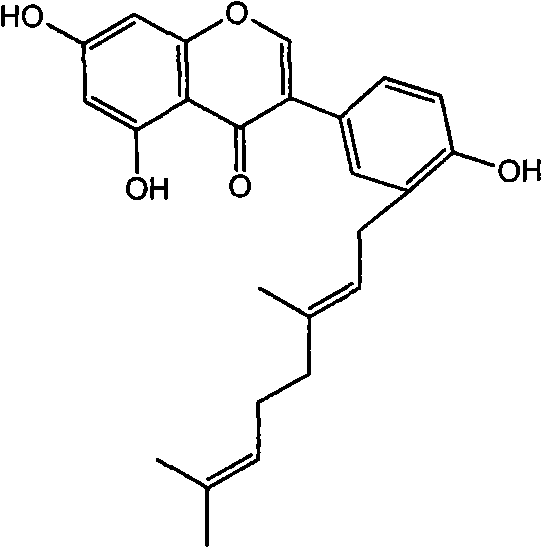
Flavonoid agricultural antibacterial compound
A flavonoid compound and chemical technology, applied in the field of pesticides, can solve the problems of excessive pesticide residues, threats to food safety and human health, etc., and achieve the effect of large output, low pressure and rapid reproduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] compound separation
[0012] Collect the aboveground part of Echinochloa chinensis, weigh 500g of the dried and crushed plant sample, place it in a 5L round bottom flask, add 3000mL methanol for reflux extraction for 3 hours, filter the supernatant, then add 1000mL methanol for reflux extraction 2 After hours, the supernatant was filtered off, and the filtrates were combined and concentrated under reduced pressure to about 300 mL. The concentrated solution was suspended in 700 mL of water, and then extracted three times with 500 mL of dichloromethane, and the dichloromethane phases were combined and concentrated to near dryness under reduced pressure. The concentrated solution was then transferred to a silica gel column (2.5×75cm), and eluted with petroleum ether / chloroform=9 / 1, 8 / 2, and 7 / 3 (volume ratio) successively, washing 1000mL for each solvent ratio, and A portion of 100 mL was collected to finally obtain 1.5 g of the compound.
Embodiment 2
[0014] Preparation of emulsifiable cream preparations
[0015] Take by weighing 5.0g (purity ≥ 98.0%) of the compound involved in the present invention and dissolve it in 85g methanol, then add anionic surfactant calcium dodecylbenzenesulfonate (DBS-Ca) 3.0g, nonionic surfactant OP -107.0g, stirred until transparent and homogeneous, namely to obtain the emulsifiable concentrate preparation of this compound as an active ingredient, wherein the active ingredient content is 5%.
Embodiment 3
[0017] Experiment on Controlling Cucumber Downy Mildew in Potted Plants
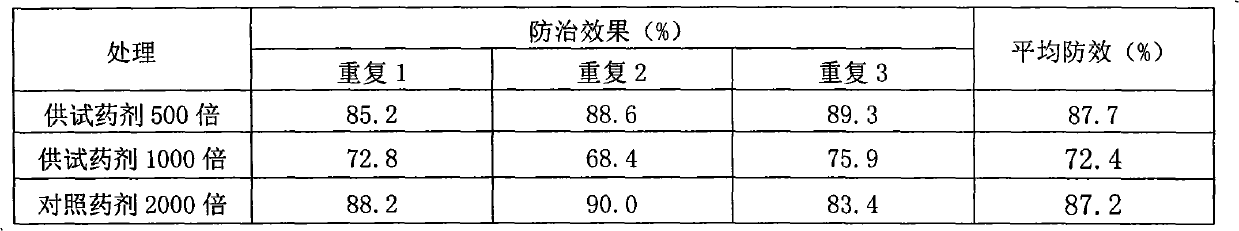
[0018] Cultivate cucumbers (the variety is Xintai Mici) in the greenhouse with nutrient soil, 2 plants per pot, and when the seedlings grow 2 true leaves, select the ones with the same growth for testing. The test agent is the emulsifiable concentrate preparation with 5% active ingredient content, and the contrast agent is 50% dimethomorph water-dispersible granule. Every 10 bowls is 1 treatment, and the test has 4 treatments in total, which are respectively 500 times and 1000 times dilution of the test agent, 2000 times dilution of the control agent and water control, and each treatment is repeated 3 times. 24 hours after spraying, the pathogenic spores of cucumber downy mildew were artificially inoculated, and the control effect was investigated one week after spraying. The results are shown in Table 1.
[0019] Table 1 Pot control test results of the tested EC preparation on cucumber downy mildew
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com