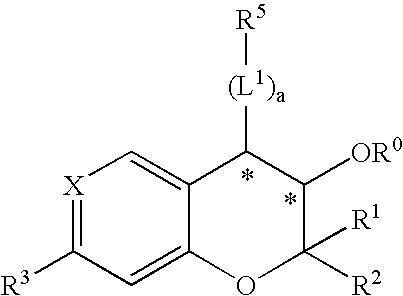
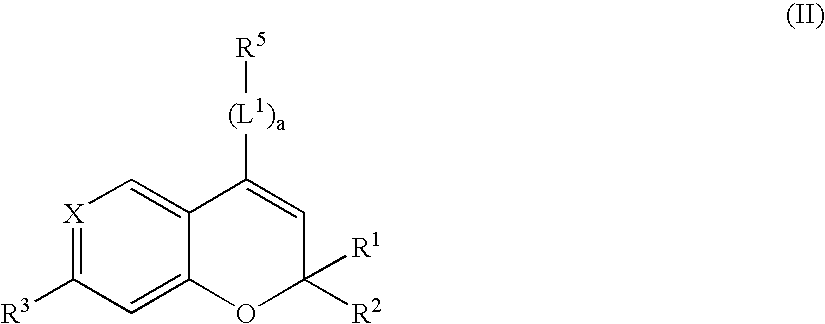
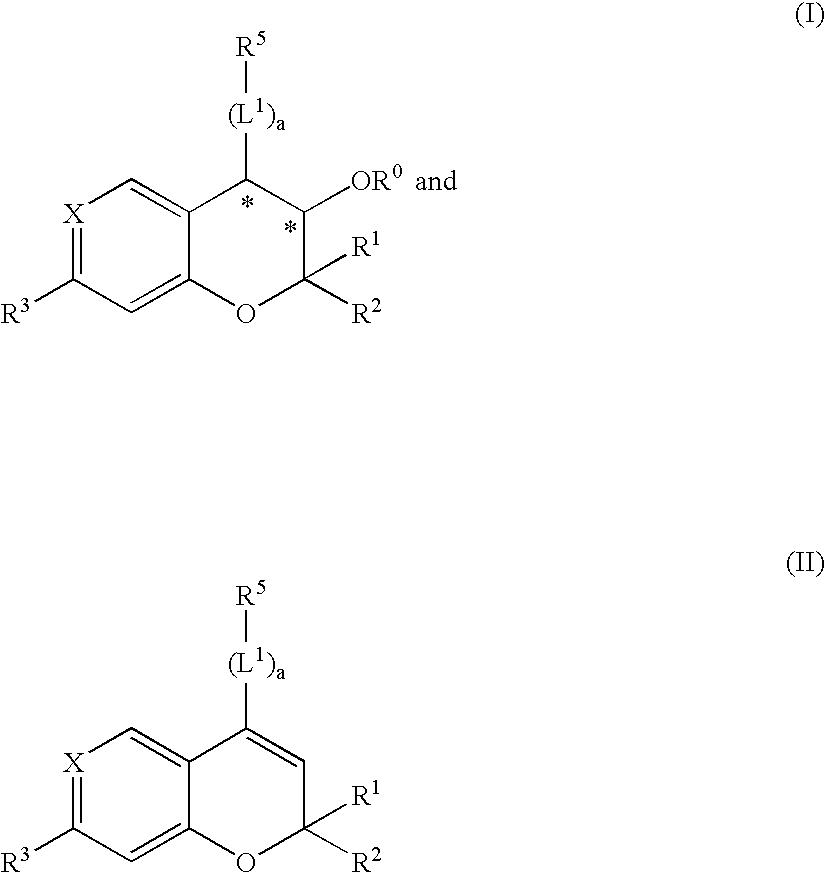
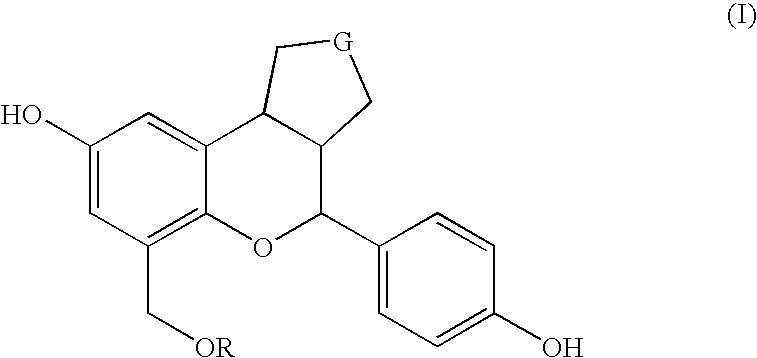
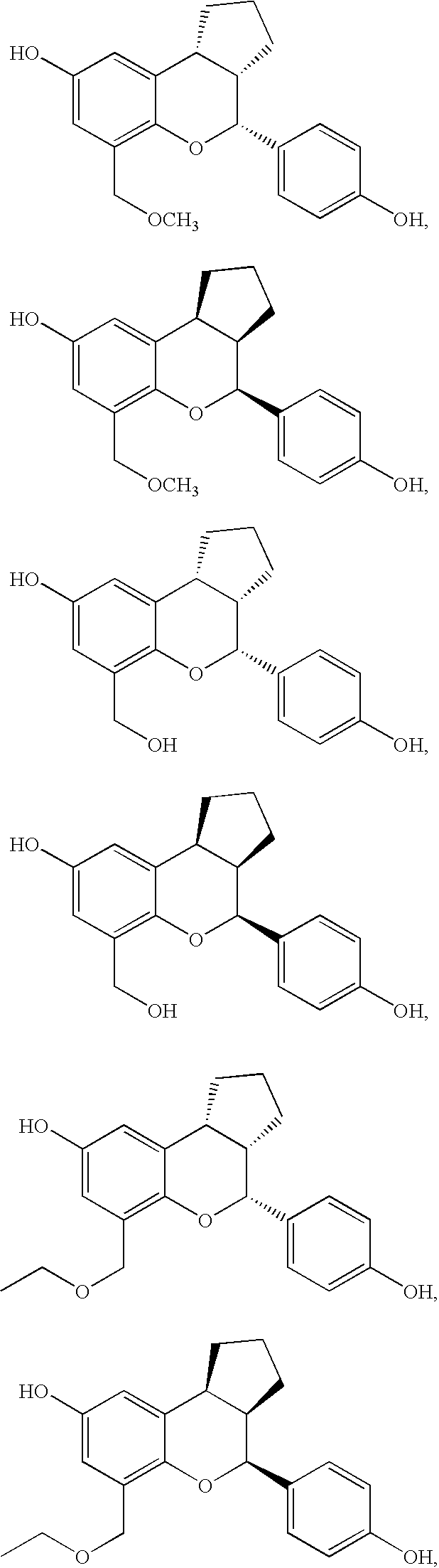
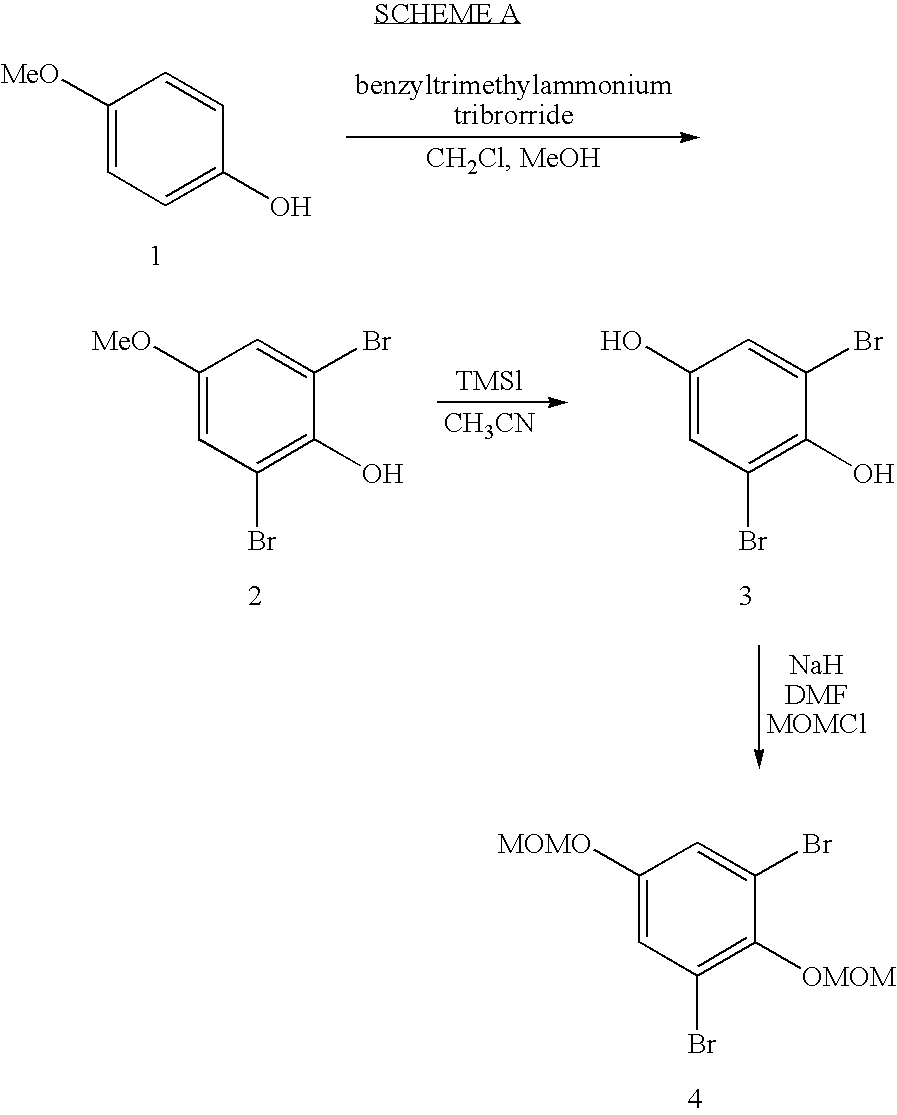
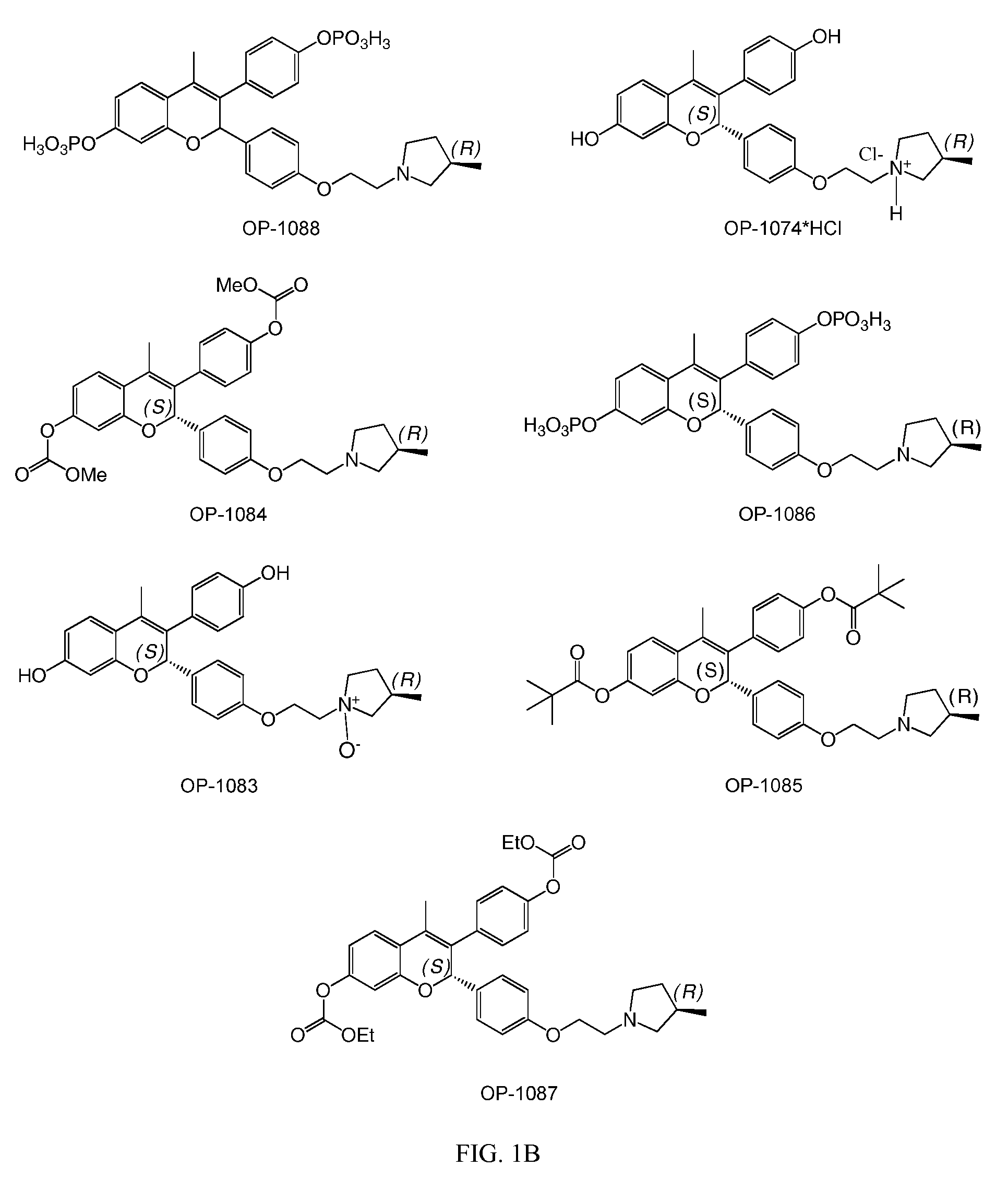
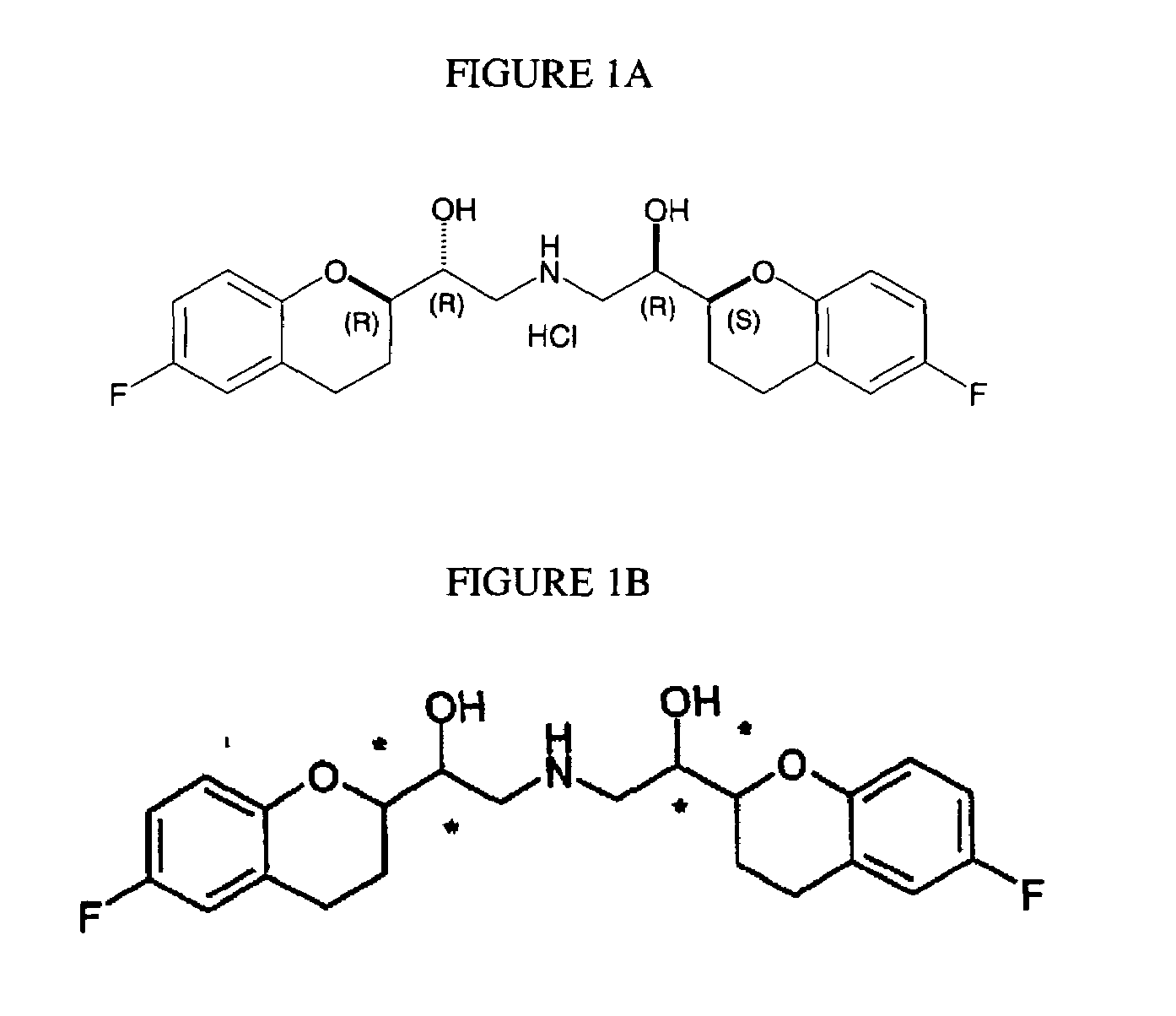
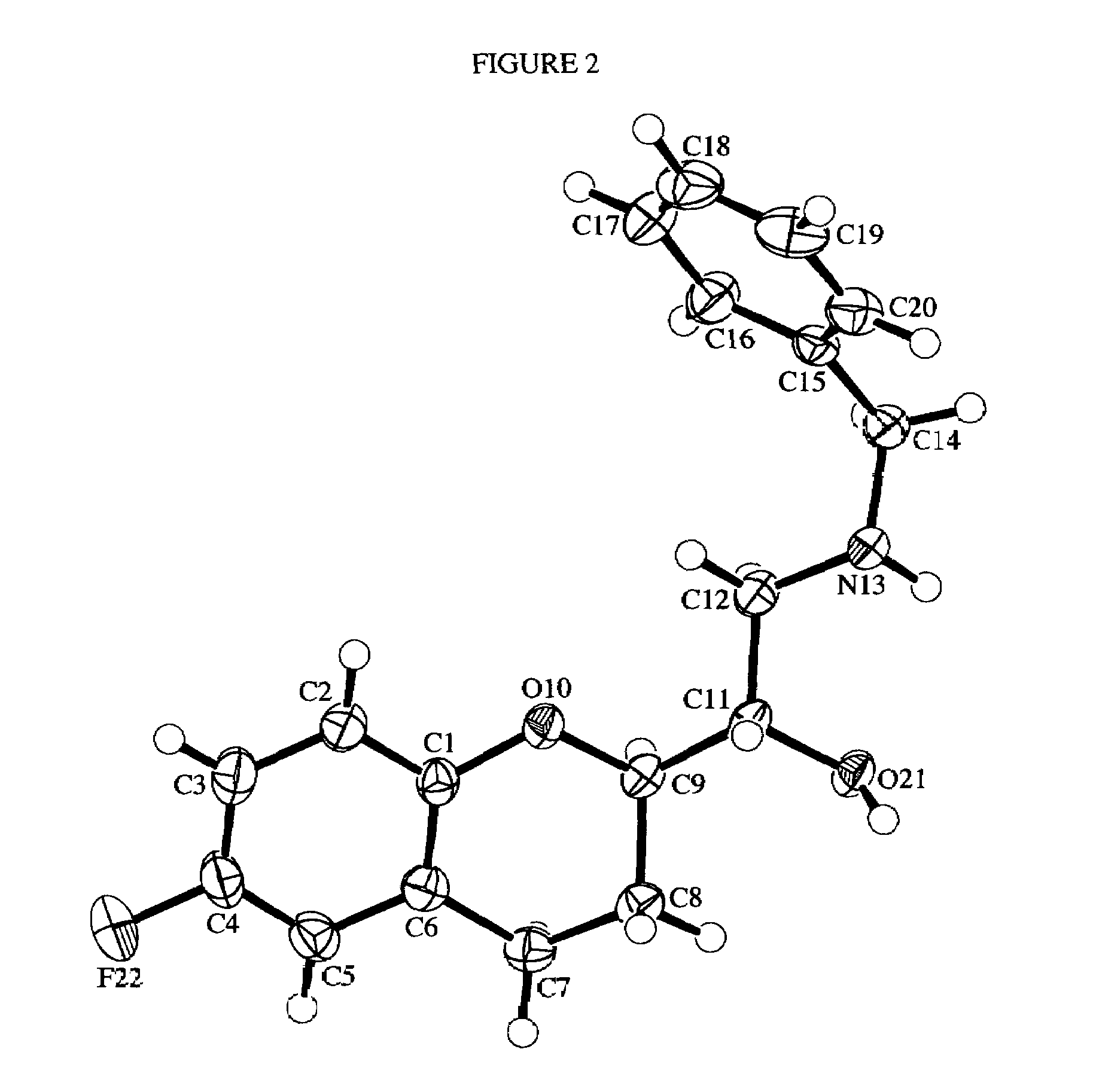
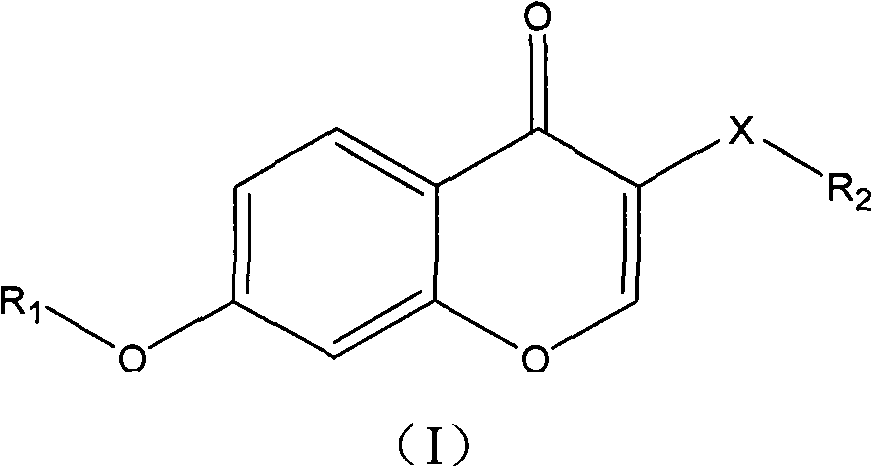
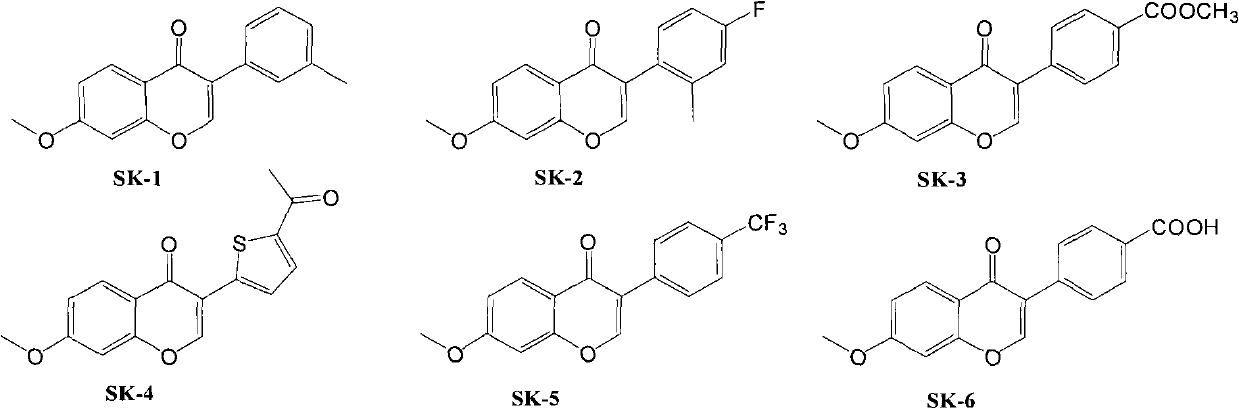
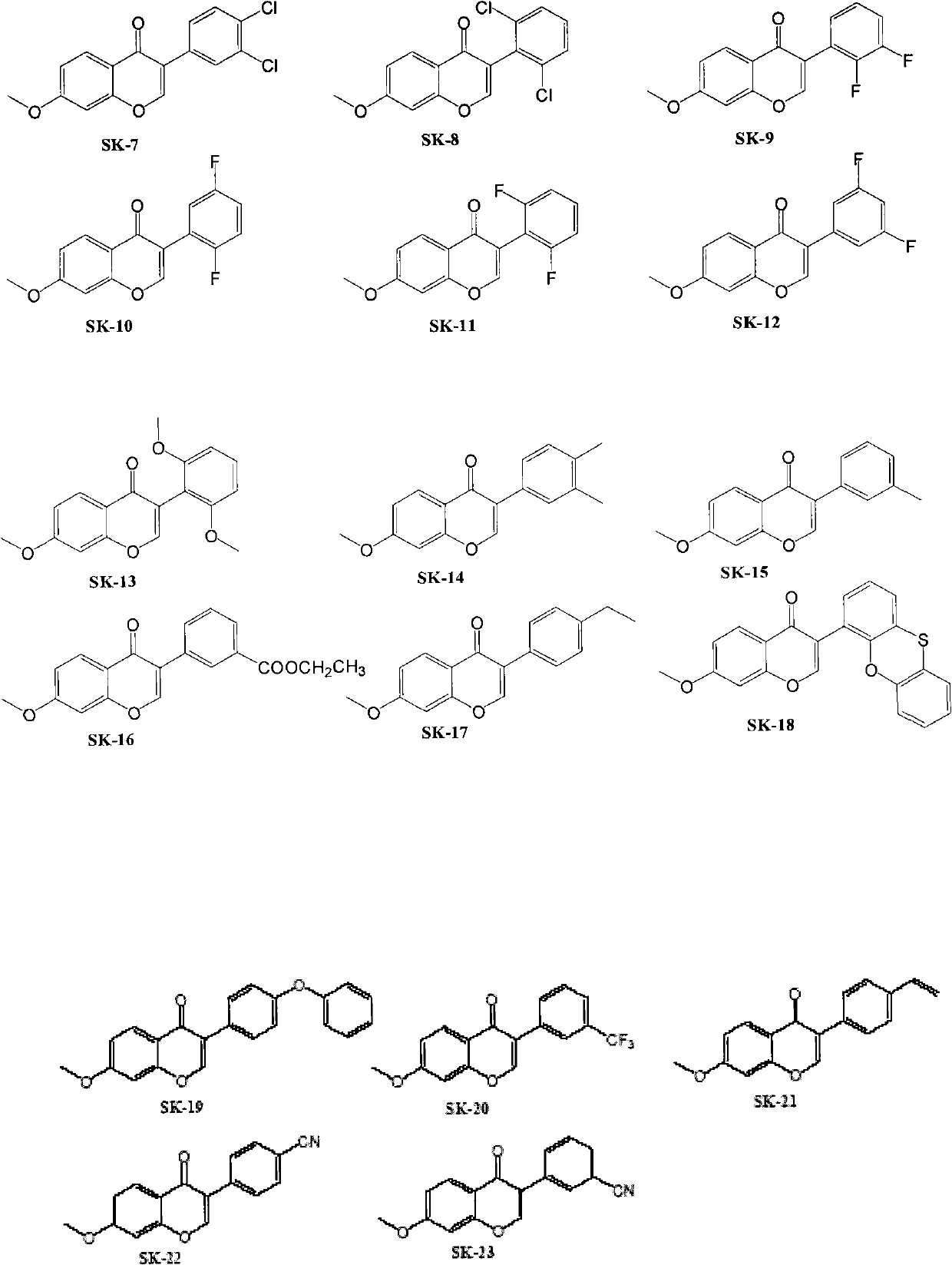
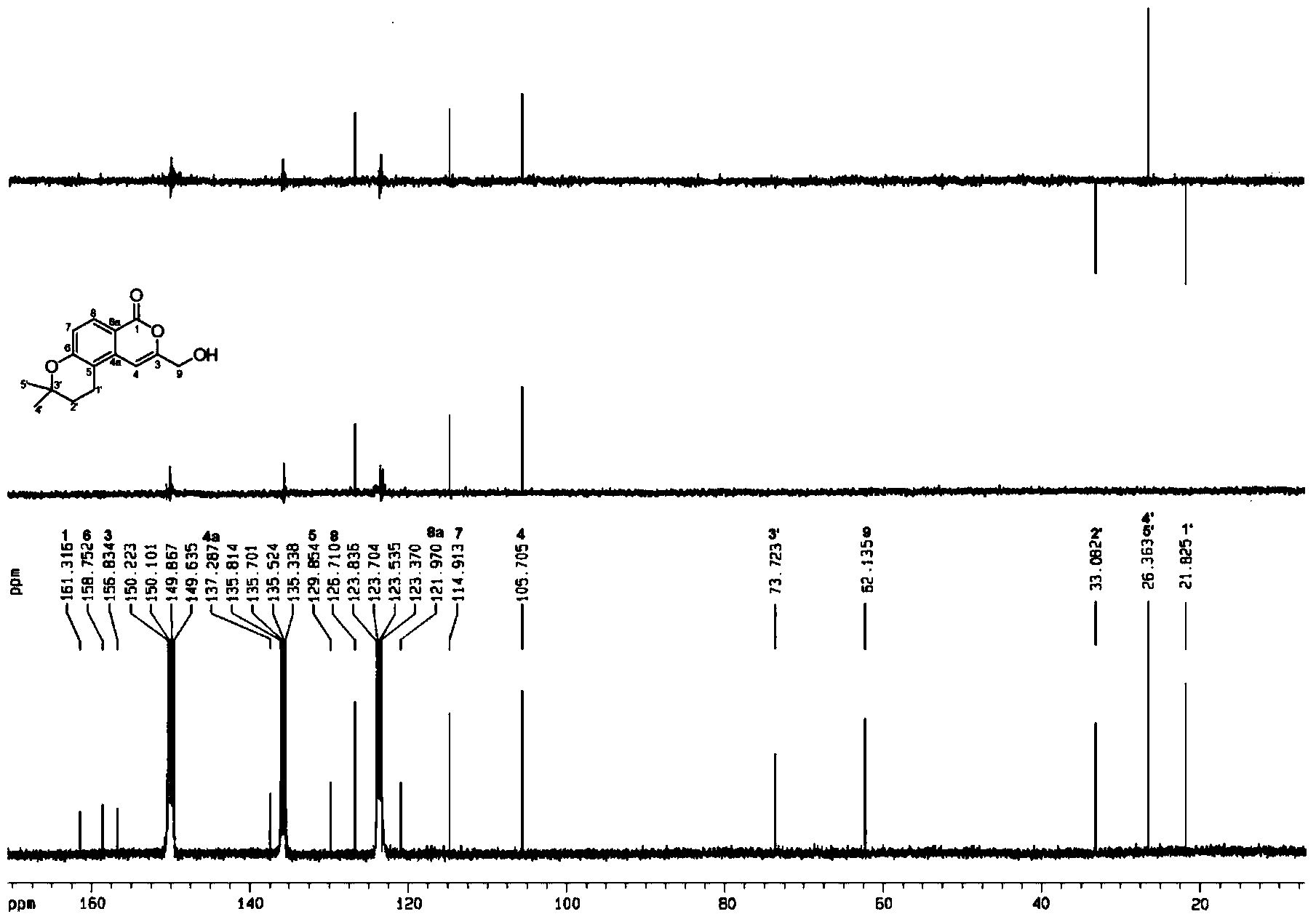
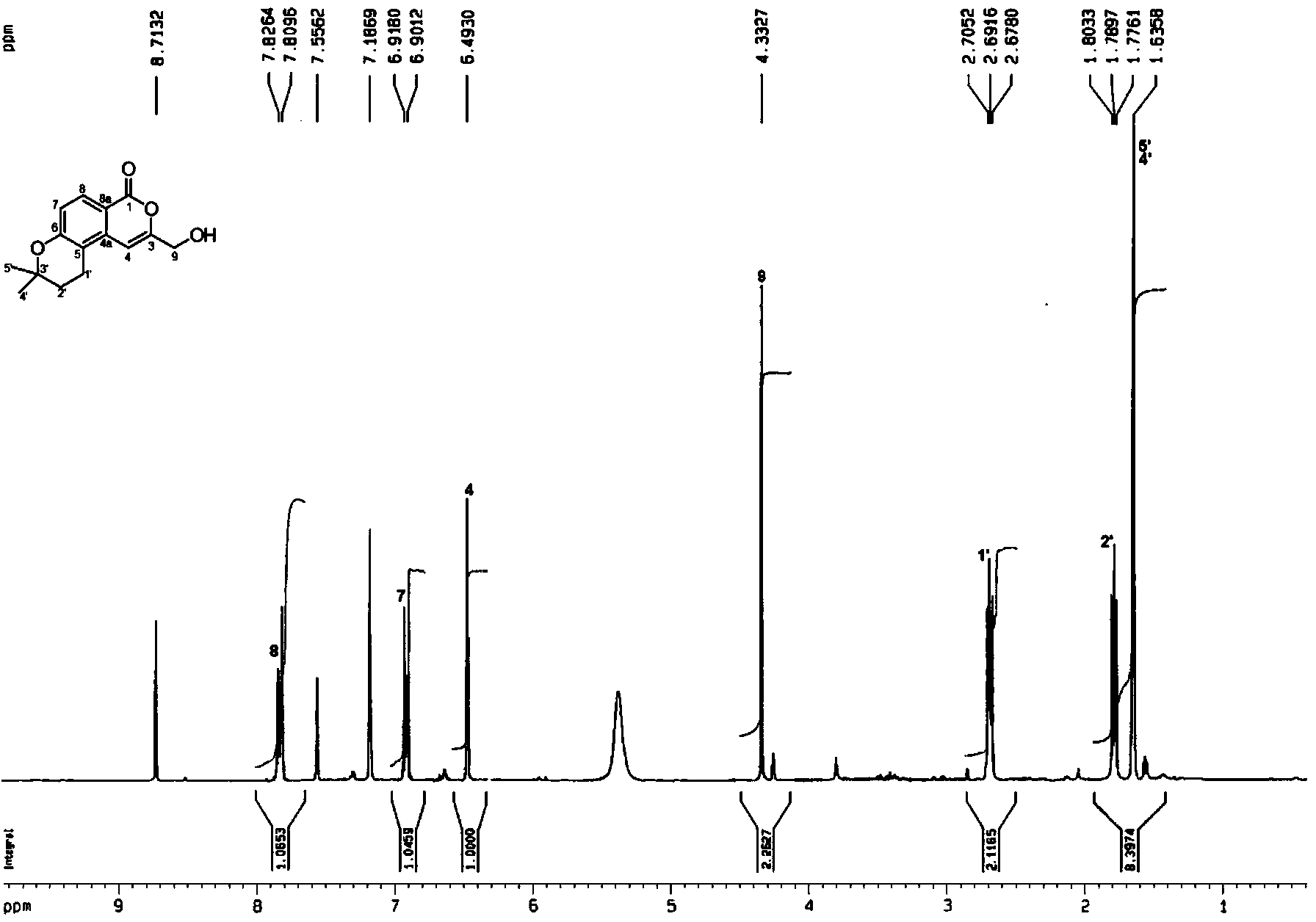
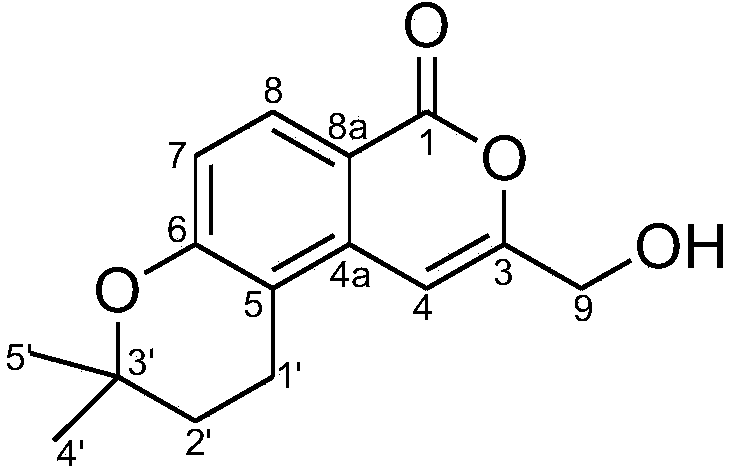
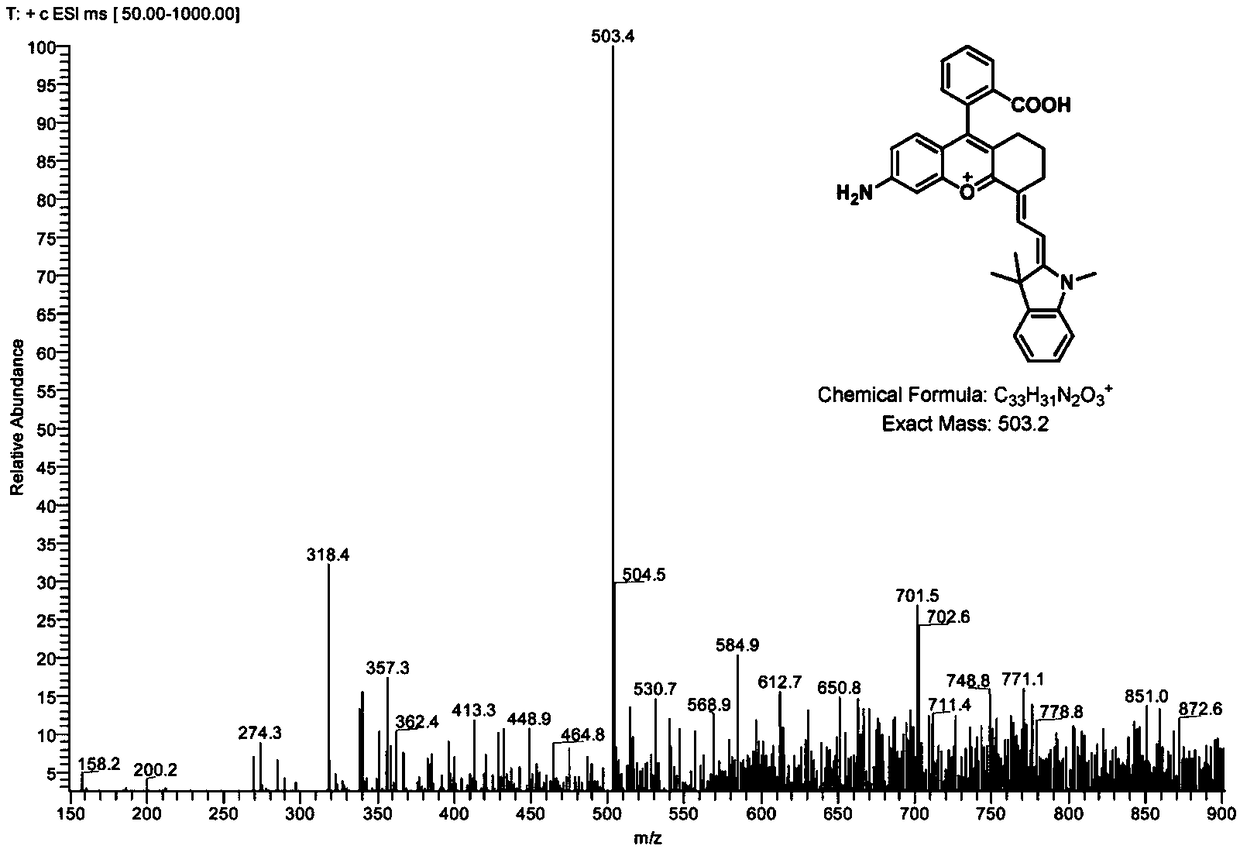
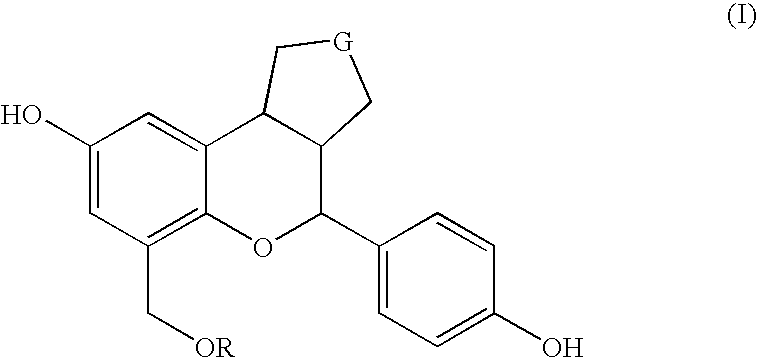
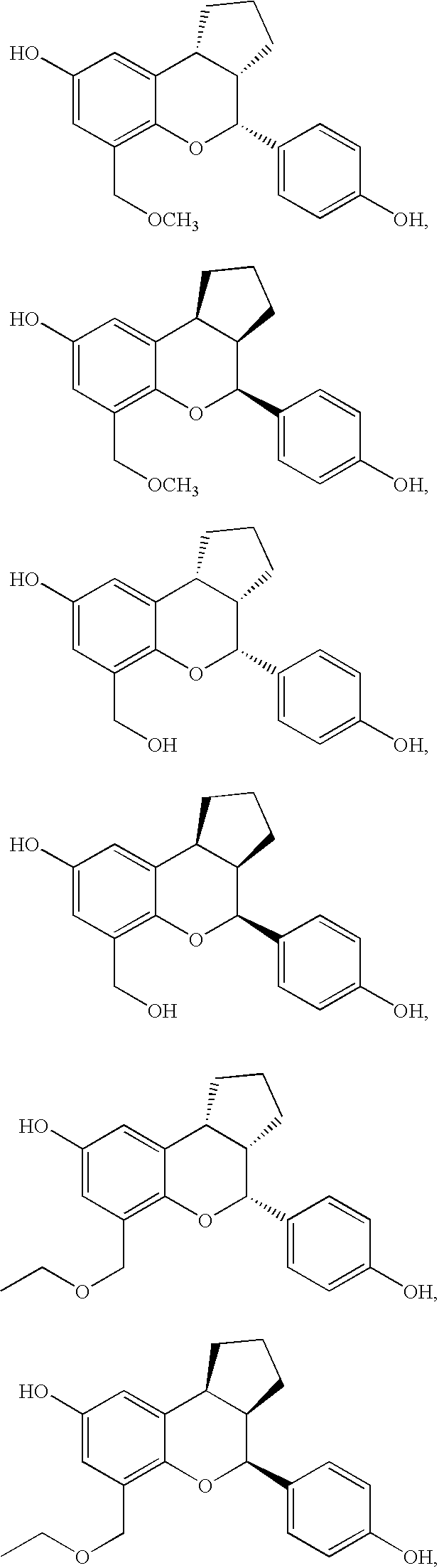
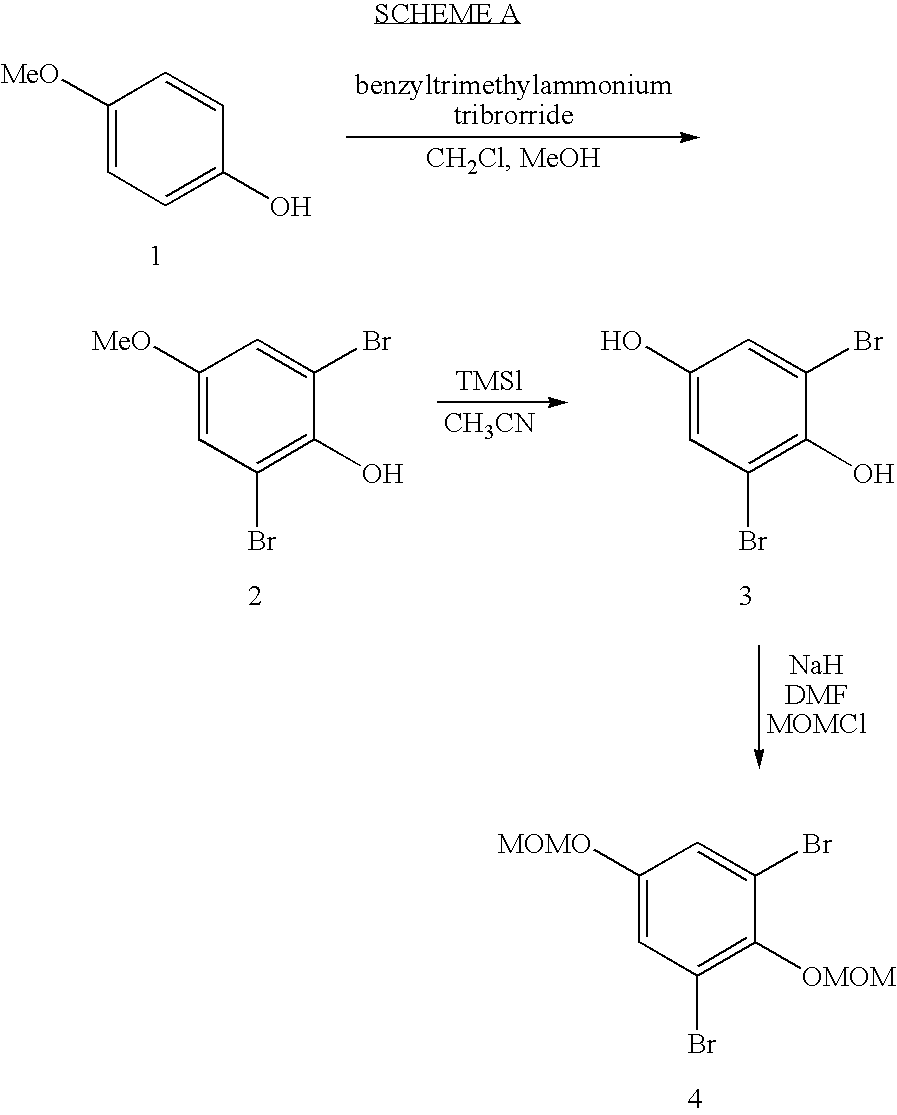
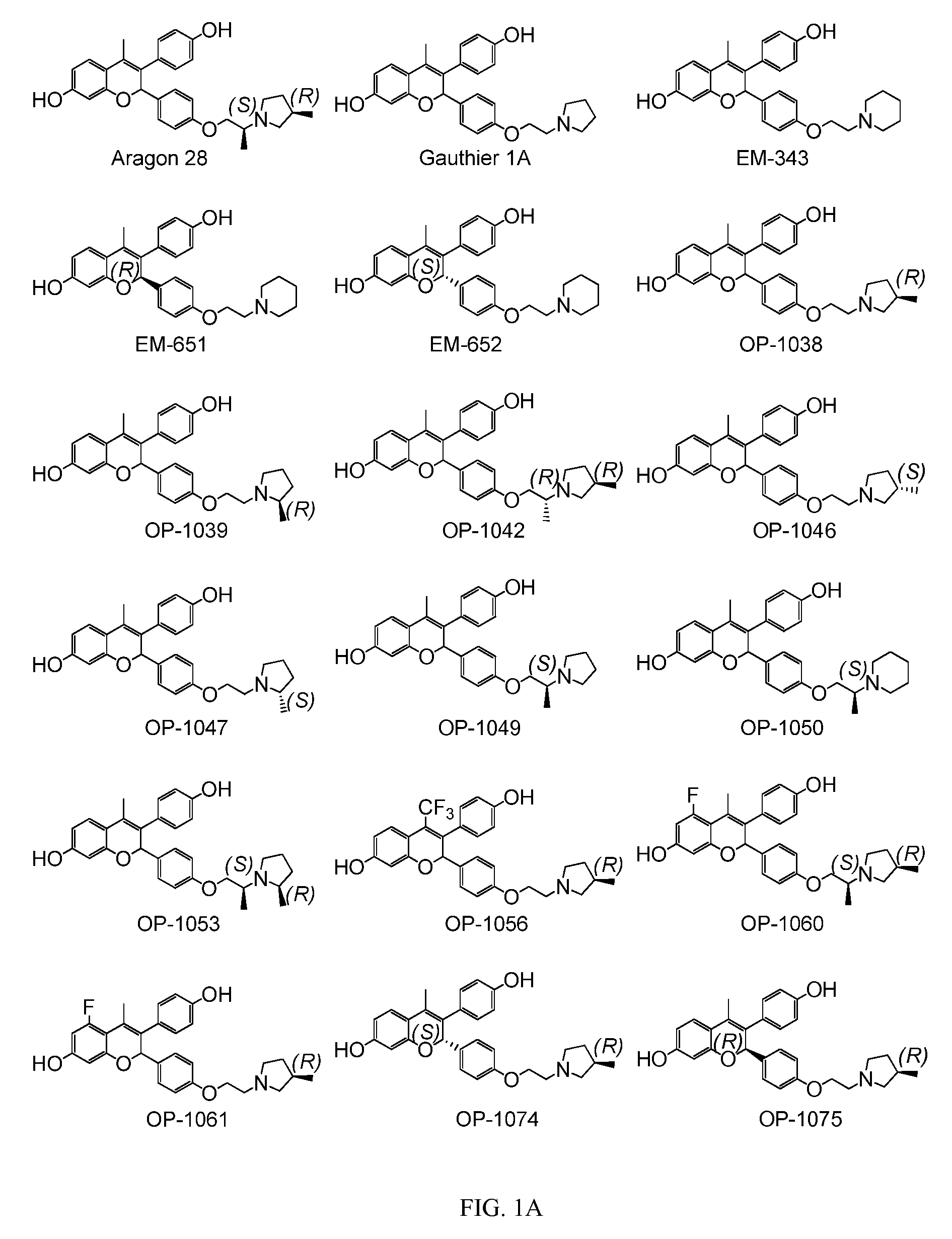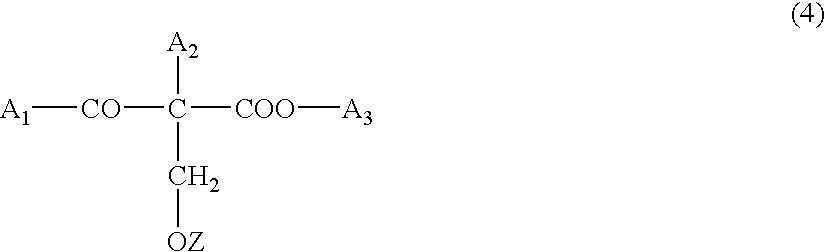Patents
