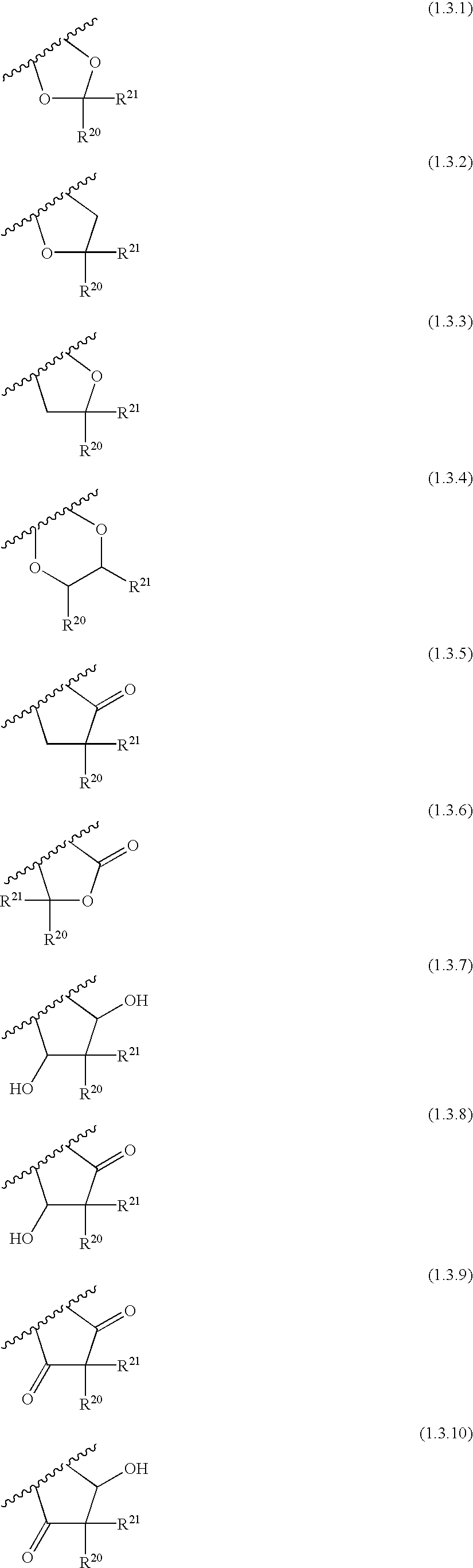
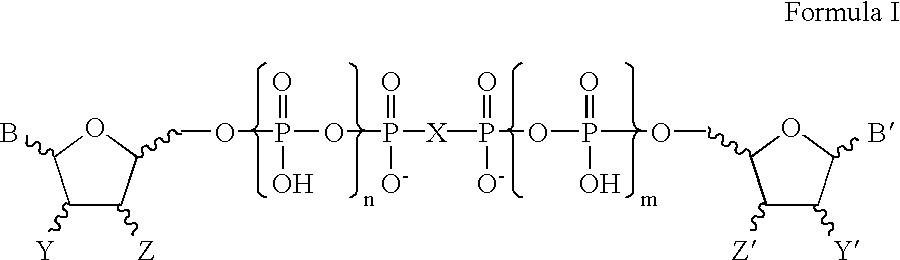
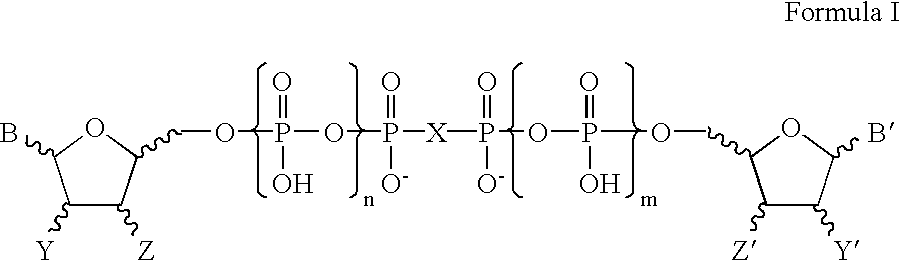
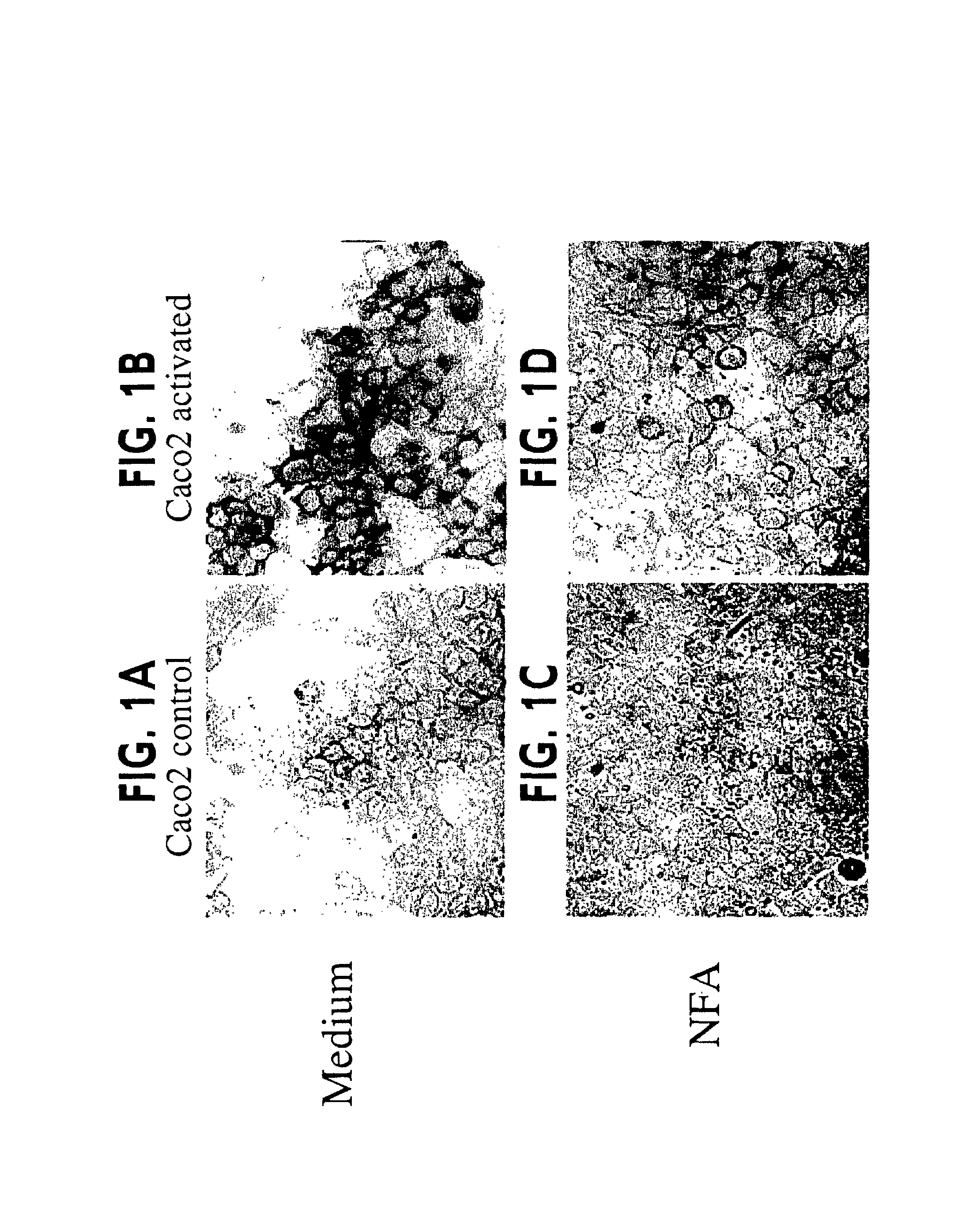
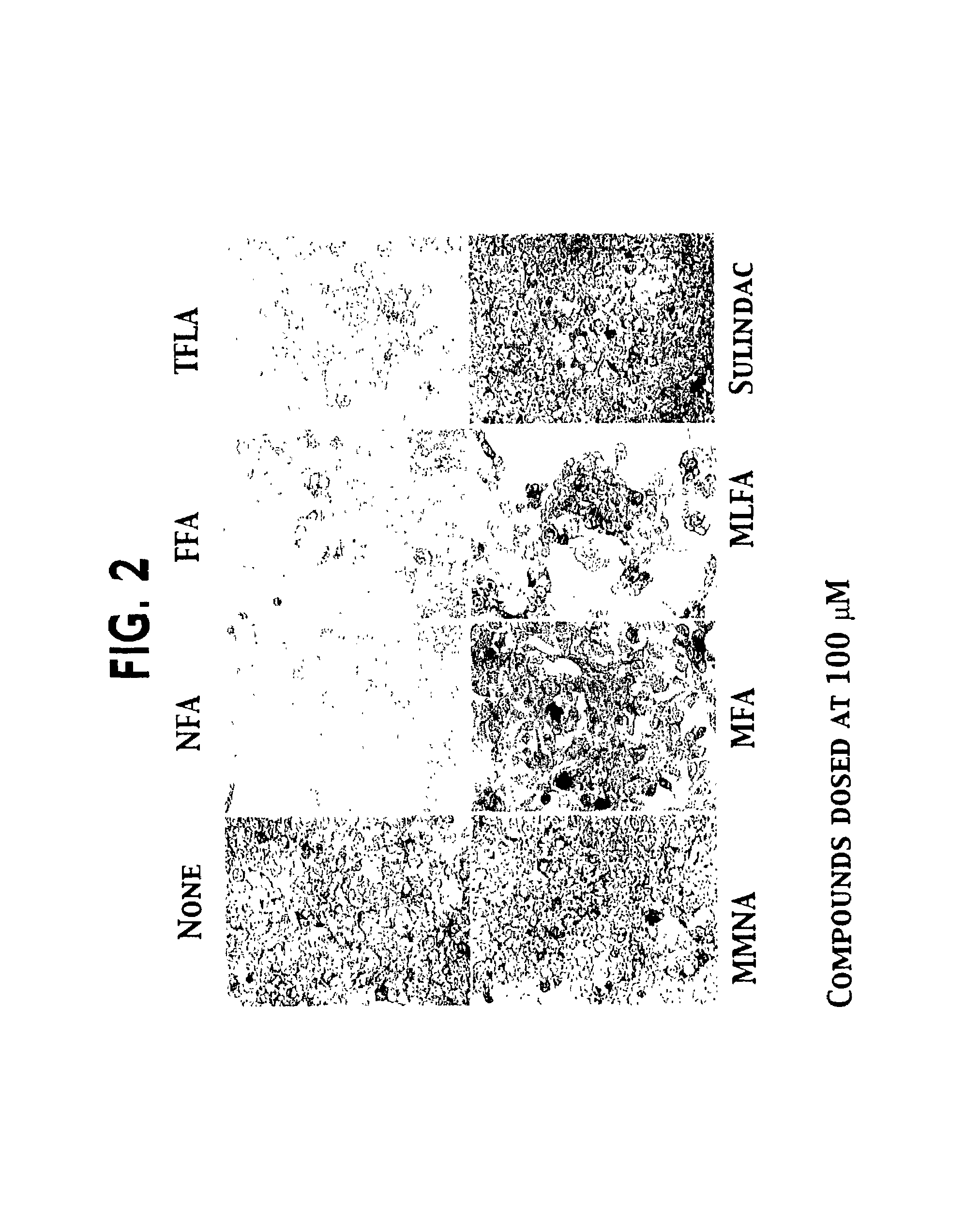
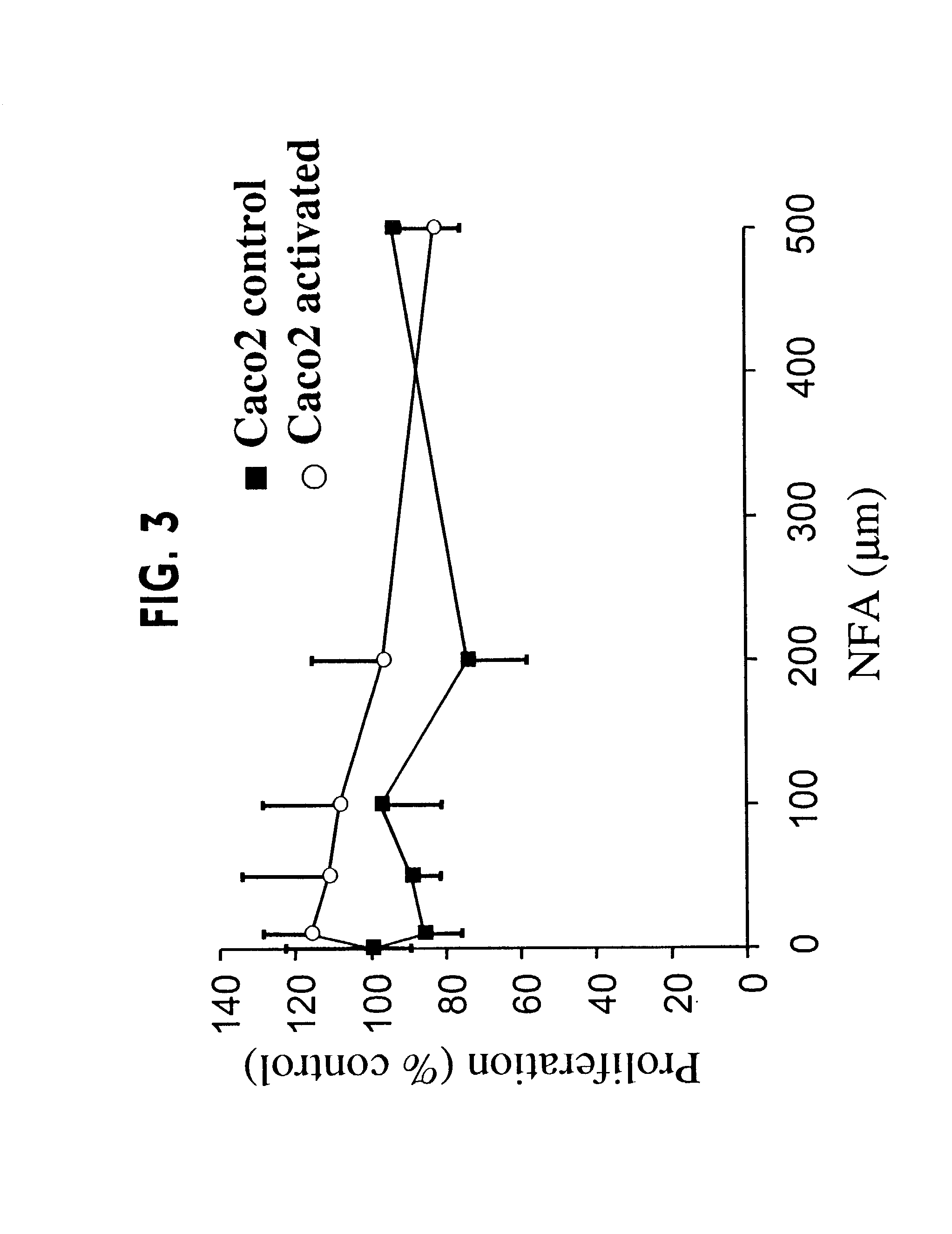
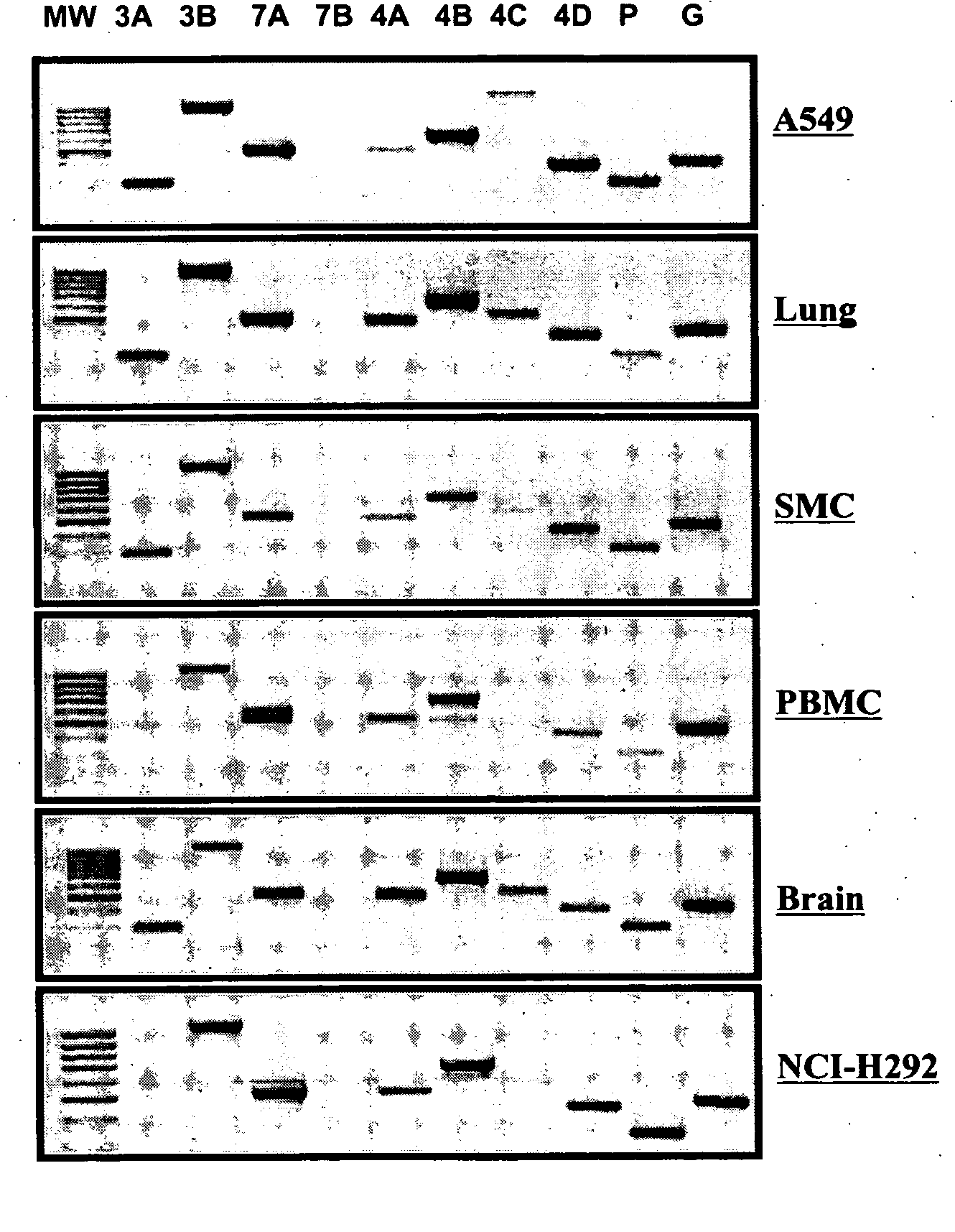
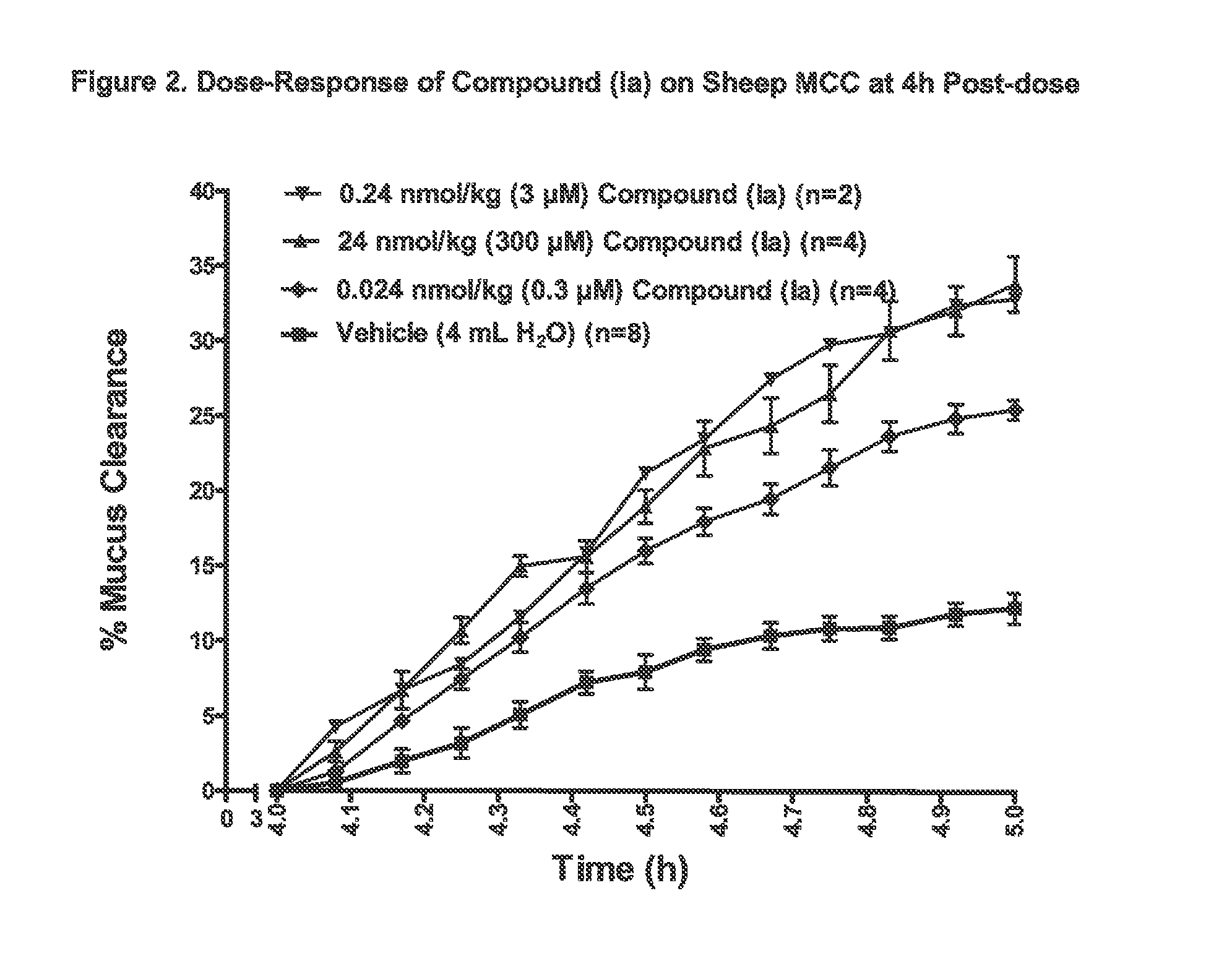
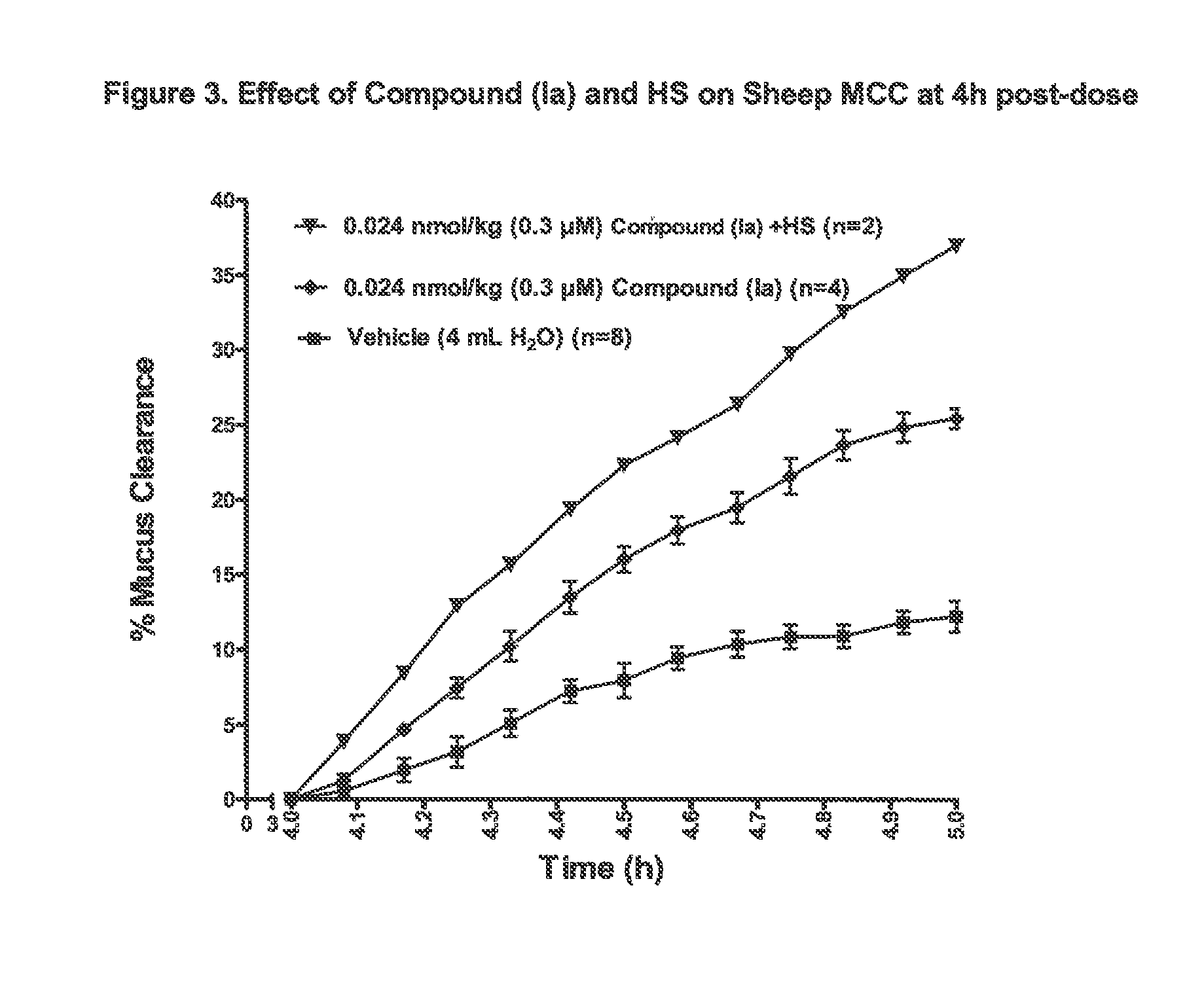
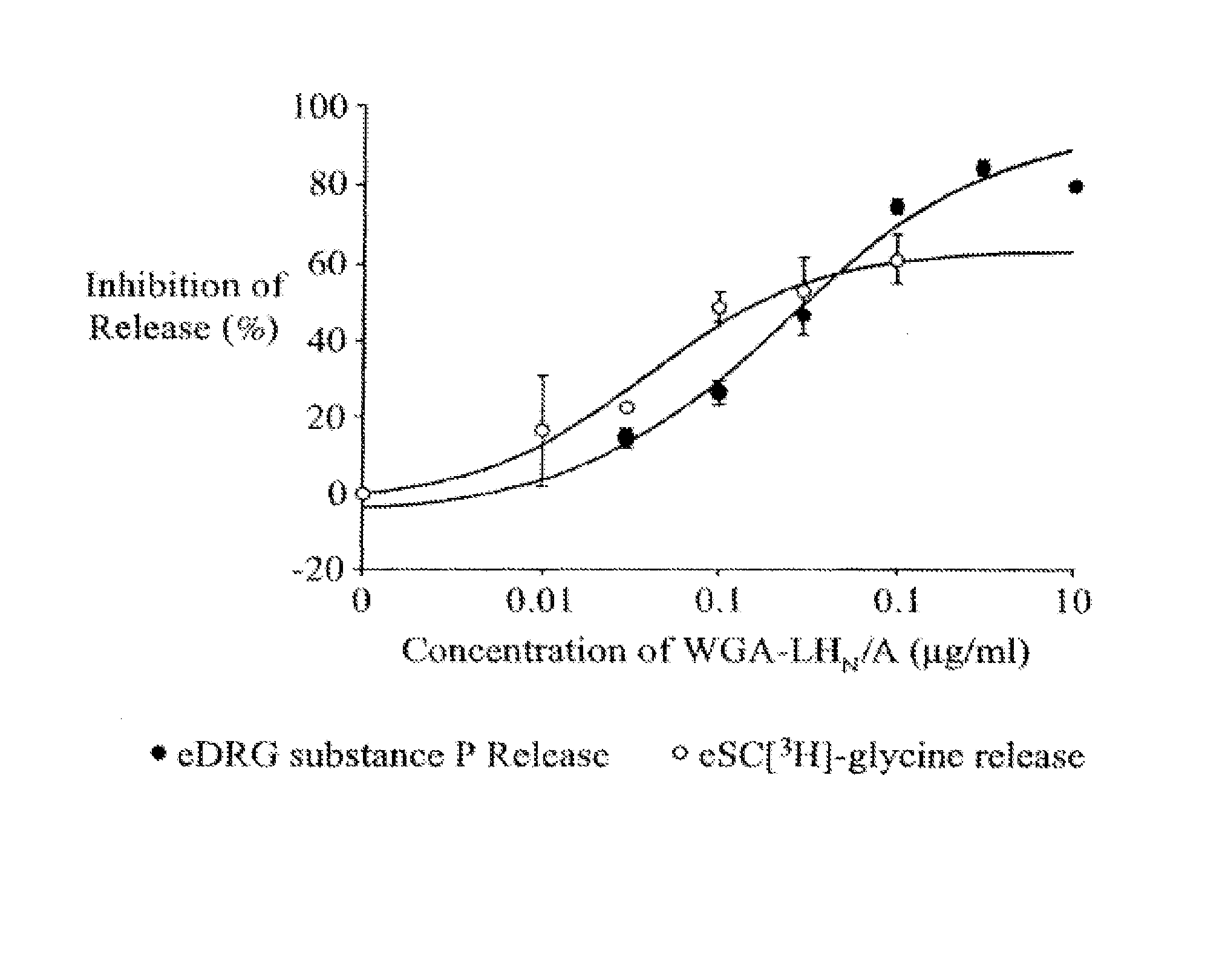
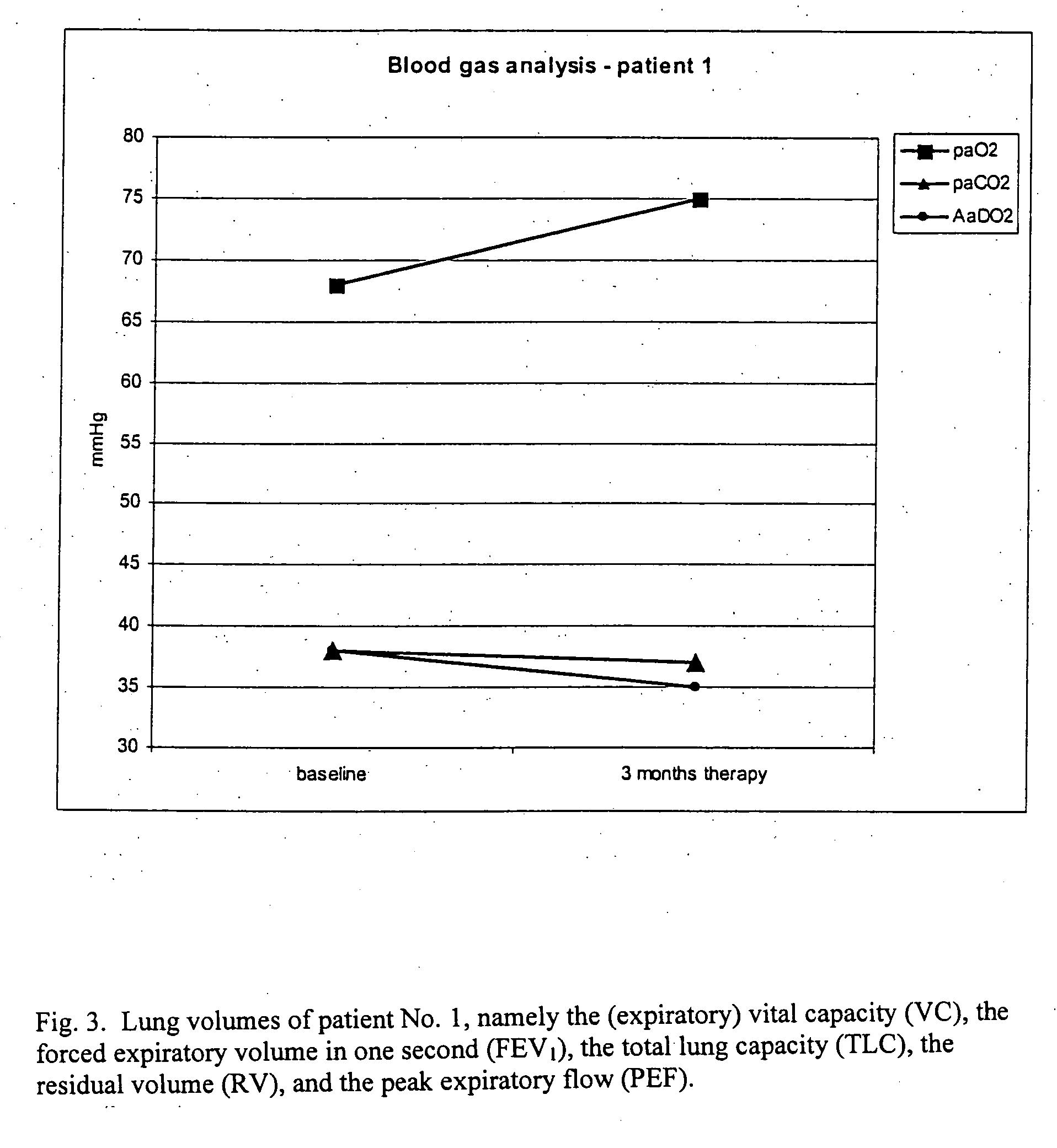
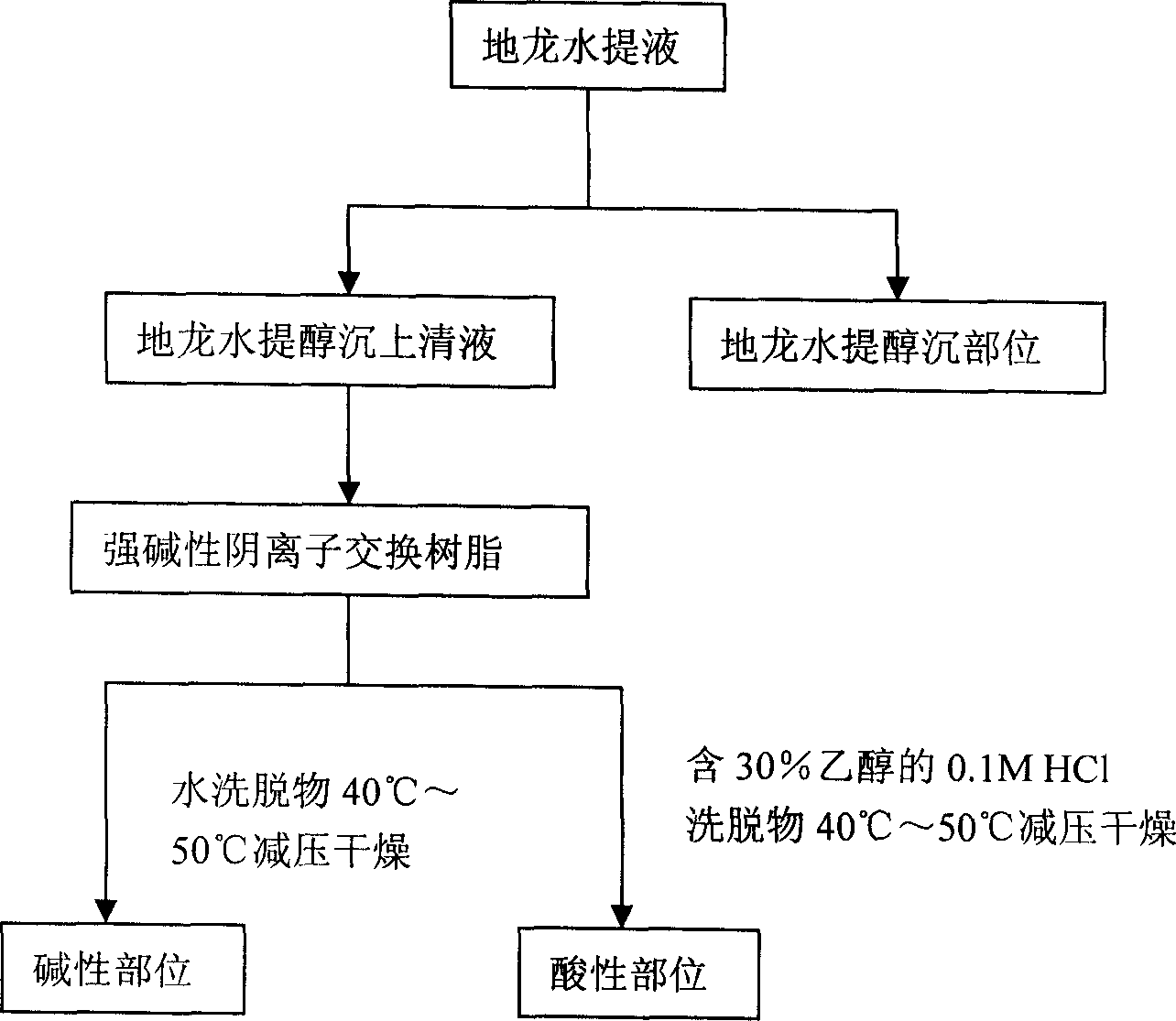
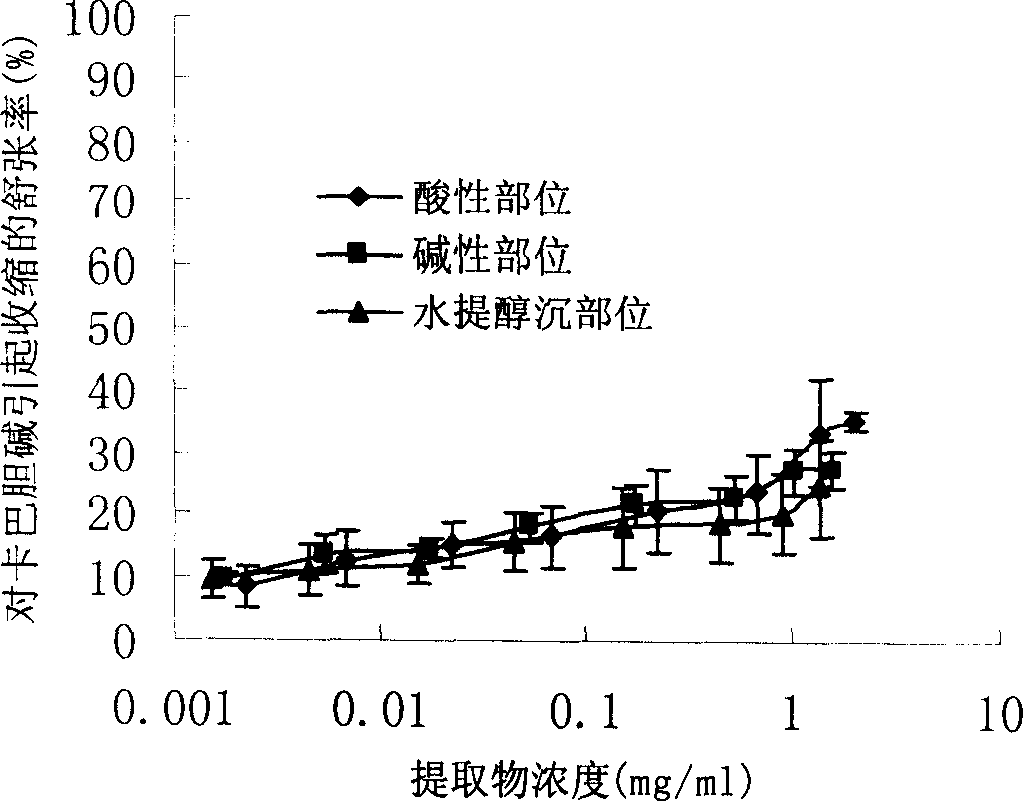
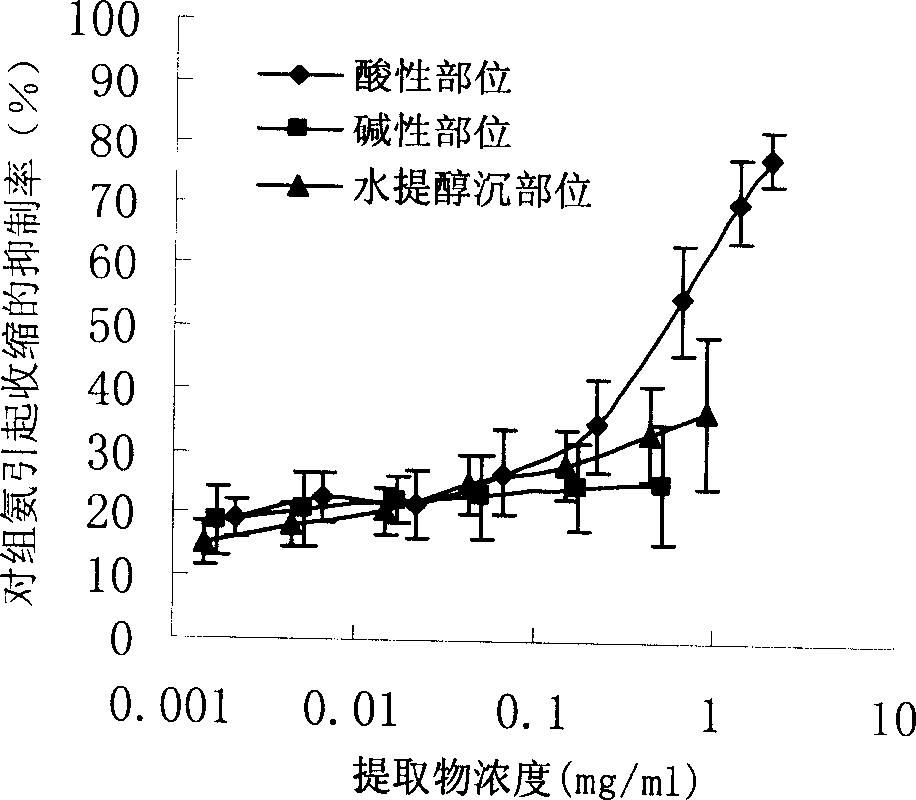
