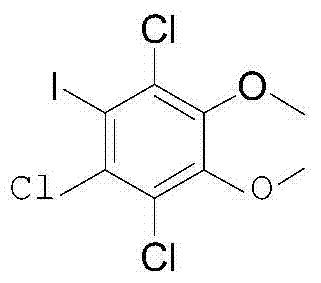
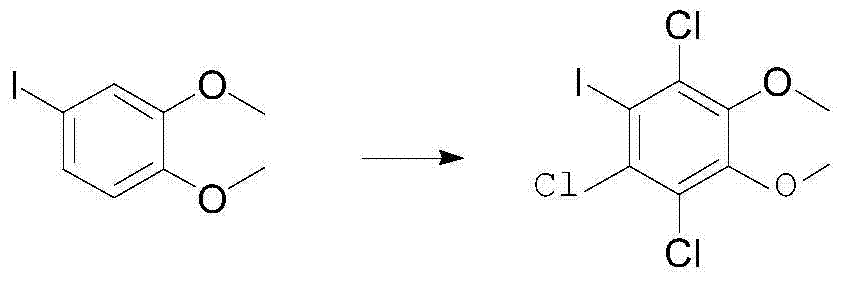
A kind of preparation method of 1,2-dimethoxy-4-iodobenzene-3,5,6-trichlorobenzene
A technology of dimethoxybenzene and trichlorobenzene, which is applied in the field of preparation of compound intermediates, can solve the problems of unfriendly environment, high cost of separation and purification, and low yield, so as to avoid column chromatography and achieve high yield , the effect of high product content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
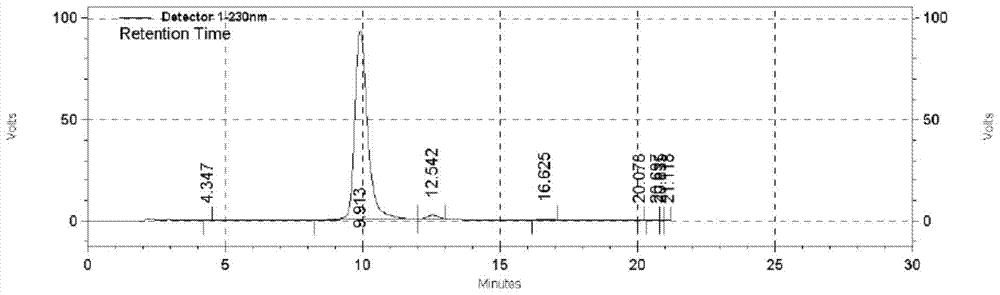
[0021] Add 2.64g of 4-iodo-1,2-dimethoxybenzene and 26ml of acetic acid into a 100ml reaction flask with a tail gas absorption device, then add 30ml of concentrated hydrochloric acid in sequence, and control the temperature of the reaction solution at 15-20 degrees. Slowly add 10ml of hydrogen peroxide diluted with 5ml of glacial acetic acid dropwise. After the dropwise addition, keep stirring at 15-25°C for 12 hours for sampling by HPLC monitoring. If the raw material is less than 1%, add about 30ml of saturated sodium bisulfite solution and filter, transfer the filter cake To a 100ml reaction bottle, add 10ml of acetic acid and 30ml of water, raise the temperature to 50-55°C and stir for 1 hour, then cool down to room temperature and filter with suction, rinse with a little water, TLC test shows a single spot. 3.01 g of white solid were obtained, yield 82%. , HPLC content greater than 97.8%.
Embodiment 2
[0023] Add 1.32g of 4-iodo-1,2-dimethoxybenzene and 6ml of formic acid and 6ml of propionic acid in a 100ml reaction flask with a tail gas absorption device, then add 15ml of concentrated hydrochloric acid in sequence, and control the temperature of the reaction solution for 25 -30°C, slowly add 5ml of hydrogen peroxide diluted with 2.5ml of formic acid dropwise, after the dropwise addition, keep stirring at 25-30°C for 12 hours for sampling by HPLC monitoring, the raw material is less than 1%, add about 15ml of saturated sodium bisulfite solution and filter , transfer the filter cake to a 100ml reaction flask, add 5ml of formic acid and 20ml of water, heat up to 50-60°C and stir for 5 hours, drop to room temperature and suction filter, rinse with a little water, TLC test shows a single spot. 1.46 g of white solid was obtained, yield 80%. HPLC content greater than 98.1%.
Embodiment 3
[0025] Add 5.28g of 4-iodo-1,2-dimethoxybenzene and 52ml of acetic acid into a 250ml reaction flask with a tail gas absorption device, then add 60ml of concentrated hydrochloric acid in sequence, and control the temperature of the reaction solution at 25-35 degrees. Slowly add 20ml of hydrogen peroxide diluted with 10ml of propionic acid dropwise. After the dropwise addition, keep stirring at 25-35°C for 12 hours for sampling by HPLC monitoring. If the raw material is less than 1%, add about 60ml of saturated sodium bisulfite solution and filter, transfer the filter cake To a 250ml reaction bottle, add 20ml of propionic acid and 100ml of water, raise the temperature to 50-55 degrees and stir for 3 hours, then cool down to room temperature and filter with suction, rinse with a little water, TLC test shows a single spot. 5.75 g of white solid was obtained, yield 80.5%. HPLC content greater than 97.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com