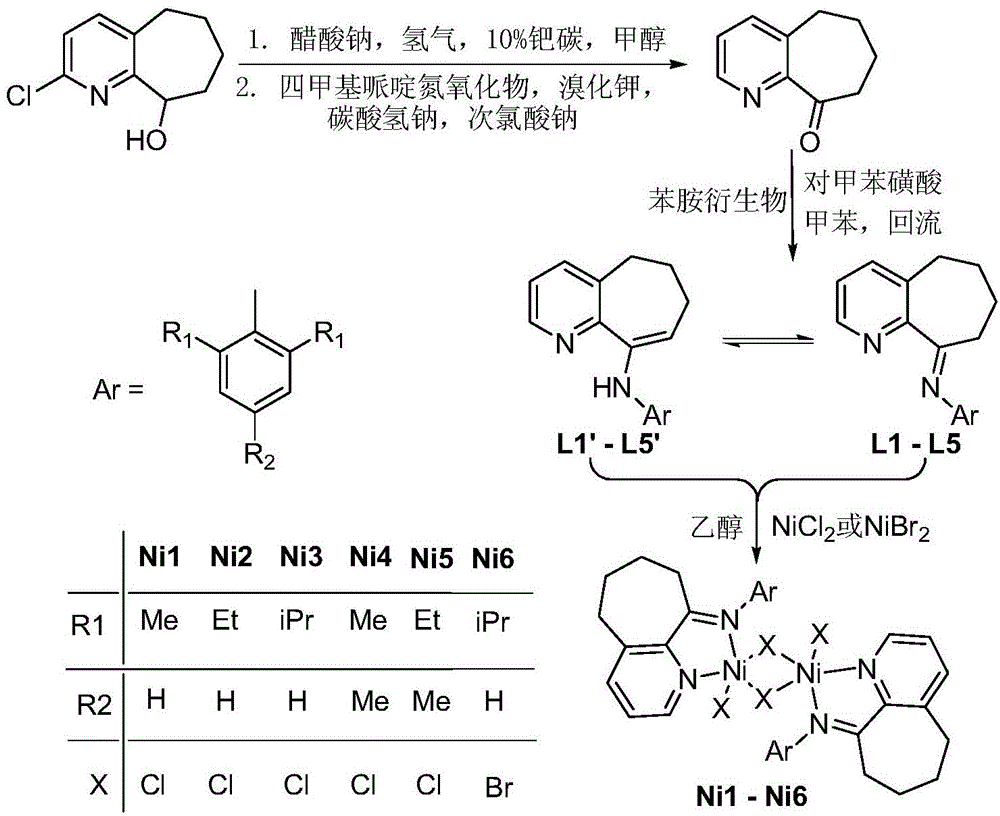
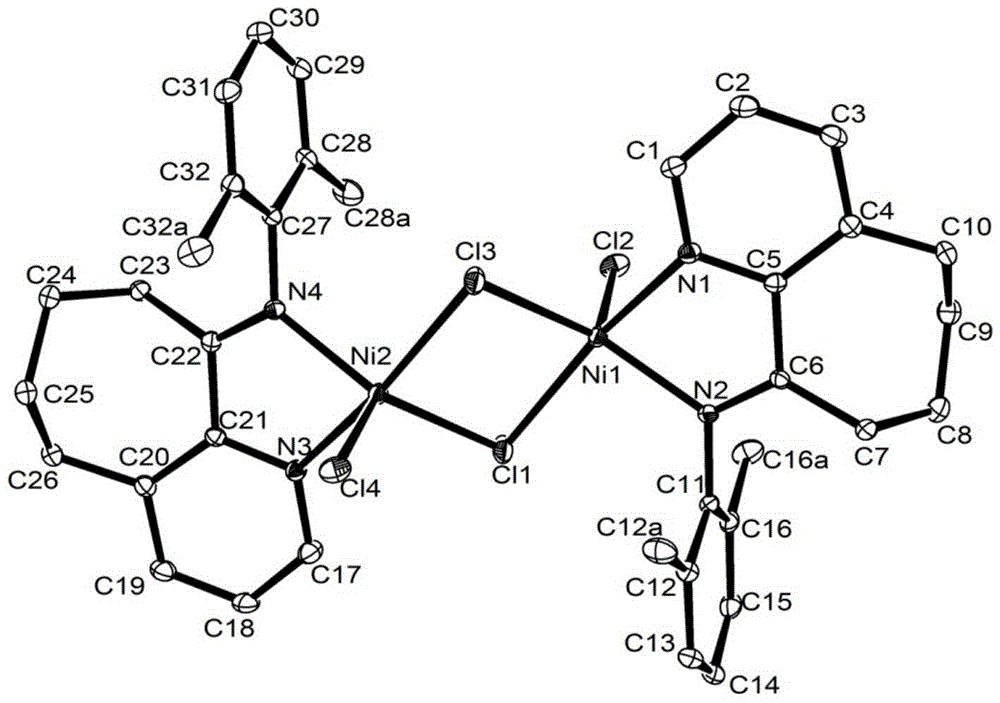
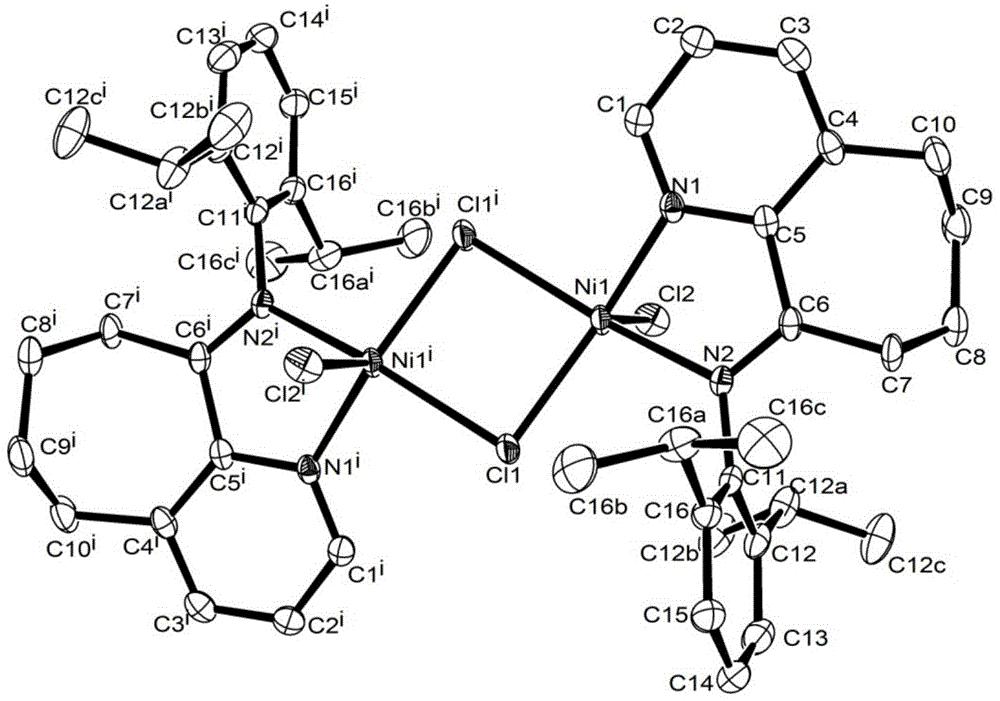
Pyridinocycloheptane imine nickel complex catalyst, preparation method and application thereof
A complex and catalyst technology, which is applied in the production of nickel organic compounds and bulk chemicals, can solve the problems of restricting the development of basic research, poor thermal stability of complexes, and affecting the application of catalysts, and achieve high catalytic activity, narrow molecular weight distribution, The effect of a wide range of industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1, prepare the synthetic method of 5,6,7,8-tetrahydrocycloheptapyridin-9-one:
[0079] Add 8.03g (40.6mmol) formula II compound in 250ml stainless steel kettle, sodium acetate 3.83g (46.7mmol), 10% palladium carbon 0.80g, methyl alcohol 35ml, after hydrogen replacement three times, hydrogen pressure is added to 1.0MPa, magnetic force stirs, pass 60°C water bath, after 12 hours of reaction, the pressure gauge index did not drop, and the reaction was stopped. The reaction solution was filtered (suction filtration under reduced pressure) to obtain 6.30 g of solid. The solid is transferred to a 250ml jacketed bottle and added, dissolved with 70ml of dichloromethane, filtered to remove insoluble matter, and then 70ml of an aqueous solution of 10.08g (120mmol) sodium bicarbonate, 0.95g of potassium bromide (20mol%), and 0.95g of potassium bromide (20mol%) are added successively and 0.06 g of tetramethylpiperidine nitrogen oxide (1 mol%), mechanically stirred, and ...
