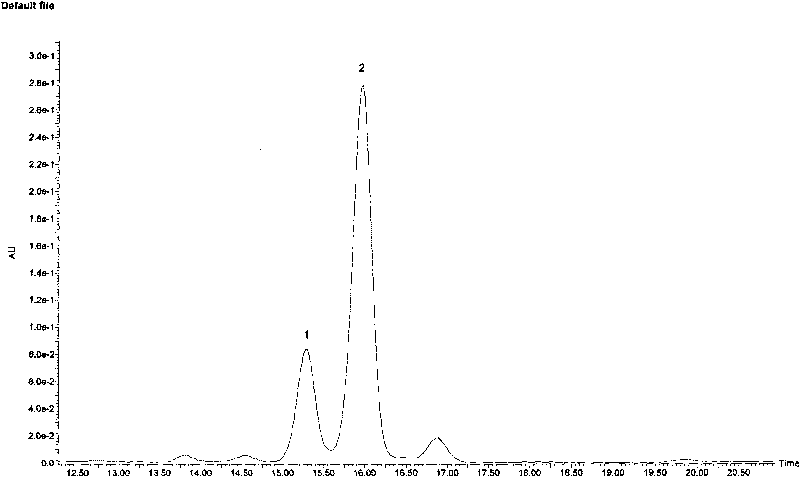
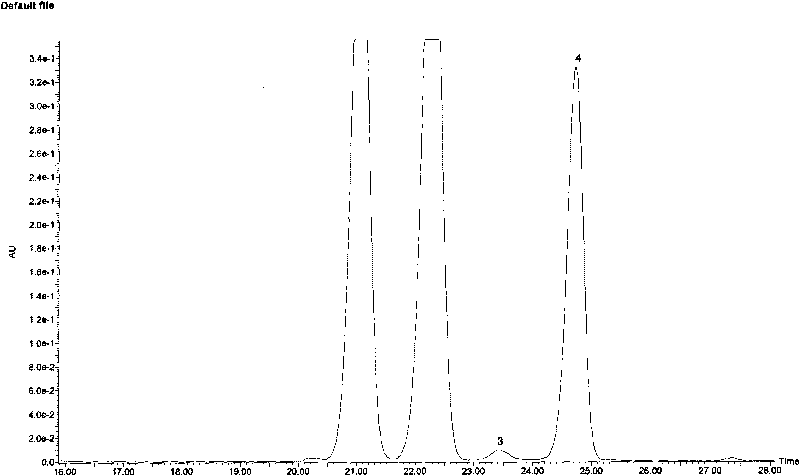
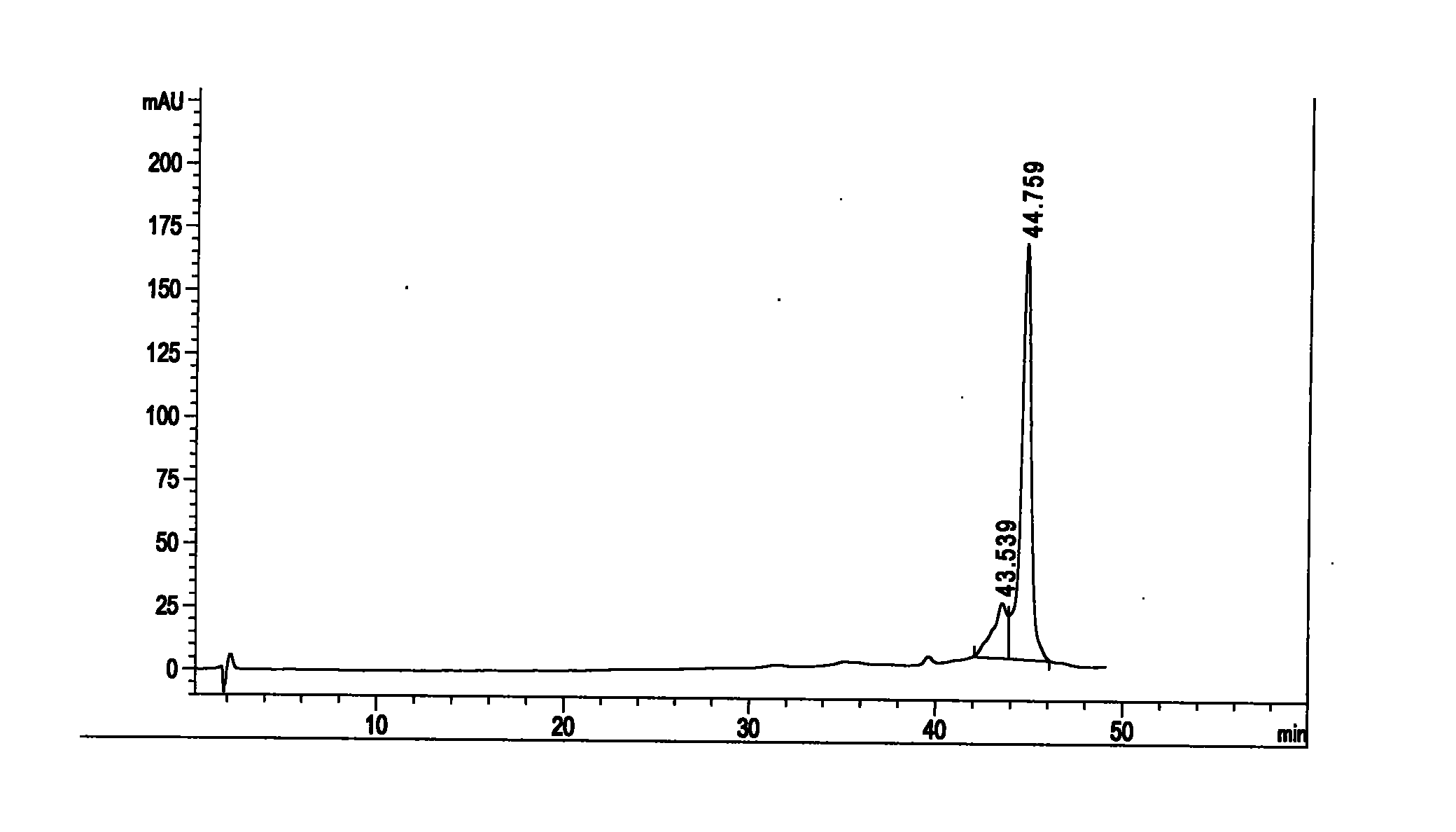
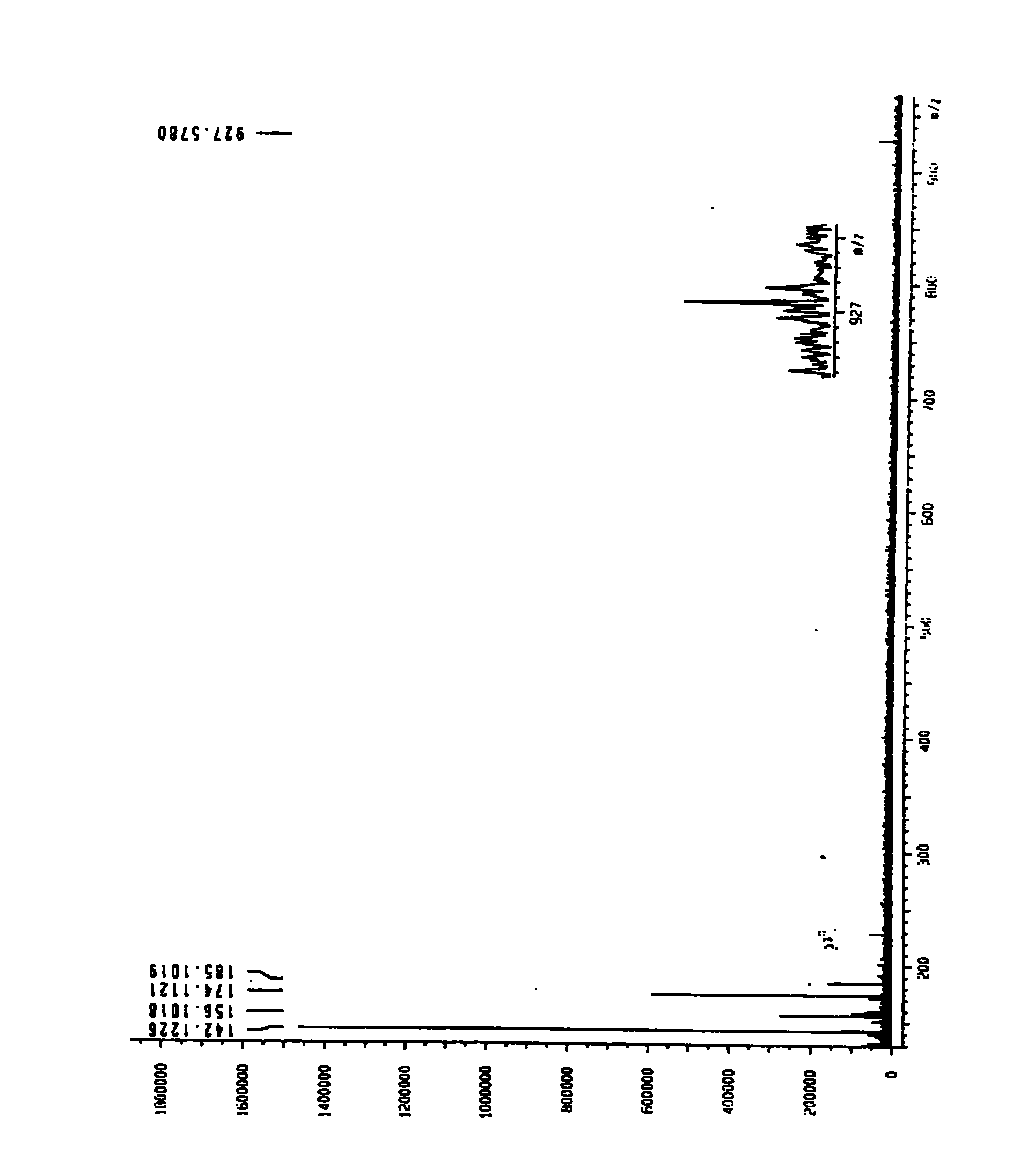
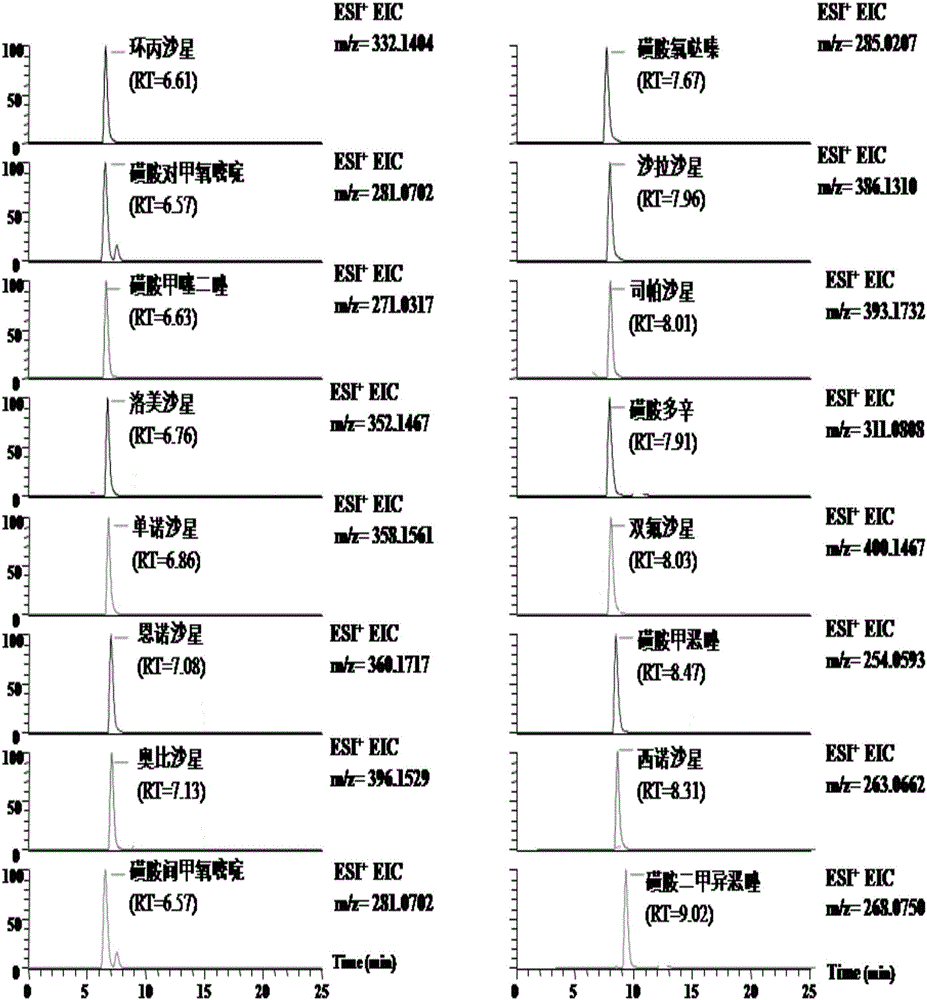
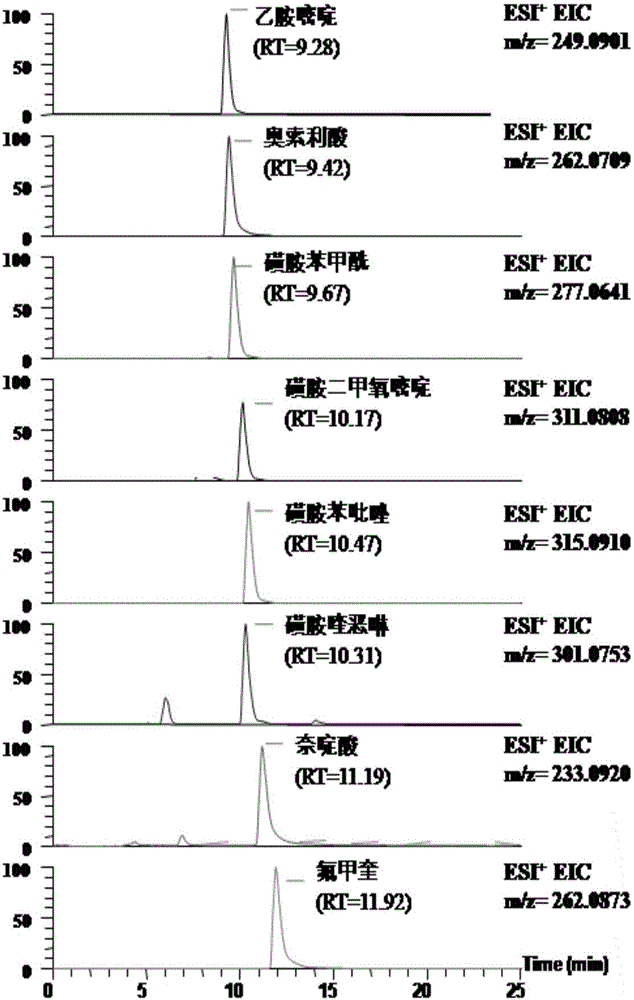
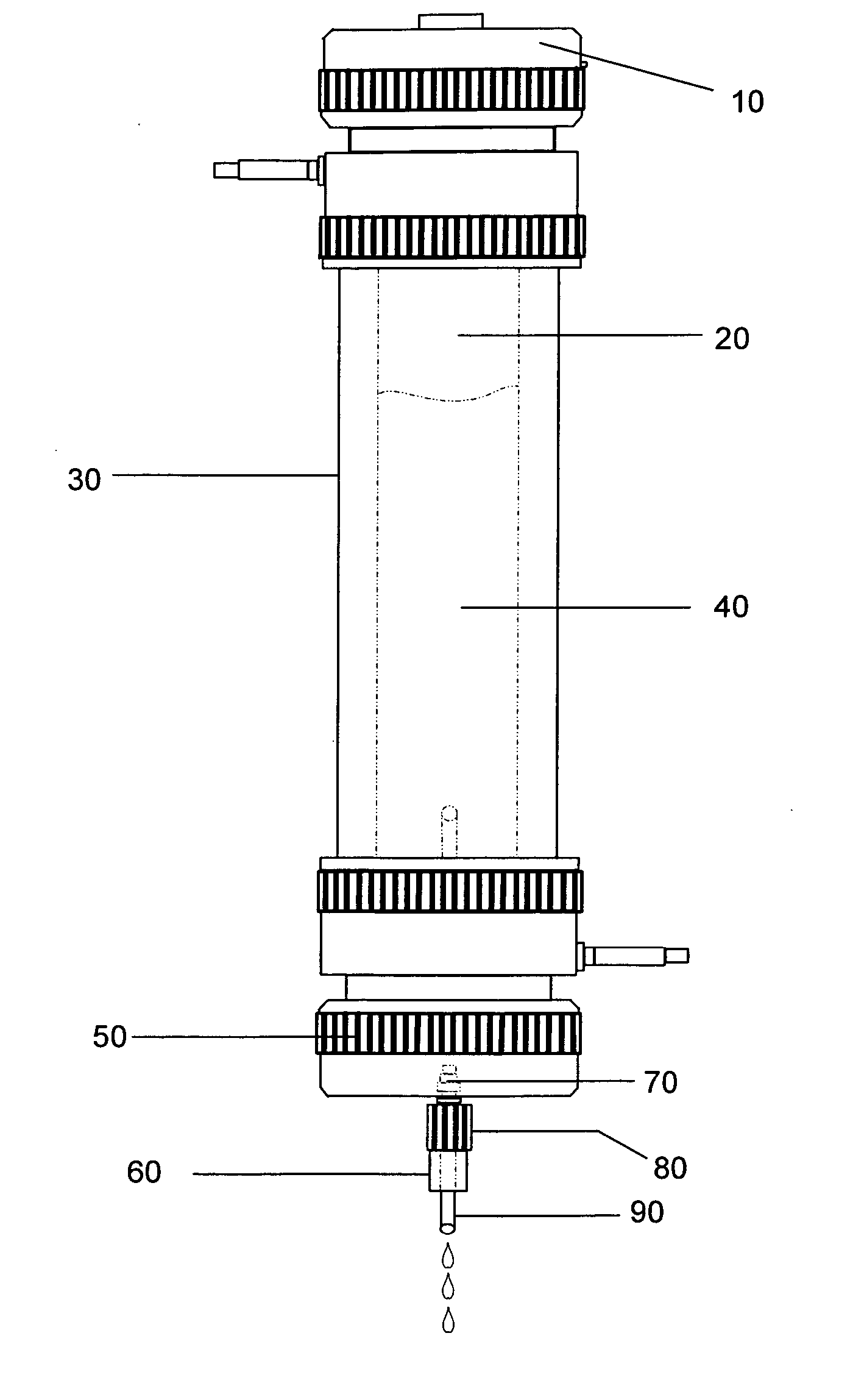
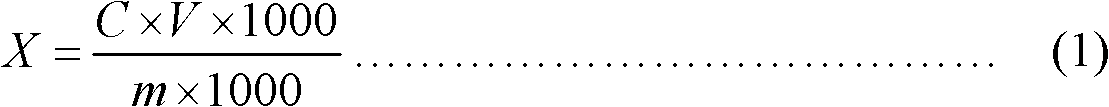Patents
Literature
8628 results about "Hplc mass spectrometry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
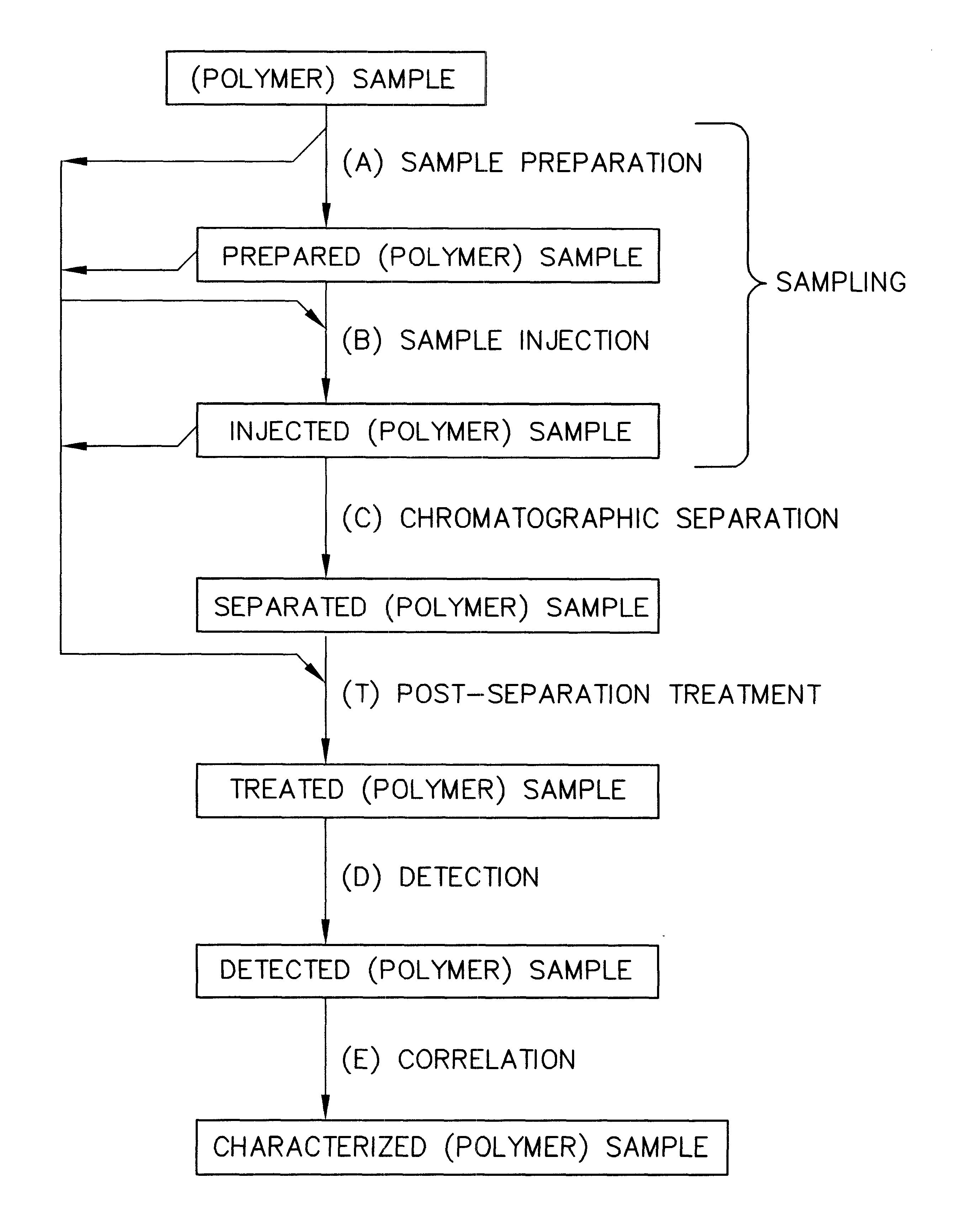
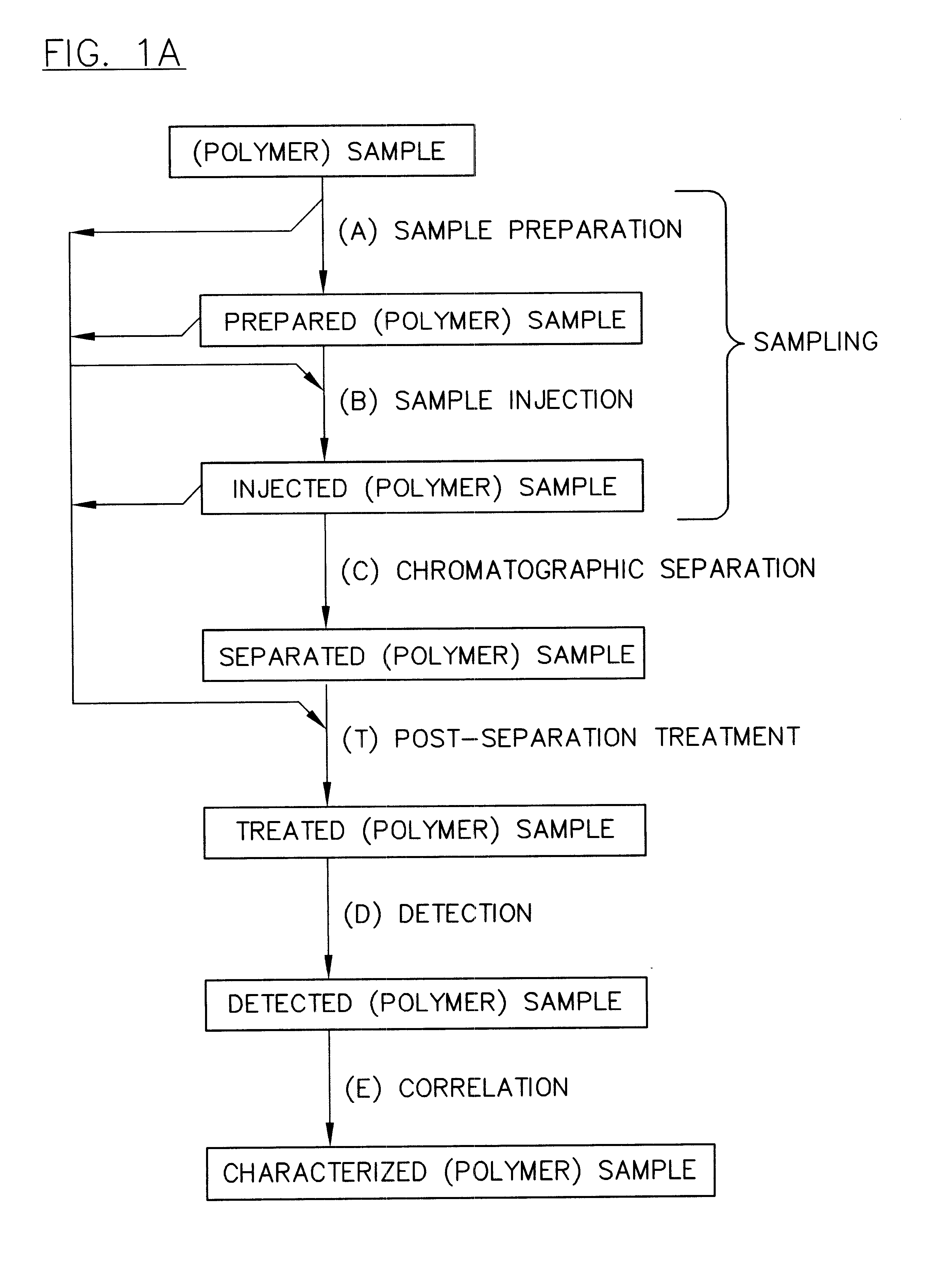
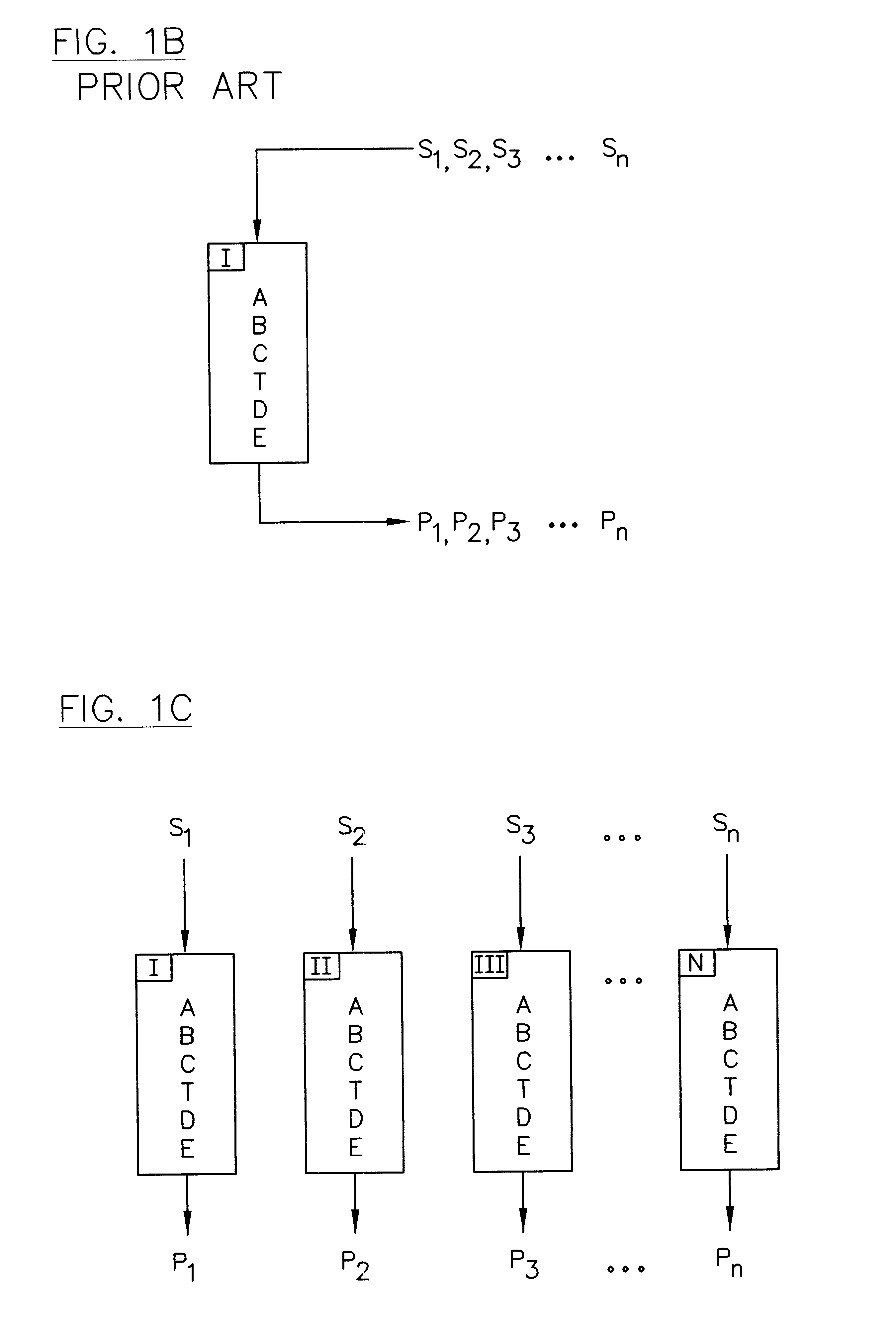
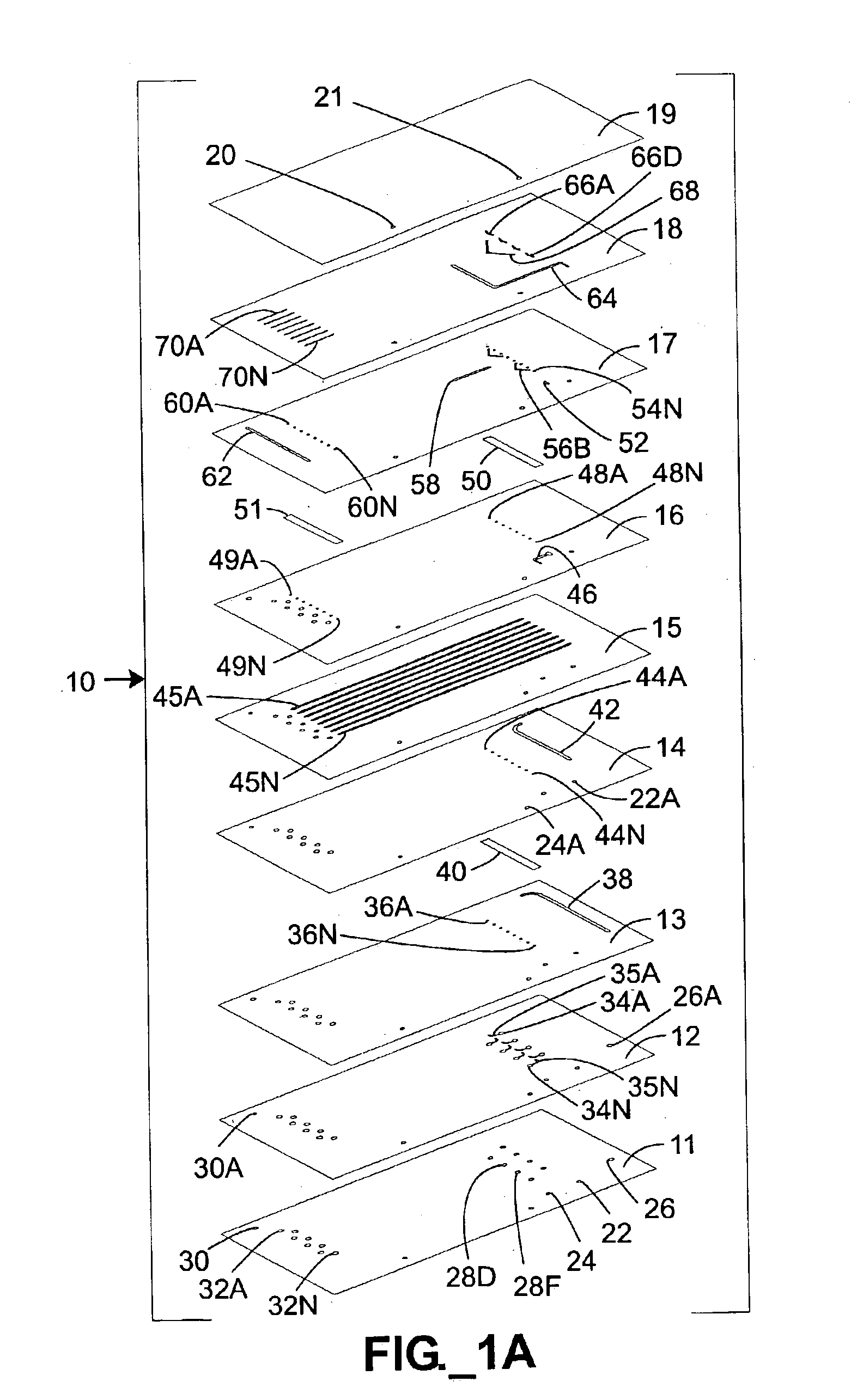
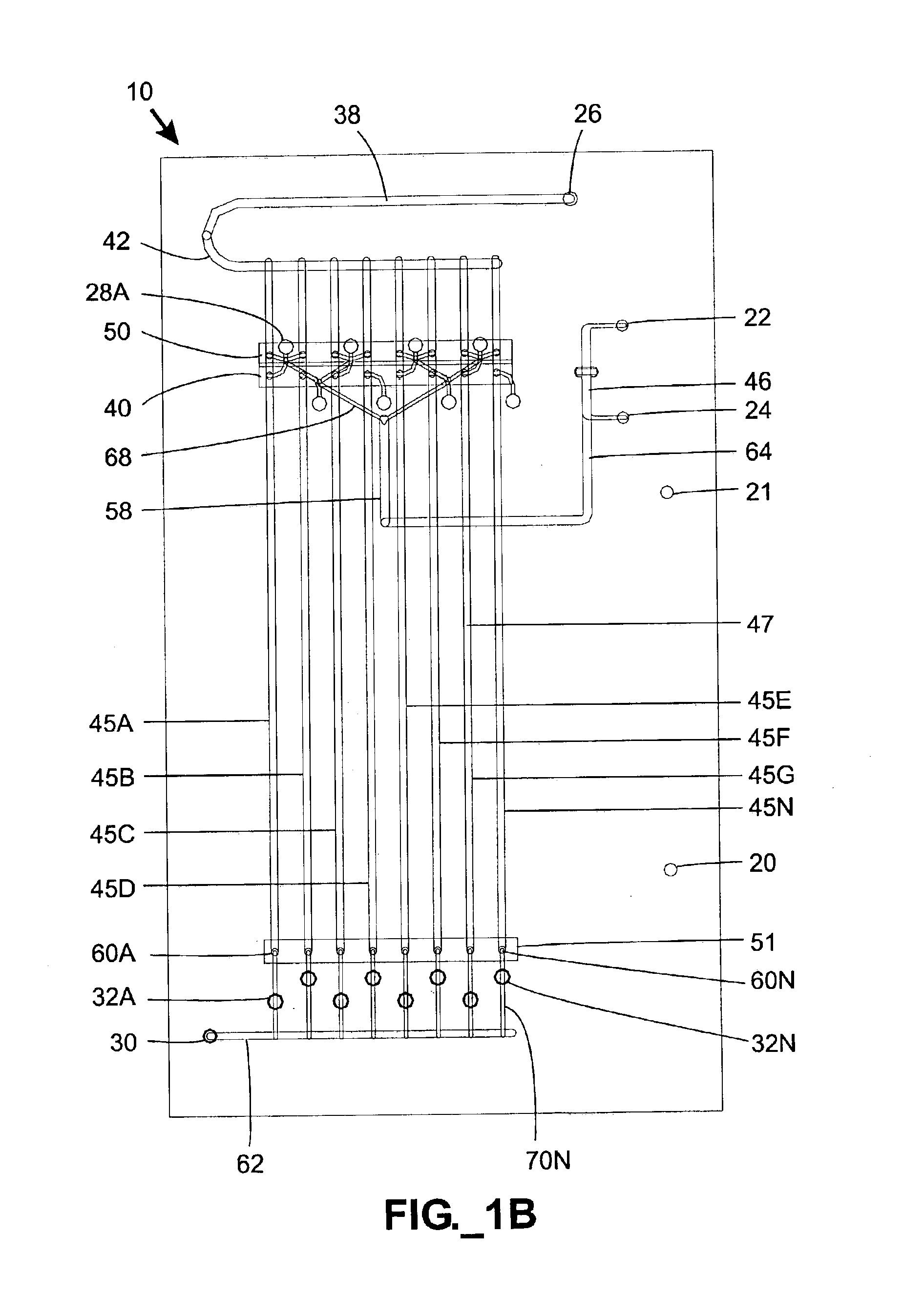
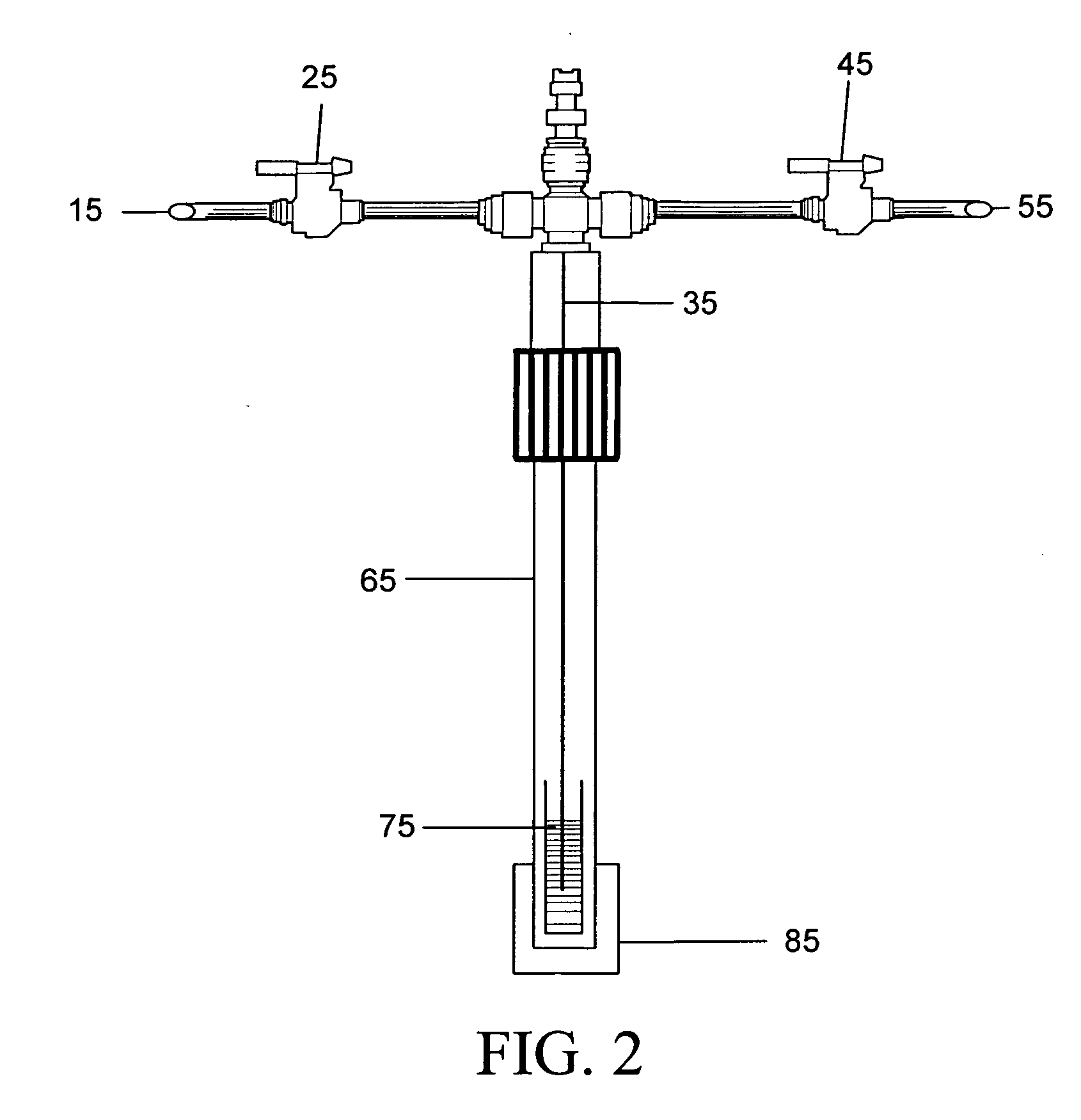
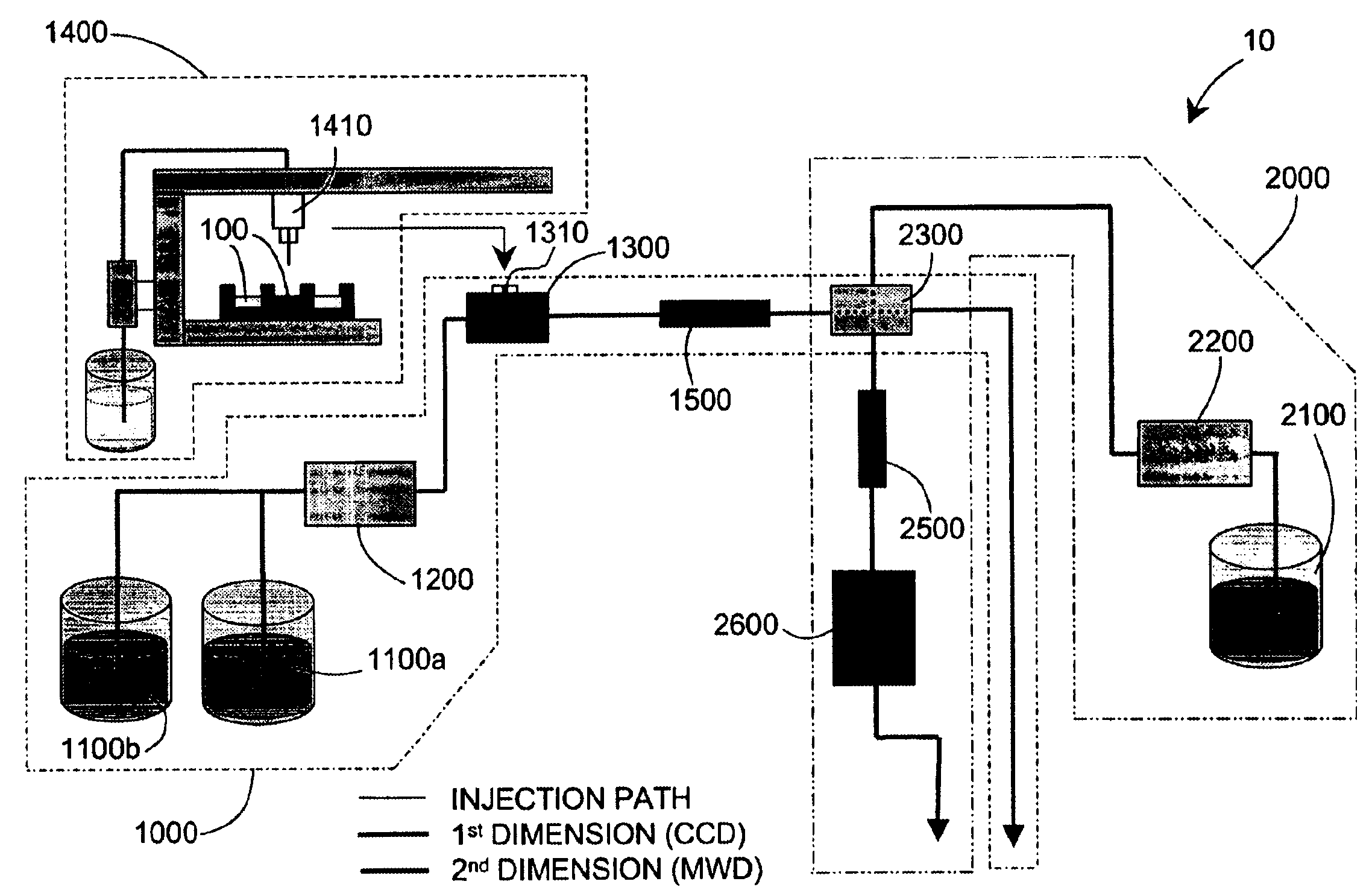
Parallel high-performance liquid chromatography with post-separation treatment
InactiveUS6436292B1High sample throughputEasy to findIon-exchange process apparatusSamplingChromatographic separationCombinatorial synthesis
High-performance liquid chromatography (HPLC) methods and systems are disclosed that combine parallel chromatographic separation of a plurality of samples with a detection technique that involves post-separation treatment of the plurality of samples to enhance one or more properties of the sample or of a component thereof, followed by detection of the one or more enhanced properties. Selective, tunable detection schemes are achievable, and are particularly advantageous as applied in connection with combinatorial chemistry, combinatorial material science and more particularly, combinatorial synthesis and screening of polymeric materials.
Owner:FREESLATE
Separation column devices and fabrication methods
Pressure-driven microfluidic separation devices, such as may be used for performing high performance liquid chromatography, are provided. Multiple separation columns may be defined in a single device and packed with stationary phase material retained by porous frits. One or more splitters may be provided to distribute slurry and / or mobile phase among multiple separation columns. In one embodiment, separation devices are substantially planar and fabricated with multiple device layers. Systems and methods employing slurry for packing separation devices are also provided.
Owner:AGILENT TECH INC
Sealing interface for microfluidic device
A threadless interface for a fluidic system includes a microfluidic device having an outer surface and an internal near-surface channel having a first width and disposed at a first depth relative to the outer surface, with the first width being less than about two times the first depth. A fluidic seal engages the outer surface and exerts an elevated contact pressure against at least a portion of the outer surface without substantially occluding the channel. A preferred seal includes a raised boss. A fault tolerant flow path design can accommodate misalignment between adjacent device layers without detrimentally affecting fluid flow capability. The interface may be used in a microfluidic system for performing parallel analyses such as high performance liquid chromatography.
Owner:AGILENT TECH INC
Highly purified tocopheryl phosphate, process for producing the same, analytical method therefor and cosmetic
InactiveUS6046181AGood water solubilityNo skin irritationOrganic active ingredientsCosmetic preparationsSolubilityCosmetic ingredient
