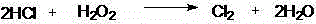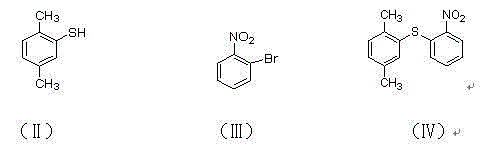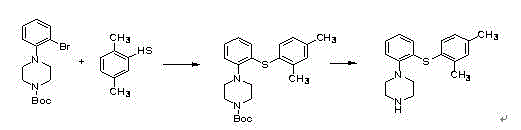Patents
Literature
65 results about "Sandmeyer reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
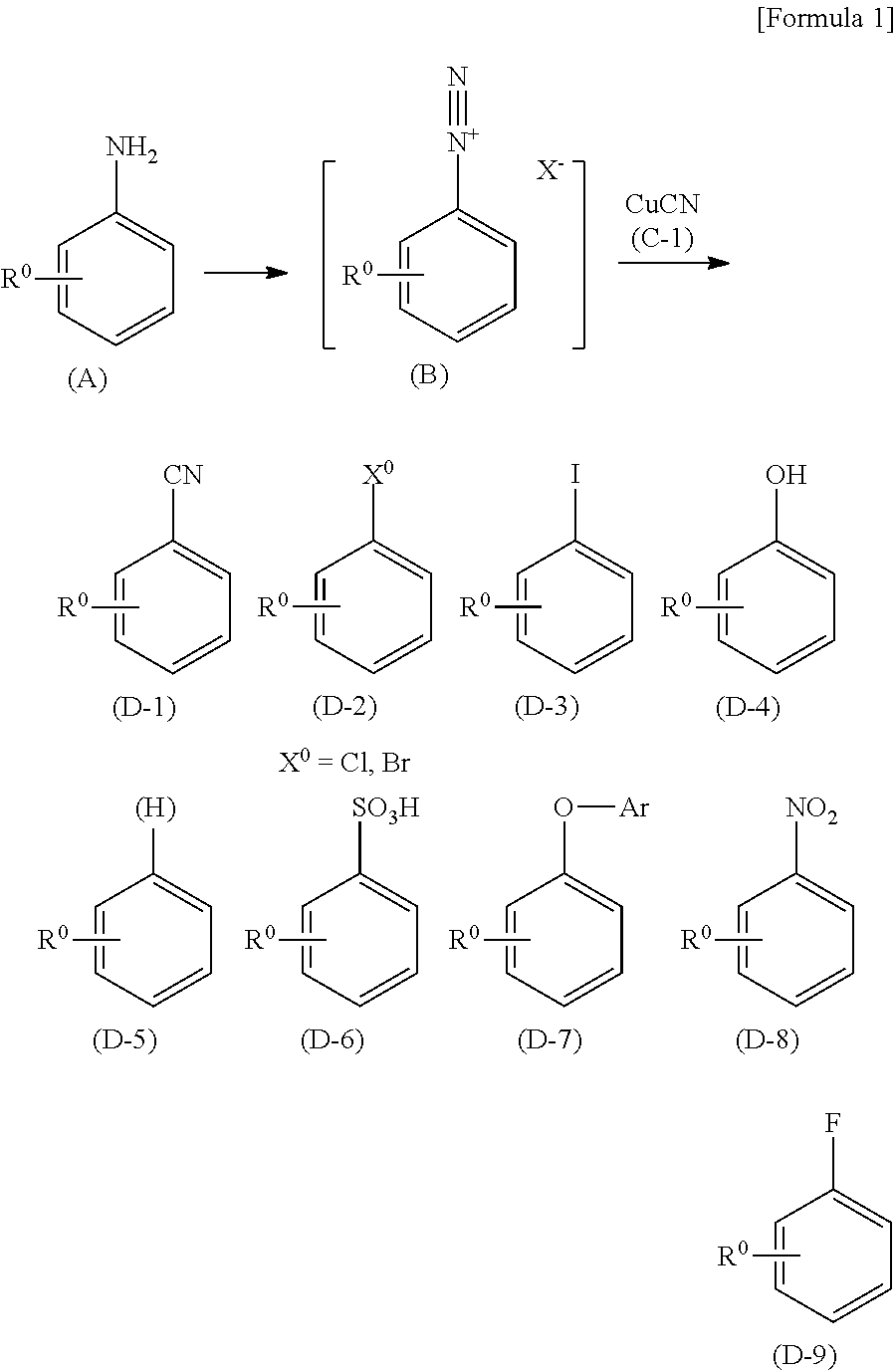
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts. It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation.
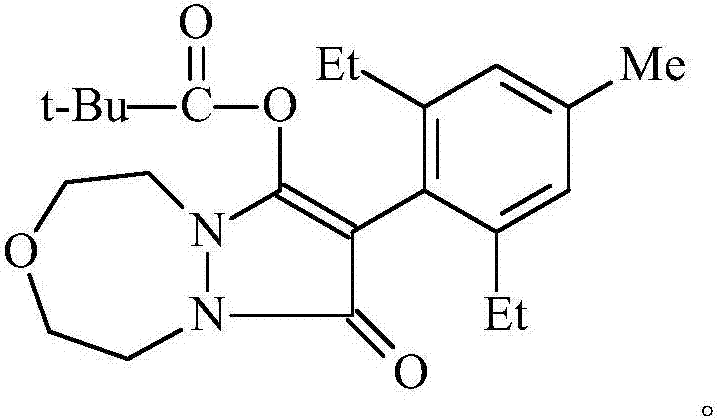
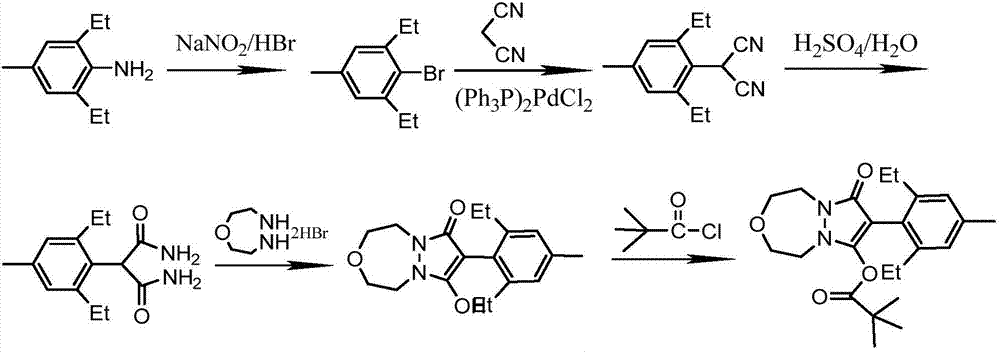
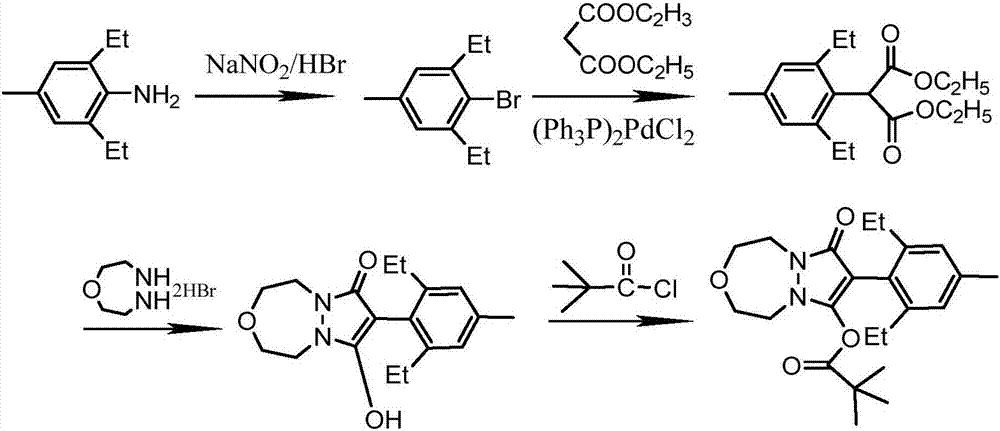
Preparation method of pinoxaden
InactiveCN106928253AReduce pollutionEmission reductionOrganic chemistrySandmeyer reactionMethylaniline
The invention relates to a method for preparing pinoxaden. The steps are as follows: 2,6-diethyl-4-methylaniline undergoes a sandmeyer reaction to obtain 2,6-diethyl-4-methylbromobenzene, and then reacts with Malononitrile is coupled under the catalysis of copper iodide to obtain 2,6-bis-ethyl-4-methylphenylmalononitrile, and finally hydrolyzed in hydrogen peroxide solution to obtain 2,6-diethyl-4 -Methylphenylmalonamide; subsequent reaction with [1,4,5]-oxadiazepine dihydrobromide in the presence of triethylamine gives 8-(2,6-diethyl- 4-methylbenzene)tetrahydropyrazole[1,2d][1,4,5]-oxadiazepine-7,9-dione, preferably reacted with pivaloyl chloride to obtain pinoxaden ester. The present invention uses low-priced catalysts to reduce production costs. In addition, hydrogen peroxide-alkali is used as the hydrolysis system in the preparation process, which greatly reduces environmental pollution.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
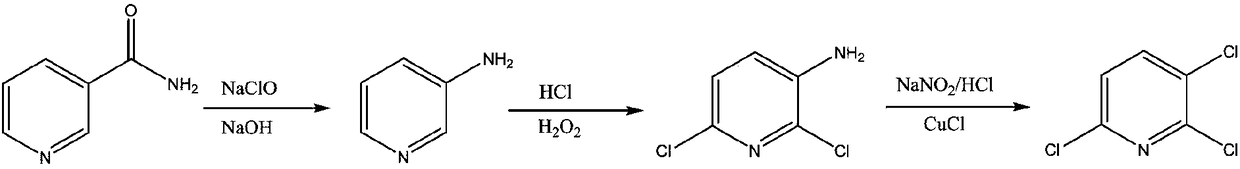
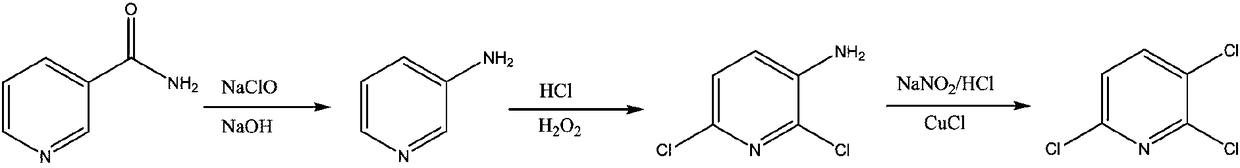
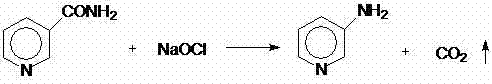
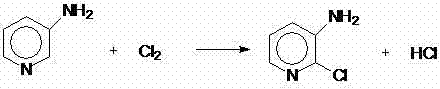
Preparation method for 2,3-dichloropyridine
ActiveCN103570609AReduce generationSave energyOrganic chemistrySandmeyer reactionBULK ACTIVE INGREDIENT
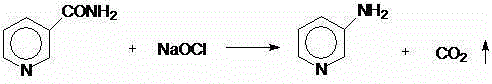
The invention discloses a preparation method for 2,3-dichloropyridine. The preparation method creatively comprises the steps of reacting nicotinamide serving as an active ingredient with sodium hypochloride to prepare 3-aminopyridine, distilling to remove water, extracting with dichloromethane to recover 3-aminopyridine, wherein the yield of the 3-aminopyridine is more than or equal to 93%; reacting the 3-aminopyridine hydrochloric acid solution with hydrogen peroxide to obtain 2-chlorino-3-aminopyridine hydrochloric acid solution, wherein the yield of the 2-chlorino-3-aminopyridine is more than or equal to 85%; then performing diazotization and sandmeyer reaction to obtain 2,3-dichloropyridine. The preparation method is less in side reaction, an intermediate product is not required for refining, and the product is high in content and yield, simple in technology and easy to operate.
