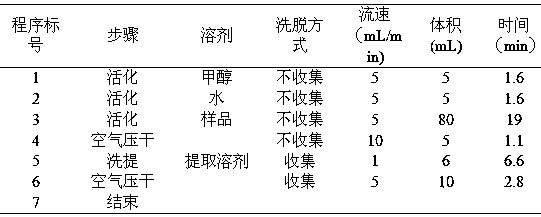
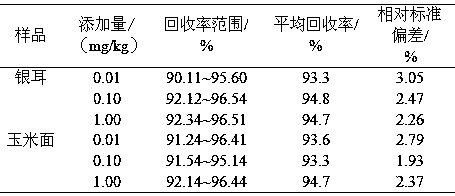
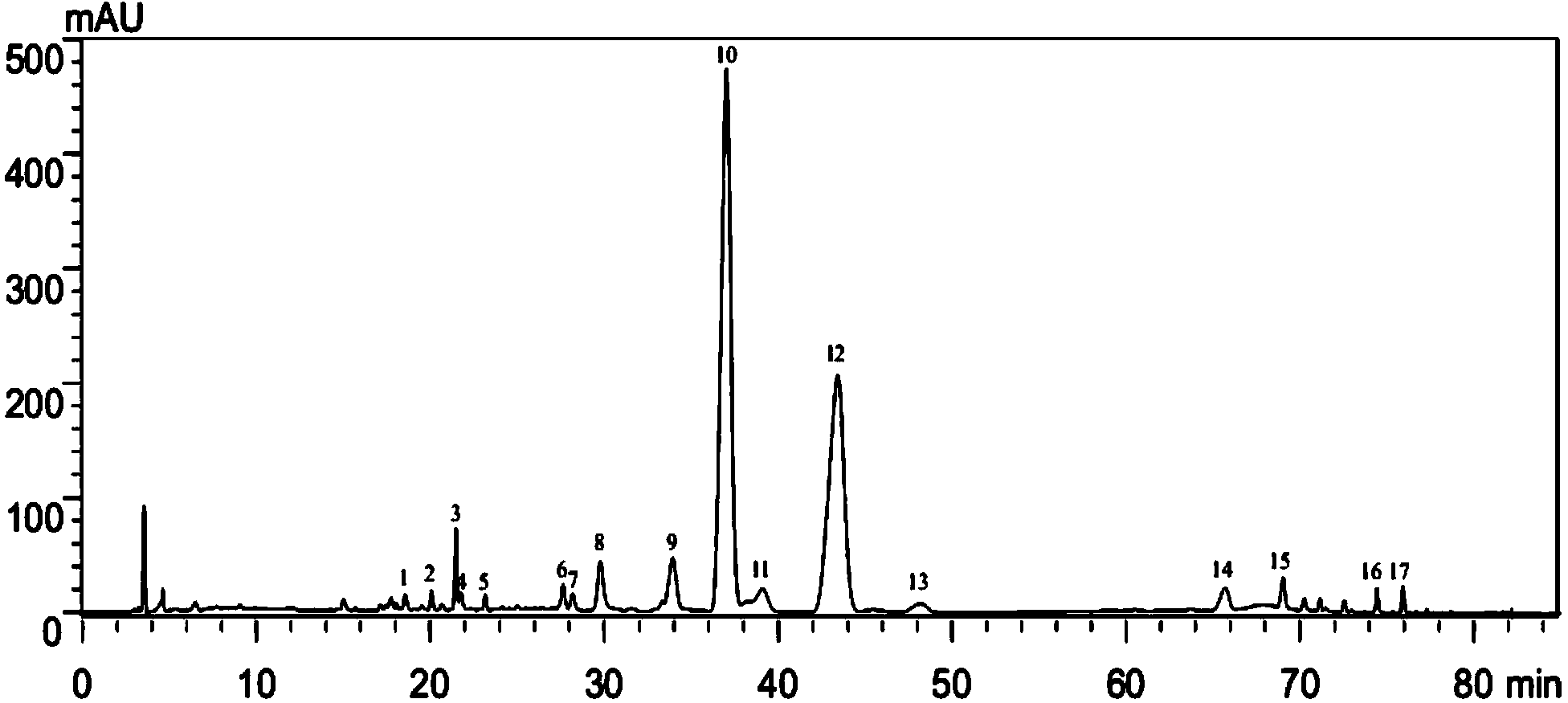
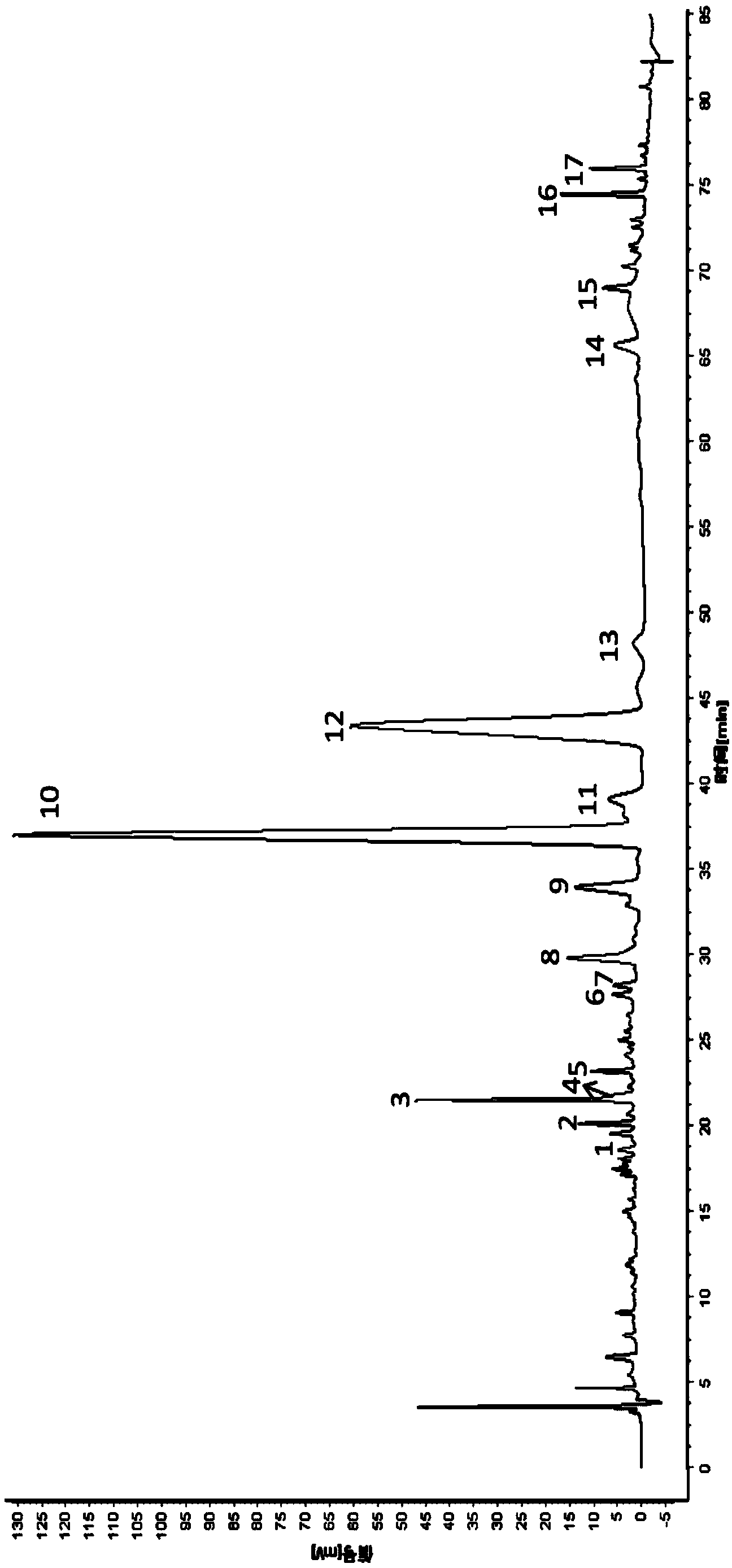
Patents
Literature
297 results about "Hplc analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HPLC is a powerful analytical technique for the separation and analysis of compounds in solution. HPLC is particularly useful for analysis of large molecules and compounds that are either not very volatile or thermally unstable. The principles of HPLC are common to other forms of chromatography where separation is based...
Qualitative and quantitative analysis method for polyoses
ActiveCN101539550AEasy to separateImprove featuresComponent separationMaterial analysis by observing effect on chemical indicatorAdditive ingredientUronic acid
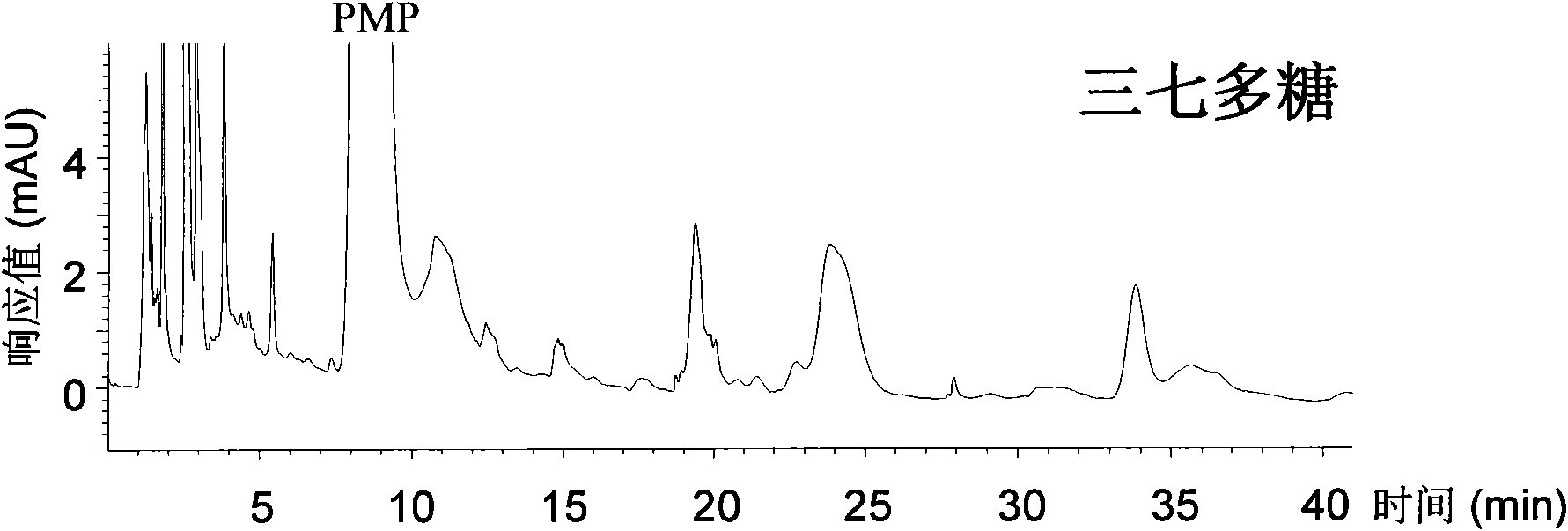
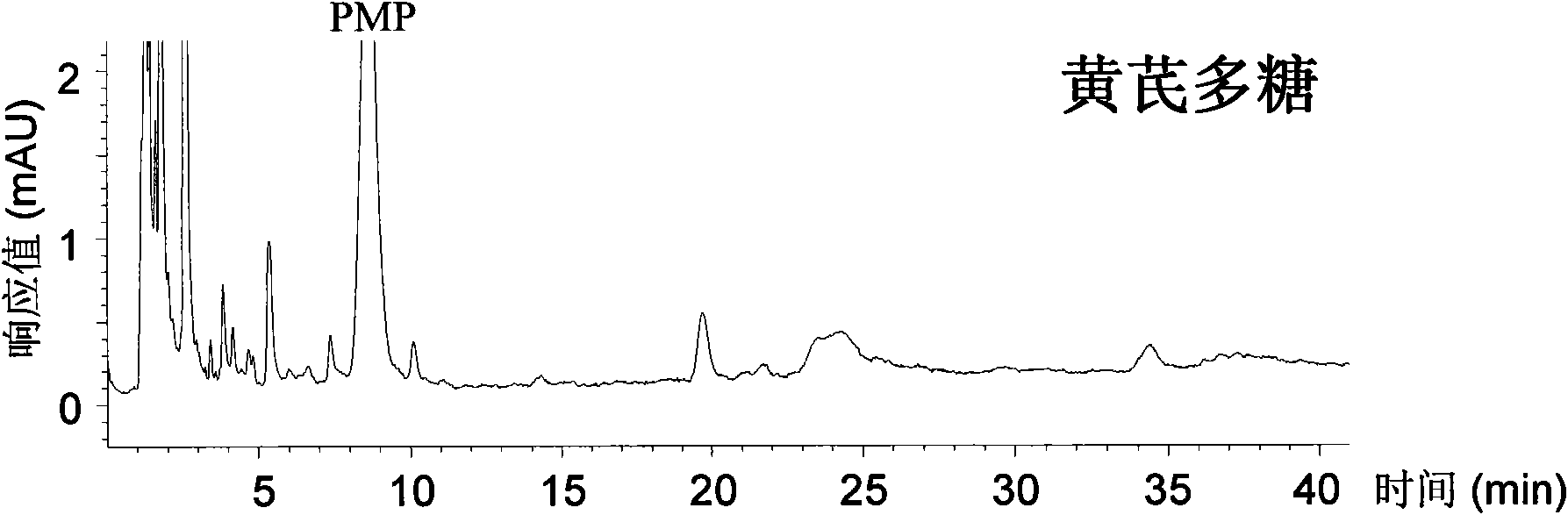
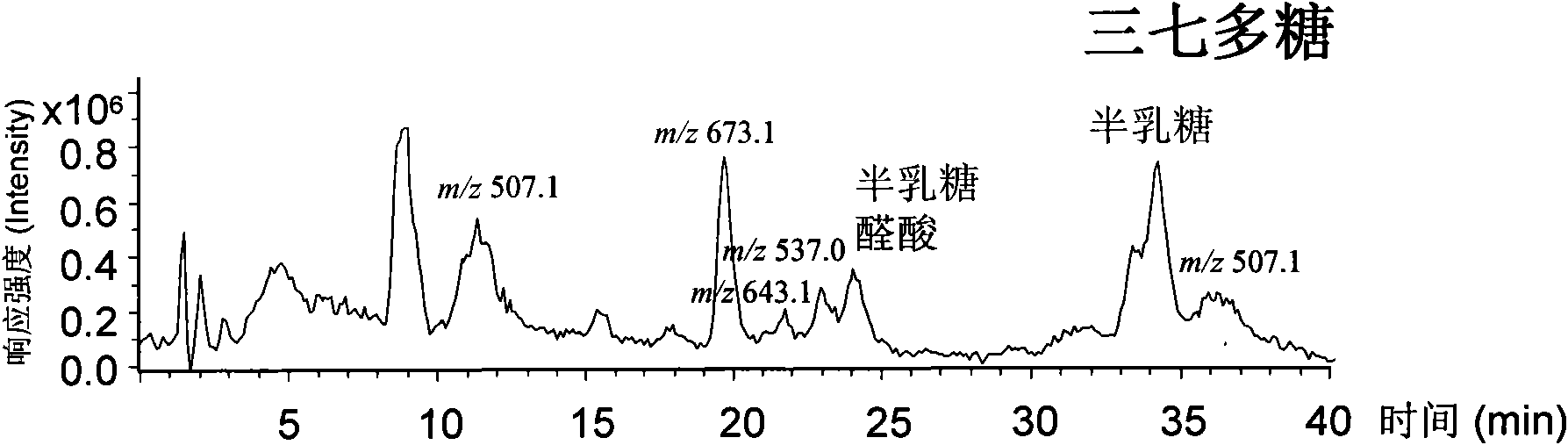
The invention relates to a qualitative and quantitative analysis method for polyoses, which comprises the following steps: (1) the zymohydrolysis and the verification of the polyoses: measuring the content of a polyoses water solution by a colorimetric method; (2) comparing the change of a polyoses mapping before and after zymohydrolysis by HPSEC-ELSD analysis; (3) saccharide ingredient direct HPLC analysis of a polyoses zymohydrolysis solution or HPLC analysis after pre-column derivatization: identifying polyoses mapping and chromatogram peaks by taking a standard monosaccharose, a uronic acid, a disaccharide, and the like as contrasts and taking a chromatogram peak mass-to-charge ratio as reference, and using stable characteristic sections as a quantitative analysis index; and (4) realizing the qualitative and quantitative analysis of the polyoses by establishing polyoses zymohydrolysis responding characteristics and polyoses zymohydrolysis mapping which are based on structural information through the independent or combining application of the steps. The qualitative and quantitative analysis method can be used for the identification and quantitative measurement of the polyoses of traditional Chinese medicine, such as panax, panax pseudoginseng, gen-seng, aweto, cordyceps, ganoderma lucidum, fomes japonica, milk veteh, angelica, and the like and provides an effective method for controlling the quality of the polyoses and the products thereof.
Owner:李绍平
Enzymolysis-HPLC method for detecting enoxaparin
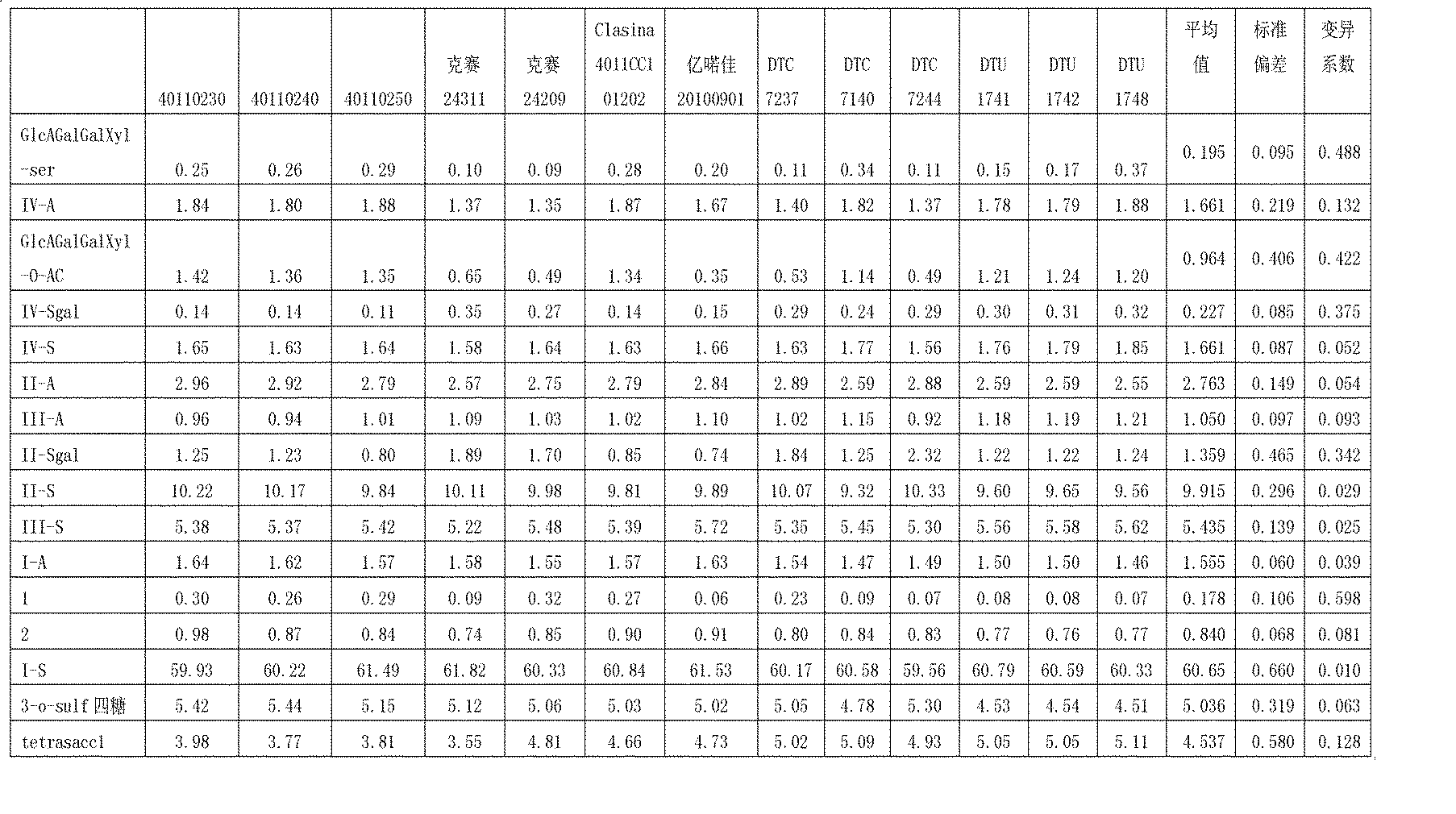
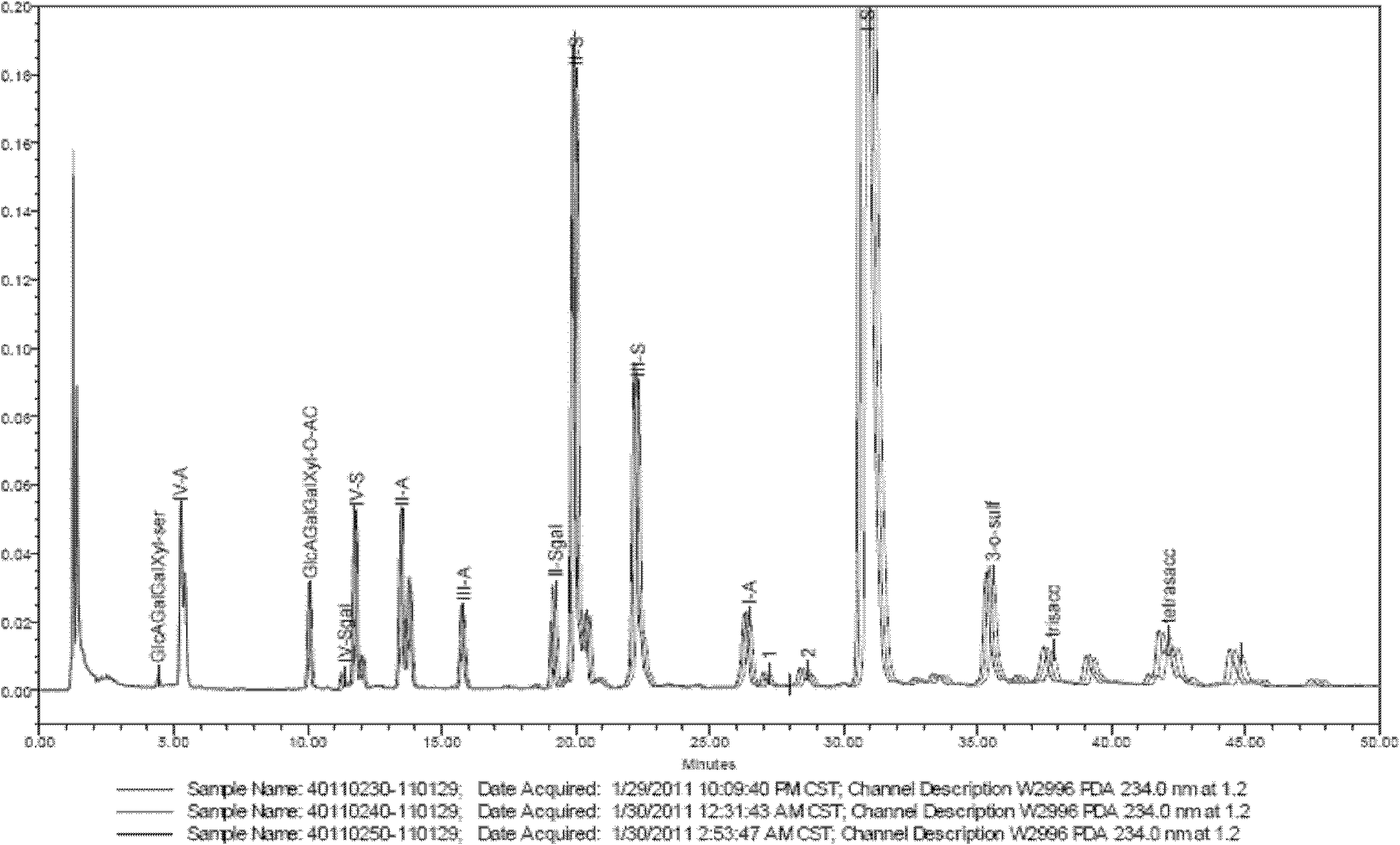
The invention discloses an enzymolysis-HPLC method for detecting enoxaparin. The method comprises steps of: a. complete enzymolysis of enoxaparin: adding a mixed enzyme solution with enzyme I, enzyme II and enzyme III in a ratio of 8:1:2 to an enoxaparin solution with a concentration of 10-200mg / ml and carrying out an enzymolysis at room temperature for 48 h. b. HPLC analysis: carrying out a SAX-HPLC analysis on the degradation products; c. calculation of variety and content of disaccharide and tetrose units: determining variety and content of disaccharide and tetrose units according to a wash out time of prior standard disaccharide and tetrose and calculating percentage content of each of the eight disaccharides and one tetrasaccharide.
