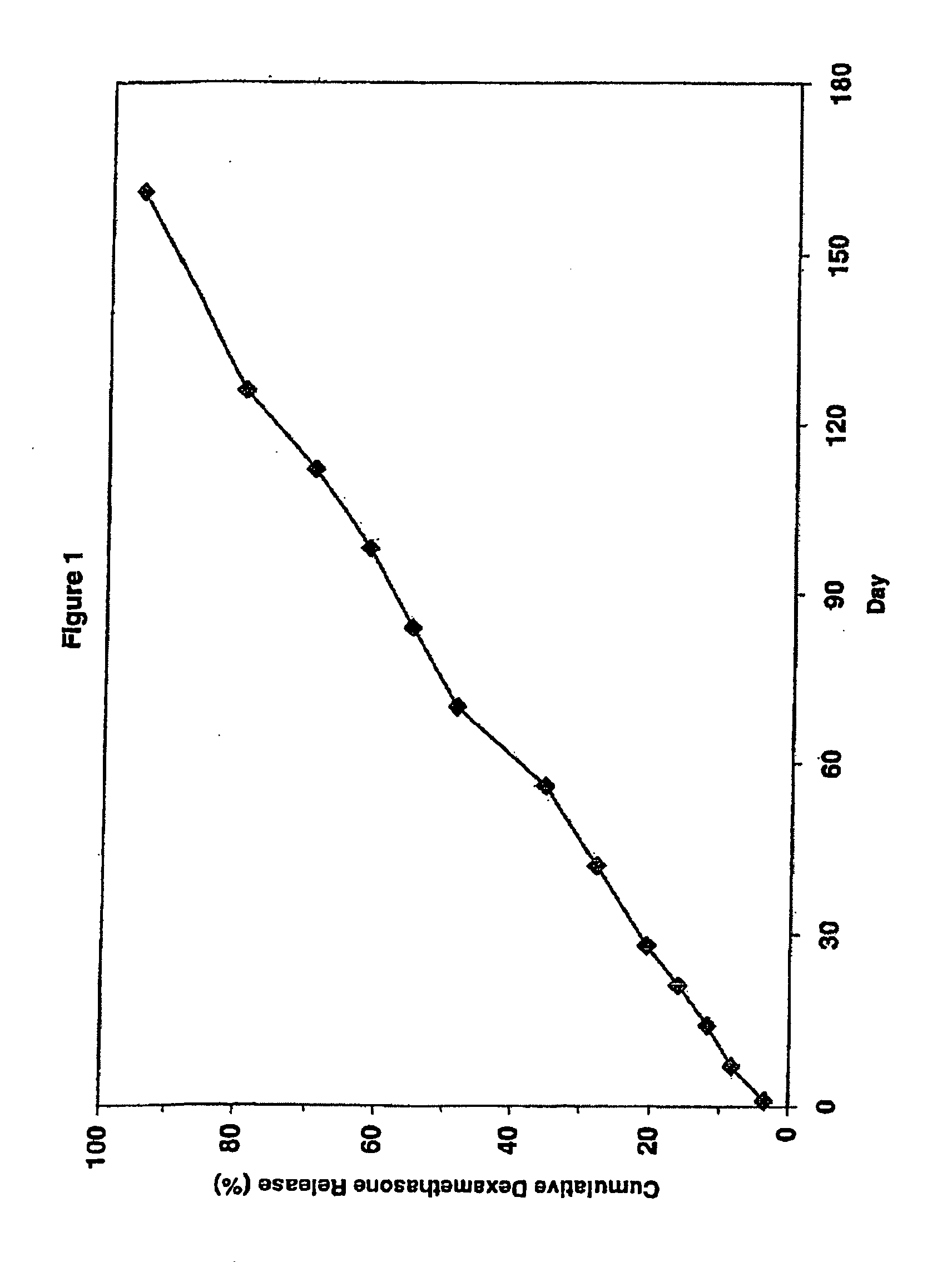
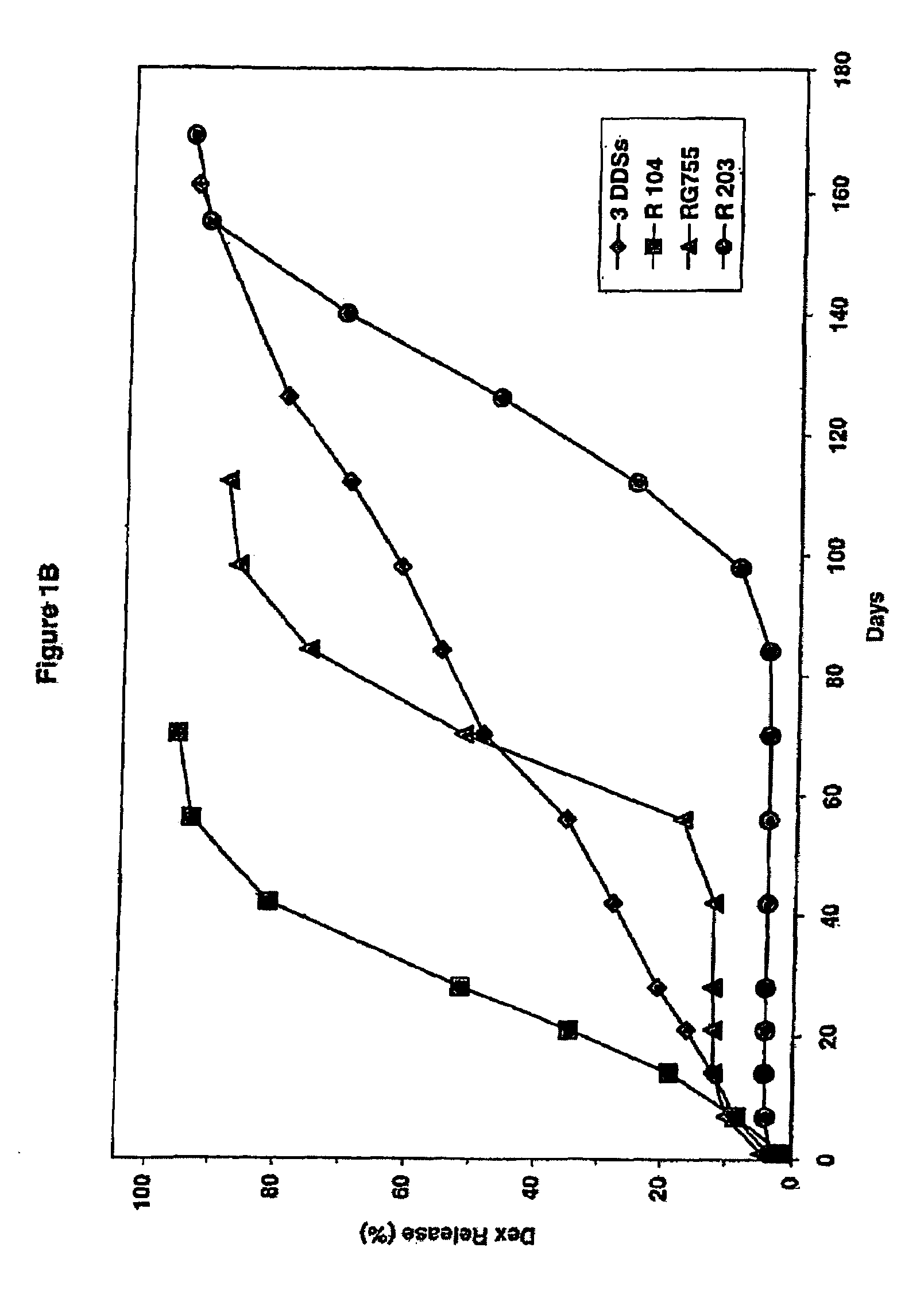
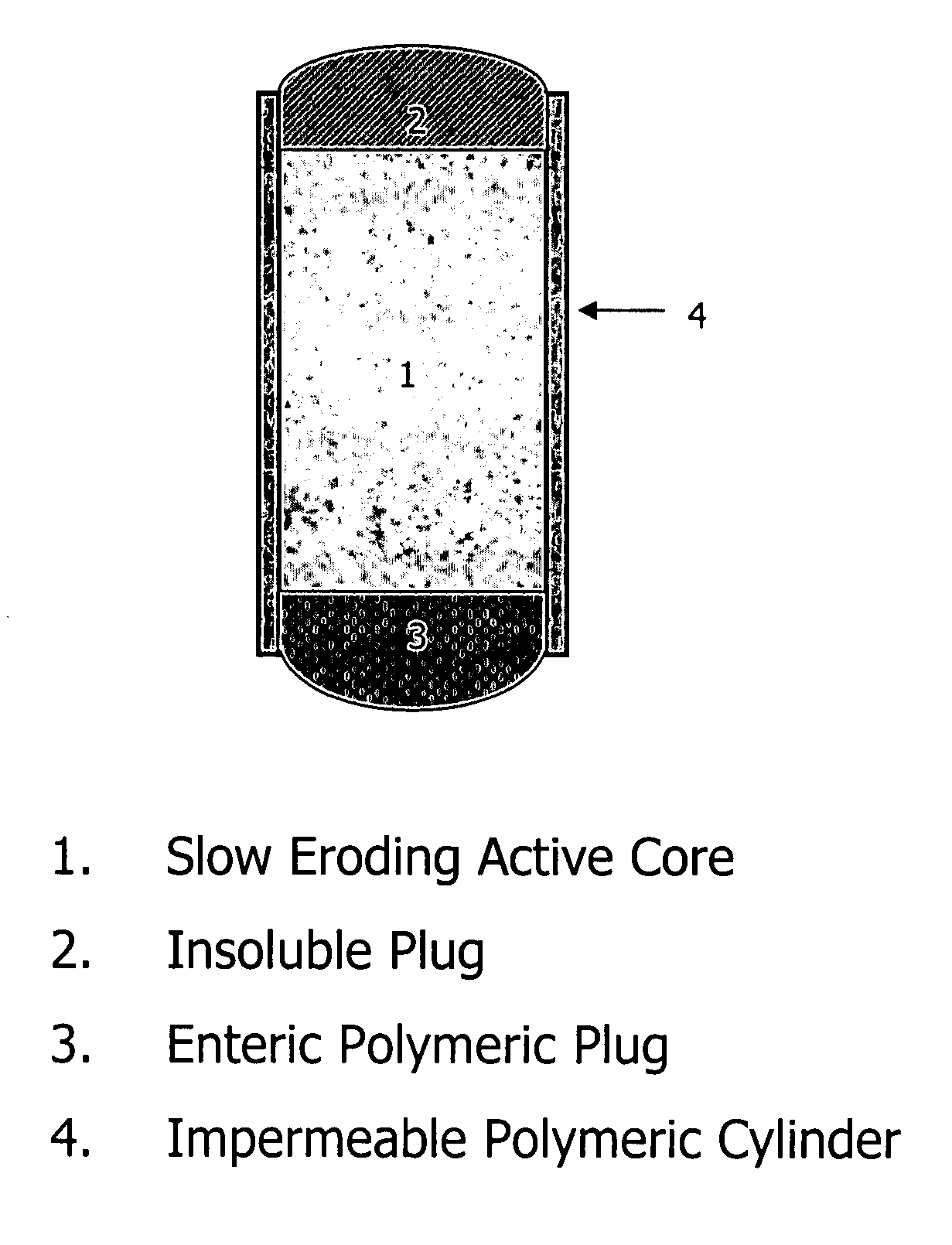
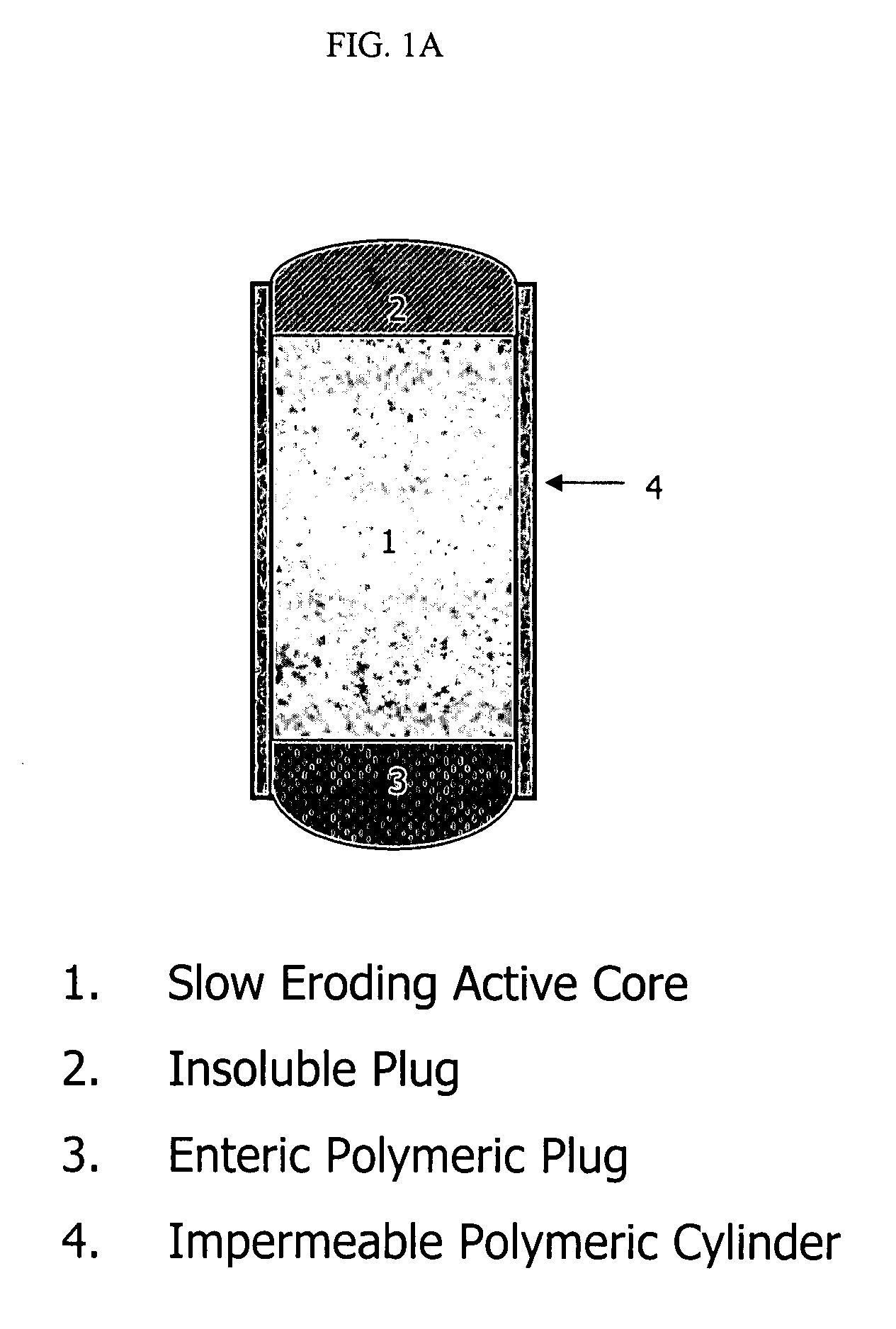
Extended release biodegradable ocular implants
Biodegradable implants sized and suitable for implantation in an ocular region or site and methods for treating ocular conditions. The implants provide an extended release of an active agent at a therapeutically effective amount for a period of time between 50 days and one year, or longer.
Owner:ALLERGAN INC
Dosage forms using drug-loaded ion exchange resins
InactiveUS20050181050A1Avoid breakingPharmaceutical non-active ingredientsPill deliveryOral medicationImmediate release
A multiparticulate, modified release composition for oral administration has been developed. The formulation is made by complexing a drug with an ion-exchange resin in the form of small particles, typically less than 150 microns. The present invention provides novel extended release coated ion exchange particles comprising drug-resin complexes, produced by binding the salt form of the drug, that do not require impregnating agents to insure the integrity of the extended release coat. To prepare a modified release formulation, one or more of the following types of particles are formulated into a final dosage form: (a) Immediate release particles, (b) Enteric coated particles, (c) Extended release particles, (d) Enteric coated-extended release particles; and (e) Delayed release particles. The various drug-containing particles described above can be further formulated into a number of different easy-to-swallow final dosage forms including, but not limited to, a liquid suspension, gel, chewable tablet, crushable tablet, rapidly dissolving tablet, or unit of use sachet or capsule for reconstitution
Owner:COLLEGIUM PHARMA INC
Powder-layered oral dosage forms
InactiveUS6077533AFacilitated releaseMinimize impactPretreated surfacesGranular deliveryImmediate releaseLactose
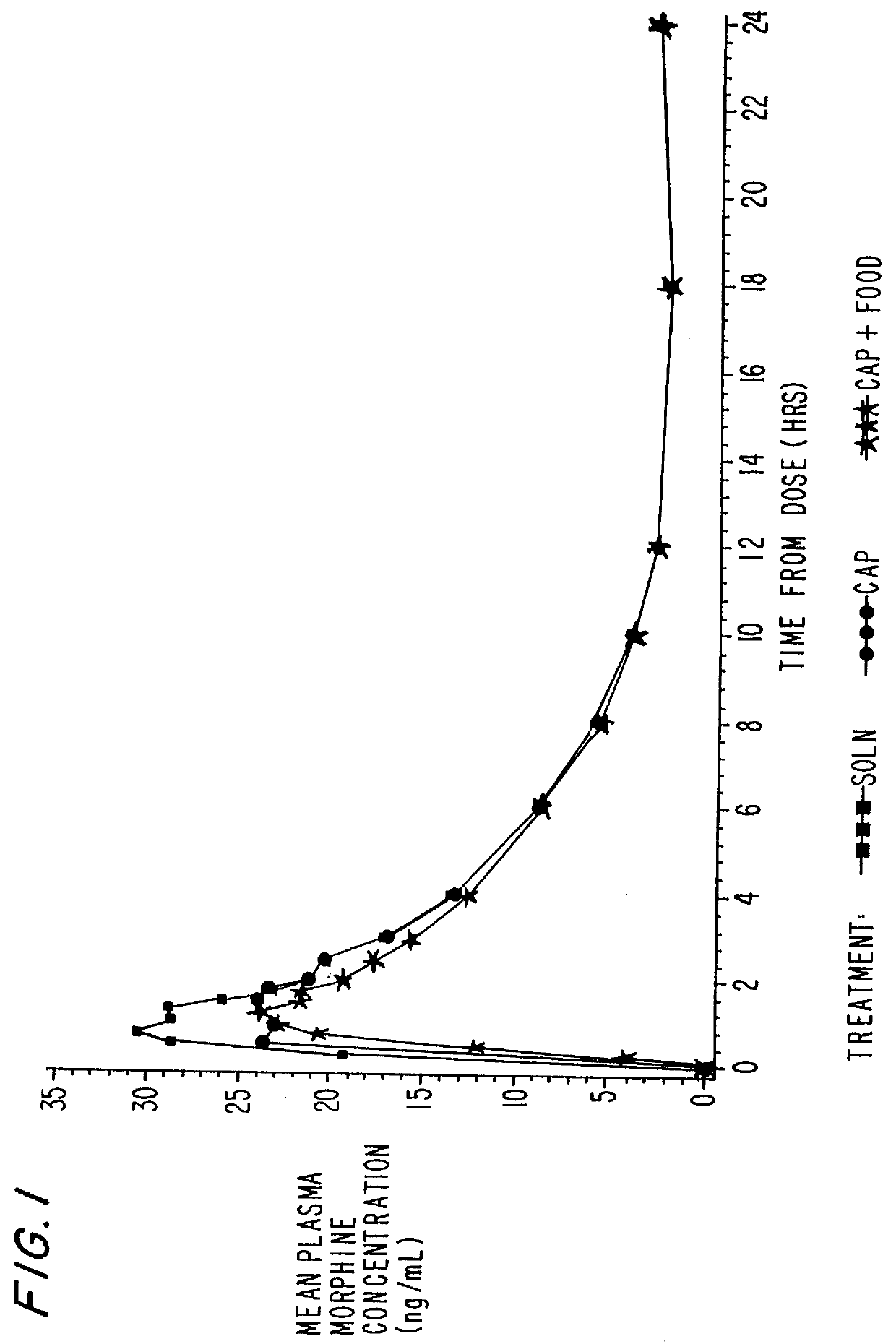
An oral dosage form of morphine is formulated by powder-layering an homogeneous mixture of morphine sulfate and hydrous lactose impalpable onto inert beads to obtain a multiparticulate product. A plurality of the powder-layered beads may be administered either in immediate release form or in an extended release form by coating with a hydrophobic material. In addition, multi-particulate oral dosage forms containing therapeutically effective agents containing a plurality of pharmaceutically acceptable inert beads powder-layered with homogeneous mixture of a therapeutically effective agent and hydrous lactose impalpable are also disclosed. A method of preparing the dosage forms as well as a method preparing spheroids containing the homogeneous mixture of therapeutically effective agent and hydrous lactose impalpable are also disclosed.
Owner:PURDUE PHARMA LP
Multimodal Abuse Resistant and Extended Release Opioid Formulations
The present invention is in the field of oral, abuse resistant pharmaceutical compositions of opioid agonists, extended release pharmaceutical compositions of opioid agonists and extended release abuse resistant pharmaceutical compositions of opioid agonists and the use thereof. The present invention is also directed to extended release pharmaceutical compositions and the use thereof for preventing or minimizing the risk of abuse and / or toxicity from either intentional or unintentional tampering. The present invention is further directed at a method of preventing or minimizing the risk of abuse and / or toxicity from either intentional or unintentional tampering.
Owner:RELMADA THERAPEUTICS
Method for improving the bioavailability of orally delivered therapeutics
The disclosed invention is a method and composition for improving the bioavailability of a pharmaceutically active ingredient comprising an oral dosage form consisting essentially of a granulation of active ingredient, amino acid, and hydrophilic polymer, wherein the granulation is dispersed in an immediate release or extended release excipient.