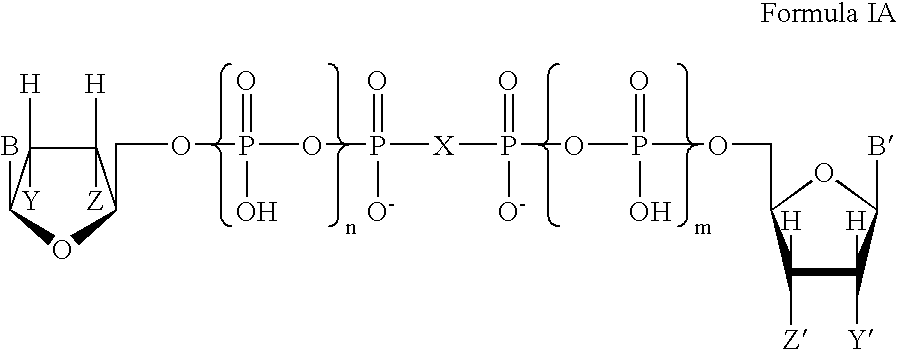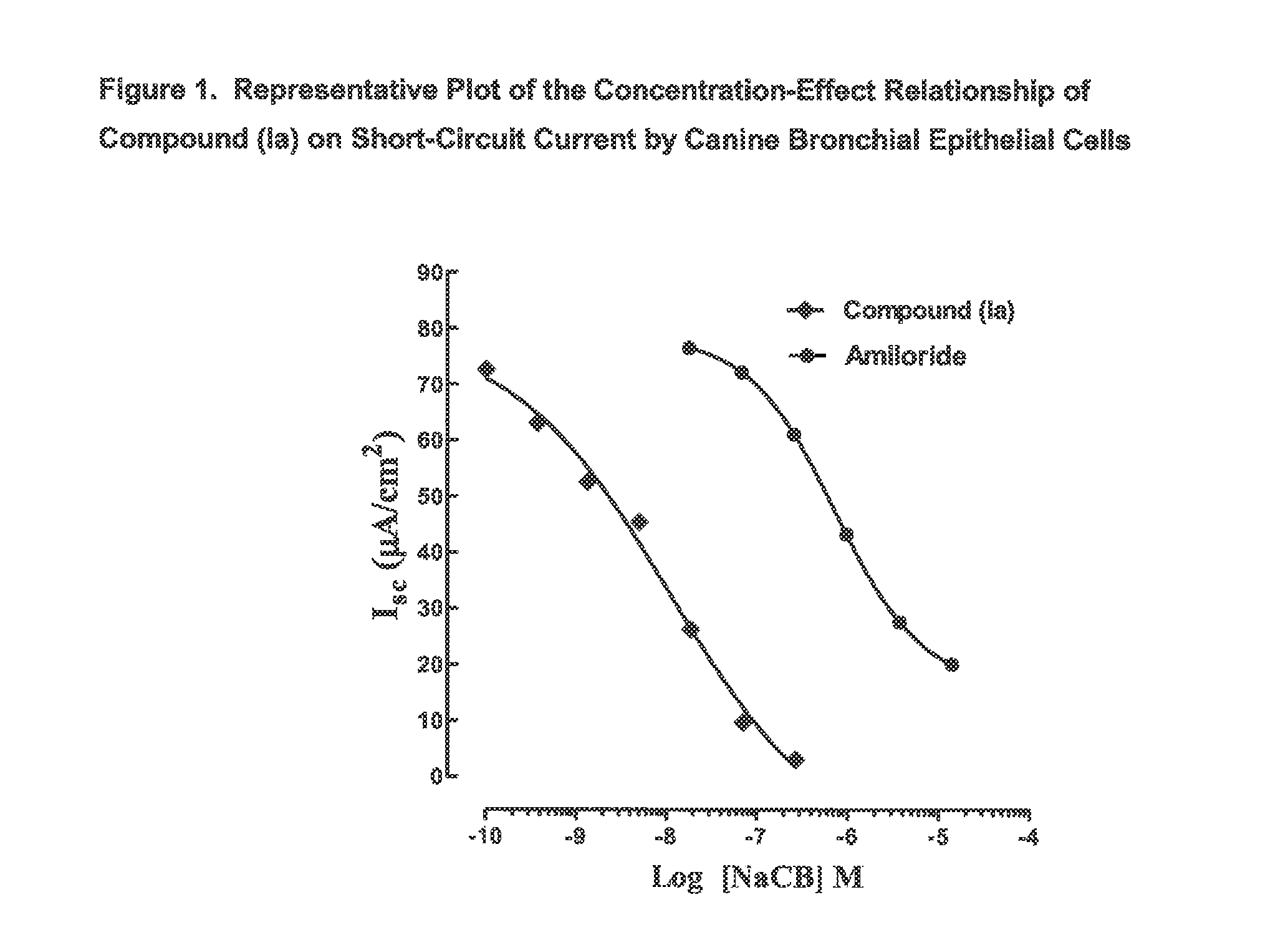Patents
Literature
1140 results about "Obstructive chronic bronchitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
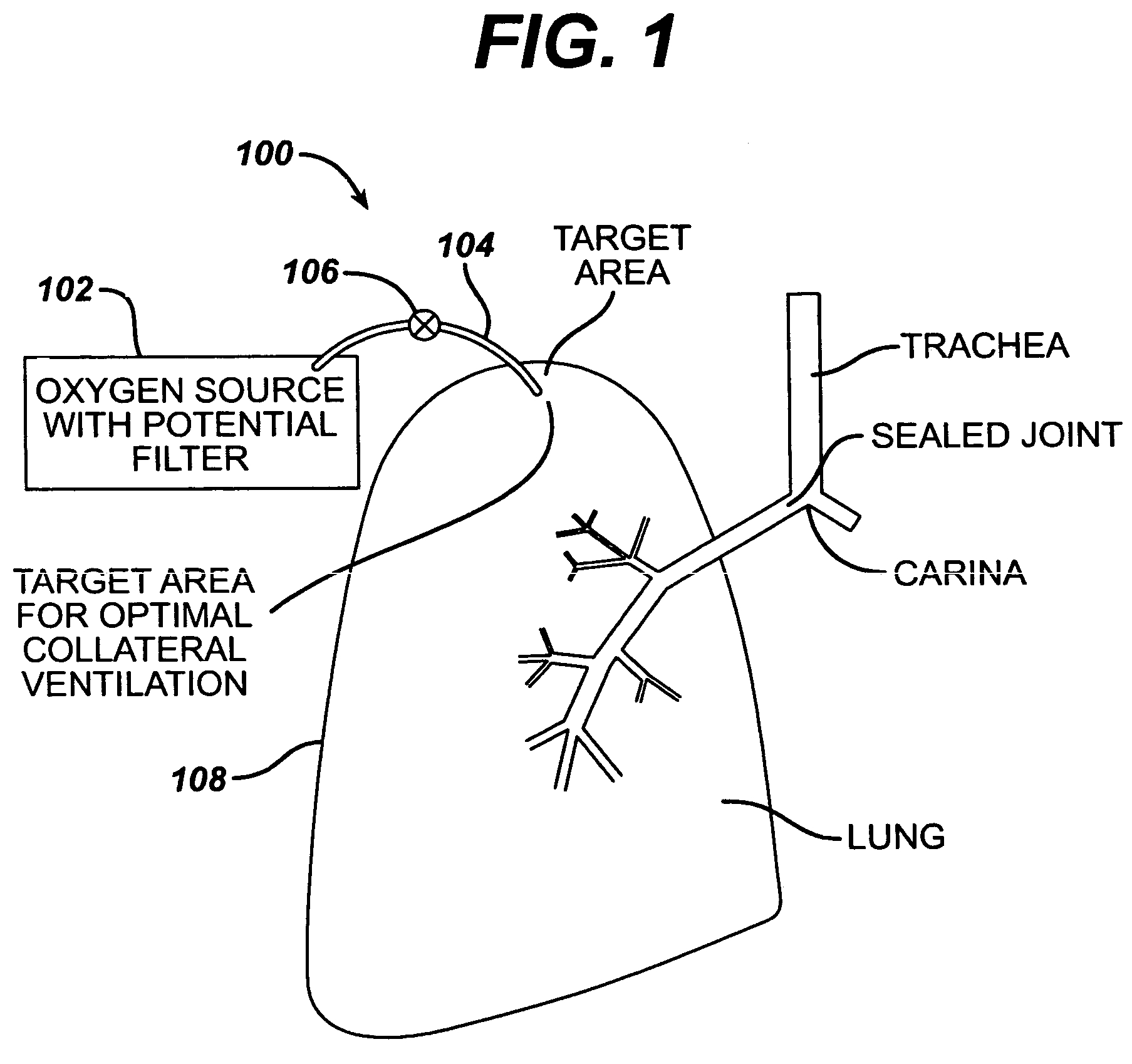
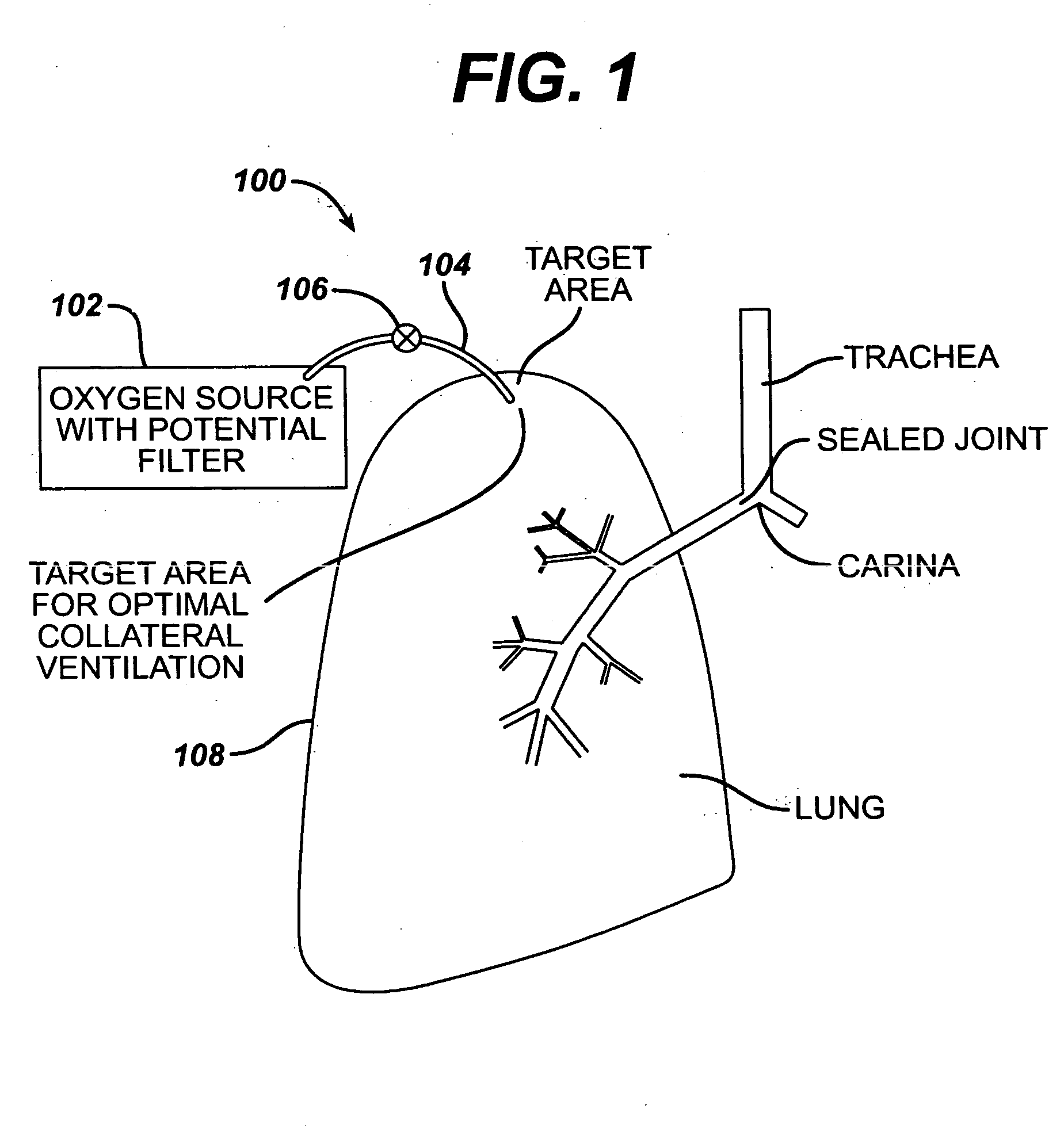
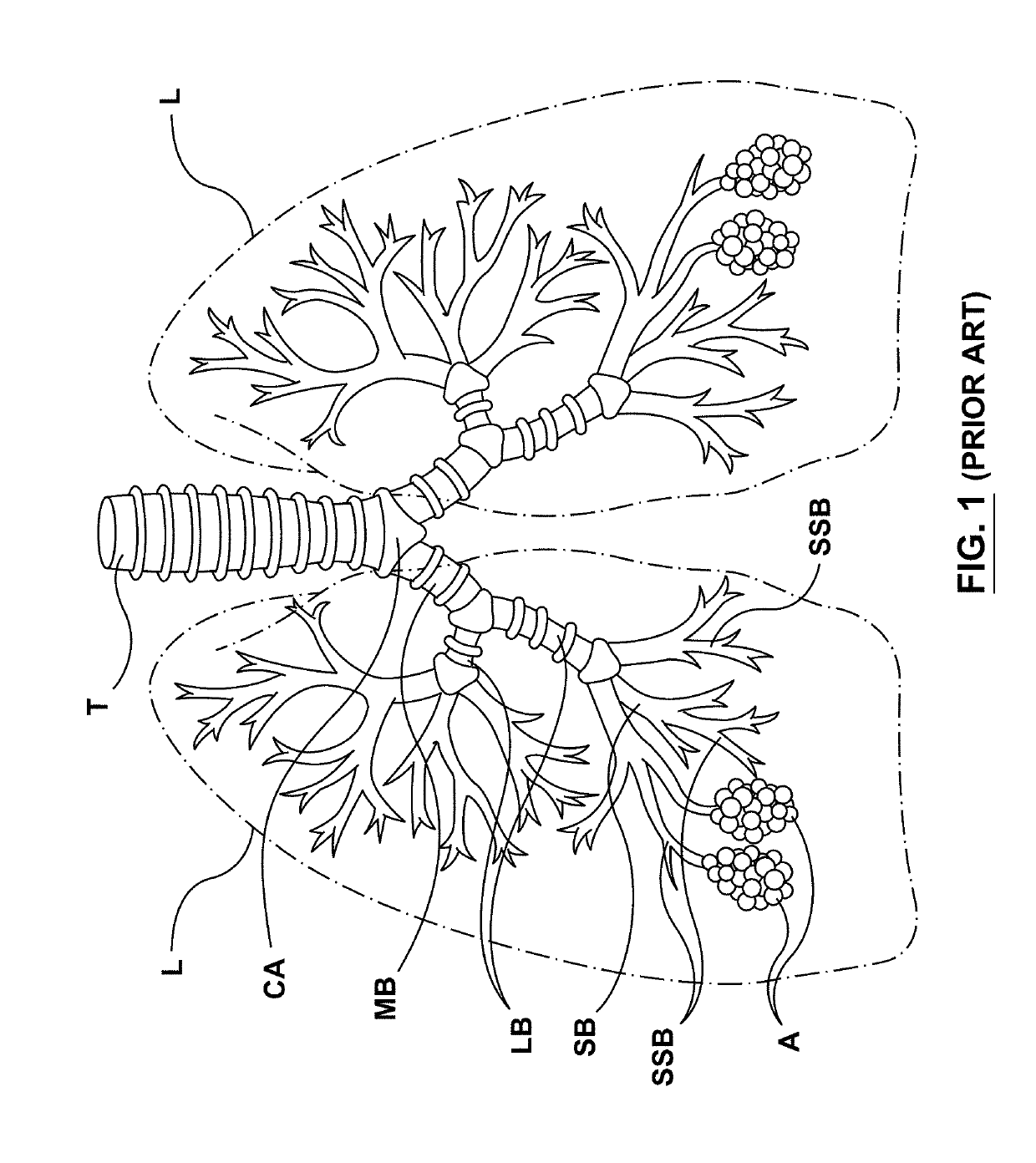
COPD is a type of obstructive lung disease in which chronic, incompletely reversible poor airflow (airflow limitation) and inability to breathe out fully (air trapping) exist. The poor airflow is the result of breakdown of lung tissue (known as emphysema) and small airways disease (known as obstructive bronchiolitis).