Owner:SHENZHEN TECHDOW PHARM CO LTD
Ethanol extract of antrodia camphorata for inducing apoptosis and preparation method thereof
InactiveUS20100210869A1Effectively inhibit growth of leukemiaIncrease polarityOrganic compound preparationFungi medical ingredientsEthyl acetateDrug biological activity
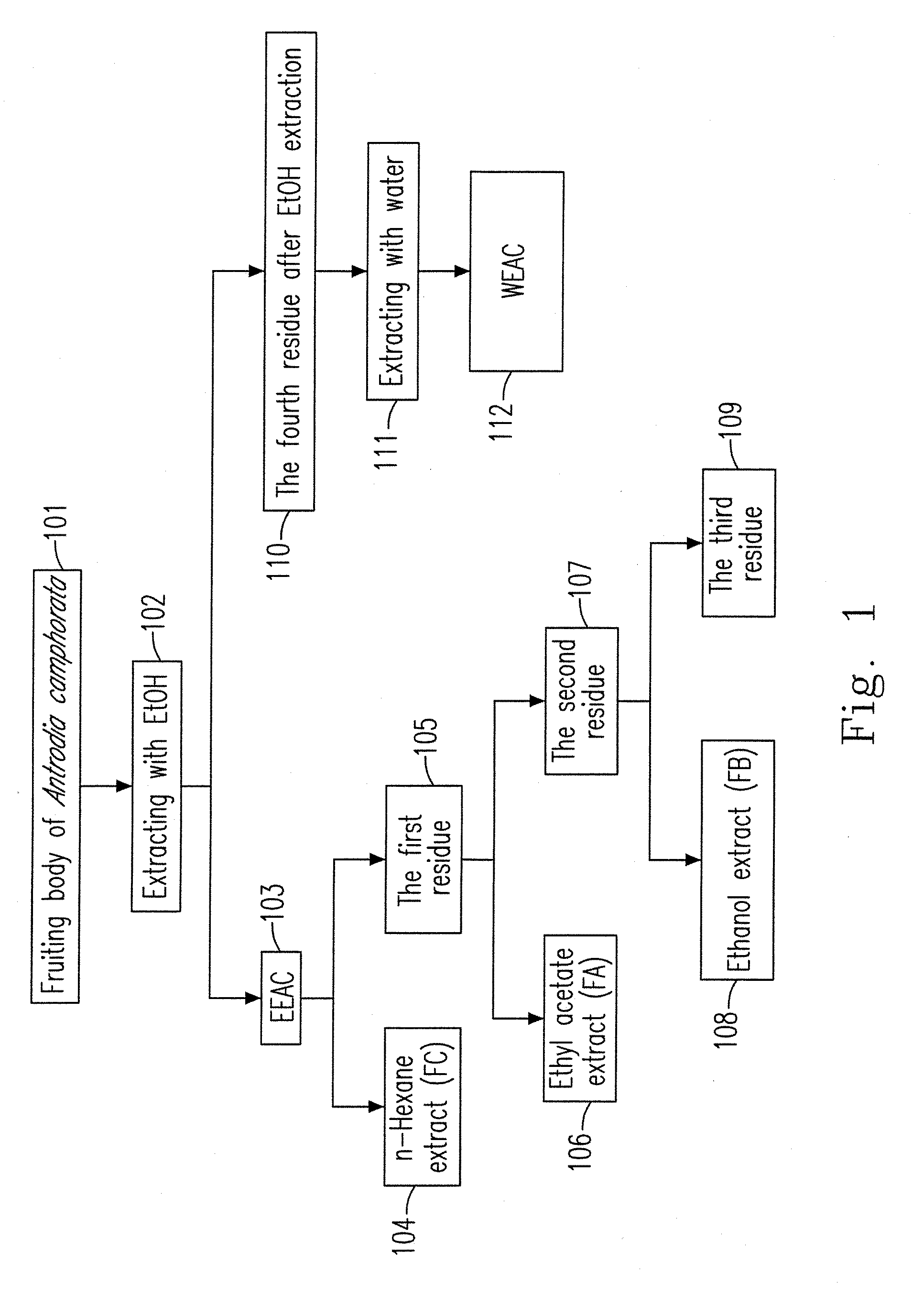
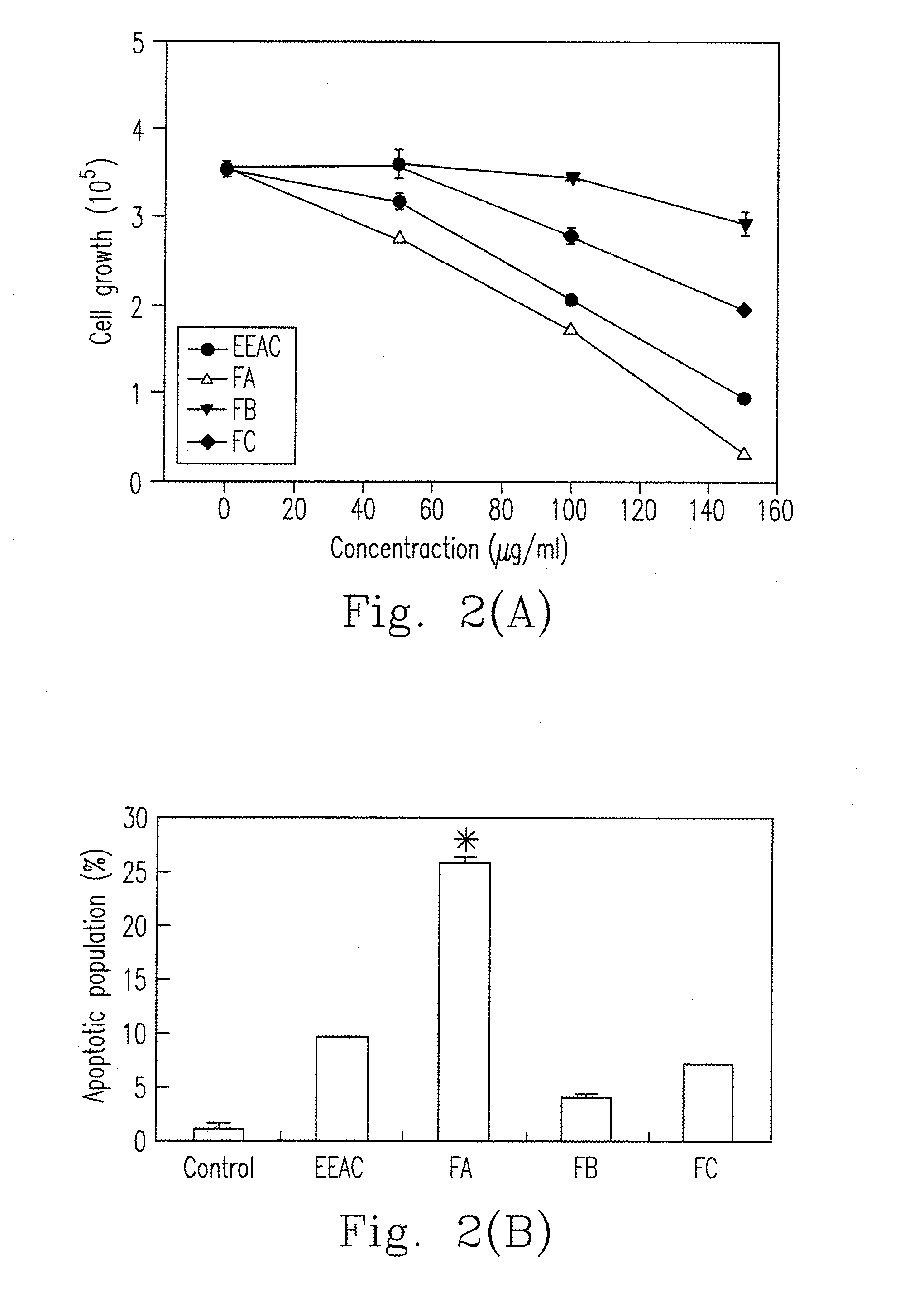
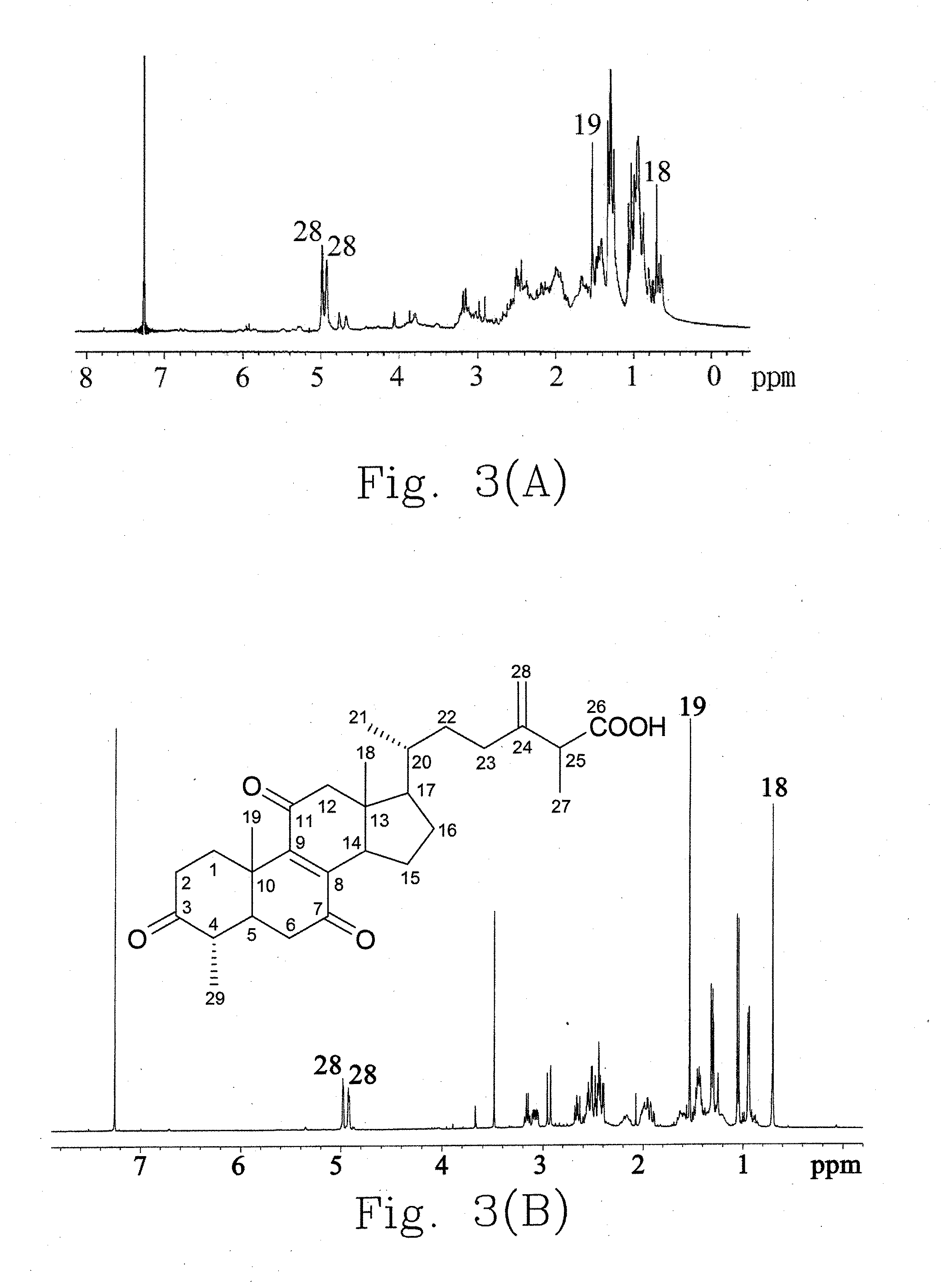
A preparation method for an ethanol extract of the fruiting body of Antrodia camphorata (EEAC) is provided. The preparation method includes steps of: (a) providing the fruiting body of A. camphorata (AC); (b) extracting the fruiting bodies with a first ethanol solution; and (c) obtaining EEAC. EEAC further can be sequentially extracted or fractioned by n-hexane, ethyl acetate and ethanol, and an n-hexane fraction (FC), an ethyl acetate fraction (FA) and an ethanol fraction (FB) respectively are generated. The growth inhibition and apoptosis induction of leukemia cell line HL 60 are effectively mediated by FA product, in which zhankuic acid A is the bioactive marker. The amount of triterpenoid in the fruiting body of AC can be determined by NMR and HPLC analysis.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
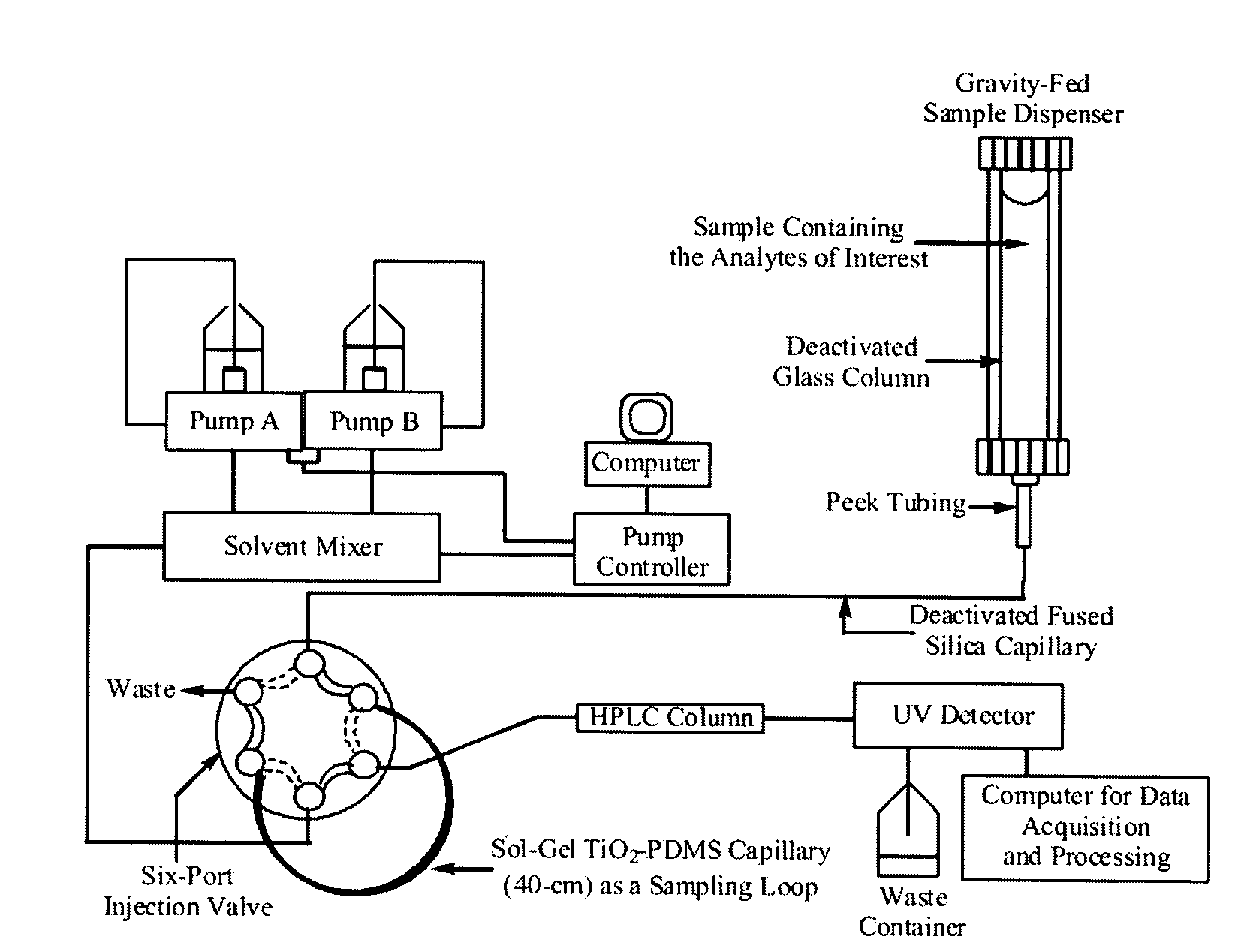
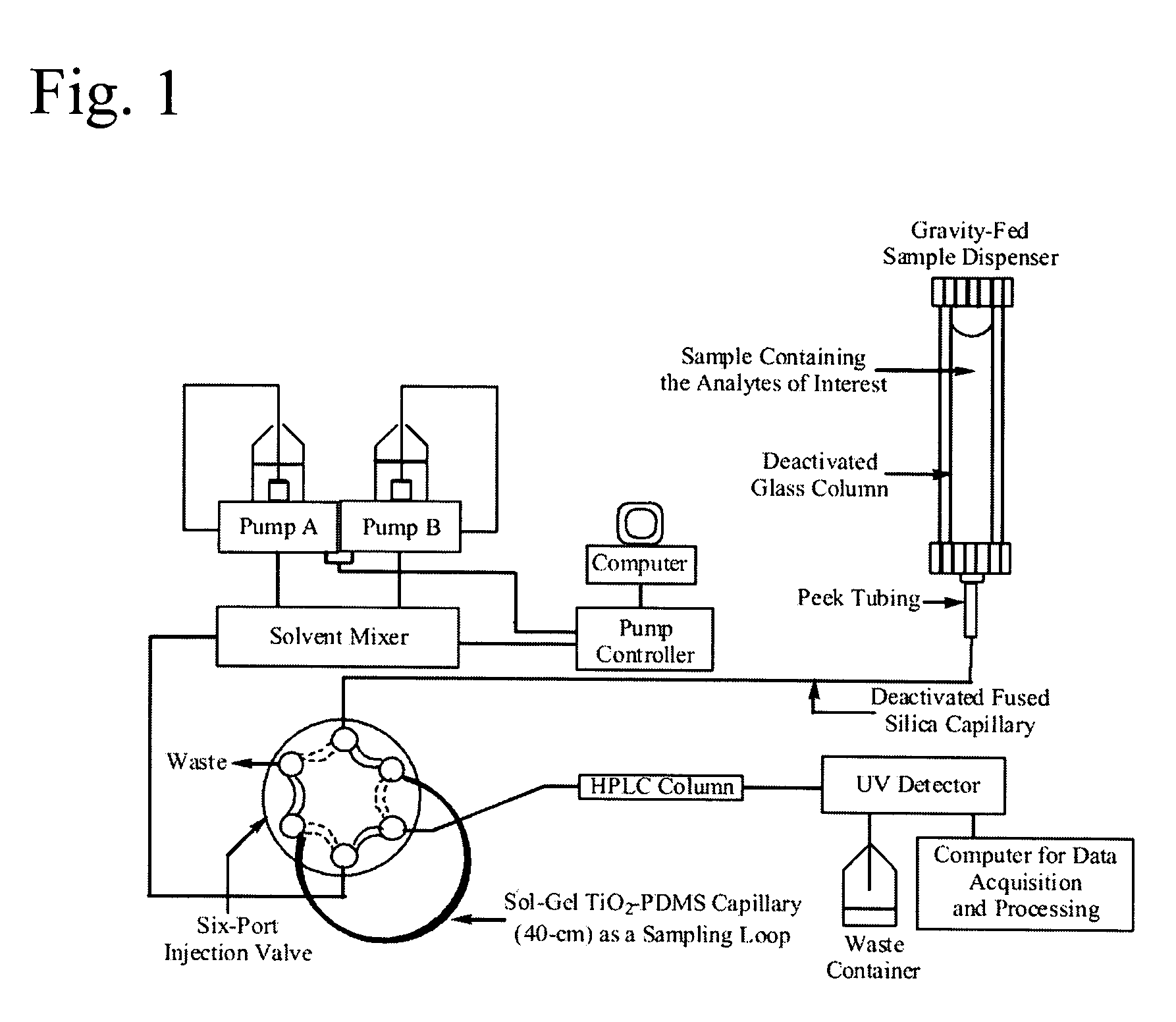
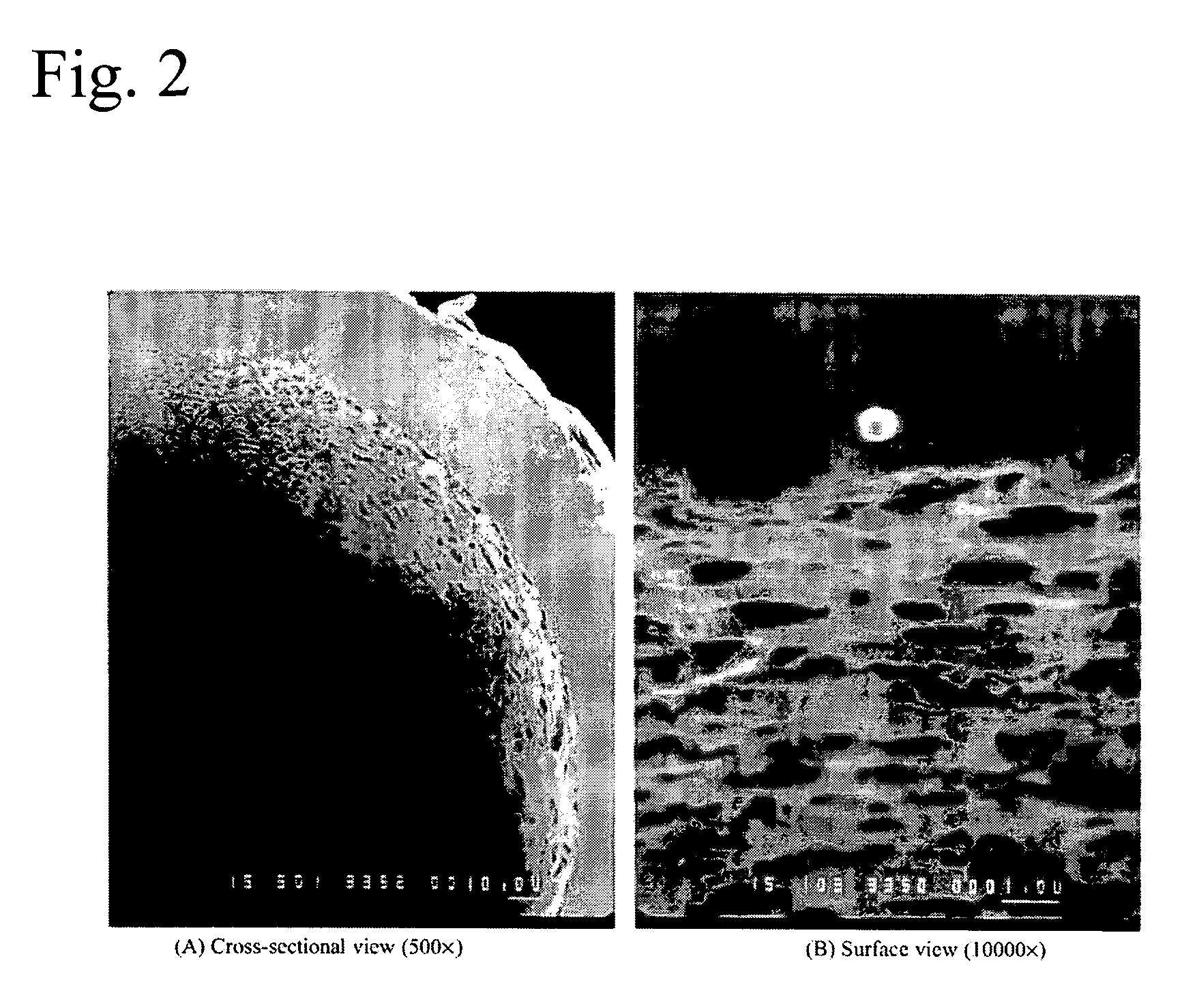
Titania-Based Coating for Capillary Microextraction
InactiveUS20060013982A1Avoid breakingRetains extraction performanceComponent separationMixing methodsSilicon dioxideDimethyl siloxane
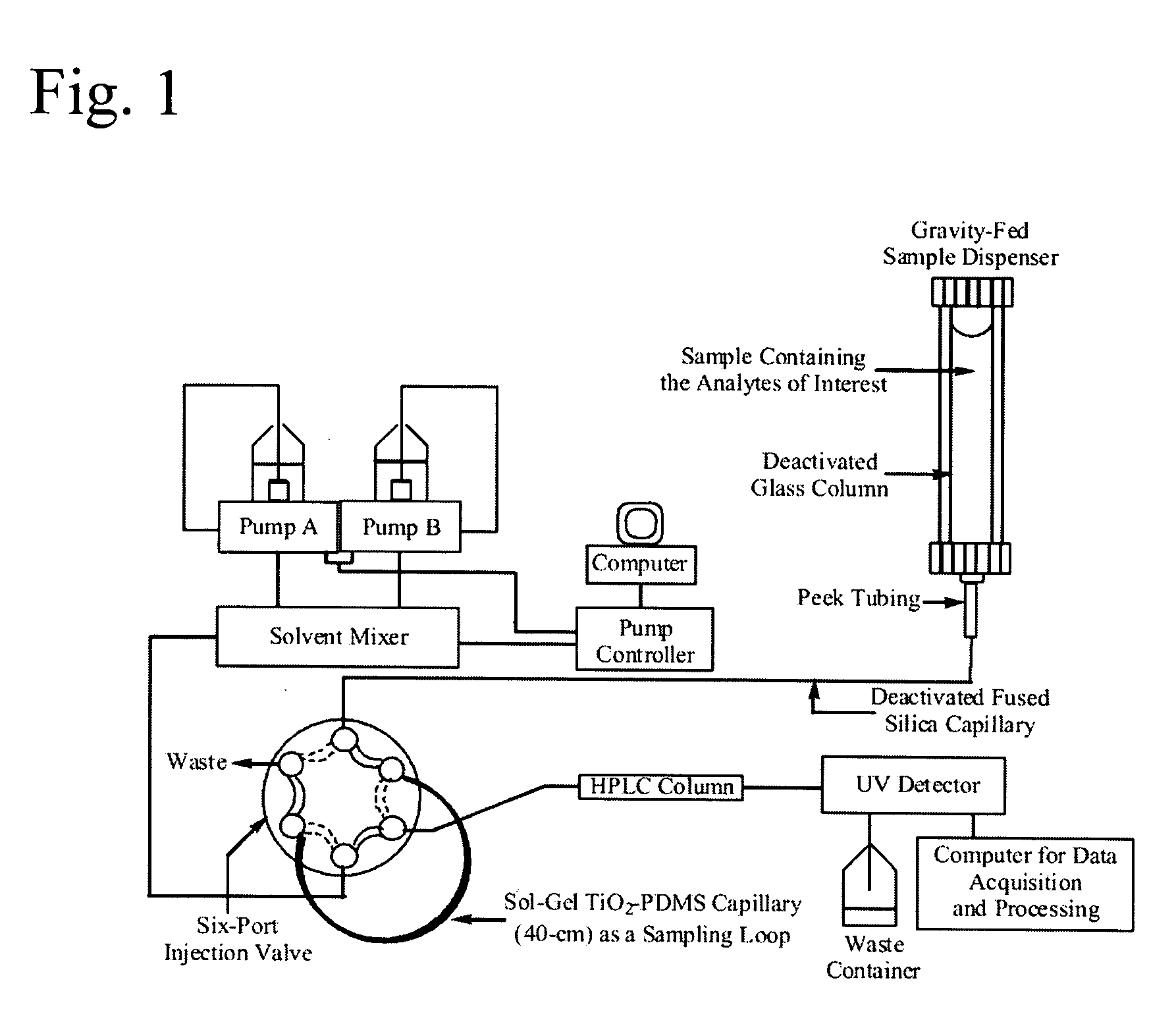
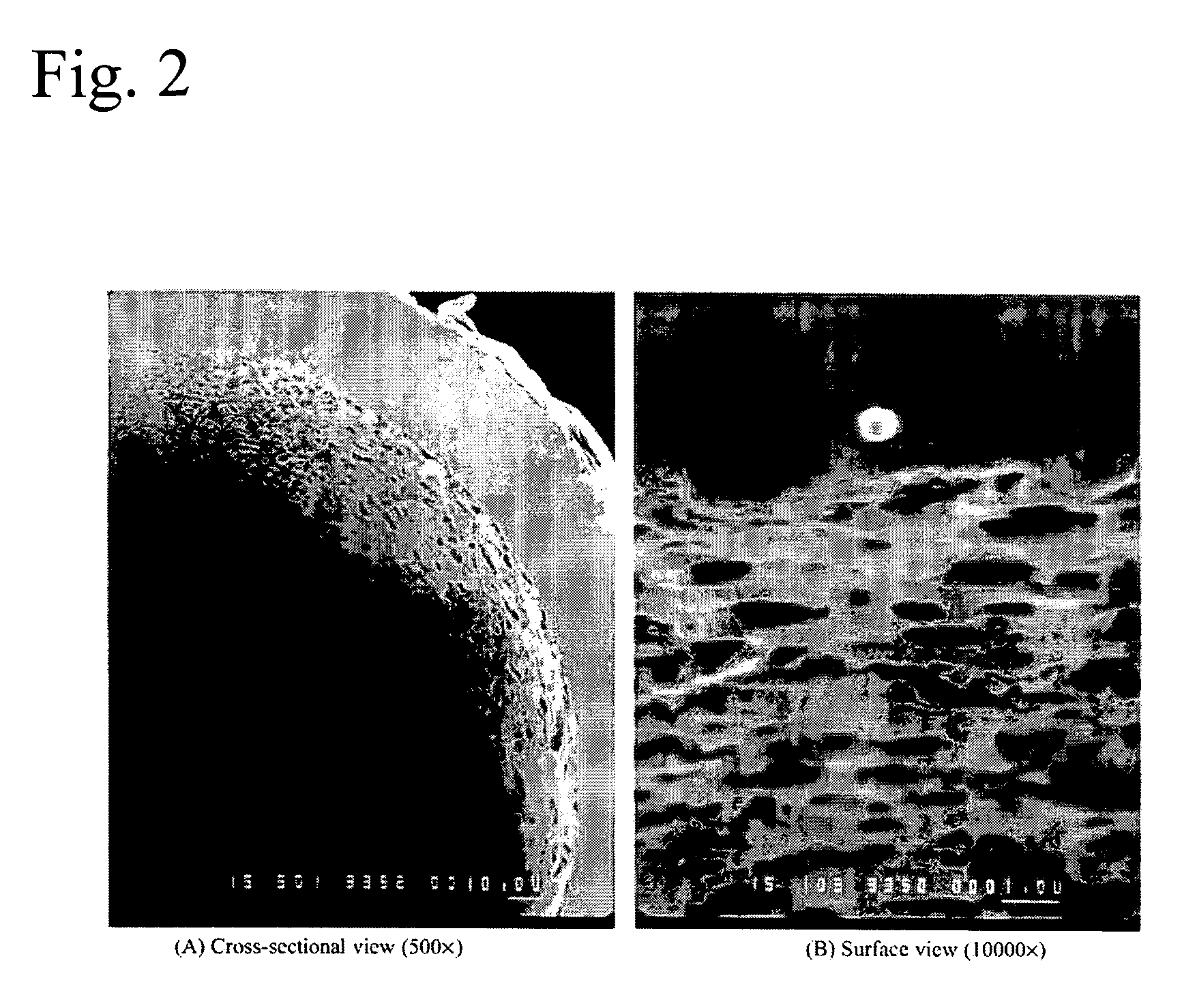
A method is presented describing in situ preparation of the titania-based sol-gel PDMS coating and its immobilization on the inner surface of a fused silica microextraction capillary. Sol-gel titania-poly (dimethylsiloxane) (TiO2-PDMS) coating was developed for capillary microextraction (CME) to perform on-line preconcentration and HPLC analysis of trace impurities in aqueous samples. The sol-gel titania-based coatings demonstrated strong pH stability and enhanced extraction capability over other commercially availble GC coatings. Extraction characteristics of a sol-gel titania-PDMS capillary remained practically unchanged after continuous rinsing with a 0.1 M NaOH solution (pH=13) for 12 hours.
