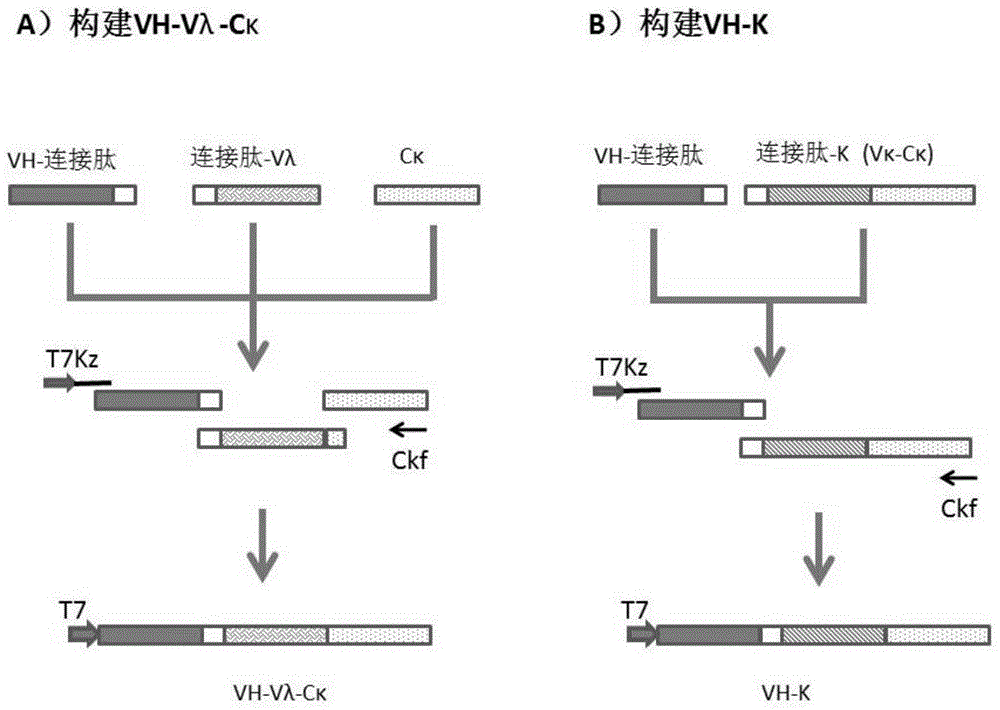
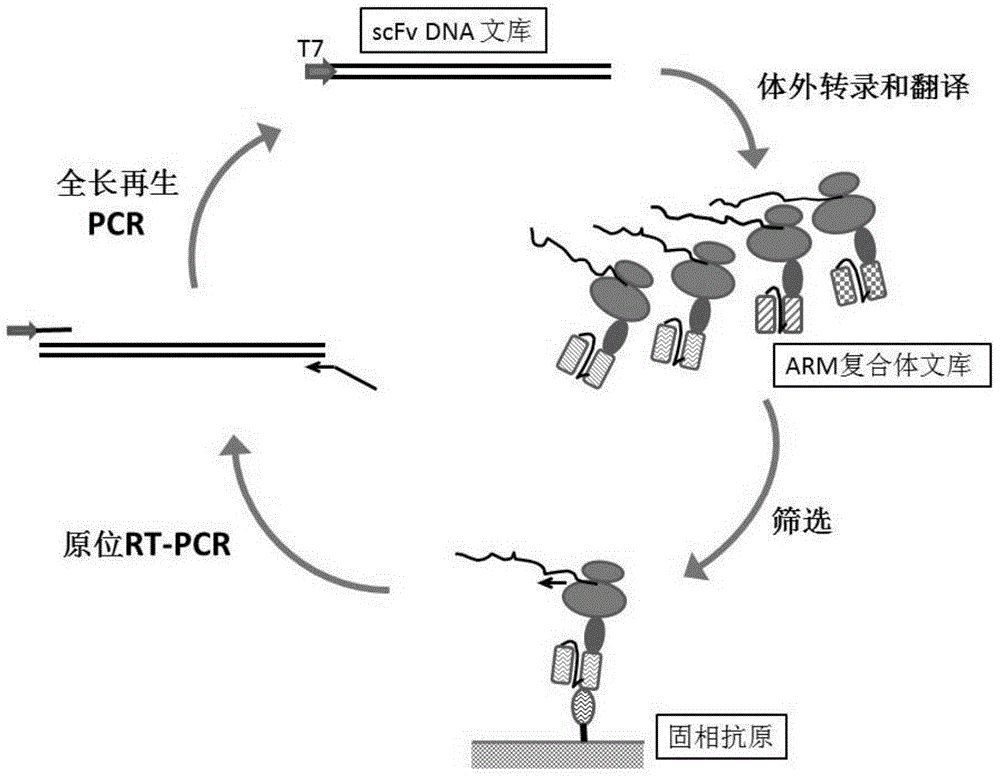
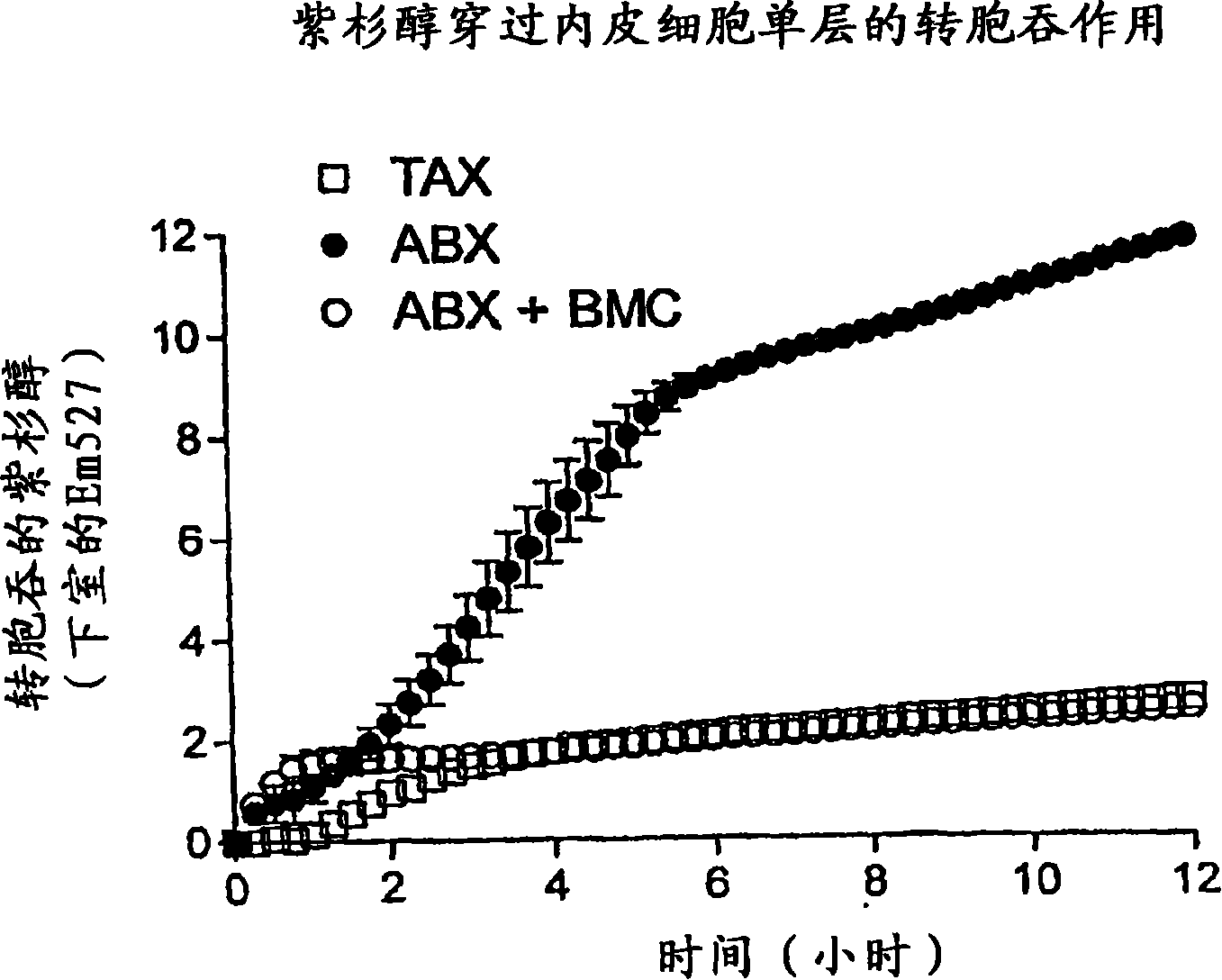
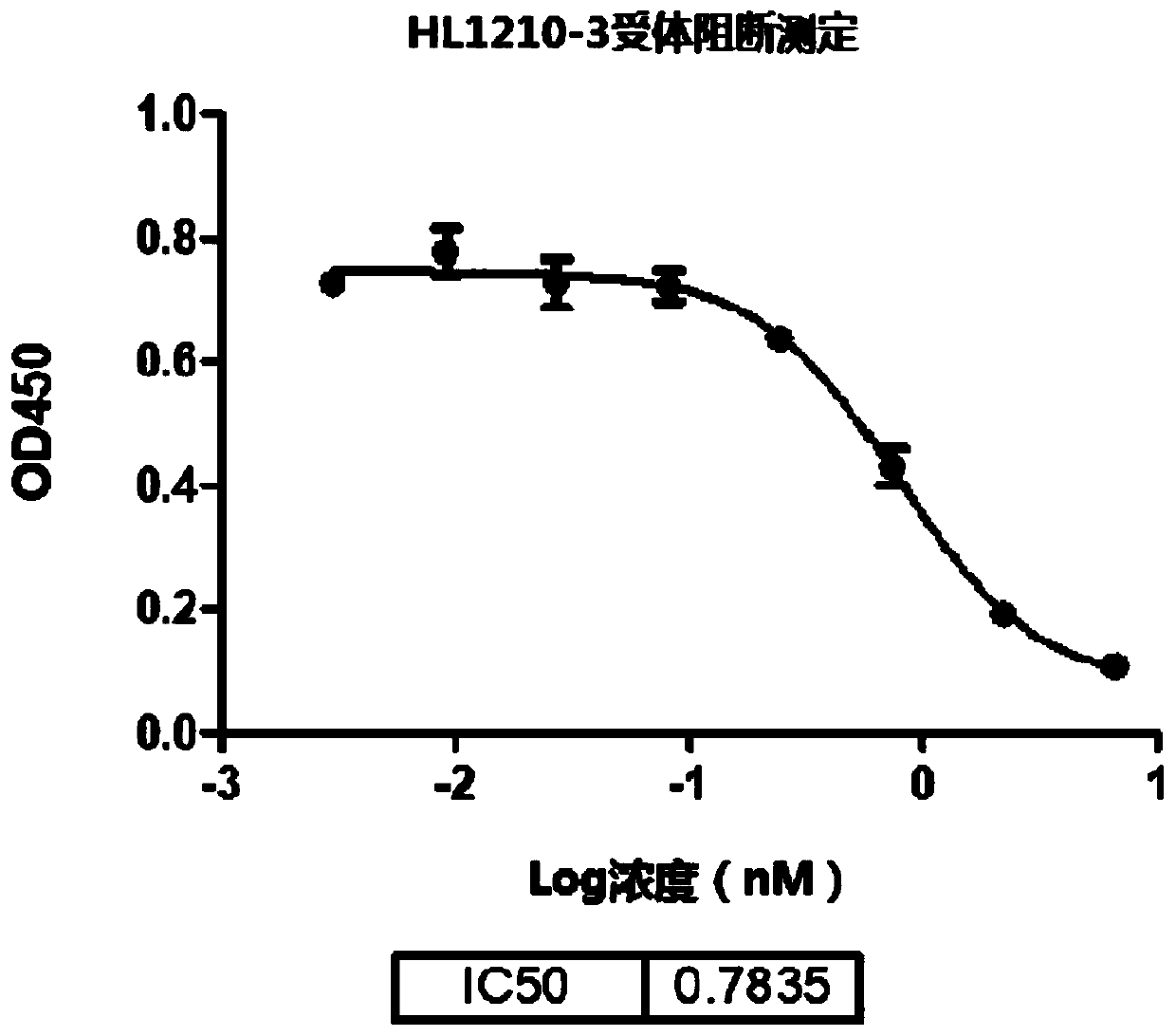
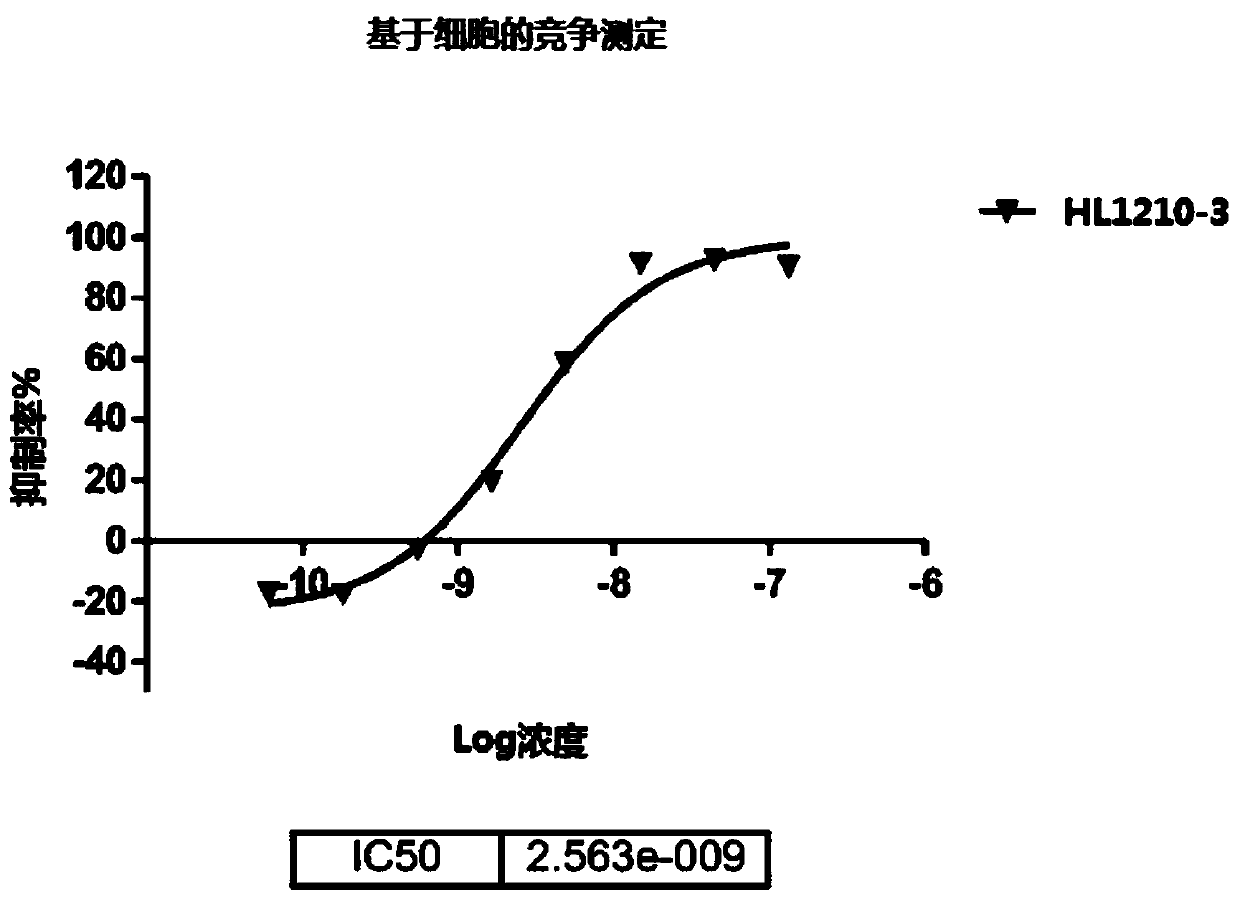
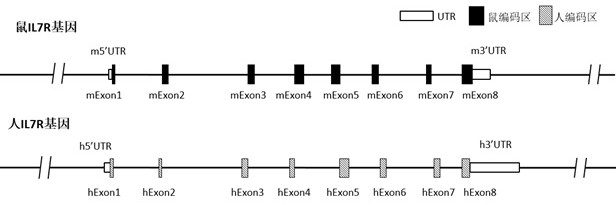
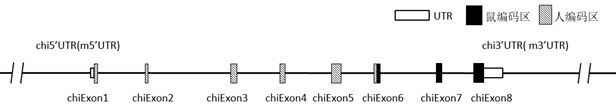
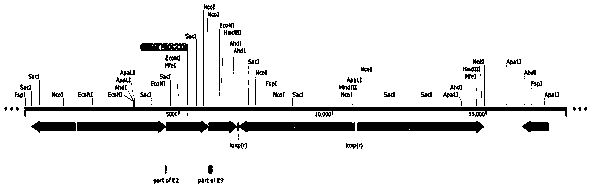
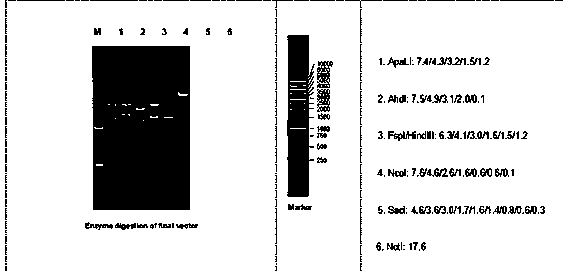
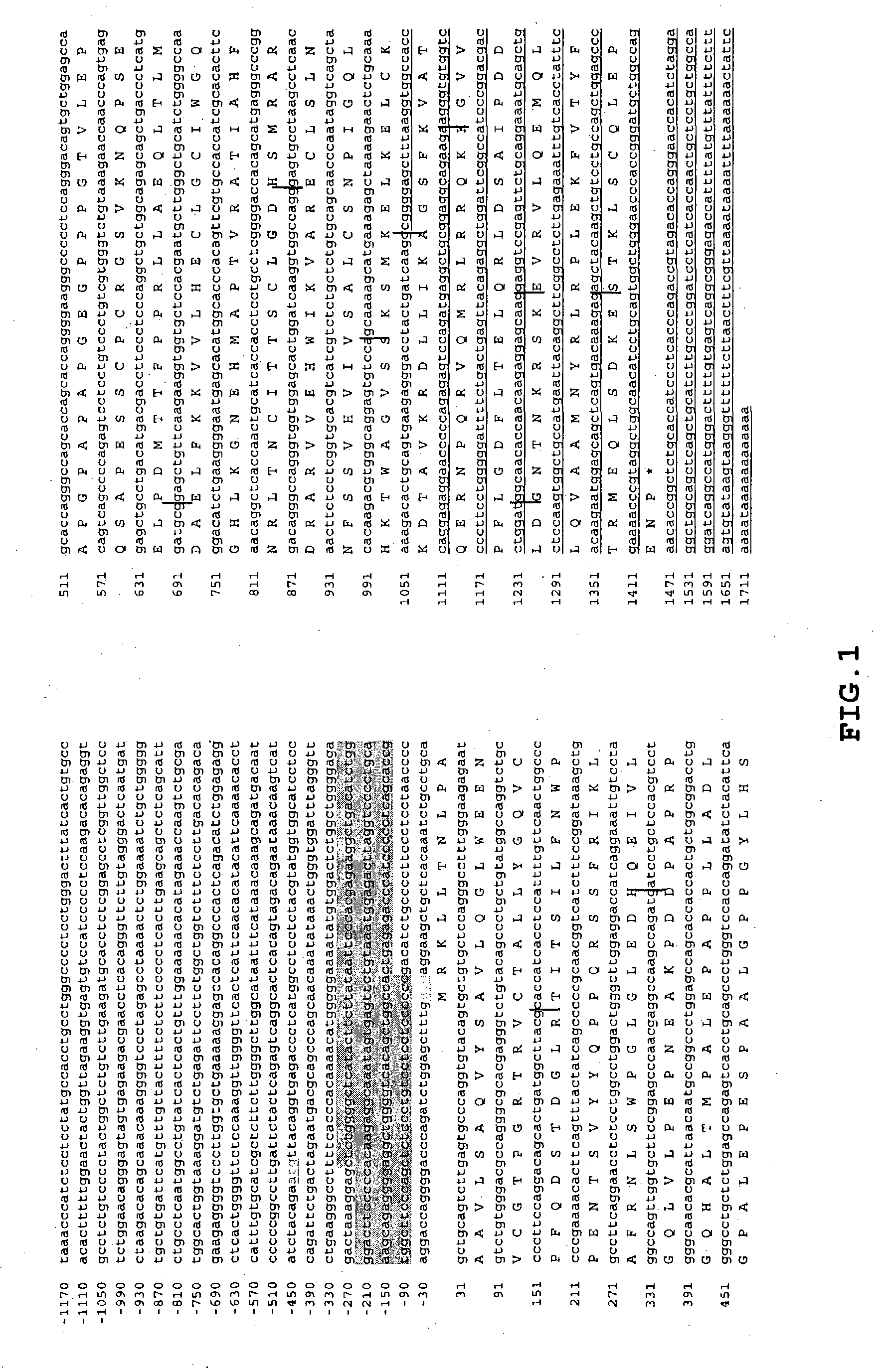
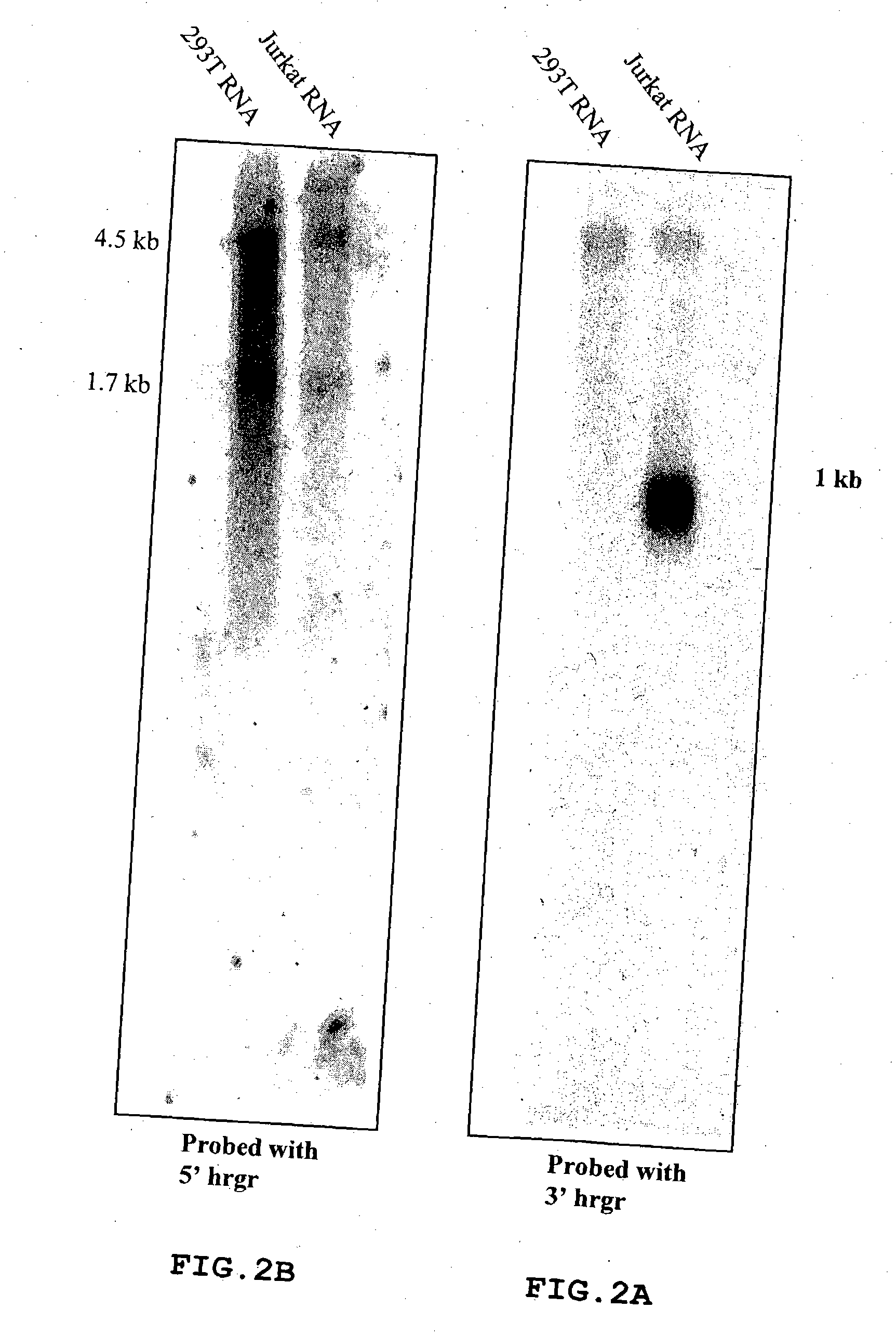
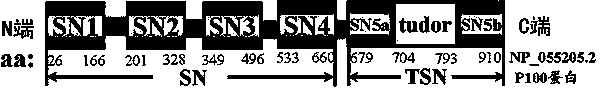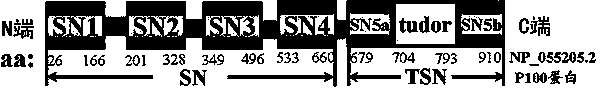Patents
Literature
162 results about "Humanin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
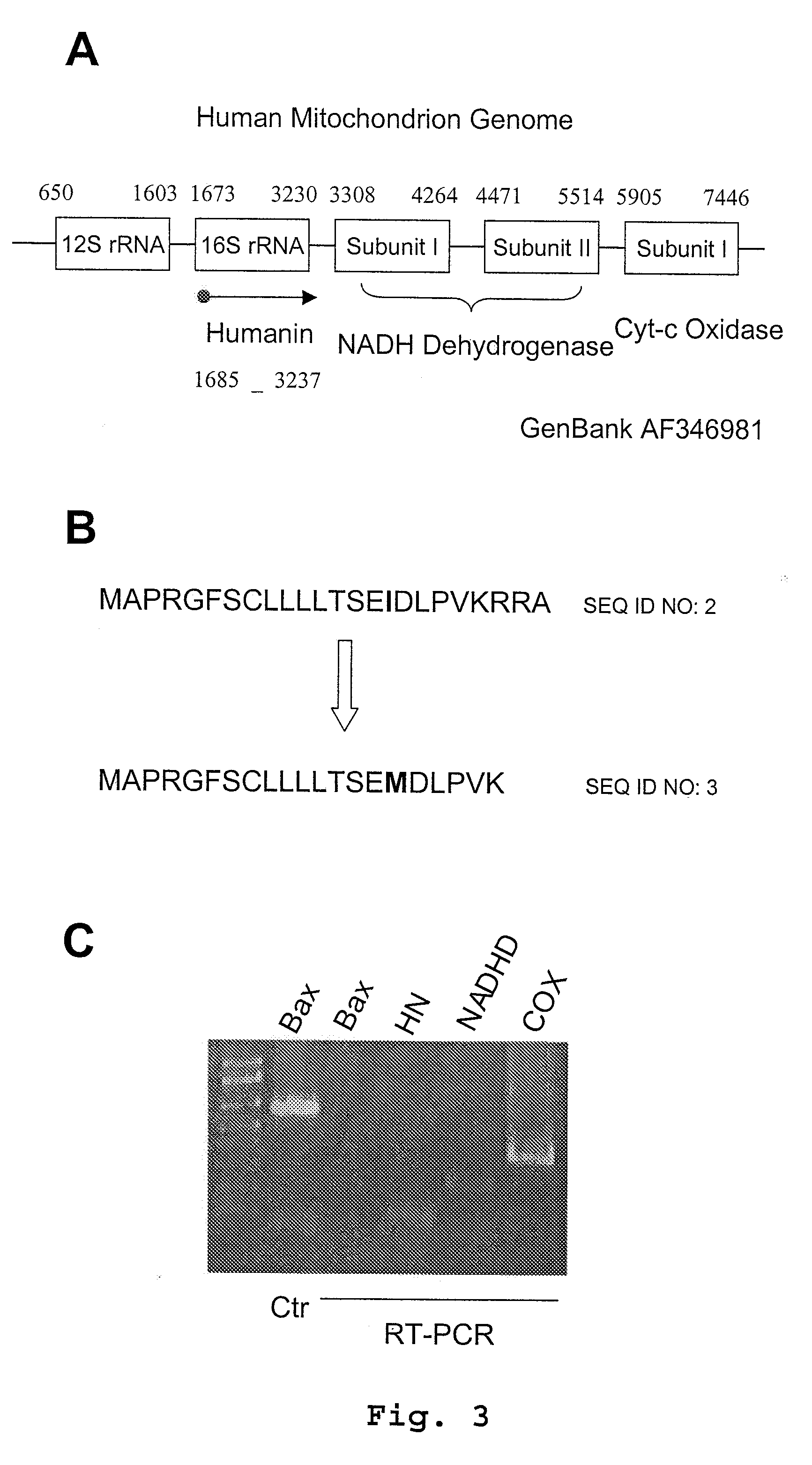
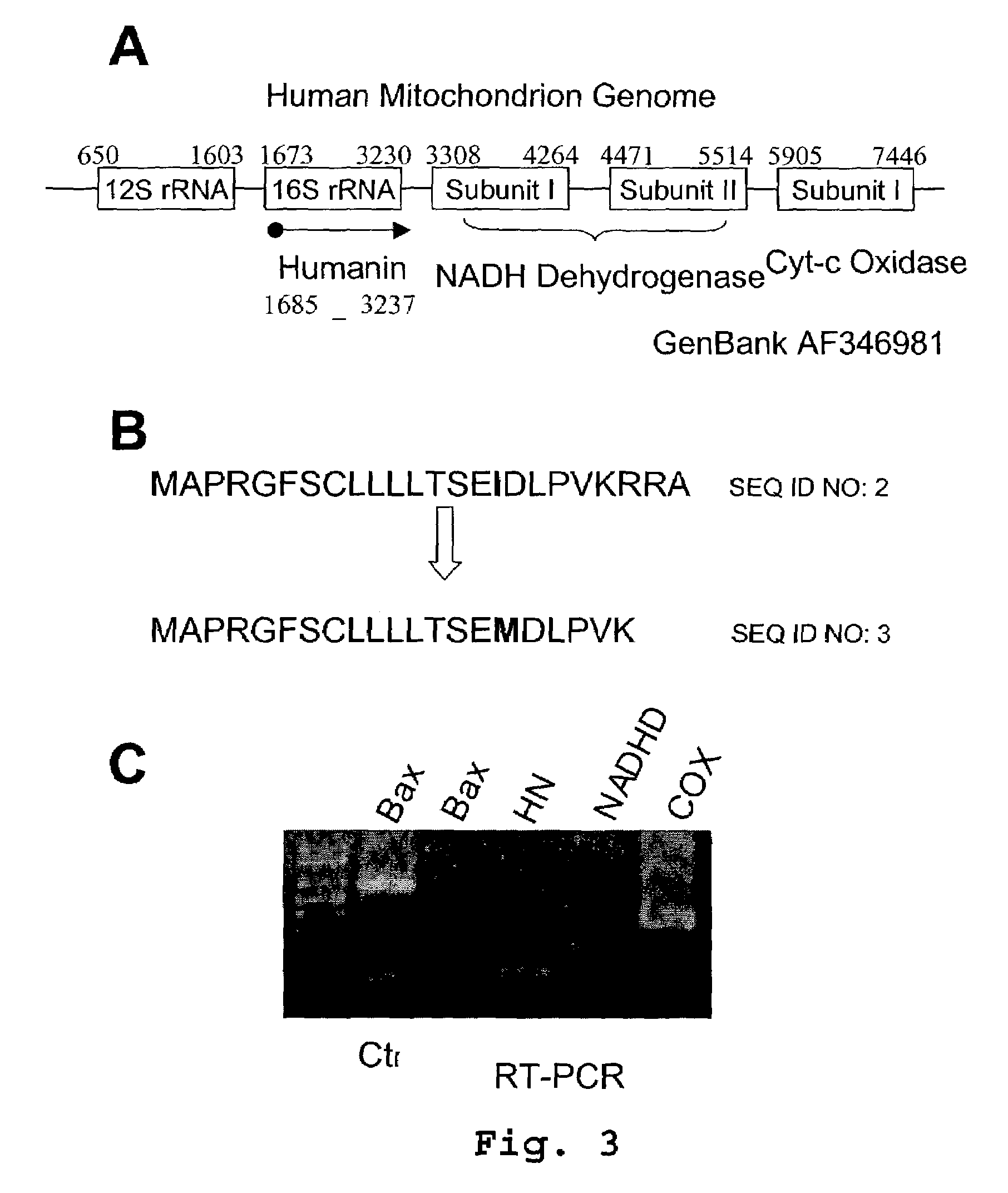
Humanin is a micropeptide encoded in the mitochondrial genome by the 16S ribosomal RNA gene, MT-RNR2. Its structure contains a three-turn α-helix, and no symmetry. In in vitro and animal models, it appears to have cytoprotective effects.
