Method for synthesizing intermediate impurities of igutimod
A technology for intermediates and impurities, applied in the field of chemical drug synthesis, can solve the problems of difficulty in separation, affecting the purity of finished products, unable to obtain products with sufficient purity, etc., and achieve the effect of high yield and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step: the preparation of 3-iodo-4-methoxy-anisole (7):
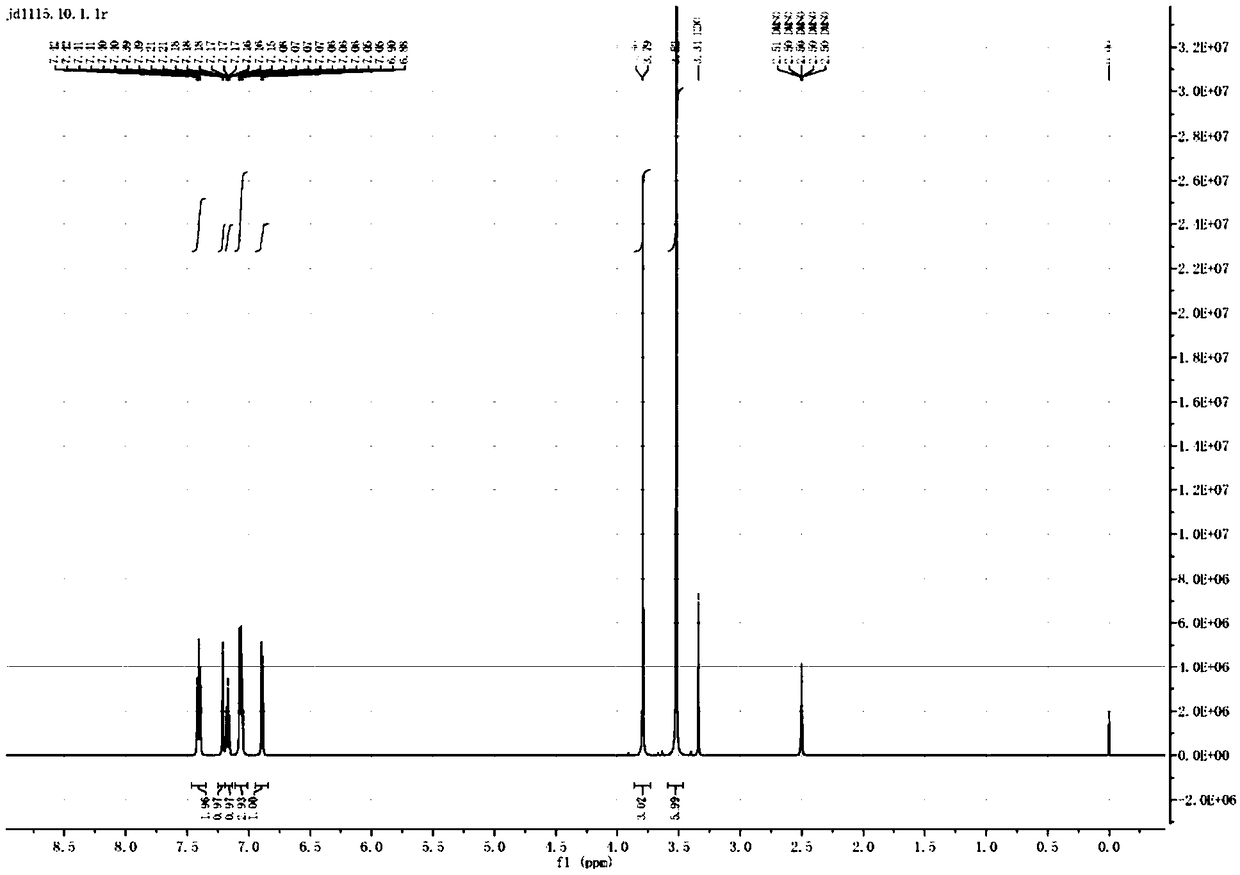
[0031] Take 4.6mmol 5-methoxy-2-phenoxyaniline and 13.9mmol p-toluenesulfonic acid, add 40mL acetonitrile to dissolve, add dropwise 10mL aqueous solution containing 9.2mmol sodium nitrite and 9.2mmol potassium iodide at room temperature. Stir at room temperature for 5 hours, and TLC monitors that the reaction is complete. Add 50 mL of water, extract with methyl tert-butyl ether (30 mL×2), wash with 10% sodium thiosulfate solution, and dry over anhydrous sodium sulfate. Suction filtration, and the filtrate was concentrated to obtain a crude product. Purified by column chromatography (petroleum ether: ethyl acetate = 10:1 elution) to obtain 0.97 g of a light yellow oily substance with a yield of 65.2%. 1 H-NMR (600MHz, CDCl 3 )δ: 7.38(dd, 1H, J=2.4Hz, Ar-H), 7.15-7.18(m, 1H, Ar-H), 6.87(d, 4H, J=2.7Hz, Ar-H), 6.85( d,1H,J=2.0Hz,Ar-H),6.83(d,1H,J=1.5Hz,Ar-H),3.81(s,3H,-OCH 3 ).
