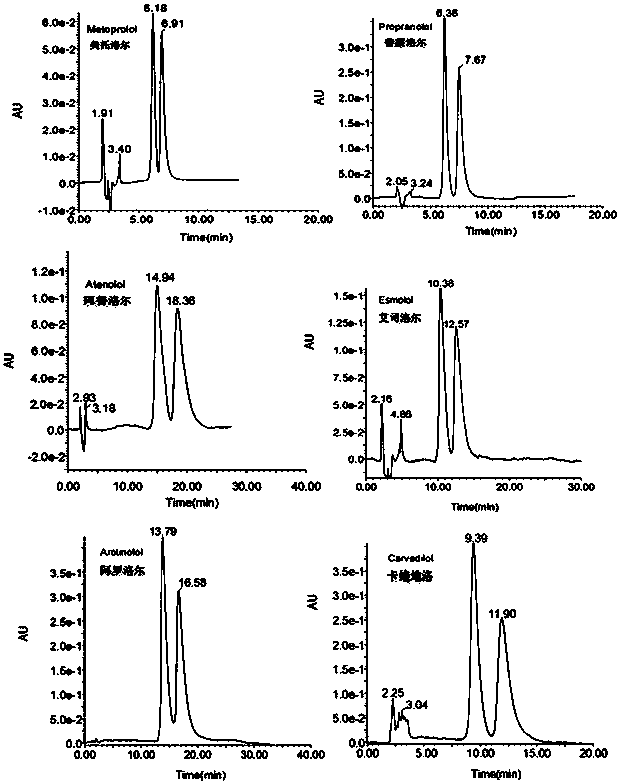
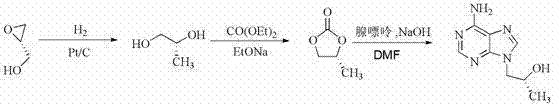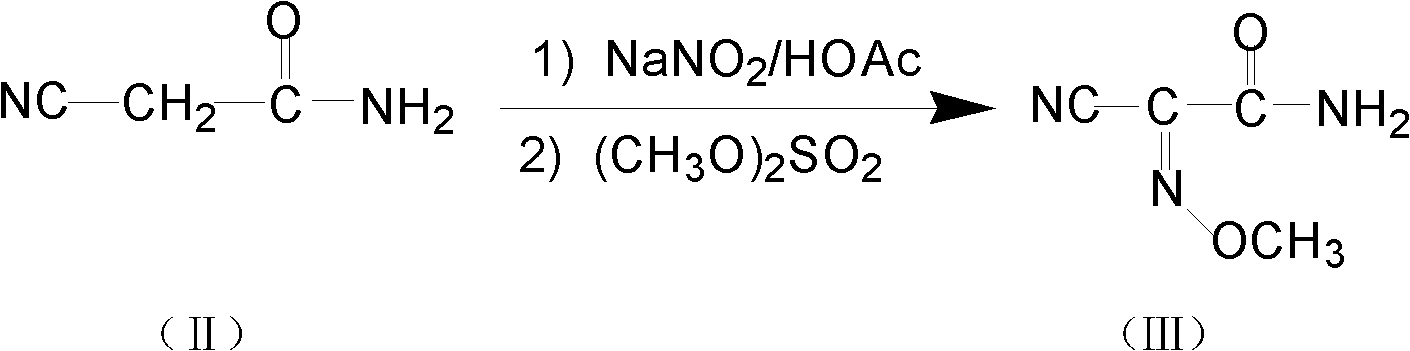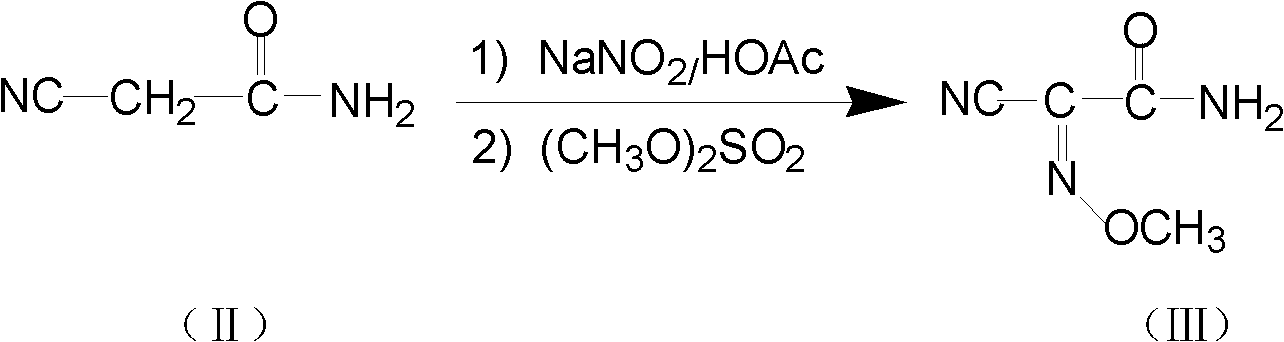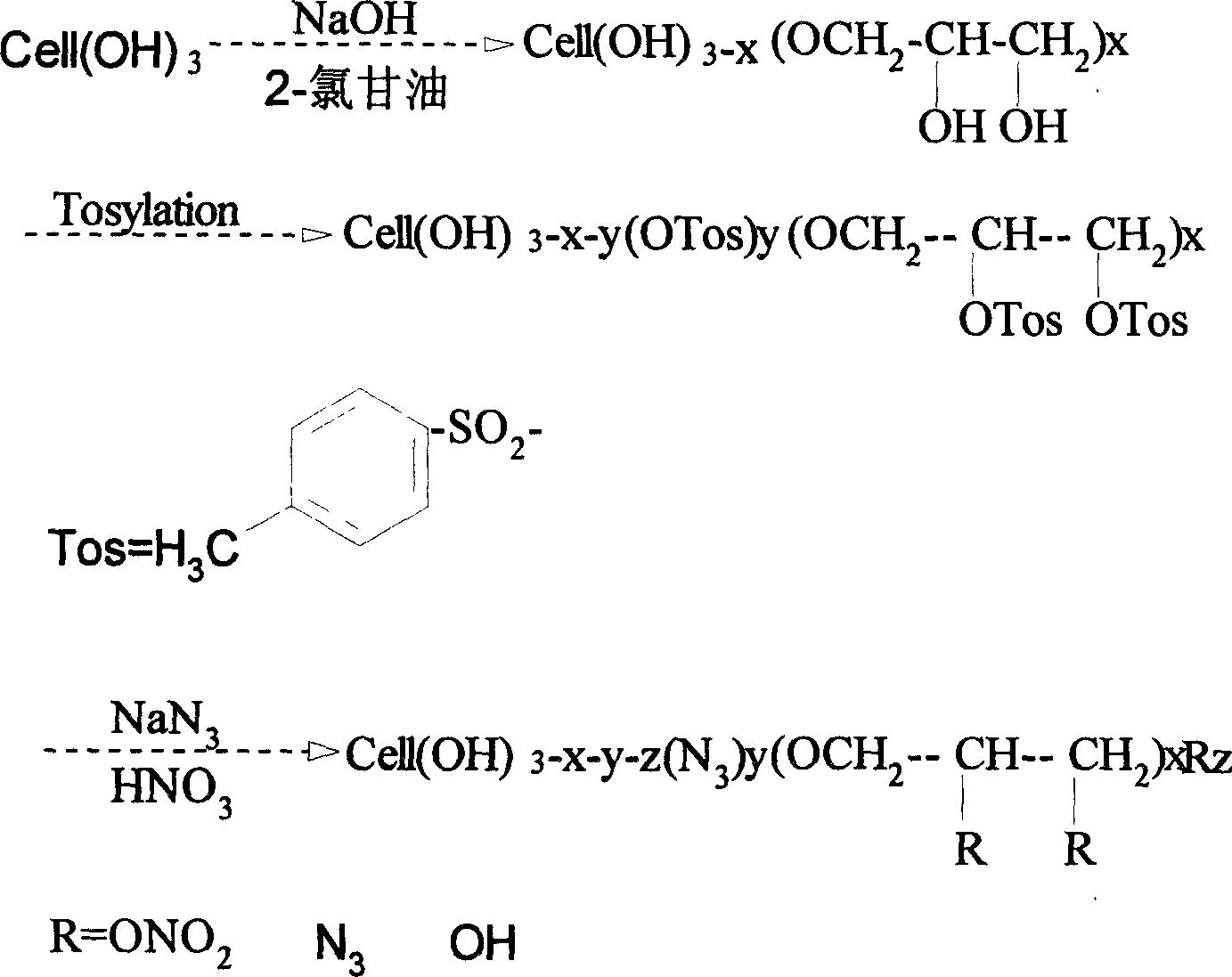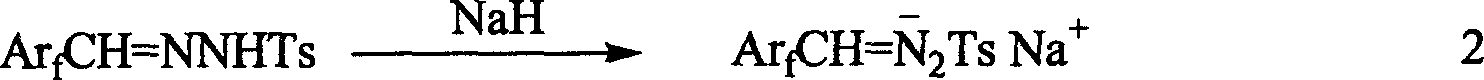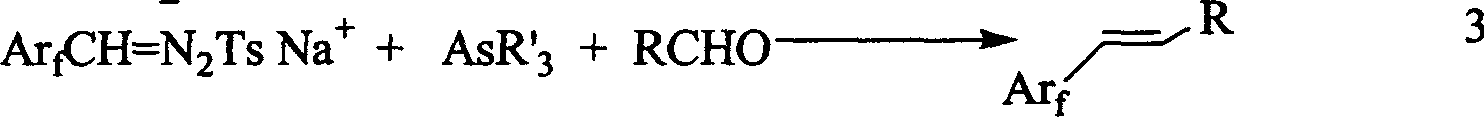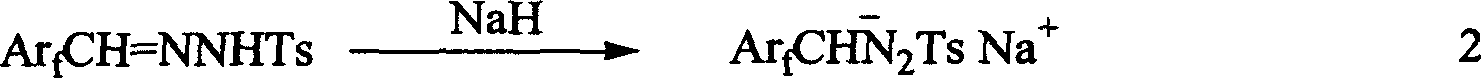Patents
Literature
351 results about "Tosylhydrazone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=N-NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.
Method for preparing sludge-based formed magnetic active carbon
ActiveCN103521180AUniform sizeHigh compressive strengthOther chemical processesAlkali metal oxides/hydroxidesMagnetic stabilitySludge
The invention provides a method for preparing sludge-based formed magnetic active carbon. The method comprises the steps as follows: sludge is used as a raw material, an activating agent is added for carbonizing and activating the sludge; nano-sized Fe3O4 is used as a magnetic source for magnetizing the sludge active carbon; and sodium carboxymethylcellulose and aluminum salt are composited to an organic-inorganic binding agent, the binding agent and a p-toluenesulfonhydrazide foaming agent are used for uniformly binding powdery magnetic active carbon into a formed magnetic active carbon precursor with abundant pore structures, and finally, the formed magnetic active carbon precursor is heated to obtain the sludge-based formed magnetic active carbon. According to the method, the sludge is used as a main raw material, the resource utilization of the sludge is achieved, the recycling is convenient, and reutilization can be achieved; and further, the sludge-based formed magnetic active carbon has a stable structure and good magnetic stability, and can be widely used in the fields such as sewage treatment, soil pollution treatment and the like.
