Patents
Literature
94 results about "Cycloheptane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cycloheptane is a cycloalkane with the molecular formula C₇H₁₄. Cycloheptane is used as a nonpolar solvent for the chemical industry and as an intermediate in the manufacture of chemicals and pharmaceutical drugs. It may be derived by Clemmensen reduction from cycloheptanone. Cycloheptane vapour is irritating to the eyes and may cause respiratory depression if inhaled in large quantity.
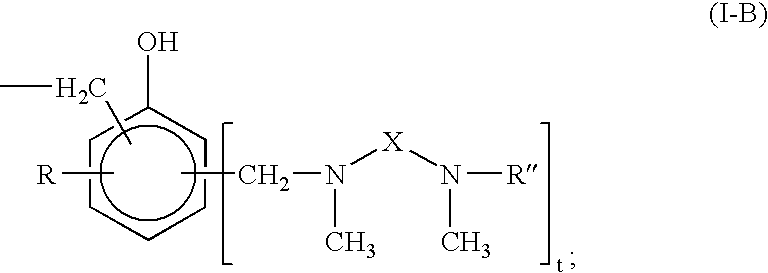
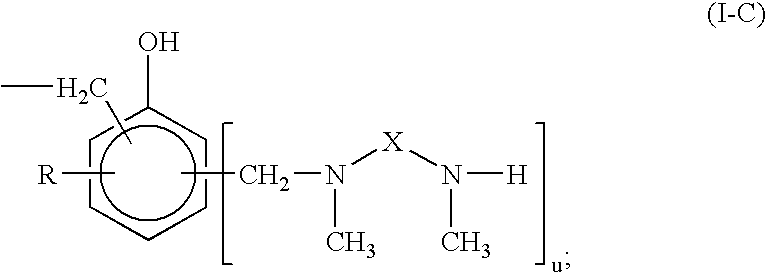
Curing agent for low temperature cure applications
InactiveUS20080194776A1Extended drying timeRapid hardness developmentThin material handlingEpoxyLow temperature curing
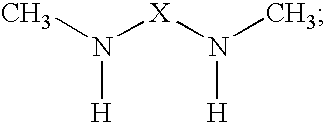
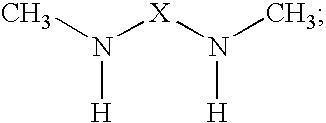
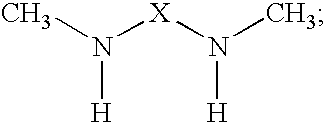
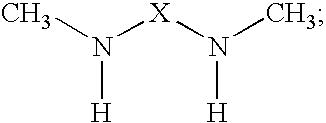
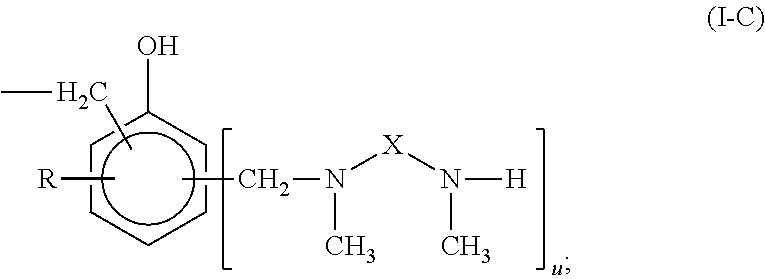
The present invention provides N,N′-dimethyl secondary diamine polymers including methylamine-terminated poly-(N-methylazetidine) and methylamine-terminated poly-(N-methylazacycloheptane). Amine compositions and amine-epoxy compositions comprising N,N′-dimethyl secondary diamine polymers are also disclosed.
Owner:EVONIK DEGUSSA GMBH
Method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine
InactiveCN102531918ASimple and fast operationMild reaction conditionsOrganic compound preparationAmino compound preparationDiisopropyl azodicarboxylateHexamethylenediamine
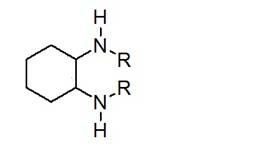
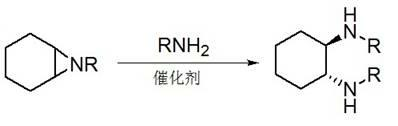
The invention discloses a method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine, belonging to the field of chemistry. The method comprises the following steps of: carrying out an airtight or reflux reaction on cyclohexene oxide and an aqueous solution of alkylamine for reacting at 80-120 DEG C for 1.5-5h to obtain 2-alkyl-amino cyclohexanol, dropwise adding DEAD (Diethyl Azodicarboxylate) or DIAD (Diisopropyl Azodicarboxylate) to triphenylphosphine, 2-alkyl-amino cyclohexanol and a solvent under an ice bath for reacting for 5-20h at room temperature to obtain N-alkyl-7-azabicyclo[4, 1, 0]heptane, adding a catalyst to the N-alkyl-7-azabicyclo[4, 1, 0]heptane and the aqueous solution of alkylamine at 100-120 DEG C to carry out the airtight or reflux reaction to obtain trans-N,N'-dialkyl-1,2 cyclohexanamine, dissolving the trans-N,N'-dialkyl-1,2 cyclohexanamine in an alcoholic solvent, and adding a tartaric acid type resolving agent with the equivalent weight of 0.5 to the alcoholic solvent to resolve so as to obtain the enantiomorphous pure symmetric trans-dialkyl cyclohexylamine.
Owner:ANYANG INST OF TECH
Epoxy resin amine curing agent of N,N′-dimethyl secondary diamine polymer
InactiveUS7666954B2Extended drying timeRapid hardness developmentThin material handlingEpoxyEndcapping
The present invention provides N,N′-dimethyl secondary diamine polymers including methylamine-terminated poly-(N-methylazetidine) and methylamine-terminated poly-(N-methylazacycloheptane). Amine compositions and amine-epoxy compositions comprising N,N′-dimethyl secondary diamine polymers are also disclosed.
Owner:EVONIK DEGUSSA GMBH
Substituted 1,2,4-trioxanes useful as antimalarial agents and a process for the preparation thereof
Owner:COUNCIL OF SCI & IND RES
Curing Agent For Low Temperature Cure Applications
ActiveUS20090259003A1Improved “ walk-on ” dry timeRapid hardness developmentIsocyanic acid derivatives preparationOther chemical processesEpoxyEndcapping
The present invention provides Mannich base derivatives of N,N′-dimethyl secondary diamine polymers including Mannich base derivatives of methylamine-terminated poly-(N-methylazetidine) and Mannich base derivatives of methylamine-terminated poly-(N-methylazacycloheptane). Amine curing agent compositions and amine-epoxy compositions containing Mannich base derivatives of N,N′-dimethyl secondary diamine polymers are also disclosed.
Owner:EVONIK OPERATIONS GMBH
Synthetic method of edible spice 1,2,3,5,6-pentathiocycloheptane
InactiveCN102260240APure aromaRealistic aromaOrganic chemistryAntibacterial activityDrug biological activity
The present invention relates to a kind of synthetic method of food spice 1,2,3,5,6-pentathiocycloheptane, the steps are as follows: firstly thiating: in a three-necked reaction flask equipped with a stirrer, a constant pressure dropping funnel, and a thermometer Add sodium tetrathiocarbonate and absolute ethanol to the mixture and stir until sodium tetrathiocarbonate is completely dissolved. Add the absolute ethanol solution of diiodomethane dropwise with a constant pressure dropping funnel within 2 hours at 25°C. Stir and react at a constant temperature of 25°C for 10 hours; second extraction: dilute the above reaction solution with water, then extract three times with cyclohexane, separate the water layer and combine the extracts, add anhydrous calcium chloride for drying; finally obtain the finished product by distillation. The invention provides an edible spice 1,2,3,5,6-pentathiacycloheptane with strong characteristic fragrance, good antibacterial activity and biological activity. The process is simple and the yield is high, and it can be safely used as a spice in It is a new type of mushroom flavor during frying, frying, cooking, frying and other cooking processes.
Owner:TIANJIN CHEM REAGENT RES INST
Pathological tissue transparentizing and dewaxing solution and preparation method thereof
ActiveCN104655460ANo irritating smellWon't become brittlePreparing sample for investigationAlkaneEther
The invention discloses a pathological tissue transparentizing and dewaxing solution and a preparation method thereof. The pathological tissue transparentizing and dewaxing solution comprises 92.5-98 parts by weight of highly branch chain saturated alkane, 0.5-5 parts by weight of a stabilizing agent and 0.1-2.5 parts by weight of synergist, wherein the highly branch chain saturated alkane is one or a mixture of more of naphthenic oil, quadricyclane, cis-1-isopropyl-4-methyl cyclohexane, and contra-1-isopropyl-4-methyl cyclohexane; the stabilizing agent is one or a mixture of more of epoxy soybean oil, trimethylolpropane, and epoxy butyl stearate; the synergist is one or a mixture of more of polyether modified heptamethyltrisiloxane, butyl ether polydimethylsiloxane, and dimethyl-3-hydroxypropyl methyl (siloxane and polysiloxane). The technical problems that a pathological tissue transparentizing and dewaxing solution is toxic and has pungent smell, and tissue becomes brittle and shrinks to be unfavorable for slicing after being soaked in the solution for a long time in the prior art are solved.
Owner:无锡市江原实业技贸有限公司 +1
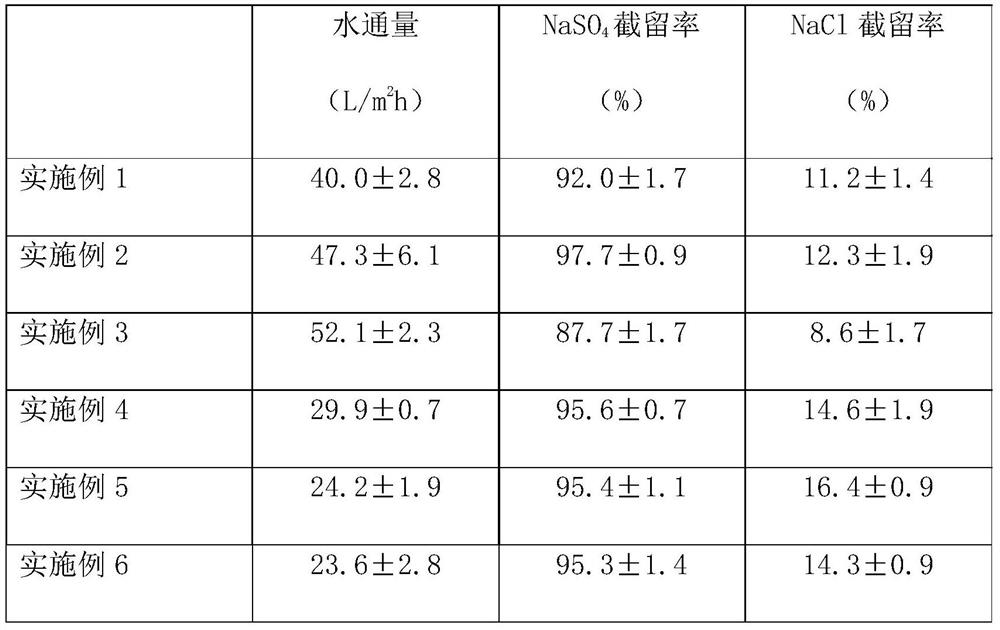
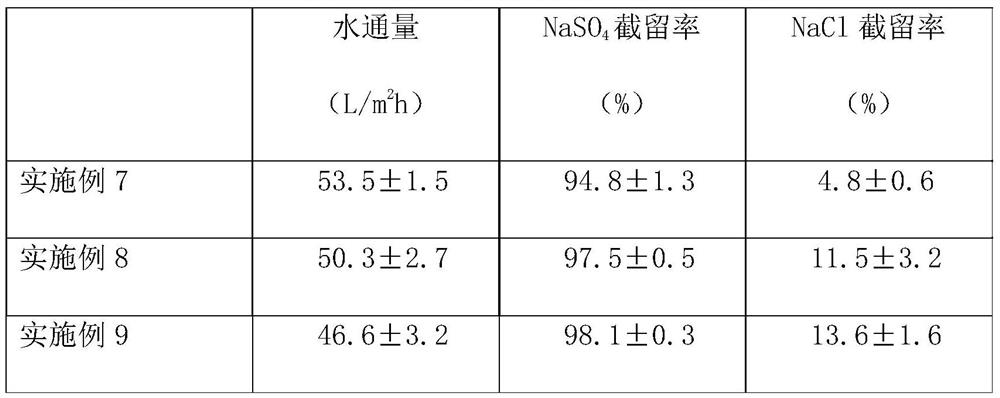
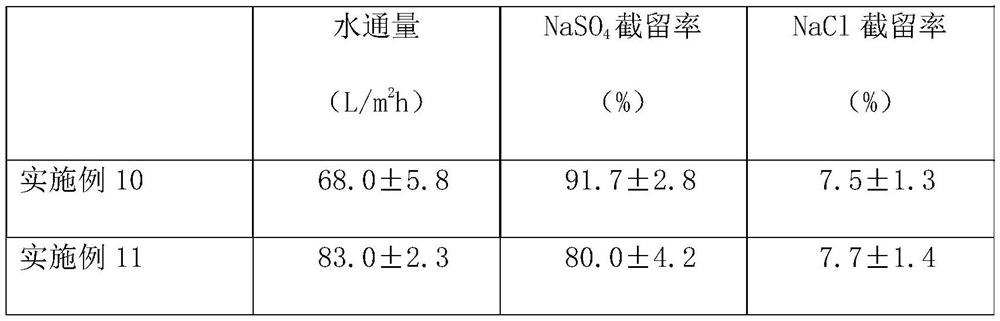
High-selectivity polyamide nanofiltration membrane and preparation method thereof
ActiveCN112090282ASimple preparation processEasy to implementGeneral water supply conservationReverse osmosisPolyamideNanofiltration
The invention provides a high-selectivity polyamide nanofiltration membrane and a preparation method thereof. The high-selectivity polyamide nanofiltration membrane is composed of a porous support base membrane and a polyamide separation layer, wherein the polyamide separation layer is prepared by carrying out interfacial polymerization on a polybasic acyl chloride organic solution and an aqueoussolution of 1, 4-diazacycloheptane or a mixture thereof on the surface of a porous support base membrane; the high-selectivity polyamide nanofiltration membrane has excellent monovalent and divalent salt selectivity and water flux, and is expected to have a wide application prospect in the fields of wastewater salt separation recycling, sea water desalination and drinking water purification.