Muscarinic receptor antagonists
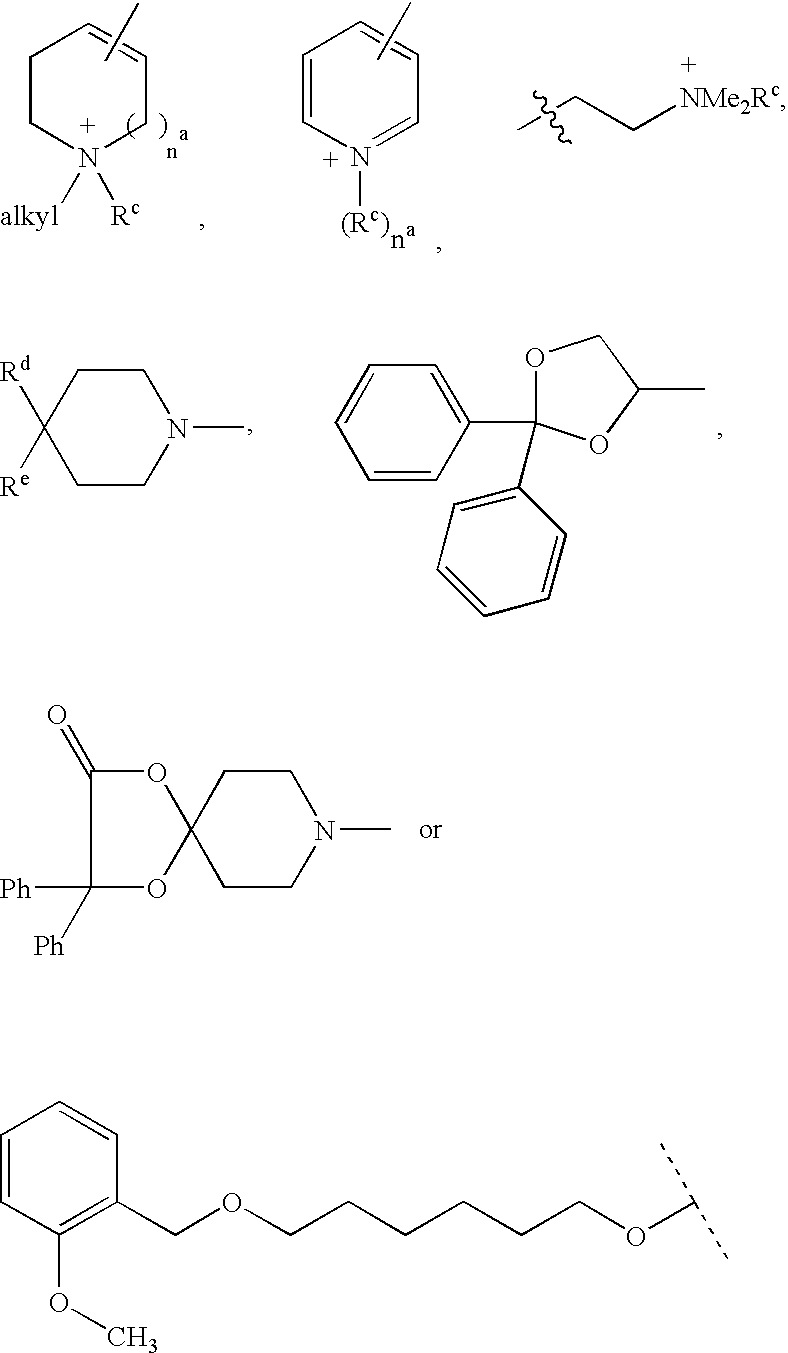
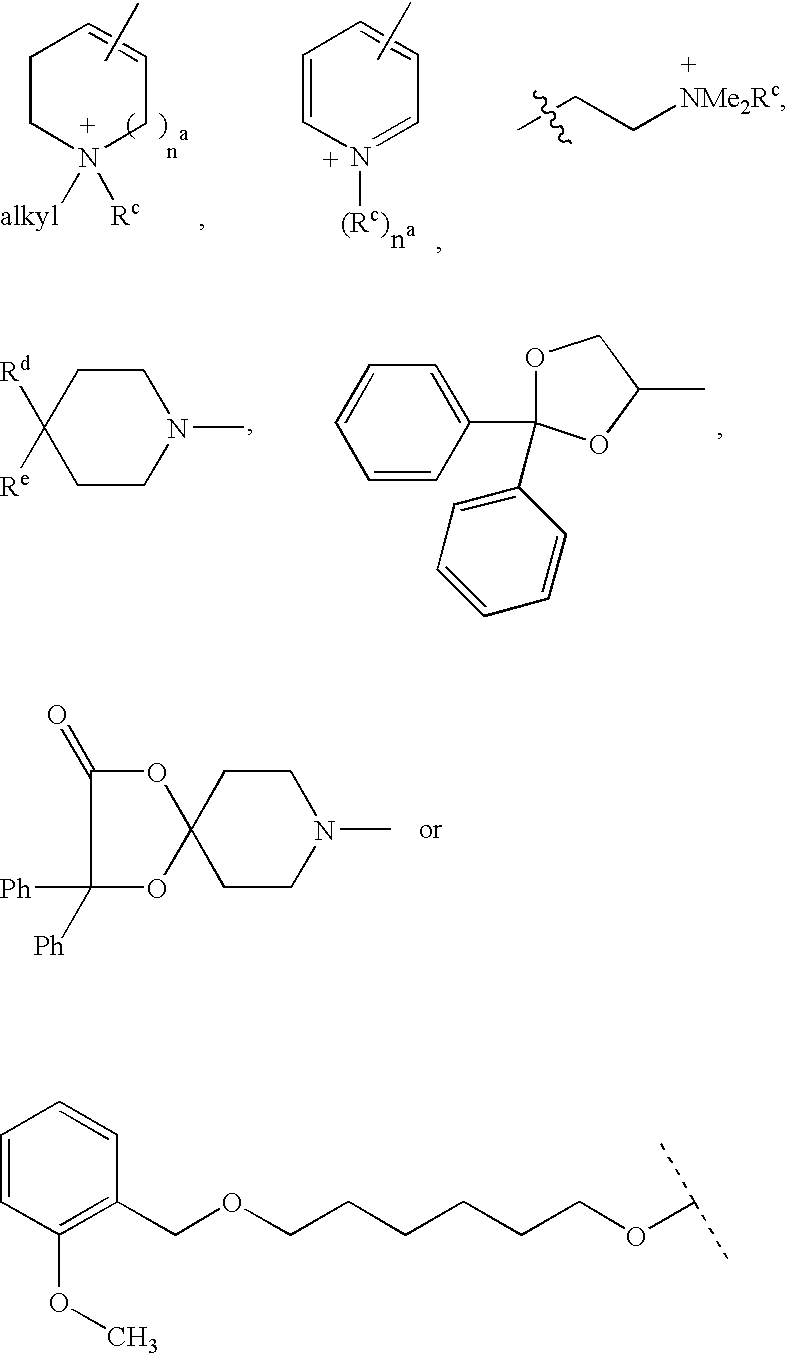
Disclosed are multibinding compounds which are muscarinic receptor antagonists. The multibinding compounds of this invention containing from 2 to 10 ligands covalently attached to one or more linkers. Each ligand is, independently of each other, a muscarinic receptor antagonist or an allosteric modulator provided that at least one of said ligand is a muscarinic receptor antagonist. The multibinding compounds of this invention are useful in the treatment and prevention of diseases such as chronic obstructive pulmonary disease, chronic bronchitis, irritable bowel syndrome, urinary incontinence, and the like.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
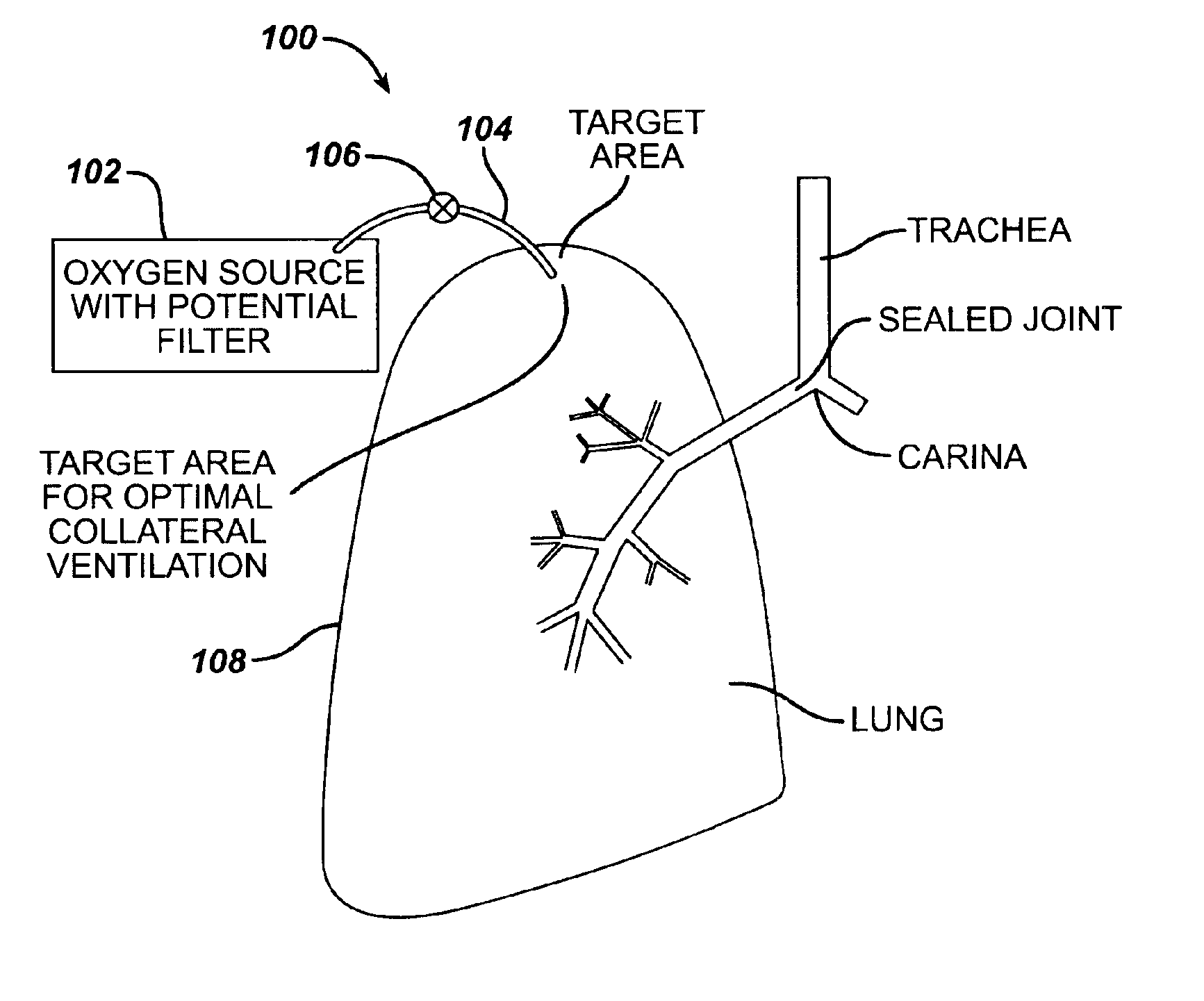
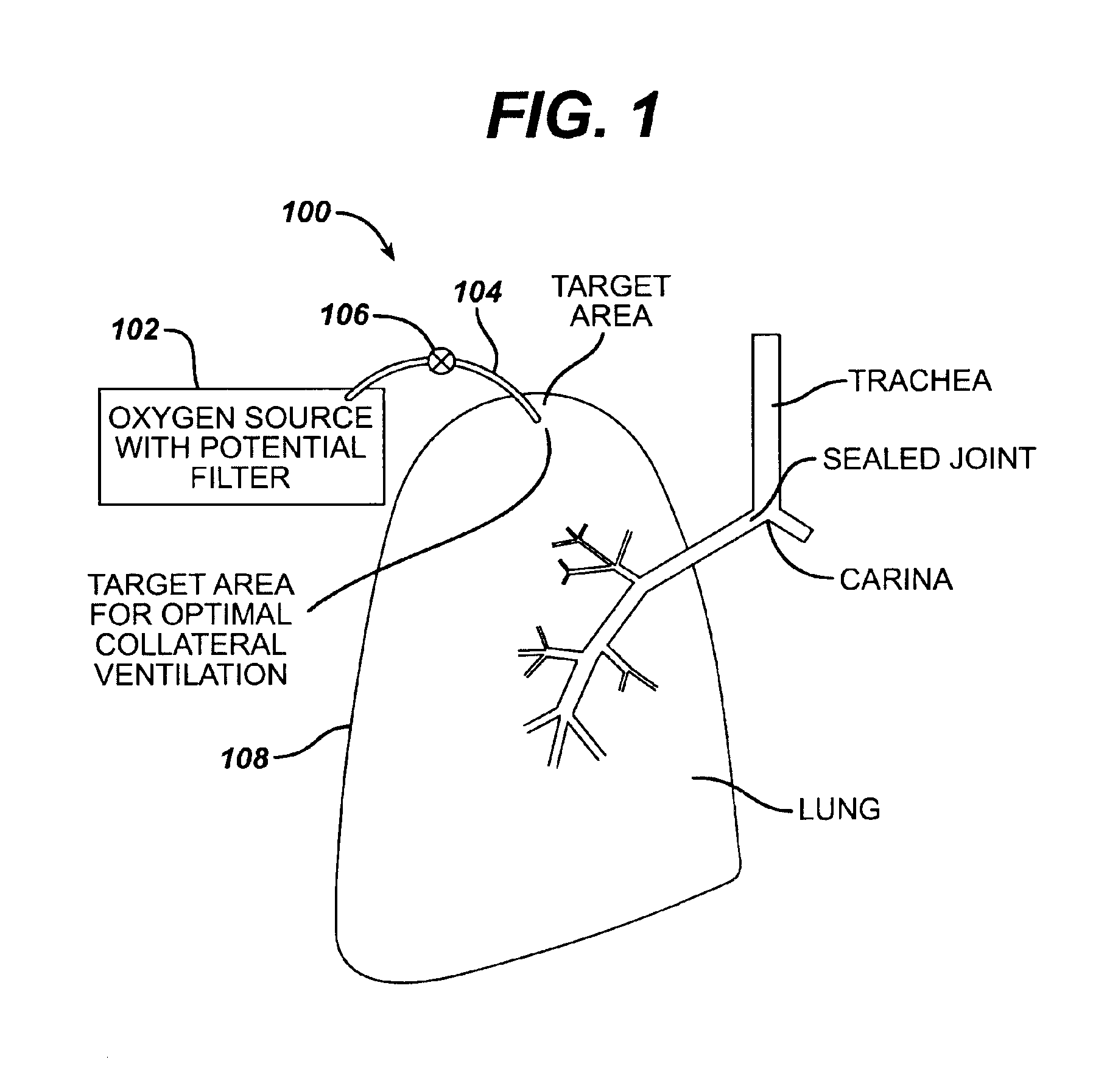
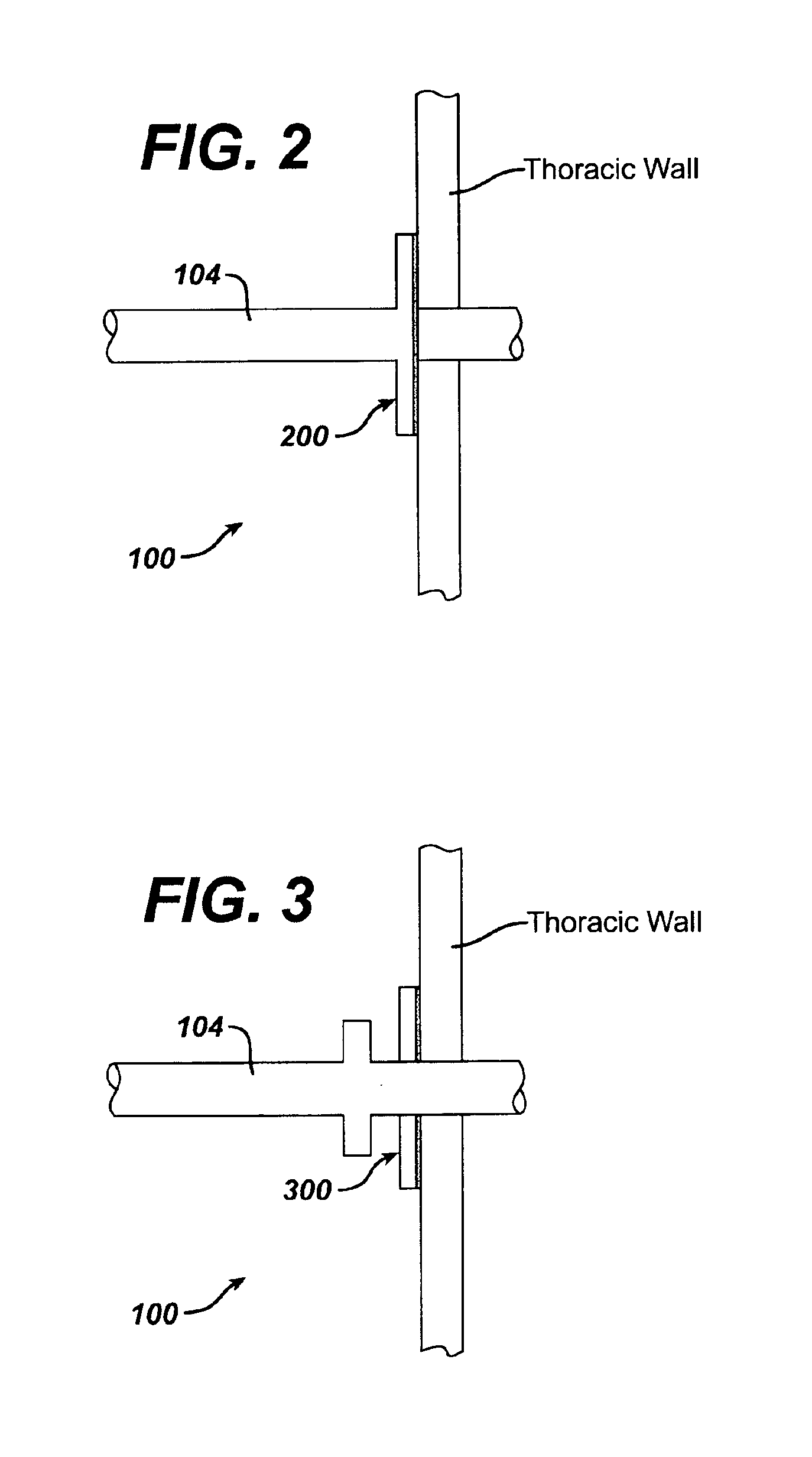
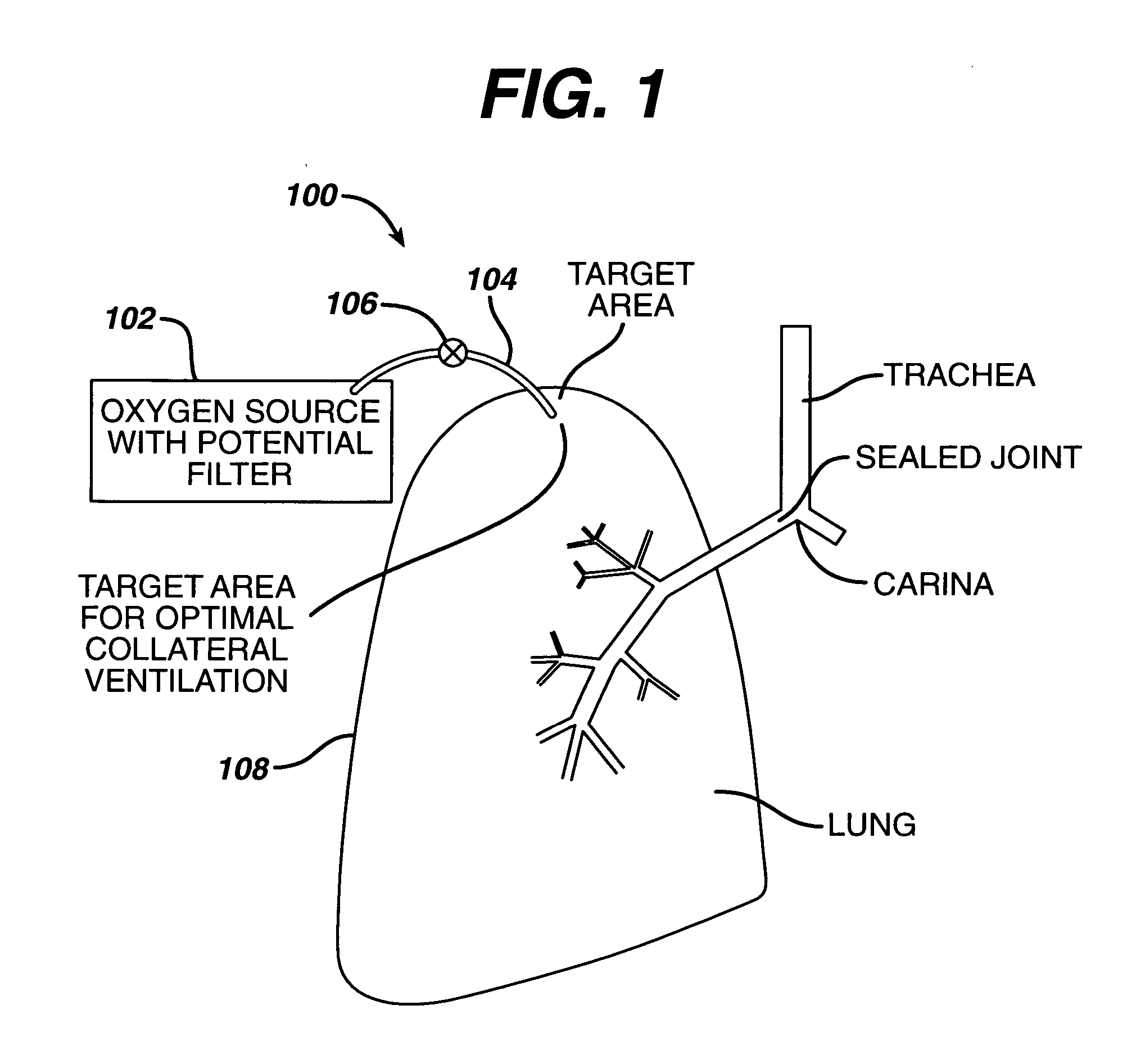
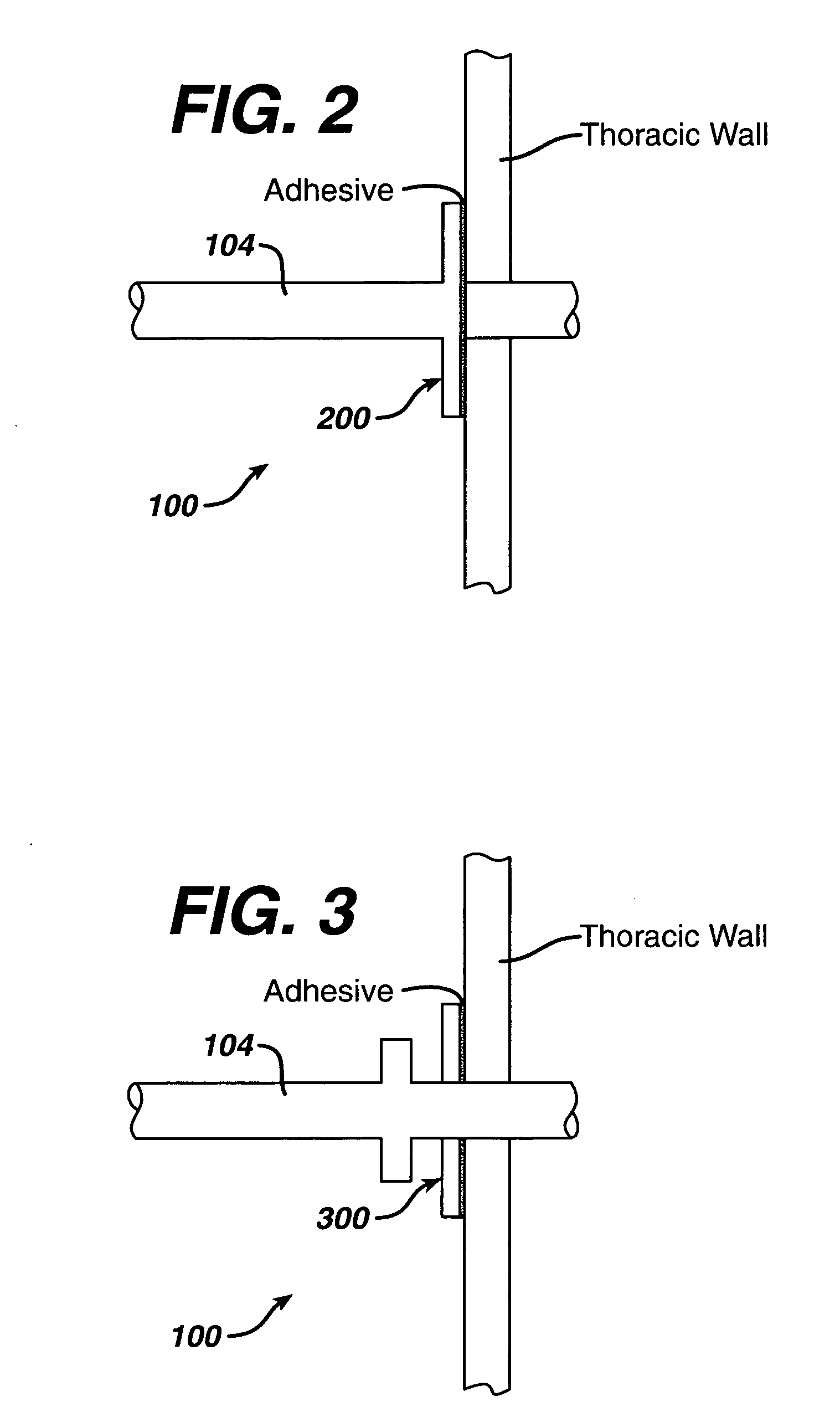
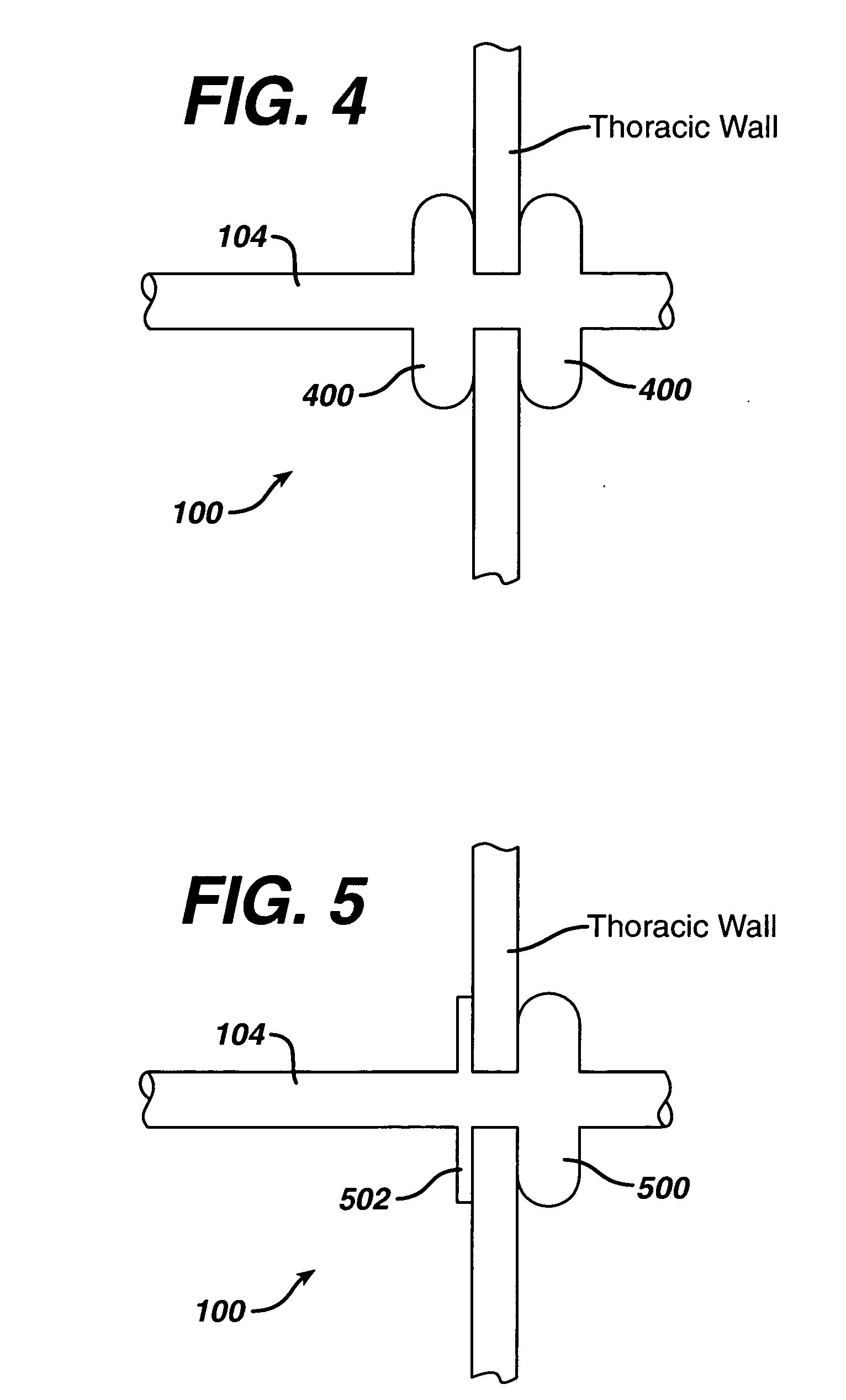
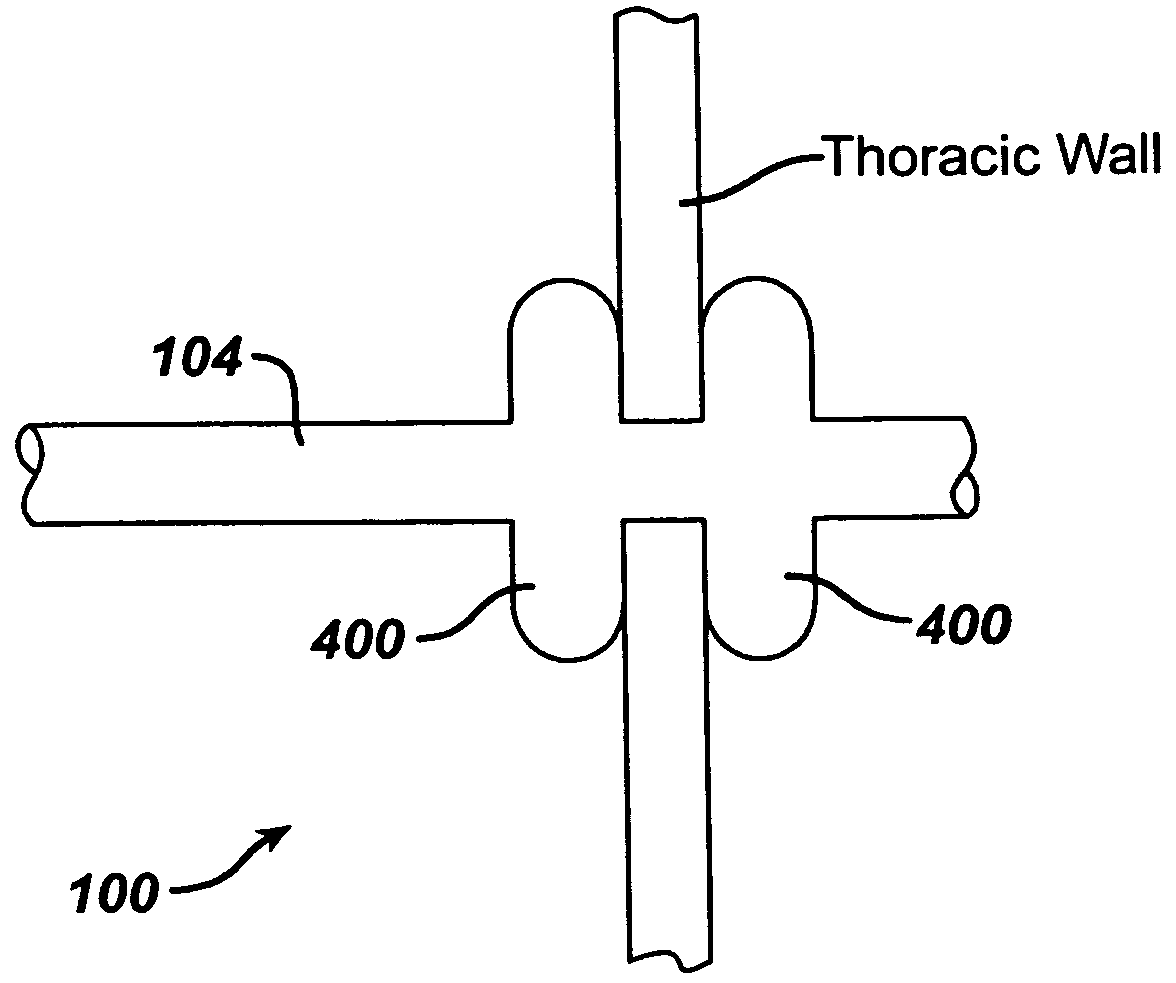
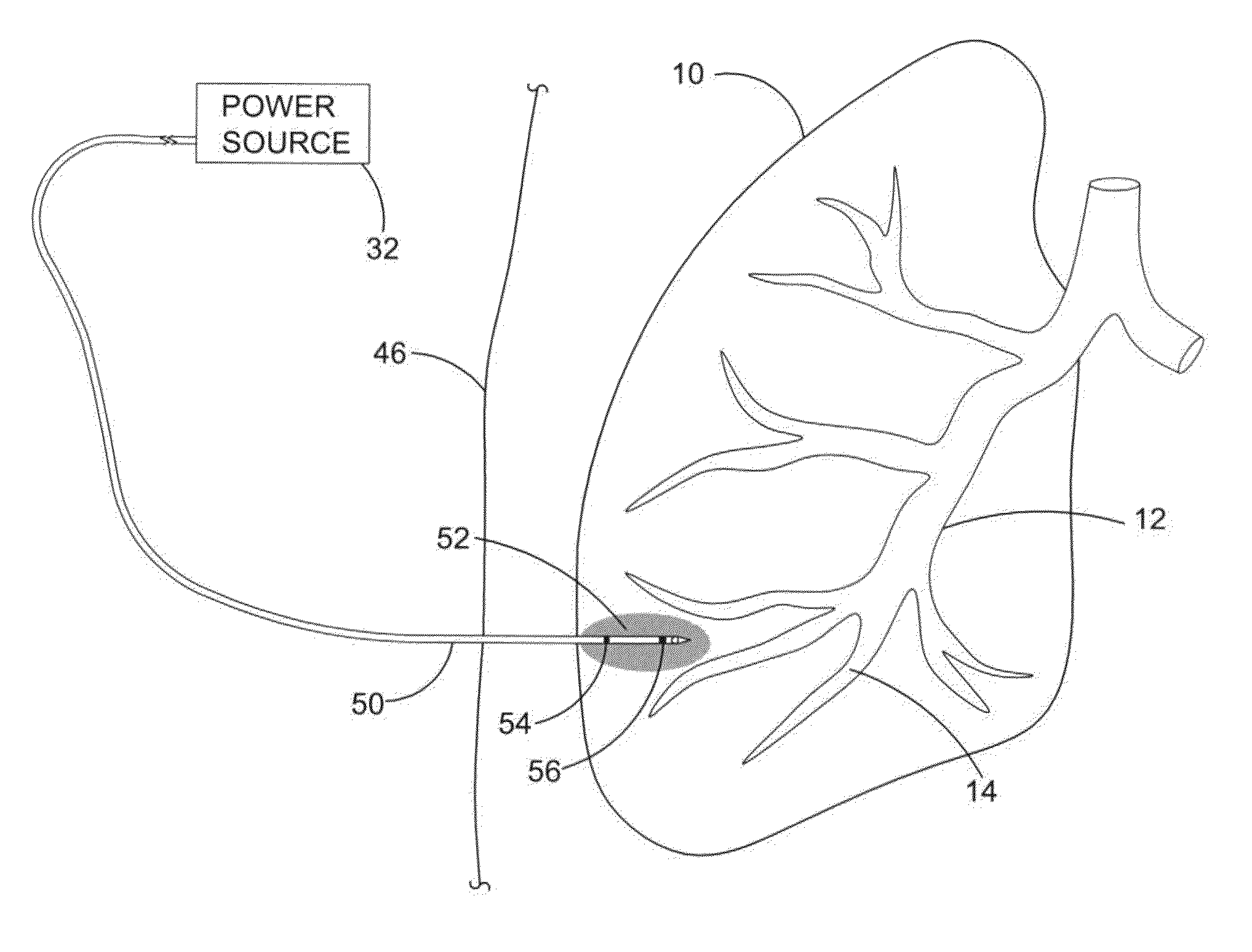
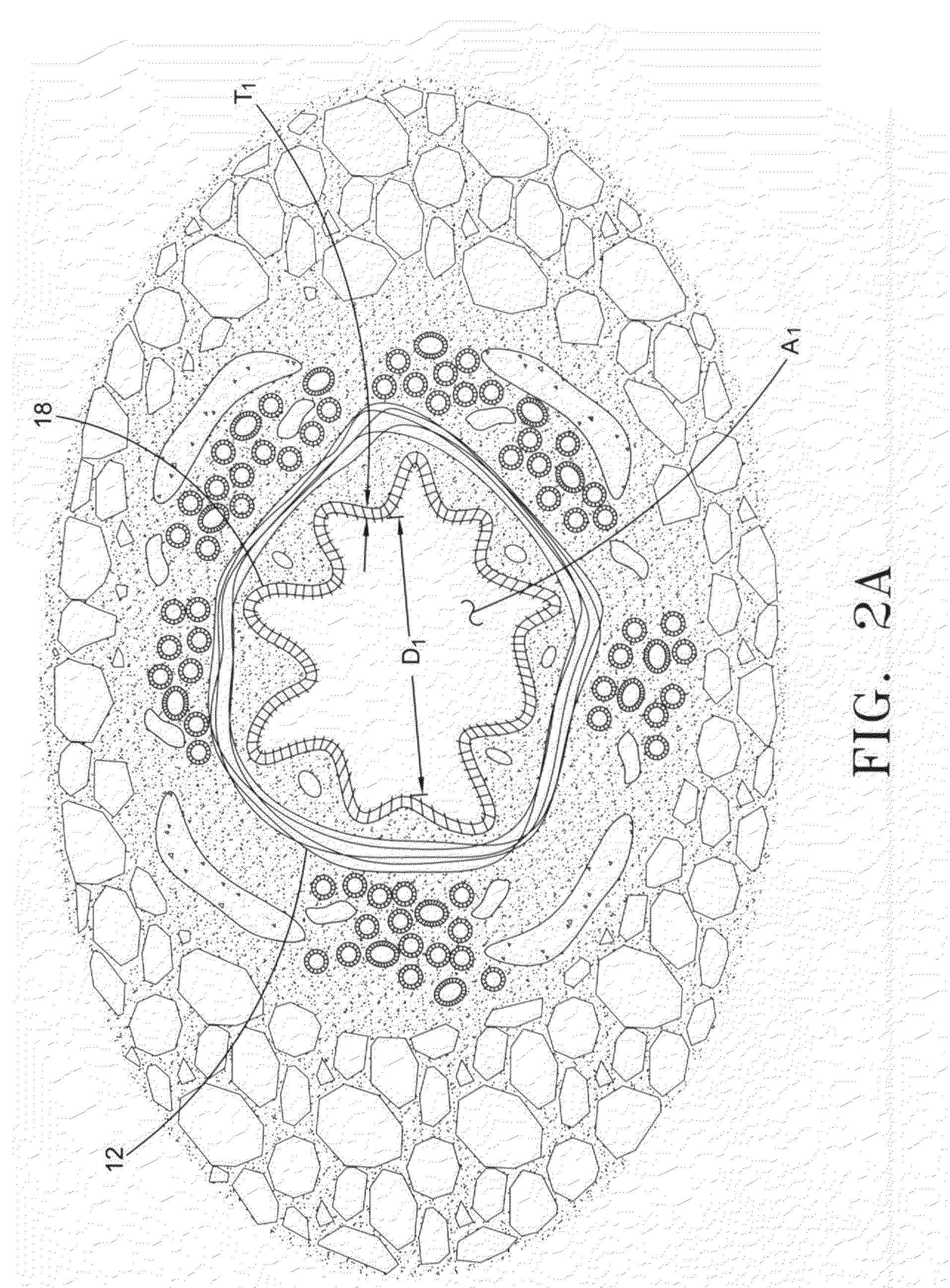
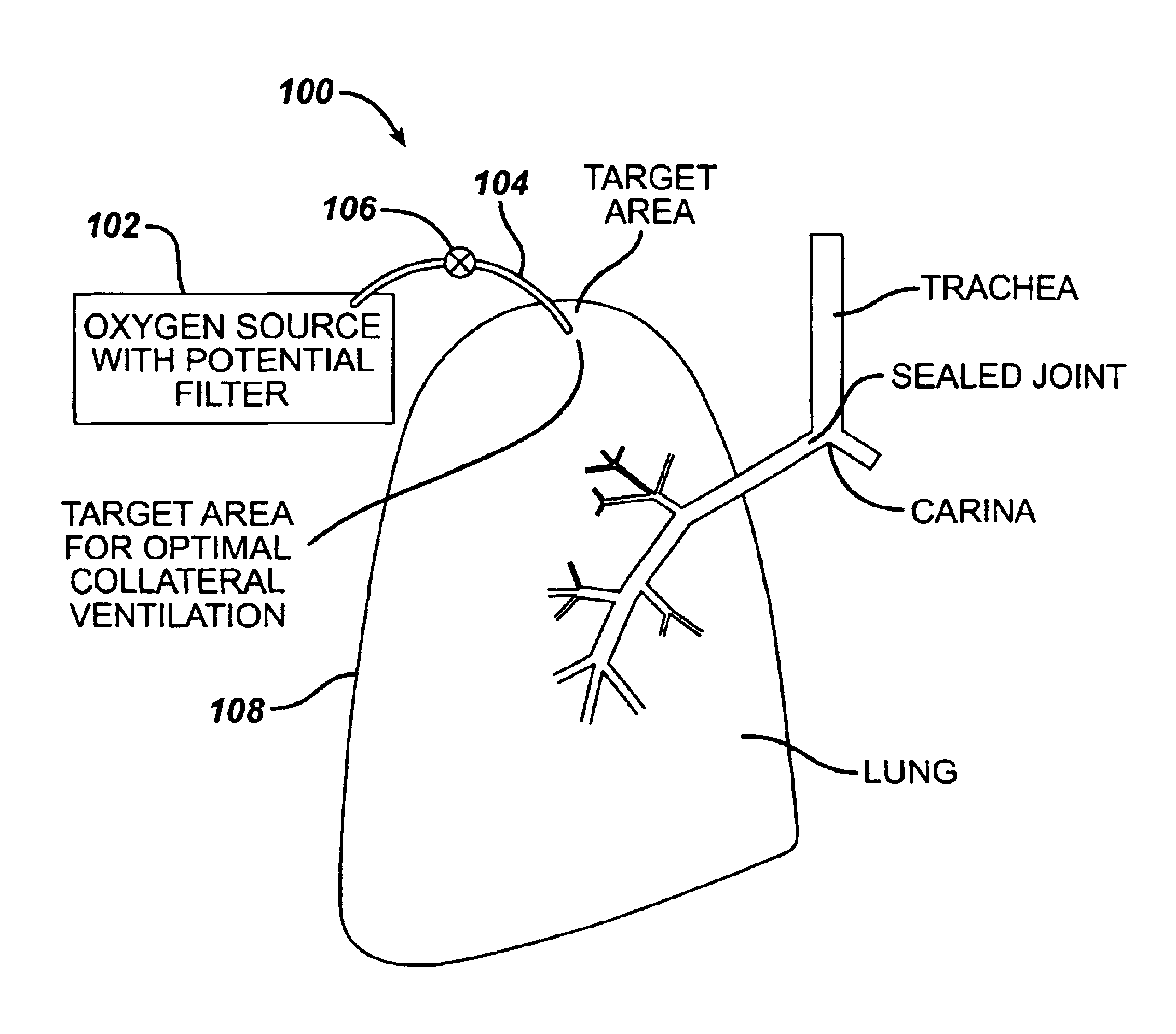
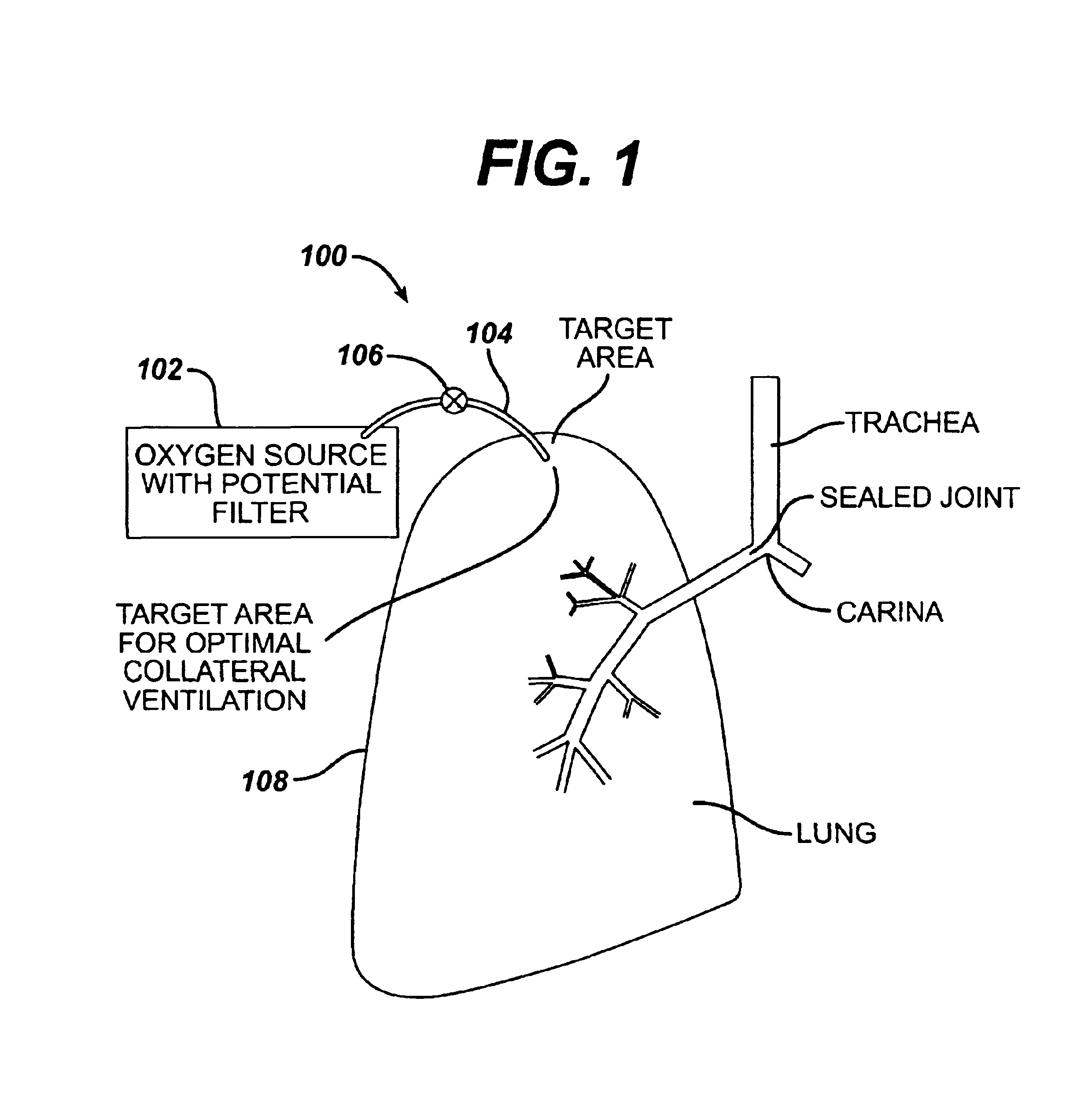
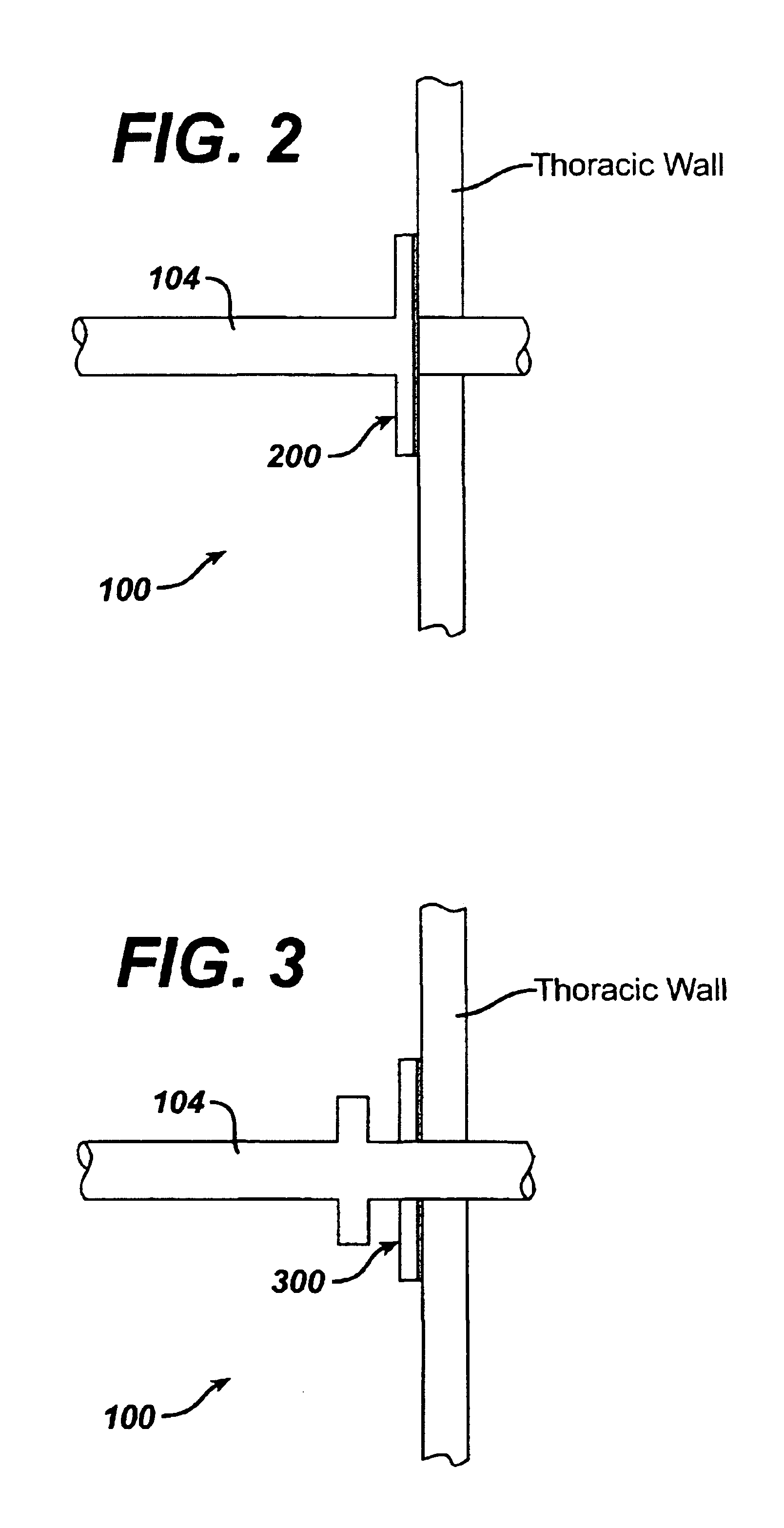
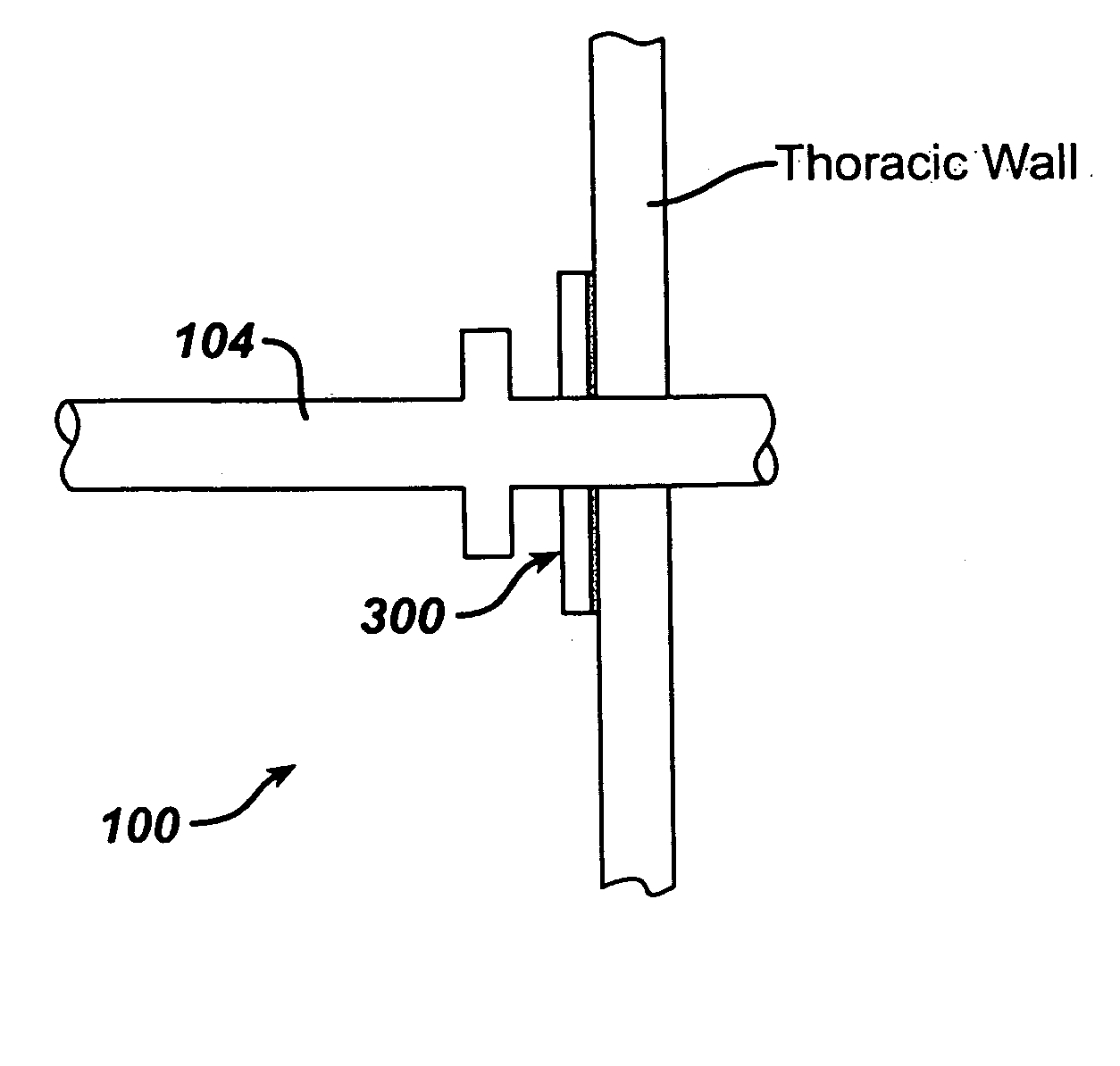
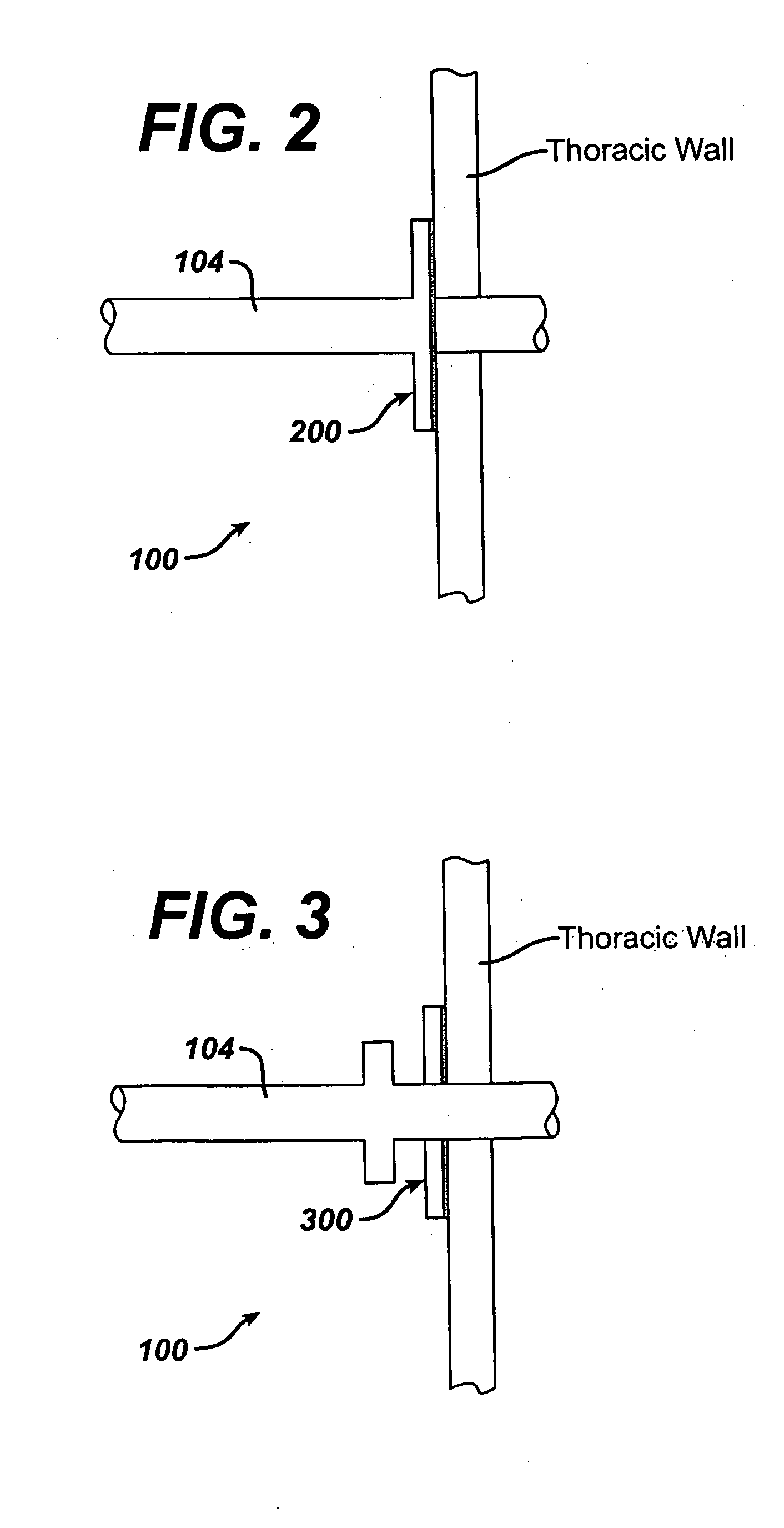
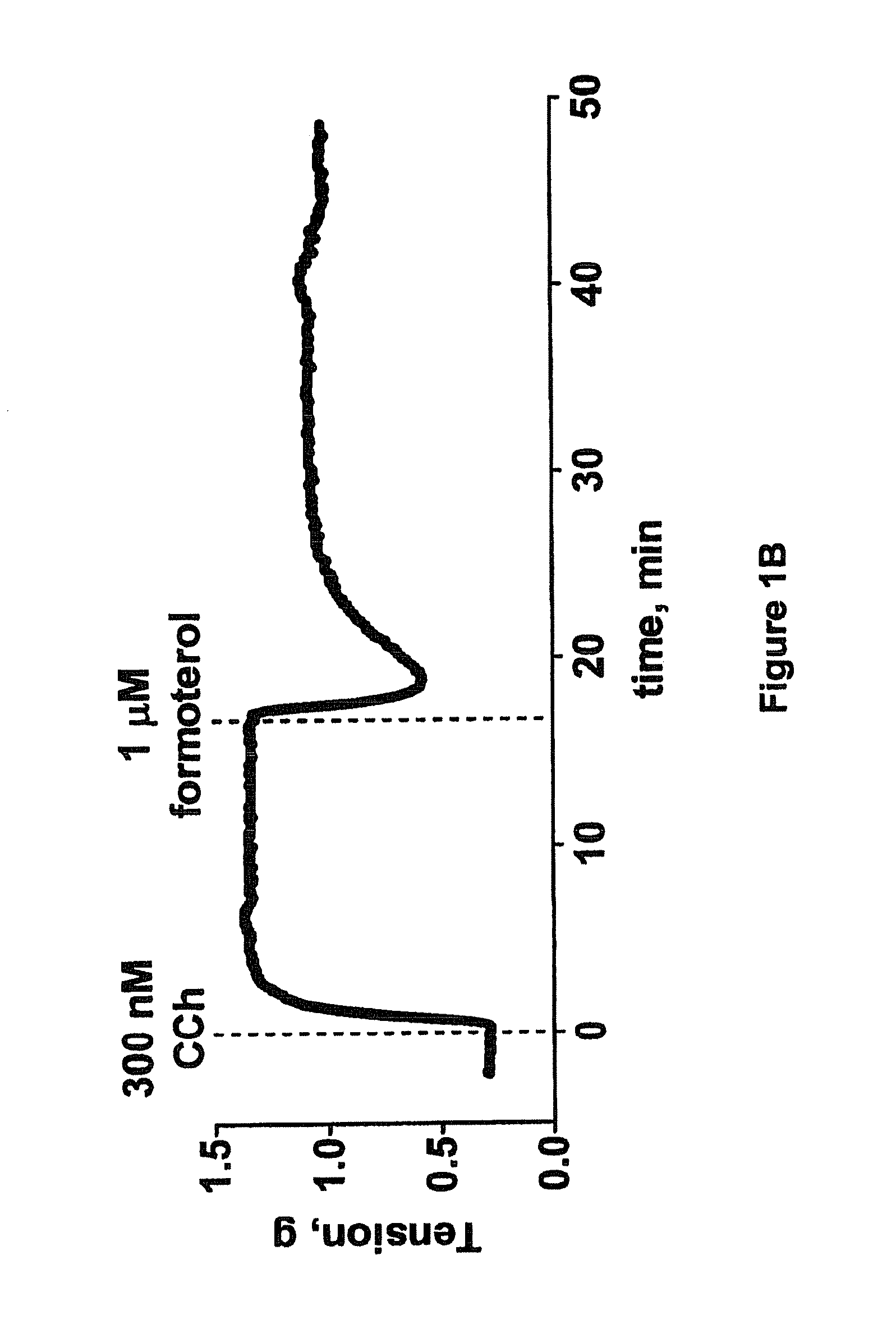
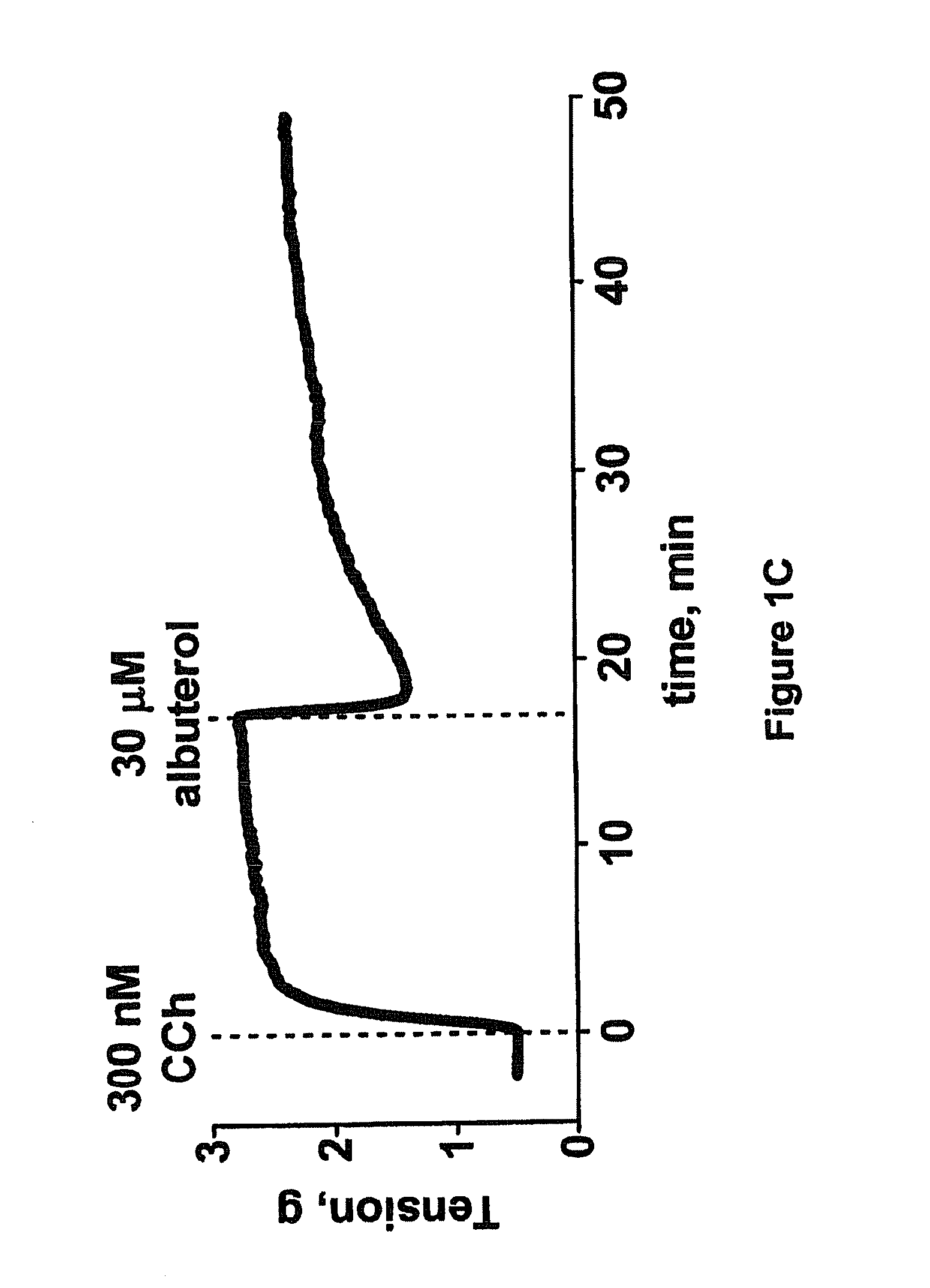
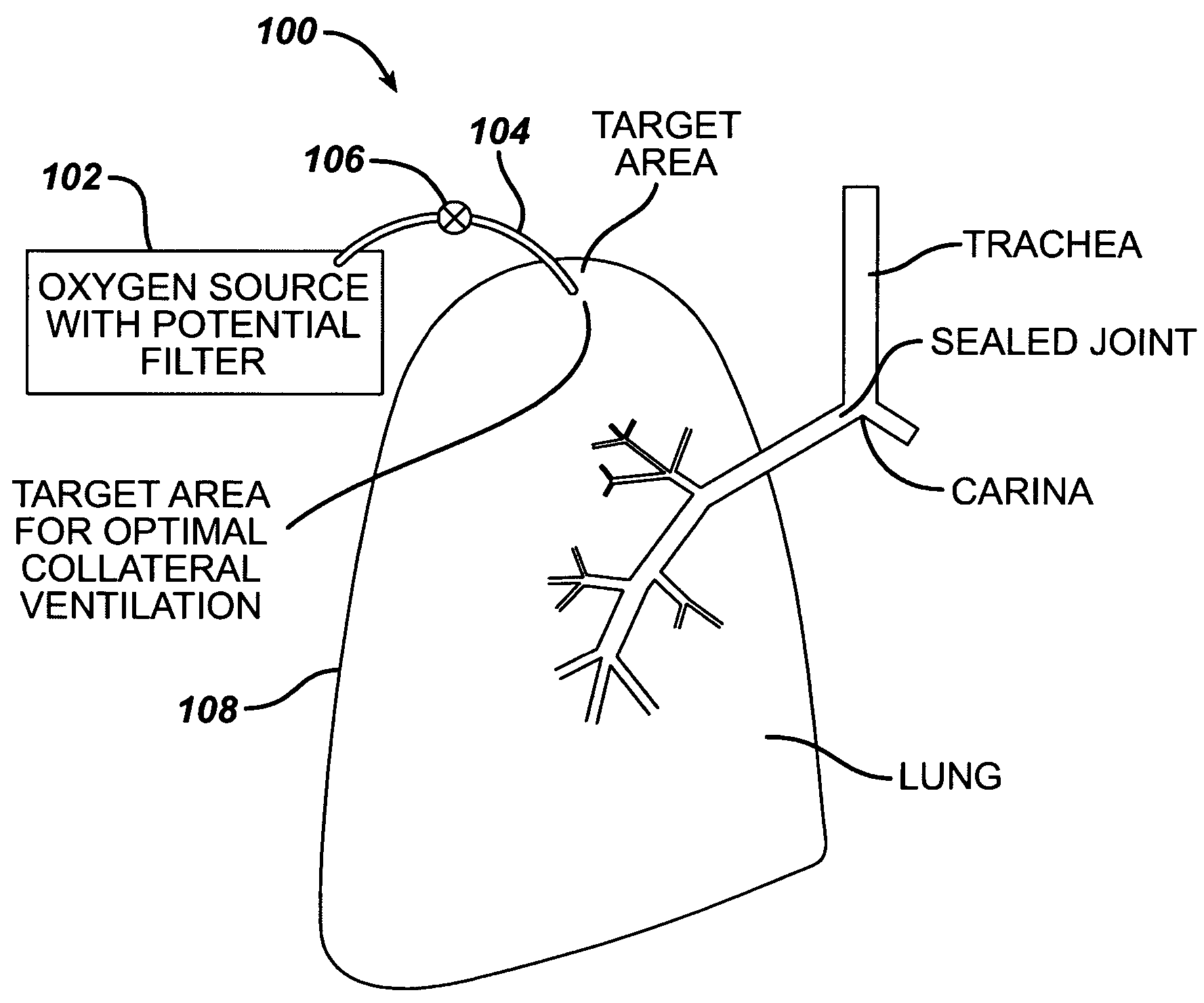
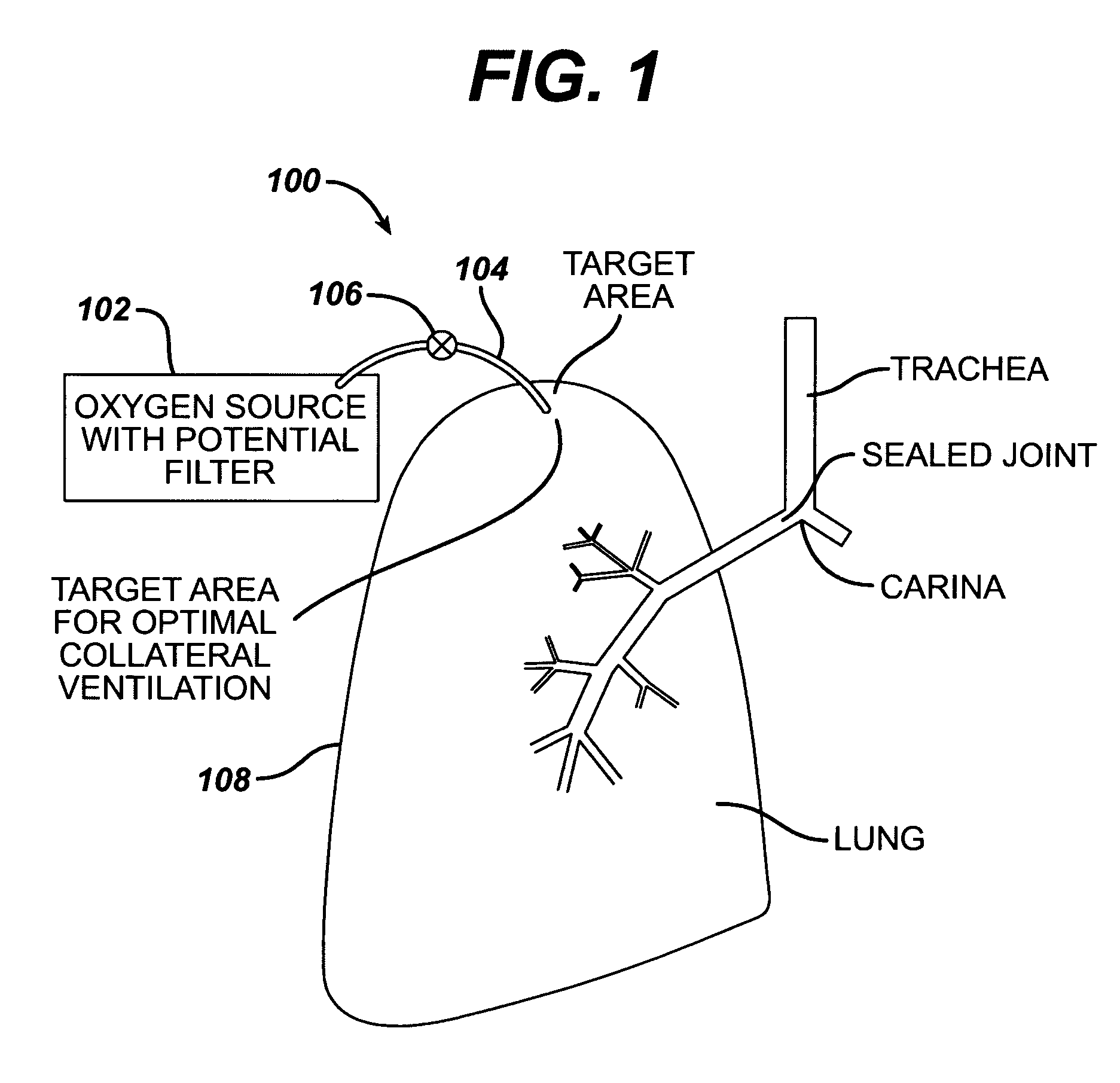
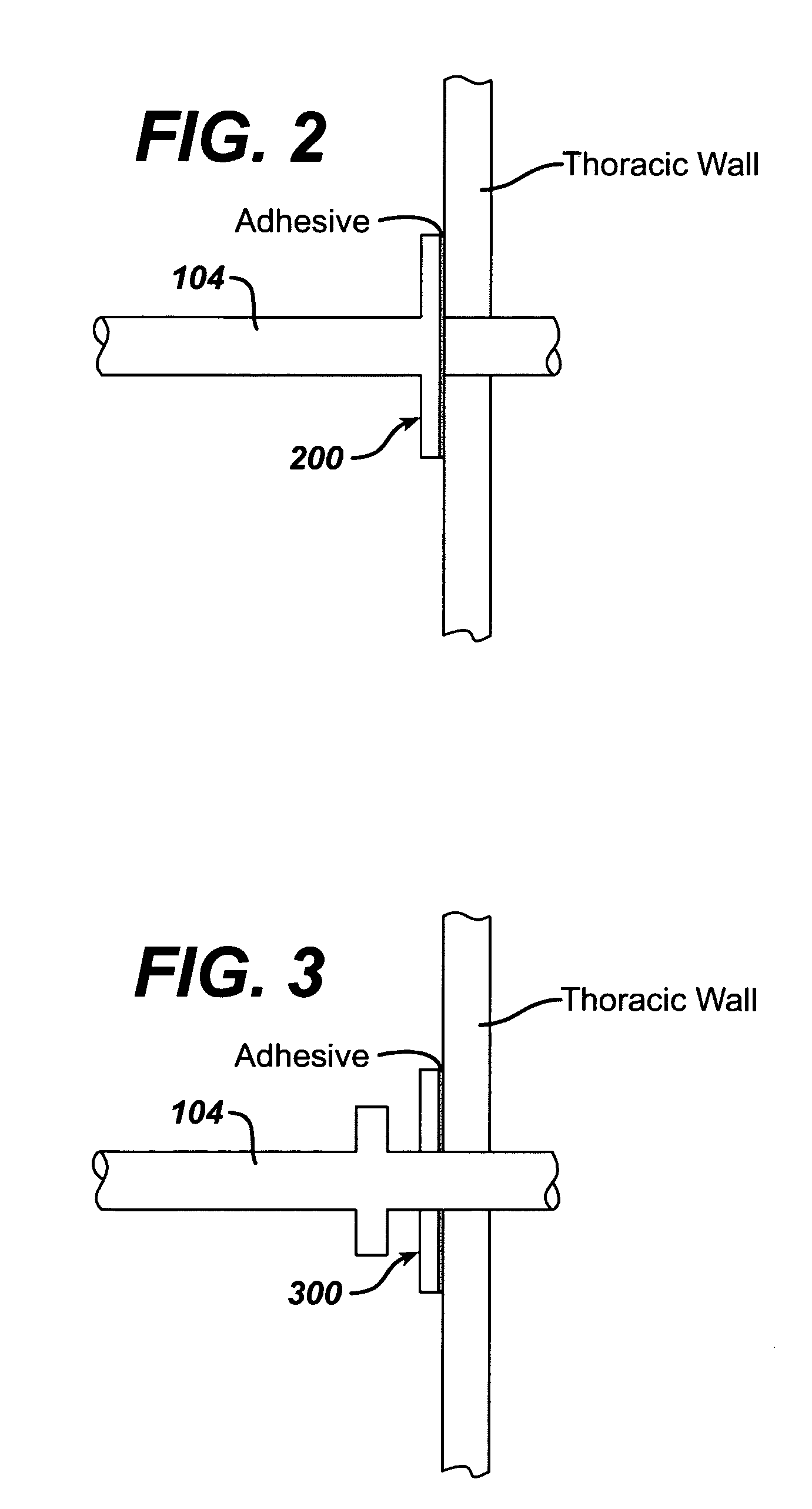
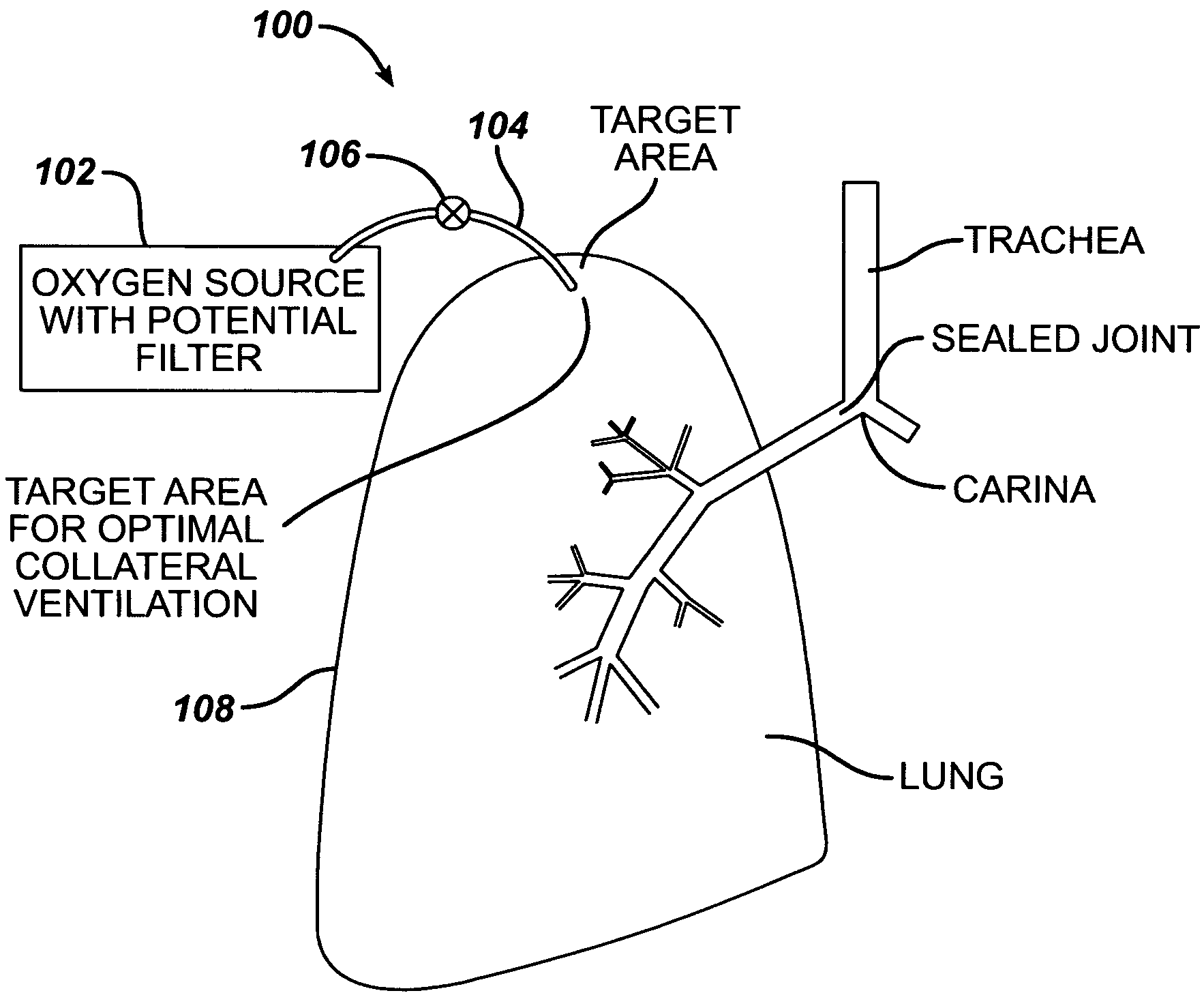
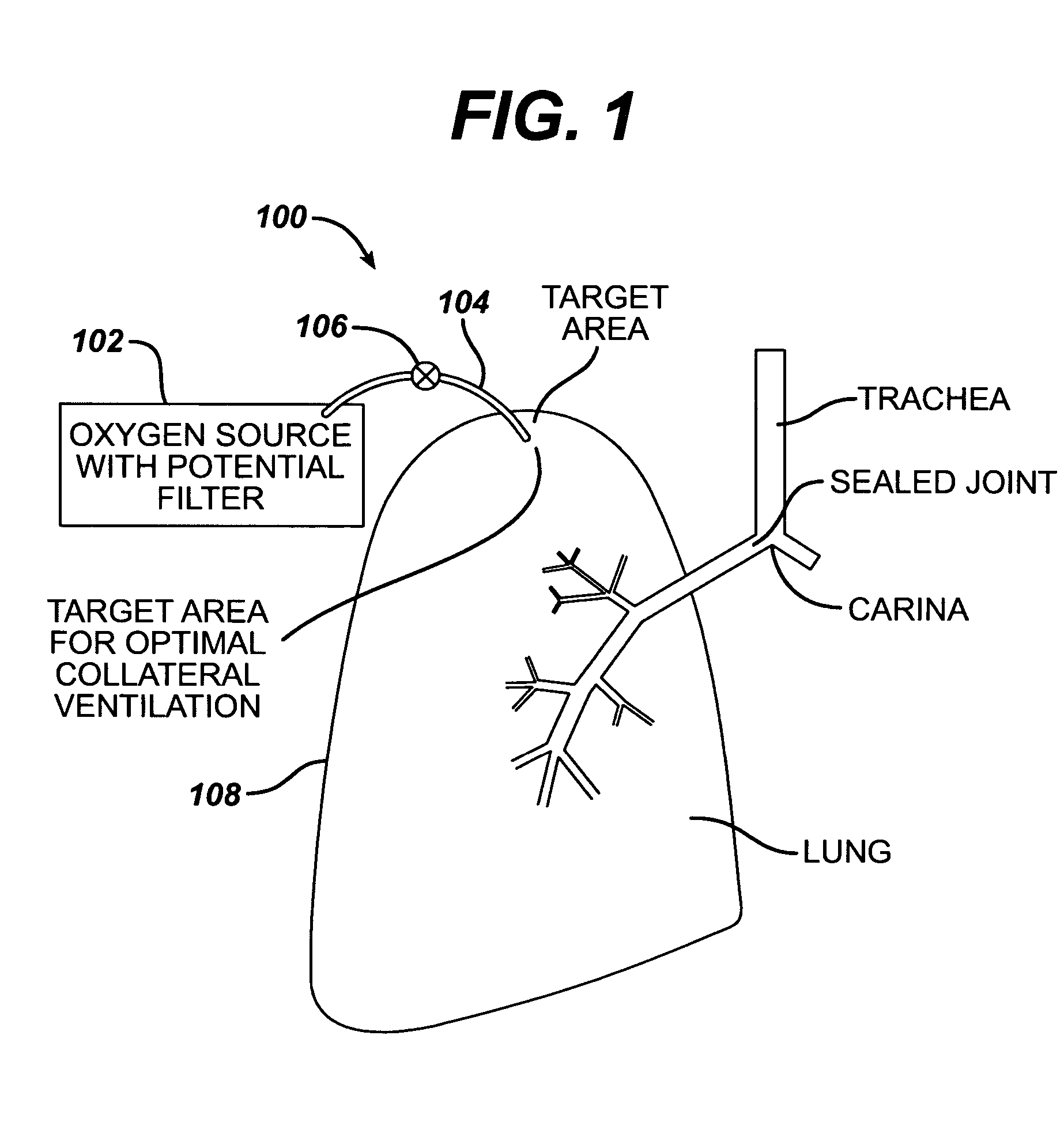
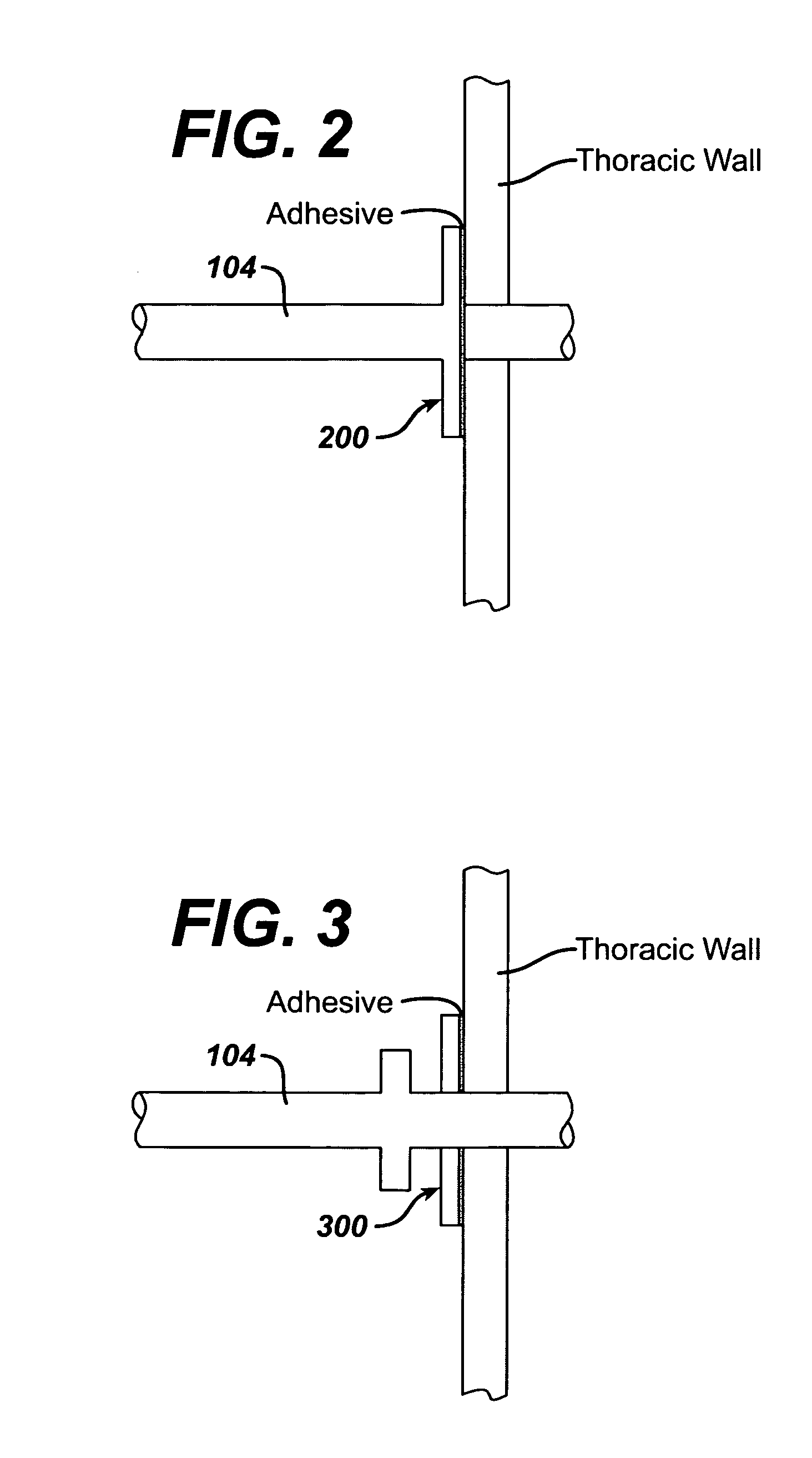
Collateral ventilation bypass trap system
InactiveUS6886558B2Increase expiratory flowSpeed up the flowTracheal tubesBreathing masksCollateral ventilationObstructive Pulmonary Diseases
A long term oxygen therapy system having an oxygen supply directly linked with a patient's lung or lungs may be utilized to more efficiently treat hypoxia caused by chronic obstructive pulmonary disease such as emphysema and chronic bronchitis. The system includes an oxygen source, one or more valves and fluid carrying conduits. The fluid carrying conduits link the oxygen source to diseased sites within the patient's lungs. A collateral ventilation bypass trap system directly linked with a patient's lung or lungs may be utilized to increase the expiratory flow from the diseased lung or lungs, thereby treating another aspect of chronic obstructive pulmonary disease. The system includes a trap, a filter / one-way valve and an air carrying conduit.
Owner:PORTAERO
Treatment of Asthma and Chronic Obstructive Pulmonary Disease With Anti-proliferate and Anti-inflammatory Drugs