Disclosed herein are a highly purified tocopheryl phosphate and / or a salt thereof (tocopheryl phosphates) wherein a P,P'-bistocopheryl hypophosphate and / or a salt thereof (P,P'-bistocopheryl diphoshates) is contained in a proportion of not higher than 3% by weight; a process for producing a highly purified tocopheryl phosphate and / or a salt thereof, which comprises the steps of reacting a tocopherol with an oxyphosphorus trihalide followed by treating with an acid or basic aqueous solution to thereby form tocopheryl phosphates (i) in which P,P'-bistocopheryl diphoshates (ii) formed as by-products are contained, hydrolyzing the P,P'-bistocopheryl diphoshates (ii) under acid condition, and, optionally, rendering the hydrolyzate neutral or basic under basic condition; and a method of analyzing tocopheryl phosphates, comprising analyzing a sample containing components (i) and (ii) with the use of a high-performance liquid chromatograph column packed with a gel of a polymethacrylate having, bonded thereto, long-chain alkyl groups. None or only an extremely minute amount of P,P'-bistocopheryl diphoshates are contained in the highly purified tocopheryl phosphates, so that the highly purified tocopheryl phosphates exhibit antioxidant and blood circulation promoting effects, have excellent water solubility, are powdery so that the handling thereof is extremely easy, are free from cutaneous irritation and allergenecity and ensure dermal safety. Therefore, the highly purified tocopheryl phosphates are useful as cosmetic ingredients. The amounts of components (i) and (ii) can be simply measured with high accuracy by the above method.
Owner:SHOWA DENKO KK
Measuring Analyte Concentrations in Liquids
InactiveUS20080137065A1Higher kinetic energy mixingSmall dropletSamplingComponent separationElectrical mobilityDroplet size
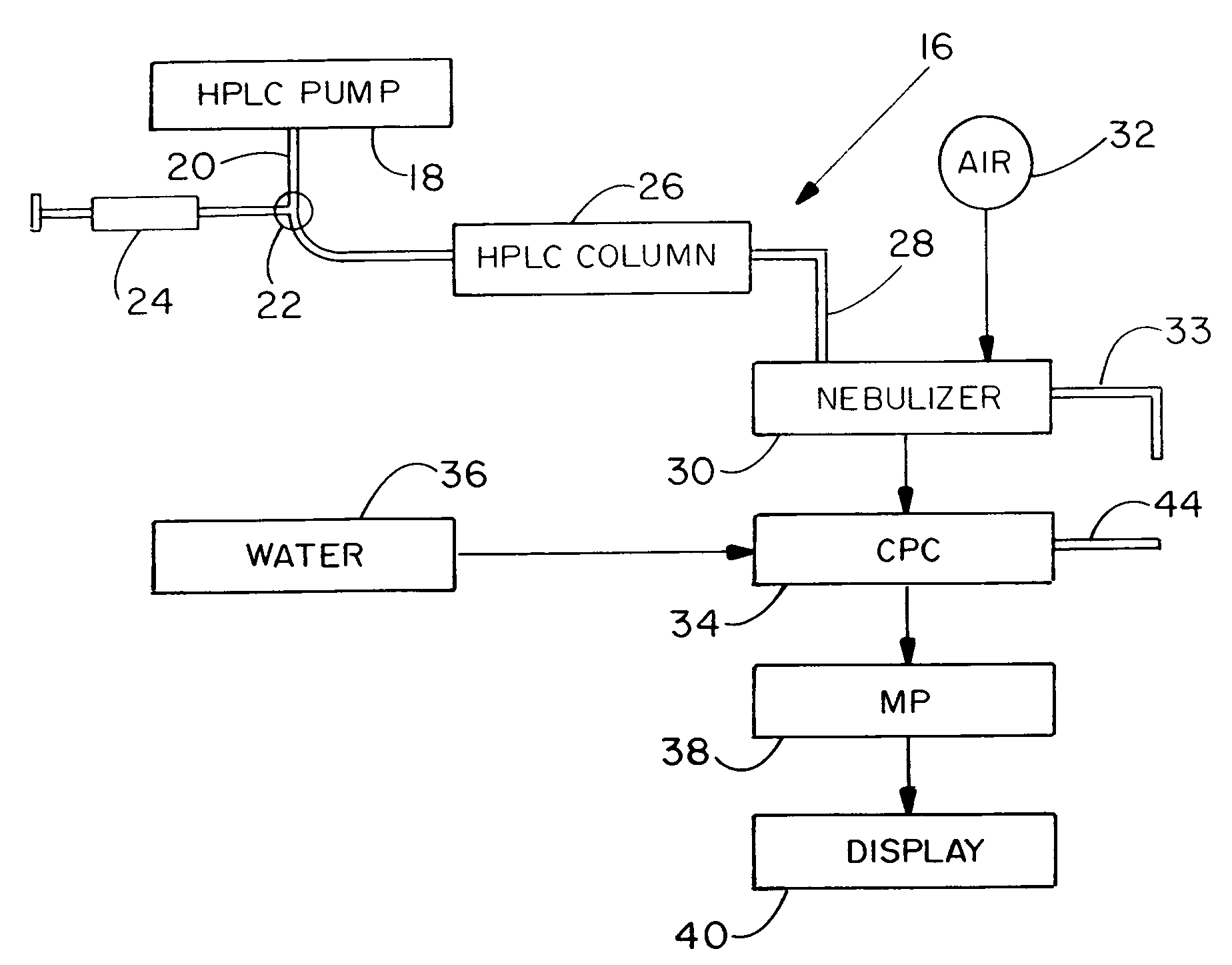
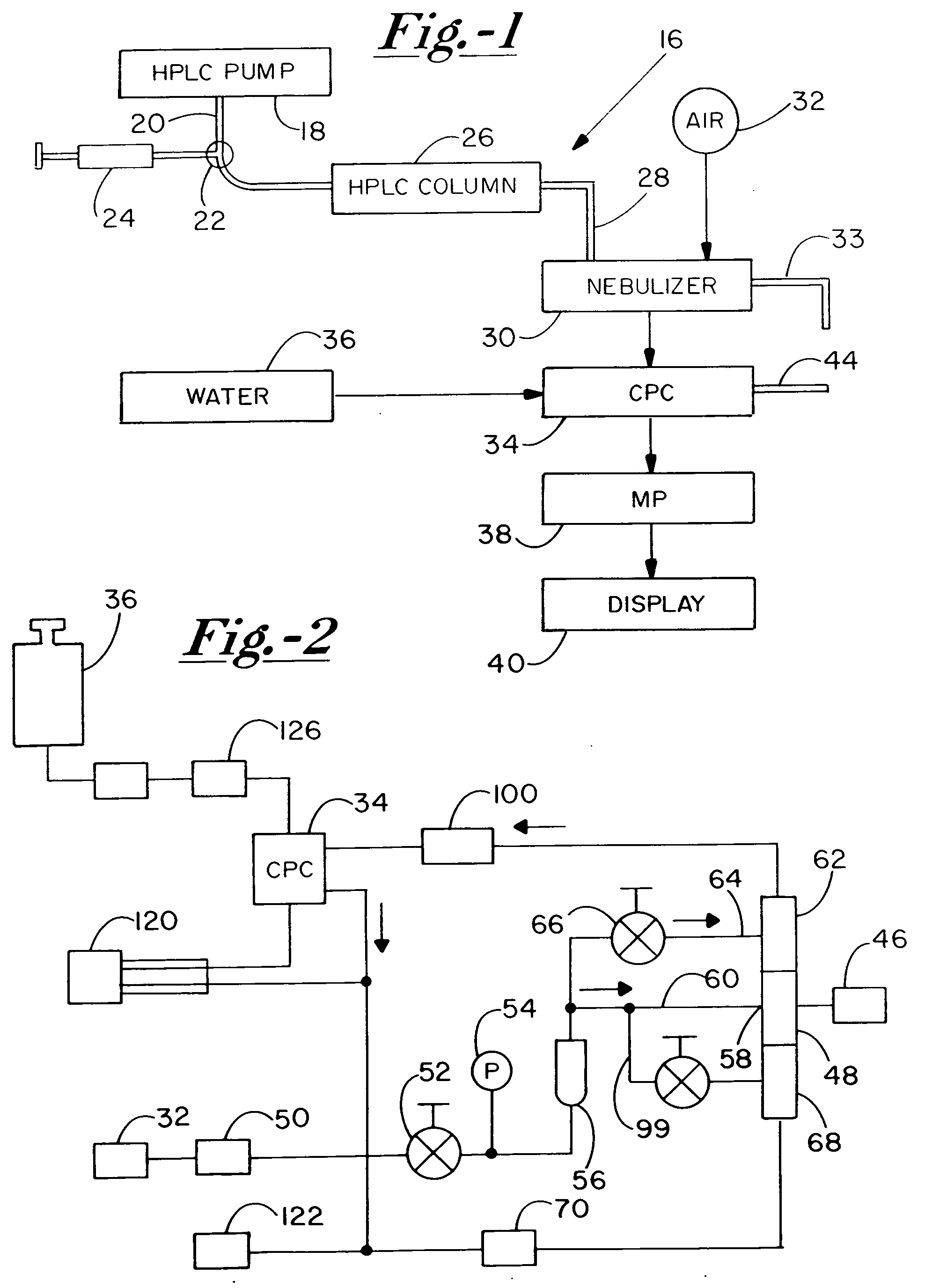
A high performance liquid chromatography system employs a nebulizer with a flow restriction at the exit of its mixing chamber to produce finer droplets, and an adjustable impactor for increased control over droplet sizes. Downstream of the mixing chamber, the nebulizer can incorporate tubing that is permeable to the sample liquid, to promote aerosol drying through perevaporation. A condensation particle counter downstream of the nebulizer uses water as the working medium, and is adjustable to control threshold nucleation sizes and droplet growth rates. A particle size selector employing diffusion, electrostatic attraction or selection based on electrical mobility, is advantageously positioned between the nebulizer and the CPC.
Owner:TSI INC
Programmable tracking pressure regulator for control of higher pressures in microfluidic circuits
InactiveUS6662818B2Low costImprove precision controlValve arrangementsControlling ratio of multiple fluid flowsCapillary networkEngineering
Regulator for precision control of pressure based on a means of measuring pressure differentials. More specifically, the present invention provides a pressure control that tracks a relatively high background pressure, and applies a positive or negative offset to create the small pressure differentials that can be utilized to transport fluids within a capillary network. The present invention is also directed to a method of controlling microfluidic elements (such as donut cavities) with a high degree of precision. In high performance liquid chromatography applications, this is accomplished using tracking pressure regulators to measure and respond to the difference between the liquid pump pressure and the regulated pneumatic pressure.
