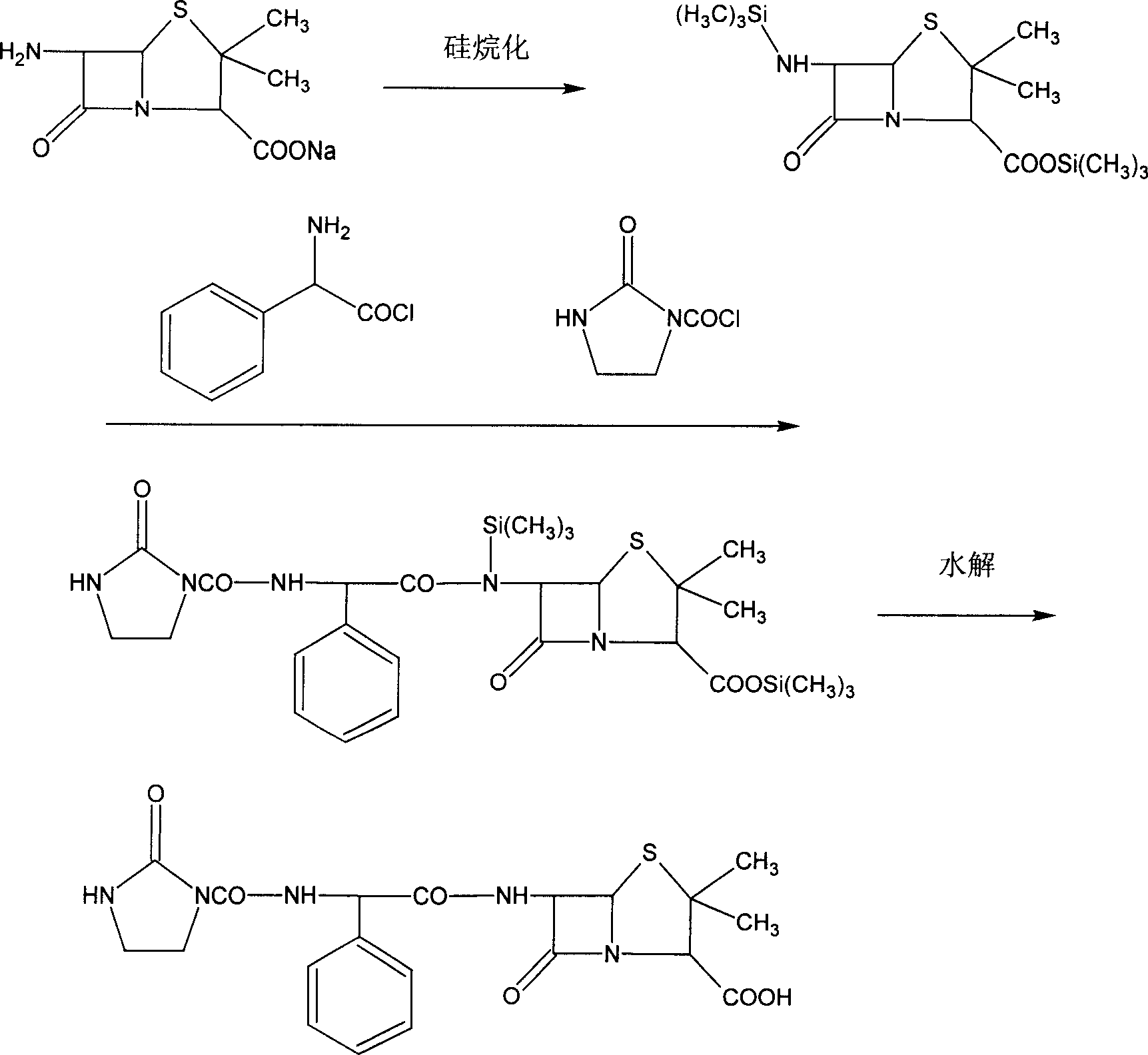
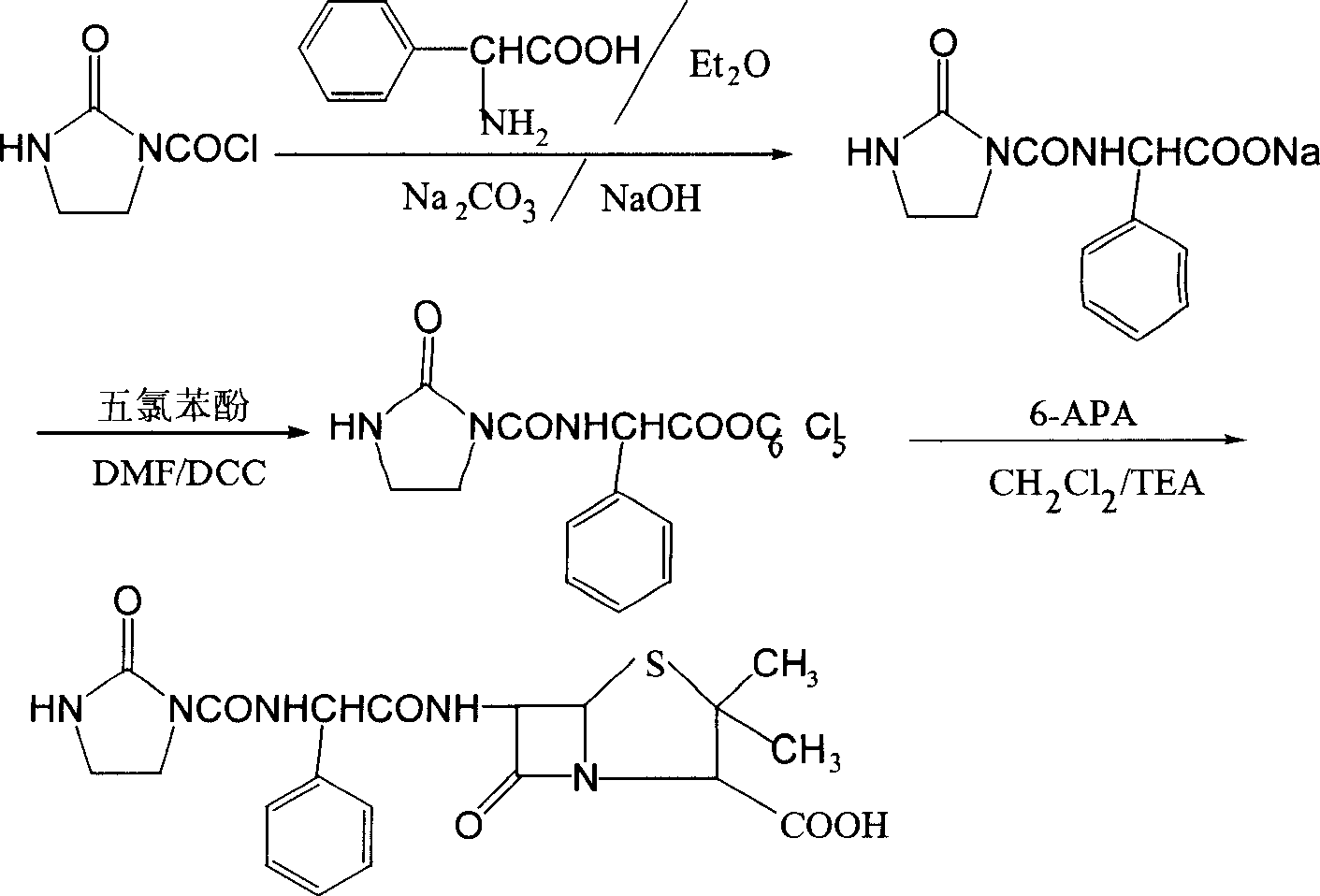
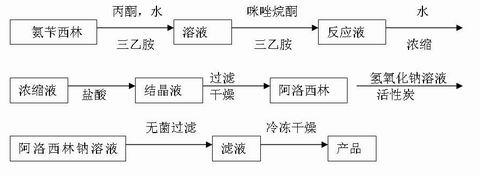
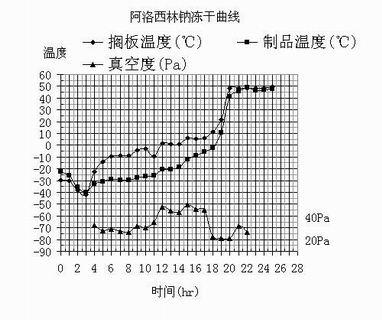
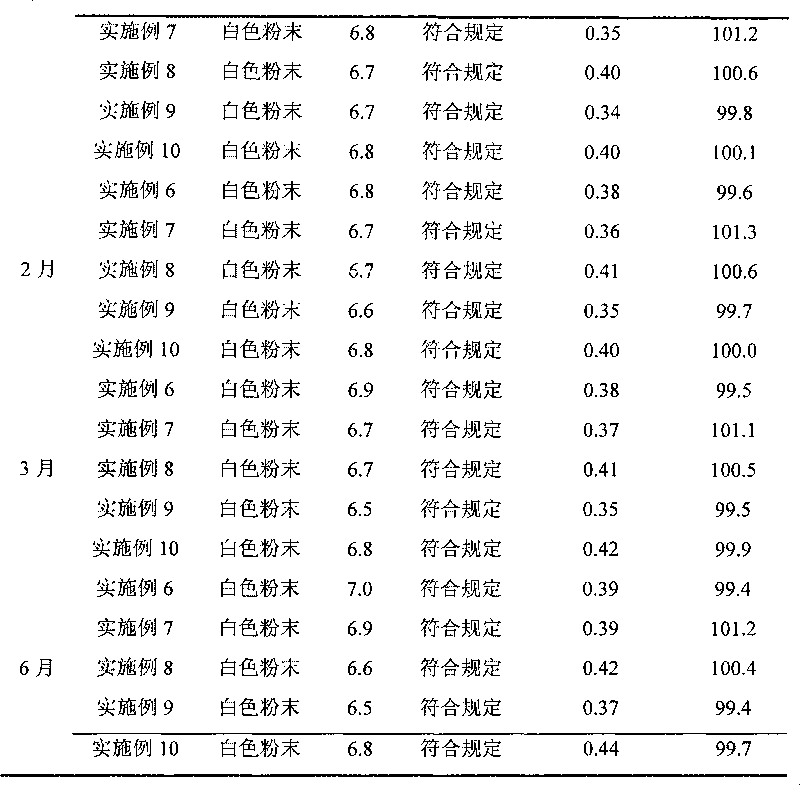
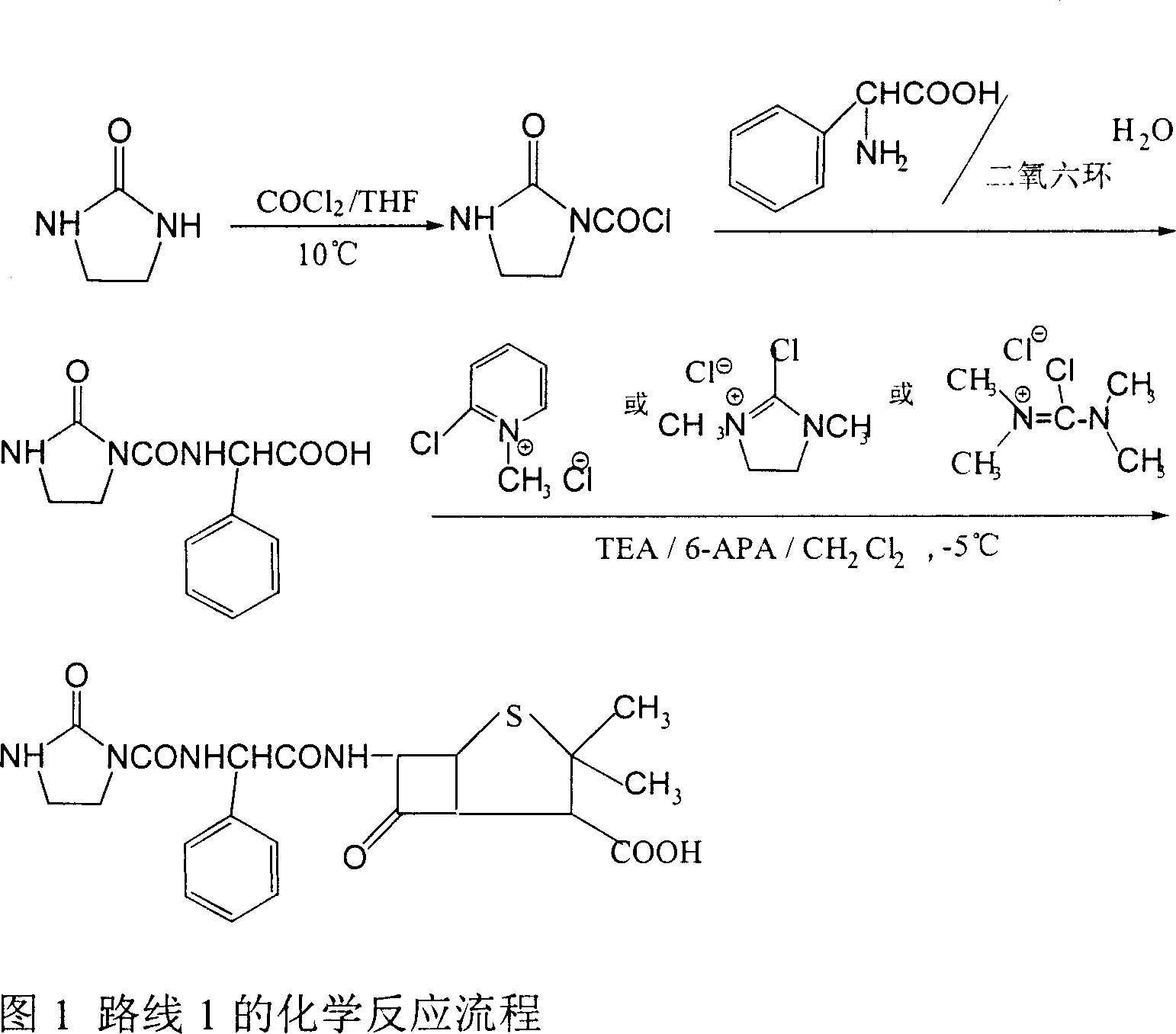
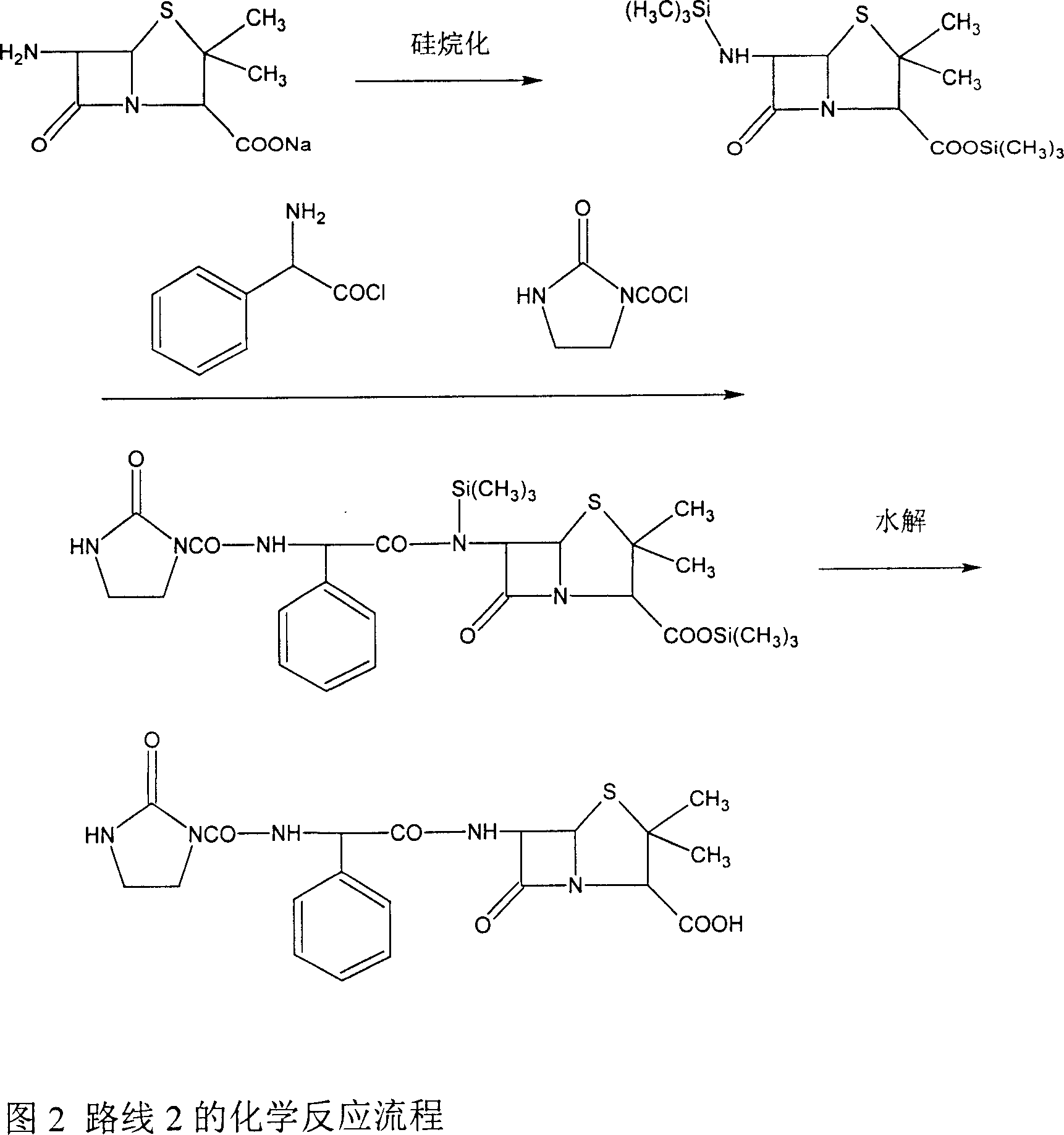
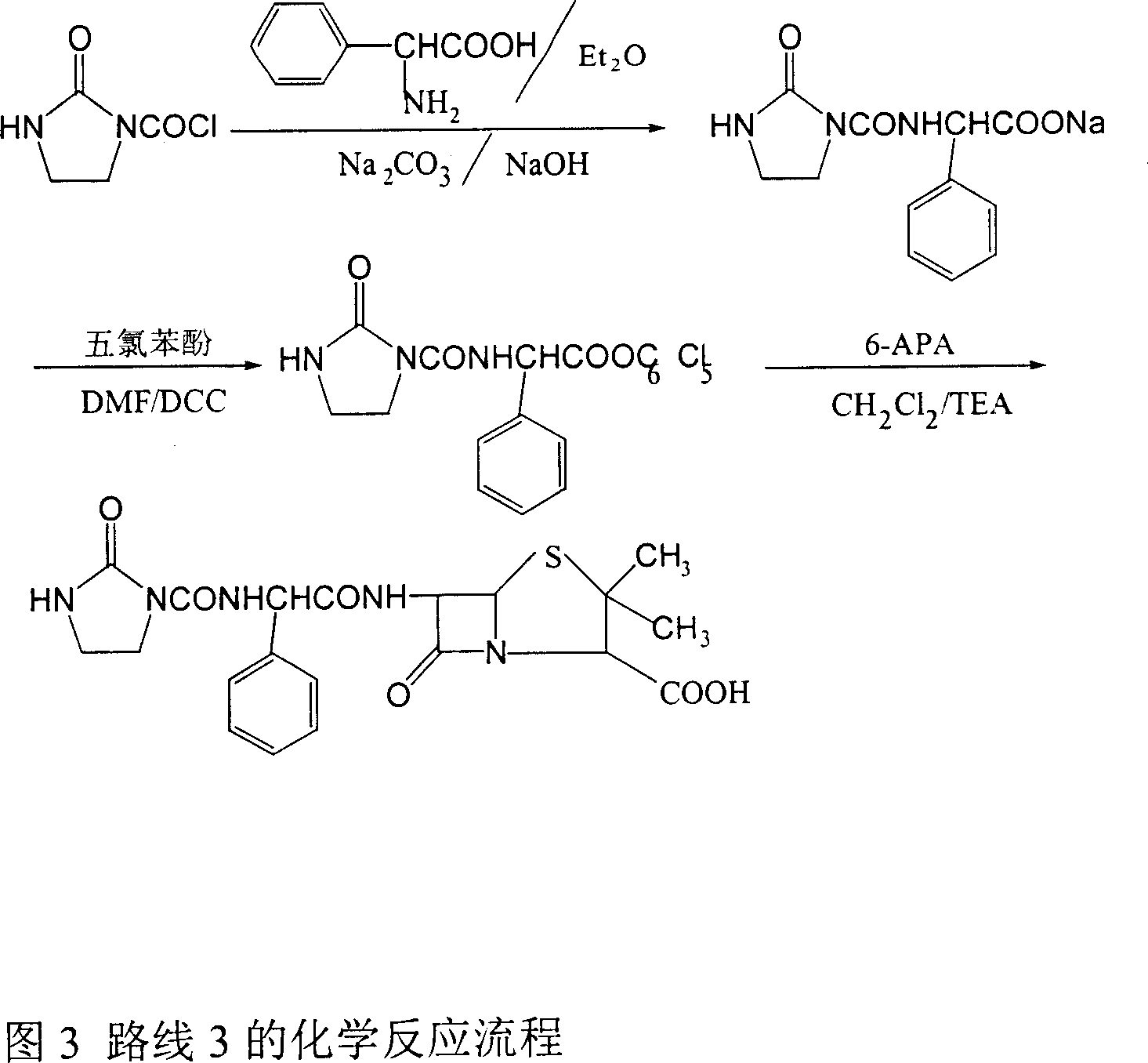
Patents
Literature
37 results about "Azlocillin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
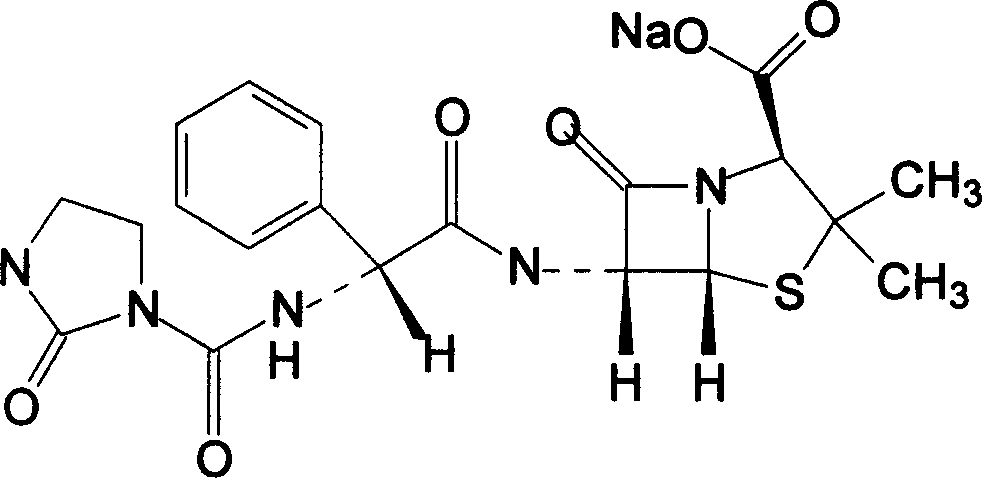
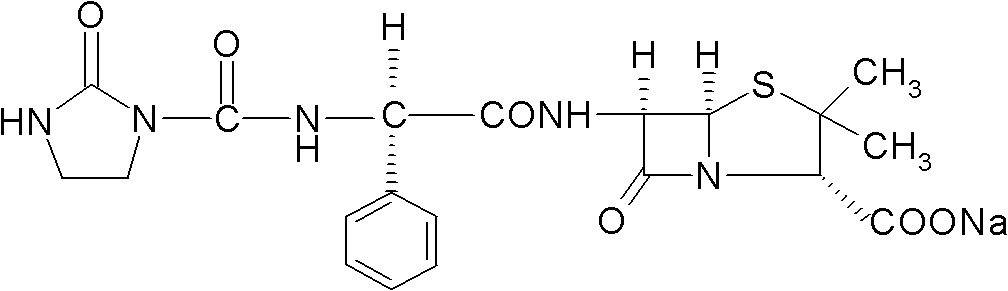
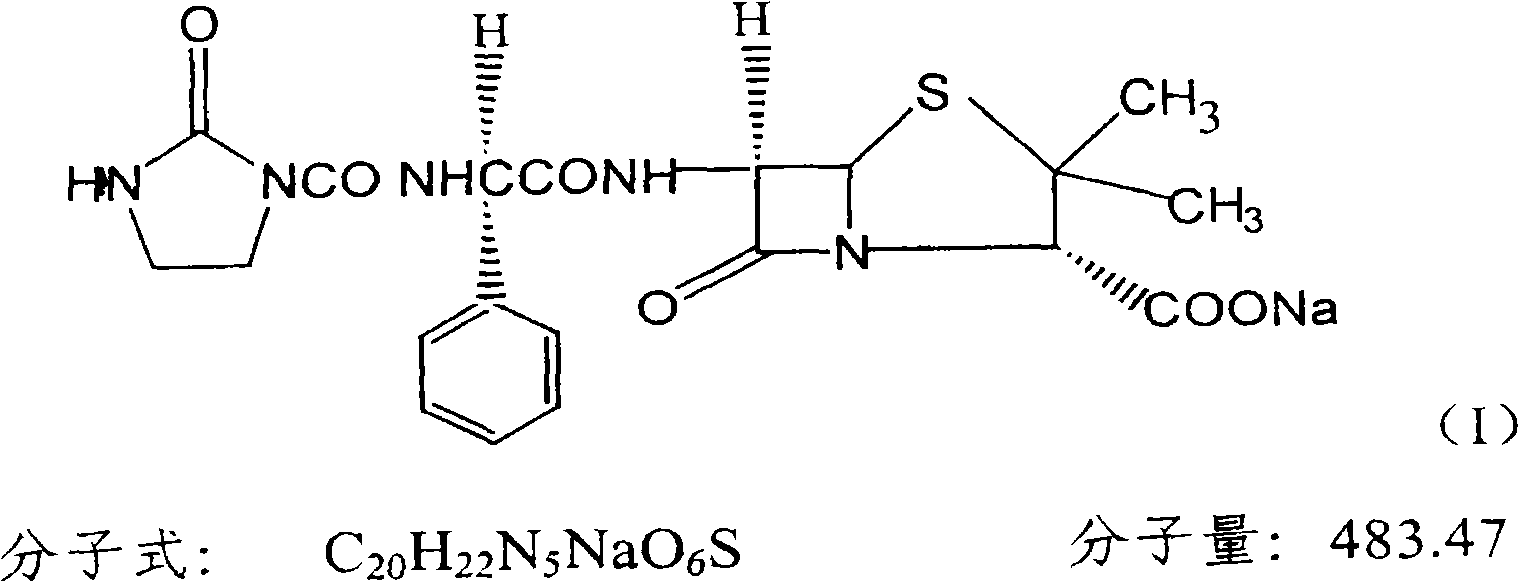
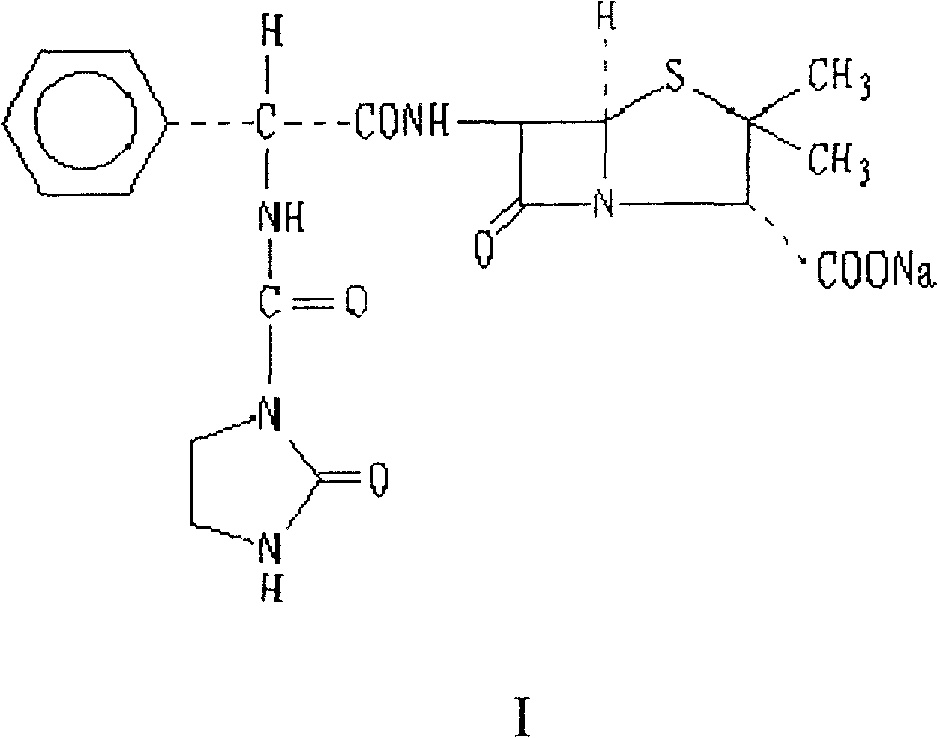
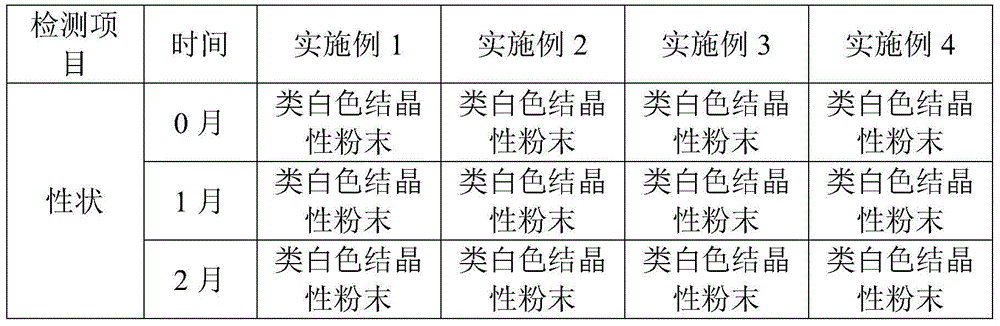
