Empagliflozin intermediate preparation method
A technology for intermediates and compounds, which is applied in the field of preparation of an intermediate of empagliflozin--3-phenoxy)tetrahydrofuran, can solve the problem of high raw material cost, and achieves reduced raw material cost, simple post-processing and little environmental pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
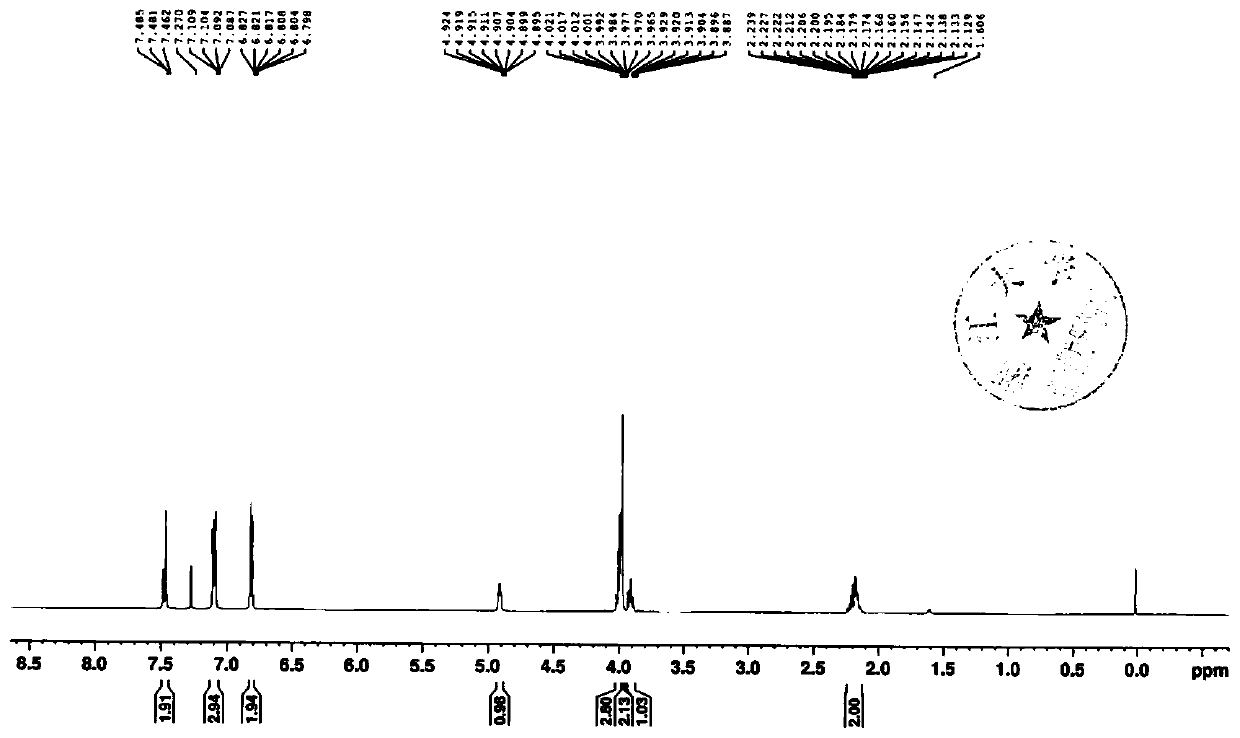
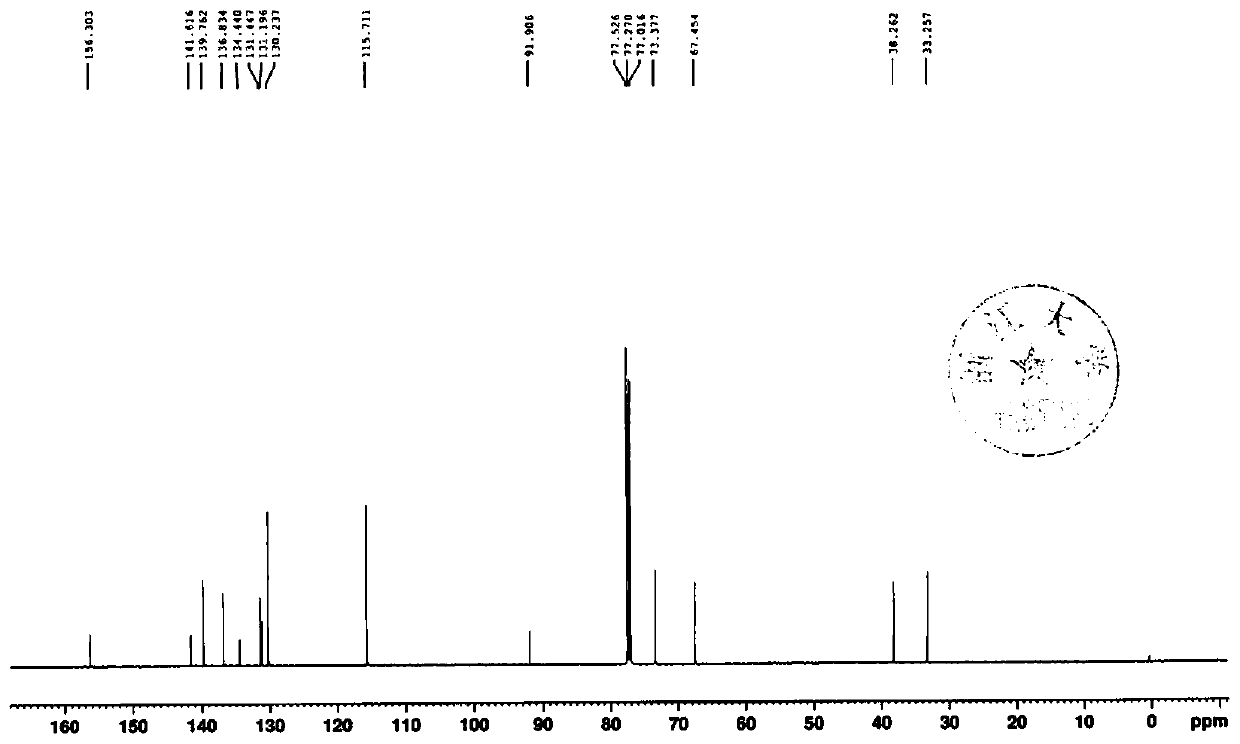
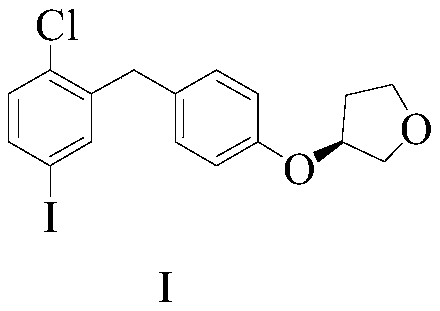
[0049] The invention provides a preparation method of an empagliflozin intermediate. The preparation method uses p-methoxybenzyl chloride and p-iodoaniline as starting materials, and obtains compound IV through substitution, and then undergoes diazotization and Sander Mayer reaction to obtain compound III, further demethylation under the action of boron tribromide to obtain compound II, and finally condensation with (S)-3-p-toluenesulfonyloxytetrahydrofuran to obtain empagliflozin intermediate compound I, specifically including Follow the steps below:
[0050] 1) With p-methoxybenzyl chloride and p-iodoaniline as raw materials, through substitution reaction, the compound 4-iodo-2-(4-methoxybenzyl)aniline shown in formula IV is obtained:
[0051]
[0052] 2) The compound shown in IV is subjected to diazotization and Sandmeyer reaction to obtain compound 1-chloro-4-iodo-2-(4-methoxybenzyl)benzene shown in formula III:
[0053]
[0054] 3) The compound shown in formula III...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com