Owner:PERSPECTIVE BIOSYST
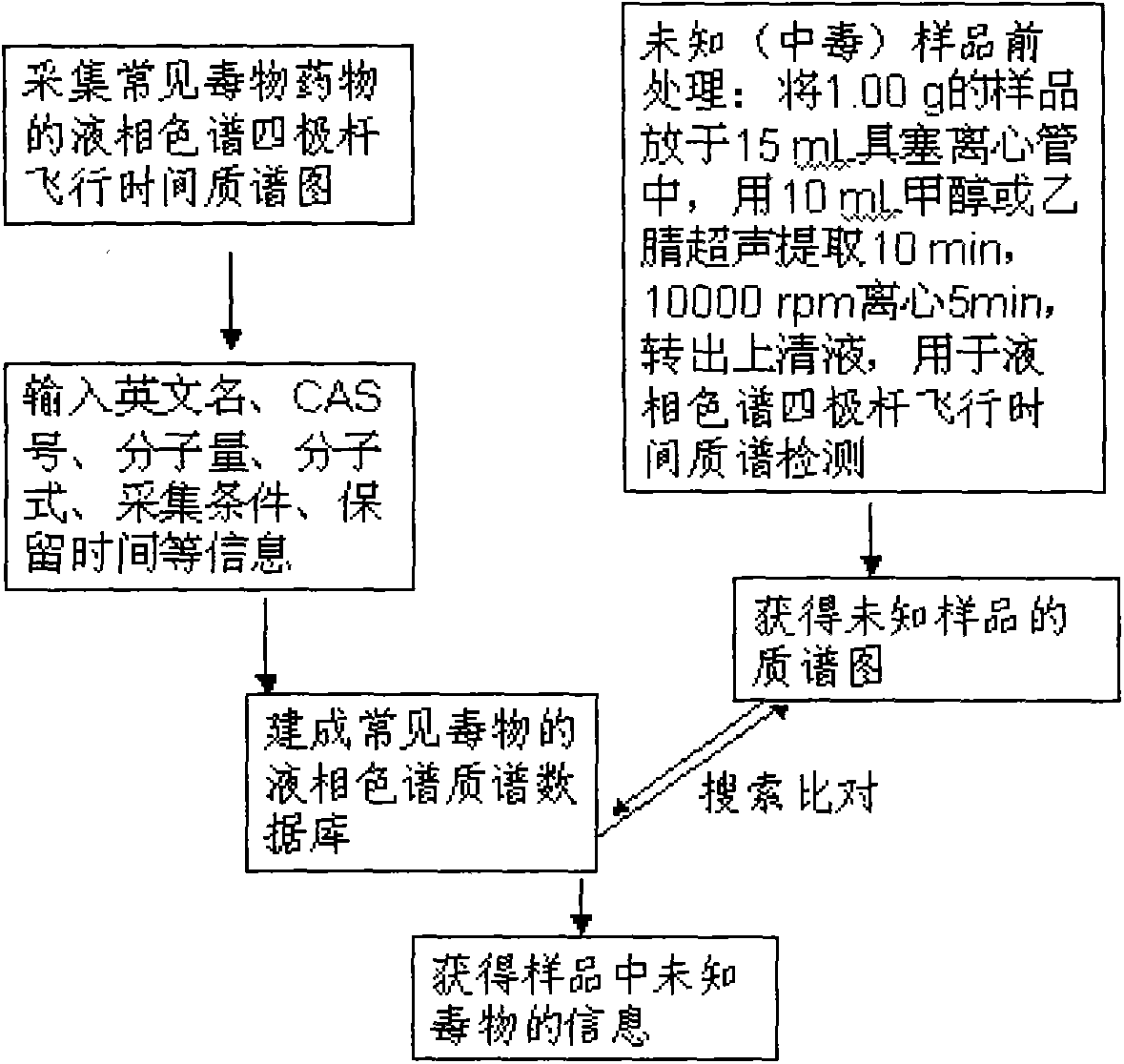
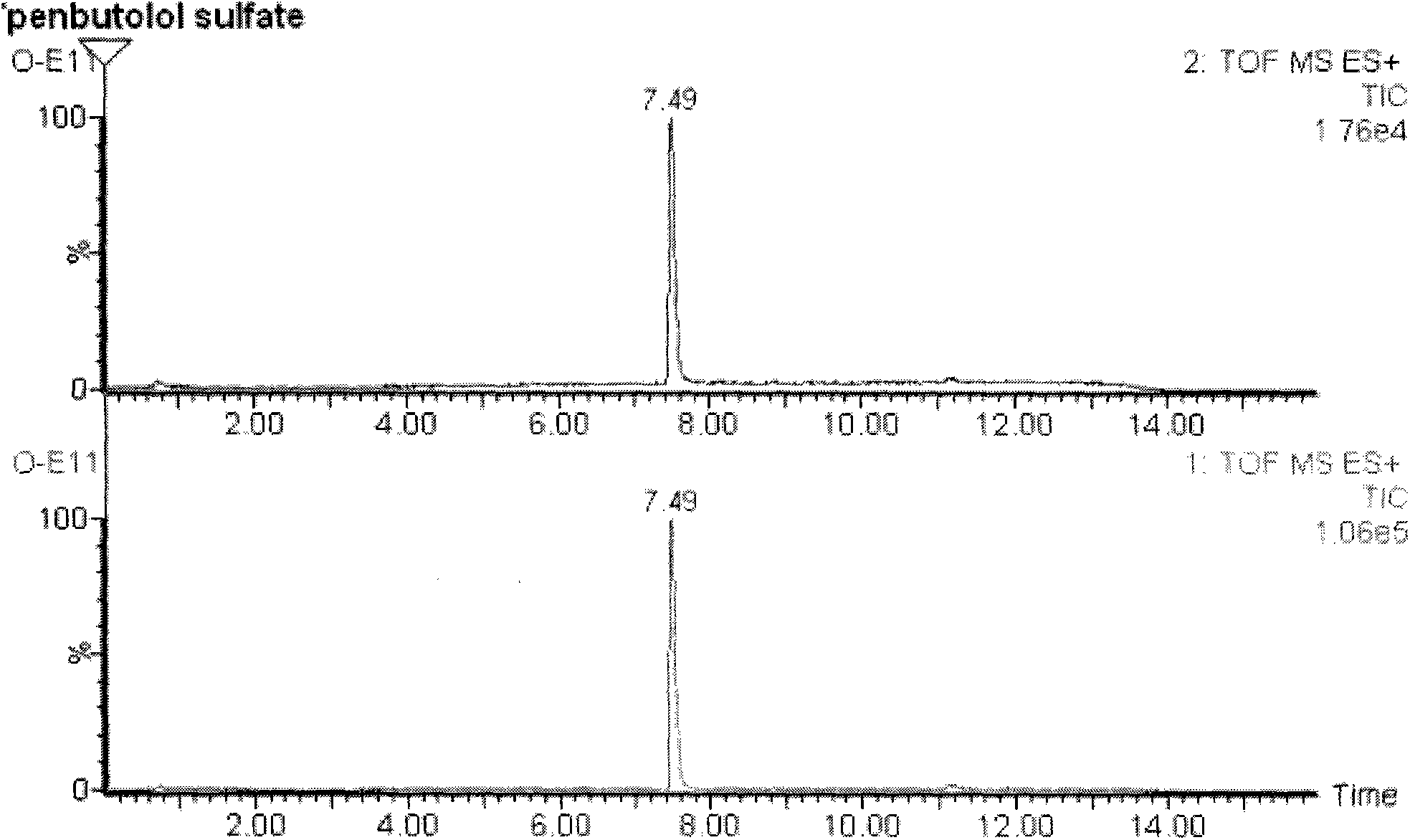
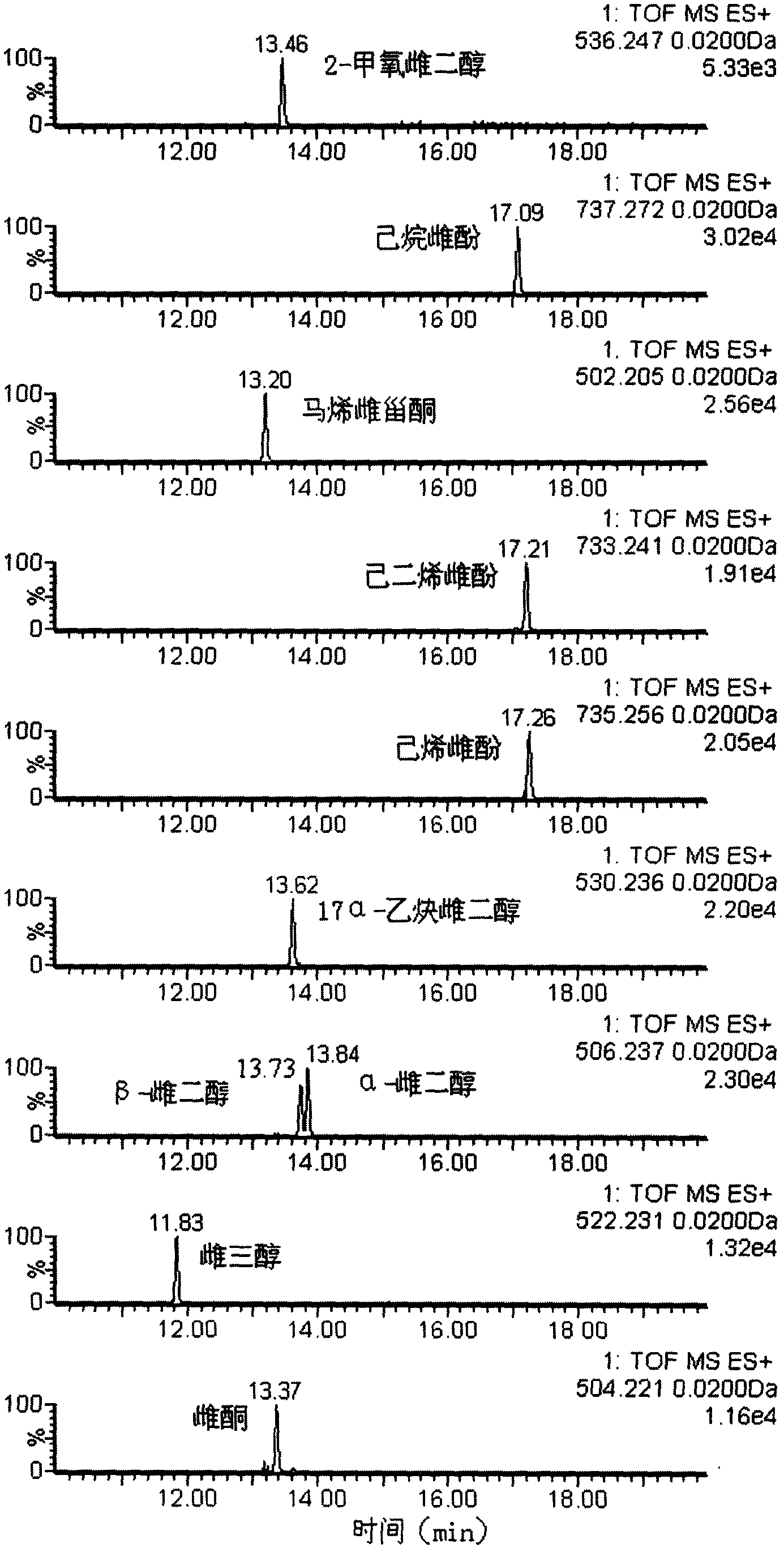
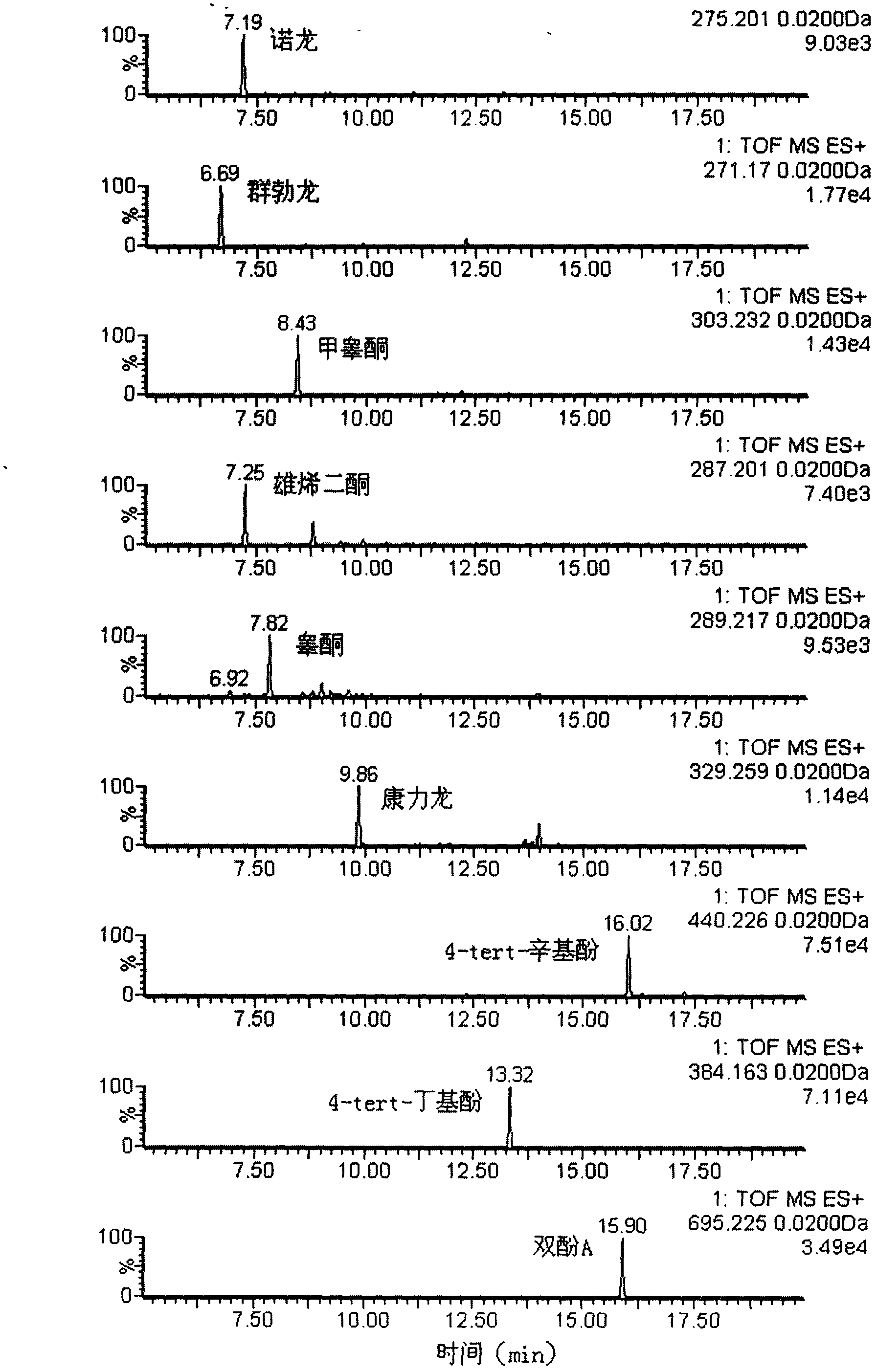
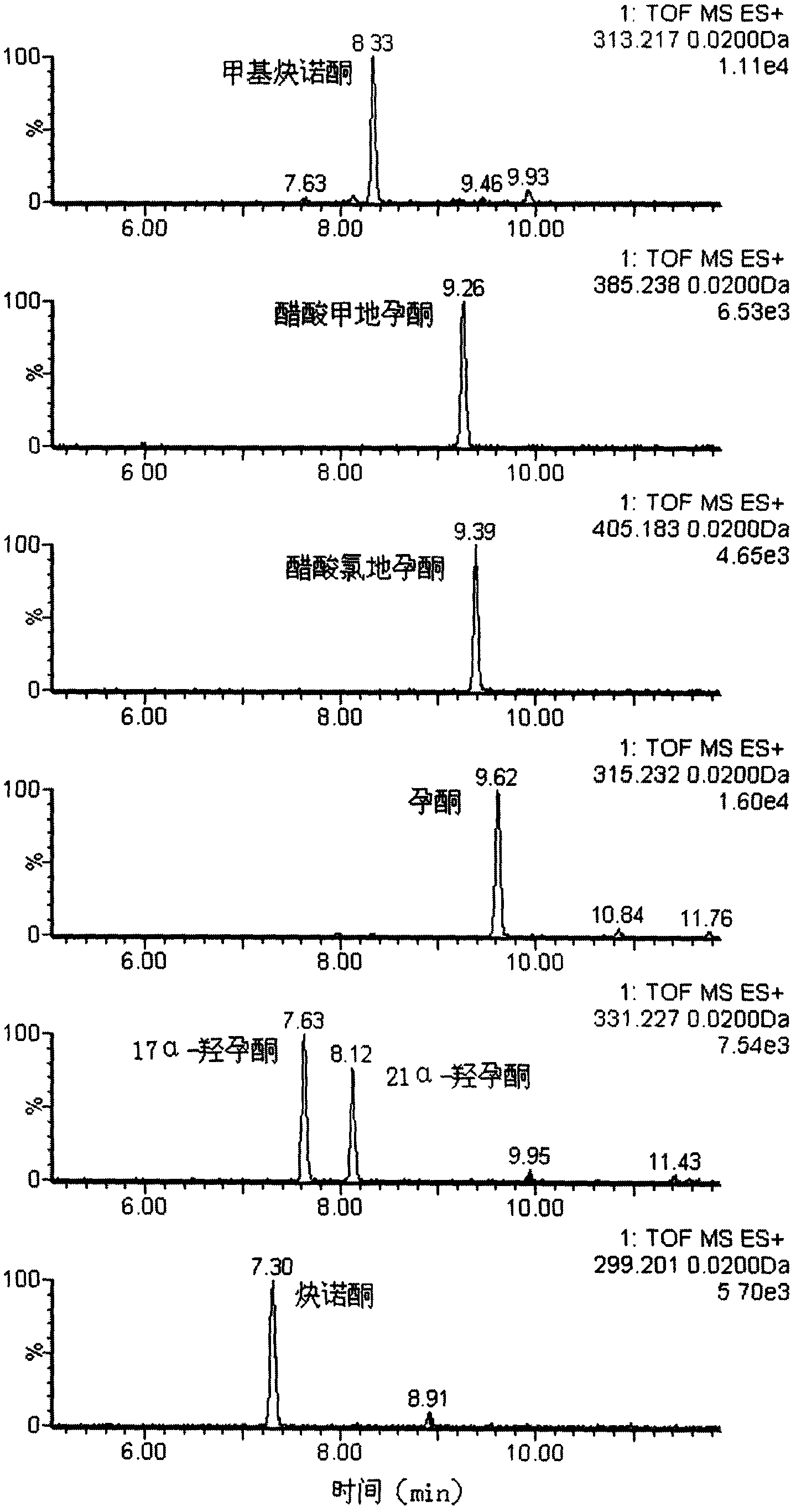
Method for detecting unknown poison by establishing liquid chromatography-mass spectrometry database
ActiveCN103823008AShorten detection timeReduce processing timeComponent separationMass spectrometry measurementToxicant
The invention relates to a method for detecting unknown poison by establishing a liquid chromatography-mass spectrometry database, in particular to a method used for detecting unknown poison during food poisoning. According to the method, firstly, an ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry method is used for establishing a liquid chromatography-mass spectrometry database of common poison; then, a sample is subjected to supersonic extraction with methyl alcohol or acetonitrile, liquid chromatography-mass spectrometry data of an extracting solution are measured similarly and searched and compared in the liquid chromatogram-mass spectrometry database of common poison according to the retention time of the sample and mass spectrometry fragments, and the variety of the unknown poison in the sample is judged; and the unknown poisoning sample is simply extracted and directly measured and compared, and a screening result can be acquired in one hour, so that the detecting and treating time of the sample is greatly shortened, the detecting efficiency is improved, and technical support is provided for related events such as food poisoning and the like caused by unknown reasons.
Owner:BEIJING CENT FOR DISEASE PREVENTION & CONTROL
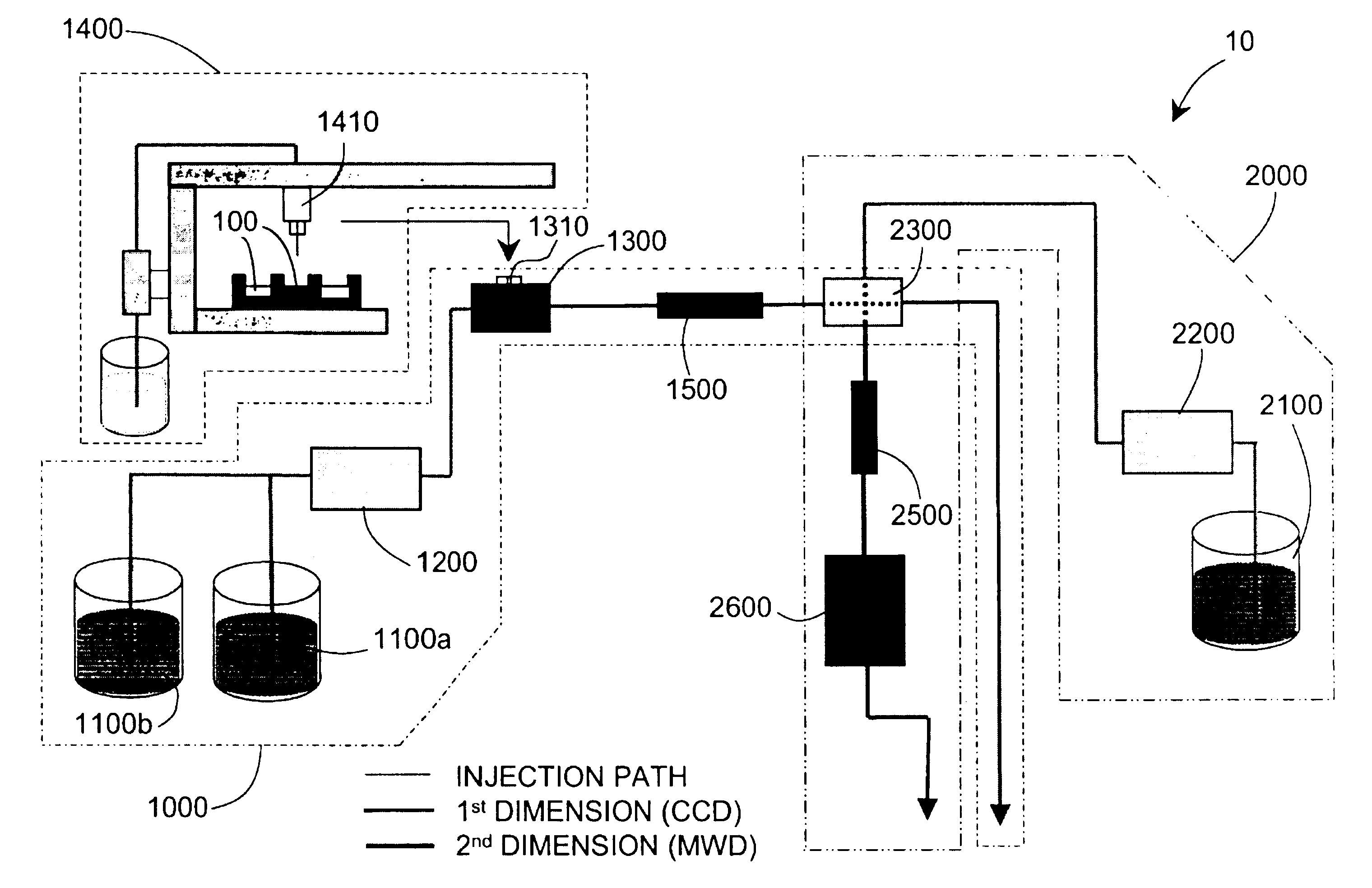
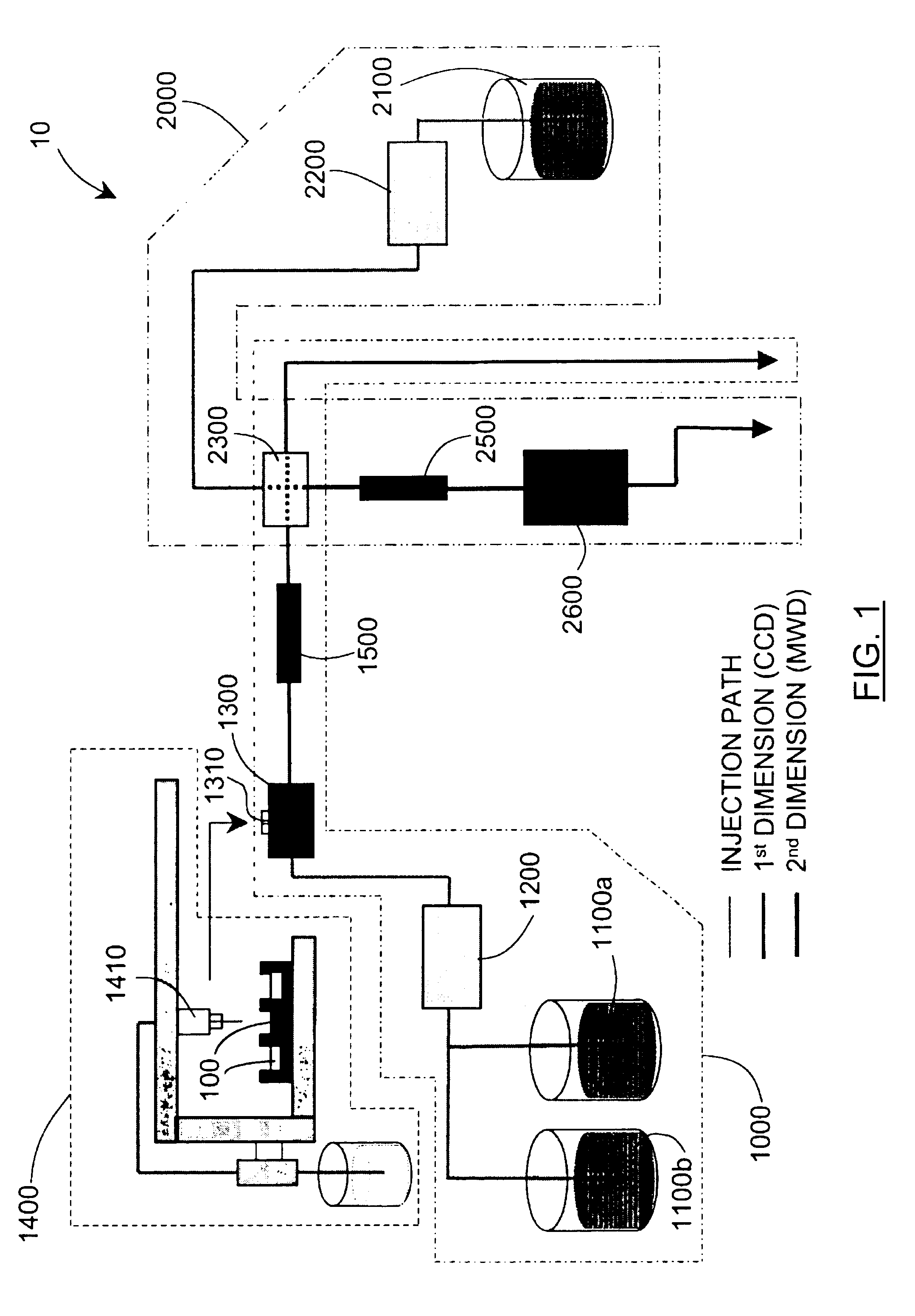
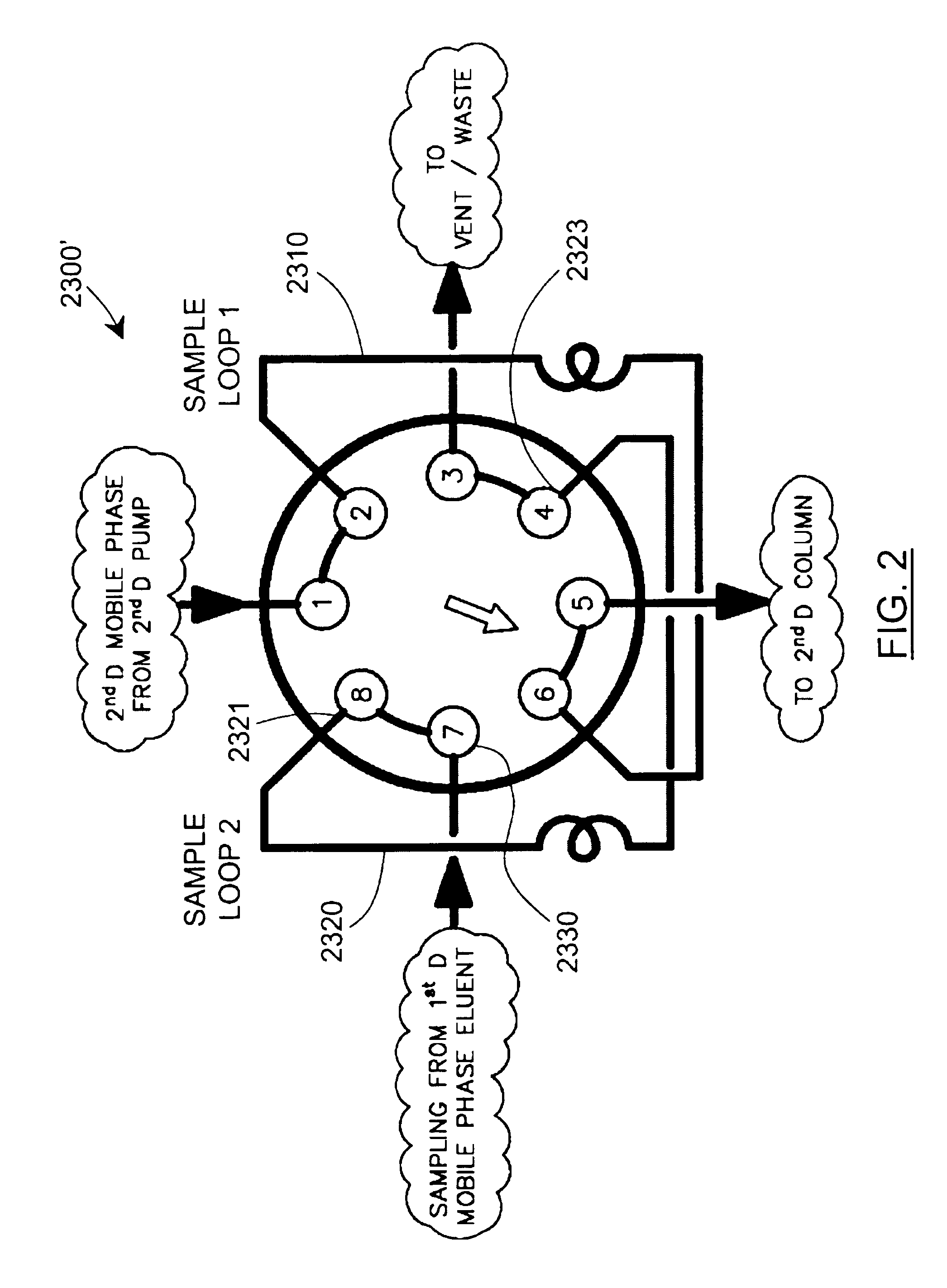
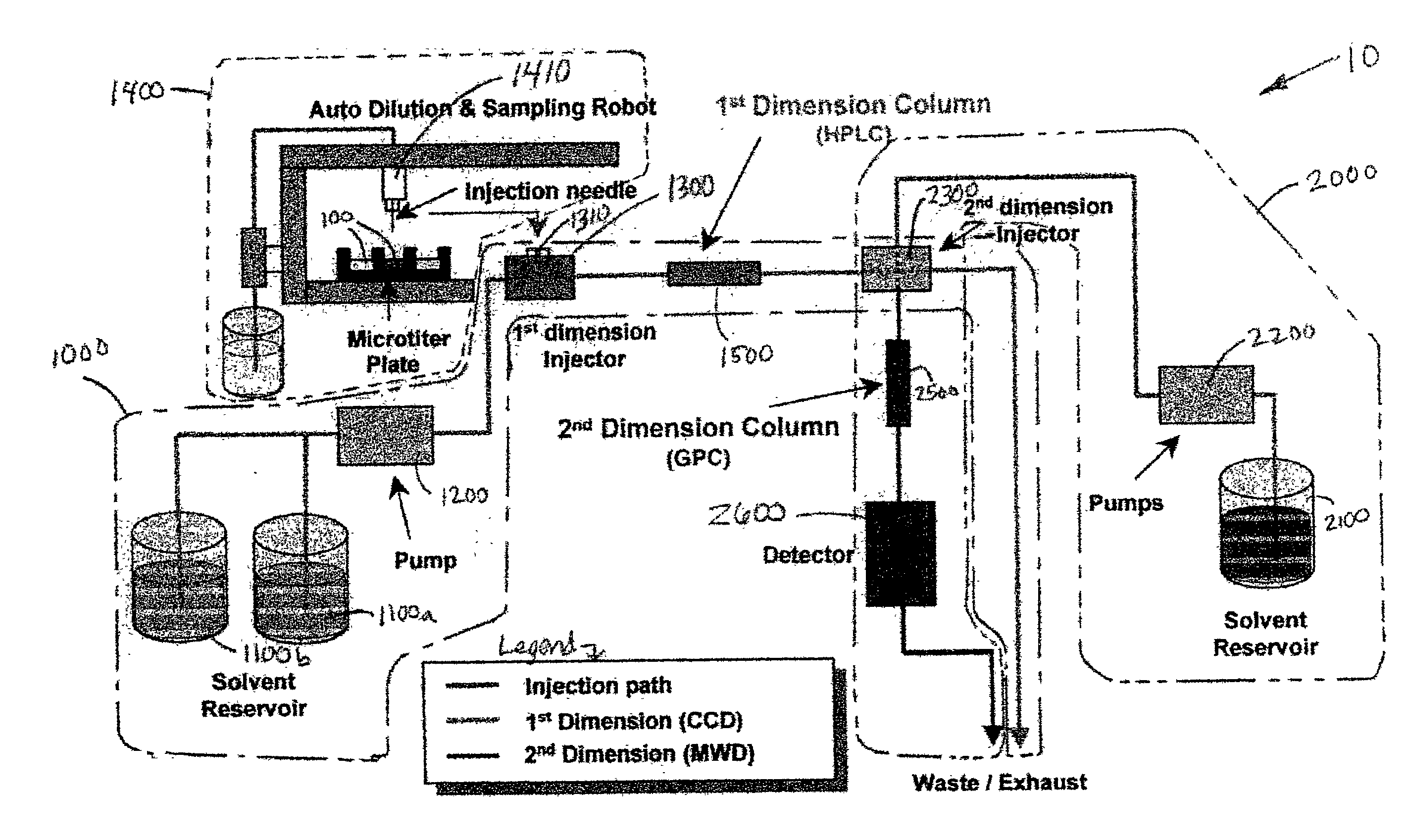
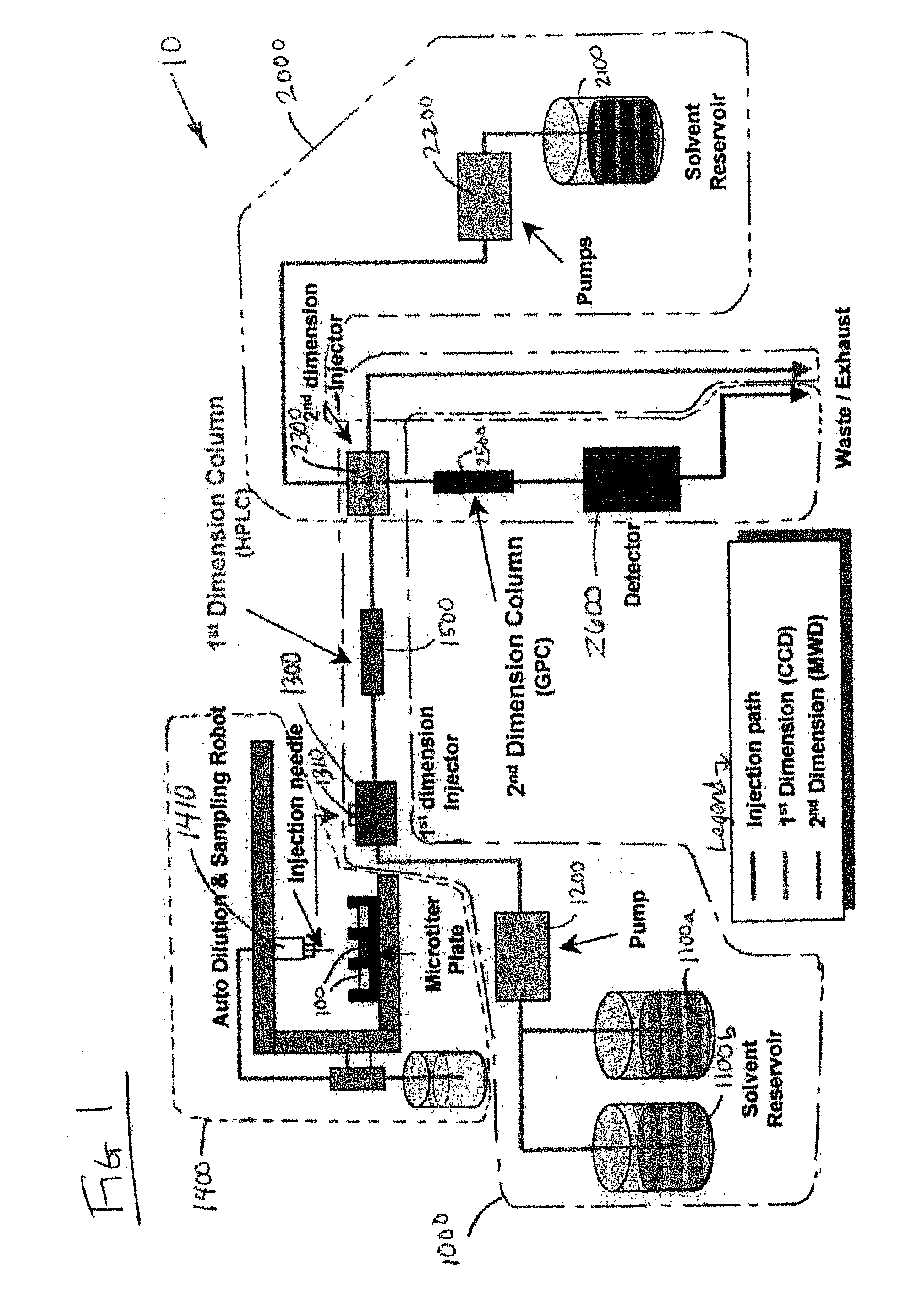
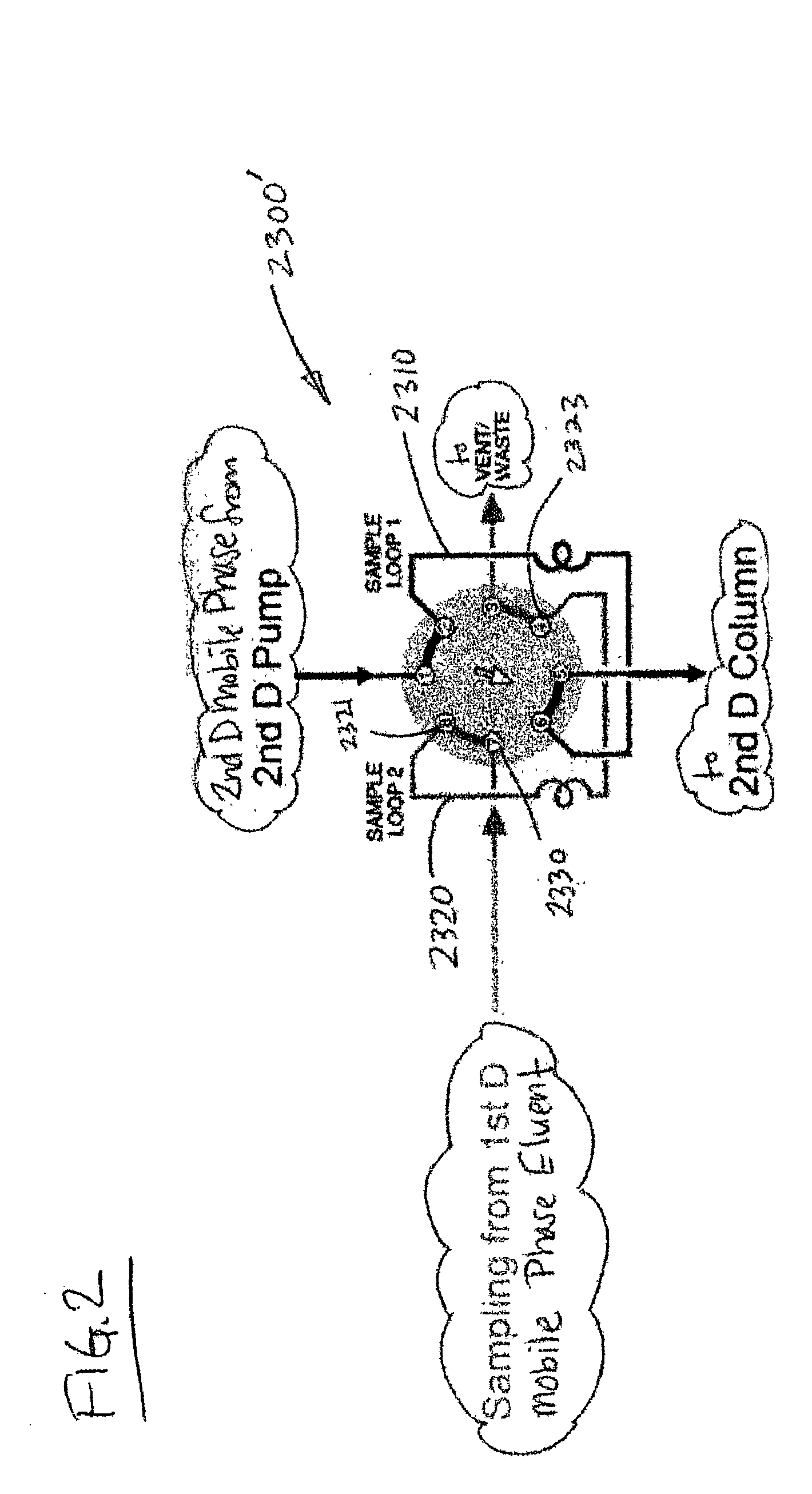
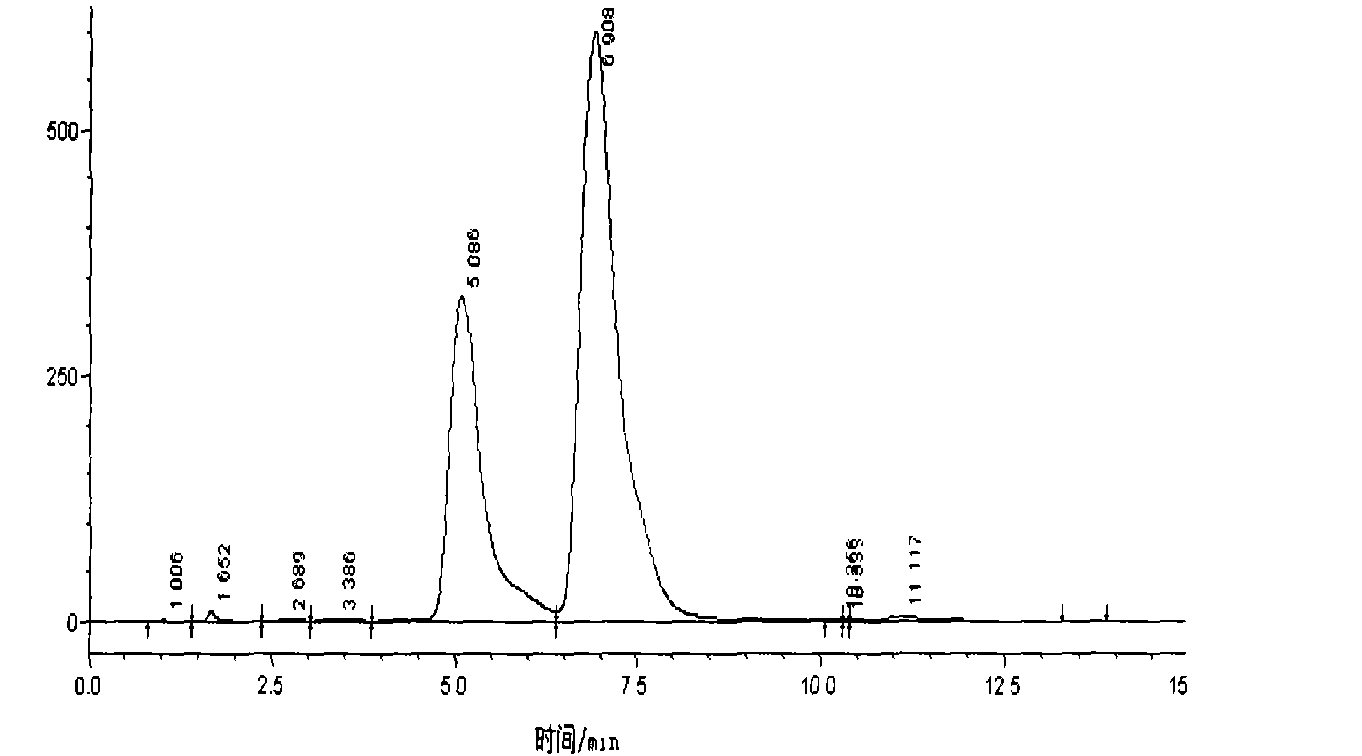
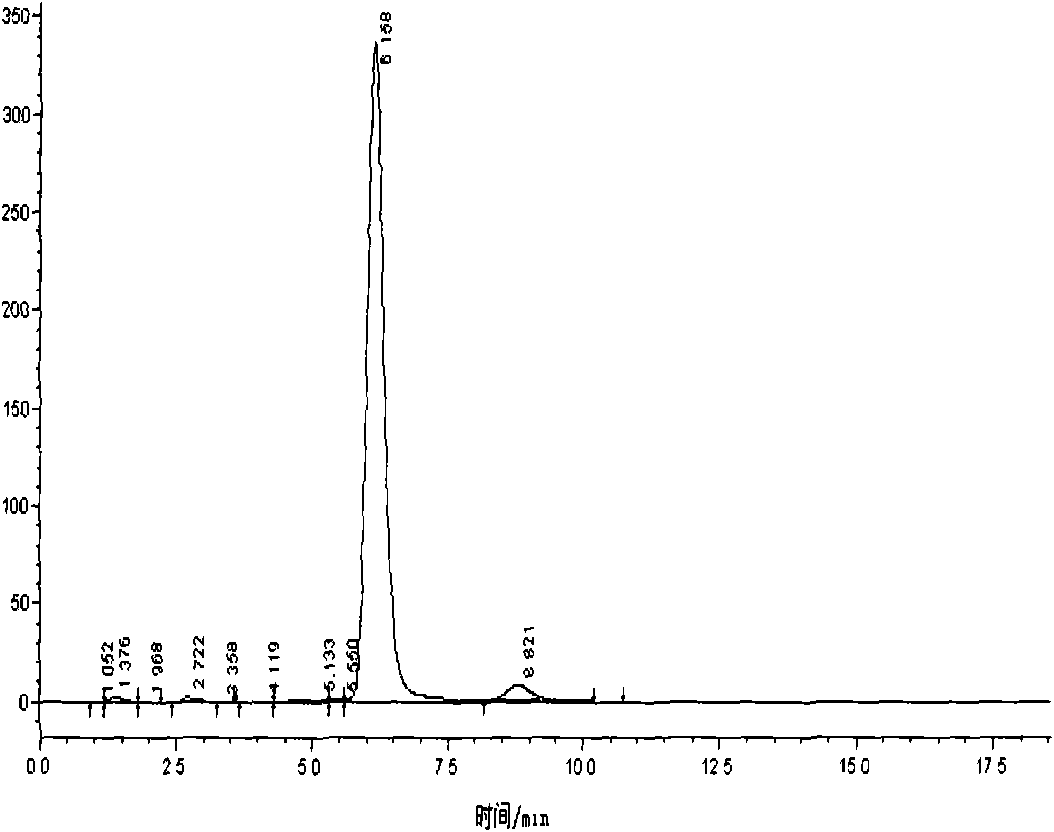
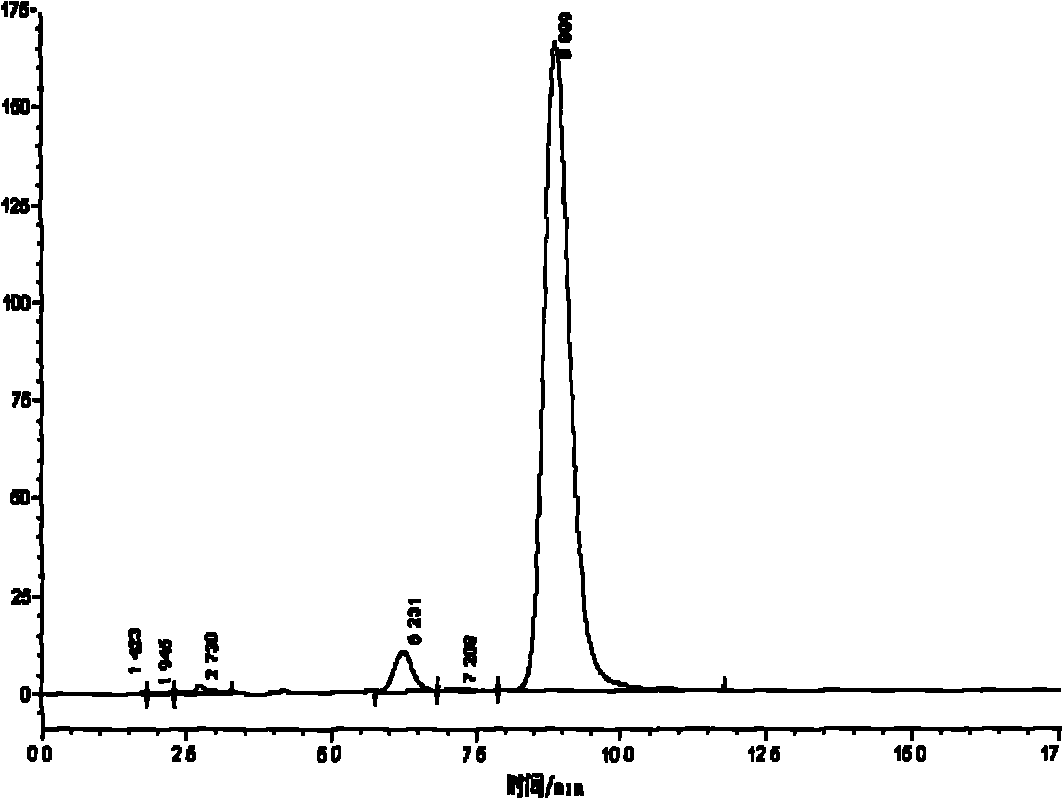
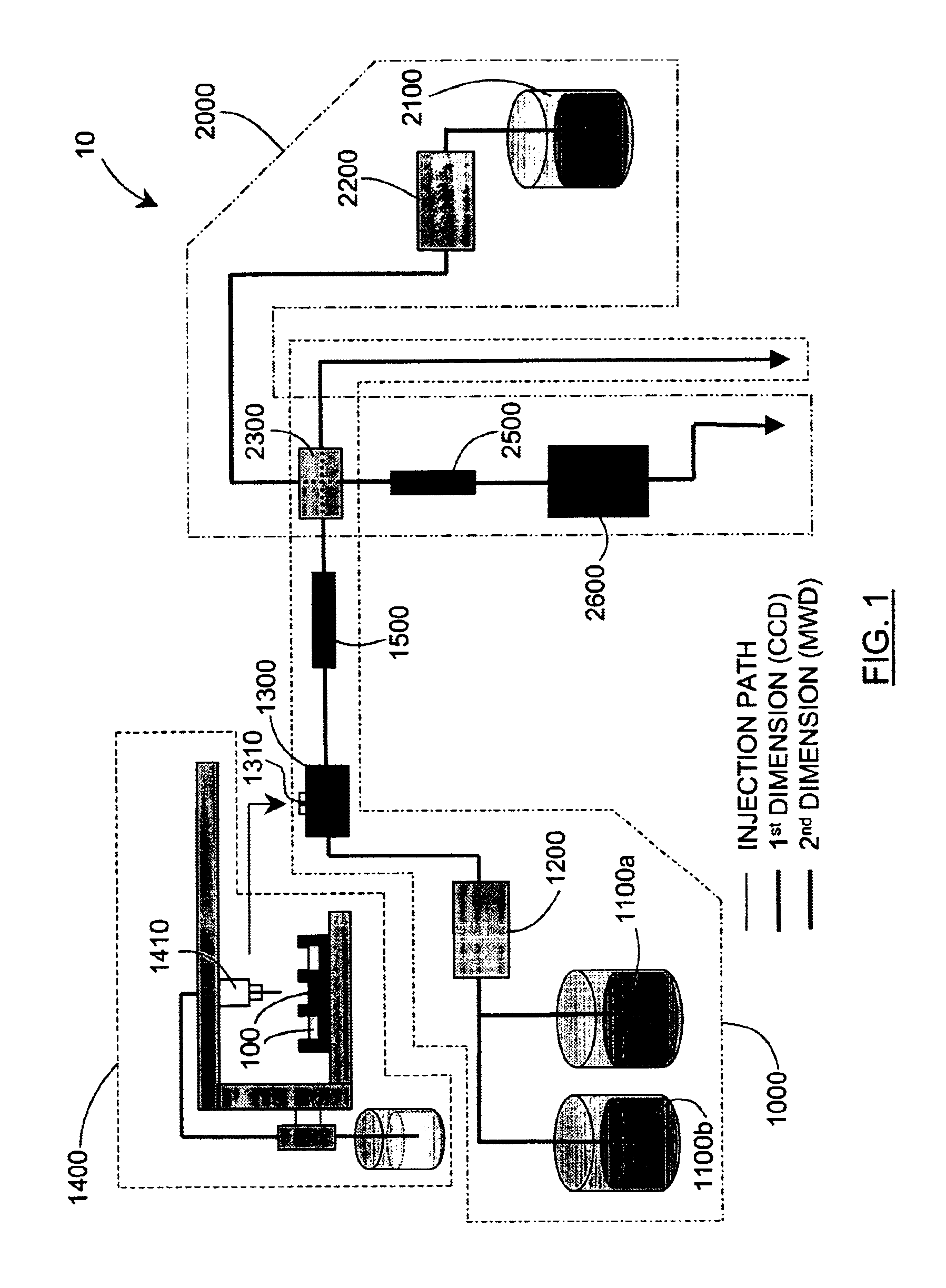