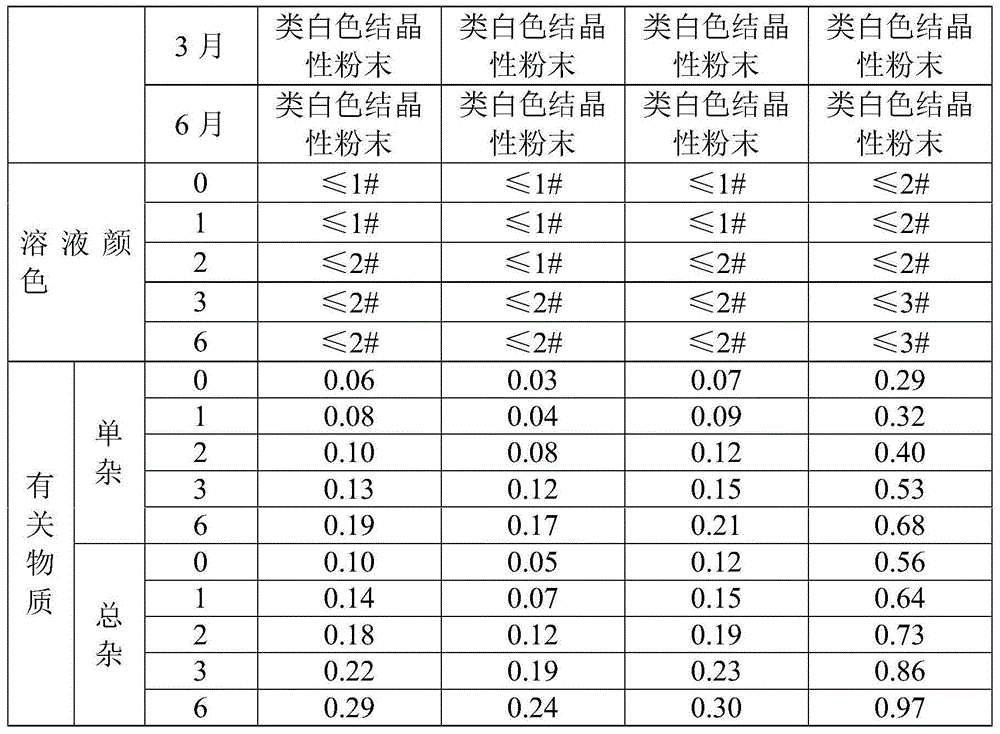
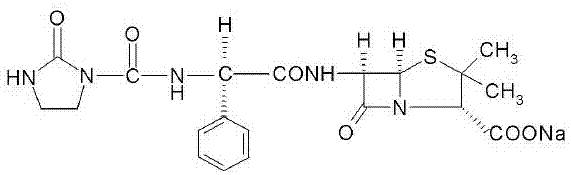
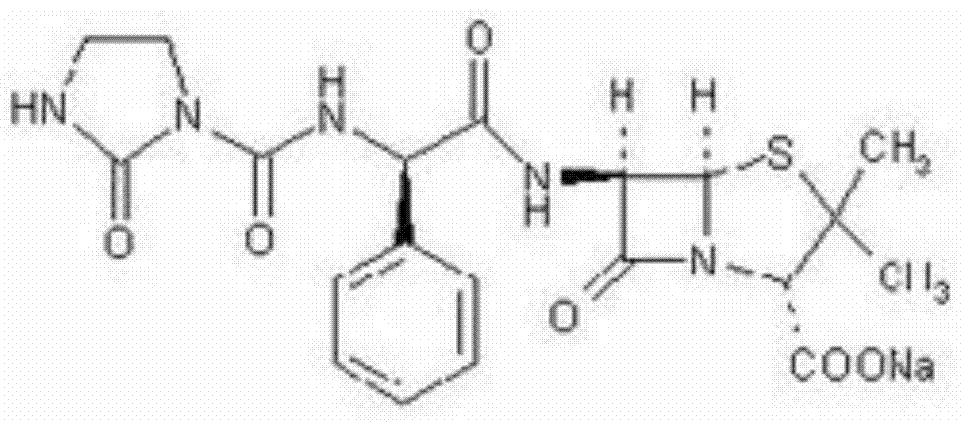
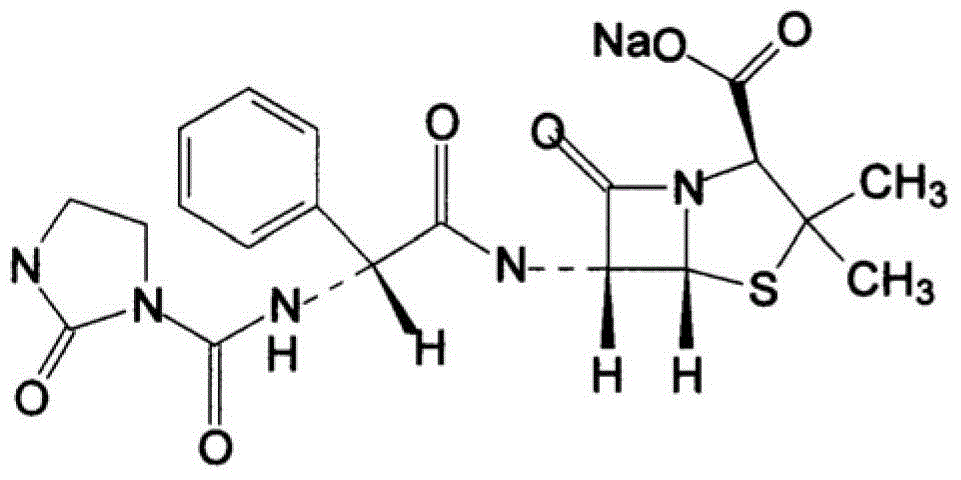
The sodium salt form of azlocillin, a semisynthetic, extended spectrum acylampicillin with antibacterial activity. Azlocillin binds to penicillin-binding proteins (PBPs) located inside the bacterial cell wall, thereby inhibiting the cross-linkage of peptidoglycans, which are critical components of the bacterial cell wall. This prevents proper bacterial cell wall synthesis, thereby results in the weakening of the bacterial cell wall and eventually leading to cell lysis.
