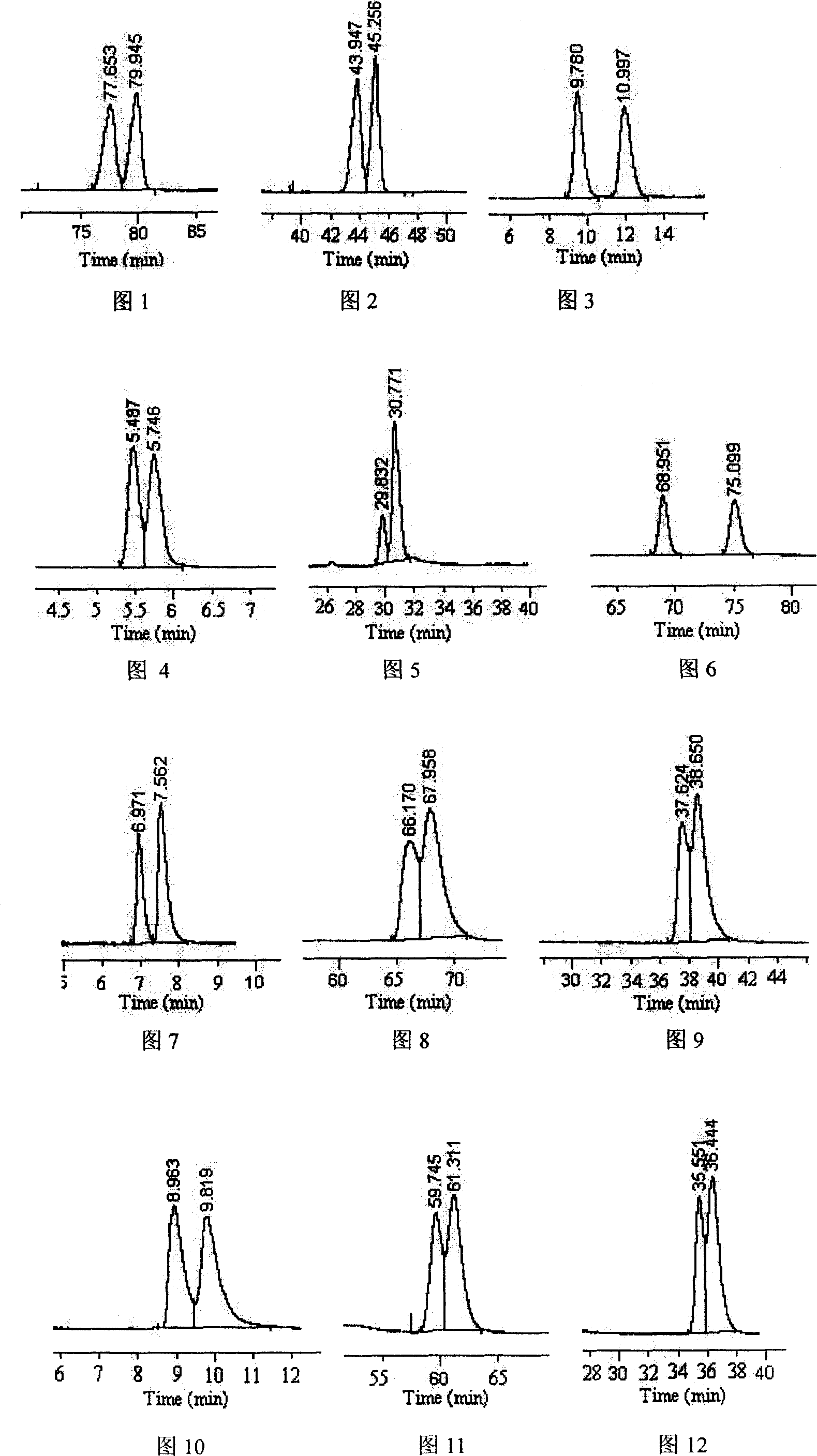
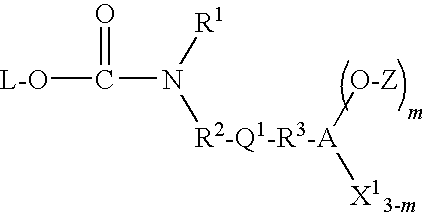
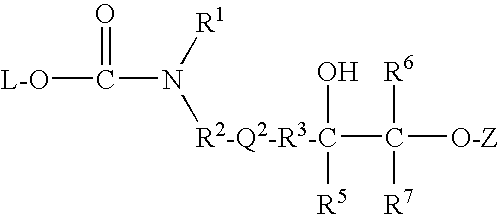
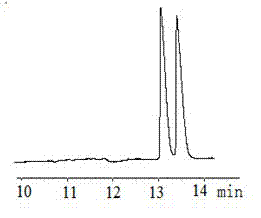
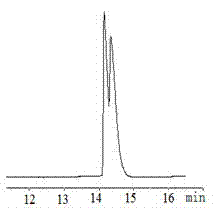
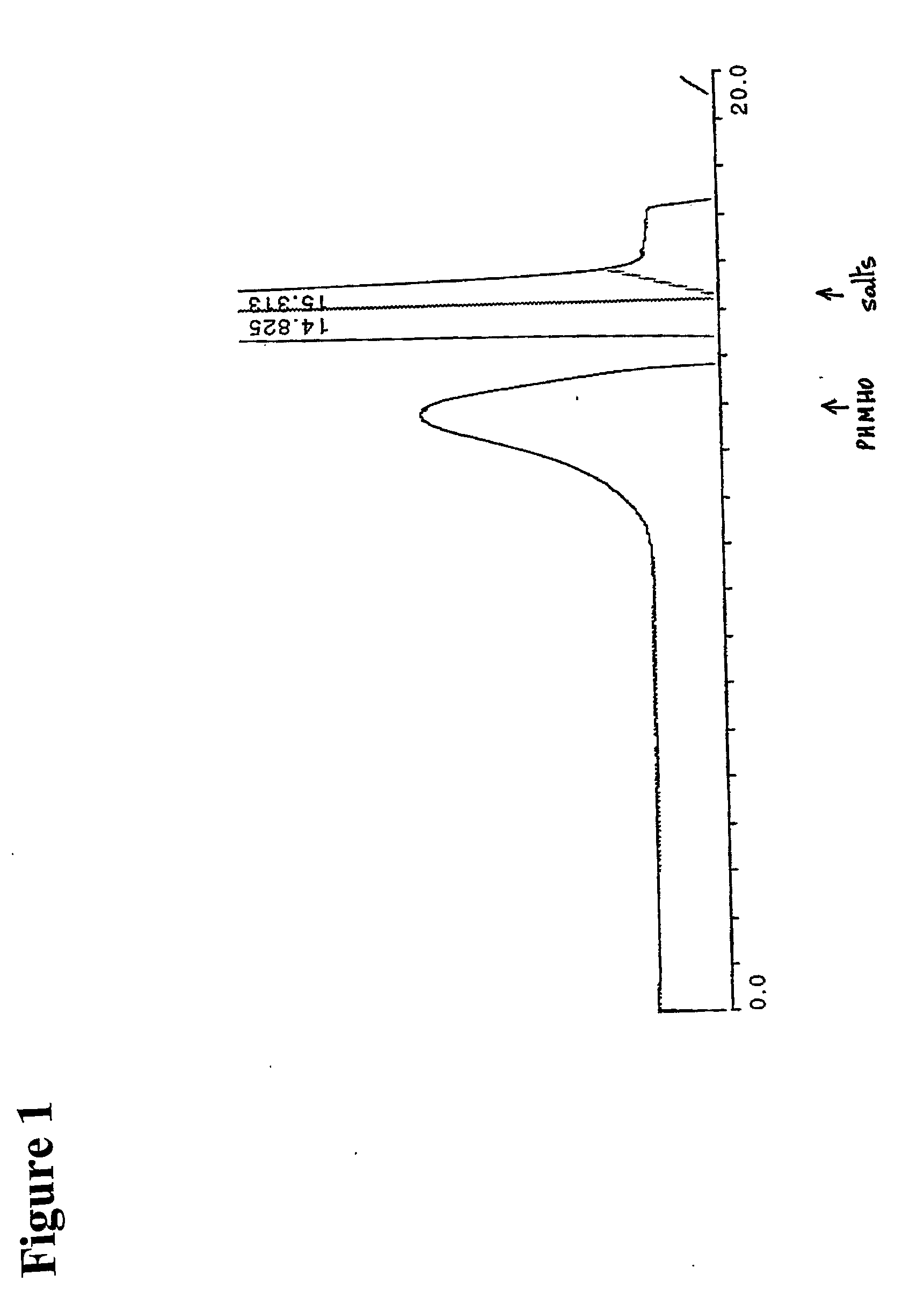
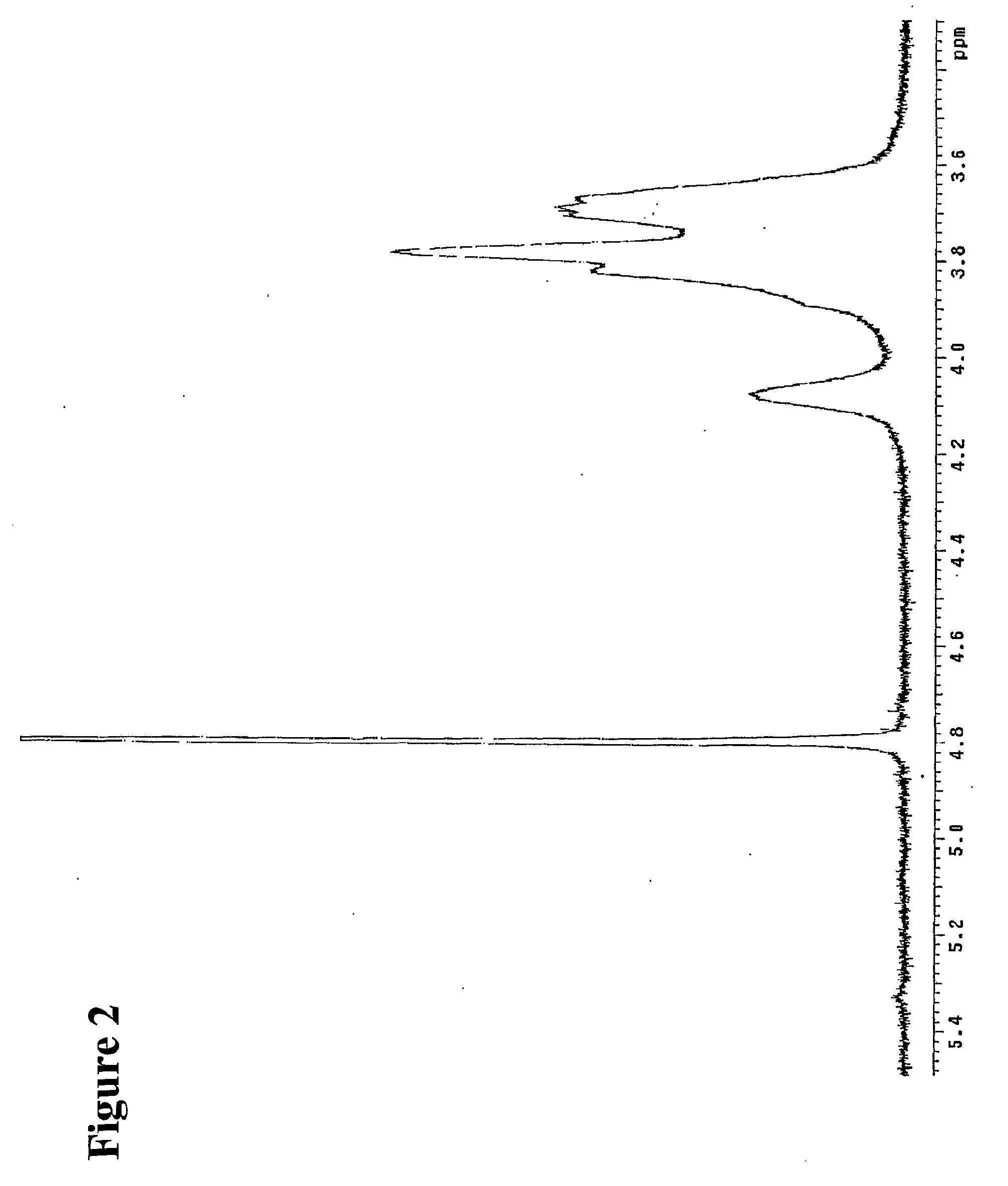
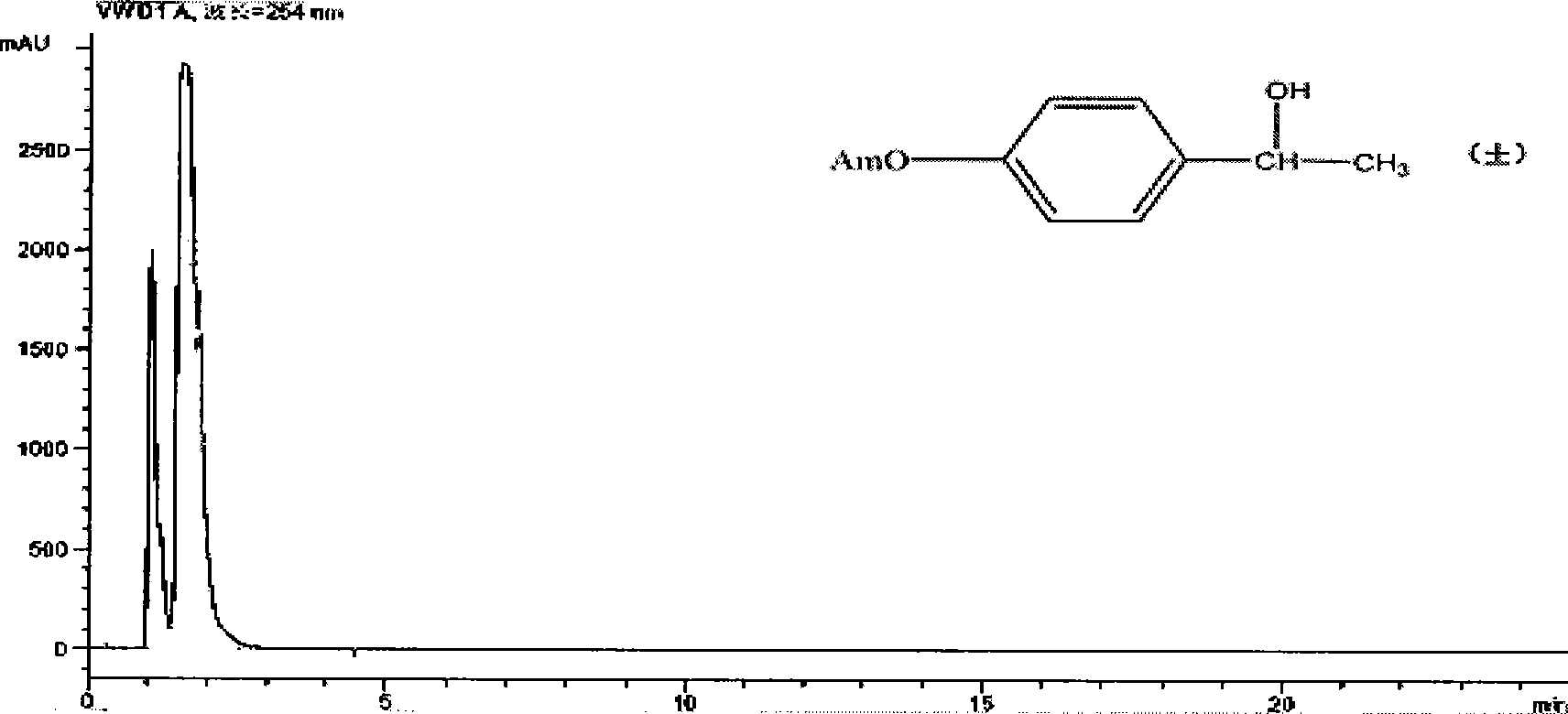
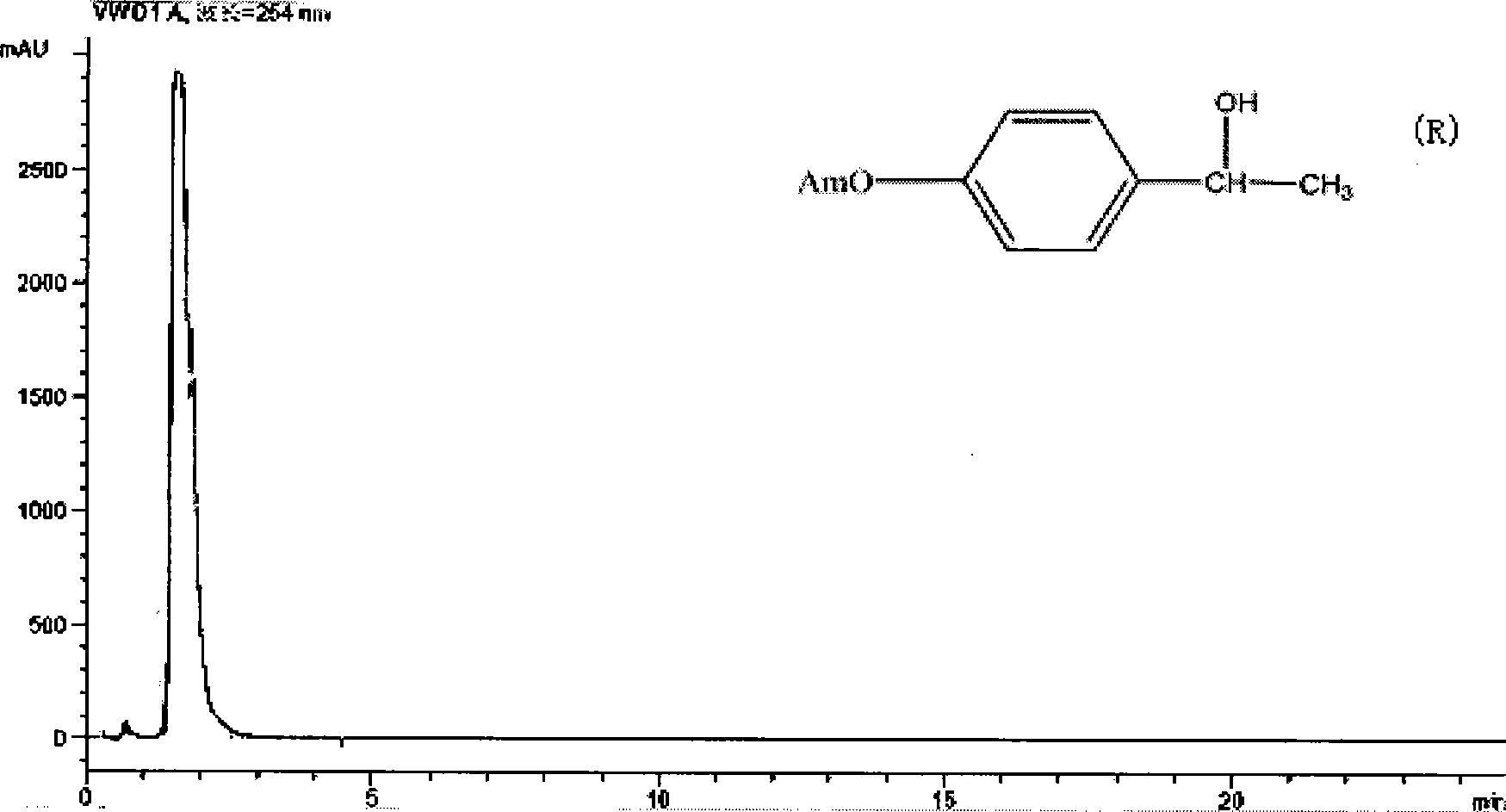
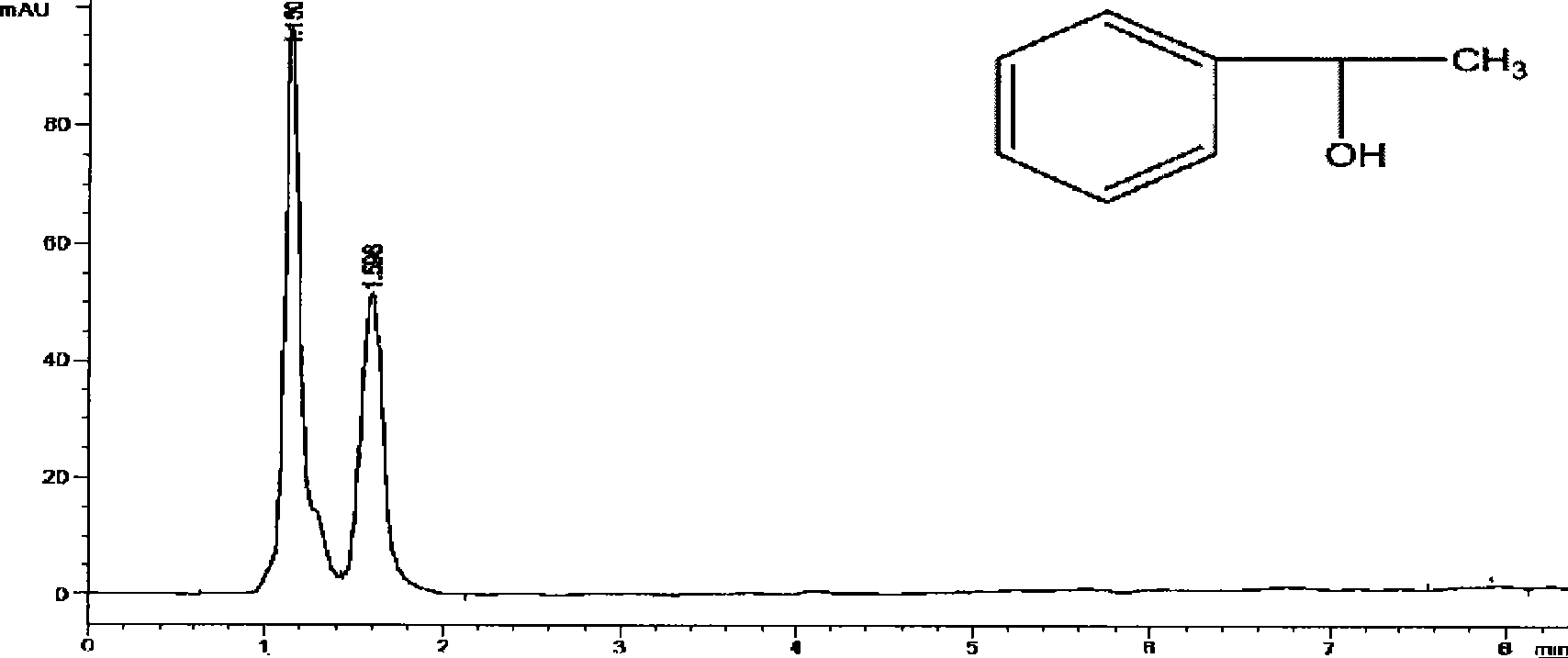
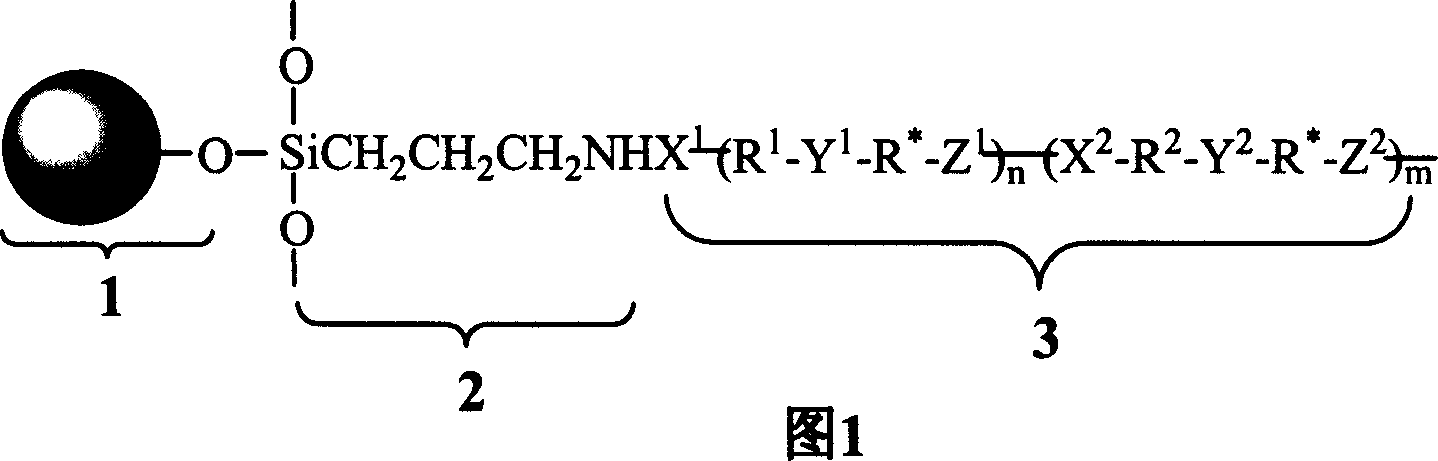
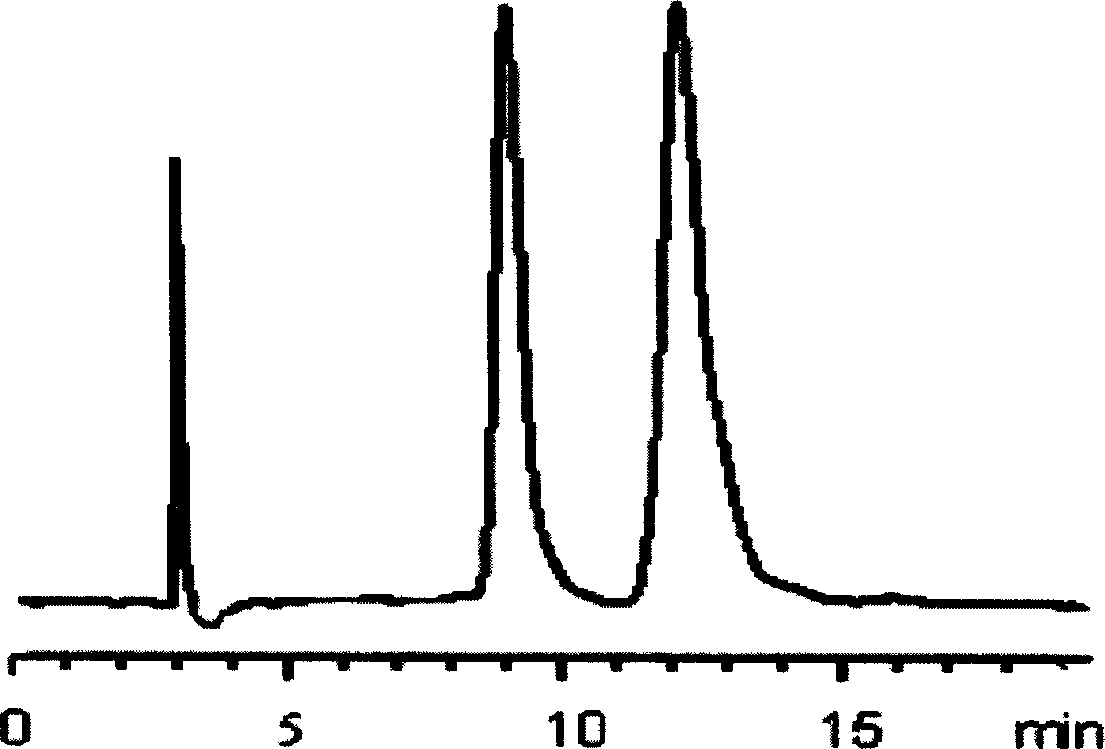
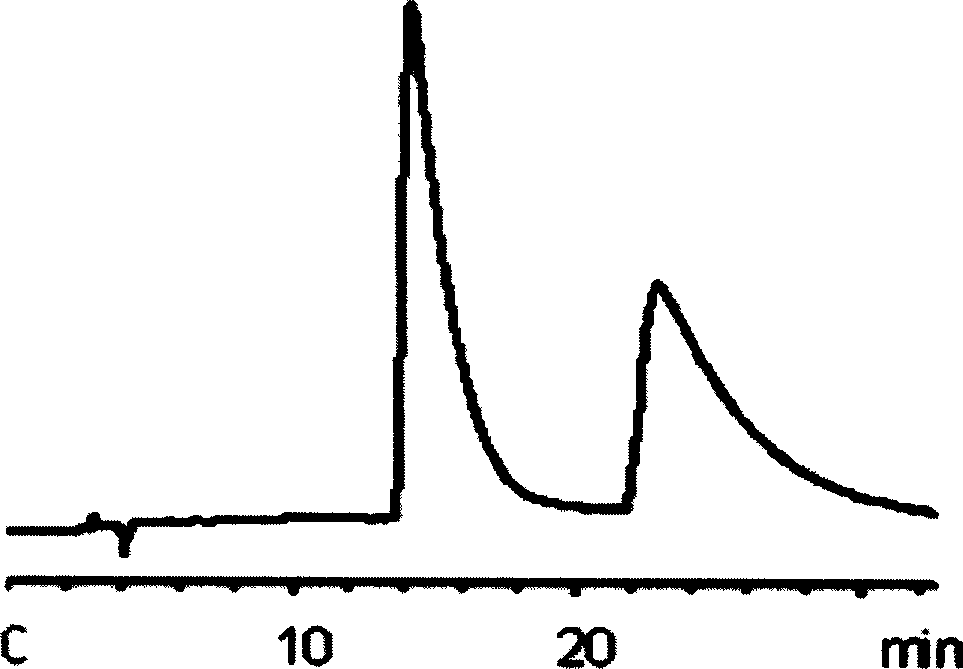
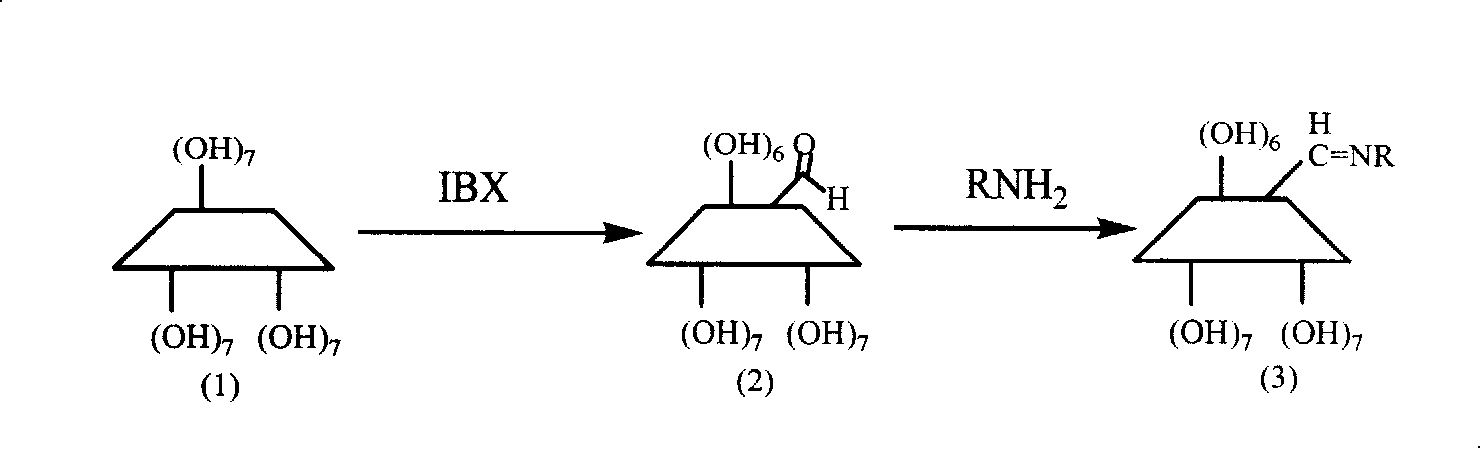
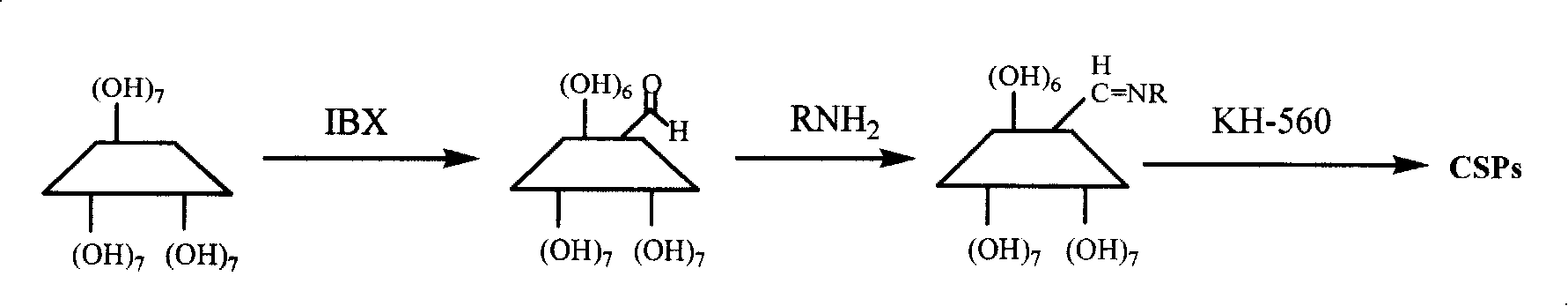Patents
Literature
297 results about "Chiral stationary phase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
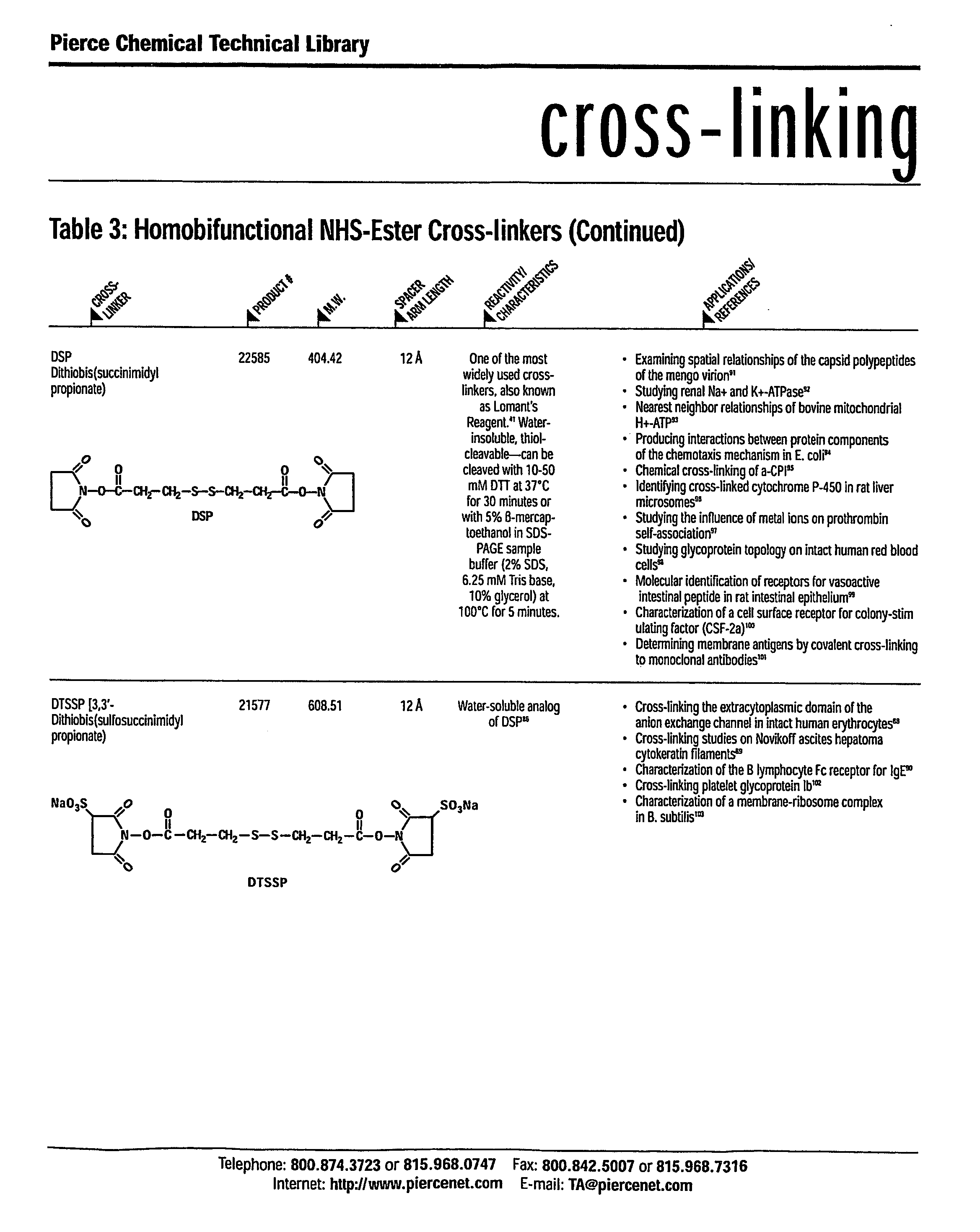
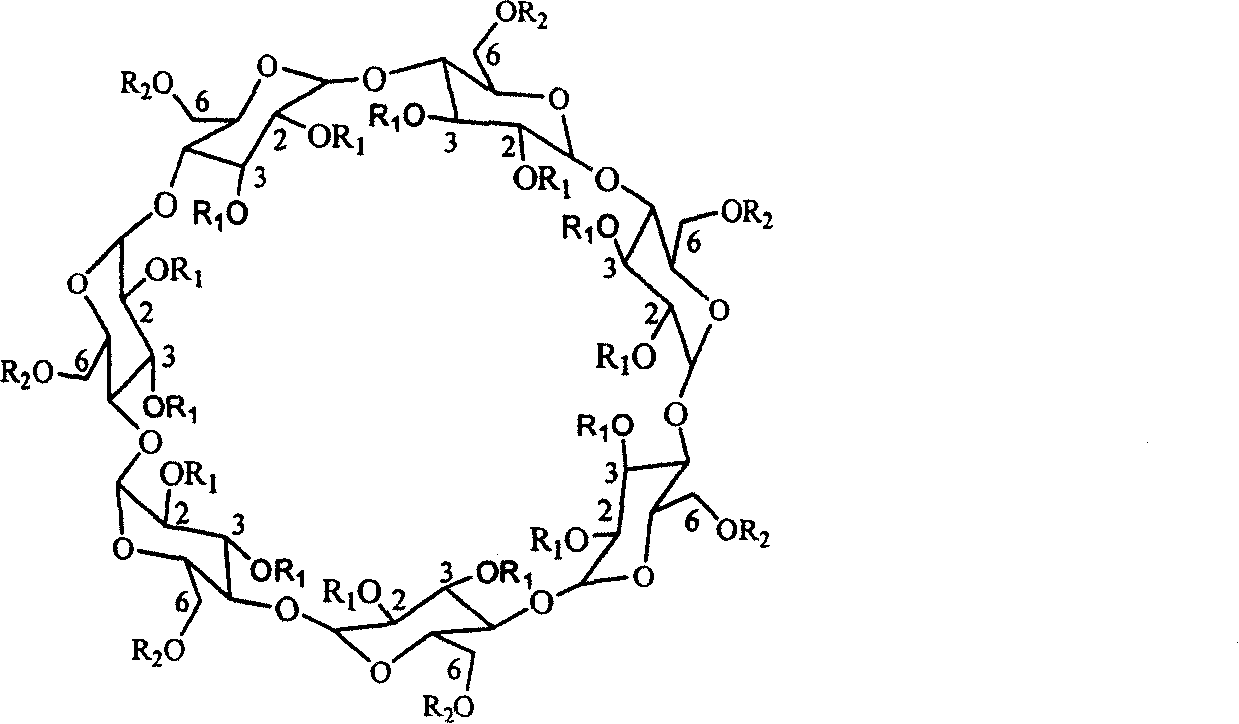
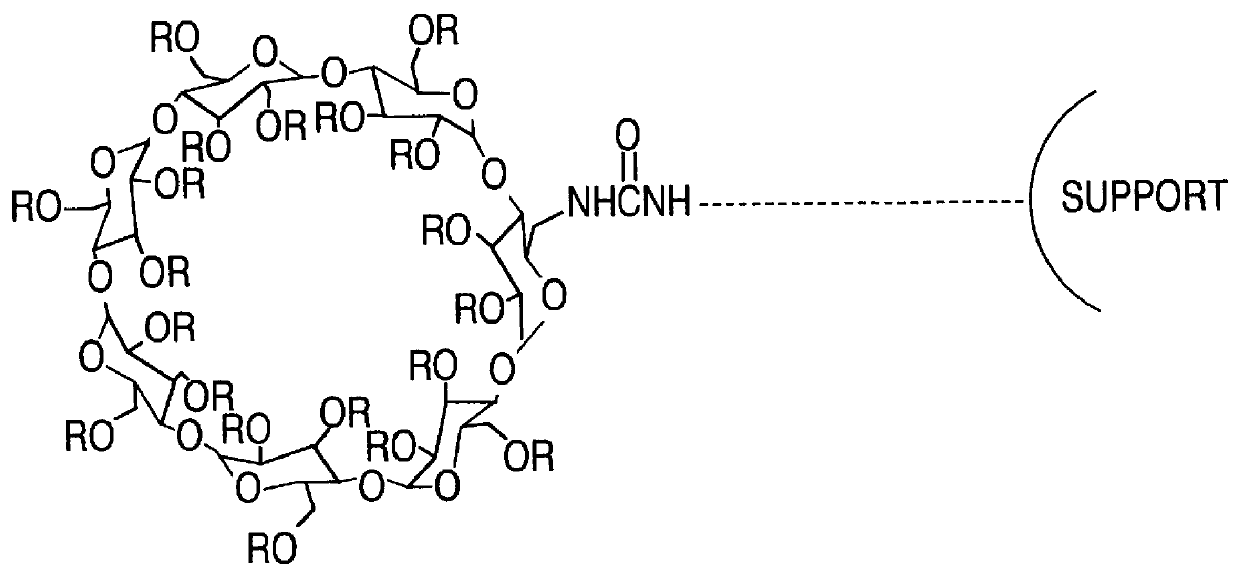
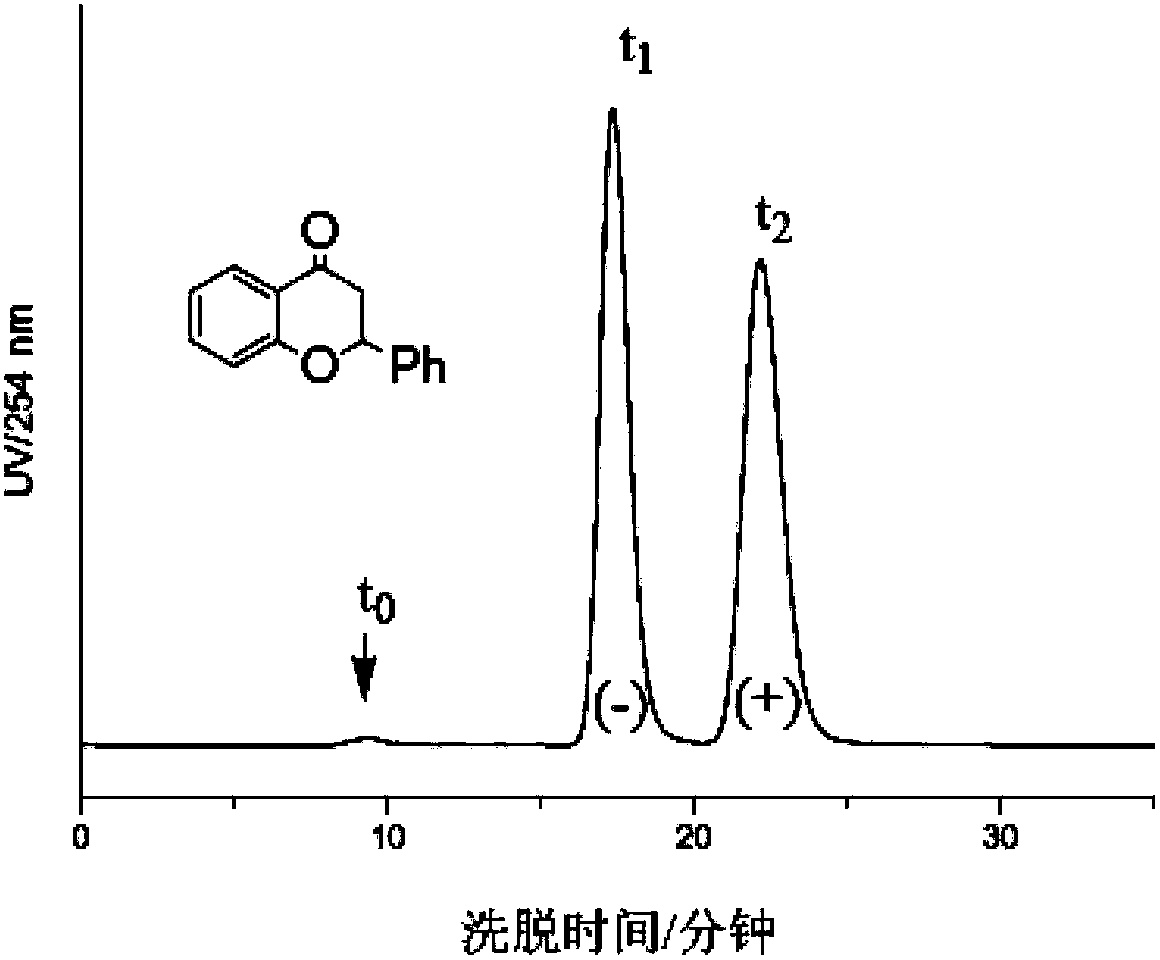
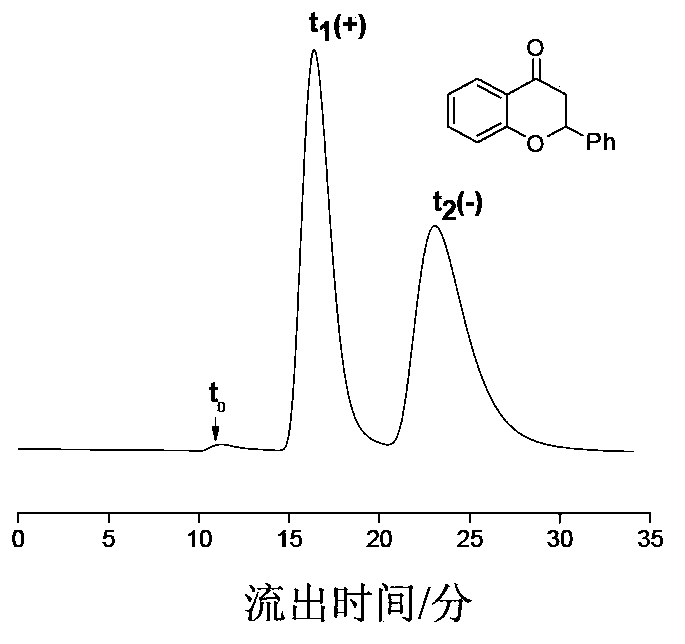
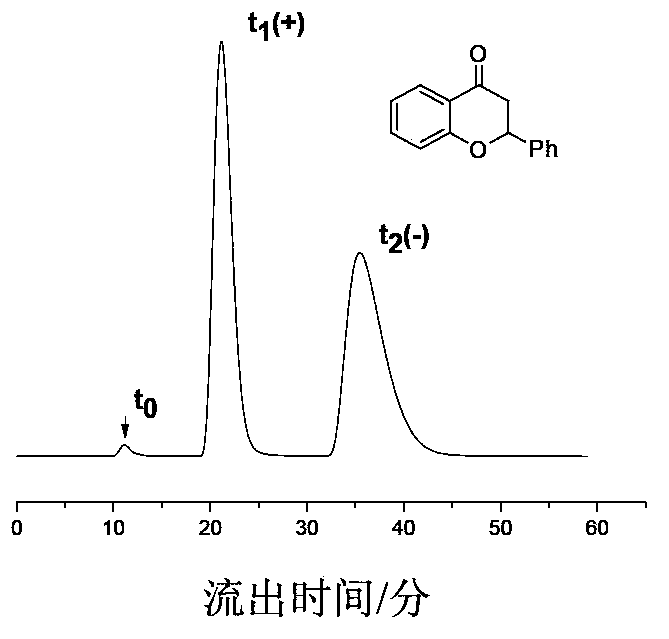
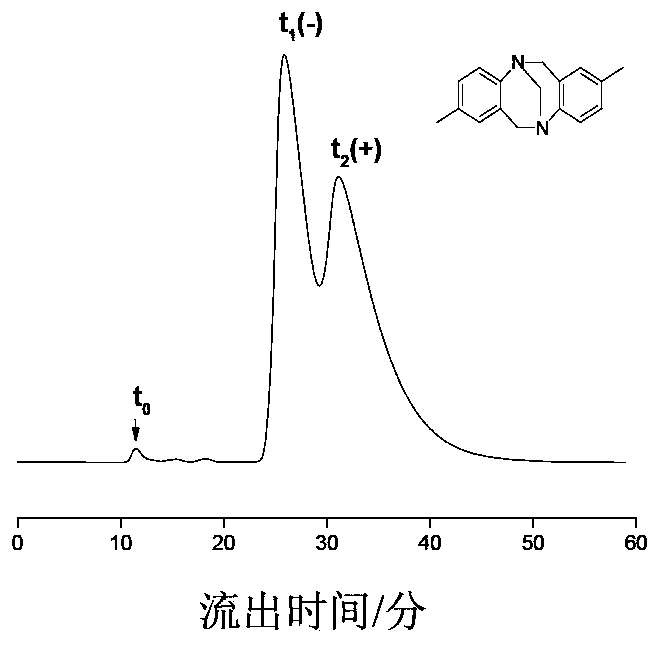
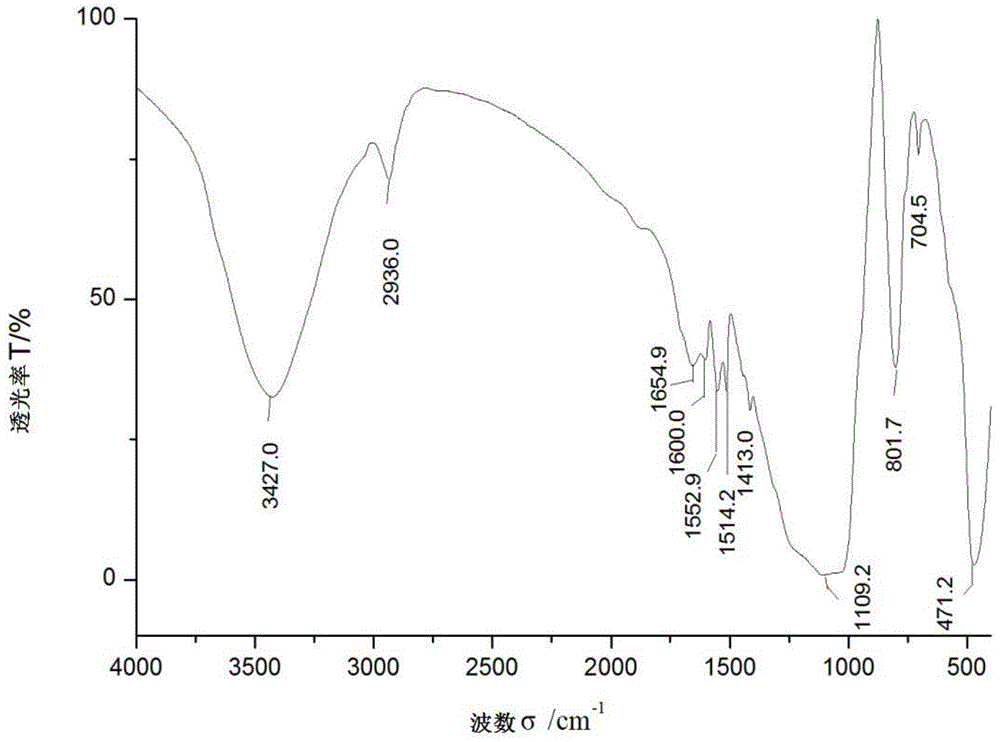
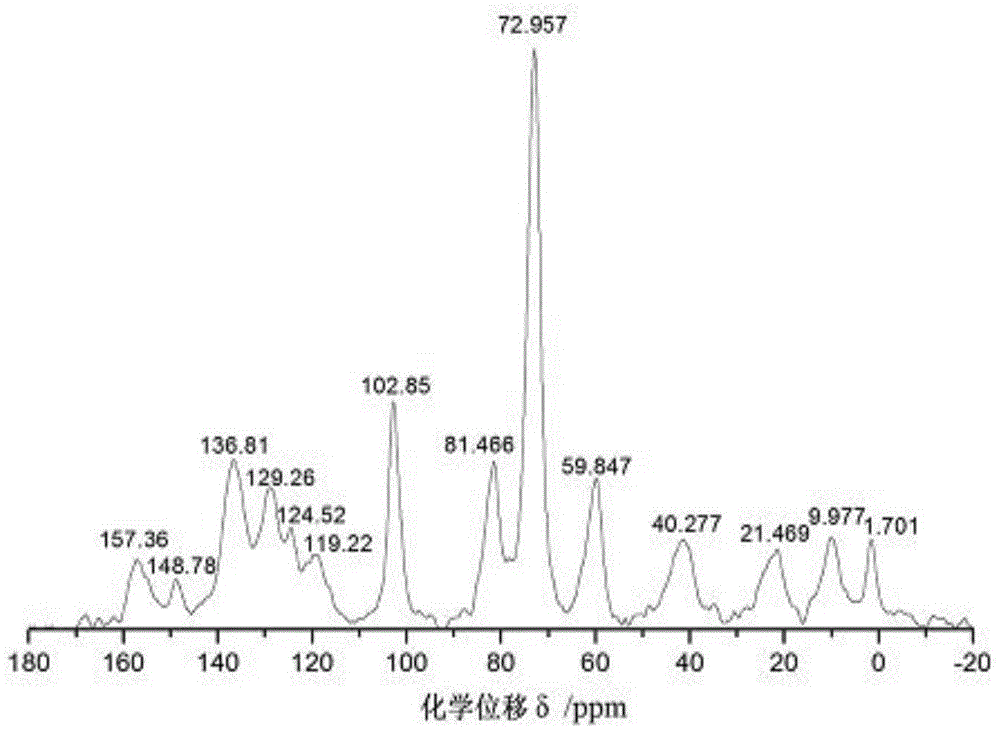
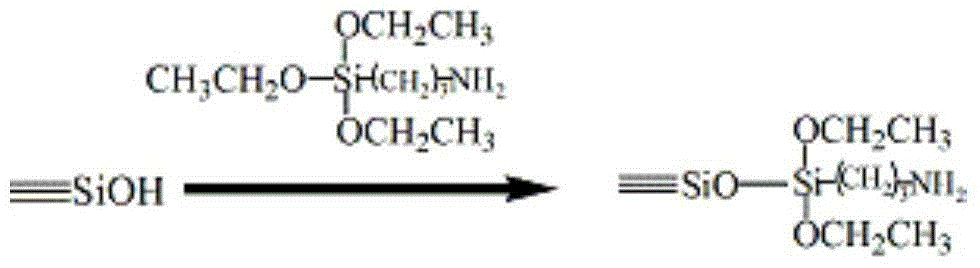
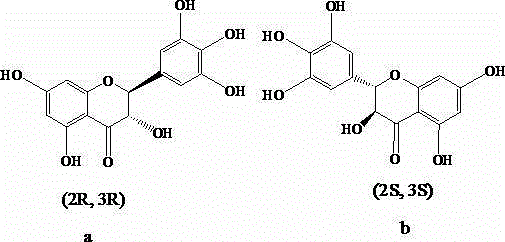
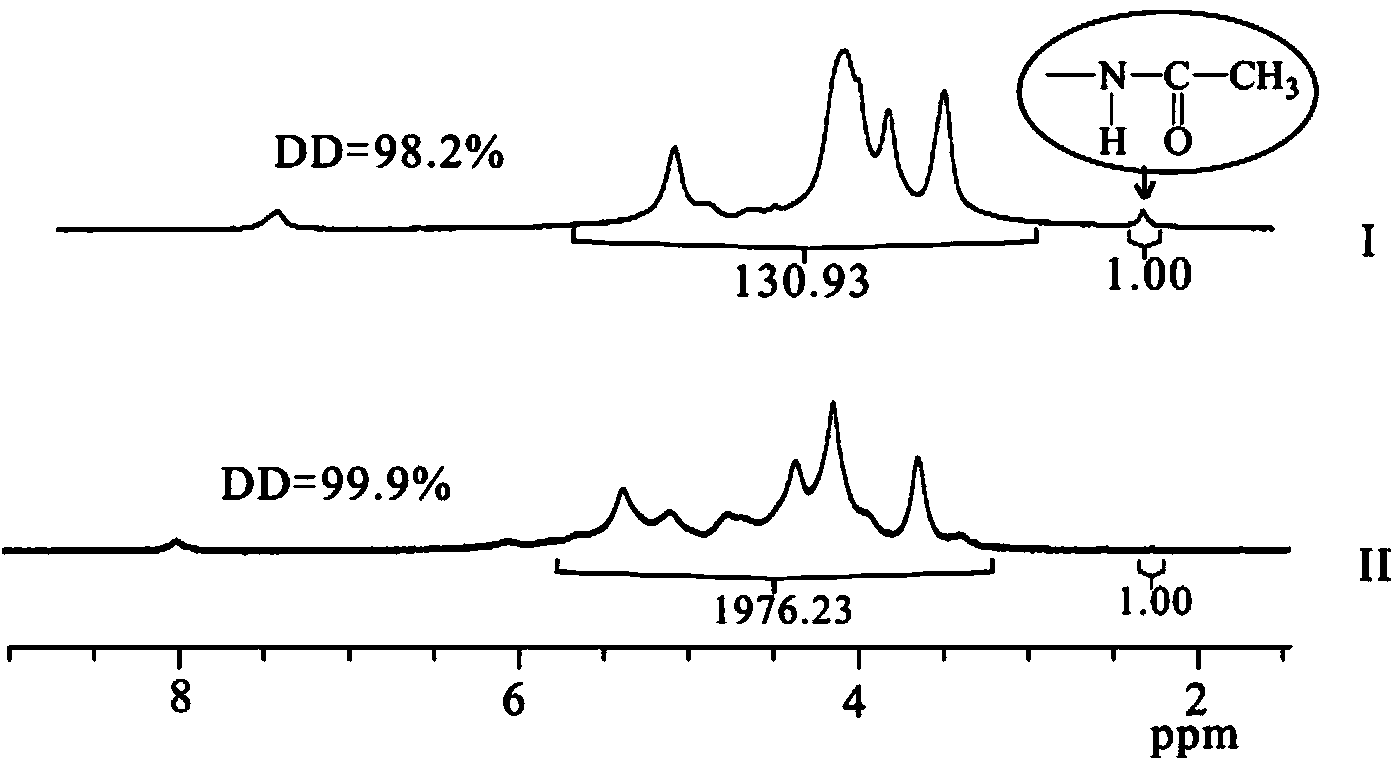
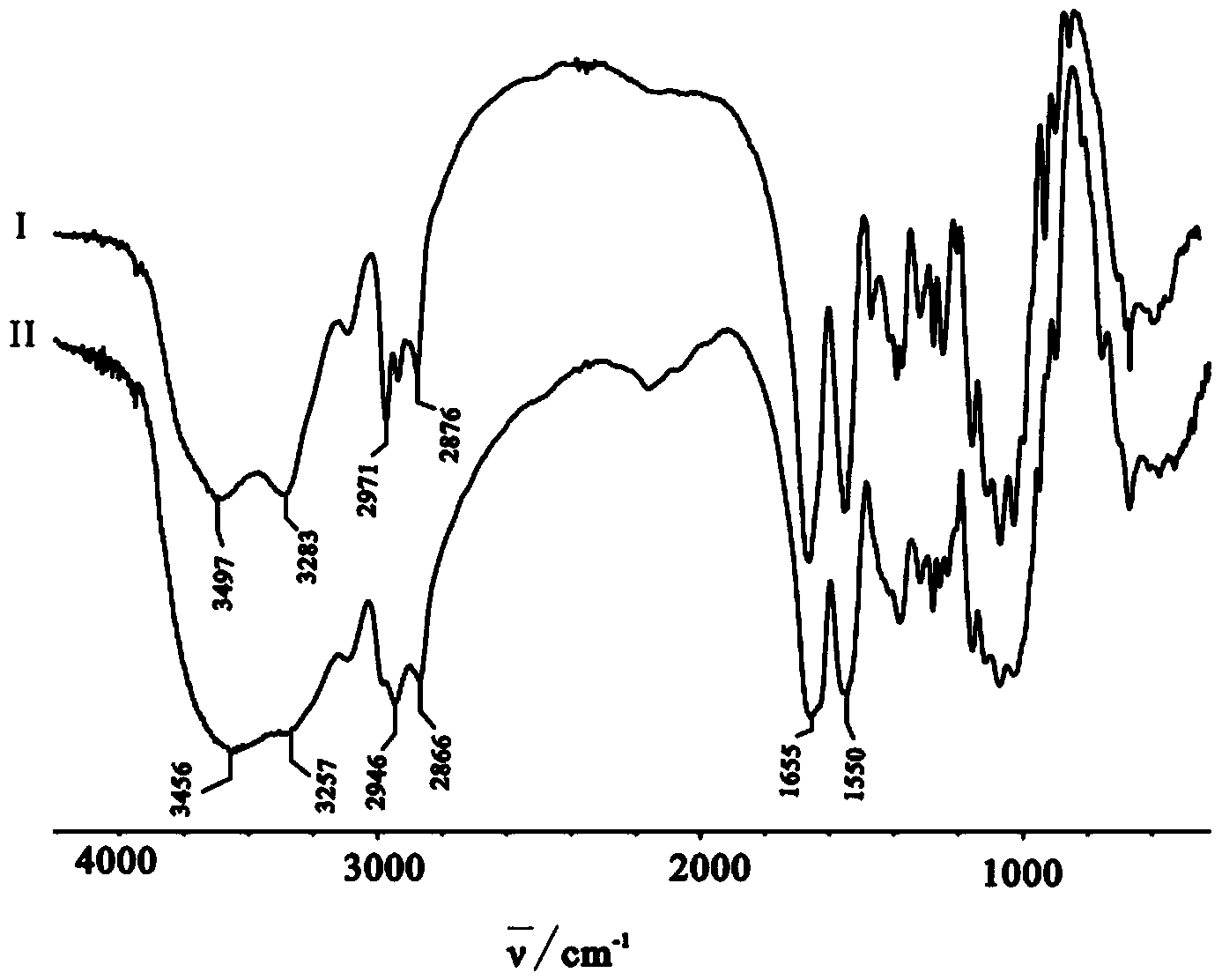
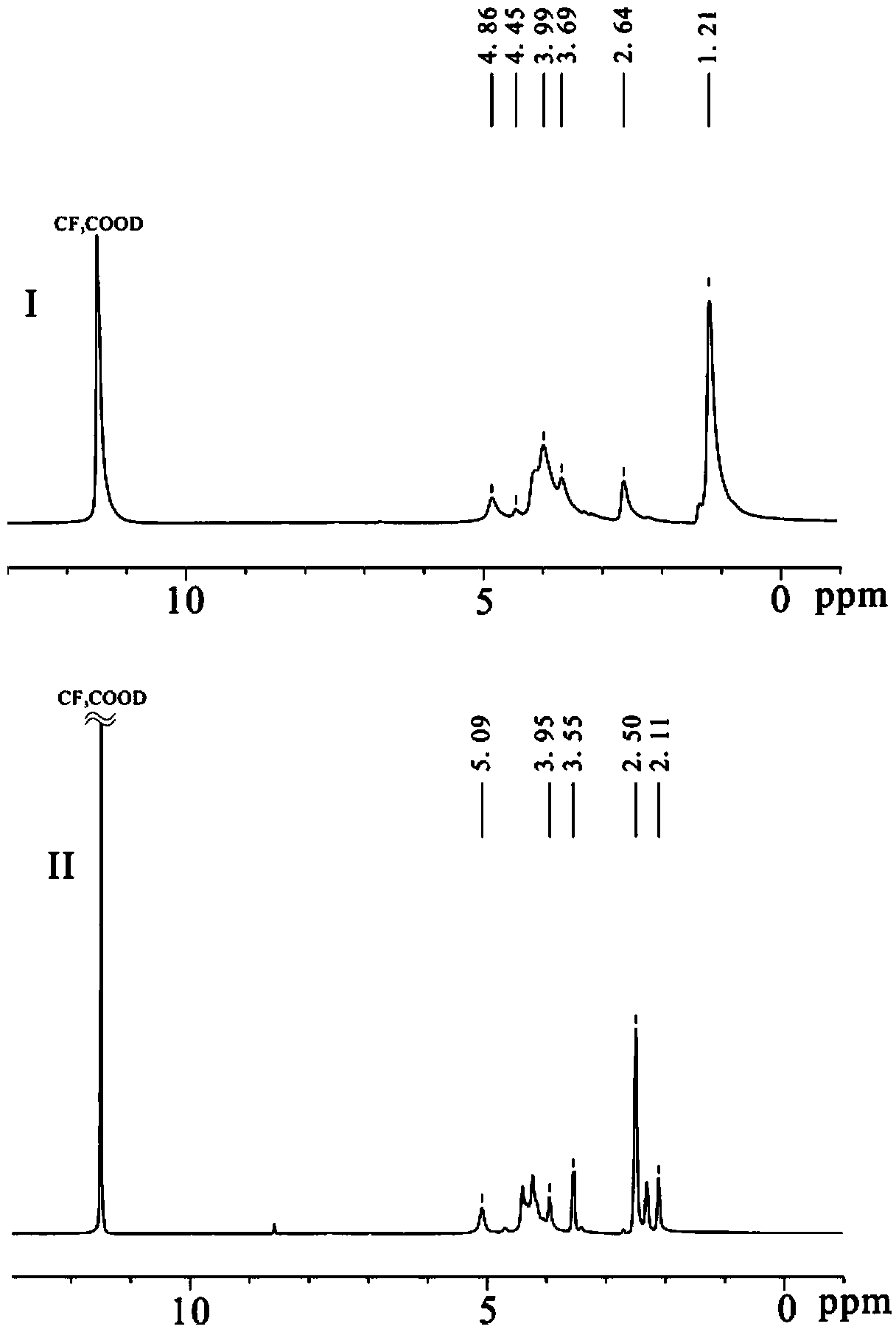
The chiral stationary phase can be prepared by attaching a suitable chiral compound to the surface of an achiral support such as silica gel, which creates a Chiral Stationary Phase (CSP). Many common chiral stationary phases are based on oligosaccharides such as cellulose or cyclodextrin...
