Synthetic method of macrocyclic inhibitor intermediate cycloheptane sulfonyl chloride
A technology of cycloheptanesulfonyl chloride and a synthesis method, which is applied in the chemical field, can solve problems such as easy pollution of the environment, high economic cost, and low yield, and achieve the effects of strong operability, simple post-treatment, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
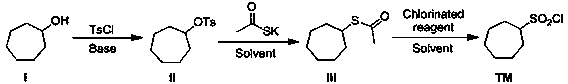
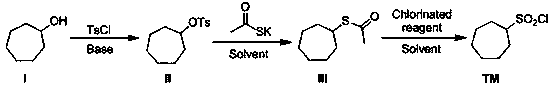
[0021] A synthetic method of macrocyclic inhibitor intermediate cycloheptanesulfonyl chloride, comprising the following reaction steps:
[0022]
[0023] Specifically: the first step: compound I cycloheptyl alcohol is reacted with p-toluenesulfonyl chloride in the first reaction solvent under the action of an acid-binding agent to obtain compound II cycloheptyl 4-methylbenzenesulfonate;
[0024] Second step: Substitution reaction occurs between compound II and potassium thioacetate in the second reaction solvent to generate compound III cycloheptylethane sulfate;
[0025] The third step: the compound III is oxidized with the chlorination reagent in the third reaction solvent to obtain the desired compound cycloheptanesulfonyl chloride.
[0026] In the first step, the first reaction solvent is one or more of dichloromethane, acetone, 1,4-dioxane, tetrahydrofuran, toluene, MTBE, and the acid-binding agent is selected from organic bases, and the acid-binding agent is One or m...
Embodiment 1
[0031] A synthetic method of macrocyclic inhibitor intermediate cycloheptanesulfonyl chloride, comprising the following reaction steps:
[0032] Step 1: Add compound I cycloheptanol (23 g, 0.20 mol, 1.0 eq.) and tetrahydrofuran (120 mL, 6 v / w) and dry pyridine (48 g, 0.60 mol, 3 eq.), under stirring, lower the temperature of the reaction system to 0°C, and slowly add p-toluenesulfonyl chloride (38 g, 0.20 mol, 1.0eq.) in the temperature range of 0~10°C , the dropwise addition is completed in half an hour; maintain the reaction temperature at 0~10°C for 2 h; at 0~-10°C, use 6% HCl aqueous solution to adjust the pH of the reaction solution to 1~2 or adjust to no pyridine odor, and use 150 Extract with ethyl acetate, separate the water phase, wash the organic phase with 200 mL of saturated aqueous sodium bicarbonate solution, 200 mL of brine, dry with 50 g of anhydrous sodium sulfate, filter and concentrate to obtain 44 g of cycloheptyl 4-methylbenzenesulfonate The base ester is...
Embodiment 2
[0036]Step 1: Add cycloheptanol (20 g, 0.17 mol, 1.0 eq.) and dichloromethane (160 mL, 8 v) and dry triethylamine (34 g, 0.34 mol, 2.0 eq.). Under stirring, the temperature of the reaction system was lowered to 0°C, and p-toluenesulfonyl chloride (48.6 g, 0.25 mol, 1.5eq.) was slowly added dropwise within the temperature range of 0-10°C, and the addition was completed in half an hour. Maintain the reaction temperature at 0-10°C for 3 h. At 0~-10°C, use 6% HCl aqueous solution to adjust the pH to 1~2 or adjust to no pyridine odor, extract with 140 mL ethyl acetate, separate the water phase, and use 150 mL saturated sodium bicarbonate for the organic phase Aqueous solution, washed with 150 mL of brine, dried with 40 g of anhydrous sodium sulfate, filtered and concentrated to obtain 40 g of cycloheptyl 4-methylbenzenesulfonate as a colorless liquid with a purity of 98% and a yield of 85%.
[0037] Step 2: In a 500 mL three-neck flask, first add potassium thioacetate (51 g, 0.45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com