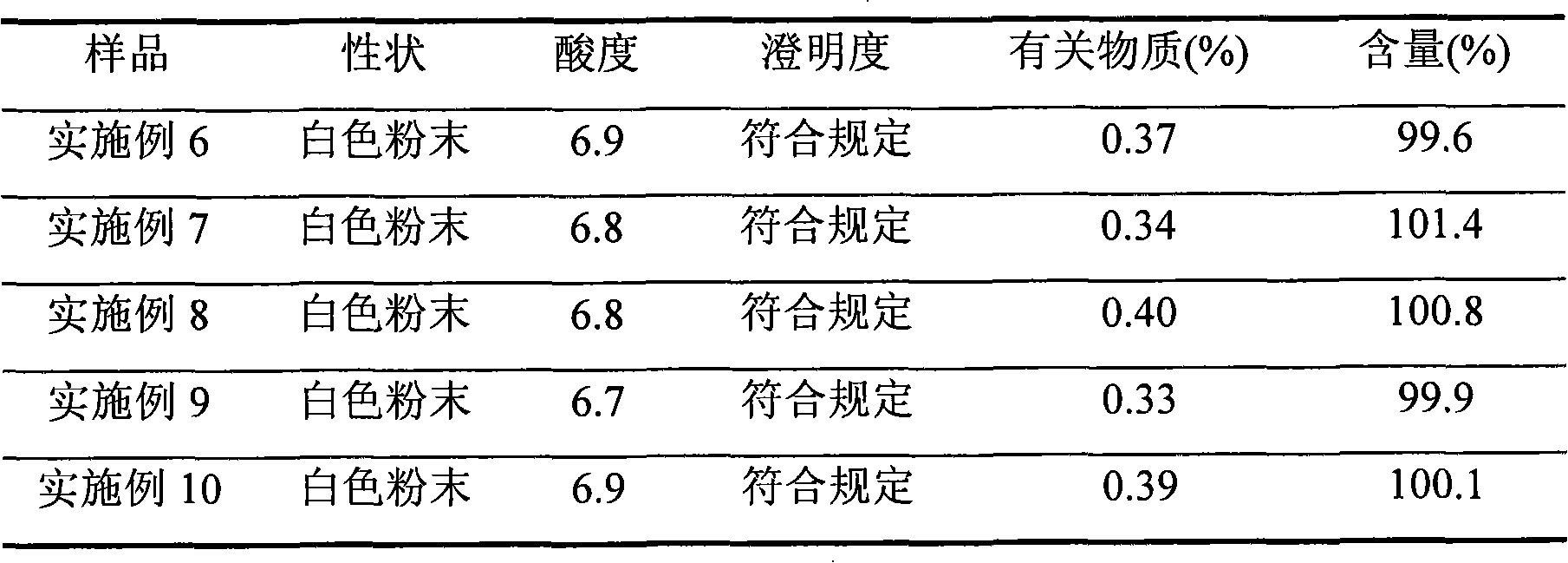
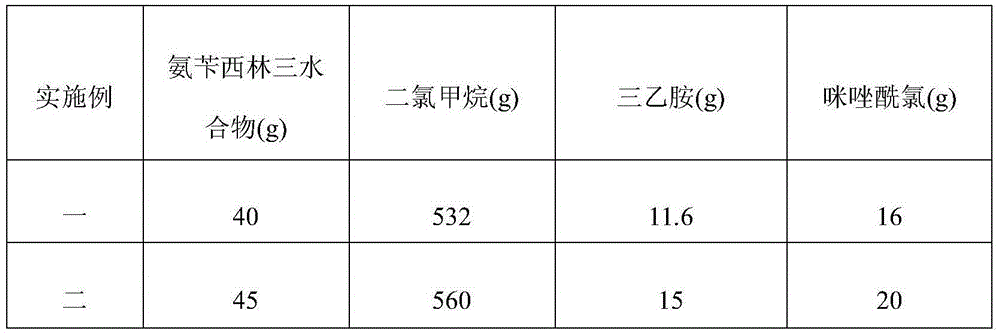
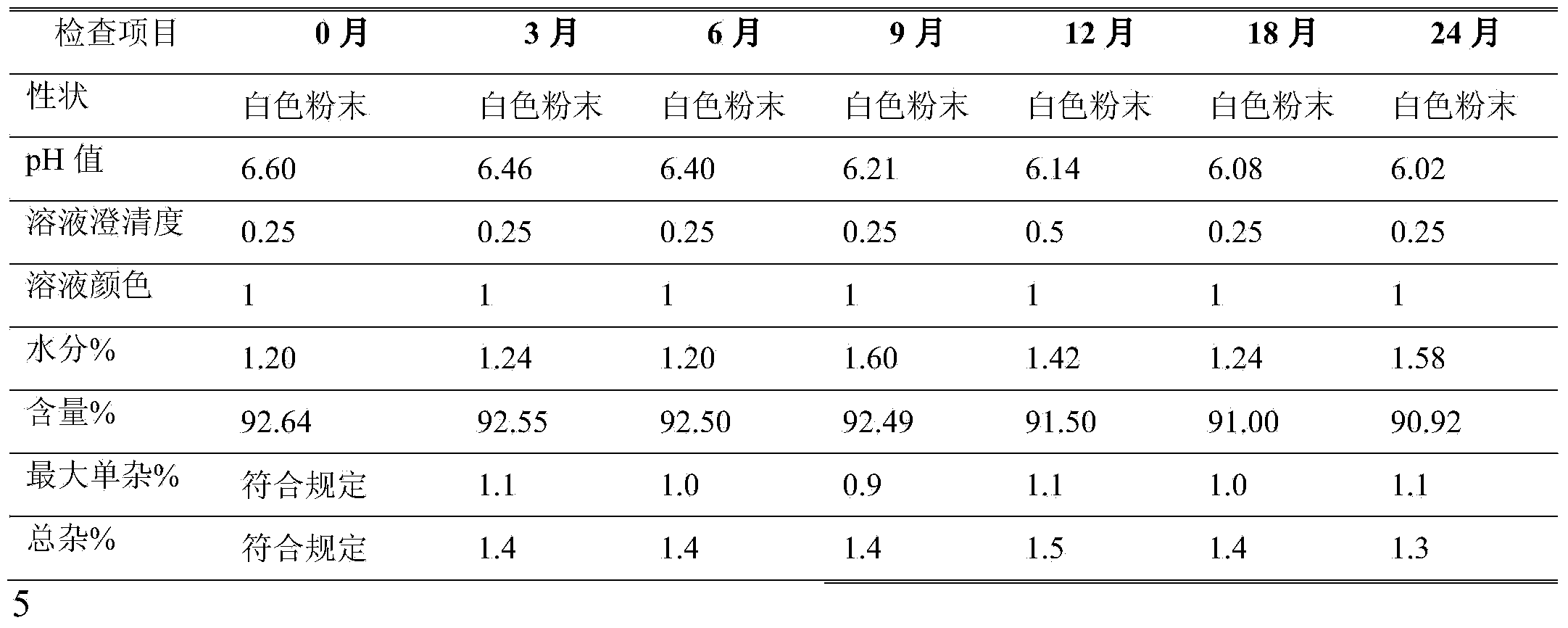
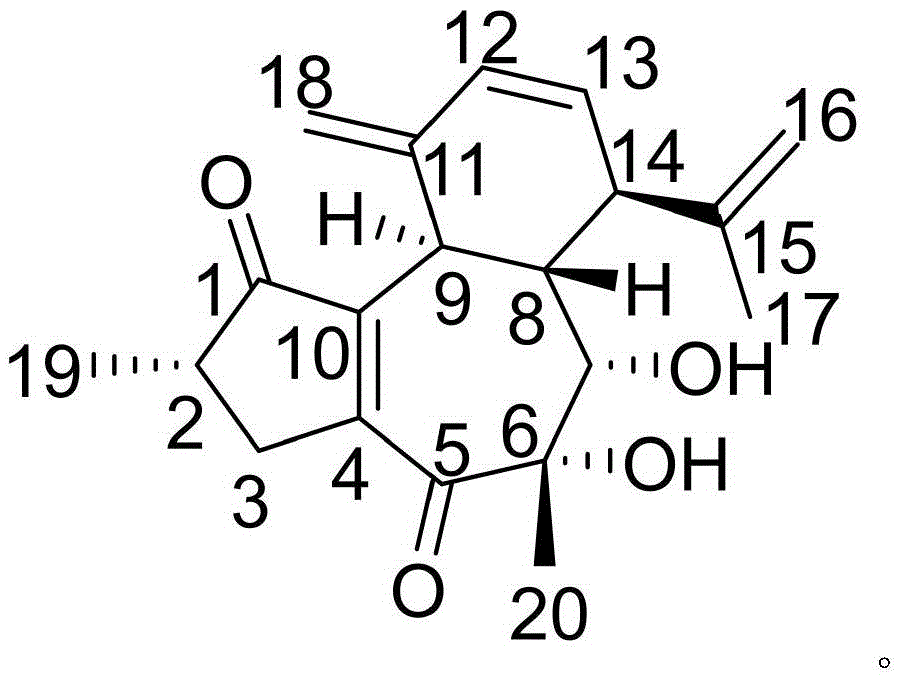
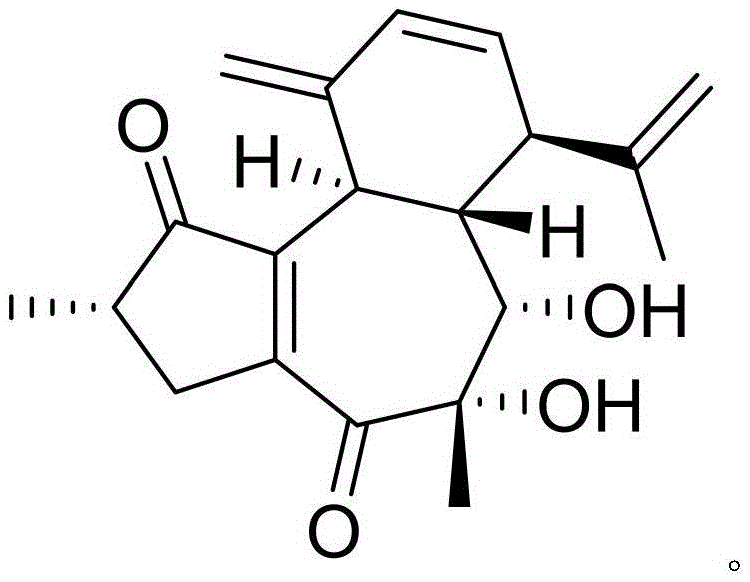
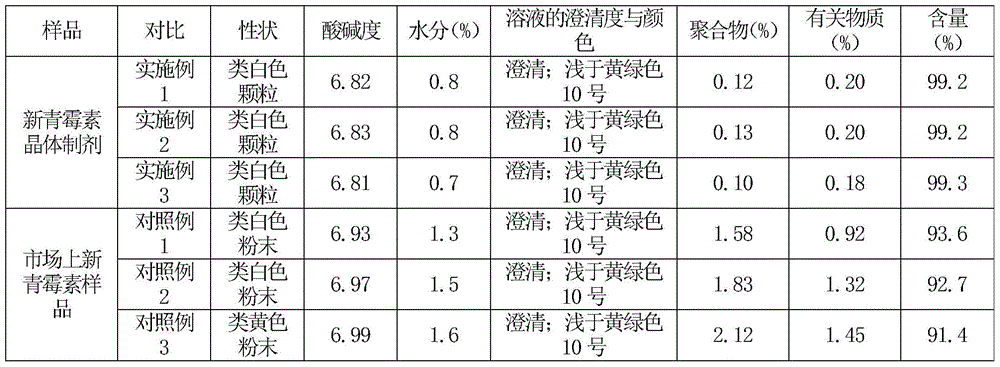
Crystalline azlocillin sodium and preparation thereof
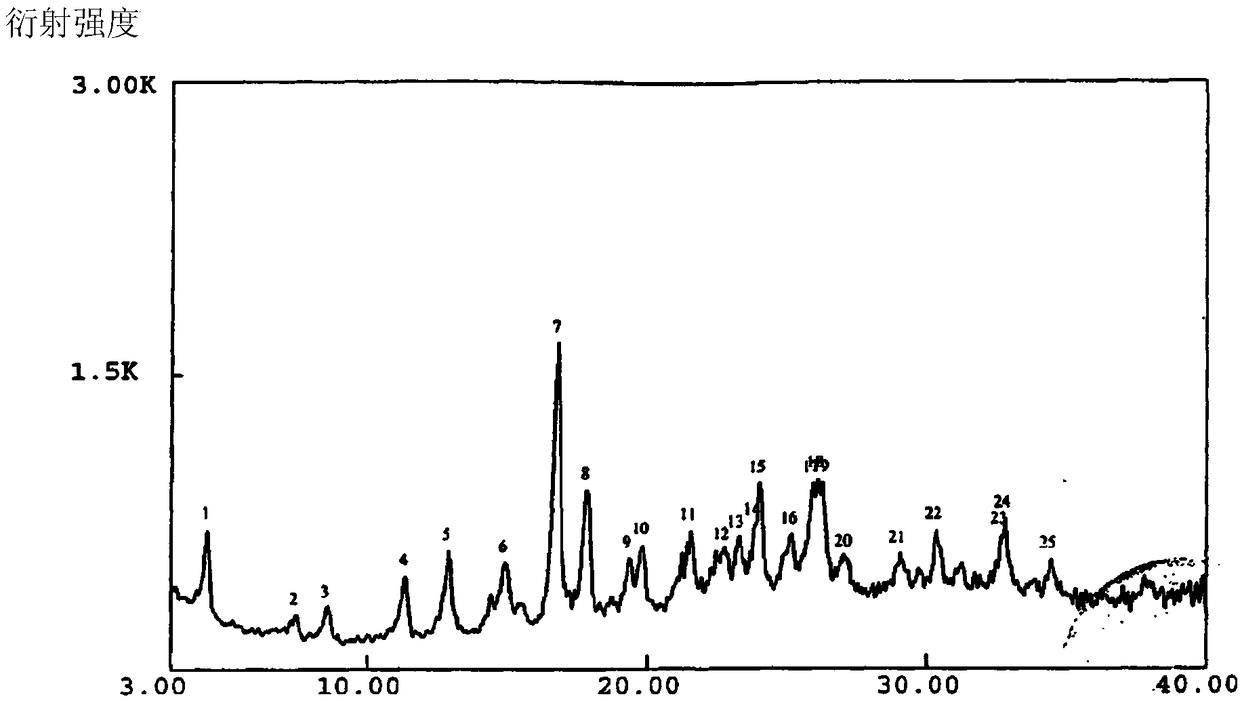
A crystalline azlocillin sodium and its production are disclosed. It is characterized by displaying the characteristic data described in specifications on differential scanning calorimeter and X-ray powdery diffraction pattern. Its advantages include simple process, low cost, short production period and high purity.
Owner:江西欧氏药业有限责任公司
Process for preparing sodium azlocillin
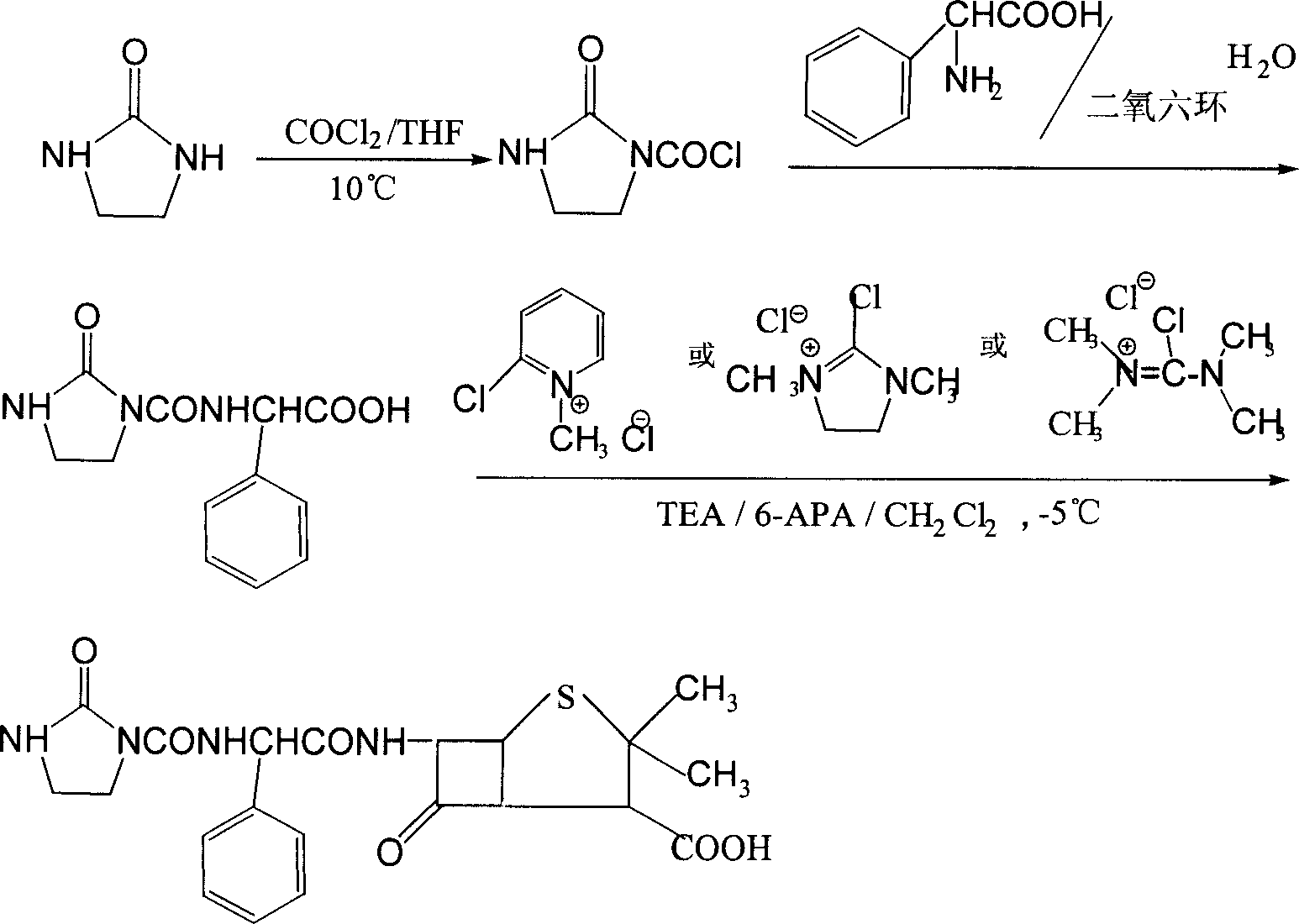
The preparation process of sodium azlocillin includes the following steps: 1. adding triethylamine slowly into water solution of ampicillin at 0-4 deg.c and stirring to clarify the reaction liquid; 2. adding 2-imidazolidinyl ketacyl chloride into the solution at 0-4 deg.c while dropping water solution of Na2CO3 or NaHCO3 to control pH 7.5-8.5 and performing condensation; 3. adding ethyl acetate into the reaction mixture at 0-5 deg.c and regulating pH value to 1.7-1.9; 4. separating to obtain organic phase, and eliminating heat source and impurity to obtain crystallization liquid; and 5. adding ethyl acetate solution of sodium isocaprylate to crystallize, separating and drying to obtain powdered white sodium azlocillin. The process has convenient operation, mild reaction condition, high yield and high product quality.
