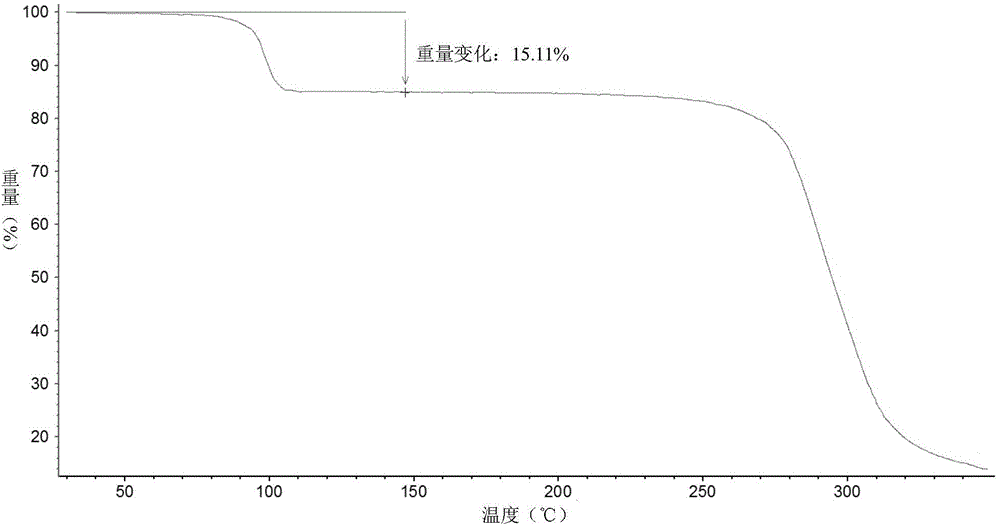
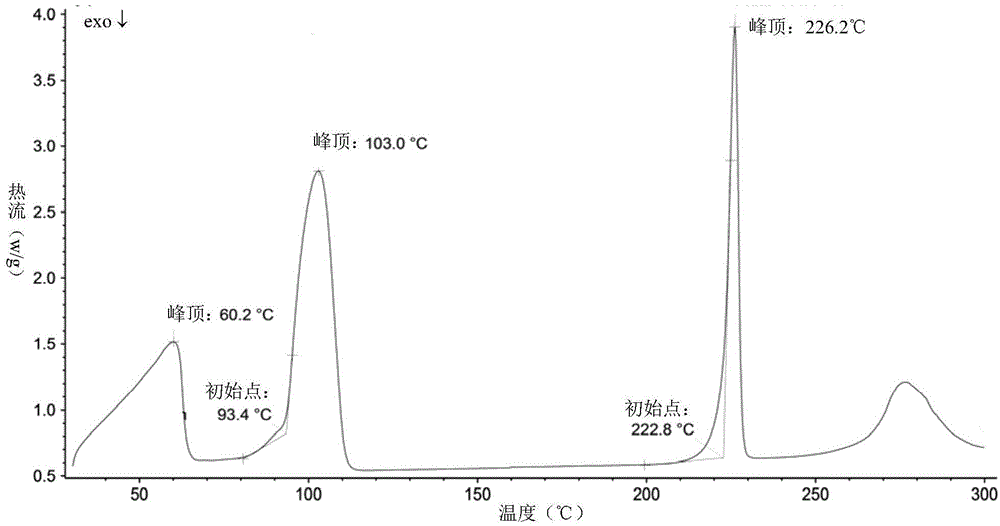
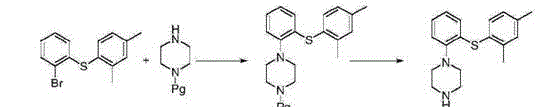
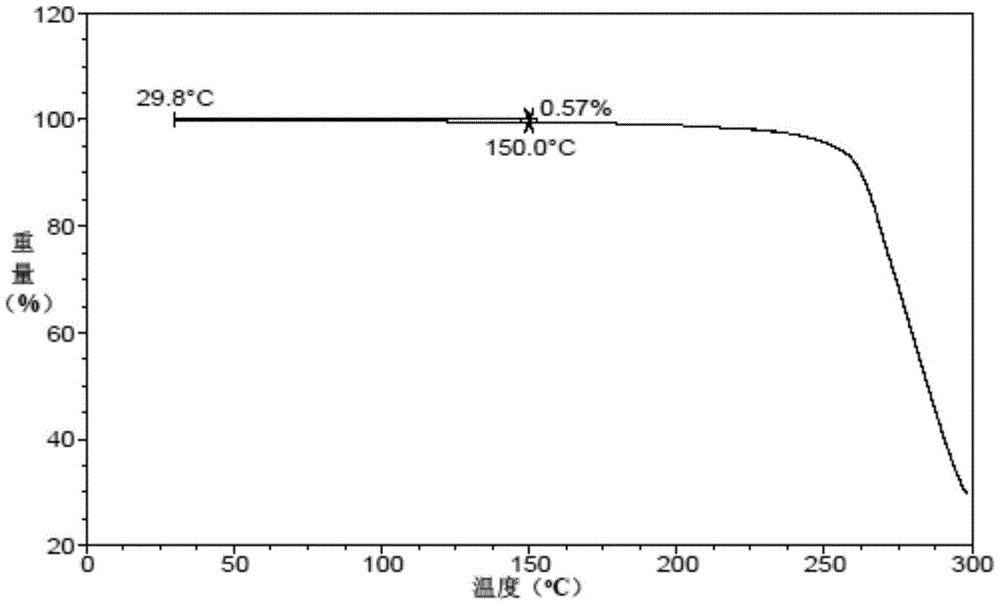
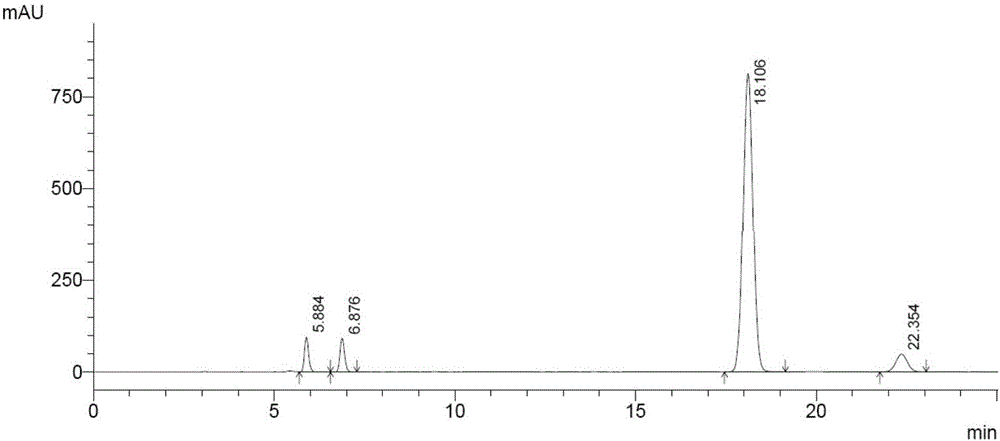
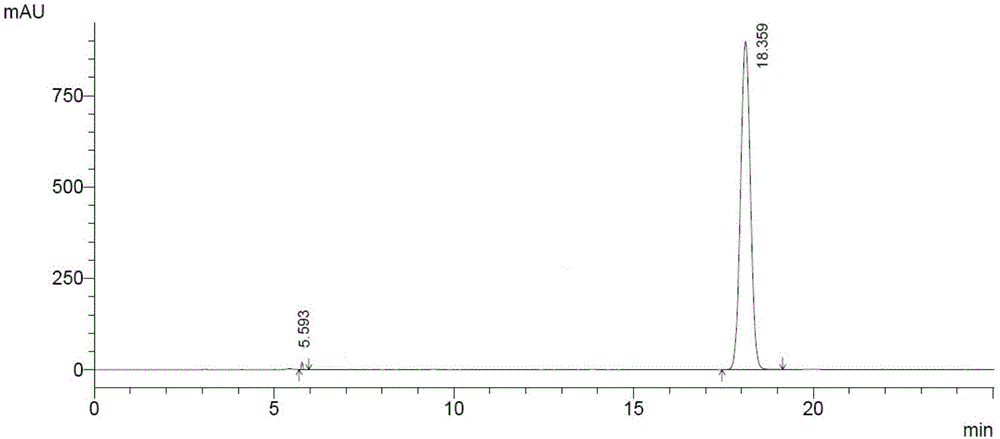
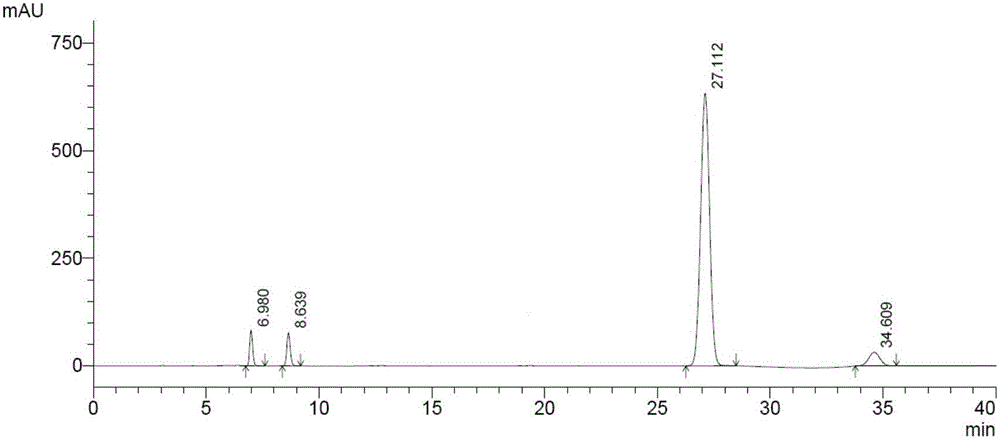
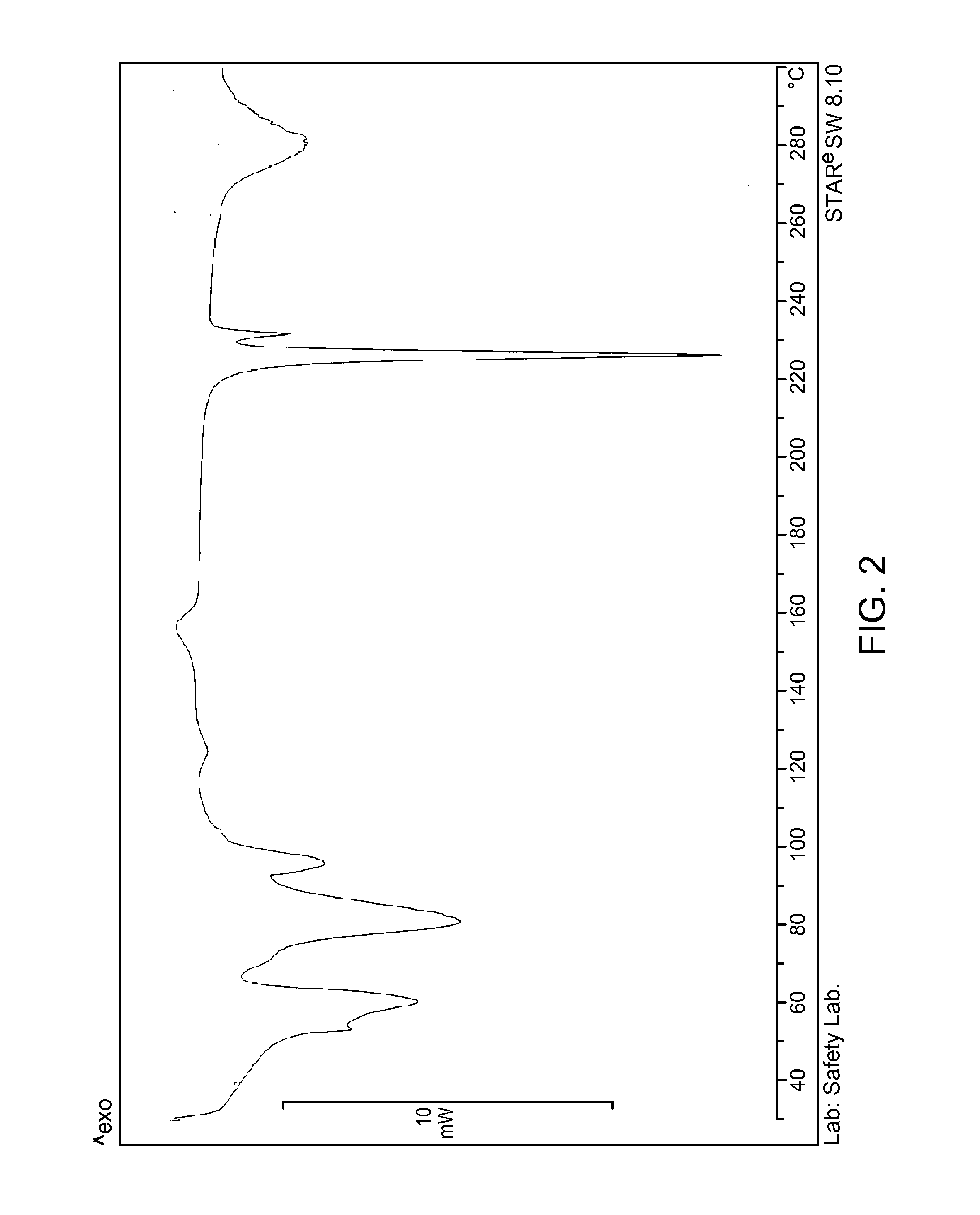
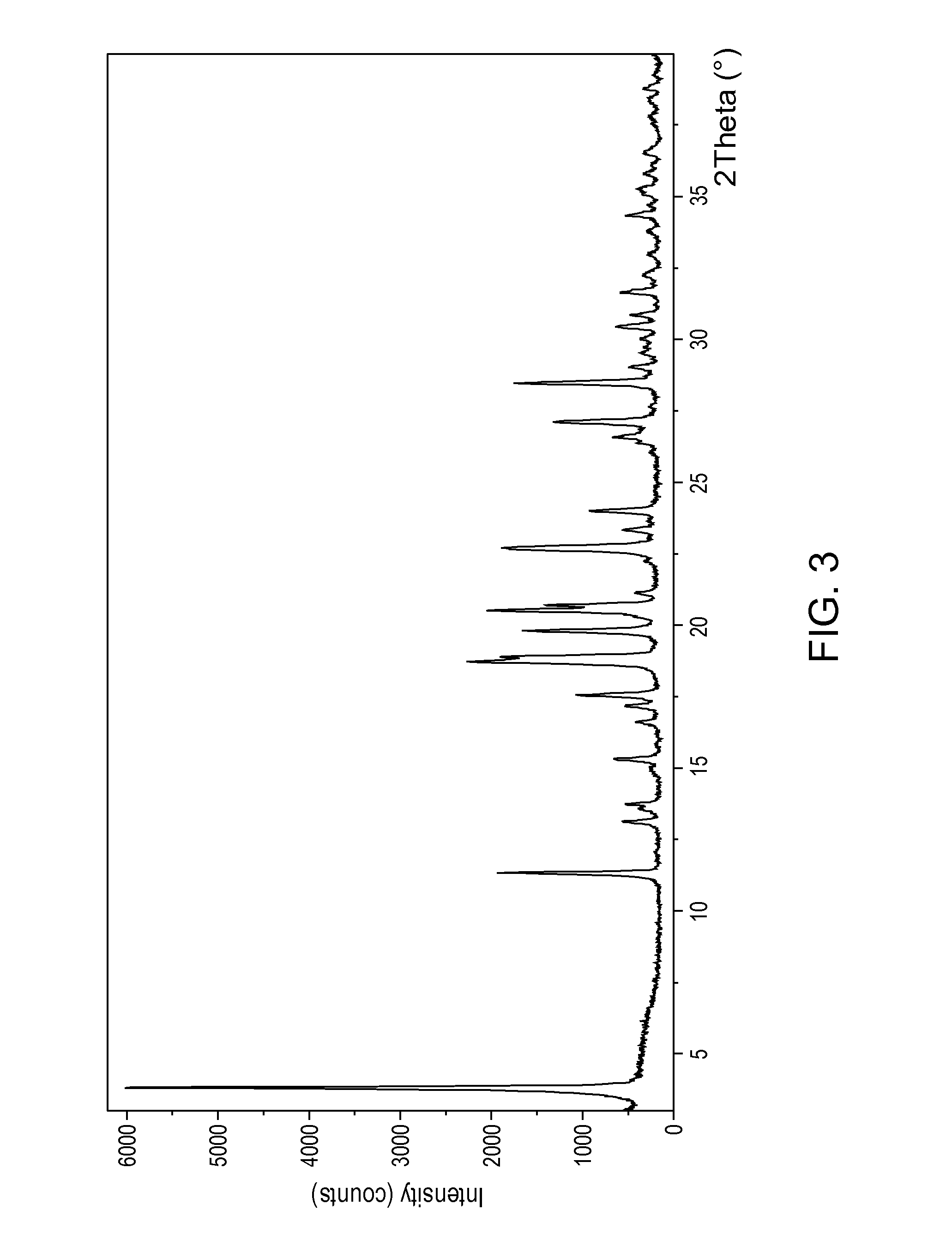
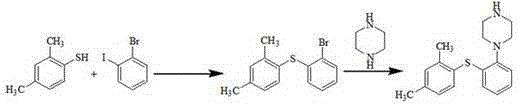
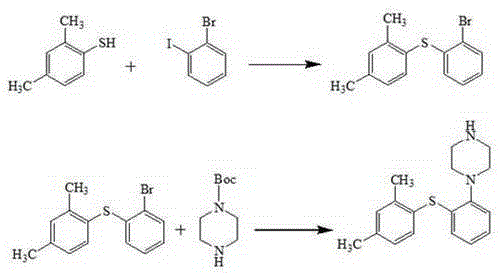
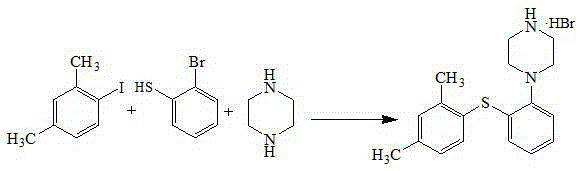
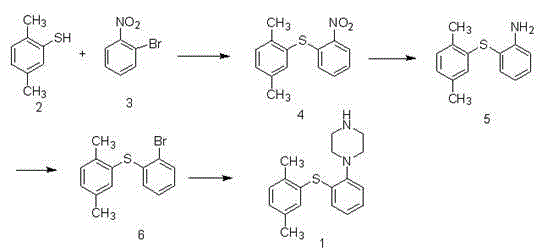
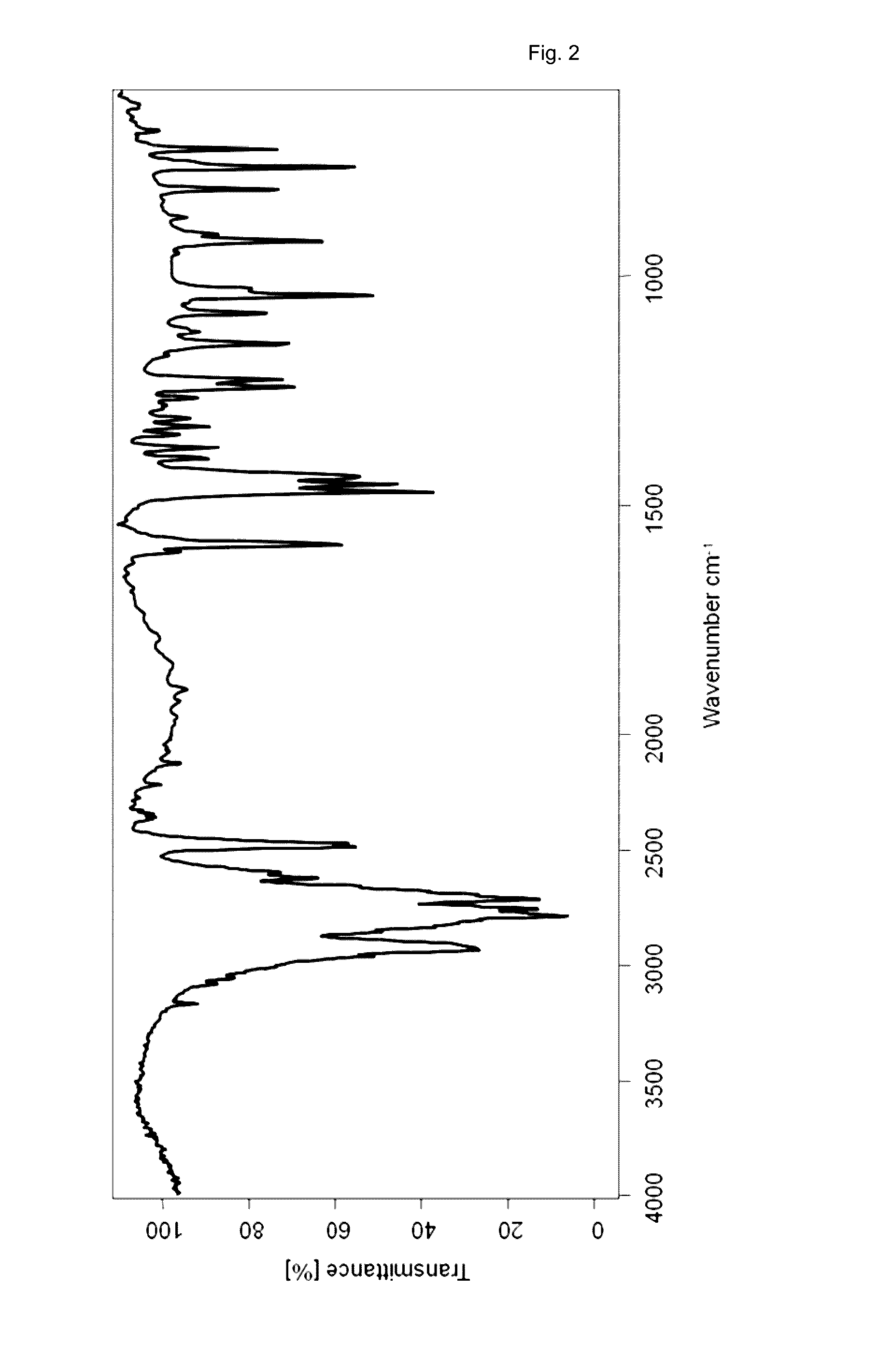
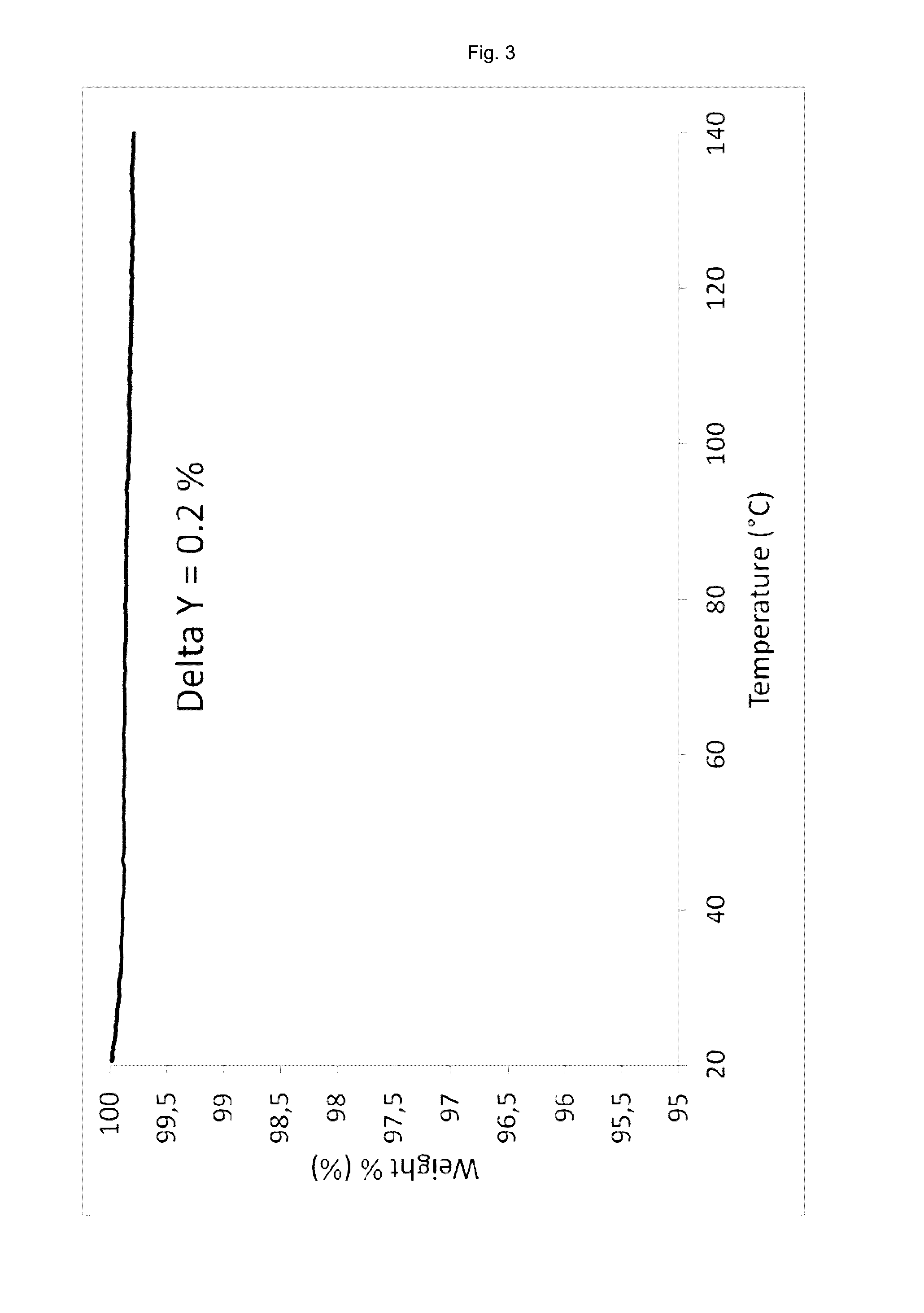
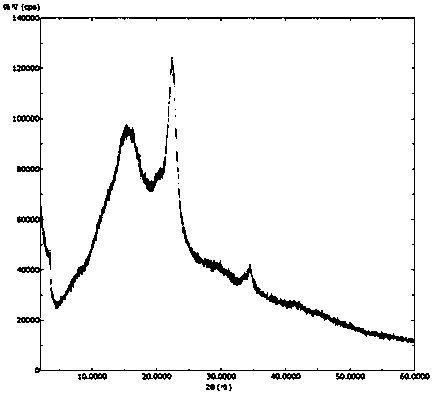
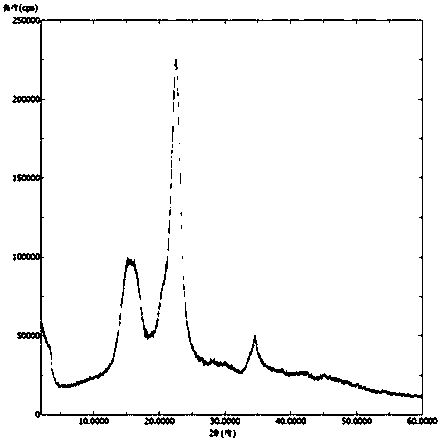
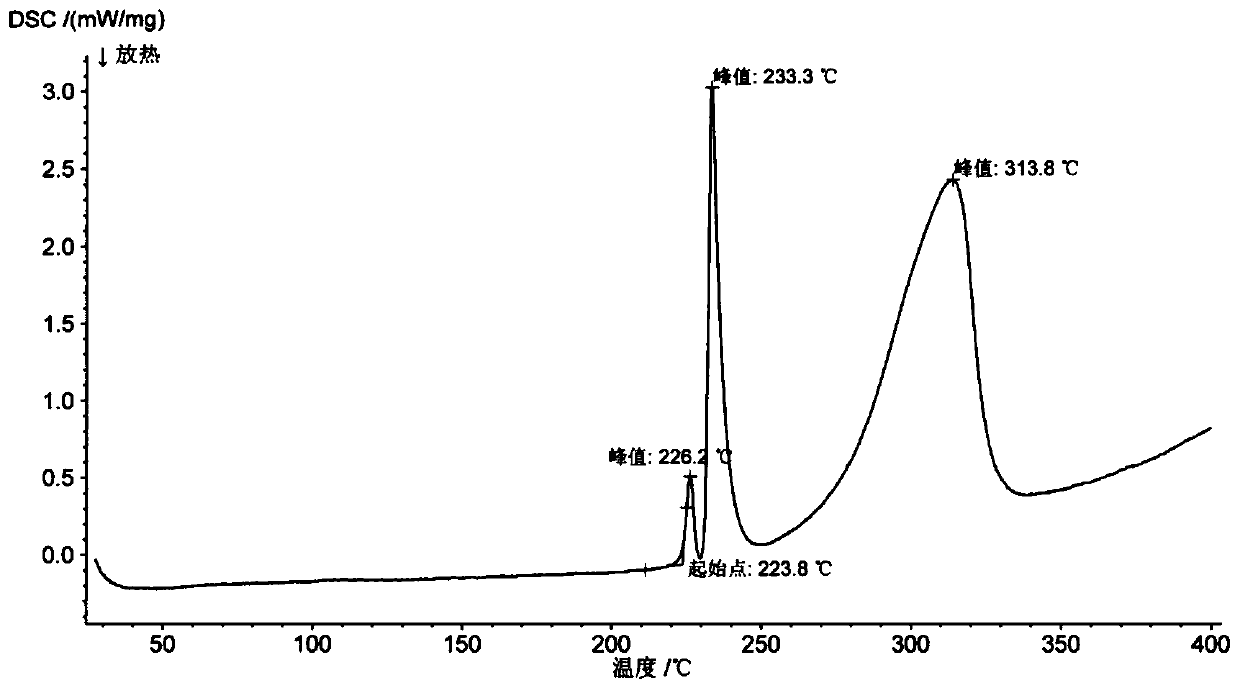
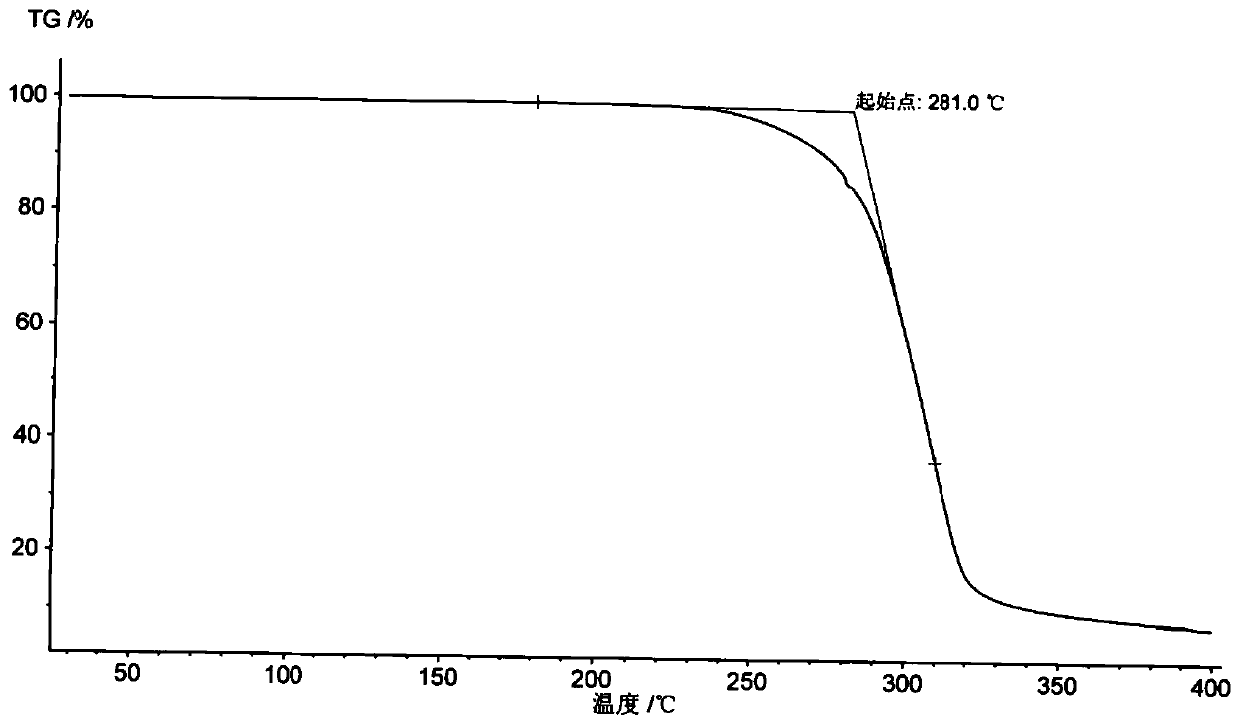
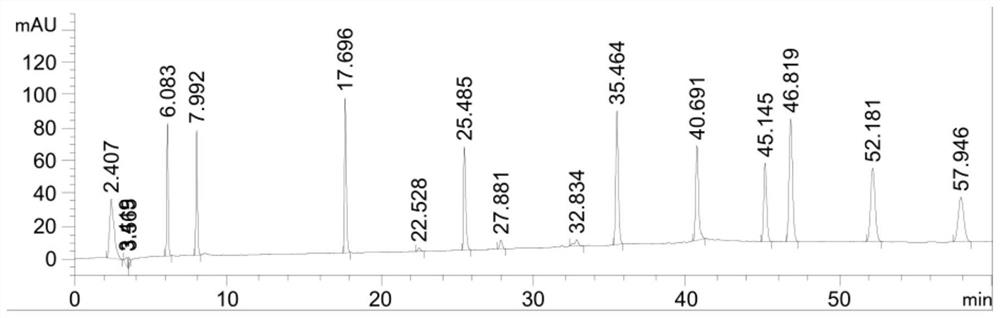
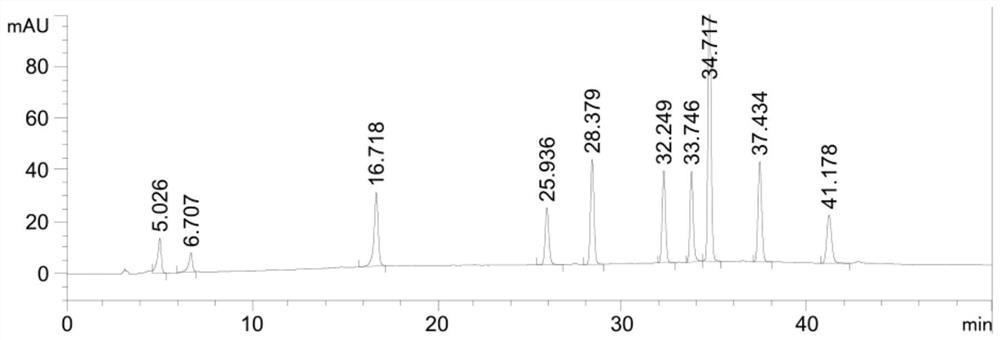