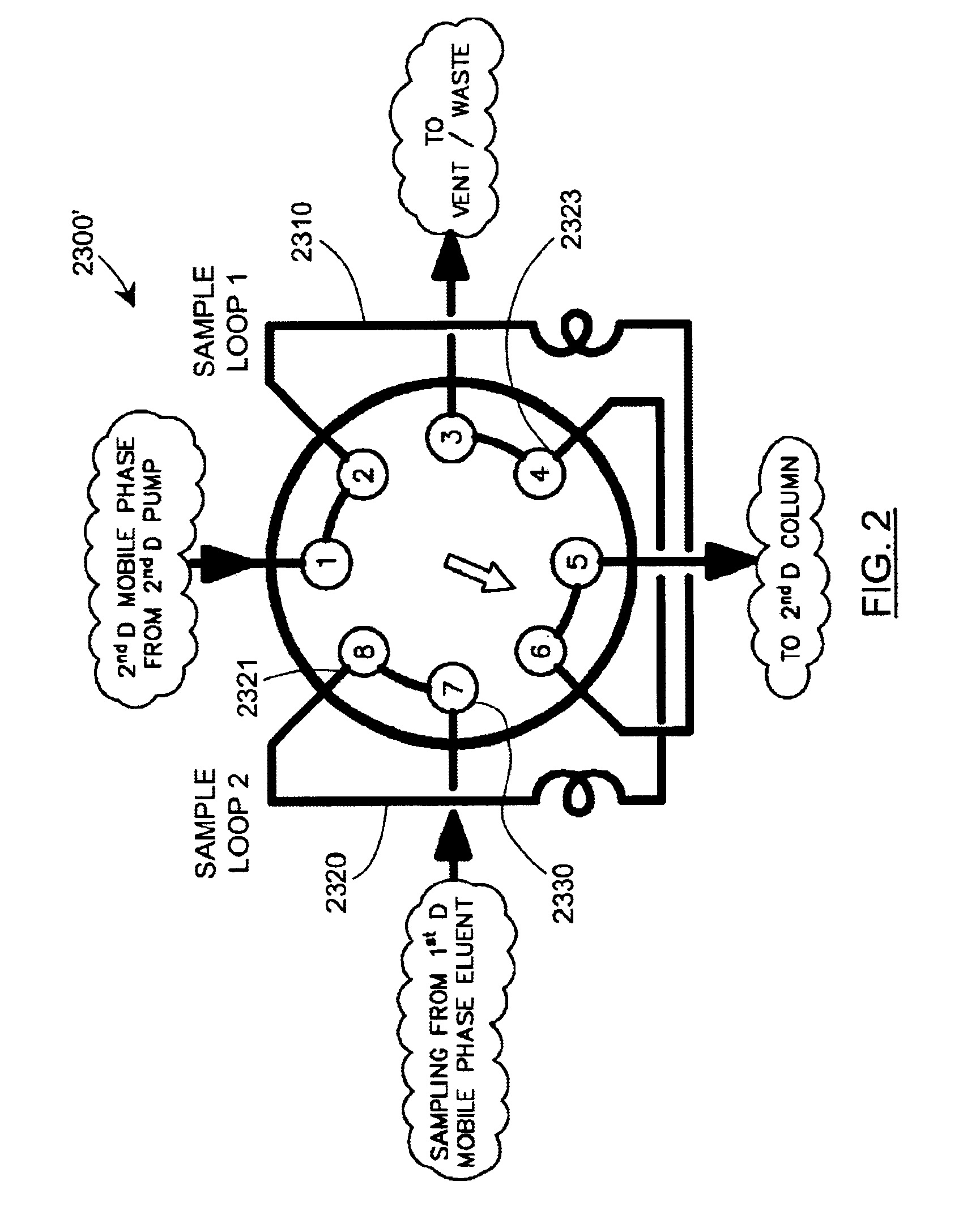
Methods and apparatus for characterization of polymers using multi-dimensional liquid chromatography with regular second-dimension sampling
InactiveUS6730228B2Less complicatedUniversal applicabilityIon-exchange process apparatusSequential/parallel process reactionsGradient elutionPhase gradient
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
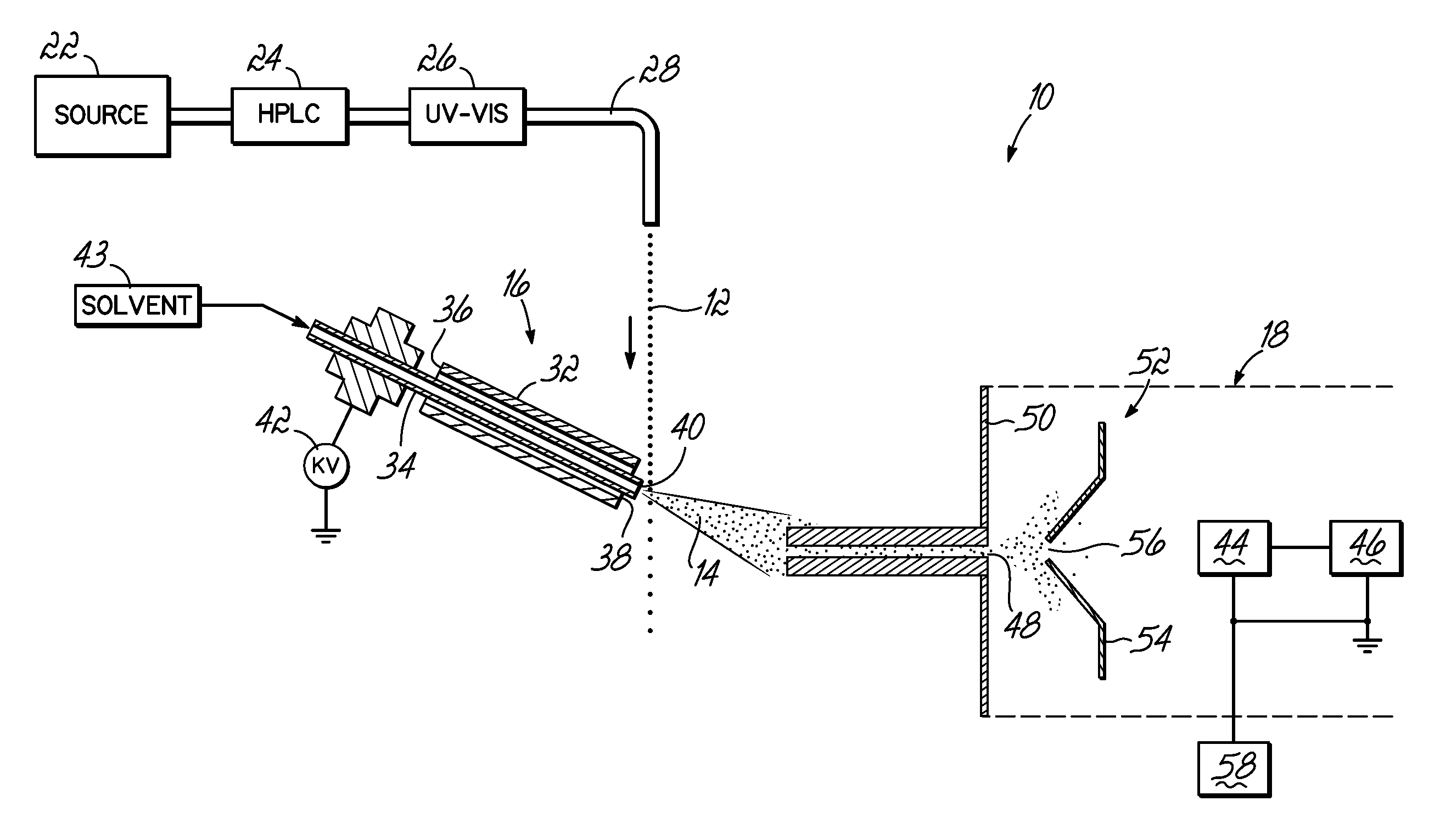
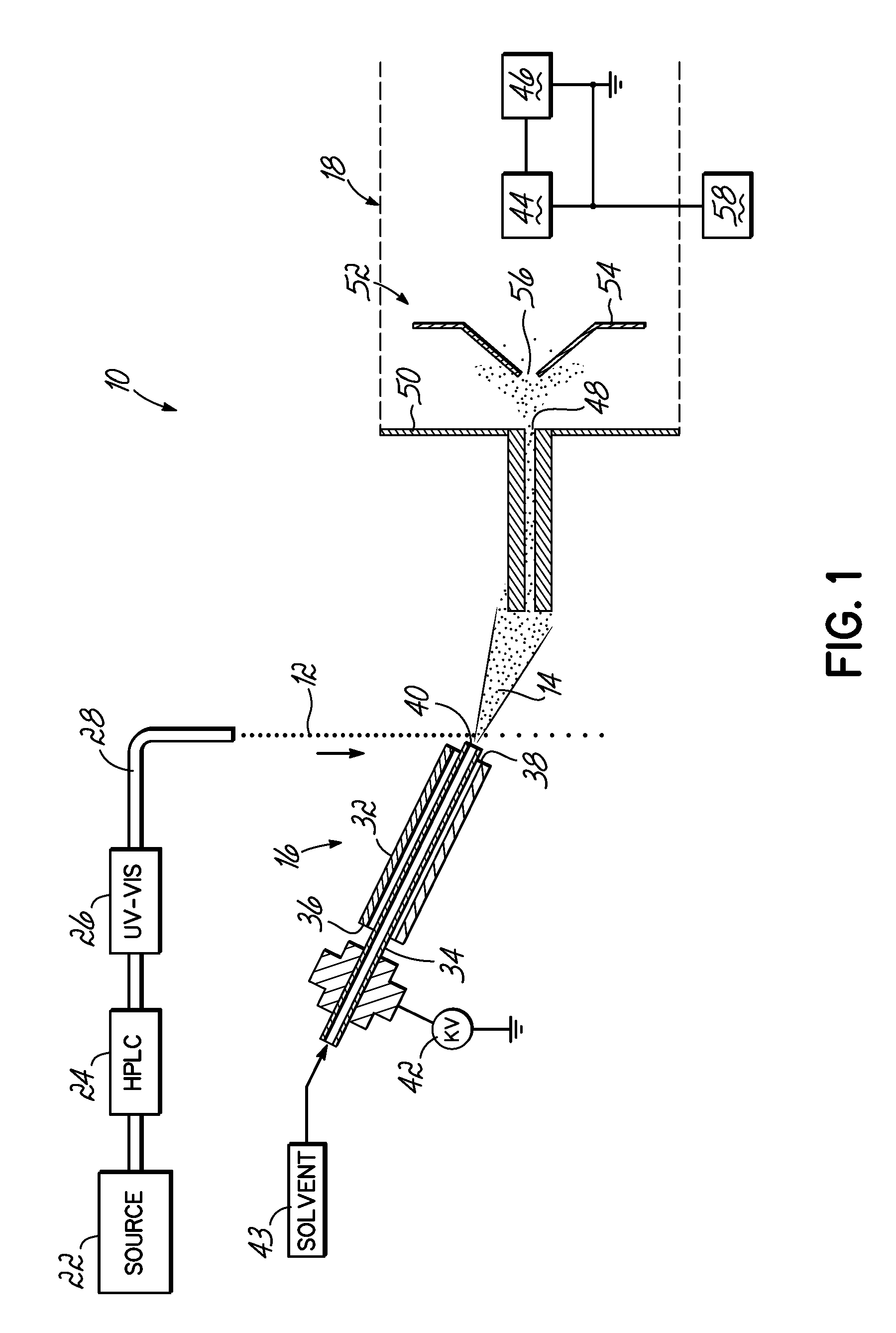
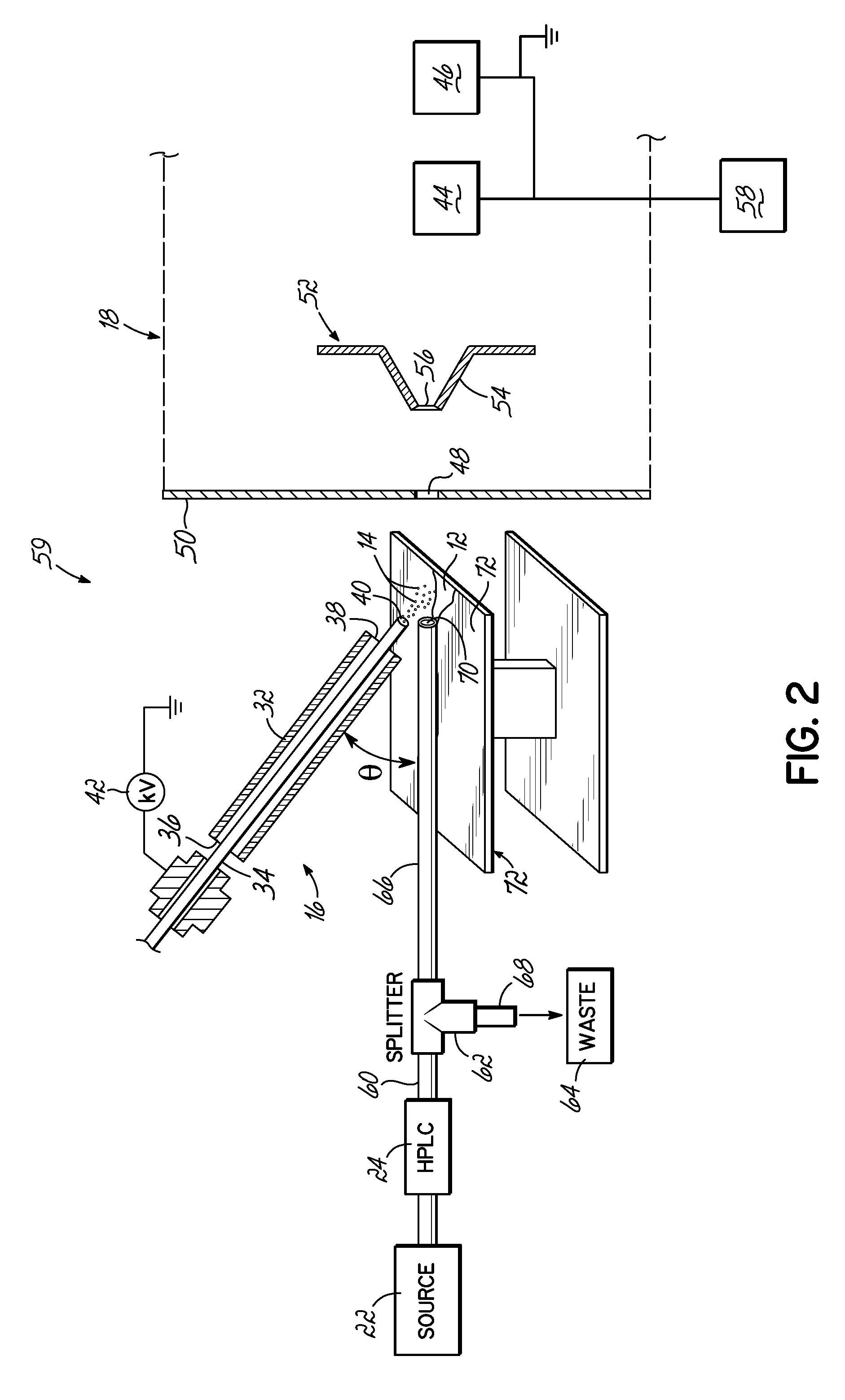
Coupling of Liquid Chromatography with Mass Spectrometry by Liquid Sample Desorption Electrospray Ionization (DESI)
ActiveUS20130023005A1Wide rangeComponent separationMicrobiological testing/measurementMass Spectrometry-Mass SpectrometryMass analyzer
An apparatus to separate and analyze components of a liquid sample include a high performance liquid chromatograph with a mass spectrometer utilizing desorption electrospray ionization. This permits separation and evaluation of different components in a liquid sample. Further, this can be combined with online derivation via reactive DESI and, further, can be used with further electrochemistry.
Owner:OHIO UNIV
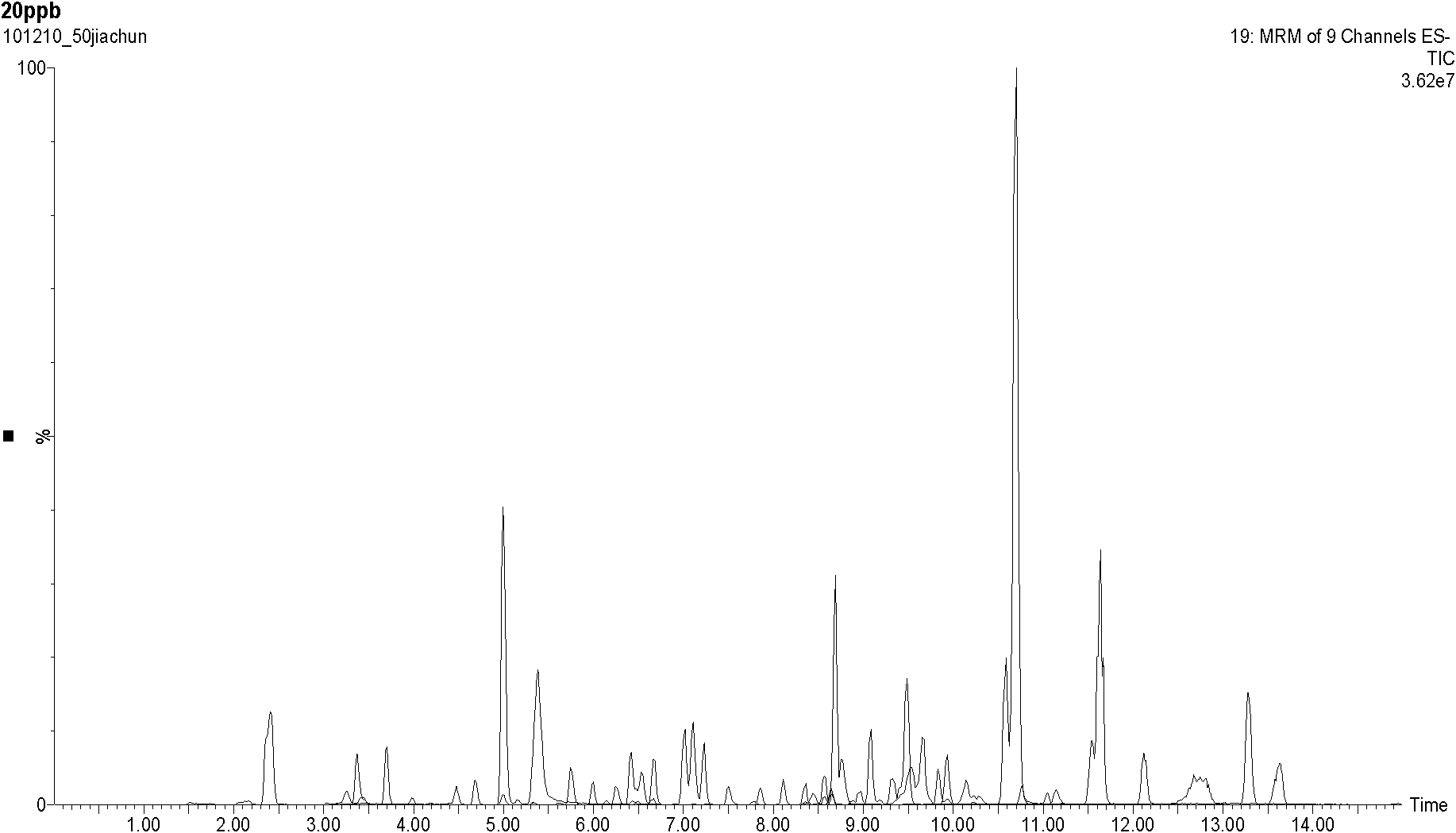
Method for simultaneously determining one hundred pesticide residuals in traditional Chinese medicine through ultrahigh performance liquid chromatography-tandem quadrupole mass spectrum
ActiveCN102735784AReduce usageReduce pollutionComponent separationHigh concentrationRelative standard deviation
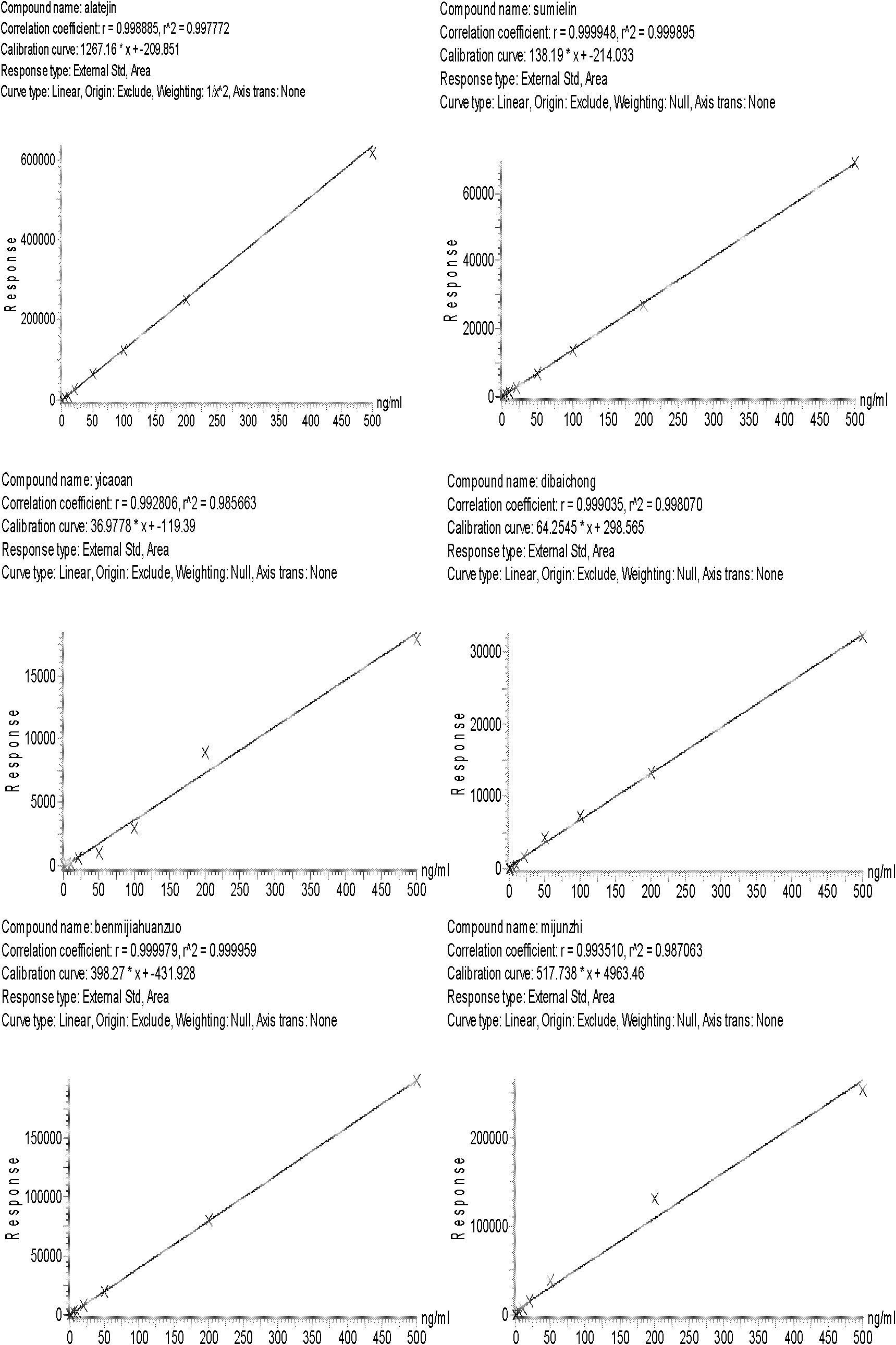
The invention provides a method for simultaneously determining one hundred pesticide residuals in a traditional Chinese medicine through ultrahigh performance liquid chromatography-tandem quadrupole mass spectrum. The method comprises the following steps: immersing traditional Chinese medicine powder with ultrapure water, extracting with acetonitrile containing 0.1% acetic acid through a homogenate method, carrying out solid phase dispersing purification with N-propylethylenediamine and graphitized carbon, detecting in a timesharing multi-reaction monitoring mode through the ultrahigh performance liquid chromatography-tandem quadrupole mass spectrum, and quantifying with an external standard curve method. 