Owner:NANTONG TENDENCI CHEM
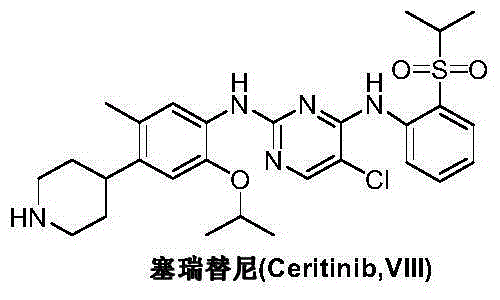
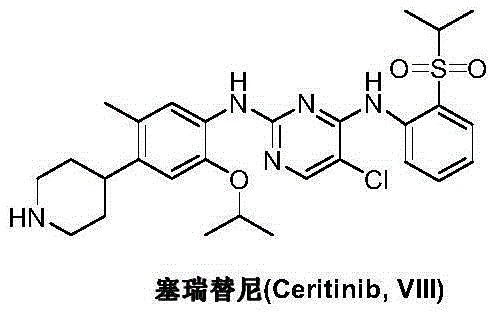
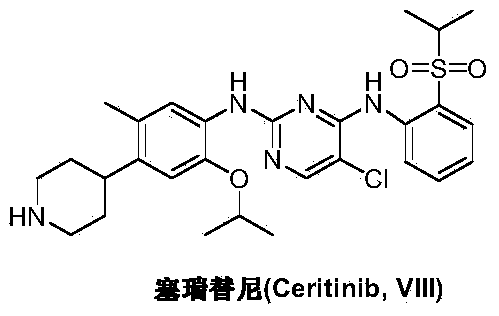
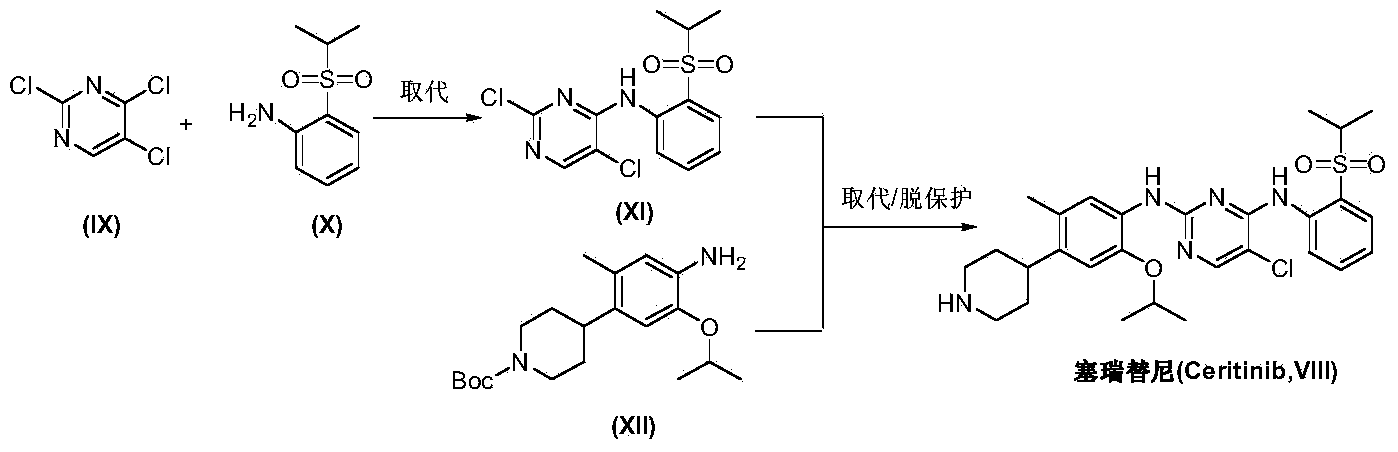
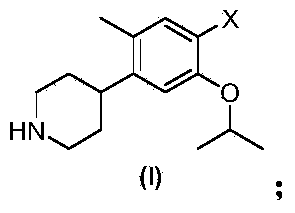
Preparation method of ceritinib and intermediate
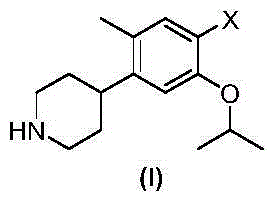
The invention discloses an intermediate 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) for preparing ceritinib and a preparation method thereof. The preparation method comprises the following steps: carrying out catalytic hydrogenation on the raw material 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine (II) to obtain 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)aniline (III); and carrying out Sandmeyer reaction on the compound (III) to obtain the 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I). The invention also discloses a preparation method of ceritinib. The 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) used as the raw material is sequentially subjected to substitution, reduction and substitution reaction to obtain the ceritinib (VIII). The preparation method has the advantages of simple technique, mild conditions and fewer side reactions, and is suitable for industrial amplification.
Owner:SCI GENERAL MATERIAL & CHEM
Preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene
InactiveCN104311532AHigh reaction yieldThree wastes lessOrganic chemistrySandmeyer reactionBoronic acid
The invention discloses a preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene. The 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene is prepared by performing twice Suzuki cross-coupling reactions between 5-nitro-2-methyl-benzoyl chloride and 5-bromothiophene-2-boronic acid first and then between a reaction product and 4-fluorophenylboronic acid, a reducing reaction and a Sandmeyer reaction. The preparation method belongs to the field of preparation of medicine intermediates. The preparation method can be used for preparing a medicine Invokana for treating type-II diabetes. The preparation method has the characteristics of simple operation, good process stability, high yield, few solid, liquid and gas wastes, low production cost and good product quality.
Owner:SHANDONG UNIV
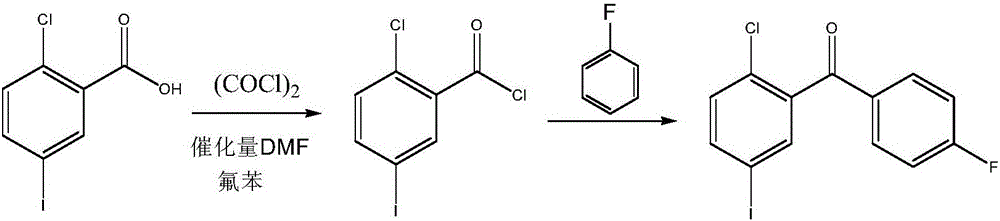
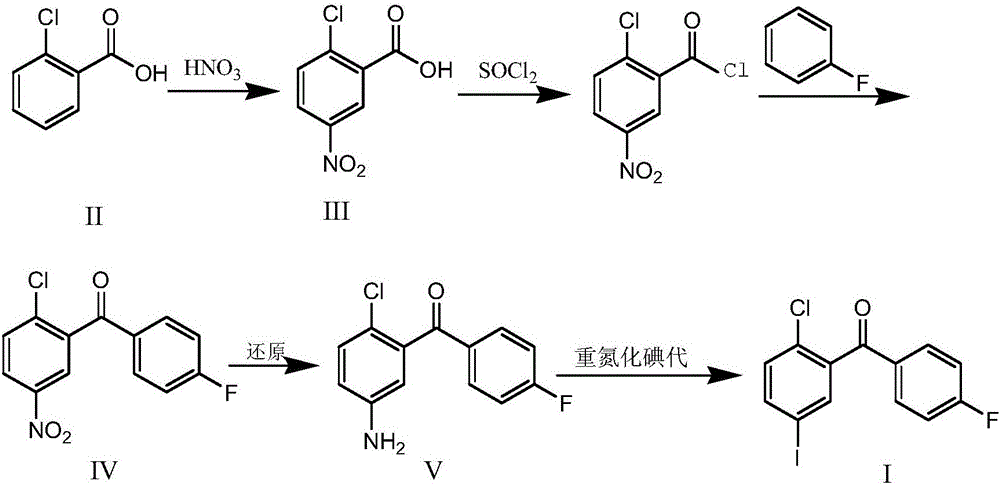
Synthesis method for (2-chloro-5-iodophenyl)(4-fluorophenyl)ketone
ActiveCN106699570AEasy to getIncrease profitOrganic compound preparationCarbonyl compound preparationSandmeyer reactionNitration
The invention discloses a synthesis method for (2-chloro-5-iodophenyl)(4-fluorophenyl)ketone. According to the method, with cheap o-chlorobenzoic acid as a starting material, (2-chloro-5-iodophenyl)(4-fluorophenyl)ketone is obtained by nitration, Friedel-Crafts acylation and reduction and finally by Sandmeyer reaction for iodination. The materials used by the method are cheap and easy to obtain, and the method adopting Sandmeyer reaction for iodination can increase the iodine utilization rate, and has the characteristics of simplicity in operation, high yield, high purity and suitability for industrialized production. (The chemical formula is shown in the specification).
Owner:SHANDONG BOYUAN PHARM CO LTD
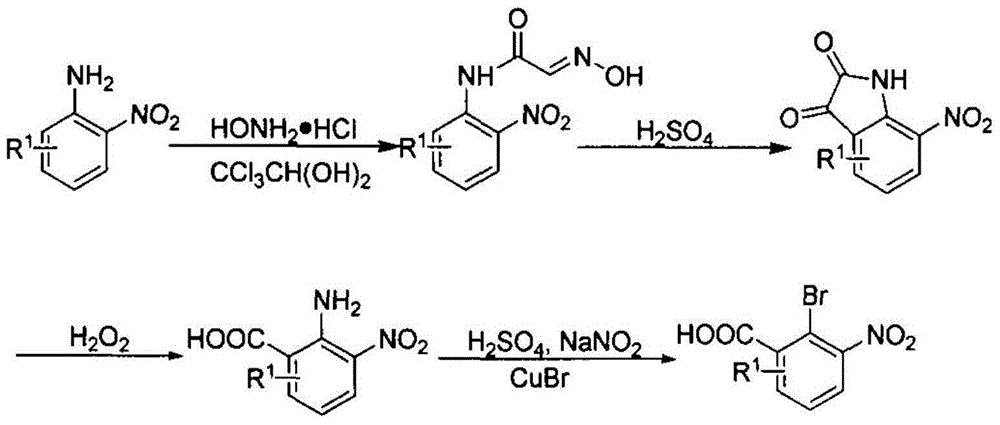
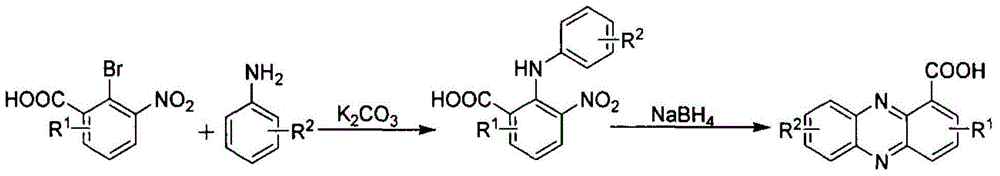
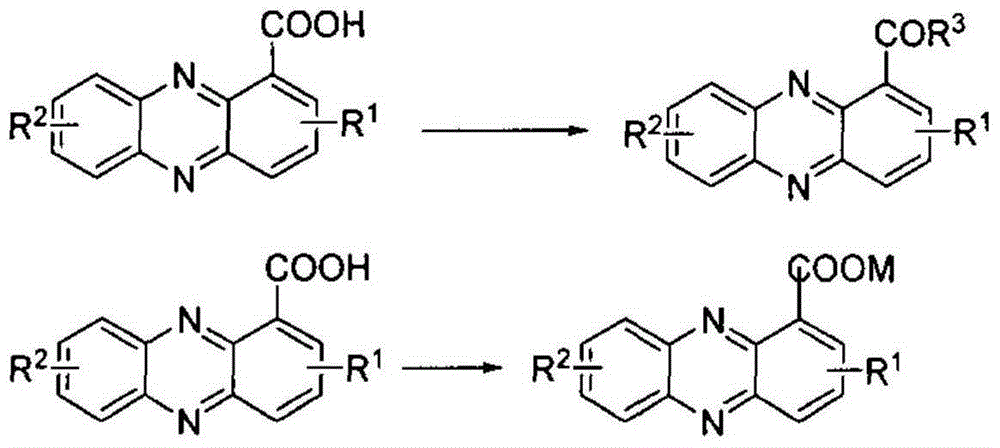
Preparation method for phenazine-1-carboxylic acid
ActiveCN104829544AReaction raw materials are readily availableHigh yieldOrganic chemistrySandmeyer reactionHydroxylamine
The invention relates to a preparation method for phenazine-1-carboxylic acid. The preparation method comprises the following steps: reacting aniline with chloral hydrate and hydroxylamine to produce alpha-oximidoacetanilide, treaing alpha-oximidoacetanilide with concentrated sulfuric acid to obtain isatin, reacting isatin with hydrogen peroxide so as to obtain 2-amino-3-nitrobenzoic acid and then carrying out Sandmeyer reaction to prepare 2-bromo-3-nitrobenzoic acid; and subjecting prepared 2-bromo-3-nitrobenzoic acid and aniline to Jourdan-Ullmann reaction so as to obtain substituted diphenylamine and carrying out ring closure to prepare phenazine-1-carboxylic acid. Compared with the prior art, the preparation method provided by the invention has the advantages of easy availability of raw materials, easy control of reaction, mild reaction conditions, easy post-treatment, high overall yield, as high as 32 to 47%, and suitability for industrial production; and compared with conventional method for production of shenqinmycin from ferment powder, the method provided by the invention enables cost to be greatly reduced.
