Preparation method and applications of vortioxetine hydrobromide degradation product
A technology of vortioxetine hydrobromide and a determination method, applied in the field of medicine, can solve problems such as complicated operation, inability to meet quality research, long reaction steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
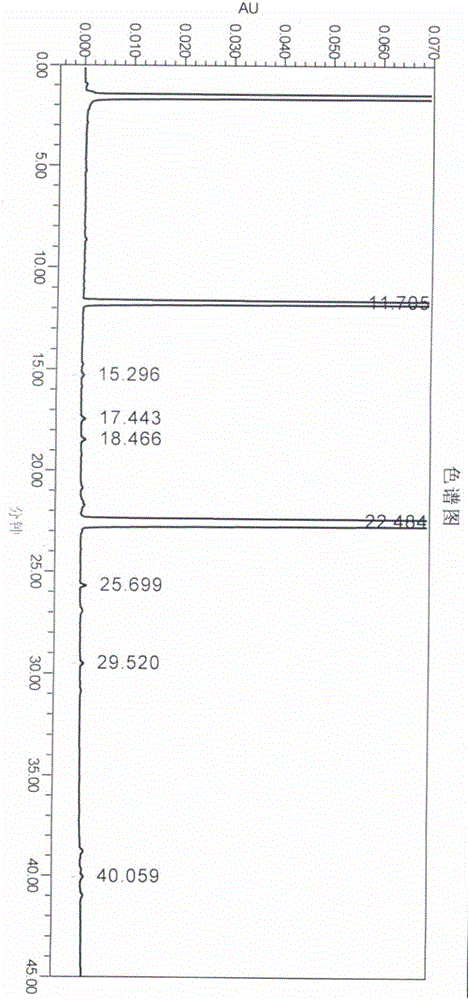
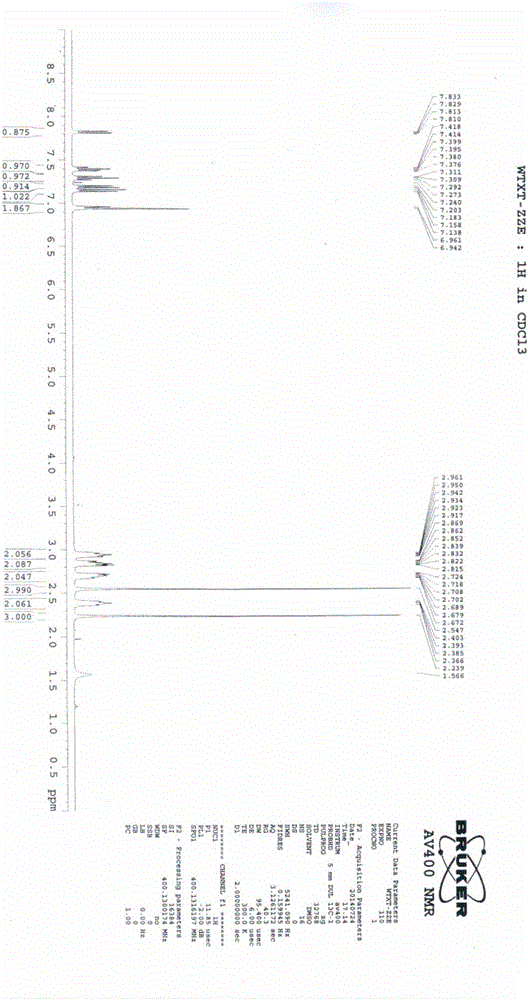
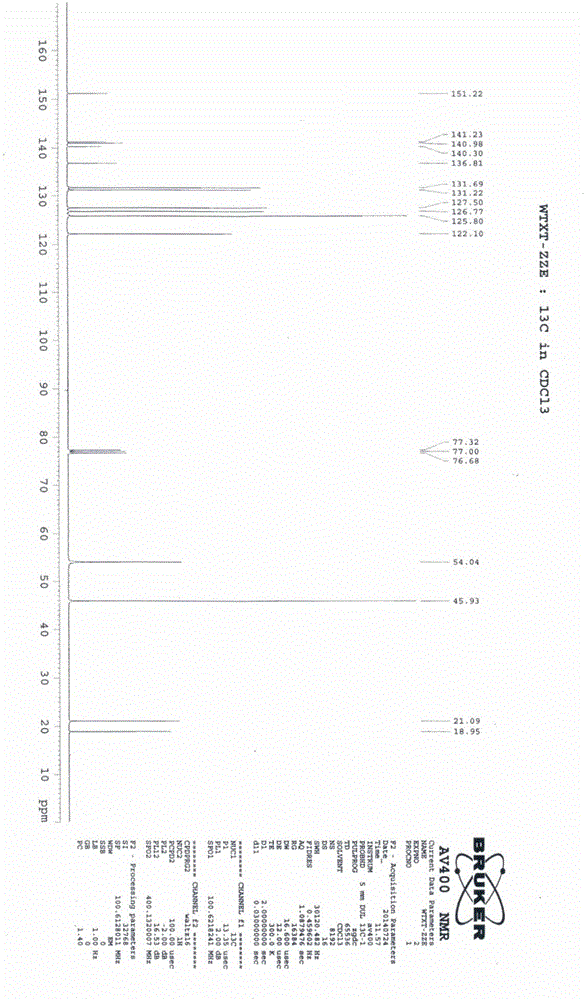
[0065] This example is used to illustrate the preparation of the compound of formula I of the present invention.
[0066] Add the compound of formula II (15g, 0.05mol) and 180ml of formic acid into a 500ml four-necked reaction flask, stir to dissolve, add hydrogen peroxide (17.1g, 0.15mol), react at 20-30°C for 6 hours, add 300ml of ammonia water dropwise, and stir for 1 After 1 hour, the crude product was obtained by filtration, and the crude product was refined with 100 ml of ethyl acetate to obtain 13.3 g of a white solid with a yield of 84.2% and an HPLC content of 99.5%.
Embodiment 2
[0068] This example is used to illustrate the preparation of the compound of formula I of the present invention.
[0069] Add the compound of formula II (15g, 0.05mol) and 180ml of acetic acid into a 500ml four-necked reaction flask, stir to dissolve, add hydrogen peroxide (11.4g, 0.1mol), react at 20-30°C for 9 hours, add 300ml of ammonia water dropwise, and stir for 1 hours, the crude product was obtained by filtration, and the crude product was purified with 100 ml of ethyl acetate to obtain 12.8 g of a white solid with a yield of 81% and an HPLC content of 99.8%.
Embodiment 3
[0071] This example is used to illustrate the preparation of the compound of formula I of the present invention.
[0072] Add the compound of formula II (15g, 0.05mol) and 180ml of trifluoroacetic acid into a 500ml four-necked reaction flask, stir to dissolve, add hydrogen peroxide (34g, 0.3mol), react at 20-30°C for 4 hours, add 300ml of ammonia water dropwise, and stir After 1 hour, the crude product was obtained by filtration, and the crude product was purified with 100 ml of ethyl acetate to obtain 13 g of a white solid with a yield of 82.3% and an HPLC content of 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com