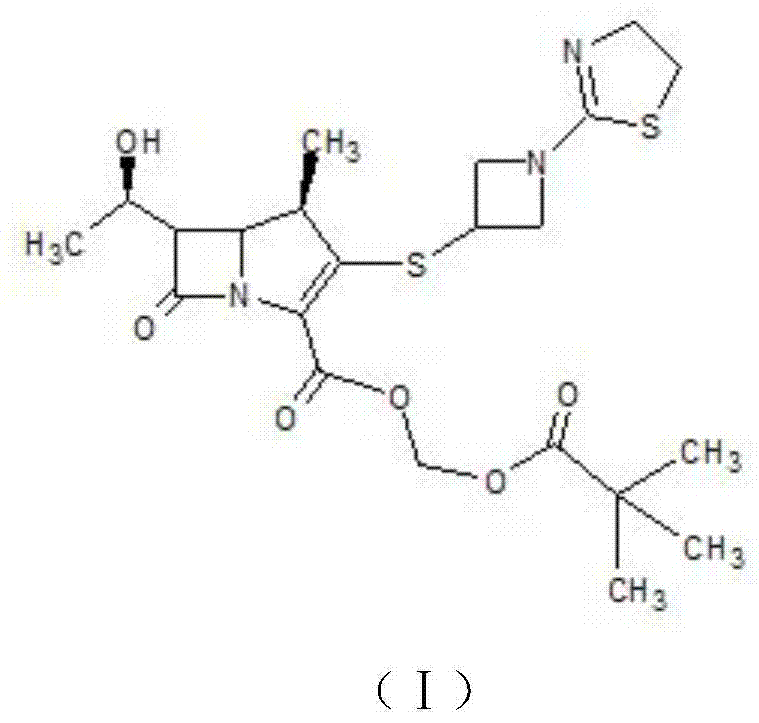
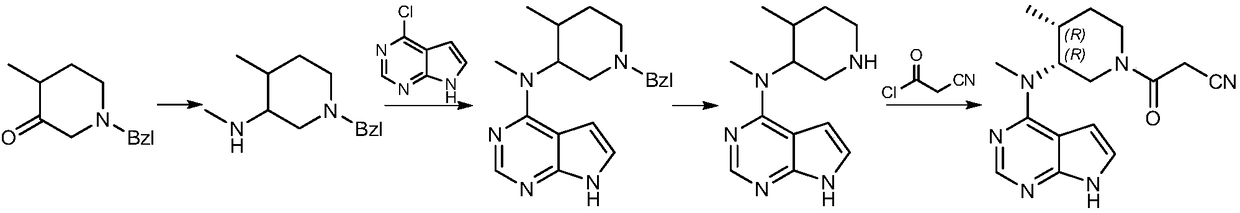
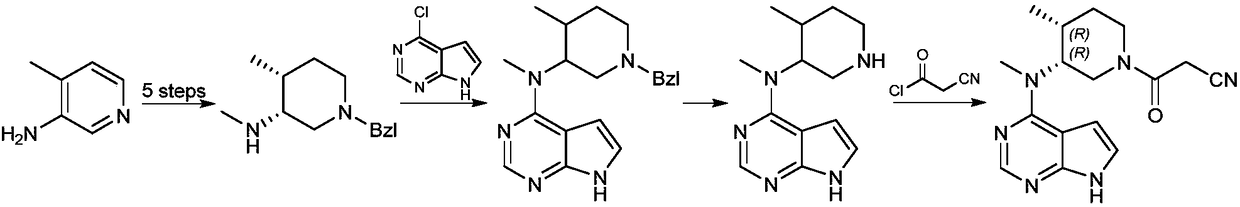
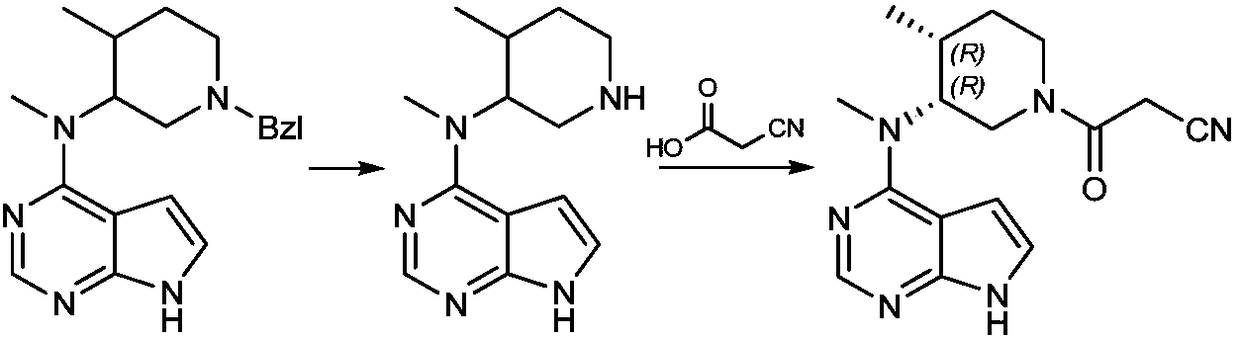
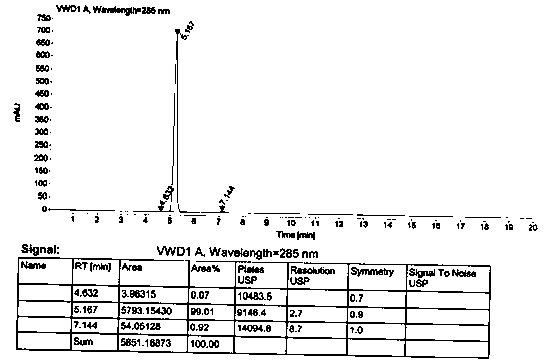
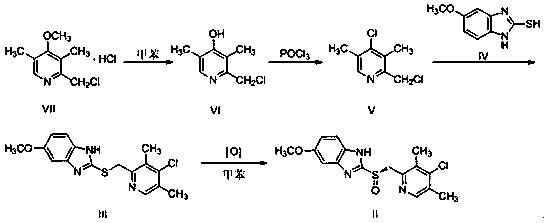
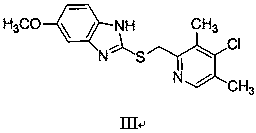
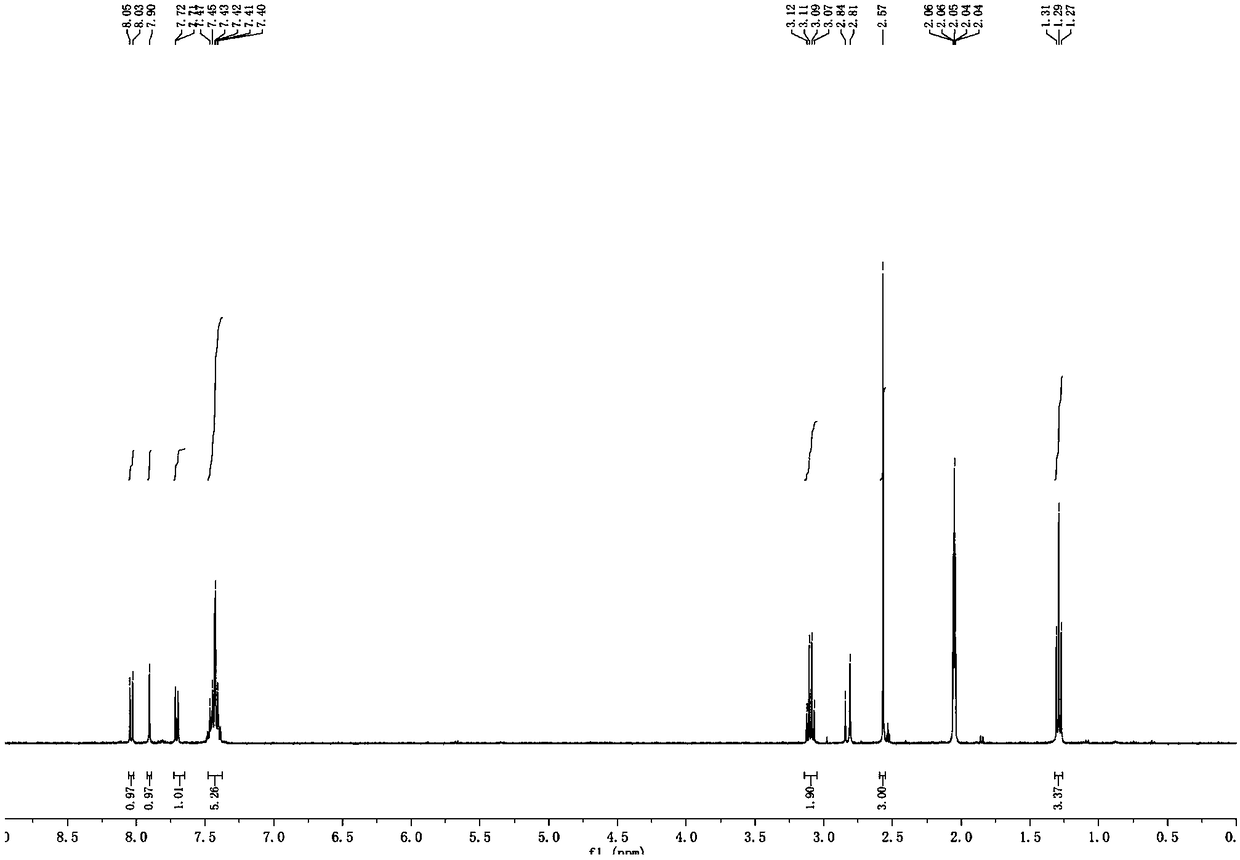
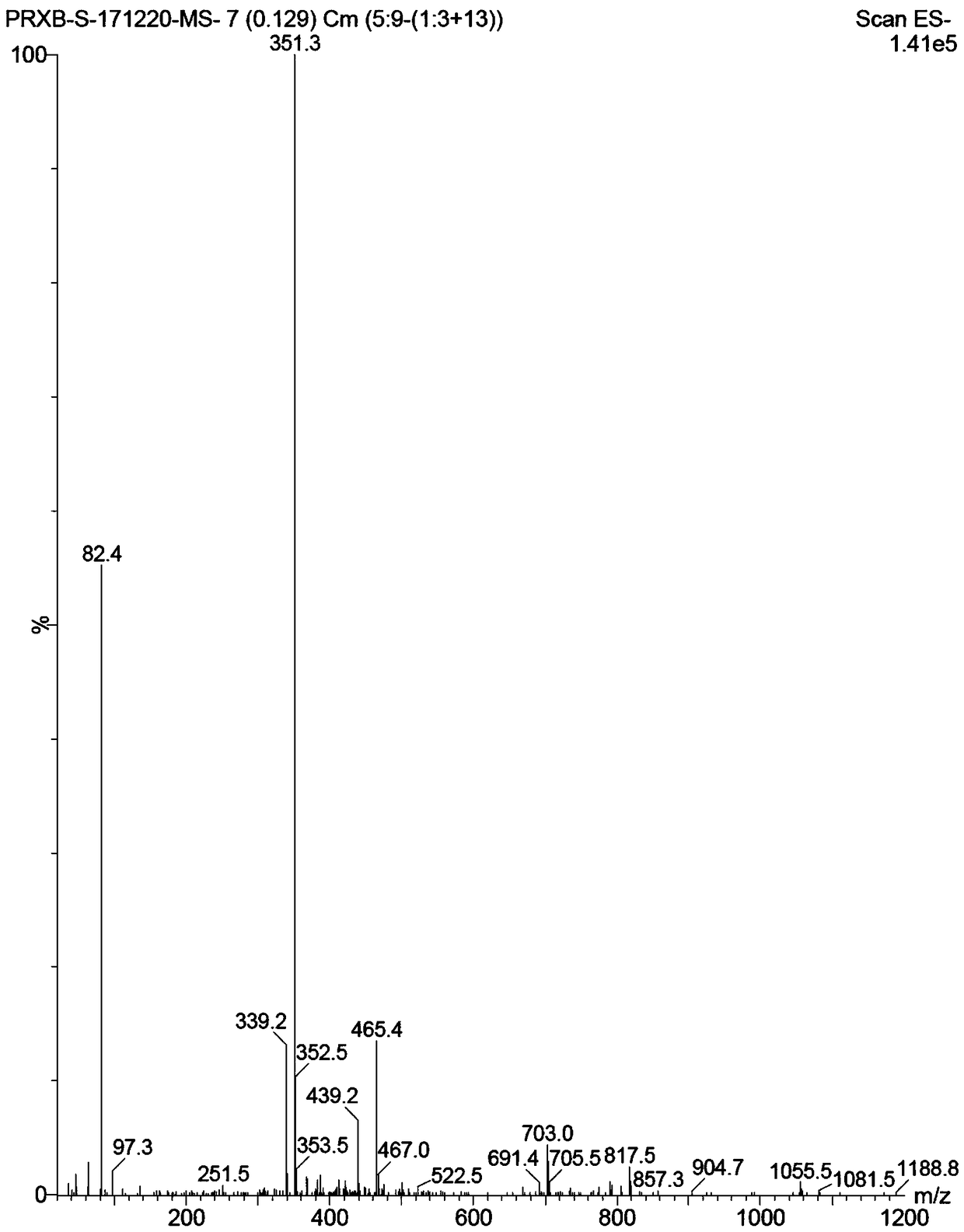
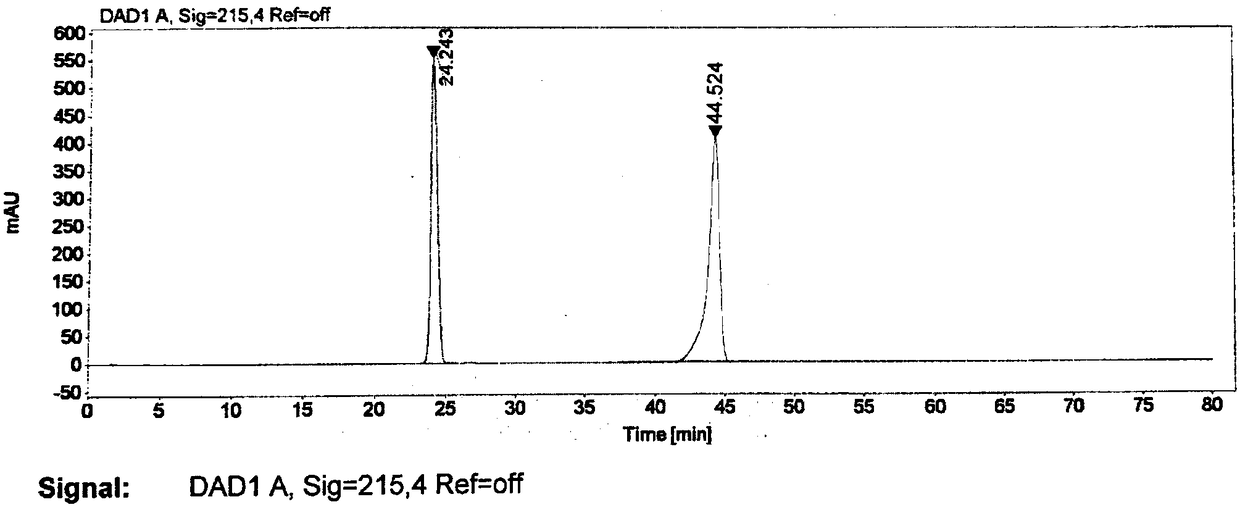
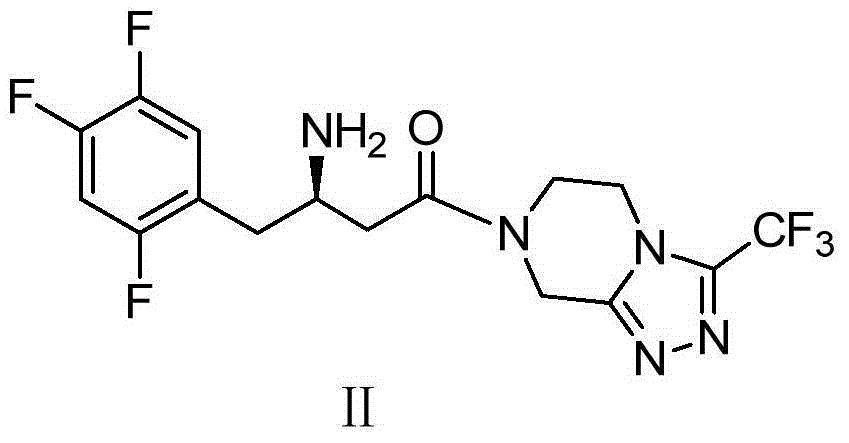
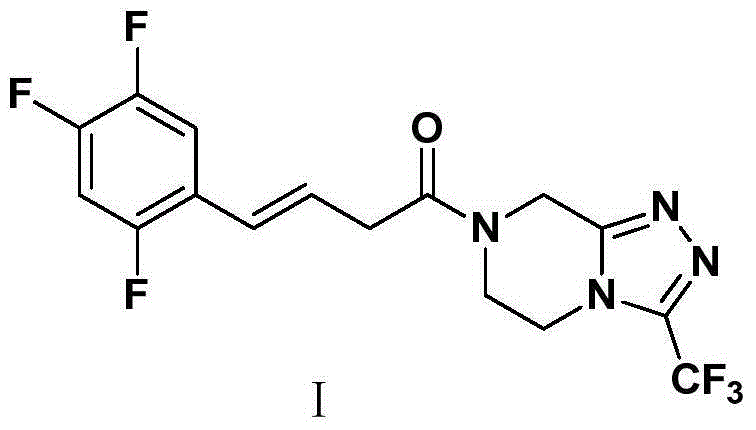
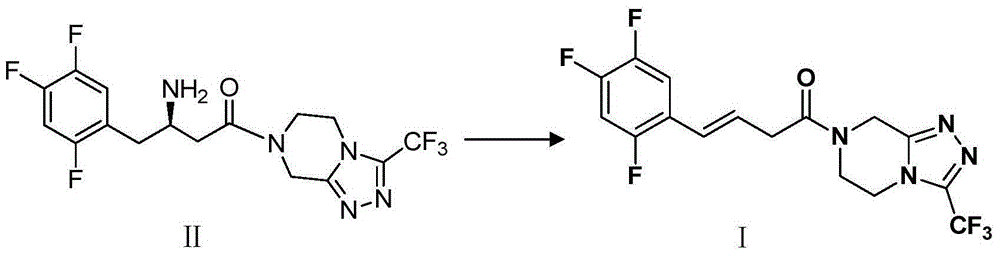
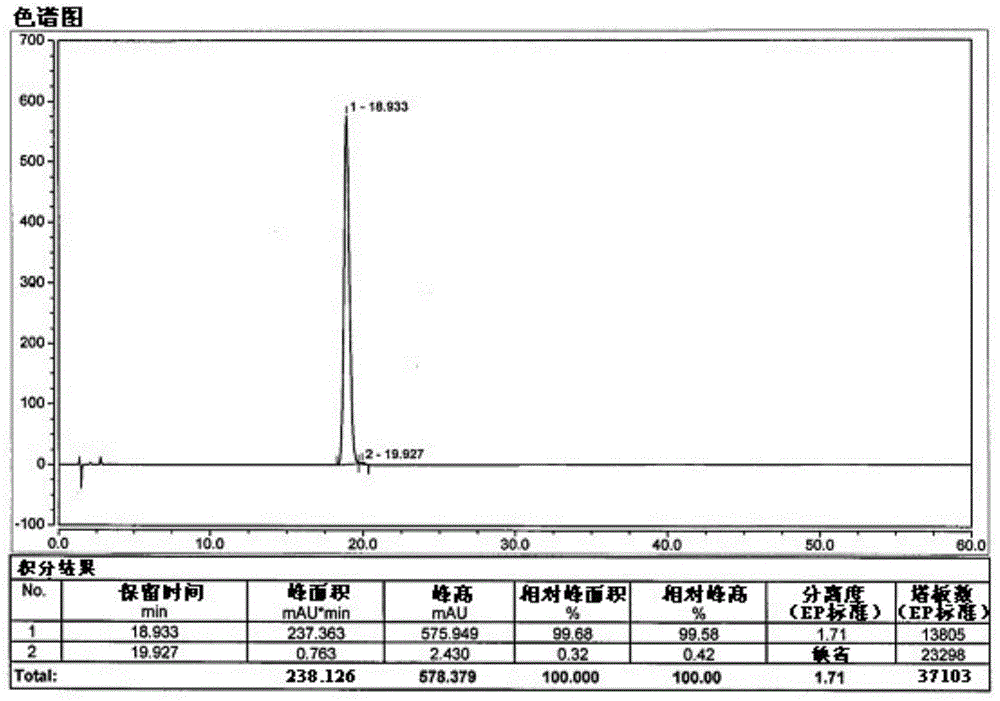
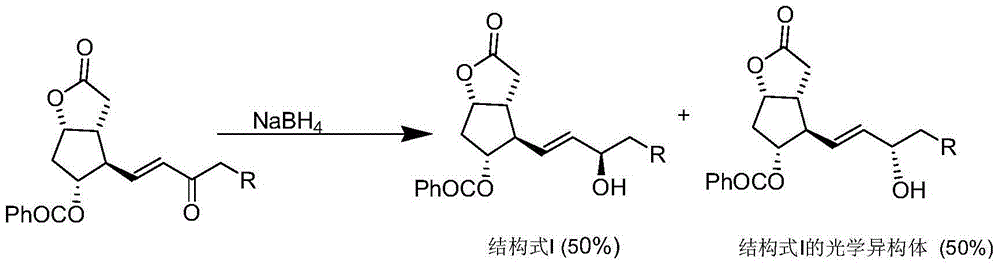
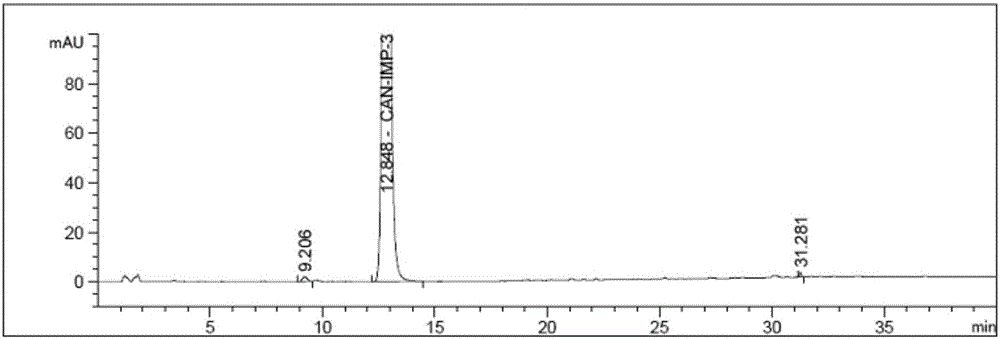
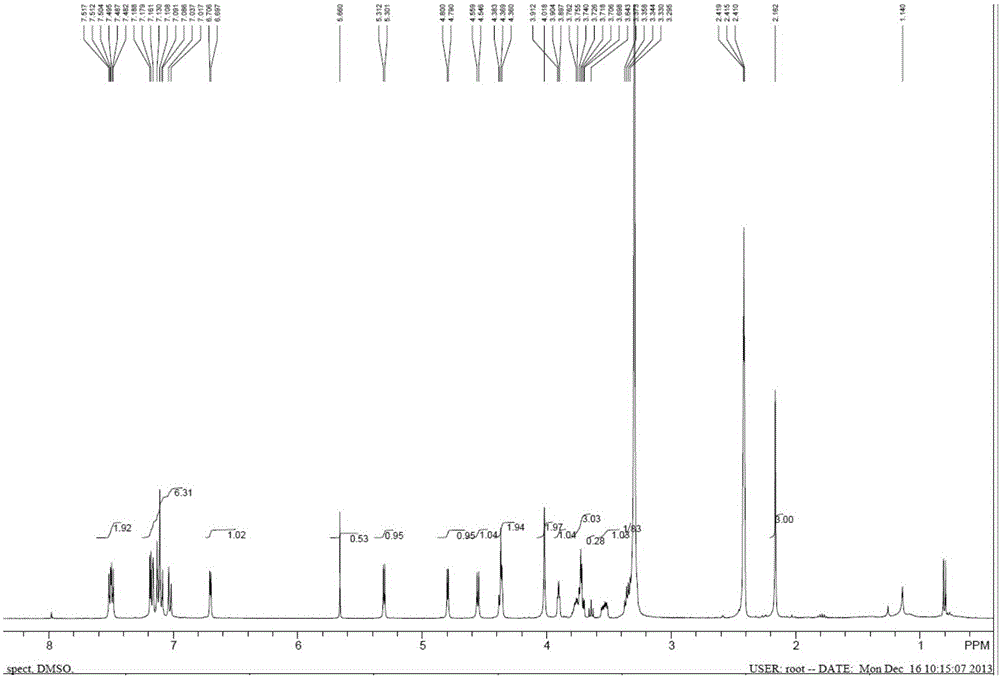
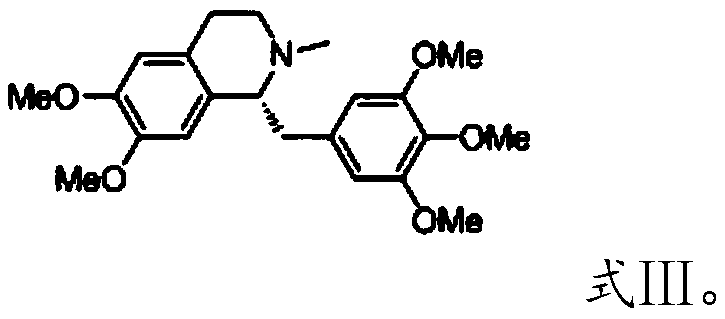
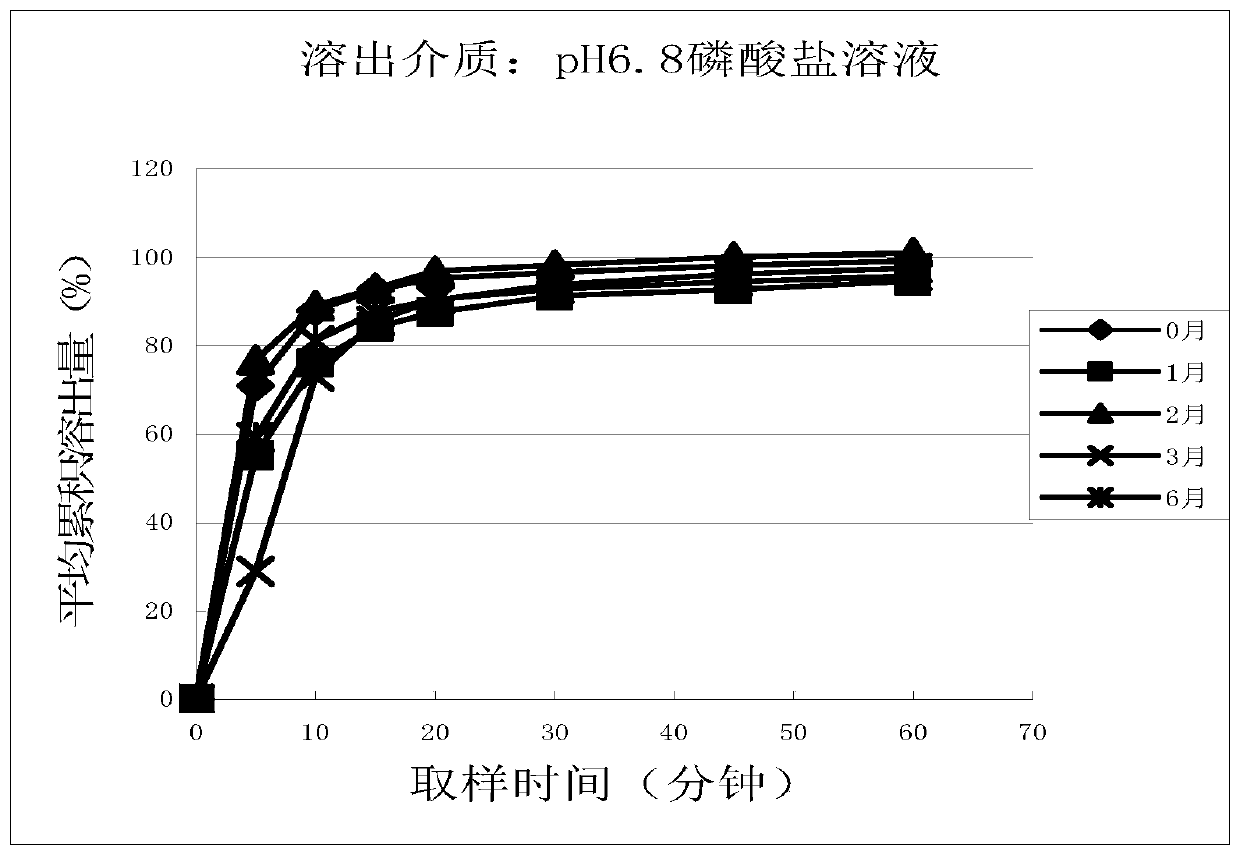
