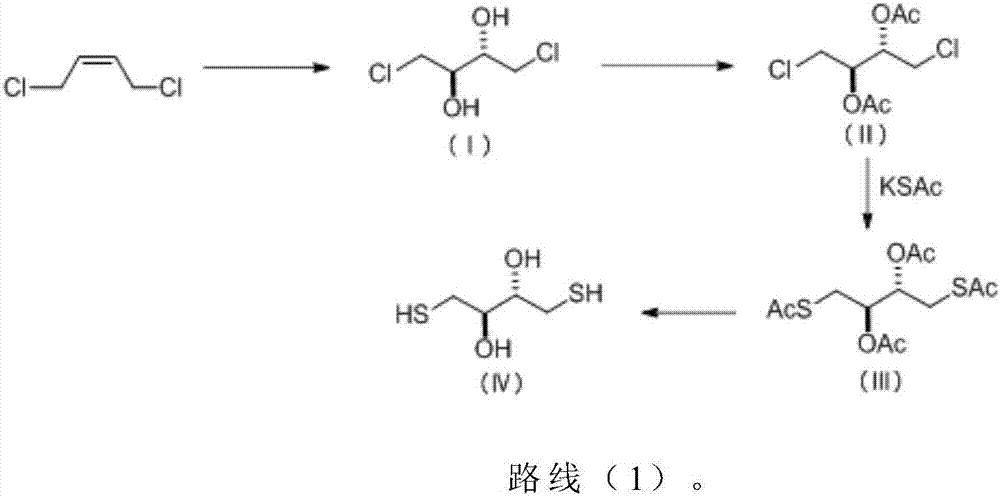
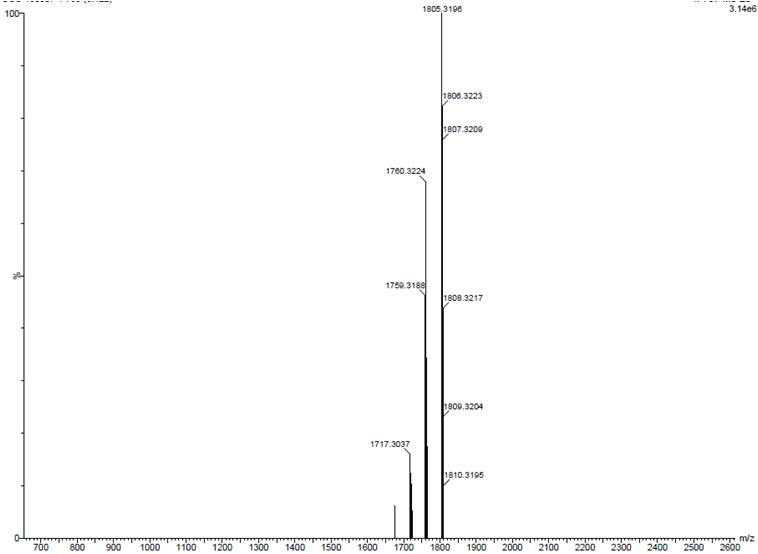
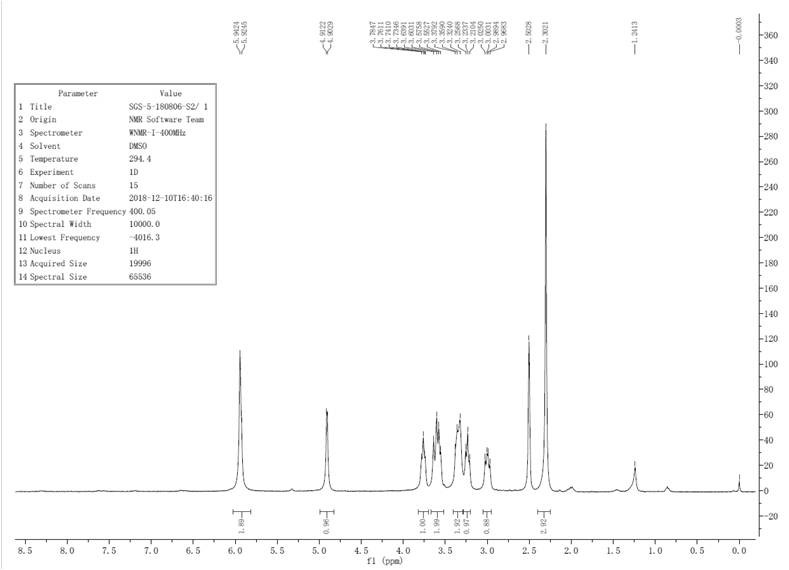
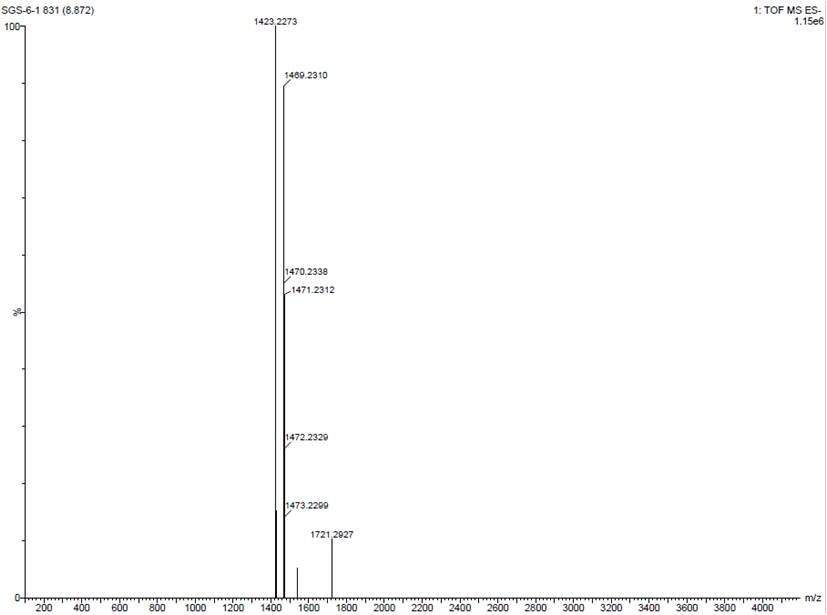
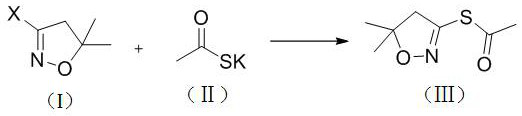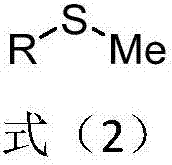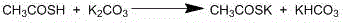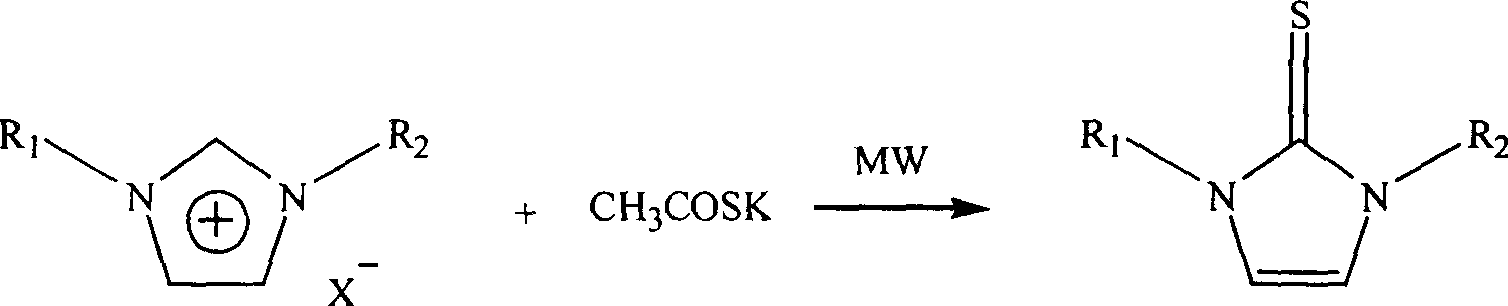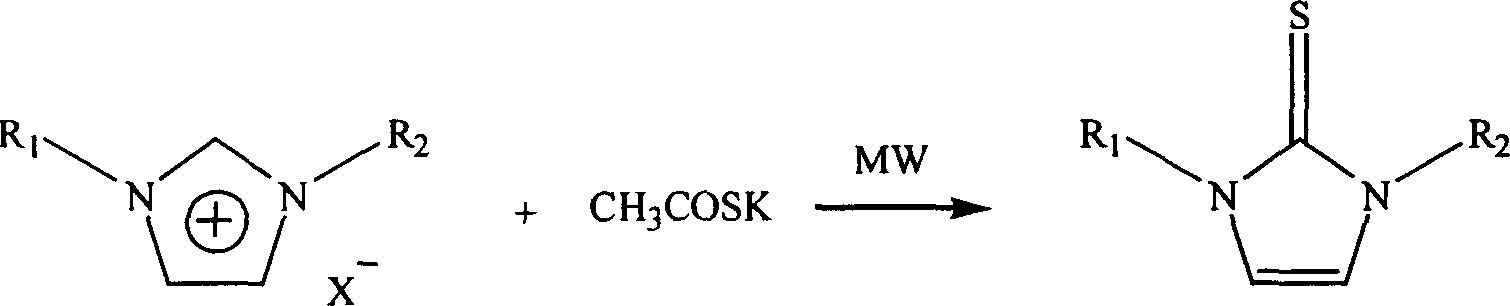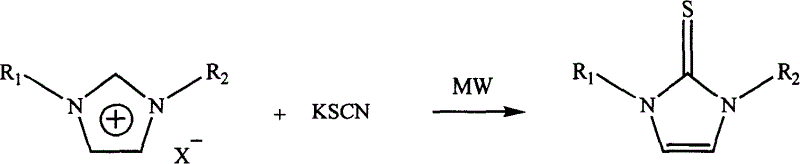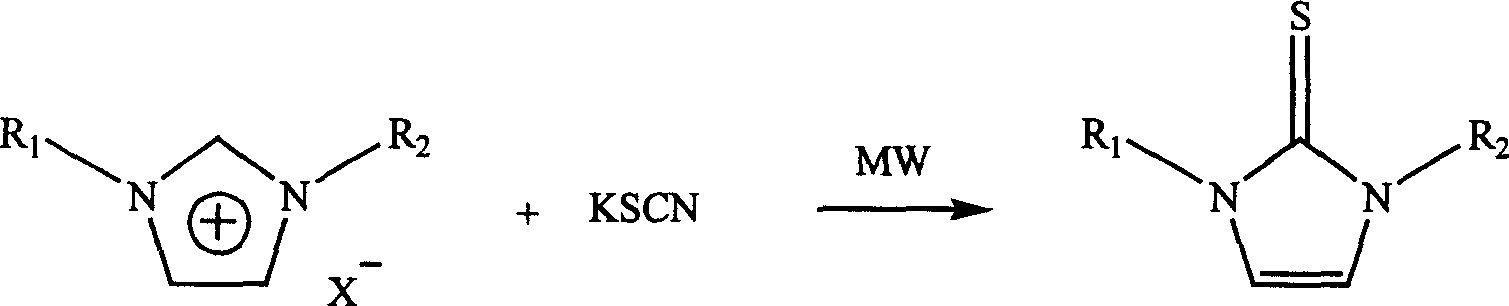Patents
Literature
42 results about "Potassium thioacetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
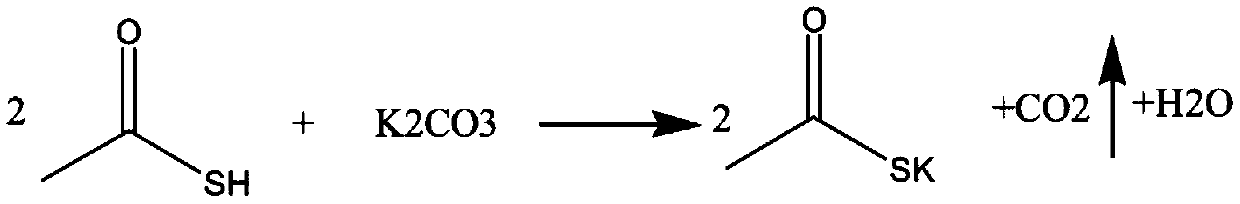
Potassium thioacetate is an organosulfur compound and a salt with the formula CH₃COS⁻K⁺. This white, water-soluble solid is used as a reagent for preparing thioacetate esters and other derivatives.
