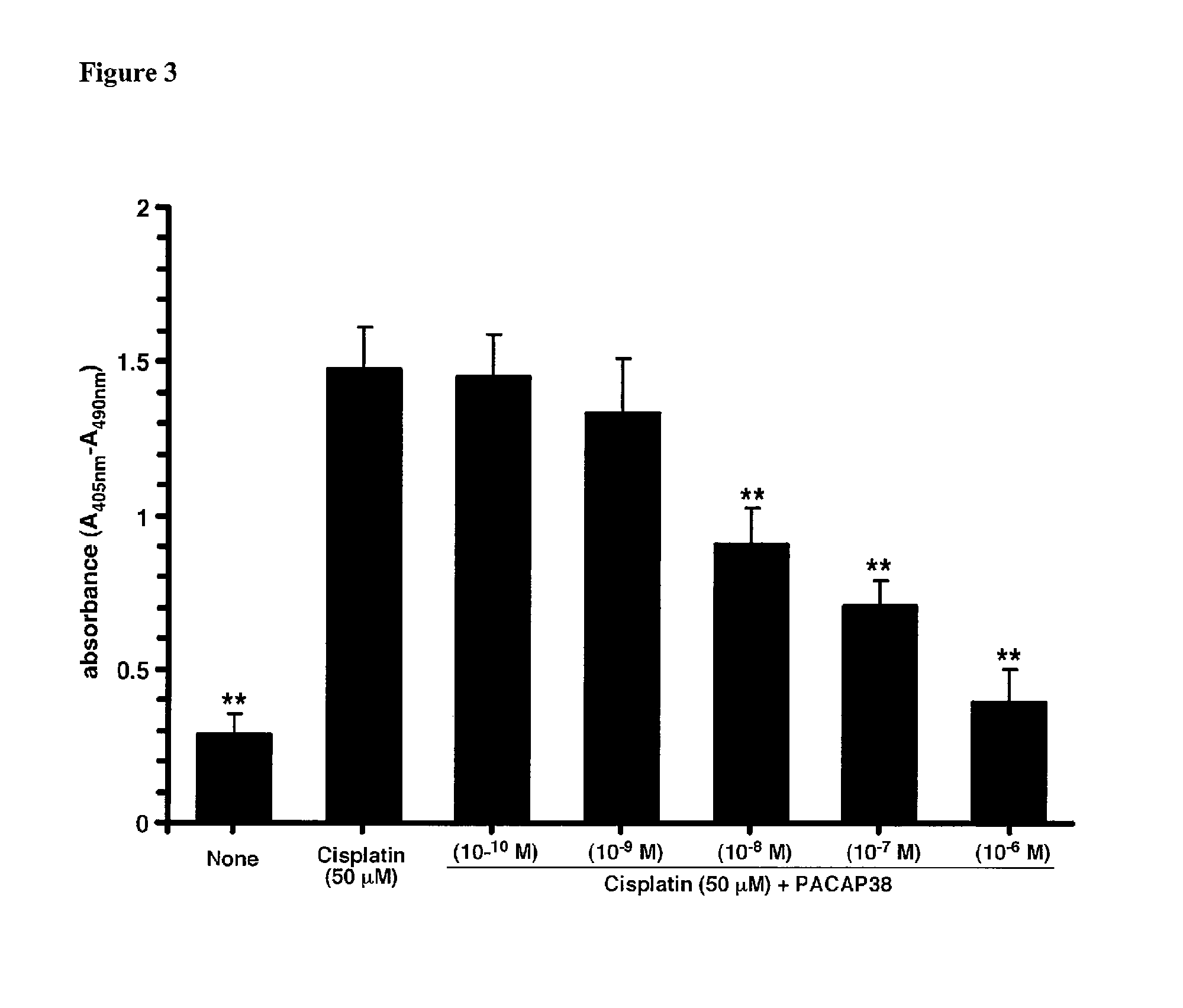
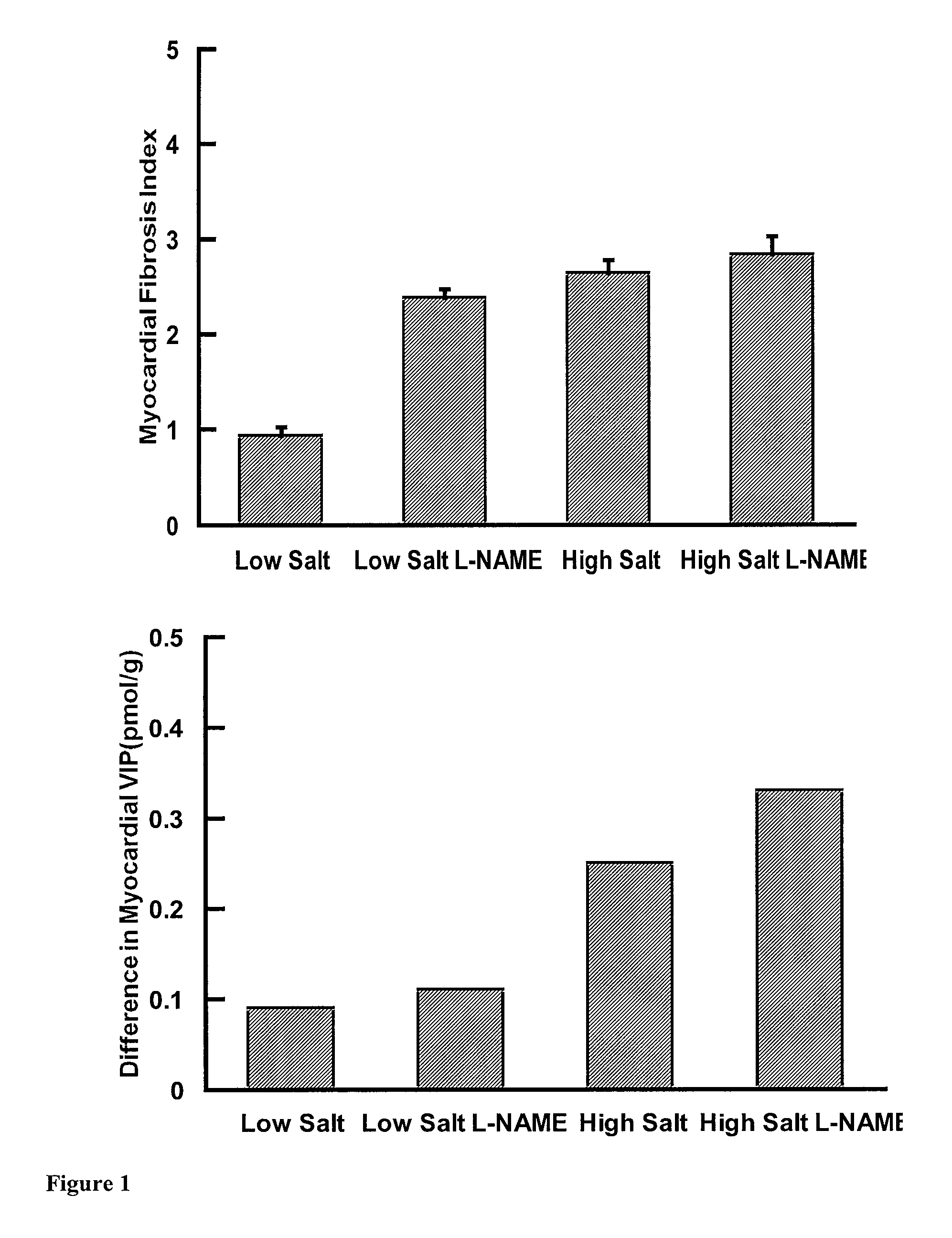
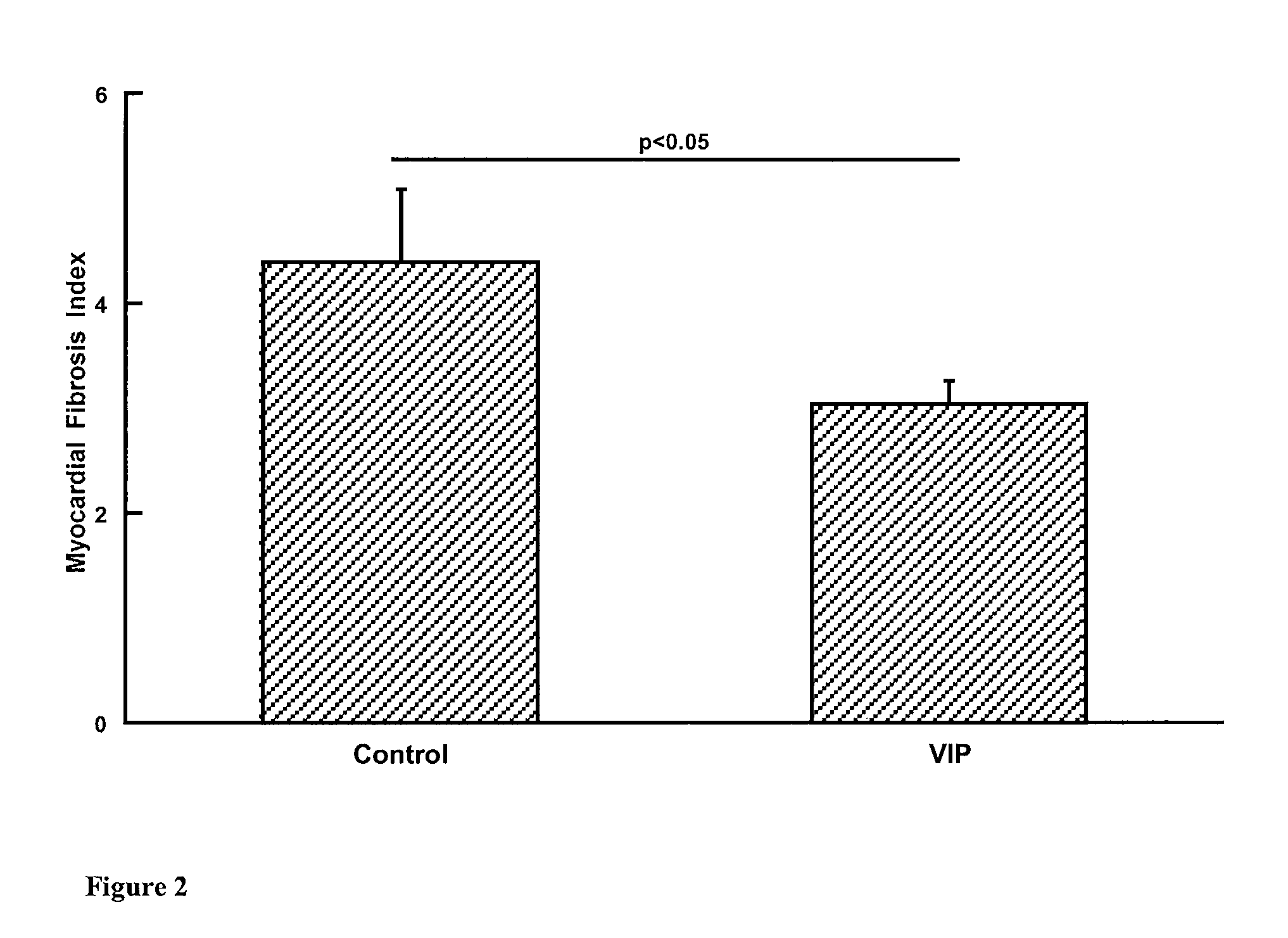
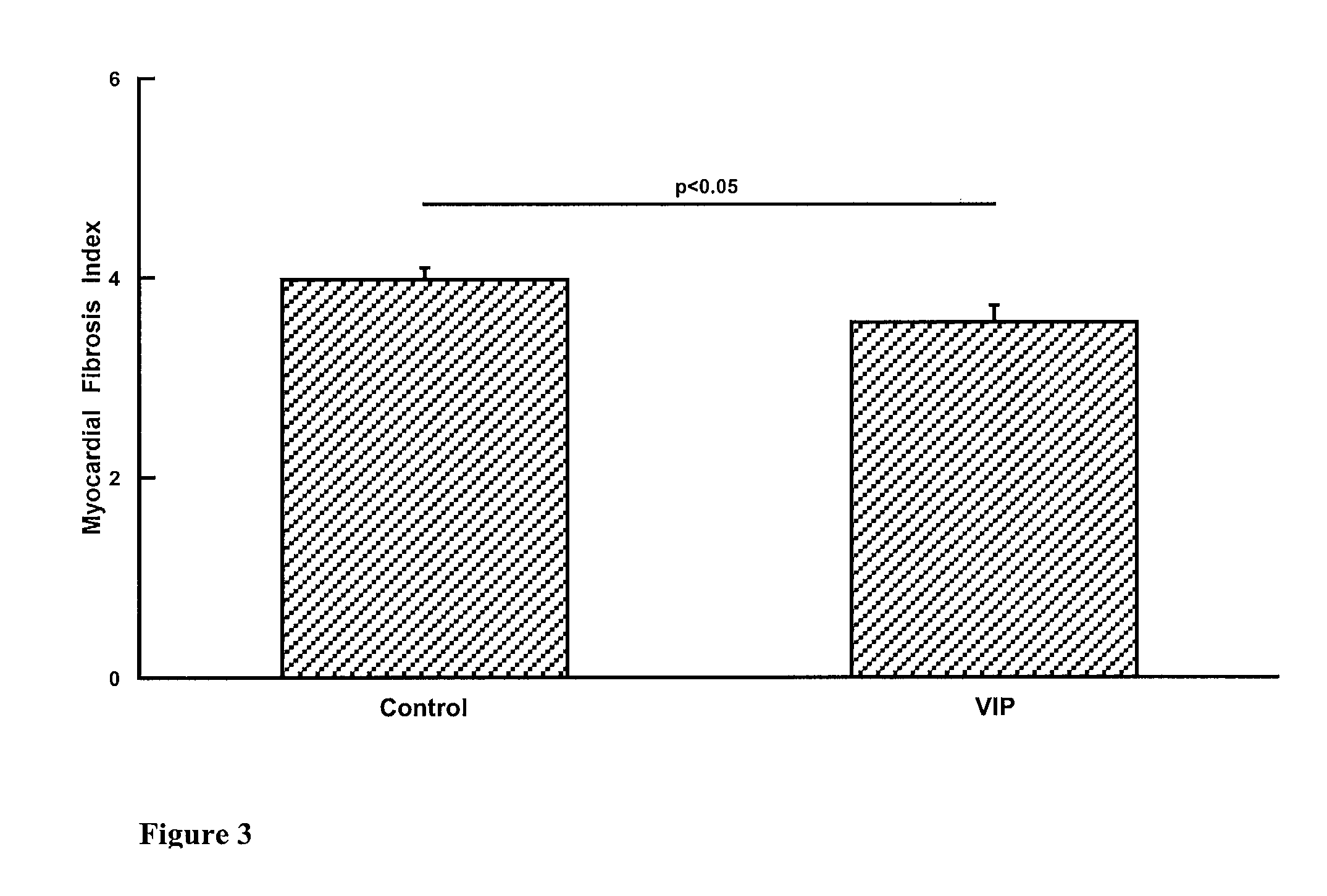
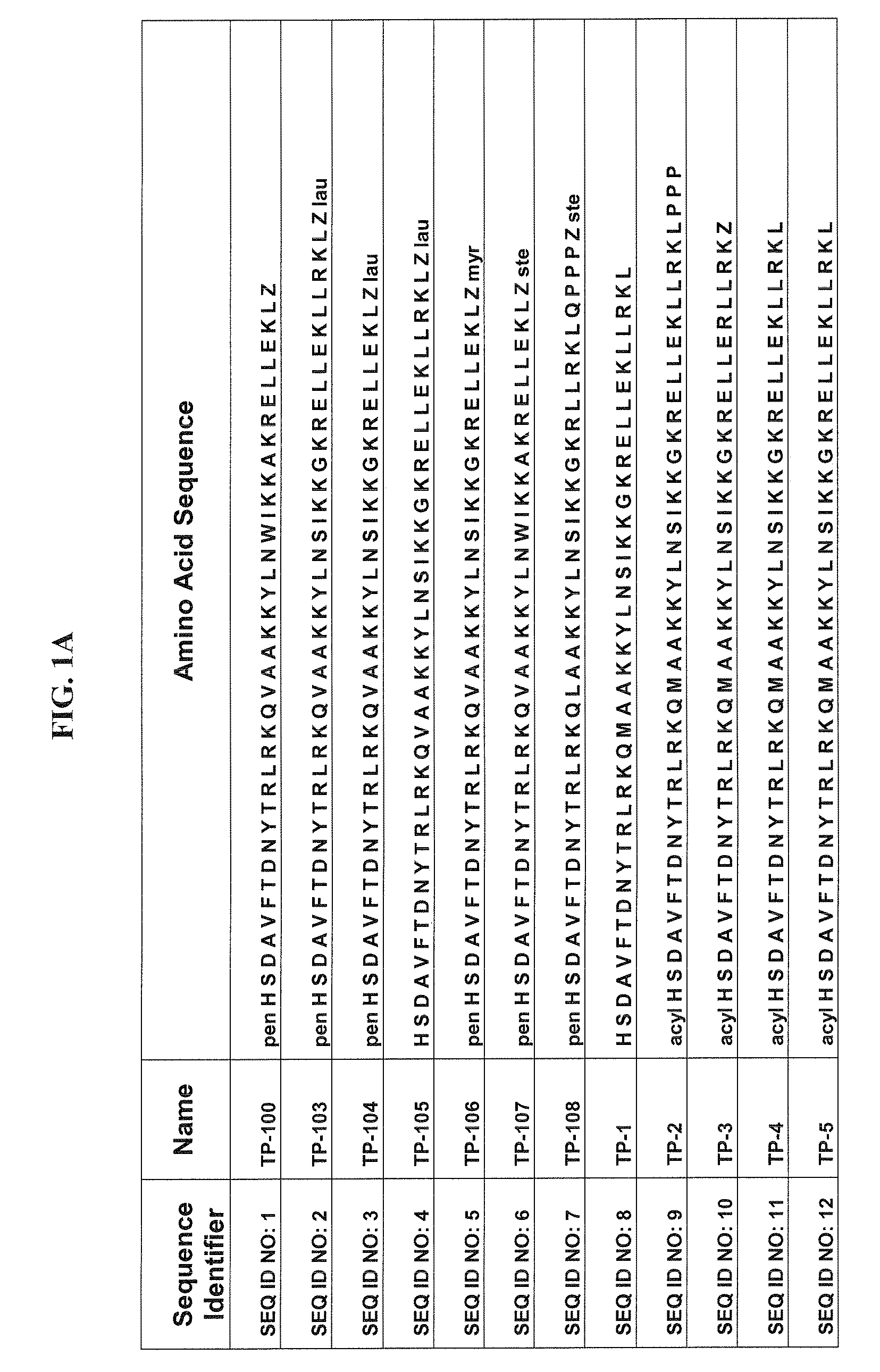
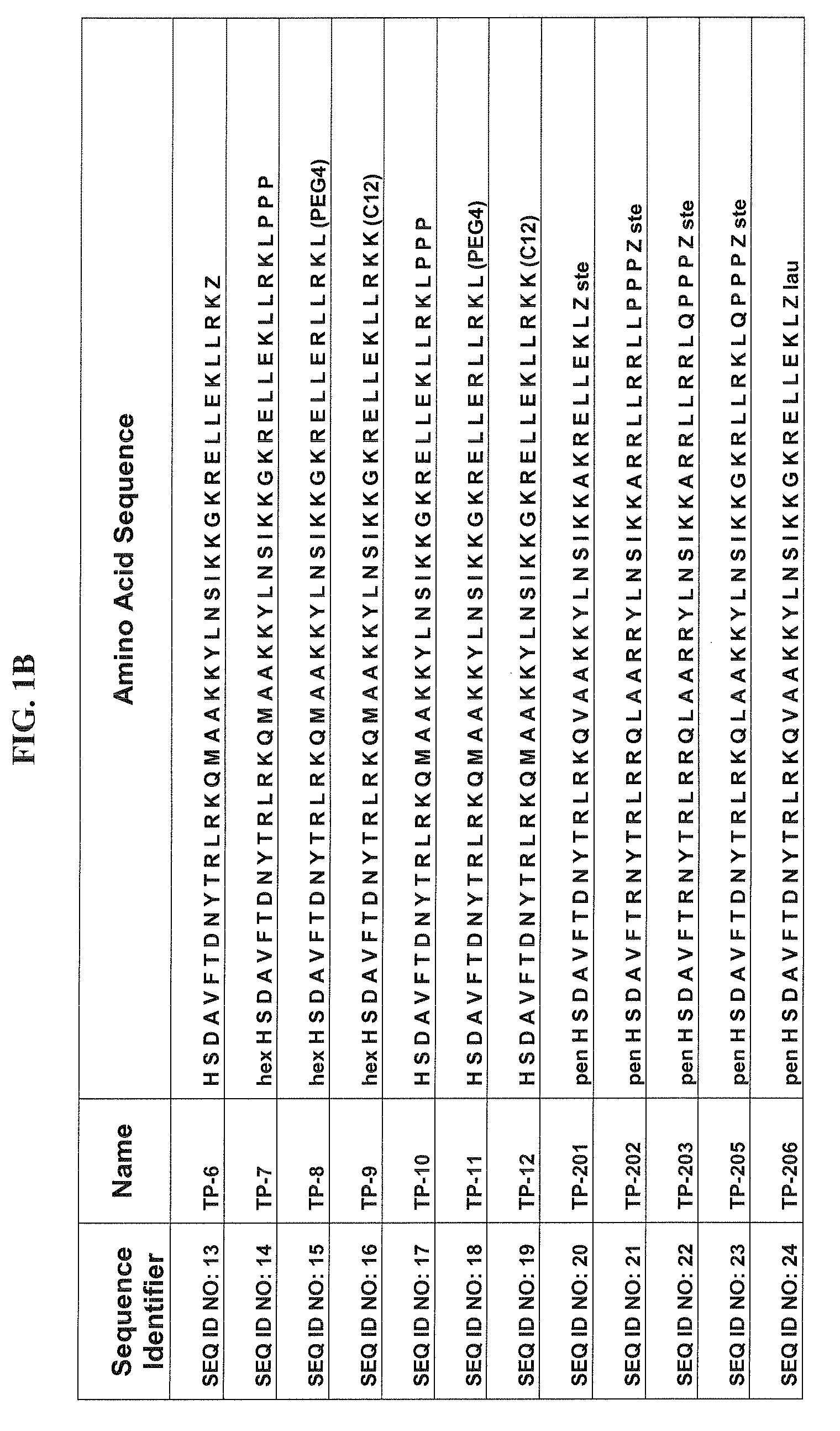
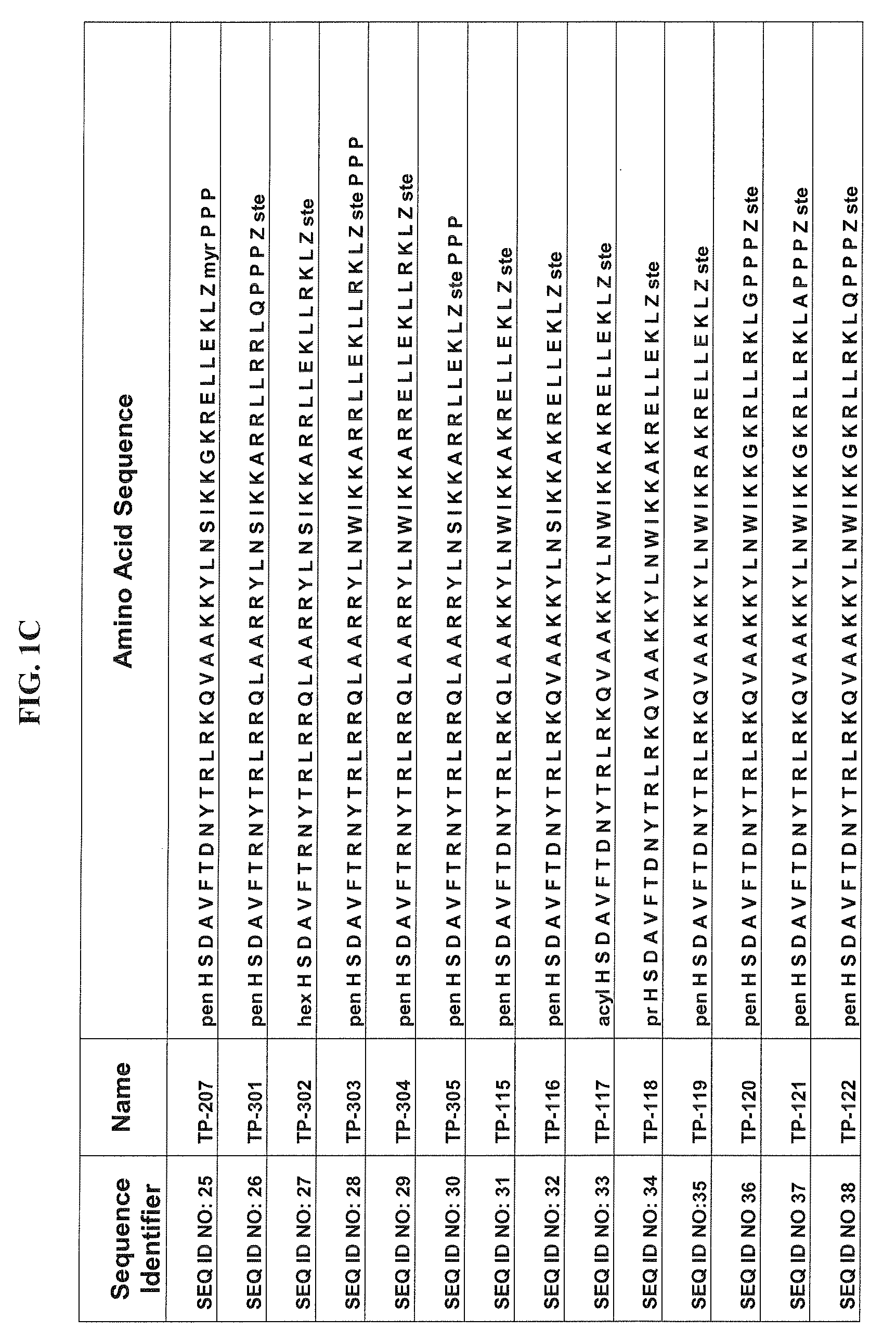
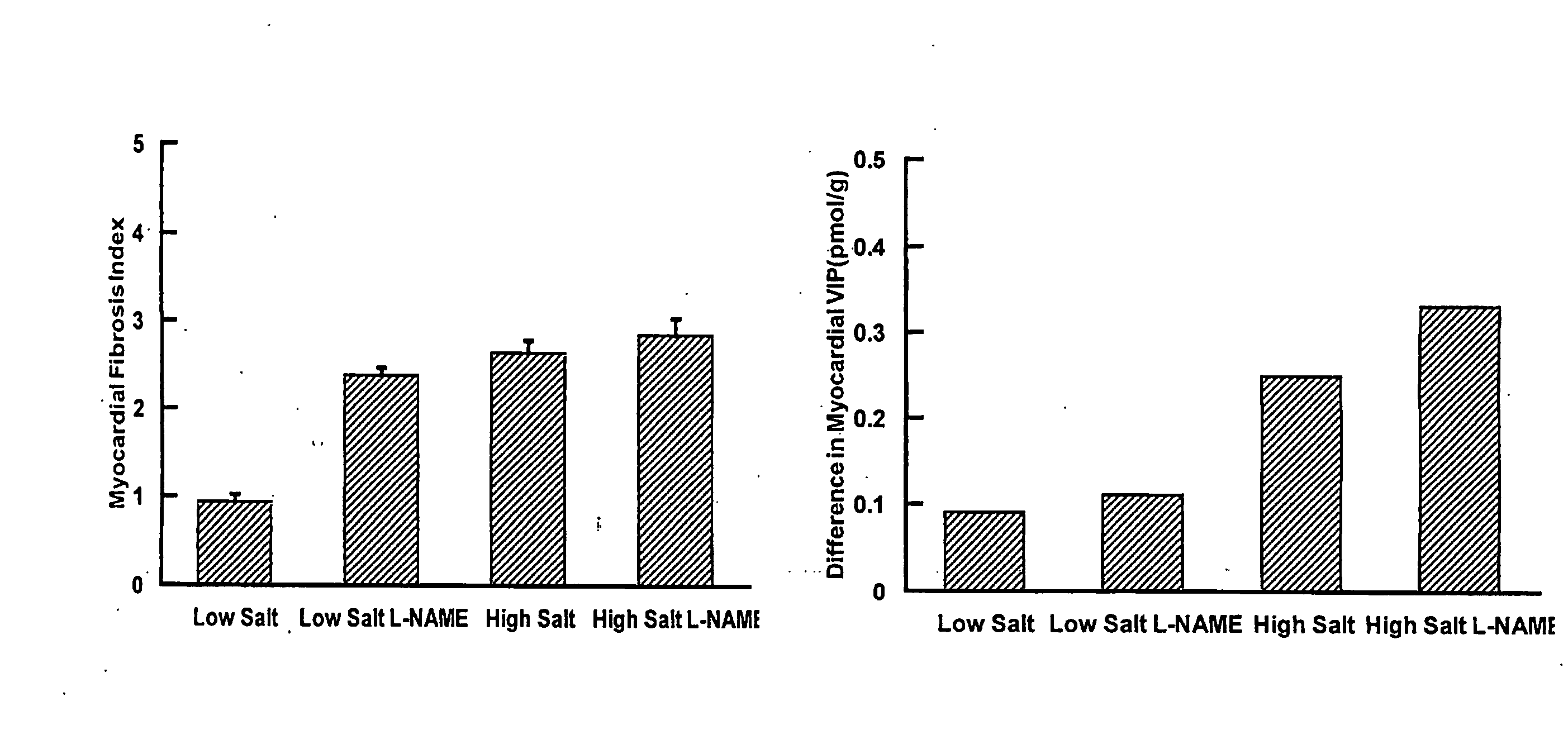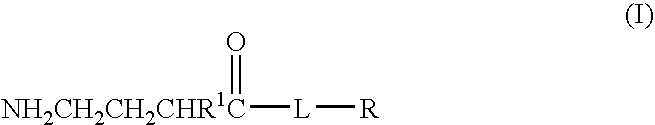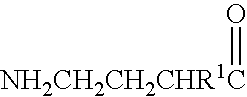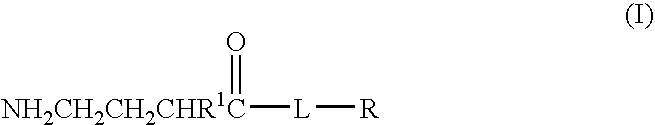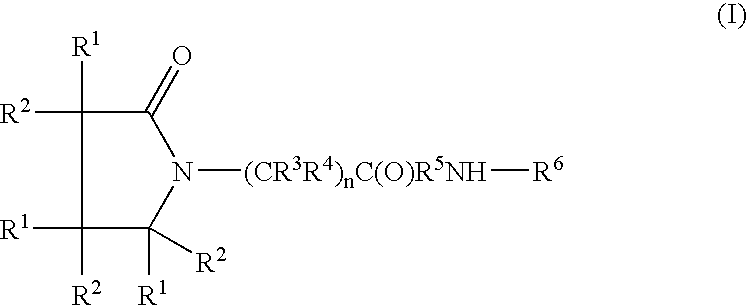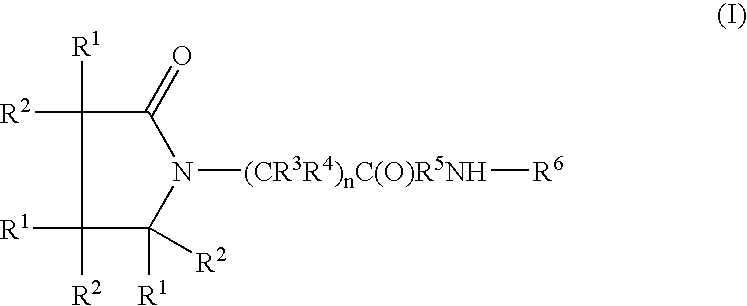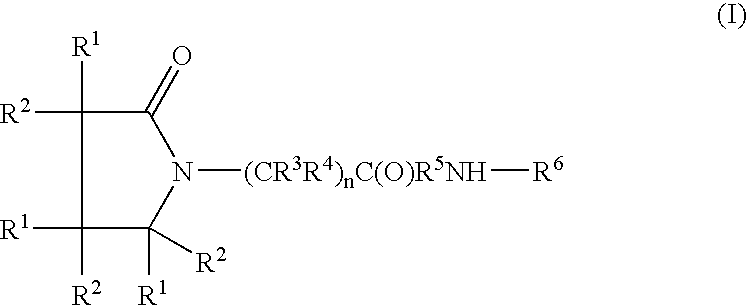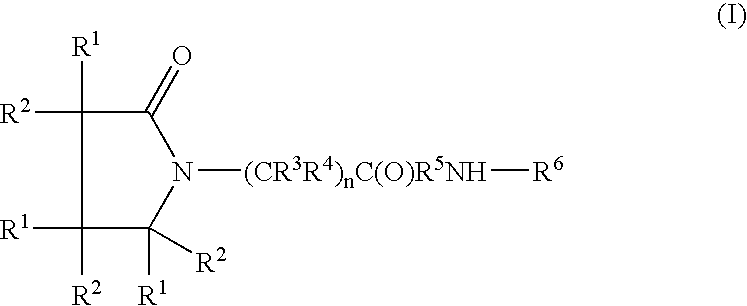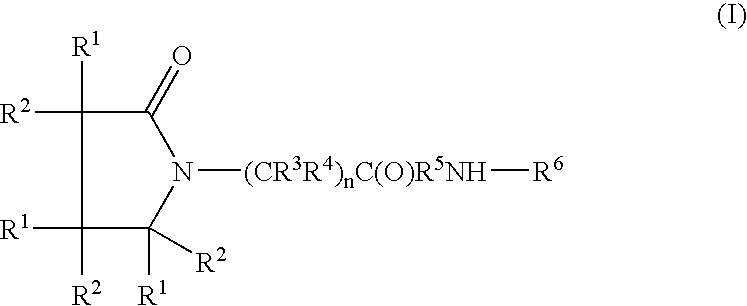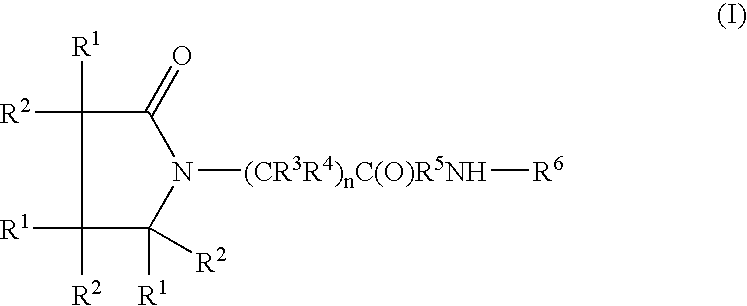Patents
Literature
85 results about "Vasoactive intestinal peptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vasoactive intestinal peptide, also known as vasoactive intestinal polypeptide or VIP, is a peptide hormone that is vasoactive in the intestine. VIP is a peptide of 28 amino acid residues that belongs to a glucagon/secretin superfamily, the ligand of class II G protein–coupled receptors. VIP is produced in many tissues of vertebrates including the gut, pancreas, and suprachiasmatic nuclei of the hypothalamus in the brain. VIP stimulates contractility in the heart, causes vasodilation, increases glycogenolysis, lowers arterial blood pressure and relaxes the smooth muscle of trachea, stomach and gall bladder. In humans, the vasoactive intestinal peptide is encoded by the VIP gene.