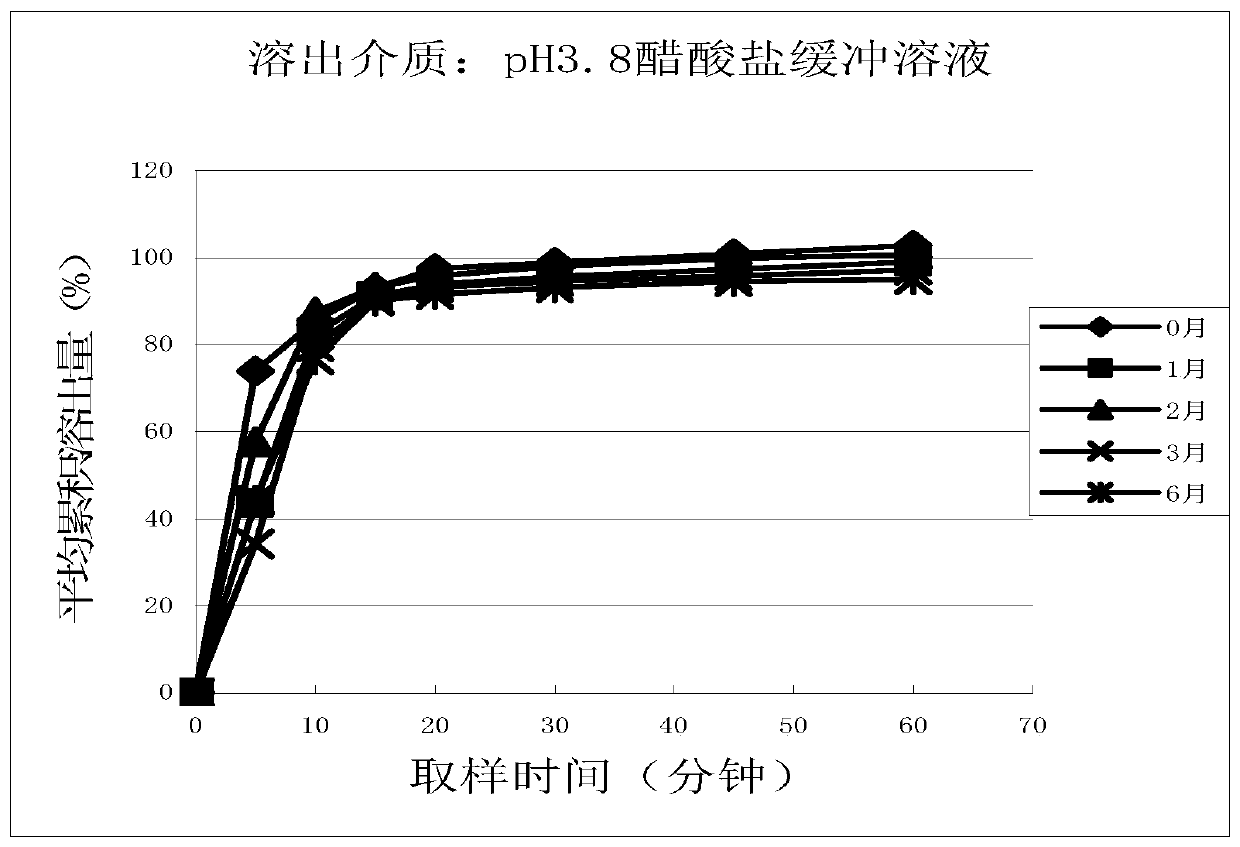
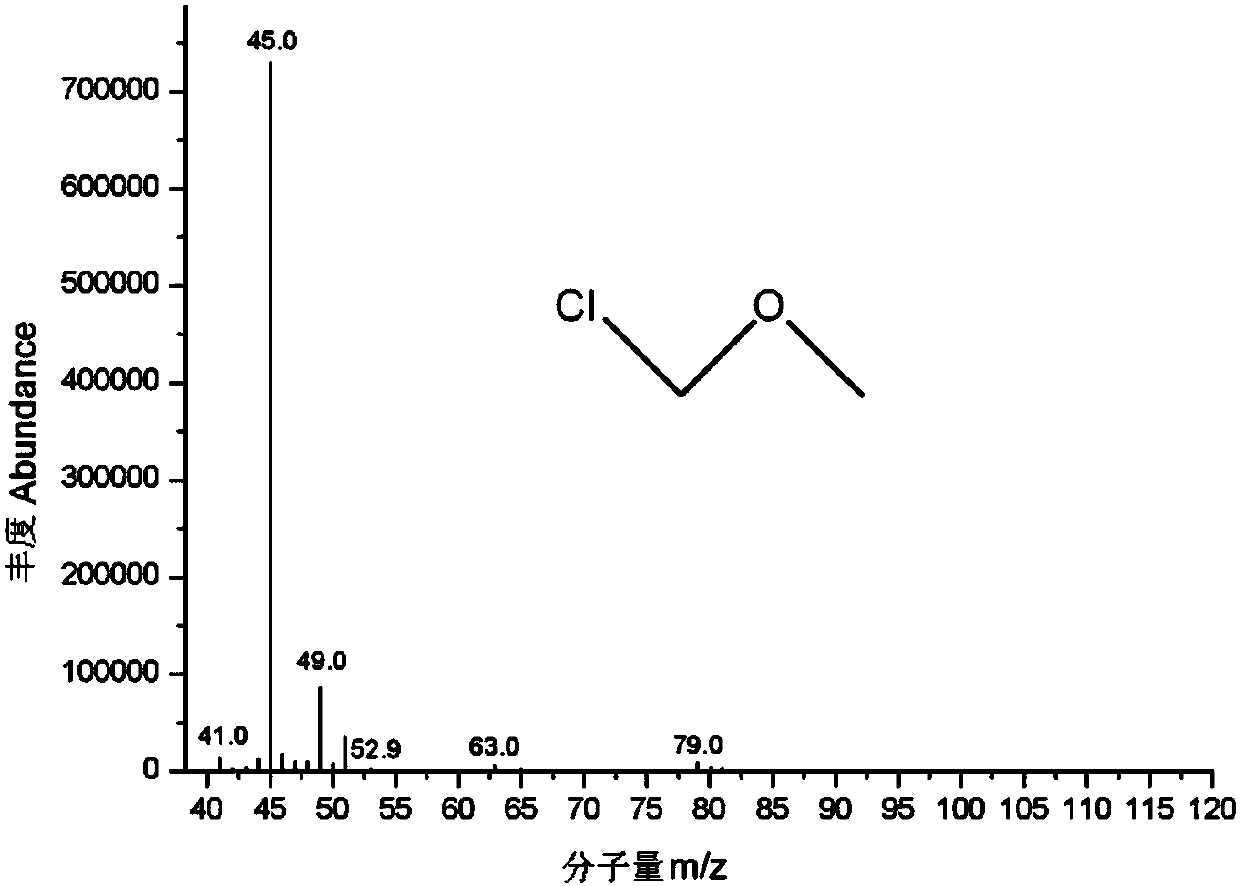
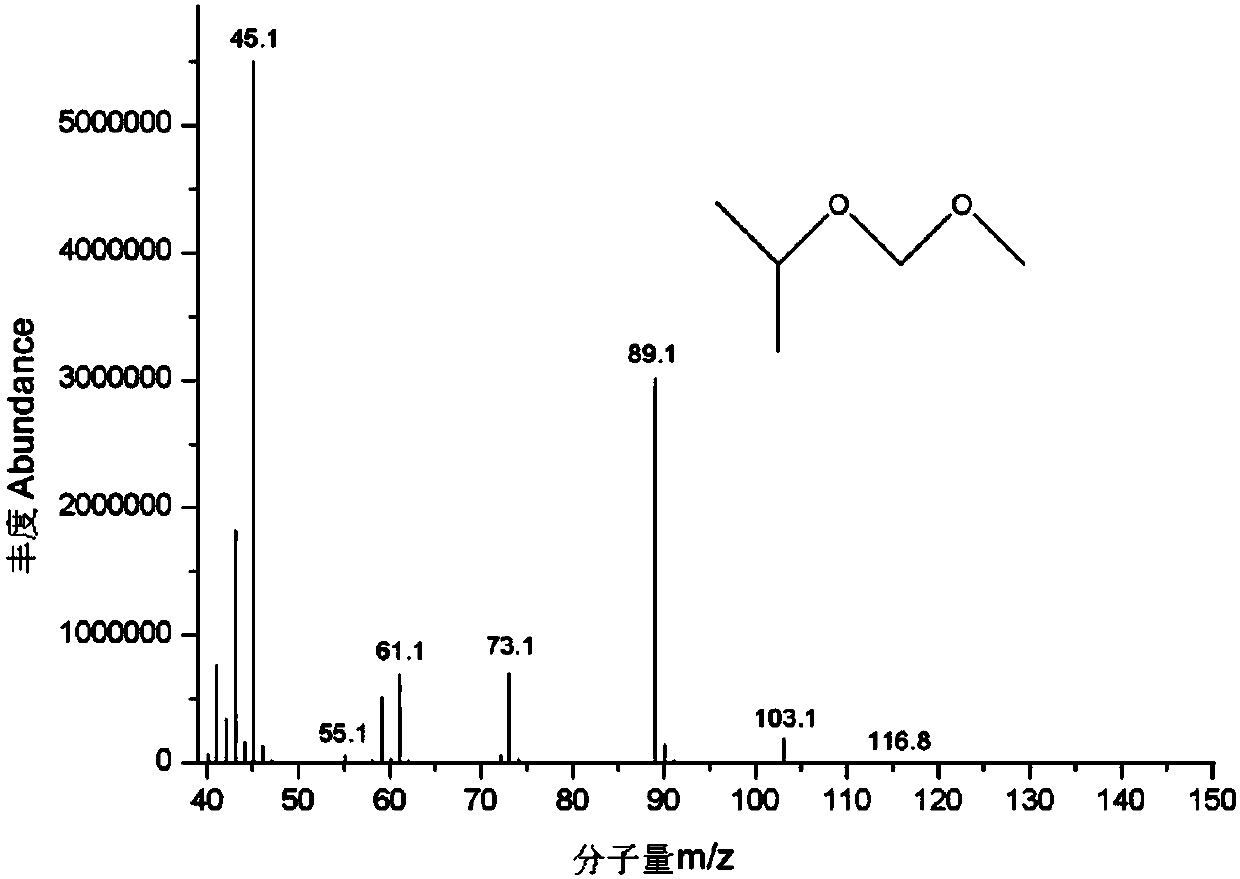
Patents
Literature
105 results about "Quality research" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Quality research most commonly denotes to the scientific process including all aspects of study design; in particular, it relates to the judgment regarding the match between the methods and questions, selection of subjects, measurement of outcomes, and protection against systematic bias, nonsystematic bias, and inferential error(Boaz & Ashby, 2003; ...
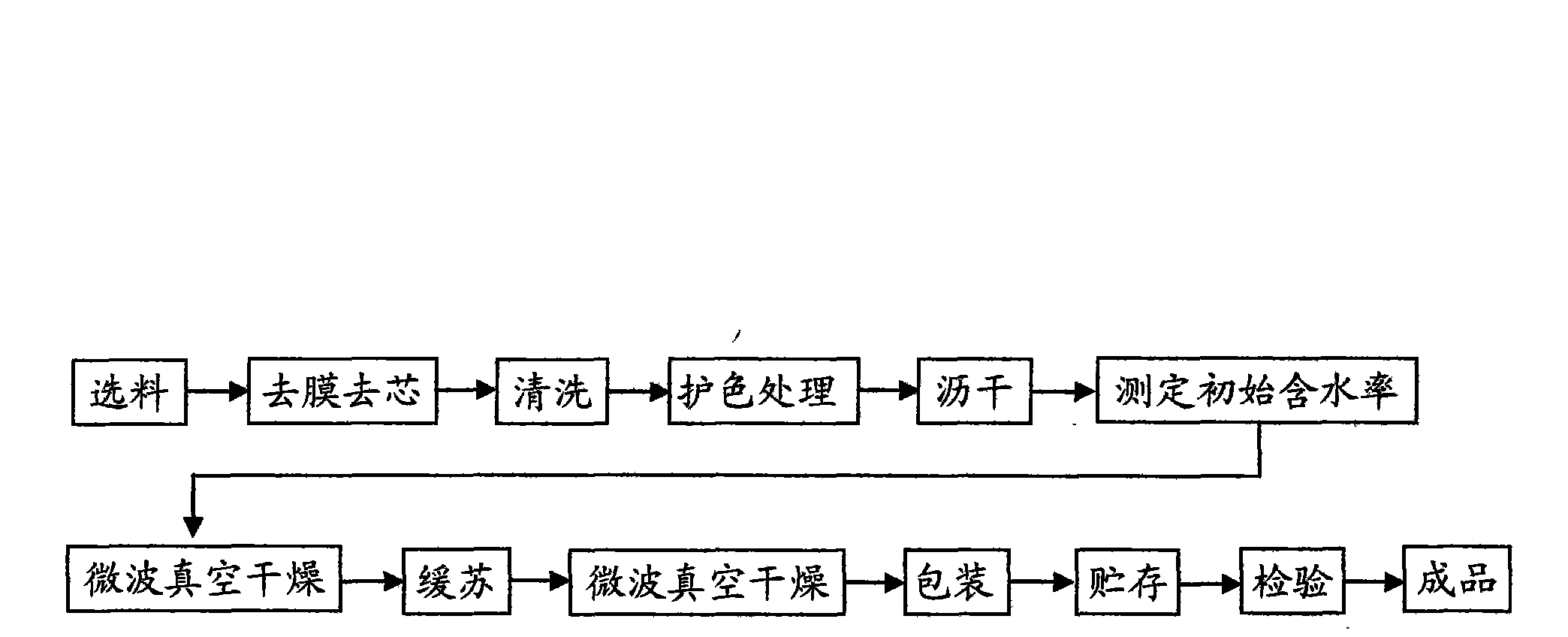
Microwave vacuum drying process for lotus seed
The invention provides a microwave vacuum drying process for lotus seeds, comprising the following steps of: selecting materials, removing films and cores, protecting color, dewatering, vacuum drying by microwave, packaging and storing, thus preparing the dry lotus seed product. The process aims at improving the quality research of the dry lotus seed and reducing the energy consumption, dries the fresh lotus seeds by microwave vacuum drying technology, improves the quality of the lotus seeds, and prolongs the shelf life of the product; the application of the high combination drying technology inaugurates another dry preparation way for drying preparation of fresh lotus seeds; the process is easy for instant control and the production is environment-protective; and the real continuous automatic clean production can be realized.