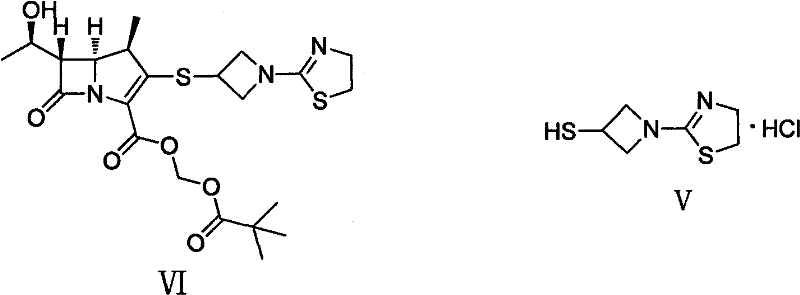
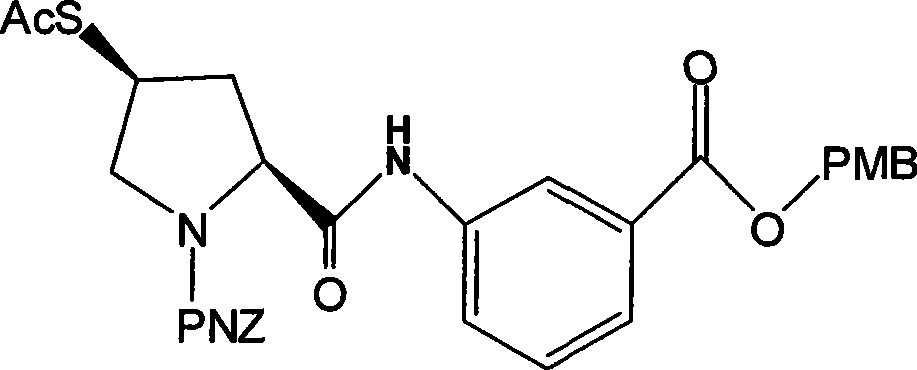
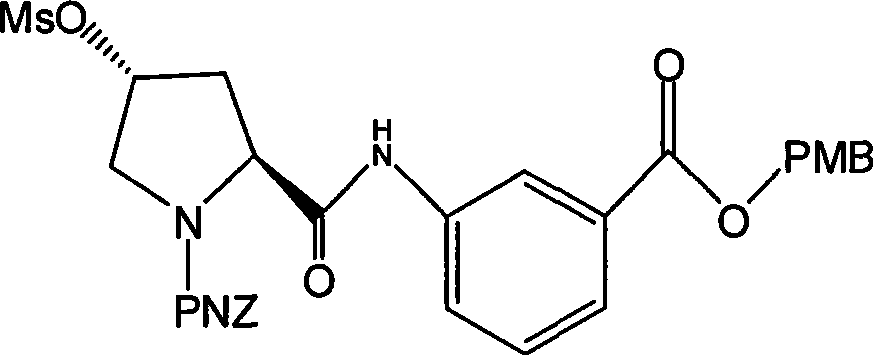
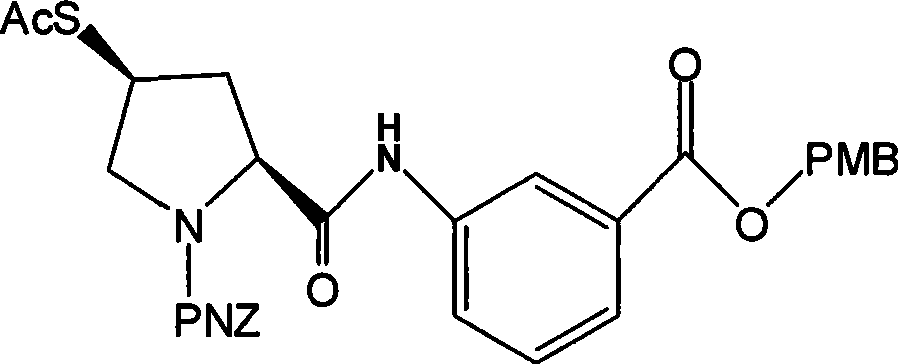
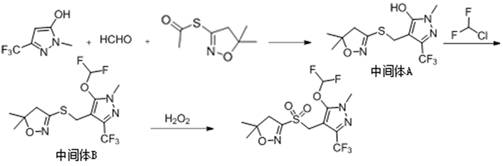
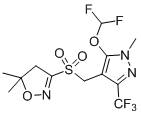
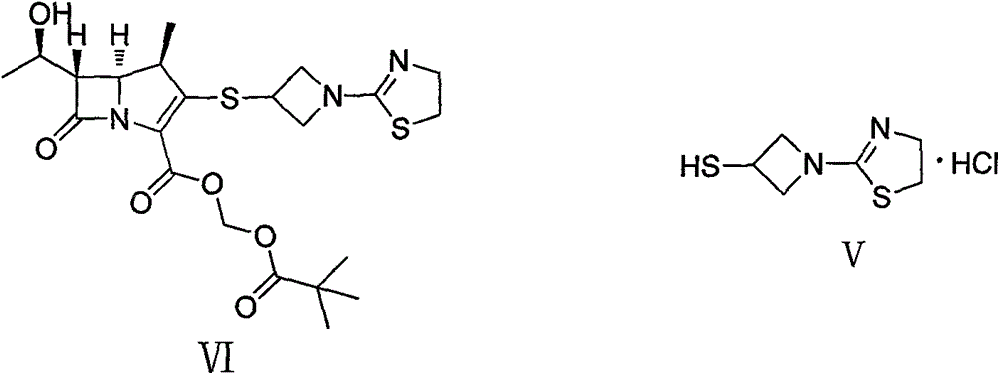
Preparation of carbapenem penicillin ertapenem intermediate
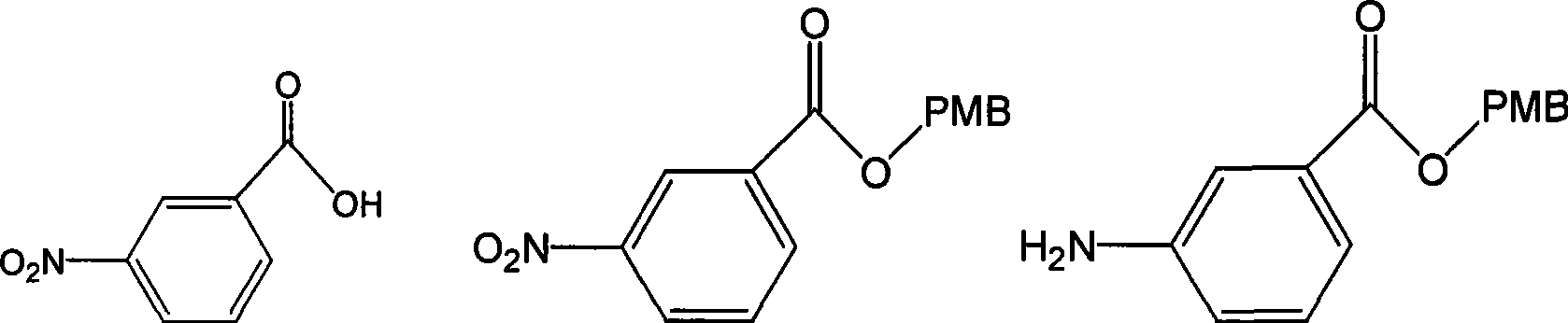
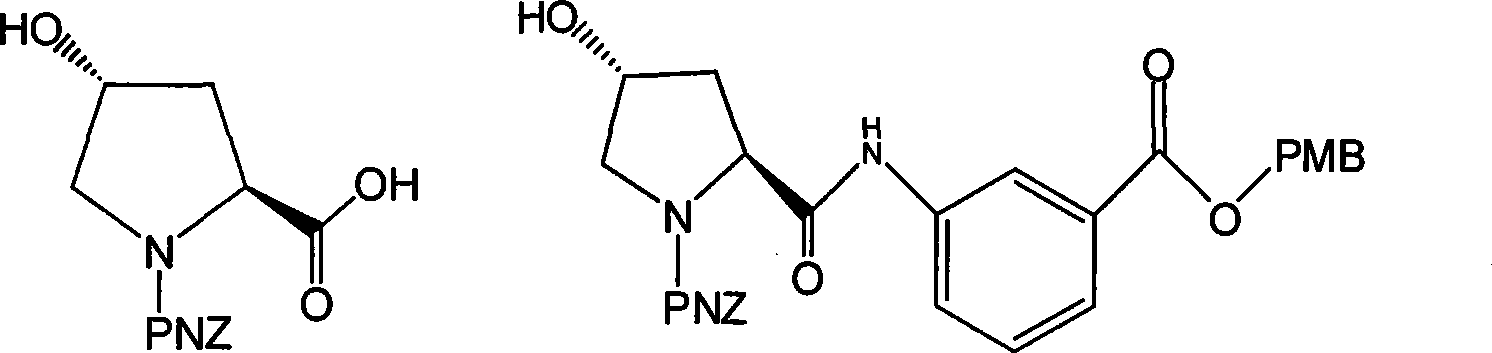
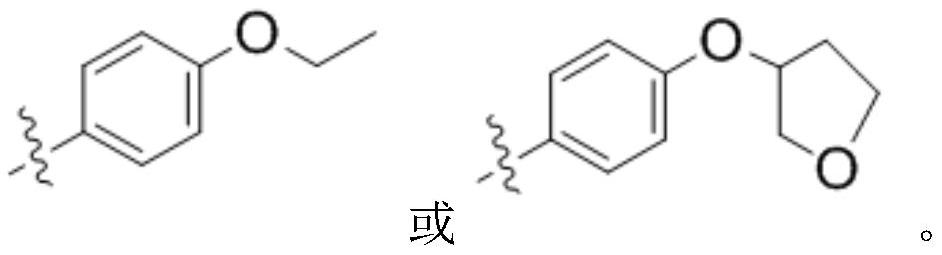
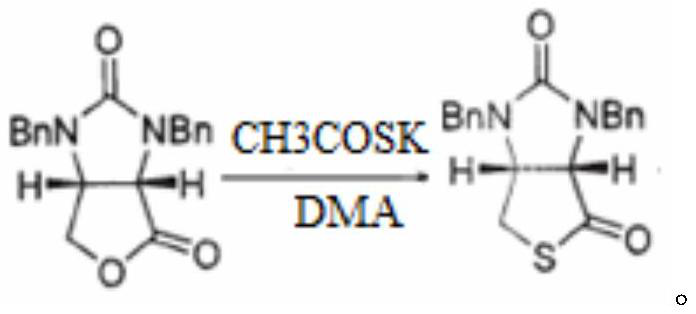
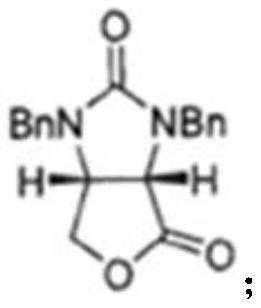
The invention discloses a preparation method of an intermediate for synthesizing carbapenems penicillin etapenem having the formula of VII. The preparation method comprises the following steps: allowing a compound having the formula of I and 4-methoxybenzyl chloride to react to obtain a product; reacting under the action of stannous chloride dehydrate, and regulating pH to 7; performing condensation reaction of above product and activated ester of PNZ L-hydroxyproline; reacting with methylsulfonyl chloride; reacting with potassium thioacetate; and hydrolyzing in acidic or alkaline condition, wherein PMB is shown in figure (1), PNZ is shown in figure (2), Ms is mesyl, and Ac is acetyl. The preparation method can be carried out at the room temperature, with the advantages of mild reaction condition, low cost, and easily realized industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
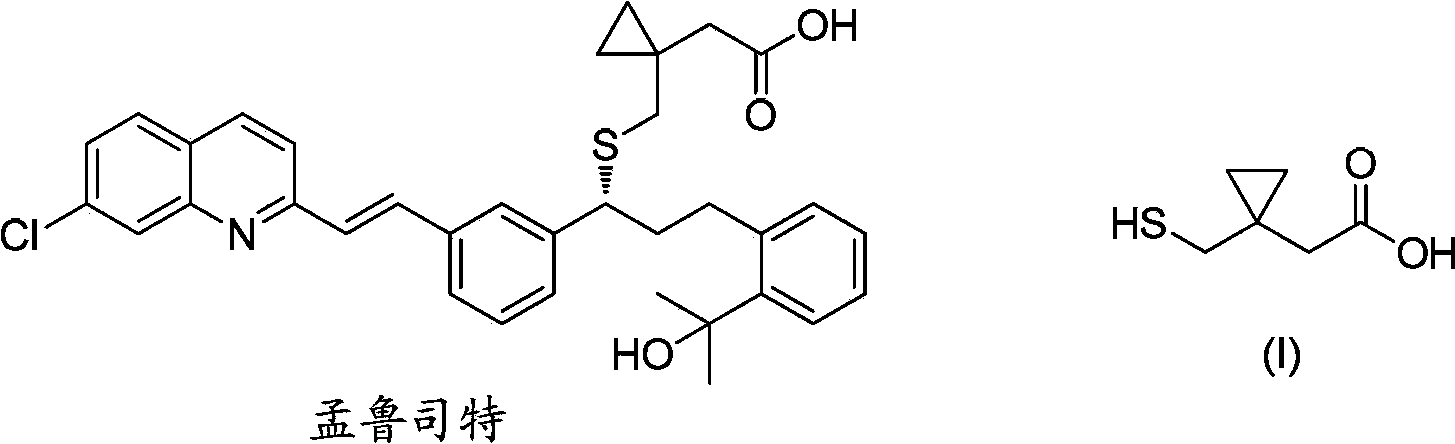
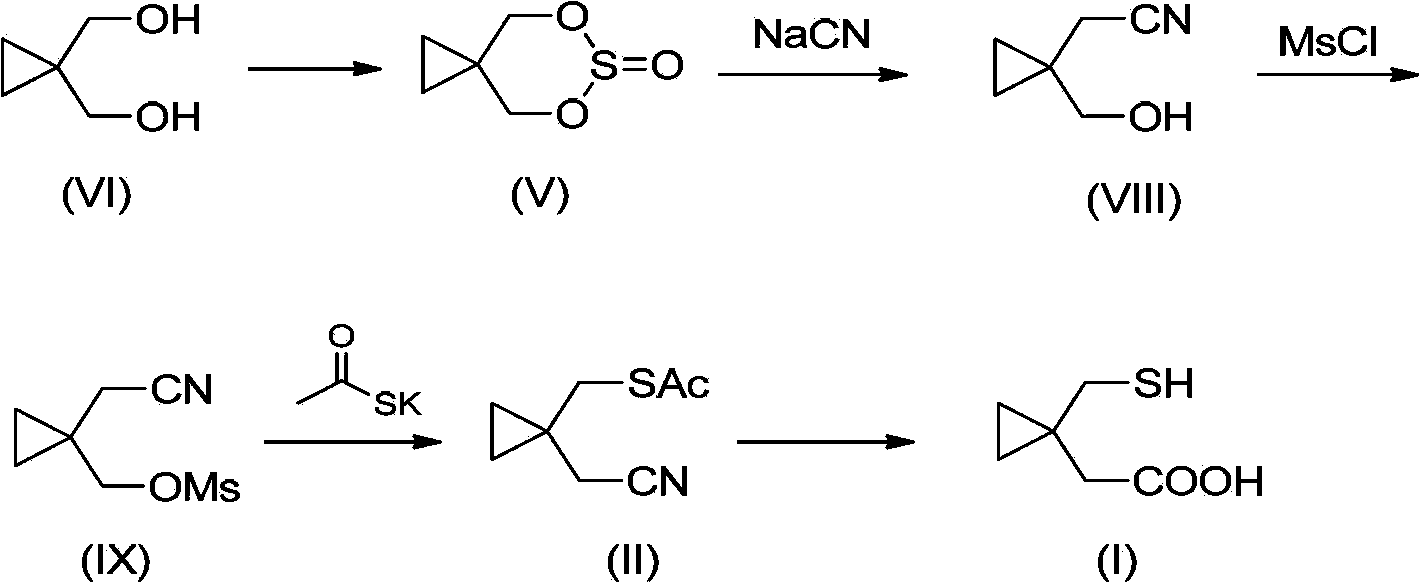