Biodegradable polyketal polymers and methods for their formation and use
InactiveUS20060069230A1Different and useful sensitivityIncrease resistancePharmaceutical non-active ingredientsChromatographic separationChiral stationary phase
The present invention relates to biodegradable biocompatible polyketals, methods for their preparation, and methods for treating animals by administration of biodegradable biocompatible polyketals. In one aspect, a method for forming the biodegradable biocompatible polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a reducing agent to form the biodegradable biocompatible polyketal. The resultant biodegradable biocompatible polyketals can be chemically modified to incorporate additional hydrophilic moieties. A method for treating animals includes the administration of the biodegradable biocompatible polyketal in which biologically active compounds or diagnostic labels can be disposed. The present invention also relates to chiral polyketals, methods for their preparation, and methods for use in chromatographic applications, specifically in chiral separations. A method for forming the chiral polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a suitable reagent to form the chiral polyketal. A method for use in chiral separations includes the incorporation of the chiral polyketals in the mobile phase during a chromatographic separation, or into chiral stationary phases such as gels. The present invention further relates to chiral polyketals as a source for chiral compounds, and methods for generating such chiral compounds.
Owner:THE GENERAL HOSPITAL CORP
Cyclodextrin chiral chromatogram fixed phase and preparation method thereof
InactiveCN101306354AHigh column efficiencyHigh selectivityOther chemical processesAzirineChemical reaction
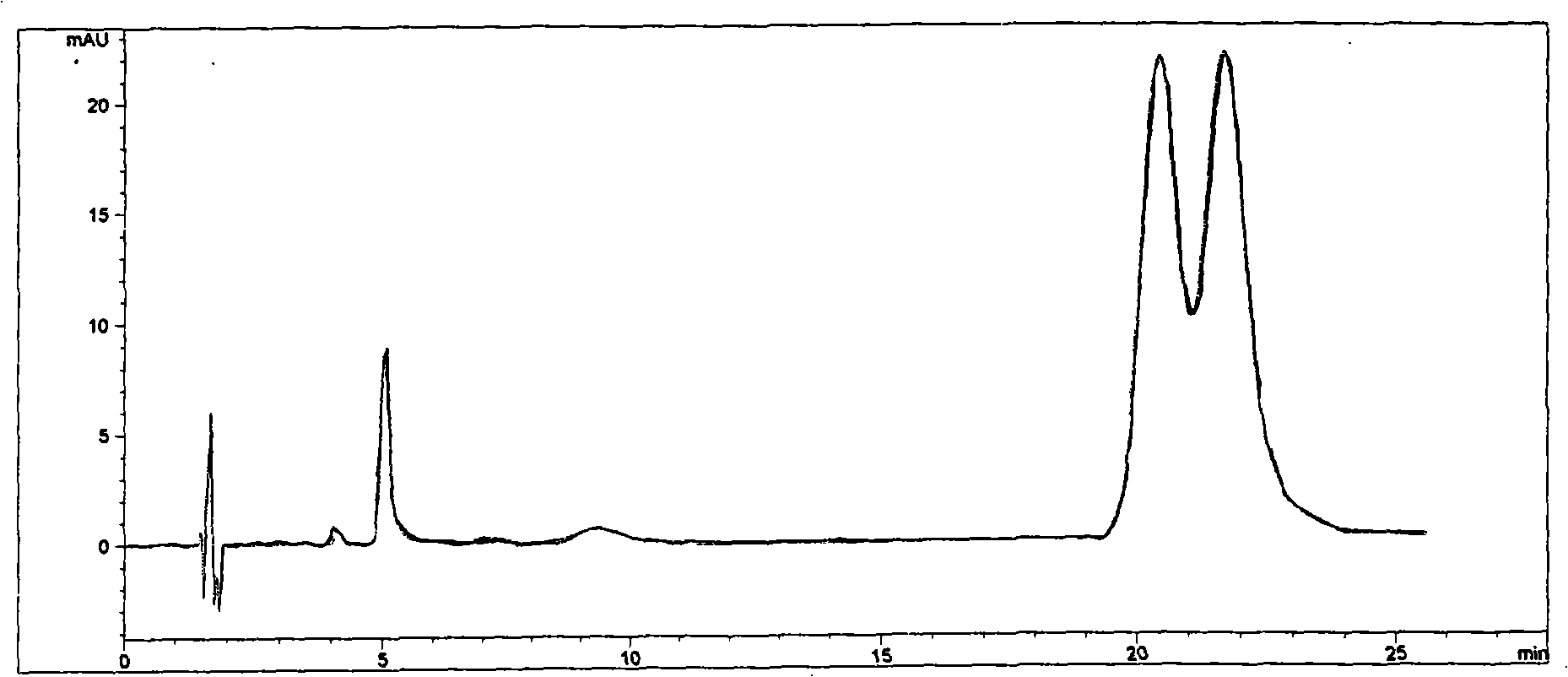
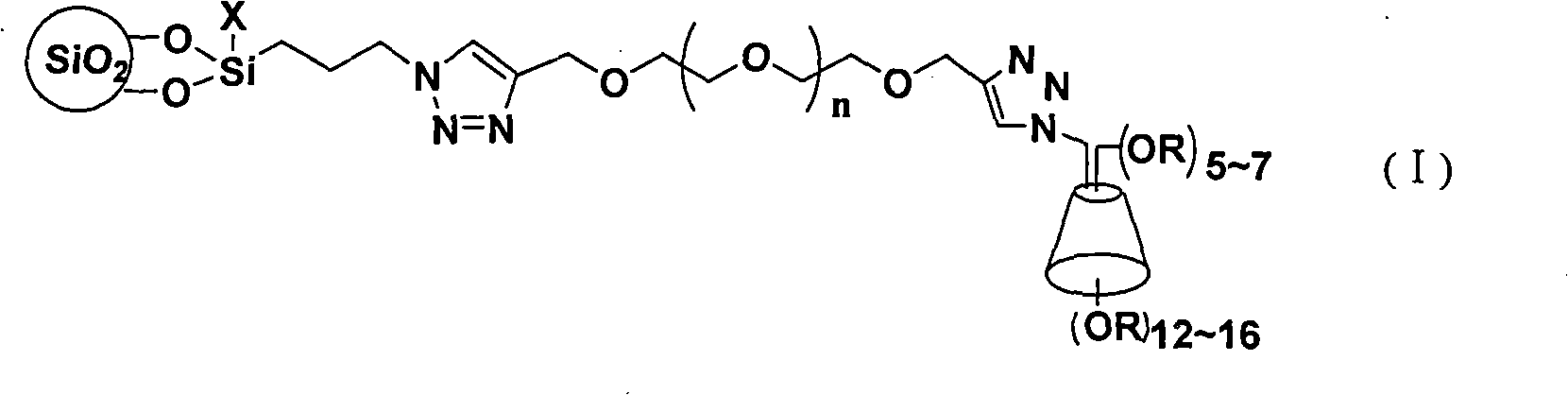
The invention discloses a cyclodextrin chiral stationary phase, the structure of which is shown in the general formula (I), wherein X is -OCH3 or -OCH2CH3, n is equal to 1-7, and R is -H, -CH3, -COCH3, -COC6H5 and -CONHC6H5. The preparation method of the stationary phase comprises the following steps: a silane coupling agent, sodium azide and a catalyst are added into an organic solvent, then spheroidal silicon is added for preparing azide silica gel derivant; oligomeric ethylene glycol, sodium hydride and propargyl bromide are added into tetrahydrofuran for preparing bialkynyl oligomeric ethylene glycol; monosubstituted nascent and derivative cyclodextrin containing azid groups is prepared; finally, the click chemistry reaction method is used for bonding the cyclodextrin. The cyclodextrin chiral stationary phase has the advantages that the selectivity of the bonding reaction is high, and the surface bonded amount is large; the chiral separation ability is strong, thereby being especially suitable for the chiral separation of a high efficiency liquid chromatography in the reversed-phase mode; the preparation method is simple and has less steps, the bonding reaction is the click chemistry reaction, the reaction condition is mild, and the reaction is carried out in the water solution.