Embodiment 2
[0083] Embodiment 2, the synthetic method of preparing (E)-2,6-diisopropyl-N-(5,6,7,8-tetrahydrocycloheptanepyridin-9-ene)aniline (L3, formula R in V 1 =i-Pr;R 2 = Compound of H):
[0084] In the compound (0.16g, 1mmol) shown in formula III and the chlorobenzene (20mL) solution of 2,6-diisopropylaniline (0.20g, 1.1mmol) shown in formula IV, add the p-toluenesulfonic acid catalyst of 30mg , stirred at 120° C. under reflux for 12 h, concentrated under reduced pressure, separated on a silica gel column, and eluted the target product with an eluent ratio of ethyl acetate:triethylamine:petroleum ether=1:1:50 to obtain 0.27g yellow oily substance, namely the pyridine enamine ligand compound L3 (wherein, R 1 is isopropyl, R 2 For hydrogen), the yield was 83.0%.
[0085] The structural confirmation data are as follows:
[0086] 1 H-NMR (400MHz; CDCl 3;TMS): δ8.66(d, J=4.4Hz, 1H, L3-Py-H), 8.54(d, J=4.4Hz, 1H, L3'-Py-H), 7.51(d, J=7.8 Hz,1H,L3-Py-H),7.34(d,J=6,8Hz,1H,L3'-Py-H)...
Embodiment 3
[0091] Example 3, the synthetic method for preparing (E)-2,6-dimethyl-N-(5,6,7,8-tetrahydrocycloheptanepyridin-9-ene)aniline (L1, formula V Middle R 1 = Me; R 2 = Compound of H):
[0092] Using the same method as in Example 1, only the 2,6-diisopropylaniline described in Example 1 was replaced by 2,6-dimethylaniline to obtain a yellow oily substance, which is pyridineenamine belonging to formula V Ligand compound L1 (wherein, R 1 = Me; R 2 =H), yield 75.0%.
[0093] The structural confirmation data are as follows:
[0094] 1 H-NMR (400MHz; CDCl 3 ;TMS): δ8.65(d, J=4.4Hz, 1H, L1-Py-H), 8.53(d, J=4.8Hz, 1H, L1'-Py-H), 7.51(d, J=7.8 Hz,1H,L1-Py-H),7.26(t,J=4.4Hz,1H,L1-Ar-H),7.15(t,J=4.8Hz,1H,L1'-Ar-H),7.12( d,J=5.2,2H,L1'-Ar-H),7.05(d,J=7.6Hz,2H,L1-Ar-H),6.92(t,J=7.8Hz,1H,L1-Py-H ), 6.15(s, 1H, L1'-NH), 4.56(t, J=6.8Hz, 1H, L1'-CH-), 2.87(t, J=6.0Hz, 2H, L1-CH 2 -),2.67(t,J=6.4Hz,2H,L1'-CH 2 -),2.35(s,6H,L1'-2xCH 3 ), 2.31(t, J=6.0Hz, 2H, L1-CH 2 -),2.16(s,6H,L1-2xC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Branching factor | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| Branching factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com