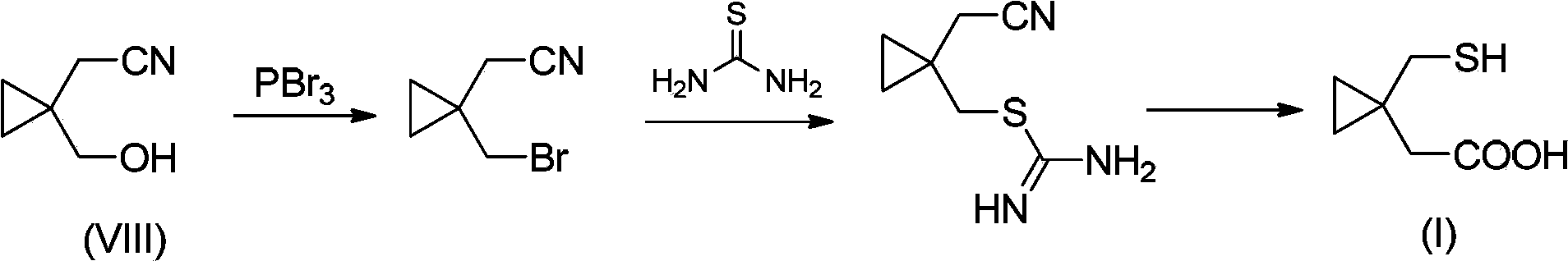
Preparation methods of 1-(mercaptomethyl)cyclopropyl acetic acid and intermediate thereof
InactiveCN103539714AIngenious designStarting materials are cheap and readily availableThiol preparationAcetic acidSulfite
The invention relates to a preparation method of 1-(mercaptomethyl)cyclopropyl acetic acid (I). The preparation method comprises the steps of carrying out ring opening on cyclopropanedimethanol cyclic sulfite (V) with potassium thioacetate, so that a compound (IV) is obtained; carrying out sulfonic acid esterification reaction on the compound (IV) and methanesulfonyl chloride or paratoluensulfonyl chloride to obtain a compound (III); carrying out cyano group substitution on the compound (III) to obtain a compound (II); and hydrolyzing the compound (II) under an alkaline condition so as to obtain the 1-(mercaptomethyl)cyclopropyl acetic acid (I), wherein R is a methyl or a p-methylphenyl. The invention further provides a preparation method of an 1-(mercaptomethyl)cyclopropyl acetic acid intermediate. The preparation methods of the 1-(mercaptomethyl)cyclopropyl acetic acid and the 1-(mercaptomethyl)cyclopropyl acetic acid intermediate are ingenious in design, initial raw materials are low in cost and easily available, and the technological process is simple and practicable, so that the production cost can be greatly reduced; the preparation methods are beneficial to industrial production and suitable for large-scale popularization and application.
