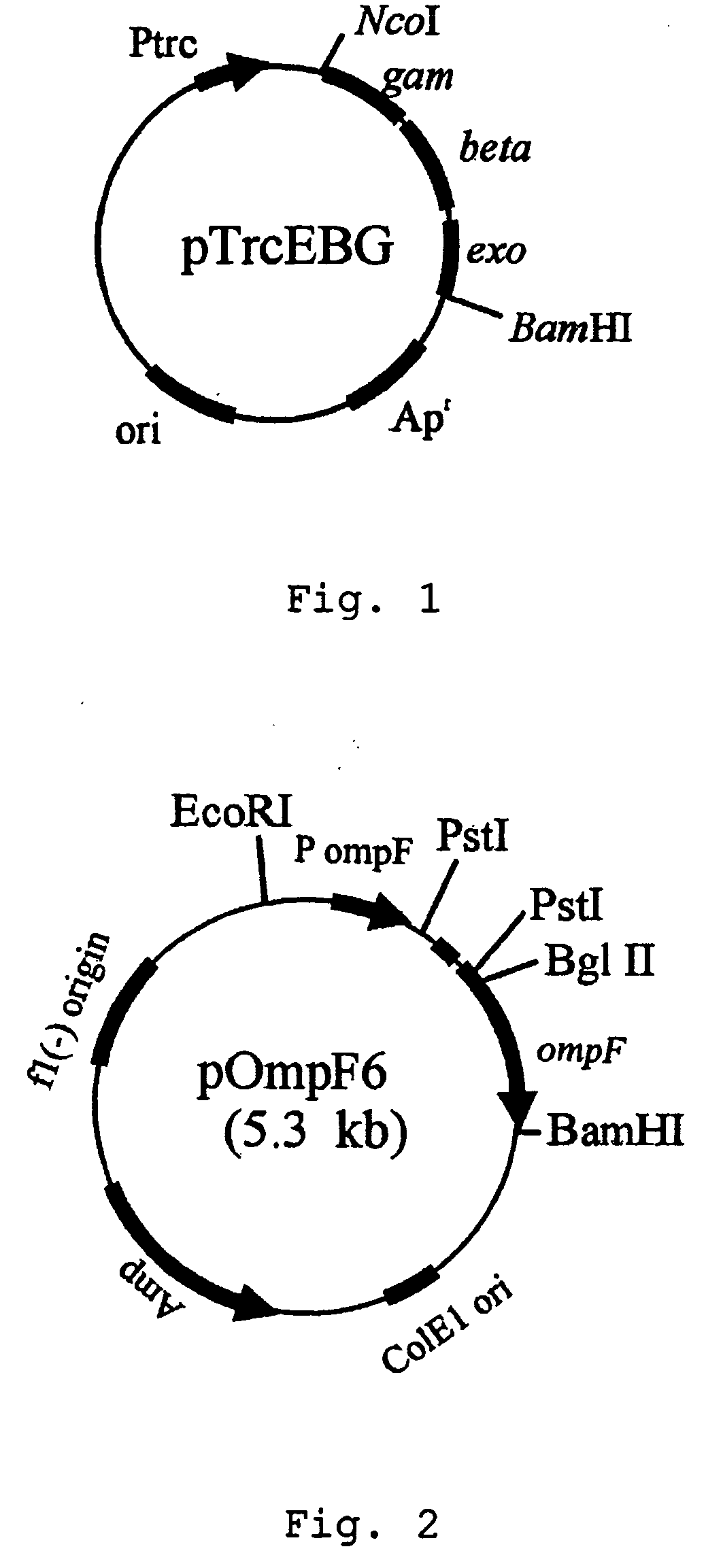
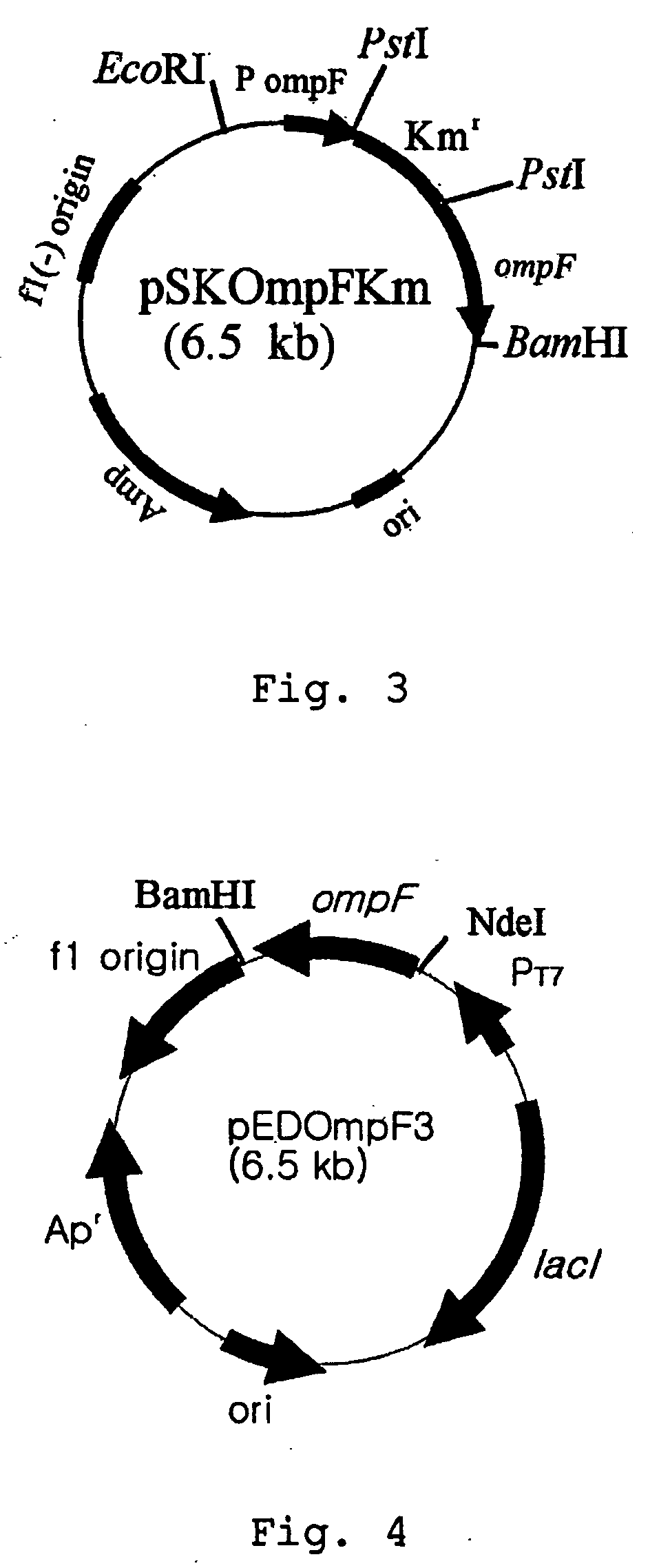
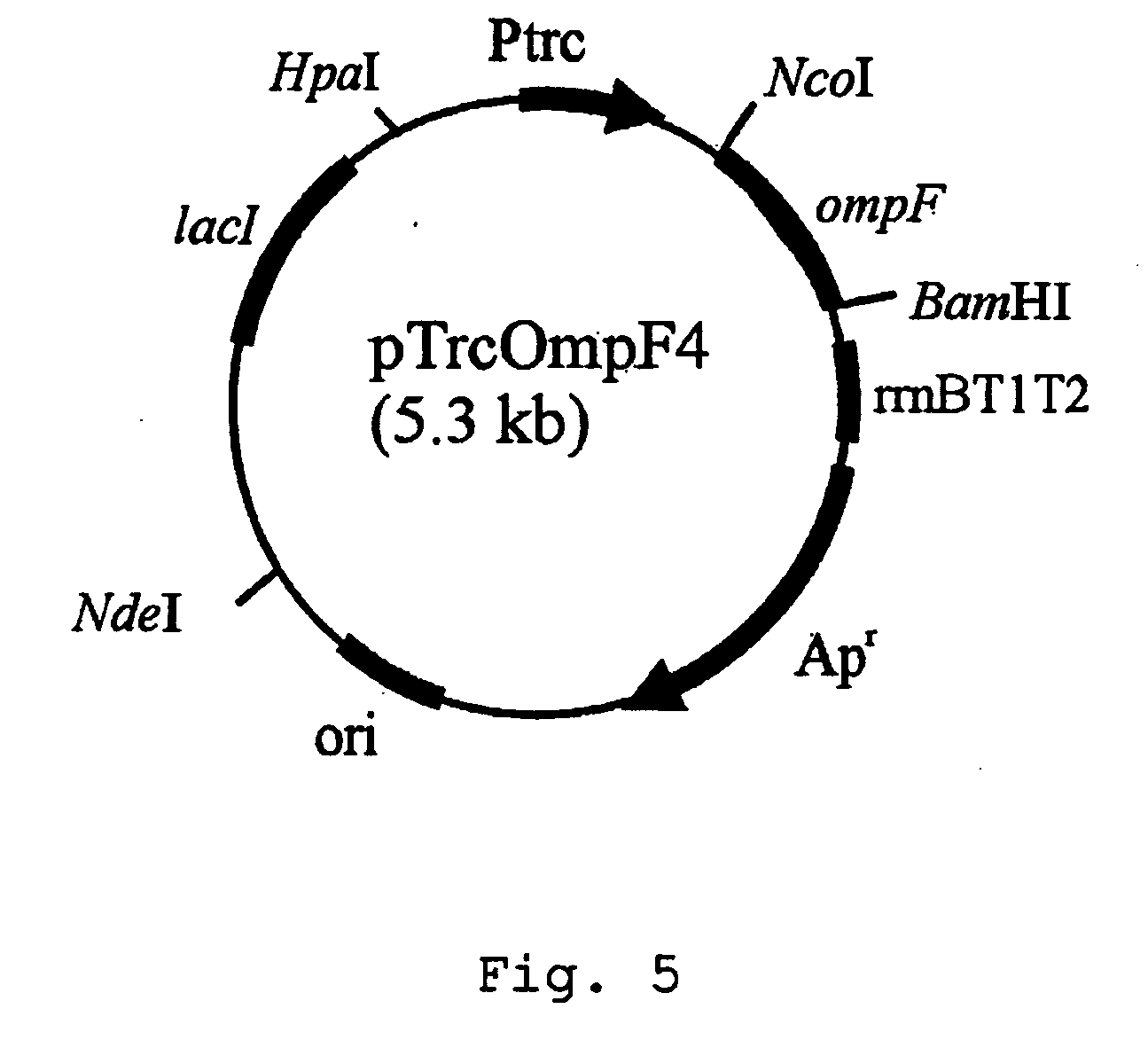
Patents
Literature
438 results about "Ampicillin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ampicillin is used to treat a wide variety of bacterial infections.
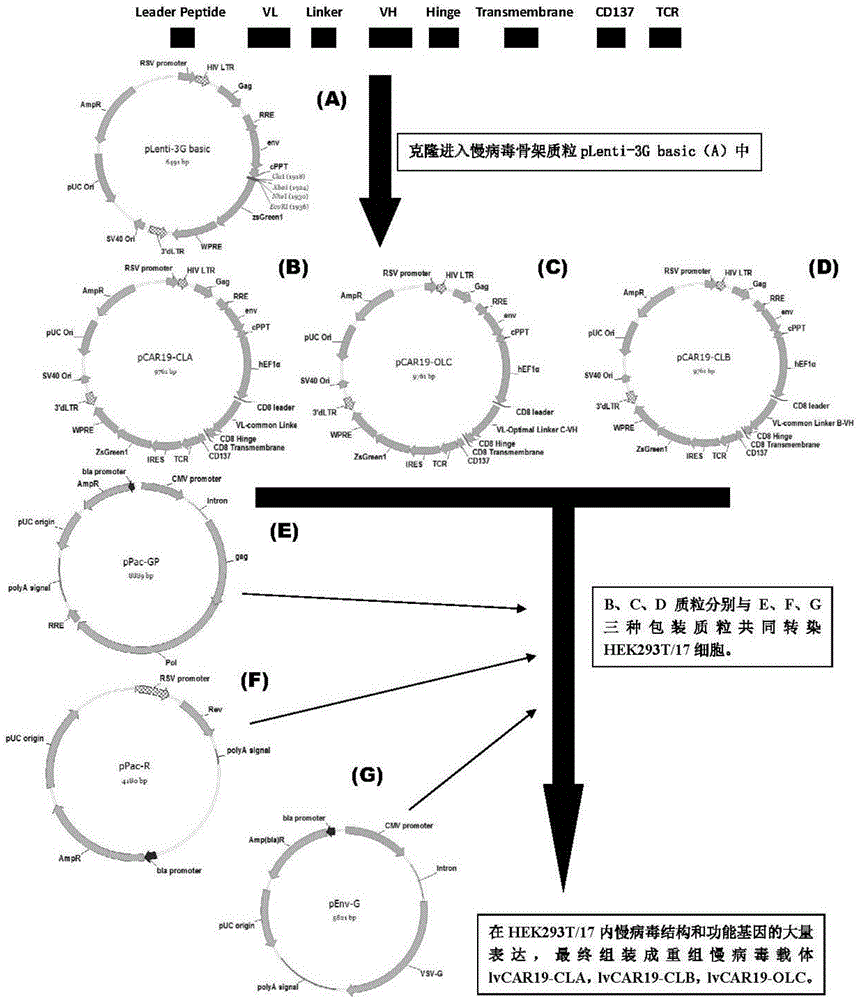
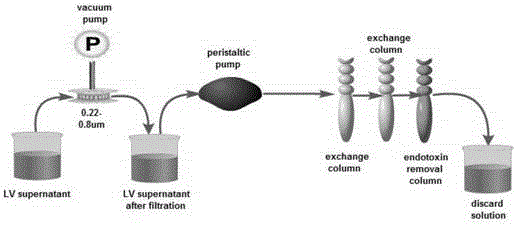
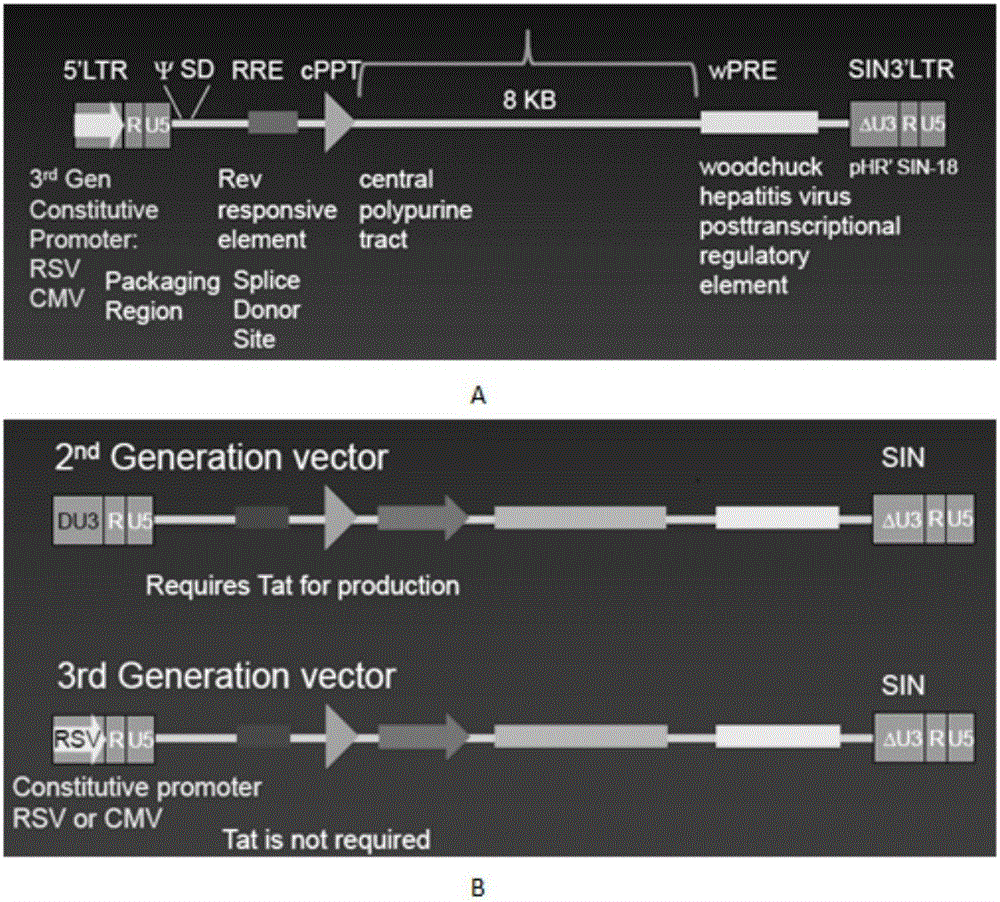
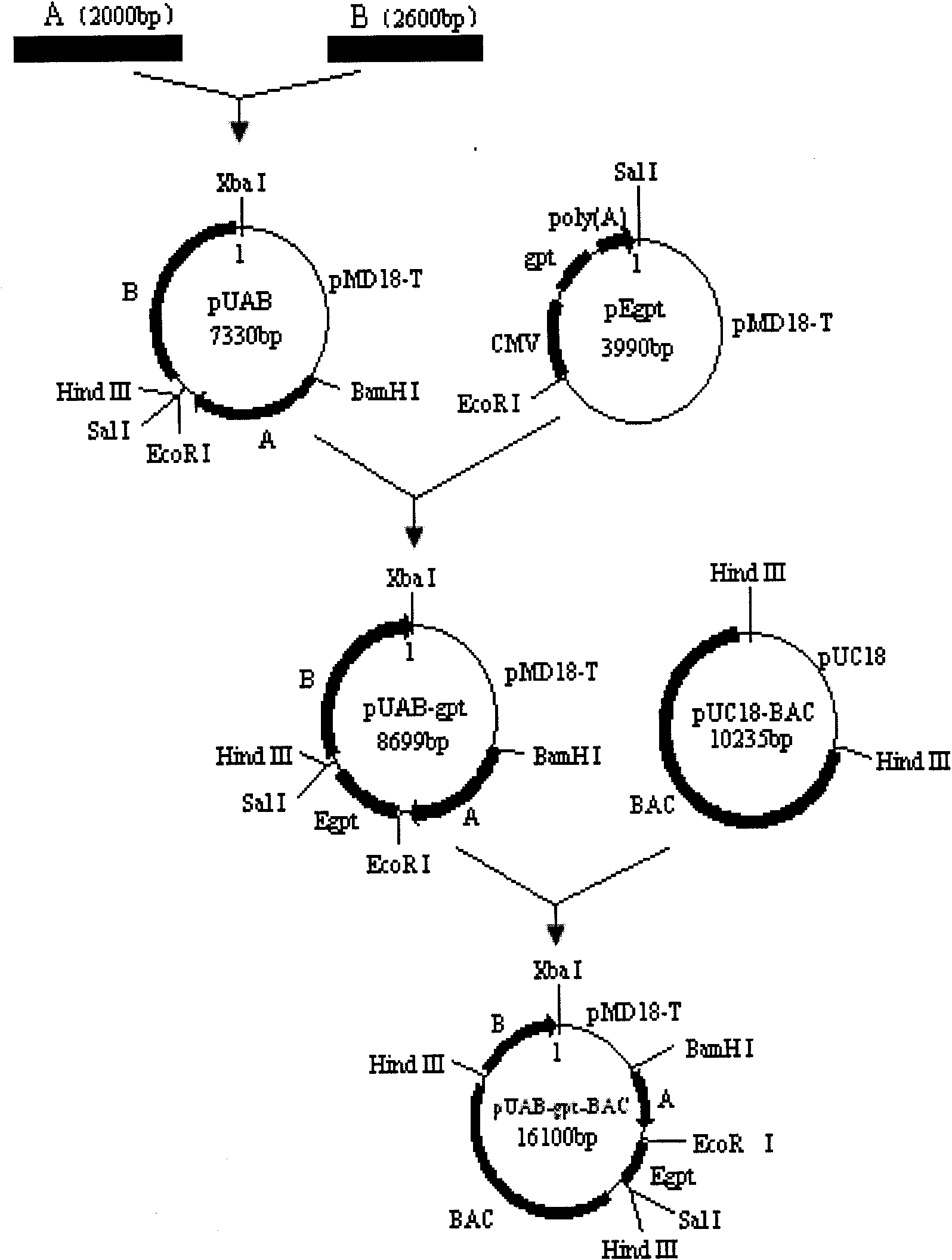
CAR-T transgene vector based on replication defective recombinant lentivirus and construction method and application of CAR-T transgene vector
ActiveCN105602992ASignificant effectPromote secretionGenetic material ingredientsFermentationEucaryotic cellAmpicillin
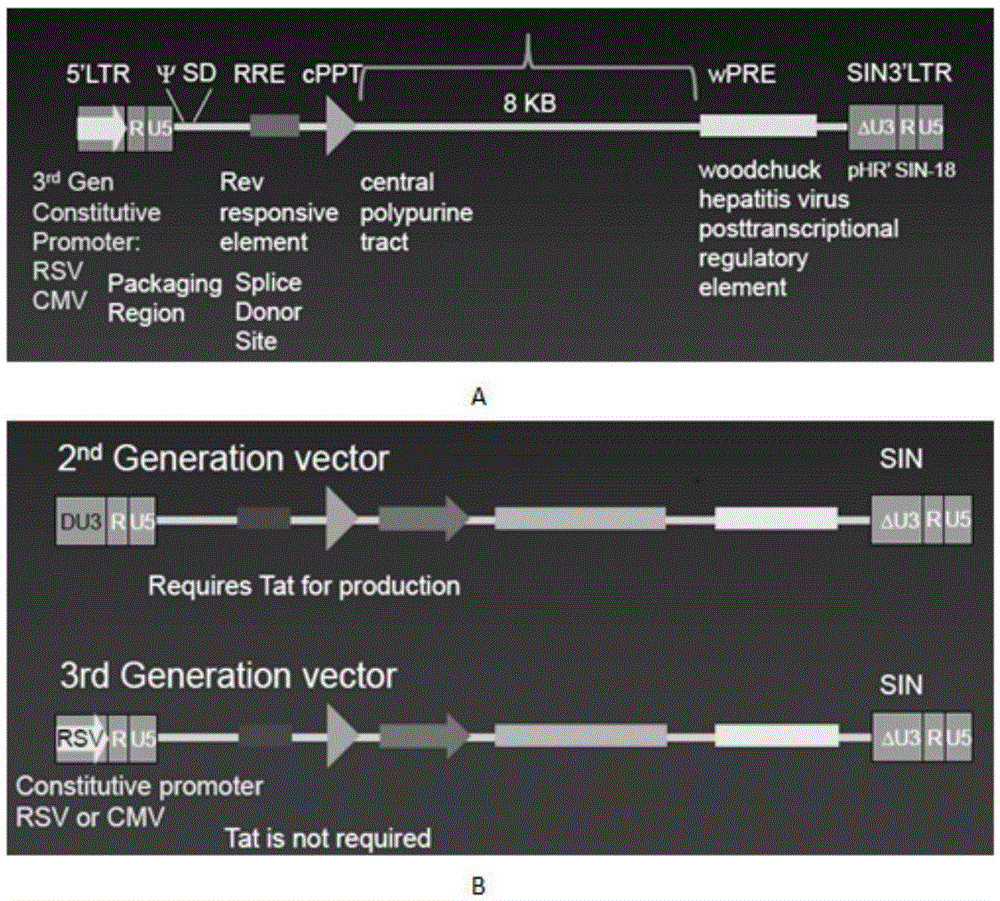
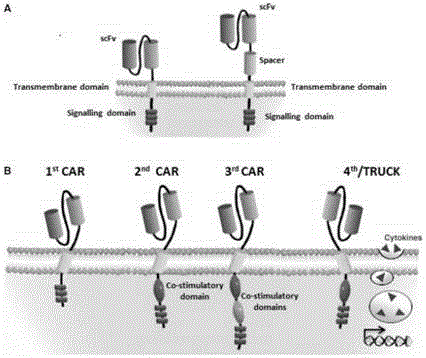
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
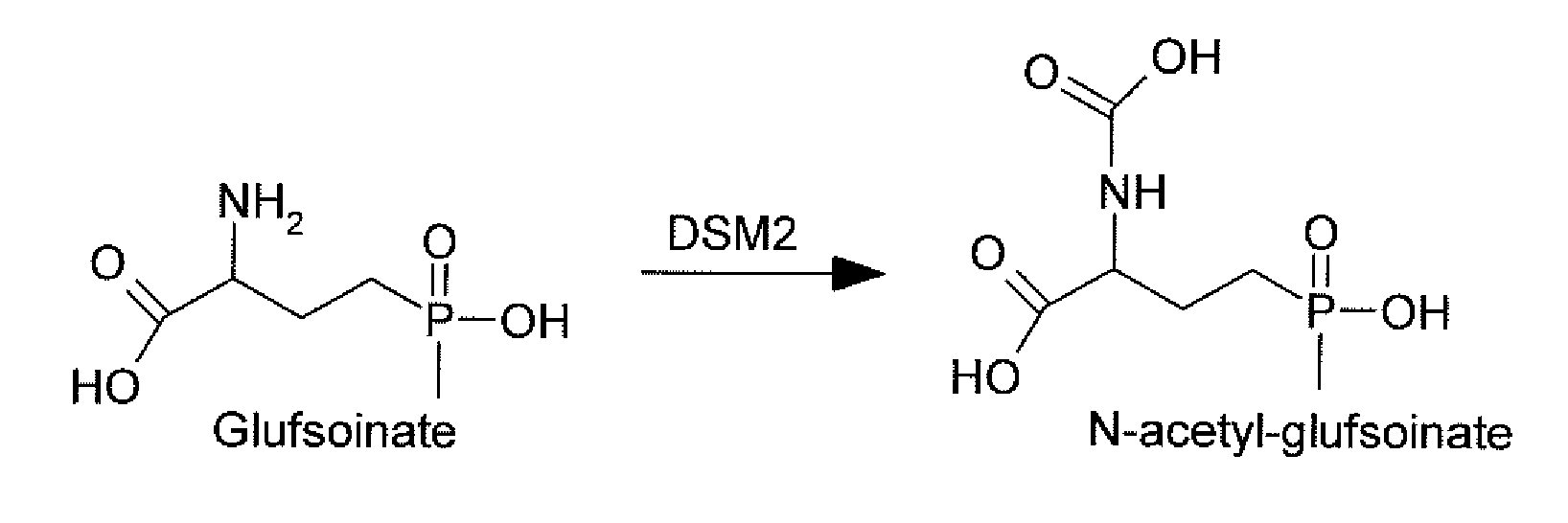
Novel Selectable Marker Genes
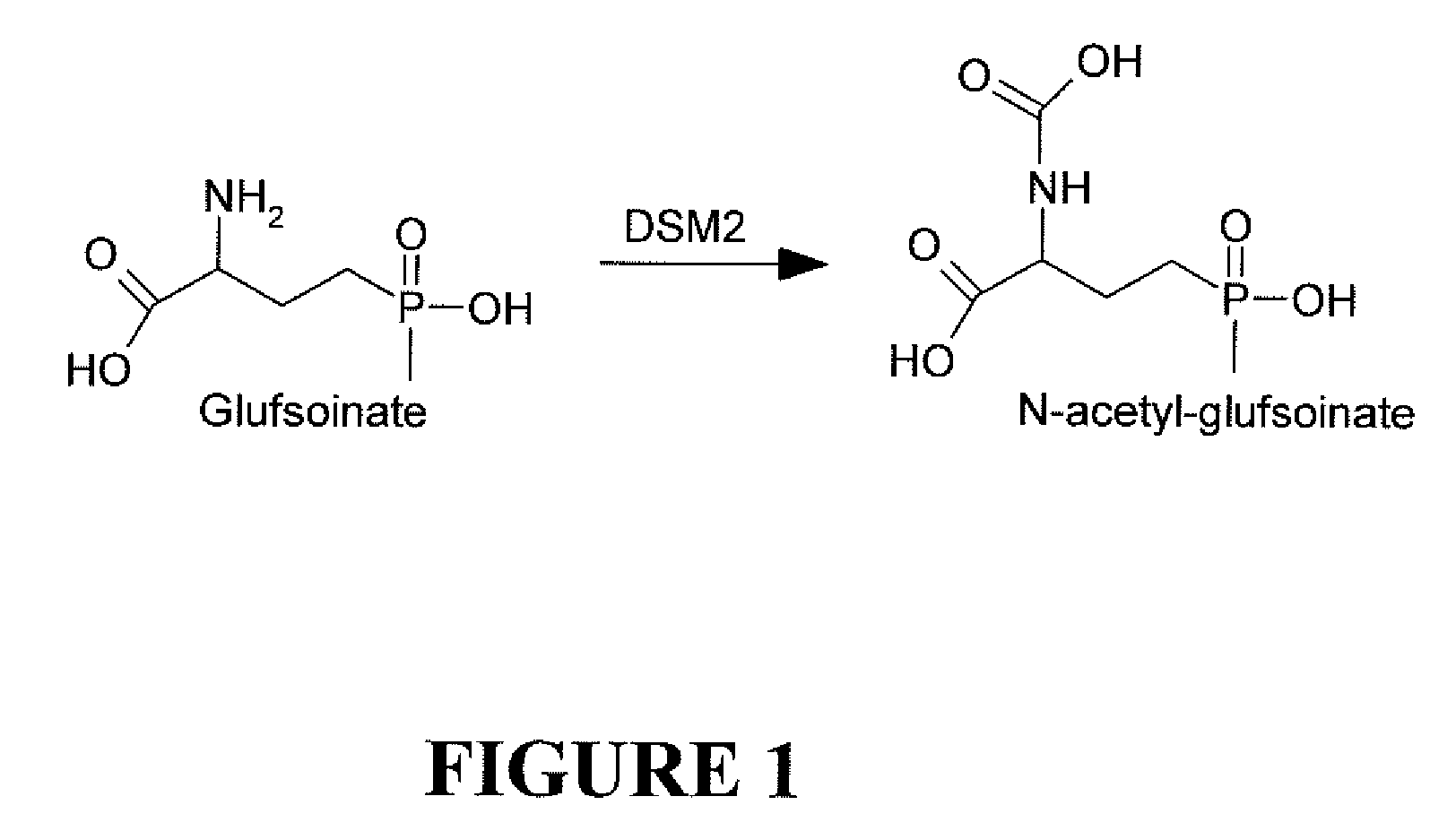
The subject invention relates to a novel gene referred to herein as DSM-2. This gene was identified in Sterptomyces coelicolor A3. The DSM-2 protein is distantly related to PAT and BAR. The subject invention also provides plant-optimized genes encoding DSM-2 proteins, DSM-2 can be used as a transgenic trait to impart tolerance in plants and plant cells to the herbicides glufosinate and bialaphos. One preferred use of the subject genes are as selectable markers. The use of this gene as a selectable marker in a bacterial system can increase efficiency for plant transformations. Use of DSM-2 as the sole selection marker eliminates the need for an additional medicinal antibiotic marker (such as ampicillin resistance) during cloning. Various other uses are also possible according to the subject invention.
Owner:CORTEVA AGRISCIENCE LLC
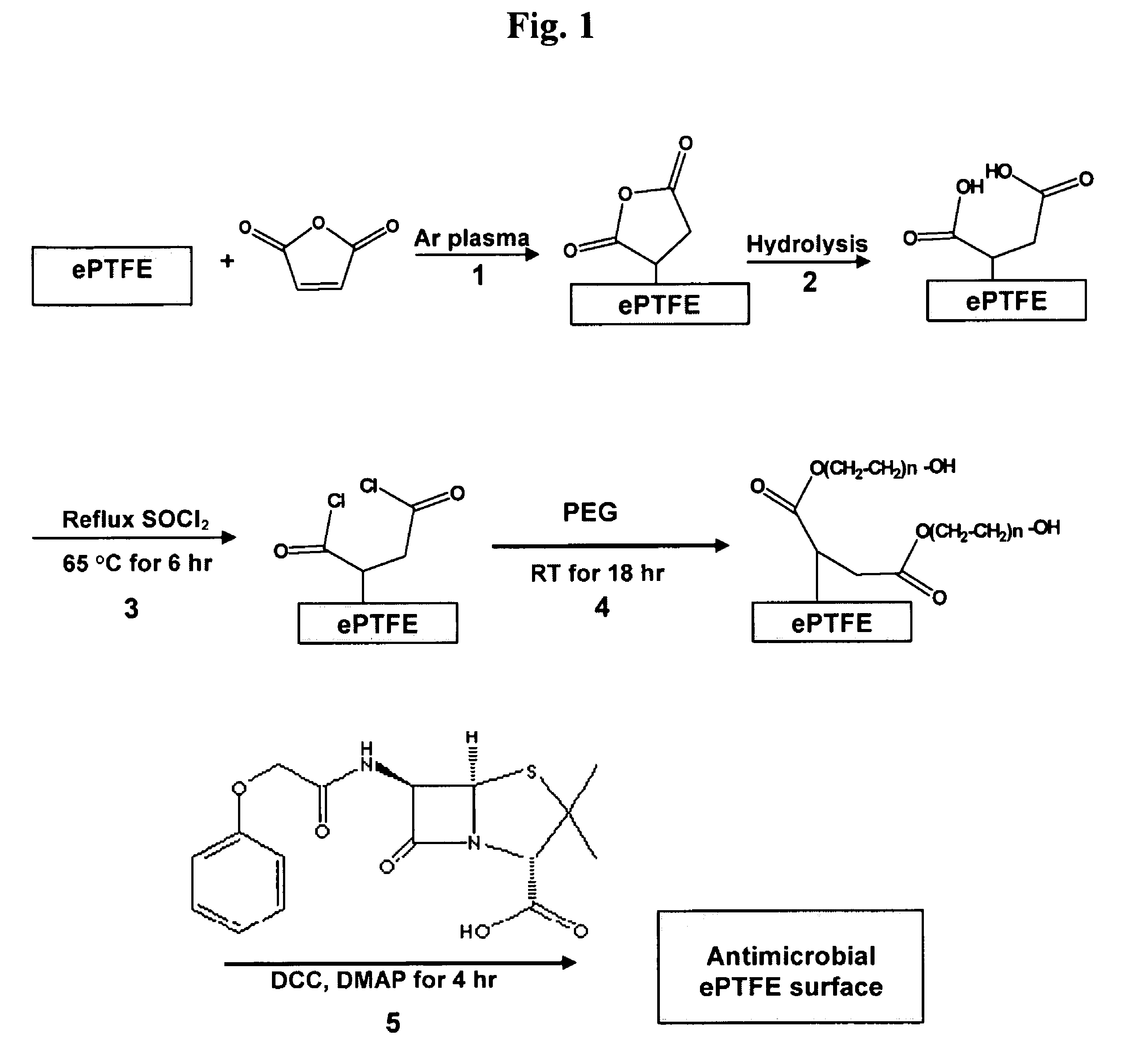
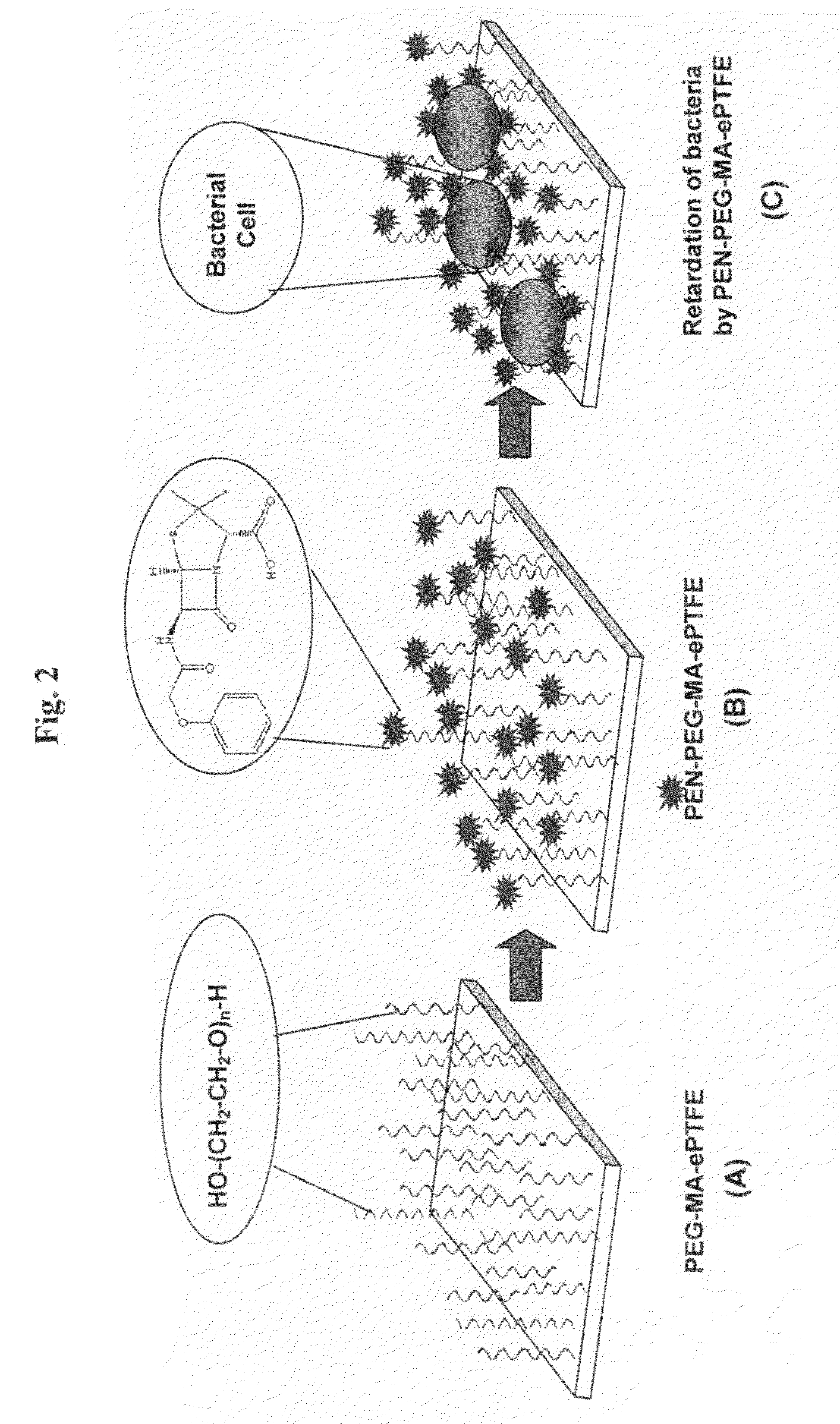
Method of attaching drug compounds to non-reactive polymer surfaces
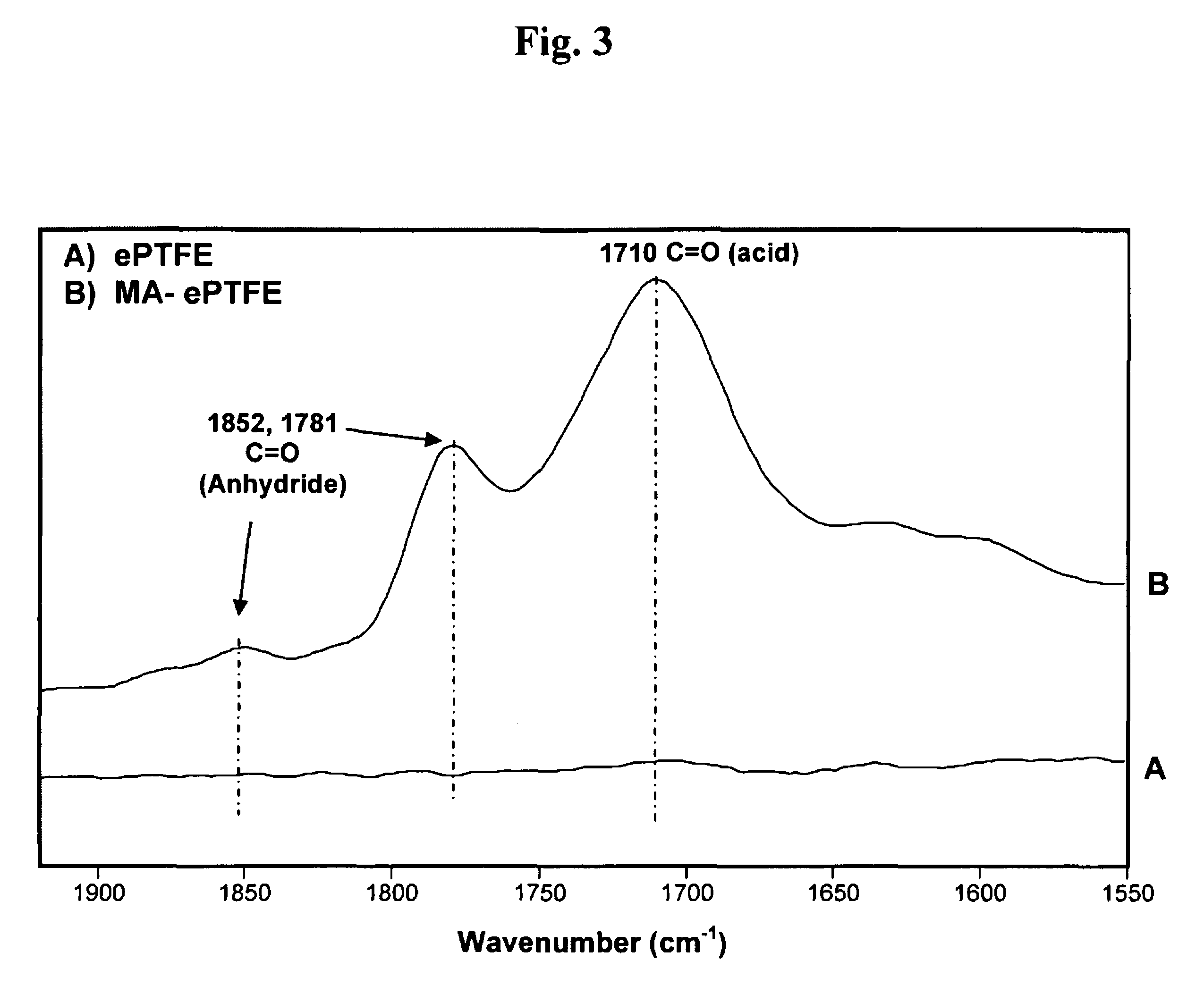
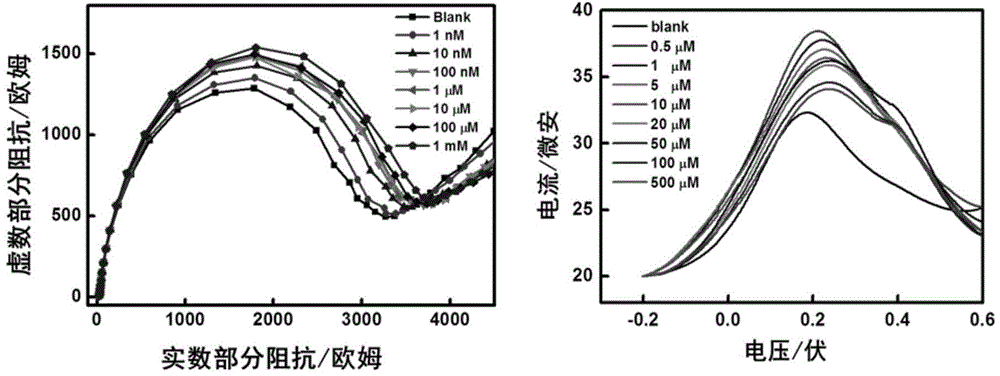
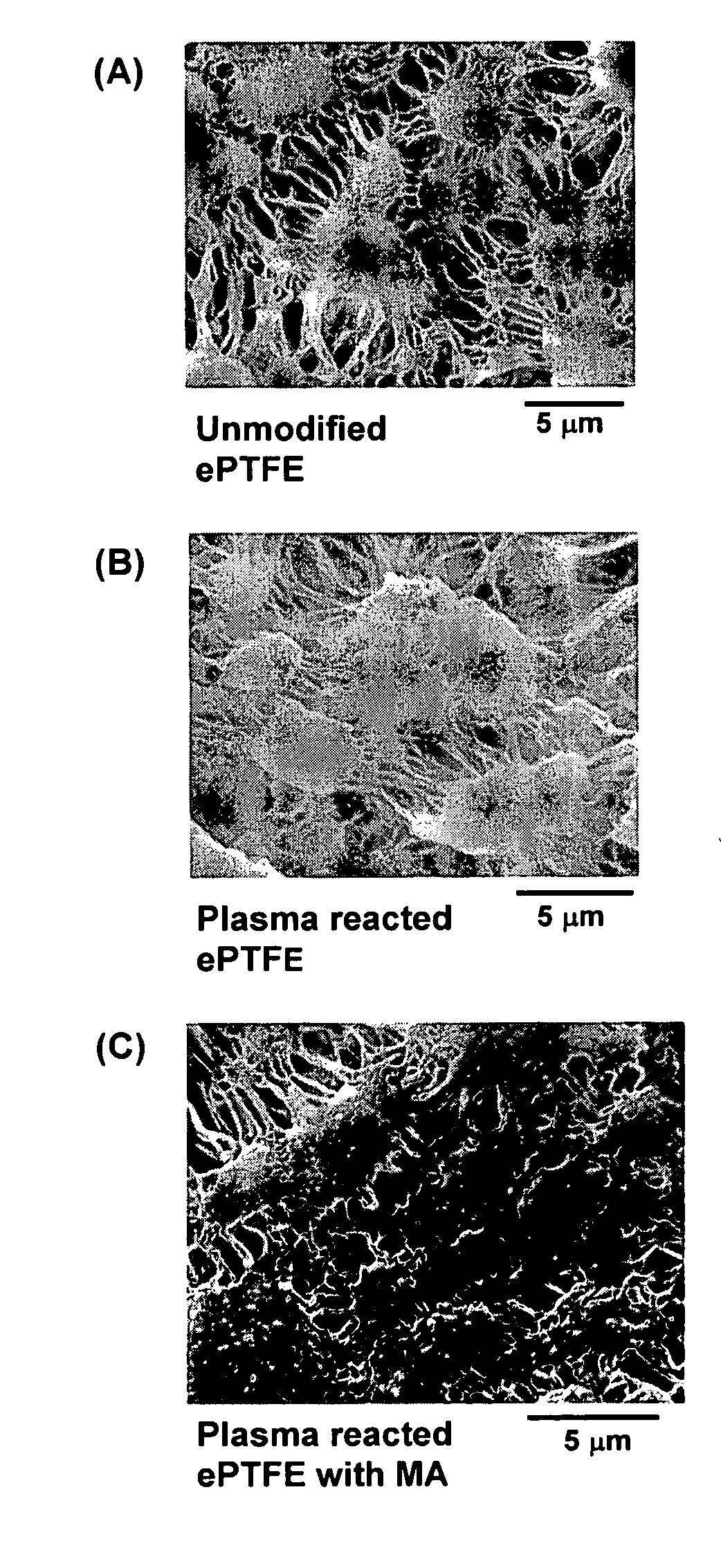
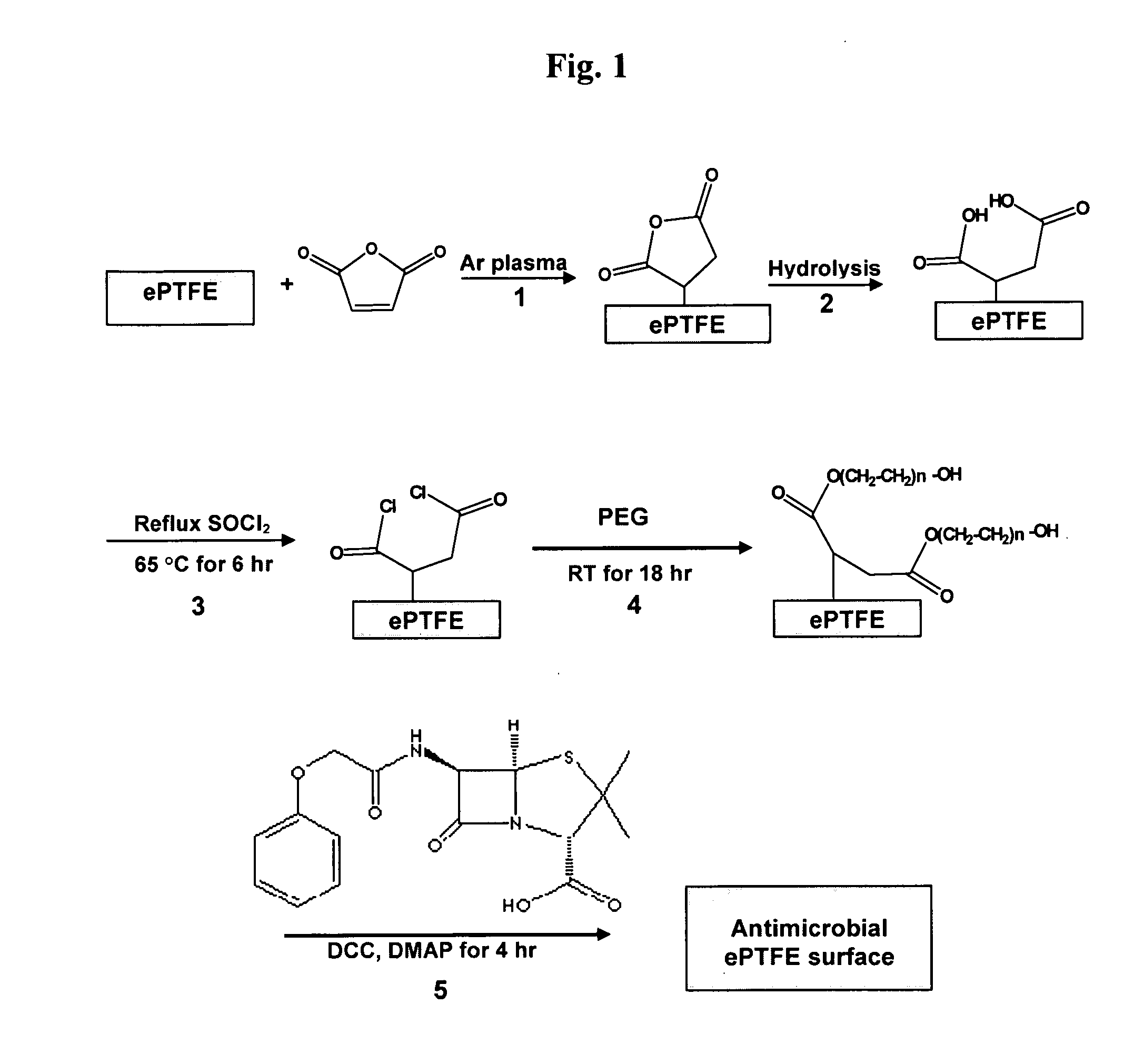
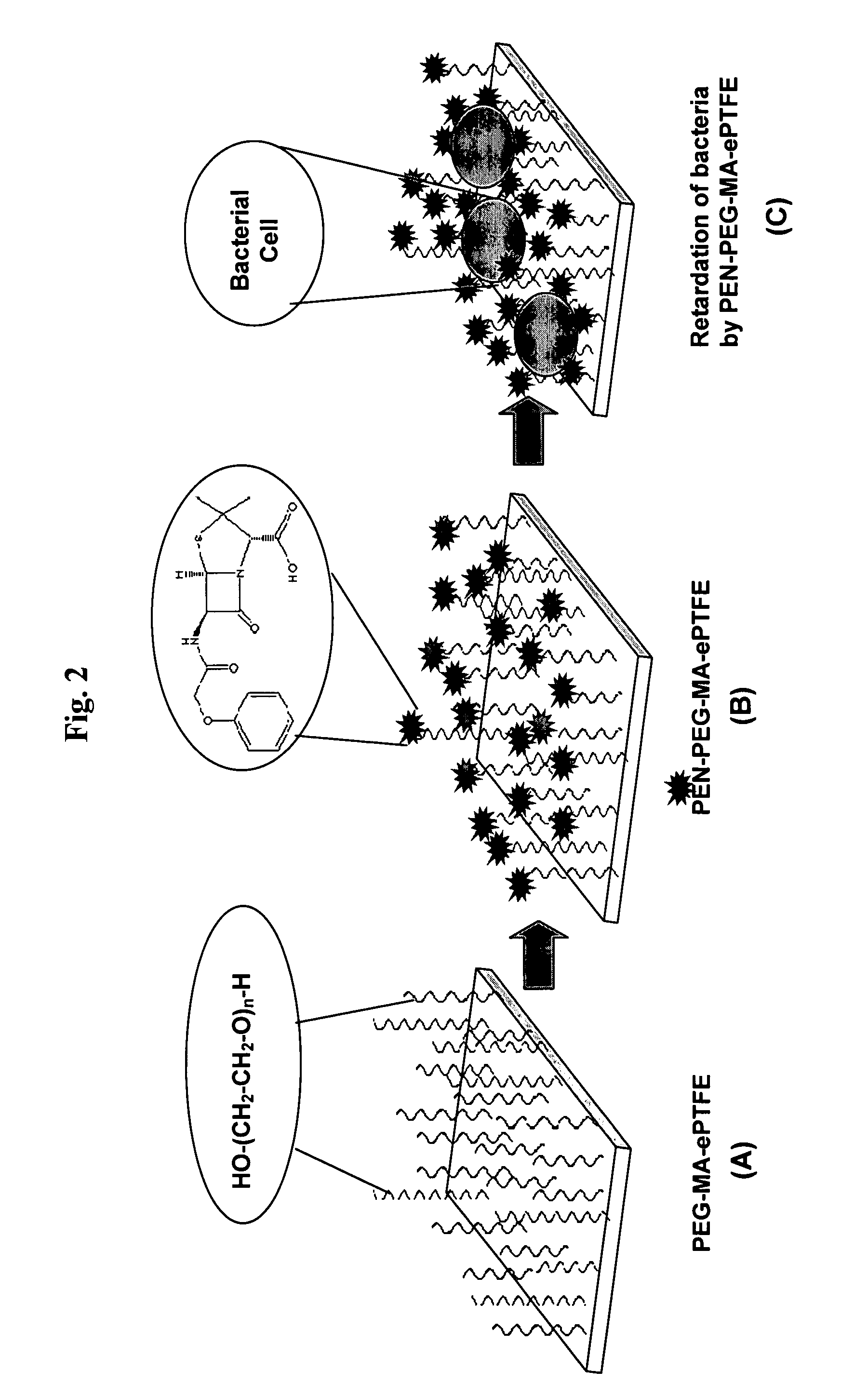