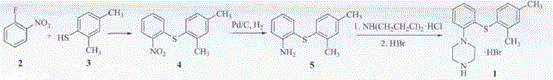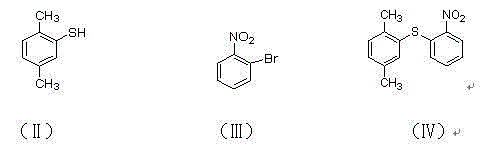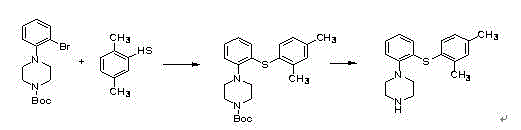Patents
Literature
80 results about "Vortioxetine Hydrobromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
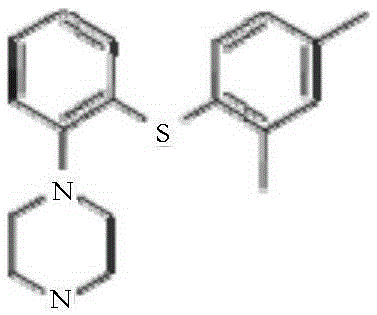
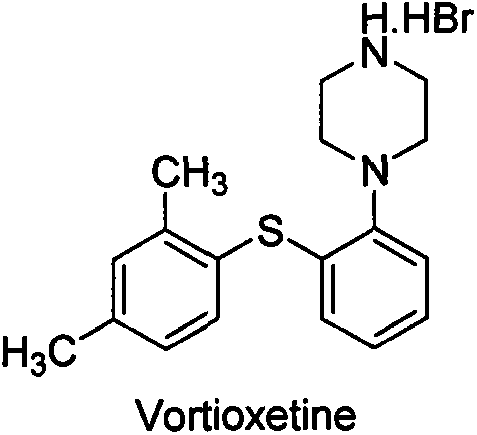
A hydrobromide salt form of vortioxetine, a serotonin (5-HT) modulator and stimulator (SMS), with antidepressant activity. Vortioxetine inhibits the reuptake of serotonin and norepinephrine from the synaptic cleft and acts variably as a serotonin receptor agonist (5-HT1A), partial agonist (5-HT1B) or antagonist (5-HT3, 5-HT1D and 5-HT7). It is not clear how this agent's purported multimodal mechanism of action contributes to its antidepressant effect; however, it is presumed to increase the synaptic availability of serotonin and norepinephrine.