88% of pesticides have good linear relations in a range of 5-500ng / mL, correlation coefficients are above 0.99, and the correlation coefficients of above 98% of the pesticides are above 0.97; the average recovery rate of the pesticides with the low concentration of 10mug / kg, the middle concentration of 50mug / kg and the high concentration of 100mug / kg is 70-130%, and the relative standard deviation is less than 0.15; and the detection limit is equal to or less than 0.01mg / kg, so routine detection requirements can be completely satisfied. The method has the advantages of strong versatility, good selectivity, high sensitivity, and rapidness and simplicity.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Methods and apparatus for characterization of polymers using multi-dimensional liquid chromatography with parallel second-dimension sampling
InactiveUS20030089663A1Less complicatedUniversal applicabilityIon-exchange process apparatusSamplingGradient elutionPhase gradient
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
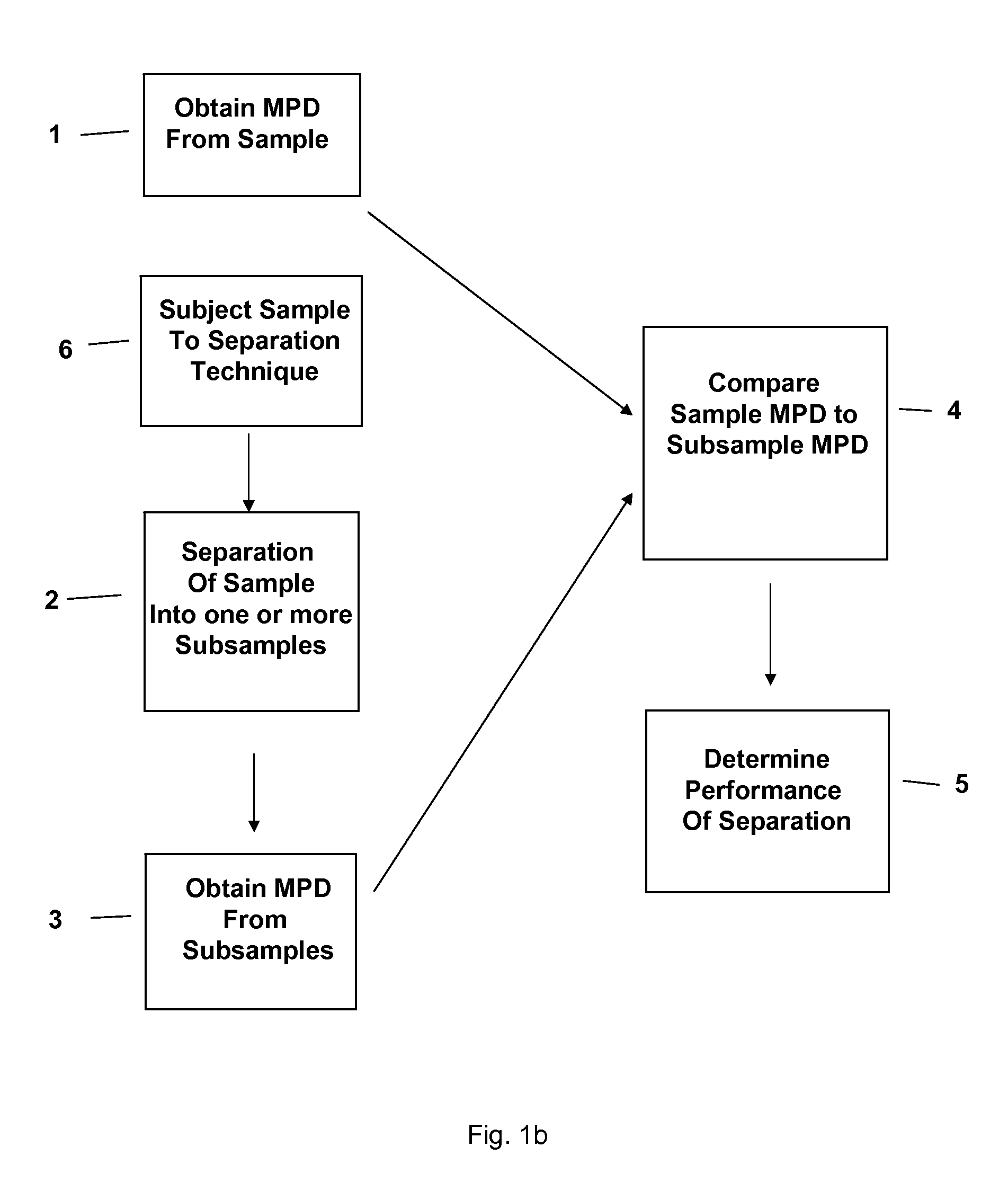
Separation technology method and identification of error
InactiveUS20100050737A1Optical radiation measurementParticle separator tubesCapillary electrophoresisElectrophoresis
The present invention relates to a method and accompanying device for separating a known or unknown sample into one or more subsamples. By comparing the subsample's measurement profile data to the sample measurement profile data, the performance of the separation can be determined. The separation could be chromatography [such as high-performance liquid chromatography (HPLC), gas chromatography (GC), or the like], electrophoresis [such as capillary electrophoresis (CE) or the like], or another separation technique. The measurement profile data could be ultraviolet / visible (UV / Vis) spectra, mass spectra (MS), or another measurement technique.
