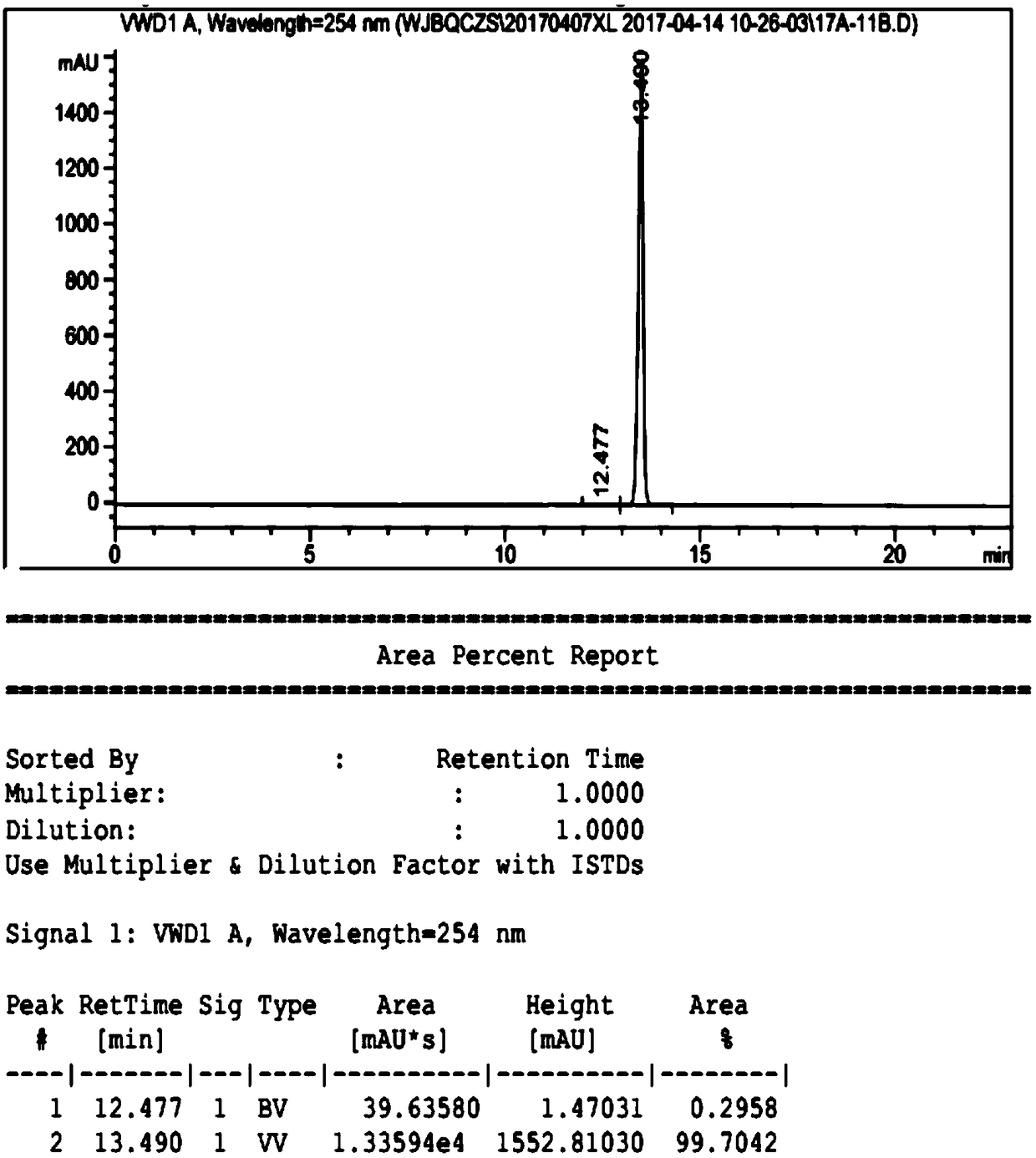
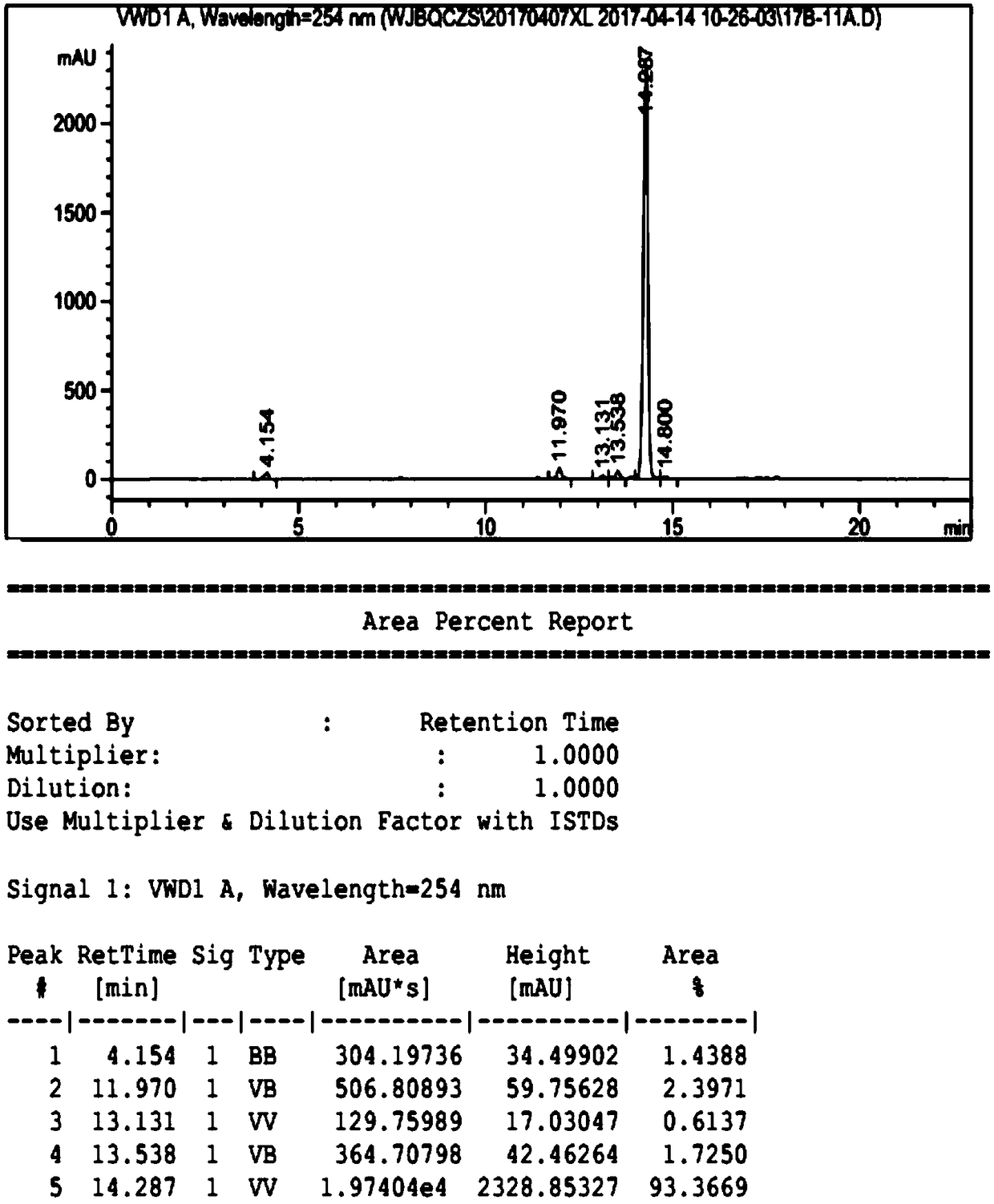
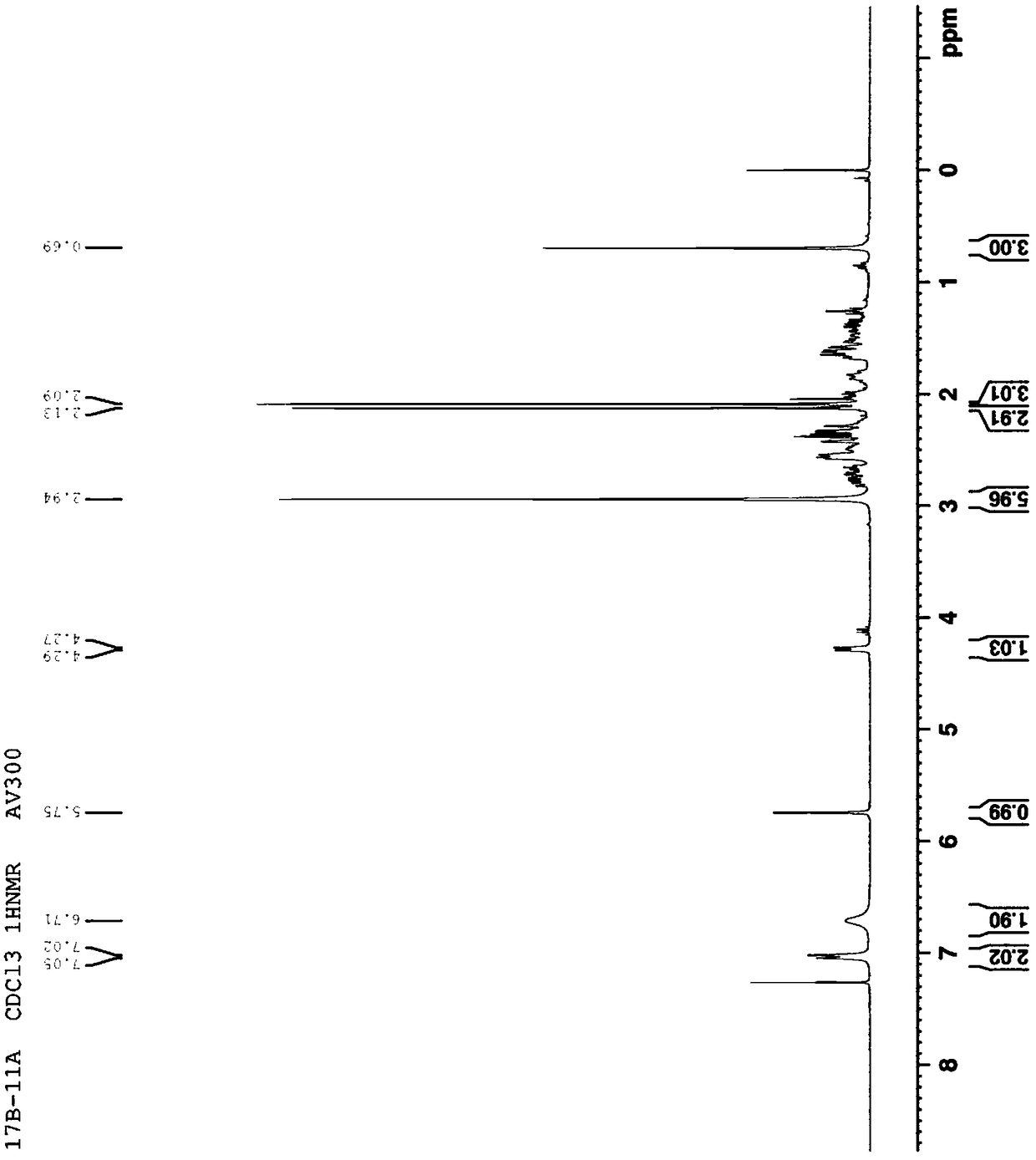
Ulipristal acetate related chiral impurities and synthetic preparation method thereof
An impurity and chirality technology, applied in the fields of organic chemistry and pharmaceutical synthesis chemistry, which can solve the problems of inability to separate thin-layer chromatography, inability to meet large-scale production, and unsuitable substrates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: the preparation of intermediate II
[0074]
[0075] Weigh 75g (668.39mmol) of potassium tert-butoxide, put it into a 1000mL three-neck flask, add 500mL of anhydrous THF, continuously feed acetylene gas, stir at room temperature for 1 hour, then cool to 0°C, add 100g of raw material I ( 318.28mmol), control the temperature below 0°C, keep the introduction of acetylene gas, monitor by TLC, add