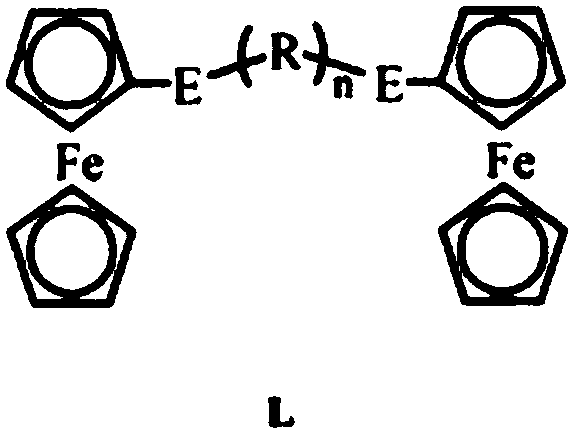
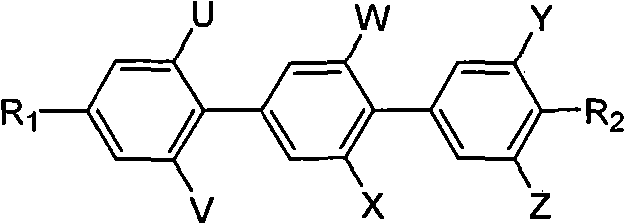
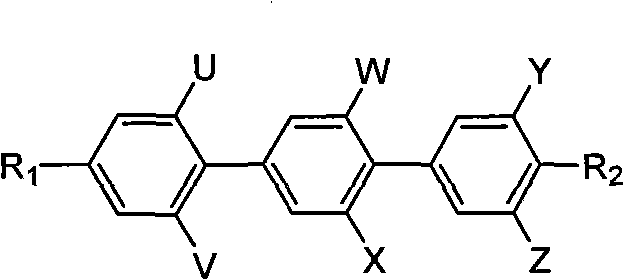
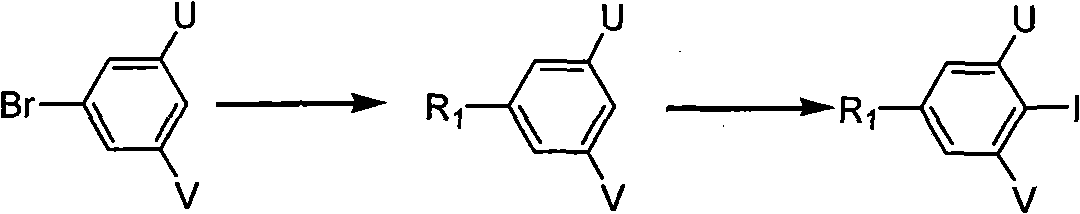
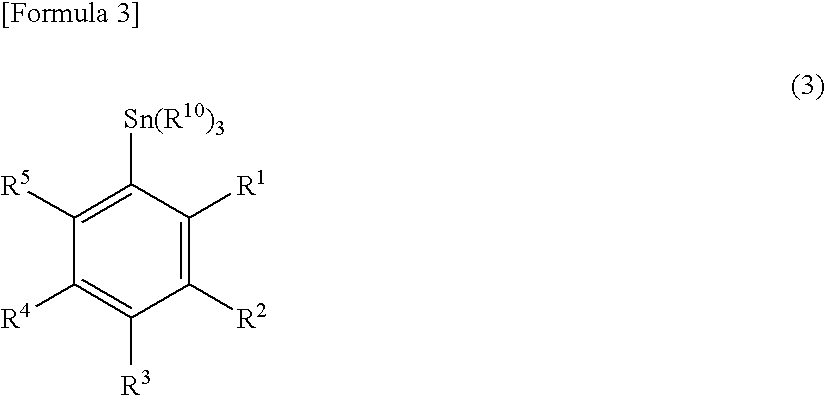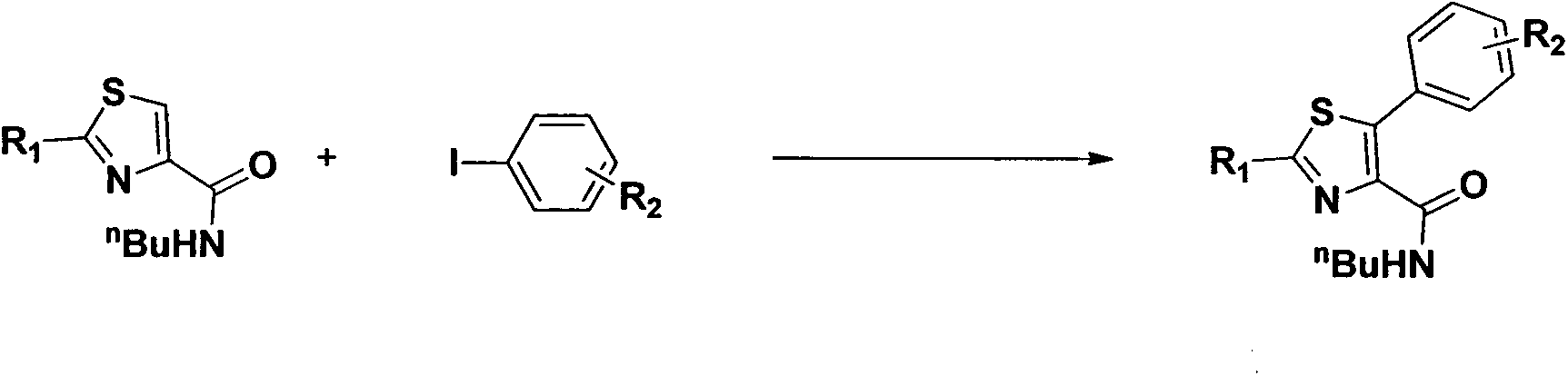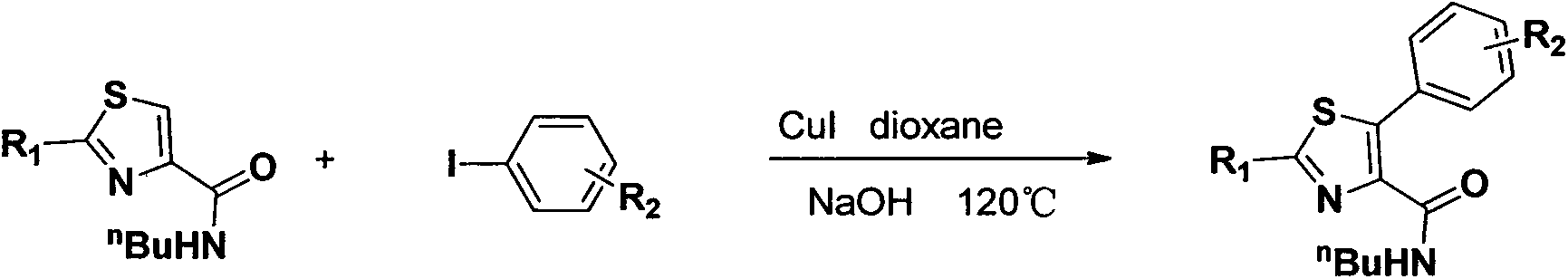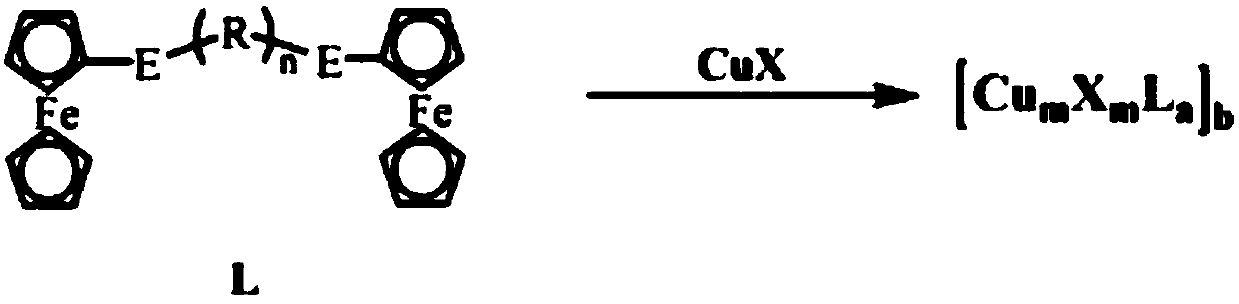Patents
Literature
276 results about "Iodobenzene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Iodobenzene is an organoiodine compound consisting of a benzene ring substituted with one iodine atom. It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorless liquid, although aged samples appear yellowish.
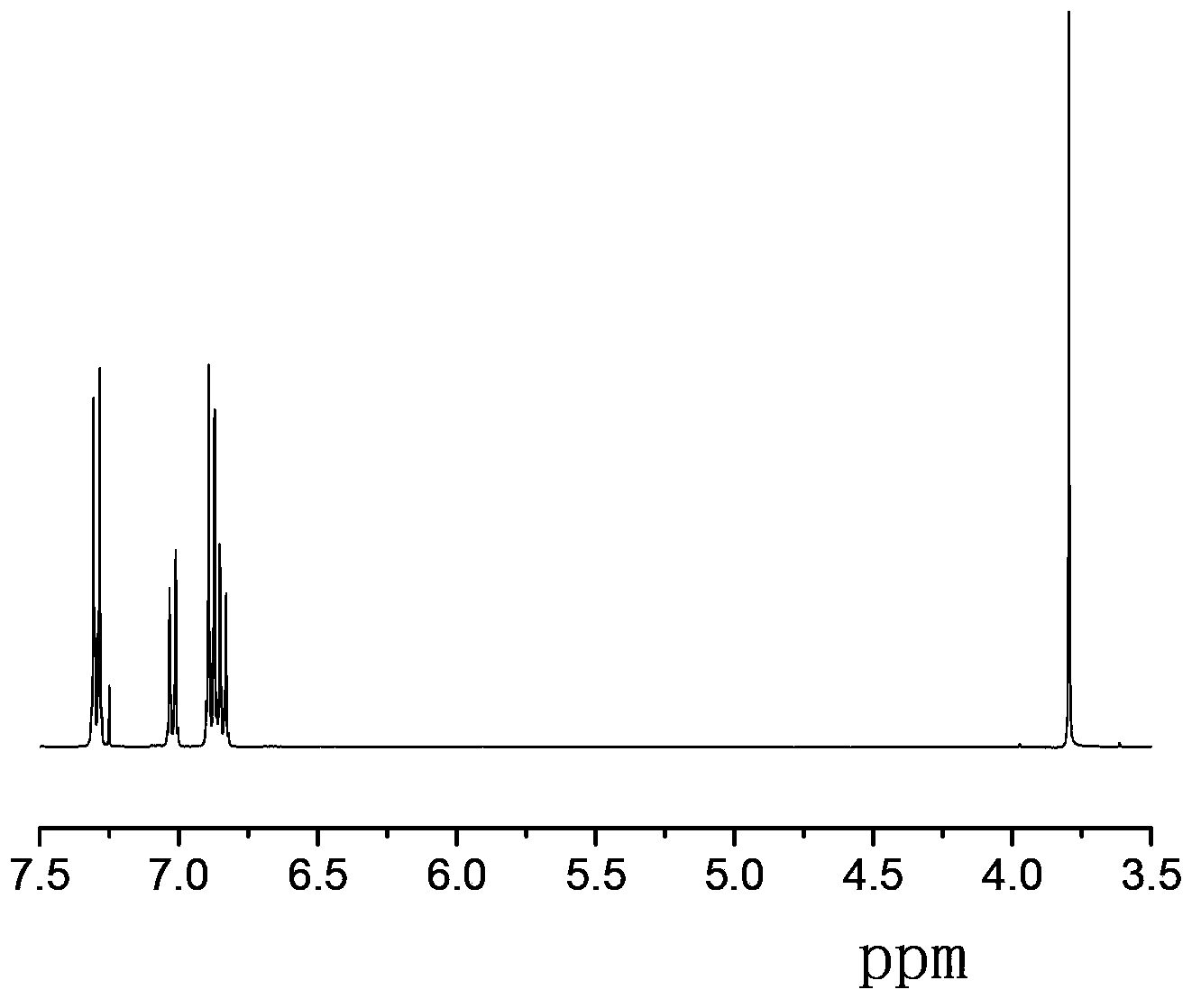
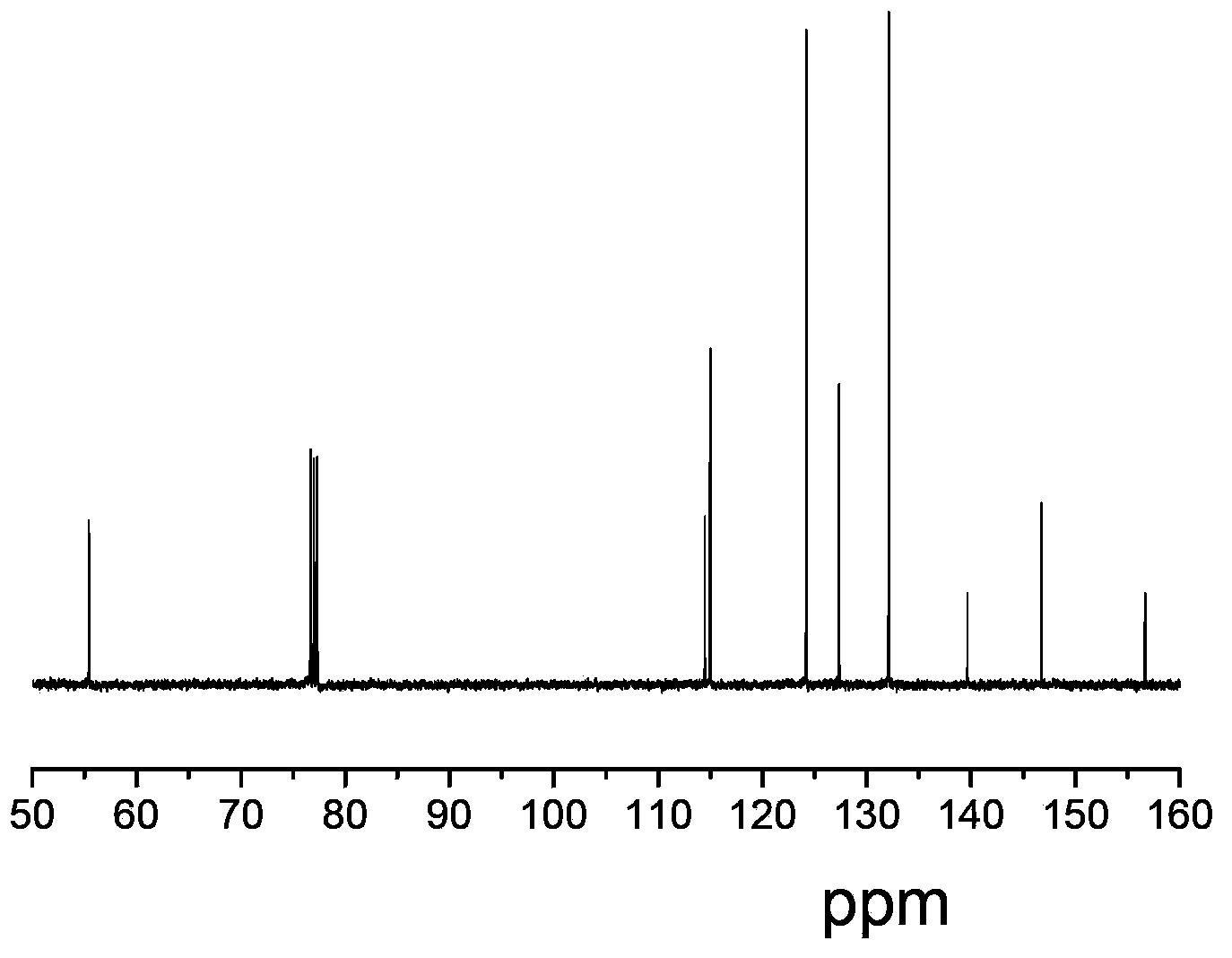
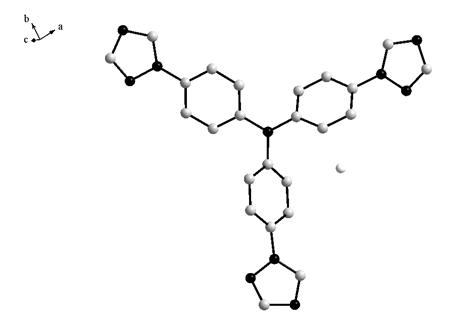
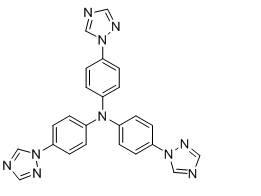
Synthesis methods of tri-(4-triazolyl phenyl) amine and tri-(4-triazolyl phenyl)amine cadmium complex
InactiveCN104193691AThe detection method is simpleHigh selectivityFluorescence/phosphorescenceGroup 2/12 organic compounds without C-metal linkagesSynthesis methodsCopper oxide
The invention discloses synthesis methods of a tri-(4-triazolyl phenyl) amine and a tri-(4-triazolyl phenyl) amine cadmium complex, and relates to the field of luminescent materials. The synthesis method of the tri-(4-triazolyl phenyl) amine takes copper oxide as a catalyst and dimethylsulfoxide as a solvent, tri-(4-iodobenzene) amine and triazole react at the temperature of 120-170 DEG C, then the mixture is diluted by the solvent, filtered, decolored, separated and purified sequentially to obtain the tri-(4-triazolyl phenyl) amine; the synthesis method of the cadmium complex is carried out by mixing 5-aminoisophthalic acid, 3-(4-triazolyl phenyl) amine and cadmium acetate into a mixed solution of water and alcohol, then the mixture is uniformly stirred under the acid or neutral condition to obtain a reaction liquid, and the reaction liquid is put into a reaction kettle to crystalize and react to obtain the tri-(4-triazolyl phenyl) amine cadmium complex. The synthesis methods obtain the tri-(4-triazolyl phenyl) amine and the tri-(4-triazolyl phenyl) amine cadmium complex, whose crystal purities are more than 95% and whose yields are more than 85%, through the simple synthesis route.