Owner:UNIV OF SOUTH FLORIDA
Method for preparing lycium barbarum lutein by HSCCC (high-speed countercurrent chromatography) separation
The invention discloses a method for preparing lycium barbarum lutein by HSCCC (high-speed countercurrent chromatography) separation. The method mainly comprises the following steps: grinding and extracting lycium barbarum berry, concentrating extract, saponifying, eluting and dissolving, and carrying out HSCCC separation to obtain a lycium barbarum lutein solution; concentrating, and freeze-drying to obtain a lutein pure product of which the purity is over 90.6 percent by means of HPLC analysis and detection. According to the method, a methanol and tetrahydrofuran composite solvent is adopted for ultrasonic extraction, and a saponification technology is combined and synergized with HSCCC, so that the problem that main carotinoid lutein and zeaxanthine in lycium barbarum belong to isomers and are difficult to separate can be successfully solved; autumn berry of lycium barbarum is utilized as a raw material, so that the production cost is greatly reduced, effective separation of lutein and zeaxanthine can be successfully realized by repeatedly regulating the solvent proportion in the counter-current chromatography separating and purifying phase, a high-purity lutein monomer can be obtained, and a new thought is provided to implementation of separating and purifying lutein from lycium barbarum and implementation of industrial production.
Owner:NINGXIA ACADEMY OF AGRI & FORESTRY SCI
Zedoary turmeric oil analysis method
InactiveCN101144802AAvoid destructionEasy to separateComponent separationOrganic solventSteam distillation
The present invention discloses a HPLC analysis method of zedoary turmeric oil and the preparation thereof. The HPLC analysis method of the present invention can be used in the assay determination on separating the compositions of the zedoary turmeric oil extracted with various preparation techniques (supercritical CO2 liquid extraction, vapor distillation, organic solvent extraction, and so on); can be used in the assay determination on separating the compositions of the zedoary turmeric oil in various preparations made of the zedoary turmeric oil; can be used in the monitoring during the zedoary turmeric oil preparing process and the monitoring of the middle sample of the zedoary turmeric oil preparation; and can be used in the examination of the matters concerned in the zedoary turmeric oil and the study of the preparation fingerprint spectrum. The HPLC analysis method of the present invention is simple and quick, has high sensitivity and excellent repeatability, and can reflect the main compositions of the zedoary turmeric oil exactly and really.
Owner:YANTAI UNIV
Fingerprint of Polygonum multiflorum and establishment method and application thereof
InactiveCN102114083AGood reproducibilityQuality improvementComponent separationDigestive systemHplc fingerprintPolygonum limbatum
The invention discloses a quality control method of Polygonum multiflorum through the fingerprint thereof. The fingerprint is HPLC (high-performance liquid chromatography) fingerprint obtained through HPLC analysis of Polygonum multiflorum. The fingerprint can be used for completely monitoring the quality of Polygonum multiflorum to ensure the stable and uniform quality of Polygonum multiflorum and related pharmaceutical preparations containing Polygonum multiflorum. The invention further discloses the establishment method and the application of the fingerprint of Polygonum multiflorum.
Owner:KANGMEI PHARMA
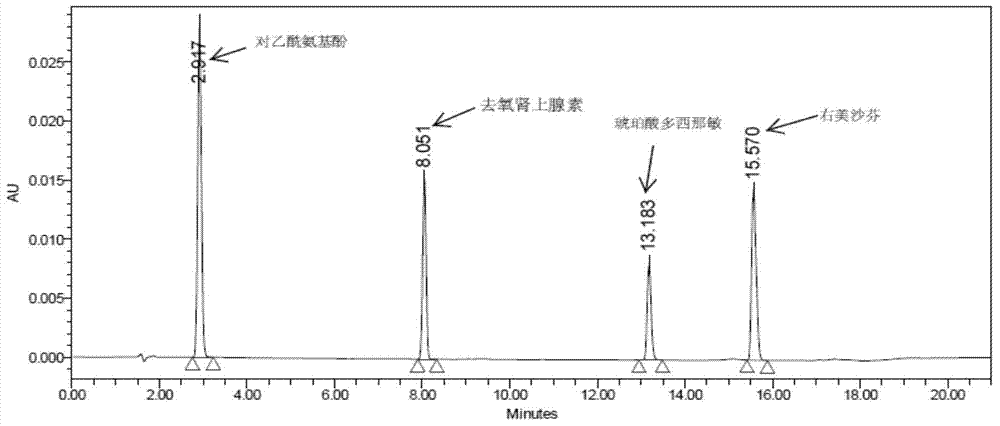
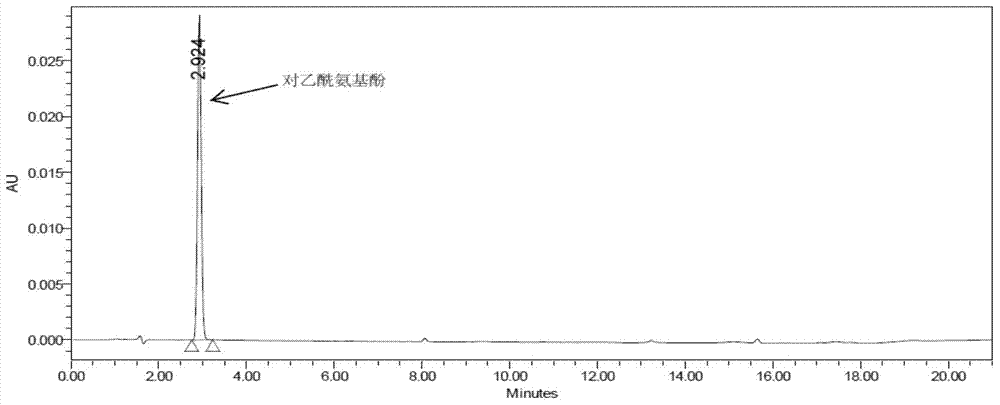
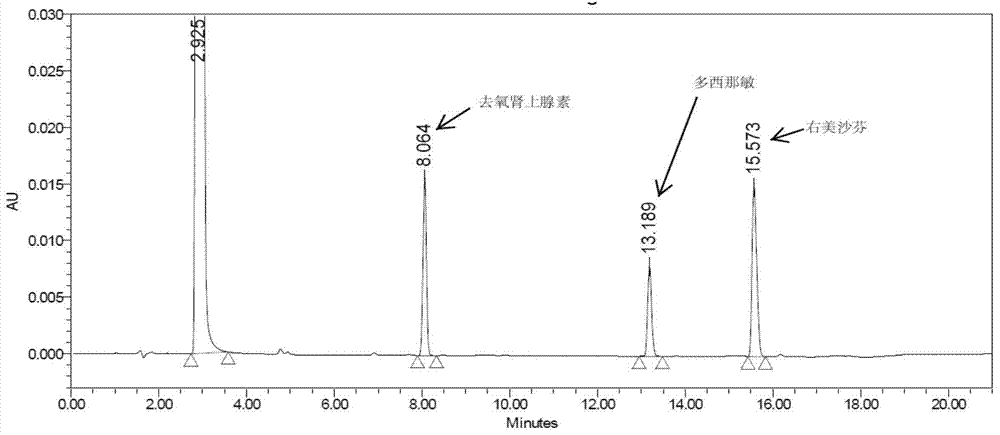
Method for analyzing night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography)
The invention discloses a method for analyzing a night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography). The night cold flu cough allergy capsule contains acetaminophen, phenylephrine hydrochloride, succinic acid doxylamine and dextromethorphan hydrobromide. In the HPLC analysis, an octadecyl silane bonded silica gel column is adopted as a chromatographic column; a sodium 1-octanesulfonate-phosphate buffer solution with the pH of 2.0-3.0 acts as a mobile phase A; acetonitrile and a mixed solution of acetonitrile and methyl alcohol act as a mobile phase B. The method can be simultaneously and effectively used for detecting four effective ingredients in the night cold flu cough allergy capsule, is simple to operate, analyzes rapidly, is good in repeatability, has favorable specificity, and can effectively and comprehensively control the product quality of the night cold flu cough allergy capsule.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Method for refining tanshinoneIIA sodium sulfonate
InactiveCN101200489AHigh purityComply with the requirements of raw materials for injectionSteroids preparationSulfonateOrganic solvent
The invention relates to a refining method of tanshinone II sulfonate. The method comprises the following procedures, firstly the crude tanshinone II sulfonate to be purified is added into the mixture solution of the organic solvent and water, heated to 40 to 60 DEG C, agitated to dissolve and filtered to remove the insoluble particles; secondly, the filtrate is slowly decreased to the temperature of 0 to 30 DEG C and crystallized and filtered; thirdly tanshinone II sulfonate with high purity is obtained after the vacuum drying. The contents of tanshinone II sulfonate can reach 99.5 percent and the quality can meet the purity requirement of raw medicine for injection by HPLC analysis. The method of the invention has the advantages of simple method, easy operation, low refining cost and being suitable for the industrialized production.
Owner:YAOPHARMA CO LTD
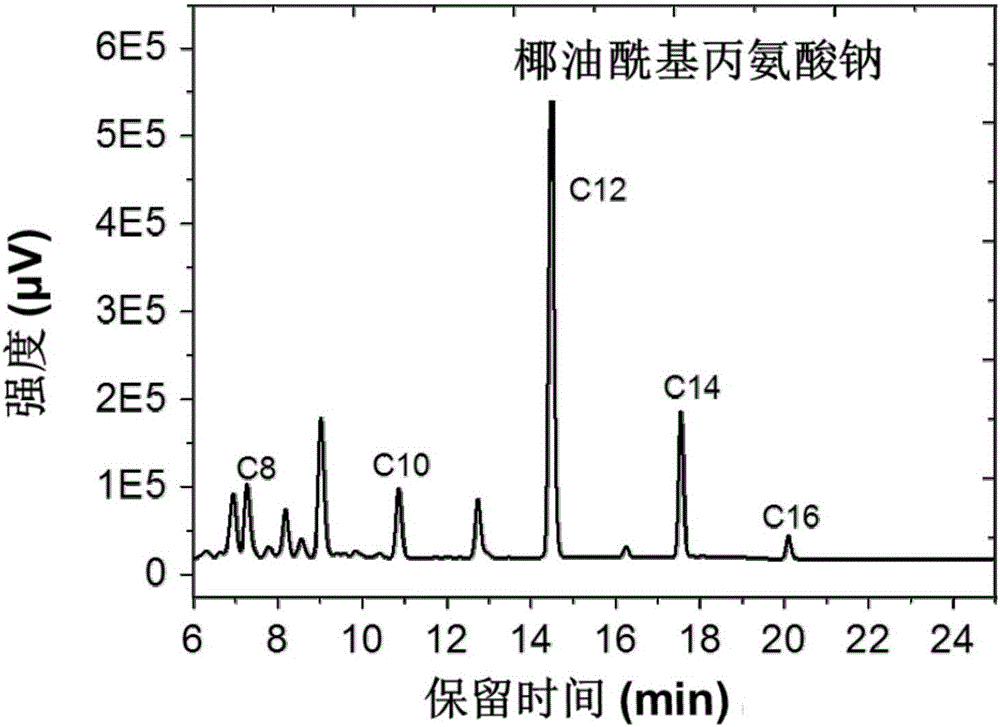
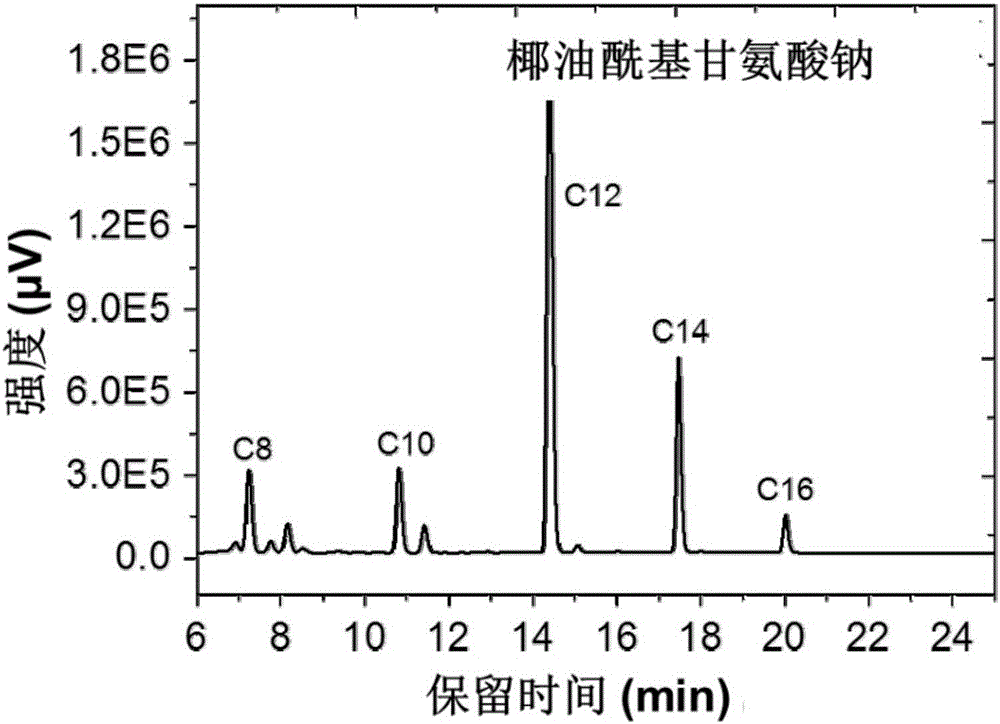
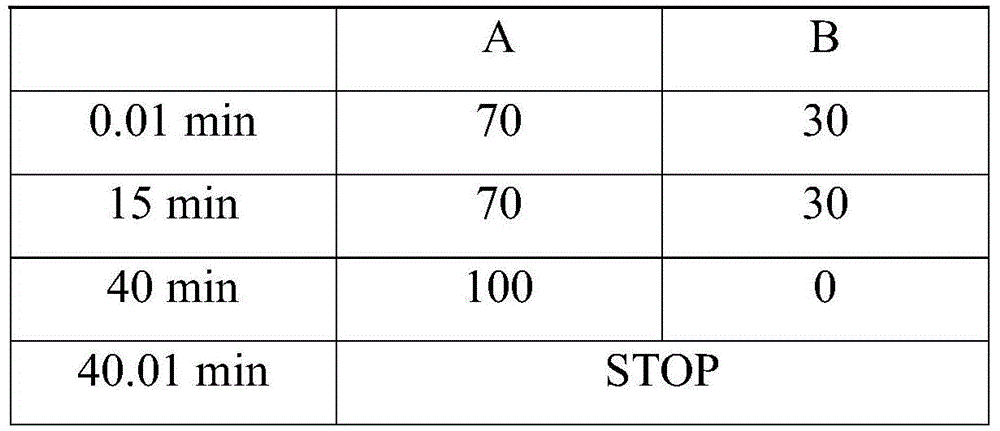
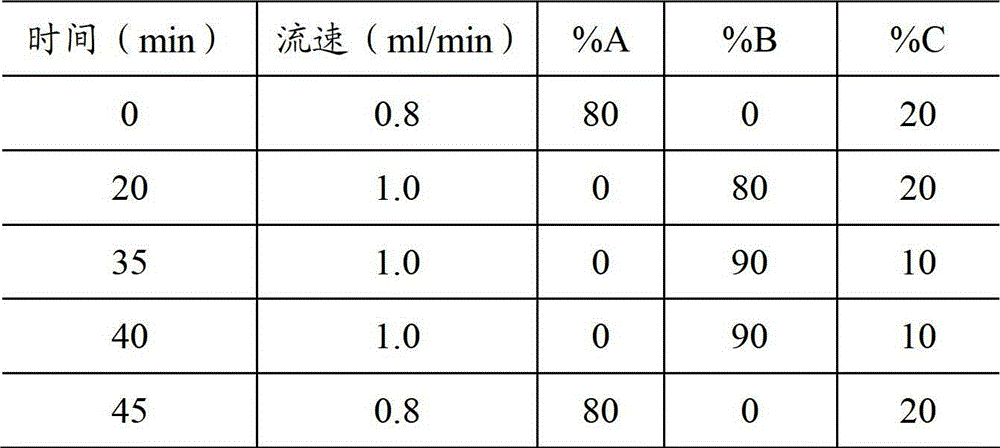
Analysis method of carbon chain composition of cocoyl amino acid surfactants
ActiveCN105675749AOvercoming the problem of missing free cocoic acid/saltEasy to operateComponent separationAcyl groupCoconut oil
The invention relates to an analysis method of carbon chain composition of cocoyl amino acid surfactants. The method comprises the following steps: hydrolysis is carried out for cocoyl amino acid / salt, coconut oil fatty acid is obtained, and HPLC analysis is carried out after derivatization. The analysis method is not influenced by kinds of amino acid surfactants, as long as the type of the surfactant accords with fatty acyl amino acid / salt; the analysis method has the advantages of simple operation, low cost, and high sensitivity, and the analysis method is not influenced by length of carbon chain.
Owner:GUANGZHOU TINCI MATERIALS TECH
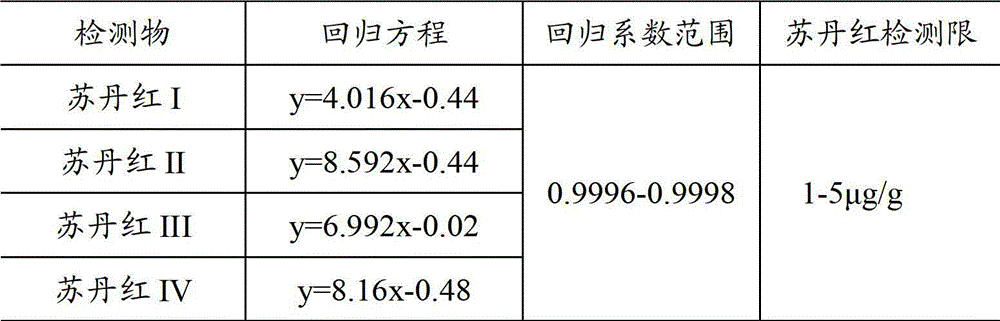
Distinguishing method for Sudan dyes in red pepper and tomatoes
The invention relates to a distinguishing method for Sudan dyes in red pepper and tomatoes, comprising the following steps of: pre-treating a red pepper sample, and then, extracting the pre-treated red pepper by using a mixture of alcohol, acetonitrile and isopropanol; then, placing the extracted red pepper into a water bath to be subjected to ultrasonic oscillation extraction; continuing to carry out oscillation extraction in a swing bed; carrying out vacuum concentration on filtrate obtained after filtering; redissolving the obtained residues by using the mixture of alcohol, acetonitrile and isopropanol; and filtering the obtained residues by using a polytetrafluoroethylene film with the size of 0.45mum to obtain a red pepper extracting liquid. A distinguished result is obtained through analysis by using a high-performance liquid chromatography (HPLC) and carrying out HPLC analysis on a standard sample of Sudan dyes. The method provided by the invention is sensitive; and the detecting limit of the Sudan dyes is only 1-5umg / g so that the method is very suitable for distinguishing whether Sudan dyes I-IV exist in melons, fruits and vegetables.