Owner:TIANJIN POLYTECHNIC UNIV
Method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine
InactiveCN102531918BSimple and fast operationMild reaction conditionsOrganic compound preparationAmino compound preparationDiisopropyl azodicarboxylateHexamethylenediamine
The invention discloses a method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine, belonging to the field of chemistry. The method comprises the following steps of: carrying out an airtight or reflux reaction on cyclohexene oxide and an aqueous solution of alkylamine for reacting at 80-120 DEG C for 1.5-5h to obtain 2-alkyl-amino cyclohexanol, dropwise adding DEAD (Diethyl Azodicarboxylate) or DIAD (Diisopropyl Azodicarboxylate) to triphenylphosphine, 2-alkyl-amino cyclohexanol and a solvent under an ice bath for reacting for 5-20h at room temperature to obtain N-alkyl-7-azabicyclo[4, 1, 0]heptane, adding a catalyst to the N-alkyl-7-azabicyclo[4, 1, 0]heptane and the aqueous solution of alkylamine at 100-120 DEG C to carry out the airtight or reflux reaction to obtain trans-N,N'-dialkyl-1,2 cyclohexanamine, dissolving the trans-N,N'-dialkyl-1,2 cyclohexanamine in an alcoholic solvent, and adding a tartaric acid type resolving agent with the equivalent weight of 0.5 to the alcoholic solvent to resolve so as to obtain the enantiomorphous pure symmetric trans-dialkyl cyclohexylamine.
Owner:ANYANG INST OF TECH
Curing agent for low temperature cure applications
ActiveUS8735512B2Extended drying timeRapid hardness developmentIsocyanic acid derivatives preparationOrganic compound preparationEpoxyLow temperature curing
The present invention provides Mannich base derivatives of N,N′-dimethyl secondary diamine polymers including Mannich base derivatives of methylamine-terminated poly-(N-methylazetidine) and Mannich base derivatives of methylamine-terminated poly-(N-methylazacycloheptane). Amine curing agent compositions and amine-epoxy compositions containing Mannich base derivatives of N,N′-dimethyl secondary diamine polymers are also disclosed.
Owner:EVONIK OPERATIONS GMBH
Acrylic acid recovery utilizing ethyl acrylate and selected co-solvents
InactiveUS20030146081A1Organic compound preparationSolvent extractionPolymer scienceMethyl palmoxirate
A method of recovering acrylic acid from a mixture comprising acrylic acid, water and acetic acid is disclosed, which includes: (a) extracting acrylic acid from the mixture with a solvent mixture comprising ethyl acrylate as the preponderant component thereof and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to form an extracted composition; and (b) azeotropically distilling the extracted composition to recover acrylic acid. Also disclosed is an alternate method of recovering acrylic acid which includes: (a) providing a feed stream containing acrylic acid, water, acetic acid, ethyl acrylate and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to a distillation column, wherein the weight ratio of ethyl acrylate to the organic co-solvent is from about 80:20 to about 95:5; and (b) azeotropically distilling said feed stream to provide an acrylic acid residue stream.
Owner:DOW GLOBAL TECH LLC
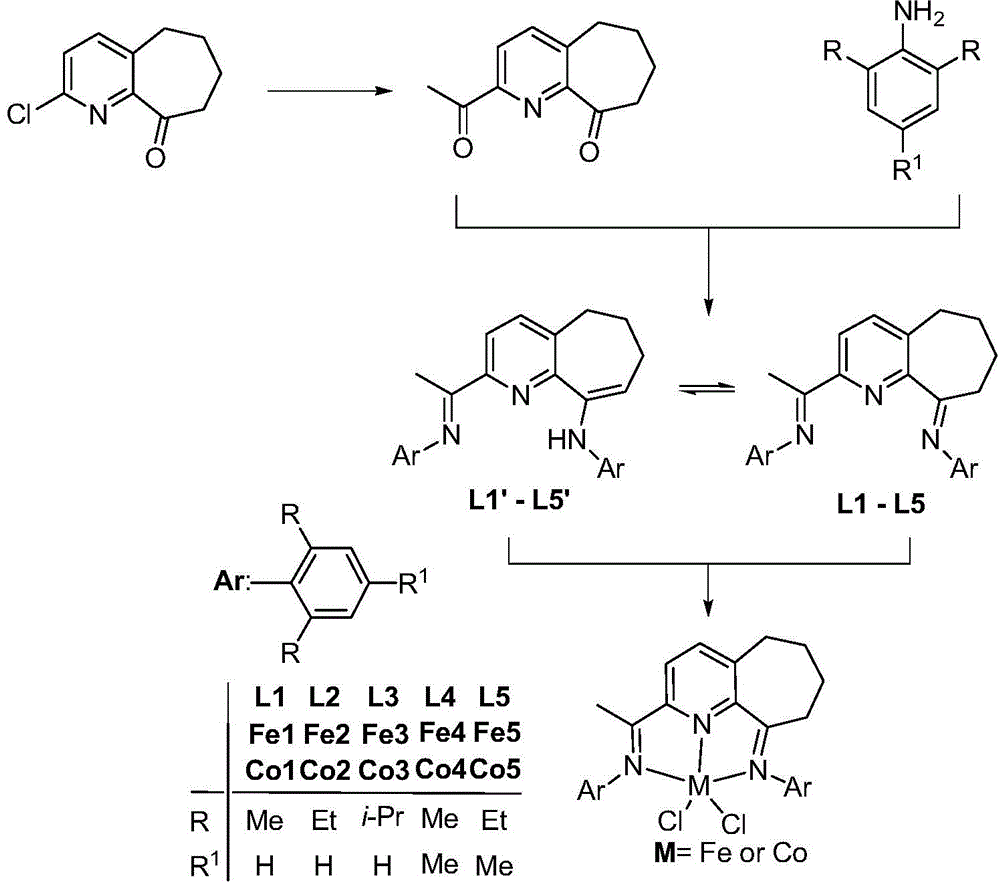
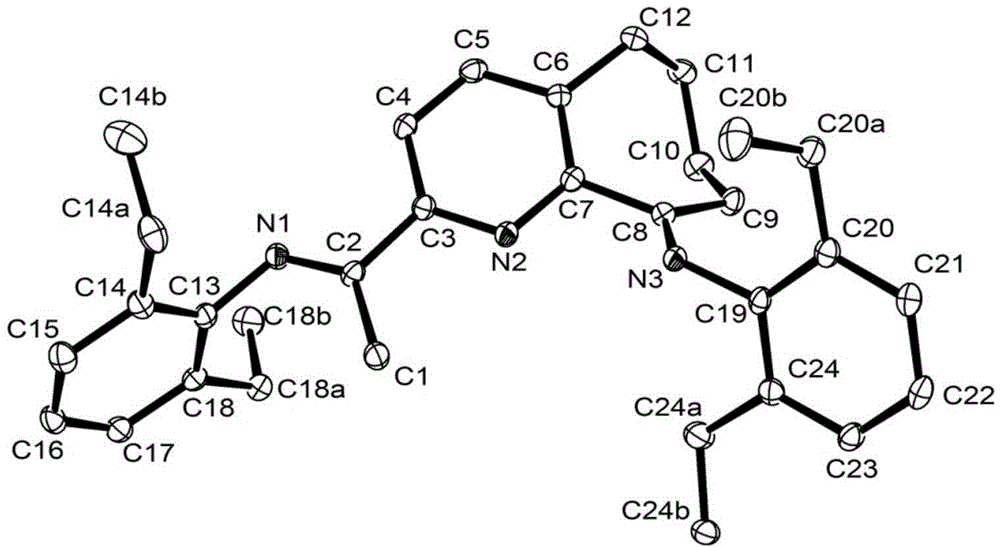
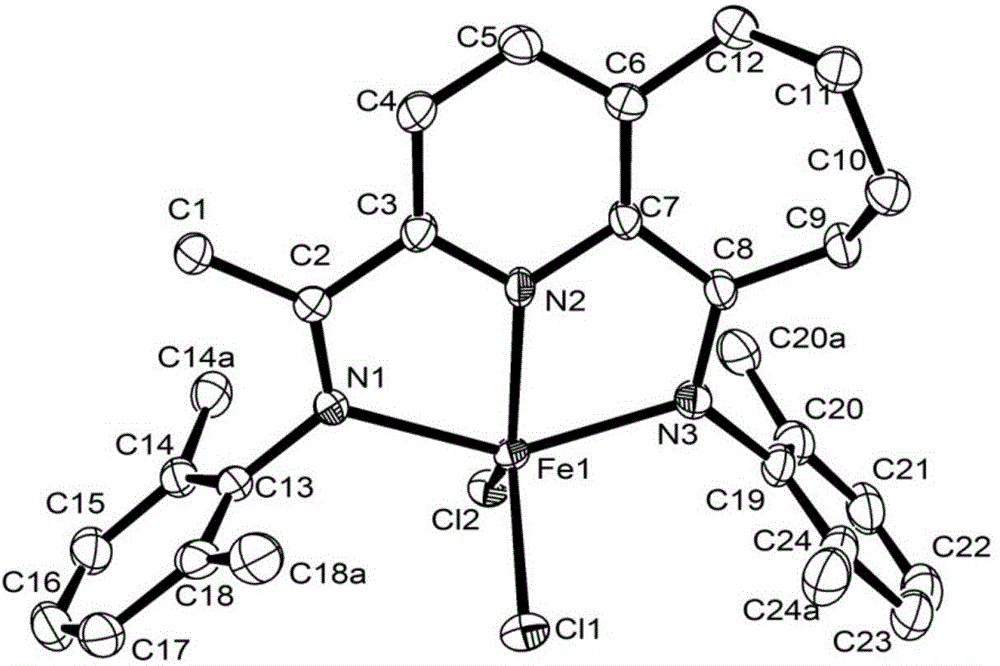
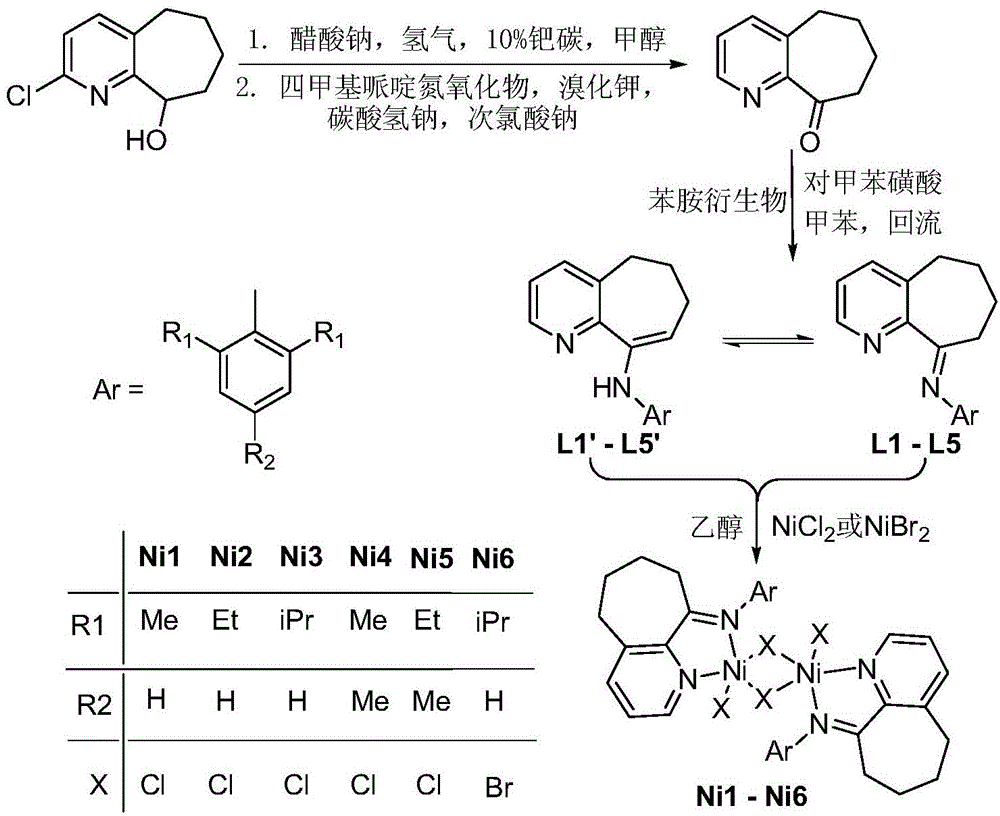
2,6-diimine pyridinocycloheptane iron and cobalt complex catalyst and preparation method therefor and application thereof
ActiveCN105315309AHigh activityHigh catalytic activityNickel organic compoundsIron organic compoundsStructural formulaMethyl group
The invention discloses a 2,6-diimine pyridinocycloheptane iron and cobalt complex catalyst and a preparation method therefor and an application thereof. The structural formula of the complex is represented by a formula I, wherein R is selected from at least one of methyl, ethyl or isopropyl; R1 is selected from methyl or hydrogen; M represents Fe or Co. The preparation method comprises the step of: carrying out a reaction on a ligand as shown in a formula V and FeCl2.4H2O or CoCl2 to obtain a complex represented by the formula I under a room temperature condition. The invention designs and synthesizes a complex which contains a 2,6-dienamine pyridine ligand and iron and cobalt metals. The series of complexes, under the action of a co-catalyst methylaluminoxane or modified methylaluminoxane, can better catalyze vinyl polymerization to obtain a polymer with high molecular weight. Meanwhile, the activity of the catalyst is further high, and the highest activities respectively reach 1.56*10<7> / mol(Fe) / h and 2.09*10<7>g / mol(Co) / h. The catalyst has a wide industrial prospect. The formula is as shown in the description, wherein Ar represents a formula shown in the description.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Oxabicycloheptanes and oxabicycloheptenes for the treatment of reperfusion injury
A method of reducing reperfusion injury in mammalian tissue comprising contacting the tissue with a protein phosphatase 2A (PP2A) inhibitor having the structure.