Owner:SHANGHAI TAIHE INT TRADE CO LTD +1
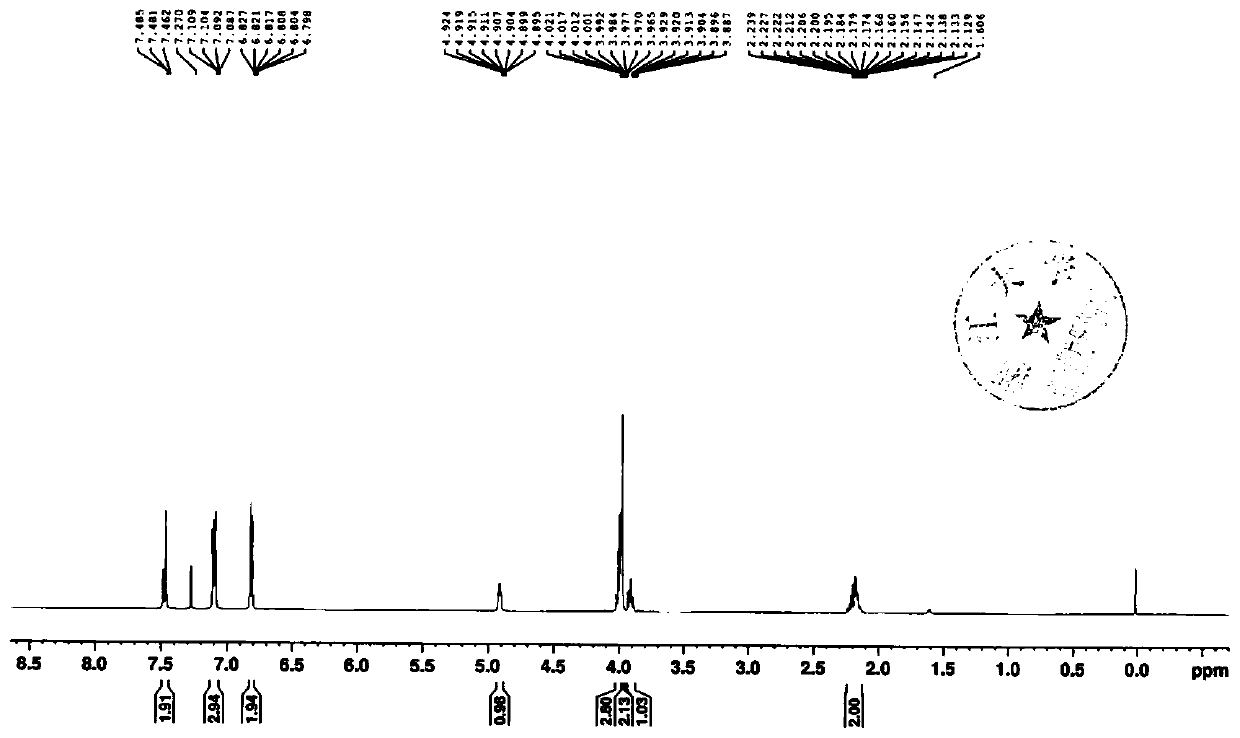
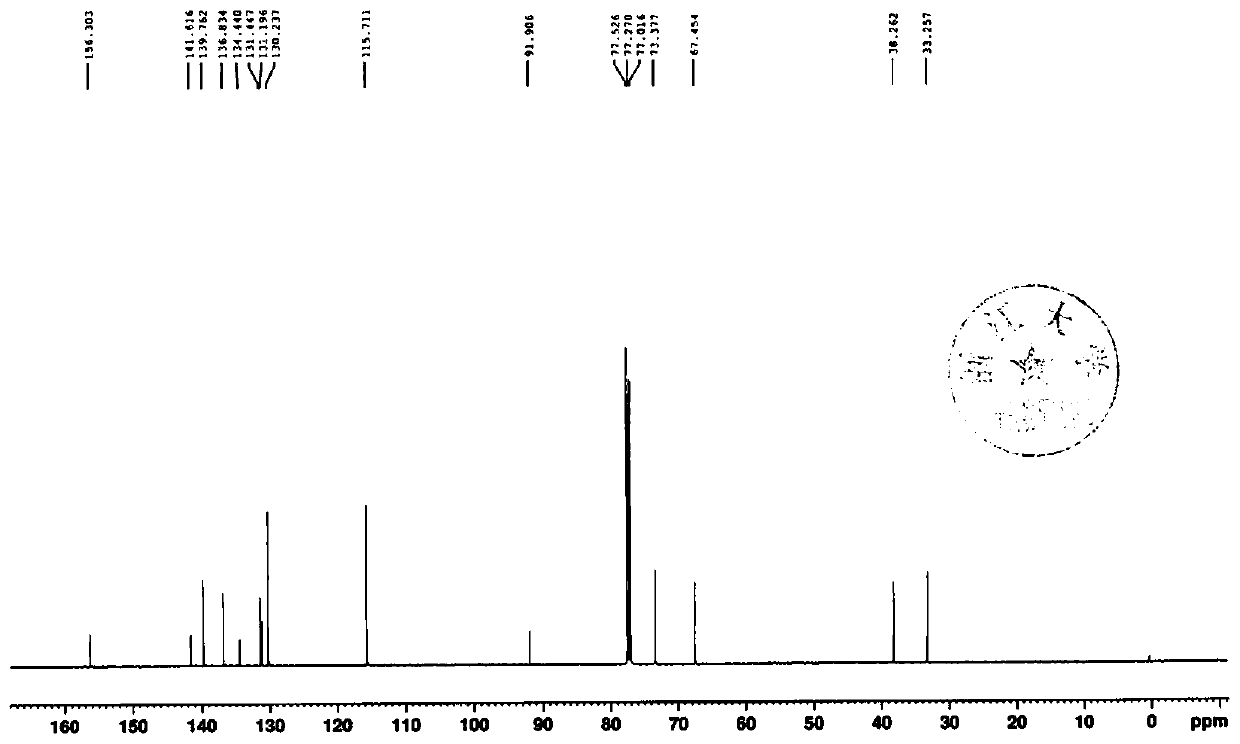
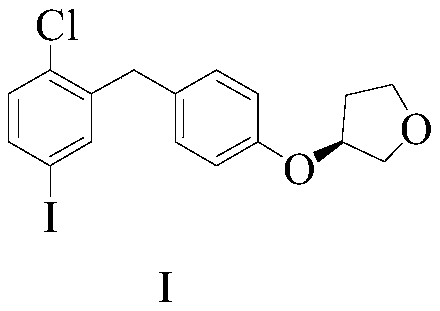
Empagliflozin intermediate preparation method
The invention discloses an empagliflozin intermediate preparation method, which comprises: carrying out substitution by using p-methoxybenzyl chloride and p-iodoaniline as starting raw materials to obtain a compound IV, performing diazotization and a Sandmeyer reaction to obtain a compound III, further performing demethylation under the action of boron tribromide to obtain a compound II, and finally performing condensation with (S)-3-p-toluenesulfonyloxy tetrahydrofuran to obtain the target compound I, wherein the product purity is greater than or equal to 99.0%. According to the invention, the preparation method has advantages of simple and easily available raw materials, low cost, simple operation steps, simple post-treatment and high product yield, and is suitable for industrial production.
Owner:ZHEJIANG HUAYI PHARMA CO LTD OF HANGZHOU HUADONG PHARMA GRP +1
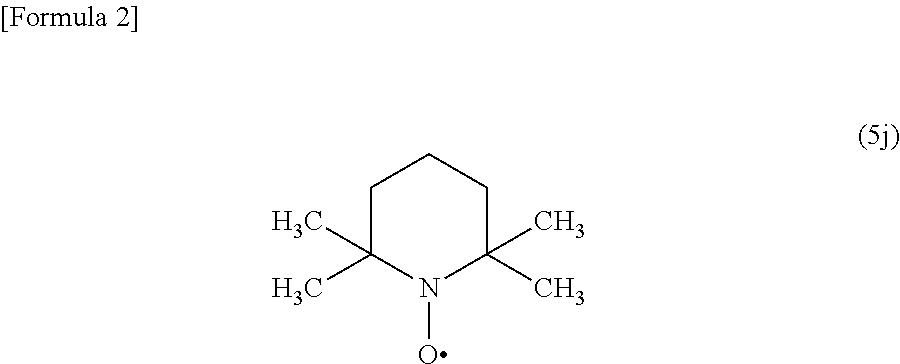
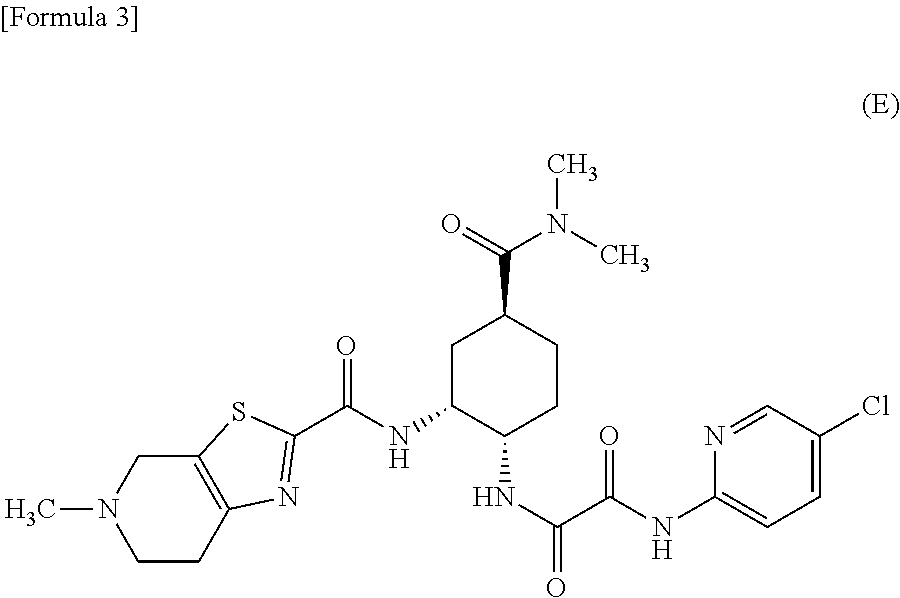
Process for preparing a compound by a novel sandmeyer-like reaction using a nitroxide radical compound as a reaction catalyst
ActiveUS9233980B2Carboxylic acid nitrile preparationOrganic compound preparationSandmeyer reactionNitroxide radical
The present invention provides a novel process for preparing a substituted aromatic compound such as an aromatic halo compound or a salt thereof through a transformation reaction of an aromatic diazonium salt from an aromatic amino compound at stable high yields utilizing a novel Sandmeyer-like reaction using a nitroxide radical compound as a reaction catalyst.
Owner:DAIICHI SANKYO CO LTD
Synthesis method of 2-bromo-5-fluorobenzotrifluoride
ActiveCN106905104ALow priceSmall market demandOrganic compound preparationAmino compound preparationSandmeyer reactionChemical synthesis
The invention relates to a synthesis method of 2-bromo-5-fluorobenzotrifluoride, belonging to the field of chemical synthesis. The method uses orthotrifluoromethyl aniline as the initial raw material, and comprises the following steps: (1) Sandmeyer bromination reaction; (2) nitration reaction; (3) catalytic hydrogenation reduction reaction; and (4) diazotization fluorination reaction. According to the method, the orthotrifluoromethyl aniline used as the raw material is subjected to Sandmeyer reaction bromination, nitration, hydrogenation reduction and diazotization fluorination to finally synthesize the 2-bromo-5-fluorobenzotrifluoride. The 2-bromo-5-fluorobenzotrifluoride product has the advantages of high purity (up to 99.0% or above), fewer impurity varieties and stable quality and properties.
Owner:ZHEJIANG WEIHUA NEW MATERIAL CO LTD
A kind of preparation method of 2,3-dichloropyridine
Owner:NANTONG TENDENCI CHEM
Method for preparing 2,3-dichloropyridine
InactiveCN104926715AThe preparation process is simpleFew stepsOrganic chemistrySandmeyer reactionAminopyridines
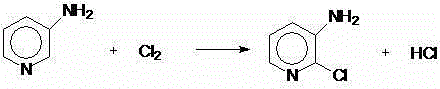
The invention provides a method for preparing 2,3-dichloropyridine. Reaction is conducted through adoption of raw auxiliary materials of 3-aminopyridine, hydrochloric acid, hydrogen peroxide, cuprous chloride, liquid caustic soda and the like, and the reaction principle is divided into three steps: firstly, 3-aminopyridine, hydrochloric acid and hydrogen peroxide react to generate 2-chlorine-3-aminopyridine, and 2-chlorine-3-aminopyridine is subjected to diazotization and Sandmeyer reaction so as to prepare the finished product. The method is simple in preparation process, less in step, relatively high in yield and high in purity.