Polymers are disclosed that are chemically modified to retard bacterial growth. Such modified polymers (e.g. ePTFE and polypropylene) are produced by first creating acid groups on the polymer surface through reactions with an anhydride. The acid groups are then linked to polyethylene glycol (PEG) through esterification or other reactions such as amidation. Preferably, at least two different molecular weight PEG species are employed. The antimicrobial surface is completed by linking antibiotics (e.g. β-lactam antibiotics) to the PEG extensions. One preferred embodiment of such a modified polymer is produced using ePTFE, maleic anhydride (MA), and penicillin (PEN) to yield PEN-PEG-MA-ePTFE, which inhibits gram-positive bacteria. The PEG spacer is critical for PEN function in this context, since PEN-ePTFE is ineffective against bacterial growth. Another preferred embodiment incorporates ampicillin (AMP) and a heterobifunctional PEG, HOOC—(CH2—CH2—O)n—NH2, to yield AMP-PEG-MA-ePTFE. This latter example inhibits both gram-negative and gram-positive bacteria.
Owner:SOUTHERN MISSISSIPPI THE UNIV OF
Novel bacterial strain for highly effective degradation of chrysanthemum ester and organophosphorus pesticide and uses thereof
The invention pertains to the technical field of biologic degrading for chemical pesticide, and relates to a bacterial strain that can efficiently degrade Pyrethroid, Organophosphorus and vermectins pesticides and the application of the bacterial strain. The bacterial strain is an Acinetobacter sp. JCX22D bacterial strain, the biologic fature of the bacterial strain is Gram's negative, free from flagella, in form of short rod, is of esterase and lipase activity, free from oxidase activity, and can take use glucose and fructose. The bacterial strain is resistant to Ampicillin and low-concentration Spectinomycin and nalectin, and not resistant to rifamide. The degrading efficiency of the bacterial straine is more than 72% for high-efficiency cypermethrin, cyhalothrin, decamethrim, bifenthrin pesticid, is more than 70% for diazinon, parathion-methyl, phoxime and chlorpyrifos, and is 90% for vermectins. The bacterial strain can be used for biologically degrading above pesticides, and biologically recovering or cleaning any water body, soil and agricultural products that are polluted by pesticides.
Owner:许雷