Owner:黄春荣
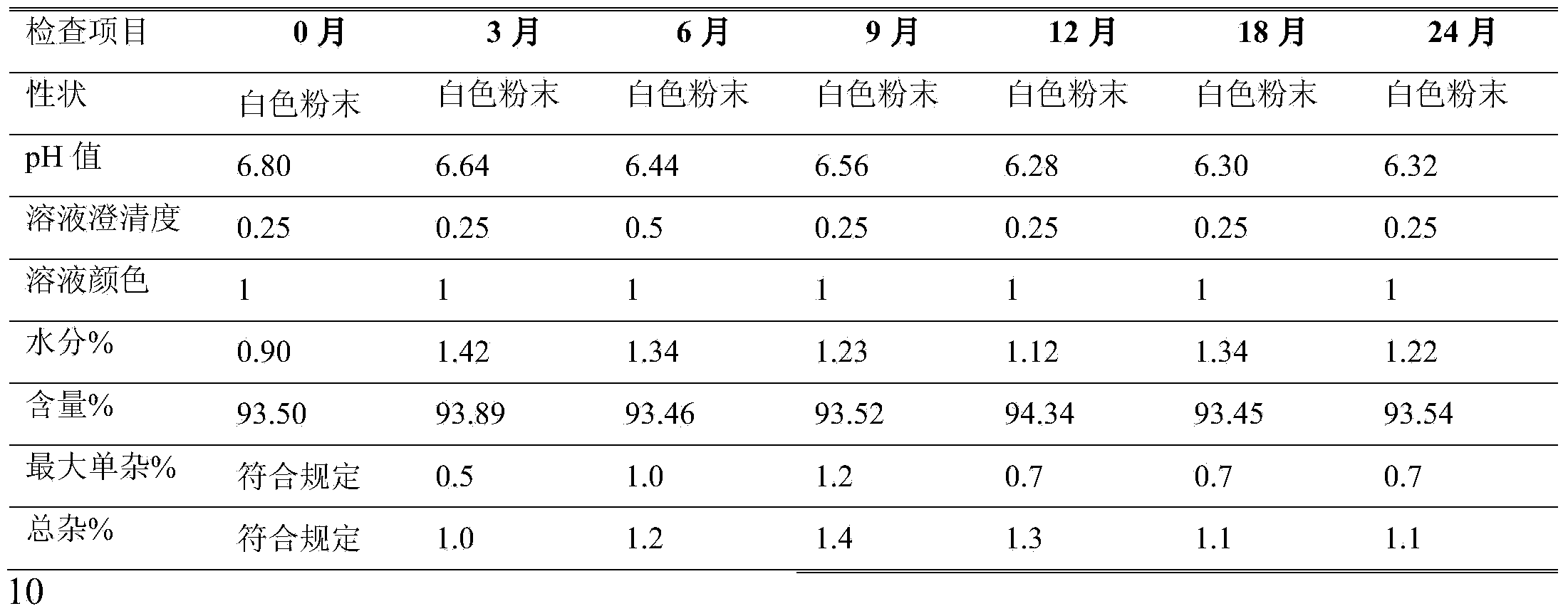
Preparation method of azlocillin sodium and azlocillin sodium used for injection
The invention provides a preparation method of azlocillin sodium and azlocillin sodium used for injection, and the azlocillin sodium and the azlocillin sodium used for injection which can be prepared by the method. The method comprises the steps of: in a new solution system, leading ampicillin trihydrate to have a reaction with 1- chloroformyl-2-imidazolone under the optimized temperature condition, and producing high-quality azlocillin acid with higher reaction efficiency and yield; and directly salifying, sterilizing and freeze-drying the prepared azlocillin acid, and obtaining the azlocillin sodium product used for injection.
Owner:SUZHOU ERYE PHARMA CO LTD
Preparation method for Azlocillin sodium
ActiveCN102311450AReduce usageReduce the risk of contaminationOrganic chemistryChemical solutionAzlocillin Sodium
The invention relates to a preparation method for Azlocillin sodium, which belongs to the synthetic technical field of antibiotics. The preparation method comprises the following steps: preparing a solution used for a condensation reaction, drawing dichloromethane into a reactor, putting ampicillin into the reactor with stirring, controlling the weight ratio of dichloromethane to ampicillin, thencooling and controlling the reduced temperature, then regulating the pH value to alkalescence by a pH regulator to obtain an solution used for condensation reaction; performing a condensation reaction; extracting and separating; crystallizing; drying; performing a salifying reaction; decoloring; freezing and drying; crushing. The invention has the advantages that the preparation method is capableof simplifying the operation, reducing the usage of the chemical solutions and the risks on environmental pollution; and has simple operation; can effectively avoid the loss caused by chromatography,raise the yield of Azlocillin sodium; and has mild salifying reaction condition, reduce the risk that Azlocillin sodium can be decomposed in alkali. The content of a single impurity of the obtained Azlocillin sodium dried product is lower than 0.6 percent and the content of total impurity of the obtained Azlocillin sodium dried product is lower than 1 percent which are obviously superior to the pharmacopoeia standard.
Owner:JIANGSU HI STONE PHARMA
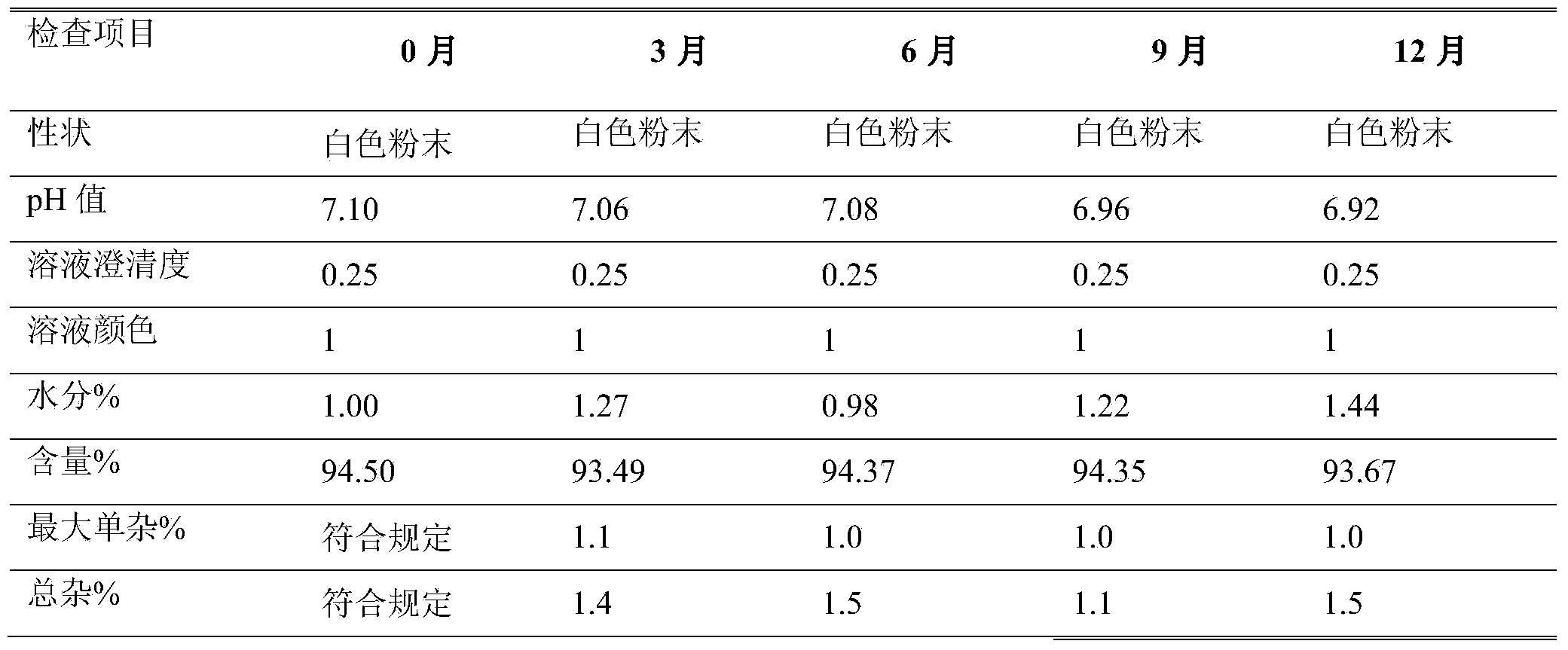
Azlocillin sodium compound, preparation method and medicine composition thereof
InactiveCN103265561ANot easy to absorb moistureEasy to storeAntibacterial agentsOrganic chemistryAzlocillin SodiumFreeze-drying
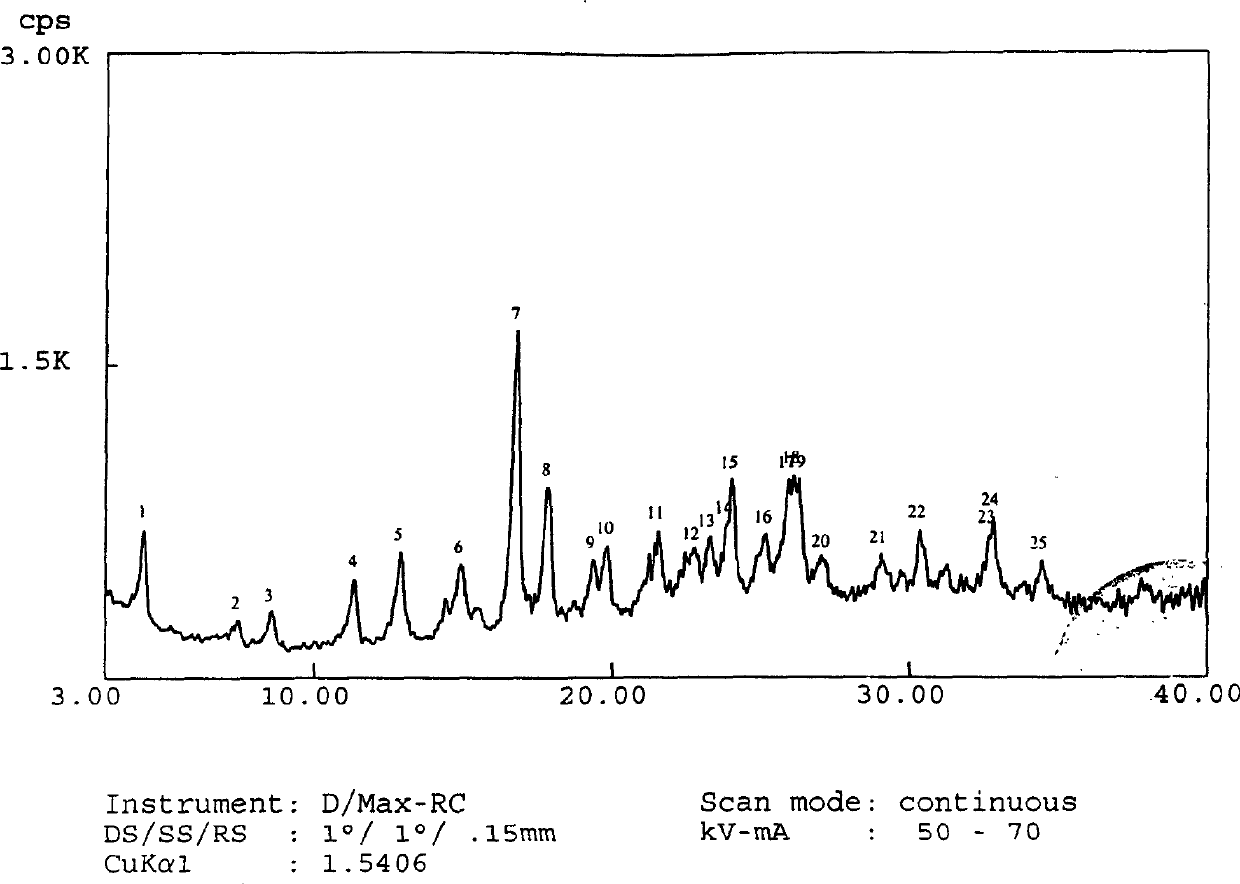
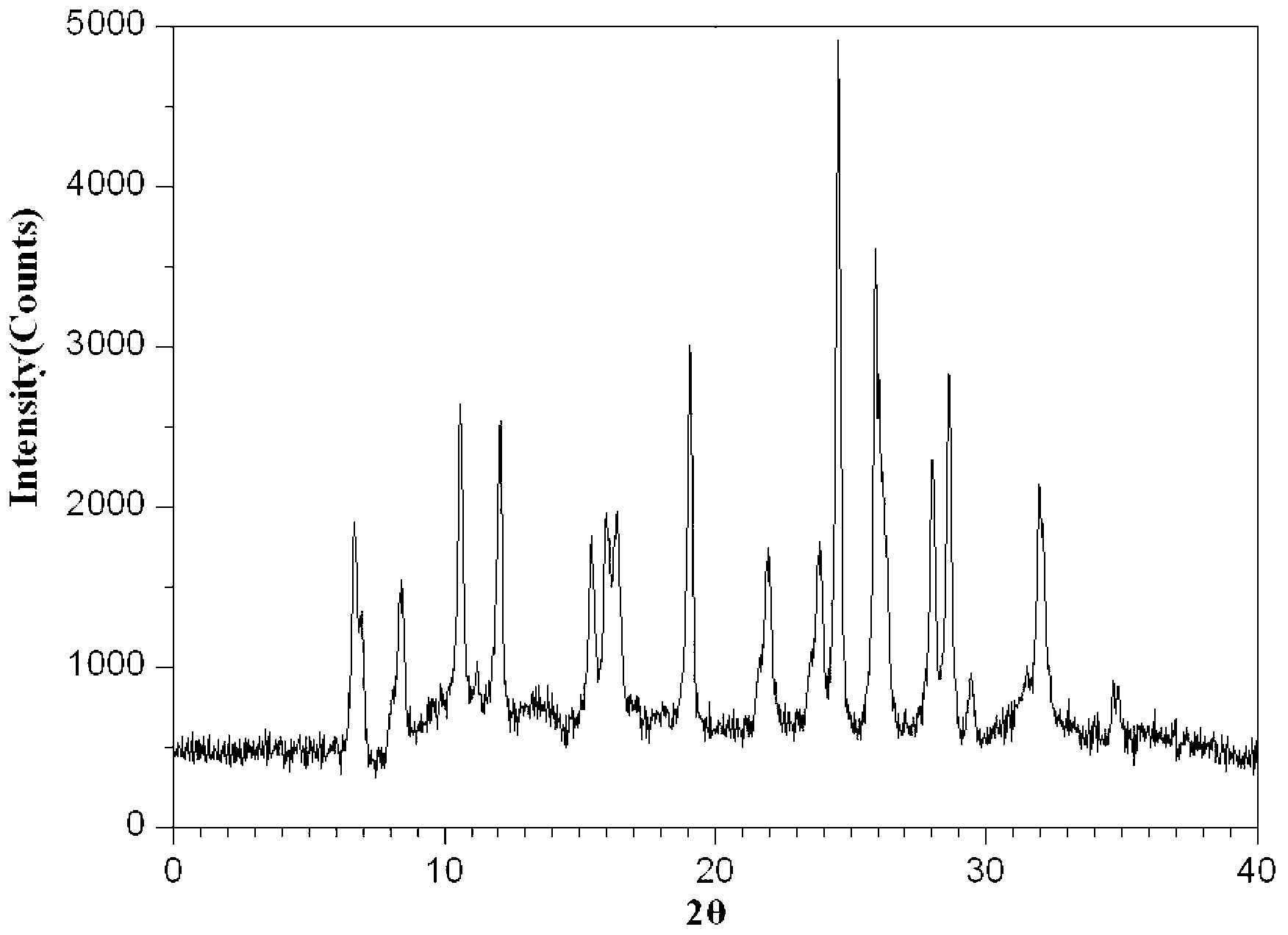
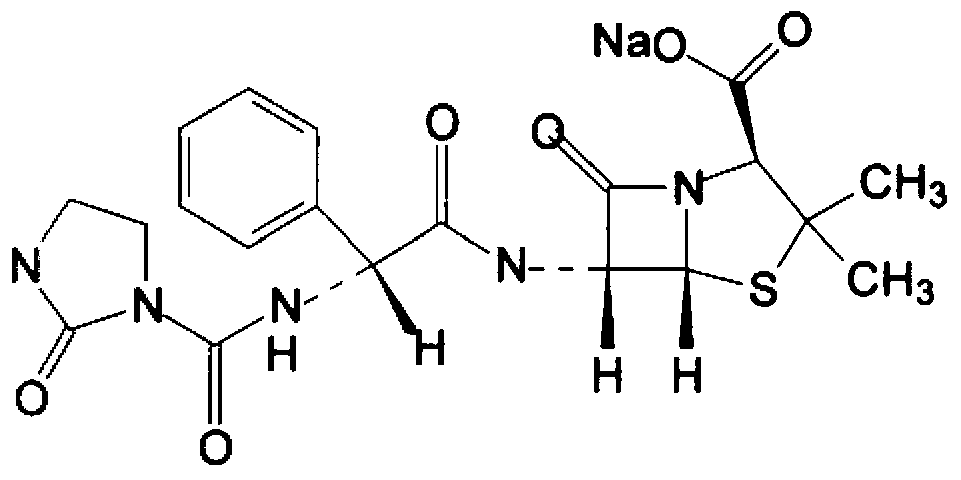
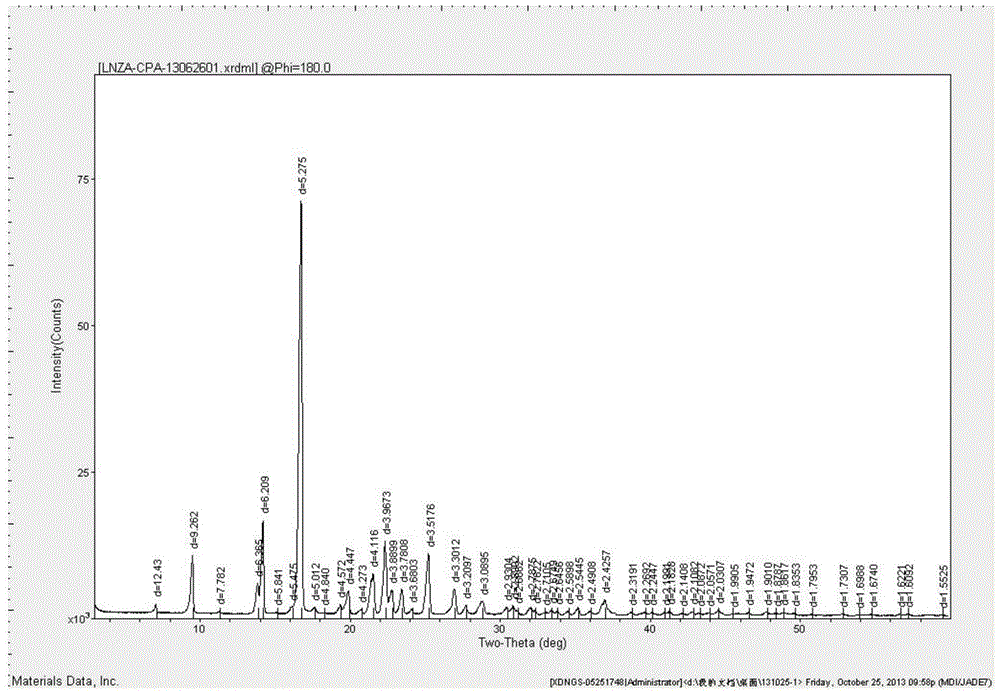
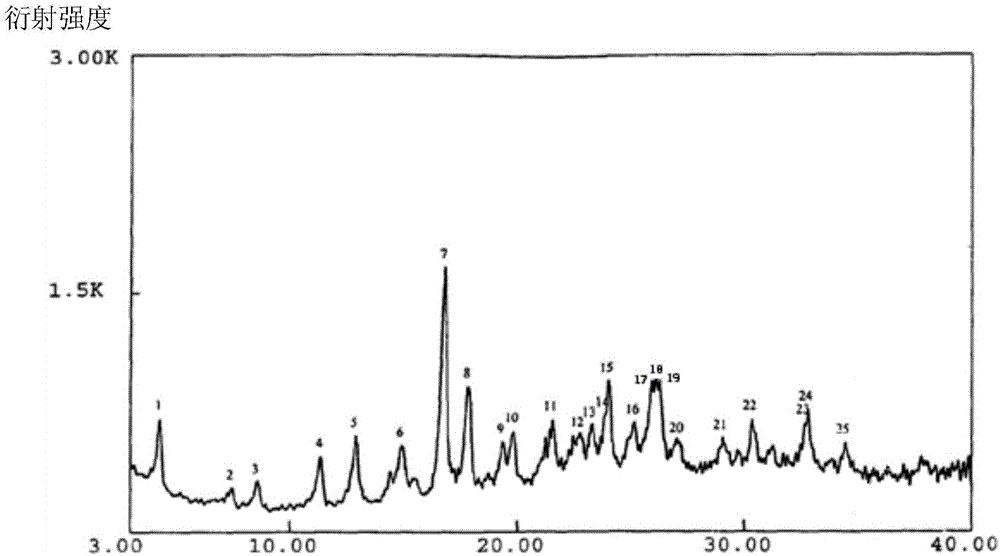
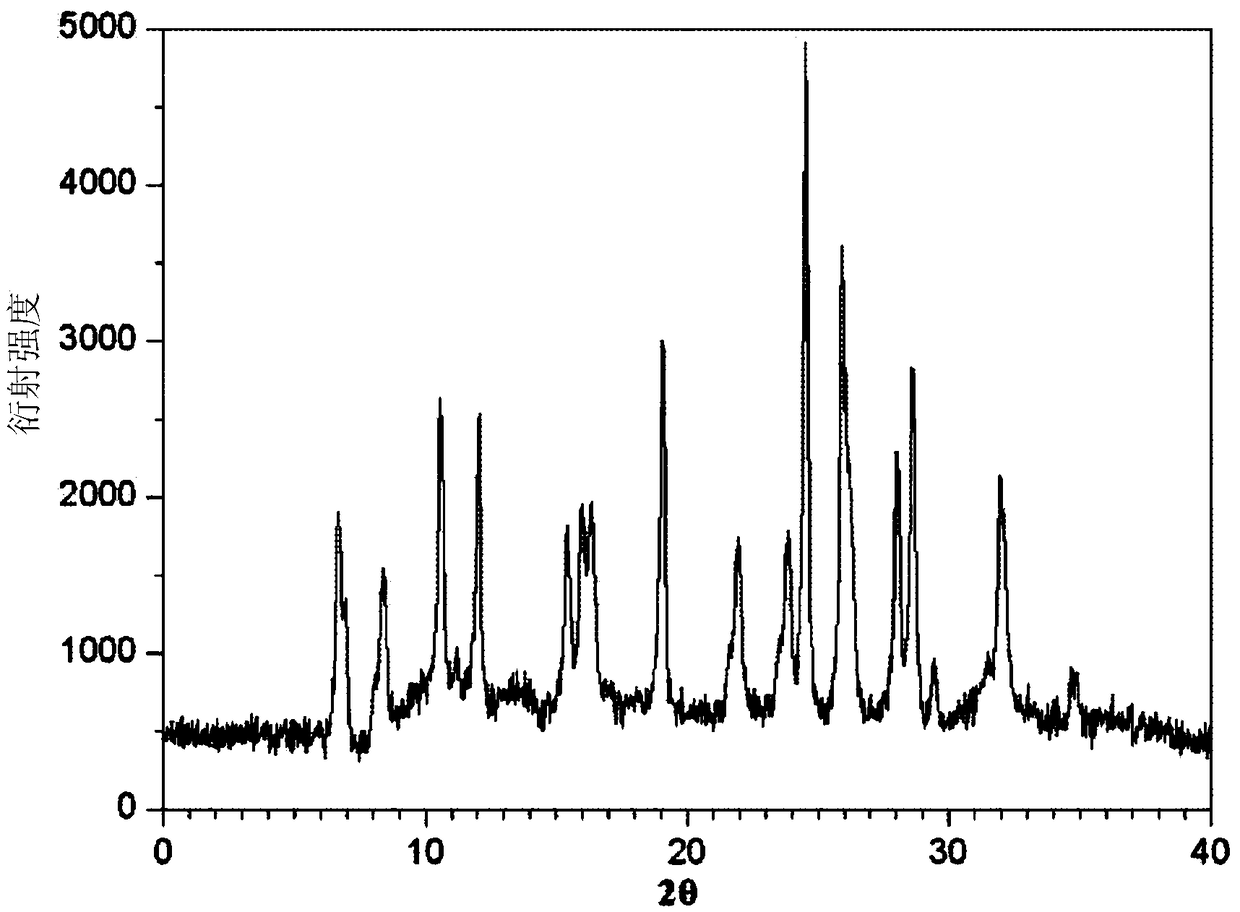
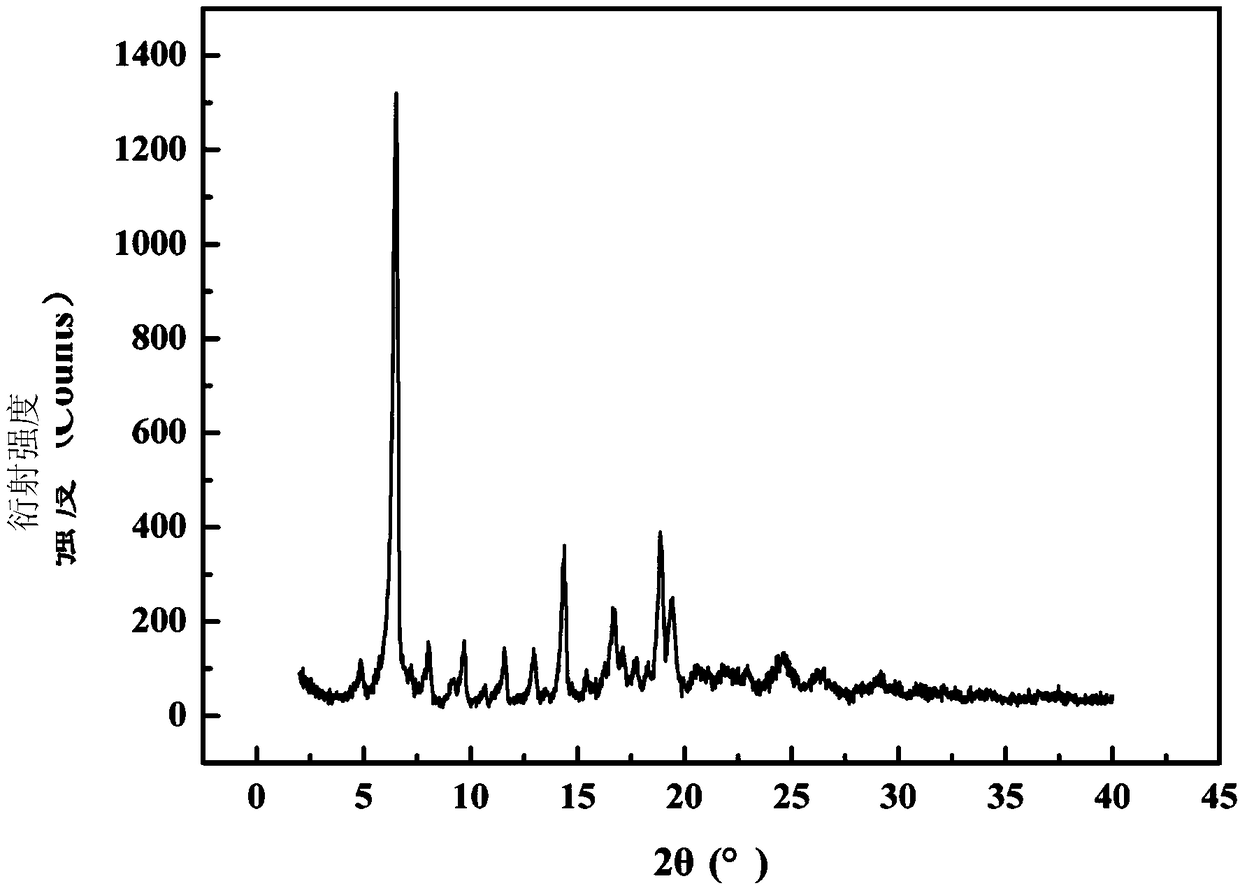
