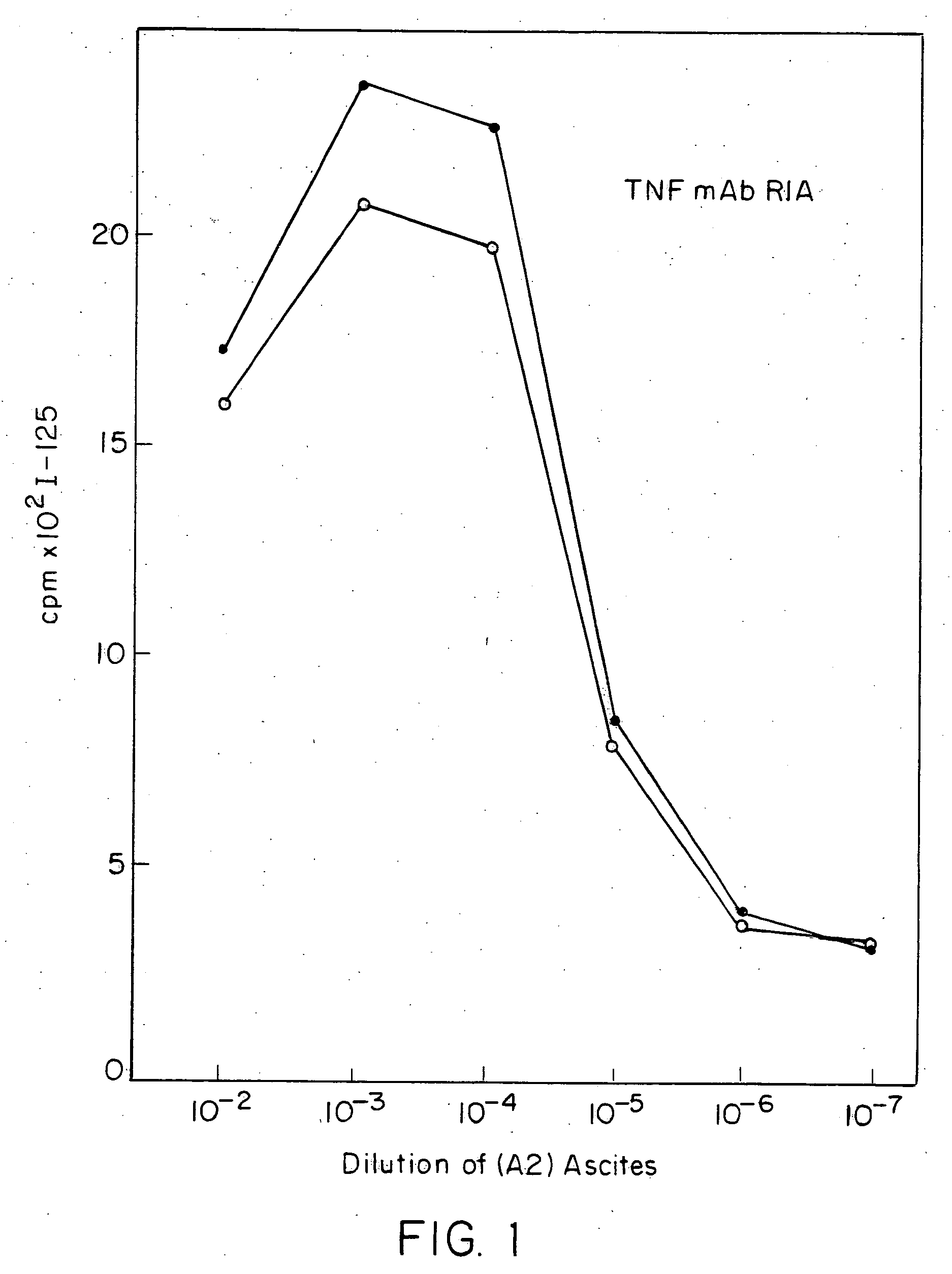
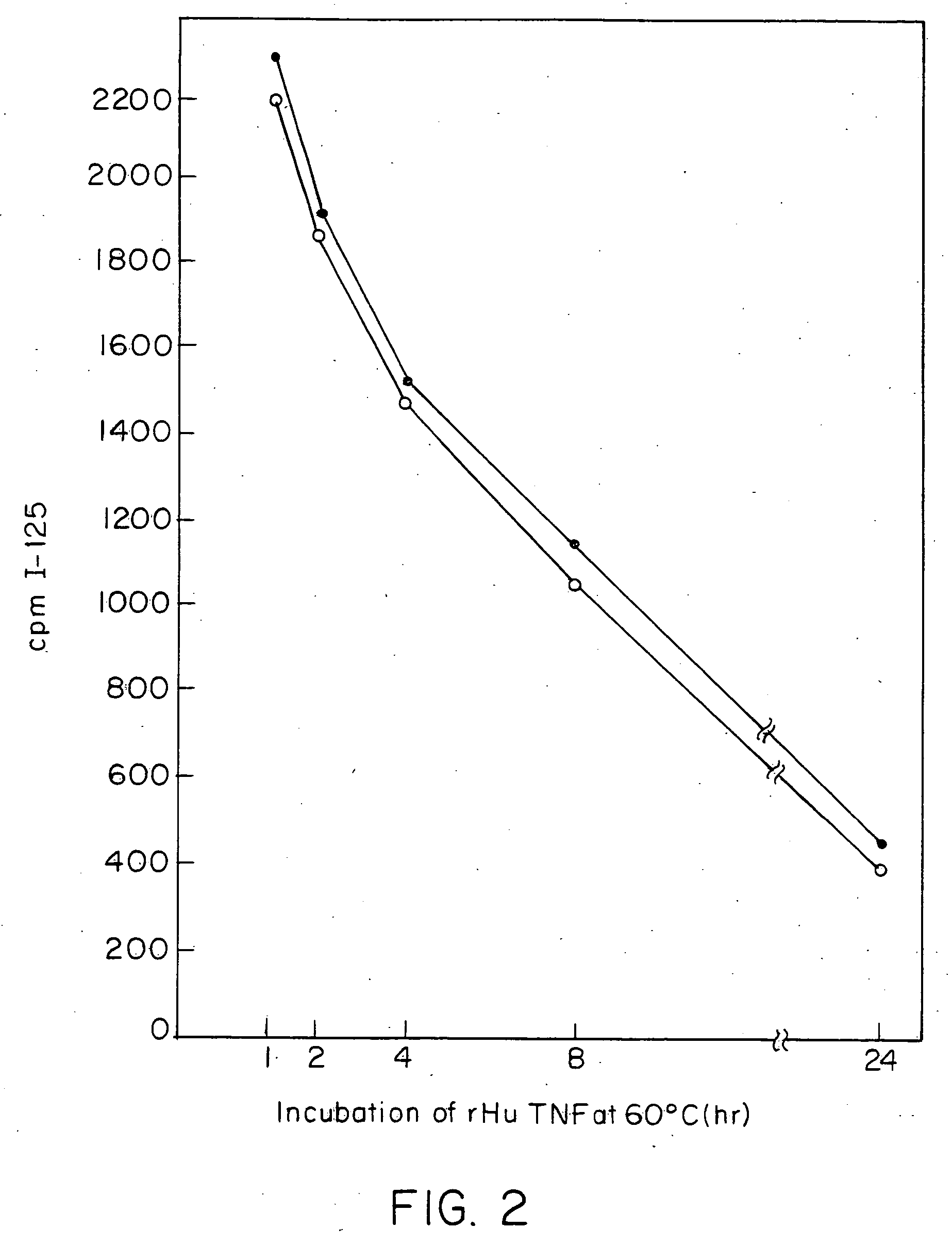
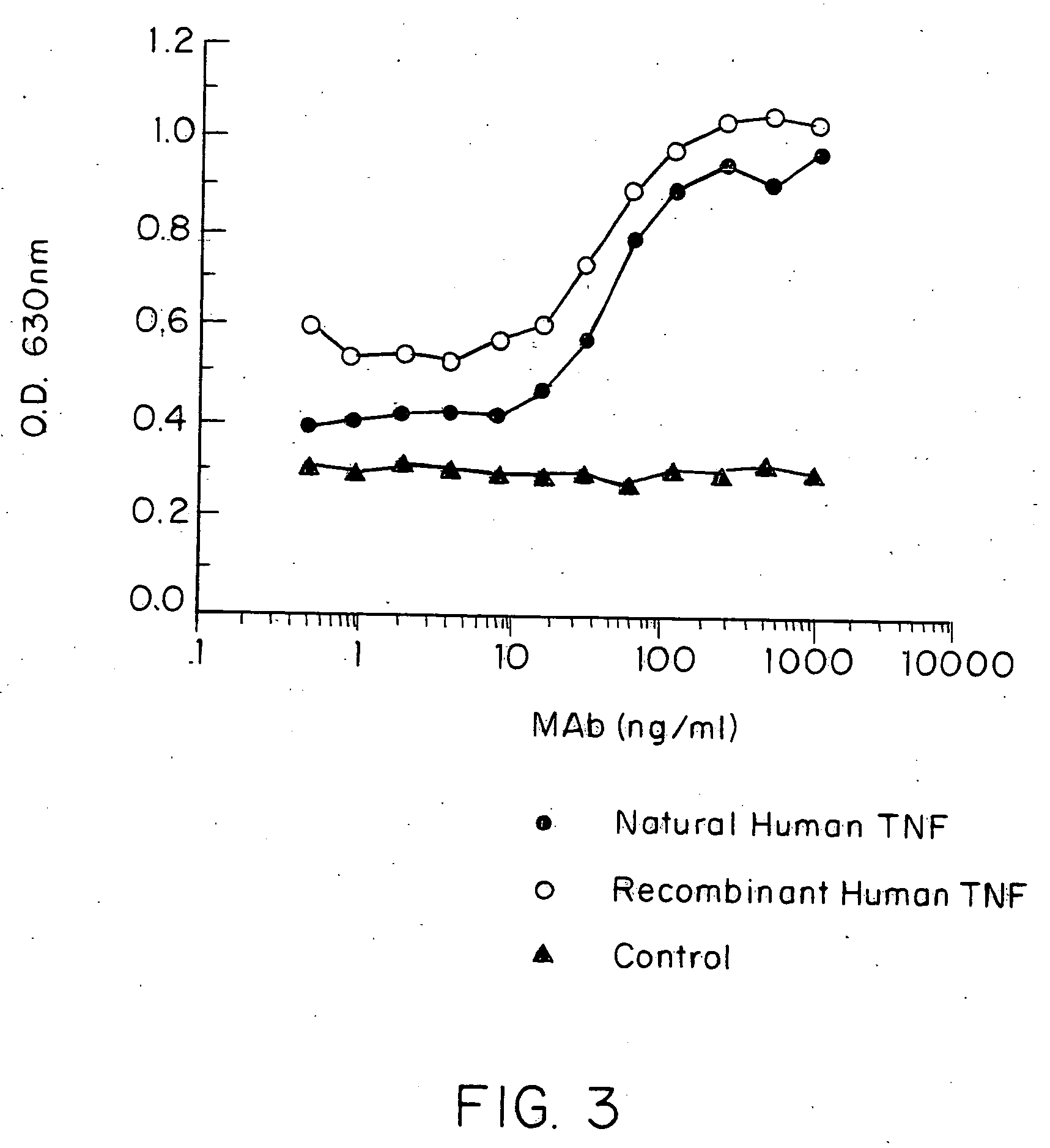
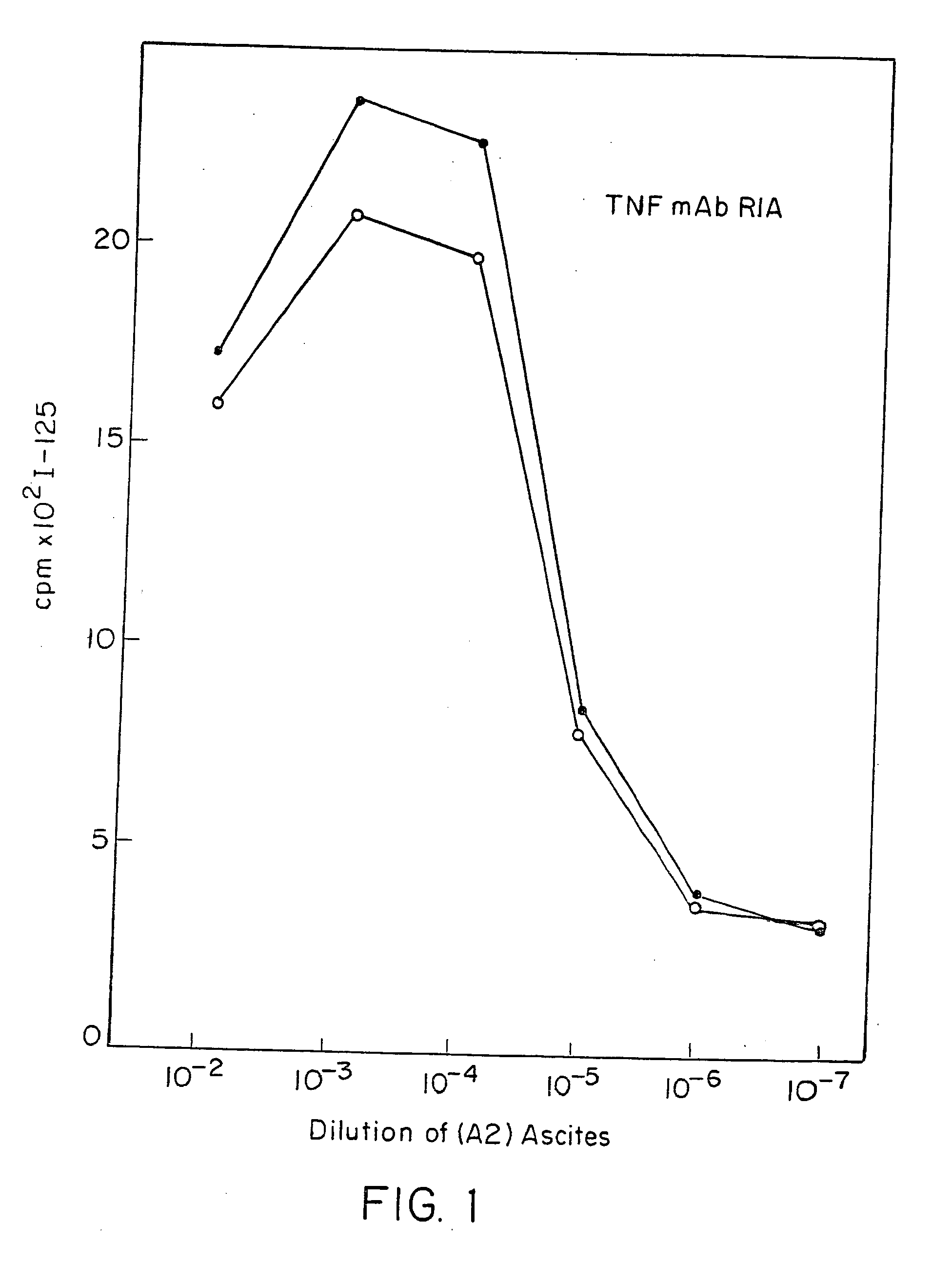
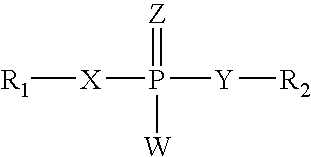Patents
Literature
872 results about "Tumor necrosis factors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The tumor necrosis factor (TNF) superfamily is a protein superfamily of type II transmembrane proteins containing TNF homology domain and forming trimers. Members of this superfamily can be released from the cell membrane by extracellular proteolytic cleavage and function as a cytokine. These proteins are expressed predominantly by immune cells and they regulate diverse cell functions, including immune response and inflammation, but also proliferation, differentiation, apoptosis and embryogenesis.