Owner:EAST CHINA UNIV OF SCI & TECH
Chiral analysis method for nicotine in tobacco juice of electronic cigarette
InactiveCN104297409AEasy to handleLow detection limitComponent separationStrength propertiesAlcoholGas liquid chromatographic
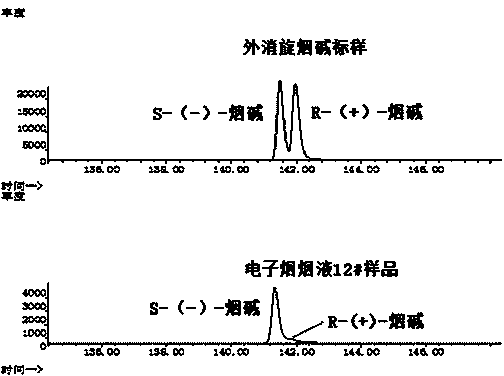
The invention discloses a chiral analysis method for nicotine in tobacco juice of an electronic cigarette. The chiral analysis method comprises the steps of diluting the tobacco juice of the electronic cigarette by a methyl alcohol / methyl tertiary-butyl ether solution, analyzing S-(-)-nicotine and R-(+)-nicotine in the tobacco juice of the electronic cigarette by using a chiral stationary phase capillary column mthrough a gas chromatograph-mass spectrometer, performing chiral analysis on the S-(-)-nicotine and the R-(+)-nicotine by comparing the retention time of a chromatographic peak and characteristic ions of nicotine in a standard sample with the retention time of a chromatographic peak and characteristic ions of nicotine in an electronic cigarette tobacco juice sample, and normalizing areas of quantitative ion peaks of the S-(-)-nicotine and the R-(+)-nicotine to quantify the proportions of the S-(-)-nicotine and the R-(+)-nicotine to the total nicotine. The chiral analysis method has the advantages that the nicotine in the tobacco juice of the electronic cigarette is separated by a chiral stationary phase capillary column, so that the S-(-)-nicotine and the R-(+)-nicotine in the nicotine can be better separated, and accurate and quantitative analysis on the nicotine can be realized. The sample treatment is simple and convenient; the chiral analysis method is low in detection limit, high in sensitivity, high in reproducibility, accurate in quantification and suitable for chiral analysis on the nicotine in a large batch of electronic cigarette tobacco juice samples.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Oxime conjugates and methods for their formation and use
ActiveUS20060058513A1Reduce molecular weightSuitable for useOrganic active ingredientsNervous disorderChromatographic separationChiral stationary phase
The present invention relates to biodegradable biocompatible polyketals, methods for their preparation, and methods for creating animals by administration of biodegradable biocompatible polyketals. In one aspect, a method for forming the biodegradable biocompatible polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a reducing agent to form the biodegradable biocompatible polyketal. The resultant biodegradable biocompatible polyketals can be chemically modified to incorporate additional hydrophilic moieties. A method for treating animals includes the administration of the biodegradable biocompatible polyketal in which biologically active compounds or diagnostic labels can be disposed. The present invention also relates to chiral polyketals, methods for their preparation, and methods for use in chromatographic applications, specifically in chiral separations. A method for forming the chiral polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a suitable reagent to form the chiral polyketal. A method for use in chiral separations includes the incorporation of the chiral polyketals in the mobile phase during a chromatographic separation, or into chiral stationary phases such as gels. The present invention further relates to chiral polyketals as a source for chiral compounds, and methods for generating such chiral compounds.
Owner:THE GENERAL HOSPITAL CORP
Oxime conjugates and methods for their formation and use
InactiveUS8030459B2Organic active ingredientsNervous disorderChromatographic separationChiral stationary phase
The present invention relates to biodegradable biocompatible polyketals, methods for their preparation, and methods for creating animals by administration of biodegradable biocompatible polyketals. In one aspect, a method for forming the biodegradable biocompatible polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a reducing agent to form the biodegradable biocompatible polyketal. The resultant biodegradable biocompatible polyketals can be chemically modified to incorporate additional hydrophilic moieties. A method for treating animals includes the administration of the biodegradable biocompatible polyketal in which biologically active compounds or diagnostic labels can be disposed. The present invention also relates to chiral polyketals, methods for their preparation, and methods for use in chromatographic applications, specifically in chiral separations. A method for forming the chiral polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a suitable reagent to form the chiral polyketal. A method for use in chiral separations includes the incorporation of the chiral polyketals in the mobile phase during a chromatographic separation, or into chiral stationary phases such as gels. The present invention further relates to chiral polyketals as a source for chiral compounds, and methods for generating such chiral compounds.
Owner:THE GENERAL HOSPITAL CORP
Beta-cyclodextrin derivative capillary gas chromatography chiral fixed phase and preparing method thereof
InactiveCN101015789AImprove stabilityCapable of chiral recognitionOther chemical processesCapillary gas chromatographyGas phase
The invention relates to a novel beta-cyclodextrin derivant capillary gas spectrum chiral fixed phase and relative preparation. The invention is characterized in that the invention leads allyl group with two keys into 2 and 3 positions of beta-cyclodextrin molecule, and leads valeryl group, enanthoyl group or caprylyl group into 6 position of beta-cyclodextrin molecule, to obtain novel beta-cyclodextrin detrivant. The inventive detrivant can be used as chiral fixed phase of capillary gas spectrum, with some chiral recognize ability to separate antimer of chiral compound.
Owner:CHINA AGRI UNIV
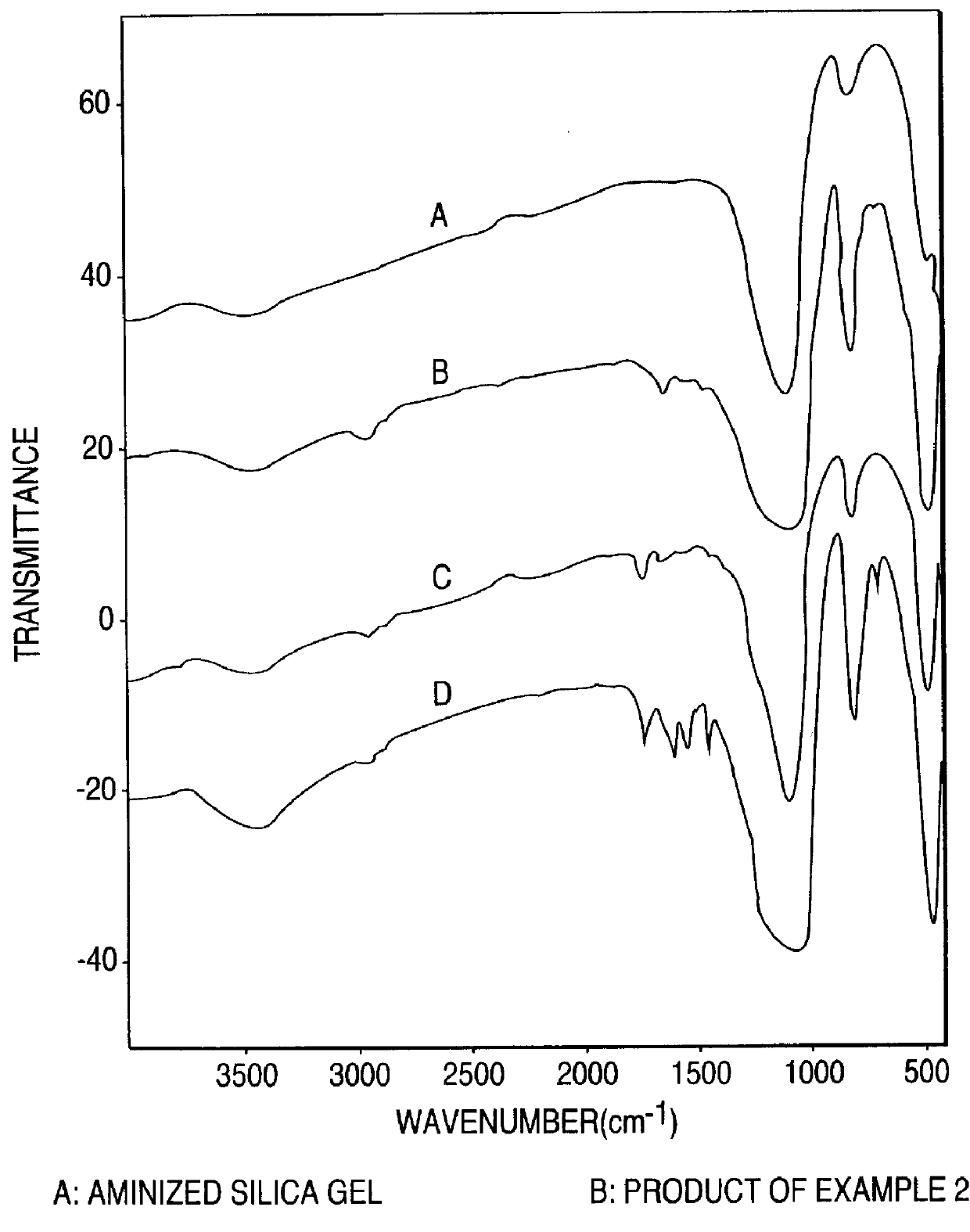
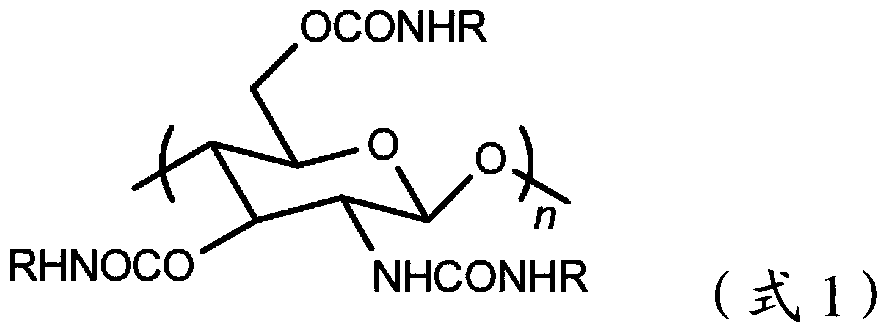
Covalently bound polysaccharide-based chiral stationary phases and method for their preparation
InactiveUS20090216006A1Extensive recognitionExtend working lifeSugar derivativesDextran coatingsChemical LinkageCellulose
The present invention relates to liquid chromatographic chiral stationary phases (CSPs) and their preparation. The CSPs are based on carbamate-derivatized polysaccharides that are covalently bound onto inorganic oxide carriers via unique linkage chemistry. The present invention also relates to methods of obtaining the said linkages, which include derivatizing and functionalizing the polysaccharides, and also chemically bonding the functionalized carbamate-derivatized polysaccharides onto inorganic oxide carriers. The polysaccharide derivatives so obtained can be used as materials for the liquid chromatographic chiral separation of enantiomers. The preferred inorganic oxides are silica, zirconium oxide, and aluminum oxide. Cellulose and amylose are the preferred chiral polysaccharides.
Owner:XU HUI +2
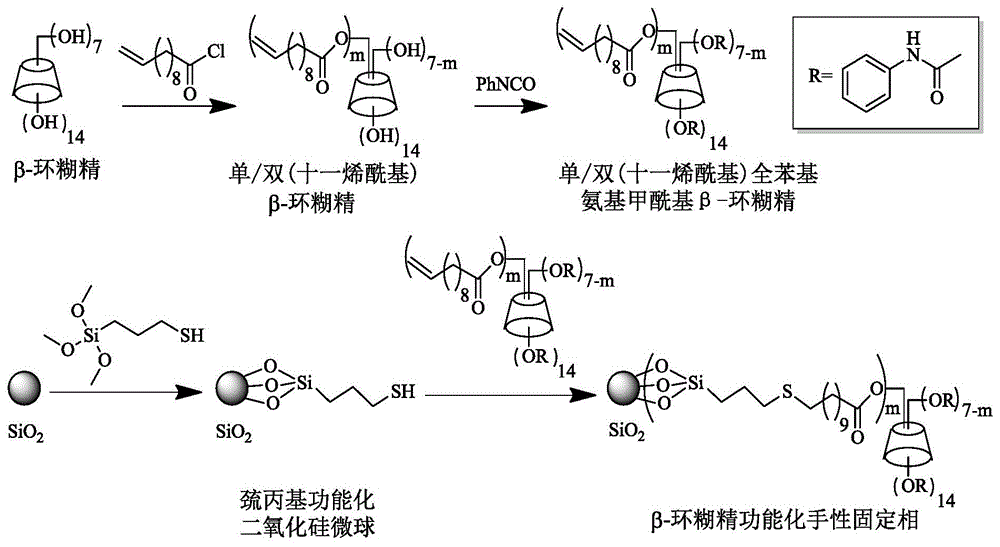
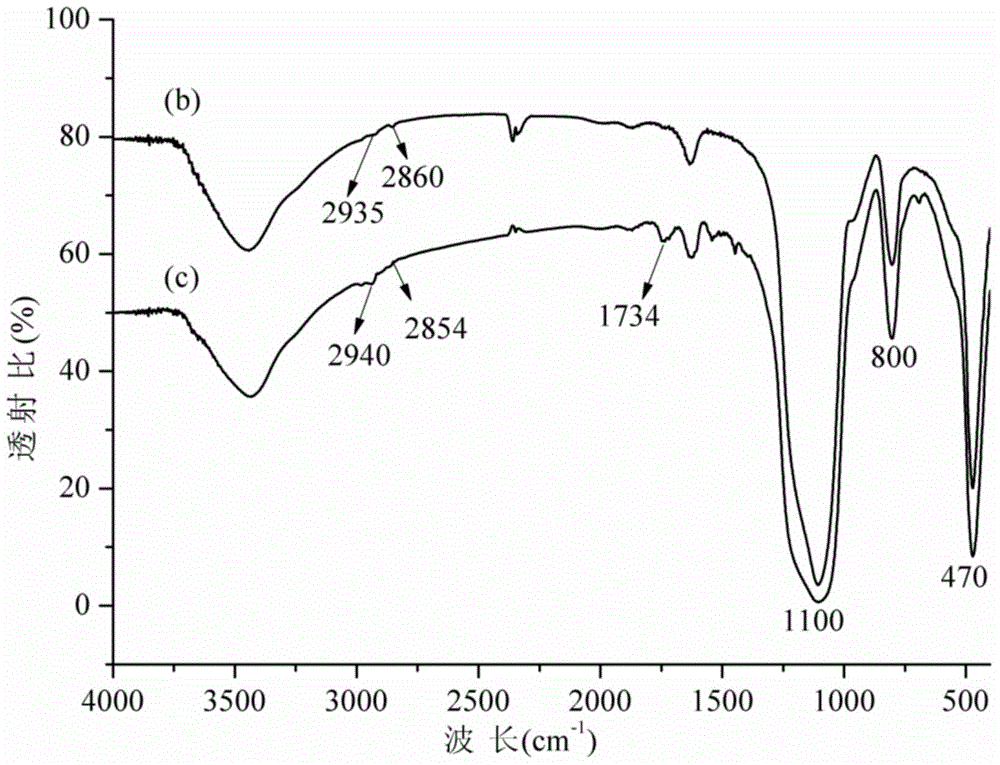
Beta-cyclodextrin functionalized chiral stationary phase, preparation and application thereof
ActiveCN105312039AFew stepsSynthesis fastOther chemical processesOptically-active compound separationFunctional monomerMicrosphere
The invention relates to a beta-cyclodextrin functionalized silica gel microsphere chiral stationary phase, preparation, and application thereof in enantiomer separation. The preparation method comprises the following steps: introducing 3-mercapto into the surface of silica gel microspheres at first, and then adding beta-cyclodextrin derivative functional monomers and an initiator, wherein the functional monomers carry out mercapto-alkene addition reactions under the regulation of the initiator so as to obtain the surface modified beta-cyclodextrin functional monomer chiral stationary phase. The preparation of chiral stationary phase is simple, the reaction conditions are mild, and the chiral stationary phase is successfully applied to the enantiomer separation.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
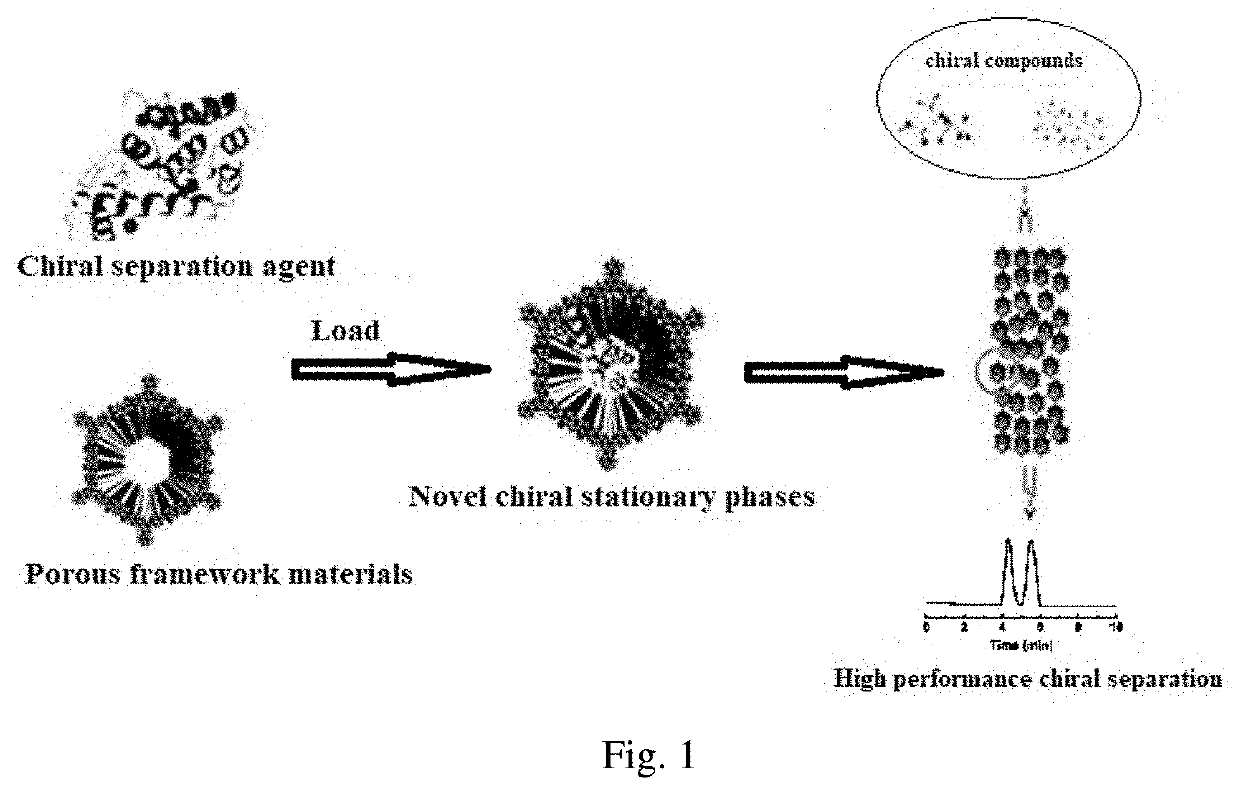
Chromatographic stationary phases prepared by taking porous frame material as matrix and used for chiral separation
ActiveCN109569026AIncreased durabilityThe synthesis method is simpleSolid sorbent liquid separationAntibiotic YChiral stationary phase
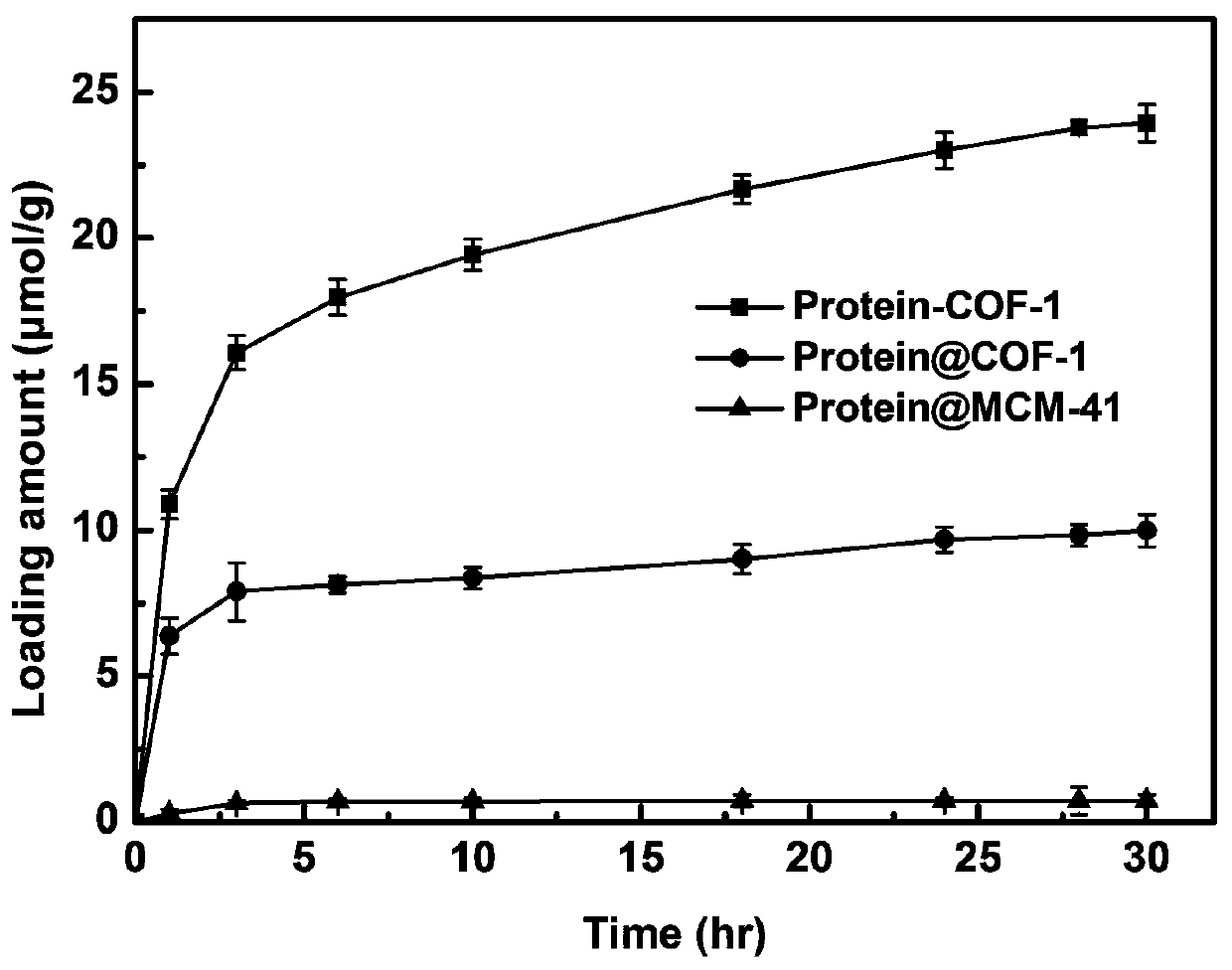
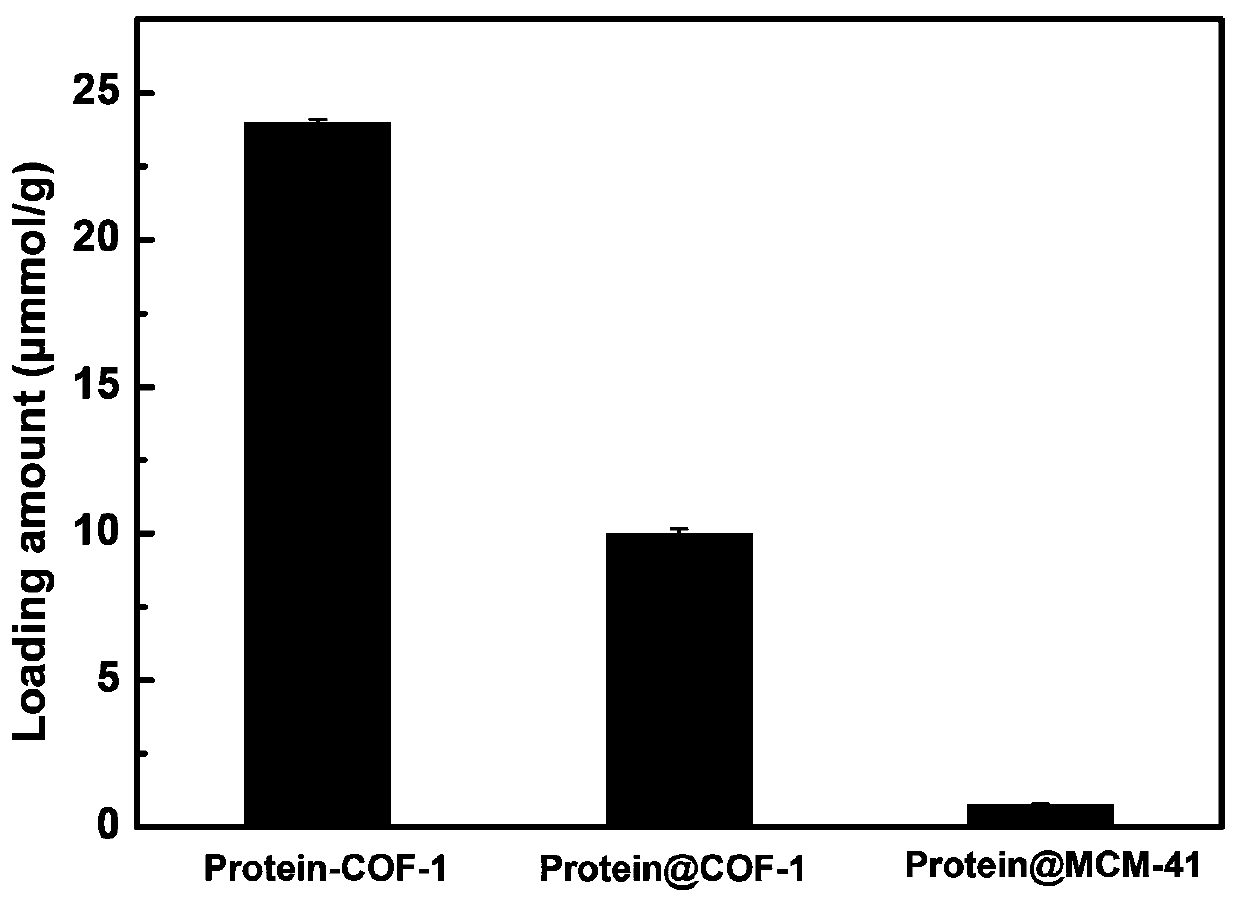
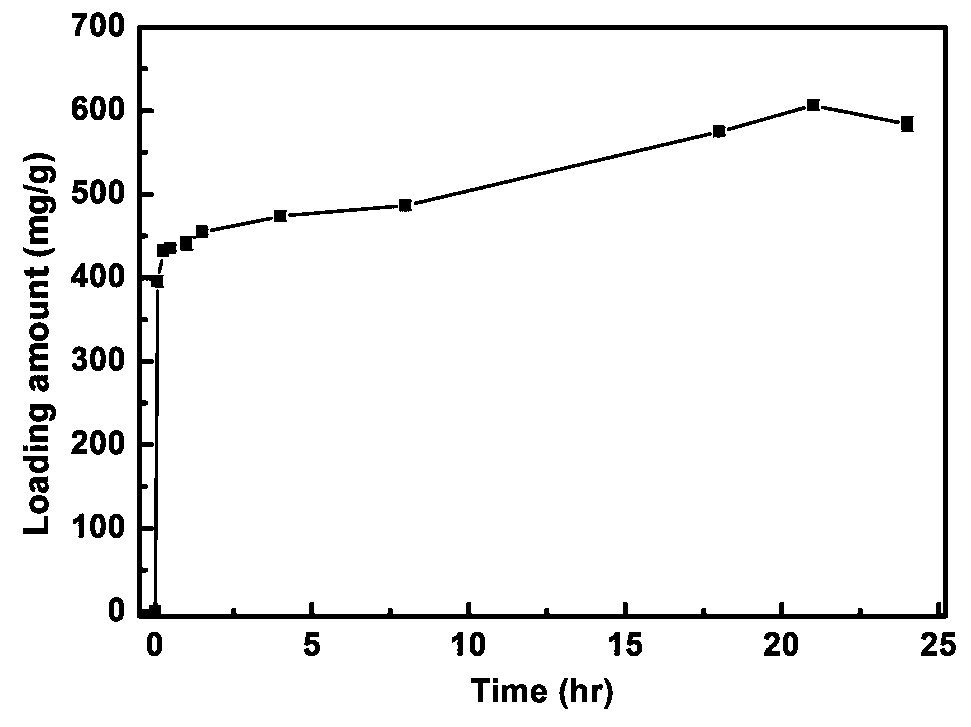
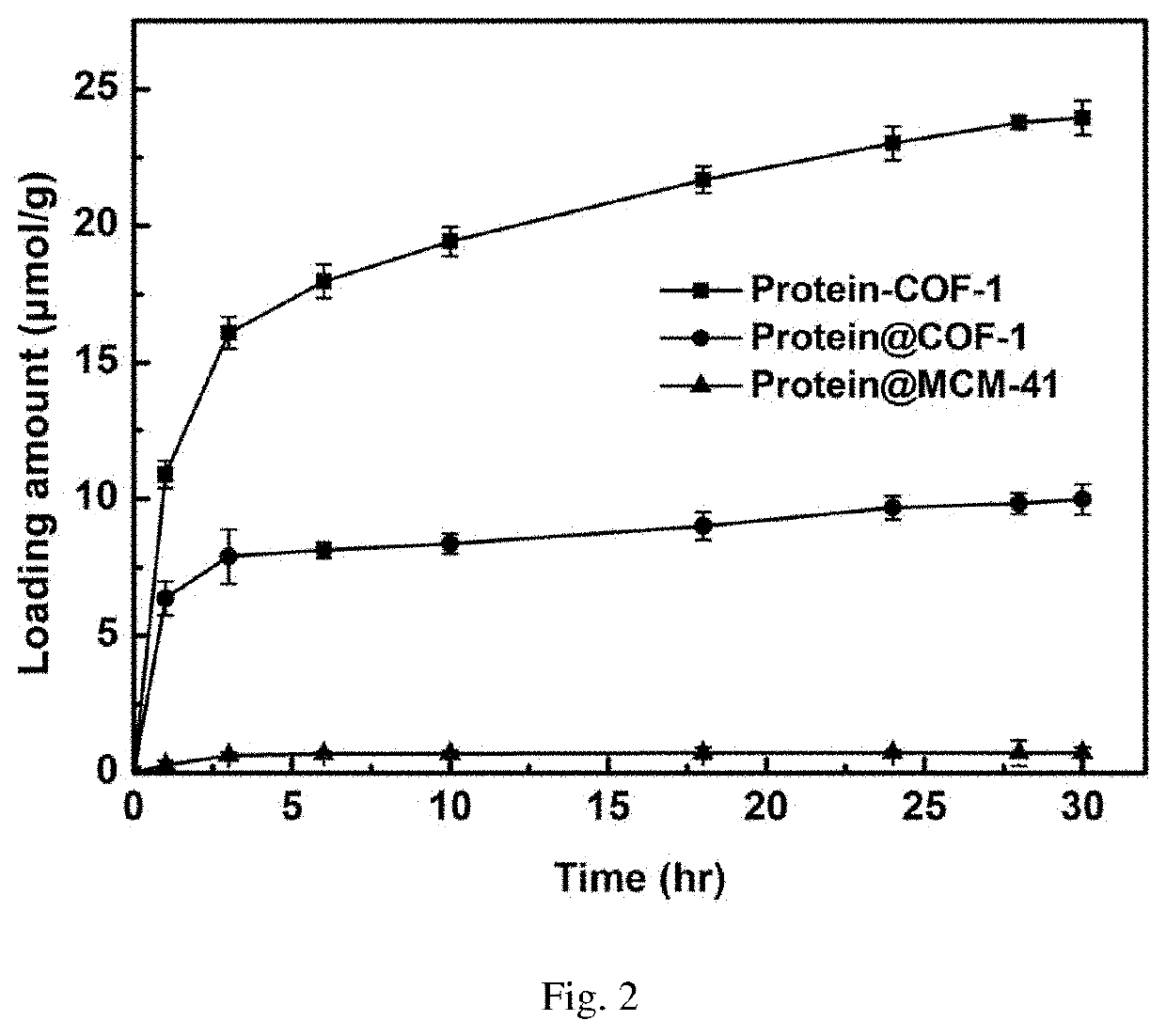
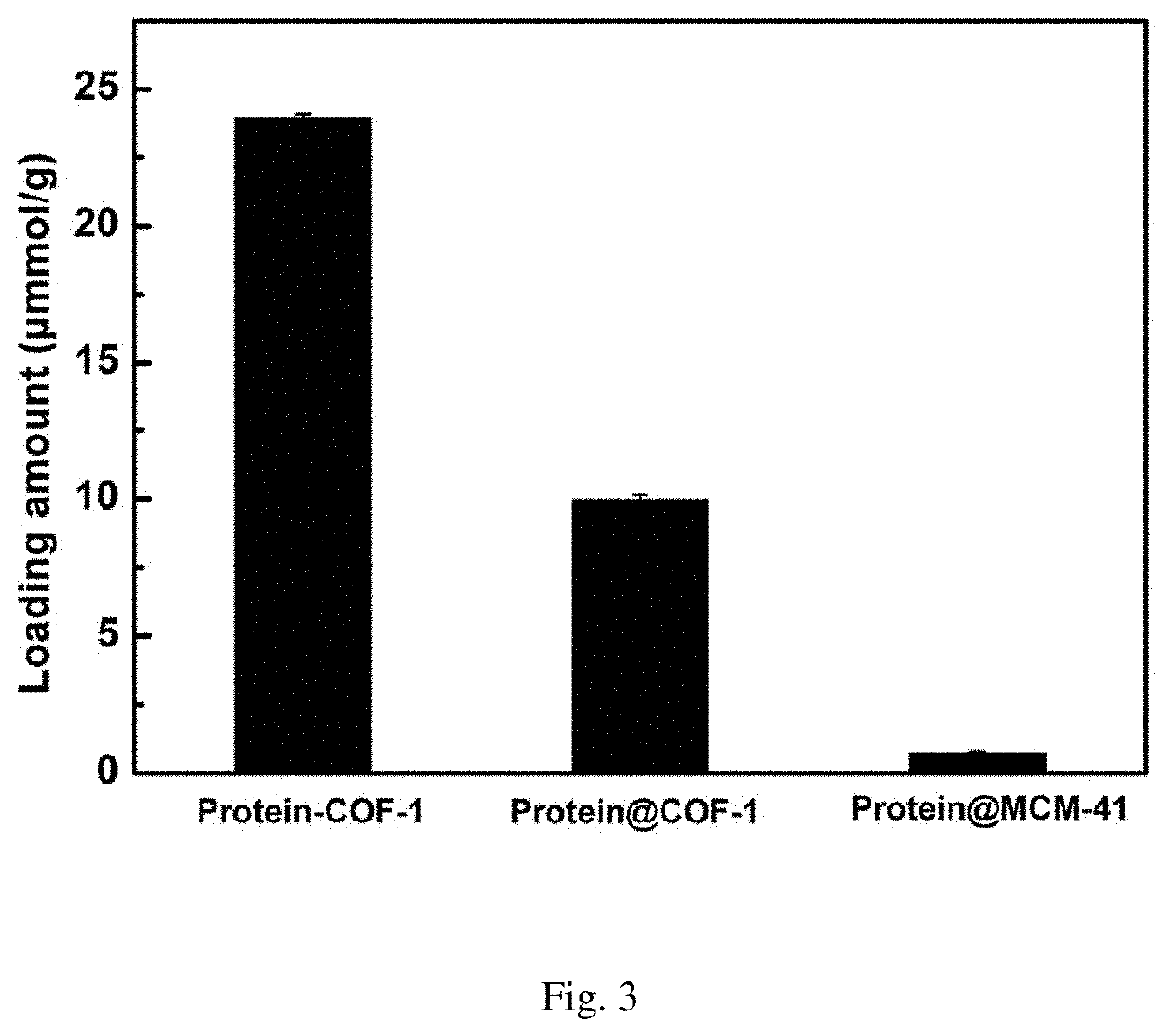