Owner:SHANGHAI PUYI CHEM CO LTD
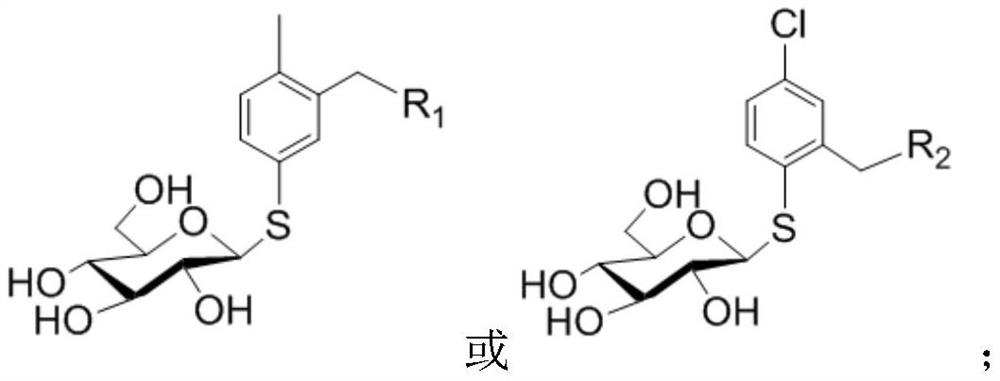
Preparation method and applications of glucosyl modified quantum dot fluorescent probe
PendingCN106566525AEasy to prepareGood water solubilityFluorescence/phosphorescenceLuminescent compositionsSolubilitySodium methoxide
The invention discloses a preparation method of a glucosyl modified quantum dot fluorescent probe. The preparation method comprises: adopting D-glucose as a raw material, carrying out hydroxyl complete protection and terminal glycosylation, adding a N,N-dimethylformamide solution and potassium thioacetate to catalyze, removing the protection group through sodium methoxide / methanol to obtain mercapto derivatized glucose, and modifying the mercapto derivatized glucose on the surface of quantum dots through ligand exchange to obtain the glucosyl modified quantum dot fluorescent probe. According to the present invention, the prepared glucosyl modified quantum dot fluorescent probe is used for detecting the pesticide fenpropathrin content; and the glucosyl modified quantum dot fluorescent probe has the specific recognition ability of sugar and the special properties of quantum dots, and further has advantages of good water solubility and long stabilization time, and the preparation method has advantages of short synthesis route of the glucosyl modified body, simple synthesis operation, simple preparation method of the modified quantum dots, and rapid preparation.
Owner:GUANGXI TEACHERS EDUCATION UNIV
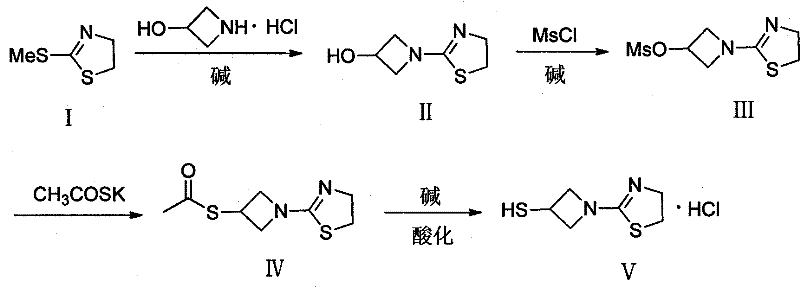
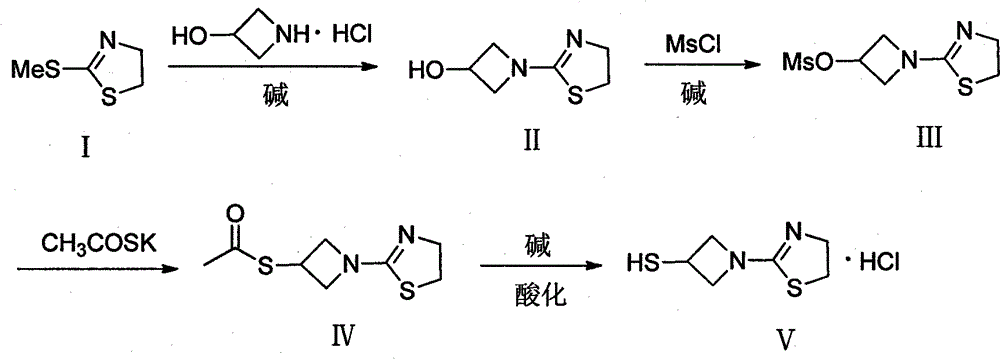
Preparation method of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride
ActiveCN102250080ARaw materials are easy to getSimple process routeOrganic chemistryHydrogenMethane sulfonate
The invention discloses a method for synthesis of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride. With 2-methylthio-2-thiazoline (I) as the raw material, the method of the invention comprises the steps of: in the presence of alkali, reacting 2-methylthio-2-thiazoline (I) with 3-hydroxyazetidine hydrochloride so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-hydroxyazetidine (II), and reacting (II) with methyl sulfonylchloride in the presence of alkali so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-methane sulfonate group azetidine (III), subjecting (III) to a reaction with potassium thioacetate so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-thioacetic acid ester group azetidine (IV), in the presence of alkali, subjecting (IV) to hydrolysis and then to acidification with dilute hydrochloric acid, thus obtaining the 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride (V). With easily available and inexpensive starting material, the method of the invention simplifies the synthesis route, improves the raw material utilization ratio and the overall yield. An intermediate obtained in the reaction can be subjected to refinement by a recrystallization method or to a next reaction directly, so that the yield is high and the "three wastes" produced during the reaction process are few. In addition, with low cost, the method of the invention is beneficial for industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
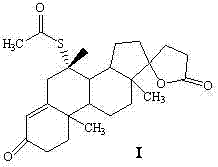
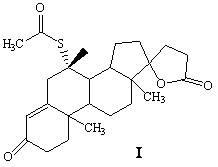
Synthetic method of spironolactone
ActiveCN102321139BReduce pollutionPromote sustainable developmentSteroidsChemical synthesisAcetic acid
A synthetic method of spironolactone belongs to the technical field of chemical synthesis. The method comprises the following steps: heating and refluxing for 3-5 hours to perform an addition reaction with canrenone and potassium thioacetate as raw materials and ethanol as a solvent in the presence of an acidic catalyst, after the reaction, cooling to -10 DEG C, performing heat preservation for 1.5-2.5 hours, filtering, treating the filter cake to obtain spironolactone, wherein the feeding molar ratio of canrenone, potassium thioacetate, and the acidic catalyst is 1:2.1:2.1. The invention adopts thiacetate instead of thioacetic acid, which effectively solves the environmental protection pressure caused by thioacetic acid, and reduces environment pollution; the method has a simple process, is easy to operate, and has high economic and environmental protection benefit; the invention mainly solves the technical problem of existing synthetic methods that the special smell of the raw material of thioacetic acid causes high environmental protection pressure for enterprises.
Owner:ZHEJIANG LANGHUA PHARMA
Halogenated-1-(4-mercaptobutyl)-3-methylimidazole ionic liquid, preparation method and application thereof
InactiveCN107501188AGood fluorescenceSave energyOrganic chemistryComponent separationPesticide residueFluorescence
The invention discloses a halogenated-1-(4-mercaptobutyl)-3-methylimidazole ionic liquid and its preparation method and application. The invention uses N-methylimidazole, potassium thioacetate, 1,4- Dihalobutane is used as a raw material, and the target product is synthesized through a three-step reaction; the reaction cycle of the present invention is short, the reaction conditions of each step are mild, the post-treatment process is simple, the repeatability and stability are good, and it is suitable for popularization and application; the ionic liquid prepared by the present invention It can be applied to the micro-extraction system of ionic liquid and used as an extractant or dispersant; the ionic liquid prepared by the present invention can greatly improve the fluorescence sensitivity of detection in the fluorescence detection of biological macromolecules, and can also be used as a fluorescent probe for pesticide residue detection , has good analytical application value.
Owner:ZHEJIANG UNIV OF TECH +1
Preparation method and application of peracetyl-protected 1-thioglucose and glucose 1-mercaptan
ActiveCN112538099AWide variety of sourcesMild conditionsEsterified saccharide compoundsSugar derivativesChemical synthesisHydrazine compound
The invention belongs to the technical field of medicine and sugar chemical synthesis, and particularly relates to a preparation method and application of peracetyl-protected 1-thioglucose and glucose1-mercaptan. The preparation method comprises the following steps of reacting peracetyl-protected glucose and potassium thioacetate in an organic solvent at the temperature of between normal temperature and 50 DEG C under the catalysis of boron trifluoride diethyl ether for 4-8 hours to obtain peracetyl-protected 1-thioglucose; and dissolving the prepared peracetyl-protected 1-thioglucose in dimethylformamide, and removing thioacetyl by using hydrazine hydrate to obtain peracetyl-protected glucose 1-mercaptan. The peracetyl-protected glucose 1-mercaptan can be used for further preparing auronofen and gliclazide thioglycoside analogues. The method disclosed by the invention is mild in reaction condition, simple and convenient to operate, low in synthesis cost, relatively green and high inyield, the auronofen is a medicine for treating rheumatic arthritis, and the gliflozin thioglycoside analogue is a potential medicine for treating type 2 diabetes mellitus.
Owner:HUAZHONG UNIV OF SCI & TECH
Synthetic method of spironolactone
ActiveCN102321139AReduce pollutionPromote sustainable developmentSteroidsChemical synthesisAcetic acid
A synthetic method of spironolactone belongs to the technical field of chemical synthesis. The method comprises the following steps: heating and refluxing for 3-5 hours to perform an addition reaction with canrenone and potassium thioacetate as raw materials and ethanol as a solvent in the presence of an acidic catalyst, after the reaction, cooling to -10 DEG C, performing heat preservation for 1.5-2.5 hours, filtering, treating the filter cake to obtain spironolactone, wherein the feeding molar ratio of canrenone, potassium thioacetate, and the acidic catalyst is 1:2.1:2.1. The invention adopts thiacetate instead of thioacetic acid, which effectively solves the environmental protection pressure caused by thioacetic acid, and reduces environment pollution; the method has a simple process, is easy to operate, and has high economic and environmental protection benefit; the invention mainly solves the technical problem of existing synthetic methods that the special smell of the raw material of thioacetic acid causes high environmental protection pressure for enterprises.