Owner:WOLTERS ANDREW MARK
Formulations of bendamustine
InactiveUS20130210879A1Improve long-term stabilityOrganic active ingredientsBiocideAntioxidantMedicine
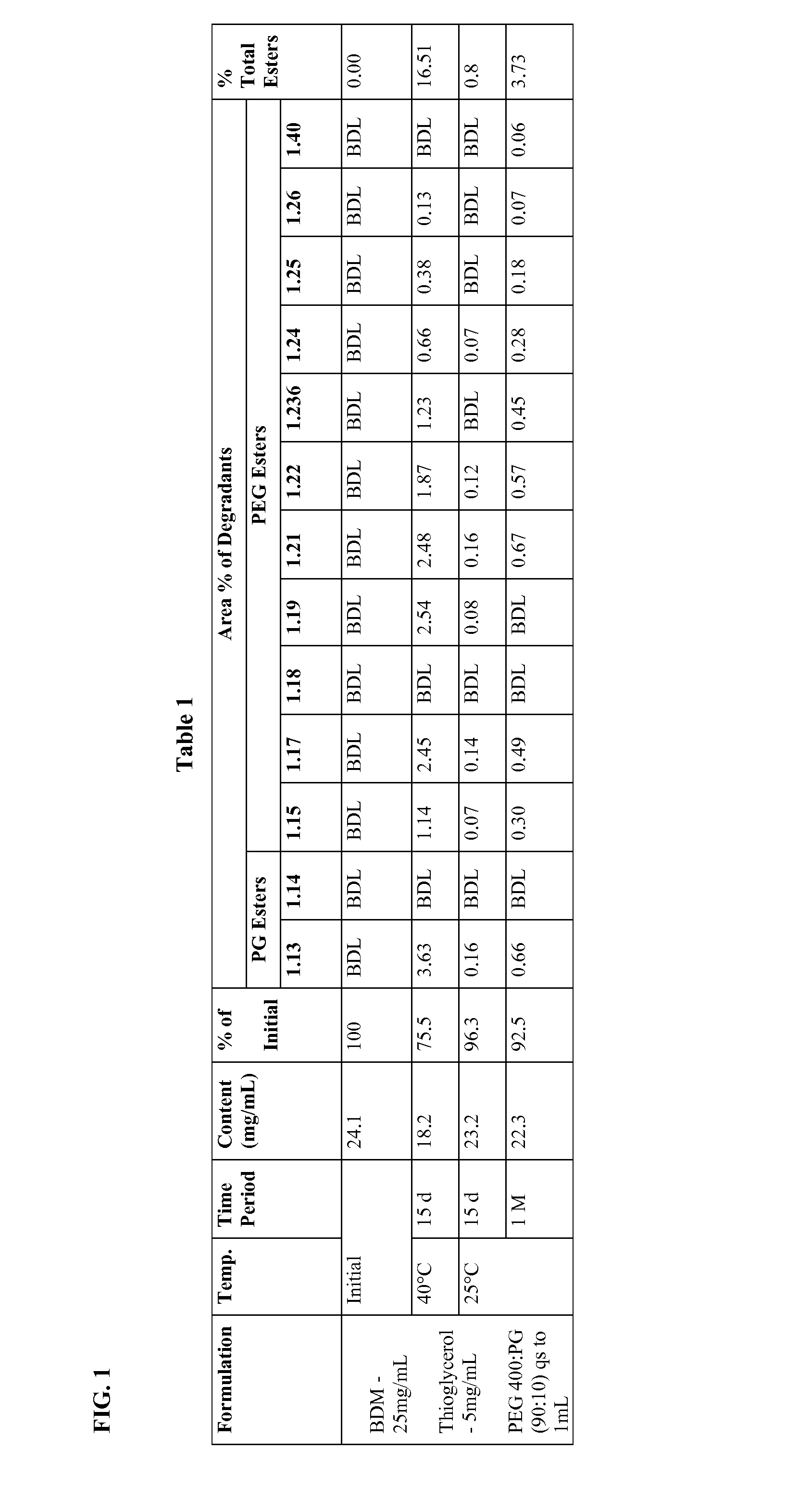
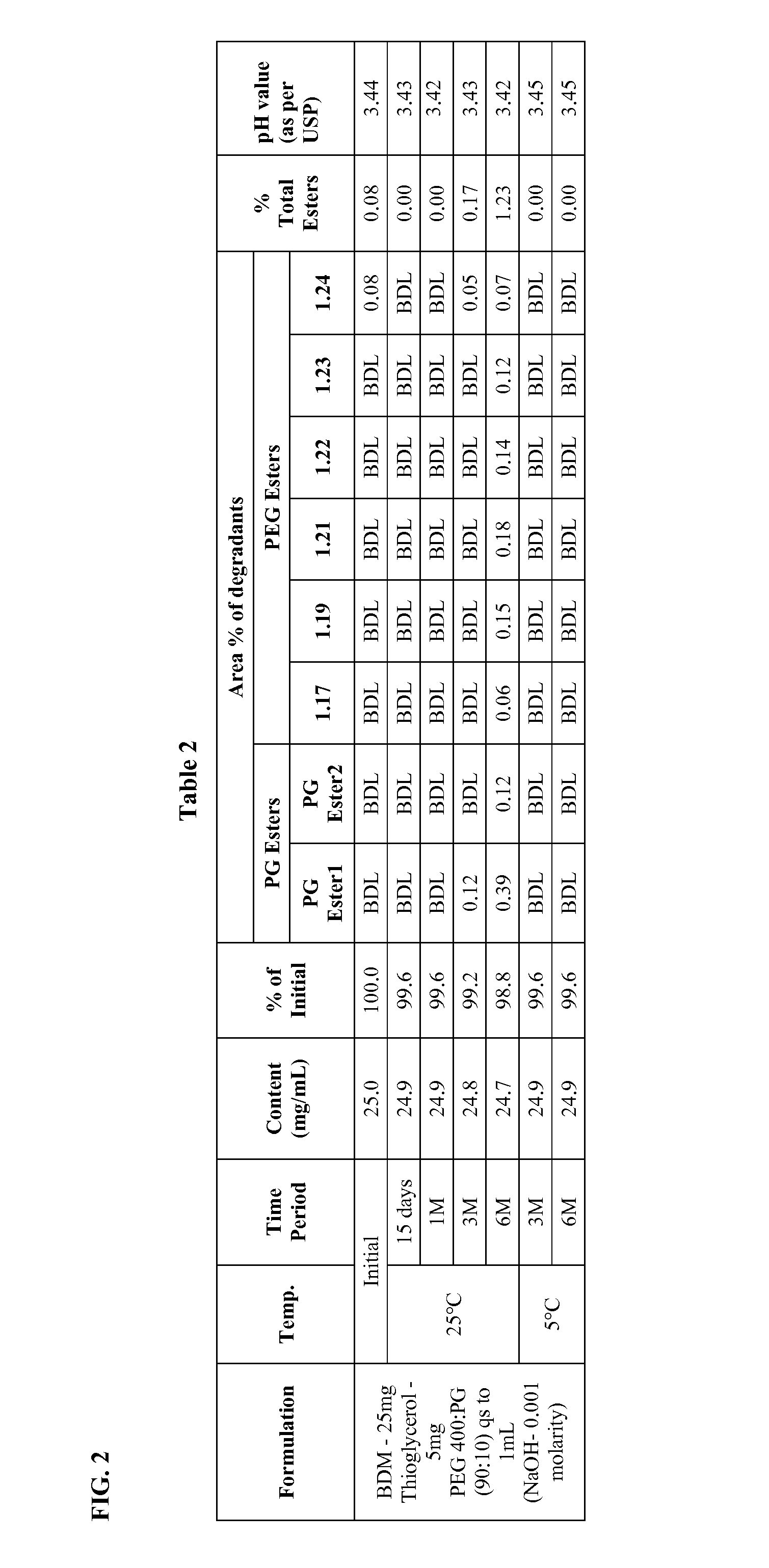
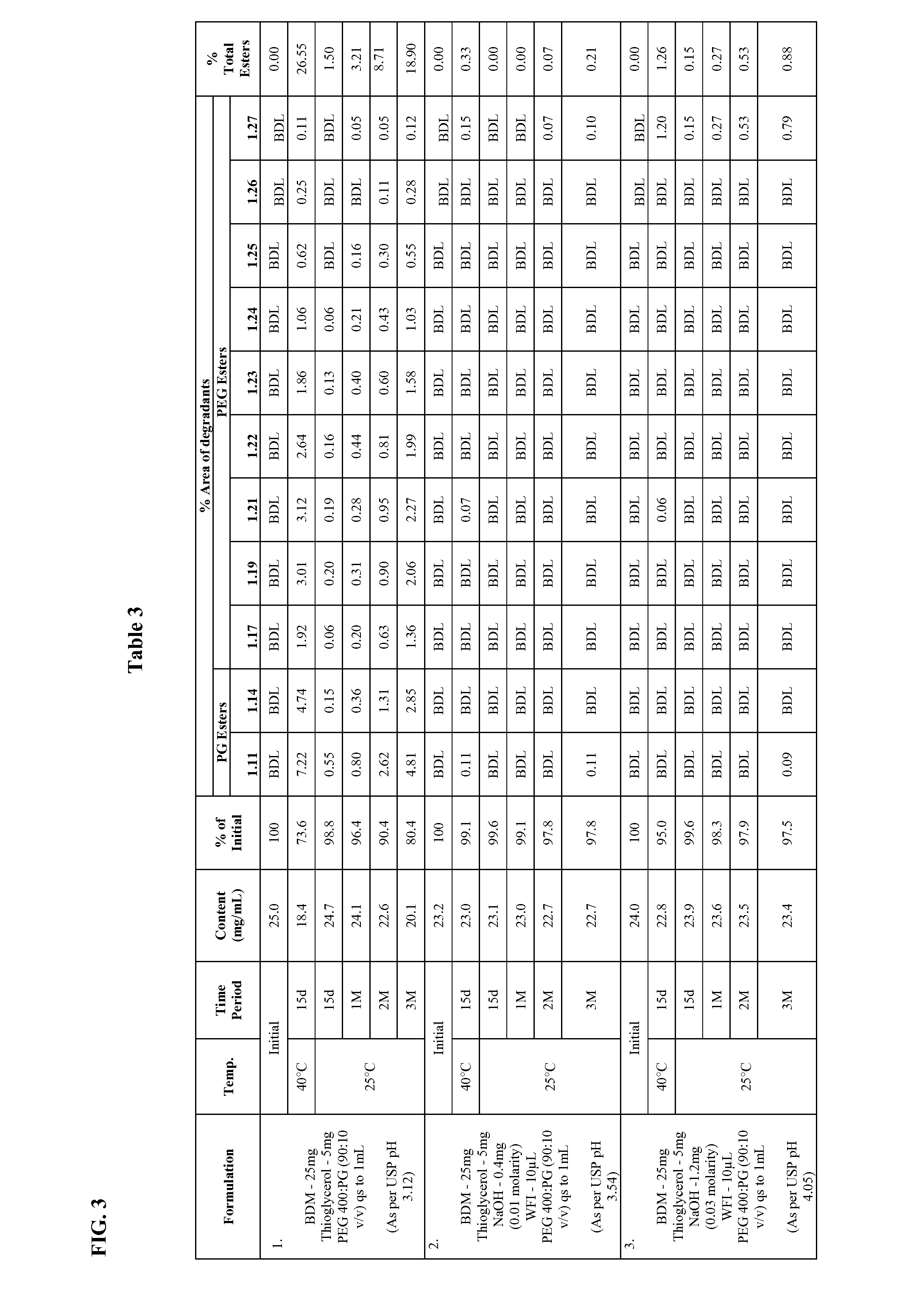
Long term storage stable bendamustine-containing compositions are disclosed. The compositions can include bendamustine or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable fluid contains a mixture of PEG and PG; an organic or inorganic compound in an amount sufficient to obtain a pH of from about 6.0 to about 11 for the polyethylene glycol, as measured using USP monograph for polyethylene glycol; and optionally an antioxidant. The bendamustine-containing compositions have less than about 5% total esters, on a normalized peak area response (“PAR”) basis as determined by high performance liquid chromatography (“HPLC”) at a wavelength of 223 nm, after at least about 15 months of storage at a temperature of from about 5° C. to about 25° C.
Owner:EAGLE PHARMACEUTICALS INC
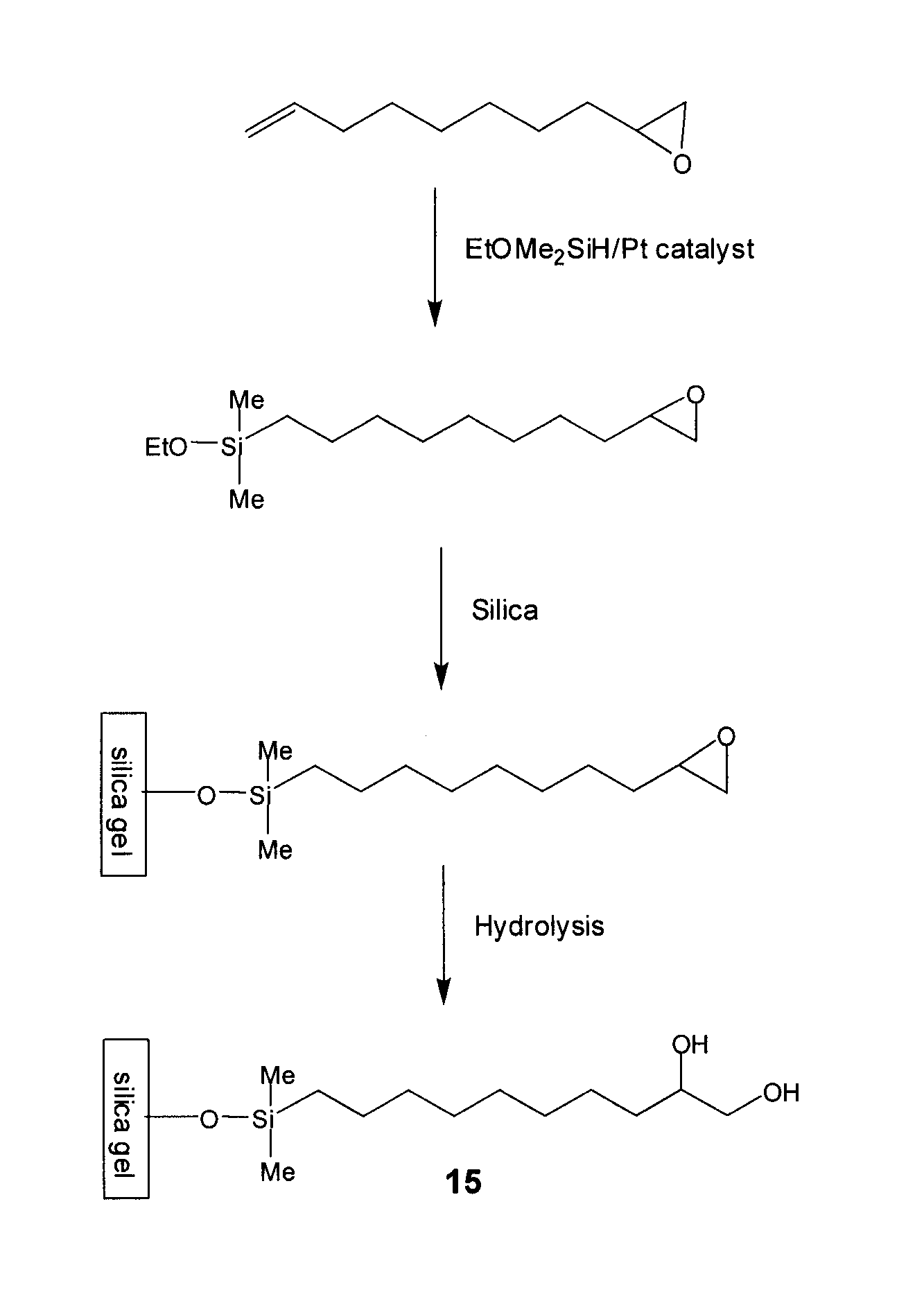
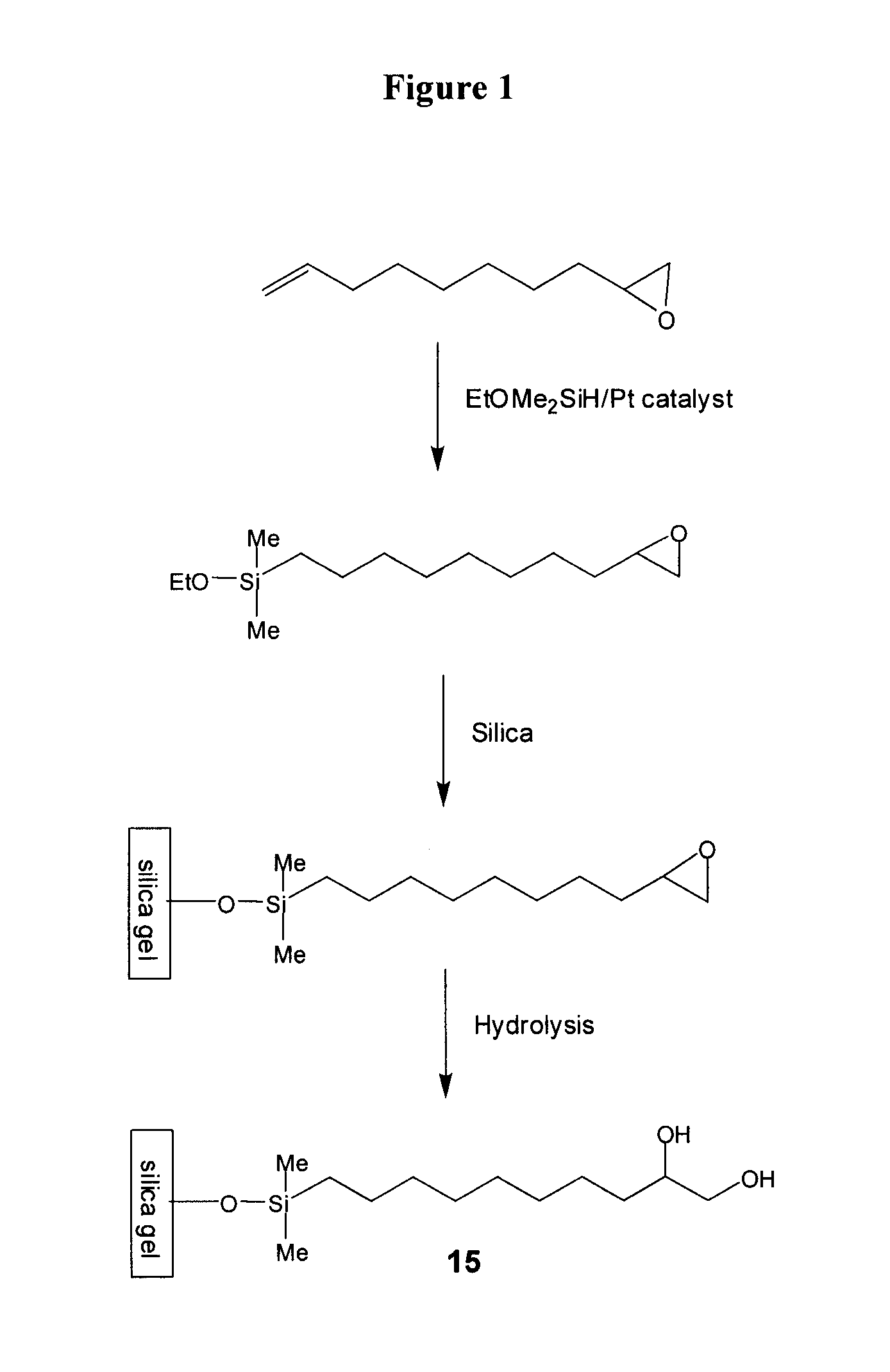
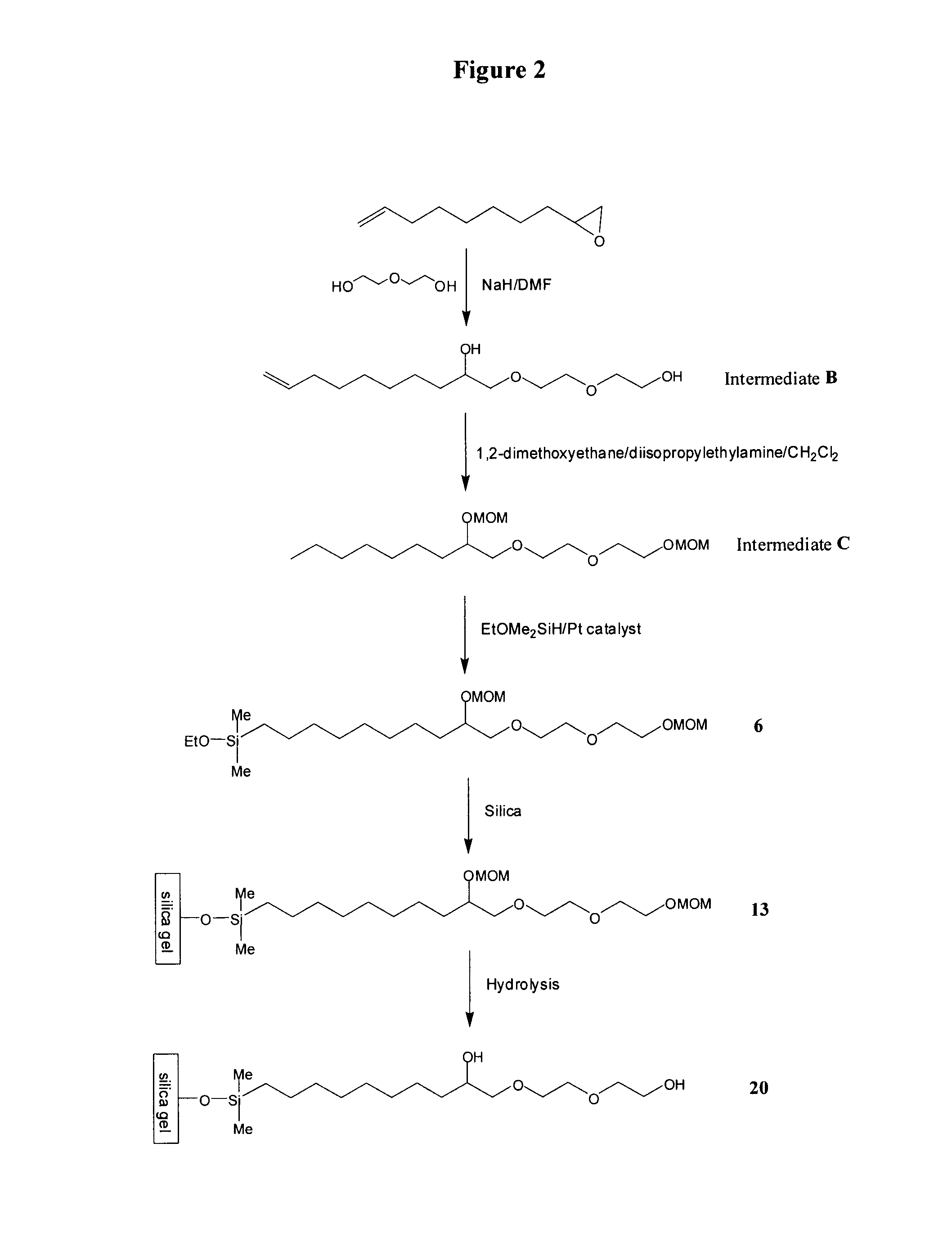
Compositions useful as chromatography stationary phases
The current invention provides compositions, which are useful as stationary phases for a variety of chromatographic applications, such as high performance liquid chromatography (HPLC). The compositions include a substrate (e.g., silica gel), covalently bound to a compound, which includes both a hydrophobic moiety and a hydrophilic moiety, which is preferably a 1,2-diol moiety. The hydrophobic moiety is sufficiently hydrophobic for the compositions to exhibit reversed phase characteristics and typically incorporates at least 5 carbon atoms in sequence. Based on having both hydrophilic and hydrophobic functionalities, the new stationary phases exhibit unique chromatographic properties. For example, these media can be used in either hydrophilic (HILIC) mode, in which the mobile phase includes a high percentage of an organic solvent, or in reversed phase mode, in which the mobile phase contains a higher percentage of an aqueous solvent. The current invention also provides methods of making and using the compounds and compositions of the invention.
Owner:DIONEX CORP
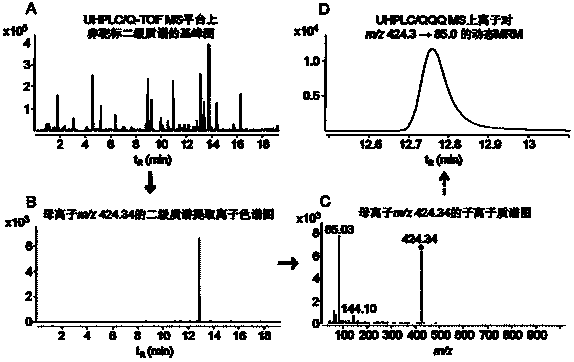
Simulative-target metabonomics analytic method based on combination of liquid chromatography and mass spectrum
ActiveCN104297355AWide detection linear rangeMeet detectionComponent separationMetaboliteRetention time
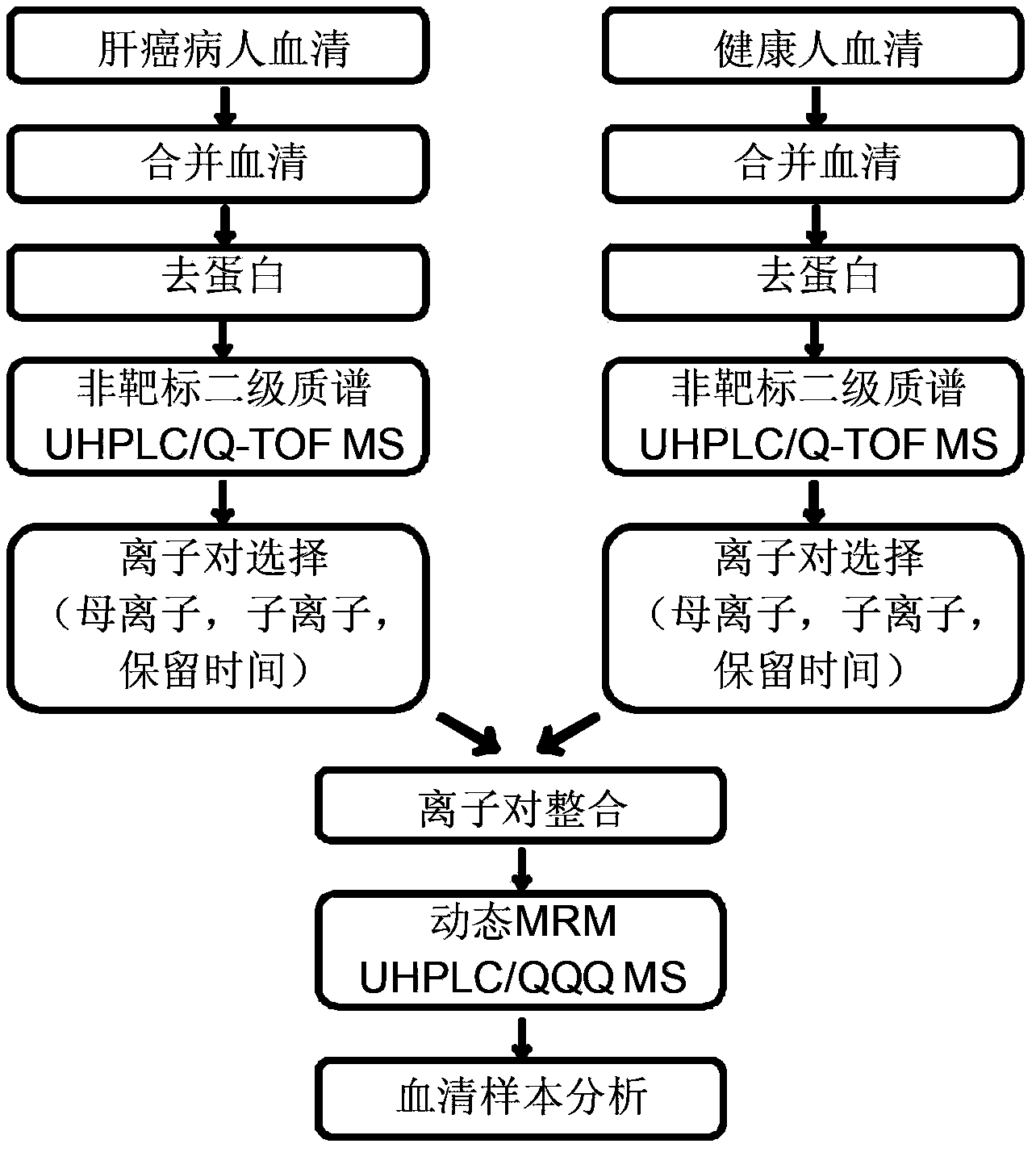
The invention provides a simulative-target metabonomics analytic method based on combination of liquid chromatography and mass spectrum. The method includes following steps: respectively manufacturing to-be-analyzed samples into merged samples according to groups; automatically collecting secondary mass spectrums of metabolites in each merged samples through data of UHPLS / Q-TOF MS with dependence on a collecting mode; extracting a retaining time and information of parent ions and daughter ions of the metabolites by qualitative analytic software; screening out characteristic ion pair information of the metabolites according to daughter ion response intensity; summing the characteristic ion pair information of each merged samples; and scanning the obtained characteristic ion pair of the metabolites by UHPLC / QQQ MS through a dynamic multi-reaction monitoring mode in an actual sample for obtaining a corresponding spectrogram; and performing integration through quantitative analytic software to obtain the metabolites in the to-be-analyzed samples and content information thereof.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for extracting iridoid active site and monomer from eucommia bark
InactiveCN101260131AEasy to separateImprove product qualitySugar derivativesPlant ingredientsAucubinSorbent
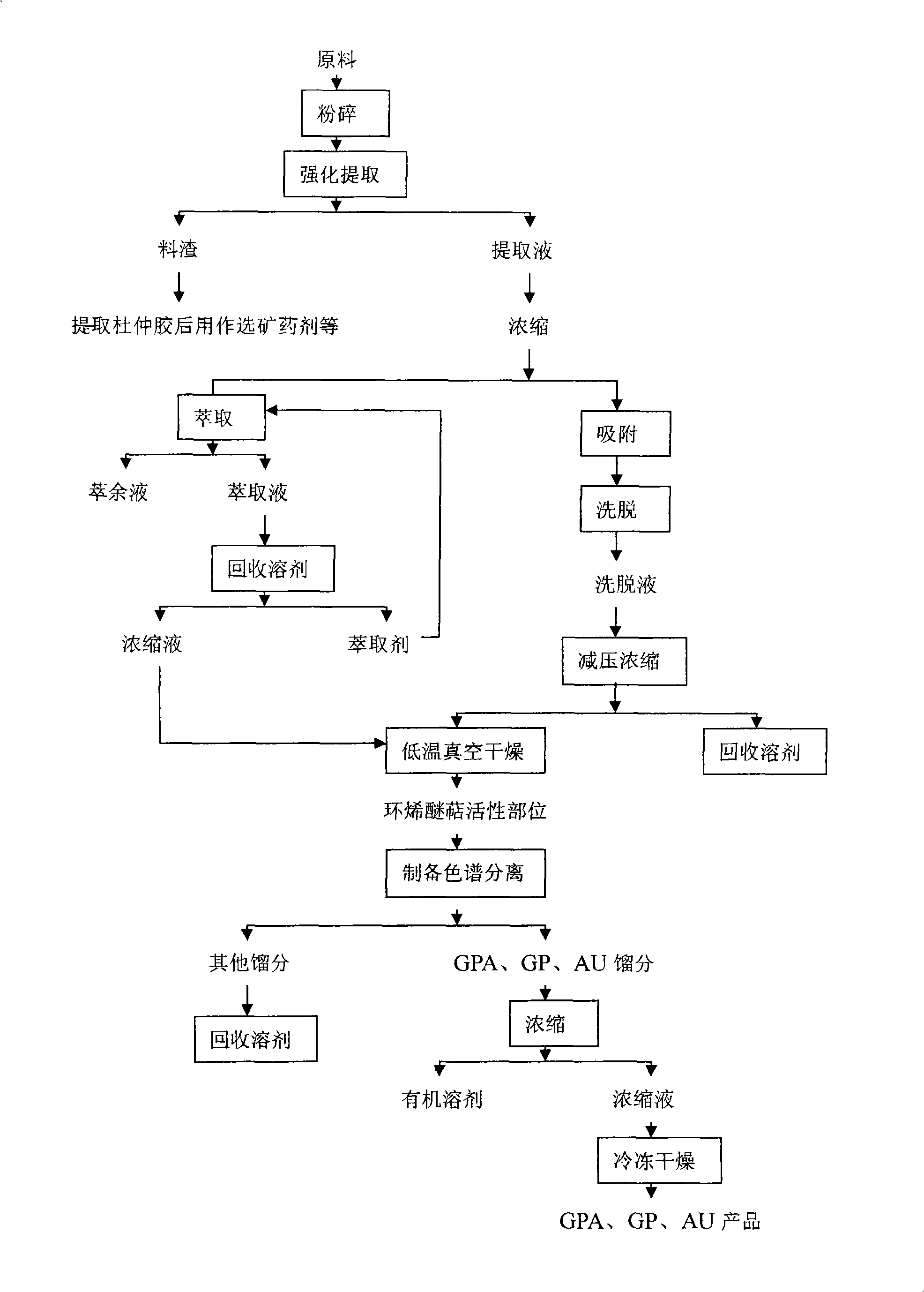
The invention discloses a method for extracting active sites of iridoid, and extracting monomer geniposidic acid (GPA), geniposide(GP) and aucubin(AU) from eucommia, wherein raw materials are extracted by taking the eucommia(husk, leaf or seed) as materials and utilizing the conventional leaching method, the ultrasonic extraction method or the microwave extraction method, and an extracting solution is preliminarily purified by methods such as the solvent extraction and the adsorption separation of special adsorbents, etc., and thus the active sites of the iridoid(the total content of the iridoid is no less than 50 percent) is obtained, and GPA, GP and AU undergo the separation, low temperature condensation, refrigeration and drying by using the preparation high pass filter technique and taking an alcohol-water solution as the mobile phase to obtain monomers of GPA, GP and AU, contents of which are all no less than 90 percent. The preparation method disclosed by the invention utilizes novel process of the extraction and separation, and can simultaneously prepare a plurality of monomer products of the eucommia, and provides scientific foundations for the comprehensive development and utilization of the eucommia; moreover, the whole process does not use solvents harmful to human bodies, thereby realizing the green preparation.