Owner:NANJING AGRICULTURAL UNIVERSITY
Methoxytriphenylamine-fluorene-containing copolymer, and preparation method and application thereof
InactiveCN103396533AGood light and heat stabilityImprove luminous efficiencySolid-state devicesSemiconductor/solid-state device manufacturingSolubility1,3-Propanediol
The invention provides a methoxytriphenylamine-fluorene-containing copolymer, and a preparation method and application thereof, relates to an organic electroluminescent material, a preparation method and application thereof, and aims to solve the problems that the existing polyfluorene electroluminescent polymer is low in light and heat stability, blue light emitted by the material is insufficient in saturation chroma and the solubility in organic solvent is poor. The methoxytriphenylamine-fluorene-containing copolymer provided by the invention is poly[2,7-(9,9-dioctylfluorene)-alternated-N-phenyl-N-(4-methoxyphenyl)aniline]. The preparation method comprises the following steps: 1, synthesizing a monomer from 4-methoxyaniline and 1-bromo-4-iodobenzene; and 2, synthesizing the methoxytriphenylamine-fluorene-containing copolymer from 9,9-dioctylfluorene-2,7-diboronic acid cis(1,3-propanediol)ester and the monomer. The polyfluorene luminescent material has favorable light and heat stability; the solubility in organic solvent can be up to 15-35%; and the material is applied to an electrogenerated blue light device, the emitted light approximates saturated deep blue.
Owner:HEILONGJIANG UNIV
Tris(4-triazole phenyl) amine compound as well as preparation method and application thereof
InactiveCN102633734AThe reaction is easy to operateHigh reaction yieldLight-sensitive devicesOrganic chemistrySingle crystalCopper oxide
The invention discloses a tris(4-triazole phenyl) amine compound and single crystal and a preparation method of tris(4-triazole phenyl) amine. The organic compound is prepared by adopting a one-pot method, namely heating tris(4-iodobenzene) amine, triazole, potassium carbonate and copper oxide. The preparation method has the characteristics of simple process operation, low production cost and low environmental pollution, and is suitable for large-scale industrial production. The prepared tris(4-triazole phenyl) amine single crystal can be applied to photoelectric materials, in particular to dyes and luminescence agents.
Owner:TIANJIN NORMAL UNIVERSITY
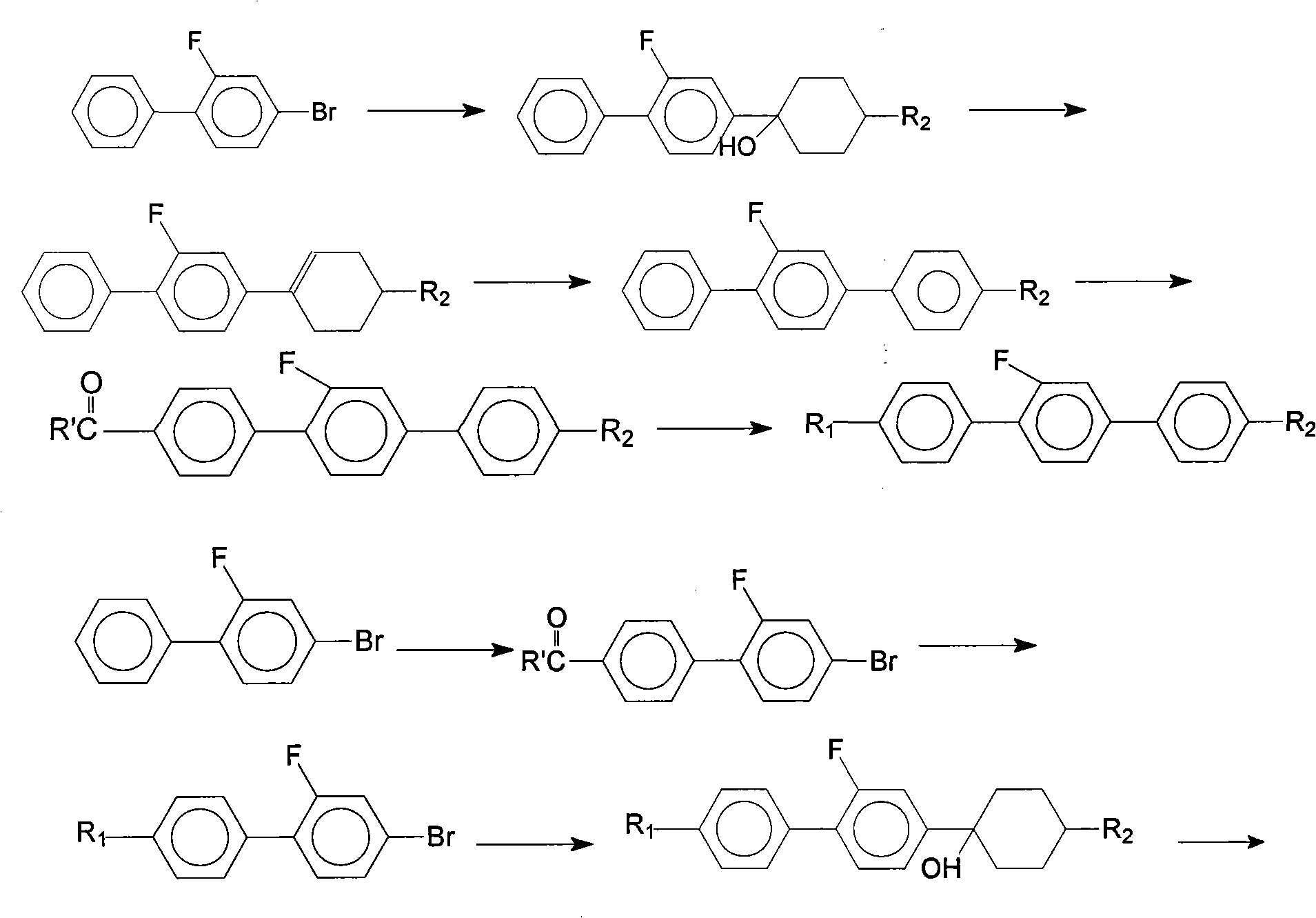
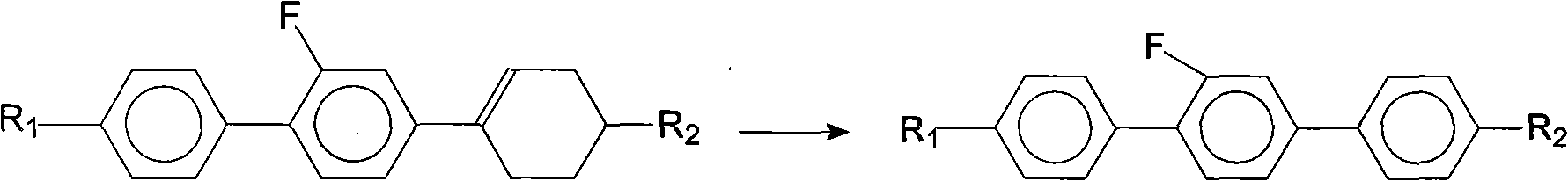
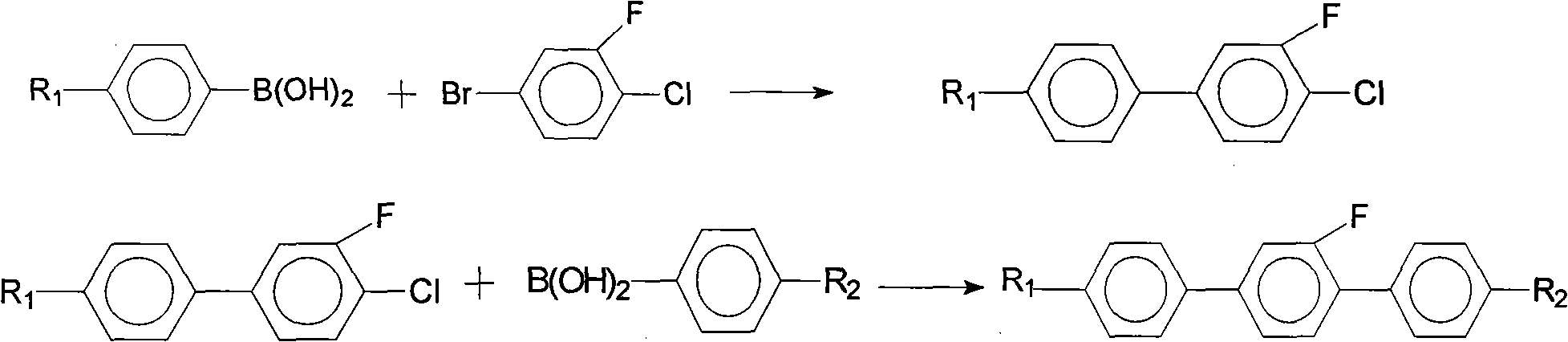
Method for synthesis of 2'-fluoro terphenyl liquid crystal
InactiveCN102399117ALow priceShort reaction pathLiquid crystal compositionsOrganic chemistry methodsBoronic acidReaction temperature
The present invention discloses a method for synthesis of 2'-fluoro terphenyl liquid crystal. With the present invention, problems of more reaction steps, low yield, heavy environmental hazard, expensive catalysts, or hazard on the human body in the prior art are solved. The technical scheme of the present invention is that: a catalyst is added to an organic solvent of the raw material to carry out a suzuki coupling reaction to realize the synthesis of the 2'-fluoro terphenyl liquid crystal. According to the present invention, 4-bromo-3-fluoro iodobenzene, a phenyl boronic acid derivative and a base are adopted as raw materials, and are subjected to two suzuki coupling reactions in the solvent under the effect of the catalyst at the reaction temperature of 70-100 DEG C to obtain the 2'-fluoro terphenyl liquid crystal, wherein the times of the two suzuki coupling reactions are respectively 4-10 hours and 10-20 hours. The method of the present invention has advantages of simple process, mild conditions, low preparation cost, high yield, environmental protection, and strong practicability.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
Chemoluminescent substrate
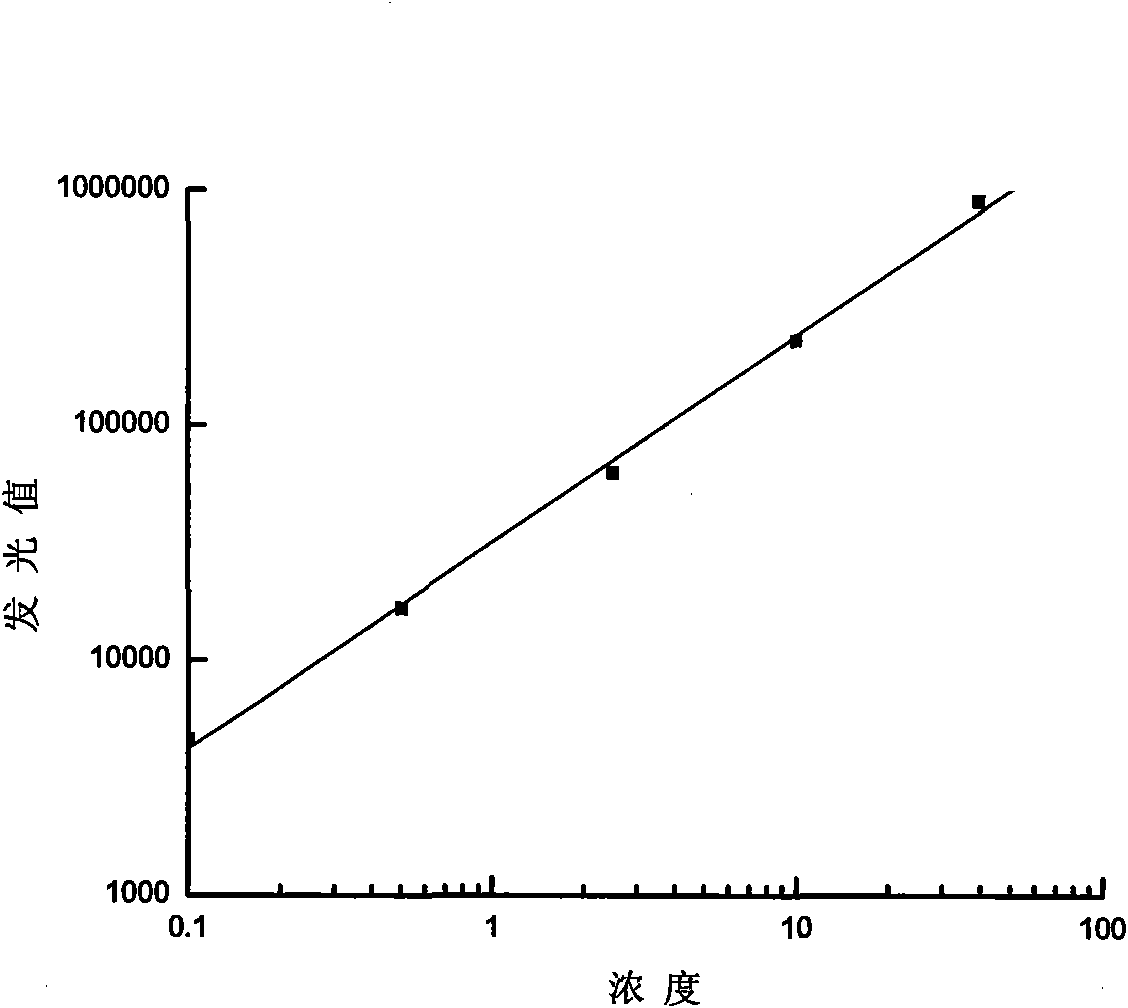
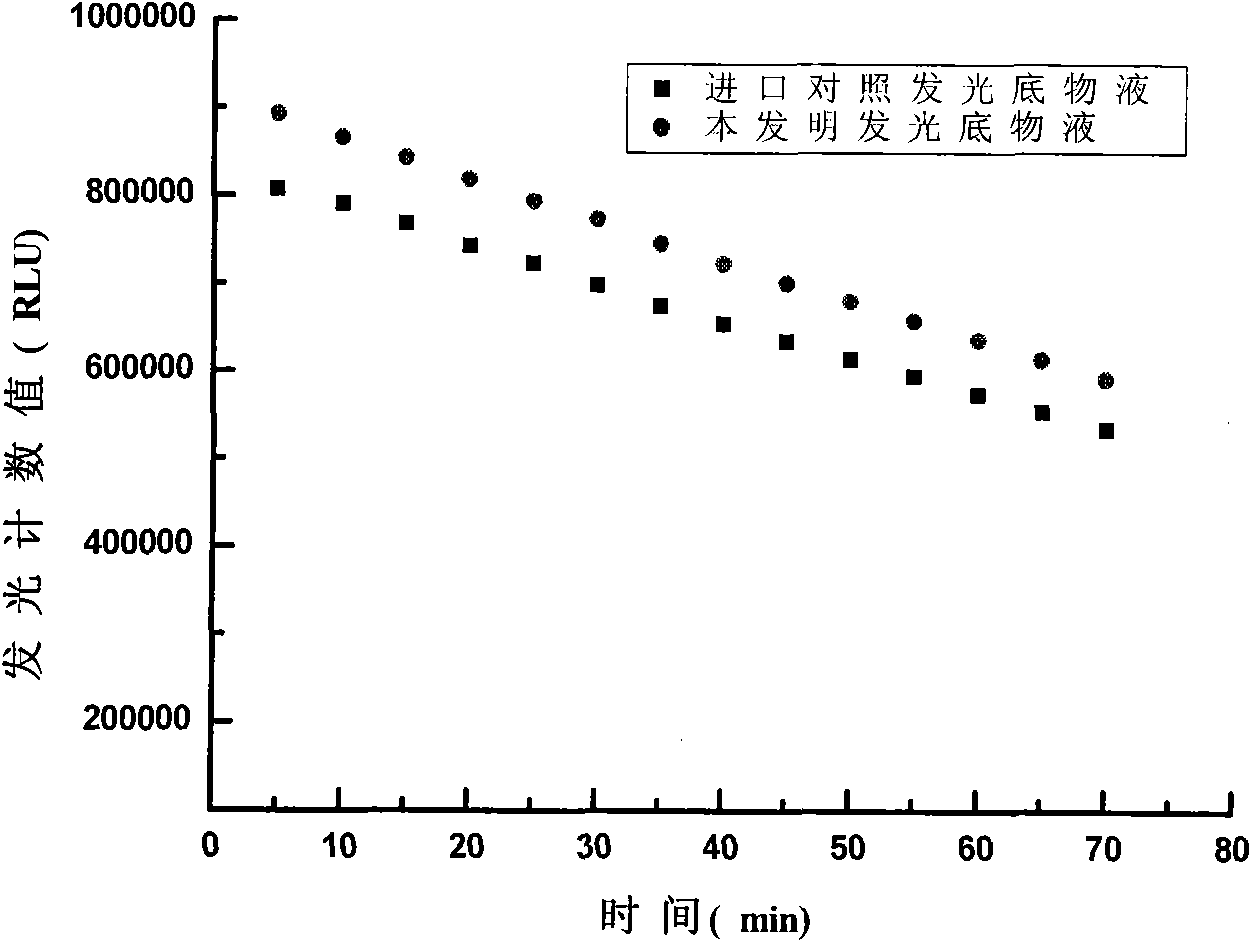
ActiveCN101571483ALow costHigh sensitivityChemiluminescene/bioluminescenceBiological testingBuffer solutionLuminescent Agents