Owner:ZHEJIANG LANGHUA PHARMA
Carbapenem penicillin ertapenem intermediate, and preparation and use thereof
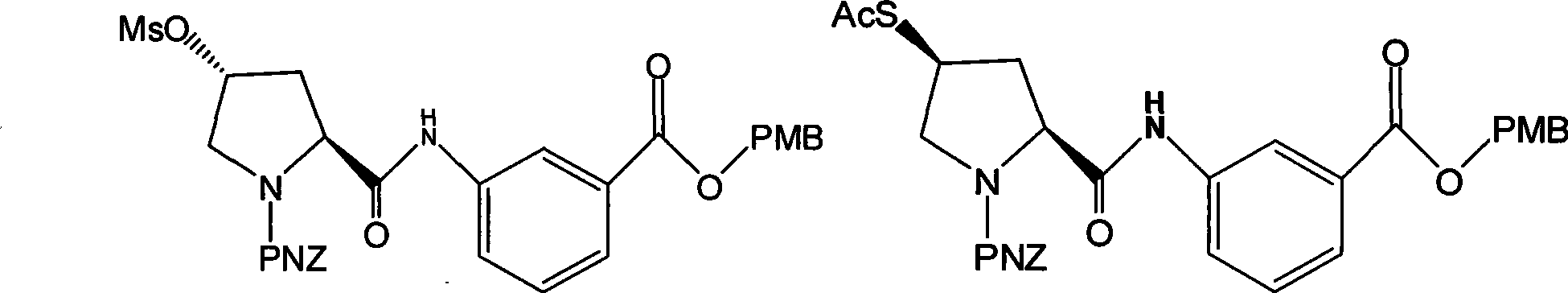
The invention discloses a preparation method of an intermediate for synthesizing carbapenems penicillin etapenem having the formula of VI, and a preparation method and application in the preparation of the carbapenems penicillin etapenem thereof. The preparation method comprises the following steps: heating a shown compound and potassium thioacetate for reacting, wherein PMB is shown in figure (1), PNZ is shown in figure (2), Ms is mesyl, and Ac is acetyl. The intermediate can be used for preparing etapenem side chain, and further etapenem. The preparation method can be carried out at the room temperature, with the advantages of mild reaction condition, low cost, simple operation, simple experiment condition, no requirement of expensive reagent, high yield, and easily realized industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
Preparation method of thioacetic acid potassium
This invention relates to a preparation method of thioacetic acid kalium. In existing method, hydrogen sulfide does not has plenary reaction, so utilization ratio is low, result in hydrogen sulfide dosage is large, cost high and three waste are too many, especially in exhaust gas, has serious pollution to environment. This invention uses ketene dimer and sodium polysulfide to inlet hydrogen sulfide gas to absolute alcohol solvent, add antioxidant, after completeness thio reaction, rectify to gain thioacetic acid and absolute alcohol solvent; add thioacetic acid or its hybrid with absolute alcohol solvent by distribution droplets to absolute alcohol solvent of potassium hydroxide, whipping, decompress and reclaim absolute alcohol solvent, and the precipitable white crystal is thioacetic acid kalium.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
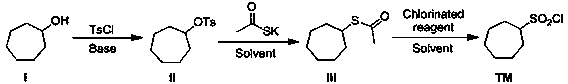
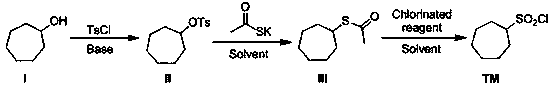
Synthetic method of macrocyclic inhibitor intermediate cycloheptane sulfonyl chloride
InactiveCN110483338AImprove responseRaw materials are easy to getSulfonic acid esters preparationSulfonic acid preparationSulfonyl chlorideSulfonate
The invention discloses a synthetic method of a macrocyclic inhibitor intermediate cycloheptane sulfonyl chloride. The synthetic method comprises the following steps of 1, reacting a compound I cycloheptanol with p-toluenesulfonyl chloride to obtain a compound II cycloheptane 4-methyl benzene sulfonate; 2, reacting a compound II with the potassium thioacetate to obtain a compound III cycloheptaneethane sulfate; and 3, oxidizing the compound III to obtain the compound cycloheptane sulfonyl chloride. The synthetic method of the macrocyclic inhibitor intermediate cycloheptane sulfonyl chloride provided by the invention is simple in reaction, simple and convenient in post-treatment, high in yield, low in cost and strong in operability, can obtain the raw materials easily, and is suitable forthe industrial production.
Owner:苏州汉德创宏生化科技有限公司
S-(5, 5-dimethyl-4, 5-dihydroisoxazole-3-yl) ethyl sulfate as well as synthesis method and application of S-(5, 5-dimethyl-4, 5-dihydroisoxazole-3-yl) ethyl sulfate
ActiveCN113135867AEasy to synthesizeEfficient synthesisOrganic chemistryBulk chemical productionThio-Combinatorial chemistry
The invention discloses S-(5, 5-dimethyl-4, 5-dihydroisoxazole-3-yl) ethyl sulfate as well as a synthesis method and application of the S-(5, 5-dimethyl-4, 5-dihydroisoxazole-3-yl) ethyl sulfate. According to the preparation method, 3-halogenated-5, 5-dimethyl-4, 5-dihydroisoxazole reacts with potassium thioacetate to prepare S-(5, 5-dimethyl-4, 5-dihydroisoxazole-3-yl) ethyl sulfate. According to the method, the synthesis process is simplified, the reaction speed is high, the product purity is high, the yield is high, the reaction condition is mild, the equipment cost is low, the synthesis process and the post-treatment process are extensive, the product is easy to separate, the obtained product can be used as a pyroxasulfone intermediate, and a plurality of defects of the 5, 5-dimethyl-4, 5-dihydroisoxazole thiamidine hydrochloride intermediate are avoided.
Owner:SHANDONG RUNBO BIOTECH CO LTD
Methyl aryl thioether compound, and synthetic method and applications thereof
InactiveCN106866327AEasy to operateWide variety of sourcesAntibacterial agentsMercapto/sulfide group formation/introductionNatural productPalladium catalyst
The invention discloses a methyl aryl thioether compound represented by formula 2, and a synthetic method and applications thereof. According to the synthetic method, in a reaction solvent, an aryl halide or an aromatic halide, dimethyl carbonate, and potassium thioacetate are taken as reaction raw materials, reaction is carried out in the presence of metal palladium catalyst under the action of a ligand and an alkali so as to obtain the methyl aryl thioether compound. The reaction conditions of the synthetic method are mild; the raw materials are cheap and easily available; reaction operation is simple; yield is relatively high. The methyl aryl thioether compound can be used for providing skeleton structures for the synthesis of a plurality of natural products and medicines, and can be widely applied in industrialized large-scale production.
Owner:EAST CHINA NORMAL UNIV
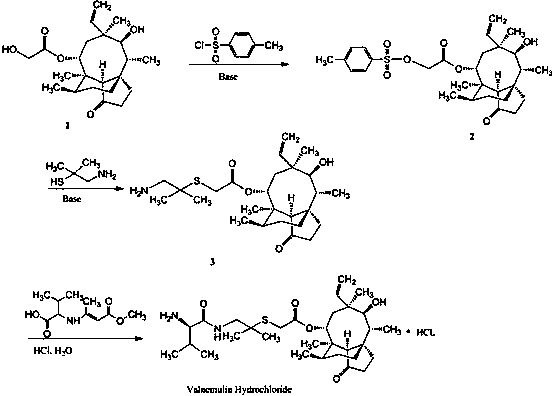
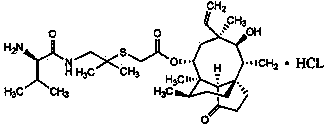
Synthesis of intermediate compound of warnemulin hydrochloride and preparation method of warnemulin hydrochloride
InactiveCN106496085BOrganic compound preparationSulfonic acid esters preparationChemical structurePropylamine
The invention discloses an intermediate compound used for synthesizing valnemulin hydrochloride. The chemical structure of the intermediate compound used for synthesizing valnemulin hydrochloride is represented by a formula in the invention. A preparation method of the intermediate compound comprises following steps: beta-hydroxyisovaleric acid is subjected to Curtius rearrangement reaction so as to obtain 2-methyl-2-hydroxy propylamine; 2-methyl-2-hydroxy propylamine and D-valine Dane salt are subjected to mixed anhydride method reaction so as to obtain an amide product; the amide product is subjected to hydroxy activation under alkaline conditions with methylsulfonyl chloride, and then is reacted with potassium thioacetate. The preparation method of valnemulin hydrochloride comprises following steps: pleuromutilin is reacted with paratoluensulfonyl chloride so as to obtain pleuromutilin p-tosylate; pleuromutilin p-tosylate is reacted with the intermediate compound, and hydrochloric acid deprotection is carried out so as to obtain valnemulin hydrochloride. In the preparation method of valnemulin hydrochloride, no dimethyl cysteamine hydrochloride is needed, production conditions are mild, no environment pollution is caused, and the preparation method is suitable for industrialized production.
Owner:盐城市优化医药化工科技有限公司
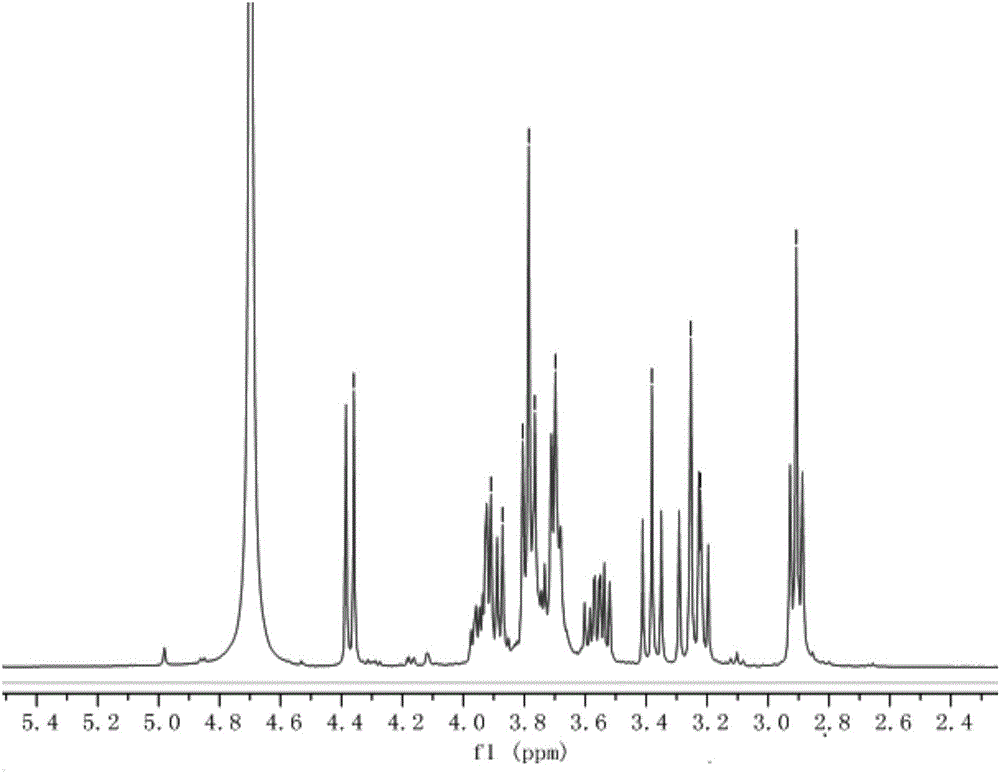
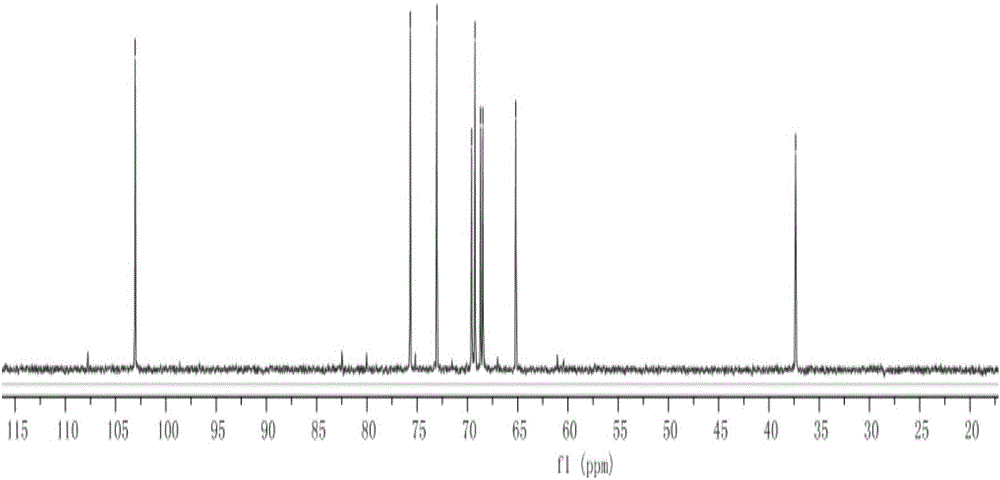
Xylose compound with terminal group containing HS-(PEG)-2-O branch chain and synthesis method of xylose compound
InactiveCN106543242AImprove responseMild conditionsEsterified saccharide compoundsSugar derivativesSodium methoxideGlycoside
The invention discloses a xylose compound with a terminal group containing a HS-(PEG)-2-O branch chain and a synthesis method of the xylose compound with the terminal group containing the HS-(PEG)-2-O branch chain. The compound is shown as the formula I (the formula is shown in the description). The synthesis method comprises the following steps that xylose is subjected to an acetylation or benzoylation reaction and terminal group glycosylation sequentially and then reacts with potassium thioacetate in a N,N-dimethylformamide solution at normal temperature, Ac or Bz protecting group removal is finally conducted with sodium methylate / methanol, and then the xylose compound with the terminal group containing the HS-(PEG)-2-O branch chain is obtained. The xylose compound with the terminal group containing the HS-(PEG)-2-O branch chain shows the good water solubility due to the fact that multiple -OH are contained and can be applied to quantum dot surface modification due to the fact that -SH is contained; the application field of the xylose compound is widened due to the fact that the xylose compound has multiple chiral centers, and therefore specific identifiability of glucose and combination of quantum dot special material properties are achieved.