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
Treatment of female sexual dysfunction with vasoactive intestinal polypeptide agonists
InactiveUS7226910B2Good for healthImprove the lubrication effectBiocidePeptide/protein ingredientsObstetricsActive agent
Owner:VIVUS
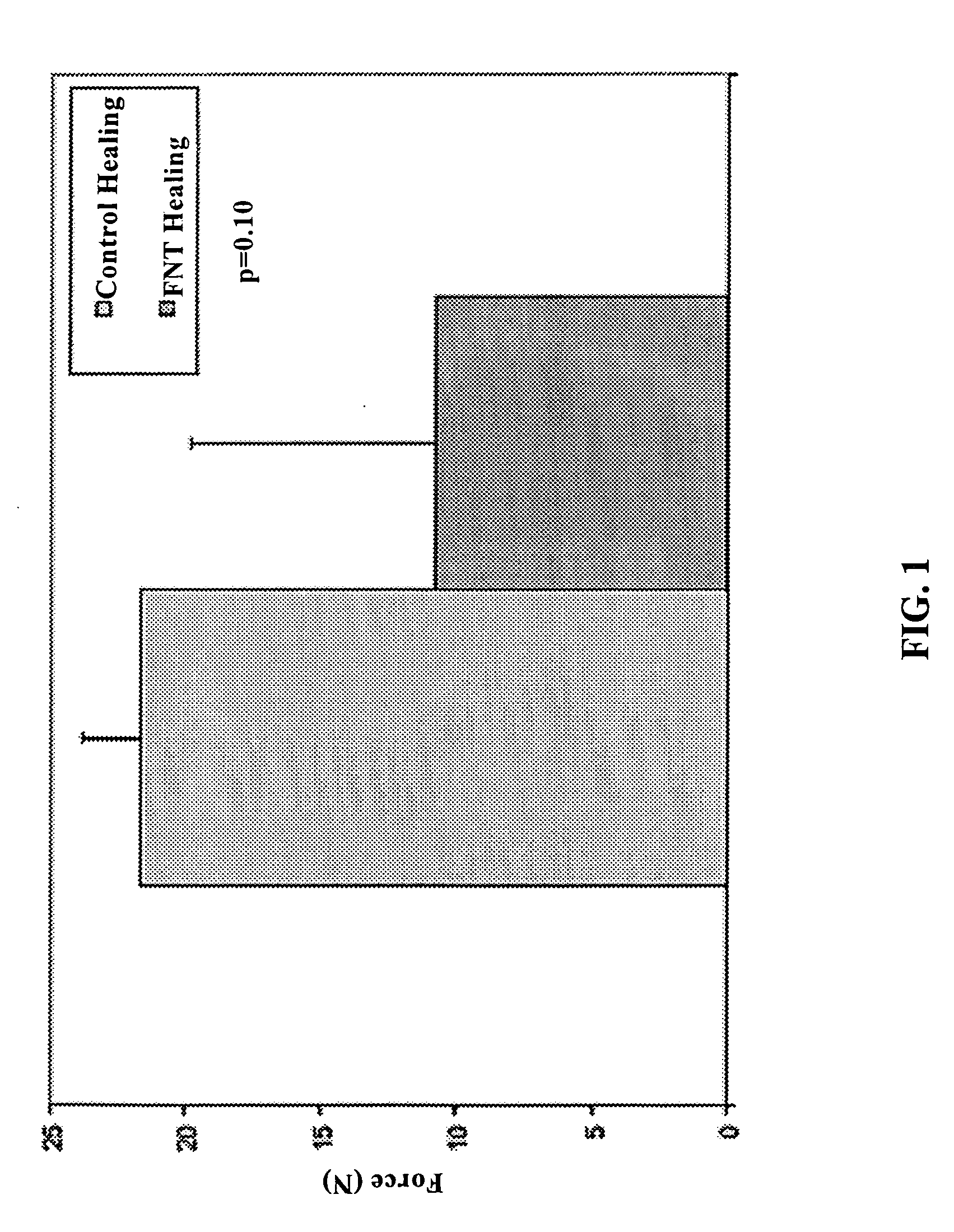
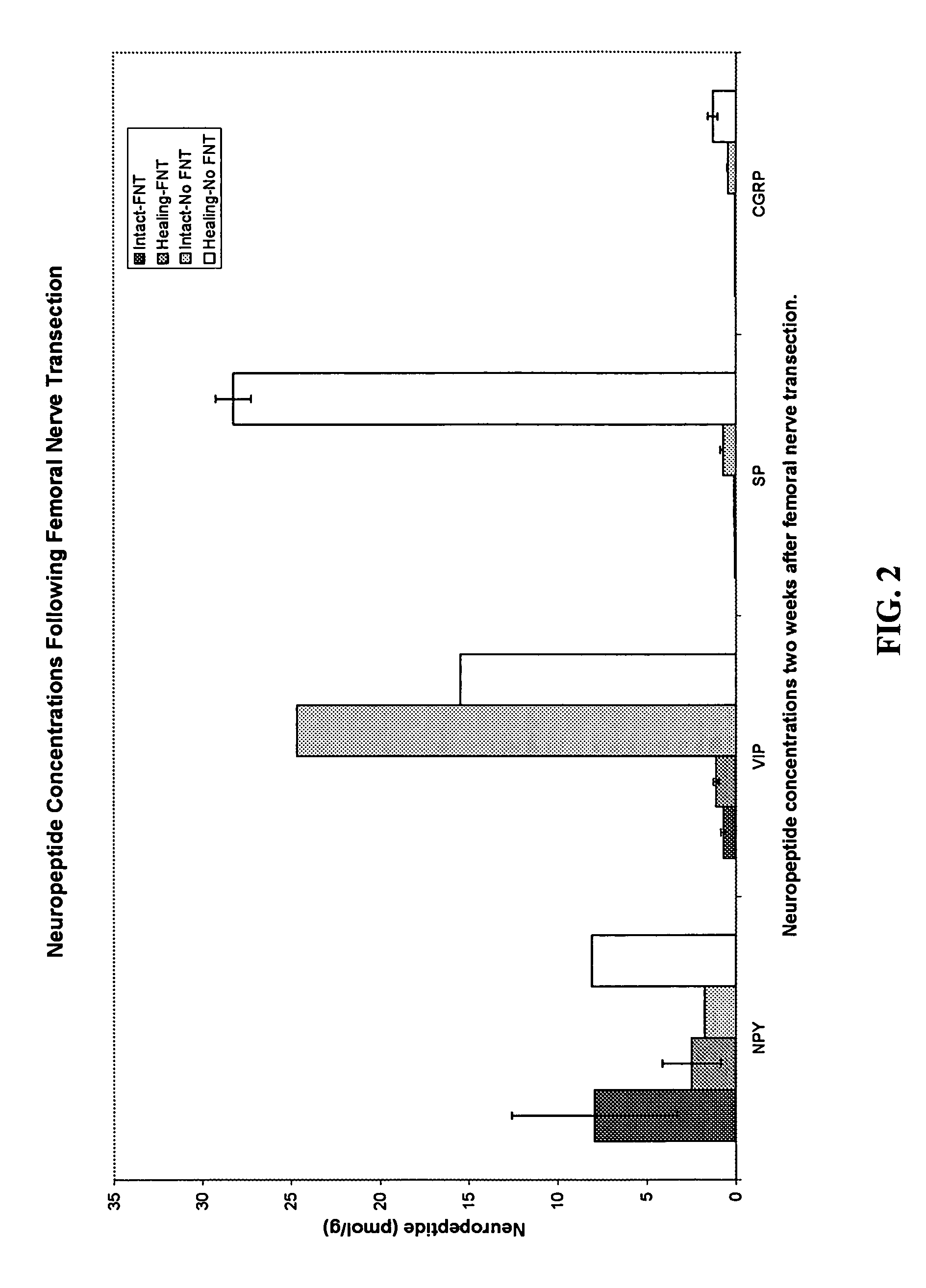
Use of neuropeptides for ligament, cartilage, and bone healing
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged cartilage and subchondral bone. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of cartilage and bone damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Compositions And Methods For Treatment Of Cardiovascular Disease
ActiveUS20080108573A1Prevent and slow down progressionDegree of reductionHormone peptidesPeptide/protein ingredientsVascular diseaseVasoactive intestinal peptide
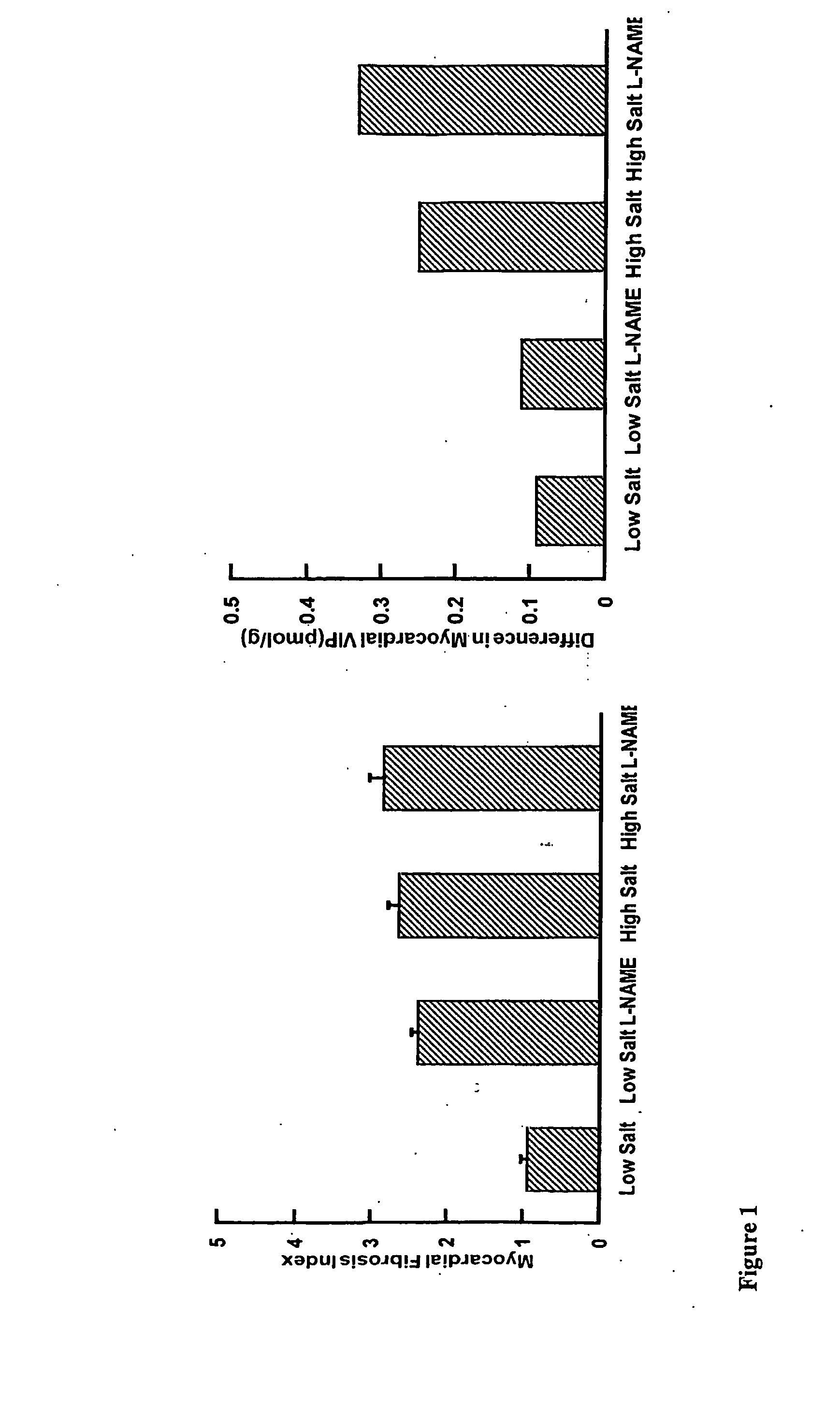
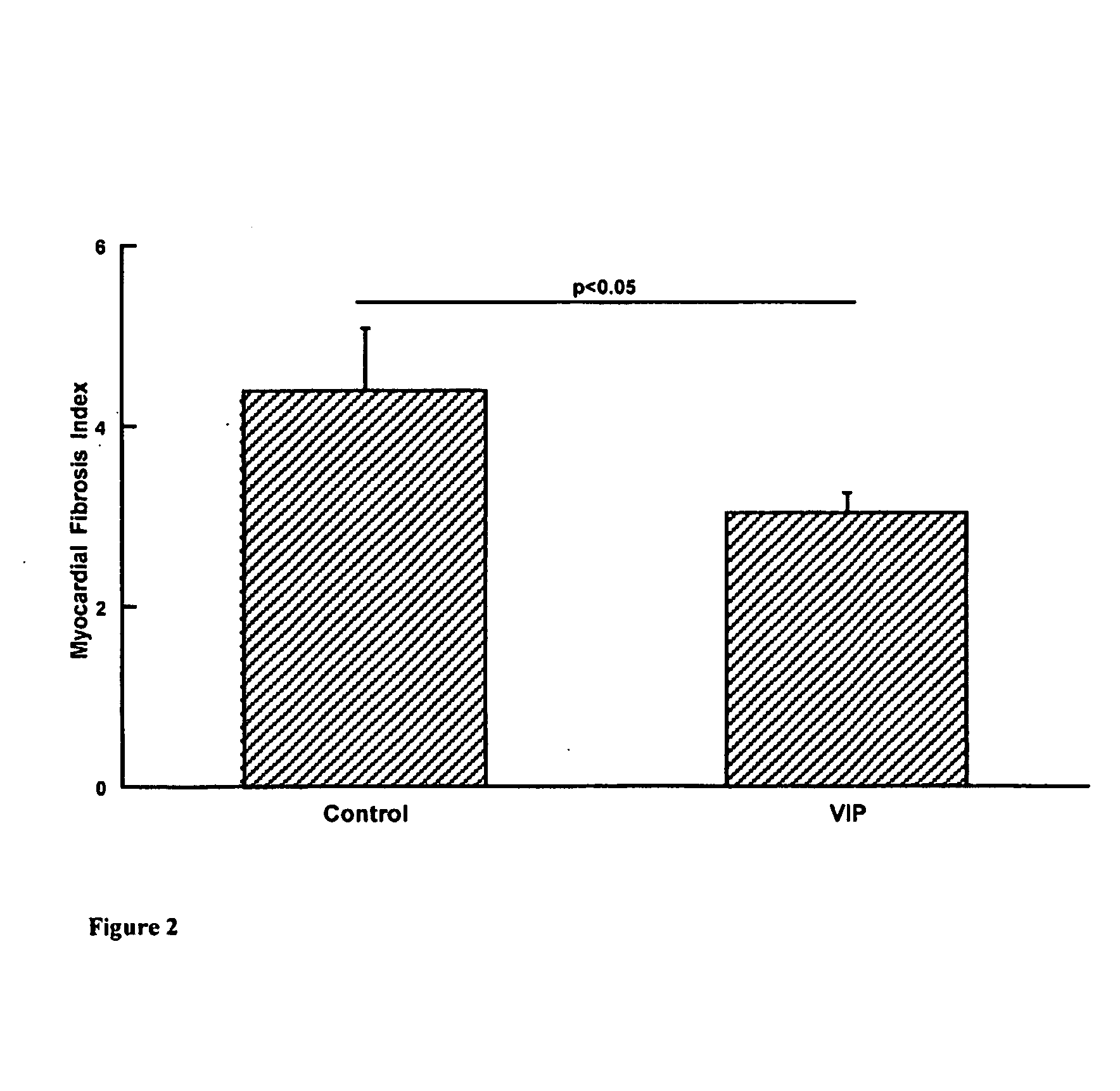
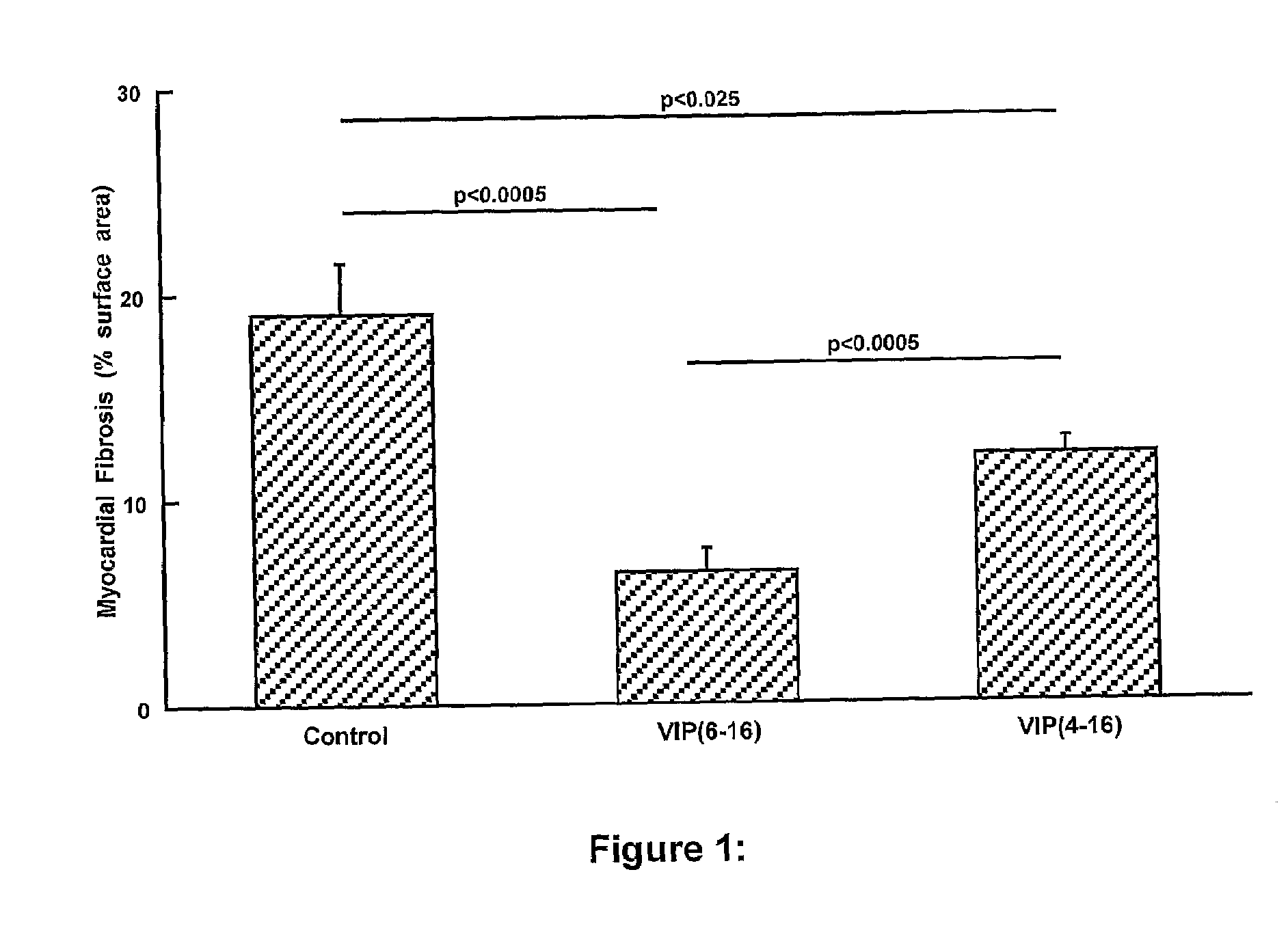
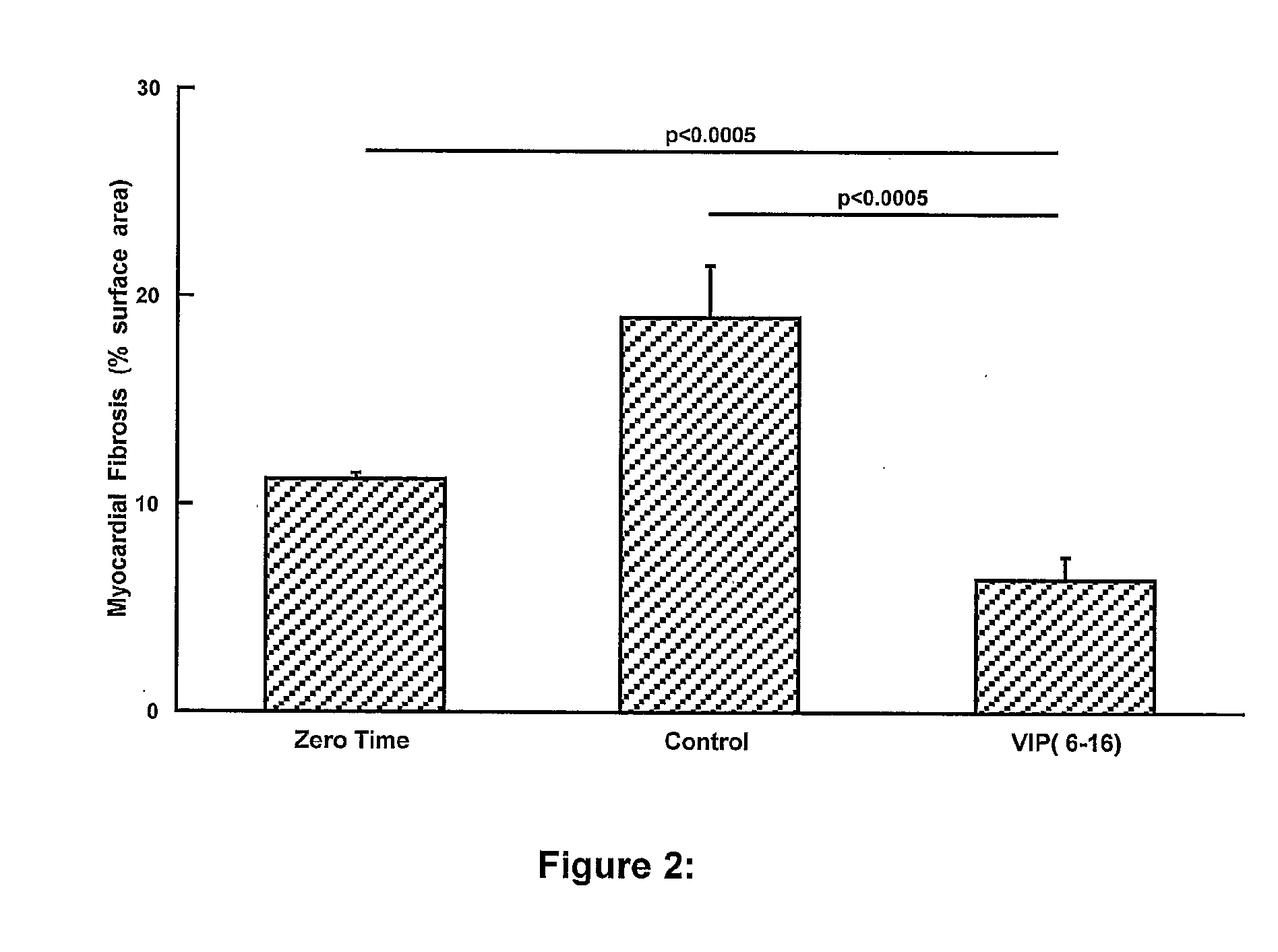
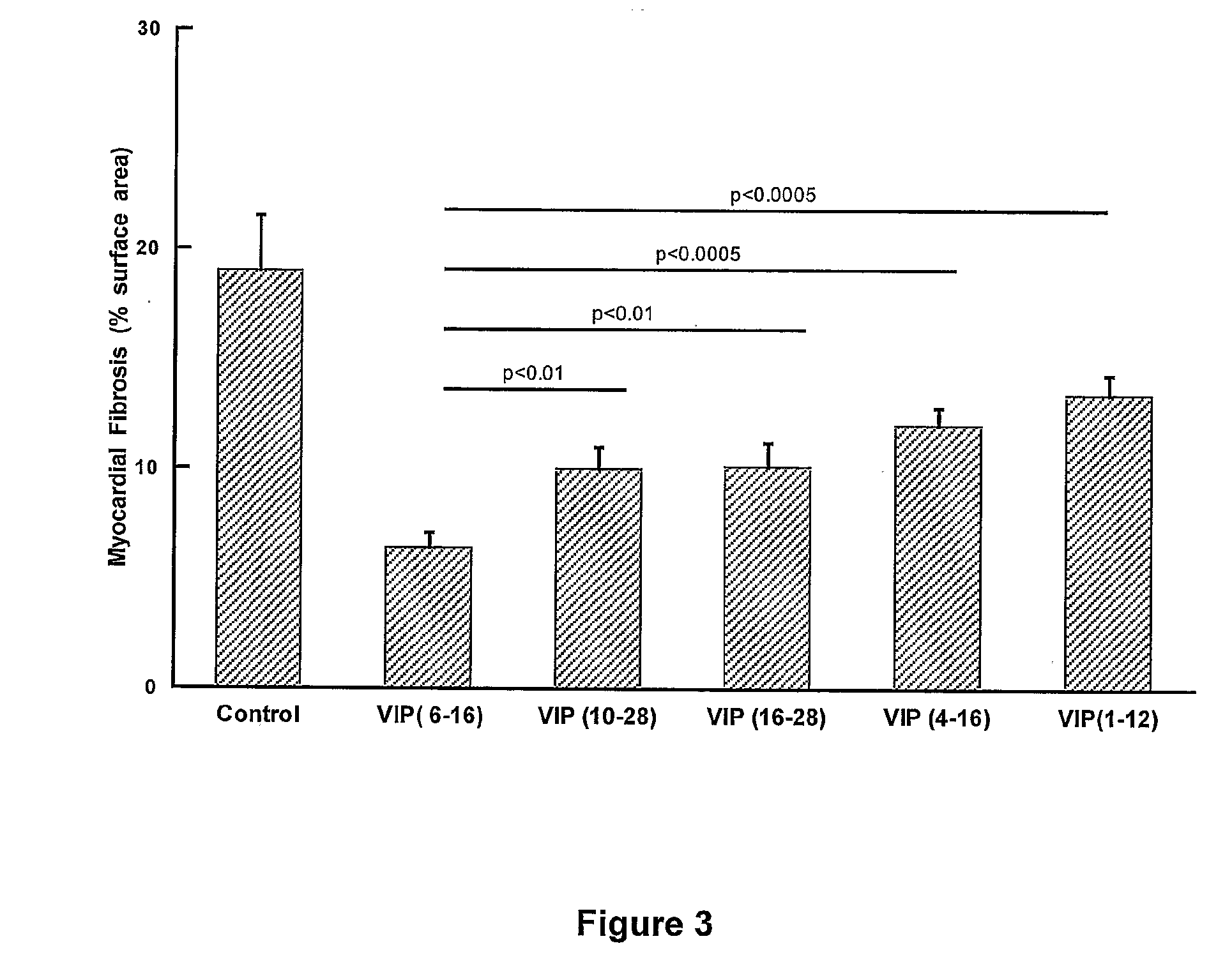
The present invention is concerned with composition and methods for treatment of certain cardiovascular conditions. In particular it is concerned with prophylactic or therapeutic treatment of myocardial fibrosis or associated conditions by administering compositions comprising vasoactive intestinal peptide (VIP) and / or active fragments) thereof.
Owner:VECTUS BIOSYSTEMS PTY LTD
Treatment of female sexual dysfunction with vasoactive intestinal polypeptide agonists
InactiveUS20060041021A1Improve vaginal muscle toneImprove tissue healthBiocidePeptide/protein ingredientsObstetricsActive agent
Methods for treating female sexual dysfunction are provided. A pharmaceutical composition containing a vasoactive agent selected from vasoactive intestinal potypeptide (VIP) and VIP agonists is administered to the vagina and / or vulvar region of the individual undergoing treatment. The formulations are also useful for improving vaginal muscle tone and tissue health, enhancing vaginal lubrication, and minimizing excess collagen deposition. Pharmaceutical formulations and kits are also provided.