Owner:ANHUI JIXI COUNTY HUIHUANG CHEM
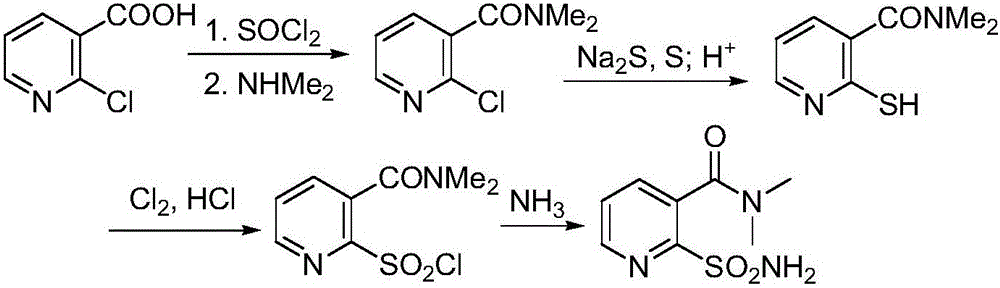
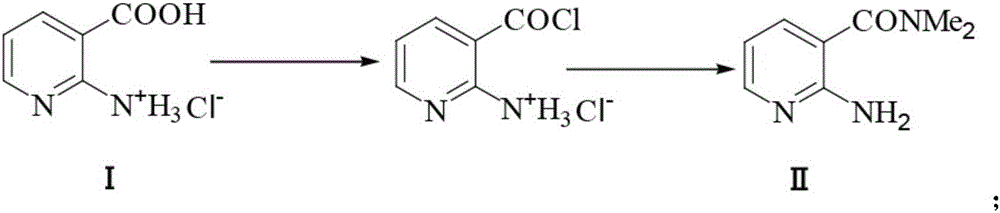
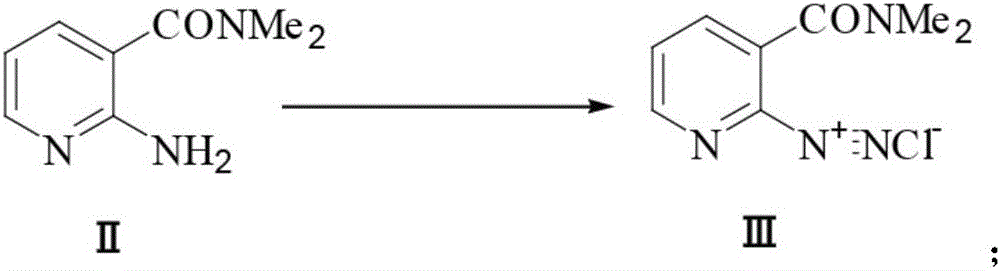
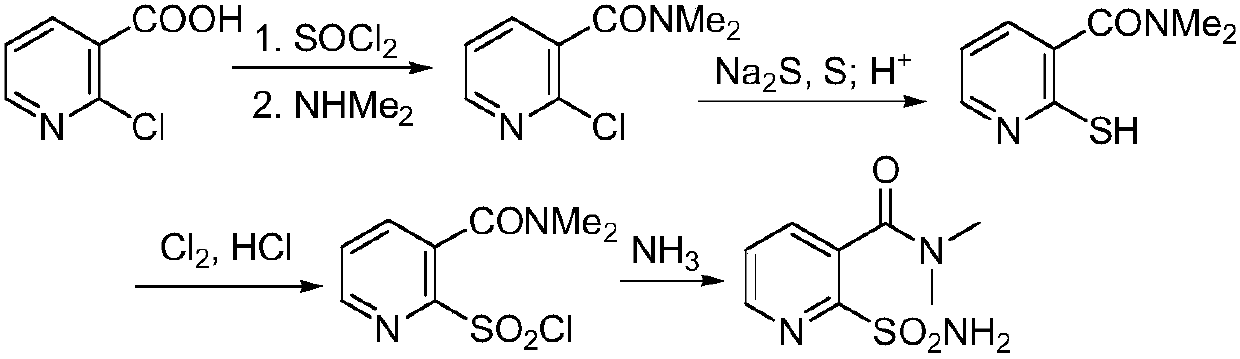
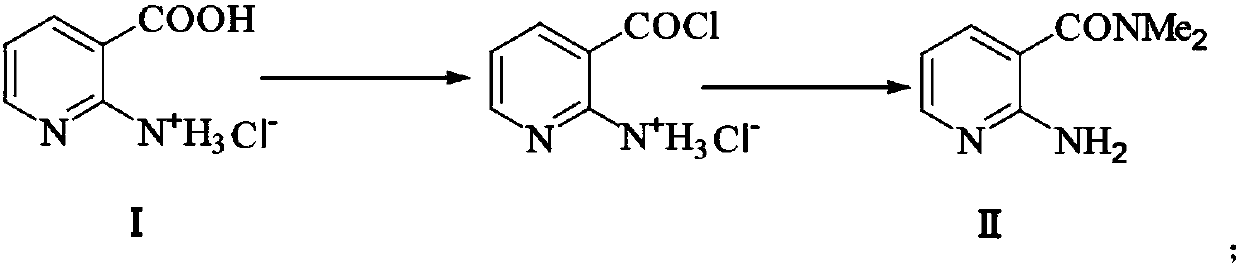
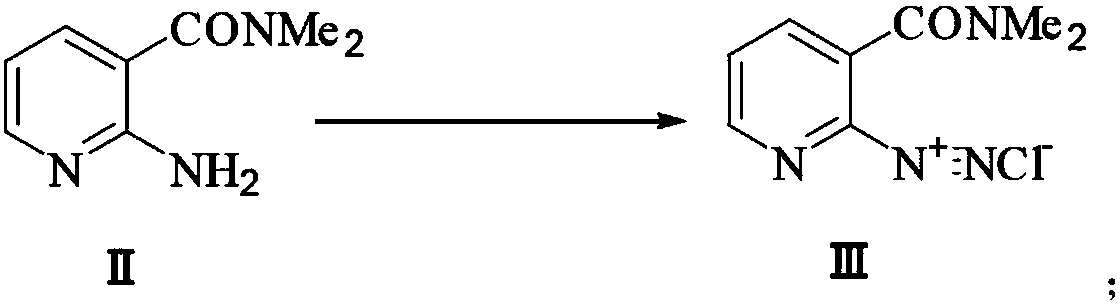
Preparation method of 2-aminosulfonyl-N,N-dimethylnicotinamide
ActiveCN106279015AEase of industrial productionLow costOrganic chemistrySandmeyer reactionReactive site
The invention provides a preparation method of 2-aminosulfonyl-N,N-dimethylnicotinamide. With 2-aminonicotinic acid serving as the raw material, 2-aminosulfonyl-N,N-dimethylnicotinamide is prepared through the first step of conducting a chloroformylation reaction and an amination reaction, the second step of conducting a diazotization reaction, the third step of conducting a Sandmeyer reaction and the fourth step of conducting an amination reaction. The preparation method has the advantages that reaction conditions are mild, stable and controllable, side reactions are not likely to occur as the number of active sites is small, and the whole route is high in yield and quality; cost can be effectively reduced as original materials can be easily obtained from the market, and the preparation method is environmentally friendly as no polysulfide is used.
Owner:ANHUI RES INST OF CHEM IND
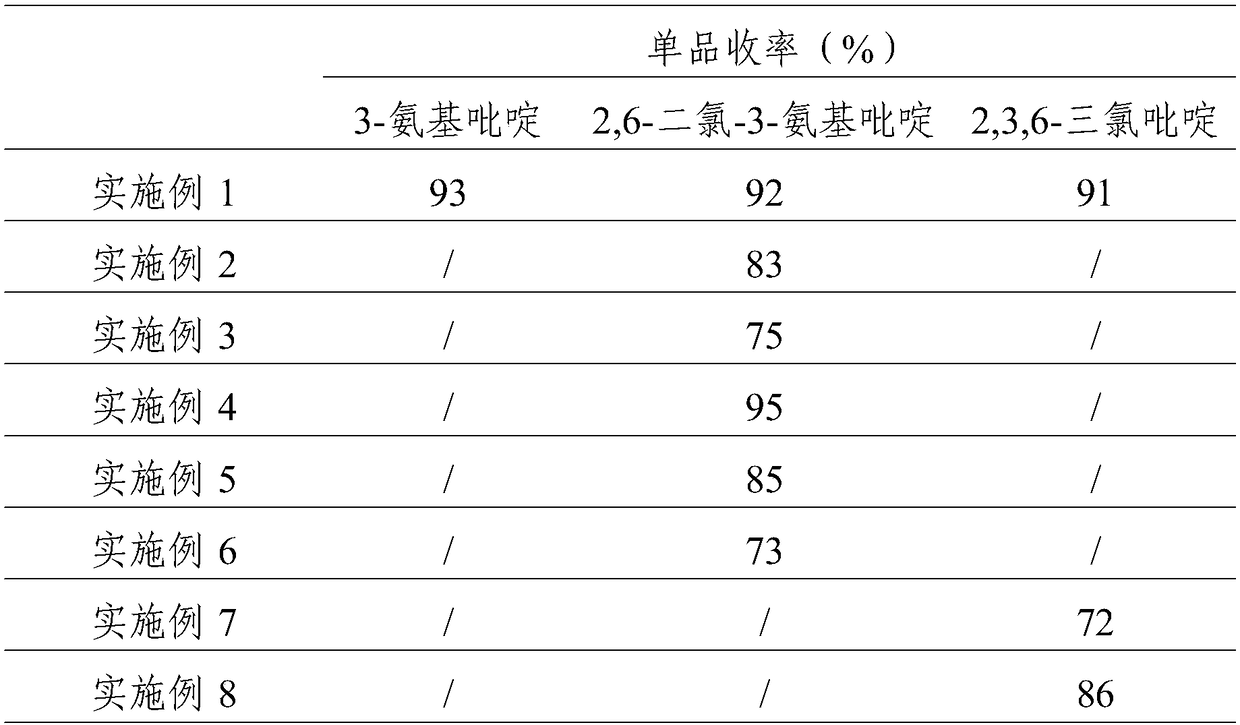
Process study for synthesizing 2,3,6-trichloropyridine from nicotinamide
The invention provides a process study for synthesizing 2,3,6-trichloropyridine from nicotinamide. The process study comprises the following steps: with nicotinamide as a raw material, adding a sodiumhypochlorite solution in an alkaline environment to carry out a Hofmann downgrading reaction to obtain 3-aminopyridine; under catalysis of a Lewis acid catalyst, performing chlorination reaction under a concentrated hydrochloric acid / hydrogen peroxide condition to obtain 2,6-dichloro-3-aminopyridine; and reacting in the presence of sodium nitrite under low temperature and strong acid conditions to form a diazonium salt solution; and finally, performing a Sandmeyer reaction to obtain the target product, 2,3,6-trichloropyridine. The process material provided by the invention is simple, easily available and cheap, the reaction condition is simple and easy to operate, the post-treatment is simple, the yield is high, and thus the process has good industrial development prospects.