Owner:GUANGXI TEACHERS EDUCATION UNIV
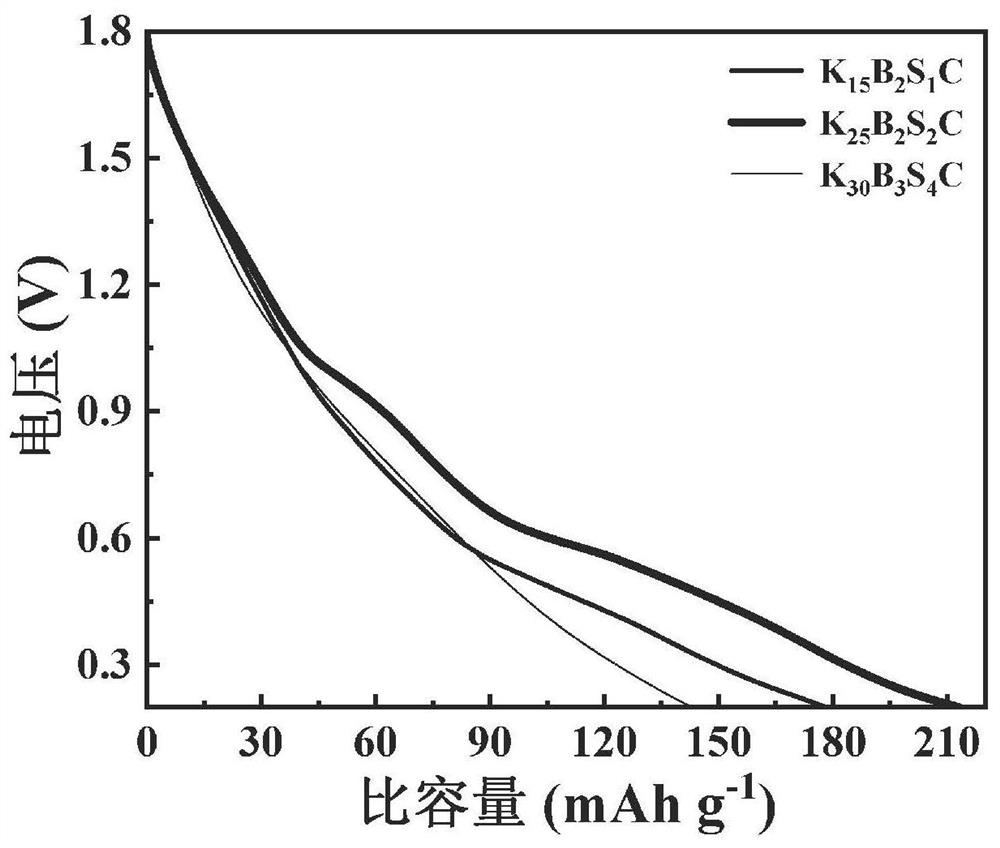
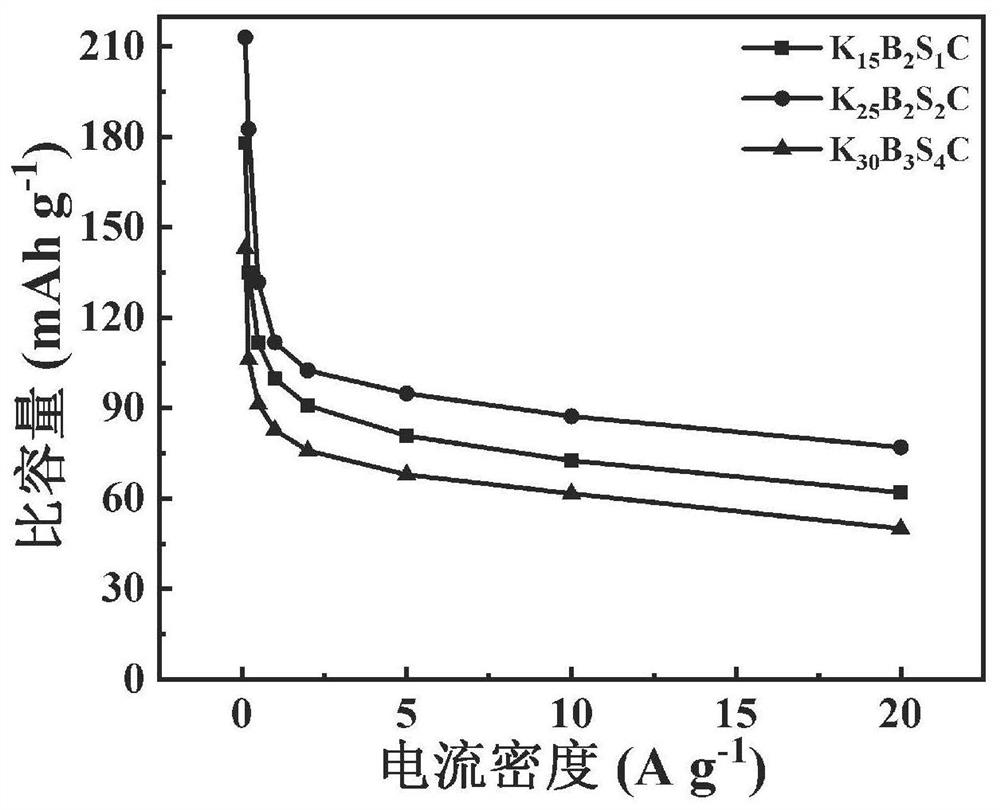
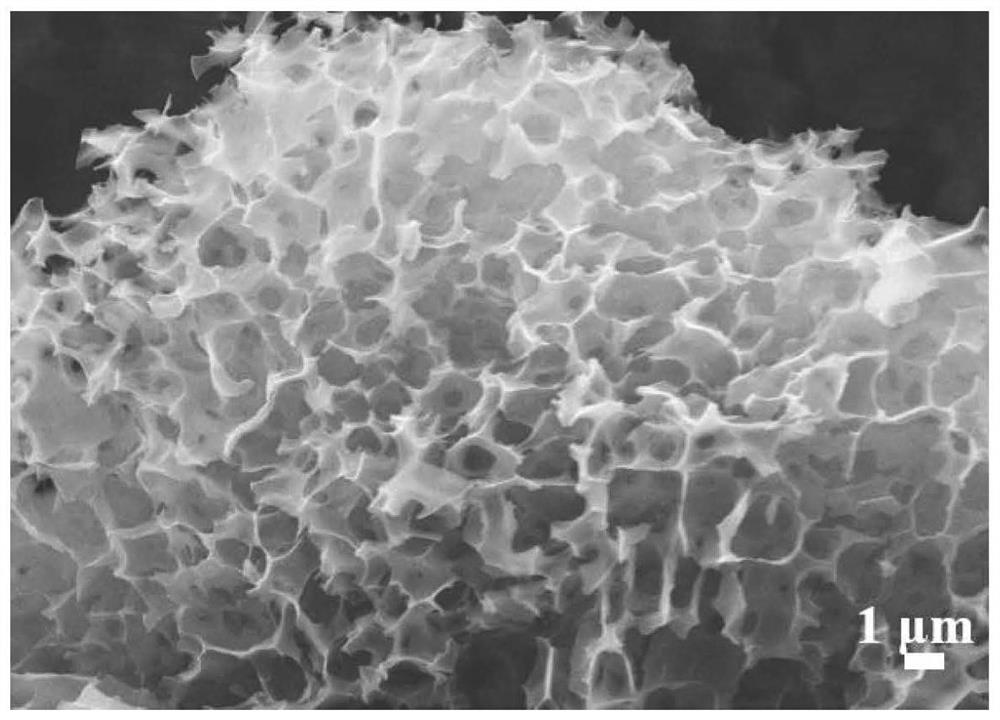
Boron-sulfur co-doped spongy porous carbon, preparation method thereof, carbon electrode and zinc ion hybrid capacitor
ActiveCN113506685AEasy to prepareImprove cycle stabilityCarbon compoundsHybrid capacitor electrodesSulfurPorous carbon
The invention provides boron-sulfur co-doped spongy porous carbon which takes carbon as a matrix and is doped with boron and sulfur, wherein the doping amount of sulfur accounts for 0.57-2.67% of the mass percent of the boron-sulfur co-doped spongy porous carbon, and the doping amount of boron accounts for 1.01-1.58% of the mass percent of the boron-sulfur co-doped spongy porous carbon; the average pore size of the boron-sulfur co-doped spongy porous carbon is 2.11-2.49 nm, and the specific surface area of the boron-sulfur co-doped spongy porous carbon is 1887-2364 m < 2 > g <-1 >; the micropore specific surface area is 205-1349 m < 2 > g <-1 >; the total pore volume is 0.67-1.12 cm < 3 > g <-1 >; and the micropore volume is 0.130-0.521 cm < 3 > g <-1 >. The boron-sulfur co-doped spongy porous carbon is prepared from bamboo sawdust, potassium carbonate, potassium tetraborate and potassium thioacetate according to the mass ratio of 5: (15-30): (2-3): (1-4).