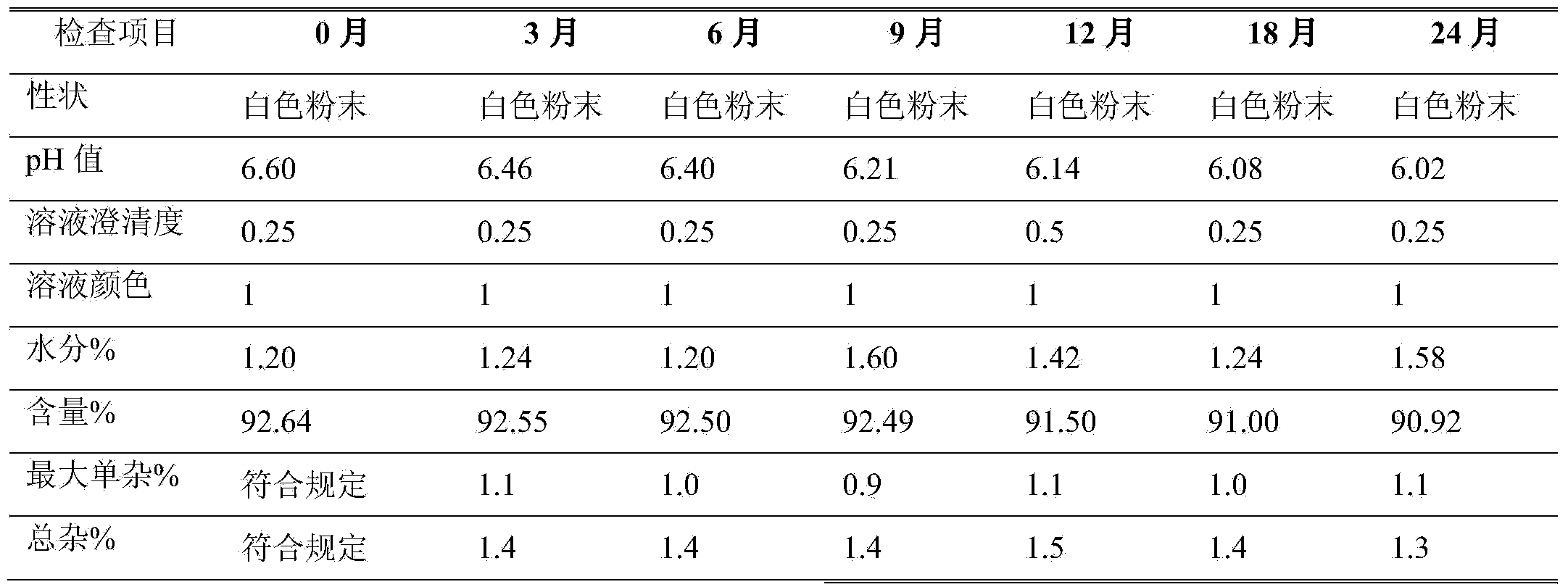
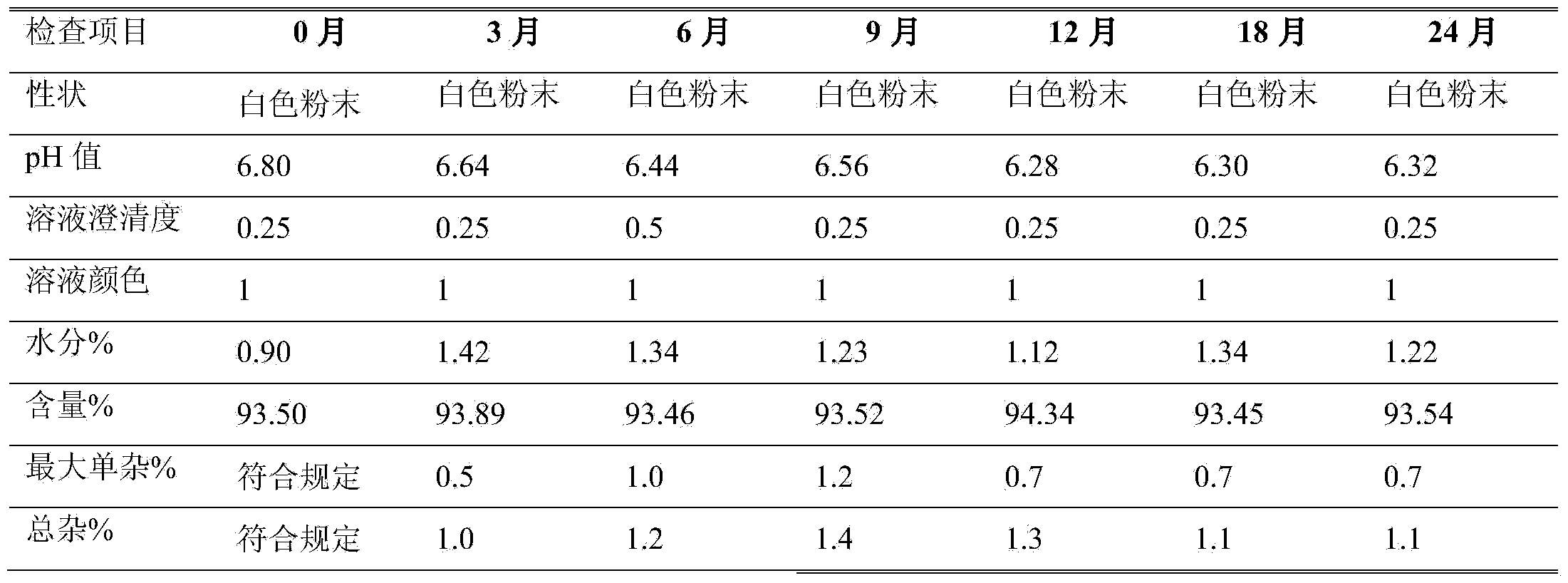
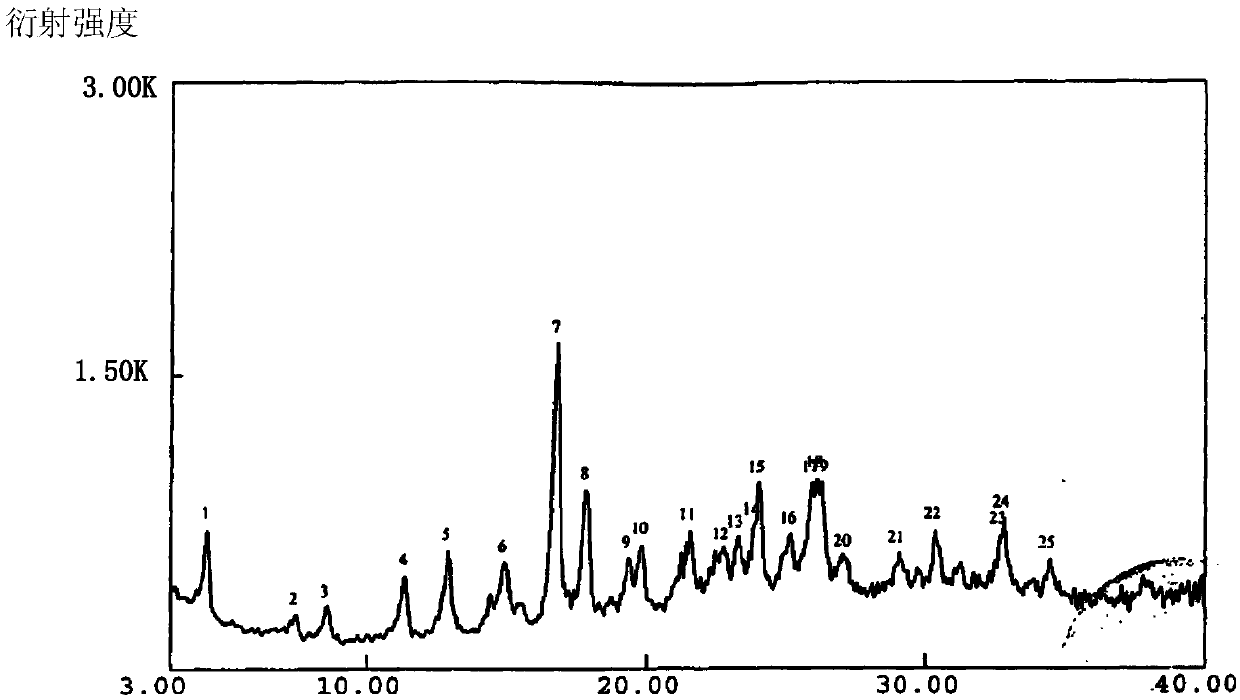
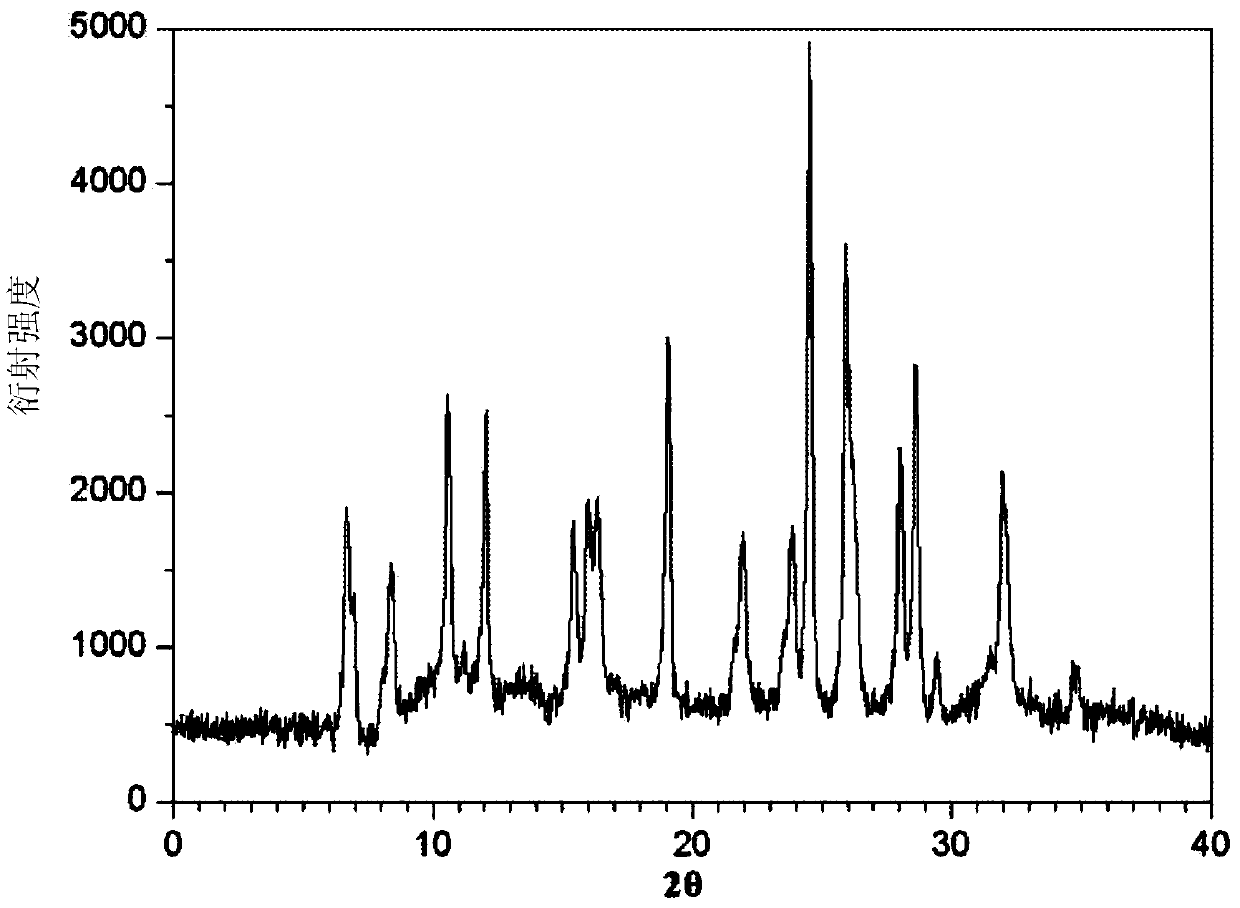
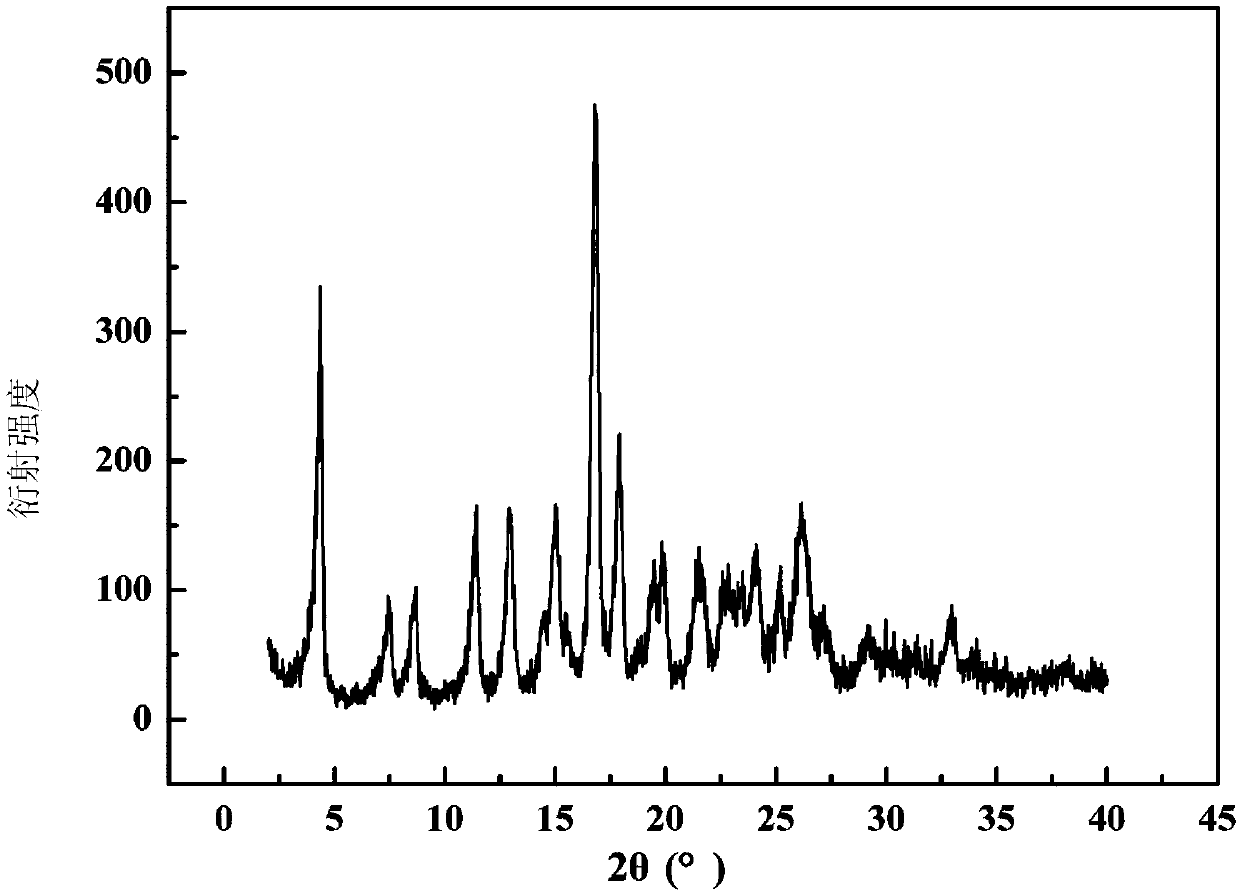
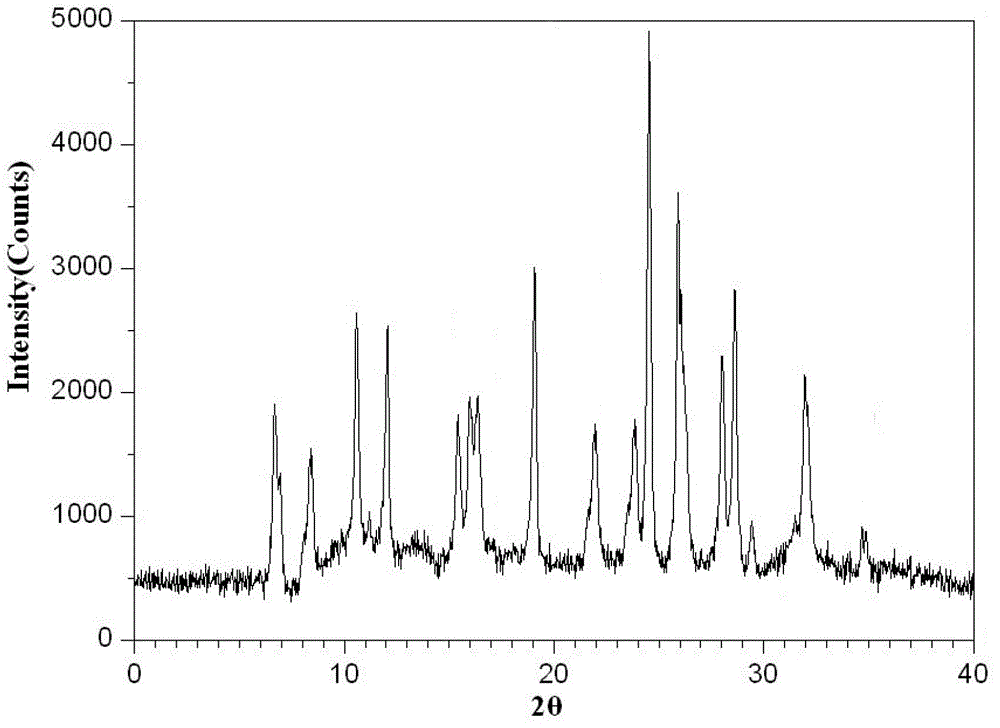
The invention belongs to the technical field of medicines, and particularly relates to an azlocillin sodium compound. The structural formula of the azlocillin sodium compound is defined in the specification, and the X-ray powder diffraction spectrogram of the azlocillin sodium compound, which is obtained by measurement by using Cu-K alpha ray, is shown in the figure 1. The invention further provides a preparation method of the azlocillin sodium compound, a medicine composition containing the azlocillin sodium compound, and a preparation method of the medicine composition. The dosage forms of the azlocillin sodium compound are sterile powder injection and freeze-dried powder injection. The azlocillin sodium compound provided by the invention almost does not absorb moisture, is easy to store and has good storage stability and low impurity content; and therefore, medication safety is greatly improved.
Owner:四川省惠达药业有限公司
Method for preparing azlocillin sodium
The invention relates to a method for preparing azlocillin sodium. An azlocillin acid is subjected to once salt-forming reaction and then is freeze-dried, thereby acquiring freeze-dried powder of the azlocillin sodium. The method has the advantages of convenient operation, low cost and short production cycle. The acquired freeze-dried powder of the azlocillin sodium is stable in quality, high in purity and excellent in water-solubility.
Owner:海南美好西林生物制药有限公司
Method for producing high-purity azlocillin sodium and powder injection thereof
InactiveCN101265265AHigh purityNo pollution in the processAntibacterial agentsOrganic active ingredientsFreeze-dryingChromatography column
The invention provided a method for preparing high-purity Mezlocillin sodium, and a method for preparing the Mezlocillin sodium powder injection. The method includes (1) dissolving Mezlocillin sodium crude product in purified water, and adjusting the pH value to less than 7 with the pH regulator; (2) extracting with organic solvent, separating the organic phase, drying with desiccant, and vacuum recovering to dry to obtain the product; (3) dissolving the product with eluting agent of mixed solution of hydrocarbon solvent and chlorine-containing solvent, eluting with alumina as filler of medium-pressure chromatography column, and collecting the fraction in a sectional manner; (4) mixing fractions with the Mezlocillin acid content of no smaller than 80%, vacuum concentrating, dissolving into ethanol, and basifying with base to separate precipitate; and (5) re-crystallizing the precipitate with ethanol, and freeze drying to obtain the high-purity Mezlocillin sodium.
Owner:海南华旗药业销售有限公司