The invention discloses chromatographic stationary phases prepared by taking a porous frame material as a matrix and used for chiral separation study. Aiming at the problem that carrier materials of conventional chiral chromatographic column stationary phases may be poor in stability, low in chiral resolving agent loading rate, prone to chiral resolving agent loss and limited in suitability, the porous frame material (like metal organic frame materials and covalent organic frame materials) is creatively designed and developed to serve as a novel carrier material having extensive suitability, and chiral resolving agents (protein, enzyme and macrocyclic antibiotics) are efficiently loaded by means of covalence, adsorption, embedding and crosslinking so as to prepare various efficient and durable chiral stationary phases to serve as novel high-performance chromatographic column filler for chromatographic chiral separation (like high-performance liquid phase chromatography and capillary chromatography). The chiral stationary phases prepared by using the technology has high separation efficiency and high stability and durability and can be successfully applied in efficient separation ofvarious chiral substances like chiral amini acid and chiral drug. The technology greatly widens application range and prolongs service life of chiral chromatographic column separation.
Owner:NANKAI UNIV
Separating materials for chromatography and electrophoresis applications comprising regiodefined functionalised cyclodextrins chemically bonded to a support via urethane functionalities
InactiveUS6017458AGood reproducibilityAdd supportIon-exchange process apparatusOther chemical processesCarbamateElectrophoresis
Novel and improved chiral stationary phases (CSP) materials comprising a support and completely regiodefined derivitized cyclodextrin chemically bonded via single or double urethane linkage(s) universally applicable in HPLC, LC, TLC, and CCE are obtained using a process based on the almost quantitative reaction of pre-synthesized regiodefined per-functionalized mono- or di- azidocyclodextrin with primary amines.
Owner:NAT UNIV OF SINGAPORE
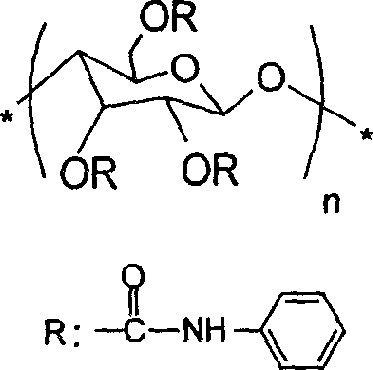
Chitosan carbanilate-carbamido derivative preparation method
ActiveCN104250312AImprove solubilitySmall structureOther chemical processesCarbamateChitosan phenylcarbamate
The invention provides a novel chitosan carbanilate-carbamido derivative synthetic method, the method employs chitosan and phenyl isocyanate with different groups to react, and then the hydroxy and amino on chitosan can be completely conversed to the chitosan carbanilate-carbamido derivative of carbamate and carbamido. According to the invention, a coating process is employed to prepare the derivative to a chiral stationary phase, and high performance liquid chromatography is used for resolution of various enantiomers, and the chiral stationary phase has high chiral identification capability.
Owner:DAICEL CHEM IND LTD
Preparation method of immobilized beta-cyclodextrin derivative type chiral stationary phase
InactiveCN103406113ASimple manufacturing methodMild reaction conditionsOther chemical processesSilanesSilicon oxygen
The invention provides a preparation method of an immobilized beta-cyclodextrin derivative type chiral stationary phase. The preparation method comprises the following steps: reacting isocyanate propyl triethoxy silane with beta-cyclodextrin; performing derivatization modification on a hydroxyl group on a beta-cyclodextrin glucose unit by using phenyl isocyanate to obtain a beta-cyclodextrin derivative; coating the beta-cyclodextrin derivative bonded with a small amount of silane coupling agent on the surface of aminopropyl silica gel, and performing condensation in ethanol / aqueous solution in the presence of trimethylchlorosilane by using the silane coupling agent in the beta-cyclodextrin derivative molecules to finally obtain the immobilized beta-cyclodextrin derivative type chiral stationary phase, wherein the phenylcarbamate beta-cyclodextrin derivative molecules are connected by using a silicon-oxygen-silicon bond to form an inclusion netlike structure which covers the surface of the aminopropyl silica gel. The preparation method has the characteristics of simplicity, few steps and high bonding efficiency, can be applied to the normal-phase high-performance liquid chromatographic condition, and has the advantages of high chromatographic column stability and high chiral separation capacity.
Owner:三亚哈尔滨工程大学南海创新发展基地
Preparation method for beta-cyclodextrin chiral stationary phase
InactiveCN103601823AHigh creativityAvoid the effects of bonding reactionsOther chemical processesMicrosphereChiral stationary phase
A disclosed preparation method for a beta-cyclodextrin chiral stationary phase comprises: taking SiO2 microballoons as a raw material, and bonding beta-cyclodextrin (beta-CD) to the surface of SiO2 microballoons by employing 3-aminopropyltriethoxysilane (KH550), trimethylchlorosilane and 4,4'-methylenediphenyl diisocyanate (MDI) to obtain the beta-cyclodextrin chiral stationary phase. The method has continuity, simple operation and short synthetic period, and the synthetic product is convenient to be subjected to derivatization; no metal ion-containing catalysts such as NaOH, NaN3, CuI(PPh3) and the like are introduced according to the method, so that the chiral stationary phase is prevented from pollution, and the method relatively accords with the separation requirement of high-purity liquid chromatogram. The method is applicable to separation of benzene ring-containing chiral medicament intermediates and pyrethroid pesticide compounds.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method of capillary electro-chromatography column taking beta-cyclodextrin as bonded stationary phase and application in chiral drug separation
InactiveCN102921193AEasy to manufactureLow priceAmino compound purification/separationOther chemical processesStationary phaseCapillary electrochromatography
The invention relates to a preparation method of a capillary electro-chromatography column taking beta-cyclodextrin as a bonded stationary phase and an application in a chiral drug separation, and can effectively solve the problems of the capillary electro-chromatography column preparation and the chiral drug separation. The solved technical proposal comprises the following steps: (1) preparing six-bit mono substituted P-toluenesulfonyl-beta-cyclodextrin, (2) preparing six-bit mono substituted ammoniated-beta-cyclodextrin, (3) preparing silylated silica gel, (4) preparing a beta-cyclodextrin bonded silica gel chiral stationary phase, and (5) preparing a capillary packing column. The preparation method is low in cost, low in toxicity and low in environment pollution, and is an innovation of the preparation method of the capillary electro-chromatography column taking the beta-cyclodextrin as the bonded stationary phase and the application in the chiral drug separation.
Owner:ZHENGZHOU UNIV
Biodegradable polyketal polymers and methods for their formation and use
InactiveUS20100150832A1Different and useful sensitivityIncrease resistanceUltrasonic/sonic/infrasonic diagnosticsPowder deliveryChromatographic separationChiral stationary phase
The present invention relates to biodegradable biocompatible polyketals, methods for their preparation, and methods for treating animals by administration of biodegradable biocompatible polyketals. In one aspect, a method for forming the biodegradable biocompatible polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a reducing agent to form the biodegradable biocompatible polyketal. The resultant biodegradable biocompatible polyketals can be chemically modified to incorporate additional hydrophilic moieties. A method for treating animals includes the administration of the biodegradable biocompatible polyketal in which biologically active compounds or diagnostic labels can be disposed. The present invention also relates to chiral polyketals, methods for their preparation, and methods for use in chromatographic applications, specifically in chiral separations. A method for forming the chiral polyketals comprises combining a glycol-specific oxidizing agent with a polysaccharide to form an aldehyde intermediate, which is combined with a suitable reagent to form the chiral polyketal. A method for use in chiral separations includes the incorporation of the chiral polyketals in the mobile phase during a chromatographic separation, or into chiral stationary phases such as gels. The present invention further relates to chiral polyketals as a source for chiral compounds, and methods for generating such chiral compounds.
