Human FGF21 mutant gene and method for preparing recombinant human FGF21 protein
A technology of FGF21 and mutant gene, which is applied in the application field of human fibroblast growth factor, mutant gene, and the preparation of recombinant human FGF21 protein, can solve the problems of low stability, high cost, poor specificity, etc., and achieve high stability , low cost, good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Construction and expression of embodiment 1mutFGF21 gene
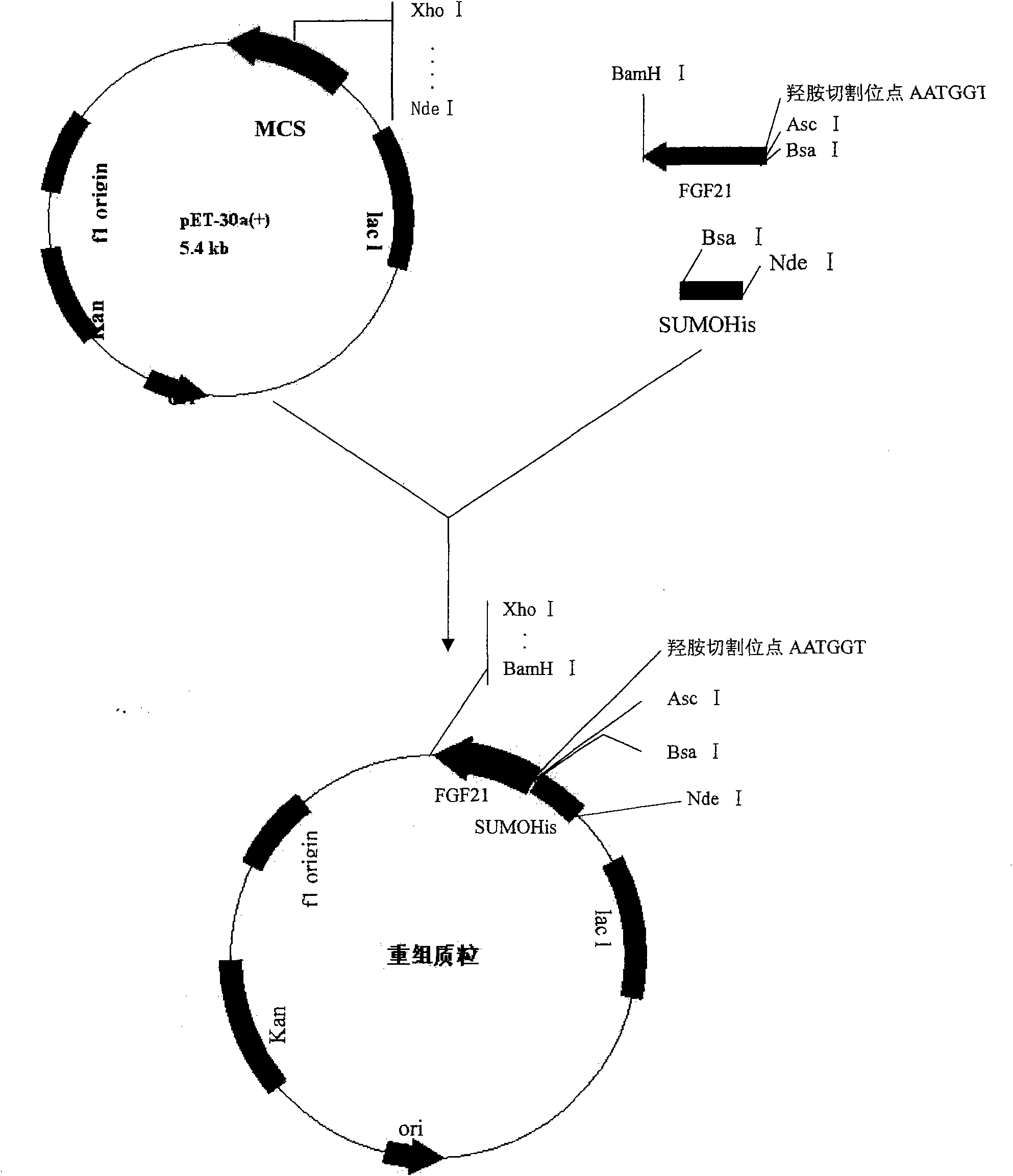
[0043] 1. Construction of recombinant expression plasmids
[0044] The SUMO sequence with 6×His tag sequence (Nde I and BsaI restriction sites introduced at both ends, respectively) was cloned into the pMD18-T vector to construct the pMD18-T-SUMOHis plasmid. The human FGF21 sequence with hydroxylamine sites (respectively introduced BsaI and BamHI restriction sites at both ends) was cloned into pMD18-T vector to construct pMD18-T-Asn-Gly-hFGF21 plasmid. The SUMOHis fragment obtained by digesting pMD18-T-SUMOHis with Nde I and BsaI was ligated with the Asn-Gly-hFGF21 fragment obtained by digesting pMD18-T-Asn-Gly-hFGF21 with BsaI and BamHI, and the ligated product was cloned again To the pET30a(+) digested with Nde I and BamHI, the recombinant expression plasmid pET30a(+)-SUMOHis-Asn-Gly-hFGF21 was obtained. For the construction process, see figure 1 .
[0045] 2. Site-directed mutation of hFGF21 gene
[0046]...
Embodiment 2
[0080] Preparation and purification of embodiment 2 human recombinant FGF21 fusion protein
[0081] 1. Conversion
[0082] Melt Rosetta-competent on ice, add 1 μl (10ng) recombinant expression plasmid, place on ice for 30 minutes, heat shock at 42°C for 45 seconds, add 200μl LB liquid (without Kan), shake at 37°C for 45 minutes, spread on the plate, and invert at 37°C Incubate overnight.
[0083] 2. Express
[0084] (1) Activation: pick a single colony in 10ml LB (50μg / ml Kan), shake overnight at 37°C;
[0085] (2) Secondary activation: 1 / 100 inoculation (1000ml inoculated with 10ml, 50μg / mlKan), shake at 37°C for 2-3 hours to OD 600 =0.3;
[0086] (3) Induction:
[0087] Two groups were set up and induced according to the following induction conditions:
[0088] Test group: Temperature: 37°C,
[0089] IPTG final concentration: 0.25mmol / L,
[0090] Speed: 100r / min,
[0091] Shaking time: 3 hours;
[0092] Control group: Temperature: 37°C,
[0093] Speed: 100r / min, ...
Embodiment 3
[0104] Example 3 Hydroxylamine Cleavage of Human Recombinant FGF21 Fusion Protein of the Present Invention and Purification of Human Recombinant FGF21
[0105] 1. Test material
[0106] Test sample: the human recombinant FGF21 fusion protein prepared and purified in Example 2;
[0107] Reagents: Hydroxylamine cleavage buffer.
[0108] 2. Test methods and results
[0109] (1) Exploration of fusion protein hydroxylamine cleavage concentration
[0110] Concentrate the fusion protein (concentration: 1 mg / ml) 5 times to a concentration of 5 mg / ml, add 2× hydroxylamine cleavage buffer at a volume ratio of 1:1, 1:2, 1:3, 1:4, 1:5 After reacting at 30°C for 1 hour, each system was added to PBS pH 7.2 to the volume before protein concentration, and samples were taken for detection by 15% SDS-PAGE to analyze the optimal concentration of hydroxylamine cleavage buffer. see results Figure 11 .
[0111] (2) Purification of human recombinant FGF21
[0112] After the fusion protein wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com