Owner:HUABAO FLAVOURS & FRAGRANCES CO LTD
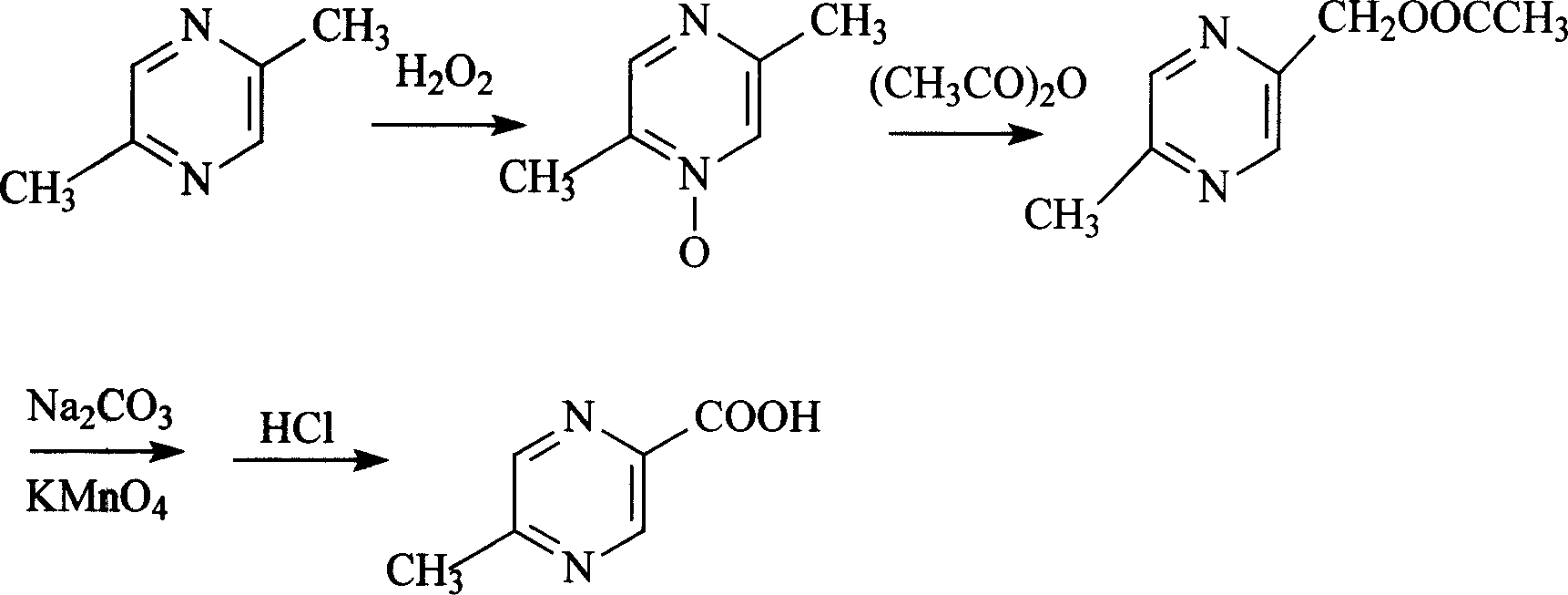
Process of selective synthesizing 5-methyl pyrazine-2-carboxylic acid using 2,5-dimethyl pyrazine
This invention use 2,5- dimethyl pyrazine as raw materials, selectively synthesize 5-methyl pyrazine-2-carboxylic acid. Its technology is use 2,5 - dimethyl pyrazine as raw material, through oxidation of nitrogen and hydrogen peroxide, acetic anhydride acid oxidation, hydrolysis and oxidation process, gain 5-methyl pyrazine-2-carboxylic acid. HPLC analysis of their content is over 99%, with 0.1 mol / L of sodium hydroxide titration, content is not less 98%, melting point is not less than of 163 deg, the quality of medicine to meet glipizide, Acipimox requirements. The process is scheme simple, high-yield, low cost and suitable for industrial production.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
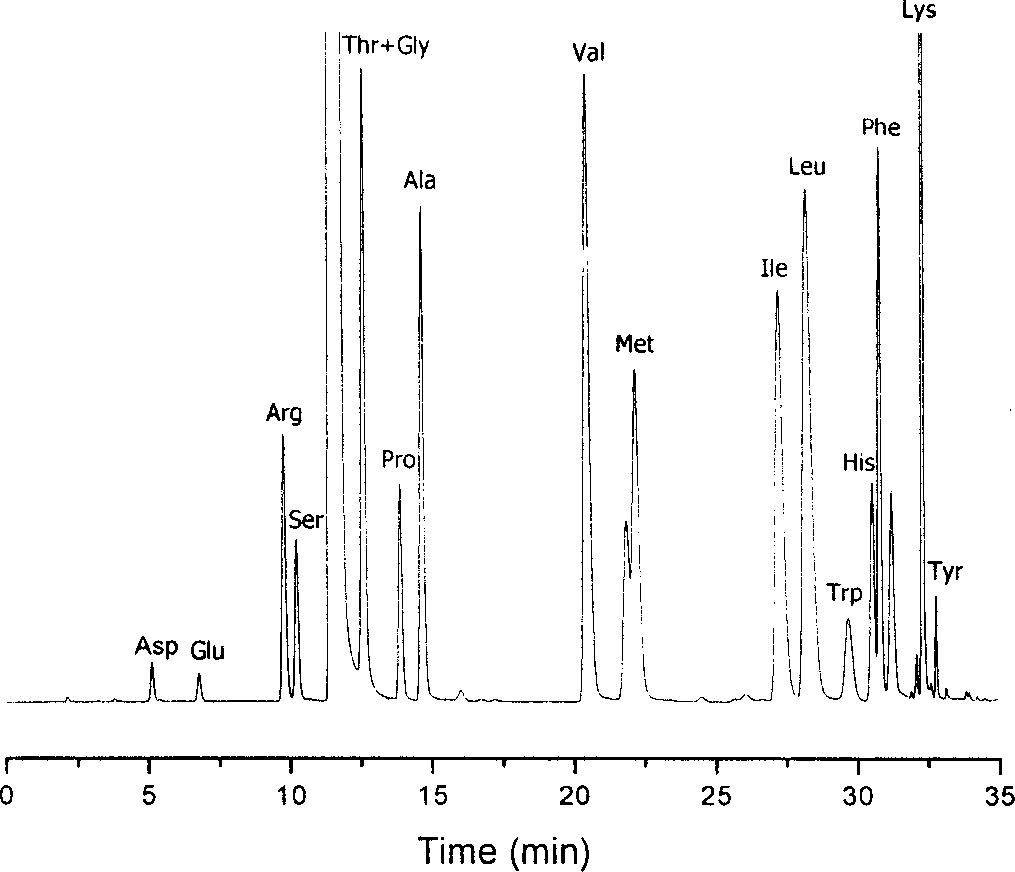
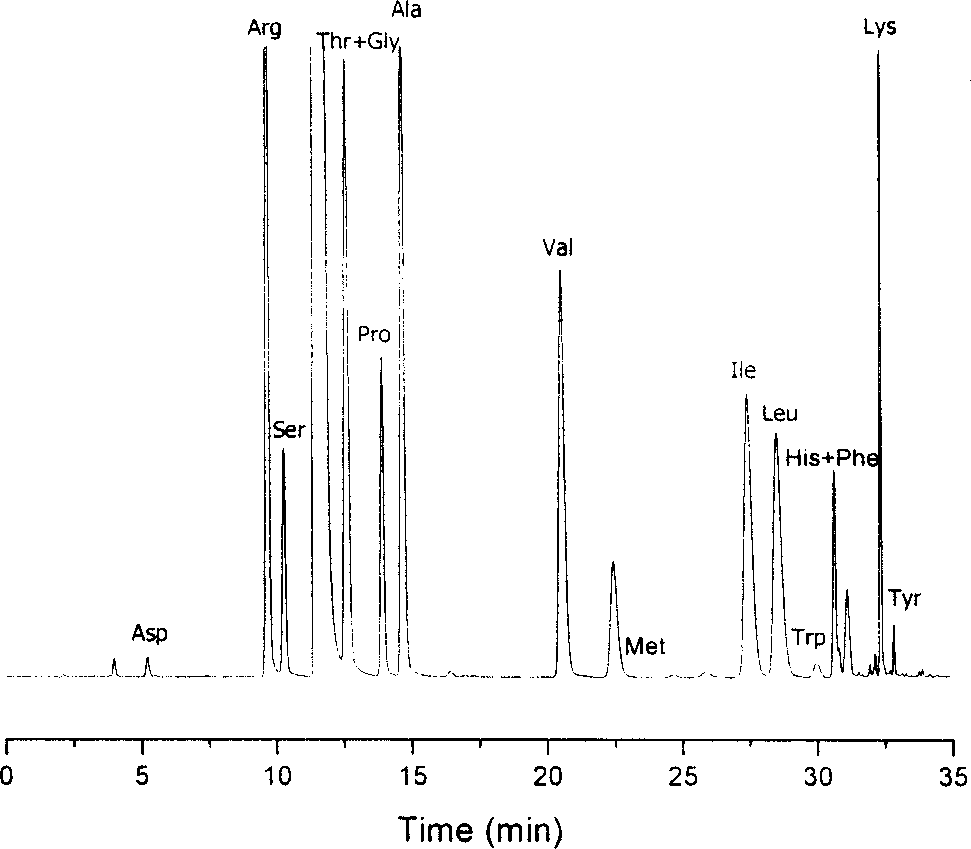
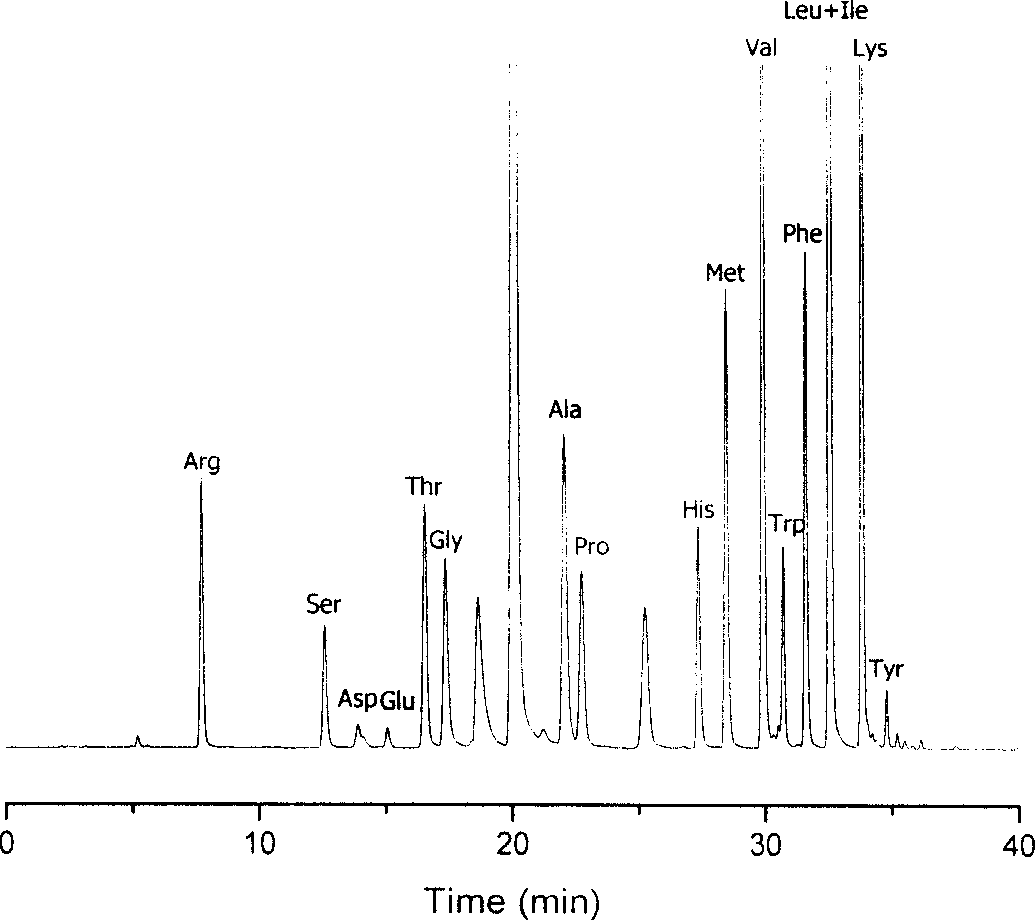
Method for analysizing amino acid
ActiveCN1749748AThe result is accurateGood reproducibilityComponent separationChromatographic separationColumn temperature
The amino acid analyzing method includes high efficiency liquid phase chromatographic separation of the 2, 4-dinitro fluoro benzene derivative of amino acid in C18 column with pH 2-3 or pH 6-7 TEAP / ACN as flowing phase; and 360nm ultraviolet detection at column temperature 35deg c. The present invention has raised separation result reproducibility, optional water phase buffering area and trimmed pH value for analysis of chromatographic column and sample, and accurate analysis result. The present invention is suitable for common HPLC analysis lab and the application range includes various amino acid analysis, indirect analysis of other matter capable of being converted into amino acid, and other matter capable of taking quantitative deriving reaction with DNFB.
Owner:TIANJIN PHARMA GROUP CORP
Dispersive liquid-liquid microextraction method for pretreatment of plant hormone
InactiveCN101762417AEfficient purificationEfficient ConcentrationComponent separationPreparing sample for investigationPlant hormoneOrganic solvent
The present invention provides a dispersive liquid-liquid microextraction method for pretreatment of plant hormone, i.e. DLLME is used for pretreatment of plant hormone. The method comprising the steps of: loading, mixing an extracting agent and dispersing agent, oscillating to form an opacifying system, centrifugal separation and drawing the organic phase to perform HPLC analysis directly. The method of the invention has high enrichment property, simple operation, less use of organic solvent and short extraction time, integrates the sampling, extraction and concentration, at the same time, avoids the potential problem of cross contamination in solid-phase microextraction, is a novel sample pretreatment technique with the advantages of simpleness, rapidness, low cost, high efficiency and environment protection, and has broad application prospect in the field of trace analysis.
Owner:FUZHOU UNIV
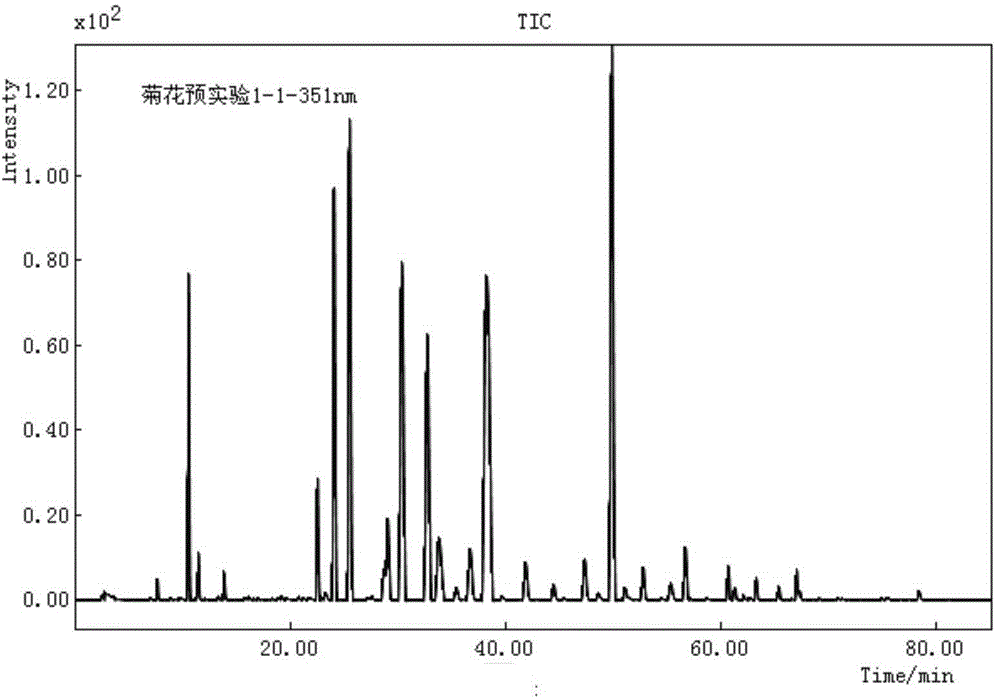
Finger-print spectrum construction method and quality detection method of chrysanthemum cell-disruption decoction pieces
ActiveCN104833749AQuality improvementControl and identify counterfeit goodsComponent separationHplc fingerprintActive component
The invention relates to a finger-print spectrum mutual mode construction method and a quality detection method of chrysanthemum cell-disruption decoction pieces. In the method, with isochlorogenic acid A as a contrast peak, mutual mode standard spectrums and determination indexes are established through HPLC analysis to not less than 10 batches of the samples under following chromatographic conditions: column temperature: 35 DEG C; wavelength: 348 nm; mobile phase: an acetonitrile-0.5% phosphoric acid solution; elution gradient: 0-8 min-24 min-50 min-75 min; and acetonitrile change: 14%-18%-18%-25%-45%. The spectrums of the samples to be detected under the same chromatographic condition are compared with the mutual mode standard spectrums to detect the quality of the samples to be detected. The invention firstly discloses the HPLC finger-print spectrum and the quality detection method aiming to the chrysanthemum cell-disruption decoction pieces. The spectrums comprehensively contain the spectrum information of main active components of the chrysanthemum cell-disruption decoction pieces. The method is strong in specificity, is quick and accurate in detection, and can effectively control the total quality of medicines, cell-disruption powders and cell-disruption decoction pieces.
Owner:ZHONGSHAN ZHONGZHI PHARMA GRP
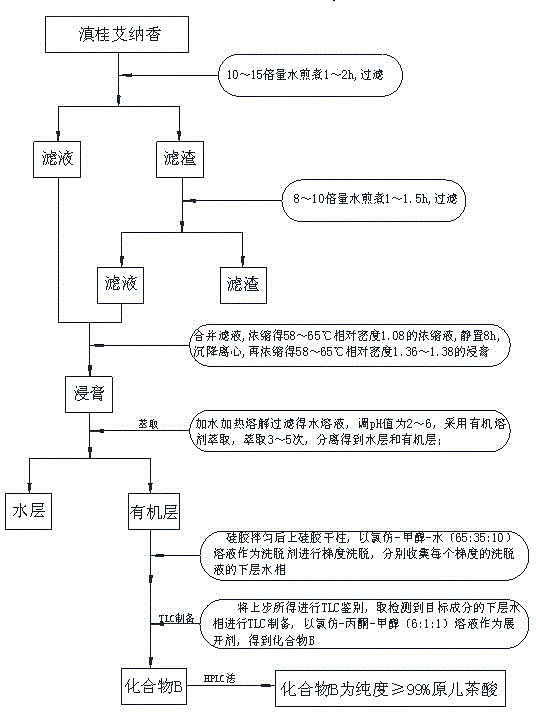
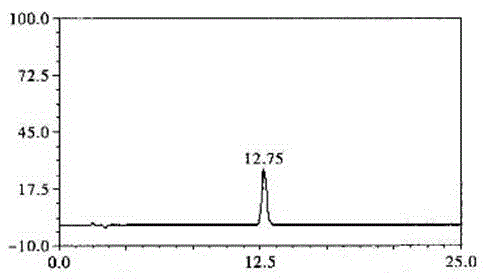
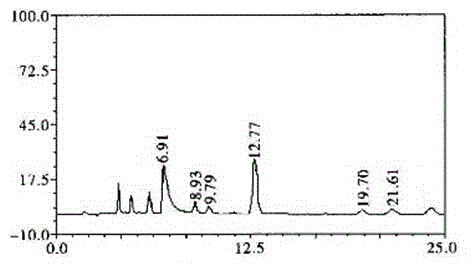
Technological method for extraction of protocatechuic acid from Blumea riparia (Bl.) DC
ActiveCN104098465AHigh purityEfficient separationCarboxylic compound separation/purificationChlorogenic acidElution
Belonging to the technical field of traditional Chinese medicine extraction, the invention discloses a technological method for extraction of protocatechuic acid from Blumea riparia (Bl.) DC. The method for preparation of a protocatechuic acid monomer consists of: water extraction, concentration, extraction, elution, and TLC preparation and purification. A compound B is determined as protocatechuic acid with a concentration of equal to or more than 99% by HPLC analysis, and is the protocatechuic acid monomer. The technological method for extraction of chlorogenic acid from Blumea riparia (Bl.) DC solves the problems of low preparation purity, small preparation quantity, complex preparation process, and difficult realization of industrialization production. By means of water extraction, concentration, extraction, elution and other processes, high extraction purity and a simple preparation process can be realized. Thus, the method is suitable for popularization, and meets the need of people for protocatechuic acid.
