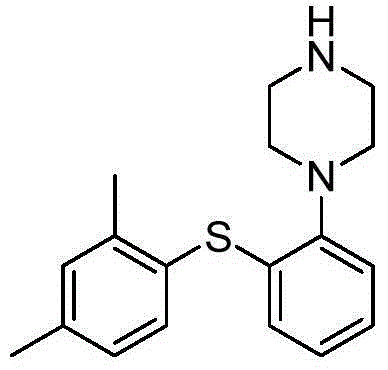
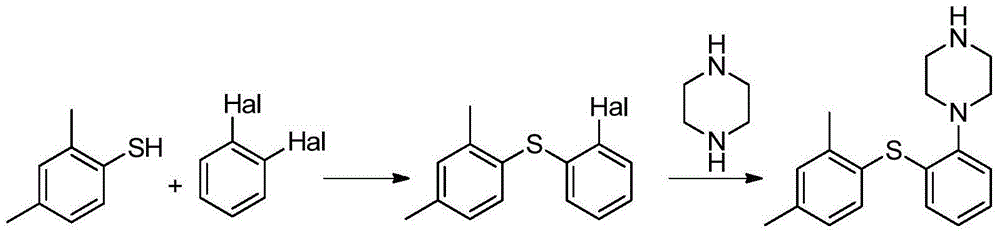
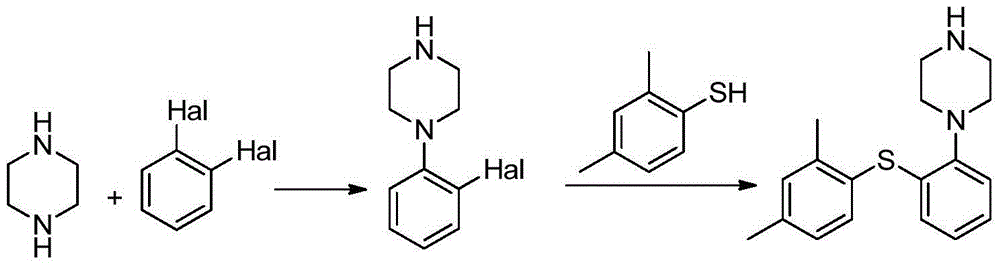
Preparation method of vortioxetine
A technology of vortioxetine hydrobromide and molar ratio, applied in organic chemistry and other directions, can solve the problems of many impurities, high purification pressure, low yield and the like, and achieve high yield, low purification pressure and high reaction selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The 100mL four-necked bottle is equipped with an electromagnetic stirrer, a condenser tube and a thermometer. Add 2,4‐dimethyliodobenzene (2.32g, 10mmol, 1.0eq), o-bromothiophenol (1.90g, 10mmol, 1.0eq) and 25mL of tetrahydrofuran, and replace with nitrogen three times. Put in Pd(dba) 2 (0.58g, 1mmol, 0.1eq), BINAP (0.93g, 1.5mmol, 0.15eq) and 1-Boc-piperazine (1.86g, 10mmol, 1.0eq), nitrogen replacement 3 times. Finally, 18‐C‐6 (7.40g, 28mmol, 2.8eq) and sodium tert-butoxide (2.69g, 28mmol, 2.8eq) were added, stirred at 30°C for 24 hours, and HPLC showed that the selectivity was 98.6%.
[0045] Throw in 0.1 g of activated carbon, and stir for 2 hours. The filtrate was precipitated to dryness, 40 mL of toluene was added, and stirred for 0.5 hours. After filtration, 48% hydrobromic acid (2.53g, 15mmol, 1.5eq) was added dropwise to the filtrate, and stirred for 0.5 hours. Filter, filter cake with toluene 10mL × 2 Rinse. Vortioxetine hydrobromide is obtained. Vacuum...
Embodiment 2
[0047] The 100mL four-necked bottle is equipped with an electromagnetic stirrer, a condenser tube and a thermometer. Add 2,4‐dimethylthiophenol (1.38g, 10mmol, 1.0eq), o-bromothiophenol (2.83g, 10mmol, 1.0eq) and 25mL of tetrahydrofuran, and replace with nitrogen three times. Put into Pd 2 (dba) 3 (0.46g, 0.5mmol, 0.05eq), DPEphos (0.27g, 0.5mmol, 0.05eq) and 1-Boc-piperazine (1.86g, 10mmol, 1.0eq), nitrogen replacement 3 times. Finally, 18‐C‐6 (7.40g, 28mmol, 2.8eq) and potassium tert-butoxide (3.37g, 30mmol, 3.0eq) were added, stirred at 40°C for 36 hours, and HPLC showed that the selectivity was 98.2%.
[0048] Throw in 0.1 g of activated carbon, and stir for 2 hours. The filtrate was precipitated to dryness, 40 mL of toluene was added, and stirred for 0.5 hours. After filtration, 48% hydrobromic acid (2.53g, 15mmol, 1.5eq) was added dropwise to the filtrate, and stirred for 0.5 hours. Filter, filter cake with toluene 10mL × 2 Rinse. Vortioxetine hydrobromide is obta...
Embodiment 3
[0050] The 100mL four-necked bottle is equipped with an electromagnetic stirrer, a condenser tube and a thermometer. Add 2,4-dimethyliodobenzene (2.32g, 10mmol, 1.0eq), o-bromothiophenol (1.90g, 10mmol, 1.0eq) and 35mL tetrahydrofuran, and replace with nitrogen three times. Put in Pd(dba) 2 (2.9g, 5mmol, 0.5eq), BINAP (3.11g, 5mmol, 0.5eq) and 1-Boc-piperazine (1.86g, 10mmol, 1.0eq), nitrogen replacement 3 times. Finally, 18‐C‐6 (11.90g, 45mmol, 4.5eq) and sodium tert-butoxide (2.69g, 28mmol, 2.8eq) were added and stirred at 25°C for 20 hours. HPLC showed that the selectivity was 98.8%.
[0051]Throw in 0.1 g of activated carbon, and stir for 2 hours. The filtrate was precipitated to dryness, 50 mL of toluene was added, and stirred for 0.5 hours. After filtration, 48% hydrobromic acid (2.53g, 15mmol, 1.5eq) was added dropwise to the filtrate, and stirred for 0.5 hours. Filter, filter cake with toluene 10mL × 2 Rinse. Vortioxetine hydrobromide is obtained. Vacuum drying ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com