Azlocillin sodium medicinal composition for injection and preparation method for azlocillin sodium medicinal composition for injection
InactiveCN102805725AImprove stabilityEncapsulation rate is smallAntibacterial agentsSolution deliveryAzlocillin SodiumNanoparticle
The invention discloses an azlocillin sodium medicinal composition, namely azlocillin sodium nanoparticle injection. The azlocillin sodium nanoparticle injection consists of azlocillin sodium, a carrier material, stabilizer and excipient, and the effective average particle size of nanoparticles is 10 to 200nm. The invention discloses a preparation method for the injection; and the method comprises the following steps of: mixing the azlocillin sodium and the stabilizer in water for injection, adjusting pH value, and adding the carrier material and the excipient to obtain the azlocillin sodium nanoparticle injection. The injection has the advantages of high redissolution performance, excellent stability, high medicine loading capacity and the like, and the process is suitable for industrial large-scale production.
Owner:海南美好西林生物制药有限公司
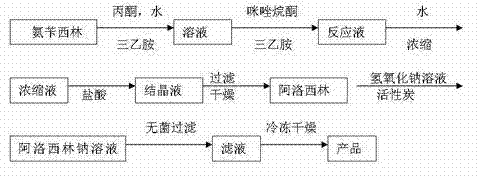
Process for preparing azlocillin sodium
InactiveCN100427489CResidue reductionReduce manufacturing costAntibacterial agentsOrganic chemistryAmpicillinAzlocillin Sodium
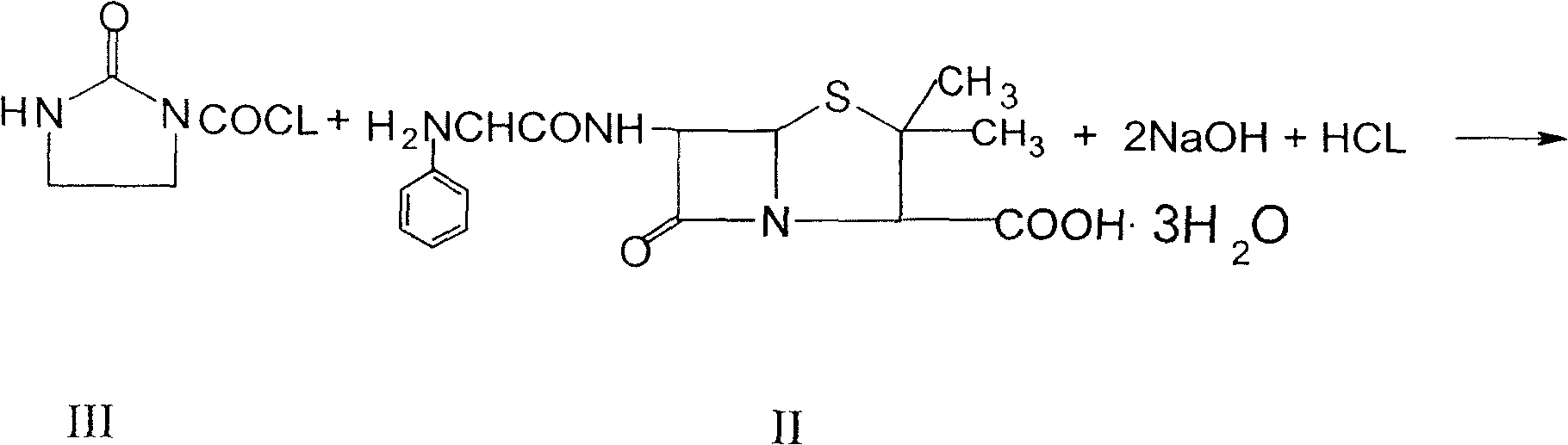
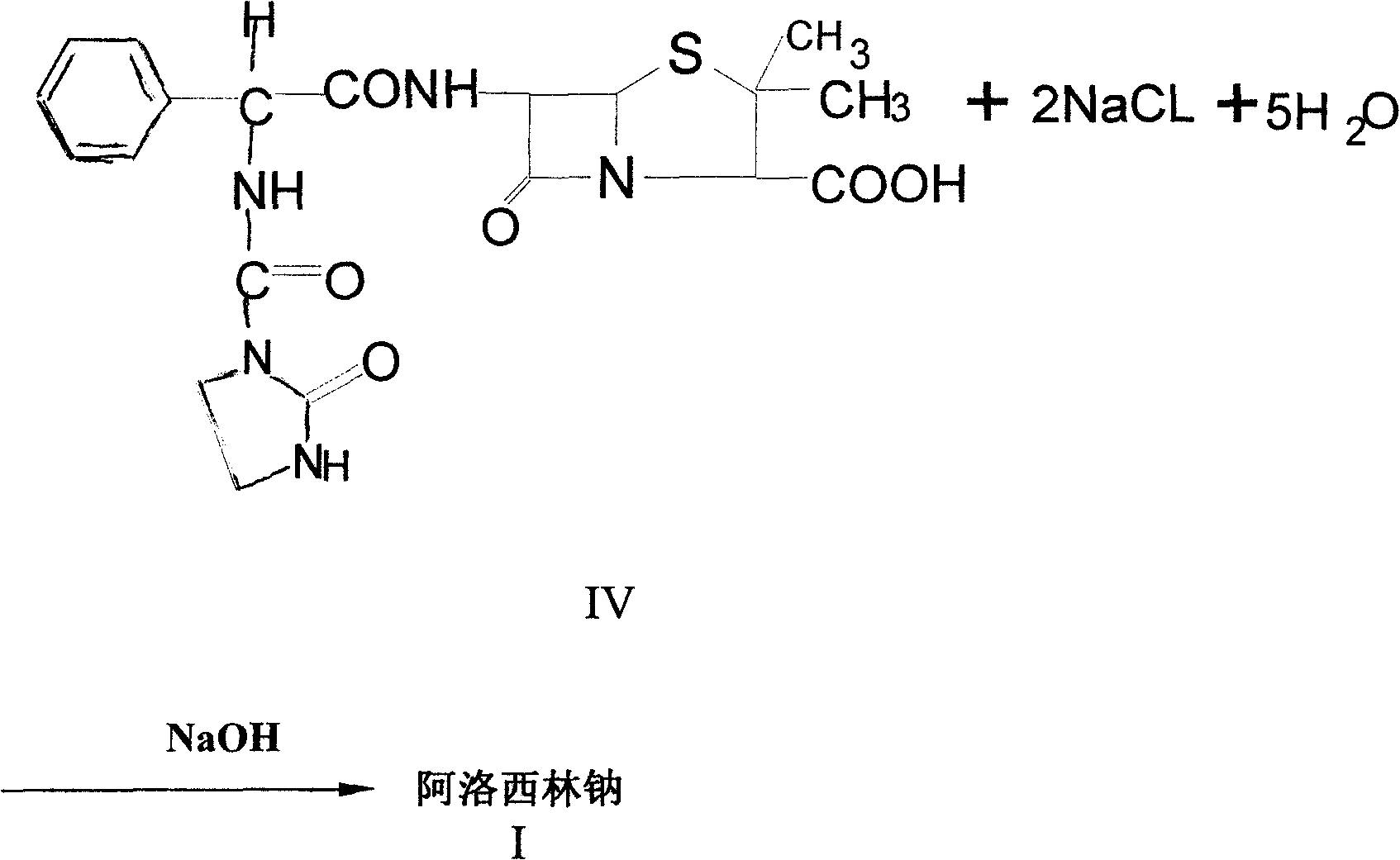
The invention relates to a process for preparing azlocillin sodium, which comprises carrying out condensation reaction between ampicillin and imidazolidinoxyl chlorine in aqueous phase, the crystallizing in acetone / water, ethanol / water or acetone / ethanol / water system so as to obtain azlocillin, then directly obtaining salts with sodium hydroxide, finally freeze-drying.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Method for producing high-purity azlocillin sodium and powder injection thereof
InactiveCN101265265BHigh purityNo pollution in the processAntibacterial agentsPowder deliveryFreeze-dryingChromatography column
Owner:海南华旗药业销售有限公司
Process for preparing sodium azlocillin
The preparation process of sodium azlocillin includes the following steps: 1. adding triethylamine slowly into water solution of ampicillin at 0-4 deg.c and stirring to clarify the reaction liquid; 2. adding 2-imidazolidinyl ketacyl chloride into the solution at 0-4 deg.c while dropping water solution of Na2CO3 or NaHCO3 to control pH 7.5-8.5 and performing condensation; 3. adding ethyl acetate into the reaction mixture at 0-5 deg.c and regulating pH value to 1.7-1.9; 4. separating to obtain organic phase, and eliminating heat source and impurity to obtain crystallization liquid; and 5. adding ethyl acetate solution of sodium isocaprylate to crystallize, separating and drying to obtain powdered white sodium azlocillin. The process has convenient operation, mild reaction condition, high yield and high product quality.
