Preparation method for soluble truncated human tumor necrosis factor-related apoptosis inducing ligand (TRAIL) active protein
An active protein, soluble technology, applied in the field of bioengineering, can solve the problems of low soluble protein yield, unsuitable for anti-tumor drugs, high cost, and achieve the effects of high yield, simple procedure and short fermentation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
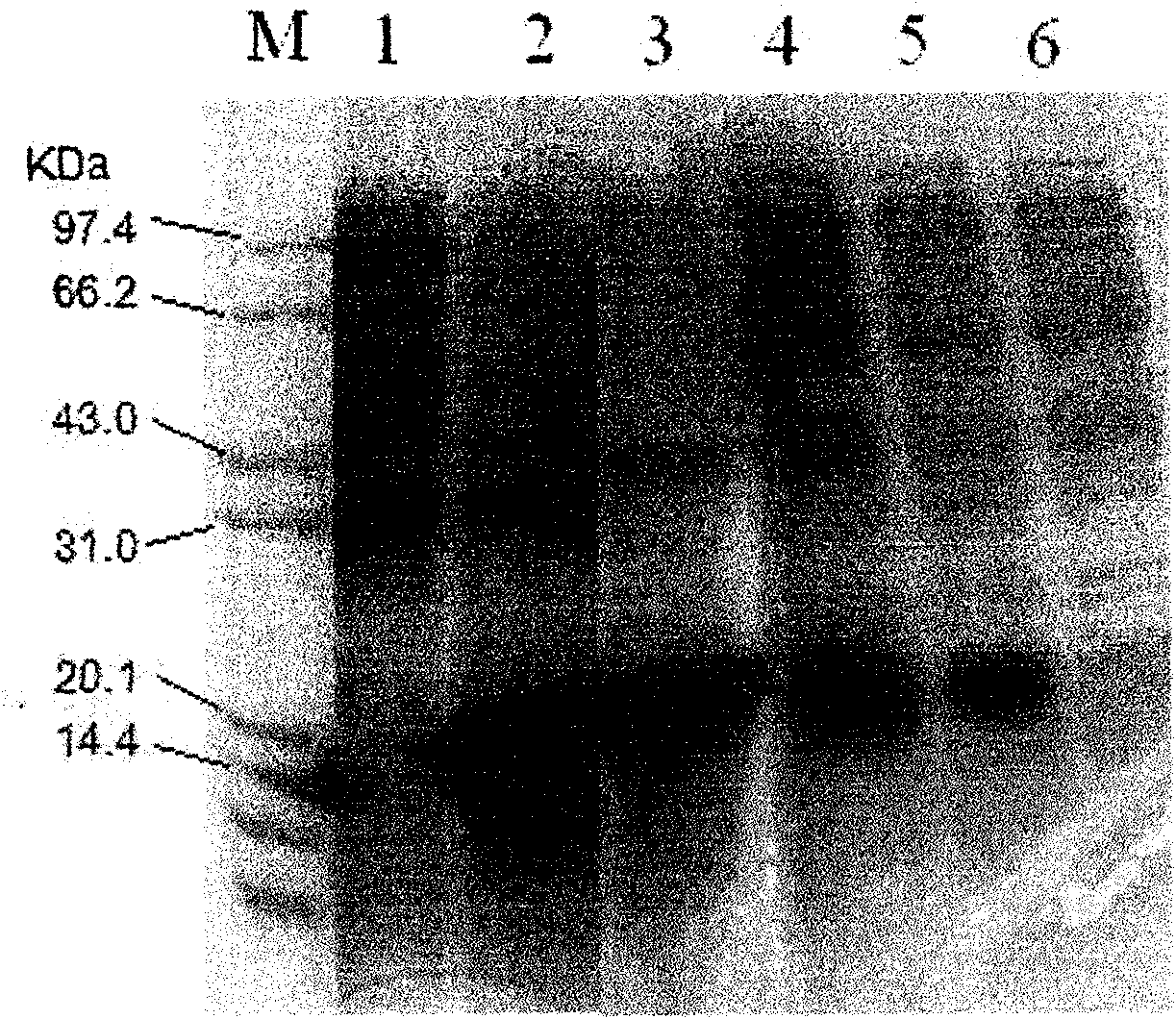
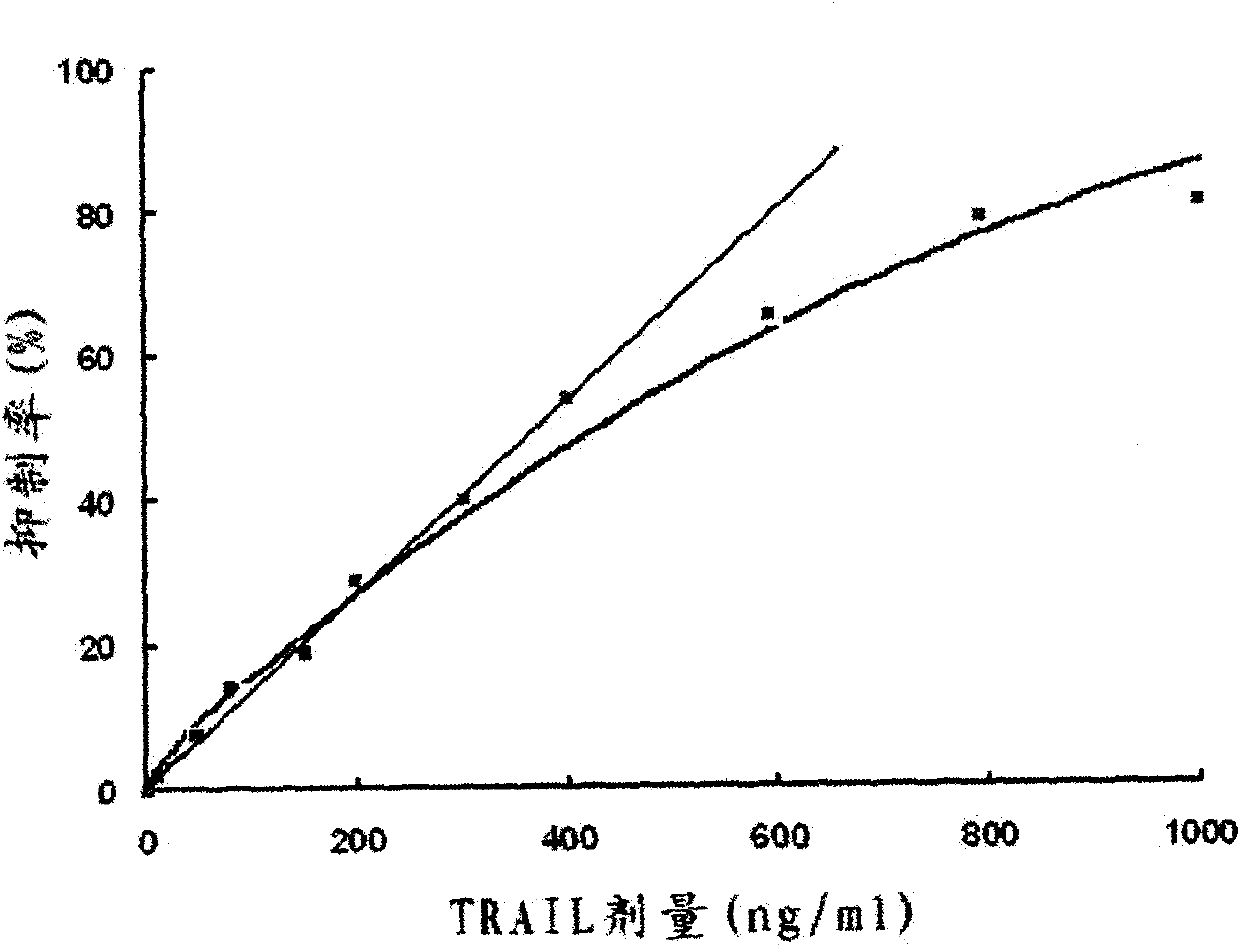