[0032] The second ste...
Embodiment 2
[0035] The first step: the preparation of 3-iodo-4-methoxy-anisole (7) is the same as the first step in Example 1.
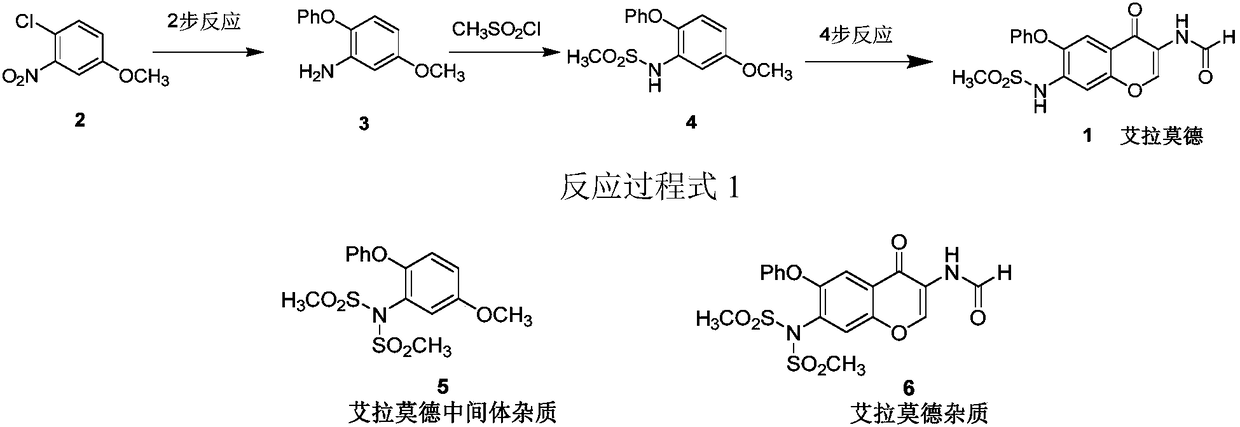
[0036] The second step: the preparation of intermediate impurity 3-(N,N-dimethylsulfonyl)amino-4-phenoxyanisole (5):
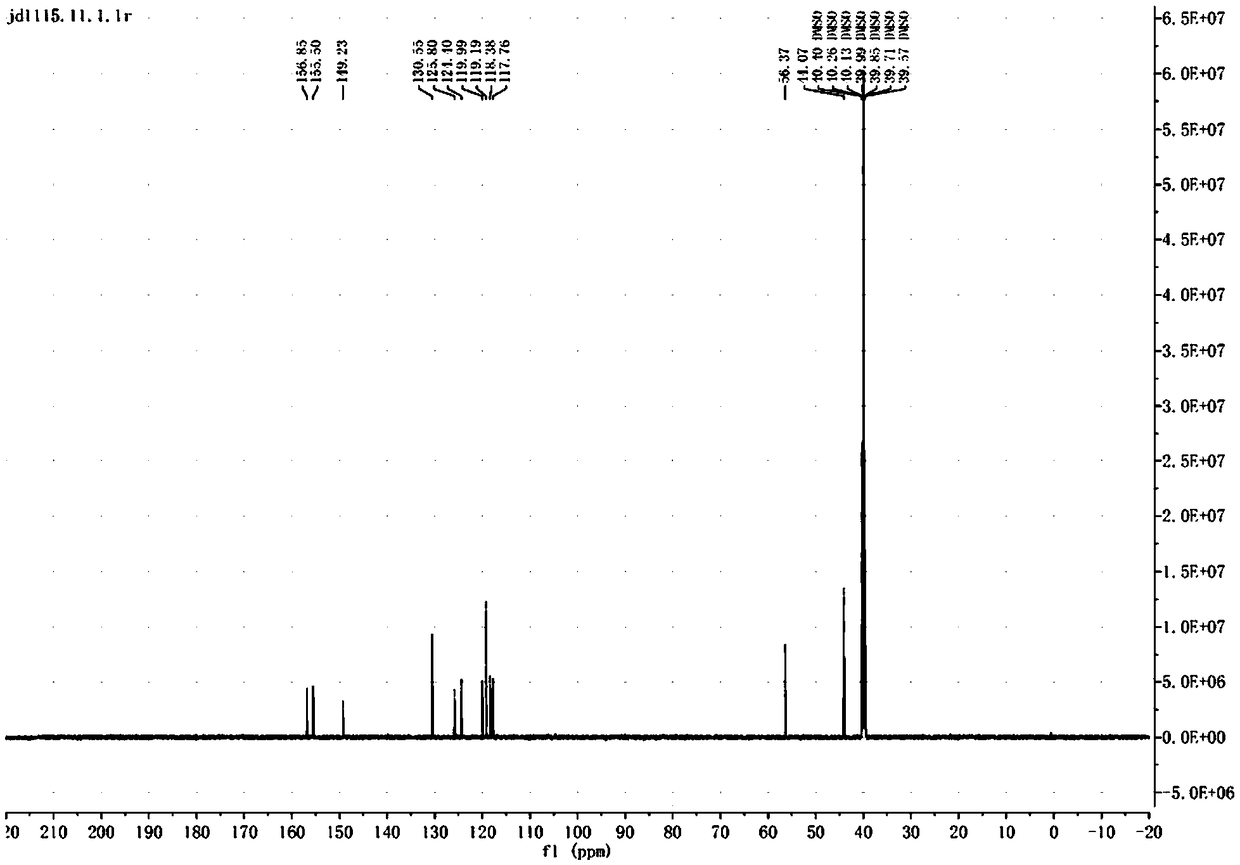
[0037] Dissolve 2.2 mmol of dimethylsulfonamide in 4 mL of solvent xylene, add 2 mmol of 3-iodo-4-methoxy-anisole, 0.2 mmol of Cu(OAc) 2 , 0.4mmol 1,10-phenanthroline, 4mmol potassium carbonate, nitrogen protection, heated to 120 ° C for 5 hours, TLC monitoring complete reaction. Cool to room temperature, add 20 mL of ethyl acetate, filter, wash the filter cake with ethyl acetate, combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate. Suction filtration, and the filtrate was concentrated to obtain a crude product. Column chromatography (petroleum ether: ethyl acetate = 3:1 elution) gave a white solid with a yield of 68%.
Embodiment 3
[0039] The first step: the preparation of 3-iodo-4-methoxy-anisole (7) is the same as the first step in Example 1.
[0040] The second step: the preparation of intermediate impurity 3-(N,N-dimethylsulfonyl)amino-4-phenoxyanisole (5):
[0041] Dissolve 2.4 mmol of dimethylsulfonamide in 3 mL of solvent DMSO, add 2 mmol of 3-iodo-4-methoxy-anisole, 0.2 mmol of Cu(OAc) 2 ·H 2 O, 0.4mmol 1,10-phenanthroline, 4mmol potassium carbonate, nitrogen protection, heated to 120°C for 5 hours, TLC monitoring complete reaction. Cool to room temperature, add 20 mL of ethyl acetate, filter, wash the filter cake with ethyl acetate, combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate. Suction filtration, and the filtrate was concentrated to obtain a crude product. Column chromatography (petroleum ether: ethyl acetate = 3:1 elution) gave a white solid with a yield of 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com