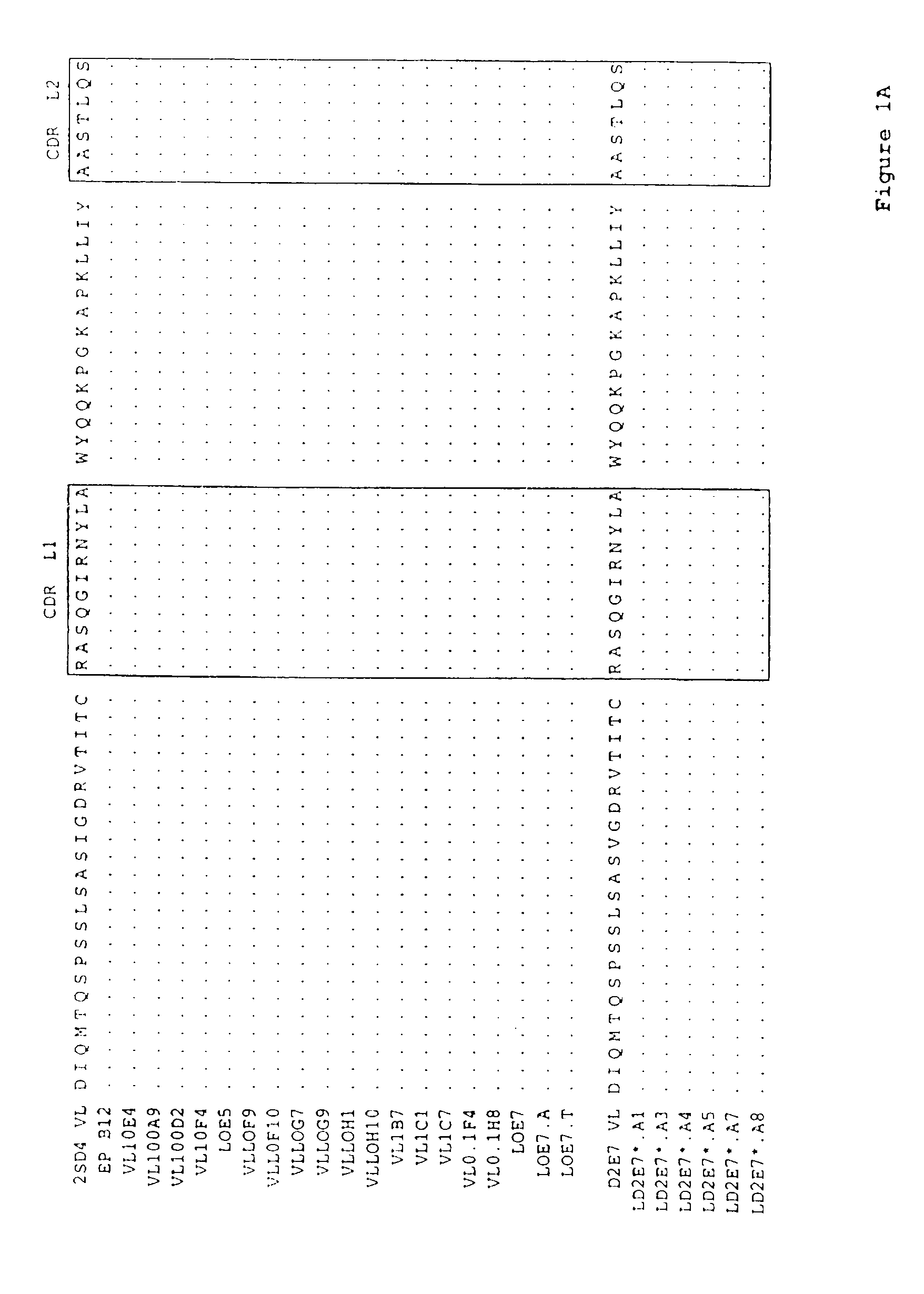
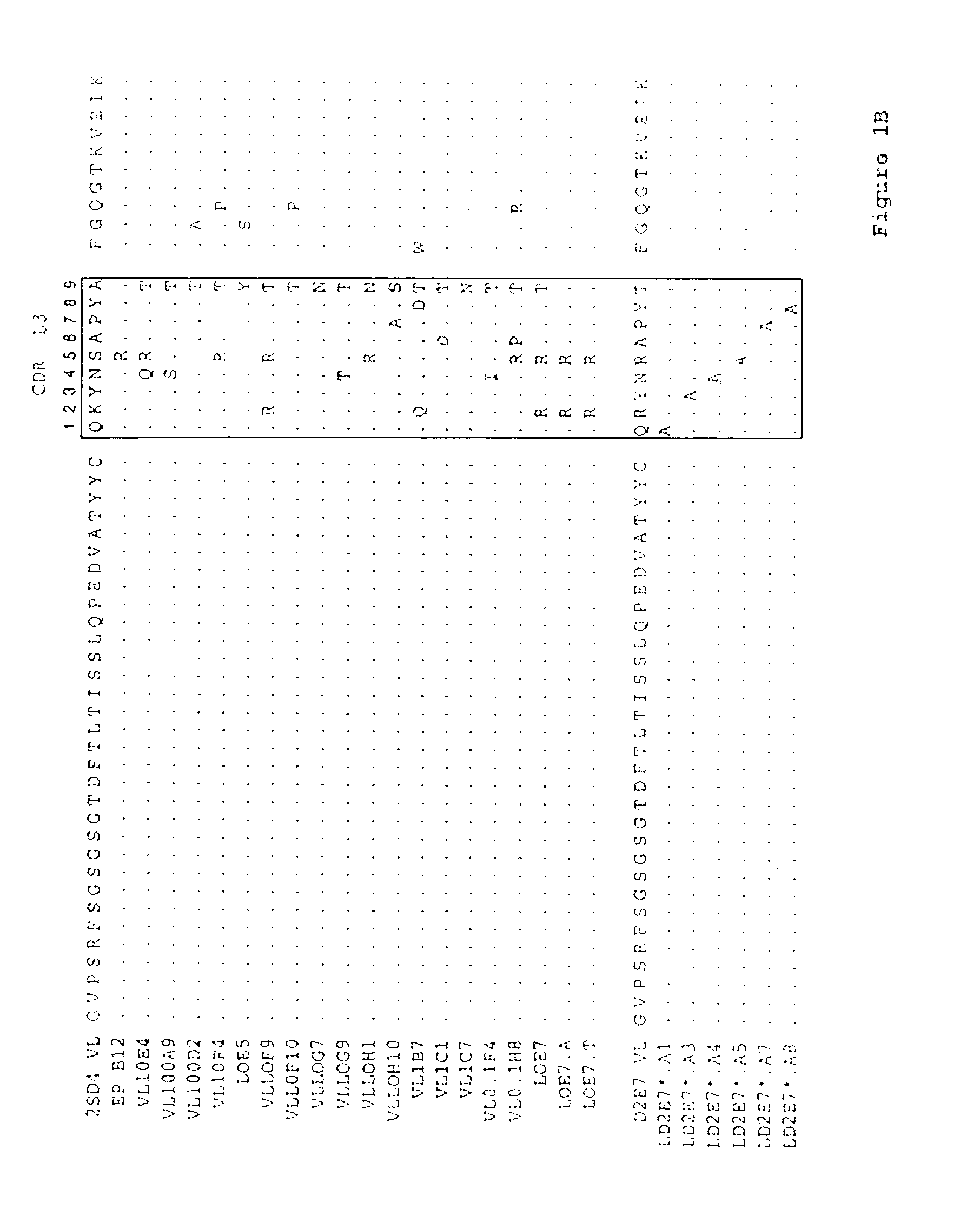
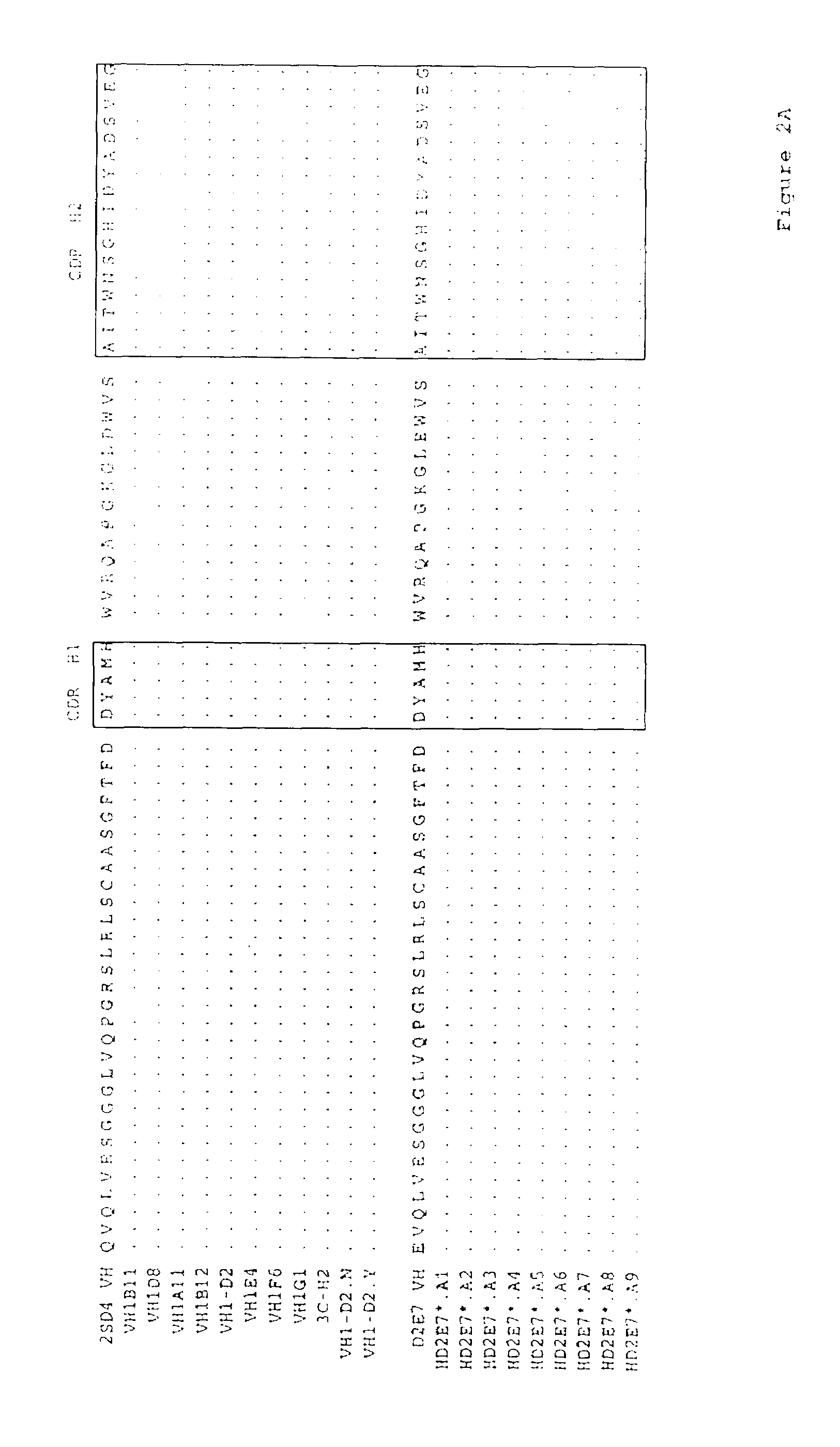
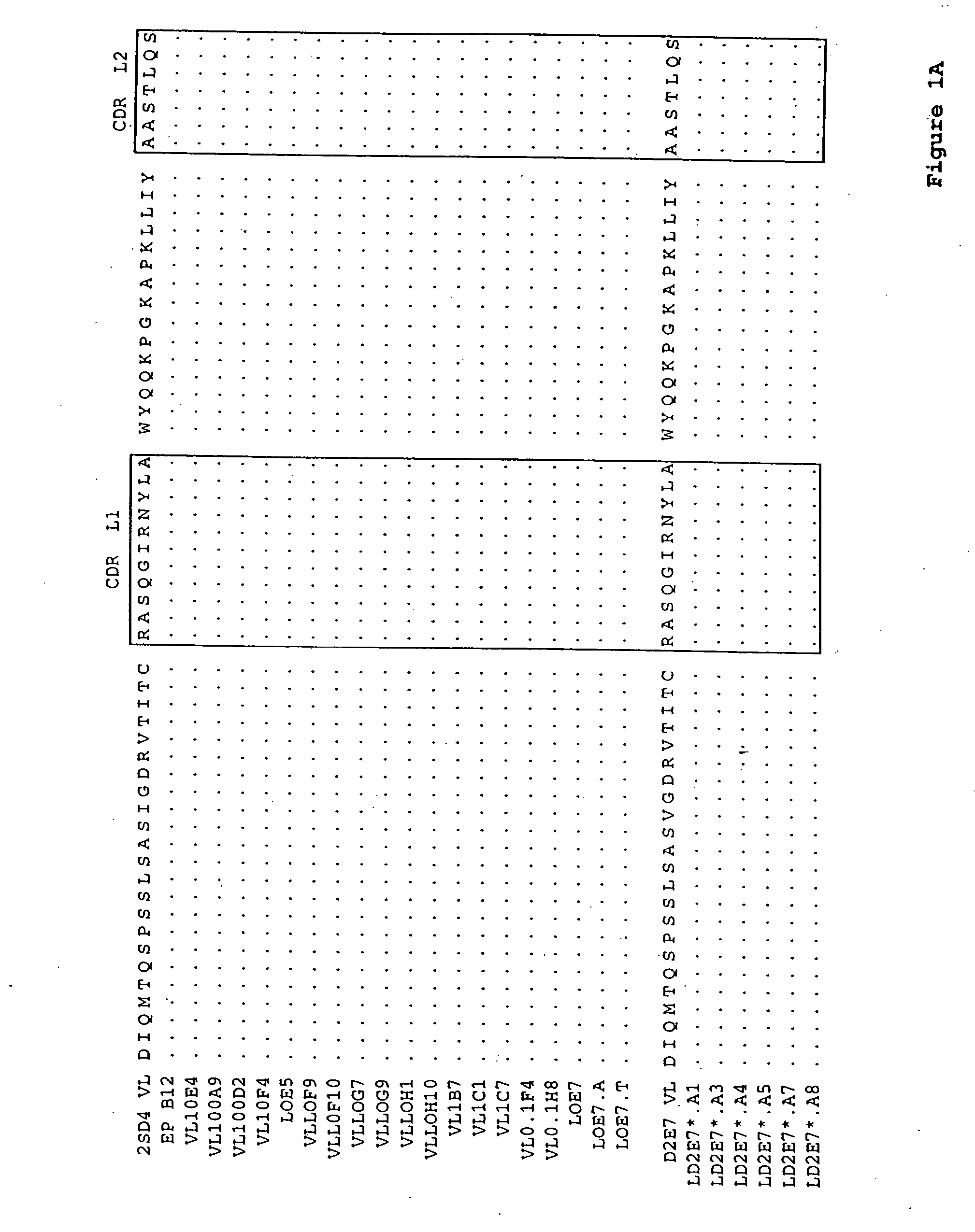
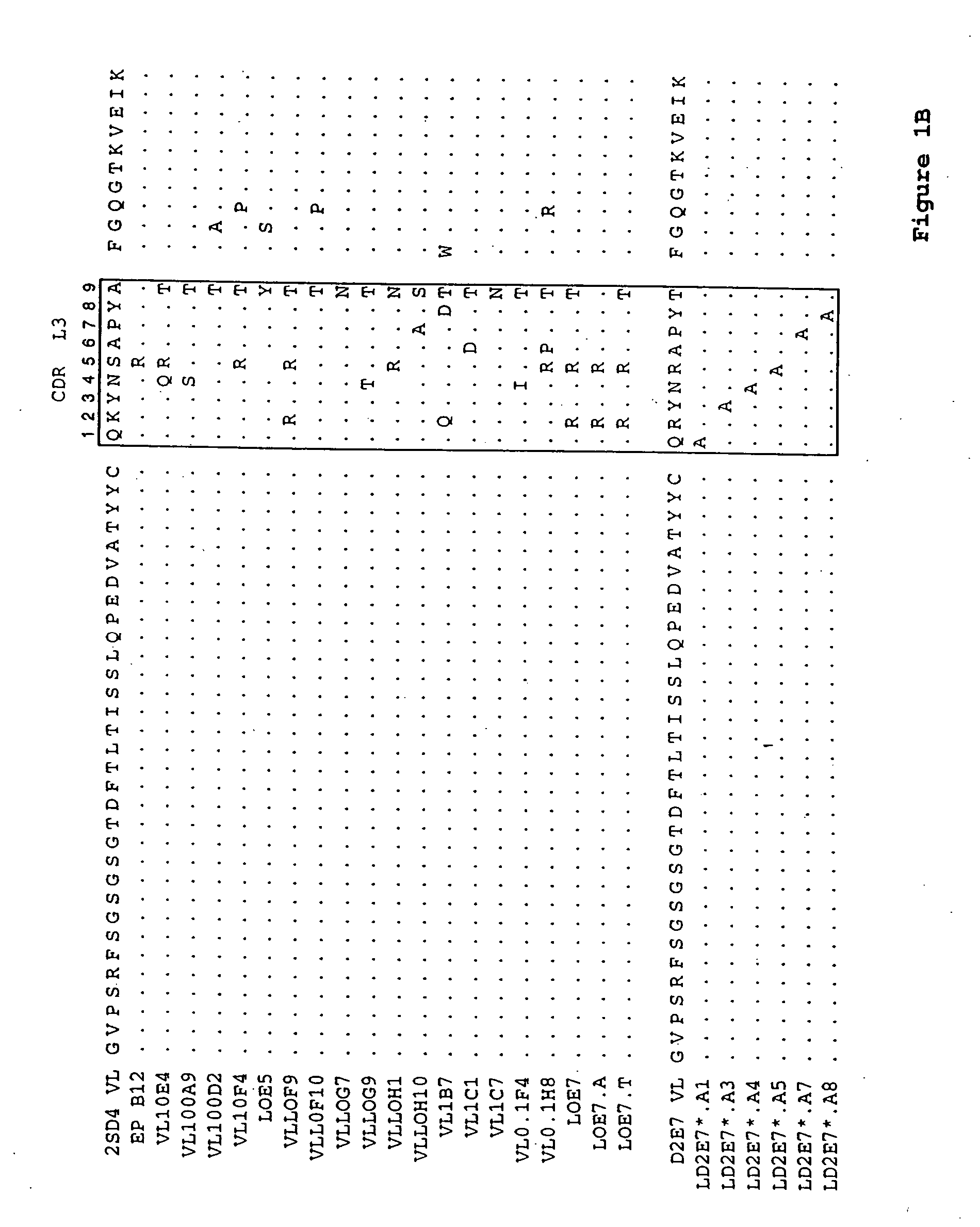
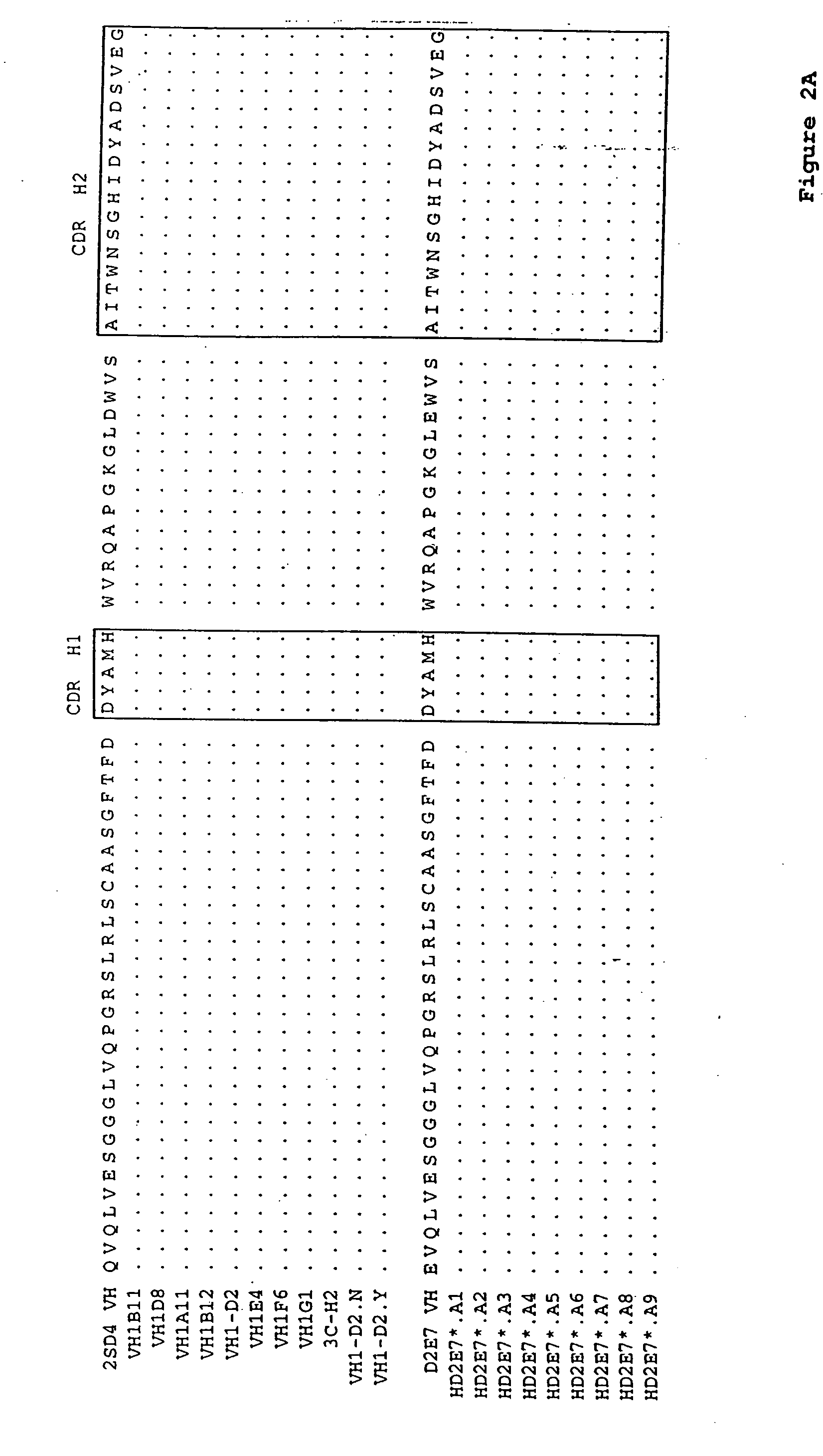
Human antibodies that bind human TNFα
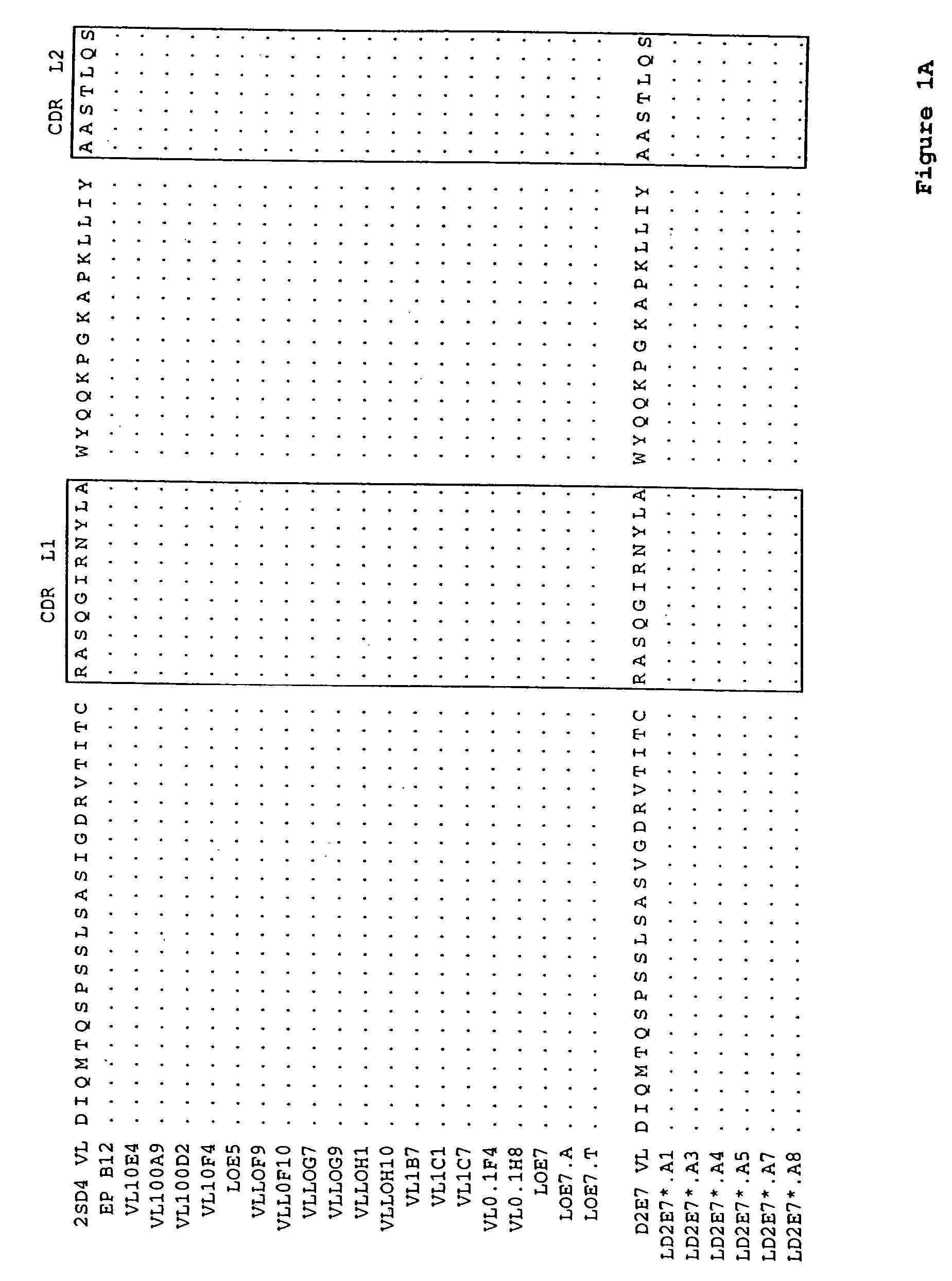
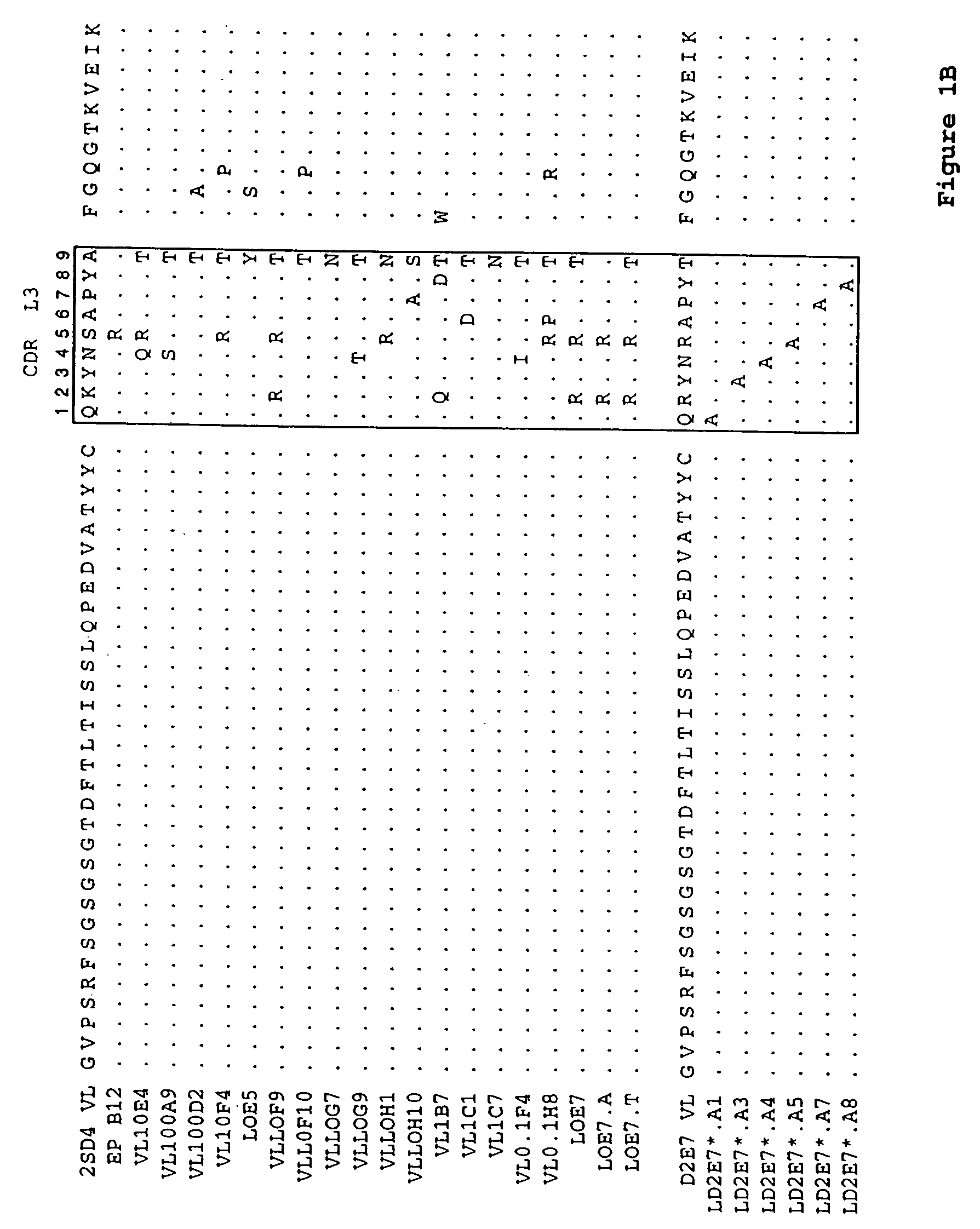
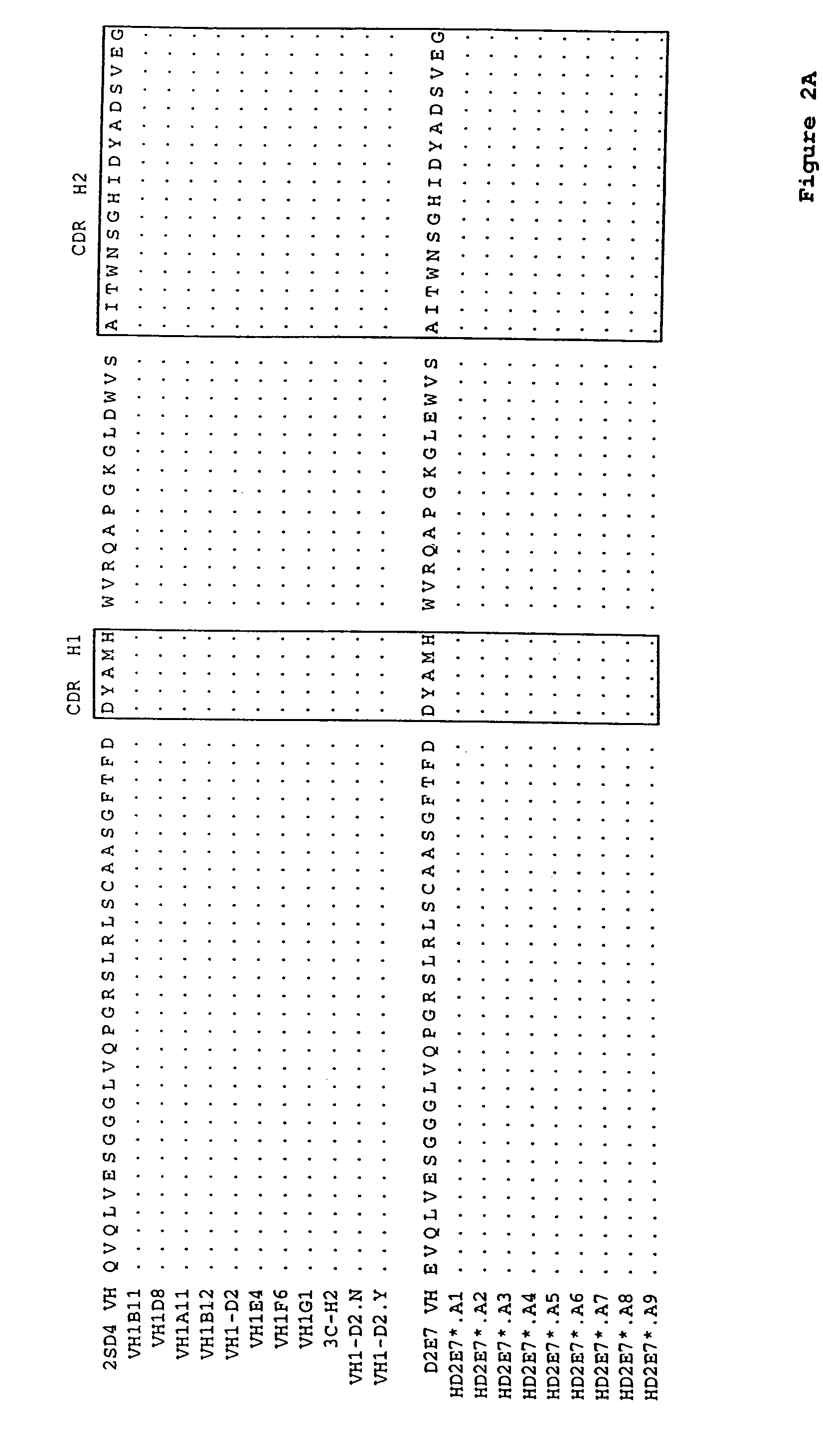
Human antibodies, preferably recombinant human antibodies, that specifically bind to human tumor necrosis factor a (hTNFα) are disclosed. These antibodies have high affinity for hTNFα (e.g., Kd=10−8 M or less), a slow off rate for hTNFα dissociation (e.g., Koff=10−3 sec−1 or less) and neutralize hTNFα activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hTNFα and for inhibiting hTNFα activity, e.g., in a human subject suffering from a disorder in which hTNFα activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE BIOTECHNOLOGY LTD
Tumor necrosis factor peptide binding antibodies
InactiveUS6593458B1Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDrug biological activityAntibody
Provided are isolated antibodies or fragments thereof which bind a peptide consisting of residues Leu63-Phe64-Lys65-Gly66-Gln67-Gly68-Cys69-Pro70-Ser71-Thr72-His73-Val74-Leu75-Leu76-Thr77-His78-Thr79-Ile80-Ser81-Arg82-Ile83 (peptide 304) of mature human TNF-alpha. The antibodies and fragments thereof may be used to identify antibodies capable of binding in the region of mature human TNF-alpha of amino acid resides 63-83. Antibodies or fragments thereof which bind particular regions of mature human TNF-alpha are shown to elicit particular biological activities dependent upon the particular region wherein binding occurs.
Owner:ARANA THERAPEUTIC LTD
RNA interference mediated inhibition of TNF and TNF receptor gene expression using short interfering nucleic acid (siNA)
InactiveUS20050227935A1Improves various propertyImprove the immunitySugar derivativesGenetic material ingredientsTumor necrosis factor receptorDouble strand