Method for preparing polymer microballons of molecular engram of nitrogen benzyl penicillin
This invention discloses a method for preparing ampicillin molecularly imprinted polymer microspheres. The method comprises: preparing 3-6% poly(vinyl alcohol) solution, mixing functional monomers, template molecules, initiator, pore-foaming agent and crosslinker, ultrasonicating, adding into poly(vinyl alcohol) 1788 solution, stirring, stirring in 70 deg.C water for 60 min, filtering, ultrasonicating, washing with double distilled water twice, methanol three times and acetone twice (10 min each time), removing the template molecules, and vacuum-drying at 50 deg.C to obtain ampicillin molecularly imprinted polymer microspheres. The method has such advantages as simple process and high efficiency, and can prepare ampicillin molecularly imprinted polymer microspheres with high molecular recognition ability. When used in solid phase extraction, the ampicillin molecularly imprinted polymer microspheres have such advantages as high column efficiency and high selectivity. The ampicillin molecularly imprinted polymer microspheres can be used for selective separation and high-efficiency enrichment of penicillin in food matrix, such as honey.
Owner:SHANGHAI JIAO TONG UNIV
Penicillin G acylase immobilized with a crosslinked mixture of gelled gelatin and amino polymer
PCT No. PCT / EP96 / 03253 Sec. 371 Date Jan. 15, 1998 Sec. 102(e) Date Jan. 15, 1998 PCT Filed Jul. 16, 1996 PCT Pub. No. WO97 / 04086 PCT Pub. Date Feb. 6, 1997Penicillin G acylase is immobilized by covalent bonding to a crosslinked mixture of a gelled gelling agent such as gelatin and a polymer containing free amino groups such as alginate amine, chitosan or polyethylene imine. The immobilized penicillin G acylase provides a higher synthesis / hydrolysis ratio as compared to immobilizing with other carriers when producing beta -lactam derivatives by a condensing reaction of an amino beta -lactam with an acylating agent. The acylating agent may be a derivative of D-phenylglycine, a derivative of D-p-hydroxyphenylglycine or a derivative of D-2,5-dihydro-phenylglycine. Examples of beta -lactam derivatives that can be produced are amoxycillin, ampicillin, cephaclor, cephadroxil, cephprozil, cephalexin and cephradine.
