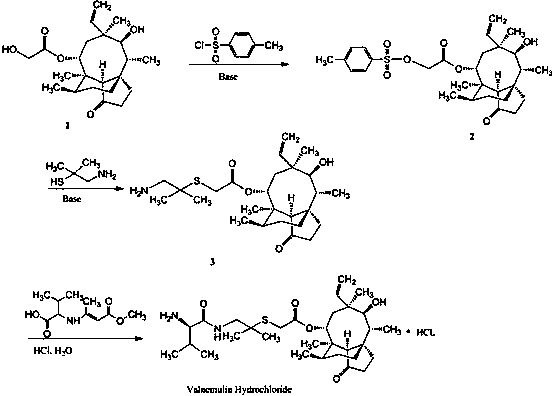
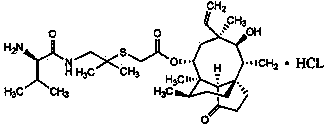
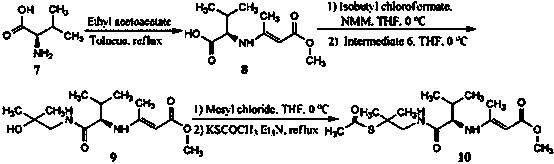
Synthesis of intermediate compound of warnemulin hydrochloride and preparation method of warnemulin hydrochloride
A technology of warnemulin hydrochloride and intermediates, which is applied in the field of medicine and can solve the problems of high preparation cost and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Synthesis of 2-methyl-2-hydroxypropylamine (intermediate 6)
[0031] Dissolve 11.81 g (0.10 mol) of β-hydroxyisovaleric acid in 250 mL of THF, add 30 mL (0.22 mol) of triethylamine, and then add 30.25 g (0.11 mol) of phosphoric acid diazide dropwise at room temperature Phenyl ester, after 15-20 min dropwise addition, the reaction was stirred at room temperature for 4 h. Then add 100 mL of distilled water, stir and reflux for 2 h. After the reaction, THF was evaporated to dryness under reduced pressure. Add 50 mL of ethyl acetate, stir, and adjust the pH to 2-3 with 5 M HCl. The two phases were separated, and the aqueous phase was extracted twice with ethyl acetate, 30 mL each time. 50 mL of ethyl acetate was added to the aqueous phase, and 5 M NaOH was added to adjust the pH to about 9. The two phases were separated, and the organic phase was extracted with water twice, 20 mL each time. Then add anhydrous Na2SO4 to dry overnight, and evaporate the solvent to ob...
Embodiment 2
[0043] (1) Synthesis of 2-methyl-2-hydroxypropylamine (intermediate 6)
[0044] Dissolve 5.91 g (50 mmol) of β-hydroxyisovaleric acid in 150 mL of THF, add 15 mL of N-methylmorpholine, and add 13.76 g (50 mmol) of diphosphodiazido dropwise at room temperature After the phenyl ester was added dropwise for 15 min, the reaction was stirred for 4 h. Then add 60 mL of distilled water, stir and reflux for 2 h. After the reaction, THF was evaporated to dryness under reduced pressure. Add 30 mL of ethyl acetate, stir, and adjust the pH to 2-3. The two phases were separated, and the aqueous phase was extracted twice with ethyl acetate, 20 mL each time. Add 30 mL of ethyl acetate to the aqueous phase to adjust the pH to about 9. The two phases were separated and extracted twice with 15 mL each. After drying with Na2SO4 and evaporating the solvent to dryness, 3.79 g of the product was obtained (85% yield).
[0045] (2) Synthesis of D-valine Deng's salt (8)
[0046] The difference ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com