Literature
367 results about "Benzopyran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
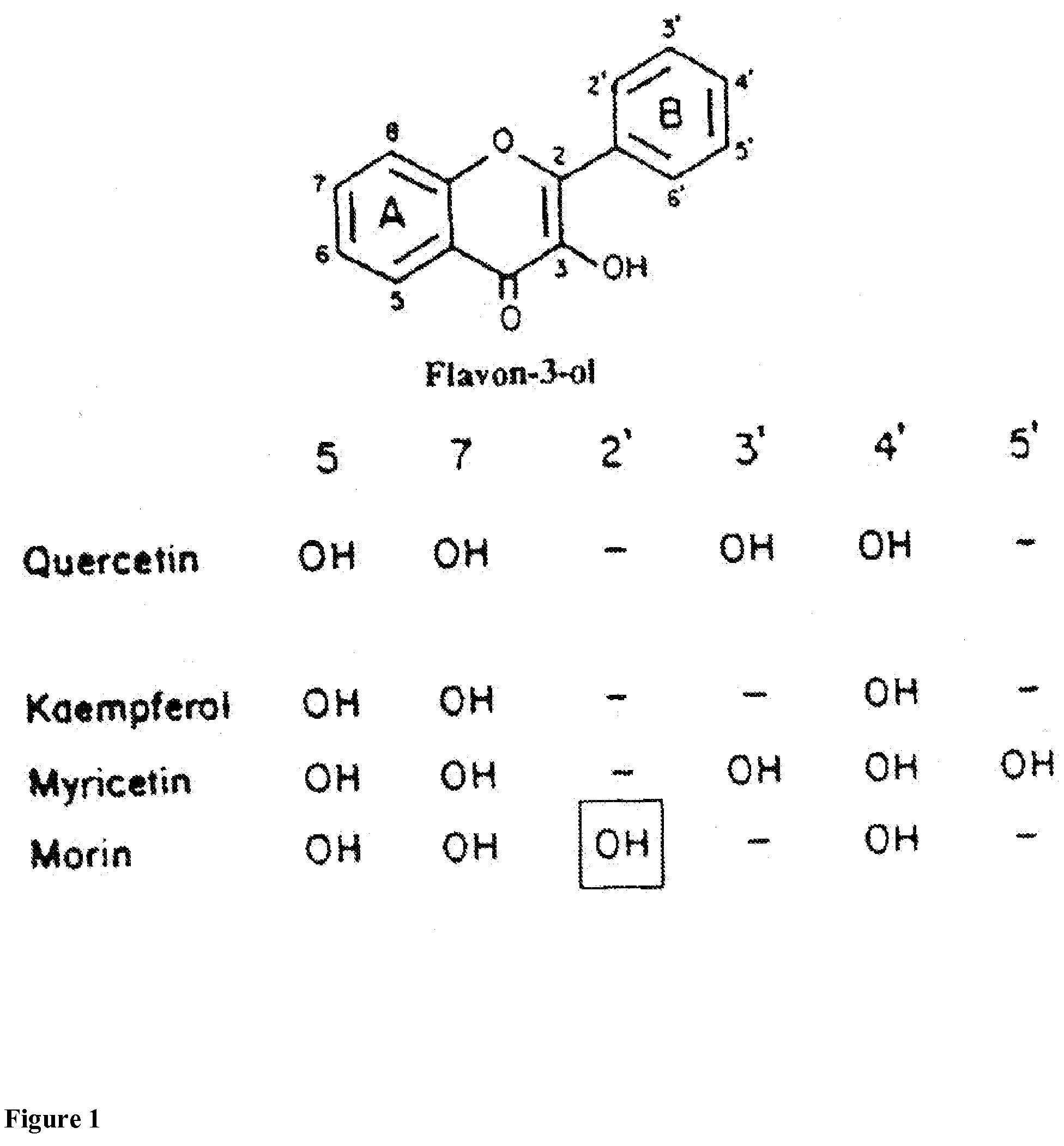
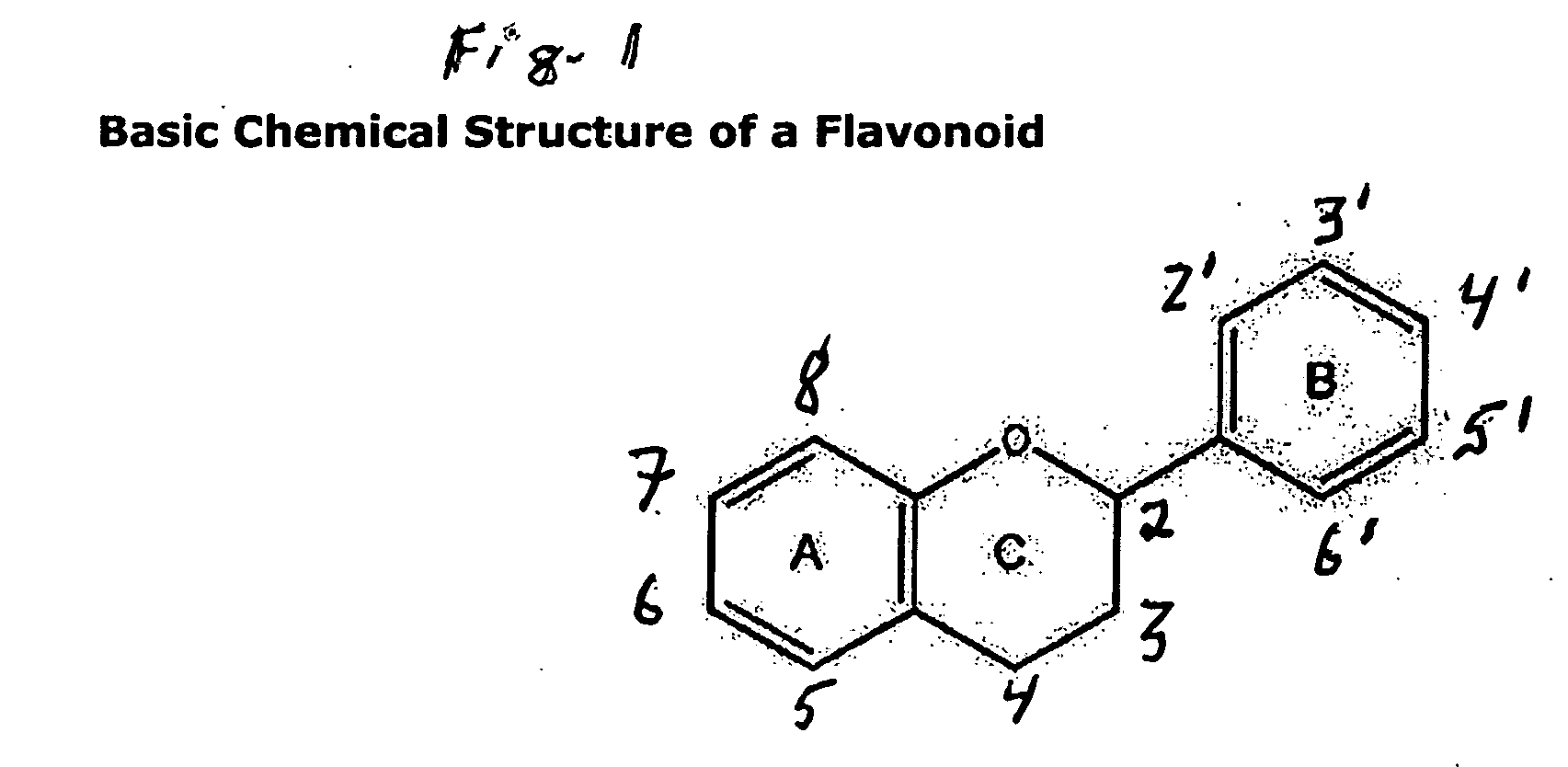
Benzopyran is a polycyclic organic compound that results from the fusion of a benzene ring to a heterocyclic pyran ring. According to current IUPAC nomenclature, the name chromene used in previous recommendations is retained; however, systematic ‘benzo’ names, for example 2H-1-benzopyran, are preferred IUPAC names for chromene, isochromene, chromane, isochromane, and their chalcogen analogues. There are two isomers of benzopyran that vary by the orientation of the fusion of the two rings compared to the oxygen, resulting in 1-benzopyran (chromene) and 2-benzopyran (isochromene)—the number denotes where the oxygen atom is located by standard naphthalene-like nomenclature.