Owner:FUJIAN AGRI & FORESTRY UNIV
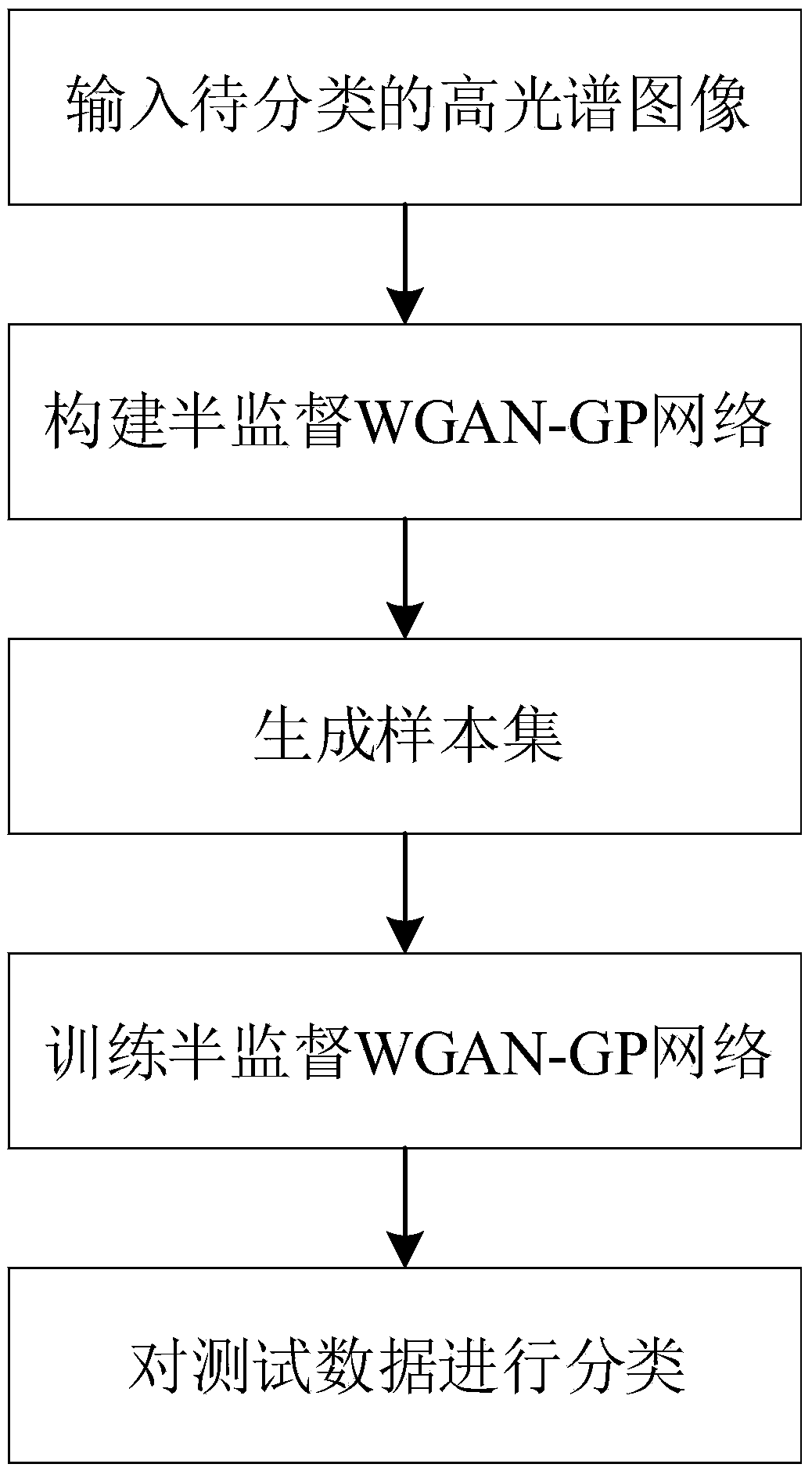
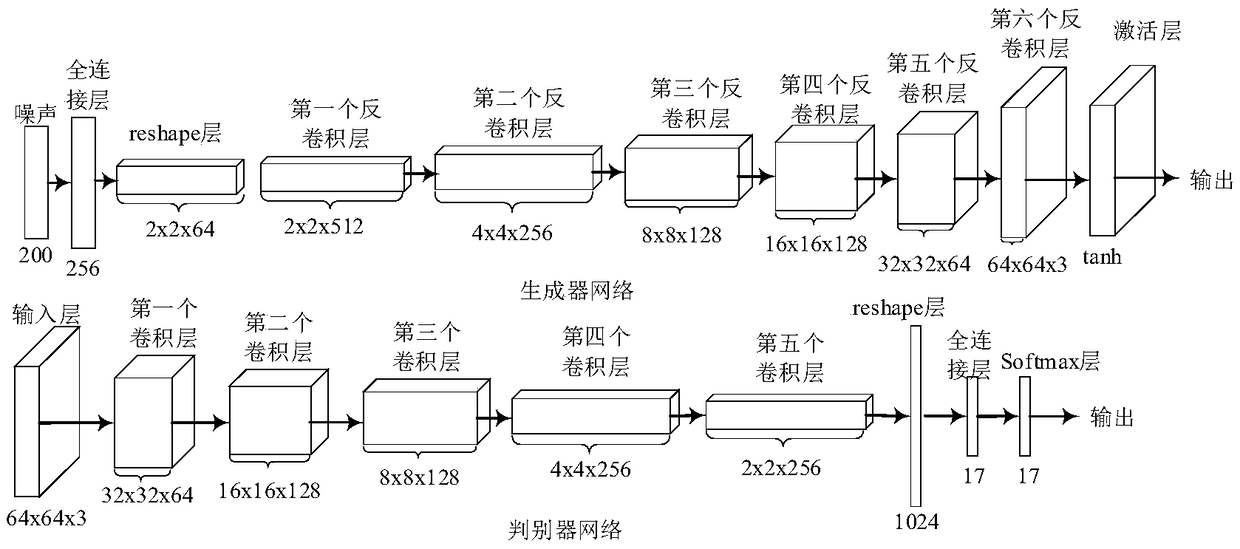
Hyperspectral image classification method based on semi-supervised WGAN-GP
ActiveCN109389080ATake advantage ofOvercome training difficulties and low classification accuracyScene recognitionNeural architecturesDiscriminatorPattern recognition
The invention discloses a hyperspectral image classification method based on semi-supervised WGAN-GP, which overcomes the problem that the existing technology is difficult to extract rich feature information under the condition of limited training data, and cannot be used to train the classifier using unlabeled samples, and the classification accuracy is low. The specific steps of the invention include: (1) inputting a hyperspectral image to be classified; (2) generating a sample set; (3) constructing semi-supervised WGAN-GP network; (4) training semi-supervised WGAN-GP network; (5) and classifying the test data. The invention can generate false hyperspectral data-assisted discriminator classification by the generator receiving noise in the semi-supervised WGAN-GP, and can fully utilize the limited sample to improve the classification precision, and can be used for hyperspectral images in the fields of fine agriculture and low quality research. Classify the target of the object.
Owner:XIDIAN UNIV
Preparation methods of lenvatinib mesylate drug impurities
The invention belongs to the field of pharmaceutical synthesis, and relates to impurities in a raw medical material production process and preparation methods of the impurities, in particular to preparation methods of process impurities A, B and C of lenvatinib mesylate, namely, 4-[3-chloro-4-(N'-cyclopropylureido) phenoxy]-7-methoxyquinoline-6-carboxamide mesylate), as a drug for treating radioiodine-refractory thyroid cancer and an application of the impurities to quality research of lenvatinib mesylate. With adoption of the methods, the process impurities A, B and C are obtained through chemical synthesis for the first time, and the target compounds shown in the description can be obtained through efficient and rapid separation.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST +2
Rice dextrinization temperature and amylose content cooperative determining method
InactiveCN101067623ASimple and fast operationLow environmental requirementsTesting starch susbtancesMaterial analysis by observing effect on chemical indicatorPhysicsQuality research
The invention discloses a method of testing the rice dextrinize temperature and amylose content which is suit to quality research and belongs to the food examination technology area, including the configuration experiment used reagent; the standard sample preparation; the standard sample and the treat measured sample dextrinize; the treat measured sample dextrinize temperature determination; the standard sample and treat measured sample dyeing; the gain standard sample and the treat measured sample chrominance information, and obtains the amylose content in the treat measured sample. The invention method has the characteristic of fast convenient, economical practical, and cost inexpensive, the sample amount used few when tests, simple operation, the high efficiency, has the good application prospect in the paddy rice quality improvement and the quality appraisal.
Owner:CHINA AGRI UNIV
Method for drying carrots by oxygen-free heat pump
InactiveCN102823639AShorten drying timeIncrease productivityFood processingFruits/vegetable preservation by dehydrationFlavorBeta-Carotene
The invention relates to a method for drying carrots, in particular to a method for drying carrots by an oxygen-free heat pump. By the method, nitrogen gas is filled into a drying chamber of the heat pump so as to generate an oxygen-free environment, and the carrots can be dried in the oxygen-free environment. On the basis of quality research on dried carrot slice products and energy reduction, the nitrogen gas is used as drying medium to dry carrot slices in the oxygen-free heat pump, so that beta-carotene in the dried carrot products is high in reservation ratio, the dried carrot products are orange in tinct and palatefull in flavor, and quality of the dried carrot slices is improved. By the method for drying carrots by the oxygen-free heat pump, drying time is shortened, and production efficiency is improved. Besides, the method for drying carrots by the oxygen-free heat pump is low in drying cost, fine in drying effect, easy to control and capable of realizing real sanitary large-scale continuous automatic production.
Owner:HENAN UNIV OF SCI & TECH
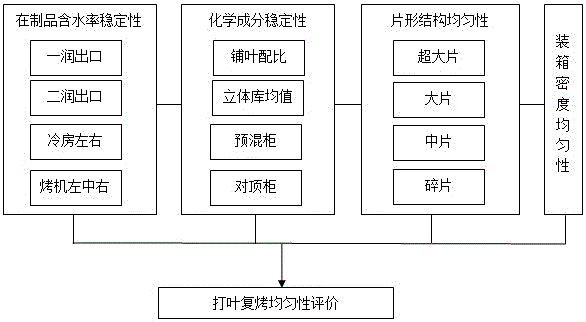
Evaluation method for threshing and redrying uniformity
InactiveCN105842402ASolving the challenge of controlling uniformity evaluationEasy to operateTesting plants/treesChemical compositionEngineering
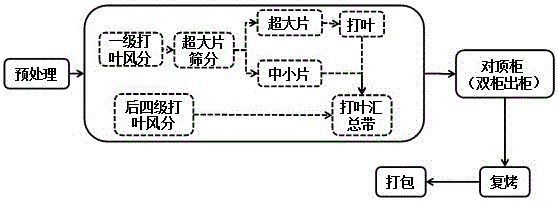
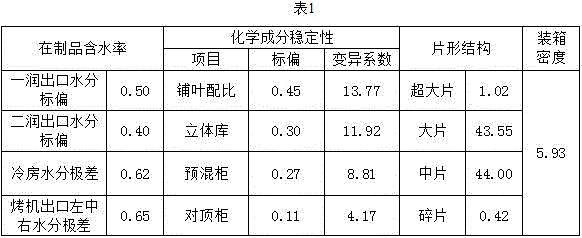
The invention relates to an evaluation method for threshing and redrying uniformity. Four-grade uniformity evaluation indexes are set, and the evaluation method suitable for threshing and redrying uniformity is formed on the four aspects of the tobacco sample product water content stability, sheet structure uniformity, chemical component stability and encasement density uniformity. According to establishment of the invention, the threshing and redrying tobacco processing capacity is brought into full play, the threshing and redrying process uniformity processing level can be accurately and objectively reflected, a gap of threshing and redrying uniformity evaluation is filled, and the method is easy and rapid to implement and scientific and objective in evaluation, and has the important significance in guiding quality research of redried tobacco leaf products.
Owner:FUJIAN WUYI TOBACCO +1
Self-compensation type free up-down pitching coupling mechanism
The invention relates to a self-compensation type free up-down pitching coupling mechanism used for carrying out a flight simulation test; the coupling mechanism comprises a free up-down pitching mechanism and a forced up-down mechanism. The coupling mechanism can greatly extend a vertical line displacement motion scope, thus realizing free up-down and pitching coupling vertical short period motions of a plane model in a horizontal wind tunnel, and satisfying vertical pneumatic / motion coupling characteristics and flight quality research demands. Compared with a flight simulator or test flightmeans, the self-compensation type free up-down pitching coupling mechanism is high in repeatability, short in period, low in risks, thus making up disadvantages of existing wind tunnel test means.