Owner:VIVUS
Compounds with the biological activity of vasoactive intestinal peptide for the treatment of pulmonary and arteriolar hypertension
InactiveUS20080221041A1High activityPromote circulationPeptide/protein ingredientsMetabolism disorderDiseaseWhole body
The present invention relates to peptides which are highly biologically and pharmacologically active as therapeutic drug for the treatment of diseases related to hypertension, especially in medical interventions involving dilatation and remodeling of arterial blood vessels, either in the pulmonary or in the systemic circulation. The peptides which can be used according to the invention for the treatment of said diseases comprise at least one specific highly conservative amino acid residue sequence which seem to play an important role in connection with pulmonary and arteriolar hypertension events. It could be shown that the known naturally occurring peptides “vasoactive intestinal peptide (VIP)” and “pituitary adenylate cyclase-activating polypeptide (PACAP)”, having these specific sequences are potent drugs which can be successfully used for treatment of primary pulmonary hypertension (PPH), secondary pulmonary hypertension (SPH), and hypertension of the systemic circulation. Furthermore, the present invention discloses pharmaceutical compositions useful for treatment of PPH, SPH, and hypertension of the systemic circulation within said methods.
Owner:MONDOBIOTECH AG
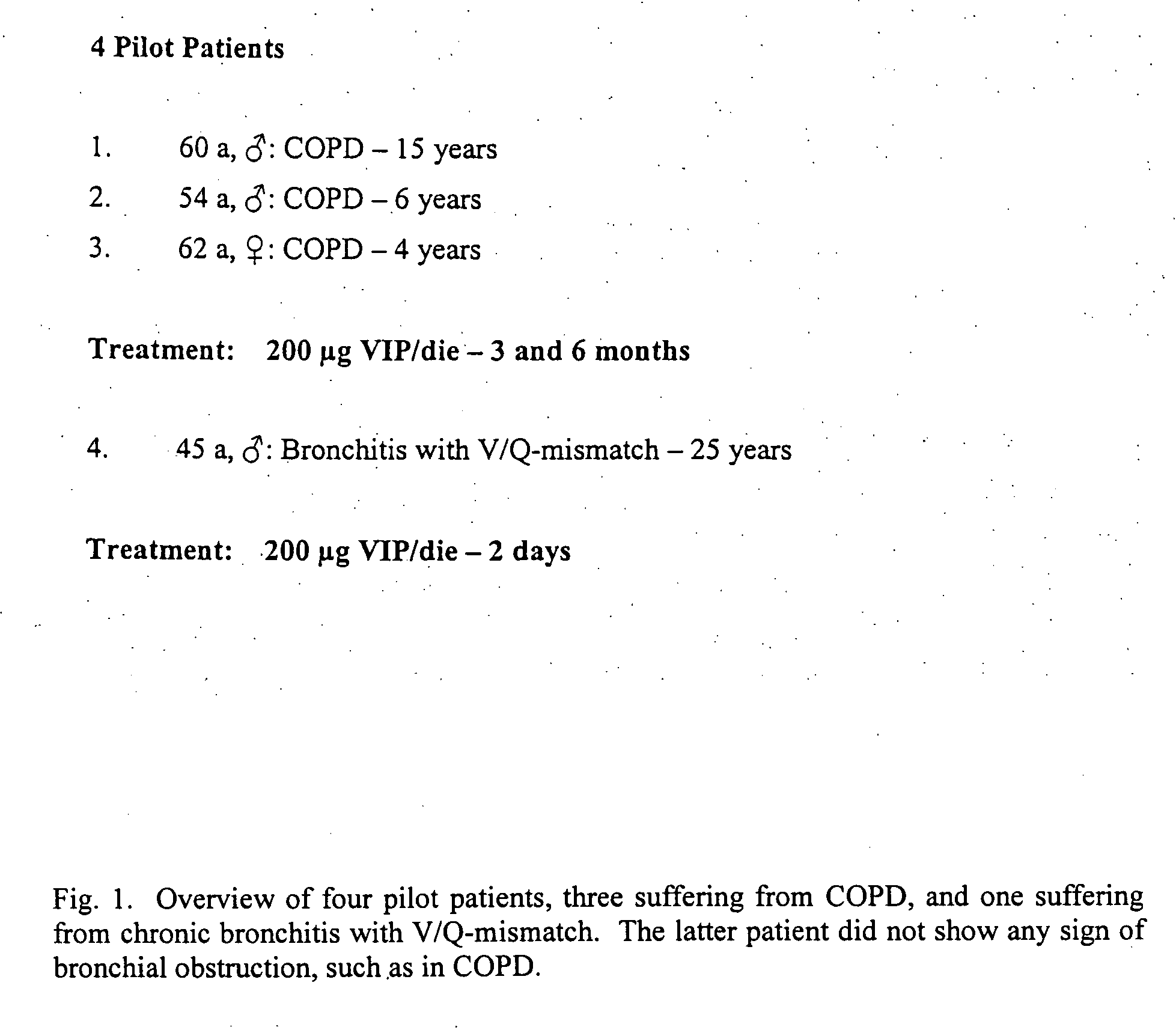
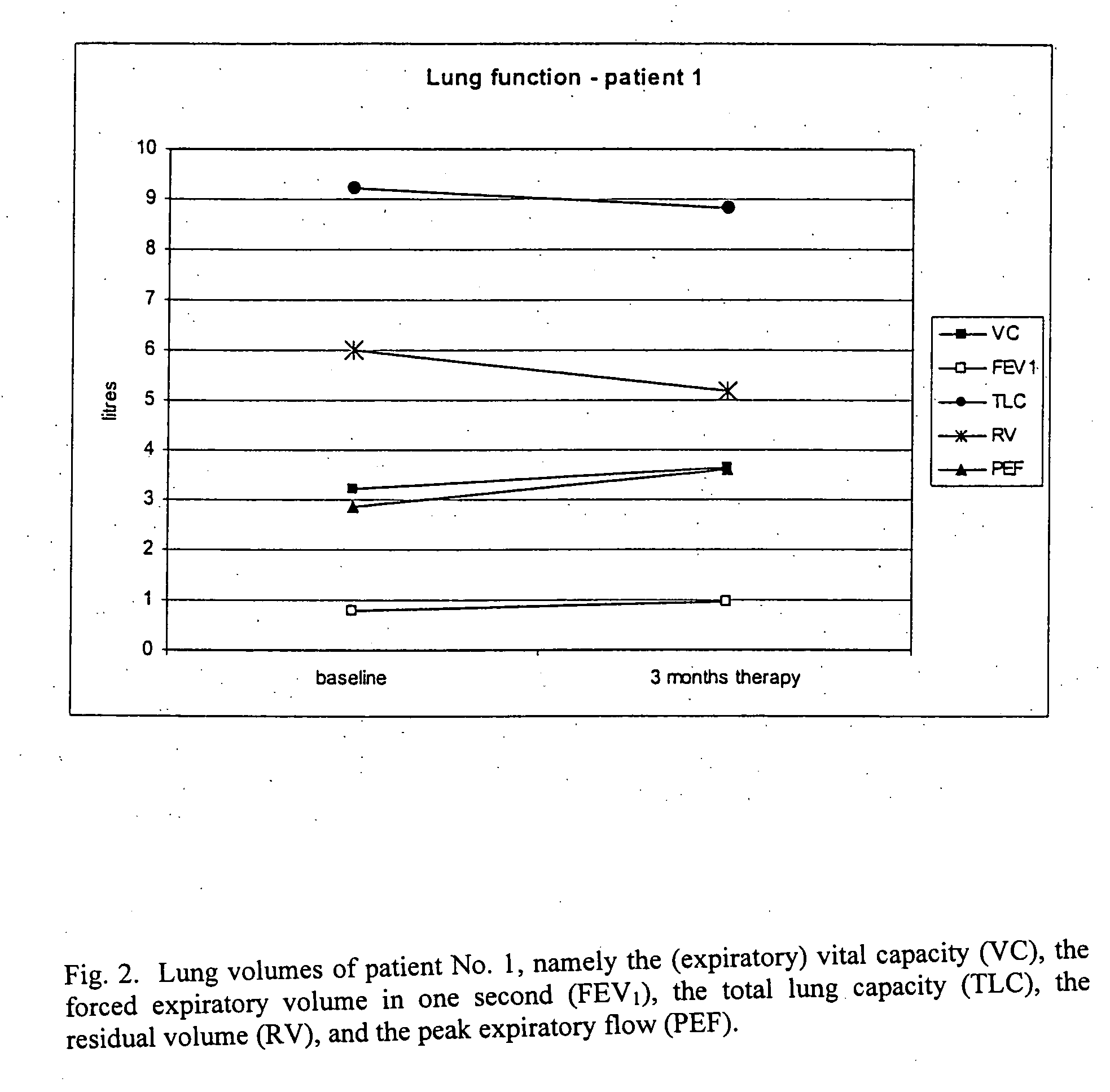
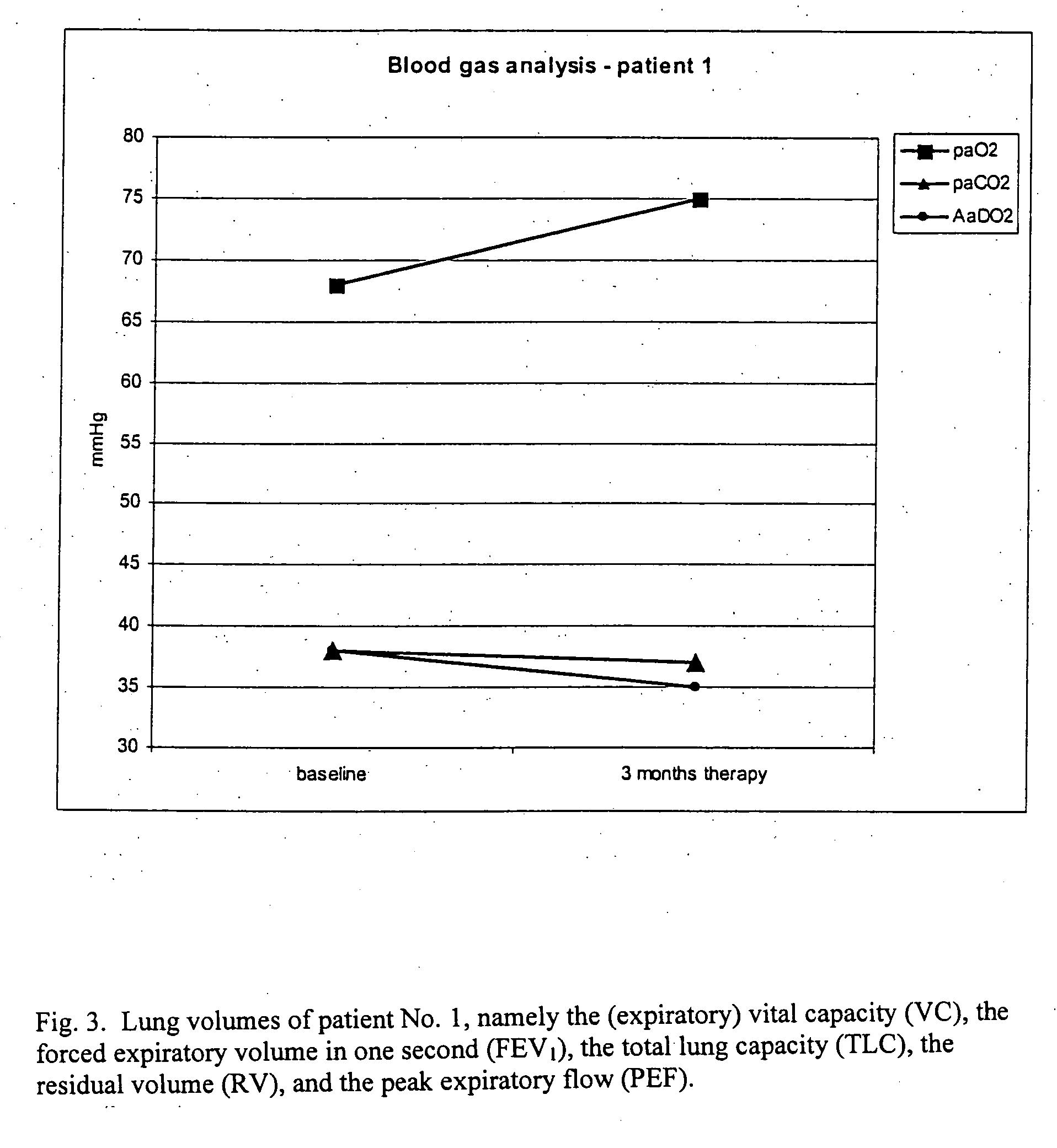
Method for treating lung diseases associated with ventilation-perfusion mismatches
InactiveUS20050118109A1Improving and recovering general state of healthAntibacterial agentsBiocideVasoactive intestinal peptideObstructive Pulmonary Diseases