saturated ammonium chloride solution after the reaction, separate the organic layer, extract the water layer with THF, combine the organic layers, wash with water, concentrate, and The concentrated solution was slowly dropped into ice water, and the solid was precipitated, filtered with suction, and slurried with water to obtain 98 g of the product intermediate II, with a yield of 91%. HRMS (ESI + ): calcd for C 22 h 30 o 3 [M+H] + 341.2111,found 341.2109.
Embodiment 2
[0076] Embodiment 2: the preparation of intermediate III
[0077]
[0078] Weigh 30g (88.18mmol) of Intermediate II, put it into a 500mL single-necked bottle, add 300mL of glacial acetic acid, stir at room temperature, slowly drop in 2.5mL (44.09mmol) of perchloric acid, and control the reaction temperature below 25°C . TLC monitoring, after the reaction was completed, the reaction solution was slowly dropped into ice water, a solid was precipitated, and filtered by suction to obtain 20 g of the product intermediate III, with a yield of 77%. HRMS (ESI + ): calcd for C 20 h 25 o 2 [M+H] + 297.1849, found 297.1847.
Embodiment 3
[0079] Embodiment 3: the preparation of intermediate IV
[0080]
[0081]Weigh 10g (33.76mmol) of intermediate III, dissolve it in 100mL of THF, add 2.5g (8.44mmol) of mercuric sulfate, then add 30mL of 30% sulfuric acid solution, stir at 60°C, monitor by TLC, after the reaction , saturated sodium bicarbonate solution to adjust the pH, dichloromethane extracted the aqueous layer, combined organic layers, washed with water, dried, and column chromatography. 4.8 g of product intermediate IV was obtained, yield: 45%. HRMS (ESI + ): calcd for C 20 h 27 o 3 [M+H] + 315.1955, found 315.1957. 1 H NMR (300MHz, CDCl 3 ):δ1.12(s,3H,–CH 3 ),2.30(s,3H,–CH 3 ),1.26-2.89(m,18H),5.70(s,1H,=CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com