Owner:里克思特生物技术有限公司
Herbicide containing fluroxypyr, nicosulfuron and atrazine and preparation method thereof
InactiveCN102972422AImprove permeabilityGood stretchabilityBiocideAnimal repellantsVegetable oilFluroxypyr
The invention provides a herbicide containing fluroxypyr, nicosulfuron and atrazine. According to the invention, fluroxypyr, nicosulfuron and atrazine are used as main components, urea is used as a stabilizing agent, and 1-dodecyl azacycloheptane-2-one is used as a synergist; addition of 1-dodecyl azacycloheptane-2-one as the synergist substantially improves the effect of the herbicide. Turpentine-based vegetable oil used as a filling material is extracted from the timber of Chinese pine; the raw material for the filling material is natural and environment-friendly, resources are renewable, and the problem that usage of considerable organic solvents in pesticide preparations poses severe pollution to the environment is thoroughly overcome. Nicosulfuron, atrazine and fluroxypyr have obvious synergistic interaction, and integral drug effect of the herbicide is obviously better than a mixture of individual preparations of nicosulfuron, atrazine and fluroxypyr. Low dosage of the herbicide provided by the invention has a minimum control effect of 80.1% on grassy weeds, a minimum control effect of 86.9% on broad-leaf weeds and a comprehensive control effect of 83.5 to 99.9%; substantial synergy and reduction in drug cost are realized.
Owner:辽宁海佳农化有限公司
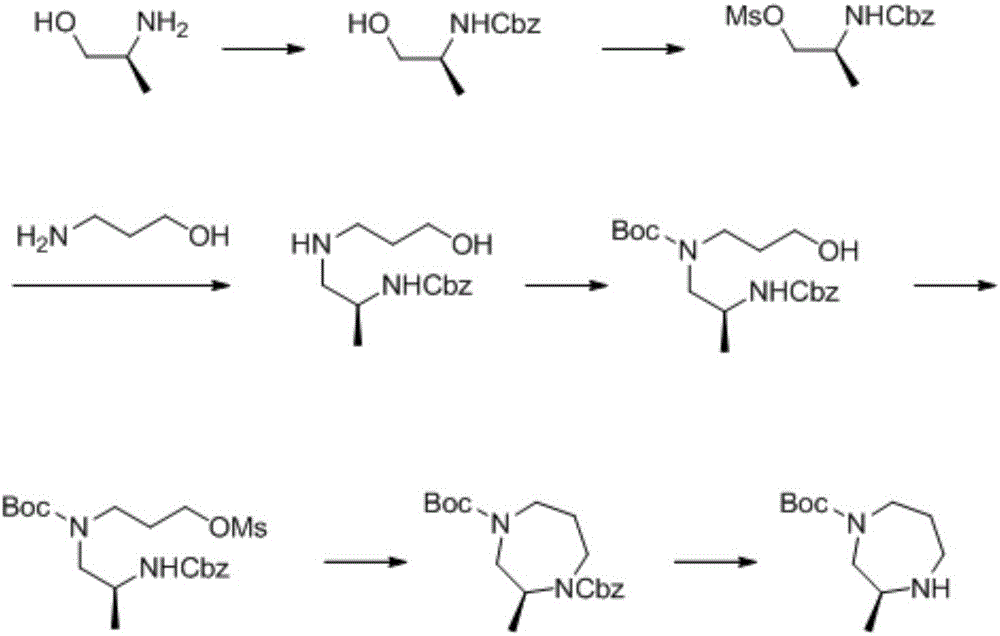
Preparation method of 1,4-dioazo-cycloheptane derivative
The invention discloses a preparation method of a 1,4-dioazo-cycloheptane derivative. The preparation method comprises the steps of carrying out multiple coupling on a compound of a formula (VI) and a compound of a formula (V) at -5-0 DEG C in aromatic hydrocarbon or a halogenated hydrocarbon solvent in the presence of alkali, after the coupling, removing hydroxyl protection from the compound of the formula (VI) by virtue of TBAF, carrying out self-cyclization by virtue of a Mitsunobu reaction to obtain a compound of a formula (II), and removing amino protection from the compound of the formula (II) by virtue of a hydrochloric acid ethyl acetate solution, so as to obtain the target compound 1,4-dioazo-cycloheptane derivative. The preparation method has the advantages that synthetic steps are few, the reaction condition of each step is mild, and the operation is simple convenient, so that the production cost is lowered; and more importantly, the yield of the 1,4-dioazo-cycloheptane derivative synthesized by the method is high.
Owner:WUCHANG UNIV OF TECH +1
Pyridinocycloheptane imine nickel complex catalyst, preparation method and application thereof
ActiveCN105646599AIncrease the degree of branchingNarrow molecular weight distributionNickel organic compoundsBulk chemical productionAluminium chlorideStructural formula
The invention discloses a pyridinocycloheptane imine nickel complex catalyst, a preparation method and application thereof. The structural formula of the complex is shown as formula I, wherein R1 is selected from methyl, ethyl or isopropyl, and R2 is selected from methyl or hydrogen. The preparation method consists of: under room temperature conditions, subjecting V-1 or V-2 shown ligand compound and NiCl2 or NiBr2 to complexation reaction in an organic solvent, thus obtaining a corresponding pyridinocycloheptane imine nickel complex shown as formula I. The invention designs and synthesizes the pyridinocycloheptane imine nickel complex, the series complex can well catalyze ethylene polymerization under the action of cocatalyst ethylaluminum sesquichloride or methylaluminoxane to obtain low molecular weight and narrow molecular weight distribution polyethylene, also has very high activity up to 7.80*10<6>g.mol<-1>(Ni).h<-1> respectively, thus having the potential for industrial application. (formula I).
Owner:INST OF CHEM CHINESE ACAD OF SCI
Acrylic acid recovery utilizing ethyl acrylate and selected co-solvents
A method of recovering acrylic acid from a mixture comprising acrylic acid, water and acetic acid is disclosed, which includes: (a) extracting acrylic acid from the mixture with a solvent mixture comprising ethyl acrylate as,the preponderant component thereof and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to form an extracted composition; and (b) azeotropically distilling the extracted composition to recover acrylic acid. Also disclosed is an alternate method of recovering acrylic acid which includes: (a) providing a feed stream containing acrylic acid, water, acetic acid, ethyl acrylate and an organic co-solvent selected from the group consisting of toluene, heptane, 1-heptene, methylcyclohexane, cycloheptane, cycloheptadiene, cycloheptatriene, 2,4-dimethyl-1,3 pentadiene, methylcyclohexene and methylenecyclohexene to a distillation column, wherein the weight ratio of ethyl acrylate to the organic co-solvent is from about 80:20 to about 95:5; and (b) azeotropically distilling said feed stream to provide an acrylic acid residue stream. A further embodiment of this invention involves directing the recovered acrylic acid stream to a distillation tower wherein a vapor or liquid side stream is obtained having a purity level of acrylic acid of at least 99%. This material can be subsequently further purified to obtain glacial acrylic acid having a purity of at least 99.8%.
Owner:DOW GLOBAL TECH LLC +1
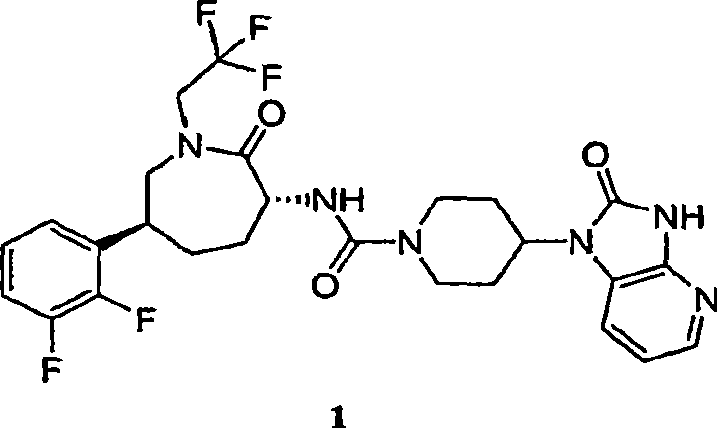
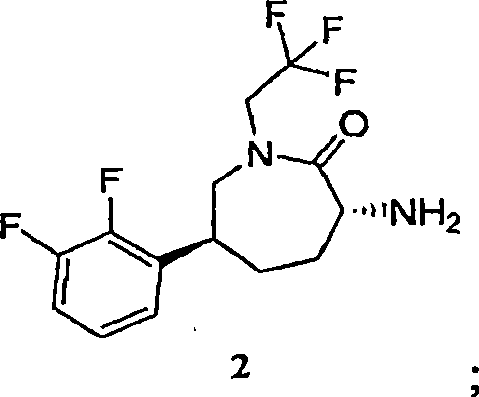
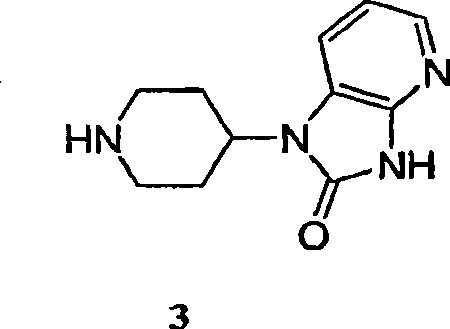
Process for the preparation of cgrp antagonist
An efficient synthesis for the preparation of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine -1-carboxamide, by coupling (3R,6S)-3-amino-6-(2,3-difluorophenyl)-1-(2,2,2-trifluoroethyl)azepan-2-one and 2-oxo-1-(4-piperidinyl)-2,3-dihydro-1H-imidazo[4,5-b]pyridine dihydrochloride with 1,1'-carbonyldiimidazole ('CDI') as carbonyl source; and an efficient preparation of the potassium salt of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine -1-carboxamide.
Owner:MERCK & CO INC
Polyester, preparation method thereof, coating containing polyester, and application of same
The invention discloses polyester, a preparation method of the polyester, a coating containing the polyester and application of the same, and relates to the technical field of coatings. The preparation method comprises the following steps: carrying out a ring-opening reaction on 2-methylene-4-phenyl-1,3-dioxolane with the molar content of 10-20%, 2-methylene-1,3-dioxoheptane with the molar contentof 10-20%, and 3,3,3',3'-tetramethylspirobiphenol with the molar content of 20-40% to obtain a hydroxyl-terminated intermediate; and then reacting with dicarboxylic acid with the molar content of 20-30% and dihydric alcohol with the molar content of 15-30% to obtain the polyester resin; and compounding the resin to obtain the coating applied to a metal coating. The product has good metal adhesion, excellent resistance and outstanding processability.