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
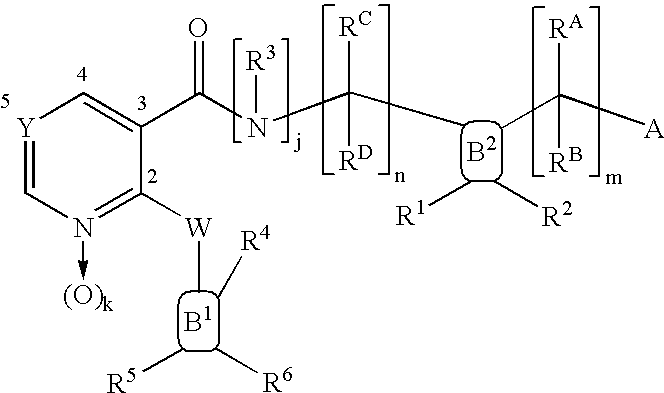
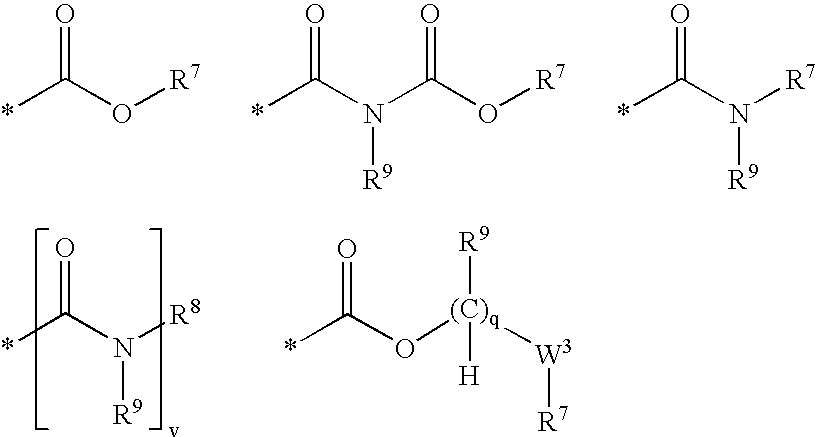
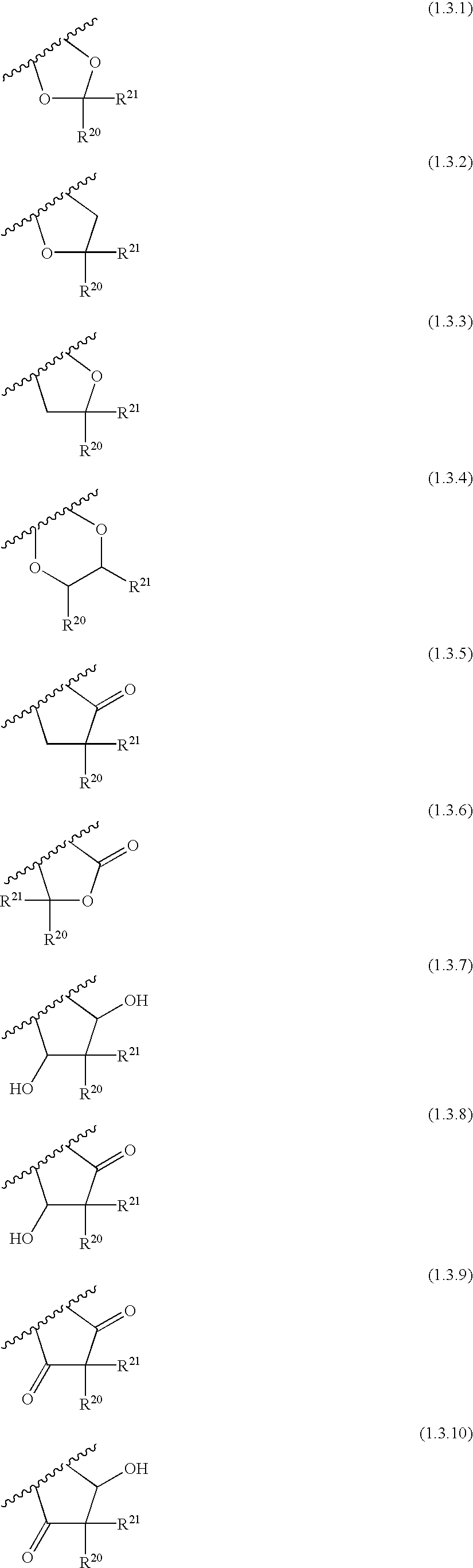
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Benzopyran-containing compounds and method for their use
InactiveUS6060503AAvoid conversionEasy to synthesizeBiocideOrganic compound preparationDiseaseBenzopyran
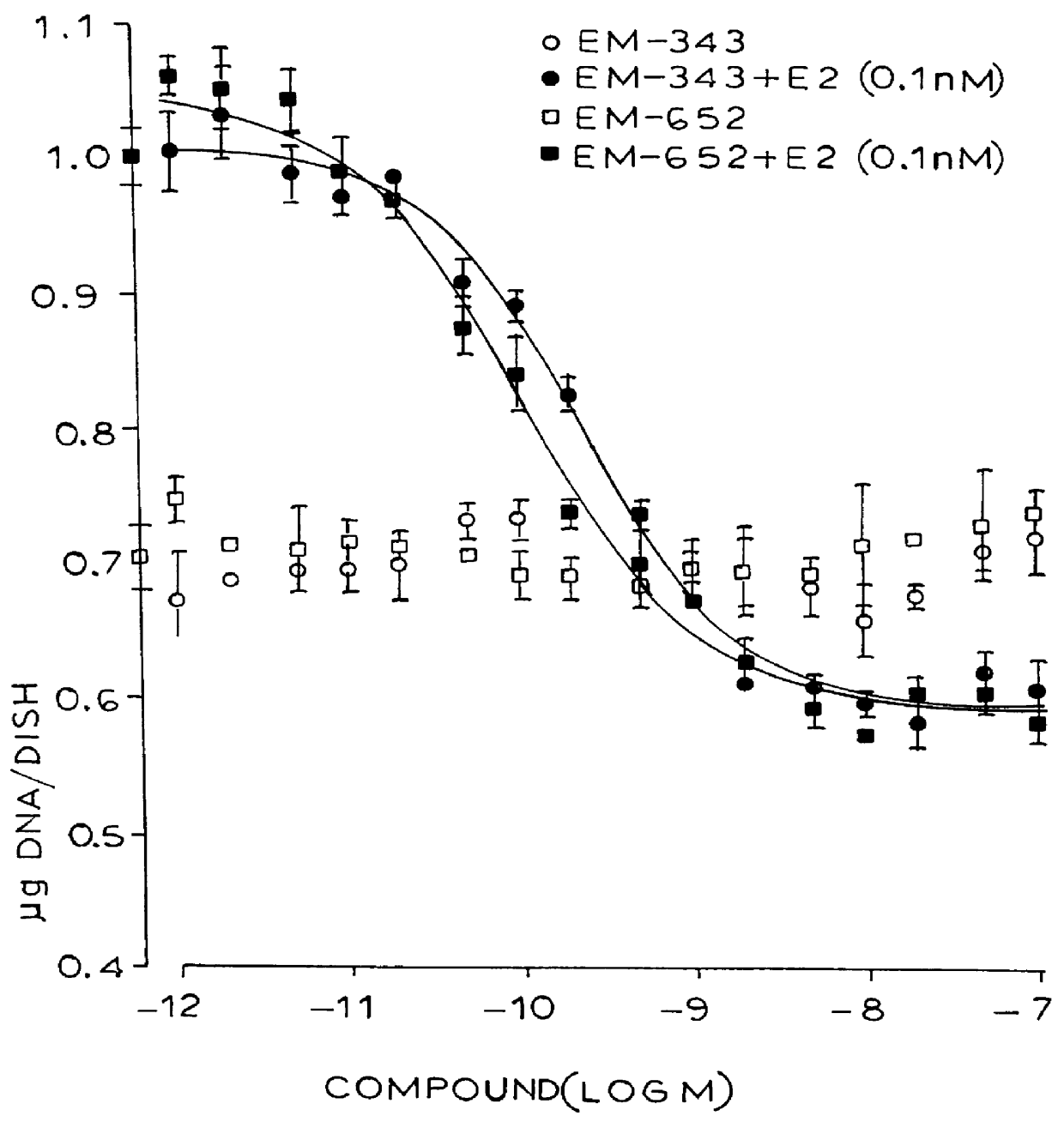
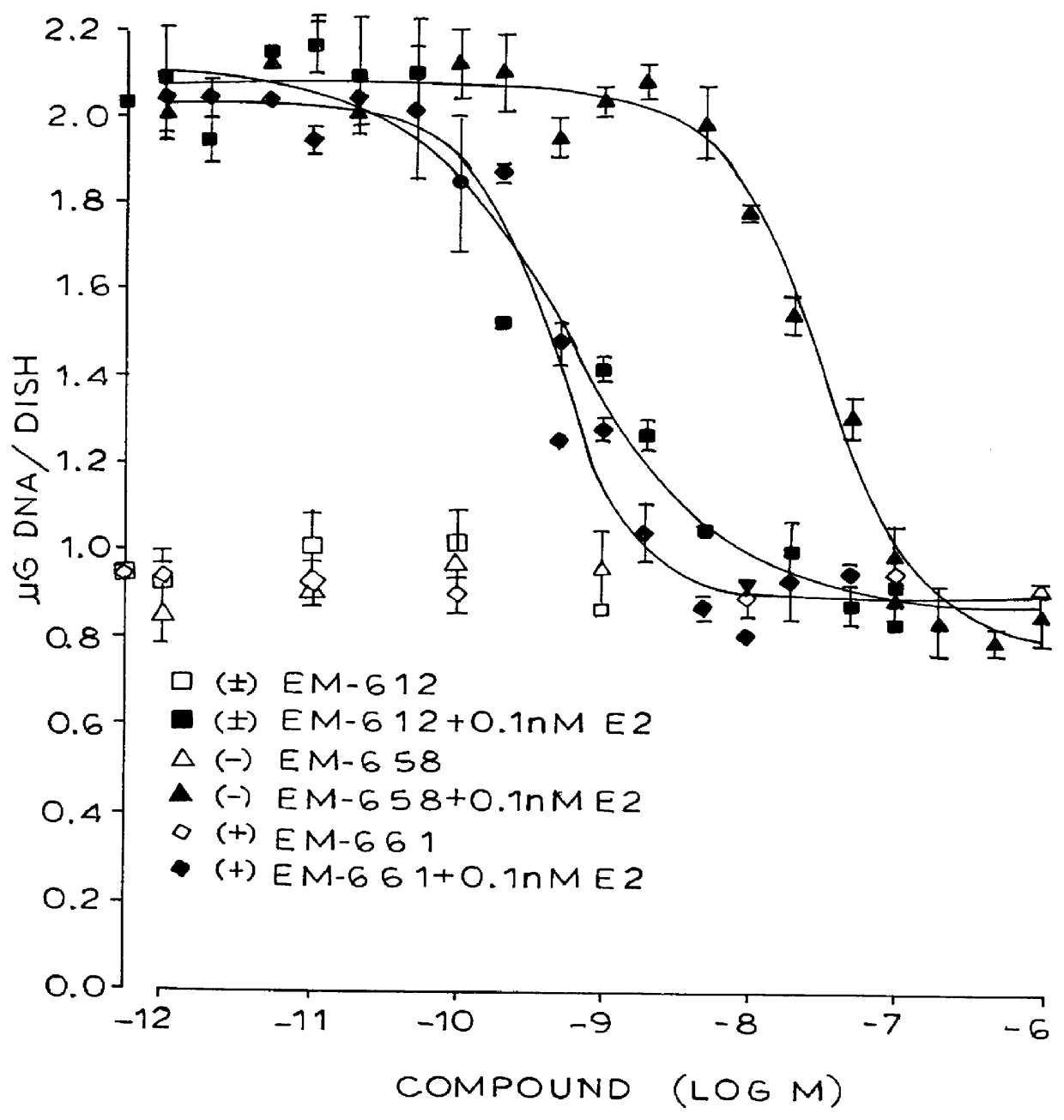
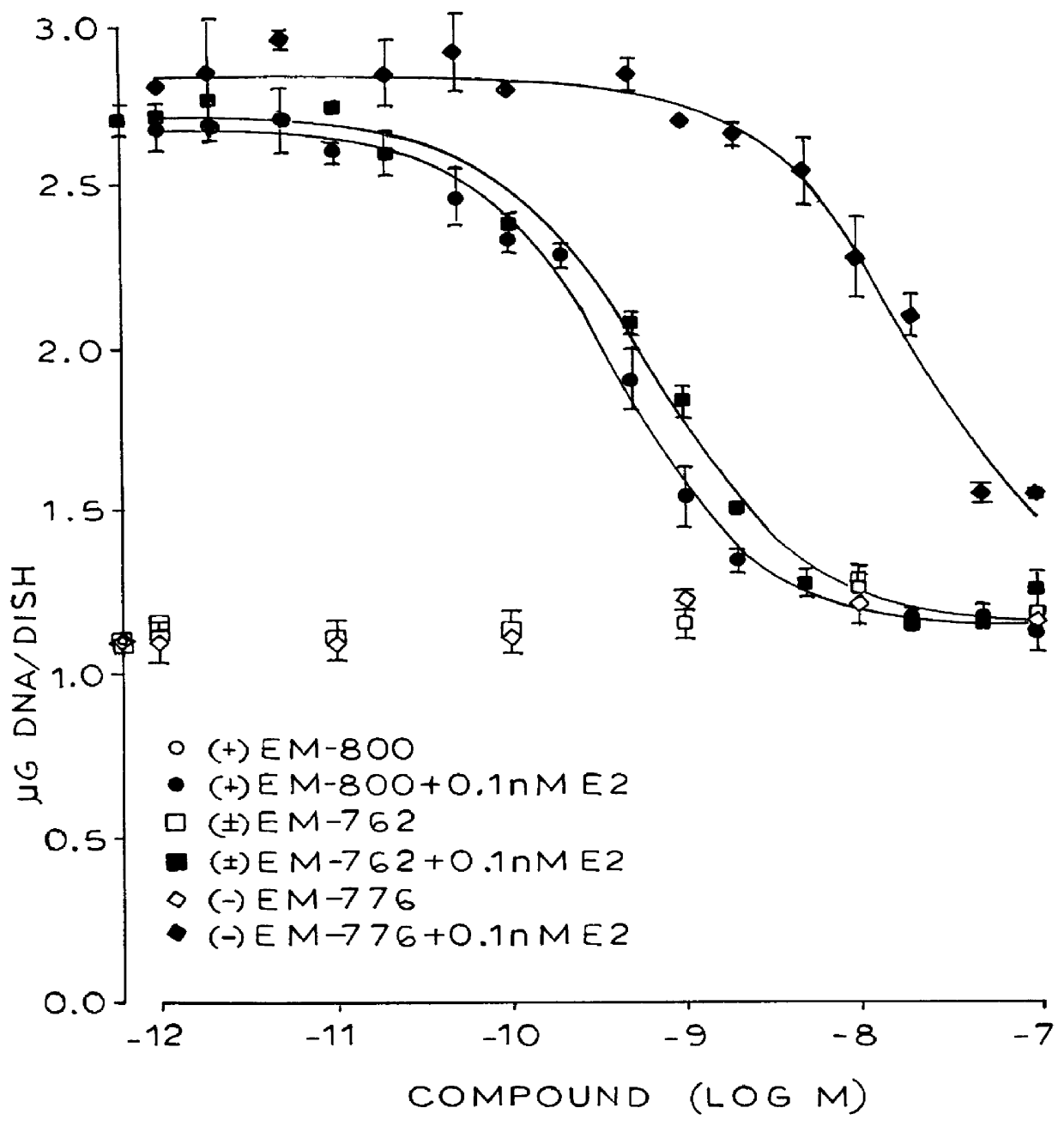
Certain benzopyran antiestrogens are disclosed for treating estrogen sensitive diseases such as breast cancer. Prodrug forms provide ease of manufacturing, good shelf life, and bioavailability, and preferred stereoisomers are shown to be more effective than racemic mixtures.