This invention relates to compounds, compositions, and methods useful for modulating tumor necrosis factor and / or tumor necrosis factor receptor gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of tumor necrosis factor and / or tumor necrosis factor receptor gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of tumor necrosis factor and / or tumor necrosis factor receptor genes, (TNF and / or TNF receptor).
Owner:SIRNA THERAPEUTICS INC
Human antibodies that bind human TNFalpha
Owner:ABBVIE BIOTECHNOLOGY LTD
Anti-TNF antibodies and peptides of human tumor necrosis factor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:CENTOCOR
Methods of treating ankylosing spondylitis using anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20050249735A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Methods of treating ankylosing spondylitis using anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20080025976A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:LE JUNMING +6
Anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20060018907A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Methods of treating seronegative arthropathy with anti-TNF antibodies
InactiveUS20070298040A1Inhibit biological activityHigh affinityAntibody ingredientsImmunoglobulinsSeronegative arthropathyHuman tumor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFαx-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
Anti-TNF antibodies and peptides of human tumor necrosis factor
InactiveUS20070196373A1Antibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsHuman tumorAnkylosing spondylitis
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-α (TNFα) and are useful in vivo diagnosis and therapy of a number of TNFα-mediated pathologies and conditions, including ankylosing spondylitis, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:LE JUNMING +6
DNA encoding tumor necrosis factor- alpha and - beta receptors
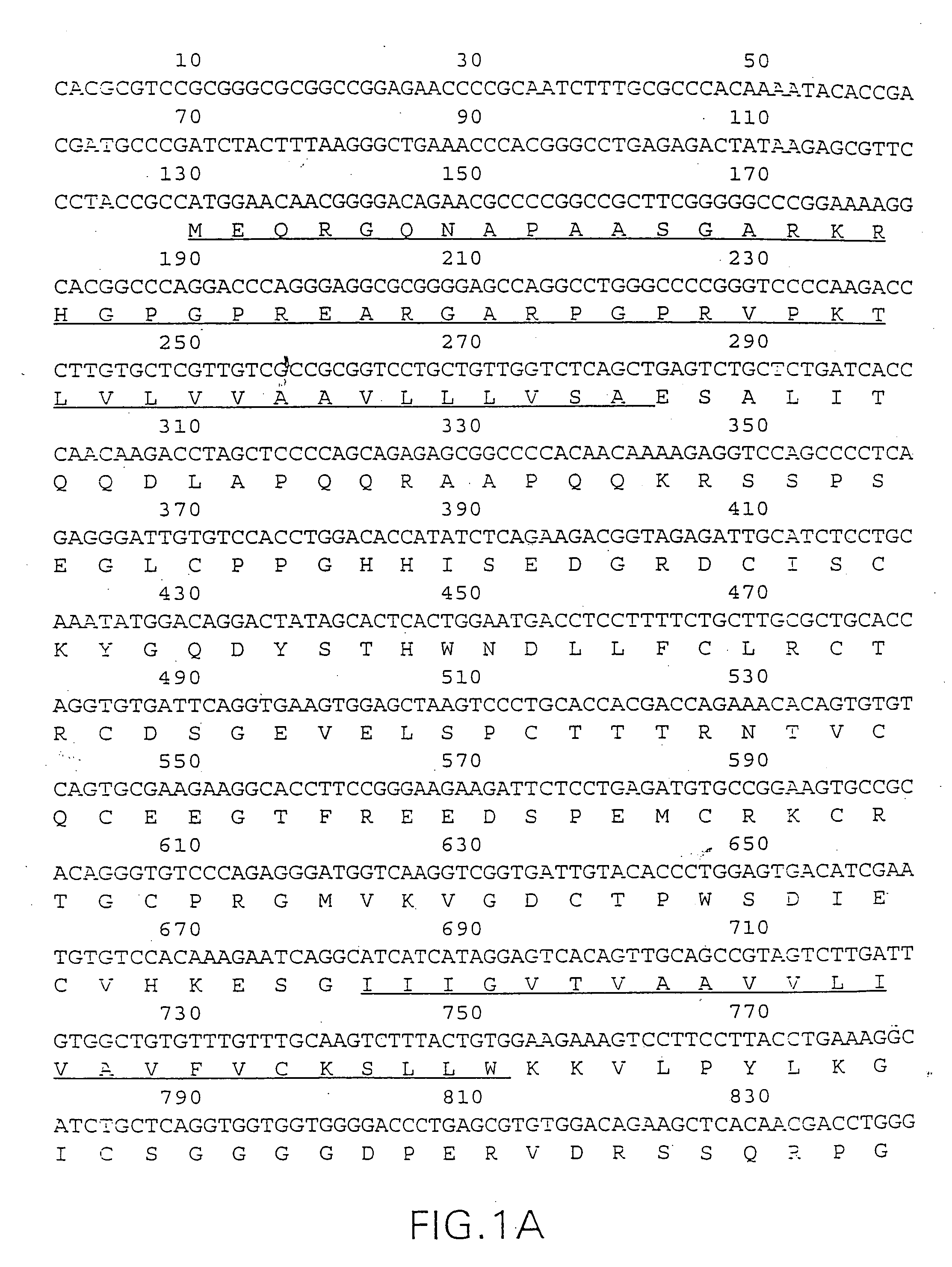
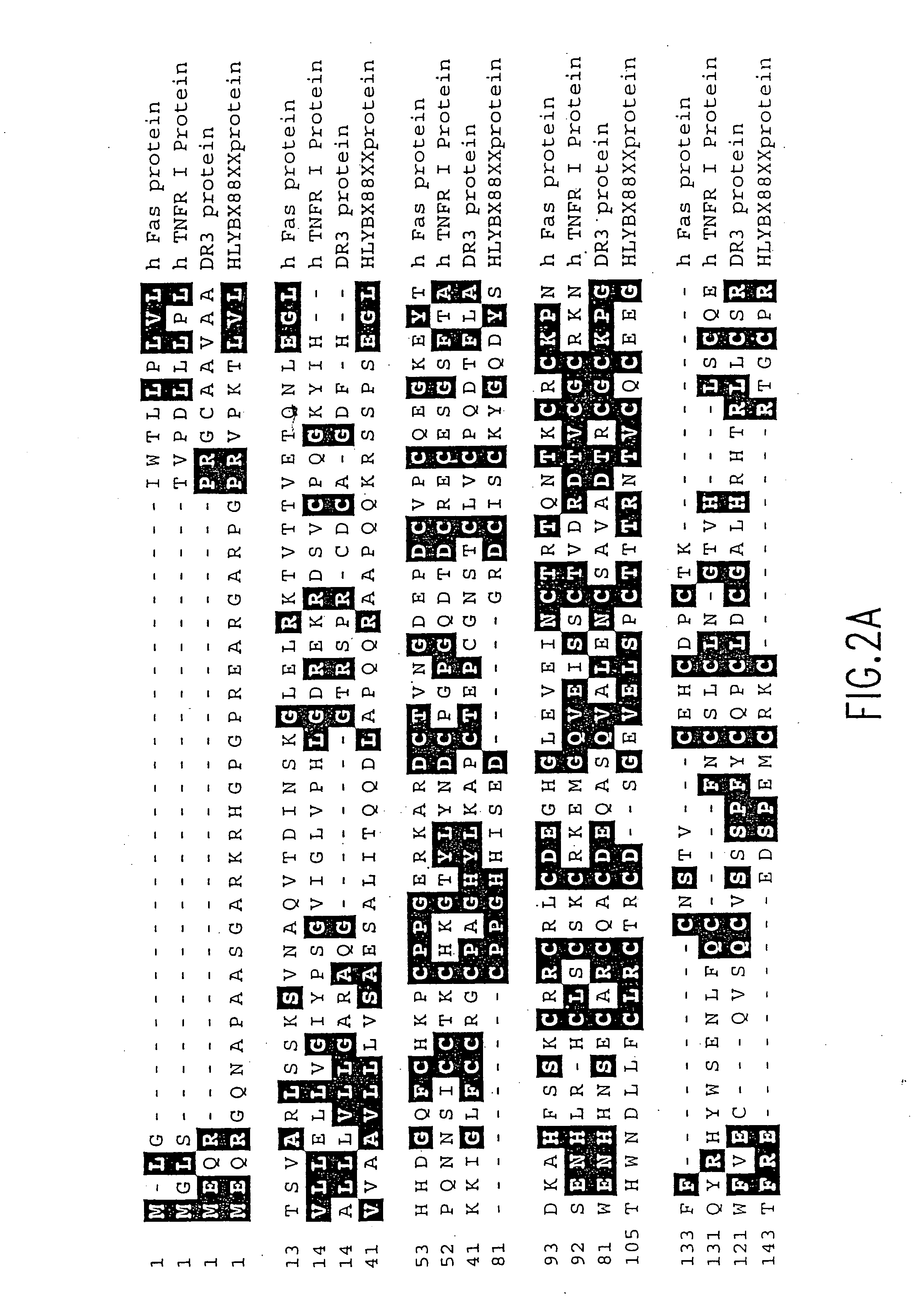
Tumor necrosis factor receptor DNAs and expression vectors encoding TNF receptors, and processes for producing TNF receptors as products of recombinant cell culture, are disclosed.