Owner:FUJIAN UNIV OF TECH
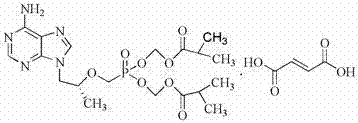
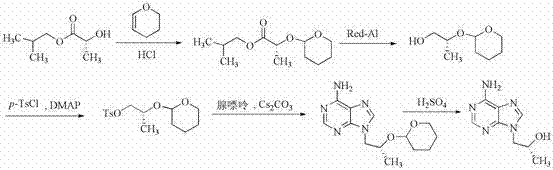
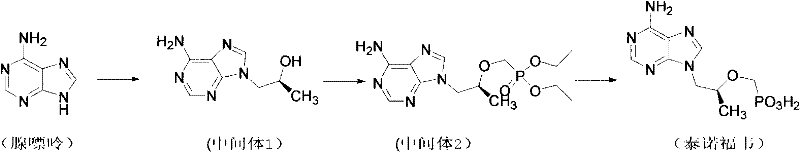
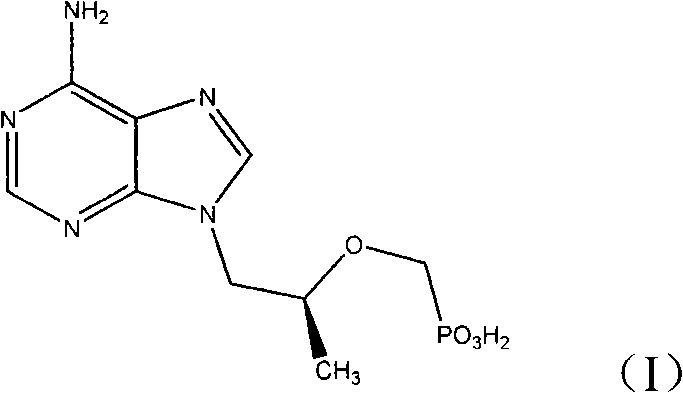
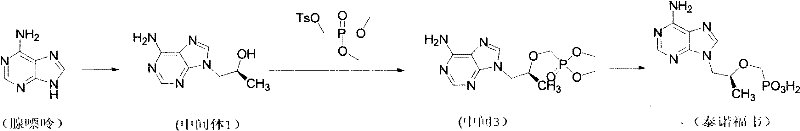
Synthetic process of tenofovir
InactiveCN102295660AHigh yieldEasy post-processingGroup 5/15 element organic compoundsDiethyl phosphatePurine
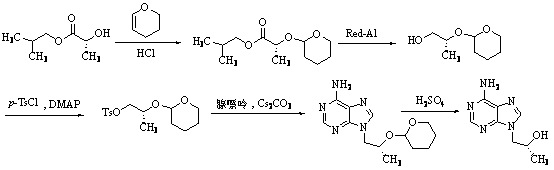
The invention relates to a synthesis technique of tenofovir, and relates to the field of medicinal chemistry. Using natural (R)-lactate as the starting material, chiral R-1,2-propanediol is obtained through reduction, and then reacted with diethyl carbonate under the action of alkali to obtain the key reaction intermediate R-propylene carbonate ester. Use adenine and R-propylene carbonate to prepare R-9-(2-hydroxypropyl)adenine; Gained R-9-(2-hydroxypropyl)adenine and diethyl p-toluenesulfonyloxyphosphate Under the catalysis of magnesium tert-butoxide, the condensation reaction is carried out to obtain R-9[(diethylphosphorylmethoxy)propyl]purine; the obtained R-9[2-(diethylphosphorylmethoxy) Propyl]purine is hydrolyzed to obtain Tenofovir. The process has short reaction steps, short required reaction time, high quality yield and good product quality, and is suitable for industrialized production.
Owner:CHANGZHOU UNIV
Novel production process of tenofovir
InactiveCN102219805ANo pollution in the processLow costGroup 5/15 element organic compoundsDimethyl methylphosphonateHydrolysis
The invention relates to a production process of drug tenofovir in the field of aids treatment. The process is a method of using adenine as a raw material, condensing toluenesulfonyloxy dimethyl methyl phosphonate by using an intermediate and then hydrolyzing by using an inorganic acid to finally prepare the tenofovir. The production process of the drug tenofovir, provided by the invention, has the characteristic of improving the hydrolysis activity of the intermediate so that the operation of the process production is largely simplified and the production cost of the tenofovir is largely reduced.
Owner:SUZHOU TENGLONG BIO PHARMA TECH
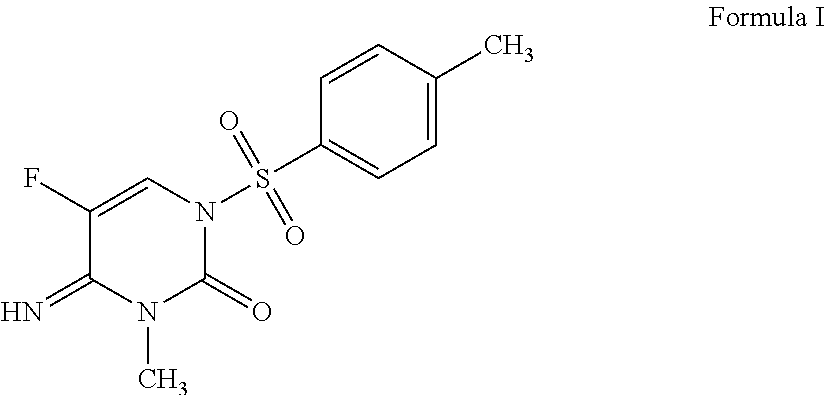
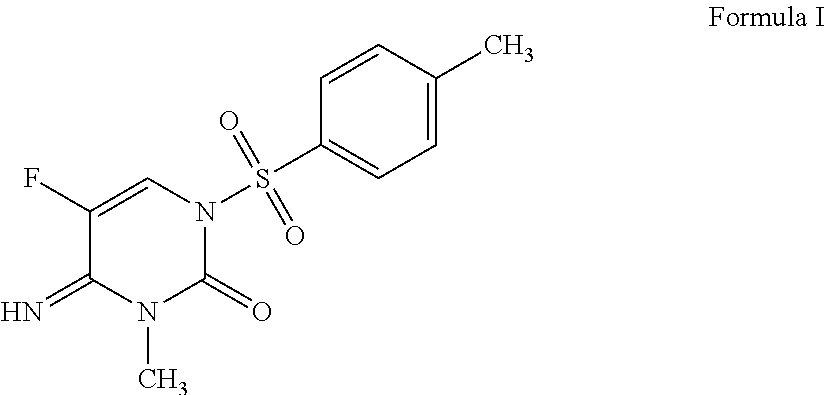
Synergistic fungicidal mixtures comprising 5-fluoro-4-imino-3-methyl-1-tosyl-3,4-dihydropyrimidin-2(1H)-one for fungal control in cereals
ActiveUS9526245B2Quality improvementHigh yieldOrganic active ingredientsBiocideEpoxiconazolePropiconazole
A fungicidal composition containing a fungicidally effective amount of the compound of Formula I:5-fluoro-4-imino-3-methyl-1-tosyl-3,4-dihydropyrimidin-2(1H)-one, and at least one fungicide selected from the group consisting of: prothioconazole, epoxiconazole, cyproconazole, myclobutanil, metconazole, difenoconazole, propiconazole, fluquinconazole, and flutriafol provides synergistic control.
Owner:ADAMA MAKHTESHIM LTD
Crosslinked polysaccharide, obtained by crosslinking with substituted polyethylene glycol, as superabsorbent
InactiveUS7365190B2Improve featuresCosmetic preparationsSugar derivativesPolyethylene glycolTriflic acid
New crosslinked polysaccharides useful as absorbents or superabsorbents alone or in a mixture are obtained by reacting polysaccharides (preferably containing carboxylates groups) with at least one crosslinker selected in the group constituted by activated polyethylene glycols such as for example halogenated (Cl, Br, I), mesylated, tosylated, or triflated activated polyethylene glycols.