The invention discloses a chemoluminescent substrate, which can be applied in the field of immunological detections, and comprises luminescent agent solution and oxidant solution, wherein the luminescence agent solution contains reinforcers, namely 4-hydroxydiphenyl and 4-iodo-borophenylic acid, a luminescent agent, namely luminal or luminal derivatives and buffer solution. The chemoluminescent substrate has high stability, good luminescence effect, long luminescent time, high sensitivity and low cost.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Fluorescent conjugated polymer silicon oxide nanoparticle preparation method and application thereof
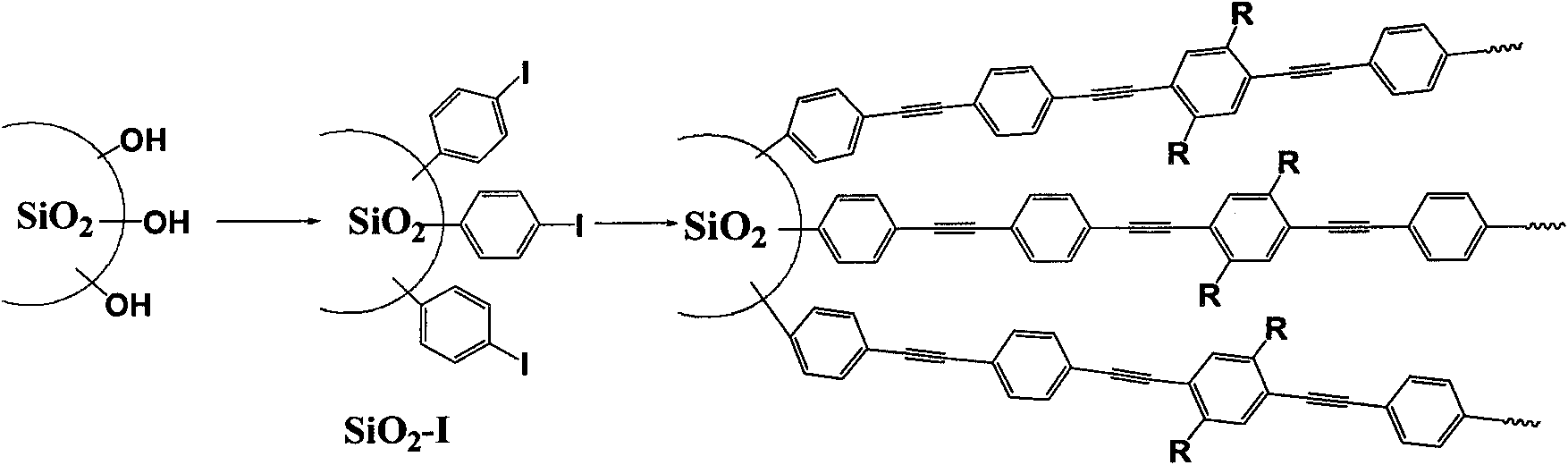
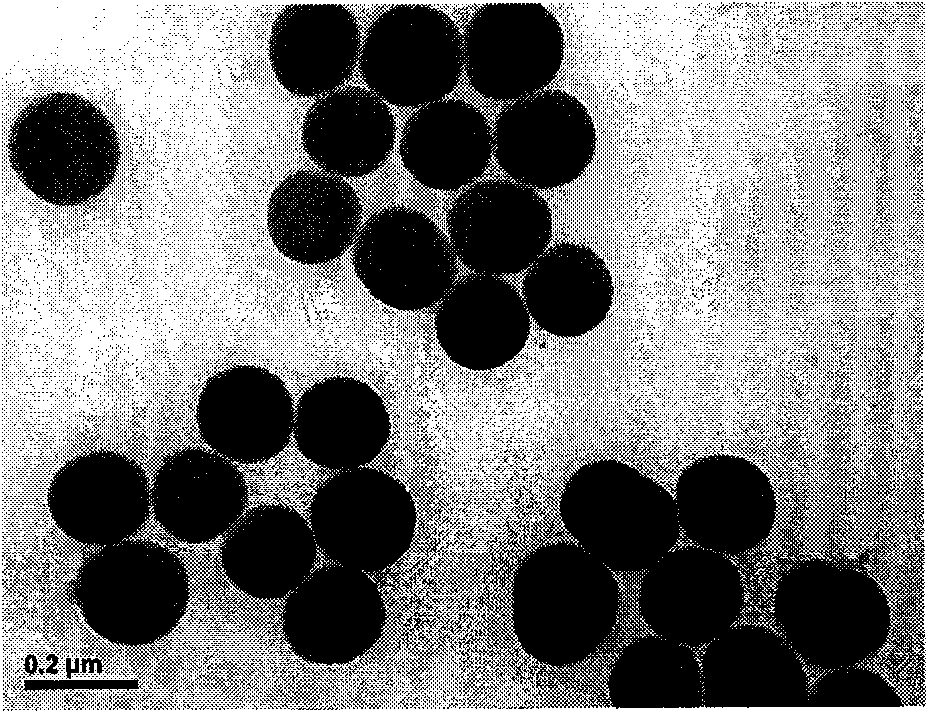
InactiveCN101597372AHigh detection sensitivityIncrease chance of contactFluorescence/phosphorescenceLuminescent compositionsSide chainSilicon oxide
The invention discloses a fluorescent conjugated polymer silicon oxide nanoparticle preparation method and an application thereof. In the method, the silicon oxide nanoparticle is used as nucleus, decorated with 4-iodo-benzo on the surface, and initiated to perform polymerization to have fluorescent conjugated polymer grafted on the surface. The preparation method comprises the two following steps: firstly, decorating the surface of the silicon oxide nanoparticle with 4-iodobenzene; secondly, initiating aromatic alkynyl benzene and aromatic monomer containing sulfonic acid side chain to perform polymerization on the surface of the silicon oxide nanoparticle to prepare the fluorescent conjugated polymer silicon oxide nanoparticle under the condition of the catalyzing by the organic metallic compound. The nanoparticle can be used to detect the content of TNT in a solution. In the method of the invention, the nanoparticle is introduced into a fluorescence sensor, thus obviously increasing the specific area and improving the contact probability of fluorescent conjugated polymer and TNT to be detected and the fluorescent quenching of conjugate polymer is enlarged, thus improving the detection sensitivity of the sensor and realizing the high sensitivity detection to the explosive TNT.
Owner:ZHEJIANG UNIV
Alpha functionalization of cyclic, ketalized ketones and products therefrom
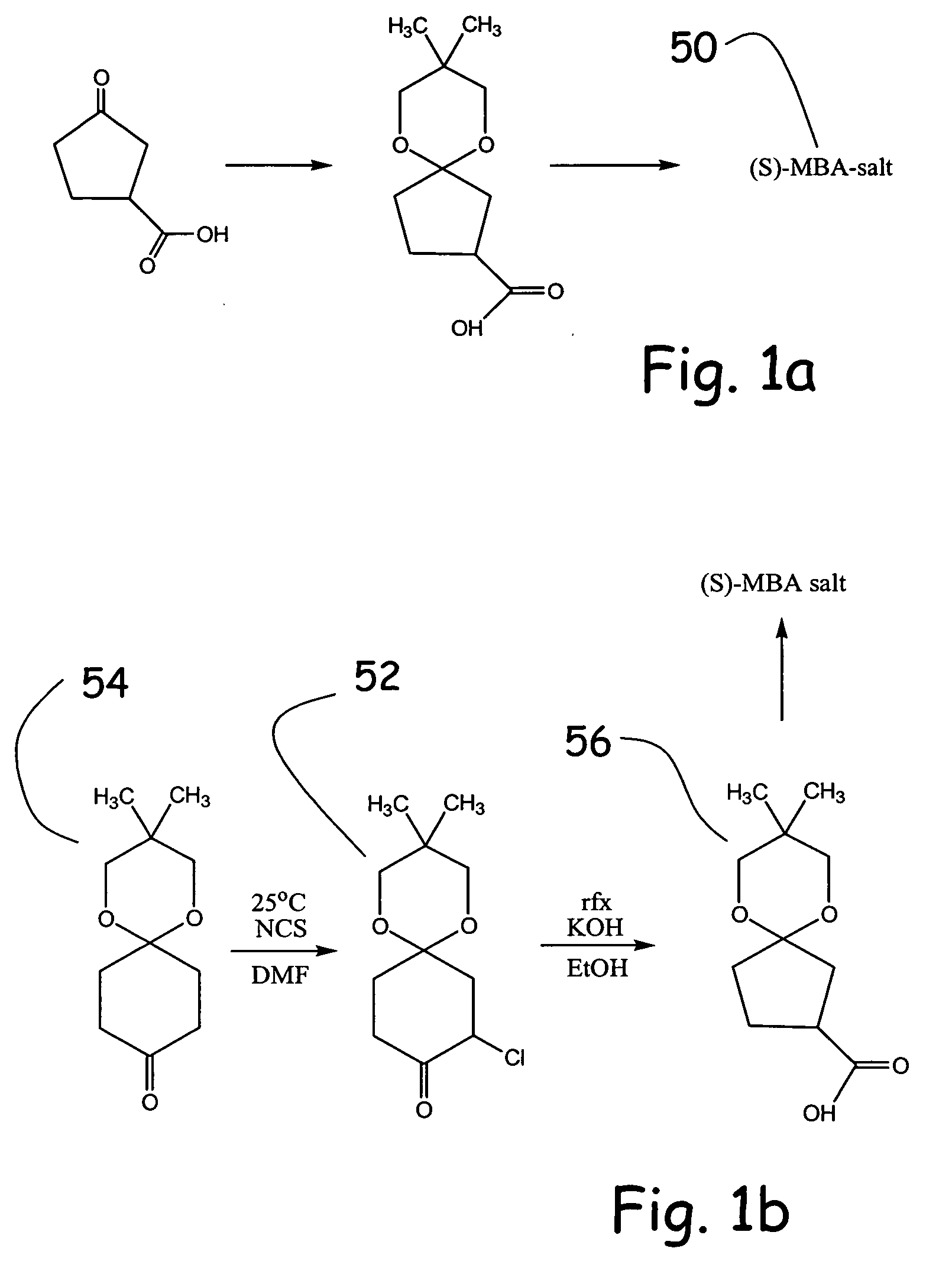
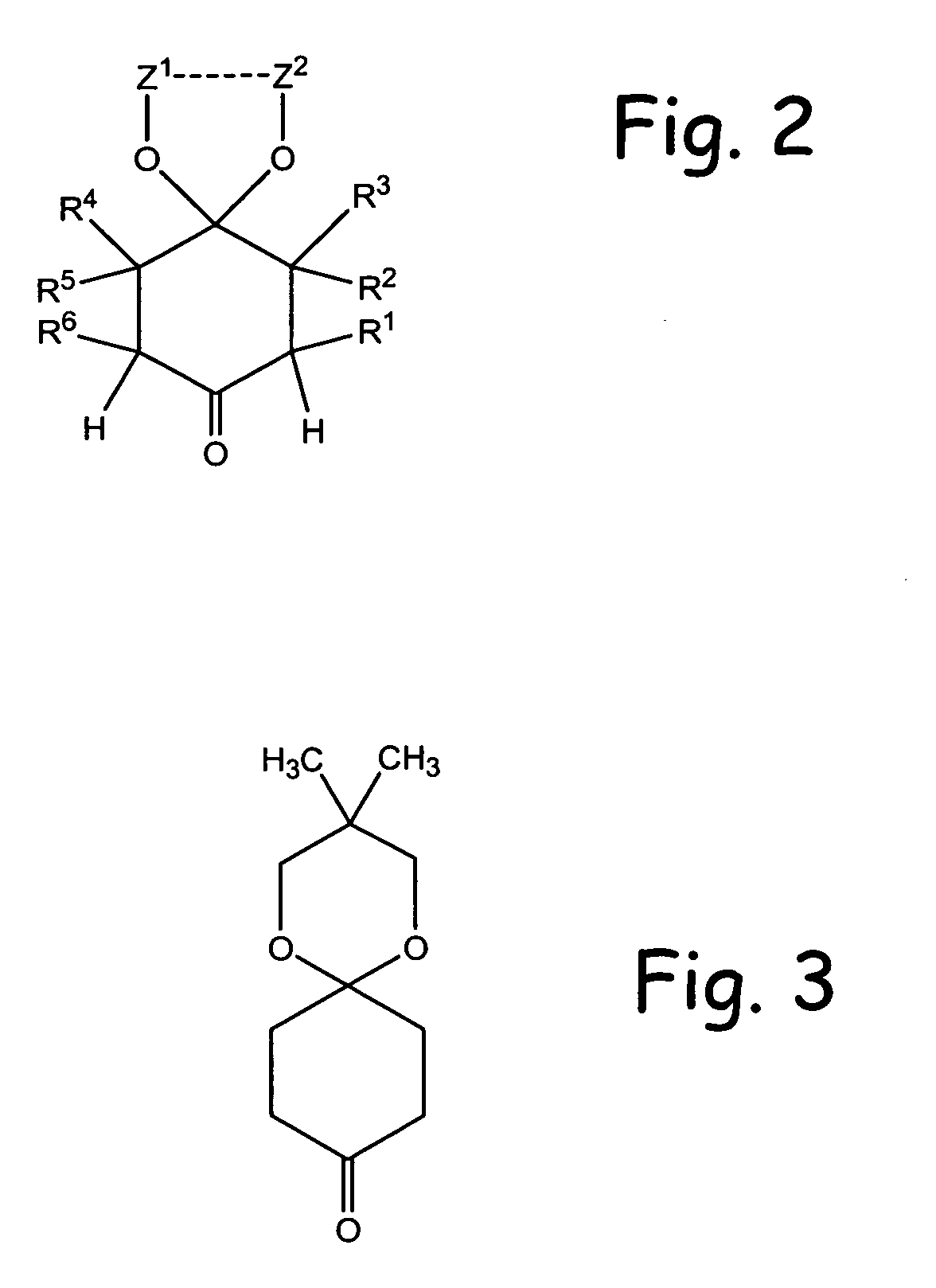
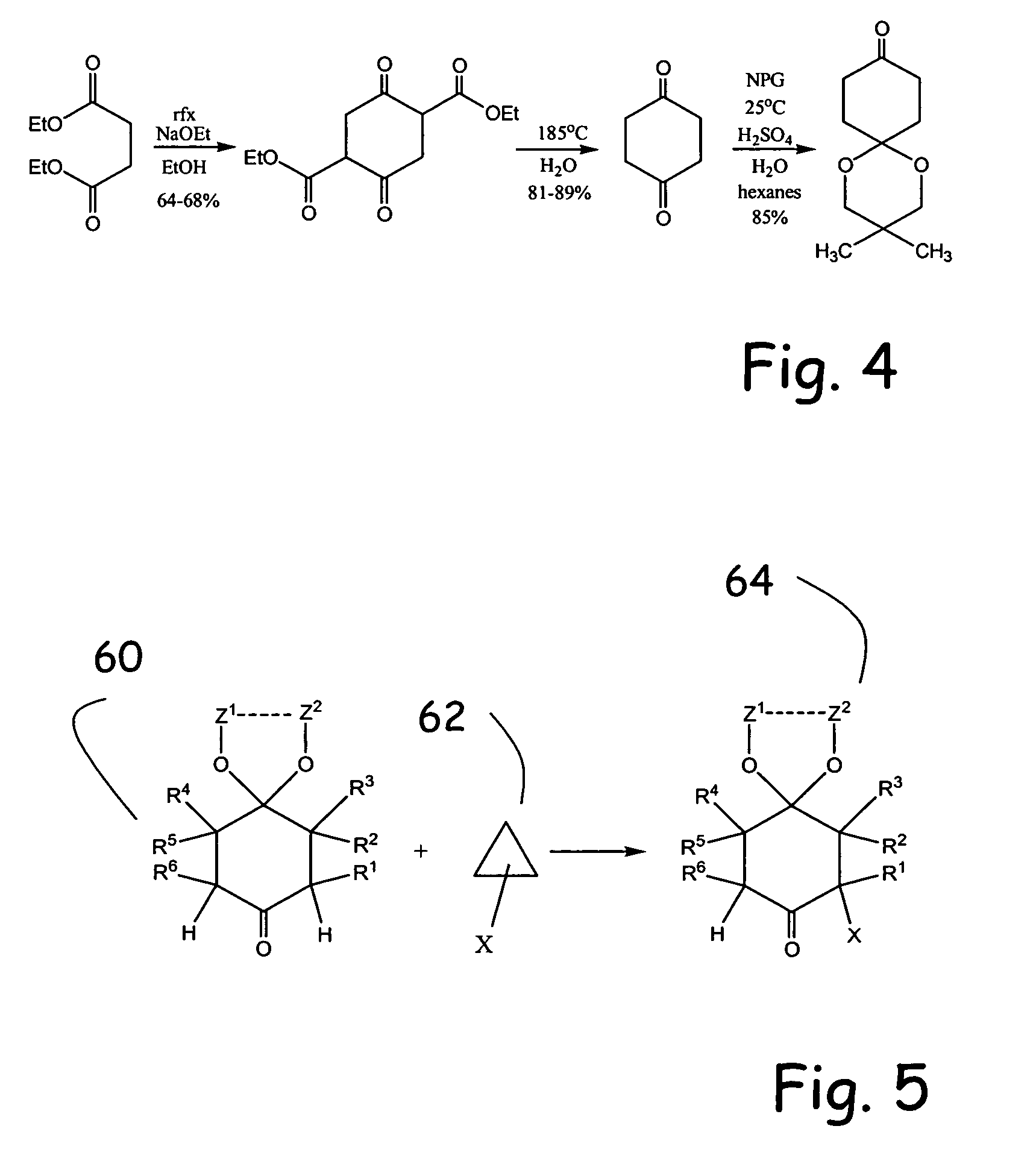
Methodologies for the alpha-monohalogenation of acid sensitive ketones, especially cyclic, acid-sensitive, ketalized ketones. As one approach, the ketone is reacted with a halogen donor compound, e.g., N-chlorosuccinimide, in anhydrous, highly polar organic reagents such as dimethylformamide (DMF). As another monohalogenation approach, it has been observed that organic salts generated from amines and carboxylic acids catalyze the monohalogenation of ketalized ketone in reagents comprising alcohol solvent (methanol, ethanol, isopropanol, etc.). The monohalogenation is fast even at −5° C. The salt can be rapidly formed in situ from ingredients including amines and / or carboxylic acids without undue degradation of the acid sensitive ketal. Aryl ketones are monooxygenated using iodosylbenzene. This methodology is applied to monohalogenation of an acid sensitive monoketal ketone. The ability to prepare monohalogenated, acid sensitive ketones facilitates syntheses using halogenated, acid sensitive ketones. As just one example, facile synthesis of halogenated, acid sensitive ketones provides a new approach to synthesize the S-ketal-acid S-MBA (S-methylbenzylamine) salt useful as an intermediate in the manufacture of a glucokinase activator. As an overview of this scheme, a monohalogenated, cyclic, ketalized ketone is prepared using monohalogenation methodologies of the present invention. The halogenated compound is then subjected to a Favorskii rearrangement under conditions to provide the racemic acid counterpart of the desired chiral salt. The desired chiral salt is readily recovered in enantiomerically pure form from the racemic mixture.