Death domain containing receptor 5
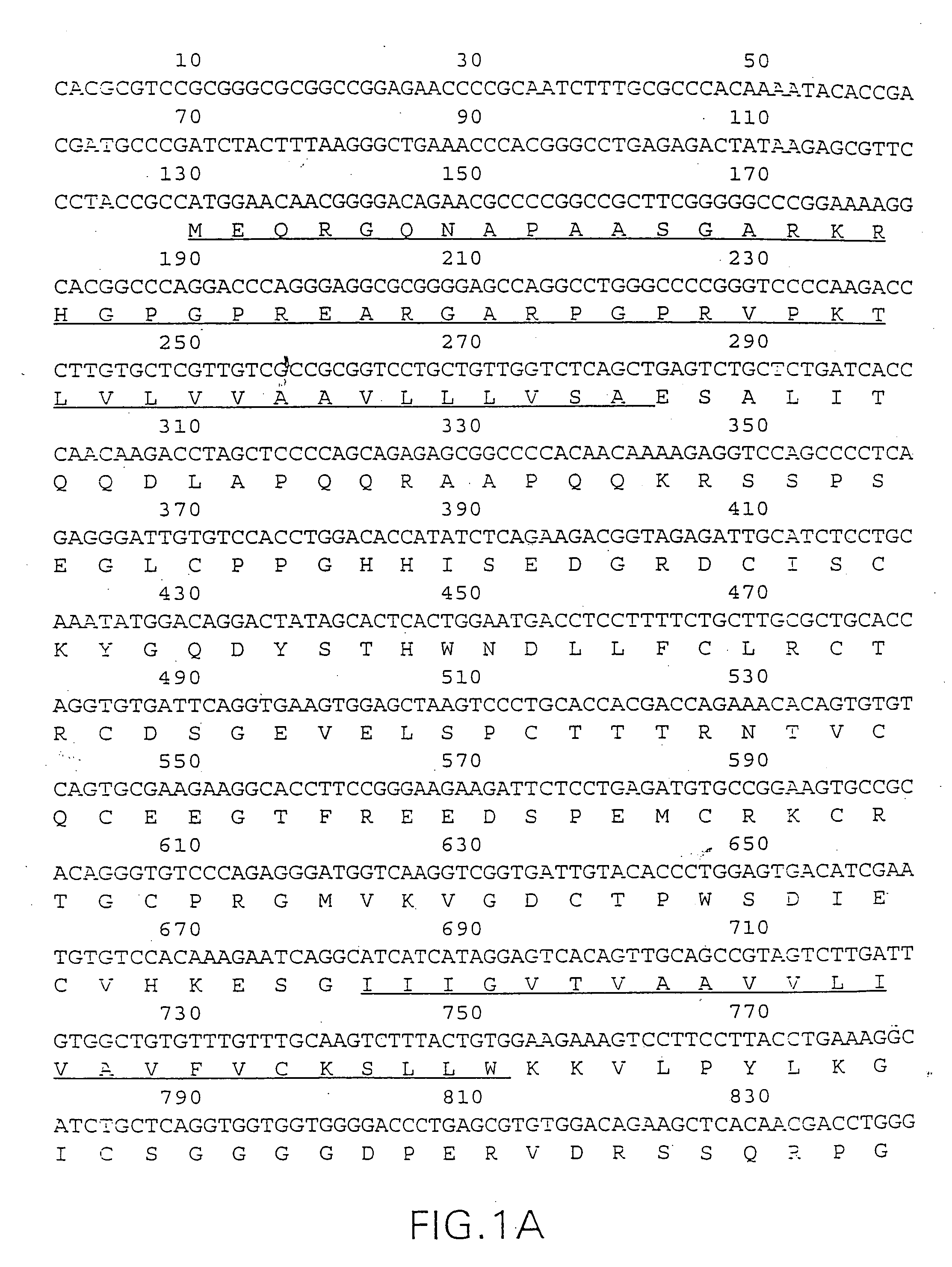
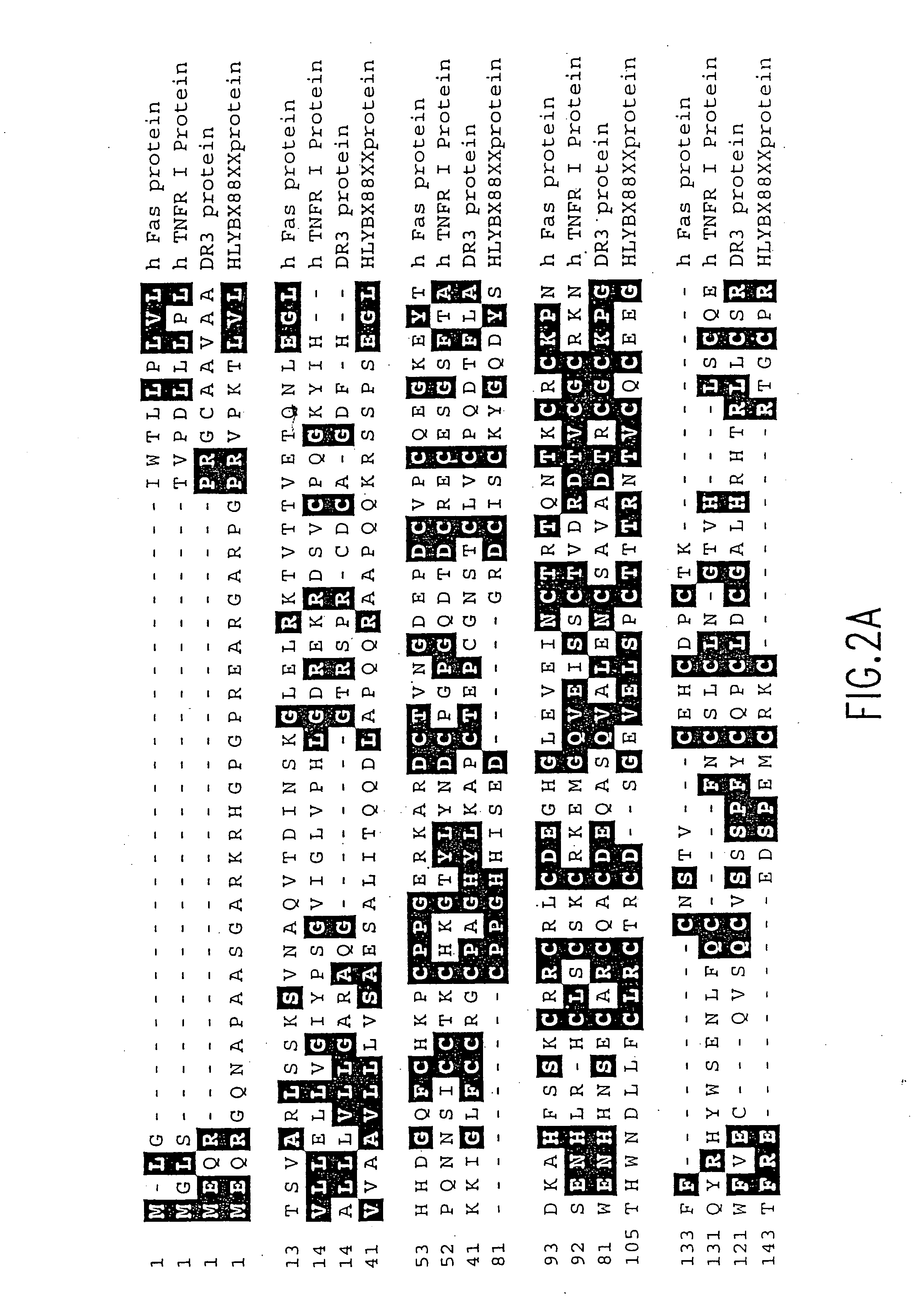
The present invention relates to novel Death Domain Containing Receptor-5 (DR5) proteins which are members of the tumor necrosis factor (TNF) receptor family, and have now been shown to bind TRAIL. In particular, isolated nucleic acid molecules are provided encoding the human DR5 proteins. DR5 polypeptides are also provided as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying antagonists and antagonists of DR5 activity. The invention also relates to the treatment of diseases associated with reduced or increased levels of apoptosis using antibodies specific for DR5, which maybe agonists and / or antagonists of DR5 activity.
