Preparation method and application of ethanediamine-bridged double-beta-cyclodextrin bonded SBA-15 chiral stationary phase
A technology of chiral stationary phase and ethylenediamine bridge, which is applied in separation methods, chemical instruments and methods, solid adsorbent liquid separation, etc., can solve the problems that the separation function of chiral chromatography has yet to be developed and utilized, and achieve the improvement of chirality Separation ability, high practicality, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take SBA-15 (400 m 2 / g) 4.0 g of activated silica gel is the substrate;
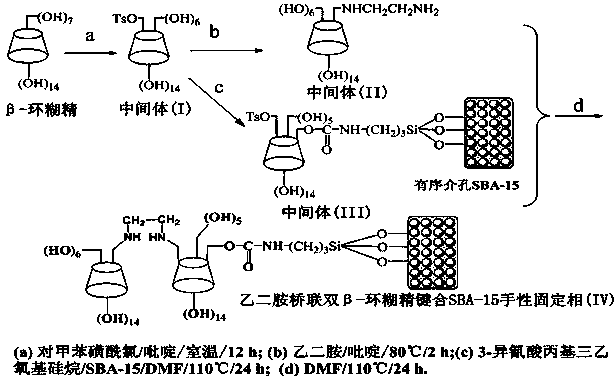
[0031] (1) Under a nitrogen atmosphere, according to the ratio of β-cyclodextrin (mmol): anhydrous pyridine A (mL): p-toluenesulfonyl chloride (mmol): anhydrous pyridine B (mL) 1:10:0.6:2.5 Dissolve β-cyclodextrin and p-toluenesulfonyl chloride in the corresponding amount of anhydrous pyridine, and add the solution of p-toluenesulfonyl chloride in anhydrous pyridine B dropwise to β-cyclodextrin within 2 h under stirring. Pyridine A solution in water, stirred and reacted at room temperature for 11 h to obtain a yellow solution, removed pyridine in vacuo, added anhydrous ether to obtain a white solid, filtered the solid and recrystallized several times with ultrapure water to obtain (6-oxo-p-toluene Sulfonyl)-β-cyclodextrin intermediate (I);
[0032] (2) Under nitrogen atmosphere, according to (6-oxo-p-toluenesulfonyl)-β-cyclodextrin intermediate (I) (mmol): anhydrous pyridine (mL): ethylenediami...
Embodiment 2
[0039] Take SBA-15 (500 m 2 / g) 4.0 g of activated silica gel is the substrate;
[0040] (1) Under a nitrogen atmosphere, according to the ratio of β-cyclodextrin (mmol): anhydrous pyridine A (mL): p-toluenesulfonyl chloride (mmol): anhydrous pyridine B (mL) at 1:12:0.9:3.0 Dissolve β-cyclodextrin and p-toluenesulfonyl chloride in the corresponding amount of anhydrous pyridine, and add the solution of p-toluenesulfonyl chloride in anhydrous pyridine B dropwise to β-cyclodextrin within 3 h under stirring. Pyridine A solution in water, stirred and reacted at room temperature for 13 h to obtain a yellow solution, removed pyridine in vacuo, added anhydrous ether to obtain a white solid, filtered the solid and recrystallized several times with ultrapure water to obtain (6-oxo-p-toluene Sulfonyl)-β-cyclodextrin intermediate (I);
[0041] (2) Under nitrogen atmosphere, according to (6-oxo-p-toluenesulfonyl)-β-cyclodextrin intermediate (I) (mmol): anhydrous pyridine (mL): ethylenedi...
Embodiment 3
[0048] Take SBA-15 (465 m 2 / g) 4.0 g of activated silica gel is the substrate;
[0049] (1) Under nitrogen atmosphere, according to β-cyclodextrin (mmol): anhydrous pyridine A (mL): p-toluenesulfonyl chloride (mmol): anhydrous pyridine B (mL) is 1:11:0.7:2.7 Dissolve β-cyclodextrin and p-toluenesulfonyl chloride in the corresponding amount of anhydrous pyridine, and add the solution of p-toluenesulfonyl chloride in anhydrous pyridine B dropwise to β-cyclodextrin within 2.5 h under stirring. Pyridine A solution in water, stirred and reacted at room temperature for 12 h to obtain a yellow solution, removed pyridine in vacuo, added anhydrous ether to obtain a white solid, filtered the solid and recrystallized several times with ultrapure water to obtain (6-oxo-p-toluene Sulfonyl)-β-cyclodextrin intermediate (I);
[0050] (2) Under nitrogen atmosphere, according to (6-oxo-p-toluenesulfonyl)-β-cyclodextrin intermediate (I) (mmol): anhydrous pyridine (mL): ethylenediamine (mL) as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com