Owner:SHANGHAI WEIKAI CHEM +2
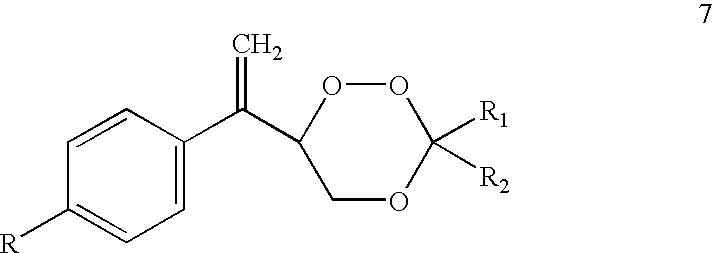
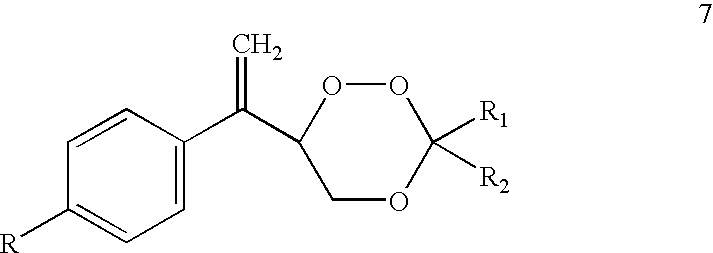
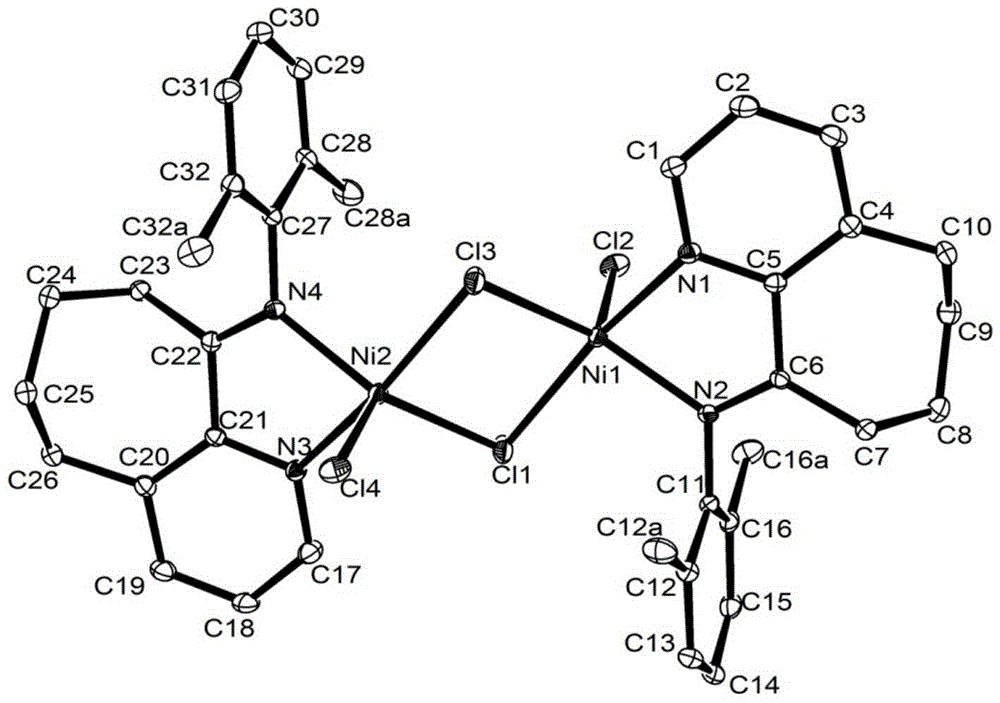
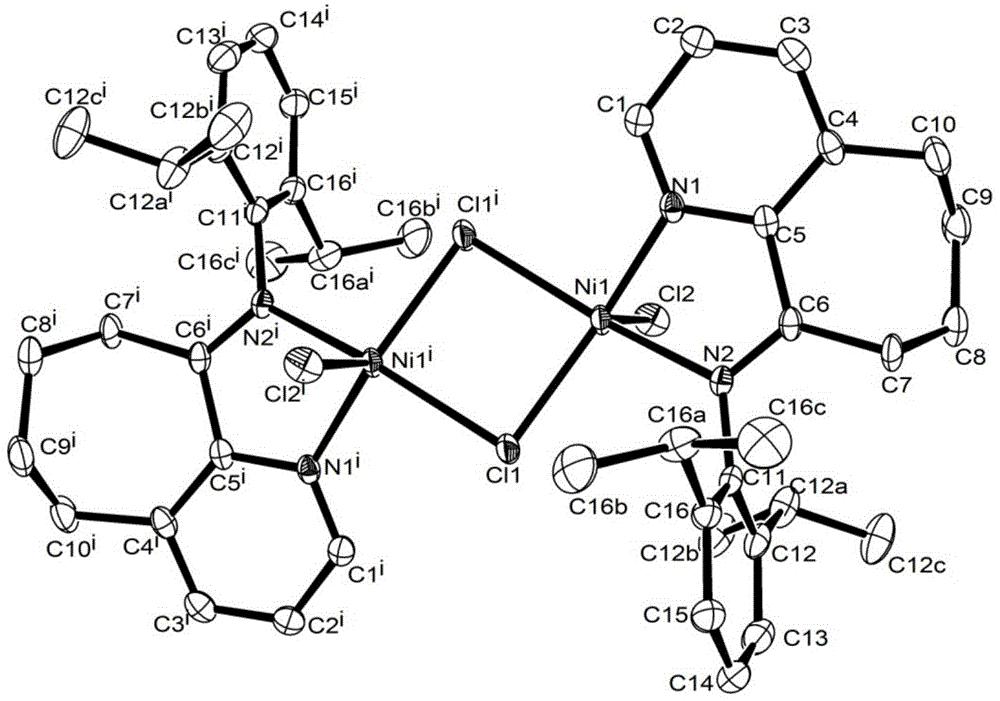
Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof
The invention discloses a diarylo[d,f]oxacycloheptane-3-ketone compound and a synthesis method thereof, relating to an oxacycloheptane-3-ketone compound and a synthesis method thereof. The invention aims to solve the problems that in the prior art, the diarylo[d,f]oxacycloheptane-3-ketone compound is difficult to synthesize, the pollution is serious and the industrial production can not be realized. The structural formula of the diarylo[d,f]oxacycloheptane-3-ketone compound is shown in the specification. The method takes 2-phenylphenoxyacetic acid derivatives or 2-naphthphenoxyacetic acid derivatives as raw materials, and comprises the following steps of: carrying out reaction between trifluoroacetic anhydride and lewis acid as catalysts in an organic solvent; pouring ice water and layering; adjusting the pH value of the organic layer to 8-9 by the aqueous solution of sodium carbonate; washing the organic layer without a water layer by saturated salt water; and concentrating to obtain a solid crude product, and recrystallizing to obtain the diarylo[d,f]oxacycloheptane-3-ketone compound. The method disclosed by the invention is mainly used for synthesizing the diarylo[d,f]oxacycloheptane-3-ketone compound.
Owner:HEILONGJIANG UNIV
Photoresist resin monomer and synthesis method thereof
PendingCN111138281AImprove corrosion resistanceImprove solubilityPreparation from carboxylic acid halidesPhotosensitive materials for photomechanical apparatusChemical synthesisAlkane
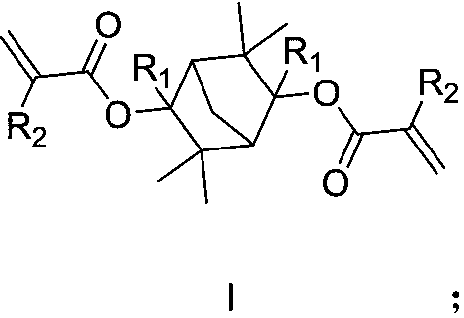
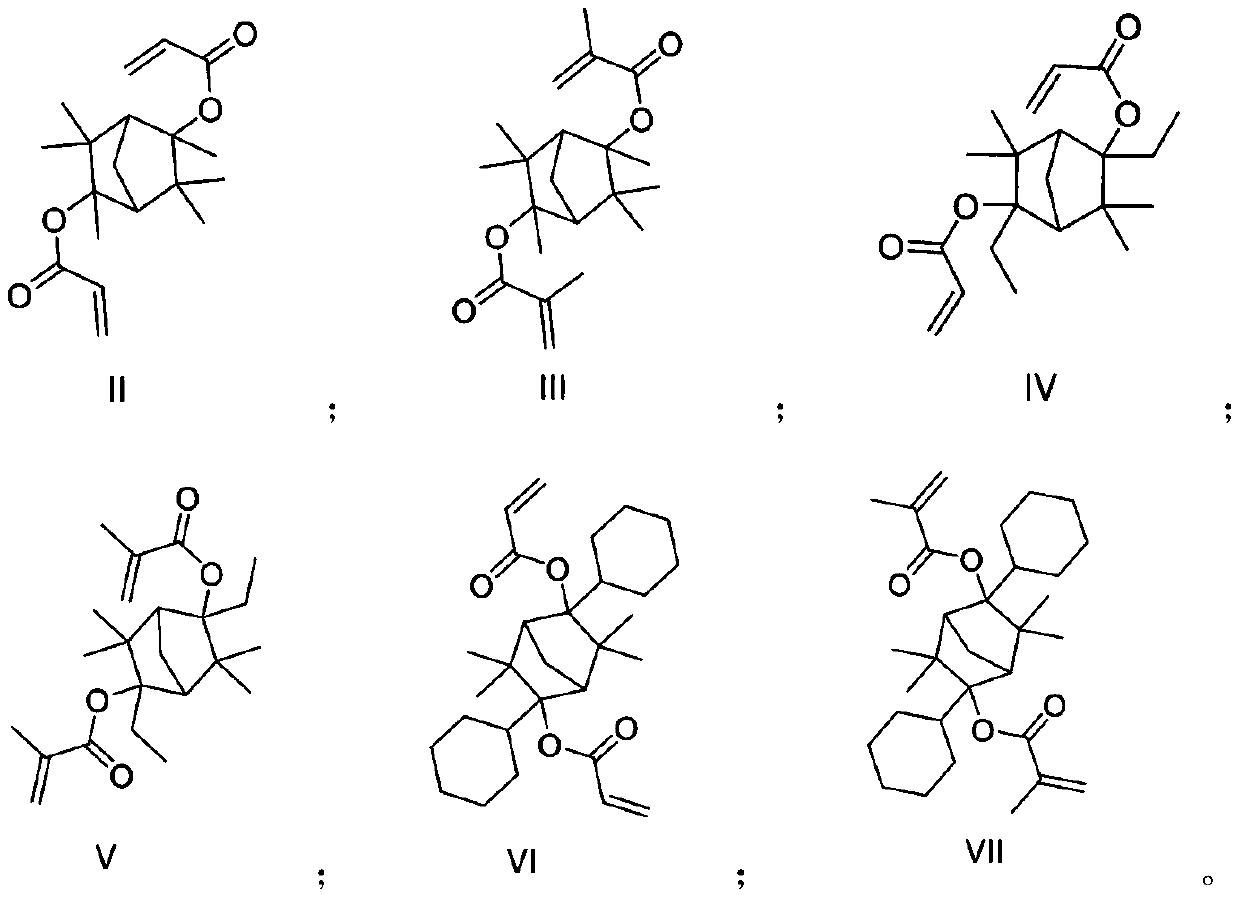
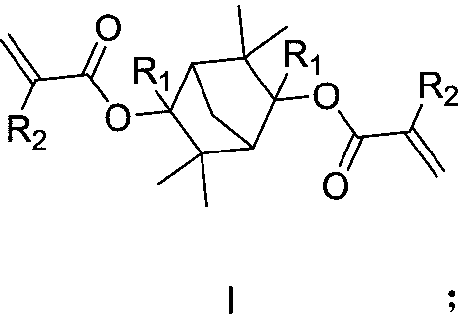
The invention discloses a photoresist resin monomer and a synthesis method thereof, and belongs to the technical fields of chemical synthesis and photoetching. The structural general formula of the photoresist resin monomer is represented by formula I shown in the description; and in the formula I, R1 is saturated alkane or cycloalkane, and R2 is hydrogen or a methyl group. The synthesis method comprises the following steps: carrying out A Grignard reaction on 3,3,6,6-tetramethylbicyclo[2.2.1]heptane-2,5-dione and an alkyl Grignard reagent or a cycloalkyl Grignard reagent under the protectionof an inert gas, adding water for quenching after the Grignard reaction is finished, and carrying out post-treatment purification to obtain an intermediate; and carrying out an esterification reactionon the intermediate and acryloyl chloride or methacryloyl chloride, and carrying out post-treatment purification after the esterification reaction is finished in order to obtain the photoresist resinmonomer. The resin monomer is a degradable resin monomer, and polymer resin containing the resin monomer has good etching resistance and can improve the resolution of photoresist photoetching patterns.
Owner:上海博栋化学科技有限公司
Liquid crystal aligning agent, liquid crystal alignment film and liquid crystal display device
ActiveCN101634778AHigh voltage retentionLow Image Sticking CharacteristicsLiquid crystal compositionsNon-linear opticsCrystallographyImide
The invention relates to a liquid crystal aligning agent, liquid crystal alignment film and liquid crystal display device. The invention provides a liquid crystal aligning agent having a high voltage retention and low image persistence property. The liquid crystal aligning agent comprises a mixture of the following polyamic acid, its imidized polymer or other imidized polymers, in which the proportion of imide coupling units ranges from 5 to 80%. The polyamic acid is obtained by incorporating 1,2,3,4-cyclobutane tetracarboxylic acid dianhydride and 1,4-diaminocyclohexane, bicyclo[2.2.1]heptan-2,6-bis(methylamine), 1,3-bis(aminomethyl)cyclohexane, isophorone or alkyl substituted compounds of these diamines, as at least a part of a tetracarboxylic acid dianhydride and of a diamine compound.
