Method for producing high-purity azlocillin sodium and powder injection thereof
A technology of azlocillin sodium powder and azlocillin sodium, applied in the field of medicine, can solve the problems of low purity, poor stability, poor clarity and the like, and achieve the effects of high purity, low cost and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
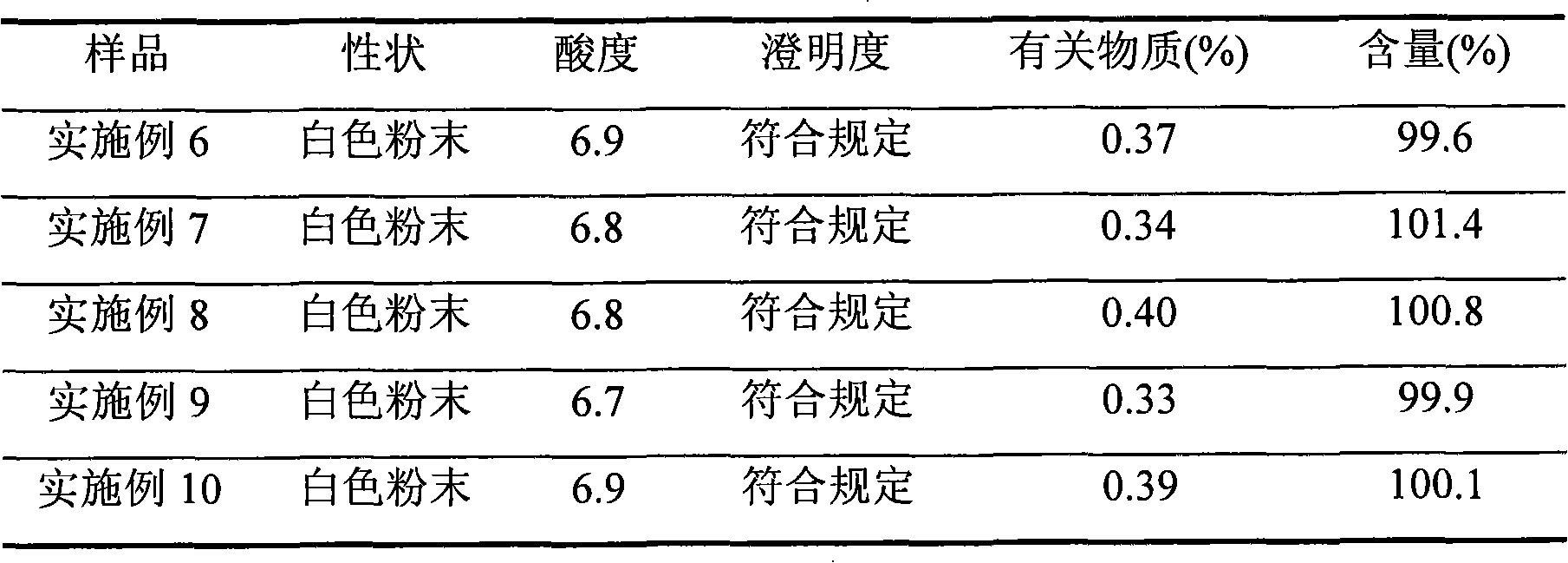
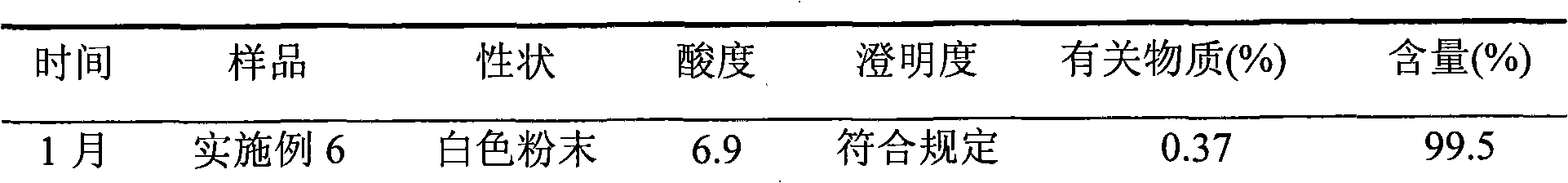
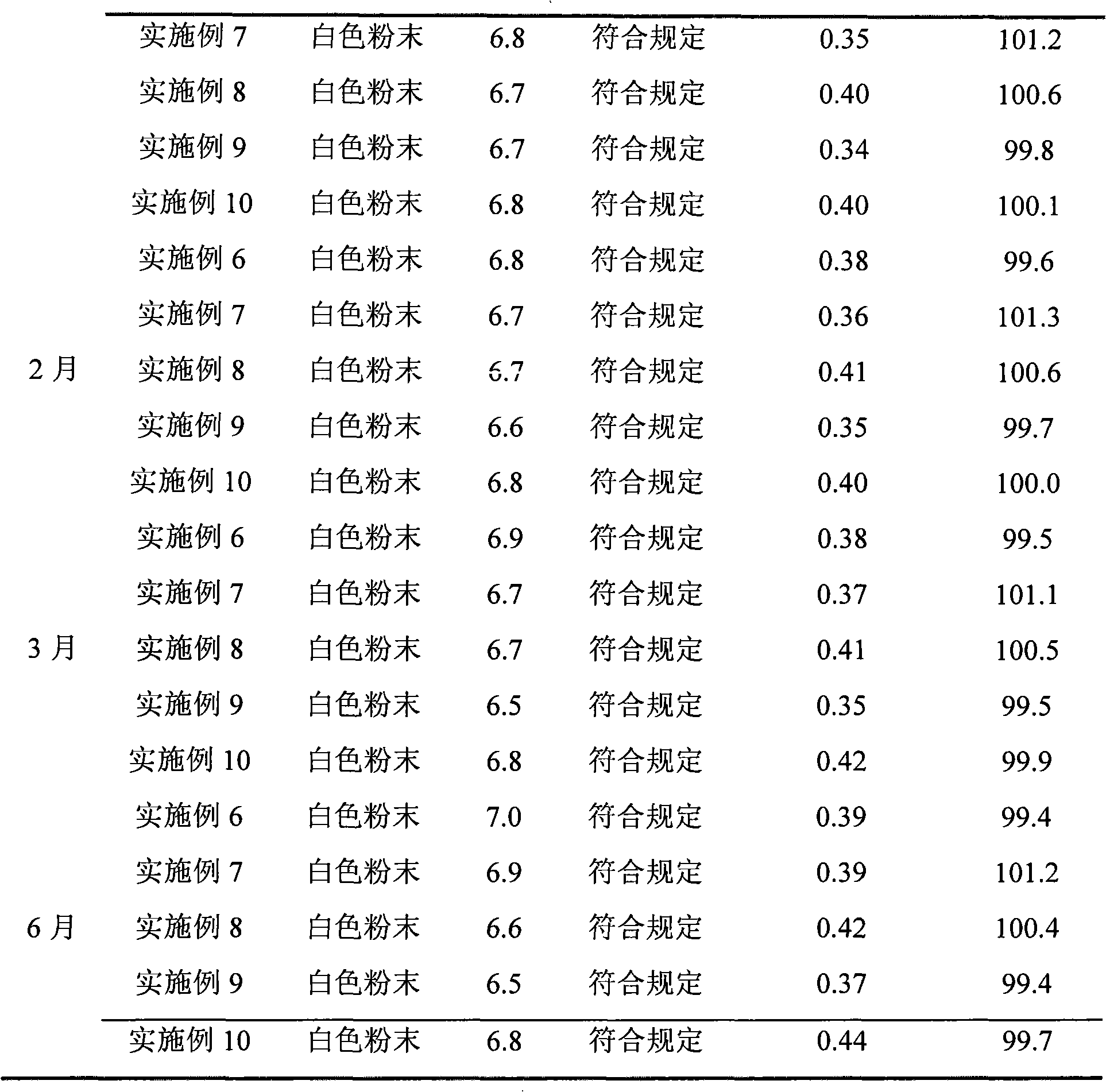
Examples
Embodiment 1
[0041] 500g of crude azlocillin sodium was stirred and dissolved in 2L of purified water, added hydrochloric acid to adjust the pH to 1, and extracted with 6L of dichloromethane three times, the extract was dehydrated through an anhydrous sodium sulfate column, and then concentrated to dryness under reduced pressure. After suspending 5kg of 50-mesh alumina with activity level II in n-hexane, remove the air bubbles, and evenly add it to a glass chromatography column with an effective column length of 20cm and an inner diameter of 5cm, and add 600ml of the crude azlocillin sodium concentrated above The eluent (the mass ratio of normal hexane to dichloromethane is 1:99) is dissolved, and the insoluble matter is filtered out and put on the column head with a plunger solvent pump, and then the eluent is pumped in (the mass ratio of normal hexane to dichloromethane is 1:99). 1:99) elution, column pressure is 0.1MPa, segmental collection fraction, TLC and HPLC detection, merge the eff...
Embodiment 2
[0043] 500g of crude azlocillin sodium was stirred and dissolved in 2L of purified water, added sulfuric acid to adjust the pH to 6, extracted with 8L of n-butanol, the extract was dehydrated through an anhydrous magnesium sulfate column, and then concentrated to dryness