InactiveUS20080175887A1Promote absorptionOrganic active ingredientsPowdered material dispensingDiseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a method for treatment of respiratory disorders such as asthma, chronic obstructive pulmonary disease, and chronic sinusitis, including cystic fibrosis, interstitial fibrosis, chronic bronchitis, emphysema, bronchopulmonary dysplasia and neoplasia. The method involves administration, preferably oral, nasal or pulmonary administration, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues).
Owner:LUTONIX INC
Methods and devices to accelerate wound healing in thoracic anastomosis applications
InactiveUS20050025816A1Increase expiratory flowOvercome disadvantagesRespiratorsElectrotherapyStapling procedureObstructive Pulmonary Diseases
A long term oxygen therapy system having an oxygen supply directly linked with a patient's lung or lungs may be utilized to more efficiently treat hypoxia caused by chronic obstructive pulmonary disease such as emphysema and chronic bronchitis. The system includes an oxygen source, one or more valves and fluid carrying conduits. The fluid carrying conduits link the oxygen source to diseased sites within the patient's lungs. A collateral ventilation bypass trap system directly linked with a patient's lung or lungs may be utilized to increase the expiratory flow from the diseased lung or lungs, thereby treating another aspect of chronic obstructive pulmonary disease. The system includes a trap, a filter / one-way valve and an air carrying conduit. In various embodiments, the system may be intrathoracic, extrathoracic or a combination thereof. A pulmonary decompression device may also be utilized to remove trapped air in the lung or lungs, thereby reducing the volume of diseased lung tissue. A lung reduction device may passively decompress the lung or lungs. In order for the system to be effective, an airtight seal between the parietal and visceral pleurae is required. Chemical pleurodesis is utilized for creating the seal and various devices and / or drugs, agents and / or compounds may be utilized to accelerate wound healing in thoracic anastomosis applications.
Owner:PORTAERO
Collateral ventilation bypass trap system
InactiveUS7195017B2Speed up the flowReduce air exchangeRespiratorsBreathing masksCollateral ventilationObstructive Pulmonary Diseases
A long term oxygen therapy system having an oxygen supply directly linked with a patients lung or lungs may be utilized to more efficiently treat hypoxia caused by chronic obstructive pulmonary disease such as emphysema and chronic bronchitis. The system includes an oxygen source, one or more valves and fluid carrying conduits. The fluid carrying conduits link the oxygen source to diseased sites within the patients lungs. A collateral ventilation bypass trap system directly linked with a patient's lung or lungs may be utilized to increase the expiratory flow from the diseased lung or lungs, thereby treating another aspect of chronic obstructive pulmonary disease. The system includes a trap, a filter / one-way valve and an air carrying conduit.