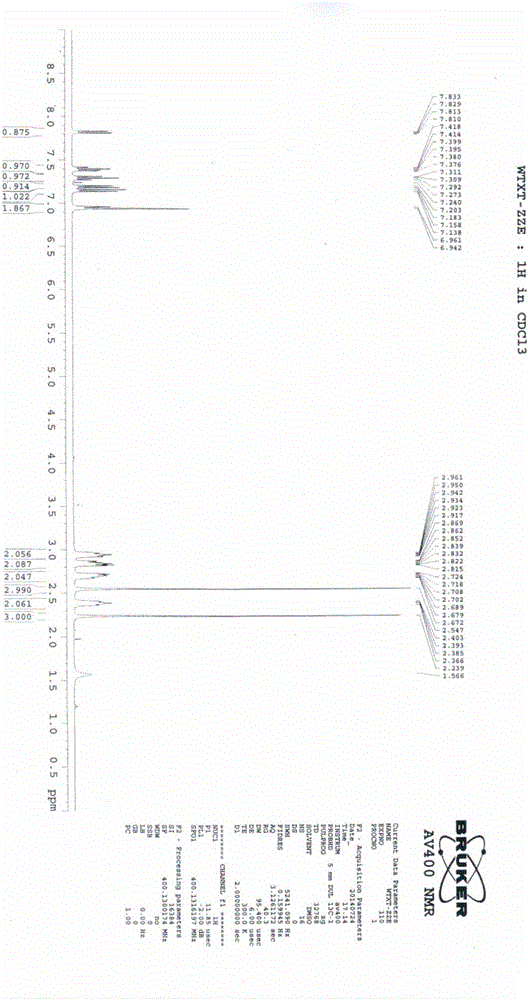
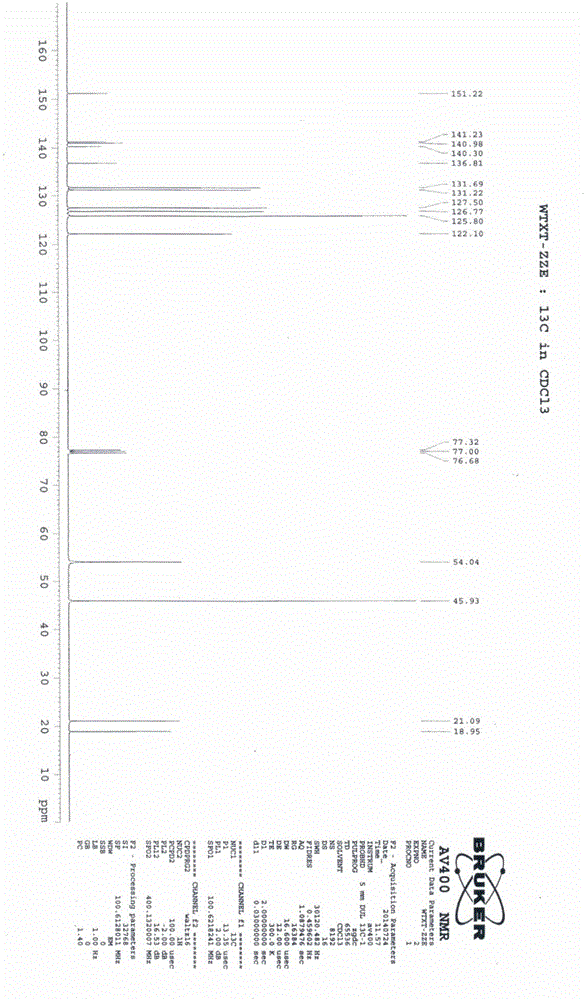
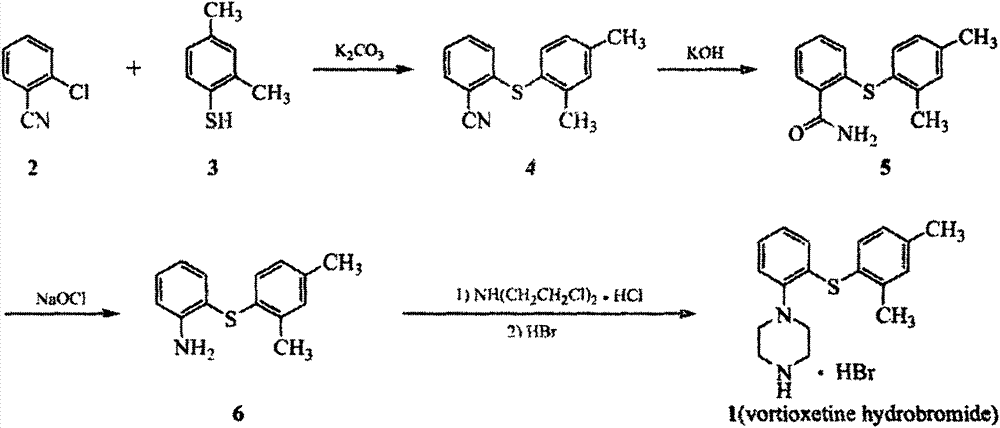
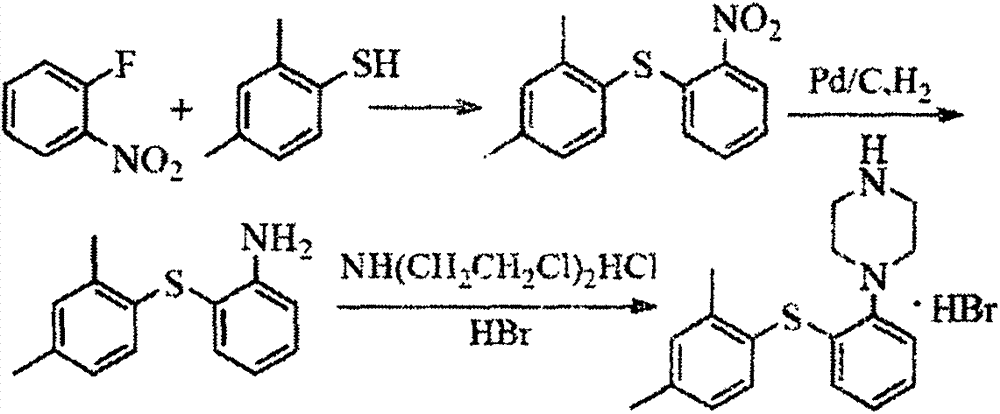
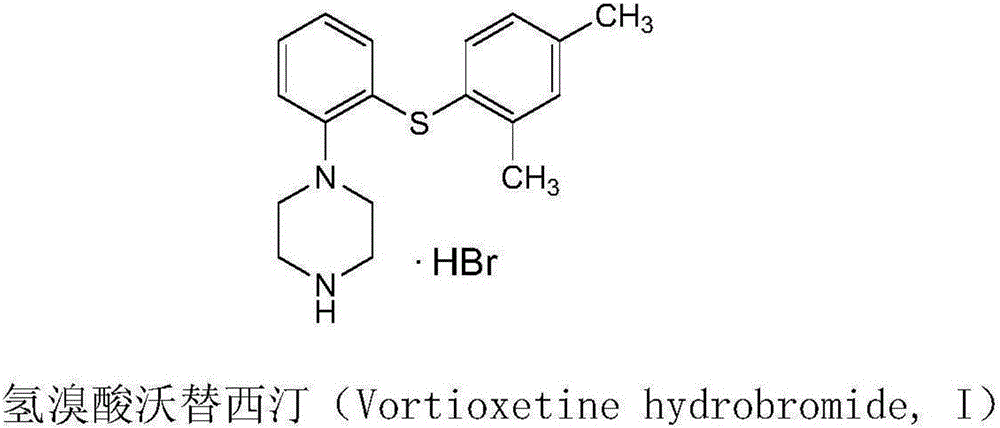
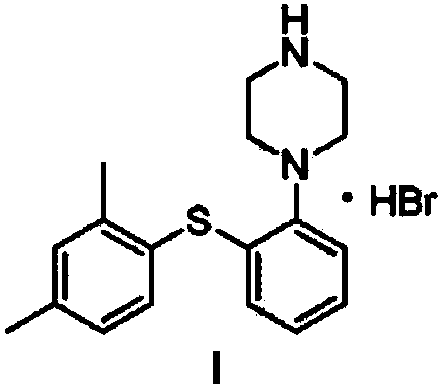
Vortioxetine hydrobromide
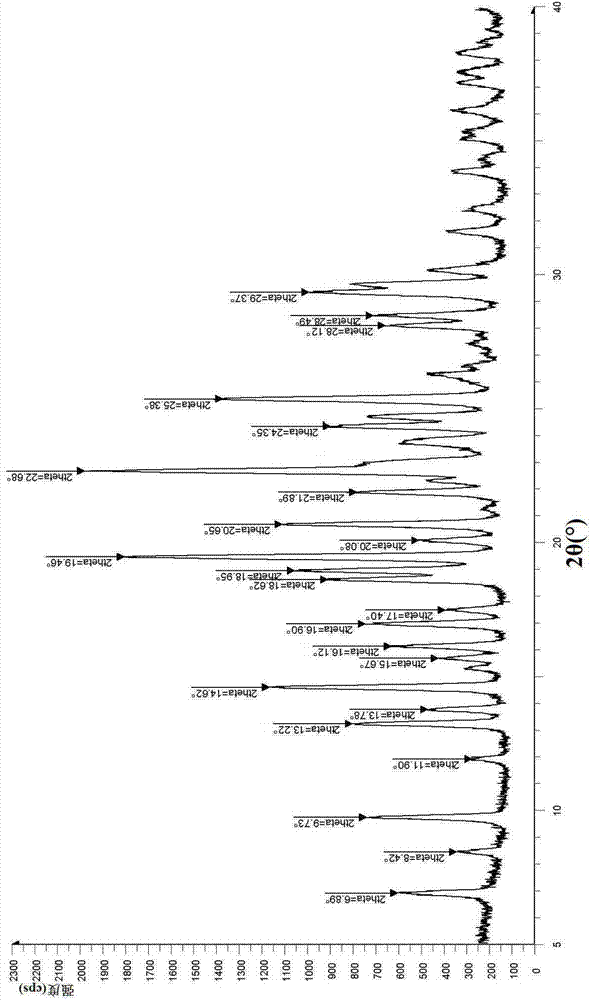
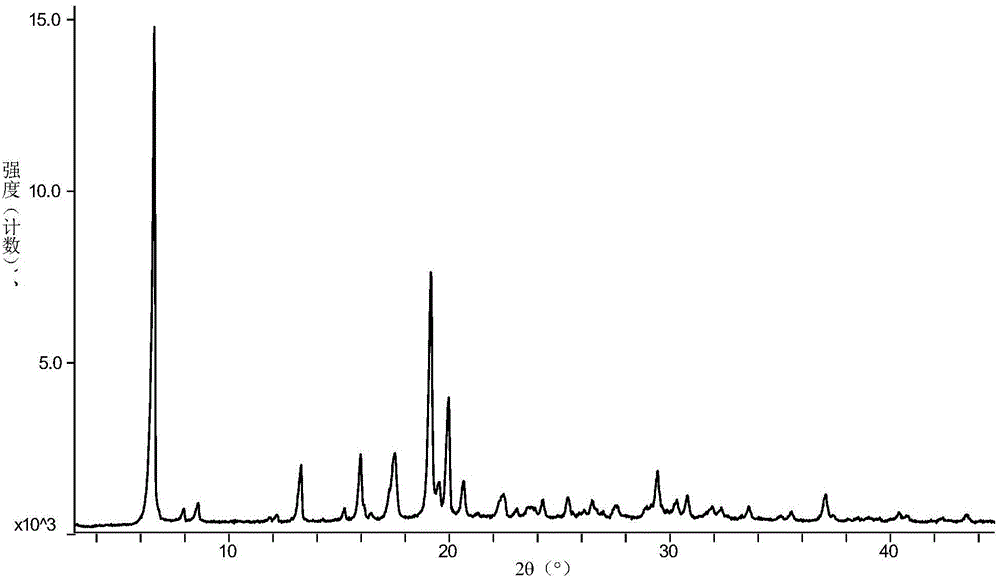
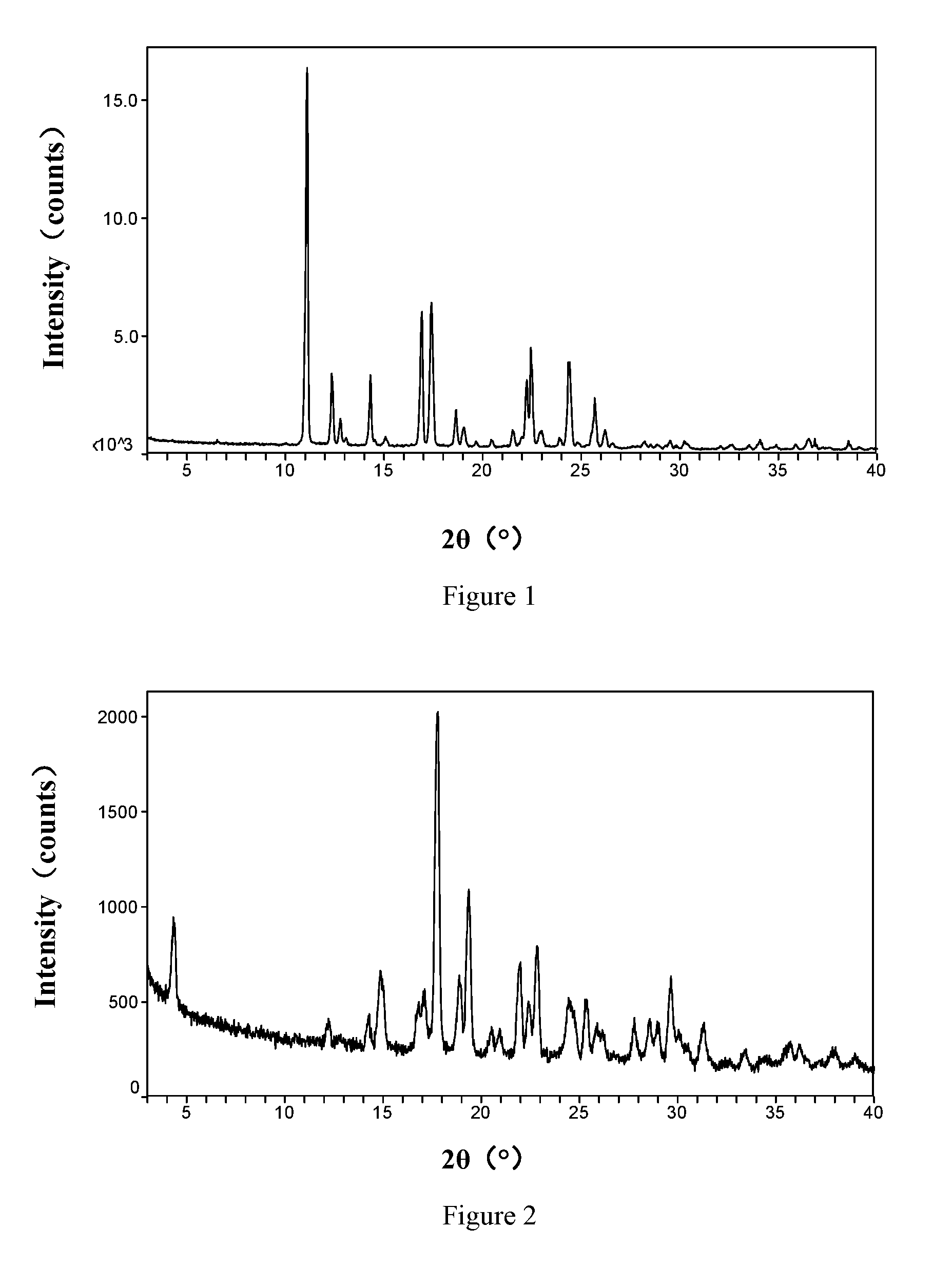
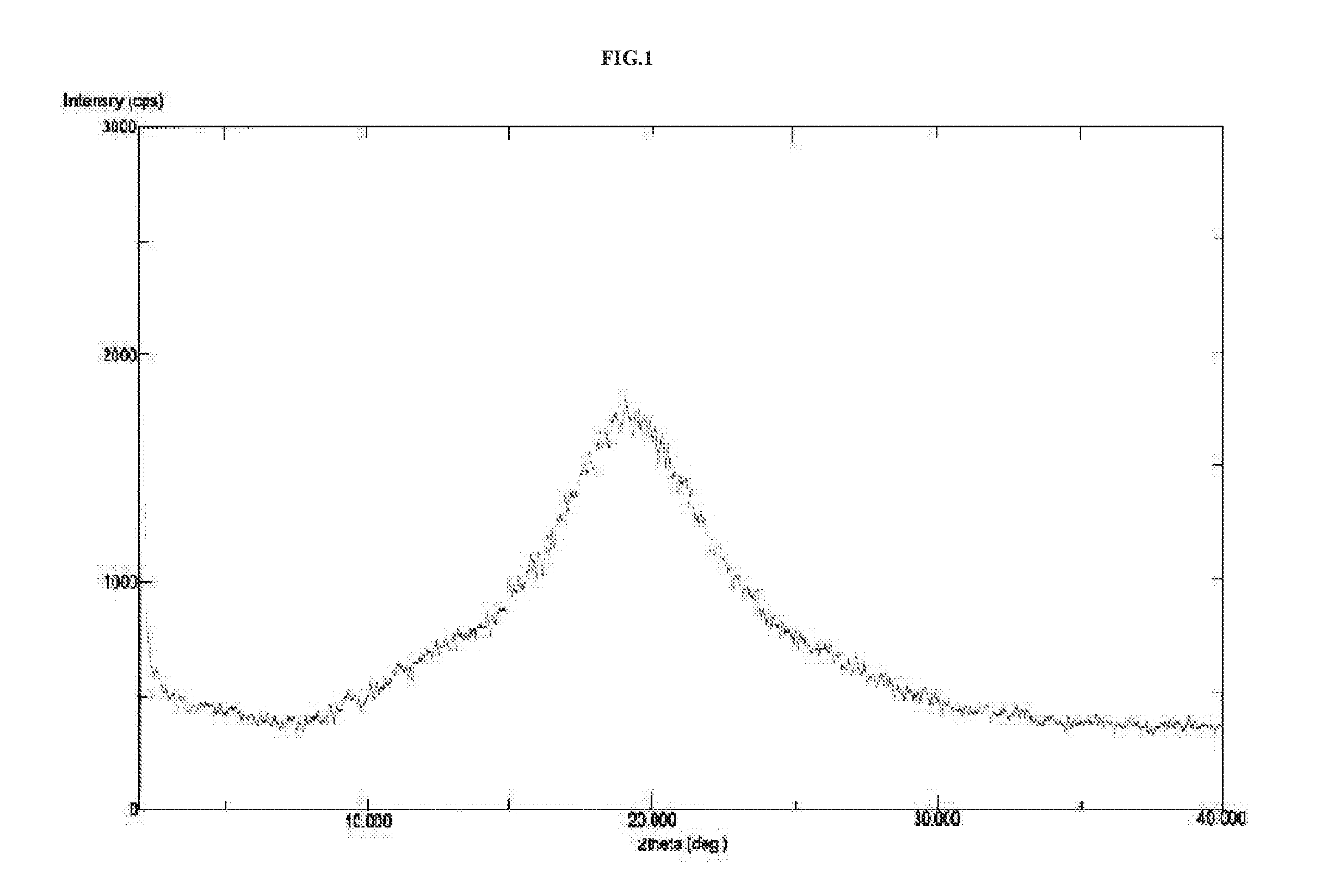
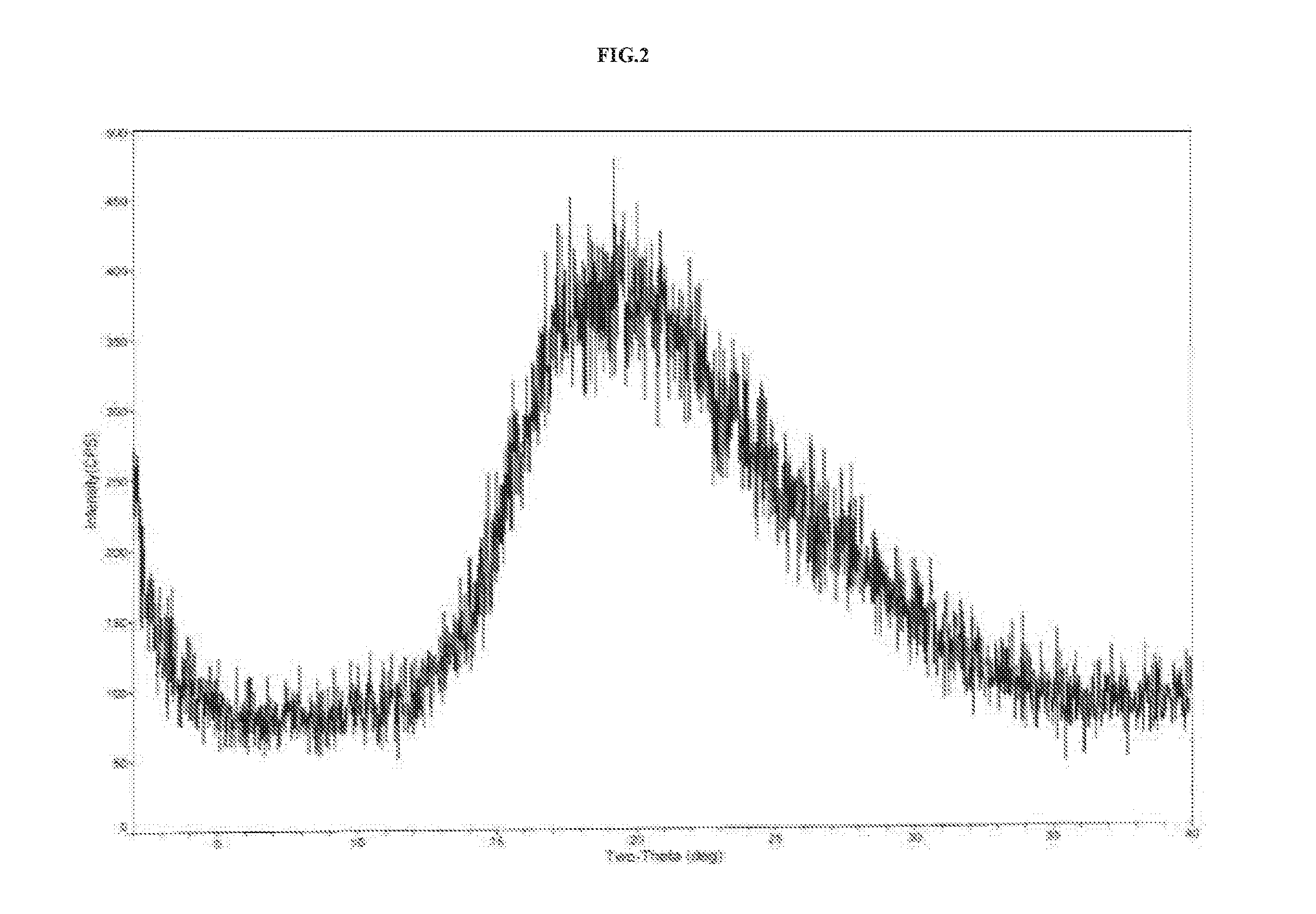
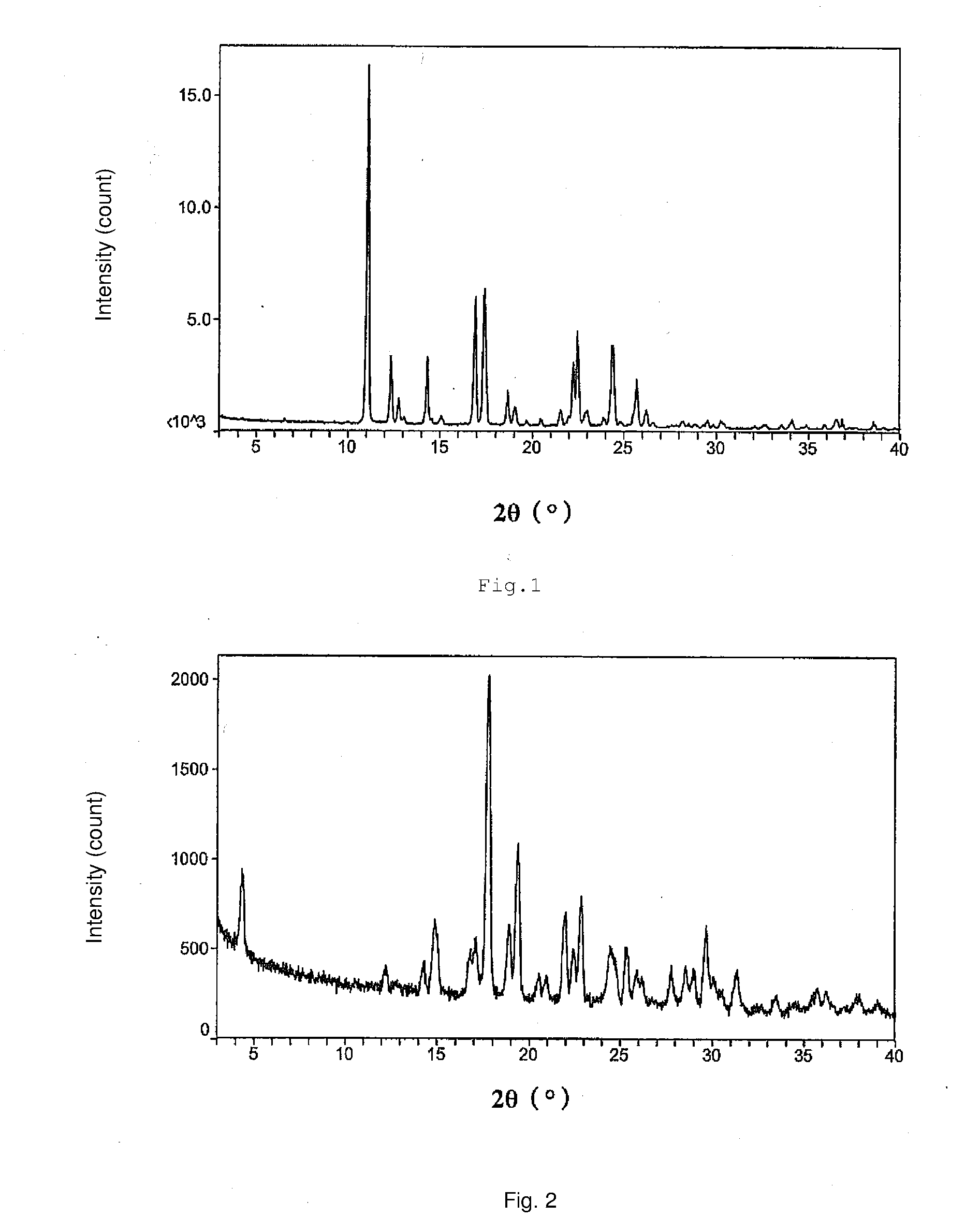
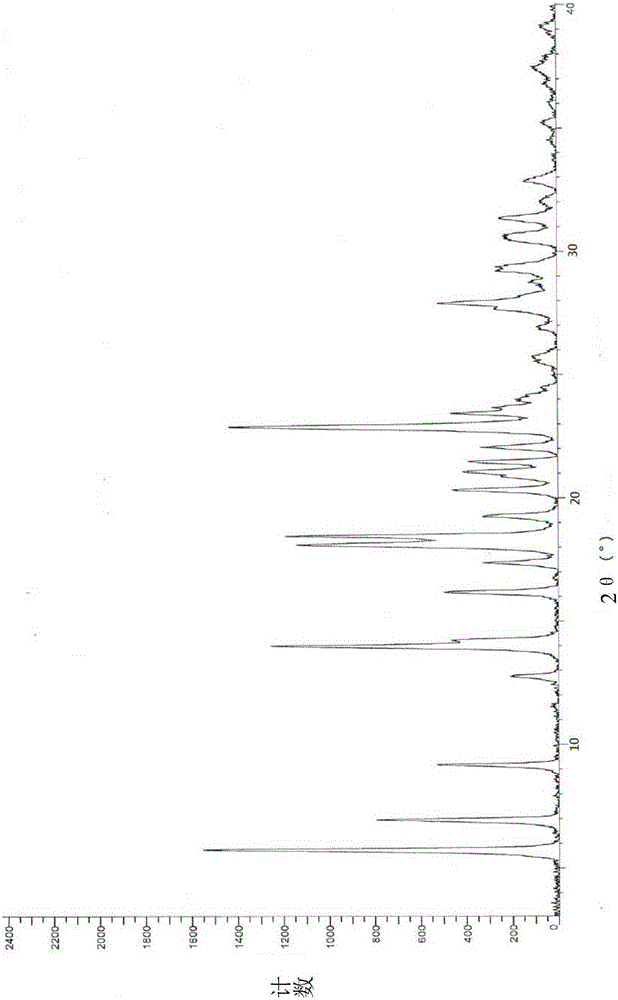
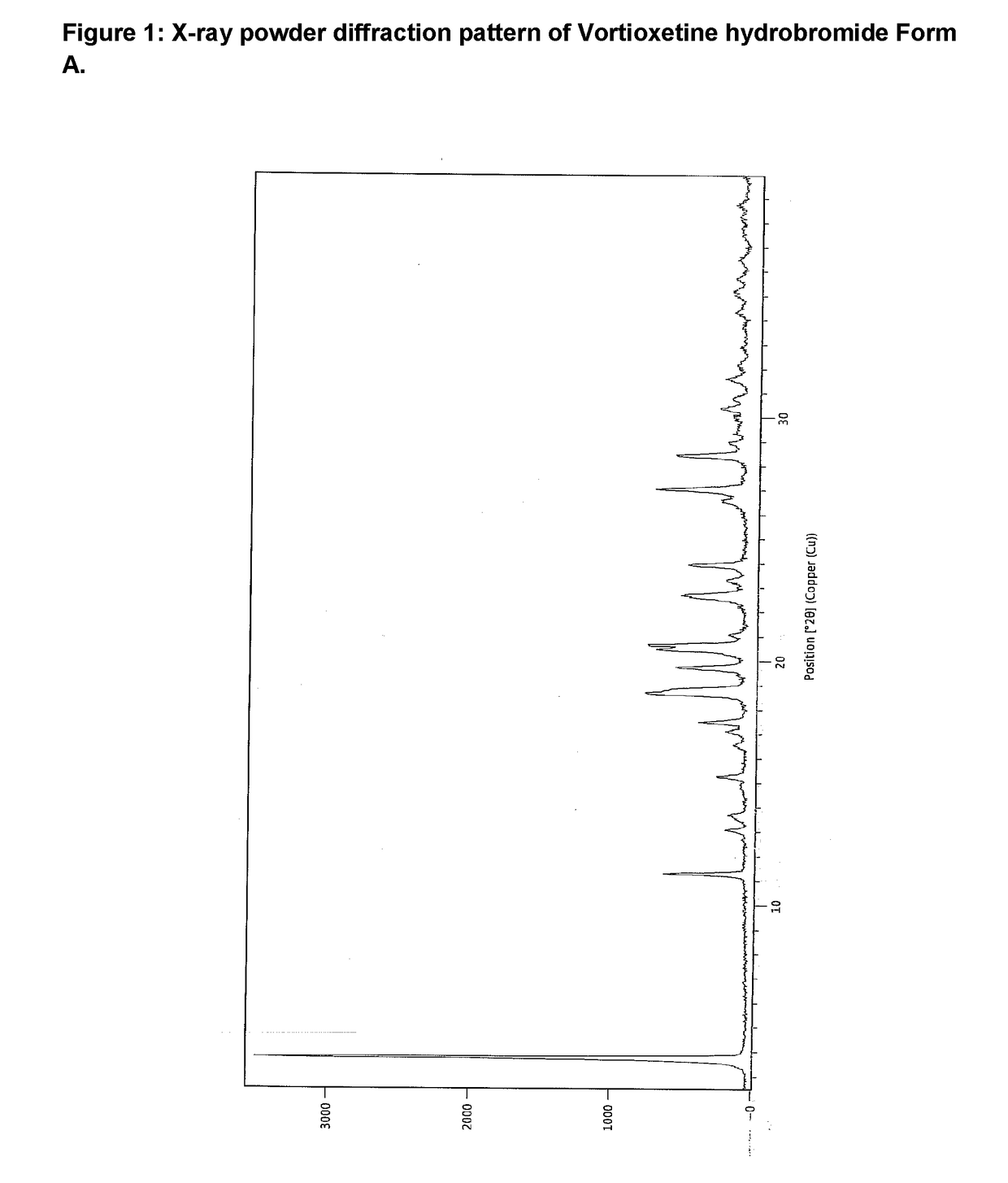
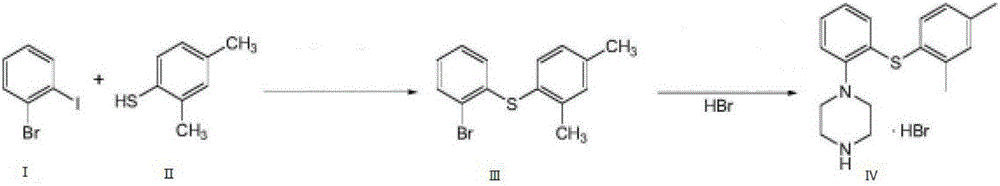
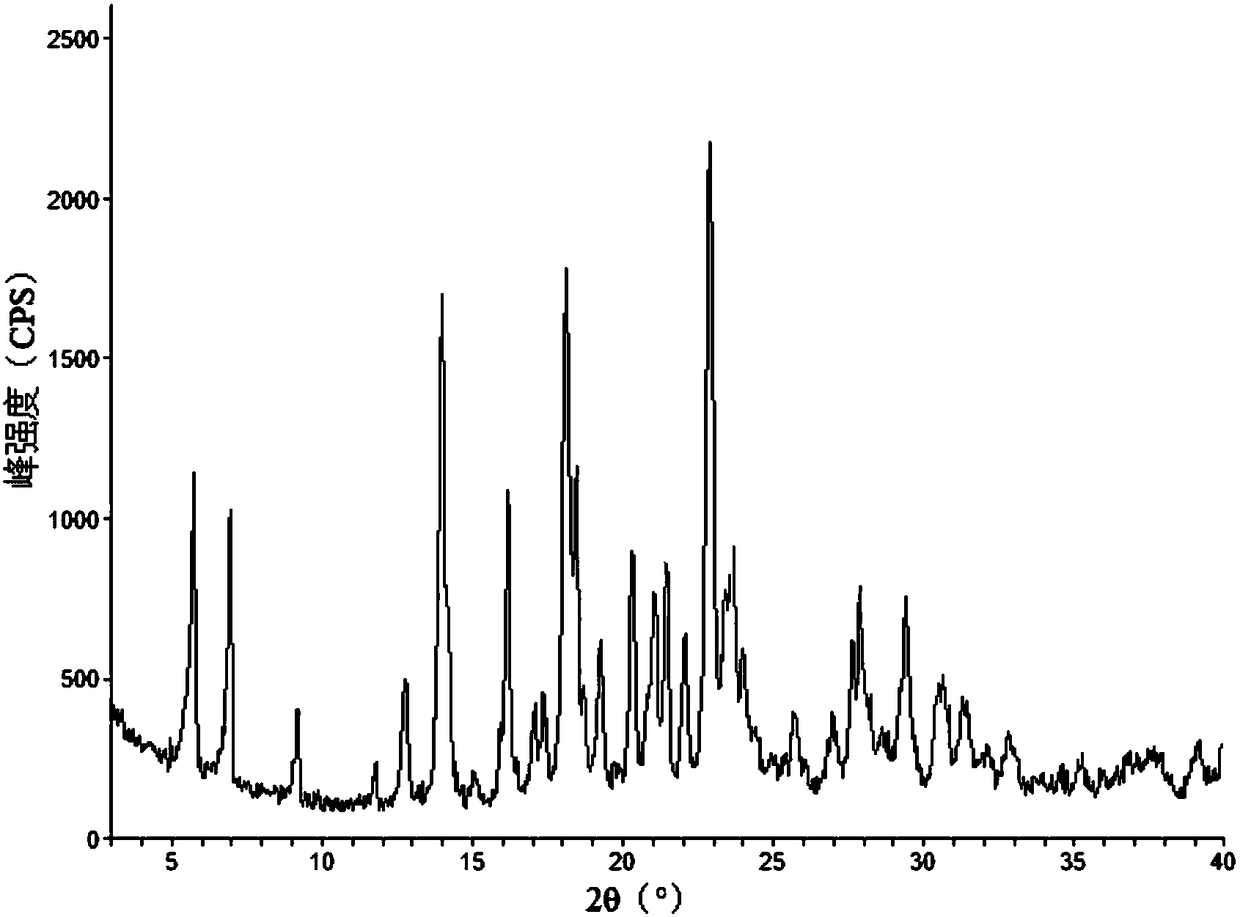
The invention relates to vortioxetine hydrobromide and in particular relates to a crystal of 1-[2-(2,4-dimethylphenylthioxo)phenyl] piperazine hydrobromide. The crystal uses Cu-K alpha radiation. In a powder X-ray diffraction pattern shown by a 2theta angle, diffraction peaks exist at about 6.89 degrees, 9.73 degrees, 13.78 degrees and 14.62 degrees. The invention also relates to a preparation method of the crystal and a drug composition containing the