Owner:ENDORES & DEV
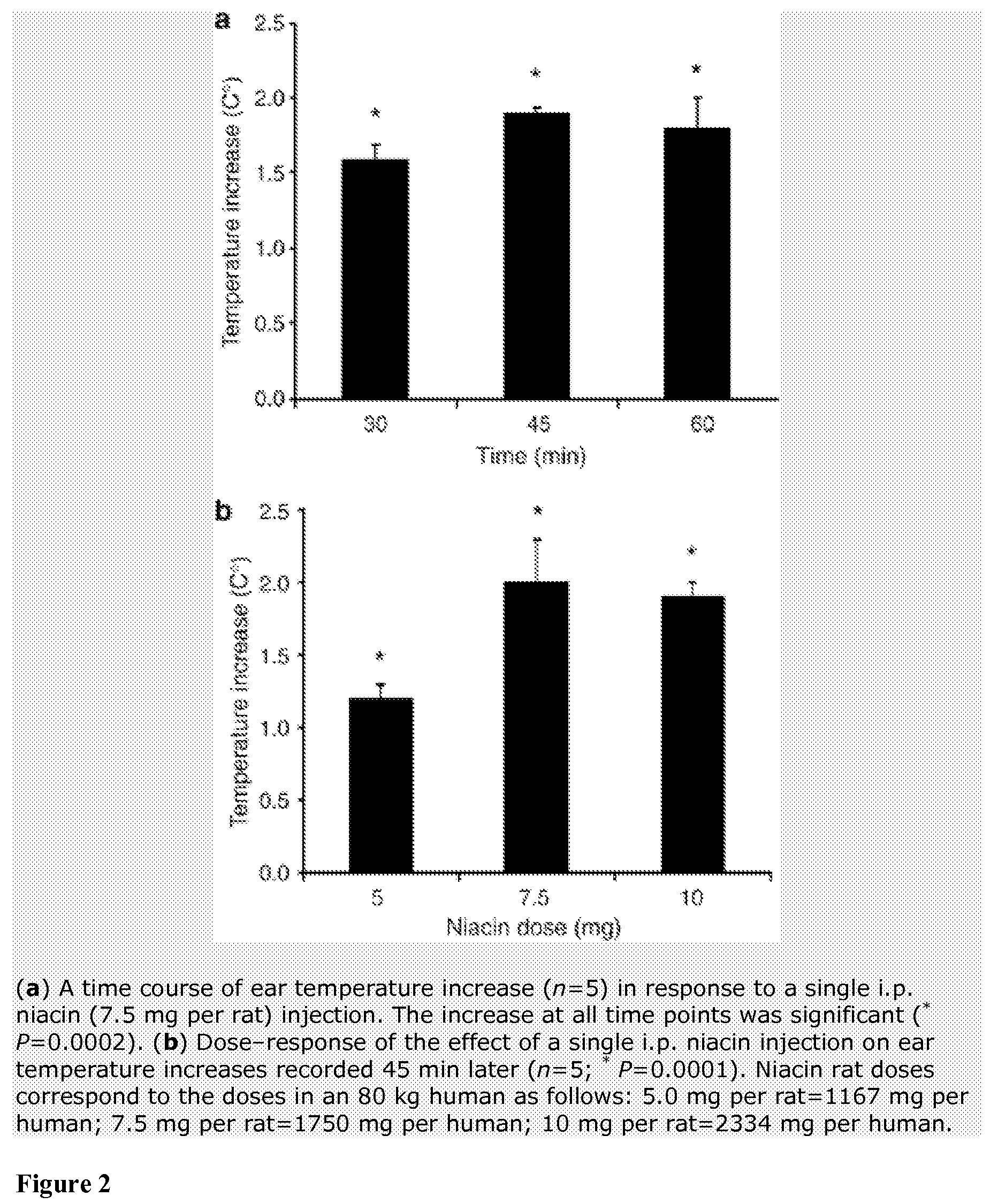
Method for protecting humans against superficial vasodilator flush syndrome,
InactiveUS20090148543A1Significant timeImprove the level ofAntibacterial agentsBiocideSulfate proteoglycanS-Adenosyl-l-methionine
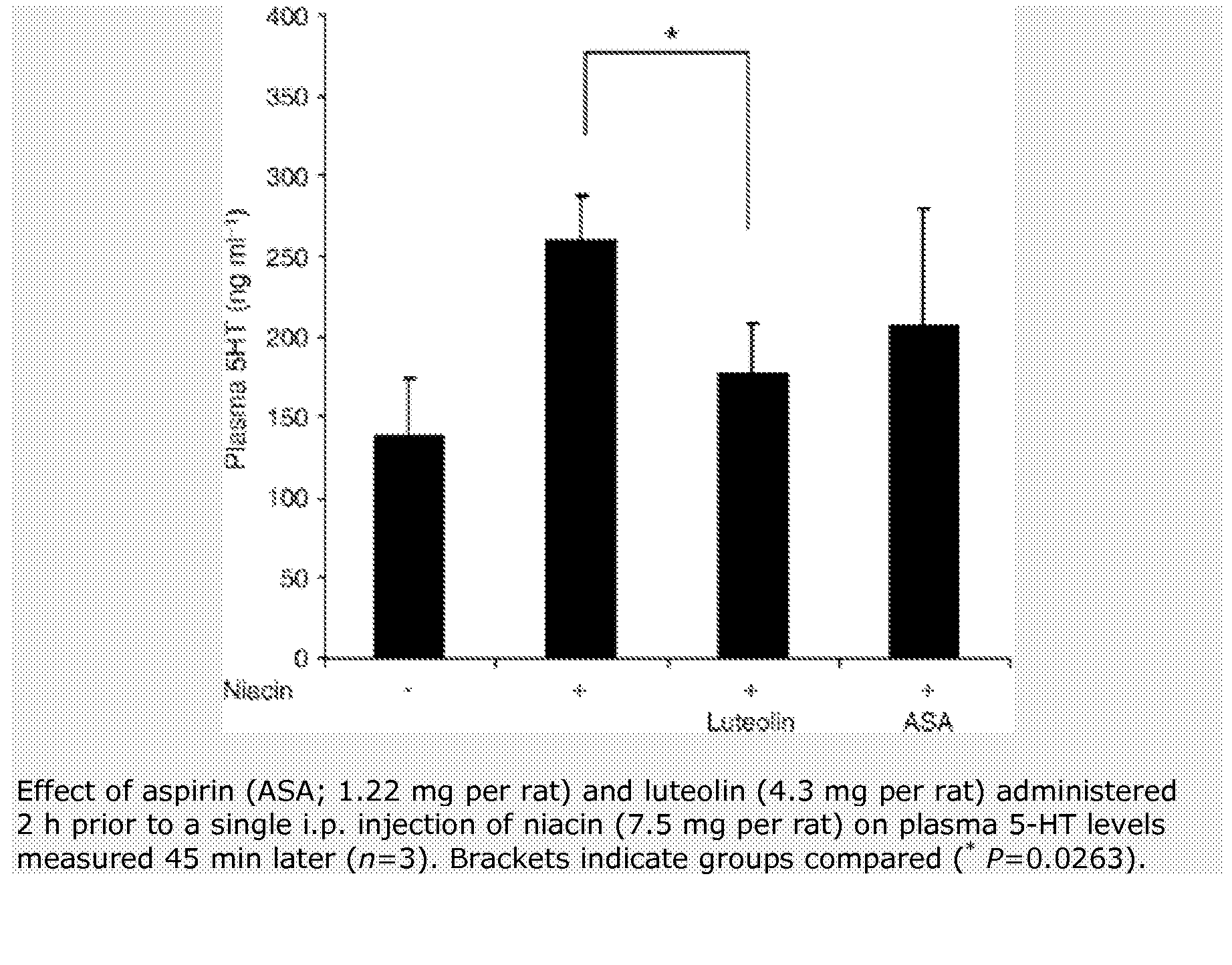
Methods for protection of a human from SVFS comprise the administration of a flavonoid compound of the basic structures 2-phenyl-4H-1-benzopyran or 2-phenyl-4-keto-1-benzopyran or glycosides thereof, alone or, optionally, together with one or more of an olive kernel extract, a non-bovine sulfated proteoglycan, bitter willow extract, a D-hexosamine sulfate, S-adenosylmethionine, folic acid, vitamin B12 and a serotonin inhibitor. Such treatment prevents, reduces or eliminates SVFS in patients receiving as much as 300-3000 mg / day of niacin therapeutically, whether administered prior to, or along with, an anti-SVFS composition.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
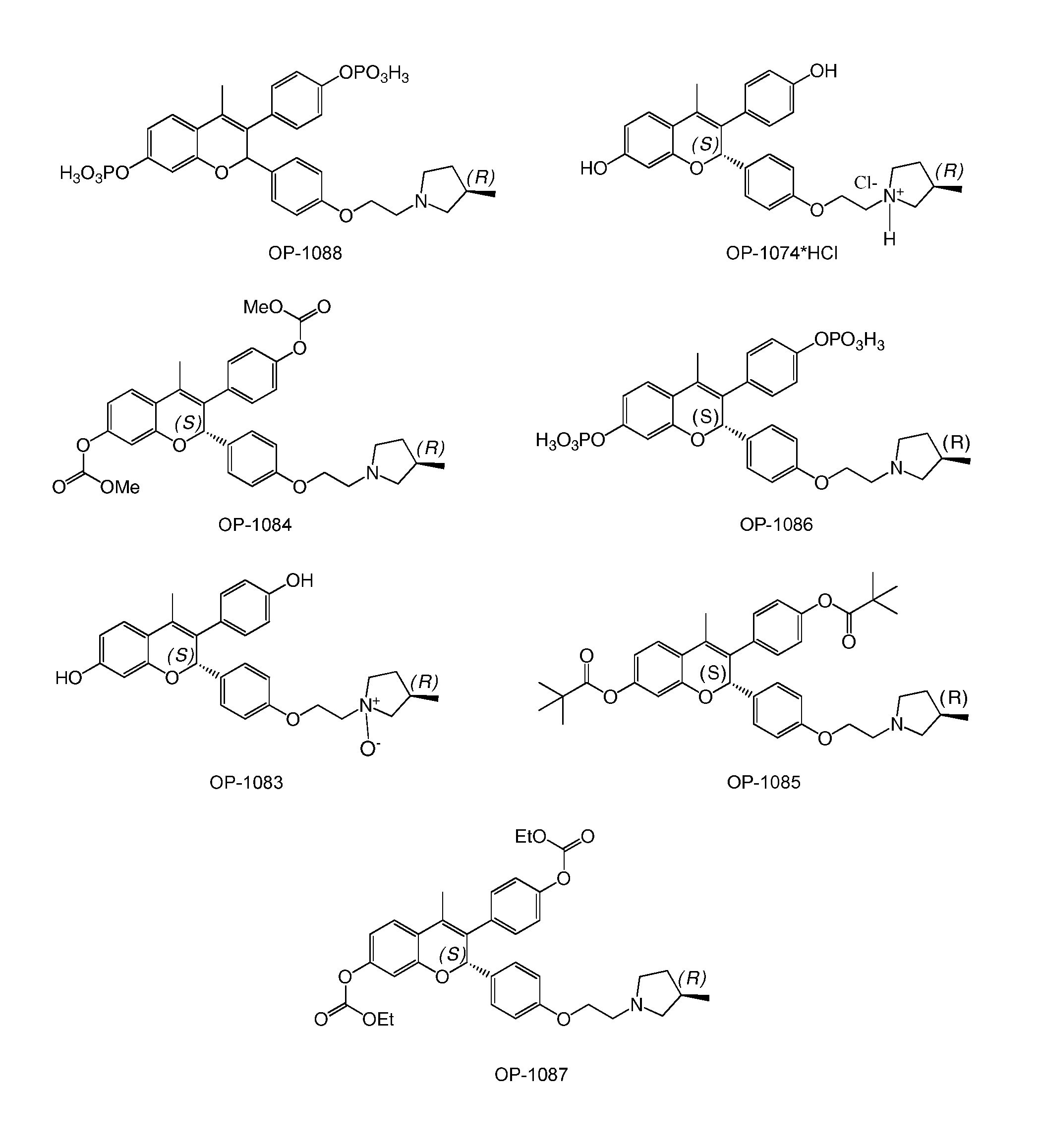
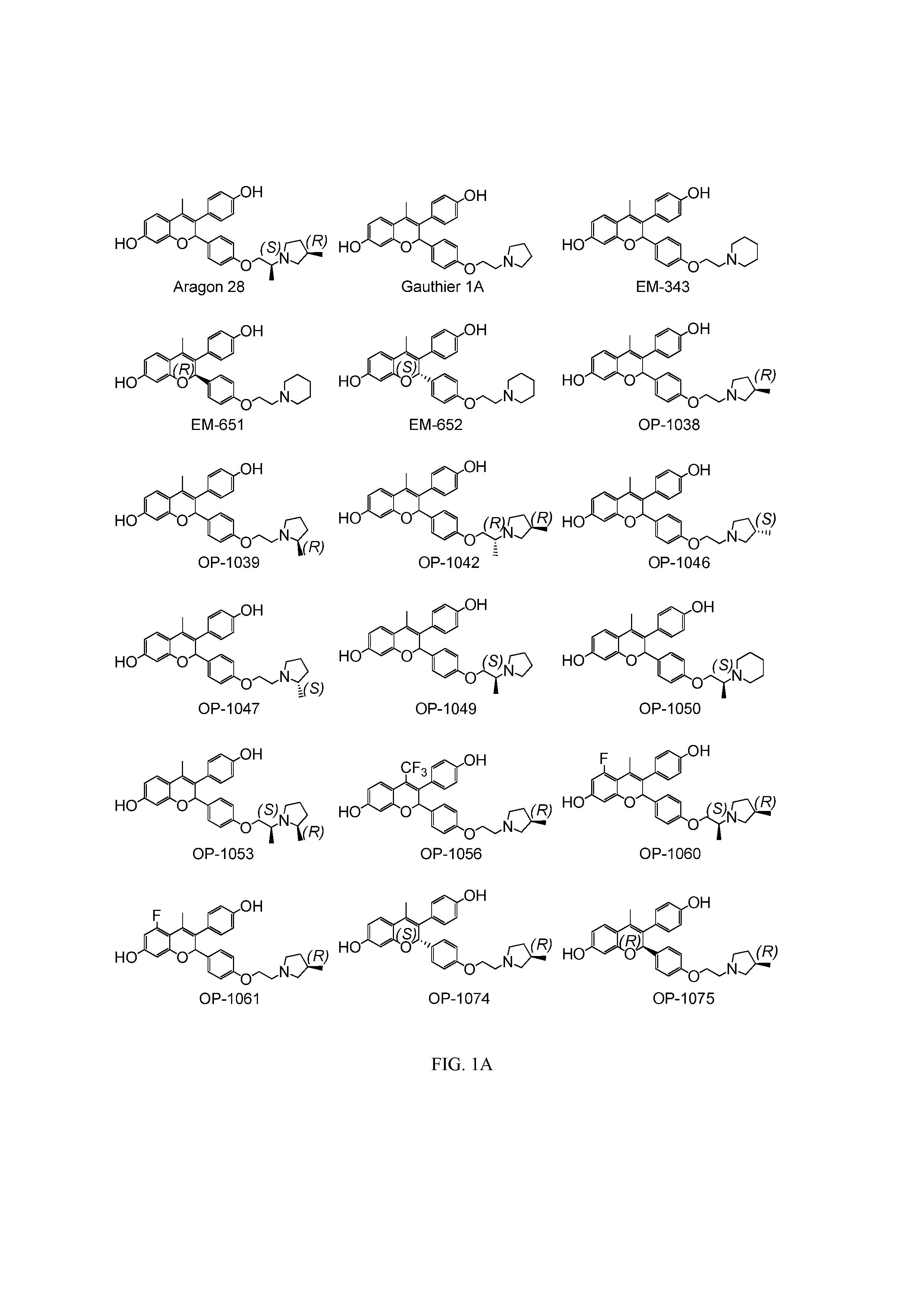
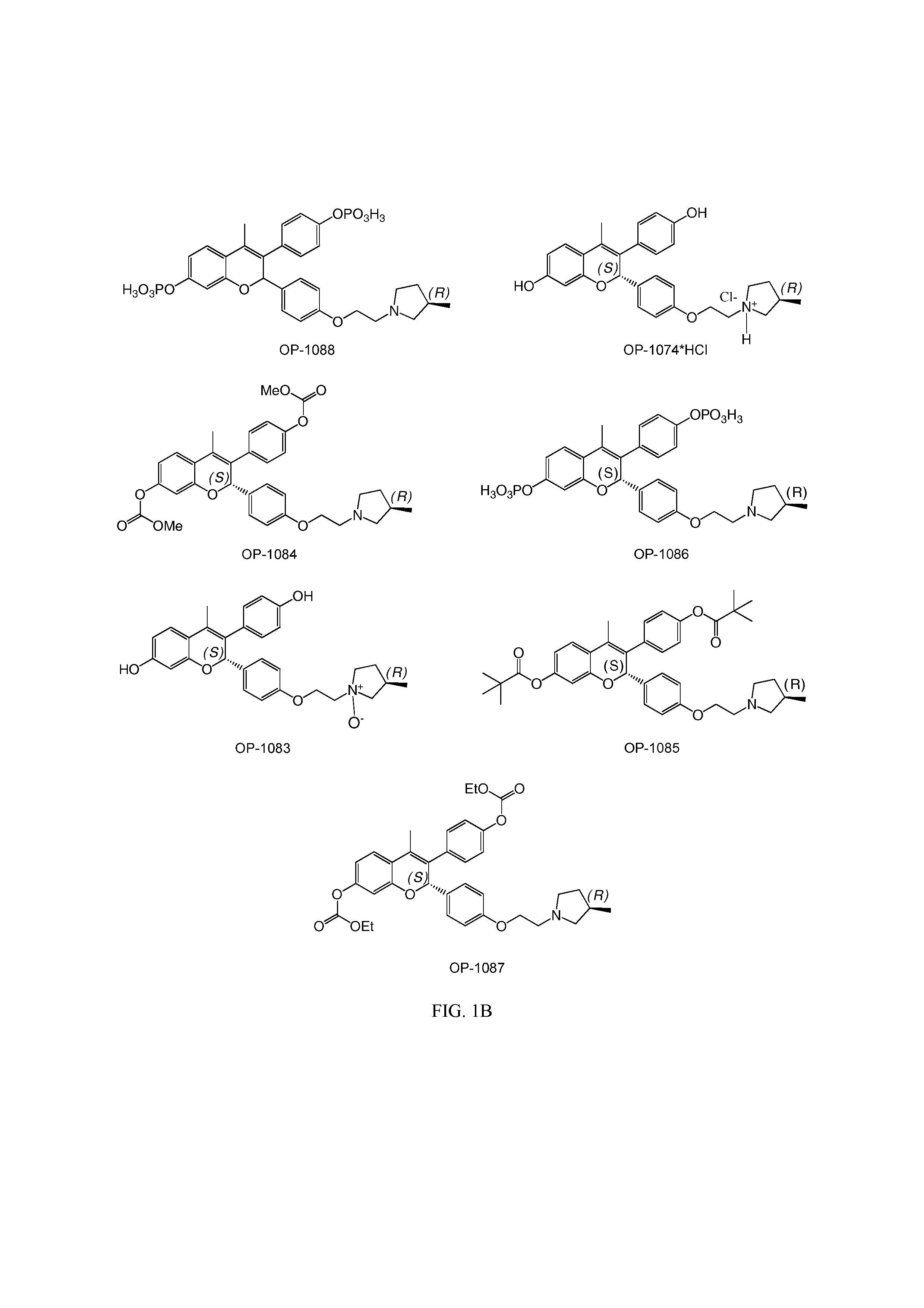
Novel Benzopyran Compounds, Compositions and Uses Thereof
Benzopyran compounds with strong anti-estrogenic activity and essentially no estrogenic activity are provided, which are OP-1038, which is 3-(4-hydroxyphenyl)-4-methyl-2-(4-{2-[(3R)-3-methylpyrrolidin-1-yl]ethoxy}phenyl)-2H-chromen-7-ol, and OP-1074, which is (2S)-3-(4-hydroxyphenyl)-4-methyl-2-(4-{2-[(3R)-3-methylpyrrolidin-1-yl]ethoxy}phenyl)-2H-chromen-7-ol. OP-1074 is a pure anti-estrogen when tested in the agonist mode and a complete anti-estrogen when tested in the antagonist mode. These compounds are useful for the treatment or prevention of a variety of conditions that are modulated through the estrogen receptor in mammals including humans.