Owner:SCOLR PHARMA
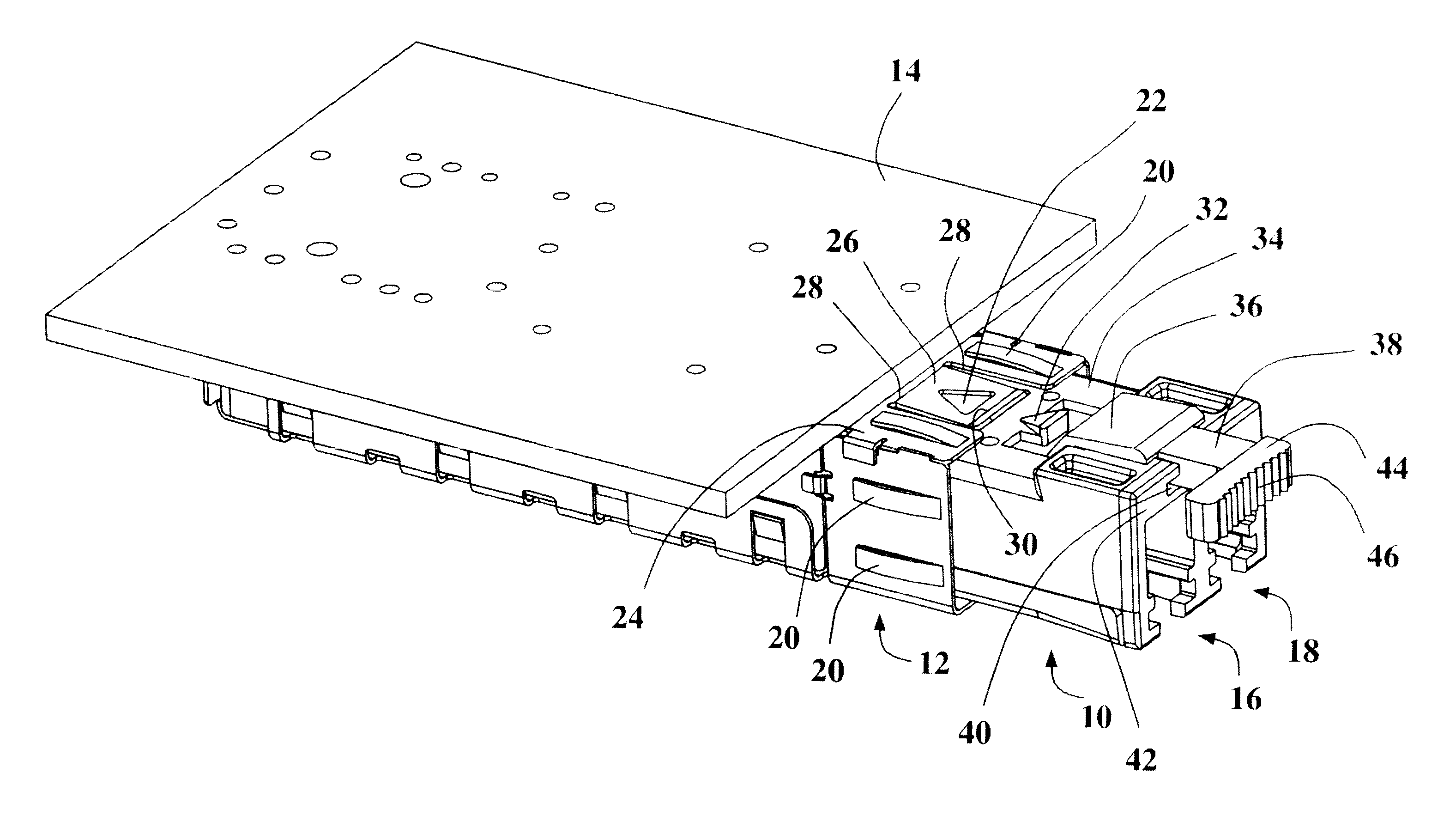
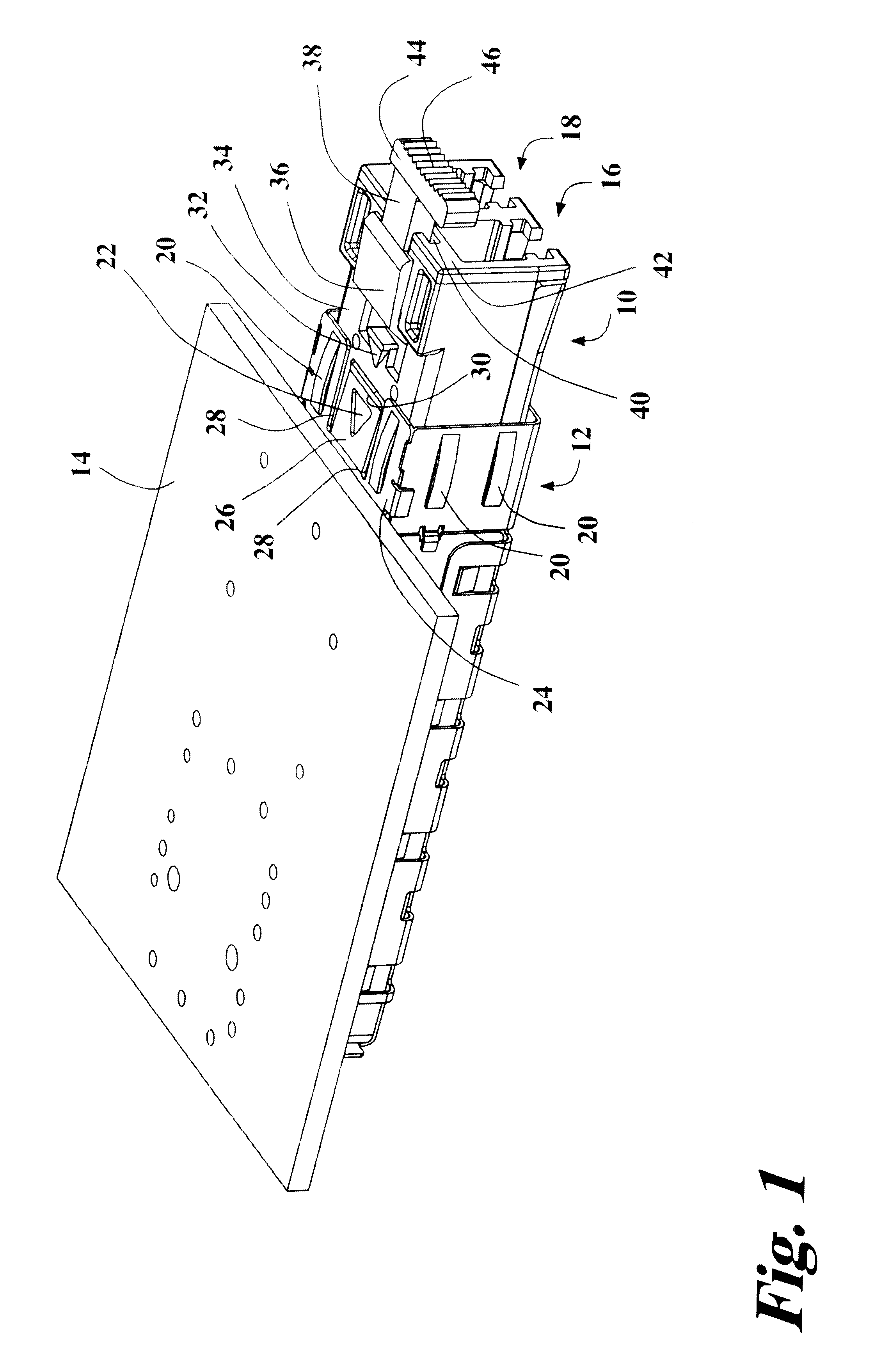
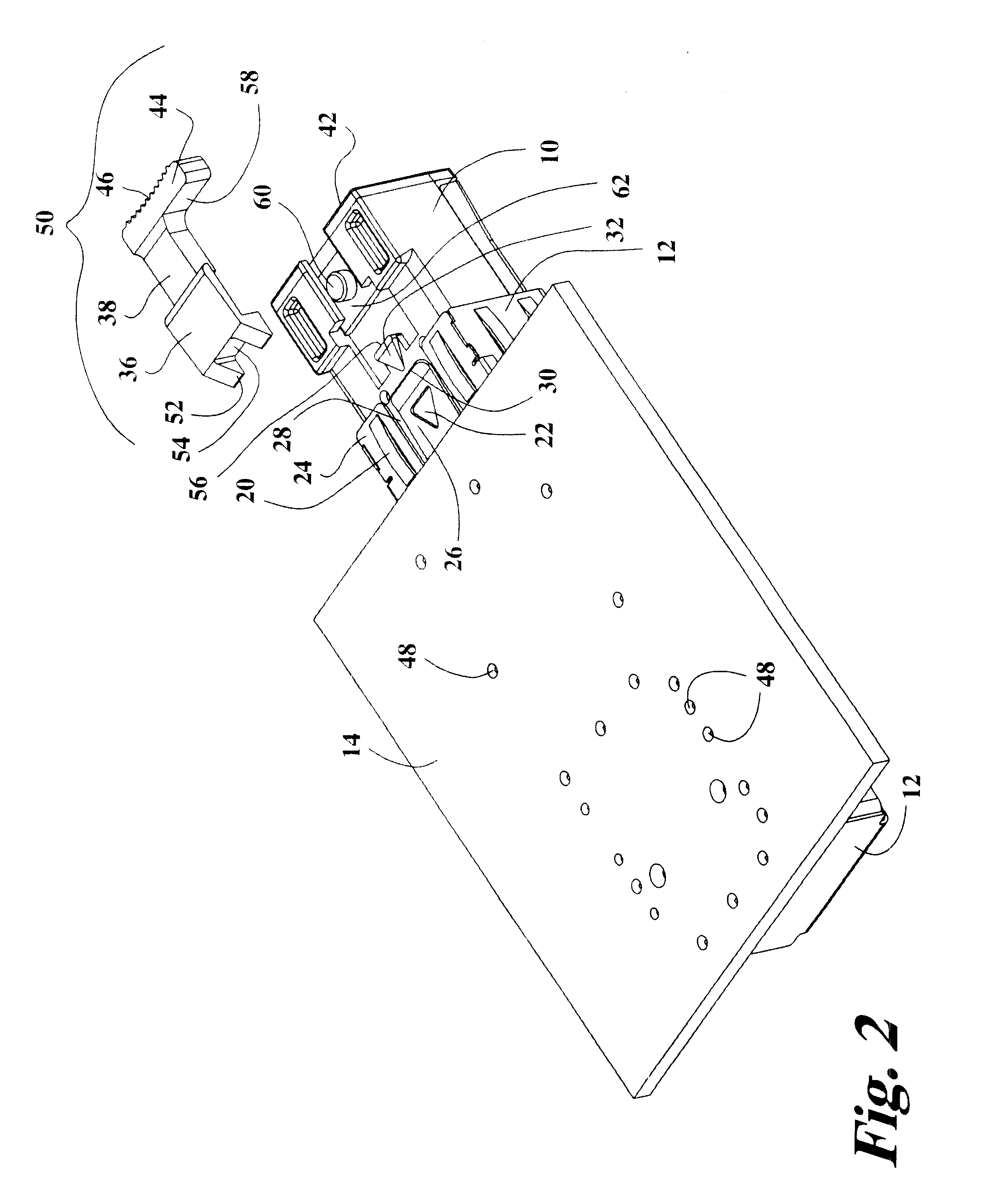
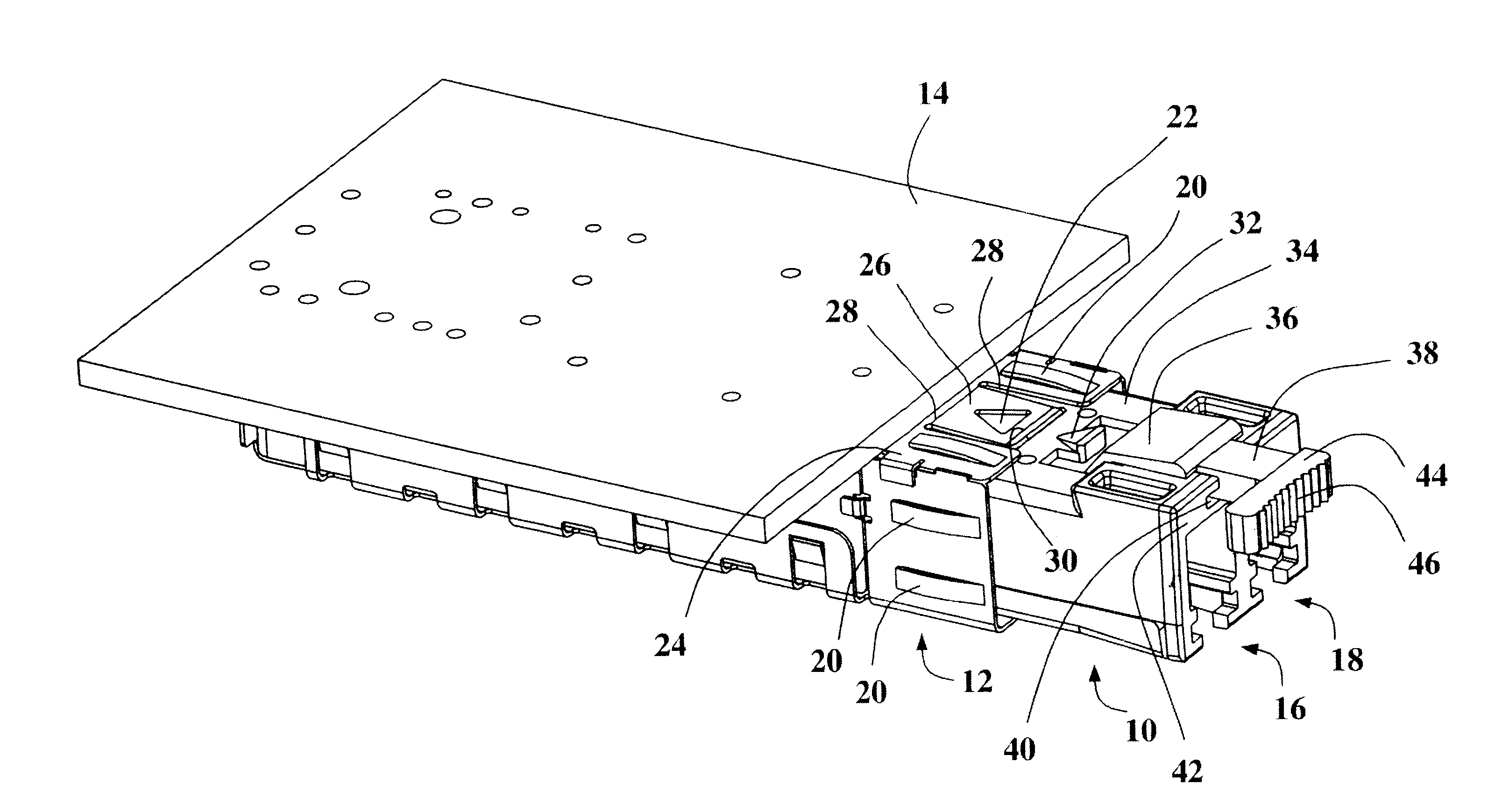
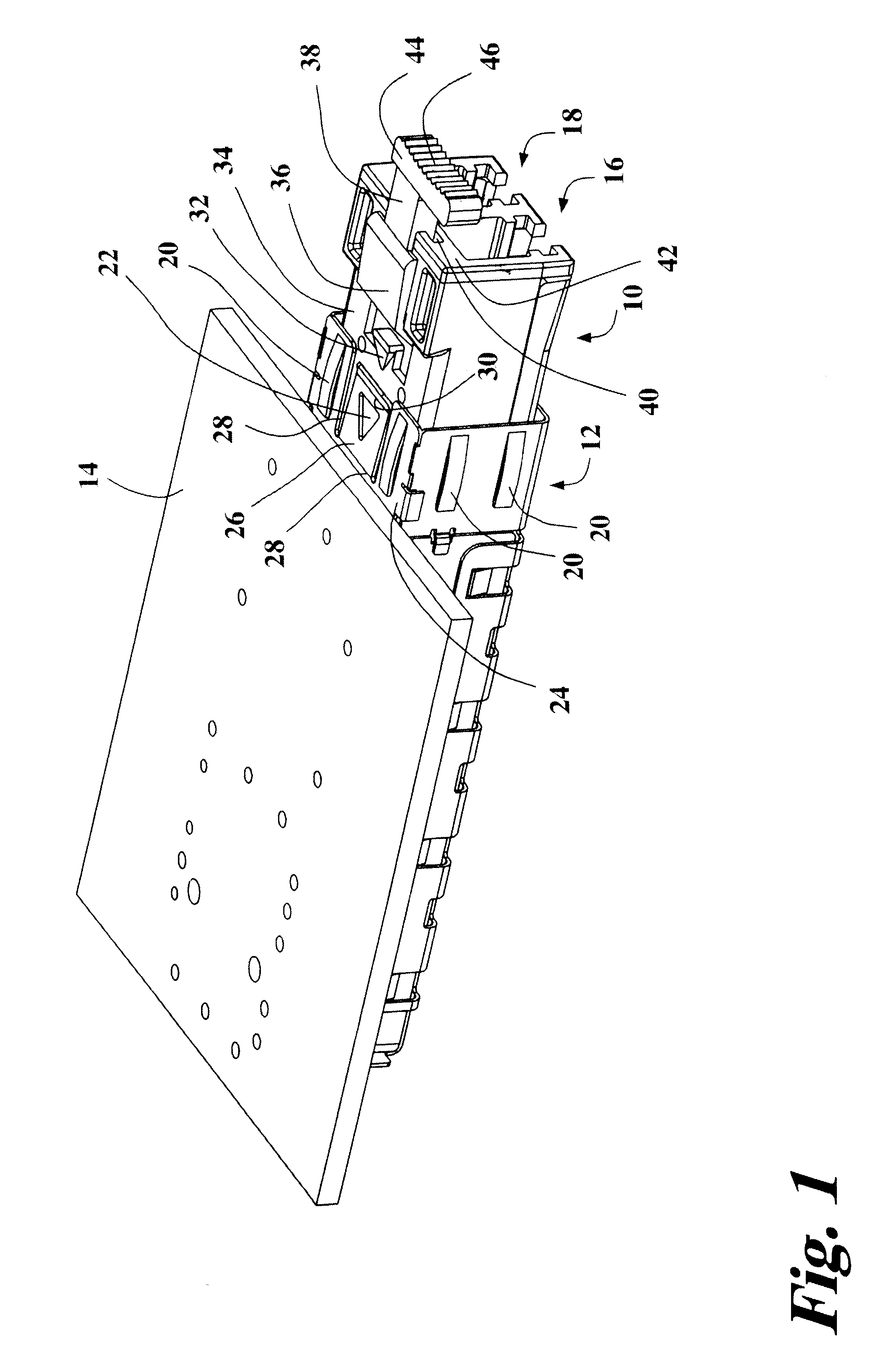
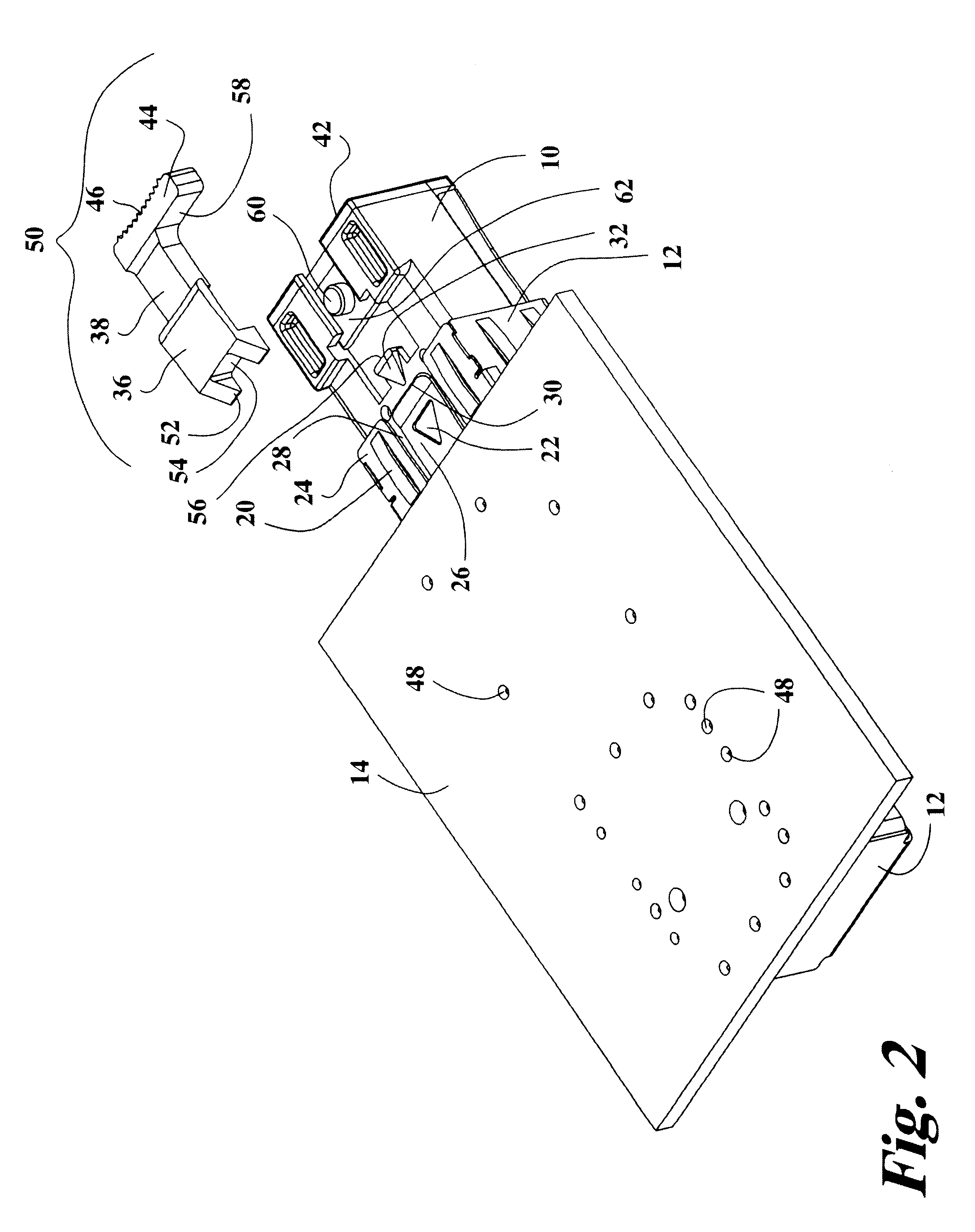
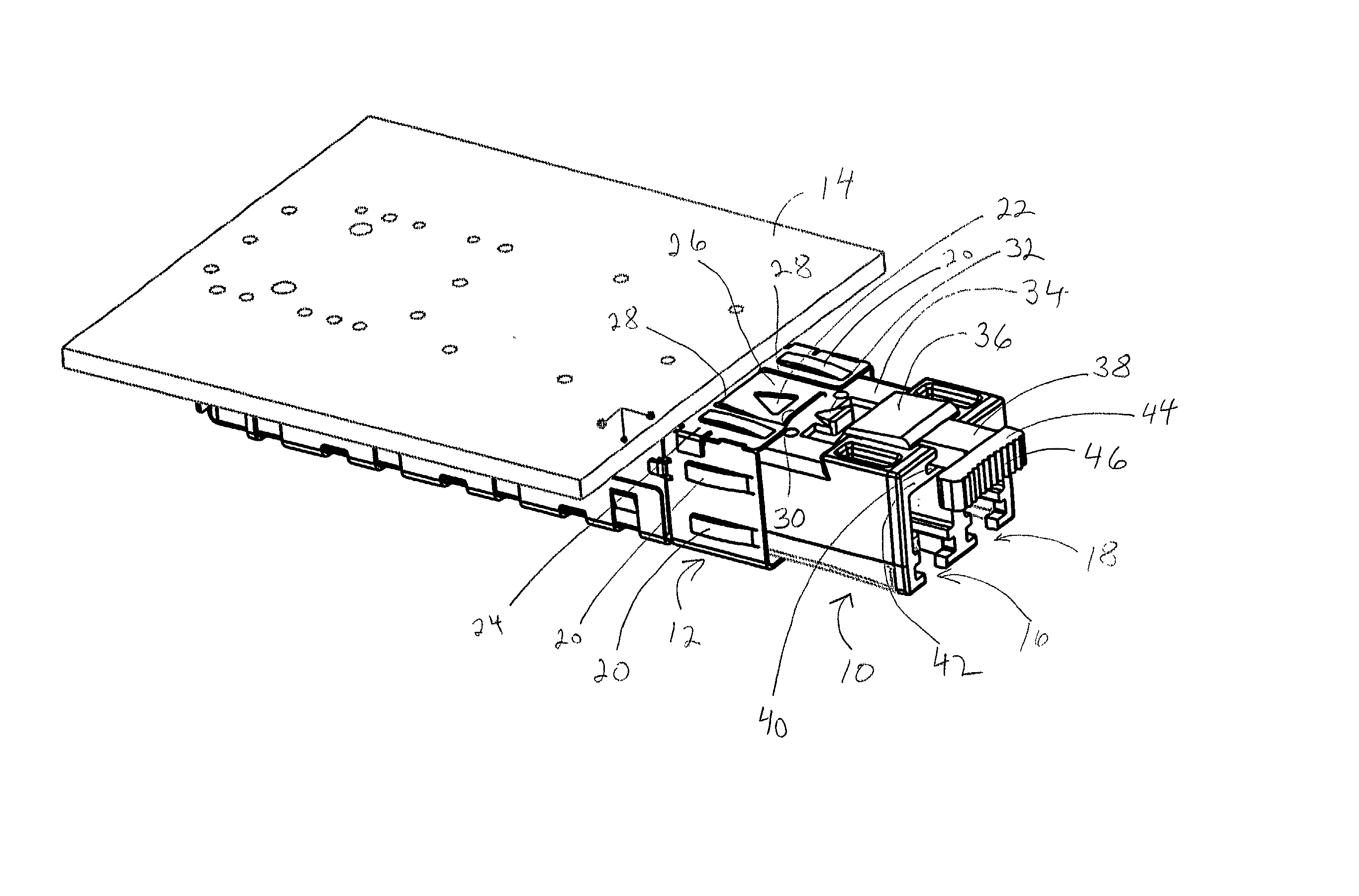
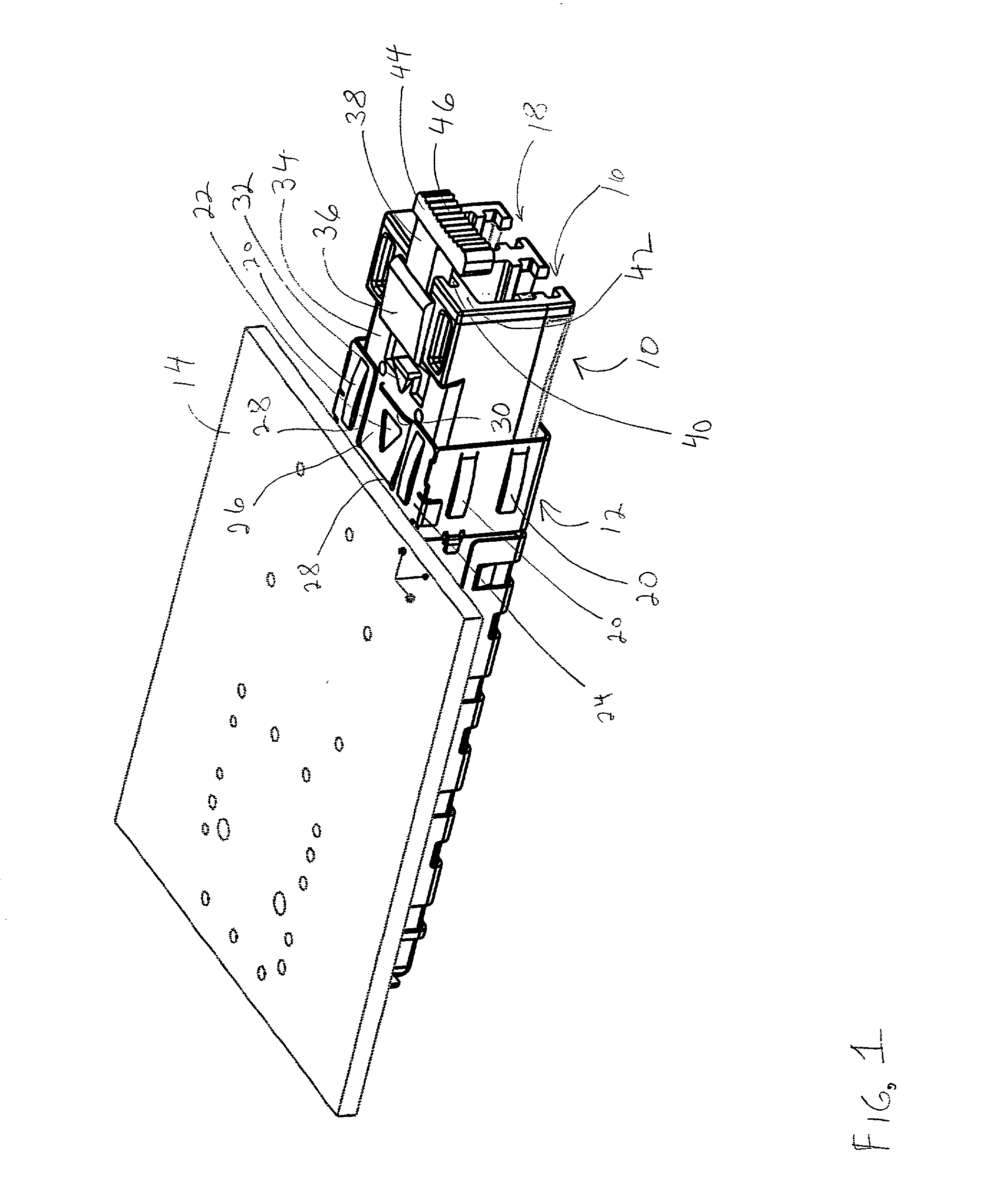
Pluggable transceiver module with extended release and removal lever
InactiveUS6570768B2Easy to operateMinimal costCoupling device detailsCoupling light guidesTransceiverEngineering
A pluggable transceiver module having a housing with a first side and a face perpendicular to the first side, and a tab extending above the surface of the first side sized to mate with a slot in a receptacle for the housing, a wedge slidably mounted on the first side proximate the tab, and a lever attached to the wedge extending beyond the face of the housing, wherein pressing the lever causes the wedge to slide between the tab and the slot on the receptacle and remove the tab from within the slot, thereby releasing the transceiver module from the receptacle, and the lever further including a recess enabling a person to at least partially insert a fingernail to easily grip and remove the pluggable transceiver module from the receptacle.
Owner:STRATOS INT
Extended release pharmaceutical composition of donepezil
The present invention relates to an extended release pharmaceutical composition for oral administration comprising donepezil or pharmaceutically acceptable salt thereof and a release-controlling agent. Further, it relates to process for preparation of said compositions.
Owner:RANBAXY LAB LTD
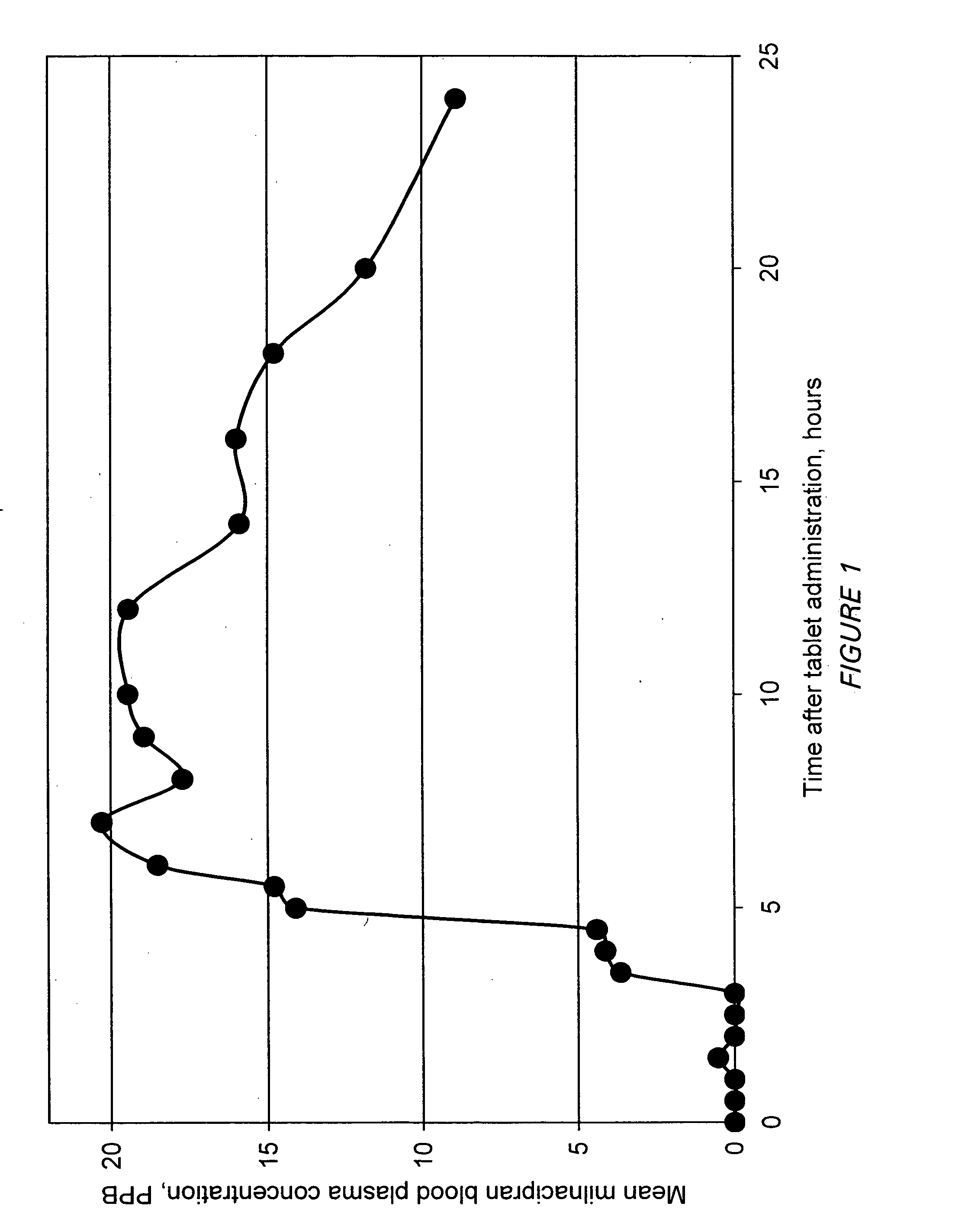
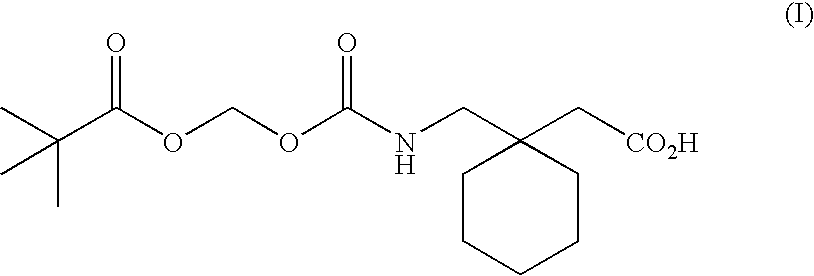
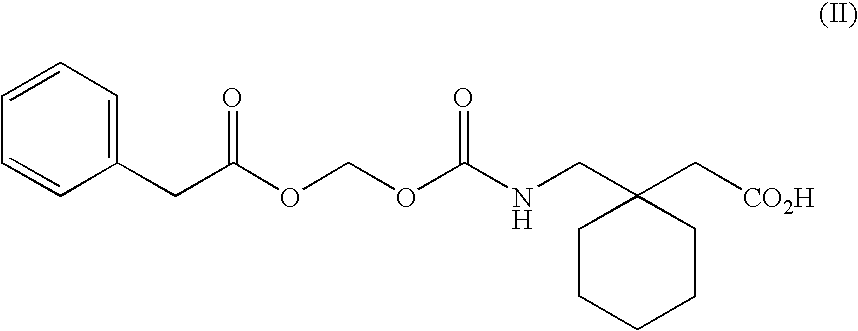
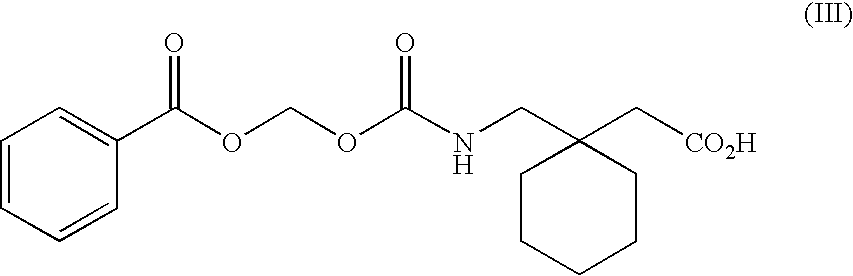
Modified release compositions of milnacipran
A once-a-day oral milnacipran modified release formulation has been developed. The formulation comprises an extended release dosage unit (optionally containing the immediate release portion) coated with delayed release coating. The milnacipran composition, when administered orally, first passes through the stomach releasing from zero to less than 10% of the total milnacipran dose and then enters the