Owner:JISHOU UNIVERSITY
Cyclodextrin chiral chromatogram fixed phase and preparation method thereof
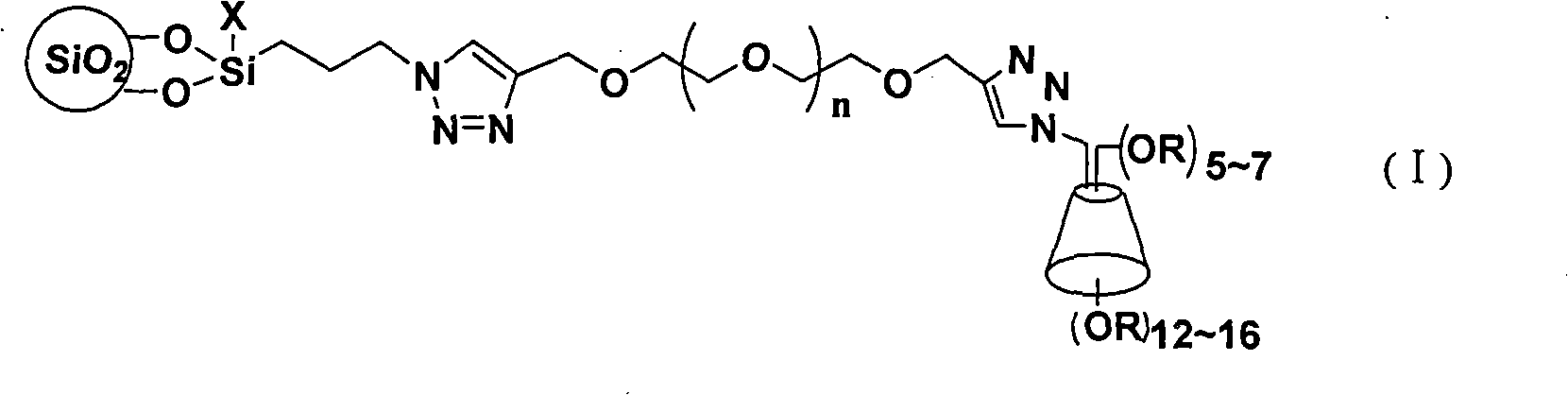
InactiveCN101306354AHigh column efficiencyHigh selectivityOther chemical processesAzirineChemical reaction
The invention discloses a cyclodextrin chiral stationary phase, the structure of which is shown in the general formula (I), wherein X is -OCH3 or -OCH2CH3, n is equal to 1-7, and R is -H, -CH3, -COCH3, -COC6H5 and -CONHC6H5. The preparation method of the stationary phase comprises the following steps: a silane coupling agent, sodium azide and a catalyst are added into an organic solvent, then spheroidal silicon is added for preparing azide silica gel derivant; oligomeric ethylene glycol, sodium hydride and propargyl bromide are added into tetrahydrofuran for preparing bialkynyl oligomeric ethylene glycol; monosubstituted nascent and derivative cyclodextrin containing azid groups is prepared; finally, the click chemistry reaction method is used for bonding the cyclodextrin. The cyclodextrin chiral stationary phase has the advantages that the selectivity of the bonding reaction is high, and the surface bonded amount is large; the chiral separation ability is strong, thereby being especially suitable for the chiral separation of a high efficiency liquid chromatography in the reversed-phase mode; the preparation method is simple and has less steps, the bonding reaction is the click chemistry reaction, the reaction condition is mild, and the reaction is carried out in the water solution.
Owner:EAST CHINA UNIV OF SCI & TECH
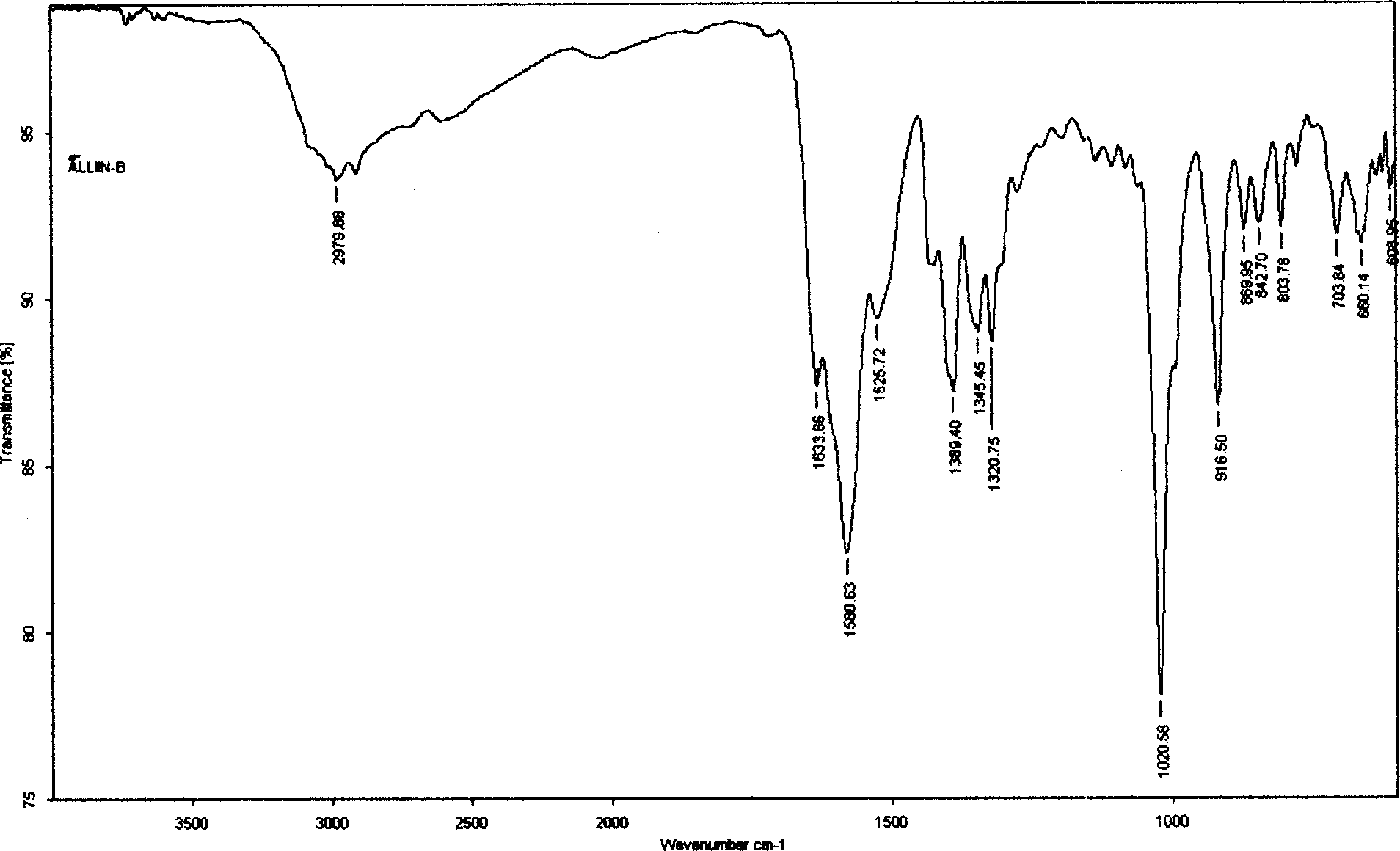
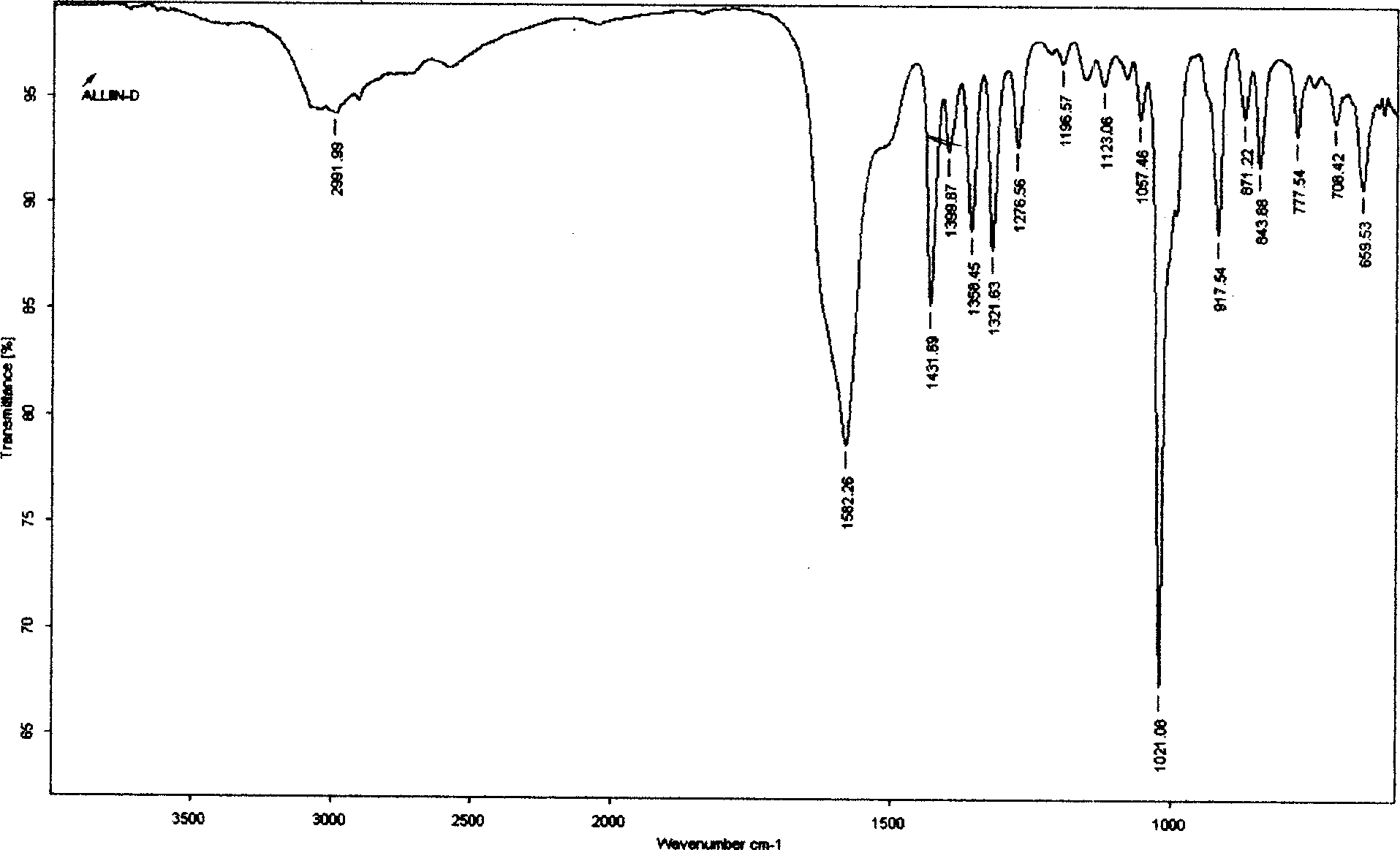
Process for extracting alliin from fresh garlic
The extraction process of alliin from fresh garlic includes the steps of: pre-treatment to kill enzyme of garlic with microwave; organic extraction through ginding garlic into slurry, ethanol extraction, separating extractive liquid and depression concentration; cationic exchange resin adsorption of alliin from concentrated extractive liquid; ammonia water desorption of alliin; and obtaining alliin crystal. The said process has alliin yield of 0.1% and product has alliin content of 91.2%.
Owner:XINJIANG AILEXIN PHARMA
Method for controlling quality of ginkgo leaves and extract thereof
InactiveCN103175912AEasy to separateEasy CalibrationComponent separationCurative effectControl quality
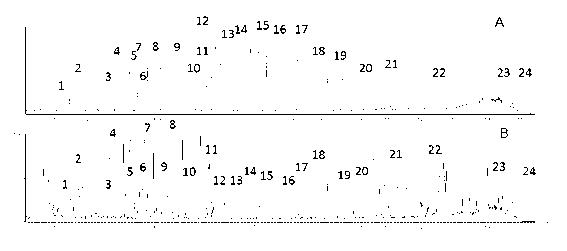
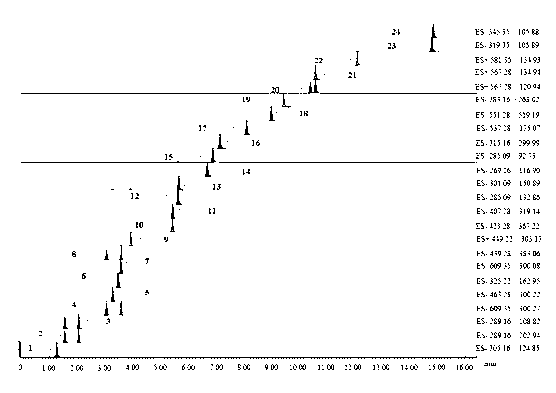
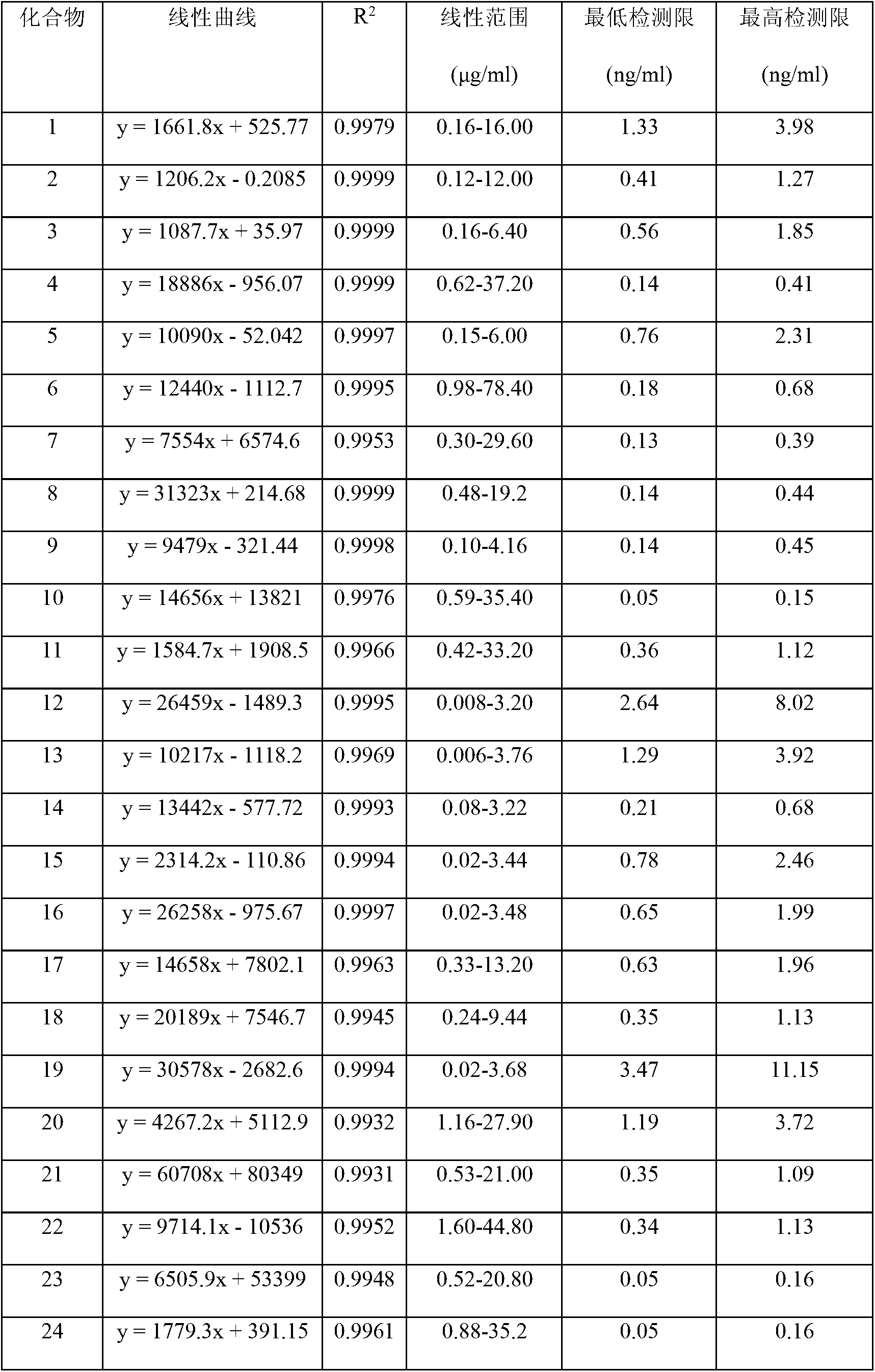
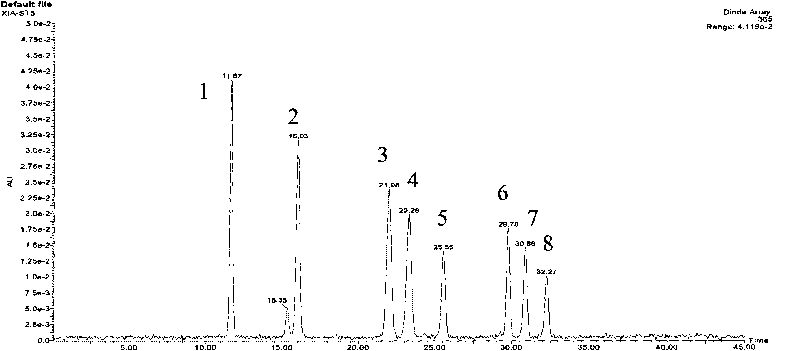
The invention discloses a method for controlling the quality of ginkgo leaves and an extract thereof. After the screening of a great quantity of experiments, the method adopts ultra-high performance liquid chromatography for detection; and the method can be used for detecting three types of components including flavone, terpene lactones and phenolic acid, twenty-four active compounds in total, in the ginkgo leaves and the extract of the ginkgo leaves. The results of the experiments prove that the method is high in detection sensitivity and good in stability, and can be used for objectively, comprehensively and accurately evaluating the quality of ginkgo leaf medicines, the extract of the ginkgo leaves and ginkgo leaf preparations, thus having important significance on controlling the quality and guaranteeing the clinical treatment effect.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for detecting tobacco-specific nitrosamines
The invention relates to a method for measuring tobacco-specific nitrosamines (TSNAs for short) by using a super efficient liquid chromatogram-tandem mass spectrum combined method, and belongs to a method for detecting TSNAs. The method comprises the following steps of: adding internal standard-containing aqueous solution of ammonium acetate into a filter sheet after trapping smoke and cigarette filters smoked completely under standard conditions or tobacco shreds, performing ultrasonic extraction, transferring 1 to 2mL of extract into a small solid-phase extraction column taking N-vinyl pyrrolidone-benzene sulfonic acid copolymer as filler, flushing the small column by using aqueous solution of methanoic acid and methanol, finally eluting the small column by using solution of aqueous ammonia and methanol solution, analyzing the eluant by using an ultra performance liquid chromatography-mass spectrometer / mass spectrometer (UPLC-MS / MS), and quantifying according to the area ratio of a component peak to an isotope internal standard peak. The detecting method can be used for detecting the TSNAs in cured and hybrid cigarette mainstream smoke, cigarette side-stream smoke, filter tip intercepting smoke and tobacco shreds, and has the advantages of simple pretreatment, accurate quantification, high instrument analysis speed, low detection limit, high sample treatment flux and the like.
Owner:HONGTA TOBACCO GRP
Method fro determining content of volatile carbonyl compound in main stream smoke of cigarette
InactiveCN101701941AEasy to separateSeparation reachedComponent separationHydrazoneChromatographic column
The invention discloses a method for determining content of volatile carbonyl compound in main stream smoke of cigarette. 2, 4-dinitrophenylhydrazine is utilized to gather volatile carbonyl compound in main stream smoke of cigarette, so as to form hydrazone compound, and the content of the hydrazone compound is determined by a high performance liquid chromatograph by adopting external standard method; and the high performance liquid chromatograph adopts chromatographic column. The determination method of the invention adopts chromatographic column, has excellent separating effect on nitro substituted arene derivative, especially on hydrazone compound formed after reaction of 2, 4-dinitrophenylhydrazine and volatile carbonyl compound, and meanwhile chromatographic analysis condition is optimized, thus achieving complete separation of volatile carbonyl compound and interfering component in main stream smoke of cigarette and improving accuracy of determination.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
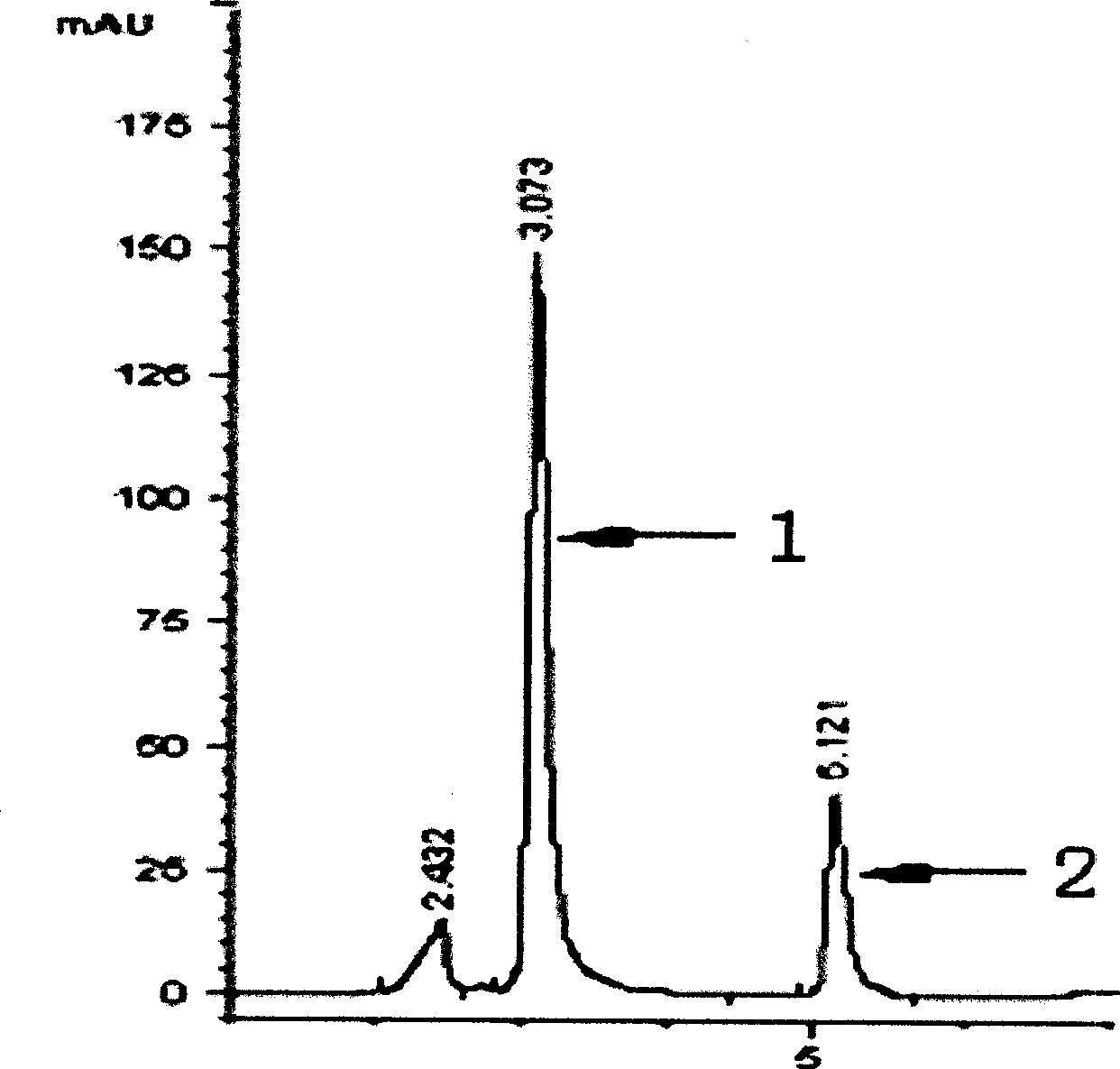
Separation and preparation of isovaleryl-spiramycin I and application thereof
InactiveCN101785778ASimple production processQuality standards are easy to controlAntibacterial agentsOrganic active ingredientsAntibiotic YAntibacterial activity
The invention relates to the separation of antibiotic and the application of the antibiotic in resisting infectious diseases, in particular to an isovaleryl-spiramycin I single-component compound. A kelimycin sample is separated by HPLC (high performance liquid chromatography) after crude separation, high-efficiency purification and post-processing, so as to obtain the pure isovaleryl-spiramycin I compound. Biological experiment results show that the antibacterial activity of the isovaleryl-spiramycin II compound is better than that of kelimycin and is much better than that of a control group. The invention lays the foundation for developing the clinical and effective isovaleryl-spiramycin II single-component antibiotic.