Owner:JSR CORPORATIOON
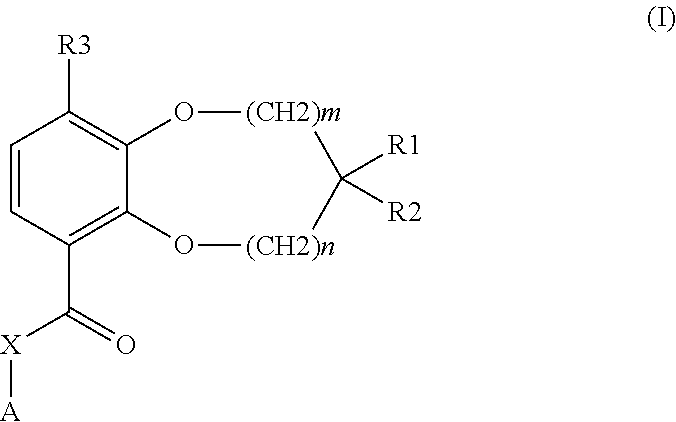
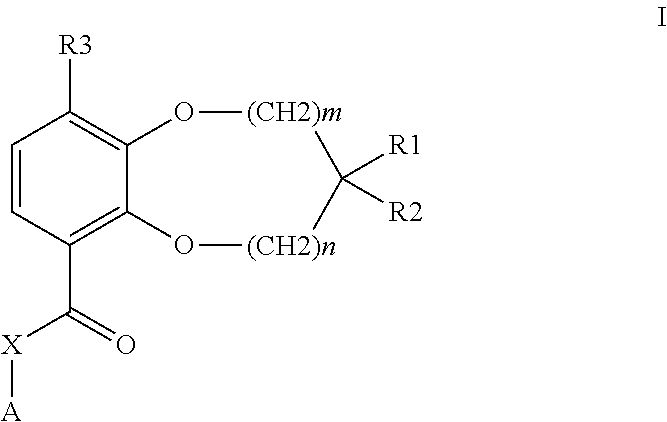
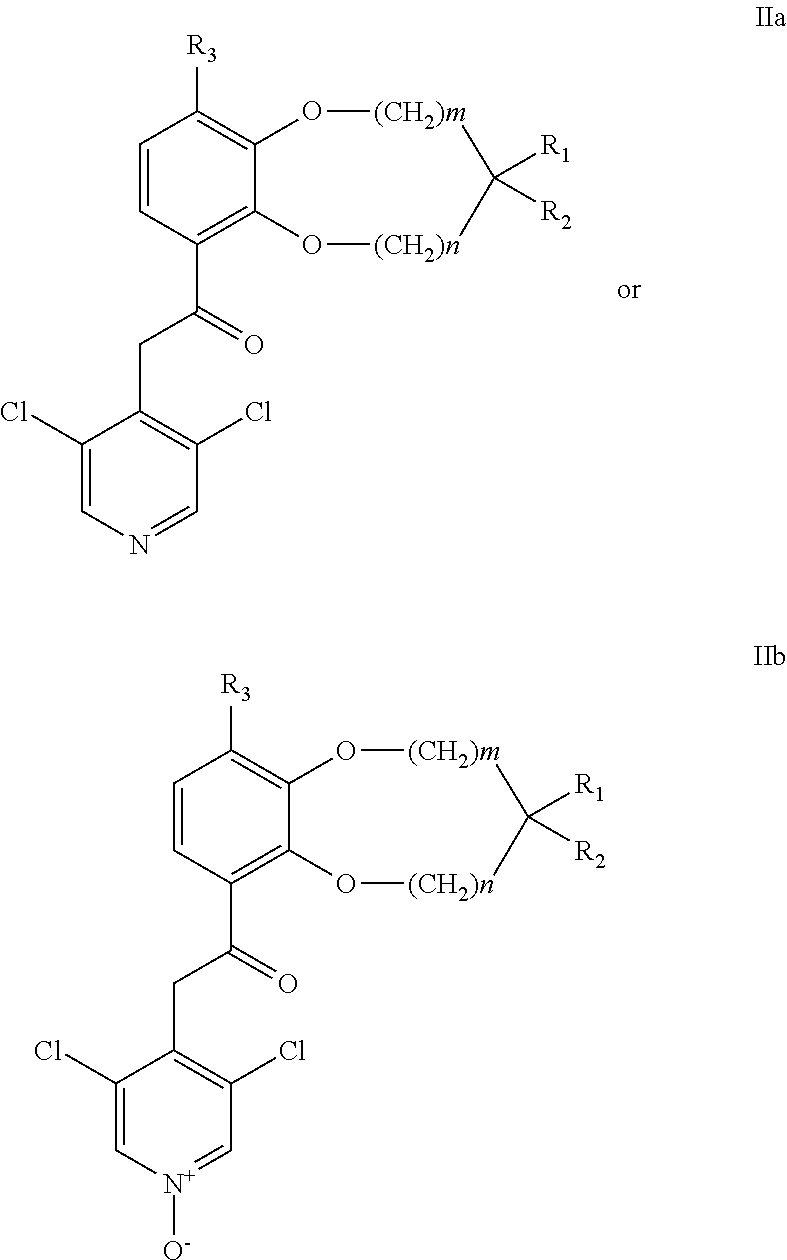
Benzodioxole or benzodioxepine heterocyclic compounds as phosphodiesterase inhibitors
Compounds of the general formula (I) wherein each of m and n is independently 0 or 1; R1 and R2, together with the carbon atom to which they are attached, form a heterocyclic ring comprising one or two heteroatoms selected from oxygen, sulfur, —S(O)— and —S(O)2—; R3 is —CHF2, —CF3, —OCHF2, —OCF3, —SCHF2 or —SCF3; X is a bond, —CH2—, or —NH—; A is aryl, cycloalkyl, cycloalkenyl, arylalkyl, heteroaryl, heteroarylalkyl, heterocycloalkyl or heterocycloalkenyl, optionally substituted with one or more, same or different substituents selected from R4; and R4 is hydrogen, amino, thioxo, alkyl, haloalkyl, hydroxyalkyl, alkoxy, haloalkoxy, halogen, oxo, thia, or hydroxy; or pharmaceutically acceptable salts, hydrates or solvates thereof, have been found to exhibit PDE4 inhibiting activity, and may therefore be useful in the treatment of inflammatory diseases and disorders.
Owner:UNION THERAPEUTICS AS
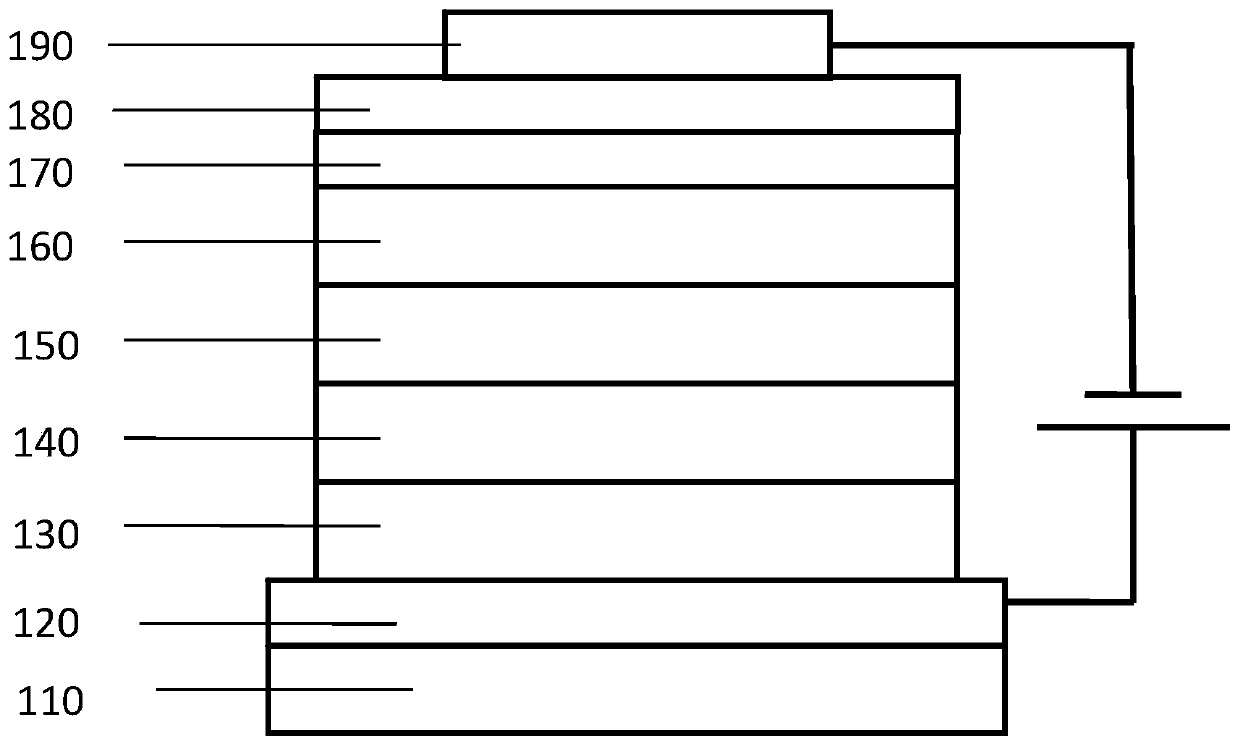
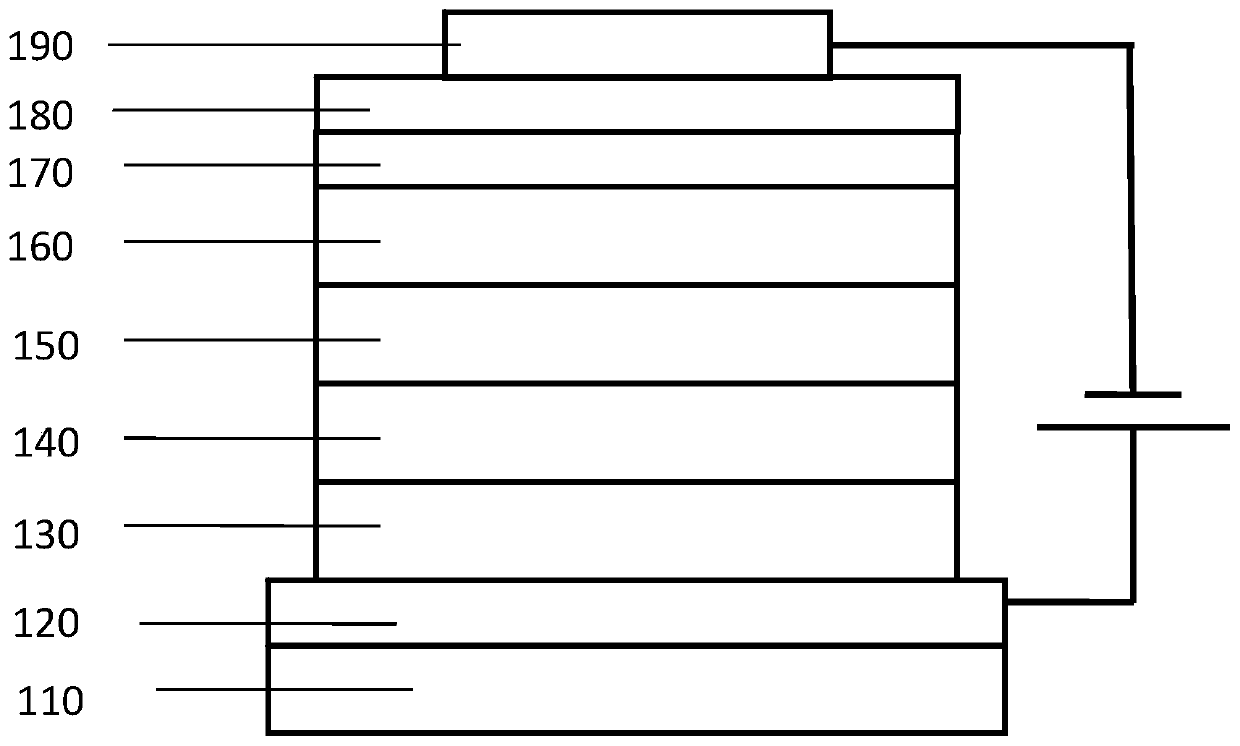
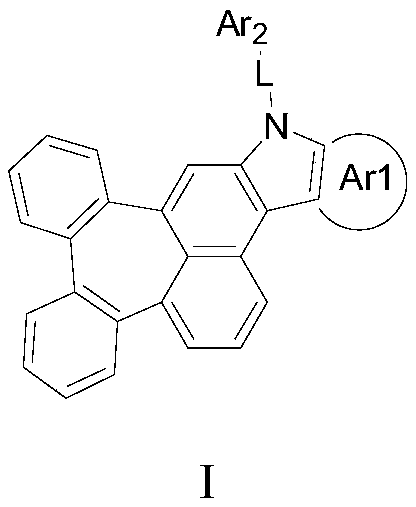
Organic electroluminescent compound containing cycloheptylnaphthalene, application thereof, and light-emitting device
The invention provides an organic electroluminescent compound containing cycloheptylnaphthalene, which is represented as the structure formula in the specification. The compound has good thermal stability, high luminous efficiency and high luminescence purity, and can be applied to the field of organic electroluminescent devices, organic solar cells, organic thin film transistors or organic photoreceptors, etc. The invention also provides an organic light-emitting device, which includes an anode, a cathode and an organic layer containing at least one of a light-emitting layer, a hole injectionlayer, a hole transport layer, a hole blocking layer, an electron injection layer and an electron transport layer. At least one layer among the organic layers contains the compound as the structure formula I. The organic light-emitting device manufactured from the compound has the advantages of high electroluminescence efficiency, excellent color purity and long life.