Owner:HARRINGTON PETER J +2
Method of semi-synthesizing artemisinin
InactiveCN103087074ARaw materials are easy to getShort processOrganic chemistryChemical reactionAlcohol
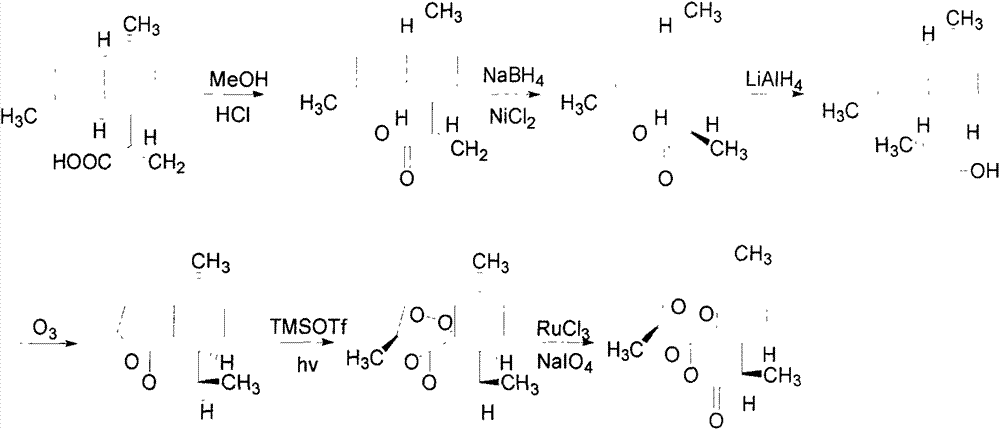
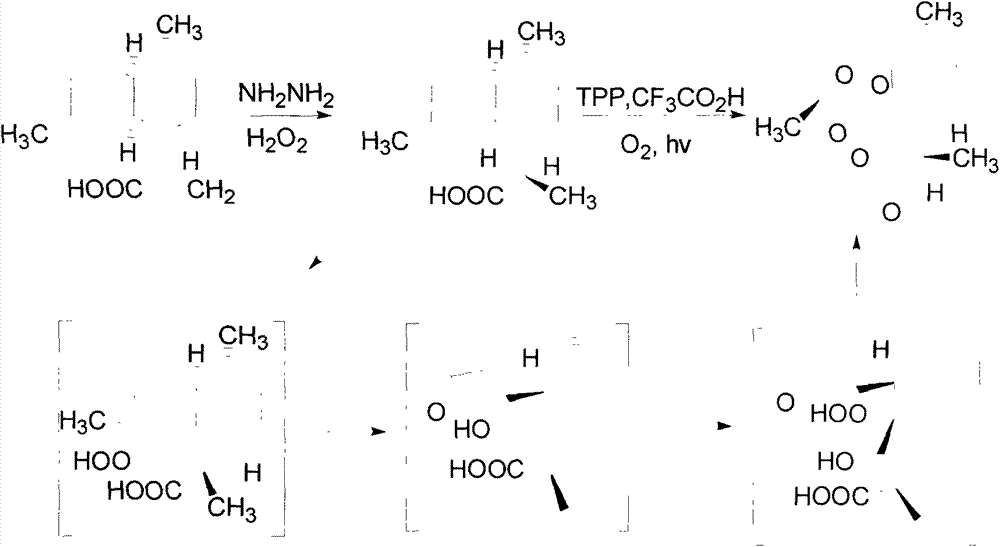
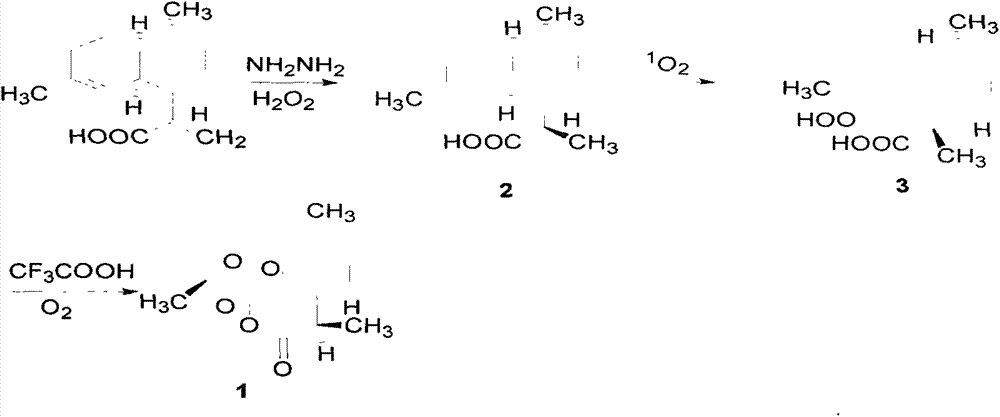
The invention relates to a method of semi-synthesizing artemisinin. The method comprises the steps of: reducing arteannuic acid into dihydro arteannuic acid by utilizing diimine produced in situ, oxidizing the dihydro arteannuic acid into peroxide alcohol by utilizing singlet oxygen produced in chemical reaction in situ, and then producing artemisinin under the action of oxygen and acid; or, obtaining dihydro arteannuic acid derivatives through protecting the dihydro arteannuic acid carboxyl, then oxidizing into corresponding peroxy alcohol under the action of the singlet oxygen produced in situ, and acting with the oxygen under the catalysis of acid to obtain artemisinin with high yield. A method of producing singlet oxygen in situ mainly comprises molybdate / peroxide, nitrile compound / peroxide, diacid iodobenzene / peroxide, peroxide-disulfate, and the like. With adoption of the method, the total yield can achieve up to 65%.
Owner:HUNAN KEYUAN BIO PRODS
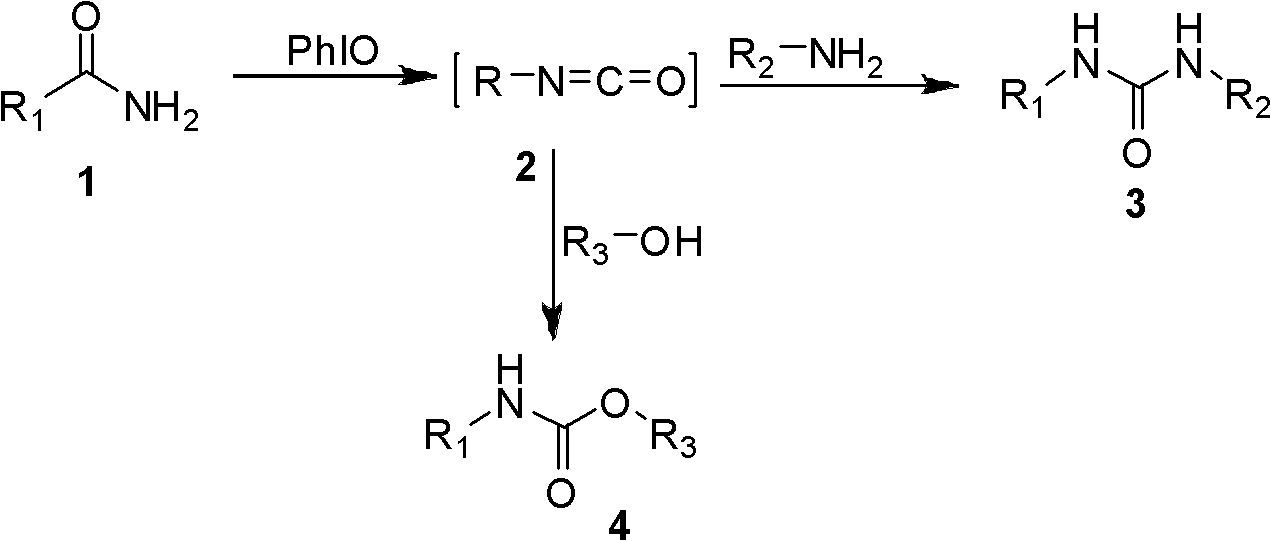
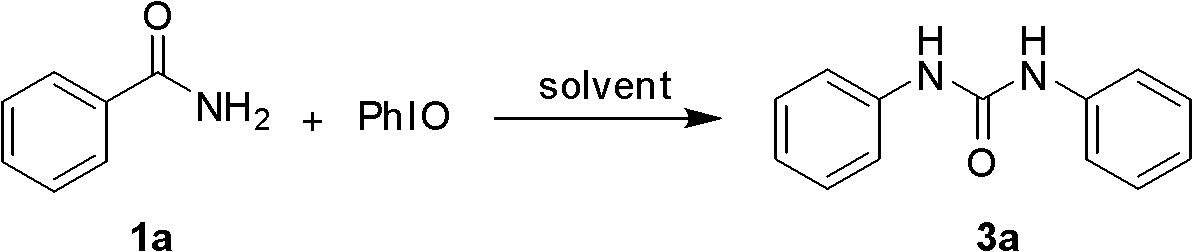
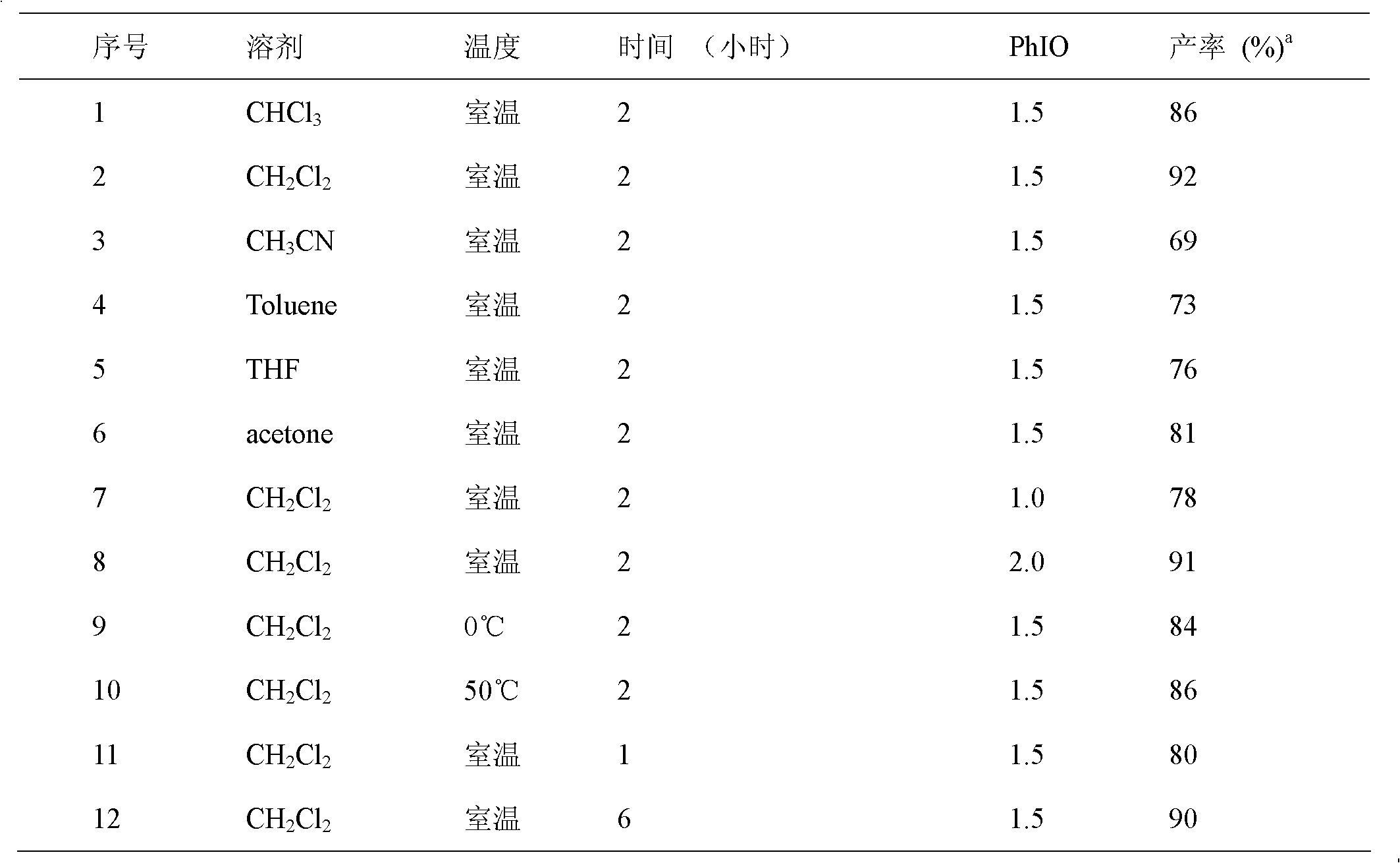
Synthetic method of 1, 3-two substituted ureas and carbamate
InactiveCN102503860AEasy to separateExperiment operation is simpleUrea derivatives preparationCarbamic acid derivatives preparationCarbamateAlcohol
The invention relates to a simple and efficient preparation method of 1, 3-two substituted ureas and carbamate. The method comprises the steps as follows: amide is taken as substrate, and the 1, 3-two substituted ureas and carbamate are prepared by using Hofmann rearrangement reaction induced by hypervalent iodine. Only acid amide and oxidization iodobenzene are added into the reaction system, stirring is performed for 2 hours in methylene dichloride at a room temperature, and symmetrical 1, 3-two substituted ureas can be obtained with high yield. When acid amide, oxidization iodobenzene and nucleophilic agent amine are in reaction, and stirring is performed for 2 hours in methylene dichloride at a room temperature, unsymmetrical 1, 3- two substituted ureas can be obtained. When acid amide, oxidization iodobenzene and nucleophilic agent alcohol are in reaction, and stirring is performed for 2 hours in alcoholic solution at a room temperature, carbamate can be prepared. The method is simple to operate, the product selectivity is strong, and the application is wide.