Owner:HUMAN GENOME SCI INC
Crystalline frap complex
InactiveUS6532437B1Reduce the numberHigh resolutionTransferasesDigital computer detailsCrystallographyTernary complex
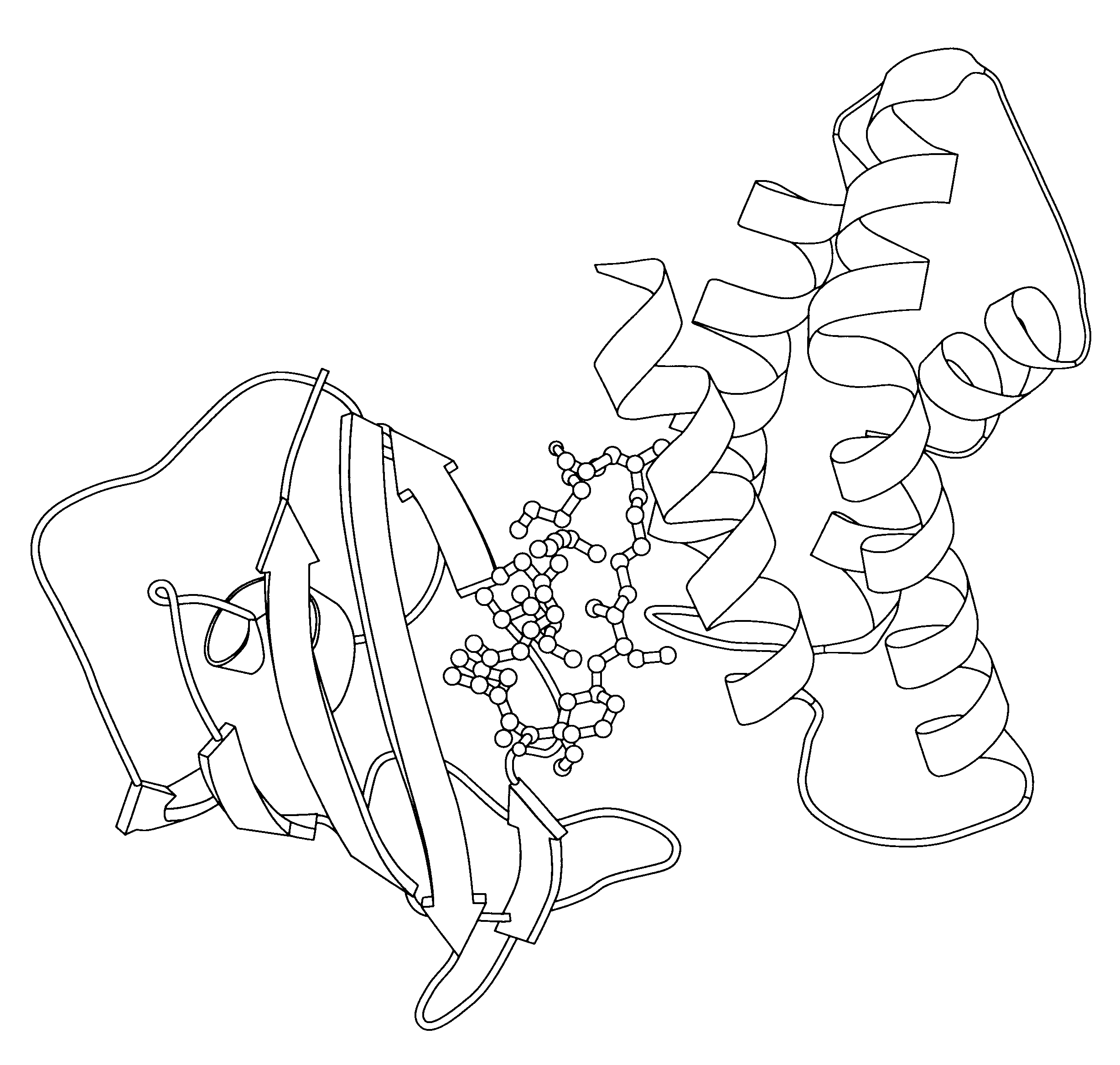
The invention relates to the human protein FRAP, and in particular to the FKBP12-rapamycin binding domain thereof and to the ternary complex formed by the FRB domain, rapamycin and FKBP12. A new crystalline composition comprising the ternary complex, coordinates defining its three dimensional structure in atomic detail, and uses thereof are disclosed.
Owner:CORNELL RES FOUNDATION INC
RNA processing protein complexes and uses thereof
InactiveUS20050053985A1High expressionInhibit and reduce expressionHydrolasesMicrobiological testing/measurementDiseasePrecursor mRNA
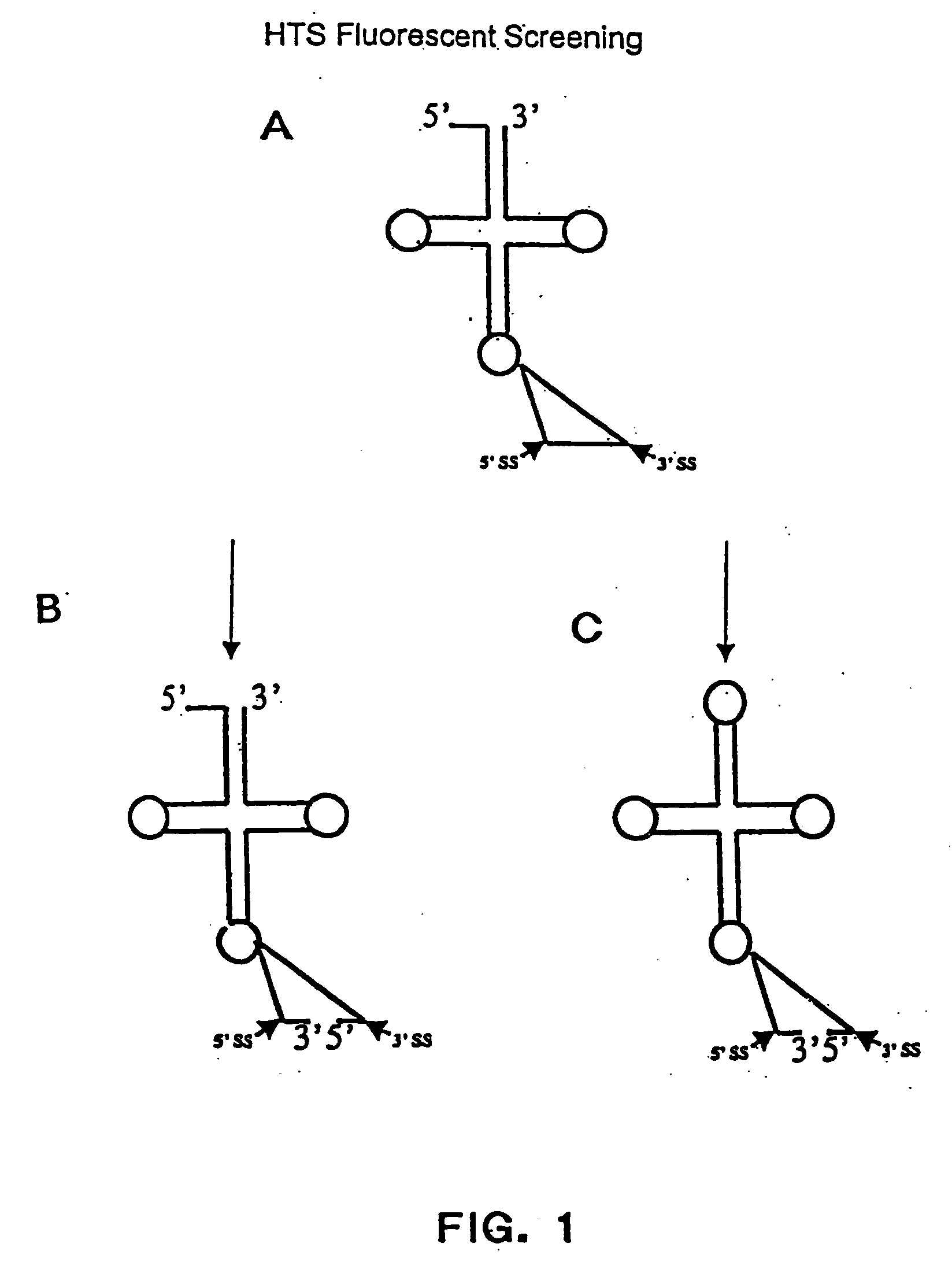
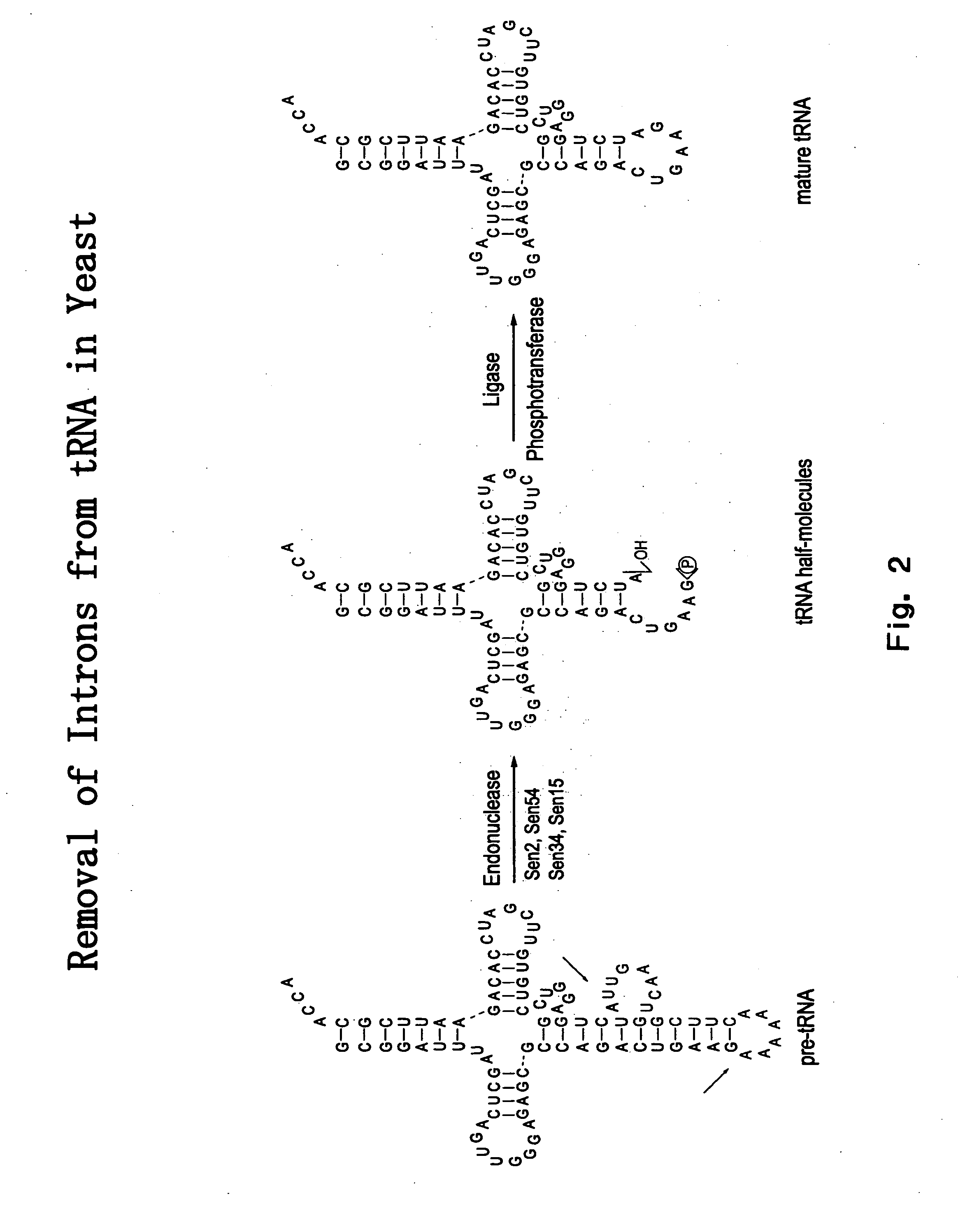
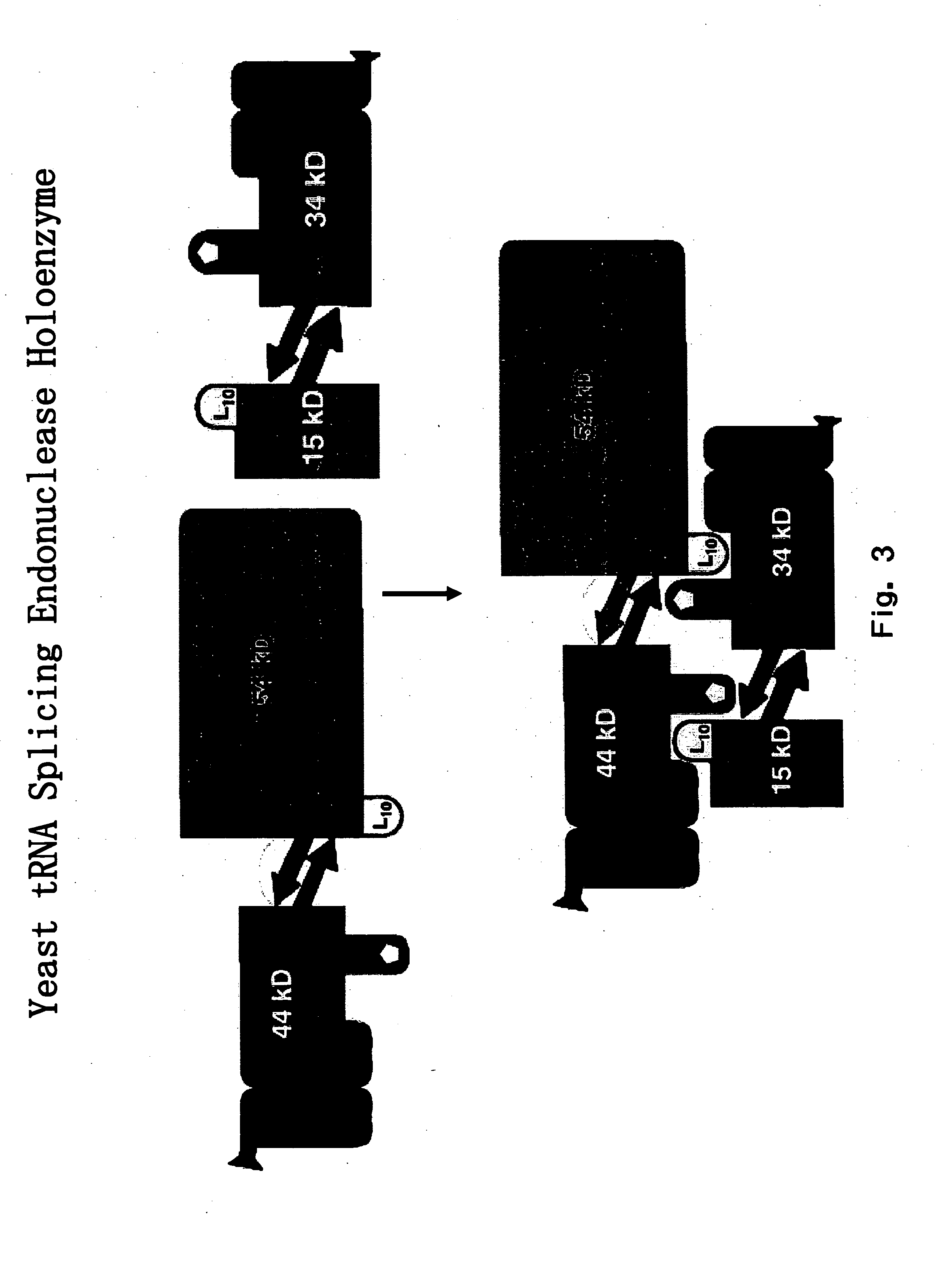
The invention provides human protein complexes with endonuclease activity. In particular, the invention provides human protein complexes with tRNA splicing endonuclease activity and / or 3' end pre-mRNA endonuclease activity. The invention also provides a splice variant of human Sen2, namely human Sen2deltaEx8, and human protein complexes comprising human Sen2deltaEx8. The human Sen2deltaEx8 complexes have pre-tRNA cleavage activity and / or 3' end pre-mRNA endonuclease activity. The invention also provides human protein complexes with pre-ribosomal RNA cleavage activity. The invention also provides antibodies that immunospecifically bind to a complex described herein or a component thereof, and methods of diagnosing, preventing, treating, managing or ameliorating a disorder utilizing such antibodies. The present invention also provides methods utilizing the complexes described herein, inter alia, in screening, diagnosis, and therapy. The invention further provides methods of preparing and purifying the complexes. The present invention further provides methods of identifying a compound that modulates the expression of a component of a complex described herein, the formation of a complex described herein or the activity of a complex described herein, and methods of preventing, treating, managing or ameliorating a disorder, such as a proliferative disorder, or a symptom thereof utilizing a compound identified in accordance with the methods.