intestines where drug is released slowly over an extended period of time. The release profile is characterized by a 0.05-4 hours lag time period during which less than 10% of the total milnacipran dose is released followed by a slow or extended release of the remaining drug over a defined period of time. The composition provides in vivo drug plasma levels characterized by Tmax at 4-10 hours and an approximately linear drop-off thereafter and Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition allows milnacipran to be delivered over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Engineering absorption of therapeutic compounds via colonic transporters
Methods of modifying therapeutic compounds such as drugs to be substrates for active transporters expressed in epithelial cells lining the lumen of the human colon are disclosed. The transporters expressed in the human colon include the sodium dependent multi-vitamin transporter (SMVT), and monocarboxylate transporters 1 and 4 (MCT 1 and MCT 4). The modified compounds can themselves be pharmacologically active, or upon cleavage of a chemical moiety after uptake from the colon, can be metabolized to form a compound that is pharmacologically active (e.g., a prodrug). The modified compounds disclosed herein are suitable for use in extended release oral dosage forms, particularly those that release drug over periods of greater than about 2-4 hours following administration.
Owner:XENOPORT
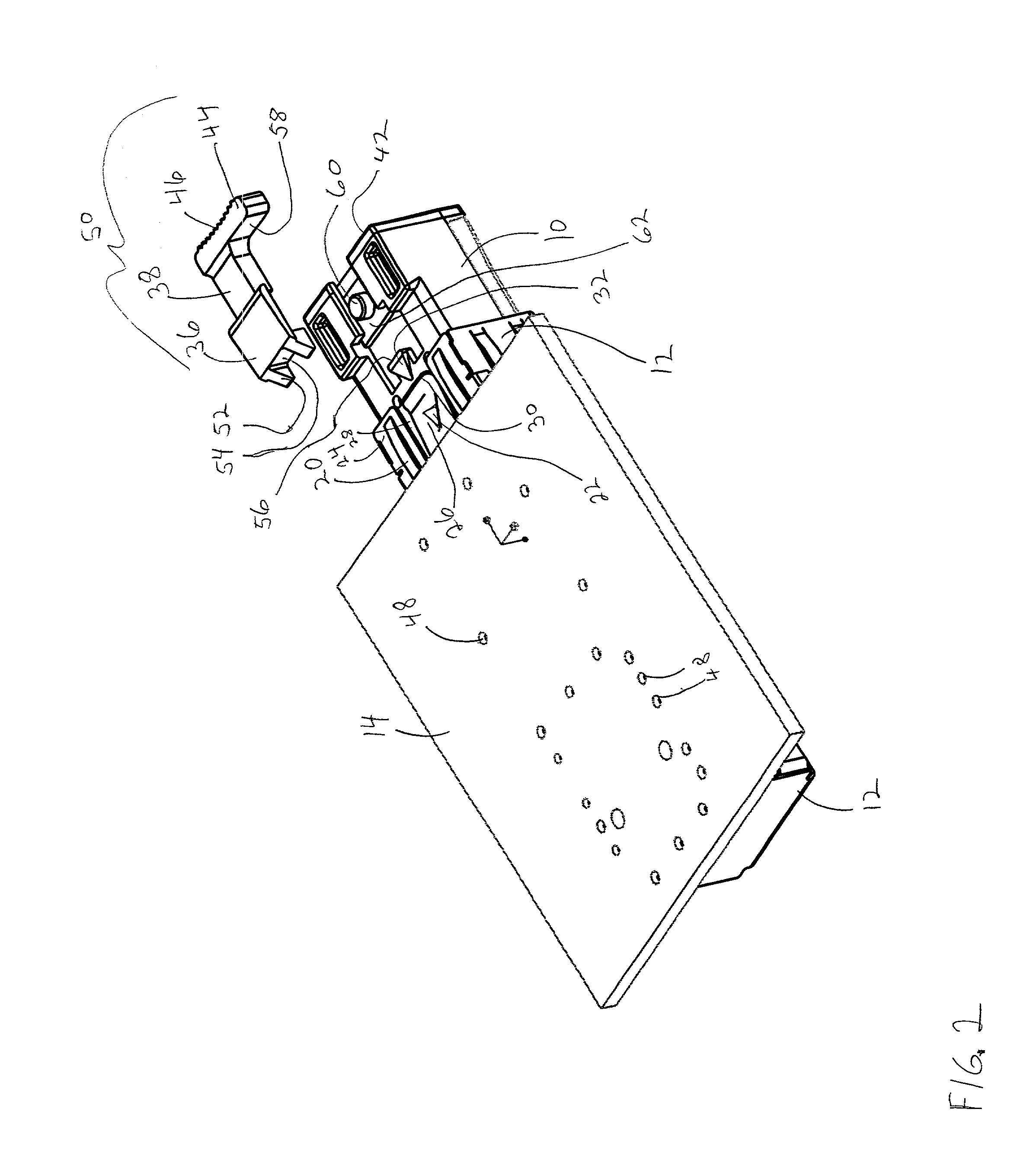
Transceiver module with extended release lever
InactiveUS6556445B2Easy to operateMinimal costCoupling light guidesElectrical apparatus contructional detailsTransceiverExtended release
A transceiver module having a housing with a first side and a face perpendicular to the first side, and a tab extending above the surface of the first side sized to mate with a slot in a receptacle for the housing, a wedge slidably mounted on the first side proximate the tab, and a release lever attached to the wedge extending beyond the face of the housing, wherein pressing the release lever causes the wedge to slide between the tab and the slot on the receptacle and remove the tab from within the slot, thereby releasing the transceiver module from the receptacle.