Owner:CHINA UNIV OF GEOSCIENCES (BEIJING)
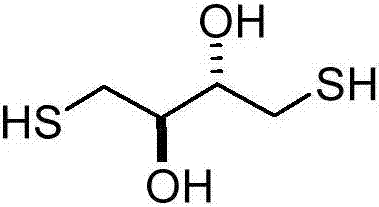
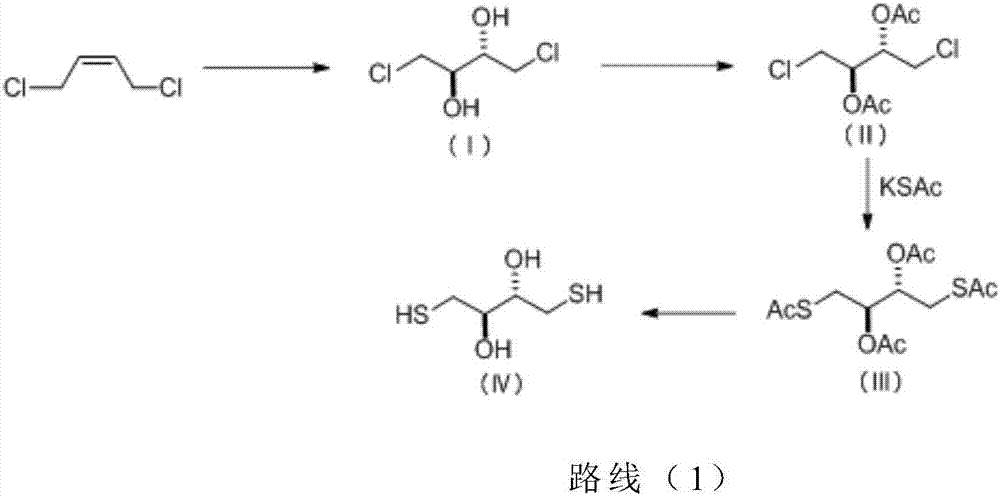
Preparation method of dithioerythritol (DTE)
InactiveCN107235872AShort synthetic routeHigh yieldPreparation by oxidation reactionsOrganic compound preparationAcetic anhydrideSodium iodide
The invention discloses a method for preparing dithioerythritol (DTE). The method comprises the following steps: (a) performing a cis-dihydroxylation reaction on the initial raw material cis-1,4-dichloro-2-butene and potassium permanganate under low temperature conditions to obtain 1,4-dichloro-2,3-butanediol disclosed as Formula (I); (b) adding the 1,4-dichloro-2,3-butanediol disclosed as Formula (I) into an organic alkali, and performing an esterification reaction with acetic anhydride to obtain 1,4-dichloro-2,3-diacetate disclosed as Formula (II); (c) performing a nucleophilic substitution reaction on the 1,4-dichloro-2,3-diacetate disclosed as Formula (II) and potassium thioacetate under the catalytic action of sodium iodide to obtain 1,4-dithioacetyl-2,3-acetate disclosed as Formula (III); and (d) hydrolyzing the 1,4-dithioacetyl-2,3-acetate disclosed as Formula (III) under acidic conditions to obtain the target product DTE disclosed as Formula (IV). The method has the advantages of low synthesis cost, short synthesis route, high safety, high product quality and high yield, is simple to operate, and conforms to the industrial production requirements.
Owner:EAST CHINA NORMAL UNIV +1
Intermediate of sugammadex sodium and preparation method thereof
The invention relates to a preparation method of sugammadex sodium serving as a muscle relaxation antagonist. The preparation method comprises the following steps: reacting gamma-cyclodextrin with potassium thioacetate after being subjected to full halogenation to prepare a key intermediate compound 6-full deoxy-6 full thioethyl ester-gamma-cyclodextrin, then hydrolyzing, and directly reacting with acrylic acid in one pot to prepare the sugammadex sodium. The method is mild in reaction condition, simple and convenient in post-treatment operation, good in process repeatability and high in yield.
Owner:BEIJING TIDE PHARMA
Novel method for preparing potassium thioacetate
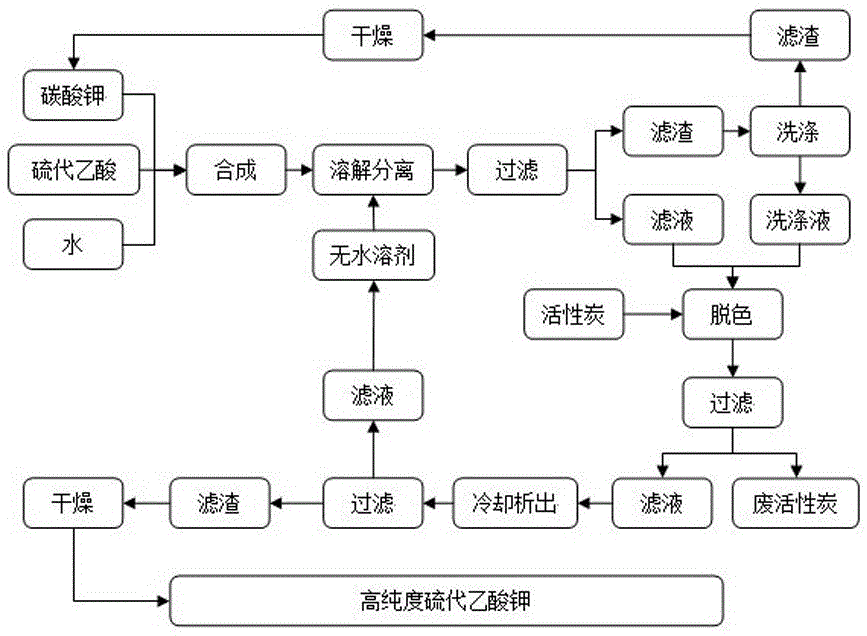
The invention discloses a novel preparation method of potassium thioacetate, and belongs to the technical field of synthesis and purification. The method comprises the following steps: by taking high-concentration ethanol as a solvent, reacting thioacetic acid and potassium carbonate and crystallizing synchronously in the same reaction kettle in a low-temperature environment, and carrying out centrifugation, leaching, vacuum drying, vacuum packaging and other operations on the reaction product to obtain high-quality potassium thioacetate. According to the invention, the preparation method is simple in operation process, the product is high in yield, good in quality and beneficial to storage, the centrifuged mother liquor treatment method is environment-friendly, the product is subjected tovacuum drying and vacuum packaging, moisture absorption, hydrolysis, oxidation, discoloration and the like can be effectively prevented, the product quality is ensured, the purity of potassium thioacetate prepared by the method can reach 99.0% or above, and in a pharmaceutical process taking potassium thioacetate as a raw material, feeding is convenient, quality guarantee period is long, and customer use effect is good.
Owner:陶陈丁
Preparation method of potassium thioacetate
PendingCN106831513AWell mixedImprove responseOrganic chemistryPotassium hydrocarbonatePotassium thioacetate
The invention discloses a preparation method of potassium thioacetate. The method comprises the following steps of adding 190-240kg of potassium carbonate or potassium bicarbonate to a 500L tapered helical tape vacuum drier, and starting helical tape stirring; adding 200kg of thioacetic acid, stirring evenly, and then adding 10-20kg of water in a spray manner; starting vacuum, wherein the vacuum degree is -0.01MPa to -0.09MPa; and heating to 40-80 DEG C for 20-30min and carrying out heat preservation reaction for 2-5h after the temperature reaches 40-80 DEG C for later use. The technical problem of how to efficiently prepare the potassium thioacetate at low cost can be solved.
Owner:绍兴市上虞区耕创化工技术服务部
Preparatino method of (2S,4S)-1-(4-nitro carbobenzoyl)-2-[(3-allyloxycarbonyl)-phenyl aminoformyl]-pyrrolidine -4-thioalcohol
InactiveCN100344613CConvenient sourceEase of industrial productionOrganic chemistryCarbonyl chlorideThiol
A process for preparing (2S, 4S)-1-(4-nitrobenzyloxycarbonyl)-2-[(3- allyloxycarbonyl)-phenylaminoformyl]-pyrrolidine-4-yl thiol includes such steps as reaction between trans-4-hydroxy-L-pyrrolidine and p-nitrobenzyl carbonyl chloride to obtain product A, the reaction of meta-nitrobenzoic acid on dichlorosulfoxide and then on allyl alcohol to obtain allyl meta-nitrobenzoate, reducing it by SnCl2 to obtain allyl meta-aminobenzoate, condensation reaction on said product A, activating the resultant by methylsulfuryl chloride, reaction on potassium thioacetate, and hydrolyzing.
Owner:SHANGHAI JIAO TONG UNIV
Microwave radiation synthesis of 1,3-substituted imidazole-2-thioketone
InactiveCN1300117CQuick responseThe reaction is easy to operateOrganic chemistryAfter treatmentOrganic solvent
Synthesis of 1,3-dibasic imidazole-2-thio-ketone by micro-wave radiation is carried out by taking 1,3-dibasic imidazole onium salt and potassium thiacetic with amount ratio 1:1-3, synthesizing 1,3-dibasic imidazole-2-thio-ketone with power 50-300W and reacting time 2-20mins, extracting, filtering, washing and concentrating. R1=alkyl(C1-4), benzyl, R2=alkyl(C1-4), benzyl, allyl, ethoxyl, and acetoxy ethyl. Its advantages include fast reacting speed, simple operation and after treatment, and no pollution.
Owner:ZHEJIANG UNIV
Coal gangue particles for immobilizing ammonia oxidizing bacteria and preparation method of coal gangue particles
InactiveCN108101232AHigh strain activityEfficient degradationTreatment using aerobic processesWater contaminantsAmmonia-oxidizing bacteriaBacterial strain
The invention discloses coal gangue particles for immobilizing ammonia oxidizing bacteria. The preparation method comprises the following steps: cleaning coal gangue particles, and modifying with mixed liquid of CuCl2, potassium thioacetate, PbCl2 and FeCl3 to obtain a substance B; modifying the substance B with mixed liquid of CoCl2, CeCl3, NaOH, NaCl and SrCl2 to obtain a substance C; modifyingthe substance C with mixed liquid of 2-pyrrole formaldehyde and glyoxylic acid to obtain a substance D; immobilizing ammonia oxidizing bacteria on the substance D to obtain the coal gangue particles for immobilizing ammonia oxidizing bacteria. The coal gangue particles and the preparation method thereof disclosed by the invention have the following beneficial effects: the prepared coal gangue particles for immobilizing ammonia oxidizing bacteria realize high activity of bacterial strains and can effectively degrade ammonia nitrogen in wastewater.