Owner:ARCHER DANIELS MIDLAND CO
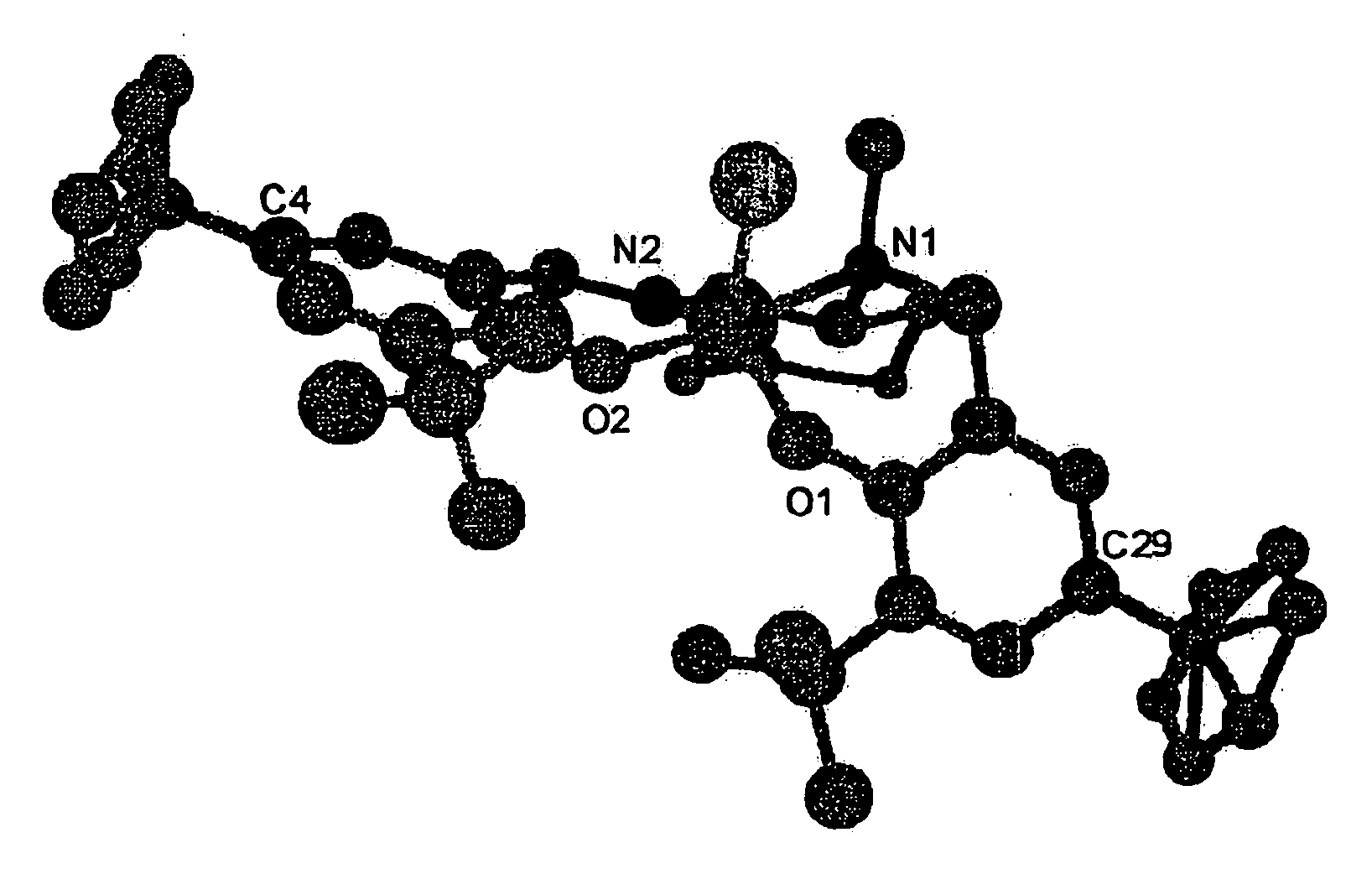
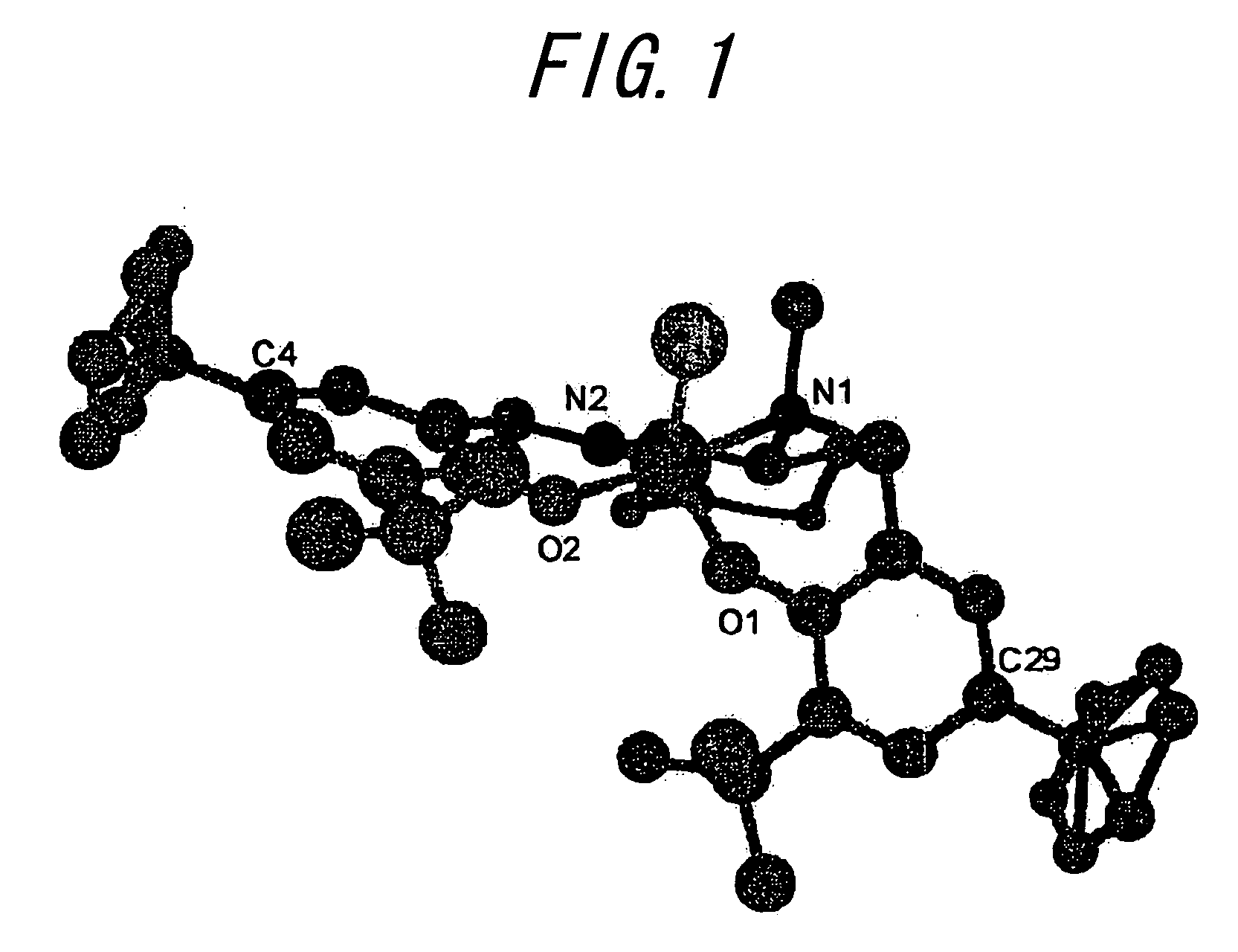
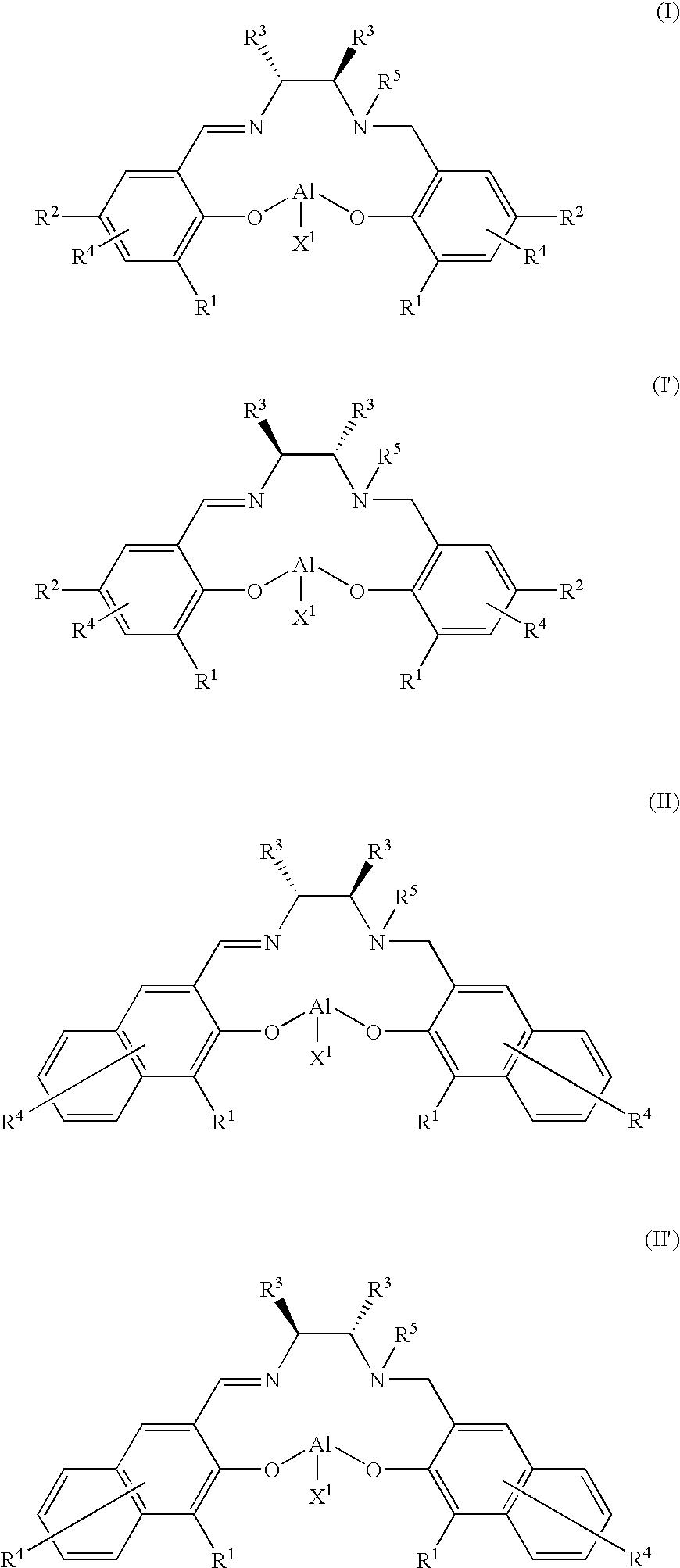
Optically Active Alpha-Hydroxyphosphonic Acid, Its Derivatives and Production Method thereof, Optically Active Aluminum (Salalen) Complex and Production Method Thereof, and Production Method of Salalen Ligand
InactiveUS20090099381A1High enantioselectivityEfficient CatalysisIsocyanic acid derivatives preparationOrganic compound preparationTosylhydrazoneEnantio selectivity
The present invention relates to a production method capable of producing an optically active α-hydroxyphosphonic acid and its derivatives with sufficiently high enantioselectivity not only for aromatic aldehydes but also for aliphatic aldehydes, and more specifically to a method of producing an optically active α-hydroxyphosphonic acid and its derivatives, characterized in that an optically active aluminum(salalen) complex represented by any one of the following formulae (I), (I′), (II) and (II′):[wherein R1s are each alkyl group or aryl group independently; R2s are each alkyl group or aryl group independently; R3s are each alkyl group or aryl group independently, and two R3s may bond with each other to form a ring; R4s are each hydrogen atom, halogen atom, alkyl group, alkoxy group, nitro group, or cyano group independently; R5 is alkyl group; and X1 is halogen atom, alkyl group, alkoxy group, acetoxy group or toluenesulfonyloxy group] is used as a catalyst to asymmetrically hydrophosphonylate an aldehyde with phosphonic acid or its derivatives.