Owner:PTC THERAPEUTICS INC
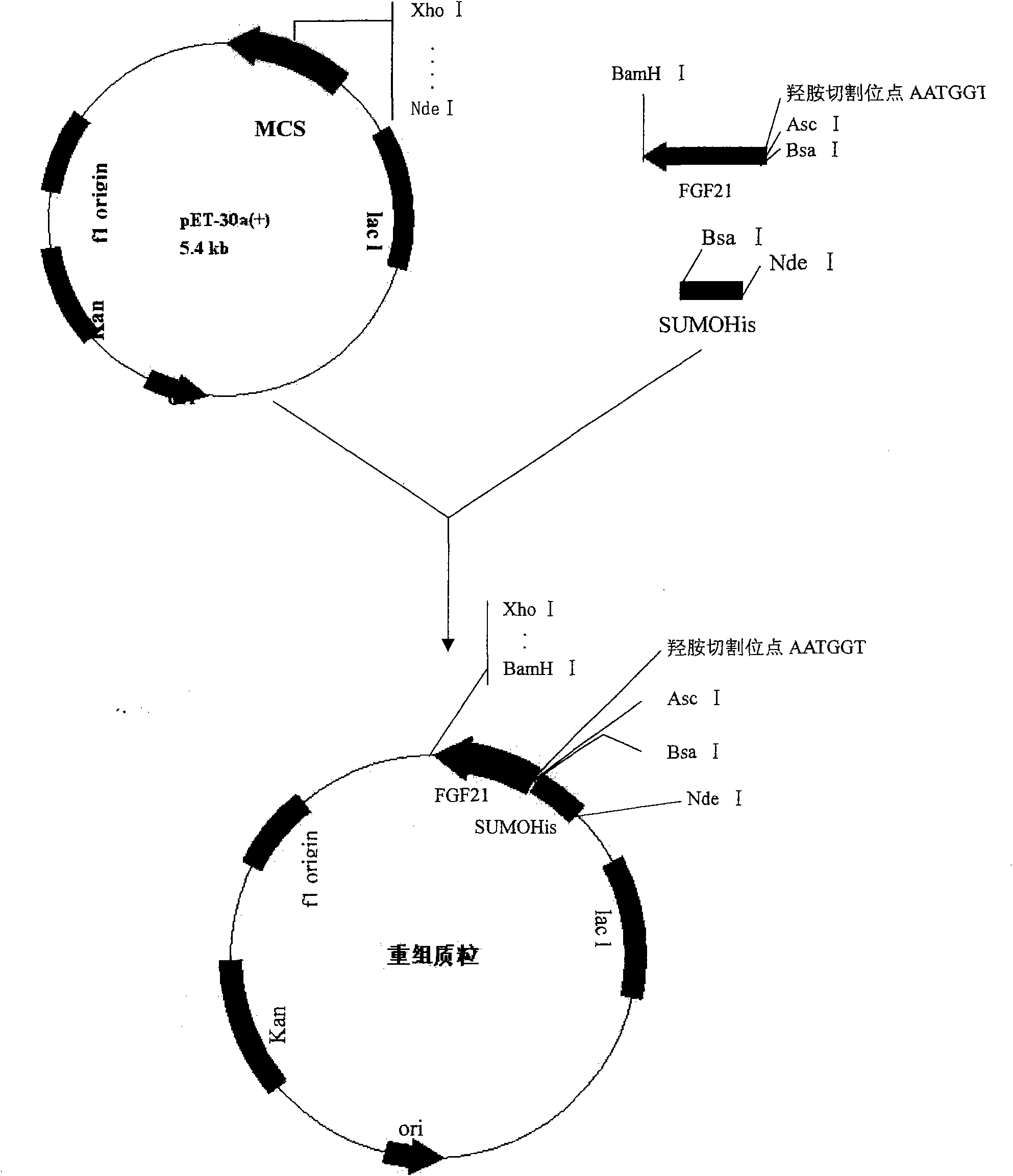
Human FGF21 mutant gene and method for preparing recombinant human FGF21 protein
InactiveCN101967485AStrong specificityImprove stabilityFungiBacteriaHydroxylamineHydroxylamine Hydrochloride
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Methods for identifying modulators of apoptosis
Owner:SANFORD BURNHAM MEDICAL RES INST
Preparation method for soluble truncated human tumor necrosis factor-related apoptosis inducing ligand (TRAIL) active protein
InactiveCN102021173ATruncated soluble expressionShort fermentation timeCell receptors/surface-antigens/surface-determinantsPeptide preparation methodsHumaninInclusion bodies
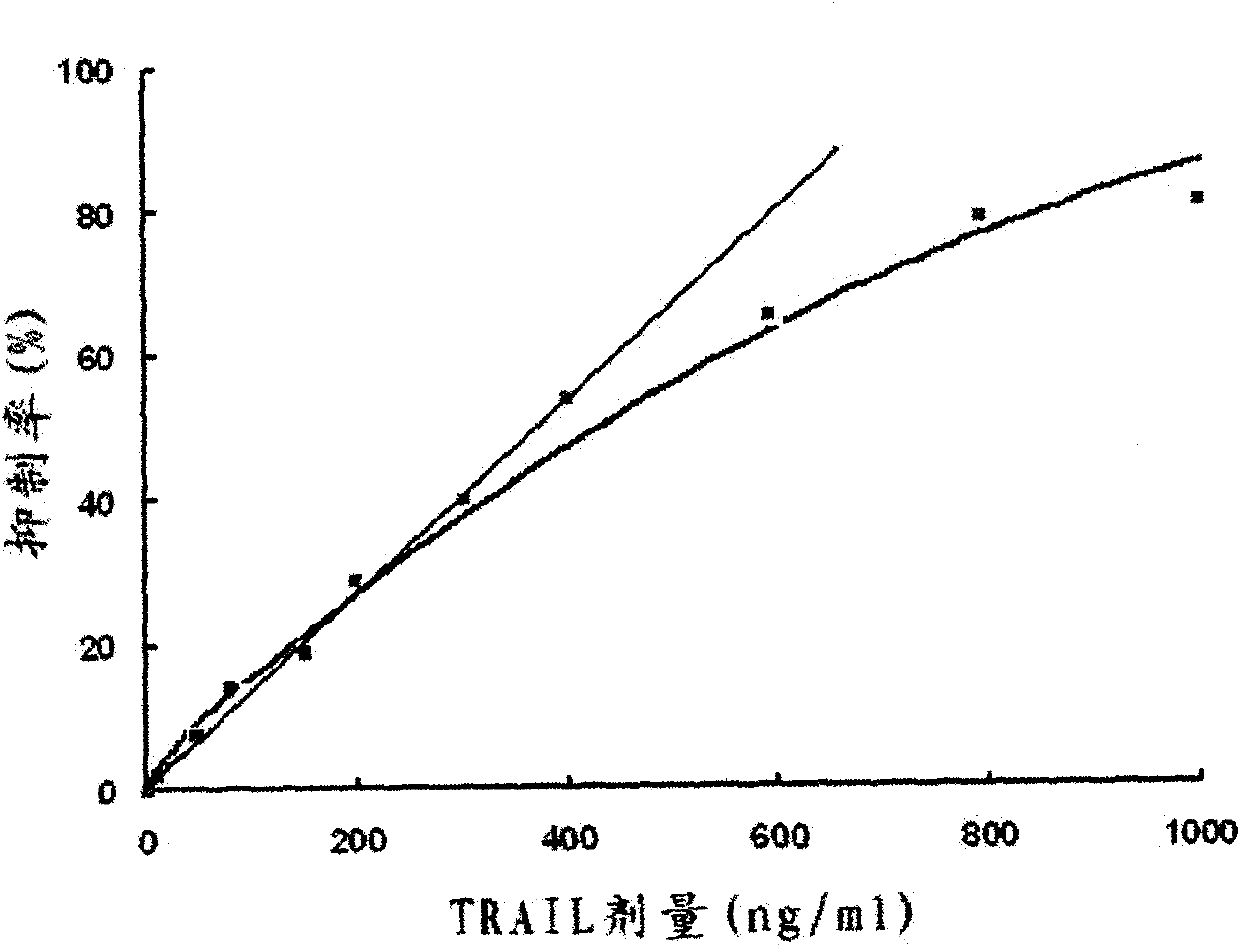
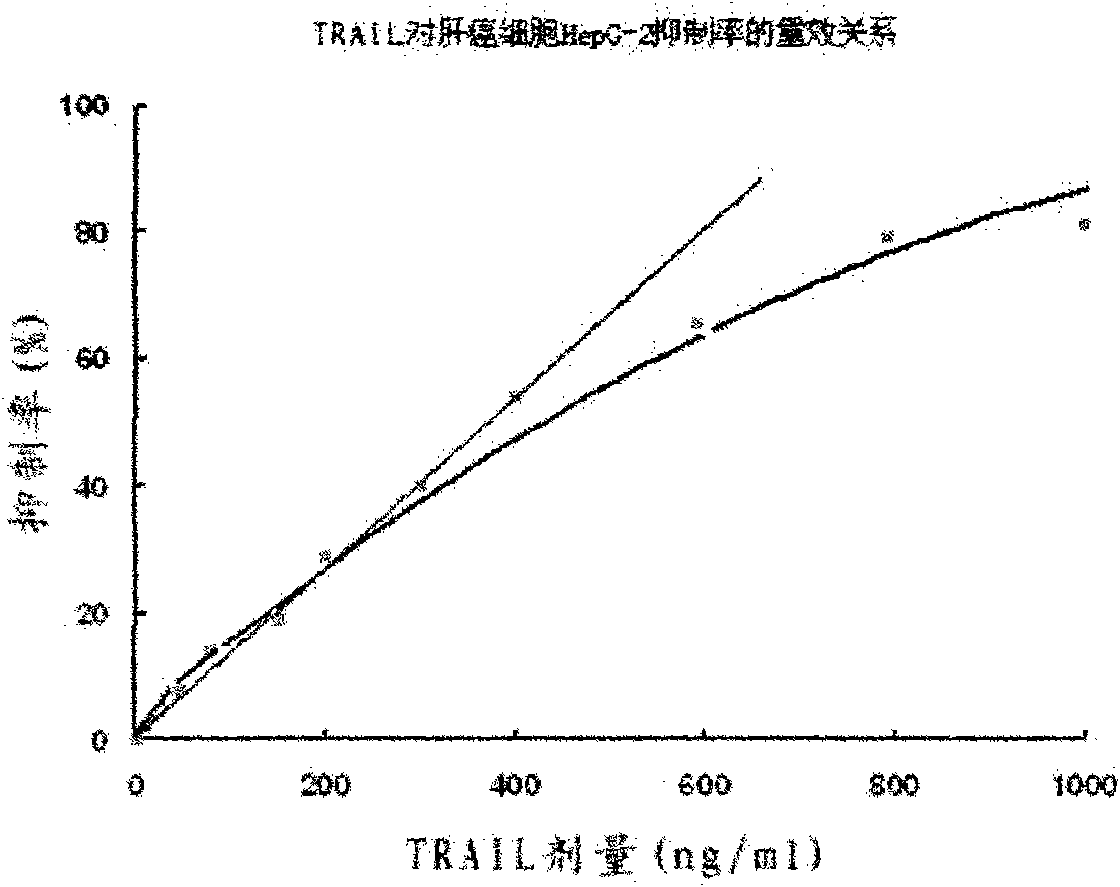
The invention provides a preparation method for a soluble truncated human tumor necrosis factor-related apoptosis inducing ligand (TRAIL) active protein, comprising the following steps of: (A) designing and synthetizing an oligomerization deoxyribonucleic acid (DNA) single-stranded segment according to the amino acid sequence of the human TRAIL protein issued by a Genebank by the preference of a bacterium genetic code; (B) synthetizing a complete double stranded DNA by three-time polymerase chain reaction (PCR); (C) carrying out amplification by utilizing a T-carrier, and inserting the amplified double stranded DNA segment into an expression carrier and screening a positive transformant; (D) expressing the truncated human TRAIL protein in escherichia coli at low temperature; (E) purifying the protein by using a three-step method; and (F) determining the truncated TRAIL protein. The invention realizes that the truncated human TRAIL protein is efficiently expressed in an escherichia coli cell and a purification preparation technique thereof and overcomes the problem that an infusible inclusion body is easy to form in the prior art. The method has the advantages of simple operation, short fermentation time and easy purification, and moreover, the protein is ensured not to have any modification, and the protein reaches electrophoretically pure. The invention can be directly used for the research work of biochemistry and molecular biology and the oncology and the preclinical stage of tumor treatment or the development of clinical drugs.
Owner:HUBEI UNIV
Anti-human PD-1 protein antibody, and coding gene and application thereof
InactiveCN106336460AEffective treatmentEffective preventionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSingle-Chain AntibodiesHeavy chain
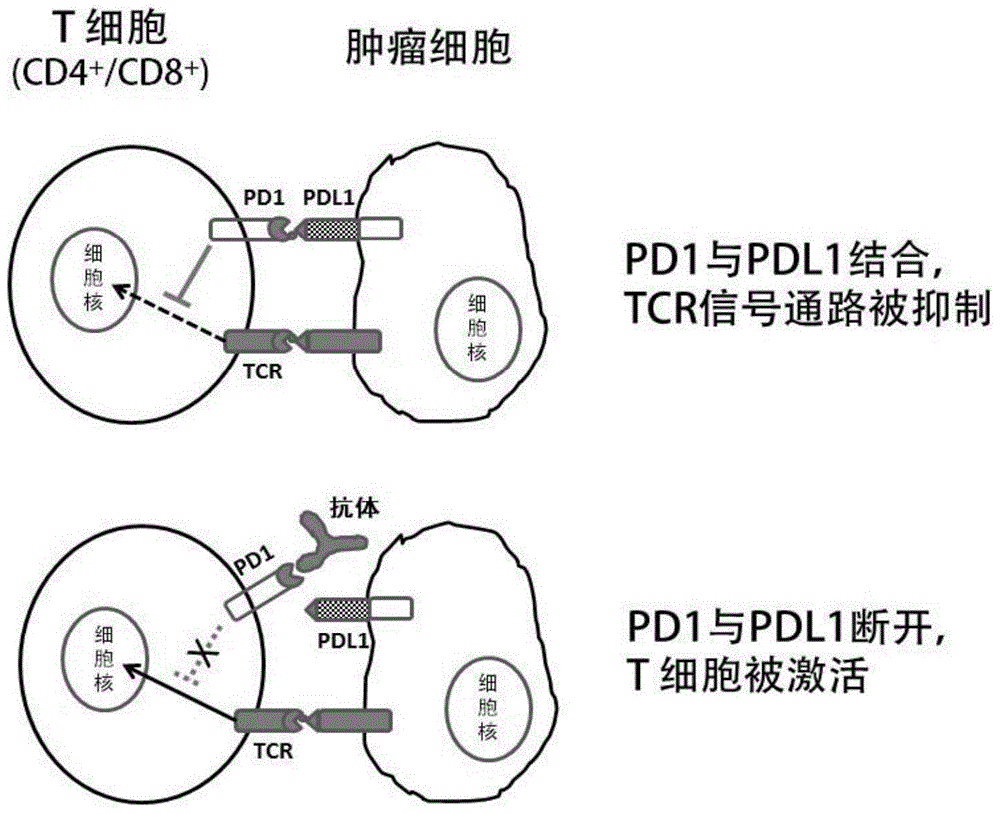
The invention discloses an anti-human PD-1 protein antibody, and a coding gene and application thereof. The antibody comprises a heavy-chain variable region and a light-chain variable region; the amino acid sequences of three hypervariable regions, i.e., CDRH1, CDRH2 and CDRH3, of the heavy-chain variable region are GGSFSGYYWS, EINHSGSTNYNPSLKS and GSPDSSRARGYYMDV, respectively; and the amino acid sequences of three hypervariable regions, i.e., CDRL1, CDRL2 and CDRL3, of the light-chain variable region are RASQGIRNDLG, AASSLQS and LQHNSYPL, respectively. According to the invention, the anti-human PD-1 protein antibody prepared by constructing a human single-chain antibody library and screening single-chain antibodies by using ribosome displaying technology can specifically bind to human PD-1 protein, has high affinity, is applicable to detection of cells expressing PD-1 ad applicable as a fully human-derived anti-human PD-1 protein antibody for treating human diseases, and effective prevents and treats tumor diseases.
Owner:杭州贝颐药业有限公司
Q3 sparc deletion mutant and uses thereof
The invention provides for SPARC polypeptides with a mutation corresponding to a deletion of the third glutamine in the mature form of the human SPARC protein, nucleic acids encoding such polypeptides, antibodies against such polypeptides, and methods of the use of such polypeptides, nucleic acids, and antibodies.