The present invention relates to pharmaceutical compositions and methods for the prevention and / or treatment of lung diseases or disorders including the bronchial tree, in an animal or human, such as chronic obstructive pulmonary disease (COPD), and diseases related to or optionally associated with COPD-like lung disorders caused by ventilation-perfusion mismatches preferably in context with chronic bronchitis. The treatment includes administration of pharmaceutical compositions comprising vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), and biologically active analogues thereof, which comprise highly conservative sequence tracks.
Owner:BLOCK LUTZ HENNING +2
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS7074775B2Improve efficiencyEliminate side effectsBiocideDipeptide ingredientsTryptophanSecretin
A compound is provided that has the formulaNH2CH2CH2CH2C(O)N—R (I)where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Vip Fragments and Methods of Use
ActiveUS20090005315A1Prevent and slow down progressionDegree of reductionPeptide/protein ingredientsMetabolism disorderDiseaseVasoactive intestinal peptide
The invention relates to composition comprising a pharmaceutically effective amount of one or more functional vasoactive intestinal peptide (VIP) fragments, and the use of those compositions in the treatment of fibrosis, hypertension and other disorders.
Owner:VECTUS BIOSYSTEMS PTY LTD
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formulaNH2CH2CH2CHR1C(O)N—R (I)where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
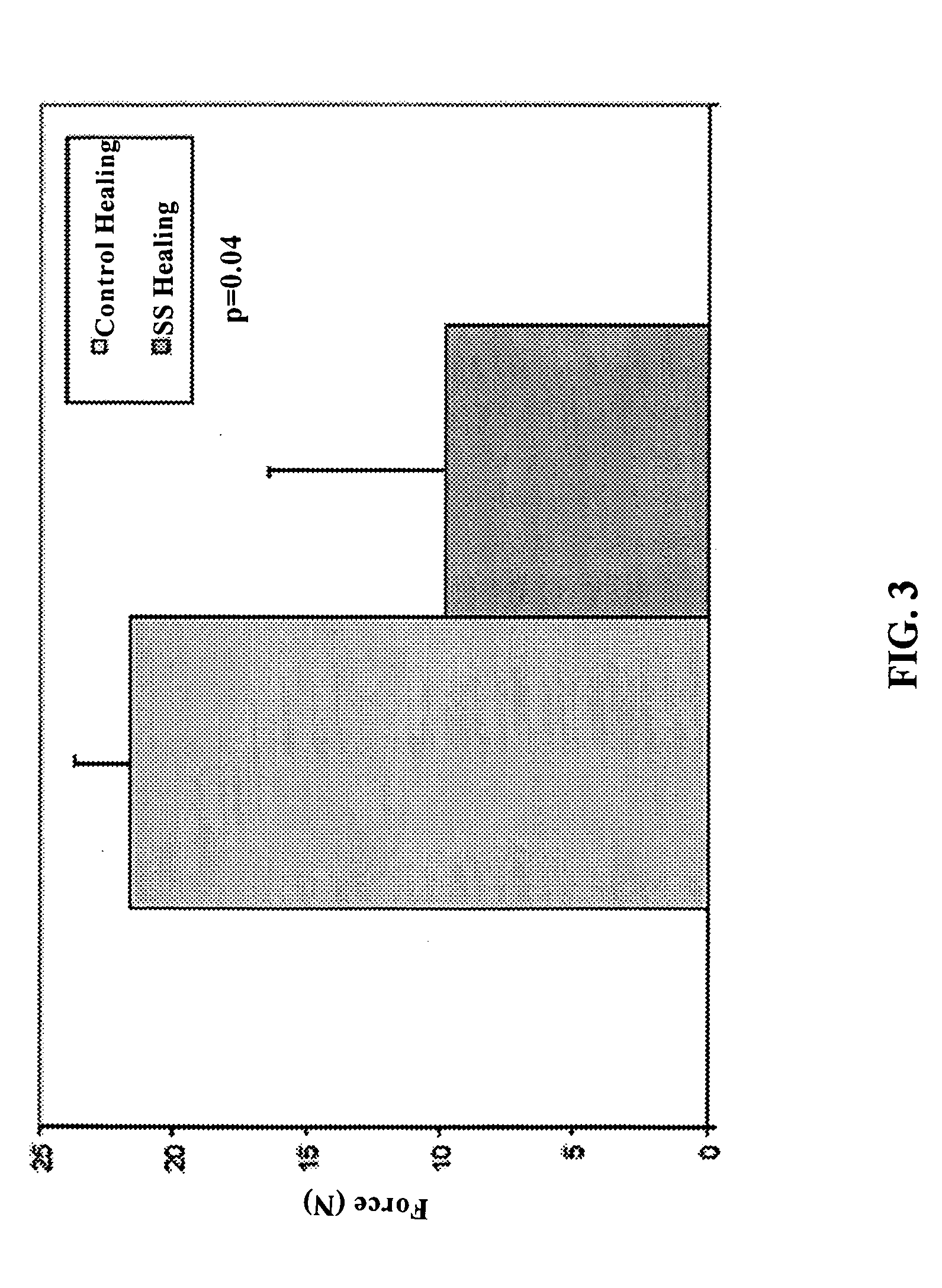
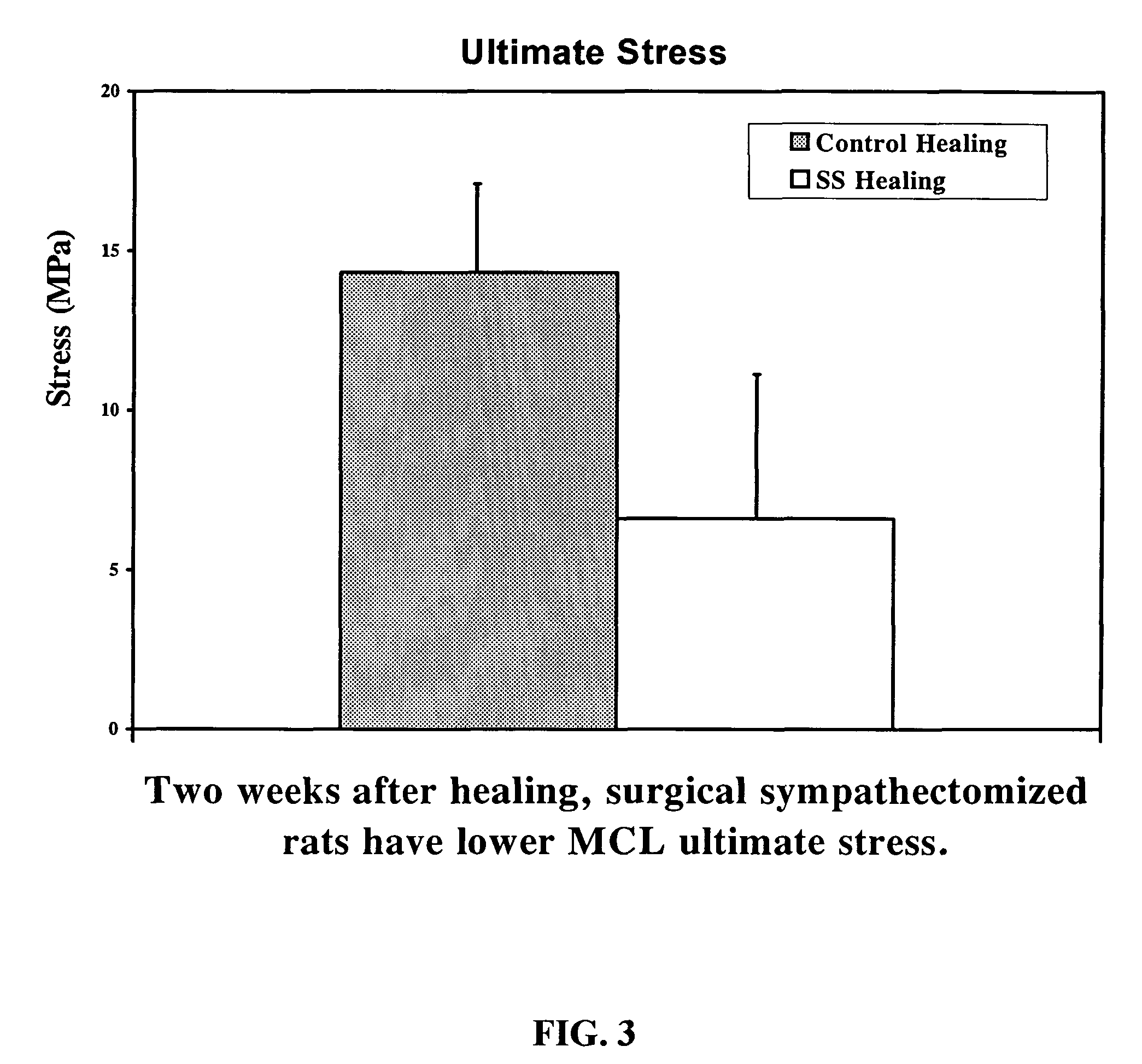
Use of neuropeptides for ligament healing
InactiveUS7776815B2High strengthDistinct utilityTachykinin ingredientsImmunoglobulinsDiseaseThyrotropin-releasing hormone
Disclosed are a method and a corresponding pharmaceutical composition for treating damaged ligaments. Neurogenic compounds in general and neuropeptides in particular have been found to be highly effective in stimulated repair of ligaments damaged due to traumatic injury, ligament disease, and disuse. Preferred active ingredients for use in the method and corresponding pharmaceutical composition include calcitonin gene-related peptide (CGRP), cholecystokinin (CCK), dynorphin, enkephalin, galanin, neuropeptide Y (NPY), neurotensin, somatostatin, substance P (SP), thyrotropin-releasing hormone (TRH), vasoactive intestinal peptide (VIP).