Owner:SHANGHIA TAOE CHEM TECH CO LTD
Preparation method of (R,S)-2-[[5-(9-fluorenemethoxycarbonylamino)dibenzo[A,D]cycloheptane-2-yl]oxyl]acetic acid
ActiveCN104761470AEasy post-processingReduce manufacturing costCarbamic acid derivatives preparationOrganic compound preparationBenzoic acidEvaporation
The invention relates to a preparation method of Ramage linker and mainly solves problems of long processes, complex post-treatment, much waste water, waste gas and solid waste, and high cost in a conventional synthetic method. The preparation method includes following steps: (A) carrying out a reaction to 2-carboxybenzaldehyde and m-methoxyphenylacetic acid to obtain an intermediate 2-(3-methoxylstyryl)benzoic acid, dissolving the intermediate with a solvent, performing hydrogenation reduction, and performing post-treatment crystallization to obtain a compound R-1; (B) carrying out a reaction to the R-1 with SOCl2 or POCl3 to obtain 2-methoxyl-10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-one, performing negative-pressure evaporation to remove the SOCl2 or the POCl3, dissolving the 2-methoxyl-10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-one in benzene, methylbenzene or 1,2-dichloroethane, performing a catalytic reaction with anhydrous AlCl3 and performing post-treatment crystallization to obtain a compound R-2; (C) carrying out a reaction to the R-2 with benzyl bromoacetate in DMF or an acetone / K2CO3 solution to obtain a compound R-3; (D) performing hydrogenation reduction to the R-3 to obtain a compound R-4; and (E) adding a catalyic amount of PTS to the R-4 in DMF and carrying out a reaction to the R-4 with Fmoc-NH2 to obtain the Ramage linker, which is an effective C-terminal linker in solid-phase synthesis.
Owner:江苏吉泰肽业科技有限公司
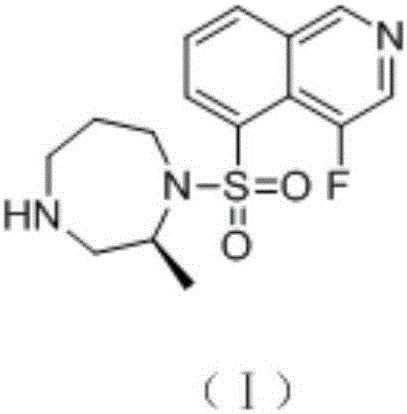
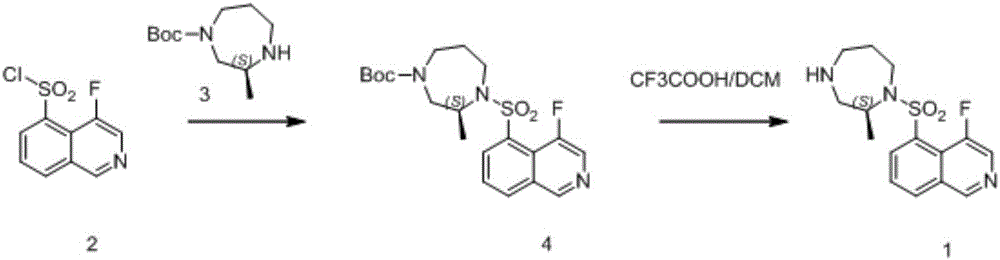
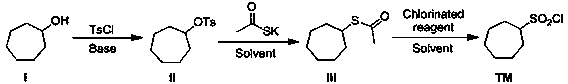
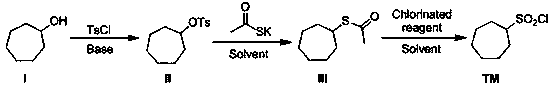
Synthetic method of macrocyclic inhibitor intermediate cycloheptane sulfonyl chloride
InactiveCN110483338AImprove responseRaw materials are easy to getSulfonic acid esters preparationSulfonic acid preparationSulfonyl chlorideSulfonate
The invention discloses a synthetic method of a macrocyclic inhibitor intermediate cycloheptane sulfonyl chloride. The synthetic method comprises the following steps of 1, reacting a compound I cycloheptanol with p-toluenesulfonyl chloride to obtain a compound II cycloheptane 4-methyl benzene sulfonate; 2, reacting a compound II with the potassium thioacetate to obtain a compound III cycloheptaneethane sulfate; and 3, oxidizing the compound III to obtain the compound cycloheptane sulfonyl chloride. The synthetic method of the macrocyclic inhibitor intermediate cycloheptane sulfonyl chloride provided by the invention is simple in reaction, simple and convenient in post-treatment, high in yield, low in cost and strong in operability, can obtain the raw materials easily, and is suitable forthe industrial production.
Owner:苏州汉德创宏生化科技有限公司
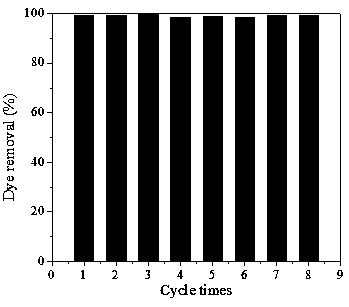
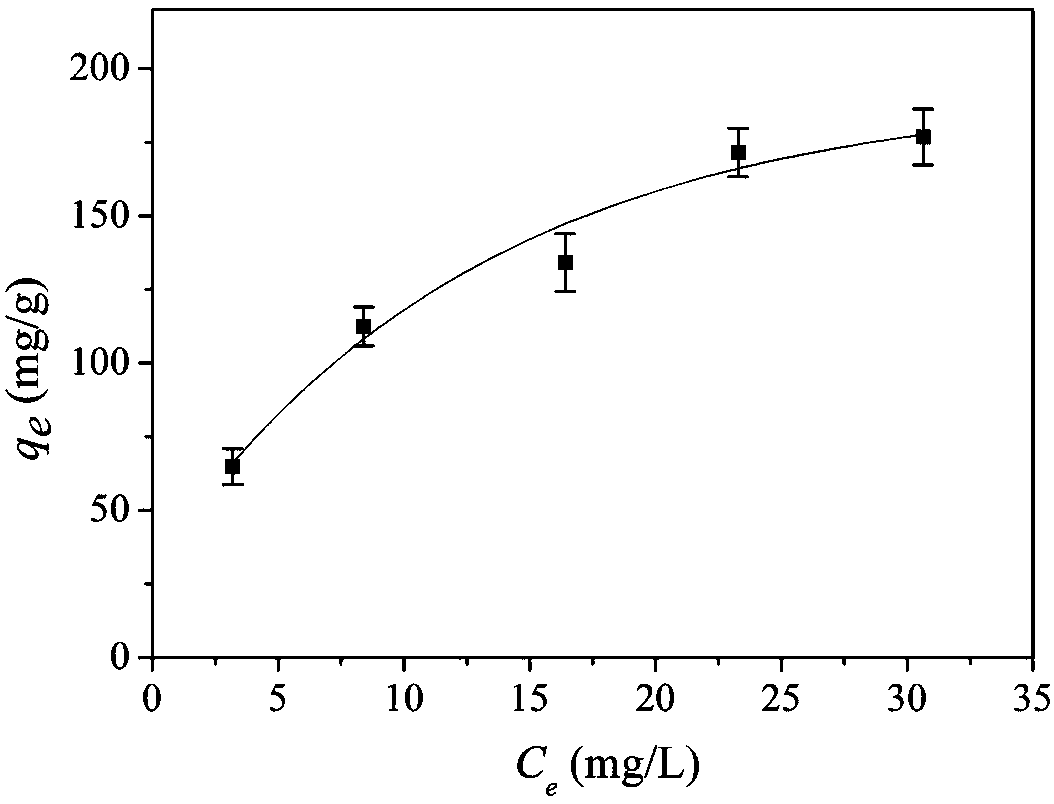
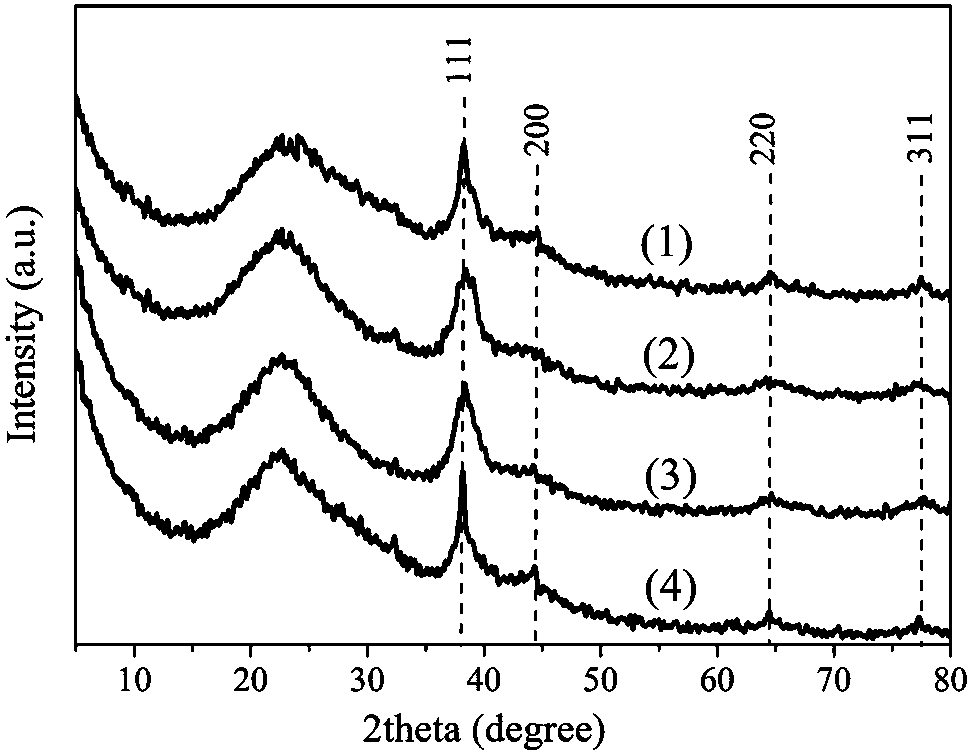
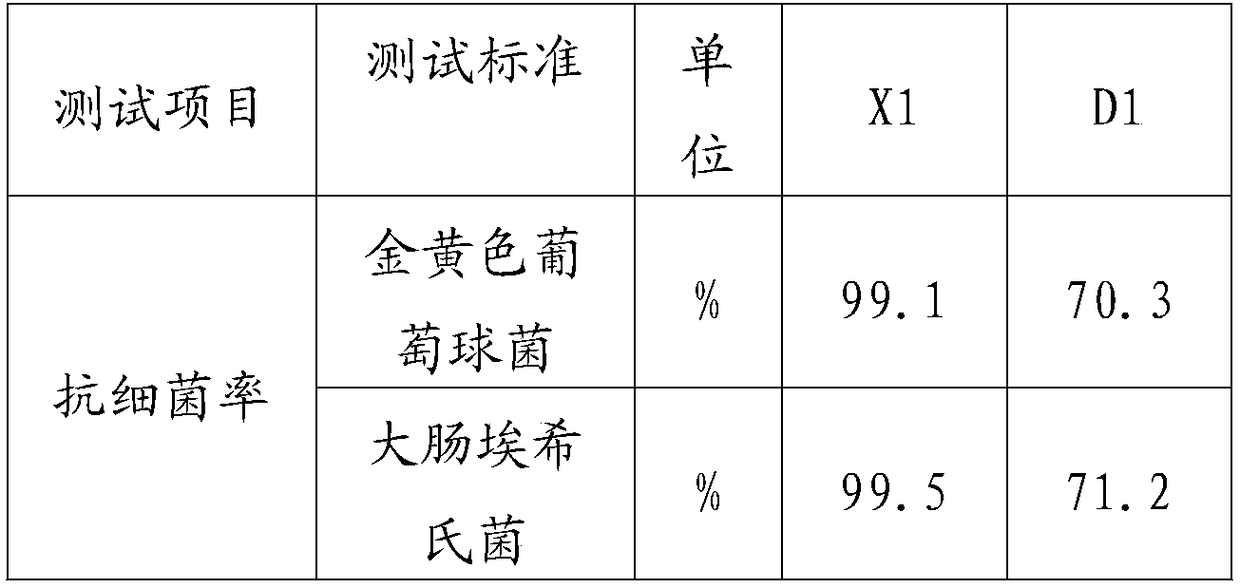
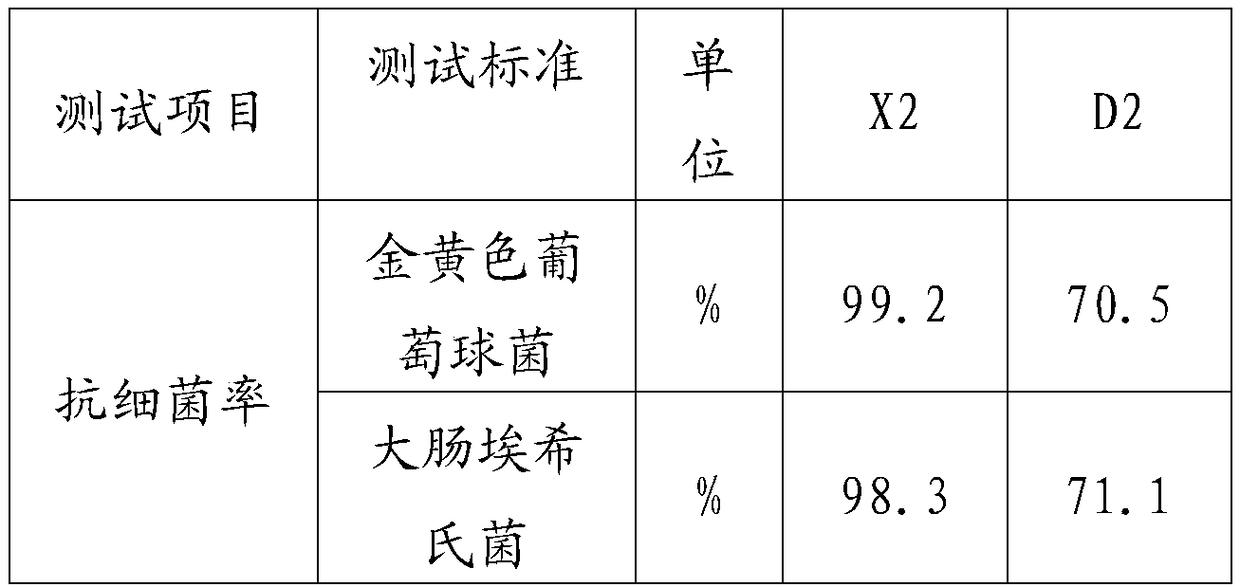
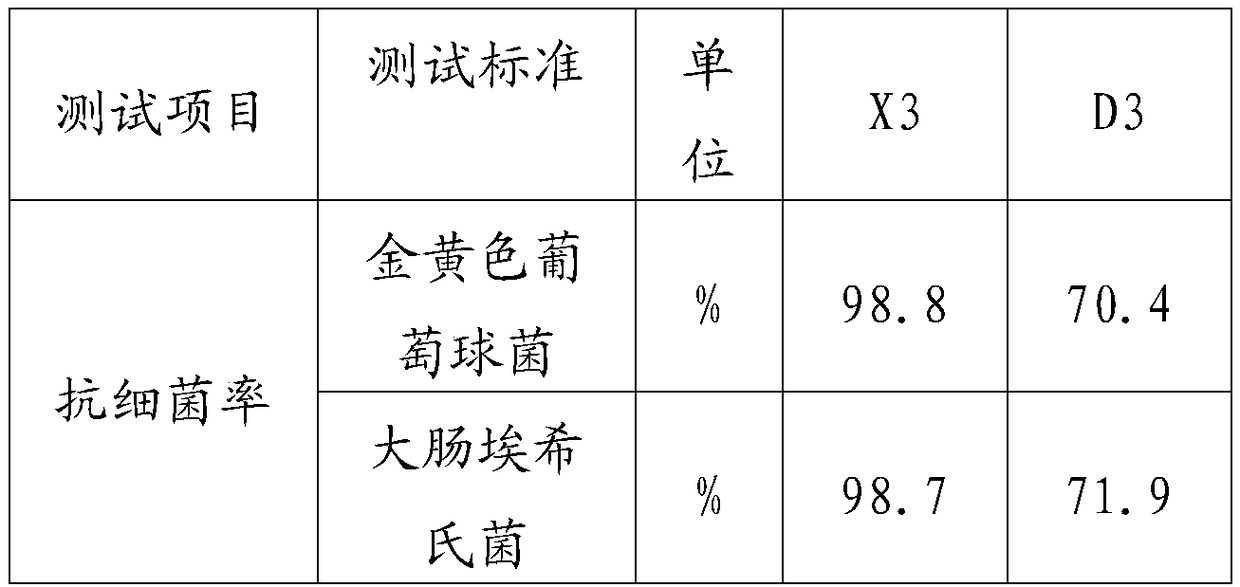
Adsorbent for eliminating methylene blue in water as well as preparation method and application thereof