Owner:ABRAXIS BIOSCI LLC
Methods for identifying modulators of apoptosis
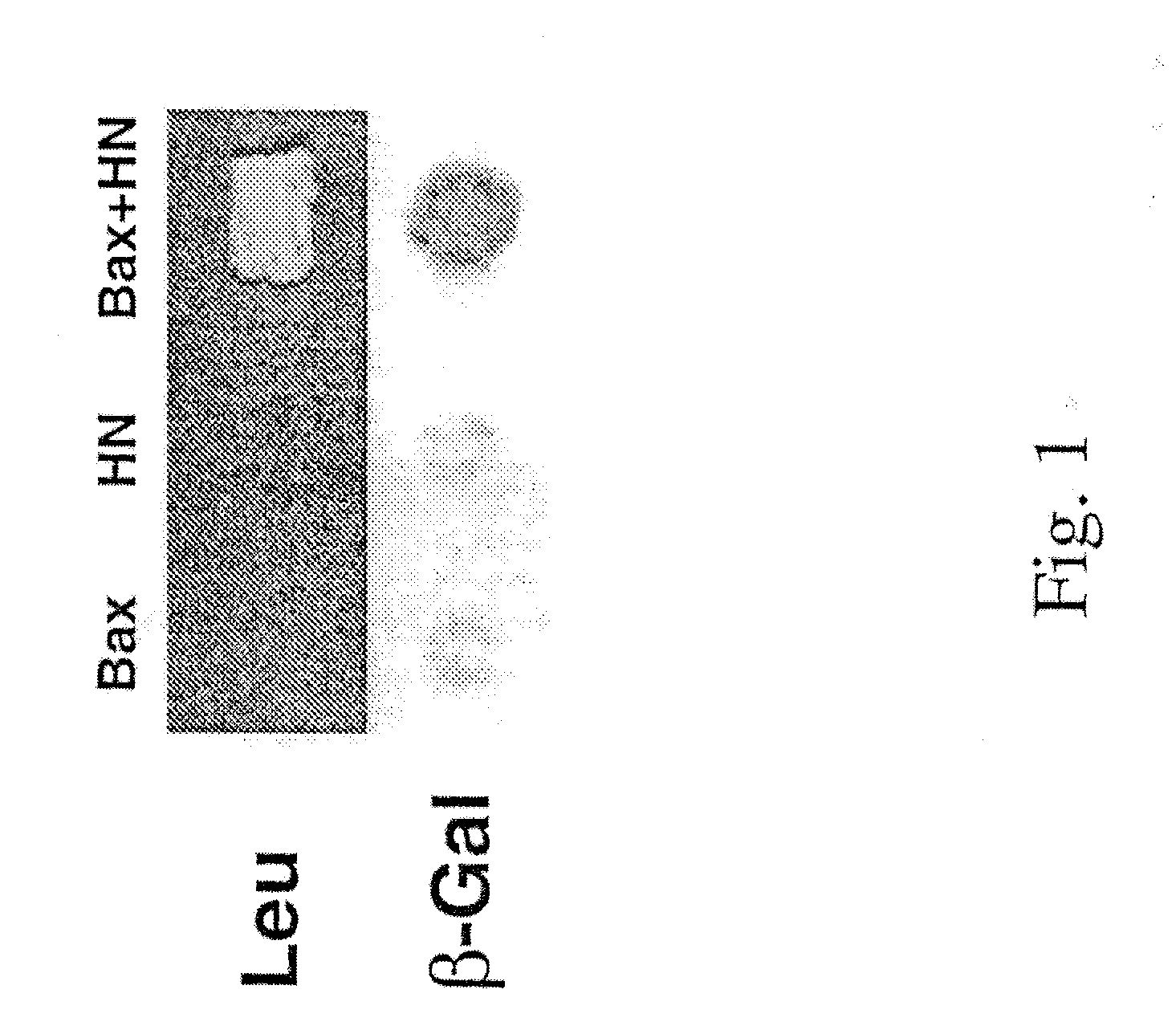
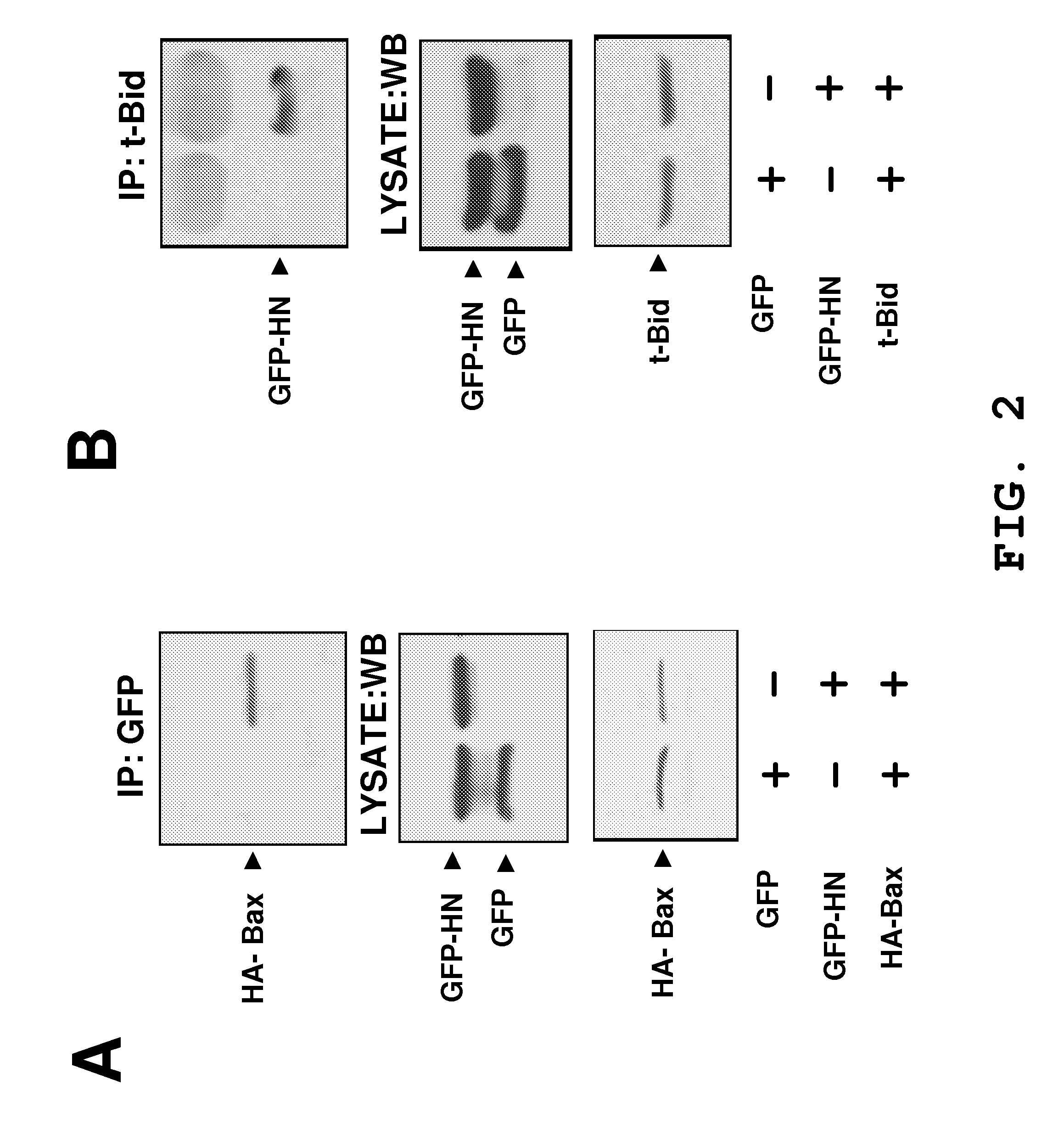
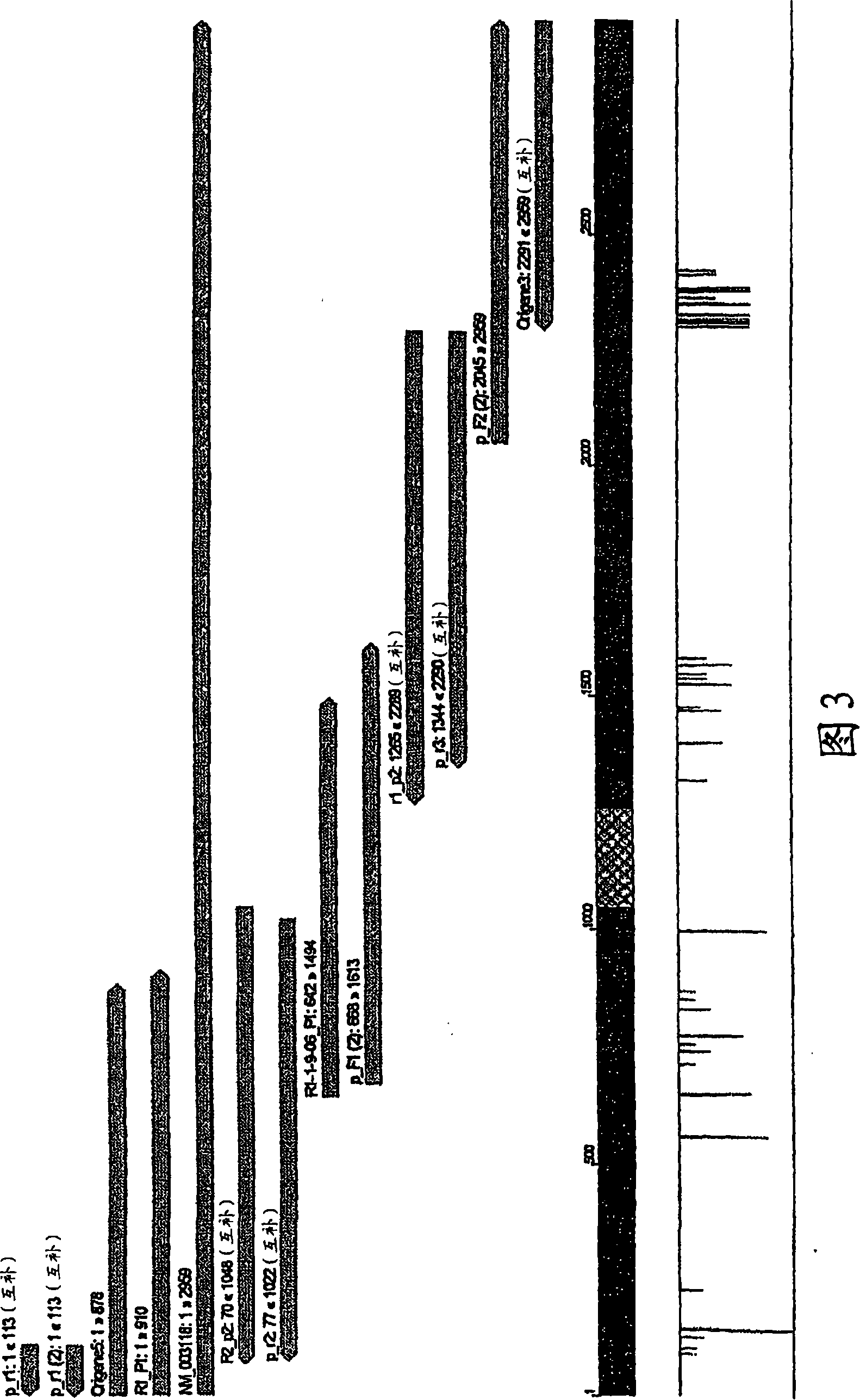
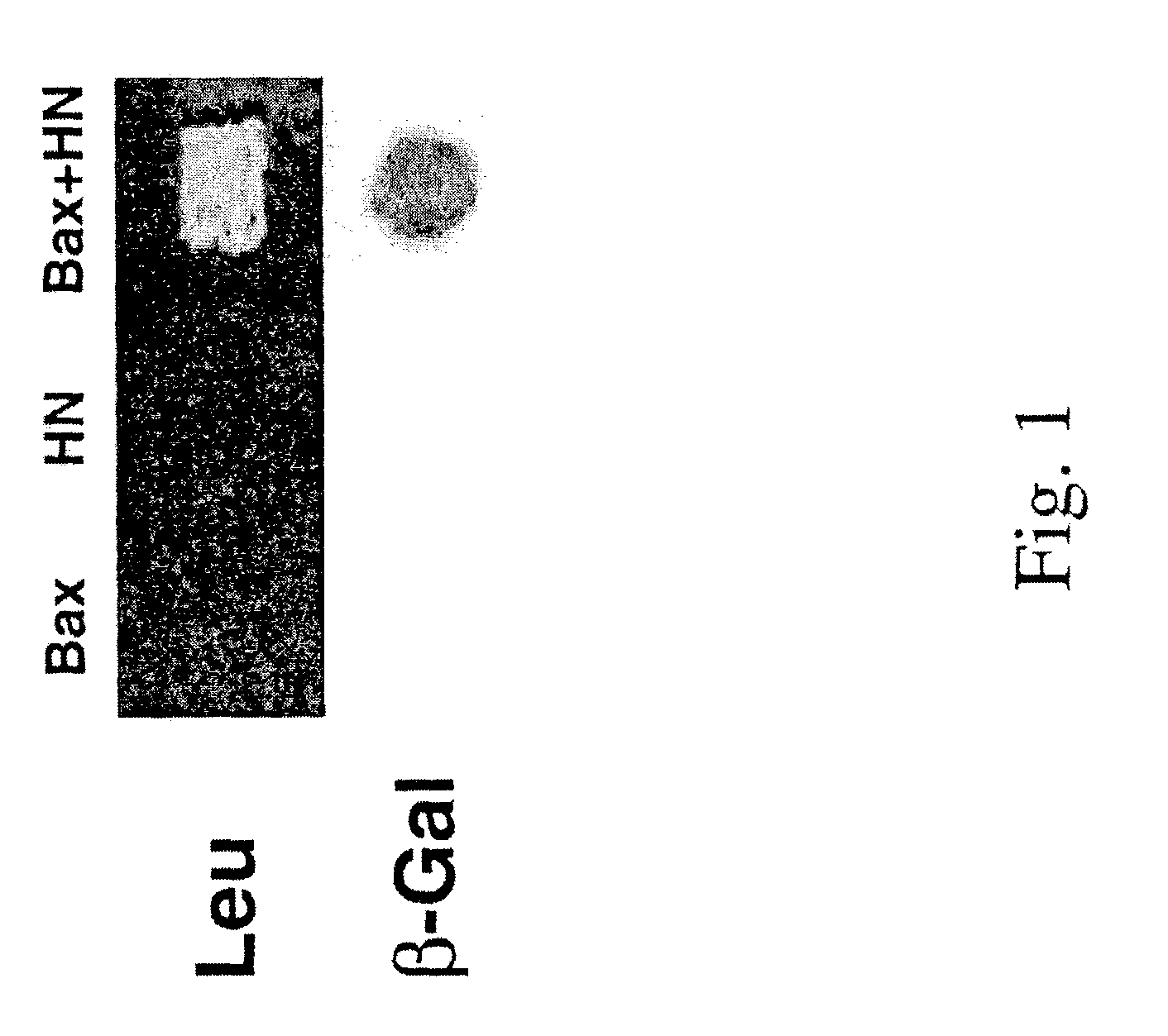
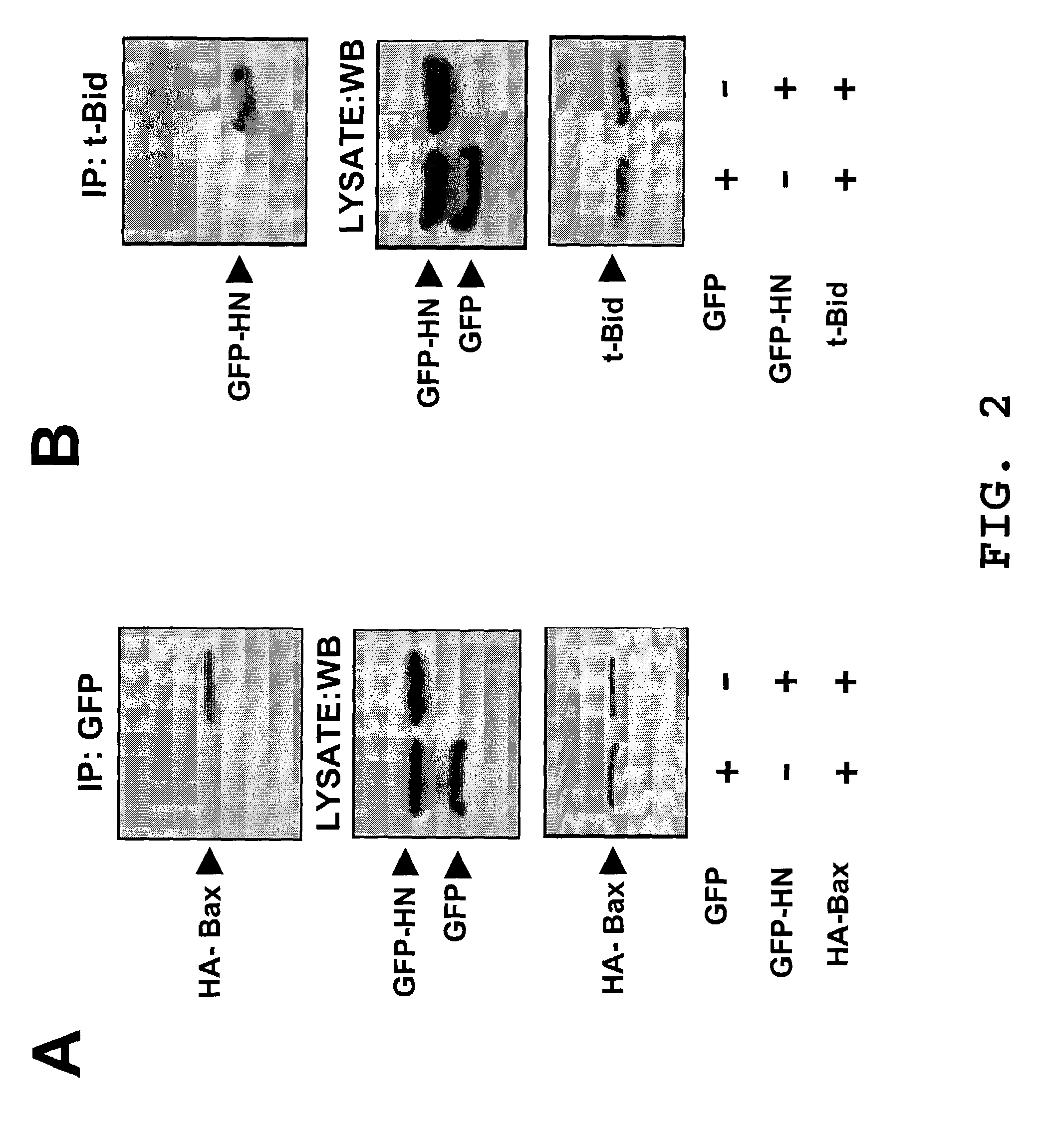
The invention provides a method of identifying an effective compound that modulates the binding of Humanin to Bax or Bid. The invention also provides a method of identifying an effective compound that modulates an activity of Bax or Bid. In addition, the invention provides a method of identifying a Humanin-like compound that binds to Bax or Bid or modulates an activity of Bax or Bid, or inhibits the apoptotic activity of Bax or Bid. The invention further provides an isolated polypeptide containing a mitochondrial-derived form of Humanin (SEQ ID NO:3) or a functional fragment thereof where the fragment contains the methionine at position 16 of SEQ ID NO:3.