Owner:WISCONSIN ALUMNI RES FOUND
Multifunctional compound microecological preparation nano-selenium-recombination expression VIP (vasoactive intestinal peptide)-lactococcus lactis and preparation method
InactiveCN108753670AMany functionsLow acute toxicityBacteriaMicroorganism based processesStaphylococcus lactisVasoactive intestinal peptide
The invention relates to multifunctional compound microecological preparation nano-selenium-recombination expression VIP (vasoactive intestinal peptide)-lactococcus lactis and a preparation method. Toxic sodium selenite can be converted into non-toxic red simple-substance nano-selenium, and synthesized nano-selenium is enriched in lactococcus lactis NZ9000 in cells. The invention also discloses amethod for preparing a multifunctional compound microecological preparation SENPs-rVIP-L.lactis NZ9000 based on lactococcus lactis NZ9000. The problems that existing selenium supplementing additives have high toxicity action and low bioavailability and easily cause environment pollution, existing production technical procedures are complicated, the cycle is long and the production cost is high andthe problems about remaining of antibiotics, drug resistance and the like are solved.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formulawhere R1 is H, C1–C4 alkyl and OH; R2 in is H, C1–C4 alkyl and OH; R3 is H and C1–C4 alkyl; R4 is H and C1–C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2–C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Method for treating the endotoxic shock in mammals
InactiveUS6429188B1Inhibit productionImprovement in evolutionBiocidePeptide/protein ingredientsToxinInterleukin 6
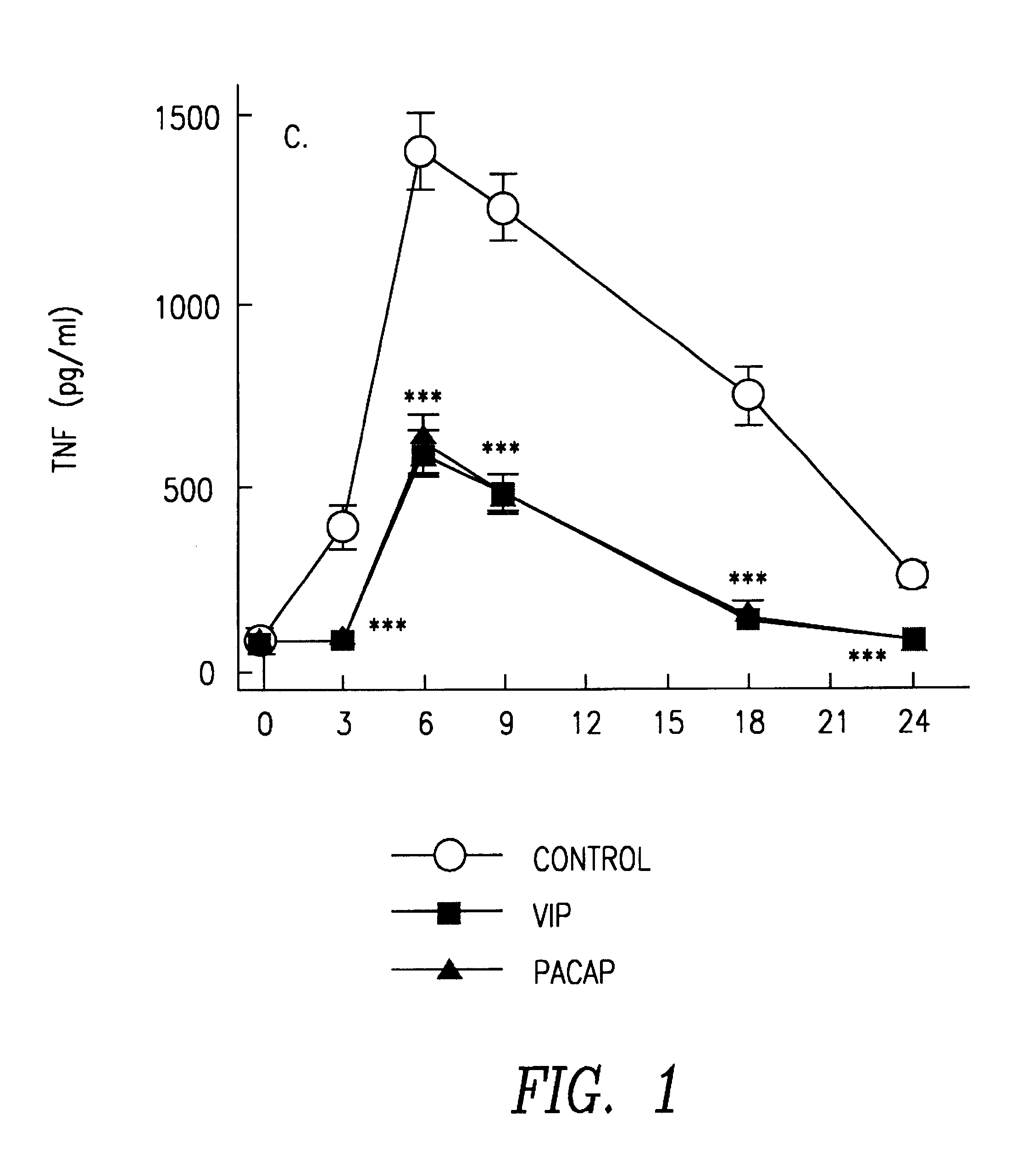
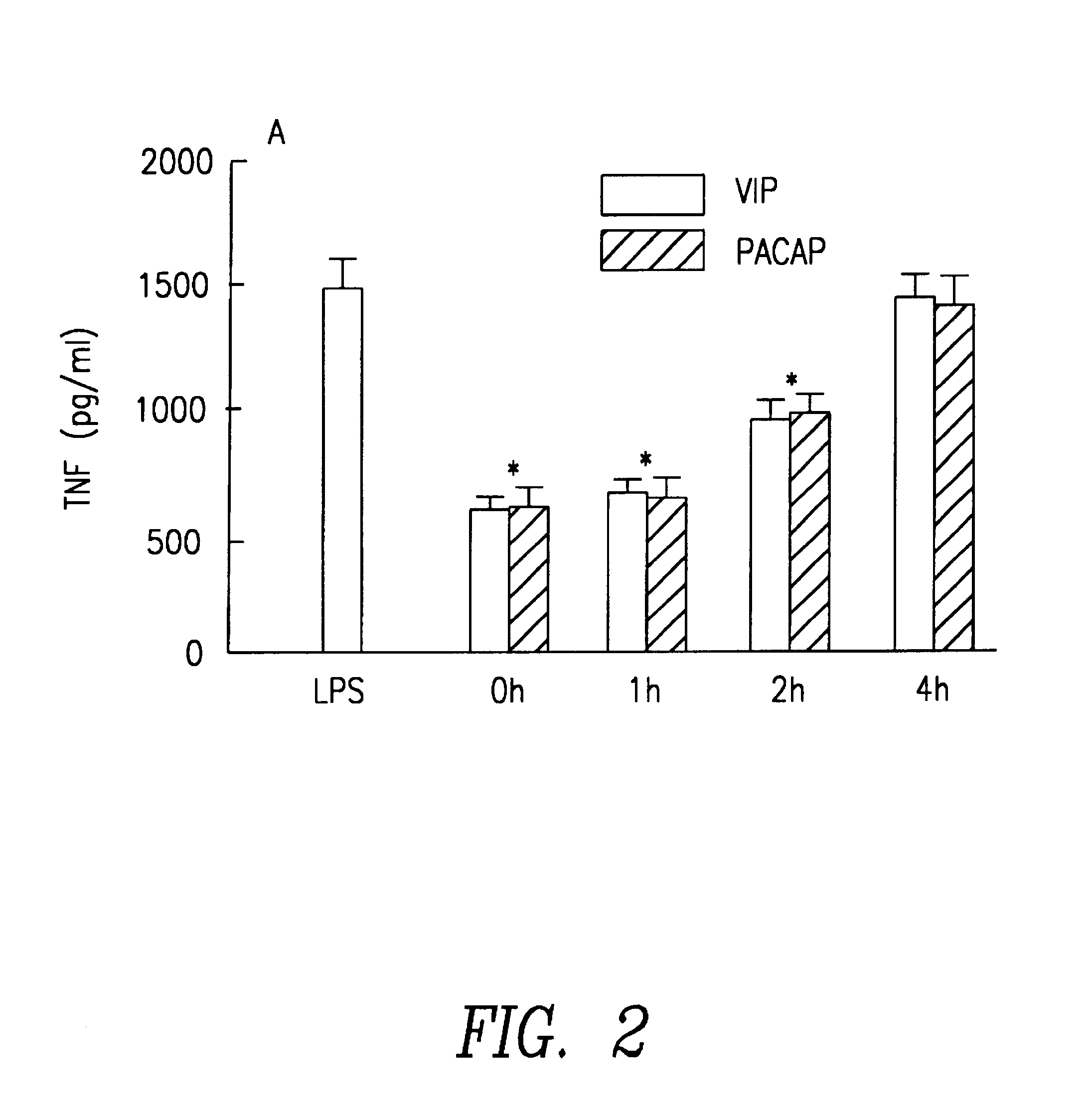
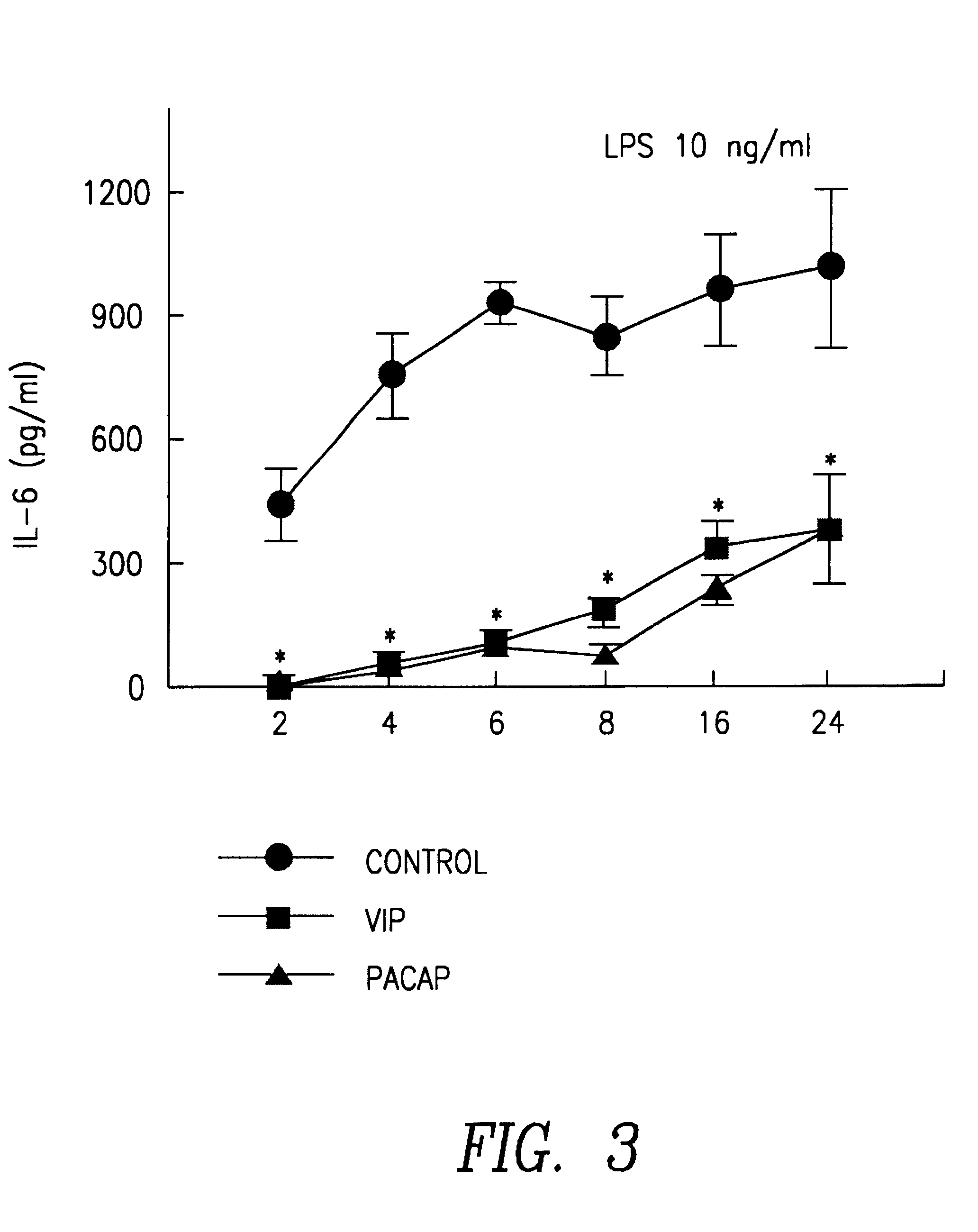
Method for the treatment of endotoxic shock in mammals. The use of vasoactive intestinal peptide (VIP) and peptide activating hypofissiary adenylate cyclase (PACAP) is described in the treatment of endotoxic shock in mammals. These substances inhibit the production of tumor necrosis factor (TNF) and interleukin 6 (IL-6).
Owner:UNIV COMPLUTENSE DE MADRID
Biologically active substance of a vasoactive intestinal peptide for treating interstitial lung diseases
InactiveUS7468353B2High activityAntipyreticAnalgesicsInterstitial lung diseaseVasoactive intestinal peptide
Owner:MONDOBIOTECH AG
Vasoactive intestinal polypeptide pharmaceuticals
InactiveUS7566691B2Facilitate duration of actionExtended durationMetabolism disorderDepsipeptidesVasoactive intestinal peptideObesity
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formula where R1 is H, C1-C4 alkyl and OH; R2 in is H, C1-C4 alkyl and OH; R3 is H and C1-C4 alkyl; R4 is H and C1-C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2-C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Use of compounds having the biological activity of vasoactive intestinal peptide for the treatment of sarcoidosis
InactiveUS20080274961A1High activityMetabolism disorderTripeptide ingredientsDiseaseVasoactive intestinal peptide
The present invention relates to peptides which are highly biologically and pharmacologically active as therapeutic drug for the treatment of diseases related to sarcoidosis. The peptides which can be used according to the invention for the treatment of said disease comprise at least one specific highly conservative amino acid residue sequence which seem to play an important role in connection with pulmonary and arteriolar hypertension events. It could be shown that the known naturally occurring peptides “vasoactive intestinal peptide (VIP)” and “pituitary adenylate cyclase-activating polypeptide (PACAP)”, having these specific sequences are potent drugs which can be successfully used for treatment of sarcoidosis. Furthermore, the present invention discloses a method for the treatment patients suffering from sarcoidosis.
