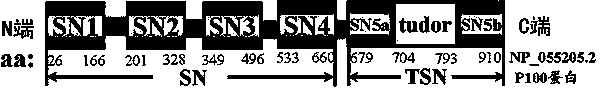Preparation method of stress phosphorylation antibody aiming at human Tudor-SN protein T73 site
A technique for preparing tudor-sn and antibodies, applied in the direction of anti-enzyme immunoglobulin, immunoglobulin from serum, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1, first determine the stress phosphorylation site of Tudor-SN protein
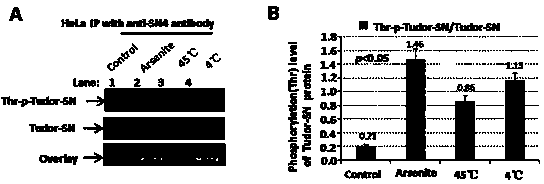
[0035] 1) We passed LI-COR Odyssey ? Near-infrared two-color laser imaging system ( figure 2 ) found that when HeLa cells were given arsenite oxidative stress, 45°C heat shock and 4°C cold shock treatment, the overall level of threonine (Thr) phosphorylation of endogenous Tudor-SN protein increased.
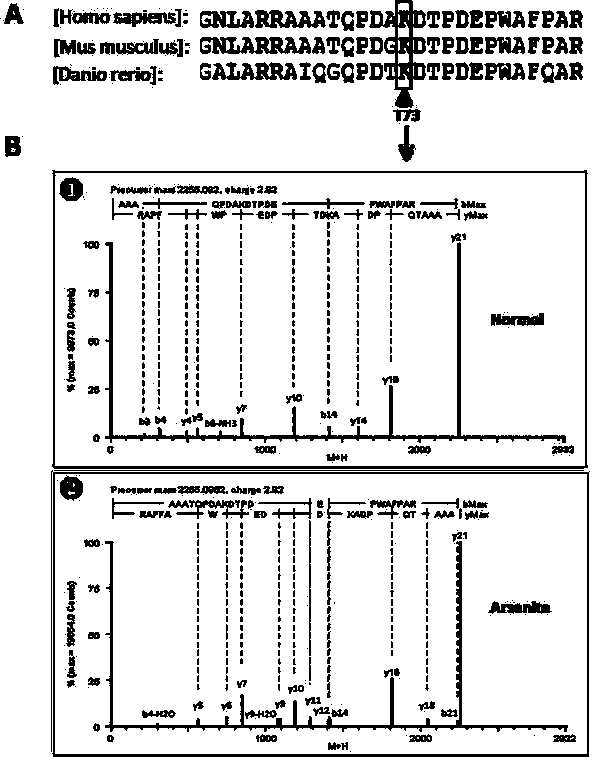
[0036] 2) Subsequent LC-MS / MS results showed that when HeLa cells were given arsenite oxidative stress, threonine at position 73 in the SN1 domain of Tudor-SN protein would be phosphorylated ( image 3). Therefore, we prepared a rabbit-derived polyclonal phosphorylated antibody against the T73 site.
[0037]
Embodiment 2
[0038] Example 2, Synthesis of Hapten Polypeptides Including T73 Phosphorylation Sites
[0039] (1) First, we predict the secondary structure of the functional region of the Tudor-SN protein containing the T73 site, including accessibility, flexibility, surface probability, antigenicity, affinity Hydrophilicity, Dipole, etc., to analyze the antigenicity of Tudor-SN. The results show that( Figure 4 ), the total antigenic score of the N-terminal part around T73 is higher (Total red line, arrow), which is predicted to be easily exposed on the molecular surface.
[0040] (2) According to the secondary structure prediction, determine the candidate amino acid sequence of the polypeptide containing T73 phosphorylation site. In order to ensure that the phosphorylation site can be recognized as much as possible, the length of the antigen polypeptide is designed to be the minimum length of the antibody, that is, 5 amino acids are selected around T73 as the center. At the same time, ...
Embodiment 3
[0044] Embodiment 3, carrying out antigen immunization animal and serum acquisition
[0045] The synthesized phosphorylated hapten polypeptide at T73 site was coupled with carrier protein (KLH) respectively to form a complete antigen. SPF grade New Zealand white rabbits were immunized four times with complete antigen and adjuvant, and a total of two New Zealand white rabbits were immunized. Note: Before immunizing animals, 1ml of pre-immune serum should be taken from each rabbit as a negative control. Day 0: Initial immunization (complete Freund's adjuvant + antigen, 600~800ug / cause); Day 21: first booster immunization (incomplete Freund's adjuvant + antigen, 400~500ug / cause); Day 35 Day: second booster immunization (incomplete Freund's adjuvant + antigen, 400~500ug / monkey); day 49: third booster immunization (incomplete Freund's adjuvant + antigen, 200ug / bird); day 56 Whole blood was collected to separate and collect about 75ml of antiserum.
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com