Owner:中国航空工业集团公司哈尔滨空气动力研究所
Automatic turnover type hot air infrared fruit and vegetable drying device and method
InactiveCN104982508AImprove qualityExtended shelf lifeFood processingFruits/vegetable preservation by dehydrationLight pipeEngineering
The invention discloses an automatic turnover type hot air infrared fruit and vegetable drying device. The device comprises a support, a conveying belt, a frame, a power motor, a drying cavity upper cover plate and a draught fan. Hot air is fed into a drying cavity through a hot air pipeline via an air inlet formed in the position of the frame of the conveying belt. The invention further discloses an automatic turnover type hot air infrared fruit and vegetable drying method. The water content of fruit and vegetable materials is calculated through the operating speed of the conveying belt, the base angle of the ridge-shaped drying cavity upper cover plate and the interval of infrared light pipes, the drying effect of the fruit and vegetable materials can be accurately controlled, the quality of dried fruits and vegetables is improved on the basis of improving quality research on the dried fruits and vegetables and reducing energy consumption, transportation is convenient, drying time is greatly shortened, production efficiency is improved, drying energy consumption is greatly reduced, energy is saved, and environment friendliness is achieved.
Owner:JIAMUSI UNIVERSITY
Blood leukocyte filtering monitoring system and method
The invention discloses a blood leukocyte filtering monitoring system which comprises a filtering device, a weight measuring device, a time measuring device and a storing device. The weight measuring device is used for collecting blood weight parameters before filtering during a filtering process in real time. The time measuring device is used for measuring time during the filtering process. The weight measuring device and the time measuring device are electrically connected. The time measuring device and the storing device are electrically connected. The weight measuring device and the time measuring device collect data of the filtering process in real time. The collected data are sent to the storing device which stores the parameters. Corresponding data are measured through a weight sensor and the time measuring device, the measured data are stored in a magnetic disk, accordingly, the objective real-time data about the influence on blood quality of the filtering process are obtained, the most primitive data are provided for blood quality researching after filtering, and the research on blood quality after filtering is promoted.
Owner:深圳市迈思特生物医学工程有限公司
Ticagrelor impurity, preparation method and application thereof
PendingCN107778312AOrganic chemistry methodsPreparing sample for investigationTicagrelorActive ingredient
The invention discloses ticagrelor impurity, namely ticagrelor impurity 7 and ticagrelor impurity 8; in addition, the invention also discloses a preparation method and application thereof. According to the ticagrelor impurity and the preparation method thereof provided by the invention, foundation is laid for the quality researches on a ticagrelor intermediate, raw medicinal materials and a composition thereof.
Owner:ZEIN BIOTECHNOLOGY CO LTD
Synthetic method for Tebipenem Pivoxil polymer impurity
The invention provides a synthetic method for Tebipenem Pivoxil polymer impurity P8. The method employs a ring opening impurity P9 of Tebipenem and Tebipenem Pivoxil as a starting material, the starting material is subjected to an etherification reaction and an esterification reaction respectively, the obtained products are subjected to a condensation reaction and esters are formed; then t-butyl dimethyl silicon group protecting group is removed, after recrystallization purification, the target compound with a purity of being more than 90% is obtained. The target compound can be employed as a reference substance and used for qualitative and quantitative research of polymer impurities in Tebipenem Pivoxil quality research, thus contents of raw material Tebipenem Pivoxil related substances can be controlled, and quality of Tebipenem Pivoxil active pharmaceutical ingredients can be ensured.
Owner:上海津力药业股份有限公司
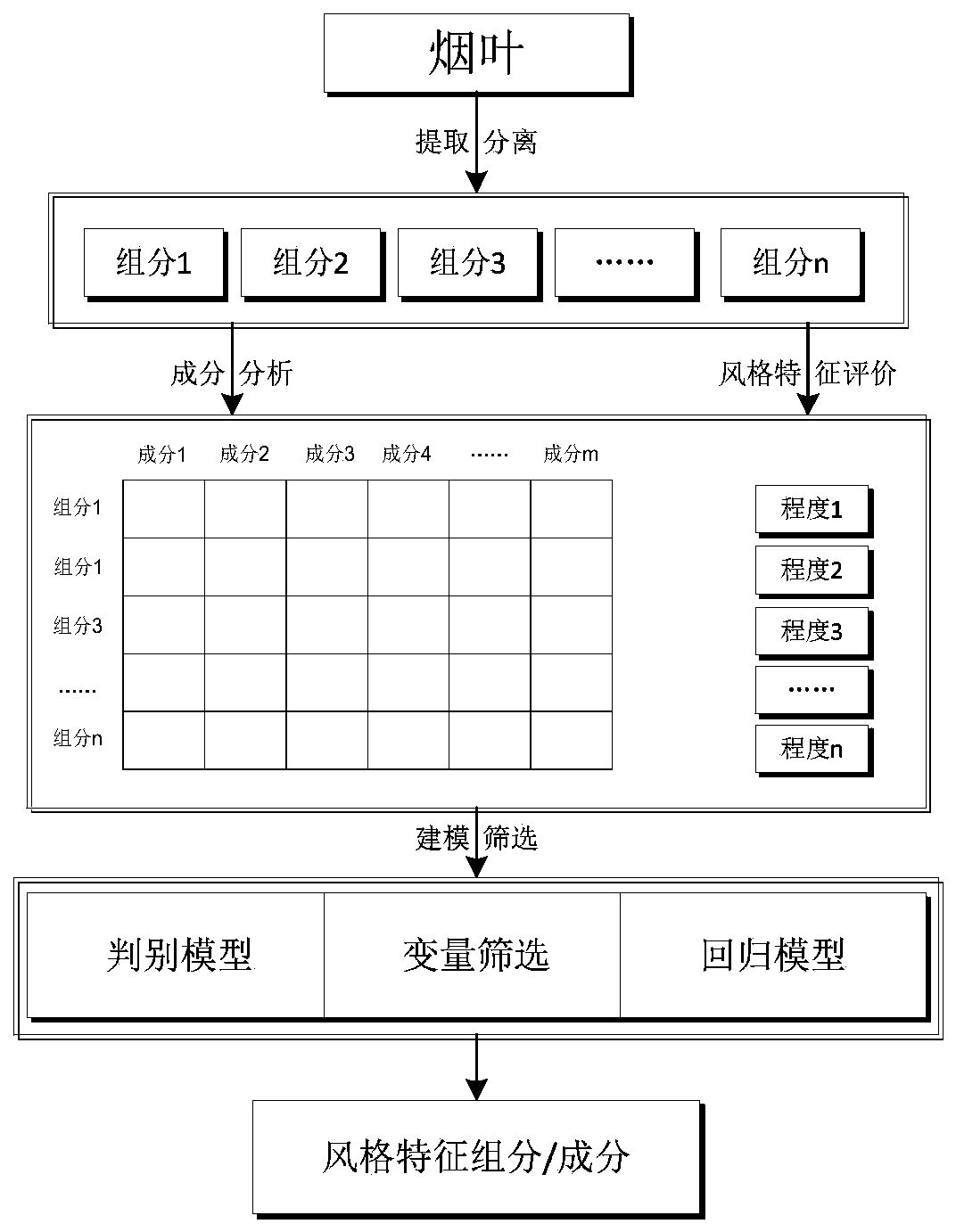
Method for screening and preparing characteristic components from tobacco and application
InactiveCN109846077AResearch Sensory Quality GreatResearch on sensory qualities has a big impactTobacco preparationTobacco treatmentBiologyOrganoleptic
The invention discloses a method for screening components having great influences on characteristic sensory effects in tobacco and application. After tobacco leaves are extracted and separated to obtain different components, the components are subjected to relative quantitative analysis, and evaluation for sensory quality research is conducted. In combination with chemometrics analysis, the components which have great influences on sensory quality research can be judged. By means of selective preparation, corresponding characteristic components can be obtained and applied to cigarette preparation. The method for screening and preparing the characteristic components from the tobacco is highly goal-oriented, so that research waste is reduced; the prominentcomponents with specific sensory quality are prepared, the particular sensory quality of cigarettes can be obviously improved, and an important effect on product quality stabilization is achieved; a new method is provided for developingcigarette essence and spice with prominent sensory quality.
Owner:CHINA TOBACCO YUNNAN IND
Preparation method of tofacitinib impurity
ActiveCN109336892AEasy to separate and purifyHigh purityOrganic chemistryQuality researchTofacitinib
The invention discloses a preparation method of tofacitinib impurity. A synthetic route is as shown in the specification. The preparation method provided by the invention has the advantages that the synthetic process is simple, the products are easy to separate and purify and the purity of the obtained products is as high as 98.0%. Therefore, the obtained compound I, II in the preparation method can be used for the quality research and process research of related products as impurity reference substances of tofacitinib raw medicinal materials and preparations.
Owner:珠海优润医药科技有限公司
Method for preparing esomeprazole impurity
The invention discloses a method for preparing an esomeprazole impurity. 5-methoxy-2-[(S)-[(4-chloro-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole (II) is obtained through demethylation, halogenation, condensation and asymmetric oxidation by taking 2-chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride as a starting raw material. According to the method disclosed by the invention,a synthesis route is simple and short, the starting raw material is easy to obtain, the reaction conditions are gentle, the operation is simple and convenient, and the impurity can be obtained withoutcolumn chromatography; the purity of the prepared impurity can be up to 99 percent or above, the ee (Enantiomeric Excess) value can be up to 99 percent or above, and the impurity can be used as reference substance in quality research.
Owner:珠海润都制药股份有限公司
Parecoxib sodium degradation impurity and preparation, detection method and application thereof
ActiveCN108164521ASimple and fast operationThe result is accurateOrganic chemistryComponent separationState of artStability study
The invention discloses a parecoxib sodium degradation impurity shown in a formula I and a preparation method thereof and aims at solving the problem of no parecoxib sodium degradation impurity research in the prior art. The invention further discloses application of the degradation impurity and a high performance liquid chromatography detection method of the degradation impurity. The parecoxib sodium degradation impurity disclosed by the invention provides basis for quality researches, standard researches, stability researches, adverse drug reaction mechanism researches on the parecoxib sodium and provides basis for production, package, storage, transportation and application condition selection of the parecoxib sodium at the same time. (The formula is shown in the description.).
Owner:CHENGDU SINO STRONG PHARMA
Sitagliptin impurity synthesis method
The invention discloses a sitagliptin impurity synthesis method. According to the method, sitagliptin is taken as a raw material, and a sitagliptin impurity is obtained through a deamination reaction under an acidic or alkali condition. The purity of the sitagliptin impurity obtained according to the method can reach 99% or higher, and thus the sitagliptin impurity can serve as a reference substance for quality research.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
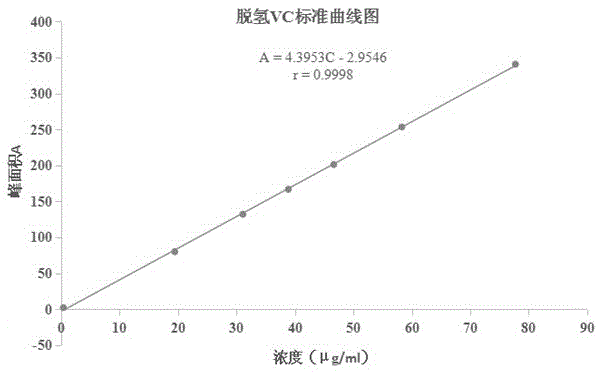
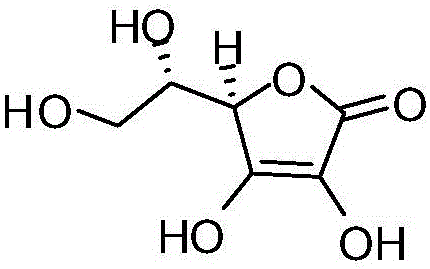
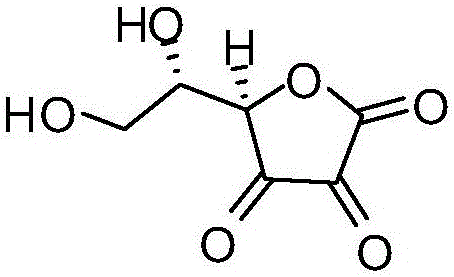
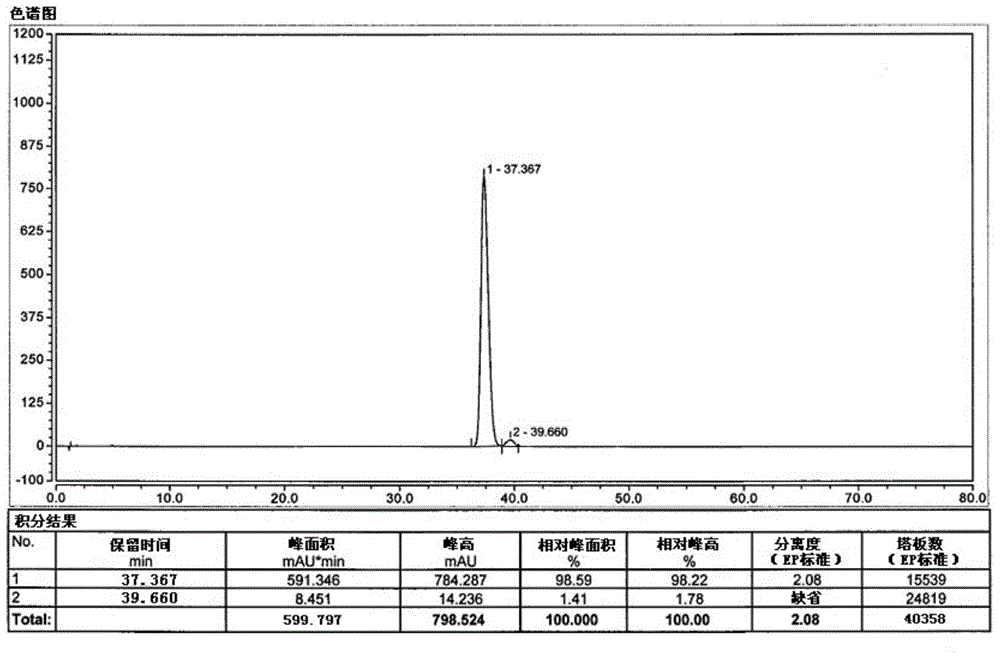
Method for detecting dehydrogenized vitamin C in vitamin C
The invention discloses a method for detecting dehydrogenized vitamin C in vitamin C and belongs to the field of analytic chemistry. The method is characterized in that the method uses liquid chromatography and an HILIC affinity chromatography column and uses acetonitrile--isopropanol-water as the mobile phase A and acetonitrile-water as the mobile phase B to perform analysis through gradient elution. The method has the advantages that by the method, the content of the dehydrogenized vitamin C can be analyzed fast and accurately; the quantification limit of the method can reach 0.38 microgram / ml, the detection limit of the method is 0.11 microgram / ml, the average yield of the method is 100.5%, and the concentration of the dehydrogenized vitamin C and peak area present a good linear relation in the range from 0.38 microgram / ml to 77.75 microgram / ml; the liquid-phase method is also applicable to vitamin quality researches such as researches on the stability and degradation of vitamin C medicine, compound vitamin (3) for injection and compound vitamin (13) injection.