Owner:WUHAN UNIV
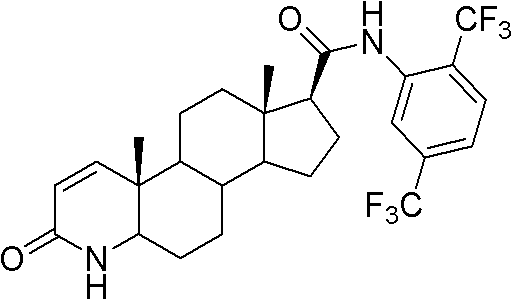
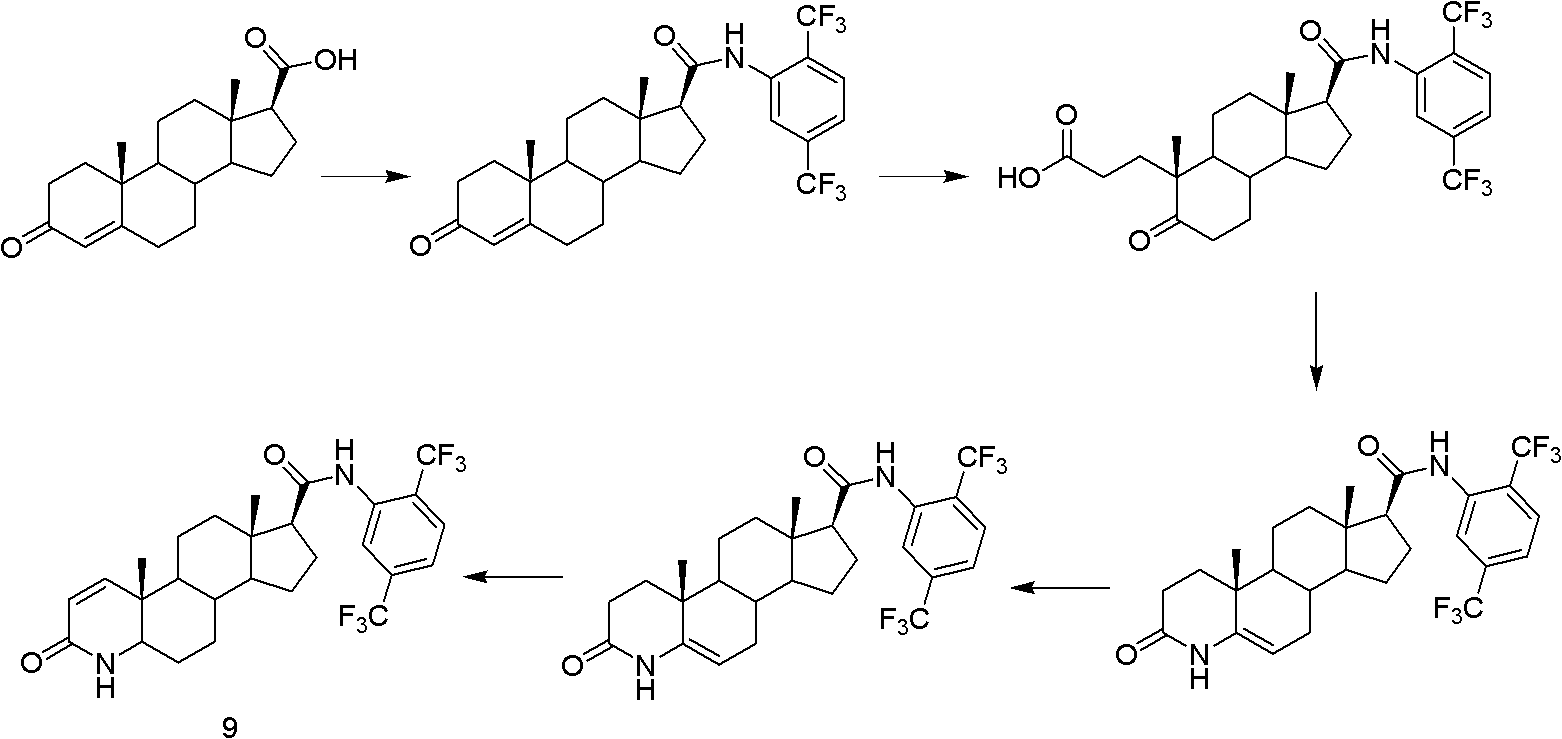
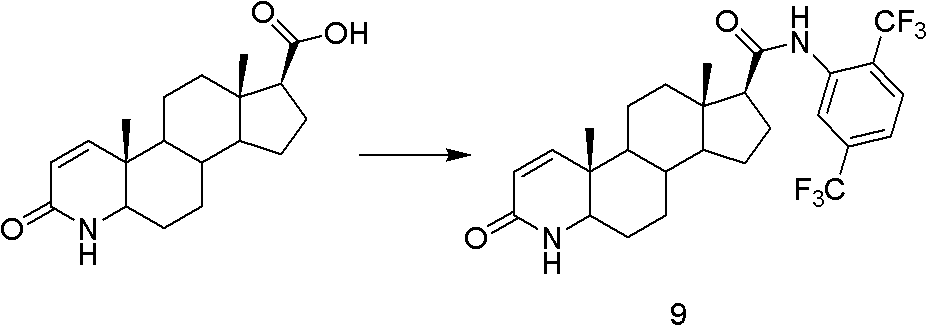
Method for preparing dutasteride
The invention discloses a method for preparing dutasteride, belonging to the field of chemical synthesis of drugs. In the invention, with androstendione as a raw material, the dutasteride is prepared by eight reaction steps of ring opening, ring closing, Grignard reaction, dehydration, reduction, oxidation, dehydrogenation and condensation. In the method disclosed by the invention, double bonds at sites 5 and 6 and double bonds at sites 16 and 27 are simultaneously hydrogenated and reduced; and amidation of a site 17 and condensation of a site 20 are continuously carried out so that the steps are saved; potassium cyanide under an acidic condition is avoided and the process safety is improved; and the expensive 4-di(trifluoromethyl)-2-iodobenzene is replaced with cheap 2,5-di(trifluoromethyl) aniline so that the cost is largely reduced.
Owner:湖南尔文水电建材有限公司
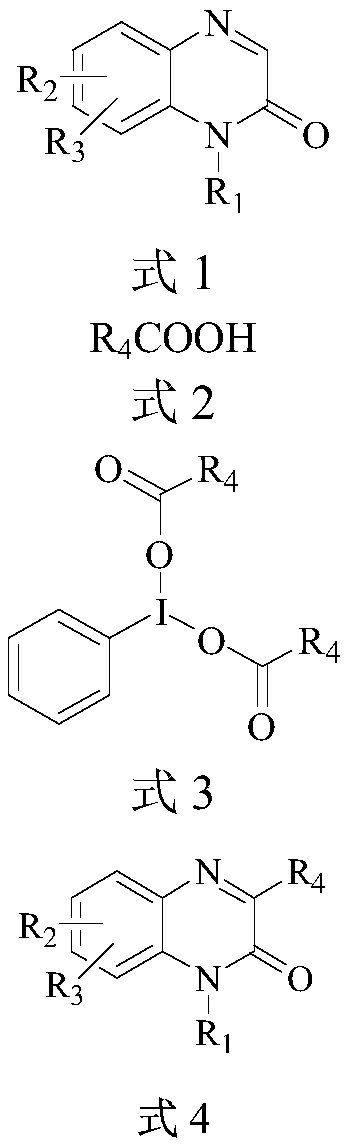
Preparation method of 3-alkyl quinoxaline-2(1H)-ketone compound
InactiveCN110117260AAvoid pollutionHigh reaction efficiencyOrganic chemistryQuinoxalineSynthesis methods
The invention discloses a preparation method of a 3-alkyl quinoxaline-2(1H)-ketone compound. According to the method, aliphatic carboxylic acid reacts with iodobenzene diacetate to obtain dialiphaticcarboxylic acid iodobenzene, and the dialiphatic carboxylic acid iodobenzene and a quinoxaline ketone compound are subjected to a visible-light catalytic reaction under the action of a photocatalyst to obtain the 3-alkyl quinoxaline ketone compound. Compared with the prior art, the synthesis method has the advantages that the raw materials such as a quinoxaline-2(1H)-ketone derivative and fatty acid in use are cheap and easy to obtain, so that the cost is reduced; lighting can be carried out at room temperature, conditions are mild, the product is obtained in one step, the reaction yield is high, the operation is environmentally friendly, and industrial production is facilitated; the method is good in functional group applicability and can obtain various 3-alkyl quinoxaline-ketone compoundderivatives, and a brand-new synthetic path is provided for the 3-alkyl quinoxaline-2(1H)-ketone compound.
Owner:HUNAN UNIV OF SCI & ENG
Method for synthesizing radioactive ligand having 18f-labeled fluorobenzene ring
InactiveUS20090069592A1High yieldShort timeBiological testingIsotope introduction to acyclic/carbocyclic compoundsTosylic acidFluorobenzene
A phenyl tin compound is synthesized by using a derivative having various functional groups and a bromo- or iodo-benzene ring as a labeling material of a radioactive ligand. On the other hand, a novel hydroxytosyl iodobenzene compound having an electron-donating group is obtained by oxidizing iodobenzene having one or more electron-donating groups and reacting it with tosylic acid. Then, a diphenyliodonium salt which is a labeling precursor is synthesized by reacting the resulting compound with various phenyl tin compounds. Finally, a 18F-labeled ligand having various functional groups and a [18F] fluorobenzene ring is synthesized by reacting the resulting diphenyliodonium salt with [18F]F−.
Owner:NAT INST FOR QUANTUM & RADIOLOGICAL SCI & TECH
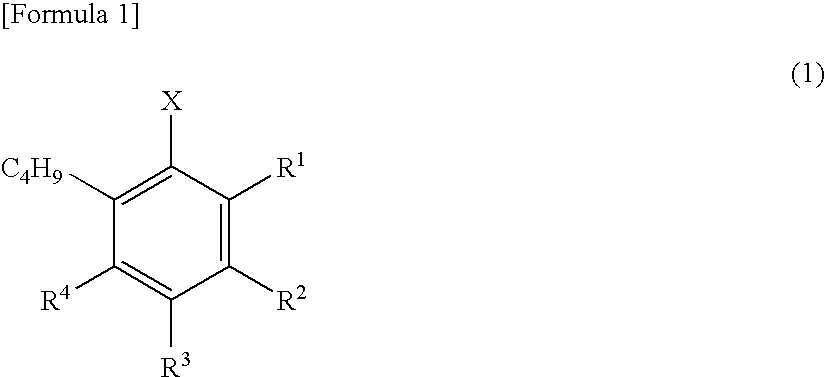
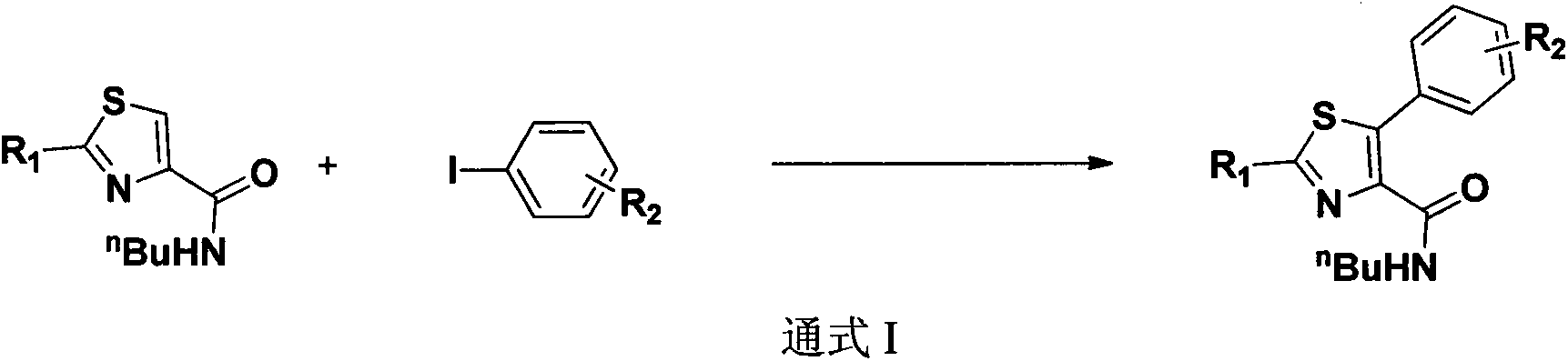
Preparation method of N-butyl-5-phenylthiazole-4-formamide derivative
The invention relates to the field of organic chemistry and in particular relates to a preparation method of an N-butyl-5-phenylthiazole-4-formamide derivative. The N-butyl-5-phenylthiazole-4-formamide derivative is prepared by using thiazole-4-formamide derivative and substituted iodobenzene as a substrate, copper iodide as a catalyst, sodium hydroxide as alkaline and dioxane as a solvent in an oil bath at 120 DEG C. The synthetic method has the advantages of high yield, easily available raw materials, small usage amount of the copper catalyst, no use of expensive organic alkali reagent, simplicity in reaction equipment, high atom economy and the like.
Owner:CHINA PHARM UNIV
Synthesis method of N-phenyl-3(4-bromophenyl) carbazole
The invention discloses a synthesis method of N-phenyl-3(4-bromophenyl) carbazole. The synthesis method is characterized in that carbazole is selected as a raw material and is subjected to Ullmann reaction with iodobenzene to prepare N-phenyl carbazole; N-phenyl-3-bromine carbazole is synthesized by NBS (N-bromosuccinimide) bromination; N-phenyl-3-boric acid base carbazole is prepared through Grignard coupling; and N-phenyl-3-boric acid base carbazole and p-bromoiodobenzene are subjected to cross coupling to obtain N-phenyl-3(4-bromophenyl) carbazole. The method provided by the invention has the characteristics of high yield, low production cost, less three-waste emission and high target compound selectivity and yield, a high-efficiency loaded catalyst is used, and isomer is reduced.