Owner:光合强化(北京)生物科技有限公司
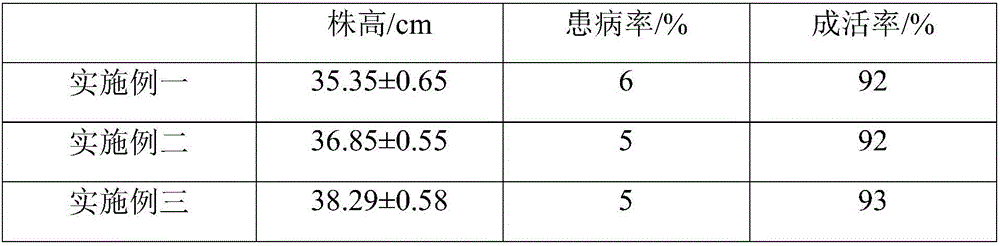
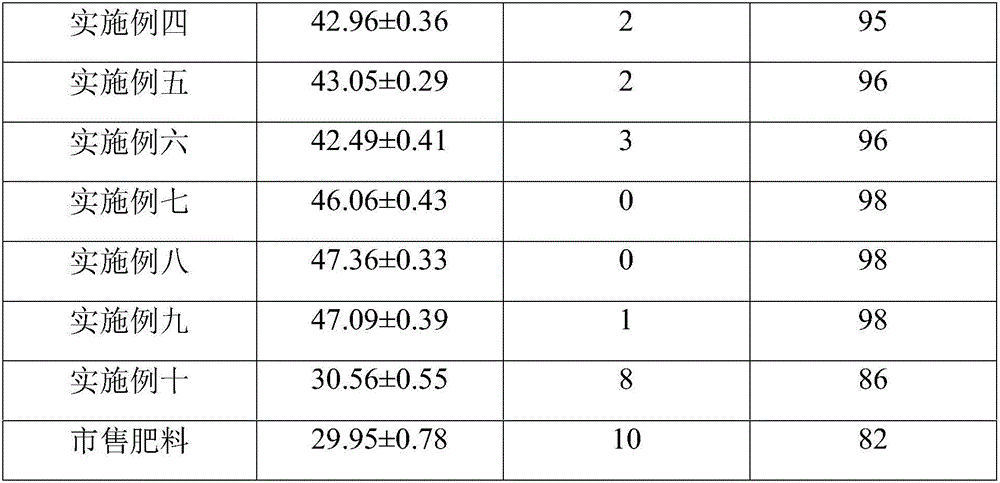
Special fertilizer having sterilization and deinsectization efficacy and used for garden flowers as well as preparation method and application of fertilizer
InactiveCN107522547AImprove survival rateIncrease profitBio-organic fraction processingAlkali orthophosphate fertiliserDiseaseRoot growth
The invention relates to special fertilizer having sterilization and deinsectization efficacy and used for garden flowers as well as a preparation method and an application of the fertilizer. The special fertilizer used for the garden flowers is prepared from components of raw materials in parts by weight as follows: 18-30 parts of monopotassium phosphate, 2-8 parts of methylphosphonic acid, 3-5 parts of tourmaline powder, 1-3 parts of potassium thioacetate, 0.5-1.5 parts of potassium acrylate, 6-8 parts of picrasma quassioides, 20-35 parts of humic acid and 0.3-0.8 parts of nicotinic acid. The provided special fertilizer contains multiple nutritional substances required in the growth process of the flowers and can provide rich oxygen for root growth of the flowers, so that the survival rate of the flowers is effectively increased. Meanwhile, the special fertilizer also has the excellent advantages of performing sterilization and deinsectization, improving soil, increasing the fertilizer utilization rate and promoting sapling growth and development, so that flower buds are full and germinate early, leaf blades are bright and thick and have clear leaf veins, the disease resistance of the flowers is notably enhanced, and the flowers grow better.
Owner:陈月桂 +1
A kind of method for preparing d-biotin thiolactone intermediate
ActiveCN107955018BReduce manufacturing costSimple operation processOrganic chemistryThio-Process engineering
The invention discloses a method for preparing a D-biotin thiolactone intermediate, which comprises the following steps: a) adding the lactone intermediate and potassium thioacetate to a reaction vessel at a mass ratio of 2:1 to 2.4:1 , heating until the reaction system is in a completely molten state, and then insulated and stirred for 1 to 5 hours; b) cooling down to 50 to 80°C, and then adding 2 to 10 milliliters of water to the reaction system according to 1 gram of lactone intermediate; c) ) lowering the temperature of the reaction system to 0-10°C while stirring, and then maintaining the temperature and continuing to stir for 1-5 hours; d) filtering, and the collected solid is the D-biotinthiolactone intermediate. The invention realizes the participation of no solvent in the reaction, not only has no problems of solvent environmental protection and recovery, but also has low preparation cost and very simple operation process, especially, the high-purity target object with HPLC purity ≥ 99.5% can be obtained only by filtering and washing with water , and the mass yield ≥ 95%.
Owner:DAFENG HEGNO PHARMA +2
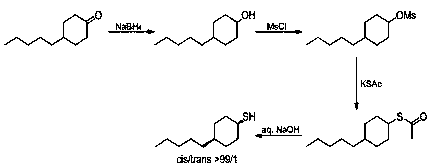
Preparation method for selectively synthesizing cis-4-n-amyl cyclohexylmereaptan
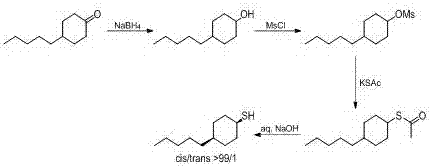
ActiveCN106916089AShort reaction timeEasy to operateThiol preparationOrganic compound preparationOrganic synthesisEthane Dichloride
The invention relates to a preparation method for selectively synthesizing cis-4-n-amyl cyclohexylmereaptan, which belongs to the field of organic synthesis. The method comprises the following steps: 4-pentylcyclohexanone and sodium borohydride are added into a tetrahydrofuran water mixed solvent, the materials are subjected to a reduction reaction to obtain the 4-amylcyclohexanol; the 4-amylcyclohexanol and methylsulfonyl chloride are subjected to a methyl sulfonylation reaction in a dichloroethanes solvent, the 4-n-amyl cyclohexyl sulfonate with content of more than 98% can be obtained through crystallization; the 4-n-amyl cyclohexyl sulfonate and potassium thioacetate are subjected to a sulfur acetylation reaction in a N,N-dimethyl formamide solvent to obtain the thiolacetic acid-4-n-amyl cyclohexyl ring; the material is subjectd to a low-temperature saponification reaction in a methanol hydrosolvent to obtain the cis-4-n-amyl cyclohexylmereaptan, and the cis products selectivity is greater than 99%. The method has the advantages of short reaction time, simple operation, and high cis products selectivity.
Owner:DALIAN JOIN KING FINE CHEM CO LTD
A kind of preparation method of selectively synthesizing cis-4-n-pentyl cyclohexanethiol
ActiveCN106916089BShort reaction timeEasy to operateThiol preparationOrganic compound preparationOrganic synthesisEthane Dichloride
The invention relates to a preparation method for selectively synthesizing cis-4-n-amyl cyclohexylmereaptan, which belongs to the field of organic synthesis. The method comprises the following steps: 4-pentylcyclohexanone and sodium borohydride are added into a tetrahydrofuran water mixed solvent, the materials are subjected to a reduction reaction to obtain the 4-amylcyclohexanol; the 4-amylcyclohexanol and methylsulfonyl chloride are subjected to a methyl sulfonylation reaction in a dichloroethanes solvent, the 4-n-amyl cyclohexyl sulfonate with content of more than 98% can be obtained through crystallization; the 4-n-amyl cyclohexyl sulfonate and potassium thioacetate are subjected to a sulfur acetylation reaction in a N,N-dimethyl formamide solvent to obtain the thiolacetic acid-4-n-amyl cyclohexyl ring; the material is subjectd to a low-temperature saponification reaction in a methanol hydrosolvent to obtain the cis-4-n-amyl cyclohexylmereaptan, and the cis products selectivity is greater than 99%. The method has the advantages of short reaction time, simple operation, and high cis products selectivity.
Owner:DALIAN JOIN KING FINE CHEM CO LTD
Method for preparing Mo-doped CdS photocatalyst by cation replacement method
ActiveCN110975890ABroaden the photoresponse rangeImprove photocatalytic activityCatalyst activation/preparationHydrogen productionEthylic acidCadmium acetate
The invention discloses a preparation method for preparing a Mo-doped CdS photocatalyst by cation replacement method. Cadmium acetate and potassium thioacetate are used as precursors, ethylene glycolis used as a solvent, CdS nanorods are synthesized through a solvothermal method, then MoCl5 is used as a precursor and ethanol is used as a medium, and cation replacement method is adopted for dopingMo ions into CdS, and the Mo-doped CdS photocatalyst is prepared. According to the photocatalyst, an impurity energy level is introduced through doping of Mo ions; vacancies are generated in crystallattices to form electron traps; therefore, the response range of CdS to visible light is expanded; the carrier migration rate is increased, photon-generated carrier separation is promoted, photon-generated carrier recombination is inhibited, more active sites are provided, and the photocatalytic activity of CdS is greatly improved; compared with the prior art, the preparation method has the advantages of simple equipment, convenience in operation and high synthesis efficiency.
Owner:FUZHOU UNIV
Process for microwave synthesis of 1,3-disubstituted imidazole-2-thioketone
The present invention discloses microwave synthesis process of 1, 3-disubstituting imidazole-2-thione. Under irradiation of microwave, 1, 3-disubstituting imidazole-onium salt and potassium thiocyanate in the equivalent ratio of 1 to 1-2 react to synthesize 1, 3-disubstituting imidazole-2-thione. The microwave radiation power is 50-200 W and microwave radiation reaction period is 2-10 min. The present invention has the advantages of short reaction period, less side reactions, high reaction yield, lower microwave radiation power, no need of dewatering, simple post-treatment, no need of organic solvent, no pollution, etc.
Owner:ZHEJIANG UNIV
Preparation method of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride
ActiveCN102250080BRaw materials are easy to getSimple process routeOrganic chemistryHydrogenMethane sulfonate
The invention discloses a method for synthesis of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride. With 2-methylthio-2-thiazoline (I) as the raw material, the method of the invention comprises the steps of: in the presence of alkali, reacting 2-methylthio-2-thiazoline (I) with 3-hydroxyazetidine hydrochloride so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-hydroxyazetidine (II), and reacting (II) with methyl sulfonylchloride in the presence of alkali so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-methane sulfonate group azetidine (III), subjecting (III) to a reaction with potassium thioacetate so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-thioacetic acid ester group azetidine (IV), in the presence of alkali, subjecting (IV) to hydrolysis and then to acidification with dilute hydrochloric acid, thus obtaining the 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride (V). With easily available and inexpensive starting material, the method of the invention simplifies the synthesis route, improves the raw material utilization ratio and the overall yield. An intermediate obtained in the reaction can be subjected to refinement by a recrystallization method or to a next reaction directly, so that the yield is high and the "three wastes" produced during the reaction process are few. In addition, with low cost, the method of the invention is beneficial for industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com