Owner:JAPAN SCI & TECH CORP
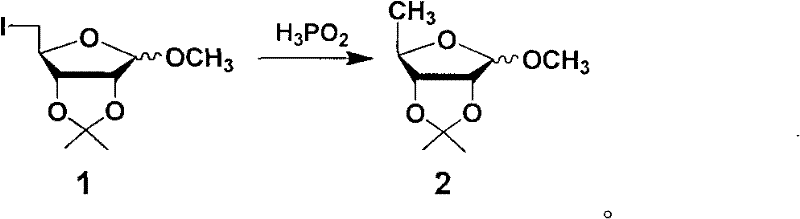
Preparation methods of capecitabine and intermediate thereof
ActiveCN102212095AAvoid it happening againReduce usageSugar derivativesSugar derivatives preparationPhosphoric acidTosylhydrazone
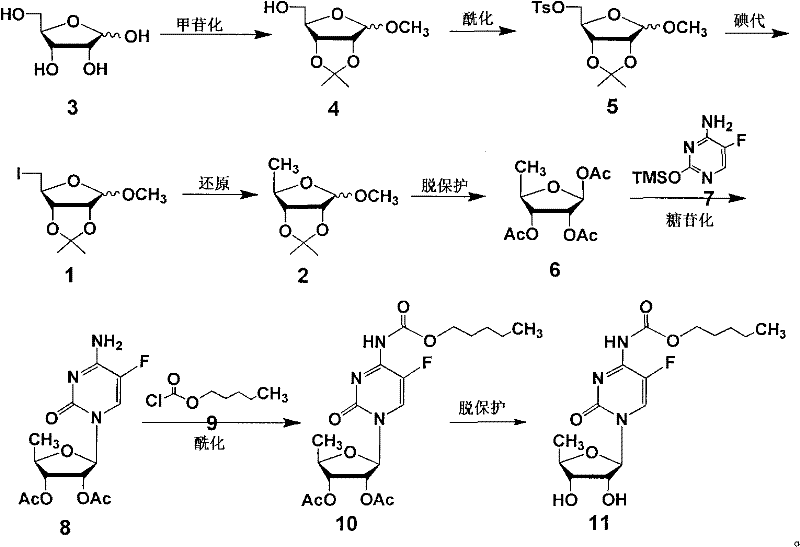
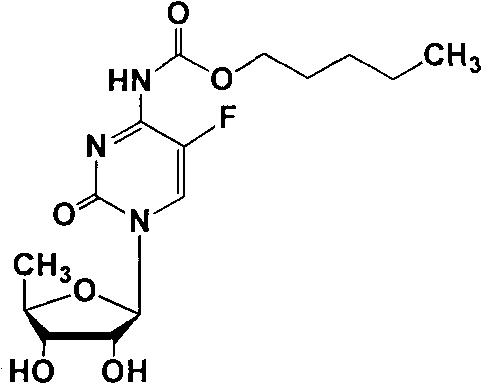
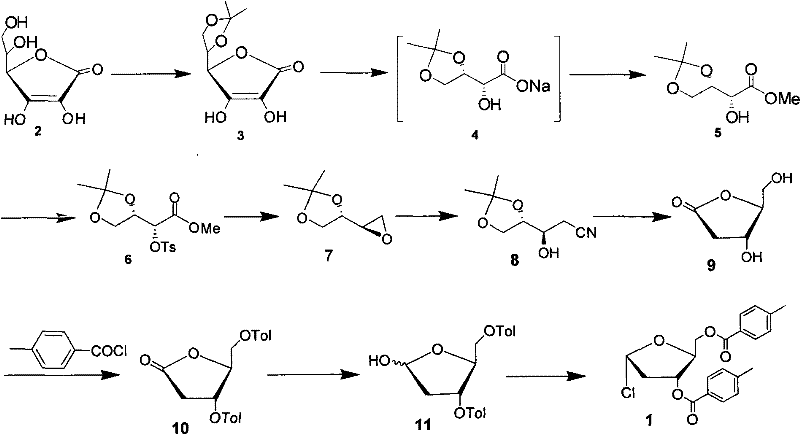
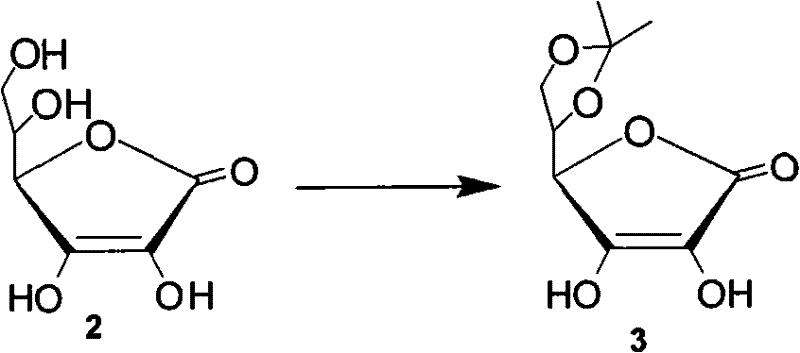
The invention discloses a preparation method of capecitabine. The method comprises the following steps: based on D-ribose serving as a starting raw material, carrying out hydroxyl protection, 5-site tosylation, iodine substitution, hypophosphorous acid deiodination and acetylation so as to obtain the key intermediate 12,3-tri-O-acetyl-5-deoxy-beta-D-ribofuranose; carrying out glycosylation on the key intermediate and 5-fluorocytosine; and finally, carrying out N-4 site acylation and deprotection so as to obtain the capecitabine. In the method, a metal catalyst dose not need to be used for participating in reaction, the reaction condition is mild, and the yield is high, thus the method is economical and effective as well as suitable for industrial production on a large scale.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +2
Preparation method of capillary electro-chromatography column taking beta-cyclodextrin as bonded stationary phase and application in chiral drug separation
InactiveCN102921193AEasy to manufactureLow priceAmino compound purification/separationOther chemical processesStationary phaseCapillary electrochromatography
The invention relates to a preparation method of a capillary electro-chromatography column taking beta-cyclodextrin as a bonded stationary phase and an application in a chiral drug separation, and can effectively solve the problems of the capillary electro-chromatography column preparation and the chiral drug separation. The solved technical proposal comprises the following steps: (1) preparing six-bit mono substituted P-toluenesulfonyl-beta-cyclodextrin, (2) preparing six-bit mono substituted ammoniated-beta-cyclodextrin, (3) preparing silylated silica gel, (4) preparing a beta-cyclodextrin bonded silica gel chiral stationary phase, and (5) preparing a capillary packing column. The preparation method is low in cost, low in toxicity and low in environment pollution, and is an innovation of the preparation method of the capillary electro-chromatography column taking the beta-cyclodextrin as the bonded stationary phase and the application in the chiral drug separation.
Owner:ZHENGZHOU UNIV
Method for performing ring-opening for cyclohexylaziridine by carboxylic acid
The invention discloses a method for performing ring-opening for cyclohexylaziridine by a carboxylic acid. The method includes that in a polar aprotic solvent system, alkali metal type inorganic base is utilized as a catalyst, a monocarboxylic acid is utilized as a nucleophilic reagent, and the cyclohexylaziridine which is activated by tosyl is subjected to a ring-opening reaction. The method has the advantages that the ring-opening reaction is simple in process, the reaction condition is mild, the solvent is environment-friendly, the carboxylic acid is utilized as the nucleophilic reagent, atom economical requirement of green chemistry is met, the catalytic agent is cheap, and the catalytic activity is high.
Owner:TAIYUAN UNIV OF TECH
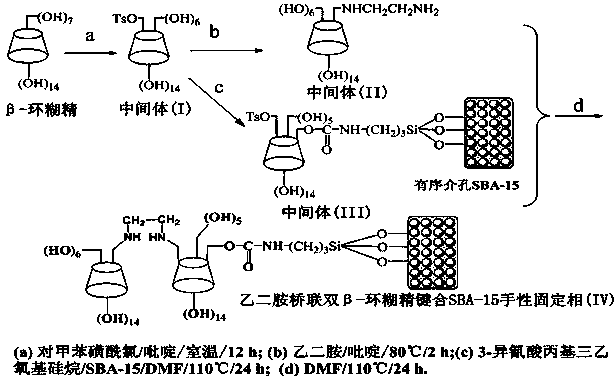
Preparation method and application of ethanediamine-bridged double-beta-cyclodextrin bonded SBA-15 chiral stationary phase
InactiveCN104289204AOther chemical processesSolid sorbent liquid separationEthylenediamineN dimethylformamide
The invention relates to a chiral stationary phase. The chiral stationary phase is prepared by the steps: in an anhydrous solvent, preparing a (6-oxy-p-toluenesulfonyl)-beta-cyclodextrin intermediate (I) from beta-cyclodextrin; reacting the (6-oxy-p-toluenesulfonyl)-beta-cyclodextrin with ethidenediamine so as to obtain a (6-deoxy-ethanediamine)-beta-cyclodextrin intermediate (II) by adopting anhydrous pyridine as a solvent; bonding the (I) onto the surface of ordered mesoporous SBA-15 silica gel by adopting anhydrous N, N-dimethylformamide as a solvent and using 3-carbimide triethoxypropylsilane as a coupling agent, so as to obtain a (6-oxy-p-toluenesulfonyl)-beta-cyclodextrin bonded SBA-15 silica gel intermediate (III); and dispersing the (III) in the anhydrous N, N-dimethylformamide by adopting a continuous reaction method and adding the (II) for reaction so as to obtain the chiral stationary phase. The preparation method is simple, relatively low in cost and wide in applicability.
Owner:NANCHANG UNIV
Non-specific adsorption inhibitor, probe-bonded particles, and method for producing the same
ActiveUS20080160167A1Easy to produceLot of noiseMicrobiological testing/measurementPharmaceutical containersSpecific adsorptionMonomethyl ether
A method for producing a non-specific adsorption inhibitor includes reacting (A) a tosylated compound of polyoxyethylene monomethyl ether with (B) a polyamine having either an amino group or imino group (—NH—), or both, in total of 3 to 12.
Owner:JSR CORPORATIOON
Method for synthesis of PMPA by combining biological technique and chemical technique
ActiveCN102899367AHigh yield of reductionEasy post-processingGroup 5/15 element organic compoundsMicroorganism based processesBiotechnologyDiethyl phosphate
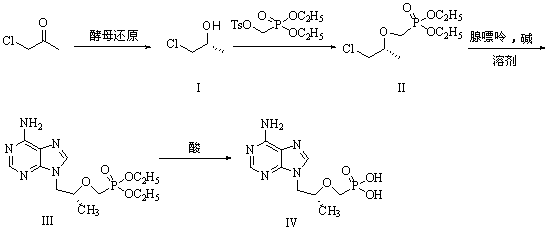
Belonging to the technical field of medicine synthesis, the invention relates to a method for synthesis of PMPA by combining a biological technique and a chemical technique. The method comprises: taking chlorinated acetone as a starting raw material, conducting yeast fermentation reduction so as to obtain chiral chloropropanol (I), then subjecting the chiral chloropropanol (I) and diethyl p-toluenesulfonyloxyphosphonate to a condensation reaction under the action of alkali so as to obtain a reaction intermediate (II); preparing R-9-(2-hydroxypropyl)adenine (III) from adenine and the reaction intermediate (II); and hydrolyzing the obtained R-9[2-(diethylphosphonomethoxy)propyl]purine, thus obtaining the PMPA (IV). The method combines the biological technique and the chemical technique and obtains a key chiral alcohol by means of a biological means, i.e. fermentation. The method has the characteristics of short reaction process, short reaction time, high mass yield, and can produce products with good quality, thus being suitable for industrialized production.
Owner:黄石福尔泰医药科技有限公司
Packing materials of starch-polypropylene foam, an preparation method
InactiveCN1563172AFast biodegradationBiodegradableSynthetic resin layered productsPack materialSodium benzoate
A biological foam degradable packing material of starch and polypropylene is made of starch, polypropylene, paramonotoluene sulfuryl semicarbazide, sodium benzoate, 2,5-dimethyl-2,5-ditert butyl peroxyhexane or diisopropylbenzene peroxide, fatty glyceride and isopentene or carbon dioxide. The preparing process includes mixing materials, heating, extruding, rolling and setting.
Owner:杨凌沃林国际降解树脂发展有限公司 +1
Technology for preparing key intermediate of telbivudine
InactiveCN102477051AReduce pollutionHigh yieldEsterified saccharide compoundsSugar derivativesVitamin CTosylhydrazone
The invention discloses a new technology for preparing a key intermediate Hoffer's chlorosugar of telbivudine. The technology is characterized in that the technology comprises the following steps: 1, obtaining an epoxide by carrying out condensation, oxidation ring-opening, methylation, tosylation, reduction and epoxidation on vitamin C which is massively supplied in China and is treated as an initial raw material; 2, carrying out ring-opening cyaniding on the obtained epoxide; 3, carrying out sulfuric acid hydrolysis and lactonization on the generated cyan butanetriol derivative; and 4, carrying out hydroxy protection with p-toluoyl chloride, reducing with sodium triacetoxyborohydride to obtain a reduction product, and directly chloridizing the reduction product to obtain the target compound Hoffer's chlorosugar without separation, wherein the Hoffer's chlorosugar is 1-choro-3,5-bis-O-p-toluyl-2-deoxy-L-ribose. According to the technology, the total route yield is 42%, and the product purity is greater than 98%.
