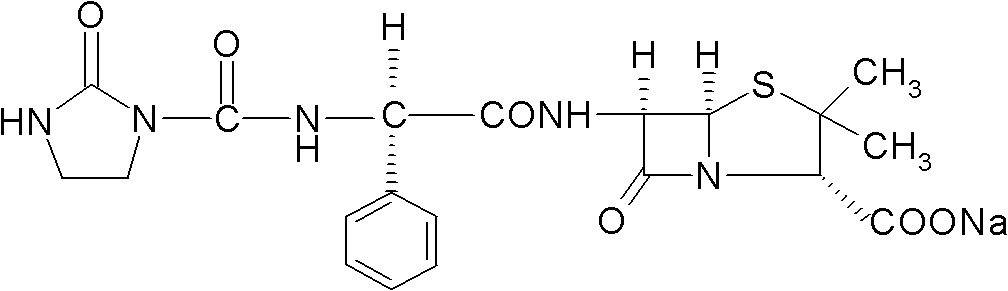
Preparation method for Azlocillin sodium
A technology of azlocillin sodium and ampicillin, applied in the field of antibiotic drug synthesis, can solve the problems of large amount of chemical solvent used, affecting product yield, loss of chromatographic operation, etc., to reduce the risk of degradation in the presence of alkali, and easy to operate , the effect of reducing risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A) prepare the condensation reaction liquid, pump 1 part of dichloromethane into the reaction tank (also called the reaction tank) by weight parts, then drop into 0.075 parts (weight parts) of ampicillin in the reaction tank under stirring state, then Open the jacket of the reaction tank and cool the frozen brine to 0°C, adjust the pH to weakly alkaline with a pH adjuster, i.e. triethylamine, that is, adjust the pH to 8.0 to obtain a solution for condensation reaction;
[0033] B) Condensation reaction, first put 1-chloroformyl-2-imidazolidinone and dichloromethane into the dissolving tank and dissolve to form a mixed solution, then the mixed solution is added dropwise and within 45min to Condensation reaction is carried out in the condensation reaction solution in the reaction tank described in step A), the temperature of the condensation reaction is 10 ° C, after the dropwise addition of the aforementioned 1-chloroformyl-2-imidazolidinone and dichloromethane After the...
Embodiment 2
[0042] A) prepare the condensation reaction liquid, pump 1 part of dichloromethane into the reaction tank (also called the reaction tank) by weight parts, then drop into 0.074 parts (weight parts) of ampicillin in the reaction tank under stirring state, then Open the jacket of the reaction tank and cool the frozen brine to 5°C, adjust the pH to weakly alkaline with a pH adjuster, i.e. triethylamine, that is, adjust the pH to 7.5 to obtain a solution for condensation reaction;
[0043] B) Condensation reaction, first put 1-chloroformyl-2-imidazolidinone and dichloromethane into a dissolving tank to dissolve and form a mixed solution, then drop the mixed solution to The condensation reaction is carried out in the condensation reaction solution in the reaction tank described in step A), the temperature of the condensation reaction is 5 ° C, after the dropwise addition of the aforementioned 1-chloroformyl-2-imidazolidinone and dichloromethane After the mixed solution of the mixed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com