Owner:GIST BROCADES NV
Antibiotic sensitive bacillus strains having antimicrobial effect against e. coli and clostridium perfringens and having high sporulation capacity
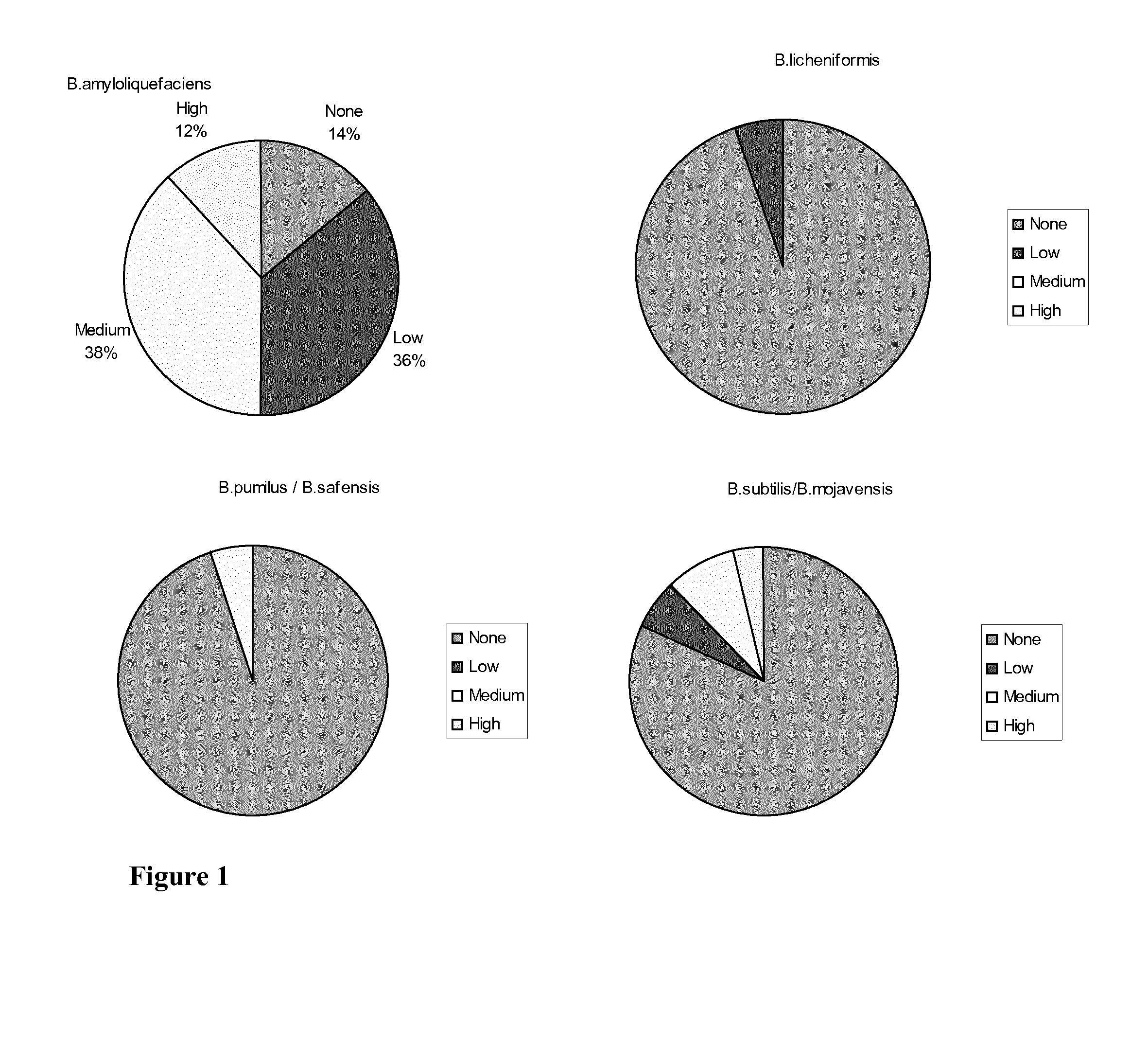
A Bacillus strain characterized by (i): sensitivity for ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline and chloramphenicol; (ii) antimicrobial activity against E. coli and Clostridium perfringens; and (iii) a sporulation percentage of at least 80 when measured after 2 days of incubation. The invention further relates to a method for selecting such strains. Many of the identified strains according to the invention are of the species Bacillus amyloliquefaciens. Some of the Bacillus amyloliquefaciens were further identified as Bacillus amyloliquefaciens subsp. amyloliquefaciens whereas others were identified as amyloliquefaciens subsp. plantarum. A Bacillus strain of the invention may be used as a feed additive to animal feed where it has a probiotic effect.
Owner:CHR HANSEN AS
Sterilizing method of spherical phaeocystis culture solution
InactiveCN102391954ASimple and fast operationImprove efficiencyUnicellular algaeMicroorganism based processesHigh concentrationKanamycin
The invention provides a sterilizing method of a spherical phaeocystis culture solution, and relates to a microalgae culture solution. The method comprises the following steps: centrifuging algae solution which grows to an exponential phase, and removing supernatant; carrying out gravity suspension on algae cells with an f / 2 culture medium, centrifuging, and removing supernatant, repeating twice, and carrying out gravity suspension on the algae cells with the f / 2 culture medium; adding SDS (sodium dodecyl sulfate) and antibiotics, wherein the antibiotics are clindamycin, azithromycin, gentamicin, kanamycin, streptomycin, cefotaxime, ampicillin and rifamycin; culturing under illumination, centrifuging the algae solution and removing supernatant during culturing, carrying out gravity suspension on the algae cells with the f / 2 culture medium, centrifuging and removing supernatant, removing residual SDS and the antibiotics, transferring into the f / 2 culture medium, and culturing under illumination; and selecting survival transferred algae solution which is treated with SDS and the antibiotics, and detecting whether bacteria exist or not after the transferred algae solution grows to the exponential phase. The method is convenient to operate, complex operations, such as bacterium separation and test on sensitivity to antibiotics and the like, are avoided, and long-term treatment of high-concentration antibiotics has good sterilization effect.
Owner:XIAMEN UNIV
Method, special kit and test paper strip for detecting beta-lactam antibiotics based on penicillin-binding protein
ActiveCN102253216AHigh sensitivityImprove accuracyBiological testingAmpicillinBeta lactam antibiotic
The invention discloses a method, a special kit and test paper strips for detecting beta-lactam antibiotics based on penicillin-binding protein. The receptor kit used for detecting beta-lactam antibiotics comprises: protein represented by SEQ ID NO: 1, enzyme labeled ampicillin and a standard substance solution, wherein the standard substance is penicillin G. The kit and colloidal gold test paper strips provided by the invention have advantages of high sensitivity, high accuracy, high precision, low cost, simple operation, short detection period, simple storage, and long shelf-life. The kit and colloidal gold test paper strips are suitable for various work units. With the kit and colloidal gold test paper strips, simultaneous and rapid detections of large batches of samples can be realized, and on-site high flux rapid detections can be realized. Therefore, the kit, the colloidal gold test paper strips and the method provided by the invention play an important role in the detections of beta-lactam antibiotics.
Owner:北京维德维康生物技术有限公司 +1
Signal peptide-free recombinant vector for exogenous gene expression in Kluyveromyces marxianus nutritional deficient strain
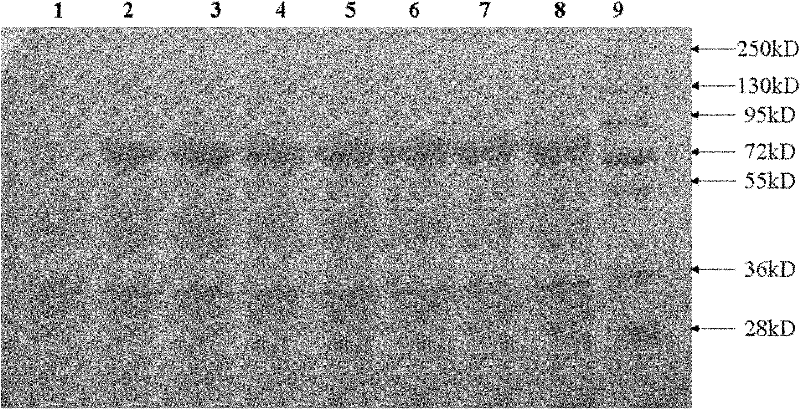
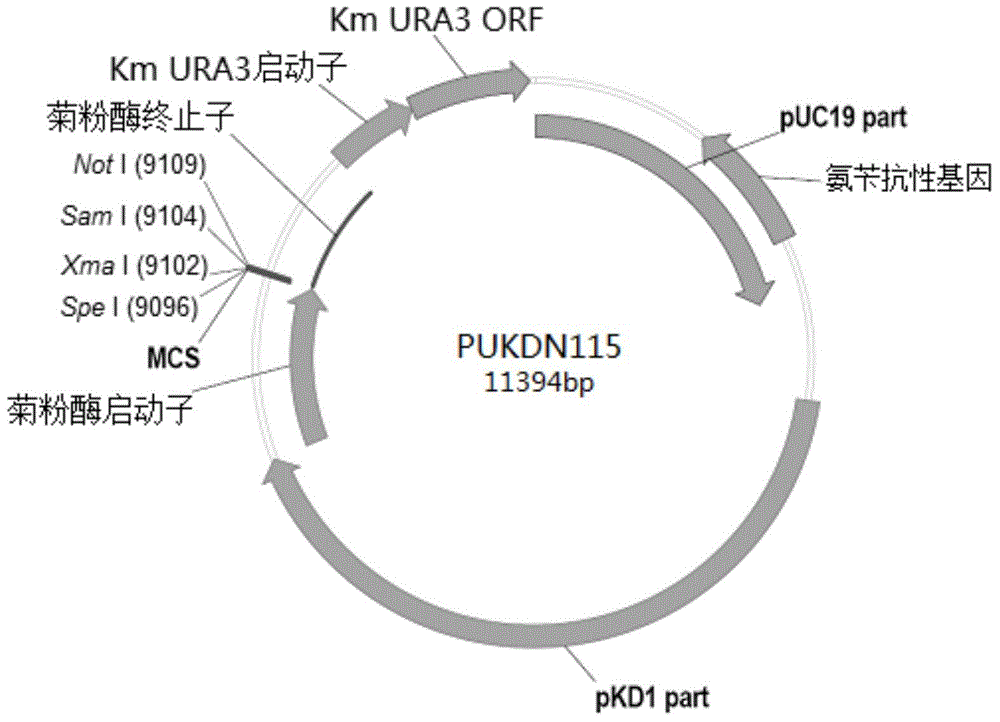
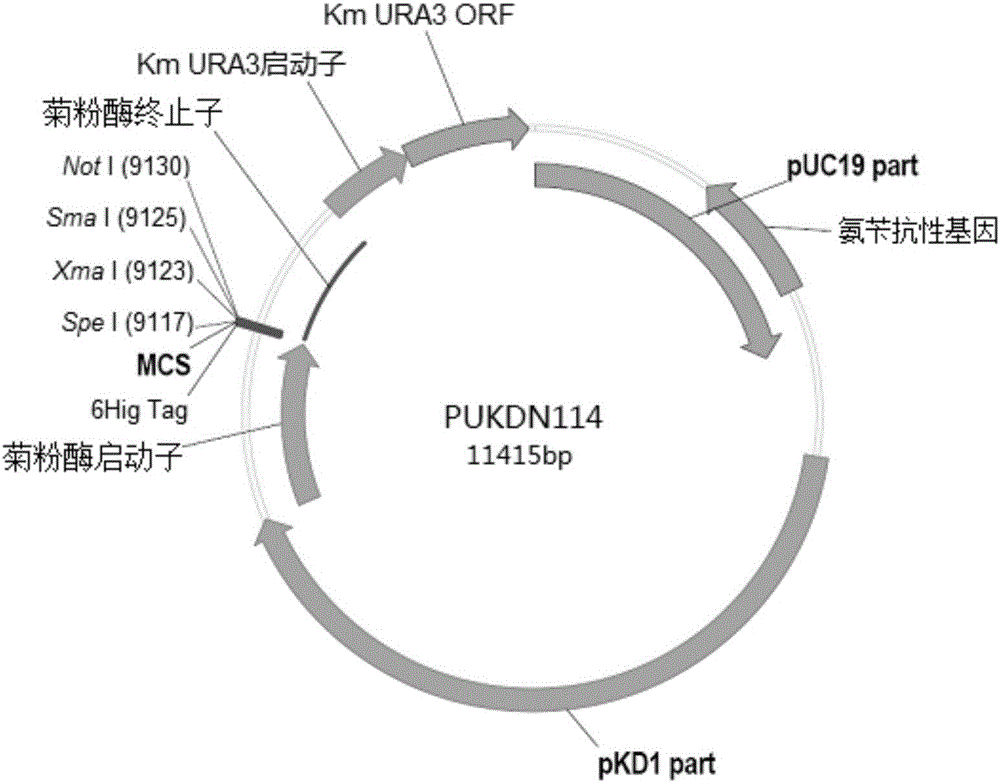
The invention provides a signal peptide-free recombinant vector for exogenous gene expression in a Kluyveromyces marxianus nutritional deficient strain as well as a preparation method and application thereof. The recombinant vector sequentially comprises an ampicillin resistance gene, a PKD1 vector, an inulase promoter, multiple cloning sites, an inulase terminator, a nutritional gene promoter and a nutritional gene open reading frame. The signal peptide-free recombinant vector and the preparation method thereof, constructed by the invention, can be used for constructing transformants to realize exogenous gene expression.
Owner:FUDAN UNIV
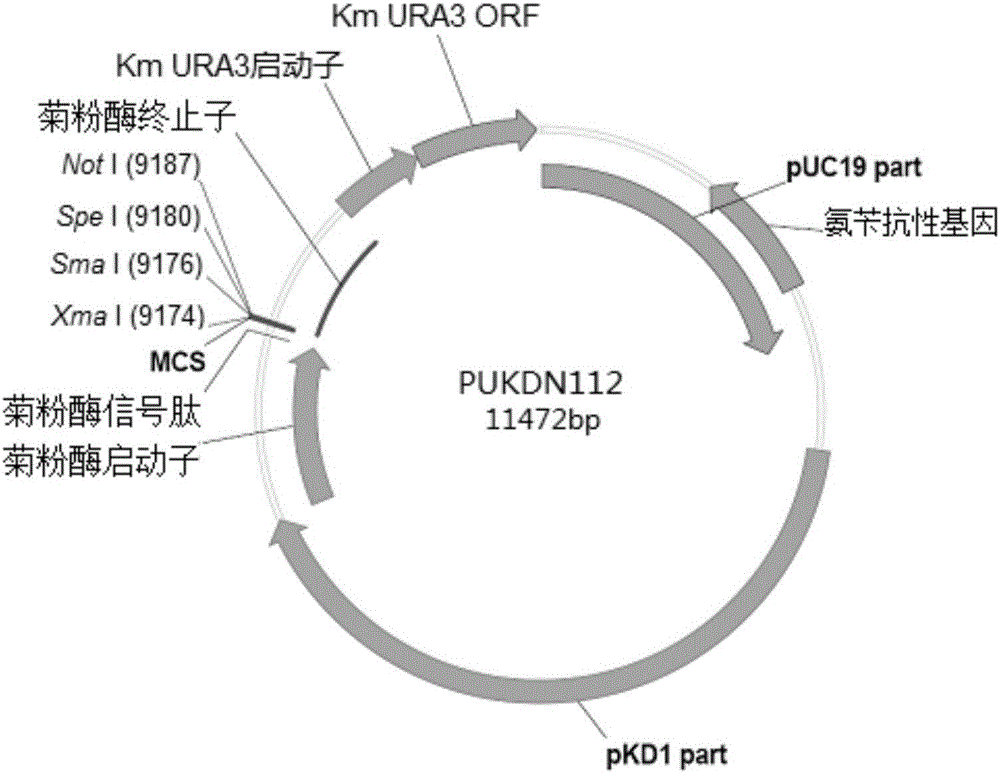
Recombinant vector used for carrying out foreign gene secretory expression in auxotrophic kluyveromyces marxianus strain
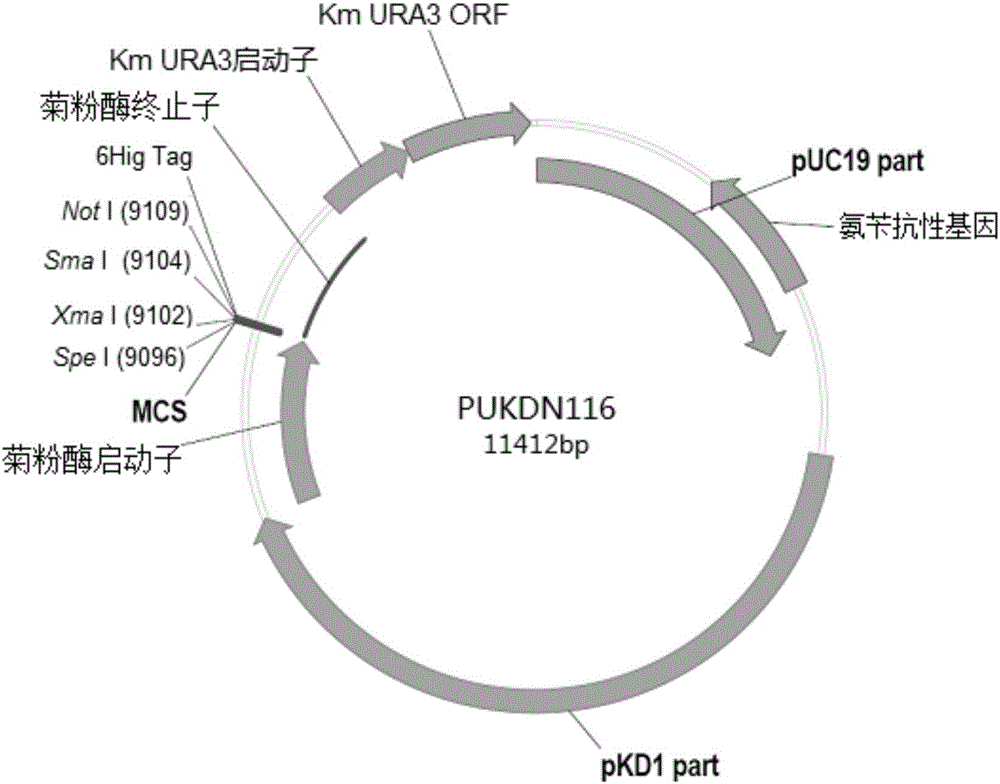
The invention provides a recombinant vector used for carrying out foreign gene secretory expression in an auxotrophic kluyveromyces marxianus strain as well as a preparation method and application of the recombinant vector. The recombinant vector comprises ampicillin resistance genes, a PKD1 vector, inulase promoters, signal peptides, multiple cloning sites, inulase terminators, nutritional gene promoters and a nutritional gene open reading flame according to the sequence. The constructed recombinant vector used for carrying out foreign gene secretory expression and the preparation method of the recombinant vector can be used for constructing a transformant to achieve secretory expression of foreign genes.