Owner:THE GENERAL HOSPITAL CORP
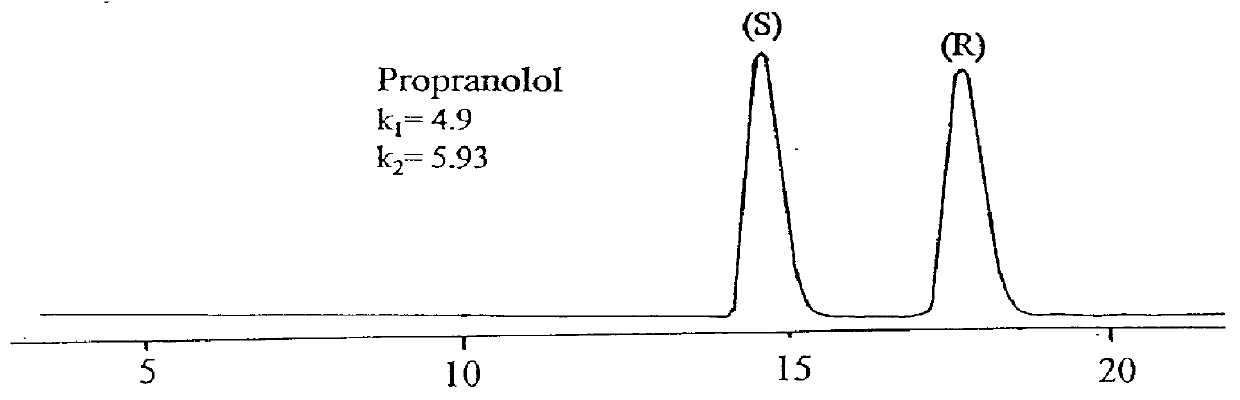
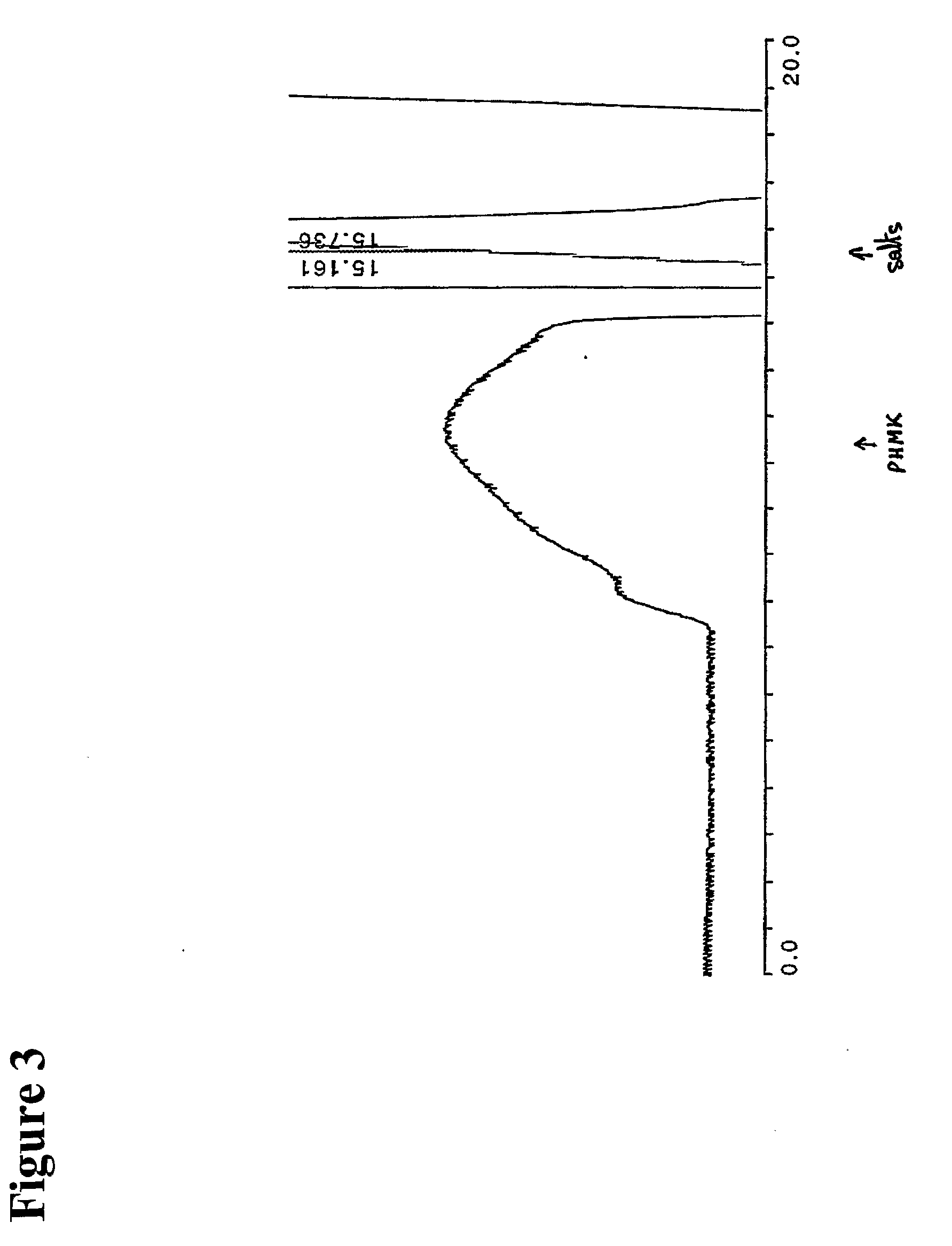
Simulated moving bed chromatography separating method of omeprazole antimer
InactiveCN1683368ASimple processContinuous productionOrganic chemistryDigestive systemCelluloseChromatographic separation
The present invention discloses the chromatographic separation process of omeprazole antimer in simulated mobile bed. In a simulated mobile bed chromatographic system with chiral fixed phase of cellulose triphenyl carbomate and flow phase of the mixture of ethanol, n-hexane and diethylamine, mixture of R-(+)-omeprazole and S-(-)-omeprazole is separated to obtain high purity S-(-)-omeprazole. Said separation process is a continuous process, so that the present invention has high automation, high production efficiency, low solvent consumption and no toxic solvent.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
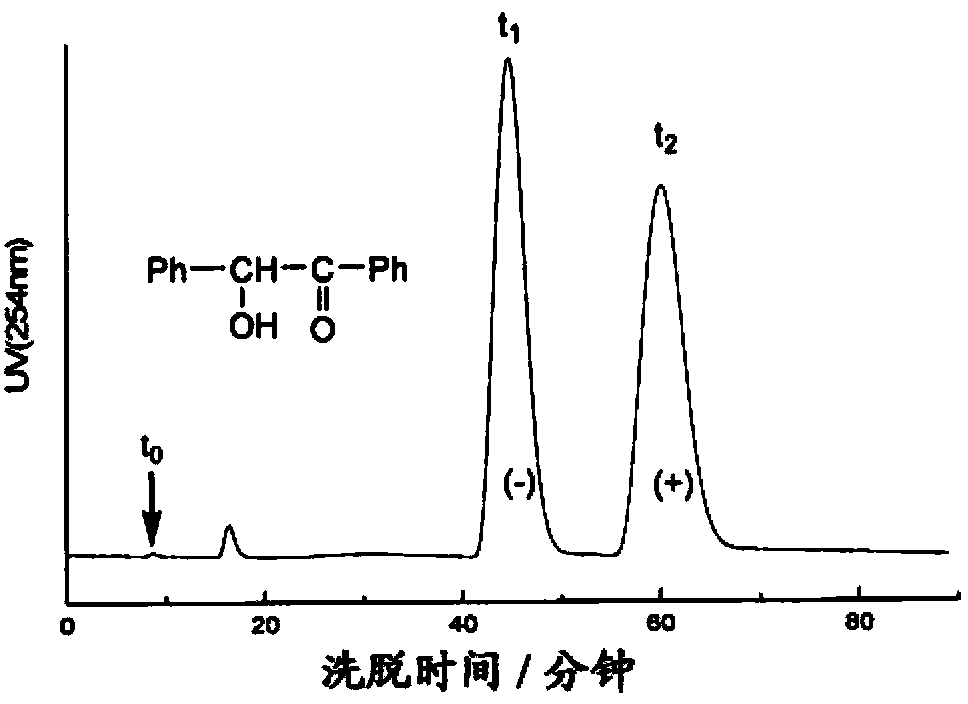
Silica gel bonded double-chirality active center chromatogram filler, preparation method and use thereof
The invention provides silica-bonded dual-chiral active central chromatography filler, a preparation method and applications thereof, belonging to the field of column filling. The preparation method comprises the following steps of: (1) activation of silica gel; (2) protection of microcrystalline cellulose 6-hydroxyl group; (3) carbonyl acyl chlorination; (4) derivative process of microcrystalline cellulose 2, 3-hydroxyl group and protection removal of 6-hydroxyl group; and (5) obtaining bonded chiral fixed phase by silica-bonded 2, 3-bonded 6-protection removal microcrystalline cellulose reaction and passivation of activated silica gel unreacted silanol group. The dual-chiral bonded chiral fixed phase is used for separating asymmetric synthesized chiral compounds, and n-hexane-isopropanol is used as flowing phase so as to separate the phenylethyl alcohol and ethanethioic acid, thus obtaining chromatograms. The filler, the preparation method and the application have high column efficiency, short separation time, and good separation effect on chiral compounds. The filling material generates no swelling even when in solvent, has good permeability performance and low column pressure; under the conditions of 100 percent n-hexane, flowing speed of 1ml / min and the chromatographic column of 150 multiplied by 4.6mm i.d, the column pressure is only 3.3MPa.
Owner:BEIJING UNIV OF CHEM TECH
Heterochain polymer chiral stationary phase and process for preparing same
The invention relates to a heterochain polymer chiral stationary phase and process for preparing same, wherein the heterochain polymer chiral stationary phase is formed by carriers and chiral macromolecules through the connection of polyamide bonds or carbamide bonds, the carrier being 3-amino propyl silica gel. The obtained chiral stationary phase has good chiral identification capability.
Owner:WUHAN CHEM COLLEGE
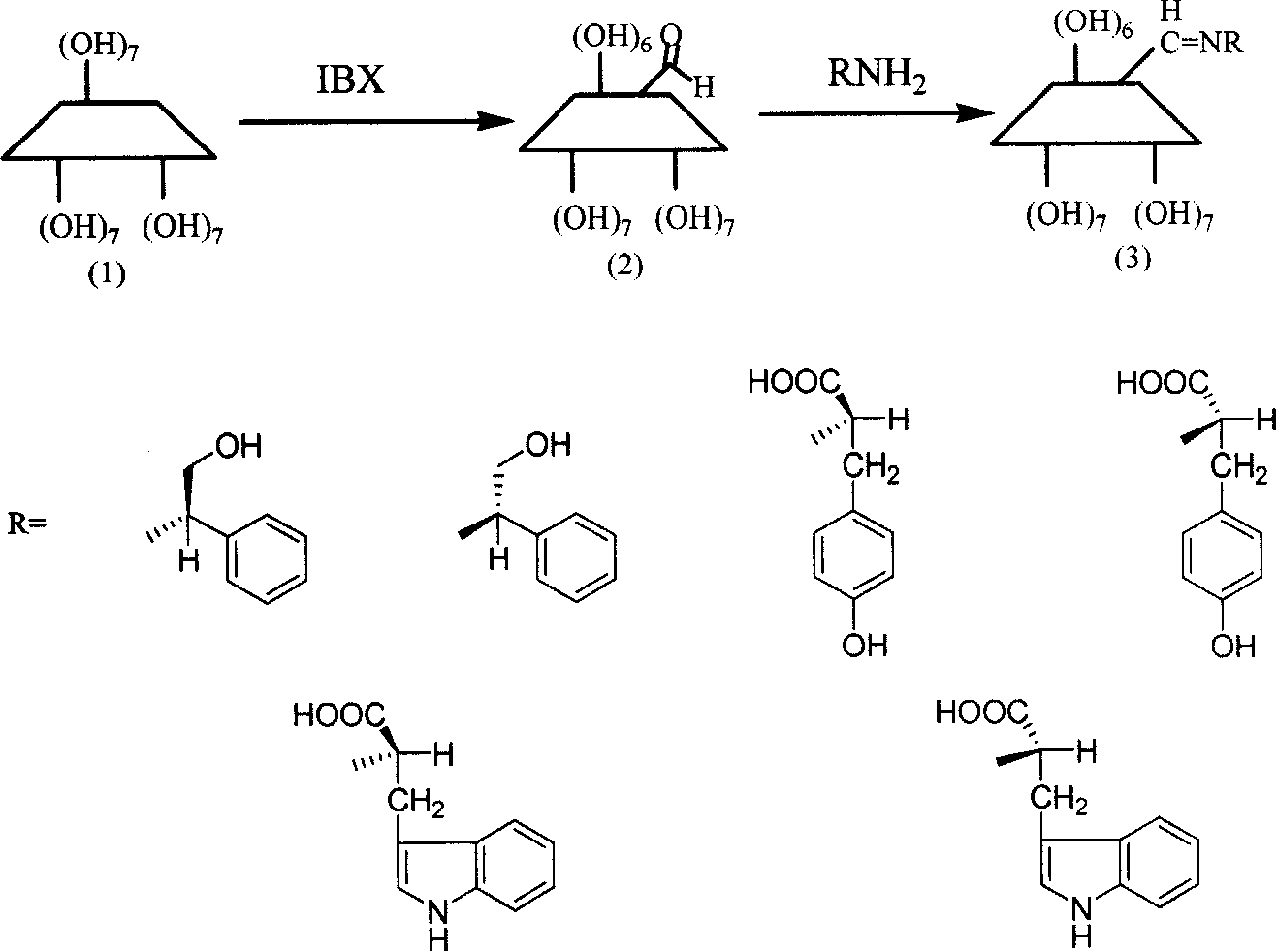
Six kinds of alpha-schiff base derived beta-cyclodextrin and use
InactiveCN101235104AIncrease varietyIncreased chiral environmentOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingAlcoholTyrosine
The invention uses beta-cyclodextrin to synthesize six kinds of new beta-cyclodextrins derived from alpha-Schiff base and six chiral stationary phases thereof, wherein the invention reacts cyclodextrin aldehyde and six amine compounds to prepare beta-cyclodextrin derived from alpha-Schiff base, the compounds reacted with cyclodextrin aldehyde are L-phenylglycinol, D-phenylglycinol, L-tyrosine, D-tyrosine, L-tryptophan and D-tryptophan. The synthesis route is simple, with high yield, simple operation and easy purification. The invention can be used in chiral separation for the qualitative and quantitative research on optical isomer of amino acid, ferrocene amine compound, drug, sulfur-containing compound, alcohol compound, amino-compound and various chiral compounds. The invention can be used in asymmetry catalysis for recovering and circulating catalyst.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
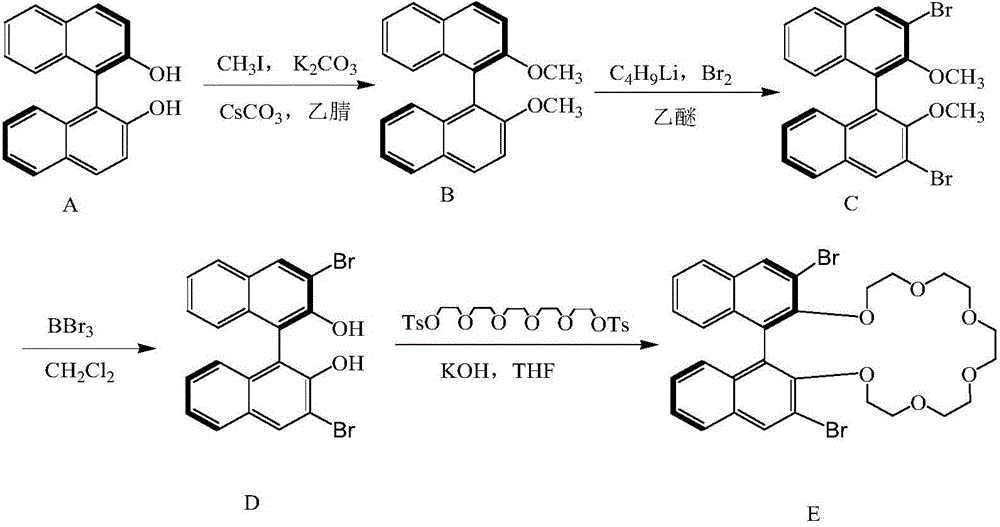
High performance liquid chromatography separating column suitable for amino acid chiral resolution
InactiveCN104475066AEfficient separationHigh resolutionOther chemical processesOrganic compound preparationEvaporationSlurry
The invention discloses a high performance liquid chromatography separating column suitable for amino acid chiral resolution. The separating column is prepared by the following steps: dissolving R(or S)-(3,3'-bromo-1,1'-dinaphthyl)-20-crown-6 in dichloromethane, uniformly dispersing the crown ether solution to C18 silica gel, carrying out rotary evaporation to remove the solvent to prepare a chiral stationary phase; and mixing the chiral stationary phase in a methanol water solution, stirring to obtain a homogenate solution, and filling the chromatographic column by a wet process by using the methanol water solution as a displacement fluid. The separating column can effectively separate all the 19 protein amino acids with chiral site at normal temperature, and can effectively separate chiral drugs with primary amine on the chiral site. The separating column has the obvious characteristics of high resolving power, high separating rate, high reproducibility, lower preparation cost and the like, and can be used repeatedly. The separating column has obviously better separating effect for amino acids than the like products at home and abroad.
Owner:YUNNAN NORMAL UNIV
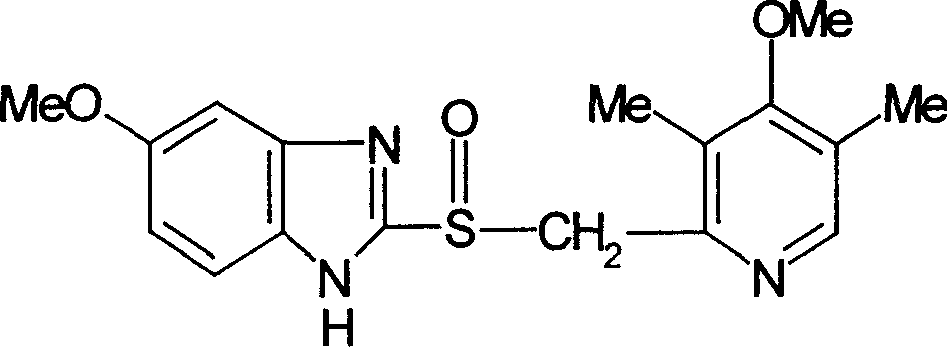
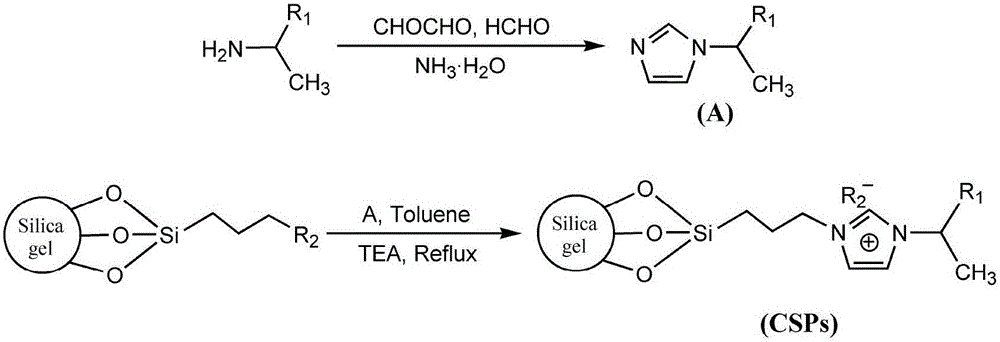
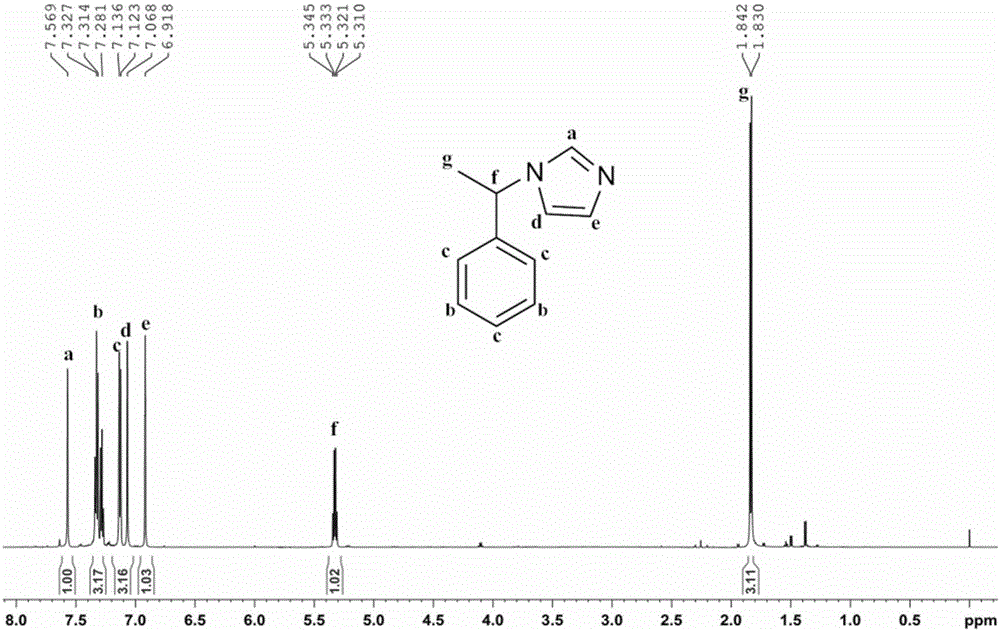
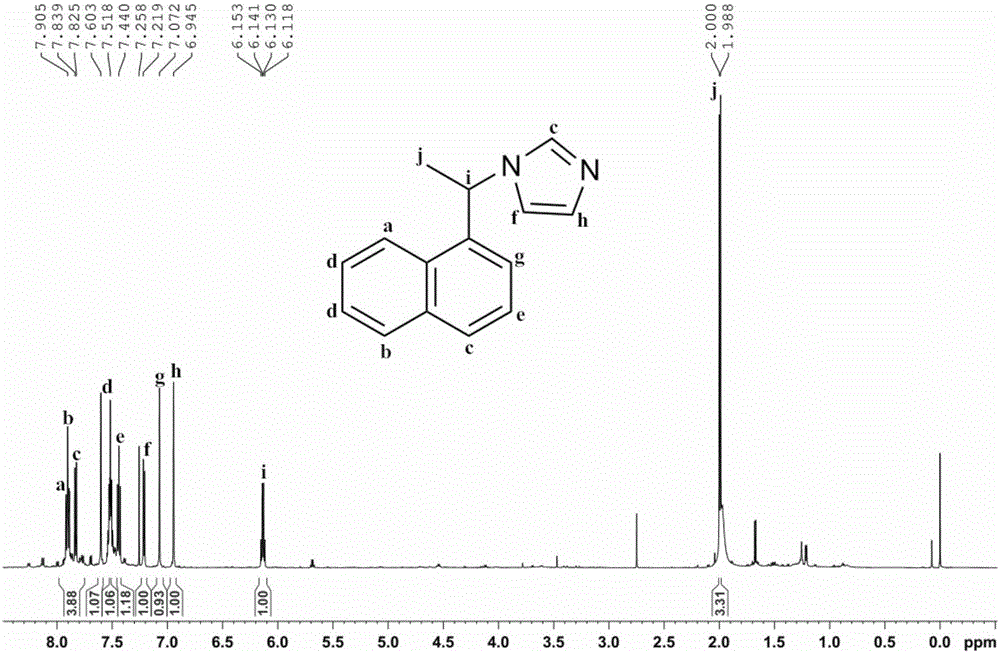
Imidazole ionic liquid chiral stationary phase and preparation method and application
ActiveCN105903457ANovel and stable structureRaw materials are easy to getOther chemical processesSolid sorbent liquid separationSilanesSilica gel
The invention belongs to an efficient stationary phase of liquid chromatography and particularly relates to an imidazole ionic liquid chiral stationary phase and a preparation method and application. The ionic liquid chiral stationary phase shown in the first formula is obtained by bonding chiral imidazole to a silica gel carrier through a silane coupling agent, wherein R1 is phenyl, benzyl, naphthyl, o-methylphenyl, m-methylphenyl or p-methylphenyl, and R2 is halogen. The preparation process includes the steps that firstly, silica gel is subjected to activating pretreatment, and activated silica gel is prepared; secondly, the activated silica gel is coupled through the silane coupling agent, and silylated silica gel with halogen at the terminal is prepared; thirdly, corresponding chiral imidazole is prepared through a chiral ethylamine raw material; fourthly, the silylated silica gel reacts with chiral imidazole to prepare the imidazole ionic liquid chiral stationary phase. According to the chiral stationary phase prepared through the method, raw materials are easy to obtain, operation steps are simple, the chiral stationary phase has the characteristic of ionic liquid and stability of silica gel in the use process, a good chiral separation effect can be achieved on various polar chiral compounds. Please see the first formula in the description.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