ActiveCN108393062AWell-developed pore structureImprove performanceOther chemical processesWater contaminantsSilicic acidSorbent
The invention discloses an adsorbent for eliminating methylene blue in water as well as a preparation method and application thereof. The adsorbent comprises a nanometer silver oxide loaded amorphoussilicon oxide material. The preparation method of the adsorbent comprises the following steps of (1) preparation of amorphous silicon oxide carrier materials: mixing cetyl trimethyl ammonium bromide and sodium hydroxide solution; performing stirring at a certain temperature; then, adding a mixture of ethyl orthosilicate, cyclopentane or cycloheptane; continuously performing stirring, centrifugation, washing and drying to obtain the amorphous silicon oxide; (2) preparation of silver oxide loaded amorphous silicon oxide material: adding silver nitrate ethanol solution and ammonium hydroxide; performing ultrasonic treatment, centrifugation, washing and drying to prepare the nanometer oxide silver loaded amorphous silicon oxide material. The method has the advantages that the preparation process is simple; the prepared adsorption material has relatively strong adsorption removal capability on methylene blue; the methylene blue in the waste water can be effectively eliminated; in addition,the adsorption material can realize the regeneration cyclic utilization.
Owner:CHINA UNIV OF MINING & TECH
Preparation method of loratadine
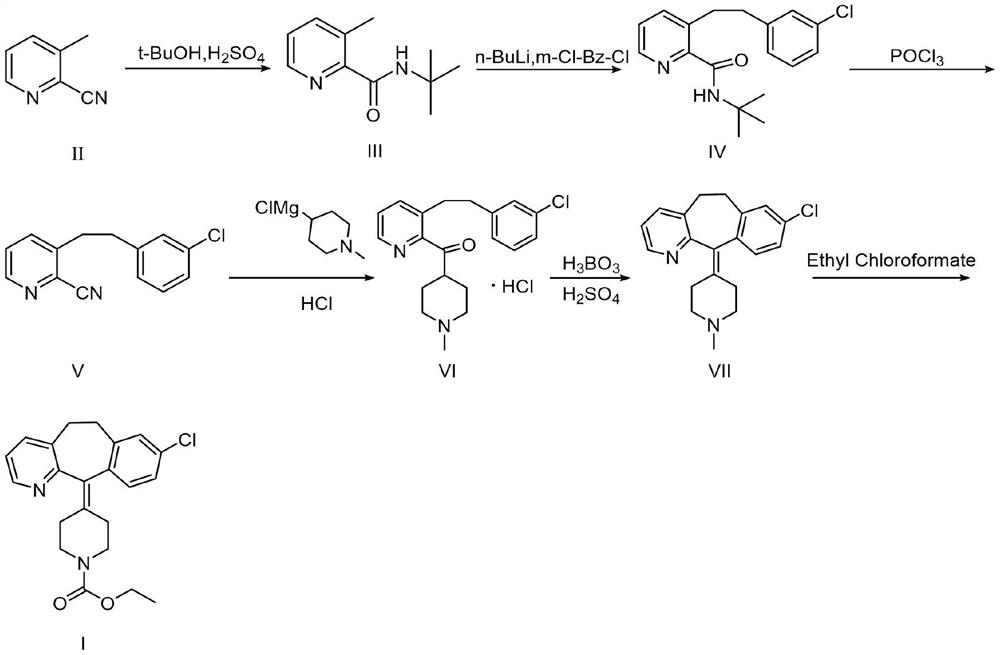
The invention provides a preparation method of loratadine. The method comprises the following steps: taking 2-cyano-3-methylpyridine as a raw material, and carrying out Ritter reaction, m-chlorobenzylchloride condensation, POCl3 deprotection group, Grignard reaction, cyclization and ethyl chloroformate substitution to obtain 4(8-chlorine-5, 6-dihydro-11H-benzo-[5, 6]cycloheptano[1, 2-b]pyridine-11-subunit)-1-piperidine carboxylic acid ethyl ester. According to the invention, a post-treatment process is innovated, and a new cyclization system is adopted to catalyze the reaction, so that the use of high-cost and high-toxicity strong acid is avoided, and a milder and more economical synthesis method is provided for industrial production.
Owner:CHENGDU UNIV
Preparation method of novel antibacterial agent
The invention relates to a preparation method of a novel antibacterial agent. A certain amount of yttria, a certain amount of deionized water and a certain amount of nitric acid are weighed, and Y(NO3)3*6H2O is formed; a certain amount of nonylphenol polyoxyethylene ether, a certain amount of glycerin, a certain amount of cycloheptane and a certain amount of Y(NO3)3*6H2O are weighed, and a solution A is formed; a certain amount of nonylphenol polyoxyethylene ether, a certain amount of glycerin, a certain amount of cycloheptane, a certain amount of 5- chloro-7-hydroxyquinoline and a certain amount of sodium o-hydroxyphenylacetate are weighed, and a solution B is formed; a certain amount of the solution A, a certain amount of the solution B and a certain amount of tetraethoxysilane are weighed, and the antibacterial agent is obtained. According to the technical scheme, the antibacterial performance of a polyolefin composite is improved through addition of the specially-made antibacterialagent, and the antibacterial agent has the great promotional value.
Owner:ANHUI JIANGHUAI AUTOMOBILE GRP CORP LTD
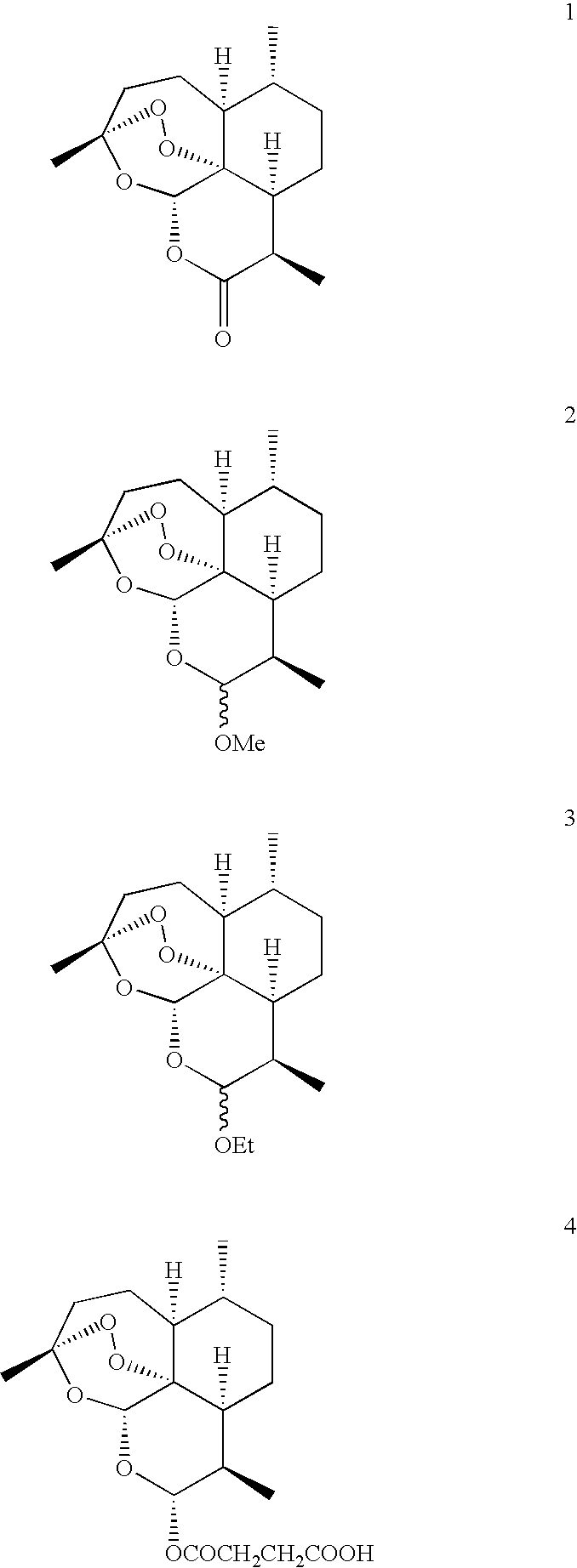