Owner:BURNHAM INST THE
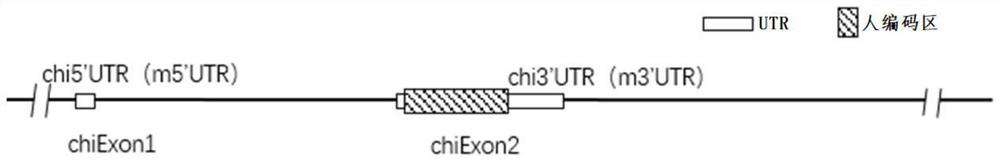
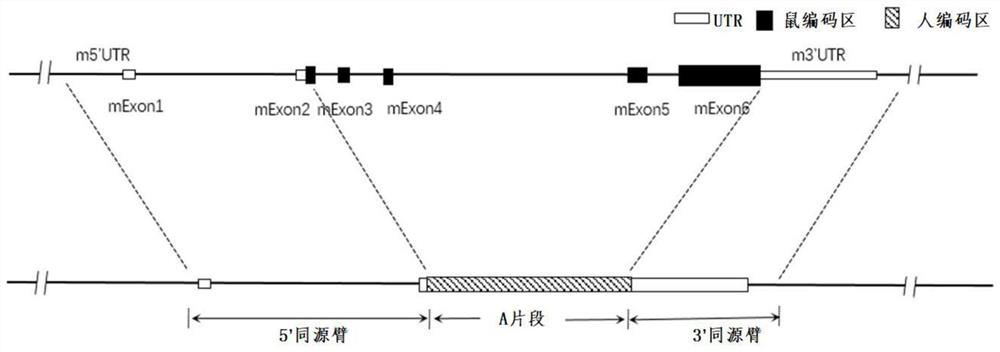
Construction method and application of humanized cell factor IL15 gene modified non-human animal
The invention provides a construction method of a humanized IL15 gene modified non-human animal. A nucleotide sequence encoding a human protein IL15 is introduced into a genome of an animal by using ahomologous recombination mode, so as to construct a gene modified humanized animal. A body of the animal can normally express the human or humanized protein IL15, promote development of human T cellsand NK cells and can serve as an animal model for human IL15 signal mechanism researching and screening of drugs for diseases such as tumors, and thus, the animal has an important application value in research and development of new drugs for immunization target points.
Owner:BIOCYTOGEN JIANGSU CO LTD +1
Anti-PD-L1/anti-LAG3 bispecific antibody and uses thereof
ActiveCN110678484AHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsHumaninAntiendomysial antibodies
The invention provides an anti-PD-L1 / anti-LAG3 bispecific antibody, which can effectively block the interaction between PD-L1 and PD-1 and the interaction between LAG3 and a ligand (MHC II molecules,FGL1, etc.). The bispecific antibody is highly affinity to PD-L1 proteins (human PD-L1 protein, for example) and LAG3 proteins (human LAG3 protein, for example). The invention also provides an antibody and segment that has specificity on PD-L1 or LAG3 proteins; or an antibody and segment that has specificity on one or more other antigens.
Owner:I MAB BIOPHARMA HANGZHOU CO LTD
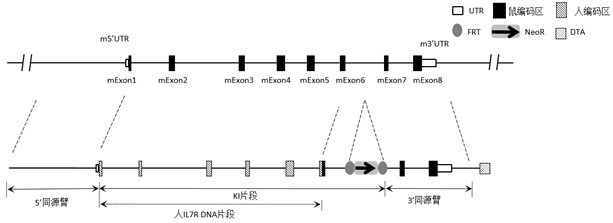
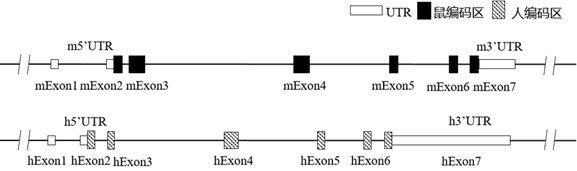
Construction method of IL7R gene humanized modified animal model and corresponding application
The invention provides a construction method of an IL7R gene humanized modified animal model, a humanized IL7R protein, a humanized IL7R gene, a targeting vector of the IL7R gene and an application ofthe targeting vector in the field of biological medicines. A part of nucleotide sequences for coding the human IL7R protein are introduced into a non-human animal genome in a homologous recombinationmanner, and the animal model can normally express the humanized IL7R protein in vivo, and can be used for human IL7R signal mechanism research, tumor and immune related disease drug screening, and important application value is realized on the research and development of new drugs of immune targets.
Owner:BIOCYTOGEN PHARMACEUTICALS (BEIJING) CO LTD
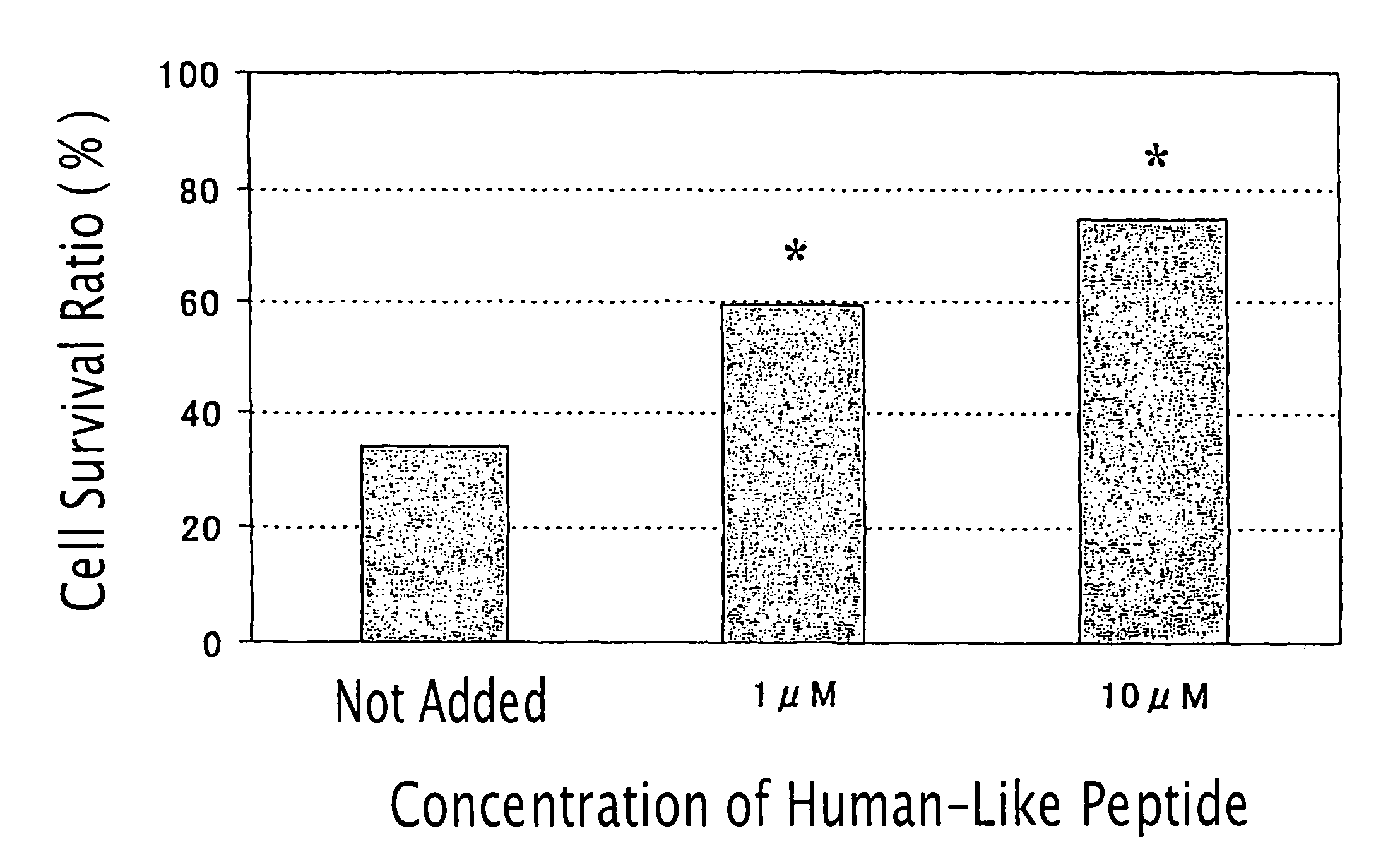
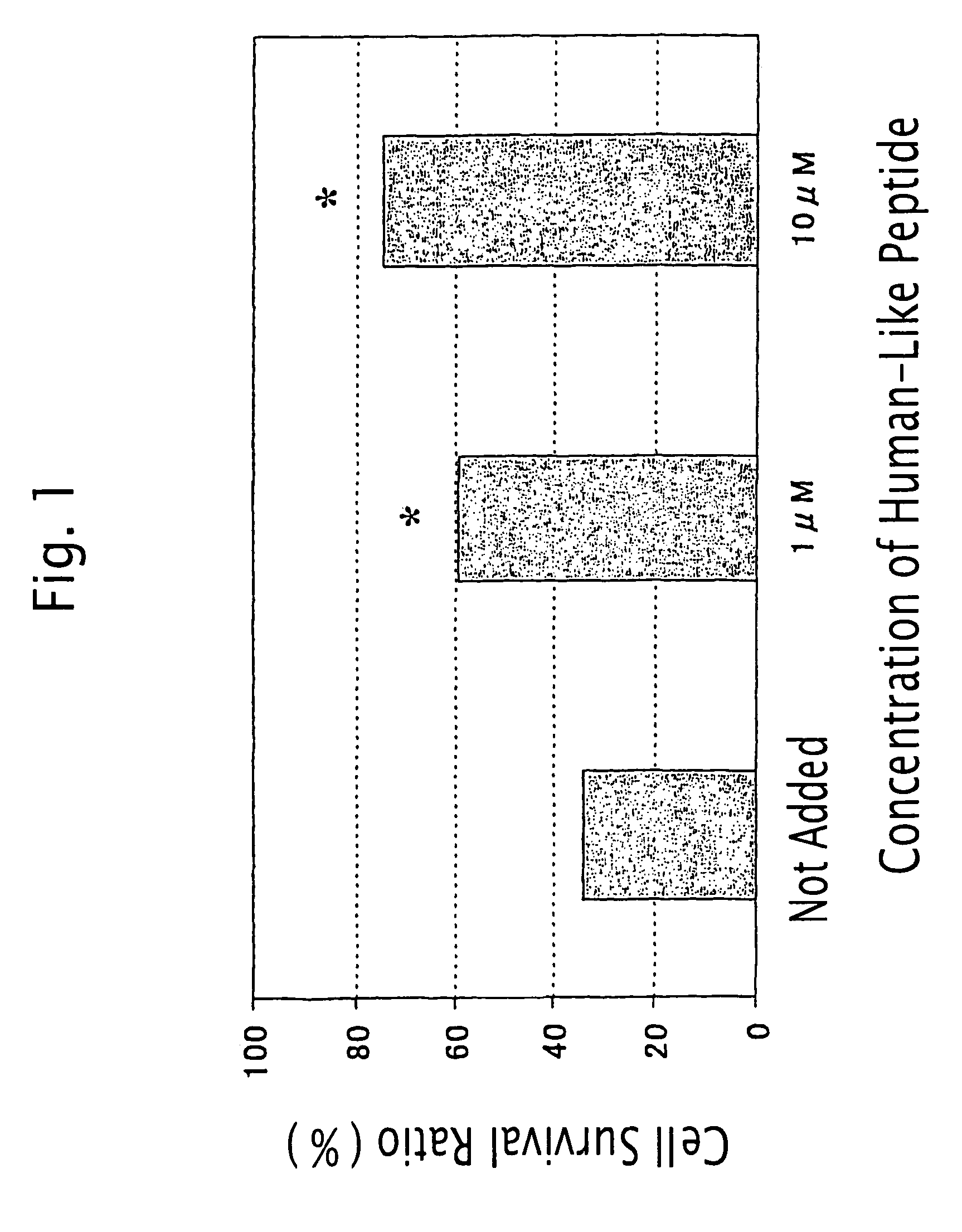
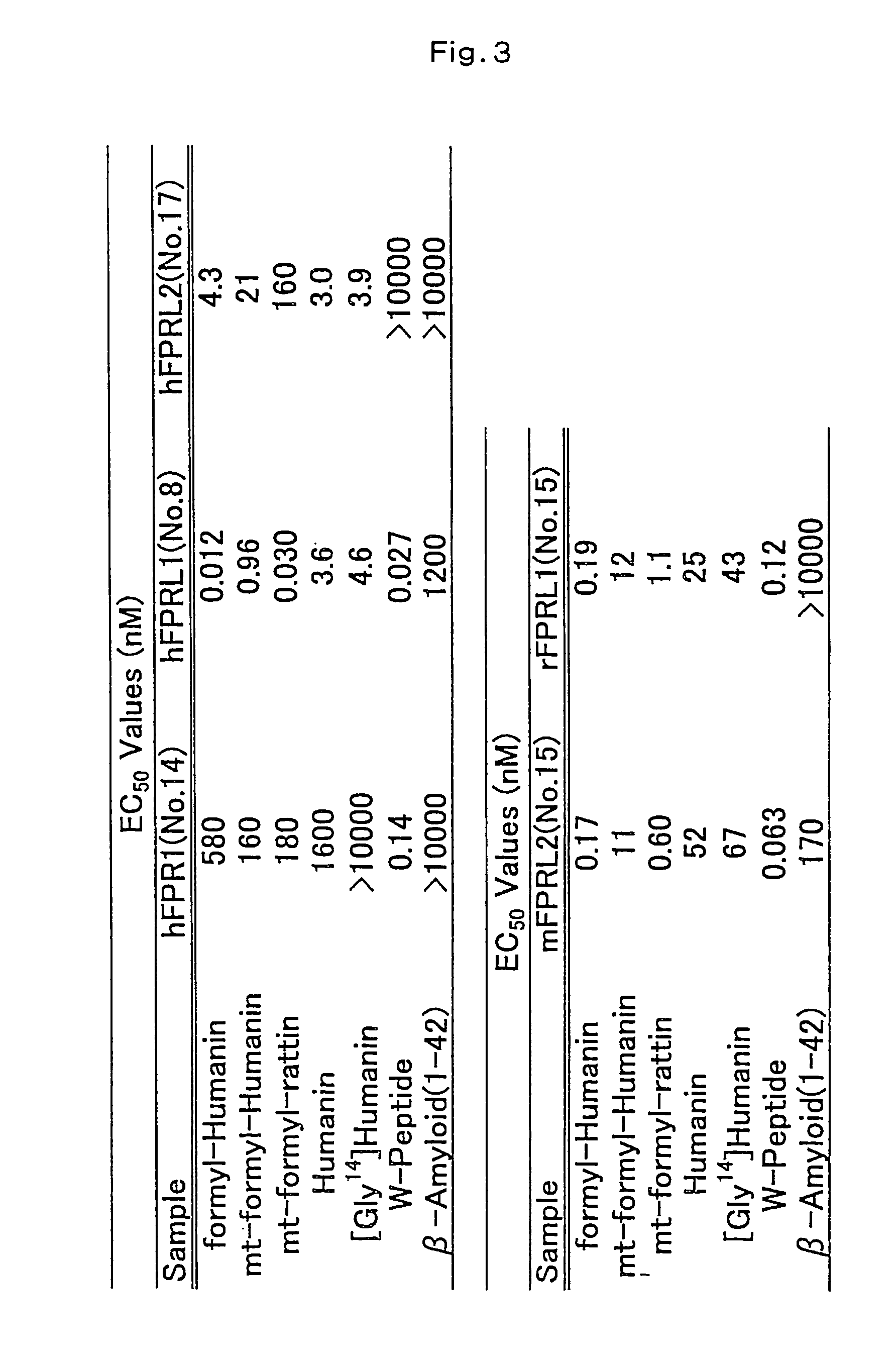
Humanin-like peptide and use thereof
A novel polypeptide having a cell death inhibitory activity and use thereof is provided. The polypeptide and the polynucleotide encoding it can be used as a diagnostic, therapeutic or prophylactic agent for various diseases and disorders. Certain suitable diseases and disorders which may be diagnosed, treated, or prevented with the polypeptide and the polynucleotide encoding it are selected from neurodegenerative diseases, brain dysfunctions, cancers, immunological disease, infections, gastrointestinal diseases, circulatory diseases, and endocrine diseases. The polypeptide and the polynucleotide encoding it can be used as a cell death inhibitor.
Owner:TAKEDA PHARMA CO LTD
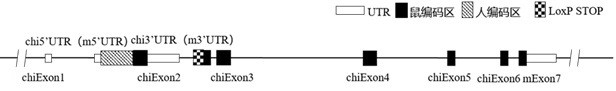
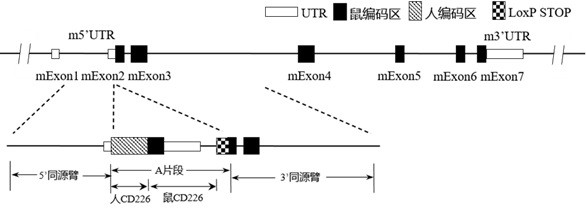
Construction method and application of CD226 gene-humanized non-human animal
ActiveCN111793647ASpeed up the R&D processShorten the timeCompounds screening/testingImmunoglobulin superfamilyBiotechnologyImmunologic disorders
The invention provides a construction method of a CD226 gene humanized modified non-human animal. By utilizing a homologous recombination manner, a nucleotide sequence for encoding a human CD226 protein is introduced into a non-human animal genome. The humanized CD226 protein can be normally expressed in an animal body, and the non-human animal can be used as an animal model for researching humanCD226 signal mechanism and screening drugs for tumor and immune diseases, and has important application value for research and development of new drugs for immune targets. The invention also providesa CD226 chimeric protein, a CD226 chimeric gene, a targeting vector of the CD226 gene, the non-human animal constructed by the construction method, and an application of the non-human animal in the field of biological medicines.
Owner:BIOCYTOGEN PHARMACEUTICALS (BEIJING) CO LTD
Anti-human cardiac troponin I antibody and applications thereof
ActiveCN111018983AHigh activityHigh affinityImmunoglobulins against animals/humansDisease diagnosisI antibodyHumanin
The invention relates to a novel isolated binding protein containing a cTnI antigen binding domain, and researches of the preparation, the applications and the like of the binding protein. The bindingprotein is high in activity, has high affinity with human cTnI protein, and can be widely applied to the field of cTnI protein detection.