compound. The compound shows the serotonin reabsorption inhibitory activity, has activities towards a serotonin receptor 1A (5-HT1A) and a serotonin receptor 3 (5-HT3), can be used for treating CNS (central nervous system)-related diseases, in particular can be used for treating depressive disorder, especially major depressive disorder of adults, and can be also used for treating other CNS-related diseases.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
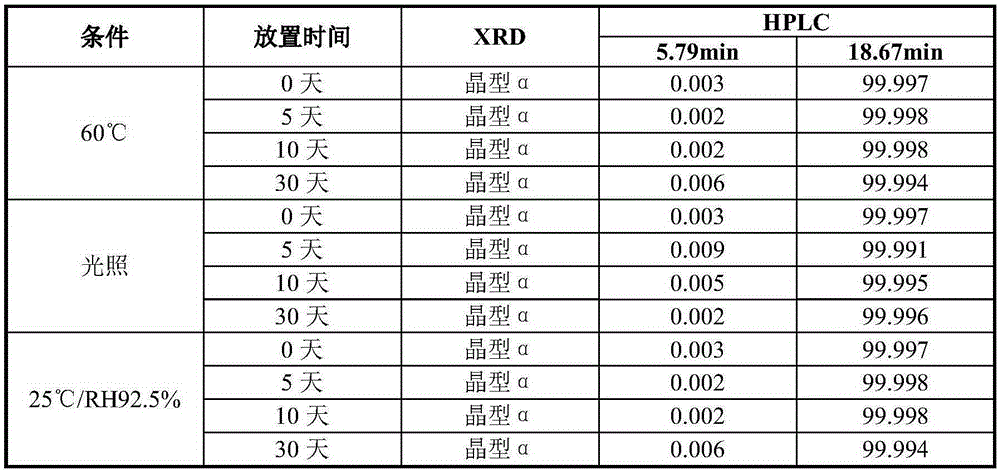
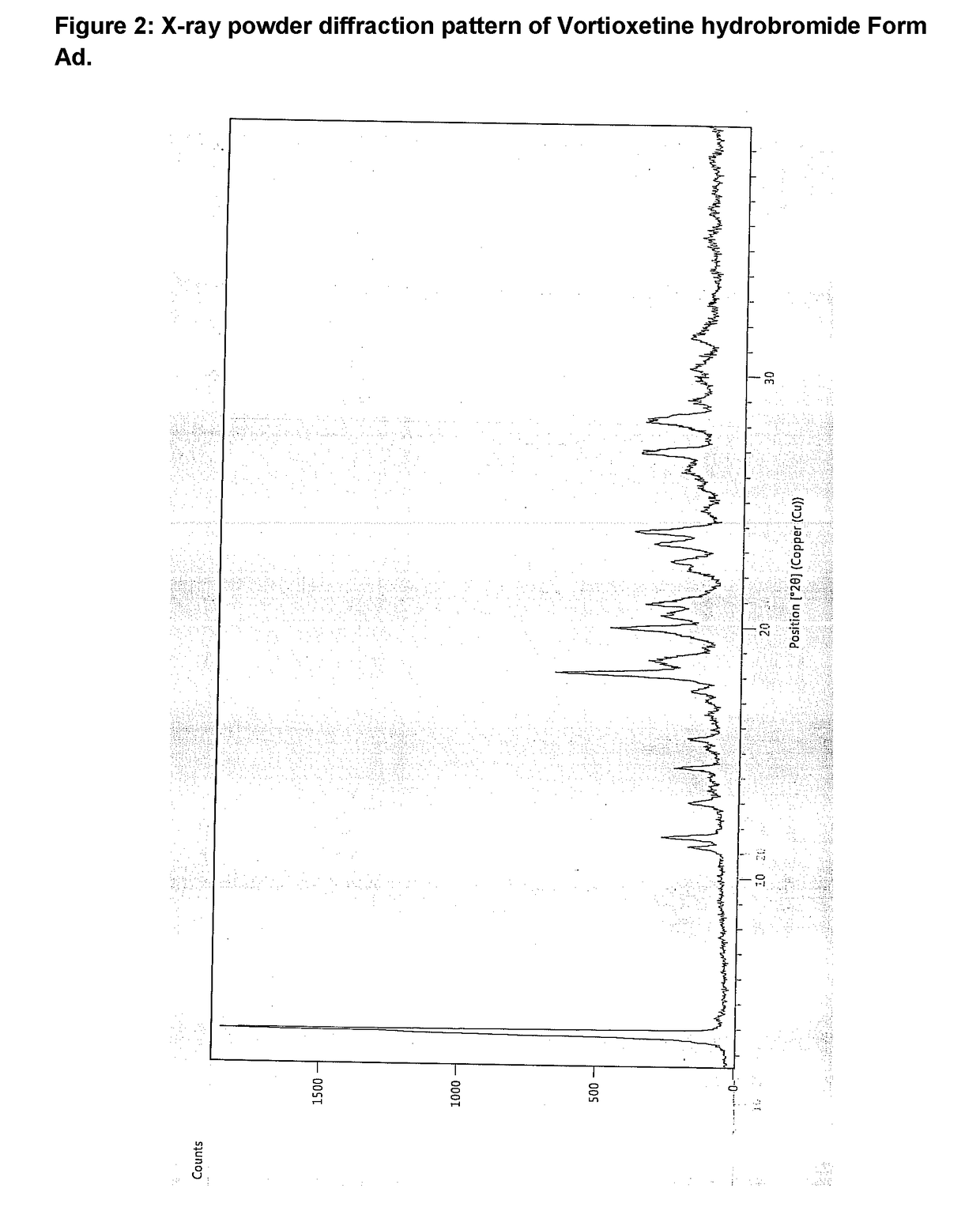
Preparing method for vortioxetine hydrobromide alpha crystal form
ActiveCN105367515AHigh crystal purityModerate granularityOrganic chemistryDesolvationSec-butyl alcohol
The invention discloses a preparing method for a vortioxetine hydrobromide alpha crystal form. The preparing method comprises the following step that sec-butyl alcohol is removed from a vortioxetine hydrobromide-sec-butyl alcohol complex to obtain the vortioxetine hydrobromide alpha crystal form. The method is low in desolvation temperature, and the prepared vortioxetine hydrobromide alpha crystal form is high in purity, suitable in particle size and suitable for industrial production.