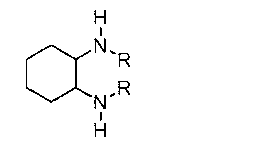
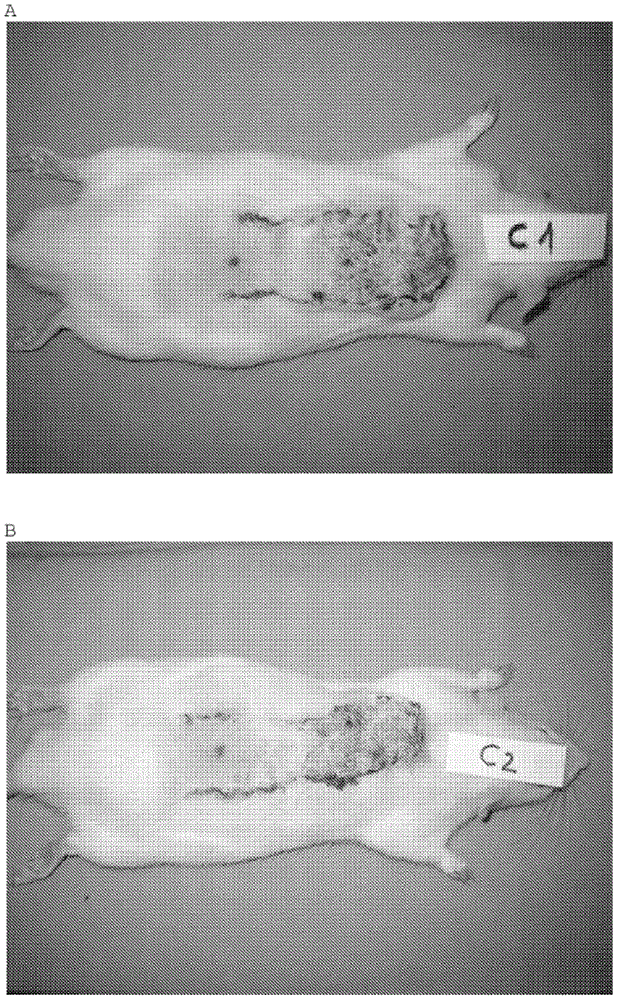
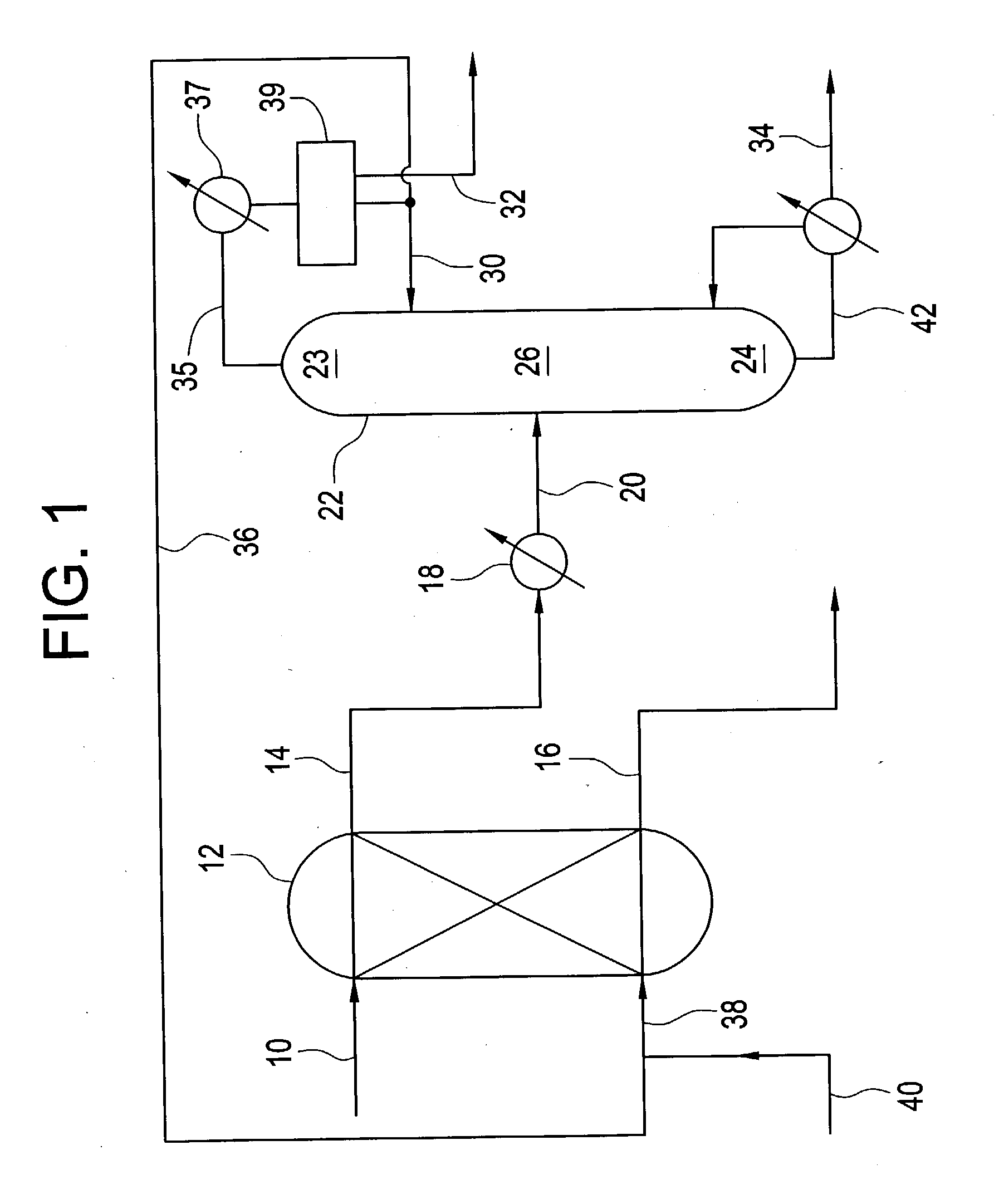
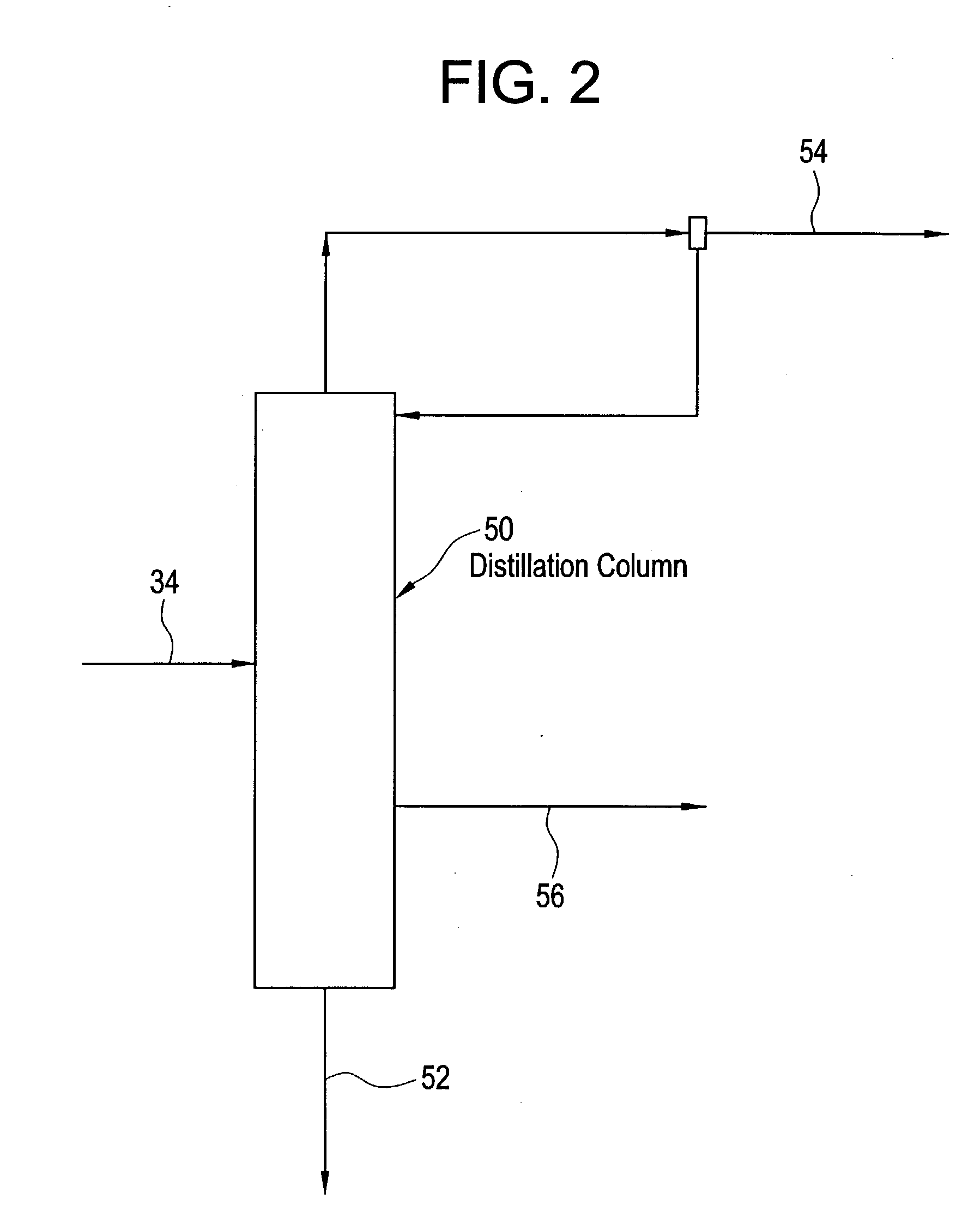
Bicyclo[4.1.0]heptane nitrosourea derivative for biological orthogonal reaction, and preparation method and application thereof
ActiveCN111138337ARelease irreversibleImprove practicalityOrganic chemistryFluorescence/phosphorescenceNitrosoNitrourea
The invention discloses a bicyclo[4.1.0]heptane nitrosourea derivative for a biological orthogonal reaction, and a preparation method and an application thereof. The structural formula of the bicyclo[4.1.0]heptane nitrosourea derivative is represented by formula I; and in the formula I, R represents an alkyl group with the carbon atom number of 1-15, an aryl group with the carbon atom number of 6-15 or H, X represents O or N, and n is a natural number between 1 and 10. A pH-initiated IEDDA reaction of trans-cycloheptene and tetrazine is provided for the first time, and can be used as a biological orthogonal reaction. A bicyclonitrosourea derivative (BNU)-bicyclo[4.1.0]heptane nitrosourea derivative is introduced as a precursor for storing and releasing trans-cycloheptene, so that the highactivity of the trans-cycloheptene is utilized, and the instability of the trans-cycloheptene is avoided as much as possible. The pH-initiated biological orthogonal reaction can be applied to in-vitroprotein labeling, living cell imaging and tissue pre-labeling imaging, and can also be applied to living animal tumor pre-labeling imaging. The novel biological orthogonal reaction has a wide application prospect.
Owner:TSINGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
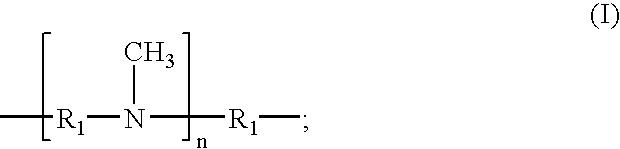

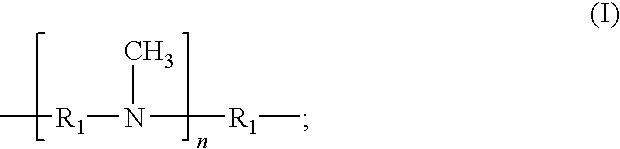




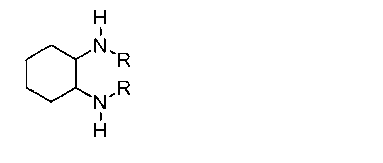
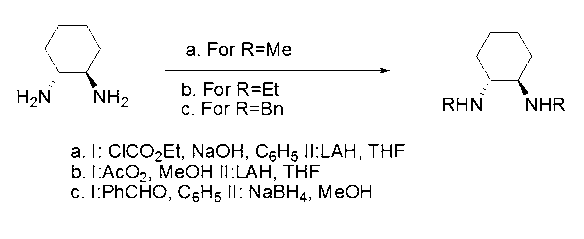


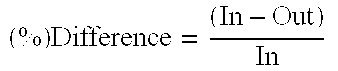



![Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/a8fd5aaa-3050-4717-8dcc-739ac7722b10/HDA00001906852900011.png)
![Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/a8fd5aaa-3050-4717-8dcc-739ac7722b10/HDA00001906852900021.png)
![Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof Diarylo[d,f]oxa-cycloheptane-3-ketone compound and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/a8fd5aaa-3050-4717-8dcc-739ac7722b10/HDA00001906852900031.png)
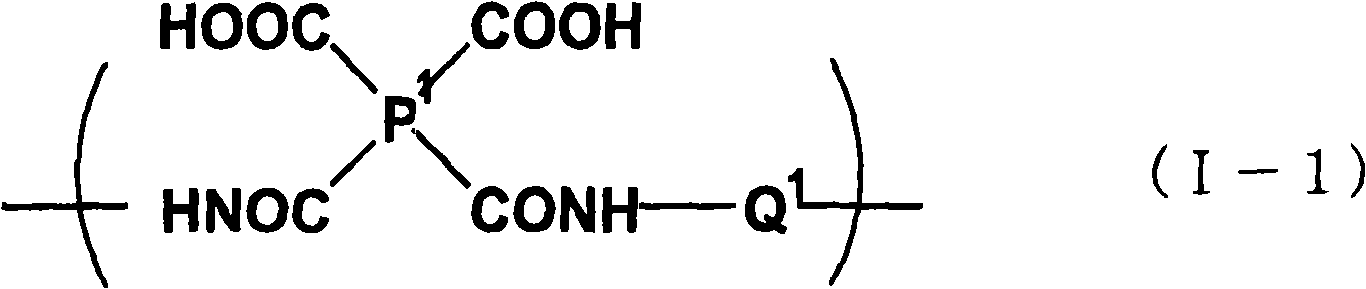

![Bicyclo[4.1.0]heptane nitrosourea derivative for biological orthogonal reaction, and preparation method and application thereof Bicyclo[4.1.0]heptane nitrosourea derivative for biological orthogonal reaction, and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/9202d0a4-7654-4e1d-9590-0509d00a81f7/HDA0002337889110000011.png)
![Bicyclo[4.1.0]heptane nitrosourea derivative for biological orthogonal reaction, and preparation method and application thereof Bicyclo[4.1.0]heptane nitrosourea derivative for biological orthogonal reaction, and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/9202d0a4-7654-4e1d-9590-0509d00a81f7/HDA0002337889110000021.png)
![Bicyclo[4.1.0]heptane nitrosourea derivative for biological orthogonal reaction, and preparation method and application thereof Bicyclo[4.1.0]heptane nitrosourea derivative for biological orthogonal reaction, and preparation method and application thereof](https://images-eureka.patsnap.com/patent_img/9202d0a4-7654-4e1d-9590-0509d00a81f7/HDA0002337889110000022.png)