Owner:JINAN KANGHE MEDICAL TECH
Preparation method of prostaglandin medicine impurity
ActiveCN103601708AMeet quality requirementsHigh yieldOrganic chemistryComponent separationBoron trichlorideKetone
The invention belongs to the field of medicine synthesis, and particularly relates to a novel preparation method of a key impurity of a prostaglandin series compound. The preparation method is characterized in that (-)alpha-pinene, sodium borohydride and boron trichloride are used as raw materials, firstly a chiral reducing agent is prepared, and then a midbody of the prostaglandin series compound, namely precursor ketone is subjected to chiral catalytic reduction, thus the target impurity is obtained. The preparation method provided by the invention has the advantages that the synthesis of the impurity is simple, convenient and feasible, the yield is high, and the optical purity is good; a relatively good impurity reference substance is supplied for the quality research and the quantitative control on the impurity of industrially produced prostaglandin series products; the figure 1 is a HPLC (High Performance Liquid Chromatography) map of the impurity A'.
Owner:WUHAN WUYAO SCI & TECH
Compound and preparation method and application thereof
InactiveCN106279072AEfficient preparationThe reaction mechanism is clearOrganic chemistryImpurityHplc mass spectrometry
The invention provides a compound shown in the formula I and a preparation method thereof and a method for detecting Gliflozin medicine through high performance liquid chromatography. According to the preparation method, 3,5,6-tribenzyl-D-glucofuranose serves as a raw material, a methylation reaction, a benzyl substitution reaction, demethylating and ketalation are carried out, 3,5,6-tribenzyl-D-glucofuranose and a halide intermediate are subjected to a nucleophilic addition, the material makes contact with triethyl silicane and boron trifluoride diethyl etherate, finally, the material makes contact with ethanethiol and boron trifluoride diethyl etherate, and the target impurity product can be obtained. By means of the method, directional preparation is achieved for synthesis of the target product; the target product provides a reliable impurity reference substance for quality research and impurity quantitative control of industrially-produced Gliflozin series diabetes treatment medicine products. The formula I is shown in the specification.
Owner:WATERSTONE PHARMA WUHAN
Canagliflozin drug impurity as well as preparation method and application thereof
ActiveCN107286143AStarting materials are cheap and readily availableReduce stepsOrganic chemistry methodsOrganic solventLithium hydroxide
The invention discloses a canagliflozin drug impurity as well as a preparation method and application thereof. The invention provides a compound as well as a preparation method and application thereof. The method comprises the following steps: (1) enabling the compound as shown in formula 2 to be in contact with an alkaline lithium hydroxide aqueous solution to obtain a coarse product containing a compound as shown in formula 3, wherein the coarse product contains a compound as shown in formula 1; (2) crystallizing and filtering the coarse product to obtain mother liquor; (3) concentrating the mother liquor to obtain residues; and (4) crystallizing and filtering the residues in an L-proline-containing organic solvent, thus obtaining the compound as shown in formula 1. The method provided by the invention can realize directed preparation of the compound as shown in formula 1, and a reliable impurity contrast is provided for quality research on industrially produced canagliflozin-series diabetes treatment drug products and quantitative control over impurities.
Owner:WATERSTONE PHARMA WUHAN
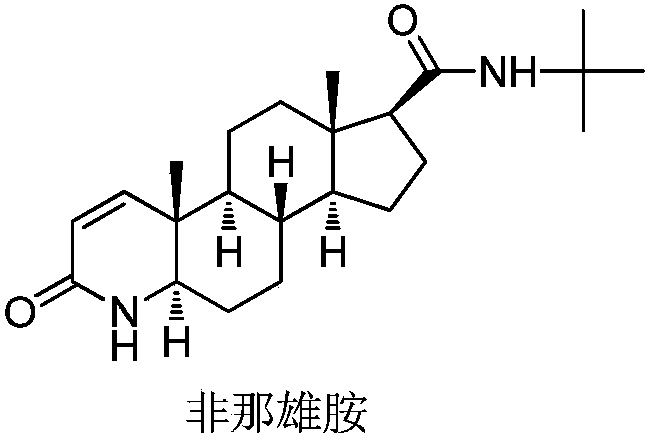
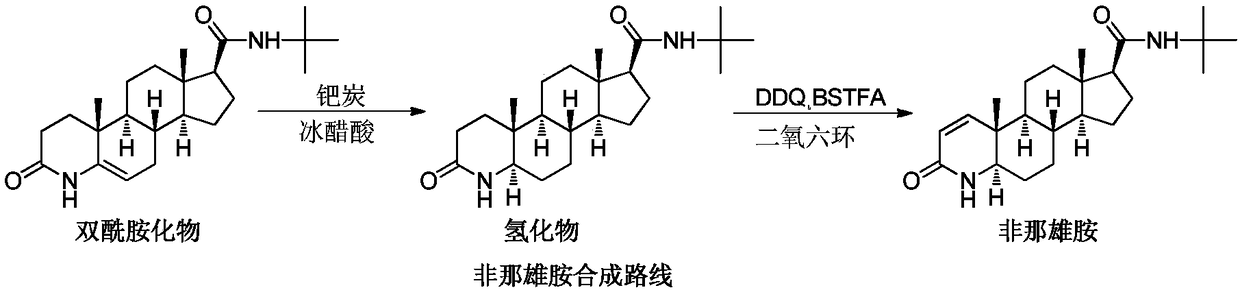
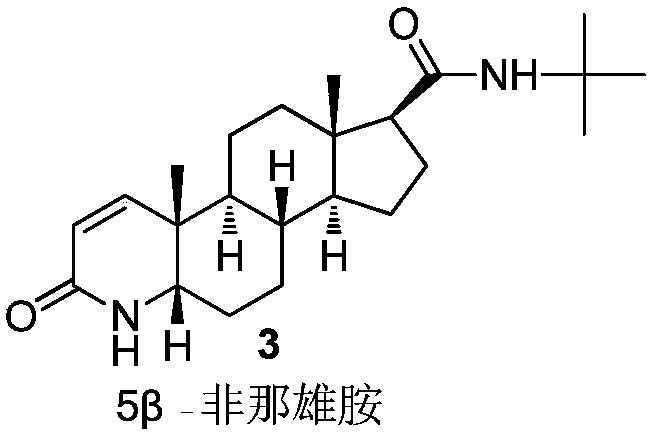
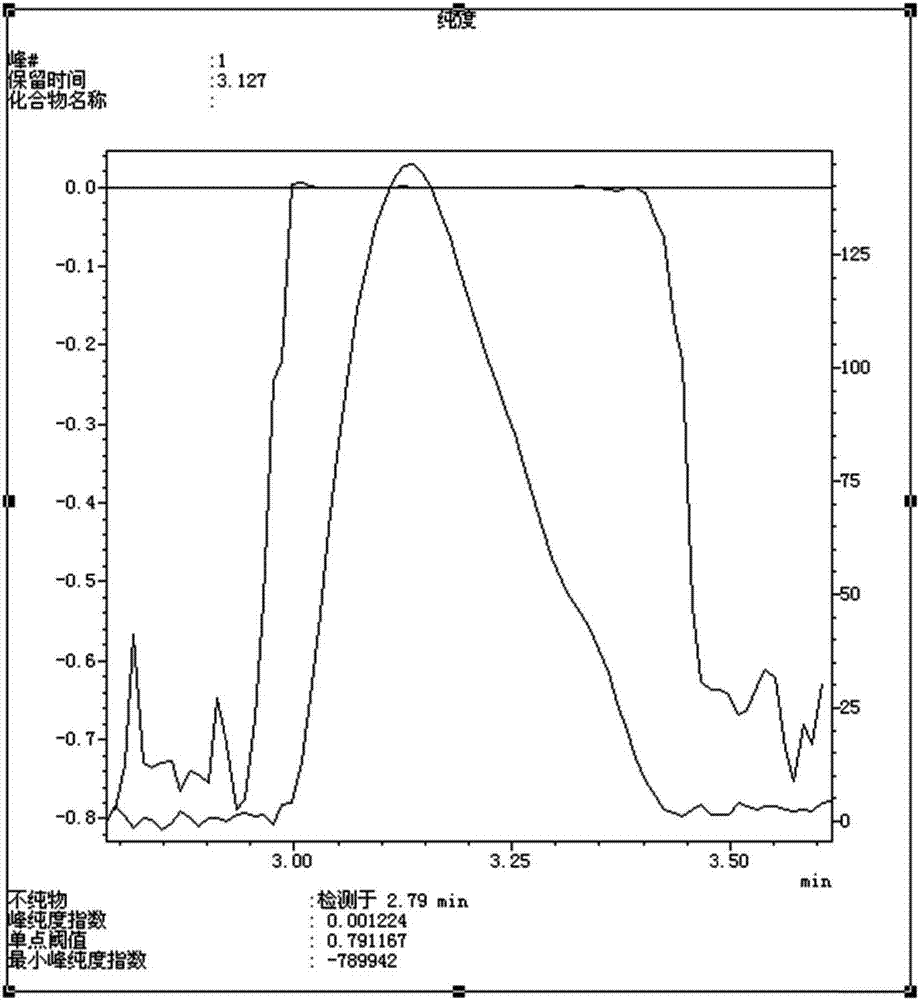
Finasteride chiral impurity (5beta-finasteride) synthesizing and purifying method
The invention discloses a finasteride chiral impurity (5beta-finasteride) synthesizing and purifying method. A diamide compound (formula 1) is used as a raw material, and a target product of formula 3is obtained through catalytic hydrogenation, refining, dehydrogenating and purifying, wherein a synthetic route is shown in the specification. The 5beta-finasteride is an unavoidable chiral impurityin a production process of finasteride, the separation and purification of the finasteride have important signification to impurity analysis of the finasteride, and provide great convenience to quality research of a finasteride active compound.
Owner:ZHEJIANG XIANJU PHARMA
Impurities of R-lipoic acid or tromethamine salts thereof, preparation method for impurities and detection method for impurities
ActiveCN107226804AAccurate and Applicable Analytical MethodsOrganic chemistryComponent separationTrifluoroacetic acidPhosphoric acid
The invention provides an impurity compound of R-lipoic acid or tromethamine salts thereof represented by a formula (I) shown in the description and a preparation method for the impurity compound. The preparation method comprises the steps of irradiating an aqueous solution of the R-lipoic acid or tromethamine salts thereof with strong light, and then, carrying out HPLC (High-Performance Liquid Chromatography) twice, thereby preparing the compound represented by the formula (I), wherein during primary preparation, a flowing phase is prepared from A: a 0.15% phosphoric acid aqueous solution and B: acetonitrile, and during secondary preparation, a flowing phase is prepared from A: a 0.1% trifluoroacetic acid aqueous solution and B: acetonitrile; and an elution mode is gradient elution. The invention further provides an HPLC detection method for related substances of the R-lipoic acid or tromethamine salts thereof, wherein a flowing phase is prepared from A: a 0.02% phosphoric acid solution and B: a 0.02% phosphoric acid solution-acetonitrile (40: 60), and an elution mode is gradient elution. The method is applied to quality research on raw materials and preparations of the R-lipoic acid or tromethamine salts thereof.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Reference substance of micurium chloride and preparation method thereof
The invention provides a reference substance of micurium chloride, and relates to the technical field of medicine and chemical industry. The reference substance has the structure shown in a formula Ior a formula II. The reference substance is close to and stabler in the nature of the micurium chloride, has low cost, and can meet the requirements of the quality study of the micurium chloride. Theresults show that the reference substance has a stable nature in comparison with the method for detecting the content of the micurium chloride by using a pure product of the micurium chloride as the reference substance, the measured content of the micurium chloride in a test sample is reasonable, and values are stable, which fully meets the testing requirements as the reference substance. The invention provides a preparation method of the reference substance of the micurium chloride. The preparation method has good stability, repeatability and high product purity, and can conduct preparing inan appropriate amplification manner, and the preparation cost is lower (3000 yuan / g), which can well meet the requirements of the quality research of the micurium chloride.