Owner:BEIJING BEILU PHARM CO LTD
Synthesis method suitable for industrialized production of vortioxetine hydrobromide
ActiveCN104130212ASimple and fast operationMild reaction conditionsOrganic chemistryBiochemical engineeringPharmaceutical drug
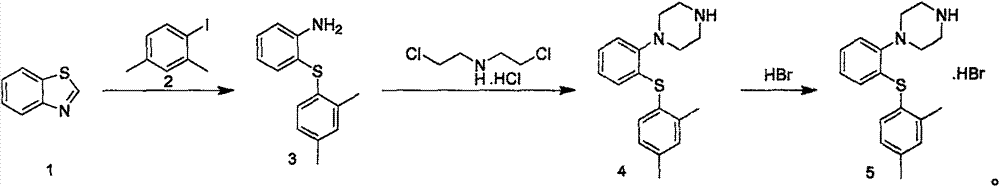
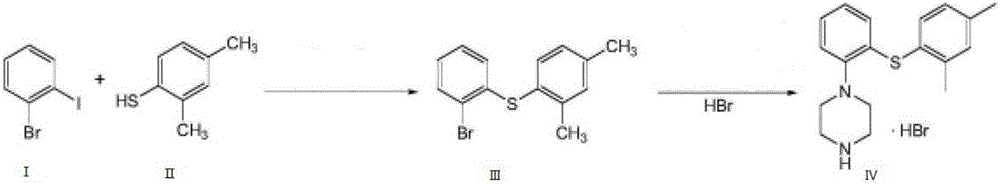
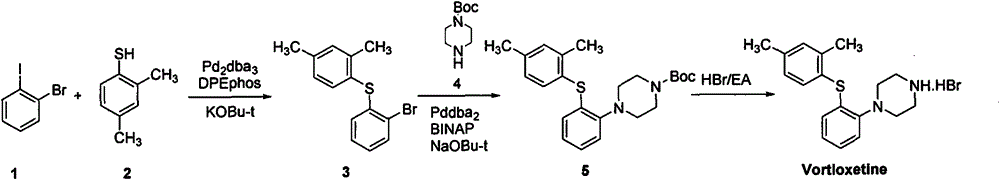
The invention provides a novel method for preparing vortioxetine hydrobromide, and belongs to the technical field of medicine synthesis. The method uses 2-fluoroaniline as a starting material to prepare the clinical medicinal vortioxetine hydrobromide by Boc protection, condensation, deprotection condensation, cyclization and other 4 steps of reaction. The method has the advantages of easy obtained raw material, low price, simple synthesis operation, mild reaction condition, easy control, no high pressure, good reaction selectivity, high yield and suitability for industrial production.
Owner:ANHUI HEALSTAR PHARM CO LTD
Vortioxetine hydrobromide crystal and preparation method thereof
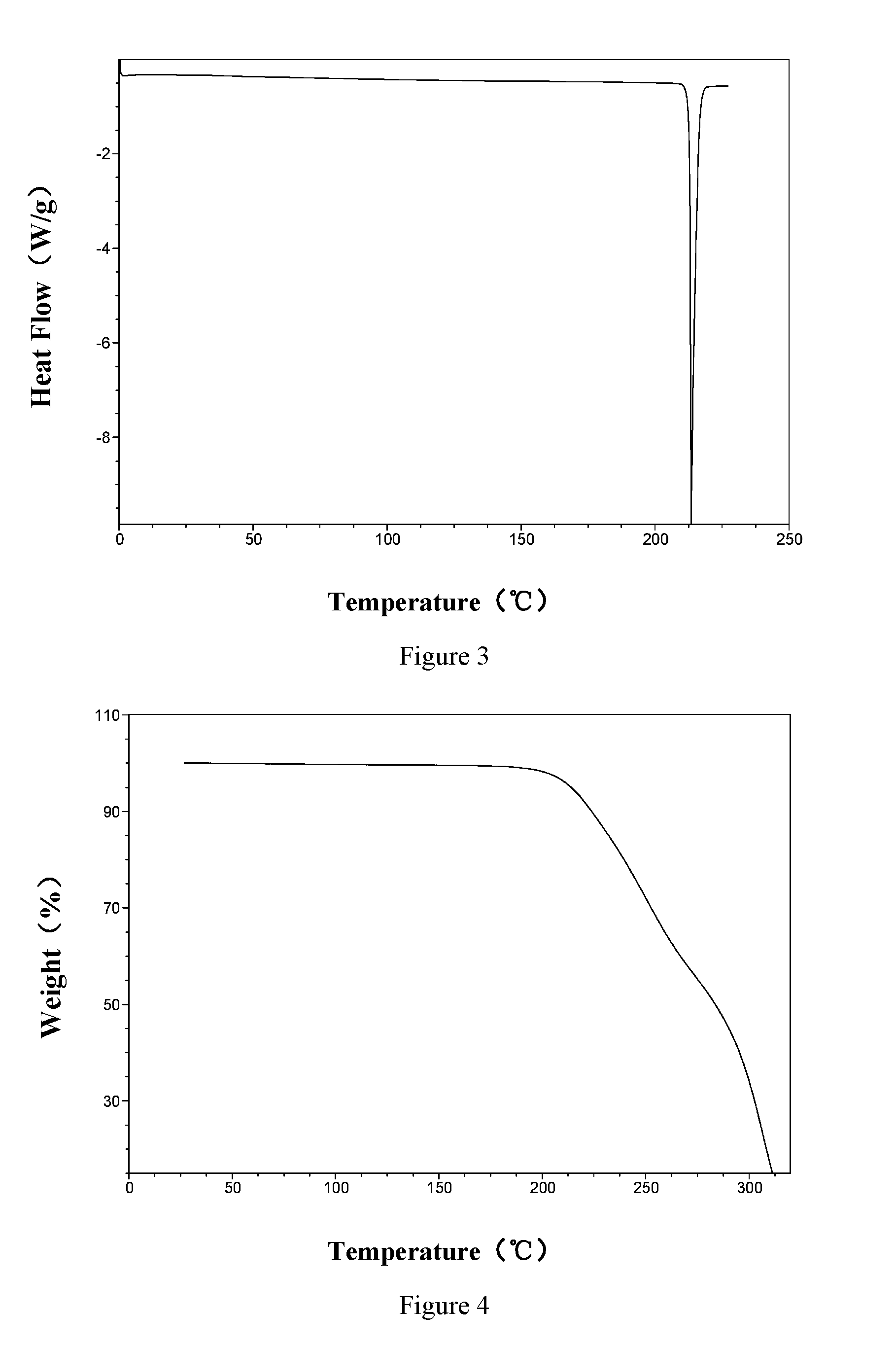