Owner:FUDAN UNIV
Genetic engineering antibiotic peptides as well as preparation method and application thereof
The invention relates to a genetic-engineering antibacterial peptide, as well as a preparation method and application thereof. The preparation method comprises the following steps: according to the characteristics of the amino acid sequence of Magainin and Cecropin P and LL-37 which are antibacterial peptide, a novel antibacterial peptide gene is designed; the sequence of the gene is synthesized and transformed to be expressed in pichia to form antibacterial-peptide gene transformation pichia engineering bacteria, and to be expressed in a fermentor to achieve the effects of high-density cultivation and efficient expression. Fermentation broth is separated and purified to be an antibacterial peptide product which can be applied to feed and food additives and be used as injection. The antibacterial peptide has the advantages of good antibacterial activity to most gram-negative bacteria and gram-positive bacteria, in particular better effects of inhibiting and killing ampicillin-resistant bacteria and kanamycin-resistant bacteria.
Owner:山东华辰制药有限公司
Escherichia coli t7 expression vector, vectors for the co-expression and co-purification of recombinant peptides in/with carrier proteins, use of expression vectors for obtaining complexes with multiple antigens and immonomodulators
InactiveUS20180135060A1Increase productionAntibacterial agentsAntibody mimetics/scaffoldsEscherichia coliAmpicillin
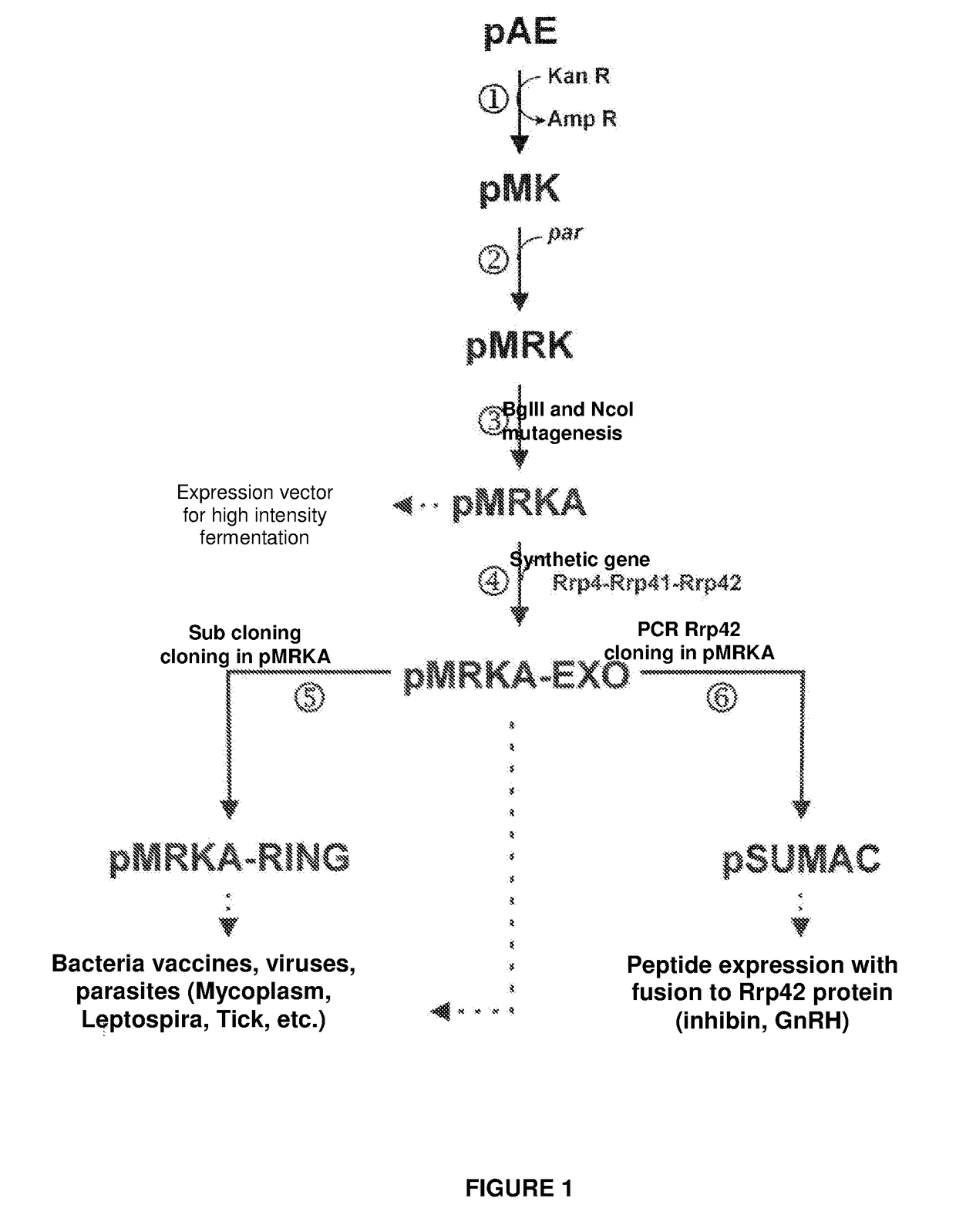
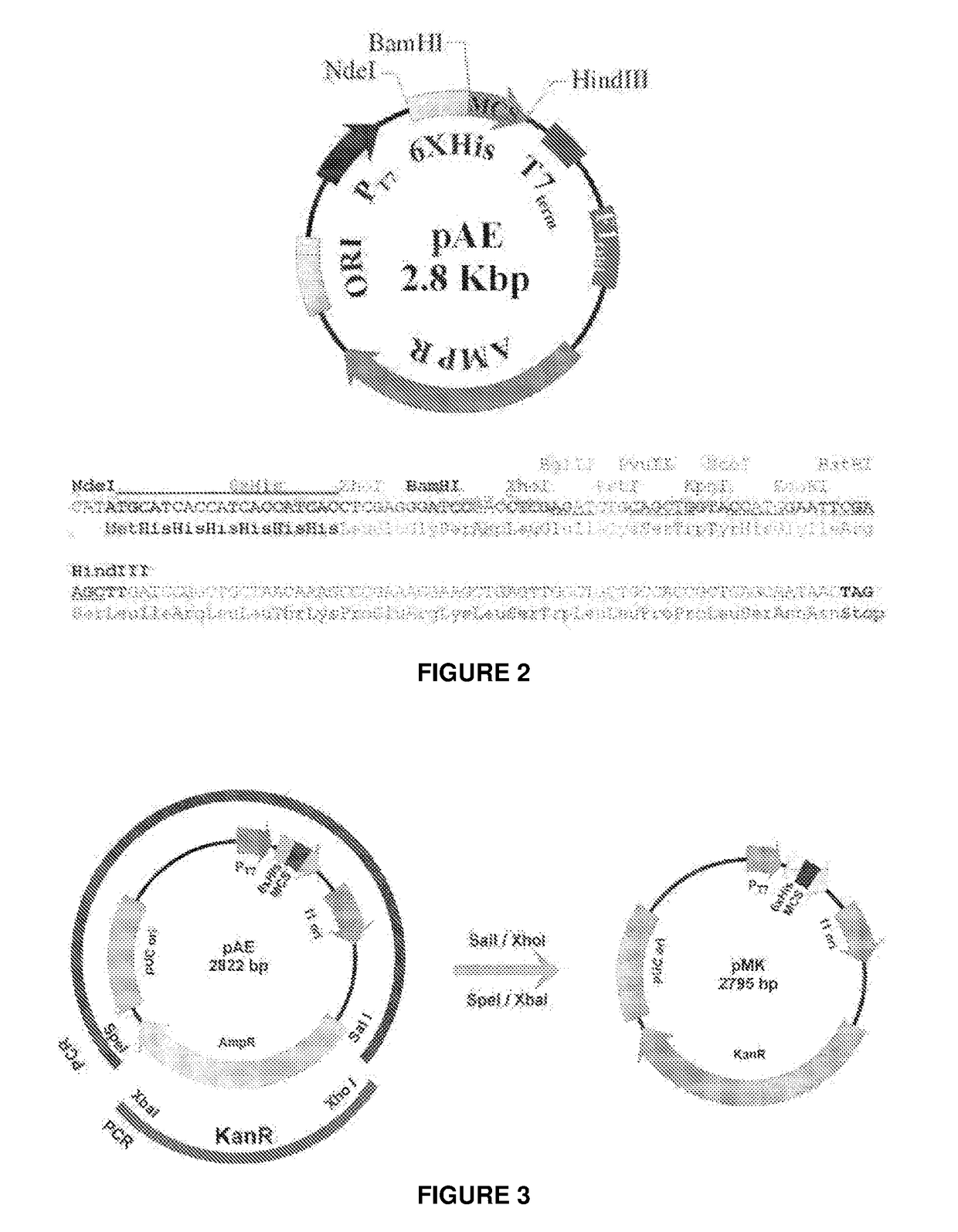
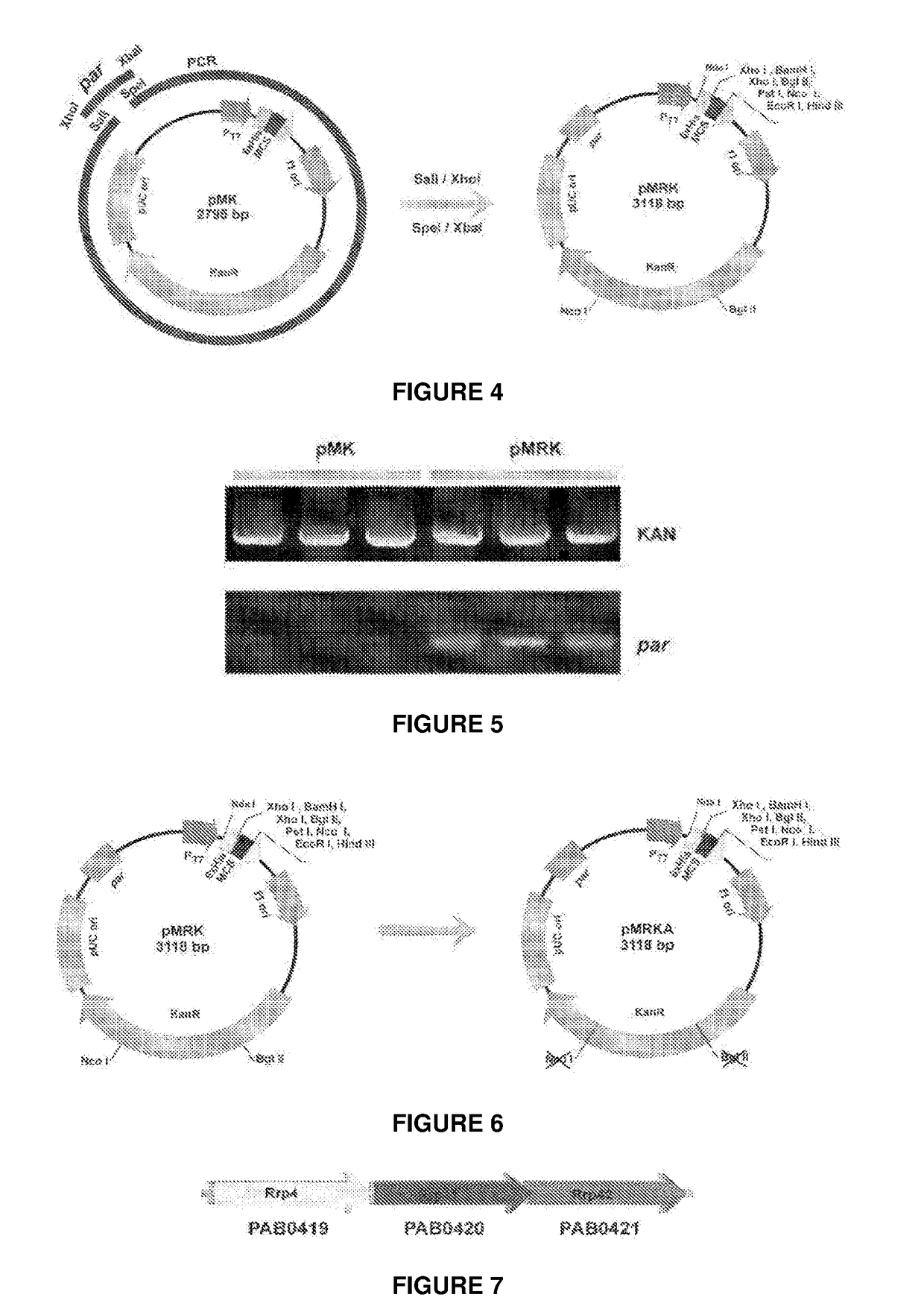
The present invention relates to a vector for the expression of recombinant proteins, antigens, pathogen-like particles and immunogenic complexes, said vector (pMRKA vector) being produced by modifying the plasmids containing the gene sequence of the T7 promoter of E. coli, this modification being mainly characterized by the substitution of the ampicillin-resistance gene by the kanamycin-resistance gene, and by the insertion of the par sequence (partition sequence which determines the efficient segregation of the plasmids in daughter cells during cell division). Also provided are expression vectors based on the pMRKA plasmid, which additionally comprise at least one of the gene sequences of the exosome of P. abyssi, which vectors are designated pMRKA-EXO, pMRKA-RING and pSUMAC. The invention also provides the vectors additionally comprising gene sequences with immunomodulatory or immunoregulatory activity, preferably the pMRKA-Z-Z-EXO and pMRKA-Z-Z-RING vectors. Other aspects of the invention include the method for producing said expression vectors and the use of the obtained vectors.
Owner:OURO FINO SAUDE ANIMAL
Forming method of ampicillin and sulfadimethoxine electrochemical sensor self-assembled passivation layer, and electrochemical sensor thereof
ActiveCN104914151ASimple designImprove general performanceMaterial electrochemical variablesElectrochemical gas sensorAmpicillin
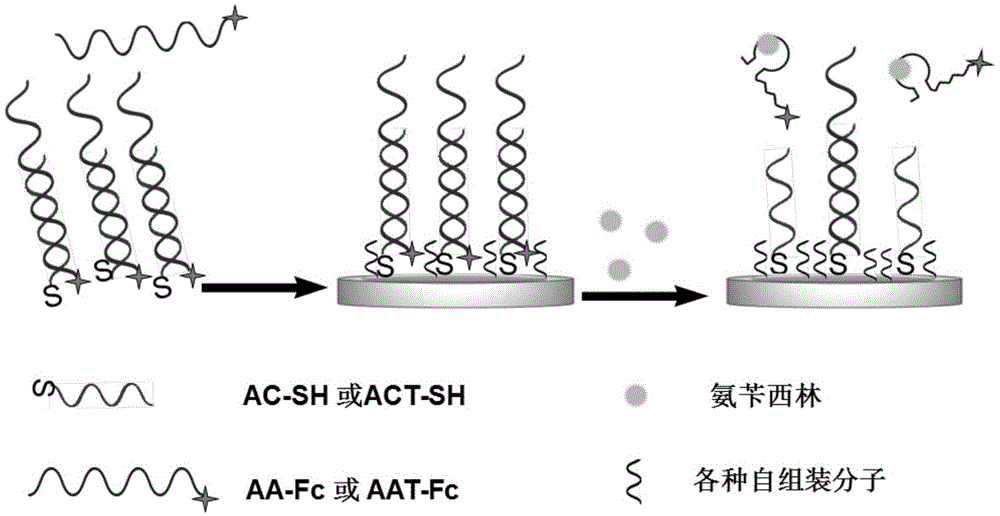
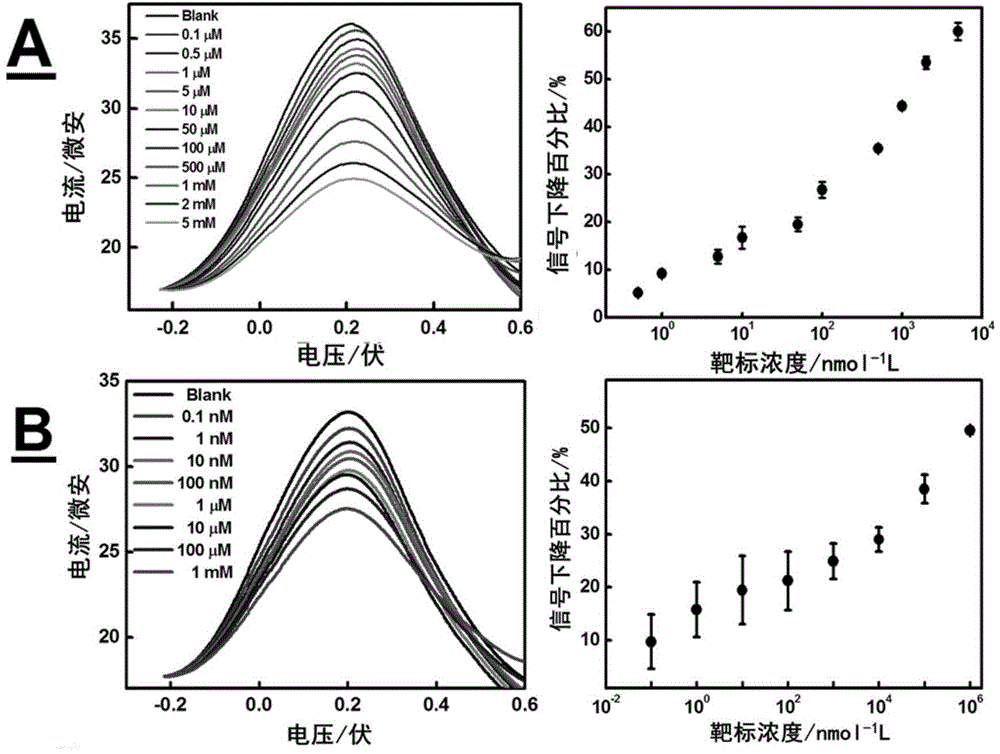
The invention relates to a forming method of a self-assembled passivation layer of a signal probe chain substitution-based aptamer electrochemical sensor (SD-EAB) for detecting ampicillin and sulfadimethoxine, and an electrochemical sensor thereof. A preparation method of ampicillin SD-EAB comprises the following steps: hybridizing a capture probe with the terminal modified by a mercapto group with a ferrocene labeled aptamer signal probe complementing with the capture probe, fixing the obtained hybridized probe on a gold electrode through self-assembling and closing the electrode by using OEG6-OMe as a self-assembled passivation layer molecule. The steps of a preparation method of sulfadimethoxine SD-EAB are similar to above steps. The problem of unable detection of ampicillin and sulfadimethoxine of MCH self-assembled passivation layers commonly used in SD-EAB in the prior art is solved in the invention.
Owner:CAPITAL NORMAL UNIVERSITY +1
Method of attaching drug compounds to non-reactive polymer surfaces
Polymers are disclosed that are chemically modified to retard bacterial growth. Such modified polymers (e.g. ePTFE and polypropylene) are produced by first creating acid groups on the polymer surface through reactions with an anhydride. The acid groups are then linked to polyethylene glycol (PEG) through esterification or other reactions such as amidation. Preferably, at least two different molecular weight PEG species are employed. The antimicrobial surface is completed by linking antibiotics (e.g. β-lactam antibiotics) to the PEG extensions. One preferred embodiment of such a modified polymer is produced using ePTFE, maleic anhydride (MA), and penicillin (PEN) to yield PEN-PEG-MA-ePTFE, which inhibits gram-positive bacteria. The PEG spacer is critical for PEN function in this context, since PEN-ePTFE is ineffective against bacterial growth. Another preferred embodiment incorporates ampicillin (AMP) and a heterobifunctional PEG, HOOC—(CH2—CH2—O)n—NH2, to yield AMP-PEG-MA-ePTFE. This latter example inhibits both gram-negative and gram-positive bacteria.