Owner:ANHUI COSTAR BIOCHEM CO LTD
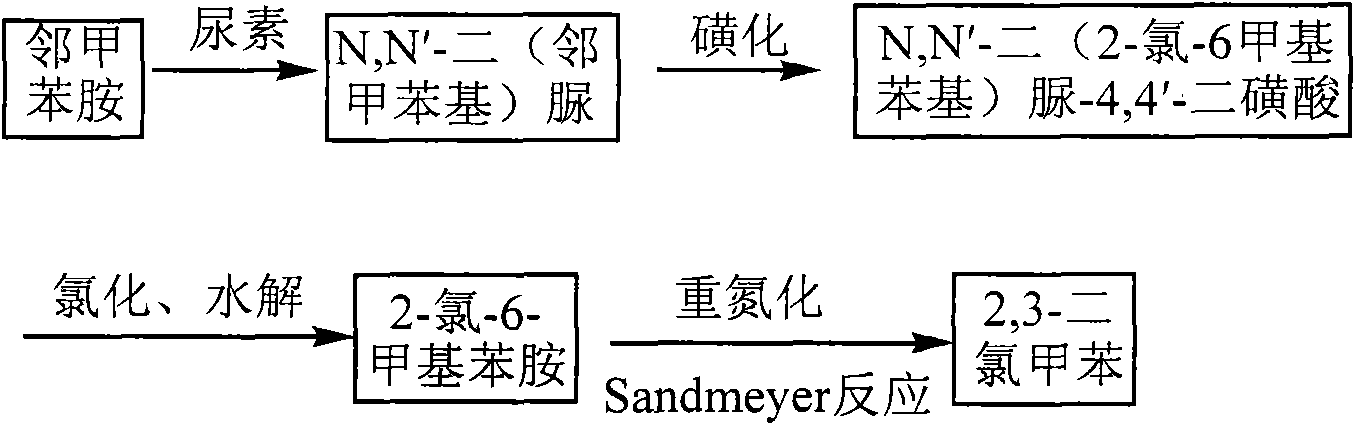
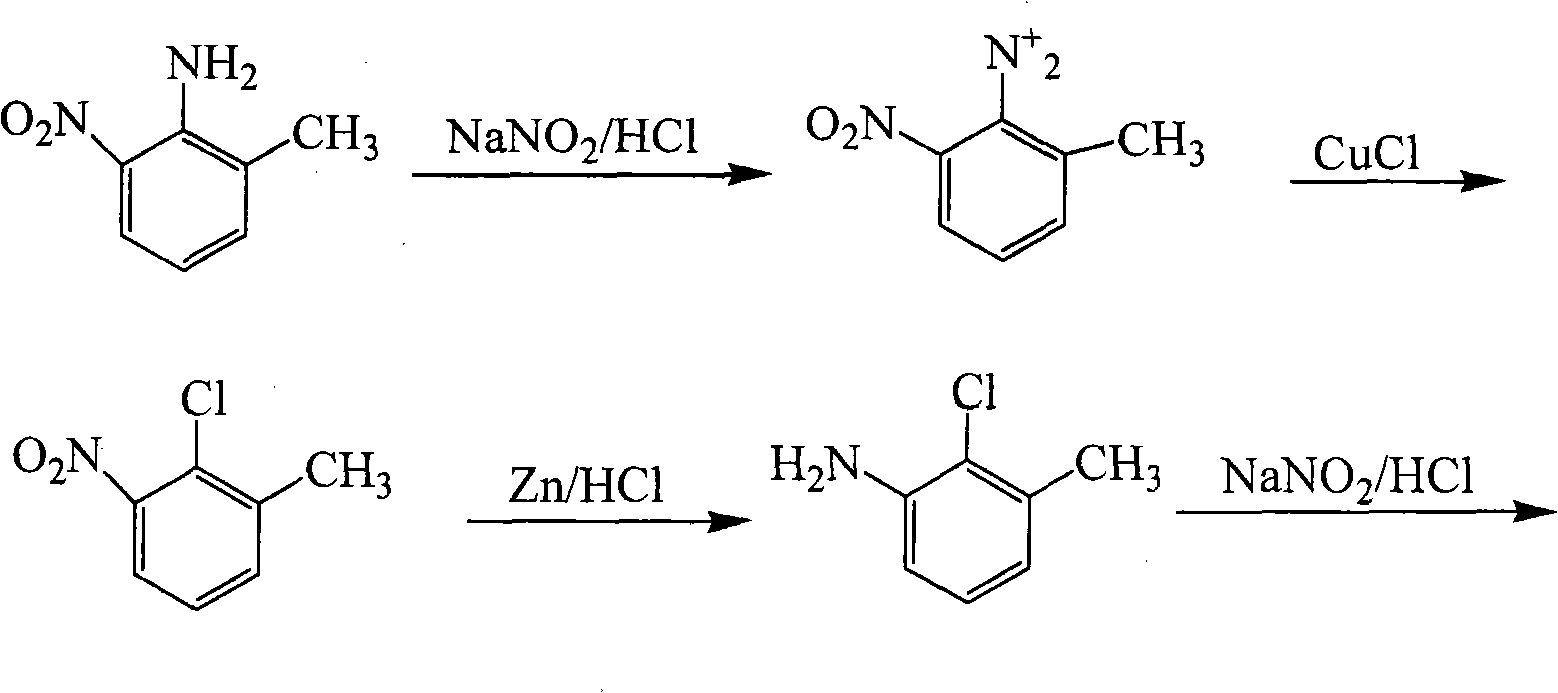
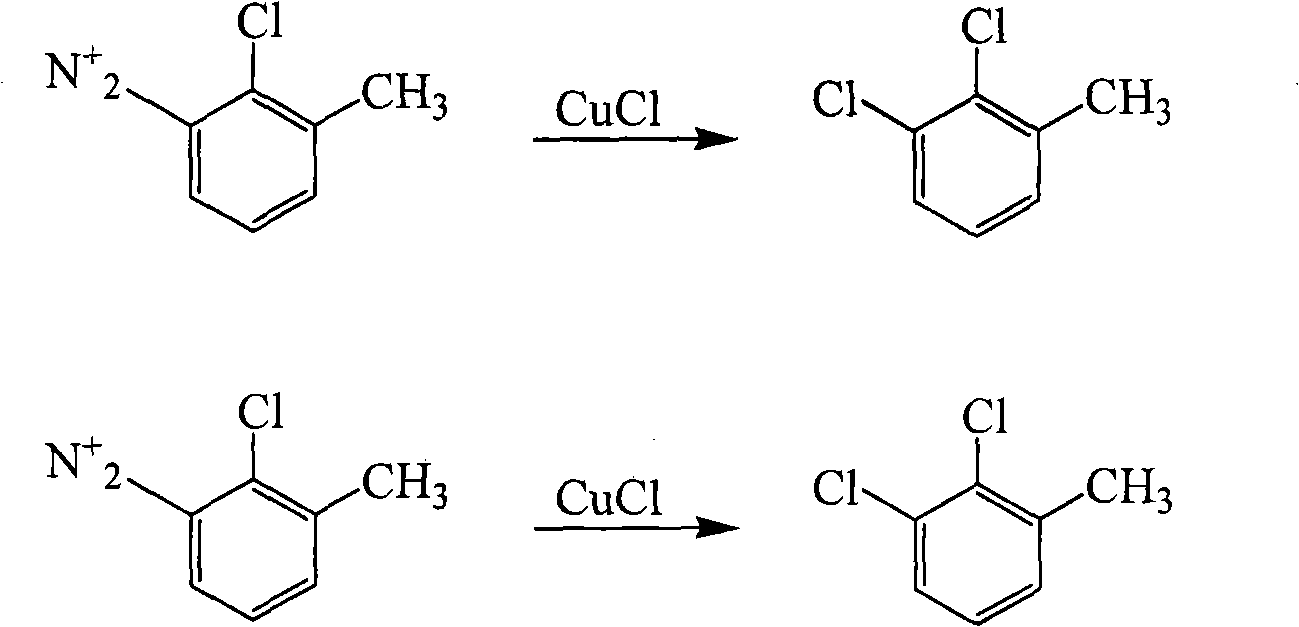
Method for preparing 2,3-dichlorotoluene
InactiveCN102079688ALow priceThe reaction operation is not cumbersomeChemical recyclingHalogenated hydrocarbon preparationSandmeyer reactionMethylaniline
The invention discloses a method for preparing 2,3-dichlorotoluene. The method comprises the following steps of: performing condensation on o-toluidine serving as an initiative raw material and urea to obtain N,N'-di(tolyl) urea; forming a sulfonic blocking group at an amino para-position through sulfonation; performing chlorination, removing carbonyl through hydrolysis and removing the blocking group through hydrolysis to obtain 2-chlorine-6-methylaniline; and performing diazotization and a Sandmeyer reaction to synthesize 2,3-dichlorotoluene to obtain the product. The method has the advantages of environmental friendliness, simple process, low cost and the like.
Owner:INNER MONGOLIA UNIV OF TECH
Preparation method of 2-fluoro-4,5-dichloronitrobenzene
ActiveCN102796003AReaction conditions are easy to controlReduce manufacturing costNitro compound preparationSandmeyer reactionNitrobenzene
The invention discloses a preparation method of 2-fluoro-4,5-dichloronitrobenzene, relating to the technical field of preparation of medicine and pesticide intermediates. The industrialized preparation method of the 2-fluoro-4,5-dichloronitrobenzene comprises the following steps: by using 4-fluoroaniline as an initial raw material, carrying out acetylation, chlorination, hydrolysis, diazotization-Sandmeyer reaction and nitrification to synthesize the 2-fluoro-4,5-dichloronitrobenzene. The 2-fluoro-4,5-dichloronitrobenzene prepared by the method disclosed by the invention is a light yellow solid, and the purity is 98.5%; the raw material conversion rate for each reaction step reaches 100%; and the total yield of the whole process is up to 27.1%.
Owner:JINING XINRUIDA INFORMATION TECH CO LTD
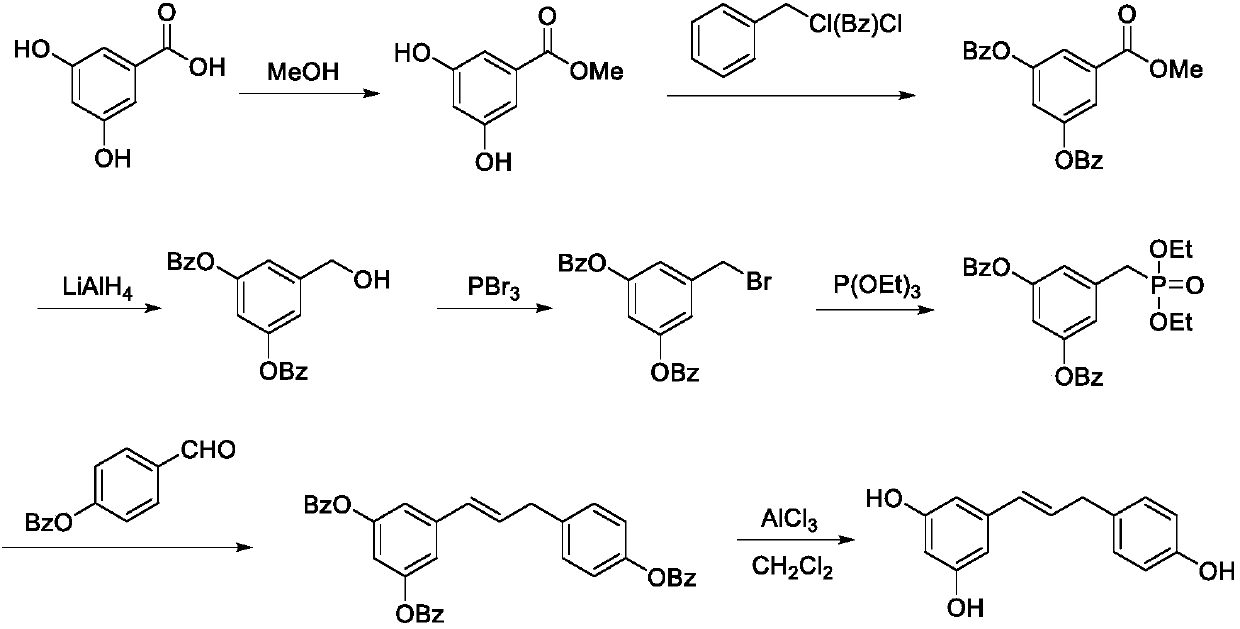
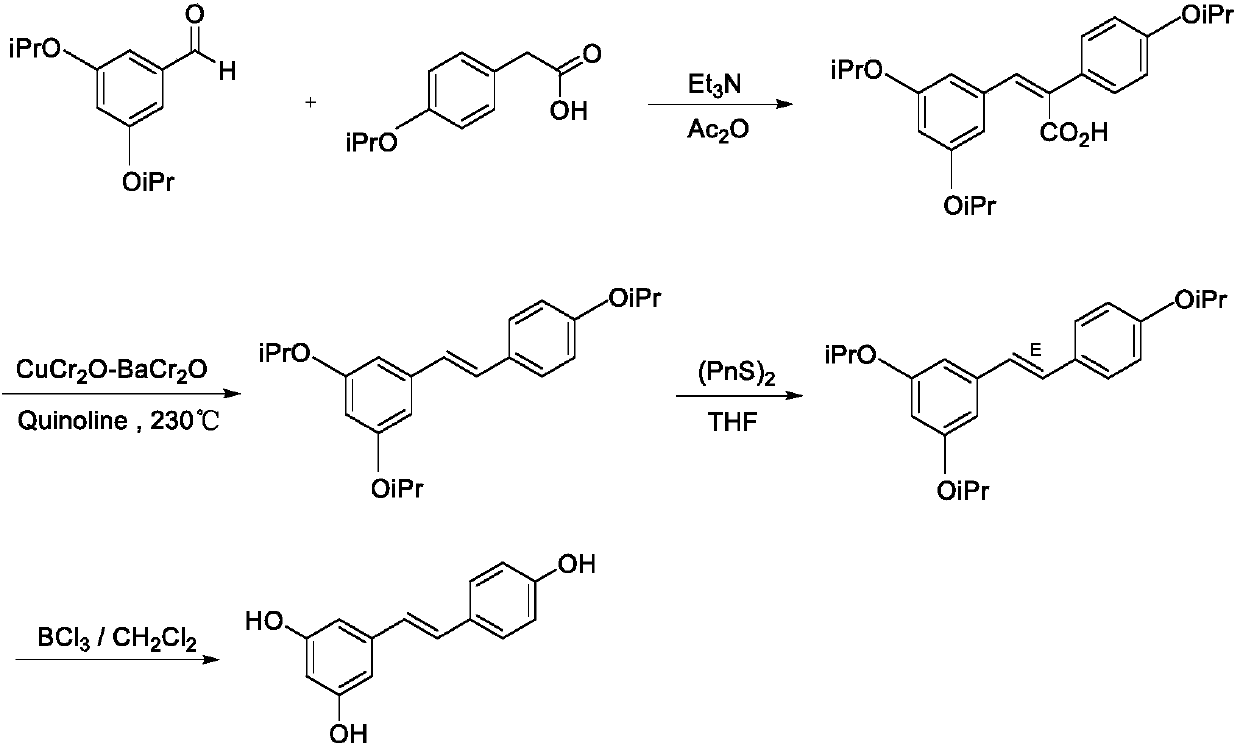
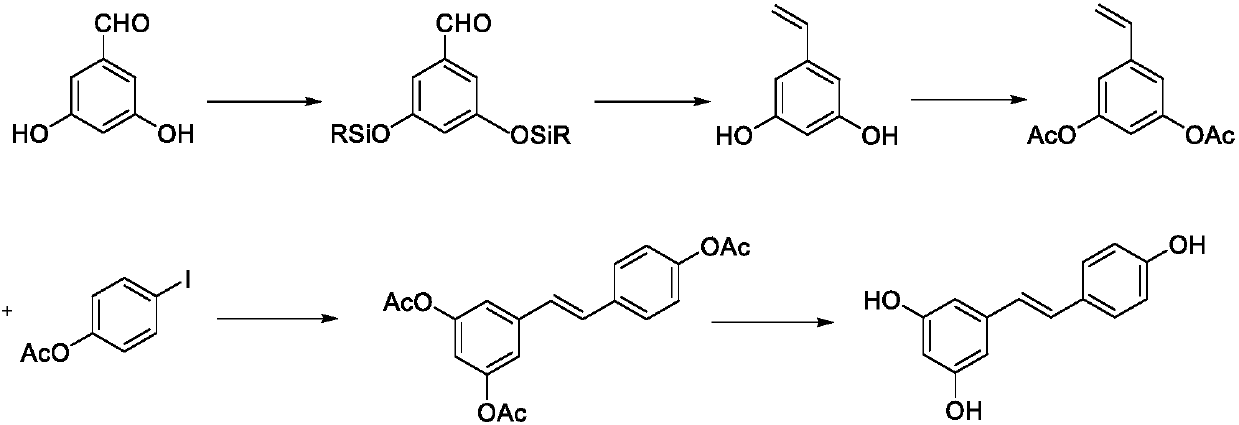
Synthesis method of resveratrol
InactiveCN107840792AHigh trans stereoselectivityMild reaction conditionsOrganic compound preparationGroup 5/15 element organic compoundsBenzoic acidSandmeyer reaction
The invention provides a synthesis method of resveratrol, and belongs to the technical field of natural product synthesis. The synthesis method comprises the following steps: with 3,5-dimethoxy benzoic acid as a raw material, generating 3,5-dimethoxy benzoyl chloride (12) through an acylating chlorination reaction; generating 3,5-dimethoxy benzamide (13) through an amidation reaction of the (12);generating 3,5-dimethoxyaniline (14) through a Hofmann degradation reaction of the (13); generating 3,5-dimethoxy iodobenzene (15) through a Sandmeyer reaction on the (14); generating 3,5,4'-trimethoxy diphenylethene (31) through a reaction between the (15) and p-methoxystyrene (23), and performing demethylation of the (31) to finally obtain resveratrol (1), wherein total yield is 23.3%. Accordingto the method, the adopted reagent is cheap and easily available, aftertreatment is simple, and two expensive intermediates for Heck reaction are synthesized by adopting cheap raw materials. A new synthesis method is provided for synthesizing resveratrol.