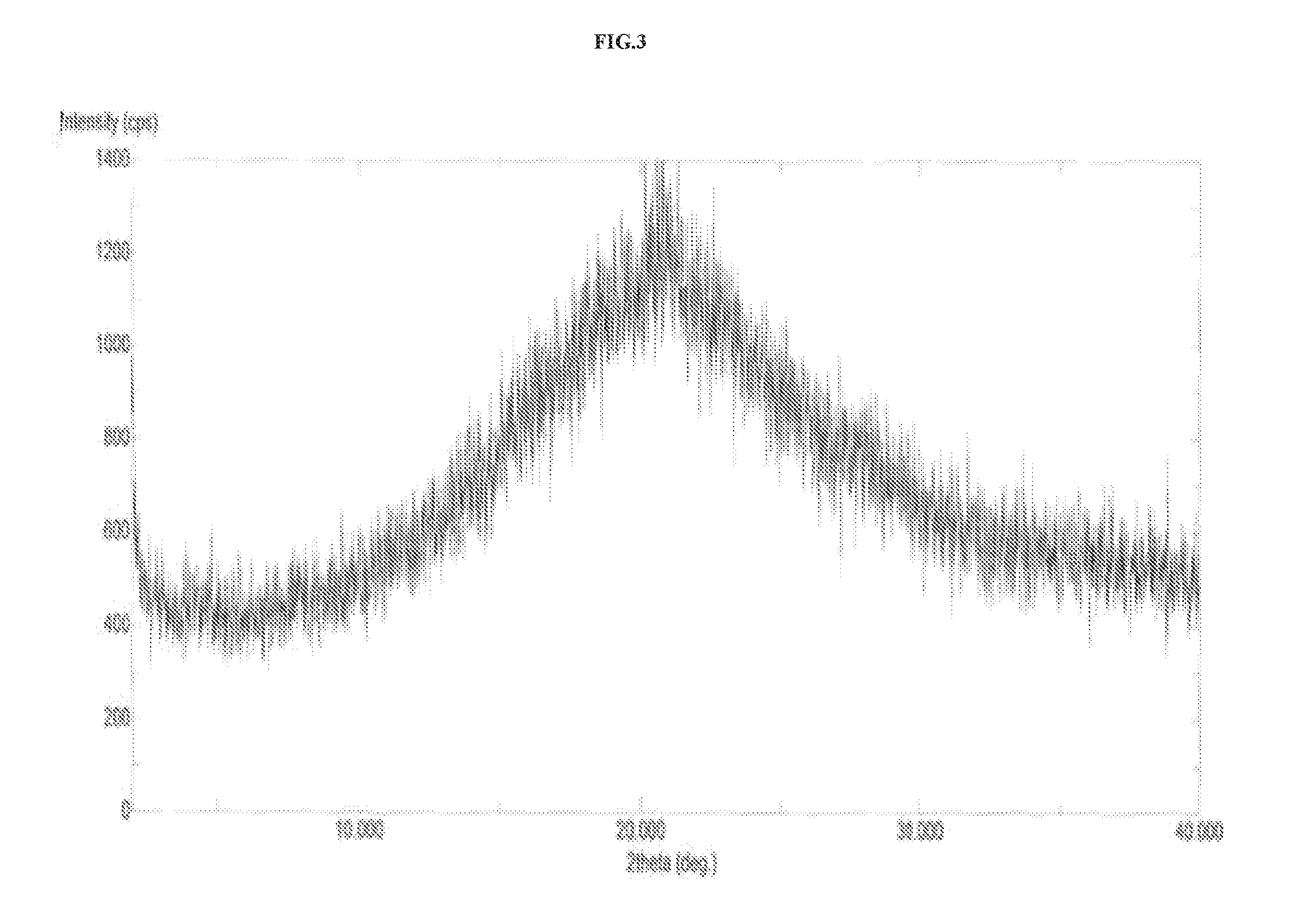
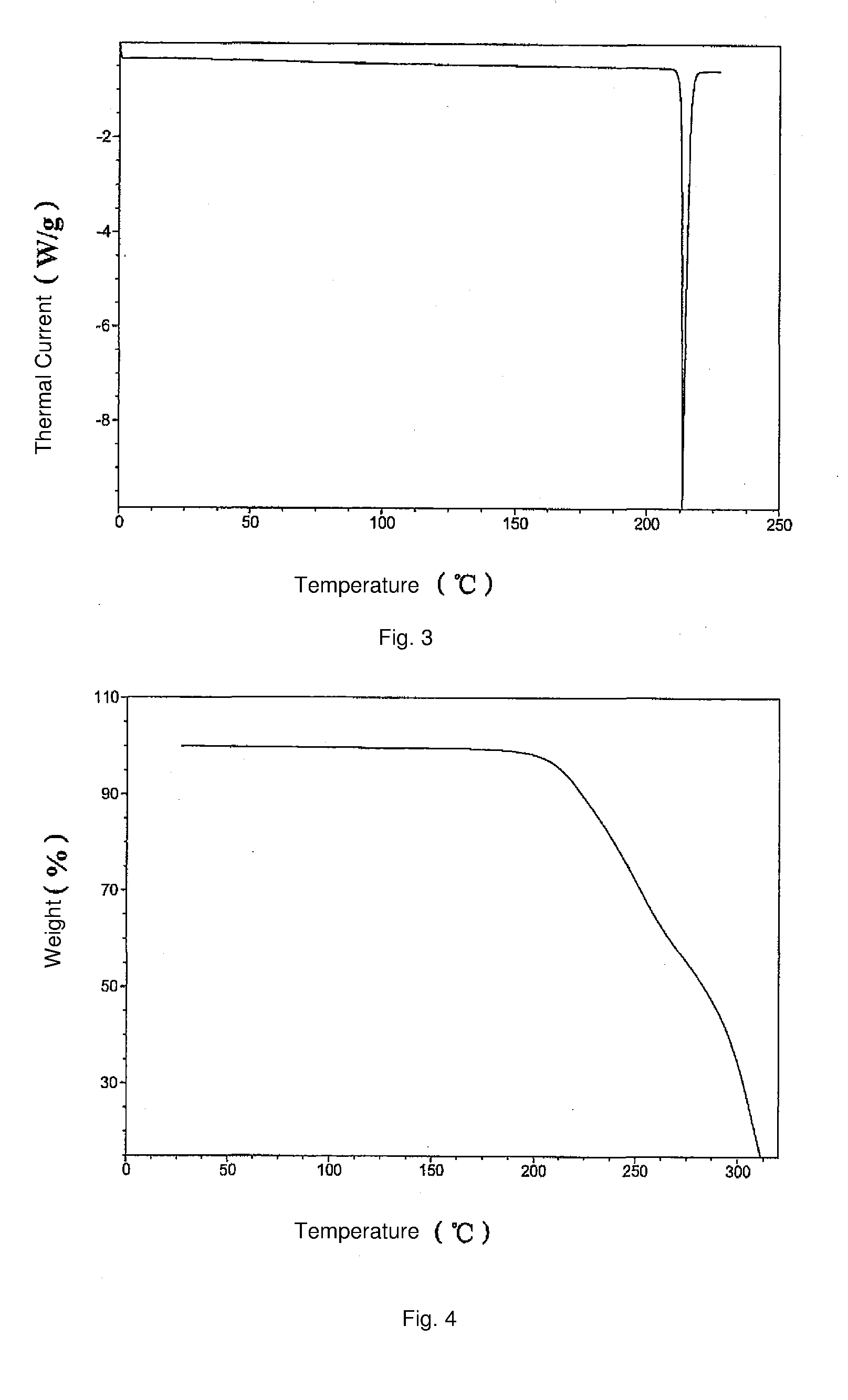
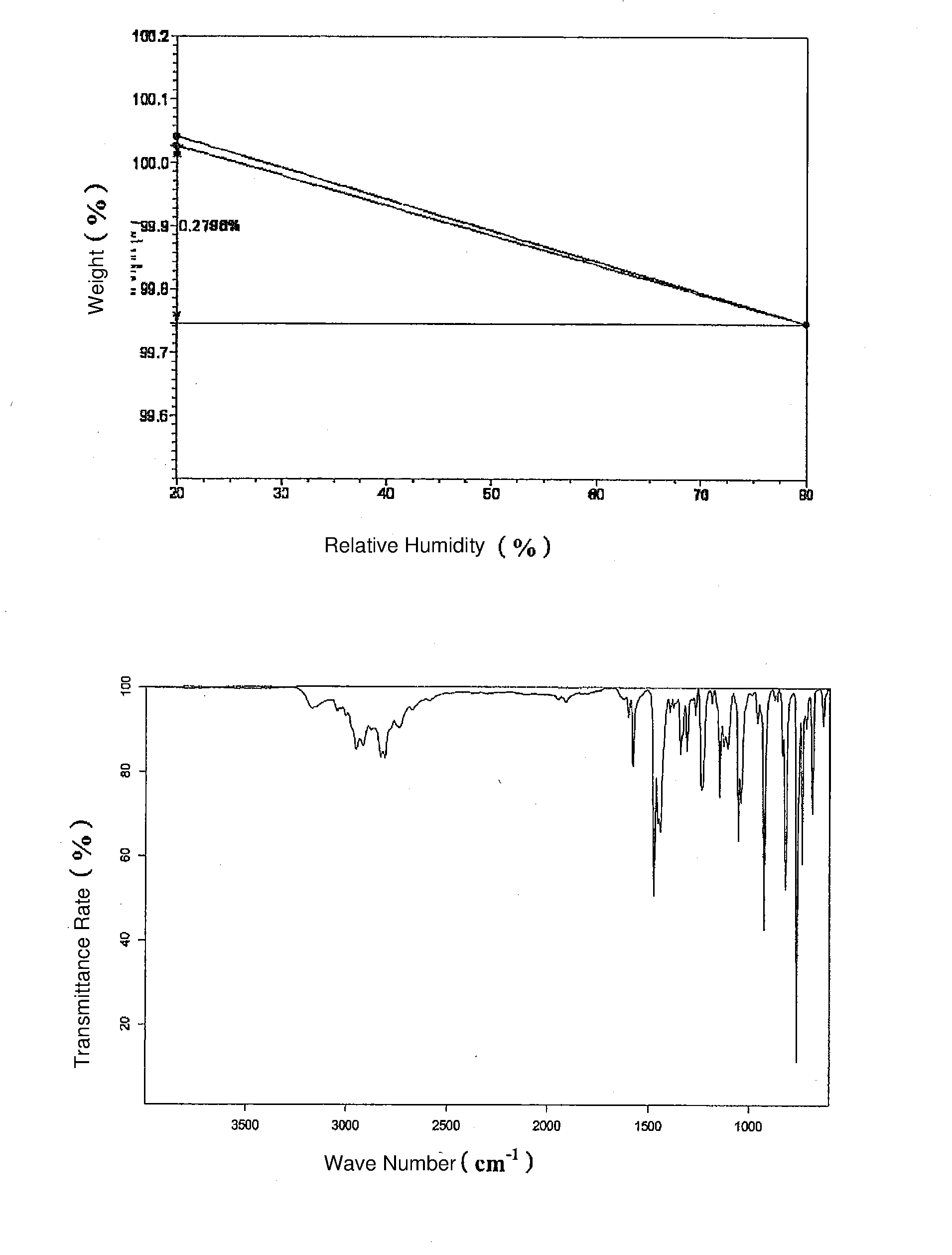
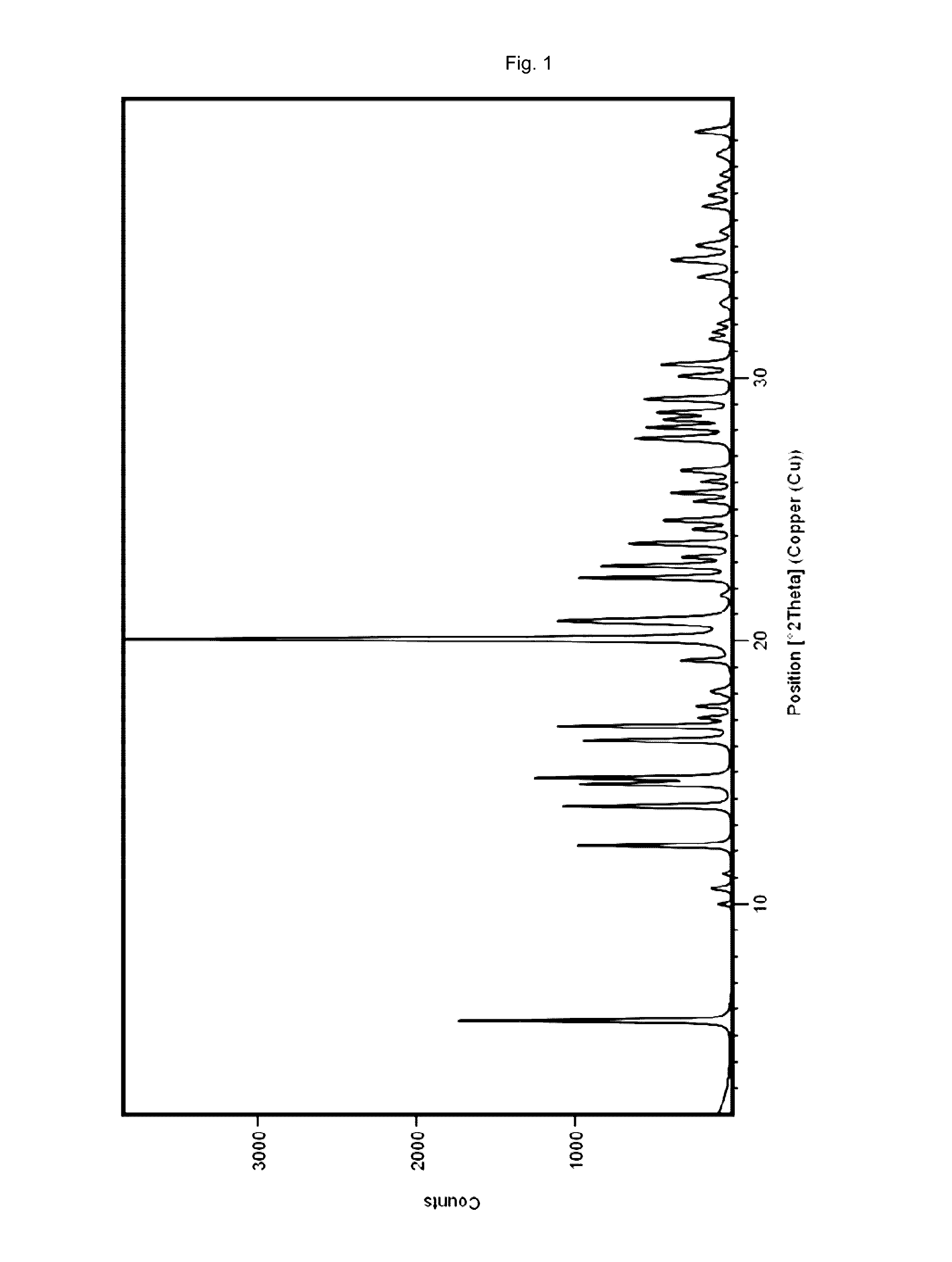
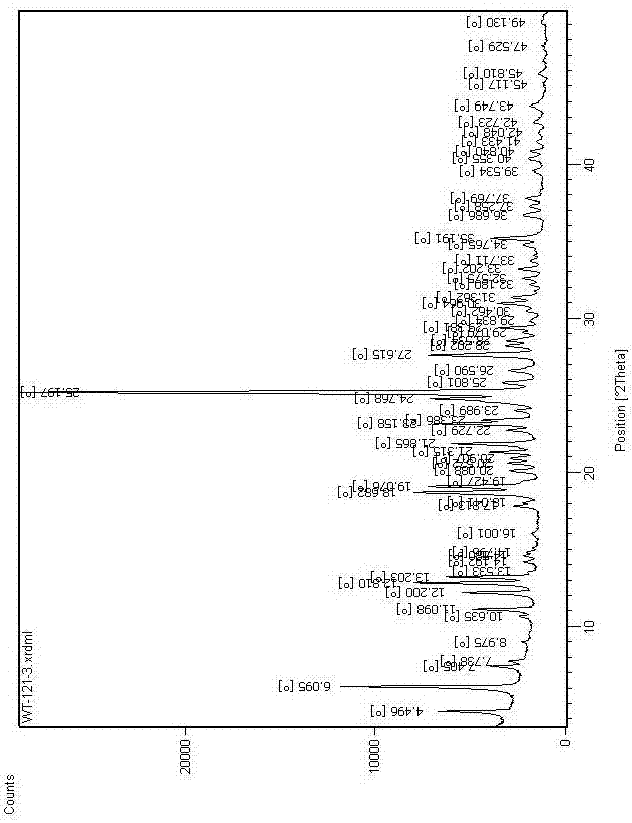
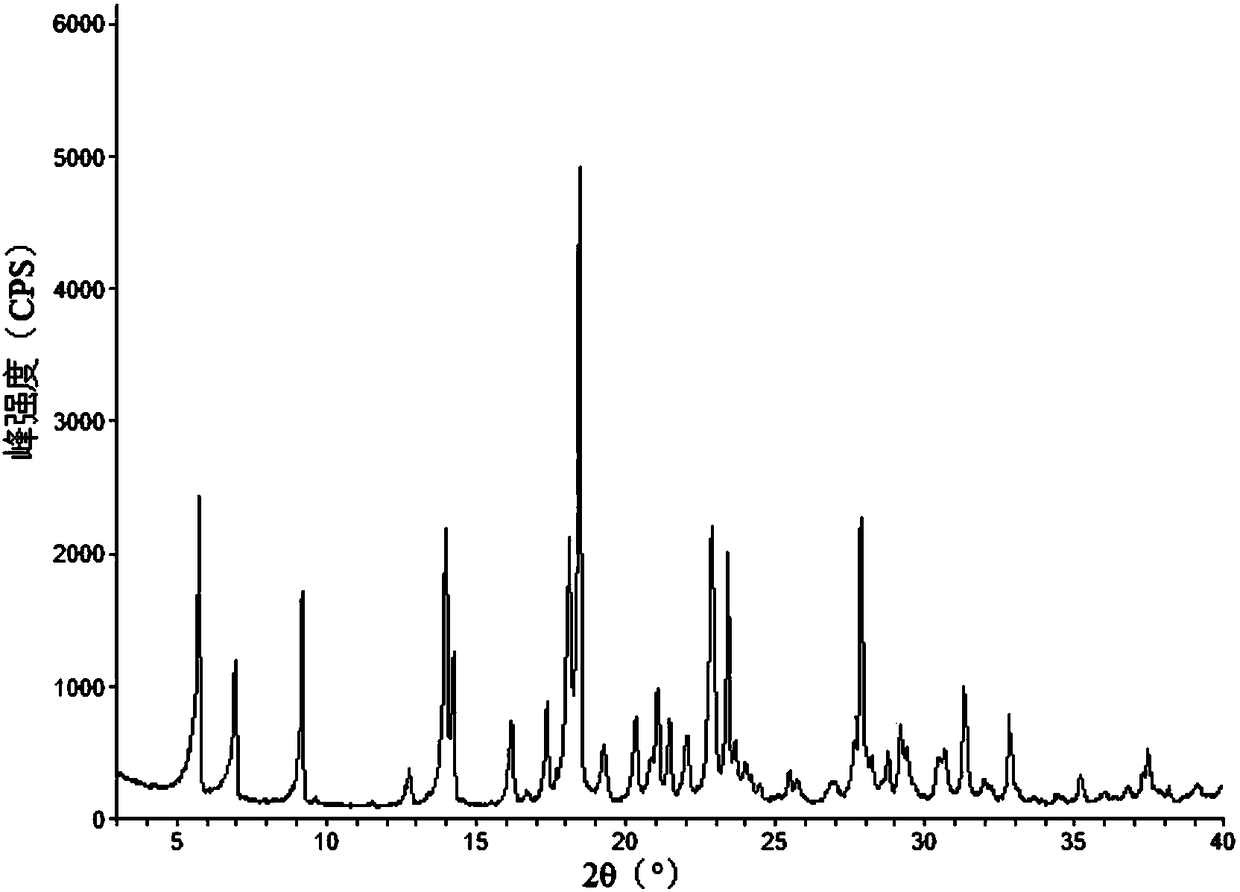
The invention discloses a vortioxetine hydrobromide crystal and a preparation method thereof. A powder X-ray diffraction pattern of the crystal shows characteristic peaks at the diffraction angle 2theta of 5.8+ / -0.2, 6.9+ / -0.2, 13.2+ / -0.2, 14.1+ / -0.2, 14.6+ / -0.2, 16.2+ / -0.2, 17+ / -0.2, 18.2+ / -0.2, 18.6+ / -0.2, 19+ / -0.2, 19.5+ / -0.2, 20.7+ / -0.2, 21.1+ / -0.2, 21.6+ / -0.2, 21.9+ / -0.2, 22.3+ / -0.2, 22.7+ / -0.2, 23+ / -0.2, 23.7+ / -0.2, 24.4+ / -0.2, 24.8+ / -0.2, 25.4+ / -0.2, 28.1+ / -0.2, 28.5+ / -0 2, and 30+ / -0.2. The vortioxetine hydrobromide crystal provided by the invention has the advantages of high purity, simple preparation method, and low hygroscopicity. The crystal is not influenced by the change of environmental humidity in raw material preparation, weighing and storage, and maintains uniform and stable quality of the final product.
