Extended release biodegradable ocular implants
a biodegradable, ocular implant technology, applied in the direction of drugs, prostheses, immunological disorders, etc., can solve the problems of more serious vision loss, slow or sudden painless loss of vision, and macula break down
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Dexamethasone Three Implant Extended Release System
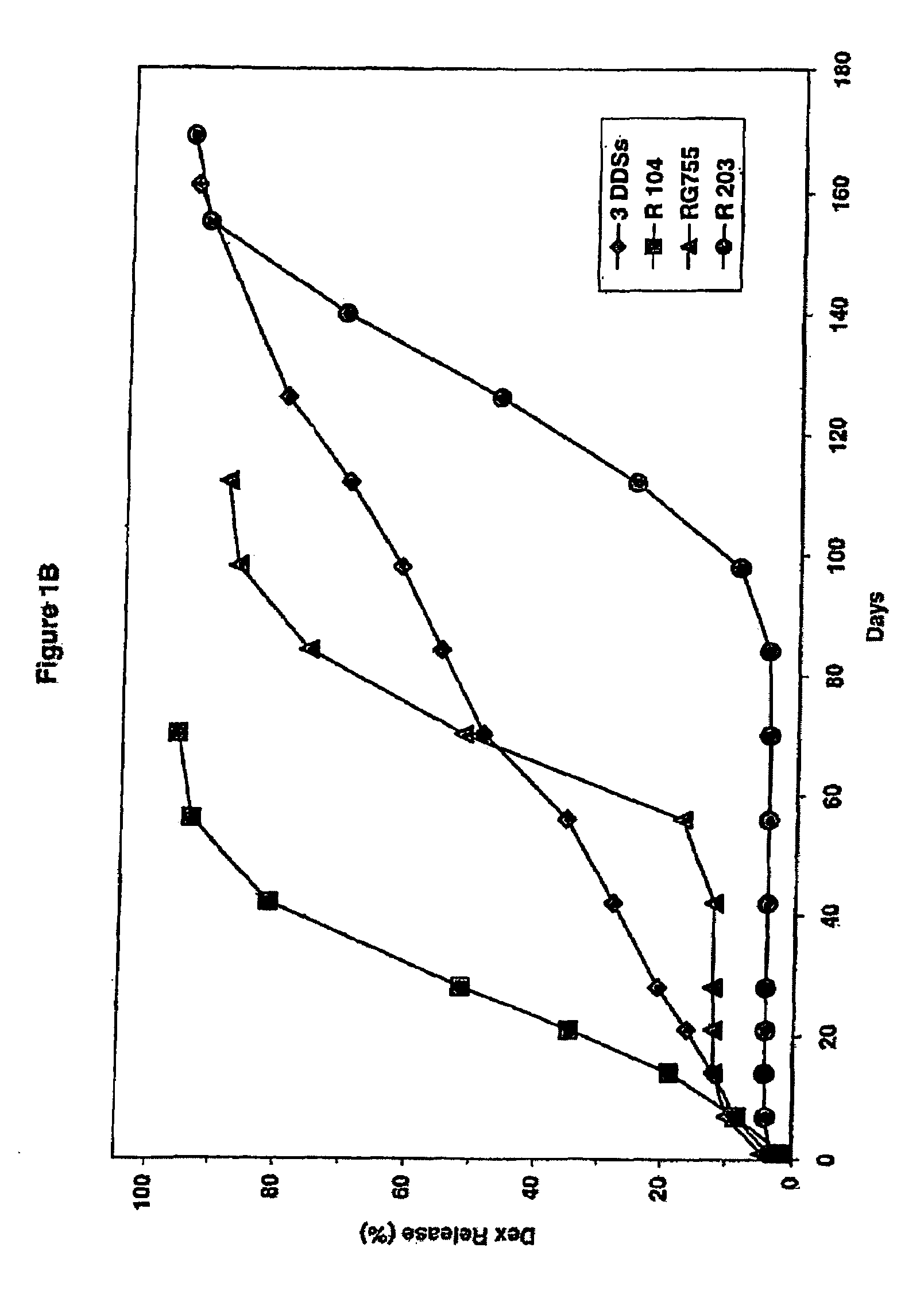
[0114]A bioerodible implant system for extended delivery of dexamethasone was made by mixing the active agent dexamethasone (Pharmacia Corp., Peapack, N.J.) separately with each of the following three different polymers:
[0115]1. poly (D,L-lactide) (R104, Boehringer Ingelheim GmbH, Germany),
[0116]2. poly(D,L-lactide-co-glycolide) as a 75:25 (wt % / wt %) blend of lactide:glycolide (RG755, Boehringer Ingelheim GmbH, Germany), and;
[0117]3. poly (D,L-lactide) (R203, Boehringer Ingelheim GmbH, Germany), so as to obtain three different dexamethasone-polymer mixes.