Owner:DONGGUAN PENGZHI BIOTECH CO LTD
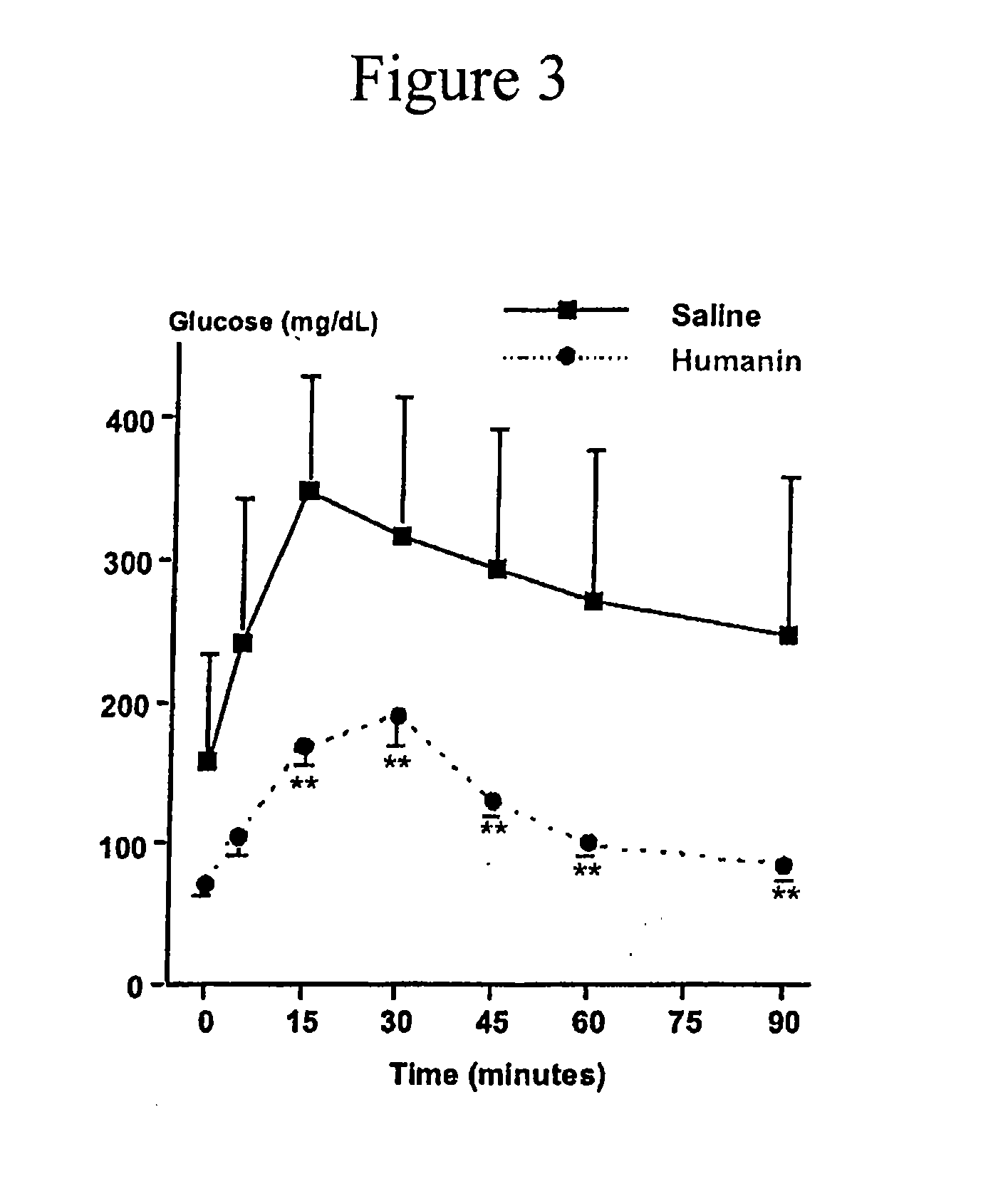
Method of treatment of type-1 diabetes with a humanin analogue
ActiveUS7998928B2Improve survivalOrganic active ingredientsPeptide/protein ingredientsHumaninApoptosis
Owner:RGT UNIV OF CALIFORNIA
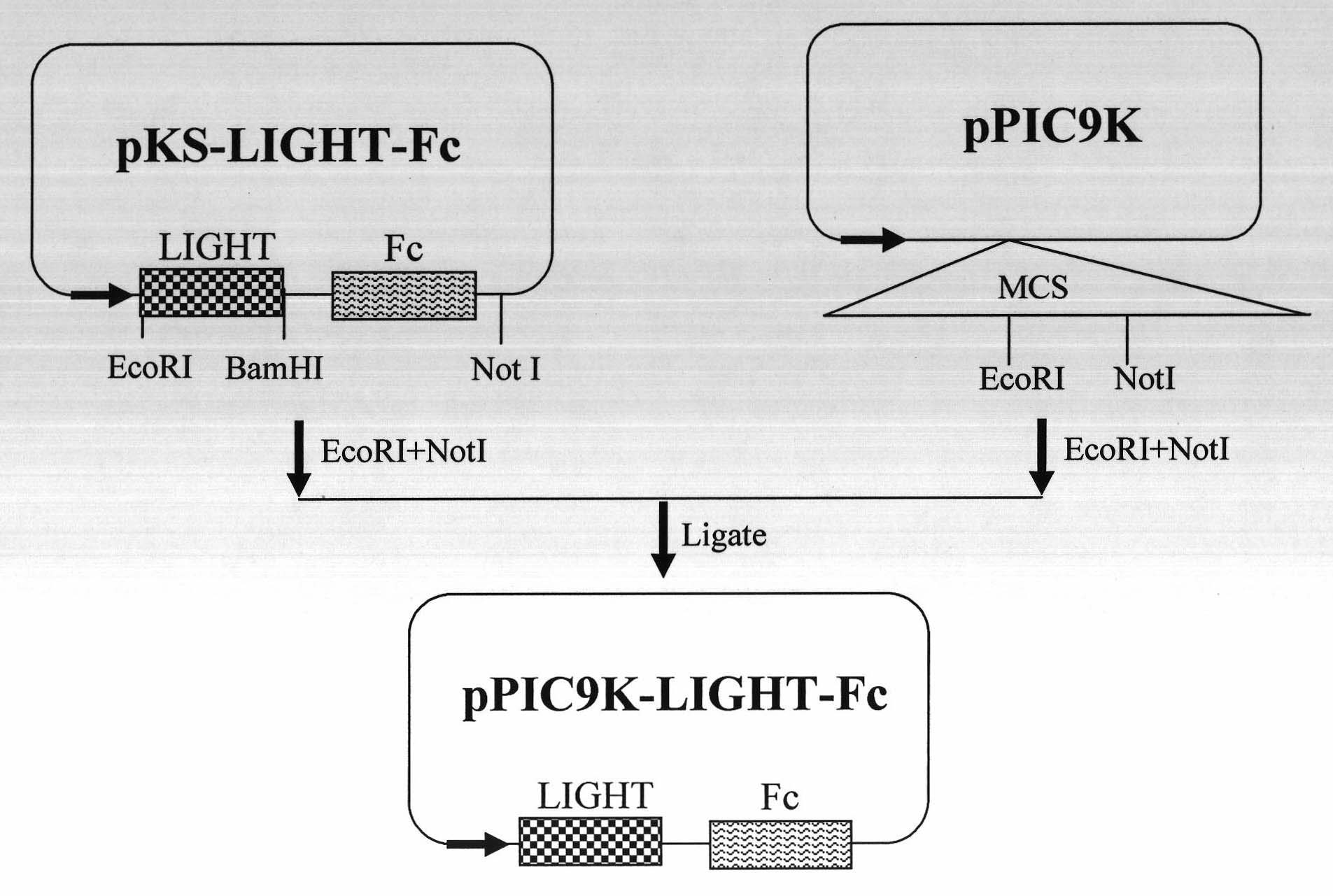
Preparation method of human LIGHT-Fc fusion protein
InactiveCN101993883AEasy to operateLow costHybrid peptidesVector-based foreign material introductionBiological bodyBiology
The invention discloses a method for preparing human LIGHT-Fc fusion protein by using a pichia expression system, comprising the following steps of: (1) constructing recombinant expression plasmid pPIC9K-LIGHT-Fc; (2) preparing and screening expression human LIGHT-Fc fusion protein yeast engineering bacteria; and (3) expressing and evaluating the human LIGHT-Fc fusion protein in pichia. The invention makes up the deficiency of the traditional human LIGHT preparation method and the expression mode of a prokaryotic expression system, otherwise, if being used for producing industrial human LIGHT protein, the invention has the advantages of simple operation, short growth cycle of raw organisms, large production scale (capable of carrying out high-density fermentation), low extraction cost, high activity of products, and the like and has important industrial application prospect and actual significance.
Owner:EAST CHINA NORMAL UNIVERSITY
Transposase for efficiently mediating exogenous gene integration, and use thereof
ActiveCN105481984AEfficient Guided AggregationIntegration mediationGenetic material ingredientsTransferasesTransposaseTransposon integration
The invention belongs to the field of molecular biology, and relates to a transposase for efficiently mediating exogenous gene integration, and a use thereof. The transposase is a fusion protein, and fuses a human c-myc pyrenoid positioning signal, and the human c-myc pyrenoid positioning signal can effectively guide aggregation of the transposase in a nucleus. The transposase is loaded in a transposon integration system, can mediate efficient integration of the exogenous gene in host cells, and can efficiently and stably express.
Owner:SHANGHAI CELL THERAPY RES INST +2
Compositions to prevent and treat type-1 diabetes
ActiveUS20100130412A1Improve survivalOrganic active ingredientsPeptide/protein ingredientsHumaninInsulin activity
Owner:RGT UNIV OF CALIFORNIA
Method for expressing human humanin protein in peanut seed
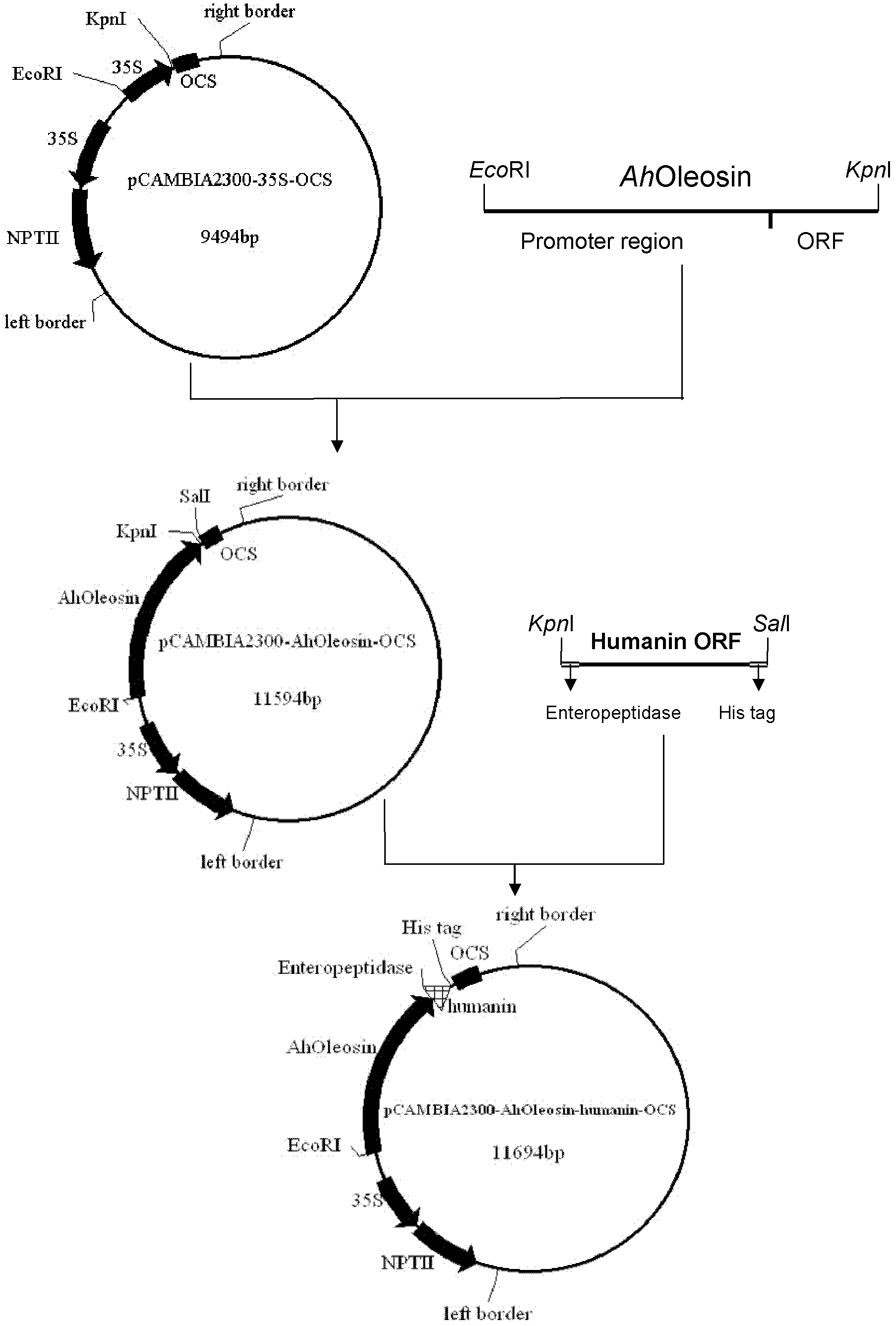
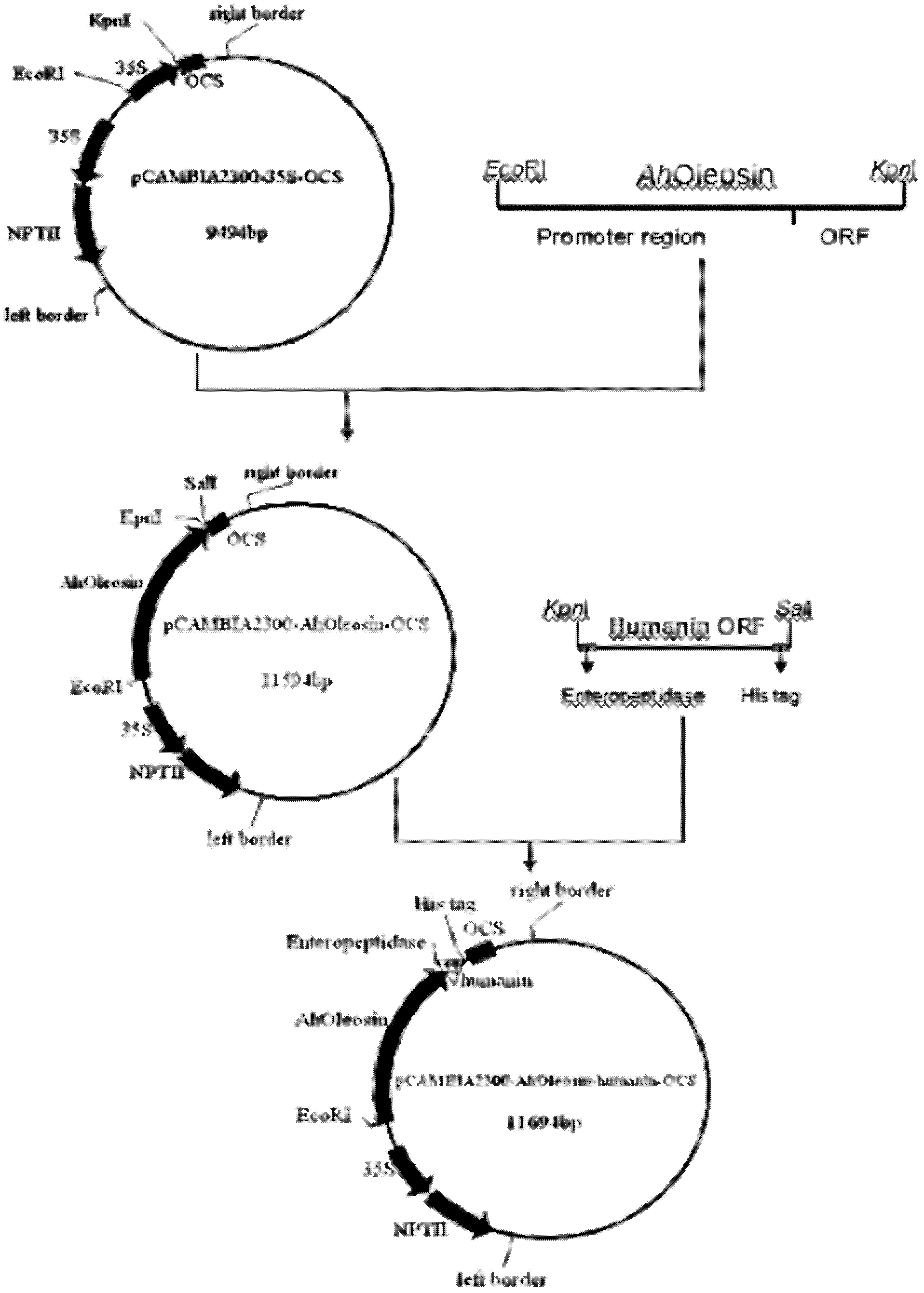
InactiveCN102321665AHigh oil contentRich in OleosinFermentationGenetic engineeringHumaninEnteropeptidase
The invention discloses a method for expressing human humanin protein in peanut seeds, which comprises the following steps: connecting humanin protein coding gene to a 3' end of an oleosin gene by a genetic engineering means, establishing an 'oleosin-erepsin-human humanin protein' plant expression vector driven by an oleosin promoter, transforming peanuts to realize high-level expression of recombinant protein in peanut seeds with the accumulation of oil body, then performing centrifugation of fusion protein, digesting the fusion protein by exo-protease, performing centrifugation, removing the oil phase and recovering water phase, performing purification to obtain human humanin protein. The recombinant protein obtained by the method of the invention is safe, has high activity, is very easy to be recovered and purified, can be stored in peanut seeds for a long term, is convenient for transportation, and greatly increases the crop added value.
Owner:山东省农业科学院高新技术研究中心
Genetically-modified non-human animal and application thereof
The invention relates to a genetically-modified non-human animal and application thereof, in particular to a transgenic mouse model and application thereof in the aspect of screening of a targeted medicine. According to the transgenic mouse model and application thereof in the aspect of screening of the targeted medicine, by targetedly knocking out and replacing a mouse protein C gene by means ofa human protein C gene expression cassette, a humanized protein C knock-in mouse is generated. The mouse has fertility, and can hybridize with another mouse disease model (such as a mouse in the deficiency of a blood coagulation factor VIII or a blood coagulation factor IX) to produce a humanized protein C mouse disease model (such as a humanized protein C factor VIII-deficient mouse model or a humanized protein C factor IX-deficient mouse model). The mouse model can be used for studying functions in vivo of human protein C and human activated protein C (APC). The mouse model is the first mouse model used for testing a therapeutic candidate medicine, which targets the human protein C or the APC, in vivo, and has very high economic value and scientific research value.
Owner:SHANGHAI RAAS BLOOD PRODUCTS CO LTD
Human RGR oncogene and truncated transcripts thereof detected in T cell malignancies, antibodies to the encoded polypeptides and methods of use
InactiveUS20040072295A1Immunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureT cellAntibody
Naturally-occurring variants of human Rgr oncogene protein, in particular, abnormally truncated variants found in T cell malignancies, as well as the human Rgr protein are encompassed by the present invention. Also included are antibodies thereto and nucleic acid molecules encoding human Rgr protein and naturally-occurring variants thereof. The present invention further provides methods for diagnosing and treating T cell malignancies associated with abnormally truncated transcripts of human rgr oncogene and / or abnormal truncation of human Rgr protein.
Owner:NEW YORK UNIVERSITY
Monoclonal antibody against CD19 as well as preparation method and application thereof
ActiveCN108840930AGood targeting and lethalitySignificant lethal effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyHeavy chainBiology
The invention provides a monoclonal antibody against CD19. The monoclonal antibody is characterized in that a complementary determining region (CDR) of a VH chain has amino acid sequences selected from the following groups: a heavy chain CDR1 shown as SEQ ID NO: 3, a heavy chain CDR2 shown as SEQ ID NO: 4 and a heavy chain CDR3 shown as SEQ ID NO: 5; a complementary determining region (CDR) of a VL chain has amino acid sequences selected from the following groups: a light chain CDR1 shown as SEQ ID NO: 6, a light chain CDR2 shown as SEQ ID NO: 7 and a light chain CDR3 shown as SEQ ID NO: 8. The invention further provides a preparation method and application of the monoclonal antibody. The monoclonal antibody can specifically recognize human CD19 proteins on the cell surface, is combined with the human CD19 by using 1*10<-7> M or lower KD and has the advantages that the immunogenicity is low, the HAMA reaction is not caused, and the like.
