Method for synthesizing high-purity non-steroidal anti-inflammatory drug loxoprofen sodium
A technology of loxoprofen sodium and a synthesis method, which can be applied to only fields, can solve problems such as being unsuitable for large-scale industrial production, high risk, difficult preparation, etc., and achieves that the purification method is simple and easy to operate, less by-products, and easy to synthesize steps. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
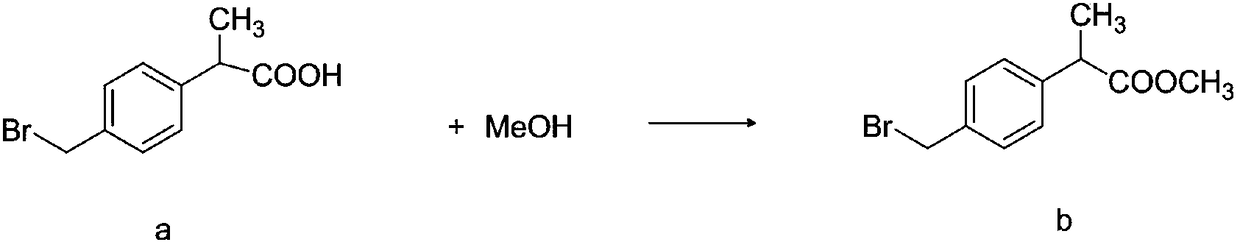
[0040] Synthesis of methyl 2-(4-bromomethylphenyl)propionate b
[0041] The reaction formula is as follows:
[0042]
[0043] Add 375ml of methanol to 1000ml of fresh toluene, slowly add 136g of concentrated sulfuric acid under stirring, the system has exotherm, keep at 35°C, then add 250g of raw material a to the reaction system, react at 281°C for 5h, detect the residue of raw material a by HPLC 0.01%, the reaction is finished, cooling the reaction solution, separating the organic layer, washing with 500ml of water, then adding 500ml of water, adding 5.5g of sodium carbonate under stirring to adjust the pH of the system to 13.5, separating the organic layer, washing with 500×2ml of water, The organic layer was dried with 25 g of anhydrous magnesium sulfate for 2 hours; the magnesium sulfate was filtered off and then concentrated under reduced pressure, a total of about 720 g of toluene was recovered, and the residue was orange-yellow transparent oil 2-(4-bromomethylphenyl...
Embodiment 2
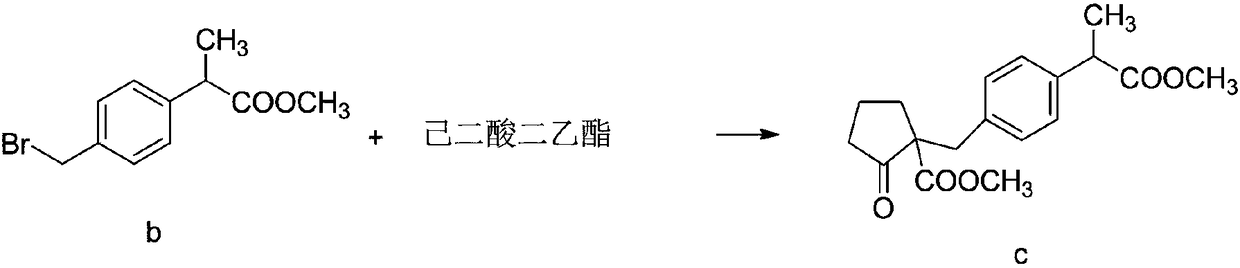
[0053] Synthesis of intermediate compound c
[0054] The reaction formula is as follows:
[0055]
[0056] 211 g of dimethyl adipate (DMA) and 71 g of DMSO were added to 870 ml of toluene, stirred and cooled to 2°C. Add 211g of 28% sodium methoxide methanol solution dropwise to the solution, control the dropping temperature not higher than 5°C, and finish the drop within about 1 hour; after the drop is finished, react at low temperature for 30 minutes, heat up, and concentrate at normal pressure to a system temperature of 112.5°C Stop; cool the reaction solution to 60°C, add 312ml of DMF, cool to 5°C, add 260g of 2-(4-bromomethylphenyl) methyl propionate b dropwise to the reaction solution, and maintain the temperature at 10°C during the dropping process; React at 18°C after dropping; after the reaction is complete (based on the disappearance of point b of methyl 2-(4-bromomethylphenyl) propionate detected by TLC), add 390ml of water and 375ml of toluene, and stir at 47°...
Embodiment 3
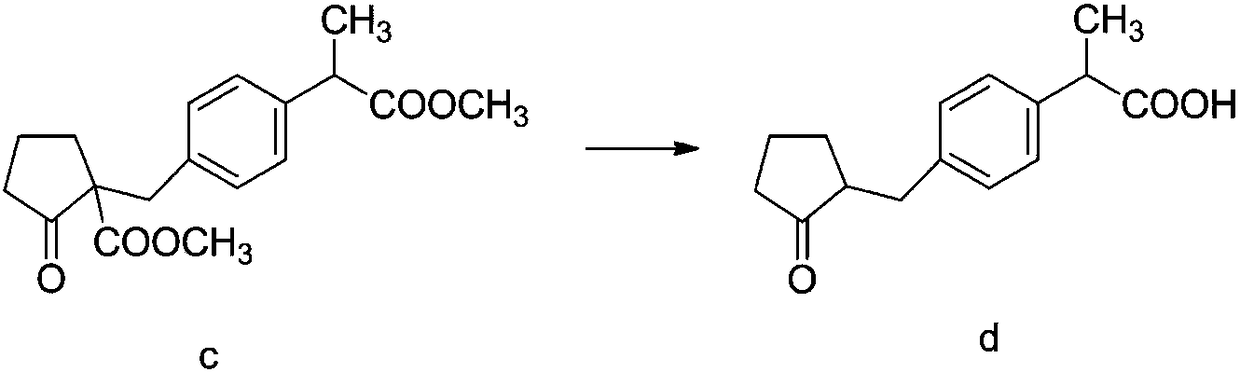
[0065] Synthesis of loxoprofen acid d
[0066] The reaction formula is as follows:
[0067]
[0068] Add 92g of compound c to the reaction flask, add 168ml of acetic acid and 108ml of water, add 48.8g of concentrated sulfuric acid, heat with a heating mantle, concentrate at normal pressure, after heating up to 110°C, maintain 110°C for reaction, and heat up to the reaction time for a total of 2h; Cool down to below 40°C, react under reduced pressure, the reaction temperature is 55°C, the reaction time is 4.5h, the reaction process is detected by HPLC, the compound c remains 0.07%, the reaction is over; after the reaction is completed, add 96ml of toluene and 96ml of water, stir and extract, after standing The organic layer is on the upper floor, separates the organic layer, washes with 150ml of water and separates the layers, adds 100ml of water to the organic layer, washes the organic layer with 5% sodium hydroxide solution to pH 5.4 (with NaOH solution 8.5g) under stirrin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com