Owner:THERAMETRICS DISCOVERY AG
Vasoactive intestinal polypeptide pharmaceuticals
InactiveUS7595294B2Facilitate duration of actionExtended durationMetabolism disorderDepsipeptidesMedicineVasoactive intestinal peptide
Owner:TRANSITION THERAPEUTICS INC
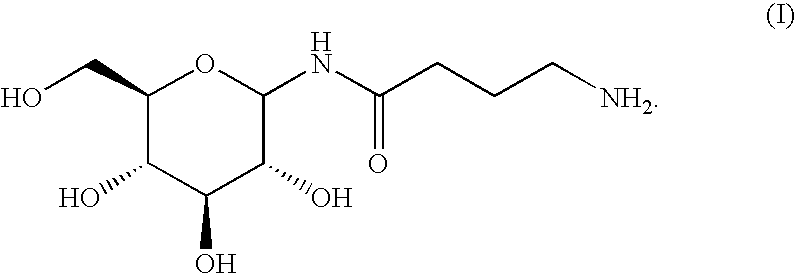
Peptides with improved properties having the biological activity of vasoactive intestinal peptide (VIP) and their use for the treatment of lung diseases
ActiveUS20100256044A1Valid testIncreased stability in pharmaceutical composition and formulationPeptide/protein ingredientsDepsipeptidesDiseaseVasoactive intestinal peptide
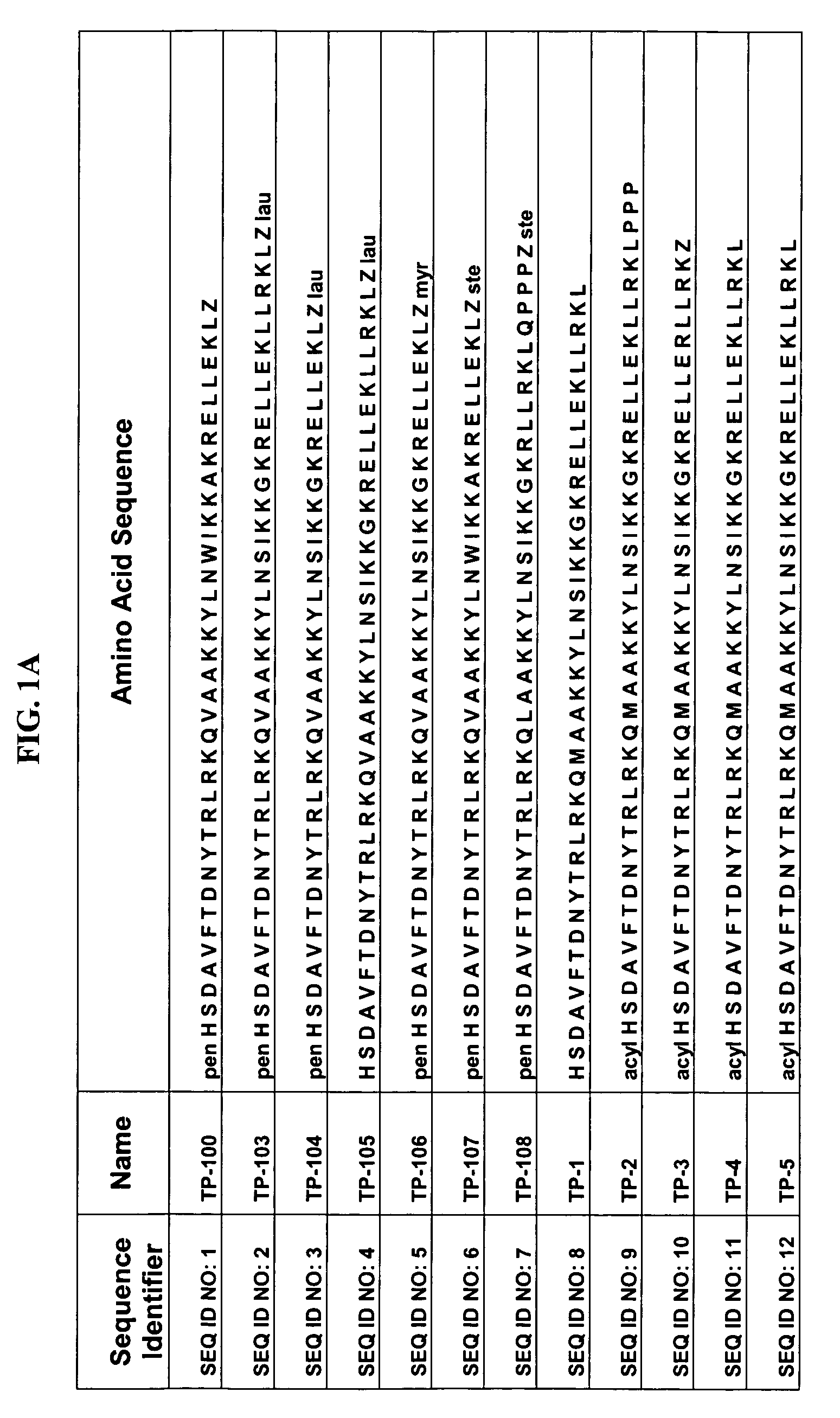
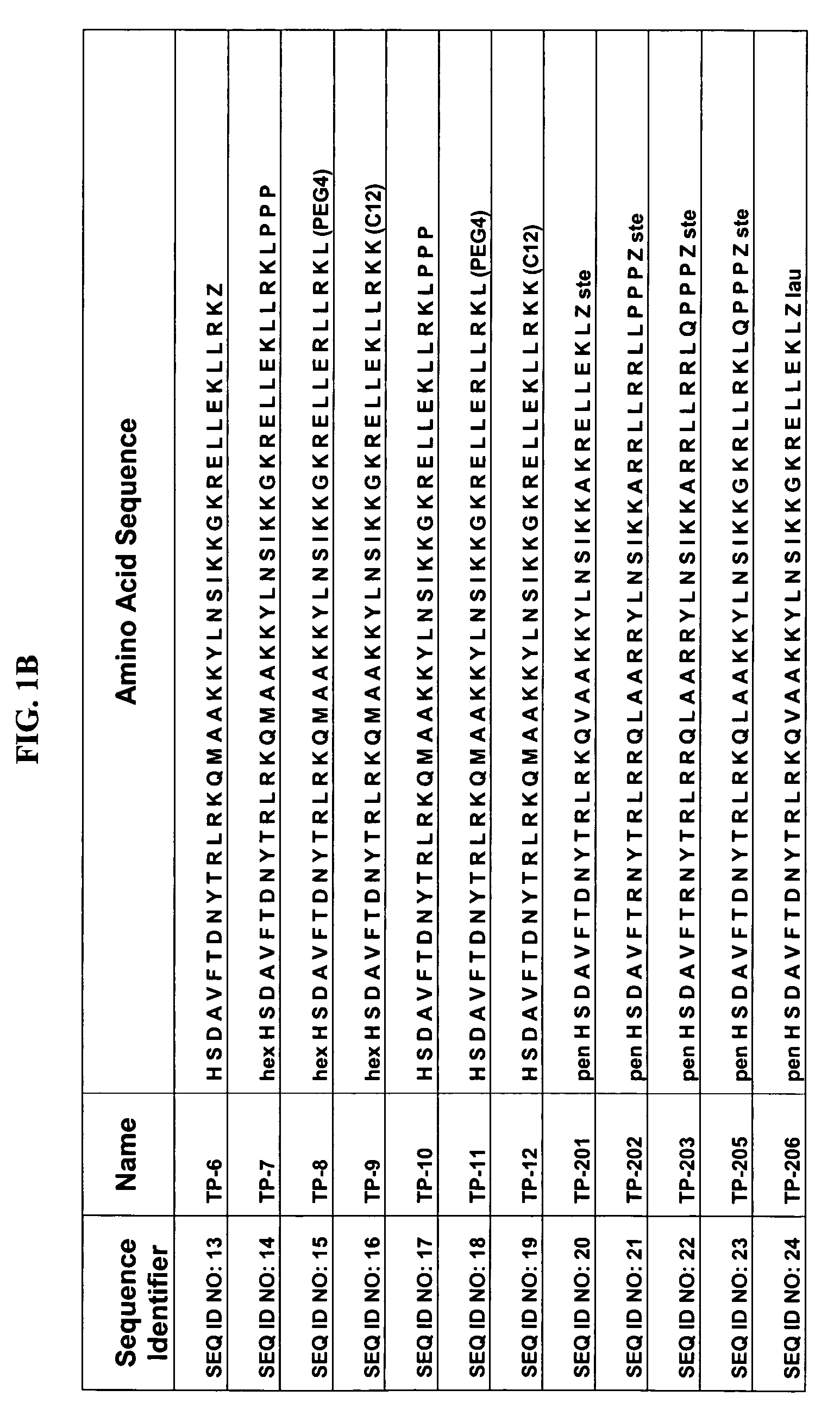
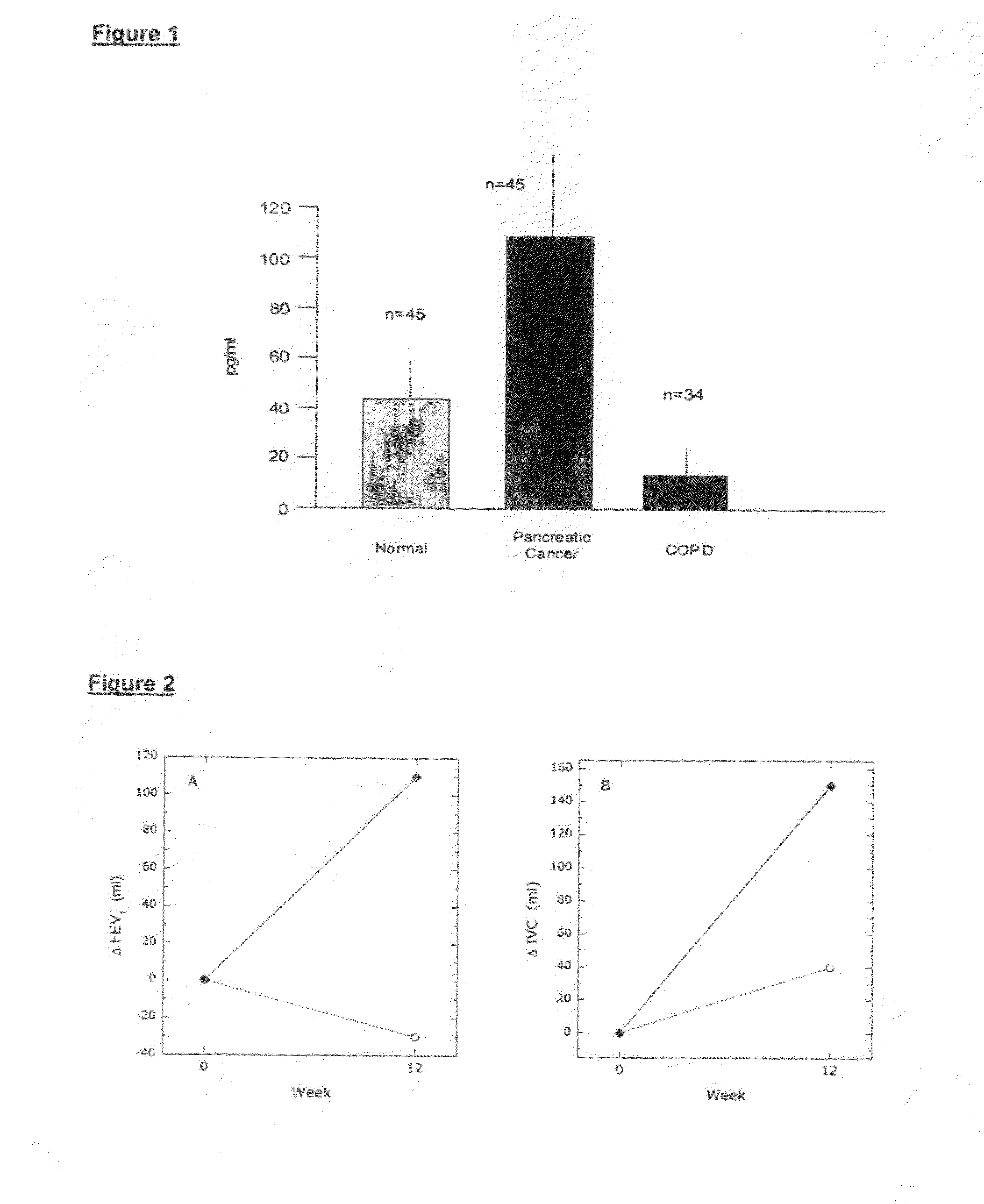
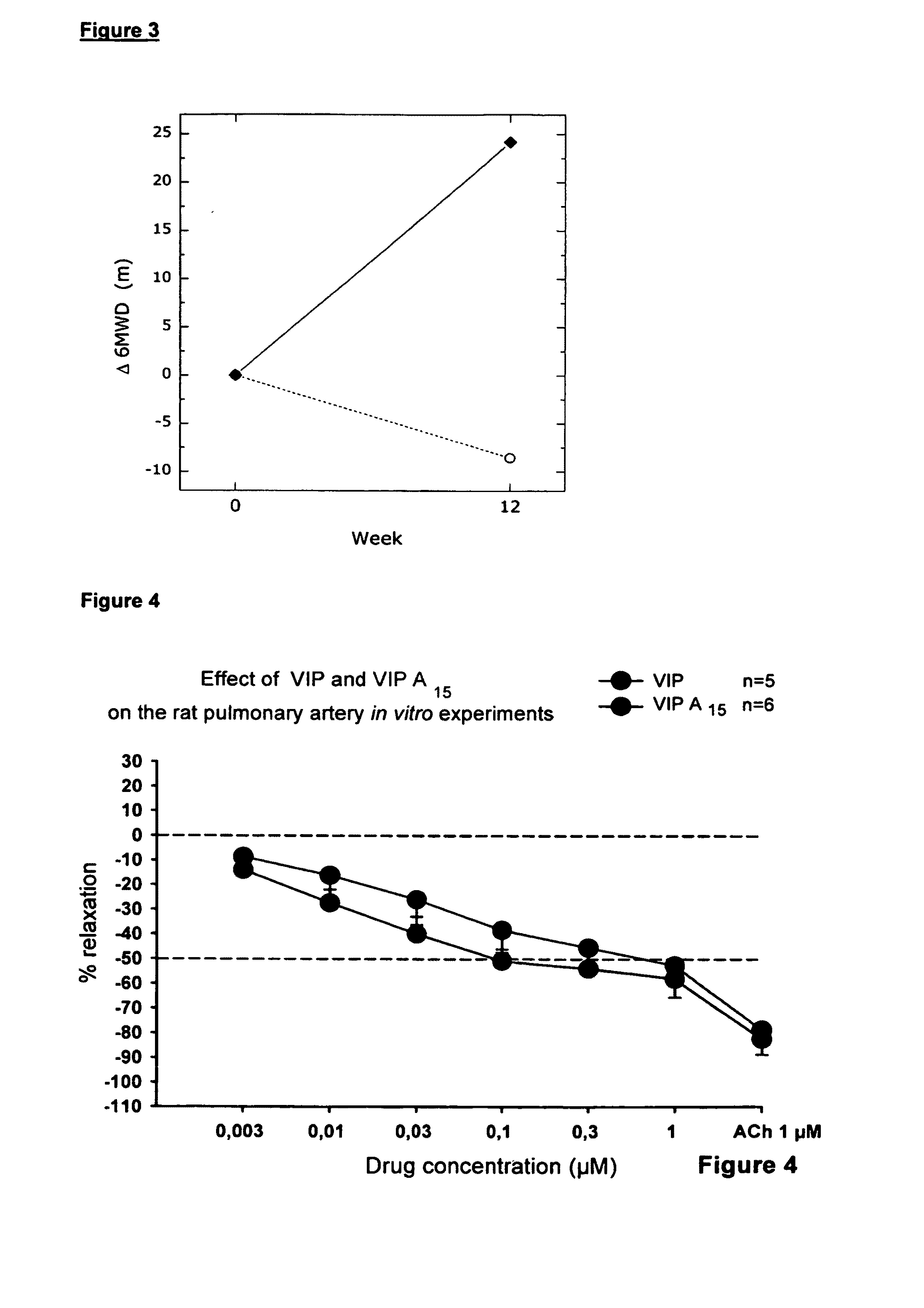
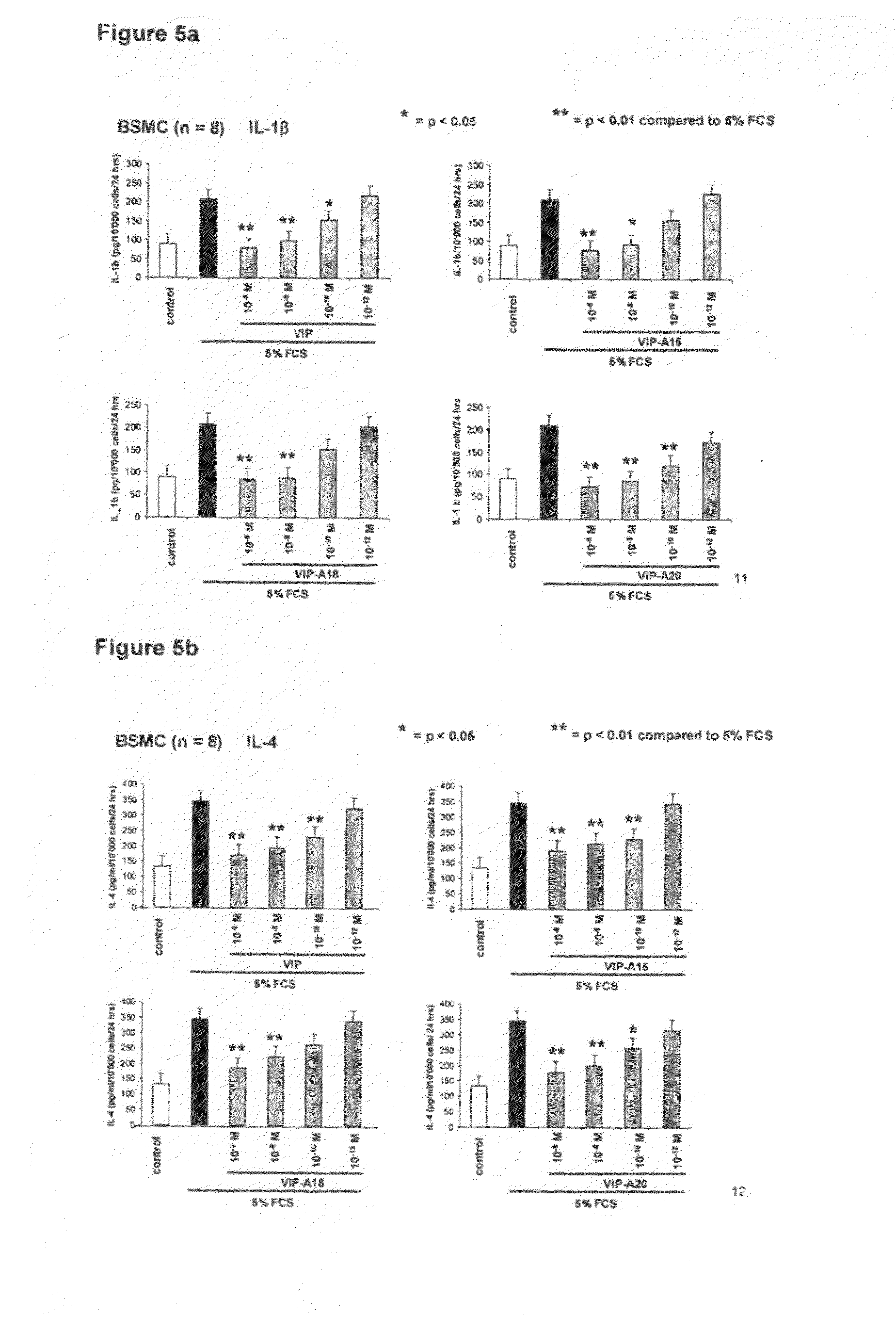
The present invention relates to novel peptides that are highly biologically and pharmacologically active as therapeutic agents for the treatment of numerous lung diseases or lung and / or bronchi related diseases, especially chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and Bronchiolitis obliterans (BO). The synthetic peptides according to the invention are derivatives of vasoactive intestinal peptide (VIP) and show enhanced physical, pharmacological and biological / therapeutic properties compared to VIP.