Owner:SOUTHERN MISSISSIPPI THE UNIV OF
O-nitrobenzaldehyde degrading bacteria and use thereof
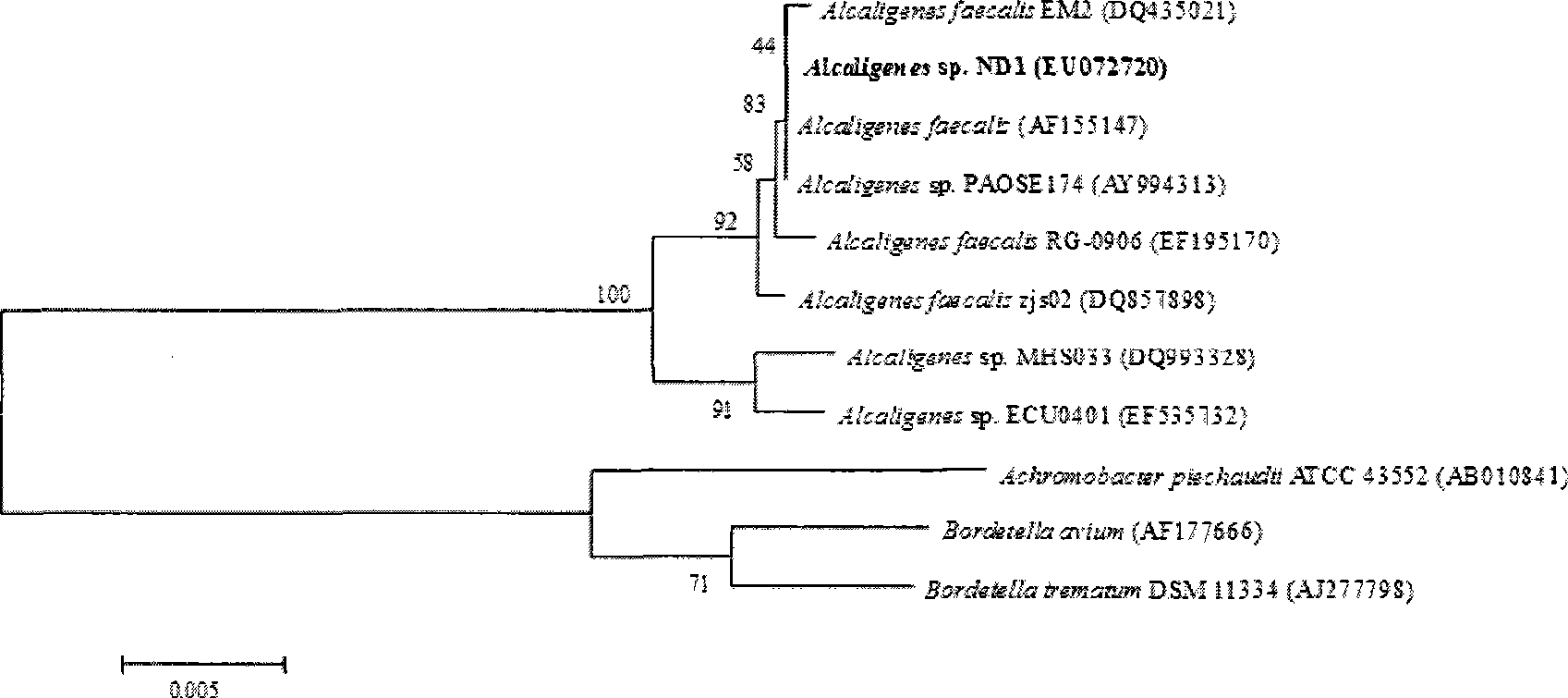
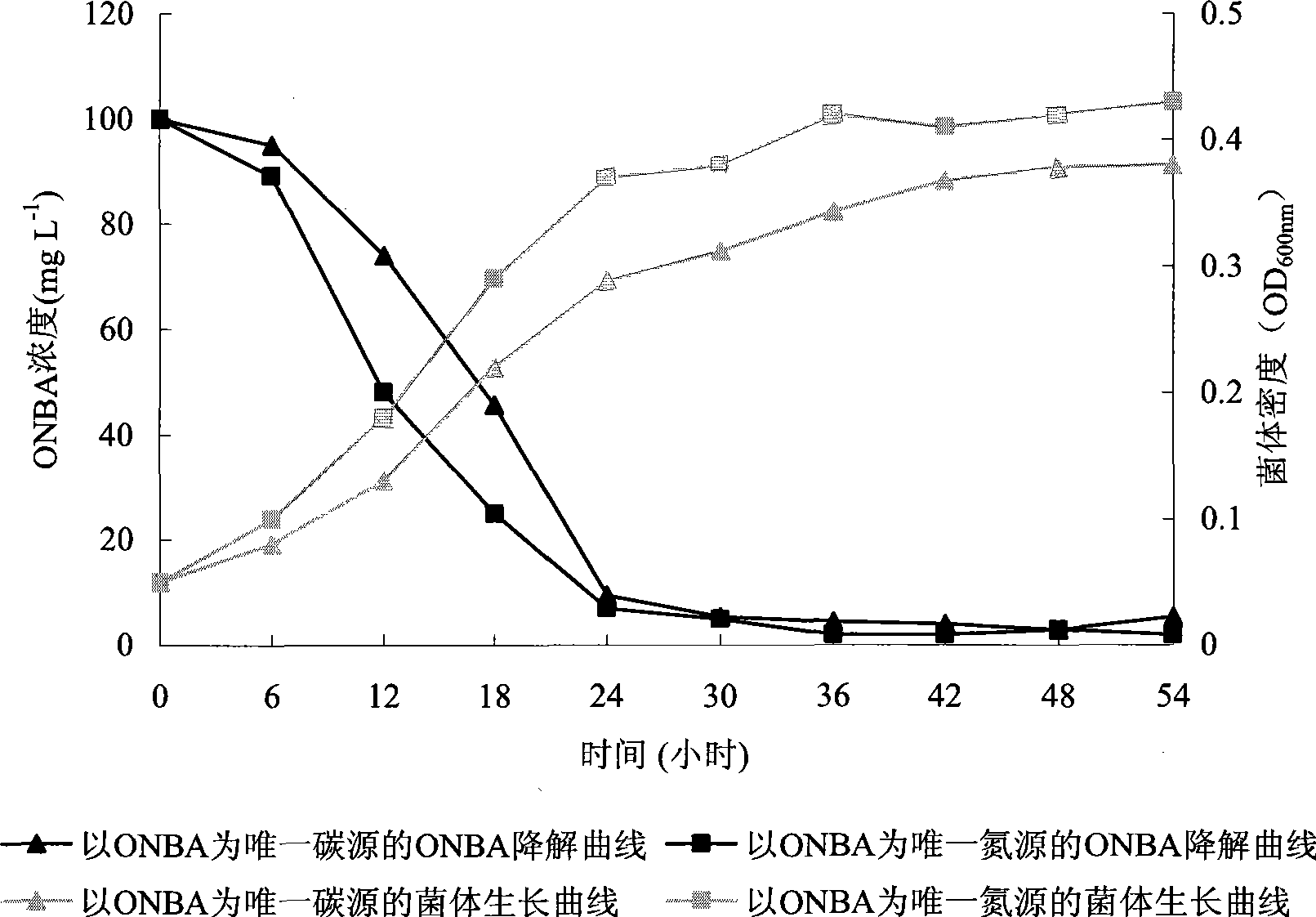
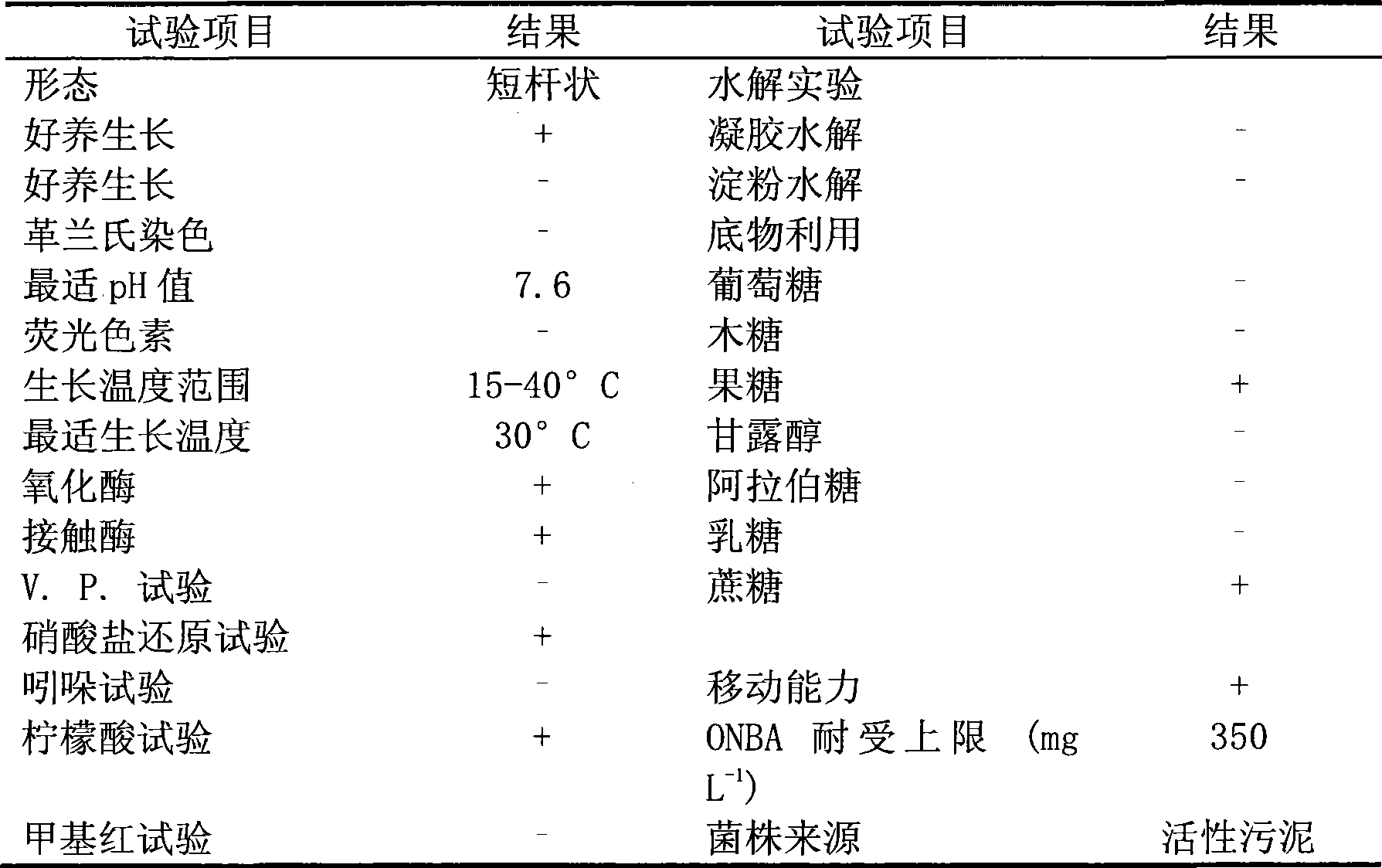
The invention pertains to the technical field of bio-treatment of environmental pollutants, particularly relates to a bacterium for degrading o-nitrobenzaldehyde and the applications thereof in bio-treatment of waste water and remediation of soil environmental pollution. The degrading bacterium of o-nitrobenzaldehyde is the strain ND1 of Alcaligenes sp. The bacterium can degrade o-nitrobenzaldehyde with high efficiency under aerobic condition, and utilize albocarbon, p-nitrophenol, o-nitrophenol, ortho-aminophenol, toluene, p-dimethylaminobenzaldehyde, 2, 4-dinitrophenol, para hydroxy benzoic acid, diphenylamine, benzoic acid, xylene and a plurality of other aromatic compounds. The Alcaligenes sp.ND1 is sensitive to five common antibiotics of tetracycline, kanamycin, streptomycin, chloromycetin and ampicillin, thus providing safe guarantees in the application thereof in the bio-treatment of waste water and remediation of soil environmental pollution.
Owner:ZHEJIANG FORESTRY UNIVERSITY
CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector as well as construction method and applications thereof
ActiveCN105950663APromote secretionImprove in vitro killing effectMammal material medical ingredientsNucleic acid vectorAntigenAmpicillin
The invention discloses a CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector. The CD 30-targeting replication-defective recombinant lentivirus CAR-T transgenic vector comprises a prokaryotic replicor pUC Ori sequence used for plasmid duplication; an ampicillin resistance gene AmpR sequence used for amplification of a large number of target strains; a virus replicor SV40 Ori sequence used for enhancing the replication in eukaryocytes; a lentivirus packaging cis element used for lentivirus packaging; ZsGreen 1 green fluorescent protein used for green fluorescence expression of the eukaryocytes; an IRES ribosome binding sequence used for co-transcription and co-expression of protein; a human EF1 alpha promoter used for eukaryotic transcription of genes of a chimeric antigen acceptor; the chimeric antigen acceptor used for forming second-generation CAR or third-generation CAR integrating recognition, delivery and promoting; and an eWPRE element used for enhancing the expression efficiency of transgenes. In addition, the invention further discloses a construction method and applications of the vector. With the vector, the secretion of the cell factors and the in-vitro lethal effect of the CAR-T cells can be obviously improved, and the effect in treating hodgkin lymphoma or non-hodgkin lymphoma clinically is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
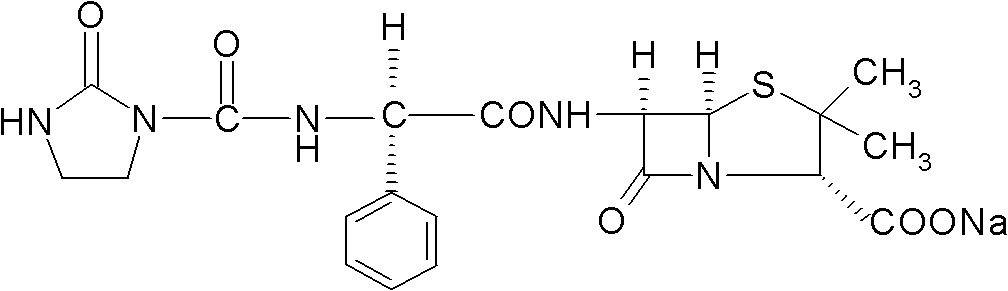
Process for preparing sodium azlocillin
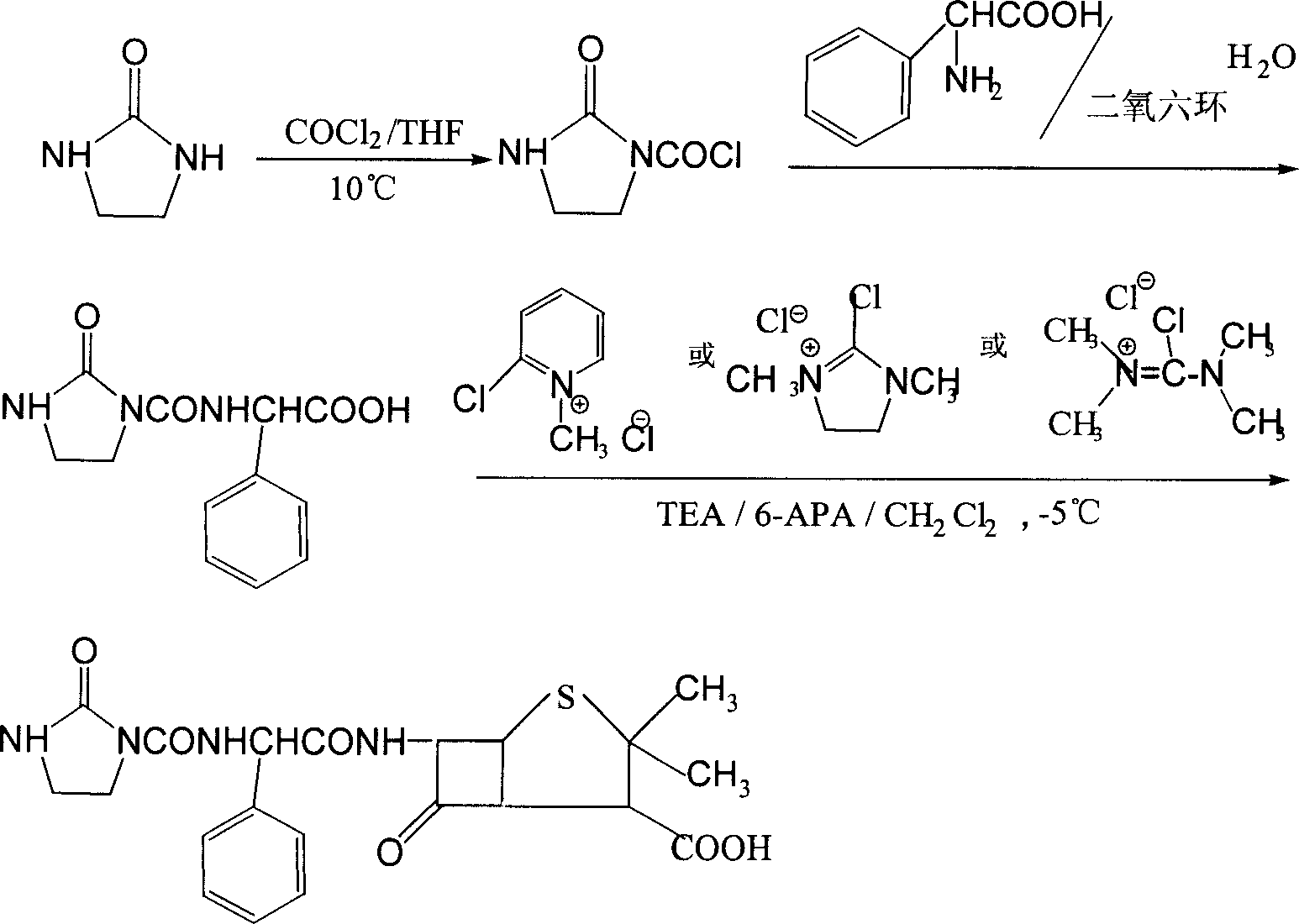
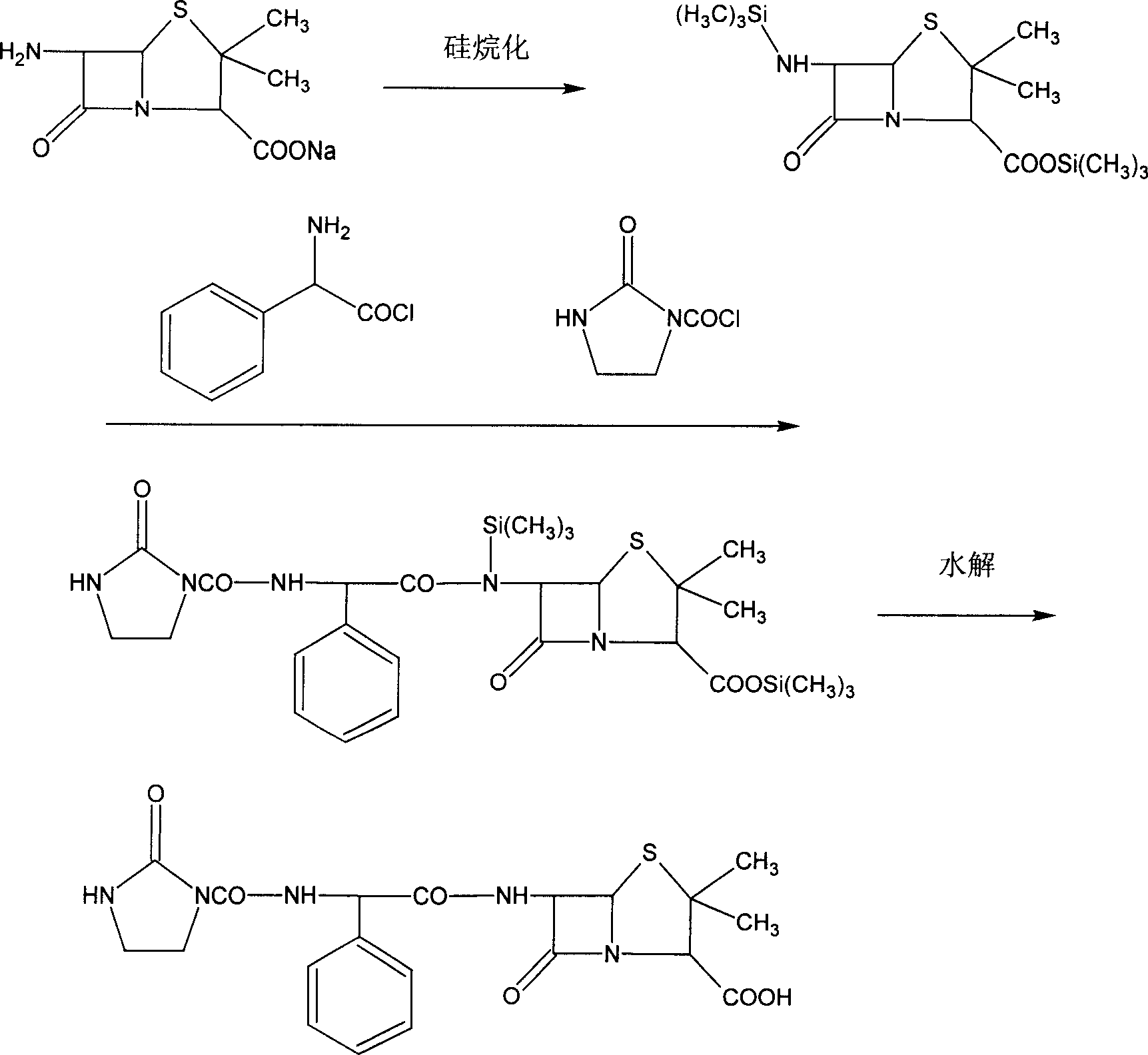
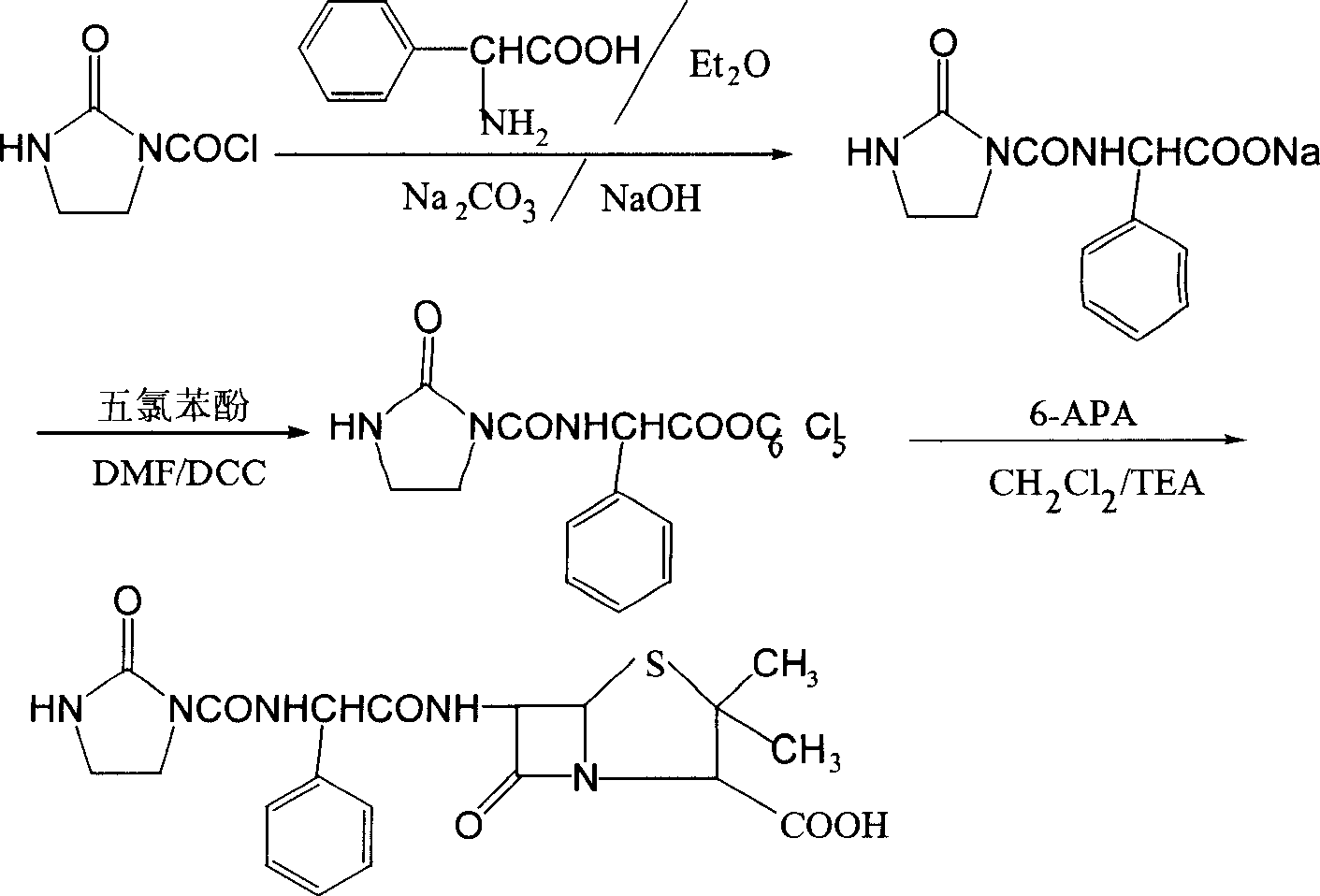
The preparation process of sodium azlocillin includes the following steps: 1. adding triethylamine slowly into water solution of ampicillin at 0-4 deg.c and stirring to clarify the reaction liquid; 2. adding 2-imidazolidinyl ketacyl chloride into the solution at 0-4 deg.c while dropping water solution of Na2CO3 or NaHCO3 to control pH 7.5-8.5 and performing condensation; 3. adding ethyl acetate into the reaction mixture at 0-5 deg.c and regulating pH value to 1.7-1.9; 4. separating to obtain organic phase, and eliminating heat source and impurity to obtain crystallization liquid; and 5. adding ethyl acetate solution of sodium isocaprylate to crystallize, separating and drying to obtain powdered white sodium azlocillin. The process has convenient operation, mild reaction condition, high yield and high product quality.
Owner:黄春荣
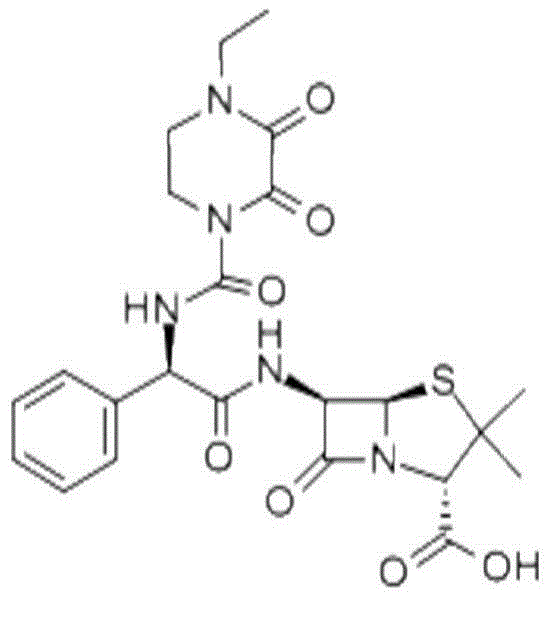
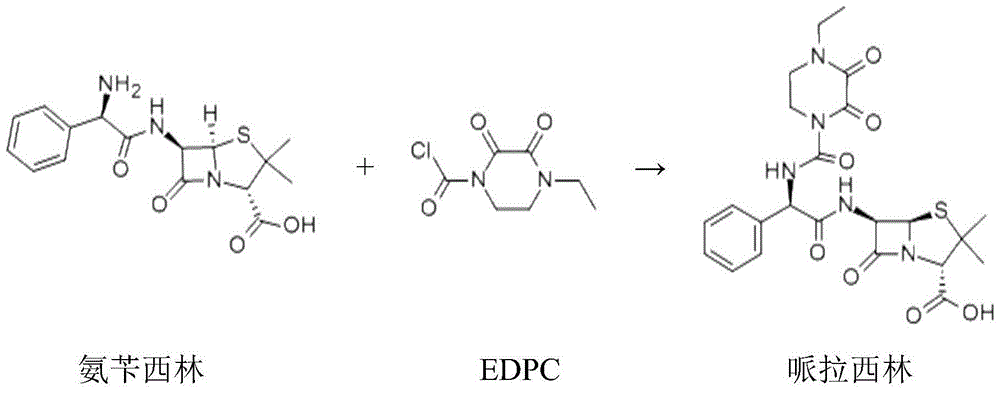
Method for preparing piperacillin acid