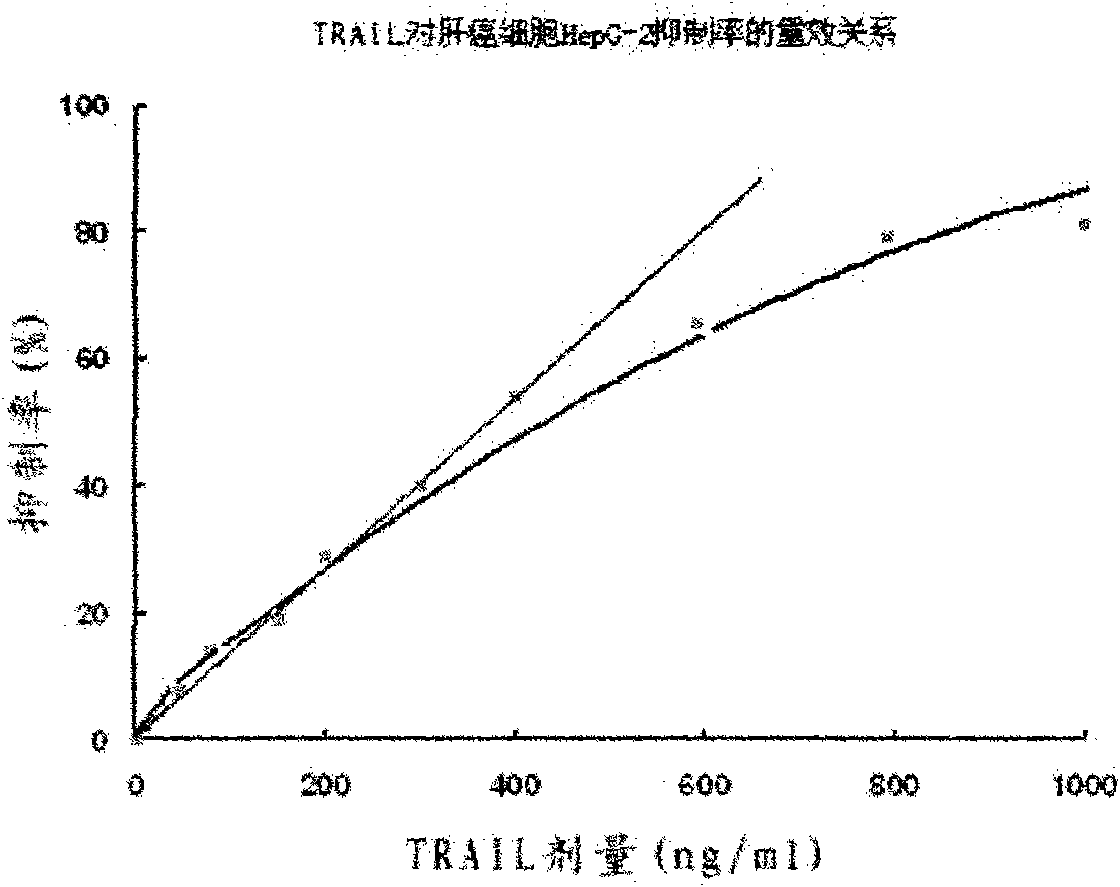
[0029] The amplified recombinant plasmid DNA and pET23a plasmid DNA were double-digested with restriction endonucleases NdeI and XhoI, and the digestion buffer was 10×H reaction buffer. Digest at 37°C for 4 hours. A 533bp DNA fragment encoding the 111-281 amino acid peptide segment of the TRAIL protein and a 3.5kb linear pET23a plasmid DNA were recovered after the digestion mixture was subjected to 1% agarose gel electrophoresis. Then the two recovered DNA fragments were mixed according to the ratio of 5 parts of the target DNA fragment: 1 part of the linear pET23a plasmid DNA, and mixed with T 4 DNA ligase was ligated overnight at 16°C. The ligation reaction mixture was then directly transformed into E.coli BL21 (DE 3 ), positive transformants were screened on LB plates containing 100 μg / ml ampicillin. Express the target protein according to the above step D at 17°C, the soluble target protein in the supernatant is 100%, and 0% in the precipitate. If expressed at 25°C or ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com