Owner:PORTAERO
Irreversible electroporation (IRE) for congestive obstructive pulmonary disease (COPD)
A method for treating Chronic Obstructive Pulmonary Disease (COPD) or chronic bronchitis to alleviate the discomforts of breathing by using non-thermal electroporation energy to ablate diseased portions of the lung including the bronchus, airways and alveoli which, in effect, opens the restrictive diseased portions thereby maximizing the overall surface area thereof causing improved airflow and uninhibited breathing.
Owner:ANGIODYNAMICS INC
Long term oxygen therapy system
InactiveUS7086398B2Promote absorptionReduce air exchangeTracheal tubesMedical devicesObstructive Pulmonary DiseasesObstructive chronic bronchitis
A long term oxygen therapy system having an oxygen supply directly linked with a patient's lung or lungs may be utilized to more efficiently treat hypoxia caused by chronic obstructive pulmonary disease such as emphysema and chronic bronchitis. The system includes an oxygen source, one or more valves and fluid carrying conduits. The fluid carrying conduits link the oxygen source to diseased sites within the patient's lungs.
Owner:PORTAERO
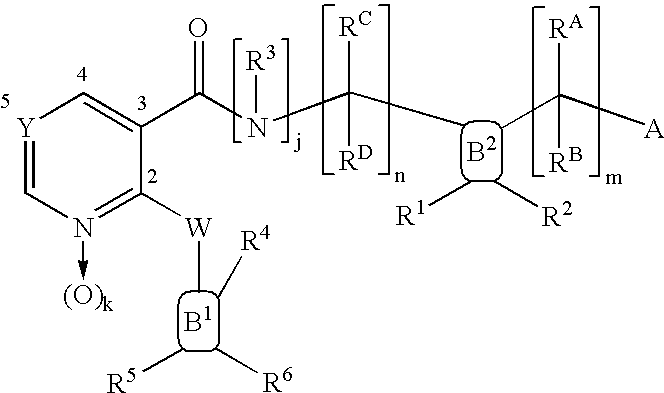
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Certain dinucleotides and their use as modulators of mucociliary clearance and ciliary beat frequency
The present invention relates to certain novel dinucleotides and formulations thereof which are highly selective agonists of the P2Y2 and / or P2Y4 purinergic receptor. They are useful in the treatment of chronic obstructive pulmonary diseases such as chronic bronchitis, PCD, cystic fibrosis, as well as prevention of pneumonia due to immobility. Furthermore, because of their general ability to clear retained mucus secretions and stimulate ciliary beat frequency, the compounds of the present invention are also useful in the treatment of sinusitis, otitis media and nasolacrimal duct obstruction. They are also useful for treatment of dry eye disease and retinal detachment as well as facilitating sputum induction and expectoration.
Owner:MERCK SHARP & DOHME CORP
Mucin synthesis inhibitors
The claimed invention relates to methods of modulating mucin synthesis and the therapeutic application of compounds of Formula II in controlling mucin over-production associated with diseases such as chronic obstructive pulmonary diseases (COPD) including asthma and chronic bronchitis, inflammatory lung diseases, cystic fibrosis and acute or chronic respiratory infectious diseases.whereinX is S, N, O or CR;Y is CRR′, O, NR6, CRR′—CRR′ or CR═CR;Z is NR6, O, S, CRR′ or CRR′—CRR′;R1-R3 are independently selected from the group consisting of H, C1-C8 alkyl, C1-C8 alkoxy, amino, hydroxy, halosubstituted alky and halo;R4 isQ is CR, NR6 orR5 is H or benzyl;R6 is H, C1-C8 alkyl, C1-C8 alkoxy, OH or halo; andR and R′ are independently H, C1-C8 alkyl, C1-C8 alkoxy, OH or halo,or a pharmaceutically acceptable salt thereof.