Owner:IMMUNEX CORP
Method for administering a cytokine to the central nervous system and the lymphatic system
InactiveUS6991785B2Provide effectModulate immune and inflammatory responseBiocideNervous disorderImmunologic disordersInterferon alpha
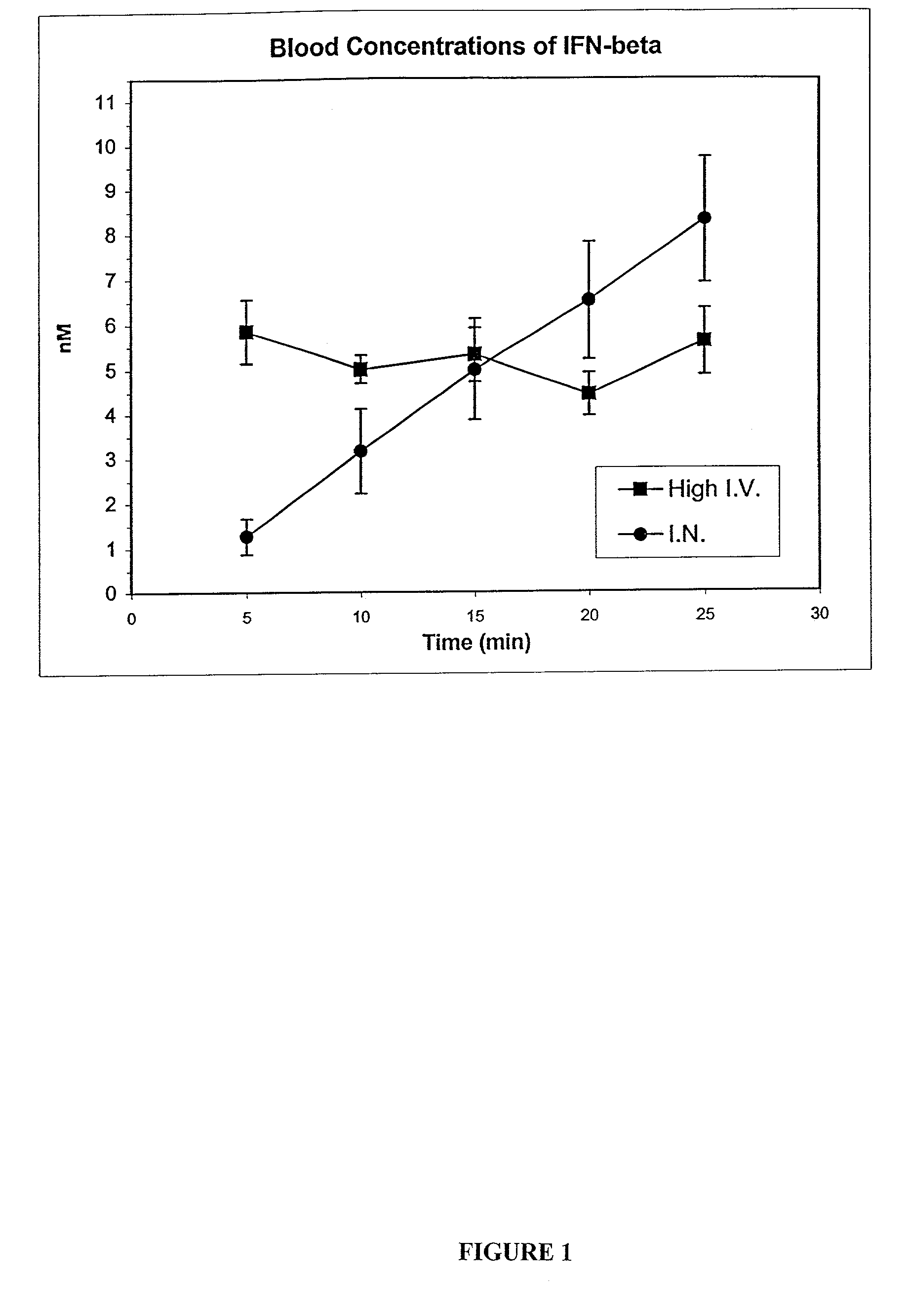
The present invention is directed to a method for delivering cytokines to the central nervous system and the lymphatic system by way of a tissue innervated by the trigeminal nerve and / or olfactory nerve. Cytokines include tumor necrosis factors, interleukins, interferons, particularly interferon-β and its muteins such as IFN-βser17. Such a method of delivery can be useful in the treatment of central nervous system disorders, brain disorders, proliferative, viral, and / or autoimmune disorders such as Sjogren's disorder.
Owner:CHIRON CORP
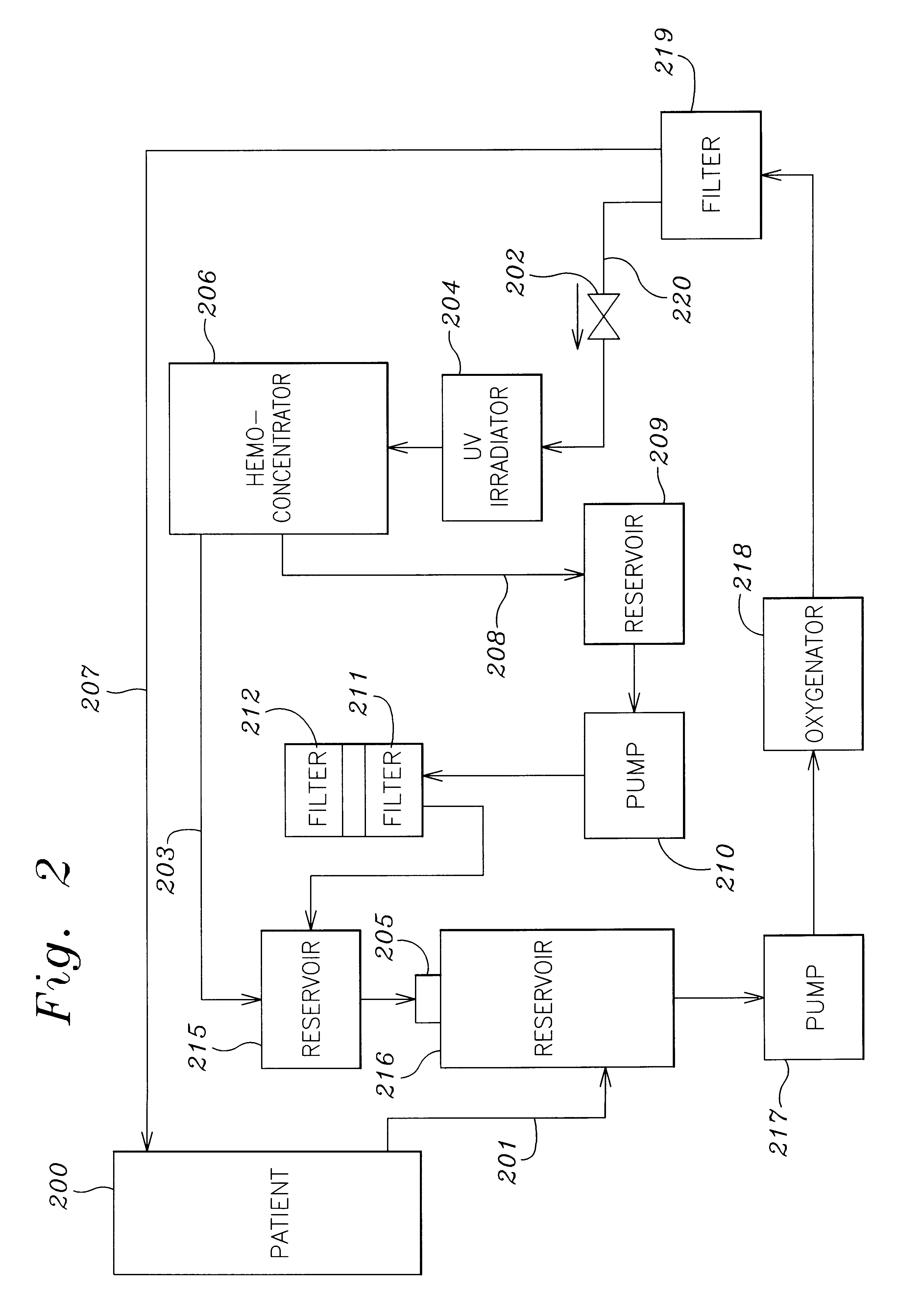
Septicemia prevention and treatment system
InactiveUS6193681B1Electrolysis componentsOther blood circulation devicesStaphylococcus cohniiFiltration
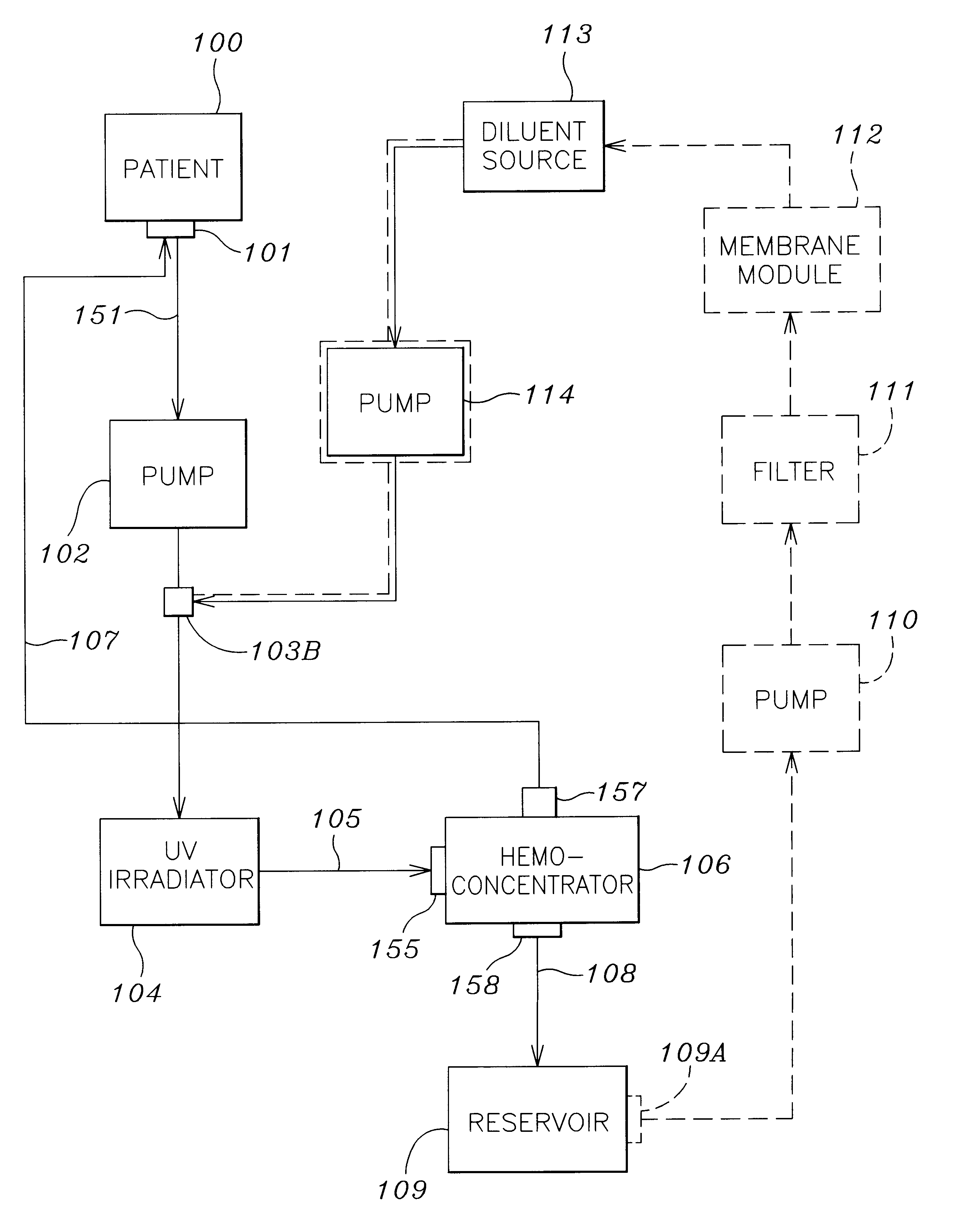
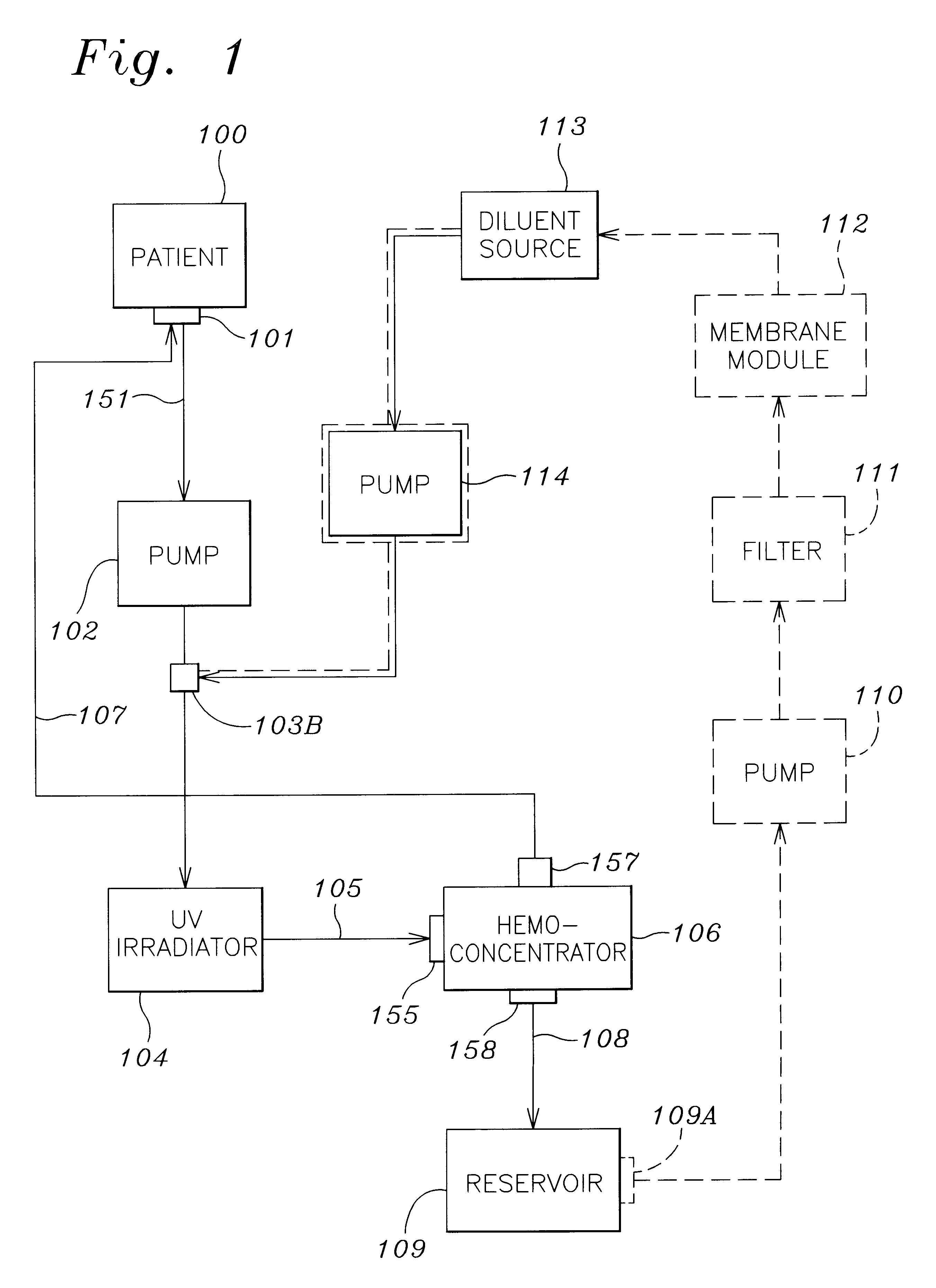
A method and apparatus for preventing and treating septicemia in patient blood. The extracorporeal system includes an anti-microbial device to kill at least 99% of bloodborne microorganisms, a hemoconcentrator / filtration unit to remove approximately 90% of target molecules from the patient blood and a filter unit to remove target molecules from patient blood from the sieved plasma filtrate. Target molecules are produced by microorganisms as well as the patient's cells and include endotoxins from gram negative bacteria, exotoxins from gram negative and gram positive bacteria, as well as RAP protein mediator from Staphylococcus aureus, and cell mediators such as tumor necrosis factor-alpha, and interleukin 1-beta, complement proteins C3a and C5a, and brandykinin.