Owner:FUAN PHARMA LYBON PHARMA TECH
Antimicrobial anticorrosive paint and preparation method thereof
ActiveCN104031442AImprove antibacterial and antiseptic effectGood adhesionAnti-corrosive paintsPolymer scienceAcrylic resin
The invention relates to an antimicrobial anticorrosive paint and a preparation method thereof. The antimicrobial anticorrosive paint is prepared from the following raw materials in parts by mass: 66-70 parts of acrylic resin, 16-23 parts of castor oil, 10-13 parts of vinyl acetate, 4-6 parts of dimethyl carbonate, 15-18 parts of nano silicon micropowder, 2-7 parts of polyacrylamide, 1-6 parts of 3-aminopropyltriethoxysilane, 1-4 parts of N-ethyl-para-toluene sulfonate, 5-10 parts of zinc oxide, 2-6 parts of bamboo charcoal powder, 90-100 parts of water, 20-30 parts of ethanol and 10-14 parts of ethyl acetate. By reasonably compounding the acrylic resin and other additives, the paint has excellent antimicrobial and anticorrosive properties and excellent adhesiveness. By adding the N-ethyl-para-toluene sulfonate and 3-aminopropyltriethoxysilane, the paint has excellent adhesive force, impact resistance and water resistance.
Owner:HEBEI CHENYANG INDAL & TRADE GROUP CO LTD +1
Compound extender for urea-formaldehyde resin
InactiveCN103589366AImprove water resistanceGood dispersionNon-macromolecular adhesive additivesMacromolecular adhesive additivesPolymer scienceTosylhydrazone
The invention discloses a compound extender for urea-formaldehyde resin, which is prepared by mixing the following raw materials in parts by weight: 30-40 parts of bean flour, 20-30 parts of wood flour, 10-15 parts of walnut shell flour, 5-10 parts of corn flour, 20-30 parts of modified attapulgite, 10-15 parts of ammonium chloride, 3-6 parts of para toluene sulfonate and 5-10 parts of desugared sodium lignosulfonate. According to the invention, the modified attapulgite contains carboxyl, so that condensation crosslinking with hydroxymethyl of urea-formaldehyde resin is greatly improved, and the dispersion property in the urea-formaldehyde resin is good, thereby reducing the number of hydrophilic groups and improving the water resistance of adhesion. The extender disclosed by the invention is convenient in adhesive mixing method, can reduce or replace extenders such as flour and the like for use and lower the cost, and has high adhesion strength, good shock strength and large elastic modulus. Through chemical bond combination and adsorption of the modified attapulgite, release of free formaldehyde is reduced.
Owner:安徽省安邦矿物股份有限公司
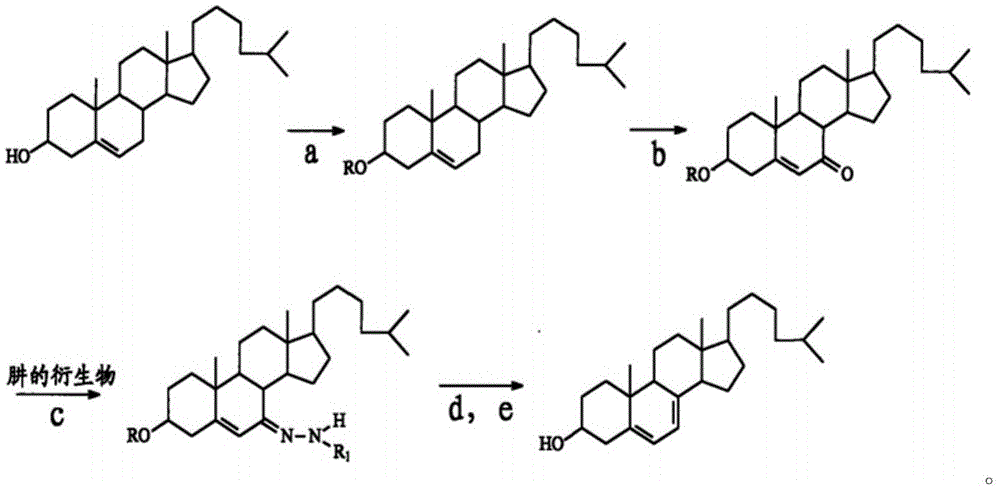
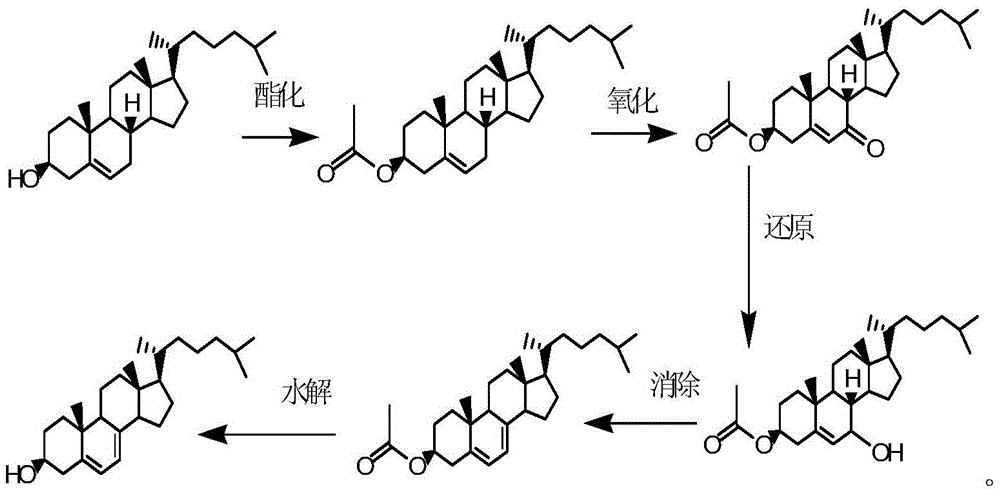
Synthesis method of 25-hydroxycholesteryl acetate-7-p-toluenesulfonylhydrazone
The invention relates to the technical field of medicine chemical engineering and particularly relates to a synthesis method of 25-hydroxycholesteryl acetate-7-p- toluenesulfonylhydrazone. The synthesis method includes following steps: (1) performing a reaction between 25-hydroxy-7-ketocholesteryl acetate and p-toluenesulfonylhydrazide under a mechanical grinding condition; and (2) performing recrystallization to a reaction product in the step (1) to obtain the 25-hydroxycholesteryl acetate-7-p-toluenesulfonylhydrazone. The method is free of an acidic catalyst, is greatly reduced in usage of organic solvent, is simple in operation, is high in yield, is less in waste gas, waste water and solid waste and is simple in post-treatment. The product is easy to separate and purify.
Owner:ZHEJIANG GARDEN BIOCHEM HIGH TECH +2
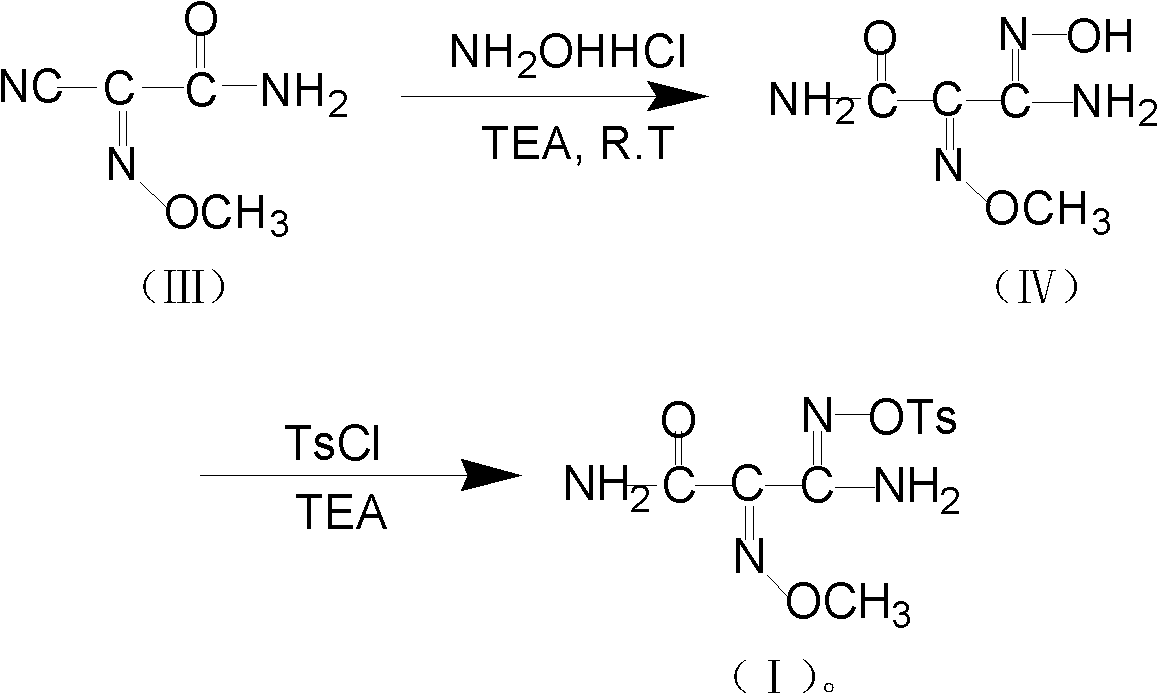
Method for preparing O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime
ActiveCN102093266AShort processReduce manufacturing costSulfonic acid esters preparationSulfonyl chlorideReaction intermediate
The invention relates to a method for preparing O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime, which comprises the following steps: (1) reacting cyanoacetamide, which is used as an initial raw material, with sodium nitrite in a nitrosation mode to obtain a reaction intermediate, and reacting the reaction intermediate with dimethyl sulfate in an esterification mode to obtain 2-cyano-2-methoxyl-imido-acetamide; and (2) under the action of hydroxylamine hydrochloride, hydrolyzing cyano groups in the 2-cyano-2-methoxyl-imido-acetamide obtained in the step (1) into amino groups, and reacting with p-methylbenzene sulfonyl chloride in an esterification mode to generate the O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime. The preparation method provided by the invention has the advantages of accessible raw materials, short technical process and high yield, and is suitable for industrial production.