Owner:GENAERA CORP
Oligonucleotide compositions and methods for treating disease including inflammatory conditions
InactiveUS20050153919A1Reduced activityReduce expressionAntibacterial agentsSenses disorderPhosphodiesteraseAllograft rejection
The invention relates to therapeutic antisense oligonucleotides directed against genes coding for phosphodiesterase (PDEs) and the use of these in combination. These antisense oligonucleotides may be used as analytical tools and / or as therapeutic agents in the treatment of disease associated with reduced cellular cAMP in a patient, such as inflammatory diseases of the respiratory tract including, for example, asthma, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome, bronchitis, chronic bronchitis, silicosis, pulmonary fibrosis, lung allograft rejection, allergic rhinitis and chronic sinusitis as well as other conditions in which an increase in cyclic AMP or a decrease in PDE levels is beneficial.
Owner:TOPIGEN PHARMA
Aliphatic pyrazinoylguanidine sodium channel blockers with beta agonist activity
ActiveUS8324218B2More potent and/or absorbed less rapidlyLess reversibleOrganic active ingredientsSenses disorderDiseaseSinusitis
The present application provides sodium channel blockers exemplified by the following structure:The compounds of the invention useful for treating chronic bronchitis, cystic fibrosis, sinusitis, vaginal dryness, dry eye, Sjogren's disease, distal intestinal obstruction syndrome, dry skin, esophagitis, dry mouth (xerostomia), nasal dehydration, ventilator-induced pneumonia, asthma, primary ciliary dyskinesia, otitis media, chronic obstructive pulmonary disease, emphysema, pneumonia, constipation, and chronic diverticulitis, for example.
Owner:PARION SCI DURHAM NC
Treatment of asthma and chronic obstructive pulmonary disease with Anti-proliferate and Anti-inflammatory drugs
ActiveUS20130261603A1Promote absorptionRespiratorsPowder deliveryDiseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a method for treatment of respiratory disorders such as asthma, chronic obstructive pulmonary disease, and chronic sinusitis, including cystic fibrosis, interstitial fibrosis, chronic bronchitis, emphysema, bronchopulmonary dysplasia and neoplasia. The method involves administration, preferably oral, nasal or pulmonary administration, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues) and an additive.