Owner:广西万寿堂药业有限公司
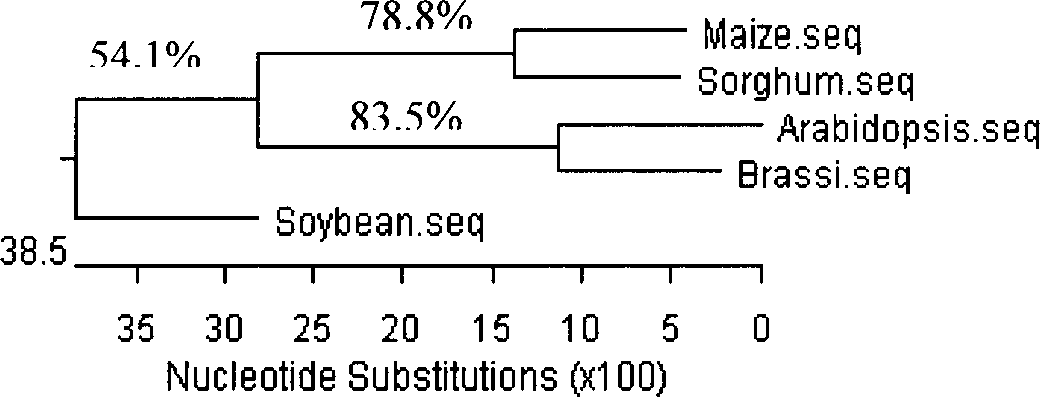
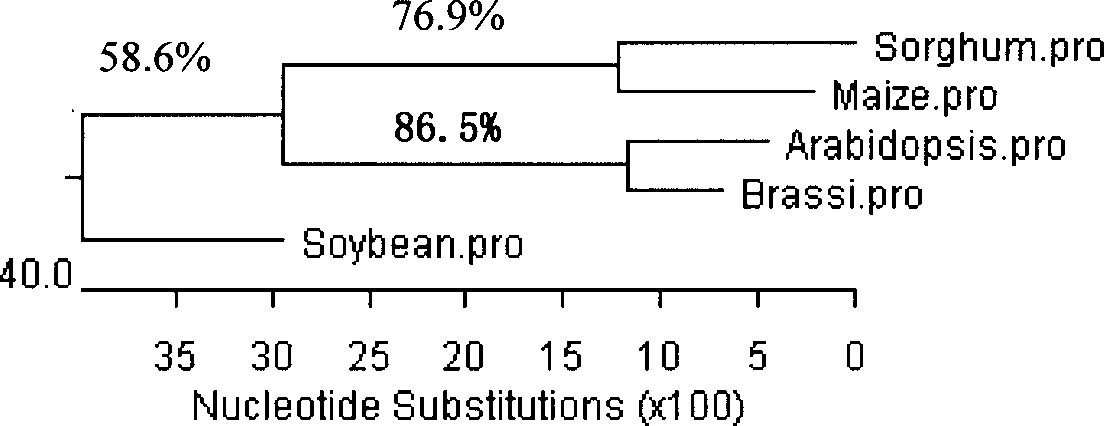
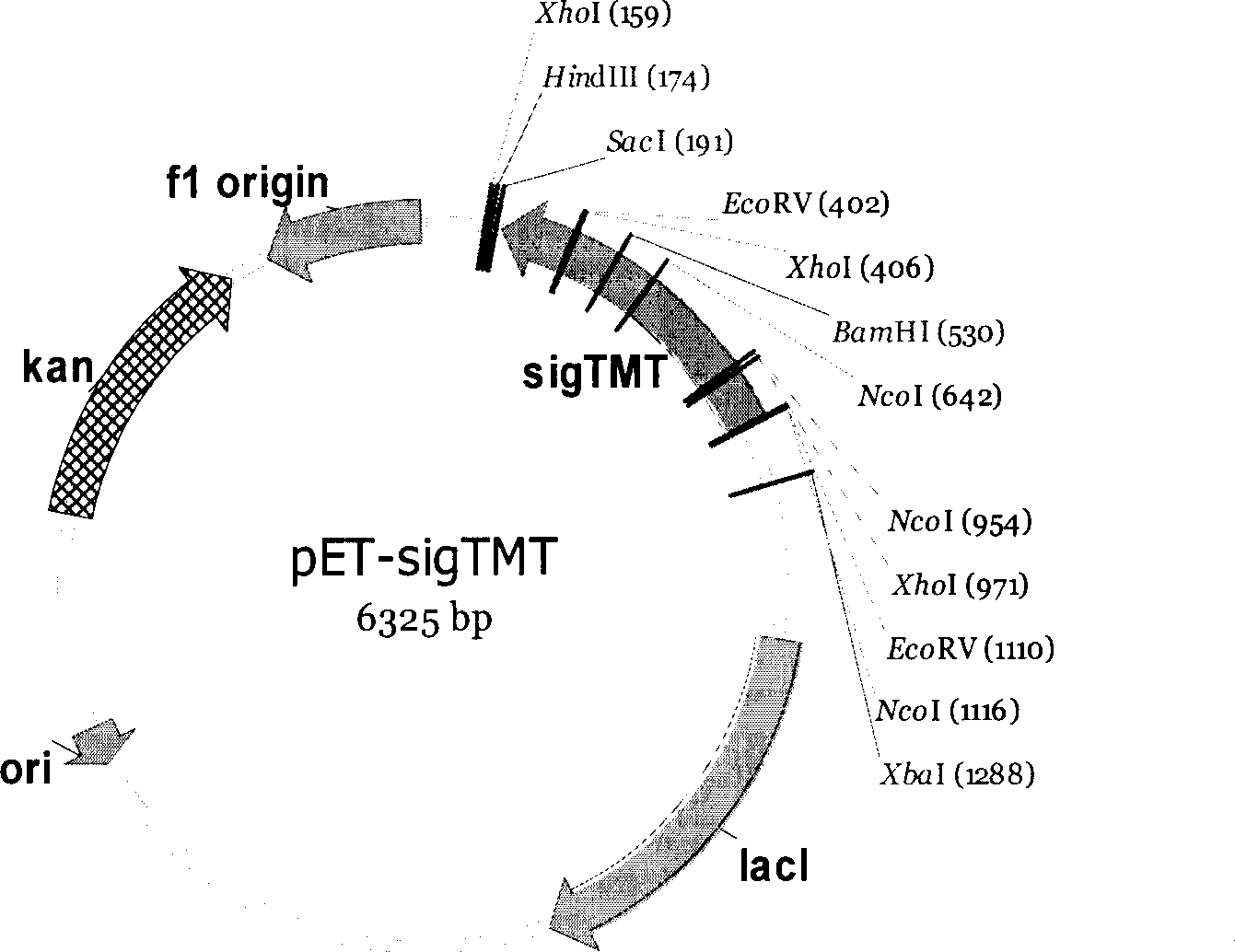
Gama-tocopherol methyl transferase gene, its coding vector and uses
This invention discloses a gamma- tocopherol methyl transfer enzyme that from corn and soybean, their code gene and their use in the plant gene project field. This invention uses RACE and RT-PCR technique, it obtains the span cDNA code sequence of gamma- tocopherol methyl transfer enzyme showed by the SEQ ID NO: 1 and SEQ ID NO: 3 from the corn of high oil 115 and soybean (9525) which all belongs to the oil plants, and it obtains the gamma- tocopherol methyl transfer enzyme with biological activity through prokaryotes expression, and it also compares with the difference of gamma-TMT enzyme activity form different sources. This invention transforms the pattern plant from the related code sequence, through HPLC analysis, the content of the alpha- tocopherol of the transfer gene plant leafage is improved by 2-5 times comparing to that of the wild leafage. This invention is to cut a new way for culturing the new plant of oil plants with high vitamin E content.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
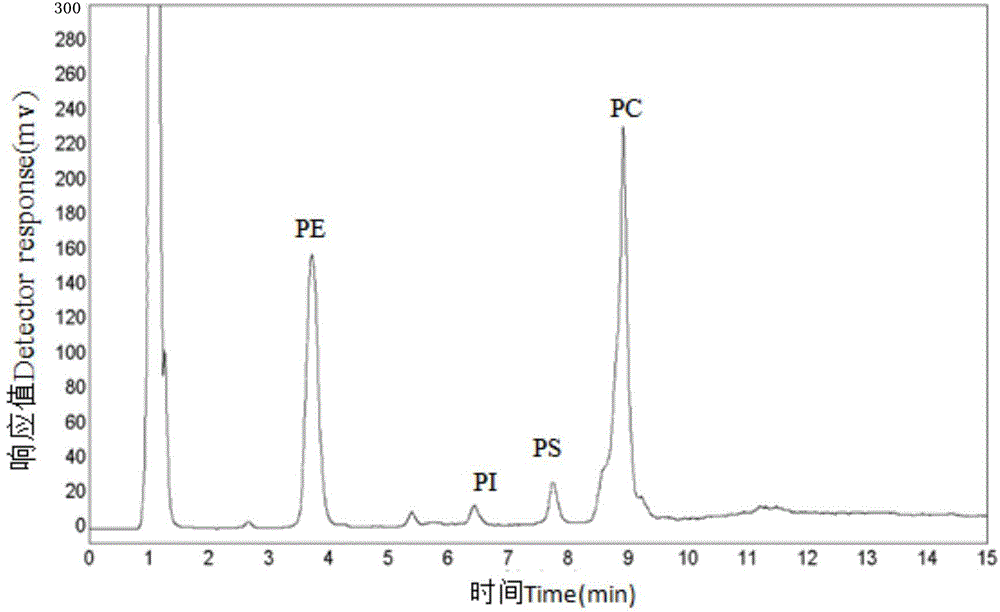
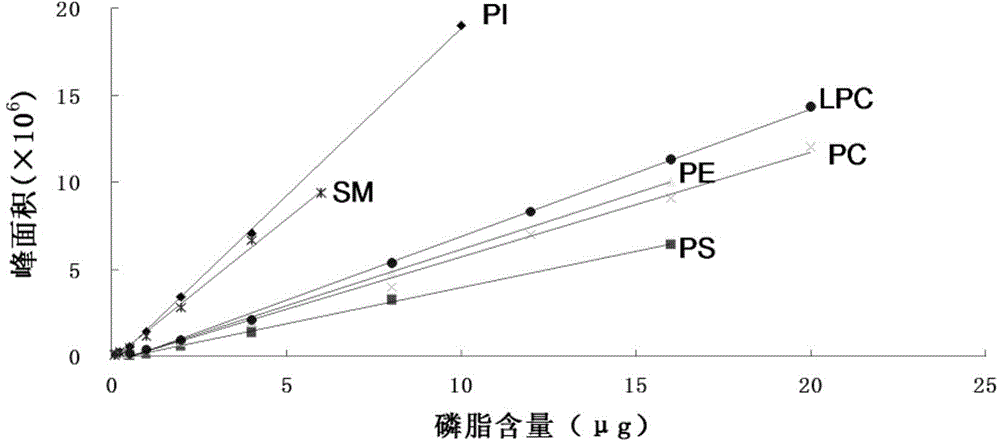
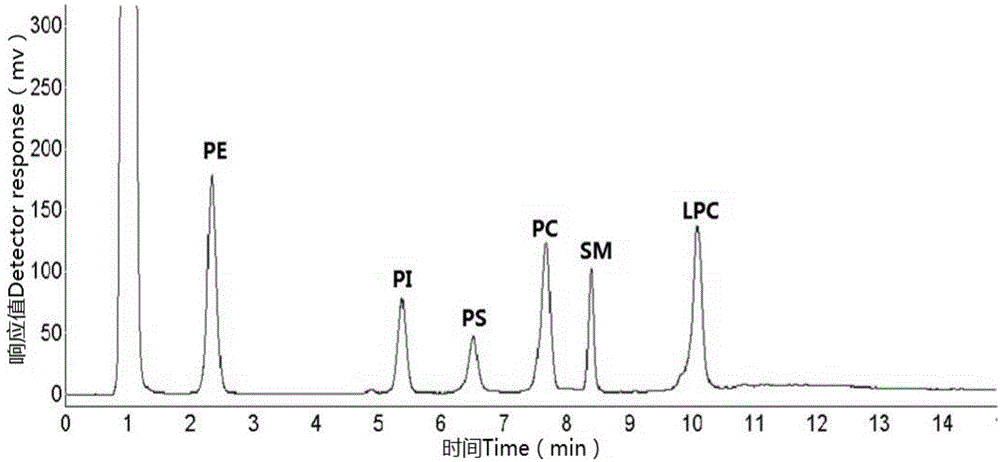
Multiple detection method for six phospholipid in complex sample
The invention discloses a multiple detection method for six phospholipid in a complex sample. The multiple detection method includes HPLC analysis, wherein specification of a positive-phase silica gel chromatography column is 100mmx4.6mm, column temperature of 25 DEG C, elution flow speed is 1.5mL / min, flowing phase of gradient elution includes 40% of A, 57% of B and 3% of C of 0.0min, 40% of A, 50% of B and 10% of C of 8.0min, 40% of A, 50% of B and 10% of C of 15.0min, 40% of A, 57% of B and 3% of C of 15.1min and 40% of A, 57% of B and 3% of C of 24.0min, flowing phase A is normal hexane containing triethylamine of 0.01-0.08%, flowing phase B is isopropanol, and flowing phase C is acetic acid solution of 10-20%. The multiple detection method is simple in operation, quick, accurate, good in component separating effect, high in repeatability and easy for application and popularization in detection practice.
Owner:卢航 +1
Stable gastrodin crystal with high bioavailability for oral administration as well as preparation method, preparation and application thereof
ActiveCN104447904AHigh purityImprove stabilityOrganic active ingredientsSugar derivativesDiseaseX-ray
The invention discloses a stable gastrodin crystal with high bioavailability for oral administration as well as a preparation method, a preparation and an application thereof. X-ray powder diffraction analysis, DSC and TG-DTA analysis and HPLC analysis show that the gastrodin crystal is a new crystal form. The preparation method comprises the steps that gastrodin crude products are dissolved with solvents for performing filtering separation, and ethyl acetate is added into the filtering liquid and is crystallized and separated to obtain a target. The preparation is a tablet or a capsule prepared by adding pharmaceutically acceptable carriers and auxiliaries into the gastrodin crystal. The application of the gastrodin crystal is an application of the stable gastrodin crystal with high bioavailability for oral administration in the preparation of medicines for preventing and / or treating cardiovascular and cerebrovascular diseases. The gastrodin crystal is high in purity, stable against light, moisture, heat and the like, high in stability in alkali solutions and high in in-vivo bioavailability; the preparation method is easy to carry out; compared with the existing crystallizing method, the used crystallized solvent has the advantages of less using amount of crystallized solvents, low production cost and easiness for industrial production; moreover, the content is more than 99 percent.
Owner:KPC PHARM INC
Analysis of Amino Compounds by Precolumn Derivatization with Dimethoxybenzenesulfonyl Chloride
InactiveCN102288707ASuitable for analysisDerivatization reaction conditions are mildComponent separationIce waterReaction temperature
The invention discloses a pre-column derivatization method for high-performance liquid chromatography for analyzing amino compounds, and belongs to the technical field of analytical chemistry. The process is as follows: prepare the sample solution with a borax buffer solution with a pH of 8.0 to 11.5 and a concentration of 0.05 to 0.35M for the primary or secondary or primary and secondary amino compound samples, and add dimethoxybenzenesulfonyl chloride Methanol or ethanol or acetonitrile solution, mix well, conduct derivatization reaction at 25-45°C for 10-40 minutes, cool in ice-water bath, filter and inject samples into full-performance liquid chromatography, use ultraviolet detector to detect the derivatization of amino compounds Derivatives were analyzed quantitatively. The invention has the advantages of simple derivation reaction operation, short reaction time, high sensitivity, simultaneous derivation of primary and secondary amine compounds, no need to remove excess reagents after derivation, stable derivatized products, low derivatization reaction temperature, and is especially suitable for the analysis of biological samples.
Owner:NANJING TECH UNIV
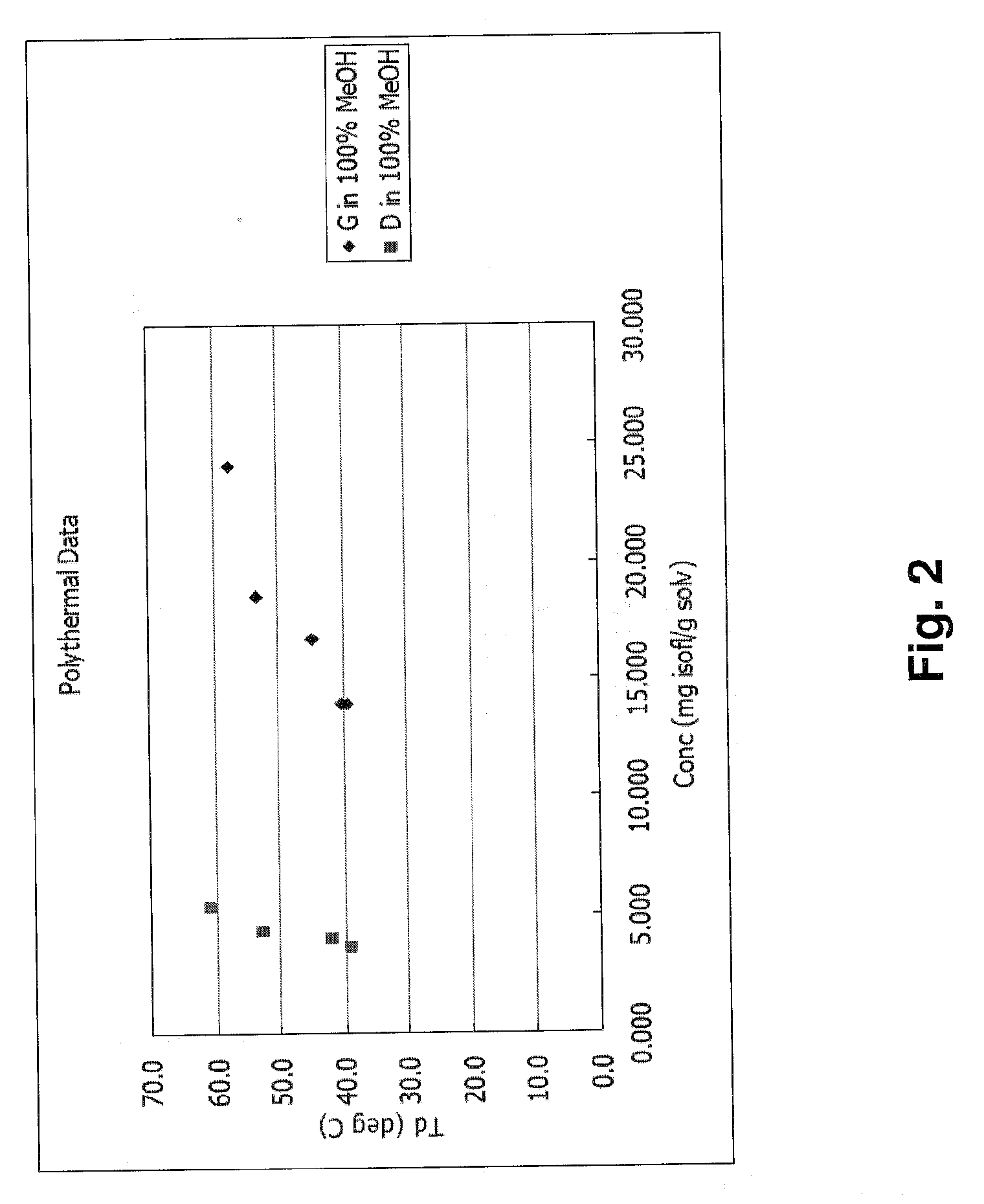
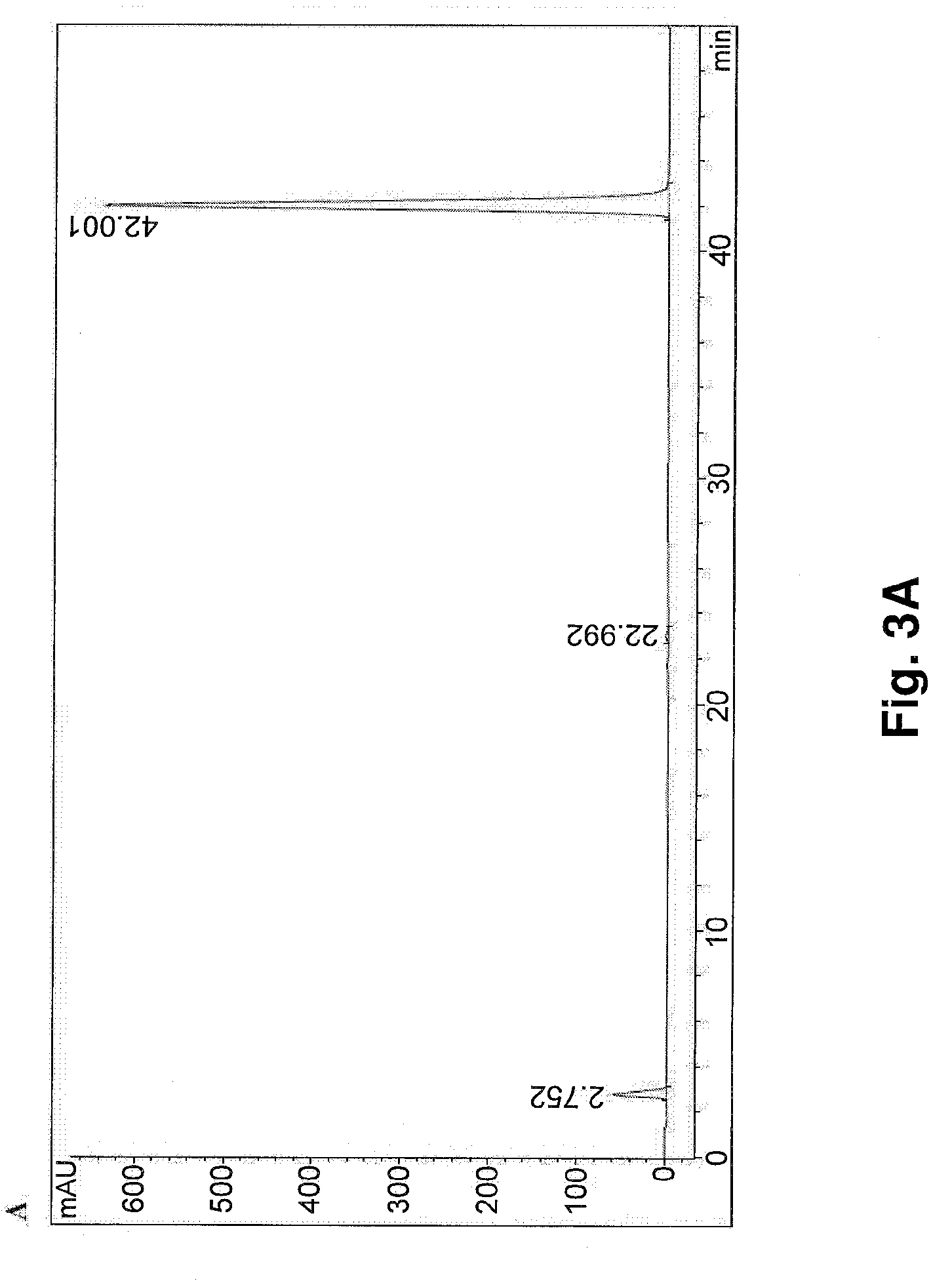
Health Care Product containing Isoflavone Aglycones and Method of Producing the Same
InactiveUS20070207224A1Easy to storeRelieving menopausal symptomBiocideCarbohydrate active ingredientsDiseasePlant Sources
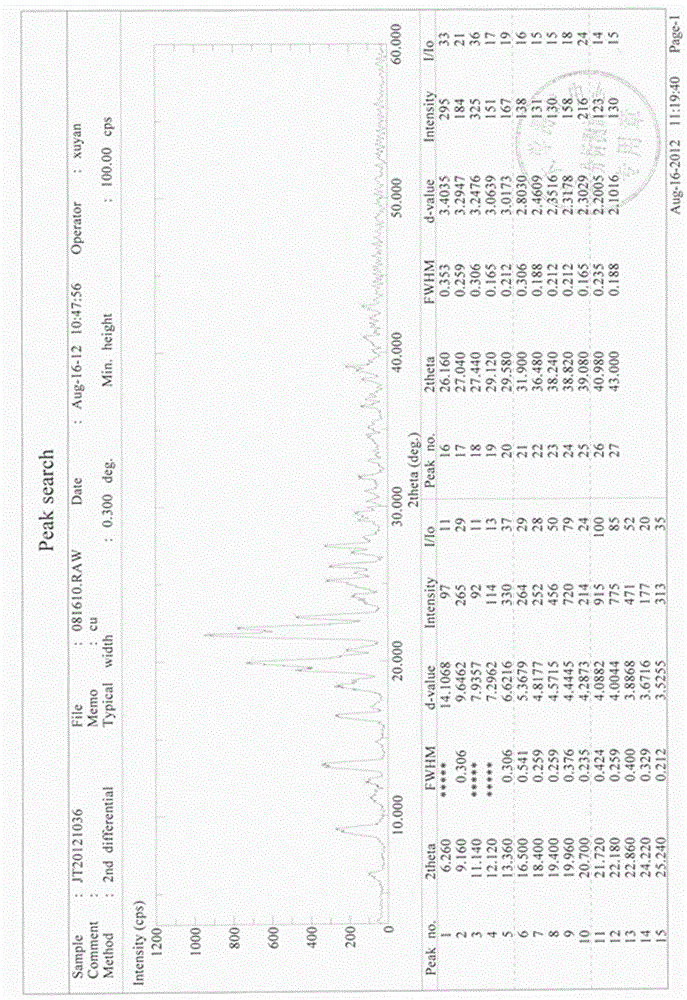
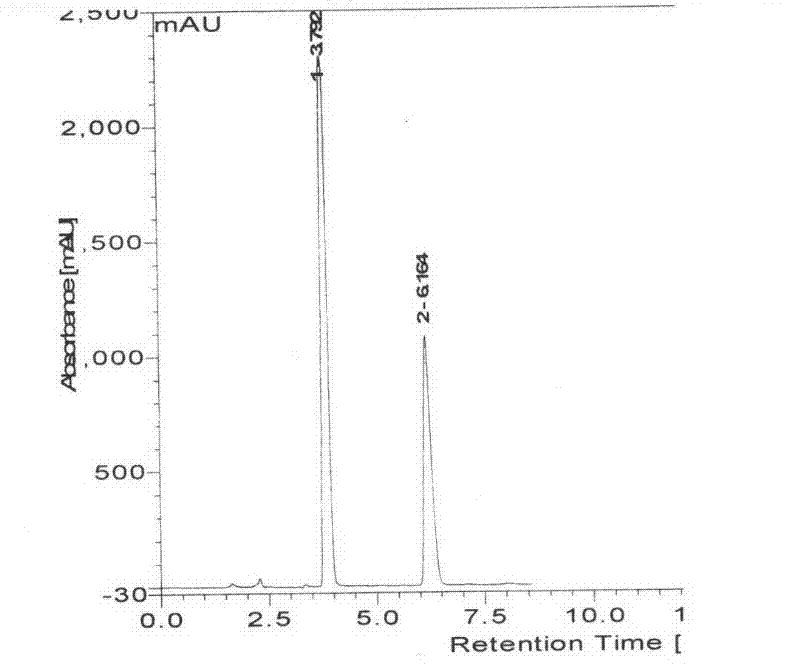
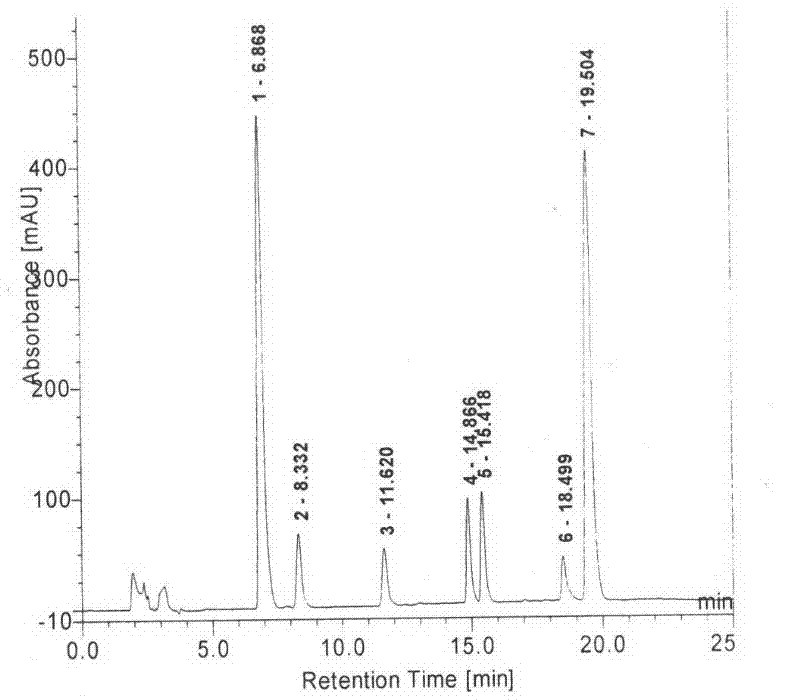
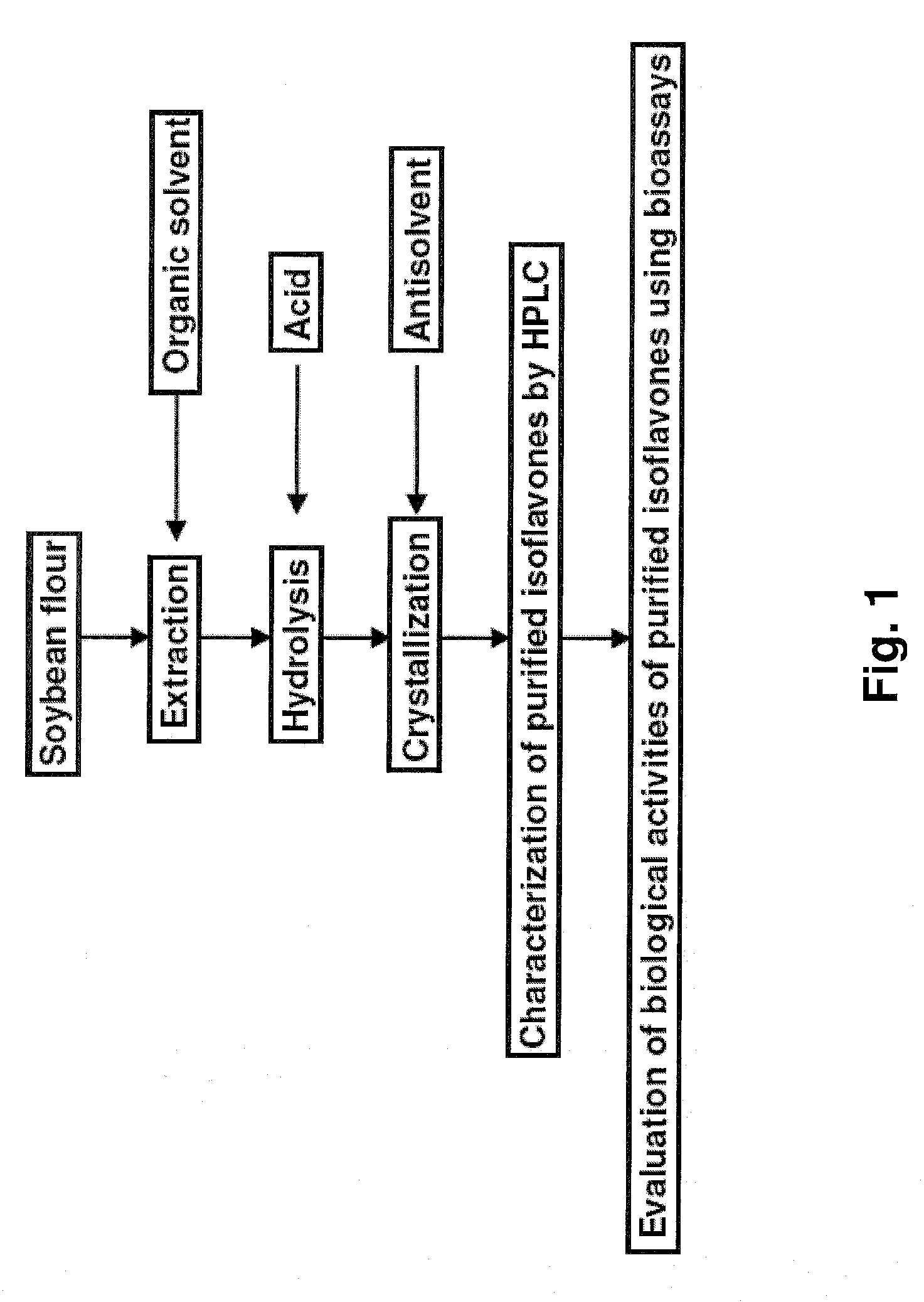
This invention relates to a novel soy isoflavone product with high purities and strong biological activities and the method of producing the same from natural soybeans, soybean materials (i.e. tofu dregs, soy molasses) and other plant sources. The method includes three steps consisting of extraction with an organic solvent, hydrolysis using an acid and crystallization using an antisolvent. The procedure is very simple and thus can be easily adapted for large-scale manufacturing. Moreover, the procedure is able to produce a high yield of total isoflavones at a lower cost. HPLC analysis and E-Screen bioassay reveal that the obtained product not only contains a high content of isoflavone aglycones by weight of dry matter but also exhibits strong estrogenic activity toward human cells. Therefore, the product should be efficacious for relieving menopausal symptoms and other estrogen-deficient diseases and can be used in health care supplements or as additives for foods, beverages or cosmetics.