Owner:HEMAVATION
Human antibodies that bind human TNFalpha
InactiveUS20060024293A1High affinitySlow dissociation kineticsAntibacterial agentsOrganic active ingredientsHuman tumorAntigen binding
Human antibodies, preferably recombinant human antibodies, that specifically bind to human tumor necrosis factor α (hTNFα) are disclosed. These antibodies have high affinity for hTNFα (e.g., Kd=10−8 M or less), a slow off rate for hTNFα dissociation (e.g., Koff=10−3 sec−1 or less) and neutralize hTNFα activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hTNFα and for inhibiting hTNFα activity, e.g., in a human subject suffering from a disorder in which hTNFα activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE BIOTECHNOLOGY LTD
Cytokine antagonists for neurological and neuropsychiatric disorders
Methods for treating neurological or neuropsychiatric diseases or disorders in humans by administering to the human a therapeutically effective dose of specific biologics are presented. The biologics of consideration include antagonists of tumor necrosis factor or of interleukin-1. The administration of these biologics is performed by specific methods, most, but not all of which fall into the category of anatomically localized administration designed for perispinal use. Anatomically localized administration involving perispinal use includes, but is not limited to the subcutaneous, intramuscular, interspinous, epidural, peridural, parenteral or intrathecal routes. Additonally, intranasal administration is discussed as a method to provide therapeutic benefit. The clinical conditions of consideration include, but are not limited to the following: diseases of the brain, including neurodegenerative diseases such as Alzheimer's Disease and Parkinson's Disease; migraine headache; spinal radiculopathy associated with intervertebral disc herniation, post-herpetic neuralgia, reflex sympathethic dystrophy, neuropathic pain, vertebral disc disease, low back pain, amyotrophic lateral sclerosis, chronic fatigue syndrome; and neuropsychiatric diseases, including bipolar affective disorder, anorexia nervosa, nicotine withdrawal, narcotic addiction, alcohol withdrawl, postpartum depression, and schizoaffective illness.
Owner:TACT IP
Methods and compositions for modulating cell proliferation and cell death
InactiveUS6599912B1Enhance in vitroImprove in vivo activityBiocidePeptide/protein ingredientsAnticarcinogenTopoisomerase-II Inhibitor
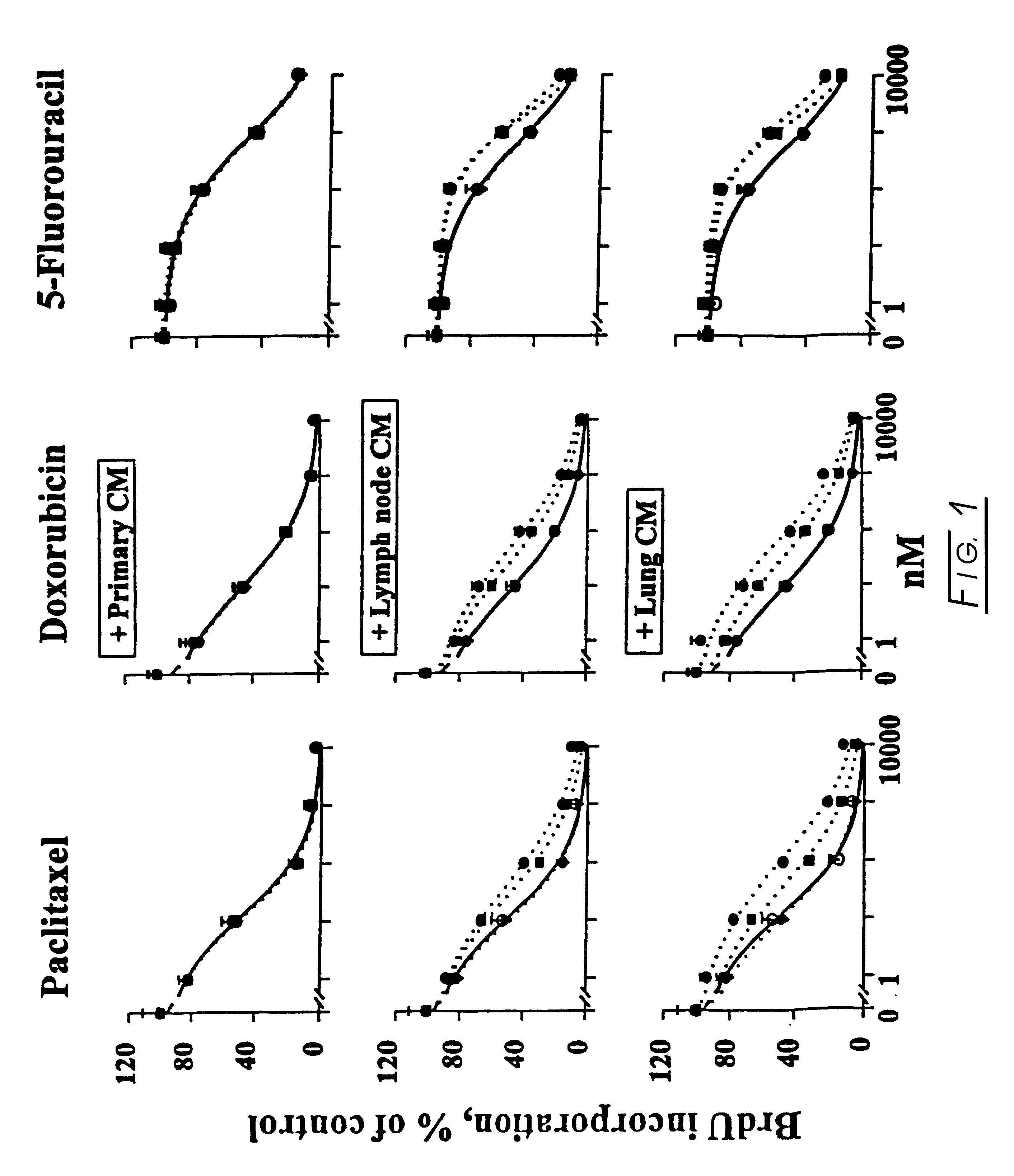
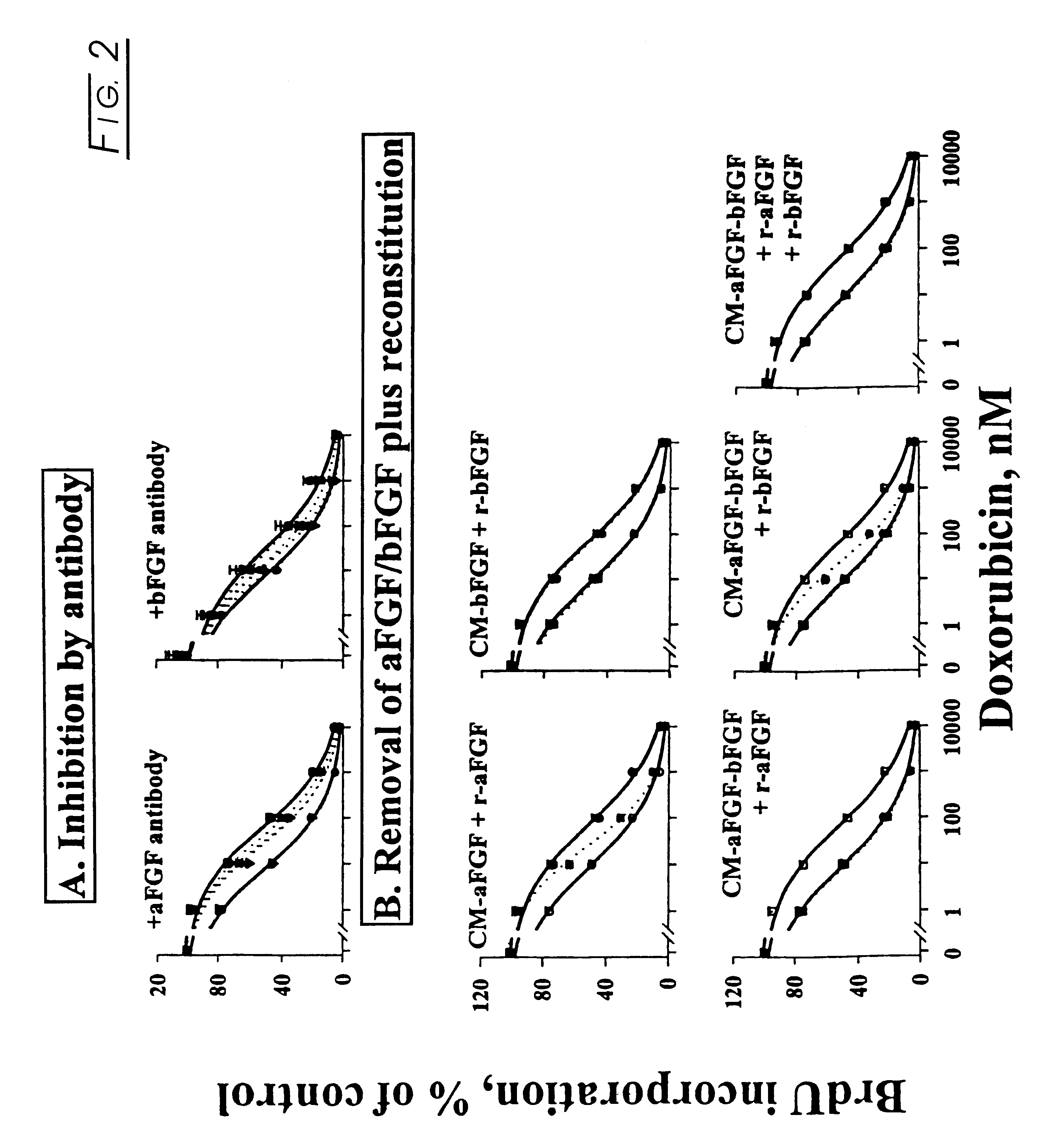
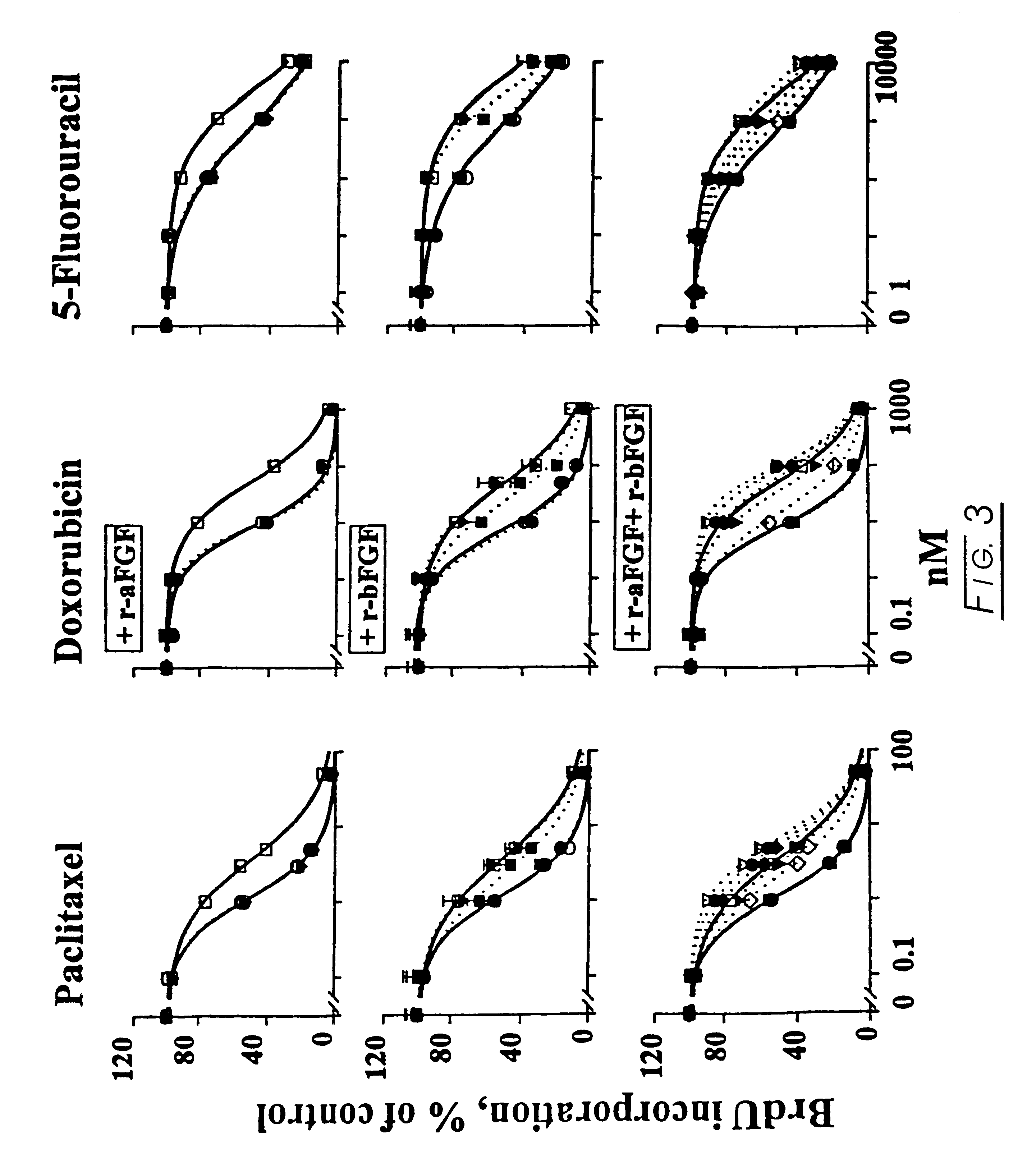
Methods and compositions for modulating the FGF effect on the sensitivity of malignant and normal cells to anticancer agents are provided. In particular, methods and compositions for inhibiting FGF-induced resistance to a broad spectrum of anticancer agents in solid and soft-tissue tumors, metastatic lesions, leukemia and lymphoma are provided. Preferably, the compositions include at least one FGF inhibitor in combination with a cytotoxic agents, e.g., antimicrotubule agents, topoisomerase I inhibitors, topoisomerase II inhibitors, antimetabolites, mitotic inhibitors, alkylating agents, intercalating agents, agents capable of interfering with a signal transduction pathway (e.g., g., a protein kinase C inhibitor, e.g., an anti-hormone, e.g., an antibody against growth factor receptors), an agent that promotes apoptosis and / or necrosis, and interferon, an interleukin, a tumor necrosis factor, and radiation. In other embodiments, methods and composition for protecting a cell in a subject, from one or more of killing, inhibition of growth or division or other damage caused, e.g., by a cytotoxic agent, are provided. Preferably, the method includes: administering, to the subject, an effective amount of at least one FGF agonist, thereby treating the cell, e.g., protecting or reducing the damage to the dividing cell from said cytotoxic agent.
Owner:AU JESSIE L S +1
Benzenesulfonamide inhibitors of PDE-IV and their therapeutic use
InactiveUS6162830ASimple structureSynthetic is simpleBiocideNervous disorderDiseasePhosphodiesterase
PCT No. PCT / US98 / 23482 Sec. 371 Date Feb. 7, 2000 Sec. 102(e) Date Feb. 7, 2000 PCT Filed Nov. 4, 1998 PCT Pub. No. WO99 / 26616 PCT Pub. Date Jun. 3, 1999The present invention provides compounds and pharmaceutical compositions thereof, and methods of using same in the treatment of diseases whose treatment benefits from the inhibition of phosphodiesterase (PDE-IV) or Tumor Necrosis Factor (TNF) including asthma, allergic diseases, rheumatoid arthritis, osteoarthritis, septic shock. The compounds provided by this invention have formula (I) wherein R1, R2, R3, R4 are as defined herein.