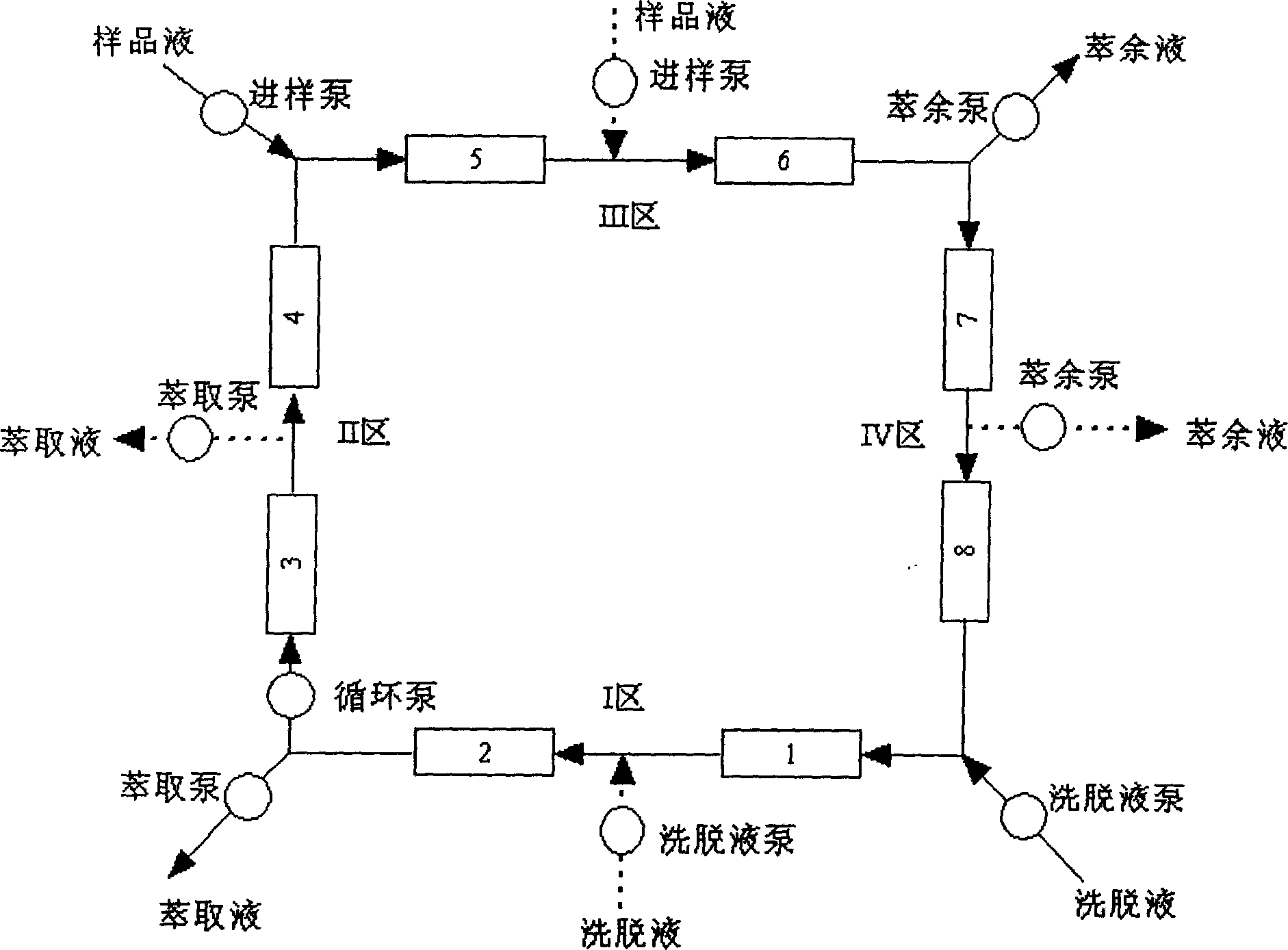
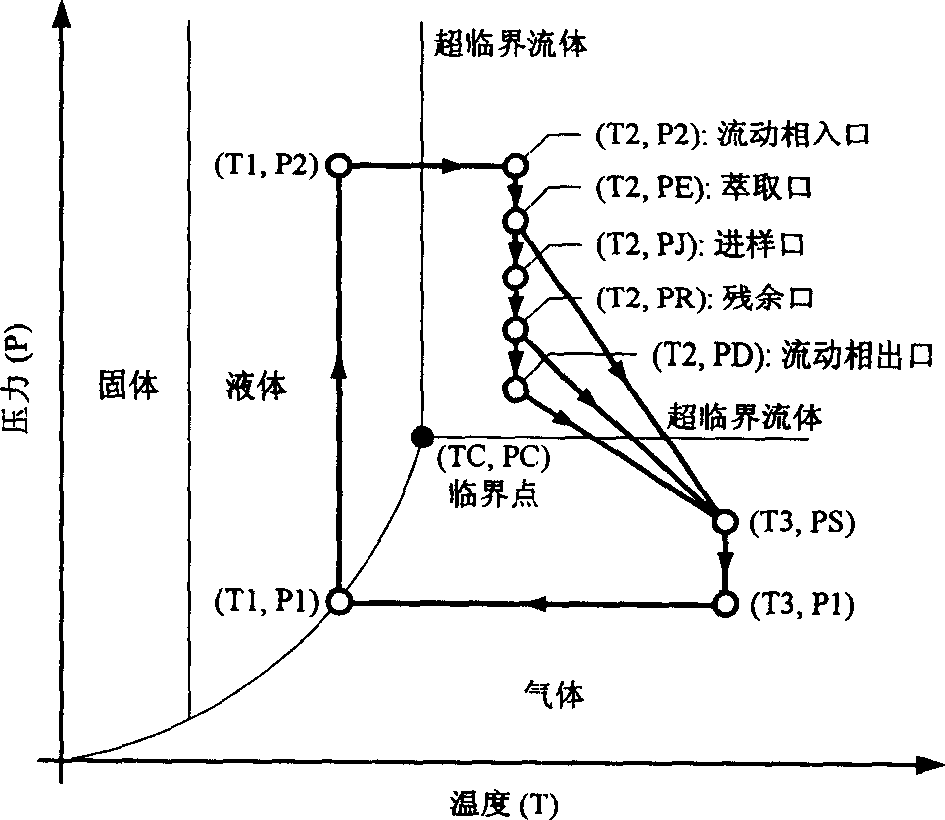
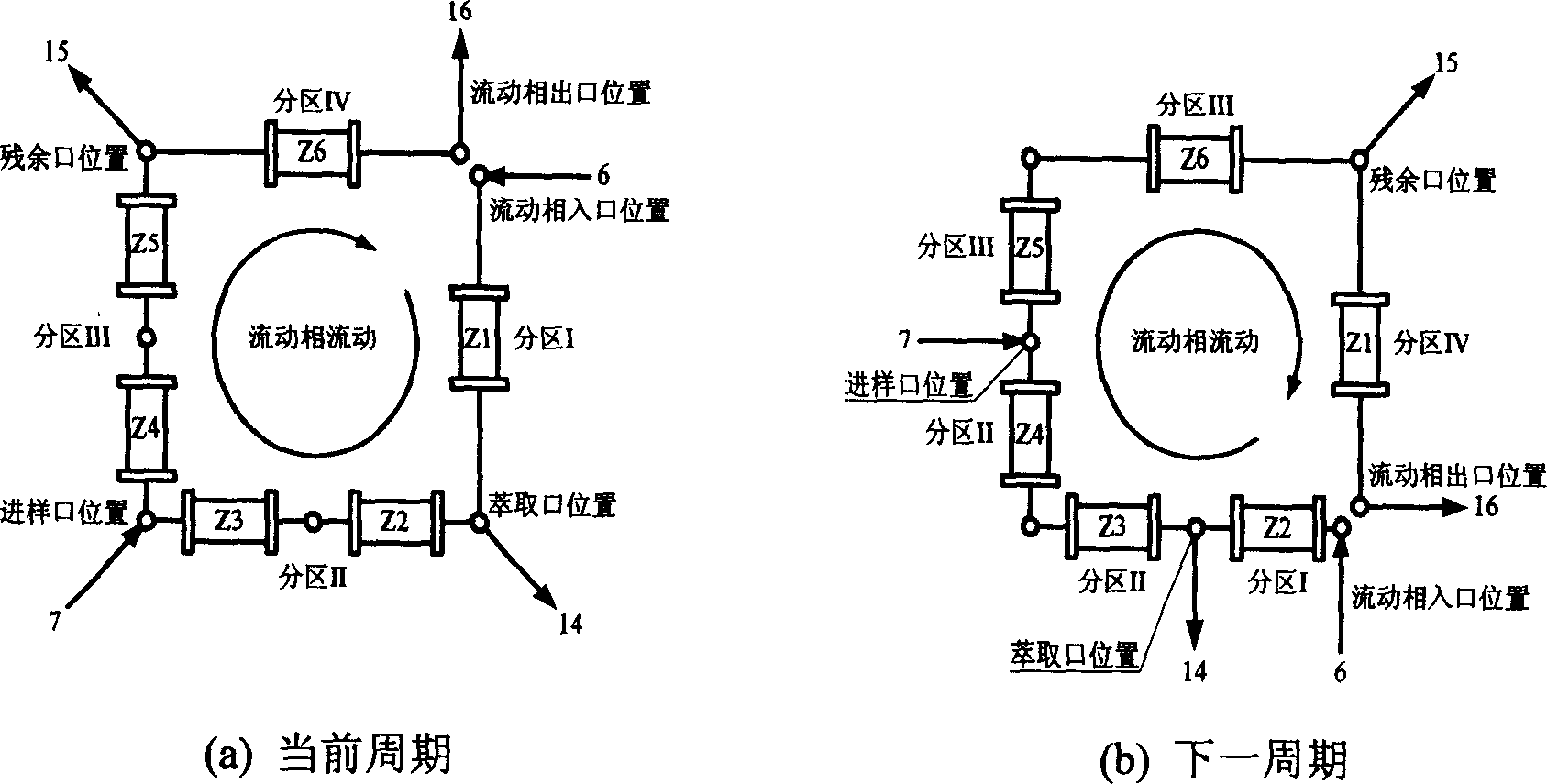
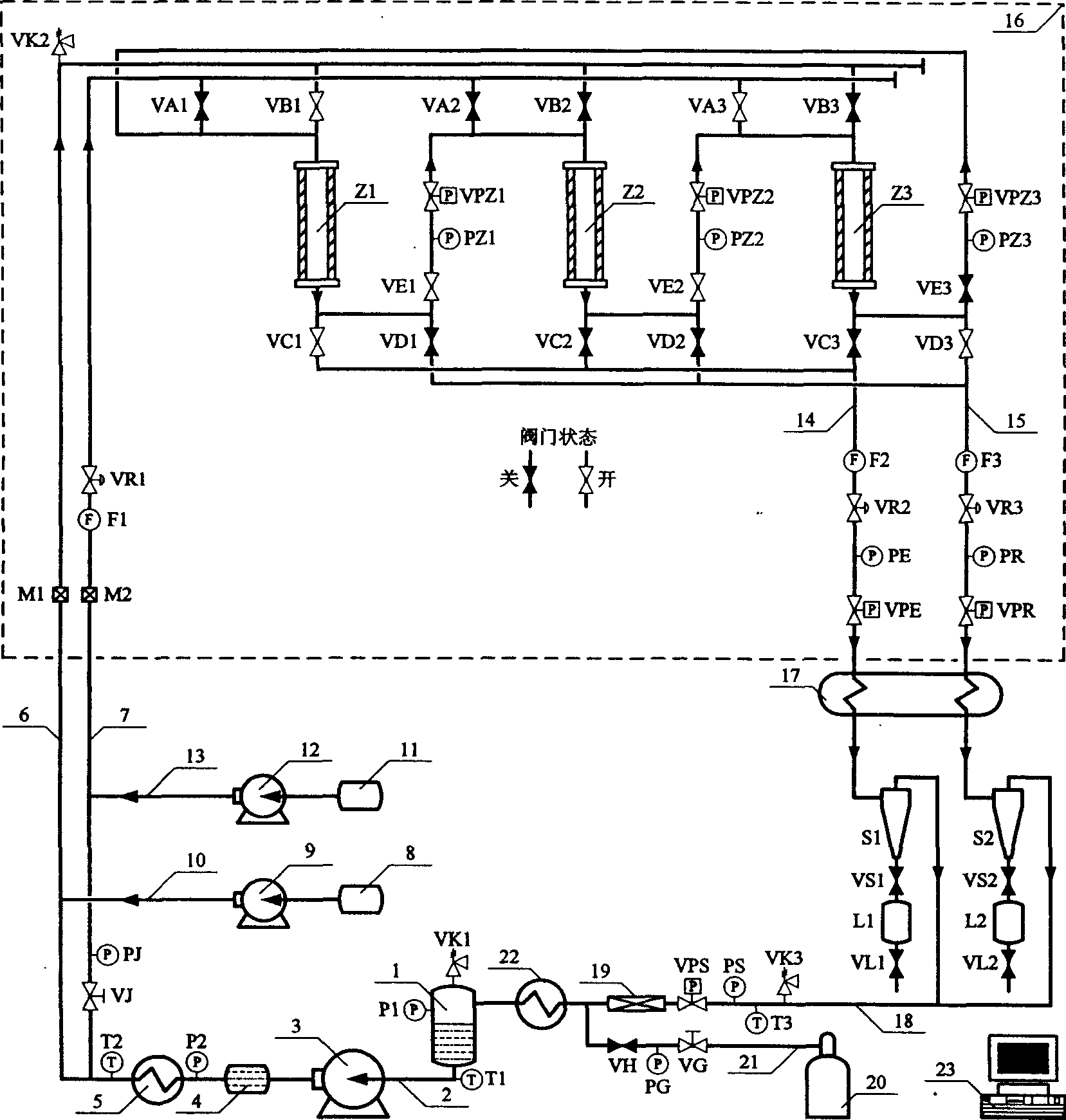
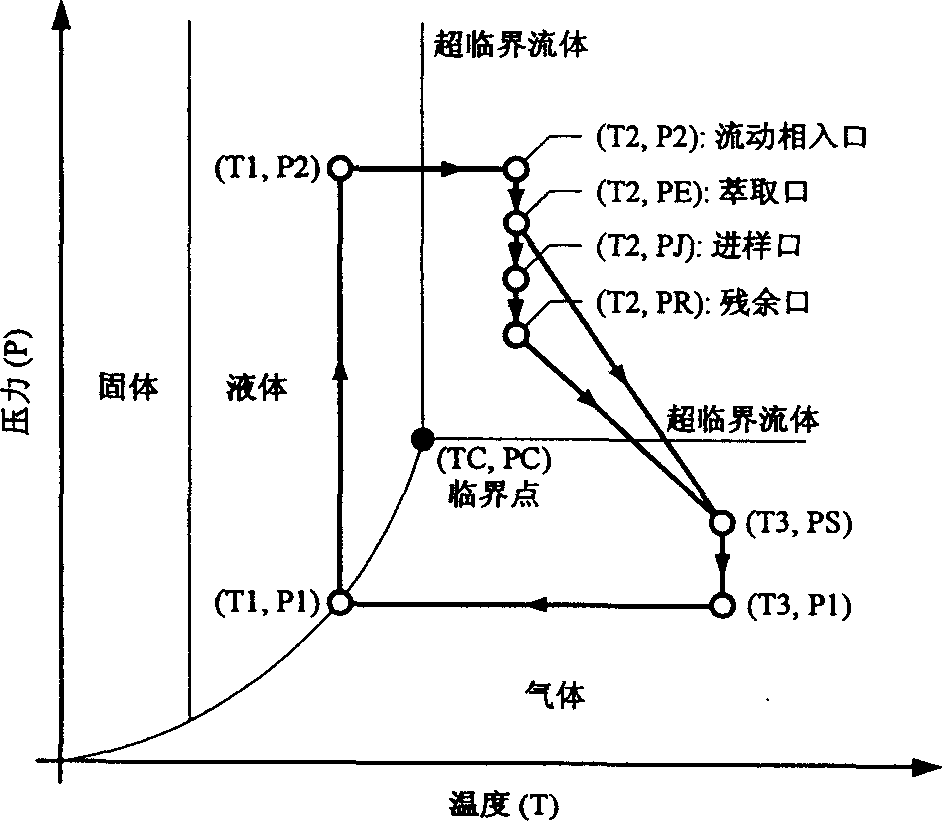
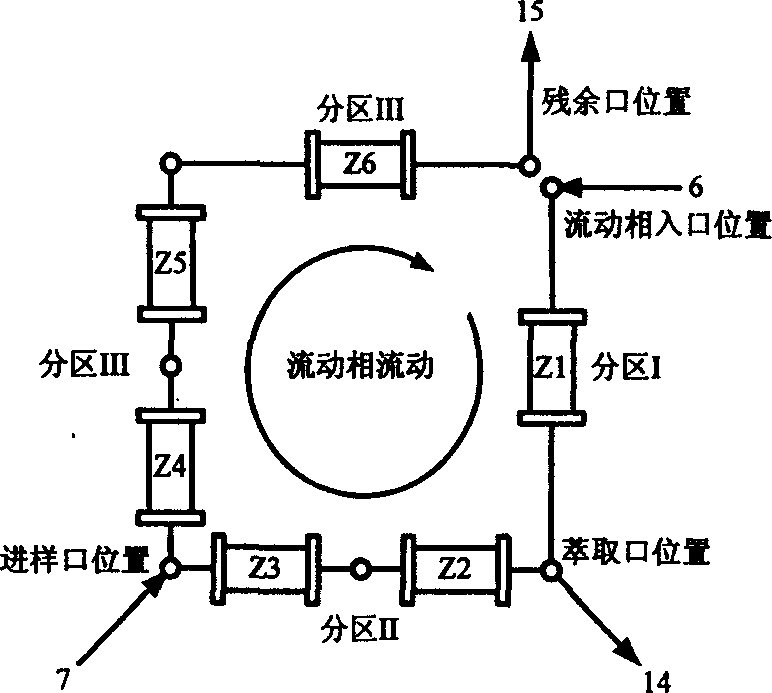
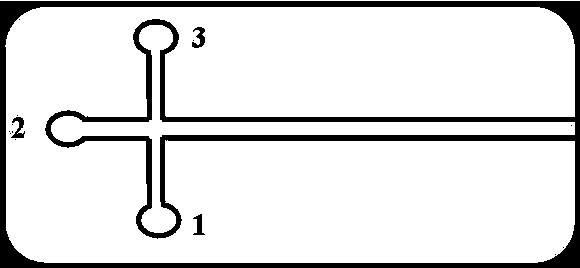
Chromatogram arrangement of supercritical fluid imitation moving bed with four subareas
InactiveCN1698925ASolving inefficienciesIon-exchange process apparatusIon-exchanger regenerationGradient operatorsInjection port
This invention discloses a quartering color spectrum device, which comprises 4~48 filling columns, wherein the exit end of the front column is connected with the entrance end of next rear column through the voltage regulator, and the exit end of the last column is connected with the entrance end of the first column; the entrance end of each column is connected with the mobile phase inlet orifice and the injection port, while the exit end of each column is connected with the extraction pipe, relict pipe and the mobile phase outlet orifice; the exit end of the extraction pipe, relict pipe and the mobile phase outlet orifice are connected with the cyclonic separator individually; the mobile phase is the supercritical fluid. The device can apply pressure gradient operator schema and the modifier gradient operator schema. Meanwhile, it uses the supercritical carbon dioxide fluid as the mobile phase, and uses the chirality fixed phase as the filler of the filling column.
Owner:ZHEJIANG UNIV
Chiral chromatographic separation and analysis method of dihydromyricetin enantiomer
ActiveCN105241982AGood chiral separationRapid Analysis DetectionComponent separationCelluloseChromatographic separation
The invention provides a chiral chromatographic separation and analysis method of dihydromyricetin enantiomer. The method comprises the following steps: dissolving a dihydromyricetin sample into the mobile phase, taking polysaccharide derivatives as the chiral stationary phase, and carrying out liquid chromatogram (LC) separation and analysis; wherein the mobile phase is a mixed solution of n-hexane and lower alcohols, and the polysaccharide derivative chiral stationary phase is one of stationary phases, which are individually covered by tri(3,5-dimethylphenylcarbamyl) straight-chain starch, tri((S)-(alpha)- phenylethylcarbamyl) straight-chain starch, tri(3,5- dimethylphenylcarbamyl)cellulose and tri(4- methylphenylcarbamyl)cellulose. According to the provided chiral separation and analysis method, the maximal separation degree of dihydromyricetin enantiomer is 1.95, and the maximal selection factor is 1.36.
Owner:GUANGDONG YANJIE PHARMA TECH CO LTD
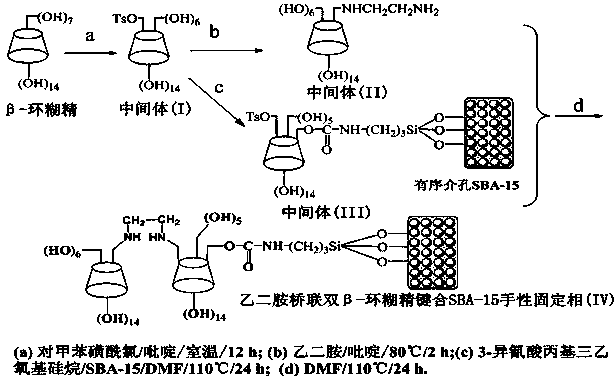
Preparation method and application of ethanediamine-bridged double-beta-cyclodextrin bonded SBA-15 chiral stationary phase
InactiveCN104289204AOther chemical processesSolid sorbent liquid separationEthylenediamineN dimethylformamide
The invention relates to a chiral stationary phase. The chiral stationary phase is prepared by the steps: in an anhydrous solvent, preparing a (6-oxy-p-toluenesulfonyl)-beta-cyclodextrin intermediate (I) from beta-cyclodextrin; reacting the (6-oxy-p-toluenesulfonyl)-beta-cyclodextrin with ethidenediamine so as to obtain a (6-deoxy-ethanediamine)-beta-cyclodextrin intermediate (II) by adopting anhydrous pyridine as a solvent; bonding the (I) onto the surface of ordered mesoporous SBA-15 silica gel by adopting anhydrous N, N-dimethylformamide as a solvent and using 3-carbimide triethoxypropylsilane as a coupling agent, so as to obtain a (6-oxy-p-toluenesulfonyl)-beta-cyclodextrin bonded SBA-15 silica gel intermediate (III); and dispersing the (III) in the anhydrous N, N-dimethylformamide by adopting a continuous reaction method and adding the (II) for reaction so as to obtain the chiral stationary phase. The preparation method is simple, relatively low in cost and wide in applicability.
Owner:NANCHANG UNIV
Supercritical fluid analog moving bed chromatograph of ternary area
InactiveCN1673736ASolving inefficienciesReduce consumptionComponent separationEngineeringOperation mode
The three-zone supercritical fluid analog movable bed chromatographic equipment includes 3-36 stuffing columns connected successively with back pressure regulator, with the output of the last stuffing column being connected to the input of the first one with back pressure regulator. All the inlets of the stuffing columns are connected with the flow phase inlet pipeline and sample feeding port pipeline, all the outlets of the stuffing columns are connected with extracting port pipeline and residue port pipeline, and the outlets of the extracting port pipeline and the residue port pipeline have cyclonic separators connected separately. The flow phase is supercritical fluid, and the present invention may operate in either pressure gradient operation mode or modifier gradient operation mode. The present invention has supercritical CO2 fluid as the flow phase and chiral fixed phase as stuffing, and may be used in splitting chiral medicine raceme in large scale.
Owner:卢建刚
Preparation of chromatographic stationary phase having porous framework material as matrix for chiral separation
ActiveUS20210023528A1Improve stabilityImprove comprehensive applicabilityOther chemical processesSolid sorbent liquid separationChromatographic separationMetal-organic framework
The novel porous framework materials (such as metal organic frameworks or covalent organic frameworks) having a wide range of applications, which was designed and developed in an inventive manner to resolve issues with respect to a carrier material in a stationary phase of a conventional chiral chromatographic column in which the carrier material has poor stability, a chiral resolving agent has a low loading rate, and the chiral resolving agent is prone to loss or is applied in a restricted manner. The porous framework material efficiently loads a chiral resolving agent (such as proteins, enzymes, or macrocyclic antibiotics) by means of covalent bonding, adsorption, embedding, and crosslinking, such that a variety of efficient and durable chiral stationary phases are prepared to serve as a novel high-performance chromatographic column filler used for chromatographic chiral separation (such as high-performance liquid chromatography or capillary chromatography). The various chiral stationary phases prepared by applying the above technique have high separation efficiency, high stability, and durability, and have been successfully applied to perform efficient separation of different kinds of chiral materials such as chiral amino acids and a chiral drug. The technique greatly widens the application range and extends the service life of a chiral chromatographic separation column.