[0118]R203 and R104 both are poly(D,L-lactide) polymers, but with different molecular weights. The molecular weight for R203 is about 14,000, while the molecular weight for R104 is about 3,500. RG755 is a poly(lactide-co-glycolide) co-polymer. The molecular weight of RG755 is about 40,000.
[0119]The dexamethasone and one of the three polymers specified above were thor...
example 2
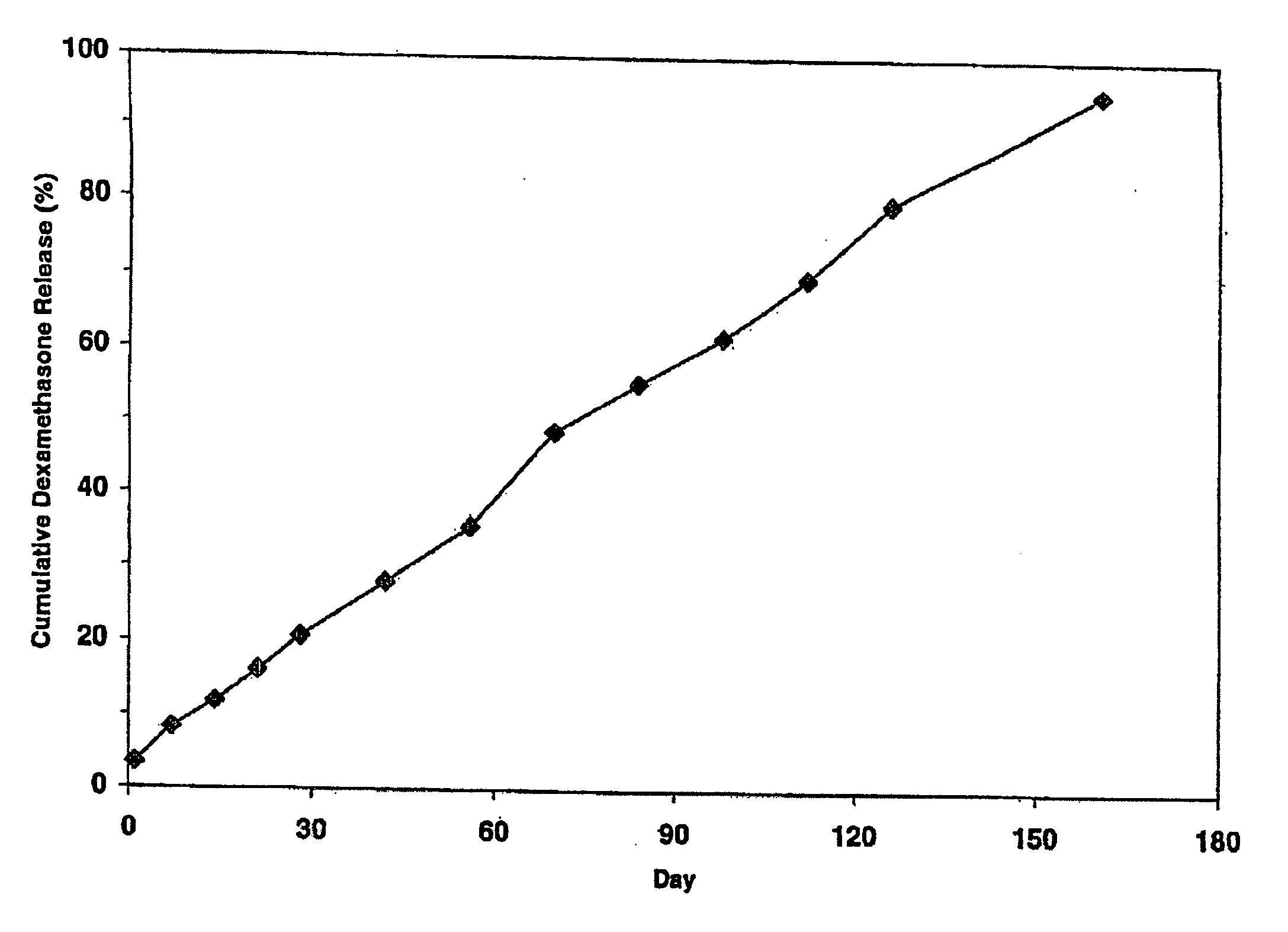
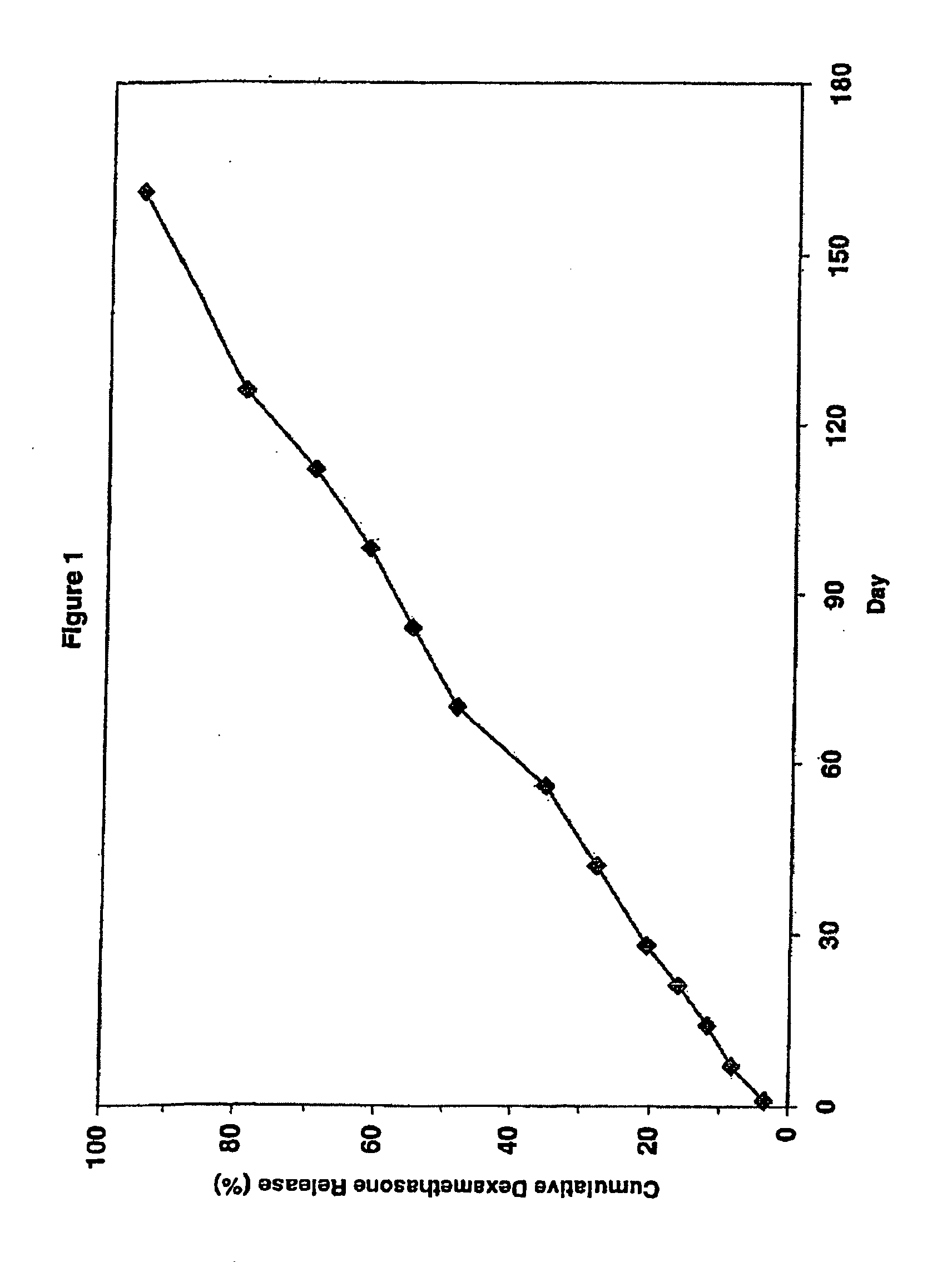
In vitro Release of Dexamethasone from Three Implant Extended Release System
[0121]Cumulative release of dexamethasone from the three implant system of Example 1 was measured in vitro. The three implant system was placed in a glass vial filled with receptor medium (0.1 M phosphate solution, pH 4.4, at 37 degrees C.). To allow for “infinite sink” conditions, the receptor medium volume was chosen so that the concentration would never exceed 5% of saturation. To minimize secondary transport phenomena, e.g. concentration polarization in the stagnant boundary layer, the glass vial was placed into a shaking Water bath at 37° C. Samples were taken for HPLC analysis from the vial at defined time points. The concentration values were used to calculate the cumulative release data, as shown in Table 1 and the corresponding FIG. 1. In Table 1 “Day” is the day of the in vitro measurement of the cumulative amount of dexamethasone released from the three implants, “Cum.” is an abbreviation for cumm...
example 3
In vivo Release of Dexamethasone from Three Implant Extended Release System
[0124]The three implant system of Example 1 was implanted into the vitreous of the eyes of eight rabbits. This was carried out by loading the three implants of Example 1 into a simple trocar with a sample holder and plunger, making an incision through the lower front sclera, inserting the trocar through the scleral incision, and depressing the trocar plunger to deposit the three implants of Example 1 into the vitreous. The in vivo vitreous concentrations of dexamethasone were monitored by vitreous sampling, using LC / MS (liquid chromatography and mass spectrometry). The dexamethasone concentrations for each eye were measured at days 7, 30, 60, 90, 120, 150, 180, 210 and 240 and 360 for the three implant system of Example 1. The averaged results of one mixed concentration measurement are set forth by the two left hand side columns of Table 2.
[0125]Comparison studies were also carried out using single (Posurdex)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com