Owner:山东盛华电子新材料有限公司
Method for measuring activity of superoxide dismutase
ActiveCN103674870AGood reproducibilityImprove accuracyColor/spectral properties measurementsTetrazoleOxyanion
The invention provides a method for measuring activity of superoxide dismutase. The technical problems of detection difficulty, low accuracy and low reproducibility existing in a method for measuring the activity of superoxide dismutase in the prior art are solved. The method comprises the following steps: selecting a pyrogallic acid alkaline auto-oxidation system, evaluating the removal effect of the superoxide dismutase on superoxide anion by combining an ultraviolet-visible light spectrophotometric method, detecting change of absorbance of a product water-soluble formazan of a trapping agent of sodium 2-(4-iodobenzene)-3-(4-nitrobenzene)-5-(2',4-bisulfophenyl)-2H-tetrazole after acting with the superoxide anion, calculating the removal rate of the superoxide dismutase on the superoxide anion, and further obtaining the activity of superoxide dismutase. The method has the advantages of high reproducibility, high accuracy and simplicity and convenience in operation.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
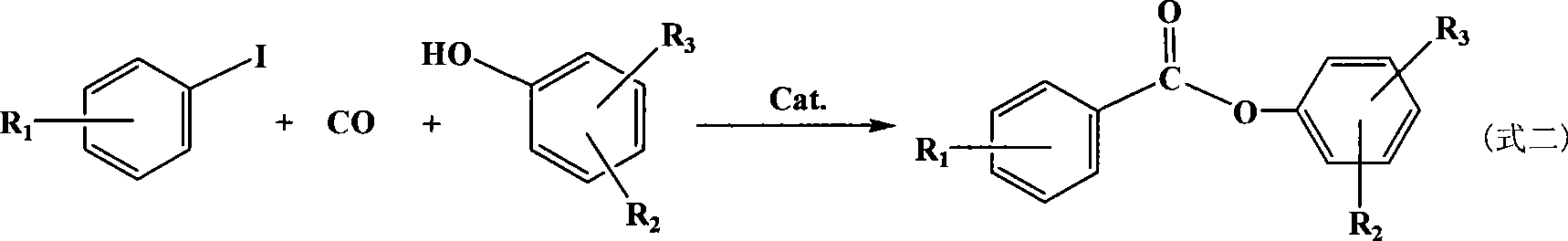
Method for synthesizing aromatic carboxylic ether by iodo aromatic hydrocarbon acarbonylation
InactiveCN101456815ARaw materials are easy to getMild reaction conditionsPreparation by carbon monoxide or formate reactionIodideSolvent
The invention discloses a method for synthesizing aromatic carboxylic ester by carbonylation of aryl iodide. The method comprises: iodobenzene and derivatives of the iodobenzene, straight chain alcohol of C1 to C4 or phenolic compound and carbon monoxide are used as reactants to react in the presence of active carbon loaded palladium as a catalyst, an auxiliary catalyst and reaction solvent at 80 to 140 DEG C under a pressure of the carbon monoxide of between 0.1 and 2.5MPa for 0.5 to 8 hours, and obtaining the finished product. The method is highly efficient, economical and environment friendly, and has quite good industrial application prospect.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
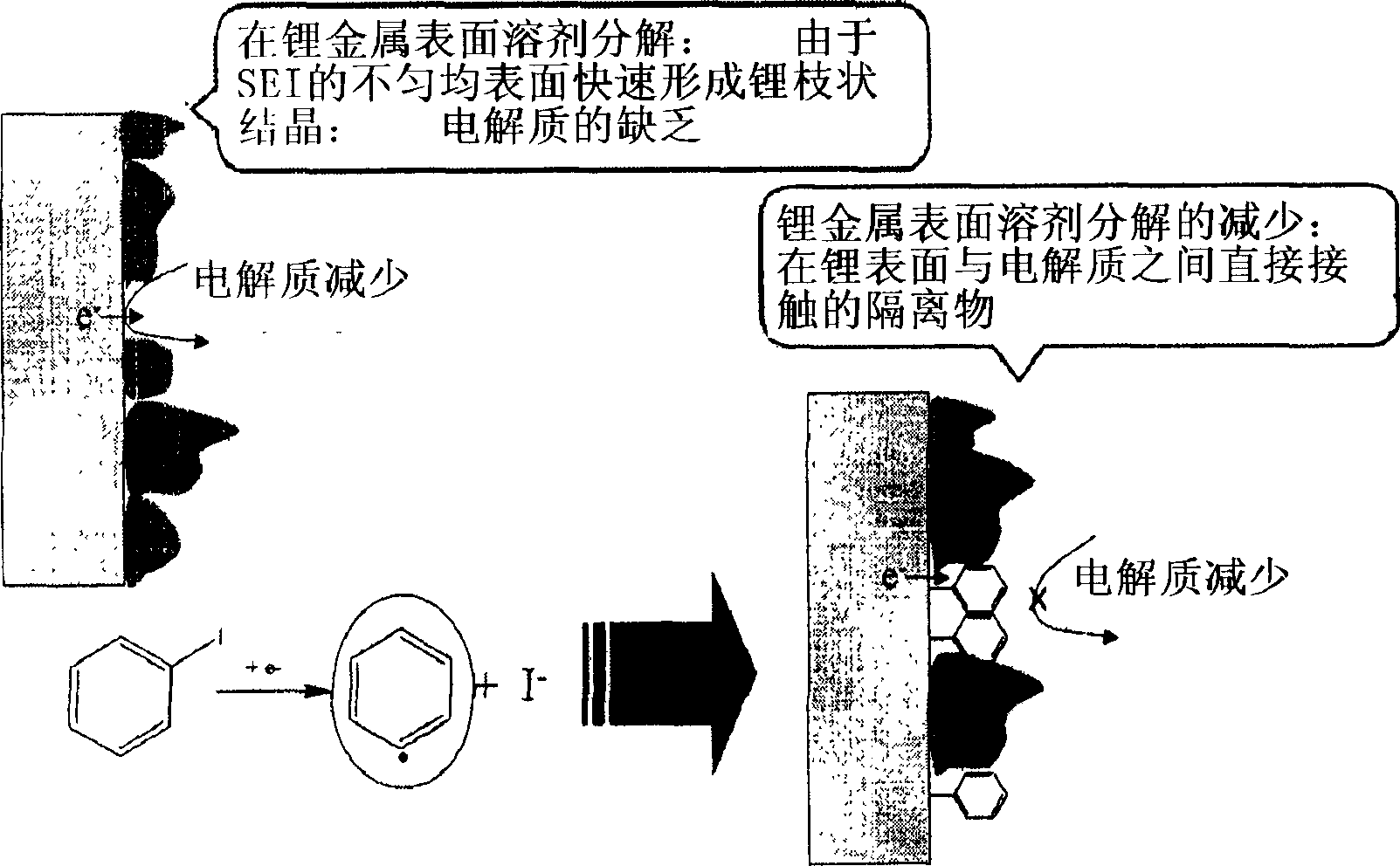
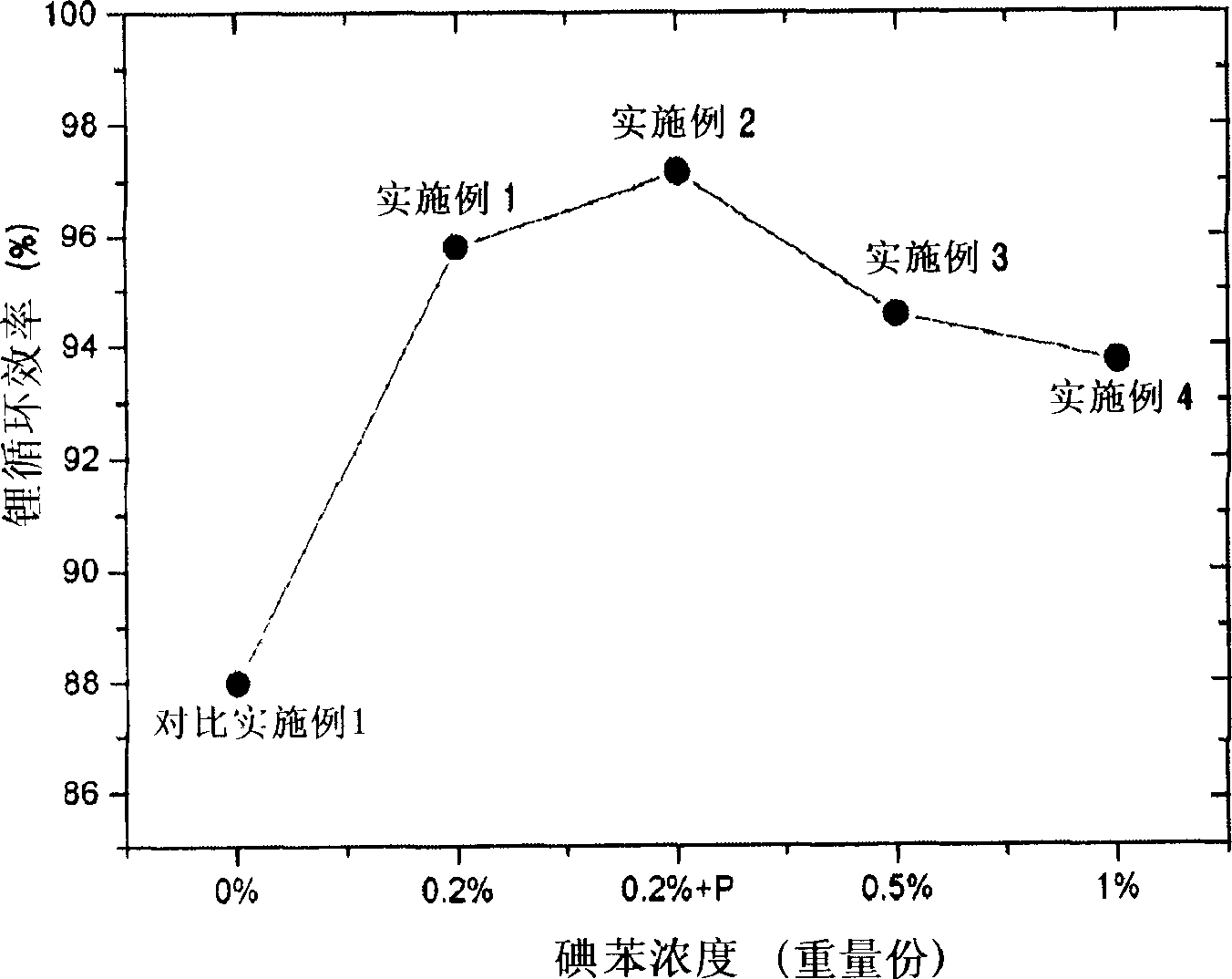
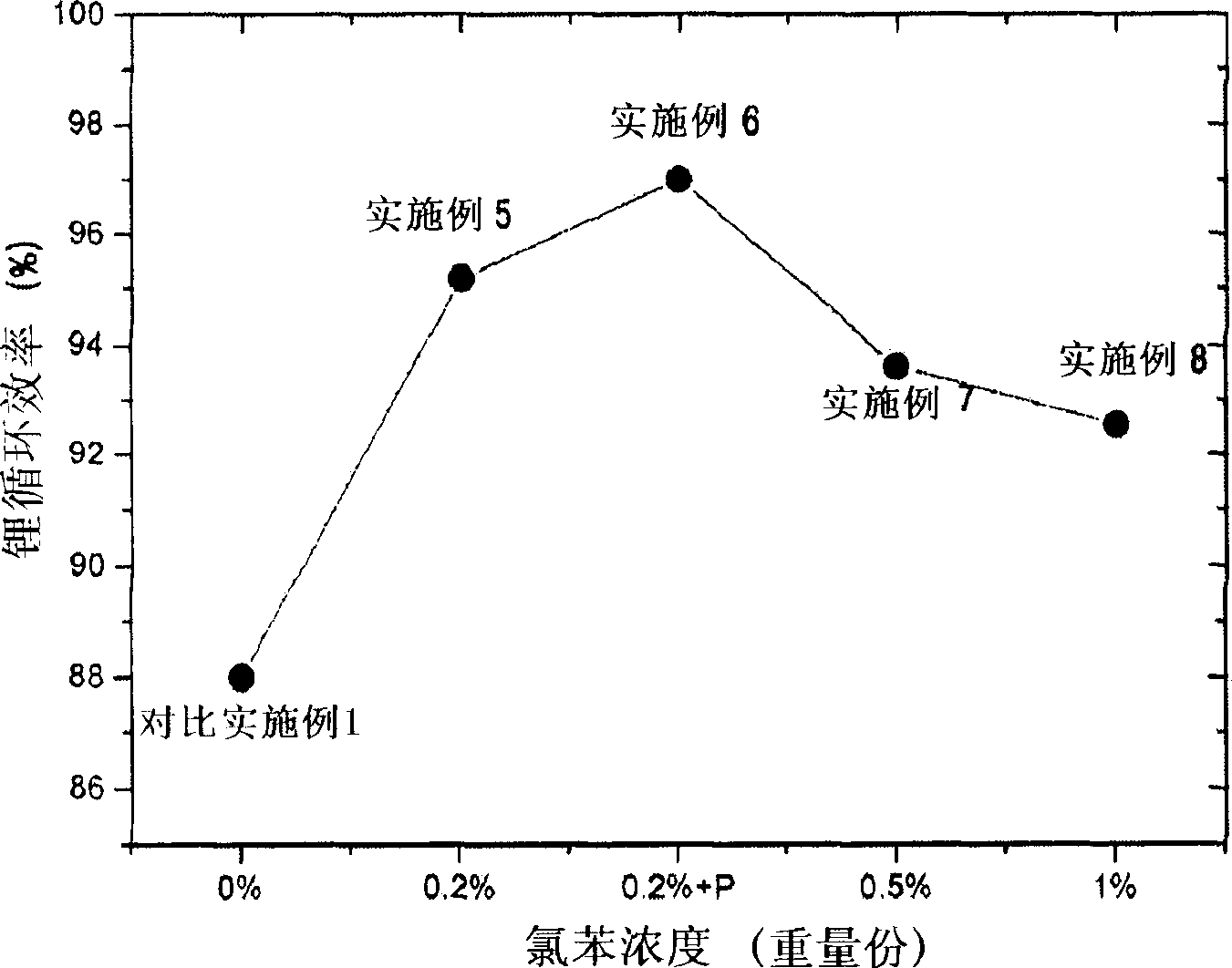
Organic electrolytic solution and lithium battery using the same
The present invention is related to an organic electrolytic solution comprising a halogenated benzene compound, such as 1-iodobenzene or 1-chlorobenzene. Specifically, the halogenated benzene compound has a high polarity and is capable of reducing the reactivity of the lithium metal surface. Due to these characteristics of the halogenated benzene compound, the lithium ions are unlikely to bond with the sulfide anions. Therefore, the solubility of the sulfide within the electrolyte is increased, thereby improving the charge / discharge efficiency characteristics of the lithium ions and the lifespan of batteries. Moreover, the organic electrolytic solution of the present invention may be used in any battery type where an anode is composed of lithium metal, and in particular, lithium sulfur batteries.
Owner:SAMSUNG SDI CO LTD
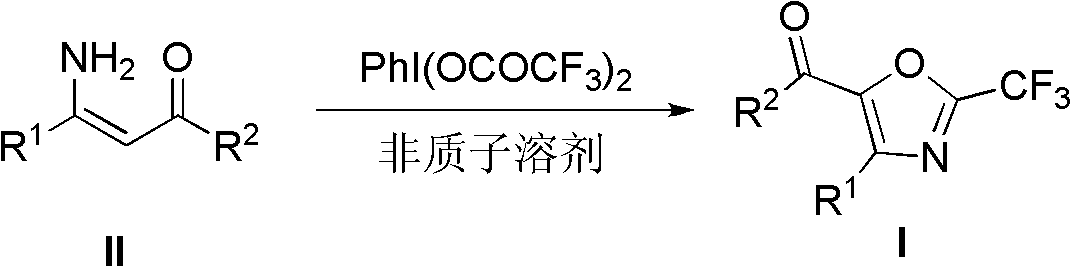
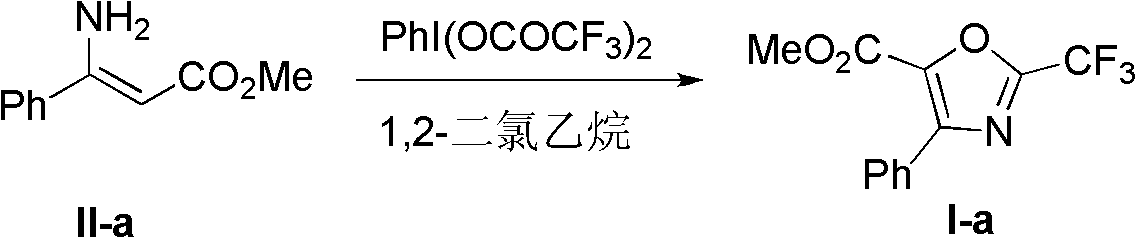
Synthesis method of 2-trifluoromethyloxazole derivatives
The invention discloses a synthesis method of 2-trifluoromethyloxazole derivatives. The method is as follows: dissolving enamine compounds of which the beta-positions are not replaced in an anhydrous aprotic solvent; and oxidizing the 2-trifluoroacetyl in the presence of bis(trifluoroacetoxy)iodobenzene to further perform intramolecular condensation with amino, thus obtaining the 2-trifluoromethyloxazole derivatives. In the method, simple and available enamine compounds of which the beta-positions are not replaced are utilized as substrates, and a nontoxic and environmentally-friendly solid reagent, namely bis(trifluoroacetoxy)iodobenzene is utilized as a fluorinating agent; and the method has the advantages that the operations are simple, the conditions are mild, and the synthetized substituted 2-trifluoromethyloxazole derivatives are difficult to prepare by virtue of other methods, and the like.