The invention discloses a method for preparing piperacillin acid. The method comprises the following steps: adding ampicillin, water and a buffer solution with the pH of 6.0-9.0 into a reactor; adding EDPC into the reactor, meanwhile, adding an alkaline regulator to control the pH to be 6.0-9.0, and reacting for 30-60 minutes in the temperature range of 0 to 10 DEG C while carrying out heat preservation; and adding a solvent to crystallize, controlling the crystallizing point to be 15+ / -2 DEG C, dropwise adding an acidic regulator to regulate the end pH to be 1.5-2.0, carrying out crystal growing for 1 hour in the temperature range of 0 to 10 DEG C, and then, filtrating, washing and drying crystals, thereby obtaining the piperacillin acid finished product. According to the method, during acylation, water is used as a solvent, and the buffer solution is added, so that synthetic reaction for piperacillin acid is inhibited from going towards a reverse reaction direction, the yield of piperacillin acid is increased, and the purity of the product is improved.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
Method for extracellular production of target proteins employing OmpF in E.coli
The present invention provides an expression vector comprising genes encoding OmpF of E. coli and a desired protein, E.coli transformed with the expression vector, and a method for extracellular production of desired proteins by employing the same. The recombinant expression vector of the invention comprises an ampicillin-resistance gene, the OmpF promoter and the OmpF gene. In accordance with the invention, a desired protein can be produced extracellularly by a simpler method than conventional methods such that: secretory production of OmpF fusion protein begins simultaneously with growth of the cells through constitutive expression employing an OmpF promoter, and as the concentration of cells increases, the amount of secretory production of the protein also increases continuously. Therefore, desired proteins can be produced in large quantities by a high concentration culture of cells.
Owner:KOREA ADVANCED INST OF SCI & TECH
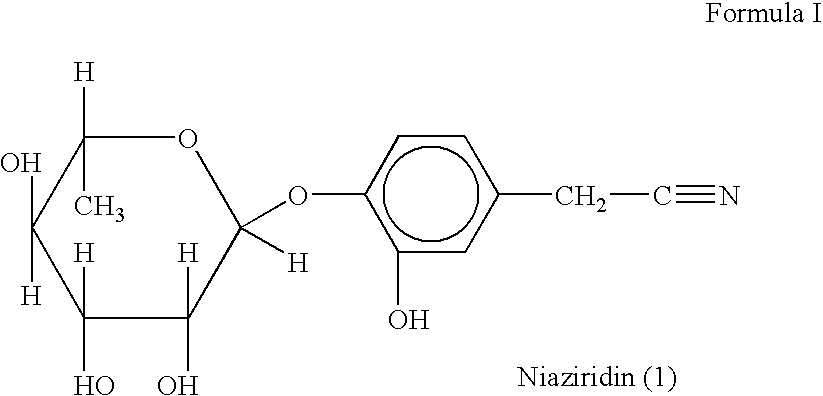
Novel nitrile glycoside useful as a bioenhancer of drugs and nutrients, process of its isolation from moringa oleifera
InactiveUS20040198669A1Improve biological activityEnhance bioavailability absorptionBiocideSugar derivativesAmpicillinBioactivity guided fractionation
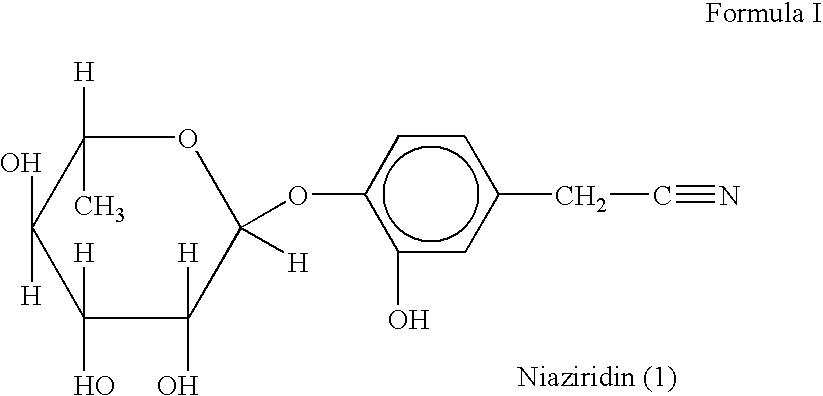
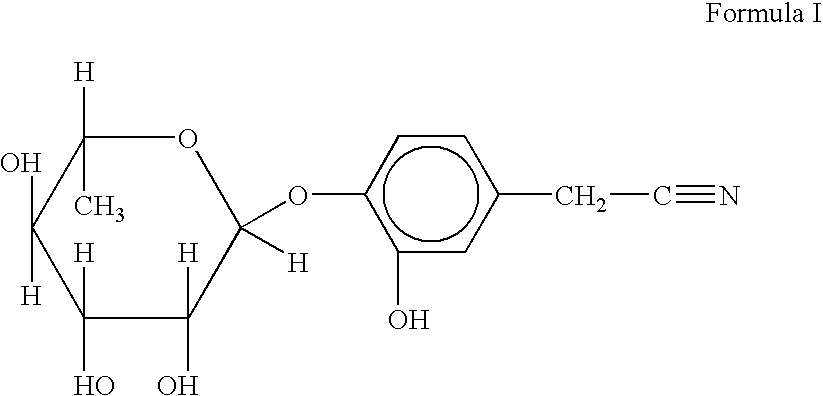
The present invention relates to a novel nitrile glycoside of Formula I named NIAZIRIDIN and to analogues and derivatives thereof. The present invention also relates to a process for the isolation of a novel nitrile glycoside of Formula I below named NIAZIRIDIN and its derivatives and analogues by bioactivity-guided fractionation from the pods of Moringa oleifera. The present invention particularly relates to the bioenhancing activity of the novel nitrile glycoside of Formula I below named NIAZIRIDIN and its derivatives and analogues in enhancing bioactivity of commonly used antibiotics such as rifampicin, tetracycline and ampicillin against Gram (+) and (-) bacteria. The biomolecule also enhances the absorption of drugs, vitamins and nutrients through the gastro-intestinal membrane increasing their bio-availability. Therefore niaziridin can be used in combination therapy with drugs and nutrients resulting in reduced drug associated toxicity, reduced cost and duration of chemotherapy.
Owner:COUNCIL OF SCI & IND RES
New lactic bacteria useful as probiotics
InactiveUS20080063666A1Pleasant flavorHealthy digestion and intestineBiocideBacterial antigen ingredientsDiseaseAmpicillin
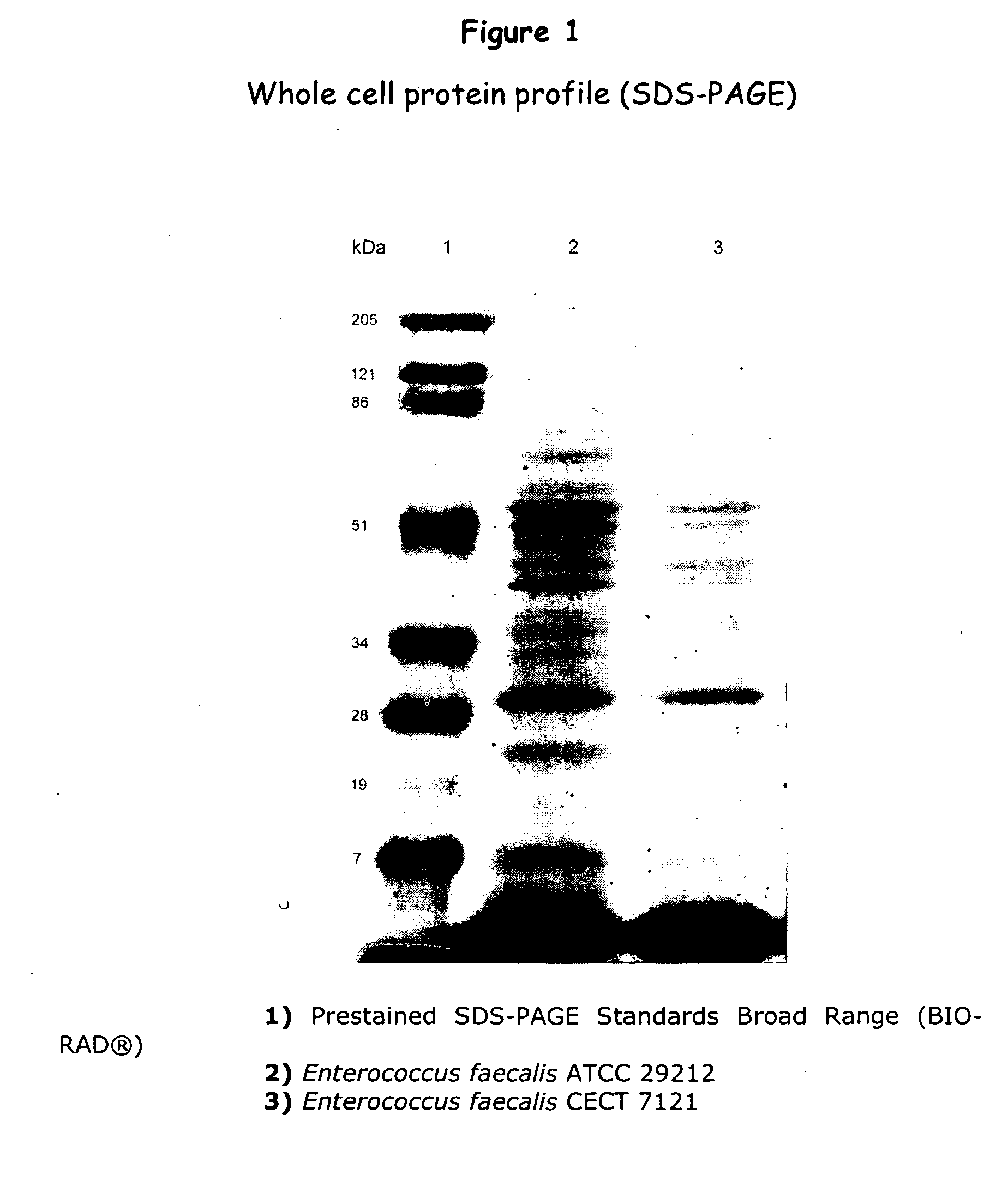
An isolated strain of Enterococcus faecalis GALT deposited under number C E C T 7121 of the group of lactic bacteria is disclosed, which is capable of surviving and colonizing the gastrointestinal tract of humans and / or animals and showing beneficial probiotic activity for the health of humans and animals. The strain E. faecalis GALT and / or a culture supernatant and / or metabolites thereof shows no in vitro multiresistance to antibiotics of common use in human clinics as glycopeptides, such as vancomycin, teicoplanine; carbapenemes, such as impipenem, meropenem; and ampicillin. The strain E. faecalis GALT contains no red blood cell-destroying hemolysins of human, ovine and equine origin; and it does not produce any gelatinase, DNase and decarboxylases. The strain E. faecalis GALT is useful for the preparation of a composition intended for the treatment and / or prophylaxis of disorders associated with colonization by pathogenic microorganisms of the gastrointestinal tract; for use as a regulator of the immune response in human and animals, as well as for the preparation of a composition. The invention is also directed to methods and uses of the strain E. faecalis GALT.