Owner:HAINAN STAR PHARM CO LTD
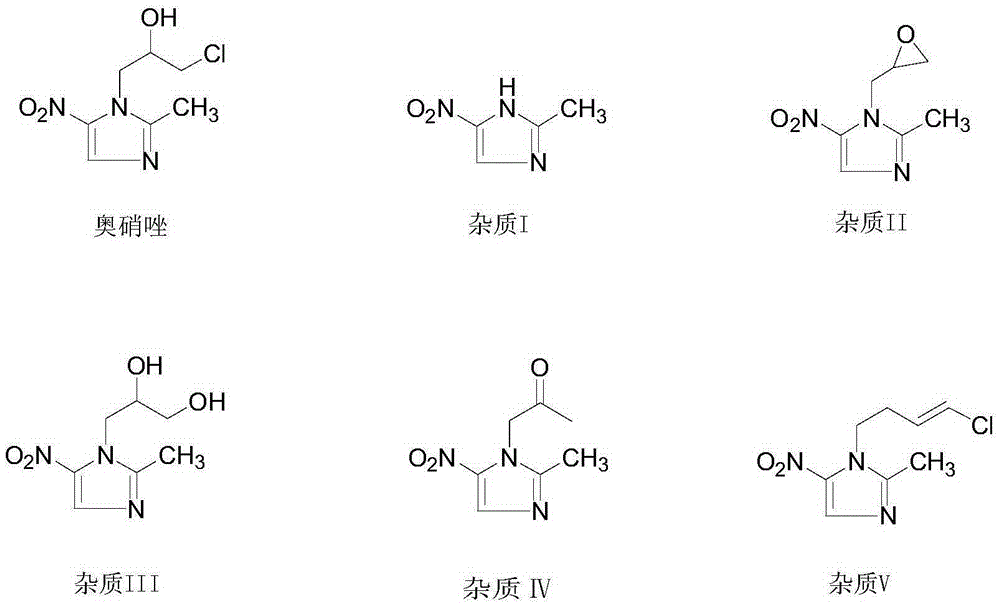
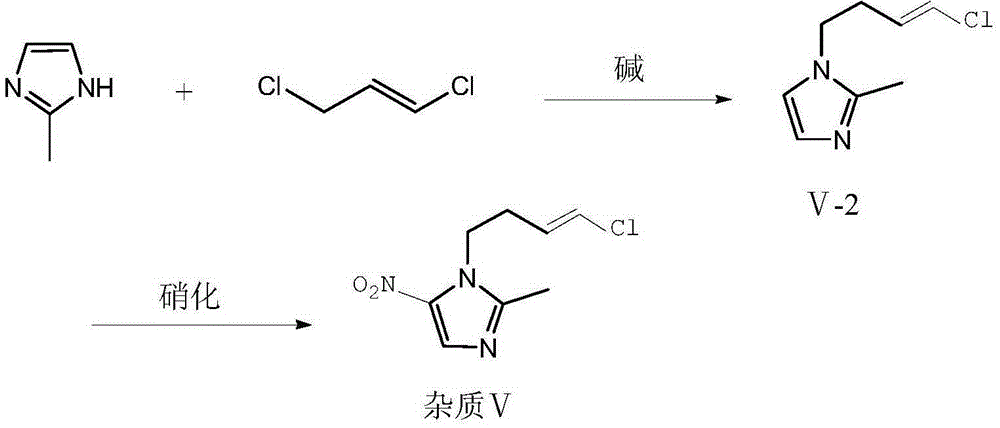
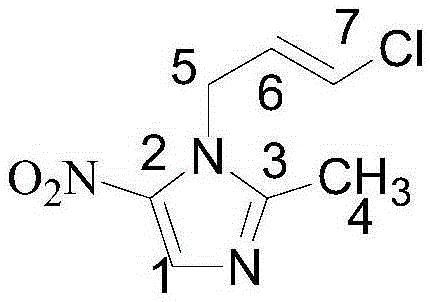
Preparation method of ornidazole injection impurity 1-(3-chloro-propenyl)-2-methyl-5-nitroimidazole
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of ornidazole injection impurity 1-(3-chloro-propenyl)-2-methyl-5-nitroimidazole. The preparation method comprises the following steps: dissolving 2-methylimidazole, 1,3-dichloropropene and alkali into a solvent, heating to backflow for performing a reaction, and after the reaction, a product, namely the 1-(3-chloro-propenyl)-2-methylimidazole is obtained through post-treatment; adding the product into a reactor containing nitrosonitric acid for performing a nitration reaction, after adding, adding a dehydrating agent in batches into a system, and obtaining 1-(3-chloro-propenyl)-2-methyl-5-nitroimidazole through post-treatment. The preparation method provided by the invention has the advantages of being simple to operate, mild in reaction, relatively high in yield, high in product purity, suitable for quality research and the like, has excellent commercial value, and provides a guarantee for the quality of ornidazole.
Owner:SHANDONG QIDU PHARMA
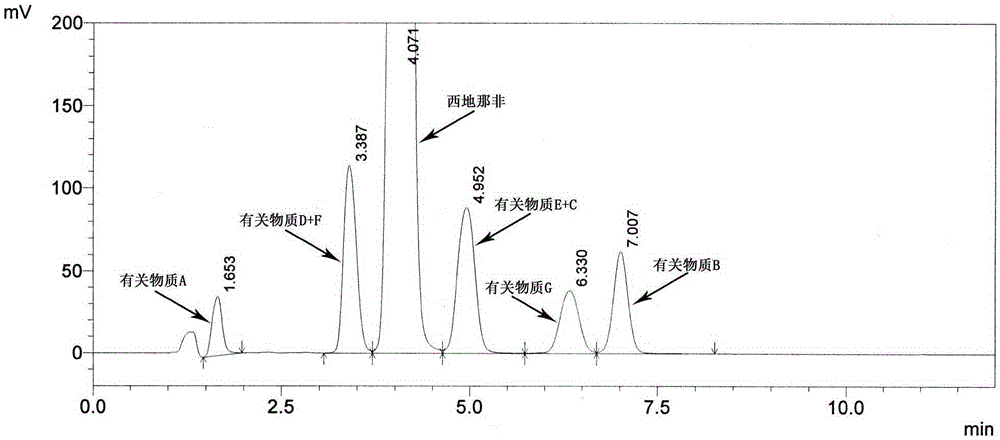
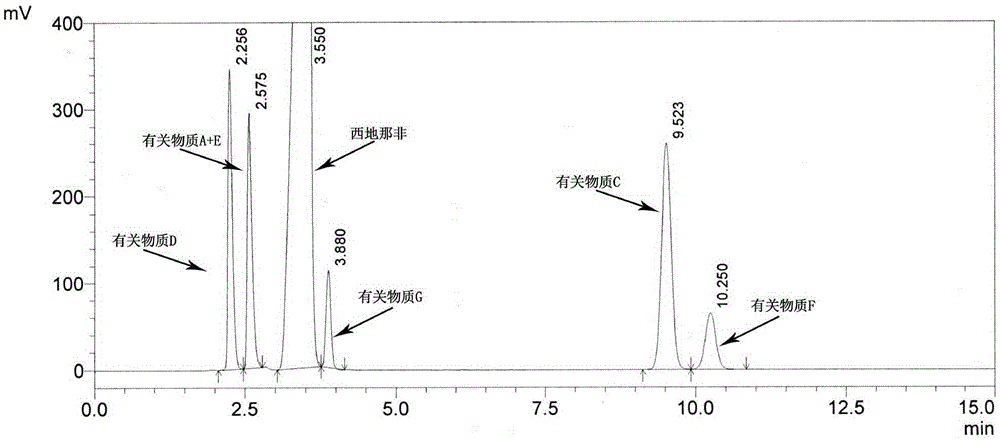
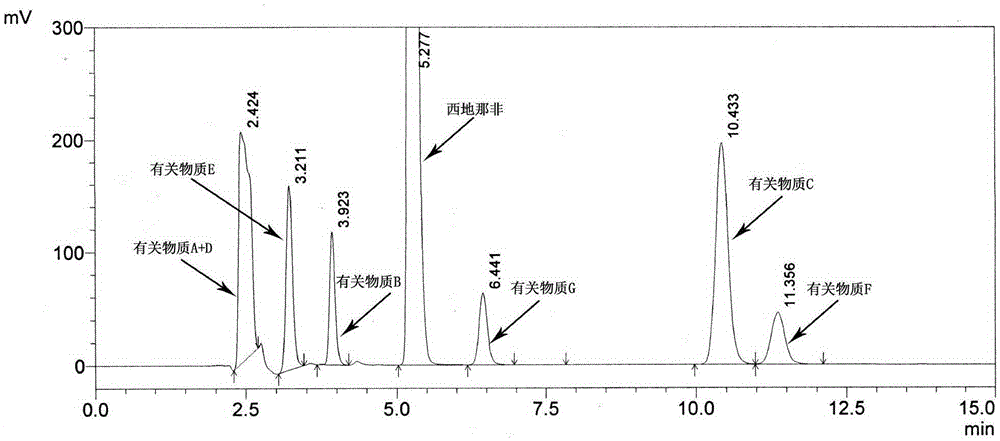
Detection method for sildenafil citrate related substances
The invention discloses a detection method for sildenafil citrate related substances. The high performance liquid chromatography is adopted in the detection method, octadecylsilane bonded silica gel is used as a chromatographic column for the stationary phase, an ammonium acetate water solution is used as the mobile phase A, an acetonitrile-methyl alcohol mixture is used as the mobile phase B, the detection wavelength is 292 nm, and gradient elution is carried out. The detection method is simple, efficient, high in resolution and capable of separating and detecting seven related substances in sildenafil citrate at the same time, and the mobile phases are suitable for mass spectrometric detection and can be used for quality research and quality control over sildenafil citrate raw material drugs and preparation products.
Owner:CHANGZHOU YABANG PHARMA +1
Lisinopril impurity and preparation method thereof
PendingCN108948136AStrong practical valuePeptide preparation methodsPeptides with abnormal peptide linkChlorideSuccinic acid
The invention provides a lisinopril impurity N6-(3-carboxypropanoyl) lisinopril (I) and a preparation method thereof. The lisinopril impurity is prepared through reaction of lisinopril and succinic acid or succinic acid derivatives in a solvent, and the succinic acid derivatives are succinic anhydride, succinyl chloride, succinyl chloride and succinimide. The prepared N6-(3-carboxypropanoyl) lisinopril (I) can be used for supporting the development works for the quality research and an analytical method of the impurity and can be used for supporting the process improvement of lisinopril.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
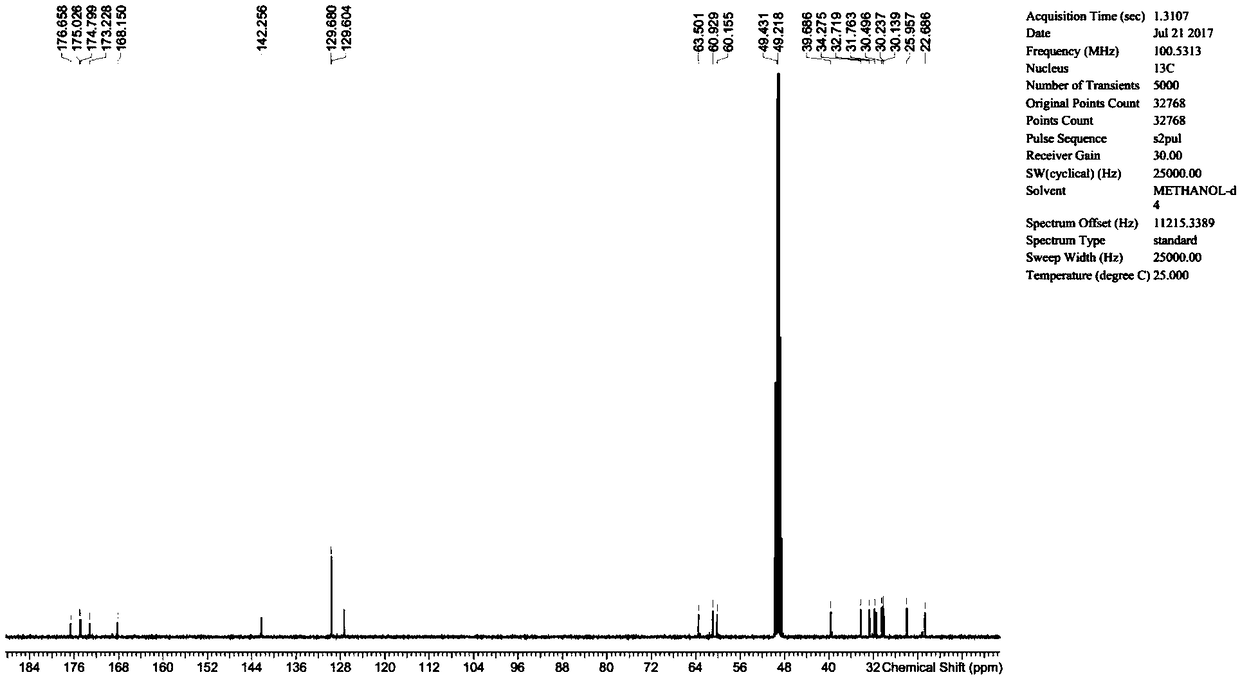
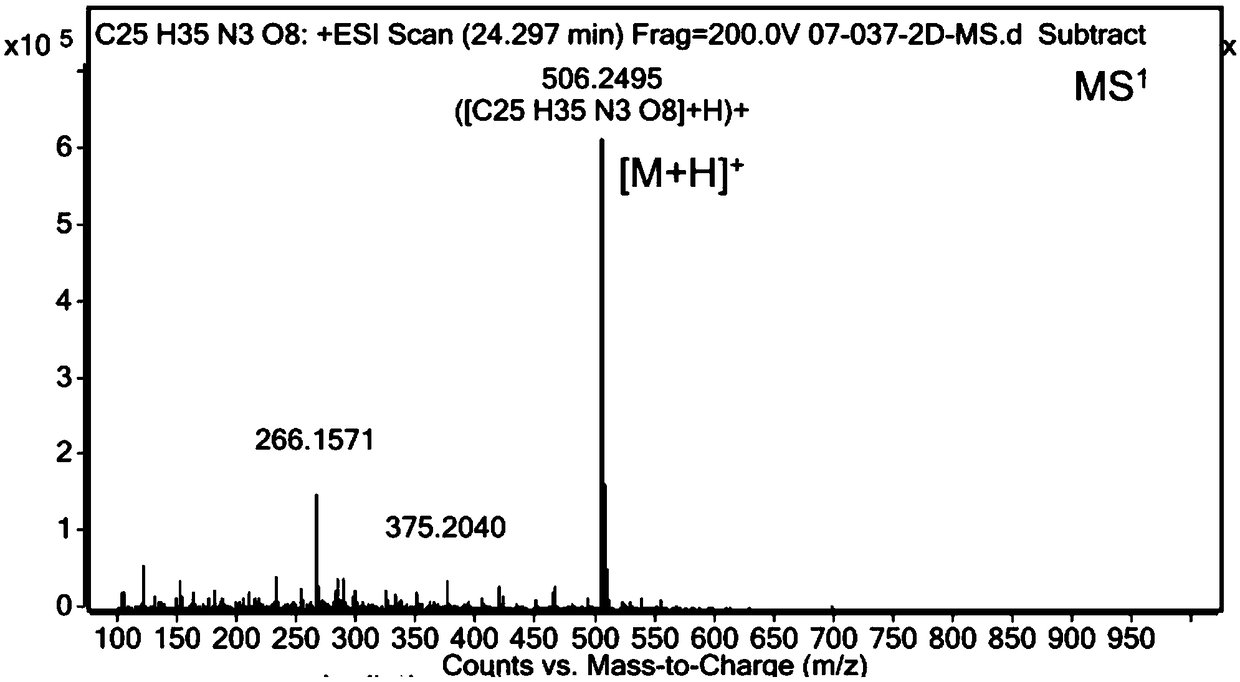
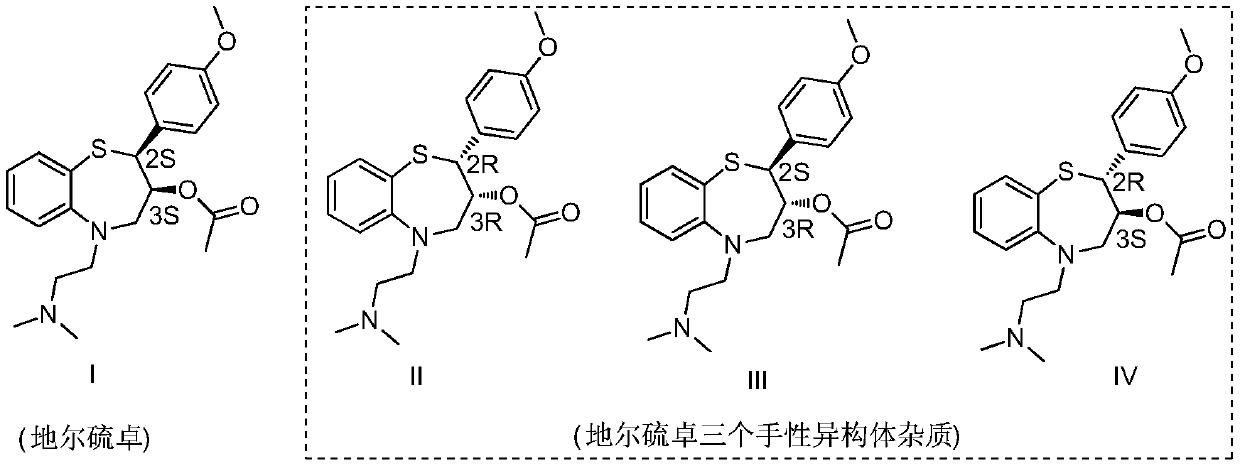
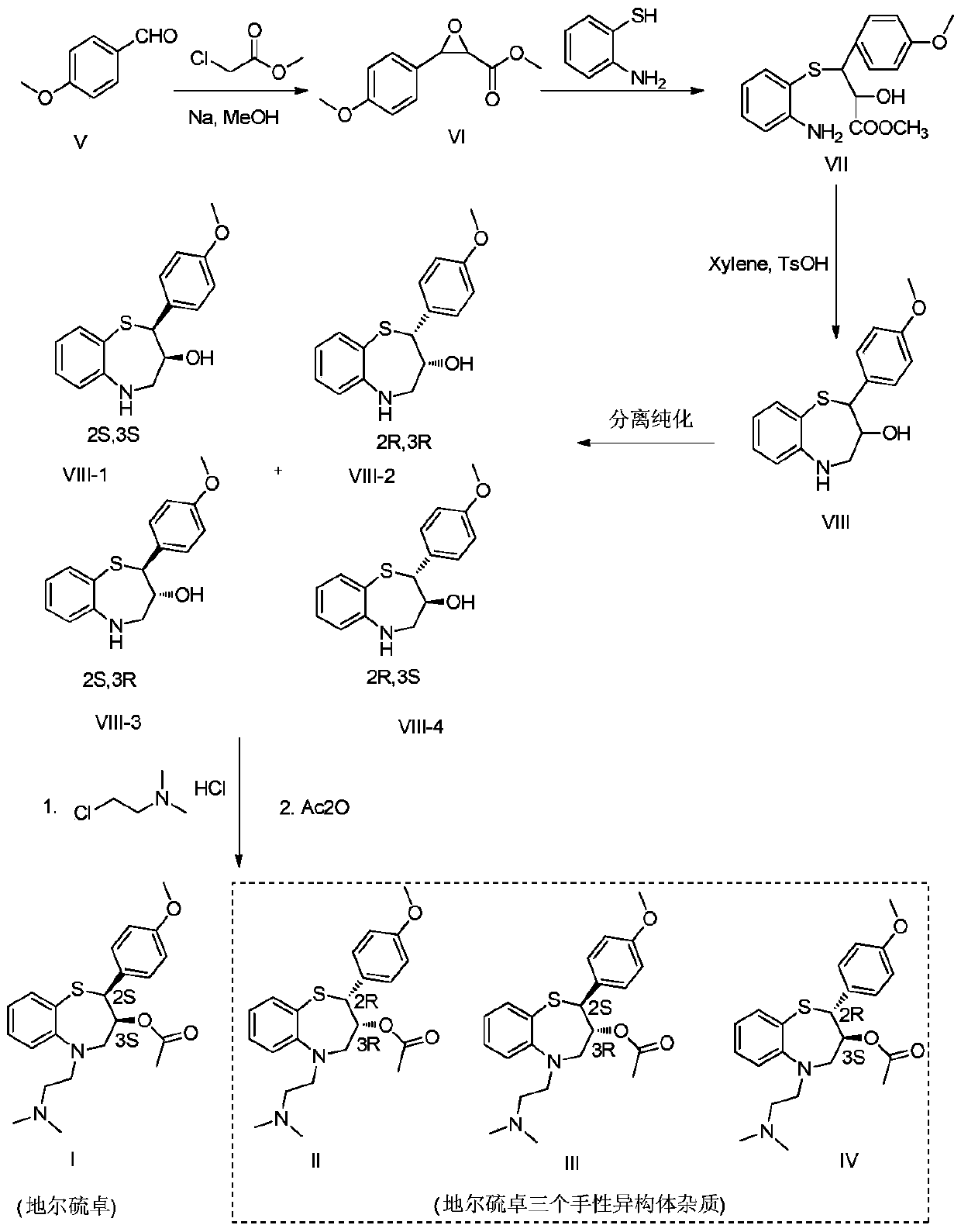
Preparation and purification method of diltiazem chiral isomer impurity
InactiveCN110407771AEasy Preparative SeparationHigh purityOrganic chemistry methodsPurification methodsDiltiazem
The invention provides a method for preparing and purifying a diltiazem chiral isomer. The method comprises the steps of taking p-methoxybenzaldehyde as a starting raw material, carrying out Darzens condensation, a reaction with o-aminobenzenethiol and a cyclization reaction to prepare an intermediate, carrying out chiral column separation on the intermediate, then carrying out an alkylation and acetylation reaction separately to obtain a diltiazem chiral isomer impurity I. The method is simple to operate and short in period, and the purity of the obtained diltiazem chiral isomer impurity is high, so that a reference substance is provided for quality research and drug synthesis process research of diltiazem drugs, and the method has important significance in control and checking of the chiral purity in the pharmaceutical research of diltiazem.