Owner:CELLYAN THERAPEUTICS WUHAN CO LTD
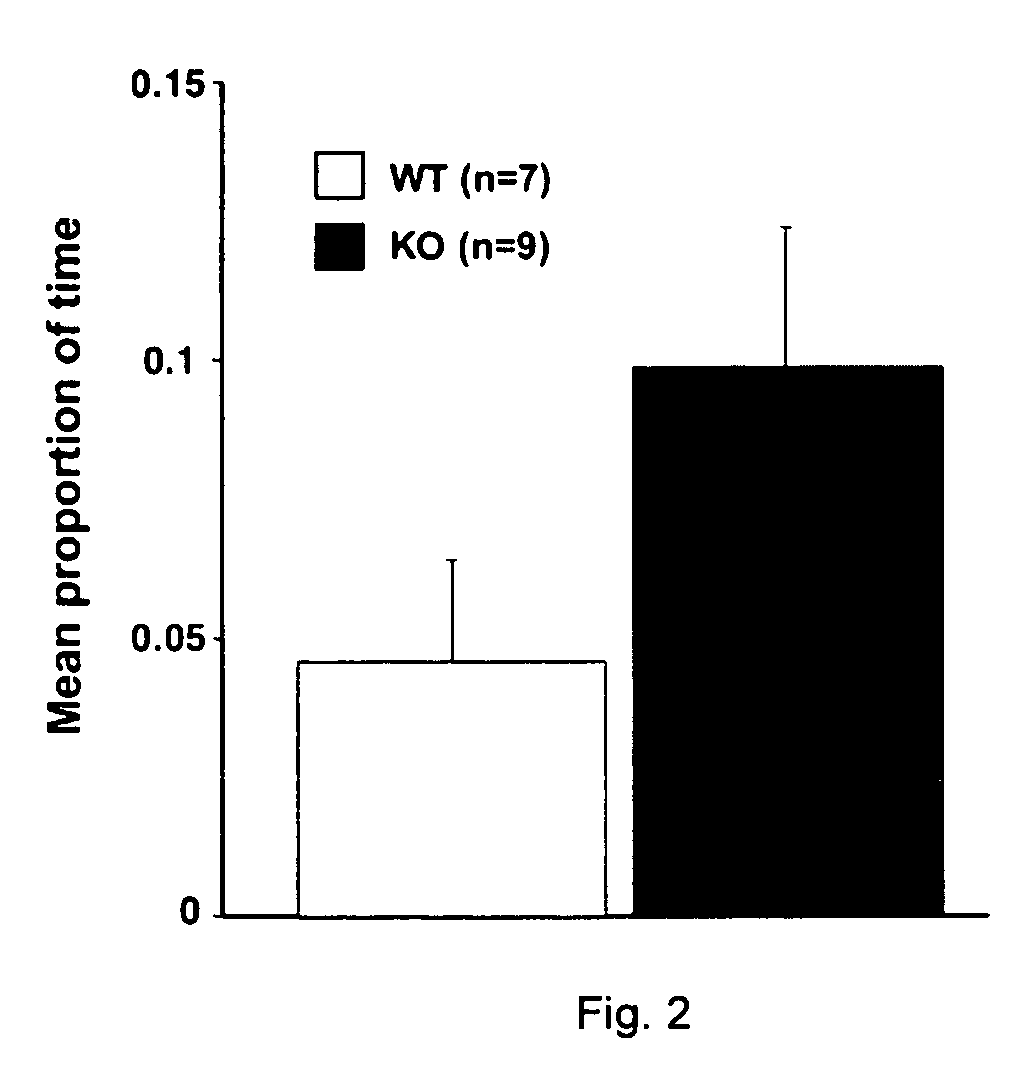
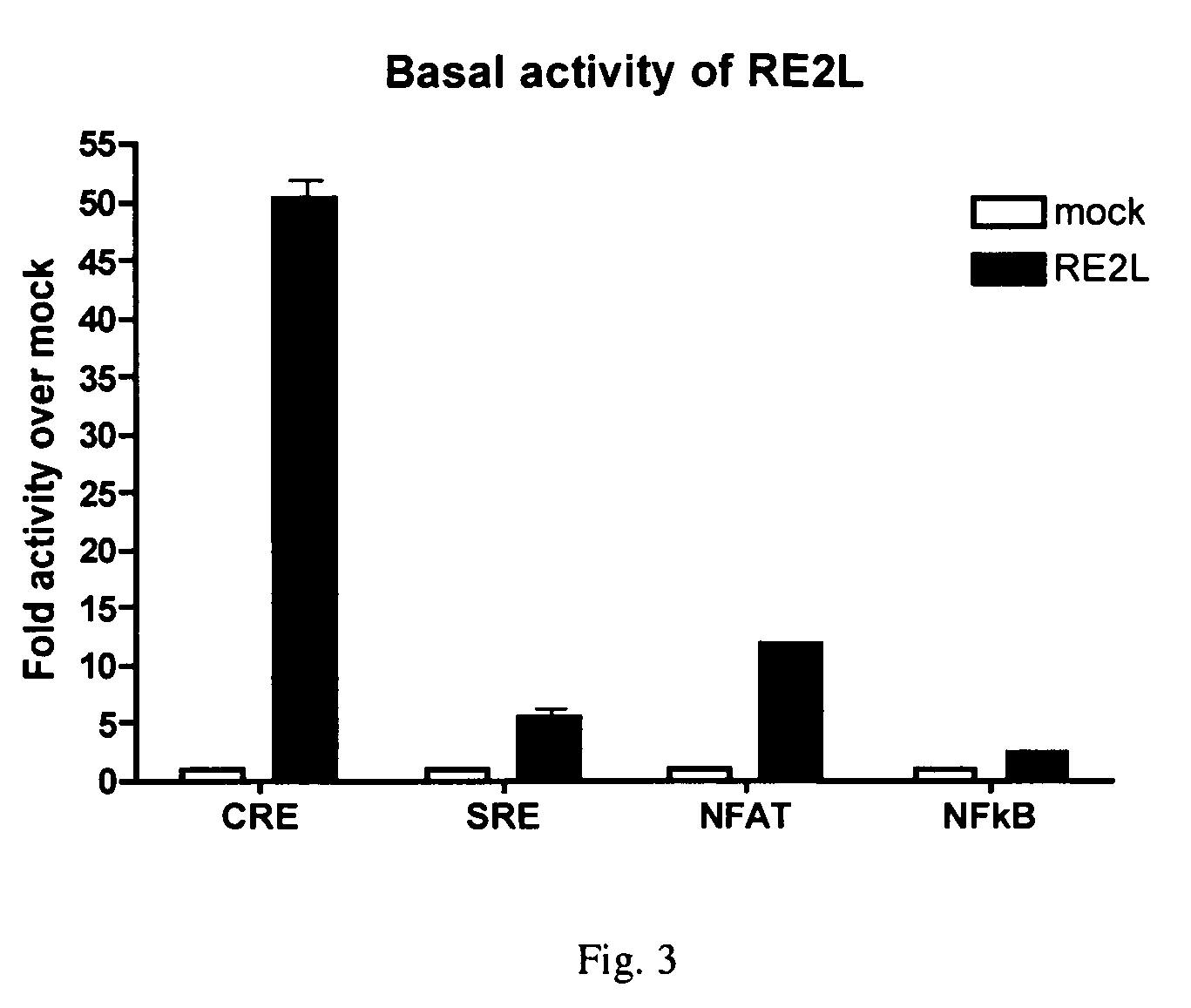
Assay methods for identifying RE2-like antagonists, methods of use, and non-human transgenic animals
InactiveUS20060123502A1Relieve pressureReduce anxietyGenetic material ingredientsAntibody ingredientsDiseaseAssay
Provided is a human RE2-L protein, as well as the encoding nucleic acid, methods for screening for agents capable of modulating RE2-L related activity and treating RE2-L-mediated conditions. Further provided are animal models useful for screening agents capable of ameliorating or reducing anxiety related disorders and obsessive-compulsive disorders.
Owner:REGENERON PHARM INC
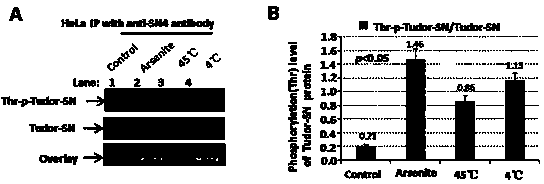
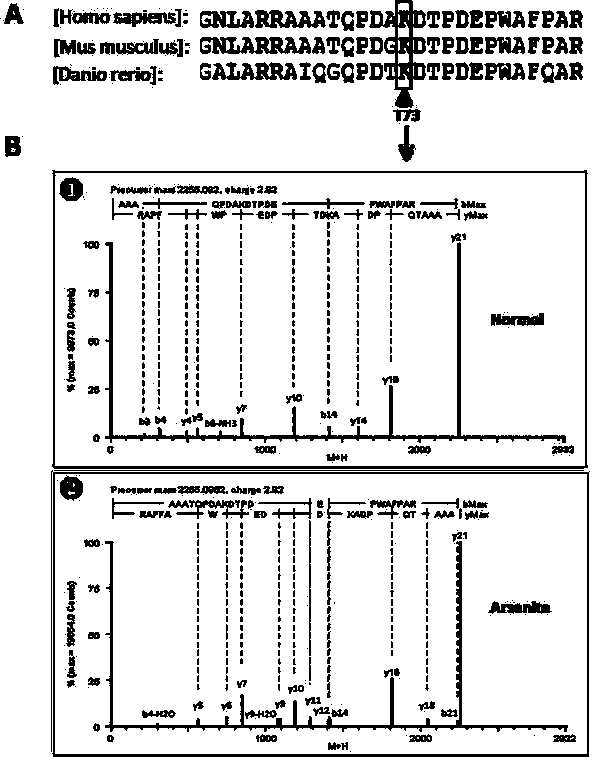
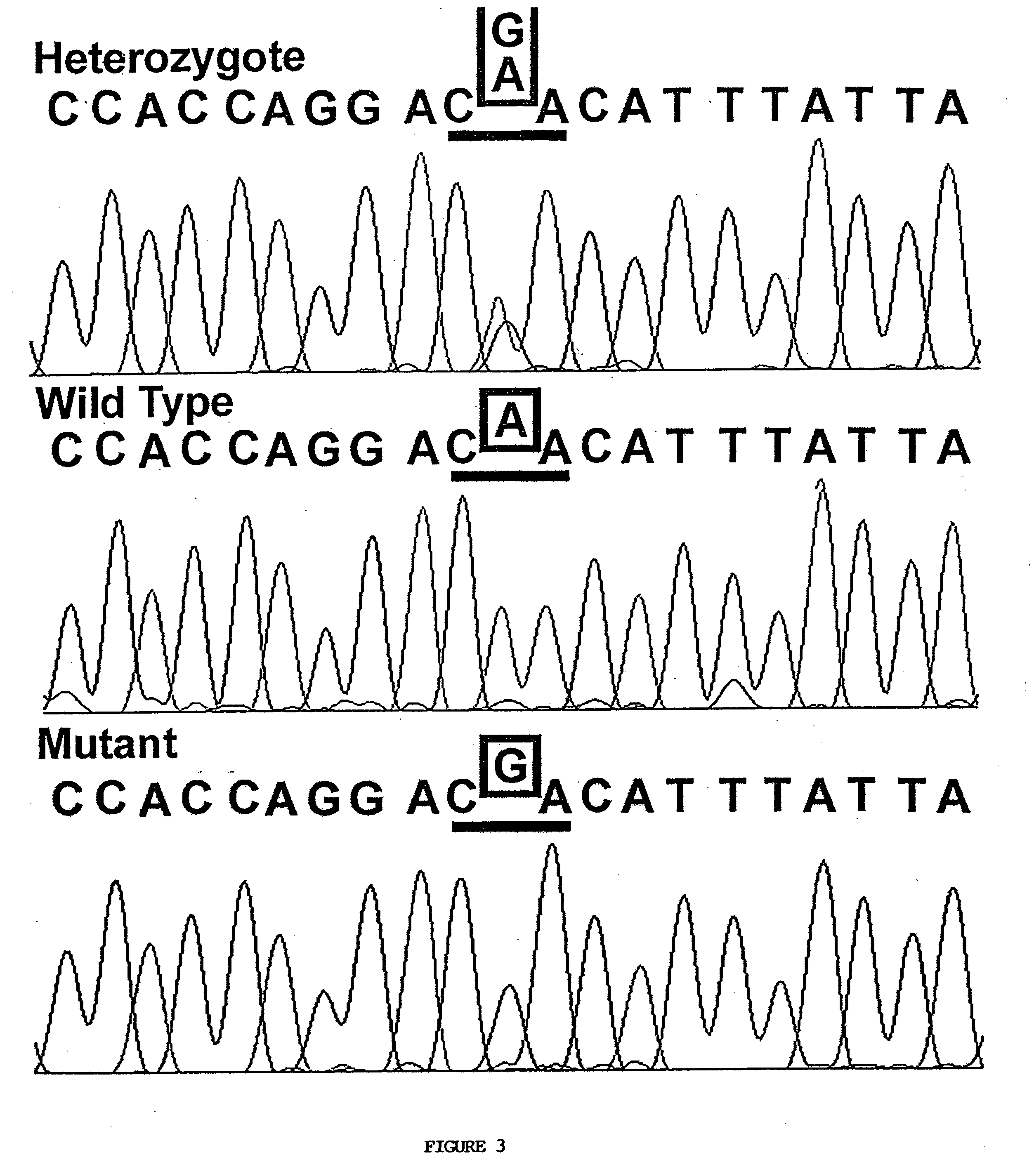
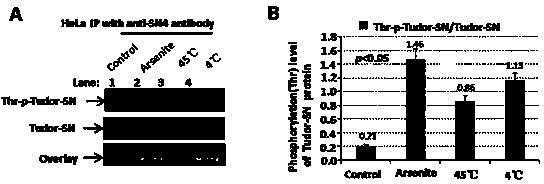
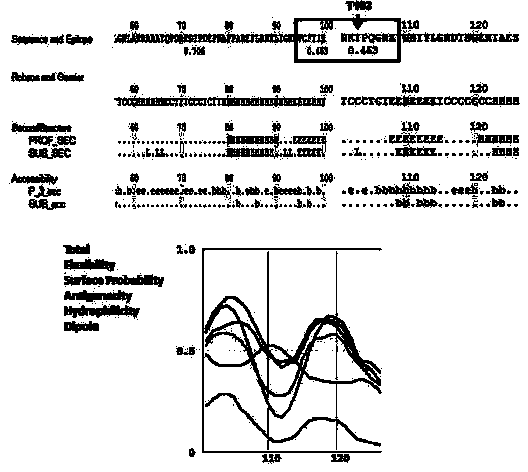
Preparation method of stress phosphorylation antibody aiming at human Tudor-SN protein T73 site
InactiveCN104277110AEasy to explore correlationSerum immunoglobulinsImmunoglobulins against enzymesDiseaseHumanin
The invention discloses a stress phosphorylation antibody aiming at a human Tudor-SN protein T73 site and a preparation method thereof. The preparation method adopts a multifunctional human Tudor-SN protein as a research object and comprises the following steps of determining if threonine at a 73 site of a human Tudor-SN protein under external environment oxidation stress is subjected to phosphorylation modification by near-infrared double-color laser imaging and mass spectrometry, synthesizing a T73 phosphorylation site-containing semiantigen polypeptide according to the secondary structure prediction result, coupling the T73 phosphorylation site-containing semiantigen polypeptide and a carrier protein (KLH) into a complete antigen, carrying out immunization on SPF-level New Zealand white rabbit by the complete antigen combined with an adjuvant four times and collecting, purifying and identifying a T73 specific stress phosphorylation polyclonal antibody. In practical application, Tudor-SN protein epigenetic phosphorylation modification in different stress environments can be detected, the effect mechanism of the Tudor-SN protein epigenetic phosphorylation modification in cell stress tumor propagation and migration can be discussed and a latent action target point for clinical tumor disease diagnosis and treatment is provided.
Owner:TIANJIN MEDICAL UNIV
Method of screening therapeutic agents for nerve degeneration associated diseases
InactiveUS7172876B2Improve expression levelCompound screeningNervous disorderHumaninNerve degeneration
Owner:TAKEDA PHARMA CO LTD
Preparation of monoclonal antibody 7H8 capable of resisting epitope at N terminal of human procalcitonin protein
ActiveCN106399294AReduce false positive rateAvoid cross reactivityImmunoglobulins against animals/humansTissue cultureBALB/cEscherichia coli
The invention discloses a hybridoma cell capable of secreting monoclonal antibodies 7H8 capable of resisting amino acid sequences at the N terminal of human PCT (procalcitonin) protein. Through the prokaryotic expression of a human PCT whole-genome sequence with codon optimization in escherichia coli, the purified human PCT protein is taken as an antigen for immunizing BALB / c mice. Through cell fusion and screening, the hybridoma cell capable of stably secreting the monoclonal antibodies aiming at epitope at the N terminal of the human PCT protein is obtained, and the hybridoma cell is named as 7H8. The 7H8 monoclonal antibody can be used for testing PCT concentration of serum, and has high sensitivity and specificity. The 7H8 antibody identifies the amino acid sequences at the N terminal of the human PCT protein, the cross reaction with the calcitonin in the body is avoided, the specificity in identifying the PCT protein is improved, a very good novel material is provided for developing a diagnostic reagent corresponding to the PCT, and the technical scheme can be widely applied to the cytobiology and immunology experiments, and has a very good application value.
Owner:HUNAN NORMAL UNIVERSITY
Construction method and application of thrombopoietin (THPO) gene humanized non-human animal
The invention provides a construction method and application of a thrombopoietin (THPO) gene humanized non-human animal. The method comprises the following steps: introducing a sequence for encoding human THPO protein into an animal genome by using a homologous recombination manner; the animal can normally express the human or humanized THPO protein in vivo, to promote the development of human T cells and NK cells; the non-human animal can be used as an animal model for human THPO signal mechanism research and drug screening for diseases including tumors and the like, and has important application value for new drug research and development of immune targets. The invention further provides a targeting vector of a THPO gene, sgRNA of the target THPO gene and application of the targeting vector and the sgRNA to preparation of a humanized THPO gene.
Owner:BIOCYTOGEN PHARMACEUTICALS (BEIJING) CO LTD
Gene mutation associated with age-related macular degeneration
InactiveUS20060127915A1Sugar derivativesMicrobiological testing/measurementGene mutationAMD - Age-related macular degeneration
The present invention describes the identification of a mutation in a human FIBL-6 protein, which mutation is associated with Familial Age-Related Macular Degeneration. Transcripts and products of this mutated gene are useful in detecting and diagnosing AMD, developing therapeutics for treatment of AMD, as well as the isolation and manufacture of the protein and the constructions of transgenic animals expressing the mutant genes.
Owner:OREGON HEALTH & SCI UNIV
Preparation method of stress phosphorylation antibody aiming at human Tudor-SN protein T103 site
InactiveCN104277109AEasy to explore correlationSerum immunoglobulinsImmunoglobulins against enzymesDiseaseHumanin
Owner:TIANJIN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com