Owner:黄春荣
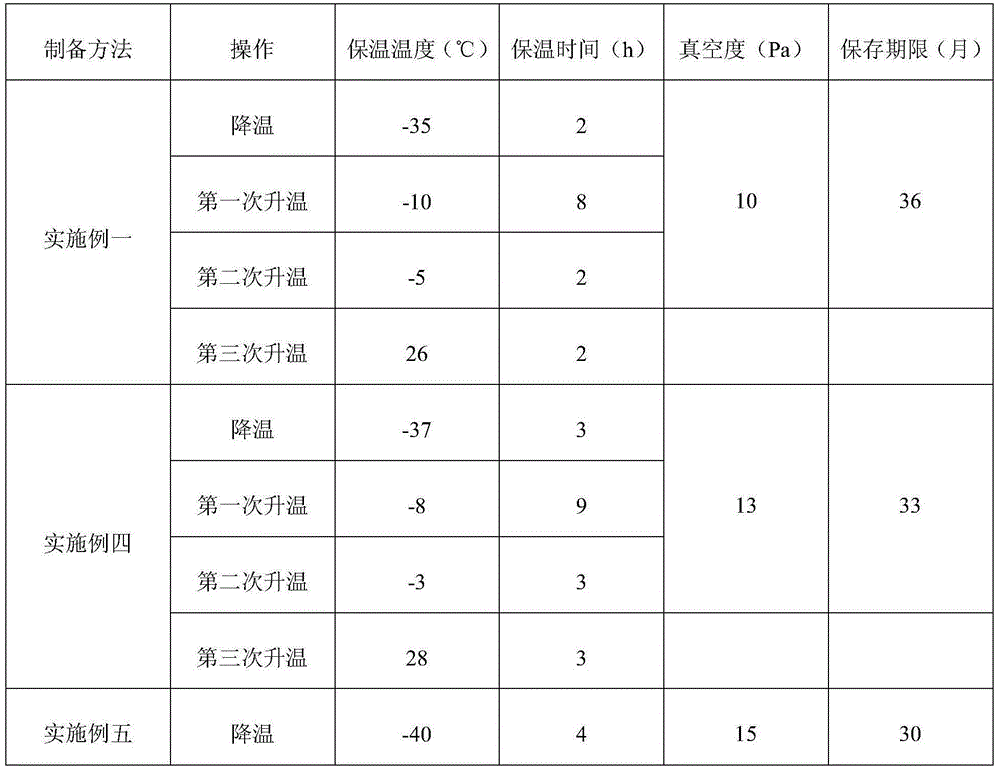
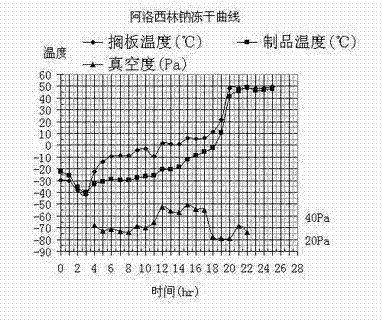
Preparation methods for azlocillin sodium and azlocillin sodium freeze-dried powder for injection
ActiveCN104478899AHigh yieldImprove qualityOrganic active ingredientsOrganic chemistryAzlocillin SodiumFreeze-drying
The invention provides preparation methods for azlocillin sodium and an azlocillin sodium freeze-dried powder for injection. The preparation methods are simple to operate and suitable for popularization. The obtained azlocillin sodium is high in yield, high in quality, good in stability and high in clinic usage safety. The provided preparation method for the azlocillin sodium freeze-dried powder for injection precisely controls warm-keeping temperature, warm-keeping time, pressure and other parameters, controls multi-layer filtering, and controls other things, so that the obtained azlocillin sodium freeze-dried powder is good in stability, and is improved in clinic usage safety.
Owner:HAINAN GENERAL & KANGLI PHARMA
Preparation process of azlocillin sodium for injection
InactiveCN104771367AAvoid performance degradationSpeed up the mixingAntibacterial agentsPowder deliveryAzlocillin SodiumNitrogen
The invention relates to a preparation process of azlocillin sodium for injection. The preparation process comprises the following steps: synthesizing azlocillin sodium; and drying the synthesized azlocillin sodium, and preparing into powder, wherein the preparation process of the azlocillin sodium powder comprises the following steps: firstly, cleaning a reagent bottle for containing the azlocillin sodium powder, then quantitatively filling the azlocillin sodium powder into the cleaned reagent bottle according to a specification, filling nitrogen, then covering the bottle by using a pretreated rubber plug, then performing cover tying treatment by using an aluminum cover to ensure that the product is sealed, then sequentially performing inner label pasting, packaging and lamp inspection on the reagent bottle subjected to cover tying, then pasting an outer label on a packing box, and then performing inspection and packaging. The preparation process provided by the invention overcomes the problem that the existing preparation process of azlocillin sodium for injection reduces the medicinal effect of the azlocillin sodium for injection.
Owner:SICHUAN PHARMA
High efficient preparation method of azlocillin sodium for injection
InactiveCN107129507AImprove product qualityLow costAntibacterial agentsPowder deliverySodium bicarbonateAmpicillin
The invention discloses a high efficient preparation method of azlocillin sodium for injection. According to the preparation method, ampicillin and imidazole acyl chloride carry out condensation reactions in a water phase, then the reaction product is crystallized in an acetone / water, ethanol / water, or acetone / ethanol / water system, then the crystals directly carry out salt forming reactions with sodium carbonate and / or sodium bicarbonate, and the reaction product is freeze-dried to obtain azlocillin sodium. The pharmaceutical effect of azlocillin sodium is effectively improved; and the problem that in a conventional technology, the pharmaceutical effect of azlocillin sodium is reduced is solved.
Owner:SICHUAN PHARMA
Preparation method of azlocillin sodium and azlocillin sodium used for injection
Owner:SUZHOU ERYE PHARMA CO LTD
High performance antibiotic medicine composition
InactiveCN1586478AHigh antibacterial activityImprove antibacterial propertiesAntibacterial agentsHeterocyclic compound active ingredientsAzlocillin SodiumMedicine
The present invention relates to high efficiency antibiotic medicine composition containing azlocillin sodium and sulbactam in the weight ratio of 0.1-10, optimally of 1-4. The medicine composition has obvious cooperative effect of azlocillin sodium and sulbactam and antibiotic capacity much higher than azlocillin sodium. The present invention has wide antibiotic spectrum, powerful bactericidal effect, high chemical stability and low toxicity, and the medicine composition is prepared into injection normally.
Owner:陶灵刚
Azlocillin compound and preparation method and preparation thereof
InactiveCN103830185AImprove stabilityImprove performanceAntibacterial agentsPowder deliveryAzlocillin SodiumMedicine
The invention discloses an azlocillin medicine composition. A preparation method of the medicine composition comprises the following steps: disinfecting and sterilizing azlocillin sodium powder after sieving to obtain sterile azlocillin sodium powder and feeding the sterile azlocillin sodium powder to a clean region for later use; cleaning and sterilizing small bottles for subpackaging the azlocillin sodium powder and then feeding to the clean region for later use; subpackaging the sterile azlocillin sodium powder into the sterilized bottles; and covering by butyl rubber stoppers and then pressing by aluminum caps to seal, so as to obtain an azlocillin sodium powder injection. By adopting the method disclosed by the invention, the azlocillin sodium particles are optimized, the defect that the azlocillin sodium is difficult to subpackage in the prior art is effectively overcome, and the stability of the preparation is remarkably improved; the product performance test shows that the azlocillin medicine composition prepared by the preparation method is good in quality, and the test indexes are free of significant changes after long-term tests for more than 12 months, and accord with the quality standard, which shows that the preparation is good in stability.
Owner:NORTH CHINA PHARMA COMPANY
Azlocillin sodium drug composition and anti-aging function thereof
InactiveCN105646170AImprove anti-aging effectHighlight substantive featuresAntinoxious agentsCarbonyl compound separation/purificationNatural productAzlocillin Sodium
The invention discloses an azlocillin sodium drug composition and an anti-aging function thereof. The azlocillin sodium drug composition comprises azlocillin sodium and a natural product compound (I), wherein the natural product compound (I) is of a novel structure and is separated from dried roots of rhizoma anemarrhenae. The azlocillin sodium drug composition has the advantages that when the azlocillin sodium and the compound (I) singly act on a D-galactose model mouse, the learning and memory capabilities of the mouse can be respectively improved; when the azlocillin sodium and the compound (I) jointly act, the improvement function is stronger; meanwhile, when the azlocillin sodium and the compound (I) singly act on the D-galactose model mouse, the SOD (superoxide dismutase) activity of the body is improved, and the function of delaying aging is realized; when the azlocillin sodium and the compound (I) jointly act, the function is more obvious, and the azlocillin sodium drug composition can be developed into an anti-aging drug.
Owner:李晨露
New penicillin crystal preparation and preparation method thereof
ActiveCN104130270BSmall particlesUniform particle size distributionAntibacterial agentsOrganic chemistry methodsSide effectFiltration
The invention discloses an azlocillin sodium crystal preparation and a preparation method thereof. The preparation method comprises the following steps: step 1, dissolving a crude azlocillin sodium product in a mixed solvent of methanol and ethyl methyl ketone, adding active carbon for adsorption, carrying out filtering, cooling the obtained filtrate to allow a crystal to be precipitated, washing the crystal with a mixed solution of methanol and ethyl methyl ketone and carrying out pressure-reduced drying so as to obtain a primary refined azlocillin sodium product; and step 2, dissolving the primary refined azlocillin sodium product in a hot ethyl methyl ketone solution, carrying out press filtration with a high-efficiency filter, subjecting obtained mixed liquor to cooling, pressure reduction and concentration, then carrying out cooling to allow a crystal to be precipitated and subjecting the precipitated crystal to filtering and pressure-reduced drying so as to obtain the azlocillin sodium crystal preparation. The azlocillin sodium crystal preparation provided by the invention has the advantages of a small particle size, uniform particle size distribution, good fluidity, simple crystallization process, low residual quantity of the organic solvent, high stability, good security, small toxic and side effects, low proneness to allergy, etc.
Owner:BAIDE LIXI HEALTH CARE
Crystal form of azlocillin sodium and crystallization preparing method thereof
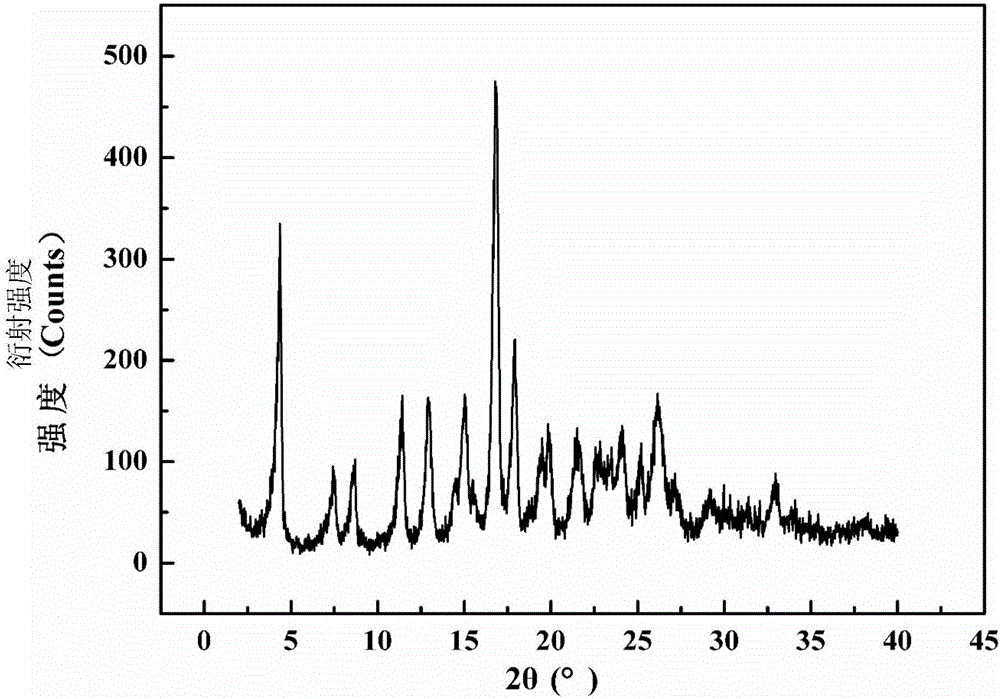
ActiveCN106518891AReduce dosageReduce energy consumptionOrganic chemistry methodsAzlocillin SodiumUltrasound - action
The invention relates to a crystal form of azlocillin sodium and a crystallization preparing method thereof. An X-ray powder diffraction spectrum is used for carrying out representation at the diffraction angle 2<theta> degrees and the characteristic peak of TGA; under the temperature ranging from 25 DEG C to 35 DEG C, an azlocillin sodium solid is added into a good solvent, stirring is carried out to enable azlocillin sodium to be dissolved completely, and a solution with the concentration being 0.05-0.2 g / mL is prepared; under the condition of applying the ultrasonic wave effect, a poor solvent is added into the solution, and a crystal is separated out, wherein the use amount of the poor solvent is 3-5 times of the volume of the good solvent; then temperature is lowered to 0 DEG C-10 DEG C, and stirring continues to be carried out for 1-3 h; crystal slurry is filtered, washed and dried to obtain an azlocillin sodium crystal-form product. The decomposition temperature of the novel crystal form is 229+ / -1 DEG C, and it shows that the stability of the product is improved. Meanwhile, the crystal-form product is of a sphere shape, is large in grain size, has higher bulk density and better mobility, and is more beneficial for the follow-up formulation process, product storage and use.
Owner:TIANJIN UNIV
Children type azlocillin sodium and low-sodium carrier pharmaceutical composition
InactiveCN104940130AHigh puritySmall side effectsAntibacterial agentsOrganic chemistryAzlocillin SodiumSodium Chloride Injection
The present invention relates to a children type azlocillin sodium pharmaceutical composition, especially to a combination application package, wherein the composition is an azlocillin sodium and low-sodium carrier infusion pharmaceutical combination preparation, which comprises azlocillin sodium for injection and a low-sodium carrier infusion, wherein the low-sodium carrier infusion contains a glucose and sodium chloride injection (15-200:1), a glucose, sodium chloride and potassium chloride injection (15-200:1:0-1) and the like. According to the present invention, the clinical application steps are simplified compared with the compatible mixing use of the azlocillin sodium and the low-sodium carrier infusion; and the clinical risk caused by the excessive sodium positioned in the blood and incapable of being metabolized due to the children kidney achieving the immature development state is reduced so as to improve the clinical application quality and the safety of the children medication are improved.
Owner:ZHEJIANG CHANGDIAN PHARMA
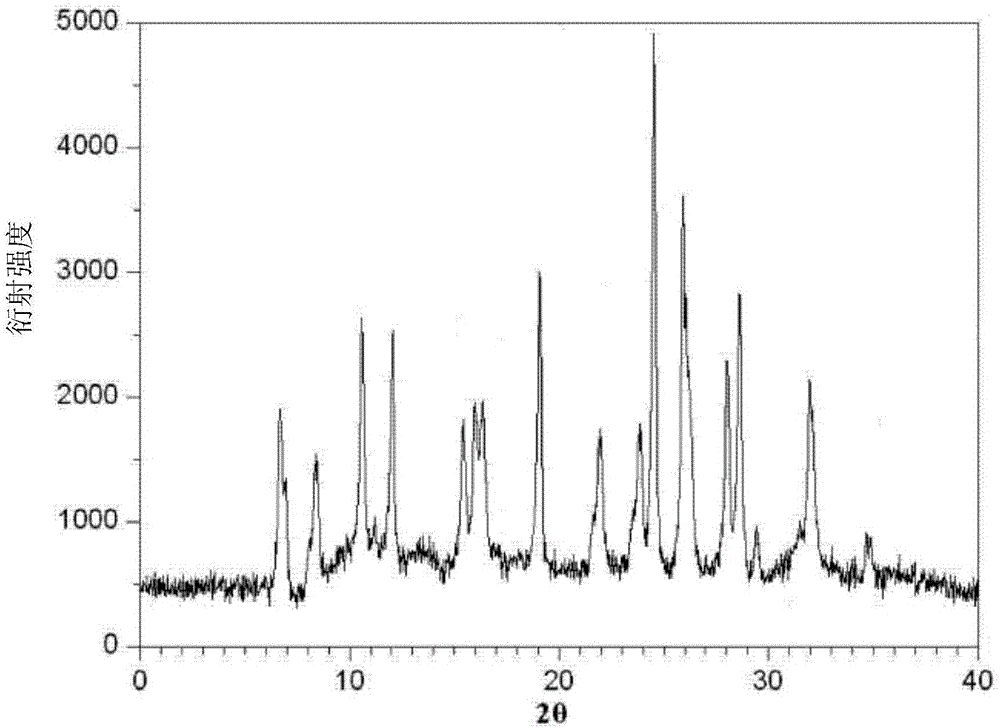
A kind of azlocillin sodium crystal and preparation method thereof
The invention relates to a crystal form of azlocillin sodium and a preparation method thereof. The crystal form is characterized by TGA characteristic peaks in the diffraction angles 2 theta in the X-ray powder diffraction spectrum; the amoxicillin sodium solid is added into a good solvent at 20-30 DEG C, the axlocillin sodium is completely dissolved by stirring, and the concentration of the solution is 0.2-0.3 g / ml, the system temperature is reduced to 0-15 DEG C, a poor solvent is added, crystals are crystallised out, and stirring is continued for 2-10 hours, the crystal pulp is subjected to filtration, washing and drying to obtain the amoxicillin sodium crystal form product. The crystal form crystal is large in particle size and good in fluidity, and the decomposition temperature is 217 + / -1 DEG C, the solubility of the product is large, the solubility of the product is improved, and the absorption and utilization of the drug are facilitated.
Owner:TIANJIN UNIV
Synthesis method of azlocillin sodium
InactiveCN107383061AIncrease productionHigh purityOrganic chemistryAzlocillin SodiumSynthesis methods
The invention discloses a synthesis method of azlocillin sodium. The synthesis method comprises the following steps: (1) dissolving raw materials; (2) carrying out condensation reaction; (3) carrying out purification treatment; (4) preparing finished-product azlocillin; (5) carrying out salt formation reaction; (6) filtering and degerming; (7) freezing and drying. According to the synthesis method of the azlocillin sodium, disclosed by the invention, a synthesis process is simplified effectively and reaction conditions are moderate; the feasibility of implementation is high and the raw materials are easily obtained; the controllability of a synthesis technology is relatively good and industrial production is convenient to realize; the obtained azlocillin sodium has the advantages of high yield, great purity and stable drug property and has a wide market prospect.
