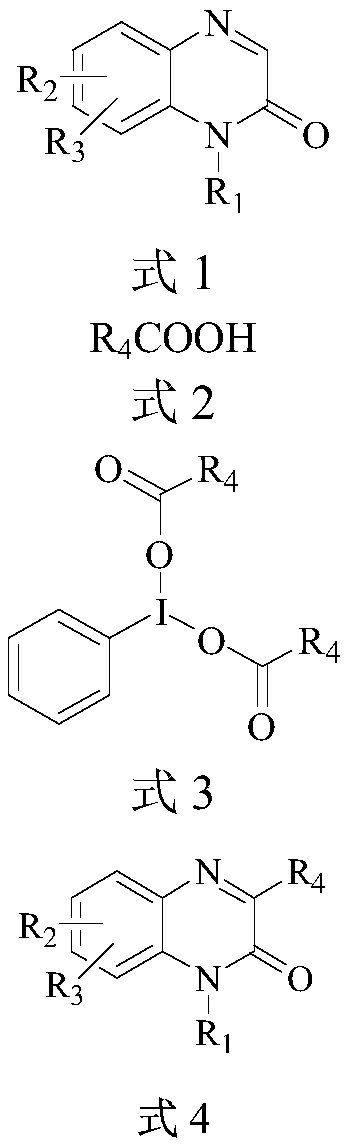
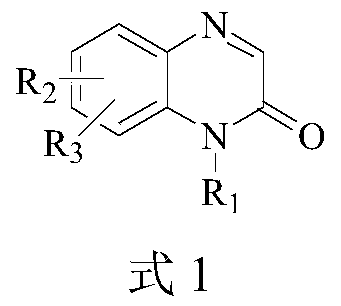
Preparation method of 3-alkyl quinoxaline-2(1H)-ketone compound
A technology of ketone compounds and alkylquinones, which is applied in the field of organic synthesis, can solve problems such as unrealized reactions, and achieve the effects of good applicability, reduced reaction costs and energy consumption, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] Add iodobenzene diacetate (10mmol) and propionic acid (20mmol) into a round-bottomed flask, dissolve them in 25ml of chloroform, and concentrate to dryness under reduced pressure at 50°C to obtain iodobenzene dipropionate 2b, which is used directly for Next reaction.
[0047] At 25°C, in a 10mL reaction tube, sequentially add 1-methylquinoxaline-2(1H)-one 1a (0.2mmol), iodobenzene dipropionate 2b (0.44mmol), Ru(bpy) 3 Cl 2 ·6H 2 O (0.002mmol), PEG-200 (1mL), mixed evenly, and then stirred and reacted for 8h under the irradiation of 3w blue LED light. After the reaction was detected by TLC, cyclopentyl methyl ether (2ml×3) was added for extraction, and the upper layer extract was taken and concentrated in vacuum at 50°C until there was no solvent to obtain a crude product, which was then extracted with petroleum ether at a volume ratio of 2:1 Wash with ethyl acetate mixed eluent, flash column chromatography on silica gel to obtain 3-ethyl-1-methylquinoxali...
Embodiment 2
[0050]
[0051] Add iodobenzene diacetate (10mmol) and cyclohexanecarboxylic acid (20mmol) in a round bottom flask, dissolve in 25ml of chloroform, and concentrate to dryness under reduced pressure at 50°C to obtain iodobenzene dicyclohexanecarboxylate 2c without purification , used directly in the next reaction.
[0052] At 25°C, in a 10mL reaction tube, sequentially add 1-methylquinoxaline-2(1H)-one 1a (0.2mmol), iodobenzene dicyclohexanecarboxylate 2c (0.44mmol), Ru(bpy) 3 Cl 2 ·6H 2 O (0.002mmol), PEG-200 (1mL), mixed evenly, and then stirred and reacted for 6h under the irradiation of 3w blue LED light. After the reaction was detected by TLC, cyclopentyl methyl ether (2ml×3) was added for extraction, and the upper layer extract was taken and concentrated in vacuum at 50°C until there was no solvent to obtain a crude product, which was then extracted with petroleum ether at a volume ratio of 2:1 Wash with ethyl acetate mixed eluent, silica gel column flash column chr...
Embodiment 3
[0055]
[0056] Add iodobenzene diacetate (10mmol) and n-octanoic acid (20mmol) into a round-bottomed flask, dissolve them in 25ml of chloroform, and concentrate to dryness under reduced pressure at 50°C to obtain iodobenzene dioctanoate 2d, which is directly used in the next step without purification. One step reaction.
[0057] At 25°C, in a 10mL reaction tube, sequentially add 1-methylquinoxaline-2(1H)-one 1a (0.2mmol), iodobenzene dioctanoate 2d (0.44mmol), Ru(bpy) 3 Cl 2 ·6H 2 O (0.002mmol), PEG-200 (1mL), mixed evenly, and then stirred and reacted for 8h under the irradiation of 3w blue LED light. After the reaction was detected by TLC, cyclopentyl methyl ether (2ml×3) was added for extraction, and the upper layer extract was taken and concentrated in vacuum at 50°C until there was no solvent to obtain a crude product, which was then extracted with petroleum ether at a volume ratio of 2:1 Wash with ethyl acetate mixed eluent, silica gel column flash column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com