Owner:WARNER-LAMBERT CO
Soluble TNF receptors and their use in treatment of disease
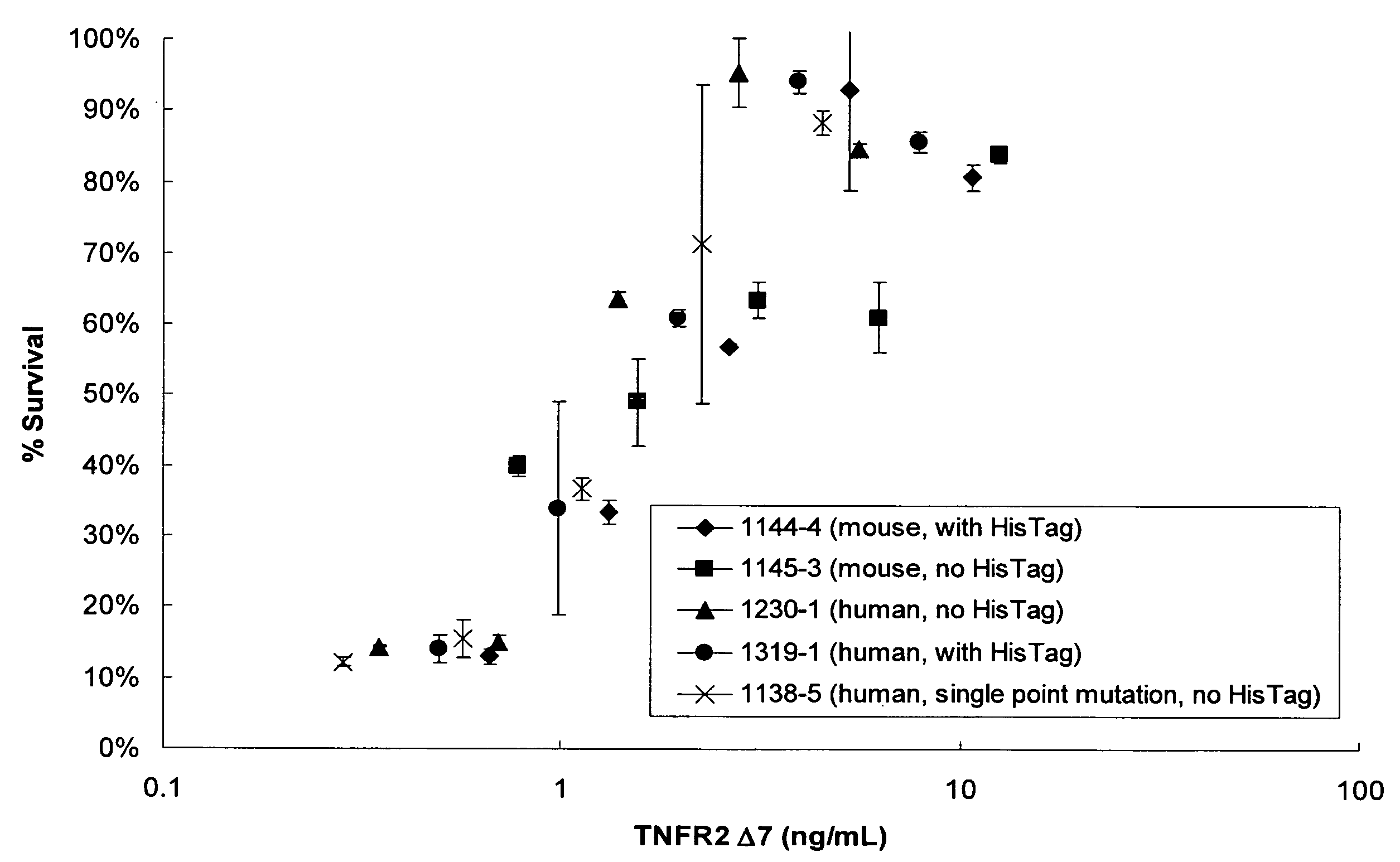
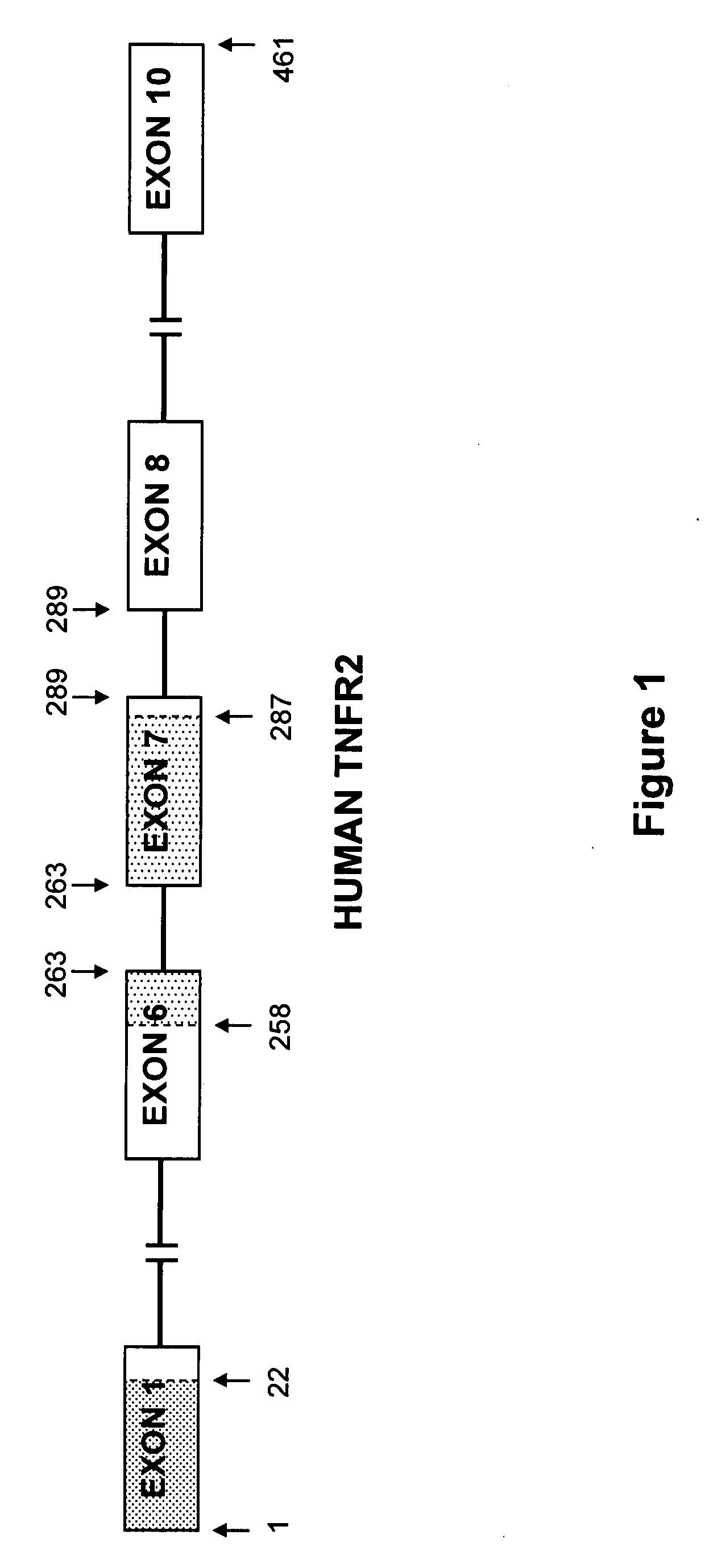
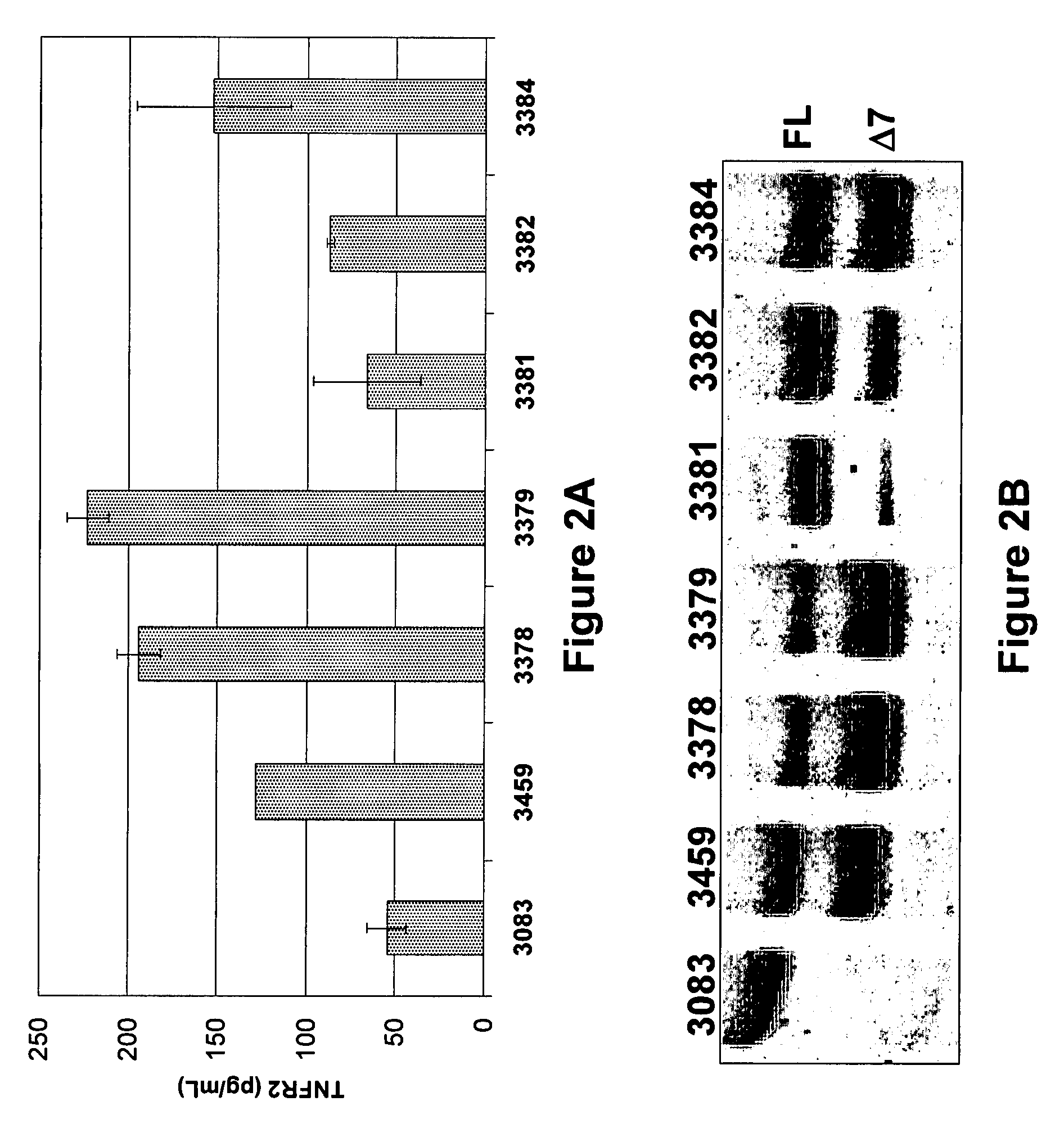
The present invention relates to tumor necrosis factor (TNF) antagonists and corresponding nucleic acids derived from tumor necrosis factor receptors (TNFRs) and their use in the treatment of inflammatory diseases. These proteins are soluble secreted decoy receptors that bind to TNF and prevent TNF from signaling to cells. In particular, the proteins are mammalian TNFRs that lack exon 7 and which can bind TNF and can act as a TNF antagonist.
Owner:NORTH CAROLINA AT CHAPEL HILL THE UNIV OF +2
Death domain containing receptor 5
The present invention relates to novel Death Domain Containing Receptor-5 (DR5) proteins which are members of the tumor necrosis factor (TNF) receptor family, and have now been shown to bind TRAIL. In particular, isolated nucleic acid molecules are provided encoding the human DR5 proteins. DR5 polypeptides are also provided as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying antagonists and antagonists of DR5 activity. The invention also relates to the treatment of diseases associated with reduced or increased levels of apoptosis using antibodies specific for DR5, which maybe agonists and / or antagonists of DR5 activity.
Owner:HUMAN GENOME SCI INC
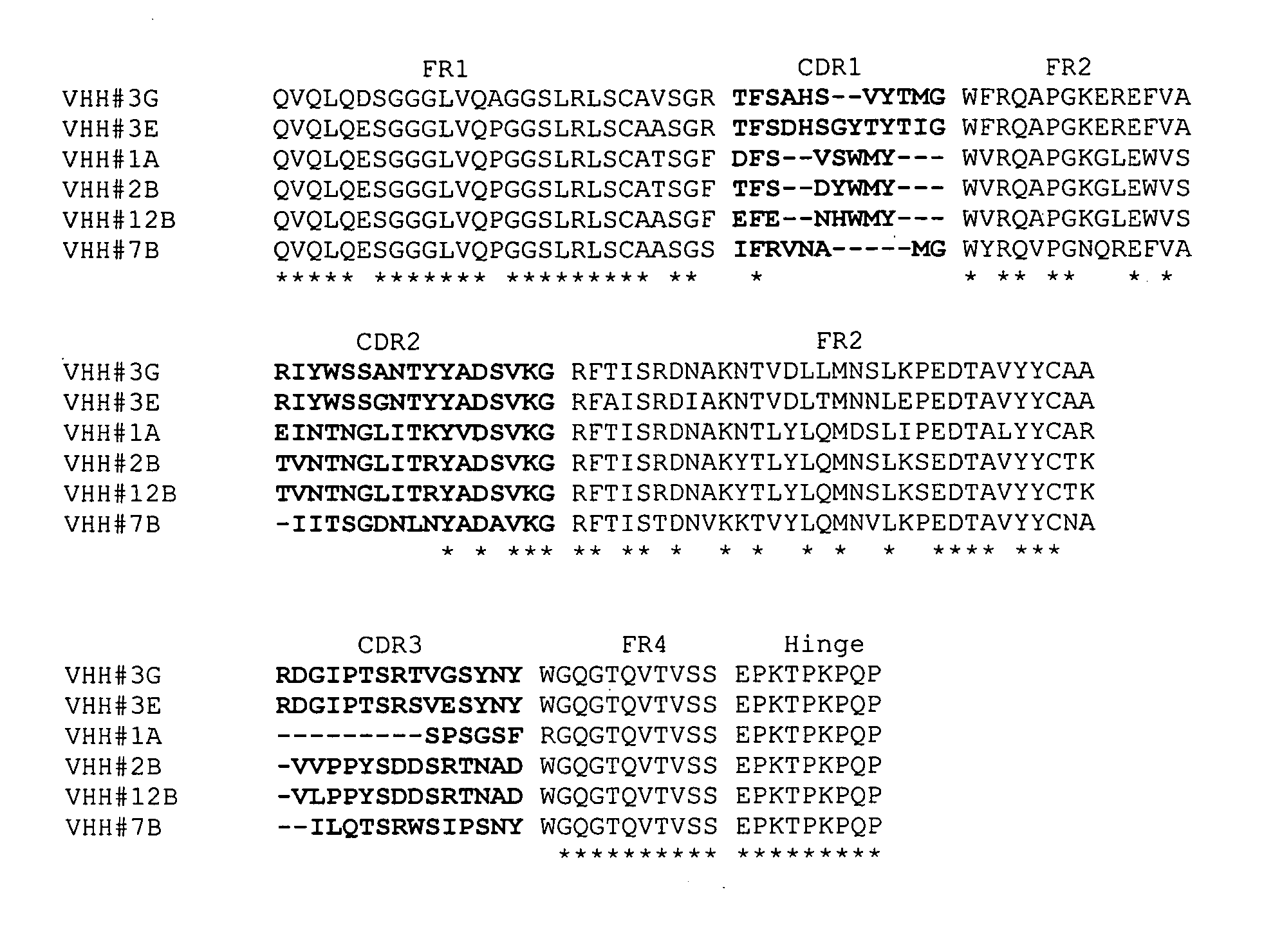
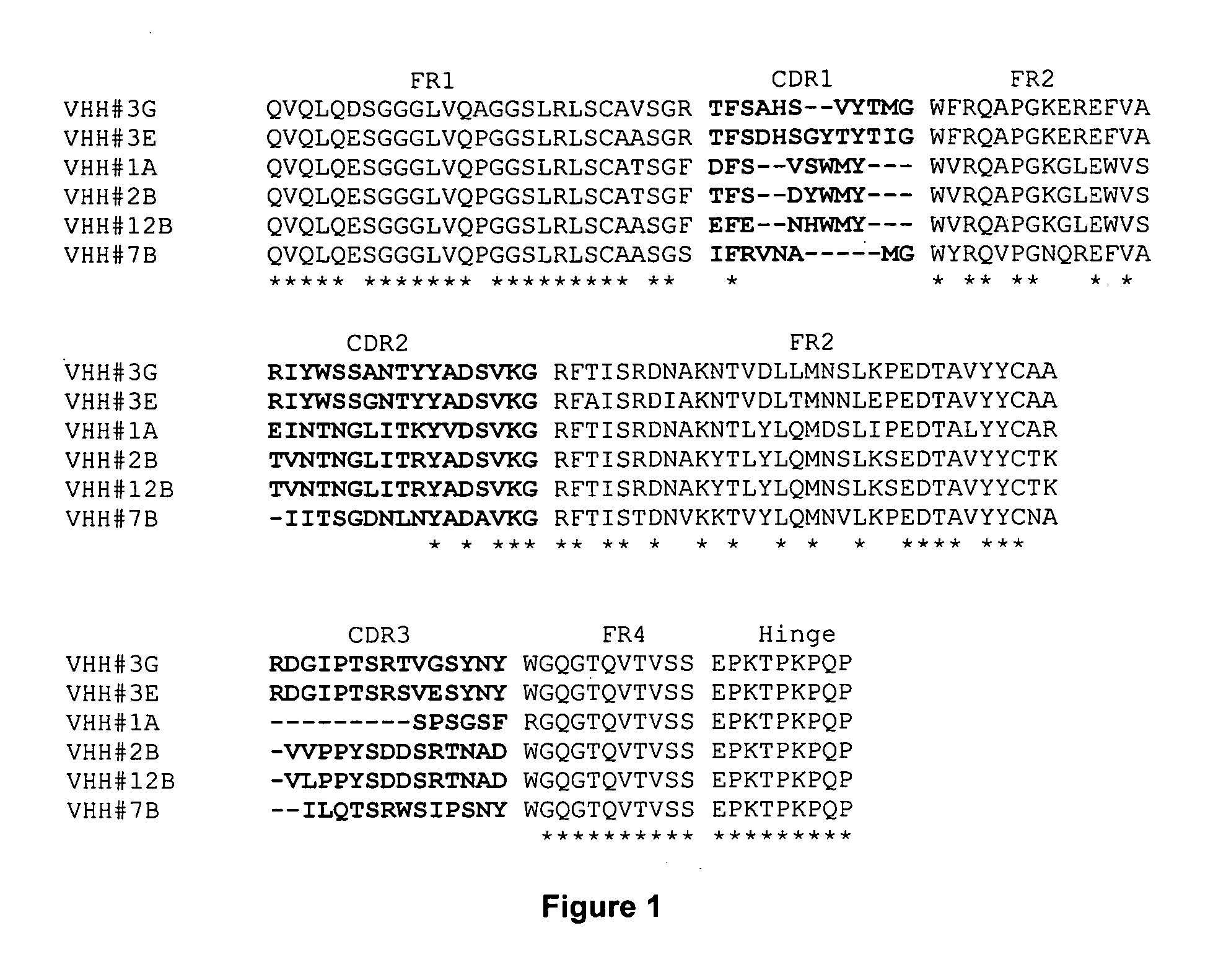
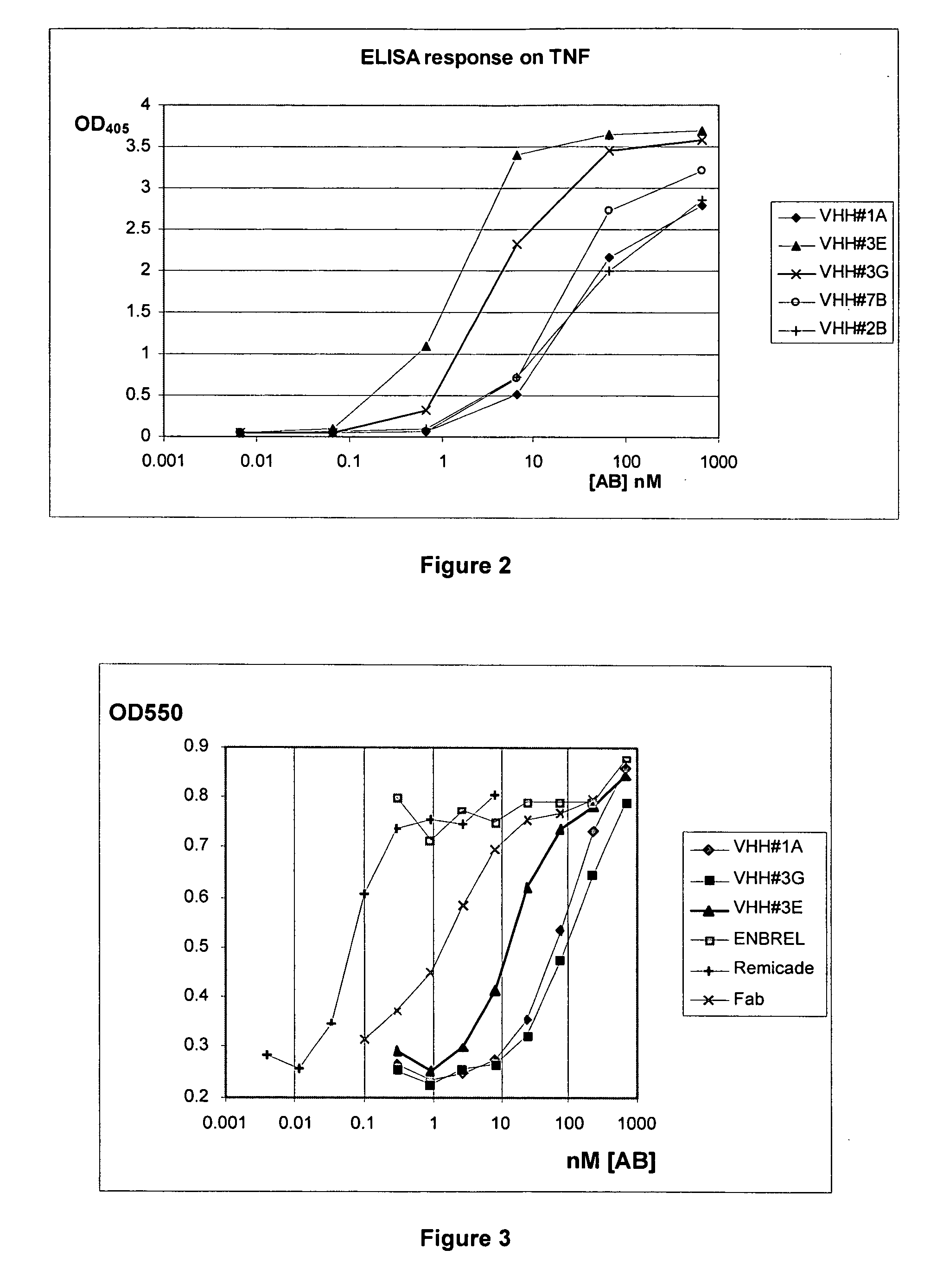
Single domain antibodies directed against tumour necrosis factor-alpha and uses therefor
InactiveUS20070077249A1Pass efficientlyImmunoglobulins against blood coagulation factorsAntibacterial agentsSingle-domain antibodyGreek letter alpha
The present invention relates to polypeptides derived from single domain heavy chain antibodies directed to Tumor Necrosis Factor-alpha. It further relates to single domain antibodies that are Camelidae VHHs. It further relates to methods of administering said polypeptides. It further relates to protocols for screening for agents that modulate the TNF-alpha receptor, and the agents resulting from said screening.