Owner:CHANGZHOU UNIV
Synthetic method for cellulose derivative with large volume liquid crystal unit side group
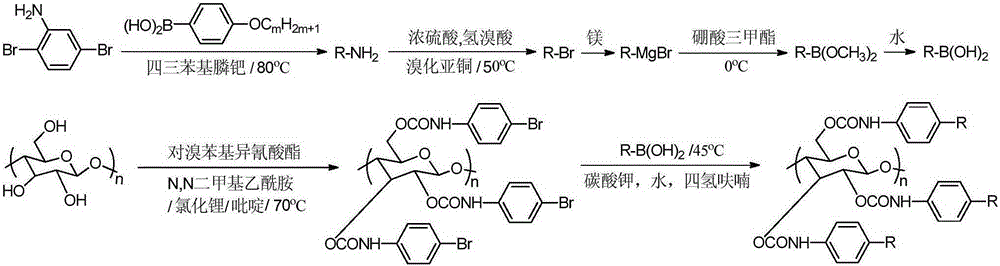
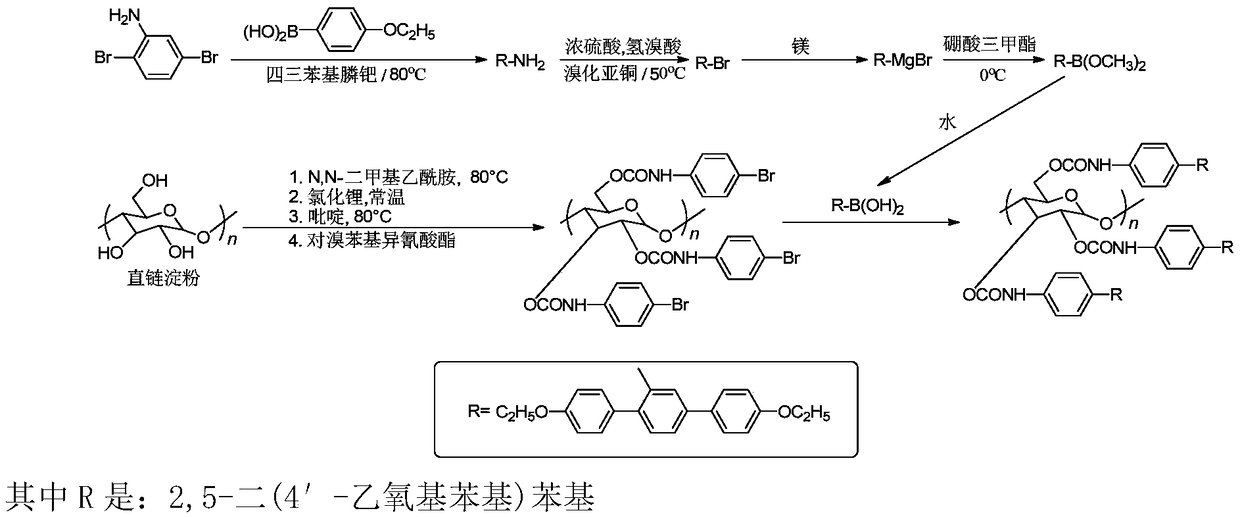
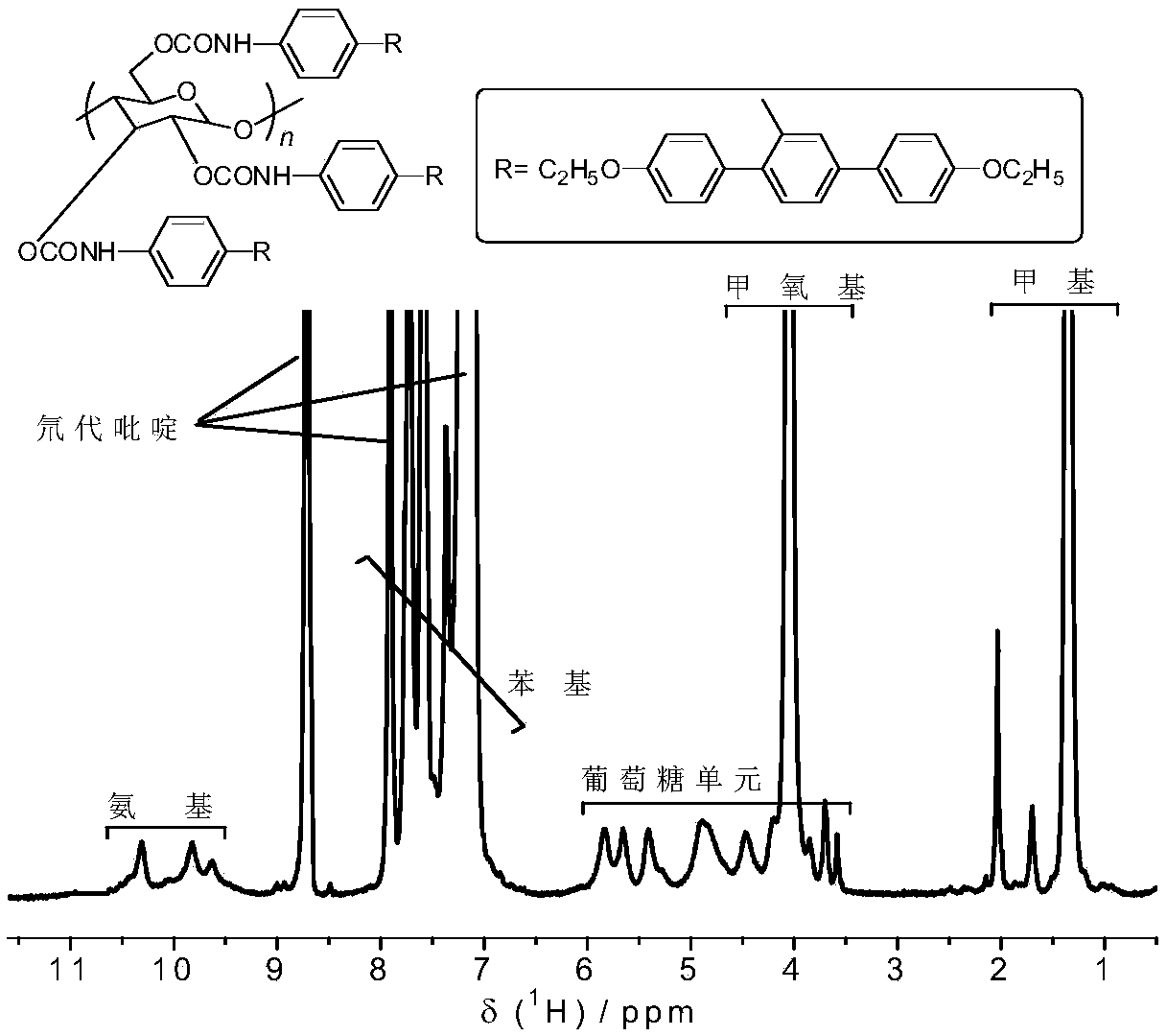
The invention provides a synthetic method for cellulose derivative with a large volume liquid crystal unit side group. The synthetic method comprises: by taking 2,5-dibromoaniline and 4-alkoxyl phenylboronic acid as raw materials, synthesizing 2,5-bi(4'-alkoxyl phenyl) phenylboronic acid through Suzuki coupling reaction, Sandmeyer reaction, Grignard and the like; then by taking microcrystal cellulose as a reaction medium, synthesizing cellulose-tri(4-bromophenyl amino formate); and carrying out Suzuki coupling reaction on the synthesized 2,5-bi(4'-alkoxyl phenyl) phenylboronic acid and cellulose-tri(4-bromophenyl amino formate) to finally synthesize the novel cellulose phenyl amino formate derivative with large volume liquid crystal unit side group, wherein a benzene ring replaces p-terphenyl in a p-position. The molecular structure of a product is represented in detail and analyzed by applying Fourier transform infrared spectrometer, a nuclear magnetic resonance hydrogen spectrum and a nuclear magnetic resonance carbon spectrum, and the liquid crystal performance of the synthesized novel cellulose derivative is further inspected by applying a differential scanning calorimeter and a polarizing microscope.
Owner:HARBIN ENG UNIV
Preparation method of vortioxetine hydrobromide
InactiveCN105985301ALow priceMild reaction conditionsOrganic chemistrySandmeyer reactionAfter treatment
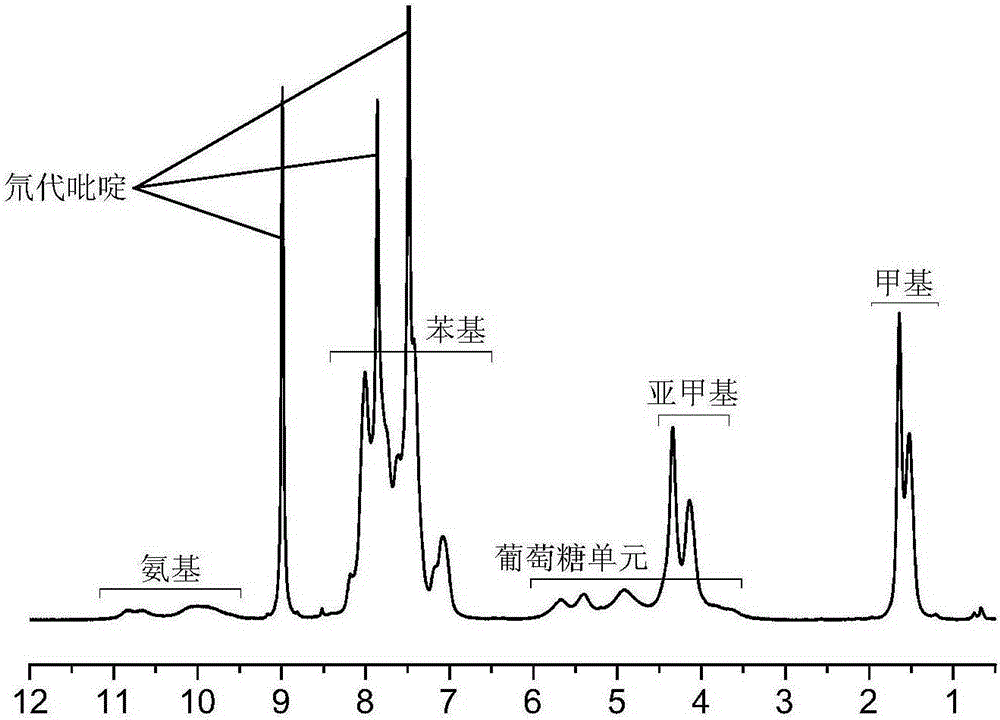
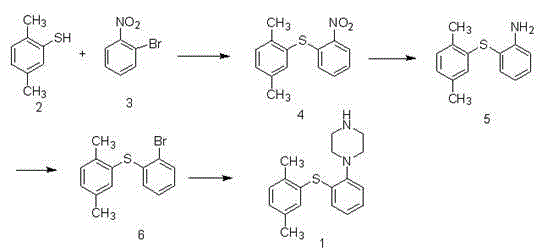
The invention relates to the technical field of preparation of vortioxetine hydrobromide, in particular to a preparation method of vortioxetine hydrobromide. The preparation method comprises the following steps: taking 2,4-dimethyl thiophenol as a raw material to react with o-bromonitrobenzene so as to generate a compound (IV); treating the compound (IV) via a normal pressure catalytic hydrogenation method to obtain a compound (V); treating the compound (V) via a Sandmeyer reaction to obtain a compound (VI); and reacting a compound (VII) with piperazine, and then performing a reaction with hydrobromic acid to generate a salt, thereby obtaining a target compound (I). The method for preparing vortioxetine hydrobromide is relatively short in route, relatively mild in reaction condition, simple, convenient and feasible in after treatment, and more suitable for industrial production requirements.