under reduced pressure. Suspend 100 kg of 800 mesh alumina with activity level II in n-hexane, remove air bubbles, and evenly add it to a glass chromatography column with an effective column length of 2000 cm and an inner diameter of 100 cm, and add the crude azlocillin sodium concentrated to dryness 700ml of eluent (cycloheptane and chloroform mass ratio is 100:1) is dissolved, and the insoluble matter is filtered off and put on the column head with a plunger solvent pump, and then pumped into the eluent (cycloheptane and chloroform The mass ratio is 100:1) elution, the column pressure is 5MPa, the fractions are collected in sections, detected by TLC and HPLC, the effluent with azlocillin acid content greater ...
Embodiment 3
[0045] 500g of crude azlocillin sodium was stirred and dissolved in 2L of purified water, added phosphoric acid to adjust the pH to 2, extracted with 7L of cyclooctane, the extract was dehydrated through an anhydrous calcium chloride column, and then concentrated to dryness under reduced pressure. After suspending 7.5kg of 100-mesh alumina with activity level II with n-hexane, remove air bubbles, and evenly add it to a glass chromatography column with an effective column length of 30cm and an inner diameter of 10cm. The crude azlocillin sodium concentrated to dry Add 650ml of eluent (the mass ratio of 1-nonene to 1,2-dichloroethane is 1:4) to dissolve, filter out the insoluble matter and put it on the column head with a plunger solvent pump, then pump the eluent ( The mass ratio of 1-nonene to 1,2-dichloroethane is 1:4), and the column pressure is 2.5MPa. The fractions are collected in sections, detected by TLC and HPLC, and the combined azlocillin acid content is greater than ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com