Owner:BENGBU BBCA MEDICINE SCI DEV
Azido dihydroxypropyl cellulose nitrate preparation method and synthesis
The invention discloses the azido dihydroxypropyl cellulose nitrate preparation method and synthesis which comprises, subjecting natural cotton and cellulose to alkalization under semi-homogeneous phase reaction condition, charging epoxy propyl alcohol for sectionwise etherification to the alkali cellulose, obtaining dihydroxy cellulose ether whose substitution degree is between 0.5-1.2, completely dissolving in dimethyl polyamide / 9% LiCl system, scouring, purifying, drying and dissolving in dimethyl sulfoxide, carrying out homogeneous phase nitrine at high temperature.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY +1
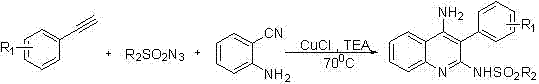
Method used for preparing 4-aminoquinoline derivative
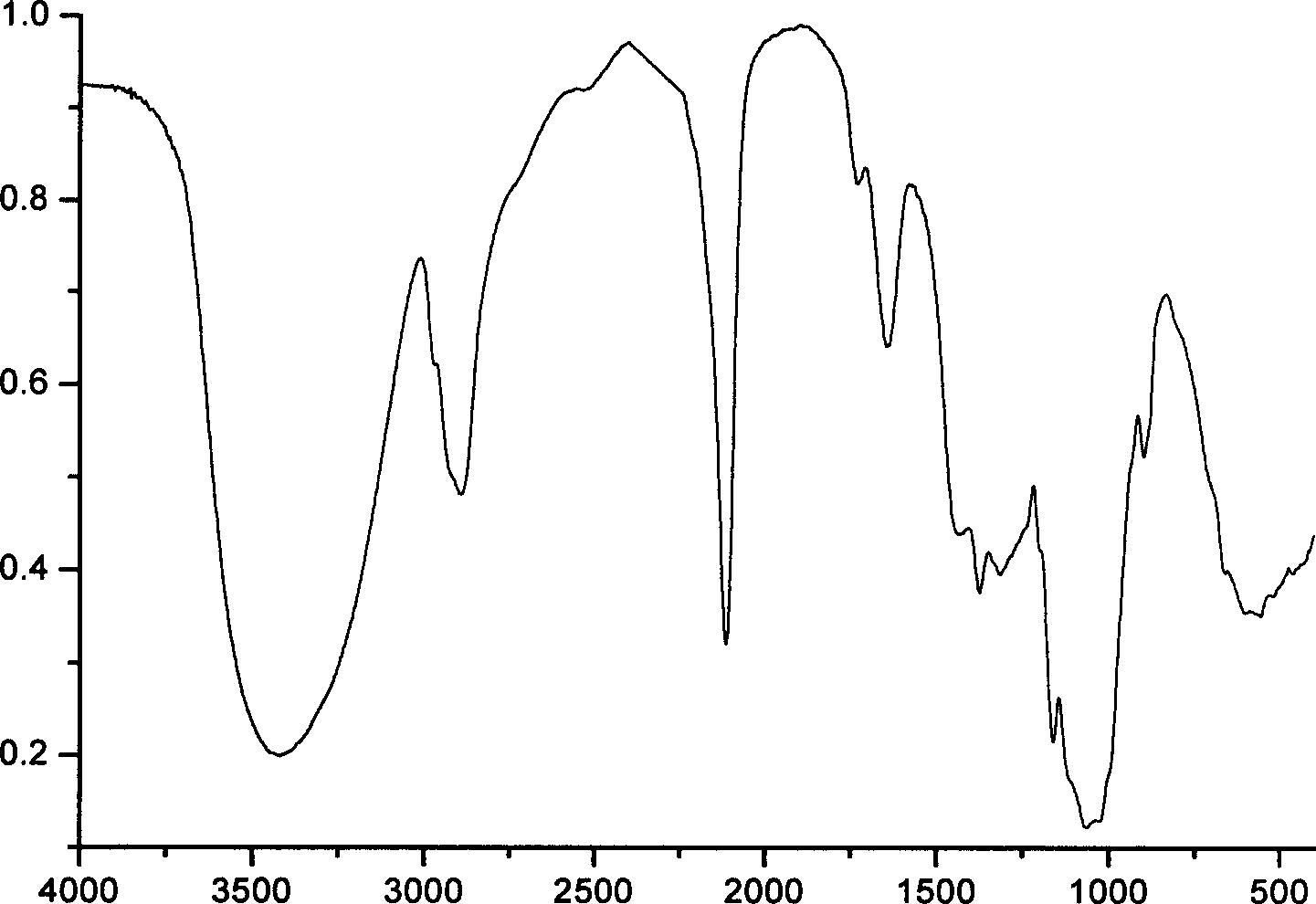
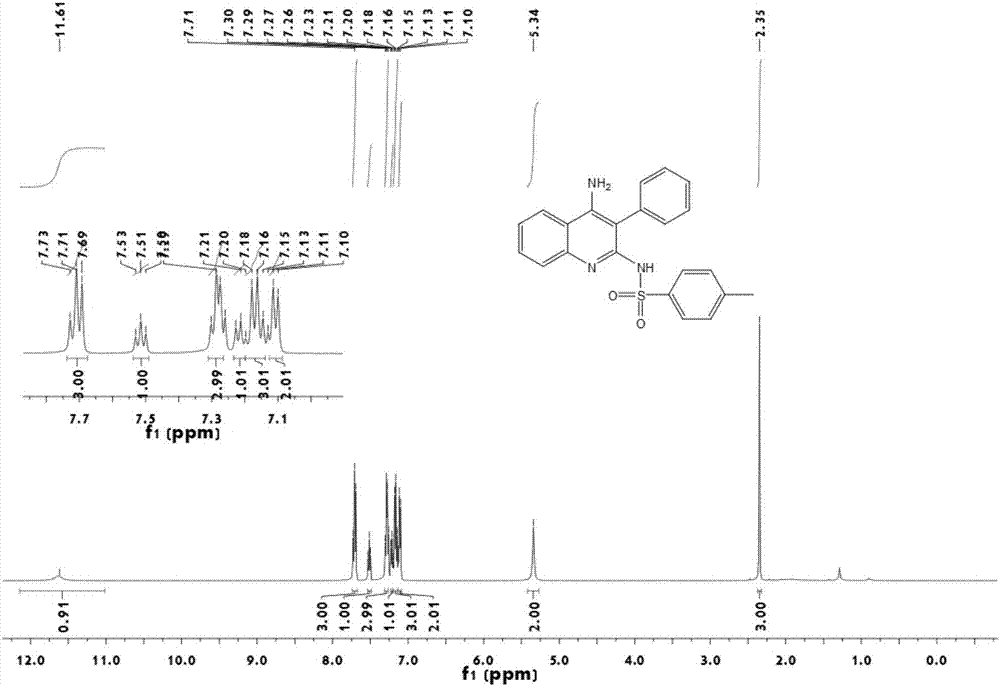
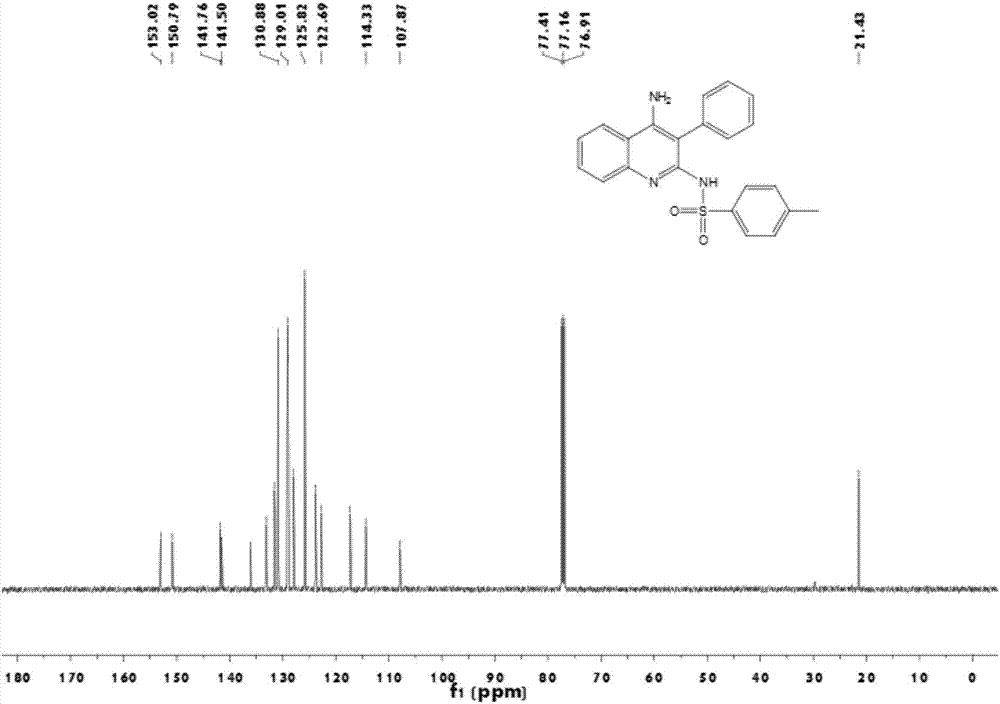
InactiveCN106866528ASignificant technological progressImprove reaction efficiencyOrganic chemistryEthylenediamineAlkyne
The invention discloses a method used for preparing a 4-aminoquinoline derivative. According to the method, an acuprous catalyst, an additive, and o-aminobenzontrile are delivered into a reaction container, wherein the additive is one ingredient selected from triethylamine, tetramethyl ethylenediamine, 1,8-diazabicyclo undec-7-ene, potassium carbonate, or cesium carbonate at will; an organic solvent is added; an alkyne-terminated compound and p-toluenesulfonyl azide are introduced into the reaction container; an obtained mixture is subjected to heating reaction for 3h to 6h at a temperature ranging from room temperature to 100 DEG C, wherein reaction process is monitoring via thin-layer chromatography, and optimal reaction conditions are obtained via selection; after reaction, an obtained mixture is filtered, obtained filter cake is washed, obtained filtrate is mixed, and is subjected to condensation and column chromatography separation so as to obtain the 4-aminoquinoline derivative. One-pot method is adopted to prepare the 4-aminoquinoline derivative, reaction efficiency is increased greatly, post-treatment is simple; and industrialized application prospect is promising.