Owner:RES INT
Modified vasoactive intestinal peptides
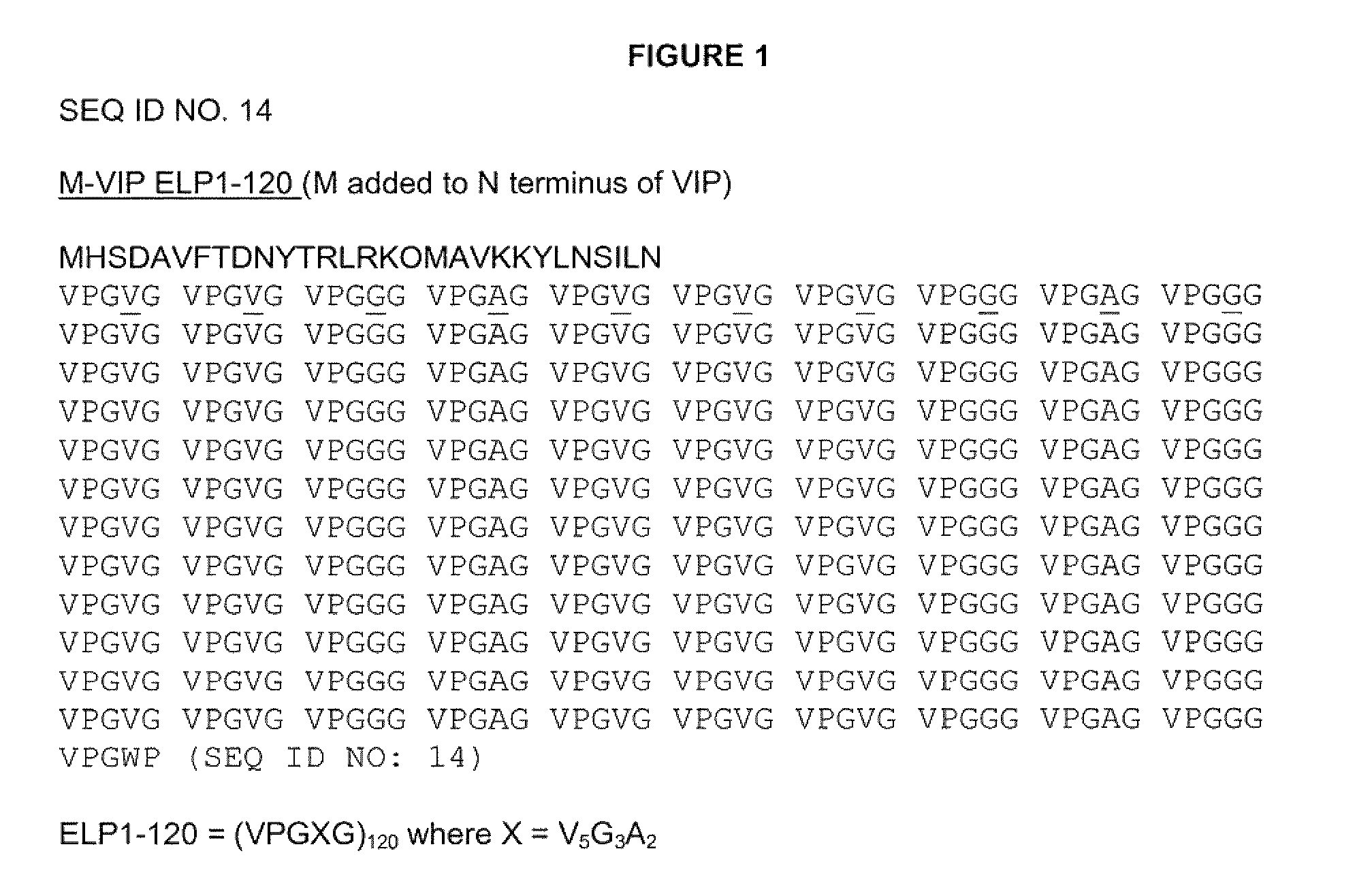
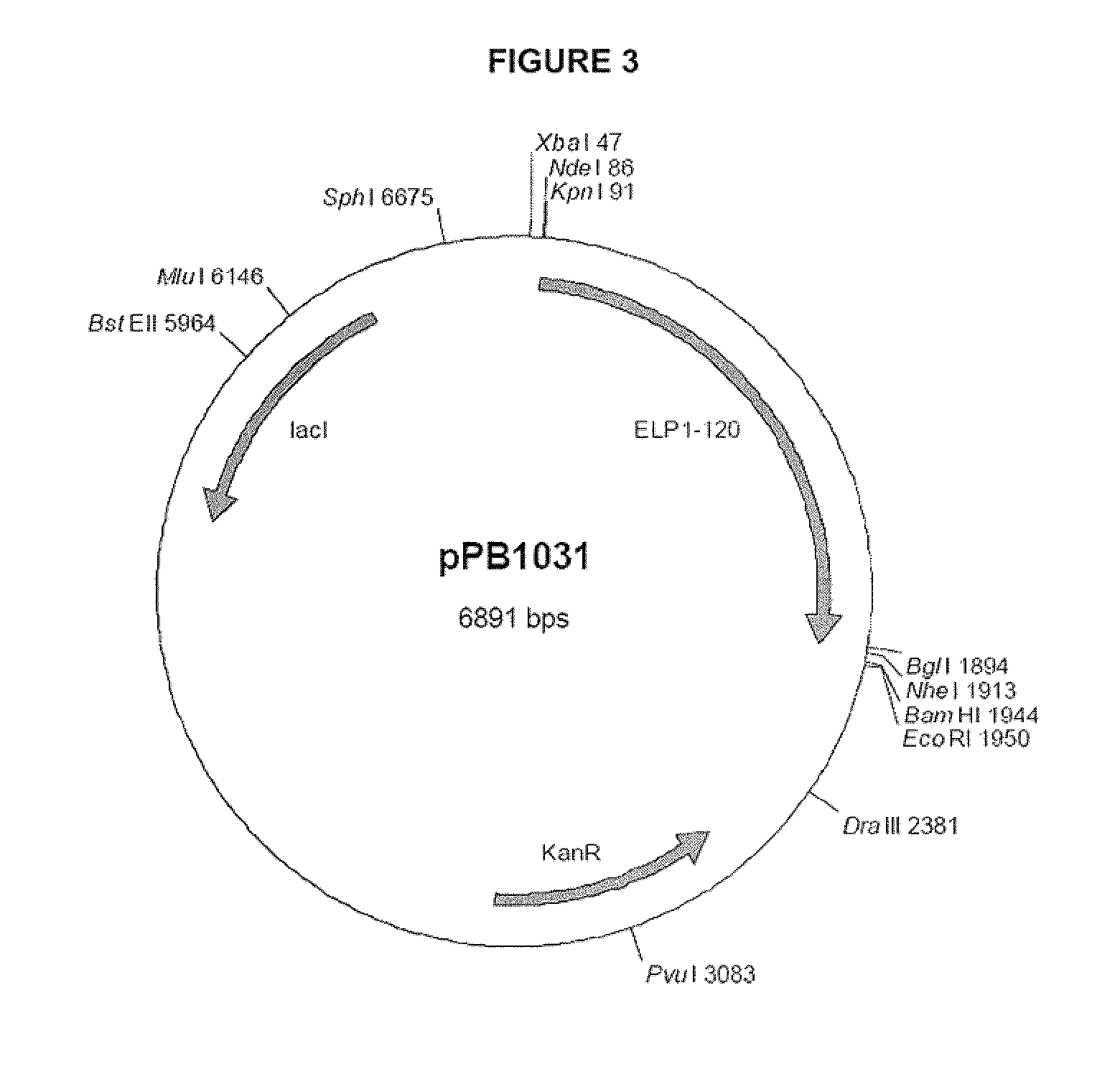
ActiveUS9029505B2Increased circulatory half-life and persistenceAltered binding preferencePeptide/protein ingredientsPharmaceutical delivery mechanismHalf-lifeVasoactive intestinal peptide
The present invention provides modified Vasoactive Intestinal Peptides (VIPs), encoding polynucleotides and vectors, as well as pharmaceutical compositions comprising the same. The invention further provides methods of making and using the modified VIP agents. In accordance with the invention the VIP exhibits an extended circulatory half-life, receptor-binding or biological potency, and / or altered receptor binding profile with respect to unmodified VIP.
Owner:PHASEBIO PHARMA INC
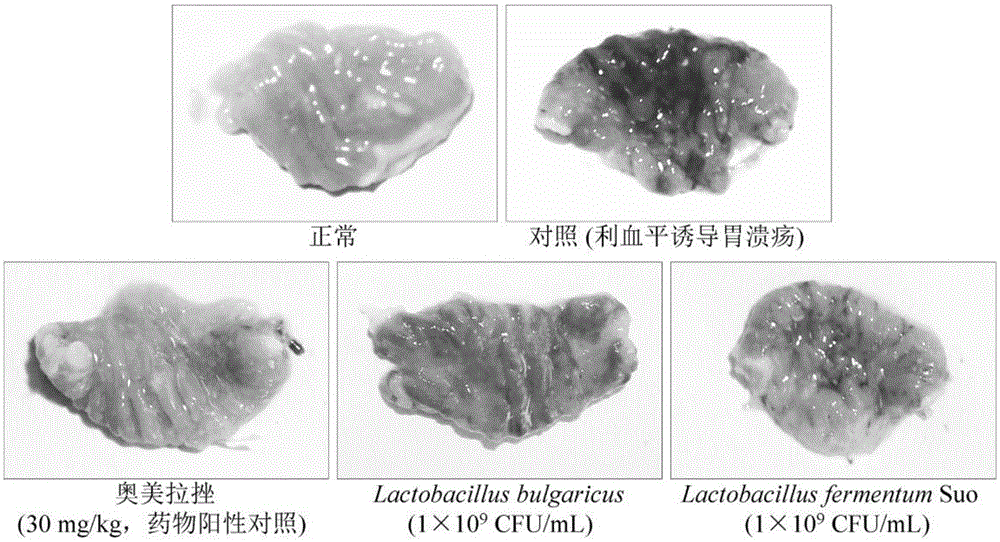
Lactobacillus fermentum strain suo capable of preventing gastric ulcers and application of lactobacillus fermentum strain suo
The invention discloses lactobacillus fermentum strain suo capable of preventing gastric ulcers and application of the lactobacillus fermentum strain suo. A bacterial strain of which the preservation number CCTCC NO is M2013511 has strong acid resistance, and the survival rate in artificial gastric juice of which the pH value is 3.0 for three hours reaches 92.46 plus or minus 4.06%, and the bacterial strain can slowly grow in cholate of which the concentration is 1.0%, and the growth efficiency reaches 17.36 plus or minus 1.19% of cholate-free cultivation; the hydrophobicity of lactobacillus fermentum strain suo cells also reaches 68.44 plus or minus 2.48%, and the lactobacillus fermentum strain suo cells can normally grow in human intestines. By adopting the lactobacillus fermentum strain suo, the areas and degrees of the gastric ulcers can be reduced; motilin (MOT) and P substances (SP) are reduced by different degrees; somatostatin (SS) and vasoactive intestinal peptides (VIP) are increased, and tumor necrosis factors-alpha (TNF-alpha), interleukin-6 (IL-6), interleukin-12 (IL-12) and interferon-gamma (IFN-gamma) can be lowered; the lactobacillus fermentum strain suo has a good gastric ulcer inhibiting effect, and the effect similar to omeprazole is achieved.
Owner:江苏新申奥生物科技有限公司
Use of modified vasoactive intestinal peptides in the treatment of hypertension
ActiveUS20150111829A1Modified receptor binding profileLong persistenceOrganic active ingredientsConnective tissue peptidesHypertension medicationsVasoactive intestinal peptide
The present invention is based on the discovery that a VIP having a binding preference for VPAC2 can provide long-acting blood pressure control synergistically with concomitant anti-hypertensive therapies. Accordingly, methods and compositions useful for the treatment and / or amelioration of hypertension are provided.
Owner:PHASEBIO PHARMA INC
Analogs of pituitary adenylate cyclase-activating polypeptide (PACAP) and methods for their use
InactiveUS20160122406A1Reduce harmPrevent restenosisCompounds screening/testingNervous disorderMedical disorderDisseminated cancer
This invention relates to analogs of pituitary adenylate cyclase-activating polypeptide (PACAP), which are agonists for the PACAP / vasoactive intestinal peptide (VIP) receptors: PAC1, VPAC1 and VPAC2 receptors. These PACAP analogs can be used as prophylactic / therapeutic agents for a wide range of medical disorders. These PACAP analogs coupled to suitable radionuclides can be used in the localization, diagnosis and treatment of disseminated cancers and metastatic tumors, and coupled to small molecule therapeutics can be used as vectors for targeted drug delivery. This invention also provides pharmaceutical compositions of one or more PACAP analogs of the invention either alone or in combination with one or more other prophylactic / therapeutic agent.
Owner:TULANE EDUCATIONAL FUND
Antibacterial peptide VIP (vasoactive intestinal peptide)-like peptide and preparation method thereof
InactiveCN104530215ASimple production processReduce manufacturing costHormone peptidesFermentationVasoactive intestinal peptideCombinatorial chemistry
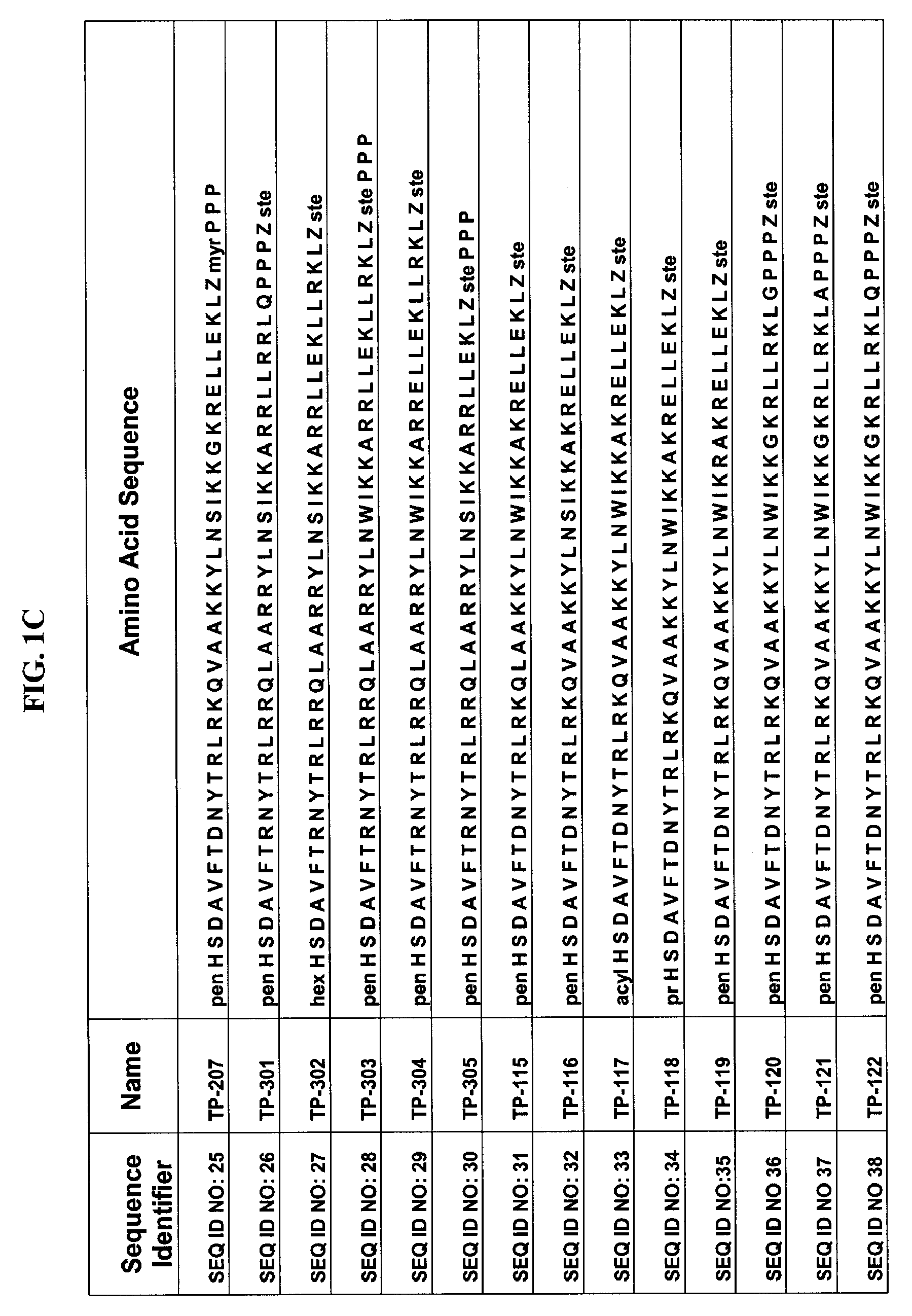
The invention discloses an antibacterial peptide VIP (vasoactive intestinal peptide)-like peptide. The antibacterial peptide VIP-like peptide is shown in the following amino acid sequence: HSKAVFTKNYTRLRKQMAVKKYLNSILN, and the invention also provides a preparation method of the antibacterial peptide VIP-like peptide. The antibacterial peptide VIP-like peptide has the advantages that a production technology is simple, production cost is low, antimicrobial activity is strong, antibacterial spectrum is broad, market prospect is broad, and the defects of complex structure, low antibacterial activity and narrow antibacterial spectrum in the prior art are overcome.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Use of pituitary adenylate cyclase-activating polypeptide (PACAP) and pacap analogs as adjunctive treatments with anticancer agents
InactiveUS20110268789A1Protecting the major organs of the bodyEffective protectionBiocidePowder deliveryVIP ReceptorsAnticarcinogen
This invention relates to methods and compositions for the treatment, management or prevention of injuries to one or more of the organs of the body, such as the brain, heart, lung, kidneys, liver, and gastrointestinal tract, of humans or other mammals caused by one or more anticancer agents. The methods of this invention consist of the administration of an effective amount of one or more pituitary adenylate cyclase-activating polypeptide (PACAP)-like compounds, which includes native human PACAP38, native human PACAP27, native human vasoactive intestinal peptide (VIP), their agonists, analogs, fragments, and derivatives, with activities toward one or more of the PACAP / VIP receptors, including all of their various isoforms. This invention also provides pharmaceutical compositions of one or more PACAP-like compounds of the invention either alone or in combination with one or more other prophylactic / therapeutic agents useful for the treatment, management or prevention of injuries to the organs of the body of humans or other mammals undergoing cancer chemotherapy. Combination therapy with one or more PACAP-like compounds plus one or more anticancer agents can be used in the treatment of hematological cancers.
Owner:TULANE EDUCATIONAL FUND
Compositions and methods for treatment of cardiovascular disease
ActiveUS7951777B2Sure easyEasy to measureHormone peptidesPeptide/protein ingredientsMyofibrosisVasoactive intestinal peptide
The present invention is concerned with composition and methods for treatment of certain cardiovascular conditions. In particular it is concerned with prophylactic or therapeutic treatment of myocardial fibrosis or associated conditions by administering compositions comprising vasoactive intestinal peptide (VIP) and / or active fragments) thereof.
Owner:VECTUS BIOSYSTEMS PTY LTD
Vasoactive intestinal polypeptide compositions
InactiveUS20090170775A1Facilitate increased duration of actionProlong the action timePeptide/protein ingredientsMetabolism disorderVasoactive intestinal peptideObesity
Pharmaceutical compositions relating to vasoactive intestinal polypeptides and methods for the treatment of metabolic disorders, including diabetes, insulin resistance, metabolic acidosis and obesity are presented. Methods of using the vasoactive intestinal polypeptide compositions are also disclosed.
Owner:TRANSITION THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com