Owner:THE HONG KONG UNIV OF SCI & TECH
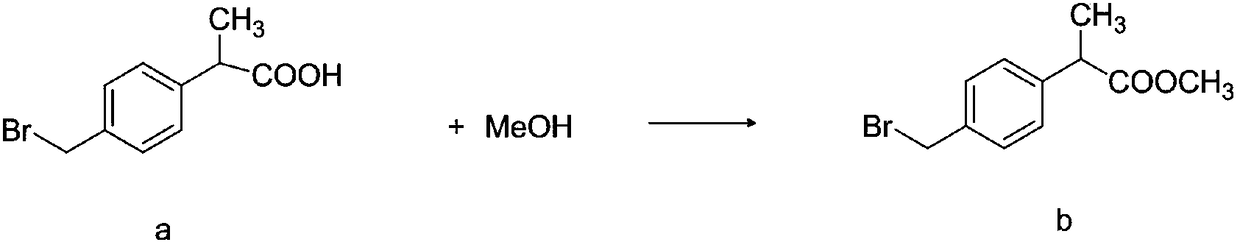
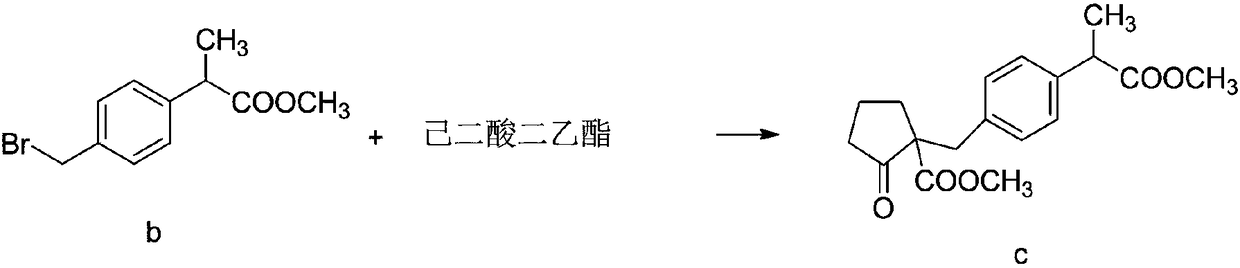
Method for synthesizing high-purity non-steroidal anti-inflammatory drug loxoprofen sodium
InactiveCN108440274AHigh HPLC contentThe reaction mechanism is simpleOrganic compound preparationCarboxylic acid esters preparationCompound cMethyl propionate
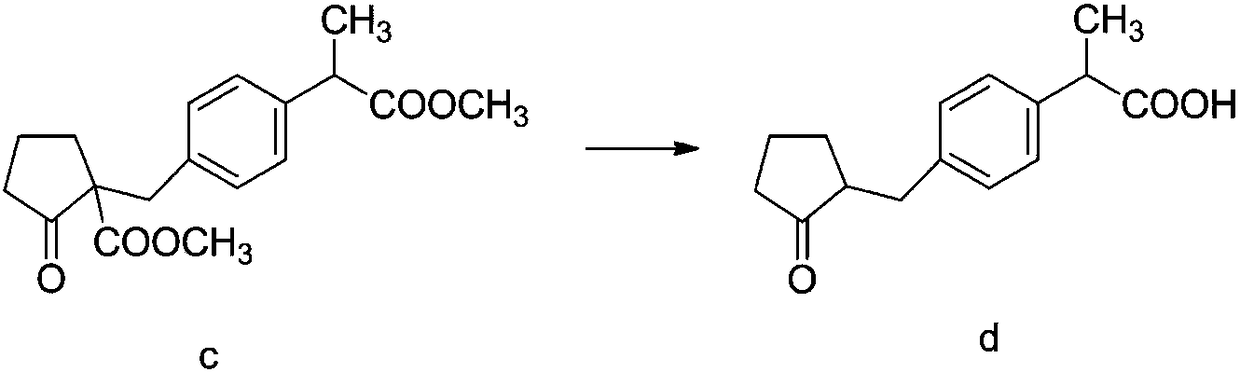
The invention discloses a method for synthesizing high-purity non-steroidal anti-inflammatory drug loxoprofen sodium, 2-(4-bromomethylphenyl) methyl propionate b is synthesized by esterification of raw material a and methanol, intermediate compound c and loxoprofen acid are synthesize sequentially, and the loxoprofen sodium is finally synthesized. The reaction mechanism is simple, by-products arefew, synthesis steps are easy to control, the raw material is easily available, and the impurity content of each step is strictly controlled. The purifying method is easy to operate and suitable for industrial production. The white flake crystal loxoprofen sodium is prepared. Finally, HPLC analysis shows that the loxoprofen sodium content detected by HPLC is as high as 99.95%.
Owner:大桐制药(中国)有限责任公司
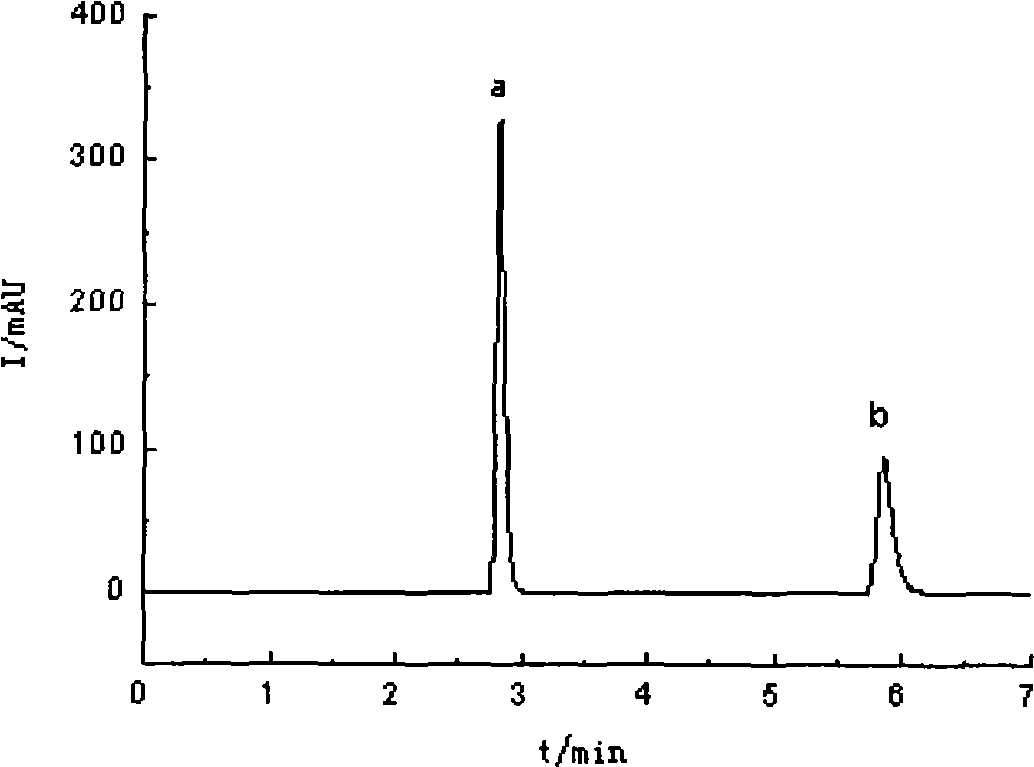
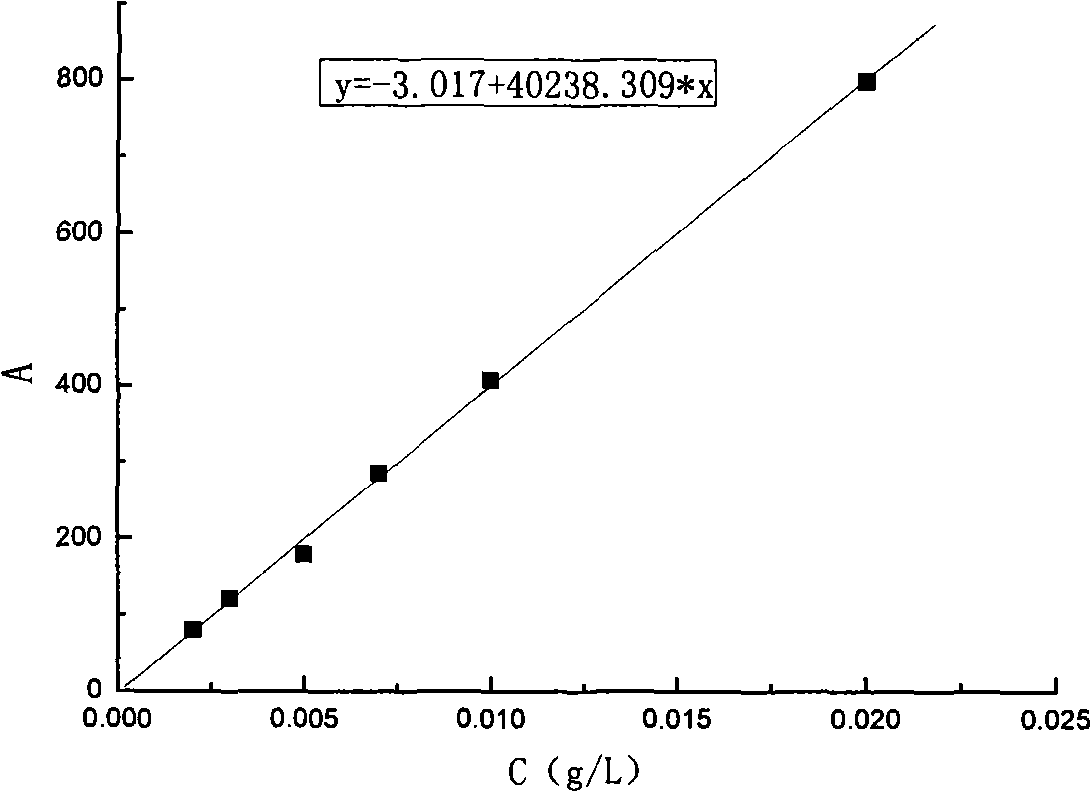
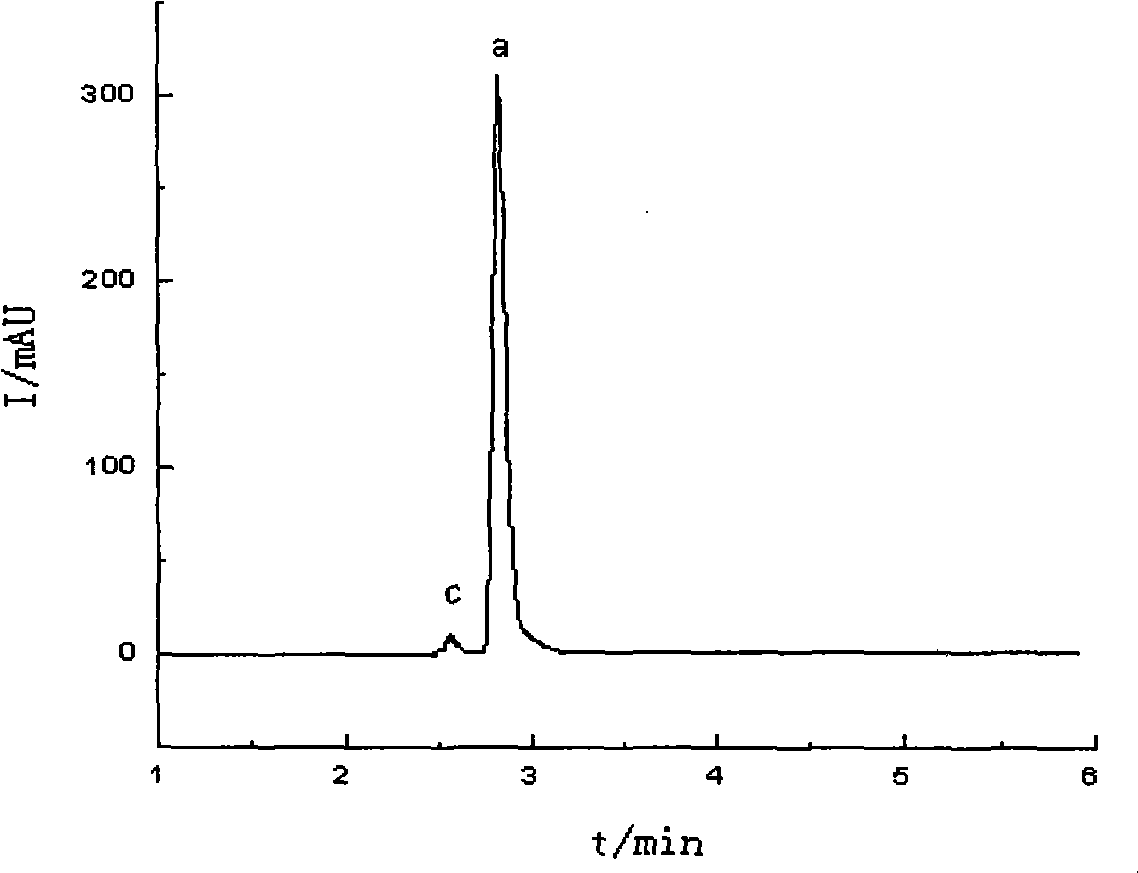
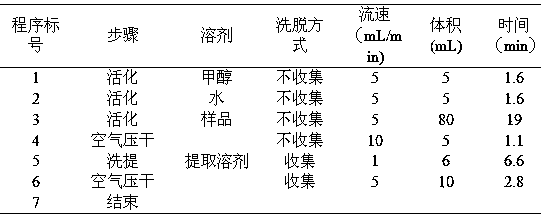
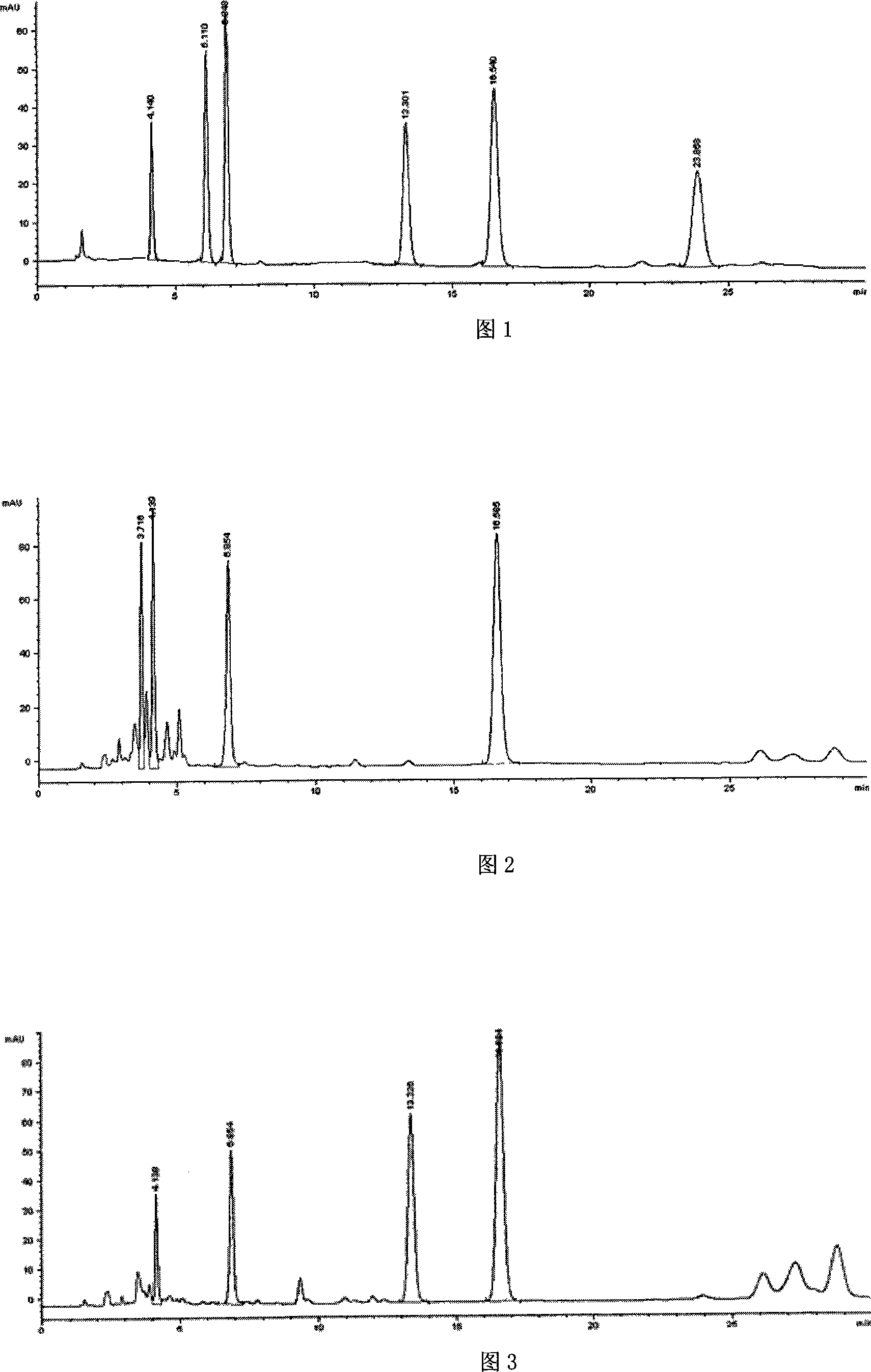
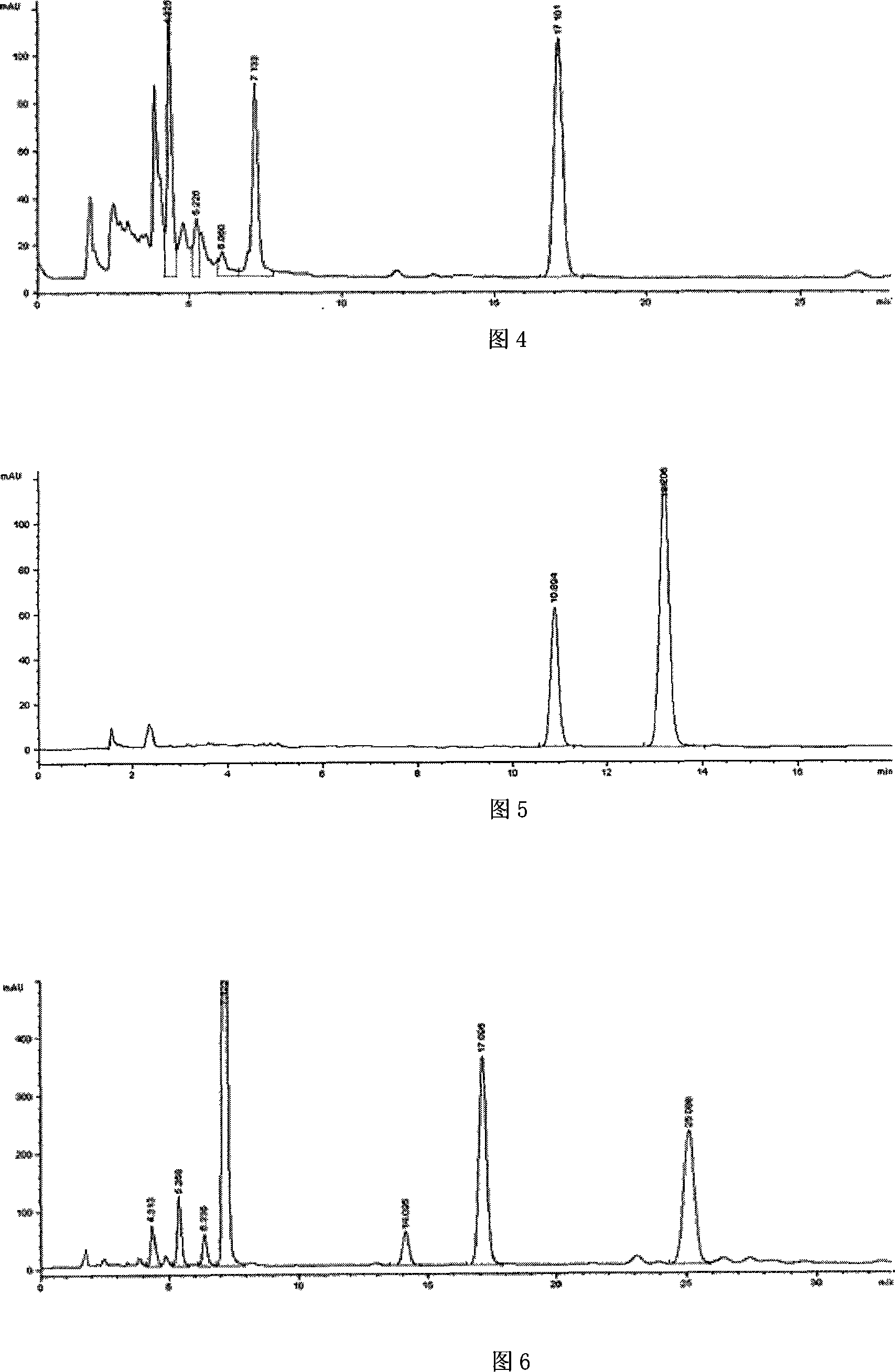
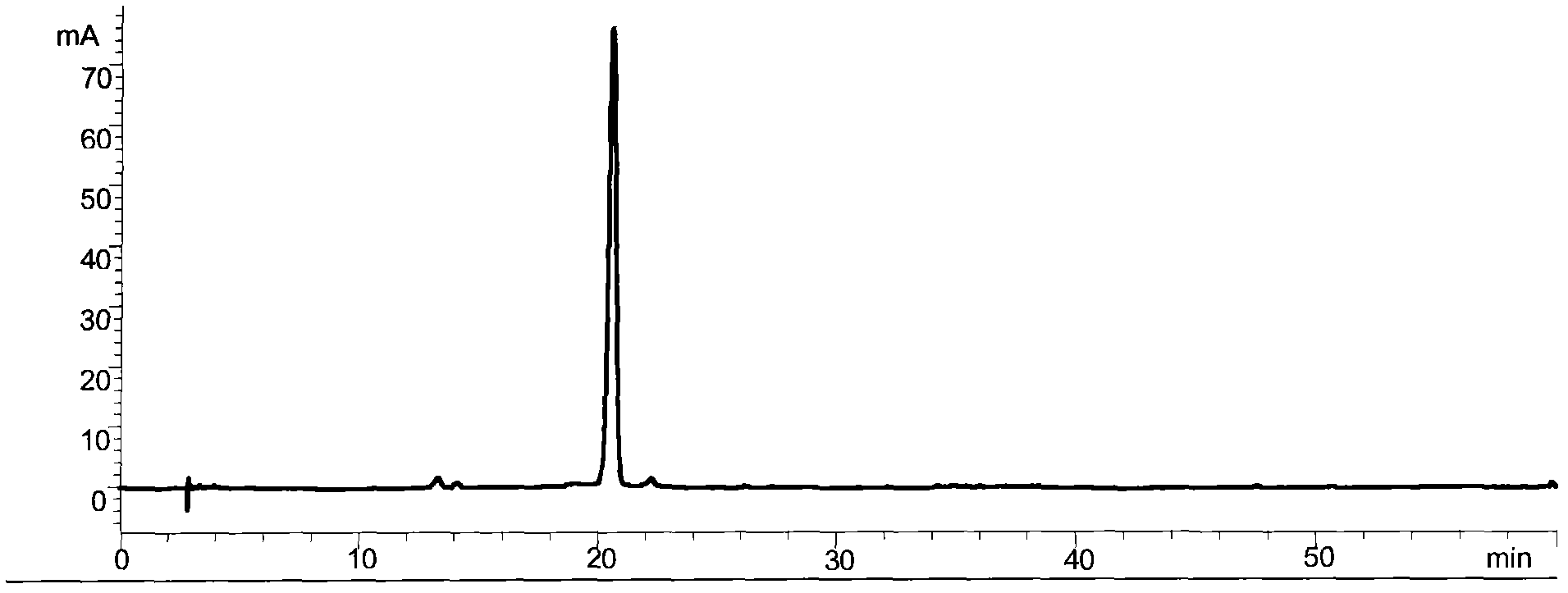
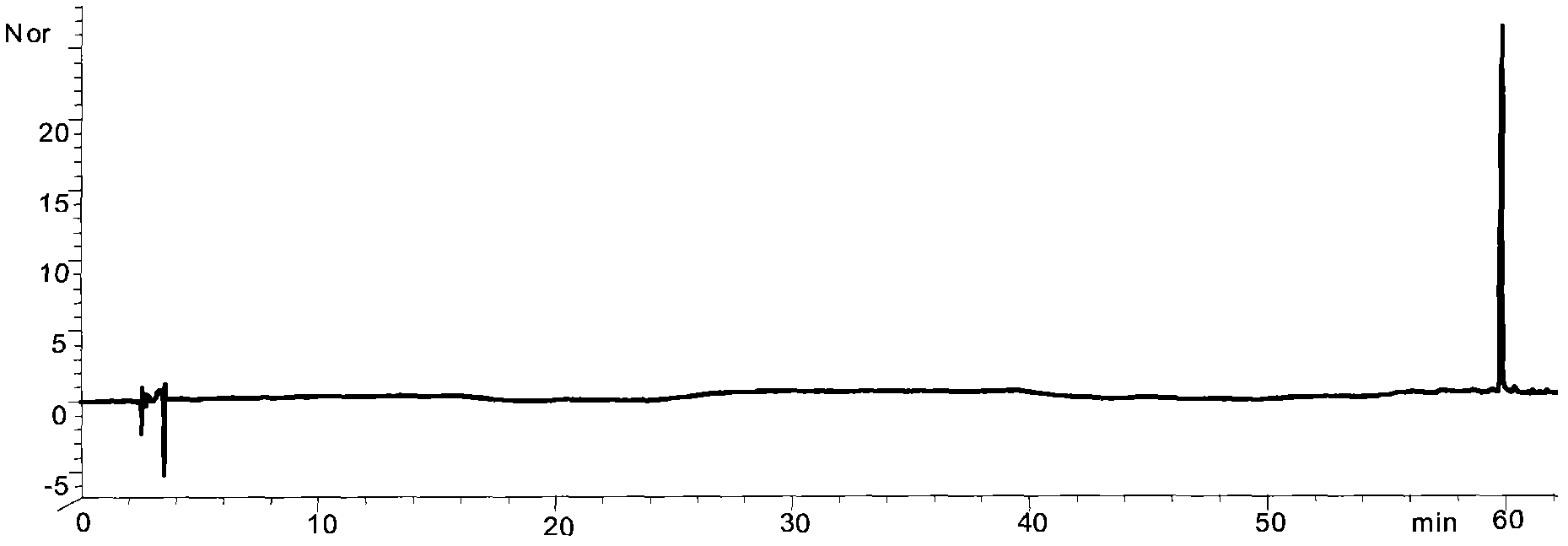
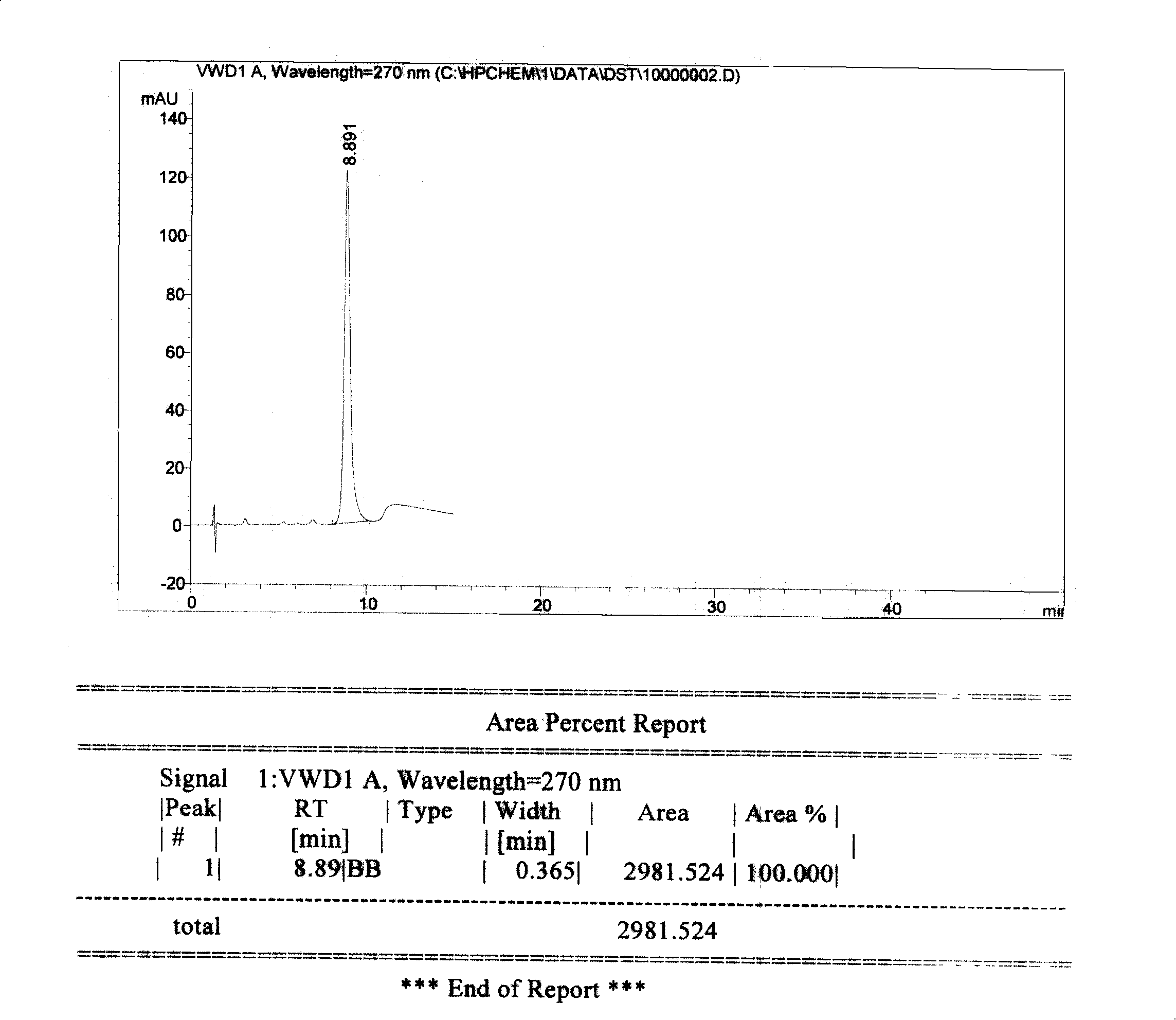
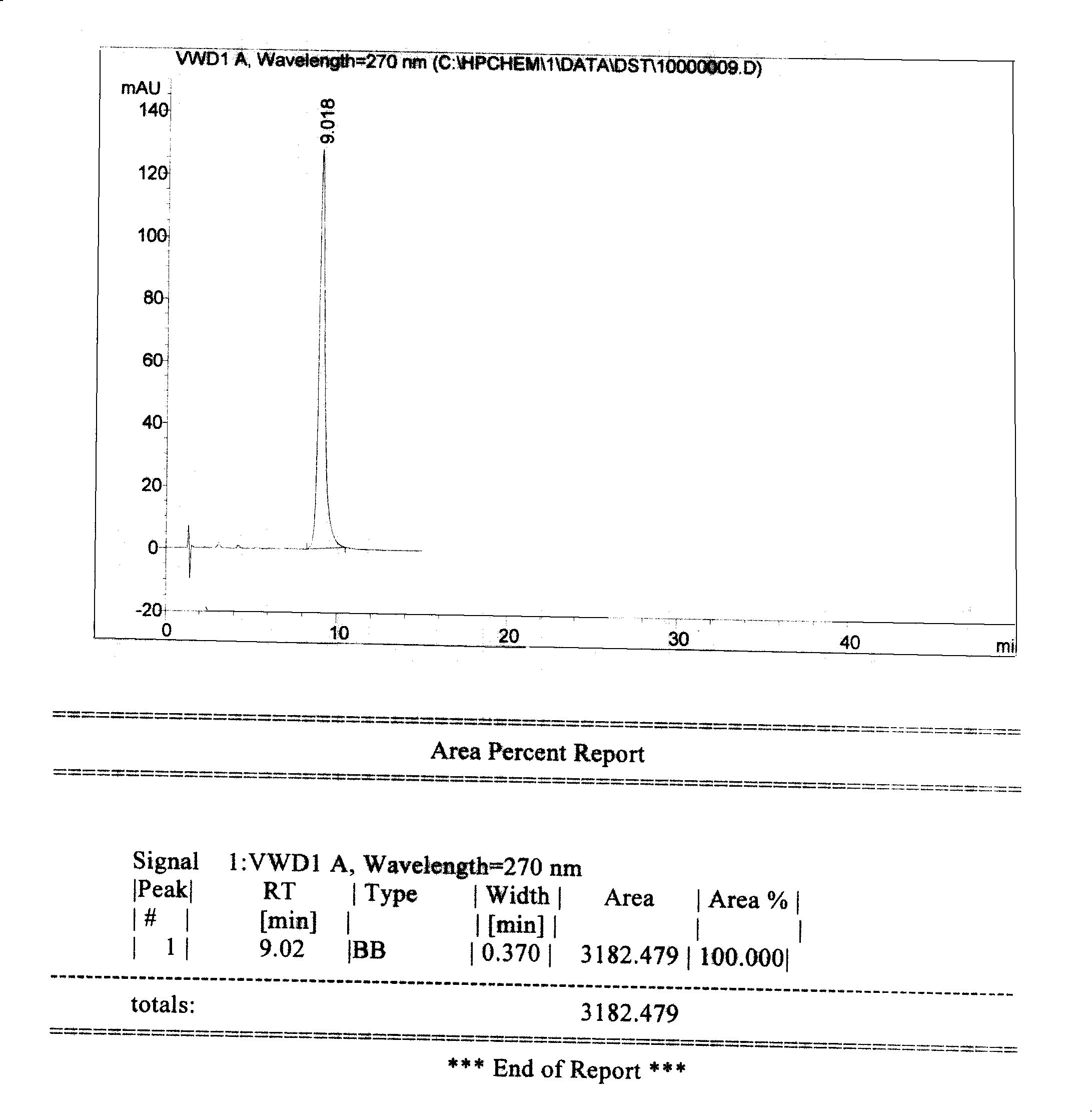
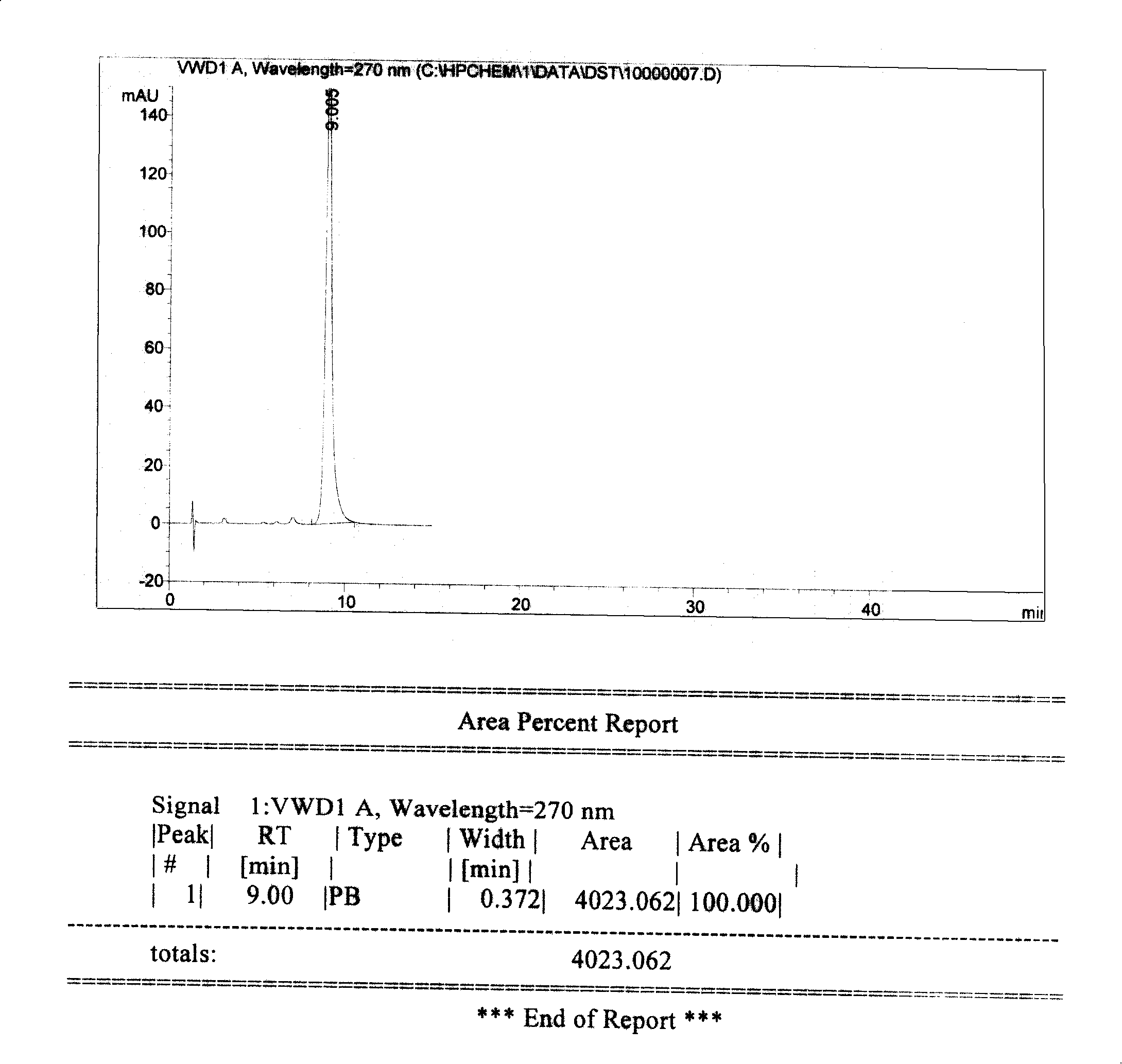
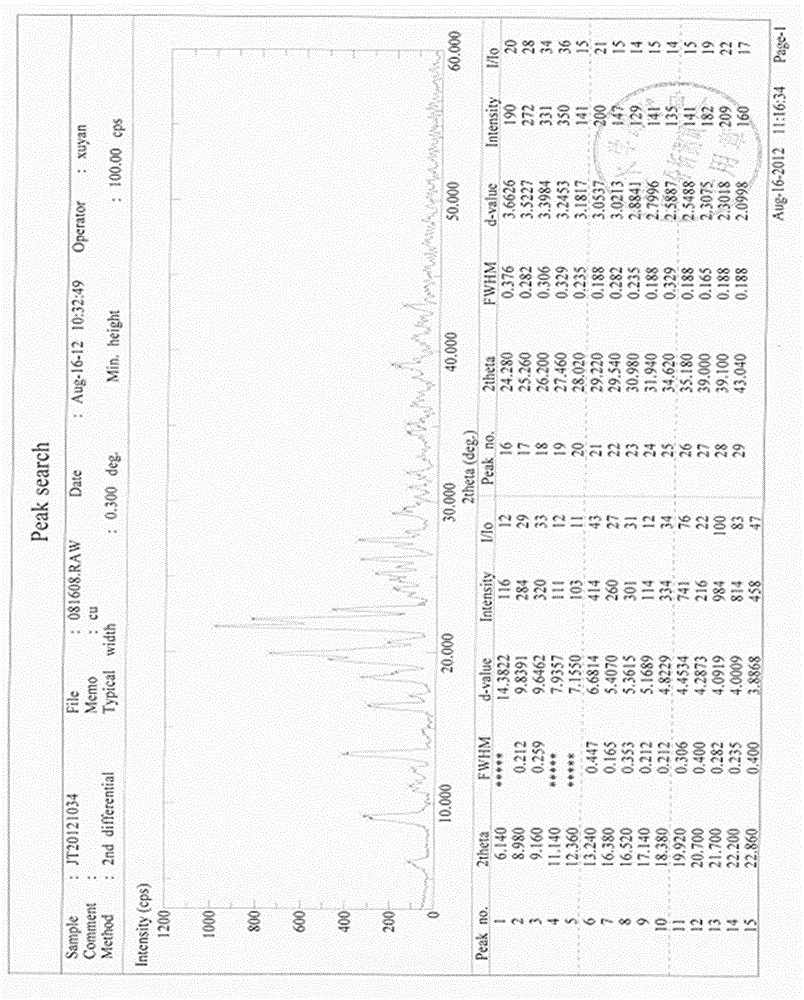
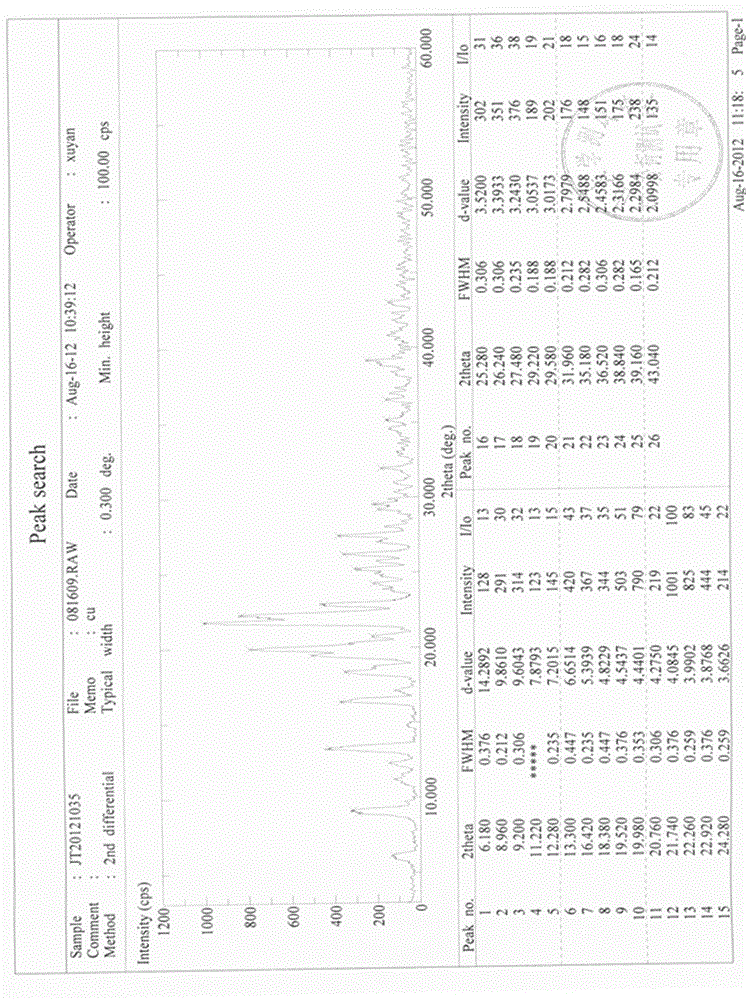
Preparation method of high-bulk density 9,9-di[3-phenyl-4-(2-hydroxyethoxy)phenyl]fluorene crystals
InactiveCN106242955AHigh bulk densityImprove liquidityEther separation/purificationOrganic chemistry methodsPtru catalystPropanoic acid
Owner:JIANGSU EVER GALAXY CHEM CO LTD
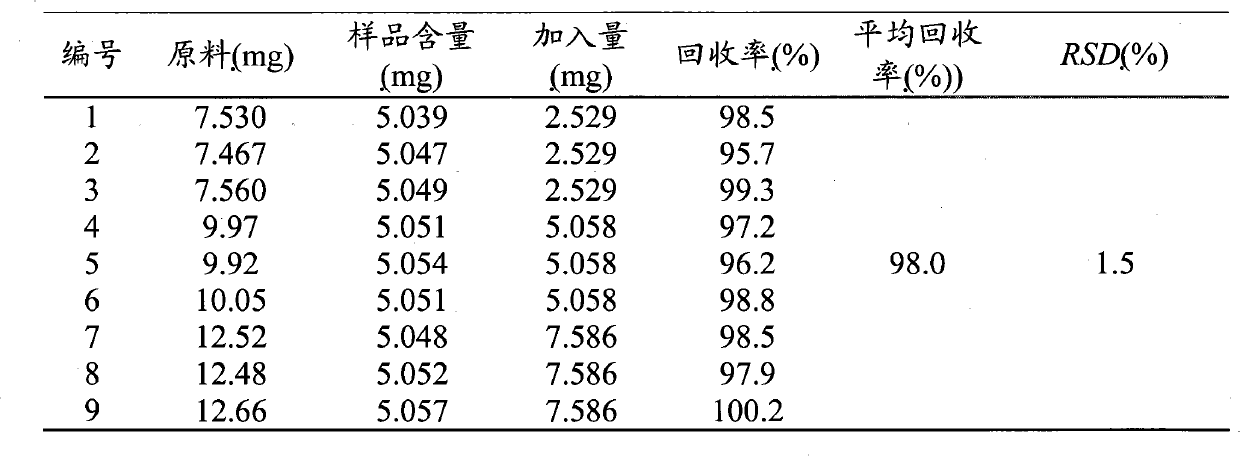
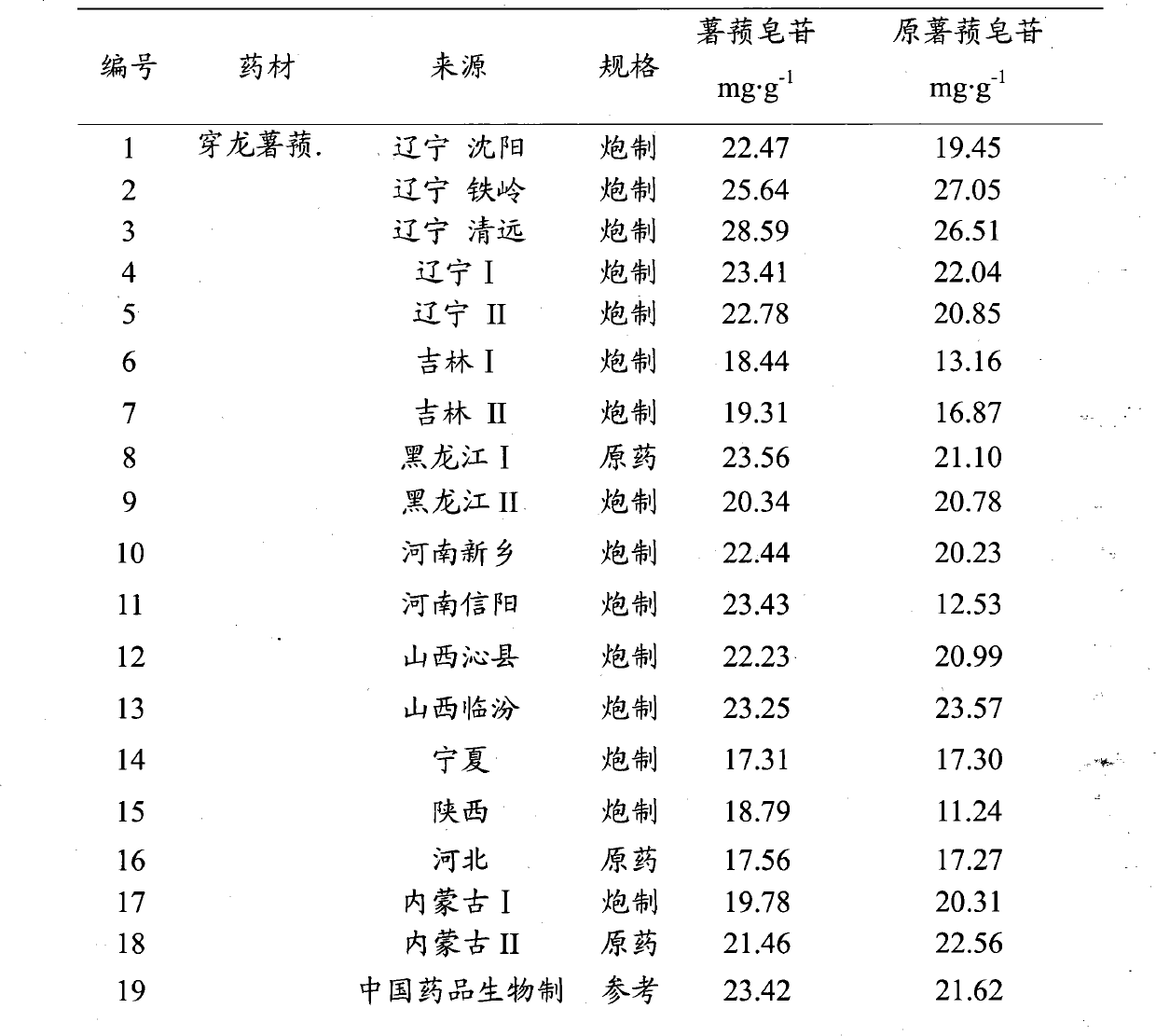
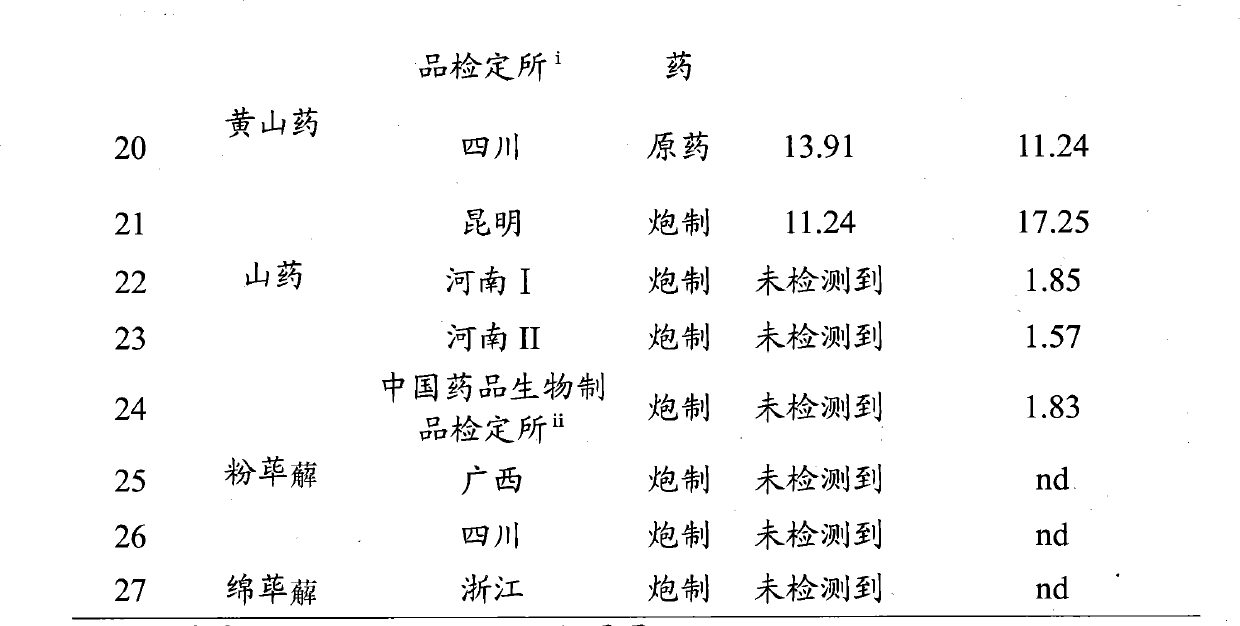
Method for testing quality of Discorea nipponica Makino in different places and medicinal materials of same genera
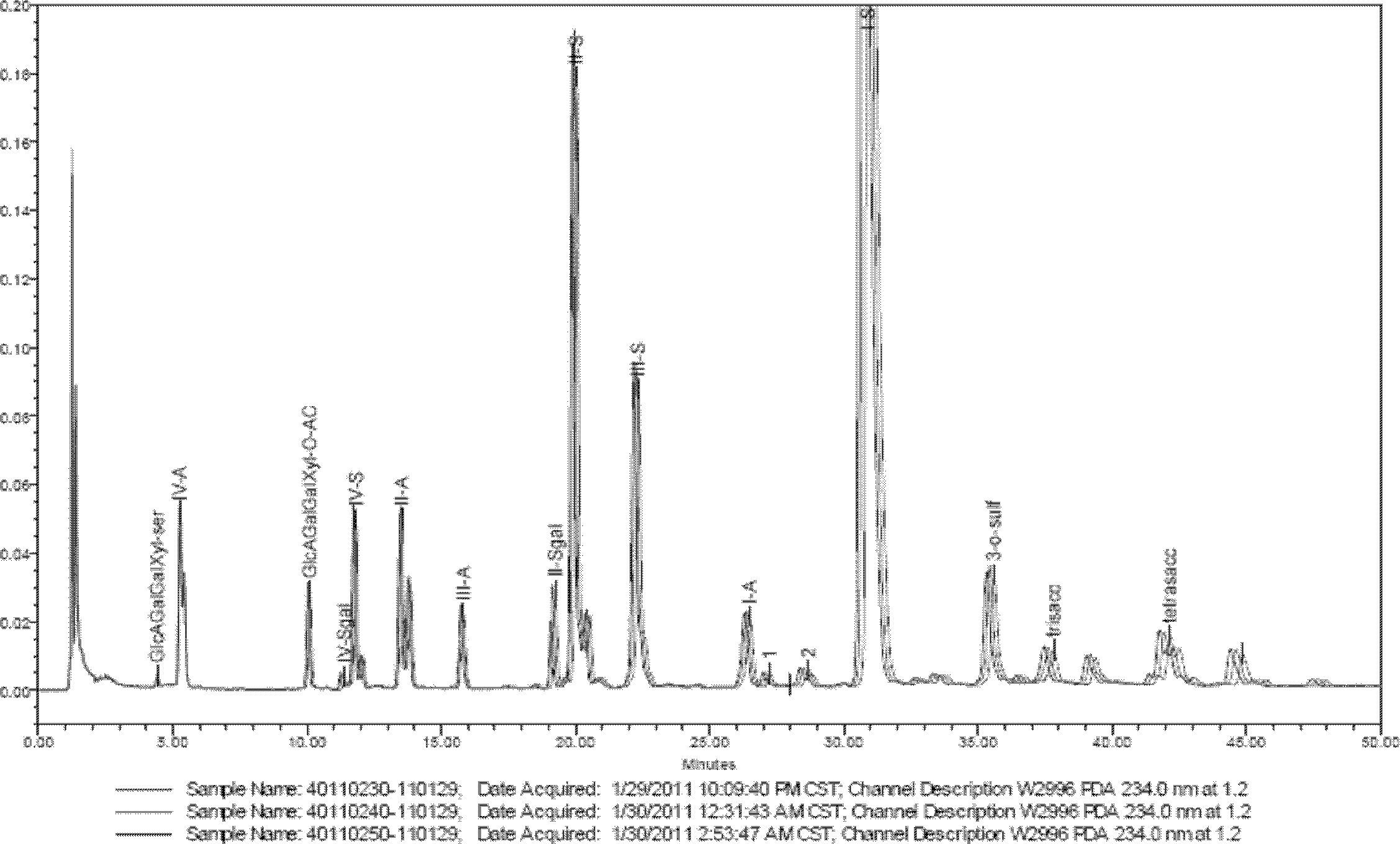
The invention provides a method for testing the quality of Discorea nipponica Makino and medicinal materials of dioscorea in different places. The method comprises the following steps: using medicinal material powder to be tested to prepare sample solutions, testing under a certain chromatographic conditions, and calculating the content of the indexes of each sample through external standard method, and the method is characterized in that dioscin and protodioscin are used as the content determination indexes. Dioscin and protodioscin are the main saponins components of dioscorea medicinal material, are active ingredients of Discorea nipponica Makino for reducing blood fat and are very important to control the quality of medicinal materials and preparations. The invention adopts RP-HPLC analysis method to test the contents of dioscin and protodioscin in Discorea nipponica Makino and medicinal materials of dioscorea in different places; and the method is sensitive, accurate and targeted and can be used to control the quality of medicinal materials, extracts and corresponding preparations.
Owner:深圳市药品检验所
Titania-based coating for capillary microextraction
InactiveUS7622191B2Avoid breakingRetain performanceComponent separationMixing methodsSilicon dioxideDimethyl siloxane
A method is presented describing in situ preparation of the titania-based sol-gel PDMS coating and its immobilization on the inner surface of a fused silica microextraction capillary. Sol-gel titania-poly (dimethylsiloxane) (TiO2-PDMS) coating was developed for capillary microextraction (CME) to perform on-line preconcentration and HPLC analysis of trace impurities in aqueous samples. The sol-gel titania-based coatings demonstrated strong pH stability and enhanced extraction capability over other commercially availble GC coatings. Extraction characteristics of a sol-gel titania-PDMS capillary remained practically unchanged after continuous rinsing with a 0.1 M NaOH solution (pH=13) for 12 hours.