Owner:NANKAI UNIV
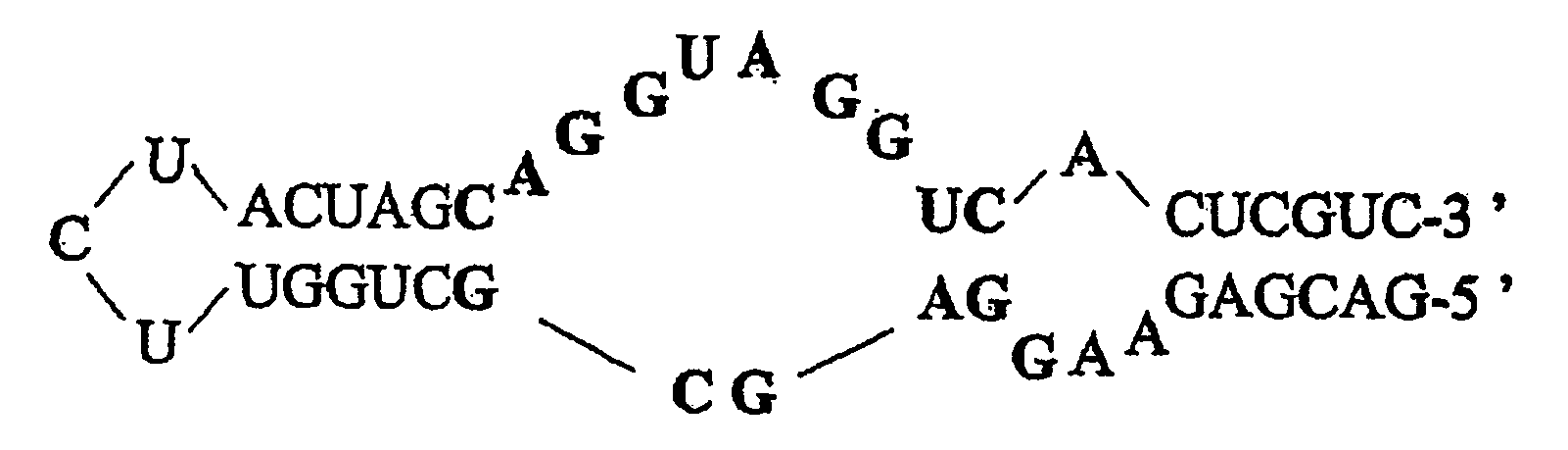
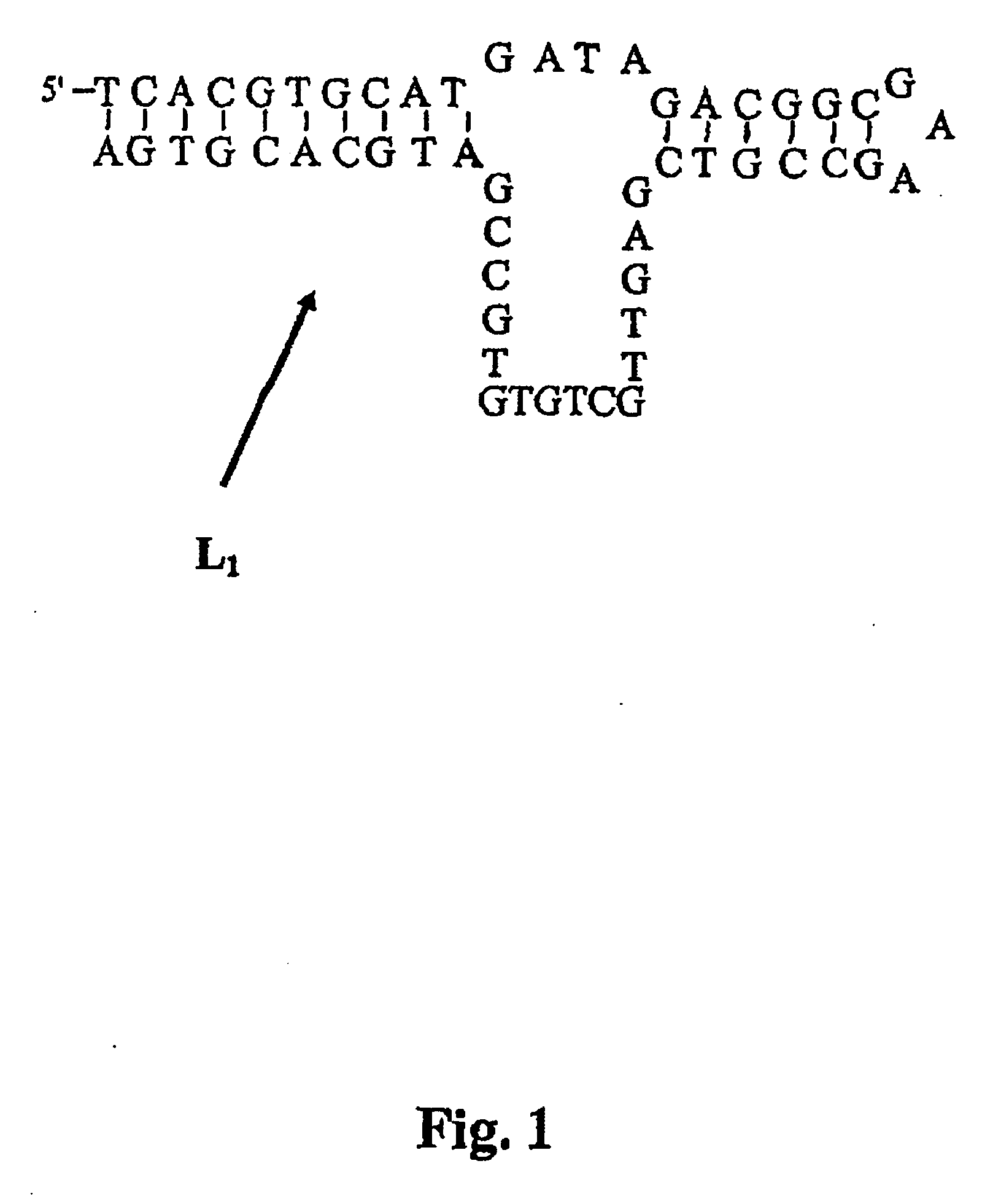
Nucleic acids in the form of specific novel chiral selectors
InactiveUS20070056908A1Strong specificityHigh affinityIon-exchange process apparatusOther chemical processesEnantiomerElectrophoresis
The invention relates to chiral separation chromatographic and electrophoretic techniques. The aim of said invention is to obtain chiral stationary and mobile phases comprising an oligonucleotide which is specifically selected by a SELEX method against an enantiomer to be separated as a special-purpose chiral selector. Methods for separating enantiomers by the chiral stationary and mobile phases are also disclosed.
Owner:UNIV JOSEPH FOURIER
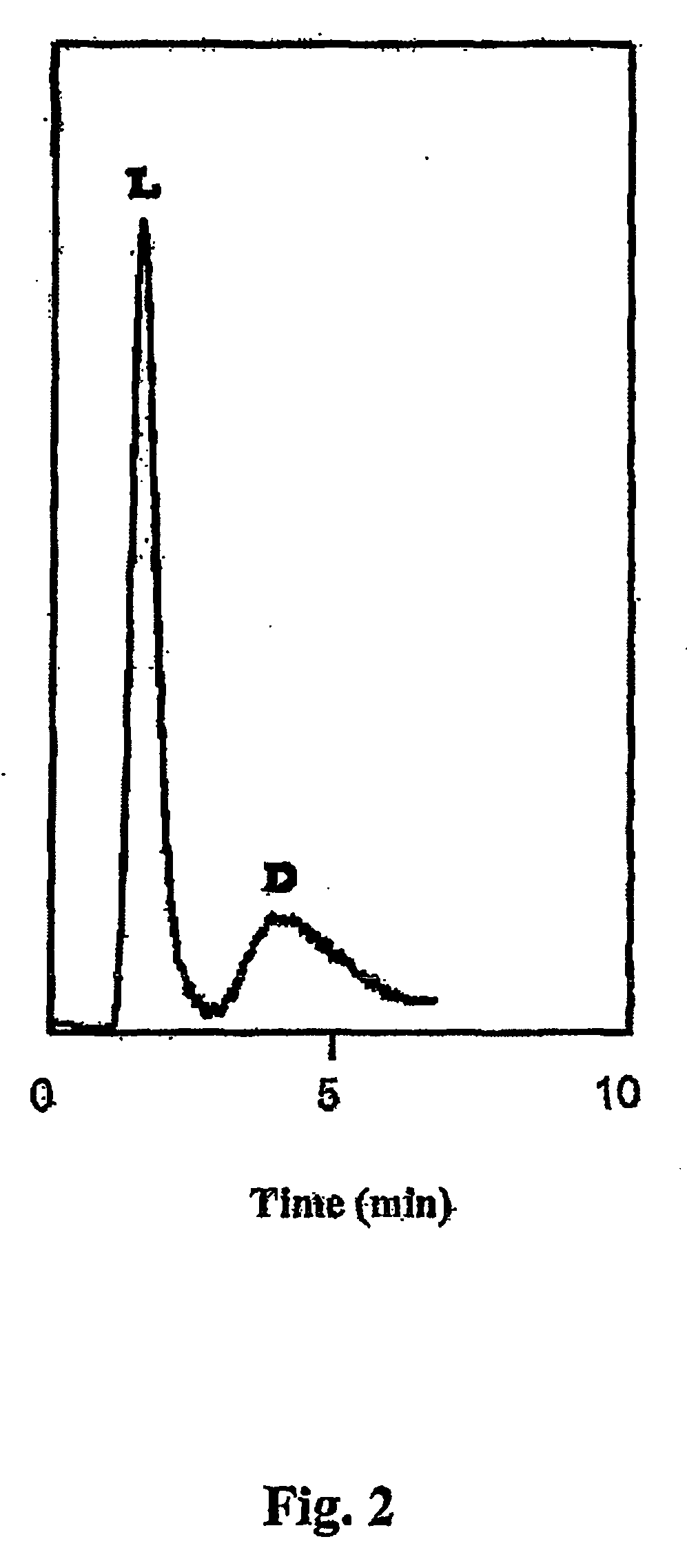
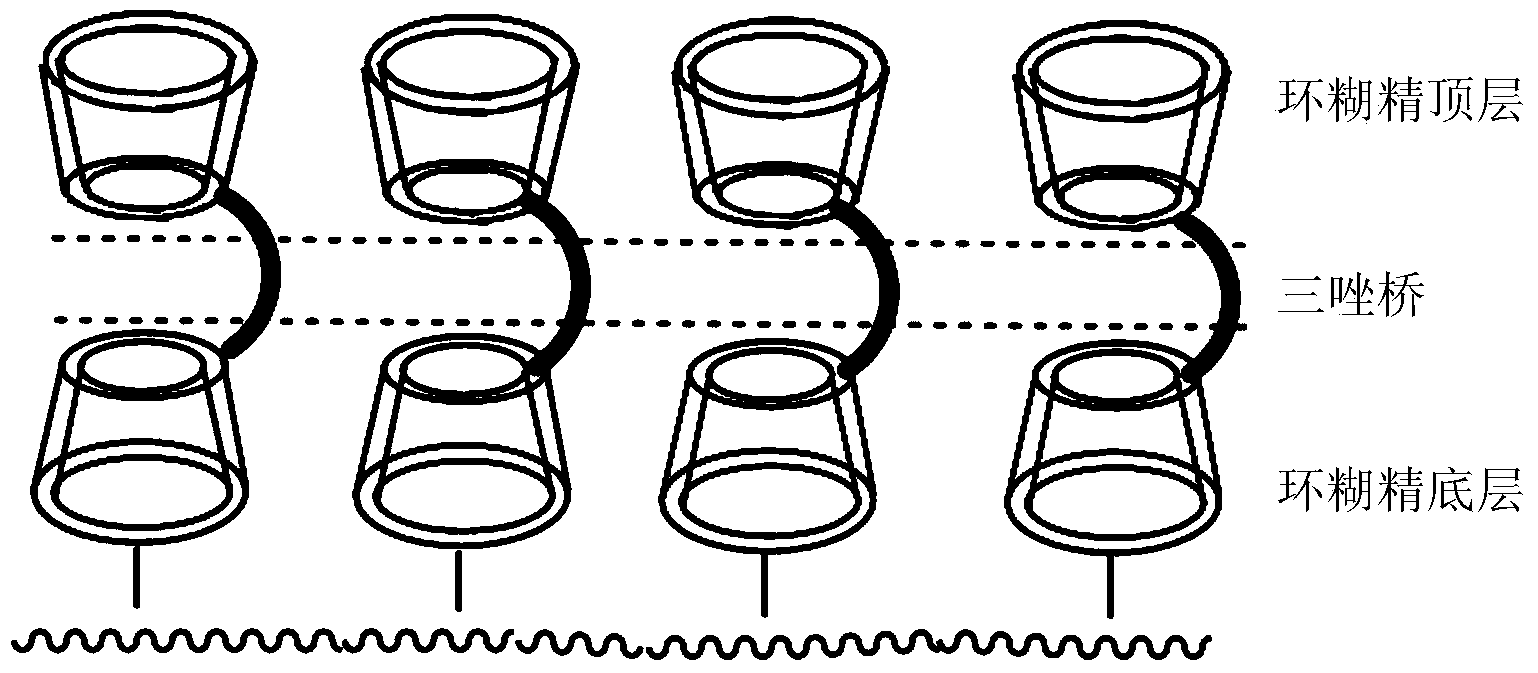
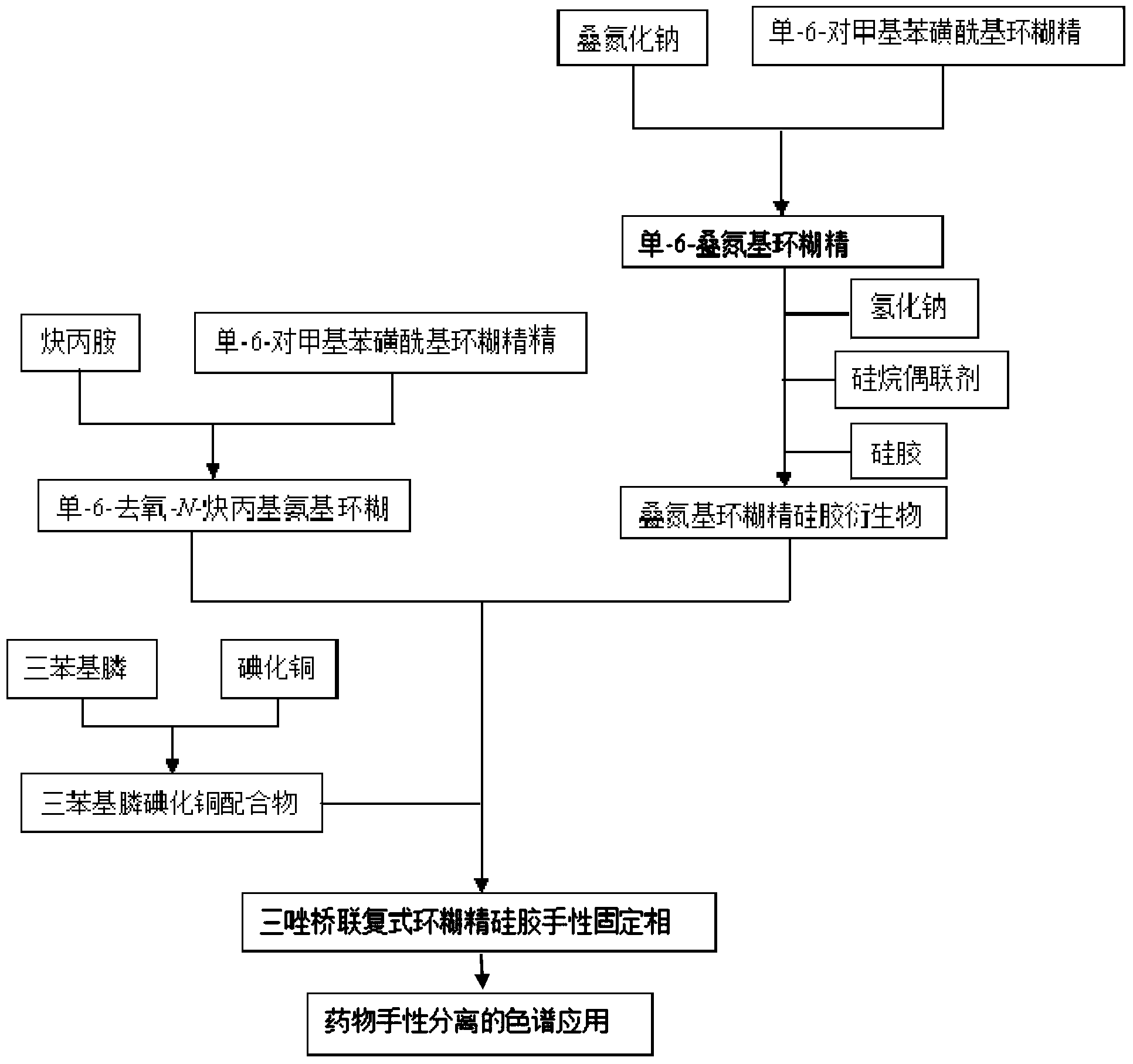
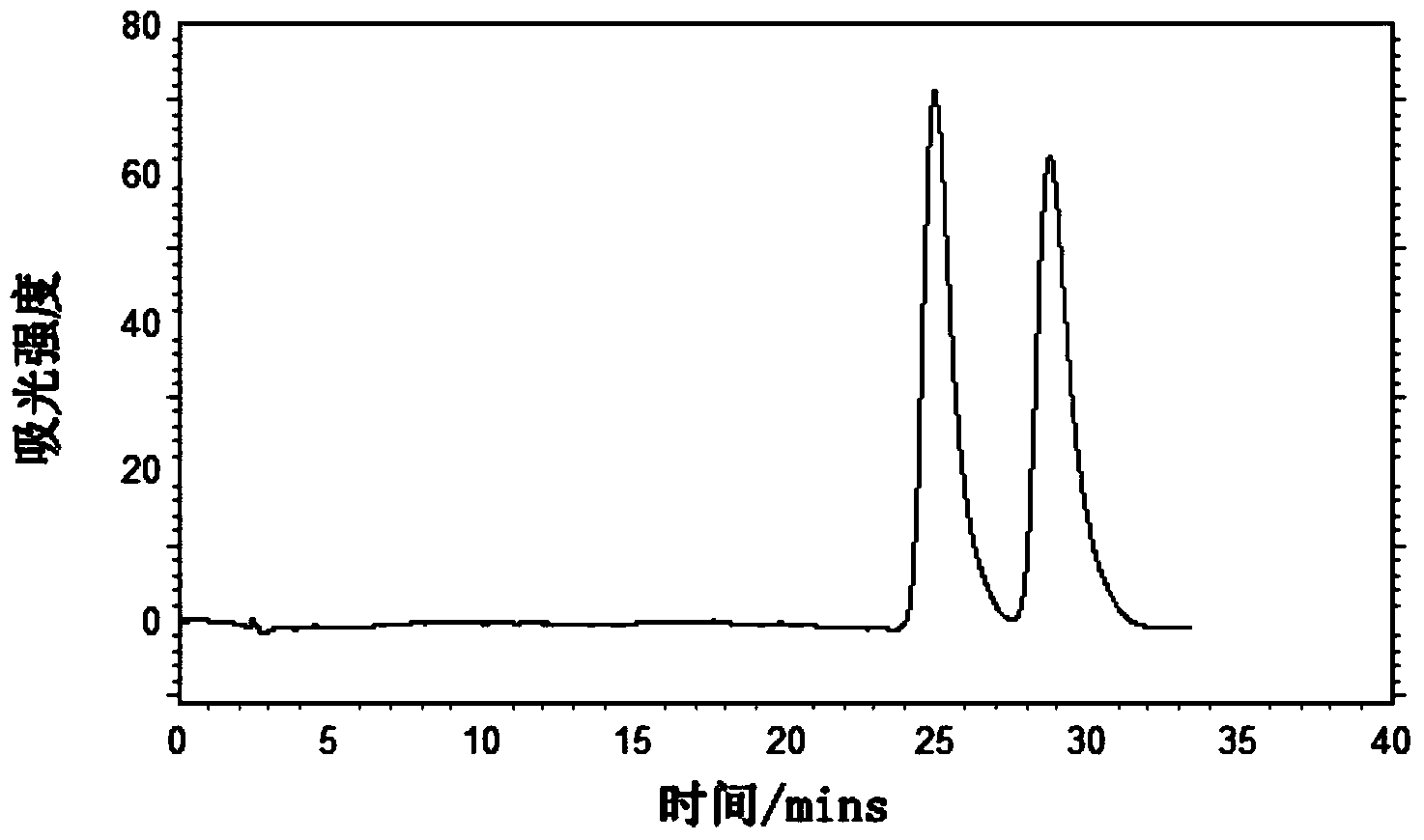
Preparation method and application of novel triazole bridging compound cyclodextrin chiral stationary phase
InactiveCN103464125AEasy to separateIncrease Separation ThroughputOther chemical processesClick chemistryCycloaddition
The invention discloses a preparation method of a novel triazole bridging compound cyclodextrin chiral stationary phase. The preparation method comprises the following two steps: firstly, introducing azido mono-substituted cyclodextrin on the surface of silica gel through an ether bond by utilizing a silane coupling agent; then, bonding alkynyl substituted cyclodextrin on the surface of azido cyclodextrin silica gel through cuprous catalyzed 1,3-dipolar cycloaddition reaction (click chemistry) so as to prepare the triazole bridging compound cyclodextrin silica gel chiral stationary phase with a good synergistic effect. The stationary phase prepared by the invention shows excellent separation ability to different drugs, such as dansyl amino acid, micromolecular aromatic acid and neutral racemic form, in liquid chromatogram, and therefore, the stationary phase prepared by the invention can be used as the chromatographic chiral stationary phase applied in the field of drug chiral separation.
Owner:TIANJIN UNIV
Chitosan-bi(aryl-carbamate)-(amide) and preparation method thereof
The invention relates to a material chitosan-bi(aryl-carbamate)-(amide) for preparing a chiral stationary phase and a preparation method of the chitosan-bi(aryl-carbamate)-(amide). The preparation method comprises the following steps: 1) acylating chitosan amino, namely enabling chitosan with the deacetylation degree of over 98 percent to be reacted with excessive anhydride, thereby obtaining acylated N-chitosan; and 2) performing carbamic acid esterification on acylated N-chitosan, namely dissolving the acylated N-chitosan in N,N-dimethylacetamide solution of lithium chloride, adding excessive isocyanate containing different substituent groups on the benzene ring, and reacting at the temperature of 80-95 DEG C for 24-36 hours, thereby generating a chitosan derivative, namely the chitosan-bi(aryl-carbamate)-(amide). The structural formula is as shown in the specification.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
Preparation method of Fe3O4@Au-BSA magnetic nano-compound and chiral separation use thereof
InactiveCN104043438AAchieve regenerationHigh efficiency loadOrganic chemistryOther chemical processesBovine serum albuminBiocompatibility
The invention discloses a synthesis method of Fe3O4 magnetic nanoparticles coated with gold and its use in chiral selector assembling, stationary phase preparation and chiral amino acid separation analysis. Through an ultrasonic chemical method, gold nanoparticles coat the surfaces of the Fe3O4 magnetic nanoparticles so that Fe3O4@Au core-shell nanoparticles having good magnetism and good biocompatibility are obtained, through gold-ammonia bond action, bovine serum albumin is fixed to the surfaces of the Fe3O4@Au nanoparticles, and under the action of an external magnetic field, Fe3O4@Au-bovine serum albumin can be controllably fixed in a separation channel of a micro-fluidic chip so that a micro-fluidic chip with Fe3O4@Au-bovine serum albumin as a chiral stationary phase is obtained.
Owner:NANCHANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com