Owner:SHANGHAI NEOSUN PHARMA TECH CO LTD
Vortioxetine salt and crystal thereof, their preparation method, pharmaceutical compositions and usage
ActiveUS9562024B2Improve thermal stabilityGood non-hygroscopicityOrganic active ingredientsNervous disorderHydrobromidePharmaceutical Substances
The present invention relates to the novel vortioxetine salts, solvates and crystalline forms thereof, specifically, vortioxetine hemihydrobromide and a crystalline form thereof, and isopropanol solvate of vortioxetine hydrobromide and a crystalline form thereof. Compared to the known vortioxetine hydrobromide, the vortioxetine salts, solvates and crystalline forms of the present invention have improved features in stability, hygroscopicity and purity. The present invention also relates to preparation methods of the vortioxetine salts, solvates and crystalline forms, pharmaceutical compositions thereof and their uses in the manufacture of antidepressant drugs.
Owner:NI YUN
An amorphous vortioxetine and salts thereof
The present invention relates to an amorphous vortioxetine and salts thereof. In particular, the invention relates to a process for the preparation of an amorphous vortioxetine hydrobromide. Further, the invention also relates to a process for preparation of amorphous vortioxetine free base. The invention also relates to pharmaceutical compositions comprising an amorphous vortioxetine or hydrobromide salt thereof for oral administration for treatment of major depressive disorder (MDD) and generalized anxiety disorder (GAD).
Owner:CADILA HEALTHCARE LTD
Vortioxetine salt and crystal thereof, their preparation method, pharmaceutical compositions and usage
InactiveUS20160200698A1High purity valueIncrease valueOrganic active ingredientsNervous disorderAntidepressants drugsMedicinal chemistry
The present invention relates to the novel vortioxetine salts, solvates and crystalline forms thereof, specifically, vortioxetine hemihydrobromide and a crystalline form thereof, and isopropanol solvate of vortioxetine hydrobromide and a crystalline form thereof. Compared to the known vortioxetine hydrobromide, the vortioxetine salts, solvates and crystalline forms of the present invention have improved features in stability, hygroscopicity and purity. The present invention also relates to preparation methods of the vortioxetine salts, solvates and crystalline forms, pharmaceutical compositions thereof and their uses in the manufacture of antidepressant drugs.
Owner:HANGZHOU PUSHAI PHARMA TECH
Preparation method of high-purity vortioxetine hydrobromide
ActiveCN104725335ARaw materials are easy to getProcess reaction conditions are mildOrganic chemistryChlorobenzene2-Chlorophenol
The invention discloses a preparation method of high-purity vortioxetine hydrobromide. The method comprises the following steps: firstly, synthesizing 2-(2,4-dimethyl phenyl sulfanyl) chlorobenzene from 2-chlorophenol and 2,4-dimethylbenzenethiol; then, adding di(dibenzylideneacetone)palladium, 1,1'-binaphthyl-2,2'-bis(diphenyl phosphine), sodium tert-butoxide, and methylbenzene into a reaction bottle to mix, and adding other materials so as to prepare vortioxetine; and dissolving the prepared vortioxetine by using 14-16 times of ethyl acetate, so that a vortioxetine hydrobromide coarse product is obtained; and finally, purifying the coarse product so as to obtain a vortioxetine hydrobromide fine-product. The method disclosed by the invention is easily-obtained in raw materials, mild in process reaction conditions, high in product yield, high in product purity, and convenient for industrial production. Prepared vortioxetine hydrobromide is white crystalline powder, and the purity is more than 99.5%.
Owner:郑州大明药物科技有限公司
Preparation method and applications of vortioxetine hydrobromide degradation product
The present invention provides a preparation method of a compound represented by a formula I, and applications of the compound in detection of the related substance content in vortioxetine hydrobromide. The preparation method comprises: dissolving a compound represented by a formula II in an organic acid, adding hydrogen peroxide, carrying out a reaction for 2-20 h, and treating to obtain the compound represented by the formula I. According to the present invention, the research results of the compound show that the compound can provide the reference substance for the qualitative and quantitative analysis of the vortioxetine hydrobromide impurity so as to effectively improve the quality control standard of vortioxetine hydrobromide, and ensure the safe medication. The formulas I and II are defined in the specification.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
New synthesis process for vortioxetine hydrobromide
InactiveCN107266390ARaw materials are easy to getSimple processOrganic chemistryVortioxetine HydrobromideBenzothiazole
The invention relates to a preparation method of vortioxetine hydrobromide. The method is characterized by: subjecting benzothiazole and dimethyliodobenzen to ring opening reaction under the catalysis of ferric trichloride, then cooperating with dichloroethylamine hydrochloride to generate vortioxetine, and then conducting hydrobromination so as to obtain the target product. The process provided by the invention has the advantages of easily available raw materials, concise technology, high overall yield, few by-product, and simple post-treatment, thus being suitable for industrial production.
Owner:山东鲁宁药业有限公司
Vortioxetine hydrobromide tablets and preparation method thereof
ActiveCN105193763AImprove stabilityAvoid degradationOrganic active ingredientsNervous disorderMannitolTableting
The invention relates to vortioxetine hydrobromide tablets and a preparation method thereof, and belongs to the technical field of medicine. Tablet cores of the vortioxetine hydrobromide tablets are prepared from vortioxetine hydrobromide, mannitol, calcium phosphate dibasic, sodium carboxymethyl starch and magnesium stearate. A coating solution of the vortioxetine hydrobromide tablets is prepared from hydroxypropyl methylcellulose, polyethylene glycol 400, titanium dioxide, iron oxide yellow and purified water. The preparation method includes the steps that the raw materials of the tablet cores are screened, evenly mixed, directly subjected to tabletting and then subjected to coating. After the tablets are subjected to gastric-soluble film coating, the bitter taste of the tablets can be well avoided when the tablets are taken orally, and therefore compliance is improved. A film coating layer contains titanium dioxide, shading can be well achieved, it is avoided that the tablets are degraded under illumination, and therefore the stability of the tablets is improved.
Owner:KAMP PHARMA
A kind of preparation method of vortioxetine hydrobromide crystal
ActiveCN104910099BComply with medicinal requirementsHigh yieldOrganic chemistry methodsHydrobromideThermal insulation
The invention discloses a vortioxetine hydrobromide crystal preparation method. The method comprises a, dissolving vortioxetine free alkali in ethyl acetate at a temperature of 20-30 DEG C, b, carrying out filtration, cooling the filtrate to a temperature of 0-10 DEG C, dropwisely adding an ethyl acetate solution of hydrobromic acid into the filtrate along with thermal insulation and then carrying out thermal insulation stirring for 2-8h, c, filtering the mixture subjected to thermal insulation stirring in the step b to obtain filter cake 1, leaching the filter cake 1 by ethyl acetate, and carrying out stirring washing in ethyl acetate at a temperature of 0-10 DEG C for 0.5-5h, d, filtering the mixture subjected to stirring washing in the step c to obtain filter cake 2, leaching the filter cake 2 by methyl tert-butyl ether / ethyl acetate pre-cooled at a temperature of 0-10 DEG C and carrying out stirring washing in methyl tert-butyl ether at a temperature of 10-30 DEG C for 15-24h, and e, filtering the mixture subjected to stirring washing in the step d to obtain filter cake 3, leaching the filter cake 3 by methyl tert-butyl ether and carrying out vacuum drying at a temperature of 40-50 DEG C to obtain the product. The method has the advantages of good repeatability, simple processes, a high yield and high product purity and is suitable for industrial production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for separation and determination of vortioxetine hydrobromide intermediate related substances by liquid chromatography
InactiveCN106568862AEfficient separationQuality is easy to controlComponent separationBenzeneHydrobromide
The invention belongs to the field of analytical chemistry, discloses a method for separation and determination of vortioxetine hydrobromide intermediate 1-bromo-2-iodo-benzene related substances by liquid chromatography. The method takes a chromatographic column with octadecylsilane bonded silica gel as a packing, takes a certain ratio of water-organic phase as a mobile phase, and can be used for quantitative determination of the contents of a vortioxetine hydrobromide intermediate and the related substances thereof, so as to effectively control the quality of the vortioxetine hydrobromide intermediate and ensure controllable quality of a vinpocetine hydrobromide final product. The method has the advantages of strong specificity, high accuracy and convenience in operation.
Owner:万全万特制药江苏有限公司
Novel method for preparation of vortioxetine hydrobromide crystal form alpha
ActiveCN106632145AImprove stabilityImprove machinabilityOrganic chemistry methodsHydrobromideChemical industry
The invention relates to a novel method for preparation of a vortioxetine hydrobromide crystal form alpha. The method includes steps: dissolving vortioxetine hydrobromide into a good solvent, dropwise adding to a poor solvent at a low temperature, and crystallizing to obtain the crystal form alpha. The novel method has advantages of high yield, high purity, low energy consumption, simplicity in operation, safety, environment friendliness, high repeatability and the like and is especially suitable for large-scale chemical production in the pharmaceutical and chemical industry.
Owner:JIANGSU HANSOH PHARMA CO LTD
Crystalline forms of an antidepressant compound
ActiveUS20160015706A1Organic active ingredientsNervous disorderMedicinal chemistryVortioxetine Hydrobromide
The present invention relates to novel crystalline forms of vortioxetine hydrobromide, in particular three crystalline forms, a process for their preparation, a pharmaceutical composition containing said novel crystalline forms, and a process for the purification of vortioxetine or a salt thereof, comprising the formation of one or more of the novel crystalline forms of vortioxetine hydrobromide described herein.
Owner:DIPHARMA FRANCIS
Synthetic method for vortioxetine hydrobromide
ActiveCN105348220AMild reaction conditionsRaw materials are easy to getOrganic chemistryNitrobenzeneVortioxetine Hydrobromide
The invention provides a synthetic method for vortioxetine hydrobromide. Firstly, 2-nitro thiophenol and 2,4-dimethyl iodobenzene are reacted, an intermediate 2-(2,4-dimethyl phenyl sulfenyl) nitrobenzene, the intermediate is subjected to reduction, 2-(2,4-dimethyl phenyl sulfenyl) phenylamine is prepared, then 2-(2,4-dimethyl phenyl sulfenyl) phenylamine and bis(2-chloroethyl)amine hydrochloride are subjected to a cyclization reaction, vortioxetine is prepared, finally, vortioxetine is reacted with hydrobromic acid and is salified, and vortioxetine hydrobromide is prepared. The total yield of vortioxetine hydrobromide can reach 65%, the method has advantages of cheap and easily available initial raw materials and simple synthetic technology, and meets requirements of large-scale industrial production.
Owner:山东川成医药有限公司
Preparation method of vortioxetine hydrobromide
InactiveCN105985301ALow priceMild reaction conditionsOrganic chemistrySandmeyer reactionAfter treatment
The invention relates to the technical field of preparation of vortioxetine hydrobromide, in particular to a preparation method of vortioxetine hydrobromide. The preparation method comprises the following steps: taking 2,4-dimethyl thiophenol as a raw material to react with o-bromonitrobenzene so as to generate a compound (IV); treating the compound (IV) via a normal pressure catalytic hydrogenation method to obtain a compound (V); treating the compound (V) via a Sandmeyer reaction to obtain a compound (VI); and reacting a compound (VII) with piperazine, and then performing a reaction with hydrobromic acid to generate a salt, thereby obtaining a target compound (I). The method for preparing vortioxetine hydrobromide is relatively short in route, relatively mild in reaction condition, simple, convenient and feasible in after treatment, and more suitable for industrial production requirements.
Owner:山东康美乐医药科技有限公司
Preparation method of orally disintegrating tablet containing vortioxetine hydrobromide
InactiveCN109589314AGreat tasteFast absorptionOrganic active ingredientsNervous disorderSide effectOrally disintegrating tablet
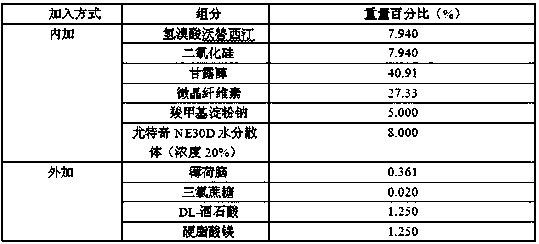
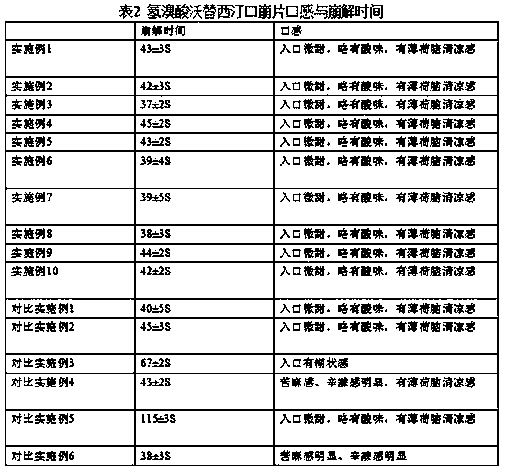
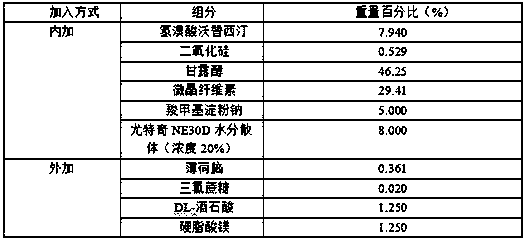
The invention provides a preparation method of an orally disintegrating tablet containing vortioxetine hydrobromide. The method comprises the steps of performing combined taste masking on vortioxetinehydrobromide by silicon dioxide and Eudragit NE30D, and then performing wet granulation to form the orally disintegrating tablet. The application of the method aims at improving the taste of the orally disintegrating tablet, overcoming the problem of unbearable bitter and numb feeling caused by crude drugs or intake of an excess of carbohydrate caused by application of an excess of sweetening agents for taste modifying, meanwhile endowing rapid disintegration time to the orally disintegrating tablet, and providing the orally disintegrating tablet containing vortioxetine hydrobromide which isfast to absorb, high in bioavailability, low in side effect and convenient to take for patients.
Owner:万全万特制药(厦门)有限公司
Novel polymorphic forms of vortioxetine and its pharmaceutically acceptable salts
ActiveUS20170189394A1Improve stabilityEasy to prepareOrganic active ingredientsNervous disorderVortioxetine HydrobromideMedicinal chemistry
The present invention provides polymorphic forms of Vortioxetine of and its pharmaceutically acceptable salts. Specifically the present invention relates to the novel crystalline forms of Vortioxetine or its pharmaceutically acceptable salts. Moreover, the present invention also provides an amorphous form of Vortioxetine hydrobromide and a stable amorphous co-precipitate of Vortioxetine hydrobromide with pharmaceutically acceptable excipients.
Owner:ALEMBIC PHARMA
Synthetic method and application of vortioxetine hydrobromide
InactiveCN106380455ASolve the problem of competing side effectsHigh yieldOrganic chemistryChemical synthesisSide reaction
The invention relates to the technical field of medicinal chemical synthesis, and concretely relates to a synthetic method of vortioxetine hydrobromide. The method comprises the following steps: carrying out a reaction on compounds comprising o-bromoiodobenzene and 2,4-dimethylthiophenol in environment containing a catalyst, an inorganic alkali and a protic solvent to obtain 2-(2,4-dimethylthiophenyl)bromobenzene; and coupling 2-(2,4-dimethylthiophenyl)bromobenzene with piperazine in environment containing a catalyst, an organic alkali and an aprotic solvent, and carrying out salt formation on the obtained coupling product and hydrobromic acid to prepare the vortioxetine hydrobromide. Compared with the prior art, the method disclosed in the invention has the advantages of solving of double-halogen competition side reactions, great reduction of generation of byproducts, high total yield, good product purity, simplicity in process operation, and suitableness for amplification and industrial production.
Owner:合肥美利康医药技术股份有限公司
Refining and crystal transformation method for vortioxetine hydrobromide
InactiveCN106279065ASuitable for industrial productionOrganic chemistry methodsInorganic saltsToluene
The invention provides a refining and crystal transformation method for vortioxetine hydrobromide, belonging to the technical field of synthesis of chemical medicines. According to the invention, a novel recrystallization solvent system is used for safe and high-efficient preparation of beta-type vortioxetine hydrobromide. The method provided by the invention overcomes the problems of toluene solvent residues, inorganic salt residues and the like in the prior art; the obtained beta-type vortioxetine hydrobromide has chemical purity and crystal form purity meeting medicinal needs; and the method is suitable for industrial production.