Owner:ABLYNX NV
Human tumor necrosis factor-immunoglobulin(TNFR1-IgG1) chimera composition
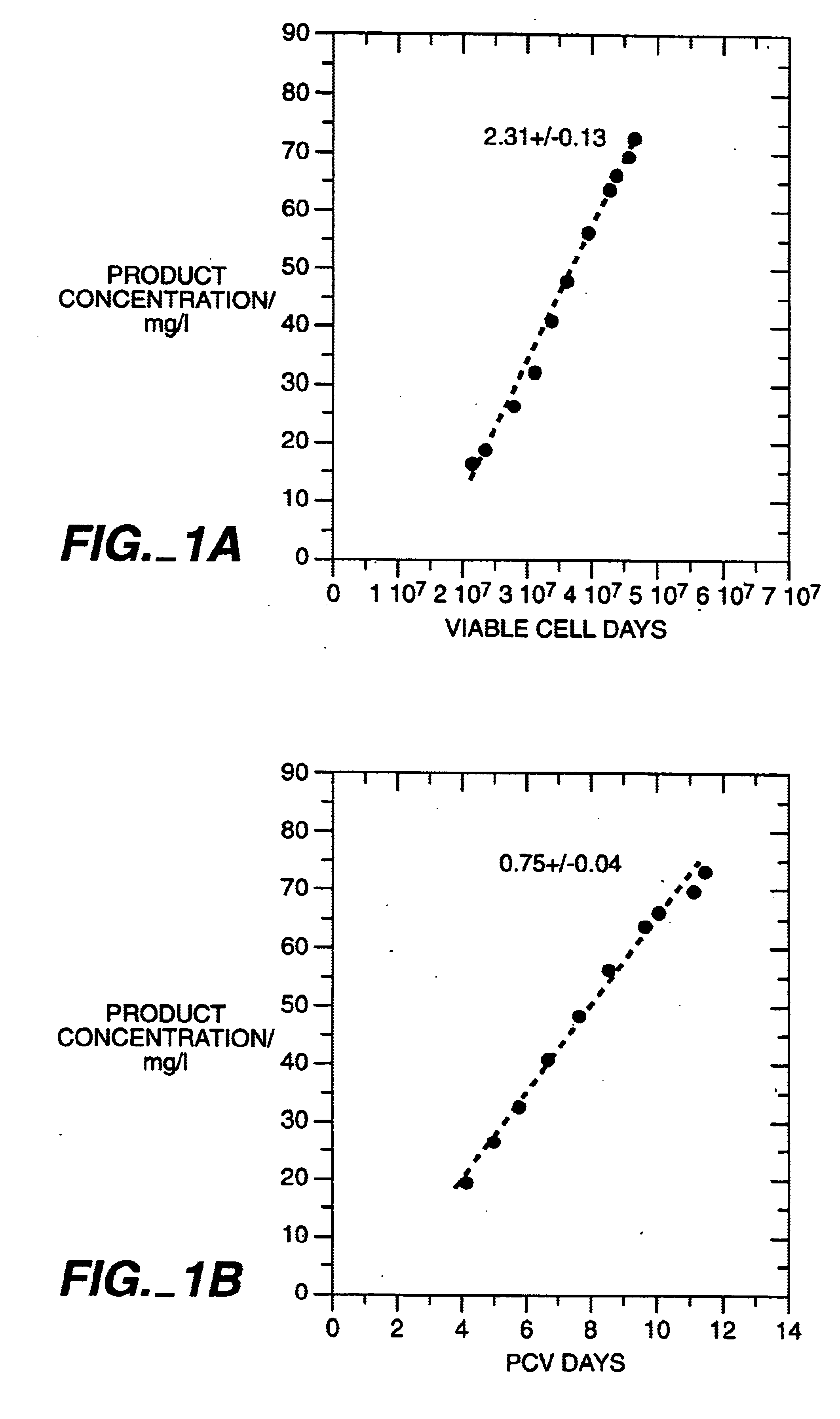
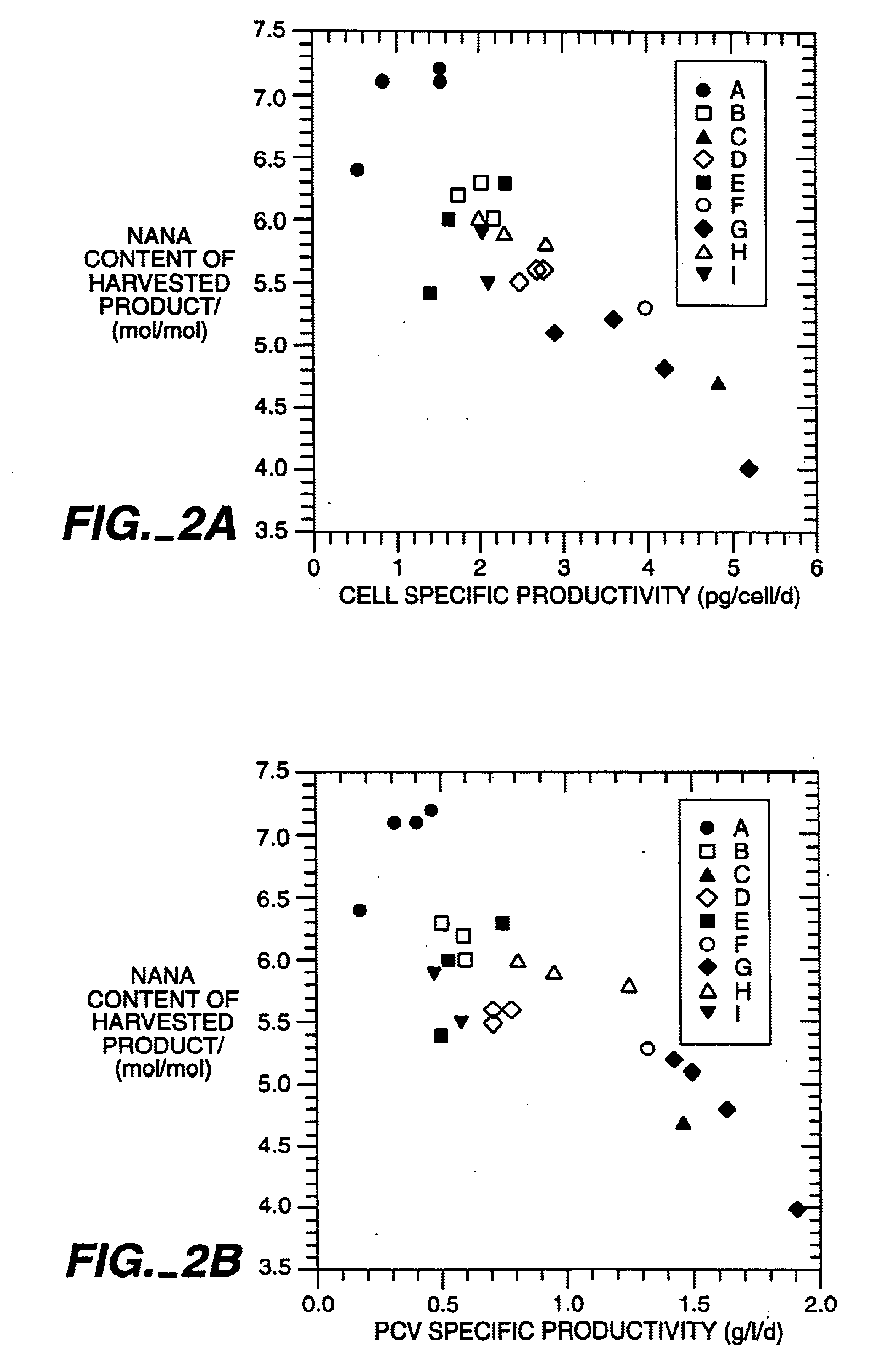
The present invention relates to novel process for the preparation of glycoproteins by mammalian cell culture wherein the sialic acid content of the glycoprotein produced is controlled over a broad range of values by manipulating the cell culture environment. The invention provides for processes in which the sialic acid content of the glycoprotein is modified by changes in cell culture parameters which affect cell specific productivity. Preferred embodiments of the invention include cell culture processes in the osmolality of the cell culture is controlled as well as the concentration of a transcription enhancer during the production phase of the cell culture. The invention further provides for novel preparations of soluble type 1 tumor necrosis factor immunoglobulin G1 and their uses in the treatment of inflammatory or immune related disorders.
Owner:GENENTECH INC
Method and composition for treatment of renal failure with antibodies and their equivalents as partial or complete replacement for dialysis
InactiveUS7504106B2Lower Level RequirementsSlow onsetBiocidePeptide/protein ingredientsInterleukin 6Creatinine rise
A method for treating patients with renal failure includes administering to them an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
Tumor necrosis factor-alpha mutants
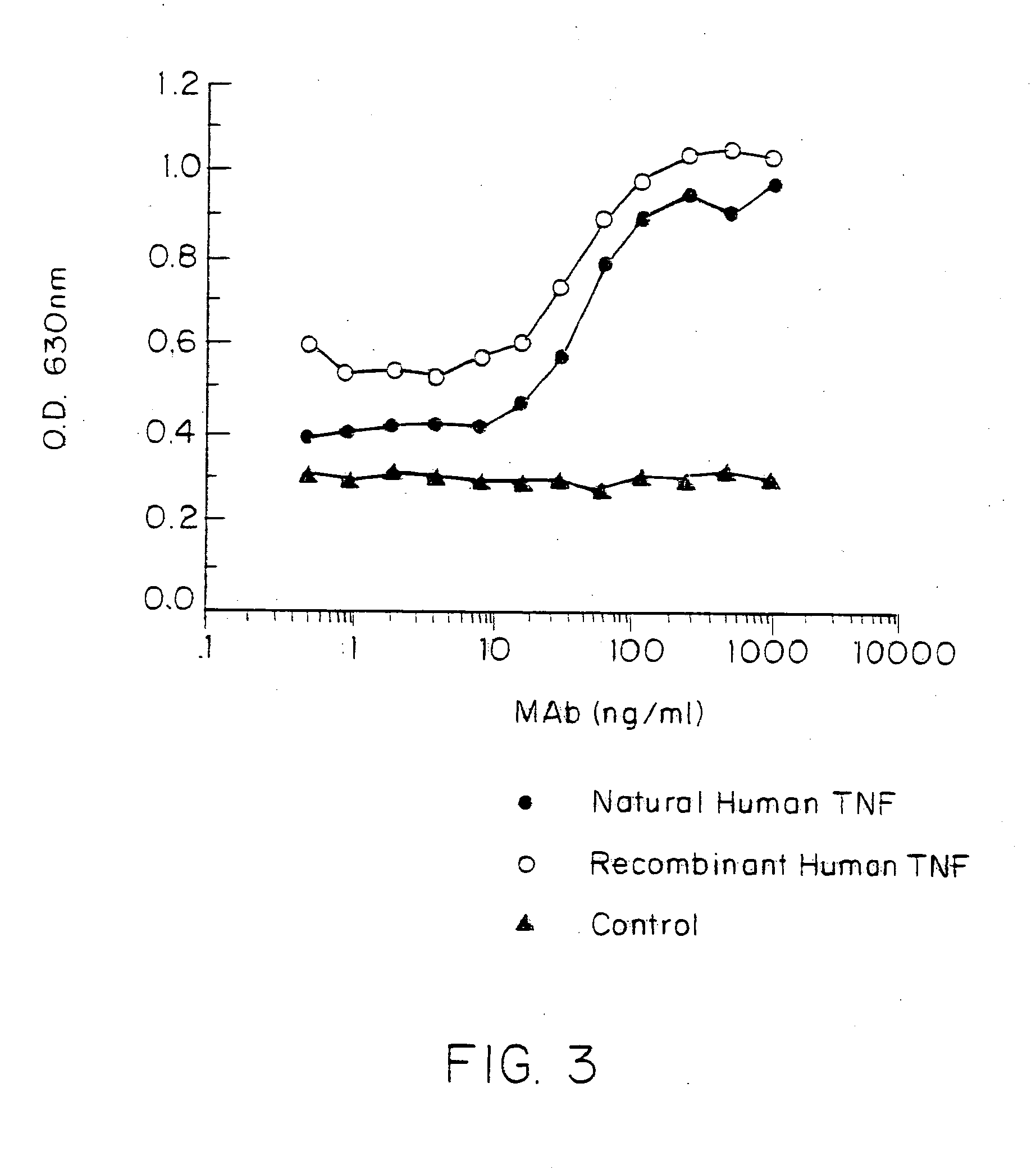
The present invention has an object to provide a tumor necrosis factor mutant protein, particularly, a tumor necrosis factor mutant protein specific to TNF-R1 or TNF-R2; tumor necrosis factor inhibitor; or tumor necrosis factor preparation containing it as an effective ingredient, and the object is solved by providing a tumor necrosis factor mutant protein where one or more amino acid residues selected from the group consisting of 29th, 31st, 32nd, 145th, 146th and 147th, or the group consisting of 84th to 89th from the N-terminal of the amino acid sequence of SEQ ID NO:1 is / are replaced with other amino acid residue(s); a tumor necrosis factor inhibitor; and a tumor necrosis factor preparation containing it as an effective ingredient.
Owner:MAYUMI TADANORI +3
Anti-idiotypic anti-TNF antibodies and related immunoassay methods
Anti-TNF antibodies and anti-TNF peptides, specific for tumor necrosis factor (TNF) are useful for in vivo diagnosis and therapy of a number of TNF-mediated pathologies and conditions, as well as polynucleotides coding for anti-TNF murine and chimeric antibodies, peptides, methods of making and using the antibody or peptides in immunoassays and immuno-therapeutic approaches are provided, where the anti-TNF peptide is selected from a soluble portion of TNF receptor, an anti-TNF antibody or structural analog thereof.
Owner:NEW YORK UNIV +1
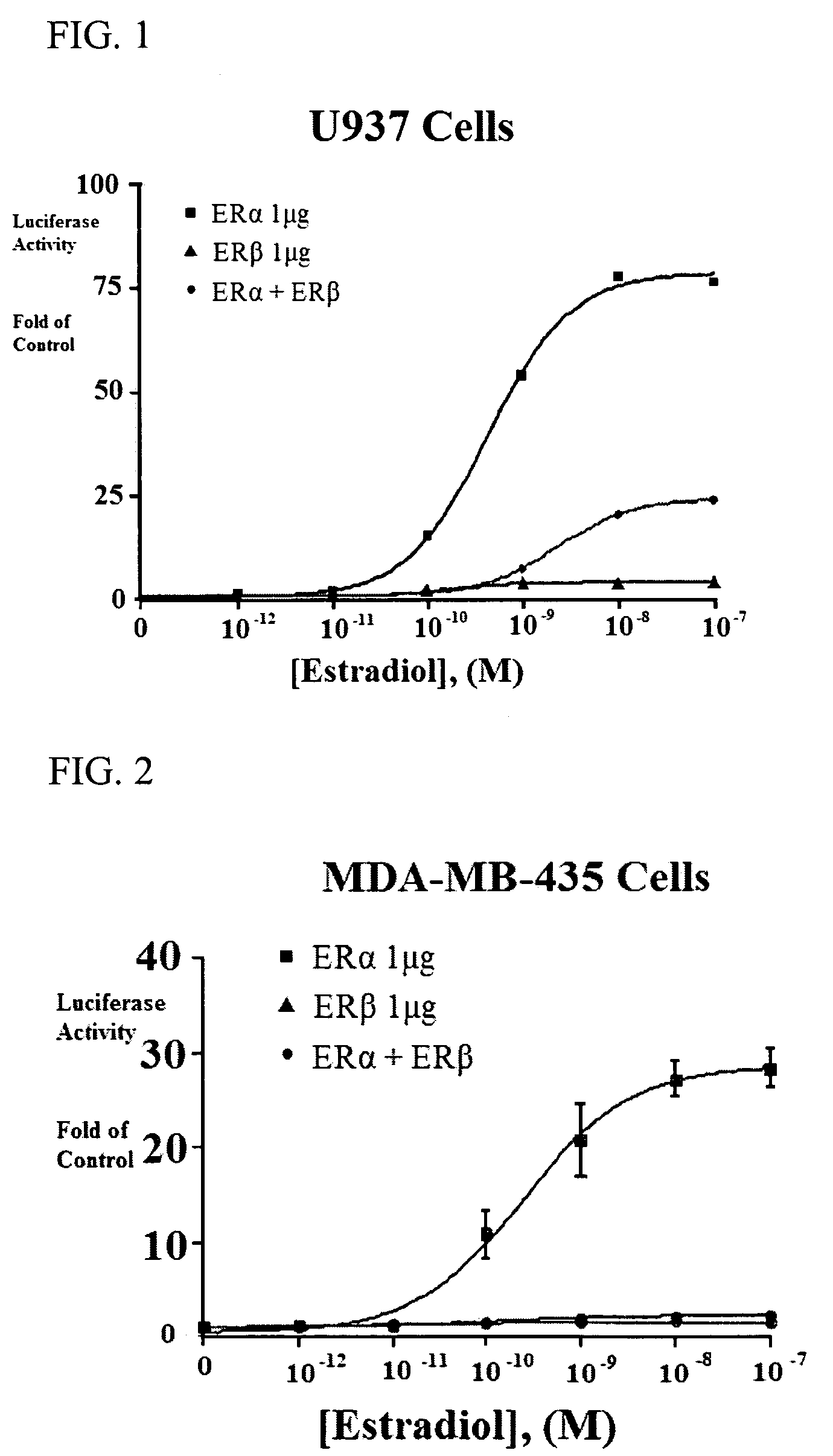
Estrogenic extracts of Morus alba and uses thereof
Extracts of various species of the Moraceae family have estrogenic properties. For example, aqueous and ethanolic extracts of Morus alba L. species possess estrogenic properties in both ERα+ and ERβ+ cells. These estrogenic effect include estrogen response element (ERE) stimulation as well as tumor necrosis factor (TNF) repression. Methods are provided for treating climacteric symptoms, breast and / or uterine cancer, and osteoporosis.