Owner:TIANJIN UNIV
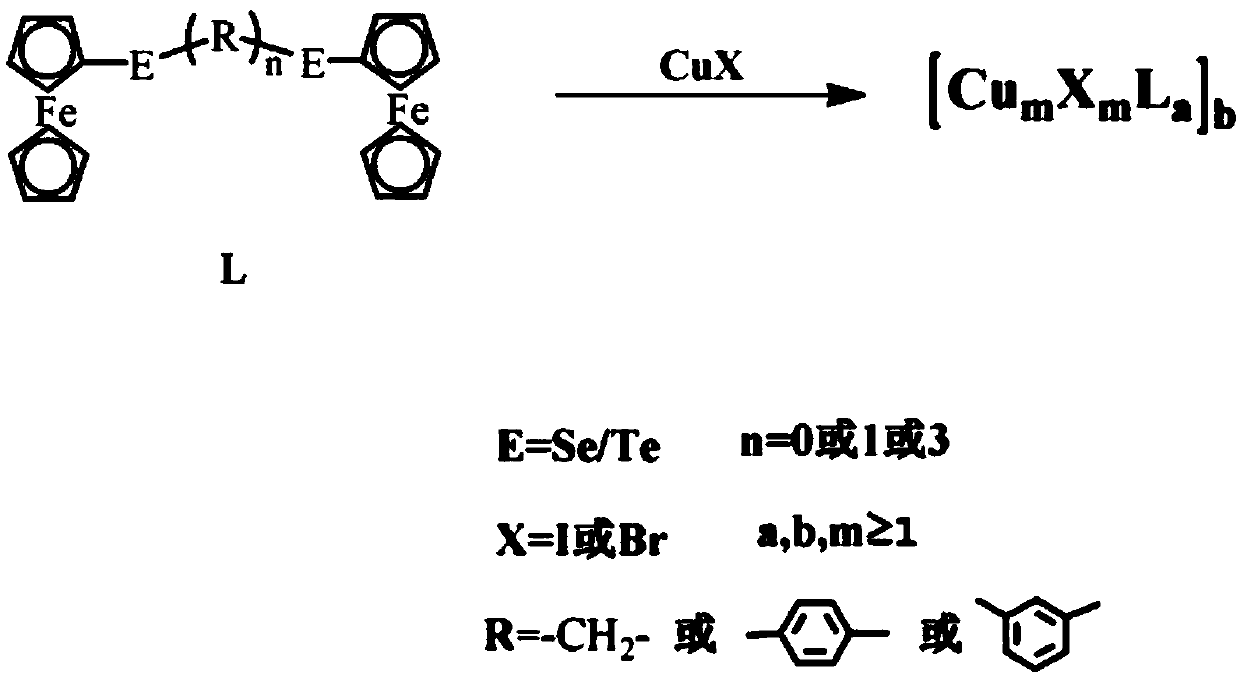
Ferrocene cuprous cluster catalyst capable of catalyzing C-N coupled reaction and preparation method thereof
InactiveCN107803223AWide variety of sourcesLow priceOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsFerroceneSolvent
The invention discloses a ferrocene cuprous cluster catalyst capable of catalyzing a C-N coupled reaction and a preparation method thereof. According to the catalyst, on the basis of cuprous cluster complex of selenium / telluride ligands of ferrocene, a coupled reaction of iodobenzene and imidazole under an alkaline condition serves as a template reaction, and the yield of a series of cuprous cluster compounds serving as the catalyst is characterized by virtue of gas chromatography. The invention further discloses a preparation method of the catalyst. The series of catalysts have the yield of 99% at a high temperature within six hours, and due to the absence of a solvent, the application of the series of catalysts in production is increased. Meanwhile, the catalyst is environmental-friendly, and pollution of the solvent to the environment is reduced. Moreover, the series of cluster compounds have high auto-stability and can be stored in air for a long time.
Owner:NANJING UNIV OF TECH
Polyfluoric terphenyl liquid crystal compound and synthesis method and use thereof
InactiveCN101768447ALow viscosityLow Optical Dielectric AnisotropyLiquid crystal compositionsOrganic chemistryCrystallographyDielectric anisotropy
The present invention discloses a polyfluoric terphenyl liquid crystal compound. The structural formula of the compound is as follows: R1 is C1-C8 alkyl, R2 is C1-C8 alkyl, fluoro, trifluoromethyl, trifluoromethoxy; U,V, W, X, Y and Z are fluorine substituent or hydrogen, at least one thereof is fluorine substituent. The synthetic process comprises the steps of preparing an intermediate 4-alkyl iodobenzene containing fluorine, preparing an intermediate phenyloboric acid containing fluorine, preparation an intermediate 4-alkyl biphenyloboric acid containing fluorine, and synthesizing target products of the polyfluoric terphenyl liquid crystal compound. The compound has the features of great dielectric anisotropy (delta e), excellent optical, heat, electrical and chemical stability, can be used for improving properties of the liquid crystal mixture, can be widely applied in TN, HTN, STN, TFT mixed liquid crystal materials, and further can be applied in a liquid crystal display.
Owner:SHIJIAZHUANG CHENGZHI YONGHUA DISPLAY MATERIALS CO LTD
Nano periodic mesoporous organic silicon oxide material and its synthetic method and its use
InactiveCN1943856AReduced solubility limitationsNo longer producedCatalyst carriersHydrocarbon from halogen organic compoundsOrganic reactionSilicon oxide
The present invention discloses one kind of nanometer periodical mesoporous organic silicon oxide material and its synthesis process and application as carrier for supporting Pd catalyst used in water phase iodobenzene coupling reaction. The material has pore size of 1.8-3.9 nm, pore volume of 0.3-1 cu cm / g and specific surface area of 800-1000 sq m / g. When the supported catalyst is used in the water phase iodobenzene coupling reaction, the supported catalyst has catalytic activity higher than that of Ph-MCM-41 supported catalyst prepared with no-phenyl radical modification MCM-41 and through copolymerization. The material is used in high efficiency heterogeneous catalyst, and has simple preparation process and no toxic and harmful matter.
Owner:SHANGHAI NORMAL UNIVERSITY
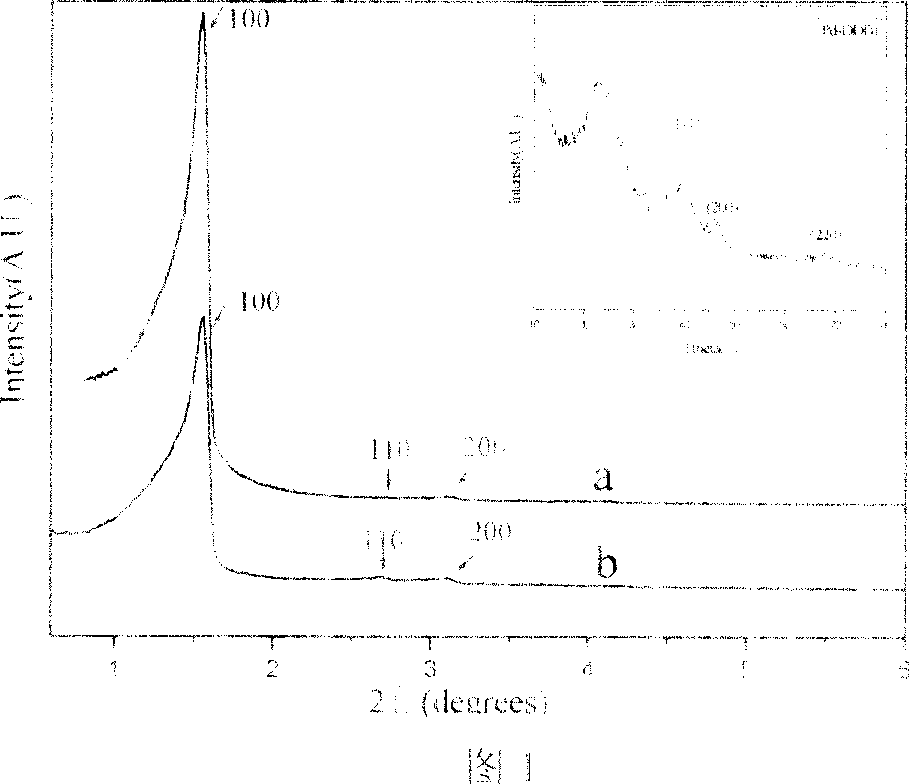
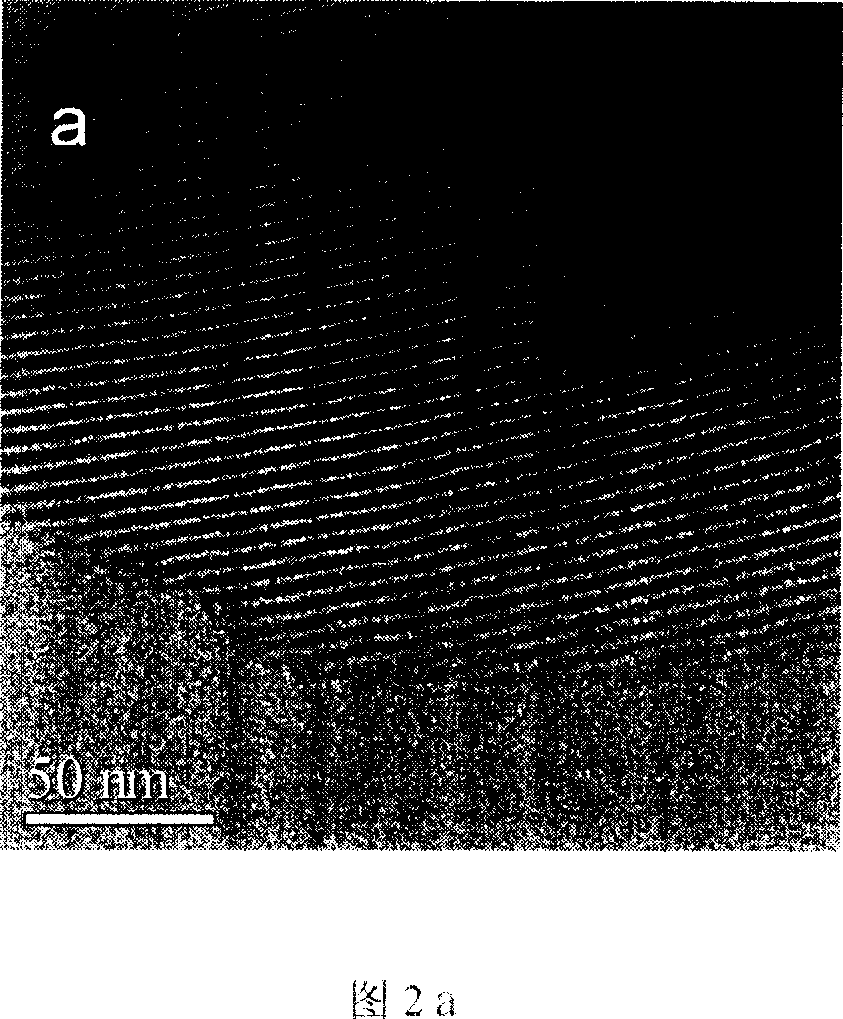
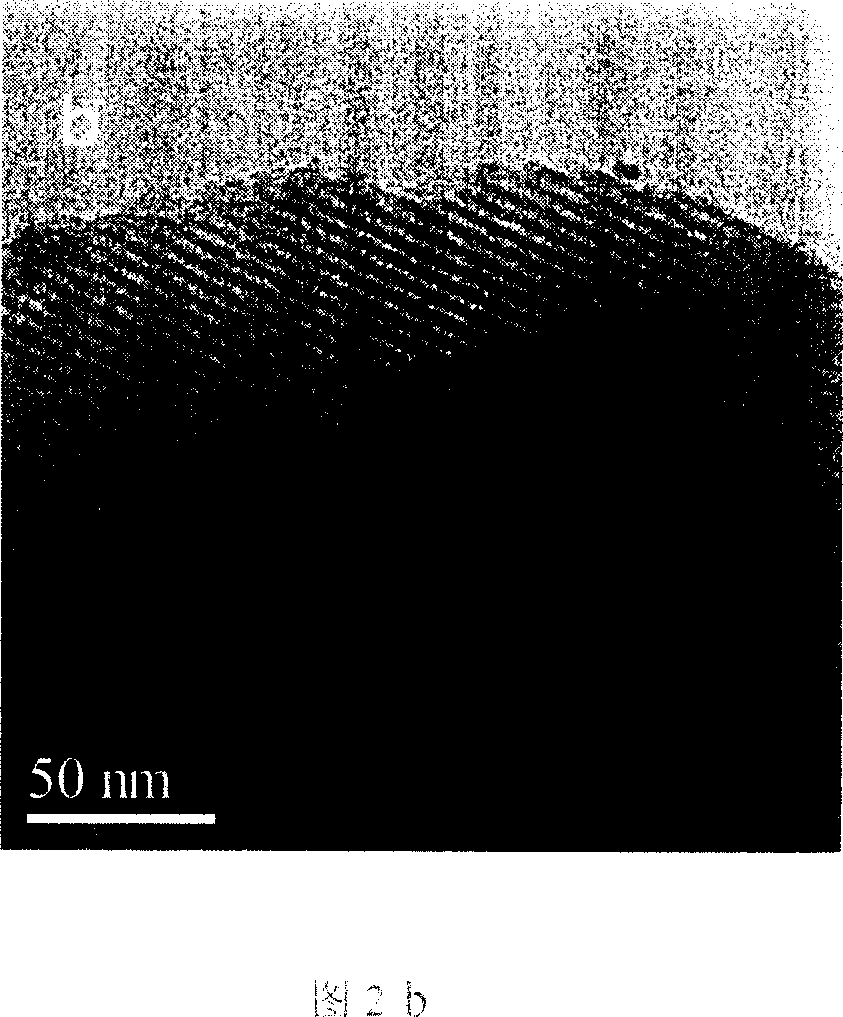
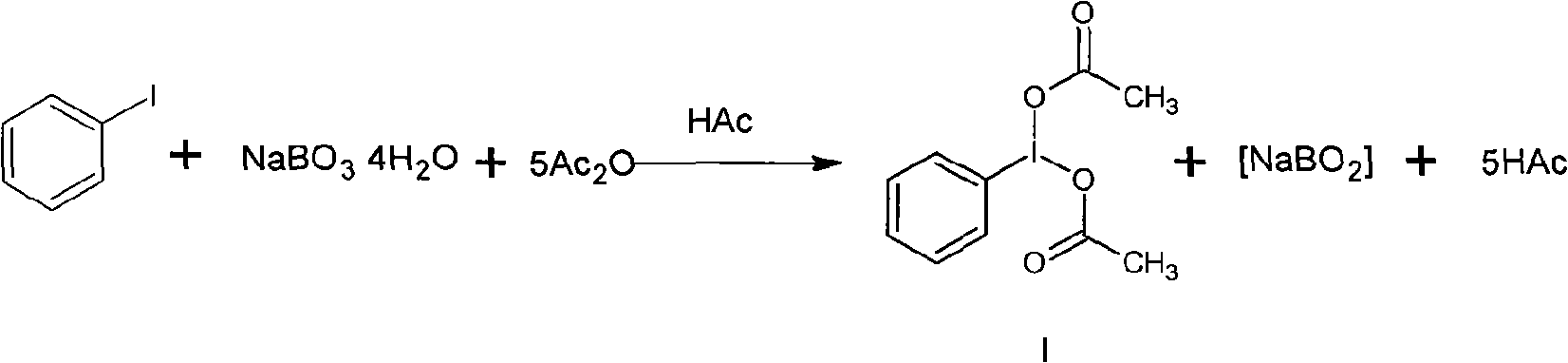
Method for preparing iodobenzene diacetate
The invention relates to a method for preparing iodobenzene diacetate, which comprises the following steps: using tetrahydrate sodium perborate as a raw material to perform acylation reaction with iodobenzene in the presence of a glacial acetic acid / acetic anhydride mixed solution, wherein the molar ratio of the tetrahydrate sodium perborate to the iodobenzene is 3-10:1, the reaction temperature is between 30 and 45 DEG C, and the reaction time is between 4 and 24 hours; after the reaction, adding ice water to obtain a crude product of the iodobenzene diacetate; and performing recrystallization to obtain the iodobenzene diacetate. Compared with the prior art, the method has the advantages of reasonable process, simple operation and safe production, is suitable for industrial mass production, and provides a favorable condition for producing the iodobenzene diacetate industrially.