Owner:BEIJING SHENLANHAI BIO PHARM TECH
Crystalline form of vortioxetine hydrobromide
ActiveUS9499504B2Suitable for preparationOrganic active ingredientsNervous disorderHydrobromideCrystallography
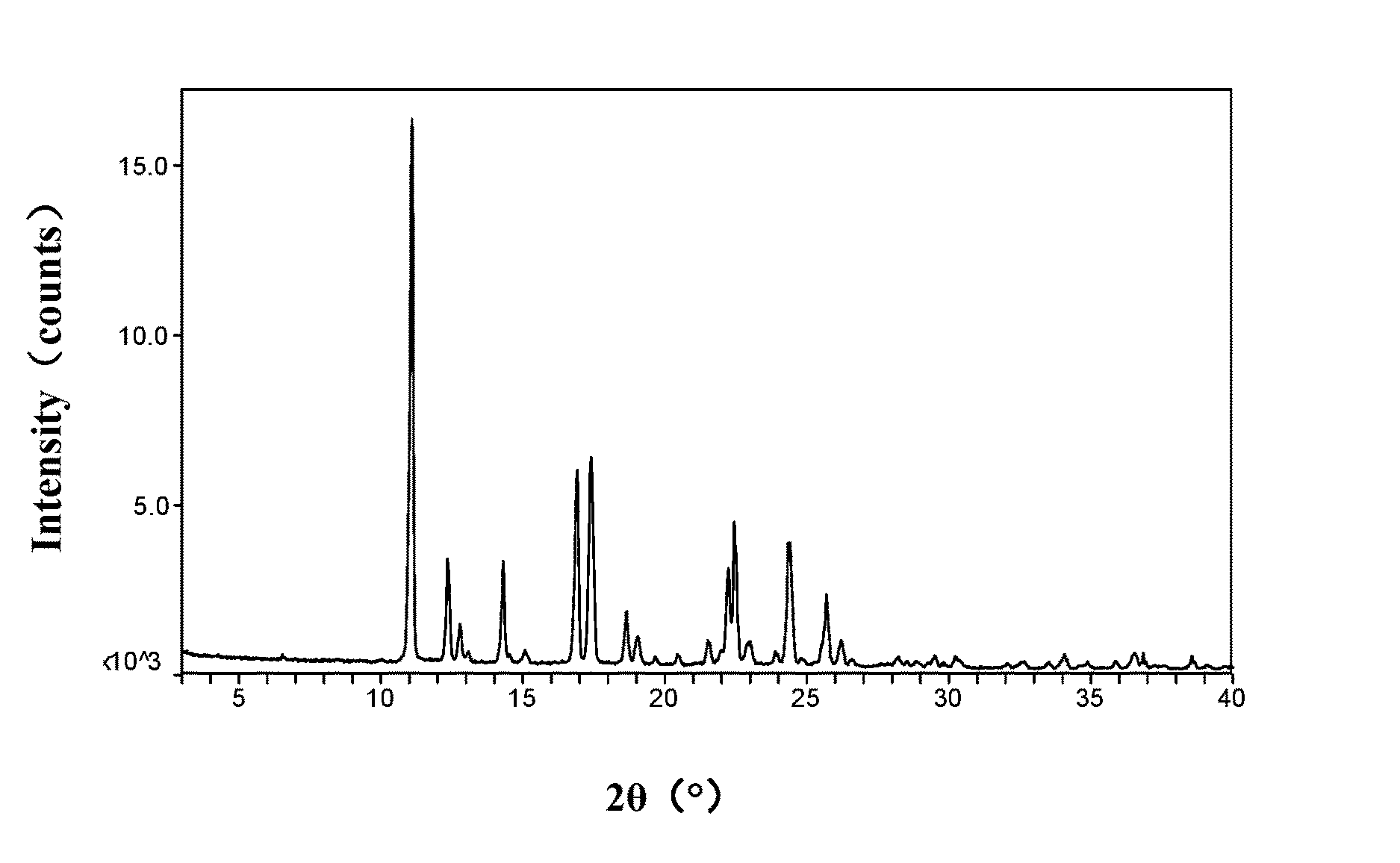
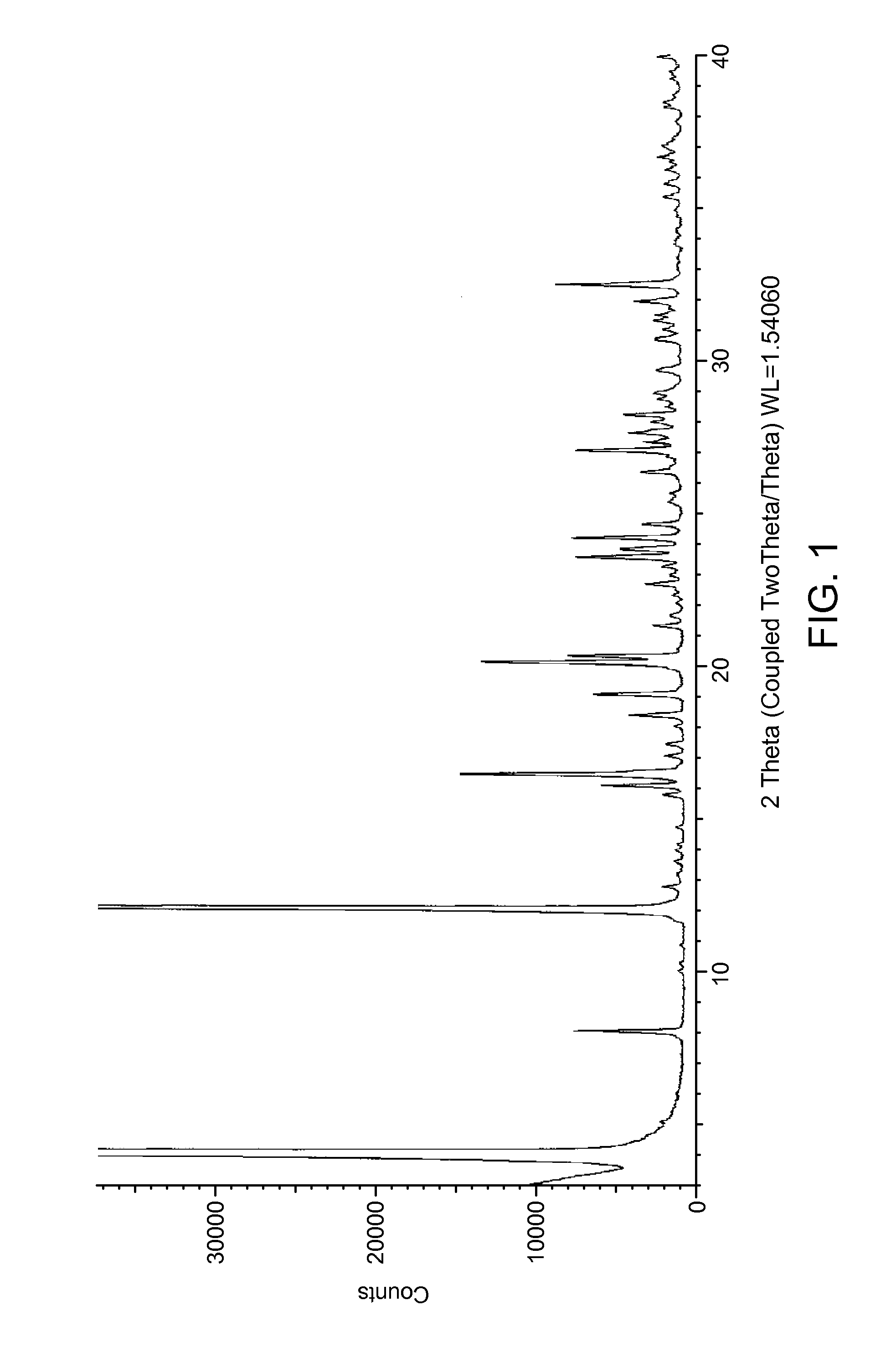
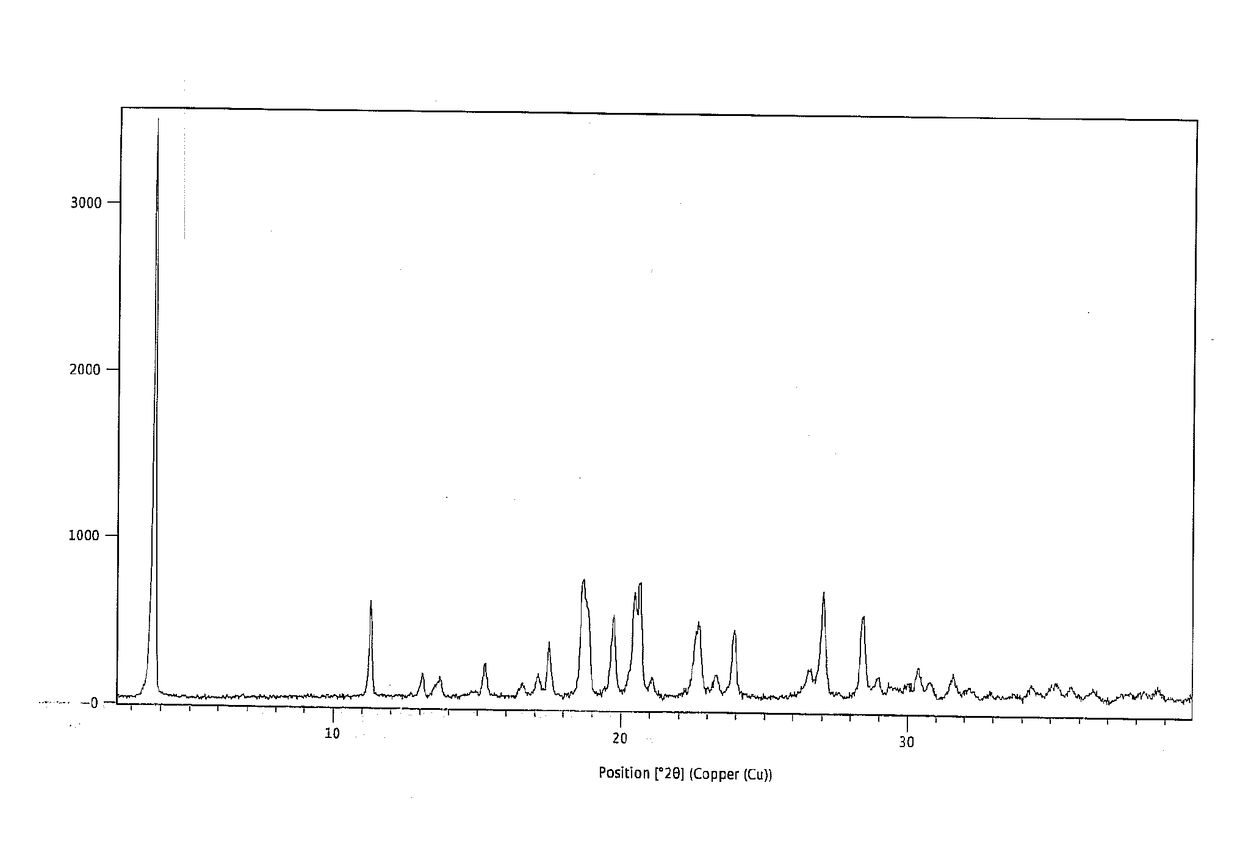
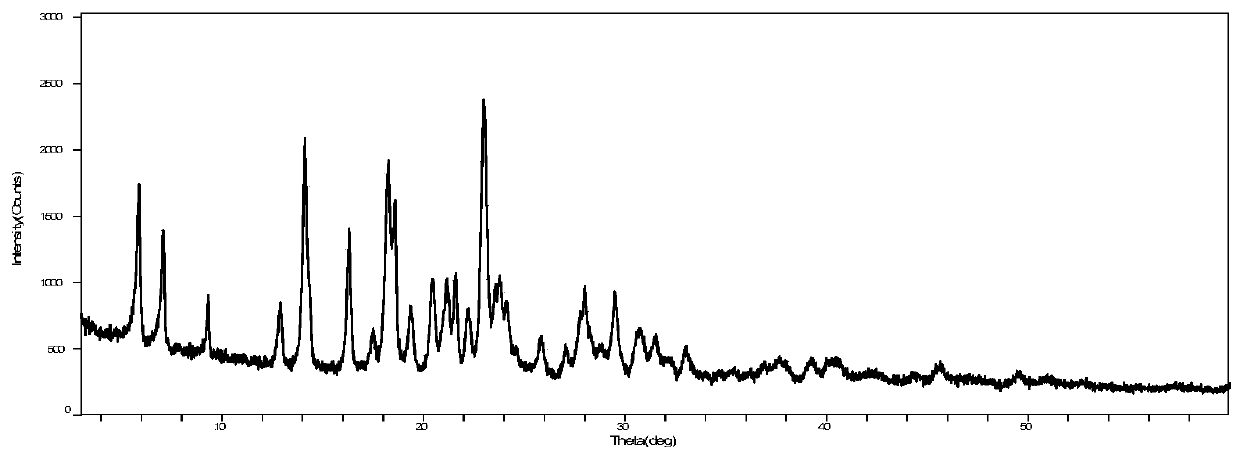
The present invention is directed to a crystalline compound comprising a hydrobromide acid (HBr) salt of a compound of formula (I) (1-{2-[(2,4-dimethylphenyl)sulfanyl]phenyl}piperazine, INN: vortioxetine), having an XRPD pattern with characteristic peaks (expressed in 2θ±0.2° 2θ (CuKα radiation)) at 5.5°, 14.8°, 16.7° and 20.0° and processes for obtaining the same.
Owner:SANDOZ LTD
Novel medicinal composition containing amorphous vortioxetine hydrobromide and preparation method of novel medicinal composition
InactiveCN107638425ASave energyIncrease surface free energyOrganic active ingredientsPowder deliveryAdjuvantMedicine
The invention relates to a medicinal composition containing vortioxetine hydrobromide. The medicinal composition contains a solid dispersion (formed by vortioxetine hydrobromide and an organic carrier), at least one adsorbent, and at least one medicinal adjuvant, wherein vortioxetine hydrobromide is amorphous, in the X-ray powder diffraction spectrum of the composition, after the background peak of the organic carrier, the adsorbent and the medicinal adjuvant is deducted, the characteristic peak of crystal of vortioxetine hydrobromide does no exist. The invention further relates to a preparation method of the medicinal composition of the amorphous vortioxetine hydrobromide. The medicinal composition of vortioxetine hydrobromide provided by the invention is good in stability and dispersibility, the dissolution rate of vortioxetine hydrobromide is increased, the bioavailability of the medicinal preparation and the absorption of the body for the medicine can be improved, and under the condition of acceleration test, good physical stability and chemical stability can be kept. The preparation method provided by the invention is easy to operate, low in cost, good in reproducibility, easyto realize, and suitable for industrial production.
Owner:SHANGHAI FANGNAN PHARMA
Vortioxetine hydrobromide crystalline form C and preparation method thereof
The invention discloses a novel crystalline form C of vortioxetine hydrobromide hemihydrate, and discloses a preparation method and a pharmaceutical composition of the crystal form C. The powder diffraction pattern of the novel crystalline form C of the vortioxetine hydrobromide hemihydrate contains characteristic peaks at diffraction angles, expressed by 2[theta] + / - 0.20 degrees, of 4.50, 6.10,7.74, 11.10, 12.20, 19.08, 25.20 and 27.62. The novel crystalline form provided by the invention has good solubility, and stable physical and chemical properties, and is suitable for various forms ofpreparations; and the preparation process of the crystalline form is simple, convenient for transformation of industrial scales, and suitable for industrialized production.
Owner:BEIJING LUNARSUN PHARMA
Preparation method of alpha crystal form of vortioxetine hydrobromide
InactiveCN108069924AHigh purityHigh crystallinityOrganic chemistry methodsHydrobromideVortioxetine Hydrobromide
The invention discloses a preparation method of an alpha crystal form of vortioxetine hydrobromide. The alpha crystal form, obtained with the method, of vortioxetine hydrobromide has high purity, goodcrystallinity, high stability and particle uniformity. The preparation process is simple, good in repeatability, high in yield and suitable for industrial production.
Owner:ZHEJIANG JINGXIN PHARMA
Crystal form of vortioxetine hydrobromide and preparation method thereof
InactiveCN111087365ACrystal stableNo crystal changeOrganic chemistry methodsPhysical chemistryVortioxetine Hydrobromide
The invention provides a crystal form of vortioxetine hydrobromide, and also relates to a preparation method for the crystal form. The crystal form of the vortioxetine hydrobromide provided by the invention has good physicochemical stability and purity, facilitates large-scale production, is simple to operate, and has wide application prospect.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Detection method and application of vortioxetine hydrobromide and related substances thereof
ActiveCN111693629AAchieve quality controlEfficient separationComponent separationPhosphoric acidGradient elution
The invention relates to the technical field of pharmaceutical analysis, in particular to a detection method and application of vortioxetine hydrobromide and related substances thereof. The detectionmethod vortioxetine hydrobromide and related substances thereof comprises the following steps: detecting a test solution by adopting high performance liquid chromatography, wherein the detection conditions of the chromatography are as follows: the detection wavelength is 202-210 nm, the mobile phase A is a phosphoric acid aqueous solution, and the mobile phase B is acetonitrile and / or methanol; and carrying out certain gradient elution by adopting the mobile phase A and the mobile phase B. According to the detection method, vortioxetine hydrobromide and nine related substances can be efficiently separated at a time, good specificity, repeatability and accuracy are achieved on the basis that efficient separation of all the related substances and effective components is guaranteed, and quality control over vortioxetine hydrobromide related preparations is better achieved.
Owner:SHANXI ZHENDONG PHARMA
Vortioxetine hydrobromide preparation method
InactiveCN105061364ASolve the problem of competing side effectsHigh yieldOrganic chemistryOrganic basePalladium catalyst
The invention provides a vortioxetine hydrobromide preparation method. The vortioxetine hydrobromide preparation method comprises the following steps: a reaction is performed between o-bromoiodobenzene and 2,4-dimethylbenzenethiol in a protonic solvent under the action of a cuprous catalyst, glycol and inorganic base to obtain 2-(2,4-dimethylthiophenyl) bromobenzene (a compound IV); the compound IV in an aprotic solvent is coupled with piperazine under the action of a palladium catalyst, a phosphine ligand and organic base; finally, salifying is performed with hydrobromic acid to obtain vortioxetine hydrobromide. Compared with the prior art, the vortioxetine hydrobromide preparation method has the advantages that competition side reactions of dual halogens are avoided, by-products are reduced, the total yield and product purity are high, and the process operation is simple, and suitable for amplification and industrialized production.
Owner:HEBEI GUOLONG PHARMA CO LTD
Preparation method of vortioxetine hydrobromide impurity
InactiveCN109438391ARaw materials are cheap and easy to getObvious cost advantageOrganic chemistryVortioxetine HydrobromidePiperazine
The invention discloses a preparation method of a vortioxetine hydrobromide impurity. The preparation method comprises the steps as follows: piperazine and o-bromoiodobenzene are subjected to a condensation reaction under the action of an acid binding agent and a catalyst, wherein when the molar ratio of piperazine to o-bromoiodobenzene is 1:(1-1.5), 2-bromophenylpiperazine is obtained through thecondensation reaction; when the molar ratio of piperazine to o-bromoiodobenzene is 1:(2-3), 1,4-bis(2-bromophenyl)piperazine is obtained through the condensation reaction. The synthesis route is short, raw materials are cheap and easy to obtain, the operation is simple, the reaction condition is mild, and a product has high purity and yield.
Owner:合肥创新医药技术有限公司
Method for determining cvortioxetine intermediate and its isomers by using liquid chromatography
InactiveCN110873760AEfficient separationSolving Separation Assay ProblemsComponent separationFluid phasePhysical chemistry
The invention belongs to the field of analytical chemistry. The invention discloses an analysis method for separating and determining a vortioxetine hydrobromide intermediate and its isomers by usingliquid chromatography. The method uses a chromatographic column filled with phenyl bonded silica gel as a filler and a certain proportion of a buffer salt solution-organic phase as a mobile phase, andcan quantitatively determinethe content of the vortioxetine hydrobromide intermediate and isomers thereof so that the mass of a vortioxetine hydrobromide starting material is effectively controlled,side reactions are reduced, and therefore it is guaranteed that the quality of a vortioxetine hydrobromide final product is controllable. The method is strong in specificity, high in accuracy and simple and convenient to operate.
Owner:万全万特制药(厦门)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com