Owner:山东康美乐医药科技有限公司
Method for synthesizing 2,3í»-dichloroacetophenone
InactiveCN101333157AOrganic compound preparationCarbonyl compound preparationM-aminoacetophenoneSandmeyer reaction
The invention relates to a synthesis method for 2,3'-dichloroacetophenone (alpha-chloro-acetophenone inter-chlorophenyl) and is characterized in that the method takes inter-amino-acetophenone as raw material to generate between inter-acetophenone diazo-hydrochloride through diazotization reaction, or generate inter-p-acetophenone through Sandmeyer reaction, or generate 2,3'-dichloroacetophenone through alpha-chloro-reaction; the synthetic way is simple and the conditions are mild, with a total yield rate more than 80%.
Owner:徐州诺特化工有限公司
A synthetic method of 2-chloro-5-iodobenzoic acid
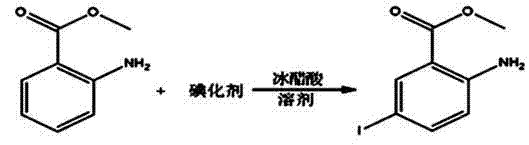
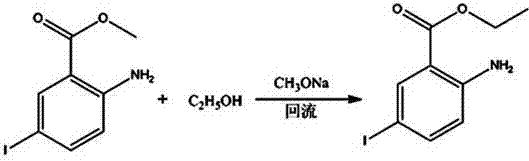
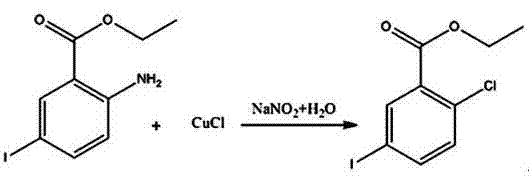
InactiveCN104193616ASimple processEasy to operateOxygen-containing compound preparationOrganic compound preparationSandmeyer reactionBenzoic acid
A synthetic method of 2-chloro-5-iodobenzoic acid is disclosed. The target product is obtained by subjecting methyl 2-aminobenzoate to iodination, substitution, a Sandmeyer reaction and hydrolysis under alkaline conditions. The method is characterized by modification or optimization of process steps and parameters based on traditional iodination, substitution, the Sandmeyer reaction and hydrolysis. The method is simple in process, easy in operation, safe in production process, free of pollution, and high in yield of each step. The purity of the product is 95-98%. The total yield of the product is 64-70%.
Owner:姜树林
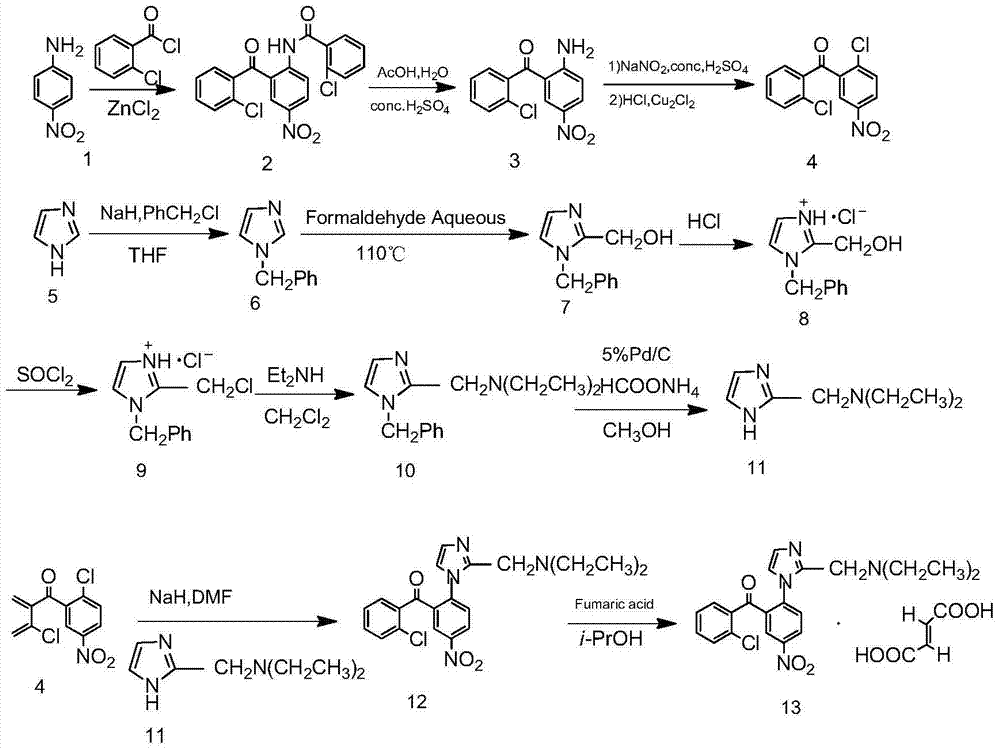
Synthesis process of nizofenone fumarate
The invention belongs to the field of medical technology, and discloses a synthesis process line of an ischemic brain dysfunction improving medicine nizofenone fumarate. The synthesis process comprises the following steps: by adopting paranitroaniline as a start raw material, performing an amidation reaction, a Friedel-Crafts acylation reaction, a hydrolysis reaction, a diazotization reaction and a Sandmeyer reaction to obtain an important intermediate 2'-chloro-2-chloro-5-nitrobenzophenone (4); by adopting imidazole as a start raw material, performing N-benzyl protection, a hydroxymethylation reaction, a chlorination reaction, an amination reaction and a transfer hydrogenation debenzylation reaction to obtain another important intermediate 2-(diethylaminomethyl)imidazole (11); catalyzing the two intermediates with sodium hydride to obtain nizofenone; salifying with fumaric acid to generate a target compound.
Owner:SHENYANG PHARMA UNIVERSITY +1
Prepn process of 4-bromo-3,5-dimethoxy benzaldehyde
InactiveCN1357525AShort processHigh yieldOrganic compound preparationCarbonyl compound preparationSandmeyer reactionSodium methoxide
The preparation of 4-bromo-3,5-demethoxy benzaldehyde, as the main intermediate for preparing brodimoprim, includes the steps of: the substitution reaction between amino methylbenzene and bromine on benzene ring in solvent A and the decompressing evaporation of solvent A; the alkoxylation between the product of the fore step and added sodium methoxide in solvent B and in the presence of catalyst, filtering, water washing of the filtering cake to eliminate solvent B and drying; and diazotization of the product, Sandmeyer reaction, photobromization and hydrolysis to obtain 4-bromo-3,5-demethoxy benzaldehyde. It has short technological process and high product yield.
Owner:昆山虹祺药业有限责任公司
Synthetic processes of intermediates for preparation of SGLT inhibitor
ActiveCN112094253AShort routeMild reaction conditionsOrganic chemistrySandmeyer reactionCombinatorial chemistry
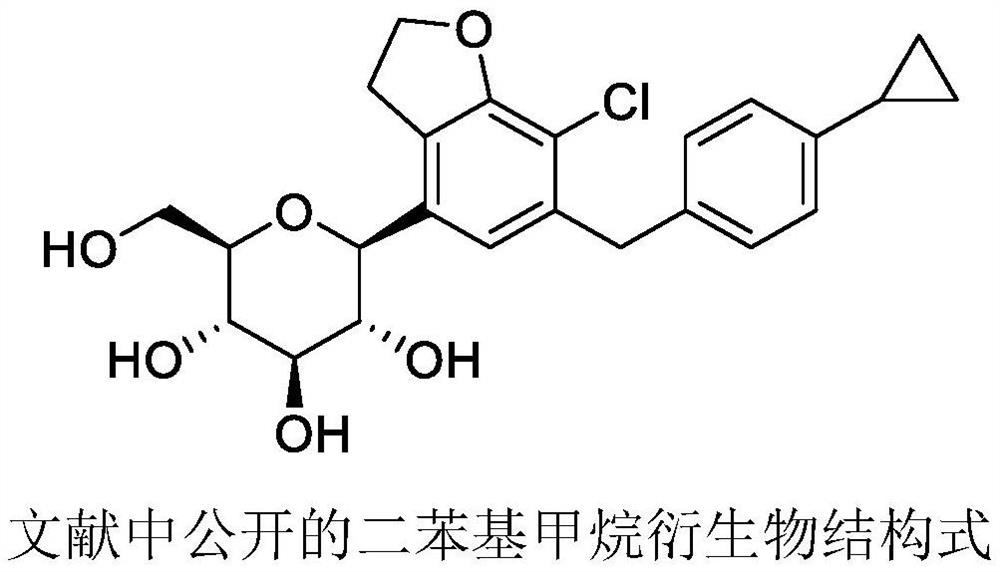
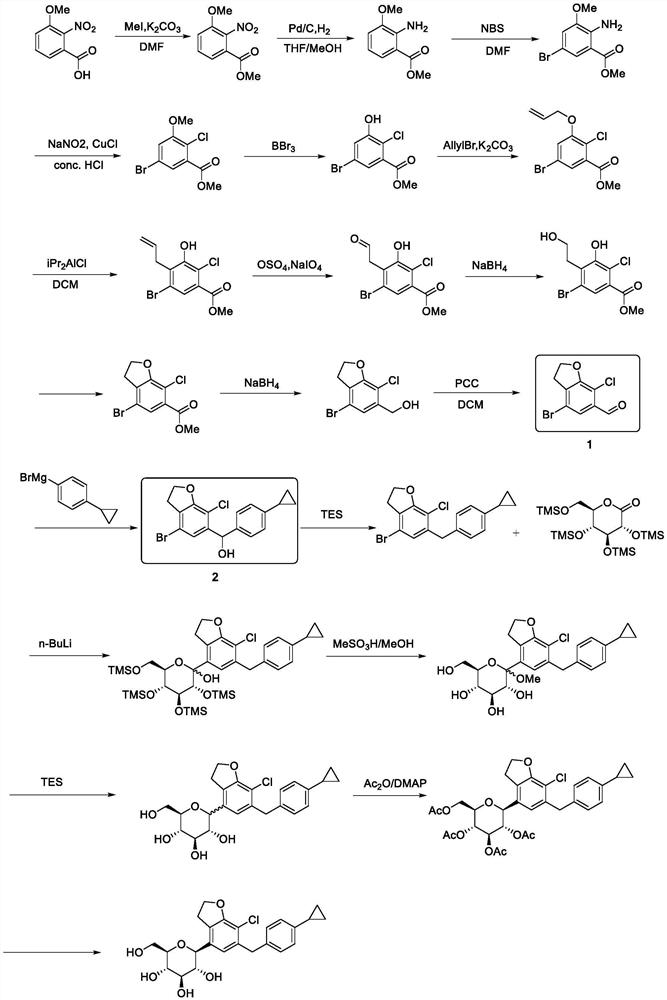
The invention discloses a method for preparing a compound 1 and a compound 2, the structure of the compound 1 and the structure of the compound 2 are shown in the specification.The method comprises the following steps: 1) carrying out selective dibromination on 2, 3-dihydrobenzofuran-7-aniline serving as a raw material by using a bromination reagent to obtain 4, 6-dibromo-2, 3-dihydrobenzofuran-7-aniline; 2) subjecting the 4, 6-dibromo-2, 3-dihydrobenzofuran-7-aniline obtained in the step 1) to a Sandmeyer reaction for chlorination to give 4, 6-dibromo-7-chloro-2, 3-dihydrobenzofuran; 3-1) performing selective bromine removal on the 4, 6-dibromo-7-chloro-2, 3-dihydrobenzofuran obtained in the step 2) by using a strong base, adding a formylation reagent to give the compound 1; and 3-2) performing selective bromine removal on the 4, 6-dibromo-7-chloro-2, 3-dihydrobenzofuran obtained in the step 2) by using a strong base and then reacting with 4-cyclopropylbenzaldehyde to give the compound 2.