Owner:SHANGHAI INST OF TECH
1-aryl-2 perluoro or polyfluoro phenyl ethylene and its derirative, its synthesis and application
InactiveCN1475471ACarboxylic acid nitrile preparationOrganic compound preparationTosylhydrazoneCarbene
A 1-aryl-2 perfluoro (or polyfluoro) phenylethene and its derivative used as fluoric organic luminescent material or electrically conductive polymer is prepared from perfluoro (or polyfluoro) phenylformaldehyde through condensating with toluenesulfonyl diamine, dehydrogenating by sodium hydride, reacting on phase-transfer catalyst to generate perfluoro (or polyfluoro) phenyldiazo, catalytic decomposing by catalyst to generate metal carbene, capturing it by triphenyl (or trialkyl) arsenic, and condensating reaction on aldehide.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
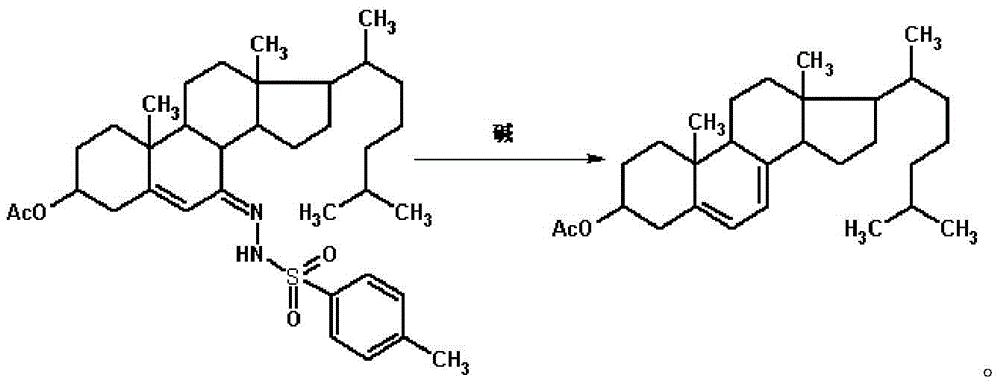
Method for preparing 7-dehydrogenized cholesteryl ester from 7-tosylhydrazones-3-cholesteryl ester
InactiveCN105646632AMild reaction conditionsShort reaction timeOrganic-compounds/hydrides/coordination-complexes catalystsSteroidsHydrazonePolystyrene
The invention discloses a method for preparing 7-dehydrogenized cholesteryl ester from 7-tosylhydrazones-3-cholesteryl ester. In an existing method, cholesteryl ester is subjected to esterification, oxidation, hydrazone compounding, hydrazone removing and hydrolyzation to obtain 7-dehydrogenized cholesteryl ester, wherein a hydrazone removing reaction is a typical heterogeneous reaction and is only carried out on the solid surface of an alkali reagent; consequently, the reaction selectivity and the product yield are not high, and thus the production cost is high. The method is characterized in that a crown ether phase transfer catalyst supported by polystyrene resin is adopted for a hydrazone removing reaction. By means of the method, a heterogeneous reaction is converted into a homogeneous reaction, the reaction selectivity is improved, and the conversion rate can be remarkably increased, and the production cost is lower while the product quality is improved. Meanwhile, crown ether obtained after modification and immobilization can be applied repeatedly, and thus the toxicity of common crown ether on the environment is avoided.
Owner:ZHEJIANG NHU CO LTD
<18>F-labeled aggregation-induced emission (AIE) fluorescent/positron emission tomography (PET) dual-mode probe, and preparation method and application thereof
ActiveCN109867591AStrong resistance to photobleachingStrong penetrating powerOrganic compound preparationSulfonic acid esters preparationSynthesis methodsDual mode
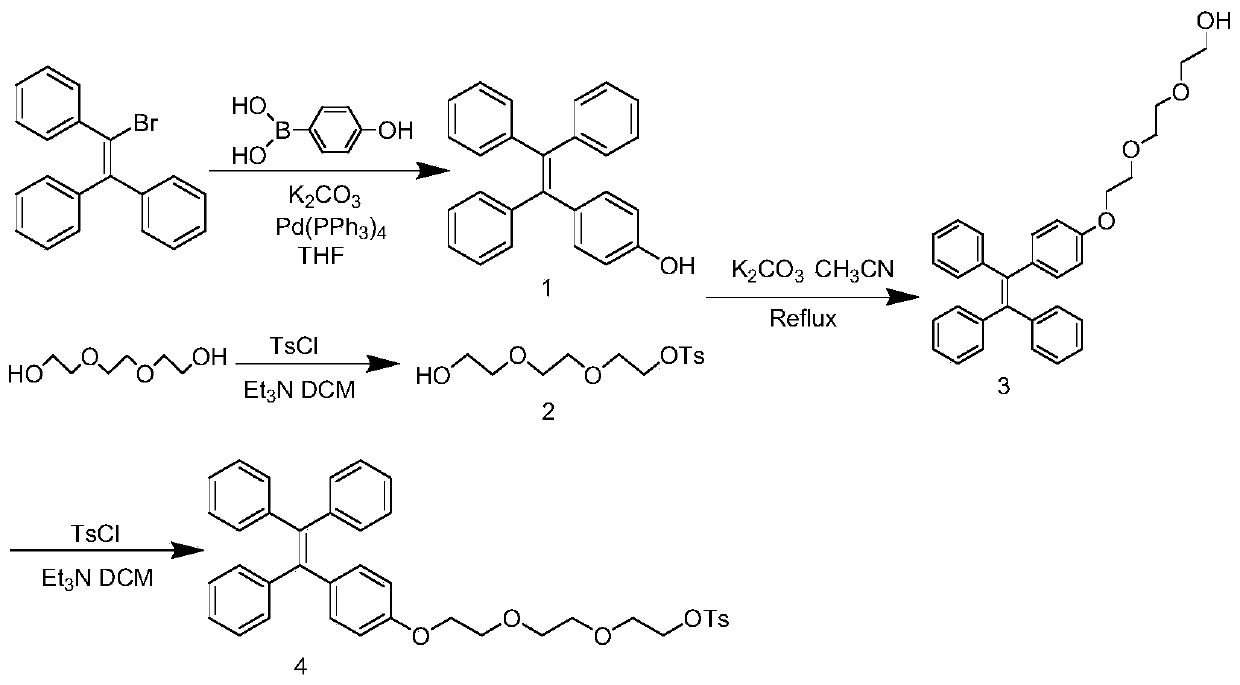
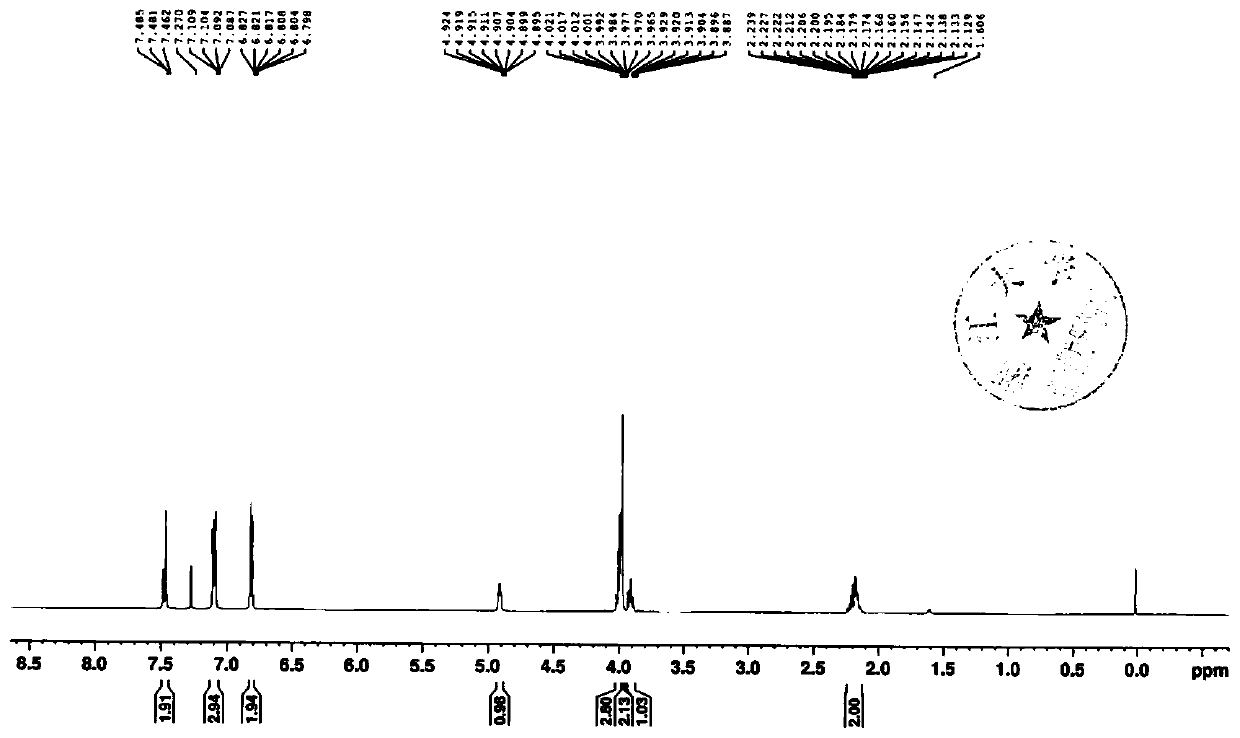
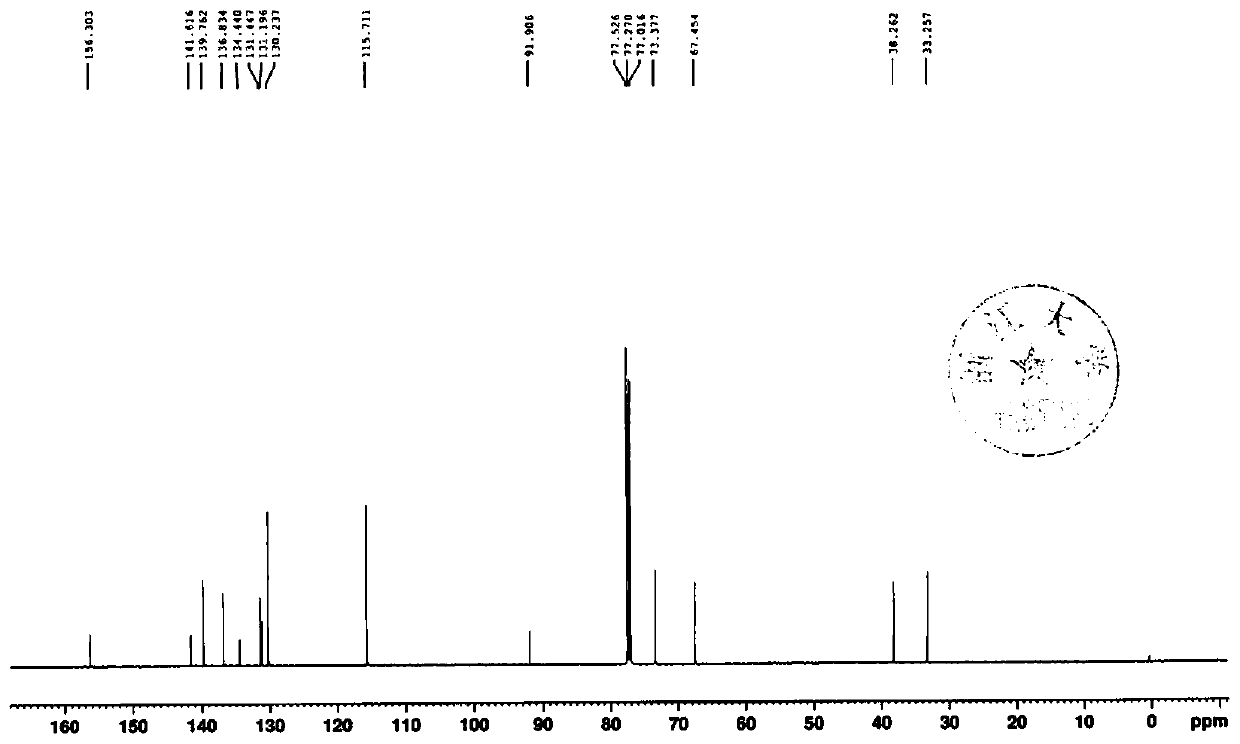
The invention discloses an <18>F-labeled aggregation-induced emission (AIE) fluorescent / positron emission tomography (PET) dual-mode probe, and a preparation method and application thereof. The compound is denoted as <18>F-TPE-TEG, and has a following structure. The synthesis method of a labelled precursor of the compound comprises the following steps: firstly, adding 2-bromo-1,1,2-triphenylethylene, 4-hydroxyphenylboronic acid and tetrabutylammonium bromide into tetrahydrofuran, and taking K2CO3 and tetrakis(triphenylphosphine)palladium as catalysts to carry out a reaction to obtain 4-hydroxytetraphenylethylene; then dissolving triethylene glycol, triethylamine and p-toluenesulfonyl chloride in dichloromethane, and carrying out a reaction to obtain 8-p-toluenesulfonyloxy-3,6-dioxyoctanol;adding the 4-hydroxytetraphenylethylene, K2CO3, the 8-p-toluenesulfonyloxy-3,6-dioxyoctanol into acetonitrile, and carrying out a reaction to obtain 8-tetraphenylethyleneoxy-3,6-dioxyoctanol; and finally, dissolving the 8-tetraphenylethyleneoxy-3,6-dioxyoctanol, p-toluenesulfonyl chloride and triethylamine in dichloromethane, and carrying out a reaction to obtain the labelled precursor. The <18>F-labeled compound can be used as an AIE fluorescent / PET dual-mode probe to be applied to tumor imaging research.