Owner:BIONOVO
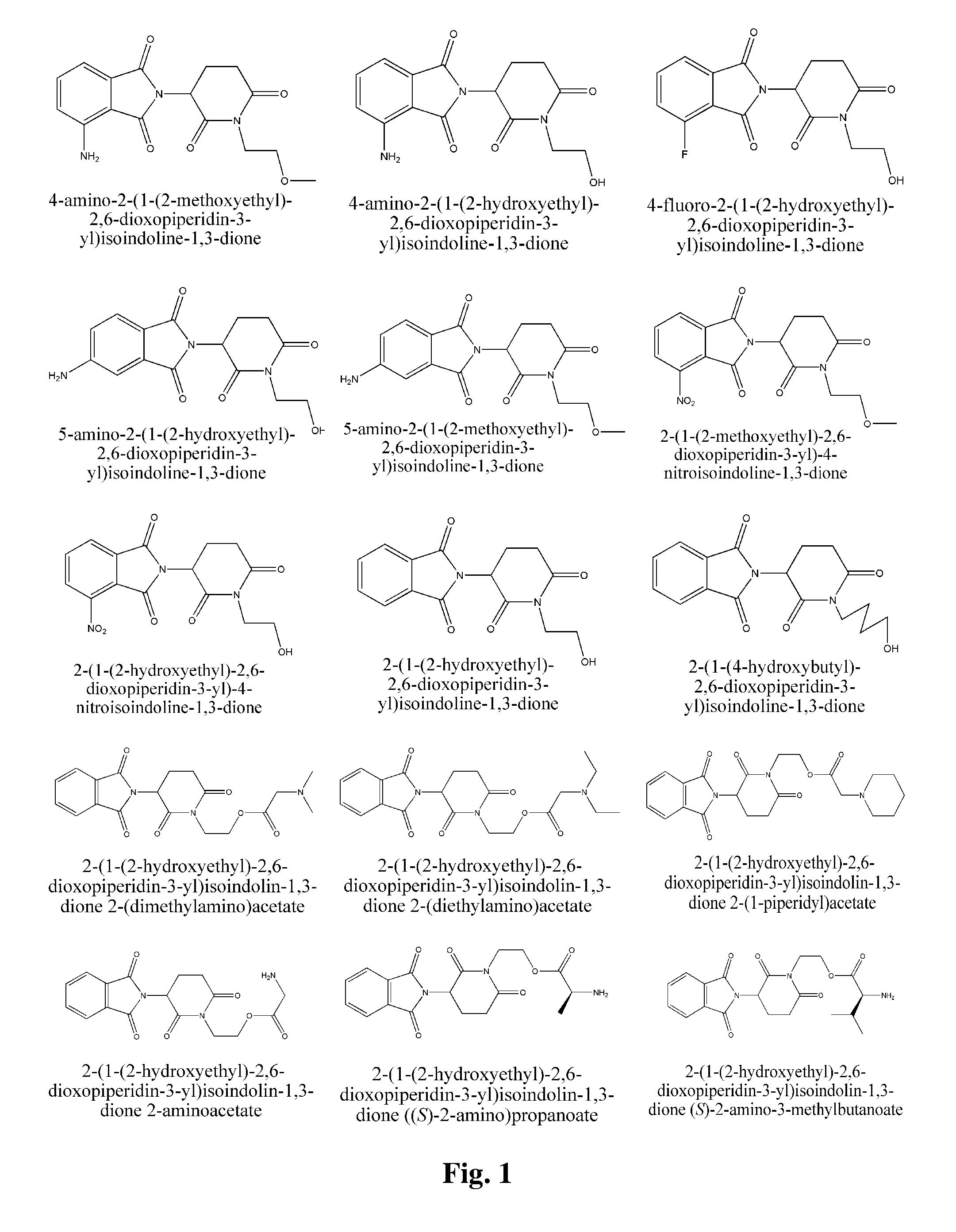
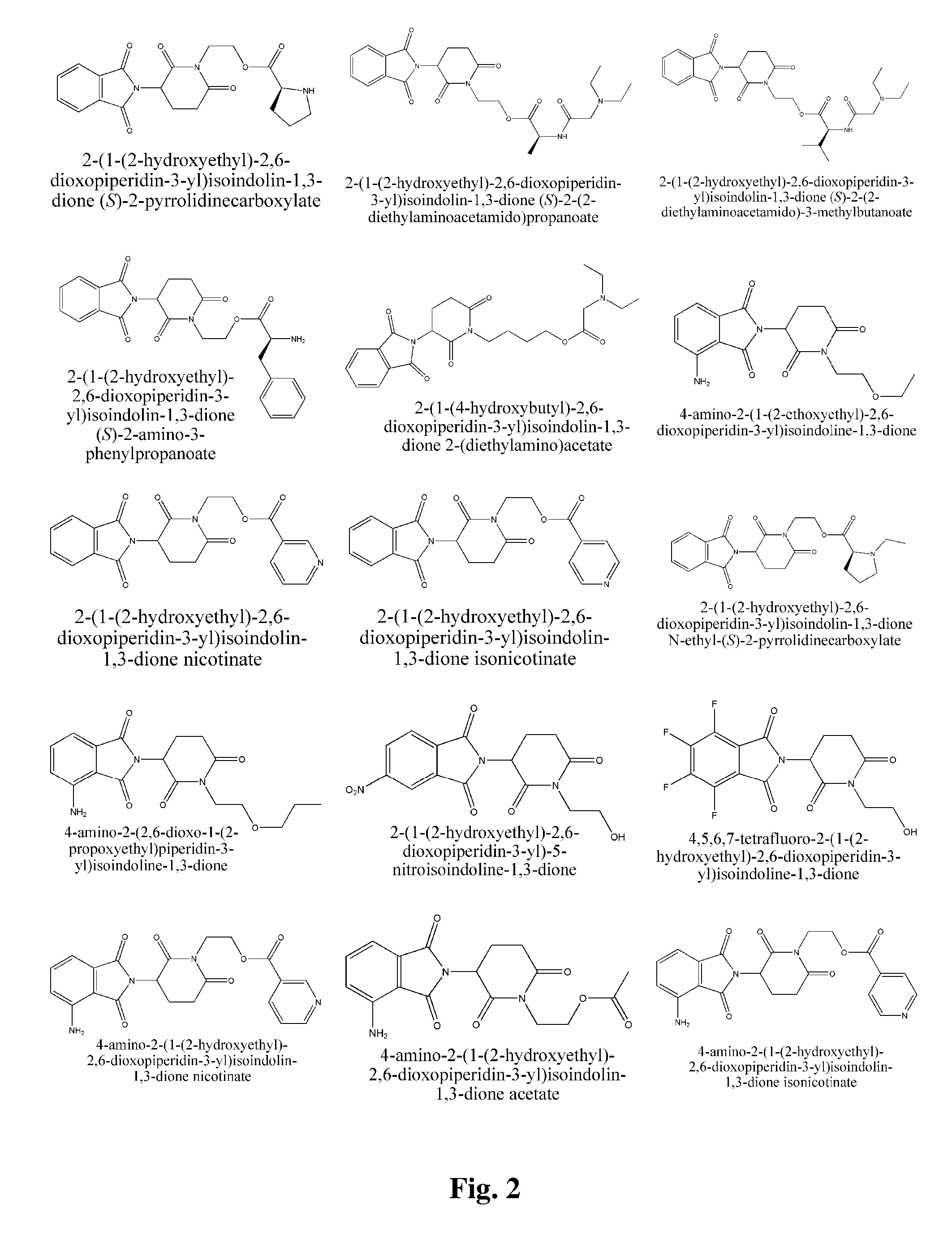
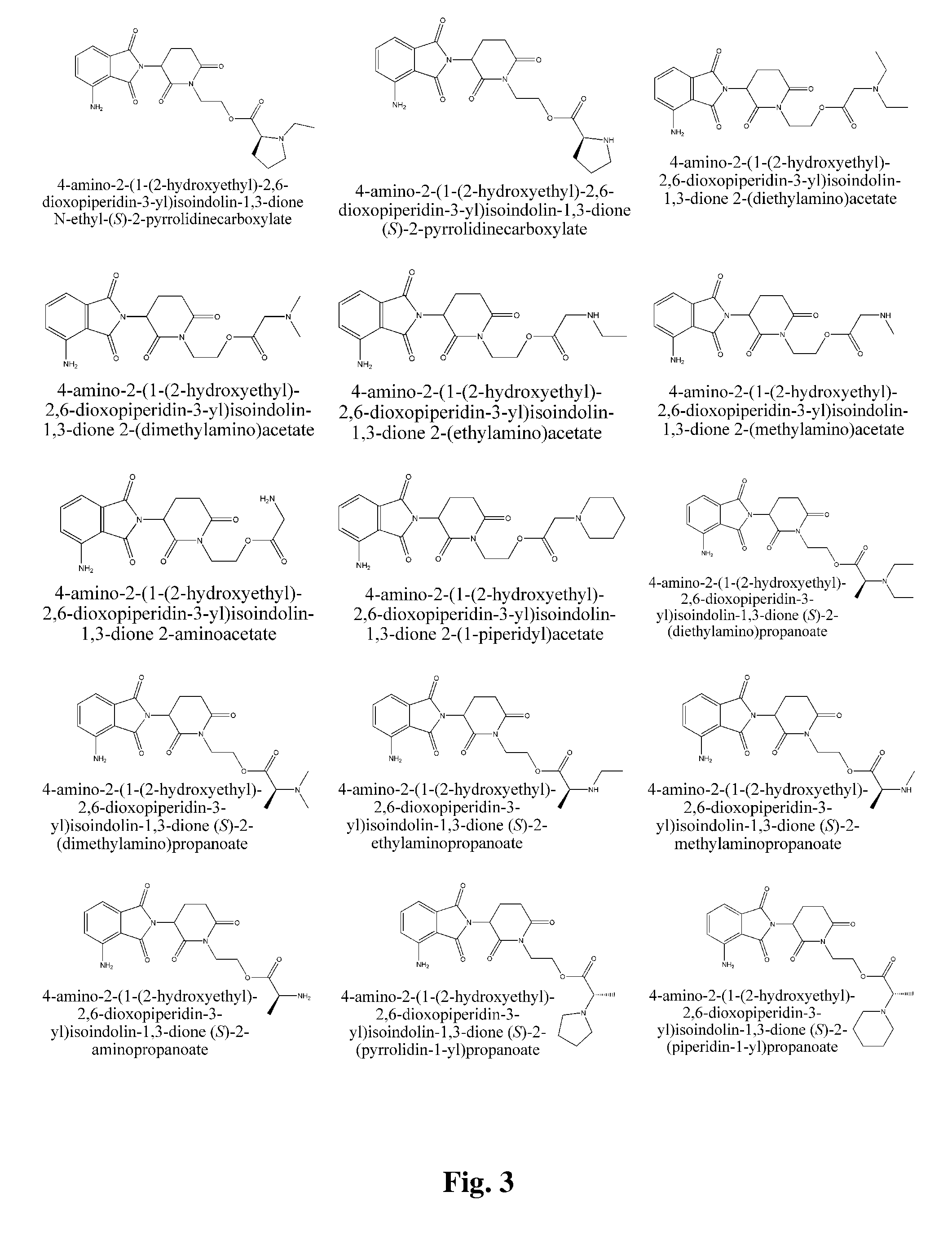
Piperidine-2, 6-dione derivatives and their use as tumor necrosis factor inhibitors
This invention is directed to derivatives of piperidine-2,6-dione, or their organic or inorganic salts thereof, a methods of synthesis of these derivatives, and their application as active pharmaceutical ingredient as inhibitors of TNFα releasing in cells, the derivative of piperidine-2,6-dione being of the general formula (I): wherein n represents 1, 2, 3, 4, 5 or 6; R1 represents from one to four of the same or different substituents selected from F, Cl, Br, C1-4 alkyl, OH, OC1-4 alkyl, NO2, NHC(O)C1-4 alkyl, NH2, NH(C1-4 alkyl), N(C1-4 alkyl)2; R2 represents OR3, NR3R4, N(R3)COR4, O2CR5; R3 and R4 represent independently and at each occurrence H or C1-4 alkyl; R5 represents CHR6NR7R8, CHR6NR9C(O)CHR10NR7R8, a heterocycle W or CHR6NR9C(O)W; R6, R9, R10 represent independently and at each occurrence H, or C1-4 alkyl; R7 and R8 represent independently and at each occurrence H, C1-4 alkyl, or R7 and R8 taken together represent 1,3-propylene, 1,4-butylene, 1,5-pentylene, or 1,6-hexylene; W represents four-membered, five-membered, six-membered, seven-membered, or eight-membered saturated or unsaturated heterocycle.
Owner:TIANJIN HEMAY PHARM SCI TECH CO LTD +1
Death domain containing receptor 5
The present invention relates to novel Death Domain Containing Receptor-5 (DR5) proteins which are members of the tumor necrosis factor (TNF) receptor family, and have now been shown to bind TRAIL. In particular, isolated nucleic acid molecules are provided encoding the human DR5 proteins. DR5 polypeptides are also provided as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying antagonists and antagonists of DR5 activity. The invention also relates to the treatment of diseases associated with reduced or increased levels of apoptosis using antibodies specific for DR5, which may be agonists and / or antagonists of DR5 activity.
Owner:HUMAN GENOME SCI INC
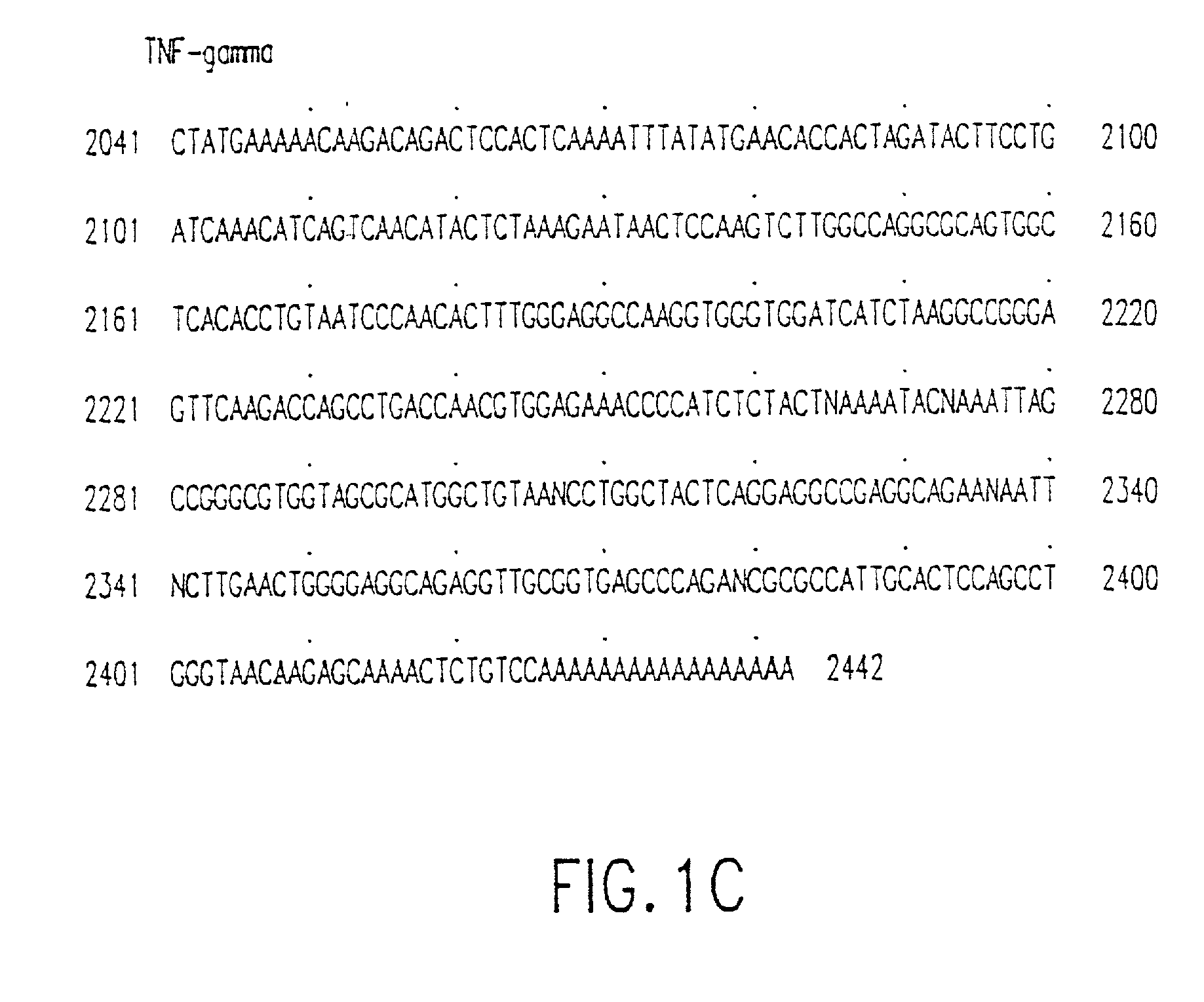
Tumor necrosis factor-gamma
InactiveUS7597886B2Increase blockingInduce inflammatory activityOrganic active ingredientsIn-vivo radioactive preparationsAbnormal tissue growthDisease
Human TNF-gamma-alpha and TNF-gamma-beta polypeptides and DNA (RNA) encoding such polypeptides and a procedure for producing such polypeptides by recombinant techniques are disclosed. Also disclosed are methods for utilizing such polypeptides to inhibit cellular growth, for example in a tumor or cancer, for facilitating wound-healing, to provide resistance against infection, induce inflammatory activities, and stimulating the growth of certain cell types to treat diseases, for example restenosis. Also disclosed are diagnostic methods for detecting a mutation in the TNF-gamma-alpha and TNF-gamma-beta nucleic acid sequences or overexpression of the TNF-gamma-alpha and / or TNF-gamma-beta polypeptides. Antagonists against such polypeptides and their use as a therapeutic to treat cachexia, septic shock, cerebral malaria, inflammation, arthritis and graft-rejection are also disclosed.
Owner:HUMAN GENOME SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com