Owner:OLEMA PHARMA
Novel benzopyran derivatives as potassium channel openers
The present invention is directed to novel benzopyran derivatives, pharmaceutical compositions containing them and their use in the treatment of disorders related to potassium channel.
Owner:JANSSEN PHARMA NV
Substituted benzopyrans as selective estrogen receptor-beta agonists
The present invention relates to substituted benzopyran derivatives, stereoisomers, and pharmaceutical acceptable salts thereof and processes for the preparation of the same. The compounds of the present invention are useful as Estrogen Receptor ? agonists. Such agonists are useful for the treating Estrogen Receptor ? mediated diseases such as prostate cancer or BPH.
Owner:ELI LILLY & CO
Benzopyran compounds, compositions and uses thereof
InactiveUS9018244B2Prevent and treat osteoporosisUseful in therapyBiocideSkeletal disorderBenzopyranAnti estrogenic
Benzopyran compounds with strong anti-estrogenic activity and essentially no estrogenic activity are provided, which are OP-1038, which is 3-(4-hydroxyphenyl)-4-methyl-2-(4-{2-[(3R)-3-methylpyrrolidin-1-yl]ethoxy}phenyl)-2H-chromen-7-ol, and OP-1074, which is (2S)-3-(4-hydroxyphenyl)-4-methyl-2-(4-{2-[(3R)-3-methylpyrrolidin-1-yl]ethoxy}phenyl)-2H-chromen-7-ol. OP-1074 is a pure anti-estrogen when tested in the agonist mode and a complete anti-estrogen when tested in the antagonist mode. These compounds are useful for the treatment or prevention of a variety of conditions that are modulated through the estrogen receptor in mammals including humans.
Owner:OLEMA PHARMA
Fluorescent retroreflective sheet
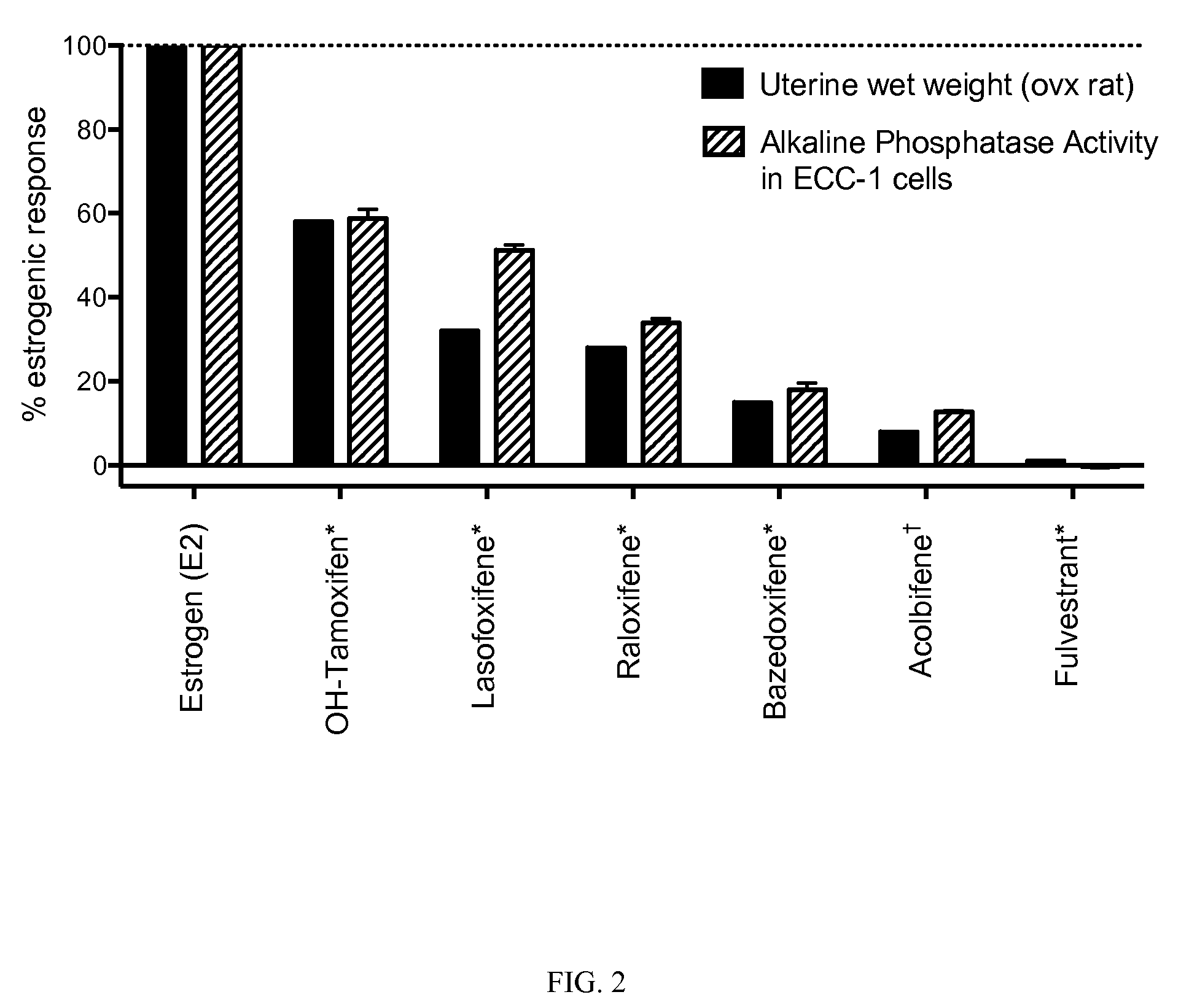
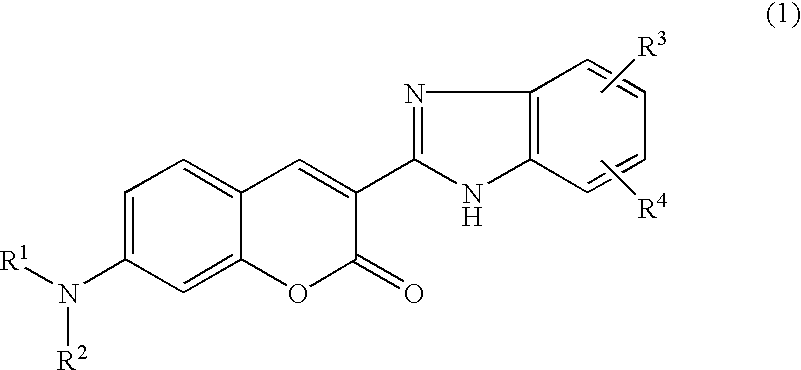
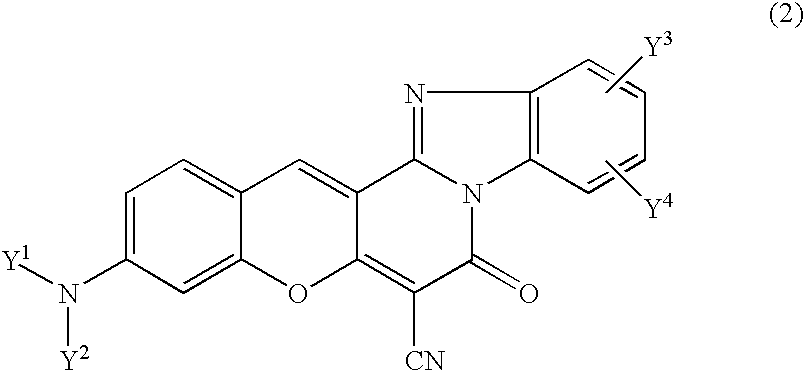
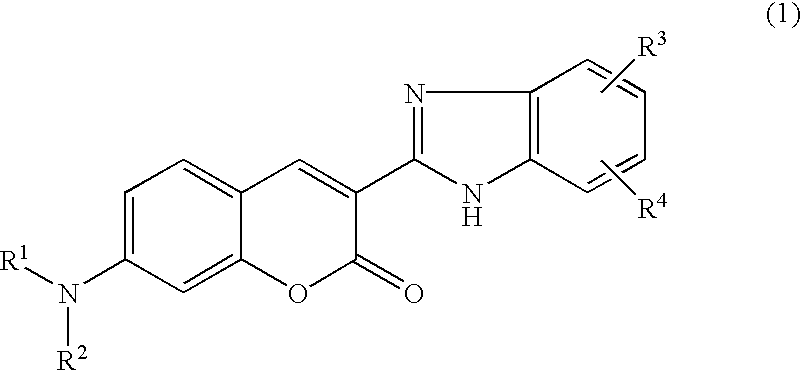
This invention provides encapsulated type fluorescent retroreflective sheeting which is excellent in appearance and weatherability, and which comprises a surface-protective layer disposed on the side on which light is to strike, a binder layer connected to the surface-protective layer through network bonding parts, an air layer which is sealed-up by the network bonding parts between the surface-protective layer and the binder layer, and a retroreflective element layer disposed between the surface-protective layer and the air layer, or between the binder layer and the air layer, wherein at least one layer located on the light-incident side of sealed-up air layer contains at least one fluorescent dye selected from the group consisting of benzimidazole coumarin type-fluorescent dyes of formula (1) as follows: and benzopyran type-fluorescent dyes of formula (2) as follows:
Owner:NIPPON CARBIDE KOGYO KK
Compositions for protection against superficial vasodilator flush syndrome, and methods of use
ActiveUS20070141187A1Promote absorptionBiocideCosmetic preparationsSulfate proteoglycanVascular dilatation
Compositions for protection against SVFS syndromes are composed of a flavonoid compound of the basic structures 2-phenyl-4H-1-benzopyran or 2-phenyl-4-keto-1-benzopyran or glycosides thereof, an inventive olive kernel extract and a non-bovine sulfated proteoglycan, and, optionally, one or more of bitter willow extract, D-glucosamine sulfate and serotonin inhibitor.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
Esculetin derivatives
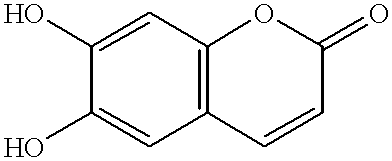
The invention concerns novel 6- or 7-substituted derivatives of esculetin (6,7-dihydroxy-2H-1-benzopyran-2-one), their synthesis, and their application as substrates for the detection of micro-organisms in samples where a derivative is enzymatically cleaved to release a colored or fluorogenic marker which has a low tendency to diffuse through agar or other aqueous environments.
Owner:LAB M
Process for preparation of racemic Nebivolol
Owner:UNIV ZURICH +1
Isoflavone compound, its preparation method, and its application in preparation of antiviral or antitumor drugs
The invention relates to an isoflavone compound, its preparation method, and its application in the preparation of antiviral or antitumor drugs, and concretely relates to a compound with a structural formula represented by formula (I). In the formula (I), R1 is hydrogen or C1-6 alkyl; X is -O-, -NH-, -CH=CH-, or ethinyl, or R2 is directly connected with benzopyran; R2 is selected from substituted or unsubstituted phenyl, substituted or unsubstituted heteroaryl, C1-16 alkyl, C1-16 alkyloxy, C1-16 alkyloxycarbonyl, or substituted or unsubstituted benzoyloxy; the heteroaryl is selected from groups derived from a five or six-membered heterocycle or a fused heterocycle containing one or two hetero atoms of N, O or S, and is selected but not limited to pyridyl, benzopyridyl, or groups derived from phenoxathiin, thiophene, oxazole and benzopyridine; and above substituent is selected from halogen, cyan, nitro, hydroxy, amino, C1-6 monosubstituted or disubstituted amino, C1-16 alkyl, halogenated C1-6 alkyl, C2-6 alkenyl, C6-12 arylalkenyl, C1-16 alkyloxy, C1-16 carboxyl, C1-16 alkyloxycarbonyl, C1-16 alkylcarbonyl, and substituted or unsubstituted phenoxyl. The invention also provides the preparation of the compound.
Owner:SHANGHAI RUIGUANG BIOCHEM TECH DEV +1
Aqueous liquid pharmaceutical composition containing as main component benzopyran derivative
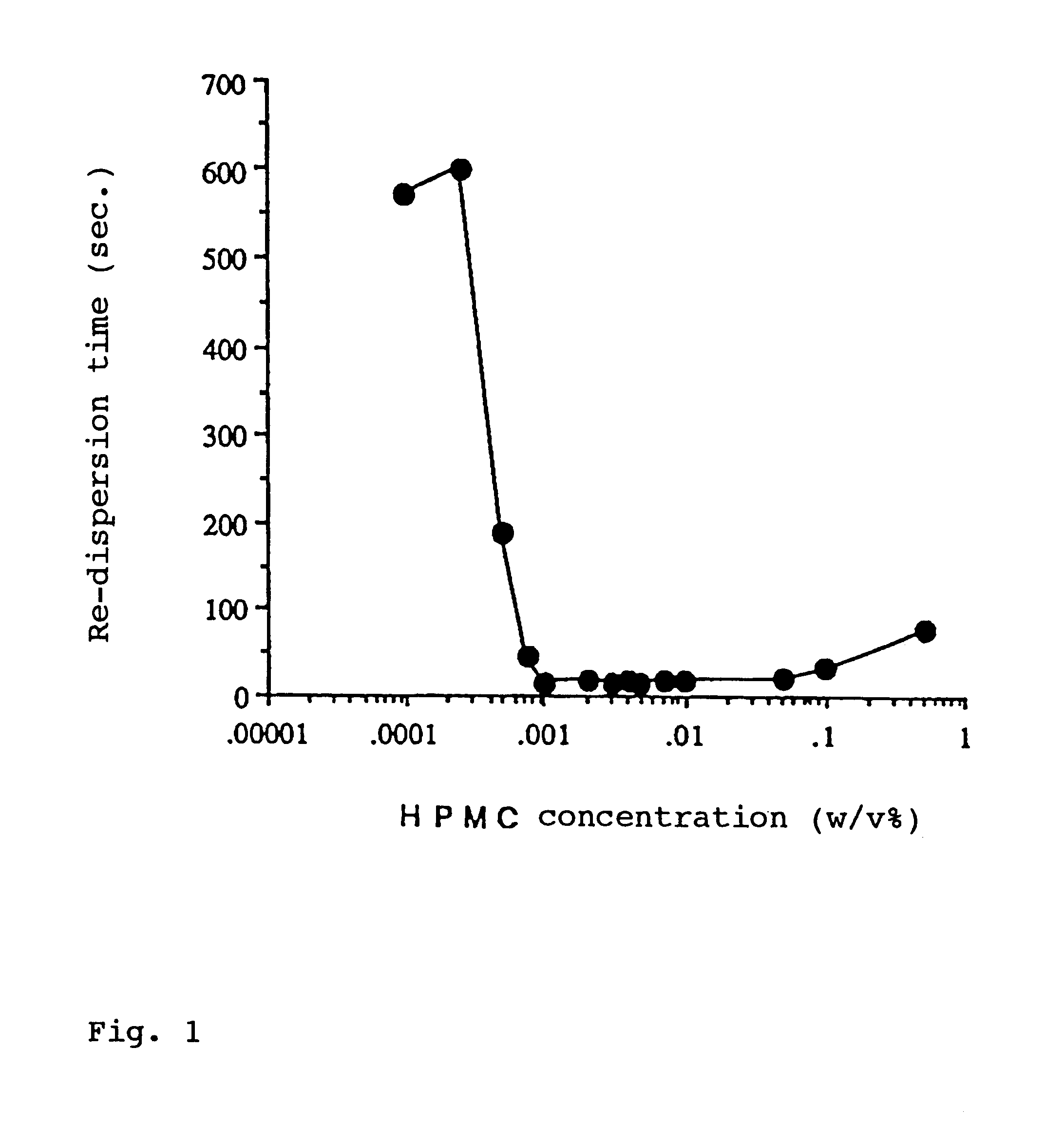
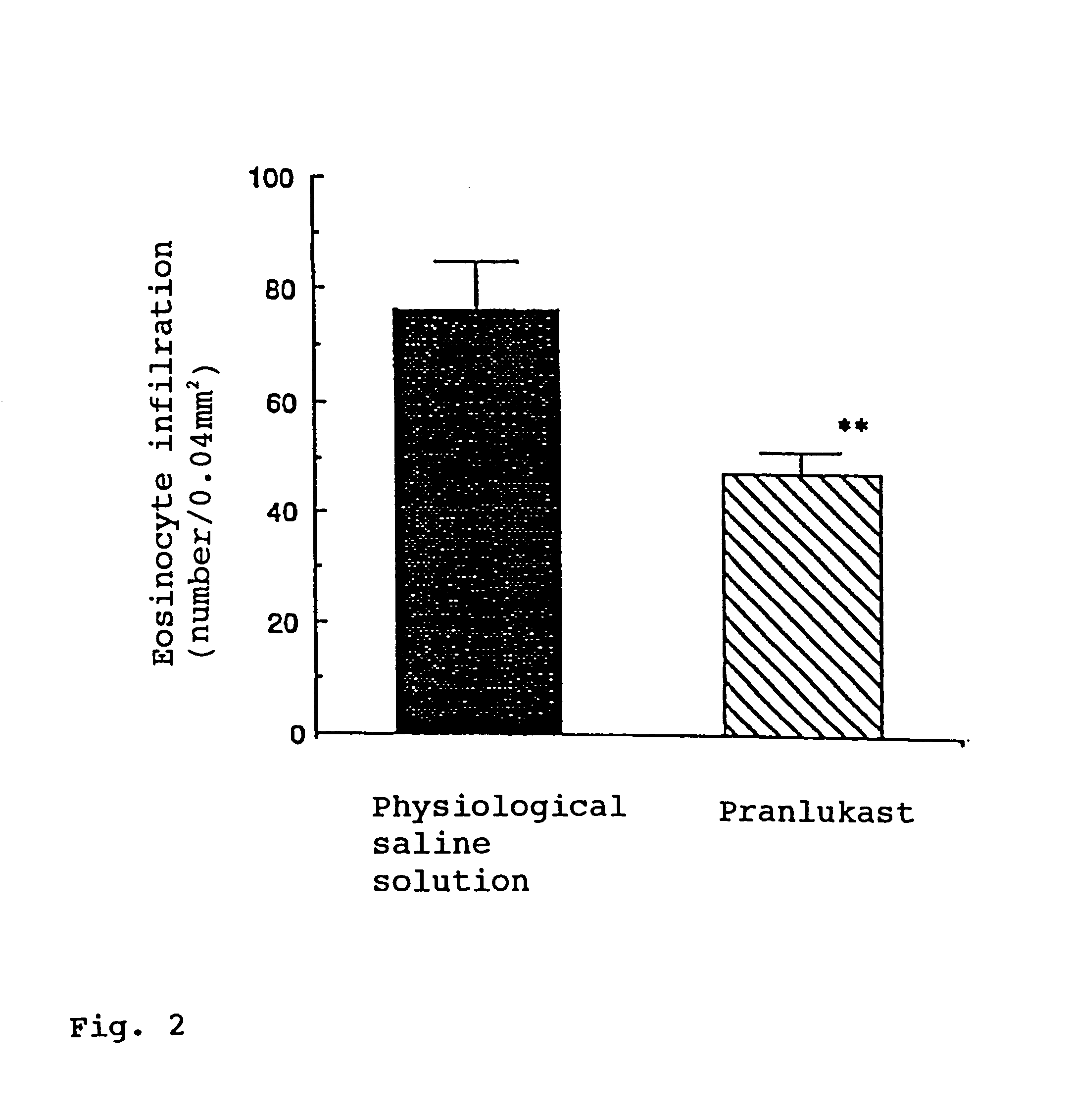
InactiveUS6274609B1Enhanced inhibitory effectPromote solubilization and suspensionBiocideSenses disorderBenzopyranHigh concentration
In order to promote solubilization or suspension of 4-oxo-8-[4-(4-phenylbutoxy)benzoylamino]-2-(tetrazol-5-yl)-4H-1-benzopyran or its hydrate (pranlukast) in water, at least one component selected from surfactants, water-soluble cellulose derivatives and water-soluble vinyl polymers is formulated together with pranlukast. Thus, it is possible to provide an aqueous liquid pharmaceutical composition containing higher concentration of pranlukast and having good properties.