Owner:JIANGSU HI STONE PHARMA
Preparation method and product of azlocillin sodium powder injection
InactiveCN103622822AImprove stabilityImprove performanceAntibacterial agentsPowder deliveryAzlocillin SodiumMedicine
The invention discloses a preparation method and a product of azlocillin sodium powder injection. The preparation method comprises the following steps of: screening and disinfecting raw powder of azlocillin sodium to obtain sterile raw powder of azlocillin sodium; transferring the sterile raw powder of azlocillin sodium into a cleaning area to standby; cleaning and sterilizing bottles used for subpackaging the raw powder of azlocillin sodium, and transferring into the clean area to standby; subpackaging the sterile raw powder of azlocillin sodium into the sterile bottles; covering butyl rubber stoppers; pressing aluminum covers to seal to obtain the azlocillin sodium powder injection. According to the method, azlocillin sodium particles are preferred, therefore, the problem of the prior art that azlocillin sodium is difficulty subpackaged is effectively overcome, and the stability of a preparation is improved obviously; the performance detection test shows that the prepared powder injection has high quality, and all detection indexes do not vary obviously after long-term test of more than 12 months and all meet the quality standard, namely, the preparation is high in stability.
Owner:NORTH CHINA PHARMA COMPANY
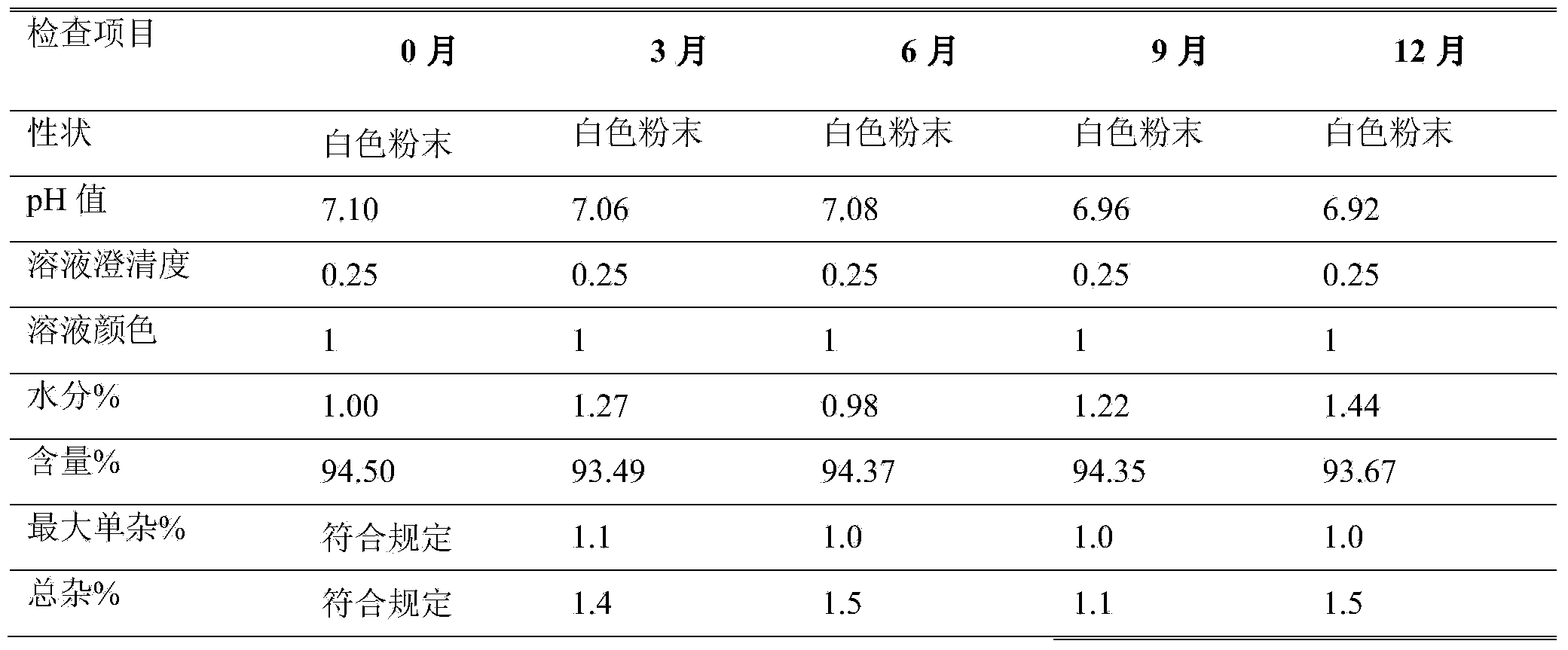
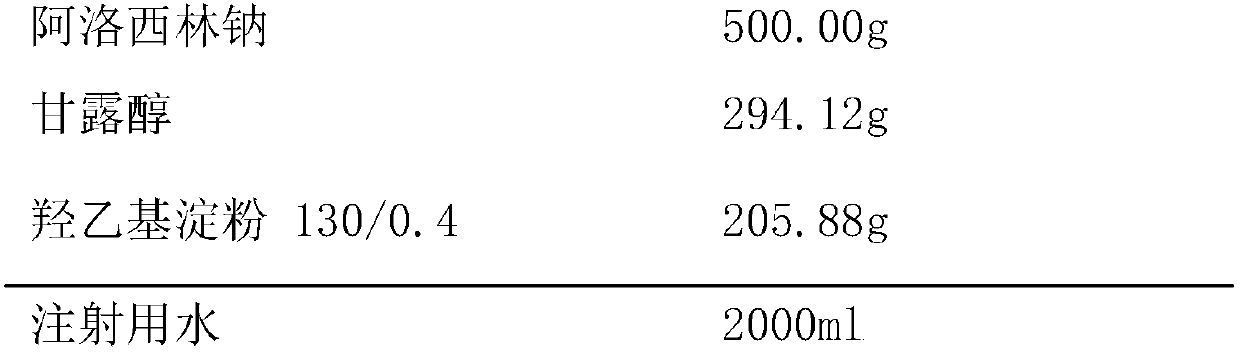
Azlocillin sodium freeze-dried powder injection for injection prepared from double excipients
InactiveCN107638401ASimple preparation processImprove product qualityAntibacterial agentsPowder deliveryHydroxyethyl starchAzlocillin Sodium
The invention provides an azlocillin sodium freeze-dried powder injection for injection prepared from double excipients, relates to the field of pharmaceutical preparation and preparation method, andmainly overcomes the defects that the excipients in a production formula of an azlocillin sodium freeze-dried powder injection for injection has long freeze-drying time, high drying temperature and high energy consumption in production in the prior art. The azlocillin sodium freeze-dried powder injection for injection uses double excipients including mannitol and hydroxyethyl starch 130 / 0.4, the main drug is azlocillin sodium, the weight ratio of the main drug to the excipients is (100:100)-(100:130), and the weight ratio of mannitol to hydroxyethyl starch 130 / 0.4 is (100:50)-(100:80). The azlocillin sodium freeze-dried powder injection for injection is prepared from the main drug and the excipients. The azlocillin sodium freeze-dried powder injection for injection provided by the invention has the advantages of stable quality, safety and effectiveness, simple product production process, short drying time, low drying temperature and low energy consumption in production.
Owner:刘兴付
A kind of crystal form of azlocillin sodium and its crystallization preparation method
ActiveCN106518891BReduce dosageReduce energy consumptionOrganic chemistry methodsUltrasound - actionDecomposition
The invention relates to a crystal form of azlocillin sodium and a crystallization preparing method thereof. An X-ray powder diffraction spectrum is used for carrying out representation at the diffraction angle 2<theta> degrees and the characteristic peak of TGA; under the temperature ranging from 25 DEG C to 35 DEG C, an azlocillin sodium solid is added into a good solvent, stirring is carried out to enable azlocillin sodium to be dissolved completely, and a solution with the concentration being 0.05-0.2 g / mL is prepared; under the condition of applying the ultrasonic wave effect, a poor solvent is added into the solution, and a crystal is separated out, wherein the use amount of the poor solvent is 3-5 times of the volume of the good solvent; then temperature is lowered to 0 DEG C-10 DEG C, and stirring continues to be carried out for 1-3 h; crystal slurry is filtered, washed and dried to obtain an azlocillin sodium crystal-form product. The decomposition temperature of the novel crystal form is 229+ / -1 DEG C, and it shows that the stability of the product is improved. Meanwhile, the crystal-form product is of a sphere shape, is large in grain size, has higher bulk density and better mobility, and is more beneficial for the follow-up formulation process, product storage and use.
Owner:TIANJIN UNIV
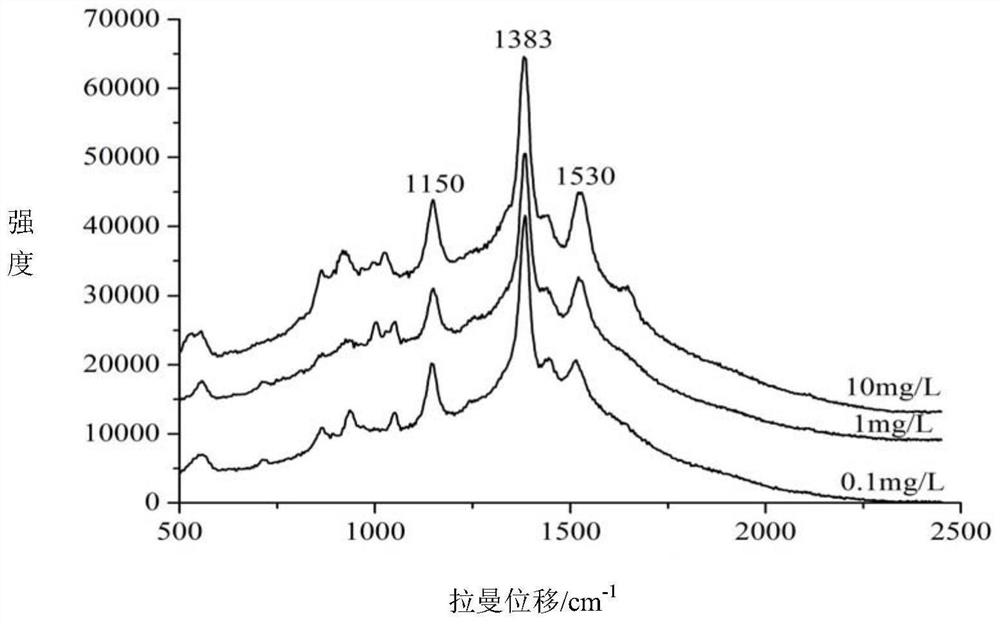
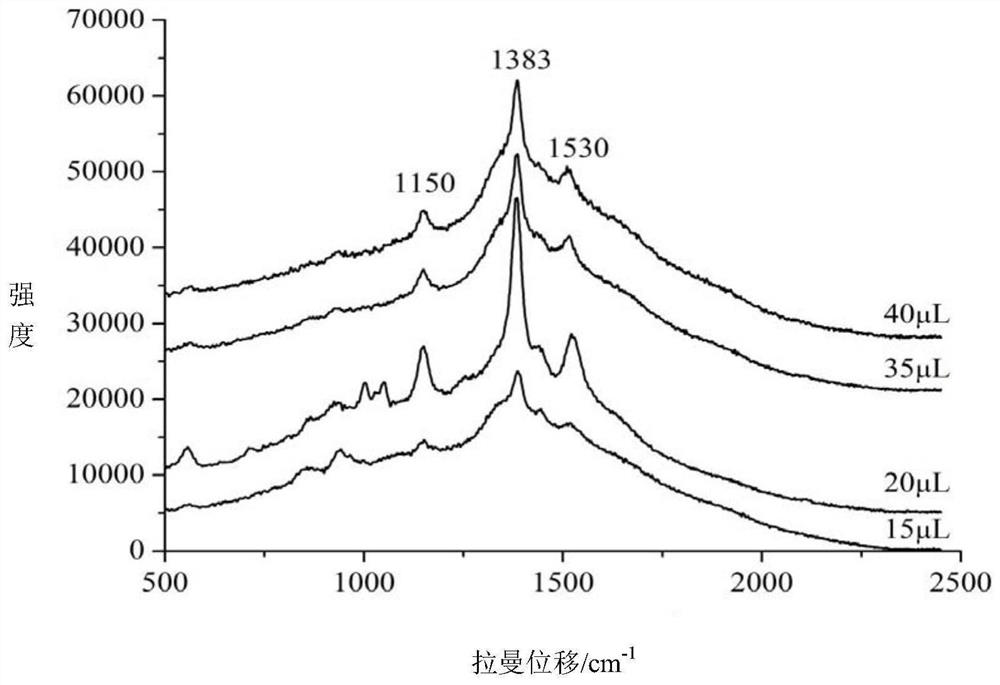
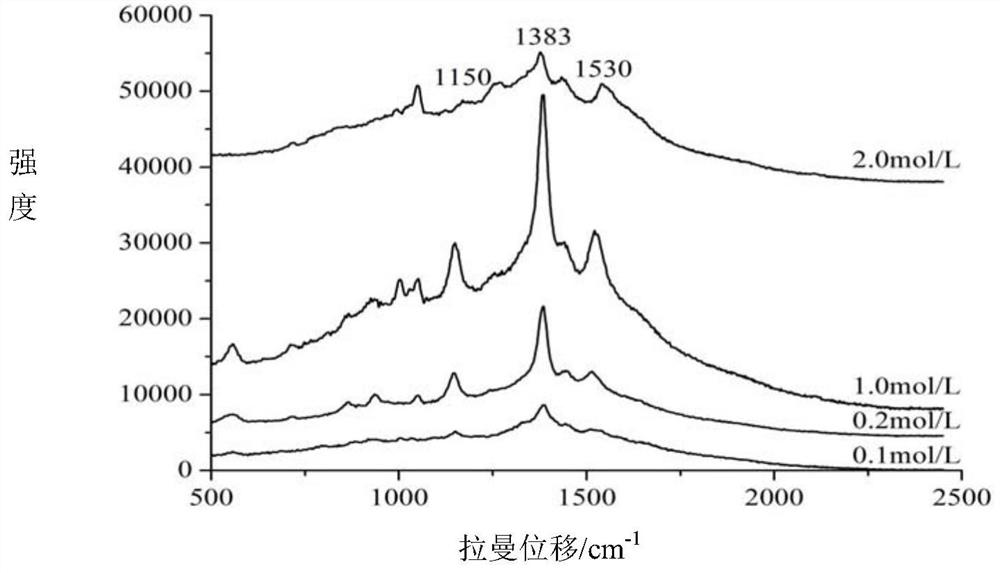
Method for quickly detecting azlocillin sodium in milk by surface enhanced Raman spectroscopy
PendingCN112179890AQuick checkHigh sensitivityRaman scatteringAzlocillin SodiumSurface-enhanced Raman spectroscopy
The invention relates to a method for quickly detecting azlocillin sodium in milk by surface enhanced Raman spectroscopy, which comprises the following steps of pretreating a sample to obtain an eluent, thereby obtaining a pretreated milk sample, preparing standard solutions, namely weighing azlocillin sodium standard substances, adding water, uniformly mixing to obtain azlocillin sodium standardsolutions, and respectively preparing azlocillin sodium standard solutions with different concentrations for later use, raman detection: respectively adding the pretreated milk sample and azlocillin sodium standard solutions with different concentrations in the previous step into nanogold sol, uniformly mixing, adding an aqueous solution of sodium chloride, uniformly mixing, and carrying out Ramanspectrum detection, and finally, conducting linear fitting. The method disclosed by the invention has the advantages of high sensitivity, short time, strong specificity, no damage, small required sample amount, simple pretreatment and the like, is suitable for detecting azlocillin sodium in milk, and has a relatively good application prospect.
Owner:武夷学院
Azlocillin sodium composition powder for injection
InactiveCN103536620AImprove stabilityGood resolubilityAntibacterial agentsPowder deliveryChitosan nanoparticlesAdditive ingredient
The invention provides an azlocillin sodium composition powder for injection, and belongs to the field of medicine and medicine preparation technology. The azlocillin sodium composition powder comprises following raw material ingredients, by weight, 7.26 to 9.17 parts of azlocillin sodium, 1.45 to 1.84 parts of chitosan nanoparticle, and 88.99 to 91.29 parts of injection water. Advantages of the azlocillin sodium composition powder are that: the size of the chitosan nanoparticle is ultra small, so that the chitosan nanoparticle is capable of passing through capillary vessels with a smallest diameter, rapid removing of the chitosan nanoparticle by macrophage is avoided, so that lasting time of the azlocillin sodium composition in blood is prolonged greatly; the azlocillin sodium composition is capable of improving antimicrobial activity of azlocillin sodium significantly, reducing dosage of azlocillin sodium in clinic, and reducing adverse reaction of azlocillin sodium; and the chitosan nanoparticle can be used as a freeze-dried skeleton agent of the freeze-dried powder injection instead of mannitol so as to avoid active effects of mannitol on human bodies.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
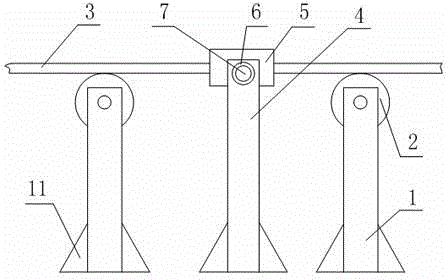
Preparation method of azlocillin sodium based on conveying device having fixing grooves
InactiveCN104816914AImprove structural stabilityPrevent dumpingConveyorsAzlocillin SodiumReagent bottle
The invention relates to a preparation method of azlocillin sodium based on a conveying device having fixing grooves. The method comprises the following steps: firstly, cleaning reagent bottles; secondly, sub-packaging, filling azlocillin sodium powders into the reagent bottles according to specifications; then, covering the reagent bottles containing the azlocillin sodium powders; and then, packaging the covered reagent bottles; finally, implementing light inspection, labeling, checking and packaging procedures, wherein each procedure is connected with the other by a conveying structure. The conveying device comprises multiple fixing structures which are matched with each other. A conveying belt is arranged on the fixing structures. Bilateral sides of the upper end face of the conveying belt are provided with two opposite vertical fixing plates. The fixing grooves are alternately arranged on the inner sides of the two vertical fixing plates. The fixing grooves on different vertical fixing plates are distributed symmetrically. A fixing rod is clamped between two symmetric fixing grooves. The preparation method of azlocillin sodium based on the conveying device having the fixing grooves solves the problem that the reagent bottles are easy to topple over in the conveying process of the conventional conveying device.
Owner:SICHUAN PHARMA
Azlocillin sodium compound, its preparation method and pharmaceutical composition thereof
InactiveCN103265561BNot easy to absorb moistureEasy to storeAntibacterial agentsOrganic chemistryAzlocillin SodiumFreeze-drying
The invention belongs to the technical field of medicines, and particularly relates to an azlocillin sodium compound. The structural formula of the azlocillin sodium compound is defined in the specification, and the X-ray powder diffraction spectrogram of the azlocillin sodium compound, which is obtained by measurement by using Cu-K alpha ray, is shown in the figure 1. The invention further provides a preparation method of the azlocillin sodium compound, a medicine composition containing the azlocillin sodium compound, and a preparation method of the medicine composition. The dosage forms of the azlocillin sodium compound are sterile powder injection and freeze-dried powder injection. The azlocillin sodium compound provided by the invention almost does not absorb moisture, is easy to store and has good storage stability and low impurity content; and therefore, medication safety is greatly improved.
Owner:四川省惠达药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com