Owner:ZHEJIANG UNIV
Empagliflozin intermediate preparation method
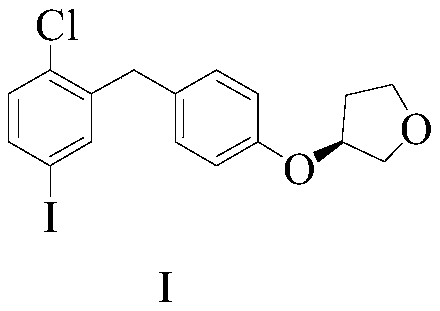
The invention discloses an empagliflozin intermediate preparation method, which comprises: carrying out substitution by using p-methoxybenzyl chloride and p-iodoaniline as starting raw materials to obtain a compound IV, performing diazotization and a Sandmeyer reaction to obtain a compound III, further performing demethylation under the action of boron tribromide to obtain a compound II, and finally performing condensation with (S)-3-p-toluenesulfonyloxy tetrahydrofuran to obtain the target compound I, wherein the product purity is greater than or equal to 99.0%. According to the invention, the preparation method has advantages of simple and easily available raw materials, low cost, simple operation steps, simple post-treatment and high product yield, and is suitable for industrial production.
Owner:ZHEJIANG HUAYI PHARMA CO LTD OF HANGZHOU HUADONG PHARMA GRP +1
Synthesis and application of L-phenylalanine derived Beta-cyclodextrin bonded silica gel for separating alanine enantiomer
InactiveCN103193898AGood chiral separation effectImprove adsorption capacityOther chemical processesOrganic compound preparationCyclodextrinOrganosolv
The invention belongs to the field of analytic chemistry, relates to synthesis of a chiral column material, and in particular relates to synthesis and an application of L-phenylalanine derived Beta-cyclodextrin bonded silica gel for separating an alanine enantiomer. According to the technical scheme, the synthesis process comprises the following steps: firstly, preparing activated silica gel; then synthesizing Beta-cyclodextrin bonded silica gel; next, synthesizing tosylated Beta-cyclodextrin bonded silica gel; and finally, derivatizing an obtained product by using L-phenylalanine. The prepared L-phenylalanine derived Beta-cyclodextrin bonded silica gel is yellow solid powder which is insoluble in water and organic solvents such as N,N-dimethyl formamide and the like as well as difficult to dissolve in acid solutions. If the synthesized L-phenylalanine derived Beta-cyclodextrin bonded silica gel is served as a chromatographic filling material to perform adsorption separation on an aqueous solution containing the alanine enantiomer at the constant temperature and under the normal pressure, the good chiral separation effect can be achieved. When the L-phenylalanine derived Beta-cyclodextrin bonded silica gel is used for separating the alanine enantiomer, the operation is simple, the cost is low and the adsorption effect is good. Therefore, the L-phenylalanine derived Beta-cyclodextrin bonded silica gel has a definite practical value.
Owner:JIANGSU UNIV
2-(1,2,4-triazole-1-methyl)-2-(coumarone-5-radical)-1,3-dioxolane and application thereof
The invention relates to 2-(1,2,4-triazole-1-methyl)-2-(coumarone-5-radical)-1,3-dioxolane with a chemical formula I shown in the description, where R is selected from C1-C2 alkyl radical and C3-C4 linear alkyl radical or branched alkyl radical, R1 selected from H and C1-C2 alkyl radical, X selected from chlorine,bromine,iodine, mesyloxy,trifluoro-mesyloxy, benzenesulfonyl oxy radical, and p-toluenesulfonyl oxy radical, and Y selected from hydrogen, C1-C2 alkoxy, C3-C4 linear alkoxy or branched alkoxy, chlorine, bromine, fluorine or iodine. The chemical formula I is prepared from a key intermediate II of a chemical formula II shown in the description; and a compound I can be applied to preparation of bactericide.
Owner:HUNAN UNIV
Foaming printing ink
The invention relates to foaming printing ink which comprises the following components in parts by mass: 5-30 parts of para toluene sulfonate, 10-25 parts of azodicarbonamide, 0.1-0.2 parts of defoaming agent, 15-30 parts of ethanol amine, 1-2 parts of cross-linking agent, 50-60 parts of polyvinyl chloride, 60-80 parts of polyvinylidene chloride emulsion, 5-10 parts of epoxy glycol, 0.5-1 part of thickener, 10-20 parts of ethylene glycol, and 1-3 parts of water dispersing pigment. The foaming printing ink is stable to store, nontoxic, odorless, difficult to damage and low in cost, has a three-dimensional effect, improves a decorative art effect, can shorten a technological flow, can lower the printing cost, and can be printed in quantity quickly.
Owner:苏州凹凸彩印厂
Pleuromutilin derivative with thiadiazole skeleton, as well as preparation method and applications thereof
ActiveCN103319437ARaw materials are easy to getLow priceAntibacterial agentsOrganic active ingredientsBiochemical engineeringChemical compound
The invention discloses a pleuromutilin derivative with a thiadiazole skeleton and a preparation method thereof. The preparation method comprises the following steps: 1, synthesizing 22-O-(4-tosyl)acetoxyl mutilin; 2, synthesizing 14-O-( iodoacetyl)mutilin; 3, synthesizing 14-O-[(4, 2-amino-1-thiadiazole-5yl)thioacetyl]mutilin; 4, synthesizing an end product. The compound synthesis method is easily available in raw materials, cheap in price, simple in operation, easy in product separation and purification and high in yield, wherein the total yield is 32-40%.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
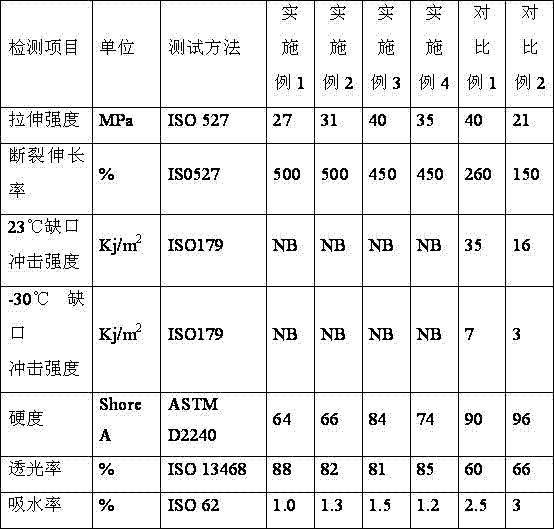
Transparent nylon elastomer and preparation method thereof
The invention discloses a transparent nylon elastomer, which is prepared from the following components in parts by weight: 35-80 parts of nylon, 8-50 parts of a polystyrene-b-poly(ethylene / ethylene / propylene)-b-polystyrene block copolymer, 1-20 parts of N,N-dimethyl-p-toluenesulfonamide, 1-35 parts of paraffin oil, 1-35 parts of naphthenic oil, 1-15 parts of maleic anhydride-grafted SEBS / SEEPS, 0.1-2 parts of an antioxidant 1098, 0.1-2 parts of a lubricant, 0.1-2 parts of a nucleating agent and 0.1-2 parts of a light stabilizer; the relative viscosity of the nylon is 2.1-4.0; the grafting rate of the maleic anhydride-grafted SEBS / SEEPS is 0.8% or more; and the lubricant is a lubricant EB-FF or polyethylene wax. The light transmittance of the transparent nylon elastomer is high, the elongation at break reaches 450 or over, the transparent nylon elastomer has the non-breaking characteristic in notch impact at -30 DEG C, and the harness reaches a relatively wide interval of 50-95 (shore A).
Owner:中广核高新核材科技(苏州)有限公司
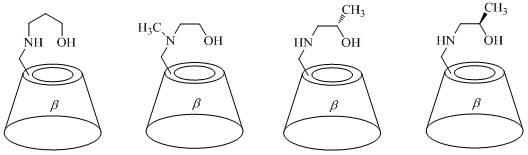
Beta-cyclodextrin derivative and preparation method and application thereof
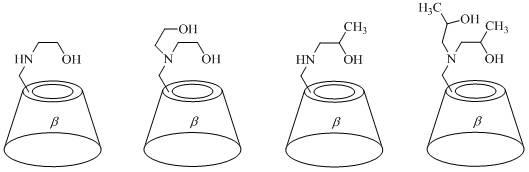
InactiveCN102627704AHigh
<i>ee</i>
%valueHigh purityAsymmetric synthesesCatalytic oxidationEthyl group
The invention discloses a beta-cyclodextrin derivative and a preparation method and application thereof. The beta-cyclodextrin derivative is a single [6-(2-hydroxy ethyl) amino-6-desoxy]-beta-cyclodextrin, single [6-di(2-hydroxy ethyl) amino-6-desoxy]-beta-cyclodextrin, single [6-(2-hydroxy propyl) amino-6-desoxy]-beta-cyclodextrin, single [ 6 di(2-hydroxy propyl ) amino-6- desoxy]-beta-cyclodextrin, single [6-(3-hydroxy propyl) amino-6-desoxy)-beta-cyclodextrin, single [6-methyl ( 2-hydroxy ethyl ) amino-6-desoxy]-beta-cyclodextrin, single {6- [(2S)-2-hydroxy propyl] amino-6-desoxy}-beta-cyclodextrin and single {6[(2R)-2-hydroxy propyl] amino-6- desoxy}-beta- yclodextrin. The beta-cyclodextrin derivative can be prepared by a nucleophilic substitution reaction of single (6-O-p-toluene sulfonyl)-beta-cyclodextrin and corresponding aminoalcohol. The synthesis method has the advantages of simple operation, mild reaction conditions, simple purification, high yield and good purity of object products. The beta-cyclodextrin derivative can be used as a water-soluble ligand of metal mimic enzyme in aqueous metallic catalytic oxidation and aqueous metallic catalytic reduction reaction.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com