Owner:STRATOS INT
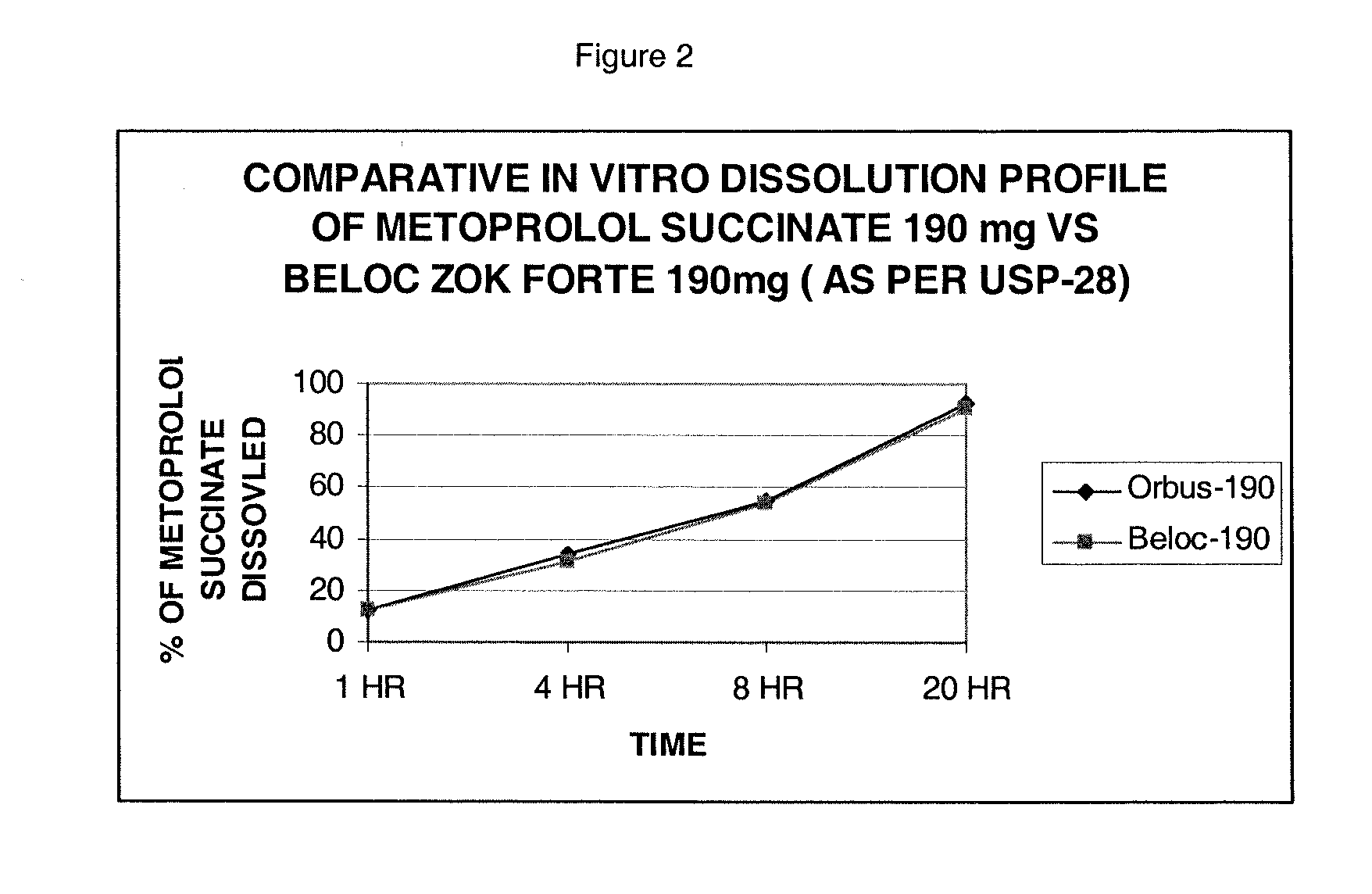
Stabilized extended release pharmaceutical compositions comprising a beta-adrenoreceptor antagonist
InactiveUS20070092573A1Well formedStrengthen matrixBiocideOrganic active ingredientsGreek letter betaAdrenergic receptor sites
The present invention is a new stable extended release drug composition particularly suitable for use as a beta-adrenoreceptor antagonist agent. The present invention is specifically a drug composition comprising a pharmaceutical, a methacrylic acid copolymer and a matrix forming agent, and a method for manufacturing same. When applied to highly soluble drugs like metoprolol succinate, the resulting drug composition is characterized by an extended-release profile.
Owner:ORBUS PHARMA INC
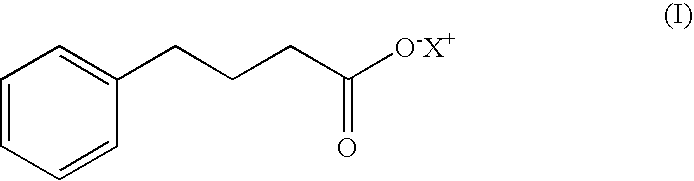
4-phenylbutyric acid controlled-release formulations for therapeutic use
InactiveUS20060045912A1Low costReduce the amount requiredBiocideNervous disorderHalf-lifeNeuro-degenerative disease
Controlled-release formulations and dosage forms containing 4-phenylbutyric acid sodium salt, or other pharmaceutically acceptable salts, esters or prodrugs, and a controlled release material for use in the treatment of diseases and disorders including neoplastic disorders and neurodegenerative diseases The formulations provide extended release and extended half-life.
Owner:LUNAMED
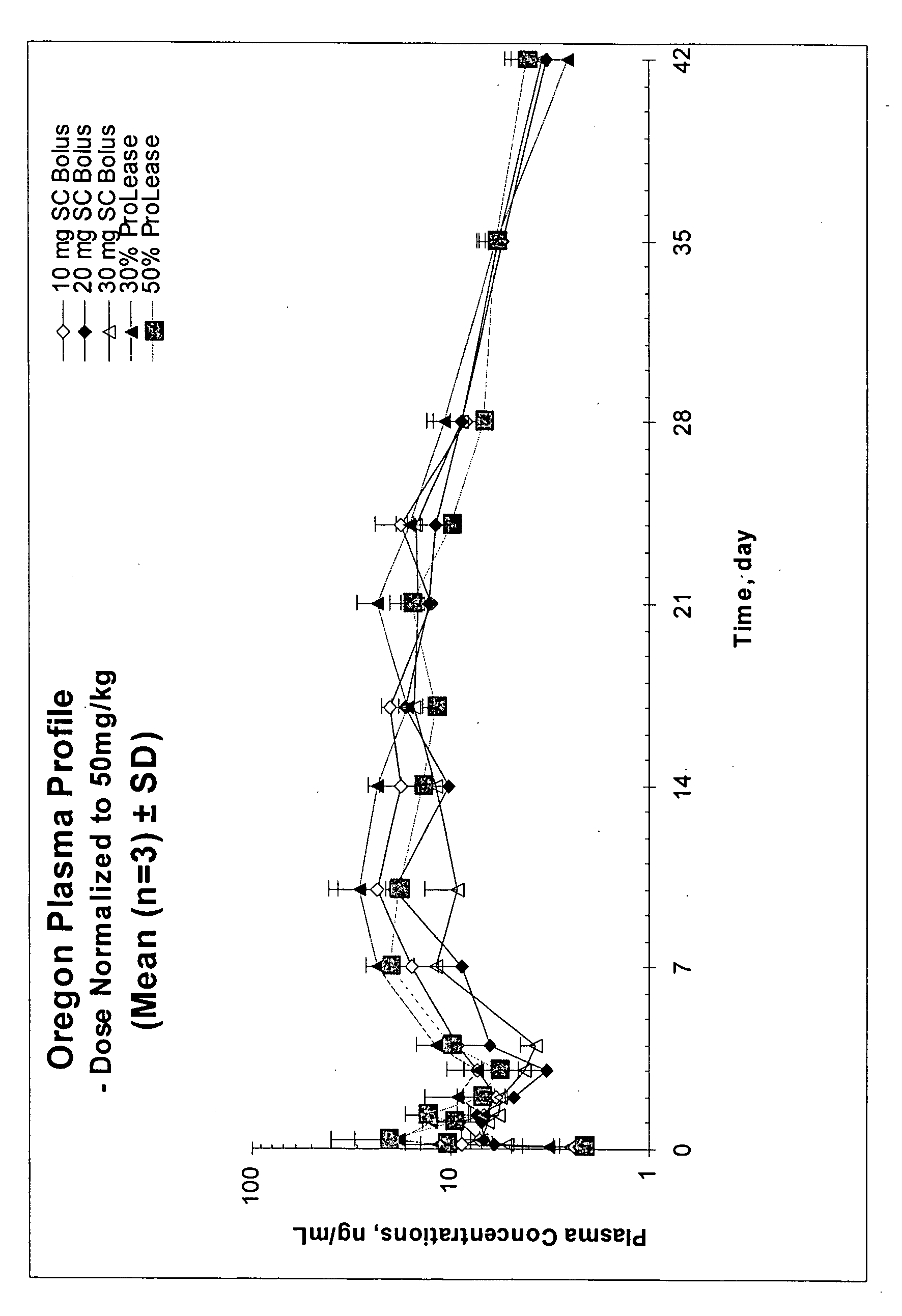
Methods for administering aripiprazole
InactiveUS20050032811A1Without complexityWithout expenseOrganic active ingredientsNervous disorderActive agentMicrosphere
The present invention relates, in part, to the discovery that a pharmaceutical composition comprising aripiprazole and a carrier administered in a bolus injection resulted in an extended release profile similar to that obtained by the injection of a poly lactide-co-glycolide microsphere formulation containing the active agent. This surprising result suggests that pharmacologically beneficial extended release formulations without the complexities and expense associated with the manufacture microspheres.
Owner:ALKERMES INC
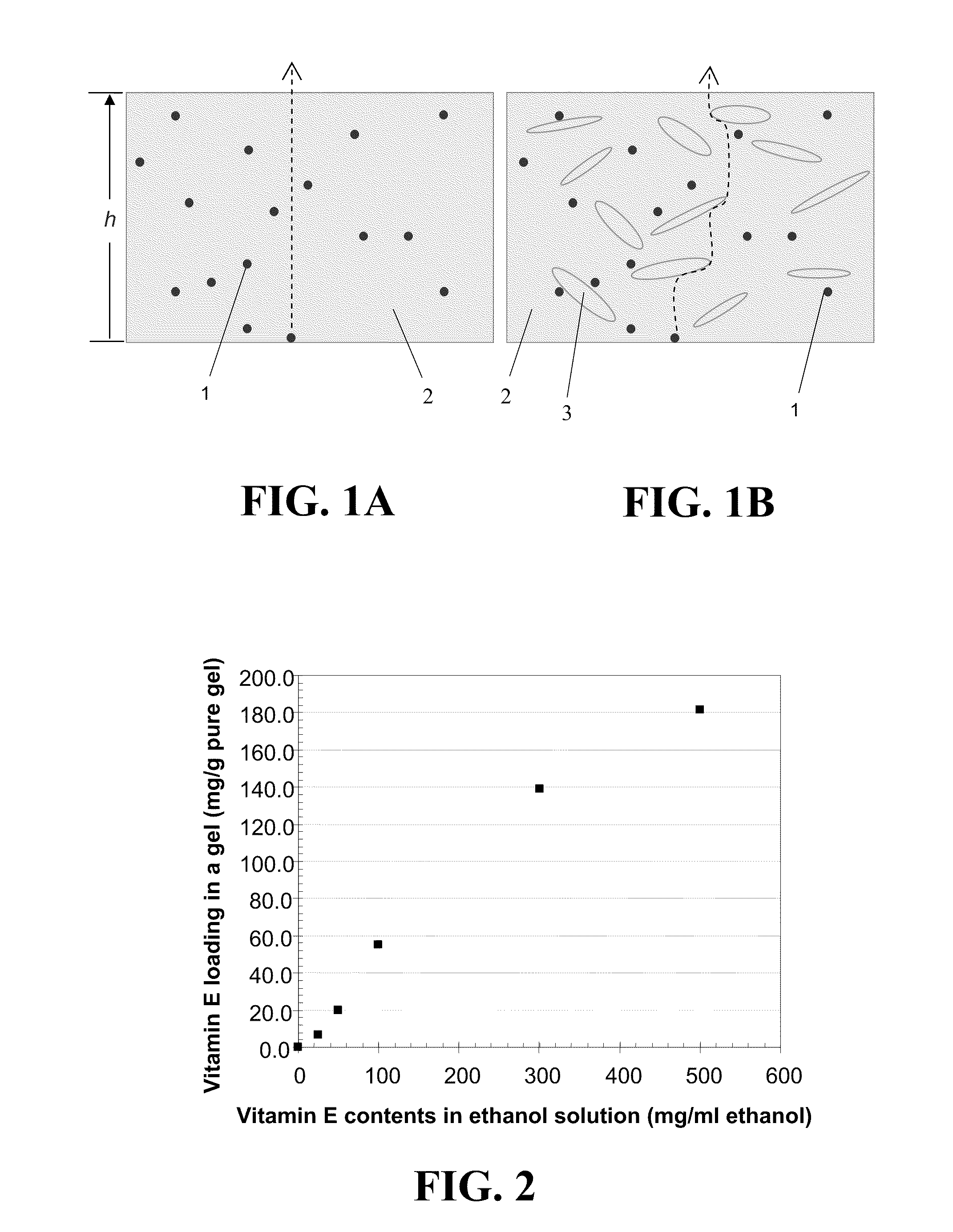
Contact lenses for extended release of bioactive agents containing diffusion attenuators
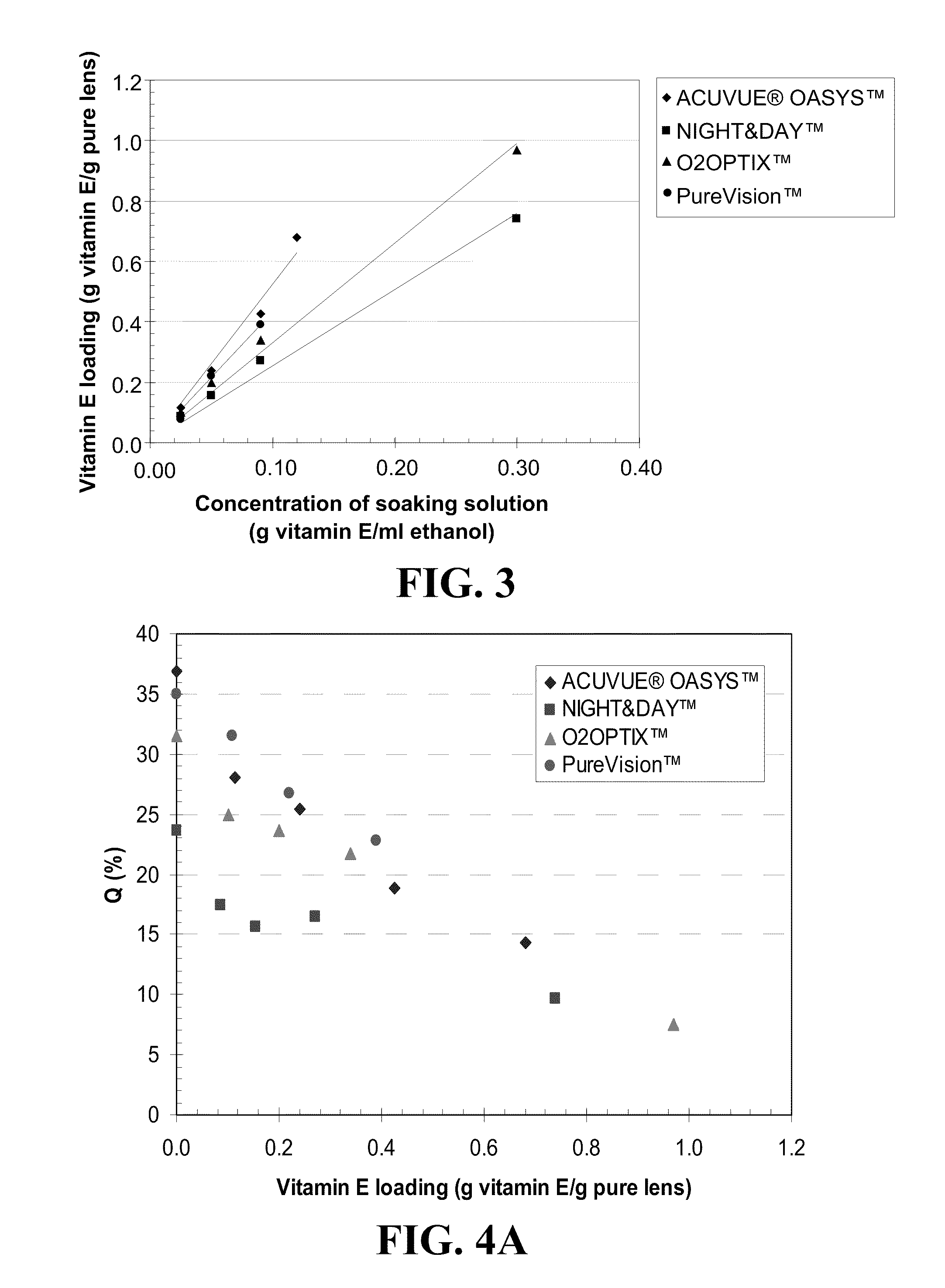
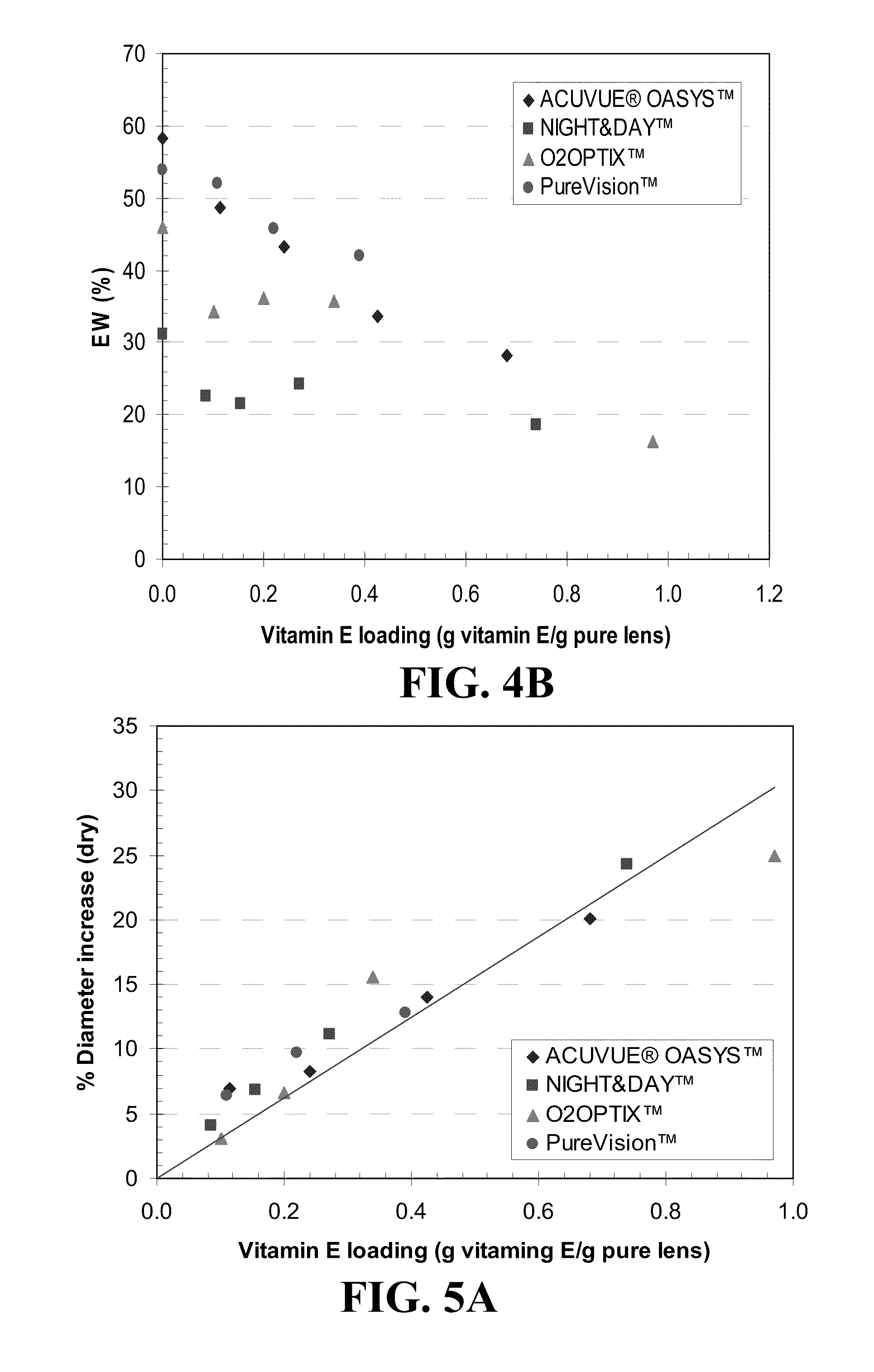
An appliance for the delivery of at least one bioactive agent to the eye has at least one diffusion attenuator within a hydrophilic or silicone-hydrogel contact lens. The bioactive agent can be a drug or a nutraceutical. The diffusion attenuator can be a plurality of solid particles or phase separated liquid aggregates within at least one continuous phase of the lens where the diffusion attenuators promote a tortuous path for the diffusion of the bioactive agent to mediate the rate by which the bioactive agent diffuses from the contact lens. The diffusion attenuator can be homogeneously dispersed throughout at least one continuous phase of the lens to modify the diffusivity of the bioactive agent through that phase. The diffusion attenuator can have little or no affinity for the bioactive agent or can be miscible with the bioactive agent. The diffusion attenuator can be incorporated while forming the contact lens by polymerization of a monomer mixture containing the diffusion attenuator. For liquid diffusion attenuators, the liquid can be co-absorbed with a solvent into the lens followed by removal of the solvent, where the bioactive agent can be co-absorbed or subsequently absorbed after the loading of the diffusion attenuator. The diffusion attenuator can be Vitamin E.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Topiramate compositions and methods of enhancing its bioavailability
ActiveUS20080085306A1Reduced adverse eventConvenient treatmentBiocideNervous disorderRegimenImmediate release
Owner:SUPERNUS PHARM INC
Controlled release formulations using intelligent polymers
InactiveUS6893661B1Promote absorptionMaintenance of therapeutically effective blood levelPowder deliveryOrganic active ingredientsSmart polymerWater contact
An extended release dosage composition of pharmaceutically active substances that have a water contact angle (θ) such that cos θ is between +0.9848 and −0.9848 presented as a matrix tablet containing the said pharmaceutically active substances, with / without suitable pharmaceutical excipients in intimate mixture with two groups of intelligent polymers having opposing wettability characteristics, one demonstrating a stronger tendency towards hydrophobicity and the other a stronger tendency towards hydrophilicity, the polymer combination being between the ratios of 1:50 and 50:1 amounts effective to control the release of said pharmaceutically active substances in a mathematically predictable manner, wherein the polymer demonstrating a stronger tendency towards hydrophobicity is not less than 5% wt / wt and preferably between 5-70% wt / wt of the final formulation composition. The intelligent polymers being ethylcellulose (EC) as a more strongly hydrophobic and hydroxyethylcellulose (HEC) and / or hydroxypropyl methylcellulose (HPMC) as more strongly hydrophilic (the ratio of HEC to HPMC being between 1:100 and 100:1). The matrix tablet is optionally coated with an enteric coat, 0-5%-15% wt / wt to prevent the initial burst effect seen in such systems and to impart gastrointestinal tract (GIT) “stealth” characteristics especially in the presence of food.