Owner:上海予利生物科技股份有限公司
Method for synthesizing nano palladium particles with superactivity
ActiveCN102205252ANot easy to reuniteGood monodispersityOrganic-compounds/hydrides/coordination-complexes catalystsSolubilityLiquid temperature
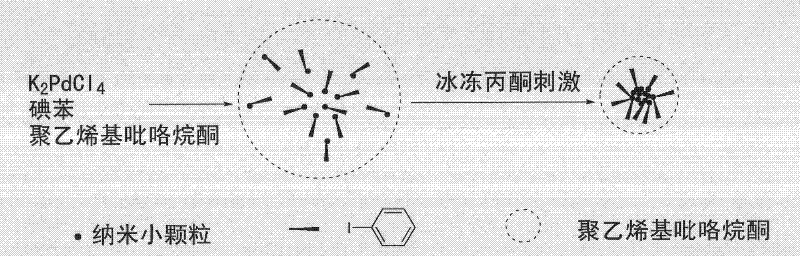
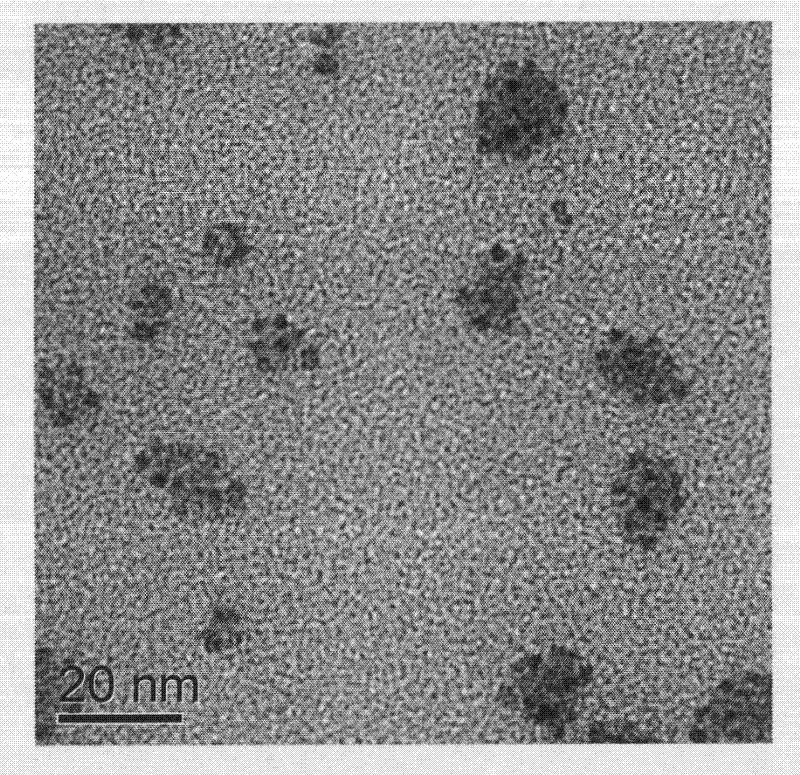
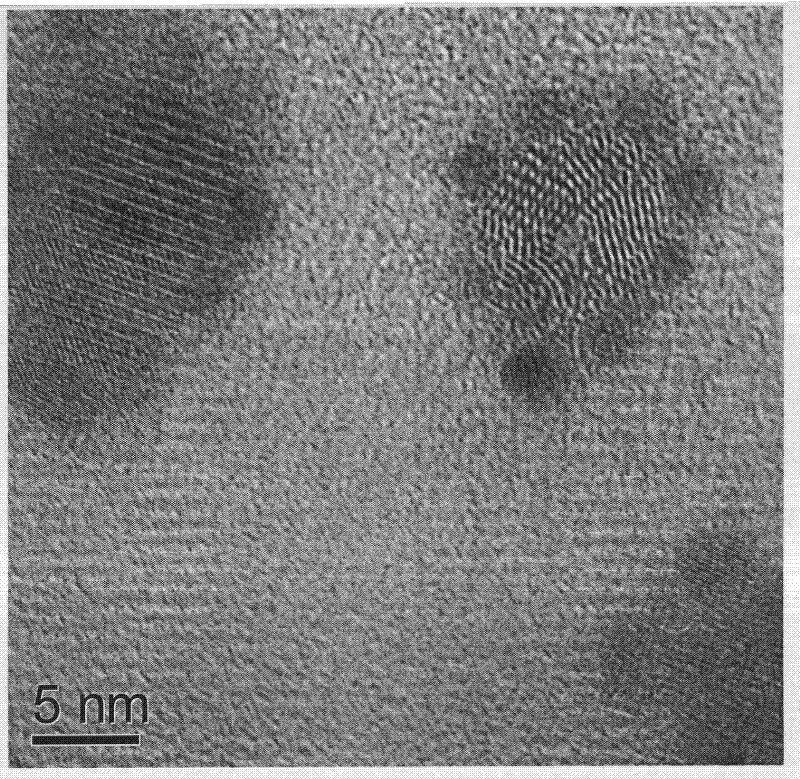
The invention provides a method for synthesizing nano palladium particles with superactivity and widens a method for preparing relevant nano particles. By creatively using iodobenzene as a surface modifying agent and adopting the characteristics that metal nano particles can be effectively absorbed and an effective steric hindrance effect can be provided by using phenyl of the iodobenzene, more stable nano palladium particles with high-content defects at the inner part and the surface are formed under the combined action of sudden reduction of reaction liquid temperature and polyvinyl pyrrolidone solubility caused by freezing acetone. The method has the characteristics of simple process, convenience for operation, controllable feature, wide application range and the like, and has a potential application value.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Method for synthesizing 2-substituted benzothiazole derivative
The invention discloses a method for synthesizing a 2-substituted benzothiazole derivative. A copper compound is used to catalyze substituted o-nitro-iodobenzene and aryl formaldehyde or aromatic heterocyclic formaldehyde by a copper compound in air or sealed environment, and the reaction is carried out in one reactor under the existence of sodium sulfide to generate the 2-substituted benzothiazole derivative. The copper catalyst adopted by the method has low cost and is easy to obtain and recover. In addition, the method can realize the synthesis of a series of 2-substituted benzothiazole and derivatives at high yield in the mild condition and is beneficial to industrial production.
Owner:HUNAN UNIV
Glycosylated serum protein detection reagent and application thereof
ActiveCN105223192AImprove anti-interference abilityHigh measurement sensitivityMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsTetrazoleDioxyethylene Ether
Belonging to the technical field of medical examination, the invention in particular relates to a glycosylated serum protein detection reagent and application thereof. The reagent comprises a reagent R1 and a reagent R2 in a volume ratio of 3:1, the reagent R1 is mainly composed of a potassium phosphate buffer solution, uricase, ascorbic acid oxidase, alkanolamide polyoxyethylene ether, polyol, calcium chloride and a preservative, and the reagent R2 is mainly composed of an MES buffer solution, alkyl polyglucoside, 2-(4-iodobenzene)-3-(2, 4-dinitrobenzene1)-5-(2, 4-di-sulfobenzene1)-2H-tetrazole and a preservative. The glycosylated serum protein detection reagent provided by the invention has the advantages of stronger anti-interference ability, high determination sensitivity and precision, and good repeatability of determination result, also the detection reagent has high stability and good water solubility, and is conducive to clinical application of the glycosylated serum protein detection reagent.
Owner:郑州金域临床检验中心有限公司
Method for determining content of binding-state glutamine in protein or protein hydrolysate
The invention discloses a method for determining the content of binding-state glutamine in protein or protein hydrolysate, belonging to the analysis technology field. In the conventional amino acid analysis, glutamine in protein or peptide molecule is converted into glutamic acid after being hydrolyzed by hydrochloric acid, only the total content of glutamic acid is displayed in an analysis result, but the content of the glutamine in the protein cannot be displayed. Based on the characteristic that binding-state glutamine in protein or peptide molecule cannot be hydrolyzed by hydrochloric acid after reaction with a special reagent (bis(trifluoacetoxy) iodobenzene) (BTI), the method is characterized in that the content of glutamic acid is determined through acid hydrolysis under the protection of the BTI reagent, and finally the content of the binding-state glutamine in protein or peptide molecule can be worked out according to the difference between the content of glutamic acid in the sample under BTI protection and the content of glutamic acid in a sample without BTI protection. The method comprises the steps of preparation of a sample to be tested, BTI protection treatment, acidolysis, derivation, amino acid chromatographic column separation, control sample preparation, and content determination. The method is simple and rapid, has high sensitivity and can timely guide the proteolysis industrial production.
Owner:郑州新威营养技术有限公司
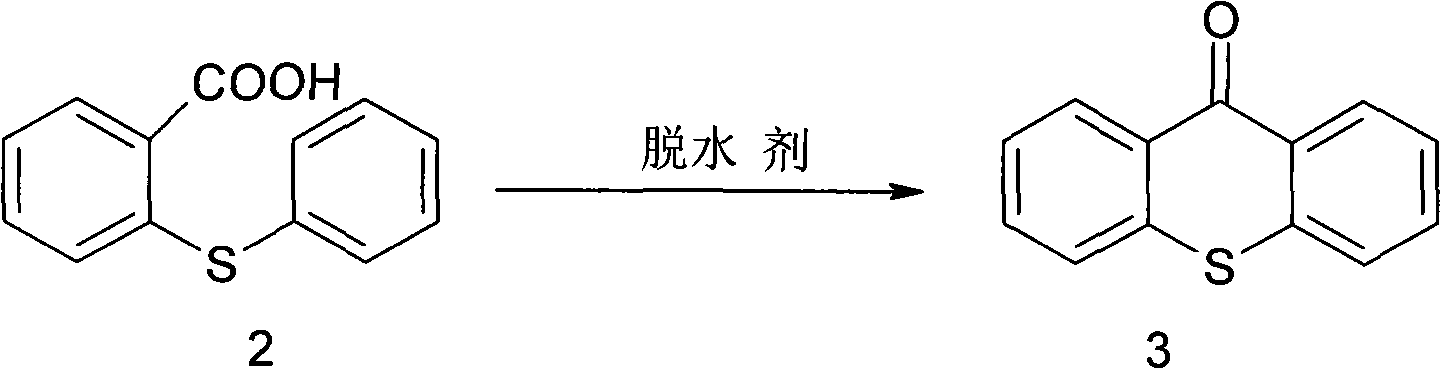
Synthesis method of metixene hydrochloride
InactiveCN101525333AWide variety of sourcesLow priceNervous disorderOrganic chemistryAntiparkinsonian drugBenzoic acid
A synthesis method of metixene hydrochloride relates to a synthesis process of an antiparkinsonian drug. The synthesis method comprises the following steps: taking thiosalicylic acid as a raw material to obtain 2-thiophenyl benzoic acid by coupling with iodobenzene; then dehydrating and cyclizing to obtain 9-thioxanthone; reducing a carbonyl to obtain thioxanthene; and finally allowing the thioxanthene to react with 3-chloromethyl-1-pipecoline to obtain the target product (5) metixene hydrochloride shown as a formula (5) below. The synthesis method has the advantages of available raw materials, low cost, short preparation course, simple operation and easy industrialization, and helps produce the metixene hydrochloride which is the antiparkinsonian drug.
Owner:EAST CHINA NORMAL UNIV
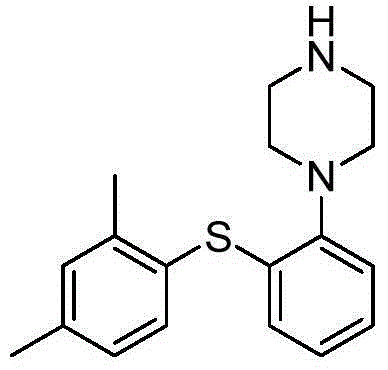
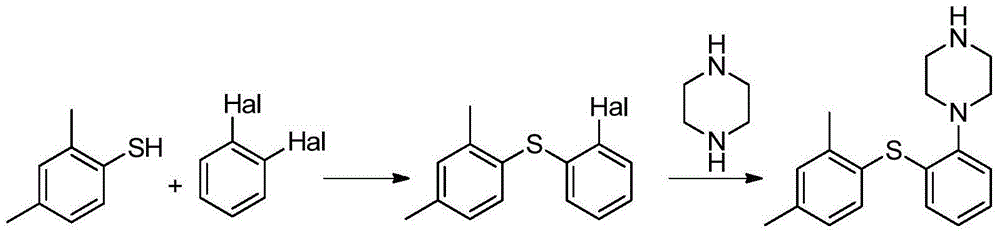
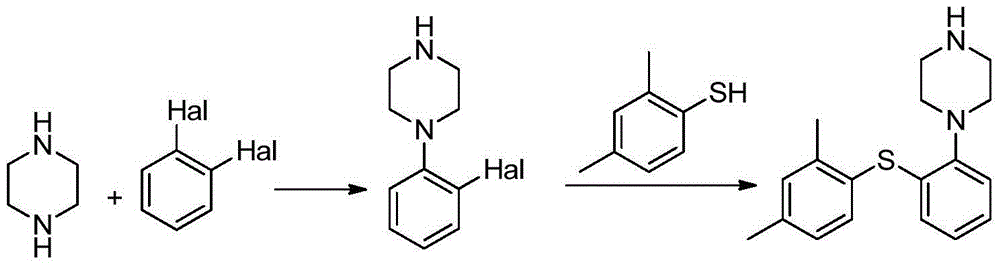
Preparation method of vortioxetine
The invention relates to a preparation method of vortioxetine. The preparation method of vortioxetine comprises the following steps: enabling 2,4-dimethyl-iodobenzene, o-bromothiophenol and 1-Boc-piperazine to react at the temperature of 25-40 DEG C in the presence of the catalysis of an appropriate amount of palladium catalyst, ligand, alkali and phase transfer catalyst to prepare a compound II and de-protecting the compound II by hydrobromic acid solution to obtain vortioxetine which shown in a formula I, or enabling 2,4-dimethylbenzenethiol, 1-bromo-2-iodobenzene and 1-Boc-piperazine to react at the temperature of 25-40 DEG C in the presence of the catalysis of an appropriate amount of palladium catalyst, alkali and phase transfer catalyst to prepare a compound II and de-protecting the compound II by the hydrobromic acid solution to obtain vortioxetine which is shown in a formula I. According to the preparation method, the preparation process is simple, the reaction selectivity is high, few impurities exist, the purification pressure is low and the yield is high.
Owner:ZHONGSHAN WANHAN PHARM CO LTD +1
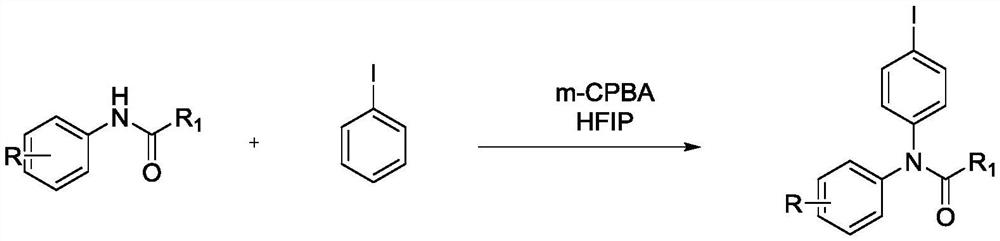
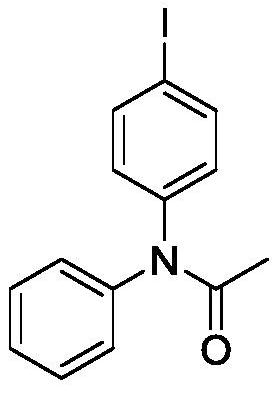
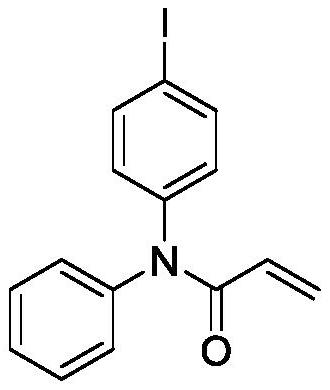
Preparation method of iodobenzene para-amination compound promoted by m-chloroperoxybenzoic acid
ActiveCN111646917ABiologically activeMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationBenzoic acidChromatographic separation
The invention relates to a preparation method of an iodobenzene para-aminated compound promoted by m-chloroperoxybenzoic acid, and the method comprises the following steps: adding substituted acetanilide, iodobenzene, m-chloroperoxybenzoic acid and hexafluoroisopropanol into a reactor, putting the reactor into an oil bath pot at 40-80 DEG C, condensing, refluxing, magnetically stirring, and heating to react for 4-10 hours; pouring the obtained reaction liquid into a separating funnel, adding distilled water, extracting for three times by using an organic solvent, carrying out reduced pressuredistillation on an organic phase to obtain a crude product, and carrying out column chromatography separation and purification to obtain the iodobenzene para-aminated compound. The method is mild in reaction condition, high in selectivity, high in yield and environmentally friendly, and the synthesized aryl amide compound has certain biological activity and can be applied to the fields of medicinesynthesis, pesticide synthesis, paint and dye synthesis and the like.
Owner:HUBEI UNIV OF TECH
Magnetic multistage nuclear @ shell structure nano-palladium catalyst and preparation method thereof
InactiveCN104815685AChemical composition controlPhysical/chemical process catalystsDivalent metalPalladium catalyst
The invention discloses a magnetic multistage nuclear @ shell structure nano-palladium catalyst and a preparation method thereof, and belongs to the field of magnetic nano-catalysis materials. The general form of the materials is Fe3O4@MAl-LDH@xPd0, wherein the MAl-LDH refers to shell hydrotalcite, the M refers to one or two divalent metal elements, and the x refers to the weight percent load of palladium. By a low-temperature double-dipping co-precipitation method, LDH nano-crystals are assembled on the surface of a Fe3O4 magnetic nuclear surface, shell LDH hexagonal nano-crystals grow in a mutually staggered mode in such a manner that ab surfaces are perpendicular to the magnetic nuclear surface, and the shell LDH hexagonal nano-crystals are honeycomb. By an impregnation reduction method, palladium nano-particles are loaded on a magnetic multistage nuclear @ shell structure carrier to obtain a primary magnetic multistage nuclear @ shell structure nano-palladium catalyst, and the palladium nano-particles are uniformly distributed at edges and staggered positions of the LDH nano-crystals. The obtained catalyst is used for iodobenzene and styrene Heck coupling reaction and has fine catalytic activity, the highest TOF value is 160.5h-1, the catalyst is recycled by an external magnetic field, and the catalytic activity is not obviously reduced after used for ten times.
Owner:BEIJING UNIV OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com