Owner:LUTONIX INC
Dynamic variable release
InactiveUS20050152967A1Improve efficiencyReduce in quantityBiocideOrganic active ingredientsDiseaseCommon cold
The present invention relates to novel mixed release pharmaceutical formulations that include a expectorant available for immediate release and a decongestant for extended release that provide for the symptomatic relief of cough associated with respiratory tract conditions such as the common cold, bronchial asthma, acute and chronic bronchitis.
Owner:NEOS THERAPEUTICS LP
Collateral ventilation bypass trap system
InactiveUS20050161040A1Speed up the flowReduce air exchangeRespiratorsBreathing masksCollateral ventilationObstructive Pulmonary Diseases
A long term oxygen therapy system having an oxygen supply directly linked with a patients lung or lungs may be utilized to more efficiently treat hypoxia caused by chronic obstructive pulmonary disease such as emphysema and chronic bronchitis. The system includes an oxygen source, one or more valves and fluid carrying conduits. The fluid carrying conduits link the oxygen source to diseased sites within the patients lungs. A collateral ventilation bypass trap system directly linked with a patient's lung or lungs may be utilized to increase the expiratory flow from the diseased lung or lungs, thereby treating another aspect of chronic obstructive pulmonary disease. The system includes a trap, a filter / one-way valve and an air carrying conduit.
Owner:PORTAERO
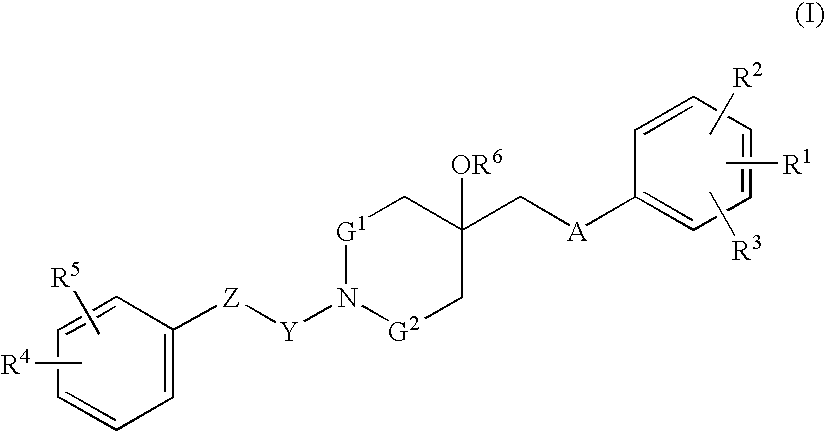
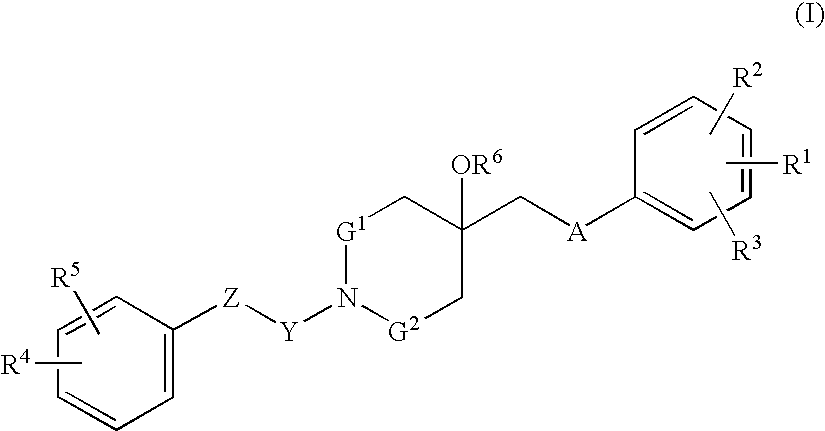
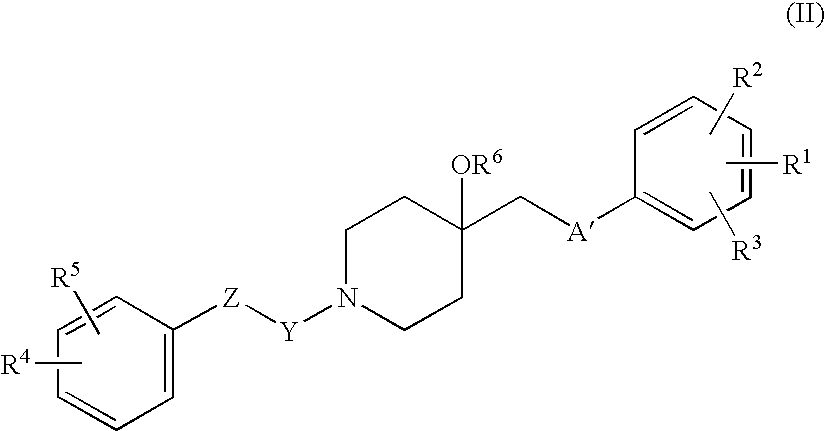
Agent for treating respiratory diseases containing 4-hydroxypiperidine derivative as active ingredient
InactiveUS7494987B2Potent antitussive actionImprove securityAntibacterial agentsBiocideDiseaseCarboxyl radical
An agent for preventing / treating respiratory diseases contains, as an active ingredient, a compound represented by following Formula (I):wherein A is a group represented by L-W [wherein L is a bond or CH2; and W is O, SOn (wherein n is 0 to 2), or —NR7— (wherein R7 is hydrogen or lower alkyl)]; each of G1 and G2 is (CH2)r (wherein r is 0 to 2), provided that when n is 1, G1 and G2 may be bridged by lower alkylene; Y is a lower alkylene or (substituted) benzylidene; Z is a bond or O, provided that when Z is a bond, Y may form a 5- or 6-membered ring with carbon on the benzene ring; R1 is, for example, NO2, a lower alkoxycarbonyl, (substituted) carbamoyl, (protected) hydroxyl group, (protected) carboxyl, or (protected) N-hydroxycarbamoyl; each of R2 and R3 is hydrogen, halogen, (halogenated) lower alkyl, (halogenated) lower alkoxy or NO2; each of R4 and R5 is, for example, hydrogen, halogen, (halogenated) lower alkyl, (halogenated) lower alkoxy, CN, or lower alkylsulfonyl; and R6 is hydrogen or lower alkyl, a salt thereof or a solvate of them. It has excellent antitussive activity when used as an agent for preventing / treating respiratory diseases such as lung cancer, common cold syndrome, pulmonary tuberculosis, pneumonia, acute bronchitis or chronic bronchitis.
Owner:MOCHIDA PHARM CO LTD
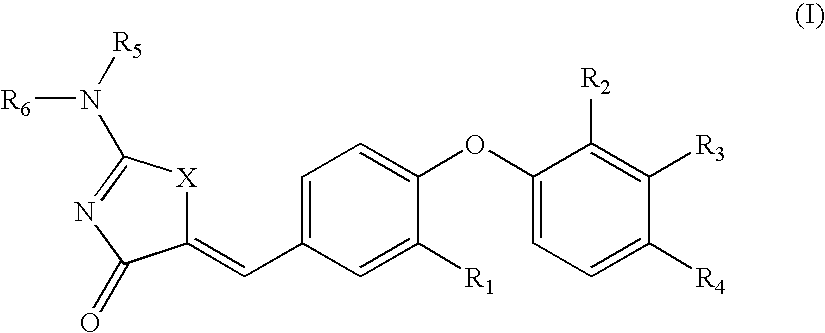
SUBSTITUTED PHENOXY AMINOTHIAZOLONES as estrogen related receptor-alpha modulators
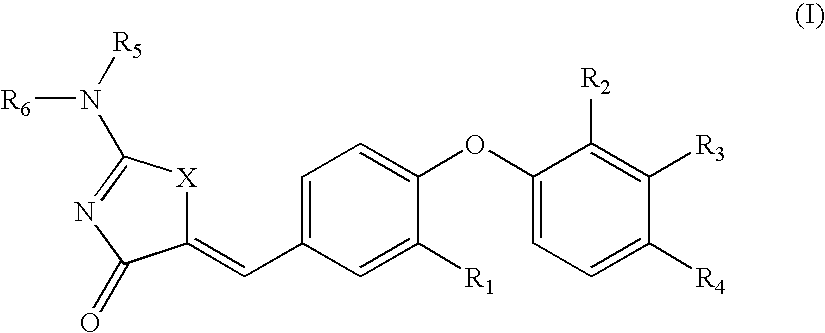
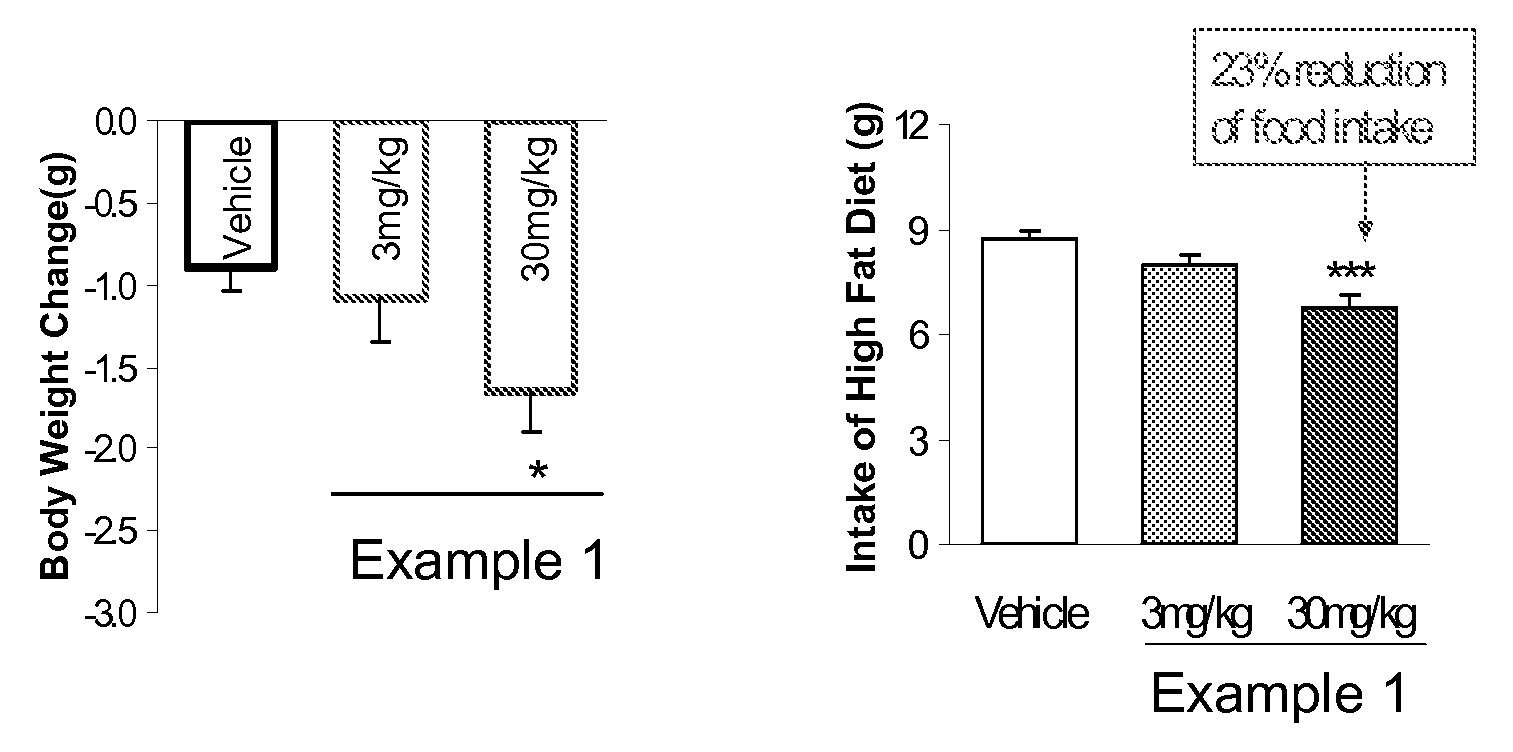
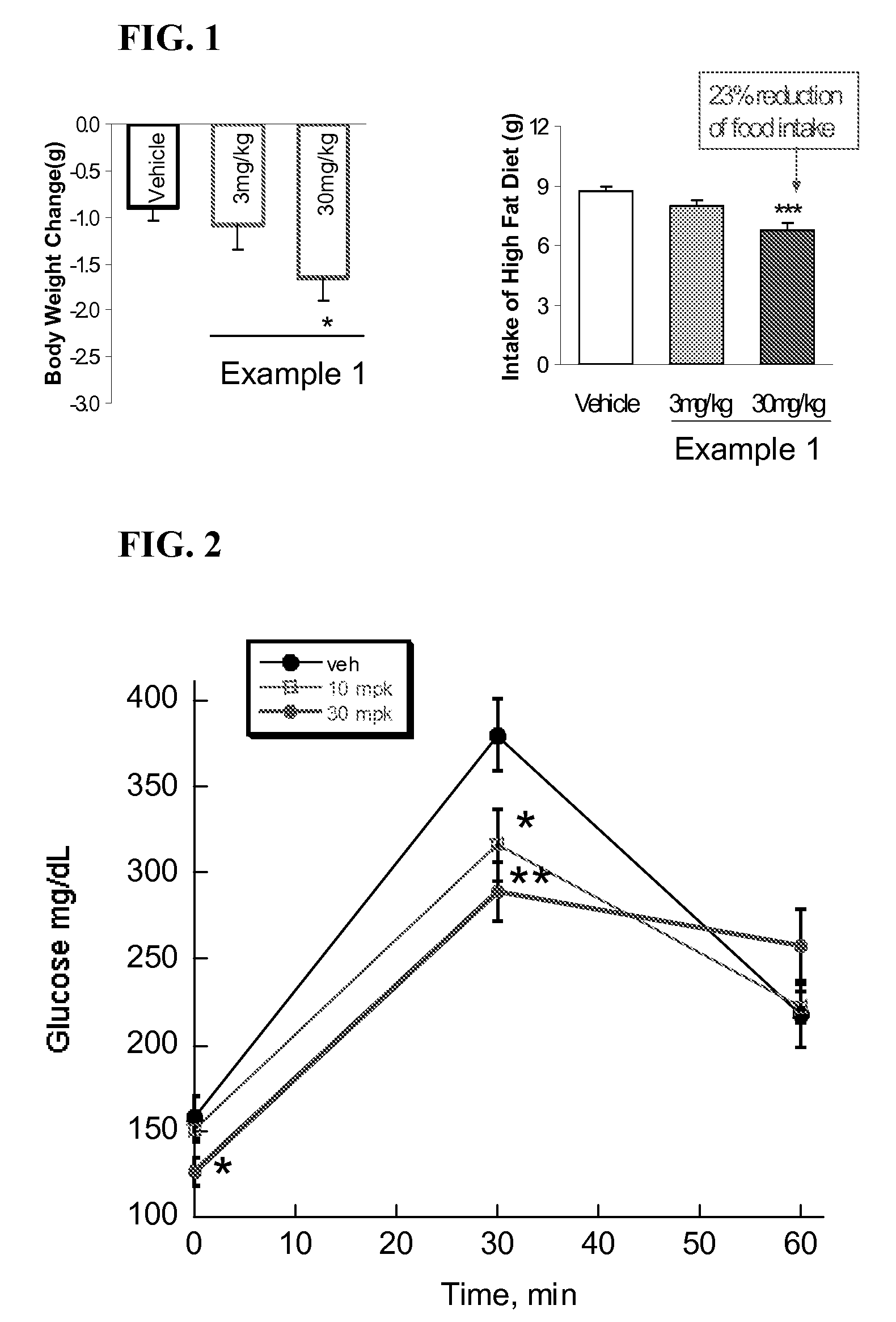
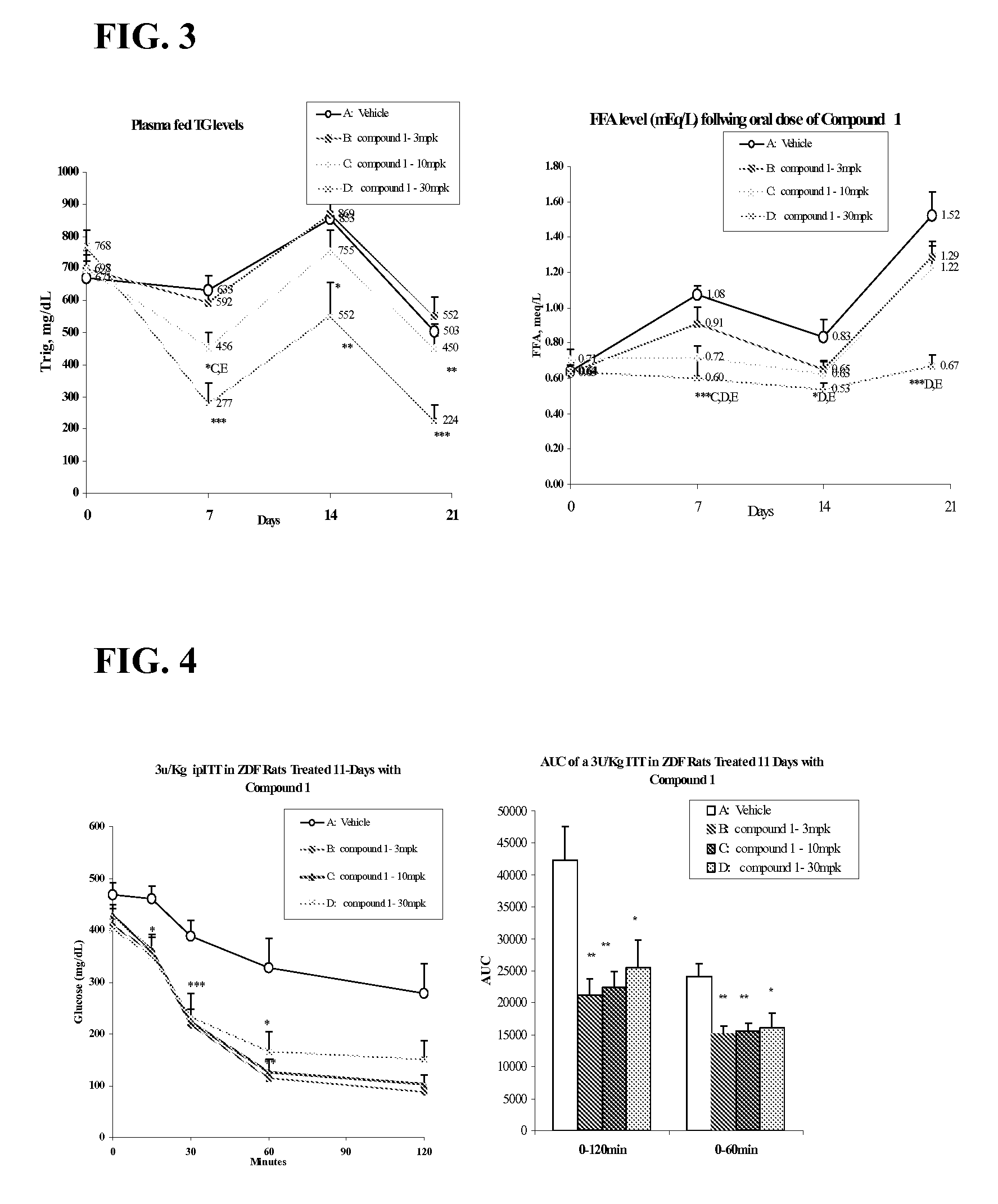
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
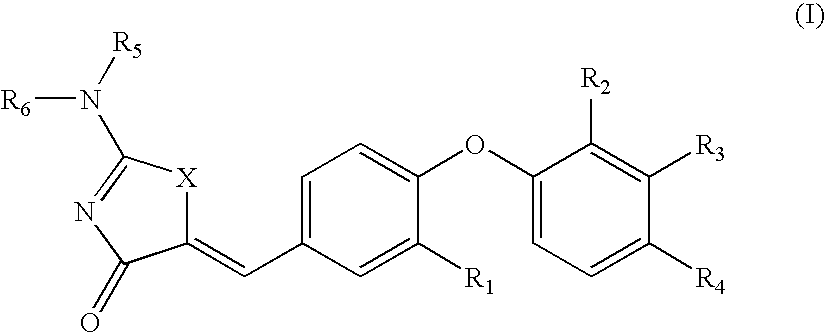
SUBSTITUTED PHENOXY THIAZOLIDINEDIONES AS ESTROGEN RELATED RECEPTOR-alpha MODULATORS
The present invention relates to compounds of Formula (I),methods for preparing these compounds, compositions, intermediates and derivatives thereof and for treating a condition including but not limited to ankylosing spondylitis, artherosclerosis, arthritis (such as rheumatoid arthritis, infectious arthritis, childhood arthritis, psoriatic arthritis, reactive arthritis), bone-related diseases (including those related to bone formation), breast cancer (including those unresponsive to anti-estrogen therapy), cardiovascular disorders, cartilage-related disease (such as cartilage injury / loss, cartilage degeneration, and those related to cartilage formation), chondrodysplasia, chondrosarcoma, chronic back injury, chronic bronchitis, chronic inflammatory airway disease, chronic obstructive pulmonary disease, diabetes, disorders of energy homeostasis, gout, pseudogout, lipid disorders, metabolic syndrome, multiple myeloma, obesity, osteoarthritis, osteogenesis imperfecta, osteolytic bone metastasis, osteomalacia, osteoporosis, Paget's disease, periodontal disease, polymyalgia rheumatica, Reiter's syndrome, repetitive stress injury, hyperglycemia, elevated blood glucose level, and insulin resistance.
Owner:JANSSEN PHARMA NV
3,5-diamino-6-chloro-N-(N-(4-(4-(2-(hexyl(2,3,4,5,6-pentahydroxyhexyl)amino)ethoxy)phenyl)butyl)carbamimidoyl)pyrazine-2-carboxamide
The present invention relates to the compound of the formula:or pharmaceutically acceptable salts thereof, as well as compositions containing the same, processes for the preparation of the same, and therapeutic methods of use therefore in promoting hydration of mucosal surfaces and the treatment of diseases including chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, acute and chronic bronchitis, cystic fibrosis, emphysema, and pneumonia.
Owner:PARION SCI DURHAM NC
Treatment of mucus hypersecretion
InactiveUS20080032928A1Effective treatmentPrevent hypersecretionCell receptors/surface-antigens/surface-determinantsSugar derivativesObstructive Pulmonary DiseasesObstructive chronic bronchitis
The present invention relates to treatment of mucus hypersecretion, to compositions therfor and manufacture of those compositions. The present invention relates particularly, though not exclusively, to the treatment of chronic bronchitis in chronic obstructive pulmonary disease (COPD), asthma and other clinical conditions involving COPD.
Owner:HEALTH PROTECTION AGENCY
Oral solid preparation containing ambroxol hydrochloride and salbutamol active components
InactiveCN101099729APlace stableEasy to carryPowder deliveryOrganic active ingredientsDiseaseRespiratory tract disease
The present invention discloses an oral solid preparation containing ambroxol hydrochloride and salbutamol active components. It is formed from ambroxol hydrochloride, salbutamol and auxiliary material according to a certain mixing ratio. Said oral solid preparation has the obvious synergistic action for remitting the symptoms of dyspnea, etc. due to respiratory tract obstruction diseases of bronchial asthma, chronic bronchitis and pulmonary emphysema, etc.
Owner:GUANGZHOU LIXIN PHARM CO LTD
Method for treating pulmonary diseases using rho kinase inhibitor compounds
InactiveUS20100204210A1Not effectiveBiocidePharmaceutical delivery mechanismDiseaseOccupational pulmonary disease
This invention relates to methods of treating pulmonary diseases in patients that beta adrenergic receptor agonist therapy is not effective. The method comprises the steps of: identifying a patient who suffers from a pulmonary disease and has reduced responsiveness to treatment with one or more beta adrenergic receptor agonists, and administering to the patient an effective amount of a Rho kinase inhibitor compound, wherein said pulmonary disease is selected from the group consisting of: asthma, chronic obstructive pulmonary disease, respiratory tract illness caused by respiratory syncytial virus infection such as RSV-induced wheezing, airway hyperreactivity, or bronchiolitis, bronchiectasis, alpha-1-antitrypsin deficiency, lymphangioleiomyomatosis, cystic fibrosis, bronchiolitis or wheezing caused by agents other than respiratory syncytial virus, chronic bronchitis, and occupational lung diseases.
Owner:INSPIRE PHARMA
Medicine capable of relieving cough and moistening lung
InactiveCN101455806ANo side effectsShort course of treatmentRespiratory disorderPlant ingredientsSide effectChinese traditional
The invention provides a drug with effects of relieving cough and moistening lung. The drug is prepared from the following Chinese medicine materials: prepared rehmannia root, semen ginkgo, bulbus fritillariae cirrhosae, rhizoma polygonati, stigmata maydis, orange peel, folia eriobotryae, angelica decursiva, fructus aristolochiae, almond, semen lepidii, bamboo juice, honeysuckle, rice, medlar, stemona root, oroxylum indicum, liquorice, fructus schizandrae, rhizoma phragmitis, corn mint, figwort, lily, aster and rhizoma polygonati officinalis. The drug uses traditional Chinese herbal medicines as raw materials and is prepared through a scientific formulation and repeated validation of clinical test and meets the theory of traditional Chinese medicine; and the drug has the advantages of no toxicity, no side effect, short treatment course and high radical treatment efficiency. The drug can be used for treating common cold or cough, dry pharynx and other symptoms caused by acute and chronic bronchitis, and has the efficacies of replenishing qi, nourishing yin, moistening lung, relieving cough and the like; therefore, the drug has good social benefits when widely popularized.