Owner:VALEANT INT BERMUDA
Sequential release pharmaceutical formulations
InactiveUS20070141147A1Efficient coordinationBiocideHydroxy compound active ingredientsControlled releaseImmediate release
A mixed-release tablet or capsule formulation including vehicles for the delivery of a plurality of drugs in various combinations of immediate release, extended release, and / or delayed release modes over a predetermined time period have been developed, which provide for controlled release not just of the drugs, but controlled release that is designed to create more effective coordination between the drugs being delivered. The drugs can be any medically and / or physiologically appropriate combination of drugs and active ingredients, preferably decongestant drugs, antihistamines, expectorants, antitussives, cough suppressants, and drying agents.
Owner:AURIGA LAB
Slow release magnesium composition and uses thereof
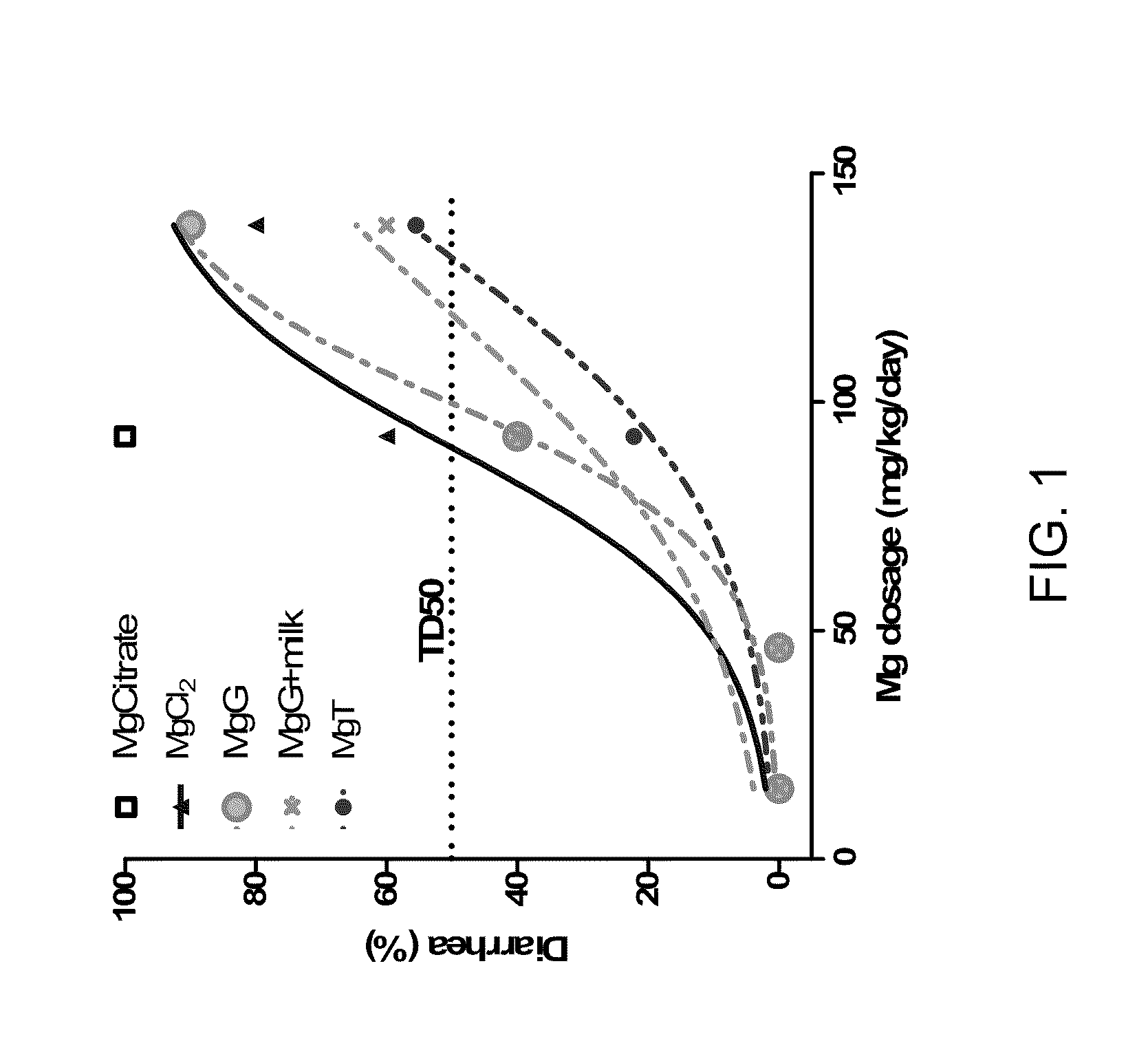
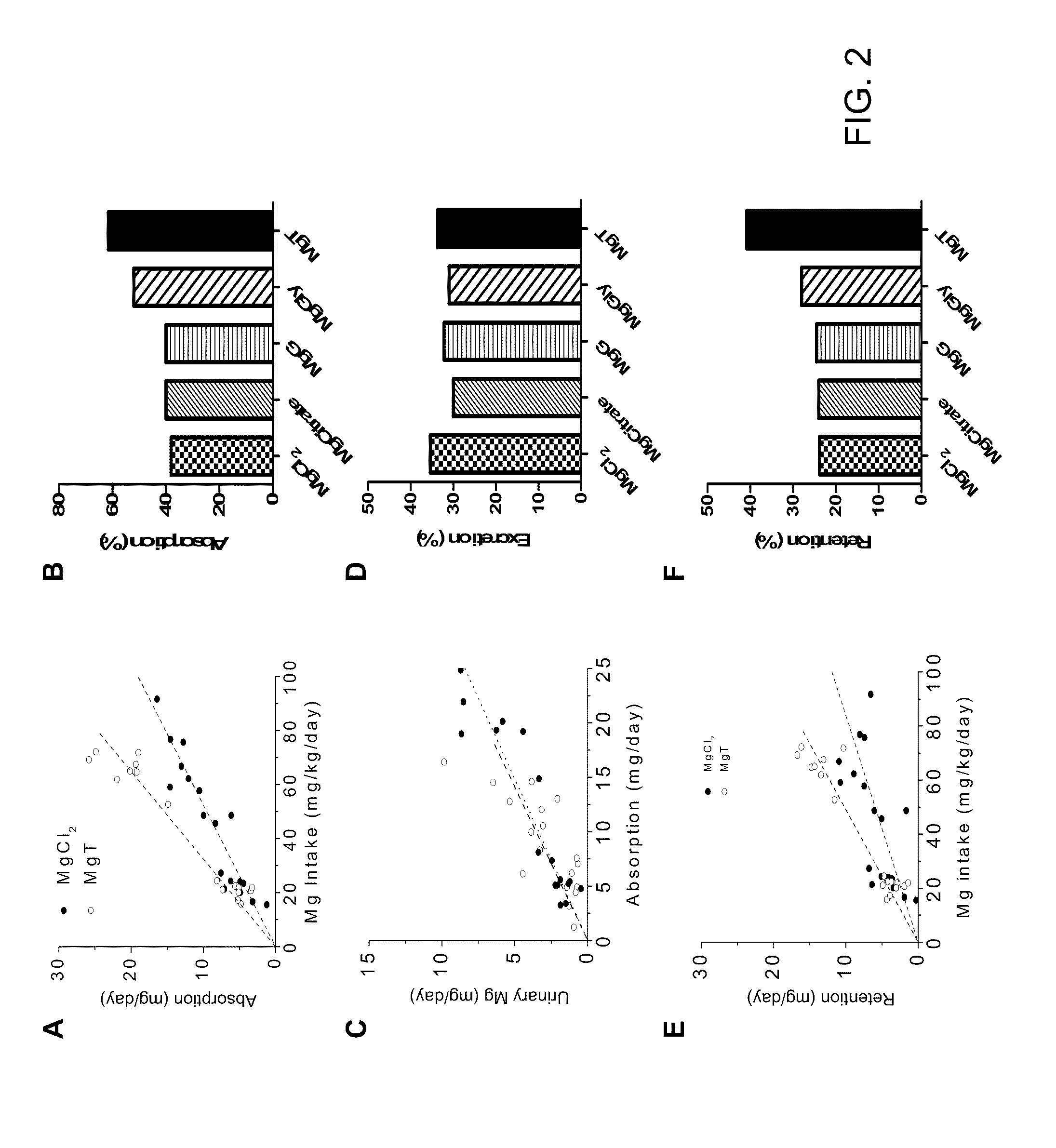
ActiveUS20110020443A1Reduce adverse effectsIncrease concentrationBiocideNervous disorderSide effectDiarrhea
The present invention provides compositions that contain magnesium and threonate, or a threonate precursor molecule, formulated for extended or modified release to provide physiological concentrations over a desired time period. The extended release or modified release form is particularly useful in providing Mg to a subject while avoiding adverse side effects such as diarrhea.
Owner:NEUROCENTRIA INC
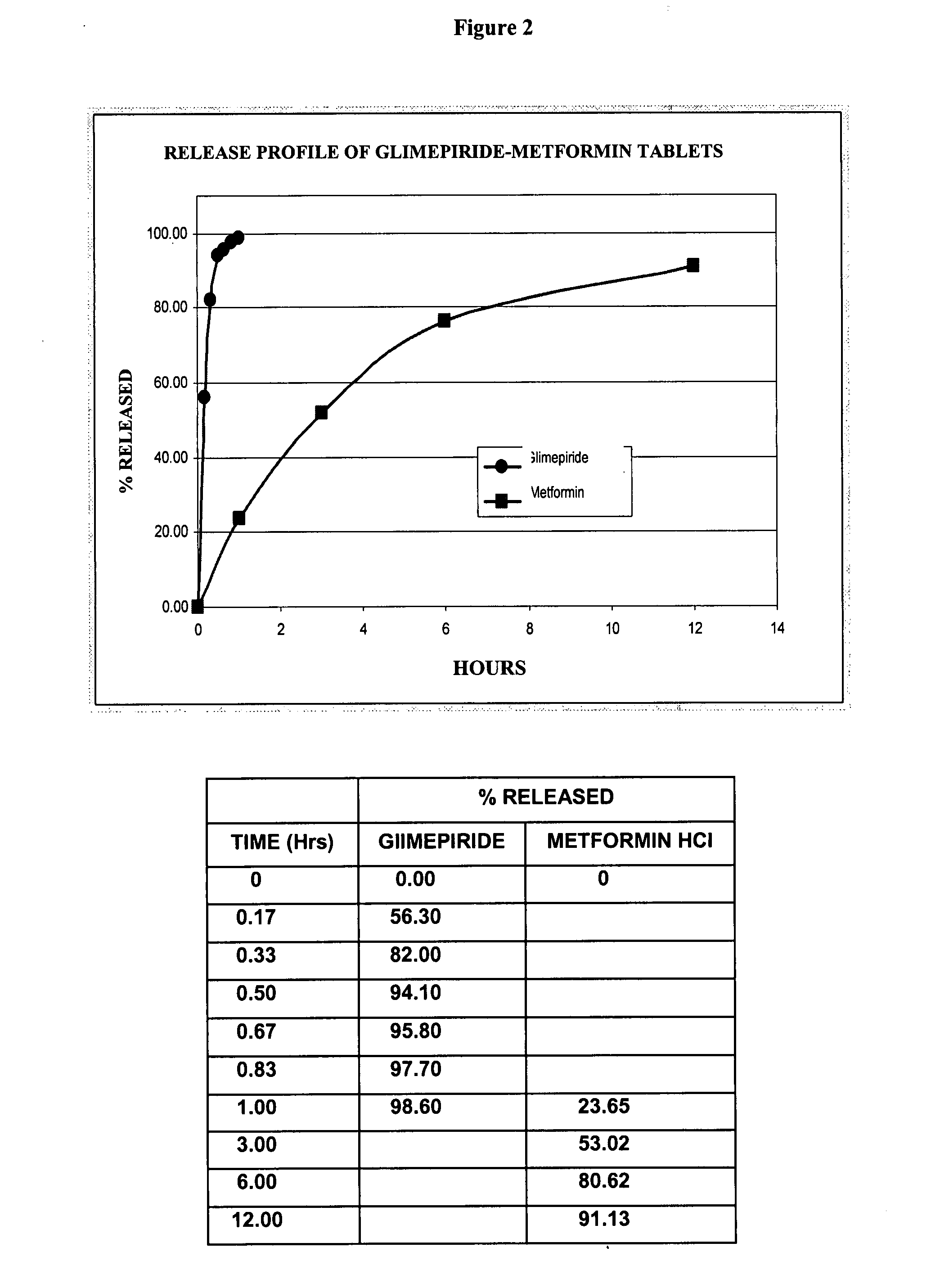
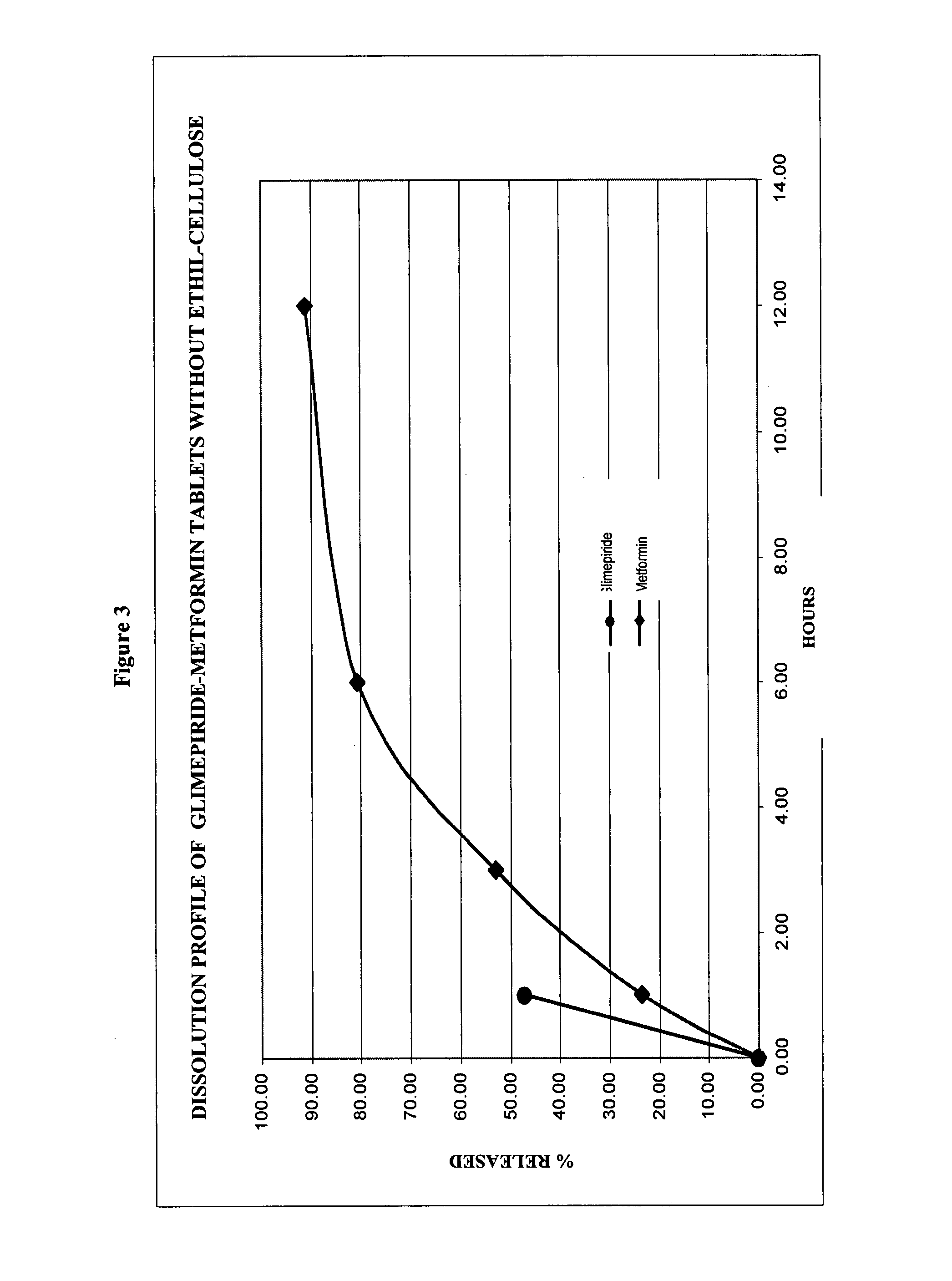
Stable pharmaceutical composition of immediate-release glimepiride and extended-release metformin
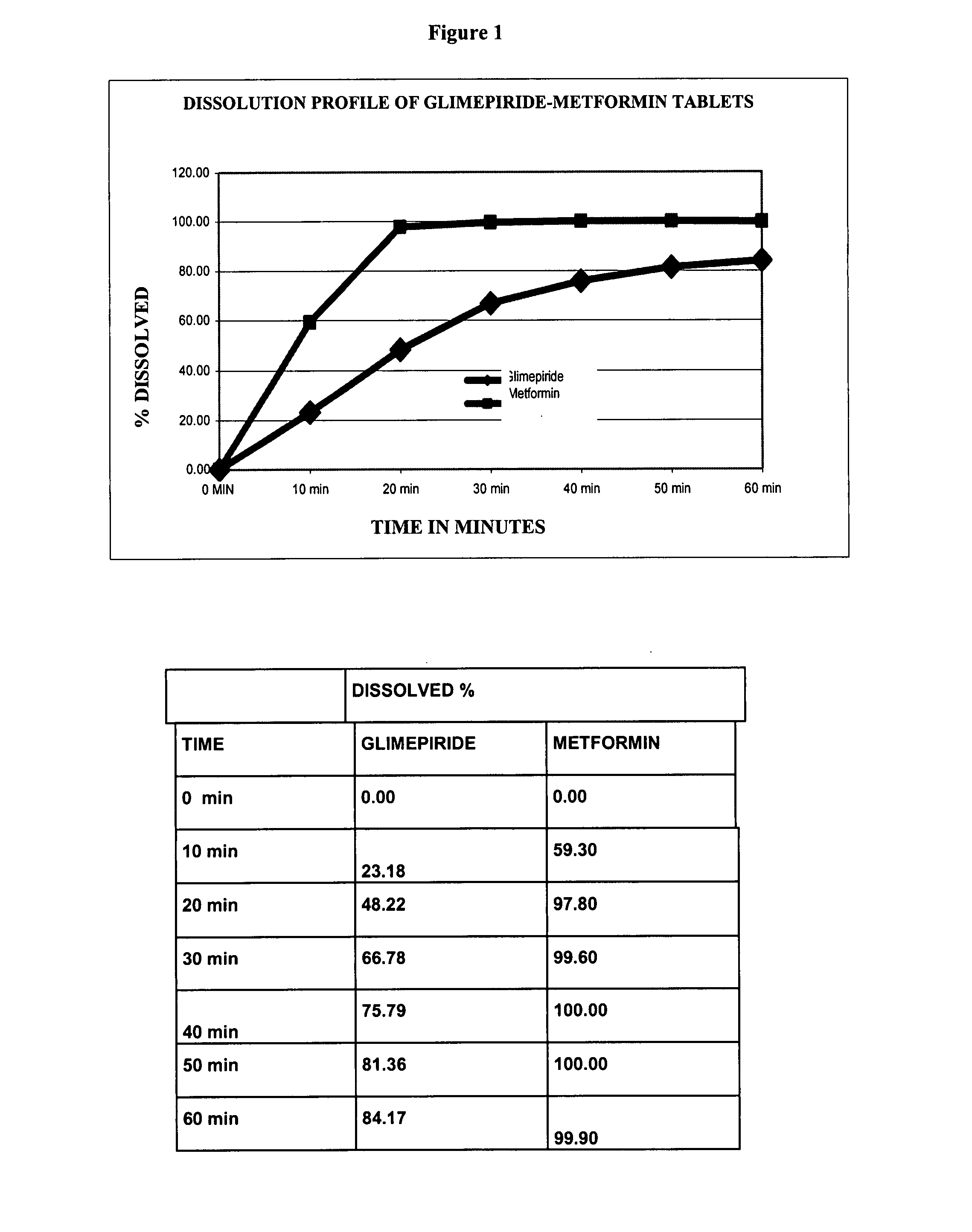
InactiveUS20070264331A1Avoid mixingBiocideMetabolism disorderImmediate releaseMetformin Hydrochloride
This invention is directed to a pharmaceutical composition in the form of a tablet with improved stability, as well as the process for obtaining said composition. The tablet of the present invention comprises two active ingredients comprising two oral hypoglycemic agents: one phase with a sulphonylurea, such as immediate release Glimepiride, and a second phase with a biguanide, such as extended-release Metformin hydrochloride (Metformin HCl). The biphasic tablet, which can include over 500 mg of Metformin HCl (i.e. up to 1,000 or 1,500 mg, depending on the daily requirements of each patient), is to be orally administered once or twice a day. The combination of these hypoglycemic agents has a synergic effect and therefore a greater effectiveness in controlling the blood glucose level in patients with diabetes mellitus, type 2.