Owner:UNIV OF SOUTH FLORIDA
Online discovery and comprehensive antioxidation activity evaluation method for natural antioxidants in fructus chaenomelis as crude medicine
ActiveCN106483215AAntioxidant activity objectiveComprehensive antioxidant activityComponent separationDPPHIc50 values
The invention discloses an online discovery and comprehensive antioxidation activity evaluation method for natural antioxidants in fructus chaenomelis as a crude medicine. The method comprises the following steps: firstly, the antioxidation activity of fructus chaenomelis extracting solutions of different batches is determined; then chemical components contained in the fructus chaenomelis are measured and determined with HPLC-ESI-TOF / MS; chemical components with antioxidation activity in the fructus chaenomelis are screened out with an HPLC-DPPH online screening method, and IC50 values of part of the active components are determined; an antioxidation activity fingerprint spectrum of the fructus chaenomelis is established by means of HPLC analysis of multiple batches of fructus chaenomelis samples, and fingerprint spectrum similarity analysis is used for evaluating the antioxidation activity of different varieties of the fructus chaenomelis; multiple antioxidation active components are selected for content determination. The antioxidation activity quality of the fructus chaenomelis is evaluated preliminarily on the basis of the antioxidation capacity of the fructus chaenomelis extracting solutions, the variety difference between different varieties of the fructus chaenomelis is evaluated preliminarily on the basis of the antioxidation activity fingerprint spectrum similarity analysis, and the antioxidation activity of the fructus chaenomelis as the crude medicine is evaluated comprehensively by analyzing the content of multiple antioxidation active components.
Owner:SHANDONG ANALYSIS & TEST CENT
High performance liquid chromatography analysis method for oxalic aldehyde and glyoxalic acid
InactiveCN101509903ASensitive detectionHigh degree of automationComponent separationWater bathsColumn temperature
The invention relates to a high-performance liquid chromatography (HPLC) analytical method of glyoxal and glyoxylate comprising the following specific detection steps of: taking proper amount of samples; adding DNPH, the mole ratio of which to the aldehyde group is 2:1 to 50:1, as a derivative reagent; adjusting the pH to 1.5 to 3 by using weak-acid salt solution; reacting in thermostatic water bath at 30 to 80 DEG C for 60 to 240 minutes; and analyzing the obtained derivative by using HPLC with the ultraviolet detection wavelength of 400-500 nm. The HPLC analysis conditions are as follows: the mobile phase is acetonitrile and aqueous solution, with the volume ratio of 95 / 5 to 50 / 50; the flow rate is 0.6 to 2ml / min; the column temperature is 20 to 50 DEG C; and the sample size is 1 to 20 Mu L. The HPLC analytical method of glyoxal and glyoxylate has the advantages of high degree of automation, high selectivity, sensitive detection, high efficiency and accuracy and the like. Experimental results show that the acetaldehyde, acetic acid and oxalic acid can not generate mutual interference to the detection of the glyoxal and glyoxylate.
Owner:NANJING UNIV OF TECH
Method for rapidly measuring content of bongkrekic acid in food by automatic solid phase extraction-ultra performance liquid chromatography
PendingCN108195954ASatisfy securityEnsure safetyComponent separationFood safetySolid phase extraction
The invention discloses a method for rapidly measuring a content of bongkrekic acid in food by automatic solid phase extraction-ultra performance liquid chromatography. The method comprises steps of pretreatment of food samples, purification of extracted solution and HPLC analysis to obtain the content of bongkrekic acid in the food samples; and results are proved. The method provided by the invention replaces a traditional manual column passing method with an automatic solid phase extraction instrument to realize automation, reduce labor costs and personal errors, realize controllability of solid phase extraction velocity, improve repeatability and accuracy of experiments and shorten a sample treatment cycle; the method optimizes extraction conditions, chromatographic conditions and the like to obtain an excellent separation degree and chromatographic peak, has advantages of high sensitivity, high recovery rate and relatively short pretreatment cycle, meets requirements on detection of the content of bongkrekic acid in food, provides a reference for a laboratory to carry out relevant detections and plays an active role in ensuring food safety.