Owner:ONO PHARMA CO LTD +1
Isocoumarin compound and preparation method and use thereof
The invention discloses a novel isocoumarin compound. The isocoumarin compound has the chemical name of 2-(hydroxymethyl)-8,8-dimethyl-9,10-dihydropyrano[4,3-f]benzopyran-4 (8H)-one, the molecular formula of C15H16O4 and the structure represented by a formula shown in the specification. The invention further discloses a preparation method for preparing the isocoumarin compound from tobacco. The invention further discloses use of the compound, and shown by activity tests, the compound plays a good role in inhibiting tobacco mosaic virus. The compound disclosed by the invention is simple in structure and relatively good in tobacco mosaic virus resisting activity and can be applied to the research and development of tobacco mosaic virus resisting pesticide preparations as a tobacco mosaic virus resisting lead compound.
Owner:CHINA TOBACCO YUNNAN IND
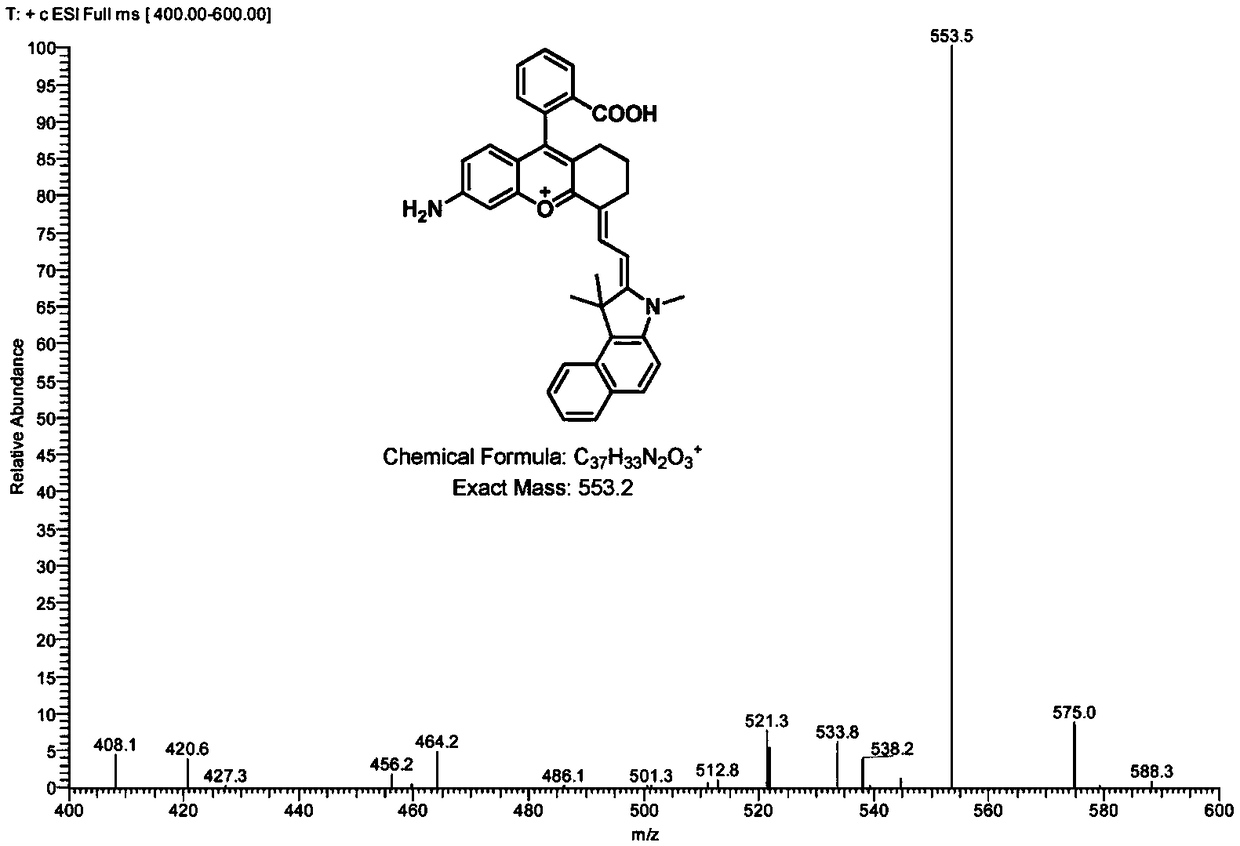
Amido benzopyran cyanine fluorochrome and probe as well as synthetic method and application thereof
ActiveCN109111915AImproved tissue penetrationStrong penetrating powerMethine/polymethine dyesFluorescence/phosphorescenceQuantum yieldStructural formula
The invention discloses an amido benzopyran cyanine fluorochrome and a probe as well as a synthetic method and application thereof. The fluorochrome is any one kind in structural formulas I-II as shown in the description. According to near infrared fluorochromes I and II with an amido benzopyran cyanine structure provided by the invention, the fluorescence quantum yield is high, the light stability is good, the fluorescence-emission spectrum ranges from 650nm to 900nm and belongs to near infrared spectrum areas, the fluorescence imaging analysis in a living body level can be realized, meanwhile, the penetrating power of biological tissue samples is enhanced, the light damage is reduced, the fluorescence of biomolecules per se is relatively weak, and the biological system can be prevented from self-emitting fluorescent interference to obtain a relatively high signal-to-noise ratio, so that the sensitivity is improved.
Owner:HUNAN UNIV
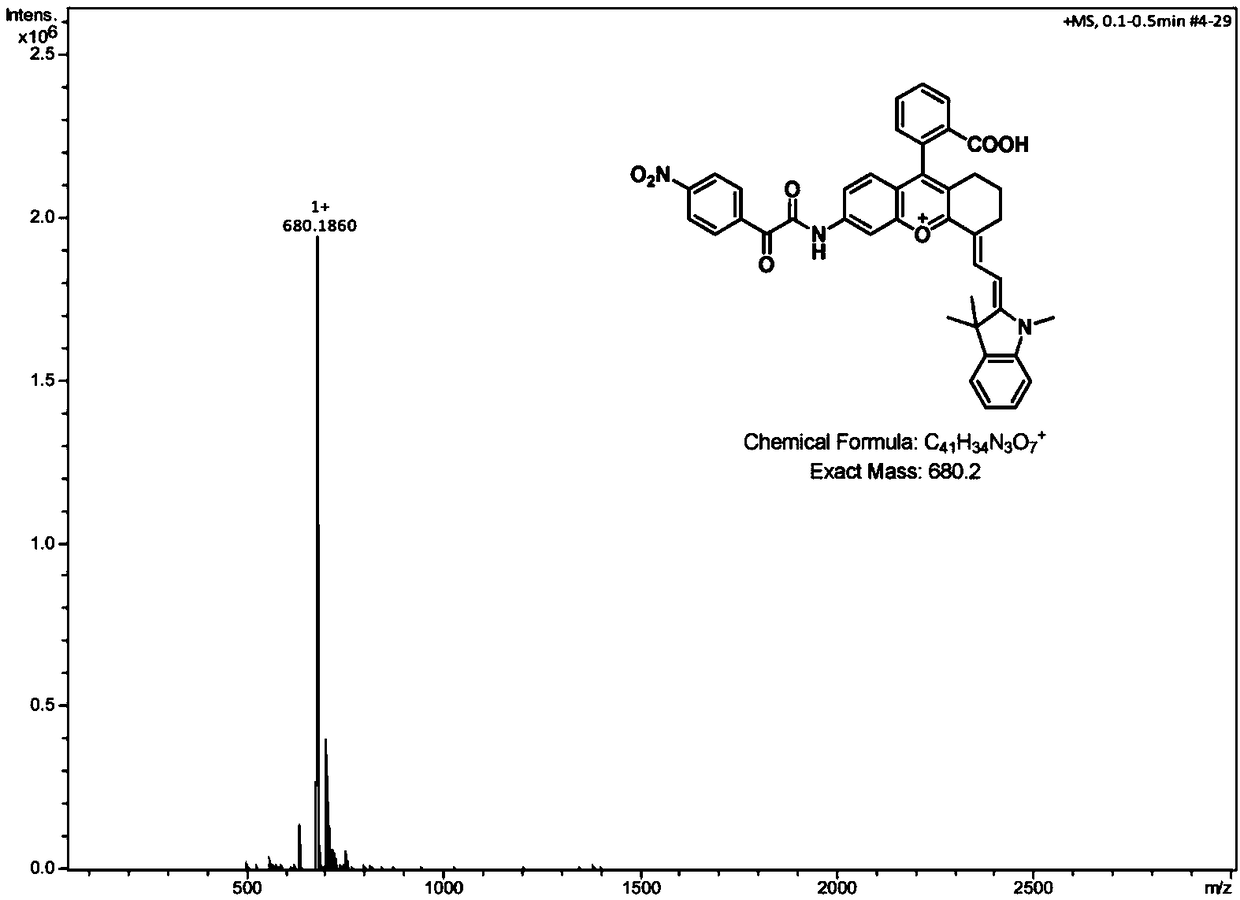
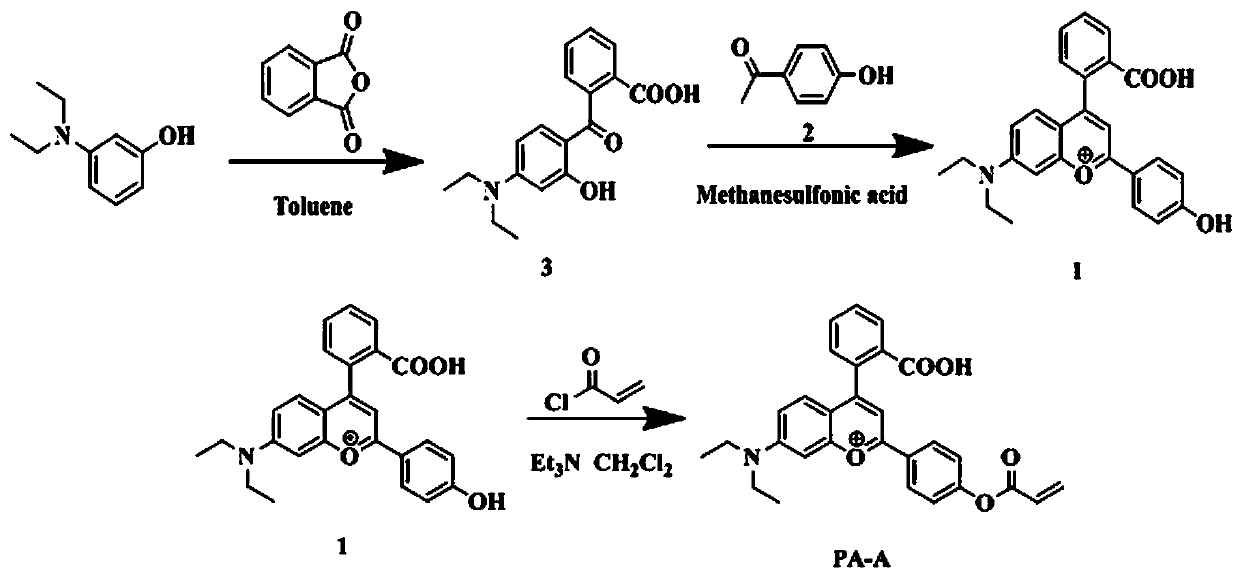
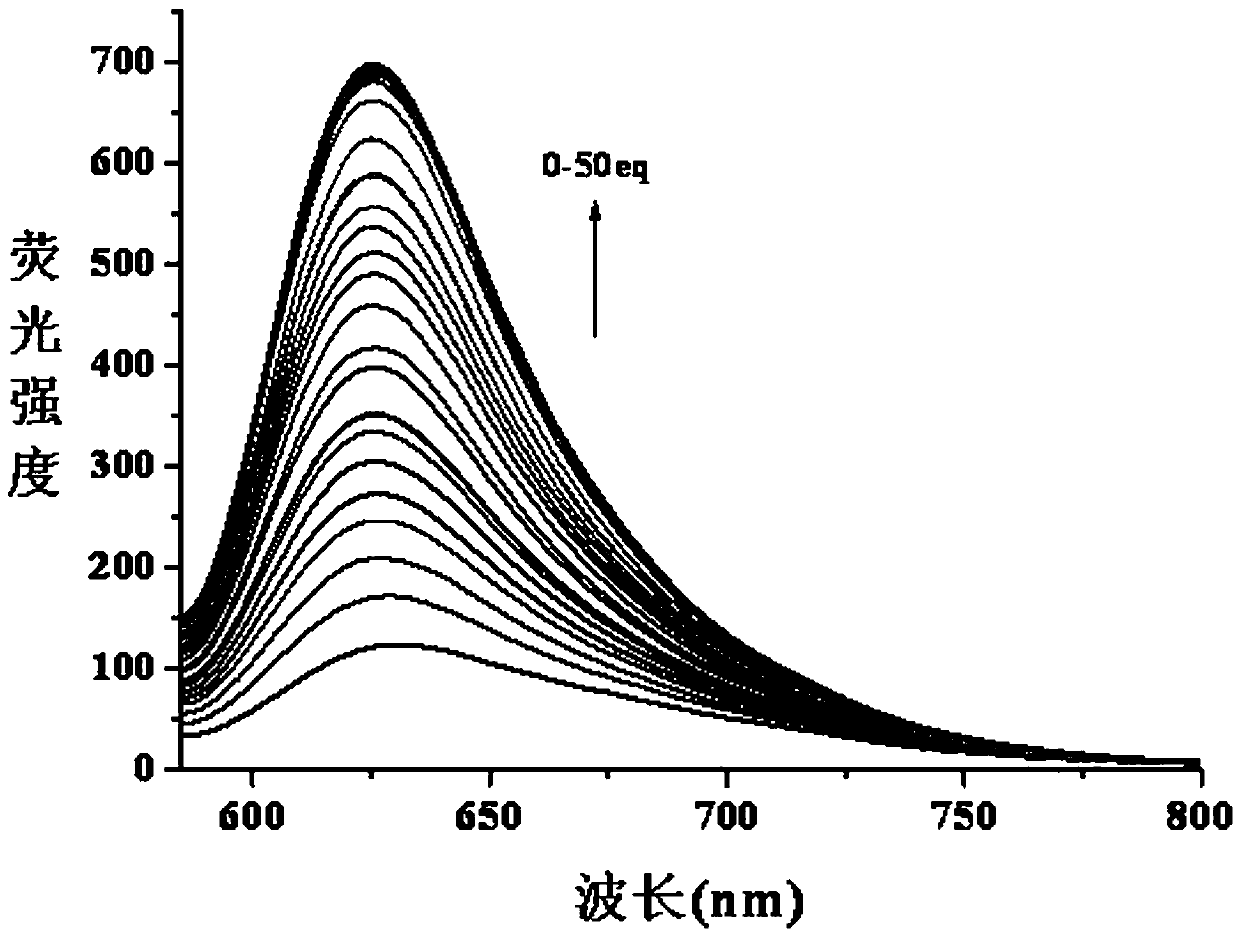
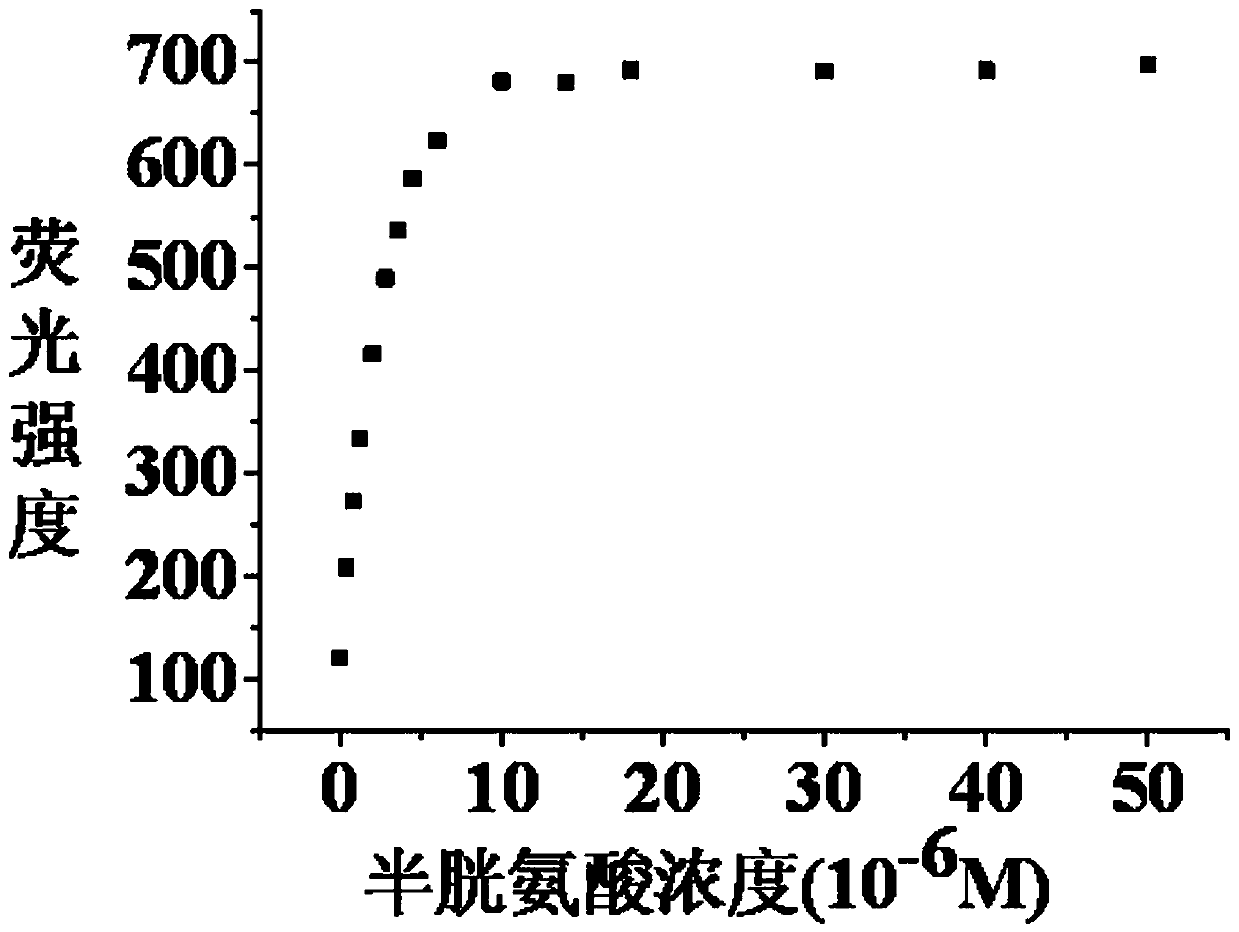
Long wavelength emitting fluorescence probe for specifically detecting cysteine in living cells and preparation method and application thereof
The invention belongs to the technical field of fluorescence probes, and provides a long wavelength emitting fluorescence probe for specifically detecting cysteine in living cells and a preparation method and an application thereof. The molecular formula of the fluorescence probe is C29H26NO5+, and the name of the probe compound is 2-(4-(acryloyloxy)phenyl)-4-(2-carboxyphenyl)-7-(diethylamino)benzopyran, abbreviated as PA-A. A fluorescent probe for specifically recognizing cysteine, is constructed with anthocyanin derivatives as fluorophore and acrylate as recognition unit. Michael addition-pyrolysis reaction of cysteine and probe is used for detecting with high selectivity. The invention belongs to the technical field of organic small molecule fluorescent probes, the probe has obvious color change and good water solubility, and can efficiently identify cysteine in water solubility environment, organic environment and cell environment. The method of the invention has the advantages ofsimple operation, high sensitivity, good selectivity and stable properties, and can be stored and used for a long time.
Owner:SHANXI UNIV
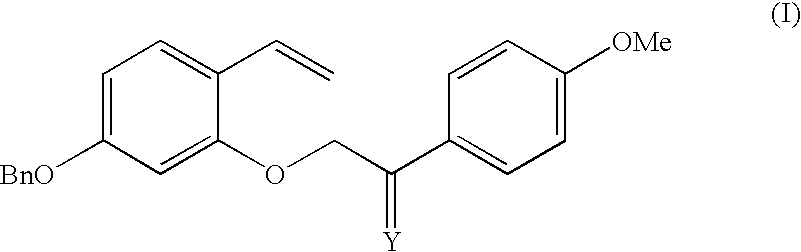
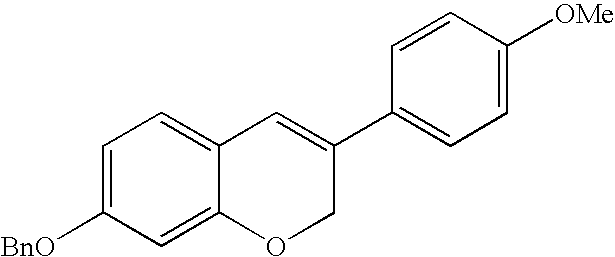
Intermediate compounds and processes for the preparation of 7-benzyloxy-3-(4-methoxyphenyl)-2H-1-benzopyran
Disclosed herein is a compound of formula (I):wherein:Bn represents benzyl;Me represents methyl; andY represents an oxygen atom or a CH2 group.The compound of formula (I) can be used in the preparation of 7-benzyloxy-3-(4-methoxyphenyl)-2H-1-benzopyran,Preparation processes of said compound of formula (I) are also disclosed herein.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Substituted benzopyrans as selective estrogen receptor-beta agonists
The present invention relates to substituted benzopyran derivatives, stereoisomers, and pharmaceutical acceptable salts thereof and processes for the preparation of the same. The compounds of the present invention are useful as Estrogen Receptor ? agonists. Such agonists are useful for the treating Estrogen Receptor ? mediated diseases such as prostate cancer or BPH.
Owner:ELI LILLY & CO
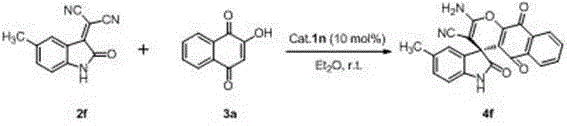
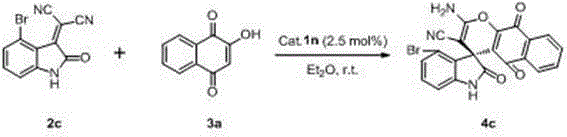
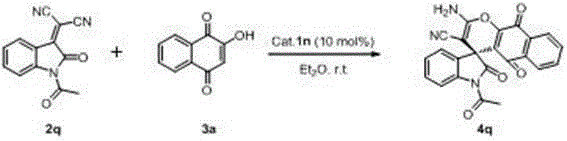
Preparation method of chiral spiro naphthoquinone benzopyran hydroxyindole compound
The invention discloses a preparation method of a chiral spiro naphthoquinone benzopyran hydroxyindole compound. The method comprises the following specific steps: reacting the condensation product of an isatin compound and propane dinitrile and a 2-hydroxyl-1,4-naphthoquinone compound in a solvent under the catalysis of quinine thiourea dihydride, thereby obtaining a product. According to the method, raw materials are simple and easily available, the reaction condition is mild, the post-treatment is simple and convenient, the applicative substrates are wide, the enantioselectivity is high, and the yield can reach 99%; therefore, the method is a novel method capable of efficiently synthesizing the chiral spiro naphthoquinone benzopyran hydroxyindole compound having important medicinal value and asymmetric synthesis value. The compound prepared by the method can be used for preparing intermediates of chiral drugs and has wide physiological and pharmacological activities.