Owner:LAB SILANES
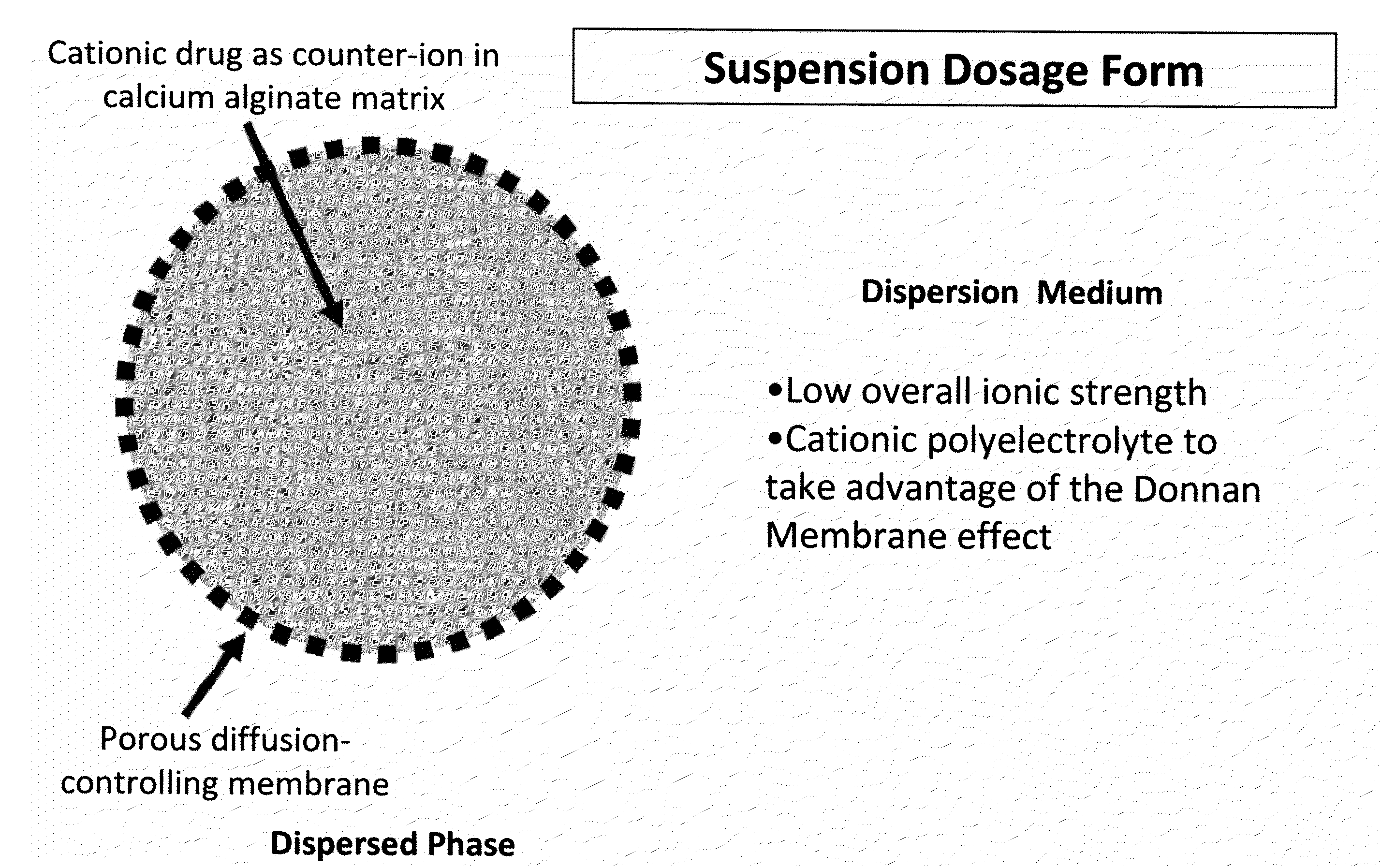
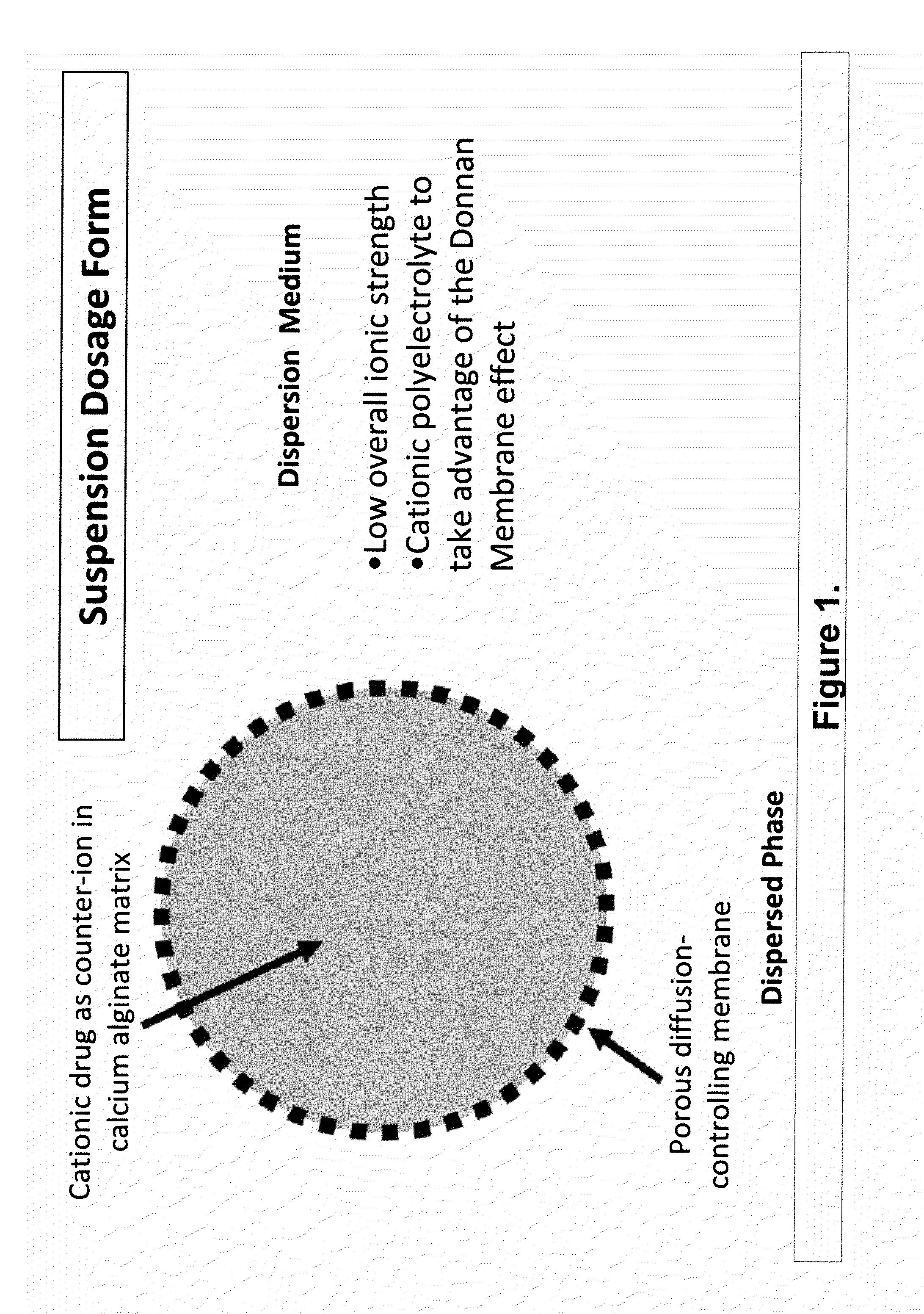
Sustained-release drug delivery compositions and methods
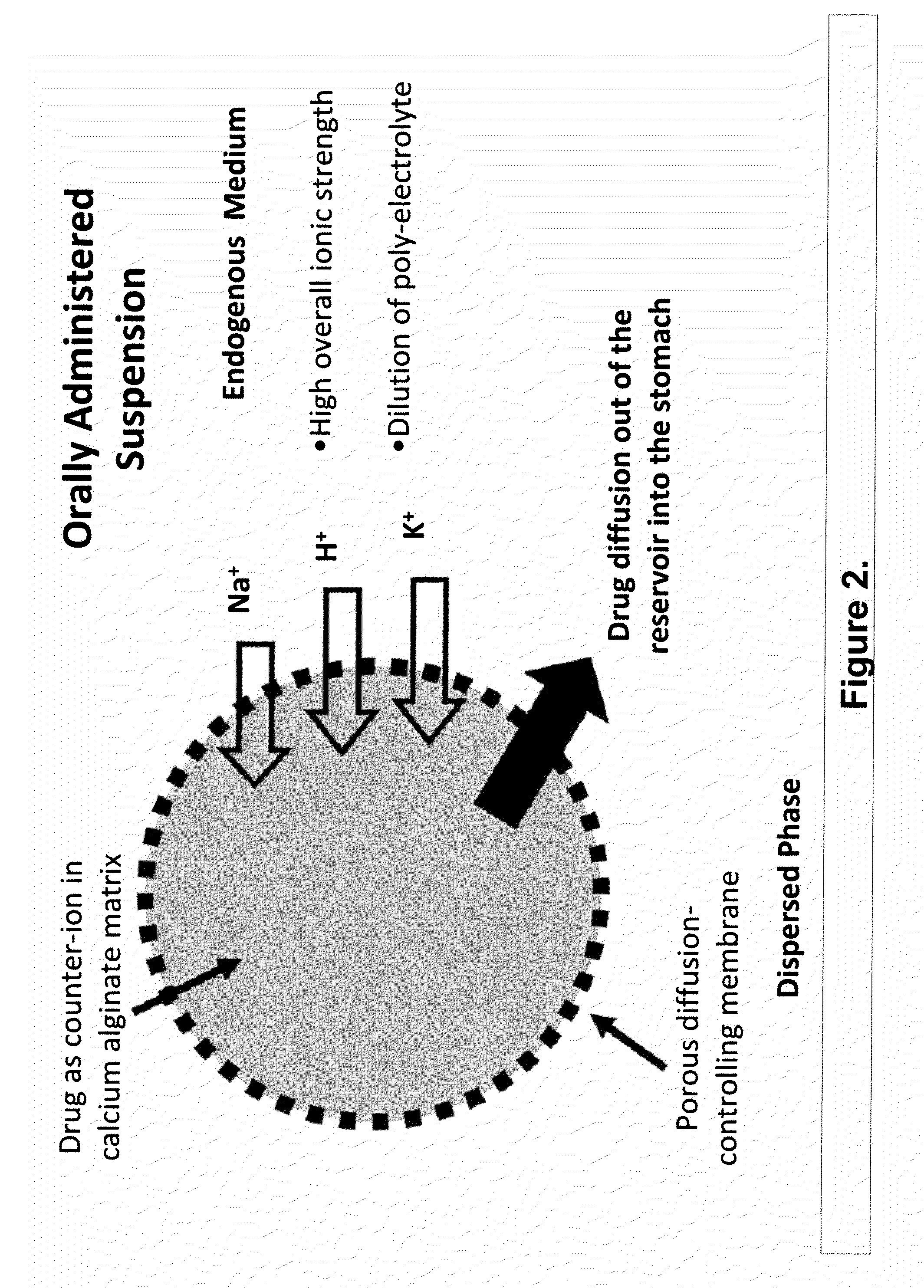
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
Oral extended-release composition
InactiveUS20050064034A1Antibacterial agentsPowder deliveryControlled-Release FormulationsExtended release
The invention is directed to controlled release formulations containing drugs which are preferably considered sparingly soluble to insoluble and which are suitable for administration to a patient in need of treatment related thereto, and methods of manufacturing the same.
Owner:ANDRX PHARMA INC
Bioadhesive progressive hydration tablets
InactiveUS20070031491A1Facilitated releasePharmaceutical delivery mechanismBioadhesiveBioavailability
A bioadhesive controlled, extended release progressive hydration composition wherein the active ingredient may be protected from water or the surrounding environment, thereby protecting it from metabolism or from other degradation caused by moisture, enzymes, or pH effects, and making it bioavailable only at a controlled rate. The active ingredient may be protected from moisture during the manufacturing process, as necessary or desired, and more importantly may be protected from moisture and the immediate septic environment until well after the patient has applied the composition, and then only at a slow and controlled rate. It is by this process of progressive hydration that the active ingredient remains protected for many hours after administration. It is also by the process of progressive hydration that controlled and sustained release is achieved because only that part of the active ingredient that is the hydrated (aqueous) fraction of the composition is available for absorption (bioavailable).
Owner:JUNIPER PHARMA INC
Pluggable transceiver module with extended release and removal lever