Owner:ZHEJIANG INST FOR FOOD & DRUG CONTROL
Fingerprint spectrum of immature bitter orange medicinal material and construction method and application of fingerprint spectrum
ActiveCN103837621AComprehensive characteristic peak informationImprove stabilityComponent separationHplc fingerprintComputer science
The invention belongs to the technical field of pharmaceutical analysis and relates to a fingerprint spectrum of an immature bitter orange medicinal material and a construction method and application of the fingerprint spectrum. Specifically, the invention relates to a high performance liquid chromatography (HPLC) fingerprint spectrum of the immature bitter orange medicinal material and a construction method of the fingerprint spectrum, as well as application of the HPLC fingerprint spectrum and / or a standard fingerprint spectrum of the immature bitter orange medicinal material in identification of the immature bitter orange medicinal material. The method for constructing the HPLC fingerprint spectrum of the immature bitter orange medicinal material specifically comprises the following steps: (1) preparing an immature bitter orange test sample; and (2) performing HPLC analysis. The invention also relates to a method for identifying the immature bitter orange medicinal material. The method is applied to identifying the authenticity and quality of the immature bitter orange medicinal material.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method for measuring content of Shenqi blood sugar reducing preparation and application thereof in overall quality control
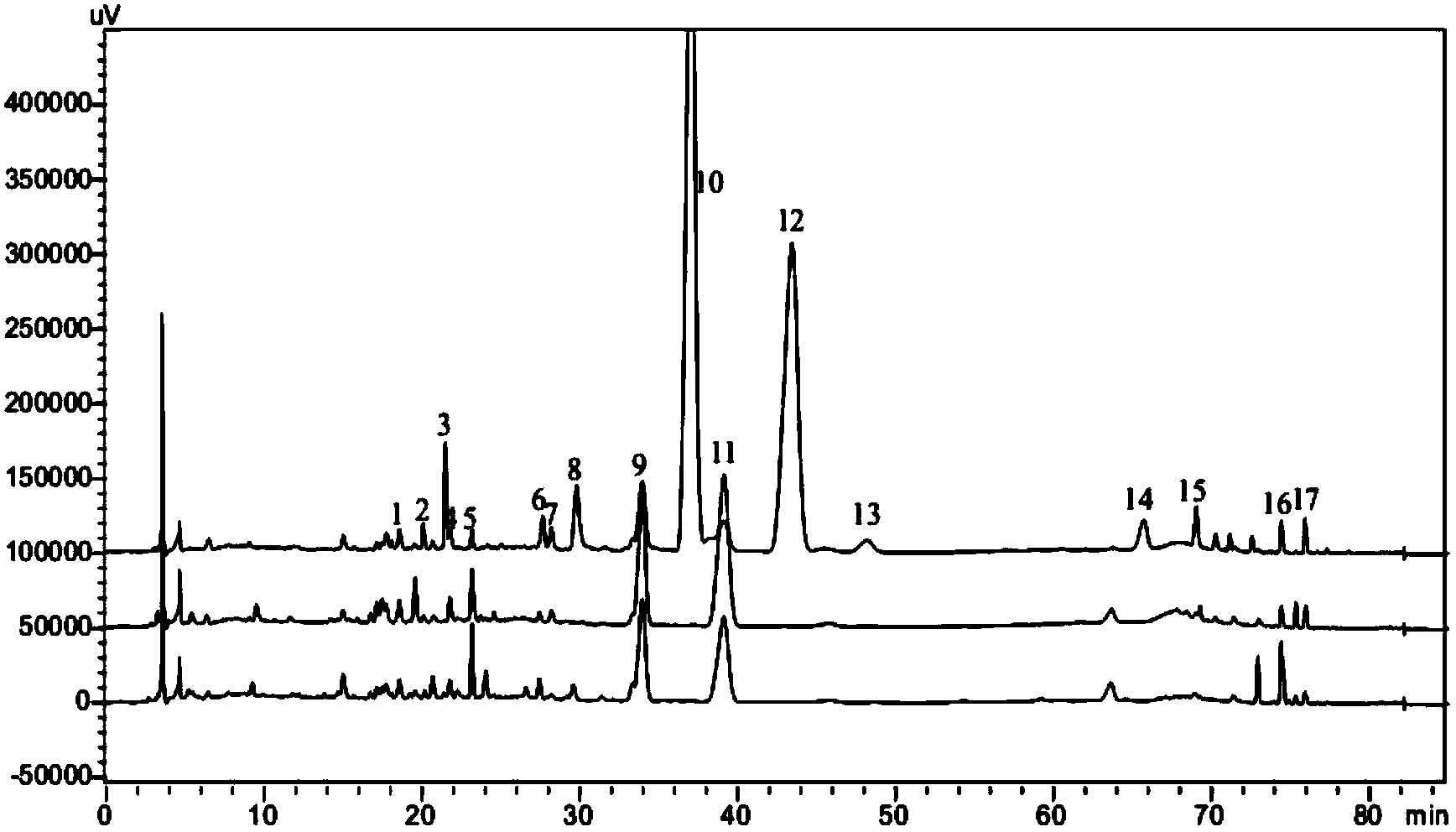
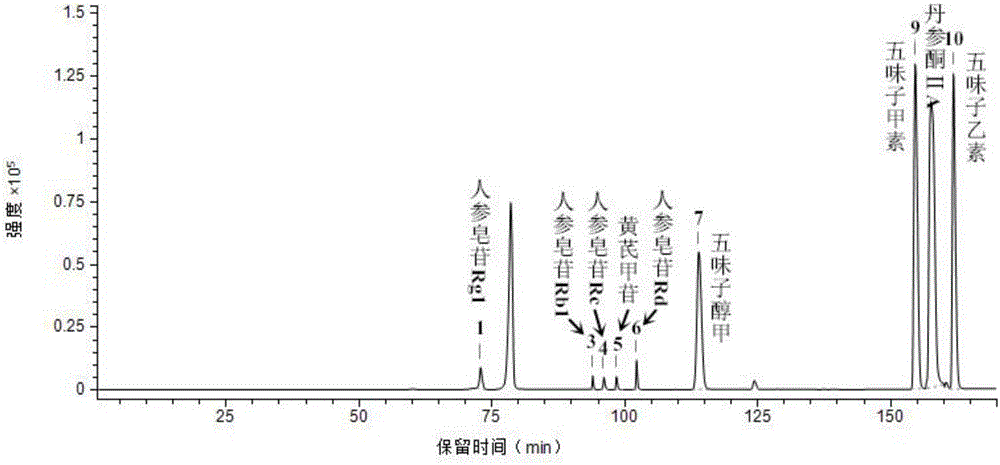
The invention provides a method for measuring the content of a Shenqi blood sugar reducing preparation. The method is used for measuring the content of 10 active ingredients in the Shenqi blood sugar reducing preparation: ginsenoside Rb1, ginsenoside Rc, ginsenoside Rd, ginsenoside Rg1, ginsenoside Re, astragaloside, deoxyschizandrin, schisandrol A, schisandrol B. In the invention, with the guidance of blood sugar reducing activity of a compound, multiple active substances in the compound are detected, and the quality level of the whole compound preparation can be reflected better; meanwhile, a Q-MS ion monitoring mode is selected for content measurement, and the mass spectrum quantification has high accuracy, specificity and sensitivity and is superior to traditional HPLC analysis method; and moreover, fingerprint is established according to the liquid phase diagrams of different batches of Shenqi blood sugar reducing preparations obtained by the method, the similarity is evaluated, the inherent quality of the Shenqi blood sugar reducing preparation product is evaluated more scientifically and comprehensively, and a basis is provided for establishing a quality standard of higher level.
Owner:ZHEJIANG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method of high-bulk density 9,9-di[3-phenyl-4-(2-hydroxyethoxy)phenyl]fluorene crystals Preparation method of high-bulk density 9,9-di[3-phenyl-4-(2-hydroxyethoxy)phenyl]fluorene crystals](https://images-eureka.patsnap.com/patent_img/8bd2fec1-d68b-4c9b-ab08-ac8d2345c592/150811172207.PNG)
![Preparation method of high-bulk density 9,9-di[3-phenyl-4-(2-hydroxyethoxy)phenyl]fluorene crystals Preparation method of high-bulk density 9,9-di[3-phenyl-4-(2-hydroxyethoxy)phenyl]fluorene crystals](https://images-eureka.patsnap.com/patent_img/8bd2fec1-d68b-4c9b-ab08-ac8d2345c592/150811172213.PNG)
![Preparation method of high-bulk density 9,9-di[3-phenyl-4-(2-hydroxyethoxy)phenyl]fluorene crystals Preparation method of high-bulk density 9,9-di[3-phenyl-4-(2-hydroxyethoxy)phenyl]fluorene crystals](https://images-eureka.patsnap.com/patent_img/8bd2fec1-d68b-4c9b-ab08-ac8d2345c592/150811172217.PNG)