Owner:DAEWOONG PHARM CO LTD
Method for preparing 2-fluoro-4-bromo trifluoromethoxyphenyl
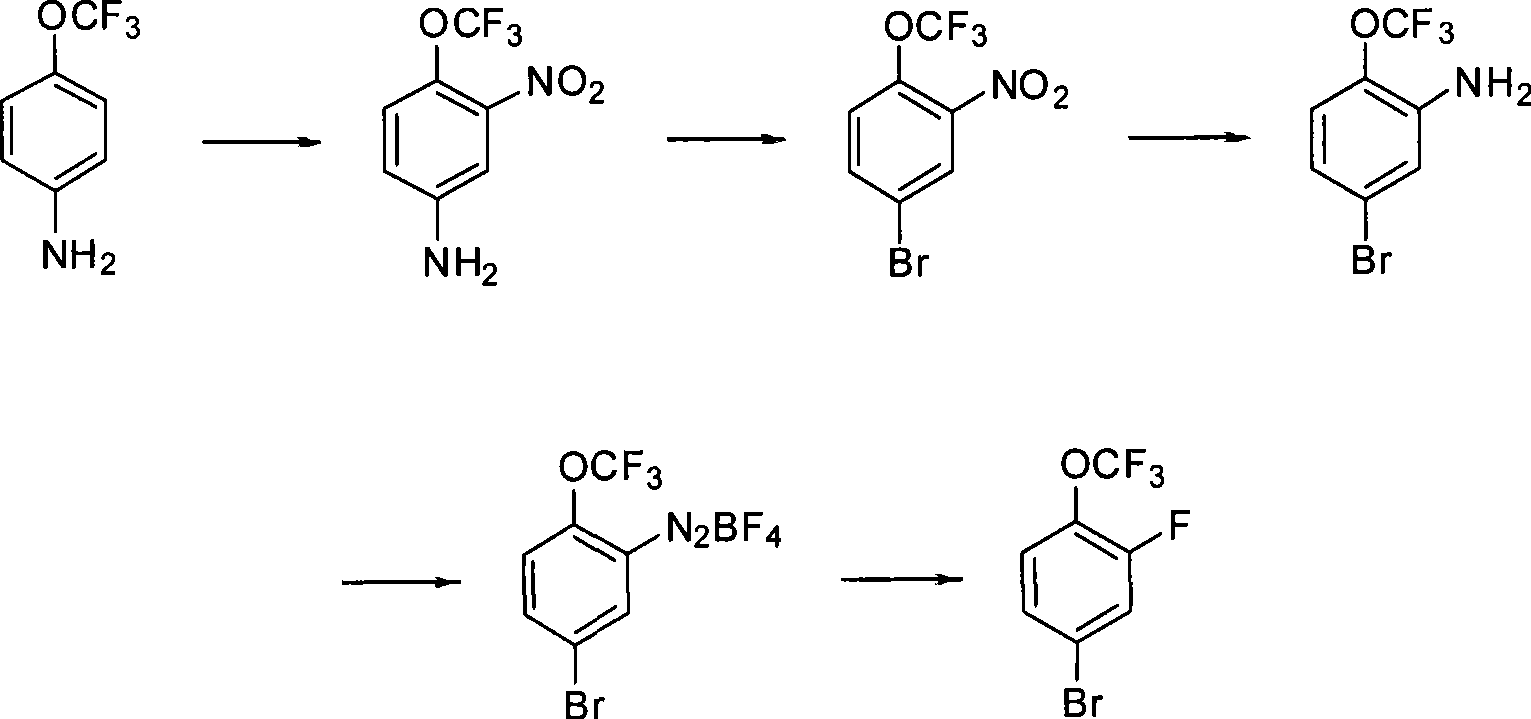
ActiveCN101450891AMeet the requirementsMild reaction conditionsEther preparationSandmeyer reactionBenzene
The invention discloses a method for preparing 2-fluorine-4-bromine(tri-fluoromethoxy)benzene. The method comprises the following steps: 1, 4-amino(tri-fluoromethoxy)benzene is nitrified to form 2-nitro-4-amino-(tri-fluoromethoxy)benzene; 2, the 2-nitro-4-amino-(tri-fluoromethoxy)benzene is subjected to diazotization and Sandmeyer reaction to form 2-nitro-4- bromine(tri-fluoromethoxy)benzene; and 3, the 2-nitro-4- bromine(tri-fluoromethoxy)benzene is subjected to reduction, diazotization and Schiemann reaction to form 2-fluorine-4- bromine(tri-fluoromethoxy)benzene. The method has the advantages of less by products on the synthetic route, mild and easily controlled reaction conditions, low cost, easy realization of industrialization, high production capability, and products obtained by the method has high purity and stable quality, and completely meets use requirements of a medicine intermediate.
Owner:SHANGHAI CHEMSPEC CORP
Preparation method of ceritinib and its intermediate
The invention discloses an intermediate 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) for preparing ceritinib and a preparation method thereof. The preparation method comprises the following steps: carrying out catalytic hydrogenation on the raw material 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine (II) to obtain 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)aniline (III); and carrying out Sandmeyer reaction on the compound (III) to obtain the 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I). The invention also discloses a preparation method of ceritinib. The 2-isopropoxy-5-methyl-4-(piperidyl-4-yl)halogeno-benzene (I) used as the raw material is sequentially subjected to substitution, reduction and substitution reaction to obtain the ceritinib (VIII). The preparation method has the advantages of simple technique, mild conditions and fewer side reactions, and is suitable for industrial amplification.
Owner:SCI GENERAL MATERIAL & CHEM
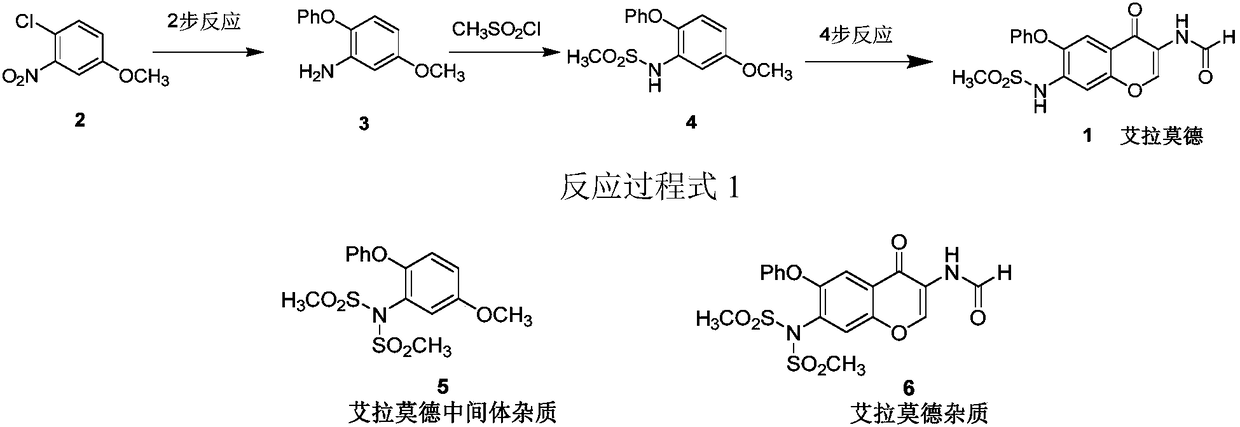
Method for synthesizing intermediate impurities of igutimod
PendingCN109400507AGood for quality control researchHigh purityOrganic compound preparationSulfonic acid amide preparationSandmeyer reactionDrugs synthesis
The invention discloses a method for synthesizing intermediate impurities of igutimod, and belongs to the field of chemical drug synthesis. The method comprises the steps that 3-iodo-4-phenoxy-anisoleis obtained through Sandmeyer reaction iodination with an intermediate 5-methoxy-2-phenoxyaniline as a starting material, and 3-(N,N-dimethylsulfonyl)amino-4-phenoxyanisole is obtained through catalytic reaction of copper. The method has the advantages of simple steps, high yield, good product purity and the like, and helps the study of the igutimod and the intermediate impurities thereof, and control over final product quality.
Owner:CHANGZHOU VOCATIONAL INST OF ENG +1
Preparation method of chiral fluorescence sensor with large-volume side group amylase derivative
PendingCN108760698AWide variety of sourcesThe synthesis process is simpleFluorescence/phosphorescenceSandmeyer reactionCarbamate
The invention provides a preparation method of a chiral fluorescence sensor with a large-volume side group amylase derivative. The preparation method comprises the following steps: taking 2,5-dibromoaniline and 4-ethoxybenzeneboronic acid as raw materials, and synthesizing 2,5-di(4'-ethyoxyl phenyl) aniline through a Suzuki coupling reaction; synthesizing 2,5-di(ethyoxyl phenyl) bromobenzene through a Sandmeyer reaction; and synthesizing 2,5-di(4'-ethyoxyl phenyl) phenylboronic acid with a one-pot method through reactions such as Grignard; then, taking amylase as a reaction substrate, taking 4-bromophenyl isocyanate as a derivatization reagent, synthesizing amylase-tri(4-bromophenyl carbamate) through a traditional esterification method, and finally synthesizing amylase-tri(4-(2'-diethoxypara-terphenyl)) phenyl carbamate) through a Suzuki coupling reaction. The preparation method is clear and practicable in synthetic route, mature in technology and easy to realize, and can be used forlarge-scale batch production.
Owner:HARBIN ENG UNIV
Method for synthesizing 7-halogenoindoles
ActiveCN105732462ARaw materials are easy to getLow priceOrganic chemistrySandmeyer reactionSynthesis methods
The invention relates to a method for synthesizing 7-halogenoindoles.The method includes the steps that o-halogenated aniline, chloral hydrate and hydroxylamine hydrochloride are subjected to a Sandmeyer reaction to synthesize 7-halogenated indirubin; the 7-halogenated indirubin is dissolved through an organic solvent, the 7-halogenoindoles is obtained in a reduction reaction mode under the reducing agent condition, and the reducing agent is an alkali metal hydroboron system or a lithium aluminum hydride system or a lithium hydride system or a triethyl-silane system.The method has the advantages that the o-halogenated aniline, the chloral hydrate and the hydroxylamine hydrochloride serve as raw materials, and the 7-halogenated indirubin is prepared with the Sandmeyer isonitroso acetanilide synthesis method, and then is reduced through the reduction system to prepare 7-halogenoindoles; the method for preparing the 7-halogenoindoles from the 7-halogenated indirubin has the advantages that raw materials are easy to obtain, cost is low, the technology yield is high, the product purity is good, operation is easy and convenient, and the method is suitable for preparing the 7-halogenoindoles in a large-scale mode.
Owner:CHANGZHOU XIAOGUO INFORMATION SERVICES
A kind of method of synthesizing 7-halogenated indoles
ActiveCN105732462BRaw materials are easy to getLow priceOrganic chemistrySandmeyer reactionSynthesis methods
Owner:CHANGZHOU XIAOGUO INFORMATION SERVICES
A kind of preparation method of 2-aminosulfonyl-n,n-dimethylnicotinamide
The invention provides a preparation method of 2-aminosulfonyl-N,N-dimethylnicotinamide. With 2-aminonicotinic acid serving as the raw material, 2-aminosulfonyl-N,N-dimethylnicotinamide is prepared through the first step of conducting a chloroformylation reaction and an amination reaction, the second step of conducting a diazotization reaction, the third step of conducting a Sandmeyer reaction and the fourth step of conducting an amination reaction. The preparation method has the advantages that reaction conditions are mild, stable and controllable, side reactions are not likely to occur as the number of active sites is small, and the whole route is high in yield and quality; cost can be effectively reduced as original materials can be easily obtained from the market, and the preparation method is environmentally friendly as no polysulfide is used.
Owner:ANHUI RES INST OF CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
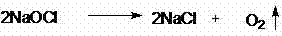
![Preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene Preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene](https://images-eureka.patsnap.com/patent_img/1179fd25-1951-492e-96be-357afd6d844f/BDA0000599254950000011.PNG)
![Preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene Preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene](https://images-eureka.patsnap.com/patent_img/1179fd25-1951-492e-96be-357afd6d844f/BDA0000599254950000012.PNG)
![Preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene Preparation method of 2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl) methyl] thiophene](https://images-eureka.patsnap.com/patent_img/1179fd25-1951-492e-96be-357afd6d844f/BDA0000599254950000021.PNG)