InactiveUS20020093796A1Easy to operateMinimal costCoupling device detailsCoupling light guidesTransceiverEngineering
A pluggable transceiver module having a housing with a first side and a face perpendicular to the first side, and a tab extending above the surface of the first side sized to mate with a slot in a receptacle for the housing, a wedge slidably mounted on the first side proximate the tab, and a lever attached to the wedge extending beyond the face of the housing, wherein pressing the lever causes the wedge to slide between the tab and the slot on the receptacle and remove the tab from within the slot, thereby releasing the transceiver module from the receptacle, and the lever further including a recess enabling a person to at least partially insert a fingernail to easily grip and remove the pluggable transceiver module from the receptacle.
Owner:STRATOS INT
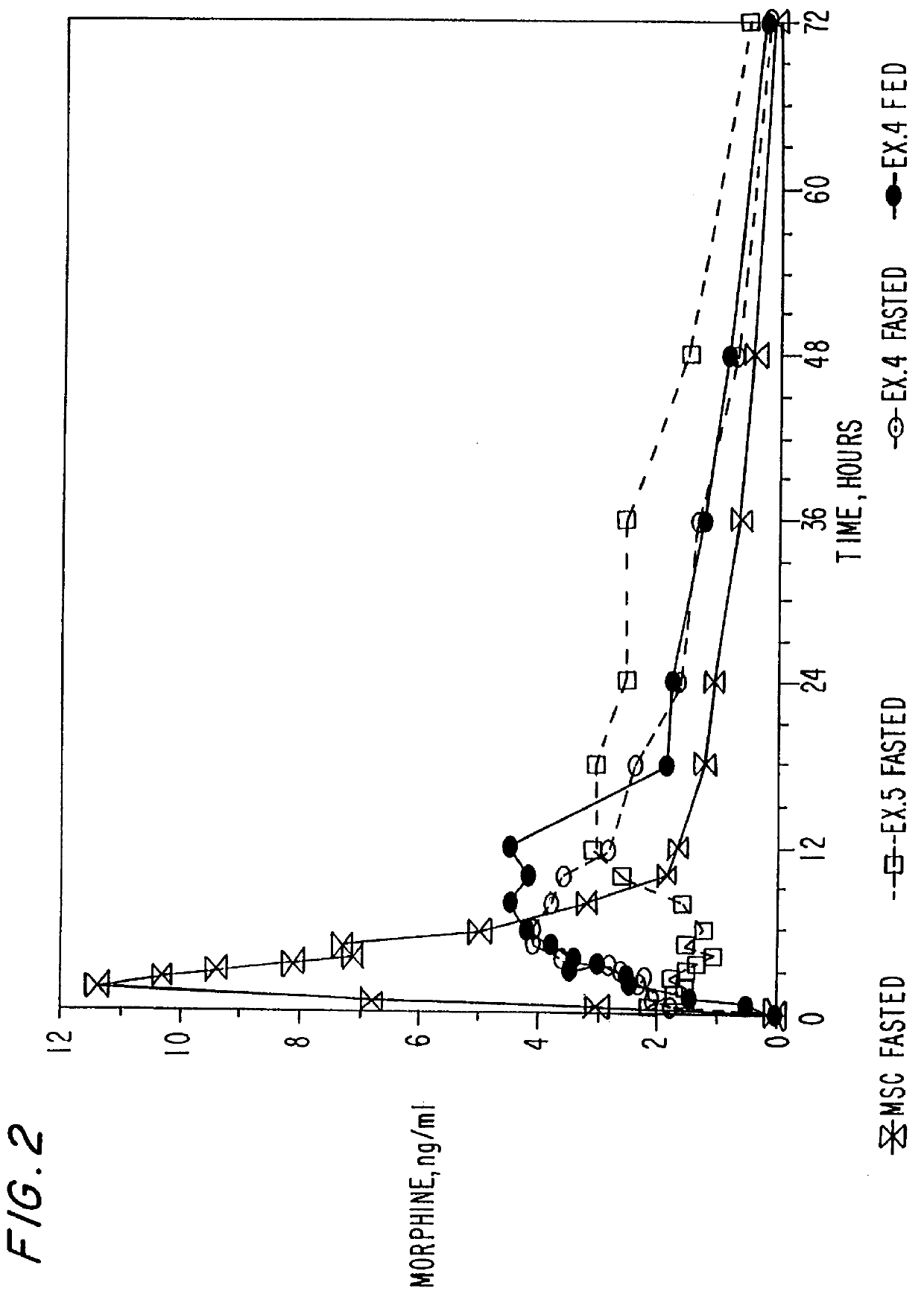
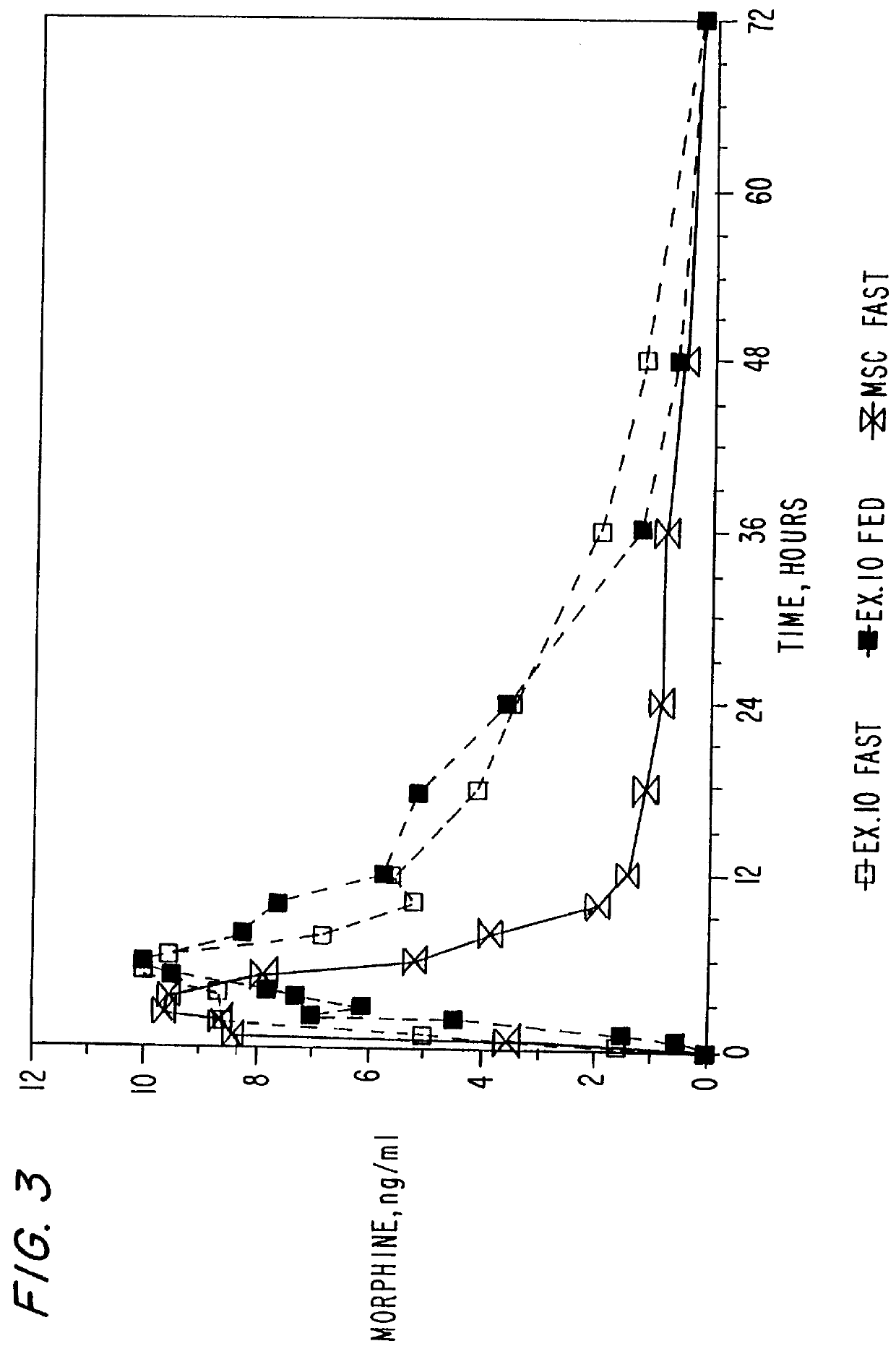
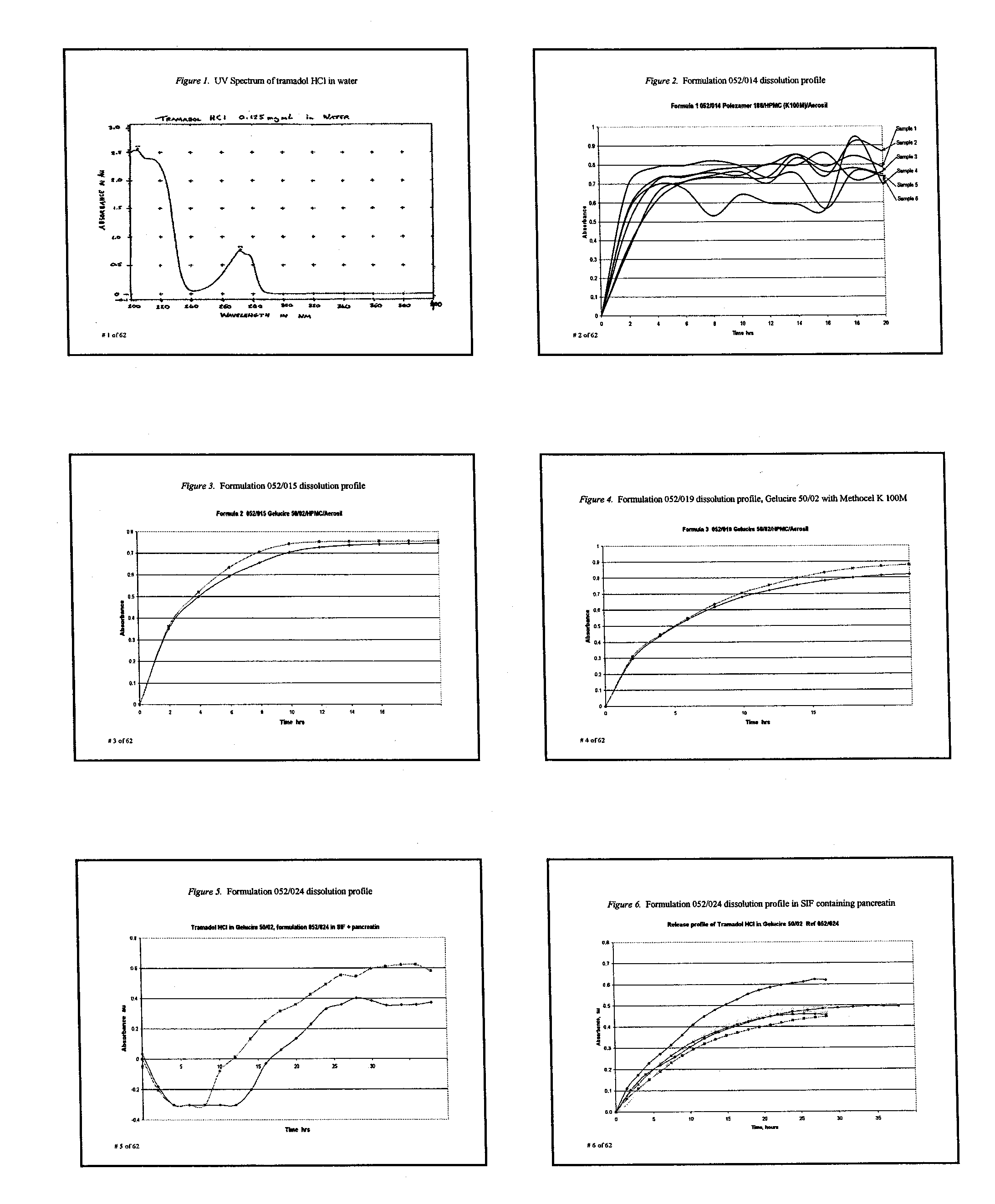
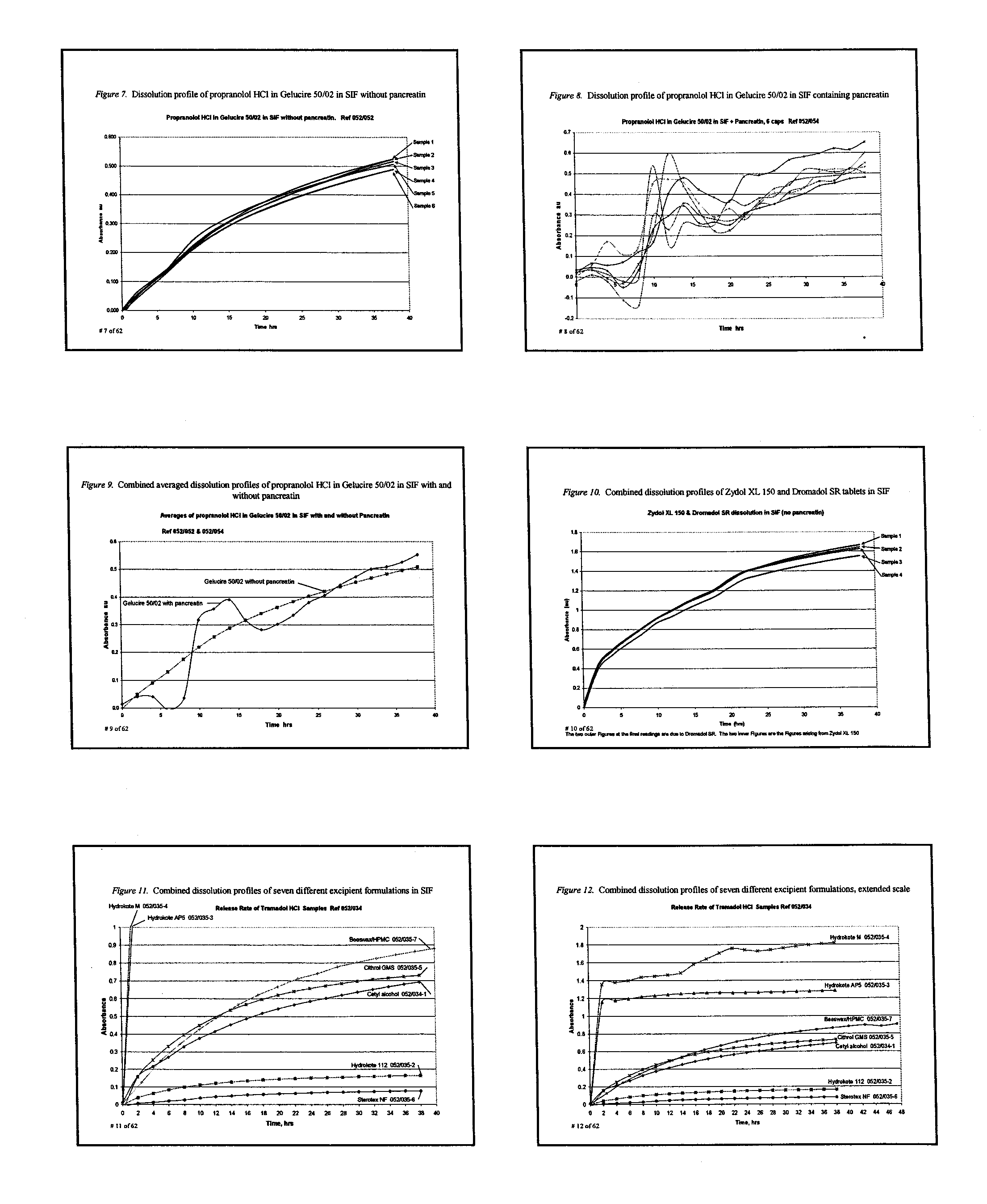
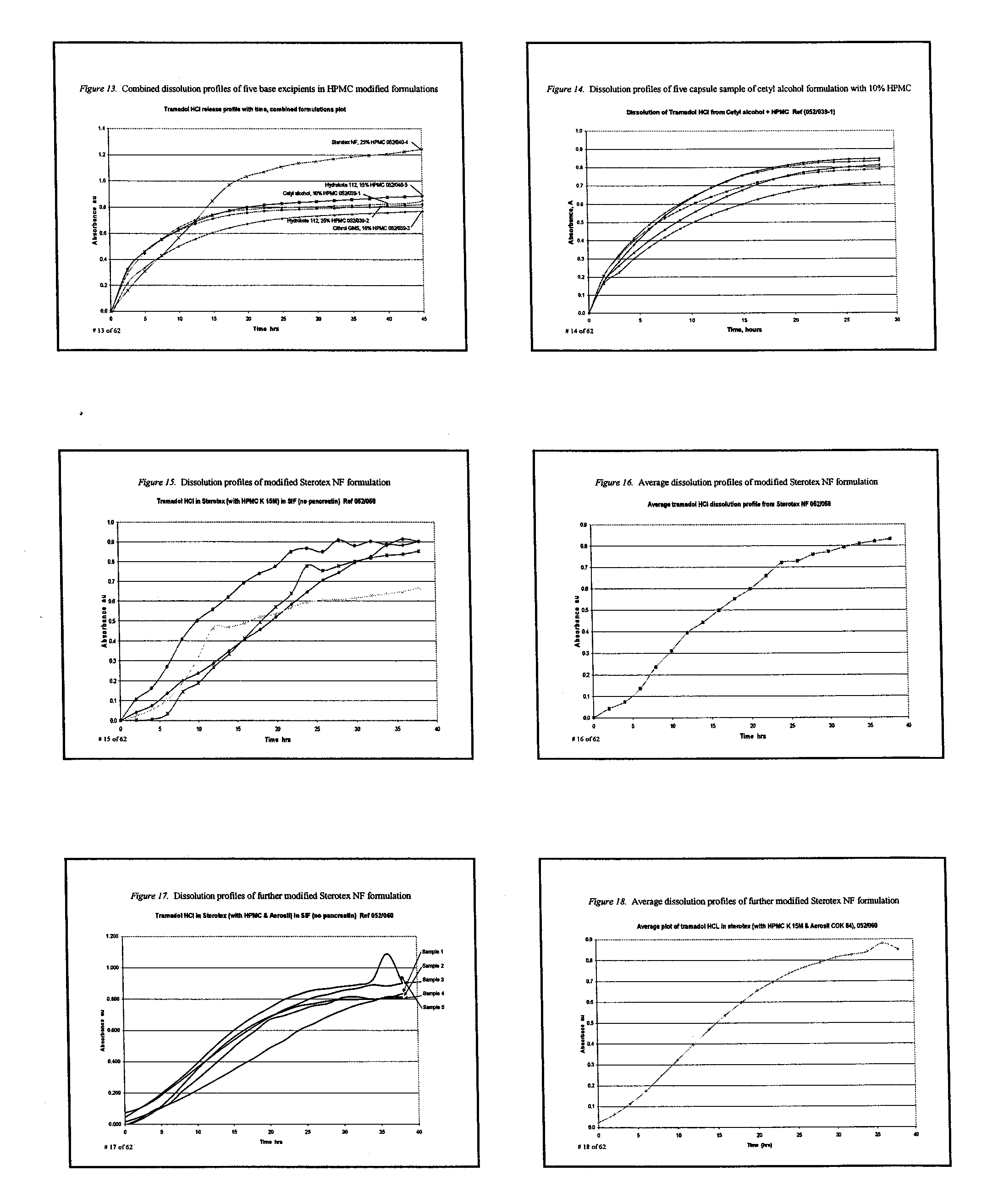
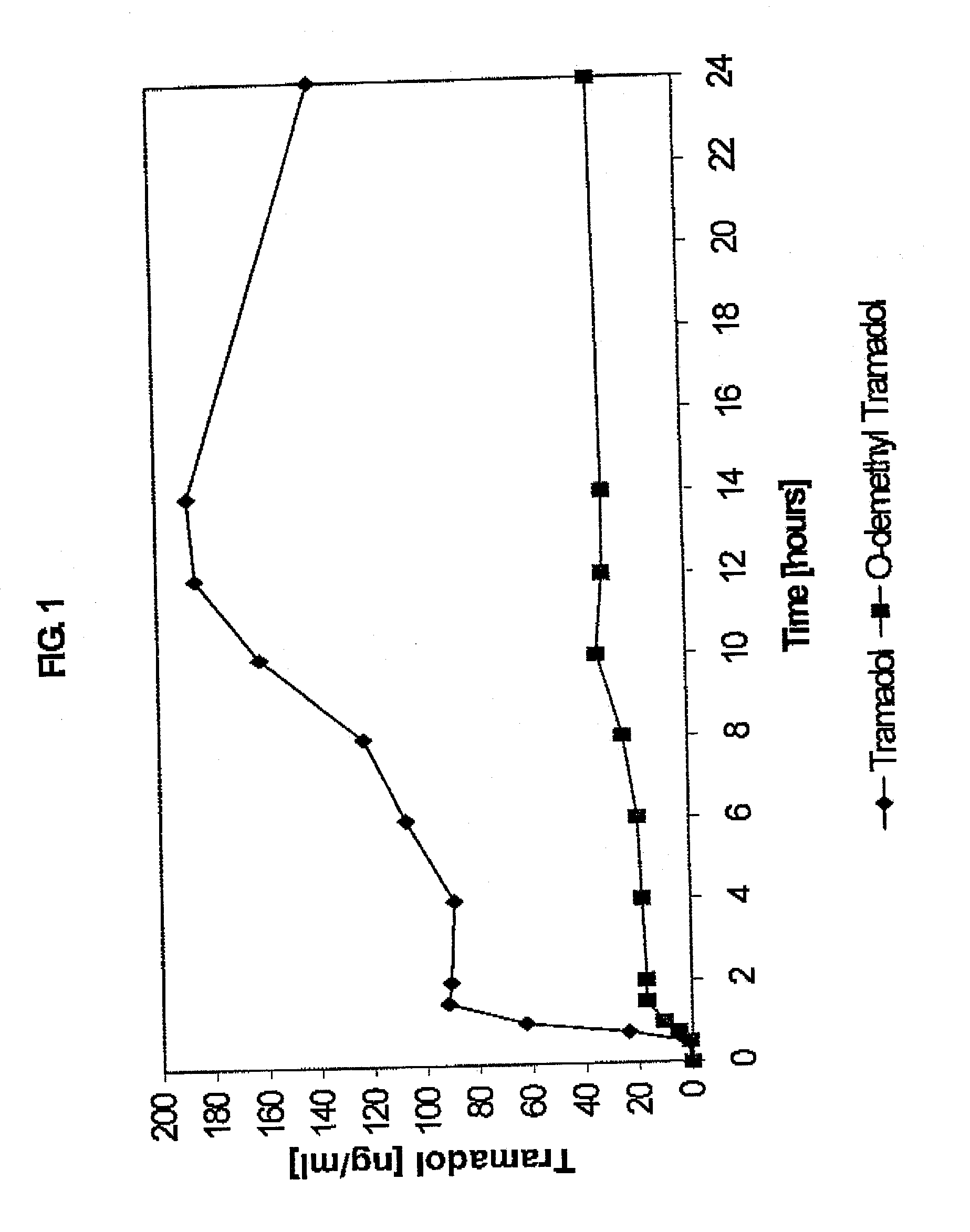
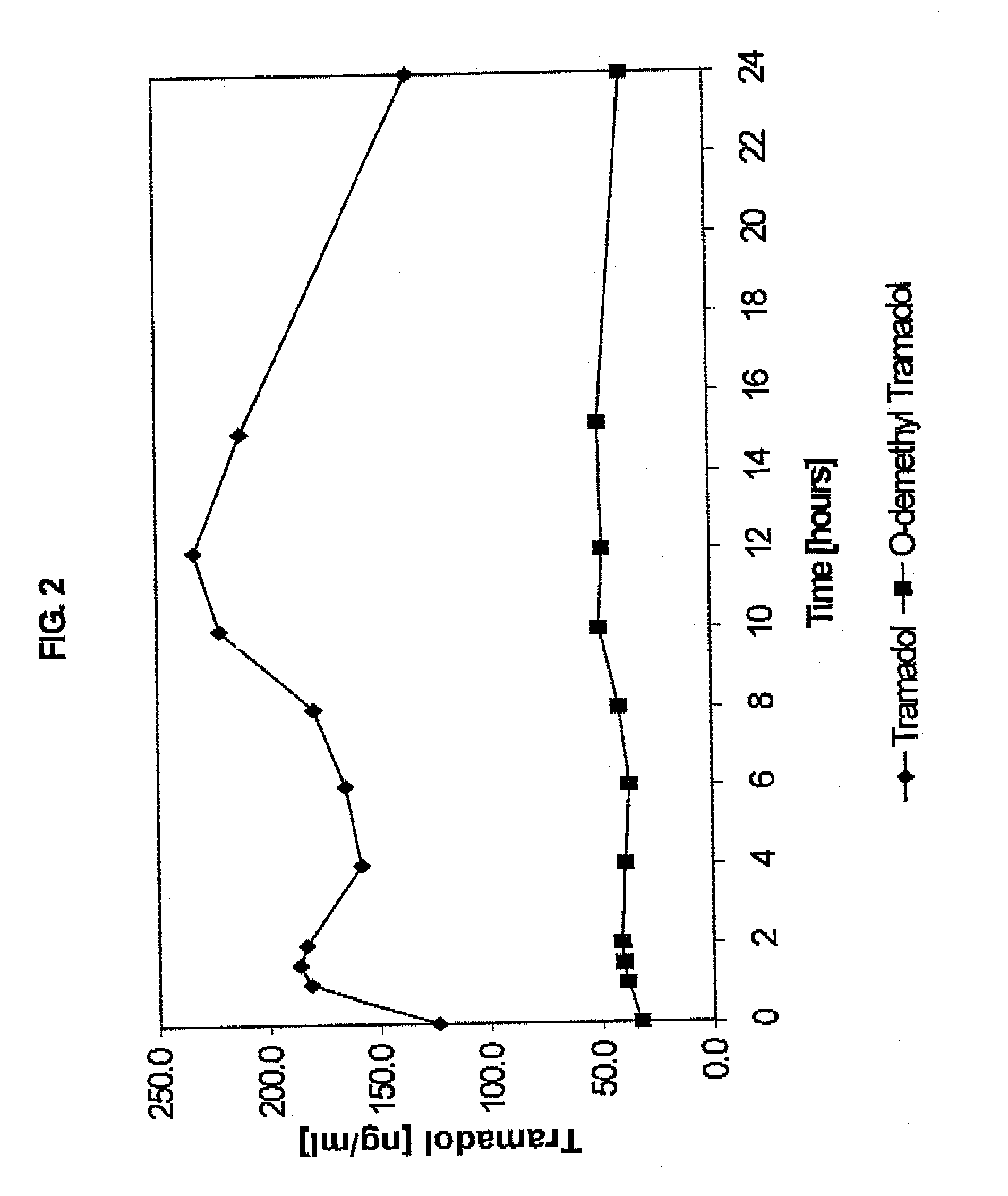
Extended release composition containing Tramadol
InactiveUS20030143270A1Effective controlRelieve painPowder deliveryBiocideBlood concentrationPeak concentration
The present invention relates to a once daily extended release pharmaceutical preparation of Tramadol or its acceptable pharmaceutical salts. The preparation provides, effective blood concentration for a period of about 24 hours with reduced peak concentrations. It is characterized that effective Tramadol levels appear within the first hours after administration, the time to maximal Tramadol content Tmax is at least 10 hours and the peak Tramadol concentration is less than three times the concentration obtained after 24 hours of said administration.
