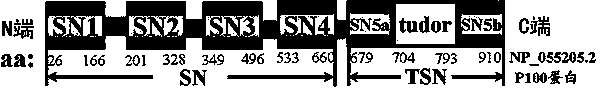Preparation method of stress phosphorylation antibody aiming at human Tudor-SN protein T103 site
A technology of tudor-sn and T103, applied in the field of antibody preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, first determine the stress phosphorylation site of Tudor-SN protein
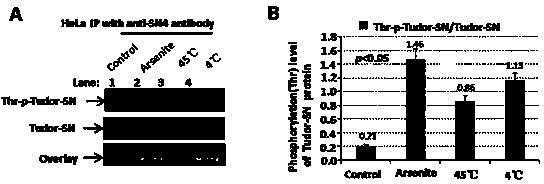
[0034] 1) We passed LI-COR Odyssey ? Near-infrared two-color laser imaging system ( figure 2 ) found that when HeLa cells were given arsenite oxidative stress, 45°C heat shock and 4°C cold shock treatment, the overall level of threonine (Thr) phosphorylation of endogenous Tudor-SN protein increased.
[0035] 2) Subsequent LC-MS / MS results showed that when HeLa cells were given arsenite oxidative stress, the threonine at position 103 in the SN1 domain of Tudor-SN protein would be phosphorylated ( image 3 ). Therefore, we prepared a rabbit-derived polyclonal phosphorylated antibody against the T103 site.
[0036]
Embodiment 2
[0037]Example 2 Synthesis of Hapten Polypeptides Comprising T103 Phosphorylation Sites
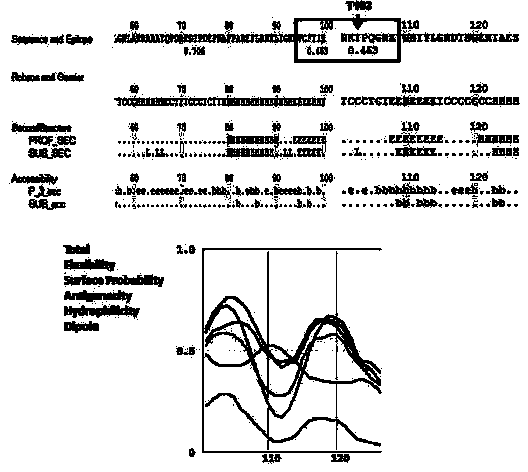
[0038] (1) First, we predict the secondary structure of the functional region of the Tudor-SN protein containing the T103 site, including accessibility, flexibility, surface probability, antigenicity, affinity Hydrophilicity, Dipole, etc., to analyze the antigenicity of Tudor-SN. The results show that( Figure 4 ), the score around T103 is low, it is possible that in the Native state protein, it is not easy to be exposed on the molecular surface.
[0039] (2) According to the secondary structure prediction, determine the candidate amino acid sequence of the polypeptide containing T103 phosphorylation site. In order to ensure that the phosphorylation site can be recognized as much as possible, the length of the antigen polypeptide is designed to be the minimum length that can obtain the antibody, that is, 5 amino acids are selected around T103 as the center. At the same time, in order to...
Embodiment 3
[0043] Embodiment 3, carrying out antigen immunization animal and serum acquisition
[0044] The synthesized phosphorylated hapten polypeptide at T103 site was coupled with carrier protein (KLH) respectively to form a complete antigen. SPF grade New Zealand white rabbits were immunized four times with complete antigen and adjuvant, and a total of two New Zealand white rabbits were immunized. Note: Before immunizing animals, 1ml of pre-immune serum should be taken from each rabbit as a negative control. Day 0: Initial immunization (complete Freund's adjuvant + antigen, 600~800ug / cause); Day 21: first booster immunization (incomplete Freund's adjuvant + antigen, 400~500ug / cause); Day 35 Day: second booster immunization (incomplete Freund's adjuvant + antigen, 400~500ug / monkey); day 49: third booster immunization (incomplete Freund's adjuvant + antigen, 200ug / bird); day 56 Whole blood was collected to separate and collect about 75ml of antiserum.
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com