Owner:SUZHOU UNIV
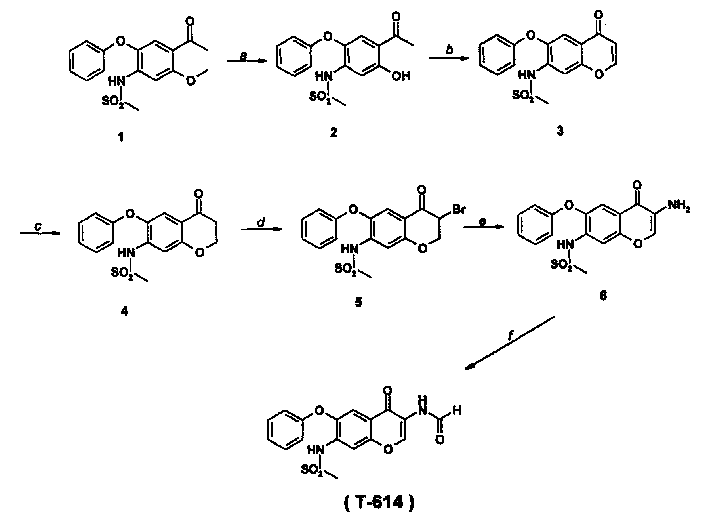
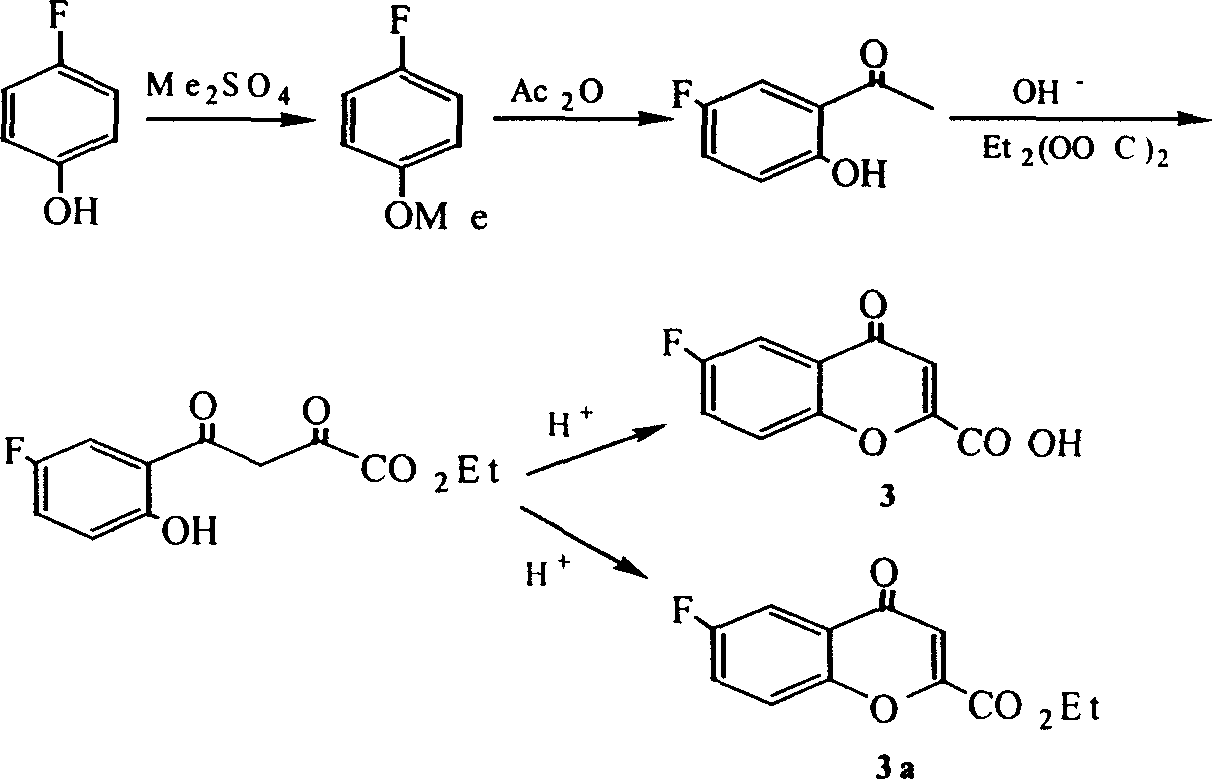
Preparation of 3-(formamide)-7-(methylsulfonyl amine)-6-(phenoxy)-4H-1-(benzopyran)-4-ketone
A process for preparing 3-(formamide)-7-(methylsulfonylamine)-6-(phenoxy) -4H-1-(benzopyran)-4-one as an anti-inflammatory from 3-phenoxy-4-methylsulfonylamine-6- methoxyacetophenone includes demethylation, cyclization, catalytic hydrogenation, bromination, vinylog and amination.
Owner:YANGTZE RIVER PHARM GRP CO LTD
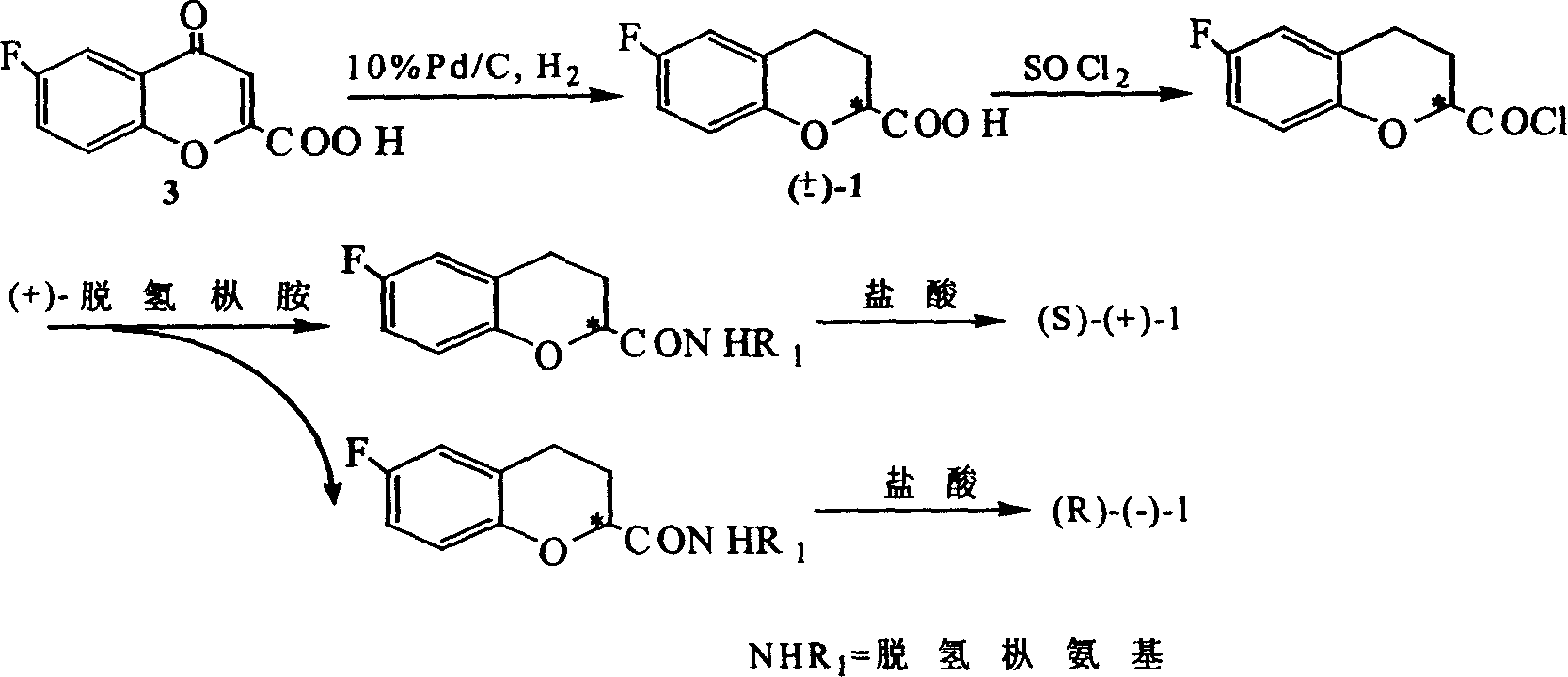
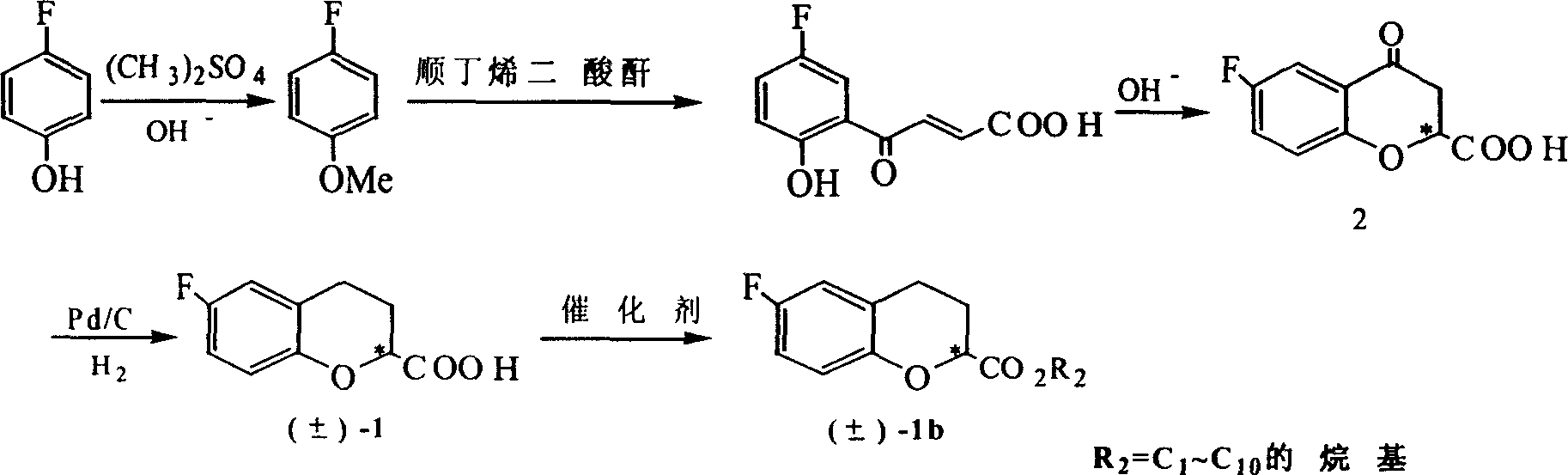
Method for synthesizing optical enantiomer 6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-carboxylic acid and 6-fluoro-3, 4-dihydro-2H-1-benzopyran-2-carboxylate
InactiveCN1629154AThe synthesis method is simpleThe synthesis method is reasonableOrganic chemistryBenzopyranAlcohol
The invention provides the method for synthesizing destination substance of (+-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid through two paths, the (+-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid and C1-C10 alcohols are subject to esterification reaction so as to synthesize the corresponding racemic ester. Then chemical dismantling is conducted to (+-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid, in order to obtain prepare optical purity of (R)-(-)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid and (S)-(+)-6-fluoine-3,4-dihydrogen-2H-1-benzopyrans-2-carboxylic acid.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
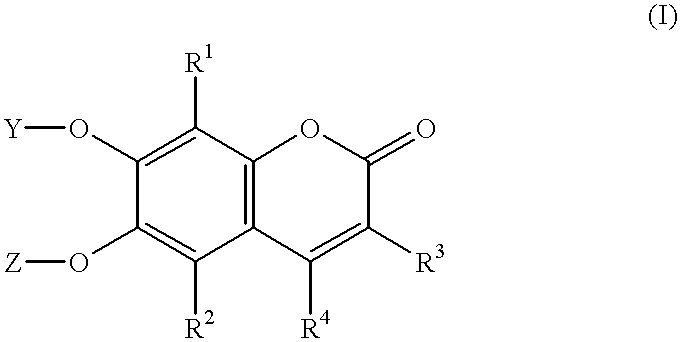
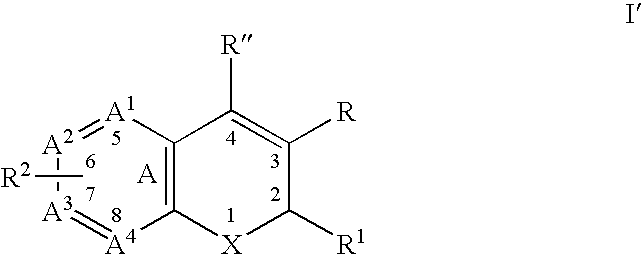
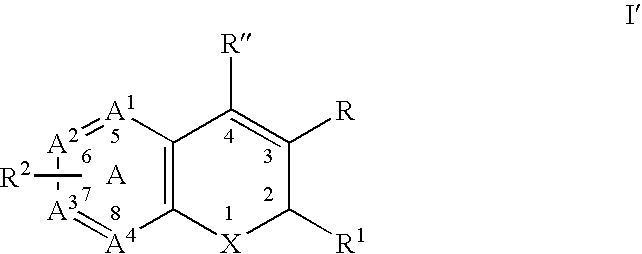
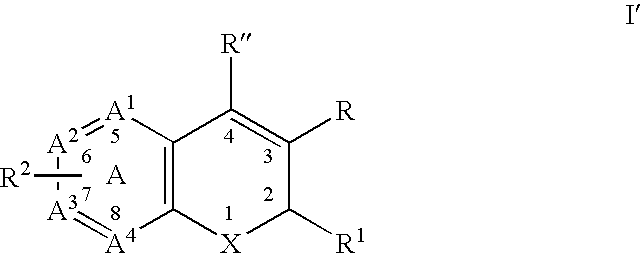
Substituted benzopyran derivatives for the treatment of inflammation
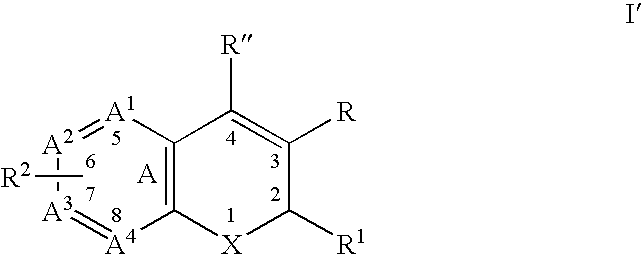
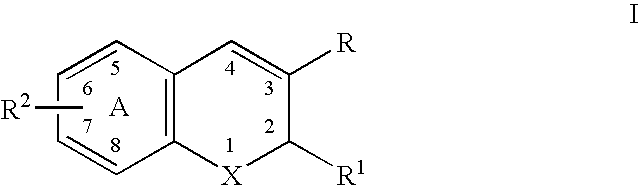
A class of benzopyrans, benzothiopyrans, dihydroquinolines, dihydronaphthalenes, and analogs thereof, is described for use in treating cyclooxygenase-2 mediated disorders. Compounds of particular interest are defined by Formula I′wherein X, A1, A2, A3, A4, R, R″, R1 and R2 are as described in the specification.
Owner:RAQUALIA PHARMA INC
Novel flavone glycoside derivatives for use in cosmetics, pharmaceuticals and nutrition
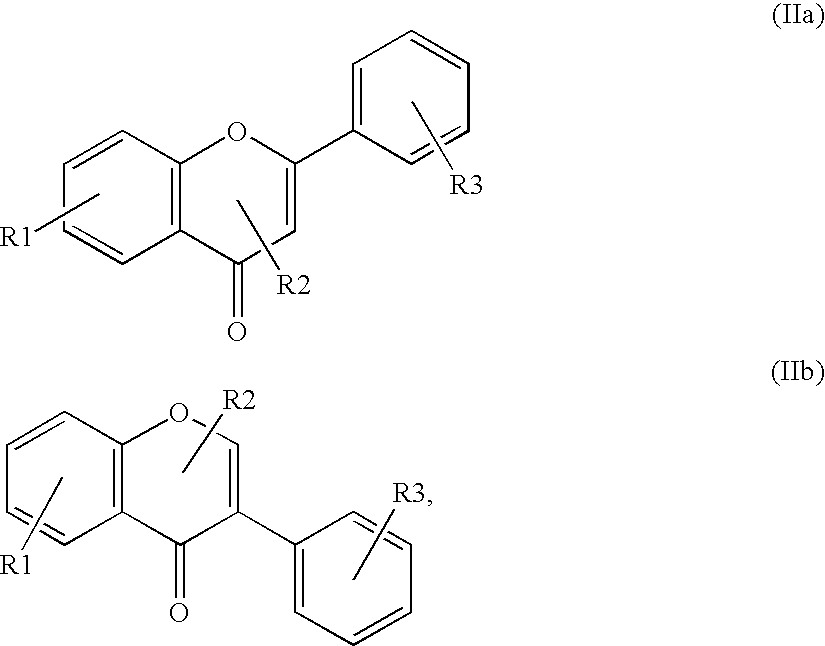
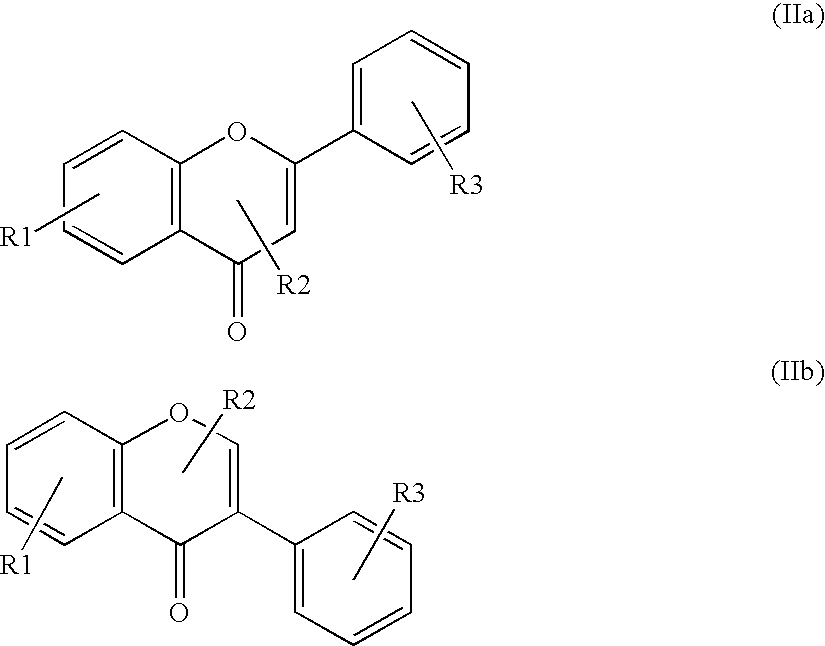
InactiveUS20080176811A1Improve usabilityBroad spectrum of actionCosmetic preparationsBiocideBenzopyranAcyl group
Substituted and unsubstituted flavone or isoflavone glycoside derivatives of the formula [A1-C(═O)O]m—[X—O-Z]—[O—C(═O)-A2]n, wherein [X—O-Z] represents a flavone or isoflavone glycoside structure, particularly a naringin residue, X is a flavone or isoflavone corresponding to formula (IIa) or formula (IIb):wherein the flavone or isoflavone residue is substituted one or more times and / or reduced one or more times; Z represents a mono-, di- or polysaccharide, which is acetally-bound at the benzopyran group to X and ester-substituted by —O—C(═O)-A2; [A1-C(═O)] is an acyl group on the flavone or isoflavone; A1 and A2 independently, represent a polyunsaturated C15-26 alkenyl group containing at least four isolated and / or at least two conjugated double bonds, or an arylaliphatic radical with 1-to-4 methylene groups between the ester group and the aromatic ring; [C(═O)A2] is an acyl group; n is an integer other than 0; m is an integer, including 0; and R1, R2 and R are hydroxyl groups or hydrogen atoms.