Owner:GALEPHAR PHARMA RES
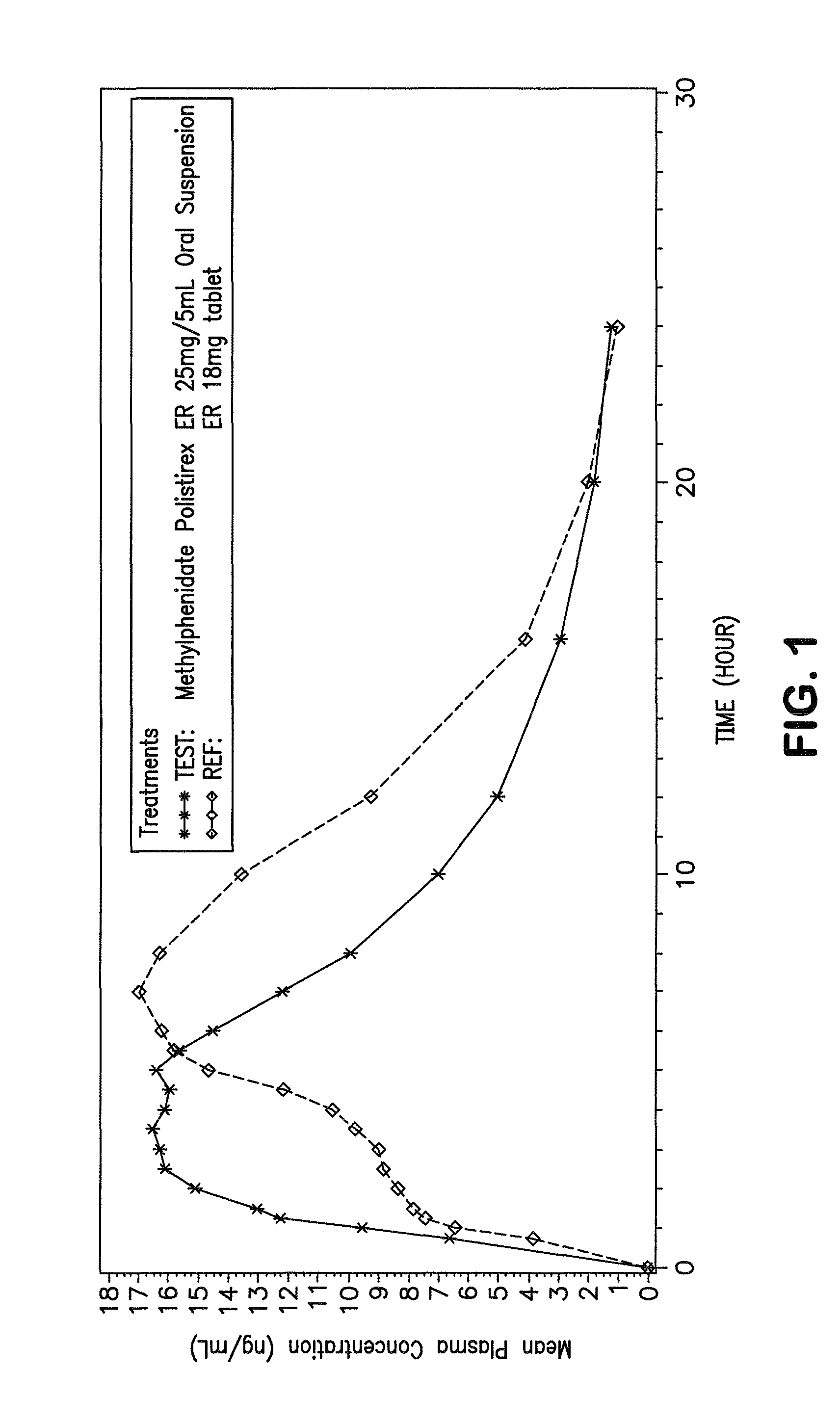
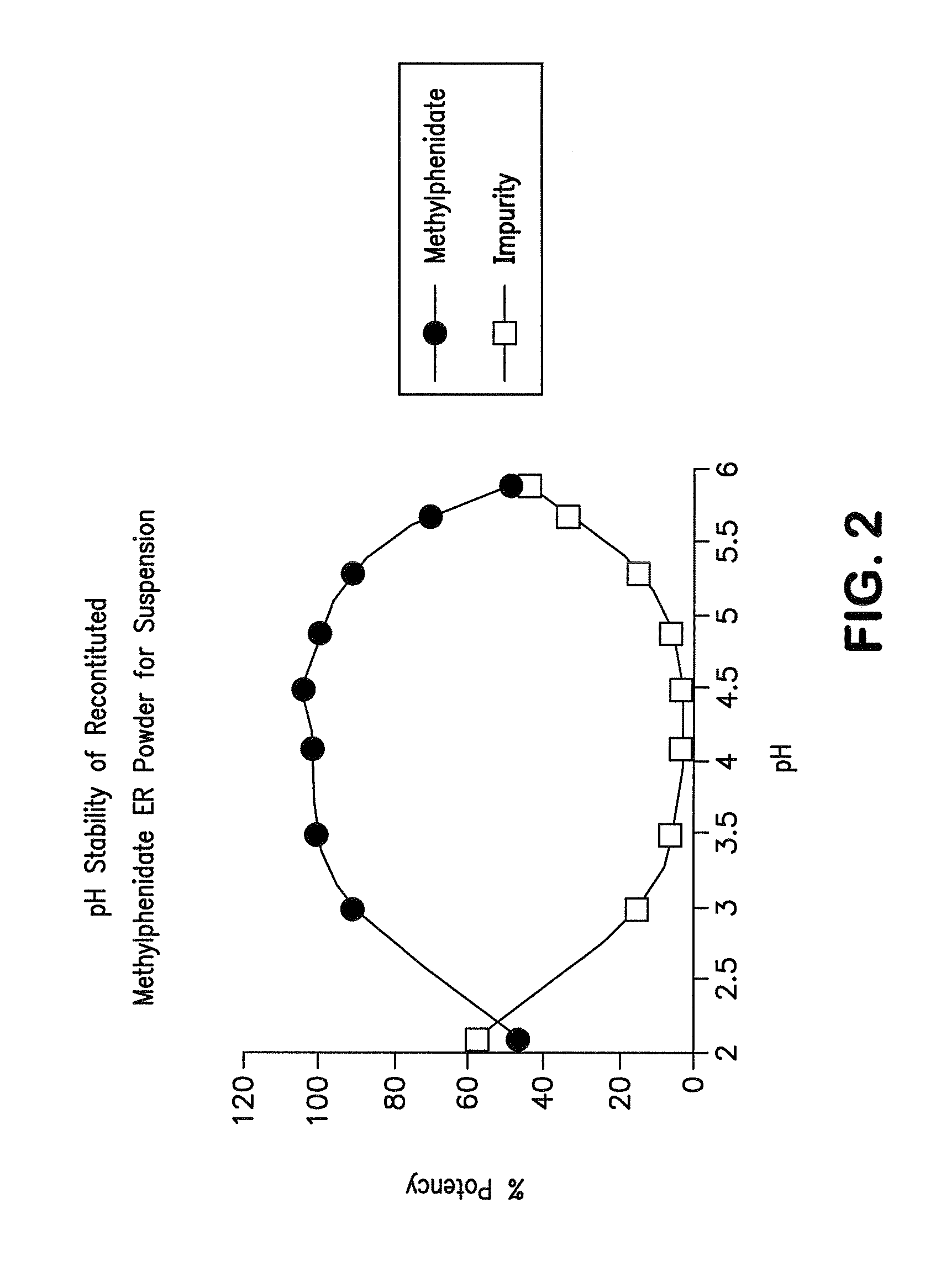
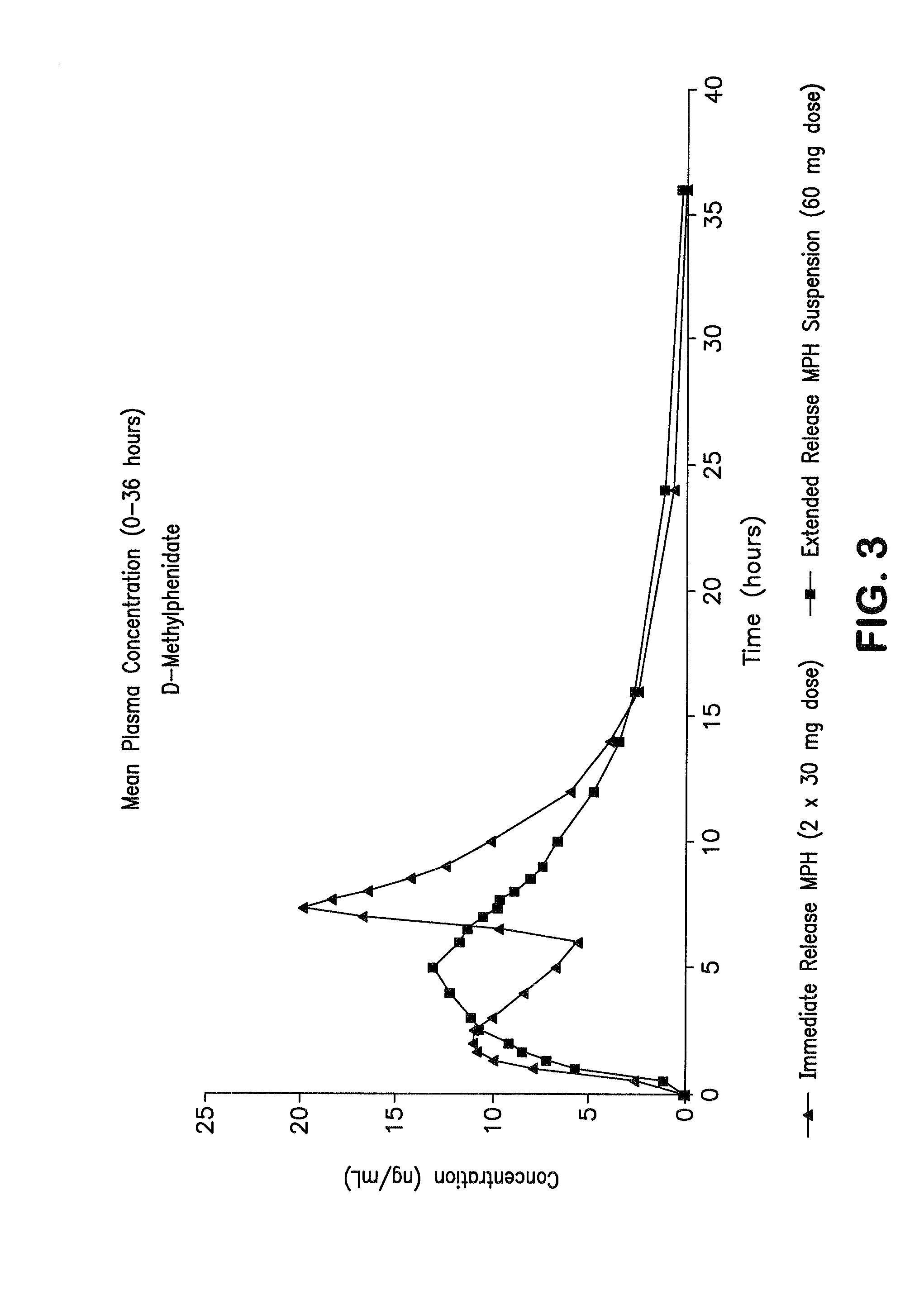
Orally effective methylphenidate extended release powder and aqueous suspension product
An oral methylphenidate powder which is reconstitutable into a final oral aqueous sustained release formulation containing at least about 50%, or at least about 80% by weight water based on the total weight of the suspension, is provided. The powder is a blend containing a combination of an uncoated methylphenidate-ion exchange resin complex, a barrier coated methylphenidate-ion exchange resin complex-matrix, and a water soluble buffering agent such that upon formed into an aqueous liquid formulation, the formulation has a pH in the range of about 3.5 to about 5, or about 4 to about 4.5. Following administration of a single dose of the oral aqueous methylphenidate suspension, a therapeutically effective amount of methylphenidate is reached in less than one hour and the composition provides a twelve-hour extended release profile.
Owner:TRIS PHARMA
Controlled extended drug release technology
ActiveUS20060003007A1Longer resident timeEasy to controlCapsule deliveryCoatingsActive agentWater insoluble
A controlled extended drug release technology for the controlled extended release of hydrophobic or hydrophilic drugs or therapeutically active agents consisting of a homogeneous blend of one or more therapeutic agents, gas generators and surrounded by one or more layers of coat made of thermoplastic water insoluble cellulose derivatives, acrylic polymers, superdisintegrants and optionally an oil, antioxidants and electrolytes. The technology platform is capable of releasing therapeutic agents via zero, first or pseudo first order release.
Owner:INTELLIPHARMACEUTICS
Novel clonidine formulation
Owner:TRIS PHARMA
Extended Release Pharmaceutical Composition Of Entacapone Or Salts Thereof
InactiveUS20110229561A1Reducing “ wearing off ” phenomenonReduce wearBiocideNervous disorderTriple combinationCarbidopa
There is provided an extended release pharmaceutical composition comprising from about 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also provides an extended release pharmaceutical composition comprising triple combination of from about 30 mg to about 300 mg of levodopa, 10 mg to about 100 mg of carbidopa and 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also relates to process of preparation of such compositions.
Owner:WOCKHARDT LTD
Combined immediate release and extended release analgesic composition
InactiveUS20060240128A1Lower potentialGood effectPowder deliveryBiocideAnalgesics drugsImmediate release
The present invention pertains to an analgesic composition comprising an analgesic drug in an extended release form in combination with an analgesia-enhancing amount of a nontoxic N-methyl-D-aspartate receptor antagonist in an immediate release form.
Owner:ENDO PHARMA INC
Pharmaceutical composition and method for treating
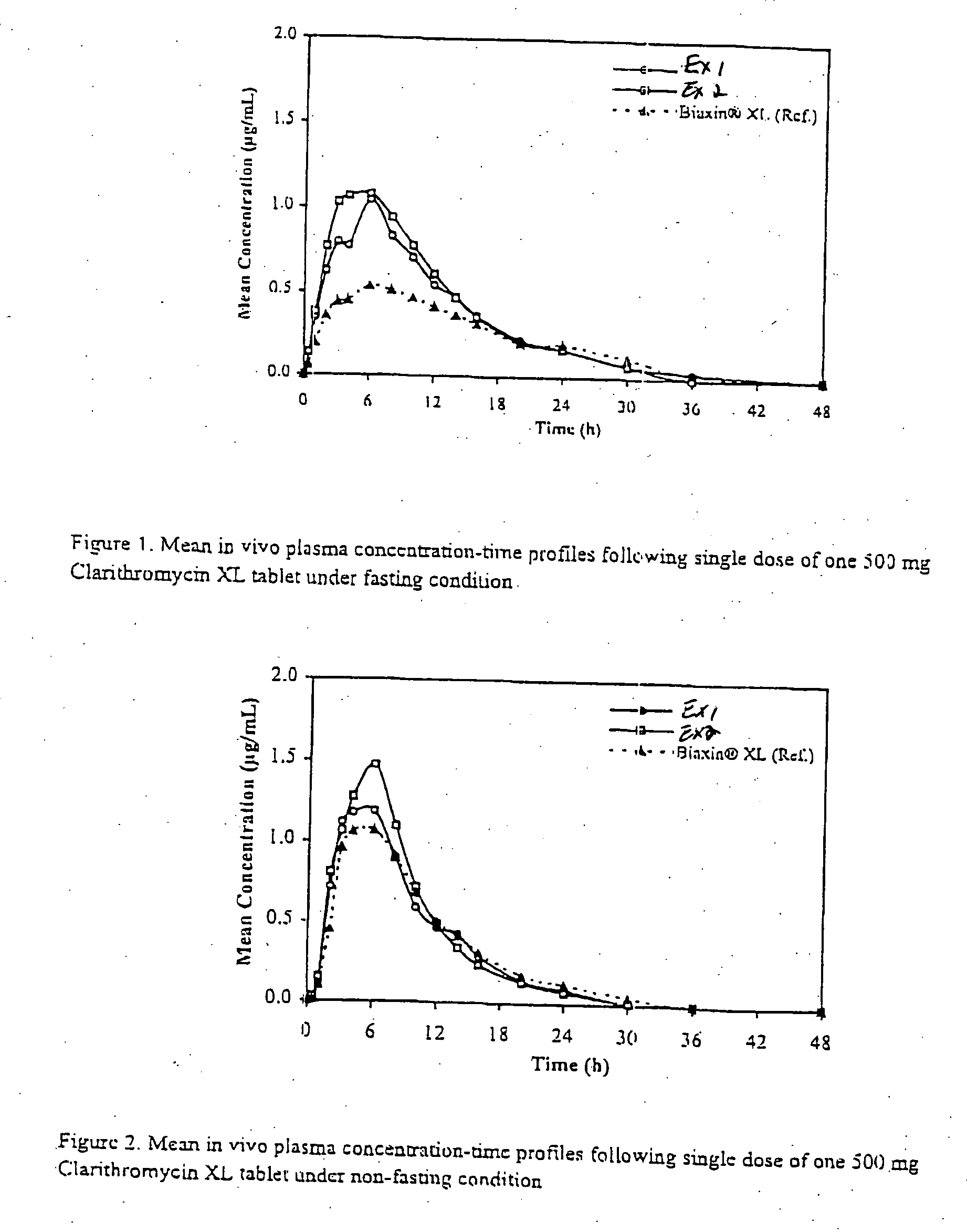
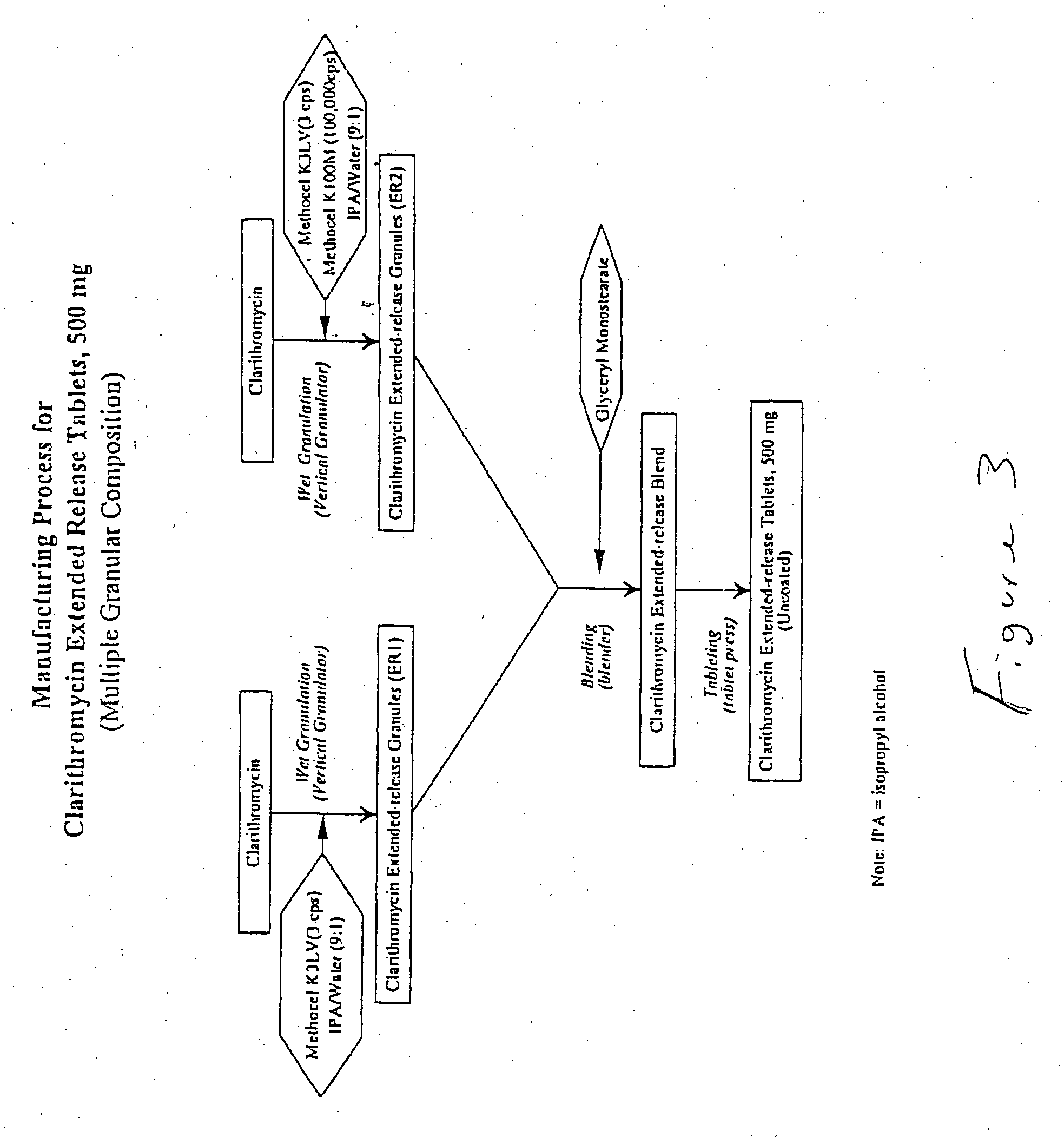
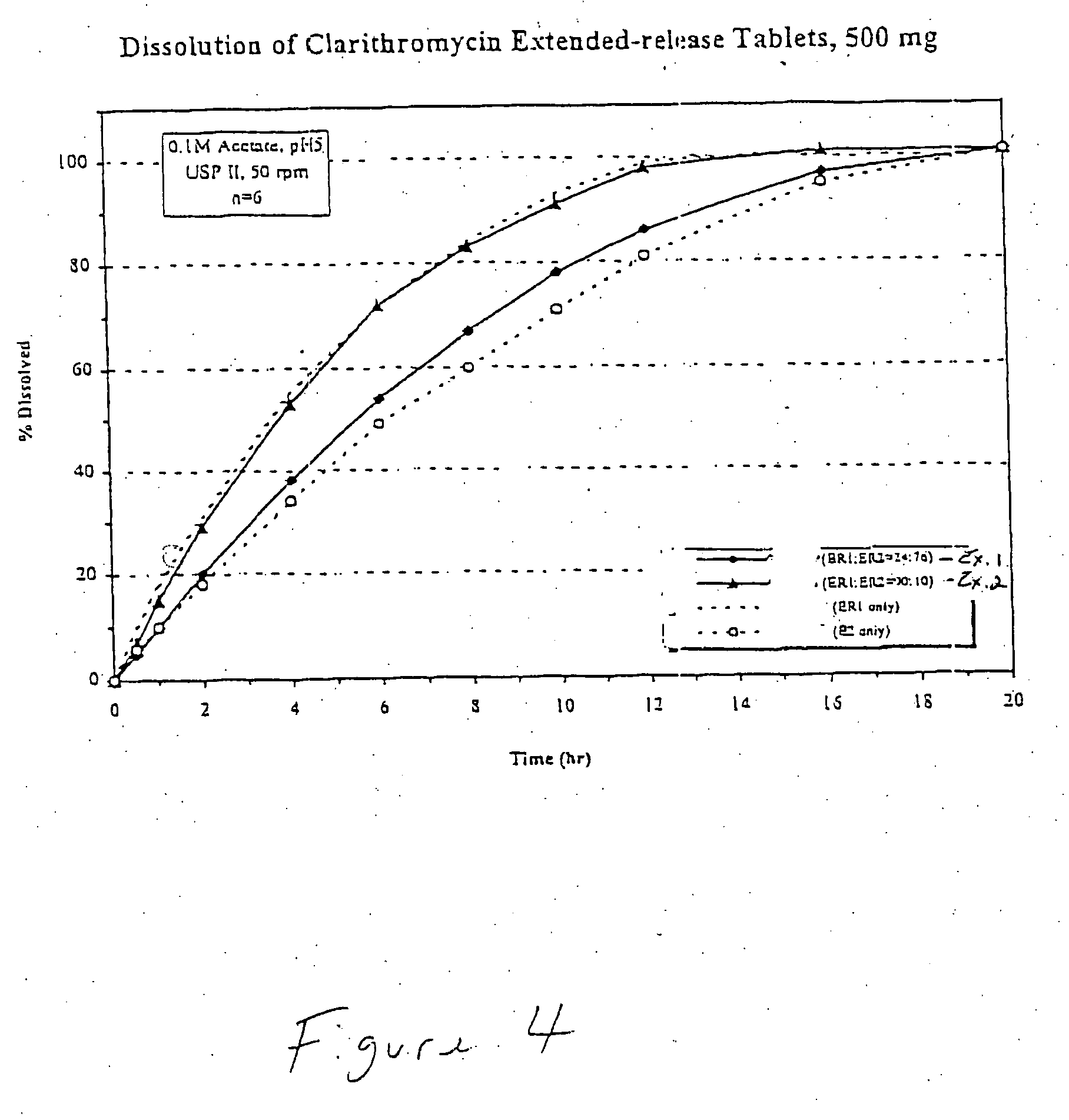
Embodiments of the invention generally provide pharmaceutical drug compositions, methods of preparing oral drug compositions, such as extended release dosage compositions, and methods for treating infection. More particularly, the invention relates to formulations containing a drug and a carrier material. In one aspect, the invention provides a pharmaceutical formulation including a therapeutically active agent, from about 0.1% to about 4.9% by weight of a pharmaceutically acceptable polymer, and from about 0.1% to about 30% by weight of a pharmaceutically acceptable acid, wherein the pharmaceutical composition have a zero order release profile of the therapeutically active agent. In another aspect, the invention provides methods for preparing and administering a pharmaceutical antibiotic composition in oral dosage form, such as a tablet.
Owner:BIOKEY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com