Owner:ALLENDE MIGUEL ANGEL GARCIA
Electrotransformation method for introducing shuttle plasmid into corynebacterium acetoacidophilum
InactiveCN103160535AReduce hindranceAvoid cutsBacteriaMicroorganism based processesEscherichia coliAmpicillin
The invention provides an electrotransformation method for introducing shuttle plasmid into corynebacterium acetoacidophilum. The method is mainly used for rapidly and effectively introducing shuttle-expression recombinant plasmid in escherichia coli and corynebacterium acetoacidophilum into corynebacterium acetoacidophilum through electrotransformation. Through controlling composition of a culture medium, cell culture time, ampicillin processing time, methylation of the plasmid, heat shock time and temperature and electric shock conditions, a high efficiency electrotransformation competent cell is prepared, a blocking effect of the cell wall of corynebacterium acetoacidophilum on the shuttle plasmid is weakened, restriction enzyme digestion of corynebacterium acetoacidophilum on the shuttle plasmid is reduced, so the shuttle plasmid can be highly efficiently introduced into corynebacterium acetoacidophilum through electrotransformation, and electrotransformation efficiency reaches 6.4 * 10<4> cfu / mu g DNA.
Owner:JIANGNAN UNIV
ELISA kit for detecting penicillin G and detection method thereof
The invention relates to an enzyme immune agent box for detecting ampicillin G, which comprises: enzyme mark plate which coats ampicillin G antibody or antigen, enzyme mark material, ampicillin G standard solution, base material color developing solution, compression cleaning liquid, ending solution, compression twin solution and antibody working solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:贵州勤邦食品安全科学技术有限公司
Multimeric protein having effect of brain targeting, and preparation method and usage thereof
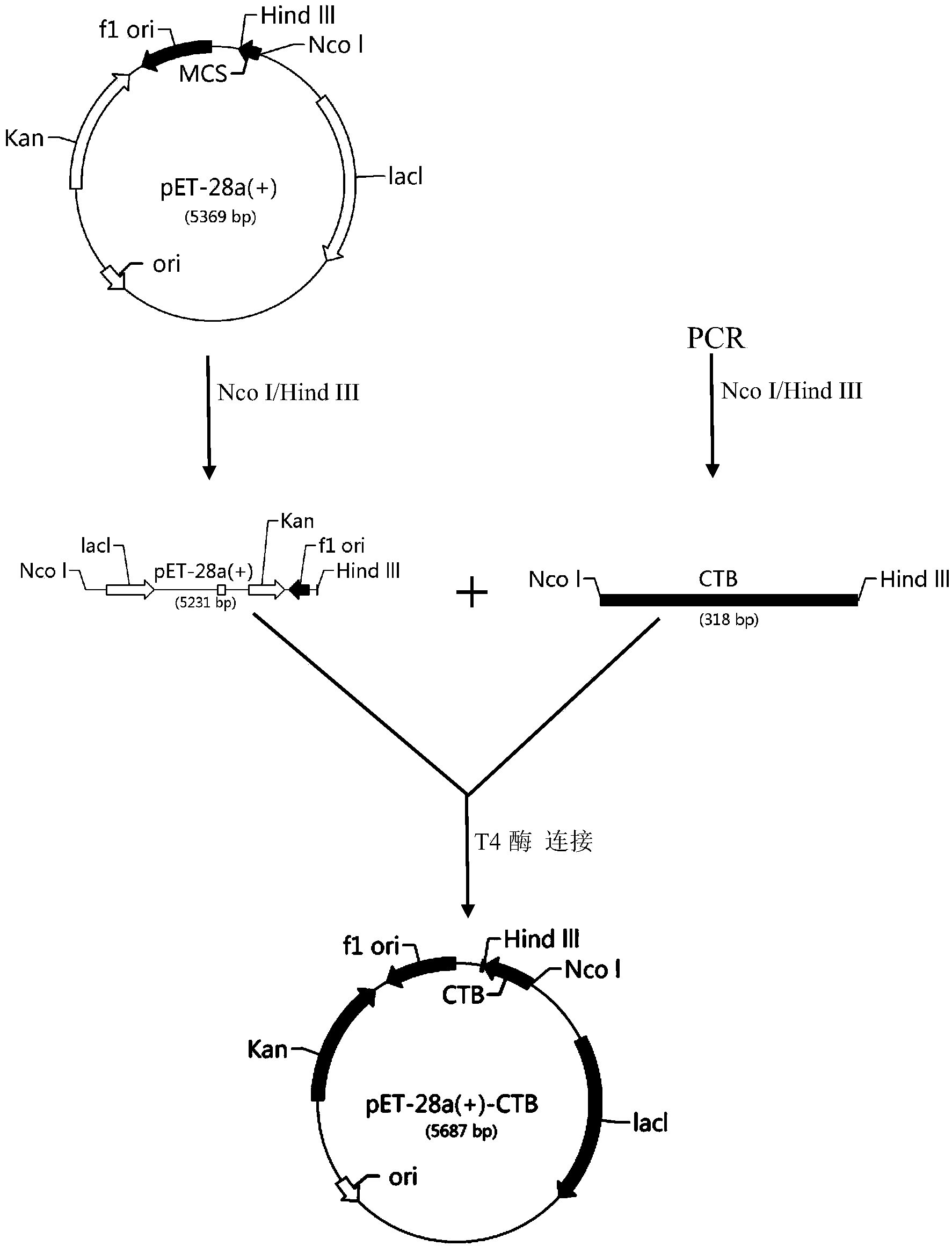
InactiveCN104387472AIncrease contentRich research methodsNervous disorderPeptide/protein ingredientsEscherichia coliKanamycin
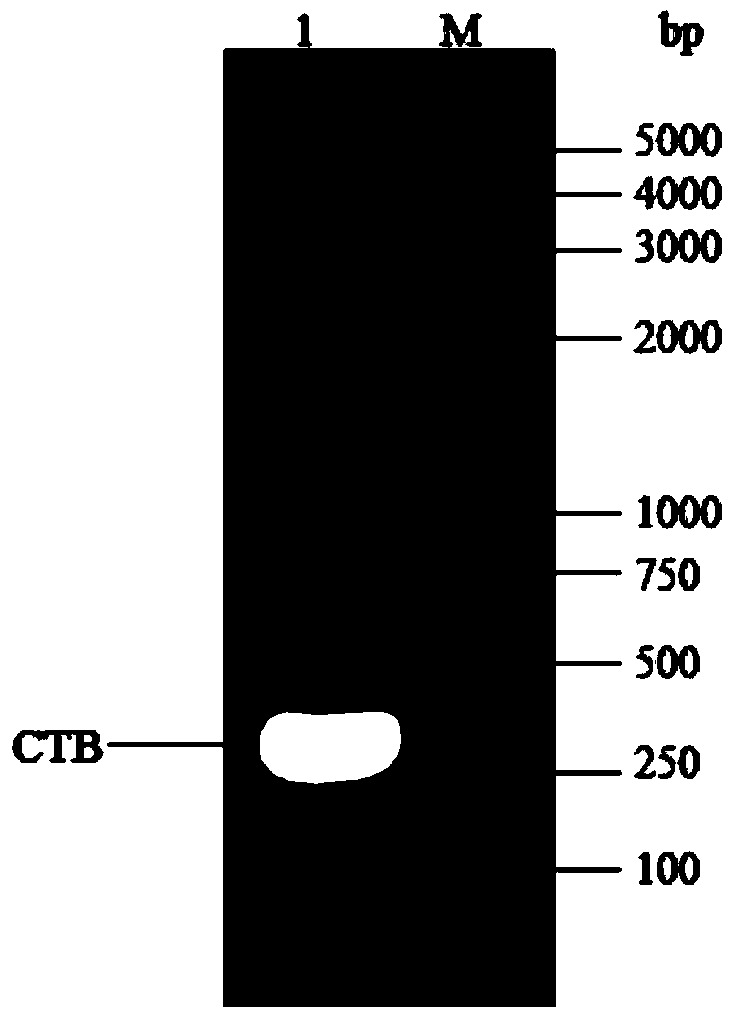
The invention discloses a multimeric protein having the effect of brain targeting, and a preparation method and usage thereof. The method comprises the steps: firstly, expressing cholera toxin B subunit and a fusion protein EGFP-CTA2-TAT of three proteins: an enhanced green fluorescent protein, a cholera toxin A subunit and cell-penetrating peptide in escherichia coli by incompatible double plasmid systems to obtain CTB gene by PCR amplification; cloning the gene segment into a carrier pET-28a to obtain a recombinant plasmid pET-28a-CTB; using wild type CTA2, EGFP and TAT amino acid sequences as templates, inserting 3 enzyme cutting sites and linkers between EGFP and CTA2, and optimizing to obtain codons suitable for expression in escherichia coli; cloning the gene segment into a carrier PET-22b (+) to obtain recombinant plasmid PET-22b-EGFP-CTA2-TAT; using different resistances of PET-28a-CTB and PET-22b-EGFP-CTA2-TAT, and co-transforming the different resistances of the PET-28a-CTB and the PET-22b-EGFP-CTA2-TAT into escherichia coli BL21, to obtain engineering bacteria after screening under double resistance selection pressure of penbritin and kanamycin. CTB5 / EGFP-CTA2-TAT chimeric proteins can be obtained after inducible expression of the engineering bacteria by IPTG. The invention further discloses the preparation method and usage of the protein.
Owner:GUANGDONG UNIV OF TECH
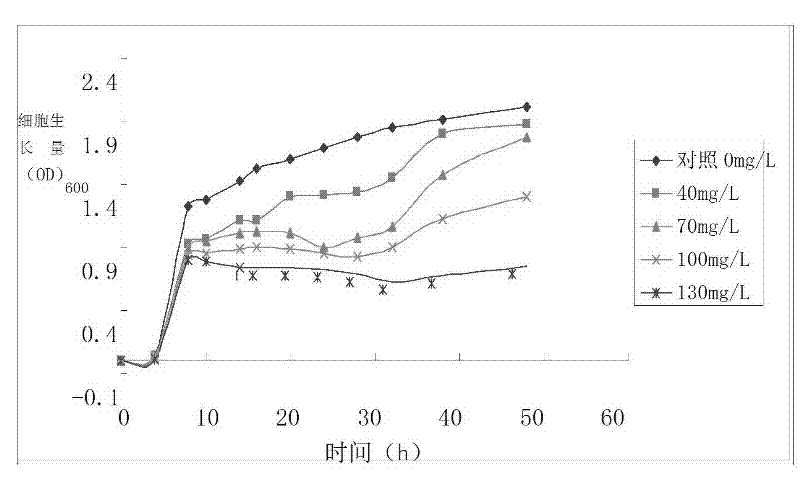
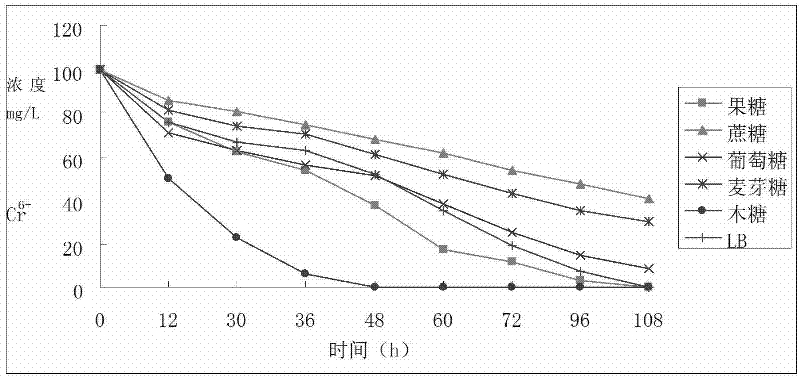
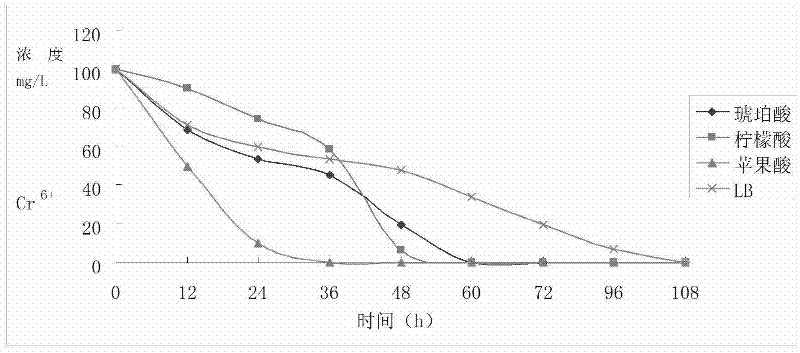
Reductive chromium ion bacillus thuringiensis YB-03 and cultivation method and application of reductive chromium ion bacillus thuringiensis YB-03
The invention relates to reductive chromium ion bacillus thuringiensis YB-03 and a cultivation method and application of the reductive chromium ion bacillus thuringiensis YB-03. According to the invention, the separated reductive chromium ion bacillus thuringiensis YB-03 strain with CGMCC (China General Microbiological Culture Collection Center) NO.5653 not only can reduce Cr<6+>, but also can generate resistance to Ag<+>, Hg<2+>, Cu<2+>, Zn<2+>, Co<2+>, Mn<2+> at different degrees, is insensitive to penbritin and streptomycin and has important application value in treatment of chromium pollution.
Owner:HENAN ACAD OF AGRI SCI
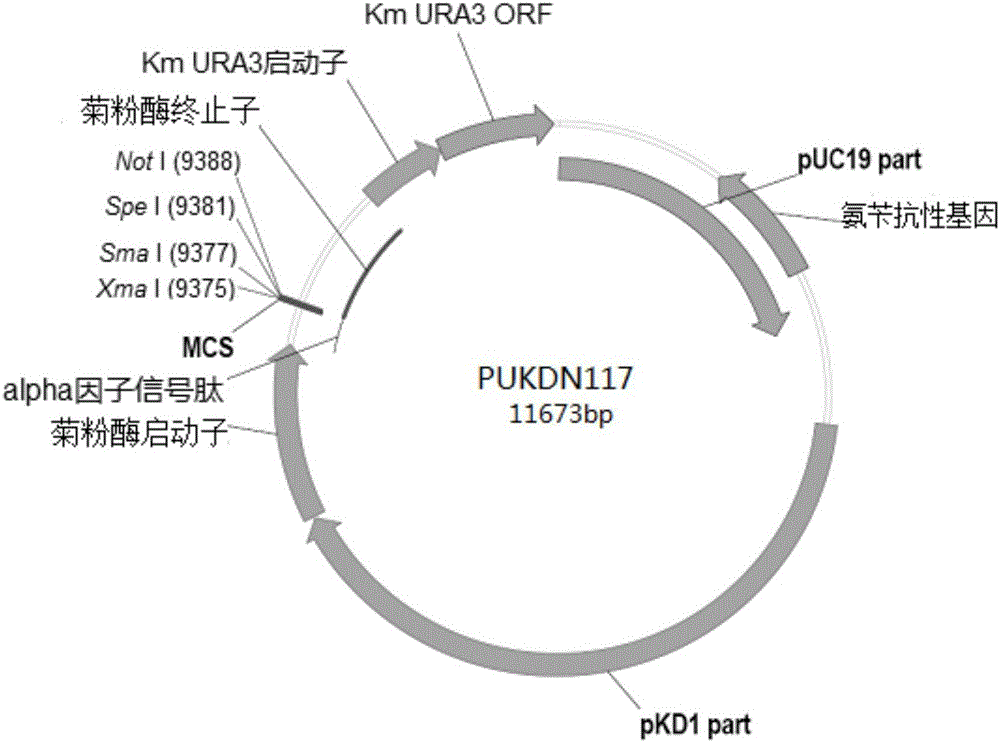
Recombinant vector for expressing histidine-tag-fused foreign gene in Kluyveromyces marxianus nutritional deficient strain
The invention provides a recombinant vector for expressing a histidine-tag-fused foreign gene in a Kluyveromyces marxianus nutritional deficient strain, and a preparation method and application of the recombinant vector. The recombinant vector sequentially comprises an ampicillin resistance gene, a PKD1 vector sequence, an inulase promotor, multiple cloning sites, an inulase terminator, a nutritional gene promotor and a nutritional gene open reading frame, and further comprises histidine tags located at C ends or N ends of the multiple cloning sites. The recombinant vector provided by the invention can be used for building a transformant to realize expression of the foreign gene.
Owner:FUDAN UNIV
Fabrication method and application of ampicillin sensor
ActiveCN109254049AEasy to makeEasy to operateMaterial electrochemical variablesAmpicillinHigh activity
The invention discloses a fabrication method of an ampicillin sensor, and belongs to the technical field of novel nanometer functional material and biological sensing analysis. A nickel-iron thermometal layered hydroxide nanosheet array is fabricated on a disposable throwable electrode, an electronic medium-containing polydopamine thin film and a molecular imprinting polymer taking ampicillin as atemplate molecule polymer are directly and sequentially fabricated on the nickel-iron thermometal layered hydroxide nanosheet array by an in-situ growth method according to large specific surface area, high-activity hydroxyl functional group and amino functional group of polydopamine. After the template molecule is eluted, the original position of the template molecule is changed to holes, and the molecule imprinting polymer of the template molecule is eluted. Therefore, the ampicillin sensor is fabricated and completed.
Owner:UNIV OF JINAN
Artificial chromosome transfer vector for recombinant herpesvirus-of-turkey bacteria
InactiveCN101864442AEasy to identifyEfficient expressionGenetic material ingredientsAntiviralsAmpicillinPhosphotransferase Gene
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Preparation method for Azlocillin sodium
ActiveCN102311450AReduce usageReduce the risk of contaminationOrganic chemistryChemical solutionAzlocillin Sodium
The invention relates to a preparation method for Azlocillin sodium, which belongs to the synthetic technical field of antibiotics. The preparation method comprises the following steps: preparing a solution used for a condensation reaction, drawing dichloromethane into a reactor, putting ampicillin into the reactor with stirring, controlling the weight ratio of dichloromethane to ampicillin, thencooling and controlling the reduced temperature, then regulating the pH value to alkalescence by a pH regulator to obtain an solution used for condensation reaction; performing a condensation reaction; extracting and separating; crystallizing; drying; performing a salifying reaction; decoloring; freezing and drying; crushing. The invention has the advantages that the preparation method is capableof simplifying the operation, reducing the usage of the chemical solutions and the risks on environmental pollution; and has simple operation; can effectively avoid the loss caused by chromatography,raise the yield of Azlocillin sodium; and has mild salifying reaction condition, reduce the risk that Azlocillin sodium can be decomposed in alkali. The content of a single impurity of the obtained Azlocillin sodium dried product is lower than 0.6 percent and the content of total impurity of the obtained Azlocillin sodium dried product is lower than 1 percent which are obviously superior to the pharmacopoeia standard.
Owner:JIANGSU HI STONE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com