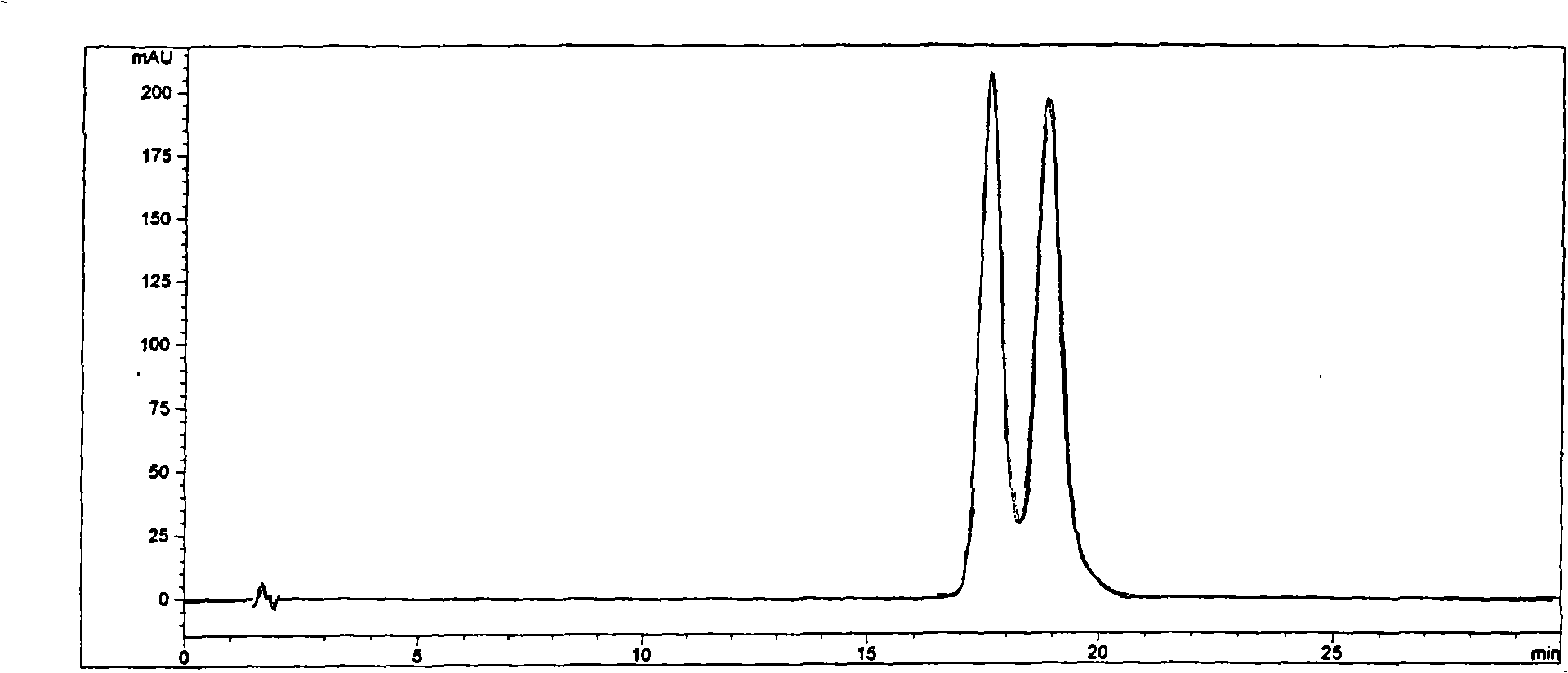
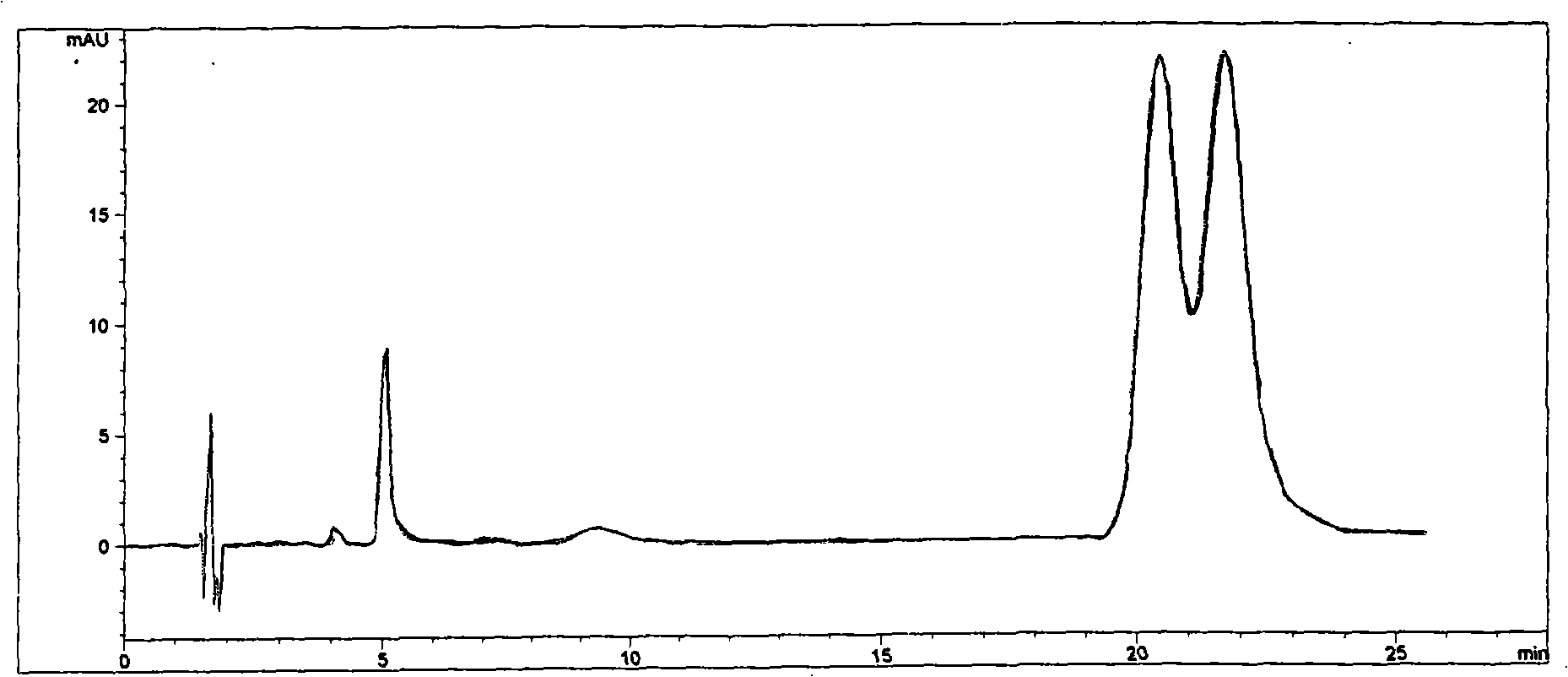
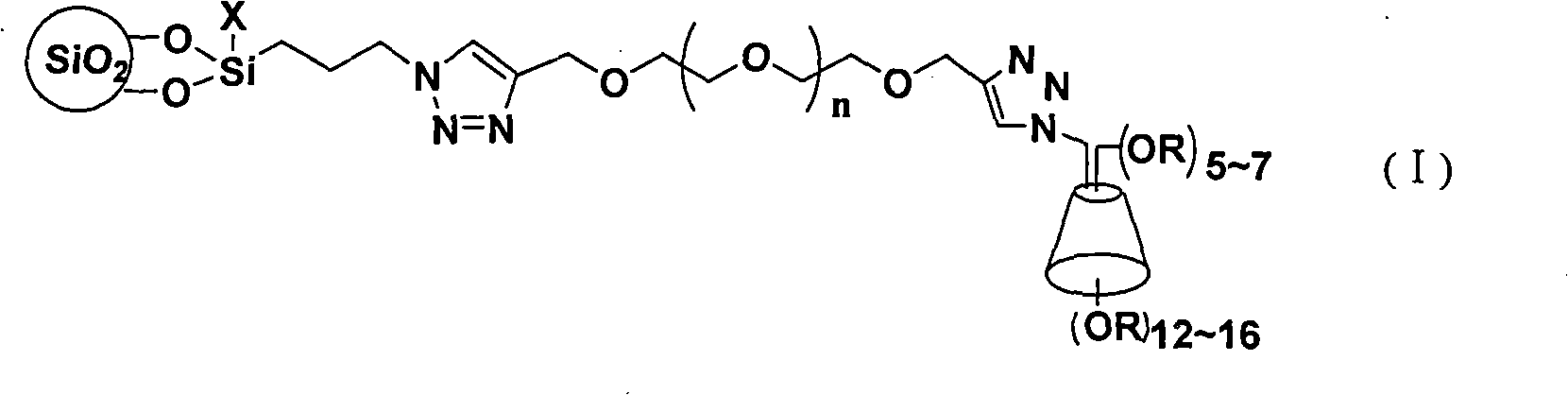
Cyclodextrin chiral chromatogram fixed phase and preparation method thereof
A chiral chromatography and cyclodextrin technology is applied in the field of cyclodextrin chiral chromatography stationary phase and its preparation, and achieves the effects of large surface bonding amount, few steps and avoiding side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Step (1) In a 1000ml three-necked flask, add 400ml of anhydrous N, N dimethylformamide, 45mmol (10ml) 3-chloropropyltriethoxysilane, 60mmol (4.0g) sodium azide, and then add 3.0 Mmol sodium iodide (0.5g) was used as a catalyst, reacted at 80°C for 24h, and then added 20g of spherical silica gel particles were reacted at 80°C for another 24h, and then washed twice with dichloromethane, ethanol, water, and acetone, 200ml each time, and dried in vacuum for 12 hours to obtain (3-propyl)azidosilica gel .
[0044] Step (2) 250ml three-necked flask was added under nitrogen protection with 100ml tetrahydrofuran, 20mmol (4.00g) tetraethylene glycol, 43mmol (1.73g) 80% sodium hydride was added at 0°C, and 45mmol (3.5ml) bromine was added propyne. React at 0-5°C for 1 hour, then react at room temperature, use n-hexane / ethyl acetate (V:V=1:1) as a developing solvent, separate by thin-layer chromatography, and use potassium permanganate solution color development method Check wh...
Embodiment 2
[0049] During the preparation of (3-propyl)azido silica gel in Step (1) of Example 1, the reaction temperature was changed from 80°C to 60°C, or to 130°C, and the other steps were performed as in Example 1, or The cyclodextrin chiral chromatography stationary phase in Example 1 was obtained.
Embodiment 3
[0051] In embodiment 1 step (1) (3-propyl) azido base silica gel preparation process, adopt 400ml toluene to replace the solvent N in embodiment 1, N-dimethylformamide, other steps are operated according to embodiment 1 , the cyclodextrin chiral chromatography stationary phase in Example 1 is also available.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com