Owner:GEERS BERNADETTE +5
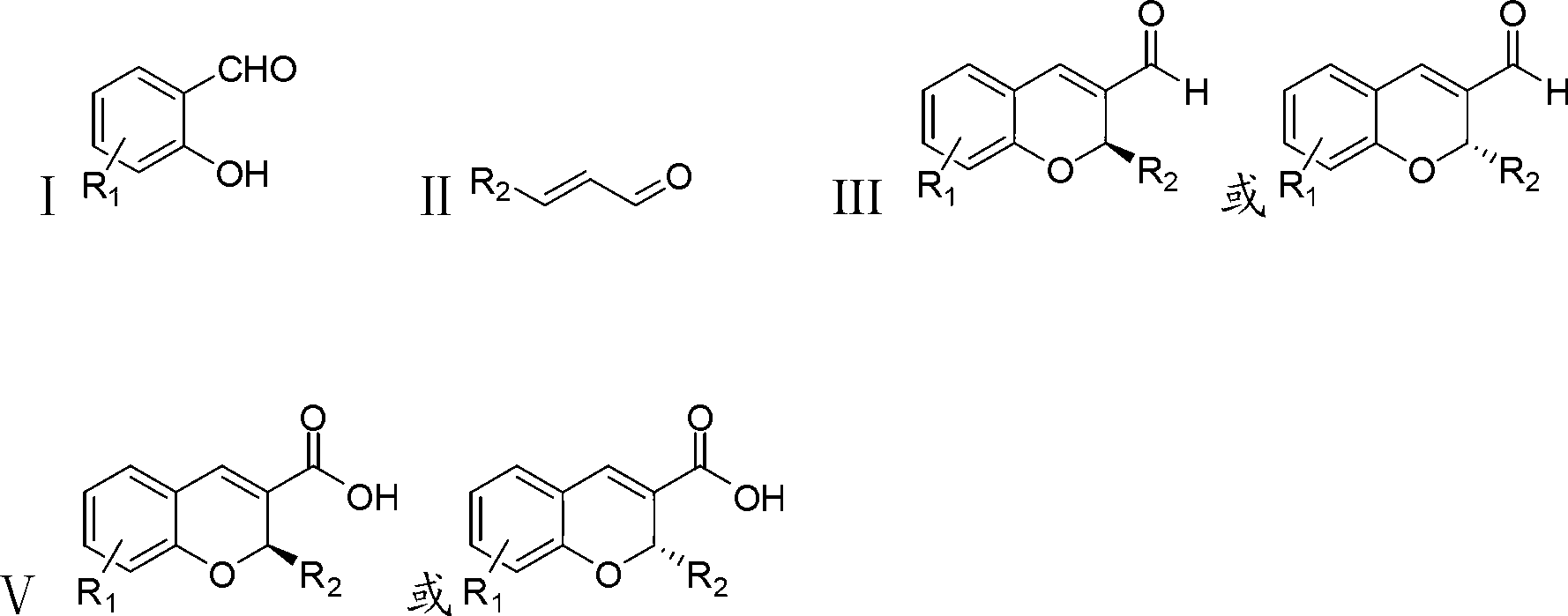
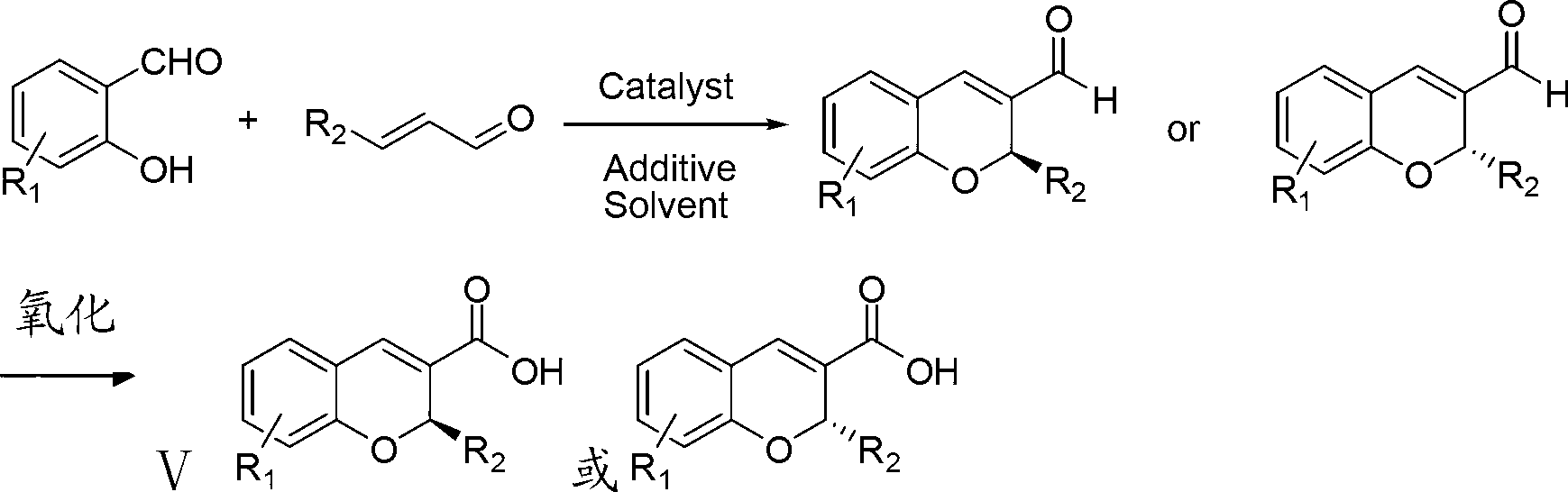
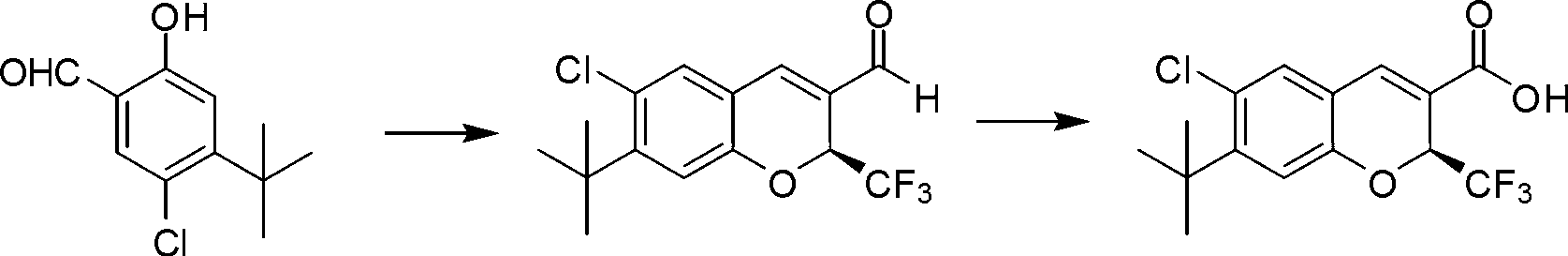
Synthetic method of benzopyran chiral compound
The invention discloses a synthetic method of a benzopyran chiral compound. The method comprises the following steps: a compound with the structural formula I and a compound with the structural formula II carry out the condensation reaction to generate a compound with the structural formula III, and the compound with the structural formula III is oxidized to obtain the benzopyran chiral compound; the chemical additive adopts a benzoic acid or a substituted benzoic acid compound; and the chiral catalyst is a diphenyl prolinol ester compound or dinaphthyl prolinol ester compound. The synthetic method has the advantages that under the mild condition, relatively cheap raw materials are used, the benzopyran chiral compound derivate is synthesized with high yield, and the derivate is high in optical purity, accessible in raw material, and beneficial for large-scale production and application.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Negative photosensitive resin composition, negative photosensitive dry film and method of forming pattern
InactiveUS6913867B2High sensitivityPhotography auxillary processesPhotosensitive materialsBenzopyranPhotosensitizer
The present invention provides a negative photosensitive resin composition comprising (A) a photocurable resin having a photosensitive group or groups crosslinkable by light irradiation, (B) a photoacid generator and (C) a photosensitizer which is a benzopyran condensed ring compound capable of increasing photosensitivity to visible light with a wavelength of 480 nm or more,a negative photosensitive dry film prepared by applying the photosensitive resin composition to a surface of support film, followed by drying, to form a photosensitive resin layer, anda method of forming a pattern using the resin composition or the dry film.
Owner:KANSAI PAINT CO LTD
Phosphatidylinositol-3-kinase inhibitor and application thereof
InactiveCN101849934AInduce apoptosisControl proliferationOrganic active ingredientsAntineoplastic agentsGackstroemiaAkt signalling
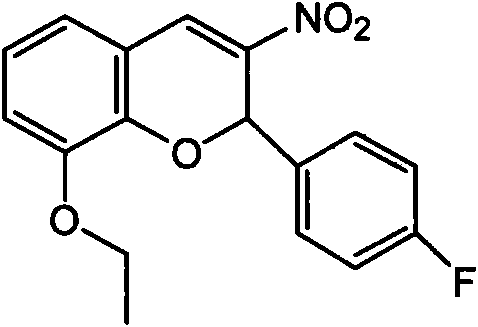
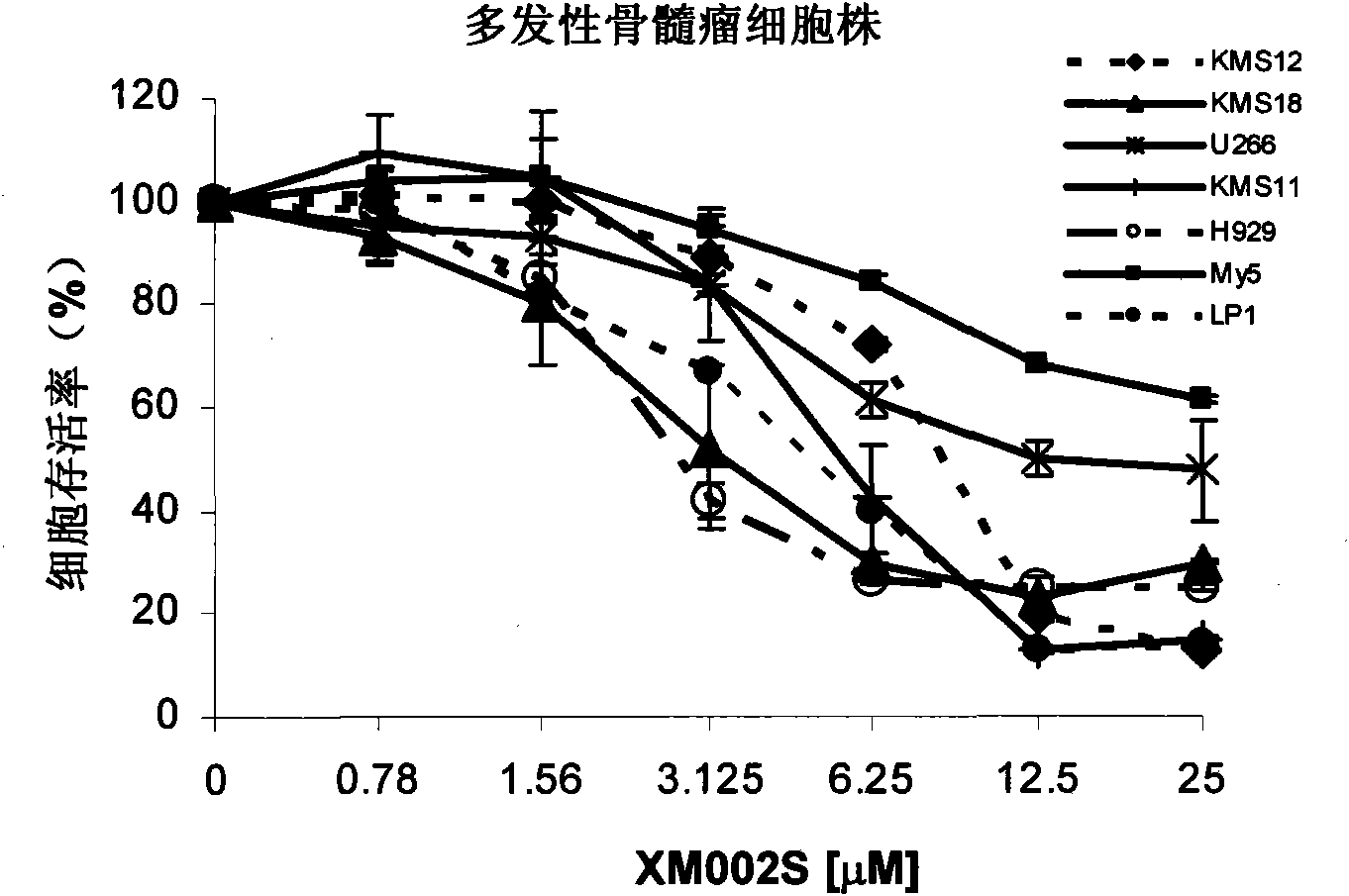
The invention discloses new application of a 2-(4-fluorophenyl)-3-nitro-8-O-ethyl-2-hydro-benzopyran compound, in particular application of the compound serving as phosphatidylinositol-3-kinase (PI3K) inhibitor. The PI3K inhibitor can inhibit PI3K and disturb a PI3K / AKT signal channel, has good treatment effect on multiple tumors, particularly malignant hematological diseases, can effectively control the propagation of tumor cells and induce the apoptosis thereof, and has the effect of inhibiting and treating the tumors; and meanwhile, the PI3K has low toxicity.
Owner:SUZHOU UNIV
Solid-phase synthesis method of coumarin and analogue thereof
InactiveCN102532015AHigh yieldOvercome the disadvantage of low yield of cyclization reactionOrganic chemistryPolystyreneKetone
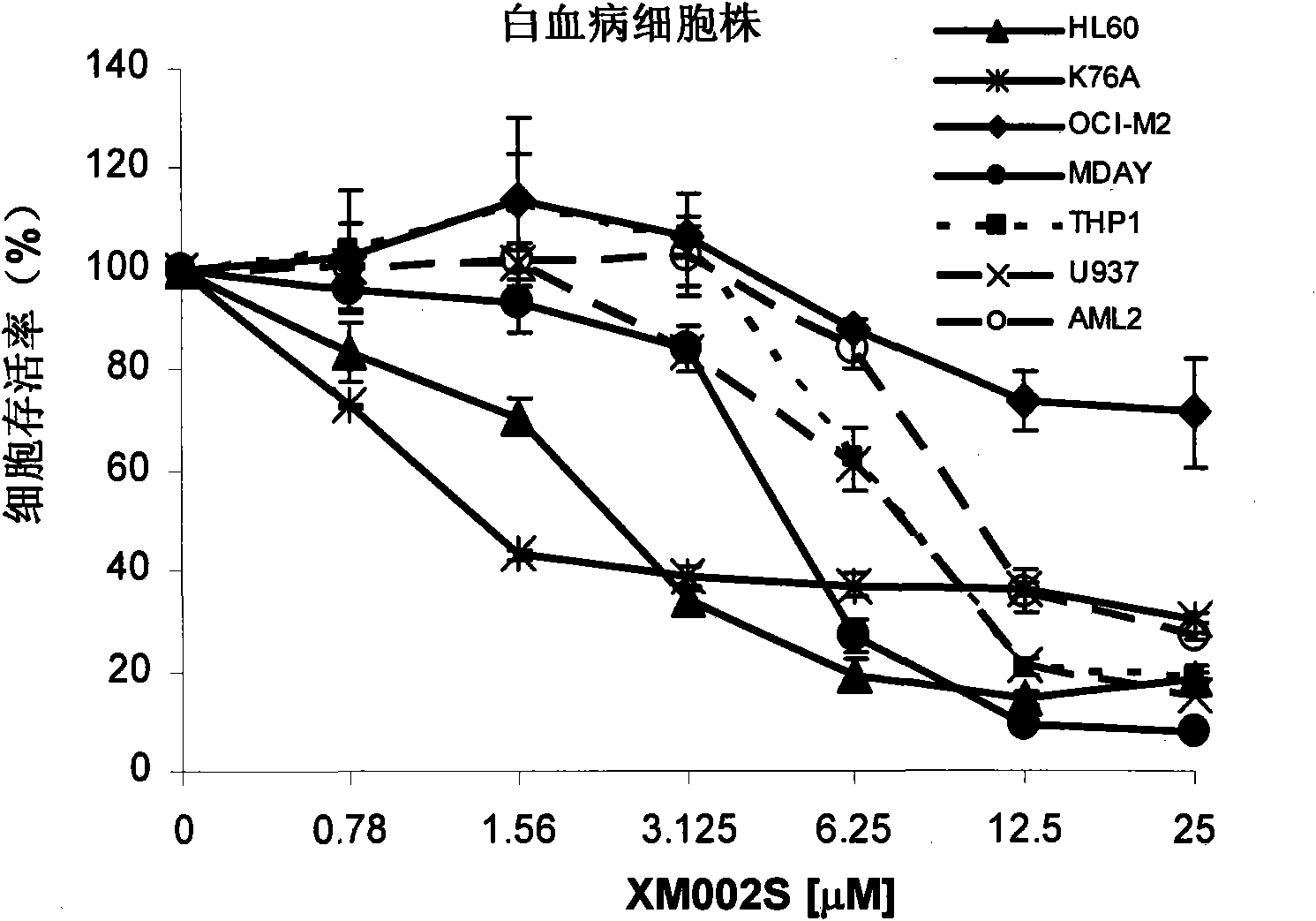
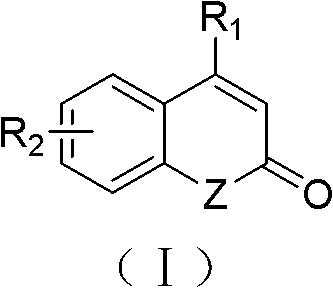
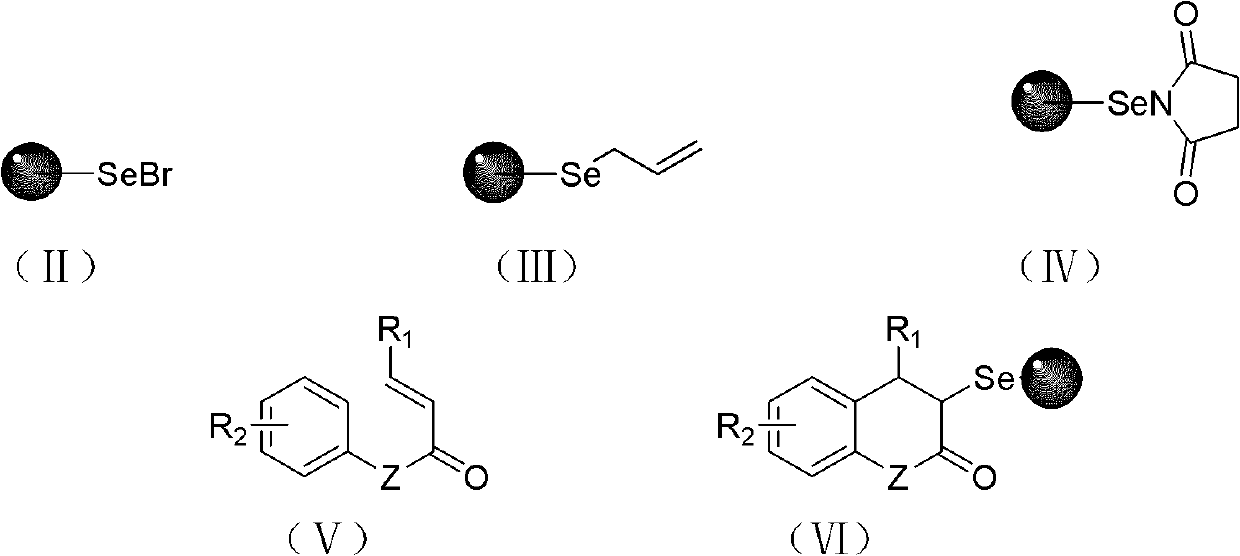
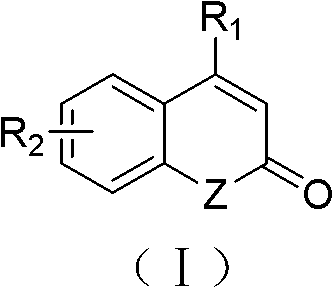
The invention relates to a solid-phase synthesis method of coumarin and an analogue (I) thereof and belongs to the field of organic chemistry. The method comprises the following steps: 1) taking 1% of cross-linked polystyrene resin as a carrier to prepare a polystyrene-supported seleno-succimide reagent (III); 2) using the III to induce phenyl acrylate (V) to perform intramolecular cyclization under the catalysis of trimethylsilyl trifluoromethanesulfonate so as to form 3-polystyrene-supported seleno-3,4-dihydro-benzopyran-2-ketone (VI); and 3) performing oxidation elimination on the VI via an oxidant so as to directly get the coumarin (I) without further separation. When the phenyl acrylate (V) is replaced by N-phenyl acrylamide, the analogue of the coumarin, namely a 2-quinolone compound, can be prepared through the same steps. The solid-phase synthesis method disclosed by the invention has the advantages of easily available raw materials, good product yield, high purity, simplicityand convenience in operation, simple post-treatment and great industrial application prospects.
Owner:YUNNAN UNIV
Synthesis and application of coumarin type dye sensitizer
InactiveCN103087051AImprove electrochemical performanceOrganic chemistryLight-sensitive devicesCyanoacetic acidElectron donor
The invention relates to a coumarin functional dye containing a thiophene bridge chain in the field of fine chemical industry and organic photoelectric material applications. The structure of the coumarin functional dye takes coumarin and a derivative thereof as an electron donor, contains a thiophene structure unit capable of adjusting an absorption spectrum and a fluorescence emission spectrum as the bridge chain and is further connected with a cyanoacetic acid electron withdrawing group. Coumarin-thiophene, POCl3 / DMF (dimethyl fumarate) are added into a reaction container by adopting general reaction for reaction so as to get a 5-(7-substiutted-2-carbonyl-2H-benzopyran-3-yl) thiophene-2-formaldehyde intermediate (II) with an aldehyde group; and the intermediate II with the aldehyde group further reacts with cyanoacetic acid to get the coumarin dye connected by the thiophene. As the coumarin is taken as a chromophore, the electron donating capability is good; the thiophene has high electron cloud density and special optical properties and electron transmission capability; and the electron withdrawing group of the cyanoacetic acid is further connected for enabling the dye to have good light, thermal and chemical stability and photoelectric properties. Therefore, the dye can be used as a photosensitive dye for dye-sensitized solar cells.
Owner:ZHEJIANG UNIV OF TECH
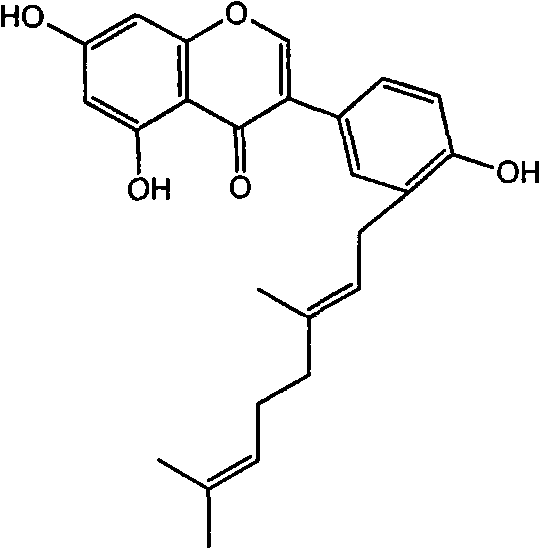
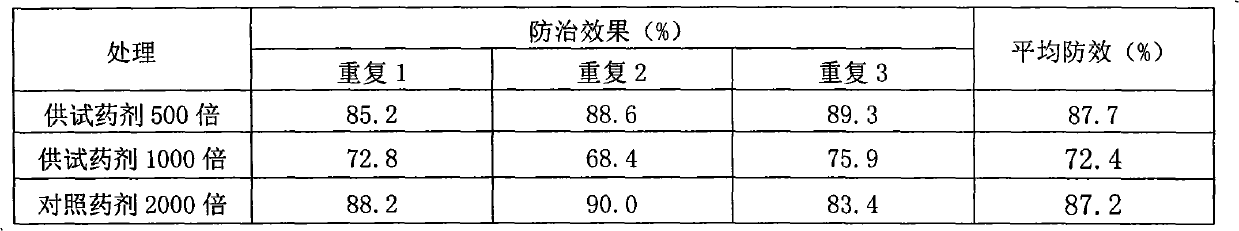
Flavonoid agricultural antibacterial compound
The invention provides a flavonoid with agricultural bacteriostatic activity and belongs to the technical field of pesticides. The compound has the following structural formula. The International Union of Pure and Applied Chemistry (IUPAC) chemical name of the compound is E-3-(3-(3,7-dimethyl-2,6-dialkylene)-4-hydroxyphenyl)-5,7-dihydroxyl-4H-benzopyran-4-ketone. The compound has high inhabitation effect on oomycetes selectively and can be processed into a pesticide preparation for preventing and controlling oomycete diseases.
Owner:PESTICIDE INST XIBEI AGRI & FORESTRY TECHUNIV
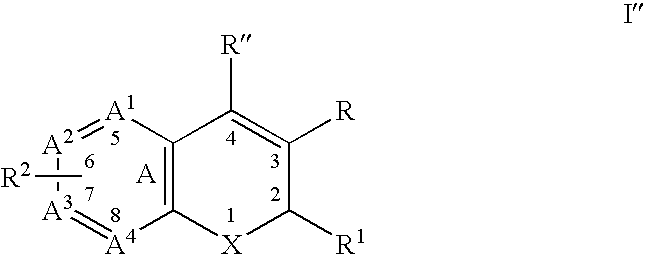
Substituted benzopyran derivatives for the treatment of inflammation
A class of benzopyran, derivatives is described for use in treating cyclooxygenase-2 mediated disorders. Compounds of particular interest are defined by Formula I′wherein X, A1, A2, A3, A4, R, R″, R1 and R2 are as described in the specification.
Owner:RAQUALIA PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com