Owner:朱新华
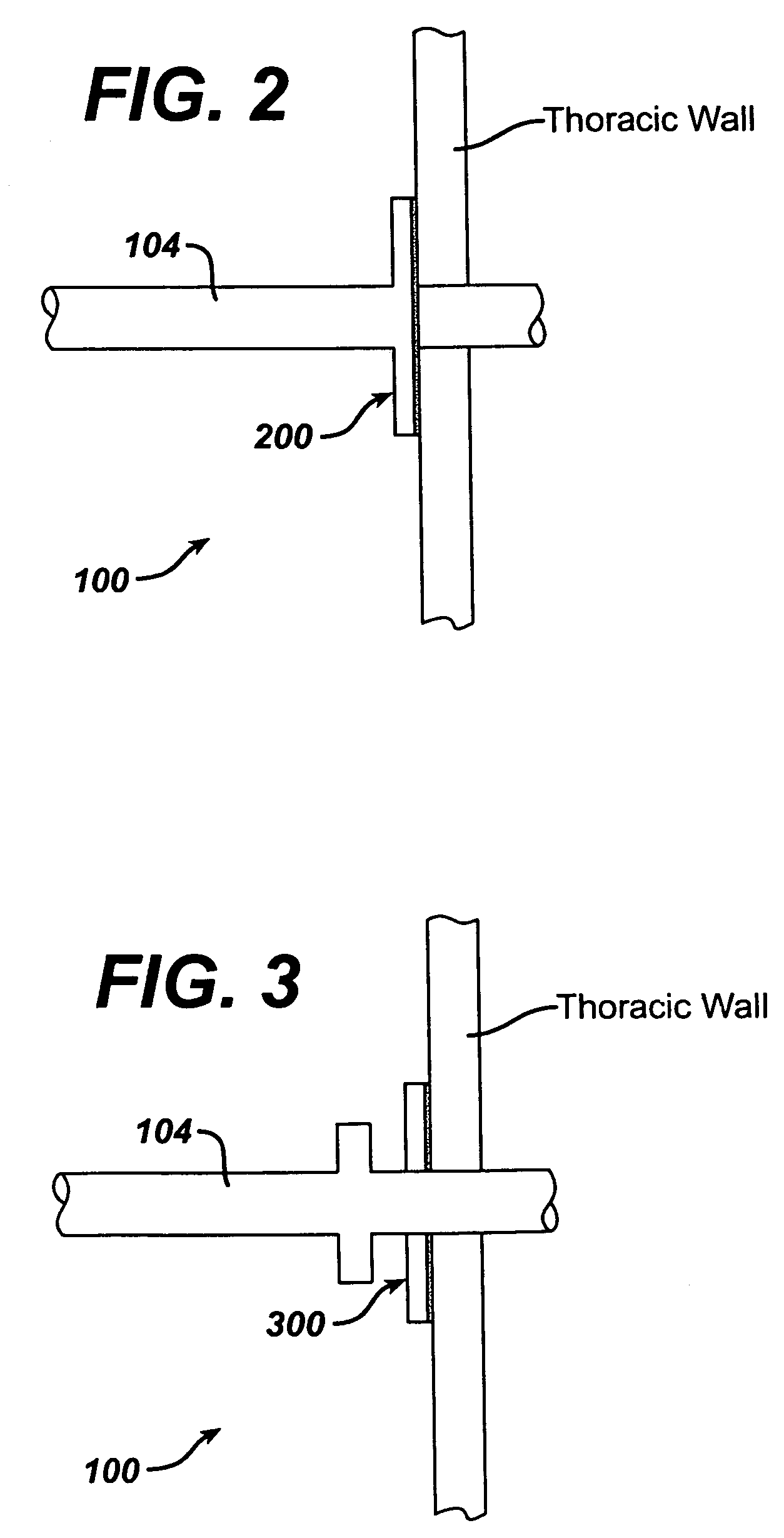
Intra/extra-thoracic collateral ventilation bypass system and method
InactiveUS7426929B2Speed up the flowPromote absorptionTracheal tubesDiagnosticsObstructive Pulmonary DiseasesObstructive chronic bronchitis
A long term oxygen therapy system having an oxygen supply directly linked with a patient's lung or lungs may be utilized to more efficiently treat hypoxia caused by chronic obstructive pulmonary disease such as emphysema and chronic bronchitis. The system includes an oxygen source, one or more valves and fluid carrying conduits. The fluid carrying conduits link the oxygen source to diseased sites within the patient's lungs. A collateral ventilation bypass trap system directly linked with a patient's lung or lungs may be utilized to increase the expiratory flow from the diseased lung or lungs, thereby treating another aspect of chronic obstructive pulmonary disease. The system includes a trap, a filter / one-way valve and an air carrying conduit. In various embodiments, the system may be intrathoracic, extrathoracic or a combination thereof. In order for the system to be effective, an airtight seal between the parietal and visceral pleurae is required. Chemical pleurodesis is utilized for creating the seal.
Owner:PORTAERO
Methods and devices to assist pulmonary decompression
InactiveUS7533667B2Increase expiratory flowSpeed up the flowRespiratorsBreathing masksObstructive Pulmonary DiseasesLung tissue
A long term oxygen therapy system having an oxygen supply directly linked with a patient's lung or lungs may be utilized to more efficiently treat hypoxia caused by chronic obstructive pulmonary disease such as emphysema and chronic bronchitis. The system includes an oxygen source, one or more valves and fluid carrying conduits. The fluid carrying conduits link the oxygen source to diseased sites within the patient's lungs. A collateral ventilation bypass trap system directly linked with a patient's lung or lungs may be utilized to increase the expiratory flow from the diseased lung or lungs, thereby treating another aspect of chronic obstructive pulmonary disease. The system includes a trap, a filter / one-way valve and an air carrying conduit. In various embodiments, the system may be intrathoracic, extrathoracic or a combination thereof. A pulmonary decompression device may also be utilized to remove trapped air in the lung or lungs, thereby reducing the volume of diseased lung tissue. In order for the system to be effective, an airtight seal between the parietal and visceral pleurae is required. Chemical pleurodesis is utilized for creating the seal.
Owner:PORTAERO
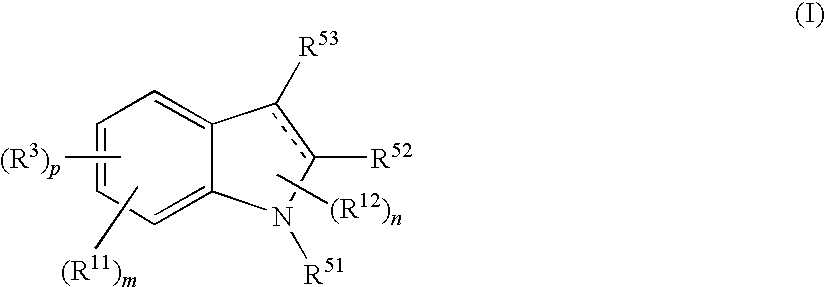
Indole compound and use thereof
InactiveUS7728023B2Increased airway hyperreactivityImprove respiratory functionBiocideSenses disorderClinical trialObstructive Pulmonary Diseases
The present invention relates to a compound represented by the formula (I),wherein all symbols are as defined in the description,a salt thereof, a solvate thereof, or a prodrug thereof, which has a leukotriene receptor antagonistic activity which is expected to be more effective than those of the leukotriene receptor antagonists currently used in clinical trials. Therefore, it is useful as an agent for the prevention and / or treatment of a leukotriene-mediated disease such as a respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, pulmonary emphysema, chronic bronchitis, pneumonia (e.g. interstitial pneumonia etc.), severe acute respiratory syndrome (SARS), acute respiratory distress syndrome (ARDS), allergic rhinitis, sinusitis (e.g. acute sinusitis, chronic sinusitis, etc.), or the like, or as an expectorant or an antiitussive.
Owner:ONO PHARMA CO LTD
Methods, apparatuses, and systems for the treatment of pulmonary disorders
ActiveUS20190231425A1Reduce in quantityElectrotherapySurgical instruments for heatingDiseaseAcute bronchitis
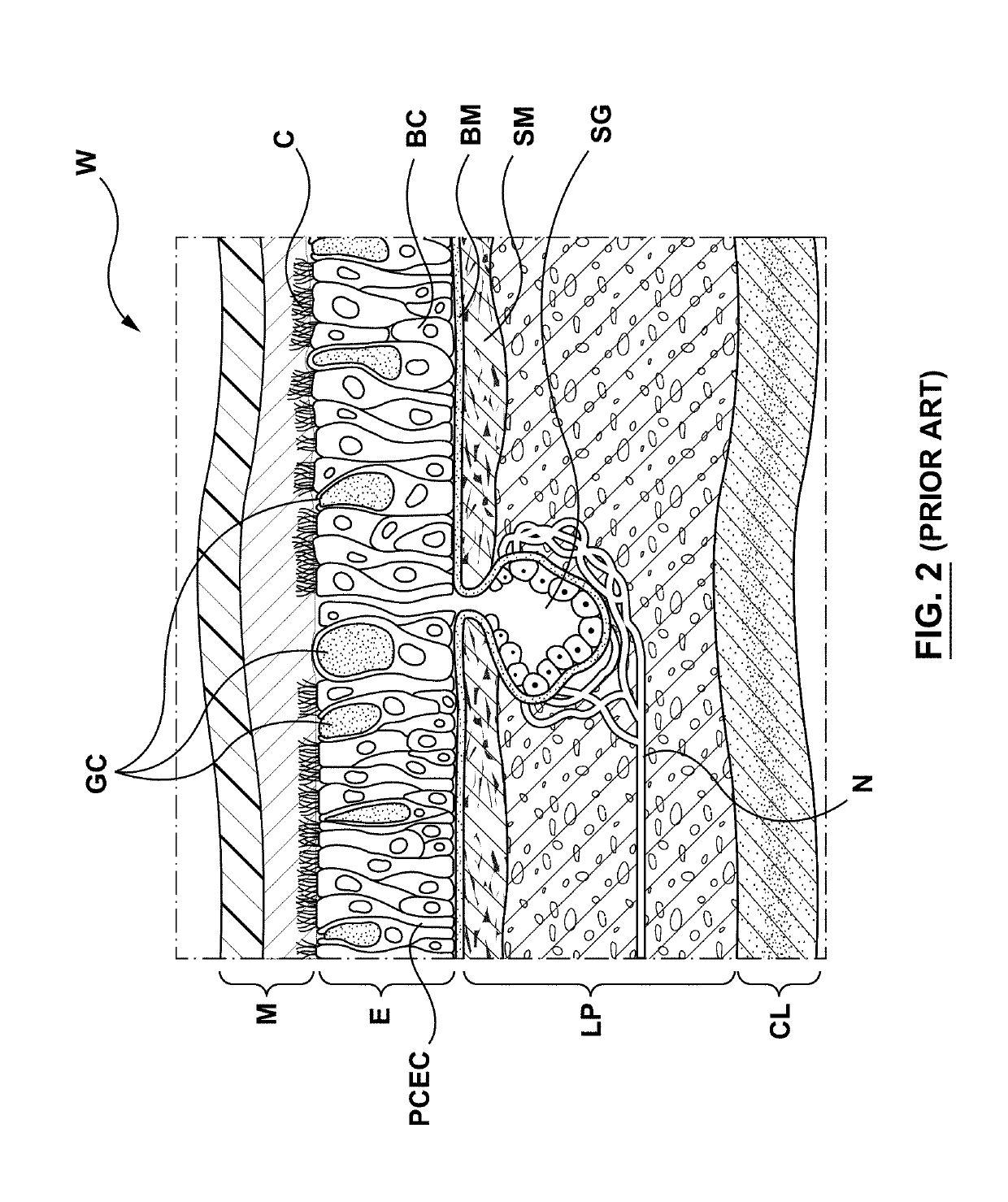
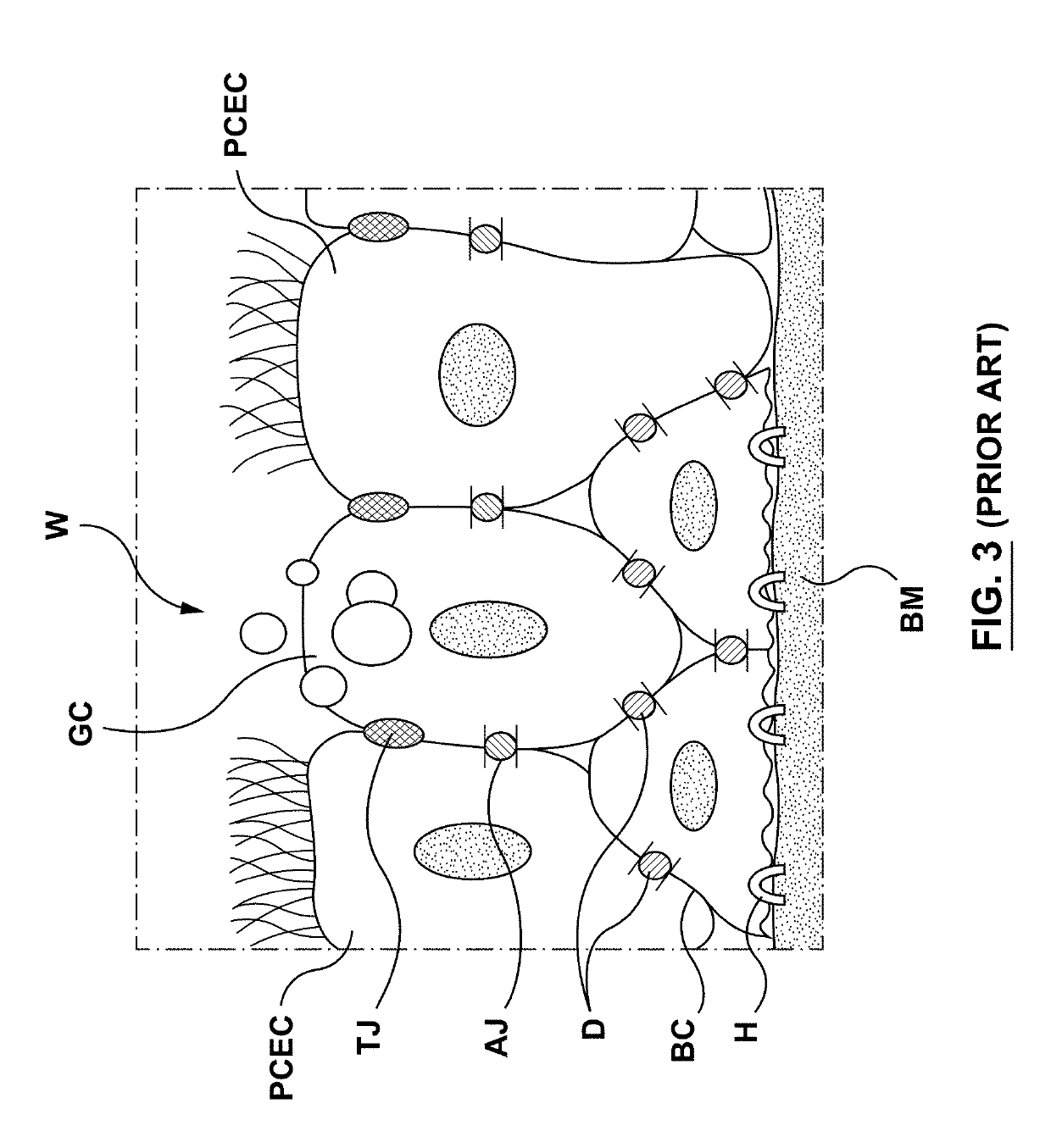
Apparatuses, systems and methods are provided for treating pulmonary tissues via delivery of energy, generally characterized by high voltage pulses, to target tissue using a pulmonary tissue modification system (e.g., an energy delivery catheter system). Example pulmonary tissues include, without limitation, the epithelium (the goblet cells, ciliated pseudostratified columnar epithelial cells, and basal cells), lamina propria, submucosa, submucosal glands, basement membrane, smooth muscle, cartilage, nerves, pathogens resident near or within the tissue, or a combination of any of these. The system may be used to treat a variety of pulmonary diseases or disorders such as or associated with COPD (e.g., chronic bronchitis, emphysema), asthma, interstitial pulmonary fibrosis, cystic fibrosis, bronchiectasis, primary ciliary dyskinesia (PCD), acute bronchitis and / or other pulmonary diseases or disorders.
Owner:GALVANIZE THERAPEUTICS INC
Method for treating lung diseases associated with ventilation-perfusion mismatches
InactiveUS20050118109A1Improving and recovering general state of healthAntibacterial agentsBiocideVasoactive intestinal peptideObstructive Pulmonary Diseases
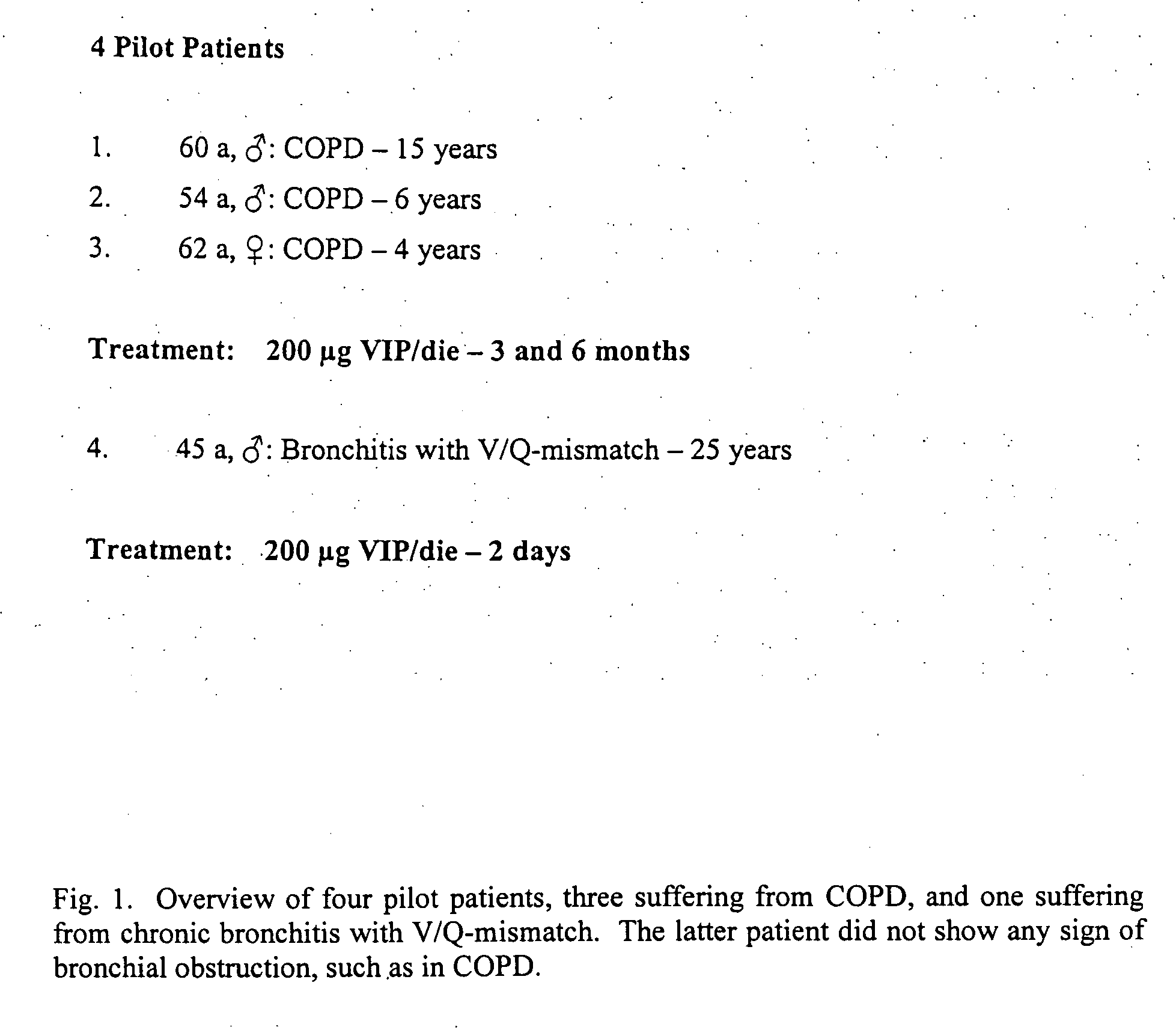
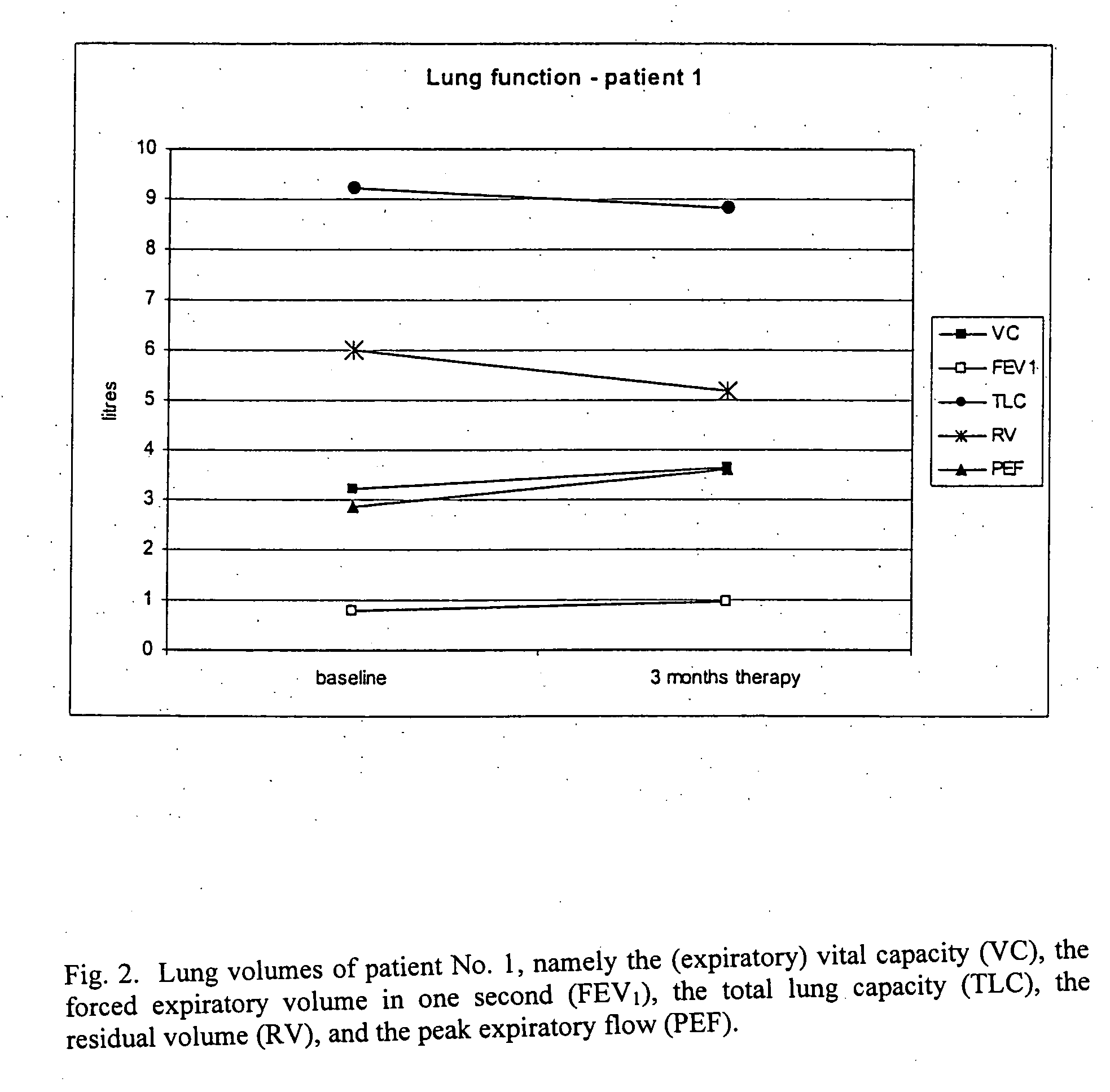
The present invention relates to pharmaceutical compositions and methods for the prevention and / or treatment of lung diseases or disorders including the bronchial tree, in an animal or human, such as chronic obstructive pulmonary disease (COPD), and diseases related to or optionally associated with COPD-like lung disorders caused by ventilation-perfusion mismatches preferably in context with chronic bronchitis. The treatment includes administration of pharmaceutical compositions comprising vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), and biologically active analogues thereof, which comprise highly conservative sequence tracks.
Owner:BLOCK LUTZ HENNING +2
Earthworm acidic-part medicine for treating cough asthma disease and preparing method
InactiveCN1850118AEasy to acceptIncreased content of anti-asthma active ingredientsRespiratory disorderLeech/worm material medical ingredientsDiseasePurine
The present invention relates to an earthworm acid region medicine for curing cough and asthma diseases and its preparation method. Said earthworm acid region medicine contains fatty acid, amino acid and purine compound, and can be used for curing the diseases of asthma and chronic bronchitis, etc. Its preparation method includes the following steps: using strong base anion-exchange resin to adsorb aqueous extract of earthworm, then using dilute hydrochloric acid or alcohol-containing dilute hydrochloric acid to make elution.
Owner:SHANGHAI JIAO TONG UNIV
Traditional Chinese medicinal composition for treating bronchitis and bronchial asthma
InactiveCN103920106AImprove antibacterial propertiesImprove immunityRespiratory disorderAluminium/calcium/magnesium active ingredientsFritillaria cirrhosaLicorice roots
The invention discloses a traditional Chinese medicinal composition for treating bronchitis and bronchial asthma. The composition is characterized in that the composition comprises, by weight, 12 parts of Cordyceps sinensis, 15 parts of Radix Codonopsis, 15 parts of Rhizoma Atractylodis Macrocephalae (stir-fried with bran), 15 parts of Poria cocos, 10 parts of Radix Ophiopogonis, 12 parts of Malaytea Scurfpea Fruit (stir-fried with salt), 10 parts of Chinese magnoliavine (processed with vinegar), 12 parts of Chinese angelica, 9 parts of Rhizoma Pinelliae Preparatum, 12 parts of snakegourd peel, 9 parts of bitter apricot kernel, 9 parts of Rhizoma Coptidis, 6 parts of jujube, 10 parts of perilla fruit, 10 parts of Aster tataricus, 10 parts of lily, 6 parts (calcined) gypsum, 9 parts of Cortex Magnoliae Officinalis, 10 parts of Radix Peucedani, 6 parts of dried orange peel, 9 parts of Chinese Eaglewood, 6 parts of ginger, 9 parts of loquat leaf, 10 parts of Fritillaria cirrhosa, 12 parts of prepared rehmannia root, 10 parts of Radix Scrophulariae, 6 parts of Arisaema Cum Bile, 10 parts of Radix Platycodonis, 10 parts of White Mulberry Root-bark (processed with honey), 9 parts of Rhizoma Anemarrhenae, 12 parts of Common Coltsfoot Flower, 15 parts of honeysuckle flower, 12 parts of Herba Schizonepetae, 12 parts of Weeping Forsythia and 6 parts of honey-fried licorice root.
Owner:赵庆军
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com