Owner:SHANDONG XINHUA PHARMA CO LTD
Ulipristal acetate related chiral impurities and synthetic preparation method thereof
InactiveCN109369766AQuality improvementEasy to controlComponent separationOrganic chemistry methodsGrignard reactionImpurity
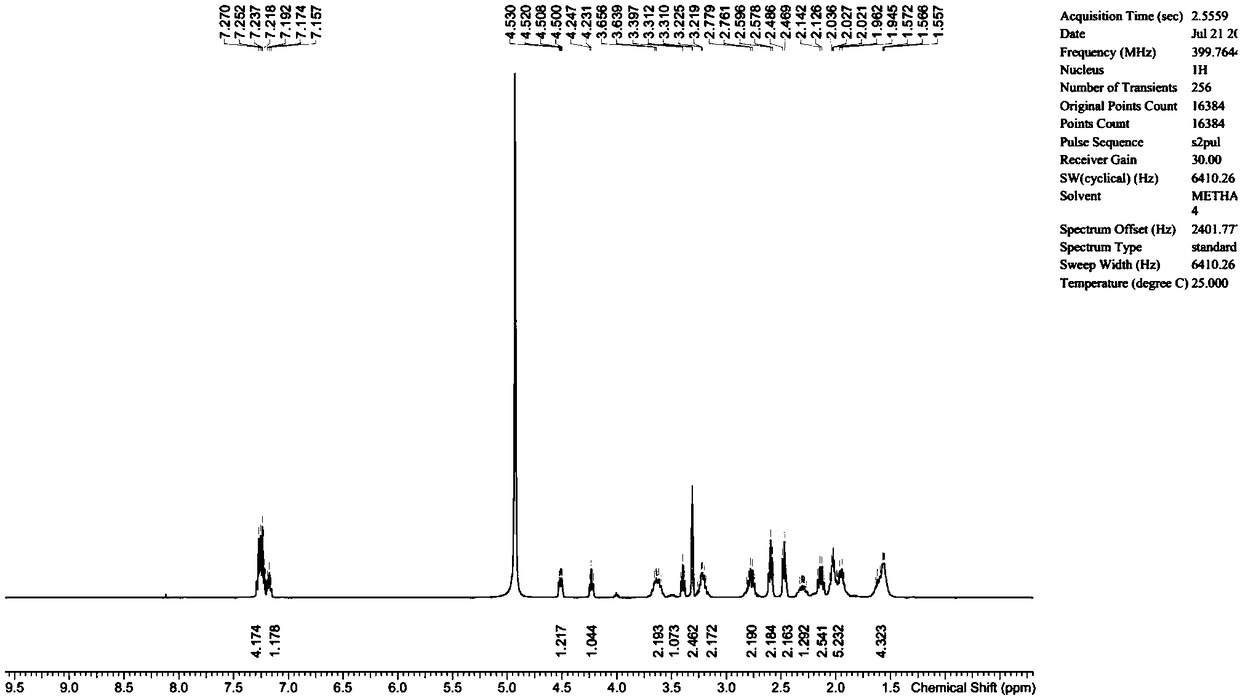
The invention discloses ulipristal acetate related chiral impurities and a synthetic preparation method thereof. Part of chiral impurities can be prepared by the following steps: by taking 3-ketal asan initial raw material, carrying out an addition reaction to obtain an intermediate II; deprotecting the intermediate II in an acidic condition to obtain an intermediate III; carrying out hydrolysisand oxidation to obtain an intermediate IV; and adding protecting groups to obtain the part of chiral impurities; or carrying out a Grignard reaction on a 3,20-diketal oxidative product to obtain thepart of chiral impurities. Structures of all the impurity compounds are confirmed by a magnetic resonance hydrogen spectrum, a high resolution mass spectrum and high performance liquid chromatography.The purities of the ulipristal acetate related chiral impurity compounds prepared by the method are over 95% and can be used as reference substances for quality research.
Owner:PHARMA CHANGZHOU PHARMA FACTORY NO 4 +1
Cetirizine hydrochloride tablet and preparation method thereof
ActiveCN108309948BImprove stabilityImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolMagnesium stearate
The invention belongs to the technical field of medicinal solid oral-taking preparation, relates to a cetirizine hydrochloride tablet and a preparation method thereof. The cetirizine hydrochloride tablet is prepared from the following raw materials and auxiliary materials of cetirizine hydrochloride, a filling agent, a flow aid, a bonding agent, a plasticizer, an opaquer, wherein the filling agentis prepared from microcrystalline cellulose and lactose; the flow aid is prepared from one or two of silicon dioxide, talcum powder and colloidal silicon dioxide; the lubricating agent is magnesium stearate; the bonding agent is prepared from one or two of hydroxypropyl methylcellulose, starch, dextrin or polyvinyl alcohol; the plasticizer is polyethylene glycol-400; and the opaquer is titanium dioxide. The product has high stability, can keep stable at high temperature and in a high-temperature environment; the product has high stability, can be stored for a longer time under the normal storage conditions, the shelf life of the product is prolonged, the product can be dissolved rapidly, bioavailability is high, as provided by external quality research, the product conforms to the regulation and standard, clinical bioequivalence can meet the requirement of the regulation. The invention further provides a preparation method, and the production prescription process is easy.
Owner:XINHUA PHARMA GAOMI CO LTD
Gas chromatgraphy-mass spectrometry detection method of residual chloromethyl methyl ether in crude drug
The invention discloses a gas chromatgraphy-mass spectrometry detection method of residual chloromethyl methyl ether in a crude drug. Alcohol is adopted as a solvent and a derivatization reagent, a capillary gas chromatography column, direct sample introduction and temperature programming are adopted, and a mass spectrometry detector is further adopted for detection. The method can effectively detect chloromethyl methyl ether in the crude drug; a spectrogram baseline is stable and free from drift; a sample treatment process of the method is simple, convenient and quick; alcohol serves as the solvent and the derivatization reagent at the same time; a derivatization reaction is mild, quick and complete. The method greatly simplifies a sample preparation process, avoids interference of a complicated derivatization reagent with detection and improves detection precision and accuracy; the method is used for determining residual chloromethyl methyl ether in the crude drug, has a good linearrelationship, high precision, high accuracy and high sensitivity and has an important research value in aspects such as crude drug quality research, and impurity analysis and control research.
Owner:上海药明康德新药开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com