Owner:SHENYANG TONGLIAN GRP CO LTD
Cancer chemopreventive agents
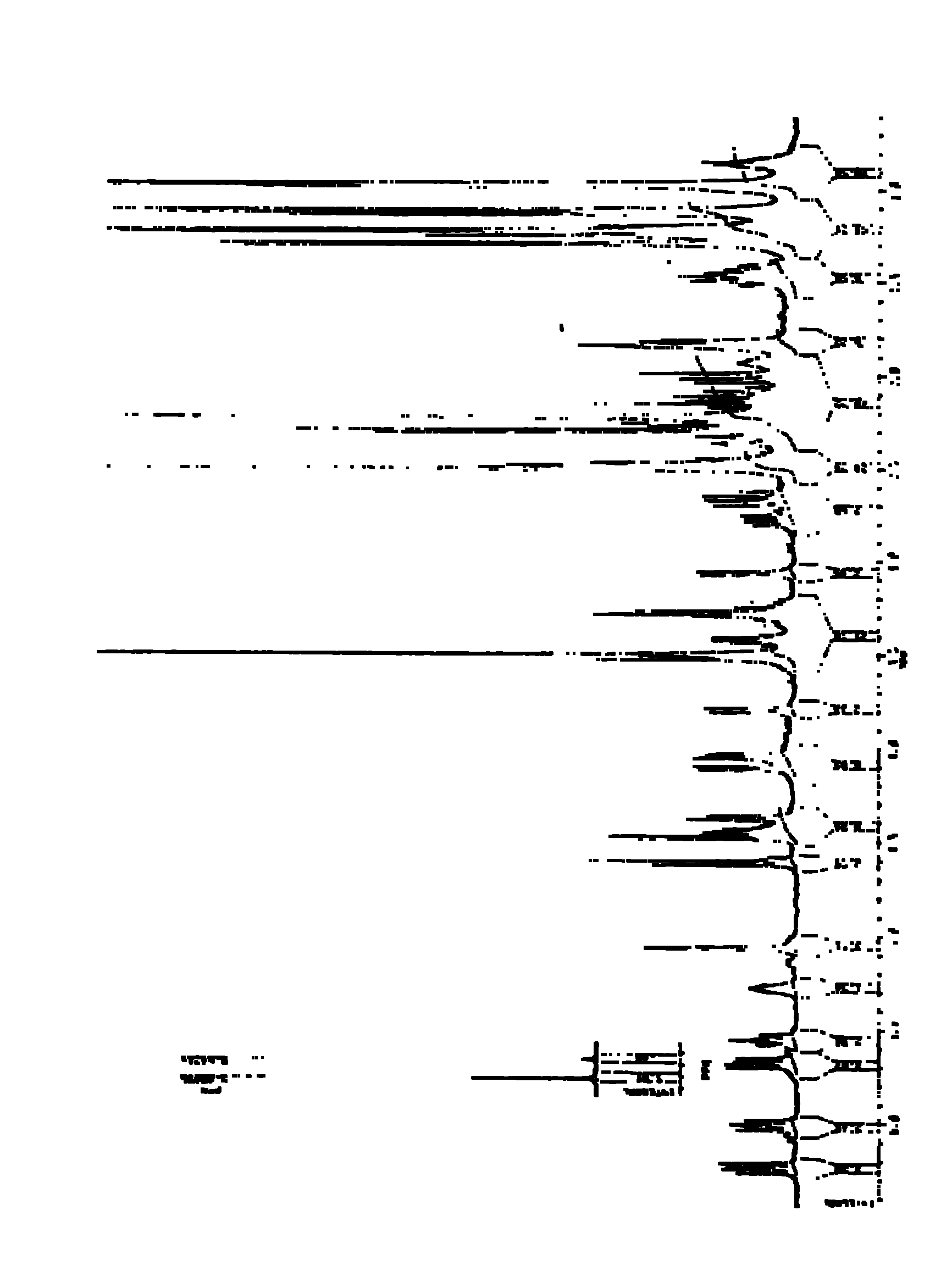
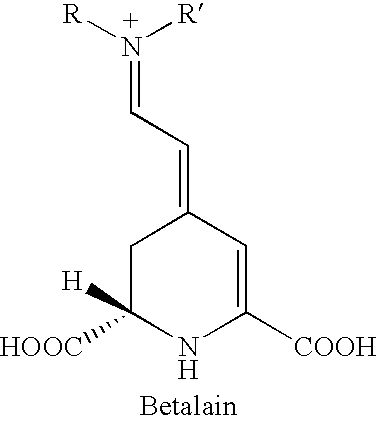
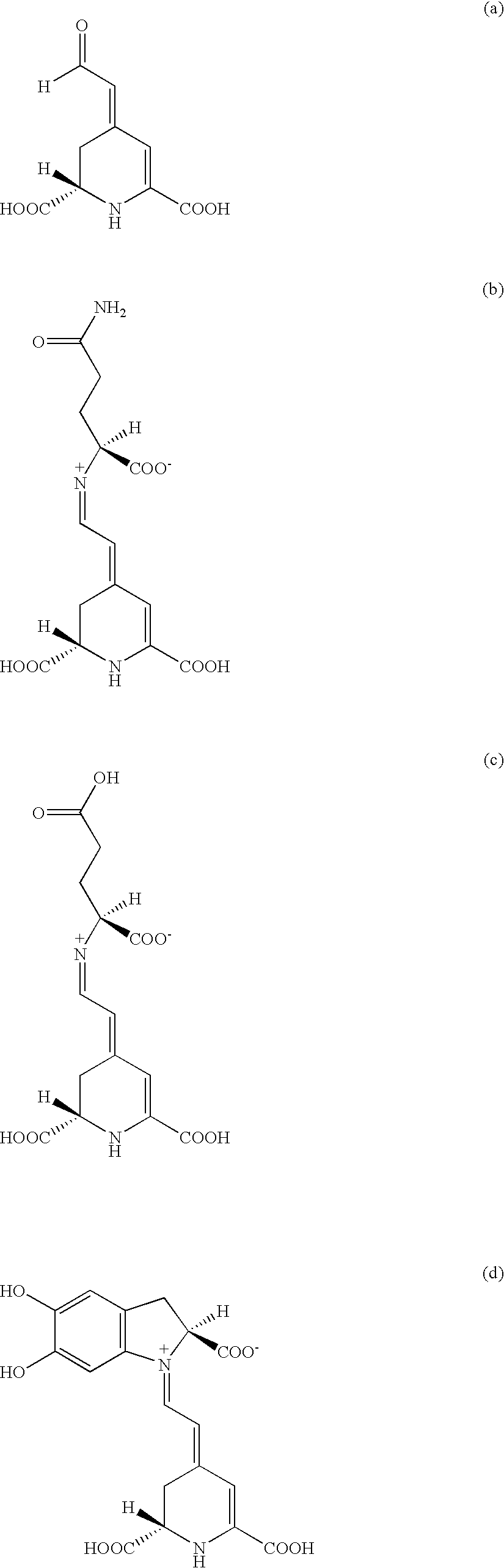
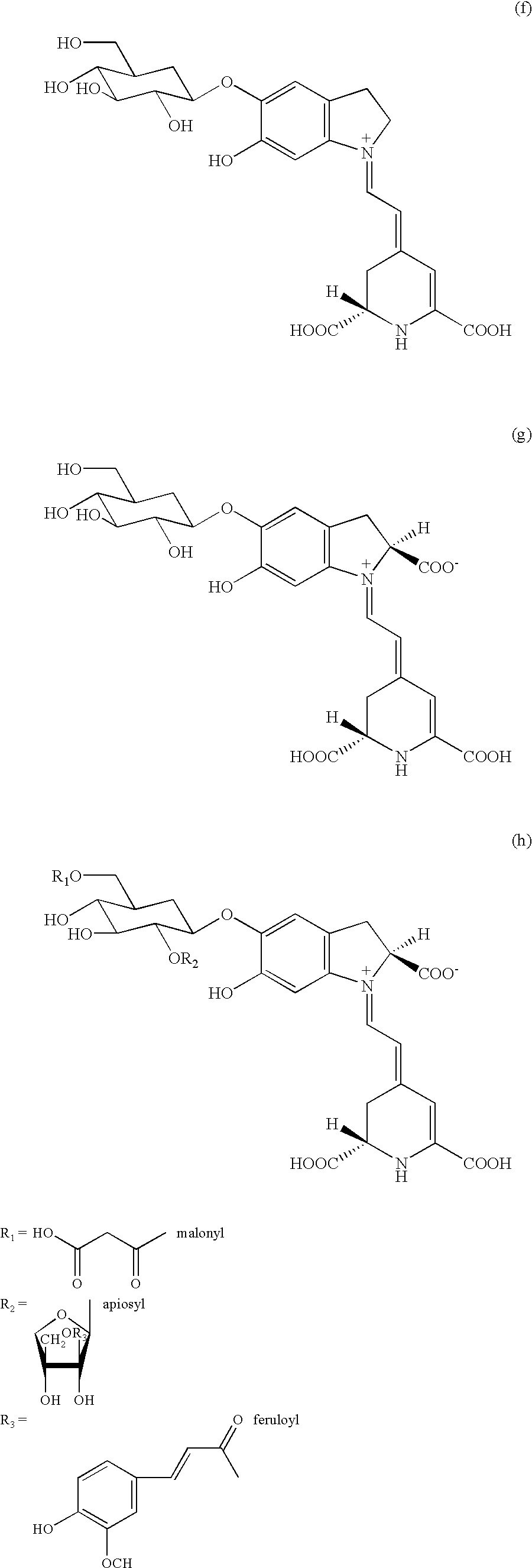
Extracts prepared from red and high-pigment beetroots (Beta vulgaris L.) with a solvent, such as a water-containing solvent, possess antioxidant activity as demonstrated by a panel of assays. These extracts also have an ability to induce quinone reductase in Murine hepatoma cell (Hepa 1c1c7) cultured in vitro. Fractions purified from the active extracts also possess antioxidant activity and retain quinone reductase-inducing activity in the Hepa 1c1c7 cell line. The active extracts, purified by column, thin layer, and high-performance liquid chromatographic techniques, include betalains. A method of extracting a betalain includes steps of freeze-drying a source containing the betalain; grinding the freeze-dried source; and extracting the betalain from the ground source with the solvent. Additionally, the betalain extract can be isolated betalain components, such as by chromatography.
Owner:WISCONSIN ALUMNI RES FOUND
Water base dispersion of fluorinated polymer and process for producing the same
The object of the present invention is a fluoropolymer aqueous dispersion showing only a moderate viscosity increase upon temperature rise and having a low fluorine-containing anionic surfactant concentration as well as a method of producing such fluoropolymer aqueous dispersion. The present invention provides a fluoropolymer aqueous dispersion comprising a particle comprising a fluoropolymer dispersed in an aqueous medium in the presence of a nonionic surfactant, wherein a supernatant for assaying as obtained by subjecting the fluoropolymer aqueous dispersion to 30 minutes of centrifugation at 25° C. and at a gravitational acceleration of 1677 G, when subjected to high-performance liquid chromatography [HPLC] under the conditions of a flow rate of 1.0 ml / minute and a column temperature of 40° C. using an acetonitrile / 0.05 M aqueous solution of phosphoric acid (60 / 40% by volume) mixture as a developing solution, followed by detection at an absorption wavelength at which the nonionic surfactant can be identified, shows a ratio (A1 / A0), which is the ratio between the total area (A0) under the detected line and the area (A1) under the detected line over a retention time period shorter than 16 minutes, of not lower than 0.4 and the supernatant for assaying has a fluorine-containing anionic surfactant content of not higher than 100 ppm.
Owner:DAIKIN IND LTD
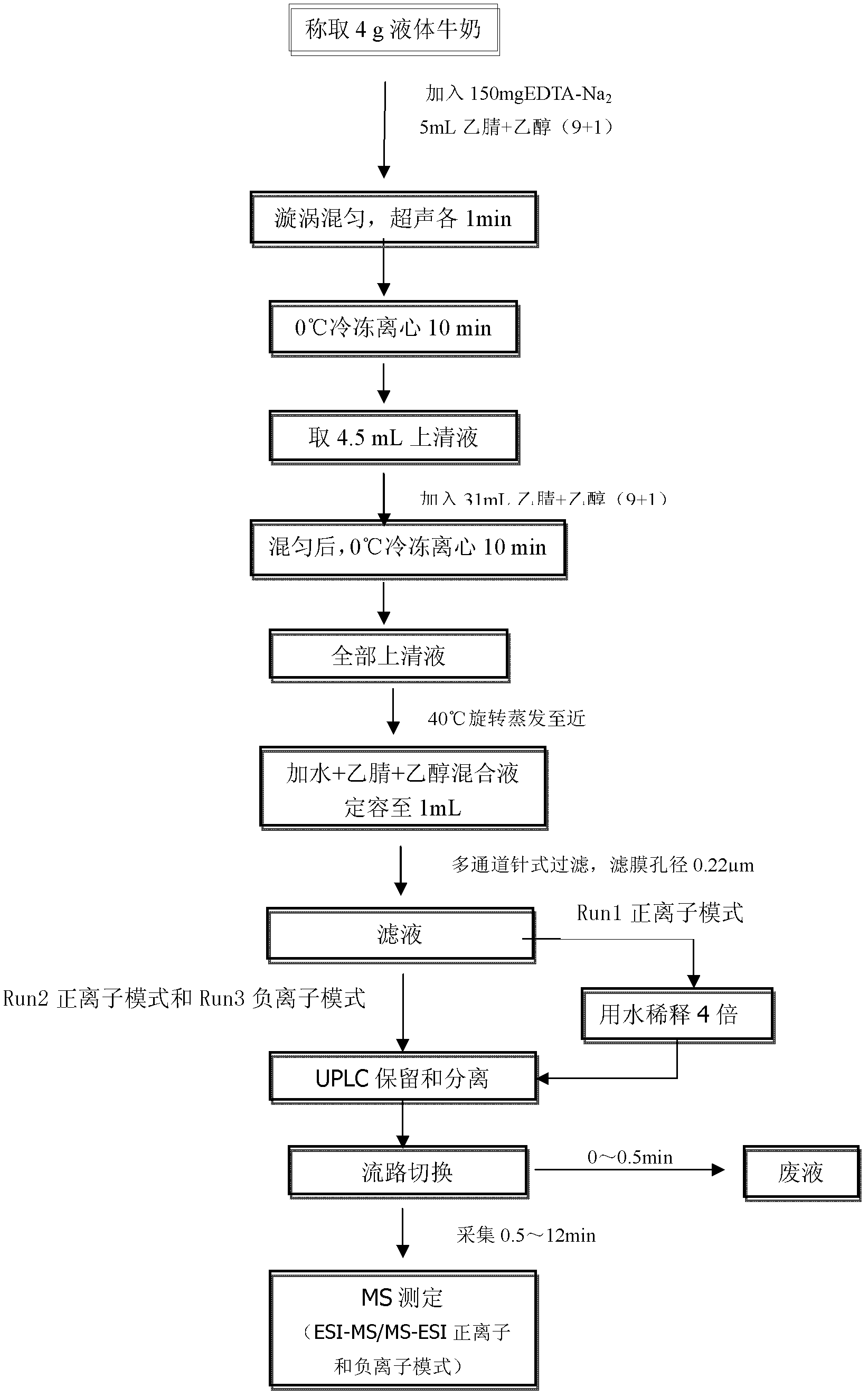
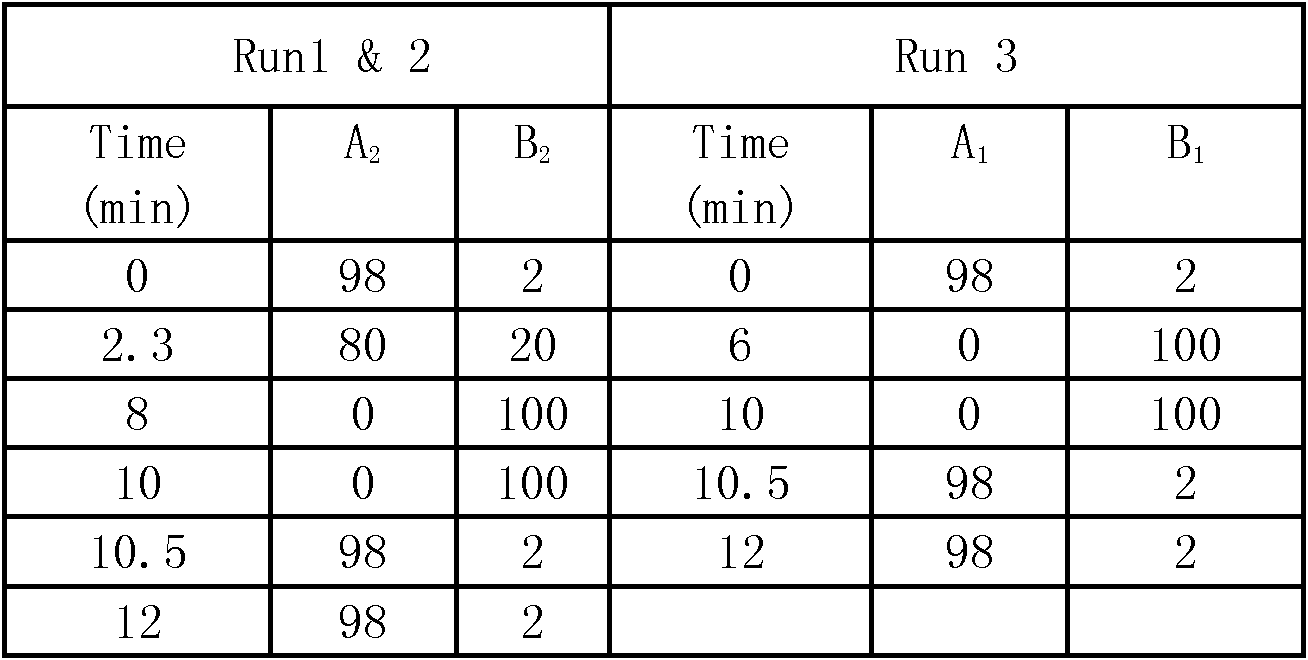
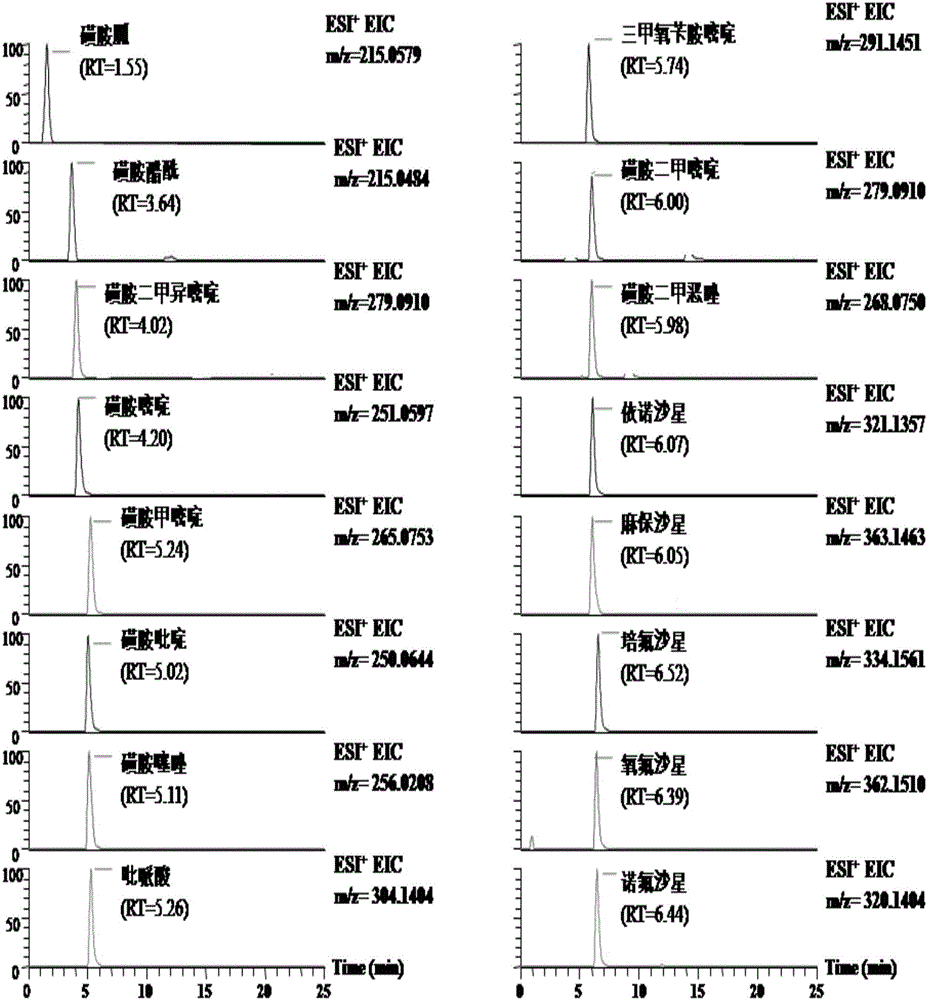
General rapid detection method for micromolecular poisonous and harmful substances in liquid milk
The invention discloses a general rapid detection method for micromolecular poisonous and harmful substances in liquid milk. The method is characterized in that the method concretely comprises the following steps: 1, carrying out simple and general extraction and purification on a liquid milk sample to obtain a loading liquid; 2, preparing a mixed standard solution and a matrix addition standard curve; 3, determining the micromolecular poisonous and harmful substances in the liquid milk through UPLC-MS / MS; and 4, carrying out concentration calculation. The method of the invention has the following advantages: the method starts from general characteristics of micromolecular compounds and fully takes the dissolvability, the thermal stability, the acidic and basic stability, the polar strength, the coexistent stability and the like of all the substances into consideration, a general rapid preprocessing technology and a UPLC-MS / MS electro-spray apparatus determination technology are developed, and the substantial efficiency improvement and the detection cost reduction effect are brought to the production process control and food detection work on condition that detection limit requirements are satisfied.
Owner:NINGBO ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
High-throughput detection method for 99 residual veterinary drugs in animal-derived food
The invention discloses a high-throughput detection method for 99 residual veterinary drugs in animal-derived food. The detection method is characterized by comprising the following concrete steps: (1) carrying out extraction and purification on 99 residual veterinary drugs having substantially different physicochemical properties and belonging to eight kinds of common veterinary drugs through one-step pretreatment based on carrier-assisted liquid-liquid extraction technology; (2) preparation of a mixed standard solution and a matrix standard curve; and (3) determining the concentrations of 99 residual veterinary drugs in a to-be-detected solution by using ultrahigh performance liquid chromatography-triple quadrupole tandem mass spectrometry. According to the invention, the pretreatment and instrumental analysis process of the method is directed at compounds with different physicochemical properties, so the method has the advantages of good compatibility, high detection efficiency, good operability and low detection cost, and the detection limit of the method can meet requirements of all the test objects.
Owner:INSPECTION & QUARANTINE TECH CENT OF NINGBO ENTRY EXIT INSPECTION & QUARANTINE BUREAU
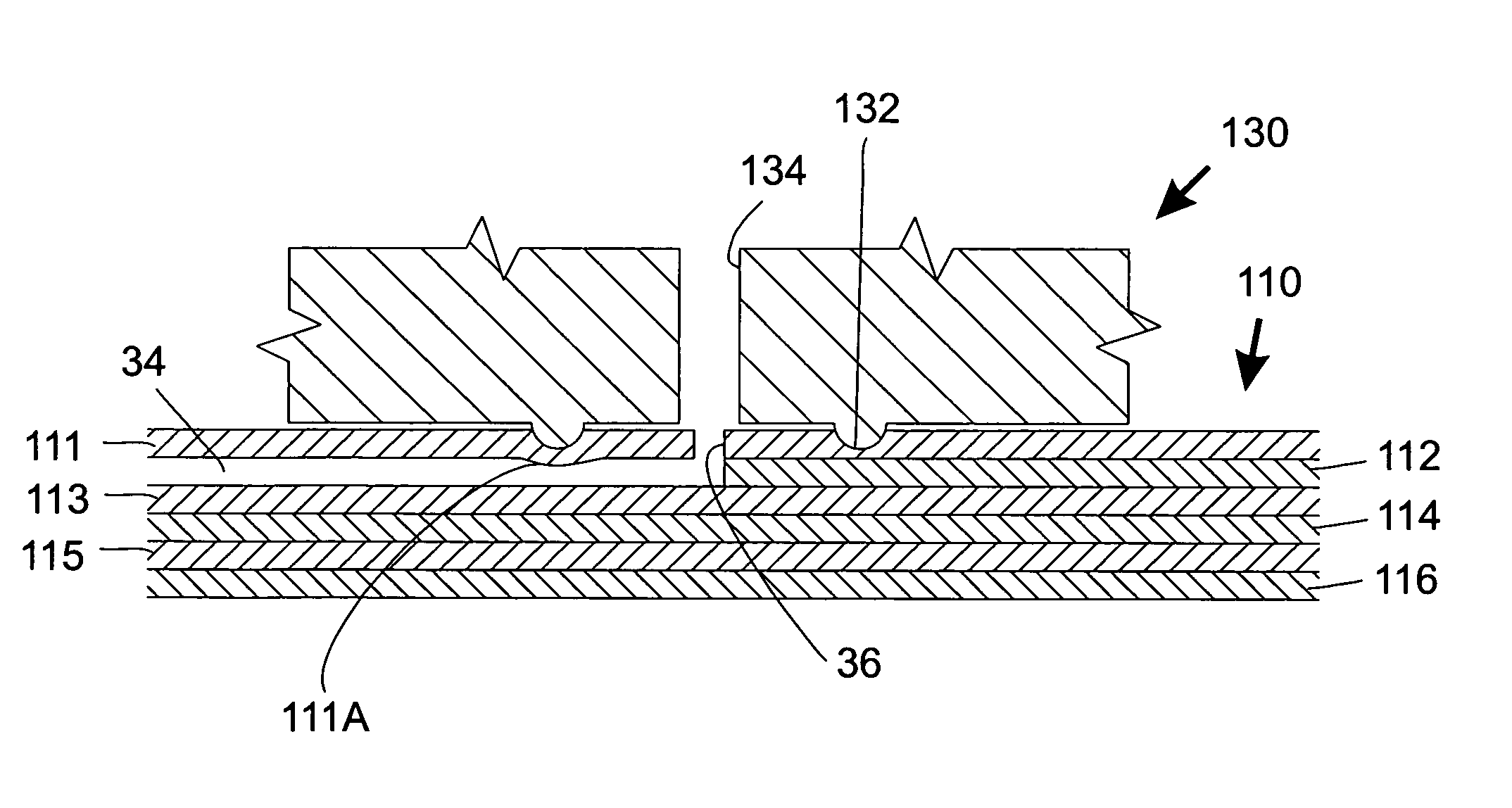
Tube structure with sol-gel zirconia coating
InactiveUS20070095736A1Improve stabilityUnique surface chemistryIon-exchange process apparatusOther chemical processesKetoneHYDROSOL
The subject invention concerns zirconia-based hybrid organic-inorganic sol-gel coating for optional use as a stationary phase in capillary microextraction (CME), gas chromatographic (GC), high performance liquid chromatography (HPLC), capillary electrophoresis (CE), capillary electrochromatography (CEC) and related analytical techniques. Sol-gel chemistry is employed to chemically bind a hydroxy-terminated silicone polymer (polydimethyldiphenylsiloxane, PDMDPS) to a sol-gel zirconia network. In one embodiment, a fused silica capillary is filled with a properly designed sol solution to allow for the sol-gel reactions to take place within the capillary. In the course of this process, a layer of the evolving hybrid organic-inorganic sol-gel polymer becomes chemically bonded to the silanol groups on the inner capillary walls. The unbonded part of the sol solution is expelled from the capillary under helium pressure, leaving behind a chemically bonded sol-gel zirconia —PDMDPS coating on the inner walls of the capillary. Polycyclic aromatic hydrocarbons, ketones, and aldehydes are efficiently extracted and preconcentrated from dilute aqueous samples followed by GC separation of the extracted analytes.
Owner:MALIK ABDUL +1
Method for analyzing and detecting a plurality of endocrine disruptors in food
ActiveCN103185762AHigh sensitivityAccurate analysisComponent separationPerturbateurs endocriniensLiquid milk
The invention belongs to the field of food inspection, relates to an assay determination method for endocrine disruptors in food, and particularly relates to a method for analyzing and determining a plurality of endocrine disruptors in milk powder and liquid milk. The method comprises the steps of dissolving a sample with water, adding an organic solvent mixable with water, extracting a to-be-detected material by ultrasonic, adding a sodium salt to make the organic solvent separated from a water phase to realize liquid-liquid extraction, taking an organic solvent containing quantitative to-be-detected material, purifying by extraction with a solid phase filled with C 18 materials, deriving by dansyl chloride, and analyzing and detecting four types of 26 endocrine disruptors in the food by using ultra-high performance liquid chromatography-quadrupole-time of flight-mass spectrometry at the same time. The method can overcome the disadvantages that a conventional technology cannot realize simultaneous analysis of the plurality of the endocrine disruptors in the food by once chromatographic sample injection, can increase sensitivity of the quadrupole-time of the flight-mass spectrometry, and realize accurate analysis of the endocrine disruptors in the food by using the quadrupole-time of the flight-mass spectrometry.
Owner:FUDAN UNIV
Preparation method for fast separating flavonoid glycosides from oil-tea-cakes with medium pressure column
ActiveCN101899070ASimple processHigh product contentSugar derivativesSugar derivatives preparationCamellia oleiferaSolvent
The invention discloses a preparation method for fast separating flavonoid glycosides from oil-tea-cakes with a medium pressure column, which comprises the following steps of: decorticating and crushing camellia oleifera abel seeds, degreasing the camellia oleifera abel seeds with non-polar solvents, performing extraction with ethanol water, filtering and condensing the extract to obtain crude extract concrete, performing fast separation with the medium pressure column to obtain an over 90 percent flavonoid glycoside mixture, and further adopting a high performance liquid chromatography to prepare over 95 percent flavonoid glycoside monomers, wherein the flavonoid glycoside monomers are kaemplerol 3-O-[2-O-beta-D-galactose-6-O-alpha-L-rhamnose]-beta-D-glucoside (I) and kaemplerol 3-O-[2-O-beta-D-xylose-6-O-alpha-L-rhamnose]-beta-D-glucoside (II) respectively. The method can be used for batch preparation, and has the advantage of providing quality raw materials for the development of flavonoid glycoside medicaments and healthcare functional products in the oil-tea-cakes.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Methods for characterization of polymers using multi-dimensional liquid chromatography with parallel second-dimension sampling
InactiveUS6855258B2Less complicatedUniversal applicabilityIon-exchange process apparatusSamplingGradient elutionPhase gradient
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com